Abstract
Objective: To evaluate the predictive value of somatosensory evoked potentials (SEPs) for the efficacy of closed reduction combined with over-extension reduction technique (PVP) in managing thoracolumbar spinal compression fractures. Methods: Data were collected from 125 patients who underwent closed reduction with PVP and SEP monitoring from February 2021 to July 2023. We evaluated surgery success rates, incidence of bone cement leakage, and patient recovery outcomes including vertebral anterior height, Oswestry Disability Index (ODI), and Cobb angle restoration. SEP results were analyzed to categorize patients into effective and ineffective treatment groups. Differences in SEP waveforms between these groups were examined, and ROC analysis was used to assess the predictive value of these differences. Multivariate logistic regression was employed to identify risk factors affecting treatment efficacy. Results: Post-treatment assessments showed significant improvements in vertebral anterior height, ODI, and Cobb angle. SEP monitoring correlated well with intraoperative findings and physical examinations. During reduction, changes in SEP latency and amplitude were noted in 37 patients, with 7 patients meeting SEP amplitude alarm criteria, which normalized after adjustments. During PVP, 28 patients exhibited SEP amplitude fluctuations and 5 experienced a 30% reduction in amplitude following initial cement injection, with no significant latency changes. Treatment was deemed effective in 93 patients and ineffective in 32. SEP amplitudes during vertebral compression and PVP were significantly lower in the effective group (P<0.05). The AUC for predicting treatment efficacy was 0.819 and 0.859, respectively. Multivariate analysis revealed low preoperative vertebral compression ratio, number of fractures, and abnormal SEP amplitudes as independent risk factors for treatment outcomes. Conclusion: SEP monitoring provides an accurate reflection of spinal cord function during closed reduction with PVP, aiding in predicting treatment safety and efficacy. The use of SEP monitoring is thus recommended for clinical application in this context.
Keywords: Somatosensory evoked potentials, closed reduction, over extension reduction technique, thoracolumbar spinal compression fracture
Introduction
With China’s aging population, the incidence of osteoporotic vertebral compression fractures (OVCF) has steadily increased annually [1]. Thoracolumbar osteoporotic compression fractures are particularly prevalent among the elderly, characterized by symptoms such as kyphosis, chest deformity, and sudden lumbar back pain. These fractures can impair cardiopulmonary function and increase the risk of upper respiratory tract and pulmonary infections, significantly affecting the quality of life in elderly patients [2,3]. Although percutaneous vertebroplasty (PVP) combined with the over-extension reduction technique has become the preferred treatment, simple PVP often does not restore the original vertebral morphology and height [4].
Traction reduction, a technique rooted in traditional Chinese medicine, has shown unique advantages in restoring vertebral height and treating compression fractures, backed by years of practical experience [5]. Recently, the integration of PVP with traction reduction has emerged as a prominent method for managing thoracolumbar spinal compression fractures. However, the effectiveness of traction reduction heavily depends on the operator’s subjective judgment and experience, lacking established objective standards for traction force, duration, and depth of vertebral compression during the procedure. Additionally, while PVP is relatively safe and minimally invasive, it can still lead to complications such as spinal cord and nerve compression [6,7]. Thus, implementing safety monitoring for PVP combined with traction reduction is crucial. Somatosensory evoked potential (SEP) monitoring, a widely recognized method for assessing spinal cord function during spinal surgeries, is used predominantly in cervical and lumbar procedures [8,9].
Despite the established value of SEP in spinal surgery monitoring, its application in hyperextension reduction manipulation combined with PVP treatment remains underexplored. Thus, this study introduces SEP monitoring to the process of PVP combined with traction reduction, aiming to monitor spinal cord function in real-time, enhance the safety of the treatment, and establish objective and effective standards for the quality control of this therapy.
Materials and methods
Clinical data
A retrospective analysis was conducted on 68 patients with thoracolumbar spinal compression fractures treated with extension reduction manipulation combined with PVP and SEP testing at the Affiliated Hospital of Gansu University of Traditional Chinese Medicine from February 2021 to July 2023.
