Abstract
BACKGROUND
The endoscopic endonasal approach to paramedian skull base lesions has garnered increasing attention in recent reports. However, it is still a challenging approach. While the primary objective of the approach is the maximal removal of tumors through a minimally invasive procedure, discussions of the approach rarely include information about the maximum preservation of nasal structures. This study aimed to retrospectively review the clinical outcomes of patients who had undergone an endoscopic endonasal approach to paramedian lesions, describe the technical and anatomical nuances related to this approach at the authors’ institution, and discuss the maximal preservation of nasal structures.
OBSERVATIONS
The authors conducted a descriptive retrospective study of 17 surgical cases of paramedian endoscopic endonasal approaches performed jointly by otolaryngologists and neurosurgeons from August 2018 to August 2022 at a tertiary hospital.
LESSONS
The approach to the paramedian region of the skull base was examined. Creating an appropriate corridor to maximize the surgical field is essential to allow a safe and accurate procedure. From an otolaryngologist’s perspective, the endoscopic modified medial maxillectomy is an essential procedure that maximizes the surgical corridor and maximally preserves nasal morphology.
Keywords: transsphenoidal approach, transpterygoid approach, EMMM, endoscopic modified medial maxillectomy
ABBREVIATIONS: AMA = accessory meningeal artery, CSF = cerebrospinal fluid, EESS = endoscopic endonasal skull base surgery, EMMM = endoscopic modified medial maxillectomy, ENS = empty nose syndrome, ICA = internal carotid artery, MRI = magnetic resonance imaging, NBCA = n-butyl-2-cyanoacrylate, SPA = sphenopalatine artery
Endoscopic endonasal skull base surgery (EESS) has made remarkable progress in recent years due to the development of endoscopes. Initially used primarily for pituitary surgery, EESS now has broader applicability.1, 2 Previous literature has described EESS as “access and visualization through the narrowest practical corridor providing maximum effective action at the target with minimal disruption of normal tissues.”3–6 In particular, the paramedian approach has spread rapidly, largely through the introduction of the transpterygoid approach as the essential procedure.7, 8 However, the paramedian approach of EESS is still challenging and not yet a procedure that can be performed at all facilities.
Nevertheless, with the formation of collaborative skull base teams and a profound anatomical understanding of the skull base, EESS can be performed safely. At our institution, we launched a skull base team in 2018, fostering collaboration on this approach. Historically, the paramedian approach has focused on what approach should be selected and how much of the tumor could be removed. Surgeons have paid less attention to how much nasal structure is preserved. We are now entering a phase where nasal structure preservation is emphasized to maintain patient quality of life, even in the paramedian approach of EESS. In this study, we retrospectively analyzed the paramedian endoscopic endonasal approaches for EESS and investigated methods to maximally protect nasal structures.
Study Description
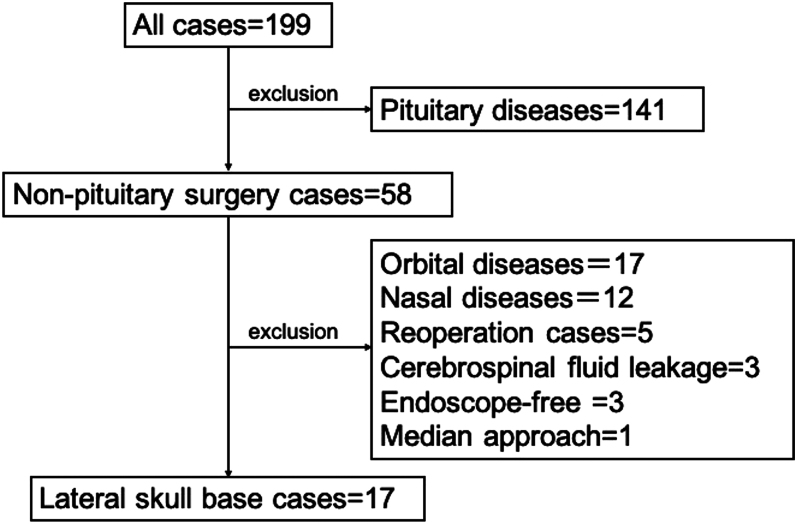
We analyzed the clinical outcomes of patients treated at Kyushu University Hospital’s Departments of Otolaryngology and Neurosurgery from August 2018 to August 2022. A skull base team comprising otolaryngologists and neurosurgeons conducted 199 surgeries. Excluding 141 pituitary tumor cases, along with others like orbital lesions, nasal lesions, reoperations, cerebrospinal fluid (CSF) leakage, endoscope-free cases, and median approaches, we retrospectively reviewed 17 cases of paramedian endoscopic endonasal approaches (Fig. 1).
