Abstract
CD4 T cells recognized posttranslationally modified peptides of the protein hen egg-white lysozyme (HEL), consisting of nitration of tyrosines and modifications of tryptophans in the T cell contact residues of the peptides. T cells were directed against modifications of a chemically dominant HEL peptide as well as a minor HEL peptide, bound to the class II histocompatibility molecule I-Ak. The modified peptides were generated in vivo after immunization with native HEL molecules or were generated ex vivo by peroxynitrite treatment of HEL. Moreover, antigen-presenting cells (APC), either macrophages or dendritic cells activated in culture or in vivo, generated the modified HEL epitopes that stimulated the T cells. In transgenic mice expressing HEL, the T cells to the modified epitopes escaped negative selection and were found, albeit fewer in number than in normal mice. Infection with Listeria monocytogenes of the transgenic HEL mice generated APC containing the modifications. T cells to modified epitopes induced by activation of APC may be a component of antimicrobial immunity and autoimmune reactions.
Keywords: histocompatability molecules, nitrotyrosine, posttranslational modification
Peptides bound to either class I or class II MHC molecules suffer posttranslational modifications of potential importance in the context of inducing unique T cell reactivities. A number of posttranslational changes were identified on peptides isolated from class I MHC proteins (1, 2), including phosphorylation of tyrosine residues (3). Unique T cell reactivities were ascribed to changes in the peptides bound to class II MHC molecules (reviewed in ref. 4). These included phosphorylations (5), conversions of asparagine or aspartic acid to isoaspartic acid (6, 7), glutamine to glutamic acid (8, 9), nitration of tyrosine (10), and addition of saccharide residues to a peptide (11–15). These results are reminiscent of early ones showing T cell reactions to proteins containing small reactive chemical groups, i.e., with “haptens,” first reported by Jones and Leskowitz (16) in delayed sensitivity reactions to arsanilic acid. Their observations were extended by others (17), including by using dinitrophenyl to derivatize class II molecules on cells (18). Clearly, T cells are highly discriminatory of large or small changes in the residues to which their receptor [T cell receptor (TCR)] establishes contact. Antibodies directed to posttranslationally modified proteins have also been identified (19, 20).
Activation of macrophages and dendritic cells (DC) takes place after their interactions with IFN-γ together with cytokines like TNF or IL-1, or microbial products like LPS from Gram-negative bacteria. The activation includes the production of nitric oxide (NO) and reactive oxygen species with the formation of strong oxidizing molecules such as peroxynitrite (21–23) or other derivatives (24, 25). These molecules induce a number of amino acid changes, including nitration of tyrosines (21, 22) and modification of tryptophans (26). The present study reports that processing of the protein antigen hen egg-white lysozyme (HEL) by activated antigen-presenting cells (APC) results in the presentation of modified peptides containing nitrotyrosines (nTyr) and altered tryptophans by the class II MHC molecule I-Ak. The posttranslational changes occur in the lymphoid tissues and alter the repertoire of the specific T cells by eliciting specific CD4 T cells.
Materials and Methods
Cellular Techniques. Most of the CD4 T cells were hybridomas made by fusing T cells obtained from popliteal nodes of mice immunized with the antigens (5 nmol of HEL or 10 nmol of peptides) in complete Freund's adjuvant. Usually cells were harvested and stimulated 3 days in culture with the antigens and fused to BW5147 α-,β-thymoma line (exactly as in ref. 27). APC cells or lines included the C3.F6 B lymphoma line (28); peritoneal macrophages, obtained from normal mice or from mice 3 days after i.p. injection with 100 μg of Con A or infection with 106 Listeria monocytogenes (strain EGD); CD11c+, CD11b+, and CD11c–, CD11b+ cells isolated from spleens by magnetic bead techniques (as in ref. 29); and bone marrow dendritic cells obtained 6–7 days after culture in granulocyte/macrophage colony-stimulating factor containing medium (as in ref. 30). On occasion, the APC were fixed in 1% paraformaldehyde for 10 min. The activation of the T cell hybridomas was measured by IL-2 production after culture with the APC and antigens by standard techniques (27). Limiting dilution analysis was done on popliteal lymph nodes cells of immunized mice (27); growth-positive wells were scored after 7 days of culture in 25 units/ml recombinant IL-2 and 1 μM antigens with 5 × 105 irradiated splenocytes. The frequency was determined by using Poisson distribution, and the growth-positive wells were expanded and then tested for specificity with the various antigens.
The antigens were unmodified HEL and peptides 48–62 or 20–35 of HEL. The same were also tested, modified by peroxynitrite treatment, 100 mM antigen with 0.25–7.5 mM peroxynitrite. The peroxynitrite was prepared in the Department of Chemistry at Washington University. Several molecular species were identified by mass spectrometry analysis carried out by Michael Gross' laboratory (Department of Chemistry, Washington University), but their identity relative to the biological effect is now being examined. Despite the molecular heterogeneity, biological responses were highly consistent and reproducible. Also used were synthetic peptides containing amino acid changes including nTyr instead of tyrosine. Presented are results representative of at least three different experiments.
