Abstract
Some osteoporosis drug trials have suggested that treatment is more effective in those with low BMD measured by DXA. This study used data from a large set of randomized controlled trials (RCTs) to determine whether the anti-fracture efficacy of treatments differs according to baseline BMD. We used individual patient data from 25 RCTs (103 086 subjects) of osteoporosis medications collected as part of the FNIH-ASBMR SABRE project. Participants were stratified into FN BMD T-score subgroups (≤−2.5, > −2.5). We used Cox proportional hazard regression to estimate treatment effect for clinical fracture outcomes and logistic regression for the radiographic vertebral fracture outcome. We also performed analyses based on BMD quintiles. Overall, 42% had a FN BMD T-score ≤ −2.5. Treatment with anti-osteoporosis drugs led to significant reductions in fractures in both T-score ≤ −2.5 and > −2.5 subgroups. Compared to those with FN BMD T-score > −2.5, the risk reduction for each fracture outcome was greater in those with T-score ≤ −2.5, but only the all-fracture outcome reached statistical significance (interaction P = .001). Results were similar when limited to bisphosphonate trials. In the quintile analysis, there was significant anti-fracture efficacy across all quintiles for vertebral fractures and with greater effects on fracture risk reduction for non-vertebral, all, and all clinical fractures in the lower BMD quintiles (all interaction P ≤ .03). In summary, anti-osteoporotic medications reduced the risk of fractures regardless of baseline BMD. Significant fracture risk reduction with treatment for 4 of the 5 fracture endpoints was seen in participants with T-scores above −2.5, though effects tended to be larger and more significant in those with baseline T-scores <−2.5.
Keywords: osteoporosis, BMD, T-score, treatment, SABRE
Introduction
A BMD value (T-score) 2.5 SDs or more below the mean for young adults is commonly used to define higher fracture risk and is incorporated into many osteoporosis guidelines. Low BMD is known to increase the likelihood of fractures including hip, vertebrae, forearm, and humerus. However, many fractures occur in patients having osteopenia (T-score between −1.0 and −2.5).1 Moreover, patients with normal BMD can also have major osteoporotic fractures.2
Many randomized trials have been conducted to assess the efficacy of antiosteoporotic medications on fracture risk. Medications included in these trials include antiresorptives (bisphosphonates [oral and parenteral], SERMs, denosumab, hormone replacement treatment [HRT]), anabolic (PTH and PTH-Related Protein Analogs [teriparatide and abaloparatide]), and more recently romosozumab [that can both increase bone formation and decrease bone resorption]. In general, treatment is most commonly recommended for people with high fracture risk assessed by risk assessment tools such as the Fracture Risk Assessment Tool (FRAX), QFracture, the Garvan Institute fracture risk calculator, or in patients with prior hip or spine fractures.3–5
Consequently, not all patients receiving antiosteoporotic medications have a T-score ≤ −2.5. Some studies have shown similar efficacy for those with baseline BMD above and below −2.5.6 However, several studies have suggested a larger effect of treatment on fractures in those with lower BMD. The FIT Clinical fracture arm (FIT II) recruited women free of vertebral fracture and showed that alendronate did not have an anti-fracture effect on non-vertebral fractures in women who do not have BMD T-score below −2.5.7 In the BONE Study (oral ibandronate), there was a significant non-vertebral fracture reduction only in those with baseline BMD T-score below −3.8 The limitations of previous studies include the issue of publication bias and the possibility of chance findings due to multiple comparisons. Moreover, several studies were performed before the introduction of the National Health and Nutrition Examination Survey (NHANES) III data as the reference standard for FN and TH T-scores.9 Given these inconsistent and incomplete findings, it is important to examine systematically whether antiosteoporosis treatments work better in those with osteoporosis based on BMD after standardizing both BMD and fracture definitions.
A recent study evaluated 69 published randomized controlled trials (RCTs) and concluded that treatments for osteoporosis were beneficial in decreasing the risk for all clinical fractures in postmenopausal women, and found that this effect was mostly independent of baseline risk indicators. The risk indicators included BMD at LS. The researchers suggested that future research should ideally be performed using individual patient data.10
This study aimed to use IPD from a large set of RCTs to determine whether anti-fracture efficacy of antiosteoporotic drugs varies by baseline BMD. These data were compiled as part of the FNIH/SABRE Project, which is using these data to apply for FDA qualification for TH BMD change as a surrogate endpoint for fracture in the RCTs.11 As a primary analysis, we stratified participants into BMD subgroups using baseline FN BMD T-score ≤ and > −2.5. We also performed a sensitivity analysis by FN BMD quintiles.
Materials and methods
Literature search and eligible studies
The studies were included as a result of a systematic review published previously.12,13
IPD and fracture definitions
When eligible studies were selected, an attempt was made to contact the sponsor and obtain the complete data files, IPD, and study documentation such as study protocol, data specifications, clinical study reports, and annotated forms. Studies where the sponsor was unable or unwilling to provide the data were not included. We were able to collect and include IPD for all of the key trials of approved antiosteoporotic medications in the US and for additional trials of medications for which approval was not sought or received.
A standard data template was created, and all the data were converted into this standard format. This included baseline demographics, standardized BMD conversions (see below), and uniform fracture definitions.
For trials where there was more than one dose of a medication, the active doses of the study medication were combined into one active arm. For trials where there was more than one active treatment arm or an arm with combinations of drugs, only the arm for the primary drug of interest was used for comparison against placebo. For the FRAME study, we included data from the first 12 mo only, when the trial was placebo-controlled. Finally, for trials where a study dose was discontinued before the end of the trial, the results for that dose were excluded from the analysis.
Standardized definitions for fracture outcomes were created and applied across all studies (radiographic vertebral, hip, and non-vertebral fractures). In addition, 2 composite fracture outcomes were created: “all clinical” and “all” fractures. “All clinical” included non-vertebral and clinical vertebral fractures, and “all” included non-vertebral, clinical vertebral, and radiographic vertebral fractures.
