Abstract
Background
Normal pressure hydrocephalus (NPH) occurs when the brain ventricles expand, causing a triad of gait, cognitive, and urinary impairment. It can occur after a clear brain injury such as trauma, but can also occur without a clear cause (termed idiopathic, or iNPH). Non‐randomised studies have shown a benefit from surgically diverting ventricular fluid to an area of lower pressure by cerebrospinal fluid (CSF)‐shunting in iNPH, but historically there have been limited randomised controlled trial (RCT) data to confirm this.
Objectives
To determine the effect of CSF‐shunting versus no CSF‐shunting in people with iNPH and the frequency of adverse effects of CSF‐shunting in iNPH.
Search methods
We searched the Cochrane Dementia and Cognitive Improvement Group's register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (Ovid SP), Embase (Ovid SP), PsycINFO (Ovid SP), CINAHL (EBSCOhost), Web of Science Core Collection (Clarivate), LILACS (BIREME), ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform on 15 February 2023.
Selection criteria
We included only RCTs of people who had symptoms of gait, cognitive, or urinary impairment with communicating hydrocephalus (Evans index of > 0.3) and normal CSF pressure. Control groups included those with no CSF shunts or those with CSF shunts that were in 'inactive' mode.
Data collection and analysis
We used standard Cochrane methodological procedures. Where necessary, we contacted study authors requesting data not provided in the papers. We assessed the overall certainty of the evidence using GRADE.
Main results
We included four RCTs, of which three were combined in a meta‐analysis. The four RCTs included 140 participants (73 with immediate CSF‐shunting and 67 controls who had delayed CSF‐shunting) with an average age of 75 years. Risk of bias was low in all parallel‐group outcomes evaluated apart from gait speed, cognitive function (general cognition and Symbol Digit Test) (some concerns) and adverse events, which were not blind‐assessed. CSF‐shunting probably improves gait speed at less than six months post‐surgery (standardised mean difference (SMD) 0.62, 95% confidence interval (CI) 0.24 to 0.99; 3 studies, 116 participants; moderate‐certainty evidence). CSF‐shunting may improve qualitative gait function at less than six months post‐surgery by an uncertain amount (1 study, 88 participants; low‐certainty evidence). CSF‐shunting probably results in a large reduction of disability at less than six months post‐surgery (risk ratio 2.08, 95% CI 1.31 to 3.31; 3 studies, 118 participants; moderate‐certainty evidence). The evidence is very uncertain about the effect of CSF‐shunting on cognitive function at less than six months post‐CSF‐shunt surgery (SMD 0.35, 95% CI −0.04 to 0.74; 2 studies, 104 participants; very low‐certainty evidence). The evidence is also very uncertain about the effect of CSF‐shunt surgery on adverse events (1 study, 88 participants; very low‐certainty evidence). There were no data regarding the effect of CSF‐shunting on quality of life.
Authors' conclusions
We found moderate‐certainty evidence that CSF‐shunting likely improves gait speed and disability in iNPH in the relative short term. The evidence is very uncertain regarding cognition and adverse events. There were no longer‐term RCT data for any of our prespecified outcomes. More studies are required to improve the certainty of these findings. In addition, more information is required regarding patient ethnicity and the effect of CSF‐shunting on quality of life.
Keywords: Aged; Humans; Bias; Cerebrospinal Fluid Shunts; Cerebrospinal Fluid Shunts/adverse effects; Cognition; Gait; Gait/physiology; Gait Disorders, Neurologic; Gait Disorders, Neurologic/etiology; Hydrocephalus, Normal Pressure; Hydrocephalus, Normal Pressure/surgery; Randomized Controlled Trials as Topic
Plain language summary
Surgery for normal pressure hydrocephalus of unknown cause
Key messages
‐ Surgery to move excessive fluid away from the brain (cerebrospinal fluid (CSF)‐shunting) likely improves walking speed and disability in the short term (less than six months post‐surgery) in people with idiopathic normal pressure hydrocephalus (iNPH).
‐ CSF‐shunting did not cause any deaths, and repeat surgery was rare, but unwanted effects were common in the studies assessed.
‐ More evidence on the effect of CSF‐shunting on quality of life is needed.
What is idiopathic normal pressure hydrocephalus?
Normal pressure hydrocephalus (NPH) is a medical condition where normal fluid‐filled brain structures (ventricles) slowly become larger over time (hydrocephalus), and the surrounding brain structures slowly change to adjust to this. Eventually, the critical brain structures become affected, causing symptoms such as difficulty walking, thinking, and with bladder control. Sometimes there is a clear reason for the ventricles getting larger, for example after a head injury, where blood can 'clog‐up' the ventricles. However, in older adults (over 60 years), NPH develops with no clear cause, known as 'idiopathic' normal pressure hydrocephalus (iNPH).
What did we want to find out?
Since 1965 there have been reports of patients with iNPH getting better when an operation is performed where a tube is inserted into the brain or spinal canal to move the cerebrospinal fluid (CSF) to an area of lower pressure such as the abdominal cavity or right atrium of the heart (CSF‐shunting). However, most of the evidence to support the use of CSF‐shunting has been of low quality, and people having CSF‐shunting were not directly compared with control groups who did not have CSF‐shunting. As such, medical practitioners differ in opinion about the benefit of CSF‐shunt surgery for iNPH. We wanted to compare the existing high‐quality studies to find out if there was evidence showing that CSF‐shunting improved walking (gait), disability, cognitive function (thinking), urinary function, and quality of life. We also wanted to know if CSF‐shunting was associated with any unwanted effects.
What did we do?
We searched for and compared all studies in which people with NPH were assigned randomly to CSF‐shunting or to a control group which was either delayed CSF‐shunting or CSF‐shunt surgery where the shunt was temporarily set to 'inactive' mode. We included only people with NPH who had problems walking, thinking, or with bladder function and no clear cause for their hydrocephalus (iNPH).
What did we find?
We included four studies in the review, but could only use data from three in the analysis. One larger study was from Japan, and three smaller studies were from Sweden, the UK, and a US‐Canadian‐Swedish collaboration. All studies included only iNPH patients (average age 75 years) who had difficulty walking with or without problems with thinking and bladder control. People were observed for 6 to 12 months. The included studies involved a total of 140 participants (73 who had active/immediate CSF‐shunting and 67 controls).
What are the conclusions?
Walking speed probably improves with CSF‐shunting compared with control. CSF‐shunting may improve walking function by an uncertain amount. CSF‐shunting probably results in a large improvement in patient disability. Only 3.4 participants needed to have CSF‐shunting to result in 1 being functionally independent (able to perform activities of daily living). It is unclear if CSF‐shunt surgery has an effect on cognitive function or unwanted effects. There was no information regarding how CSF‐shunting affects quality of life.
What are the limitations of the evidence?
Our confidence in the results for walking speed and patient disability is moderate. Our confidence in the other results is low to very low. The included studies were very small, which means that more studies are needed to increase our confidence in the evidence. Due to the design of the studies we assessed, there was little information about unwanted effects occurring with CSF‐shunting compared to no surgery at all. In participants who had CSF‐shunt surgery, 52% had an unwanted effect of any kind, but the need for repeat surgery after having a shunt was infrequent (8.9%), and there were no deaths that were clearly related to CSF‐shunt surgery. Strokes occurred more commonly than expected in the 12 months following shunt surgery (8%); more research is needed to confirm this finding.
How up‐to‐date is this evidence?
The evidence is current to February 2023.
Summary of findings
Summary of findings 1. Summary of findings table ‐ CSF‐shunting compared to no or inactive CSF‐shunting for idiopathic normal pressure hydrocephalus.
| CSF‐shunting compared to no or inactive CSF‐shunting for idiopathic normal pressure hydrocephalus | ||||||
| Patient or population: idiopathic normal pressure hydrocephalus Setting: secondary care Intervention: CSF‐shunting Comparison: no or inactive CSF‐shunting | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no or inactive CSF‐shunting | Risk with CSF‐shunting | |||||
| Gait speed (< 6 months post‐surgery) | ‐ | SMD 0.62 SD higher (0.24 higher to 0.99 higher) | ‐ | 116 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | CSF‐shunting probably improves gait speed at < 6 months post‐surgery. |
| Qualitative gait function (< 6 months post‐surgery) | 2 studies assessed qualitative gait function. Kazui and colleagues showed a reduction in 0.9 points of iNPHGS‐gait scale (CI 0.6 to 1.2, P < 0.001). Tisell and colleagues showed a 30% improvement in gait, but did not provide standard deviations, thereby preventing meta‐analysis. | 88 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | CSF‐shunting may improve qualitative gait function at < 6 months post‐surgery. | ||
| Patient disability (functionally independent, < 6 months post‐surgery) | 268 per 1000 | 557 per 1000 (351 to 887) | RR 2.08 (1.31 to 3.31) | 118 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | CSF‐shunting probably results in a large increased chance of having a disability status of "good or better" at < 6 months after surgery. |
| Cognitive function (< 6 months post‐surgery) | ‐ | SMD 0.35 SD higher (0.04 lower to 0.74 higher) | ‐ | 104 (2 RCTs) | ⊕⊝⊝⊝ Very lowc,d | CSF‐shunting may increase/have little to no effect on cognitive function, but the evidence is very uncertain. |
| Adverse events (< 3 months post‐surgery) | Kazui and colleagues assessed adverse events for the comparison LP‐shunting vs no shunting. 41% of adverse events occurred in the shunted group (n = 46, 15% major) vs 2% in the non‐shunted group (n = 43, all major) at 3 months.There was no 3‐month mortality.There were no controlled data detected for VP‐shunting vs no shunting.There were no controlled adverse event data at 12 months' post‐shunt. | 88 (1 RCT) | ⊕⊝⊝⊝ Very lowe,f | Adverse events are common post‐LP‐shunt surgery, but there were no controlled data after 3 months, and it is unclear if the reported adverse events were likely the result of shunt surgery. The certainty of the evidence is very low. There were no controlled adverse event data regarding VP‐shunt surgery.e,f | ||
| Quality of life (< 6 months post‐surgery) | No study assessed quality of life. | (0 studies) | ‐ | We did not find any data on the impact of shunting on quality of life in iNPH. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_440424780366411424. | ||||||
a For gait speed, CIs were narrow. The study was larger than the minimum power calculations for a single trial (Kazui estimated the need for 50 participants per arm). However, the overall participant number was < 200 per group, and in keeping with GRADE Handbook we consider there to be serious concerns of imprecision and have downgraded by one level. b Tisell and colleagues did not publish their protocol or data analysis plan, resulting in a risk of bias assessment of some concerns. We downgraded the certainty of evidence by one level. c I² = 75%, suggesting a large degree of heterogeneity. We downgraded the certainty of evidence by one level. d The sample size was very low for this outcome (104), and the CI crossed 0, resulting in concerns about precision. We downgraded the certainty of evidence by two levels. e There was often no description of who screened for adverse events; it is likely this was done in part by the attending surgeons, who would have been aware of the intervention. Participants and carers were also aware of group assignment. Despite this, numerous adverse events were reported in the intervention group and very few in the control group, suggesting that there was no problem with event reporting, although non‐serious adverse events may not have been reported. We downgraded the certainty of evidence by two levels. f The sample size for adverse event assessment was small, which would result in some imprecision. Adverse event assessors were not all blinded to treatment group, so there was serious potential for bias.
