Abstract
Variability in urine dilution complicates urine cannabinoid test interpretation. Normalizing urine cannabinoid concentrations to specific gravity (SG) or creatinine was proposed to account for donors’ hydration states. In this study all urine voids were individually collected from 8 frequent and 8 occasional cannabis users for up to 85 h after each received on separate occasions 50.6 mg Δ9-tetrahydrocannabinol (THC) by smoking, vaporization and oral ingestion in a randomized, within-subject, double-blind, double-dummy, placebo-controlled protocol. Each urine void was analyzed for 11 cannabinoids and phase I and II metabolites by LC-MS/MS, SG and creatinine. Normalized urine concentrations were log10 transformed to create normal distributions, and Pearson correlation coefficients determined the degree of association between the two normalization methods. Repeated-measures linear regression determined if the degree of association differed by frequent or occasional cannabis use, or route of administration after adjusting for gender and time since dosing. Of 1880 urine samples examined, only 11-nor-9-carboxy-THC (THCCOOH), THCCOOH-glucuronide, THC-glucuronide and 11-nor-9-carboxy-Δ9-tetrahydrocannabivarin (THCVCOOH) were greater than the method’s limits of quantification. Associations between SG- and creatinine-normalized concentrations exceeded 0.90. Repeated-measures regression analysis found small but statistically significant differences in degree of association between normalization methods for THCCOOH and THCCOOH-glucuronide in frequent vs. occasional smokers, and in THCVCOOH and THC-glucuronide by route of administration. For the first time, SG- and creatinine-normalized urine cannabinoid concentrations were evaluated in frequent and occasional cannabis users and following oral, smoked and inhaled cannabis. Both normalization methods reduced variability improving interpretation of urine cannabinoid concentrations and methods were strongly correlated.
Keywords: Specific gravity, creatinine, urine cannabinoids
Graphical Table of Contents
The correlation of specific gravity and creatinine normalized phase I and II urine cannabinoids following controlled administration of THC by oral, smoked and vaporized routes of administration was strong despite being statistically influenced by gender and time since dosing.
Introduction
Urinary excretion of drugs and metabolites enables clinical and forensic assessment and monitoring of drug use. An individual’s hydration state can greatly affect drug concentration. As donors ingest liquids, the urine becomes more dilute, reducing drug concentrations, sometimes below specified cutoff concentrations. As early as 1945, Levine and Fahy reported that dilution caused the largest fluctuations in urine lead concentrations during environmental monitoring. They recommended normalizing lead concentrations in single urine samples with the sample’s specific gravity (SG), demonstrating a reduction in the coefficient of variation from greater than 25% to less than 7% for normalized concentrations for some specimens.1 Urine density increases with higher salt and other compound concentrations; SG compares the urine sample’s density to that of water providing a measure of dilution. This normalization method converts concentrations to that expected for a single, selected SG reference value, improving clinical interpretation. Urine analyte concentrations corrected for SG were utilized for exposure monitoring, hormones for endocrine assessment, and urine drug testing.2–4 The World Antidoping Agency establishes thresholds for drugs and endogenous compounds in sports and selected SG correction among several normalization options available to account for an athlete’s state of hydration.5
Later investigators recommended normalization to urine creatinine concentrations, since there was evidence that creatinine, a product of muscle metabolism, was excreted at a steady rate in normally functioning kidneys.6 Haddow et al. compared creatinine and SG corrected cotinine concentrations and reported that both were acceptable to improve clinical assessment.4 Several scientific studies reported improved interpretation with creatinine normalized urine concentrations. Examples included uranium for exposure monitoring7, hormones for endocrine assessments2, and urine drug testing.8,9 Creatinine-normalization was especially helpful in drug metabolism studies where sequential urine samples were collected and analyzed to determine pharmacokinetic parameters, such as half-life and predicted values.10 Huestis and Cone examined sequential urine 11-nor-Δ9-tetrahydrocannabinol-9-carboxylic acid (THCCOOH) concentrations after a single smoked cannabis cigarette (placebo, 1.75% or 3.55% Δ9-tetrahydrocannabinol [THC]).11 After peak urine THCCOOH concentration, consistent concentration decreases were expected with time, but subsequent urine THCCOOH concentrations were occasionally higher, even when samples were separated by 24 h. Creatinine-normalization smoothed the elimination curve and subsequent increases rarely occurred. Creatinine-normalization was recommended for identifying cannabis relapse in patients in drug treatment programs utilizing new use prediction models.11,12
Creatinine- and SG-corrected concentrations of 28 analytes in 10,899 urine samples submitted for pain management testing were compared.13 Linear regression r values for nearly all analytes were >0.9. When additional data from heroin abusers in a clinical study and cannabis/cocaine users in a dilution study were added, the mean r value was lower, 0.84, but still statistically significant.