FIG. 1.
A total of 199 skull base surgeries performed by the Departments of Otolaryngology and Neurosurgery at Kyushu University Hospital from August 2018 to August 2022 were included in our analysis. We excluded 141 cases of pituitary tumors. Seventeen cases of orbital lesions, 12 cases of nasal lesions, 5 cases of reoperation, 3 cases of CSF leakage, 3 endoscope-free cases, and 1 median approach were also excluded. We retrospectively reviewed 17 cases of paramedian endoscopic endonasal approaches in the present study.
Surgical Ports
Surgical ports included ipsilateral and/or contralateral nostrils and/or ipsilateral/contralateral sublabial ports. Transoral ports were also utilized for accessing paramedian skull base lesions such as those in the jugular foramen and parapharyngeal space, with incisions made between the palatoglossal arch and retromolar trigone. Conley and Price9 and Anand and Conley10 have described the sublabial approach, involving incisions at gingivobuccal and gingivolabial grooves to release soft tissues, allowing access to the middle third of the face and nose. However, we opted for a smaller incision, removing the anterior maxilla wall to create a window for endoscope insertion or instrument use, which facilitates 3- and 4-handed surgeries, minimizing tool interference.
Surgical Approaches
Transsphenoidal Approach
Jankowski et al.1 reported the first complete endoscopic resection of a pituitary adenoma by accessing the sphenoid sinus recess and right sphenoid sinus through a transnasal incision. We utilized a similar approach, making incisions from below the natural foramen of the sphenoid sinus to the anterior nasal septal mucosa, with a slightly shorter incision on the contralateral side. Dissecting the mucosa exposed the anterior wall of the sphenoid sinus, allowing full observation of its interior upon removal of the bony wall. This corridor is crucial for manipulating structures like the petrous apex, cavernous sinus, and clivus. Additionally, we extended the transsphenoidal approach posteriorly and laterally, known as the “extended transsphenoidal approach,” for conditions such as giant pituitary adenomas, craniopharyngiomas, meningoencephaloceles, and cholesterol granulomas.11–13
Transpterygoid Approach
The approach to the infratemporal fossa is useful for extracranial lesions with intracranial extension.14–16 Initially, the mucosa, bone, andperiosteum of the posterior maxillary sinus wall are removed to expose the pterygopalatine fossa. Resectioning or clipping the maxillary artery and resecting the base of the pterygoid process of the sphenoid sinus allow access to areas like the infratemporal fossa. Recently, Pinheiro-Neto et al. proposed transpositioning the pterygopalatine fossa contents for the paramedian skull base approach, preserving neurovascular structures.17 Our team employs this technique along with endoscopic modified medial maxillectomy (EMMM) for safer surgical manipulation.
Endoscopic Modified Medial Maxillectomy
Nakayama et al.18 reported the first EMMM to manipulate a broad area within the maxillary sinus while preserving the inferior turbinate and nasolacrimal duct. This technique is a modified version of endoscopic medial maxillectomy,19 in which the inferior turbinate and nasolacrimal duct are excised, allowing an approach into the maxillary sinus. The EMMM is a revolutionary surgical technique that allows fine manipulation of the maxillary sinus and maximally preserves nasal structures. This technique can also be applied to areas beyond the maxillary sinus.
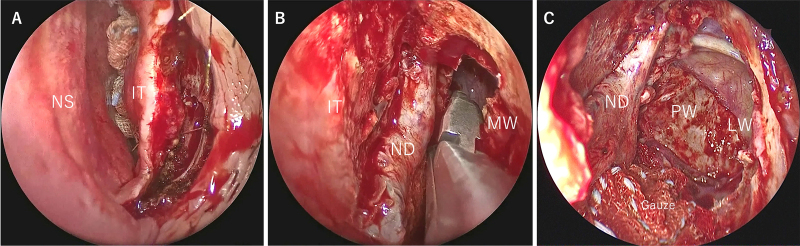
Surgical techniques are presented along with actual surgical images. The anterior end of the inferior turbinate is incised (Fig. 2A), and the medial wall of the maxillary sinus is placed in a clear view (Fig. 2B). Then, the nasolacrimal duct is identified, and the bony nasolacrimal duct is drilled out. The nasolacrimal duct and inferior turbinate are swung inward to expose the medial wall of the maxillary sinus. The medial wall of the maxillary sinus is then drilled out to open the maxillary sinus. This facilitates the approach to the lateral and posterior wall of the maxillary sinus and allows for a safe transpterygoid and maxillary sinus approach (Fig. 2C). To create a broader corridor, the anterior wall of the maxillary sinus can be drilled, in addition to the removal of the medial wall of the maxillary sinus, a technique that has been described as a “direct approach to the anterior and lateral part of the maxillary sinus with an endoscope” (DALMA) by Omura et al.20
FIG. 2.