Fractures due to major trauma (ie, trauma sufficient to cause a fracture in a young, normal individual) were excluded. When trauma information was not available, the fractures were included. Trauma status was provided for the Fracture Prevention Trial (trial of teriparatide),14 yet all traumatic fractures were included since excluding these fractures would have eliminated 49% of the non-vertebral fractures in the trial (usually <10% of fractures were traumatic in the other trials). Fractures of the fingers, toes, face, skull, and cervical spine were excluded.
For radiographic vertebral fractures, the individual study definitions were used; these were based on comparisons of the baseline lateral spine radiographs with one or more of the follow-up radiographs. The definitions of an incident vertebral fracture differed across studies, as some used quantitative morphometry, semiquantitative assessment, or a combination of these criteria. Some studies evaluated radiographic vertebral fractures on more than one occasion; in these cases, the data from the final study evaluation were used.
BMD data
BMD was measured using various devices across studies (Hologic; GE Lunar; and Norland Corporation). Therefore, standard equations were used to convert BMD from Lunar and Norland to Hologic BMD for the TH, FN, and LS.15,16 This created Hologic-standardized BMD values comparable across DXA devices. When available, the LS vertebrae L1–4 were used, otherwise L2–4 were used. The non-Hispanic white female NHANES III database was used to calculate the FN BMD T-score,17 and Hologic reference values for young non-Hispanic white females were used to calculate the LS BMD T-score.
Statistical analysis
The primary aim of this analysis was to determine whether the anti-fracture efficacy of osteoporosis treatments differs by baseline FN BMD. For the primary analysis, participants were stratified into 2 subgroups, those with FN BMD T-score ≤ −2.5 and those with T-score > −2.5 as these are the thresholds used in clinical practice. We used FN BMD rather than TH BMD T-score since FN BMD is used in calculation tools like FRAX, and was proposed by the working party of the WHO for screening. Since FN region is a subregion of TH, the measurements are highly correlated. We reasoned that using the FN region to categorize at baseline would lessen the correlation with TH for the analyses of change in BMD. In our datasets, most participants who had TH also had FN but TH had more missing data since some of the older studies used densitometers that could not assess this region.
Baseline characteristics of the 2 subgroups were compared using t-tests for continuous characteristics and chi-square tests for categorical characteristics. For each BMD subgroup, we estimated the treatment effect on fracture reduction across all trials for the following fracture outcomes: radiographic vertebral, non-vertebral, hip, all clinical, and all fractures. We used Cox proportional hazard models to estimate the treatment effect in each FN BMD subgroup for time to first fracture for non-vertebral, hip, all clinical, and all fractures, with results reported as hazard ratios (HRs) and 95% CIs. We used logistic regression models for the incident radiographic vertebral fracture outcome, where exact time to event was unknown, to estimate the treatment effect in each FN BMD subgroup, with results reported as odds ratios (ORs) and 95% CIs. All analyses were by intention-to-treat.
We tested for interaction between treatment and BMD subgroup; the interaction models included indicators for trial, treatment, BMD subgroup, and the interaction between treatment and BMD subgroup. We first estimated the effect using data from all trials, then limited to bisphosphonate trials only because these are the most commonly used medications in clinical practice.
To estimate the effects of treatment on fracture risk reduction within each trial, we stratified participants into those with T-score ≤ −2.5 and > −2.5 and used the methods described above for the overall analysis. For some studies, the fracture reductions (OR or HR) differed from published results for various reasons such as the use of different fracture definitions, the degree of trauma excluded, or updates to the final dataset after the study was published.
We also checked for interaction between treatment and FN BMD subgroup within each trial. We present the results of the all-fracture outcome as a forest plot. To evaluate evidence for heterogeneity across studies, we used the overall pooled IPD in Cox and logistic models, including indicators for study, treatment, and BMD subgroup, and all 2-way and 3-way interactions between these factors. The test for 3-way interaction assesses heterogeneity in anti-fracture treatment efficacy for those with low vs high BMD across the studies. We have analyzed the results by stratifying by baseline vertebral fracture status (ie, BMD ≤ −2.5 with and without VFx and BMD > −2.5 with and without VFx). We focused on vertebral fractures for 2 reasons. First, inclusion criteria for many of the studies was based partially or fully on the presence of vertebral fracture. Second, we only have data on prior non-vertebral fracture in fewer than half the patients.
As a secondary aim, we tested the equality of the treatment effect on 24-mo change in TH, FN, and LS BMDs for each BMD subgroup across all trials. Trials without 24-mo BMD change data were not included. The active-placebo difference in mean percentage change in BMD at 24 mo was calculated and presented as mean (95% CI). The active-placebo difference in mean absolute change in BMD at 24 mo was also calculated. We first estimated the effect using the data from all studies, then limited to bisphosphonate trials only. Linear regression models were used to test the interaction between treatment assignment and BMD subgroup.
A further analysis was performed in which we divided FN BMD T-score into quintiles and assessed the effect on the same fractures as mentioned above. We also tested the treatment effect on 24-mo change in TH, FN, and LS BMDs for each BMD quintile across all trials and across bisphosphonate trials only as mentioned above. We tested for interaction between treatment and BMD quintiles; the interaction models included indicators for trial and treatment, BMD quintile as a linear term, and the interaction between treatment and linear BMD quintile.
We used SAS software (version 9.4, SAS Institute Inc.) for the analyses and RStudio (2022.07.1) for creating the forest plots.
Results
Analysis using 2 subgroups: BMD T-score ≤ −2.5 and > −2.5
Included studies
The studies included in the fracture analyses are shown in Table 1 and study inclusion criteria are provided in Table S1. The fracture analyses included 25 RCTs (13 of bisphosphonates [5 alendronate, 1 clodronate, 2 ibandronate of which 1 was intravenous, 3 risedronate and 2 zoledronate], 1 of odanacatib, 3 of anabolic medications [1 PTH (1-84), 1 abaloparatide, 1 teriparatide], 1 of denosumab, 1 of romosozumab, 2 of HRT, and 4 of SERMs).
