Abstract
BACKGROUND
Traumatic high cervical spinal cord injury (SCI) can result in a devastating loss of functional respiration, leaving patients permanently dependent on mechanical ventilation. Nerve transfer is a promising reinnervation strategy that has the potential to restore connectivity in paralyzed distal muscles. The spinal accessory nerve (SAN) remains functional in most cases after high cervical SCI and can serve as a donor to reinnervate the phrenic nerve (PN), thereby improving diaphragmatic function.
OBSERVATIONS
Information regarding thorough physical, electrodiagnostic, and pulmonary assessments to establish candidacy for nerve transfer, as well as the surgical procedure, was summarized with an illustrative case. The patient demonstrated improvement in pulmonary function testing but did not achieve independent respiration. A systematic literature review identified 3 studies with 9 additional patients who had undergone SAN-to-PN transfer. The nerve transfer meaningfully restored diaphragmatic function, improving pulmonary function tests and reducing ventilator dependency.
LESSONS
Respiratory dependency significantly impacts the quality of life of patients with a high cervical SCI. The use of the lower SAN motor branch for PN transfer is safe and does not result in a meaningful downgrade in trapezius function. Outcomes following this procedure are promising but heterogeneous, indicating a need for significant innovation and improvement for future therapies.
Keywords: nerve transfer, phrenic nerve, spinal cord injury, spinal accessory nerve, respiration, diaphragm reinnervation
ABBREVIATIONS: AIS = ASIA Impairment Scale, ASIA = American Spinal Injury Association, CAMP = compound motor action potential, CT = computed tomography, EMG = electromyography, LMN = lower motor neuron, MRC = Medical Research Council, PN = phrenic nerve, PFT = pulmonary function testing, SAN = spinal accessory nerve, SCI = spinal cord injury, SCM = sternocleidomastoid.
Respiratory support via mechanical ventilation is required in the initial course of injury in approximately 40% of patients with high tetraplegia following high cervical spinal cord injury (SCI).1 Approximately 5% of these patients remain ventilator dependent permanently.2 Therefore, these patients suffer from high morbidity and mortality, with limited mobility, speech restriction, and an increased predisposition to pulmonary infections, resulting in only 10 years of life expectancy.3, 4 These data suggest an urgent need for interventions that could return independent respiratory function to these patients.
After high cervical SCI, diaphragmatic control is lost secondary to absent phrenic nerve (PN) function. Depending on the level of SCI, 2 distinct PN deficits can occur.5, 6 SCI above C3 results in PN upper motor neuron injury. In this case, the lower motor neurons (LMNs) remain intact, and the diaphragm muscles do not undergo degeneration and atrophy.7, 8 Therefore, electrical stimulation pacing can be performed to activate the diaphragm.9 Alternatively, SCI at C3, C4, or C5 can result in PN LMN injury, resulting in denervation atrophy of the phrenic-diaphragm nerve-muscle unit. This results in an inability to use electrical stimulation pacing to activate the diaphragm.9 Both types of injuries result in either partial or complete ventilator dependency.
To date, 2 treatment options are available to restore respiration in patients with high cervical SCI.10 First, a lifelong portable ventilator can be used with adequate pulmonary care. Second, a PN pacer device, which artificially activates the diaphragm at regular intervals to reduce ventilator dependence, can be used for SCI above C3. Both therapies provide artificial means of respiration associated with respiratory dependency.10 Recently, nerve transfer surgery has shown promise in the reanimation of muscles paralyzed by SCI.5, 11 Nerve transfer surgery bypasses the SCI by the redirection of intact axons above the SCI to reinnervate the paralyzed distal nerve muscles below the SCI.7 After high cervical SCI, all peripheral motor nerves are paralyzed, but the spinal accessory nerve (SAN), innervated by the spinal and cranial portions, remains intact. The SAN has shown promise in restoring upper limb function after high cervical SCI,11 and it may serve as a potential donor nerve to reinnervate the PN and reanimate a paralyzed diaphragm. This nerve transfer was first demonstrated in a patient with a high cervical SCI by Yang et al.12
The aim of this report was to describe the rationale, indications, and operative technique of SAN-to-PN transfer surgery through an illustrative case and synthesize evidence regarding this surgery through a systematic review of the literature.