Background
Description of the condition
Normal pressure hydrocephalus (NPH) is a clinical syndrome of gait apraxia, cognitive impairment, and urinary incontinence (Adams‐Hakim triad) due to communicating hydrocephalus with normal cerebrospinal fluid (CSF) pressure (Hakim 1965). Before Hakim and Adams' original description in 1965, hydrocephalus was only recognised to occur due to acute intracranial illness, such as an expanding tumour or bleeding, where people presented acutely with signs and symptoms of raised CSF pressure, such as headache and visual loss.
In NPH, it is thought that an initial increase in intracranial pressure causes the intracerebral ventricles to expand and change shape, until a new compensated state occurs, where the CSF pressure is relatively normalised (Hakim 1965). When intracranial pressure rises further, this equilibrium decompensates and leads to a subacute presentation of NPH clinical syndrome (Hakim 1965).
NPH can occur when there is a clear cause for the initial rise in intracranial pressure, such as after brain trauma or central nervous system (CNS) inflammation (Hakim 1965). It can also occur in the elderly population (> 60 years) without a clear cause (termed idiopathic NPH, or iNPH) (Adams 1965). Even in iNPH, reducing the intraventricular pressure by permanent CSF diversion (CSF‐shunting) has been reported to improve symptoms (Adams 1965; Kazui 2015; Tisell 2011).
However, there are no known pathognomonic histological features to characterise the disease (Espay 2017). Problematically, the current diagnostic gold standard is a (variably defined) positive response to definitive CSF‐shunting, which is also the proposed treatment (Espay 2017). Controversy regarding iNPH as a clinical entity remains, as not everyone with iNPH responds to CSF‐shunting (Malm 2006).
Enlargement of the cerebral ventricles (ventriculomegaly) was historically the sole radiological indicator of NPH (Kitagaki 1998), but this is now understood to be common in normal ageing individuals; more than 20% of those over 70 years fulfil the criteria for ventriculomegaly (Jaraj 2017). Several groups noticed specific morphological changes in those with shunt‐responsive iNPH, such as disproportional enlargement of the subarachnoid space hydrocephalus (DESH) and a narrow callosal angle (measured in the coronal plane at the level of the posterior commissure) (Hashimoto 2010; Kitagaki 1998; Kockum 2018). These iNPH‐specific magnetic resonance imaging (MRI) features have been further developed and have been incorporated into recent diagnostic criteria for iNPH (Nakajima 2021). iNPH radiological grading scales are used to identify those who definitely do not have shunt‐responsive iNPH (Kockum 2020).
Potential disease mechanisms in iNPH are poorly understood, but reduced CSF conductance, reduced pulse pressure across the cerebral aqueduct, reduced CSF production and turn‐over, impaired regional cerebral perfusion, impaired glymphatic drainage, and build‐up of toxic metabolites have all been reported in iNPH (Bradley 2015; Momjian 2004; Ringstad 2017; Silverberg 2003).
Cilia are present in the CNS, and have an active role in the development of choroid and ventricular function (Banizs 2005). It is well understood that dysfunction of CNS cilia is associated with hydrocephalus (Banizs 2005; Louvi 2011). Autosomal dominant mutations in the cilia and flagella associated protein 43 (CFAP43) gene, which encodes a cilial protein, are usually seen in primary cilial dyskinesia, but were recently found in a Japanese person with familial NPH (Morimoto 2019). The further discovery that cell wall biogenesis 43 C‐terminal homolog (CWH43) mutations can induce hydrocephalus in mice, which have reduced ventricular cilial density, and that these mutations are over‐represented in people with purported idiopathic NPH, suggest that CNS cilial function is important in the development of NPH (Yang 2021).
Diabetes mellitus, obstructive sleep apnoea, and schizophrenia are all more common in people with iNPH than in their age‐matched controls, but the nature of these relationships is not known (Hudson 2019; Román 2018; Vanhala 2019).
Comorbid neurodegenerative disease is also common in people with iNPH; there will usually be evidence of neurodegenerative disease at postmortem (Cabral 2011). However, people with possible iNPH who have CSF or neuropathological findings consistent with Alzheimer’s disease do not respond differently to CSF removal or shunting (Müller‐Schmitz 2020; Yasar 2017). As the radiological features specific to iNPH are thought to develop over time in ageing individuals, from an asymptomatic to symptomatic stage (Kimihira 2020), it is not surprising that they are also seen in the elderly population who present with symptoms of neurodegenerative disease (Ohara 2020). The relationship between NPH, ageing, and neurodegenerative disease is likely to be complex.
Description of the intervention
CSF‐shunting is the process during which the CSF volume is reduced by surgically inserting a catheter to divert CSF to an area of lower pressure. Initial studies in NPH used ventriculoatrial (VA) shunts, which relocated CSF to the right atrium of the heart (Hakim 1965). Due to potentially serious cardiac complications of VA shunting (Lam 1997), lumboperitoneal (LP) or ventriculoperitoneal (VP) shunts are now used routinely, diverting CSF to the peritoneum (Kazui 2015; Tisell 2011). Ventriculopleural (VPl) shunts may be considered when VP or LP shunts are contraindicated (Craven 2016). Third ventriculostomy is another diversion procedure, during which a hole is created to divert CSF from the third ventricle of the brain to an extra‐parenchymal CSF space.
How the intervention might work
In NPH, cerebral ventricles change shape to accommodate for a rise in pressure, and the frontal lobes become compressed, causing a classical appearance with a tight, high convex, and a reduced callosal angle, with widening of the Sylvian fissures (Kitagaki 1998). Pathology in the frontal cortical and subcortical regions is known to cause cognitive decline, gait apraxia, and urinary symptoms, which are seen in iNPH (Hakim 1976; Ogino 2006; Sakakibara 2008). The amount of mass effect (or change in shape due to pressure effects) seen in the superior cortical structures appears to correlate with the effect of CSF‐shunting (Narita 2016). In people with NPH, a reduction of CSF conductance and periventricular cerebral perfusion are also seen with lumbar infusion testing (Børgesen 1982; Momjian 2004); the former has an inverse relationship with the effect of CSF‐shunting (Børgesen 1982). Consequently, there are plausible mechanisms to explain how decompensation of an NPH state can cause Hakim's triad of symptoms, and how permanent CSF diversion can help normalise the effects of pressure and improve symptoms of people with iNPH.
Why it is important to do this review
Because of the evolution of clinical and radiological definitions of iNPH over time, there is a lack of high‐quality, standardised epidemiological data (Zaccaria 2020). The prevalence has been estimated as 29/100,000, with an incidence of 7.3/100,000/year, rising to 1.2/1000 in those over 70 years of age (Zaccaria 2020). However, cardinal symptoms of iNPH are both non‐specific and common (Macki 2020). High‐quality, community‐based, prospective studies show that up to 3.7% of those over 65 years of age fulfil the clinical criteria for iNPH, and on cranial imaging show radiological features specific to iNPH (Andersson 2019). In the UK and Ireland, between 2004 and 2013, 14% of CSF shunts (2173) were inserted for iNPH, and the number seems to be rising (Fernández‐Méndez 2019). People commonly present to clinicians with possible iNPH, and many have surgery. There is a need for clear evidence about the role of CSF‐shunting.
Several reviews have concluded that there is a role for CSF‐shunting in iNPH (Giordan 2018; Halperin 2015; Hebb 2001; Toma 2013). However, there are few meta‐analytic data regarding the effect size, and systematic reviews restricted to randomised controlled trials (RCTs) have not reached similar conclusions (Esmonde 2002). As such, there remains uncertainty in the neurological community regarding iNPH management.
Since the last Cochrane review of shunting in NPH (Esmonde 2002), there have been advances in understanding specific radiological features of iNPH (Kockum 2020; Narita 2016), and an evolution of clinical criteria for iNPH (Nakajima 2021; Relkin 2005). There have also been new RCTs that assess the effect of CSF‐shunting on iNPH, which have taken these updated criteria and imaging features into account. We thus considered it timely to conduct a systematic review on the effects of CSF‐shunting on people with iNPH, in order to help guide management decisions.
Objectives
To determine the effect of cerebrospinal fluid (CSF)‐shunting versus no CSF‐shunting in people with idiopathic normal pressure hydrocephalus (iNPH) and the frequency of adverse effects of CSF‐shunting in iNPH.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs evaluating the effect of shunting on iNPH.
People may be reluctant to participate in clinical trials in which they are at risk of not being shunted. As anticipated, we encountered only trials with a one‐arm, cross‐over design, in which half of the cases had the intervention initially, the other having 'delayed' intervention at a later time point (Saper 2017). In trials of this design, there is an early post‐randomisation time period when there are parallel intervention and control groups. These early results are included here, to investigate the effects of the intervention. We included both blinded and unblinded studies.
Types of participants
We included participants with at least one symptom of the Adams‐Hakim triad: gait apraxia, dementia, or urinary incontinence, and an Evans index of > 0.3 on cranial imaging. Participants were required to be at least 60 years of age, and have normal CSF opening pressure.
We excluded participants with potential secondary causes of NPH, such as previous head trauma, meningitis, or subarachnoid haemorrhage.
These criteria are consistent with the Japanese Society of Normal Pressure Hydrocephalus Guidelines (Nakajima 2021), except that we defined elevated opening pressure as > 24.5 cm of water, which is consistent with international diagnostic guidelines in iNPH, and normal CSF reference ranges used in other neurological fields (Mollan 2018; Relkin 2005). We included studies in which participants may have had only one of Hakim's triad of symptoms, to ensure we did not exclude studies conducted before current diagnostic guidelines had become available. We performed a sensitivity analysis to determine the effect of including these studies (Ishikawa 2004; Relkin 2005). Similarly, we did not restrict participants to those who had tight, high convexity and enlarged Sylvian fissures on cranial imaging, or those who had positive 'provocative' CSF testing. Instead, we performed sensitivity analyses to understand the effect of including only studies with these restricted criteria.