A: In the EMMM, the anterior end of the inferior turbinate is incised. B: The medial wall of the maxillary sinus is placed in a clear view. The nasolacrimal duct is identified, and the bony nasolacrimal duct is drilled out. C: The nasolacrimal duct and inferior turbinate are swung inward to expose the medial wall of the maxillary sinus. The medial wall of the maxillary sinus is drilled out to open the maxillary sinus. When the medial wall of the maxillary sinus is sufficiently expanded, observation of the posterior and lateral walls of the maxillary sinus becomes easier. IT = inferior turbinate; LW = lateral wall; MW = medial wall; ND = nasolacrimal duct; NS = nasal septum; PW = posterior wall.
Case Profiles
The cases included 7 males and 10 females with a mean age of 42.8 years (13–82 years). There were 3 cases each of chondrosarcoma, schwannoma, and cholesterol granuloma. There were 2 cases each of angiofibroma and adenoid cystic carcinoma. In addition, there was 1 case each of meningoencephalocele, meningioma, lymphoma, and optic nerve compression. The localization of the lesions varied widely and is described in Supplemental Table 1.
EMMM18 was performed in 9 of 17 cases. One case was treated with a transoral approach and another with an ipsilateral nostril port. In all but 1 case, a contralateral nostril port was also used. Five patients had an ipsilateral sublabial port and 1 a contralateral sublabial port. Case 13 was also treated with the combination of an orbitozygomatic approach. The procedure details for each approach are shown in Supplemental Table 1. We tried to maximally preserve intranasal structures. The total resection of the ipsilateral superior turbinate was performed in 3 cases. The middle turbinate was resected in 8 cases; 5 were total resections, and 3 were partial resections. Partial resection of the inferior turbinate was performed in 3 cases. Importantly, all nasal structures on the contralateral side were preserved.
Nasal mucosal flaps were used in 8 of 17 patients. The middle turbinate mucosal flap was used in 3 cases, the nasal septum flap in 4, the posterior septal nasal floor flap in 1, and the sphenoid sinus mucosa in 1. Two flaps were used in case 5 and case 16. In addition, cases 3 and 10 received 70 and 56 Gy of adjuvant radiotherapy, respectively. Complications were seen in 5 of the 17 patients (29.4%), including otitis media with effusion, palatal numbness, dry eye, cranial nerve V1 and V2 palsy, and dysomnia. However, almost all complications were temporary, and no cases developed empty nose syndrome (ENS) after surgery.
Illustrative Cases
Case 7
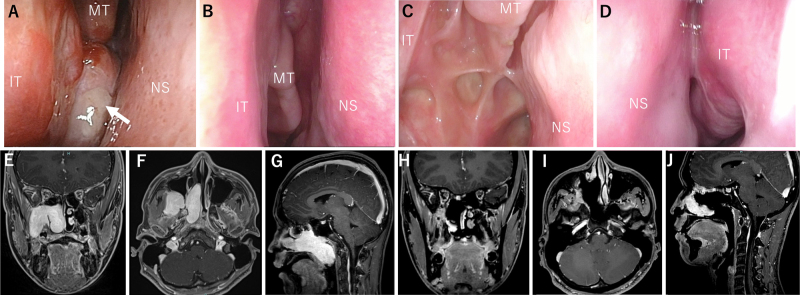
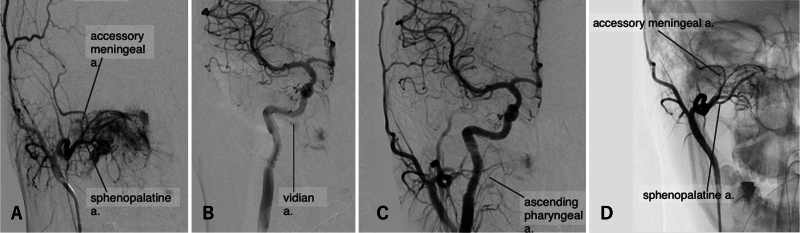
