Abstract
Objective: To evaluate the efficacy of exercise interventions combined with Selegiline in ameliorating freezing of gait (FOG) in Parkinson’s disease (PD) patients. Methods: A total of 60 PD patients with FOG treated in the First People’s Hospital of Fuyang District from January 2020 to January 2023 were retrospectively collected and analyzed. Patients were divided into a control group (n = 28, treated with Selegiline alone) and an observation group (n = 32, treated with Selegiline and exercise interventions). Gait parameters, FOG indices, motor and balance functions, Berg Balance, psychological status, and quality of life were compared between the groups pre- and post-treatment. Results: After treatment, the observation group exhibited longer step length, higher step speed, and lower step frequency (P = 0.000, 0.003, 0.001, respectively), with enhanced balance as indicated by lower Timed Up and Go Test and higher Berg Balance Scale scores than the control group (P = 0.000, 0.000, respectively). The Beck Depression Inventory and Beck Anxiety Inventory scores were notably lower in the observation group than those in the control group (P = 0.000, 0.004, respectively). Additionally, the observation group showed better quality of life across several dimensions of the Parkinson’s Disease Quality of Life Questionnaire, including mobility, activities of daily living, emotional well-being, stigma, social support, cognition, communication, and bodily discomfort (P = 0.000, 0.000, 0.000, 0.000, 0.017, 0.000, 0.000, 0.000, respectively) than the control group. Conclusion: The combination of exercise interventions and Selegiline effectively rectifies the gait parameters, enhances the balance function, alleviates psychological distress, and improves the overall quality of life in PD patients experiencing FOG.
Keywords: Exercise intervention, Selegilin, Parkinson’s disease, freezing of gait, balance function
Introduction
Parkinson’s disease (PD), often referred to as a common neurodegenerative disorder in the elderly, increases in incidence notably after the age of 60 [1]. PD results from a combination of aging, genetic factors, environmental toxins, oxidative stress abnormalities, and mitochondrial dysfunction [2]. It is marked by the progressive degeneration of nigrostriatal dopaminergic neurons and the presence of Lewy bodies. Clinically, PD is characterized by several core symptoms: resting tremor, bradykinesia, rigidity, and impairments in posture and gait, all of which progressively worsen over time. In more advanced stages, over 50% of patients may experience freezing of gait (FOG) after living with PD for a decade or longer [3]. FOG significantly hinders the mobility of PD patients and increases their risk of falls and associated disabilities, thereby severely affecting their daily activities and social participation. This highlights the importance of addressing FOG in the diagnosis and treatment of PD [4,5].
The etiopathogenesis of FOG is primarily associated with reduced dopamine secretion in the basal ganglia, leading to an imbalance between dopaminergic and cholinergic neurotransmission [6]. Selegiline, a monoamine oxidase B (MAO-B) inhibitor, acts as a disease-modifying agent for FOG by reducing dopamine degradation and enhancing its availability [7]. This adjustment helps restore the balance between dopaminergic and cholinergic systems, thus alleviating symptoms of FOG. Despite the symptomatic relief offered by pharmacotherapy, its long-term use may lead to adverse effects, increasing interest in non-pharmacological adjunct therapies [8]. Studies have shown that complementary therapies such as Tai Chi, Qigong, and Yoga can alleviate both motor and non-motor symptoms of PD [9]. Visual and auditory cues have been demonstrated to regulate stride rhythms, improve gait parameters, and enhance mobility [10]. Additionally, physical therapy has been shown to reduce motor symptoms and medication dosage in PD patients [11]. While various studies confirm the benefits of exercise therapy for FOG, the methods and their impact on FOG vary.
Our study retrospectively analyzed the effects of exercise interventions focused on muscle strength, balance, and gait training using treadmills in 60 PD patients with FOG. We utilized quantifiable measures such as the Freezing of Gait Questionnaire (FOG-Q) and the Timed Up and Go Test (TUGT) to assess the impact on gait freezing and balance. The study also examined improvements in the psychological state of patients post-intervention.
Materials and methods
Study design and grouping
This retrospective analysis received approval from the Ethics Committee of The First People’s Hospital of Fuyang District. We conducted a search in the hospital’s electronic medical record system from January 2020 to January 2023 using the keywords “Parkinson’s disease” and “freezing of gait”, identifying 103 potential participants. Following the inclusion and exclusion criteria, 60 PD patients with FOG were selected for inclusion in the study. The process of case selection is illustrated in Figure 1.
Figure 1.
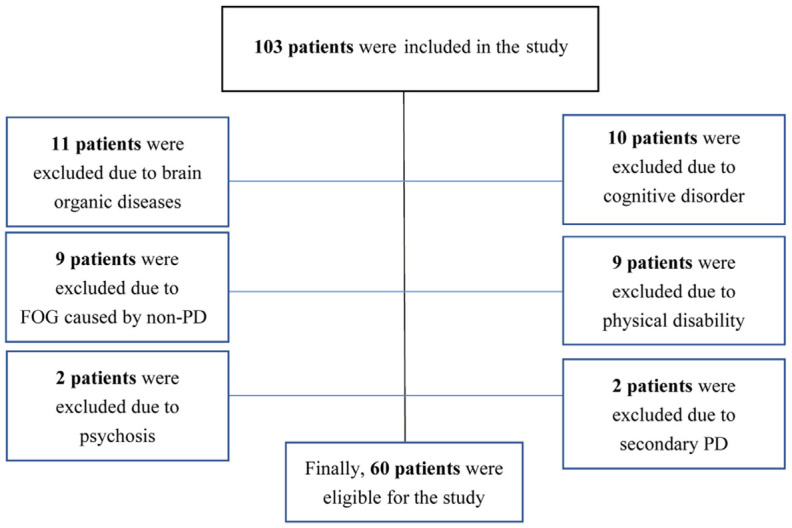
Flow chart of case selection.