Table 1.
Baseline FN BMD T-score status by trial in SABRE study.
| Trial | Drug class | Study drug | N (%) in FN BMD T-score > −2.5 | N (%) in FN BMD T-score ≤ −2.5 |
|---|---|---|---|---|
| ALN Phase 322 | Bisphosphonate | Alendronate | 608 (68.4%) |
281 (31.6%) |
| FIT I23 | Bisphosphonate | Alendronate | 1133 (55.9%) |
893 (44.1%) |
| FIT II7 | Bisphosphonate | Alendronate | 3239 (73.1%) |
1191 (26.9%) |
| FOSIT24 | Bisphosphonate | Alendronate | 1381 (76.0%) |
436 (24.0%) |
| MENs25 | Bisphosphonate | Alendronate | 174 (72.2%) |
67 (27.8%) |
| CLODRONATE26 | Bisphosphonate | Clodronate | 4008 (77.5%) |
1165 (22.5%) |
| BONE8 | Bisphosphonate | Ibandronate | 2040 (70.4%) |
856 (29.6%) |
| IBAN IV27 | Bisphosphonate | Ibandronate (intravenous) |
1966 (69.6%) |
860 (30.4%) |
| HIP28 | Bisphosphonate | Risedronate | 545 (31.7%) |
1173 (68.3%) |
| VERT-MN29 | Bisphosphonate | Risedronate | 409 (52.3%) |
373 (47.7%) |
| VERT-NA30 | Bisphosphonate | Risedronate | 971 (61.4%) |
611 (38.6%) |
| HORIZON PFT31 | Bisphosphonate | Zoledronate (intravenous) |
2643 (34.3%) |
5053 (65.7%) |
| HORIZON RFT32 | Bisphosphonate | Zoledronate (intravenous) |
976 (52.3%) |
891 (47.7%) |
| LOFT33 | Cathepsin K inhibitor | Odanacatib | 4719 (30.7%) |
10 635 (69.3%) |
| ACTIVE34 | Anabolic | Abaloparatide | 1168 (71.1%) |
474 (28.9%) |
| TOP35 | Anabolic | PTH (1-84) | 1637 (64.7%) |
894 (35.3%) |
| FPT14 | Anabolic | Teriparatide | 949 (61.2%) |
602 (38.8%) |
| FRAME36 | Anabolic | Romosozumab (subcutaneous) |
799 (11.1%) |
6380 (88.9%) |
| WHI-E37 | Hormone therapy | Estrogen | 865 (92.6%) |
69 (7.4%) |
| WHI-EP38 | Hormone therapy | Estrogen and progestin | 940 (91.8%) |
84 (8.2%) |
| FREEDOM39 | RANKL inhibitor | Denosumab (subcutaneous) |
5210 (67.1%) |
2559 (32.9%) |
| GENERATIONS40 | SERMs | Arzoxifene | 7555 (81.0%) |
1773 (19.0%) |
| BZA41 | SERMs | Bazedoxifene | 4582 (81.3%) |
1054 (18.7%) |
| PEARL42 | SERMs | Lasofoxifene | 5696 (66.7%) |
2845 (33.3%) |
| MORE43 | SERMs | Raloxifene | 5074 (66.3%) |
2580 (33.7%) |
Abbreviations: ACTIVE, Abaloparatide Comparator Trial in Vertebral Endpoints; ALN, alendronate; BONE, Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe; BZA, bazedoxifene; FIT, Fracture Intervention Trial; FOSIT, Fosamax International Trial; FPT, Fracture Prevention Trial; FRAME, Fracture Study in Postmenopausal Women with Osteoporosis; FREEDOM, fracture reduction evaluation of denosumab in osteoporosis every 6 mo; HIP, Hip Intervention Program Study Group; HORIZON PFT, Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly Pivotal Fracture Trial; HORIZON RFT, Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly Recurrent Fracture Trial; IBAN, ibandronate; LOFT, Long-term Odanacatib Fracture Trial; MORE, Multiple Outcomes of Raloxifene Evaluation; PEARL, Postmenopausal Evaluation and Risk-Reduction with Lasofoxifene Study; TOP, Treatment of Osteoporosis with Parathyroid hormone; VERT-MN, Vertebral Efficacy with Risedronate Therapy, Multinational Trial; VERT-NA, Vertebral Efficacy with Risedronate Therapy, North American Trial, WHI-E, Women’s Health Initiative, Estrogen Arm; WHI-EP, Women’s Health Initiative, Estrogen-Progestin Arm.
Baseline characteristics by FN BMD T-score subgroup are shown in Table 2. The analysis included a total of 103 086 subjects (99% female), with 42% having FN BMD T-score ≤ −2.5 (n = 43 799). On average, participants with low BMD were older and had lower BMI. The percentage of participants with a history of non-vertebral fracture was significantly higher in the osteoporosis subgroup.
Table 2.
Baseline characteristics (mean ± SD, or %) by baseline FN BMD T-score status.
| FN BMD T-score | |||
|---|---|---|---|
| >−2.5 (N = 59 287) |
≤ −2.5 (N = 43 799) |
P-value | |
| Age (yr) | 68.5 ± 7.2 | 71.6 ± 6.7 | <.0001 |
| Female (%) | 99.3 | 99.4 | .008 |
| BMI (kg/m2) | 26.6 ± 4.3 (n = 59 070) |
24.5 ± 4.2 (n = 43 653) |
<.0001 |
| Prevalent vertebral fracture (%) | 39.8 (n = 54 771) |
39.4 (n = 41 844) |
.18 |
| History of non-vertebral fracture (%) | 32.1 (n = 21 453) |
35.2 (n = 15 415) |
<.0001 |
| TH BMD T-score | −1.49 ± 0.78 (n = 54 392) |
−2.60 ± 0.60 (n = 40 106) |
<.0001 |
| FN BMD T-score | −1.80 ± 0.59 (n = 59 287) |
−2.91 ± 0.34 (n = 43 799) |
<0.0001 |
| LS BMD T-score | −2.38 ± 1.11 (n = 51 323) |
−2.96 ± 1.08 (n = 35 912) |
<0.0001 |
Abbreviation: n, number of participants with data.
