Abstract
Background:
Glioblastoma (GBM) is the most common primary brain tumor in adults. Despite extensive research and clinical trials, median survival post-treatment remains at 15 months. Thus, all opportunities to optimize current treatments and improve patient outcomes should be considered. A recent retrospective clinical study found that taking TMZ in the morning compared to the evening was associated with a 6-month increase in median survival in patients with MGMT-methylated GBM. Here, we hypothesized that TMZ efficacy depends on time-of-day and O6-Methylguanine-DNA Methyltransferase (MGMT) activity in murine and human models of GBM.
Methods and Results:
In vitro recordings using real-time bioluminescence reporters revealed that GBM cells have intrinsic circadian rhythms in the expression of the core circadian clock genes Bmal1 and Per2, as well as in the DNA repair enzyme, MGMT. Independent measures of MGMT transcript levels and promoter methylation also showed daily rhythms intrinsic to GBM cells. These cells were more susceptible to TMZ when delivered at the daily peak of Bmal1 transcription. We found that in vivo morning administration of TMZ also decreased tumor size and increased body weight compared to evening drug delivery in mice bearing GBM xenografts. Finally, inhibition of MGMT activity with O6-Benzylguanine abrogated the daily rhythm in sensitivity to TMZ in vitro by increasing sensitivity at both the peak and trough of Bmal1 expression.
Conclusion:
We conclude that chemotherapy with TMZ can be dramatically enhanced by delivering at the daily maximum of tumor Bmal1 expression and minimum of MGMT activity and that scoring MGMT methylation status requires controlling for time of day of biopsy.
Keywords: GBM, circadian rhythms, TMZ, DNA repair, circadian medicine, chronotherapy
Graphical Abstract
Introduction
Gliomas are the most common brain malignancies, consisting largely of cells that resemble astrocytes, oligodendrocytes, oligodendrocyte precursor cells, and earlier neural stem cells [1], [2]. Glioblastoma (GBM) is the most common and aggressive glioma in adults, accounting for 54% of all gliomas, and 16% of all primary brain tumors [3]. In the United States, 12,000 adults are diagnosed annually at a median age of 64 years [4]. The current standard of care for GBM consists of maximal safe surgical resection, followed by radiation and chemotherapy with the DNA alkylator, Temozolomide (Temodar®, TMZ), and tumor-treating fields [5], [6]. When introduced into the standard of care for GBM approximately 20 years ago, TMZ extended median survival by 2.5 months, which was heralded as a dramatic improvement in treatment [7], [8]. In clinical applications, however, progression-free survival at 6 months in patients receiving TMZ was only 46% and even lower for recurrent GBM (17%) [9]. Despite extensive research and efforts to improve outcomes, median survival time remains approximately 15 months, and 5-year survival is less than 5%, after diagnosis [3]. Thus, the importance of further research to optimize current, and develop new, treatments against GBM remains highly significant and all avenues to lengthen survival should be pursued.
Among first line chemotherapy drugs used to treat GBM patients, TMZ has many advantages including oral administration, easy penetration through the blood-brain barrier, and no known toxic interactions with other drugs used in the clinic [10]. TMZ is a DNA alkylating agent that triggers the death of GBM cells by attaching a methyl group to purine bases of DNA (O6-guanine; N7-guanine and N3-adenine) [10]. The most common TMZ-induced cytotoxic lesion is O6-methylguanine (O6-MeG), which can be removed by the DNA repair enzyme O6-Methylguanine-DNA Methyltransferase (MGMT) in tumors that express this protein [10]. Expression of this DNA repair enzyme confers resistance to chemotherapy and represents a challenge in treating patients with unmethylated MGMT promoter sequences [10], [11]. In contrast, patients with MGMT-promoter methylated tumors (i.e., silenced MGMT) respond better to chemotherapy and have a better prognosis. In a retrospective study of morning versus evening TMZ, efficacy was greater in patients with MGMT-methylated tumors, who exhibited a longer median survival of 6 months when receiving morning TMZ compared to evening [12]. Thus, MGMT expression and activity confers resistance to TMZ in patients and, yet may vary with time of day in GBM.
Circadian rhythms in gene expression and physiology are ubiquitous across cell types and species [13], [14]. Unlike some other cancers where circadian rhythms tend to be disrupted, well-studied GBM models have reliable circadian rhythms in gene expression [15]–[17]. Furthermore, expression of the MGMT protein has been shown to oscillate daily in healthy mouse liver cells, peaking during the subjective night (Circadian Time, CT19) [18]. It is unknown whether MGMT expression varies with time of day in GBM cells. Previous research has found that primary isolates from GBM patients and immortalized human and murine GBM cell lines, have high-amplitude daily rhythms in Per2 and Bmal1 clock gene expression, and in sensitivity to chemotherapy in vitro [15]. Thus, GBM biology and response to chemotherapeutic agents can vary with time of day. However, we do not know the mechanisms driving daily rhythms in TMZ efficacy or whether these daily rhythms in sensitivity are conserved across the diversity of gliomas. Several additional factors that could influence TMZ efficacy in the clinic also vary with time of day. For example, absorption and excretion of drugs in the blood varies by time of day, as does permeability of the blood-brain barrier [19]–[21]. Activation of checkpoint kinases that trigger apoptosis after induced DNA damage has also been shown to change based on time of day via an interaction with the clock genes Per1 and Per3 [22]–[24]. It is unknown if drug resistance in GBM can be ameliorated through strategically timed drug delivery to maximize tumor death while minimizing side effects.
Here, we aimed to maximize tumor cell death by targeting circadian molecules in GBM models. We found that human and murine GBM cell lines exhibit daily rhythms in TMZ sensitivity with the highest efficacy observed at the peak of Bmal1 and trough of MGMT in vitro, and morning administration in vivo. This correlates with daily rhythms in MGMT methylation that peak before its transcription. Inhibiting MGMT activity in vitro enhances TMZ sensitivity as a function of circadian time. We conclude that TMZ efficacy and GBM outcomes can be improved by targeting daily rhythms in MGMT expression and activity.
Results
GBM sensitivity to TMZ increases around the daily peak of Bmal1 expression
To test whether models of GBM differ in their sensitivity to TMZ over the day, we transduced human LN229 and murine GL261 cells with luciferase reporters of Bmal1 or Per2 transcriptional activity (B1L or P2L, respectively). In human LN229 cells, we found intrinsic daily rhythms in Bmal1 and Per2 expression over 72 h in culture, with Bmal1 and Per2 peaking in antiphase at CT4 or CT16, respectively (Fig. 1a, all recordings had cosine fits with correlation coefficients, CC>0.9). We next treated cultures with TMZ at one of eight circadian phases of Bmal1 expression and found 100μM TMZ was more toxic to LN229 cells at the daily peak of Bmal1 (Fig. 1b, *p=0.03; 72 h after a single-dose TMZ treatment, average trace scored circadian by JTK cycle p < 0.05). Specifically, we found that 21% of LN229 cells survived TMZ treatment around peak Bmal1, while 69% survived when treated at the trough. This time-of-day sensitivity reproduced in three different LN229 and GL261 biological replicates (Fig. 3). TMZ dose-dependently killed LN229 cells, dependent also on circadian time of administration (Supplementary Fig. 1, IC50 at CT4=195μM vs. IC50 at CT12= 321μM). The daily rhythm in TMZ sensitivity was not detectable at 10, 200 or 1000μM TMZ (Fig. 1b, p>0.05, average traces not scored circadian by JTK cycle p > 0.05). Together, these results demonstrate that an intermediate dose of TMZ aligned to the peak of Bmal1 in GBM cells results in greater cell death.