Inclusion criteria: (1) Diagnosis of primary PD according to the 2015 criteria of the International Parkinson and Movement Disorder Society (MDS) [12]. (2) Evidence of FOG as assessed by the FOG-Q. (3) Disease duration of at least one year, with no medication. (4) Hoehn and Yahr (H-Y) staging between II and III. (5) Completion of 12 weeks of continuous treatment.
Exclusion criteria: (1) Presence of organic cranio-cerebral disease. (2) Diagnosis of secondary PD. (3) Cognitive impairment. (4) FOG not caused by PD. (5) Severe concurrent cardio-cerebral and cerebrovascular diseases. (6) Psychiatric disorders. (7) Physical disability precluding participation.
Participants were assigned to treatment groups based on their therapeutic regimen. Patients treated exclusively with Selegiline were placed in the control group (n = 28), while those who received combined physical exercise interventions along with Selegiline were placed in the observation group (n = 32).
Treatment methods
Patients in the control group were administered Selegiline hydrochloride tablets (Midolpy, Orion Corporation; 5 mg, 100 tablets; HJ20160342). This medication is routinely used for treating FOG. The treatment regimen began with one tablet each morning during the first week, escalating to two tablets daily - one in the morning and another in the afternoon - from the second week through the twelfth week [13].
In addition to the same regimen of Selegiline hydrochloride as the control group, patients in the observation group participated in a structured exercise intervention program, which included.
Plyometric and muscle training
Upper limb and shoulder muscle training: Patients performed five sets of swinging their arms backward, clasping hands, and extending upwards. Additionally, with assistance for shoulder stabilization, patients exerted reverse force in a lateral position, holding for 5 seconds in two sets.
Lumbar and hip muscle training: In a supine position, patients alternately performed hip flexion and knee holding in ten sets. In the prone position, with arms at their sides, they lifted their lower limbs using their waist as a pivot, maintaining the position for 5 seconds in five sets.
Lower limb muscle training: Patients performed squats with their feet shoulder-width apart, descending to the lowest comfortable point and holding for 5 seconds in five sets.
Movement training
Lift-off training: Holding a gymnastic bar, patients swung it to one side, rotated their bodies to align with the bar, and shifted their weight onto the corresponding foot, naturally lifting the opposite foot off the ground. This exercise was repeated in ten sets.
Turning training: Patients navigated around obstacles, alternating between left and right turns, with each direction repeated in five sets.
Balance training: Standing with feet shoulder-width apart, patients swayed their bodies front-to-back and side-to-side, shifting their center of gravity to maintain balance for five minutes.
Single-leg standing: Concentrating their center of gravity on one foot, patients tried to maintain balance for five minutes.
Gait training: Patients used a treadmill with the speed adjusted from slow to fast, targeting a steady speed of 1.0-1.5 m/s. Forward movement exercises lasted two minutes and were repeated in eight sets.
Backward walking: With hands on the hips and maintaining a steady center of gravity, the speed was gradually increased to between 0.5-1.0 m/s. Backward walking exercises also lasted two minutes and were repeated in eight sets.
Considering the physical condition of patients, the exercise regimen was structured as follows: Exercises 1 and 2 were conducted one to two times daily, and exercise 3 was performed twice daily. The entire intervention lasted 12 weeks [14].
Data collection and scale scoring methods
Data were systematically collected one day before treatment initiation and at the 12-week post-treatment mark, extracted from the diagnostic and evaluation records of the patients. The collected data encompassed several indicators:
Gait parameters
These were assessed through footprint analysis, whereby patients’ feet were dusted with white powder, and they were then asked to walk on a long, smooth platform for five minutes. The measured parameters included average stride length, stride frequency, and stride speed.
FGO-related indices
Freezing of gait was evaluated using the FOG-Q and UPDRS III focused on motor examination. The FOG-Q, which assesses perceptions of freezing, its impact, frequency, duration, initiation, and turning, comprises six items scored from 0 to 4, with a total possible score of 24 points - a higher score indicates more severe FOG. The UPDRS, a globally recognized scale for PD, comprises four sections: I (mental, behavioral, and mood disorders), II (activities of daily living), III (motor examination), and IV (complications of therapy). This study specifically utilized part III, which includes 14 items that rate speech, limb movements, gait, and stability, each scored from 0 to 4, yielding a maximum of 56 points, with higher scores indicating more severe motor symptoms [15].
Gait and balance-related indicators
The Timed Up and Go Test (TUGT) and the Berg Balance Scale (BBS) were employed. The TUGT required patients to rise from a chair, walk to a line 3 meters away, return, and sit down, with the sequence repeated three times to obtain an average time. The BBS is a comprehensive measure of balance and walking capability, consisting of 14 progressively challenging movements scored from 0 to 4, with a total possible score of 56 points, where higher scores reflect better balance [16].
Psychological state-related indicators
The Beck Depression Inventory (BDI) and the Beck Anxiety Inventory (BAI) were used to assess psychological states. Both inventories contain 21 items scored from 0 to 3, with total scores up to 63 points. BDI scores above 17 suggest depression, and BAI scores above 15 indicate anxiety.