We planned to include studies with some participants who fulfilled our criteria if we were able to extract controlled data for those individuals, or if the majority of participants met our entry criteria, suggesting that the average participant studied met our eligibility criteria. In the latter case, we would apply sensitivity analysis to understand the impact of including such studies.
Types of interventions
Experimental interventions included any permanent CSF‐shunting technique for the treatment of iNPH, including VP shunt, LP shunt, VA shunt, VPl shunt, or third ventriculostomy.
Comparator interventions included no CSF‐shunting, or the insertion of a shunt, but with the programmable valve not yet activated to a draining position (placebo shunt).
Types of outcome measures
Broadly, the outcome categories were as follows.
Gait function
Cognitive function
Urinary function
Disability
Quality of life
Adverse events
We assessed non‐adverse outcomes after CSF‐shunting in the short term (< 12 months). Due to the preferred cross‐over trial design in the field of study, there were no parallel control data for long‐term outcomes (> 12 months). We provided a narrative description of available non‐random long‐term data (> 12 months).
We considered gait function the primary efficacy outcome, along with disability and quality of life, because this is usually pivotal in the decision to conduct CSF‐shunting.
Primary outcomes
Gait speed (short term), measured with validated global gait tools, e.g. the timed up and go (TUG) test, or 10‐metre walk test (10 MWT).
Qualitative gait function (short term), measured with validated qualitative gait tools, e.g. the Tinetti score, or the Kubo NPH grading scale.
Patient disability (short term), measured on global disability scales, e.g. modified Rankin Scale (mRS).
Quality of life (QoL; short term), measured with validated QoL scales.
Adverse events (short term), documented separately for serious complications and death. Where possible, we tried to categorise adverse events in keeping with international guidance on adverse event reporting if they had not already been categorised this way (ICH 1994).
Adverse events (long term), documented separately for serious complications and death.
Secondary outcomes
Gait speed (long term), measured with validated global gait tools, e.g. the TUG test or 10 MWT.
Cognitive function (short term), measured with validated global cognitive screening tools, such as the Montreal Cognitive Assessment (MoCA), the Mini‐Mental State Examination (MMSE), or the idiopathic normal‐pressure hydrocephalus grading scale (iNPHGS) (Hellström 2012).
Qualitative cognitive function (short term), measured with validated scales (e.g. Keifer scale or iNPHGS).
Cognitive function (long term), measured with validated global cognitive screening tools, such as the MoCA, MMSE, or iNPHGS (Hellström 2012).
Urinary function (short term), measured on any appropriate scale.
Search methods for identification of studies
We searched the reference lists of full‐text papers, including relevant published systematic reviews, for further references, and review authors searched for personal holdings of references to reports and trials.
Electronic searches
We searched the Cochrane Dementia and Cognitive Improvement Group’s Specialised Register. The Register was maintained by the Information Specialists of the Cochrane Dementia and Cognitive Improvement Group and contained studies in the areas of dementia (prevention and treatment), mild cognitive impairment and cognitive improvement. We performed additional searches in the following databases to cover the time frame from the last searches of the Register to ensure that the search for the review was as up‐to‐date and as comprehensive as possible.
Monthly searches of a number of major healthcare databases: MEDLINE, Embase, CINAHL (Cumulative Index to Nursing and Allied Health Literature), PsycINFO, and LILACS (Latin American and Caribbean Health Science Information database).
Monthly searches of the following trial registers: the World Health Organization International Clinical Trials Registry Platform (which covers ClinicalTrials.gov, ISRCTN, the Chinese Clinical Trials Register, the German Clinical Trials Register, the Iranian Registry of Clinical Trials, and the Netherlands National Trials Register, plus others) and ClinicalTrials.gov.
Quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL).
Six‐monthly searches of a number of grey literature sources from Web of Science Core Collection.
We used the Cochrane Highly Sensitive Search Strategy in MEDLINE, Embase, and CINAHL Plus. The search strategies are described in Appendix 1. The most recent search was carried out on 15 February 2023.
Searching other resources
We searched the reference lists of full‐text papers, including relevant published systematic reviews, for further references. We emailed the authors of included RCTs asking for further information that was not available in the publication. Additional data were provided by the study authors (Table 2).
1. Additional data requests*.
| Study | Contact | Date | Data requested | Replied | Data provided |
| Tisell 2011 | Magnus Tisell | 19 August 2022 | IPD | Yes | No longer available |
| Toma 2016 | Ahmed Toma | 13 March 2022 | Full IPDs were requested as the study was only formally published in truncated form. | Yes | Full available data set provided. |
| Kazui 2015 | Hiroaki Kazui | 22 August 2022 | Inclusion/exclusion criteria. Disability data by mRS score group | Yes | Requested data provided. |
| Luciano 2023 | Marc Luciano | 26 August 2022 | Pre‐publication of PENS we requested IPDs and disability data by mRS score group. Raw OAB scores in delayed‐shunt group | Yes | Data provided. |
Abbreviations: IPD: individual patient data set; mRS: modified Rankin Scale; OAB: Overactive Bladder Questionnaire
*Table documenting requests and additional data provided by the authors of randomised controlled trials identified in our search.
Data collection and analysis
Following de‐duplication, we imported into Covidence all references identified in the searches. Two of three review authors (RP, CC, or AM) independently examined titles and abstracts of citations obtained from the searches, excluding any clearly ineligible or duplicate articles, using Covidence (Covidence).
Selection of studies
Following the initial screening, we independently assessed the full‐text articles of potentially relevant studies for inclusion in the review based on our predefined inclusion and exclusion criteria. A third review author arbitrated any disagreements to reach consensus. We identified and recorded reasons for exclusion of the ineligible studies. We recorded the study selection process in a PRISMA flow diagram (see Figure 1) (Moher 2009). The review authors were not blind to trial authors, institutions, or journals.
1.
PRISMA flow diagram.
Data extraction and management
We piloted the extraction process on two studies in the review. We used Covidence to manage study selection (Covidence).
Two review authors (AG, AM, CC, or RP) independently extracted study characteristics and outcome data from the included studies. Study characteristics included: study design, setting, characteristics of participants (e.g. gender, age, ethnicity, disease severity, number of Hakim's triad of symptoms needed to fulfil NPH criteria for the study), randomisation, eligibility criteria, intervention details, type of control, outcomes assessed, and source of study funding. We assessed conflicts of interest by searching the published article and where available the study protocol. Any disagreements were moderated by a third review author.
For each outcome of interest, we extracted mean scores and standard deviations (SDs). We exported data from Covidence to RevMan software (RevMan 2024). When continuous outcome measures were reported using different scales for the same construct, the lead review author (CC) derived standardised mean differences, as outlined in Section 6.6.2.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
Assessment of risk of bias in included studies
Two review authors (AM, CC, or RP) independently assessed risk of bias in the included studies using the RoB 2 tool (Higgins 2023; Sterne 2019). Any disagreements were resolved by discussion with a third review author (AG) to reach consensus. The effect of interest was the intention‐to‐treat (ITT) effect.
We assessed risk of bias for each study outcome for which there were RCT data, using the following Cochrane RoB 2 criteria.
Bias arising from the randomisation process
Bias due to deviations from intended interventions
Bias due to missing outcome data
Bias in measurement of the outcome
Bias in selection of the reported result
For each domain, we answered a series of signalling questions with yes, probably yes, no information, probably no, or no, to determine the risk of bias (low risk, some concerns, or high risk). We included text alongside our risk of bias judgements to support our decisions.
We judged risk of bias for each outcome (e.g. gait speed (short term)) by its performance in each risk of bias domain. In keeping with Section 8.2.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2023), we considered an outcome to be at low risk of bias if all domains for the result were judged to be low risk. We judged an outcome to be at overall some concerns if at least one domain was some concerns, but no domains were at high risk of bias for the result. We judged an outcome to be at high risk of bias if at least one domain was at high risk of bias for the result, or if there were some concerns for multiple domains such that our confidence in the result was substantially lowered. We have summarised the risk of bias in traffic lights on the relevant forest plots.
Measures of treatment effect
We calculated effect estimates with 95% confidence intervals (CIs), using time point scores for each trial outcome. Ordinal outcome rating scales with more than 10 categories were treated as continuous scales arising from a normal distribution. When outcomes were measured on a single continuous scale, the measure of treatment effect was the mean difference (MD). When the same outcome was measured on different scales, we used the standardised mean difference (SMD). We used 'Guiding rules' for interpreting SMDs (or Cohen’s effect sizes), as outlined in Section 15.5.3.1 of the Cochrane Handbook for Systematic Reviews of Interventions, where a difference of 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Schünemann 2021b).
Unit of analysis issues
There were several unit of analysis issues to contend with in this field of study.
One‐arm cross‐over trial design
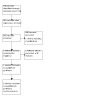
There is a favoured one‐arm cross‐over trial design in iNPH, in which control participants are initially given CSF shunts that are not yet set to a draining position or no treatment (Saper 2017) (Figure 2). At an early time point (usually three to four months), the control group has delayed CSF‐shunting if they have not had a shunt, or the inactive shunts are turned to the active position. In this paradigm, there are no long‐term controls for parallel‐group assessment, as all participants end the trial in the treatment intervention group.
2.

Schematic of a 'one‐arm, cross‐over' randomised controlled trial design. Half of participants start the study with no cerebrospinal fluid (CSF) shunt and half with an inactive CSF shunt. All participants conclude the study with active CSF shunting. Schematic produced by C Carswell.
Consequently, in one‐arm cross‐over trials, we compared short‐term data between the intervention group (CSF shunt) and the control group (no shunt or inactive shunt), as for a parallel RCT design.
With a cross‐over trial design, the groups assigned to the control have delayed CSF‐shunting, and it is possible to analyse the effect of shunting in this group in a paired fashion (a pre‐post, or 'before‐and‐after' study design). These are randomised data, even though all the cases have the control intervention first, but we did not include these data in the main analysis/summary of findings tables as a three‐month delayed CSF‐shunt is an artificial construct which is not encountered in clinical practice. We did conduct analysis of these groups to compare them with unpaired parallel‐group assessments.
Outcomes
For some outcomes (e.g. disability), we anticipated that we would encounter commonly applied, non‐linear ordinal rating scales with few categories (e.g. mRS). In this scenario, we initially intended to treat the data as continuous data, contextualising the effect size by referring to the original scale. In some cases, we had independent patient data sets (IPDs) and were able to binarise the scale to compare outcome effects in a tangible way.
We anticipated that there would be multiple measures of the same outcome. When this was the case, we used the following principles to guide the selection of measures for data extraction, which are similar to those used by Bahar‐Fuchs (Bahar‐Fuchs 2019).
We used common and preferred outcome measures if reported by studies (e.g. TUG test or gait speed). When these were not available for a given study, we used the most similar test reported.