Comparison of anti-fracture efficacy of osteoporosis treatment by baseline FN BMD T-score subgroup (FN BMD T-score ≤ −2.5 and > −2.5)
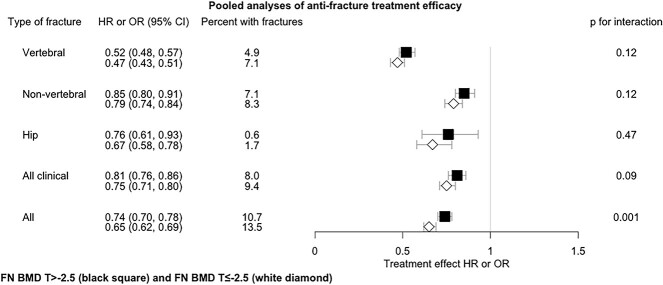
Figure 1 and Table S2 provide the HR or OR for fracture reduction in response to treatment in those with T-score above and below −2.5 across the combined set of 25 trials. As expected, incident fracture risk was higher in those with T-score ≤ −2.5 compared to > −2.5 participants. Treatment efficacy was greater in the T-score ≤ −2.5 subgroup than the > −2.5 subgroup for all 5 fracture types (eg, reduction of 53% vs 48% for vertebral, 25% vs 19% for all clinical), but only the all-fracture outcome reached conventional levels of statistical significance (reduction of 35% vs 26%, P-value for interaction = .001).
Figure 1.
Pooled analyses of anti-fracture treatment efficacy by baseline FN BMD T-score status across all studies. All results are adjusted for trial. 2-way interaction: Treatment * T-score status. Abbreviations: HR, hazard ratio; OR, odds ratio.
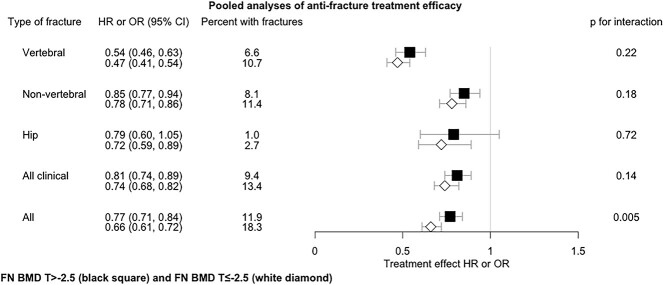
Figure 2 and Table S2 provide the anti-fracture treatment efficacy in those with and without T-score ≤ −2.5 across the combined set of 13 trials evaluating bisphosphonate treatments. Once again, the fracture risk reduction was greater in those with T-score ≤ −2.5 for all-fracture outcomes, but only the all-fracture outcome reached statistical significance (P-value for interaction = .005).
Figure 2.
Pooled analyses of anti-fracture treatment efficacy by baseline FN BMD T-score status across 13 bisphosphonate trials. All results are adjusted for trial. 2-way interaction: Treatment * T-score status. Abbreviations: HR, hazard ratio; OR, odds ratio.
Figure S1 displays the anti-fracture efficacy of osteoporosis treatment for the all-fracture outcome in the osteoporotic and non-osteoporotic subgroups for each trial, as well as the P-value for the 2-way interaction between treatment and FN BMD T-score status in each trial (Figure 1). In most trials, there was no significant interaction of treatment with T-score status. There were some exceptions: bazedoxifene showed higher efficacy in subjects with T < −2.5 vs. >−2.5 (HR 0.52 [95% CI, 0.34–0.81] vs HR 0.94 [95% CI, 0.75–1.20], interaction P = .02). In the FIT II trial, alendronate demonstrated a significant decrease in fracture risk in subjects with T < −2.5, whereas this was not shown for subjects with T > −2.5 (HR 0.61 [95% CI, 0.46–0.81] vs HR 1.02 [95% CI, 0.83–1.24]) (interaction P < .01). All other trials of alendronate and other bisphosphonates had similar effects in the 2 subgroups. The overall efficacy of bisphosphonates was greater in the subjects with T < −2.5 vs. >−2.5 (interaction P < .01). The 3-way interaction was not significant (P = .36), indicating that the 2-way interactions between treatment and the FN BMD T-score subgroup were similar across the trials.
For those with BMD T-score < −2.5, fracture reductions are generally similar in those with and without vertebral fractures. For those with BMD T-score > −2.5, reductions were larger for those with baseline vertebral fractures than for those without fractures; the reductions with treatment were significantly different in those with and without baseline vertebral fractures for the all-fractures and all-clinical fracture outcomes (Appendix Figure S2).
Comparison of treatment-related increases in BMD by baseline FN BMD T-score subgroup (FN BMD T-score ≤ −2.5 and > −2.5)
Across all trials, the T-score ≤ −2.5 subgroup had greater increases at all 3 BMD sites, whether expressed as a percentage or as an absolute change. For example, participants with T-score ≤ −2.5 receiving treatment increased their TH BMD by a mean of 4.17% compared to placebo vs 2.97% in the T-score > −2.5 subgroup (Table S3).
Across the combined set of 12 bisphosphonate trials with BMD data at 24 mo, the T-score ≤ −2.5 subgroup had greater treatment-related percentage increases in TH and FN BMD, but not LS BMD. When change was expressed as absolute change, only BMD change at the TH was significantly greater in the T-score ≤ −2.5 subgroup (Table S4).
Analysis by FN BMD T-score quintiles
Comparison of anti-fracture efficacy of osteoporosis treatment
The number of participants included in each FN BMD T-score quintile for each study is shown in Table S5, and the baseline characteristics by T-score quintiles are provided in Table S6. In general, there was a linear relationship between quintiles, age, and BMI: the lower the T-score, the lower the BMI, and the older the age.