Quality of life-related indicators
The Parkinson’s Disease Quality of Life Questionnaire (PDQ-39), tailored for PD patients, was employed. It covers eight domains: mobility (10 items), activities of daily living (ADL, 6 items), emotional well-being (6 items), stigma (4 items), social support (3 items), cognition (4 items), communication (3 items), and bodily discomfort (3 items). Each item is scored from 0 to 4, with a total score of 156 points; higher scores indicate poorer quality of life [17].
Treatment efficacy
Treatment efficacy was determined by changes in FOG-Q scores, calculated using the formula: (FOG-Qbefore treatment - FOG-Qafter treatment)/FOG-Qbefore treatment × 100%. An improvement rate exceeding 50% was considered markedly effective, 20%-50% effective, and below 20% ineffective. The overall treatment efficacy rate was calculated as (number of markedly effective + number of effective)/total number of cases × 100%.
Outcome measures
Primary outcomes included gait parameters, freezing of gait-related indices (FOG-Q, UPDRS III), gait and balance-related indicators (TUGT, BBS), and treatment efficacy. Secondary outcomes encompassed psychological state-related indicators (BDI, BAI) and quality of life-related indicators (PDQ-39).
Statistical analysis
Data were analyzed using SPSS version 21.0. Continuous variables were presented as means ± standard deviations. The paired sample t-test was utilized for intra-group comparisons, and the independent sample t-test for inter-group comparisons. Categorical variables were expressed as rates, and analyzed using the chi-square test. A p-value of < 0.05 was considered statistically significant.
Results
Comparison of general data between the two groups
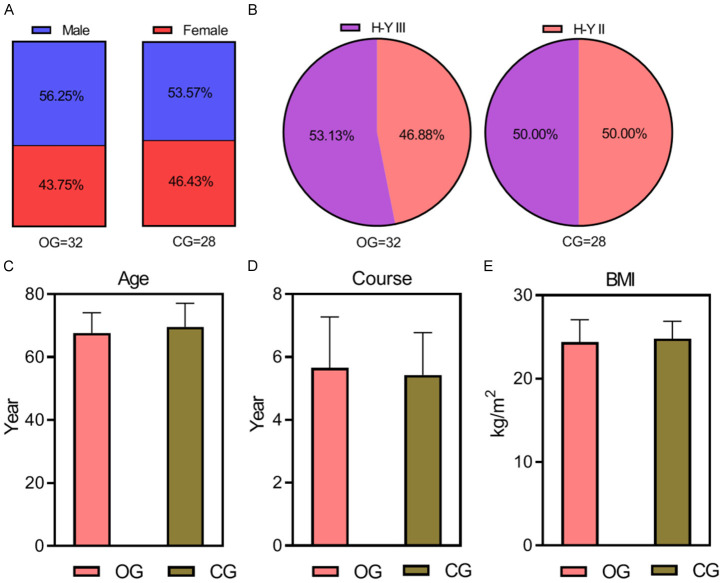
Baseline demographic and clinical characteristics, such as gender, age, PD duration, body mass index (BMI), and Hoehn-Yahr staging, were collected and analyzed for both groups. Statistical analysis indicated no significant differences in these baseline characteristics between the groups (P > 0.05), as detailed in Table 1 and illustrated in Figure 2.
Table 1.
Comparison of baseline data between the two groups (x̅ ± s)/[n (%)]
| Group | Number of cases | Gender (male/female) | Age (years) | Duration of PD (years) | BMI (kg/m2) | H-Y staging (II/III) |
|---|---|---|---|---|---|---|
| Observation group | 32 | 18/14 | 66.78±7.56 | 5.57±1.63 | 23.86±2.56 | 15/17 |
| Control group | 28 | 15/13 | 67.14±7.35 | 5.69±1.68 | 23.80±2.62 | 14/14 |
| t/χ2 | - | 0.043 | 0.176 | 0.280 | 0.090 | 0.058 |
| P | - | 0.835 | 0.853 | 0.780 | 0.929 | 0.809 |
BMI: body mass index; H-Y staging: Hoehn-Yahr staging.
Figure 2.
Comparison of baseline data. The difference between the two groups in terms of gender (A), H-Y staging information (B), age (C), disease duration (D), and BMI (E) is not statistically significant (all P > 0.05). BMI: body mass index; H-Y staging: Hoehn-Yahr staging. OG, observation group; CG, control group.
Comparison of gait parameters between the two groups
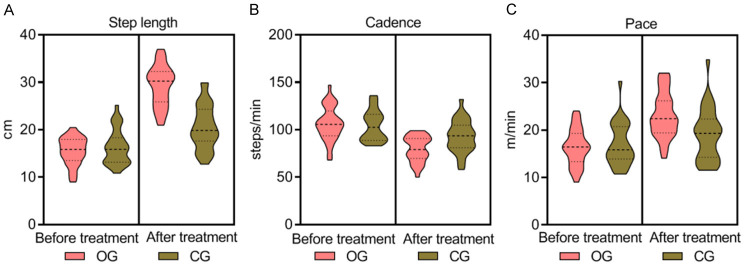
Before treatment, no significant differences were observed in gait parameters (step length, step frequency, step speed) between the groups (P > 0.05). After 12 weeks of treatment, the observation group exhibited statistically significant improvements, with longer step lengths, higher step speeds, and lower step frequencies compared to the control group (P = 0.000, 0.003, 0.001 respectively), as presented in Table 2 and Figure 3.
Table 2.