An anti-nTyr-specific mouse monoclonal antibody, ANY590, was produced in our laboratory and used to detect nTyr products in smears and cell suspensions of peritoneal macrophages. The antibody was used with the Zenon Mouse Ig Labeling procedure (Molecular Probes). Cell suspensions were examined by flow cytometry by using FACSCalibur, analyzing the data by using cellquest software (BD Pharmingen). Reactive oxygen species were investigated in cell smears by using 2 μM dichlorodihydrofluorescein diacetate (C-6827, from Molecular Probes).
Mice and Reagents. B10.BR mice were obtained from The Jackson Laboratory, of either sex, usually 6–12 weeks of age. B10.BR mice containing a membrane form of HEL (mHEL) under the class II Eα promoter were produced and maintained by our laboratory (27). HEL was obtained from Sigma and purified to eliminate ≈2% contaminant proteins. Synthetic peptides were produced by Fmoc techniques. Aminoguanidine, which inhibits NO production (31), was obtained from Sigma and used at concentrations of 1 mM. The superoxide scavenger manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin pentachloride (32) was obtained from Alexis Biochemical (San Diego), used at 100 μM. Recombinants IFN-γ and IL-1 were obtained from PeproTech (Rocky Hill, NJ) and used at 100 units/ml; Escherichia coli LPS was from Sigma and used at 1 or 10 μg/ml culture.
Results
Generation of T Cells to Modified Peptides. HEL is processed by APC into several families of peptides (28, 33–35). The major peptide family presented by I-Ak molecules is centered on the 52–60 core segment, usually encompassing from residues 48–62 or 63 (33, 36). It is highly represented in the I-Ak molecules after HEL processing, sometimes as high as 10% of the total peptides (37) (48–63, DGST DYGILQINS RWW). The core sequence is in bold, and its main MHC anchor residue is Asp-52, which is P1 (33, 36). The TCR contact residues are Tyr-52, Leu-56, and Asn-59 (underlined). A tryptophan at residue 62 also serves as a TCR contact to some T cells (38). There is minor peptide family, expressed at ≈1/200th the amount as those of the 52–60 family, encompassing residues 20–35 (YTGY SLGNWVCAA KFE) (34, 37). The core segment centers on residues 24–32 are in bold. The main MHC anchor is Asn-27, which is P4; and the main TCR contact residues are Leu-25, Trp-28, and also the Tyr-23, which is P1 (Fig. 1).
Fig. 1.
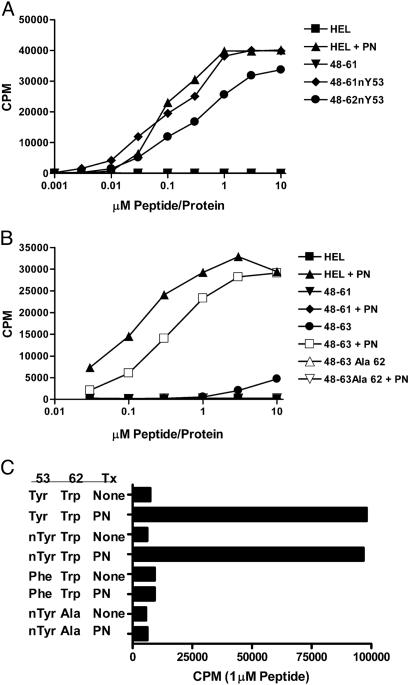
The AJ.S7 T cells react with nTyr-53 residue of the 52–60 family, and the 2–5 T cells also react with Trp-62. The C3.F6 APC line was used to present the antigens. HEL+PN refers to HEL treated with peroxynitrite. (A) The response of the AJ.S7 T cell hybridoma. In other experiments, peptide 48–61 treated with peroxynitrite stimulated at a half-maximal response of 0.01 μM (and a top response of 21,500 cpm), but the treatment of the peptide in which the Tyr-53 was changed to alanine did not stimulate. AJ.S7 did not respond when tested with an unrelated peptide but had a nTyr (a transferrin peptide 103–117: KGTDY*QLNQLEGKKG0). (B) The response of the 2–5 hybridoma that recognizes nTyr-53 and changes in the Trp-62. This hybridoma recognizes HEL, the 48–62 or 48–63 peptides treated with peroxynitrite, but not the 48–61 peptide (which lacks the Trp-62) or the 48–62 peptide, in which the Trp-62 has been changed to alanine. (C) The response of the 2–5 hybridoma to 48–62. Indicated are the peptides containing the residues at positions 52 and 62, all tested at 1 μM. Tx, treatment; PN, peroxynitrite.
Three issues were examined. First, whether specific T cells could be elicited against the two well characterized peptide families when HEL was modified by an oxidative/NO reaction, and, if so, the nature of their specificity. Second, whether activated APC presented the modified peptides in both in vivo and ex vivo conditions. Third, whether T cells to the modified peptides could be found in mice after HEL immunization or in HEL-transgenic mice, in which HEL behaves as a self-protein. [In standard binding assays with baculovirus-purified molecules (28, 33–36), all peptides examined here bound to purified I-Ak molecules with the same affinity as the unmodified peptide.]
The CD4 T cells were first obtained from B10.BR mice immunized with HEL or the 48–62 peptide treated with peroxynitrite. Seven days later the CD4 T cells were isolated and either cloned or made into T cell hybridomas. Three sets of T cells were found against modified peptides; one set, the most frequent, was reactive with the 48–62 peptide bearing a nTyr at the TCR P2 residue. A second set reacted with modifications of the Trp-62 of the same peptide sequence. A third set was found directed to the 20–35 peptide family centered on a modification of Trp-27.