Fig. 1.
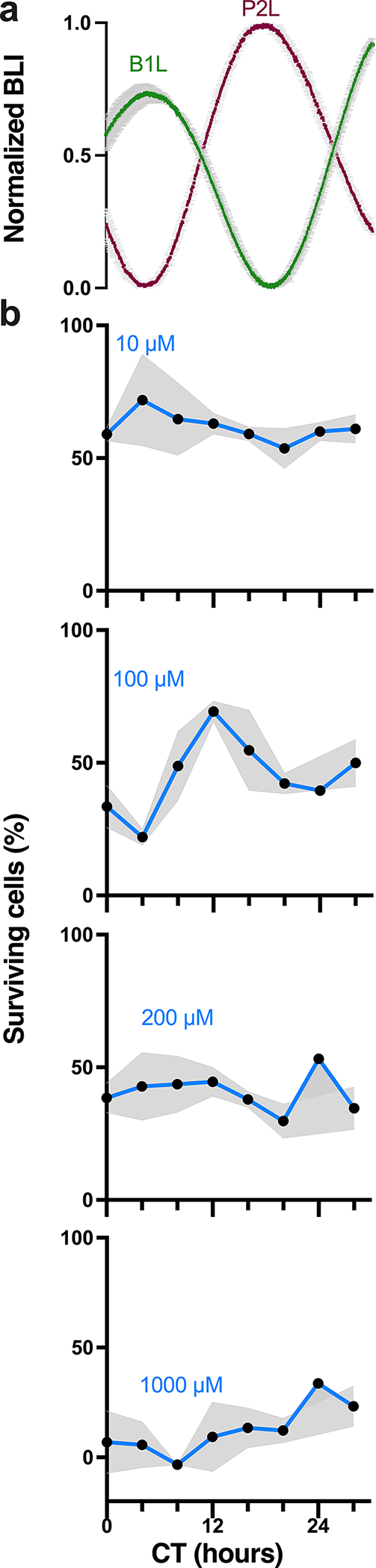
TMZ maximally inhibits growth at the daily peak of Bmal1 expression in GBM cells in vitro. a) Circadian rhythms in clock gene expression recorded as bioluminescence from human LN229 GBM cells transduced with a Per2- or Bmal1-driven luciferase reporter (P2L or B1L, n=2 cultures each, mean ± SEM). b) Survival of LN229-B1L cells varied with the circadian time (CT) and dose of TMZ treatment in vitro. For example, more cells survived 100μM TMZ when administered around the trough of Bmal1 (CT12) than around the peak (CT4). Higher doses of TMZ yielded more cell death and less circadian dependence (n=3 cultures per dose at each time, mean ± SEM, 100μM JTK cycle p = 0.001, ns for 10, 200, 1000μM)
Fig. 3.
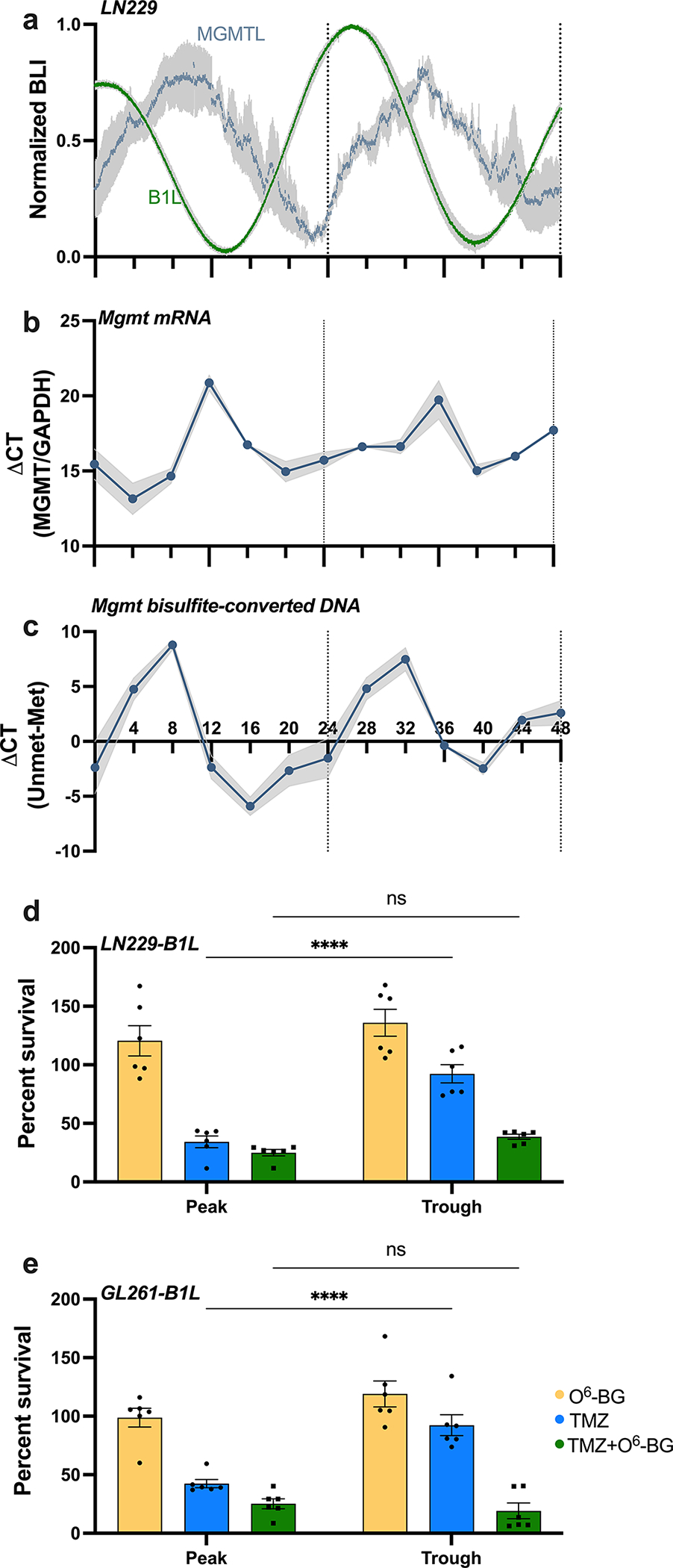
TMZ sensitivity depends on MGMT activity and correlates with daily rhythms in MGMT mRNA abundance and promoter methylation in vitro. a) Circadian rhythms in MGMT expression peak approximately 10 h after peak Bmal1 expression in human LN229 GBM cells. Bioluminescence of cells transduced with either MGMT-luciferase (MGMTL) or Bmal1-luciferase (B1L) was recorded for 48 h in vitro (n=5 cultures each, mean ± SEM). b) LN229 cells collected every 4 h over 48 h show daily rhythms in MGMT mRNA (n=6 cultures each, mean ± SEM, JTK cycle p = 0.05) and c) MGMT promoter methylation (n=3 cultures each, mean ± SEM, JTK cycle p = 0.002). d) LN229-B1L and e) GL261-B1L cells show decreased cell number when treated with 100μM TMZ at either the peak or trough of Bmal1, with highest sensitivity at the peak. This time-of-day-dependent sensitivity is abrogated when co-treating with the MGMT inhibitor O6-BG (n=6 per group; mean±SEM; ****p < 0.0001, ns indicates p > 0.05)
To evaluate in vivo TMZ sensitivity as a function of time of day, we implanted immunocompromised nude mice with human LN229 cells, and C57 mice with murine GL261 cells (Fig. 2a). These orthotopic GBM models expressed either circadian (Per2-luciferase) or constitutive (Ef1α-luciferase) reporters to monitor tumor growth as bioluminescence after implantation. We delivered 100mg/kg TMZ or vehicle (10% HPMC) by oral gavage in the morning (11:00 a.m., 4 h after daily light onset) or evening (6:00 p.m., 1 h before daily light offset) for five days starting 11–13 days after implantation. We found no significant difference in tumor growth between mice treated with vehicle at each time point tested, and thus data were combined. Further, we found no significant difference in bioluminescence counts between