If multiple relevant scales were presented to measure the same outcome, we considered creating a composite outcome score, as described in Bahar‐Fuchs 2019. However, we did not encounter this situation and did not have to create composite outcome scores.
Dealing with missing data
We contacted study investigators to obtain missing outcome or baseline characteristic data, when needed.
Where change in baseline data were not available, we compared time point data.
When we acquired the data it became apparent that one study had performed ITT analysis by carrying forward the last known value for disability on those lost to follow‐up (Kazui 2015). This will underestimate any positive effect of shunting and seemed reasonable. As we had near full data sets for parallel assessment of post‐shunt disability status, one review author (CC) computed similar values for missing data from Luciano and Toma to present similar ITT analysis for this outcome (Luciano 2023; Toma 2016). Two review authors (AG and RP) reviewed these computations to ensure accuracy.
Assessment of heterogeneity
We assessed statistical heterogeneity using a standard Chi² statistic and the associated l² statistic. Consistent with recommendations from Deeks 2021, for studies with a small sample size, we deemed heterogeneity to be present when the Chi² statistic was significant at the P = 0.1 level, or when l² suggested that more than 40% of the variability in the effect estimate was due to heterogeneity. We initially planned to visually examine forest plots to identify heterogeneity, but we identified too few studies to make this method valid.
Assessment of reporting biases
We assessed within‐trial reporting bias as part of our risk of bias assessment, by evaluating whether outcomes specified in the methods section of the included studies were reported in the results. When our searches identified unpublished studies, we attempted to contact the investigators for results and a status update (Table 2).
Data synthesis
We used RevMan software to perform meta‐analysis (RevMan 2024). We used fixed‐effect meta‐analysis, as the studies were small and had very similar designs.
Subgroup analysis and investigation of heterogeneity
As anticipated, we identified only a few studies with relatively low sample sizes, and thus did not undertake subgroup analysis.
Sensitivity analysis
We performed the following sensitivity analyses.
We removed studies with high risk of bias from the analysis for the major outcomes.
We removed studies that used no‐shunt comparator groups (as opposed to placebo‐shunt comparator groups).
We planned to remove studies in which the majority of participants had only one of the Hakim‐Adam's triad of symptoms, but did not detect any such studies.
We re‐ran the analyses using a random‐effects model to test the robustness of findings with the meta‐analytic model used.
We found that some studies included specific NPH‐radiological findings, or else used positive 'provocative' tests as entry requirements; where possible, we performed sensitivity analysis of these entry criteria.
Summary of findings and assessment of the certainty of the evidence
We assessed the certainty of evidence for each outcome based on the GRADE approach, as described in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2021a).
Two review authors (CC and AG) independently applied the GRADE approach, assessing the certainty of evidence as high, moderate, low, or very low. We discussed the certainty of evidence ratings for each outcome with other members of the review team. A third review author (RP) arbitrated any disagreements to reach consensus for final decisions on the ratings.
We considered the following factors when deciding whether to downgrade the certainty of evidence in relation to each outcome.
Risk of bias
Inconsistency of results
Indirectness of evidence
Imprecision of results
Publication bias
Since we only included RCTs, we started with a high‐certainty rating for each outcome. We downgraded the certainty of the evidence by one level if we considered there to be a serious limitation in relation to a particular factor, or by two levels if we considered there to be a very serious limitation. We documented our reason(s) for downgrading the certainty of the evidence in footnotes.
We generated a summary of findings table using GRADEpro GDT software (GRADEpro GDT), which compared CSF shunt to no shunt or a placebo shunt (using randomised parallel‐group data only) for the following outcomes.
Gait speed (short term)
Qualitative gait function (short term)
Patient disability (short term)
Cognitive function (short term)
Adverse events (short term)
We intended to consider the following outcome but did not find sufficient data to analyse.
QoL (short term)
Results
Description of studies
Results of the search
We identified 942 studies in our search (Figure 1; Appendix 1). After removal of 40 duplicates, 902 studies were imported to Covidence. Of these, 896 were excluded as they did not meet our Population, Intervention, Comparison, Outcomes, and Study (PICOS) criteria (Figure 1). The main reason for exclusion was irrelevance or non‐RCT design. One study, NCT01798641, was an RCT with a traditional cross‐over trial design and fulfilled our inclusion criteria. Although some results were listed in ClinicalTrials.gov, it has not yet been published in peer‐reviewed literature. The study author did not answer our request for further information, and we could not include this study (Figure 1; Characteristics of studies awaiting classification) (NCT01798641). One RCT that aimed to assess the effect of gradual pressure lowering in iNPH in the control group was excluded at the full‐text stage because it was unsuitable for further comparison with the other included studies (Figure 1; Characteristics of excluded studies) (Saehle 2014).
We included four studies in the review (Figure 1; Characteristics of included studies) (Kazui 2015; Luciano 2023; Tisell 2011; Toma 2016). The Kazui study compared immediate lumboperitoneal (LP)‐shunting with delayed LP‐shunting. The remaining studies all compared active versus inactive ventriculoperitoneal (VP)‐shunting; for inactive VP‐shunting, Tisell tied ligatures to the shunt catheter to prevent active draining (the ligatures were later untied in these individuals), and Luciano and Toma both initially set the shunt valve to a pressure setting that was so high that CSF drainage was unlikely (Luciano 2023; Tisell 2011; Toma 2016). We included data from Tisell in our qualitative analysis but did not have data with information about population variation to permit inclusion in formal meta‐analysis (Tisell 2011). Toma was not published in full, but Mr Ahmed Toma gave us access to his peer‐reviewed thesis containing a near‐complete IPD (Toma 2016). Likewise, Mr Marc Luciano, Mr Hiroaki Kazui, and Mr Magnus Tisell all responded to our request for information (Kazui 2015; Luciano 2023; Toma 2016). Original raw data sets were not available for the Tisell study (Table 2) (Tisell 2011).
The key characteristics of RCTs identified in our search are summarised in Table 3. We are aware of one ongoing study that meets our PICOS criteria (Characteristics of ongoing studies) (NCT05081128).
2. Summary of key characteristics of iNPH randomised controlled trials.
| Study | Study design | Location | Blinding | Participants | CSF testing entry criteria | Radiological criteria | Number randomised/shunted | Intervention | Published in medical literature |
| Tisell 2011 | 1‐arm, cross‐over RCT | Single centre, Sweden | Double (participant & assessor) | Symptomatic iNPH > 60 years with Binswanger's | Negative CSFTT and LIS | Evans index > 0.3, Binswanger's disease* | 14/14 | VP shunt | Full |
| Toma 2016 | 1‐arm, cross‐over RCT | Single centre, UK | Double (participant & assessor) | Symptomatic iNPH > 60 years | Positive ELD | Evans index > 0.3 | 15/15 | VP shunt | Partial |
| Kazui 2015 | 1‐arm, cross‐over RCT | Multicentre, Japan | Not blinded | Symptomatic iNPH > 60 years | Normal CSF opening pressure | Evans index > 0.3 and high‐convexity and medial subarachnoid space tightness | 93/88 | LP shunt | Full |
| Luciano 2023 | 1‐arm, cross‐over RCT | Multicentre: USA, Canada, Sweden | Double (participant & assessor) | Symptomatic iNPH > 60 years | Positive CSFTT or ELD | Evans index > 0.3 | 18/18 | VP shunt | Full |
Abbreviations: CSF: cerebrospinal fluid; CSFTT: CSF tap‐test; ELD: external lumbar drain; iNPH: idiopathic normal pressure hydrocephalus; LIS: lumbar infusion study; LP: lumboperitoneal; RCT: randomised controlled trial; VP: ventriculoperitoneal
*Binswanger's disease defined by a score of 2 to 3 on Wahlund scale.
Included studies
For details, see Characteristics of included studies.
The four included studies enrolled a total of 140 participants (73 in the shunt group and 67 in the control group who had delayed CSF‐shunting). One large study with 93 participants took place across 20 sites in Japan (Kazui 2015). The other three studies were small or pilot trials: 18 participants were enrolled in a multisite US, Canadian, and Swedish study (Luciano 2023); one study enrolled 15 participants at a single centre in the UK (Toma 2016); and 14 participants were enrolled at a single centre in Sweden (Tisell 2011). The pooled study population was small, and only 135 participants in total received shunt surgery in the four studies: 88 had lumbar surgery, and 47 had VP shunt surgery (Kazui 2015; Luciano 2023; Tisell 2011; Toma 2016).
Participants
All studies investigated adults over 60 years of age with iNPH only. No study attempted to distinguish between those with macrocephaly and chronic hydrocephalus and those with adult‐onset hydrocephalus. The mean age (SD) was 75.1 (1.2) in the shunt group and 75.0 (1.0) in the control group in the three studies reporting summary statistics for age. Three studies reported participant sex: 46%, 44%, and 19% of participants were female in Kazui, Luciano, and Toma respectively (Kazui 2015; Luciano 2023; Toma 2016). No data were reported on ethnicity in any of the studies. On average, 63% of participants were hypertensive (range 55% to 71%). A full set of vascular risk factors was only presented by Kazui, where 26% were diabetic, 25% had hyperlipidaemia, and 8% were current smokers (Kazui 2015).
Toma and Luciano required gait impairment and one other of Hakim's triad symptoms to be eligible (Luciano 2023; Toma 2016). Kazui required only one of Hakim's triad symptoms for eligibility, but 90% of enrolled cases had two of Hakim's triad symptoms, and 91% had gait impairment (Table 3) (Kazui 2015), so the populations were similar in symptom profile. Tisell did not specify which NPH symptoms were required for study entry.
There were key differences in study eligibility criteria for brain imaging in the included studies (Table 3). Luciano and Toma required all participants to have an Evans index > 0.3 (Luciano 2023; Toma 2016). Kazui also required all participants to have "high‐convexity and medial subarachnoid space tightness" on coronal sequences (Kazui 2015). Tisell designed a study to investigate patients with radiological features of both iNPH (Evans index > 0.3) and cerebral small vessel changes (Binswanger's disease), scoring 2 to 3 on the Wahlund scale (Table 3) (Tisell 2011).
There were also key differences in the study eligibility requirements regarding CSF testing (Table 3). Kazui was the only study to require no positive 'provocative' CSF test; they required only normal CSF contents and opening pressure (Kazui 2015). Conversely, Tisell investigated a CSF tap test (CSFTT) unresponsive population who also had a normal lumbar infusion test (LIS) (Tisell 2011). Toma required participants to have a positive response to external lumbar drain (ELD) (Toma 2016), and Luciano required participants to have a positive response to CSFTT or ELD (Luciano 2023).