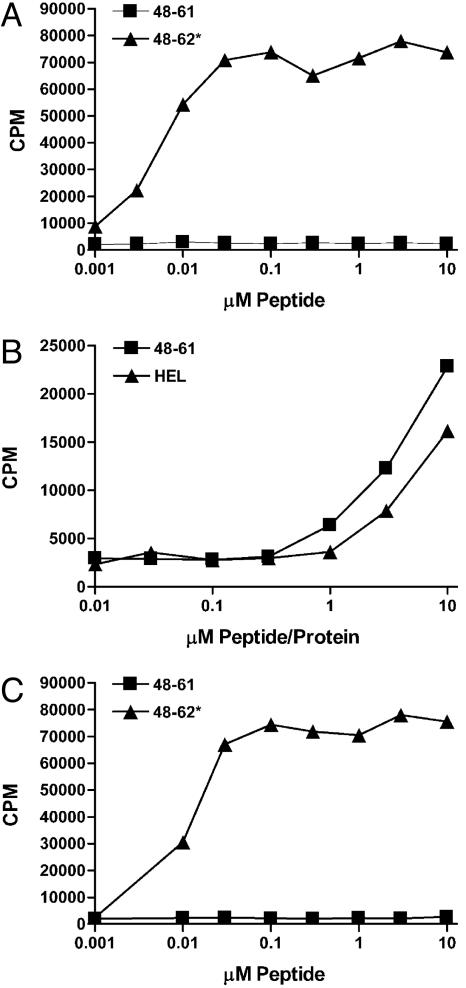
Figs. 1 and 2 show representative CD4 T cells that recognize the nTyr-53 of the 52–60 peptide family. The T cells were tested with synthetic peptides containing the nTyr or with the regular peptides but treated with peroxynitrite. AJ.S7 recognized neither HEL nor the 48–61 peptide. It recognized HEL treated by peroxynitrite (HEL+PN, Fig. 1A) and the synthetic 48–61 peptide containing a nTyr-53. An unrelated peptide, from the transferrin receptor, containing nTyr at P1 was not recognized (Fig. 1 legend).
Fig. 2.
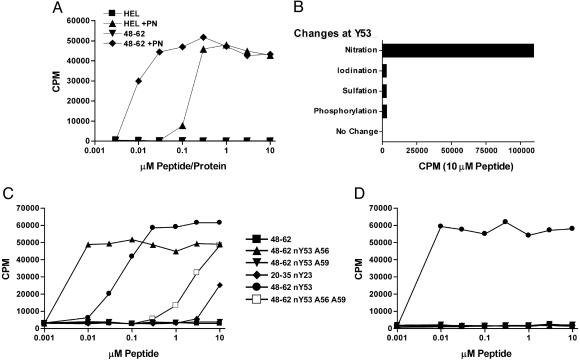
Analysis of two other T cells reactive with nTyr-53 of the 52–60 peptide family. The APC was the C3.F6 line. (A) Clone H recognizes peroxynitrite-treated 48–62. Clone H recognizes equally 48–61 or 48–62; i.e., the Trp-62 is irrelevant. (B) Recognition of 48–61 in which the tyrosine has been modified, as indicated. The recognition of nTyr is specific. (C and D) The response of NO75 and clone H, respectively, to a number of peptides, explained in the text.
A T cell similar to AJ.S7 is “clone H,” shown in Fig. 2, tested as a T cell hybridoma in all experiments shown here. Clone H is more sensitive that AJ.S7 and was used most frequently to examine the display of the modified peptide by activated APC. (In Figs. 1, 2, 3, the APC was the C3.F6 line, a B cell-presenting lymphoma cell that does not release NO·.)
Fig. 3.
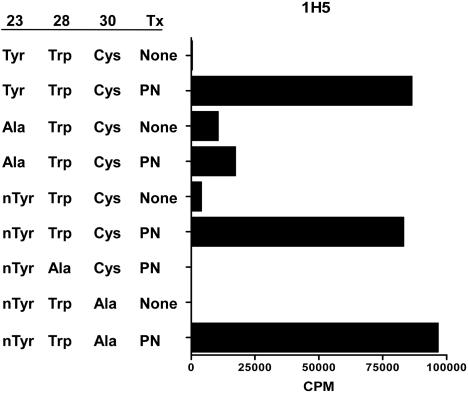
Hybridoma 1H5 responds to Trp-28 of the 20–35 peptide. A representative experiment of three in which 1H5 was tested against 20–35 modified peptides (YRGYSLGNWVCAA; see Results). Underlined are the amino acids subject to modification. Peptides were tested at 1 μM. Tx, treatment; PN, peroxynitrite. The APC was C3.F6. In a different experiment, the T cells responded to a peroxynitrite-treated peptide in which the Tyr-23 was changed to phenylalanine (46,500 cpm vs. 51,250 in controls).
