Abstract
Objective: Kidney-tonifying formulas are frequently used in clinical practices to enhance follicular development and maturation. This research explored the impacts of the Bushen Tiaojing formula (BSTJF) on the development of mouse preantral follicles in vitro and its relationship with granulosa cells and gonadotropins. Methods: Preantral follicles were extracted from mice and cultured with or without serum from rats that were previously treated with or without BSTJF. During cultivation, the follicles were monitored for morphological changes and developmental maturation. Exhausted medium was collected every other day for the measurement of progesterone and estradiol (E2) levels by ELISA. Granulosa cells in in-vitro medium were collected on days 8, 10, and 12 and analyzed for determining the expressions of apoptosis-associated genes (Bax, Bcl-2, and Caspase-3). Propagation and apoptosis rates of collected granulosa cells were measured by CCK-8 assay and flow cytometry. Results: Compared with control follicles, follicles cultured with serum from BSTJF-treated rats had a higher survival rate, larger follicle diameter, higher Bcl-2 expression, and lower Bax and Caspase-3 expressions (all P ≤ 0.05). In addition, their granulosa cells presented substantially elevated proliferation (P ≤ 0.05) and a lower rate of apoptosis (P ≤ 0.05) compared with granulosa cells from control follicles. The level of E2 in the culture media of all groups increased slowly in the first 6 days. Subsequently, after formation of the antrum, the levels of E2 and progesterone were enhanced in the medium of follicles cultured with serum from BSTJF-treated rats compared with those in the media of control follicles (all P ≤ 0.05). Conclusion: Serum from BSTJF-treated rats facilitated the in vitro development and maturation of mouse follicles by increasing the expression of anti-apoptotic gene Bcl-2, reducing the expressions of pro-apoptotic genes Bax and Caspase-3 as well as the apoptosis of granulosa cells, promoting the proliferation of granulosa cells and increasing the secretion of E2 and progesterone in the cells.
Keywords: Kidney-tonifying formulas, Bushen Tiaojing, in vitro culture, preantral follicles, granulosa cells
Introduction
The incidence of infertility worldwide is approximately 15% [1]. Follicles are the basic units of female reproductive biology, and poor follicular growth is a prevalent etiological factor contributing to infertility. Thousands of follicles initially present in the ovaries, of which only a small number would grow mature and ovulate. The rest of them undergo atresia and degeneration during the preantral stage. About 99.9% of oocytes disappear because of follicular atresia over an individual’s lifetime. Previous research indicated that follicular atresia was primarily influenced by granulosa cell apoptosis [2,3], and that enhanced granulosa cell apoptosis was linked with low oocyte yield and reduced fertilization and pregnancy rates [4-7]. If more preantral follicles were able to reach maturity, it would improve the pregnancy outcomes of assisted reproductive technologies such as in vitro fertilization-embryo transfer (IVF-ET). Therefore, it is important to study the process of follicular growth and development to better preserve female reproductive capacity and treat infertility.
Follicles comprise follicular membrane cells, granulosa cells, and oocytes. These cells produce autocrine and paracrine factors that allow granulosa cells and oocytes to regulate one another and influence the growth, development, and maturation of the follicles and oocytes. During the late preantral stage of follicular development, granulosa cells are stimulated by follicle-stimulating hormone (FSH) to express FSH receptors (FSHRs). FSH-FSHR binding induces aromatase synthesis in granulosa cells, leading to the conversion of androgens to estrogens, which promotes follicular development and the formation of dominant follicles. In the antral stage, luteinizing hormone (LH) binds to its receptor (LHR) on granulosa cells, causing the oocyte to undergo the first meiotic division. Apoptosis of granulosa cells is the direct cause of follicular atresia during this stage. Previous research showed that the level of granulosa cell apoptosis was an indicator of the developmental potential of oocytes [8]. Caspase-3 is the primary effector enzyme in the process of apoptosis. Bcl-2-linked X protein (Bax), a member of the Bcl-2 gene family, induces caspase activation by altering the permeability of the mitochondrial inner membrane and suppressing the mitochondrial transmembrane potential, leading to the release of cytochrome c and other pro-apoptotic factors. Conversely, B-cell lymphoma 2 (Bcl-2) exhibits anti-apoptotic characteristics due to its ability to stabilize calcium ion levels in the mitochondria and inhibit the release of cytochrome c and other pro-apoptotic factors.
Traditional Chinese Medicine (TCM)-related literature does not contain descriptions of “poor follicular development”. However, poor follicular development can lead to conditions such as menstrual irregularities and infertility. In TCM, the kidneys store essence and govern reproduction. Therefore, follicles can be considered as a form of “reproductive essence” governed by the kidneys. The growth and maturation of follicles require three components: essence, nourishment from kidney yin, and blood and warmth from kidney yang. The production of high-quality oocytes is thus based on an abundance of kidney essence and qi as well as a balanced state of yin and yang in the body. In clinical practice, kidney-nourishing and menstruation-regulating formula is commonly prescribed to nourish the kidneys of patients undergoing IVG-ET for the treatment of infertility. Preliminary findings suggest that kidney-nourishing and menstruation-regulating formula can increase the survival of preantral follicles during their transition to the antral stage by regulating the expressions of genes and pathways linked to preantral follicle secretion, thereby enhancing oocyte quality [9-11]. This study aimed to investigate the effects of the kidney-tonifying formula, the Bushen Tiaojing Fang (BSTJF), on granulosa cells and the development and maturation of preantral follicles.
Materials and methods
Experimental animals
A total of 120 female 10-day-old Institute of Cancer Research (ICR) mice (No. 1100111911065705) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Thirty female 6-week-old Sprague-Dawley (SD) rats (No. 211002300052527) were obtained from Liaoning Changsheng Biotechnology Co., Ltd. (Liaoning, China). Ethical approval for animal experimental procedures was obtained from the Ethics Committee of the Institute of Hebei University of Chinese Medicine (DWLL2019015, China). The study was performed based on the guidance for the Care and Use of Laboratory Animals (National Academy of Sciences, 1996).
Experimental drugs
The BSTJF, obtained from Shijiazhuang Lerentang Pharmaceutical Co., Ltd., was composed of 15 g Shudihuang (Radix Rehmanniae Preparata), 15 g Shanzhuyu (Fructus Corni), 12 g Shanyao (Rhizoma Dioscoreae), 12 g Gouqizi (Tructus Lycii), 9 g Nvzhenzi (Fructus Ligustri Lucidi), 3 g Ziheche (Placenta Hominis), 10 g Yinyanghuo (Herba Epimedii), 12 g Tusizi (Semen Cuscutae), 10 g Fupenzi (Fructus Rubi), 6 g Xiangfu (Rhizoma Cyperi), 9 g Baishao (Radix Paeoniae Alba), 10 g Roucongrong (Herba Cistanches), 10 g Danshen (Radix Salviae Miltiorrhizae), and 9 g Danggui (Radix Angelicae Sinensis). The BSTJF solution containing 2.31 g/ml of drugs was sterilized with UV light for 1 h, and stored at 4°C until perfusion in rats.