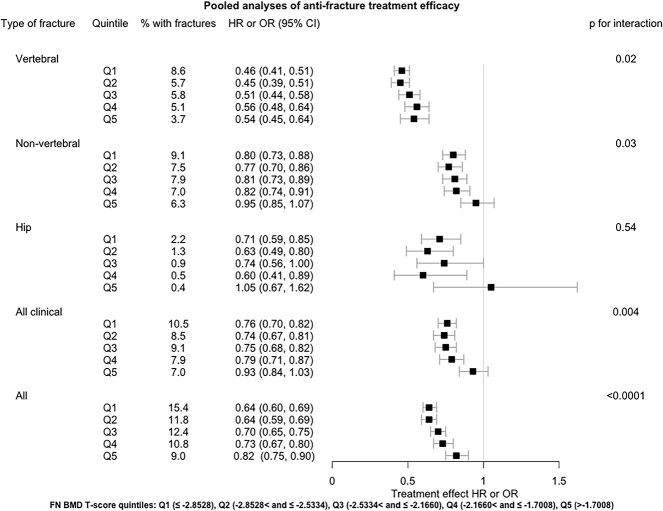
There was a treatment-related reduction in fracture risk within each quintile for each fracture type assessed, generally more pronounced in lower T-score quintile (Figure 3 and Table S7). There was no significant interaction between treatment and BMD quintile for the hip fracture outcome, but the number of hip fractures was small (eg, n = 82 at Q5). Interactions were significant for the other 4 fracture outcomes (P-value for interaction ≤.03). The CI for the highest T-score quintile crossed 1.0 for the non-vertebral and all clinical fractures outcomes, suggesting that antiosteoporotic medications might not be effective against these fracture types among those with higher BMD (Figure 3). The change by quintile was steeper for some fracture types for example non-vertebral and all clinical fractures than others for example vertebral fracture.
Figure 3.
Pooled analyses of anti-fracture treatment efficacy by baseline FN BMD T-score quintiles across 25 trials.
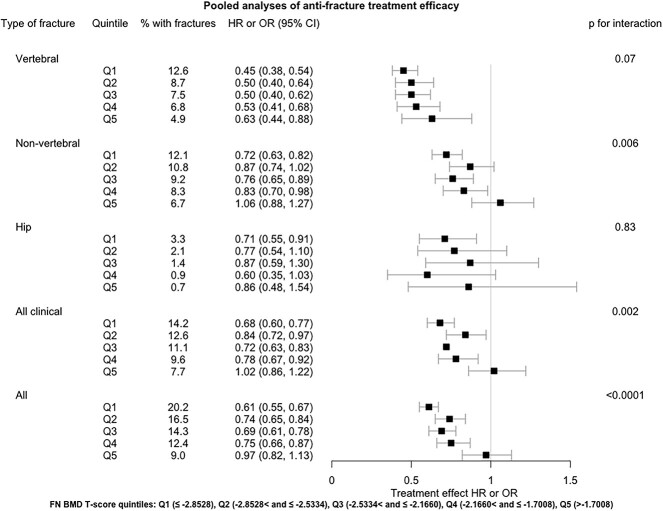
Bisphosphonates tended to reduce risk more in the lower 4 quintiles than in the fifth quintile (Figure 4 and Table S8).
Figure 4.
Pooled analyses of anti-fracture treatment efficacy in baseline FN BMD T-score quintiles across 13 bisphosphonate trials.
We also observed that treatment-related BMD increases were more pronounced in the lower baseline BMD quintiles among all trials and when limited to bisphosphonate trials (Table S9 and Table S10).
Discussion
Our study used individual patient data from 25 randomized, placebo-controlled trials of anti-osteoporosis therapies to evaluate whether the effect of treatment on fracture risk differed based on the baseline FN BMD T-score. We assessed the effect on vertebral, non-vertebral, hip, all clinical, and all fractures (combination of non-vertebral and clinical or radiographic vertebral fractures).
In our primary analysis, we stratified the groups into the ones with T-score ≤ −2.5 and > −2.5 as this is the clinically-used threshold; this was based on FN BMD, as most studies had data on this site; moreover, this is the T-score used in FRAX. Antiosteoporotic medications effectively reduced fractures in both groups. Still, there was a consistent trend toward greater efficacy in the lower BMD group, although it only reached statistical significance for the all fracture outcomes (P = .001), which could be due to the larger number of fractures. The results were similar when studying bisphosphonate-only trials. In a sensitivity analysis, we divided FN BMD T-score into quintiles. When studying all medications together, treatment significantly reduced fracture risk at all fracture outcomes assessed, apart from the hip, but the number of hip fractures was small.
In an additional analysis, we checked whether the presence of vertebral fractures at baseline affects the results. We found that for those with BMD T-score ≤ −2.5, the fracture reductions as similar, independent of the presence of fractures. However, in BMD T-score > −2.5, the reductions in fractures were greater when there was a history of vertebral fractures; this difference was significant for all clinical and all fractures.