tumors expressing Per2:luc or Ef1α:luc (Fig. 2b–d), suggesting that both reporters can be used to track tumor growth in vivo. We found that tumor bioluminescence declined faster in mice treated with TMZ in the morning, compared to evening and vehicle treatment regardless of the reporter (Figs. 2b and c, ****p < 0.0001 in LN229-Ef1α:luc, *p = 0.05 in LN229-P2L) or cell line (Fig. 2d, *p = 0.03). In parallel, we found that body weight increased for mice treated with TMZ in the morning but decreased in mice treated with TMZ in the evening or with vehicle (Fig. 2e–g, *p = 0.02 in LN229-Ef1α:luc, *p = 0.01 in LN229-P2L, *p = 0.02 in GL261-Ef1α:luc). Together, these data demonstrate that TMZ reduced GBM tumor size and slowed disease progression more when administered in the morning, compared to evening, in both human and murine models of GBM.
Fig. 2.
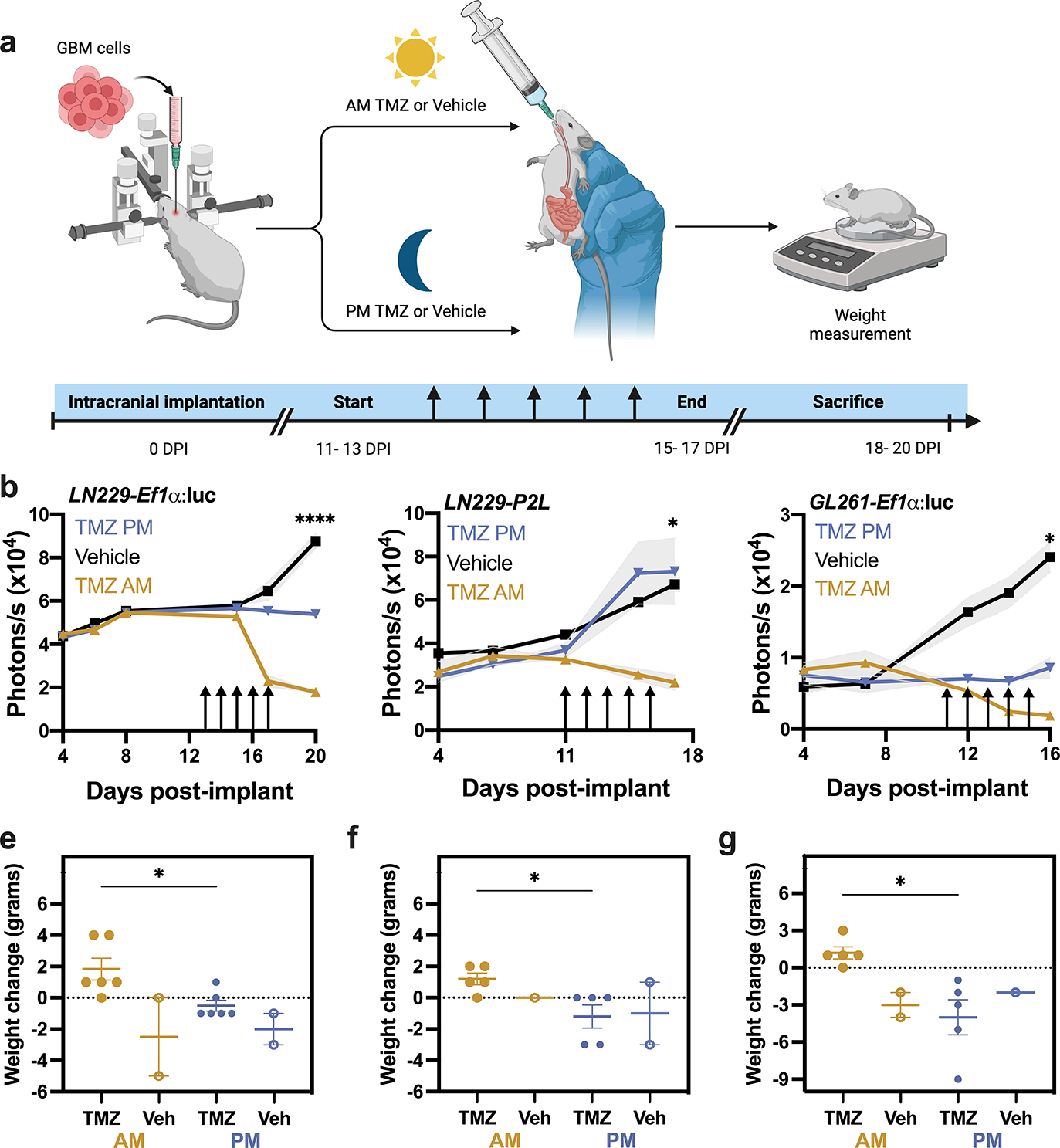
GBM xenografts show elevated sensitivity to TMZ in the morning, compared to evening. a) Experimental paradigm testing TMZ chronotherapy in GBM orthotopic xenograft models. b) LN229-Ef1α implants grew less following five daily doses (arrows) of 100mg/kg TMZ in the morning (ZT4, mean ± SEM) compared to those treated with TMZ in the evening (ZT11) or vehicle (****p < 0.0001 AM vs. PM at 20 days post-implantation, DPI). We quantified tumor size as total bioluminescence at each 11am measurement. c) Mice bearing LN229-P2L xenografts also showed less tumor growth when treated in the morning compared to vehicle or evening TMZ (TMZ PM, *p < 0.05 AM vs. PM at 17 DPI). d) Similarly, GL261-Ef1α xenografts grew less when treated in the morning compared to vehicle or evening TMZ (*p < 0.05 AM vs. PM at 16DPI). e-g) Mice bearing LN229 or GL261implants treated with TMZ in the morning lost less weight from start to the end of TMZ treatment compared to those treated in the evening or with vehicle (mean ± SEM; *p < 0.05)
We also tested TMZ at other times of day, doses, and disease state (Supplementary Fig. 2a–b). Mice were implanted with GL261-P2L or LN229-P2L cells and, immediately after implantation (4 days post-implant), started receiving 70mg/kg TMZ by oral gavage for two weeks, 5 days on and 2 days off, at either 8:00 a.m. or 7:00 p.m. (Zeitgeber Time, ZT1 or ZT12). After a total of 10 treatments with a lower TMZ dose, we found no significant difference between groups (Supplementary Fig. 2a–b, p>0.99). These results indicate that time-dependent TMZ sensitivity depends on disease progression status at start of treatment, dose, and circadian time.