Creatinine-normalization was criticized for some monitoring applications because muscle metabolism may be altered, producing increased creatinine intra- and inter-subject variability.14,15 This criticism led others to recommend SG correction.2,3 Questions remain about which factors change dilution and influence correlation between creatinine and SG-normalization. For example, chronic frequent cannabis users excrete THCCOOH into urine over extended periods of drug abstinence. Whether there are differences in creatinine or SG-normalization between these individuals and occasional cannabis users was not investigated. Also, does route of cannabis administration with different rates of THC absorption, distribution, metabolism and elimination affect normalization methods? Are there differences in creatinine and SG correlations for phase I and phase II metabolites?
Newmeyer et al. conducted a placebo-controlled, within-subject, randomized study of 8 chronic frequent and 8 occasional cannabis users following administration of a single 50.6 mg THC dose by oral, smoked, and vaporized routes.16 This published study examined blood concentrations and reported free and glucuronide cannabinoid concentrations as well as pharmacokinetic parameters. As part of this study, we analyzed urine samples collected up to 85 h after cannabis dosing and quantified 11 urinary cannabinoids and metabolites concentrations, creatinine and SG. Each cannabinoid analyte’s concentration was normalized to urine creatinine and SG. Correlations between the two normalization methods were determined and results examined for strength of correlation and differences between frequent and occasional cannabis user groups, routes of administration and phase I and II metabolites.
Materials and Methods
PARTICIPANTS
Healthy adults between ages 18 and 50 years were recruited for this study, which was approved by the National Institute on Drug Abuse Intramural Research Program Institutional Review Board, Food and Drug Administration and Drug Enforcement Administration. Individuals received a comprehensive medical and psychological evaluation and were accepted if meeting criteria for good health. Inclusion criteria included a mean self-reported cannabis intake frequency ≥2x per month but ≤3x per week (occasional users) or ≥5x per week (frequent users) over the previous 3 months and a positive urine cannabinoid screen for frequent smokers. Individuals provided written, informed consent.
STUDY DESIGN
The study design was within-subject, randomized, double-blind, double-dummy and placebo-controlled, with each participant receiving active drug by all three routes of administration and placebo oral and inhaled doses within four sessions as previously described.16 In each session participants ingested a brownie (Duncan Hines® Double Fudge brownie mix) and either smoked a cigarette or inhaled from a vaporizer (210 °C, Volcano Medic, Storz & Bickel). Active drug, THC, was present in only one route of administration and the other was placebo. An optional fifth session followed the same administration protocol except without any inhaled cannabis (Oral 5). The purpose of Oral 5 was to evaluate cannabinoids in oral fluid after oral cannabis without potential contribution from inhaled active or placebo cannabis. Urine samples collected in the Oral 5 sessions provided additional urine samples for measurement of urine creatinine and SG. Participants entered the secure research unit approximately 19 h before dosing to preclude acute intoxication. Placebo and active cannabis cigarettes were obtained from the National Institute on Drug Abuse, Chemistry and Physiological Systems Research Branch. Active cigarettes [0.734±0.05g] contained 6.9±0.95% (50.6 mg) THC, 0.20±0.01% (1.5 mg) cannabidiol (CBD) and 0.44±0.08% (3.3 mg) cannabinol (CBN). Placebo cigarettes [0.713±0.05 g] contained 0.001±0.000% THC, no detectable CBD, and 0.004±0.000% CBN. Frequent smokers remained on the unit up to 85 h post-dose and were required to leave the unit for 72 h before the next session admission to minimize acute withdrawal symptoms. Occasional smokers remained on the unit up to 61 h post-dose and were allowed to remain on the unit for multiple sessions, if they chose to stay; they were not dosed more frequently than their self-reported intake frequency. Every urine sample was collected from admission to the end of the study period and analyzed separately.