Inclusion criteria: (1) Diagnosis of thoracolumbar vertebral compression fractures confirmed by X-ray; (2) Disease course within one week; (3) Suitability for PVP surgery; (4) Complete medical records; (5) Received PVP treatment and SEP monitoring, with outcomes assessed post-surgery.
Exclusion criteria: (1) Patients with chronic thoracolumbar vertebral compression fractures; (2) Patients with concurrent malignant bone tumors; (3) Patients with symptoms of spinal cord and nerve root compression; (4) Non-compliance or loss to follow-up. The study flowchart is detailed in Figure 1. Ethical approval was granted by the ethics Committee of the Affiliated Hospital of Gansu University of Traditional Chinese Medicine.
Figure 1.
Flow chart of patient inclusion.
Treatment and monitoring
SEP monitoring [10]
SEP monitoring was conducted using the Oxford Medilogic Muscle Evoked Potential Instrument, with room temperature maintained between 22°C and 25°C. Surface electrodes were used to stimulate the medial malleolus of the lower limbs using square wave stimuli, with current intensities ranging from 30 to 60 mA and toe twitch as the standard response. Stimulation frequencies varied between 1.9 and 5.7 Hz, with pulse widths from 0.2 to 0.3 ms, repeated 100 times. The recording electrode was placed at the Cz point on the scalp, with the reference electrode at the Fz point. SEP examinations were performed routinely on all patients prior to and during various phases of the procedure: before reduction maneuvers, prior to surgery, during horizontal and vertical traction, during vertical compression of the fractured vertebra, and upon reduction completion. Continuous monitoring occurred from before anesthesia induction through surgery, until treatment completion. Needle electrodes were used to provide continuous electrical stimulation to the bilateral tibial nerves at the posterior lower limbs to consistently elicit toe twitches throughout the monitoring. SEP monitoring indices included latency and amplitude, with P37 set as the standard evoked potential. The interval from stimulus onset to the first evoked potential waveform and the amplitude from peak to trough were measured. Baseline latency and amplitude were established after full exposure of the vertebral body following paraspinal muscle dissection. Abnormal criteria were defined as a decrease in amplitude greater than 50% or an extension of latency exceeding 10%. Upon detecting such abnormalities, immediate investigation and intervention were undertaken.
Over extension reduction technique
The patient is placed in the prone position. An assistant stands by the patient’s head, gripping the axillae, while two other assistants stand by the patient’s feet, holding the ankles (ensuring avoidance of the SEP stimulation electrodes). Both groups exert simultaneous horizontal traction in opposite directions for 3 to 5 minutes. Following this, the foot-side assistants gradually lift the lower limbs to elevate the spine into a hyperextension position. Concurrently, the operator applies pressure to the injured vertebrae with overlapping hands. The procedure is conducted under fluoroscopic guidance, aiming to restore the vertebral height to 90% or more.
PVP procedure
After reducing the fracture, a C-arm X-ray machine is used to identify the operative vertebra, pedicle directions, and the “owl’s eye” projection to correct any vertebral rotation. Under C-arm guidance, the puncture needle is inserted through the pedicle into the junction of the pedicle and vertebral body. A lateral view confirms the needle’s position. The pedicle is manipulated in all directions to widen the entry point, and the angle of the needle is adjusted to avoid breaching the pedicle’s inner wall. The needle is advanced to the anterior two-thirds of the vertebral body, with the tip nearing or crossing the midline. After withdrawing the stylet, a contrast medium is injected to check for leaks. Type III acrylic resin bone cement is then prepared to a toothpaste-like consistency and slowly injected at a rate of 2 ml initially, followed by an additional 2-6 ml after a 2-minute wait. The distribution and diffusion of bone cement within the vertebral body are closely monitored for any signs of leakage into the vertebral veins, epidural space, or neural foramina, with the injection halted immediately if leakage is detected. On average, 4.8±0.4 ml of bone cement is injected per vertebral body. Postoperatively, patients are required to remain bedridden for 24 to 72 hours.