Comparison of gait parameters (x̅ ± s)
| Group | Number of cases | Step length (cm) | Step frequency (steps/min) | Step speed (m/min) | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Before treatment | After 12 weeks of treatment | Before treatment | After 12 weeks of treatment | Before treatment | After 12 weeks of treatment | ||
| Observation group | 32 | 15.49±2.89 | 29.29±4.25 | 106.38±17.99 | 79.54±13.34 | 16.38±3.82 | 23.19±4.77 |
| Control group | 28 | 16.38±3.69 | 20.52±4.62 | 104.35±16.27 | 92.85±17.13 | 17.04±4.51 | 19.03±5.58 |
| t | - | 1.046 | 7.657 | 0.456 | 3.379 | 0.614 | 3.113 |
| P | - | 0.300 | 0.000 | 0.650 | 0.001 | 0.542 | 0.003 |
Figure 3.
Comparison of gait parameters between the two groups. Before treatment, no significant differences were observed in stride length (A), cadence (step frequency) (B), and walking speed (C) between the two groups (P > 0.05). After 12 weeks of treatment, the observation group exhibited longer stride length, higher walking speed, and lower step frequency compared to the control group, with these differences being statistically significant (P = 0.000, 0.003, 0.001). OG, observation group; CG, control group.
Comparison of FOG indices between the two groups
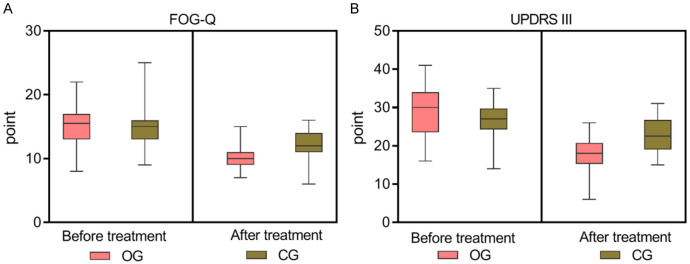
Initially, the FOG indices, including the FOG-Q and Part III of the UPDRS III, showed no significant differences between the groups (P > 0.05). After 12 weeks of treatment, the observation group demonstrated significantly lower FOG-Q and UPDRS III scores than the control group (both P = 0.000), indicating marked improvements, as shown in Table 3 and Figure 4.
Table 3.
Comparison of freezing of gait-related indices between the two groups (x̅ ± s, points)
| Group | Number of cases | FOG-Q | UPDRS III | ||
|---|---|---|---|---|---|
|
|
|
||||
| Before treatment | After 12 weeks of treatment | Before treatment | After 12 weeks of treatment | ||
| Observation group | 32 | 15.07±3.32 | 9.93±1.64 | 28.65±6.04 | 17.47±4.76 |
| Control group | 28 | 14.69±3.36 | 12.11±2.41 | 26.52±4.07 | 22.67±4.58 |
| t | - | 0.440 | 4.140 | 1.508 | 4.296 |
| P | - | 0.662 | 0.000 | 0.137 | 0.000 |
FOG-Q: Freezing of Gait Questionnaire; UPDRS III: part III of the Unified Parkinson’s Disease Rating Scale.
Figure 4.
Comparison of freezing of gait indices between the two groups. Before treatment, no significant difference was found in the FOG-Q (A) and UPDRS III (B) scores between the two groups (Both P > 0.05). After 12 weeks of treatment, the observation group had lower FOG-Q and UPDRS III scores compared to the control group, showing statistically significant differences (P = 0.000, 0.000). OG, observation group; CG, control group. FOG-Q: Freezing of Gait Questionnaire; UPDRS III: part III of the Unified Parkinson’s Disease Rating Scale.
Comparison of gait balance-related indices between the two groups
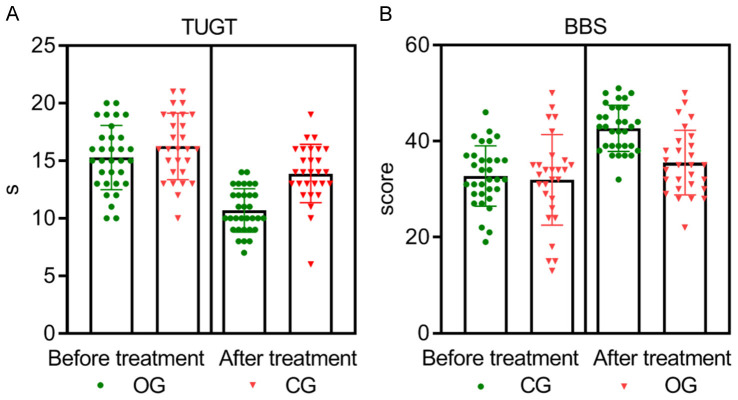
At the outset, there were no significant differences in gait and balance-related indices, specifically the TUGT and BBS, between the two groups (both P > 0.05). However, after 12 weeks of treatment, the observation group achieved statistically significant better outcomes, with lower TUGT times and higher BBS scores than the control group (both P = 0.000), as depicted in Table 4 and Figure 5.
Table 4.
Comparison of gait and balance parameters between the two groups (x̅ ± s, points)
| Group | Number of cases | TUGT (s) | BBS (points) | ||
|---|---|---|---|---|---|
|
|
|
||||
| Before treatment | After 12 weeks of treatment | Before treatment | After 12 weeks of treatment | ||
| Observation group | 32 | 15.25±2.74 | 10.67±1.93 | 32.65±6.29 | 42.68±4.88 |
| Control group | 28 | 16.29±2.92 | 13.93±2.48 | 31.93±9.38 | 35.59±6.66 |
| t | - | 1.423 | 5.718 | 0.353 | 4.742 |
| P | - | 0.160 | 0.000 | 0.725 | 0.000 |
TUGT: Timed Up and Go Test; BBS: Berg Balance Scale.