The studies varied in their exclusion criteria (Characteristics of included studies), with Toma and Luciano including only patients who were ambulant after provocative testing (Luciano 2023; Toma 2016). Luciano also limited their study to patients who walked slower than 1 m/s, and excluded those who had medical conditions that would interfere with the assessment of gait (Luciano 2023). Toma excluded patients with evidence of concomitant Alzheimer’s disease or vascular dementia, but did not define diagnostic criteria for these conditions (Toma 2016). Luciano initially excluded individuals with a MoCA level (< 18/30) or those with psychiatric or movement disorders, but these restrictions were lifted part way through the study as long as these disorders were not thought to be the cause of the patient's symptoms (Luciano 2023; Toma 2016). Luciano excluded patients on anticoagulation (Luciano 2023). The Kazui study had a highly refined population and excluded patients who had severe spinal disease, back pain, history of cancer, bleeding, liver or renal disease, coagulopathy, concomitant disorders that may explain NPH symptoms, and patients who were not thought to be appropriate for study entry (Kazui 2015).
Study designs and types of interventions
All studies had the anticipated one‐arm, cross‐over trial design (Figure 2), but there were other important differences in trial design and interventions. Kazui performed LP‐shunt surgery in the intervention group and delayed LP‐shunt surgery three months after trial onset in the control group (Kazui 2015). This study was randomised and controlled but was not participant or assessor blinded. Despite the lack of participant blinding, 88 of the 93 enrolled participants had their intended intervention. The other studies all had a similar design where participants all had VP‐shunt surgery, but the control group had their shunt turned to an active position after three months (Tisell 2011; Toma 2016) or four months (Luciano 2023) post‐surgery. Tisell, Toma, and Luciano were participant and assessor blinded. All four studies had early time point data in which the active intervention and control arms could be assessed in a standard parallel fashion.
Also, as anticipated, all trials could also be analysed using paired assessment in the control group which had an initial 'inactive shunt' or 'no shunt' period before crossing over to delayed active‐shunting. The post‐shunt assessment times in the paired analysis varied between 8 months (Luciano 2023), 3 or 9 months (Toma 2016), and 12 months (Kazui 2015) (Figure 2). As all these participants in all studies ended the study with an active shunt, there were no true 'long‐term', controlled outcome data. All studies had follow‐up data up to 12 months from study onset, apart from Tisell, who performed the last assessment at 6 months with qualitative follow‐up at a more remote time point (Tisell 2011).
Types of outcomes
All studies measured gait and cognitive function outcomes (Kazui 2015; Luciano 2023; Tisell 2011; Toma 2016). All studies except Tisell included urinary function and disability as outcomes (Tisell 2011). Additionally, Kazui measured independence using the level of independence in activities of daily living in the long‐term care insurance system in Japan (Kazui 2015), and Luciano measured independence using the Lawton Activities of Daily Living Questionnaire (Luciano 2023); Kazui measured carer burden (Kazui 2015); and Luciano measured levels of depression using the Beck Depression Inventory (Luciano 2023). No study measured quality of life (Table 1). Situations where outcomes were measured using multiple scales or in non‐standard ways are described in more detail below.
Gait
Kazui measured qualitative gait function using a semi‐ordinal scale, the iNPHGS‐gait scale. Tisell used a composite score of a series of gait scales including the 10 MWT, TUG, time to sit from lying, time to ascend and descend six flights of stairs, number of steps to turn 180°, number of steps to walk 3 m backwards (Kazui 2015; Tisell 2011).
All four included studies measured gait speed in participants who had active CSF‐shunting versus those who did not (Kazui 2015; Luciano 2023; Tisell 2011; Toma 2016). Toma measured the time to walk 10 m, and Luciano measured 10‐metre velocity (Luciano 2023; Tisell 2011). Kazui measured the time to complete a 3‐metre TUG and also a 3‐metre reciprocating walking test (WT) (Kazui 2015). We did not have IPD for Kazui for these outcomes and were unable to create a composite score for these two ways of measuring gait speed. In keeping with our original protocol, we used the TUG, as it is a more established scale for this outcome in NPH research and also had a more complete data set (Bluett 2023). We performed sensitivity analysis using reciprocated 3‐metre WT instead of 3‐metre TUG in Kazui, but it did not change the results (Kazui 2015). Tisell measured gait speed in multiple ways but did not provide individual data for gait speed subcategories, and we could not directly compare these data with those from other studies (Tisell 2011).
Disability
Luciano and Kazui measured disability using the mRS scale; Toma used the Stein‐Langfitt scale, but only for up to six months post‐study onset (Kazui 2015; Luciano 2023; Toma 2016).
Cognitive function
Kazui was the only group to measure cognitive function using a semi‐ordinal scale, iNPHGS‐cog. They also measured cognitive function using the MMSE. Furthermore, they measured cognitive subdomains of executive function using the Frontal Assessment Battery, attention and executive function (task‐switching) using the Symbol Digit Test of the Wechsler Adult Intelligence Scale, Third Edition (WAIS‐III), and attention using Trails‐A (Kazui 2015). Luciano measured cognitive function using the MoCA, and also measured attention and task‐switching using the Symbol Digit Modalities Test (SDMT) (Luciano 2023). Toma measured cognitive function by formal neuropsychological assessment without a clear standardised battery or general screening tool (Toma 2016). Tisell measured cognition using a composite score, including the Bingley Test, Rey Auditory Verbal Learning Test, Reaction Time Test, Identical Forms Test, Stroop Test, Grooved Pegboard Test, and Tracks Test. For gait function, they created a maximum ceiling to this composite scale (which was the basis for the development of the iNPHGS) (Hellström 2012; Tisell 2011). The scale‐ceiling was part of a predetermined plan (personal communication from Mr Magnus Tisell). No individual data for each subscale were provided.
Adverse events
Adverse events (AEs) were recorded for all included studies, but the definitions and methods for observing and recording them varied between studies.
Adverse events (short term)
Controlled data
Kazui recorded all AEs regardless of whether they were suspected to be related to the treatment (Kazui 2015). AEs were subdivided by severity into serious adverse events (SAEs) and "non‐serious" AEs using traditional nomenclature; it is reasonable to assume that definitions were standard for clinical trials (ICH 1994). AEs were documented for both immediate LP‐shunt and delayed LP‐shunt groups at an early time point and after 12 months of active shunting, which allowed comparison of LP shunt versus no shunt and also a comparison of AEs in groups with immediate and delayed LP shunts. AEs were assessed in terms of "relatedness", which was summarised in their manuscript. They did not publish details regarding how AEs were screened for, by whom, or whether they were blinded to the treatment group. There were no data regarding the 'expectedness' of AEs.
Non‐controlled data
Luciano defined AEs as "untoward medical occurrences experienced by a subject" (Luciano 2023). Falls were not considered an AE, unless the fall led to the requirement of medical care. For this study, AEs included an empirical drop in MoCA scores by 2 points. SAEs were defined in keeping with standard definitions (ICH 1994). Study sites recorded all new or worsening symptoms or events recorded in the medical notes or reported by participants up to 30 days' post‐surgery. Participating sites then reported all neurological, urological, and SAEs throughout the course of the trial (meaning that all non‐serious AEs reported in this trial occurred in the first 30 days' post‐CSF shunt). AEs were monitored by an independent data and safety monitoring board. All AEs were assessed for "relatedness" by the study investigators as not related, possibly related, or probably related. Equally, all AEs were assessed for expectedness. AEs between active and inactive shunts were only described at the conclusion of the study when all participants had received active CSF‐shunting (Luciano 2023). Selected "events of interest" were reported in Table 3 of the published report comparing those with initially active and inactive CSF shunts (at study endpoint), and a full report of all AEs was published in appendices with full details of severity of AEs and relation to NPH surgery.
Toma described seven "procedural complications" in his thesis in narrative form, which all appeared to fulfil the criteria for SAEs. He states that three events were unrelated to shunt surgery, but does not specify the 'seriousness' or 'expectedness' of the events. Non‐serious AEs were not reported in this study. There was no information about how AEs were screened for or blinding of reporters.
Tisell published a narrative of "procedural complications". They did not report using AE terminology and did not subcategorise events formally in terms of SAE or AE. They described the narrative severity of each complication. No non‐serious symptomatic AEs were reported in Tisell, which is notable as all 14 participants had repeated lumbar infusion studies to confirm shunt status three and six months after shunt surgery. The active VP‐shunt group participants also had a sham operation three months after surgery where the original surgical incision was re‐incised and sutured up again, whereas the initially inactive VP‐shunt group had an operation to untie the ligature that was preventing active CSF‐shunting (Tisell 2011). Tisell did not publish details about how AEs/complications were screened for or whether these participants were blinded to the treatment group. They did not always say if procedural complications occurred in those with active or initially inactive shunts.
Adverse events (long term)
Tisell reported long‐term outcomes of 13 (out of 14) participants between 15 and 61 months post‐CSF‐shunt surgery (Tisell 2011).
Urinary function
Luciano measured urinary function using the Overactive Bladder Questionnaire ‐ Short Form (Luciano 2023). Kazui measured urinary function using the semi‐ordinal scale iNPHGS‐urine (Kazui 2015); Toma used a similar bespoke semi‐ordinal scale (Toma 2016). Kazui and Toma did not break data down by numbers of participants in each score group, so we were unable to directly compare the results or binarise the data.
Method of analysis
Tisell had a full data set for all outcomes measured in all participants and performed a true ITT analysis (Tisell 2011). The adherence to group assignment for all other studies was very high at early time point parallel assessment (three or four months post‐study onset); these studies performed a modified ITT analysis. The one exception to this was for Kazui for the outcome parallel assessment of disability, where missing data were computed by carrying forward the last known measure, and a true ITT analysis was performed (Kazui 2015).
Excluded studies
Saehle was a prospective double‐blinded, randomised, controlled, dual‐centre study, where a VP shunt with an adjustable valve was implanted in 68 participants with iNPH, randomised into two groups. In one group (the 20‐4 group), the valve setting was initially set to 20 cm H₂O and gradually reduced to 4 cm H₂O over the course of the six‐month study period. In the other group (the 12 group), the valve was kept at a medium pressure setting of 12 cm H₂O during the whole study period (Saehle 2014). The primary goal of this study was to compare a medium shunt setting with gradual shunt setting reduction, rather than to compare CSF‐shunting versus no shunting, and none of the participants fulfilled the entry criteria for this study (Saehle 2014).
Risk of bias in included studies
Details of our risk of bias judgements relating to each analysis can be found in the risk of bias tables (Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 17; Table 18 ; Table 20; Table 16). Our consensus answers to risk of bias signalling questions are available in an online repository (Carswell 2024).