Illustrative Case
Baseline Clinical Evaluation
A 36-year-old male patient incurred a C1 American Spinal Injury Association (ASIA) Impairment Scale (AIS) grade A complete SCI following multiple gunshots to the neck in the summer of 2016. On neurological examination, the patient had a sensory level at the occipital region, with no distal motor activity below C1. Computed tomography (CT) scanning performed at an outside hospital demonstrated minimally displaced bilateral C3 pedicle fractures and a left C3 inferior articular fracture with no malalignment. There was evidence of retained bullet fragments within the posterior fossa, in the spinal canal at C2–3, and in the soft tissues of the neck. CT angiography showed evidence of segmental occlusion of the left vertebral artery. The patient was maintained in a hard cervical collar, and after a few weeks of acute care, he was unable to be weaned off the ventilator, requiring a tracheotomy. In the fall, bilateral phrenic pacing systems were implanted to begin ventilator weaning. However, he remained ventilator dependent until early 2020 when he was referred to our service for potential diaphragmatic reinnervation.
Surgical and Biological Rationale
The SAN is the 11th cranial nerve and originates from the C1 to C4 nerve roots, with a spinal component and brainstem cranial component.13 After exiting the jugular foramen, the SAN innervates the sternocleidomastoid (SCM) and trapezius muscles. The SAN enters the SCM muscle approximately 5 cm inferior to the mastoid process and gives off motor branches.13 Following this, the SAN travels to the posterior triangle of the neck and gives off motor branches to the upper trapezius muscle, followed by terminal divisions innervating the middle and lower trapezius.13 Multiple studies have demonstrated the crucial importance of upper trapezius branches in preserving motor function of the trapezius muscle.9, 14 The division of the distal SAN below the upper trapezius has been shown to be a safe and feasible approach with minimal downgrading in the motor function of the trapezius muscle.15, 16
The PN originates bilaterally from the C3 to C5 nerve roots and exits the spinal canal at the neck root. It travels through the posterior triangle of the neck, superficially to the anterior scalene, before entering the superior mediastinum and running along the pericardium to innervate the diaphragm.17, 18
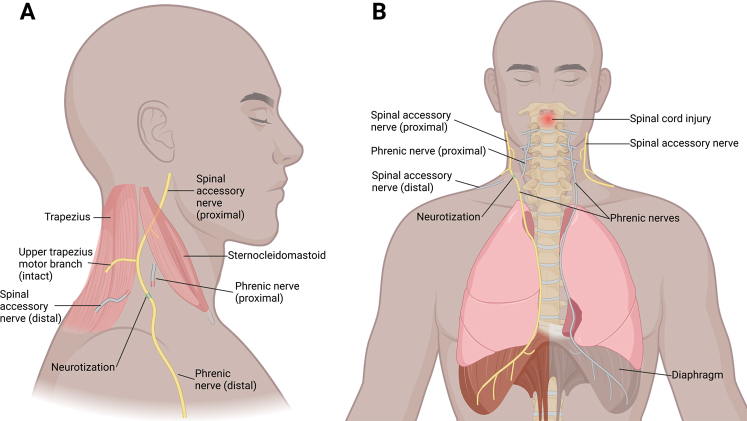
The transfer of the SAN to the PN is feasible both anatomically and biologically.18, 19 The distal segment of the SAN contains approximately 1102 ± 216 myelinated axons, and the proximal segment of the PN contains approximately 911 ± 321 myelinated axons, making a donor/recipient ratio of 1.2.18 An ideal donor/recipient ratio has been established to be approximately 0.7, as up to 30% of axonal fibers are lost due to axonal disposition after transposition.16, 20 However, only 30% of intact axons are required to reinnervate the recipient muscle, as intact axons have the capacity to expand in a 1:5 ratio via collateral axonal sprouting.21 In addition, both the SAN and PN are pure motor nerves, which may limit the loss of axons in sensory targets during regeneration and facilitate motor learning of new functions. In an anatomical study, the full length of the PN was measured to be 24.6 ± 1.7 cm on the right and 30.6 ± 1.8 cm on the left.22 Therefore, with the given regeneration speed of 1 mm/day,23 it would require approximately 12 months to expect any signs of reinnervation. The SAN-to-PN transfer surgery is illustrated in Fig. 1.