Outcome measure
Primary outcome measures
Patients were classified into either a good recovery group or a poor recovery group based on the percentage of height loss restoration of the anterior edge of the injured vertebra, Oswestry Disability Index (ODI), and the recovery of the Cobb angle of the thoracolumbar spine sagittal plane post-treatment. The criteria for good recovery included: restoration of the anterior vertebral height to less than 25% loss, ODI improved to less than 40%, and a Cobb angle of less than 10°. SEP monitoring waveforms at different treatment stages were compared between the two groups, and the predictive value of significant waveform differences was analyzed using ROC analysis. Multivariate logistic regression was used to identify risk factors impacting patient outcomes.
Secondary outcome measures
(1) Postoperative metrics included surgical success rates and incidences of bone cement leakage. (2) Two months post-surgery, patient outcomes were evaluated, including the restoration of anterior vertebral body height, preoperative ODI, and Cobb angle. (3) Results from somatosensory evoked potentials (SEP) monitoring were analyzed.
Statistical analysis
Data were organized using Excel and analyzed using SPSS 20.0. Measurement data were expressed as mean ± standard deviation (x̅ ± sd), with independent t-tests comparing groups with differing recovery outcomes and paired t-tests assessing pre- and post-treatment changes within groups. Categorical data were analyzed using the Chi-square test. ROC analysis was conducted to evaluate the predictive value of significant waveform amplitude differences, and multivariate logistic regression analysis was employed to investigate risk factors affecting patient outcomes. A p-value <0.05 was considered statistically significant.
Result
Patient demographics and baseline characteristics
All patients met the inclusion criteria and were enrolled in the study. Basic demographic and clinical characteristics are summarized in Table 1.
Table 1.
Patient demographics and baseline characteristics
| Item | n=68 |
|---|---|
| Gender | |
| Male | 66 (52.94) |
| Female | 59 (47.06) |
| Age | |
| ≤60 | 45 (36.76) |
| >60 | 85 (63.24) |
| Body mass index (kg/m2) | |
| ≤23 | 64 (51.47) |
| >23 | 61 (48.53) |
| Number of cone fractures | |
| Single cone | 75 (60.29) |
| Double cone | 44 (35.29) |
| 3 cones | 6 (4.41) |
| Fracture site | |
| Thoracic | 55 (44.00) |
| Lumbar spine | 70 (56.00) |
| Comorbidities | |
| Diabetes | 37 (29.60) |
| Hypertension | 37 (29.60) |
| Others | 51 (40.80) |
Patient surgical success rate and bone cement leakage
A total of 181 vertebral bodies in 125 patients were treated successfully, with an average surgical duration of 54.89±4.03 minutes. There were no intraoperative deaths or adverse events related to cardiovascular or cerebrovascular systems. Bone cement leakage occurred anteriorly in 4 cases and laterally in 2 cases, with no posterior leakage observed. Detailed results are provided in Table 2.
Table 2.
Patient surgical success rate and bone cement leakage
| Project | n=125 |
|---|---|
| Surgery success rate | 125 (100.00) |
| Surgical time (min) | 54.89±4.03 |
| Bone cement leakage | |
| Leakage anterior to cone | 7 (5.60) |
| Cone side leakage | 4 (3.20) |
| Leakage behind the cone | 0 |
| Bleeding volume (ml) | 6.92±1.27 |
| Hospital stay (days) | 8.61±1.18 |
Assessment of patient recovery outcomes
Preoperatively, the average percentage of anterior vertebral body height loss in patients was 45.7±10.74%. One week postoperatively, X-ray evaluations showed that this had improved to 24.95±4.11%, a statistically significant recovery (P<0.05). The ODI decreased from 69.97±6.01% preoperatively to 38.83±2.25% postoperatively, also showing significant improvement (P<0.05). The preoperative Cobb angle of the thoracolumbar spine in the sagittal plane was 27.04±1.89°, which recovered to 8.64±1.3° postoperatively, indicating significant correction (P<0.05). These results are depicted in Figure 2.