URINE CANNABINOID ANALYSIS
Urine samples were analyzed for THC, 11-hydroxy-THC (11-OH-THC), THCCOOH, CBN, CBD, THCCOOH-glucuronide, cannabigerol (CBG), Δ9-tetrahydrocannabinolic acid (THCAA), Δ9-tetrahydrocannabivarin (THCV), 11-nor-THCV-9-carboxylic acid (THCVCOOH), and THC-glucuronide with a previously reported LC-MS/MS method.17 Briefly, sample preparation consisted of disposable pipette extraction (WAX-S) of 200μL urine. Separation was achieved on a Kinetex C18 column using gradient elution with a 0.5 mL/min flow rate, mobile phase A (10 mM ammonium acetate in water) and mobile phase B (15% methanol in acetonitrile) with identification and quantification by tandem mass spectrometry. 11-OH-THC-glucuronide and THCAA-glucuronide were not examined since no reference standard was available. Linear ranges were 0.5–100 μg/L for THC and THCCOOH; 0.5–50 μg/L for 11-OH-THC, CBD, CBN, THCAA, and THC-glucuronide; 1–100 μg/L for CBG, THCV, and THCVCOOH; and 5–500 μg/L for THCCOOH-glucuronide (R2 > 0.99). Analytical biases were 88.3–113.7 %, imprecisions 3.3–14.3 %, extraction efficiencies 42.4–81.5 %, and matrix effects −10 to 32.5 %. Deuterated internal standards were commercially-available for the first six analytes.
SPECIFIC GRAVITY (SG) ANALYSIS
Urine was collected and allowed to cool to room temperature. Specific gravity was measured to three decimals using an Atago-10S 3-digit refractometer (Cole-Palmer, Vernon Hills, IL). The analytical bias was zero for two controls with expected SG of 1.0016 and 1.0177. The mean deviation from the expected value for these controls was 0.000 and +0.0007, respectively. The refractometer was cleaned with distilled water between each measurement and the specific gravity checked to ensure that the SG of distilled water was 1.000 prior to reading the next urine sample.
CREATININE ANALYSIS
Creatinine was measured in urine that was frozen at −20 °C within 2 h of collection, shipped on dry ice to the Centers for Disease Control and Prevention laboratory and brought to room temperature prior to analysis. The analyzer was a Roche/Hitachi Modular P instrument using a Roche Diagnostics Creatinine Plus ver.2 enzymatic assay (Roche Diagnostics, Indianapolis, IN) as described previously.18 Briefly, the linear range was 0.006–6.00 g/L (R2 > 0.99), intra-day imprecision 0.79–1.23 % and inter-day imprecision 1.22–1.41%.
DATA ANALYSIS
Data were compartmentalized by each administration route session for a participant. Analyte concentrations greater than or equal to the limit of quantification (LOQ) were normalized as follows:
Concentrations were normalized to SG as previously reported.5
CSG normalized = Csample ∗ (1.0200–1)/(SGsample −1)
(CSG normalized, SG-normalized analyte concentration; Csample, measured analyte concentration; SGsample, SG of the urine specimen)
Concentrations were also normalized using creatinine concentrations.
CCreatinine normalized = (Csample in μg/L)/ creatinine in g/L
(CCreatinine normalized, creatinine-normalized analyte concentration)
STATISTICS
Observations were limited to those for which non-zero normalized concentrations were available for each normalization method. Concentrations used for comparison were normalized by SG and creatinine on the same sample, i.e. they were paired data. The normalized concentration data were transformed using log10 to create a Gaussian distribution. Two repeated measures regression models were run for each of the four analytes with sufficient non-zero numbers for the analysis (THCCOOH, THCVCOOH, THC-glucuronide, and THCCOOH-glucuronide): one to assess whether the association between normalization methods varied by smoking frequency group, and the other to assess whether this association varied by route of administration. In each model, the SG-normalized value was the dependent variable, and the creatinine-normalized value and its interaction with the variable of interest, group or route, were the independent variables. Covariates gender and time after dosing were also included in each model to control for any influence these variables may have had on the normalization methods.
Pearson correlation coefficients were calculated between the two normalization methods for each of the four analytes (1) for the sample as a whole, (2) by occasional and frequent smoking groups if regression analyses indicated that the two groups differed in terms of the association between the two normalization methods, and (3) by route if regression analyses indicated differences in association between the two normalization methods by route. All statistical analyses were conducted using R version 3.4.2.
Results
Eight frequent and 8 occasional cannabis smokers of ages 19–46 years, 75% male, 69% African American (31% self-reported as Caucasian) completed the study. Every urine sample produced was collected bringing the total examined to 1880.