Most T cells recognized the modified residue plus the other unmodified TCR contact residues, Leu-56 and Asn-59. Note the response of clone H in Fig. 2C; the peptide 48–62 nTyr-53 having alanine substitutions, instead of leucine and asparagine at residues 56 and 59, respectively, did not stimulate. The position of the modified residue was critical. Peptides where the nTyr was placed in a different position as P5 or P9 did not stimulate. That clone H required the contact with the other two TCR residues is also noted by its lack of reactivity against 20–35 nTyr-23 peptide. The recognition of the nTyr-53 is specific; it did not recognize the iodinated, sulfated or phosphorylated Tyr-53 (Fig. 2B).
Fig. 1 B and C shows the second set of T cells reactive to the 52–60 family, this one recognizing both nTyr-53 and the modifications of the Trp-62. The 2–5 hybridoma recognized only the peroxynitrite-treated HEL, 48–62, 48–63 peptide, and 48–63 Ala-63, but not when the Trp-62 was substituted by alanine (Fig. 2 shows only the results with 48–63). Fig. 1C shows that the 48–62 peptide with nTyr-53 did not stimulate 2–5 unless changes in the tryptophan were induced by treatment with peroxynitrite. Moreover, a peptide having phenylalanine at 53 treated with peroxynitrite did not stimulate.
We searched extensively for T cells that would react with nTyr independent of the carrier peptide, but with limited success, i.e., for every isolation, T cells were tested against peptides different from the ones used for immunization. In one special trial, T cells induced by peroxynitrite-treated 48–62 were cultured with the same peptide and then developed into hybridomas that were screened on peptides lacking the two TCR contacts, Leu-56 and Asn-59. NO75 is the only T cell of >300 tested that recognized a nTyr in a different carrier peptide, albeit to a small degree; see the small response to 20–35 nTyr-23 in Fig. 2C. However, NO75 did not recognize nTyr in the context of other carrier peptides. (These results have been repeated several times with hybridomas that were subcloned.) Another interesting feature of this T cell is that it recognized the 48–62 peptide nTyr-53 peptide with Ala substitutions of both TCR contact residues; moreover, the recognition by NO75 was actually markedly improved with a peptide having a single Ala-56 substitution. In brief, T cells that recognize selectively a single peptide modification independent of the carrier are very rare.
Fig. 3 shows the results of a representative T cell, 1H5, that reacted with the modified 20–35 peptide family. Modifications could occur at residues Tyr-23, Trp-28, and Cys-30. By examining a number of modified peptides, the tryptophan was identified as the target of the modification. 1H5 recognized the modified Trp-28 but also recognized a nonmodified phenyl ring at P1.
Presentation by Activated APC. Peritoneal macrophages were obtained after in vivo injection of the mice with Con A; this procedure results in a set of macrophages with high levels of class II MHC molecules brought about by the production of IFN-γ in vivo. The macrophages were reactive by immunofluorescence with a specific nTyr monoclonal antibody, although there was no detectable nitrate released into the medium testing by the Griess reaction (Fig. 4 E and F). Macrophages were also strongly positive testing with dichlorodihydrofluorescein, indicating reactive oxygen derivatives.
Fig. 4.
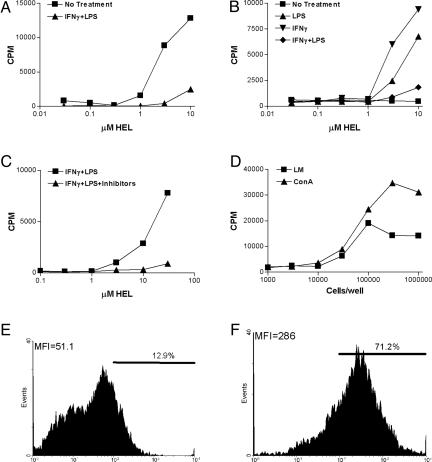
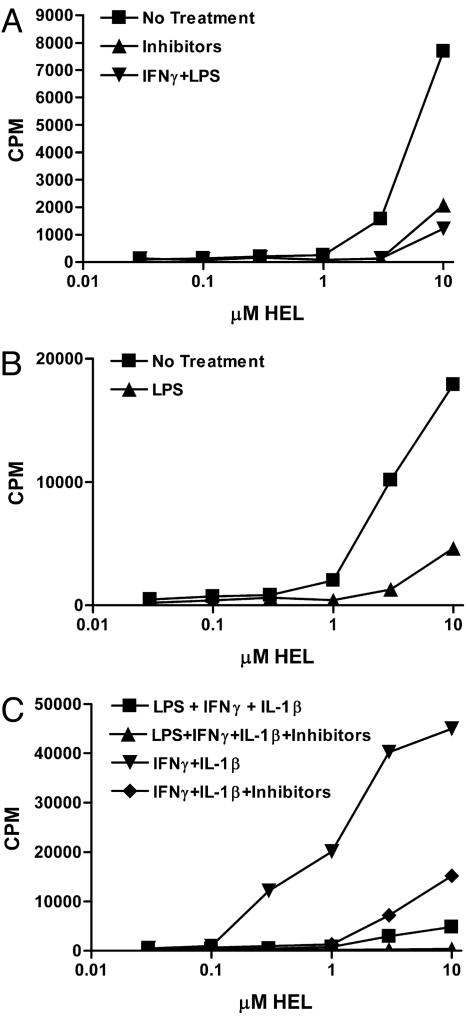
APC present the modified peptides. For each of these experiments, the T cell hybridoma used was clone H cultured for 24 h with the indicated amounts of HEL. (A) Peritoneal macrophages harvested 3 days after Con A injection of mice. (B) The same cells after 3 days of culture treated as explained in the key. (C) Peritoneal macrophages from normal mice cultured, as indicated. (D) Exudates from mHEL transgenic mice after injection of Con A or infection with L. monocytogenes (LM), both i.p. (E and F) Flow cytometry plots of peritoneal exudate cells obtained 3 days after Con A. (E) An irrelevant monoclonal antibody, clone A4-8 reactive with listeriolysin O. (F) Anti-nTyr clone ANY590.