Figure 2.
Assessment of patient recovery outcomes. ODI: Oswestry Disability Index. * indicates P<0.05.
SEP monitoring results
Preoperative SEP monitoring aligned with intraoperative electrophysiological examination findings and physical examination results. All 125 patients underwent successful SEP monitoring during treatment. During the overextension reduction technique, 88 patients showed no significant changes in SEP. However, 37 patients exhibited variations in SEP latency and amplitude, with 7 experiencing significant transient prolongations (>2.5 ms) in SEP latency and reductions in amplitude exceeding 50% during vertebral compression, triggering alarm criteria. Upon temporarily halting the reduction and adjusting the pressure, SEP waveforms normalized. No other abnormal changes in SEP waveforms were noted during the overextension reduction in the remaining patients. During the PVP procedure, 28 patients showed intraoperative waveform fluctuations, and 5 of these experienced a 30% decrease in SEP amplitude following the initial 2 ml bone cement injection, without significant latency changes. No corrective action was taken, and the procedure continued with the remaining bone cement injection under ongoing SEP monitoring. Postoperatively, no neurological dysfunction was reported, consistent with the preoperative and intraoperative SEP monitoring results. The detailed findings are presented in Table 3.
Table 3.
SEP monitoring values of patients at different monitoring stages
| Monitoring phase | Amplitude (μv) | Latency (ms) |
|---|---|---|
| Before reduction (prone position) | 1.65±0.19* | 34.49±1.26 |
| Longitudinal traction | 1.98±0.26 | 36.78±1.16 |
| Hyperextension traction | 2.01±0.21 | 36.56±1.39 |
| Press the injured vertebra | 2.21±0.21# | 36.84±1.28 |
| After reduction/before PVP (prone position) | 1.6±0.18* | 34.33±1.16 |
| During PVP surgery | 2.07±0.21# | 36.78±2.02 |
| After PVP surgery | 1.57±0.17 | 34.39±1.45 |
SEP: somatosensory evoked potential; PVP: percutaneous vertebroplasty. Note: * compared with #, P<0.05.
Comparison of SEP monitoring amplitudes
Patients were divided into an effective group (93 cases) and an ineffective group (32 cases) based on the percentage of anterior edge height loss of the injured vertebra, ODI, and recovery of the sagittal Cobb angle of the thoracolumbar spine. SEP amplitudes were compared across various monitoring stages between these groups. No significant differences in SEP amplitudes were observed before reduction (in prone position), during longitudinal traction, during hyperextension traction, after reduction/PVP preoperatively (in prone position), and post-PVP surgery (P>0.05). However, SEP amplitudes during vertebral compression and PVP surgery were significantly lower in the effective group compared to those in the ineffective group (P<0.05), as shown in Table 4.
Table 4.
Comparison of SEP monitoring amplitudes
| Monitoring phase | Effective group | Ineffective group | t | P |
|---|---|---|---|---|
| n=93 | n=32 | |||
| Before reduction (prone position) | 1.68±0.19 | 1.62±0.23 | 1.458 | 0.147 |
| Longitudinal traction | 1.95±0.21 | 1.97±0.26 | 0.436 | 0.663 |
| Hyperextension traction | 2.06±0.24 | 2.01±0.21 | 1.048 | 0.297 |
| Press the injured vertebra | 2.12±0.16 | 2.35±0.22 | 6.339 | <0.001 |
| After reduction/before PVP (prone position) | 1.61±0.15 | 1.63±0.14 | 0.661 | 0.510 |
| During PVP surgery | 2.01±0.15 | 2.31±0.21 | 8.757 | <0.001 |
| After PVP surgery | 1.62±0.17 | 1.57±0.16 | 1.456 | 0.148 |
SEP: somatosensory evoked potential; PVP: percutaneous vertebroplasty.