Preparation of rat serum
The female SD rats were maintained in a clean-grade animal facility with free access to food and water. The animal facility was controlled with ~50% relative humidity, ~20°C ambient temperature, and a 12 h light/dark alternative cycle. The rats were evenly divided into two groups using a random number table after 1 week of acclimatization. After grouping, rats in test group received oral administration of 1 ml BSTJF solution/100 g body weight (BW) twice daily (0800 h and 1800 h) for 4 days, while those in control group received oral administration of 1 ml distilled water/100 g BW. On day 5, after the 9th treatment, the dose was doubled to 2 ml/100 g BW, and the treatment was continued until day 7. One hour after the last (18th) treatment, the rats were anesthetized with CO2, and blood was collected from the femoral artery. After blood sample collection, the rats had no breathing and did not respond to any pressure with a forcep on their toes. They were confirmed dead.
After the blood was allowed to clot for 2 h, the samples were centrifuged for 10 min at 3000 rpm under 4°C. The serum was collected, heat-inactivated at 56°C for 30 min, filtered through a sterile 0.22 μm filter, and stored at -80°C for future use.
Isolation and in vitro culture of preovulatory follicles
The ICR mice were evenly divided into three groups using a random number table and euthanized with CO2. Ovaries were isolated immediately after euthanasia, and preovulatory follicles were dissected under an inverted microscope using a sterile 1 ml syringe needle. The follicles were then transferred to different culture media depending on their group assignment. Follicles in the normal control group were cultured in growth medium consisting of Minimal Essential Medium α (α-MEM, Gibco, Logan, UT, USA) with 10% fetal bovine serum (FBS, Gibco, Logan, UT, USA), 100 IU/ml penicillin, 100 μg/ml streptomycin, 100 mIU/ml rFSH, 10 mIU/ml rLH, and 1% ITS. Follicles in the serum control group were cultured in the same growth medium supplemented with serum from control rats. Follicles in the BSTJF group were cultured in growth medium supplemented with serum from rats that were treated with BSTJF. The culture medium for each group was replaced every other day, and the contents of the wasted media were analyzed. On days 8, 10, and 12, granulosa cells were collected for analysis. FBS and α-MEM were purchased from Gibco (USA), and ITS was purchased from Sigma (USA).
Observation of follicular morphology and survival
After the introduction of preantral follicles into the culture media, the media were replaced every other day. Next, an inverted microscope was used to observe the dynamic changes of the follicles, including changes in morphology and enclosure of the follicles, and the diameter, atrophy, deformation, and disintegration of the oocytes.
Detection of estradiol (E2) and progesterone levels in culture media
Samples of wasted culture media were subjected to ELISA using the Mouse Estradiol (E2) ELISA Kit and the Mouse Progesterone (PROG) ELISA Kit (both from Wuhan Huamei Company) according to the manufacturer’s guidelines. E2 and progesterone levels on days 0, 2, 4, 6, 8, 10, and 12 were measured with inter-batch variability (CV) < 15% and intra-batch variability (CV) < 15%.
Detection of the proliferation of granulosa cells by CCK-8 assay
Oocytes and granulosa cells were separated on days 8, 10, and 12 from the culture media with follicles. The collected granulosa cells were transferred to a culture well plate, which was added with 10 μl/well CCK-8 solution. The plate was maintained at 37°C with 5% CO2 for 1.5 h, and the optical density (OD) was detected using an ELISA reader. The percentage of viable cells was calculated as [(OD of the experimental group - OD of the blank well) ÷ (OD of the control group - OD of the blank well)] × 100.
Detection of the apoptosis of granulosa cells by flow cytometry
Oocytes and granulosa cells were separated and collected on days 8, 10, and 12 from the extracorporeal follicle culture. Granulosa cells were transferred to 1.5 ml EP tubes and washed thrice with pre-cooled PBS. Each washing involved centrifugation of the cells at 2000 rpm for 5 min. Following the instructions of the Cell Counting Kit-8 (CCK-8) (Seven Biotechnology Co., Ltd., Beijing), the cells were reacted at room temperature in the dark for 10-15 min and assessed for apoptosis using a flow cytometer (Beckman Coulter Commercial Enterprise Co., Ltd.).
Quantitative real-time polymerase chain reaction (qRT-PCR)
QRT-PCR was used to detect Bax, Bcl-2, Caspase-3, FSHR, and LHR mRNA levels in granulosa cells. Total RNA was extracted using a total RNA Kit II (Omega, USA) according to the manufacturer’s instructions. The purity of the RNA was assessed using a Nanodrop 2000C spectrophotometer (Thermo, USA), with an OD260/OD280 ratio within the range of 1.8-2.0, which is widely accepted as an indicator of high purity. After removal of genomic DNA, cDNA was synthesized using a Monarch RNA-to-cDNA Kit (New England Biolabs) according to the manufacturer’s instructions. The obtained cDNA was subjected to qRT-PCR on a 7500 Fast Real-Time PCR System (Thermo Scientific, USA). The thermal cycling parameters comprised an initial denaturation at 95°C for 10 min, followed by 40 amplification cycles at 95°C for 10 s and 60°C for 30 s. The melt curve originated using the default program of the instrument. Primers were designed and synthesized by Shanghai Sangon Biotech. The primer sequences are shown in Table 1. After amplification, Ct values were analyzed for data processing. Using GAPDH as the internal reference gene and the samples from the normal control group as standards, the relative quantitative value (RQ value) of the expressions of target genes was determined using the formula RQ = 2-ΔΔCt.
Table 1.