The activated macrophages cultured with HEL immediately after their isolation stimulated nTyr specific T cells, indicating that the modified peptide was generated. Results with clone H are shown in Fig. 4A, which also shows that reactivation of the macrophages by adding IFN-γ and LPS inhibited the generation of the modified epitope. Such treatment also decreased the amount of class II MHC molecules biosynthetically produced by the macrophages (not shown). Fig. 4B shows that macrophages did not stimulate the T cells if rested for 3 days and then cultured with HEL. The macrophages that had been rested for 3 days had no significant loss of I-Ak molecules and presented the 48–62 nTyr-53 peptide to clone H (not shown). However, addition to the 3-day-cultured macrophages of either IFN-γ or LPS for 24 h together with HEL resulted again in the stimulation of the T cells (Fig. 4B). Addition of both again resulted in a negative effect. The results indicated a fine balance between the requirements for activation that generated the modified epitopes and the extent or degree of activation.
Fig. 4C shows that untreated resident macrophages from mice not given Con A required the addition of IFN-γ and LPS to generate the modified epitope. The generation of modified HEL epitope for the stimulation of the T cells was inhibited by the addition of aminoguanidine and manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin pentachloride, both of which inhibited NO and an oxidative burst.
Peritoneal exudate cells from the mHEL transgenic mice stimulated clone H without the need, as expected, of adding HEL to the culture. Cells taken 3 days after Con A injection or infection with L. monocytogenes activated clone H; see, for example, Fig. 4D.
APC were isolated from spleen and separated into two sets: those DC that are CD11c+ and the spleen macrophages that are CD11b+, CD11c–. Each was tested in culture with HEL and clone H. The Cd11b+ presented the modified peptide to clone H, albeit to a small extent and without any need for stimulation (Fig. 5A). The results with the Cd11c+ were variable, usually with low levels of stimulation. Whether the cells were stimulated during the manipulations of isolation and culture is a possibility that has not been ruled out. DC generated in culture by granulocyte/macrophage colony-stimulated factor presented the modified peptide to clone H (Fig. 5 B and C). We found a basal stimulation that was increased by addition of IFN-γ and IL-1, but not if LPS was added with the two stimuli. The response was markedly ablated by using the inhibitors.
Fig. 5.
Spleen APC and cultured DC present the modified peptides. Clone H was tested after 24 h of incubation. (A) The response of CD11b+ cells harvested from spleens of normal mice. (B and C) The responses of DC cultured from bone marrow with granulocyte/macrophage colony-stimulated factor and treated as indicated in the keys.
Fixed macrophages, as expected, did not present the unmodified 48–61 peptide to clone H (Fig. 6). However, the live macrophages did stimulate, indicating that the activated peritoneal macrophages could also modify the peptide 48–61. The B cell line C3.F6, which did not express NO, did not present the peptide. All three sets presented the 48–61 peptide with a nTyr-53.
Fig. 6.
Peritoneal macrophages present the modified peptides when cultured with either peptide 48–61 or HEL. In all experiments, the hybridoma is clone H added for 24 h to the culture of the APC. (A) Peritoneal macrophages obtained after Con A injection of the mice and fixed before addition of the peptide. 48–61* is peptide 48–61 with nTyr-53. (B) The same cells, but tested without fixation. (C) The response of B cell lymphoma C3.F6 to the indicated peptides.
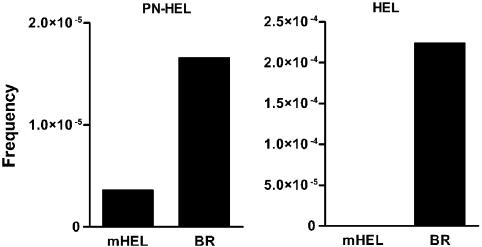
Analysis of T Cells from Mice Immunized with Peroxynitrite-Treated HEL or HEL. B10.BR mice immunized with HEL contained one reactive T cell against unmodified HEL per 4,500 lymph node cells examined by limiting dilution analysis, in accordance with our previous reports (27, 39) (Fig. 7 and Table 1). Those immunized with peroxynitrite-treated HEL generated ≈10-fold fewer T cells, all specifically to modified HEL, on the average 1 per 63,000 T cells. In agreement with previous studies, immunization of mHEL transgenic mice, where the HEL is expressed heavily as a membrane protein in APC, resulted in a complete absence of T cells when immunized with HEL (27). The mHEL transgenic mice did contain T cells reactive to peroxynitrite-treated HEL, but fewer in number than the B10.BR mice, 1 per 280,000 T cells.
Fig. 7.