SEP abnormal amplitude for efficacy prediction ROC analysis
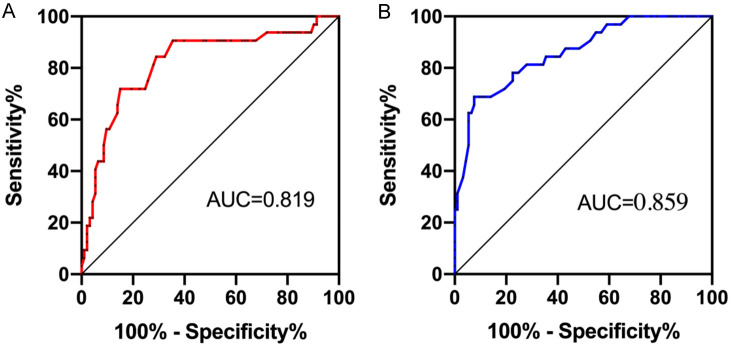
ROC analysis was conducted on SEP monitoring amplitudes during vertebral compression and PVP surgery. The amplitudes at these stages demonstrated a high predictive value for treatment efficacy. The sensitivity of amplitude prediction during vertebral compression was 84.38%, with a specificity of 72.04% and an AUC of 0.819. During PVP surgery, the sensitivity was 78.13%, with a specificity of 75.27% and an AUC of 0.859. These results are illustrated in Figure 3.
Figure 3.
SEP abnormal amplitude for efficacy prediction ROC. A: Prediction ROC of the therapeutic effect of the amplitude when pressing the injured vertebrae. B: Prediction ROC of time amplitude on efficacy during PVP surgery. SEP: somatosensory evoked potential; ROC: receiver operating characteristic; PVP: percutaneous vertebroplasty.
Multifactorial analysis of treatment efficacy
Based on postoperative outcomes, patients were categorized into effective and ineffective groups. Single-factor analysis identified factors influencing treatment efficacy as age, low preoperative vertebral compression ratio, number of vertebral body fractures, and the improvement rate of SEP amplitude (Table 5). Logistic regression analysis further revealed that a high percentage of preoperative vertebral anterior height loss, a high number of vertebral body fractures, and abnormal SEP amplitude were independent risk factors affecting patient prognosis (Table 6, P<0.05).
Table 5.
Univariate analysis
| Item | Effective group | Ineffective group | X2 | P |
|---|---|---|---|---|
| n=93 | n=32 | |||
| Age | 10.02 | 0.001 | ||
| ≤60 (n=45) | 42 (45.16) | 4 (12.50) | ||
| >60 (n=85) | 51 (54.84) | 28 (87.50) | ||
| BMI (kg/m2) | 0.025 | 0.875 | ||
| ≥23 kg/m2 (n=64) | 48 (51.61) | 16 (50.00) | ||
| <23 kg/m2 (n=61) | 45 (48.39) | 16 (50.00) | ||
| Number of cone fractures | 18.21 | <0.001 | ||
| Single cone (n=75) | 66 (70.97) | 9 (28.13) | ||
| 2-3 cones (n=50) | 27 (20.93) | 23 (71.88) | ||
| Percentage of height loss at the front edge of the injured vertebral body before surgery (%) | 15.30 | <0.001 | ||
| ≥50% (n=53) | 30 (32.26) | 23 (71.88) | ||
| <50% (n=72) | 63 (67.74) | 9 (28.13) | ||
| Is there any abnormality in SEP monitoring? | 16.62 | <0.001 | ||
| Yes (n=55) | 28 (30.11) | 27 (77.27) | ||
| No (n=70) | 65 (69.89) | 5 (22.73) |
SEP: somatosensory evoked potential.
Table 6.
Multi-factor analysis
| Factor | B | S.E. | Wals | P | Exp (B) | 95% C.I. | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Lower limit | Upper limit | ||||||
| Number of cone fractures | 1.622 | 0.692 | 5.334 | 0.011 | 4.911 | 1.320 | 11.345 |
| Percentage of height loss at the front edge of the injured vertebral body before surgery (%) | 3.412 | 0.921 | 7.329 | 0.002 | 10.4552 | 2.872 | 16.592 |
| SEP monitoring abnormality | 2.579 | 0.752 | 6.287 | 0.003 | 9.021 | 1.923 | 14.423 |
SEP: somatosensory evoked potential.