The primer sequences
| Primer | Sequence | Fragment |
|---|---|---|
| GAPDH | F: 5’-CCTCGTCCCGTAGACAAAATG-3’ | 133 bp |
| R: 5’-TGAGGTCAATGAAGGGGTCGT-3’ | ||
| Bax | F: 5’-CCGGCGAATTGGAGATGAAC-3’ | 159 bp |
| R: 5’-AAGTAGAAGAGGGCAACCACGC-3’ | ||
| Bcl-2 | F: 5’-TGACTTCTCTCGTCGCTACCGT-3’ | 112 bp |
| R: 5’-CCTGAAGAGTTCCTCCACCACC-3’ | ||
| Caspase-3 | F: 5’-TGGAATGTCATCTCGCTCTGGT-3’ | 298 bp |
| R: 5’-GAAGAGTTTCGGCTTTCCAGTC-3’ | ||
| FSHR | F: 5’-GTGTCATTGCTCTAACAGGGTC-3’ | 206 bp |
| R: 5’-ACATCTGCCTCTATTACCTCCAAG-3’ | ||
| LHR | F: 5’-GGTGCTTTTACAAACCTCCCTC-3’ | 255 bp |
| R: 5’-CGAGATTAGCGTCGTCCCA-3’ |
Immunoblot analysis
Granulocytes were harvested, and proteins were extracted on ice using RIPA lysis buffer (Beijing Solarbio Science and Technology Co., Ltd., Beijing, China). The protein extraction solution was added with 1% PMSF (Beijing Solarbio Science and Technology Co., Ltd.) and 1% phosphatase inhibitors (MCE, NJ, USA). The concentration of extracted protein was detected using a BCA protein assay kit (Beijing Solarbio Science and Technology Co., Ltd.) according to the manufacturer’s instructions. Based on the results of protein quantification, the appropriate volume of the total protein sample was mixed with 4× protein loading buffer, denatured at 95°C for 10 min, and cooled to RT. The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene difluoride membrane via semi-dry transfer. The membrane was blocked with 5% skim milk at RT for 2 h and washed with Tris-buffered saline and Tween 20 (TBST). Primary antibodies against Bax (1:500), Caspase-3 (1:500), Bcl-2 (1:1000), and Actin (1:1000) were added to the respective membranes and incubated at 4°C for 24 h. The membranes were then probed with secondary antibodies at RT for 1 h. Protein bands were detected using an enhanced chemiluminescence (ECL) detection kit (Wuhan Servicebio Biotechnology Co., Ltd.) and visualized using an Image Quant LAS 4000 chemiluminescence imaging system (GE Company, USA). Actin expression was used as the loading control and quantified using an ImageQuant TL image analysis system.
Data analysis
All presented data were analyzed using SPSS 22.0 (IBM SPSS Statistics, IBM Corporation). Continuous data from multiple groups were compared by one-way ANOVA, followed by least squares difference (LSD) or Dunnett’s T3 tests. Categorical data were examined by the χ2 test. For all hypothesis tests, p-values less than 0.05 were considered indicative of statistical significance.
Results
Comparison of morphological changes and survival rate of ovarian follicles among different groups
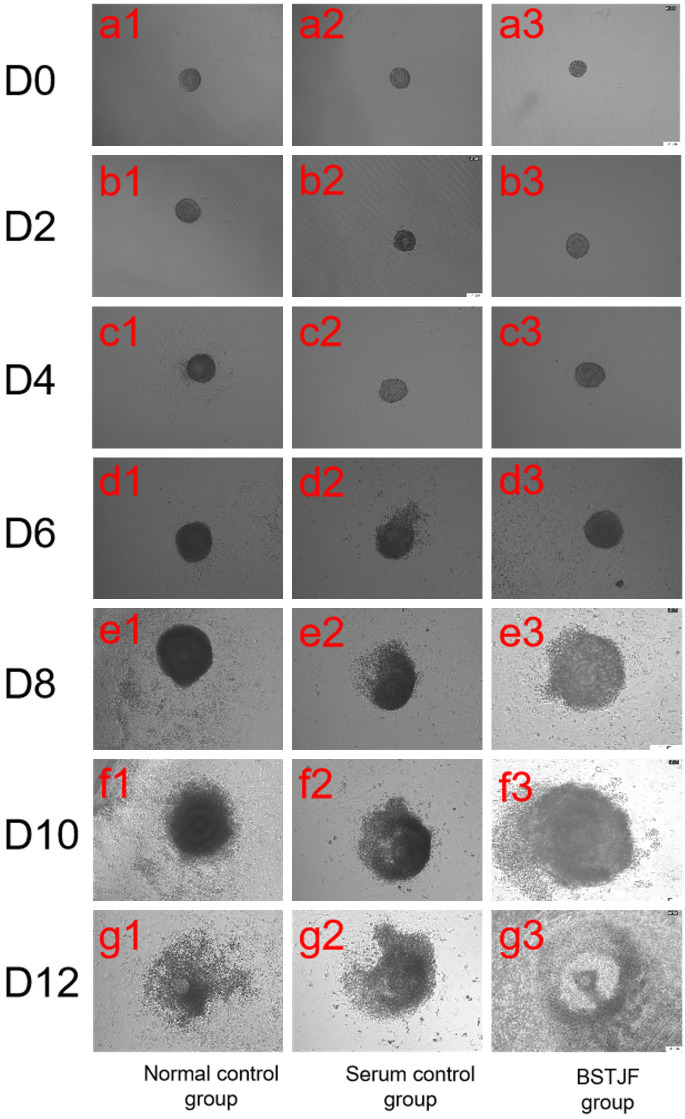
Ovarian follicles underwent a series of morphological changes from the preantral stage to the preovulatory stage, including the transition from preantral follicle to antral follicle, expulsion of cumulus-oocyte complex, extrusion of the first polar body, and oocyte maturation. On day 0 of cultivation, the follicles had not yet adhered to the culture surface (Figure 1, a1-a3). On day 2, the follicles were adhered to the culture surface, but their growth was insignificant (Figure 1, b1-b3). On day 4, the follicle volume and the number of granulosa cells were increased (Figure 1, c1-c3). On day 6, the follicle volume continued to increase, and granulosa cells had notably breached the basal lamina and grown, with oocytes slightly observed, leading to the loss of a three-dimensional structure (Figure 1, d1-d3). On day 8, some follicles exhibited a cavity-like structure, with sparse granulosa cells and a semi-transparent cavity-like appearance (Figure 1, e1-e3). On day 10, the follicle volume and the number of granulosa cells continued to increase, with enlarged cavity observed (Figure 1, f1-f3). On day 12, cumulus-oocyte complexes became visible (Figure 1, g1-g3).
Figure 1.
Morphological structure of preantral follicles in all mouse groups at different time points. D0-D12: The follicles were observed on days 0, 2, 4, 6, 8, 10, and 12 of in vitro cultivation. Scale bar = 100 μm.
There was no significant difference in follicular survival rate between the normal control and serum control groups on days 8, 10, and 12; however, the BSTJF group showed a higher follicular survival rate compared with the other two groups (all P ≤ 0.05, Table 2).