FIG. 1.
A: Lateral view showing the distal SAN motor branch transferred to the PN. B: Anterior view of the right SAN-to-PN transfer. The contralateral gray nerve and muscle indicate a lack of innervation and atrophy. Created with BioRender.com.
Preoperative Evaluation Considerations
Clinical evaluation focuses on ruling out the potential for spontaneous recovery, preserving residual function, and ensuring the integrity of donor and recipient nerves. Detailed assessment of the SCI and evaluation of preserved neurological function are critical. In patients with high cervical SCI, there is a complete lack of upper-limb motor function below the level of SCI (i.e., C1–4 for high cervical SCI). Standardized preoperative assessment of SCI is performed per the International Standards for Neurologic Classification of Spinal Cord Injury evaluation.24 Neurological level of injury and right and left motor and sensory levels are documented. Motor strength of the trapezius muscle is evaluated using manual muscle testing for Medical Research Council (MRC) grades, and only the SAN with trapezius muscle strength of at least MRC grade 4 or greater is considered for nerve transfer surgery.
Timing of Nerve Transfer Surgery
The decision to perform SAN-to-PN transfer surgery depends on the time interval after SCI and the integrity of the recipient nerve (i.e., PN) LMNs.25 To evaluate LMNs, nerve conduction studies and needle electromyography (EMG) are performed. There can be 2 distinct time windows to perform nerve transfer surgery.
First, if the SCI involves PN LMNs (C3–5 injury), then nerve transfer should be performed subacutely (within 12 months of SCI), as the nerve-muscle unit will undergo denervation and atrophy within 12–18 months.7, 8, 26 The signs of LMN injury will be apparent on electrodiagnosis, including abnormal compound motor action potential (CMAP), presence of spontaneous activity (e.g., fibrillations/positive sharp waves/fasciculations), and abnormal motor unit potential morphology indicating an LMN injury pattern.25
Second, if the SCI does not involve PN LMNs (C1–3 injury), the nerve transfer can potentially be performed in a delayed fashion (after 12 months of SCI), as intact LMNs will maintain connectivity with the diaphragm, preventing degeneration and atrophy.25 The integrity of LMNs will exhibit the following signs: normal CMAP, lack of spontaneous activity (e.g., fibrillations/positive sharp waves/fasciculations), and absent volitional motor unit potentials indicating a predominant upper motor neuron injury pattern with intact LMN connectivity.25
Pulmonary Outcome Measures
Pulmonary function testing (PFT) evaluates the residual diaphragmatic function and improvement postoperatively. In the case of a partially functioning diaphragm, nerve transfer surgery should not be performed due to the risk of losing residual respiratory function. Patients with pulmonary function tests demonstrating the complete absence of diaphragmatic activity are eligible for nerve transfer surgery. Reinnervation of the diaphragm before the evidence of pulmonary function can also be evaluated using the EMG of the diaphragm (not performed in this case).
Surgical Evaluation
On evaluation, our patient had no motor/sensory recovery below C1. Electrodiagnostic assessment showed evidence of upper motor neuron injury and relative sparing of nerve conduction studies below the level of the SCI (Table 1). The patient was referred for PFT; on attempted spirometry, no effort was observed, and no pulmonary function was observed (Table 2). On physical examination, good trapezius muscle strength (MRC grade > 4/5) was observed bilaterally. Therefore, elective right SAN-to-PN transfer was planned. At surgery, the patient was 48 months post-SCI.
TABLE 1.