Discussion
Thoracolumbar compression fractures are a prevalent type of spinal injury, often leading to vertebral collapse due to the compression of cancellous bone [11]. Over years of clinical practice, the over-extension reduction technique has demonstrated significant advantages in restoring vertebral height and addressing compression fractures. PVP, a minimally invasive technique, involves injecting polymethylmethacrylate bone cement into the vertebra to enhance vertebral height and compressive strength, thereby swiftly alleviating pain [12-14]. However, traditional over-extension reduction techniques primarily depend on the surgeon’s experience and lack an objective assessment of spinal cord function during the procedure, raising concerns about potential severe complications [15]. Although generally considered safe, PVP may still pose risks such as spinal cord or nerve compression. Additionally, during cement injection, temperatures can reach up to 113°C in the vertebral cortex and 112°C centrally, presenting a risk of thermal injury which could lead to suboptimal outcomes [16,17]. Thus, developing methods to prevent complications related to spinal cord and nerve compression and accurately predicting treatment efficacy is crucial in clinical settings.
In our study, we observed that SEP latency prolongation was more sensitive than amplitude changes during the over-extension reduction technique. Four patients exhibited transient prolongation of SEP latency (>2.5 ms) and a decrease in amplitude exceeding 50%, triggering the alarm criteria. Upon halting the reduction maneuver and adjusting the pressure on the injured vertebrae, waveforms returned to normal. During subsequent PVP treatment, although some patients experienced amplitude decreases, these did not meet the alarm threshold, thus no adjustments were made. Overall, the surgery was successful for all patients, who subsequently recovered well.
Previous studies have indicated that for treating elderly patients with osteoporotic spinal compression fractures, both PVP and percutaneous kyphoplasty (PKP) offer benefits such as minimal trauma and rapid recovery. PVP, in particular, has advantages over PKP, including significantly shorter operation times, a lower risk of bone cement venous leakage and adjacent vertebral fractures, and higher surgical safety [18]. These findings align with existing research demonstrating that combining PVP with over-extension reduction effectively manages osteoporotic thoracolumbar compression fractures by providing rapid pain relief, restoring vertebral height, and correcting kyphotic deformities [19].
Postoperative patient assessments correlated with SEP monitoring results, affirming that SEP monitoring assists surgeons in adjusting treatments to enhance efficacy. SEP is a well-established tool in spinal surgeries; amplitude of evoked potentials reflects the number of responding neurons. During surgery, traction or compression on the spinal cord or nerve roots can cause neural ischemia, evidenced by decreased evoked potential amplitude. The latency period indicates the conduction speed of nerve fibers. Persistent ischemia can reduce the number of responsive nerve fibers and cause irreversible nerve conduction changes, thus prolonging latency [20,21]. Therefore, a 50% reduction in amplitude suggests significant pathological damage, while a 10% increase in latency also signals harm [22].
SEP monitoring is commonly employed in cervical and lumbar surgeries, and its value is widely recognized. This study represents the first application of SEP monitoring during the combination of over-extension reduction and PVP treatment, providing valuable insights into its effectiveness and safety in this specific clinical context.
It is crucial to note that during the injection of bone cement into the vertebral body, unlike conventional spinal exposure surgeries, any complications such as spinal cord or nerve compression can have irreversible consequences [23]. To mitigate this risk, we implemented a staged injection protocol. Initially, after the bone puncture needle enters the vertebral body, an SEP assessment is conducted. If no abnormalities are detected, 1-2 ml of bone cement is injected, followed by an immediate SEP reassessment within one minute. This staged approach not only complements the surgical procedure but also maximizes the acquisition of crucial neural and spinal cord function information. If SEP abnormalities are detected after the initial 2 ml of bone cement, immediate investigation and response can minimize potential complications.