Table 2.
Comparison of follicular survival rates in vitro
| Number of follicles | Follicular survival rate (%) | ||||
|---|---|---|---|---|---|
|
| |||||
| Day 0 | Day 8 | Day 10 | Day 12 | ||
| Normal control | 100 | 100 | 74 ± 0.89 | 72.67 ± 1.03 | 64.17 ± 1.17 |
| Serum control | 100 | 100 | 74.83 ± 1.17 | 73.67 ± 0.82 | 65.33 ± 0.82 |
| BSTJF | 100 | 100 | 87.83 ± 1.47*,# | 85.5 ± 0.84*,# | 80.5 ± 1.05*,# |
P ≤ 0.05 compared with the normal control group;
P ≤ 0.05 compared with the serum control group.
Diameter of oocytes in ovarian follicles in different groups
No significant differences were observed in the diameter of the ovarian follicles across all groups on day 0. After 12 days, there was still no significant difference in the follicular diameter between the normal control group and the serum control group; however, the follicular diameter was remarkably larger in the BSTJF group compared with that in either control group (all P ≤ 0.05, Table 3).
Table 3.
Comparison of oocyte diameters in vitro
| Oocyte diameter (μm) | ||
|---|---|---|
|
| ||
| Day 0 | Day 12 | |
| Normal control | 51.82 ± 1.00 | 66.48 ± 1.44 |
| Serum control | 51.96 ± 0.66 | 66.52 ± 1.28 |
| BSTJF | 52.3 ± 0.69 | 67.88 ± 1.08*,# |
Note: Data are expressed as mean ± SD (n = 9).
P ≤ 0.05 compared with the normal control group;
P ≤ 0.05 compared with the serum control group.
E2 and progesterone levels in the follicular culture medium of mice at different time points
During the first 6 days of the in vitro culture of follicles, E2 levels grew relatively slowly in all groups; however, significant growth in E2 levels was observed starting from day 8, after the formation of antral cavities. On day 0, there was no significant difference in E2 levels among the three groups. During subsequent culture from day 2 to day 12, no significant difference was observed in E2 levels between the normal control group and the serum control group; however, the E2 levels in the BSTJF group were elevated compared with those in either control group (all P ≤ 0.05, Table 4). The levels of progesterone increased slowly in the first 10 days of in vitro cultivation. On day 12, the progesterone levels remarkably increased. During this process, there was no significant difference in progesterone levels among the three groups from day 0 to day 2. From day 4 to day 12, no significant difference was noted in progesterone levels between the two control groups; however, the progesterone level was higher in the BSTJF group compared with that in either control group (all P ≤ 0.05, Table 5).
Table 4.
The levels of E2 in the culture media at different time points
| The levels of E2 in the culture media (pg/ml) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Day 1 | Day 2 | Day 4 | Day 6 | Day 8 | Day 10 | Day 12 | |
| Normal control | 4203.42 ± 7.60 | 4374.60 ± 13.90 | 5172.25 ± 63.08 | 8066.37 ± 32.72 | 15498.18 ± 82.71 | 17096.21 ± 95.24 | 23515.19 ± 87.46 |
| Serum control | 4212.20 ± 13.18 | 4402.57 ± 14.02 | 5673.64 ± 445.24 | 8121.24 ± 50.39 | 15835.47 ± 224.56 | 17917.35 ± 117.35 | 24028.36 ± 237.91 |
| BSTJF | 4221.04 ± 17.30 | 4615.30 ± 47.95*,# | 6474.79 ± 13.89*,# | 8662.89 ± 41.70*,# | 16969.73 ± 54.33*,# | 20258.26 ± 252.40*,# | 29405.92 ± 121.53*,# |
Note: Data are expressed as mean ± SD (n = 3).
P ≤ 0.05 compared with the normal control group;
P ≤ 0.05 compared with the serum control group.
Table 5.
The levels of progesterone in the culture media at different time points
| Group | The levels of progesterone in the culture media (ng/ml) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Day 1 | Day 2 | Day 4 | Day 6 | Day 8 | Day 10 | Day 12 | |
| Normal control | 40.64 ± 0.11 | 42.58 ± 0.31 | 44.29 ± 0.21 | 54.89 ± 0.16 | 60.95 ± 0.38 | 97.79 ± 0.35 | 204.53 ± 1.86 |
| Serum control | 40.70 ± 0.19 | 42.71 ± 0.24 | 44.09 ± 0.91 | 54.99 ± 0.59 | 61.28 ± 0.50 | 99.65 ± 0.36 | 208.87 ± 1.90 |
| BSTJF | 40.26 ± 0.10 | 44.42 ± 0.32 | 45.08 ± 0.33*,# | 60.41 ± 0.49*,# | 68.70 ± 0.96*,# | 107.79 ± 0.4*,# | 276.21 ± 11.76*,# |
Note: Data are expressed as mean ± SD (n = 3).
P ≤ 0.05 compared with the normal control group;
P ≤ 0.05 compared with the serum control group.
Effects of BSTJF on the proliferation and apoptosis of oocyte granulosa cells
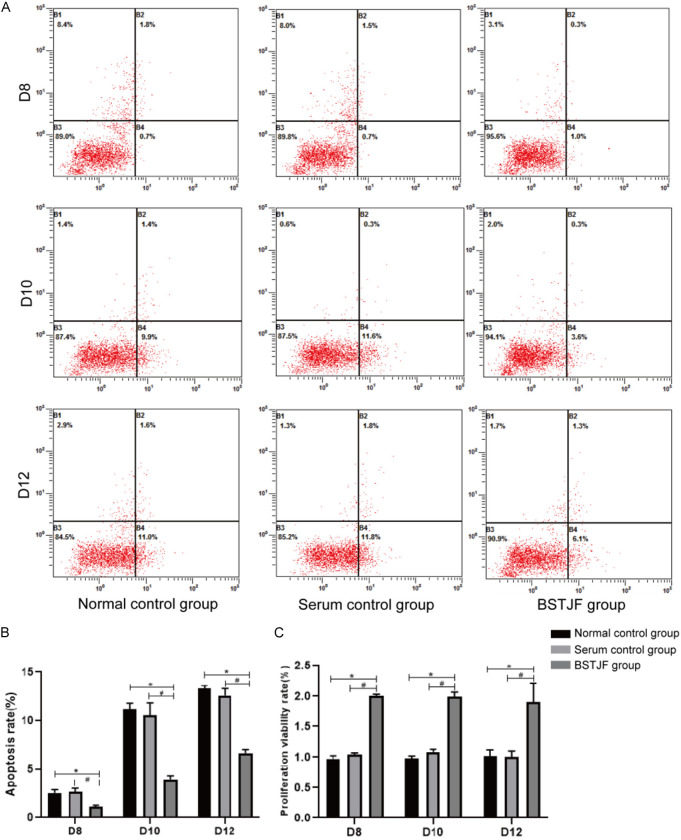
Flow cytometry analysis revealed that there was no marked difference in the apoptosis rate of granulosa cells between the normal control group and the serum control group; however, the apoptosis rate of granulosa cells was lower in the BSTJF group than that in either control group (all P < 0.05, Figure 2A and 2B). Likewise, CCK-8 assay results showed that the proliferation of granulosa cells did not differ between the normal control group and the serum control group, but higher in the BSTJF group (all P ≤ 0.05, Figure 2C).