Risk of bias for analysis 1.1 Gait speed ‐ unpaired, parallel assessment < 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kazui 2015 | Low risk of bias | Yes random block design, concealed. Both groups were well matched for age and symptom profile.. | Some concerns | Participants were aware if they had had lumbar shunting or not. Surgeons and carers were also all aware.
No patient changed their assigned intervention. One patient from 44 withdrew from active intervention arm and two from 44 control intervention post randomisation which are similar numbers. Per protocol analysis for gait speed. Very few participants did not have follow up data for this outcome. |
Low risk of bias | 93 were randomised. 2 withdrew consent in control arm and 1 in shunt arm. 2 became unwell in shunt arm before surgery. 46 of remaining 46 were analysed in shunt group and 41 of 42 remaining were analysed. Overall 93% of randmised patients were assessed but he majority of drop outs had good expalnation. | Low risk of bias | Therapist assessed TUG was appropriate. Standardised assessments performed. The published paper suggests that assessors were not blinded. UMIN trials registry suggests assessors were blinded. The assessments were standardised and performed by appropriate therapists outside of the clincial surgical team. Low level of judgement required. | Low risk of bias | The trial was registered with the IUMIN‐CTR trial registry well before publication. TUG was used by more centres. 3 reciprocal walking test 10mWT also used. Data similar for both. | Some concerns | Trial participants were aware of group assignmen. Despite this only 3 of 93 (3%) dropped out because of this. Adherence to group allocation was very high. |
| Luciano 2023 | Low risk of bias | No additional comments. | Low risk of bias | There were no deviations from intended intervention | Low risk of bias | Data was available for 16 of 18 patients for this outcome (89%). There was one missing from analysis on each intervention arm. | Low risk of bias | assessor‐blinded. | Low risk of bias | Reported outcomes at protocol stage reported on. | Low risk of bias | low risk for all domains |
| Toma 2016 | Low risk of bias | Randomised in blocks by statastician. | Low risk of bias | One patient from a population of 15 (7%) withdrew consent after shunt operation but the data was not included in an ITT analysis in the presented work. | Low risk of bias | 93% of randomised cases had outcome data. | Low risk of bias | Outcome was measured in accordance with pre‐specified criteria. Modified ITT was performed as data not available for one patient who withdrew consent post intervention. | Low risk of bias | Full data set transparent. | Low risk of bias | Low risk of bias for all domains |
Risk of bias for analysis 1.2 Gait speed ‐ paired assessment < 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kazui 2015 | Low risk of bias | Yes random block design, concealed. Both groups were well matched for age and symptom profile.. | Some concerns | No true deviations. No patient assigned to one intervention received the other. There were a few patients (2 controls and 1 active intervention) who did not receive the intervention. Per protocol used but there were 41 vs 35 participants post surgery. Some unexplained. The participants who were not analysed suffered from complications/illness. Difficult to attribute to shunting itself. |
Some concerns | 444 were randomised. 42 analysed. 41 had initial (control) data for this outcome (93%). 35 had follow up data but 6 had explanations and only 1 did poorly potentially because of the shunt (stroke). No sensitivity analysis was performed to assess impact of missing data. Most of the cases of lost follow up were explained. | Low risk of bias | 10m reciprocal walking test was used also but the result is similar and TUG had larger samples as it is more accepted and was probably used by more of the 26 participating centres. | Low risk of bias | TUG was used mosre often but 10m reciprocal WT also used. Similar results were seen with both methods however. | Some concerns | Some concerns due to missing outcome data and per protocol analysis. |
| Toma 2016 | Low risk of bias | Randomised in blocks by statastician. | Low risk of bias | One patient withdrew post shunt insertion but data was not included in results. | Low risk of bias | 14 of 15 patients randomised (93%) of patients had follow up data. | Low risk of bias | Measurement appropriate. Assessors not aware of group allocation. | Low risk of bias | Appropriate results selected. | Low risk of bias | Low risk of bias for all domains |
| Luciano 2023 | Low risk of bias | No additional comments. | Low risk of bias | No patient received the wrong intervention. | Some concerns | 8 fof 9 randomised had initial "control" data for this outcome (89%). 7 of 9 (78%) had intervention "shunt data". No specific reason was given for the additional patient without cognitive data. The missingless overall is small and only 1 case is unaccounted for and could have been due to true value. | Low risk of bias | Measurement of outcome was appropriate. | Low risk of bias | Reported results appropriate. Outcome assessors not aware of group allocation. | Some concerns | Some concerns due to missing outcome data. |
Risk of bias for analysis 2.1 Unpaired parallel assessment < 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kazui 2015 | Low risk of bias | Yes random block design, concealed. Both groups were well matched for age and symptom profile.. | Some concerns | No true deviations. Per protocol effect used. Near entire dataset however so littel cahnce that per prtocol anlysis could alter overall effect. | Low risk of bias | Near entire dataset | Low risk of bias | Appropriate scale measured by experts with little judgement required even if no blinding. | Low risk of bias | Only one analysis performed. | Some concerns | Some concerns as per protocol analysis used for this outcome analysis |
Risk of bias for analysis 3.1 Patient disability ‐ number of participants who improved (unpaired, parallel assessment < 12 months).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kazui 2015 | Low risk of bias | Yes random block design, concealed. Both groups were well matched for age and symptom profile.. | Low risk of bias | The vast majority of cases randomised had the intended intervention (46 of 49 shunt arm and 42 of 44 control arm). | Low risk of bias | Minimal loss of data. | Low risk of bias | Outcome assessors were blind. | Low risk of bias | Result reported was appropriate. | Low risk of bias | Low risk of bias in all domains. |
| Luciano 2023 | Low risk of bias | No additional comments. | Low risk of bias | No participant received the wrong intervention. | Low risk of bias | ITT analysis of disability has full data set. | Low risk of bias | Outcome assessors were blind. | Low risk of bias | Reported result was appropriate. | Low risk of bias | Low risk of bias in all domains. |
| Toma 2016 | Low risk of bias | Randomised in blocks by statastician. | Low risk of bias | No deviations from intended interventions. | Low risk of bias | No missing data in ITT analysis. | Low risk of bias | Outcome assessors were blind to group allocation. | Low risk of bias | Reported resut was appropriate. | Low risk of bias | Low risk of bias in all domains. |
Risk of bias for analysis 3.2 Patient disability ‐ functionally independent (unpaired, parallel assessment < 12 months).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kazui 2015 | Low risk of bias | Yes random block design, concealed. Both groups were well matched for age and symptom profile.. | Low risk of bias | There were no changes in intervention after group assignment. No cases assigned to a given intervention arm received the other. A few patients did not receive the intended intervention at all. Two of 43 withdrew from control arm. One from 49 withdrew from shunt arm. | Low risk of bias | 88 of 93 randomised patients (95%) were analysed. | Low risk of bias | Measurement was appropriate and outcome assessors unaware of group allocation. | Low risk of bias | Results appropriately selected. Could nto have differed between groups. | Low risk of bias | Low risk of bias in all domains. |
| Luciano 2023 | Low risk of bias | No additional comments. | Low risk of bias | No deviations. | Low risk of bias | 89% of randomised cases analysed. One missing case in each group suggesting missing data not an effect of either treatment or control. | Low risk of bias | Outcome assessors were blind. | Low risk of bias | Result reported was approriate. | Low risk of bias | Low bias in all domains. |
| Toma 2016 | Low risk of bias | Randomised in blocks by statastician. | Low risk of bias | No deviation from intended intervnetion | Low risk of bias | 93% of randmoised cases had data. | Low risk of bias | Outcome assesors were blind. | Low risk of bias | Result reported was appropriate. | Low risk of bias | Low risk of bias in all domains. |
Risk of bias for analysis 3.3 Patient disability ‐ number of participants who improved (paired assessment < 12 months).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Luciano 2023 | Low risk of bias | No additional comments. | Low risk of bias | No deviations | Low risk of bias | ITT analysis. | Low risk of bias | Outcome assessors were blind to group allocation. | Low risk of bias | Low risk of bias in all domains. | Low risk of bias | Low risk of bias for all domains for this outcome. |
| Toma 2016 | Low risk of bias | Randomised in blocks by statastician. | Low risk of bias | No deviations | Low risk of bias | ITT analysis | Low risk of bias | Outcome assessors were blind. | Low risk of bias | Reported result is appropriate. | Low risk of bias | Low risk of bias for all domains for this outcome. |
Risk of bias for analysis 3.4 Patient disability ‐ functionally independent (paired assessment < 12 months).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Luciano 2023 | Low risk of bias | No additional comments. | Low risk of bias | There were no deviations from the intended intervention. | Low risk of bias | No missing data for this outcome. | Low risk of bias | Outcome assessors were blind. | Low risk of bias | Reported result appropriate. | Low risk of bias | Low risk of bias in all domains. |
| Toma 2016 | Low risk of bias | Randomised in blocks by statastician. | Low risk of bias | No participant had the incorect intervention. | Low risk of bias | No missing outcome data for this outcome. | Low risk of bias | Outcome assessors were blind to group allocation. | Low risk of bias | Reported result was appropriate. | Low risk of bias | Low risk of bias in all domains. |
Risk of bias for analysis 5.1 General cognitive screening ‐ unpaired, parallel assessment < 6 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kazui 2015 | Low risk of bias | Yes random block design, concealed. Both groups were well matched for age and symptom profile.. | Some concerns | No patient randomsied to one intervention arm received the other. 95% of randomised cases received the intervention. 3 inactive intervention arm (2 ill, 1 withdrew) and 2 control arm (2 withdrew). Per protocol analysis but there were very missing data points meaning it is unlikely to have affected overall effect . | Low risk of bias | 95% cases assigned to control had this intervention. 90% of active arm had intervention. 2 of 3 who didn't had illness unrelated to NPH. | Low risk of bias | MMSE is appropriate. Same standardised assessment performed in all participants. Assessors were neurologists, therapists or neuropsychologists rather than attending surgeons but it seems likely that they were aware of group allocation (the patient would have been able to inform them). Same standardised assessment performed on all by neuropsychologists wiht little use of judgement in assessment. | Low risk of bias | There was only one way in which overall cognition was screened. | Some concerns | Some concerns as per protocol anallysis used. |
| Luciano 2023 | Low risk of bias | No additional comments. | Low risk of bias | No participant received an unintended intervention. | Low risk of bias | Outcome data available for 16 of 18 patients. One case was missing from both arm so missiness was not dueto true value. | Low risk of bias | Assessors were blind to group allocation. | Low risk of bias | Reported result was appropriate. There was only one way of reporting this result. | Low risk of bias | Low risk of bias for all domains for this outome. |
Risk of bias for analysis 5.2 General cognitive screening ‐ paired assessment < 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kazui 2015 | Low risk of bias | Yes random block design, concealed. Both groups were well matched for age and symptom profile.. | Some concerns | No deviations but there were 5 patients from 93 who did not receive the intended interevention as they withdrew or became ill. Per protocol analysis used. Drop‐outs were few and there was 86% follow up available with reasonable explanation for drop outs. Is not a near total dataset though. | Some concerns | 36 of 44 (86%) randomised had follow up data for this outcome. The vast majority of cases had reasonable non‐shunt explanation for their lack fo outcome data. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | Selected result was appropriate. | Some concerns | Some concerns of bias due to missing data adn use of per protocol analysis. |
| Luciano 2023 | Low risk of bias | No additional comments. | Low risk of bias | No deviations. | Low risk of bias | 8 of 9 cases in each group had data for this outcome. | Low risk of bias | Oucome assessors were blinded to group allocation. | Low risk of bias | Selected result was appropriate. | Low risk of bias | Low risk of bias for all domains of this outcome. |
Risk of bias for analysis 5.4 Symbol Digit Tests ‐ paired assessment < 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kazui 2015 | Low risk of bias | Yes random block design, concealed. Both groups were well matched for age and symptom profile.. | High risk of bias | No patient received the wrong intervention. 3 patients of 44 (7%) assigned to delayed shunt did not get it. Two withdrew and one developed ascites before drain insertion. Per protocol with many drop outs that were not fully explained. If the drop‐outs all had negative results the effect may be different. | Some concerns | 30 of 44 cases had follow up data compared with 34 of 46 who had initial data. The missingness was clearly not all due to shunting (the intervention). It was realtively balanced between groups also. | Low risk of bias | Assessors were blind to group allocation. | Low risk of bias | SDT and FAB both test executive function. Both yielded positive results however. We were not able to generate a composite without IPDs. | High risk of bias | High risk of bias due to per prtocol effect where missing data was not fully explained for this outcome. |
| Luciano 2023 | Low risk of bias | No additional comments. | Low risk of bias | No deviations | Low risk of bias | 8 of 9 randomised cases had data in both groups. | Low risk of bias | Outcome assessors were blind to group allocation. | Low risk of bias | Reported result is appropriate. | Low risk of bias | Low risk of bias for this outcome. |
Risk of bias for analysis 4.1 Adverse events < 3 months post‐surgery.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kazui 2015 | Low risk of bias | Yes random block design, concealed. Both groups were well matched for age and symptom profile.. | Low risk of bias | Almost all participants had the intended interventino and there are good explanations for those who did not. | Low risk of bias | All participants are noted to have data for this. | High risk of bias | AEs were documented by treating surgeons who were aware of group assignment. It is uncertain how AEs were measured in controls. | Low risk of bias | The actual way AEs were measured was probably appropriate and only one possible analysis for this outcome. | High risk of bias | High risk of bias due to the way it was measured. |
In this section we provide brief summaries of the risk of bias assessments for our primary and summary of findings table outcomes for the time points at which we had numerical data.