To further assess the predictive value of SEP monitoring on patient outcomes and factors influencing efficacy, we categorized patients into effective and ineffective groups based on post-treatment measures such as the percentage of anterior vertebral height loss, ODI, and thoracolumbar sagittal Cobb angle restoration. Results indicated that SEP amplitudes during vertebral compression and PVP procedures were significantly lower in the effective group, suggesting a strong predictive value of amplitude changes at these stages. Multivariate analysis identified a high preoperative percentage of anterior vertebral height loss, a greater number of vertebral fractures, and abnormal SEP amplitudes as independent risk factors affecting patient prognosis.
However, challenges remain with SEP, such as enhancing monitoring accuracy, reducing false positives and negatives, and establishing uniform alarm standards for diverse patients. The feasibility of relying solely on SEP technology during procedures also warrants further investigation. Additionally, our study faces certain limitations. First, the relatively small sample size may limit the generalizability of our findings. Larger, multicenter studies are needed to validate these results. Second, potential selection bias in the retrospective analysis could affect the external validity of our findings. Lastly, while SEP was utilized to monitor the combined over-extension reduction and PVP procedure, it may not capture all dimensions of the treatment process. Combining multiple measurement tools and methods could provide a more comprehensive assessment of treatment efficacy.
In conclusion, SEP monitoring accurately reflects spinal cord function during the combined over-extension reduction and PVP procedures, supporting its utility for predicting treatment safety and efficacy. This monitoring technique is recommended for clinical application.
Disclosure of conflict of interest
None.
References
- 1.Dai C, Liang G, Zhang Y, Dong Y, Zhou X. Risk factors of vertebral re-fracture after PVP or PKP for osteoporotic vertebral compression fractures, especially in Eastern Asia: a systematic review and meta-analysis. J Orthop Surg Res. 2022;17:161. doi: 10.1186/s13018-022-03038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou T, Lin H, Wang H, Chen X, He F. Comparative study on the biomechanics between improved PVP and traditional PKP in the treatment of vertebral peripheral wall damage-type OVCF. Exp Ther Med. 2017;14:575–580. doi: 10.3892/etm.2017.4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long Y, Yi W, Yang D. Advances in vertebral augmentation systems for osteoporotic vertebral compression fractures. Pain Res Manag. 2020;2020:3947368. doi: 10.1155/2020/3947368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma YH, Tian ZS, Liu HC, Zhang BY, Zhu YH, Meng CY, Liu XJ, Zhu QS. Predictive risk factors for recollapse of cemented vertebrae after percutaneous vertebroplasty: a meta-analysis. World J Clin Cases. 2021;9:2778–2790. doi: 10.12998/wjcc.v9.i12.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang J, Zhou L, Yan Z, Zhou Z, Gou X. Effect of manual reduction and indirect decompression on thoracolumbar burst fracture: a comparison study. J Orthop Surg Res. 2020;15:532. doi: 10.1186/s13018-020-02075-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Song J, Hou Y, Zhang G, Ding L. Clinical research about the improved PVP method in treatment of acute osteoporotic vertebral compression fractures. J Orthop Surg (Hong Kong) 2019;27:2309499019864667. doi: 10.1177/2309499019864667. [DOI] [PubMed] [Google Scholar]