Preoperatively, fiberscope findings showed that the tumor occupied the right nasal cavity, and the posterior nostril was not visible (Fig. 3A). Magnetic resonance imaging (MRI) findings showed that the tumor extended from the right pterygopalatine and infratemporal fossae to the right middle and lower nasal passages and nasopharynx (Fig. 3E– G). A pathological diagnosis of juvenile angiofibroma was made. After discussing the treatment plan, it was decided to embolize the tumor’s feeding artery the day before surgery. The main tumor-feeding arteries were the sphenopalatine artery (SPA) feeder and the accessory meningeal artery (AMA; Fig. 4A–C). The SPA feeder and AMA were embolized with n-butyl-2-cyanoacrylate (NBCA), and 3 coil embolizations (i-ED Coil, Kaneka Medical Products) were performed on the main SPA duct. This almost eliminated the external carotid feeder (Fig. 4D). NBCA was injected directly into the tumor to reduce bleeding when the tumor was divided intraoperatively. Transsphenoidal, EMMM, and transpterygoid approaches through the bilateral nostril and ipsilateral sublabial ports were used. The right inferior turbinate was divided and translocated to widen the surgical field. Total ethmoidectomy and a superior and middle turbinectomy on the right side were performed to expose the medial anterior aspect of the tumor, mainly to identify the vidian artery, which was not embolized before surgery because of the branch of the internal carotid artery (ICA). A wide field of view was created at the EMMM to allow access to the pterygoid fossa through the maxillary sinus. The transpterygoid approach facilitated the approach to the pterygoid process and the infratemporal fossa. The sublabial port mainly served as an essential port for endoscopic observation.
FIG. 3.
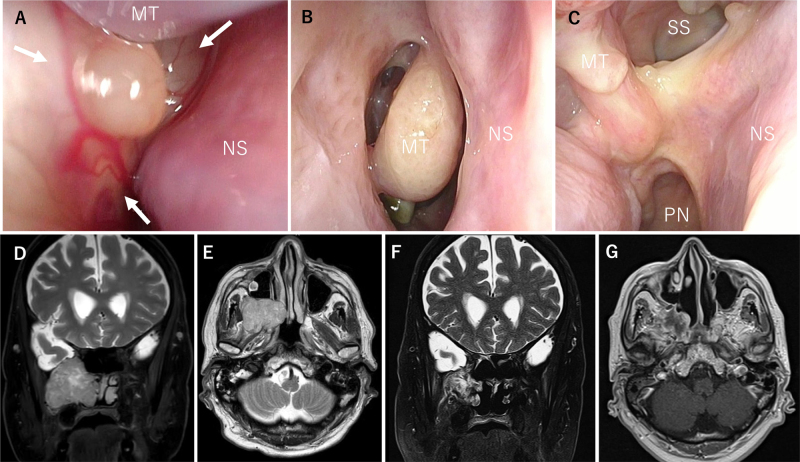
Case 7. A: Fiberscopy findings showed that the tumor (white arrow) occupied the right nasal cavity, and the posterior nostril could not be seen. BandC: Fiberscopy findings at 18 months postoperatively showed no apparent recurrence, and epithelialization was also uneventful. D: The structure of the left nasal cavity was also preserved. E–G: MRI showed that the tumor extended from the right pterygopalatine fossa to the right middle and lower nasal passages and nasopharynx. H–J: Contrast-enhanced MRI 2 years after surgery showed no evident recurrence. MT = middle turbinate.
FIG. 4.
Case 7. A: External carotid angiography showed that the tumor received its primary blood supply from the SPA and the AMA. B: Internal carotid angiography was performed and showed limited supply to the tumor. C: Common carotid angiography was performed and showed limited supply to the tumor. D: Significant reduction in blood flow to the tumor on external carotid angiography after embolization. a. = artery.
For tumor resection, the tumor was first divided with a coblator between the tumor mass in the nasal cavity and the pterygoid and infratemporal fossa during the cauterizing and cutting of the SPA. The tumor in the common nasal passage was removed. The next step was to control the vidian artery. Coagulating and cutting the vidian artery, which was the last one of the main feeding arteries, dramatically reduced bleeding from the tumor and allowed the removal of tumors extending into the pterygoid fossa and infratemporal fossa with bleeding control. Finally, we achieved total removal of the tumor. The incision sites of the left nasal mucosa and the right divided inferior turbinate’s anterior end were sutured after the tumor’s removal.