Limiting dilution analysis of popliteal nodes after immunization with 10 nmol of HEL. Two sets of mice mHEL transgenic or normal were immunized with peroxynitrite-treated HEL (PN-HEL) or HEL. See Table 1.
Table 1. Frequency of positive cells.
| Mice | HEL | PN-HEL |
|---|---|---|
| mHEL | 0 | 1:2.8 × 105 |
| B10.Br | 1:4.5 × 103 | 1:6.3 × 104 |
PN-HEL, peroxynitrite-treated HEL. Calculations are based on results from Fig. 7.
In a different manipulation, B10.BR or mHEL transgenic mice were immunized with regular HEL and examined for the generation of T cells to peroxynitrite-treated HEL. Mice were immunized with 10 nmol HEL, and 7 days later, the lymph nodes were isolated, and the cells were stimulated with peroxynitrite–HEL for 3 days and then fused to produce hybridomas. Twenty-one of 226 clones reacted only to the peroxynitrite-treated HEL, i.e., 9.3%. Of these, 12 reacted with the peptide 48–62 and 1 against 20–35, but only when they were peroxynitrite-treated; 8 did not react with the two peptides, and their specificity is unknown. The mHEL transgenic contained 1% positive T cells to peroxynitrite treated 48–62 (1 of 87 clones tested).
Discussion
These experiments indicate a range of T cells specificities directed to peptides presented by APC undergoing an activated state in which reactive oxygen and nitrogen intermediaries are produced in the cell. The T cells recognized nTyr as well as tryptophan modifications of the MHC-bound peptide, the nature of which has yet to be determined. Thus a nitration and oxidative burst results in epitopes that adds to the T cell repertoire to a given protein antigen.
We speculate that the response to pathogenic microorganisms should contain T cells to modified epitopes. Their relative contribution to antimicrobial immunity is now being evaluated. Already the results indicate that the administration of HEL in adjuvant, or the infection of mHEL transgenic mice, generated modified epitopes. Most likely the APC were activated by the lipid-mycobacteria in the adjuvant or the Listeria organisms, respectively.
Another important biological context for these results is autoimmunity, as others have speculated, that is, in situations in which the self protein is presented by an activated cell such as in the experiments in mHEL transgenic mice. The three findings relevant to this point are as follows. (i) The transgenic mHEL mice contained T cells to the modified peptides but none to the native peptides (27). The mHEL expressed a large number of peptide–MHC complex, and negative selection to them was complete (27). The presence of the T cells to the modified epitopes indicates that autoreactivity could take place to peptide–MHC complexes if the proteins or the peptides are posttranslationally modified. (ii) The mHEL transgenic mice contained a lower number of T cells to the modified epitopes in peripheral lymph nodes compared with normal mice. Whether some of the T cells were centrally thymic-deleted or affected in the periphery was not determined, but it suggests that modifications take place in the APC-bearing mHEL during the life of the mouse. (iii) After infection with L. monocytogenes of transgenic mHEL mice in which HEL is a self protein, the modifications were generated. The results suggest a scenario conducive to autoimmunity; infection generates the neoepitope, to which autoreactive T cells are available.
Our studies indicated that either macrophages or DC modified the peptides. The role of different states of DC or macrophages, or subsets of them (for example, ref. 40), in generating the modifications will need to be addressed in future experiments. Quite striking was the state of activation and the generation of the modified peptides. Although macrophages had to be activated, as were DC, and inhibitors of nitration were effective, a fine balance between activation and generation of the modified peptide was required. The extent of macrophage activation was of major importance, because a high level resulted in less presentation of the peptide–MHC complex. The induction of peroxynitrite changes a number of cellular proteins, including key critical transcription factors (21, 22, 41–44). I-Ak molecules of peritoneal macrophages metabolically labeled in the presence of a strong activation signal like IFN-γ plus LPS treatment, as was done in the experiments indicated in Figs. 1, 2, 3, 4, 5, 6, 7, had a much-reduced synthesis of I-Ak molecules (our unpublished results). Thus, the presentation of modified peptides involves a critical state of activation, preferably one in which the modifying agent, whether peroxynitrite and/or other moieties, is limiting.
Concerning the experiments shown in Fig. 4, previous studies have found that contact with the culture dish of the activated macrophages resulted in the brief production of a number of cytokines, particularly IL-1 (45). After 24 h, the macrophages returned to a basal state. The response of the macrophage cultured after 3 days, where the presentation was no longer evident, is compatible with a two-component reaction, i.e., production of NO plus an oxidative burst.
The nature of the chemical moiety in the activated APC that induces the changes is not known, although peroxynitrite is a likely component. Certainly the T cells induced by immunizing with peroxynitrite HEL or peptides also reacted with the activated APC. However, products derived from iNOS and myeloperoxidase show nitrating activity (24, 25, 46, 47). A final point is that modifications are not taking place in a single vesicular compartment; they took place in both HEL, which is processed in a late vesicular compartment, and the 48–62 peptide, processed in a recycling early vesicle (48).
Acknowledgments
This work was supported by grants from the National Institutes of Health and the Kilo Diabetes and Vascular Research Foundation.
Author contributions: J.H. and E.R.U. designed research; J.H., Y.M., T.P.C., B.S.I., and E.R.U. performed research; J.H. and E.R.U. contributed new reagents/analytic tools; J.H. and E.R.U. analyzed data; and E.R.U. wrote the paper.