Time-dependent sensitivity to TMZ does not depend on blood-brain barrier penetration
Unlike molecules that are actively transported across the blood brain barrier, and thus subject to daily rhythms in efflux transporter activity, TMZ passively diffuses [25]. We quantified TMZ and its catabolite, 4-amino-5-imidazole-carboxamide (AIC), via mass spectrometry after morning or evening gavage (70mg/kg) in mice. We found similar levels of TMZ and AIC in blood and brain after morning or evening TMZ delivery (Supplementary Fig. 3a–d). Consistent with prior reports and modeling [25], [26], TMZ and AIC levels were lower in brain than blood and rapidly declined within 4 h (****p < 0.0001). We conclude that time-of-day differences in response to TMZ are likely due to tumor-intrinsic circadian rhythms and not differences in drug distribution.
Daily rhythms in MGMT expression determine phase-dependent TMZ sensitivity
To test the hypothesis that TMZ sensitivity relates to circadian regulation of the DNA repair enzyme MGMT, we first assessed whether GBM cells exhibit daily rhythms in MGMT gene expression. Analysis of the MGMT promoter sequence (NG_052673, −2591 bp to +123 bp) yielded a total of 12 E-box sequences (CACGTG), highlighting the potential for transcription of this gene to be modulated by the clock protein BMAL1. To record MGMT gene activity in real-time, we transduced GBM cell lines with an MGMT-luciferase reporter (MGMT:luc, courtesy of Dr. Markus Christmann [27]) and recorded its transcription every 3 min for 48 h. We found daily rhythms in MGMT:luc expression in LN229 GBM cells in vitro, with expression peaking around CT12, corresponding to the trough of Bmal1 (Fig. 3a, CC>0.9). We next evaluated MGMT mRNA levels and promoter methylation on LN229 cells collected every 4 h over 48 h. Using qPCR and qMSP, we found daily rhythms in MGMT gene expression and methylation status in LN229 cells, with MGMT transcription peaking at CT12, and methylation being at its lowest point at CT8 (Fig. 3b–c, average trace scored circadian by JTK cycle, p < 0.05), consistent with the MGMT:luc reporter and indicating circadian regulation of DNA repair.
To test if daily rhythms in TMZ efficacy depend on MGMT activity, we treated LN229-B1L and GL261-B1L cultures with 100 μM TMZ and the MGMT inhibitor, O6-benzylguanine (20μM O6-BG) or vehicle. As in previous experiments, we found that the day-night difference in TMZ was abrogated such that TMZ killed more cells at the peak of Bmal1 (42% survival for GL261; 34% survival for LN229 cells) than at the trough (92% survival for both GL261 and LN229 cells) (****p < 0.0001), but not when co-delivered with O6-BG (Fig. 3d–e, p>0.05). Together, these results demonstrate that daily rhythms in TMZ sensitivity in GBM cells depend upon circadian MGMT expression. We conclude that chemotherapy with TMZ can be dramatically enhanced by delivering when Bmal1 expression in the tumor reaches its daily maximum and MGMT transcription is at its minimum.
Discussion
MGMT promoter methylation status varies with time of day of GBM sample collection
Our findings have implications for both diagnosis and treatment of GBM. We found lower MGMT methylation levels during the subjective day (CT8) than at night (CT16), suggesting epigenetic regulation of MGMT transcription based on circadian time, resulting in a daily peak in MGMT mRNA around subjective dusk. As MGMT promoter methylation exhibits strong positive prognostic value in treatments with TMZ [28]–[31] and other alkylating agents such as procarbazine and CCNU [30], [32], [33], it will be critical to consider time of tumor biopsy for the most accurate determination of MGMT promoter methylation status. Timed MGMT promoter methylation testing might improve correlated response and survival analyses prospectively and resolve the apparent contradiction with a few studies that found no relationship between methylated MGMT and favorable response to TMZ [34], [35]. This could become standard in the field. Additionally, these data suggest that uniform administration of alkylators in the morning might be best across cancer types, which could change the standard of care for patients.
The LN229 model is considered to be MGMT deficient (i.e., MGMT-methylated) [30], [34], [35]. Our evidence of circadian rhythms in MGMT promoter methylation in LN229 cells indicates that these cells are an example of a more dynamic form of promoter methylation and MGMT expression than a clinical diagnosis of MGMT silenced with hypermethylated MGMT promoter. These data emphasize the importance of timed assessment of MGMT promoter methylation status.
TMZ efficacy against GBM can be enhanced by delivery at the best time of day
We found that delivering chemotherapy in the subjective morning can significantly decrease tumor growth and improve disease outcomes for human and mouse models of GBM. Specifically, morning administration of TMZ reduced tumor bioluminescence by 3-fold compared to evening TMZ in vivo and increased tumor cell survival by 4-fold in vitro, with greatest sensitivity around the peak of Bmal11 and trough of MGMT expression in the tumor cells. Morning TMZ also slowed disease progression based on healthier gains in body weight compared to evening TMZ or vehicle treatments. Consistent with these findings, another glioma cell line, U87, shows circadian rhythms in TMZ-induced DNA damage (phosphorylated γH2AX) and apoptosis (caspase3/7 activation) [15] and a recent retrospective clinical study found that patients receiving TMZ in the morning had an increased median survival of 6 months, compared to those receiving it in the evening [12]. A prospective trial designed to test compliance with morning vs. evening dosing of TMZ in 35 patients with gliomas demonstrated feasibility of timed dosing and no differences in side-effects. The study was underpowered to evaluate overall survival. Based on the convergent evidence for daily rhythms in sensitivity to TMZ in various cellular and orthotopic models of glioma and in human GBM patients [15], we conclude that TMZ efficacy can be enhanced by administering it in the morning and in alignment with low MGMT and high Bmal1 gene expression.