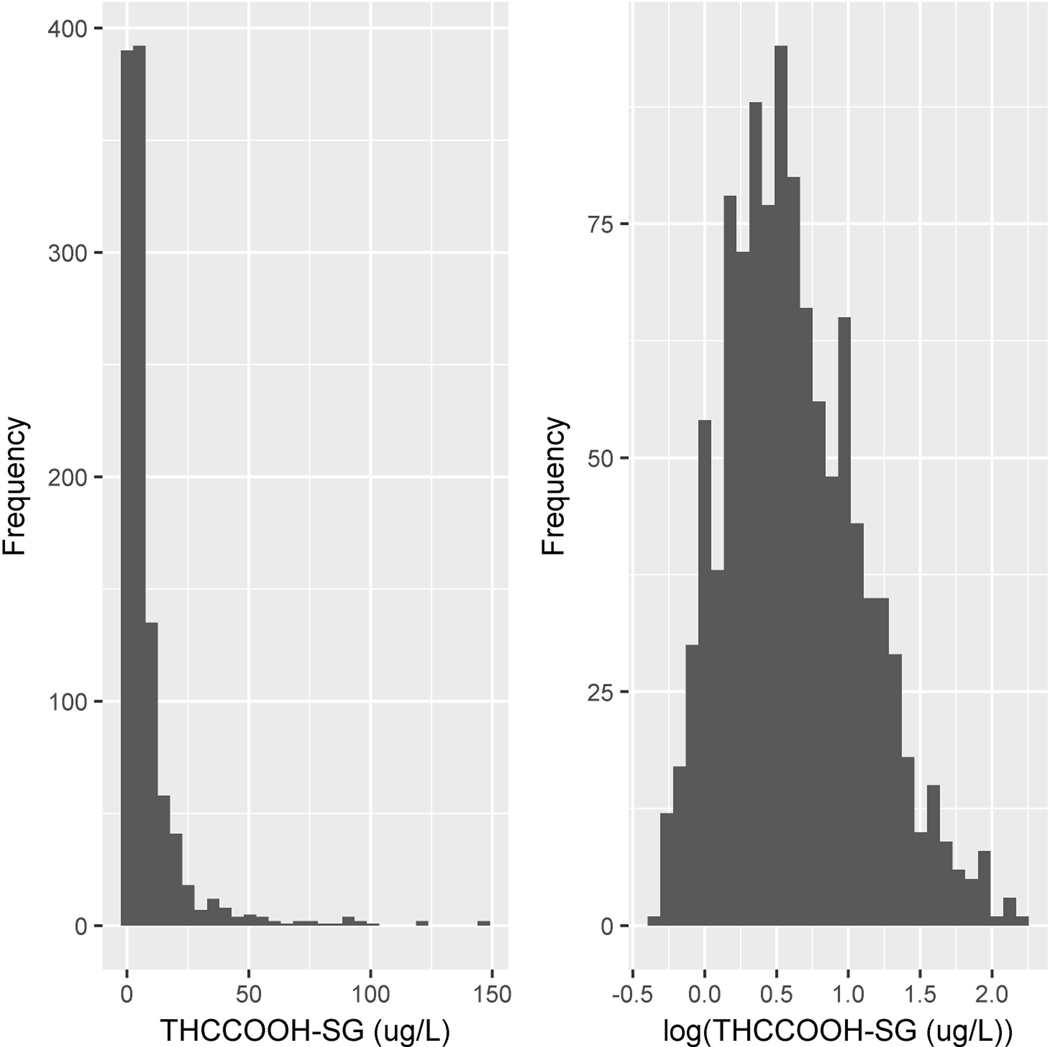
Of the eleven analytes investigated, THC, 11-OH-THC, CBD, CBN, CBG, THCV and THCAA were not detected in any occasional or frequent cannabis user’s urine sample. Therefore, correlations were determined for the four detected analytes, THCCOOH, THCVCOOH, THC-glucuronide and THCCOOH-glucuronide. The distribution of normalized concentrations for each of these four analytes was skewed towards higher concentrations; therefore, data were transformed using log10 to create a Gaussian distribution prior to conducting statistical analyses. Figure 1 displays the raw and transformed data for THCCOOH as an example. The range of SG was 1.001–1.036 and for creatinine 0.040–4.79 g/L.
Figure 1.
Histograms showing the distributional shape of the untransformed values (left panel) and log10-transformed values (right panel) of specific gravity-normalized 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THCCOOH-SG).
The correlations between SG- and creatinine-normalized concentrations were high for all four analytes with Pearson correlation coefficients exceeding 0.90 in each case (Table 1). There were small but statistically significant differences in the degree of association between normalization methods, as evidenced by statistically significant interaction terms in the regression models. The associations differed by smoking group for two analytes: THCCOOH and THCCOOH-glucuronide. For each of these analytes, the interaction term was statistically significant (THCCOOH chi square1=6.9, p=0.0086; THCCOOH-glucuronide chi square1=17.5, p<0.0001) after adjusting for participant gender and time since dosing (Table 1A, Figures 2 and 3). Regression analyses also indicated that associations differed by route of administration for the other two analytes: THCVCOOH and THC-glucuronide. For THCVCOOH, the interaction term was statistically significant (chi square1=14.9, p=0.0050) and for THC-glucuronide showed a trend toward statistical significance (chi square1=8.36, p=0.079) after adjusting for participant gender and time since dosing (Table 1B, Figure 4 and 5).