Abbreviations: APC, antigen-presenting cells; DC, dendritic cells; HEL, hen egg-white lysozyme; mHEL, membrane HEL; nTyr, nitrotyrosine; TCR, T cell receptor.
References
- 1.Skipper, J. C., Hendrickson, R. C., Gulden, P. H., Brichard, V., Van Pel, A., Chen, Y., Shabanowitz, J., Wolfel, T., Slingluff, C. L., Jr., Boon, T., et al. (1996) J. Exp. Med. 183, 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson, L. W., Hogan, K. T., Caldwell, J. A., Pierce, R. A., Hendrickson, R. C., Deacon, D. H., Settlage, R. E., Brinckerhoff, L. H., Engelhard, V. H., Shabanowitz, J., et al. (2004) J. Immunother. 27, 177–183. [DOI] [PubMed] [Google Scholar]
- 3.Zarling, A. L., Ficarro, S. B., White, F. M., Shabanowitz, J., Hunt, D. F. & Engelhard, V. H. (2000) J. Exp. Med. 192, 1755–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderton, S. M. (2004) Curr. Opin. Immunol. 16, 1–6. [DOI] [PubMed] [Google Scholar]
- 5.van Stipdonk, M. J., Willems, A. A., Amor, S., Persoon-Deen, C., Travers, P. J., Boog, C. J. & van Noort, J. M. (1998) Int. Immunol. 10, 943–950. [DOI] [PubMed] [Google Scholar]
- 6.Mamula, M. J., Gee, R. J., Elliott, J. I., Sette, A., Southwood, S., Jones, P. J. & Blier, P. R. (1999) J. Biol. Chem. 274, 22321–22327. [DOI] [PubMed] [Google Scholar]
- 7.Cirrito, T. P., Pu, Z., Deck, M. B. & Unanue, E. R. (2001) J. Exp. Med. 194, 1165–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molberg, O., McAdam, S. N., Korner, R., Quarsten, H., Kristiansen, C., Madsen, L., Fugger, L., Scott, H., Noren, O., Roepstorff, P., et al. (1998) Nat. Med. 4, 713–717. [DOI] [PubMed] [Google Scholar]
- 9.Arentz-Hansen, H., Korner, R., Molberg, O., Quarsten, H., vader, W., Kooy, Y. M., Lundin, K. E., Koning, F., Roepstorff, P., Sollid, L. M., et al. (2000) J. Exp. Med. 191, 603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birnboim, H. C., Lemay, A. M., Lam, D. K., Goldstein, R. & Webb, J. R. (2003) J. Immunol. 171, 528–532. [DOI] [PubMed] [Google Scholar]
- 11.Harding, C. V., Kihlberg, J., Elofsson, M., Magnusson, G. & Unanue, E. R. (1993) J. Immunol. 151, 2419–2425. [PubMed] [Google Scholar]
- 12.Ishioka, G. Y., Lamont, A. G., Thomson, D., Bulbow, N., Gaeta, F. C. A., Sette, A. & Grey, H. M. (1992) J. Immunol. 148, 2446–2453. [PubMed] [Google Scholar]
- 13.Michaelsson, E., Malmstrom, V., Reis, S., Engstrom, A., Burkhardt, H. & Holmdahl, R. (1994) J. Exp. Med. 180, 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deck, B., Elofsson, M., Kihlberg, J. & Unanue, E. R. (1995) J. Immunol. 155, 1074–1078. [PubMed] [Google Scholar]
- 15.Backlund, J., Carlsen, S., Hoger, T., Holm, B., Fuger, L., Kihlberg, J., Burkhardt, H. & Holmdahl, R. (2002) Proc. Natl. Acad. Sci. USA 99, 9960–9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones, V. E. & Leskowitz, S. (1965) Nature 207, 596–597. [DOI] [PubMed] [Google Scholar]
- 17.Rao, A., Faas, S. J. & Cantor, H. (1984) J. Exp. Med. 159, 479–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas, D. W. & Shevach, E. M. (1978) J. Immunol. 121, 1152–1156. [PubMed] [Google Scholar]
- 19.Brahms, H., Raymackers, J., Union, A., de Keyser, F., Meheus, L. & Luhrmann, R. (2000) J. Biol. Chem. 275, 17122–17129. [DOI] [PubMed] [Google Scholar]
- 20.Schellekens, G. A., de Jong, B. A., van den Hoogen, F. H., van de Putte, L. B. & van Venrooij, W. J. (1998) J. Clin. Invest. 101, 272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beckman, J. S. & Koppenol, W. H. (1996) Am. J. Physiol. 271, C1424–C1437. [DOI] [PubMed] [Google Scholar]
- 22.Ischiropoulos, H., Zhu, L. & Beckman, J. S. (1992) Arch. Biochem. Biophys. 298, 446–451. [DOI] [PubMed] [Google Scholar]