Figure 5.
Comparison of gait and balance parameters between the two groups. Before treatment, there were no significant differences in the gait and balance parameters (TUGT and BBS) between the two groups (P > 0.05). After 12 weeks of treatment, the observation group had lower TUGT scores (A) and higher BBS scores (B) compared to the control group, with statistically significant differences (P = 0.000, 0.000). TUGT: Timed Up and Go Test; BBS: Berg Balance Scale.
Comparison of psychological state indices between the two groups
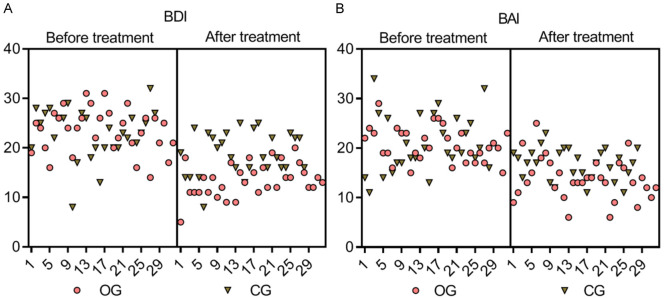
Prior to treatment, no significant differences were observed in the psychological state indices (BDI and BAI scores) between the two groups (both P > 0.05). After 12 weeks of treatment, the observation group displayed significantly lower BDI and BAI scores compared to the control group, suggesting improvements in psychological well-being (P = 0.000 for BDI and P = 0.004 for BAI). These results are presented in Table 5 and illustrated in Figure 6.
Table 5.
Comparison of psychological state indices between the two groups (x̅ ± s, points)
| Group | Number of cases | BDI | BAI | ||
|---|---|---|---|---|---|
|
|
|
||||
| Before treatment | After 12 weeks of treatment | Before treatment | After 12 weeks of treatment | ||
| Observation group | 32 | 23.36±4.38 | 13.46±3.30 | 20.72±3.34 | 13.84±4.26 |
| Control group | 28 | 23.06±5.08 | 18.84±4.32 | 21.02±5.86 | 16.84±3.23 |
| t | - | 0.246 | 5.458 | 0.247 | 3.039 |
| P | - | 0.807 | 0.000 | 0.805 | 0.004 |
BDI: Beck Depression Inventory; BAI: Beck Anxiety Inventory.
Figure 6.
Comparison of psychological state indices between the two groups. At baseline, the BDI (A) and BAI (B) scores, which assessed psychological state, did not significantly differ between the two groups (P > 0.05). After 12 weeks of treatment, the observation group demonstrated lower BDI and BAI scores than the control group, exhibiting statistically significant differences (P = 0.000, 0.004). BDI: Beck Depression Inventory; BAI: Beck Anxiety Inventory.
Comparison of quality of life-related indices between the two groups
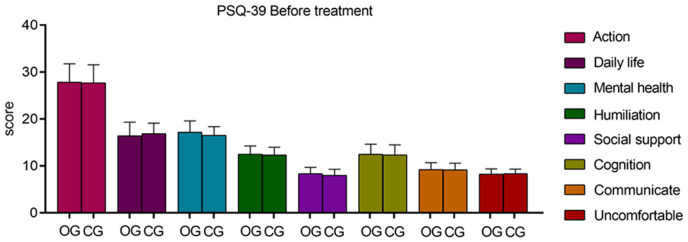
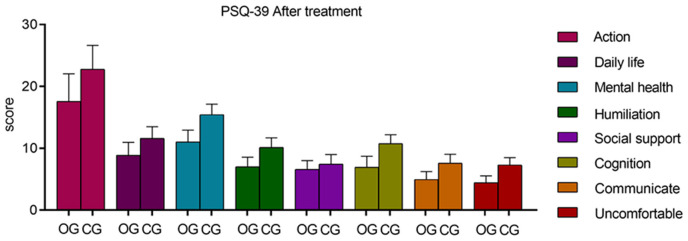
Before treatment, there were no significant differences in the PDQ-39 scores across dimensions between the two groups (P > 0.05). Post-treatment, the observation group demonstrated significantly lower scores in all PDQ-39 dimensions, including mobility, activities of daily living, emotional well-being, stigma, social support, cognition, communication, and bodily discomfort, indicating a substantial improvement in quality of life (all P = 0.000 except for social support P = 0.017). These results are depicted in Tables 6 and 7, and Figures 7 and 8.
Table 6.
Comparison of PDQ-39 scores before treatment between the two groups (x̅ ± s, points)
| Group | Number of cases | Mobility | ADL | Emotional Well-being | Stigma | Social support | Cognition | Communication | Bodily Discomfort |
|---|---|---|---|---|---|---|---|---|---|
| Observation group | 32 | 27.90±3.83 | 16.40±2.86 | 17.26±2.37 | 12.54±1.68 | 8.33±1.36 | 12.42±2.12 | 9.24±1.37 | 8.35±0.97 |
| Control group | 28 | 27.68±3.81 | 16.79±2.21 | 16.56±1.71 | 12.30±1.58 | 7.94±1.18 | 12.39±2.13 | 9.19±1.36 | 8.24±0.93 |
| t | - | 0.223 | 0.585 | 1.295 | 0.568 | 1.178 | 0.055 | 0.142 | 0.447 |
| P | - | 0.825 | 0.561 | 0.200 | 0.573 | 0.244 | 0.957 | 0.888 | 0.657 |
PDQ-39: Parkinson’s Disease Quality of Life Questionnaire; ADL: activities of daily living.