One caveat to these results is that we do not know if all GBM tumors synchronize to the host in the same way and thus, if daily MGMT expression and TMZ sensitivity vary depending on tumor or host genetics. Because GBM is a highly heterogenous disease, it is possible that intrinsic clock gene expression, synchronization of circadian rhythms to the host, and time of peak sensitivity to TMZ vary depending on tumor type, status, localization, and the patient’s individual circadian rhythm. Thus, to incorporate TMZ chronotherapy into the standard of care for GBM patients, it will be important to understand when MGMT and Bmal1 peak in individual human GBM tumors, whether this varies between patients, and the optimal dose and time required to obtain maximum drug effects.
Circadian regulation appears to be common to a variety of GBM models
A broad range of GBM cells and GBM stem cells have been found to exhibit intrinsic circadian rhythms in clock gene and protein expression, and in sensitivity to therapeutic drugs [15]–[17]. Our data further expands on these results revealing 24-hour rhythmic expression profiles in Per2 and Bmal1 transcription in murine (GL261) and human (LN229) GBM models. We found that Bmal1 and Per2 transcription peak at similar subjective times of day in GBM in vitro compared to the SCN, prefrontal cortex, hippocampus, amygdala, among other tissues [38]. Consistent with previous studies [15], we found daily rhythms in sensitivity to TMZ with the highest efficacy observed at the peak of Bmal1 in vitro and in the morning in vivo. While studies have shown circadian regulation of TMZ in vitro, there has been no mechanistic exploration as to why efficacy of this chemotherapeutic drug varies with time of day. Here we found circadian rhythms in the transcription and promoter methylation of the DNA repair enzyme MGMT that peak in anti-phase to Bmal1 expression. MGMT peak expression was found to be at CT12, consistent with previous studies that have described daily rhythms in MGMT expression in mouse liver tissue [18]. This daily anti-phase variation in Bmal1 and MGMT expression suggests that the circadian clock may modulate rhythms in DNA repair and thus, provides a time-sensitive window when TMZ can induce greater DNA damage without competing with MGMT. This model is supported with results showing that inhibiting MGMT activity with O6-BG abrogates time-dependent TMZ sensitivity. Altogether, our findings suggest a potential mechanism modulating daily sensitivity to chemotherapy and introduce the concept of combination therapy in which MGMT inhibition is combined with TMZ to maximize efficacy and increase tumor death.
Importantly, our findings raise the question of whether other chemotherapeutic drugs targeting similar mechanisms may do better when chronomodulated. For example, Lomustine (CCNU), is an alkylating agent used to treat brain tumors, particularly upon relapse. One of the most relevant lesions induced by CCNU is the formation of O6-chloroethylguanine, which can be repaired by MGMT activity. Thus, MGMT represents a common mechanism of resistance to both TMZ and CCNU. Based on our findings identifying the role for daily regulation of MGMT in TMZ efficacy, CCNU efficacy may also vary with time-of-day. As with TMZ, chronomodulation of CCNU administration has not been studied. Future research could explore whether timing CCNU to the morning, like TMZ, results in slower disease progression, and better patient prognosis.
We conclude that circadian rhythms in gene expression and response to therapies are prevalent across multiple GBM models. We present two convergent mechanisms (MGMT promoter methylation and mRNA abundance) modulating sensitivity to TMZ and highlight the importance of circadian regulation in both the diagnosis and treatment of GBM. It remains unknown whether daily rhythms persist in vivo, if they synchronize to the hosts circadian rhythm, and whether circadian signals entrain rhythms in GBM to coordinate gene expression between the tumor and the body. Our results set the stage for future studies in tumor synchrony to the host, circadian medicine, and studies that translate to clinical care by using human cell lines and human data. These studies will need to account for details such as tumor location, sex of the patient, available sequencing data, and times of surgery or tissue fixation. Future work will elucidate whether circadian rhythms in GBM can be leveraged to improve current therapies and if personalizing treatments based on a patient’s intrinsic circadian rhythm can prolong their survival.
Implementation of chronotherapy into the standard of care for GBM requires no additional approvals and is consistent with recent retrospective chart study
Since the FDA approval of TMZ in 1999 and tumor-treating fields in 2011, no new treatments have been introduced into the standard of care for GBM. Even with aggressive therapy, patient prognosis and survival remain very poor. Here we reveal daily rhythms in sensitivity to chemotherapy modulated by the daily expression of the DNA repair enzyme MGMT. If validated in patients, these findings can be rapidly translated to prescribe TMZ and MGMT inhibitors at the best time of day for GBM patients. In addition to MGMT activity, drug-resistance to TMZ in GBM patients has been attributed to deficiencies in mismatch repair (MMR) following DNA damage [13] [14] [16] [41]. TMZ induces O6-MeG DNA lesions, which in the absence of MGMT persist, resulting in MMR complex activation and the formation of single- and double-strand DNA breaks and ultimately, apoptosis [15]. Thus, high MMR complex expression is correlated with MGMT silencing and low TMZ IC50 in the GBM A172 cell line [28], whereas decreased expression of MSH6, an MMR complex protein, is correlated with TMZ resistance [38]. Intriguingly, GBM cell lines with higher MGMT expression and TMZ IC50 had reduced MMR expression [39]. As MMR expression appears to be anti-correlated with MGMT expression it will be important to determine whether it too exhibits circadian regulation. Taken together, these data support the potential for TMZ to be chronomodulated and identify a targetable mechanism promoting resistance to timed chemotherapy. While largely understudied for TMZ and GBM, the concept of chronotherapy (i.e., the practice of considering time of day in treating a disease) has been shown to improve outcomes in several cancers, including acute lymphoblastic leukemia, colorectal, ovarian, and other gynecological cancers [20], [40]–[42]. Importantly, novel therapeutic drugs for GBM, such as the inhibitor of Rac family small GTPase 1, 1A-116, showed daily rhythms in efficacy in vitro and in vivo [17]. In addition to tumor sensitivity to TMZ, in a phase I clinical trial administering TMZ at morning or evening times, adverse events were equally common in both patient groups, indicating that host side effects to TMZ are not different at the two administration times tested [43]. Incorporating chronotherapy into the standard of care for GBM patients requires no additional approvals or clinical trials, as opposed to the development of new therapies that can take decades. Our data are consistent with a previous study that associated longer patient overall survival with morning TMZ administration [12]. Because TMZ is taken orally at home, translation of these findings to patients is relatively simple. Future studies should focus on evaluating circadian medicine personalized to the tumor type and daily sleep patterns of the patient in addition to controlling for factors such as age, sex, socioeconomic status, and time since diagnosis.
Methods
Cell culture, lentiviral transductions, and bioluminescence recordings in vitro
LN229 and Glioma 261 (GL261) cells were grown in DMEM (Gibco) or RPMI-1640 (Sigma-Aldrich), respectively, supplemented with FBS (Fisher) and 1% Pen/Strep (Thermo Fisher). Cells were grown in a 37°C incubator with a 5% CO2 environment. Passage number in all experiments ranged from four to eight. GBM cells were transduced with lentiviral reporters expressing firefly luciferase driven by the mouse Bmal1 (Bmal1-luc [44], [45]), Period2 (Per2-luc [46]), Ef1α (Ef1α-luc, obtained from GenTarget Inc.), or O6-Methylguanine-DNA methyltransferase (MGMT-luc [27]). After 24h of transduction, infected cells were selected using blasticidin (1.25 μg/mL, Thermo Fisher). Luciferase expression was confirmed by recording bioluminescence as photons per 180 seconds in vitro. Cells were cultured in media containing 0.1mM D-luciferin (Goldbio) and placed under photomultiplier tubes (PMTs) (Hamamatsu Photonics). See Extended Materials and Methods.