Preoperative electrodiagnosis
| Right | Left | |
|---|---|---|
| Nerve conduction study: CMAP | ||
| Median nerve | 8.2 mV, 44 m/sec | 6.7 mV, 48 m/sec |
| Ulnar nerve | 5.6 mV, 50 m/sec | 4.9 mV, 56 m/sec |
| Radial nerve | Not tested | 0.9 mV |
| Nerve conduction study: SNAP | ||
| Median nerve | 14 µV, 54 m/sec | 19 µV, 54 m/sec |
| Ulnar nerve | 5 µV, 60 m/sec | 16 µV, 57 m/sec |
| Radial nerve | Not tested | 10 µV, 58 m/sec |
| EMG of myotomes | ||
| C5–biceps | Sp (1+/1+/none), MUP (none), rec (none) | Sp (normal), MUP (none), rec (none) |
| C6–triceps | Sp (1+/1+/none), MUP (none), rec (none) | Sp (normal), MUP (none), rec (none) |
| C7–extensor digitorum | Sp (normal), MUP (none), rec (none) | Sp (normal), MUP (none), rec (none) |
| C8–flexor digitorum | Not tested | Sp (none/1+/none), MUP (none), rec (none) |
| T1–1st dorsal interossei | Sp (normal), MUP (none), rec (none) | Sp (1+/2+/none), MUP (none), rec (none) |
MUP = motor unit potential (duration/amplitude/polyphasia); rec = recruitment (normal/slightly reduced/moderate–severely reduced/none); SNAP = sensory nerve action potential; Sp = spontaneous activity (fibrillations/positive sharp waves/fasciculations).
TABLE 2.
Pre- and postoperative pulmonary function tests
| Parameter | Preop | 6 mos | 14 mos | 20 mos |
|---|---|---|---|---|
| FVC | None | 0.30 | 0.29 | 0.27 |
| FEV1 | None | 0.25 | 0.26 | 0.23 |
| FEV1/FVC | None | 82 | 89 | 85 |
| Respiratory effort* | None | Minimal | Good w/ SpO2 97% | Good w/ SpO2 99% |
FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; SpO2 = oxygen saturation.
* All tests were performed after turning off the ventilator with the cuff down and occluding tracheotomy breathing from the mouth.
Operative Procedure
The nerve transfer procedure is performed under general anesthesia. The patient is placed supine with their neck turned toward the contralateral side, and a block is placed on the contralateral interscapular region to elevate the operative neck, providing easier access and exposure to the SAN. To facilitate intraoperative electrical stimulation and identification of donor SAN branches, the use of a muscle relaxant is avoided.
A horizontal skin incision along the natural skin crease, starting from mid-clavicle to the acromioclavicular joint, is used to expose the upper trapezius, followed by division and retraction posteriorly (Fig. 2A).5 The posterior border of the SCM is dissected, and Erb’s point is identified. Following dissection along the posterior border of the SCM at Erb’s point, the SAN is usually identified lying on the anterior surface of the trapezius muscle. Identification is facilitated with electrical stimulation, and a strong trapezius motor response is verified. Neurolysis of the distal SAN is performed, and the nerve is isolated in a vessel loop. Following exposure of the SAN, the supraclavicular fat pad is dissected, and along the anterior scalene muscle, the PN is identified and protected in a vessel loop (Fig. 2B). Extensive neurolysis is performed along the PN, both proximally and distally, until an adequate length is available for nerve transfer (Fig. 2C). To facilitate tension-free nerve transfer, the SAN is sharply divided distally, and the PN is divided proximally. Under microscopic visualization, both donor and recipient nerves are placed on a blue background and approximated together (Fig. 2D). Nerve transfer is performed using interrupted 9-0 nylon sutures to coapt the epineurium of the donor and recipient nerves (Fig. 2E). The nerve transfer is reinforced by fibrin glue (Fig. 2F). Following nerve transfer, meticulous hemostasis is obtained, and the wound is closed with 3-0 intradermal and 4-0 subcuticular resorbable sutures.
FIG. 2.
A: Photograph of a planned supraclavicular incision to expose the anterior triangle of the neck and identify the SAN and PN. B: Following exposure, the SAN and PN are identified and isolated in vessel loops. C: Both the SAN and PN underwent extensive neurolysis until an adequate length was available for tension-free nerve transfer. D: Microscopic view of both the donor SAN and recipient PN, sharply transected and placed on a blue background for planned neurotization. E: Neurotization and nerve transfer were performed by coapting donor and recipient segments with 9-0 epineurial sutures (black arrow). F: Nerve transfer reinforced by fibrin glue (black arrow). Cr = cranial; Rt = right side.
Unlike upper-extremity nerve transfers,5 brief immobilization is not required, and patients are discharged on the same day. Patients are educated to expect a long reinnervation period until they observe any effects of nerve transfer surgery.