We preserved the ipsilateral nasal structures as much as possible, and the contralateral nasal cavity was fully preserved. Postoperatively, we observed cranial nerve V2 palsy as a complication. Fiberscopy findings at 18 months postoperatively showed no apparent recurrence, and epithelialization was also uneventful (Fig. 3B and C). Furthermore, the structure of the left nasal cavity was also preserved (Fig. 3D). Contrast-enhanced MRI showed no evident recurrence 2 years after surgery (Fig. 3H–J).
Case 17
Preoperative fiberscope findings showed that the tumor was located in the pterygopalatine fossa and extended into the infratemporal fossa and common nasal cavity (Fig. 5A). MRI findings showed that the tumor was centered in the pterygopalatine fossa and extended into the right nasal cavity. There was no apparent intracranial invasion (Fig. 5D and E). Preoperatively, the most likely diagnosis of this tumor was trigeminal schwannoma (V2). The tumor had gradually grown, and the patient decided to undergo surgery for tumor removal.
FIG. 5.
Case 17. A: Preoperative fiberscopy findings showed that the tumor (white arrows) was posterior and occupied the right nasal cavity. It took much work to observe the posterior nostril. B and C: Postoperative fiberscopy findings at 3 months showed no apparent recurrence and no complications. D and E: MRI findings showed that the tumor was centered in the pterygopalatine fossa and extended into the right nasal cavity. There was no apparent intracranial invasion. F and G: MRI 6 months postoperatively showed no apparent recurrence. PN = posterior nares; SS = sigmoid sinus.
EMMM and transpterygoid approaches were used through the ipsilateral port. Total ethmoidectomy and turbinectomy were performed to expose the medial surface of the tumor. A wide surgical view field was created at the EMMM to allow access to the pterygoid and infratemporal fossae through the maxillary sinus. The tumor was exposed using a transpterygoid approach, and the internal maxillary artery was clipped and resected. We identified the tumor capsule after cutting the epineurium and perineurium. An intraoperative histopathological diagnosis of a benign schwannoma was made; therefore, we began with internal debulking to reduce the tumor’s volume. After the internal debulking of the tumor, we performed an intercapsular resection of the tumor.21, 22 The tumor in the infratemporal fossa was also completely removed. Postoperative fiberscopy findings at 3 months showed no apparent recurrence and no complications (Fig. 5B and C). MRI 6 months postoperatively showed no apparent recurrence (Fig. 5F and G), and the left nasal structures were fully preserved.
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
With the development of EESS, there is a need for less invasive and more extensive approaches. We have retrospectively reviewed our cases of the paramedian endoscopic endonasal approach, which is still one of the more challenging approaches. Our surgical team tried to maximally preserve nasal structures by combining different procedures and ports depending on the case to prevent the reported complications, such as ENS, caused by excessive inferior and middle turbinate surgery or anosmia associated with postoperative mucosal adhesions.23, 24
The sublabial approach was performed in 7 patients (41%): 6 (86%) ipsilateral and 1 (14%) contralateral. It was often used in cases with large tumor sizes or those requiring 4-handed surgery.
Butler et al.,25 Bhatia et al.,26 and Sardana27 described a sublabial approach combined with a transnasal approach to facilitate the removal of the lateral extension of a juvenile angiofibroma. This technique provides a broad field of view and allows direct observation of the lesion during its removal. If the tumor is large, surgical instruments can collide with each other in the nasal cavity, and the maneuvering of these instruments can damage nasal structures. This corridor makes it easier to perform surgical operations and prevents unnecessary sacrifices to the nasal structure. We used the sublabial approach for 7 cases, including case 7, allowing for a minimal incision as the observation port of the endoscope. There were no postoperative complications caused by the creation of the sublabial port.
The transsphenoidal approach is used for tumors extending into the sphenoid sinus and pituitary surgeries. Ceylan et al.28 found that the endoscopic transsphenoidal approach is more likely to allow the removal of adenomas in the cavernous sinus compared to transcranial or microscopic transsphenoidal surgery. The extended transsphenoidal approach, developed from the transsphenoidal approach, is effective for lesions around the cavernous sinus, the anterior skull base, the clivus, the petrous apex, and the lateral recess of the sphenoid sinus. It has been reported to benefit surgeries for residual or recurrent craniopharyngiomas, retrochiasmatic craniopharyngiomas, and sphenoid lateral recess encephaloceles.12, 29, 30 The present study used the transsphenoidal and extended transsphenoidal approaches in 13 cases (76%). It should be noted that identifying the vidian canal, a necessary landmark of the ICA, is essential in this procedure.31 When the nasal cavity is filled with a tumor, a lateral extended transsphenoidal approach is necessary to control the vidian artery.