Table 1.
Pearson correlation coefficients for the association between urine cannabinoid concentrations normalized to specific gravity (SG) and creatinine concentration for analytes for which this association differed by smoking group (A), and for which this association differed by route of administration (B).
| A Group | THCCOOH | THCCOOH-glucuronide |
|---|---|---|
| Frequent Smokers | 0.97 | 0.93 |
| Occasional Smokers | 0.94 | 0.95 |
| Overall | 0.97 | 0.95 |
| B Route of Administration | THCVCOOH | THC-glucuronide |
| Oral | 0.91 | 0.94 |
| Smoked | 0.96 | 0.95 |
| Vaporized | 0.92 | 0.94 |
| Oral 5 | 0.95 | 0.96 |
| Placebo | 0.96 | 0.96 |
| Overall | 0.93 | 0.95 |
THCCOOH, 11-nor-9-carboxy-Δ9-tetrahydrocannabinol; THCVCOOH; Δ9-tetrahydrocannabivarin carboxylic acid; THC-glucuronide, -Δ9-tetrahydrocannabinol glucuronide
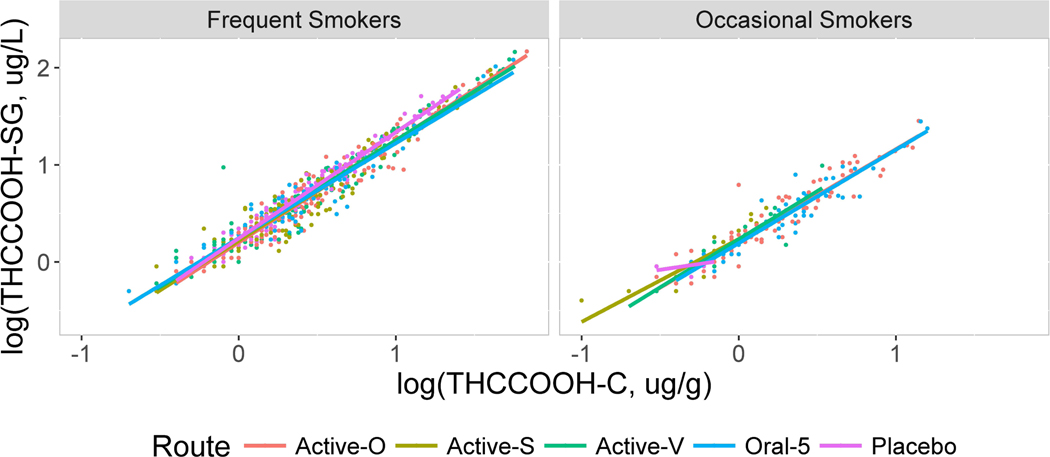
Figure 2.
Scatterplots showing the relationship between log10 THCCOOH normalized to specific gravity (SG, y axis) vs. normalized to creatinine (x axis) for frequent and occasional smokers by routes oral (Active-O), smoked (Active-S), vaporized (Active-V), oral 5 and placebo.
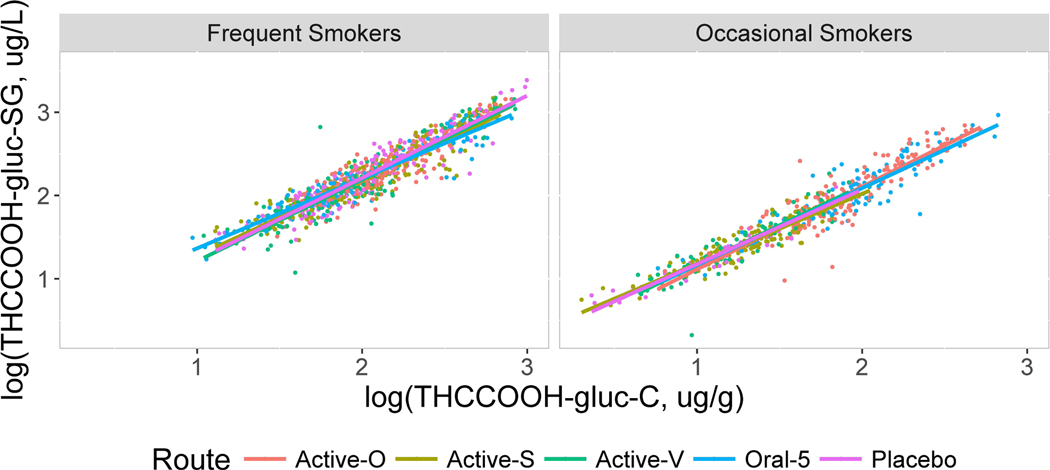
Figure 3.