Outcome
The patient had an uneventful recovery with no complications. PFT performed at 6 months after nerve transfer demonstrated minimal response in functional curves with good effort on spirometry with the cuff off the tracheotomy tube. PFT at 14 months of follow-up demonstrated enhanced respiratory effort with improvement in pulmonary functional curves and brief maintenance of oxygenation. After 20 months of follow-up, the patient had regained some pulmonary function on PFTs but remained unable to breathe independently (Table 2). Trapezius strength remained 4/5 bilaterally.
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Systematic Review of the Literature
To synthesize contemporary evidence regarding SAN-to-PN transfer surgery, a systematic search was conducted in accordance with the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses to identify all relevant studies up to January 2024. The search strategy was created by a medical librarian to capture all relevant studies that described the technique and surgical outcomes of SAN-to-PN transfer in tetraplegic patients with SCI. The relevant keywords included “phrenic nerve,” “reinnervation,” “diaphragm nerve,” “nervus phrenicus,” “reinnervate,” “neurotization,” and “transfer.” The full search strategy for databases including MEDLINE, Embase, Scopus, Cochrane, Web of Science, and ClinicalTrials.gov is provided in the Supplemental Methods. We did not submit our protocol to PROSPERO.
One reviewer (S.J.) screened the relevant titles and abstracts extracted from database searching, followed by a full-text review of screened citations. Eligibility criteria were defined based on the population, intervention, comparison, and outcome method. We included studies that investigated PN reinnervation using SAN transfer in patients with high cervical (C1–4) AIS grade A–C SCI. Studies that were available in the English language, conducted on humans, and reported patient-level data were eligible. Given the scarcity of the literature, we modified the criteria to include any of the following study designs: clinical trials, cohort studies, case series, and technical case reports. Narrative reviews and anatomical studies were excluded. Disagreements and uncertainties in the selection process were resolved via discussions with the corresponding author.
A data extraction form was developed, and the first author (S.J.) extracted individual patient-level data for each study. Extracted data included age, sex, injury etiology, level of SCI, AIS grade, and intervention-related variables including nerve transfer surgery side and type, prior electrical pacing, outcome measures, and follow-up duration. Given the heterogeneous nature of the reports and the small amount of patient data, only a qualitative synthesis was performed. Given that all selected studies were case series and reports, the risk of bias assessment and Grading of Recommendations Assessment, Development and Evaluation system could not be used to assess the quality of evidence.
Discussion
Observations
The primary objective of nerve transfer surgery in patients with high cervical SCI who are ventilator dependent is the restoration of independent respiration. The SAN transfer provides an opportunity to reinnervate the PN and reanimate the paralyzed diaphragm. The diaphragm is the major contributor to normal ventilation, and restoring its movement is critical for normal ventilation in patients with high cervical SCI .27 Importantly, the SCM and trapezius muscles are part of the accessory respiratory muscles with minor roles in ventilation.27 SAN-to-PN transfer, in combination with cortical motor learning, could enable patients to adapt the use of the SAN for a new function.
Our systematic search yielded 246 unique studies. After screening, 22 full-text articles were assessed. Among these, 3 anatomical studies were removed, 5 studies were excluded as they did not report on not SAN donors, and 11 were removed as they focused on brachial plexus reinnervation (Supplemental Figure 1). Ultimately, 3 studies totaling 9 patients included patient-level data for SAN-to-PN transfer in high cervical SCI were included.9, 12, 28 Patients were, on average, 36 (range 10–66) years of age, and 7 were male. In total, 16 SAN-to-PN transfer surgeries were performed an average of 31 (range 5–90) months after SCI (Table 3). Six patients underwent bilateral SAN-to-PN transfer, while the other 3 underwent unilateral SAN-to-PN transfer. There were variable outcome measures in each study, including diaphragm EMG activity, diaphragm mobilization on fluoroscopy, and pulmonary function tests. Seven patients had follow-up outcome measures available. Follow-up ranged from 6 to 40 months. The mean time from surgery to the first diaphragmatic response was 6 (range 3–10) months. Two patients achieved 24 hrs/day of independent respiration without a ventilator or electrical stimulation pacing after at least 1 year of surgery. The other patients achieved a mean ventilator-free time of 9 (range 1–24) hrs/day with electrical pacing.