Figure 2.
Apoptosis rate and proliferation of granulosa cells on days 8, 10, and 12 of in vitro cultivation. A. Cell apoptosis rate measured by flow cytometry. B. Quantification of apoptosis rate in each group. Column height and error bars represent the mean ± SD, respectively (n = 9). C. Proliferation in each group. Column height and error bars represent the mean ± SD, respectively (n = 9). *P ≤ 0.05 compared with the normal control group; #P ≤ 0.05 compared with the serum control group.
Expressions of Bax, Bcl-2, Caspase-3, FSHR, and LHR in granulosa cells at different time points
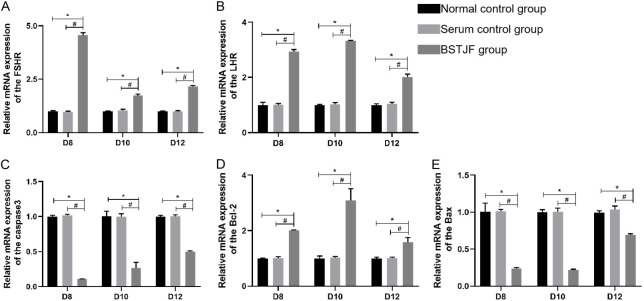
There were no noted differences in the mRNA expressions of Bcl-2, Bax, Caspase-3, FSHR, and LHR between the normal control group and the serum control group on days 8, 10, and 12, whereas the BSTJF group showed higher mRNA expressions of Bcl-2, FSHR, and LHR and lower mRNA expressions of Bax and Caspase-3 compared with those in either control group (all P < 0.05, Figure 3A-E).
Figure 3.
qRT-PCR analysis of Bcl2, Bax, Caspase 3, FSHR, and LHR mRNA expressions on days 8, 10, and 12 of in vitro cultivation. The column height represents the mean mRNA expression level, and the error bars represent the SD (n = 3). *P ≤ 0.05 compared with the normal control group; #P ≤ 0.05 compared with the serum control group.
Analysis of protein expressions in granulosa cells at different time points
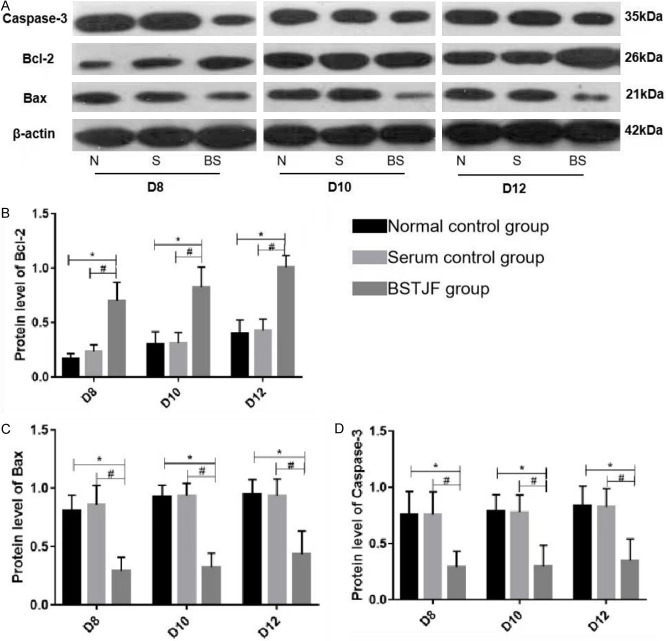
Bax, Bcl-2, and Caspase-3 protein expressions were measured in granulosa cells isolated on days 8, 10, and 12. There was no significant difference in the expressions of these proteins at any time point between the normal control group and the serum control group. Compared with both control groups, the BSTJF group showed higher Bcl-2 expression and lower Bax and Caspase-3 expressions at all three time points (all P ≤ 0.05, Figure 4).
Figure 4.
Western blot analysis of Caspase-3, Bcl-2, and Bax protein expressions on days 8, 10, and 12 of in vitro cultivation. Top panel: N = Normal control; S = Serum control; BS = BSTJF. Bottom panel: column height and error bars represent the mean ± SD (n = 3), respectively. *P ≤ 0.05 in contrast to the normal control group; #P ≤ 0.05 in contrast to the serum control group.
Discussion
The Yellow Emperor’s Inner Canon • Treatise on the Supreme Origin states that “the kidneys are the masters of water, receiving the essence of the five zang organs and six fu organs and storing it”. The five zang organs contain the heart, liver, spleen, lungs and kidneys. Six fu organs include the gallbladder, stomach, large intestine, small intestine, urinary bladder, and triple energizer. In this context, “essence” includes both pre-natal essence and post-natal essence. For females, follicles and oocytes can be classified as reproductive essences. The reproductive essence is inherited from the parents and gradually thrives under the nourishment of post-natal essence derived from water and grains, leading to the enrichment of qi and blood. In Fu Qing Zhu Gynecology, the conception of a couple originates from the abundance of kidney qi. Kidney essence is yin in nature, serving as the foundational substance that promotes the formation and development of follicles and oocytes. Abundant kidney essence, combined with the arrival of Tian Gui and the smooth flow of Ren and Chong channels, fosters growth of the follicles. Essence can transform into qi, and when kidney essence is ample, kidney qi becomes sufficient. The stimulation and propulsion of kidney yang facilitate the maturation and release of follicles and oocytes. In cases of deficiency of kidney essence and qi, the development of follicles and oocytes might get compromised, leading to their immaturity and inability to be released. This can result in poor follicular development, abnormal maturation, and ultimately, infertility in females. Therefore, kidney-tonifying formulas are commonly used in infertility treatment. These formulas primarily regulate the expression of relevant mRNAs and proteins through various pathways in oocytes, or they regulate granulosa cell-related factors and improve mitochondrial function, ultimately influencing oocytes [12]. Early-stage experimental research indicated that kidney-tonifying treatments could induce ovulation, increase the number of high-quality follicles, enhance the survival of preovulatory follicles, and improve the quality of the oocytes [13,14].