Table 7.
Comparison of 12-Week PDQ-39 scores after treatment between the two treatment groups (x̅ ± s, points)
| Group | Number of cases | Mobility | ADL | Emotional Well-being | Stigma | Social support | Cognition | Communication | Bodily Discomfort |
|---|---|---|---|---|---|---|---|---|---|
| Observation group | 32 | 17.61±4.45 | 8.82±2.12 | 11.00±1.85 | 7.15±1.51 | 6.57±1.37 | 6.86±1.74 | 5.06±1.26 | 4.53±1.01 |
| Control group | 28 | 22.81±3.91 | 11.57±1.93 | 15.39±1.71 | 10.16±1.44 | 7.49±1.52 | 10.72±1.44 | 7.62±1.30 | 7.31±1.18 |
| t | - | 4.776 | 5.225 | 9.498 | 7.871 | 2.466 | 9.280 | 7.736 | 9.834 |
| P | - | 0.000 | 0.000 | 0.000 | 0.000 | 0.017 | 0.000 | 0.000 | 0.000 |
PDQ-39: Parkinson’s Disease Quality of Life Questionnaire; ADL: activities of daily living.
Figure 7.
Comparison of quality of life between the two groups before treatment. Before treatment, the difference between the PDQ-39 dimension scores of the two groups was not significant (P > 0.05). PDQ-39: Parkinson’s Disease Quality of Life Questionnaire.
Figure 8.
Comparison of quality of life between the two groups after treatment. After 12 weeks of treatment, the observation group demonstrated significantly lower scores in the dimensions of mobility, daily life activities, emotional well-being, stigma, social support, cognitive function, communication, and bodily discomfort compared to the control group, showing statistically significant differences (P = 0.000, 0.000, 0.000, 0.000, 0.017, 0.000, 0.000, 0.000). PDQ-39: Parkinson’s Disease Quality of Life Questionnaire.
Comparison of treatment efficacy between the two groups
The treatment efficacy, defined by the rate of improvement in FOG-Q scores, was higher in the observation group (75.00%) compared to the control group (57.14%). However, this difference was not statistically significant (P = 0.143), as detailed in Table 8 and Figure 9.
Table 8.
Comparison of the treatment efficacy between the two groups [n (%)]
| Group | Number of cases | Markedly effective | Effective | Ineffective | Effective rate |
|---|---|---|---|---|---|
| Observation group | 32 | 7 (21.88) | 17 (53.12) | 8 (25.00) | 24 (75.00) |
| Control group | 28 | 2 (7.14) | 14 (50.00) | 12 (42.86) | 16 (57.14) |
| χ2 | - | - | - | - | 2.143 |
| P | - | - | - | - | 0.143 |
Figure 9.
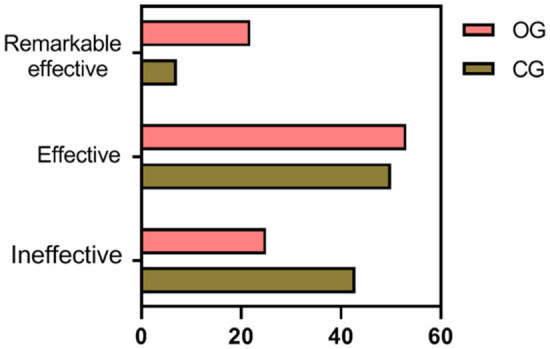
Comparison of treatment efficacy between the two groups. The treatment efficacy in the observation group was slightly higher than that in the control group, but the difference was not statistically significant (P = 0.143).
Discussion
In PD, the loss and degeneration of nigrostriatal dopaminergic neurons impair both voluntary and reflexive motor control, as well as postural regulation. The side effects of medications such as levodopa and amantadine can exacerbate motor dysfunctions in PD, with FOG being one of the most severe gait disorders [18].
In this study, the observation group, which received exercise-based physical interventions in addition to standard pharmacotherapy, showed significant improvements in gait parameters post-treatment compared to the control group. The interventions included the use of a gait plate to train specific walking frequencies and speeds, enhancing step rhythm. Additionally, muscle-strengthening exercises were employed to enhance core, upper, and lower limb muscle strength. This combination improved the dynamic interaction between active joints and muscles, leading to enhanced gait parameters in patients experiencing FOG.
FOG episodes in PD typically occur during the “off” phase and show a notable response to dopaminergic therapy, aligning with the management of the end-of-dose phenomenon. However, during the “on” phase, or when FOG persists in both “on” and “off” phases, dopaminergic medications often fail to provide adequate relief, highlighting the necessity for incorporating non-pharmacological treatments [19].
In this study, after 12 weeks of treatment, the FOG indices, specifically the FOG-Q and UPDRS III scores, were significantly lower in the observation group compared to the control group. This indicates that the combined regimen of exercise interventions and Selegiline administration effectively reduces FOG symptoms, particularly enhancing initiation and turning speeds. Selegiline, known for its neuroprotective effects through the reduction of free radicals, plays a crucial role in alleviating FOG symptoms.
Previous research has demonstrated the benefits of complex exercise regimens on FOG. For instance, adaptive resistance training has been shown to improve FOG and facilitate neural remodeling [20]. A meta-analysis of 19 studies involving 913 patients indicated that physical therapy offers short-term effectiveness in improving FOG [21]. Exercise interventions not only stimulate neural activity in the brain but also promote remodeling of the central nervous system. Rhythmic adjustments during gait plate training help patients focus more on walking mechanics, thus correcting gait disturbances. Additionally, strength training exercises combat muscle atrophy and enhance patients’ ability to control their movements more effectively.