In vitro cell growth assays and pharmacology
We calculated the fraction of cell survival as the number of living cells treated with TMZ divided by the number of living cells treated with vehicle. We replaced the culture media 72-h after treatment to remove dead cells and counted intact cells based on DAPI labeling. We chose to measure survival 3 days after TMZ administration to allow for approximate 2–3 cycles of cell division and TMZ-induced DNA lesions. Specifically, cells were plated and synchronized via serum shock with 50% FBS for 2hrs, followed by a media change, every 4hrs so that at one treatment time, plates spanned 0-, 4-, 8-, 12-, 16-, 20-, 24-, and 28-hrs post-serum shock (HPS). Cells were treated with one of four TMZ concentrations or vehicle (DMSO, 0.2%). To assess whether daily rhythms in TMZ sensitivity depend upon MGMT activity, cells were treated with 20μM of the MGMT inhibitor O6-BG or vehicle (DMSO, 0.2%) at two different time points. In all experiments, cells were fixed after 72hrs with cold methanol and stained cells with 4′,6-diamidino-2-phenylindole (DAPI, 2μg/mL). DAPI fluorescence was quantified with the Infinite 200 PRO plate reader (V_3.37_07/12_Infinite, Tecan Lifesciences). See Extended Materials and Methods.
Quantitative real-time PCR (qRT-PCR) and Methylation Specific PCR (qMSP)
RNA was extracted from 500,000 human LN229 GBM cells collected every 4 hours for a period of 48 hours using TRIzol reagent. RNA was purified using the Direct-zol RNA MiniPrep Plus kit (Zymo) and cDNA was generated by RT-PCR using SuperScript® III First-Strand Synthesis System (Thermo Fisher). For qMSP, bisulfite DNA conversion was performed with the EZ DNA Methylation-Gold kit following their standard protocol. Gene expression changes were further probed using iTaq™ Universal SYBR® Green Supermix (Bio-Rad). The primer sequences for qRT-PCR are listed in Table S1 and for qMSP in Table S2, and were obtained from previous publications (Yoshioka M., et al. (2018), Kreth S., et al. (2011)). PCR amplification was carried out at 40 cycles with 10 ng of template DNA in triplicates. Protocol is as follows: Cycle 1: 95°C for 3 min; Cycle 2: 95°C for 30 sec; Cycle 3: 60°C for 30 sec; repeat step 2–3 for 39 more times; Cycle 4: 72°C for 1 min. Negative controls included no reverse transcriptase reactions and no template DNA samples. All procedures were done in triplicate in two biological replicates.
Orthotopic xenografting
200,000 GBM cells were stereotactically implanted into the right caudate putamen of 10-week-old male and female NU/J (The Jackson Laboratory, Strain #002019) for human models, or C57BL/6J (The Jackson Laboratory, Strain #000664) for murine models. Following surgery, mice were housed in individual cages, monitored, and treated with analgesic for three days post-implant. Cells were allowed to engraft for 7 days before performing in vivo bioluminescence imaging to measure tumor size.
In vivo bioluminescence imaging
Mice were housed in standard 12h light/12h dark conditions in wheel-cages to record locomotor activity in one-minute bins. Following orthotopic xenografting, tumor size was measured every 2 days at 1:00 p.m. by subcutaneously injecting mice with 15mg/mL of D-luciferin, allowing 10 min for it to access the brain, and imaging bioluminescence with 5 min exposure time. All imaging was performed using an In Vivo Imaging System Lumina III (IVIS, Perkin Elmer). Bioluminescence images were analyzed using Living Image Software (Perkin Elmer).
TMZ gavage in vivo
We prepared a fresh 50 mg/ml stock of TMZ in HPMC each day approximately 10 minutes prior to the time of dosing (i.e., AM and PM). At the time of gavage, TMZ was diluted in 1X PBS based on mouse weight to achieve a dose of 100mg/kg with <10% HPMC. For gavage, mice were briefly anesthetized with 2% isoflurane and received between 100–200μL solution depending on mouse weight. TMZ or vehicle was administered at either ZT4 (morning) or ZT11 (evening) for 5 consecutive days after tumor growth was established at 11–13 days post-implant.
Body weight measurements
To assess tumor burden and disease progression, mice were weighed daily starting on the day of implant. At the end of the experiments, mice were sacrificed in accordance with IACUC protocols.
Mass spectrometry
Mice were anesthetized using 2% isoflurane 1 or 4hrs after receiving one dose of TMZ in the morning or evening. Blood was collected via cardiac puncture followed by decapitation and brain dissection. Tissue processing is described in Extended Materials and Methods. Ultra-high-performance LC (UHPLC)/MS/MS was performed with a Thermo Scientific Vanquish UHPLC system interfaced with a Thermo Scientific TSQ Altis Mass Spectrometer (Waltham, MA). LC/MS/MS data were processed and analyzed with the open-source Skyline software. See Extended Materials and Methods.
Statistical Analysis
The correlation coefficient (CC) of a best-fit circadian cosine function was calculated using ChronoStar 1.0 to assess circadian rhythmicity in GBM cells (See Extended Materials and Methods). For qRT-PCR and qMSP data, JTK cycle was used to assess circadian rhythmicity. Unpaired, two-tailed Student’s t tests were used for analysis of cell proliferation and tumor size among experimental groups. Group mean differences were assessed using one-way analysis of variance (one-way ANOVA) with Tukey post hoc tests to further examine pairwise differences. A level of p < 0.05 was used to designate significant differences. All the statistical analyses were performed in Prism (version 10.0.1).
Supplementary Material
Acknowledgements
We thank the members of the Herzog lab for discussion and comments on the manuscript. This work was supported by National Institutes of Health Grants NINDS R21NS120003 and the Washington University Siteman Cancer Center.
Funding:
This work was supported by the National Institutes of Health Grants NINDS R21NS120003 and the Washington University Siteman Cancer Center. Author MFGA was supported by the Washington University Neuroscience Program T32-Training Grant NIH (T32NS121881–01) and the Initiative for Maximizing Student Development (IMSD) Program Training Grant NIH (R25GM103757–10). Author ARD was supported by the National Institutes of Health National Cancer Institute (F31CA250161). Author LLT was supported by grants from Universidad Nacional de Quilmes (PUNQ 2285/22) and by Agencia Nacional de Promoción Científica y Tecnológica de Argentina (PICT 1745–2017).
Footnotes
Statements and Declarations
Competing Interests: The authors have no relevant financial or non-financial interests to disclose.
Ethics approval: All vertebrate animals in this study were used in accordance with the guidelines established by the Washington University Department of Comparative Medicine (protocol 19–1136, expiration date 05/21/2023, and protocol 23–0105, expiration date 05/17/2026).