Gait speed (short term)
We have some concerns for the domain 'deviations from intended interventions' in the unpaired parallel assessment of gait speed (short term) in one of three studies included in meta‐analysis. Kazui used a per‐protocol analysis (for all outcomes except patient disability (short term)), but adherence to group assignment was actually very high with available data for 94% and 95% of participants assigned to the intervention and control groups, respectively (Table 9) (Kazui 2015).
In addition, we have some concerns for the domain 'missing outcome data' for both Kazui and Luciano in the paired assessment gait speed (short term) for the delayed‐shunt group (Table 10) (Kazui 2015; Luciano 2023). In Luciano, one participant of nine did not have control data and two of nine did not have intervention data, and in Kazui, 35 of 44 cases did not have intervention data; in both cases the 'missingness' was well explained (Kazui 2015; Luciano 2023).
Qualitative gait function (short term)
We again have some concerns for the domain 'deviations from intended interventions' in the unpaired parallel assessment of qualitative gait function (short term) in Kazui, for similar reasons to gait speed (short term), but no additional concerns (Table 11) (Kazui 2015).
Patient disability (short term)
As Kazui used modified ITT analysis for this outcome, we assessed disability (short term) for both paired and unpaired analyses as low risk of bias (Table 12; Table 13; Table 14; Table 15) (Kazui 2015).
Cognitive function (short term)
Only two studies assessed this outcome. We considered Luciano as at low risk of bias. We had some concerns for Kazui for both the paired and unpaired analysis of the delayed‐shunt group for similar reasons to gait speed (short term) and the cognitive subdomain of Symbol Digit Tests (SDTs). (Table 17; Table 18; Table 19) (Kazui 2015; Luciano 2023). We judged the paired assessment of the delayed‐shunt group for SDTs to be at high risk of bias, as the per‐protocol analysis Kazui performed was consequential, with unexplained 'missingness' in outcome data (Table 20) (Kazui 2015).
Risk of bias for analysis 5.3 Symbol Digit Tests ‐ unpaired, parallel assessment < 6 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kazui 2015 | Low risk of bias | Yes random block design, concealed. Both groups were well matched for age and symptom profile.. | High risk of bias | One patient from 44 randomised to dealyed shunt did not receive the shunt due to illness but no patient assigned to an given interevention had a different intereventiion. There was not maximal data set for this and analysis was per protocol. This is unexpalined. | Some concerns | 34 of 44 patients (77%) had control data for this outcome compared with 37 of 46 (80%) who had pre‐shunt data. No sensitivity analysis was performed. The missingness was balanced between immediate and delayed shunt groups so it seems unlikely that it is missing because of true value. Likely there is missing data because not all study sites were doing the test of patients became fatigued of lots of cognitive tests. | Low risk of bias | Same assessment by same assessors. Assessors were probably aware (some dispute between study publicaiton and protocol) Standardised assessment in both groups. | Low risk of bias | Study protocol published on Trials Registry. Kazui et al tested FAB and trails which also test fronta/executive function but there was no comparator with Luciano. We did not have IPD so could not generate an appropriate "composite" frontal test. The outcome from FAB was similar to SDT. | High risk of bias | Unexplained loss of participant data with per‐protocol analysis. |
| Luciano 2023 | Low risk of bias | No additional comments. | Low risk of bias | Surgeons were aware of the intervention. All patients had the intended intervention. | Low risk of bias | No additional comments | Low risk of bias | No additional comments | Low risk of bias | No additional comments | Low risk of bias | Low risk of bias for this outcome in this study. |
Adverse events (short term)
Only Kazui assessed AEs in a controlled way with a parallel assessment at an early time point post‐intervention (Kazui 2015). Assessors in this study were not blinded to the intervention group, and no details were given about who documented AEs. As such, we judged this domain as at high risk of bias, as knowing if a participant had undergone CSF‐shunt surgery has a high potential for bias in documenting AEs (Table 16). For non‐controlled data for AEs from the other three included studies, we note that there were no non‐serious AEs in Toma and Tisell, which could lead to underreporting of these events. We also note that there was inherently a lack of blinding of AE reporters (the attending surgeons), and thus AE results have the potential for bias. It seems unlikely that this would have a major impact on absolute or serious outcomes such as death and repeated surgery, which are not subjective (Luciano 2023; Tisell 2011; Toma 2016).
Effects of interventions
See: Table 1
See Table 1 for summary of outcomes and certainty of the evidence for CSF‐shunting versus inactive or no shunting in parallel‐assessed groups.
Primary outcomes
Gait speed (short term)
Unpaired, parallel‐group analysis of gait speed from Kazui, Toma, and Luciano included 61 participants who had immediate CSF‐shunting and 55 randomised to control. We compared studies using standardised mean difference (SMD) to account for different scales used (the TUG test and 10 MWT). We assessed Luciano and Toma as at low risk of bias for this outcome (Luciano 2023; Toma 2016), and had some concerns about Kazui (Kazui 2015). We found that immediate CSF‐shunting resulted in a positive effect, increasing gait speed at less than six months post‐surgery with a moderate effect size (SMD 0.62, 95% confidence interval (CI) 0.24 to 0.99; 3 studies, 116 participants; moderate‐certainty evidence; Analysis 1.1; Table 1).
1.1. Analysis.

Comparison 1: Gait speed, Outcome 1: Gait speed ‐ unpaired, parallel assessment < 12 months
Paired assessment of gait speed in the delayed CSF‐shunting group showed strikingly similar data to the parallel assessment with a similar sample size (SMD 0.68, 95% CI 0.28 to 1.08; P < 0.001; 3 studies, 103 participants; Analysis 1.2). The main difference between this and the parallel data set is that the post‐shunt assessment in the paired analysis was carried out between 8 to 12 months post‐shunting rather than 3 or 4 months, and risk of bias was greater due to unexplained participant loss to follow‐up (Analysis 1.2). Both unpaired parallel and paired analyses followed a broad trend, where the one large study had narrower CIs for this outcome (Kazui 2015), and smaller studies had the same direction of effect with larger CIs (Luciano 2023; Toma 2016).
1.2. Analysis.

Comparison 1: Gait speed, Outcome 2: Gait speed ‐ paired assessment < 12 months
Qualitative gait function (short term)
Two studies reported qualitative or composite gait outcomes (Kazui 2015; Tisell 2011), but Tisell did not describe the data with means and SDs, thereby preventing formal meta‐analysis. Using the iNPHGS for gait, Kazui showed a modest reduction (improvement) of 0.9 points in the 5‐point iNPHGS gait score (95% CI 0.57 to 1.23; P < 0.001; 88 participants) in parallel‐group assessment at an early time point (Analysis 2.1). Tisell reported a 30% improvement in gait function with their composite scale in parallel assessment at a three‐month time point, but this effect could have been a chance finding (P < 0.1; 14 participants) (Tisell 2011). We assessed the certainty of the evidence as low (Table 1). Tisell showed an 18% improvement in gait function in the paired analysis of the group randomised to delayed CSF‐shunting (Tisell 2011). Kazui also demonstrated a reduction (improvement) by 0.6 points in their paired assessment of qualitative gait function with CIs that did not pass the null effect (95% CI 0.28 to 0.92; P < 0.001; 80 participants) in paired analysis of the delayed‐shunt group (Analysis 2.2).
2.1. Analysis.

Comparison 2: Qualitative gait function, Outcome 1: Unpaired parallel assessment < 12 months
2.2. Analysis.

Comparison 2: Qualitative gait function, Outcome 2: Paired assessment longitudinal < 12 months
Patient disability (short term)
Kazui, Toma, and Luciano all measured disability. Kazui and Luciano used the mRS, while Toma used a Stein‐Langfitt scale. Both scales are semi‐ordinal with a few categories and are challenging to compare directly. We were able to binarise change score data into 'number of participants who improved', to indicate the direction of effect of shunting. Analysis of unpaired, parallel groups showed a large effect size favouring shunting (risk ratio (RR) 9.02, 95% CI 3.61 to 22.53; 3 studies, 121 participants; Analysis 3.1). Sensitivity analysis removing Kazui as the one unblinded study and also the only LP‐shunt study did not change the direction of effect, and the RR did not cross the null effect (data not shown).