The transpterygoid approach is helpful for lateral lesions such as those in the medial skull base and infratemporal fossa. If the tumor arises from content in the pterygopalatine fossa, such as a V2 schwannoma, exposing the content is necessary. On the other hand, for tumors located behind the pterygopalatine fossa, the technique of transposition of the pterygopalatine fossa contents, as reported by Pinheiro-Neto et al., is feasible and desirable for the paramedian approach to the skull base because it does not expose the content of the pterygopalatine fossa, thereby preserving the neurovascular structures in the pterygopalatine fossa.17
The transpterygoid procedure was applied in 9 cases (53%), and the contents of the pterygopalatine fossa were exposed after cutting the periosteum surrounding the pterygopalatine fossa in 4 cases: cases 5, 10, 13, and 17. In case 17, this approach, in combination with EMMM, allowed for the removal of the V2 schwannoma while preserving the nasal morphology. Problems related to mastication and hypesthesia along the trigeminal distribution can result from this approach.29 In this study, 2 (29%) complications were observed in cases that underwent this approach: 1 case of V1 and 1 case of V2 nerve palsy. The V2 palsy was attributed to the transection of the greater palatine nerve for tumor resection. Preserving the greater palatine nerve during the resection of the pterygoid process and the palatine bone is crucial for functional preservation. However, regrettably, in case 7, the tumor was firmly adhered to the greater palatine nerve, necessitating its transection. It is also essential to provide adequate preoperative explanations when using this approach. Even when extracranial lesions extend intracranially, this approach plays a vital role.14, 32, 33
EMMM is an essential approach in this review, as it helps manipulate the area beyond the maxillary sinus in addition to the anterior wall and base of the maxillary sinus. Furthermore, it can also be used with the previously described approaches to maximize the operative field while preserving nasal morphology. EMMM has primarily been used for maxillary sinus diseases.34, 35 However, it is increasingly being applied to skull base conditions involving lateral extensions, such as the pterygopalatine fossa and infratemporal fossa. Despite its significance, which provides evidence that EMMM can play a crucial role in preserving nasal cavity structures, this detail has received little attention. Furthermore, it is also indicated for the resection of advanced tumors.36 EMMM was performed in 9 cases, and the only complication from this approach was temporal palatal numbness (case 5). While substantial resection of the inferior turbinate is a viable option, it is crucial to prioritize the preservation of nasal structures due to concerns regarding postoperative complications, such as ENS.37 Overall, EMMM is an approach causing fewer complications if performed appropriately.
Observations
The combination of the approaches described in this report may allow for the safe maximal preservation of nasal structures. It is essential to consider the appropriate corridor before surgery for each case. EMMM is highly effective for this purpose. Although the paramedian approach has recently developed rapidly, attention needs to be drawn toward preserving nasal structures. We are entering an era in which nasal structures must be maximally preserved to achieve maximum tumor resection.
EMMM is essential to achieve maximum tumor resection with a paramedian approach while preserving nasal structures, and an appropriate combination of EMMM with other procedures can accomplish this goal.
Lessons
In this study, we retrospectively reviewed 17 cases in which a paramedian endoscopic endonasal approach was performed. For the paramedian endoscopic endonasal approach, combining the appropriate corridor according to the extent of the lesion is essential. Furthermore, otolaryngologists are responsible for preserving maximum nasal morphology to prevent postoperative complications related to nasal cavity deformity.
This study is a retrospective chart review with a limited number of cases, although it appears to be sufficient for a retrospective review. The observation period was short, and there are no objective data such as in Sino-Nasal Outcome Test.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Komune, Suzuki, Murakami, Yoshimoto, Nakagawa. Acquisition of data: Suzuki, Kuga, Sangatsuda, Murakami. Analysis and interpretation of data: Suzuki, Yoshimoto. Drafting the article: Suzuki. Critically revising the article: Komune. Reviewed submitted version of manuscript: Komune, Miyamoto. Approved the final version of the manuscript on behalf of all authors: Komune. Administrative/technical/material support: Komune, Murakami. Study supervision: Komune, Murakami, Yoshimoto, Nakagawa.
Supplemental Information
Online Only Content
Supplemental Table 1. https://thejns.org/doi/suppl/10.3171/CASE24218.
Previous Presentations
Presented at the 35th Annual Meeting of Japanese Society for Skull Base Surgery held in Tokyo, Japan, on July 6–7, 2023.
Correspondence
Noritaka Komune: Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan. norikomu007@gmail.com.