Data availability:
The data generated in this study are available from the corresponding author upon reasonable request.
References
- [1].Alcantara Llaguno S et al. , “Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model,” Cancer Cell, vol. 15, no. 1, pp. 45–56, Jan. 2009, doi: 10.1016/j.ccr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zong H, Parada LF, and Baker SJ, “Cell of Origin for Malignant Gliomas and Its Implication in Therapeutic Development,” Cold Spring Harb. Perspect. Biol, vol. 7, no. 5, p. a020610, May 2015, doi: 10.1101/cshperspect.a020610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tamimi AF and Juweid M, “Epidemiology and Outcome of Glioblastoma,” in Glioblastoma S. De Vleeschouwer, Ed., Brisbane (AU): Codon Publications, 2017. Accessed: Jun. 01, 2021. [Online]. Available: http://www.ncbi.nlm.nih.gov/books/NBK470003/ [PubMed] [Google Scholar]
- [4].“Cancer of the Brain and Other Nervous System - Cancer Stat Facts,” SEER. Accessed: Mar. 24, 2022. [Online]. Available: https://seer.cancer.gov/statfacts/html/brain.html [Google Scholar]
- [5].Fernandes C et al. , “Current Standards of Care in Glioblastoma Therapy,” in Glioblastoma S. De Vleeschouwer, Ed., Brisbane (AU): Codon Publications, 2017. Accessed: Sep. 01, 2023. [Online]. Available: http://www.ncbi.nlm.nih.gov/books/NBK469987/ [PubMed] [Google Scholar]
- [6].Hottinger AF, Pacheco P, and Stupp R, “Tumor treating fields: a novel treatment modality and its use in brain tumors,” Neuro-Oncol, vol. 18, no. 10, pp. 1338–1349, Oct. 2016, doi: 10.1093/neuonc/now182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hegi ME et al. , “MGMT gene silencing and benefit from temozolomide in glioblastoma,” N. Engl. J. Med, vol. 352, no. 10, pp. 997–1003, Mar. 2005, doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- [8].Stupp R et al. , “Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma,” N. Engl. J. Med, vol. 352, no. 10, pp. 987–996, Mar. 2005, doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- [9].García M, Clopés A, Bruna J, Martínez M, Fort E, and Gil M, “Critical appraisal of temozolomide formulations in the treatment of primary brain tumors: patient considerations,” Cancer Manag. Res, vol. 1, pp. 137–150, Oct. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ortiz R et al. , “Temozolomide: An Updated Overview of Resistance Mechanisms, Nanotechnology Advances and Clinical Applications,” Curr. Neuropharmacol, vol. 19, no. 4, pp. 513–537, Apr. 2021, doi: 10.2174/1570159X18666200626204005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang J, Stevens MFG, and Bradshaw TD, “Temozolomide: mechanisms of action, repair and resistance,” Curr. Mol. Pharmacol, vol. 5, no. 1, pp. 102–114, Jan. 2012, doi: 10.2174/1874467211205010102. [DOI] [PubMed] [Google Scholar]
- [12].Damato AR et al. , “Temozolomide chronotherapy in patients with glioblastoma: a retrospective single-institute study,” Neuro-Oncol. Adv, vol. 3, no. 1, p. vdab041, Dec. 2021, doi: 10.1093/noajnl/vdab041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schibler U, “The daily rhythms of genes, cells and organs. Biological clocks and circadian timing in cells,” EMBO Rep, vol. 6 Spec No, pp. S9–13, Jul. 2005, doi: 10.1038/sj.embor.7400424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Saini R, Jaskolski M, and Davis SJ, “Circadian oscillator proteins across the kingdoms of life: structural aspects,” BMC Biol, vol. 17, no. 1, p. 13, Feb. 2019, doi: 10.1186/s12915-018-0623-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Slat EA et al. , “Cell-intrinsic, Bmal1-dependent Circadian Regulation of Temozolomide Sensitivity in Glioblastoma,” J. Biol. Rhythms, vol. 32, no. 2, pp. 121–129, Apr. 2017, doi: 10.1177/0748730417696788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dong Z et al. , “Targeting Glioblastoma Stem Cells through Disruption of the Circadian Clock,” Cancer Discov, vol. 9, no. 11, pp. 1556–1573, Nov. 2019, doi: 10.1158/2159-8290.CD-19-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Trebucq LL, Cardama GA, Lorenzano Menna P, Golombek DA, Chiesa JJ, and Marpegan L, “Timing of Novel Drug 1A-116 to Circadian Rhythms Improves Therapeutic Effects against Glioblastoma,” Pharmaceutics, vol. 13, no. 7, p. 1091, Jul. 2021, doi: 10.3390/pharmaceutics13071091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Martineau-Pivoteau N et al. , “Circadian variation in O6-methylguanine-DNA methyltransferase activity in mouse liver,” Anticancer. Drugs, vol. 7, no. 6, p. 703, Aug. 1996. [DOI] [PubMed] [Google Scholar]
- [19].Gachon F, Nagoshi E, Brown SA, Ripperger J, and Schibler U, “The mammalian circadian timing system: from gene expression to physiology,” Chromosoma, vol. 113, no. 3, pp. 103–112, Sep. 2004, doi: 10.1007/s00412-004-0296-2. [DOI] [PubMed] [Google Scholar]
- [20].Lévi F, Okyar A, Dulong S, Innominato PF, and Clairambault J, “Circadian timing in cancer treatments,” Annu. Rev. Pharmacol. Toxicol, vol. 50, pp. 377–421, 2010, doi: 10.1146/annurev.pharmtox.48.113006.094626. [DOI] [PubMed] [Google Scholar]
- [21].Zhang SL, Yue Z, Arnold DM, Artiushin G, and Sehgal A, “A Circadian Clock in the Blood-Brain Barrier Regulates Xenobiotic Efflux,” Cell, vol. 173, no. 1, pp. 130–139.e10, Mar. 2018, doi: 10.1016/j.cell.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Uchida Y, Hirayama J, and Nishina H, “A common origin: signaling similarities in the regulation of the circadian clock and DNA damage responses,” Biol. Pharm. Bull, vol. 33, no. 4, pp. 535–544, 2010, doi: 10.1248/bpb.33.535. [DOI] [PubMed] [Google Scholar]
- [23].Im J-S, Jung B-H, Kim S-E, Lee K-H, and Lee J-K, “Per3, a circadian gene, is required for Chk2 activation in human cells,” FEBS Lett, vol. 584, no. 23, pp. 4731–4734, Dec. 2010, doi: 10.1016/j.febslet.2010.11.003. [DOI] [PubMed] [Google Scholar]
- [24].Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, and Koeffler HP, “The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells,” Mol. Cell, vol. 22, no. 3, pp. 375–382, May 2006, doi: 10.1016/j.molcel.2006.03.038. [DOI] [PubMed] [Google Scholar]