Scatterplots showing the relationship between log10 THCCOOH-glucuronide normalized to specific gravity (SG, y axis) vs. normalized to creatinine (x axis) for frequent and occasional smokers by routes oral (Active-O), smoked (Active-S), vaporized (Active-V), oral 5 and placebo.
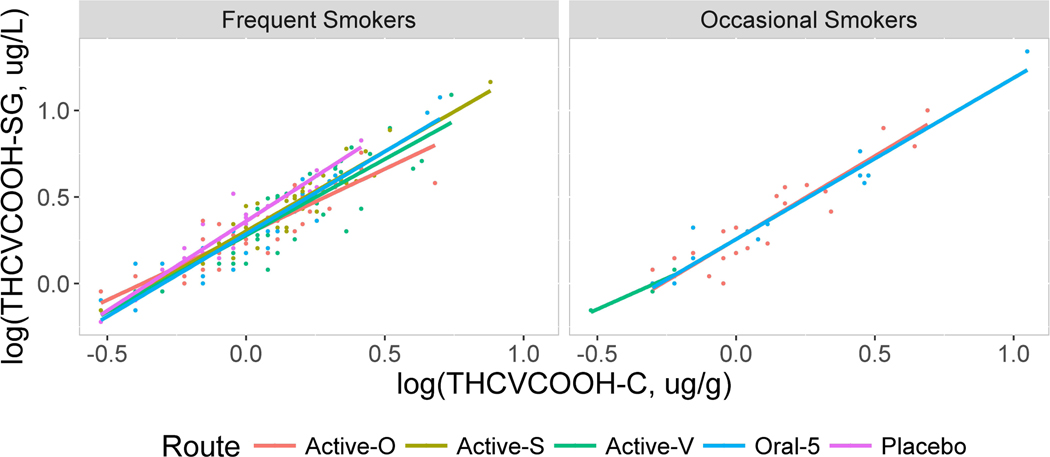
Figure 4.
Scatterplots showing the relationship between log10 THCVCOOH normalized to specific gravity (SG, y axis) vs. normalized to creatinine (x axis) for frequent and occasional smokers by routes oral (Active-O), smoked (Active-S), vaporized (Active-V), oral 5 and placebo.
Figure 5.
Scatterplots showing the relationship between log10 THC-glucuronide normalized to specific gravity (SG, y axis) vs. normalized to creatinine (x axis) for frequent and occasional smokers by routes oral (Active-O), smoked (Active-S), vaporized (Active-V), oral 5 and placebo.
The scatter plots displayed in Figures 2–5 contain linear regression lines for each route of administration. Frequent and occasional user groups are presented separately with relative SG-normalized concentrations on the y axis and creatinine-normalized concentrations on the x axis. The figures show consistently strong correlations between the two normalization methods, regardless of smoking group or route of administration. The larger number of data points for the frequent compared to occasional smokers is due to their remaining in each session for a longer period of time, as well as the occasional smokers having more urine specimens with analytes below the LOQ. Even though some best-fit regression lines differ in slope, the differences by route of administration were small for each analyte. Occasionally lines were indistinguishable for THCCOOH and THCCOOH-glucuronide and small but more prominent for THCVCOOH and THC-glucuronide.
Analytes were present in some samples following placebo due to the presence of residual drug concentrations and study design. Participants were not required to be drug-free before dosing. The monitored abstinence period prior to dosing was approximately 19 h. Metabolites were sometimes present from cannabis self-administration prior to the session, and for occasional users, from an immediately preceding dosing session. When present in sufficient numbers, normalized concentrations for these sessions were included separately in the figures and also combined in the overall comparisons. Correlations for these later stage metabolites following placebo were not significantly different from those after a cannabis dose. As expected, both types of oral dosing sessions, Oral and Oral 5, gave similar, strong correlations, R > 0.90.
Discussion
This study builds on a long history of work by other investigators that has evaluated methods for normalizing urine specimens to account for individual differences in hydration.
Beginning with Levine and Fahey in 19451, many studies have established that normalizing urine specimens, using either SG or creatinine, improves the interpretability and clinical utility of monitoring urine specimens for environmental contaminants, medications, and drugs of abuse. Despite decades of research, some controversies remain regarding which method is to be preferred. Pertaining to urine testing for drugs of abuse, some research questions have gone unanswered. When using normalized urine specimens to screen for cannabis use, it is important to know whether the results are affected by frequency of cannabis use and route of cannabis administration, and how results differ for phase I and phase II metabolites. The current study is the first to report SG- and creatinine-normalized urine cannabinoid correlations for both phase I and phase II cannabinoid metabolites and evaluate how these associations varied as a function of frequency of cannabis use, and route of administration.