TABLE 3.
Summary of ventilator-dependent high cervical SCI patients with SAN-to-PN transfer
| Authors & Year | Case No. | Age (yrs) | Sex | Injury Etiology | SCI Level | AIS Grade | Prior Pacing Attempt | Nerve Transfer | Injury to Surgery Interval (mos) | Outcome Measure | Surgery to First Response Interval (mos) | FU (mos) | Ventilator-Free Time (hrs) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pacing | Non-Pacing | |||||||||||||
| Yang et al., 201112 | 9 | 44 | M | Sporting accident | C2 | A | — | SAN-PN unilat | 11 | Diaphragm motion approx 1 rib on fluoroscopy, tidal vol increased from 250 to 670 ml, improved oxygenation | 6 | — | 12 | 24 |
| Kaufman et al., 20159 | 1 | 39 | M | — | C4 | A | No | SAN-PN bilat | 90 | Transtelephonic diaphragm EMG activity | 6 | 24 | 24 | — |
| 2 | 63 | M | — | C2 | B | No | SAN-PN bilat | 22 | 9 | 40 | — | 24 | ||
| 3 | 66 | M | — | C4 | A | Yes | SAN-PN bilat | 6 | 6 | 13 | 4 | — | ||
| 4 | 28 | M | — | C2 | A | No | SAN-PN bilat | 20 | 3 | 9 | — | 12 | ||
| 5 | 46 | M | — | C2 | A | Yes | SAN-PN bilat | 16 | 10 | 26 | 3 | — | ||
| 6 | 10 | F | — | C1 | A | Yes | SAN-PN bilat | 58 | 4 | 6 | 1 | — | ||
| 7 | 19 | F | — | C2 | A | Yes | SAN-PN unilat | 54 | 6 | 6 | 5 | — | ||
| Heredia Gutiérrez et al., 202028 | 8 | 12 | M | Iatrogenic | C1 | A | — | SAN-PN unilat | 5 | Complete diaphragm mobilization on fluoroscopy | 6 | — | 12 | — |
| Present case | 10 | 36 | M | Gunshot | C1 | A | Yes | SAN-PN unilat | 48 | Pulmonary function test, improvement in respiratory effort & sustained oxygenation | 6 | 20 | 0 | 0 |
Approx = approximately; FU = follow-up.
Our experience in this case, along with data from the literature, supports the feasibility of this strategy in restoring diaphragmatic activity via SAN-to-PN transfer in patients with high tetraplegia. For those who do not fully recover diaphragmatic movement, the reinnervated function in the PN can be used for electrical stimulation pacing, even in patients with damage to PN LMNs.9, 10, 29 Although marginal functional outcomes have been observed, this nerve transfer strategy has shown promise, and larger studies are needed to establish the efficacy of this intervention. So far, only 2 patients have achieved completely independent respiration. One patient who gained independent respiration had C2 AIS grade B SCI and underwent bilateral SAN-to-PN transfer after 22 months of SCI, gaining independent respiration after 40 months. The second patient had C2 AIS grade A SCI and underwent unilateral SAN-to-PN transfer after 11 months of SCI.9 Our patient had similar injury characteristics, but these 2 patients underwent nerve transfer surgery relatively early, at 11 and 22 months after SCI, while nerve transfer surgery in our patient was performed at 48 months. The suboptimal outcome in our patient could be attributed to disuse atrophy of the diaphragm secondary to long-term ventilator dependency.30 These findings warrant further development of this novel technique to improve the functional outcomes in patients with high cervical SCI suffering from debilitating ventilator dependency.
Lessons
This report demonstrates the feasibility of SAN-to-PN transfer to restore respiratory function in patients with high cervical SCI. However, the outcomes remain marginal, indicating a need for optimization of surgical technique and patient selection for PN reinnervation.