What is the likely cause for the importance of baseline BMD as a predictor of fracture benefit? One possible explanation for this the relationship between lower baseline BMD and larger fracture reduction is that there is a larger increase in BMD with treatment in those with lower baseline BMD. We explored this possibility by considering the relationship between baseline BMD and changes with treatment. We were aware of 2 issues to avoid in this analysis, namely the use of the same BMD region in the prediction and the outcome of this analysis (due to common variable effect), and the calculation of percent change in BMD being related to baseline BMD. Thus, we used FN BMD as the predictor and change in TH BMD as the outcome; we also calculated the absolute change in TH BMD as well as the percentage change in TH BMD. The greater TH BMD increase in patients with low baseline FN BMD is a possible cause of the greater fracture risk reduction in this group. We have proposed a 2-yr change in TH BMD as a surrogate for fracture risk reduction in future clinical trials of antiosteoporosis medications.11
To our knowledge, this is the first study that systematically combined multiple studies with multiple drugs and addressed whether anti-fracture efficacy varies across BMD subgroups using IPD. This is a significant clinical issue since we use treatment to prevent fractures in osteoporotic and non-osteoporotic patients as defined by the T-score. This is also an important finding for policymakers. A recent study evaluated all clinical, vertebral, non-vertebral, and hip fractures and concluded treatments for osteoporosis were beneficial in decreasing the risk in postmenopausal women. They evaluated a series of baseline risk indicators and found that this effect mainly was independent of these factors.10 Their analysis only evaluated LS BMD using grouped data only, whereas we included FN BMD and IPD. Thus, we have found that baseline BMD does relate to fracture outcomes, whereas Handel et al.10 found that baseline LS BMD did not relate to such outcomes. Τhere are a number of factors that may explain these differences; our study included individual patient data and we have a separate fracture outcome as all fractures, where we found some significant differences.
Antiosteoporotic medications did not significantly reduce non-vertebral or clinical fracture risk in the highest BMD quintile (T-scores above −1.7) either for all studies or when limiting to bisphosphonates. Prior to this study, there was a concern that treatments do not affect non-vertebral fractures in patients with T-score above −2.5. This was shown in the FIT II trial, where alendronate did not reduce the risk of non-vertebral and all fractures in the group with FN BMD T-score > −2.5.7 These trials are mostly antiresorptive medications and these work by lowering bone turnover and a high bone turnover increases the risk for vertebral fractures by increasing the number of stress risers and this is why antiresorptives are particularly effective for this fracture type.18 In general, the magnitude of the risk reduction is much larger for vertebral fractures than for non-vertebral fractures. This is also true for non-antiresorptive medications. Also, many of the studies included criteria related to existing vertebral fractures thus increasing the risk for additional vertebral fractures during follow-up. For both of these reasons, the precision error of the estimates of vertebral fractures reduction is much smaller which could be an important contributor to the additional consistency in vertebral fracture reductions.
Our analysis suggests that the BMD T-score threshold for treatment could be higher than −2.5 that is −1.7. However, many clinical guidelines for starting antiosteoporosis medications incorporate other fracture risk factors or fracture risk estimates (eg, FRAX) together with BMD. An example is the Bone Health and Osteoporosis Foundation19 recommendations that BMD T-score < −2.5 is sufficient alone for treatment but in those with BMD T-score between −2.5 and −1.0, a 3% 10-yr FRAX risk for hip fractures or 20% for major osteoporotic fractures is also required for recommending pharmacologic treatment. The attraction of using estimates like FRAX is that it estimates absolute fracture risk.
There are several strengths of our study. It is a large, comprehensive study which used IPD from all major osteoporosis trials to create a large database including a very large number of participants. Moreover, a variety of medications were evaluated. We used harmonized fracture definitions across the trials and standardized DXA BMD values across manufacturers.
A few things should be considered as limitations when interpreting our results. Some medications only have one placebo-controlled trial for example denosumab, odanacatib, teriparatide; results can be due to chance finding. There is overlap between the bisphosphonate-only group and the all-studies group. Most of the studies included only women so results might be different in men. Moreover, we have not analyzed other potential subgrouping variables. Importantly, we did not consider baseline fracture risk scores such as FRAX which is often an important part of the clinical decision making.3 We acknowledge that the FN is a small region of the TH, so the measurements are not independent of change in TH BMD. However, the principal contribution to TH BMD is from the intertrochanteric region of interest, not the FN.20 The lack of independence could mean that the greater increase in TH BMD in patients with low FN. BMD is a consequence in part to regression to the mean.
There were other significant differences between trials in our analytic groups, such as age, BMI, and prior fractures which could have affected the outcome, but we did not explore those interactions. Lastly, the study was underpowered for evaluating interaction.21
In summary, all antiosteoporotic medications studied reduced the risk of fractures in those with FN BMD T-scores above and below −2.5. Still, somewhat greater reductions were observed for all fractures in women having a BMD T-score ≤ −2.5 and in those with a history of vertebral fractures if BMD is above −2.5. These are important findings with potential impact on patient treatment.
Author contributions
Marian Schini (Conceptualization, Investigation, Supervision, Visualization, Writing—original draft, Writing—review & editing), Tatiane Vilaca (Conceptualization, Investigation, Visualization, Writing—original draft, Writing—review & editing), Li-Yung Lui (Data curation, Formal analysis, Methodology, Project administration, Software, Validation, Visualization, Writing—original draft, Writing—review & editing), Susan K. Ewing (Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Writing—original draft, Writing—review & editing), Austin Thompson (Project administration, Writing—review & editing), Eric Vittinghoff (Formal analysis, Methodology, Project administration, Software, Writing—original draft, Writing—review & editing), Douglas C. Bauer (Conceptualization, Project administration, Visualization, Writing—original draft, Writing—review & editing), Mary L. Bouxsein (Conceptualization, Funding acquisition, Project administration, Supervision, Visualization, Writing—original draft, Writing—review & editing), Dennis M. Black (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing), and Richard Eastell (Conceptualization, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing)
Funding
FDA grant #1U01FD007772-01.Grant from the American Society for Bone Mineral Research.
Conflicts of interest
M.S. received consultancy from Kyowa Kirin International.
T.V. received consultancy funding from Pharmacosmos.
E.V. None.
L.L. None.
S.E. None.
A.T. None.
D.B. None.
M.B. Advisory Board/Consulting: Angitia, Beryl Therapeutics. Lecture honoraria: Alexion.
D.B. Denosumab membership for Eli Lilly.
R.E. receives consultancy funding from Immunodiagnostic Systems, Sandoz, Samsung, CL Bio, CureTeQ, Biocon, Takeda, UCB, meeting presentations for Pharmacosmos, Alexion, UCB and Amgen, and grant funding from Alexion and Osteolabs.