The results reported in this study are consistent with those previously reported13, which found high correlations between SG- and creatinine-normalized concentrations. That study, like the current study, found correlations between the two methods that were consistently high regardless of analyte.
A strength of this study was the rigor of the statistical analyses. The concentration data were log10-transformed to reduce the influence of high values and create distributions that were more consistent with the assumptions that underlie linear regression and correlation methods.
In addition to calculating correlations, repeated-measures regression was used to assess the degree of association between the two normalization methods, and if this association differed by smoking group or route of administration. Regression modeling allows for inclusion of interaction terms and covariates. The interaction terms were necessary to test the hypothesis of parallelism (different slopes), i.e. the relationship between normalization methods being modified by another variable. It was necessary to include the covariates gender and time after dosing in order to control for these variables, since prior investigations have noted gender differences for creatinine concentration (2,14) and intra-subject variations in creatinine concentrations with time (19,20).
A weakness of the current study is that the number of analytes and number of participants is smaller than for some previous studies, for example that cited with 10,899 specimens.13 However, in those studies little was known about the time since dosing and donor characteristics to include their individual variability. The repeated-measures regression models used in the current analyses accounted for within-subject correlation that occurs with multiple samples taken on each subject. Failure to account for within-subject correlation can lead to biased hypothesis test results.
Since SG and creatinine produced similar normalization results, clinicians and investigators may consider other factors in making a choice of methods. For example, creatinine concentration is more easily determined on high volume autoanalyzers within the same batches that analyze for drug concentrations. SG might be preferred allowing accurate, inexpensive analysis when drug concentrations on a smaller number of samples are determined separately by chromatographic methods.
Our study found that for sequentially collected urine samples, creatinine- and SG-normalization were very strongly correlated. These correlations were consistently high, regardless of smoking frequency or route of administration, after adjusting for gender and time since dosing. The implication of these findings is that both creatinine- and SG-normalization are valid methods to reduce urine drug metabolite concentration variability that results from an individual’s state of hydration and improves interpretation of urine cannabinoid concentrations. We extended the evaluation of creatinine- and SG-normalization to phase I and phase II cannabinoid metabolites after oral, smoked or vaporized cannabis and in two distinct cannabinoid user groups, frequent and occasional cannabis users, and demonstrated the concordance of these two normalization methods for cannabinoid metabolites in urine specimens.
Acknowledgements
The authors wish to thank Dr. David A. Gorelick and Dr. Osama A. Abulseoud, Chemistry and Drug Metabolism Section, Intramural Research Program, National Institute on Drug Abuse for contributing to the study design and medical care of participants, Dr. Karl B. Scheidweiler and Allan J. Barnes for excellent oversight of data analysis and quality control, and John Etter and Janeen Nichels for outstanding technical assistance and for performing urine specific gravity measurements. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position or policy of the Centers for Disease Control and Prevention.
Nonstandard abbreviations:
- THC
Δ9-tetrahydrocannabinol
- THCCOOH
11-nor-9-carboxy-THC
- THCVCOOH
11-nor-9-carboxy-Δ9-tetrahydrocannabivarin
- CBD
cannabidiol
- CBN
cannabinol
- 11-OH-THC
11-hydroxy-THC
- CBG
cannabigerol
- THCAA
Δ9-tetrahydrocannabinolic acid
- THCV
Δ9-tetrahydrocannabivarin
- SG
specific gravity
References
- 1.Levine L, Fahy JP. Evaluation of urinary lead determinations. J. Ind. Hyg. Toxicol. 1945;27:217–223. [PubMed] [Google Scholar]
- 2.Miller RC, Brindle E, Holman DJ, Shofer J, Klein NA, Soules MR, et al. Comparison of specific gravity and creatinine for normalizing urinary reproductive hormone concentration. Clin. Chem. 2004;50(5):924–932. [DOI] [PubMed] [Google Scholar]