The BSTJF formula employed in this study to tonify the kidneys and regulate menstruation is a modification of the “Bushen Tiaojing Fang” (Tonifying Kidney and Regulating Menstruation Formula), which is based on the combination of Wuzi Yanzong Wan and Yang Jing Zhong Yu Tang. Wuzi Yanzong Wan is a classic formula with functions encompassing tonifying the kidneys, replenishing essence, and promoting the propagation of offspring. Pharmacological research indicated that Wuzi Yanzong Wan regulated the hypothalamic-pituitary-gonadal axis, exhibiting effects similar to those of sex hormones, gonadotropic hormones, and ovulation-promoting hormones [15,16]. Yang Jing Zhong Yu Tang is the first formula among the “Ten Formulas for Nourishing Seeds”, with effects of nourishing essence and enriching the blood. In Fu Qing Zhu Gynaecology, fetal formation depends on the essence of the kidneys, and the nourishment of the fetus relies on the blood of the five zang organs and six fu organs. Therefore, Yang Jing Zhong Yu Tang is the foundational formula for treating infertility. Clinical application of BSTJF has shown ideal results in treating infertility [8]. The composition of BSTJF includes prepared Rehmannia root, Chinese yam, dogwood fruit, purple Cornelian cherry, Epimedium, Chinese angelica, white peony root, goji berries, glossy privet fruit, dodder seed, raspberry, and Cyperus rhizome. The chief herb, prepared Rehmannia root, possesses a gustatory profile characterized by sweetness and warm nature. It effectively targets the hepatic and renal meridians and has the functions of nourishing kidney yin, replenishing essence, and enriching the marrow. Chinese yam, dogwood fruit, glossy privet fruit, and goji berries are deputy herbs that assist the chief herb in nourishing kidney yin and replenishing essence. Among them, Chinese yam, which is sweet in taste and neutral in nature, targets the lung, spleen, and kidney meridians. It fortifies the spleen, nourishes the lungs, stabilizes the kidneys, and nourishes essence. Purple Cornelian cherry has a sweet, salty taste and warm nature. It enters the lungs, liver, and kidney meridians, which warms the kidneys, nourishes essence, invigorates qi, and nourishes blood. It is also a substance that benefits both blood and flesh, addressing deficiencies of both yin and yang within human bodies. Raspberry has a sweet and sour taste as well as warm nature. The composition targets the liver and kidney meridians. It supplements the liver and kidneys, stabilizes essence, and contracts urine. Dodder seed nourishes the liver and kidneys and enhances essence and marrow. Chinese angelica possesses a palatable flavor characterized by a combination of sweetness and spiciness. It has a warm nature with access to the meridians associated with the liver, heart, and spleen. It contributes to the nourishment of blood, enhancement of blood circulation, regulation of the menstrual cycle, and mitigation of pain. White peony root has a bitter and sour taste with a slightly cold nature. It enters the liver and spleen meridians, softens the liver, nourishes blood, and harmonizes blood circulation. Therefore, the entire BSTJF nourishes the kidneys, enriches yin, replenishes essence, and nourishes blood to support the uterus and promote the development of ovarian follicles and oocytes.
The development of ovarian follicles results from interactions among the oocytes, surrounding granulosa cells, and follicular membrane cells. During the process of follicular development, most follicles undergo degeneration and atresia [17], which is thought to be caused by the apoptosis of granulosa cells [18,19]. Hence, granulosa cells must possess certain anti-apoptotic abilities to ensure that follicles can grow and develop to the ovulation stage. Studies have shown that Bcl-2 gene silencing in mice led to a reduction in the number of oocytes and primordial follicles [20], whereas overexpression of Bcl-2 increased the maturation rates of oocytes and reduced the apoptosis of granulosa cells in large antral follicles [21]. Conversely, the expressions of Bax and Caspase-3 are positively linked with follicular atresia and granulosa cell apoptosis [22,23]. Previous pharmacological research suggested that Wuzi Yanzong Pill had certain anti-apoptotic effects [24-26]. The results of the present study showed that treatment of the cultivated follicles with serum from BSTJF-treated rats resulted in increased Bcl-2 expression and downregulated Bax and Caspase3 expressions. Moreover, the serum from BSTJF-treated rats increased the follicle survival rate, follicular diameter, and the formation of antrum of the cultivated follicles. These results suggested that the kidney-tonifying formula promotes the proliferation of granulosa cells and reduces the apoptosis of granulosa cells, thereby reducing follicular atresia and promoting follicular growth as well as the development and maturation of the oocytes.
FSHR belongs to the G protein-coupled receptor family and is expressed in ovarian granulosa cells. It is involved in granulosa cell proliferation and differentiation, follicular development, and the maturation of the oocytes. Research has shown that FSHR levels decrease significantly when follicles and granulosa cells begin apoptosis [27,28], and ovarian granulosa cells with low FSHR expression or single-nucleotide polymorphism of FSHR gene showed poor ovarian reaction and could not obtain satisfactory results during superovulation [29-32]. Those findings suggest that the expression of a sufficient amount of FSHR can reduce the occurrence of follicular atresia and thus promote follicular growth and development. The LHR also belongs to the G protein-coupled receptor family. Enhanced LHR expression was found specifically in granulosa cells of large follicles before ovulation. The binding of LH to LHR promotes follicle growth and development, and their sensitivity to LHR increases as dominant follicles develop [38]. As a result, follicles eventually mature and release oocytes. In this study, FSHR and LHR expressions in the granulosa cells of follicles cultured with serum from rats treated with kidney-tonifying BSTJF were higher than those in control granulosa cells, which finding is consistent with that of previous studies [33,34].
Estrogen and progesterone play crucial roles in the maturation of ovarian follicles. Estrogen can promote granulosa cell differentiation, stimulate follicular maturation, inhibit follicular atresia, increase FSHR expression, and further enhance follicular development and maturation by regulating its own synthesis within the follicles [35]. Studies have shown that E2 treatment in rats can inhibit granulosa cell apoptosis in preovulatory and antral follicles, thereby increasing follicular survival rates. Furthermore, exogenous elevation of estrogen levels in rats after pituitary removal can inhibit granulosa cell apoptosis in antral follicles [36]. Moreover, progesterone can affect follicular growth and development and inhibit granulosa cell apoptosis. A previous study found that the progesterone antagonist RU486 stimulated follicular atresia and promoted granulosa cell apoptosis [37], highlighting the role of progesterone in inhibiting granulosa cell apoptosis. In this study, follicles cultured with serum from BSTJF-treated rats had higher E2 levels than the control follicles. These results support the hypothesis that BSTJF treatment promotes follicular development and oocyte maturation by accelerating the secretion of E2 and progesterone, thus reducing the apoptosis of granulosa cells.