References
- 1.Jankowski R, Auque J, Simon C, Marchal JC, Hepner H, Wayoff M. Endoscopic pituitary tumor surgery. Laryngoscope. 1992;102(2):198-202. [DOI] [PubMed] [Google Scholar]
- 2.Verillaud B, Bresson D, Sauvaget E, et al. Endoscopic endonasal skull base surgery. Eur Ann Otorhinolaryngol Head Neck Dis. 2012;129(4):190-196. [DOI] [PubMed] [Google Scholar]
- 3.Cinalli G, Cappabianca P, de Falco R, et al. Current state and future development of intracranial neuroendoscopic surgery. Expert Rev Med Devices. 2005;2(3):351-373. [DOI] [PubMed] [Google Scholar]
- 4.Jho HD. Endoscopic transsphenoidal surgery. J Neurooncol. 2001;54(2):187-195. [DOI] [PubMed] [Google Scholar]
- 5.Liu CY, Wang MY, Apuzzo MLJ. The evolution and future of minimalism in neurological surgery. Childs Nerv Syst. 2004;20(11-12):783-789. [DOI] [PubMed] [Google Scholar]
- 6.Prevedello DM, Doglietto F, Jane JA, Jagannathan J, Han J, Laws ER. History of endoscopic skull base surgery: its evolution and current reality. J Neurosurg. 2007;107(1):206-213. [DOI] [PubMed] [Google Scholar]
- 7.Klossek JM, Ferrie JC, Goujon JM, Fontanel JP. Endoscopic approach of the pterygopalatine fossa: report of one case. Rhinology. 1994;32(4):208-210. [PubMed] [Google Scholar]
- 8.Bolger WE. Endoscopic transpterygoid approach to the lateral sphenoid recess: surgical approach and clinical experience. Otolaryngol Head Neck Surg. 2005;133(1):20-26. [DOI] [PubMed] [Google Scholar]
- 9.Conley J, Price JC. Sublabial approach to the nasal and nasopharyngeal cavities. Am J Surg. 1979;138(4):615-618. [DOI] [PubMed] [Google Scholar]
- 10.Anand VK, Conley JJ. Sublabial surgical approach to the nasal cavity and paranasal sinuses. Laryngoscope. 1983;93(11Pt 1):1483-1484. [PubMed] [Google Scholar]
- 11.Dumont AS, Kanter AS, Jane JAJ, Laws ERJ. Extended transsphenoidal approach. Front Horm Res. 2006;34:29-45. [DOI] [PubMed] [Google Scholar]
- 12.Govindaraju R, Tang IP, Prepageran N. Management of sphenoid lateral recess encephalocoeles. Curr Opin Otolaryngol Head Neck Surg. 2019;27(1):37-46. [DOI] [PubMed] [Google Scholar]
- 13.Kalinin P, Sharipov O, Kutin M, et al. Amygdalohippocampectomy via the lateral extended transsphenoidal endoscopic approach through the pterygopalatine fossa: an anatomic study. World Neurosurg. 2017;103:457-464. [DOI] [PubMed] [Google Scholar]
- 14.Browne JD, Jacob SL. Temporal approach for resection of juvenile nasopharyngeal angiofibromas. Laryngoscope. 2000;110(8):1287-1293. [DOI] [PubMed] [Google Scholar]
- 15.Hadley KS, Shelton C. Infratemporal fossa approach to the hypoglossal canal: practical landmarks for elusive anatomy. Laryngoscope. 2004;114(9):1648-1651. [DOI] [PubMed] [Google Scholar]
- 16.Hegazy HM, Carrau RL, Snyderman CH, Kassam A, Zweig J. Transnasal endoscopic repair of cerebrospinal fluid rhinorrhea: a meta-analysis. Laryngoscope. 2000;110(7):1166-1172. [DOI] [PubMed] [Google Scholar]
- 17.Pinheiro-Neto CD, Fernandez-Miranda JC, Prevedello DM, Carrau RL, Gardner PA, Snyderman CH. Transposition of the pterygopalatine fossa during endoscopic endonasal transpterygoid approaches. J Neurol Surg B Skull Base. 2013;74(5):266-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakayama T, Asaka D, Okushi T, Yoshikawa M, Moriyama H, Otori N. Endoscopic medial maxillectomy with preservation of inferior turbinate and nasolacrimal duct. Am J Rhinol Allergy. 2012;26(5):405-408. [DOI] [PubMed] [Google Scholar]
- 19.Kamel RH. Transnasal endoscopic medial maxillectomy in inverted papilloma. Laryngoscope. 1995;105(8Pt 1):847-853. [DOI] [PubMed] [Google Scholar]