Patients with PD exhibit abnormal limb motor function, reduced limb muscle strength, and impaired balance [22]. It has been established that FOG compromises posterior visual perception and increases the risk of falls. FOG patients often face postural instability and balance issues due to their condition and prolonged medication use [23].
In this study, the TUGT values in the observation group were lower than those in the control group after 12 weeks of treatment, and the BBS scores were higher. This suggests that physical exercise interventions enhance balance function in PD patients with FOG. These interventions improve limb mobility, promote joint and muscle balance, reinforce the memory of specific balance postures, and enable adaptive posture adjustments during walking or unexpected situations to prevent falls.
A study involving 35 PD patients showed that resistance training could bolster muscle strength and balance, positively impacting patients [24]. It was also noted that balance and resistance group training significantly improved reactive postural responses in PD patients with FOG, thereby enhancing balance function [25]. These outcomes are consistent with the results of the current study, supporting the beneficial effects of physical exercise interventions on balance function in PD patients with FOG.
Depression in PD patients is associated with the progression of the illness and disruption of dopamine secretion. It has been found that non-motor symptoms induced by PD, such as anxiety and depression, are independent risk factors for exacerbating FOG in patients [26].
In this study, the reductions in BDI and BAI scores after 12 weeks of treatment were greater in the observation group than in the control group. This indicates that the combination of exercise and Selegiline interventions may alleviate adverse psychological emotions in PD patients with FOG. Selegiline blocks dopamine degradation and inhibits its reuptake, extending the action duration of both endogenous and exogenous dopamine. Additionally, exercise interventions actively engage patients, fostering enjoyment and stimulating endogenous dopamine secretion, thereby relieving negative emotions.
Quality of life assessments revealed that the improvement in of PDQ-39 scores after 12 weeks of treatment in the observation group surpassed that in the control group. This suggests that the synergistic effect of exercise and Selegiline not only mitigates physical symptoms of FOG but also enhances overall life quality.
The study concluded with an analysis of treatment effects based on improvements in FOG-Q scores, revealing higher efficacy in the observation group compared to the control group, though without statistical significance. This is attributed to the limited criteria for evaluating treatment effectiveness and the small sample size, highlighting a limitation of this study. Future research should establish more comprehensive criteria for treatment efficacy and include a larger patient cohort to more accurately assess the therapeutic impact.
In summary, the combination of exercise interventions and Selegiline in PD patients with FOG can rectify gait abnormalities, significantly improve balance and FOG functions, alleviate psychological distress, and enhance quality of life.
Disclosure of conflict of interest
None.
References
- 1.Rahimpour S, Gaztanaga W, Yadav AP, Chang SJ, Krucoff MO, Cajigas I, Turner DA, Wang DD. Freezing of gait in Parkinson’s disease: invasive and noninvasive neuromodulation. Neuromodulation. 2021;24:829–842. doi: 10.1111/ner.13347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss D, Schoellmann A, Fox MD, Bohnen NI, Factor SA, Nieuwboer A, Hallett M, Lewis SJG. Freezing of gait: understanding the complexity of an enigmatic phenomenon. Brain. 2020;143:14–30. doi: 10.1093/brain/awz314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui CK, Lewis SJG. Future therapeutic strategies for freezing of gait in Parkinson’s disease. Front Hum Neurosci. 2021;15:741918. doi: 10.3389/fnhum.2021.741918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bardakan MM, Fink GR, Zapparoli L, Bottini G, Paulesu E, Weiss PH. Imaging the neural underpinnings of freezing of gait in Parkinson’s disease. Neuroimage Clin. 2022;35:103123. doi: 10.1016/j.nicl.2022.103123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capecci M, Pournajaf S, Galafate D, Sale P, Le Pera D, Goffredo M, De Pandis MF, Andrenelli E, Pennacchioni M, Ceravolo MG, Franceschini M. Clinical effects of robot-assisted gait training and treadmill training for Parkinson’s disease. A randomized controlled trial. Ann Phys Rehabil Med. 2019;62:303–312. doi: 10.1016/j.rehab.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Kwok JYY, Smith R, Chan LML, Lam LCC, Fong DYT, Choi EPH, Lok KYW, Lee JJ, Auyeung M, Bloem BR. Managing freezing of gait in Parkinson’s disease: a systematic review and network meta-analysis. J Neurol. 2022;269:3310–3324. doi: 10.1007/s00415-022-11031-z. [DOI] [PubMed] [Google Scholar]