- 3.Sauve JF, Levesque M, Huard M, Drolet D, Lavoue J, Tardif R, et al. Creatinine and specific gravity normalization in biological monitoring of occupational exposures. J. Occup. Environ. Hyg. 2015;12(2):123–129 doi: 10.1080/15459624.2014.955179. [DOI] [PubMed] [Google Scholar]
- 4.Haddow JE, Knight GJ, Palomaki GE, Neveux LM, Chilmonczyk A. Replacing creatinine measurements with specific gravity values to adjust urine cotinine concentrations. Clin. Chem. 1994;40(4):562–564. [PubMed] [Google Scholar]
- 5.World Anti-Doping Agency. Decision limits for the confirmatory quantification of threshold substances. Technical Document TD2018DL. 1 March 2018. [Google Scholar]
- 6.Boeniger MR, Lowry LK, Rosenberg J. Interpretation of urine results used to asses chemical exposure with emphasis on creatinine adjustments: a review. Am. Ind. Hyg. Assoc. J. 1993;54(10):615–627. [DOI] [PubMed] [Google Scholar]
- 7.Karpas Z, Lorber A, Elish E, Marcus P, Roiz Y, Marko R, et al. Uranium in urine-normalization to creatinine. Health Phys. 1998; 74(1): 86–90. [DOI] [PubMed] [Google Scholar]
- 8.Taracha E, Habrat B, Chmielewska K, Baran-Furga H. Excretion profile of opiates in dependent patients in relation to route of administration and type of drug measured in urine with immunoassay. J. Anal. Toxicol. 2005:29(1):15–21. [DOI] [PubMed] [Google Scholar]
- 9.Berthet A, De Cesare M, Favrat B, Spokert F, Augsburger M, Thomas A, et al. A systematic review of passive exposure to cannabis. Forensic Sci. Int. 2016;269:97–112 doi: 10.1016/j.forsciint.2016.11.017. Epub 2016 Nov 16. [DOI] [PubMed] [Google Scholar]
- 10.Beardsley GD, Christensen JM. Elimination of 11-nor-9-carboxy-delta-9-tetrahydrocannabinol when normalized to urinary creatinine. Res. Commun. Mol. Pathol. Pharmacol. 2007-2008;120–121(1–6):67–78. [PubMed] [Google Scholar]
- 11.Huestis MA, Cone EJ. Differentiating new marijuana use from residual excretion in occasional marijuana users. J. Anal. Toxicol. 1998;22(6):445–454. [DOI] [PubMed] [Google Scholar]
- 12.Smith ML, Barnes AJ, Huestis MA. Identification of new cannabis use with urine creatinine-normalized THCCOOH concentrations and time intervals between specimen collections. J. Anal. Toxicol. 2009;33(4):185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cone EJ, Caplan YH, Moser F, Roberts T, Shelby MK, Black DL. Normalization of urinary drug concentrations with specific gravity and creatinine. J. Anal. Toxicol. 2009;33:1–7. [DOI] [PubMed] [Google Scholar]
- 14.Alessio L, Berlin A, Dell’Orto A, Toffoletto F, Ghezzi I. Reliability of urinary creatinine as a parameter used to adjust values of urinary biological indicators. Int. Arch. Occup. Environ. Health. 1985;55(2):99–106. [DOI] [PubMed] [Google Scholar]
- 15.Robinson-Cohen C, Ix JH, Smits G, Persky M, Chertow GM, Block GA, et al. Estimation of 24-hour urine phosphate excretion from spot urine collection: development of predictive equation. J. Ren. Nutr. 2014;24(3):194–199 doi: 10.1053/j.jrn.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Newmeyer MN, Swortwood J, Barnes AJ, Abulseoud OA, Scheidweiler KB, Huestis MA. Free and glucuronide whole blood cannabinoids’ pharmacokinetics after controlled smoked, vaporized, and oral cannabis administration to frequent and occasional cannabis users: identification of recent cannabis use. Clin. Chem. 2016;62(12):1579–1592. [DOI] [PubMed] [Google Scholar]
- 17.Andersson M, Scheidweiler KB, Sempio C, Barnes AJ, Huestis MA. Simultaneous quantification of 11 cannabinoids and metabolites by liquid chromatography tandem mass spectrometry using WAX-S tips. Anal. Bioanal. Chem. 2016;408(23):6461–6471 doi: 10.1007/s00216-016-9765-8. Epub 2016 Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Junge W, Baerbel W, Halabi A, Klein G. Determination of reference intervals for serum creatinine, creatinine excretion and creatinine clearance with an enzymatic and a modified Jaffe method. Clin. Chim. Acta. 2004;344:137–148. [DOI] [PubMed] [Google Scholar]
- 19.Tan SJ, Smith ER, Cai MM, Holt SG, Hewitson TD, Toussaint ND. Relationship between timed and spot urine collections for measuring phosphate excretion. Int. Urol. Nephrol. 2016;48(1);115–124 doi: 10.1007/s11255-015-1149-z. Epub 2015 Nov 14. [DOI] [PubMed] [Google Scholar]
- 20.Sawant PD, Kumar SA, Wankhede S, Rao DD. Creatinine as a normalization factor to estimate the reprentativeness of urine sample-intrasubject and inter-subject variability studies. Appl. Radiat. Isot. 2018;136:121–126 doi: 10.1016/j.apradiso.2018.02.007. Epub 2018 Feb 6. [DOI] [PubMed] [Google Scholar]