Data availability
All study data were acquired by requesting IPD from study sponsors. An overarching data use agreement was created between all parties and individual data use agreements were created between individual study sponsors, FNIH, and University of California, San Francisco (UCSF). Per the data sharing agreements that we have with each sponsor, the data can be used for surrogate marker analyses, including any surrogate qualification processes with regulatory authorities. However, other uses of the data are restricted by this agreement, and UCSF is not allowed to share the data.
Supplementary Material
Contributor Information
Marian Schini, Division of Clinical Medicine, School of Medicine and Population Health, University of Sheffield, Sheffield, S10 2TN, United Kingdom.
Tatiane Vilaca, Division of Clinical Medicine, School of Medicine and Population Health, University of Sheffield, Sheffield, S10 2TN, United Kingdom.
Li-Yung Lui, California Pacific Medical Center, Research Institute, San Francisco, 94158, CA, United States.
Susan K Ewing, Department of Epidemiology & Biostatistics, University of California, San Francisco, 94158, CA, United States.
Austin Thompson, Department of Epidemiology & Biostatistics, University of California, San Francisco, 94158, CA, United States.
Eric Vittinghoff, Department of Epidemiology & Biostatistics, University of California, San Francisco, 94158, CA, United States.
Douglas C Bauer, Department of Epidemiology & Biostatistics, University of California, San Francisco, 94158, CA, United States; Department of Medicine, University of California, San Francisco, 94158, CA, United States.
Mary L Bouxsein, Department of Orthopedic Surgery, Harvard Medical School, Center for Advanced Orthopedic Studies, Beth Israel Deaconess Medical Center, Boston, 02215, MA, United States.
Dennis M Black, Department of Epidemiology & Biostatistics, University of California, San Francisco, 94158, CA, United States.
Richard Eastell, Division of Clinical Medicine, School of Medicine and Population Health, University of Sheffield, Sheffield, S10 2TN, United Kingdom.
References
- 1. Kanis JA, Melton LJ, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis (in Eng). J Bone Miner Res. 1994;9(8):1137–1141. 10.1002/jbmr.5650090802 [DOI] [PubMed] [Google Scholar]
- 2. Kadri A, Binkley N, Daffner SD, Anderson PA. Fracture in patients with normal bone mineral density: an evaluation of the American Orthopaedic Association's Own the Bone registry (in Eng). J Bone Joint Surg Am. 2023;105(2):128–136. 10.2106/JBJS.22.00012 [DOI] [PubMed] [Google Scholar]
- 3. Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological Management of Osteoporosis in postmenopausal women: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2019;104(5):1595–1622. 10.1210/jc.2019-00221 [DOI] [PubMed] [Google Scholar]
- 4. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2020 update. Endocr Pract. 2020;26(Suppl 1):1–46. 10.4158/gl-2020-0524suppl [DOI] [PubMed] [Google Scholar]
- 5. Kanis JA, Johansson H, Harvey NC, et al. An assessment of intervention thresholds for very high fracture risk applied to the NOGG guidelines. Osteoporos Int. 2021;32(10):1951–1960. 10.1007/s00198-021-05942-2 [DOI] [PubMed] [Google Scholar]
- 6. Eastell R, Black DM, Boonen S, et al. Effect of once-yearly zoledronic acid five milligrams on fracture risk and change in femoral neck bone mineral density (in eng). J Clin Endocrinol Metab. 2009;94(9):3215–3225. 10.1210/jc.2008-2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low Bone density but without vertebral fractures results from the fracture intervention trial. JAMA. 1998;280(24):2077–2082. 10.1001/jama.280.24.2077 [DOI] [PubMed] [Google Scholar]
- 8. Chesnut CH, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19(8):1241–1249. 10.1359/jbmr.040325 [DOI] [PubMed] [Google Scholar]
- 9. Watts NB, Leslie WD, Foldes AJ, Miller PD. 2013 International Society for Clinical Densitometry Position Development Conference: task force on normative databases (in eng). J Clin Densitom. 2013;16(4):472–481. 10.1016/j.jocd.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 10. Händel MN, Cardoso I, Von Bülow C, et al. Fracture risk reduction and safety by osteoporosis treatment compared with placebo or active comparator in postmenopausal women: systematic review, network meta-analysis, and meta-regression analysis of randomised clinical trials. BMJ. 2023;381:e068033. 10.1136/bmj-2021-068033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Black DM, Bauer DC, Vittinghoff E, et al. Treatment-related changes in bone mineral density as a surrogate biomarker for fracture risk reduction: meta-regression analyses of individual patient data from multiple randomised controlled trials (in eng). Lancet Diabetes Endocrinol. 2020;8(8):672–682. 10.1016/S2213-8587(20)30159-5 [DOI] [PubMed] [Google Scholar]
- 12. Bouxsein ML, Eastell R, Lui L-Y, et al. Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res. 2019;34(4):632–642. 10.1002/jbmr.3641 [DOI] [PubMed] [Google Scholar]
- 13. Bauer DC, Black DM, Bouxsein ML, et al. Treatment-related changes in bone turnover and fracture risk reduction in clinical trials of anti-resorptive drugs: a meta-regression. J Bone Miner Res. 2018;33(4):634–642. 10.1002/jbmr.3355 [DOI] [PubMed] [Google Scholar]
- 14. Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–1441. 10.1056/nejm200105103441904 [DOI] [PubMed] [Google Scholar]
- 15. Genant HK, Grampp S, Glüer CC, et al. Universal standardization for dual x-ray absorptiometry: patient and phantom cross-calibration results (in eng). J Bone Miner Res. 1994;9(10):1503–1514. 10.1002/jbmr.5650091002 [DOI] [PubMed] [Google Scholar]
- 16. Hanson J. Standardization of femur BMD. J Bone Miner Res. 1997;12(8):1316–1317. 10.1359/jbmr.1997.12.8.1316 [DOI] [PubMed] [Google Scholar]