Acknowledgments
We would like to acknowledge Ms. Linda Koester and colleagues at Becker Medical library for research support and assistance on this project.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Ray, Javeed, Hardi, Faraji. Acquisition of data: Javeed, Benedict, Zhang, Hardi, Faraji. Analysis and interpretation of data: Javeed, Benedict, Greenberg, Faraji. Drafting the article: Javeed, Benedict, Zhang, Kaleem, Hardi, Faraji. Critically revising the article: Javeed, Benedict, Dibble, Greenberg, Kaleem, Faraji. Reviewed submitted version of manuscript: Ray, Javeed, Benedict, Zhang, Greenberg, Faraji. Approved the final version of the manuscript on behalf of all authors: Ray. Statistical analysis: Javeed. Administrative/technical/material support: Dibble, Zhang, Faraji. Study supervision: Ray, Faraji.
Supplemental Information
Online-Only Content
Supplemental Methods and Figure 1. https://thejns.org/doi/10.3171/CASE24236.
Correspondence
Wilson Z. Ray: Washington University, St. Louis, MO. rayz@wustl.edu.
References
- 1.Como JJ, Sutton ER, McCunn M, et al. Characterizing the need for mechanical ventilation following cervical spinal cord injury with neurologic deficit. J Trauma. 2005;59(4):912-916. [DOI] [PubMed] [Google Scholar]
- 2.DeVivo MJ, Chen Y. Trends in new injuries, prevalent cases, and aging with spinal cord injury. Arch Phys Med Rehabil. 2011;92(3):332-338. [DOI] [PubMed] [Google Scholar]
- 3.Shavelle RM, DeVivo MJ, Strauss DJ, Paculdo DR, Lammertse DP, Day SM. Long-term survival of persons ventilator dependent after spinal cord injury. J Spinal Cord Med. 2006;29(5):511-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Javeed S, Greenberg JK, Zhang JK, et al. Association of upper-limb neurological recovery with functional outcomes in high cervical spinal cord injury. J Neurosurg Spine. 2023;39(3):355-362. [DOI] [PubMed] [Google Scholar]
- 5.Javeed S, Dibble CF, Greenberg JK, et al. Upper limb nerve transfer surgery in patients with tetraplegia. JAMA Netw Open. 2022;5(11):e2243890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javeed S, Greenberg JK, Zhang JK, et al. Derivation and validation of a clinical prediction rule for upper limb functional outcomes after traumatic cervical spinal cord injury. JAMA Netw Open. 2022;5(12):e2247949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Senjaya F, Midha R. Nerve transfer strategies for spinal cord injury. World Neurosurg. 2013;80(6):e319-e326. [DOI] [PubMed] [Google Scholar]
- 8.Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged axotomy. J Neurosci. 1995;15(5Pt 2):3876-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufman MR, Elkwood AI, Aboharb F, et al. Diaphragmatic reinnervation in ventilator-dependent patients with cervical spinal cord injury and concomitant phrenic nerve lesions using simultaneous nerve transfers and implantable neurostimulators. J Reconstr Microsurg. 2015;31(5):391-395. [DOI] [PubMed] [Google Scholar]
- 10.Wijkstra PJ, van der Aa H, Hofker HS, et al. Diaphragm pacing in patients with spinal cord injury: a European experience. Respiration. 2022;101(1):18-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dibble CF, Khalifeh JM, VanVoorhis A, Rich JT, Ray WZ. Novel nerve transfers for motor and sensory restoration in high cervical spinal cord injury. World Neurosurg. 2019;128:611-615.e1. [DOI] [PubMed] [Google Scholar]
- 12.Yang ML, Li JJ, Zhang SC, et al. Functional restoration of the paralyzed diaphragm in high cervical quadriplegia via phrenic nerve neurotization utilizing the functional spinal accessory nerve. J Neurosurg Spine. 2011;15(2):190-194. [DOI] [PubMed] [Google Scholar]
- 13.Johal J, Iwanaga J, Tubbs K, Loukas M, Oskouian RJ, Tubbs RS. The accessory nerve: a comprehensive review of its anatomy, development, variations, landmarks and clinical considerations. Anat Rec (Hoboken). 2019;302(4):620-629. [DOI] [PubMed] [Google Scholar]