Conclusions
Treatment of cultured ovarian follicles with serum from rats that received BSTJF, a kidney-tonifying and menstruation-regulating formula, resulted in reduced apoptosis and Bax and Caspase-3 expressions, and increased Bcl-2, FSHR, and LHR expressions and progesterone and E2 secretions in granulosa cells. These effects were accompanied by enhanced follicular development and maturation. This study provides insight into in vitro cultures of mouse follicles, mainly focused on granule cells. We know that the follicle is mainly composed of granulosa cells, oocyte, and follicular fluid. Therefore, further research is needed to determine how the kidney-tonifying formula affects oocyte and follicular fluid in anterior sinus follicles in vitro.
Acknowledgements
The authors thank the Hebei University of Chinese Medicine and Hebei Key Laboratory of Integrative Medicine on Liverkidney Patterns for technical support. This study was supported by grants from the National Natural Science Foundation of China (No. 81774359) and Scientific Research Plan Project of Hebei Province Administration of Traditional Chinese Medicine (No. 2023124).
Disclosure of conflict of interest
None.
References
- 1.Homan GF, Davies M, Norman R. The impact of lifestyle factors on reproductive performance in the general population and those undergoing infertility treatment: a review. Hum Reprod Update. 2007;13:209–223. doi: 10.1093/humupd/dml056. [DOI] [PubMed] [Google Scholar]
- 2.Baker TG. A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond B Biol Sci. 1963;158:417–433. doi: 10.1098/rspb.1963.0055. [DOI] [PubMed] [Google Scholar]
- 3.Matsuda F, Inoue N, Manabe N, Ohkura S. Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. J Reprod Dev. 2012;58:44–50. doi: 10.1262/jrd.2011-012. [DOI] [PubMed] [Google Scholar]
- 4.Xu SX, Zhao ZM, Shen HM, Zuo YP, Song SL. Study of the relationship between the expression of follicle stimulating hormone receptor on granulosa cells and in vitro fertilization and embryo transfer (IVF-ET) Chin J Birth Health Hered. 2004;12:70–71. 92. [Google Scholar]
- 5.Lee KS, Joo BS, Na YJ, Yoon MS, Choi OH, Kim WW. Cumulus cells apoptosis as an indicator to predict the quality of oocytes and the outcome of IVF-ET. J Assist Reprod Genet. 2001;18:490–498. doi: 10.1023/A:1016649026353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Algeciras-Schimnich A, Barnhart BC, Peter ME. Apoptosis-independent functions of killer caspases. Curr Opin Cell Biol. 2002;14:721–726. doi: 10.1016/s0955-0674(02)00384-8. [DOI] [PubMed] [Google Scholar]
- 7.Corn CM, Hauser-Kronberger C, Moser M, Tews G, Ebner T. Predictive value of cumulus cell apoptosis with regard to blastocyst development of corresponding gametes. Fertil Steril. 2005;84:627–633. doi: 10.1016/j.fertnstert.2005.03.061. [DOI] [PubMed] [Google Scholar]
- 8.Chang XF. Clinical researches on the effects of Bushen Tiaojing Recipe on outcome of IVF-ET and its effects on apoptosis of granulosa cell and oocyte-secreted factors. Hebei Med Univ. 2011 [Google Scholar]
- 9.Sun XH, Fan LJ, Li L, Wei XC, Liu YH, Geng DD, Gu XX, Du HL. Bushen Tiaojing Recipe regulated expressions of BMPRII/ALK6-Smads in mouse oocytes culture in vitro. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2016;36:1241–1246. [PubMed] [Google Scholar]
- 10.Fan LJ. Comparison of GDF-9 and Smads signal pathway between Bushen treatment and Shugan treatment in vitro culture oocytes. Hebei Med Univ. 2014 [Google Scholar]
- 11.Liu YH, Duan YC, Li L, He M, Fan LJ, Liang X, Du HL. Comparison of effects of Bushen Tiaojing formula and Xiaoyao Pills on expression of ADAM8 and ADAMTS-1 in vitro cultured follicles of mice. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2021;41:736–741. [Google Scholar]
- 12.Liu WL, Yin QZ. Study on quality regulation of oocytes by traditional Chinese medicine. Liaoning J Tradit Chin Med. 2020;47:34–36. [Google Scholar]
- 13.Bai J, Du HL, Yang LY. Effects of Bushen treatment and Shugan treatment on oocyte mass and bone morphogenetic protein-15 of mice. Chin J Chin Med. 2020;35:3591–3594. [Google Scholar]
- 14.Song CM, Duan YC, He M, Xu HZ, Du HL. Effects of Bushen Tiaojing Recipe and Xiaoyao Pill on ovulation key factor HIF-1α and EDN2 of gonadotropin pretreatment mice. Chin J Chin Med. 2020;35:1714–1718. [Google Scholar]
- 15.Chai Z, Wang XH, Fan HJ, Wang YH, Li YY, Zhou R. Wuzi Yanzong Pill’s protective effect on the reproductive system and progress in its clinical research. Chin Tradit Patent Med. 2016;38:1579–1583. [Google Scholar]
- 16.Chen WQ, Ding CF, Yu J, Wang CY, Wan LY, Hu HM, Ma JX. Corrigendum: Wuzi Yanzong pill-Based on network pharmacology and In Vivo evidence-Protects against spermatogenesis disorder via the regulation of the apoptosis pathway. Front Pharmacol. 2023;13:1129448. doi: 10.3389/fphar.2022.1129448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manabe N, Goto Y, Matsuda-Minehata F, Inoue N, Maeda A, Sakamaki K, Miyano T. Regulation mechanism of selective atresia in porcine follicles: regulation of granulosa cell apoptosis during atresia. J Reprod Dev. 2004;50:493–514. doi: 10.1262/jrd.50.493. [DOI] [PubMed] [Google Scholar]