- 17. Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur Bone mineral levels of US adults. Osteoporos Int. 1998;8(5):468–490. 10.1007/s001980050093 [DOI] [PubMed] [Google Scholar]
- 18. Hernandez CJ. How can bone turnover modify bone strength independent of bone mass? Bone. 2008;42(6):1014–1020. 10.1016/j.bone.2008.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leboff MS, Greenspan SL, Insogna KL, et al. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2022;33(10):2049–2102. 10.1007/s00198-021-05900-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blake G, Wahner H, Fogelman I. The Evaluation of Osteoporosis. 2nd ed. CRC Press; 1999. [Google Scholar]
- 21. Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine — reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357(21):2189–2194. 10.1056/nejmsr077003 [DOI] [PubMed] [Google Scholar]
- 22. Liberman UA, Weiss SR, Bröll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med. 1995;333(22):1437–1444. 10.1056/nejm199511303332201 [DOI] [PubMed] [Google Scholar]
- 23. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture intervention trial research group (in eng). Lancet. 1996;348(9041):1535–1541. 10.1016/s0140-6736(96)07088-2 [DOI] [PubMed] [Google Scholar]
- 24. Pols HAP, Felsenberg D, Hanley DA, et al. Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Osteoporos Int. 1999;9(5):461–468. 10.1007/pl00004171 [DOI] [PubMed] [Google Scholar]
- 25. Orwoll E, Ettinger M, Weiss S, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343(9):604–610. 10.1056/nejm200008313430902 [DOI] [PubMed] [Google Scholar]
- 26. Mccloskey E, Selby P, Davies M, et al. Clodronate reduces vertebral fracture risk in women with postmenopausal or secondary osteoporosis: results of a double-blind, placebo-controlled 3-year study. J Bone Miner Res. 2004;19(5):728–736. 10.1359/jbmr.040116 [DOI] [PubMed] [Google Scholar]
- 27. Recker R, Stakkestad JA, Chesnut CH, et al. Insufficiently dosed intravenous ibandronate injections are associated with suboptimal antifracture efficacy in postmenopausal osteoporosis (in Eng). Bone. 2004;34(5):890–899. 10.1016/j.bone.2004.01.008 [DOI] [PubMed] [Google Scholar]
- 28. Mcclung MR, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med. 2001;344(5):333–340. 10.1056/nejm200102013440503 [DOI] [PubMed] [Google Scholar]
- 29. Reginster J-Y, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int. 2000;11(1):83–91. 10.1007/s001980050010 [DOI] [PubMed] [Google Scholar]
- 30. Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA. 1999;282(14):1344–1352. 10.1001/jama.282.14.1344 [DOI] [PubMed] [Google Scholar]
- 31. Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis (in Eng). N Engl J Med. 2007;356(18):1809–1822. 10.1056/NEJMoa067312 [DOI] [PubMed] [Google Scholar]
- 32. Lyles KW, Colón-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture (in Eng). N Engl J Med. 2007;357(18):1799–1809. 10.1056/NEJMoa074941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McClung MR, O'Donoghue ML, Papapoulos SE, et al. Odanacatib for the treatment of postmenopausal osteoporosis: results of the LOFT multicentre, randomised, double-blind, placebo-controlled trial and LOFT Extension study (in Eng). Lancet Diabetes Endocrinol. 2019;7(12):899–911. 10.1016/S2213-8587(19)30346-8 [DOI] [PubMed] [Google Scholar]
- 34. Miller PD, Hattersley G, Riis BJ, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis. JAMA. 2016;316(7):722–733. 10.1001/jama.2016.11136 [DOI] [PubMed] [Google Scholar]
- 35. Greenspan SL, Bone HG, Ettinger MP, et al. Effect of recombinant human parathyroid hormone (1-84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med. 2007;146(5):326–339. 10.7326/0003-4819-146-5-200703060-00005 [DOI] [PubMed] [Google Scholar]
- 36. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532–1543. 10.1056/NEJMoa1607948 [DOI] [PubMed] [Google Scholar]
- 37. Jackson RD, Wactawski-Wende J, Lacroix AZ, et al. Effects of conjugated equine estrogen on risk of fractures and BMD in postmenopausal women with hysterectomy: results from the Women's Health Initiative Randomized Trial. J Bone Miner Res. 2006;21(6):817–828. 10.1359/jbmr.060312 [DOI] [PubMed] [Google Scholar]
- 38. Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and Bone mineral density : the Women's Health Initiative randomized trial. JAMA. 2003;290(13):1729–1738. 10.1001/jama.290.13.1729 [DOI] [PubMed] [Google Scholar]
- 39. Cummings SR, Martin JS, Mcclung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–765. 10.1056/nejmoa0809493 [DOI] [PubMed] [Google Scholar]
- 40. Cummings SR, Mcclung M, Reginster J-Y, et al. Arzoxifene for prevention of fractures and invasive breast cancer in postmenopausal women. J Bone Miner Res. 2011;26(2):397–404. 10.1002/jbmr.191 [DOI] [PubMed] [Google Scholar]
- 41. Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res. 2008;23(12):1923–1934. 10.1359/jbmr.080710 [DOI] [PubMed] [Google Scholar]
- 42. Cummings SR, Ensrud K, Delmas PD, et al. Lasofoxifene in postmenopausal women with osteoporosis. N Engl J Med. 2010;362(8):686–696. 10.1056/nejmoa0808692 [DOI] [PubMed] [Google Scholar]
- 43. Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators (in eng). JAMA. 1999;282(7):637–645. 10.1001/jama.282.7.637 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data were acquired by requesting IPD from study sponsors. An overarching data use agreement was created between all parties and individual data use agreements were created between individual study sponsors, FNIH, and University of California, San Francisco (UCSF). Per the data sharing agreements that we have with each sponsor, the data can be used for surrogate marker analyses, including any surrogate qualification processes with regulatory authorities. However, other uses of the data are restricted by this agreement, and UCSF is not allowed to share the data.