- 20.Omura K, Nomura K, Aoki S, Otori N, Tanaka Y. Direct approach to the anterior and lateral part of the maxillary sinus with an endoscope. Auris Nasus Larynx. 2019;46(6):871-875. [DOI] [PubMed] [Google Scholar]
- 21.Battoo AJ, Sheikh ZA, Thankappan K, Hicks W, Iyer S, Kuriakose MA. Nerve-sparing subcapsular resection of head and neck schwannomas: technique evaluation and literature review. J Laryngol Otol. 2013;127(7):685-690. [DOI] [PubMed] [Google Scholar]
- 22.Park SH, Kim J, Moon IS, Lee WS. The best candidates for nerve-sparing stripping surgery for facial nerve schwannoma. Laryngoscope. 2014;124(11):2610-2615. [DOI] [PubMed] [Google Scholar]
- 23.Kuan EC, Suh JD, Wang MB. Empty nose syndrome. Curr Allergy Asthma Rep. 2015;15(1):493. [DOI] [PubMed] [Google Scholar]
- 24.May M, Levine HL, Mester SJ, Schaitkin B. Complications of endoscopic sinus surgery: analysis of 2108 patients–incidence and prevention. Laryngoscope. 1994;104(9):1080-1083. [DOI] [PubMed] [Google Scholar]
- 25.Butler RM, Nahum AM, Hanafee W. New surgical approach to nasopharyngeal angiofibromas. Trans Am Acad Ophthalmol Otolaryngol. 1967;71(1):92-104. [PubMed] [Google Scholar]
- 26.Bhatia ML, Mishra SC, Prakash J. Lateral extensions of nasopharyngeal fibroma. J Laryngol Otol. 1967;81(1):99-106. [DOI] [PubMed] [Google Scholar]
- 27.Sardana DS. Nasopharyngeal fibroma: extension into cheek. Arch Otolaryngol. 1965;81:584-588. [DOI] [PubMed] [Google Scholar]
- 28.Ceylan S, Koc K, Anik I. Endoscopic endonasal transsphenoidal approach for pituitary adenomas invading the cavernous sinus. J Neurosurg. 2010;112(1):99-107. [DOI] [PubMed] [Google Scholar]
- 29.Cavallo LM, Prevedello DM, Solari D, et al. Extended endoscopic endonasal transsphenoidal approach for residual or recurrent craniopharyngiomas. J Neurosurg. 2009;111(3):578-589. [DOI] [PubMed] [Google Scholar]
- 30.Sankhla SK, Jayashankar N, Khan GM. Extended endoscopic endonasal transsphenoidal approach for retrochiasmatic craniopharyngioma: surgical technique and results. J Pediatr Neurosci. 2015;10(4):308-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kassam AB, Vescan AD, Carrau RL, et al. Expanded endonasal approach: vidian canal as a landmark to the petrous internal carotid artery. J Neurosurg. 2008;108(1):177-183. [DOI] [PubMed] [Google Scholar]
- 32.Cass SP, Hirsch BE, Stechison MT. Evolution and advances of the lateral surgical approaches to cranial base neoplasms. J Neurooncol. 1994;20(3):337-361. [DOI] [PubMed] [Google Scholar]
- 33.Fukushima T, Day JD, Hirahara K. Extradural total petrous apex resection with trigeminal translocation for improved exposure of the posterior cavernous sinus and petroclival region. Skull Base Surg. 1996;6(2):95-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anzai T, Ito S, Yamashita A, et al. Surgical management of bilateral venous malformation (cavernous hemangiomas) of the maxillary sinus. Case Rep Otolaryngol. 2020;2020:8606103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wada K, Ishigaki T, Ida Y, Yamada Y, Hosono S, Edamatsu H. Endoscopic modified medial maxillectomy for resection of an inverted papilloma originating from the entire circumference of the maxillary sinus. Case Rep Otolaryngol. 2015;2015:952923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harvey RJ, Sheehan PO, Debnath NI, Schlosser RJ. Transseptal approach for extended endoscopic resections of the maxilla and infratemporal fossa. Am J Rhinol Allergy. 2009;23(4):426-432. [DOI] [PubMed] [Google Scholar]
- 37.Coste A, Dessi P, Serrano E. Empty nose syndrome. Eur Ann Otorhinolaryngol Head Neck Dis. 2012;129(2):93-97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.