- 14.Kierner AC, Zelenka I, Heller S, Burian M. Surgical anatomy of the spinal accessory nerve and the trapezius branches of the cervical plexus. Arch Surg. 2000;135(12):1428-1431. [DOI] [PubMed] [Google Scholar]
- 15.Vathana T, Larsen M, de Ruiter GC, Bishop AT, Spinner RJ, Shin AY. An anatomic study of the spinal accessory nerve: extended harvest permits direct nerve transfer to distal plexus targets. Clin Anat. 2007;20(8):899-904. [DOI] [PubMed] [Google Scholar]
- 16.Narakas AO, Hentz VR. Neurotization in brachial plexus injuries. Indication and results. Clin Orthop Relat Res. 1988;(237):43-56. [PubMed] [Google Scholar]
- 17.Wang J, Li J, Liu G, Deslauriers J. Nerves of the mediastinum. Thorac Surg Clin. 2011;21(2):239-249. [DOI] [PubMed] [Google Scholar]
- 18.Wang C, Zhang Y, Nicholas T, et al. Neurotization of the phrenic nerve with accessory nerve for high cervical spinal cord injury with respiratory distress: an anatomic study. Turk Neurosurg. 2014;24(4):478-483. [DOI] [PubMed] [Google Scholar]
- 19.Tubbs RS, Pearson B, Loukas M, Shokouhi G, Shoja MM, Oakes WJ. Phrenic nerve neurotization utilizing the spinal accessory nerve: technical note with potential application in patients with high cervical quadriplegia. Childs Nerv Syst. 2008;24(11):1341-1344. [DOI] [PubMed] [Google Scholar]
- 20.Schreiber JJ, Byun DJ, Khair MM, Rosenblatt L, Lee SK, Wolfe SW. Optimal axon counts for brachial plexus nerve transfers to restore elbow flexion. Plast Reconstr Surg. 2015;135(1):135e-141e. [DOI] [PubMed] [Google Scholar]
- 21.Gordon T, Yang JF, Ayer K, Stein RB, Tyreman N. Recovery potential of muscle after partial denervation: a comparison between rats and humans. Brain Res Bull. 1993;30(3-4):477-482. [DOI] [PubMed] [Google Scholar]
- 22.Jiang S, Xu WD, Shen YD, Xu JG, Gu YD. An anatomical study of the full-length phrenic nerve and its blood supply: clinical implications for endoscopic dissection. Anat Sci Int. 2011;86(4):225-231. [DOI] [PubMed] [Google Scholar]
- 23.Gordon T, Sulaiman O, Boyd JG. Experimental strategies to promote functional recovery after peripheral nerve injuries. J Peripher Nerv Syst. 2003;8(4):236-250. [DOI] [PubMed] [Google Scholar]
- 24.Kirshblum S, Snider B, Rupp R, Read MS, International Standards Committee of ASIA and ISCoS. Updates of the International Standards for Neurologic Classification of Spinal Cord Injury: 2015 and 2019. Phys Med Rehabil Clin N Am. 2020;31(3):319-330. [DOI] [PubMed] [Google Scholar]
- 25.Dibble CF, Javeed S, Khalifeh JM, et al. Optimizing nerve transfer surgery in tetraplegia: clinical decision making based on innervation patterns in spinal cord injury. J Neurosurg Spine. 2021;36(3):498-508. [DOI] [PubMed] [Google Scholar]
- 26.Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J Neurosci. 1995;15(5Pt 2):3886-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terson de Paleville DG, McKay WB, Folz RJ, Ovechkin AV. Respiratory motor control disrupted by spinal cord injury: mechanisms, evaluation, and restoration. Transl Stroke Res. 2011;2(4):463-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heredia Gutiérrez A, Cachón Cámara GE, González Carranza V, Torres García S, Chico Ponce de León F. Phrenic nerve neurotization utilizing half of the spinal accessory nerve to the functional restoration of the paralyzed diaphragm in high spinal cord injury secondary to brain tumor resection. Childs Nerv Syst. 2020;36(6):1307-1310. [DOI] [PubMed] [Google Scholar]
- 29.Nandra KS, Harari M, Price TP, Greaney PJ, Weinstein MS. Successful reinnervation of the diaphragm after intercostal to phrenic nerve neurotization in patients with high spinal cord injury. Ann Plast Surg. 2017;79(2):180-182. [DOI] [PubMed] [Google Scholar]
- 30.DiMarco AF. Phrenic nerve stimulation in patients with spinal cord injury. Respir Physiol Neurobiol. 2009;169(2):200-209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.