- 23.Whiteman, M., Armstrong, J. S., Cheung, N. S., Siau, J. L., Rose, P., Schantz, J. T., Jones, D. P. & Halliwell, B. (2004) FASEB J. 18, 1395–1397. [DOI] [PubMed] [Google Scholar]
- 24.Pfeiffer, S., Lass, A., Schmidt, K. & Mayer, B. (2001) J. Biol. Chem. 276, 34051–34058. [DOI] [PubMed] [Google Scholar]
- 25.Baldus, S., Eiserich, J. P., Brennan, M. L., Jackson, R. M., Alexander, C. B. & Freeman, B. A. (2002) Free Radic. Biol. Med. 33, 1010. [DOI] [PubMed] [Google Scholar]
- 26.Kato, Y., Kawakishi, S., Aoki, T., Itakura, K. & Osawa, T. (1997) Biochem. Biophys. Res. Commun. 234, 82–84. [DOI] [PubMed] [Google Scholar]
- 27.Peterson, D. A., DiPaolo, R. J., Kanagawa, O. & Unanue, E. R. (1999) Immunity 11, 453–462. [DOI] [PubMed] [Google Scholar]
- 28.Gugasyan, R., Vidavsky, I., Nelson, C. A., Gross, M. L. & Unanue, E. R. (1998) J. Immunol. 161, 6074–6083. [PubMed] [Google Scholar]
- 29.Byersdorfer, C. A., DiPaolo, R. J., Petzold, S. J. & Unanue, E. R. (2004) J. Exp. Med. 173, 6627–6634. [DOI] [PubMed] [Google Scholar]
- 30.Veeraswamy, R., Cella, M., Colonna, M. & Unanue, E. R. (2003) J. Immunol. 170, 5367–5372. [DOI] [PubMed] [Google Scholar]
- 31.Corbett, J. A., Tilton, R. G., Chang, K., Hasan, K. S., Ido, Y., Wang, J. L., Sweetland, M. A., Lancaster, J. R., Jr., Williamson, J. R. & McDaniel, M. L. (1992) Diabetes 41, 552–556. [DOI] [PubMed] [Google Scholar]
- 32.Gardener, P. R., Nguyen, D. D. & White, C. W. (1996) Arch. Biochem. Biophys. 325, 20–28. [DOI] [PubMed] [Google Scholar]
- 33.Nelson, C. A., Roof, R. W., McCourt, D. W. & Unanue, E. R. (1992) Proc. Natl. Acad. Sci. USA 89, 7380–7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Velazquez, C., Vidavsky, I., van der Drift, K., Gross, M. L. & Unanue, E. R. (2002) J. Biol. Chem. 277, 42514–42522. [DOI] [PubMed] [Google Scholar]
- 35.Gammon, G., Shastri, N., Cogswell, J., Wilbur, S., Sadegh-Nasseri, S., Krzych, U., Miller, A. & Sercarz, E. (1987) Immunol. Rev. 98, 53–73. [DOI] [PubMed] [Google Scholar]
- 36.Fremont, D. H., Monnaie, D., Nelson, C. A., Hendrickson, W. A. & Unanue, E. R. (1998) Immunity 8, 305–317. [DOI] [PubMed] [Google Scholar]
- 37.Velazquez, C., DiPaolo, R. & Unanue, E. R. (2001) J. Immunol. 166, 5488–5494. [DOI] [PubMed] [Google Scholar]
- 38.Carson, R. T., Vignali, K. M., Woodland, D. L. & Vignali, D. A. (1997) Immunity 7, 387–399. [DOI] [PubMed] [Google Scholar]
- 39.DiPaolo, R. J. & Unanue, E. R. (2002) J. Immunol. 169, 1–4. [DOI] [PubMed] [Google Scholar]
- 40.Serbina, N. V., Salazar-Mather, T. P., Biron, C. A., Kuziel, W. A. & Pamer, E. G. (2003) Immunity 19, 59–70. [DOI] [PubMed] [Google Scholar]
- 41.Beckman, J. S., Chen, J., Ischiropoulos, H. & Crow, J. P. (1994) Methods Enzymol. 233, 229–240. [DOI] [PubMed] [Google Scholar]
- 42.Vazquez, M. A., Sicher, S. C., Proctor, M. L., Crowley, J. C. & Lu, C. Y. (1996) J. Leukocyte Biol. 59, 683–690. [DOI] [PubMed] [Google Scholar]
- 43.Kielar, M. L., Sicher, S. C., Penfield, J. G., Jeyarajah, D. R. & Lu, C. Y. (2000) Inflammation 24, 431–445. [DOI] [PubMed] [Google Scholar]
- 44.Llovera, M., Pearson, J. D., Moreno, C. & Riveros-Moreno, V. (2001) Brit. J. Pharmacol. 132, 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hogquist, K. A., Unanue, E. R. & Chaplin, D. D. (1991) J. Immunol. 147, 2181–2186. [PubMed] [Google Scholar]
- 46.Podrez, E. A., Abu-Soud, H. M. & Hazen, S. L. (2000) Free Radic. Biol. Med. 15, 1717–1725. [DOI] [PubMed] [Google Scholar]
- 47.Hazen, S. L., Zhang, R., Shen, Z., Wu, W., Podrez, E. A., MacPherson, J. C., Schmitt, D., Mitra, S. N., Mukhopadhyay, C., Chen, Y., et al. (1999) Circ. Res. 85, 950–958. [DOI] [PubMed] [Google Scholar]
- 48.Pu, Z., Lovitch, S. B., Bikoff, E. K. & Unanue, E. R. (2004) Immunity 20, 467–476. [DOI] [PubMed] [Google Scholar]