- [25].Ballesta A, Zhou Q, Zhang X, Lv H, and Gallo JM, “Multiscale design of cell-type-specific pharmacokinetic/pharmacodynamic models for personalized medicine: application to temozolomide in brain tumors,” CPT Pharmacomet. Syst. Pharmacol, vol. 3, no. 4, p. e112, Apr. 2014, doi: 10.1038/psp.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rosso L et al. , “A new model for prediction of drug distribution in tumor and normal tissues: pharmacokinetics of temozolomide in glioma patients,” Cancer Res, vol. 69, no. 1, pp. 120–127, Jan. 2009, doi: 10.1158/0008-5472.CAN-08-2356. [DOI] [PubMed] [Google Scholar]
- [27].“Repair gene O6-methylguanine-DNA methyltransferase is controlled by SP1 and up-regulated by glucocorticoids, but not by temozolomide and radiation”, doi: 10.1111/jnc.14262. [DOI] [PubMed] [Google Scholar]
- [28].Kaina B, Christmann M, Naumann S, and Roos WP, “MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents,” DNA Repair, vol. 6, no. 8, pp. 1079–1099, Aug. 2007, doi: 10.1016/j.dnarep.2007.03.008. [DOI] [PubMed] [Google Scholar]
- [29].Sarkaria JN et al. , “Mechanisms of chemoresistance to alkylating agents in malignant glioma,” Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res., vol. 14, no. 10, pp. 2900–2908, May 2008, doi: 10.1158/1078-0432.CCR-07-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Switzeny OJ, Christmann M, Renovanz M, Giese A, Sommer C, and Kaina B, “MGMT promoter methylation determined by HRM in comparison to MSP and pyrosequencing for predicting high-grade glioma response,” Clin. Epigenetics, vol. 8, no. 1, p. 49, May 2016, doi: 10.1186/s13148-016-0204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Weller M et al. , “MGMT Promoter Methylation Is a Strong Prognostic Biomarker for Benefit from Dose-Intensified Temozolomide Rechallenge in Progressive Glioblastoma: The DIRECTOR Trial,” Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res., vol. 21, no. 9, pp. 2057–2064, May 2015, doi: 10.1158/1078-0432.CCR-14-2737. [DOI] [PubMed] [Google Scholar]
- [32].van den Bent MJ et al. , “MGMT-STP27 methylation status as predictive marker for response to PCV in anaplastic Oligodendrogliomas and Oligoastrocytomas. A report from EORTC study 26951,” Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res., vol. 19, no. 19, pp. 5513–5522, Oct. 2013, doi: 10.1158/1078-0432.CCR-13-1157. [DOI] [PubMed] [Google Scholar]
- [33].Yamamuro S et al. , “Lomustine and nimustine exert efficient antitumor effects against glioblastoma models with acquired temozolomide resistance,” Cancer Sci, vol. 112, no. 11, pp. 4736–4747, Nov. 2021, doi: 10.1111/cas.15141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yin A et al. , “The predictive but not prognostic value of MGMT promoter methylation status in elderly glioblastoma patients: a meta-analysis,” PloS One, vol. 9, no. 1, p. e85102, 2014, doi: 10.1371/journal.pone.0085102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hegi ME et al. , “Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity,” J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol., vol. 26, no. 25, pp. 4189–4199, Sep. 2008, doi: 10.1200/JCO.2007.11.5964. [DOI] [PubMed] [Google Scholar]
- [36].Köritzer J et al. , “Restoration of Sensitivity in Chemo — Resistant Glioma Cells by Cold Atmospheric Plasma,” PLoS ONE, vol. 8, no. 5, p. e64498, May 2013, doi: 10.1371/journal.pone.0064498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sciuscio D et al. , “Extent and Patterns of MGMT Promoter Methylation in Glioblastoma- and Respective Glioblastoma-Derived Spheres,” Clin. Cancer Res, vol. 17, no. 2, pp. 255–266, Jan. 2011, doi: 10.1158/1078-0432.CCR-10-1931. [DOI] [PubMed] [Google Scholar]
- [38].Chun LE, Woodruff ER, Morton S, Hinds LR, and Spencer RL, “Variations in phase and amplitude of rhythmic clock gene expression across prefrontal cortex, hippocampus, amygdala, and hypothalamic paraventricular and suprachiasmatic nuclei of male and female rats.,” J. Biol. Rhythms, vol. 30, no. 5, pp. 417–436, Oct. 2015, doi: 10.1177/0748730415598608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Perazzoli G et al. , “Temozolomide Resistance in Glioblastoma Cell Lines: Implication of MGMT, MMR, P-Glycoprotein and CD133 Expression,” PLOS ONE, vol. 10, no. 10, p. e0140131, Oct. 2015, doi: 10.1371/journal.pone.0140131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Giacchetti S et al. , “Phase III Trial Comparing 4-Day Chronomodulated Therapy Versus 2-Day Conventional Delivery of Fluorouracil, Leucovorin, and Oxaliplatin As First-Line Chemotherapy of Metastatic Colorectal Cancer: The European Organisation for Research and Treatment of Cancer Chronotherapy Group,” J. Clin. Oncol, vol. 24, no. 22, pp. 3562–3569, Aug. 2006, doi: 10.1200/JCO.2006.06.1440. [DOI] [PubMed] [Google Scholar]
- [41].Rivard GE, Infante-Rivard C, Dresse MF, Leclerc JM, and Champagne J, “Circadian time-dependent response of childhood lymphoblastic leukemia to chemotherapy: a long-term follow-up study of survival,” Chronobiol. Int, vol. 10, no. 3, pp. 201–204, Jun. 1993, doi: 10.3109/07420529309073888. [DOI] [PubMed] [Google Scholar]
- [42].Kobayashi M, Wood PA, and Hrushesky WJM, “Circadian chemotherapy for gynecological and genitourinary cancers,” Chronobiol. Int, vol. 19, no. 1, pp. 237–251, Jan. 2002, doi: 10.1081/cbi-120002600. [DOI] [PubMed] [Google Scholar]
- [43].Damato AR et al. , “A randomized feasibility study evaluating temozolomide circadian medicine in patients with glioma,” Neuro-Oncol. Pract, vol. 9, no. 3, pp. 193–200, May 2022, doi: 10.1093/nop/npac003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Liu AC, Tran HG, Zhang EE, Priest AA, Welsh DK, and Kay SA, “Redundant Function of REV-ERBα and β and Non-Essential Role for Bmal1 Cycling in Transcriptional Regulation of Intracellular Circadian Rhythms,” PLOS Genet, vol. 4, no. 2, p. e1000023, Feb. 2008, doi: 10.1371/journal.pgen.1000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang EE et al. , “A Genome-Wide RNAi Screen for Modifiers of the Circadian Clock in Human Cells,” Cell, vol. 139, no. 1, pp. 199–210, Oct. 2009, doi: 10.1016/j.cell.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ramanathan C, Khan SK, Kathale ND, Xu H, and Liu AC, “Monitoring cell-autonomous circadian clock rhythms of gene expression using luciferase bioluminescence reporters,” J. Vis. Exp. JoVE, no. 67, p. 4234, Sep. 2012, doi: 10.3791/4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are available from the corresponding author upon reasonable request.