- 7.Conde CI, Lang C, Baumann CR, Easthope CA, Taylor WR, Ravi DK. Triggers for freezing of gait in individuals with Parkinson’s disease: a systematic review. Front Neurol. 2023;14:1326300. doi: 10.3389/fneur.2023.1326300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marquez JS, Hasan SMS, Siddiquee MR, Luca CC, Mishra VR, Mari Z, Bai O. Neural correlates of freezing of gait in Parkinson’s disease: an electrophysiology mini-review. Front Neurol. 2020;11:571086. doi: 10.3389/fneur.2020.571086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deuel LM, Seeberger LC. Complementary therapies in Parkinson disease: a review of acupuncture, Tai Chi, Qi Gong, Yoga, and Cannabis. Neurotherapeutics. 2020;17:1434–1455. doi: 10.1007/s13311-020-00900-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosentino C, Putzolu M, Mezzarobba S, Cecchella M, Innocenti T, Bonassi G, Botta A, Lagravinese G, Avanzino L, Pelosin E. One cue does not fit all: a systematic review with meta-analysis of the effectiveness of cueing on freezing of gait in Parkinson’s disease. Neurosci Biobehav Rev. 2023;150:105189. doi: 10.1016/j.neubiorev.2023.105189. [DOI] [PubMed] [Google Scholar]
- 11.Okada Y, Ohtsuka H, Kamata N, Yamamoto S, Sawada M, Nakamura J, Okamoto M, Narita M, Nikaido Y, Urakami H, Kawasaki T, Morioka S, Shomoto K, Hattori N. Effectiveness of long-term physiotherapy in Parkinson’s disease: a systematic review and meta-analysis. J Parkinsons Dis. 2021;11:1619–1630. doi: 10.3233/JPD-212782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30:1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 13.Gao C, Liu J, Tan Y, Chen S. Freezing of gait in Parkinson’s disease: pathophysiology, risk factors and treatments. Transl Neurodegener. 2020;9:12. doi: 10.1186/s40035-020-00191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rutz DG, Benninger DH. Physical therapy for freezing of gait and gait impairments in Parkinson disease: a systematic review. PM R. 2020;12:1140–1156. doi: 10.1002/pmrj.12337. [DOI] [PubMed] [Google Scholar]
- 15.Dos Santos Delabary M, Komeroski IG, Monteiro EP, Costa RR, Haas AN. Effects of dance practice on functional mobility, motor symptoms and quality of life in people with Parkinson’s disease: a systematic review with meta-analysis. Aging Clin Exp Res. 2018;30:727–735. doi: 10.1007/s40520-017-0836-2. [DOI] [PubMed] [Google Scholar]
- 16.Qiu Y, Yin Z, Wang M, Duan A, Xie M, Wu J, Wang Z, Chen G. Motor function improvement and acceptability of non-invasive brain stimulation in patients with Parkinson’s disease: a Bayesian network analysis. Front Neurosci. 2023;17:1212640. doi: 10.3389/fnins.2023.1212640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore O, Peretz C, Giladi N. Freezing of gait affects quality of life of peoples with Parkinson’s disease beyond its relationships with mobility and gait. Mov Disord. 2007;22:2192–2195. doi: 10.1002/mds.21659. [DOI] [PubMed] [Google Scholar]
- 18.Nonnekes J, Bereau M, Bloem BR. Freezing of gait and its levodopa paradox. JAMA Neurol. 2020;77:287–288. doi: 10.1001/jamaneurol.2019.4006. [DOI] [PubMed] [Google Scholar]
- 19.Cupertino L, Dos Reis TG, Los Angeles E, Costa TM, Shokur S, Bouri M, de Lima-Pardini AC, Coelho DB. Biomechanical aspects that precede freezing episode during gait in individuals with Parkinson’s disease: a systematic review. Gait Posture. 2022;91:149–154. doi: 10.1016/j.gaitpost.2021.10.021. [DOI] [PubMed] [Google Scholar]
- 20.Silva-Batista C, de Lima-Pardini AC, Nucci MP, Coelho DB, Batista A, Piemonte MEP, Barbosa ER, Teixeira LA, Corcos DM, Amaro E Jr, Horak FB, Ugrinowitsch C. A randomized, controlled trial of exercise for Parkinsonian individuals with freezing of gait. Mov Disord. 2020;35:1607–1617. doi: 10.1002/mds.28128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosentino C, Baccini M, Putzolu M, Ristori D, Avanzino L, Pelosin E. Effectiveness of physiotherapy on freezing of gait in Parkinson’s disease: a systematic review and meta-analyses. Mov Disord. 2020;35:523–536. doi: 10.1002/mds.27936. [DOI] [PubMed] [Google Scholar]
- 22.Kim H, Kim E, Yun SJ, Kang MG, Shin HI, Oh BM, Seo HG. Robot-assisted gait training with auditory and visual cues in Parkinson’s disease: a randomized controlled trial. Ann Phys Rehabil Med. 2022;65:101620. doi: 10.1016/j.rehab.2021.101620. [DOI] [PubMed] [Google Scholar]
- 23.Korkusuz S, Seçkinoğulları B, Özcan A, Demircan EN, Çakmaklı GY, Armutlu K, Yavuz F, Elibol B. Effects of freezing of gait on balance in patients with Parkinson’s disease. Neurol Res. 2023;45:407–414. doi: 10.1080/01616412.2022.2149510. [DOI] [PubMed] [Google Scholar]
- 24.Strand KL, Cherup NP, Totillo MC, Castillo DC, Gabor NJ, Signorile JF. Periodized resistance training with and without functional training improves functional capacity, balance, and strength in Parkinson’s disease. J Strength Cond Res. 2021;35:1611–1619. doi: 10.1519/JSC.0000000000004025. [DOI] [PubMed] [Google Scholar]
- 25.Ribeiro de Souza C, Ávila de Oliveira J, Takazono PS, da Silva Rezende L, Silva-Batista C, Coelho DB, Teixeira LA. Perturbation-based balance training leads to improved reactive postural responses in individuals with Parkinson’s disease and freezing of gait. Eur J Neurosci. 2023;57:2174–2186. doi: 10.1111/ejn.16039. [DOI] [PubMed] [Google Scholar]
- 26.Pimenta M, Moreira D, Nogueira T, Silva C, Pinto EB, Valenca GT, Almeida LRS. Anxiety independently contributes to severity of freezing of gait in people with Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2019;31:80–85. doi: 10.1176/appi.neuropsych.17090177. [DOI] [PubMed] [Google Scholar]