- 7.Xie L, Zhao ZG, Zhang SJ, Hu YB. Percutaneous vertebroplasty versus conservative treatment for osteoporotic vertebral compression fractures: an updated meta-analysis of prospective randomized controlled trials. Int J Surg. 2017;47:25–32. doi: 10.1016/j.ijsu.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Li J, Yang H, Luo Z, Zou J. Histological evaluation of bone biopsy results during PVP or PKP of vertebral compression fractures. Oncol Lett. 2013;5:135–138. doi: 10.3892/ol.2012.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, Wang Y, Luo T, Qi H, Cai L, Yuan Y, Li J. Dermatomal somatosensory evoked potentials and cortical somatosensory evoked potentials assessment in congenital scoliosis. BMC Neurol. 2022;22:58. doi: 10.1186/s12883-022-02579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lachance B, Wang Z, Badjatia N, Jia X. Somatosensory evoked potentials and neuroprognostication after cardiac arrest. Neurocrit Care. 2020;32:847–857. doi: 10.1007/s12028-019-00903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng Y, Cheng X, Wu H. Risk factors of new vertebral compression fracture after percutaneous vertebroplasty or percutaneous kyphoplasty. Front Endocrinol (Lausanne) 2022;13:964578. doi: 10.3389/fendo.2022.964578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trumm CG, Jakobs TF, Zech CJ, Weber C, Reiser MF, Hoffmann RT. Vertebroplasty in the treatment of back pain. Radiologe. 2006;46:495–505. doi: 10.1007/s00117-006-1382-7. [DOI] [PubMed] [Google Scholar]
- 13.Sezer C, Sezer C. Pedicle screw fixation with percutaneous vertebroplasty for traumatic thoracolumbar vertebral compression fracture. Niger J Clin Pract. 2021;24:1360–1365. doi: 10.4103/njcp.njcp_47_20. [DOI] [PubMed] [Google Scholar]
- 14.Chou KN, Lin BJ, Wu YC, Liu MY, Hueng DY. Progressive kyphosis after vertebroplasty in osteoporotic vertebral compression fracture. Spine (Phila Pa 1976) 2014;39:68–73. doi: 10.1097/BRS.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 15.Gu Y, Zhang F, Jiang X, Jia L, McGuire R. Minimally invasive pedicle screw fixation combined with percutaneous vertebroplasty in the surgical treatment of thoracolumbar osteoporosis fracture. J Neurosurg Spine. 2013;18:634–640. doi: 10.3171/2013.3.SPINE12827. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann TJ, Jensen ME, Schweickert PA, Marx WF, Kallmes DF. Age of fracture and clinical outcomes of percutaneous vertebroplasty. AJNR Am J Neuroradiol. 2001;22:1860–1863. [PMC free article] [PubMed] [Google Scholar]
- 17.Yokoyama K, Kawanishi M, Yamada M, Tanaka H, Ito Y, Hirano M, Kuroiwa T. Validity of intervertebral bone cement infusion for painful vertebral compression fractures based on the presence of vertebral mobility. AJNR Am J Neuroradiol. 2013;34:228–232. doi: 10.3174/ajnr.A3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang B, Li T, Wang Z. Efficacy and complications of different surgical modalities of treating osteoporotic spinal compression fracture in the elderly. Am J Transl Res. 2022;14:364–372. [PMC free article] [PubMed] [Google Scholar]
- 19.Kim BS, Hum B, Park JC, Choi IS. Retrospective review of procedural parameters and outcomes of percutaneous vertebroplasty in 673 patients. Interv Neuroradiol. 2014;20:564–575. doi: 10.15274/INR-2014-10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li R, Huang ZC, Cui HY, Huang ZP, Liu JH, Zhu QA, Hu Y. Utility of somatosensory and motor-evoked potentials in reflecting gross and fine motor functions after unilateral cervical spinal cord contusion injury. Neural Regen Res. 2021;16:1323–1330. doi: 10.4103/1673-5374.301486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain N, Alluri R, Phan K, Yanni D, Alvarez A, Guillen H, Mnatsakanyan L, Bederman SS. Saphenous nerve somatosensory-evoked potentials monitoring during lateral interbody fusion. Global Spine J. 2021;11:722–726. doi: 10.1177/2192568220922979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar A, Bhattacharya A, Makhija N. Evoked potential monitoring in anaesthesia and analgesia. Anaesthesia. 2000;55:225–241. doi: 10.1046/j.1365-2044.2000.01120.x. [DOI] [PubMed] [Google Scholar]
- 23.Pongsoipetch B. Pain reduction in patients with painful vertebral compression fractures undergoing percutaneous vertebroplasty. J Med Assoc Thai. 2007;90:479–484. [PubMed] [Google Scholar]