- 18.Choi JY, Jo MW, Lee EY, Yoon BK, Choi DS. The role of autophagy in follicular development and atresia in rat granulosa cells. Fertil Steril. 2010;93:2532–2537. doi: 10.1016/j.fertnstert.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 19.Jiang JY, Cheung CK, Wang Y, Tsang BK. Regulation of cell death and cell survival gene expression during ovarian follicular development and atresia. Front Biosci. 2003;8:d222–237. doi: 10.2741/949. [DOI] [PubMed] [Google Scholar]
- 20.Ratts VS, Flaws JA, Kolp R, Sorenson CM, Tilly JL. Ablation of bcl-2 gene expression decreases the numbers of oocytes and primordial follicles established in the post-natal female mouse gonad. Endocrinology. 1995;136:3665–3668. doi: 10.1210/endo.136.8.7628407. [DOI] [PubMed] [Google Scholar]
- 21.Hsu SY, Lai RJ, Finegold M, Hsueh AJ. Targeted overexpression of Bcl-2 in ovaries of transgenic mice leads to decreased follicle apoptosis, enhanced folliculogenesis, and increased germ cell tumorigenesis. Endocrinology. 1996;137:4837–43. doi: 10.1210/endo.137.11.8895354. [DOI] [PubMed] [Google Scholar]
- 22.Robles R, Tao XJ, Trbovich AM, Maravel DV, Nahum R, Perez GI, Tilly KI, Tilly TI. Localization, regulation and possible consequences of apoptotic protease-activating factor-1 (Apaf-1) expression in granulosa cells of the mouse ovary. Endocrinology. 1999;140:2641–2644. doi: 10.1210/endo.140.6.6931. [DOI] [PubMed] [Google Scholar]
- 23.Uma J, Muraly P, Verma-Kumar S, Medhamurthy R. Determination of onset of apoptosis in granulosa cells of the preovulatory follicles in the bonnet monkey (Macaca radiata): correlation with mitogen-activated protein kinase activities. Biol Reprod. 2003;69:1379–1387. doi: 10.1095/biolreprod.103.017897. [DOI] [PubMed] [Google Scholar]
- 24.Li RX, Fan HJ, Chai Z, Li YR, Sun MY, Xiao WS, Wei JZ, Li YH, Zhang B, Ma CG, Zhou R. Effects of Wuzi Yanzong Pills on the apoptotic pathway of neural tube defects cell model. J Tradit Chin Med. 2019;60:1134–1141. [Google Scholar]
- 25.Qin M, Ke MH, Liu BX, Zhang XP, Cui TW, Ma WJ, Gao YX, Ke ZH. Effects of Wuzi Yanzong Pill on spermatogenesis by modulating expression of cytoskeleton protein in Sertoli cells in rats with kidney essence deficiency pattern. J Beijing Univ Tradit Chin Med. 2019;42:490–495. [Google Scholar]
- 26.Li L, Dai N, Na S, Jia HY, Zhou XC, Zhou DD, Wang TS. Protective effect of Wuziyanzong Pills on rats with experimental oligoasthenospermia and its action mechanism. Zhonghua Nan Ke Xue. 2016;22:827–833. [PubMed] [Google Scholar]
- 27.Yamoto M, Shima K, Nakano R. Gonadotropin receptors in human ovarian follicles and corpora lutea throughout the menstrual cycle. Horm Res. 1992;37(Suppl 1):5–11. doi: 10.1159/000182335. [DOI] [PubMed] [Google Scholar]
- 28.Xu Z, Garverick HA, Smith GW, Smith MF, Hamilton SA, Youngquist RS. Expression of follicle-stimulating hormone and luteinizing hormone receptor messenger ribonucleic acids in bovine follicles during the first follicular wave. Biol Reprod. 1995;53:951–957. doi: 10.1095/biolreprod53.4.951. [DOI] [PubMed] [Google Scholar]
- 29.Kolibianakis EM, Venetis CA, Diedrich K, Tarlatzis BC, Griesinger G. Addition of growth hormone to gonadotrophins in ovarian stimulation of poor responders treated by in-vitro fertilization: a systematic review and meta-analysis. Hum Reprod Update. 2009;15:613–622. doi: 10.1093/humupd/dmp026. [DOI] [PubMed] [Google Scholar]
- 30.Jun JK, Yoon JS, Ku SY, Choi YM, Hwang KR, Park SY, Lee GH, Lee WD, Kim SH, Kim JG, Moon SY. Follicle-stimulating hormone receptor gene polymorphism and ovarian responses to controlled ovarian hyperstimulation for IVF-ET. J Hum Genet. 2006;51:665–670. doi: 10.1007/s10038-006-0005-5. [DOI] [PubMed] [Google Scholar]
- 31.Detti L, Williams DB, Robins JC, Maxwell RA, Thomas MA. A comparison of three downregulation approaches for poor responders undergoing in vitro fertilization. Fertil Steril. 2005;84:1401–1405. doi: 10.1016/j.fertnstert.2005.04.053. [DOI] [PubMed] [Google Scholar]
- 32.Sunkara SK, Coomarasamy A, Arlt W, Bhattacharya S. Should androgen supplementation be used for poor ovarian response in IVF? Hum Reprod. 2012;27:637–640. doi: 10.1093/humrep/der464. [DOI] [PubMed] [Google Scholar]
- 33.Shen KJ, Xiong J, You ZL, Lei L, Fu LM, Yin XH, Liu Y. Effects of Huluan decoction on the expression of FSHR and LHR in rats’ ovary under GnRHa controlled ovarian hyperstimulation. J Hunan Univ Tradit Chin Med. 2013;33:26–29. [Google Scholar]
- 34.Zhang BJ, Feng YH, Zhang L, Xia HF, Zhao LH, Gong YP, Tian Y, Cao XP, Pan K, Sun LN, Wang JL. Influence of Bushen Cupai formula on the expression of lu teinizing hormone receptor and follicle stimulating hormone receptor in rat ovarian granulosa cells. Mod J Integr Tradit Chin West Med. 2017;26:807–809. [Google Scholar]
- 35.Sarraj MA, Drummond AE. Mammalian foetal ovarian development: consequences for health and disease. Reproduction. 2012;143:151–163. doi: 10.1530/REP-11-0247. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology. 2007;148:3580–3590. doi: 10.1210/en.2007-0088. [DOI] [PubMed] [Google Scholar]
- 37.Billig H, Furuta I, Hsueh AJ. Estrogens inhibit and androgens enhance ovarian granulosa cell apoptosis. Endocrinology. 1993;133:2204–2212. doi: 10.1210/endo.133.5.8404672. [DOI] [PubMed] [Google Scholar]
- 38.Menon KM, Clouser CL, Nair AK. Gonadotropin receptors: role of post-translational modifications and post-transcriptional regulation. Endocrine. 2005;26:249–257. doi: 10.1385/ENDO:26:3:249. [DOI] [PubMed] [Google Scholar]