3.1. Analysis.

Comparison 3: Patient disability, Outcome 1: Patient disability ‐ number of participants who improved (unpaired, parallel assessment < 12 months)
To try to quantify the magnitude of the effect size, we equated an mRS score of 2 with a Stein‐Langfitt score of 1 and considered those with this score or less as being functionally independent (this is in keeping with stroke thrombectomy trial definitions) (Badhiwala 2015). We then assessed how many participants in each group had functional independence post‐surgery. Again, we found a positive effect of shunting with a large effect size (RR 2.08, 95% CI 1.31 to 3.31; P < 0.002; 3 studies, 118 participants; moderate‐certainty evidence; Analysis 3.2; Table 1). The number needed to treat for an additional beneficial outcome (NNTB) with shunting to gain one patient with functional independence was 3.4.
3.2. Analysis.

Comparison 3: Patient disability, Outcome 2: Patient disability ‐ functionally independent (unpaired, parallel assessment < 12 months)
We had complete IPD for disability for both Toma and Luciano studies (but not Kazui) and were able to assess patient disability in a paired assessment in the group who had delayed CSF‐shunting (Kazui 2015; Luciano 2023; Toma 2016). As Toma only assessed disability at zero and six months post‐study start (three months post‐shunt activation in the control group), the time point of this assessment was considerably different from the Luciano data, which was eight months post‐shunt. However, the results were similar to those for the parallel‐group assessments showing a large overall effect size (RR 1.86, 95% CI 0.65 to 5.27; 2 studies, 30 participants; Analysis 3.3) for 'number of participants who improved', and for 'functional independence' (RR 3, 95% CI 0.54 to 16.53; 2 studies, 30 participants; Analysis 3.4). Without the additional participant data from Kazui for these assessments, the overall sample size of Toma and Luciano was very small (n = 30), and CIs crossed the null effect (RR 1), so we cannot be certain the effect is true.
3.3. Analysis.

Comparison 3: Patient disability, Outcome 3: Patient disability ‐ number of participants who improved (paired assessment < 12 months)
3.4. Analysis.

Comparison 3: Patient disability, Outcome 4: Patient disability ‐ functionally independent (paired assessment < 12 months)
Quality of life (short term)
No study assessed this outcome.
Adverse events (short term)
LP‐shunting versus no shunt at three months post‐surgery
Kazui was the only study to document AEs for the comparison CSF‐shunted group versus no‐shunt group at the three‐month time point, and reported no deaths in either group (Table 4) (Kazui 2015). There are AEs specifically associated with LP‐shunt surgery. However, serious AEs occurred in 15% of initially LP‐shunted participants (seven events: two subdural haematomas requiring surgery, four shunt tube migrations, and one rupture) versus 2% (one lumbar compression fracture) in the non‐shunted group (Table 4) (Kazui 2015). Minor AEs occurred in 26% of LP‐shunted participants (15 events in 12 participants: 10 cases of postural headache, two asymptomatic subdural haematomas, and two subdural haematomas not requiring surgery) (Kazui 2015). There were no minor AEs in the control group at three months. LP‐shunt surgery was associated with many more AEs compared to no shunt surgery (Analysis 4.1). Given that there was only one study comparing surgery with no surgery, the low sample size, and the lack of assessor blinding for this outcome, we assessed the certainty of the evidence as very low (Table 1).
3. Adverse events at study endpoint.
| Study | Study period | Total adverse events (% population) | Deaths (% population) | Serious adverse events | Non‐serious adverse events |
| Tisell 2011 | 6 months | 8 events in 7 participants (50%) | 0 (0) | 1 asymptomatic subdural haematoma needing surgery 3 ischaemic strokes (1 major and 2 minor) |
4 asymptomatic subdural haematomas treated with shunt setting adjustment |
| Kazui 2015 | 12 months | 50 events in 43 participants (49%) | 1 lung cancer, 1 suicide (2%) | 2 deaths 3 subdural haematomas needing surgery 6 shunt tube migration/rupture 1 meningitis 6 ischaemic strokes 2 fall and femoral fracture 1 lumbar fracture |
21 postural headaches 5 asymptomatic subdural effusion 2 subdural haematomas not requiring surgery 1 asthma |
| Toma 2016 | 12 months | 7 events in 7 participants (47%) | 0 (0) | 2 distal shunt tube relocation 1 angina 1 shunt valve replacement 1 ischaemic stroke 1 fall and femoral fracture 1 fall and traumatic brain injury |
Nil reported |
| Luciano 2023 | 12 months | 24 events in 12 participants (67%) | 1 contralateral stroke, 1 gallbladder necrosis (11%) | 2 deaths 1 coagulopathy 1 transient generalised muscle weakness 1 urinary tract infection 1 postoperative subdural hygroma |
1 Crohn's disease 3 transient headache 2 imbalance/dizziness 3 transient abdominal pain 1 fever of uncertain cause 1 minor head trauma 7 decrease in Montreal Cognitive Assessment (MoCA) |
4.1. Analysis.

Comparison 4: Adverse events (< 12 months), Outcome 1: Adverse events < 3 months post‐surgery
Narrative description of all adverse events (short term)
No study reported AEs in active VP‐shunting groups versus inactive VP‐shunting groups at an early time point. All groups documented procedural complications at the end of the study follow‐up, which was six months post‐CSF‐shunting for Tisell and 12 months for the other studies which we considered 'short term' for the purposes of this review (Table 4) (Kazui 2015; Luciano 2023; Tisell 2011; Toma 2016). Luciano and Kazui categorised AEs as serious AEs (SAEs) or non‐serious AEs (Kazui 2015; Luciano 2023).
Overall, AEs were reported for 69 of 135 (51%) participants who had CSF surgery, of which 38 were SAEs and 31 were non‐serious AEs (Table 4). There were four deaths (3%) at 12‐month follow‐up, none of which were attributed to shunt surgery (lung cancer, suicide, gallbladder necrosis, and stroke contralateral to shunt site) (Table 4) (Kazui 2015; Luciano 2023). Repeat surgery related to CSF‐shunting occurred in 12 participants (8.9%), with eight needing abdominal surgery (5.9%) and four needing repeat cranial surgery (3%), three of whom had LP shunts (Table 4) (Kazui 2015; Tisell 2011).
Twenty‐four (18%) participants had headaches with 21 of these (88%) occurring in the 88 participants who had LP shunts (24%), versus three of the 18 participants who had VP shunts in whom non‐serious AEs were documented (17%) (Table 4). Eleven ischaemic strokes were observed (8%), with at least one stroke occurring in each study; six of 88 participants with LP‐shunt surgery had strokes (7%), and five of 47 participants with VP‐shunt surgery had strokes (10%). Six falls were documented (4%) resulting in three fractures of the femur (2%) and one traumatic brain injury needing cranial surgery more than 21 months post‐CSF‐surgery (1%).
Toma reported that two of 15 participants (13%) in the active VP‐shunt group needed replacement of the distal shunt tube after a routine postoperative x‐ray. Following these incidents, surgical technique was modified: purse string closure of peritoneum using non‐absorbable suture material was introduced as part of the surgical protocol, and there were no similar events thereafter (Table 4) (Toma 2016).
At study endpoint there was no statistical difference in AE rate between initially active and inactive shunt groups in either the Kazui or Luciano studies (Kazui 2015; Luciano 2023).
Adverse events (long term)
Tisell was the only study to evaluate long‐term AEs. They contacted all participants 18 months following the final six‐month follow‐up period. This was a median of 42 months from study onset (range 15 to 61 months). Of the original 14 cases, one was lost to follow‐up, and three had died (stroke, malignancy, and pneumonia). Four of the 10 participants had deteriorated with time (Tisell 2011).
Secondary outcomes
Gait speed (long term)
No study assessed this outcome.
Cognitive function (short term)
Only Kazui and Luciano measured cognitive function using continuous screening tools that were comparable (Kazui 2015; Luciano 2023). Kazui showed an improvement in the MMSE at an early time point in the unpaired, parallel assessment of shunted group versus delayed‐shunt group, but Luciano found a slight deterioration in the MoCA in their active VP‐shunt versus inactive VP‐shunt group. Overall, there was a 'mild' beneficial effect of shunting on cognitive function at the early time point, but CIs were wide and crossed the null effect (SMD 0.35, 95% CI −0.04 to 0.74; P = 0.08; 2 studies, 104 participants; very low‐certainty evidence; Analysis 5.1; Table 1). Heterogeneity was high (I² = 75%), so we are not confident that this result reflects the true effect. The paired assessment of cognitive function in groups with delayed CSF‐shunting also showed a mild beneficial effect of shunting (SMD 0.4, 95% CI −0.01 to 0.82; 2 studies, 93 participants; Analysis 5.2), and although there was far less heterogeneity between the studies (I² = 0%), CIs crossed the null effect, so we are not confident in this finding.
5.1. Analysis.

Comparison 5: Cognitive function, Outcome 1: General cognitive screening ‐ unpaired, parallel assessment < 6 months
5.2. Analysis.

Comparison 5: Cognitive function, Outcome 2: General cognitive screening ‐ paired assessment < 12 months
Tisell assessed cognition using a composite scale of combined measures but failed to provide population means or SDs, so these results were not comparable with the other studies. They found an improvement in cognitive scores in both unpaired parallel assessment (23%; P < 0.05; 14 participants) and paired assessment in the delayed‐CSF‐shunt group (18%; P < 0.05; 14 participants) (Tisell 2011).
Symbol Digit Tests (short term)
Kazui and Luciano both performed Symbol Digit Tests (Symbol Digit Test (SDT) and Symbol Digit Modalities Test (SDMT)), which assess the same cognitive subdomains and were directly comparable, permitting data analysis (Kazui 2015; Luciano 2023). There was a mild improvement in SDT performance in the parallel assessment (SMD 0.45, 95% CI 0.03 to 0.88; 2 studies, 87 participants; Analysis 5.3) with reasonable agreement between studies (I²= 15%). The paired assessment of delayed‐CSF‐shunt groups for this measure was very similar (SMD 0.46, 95% CI 0.01 to 0.91; 2 studies, 79 participants; Analysis 5.4). As many of the tests that make up the Frontal Assessment Battery (FAB) also assess attention, concentration, and executive function, we compared FAB from Kazui with SDT from Luciano; the results were almost the same in effect size and heterogeneity as comparing SDTs. There was no effect of shunting when comparing Trails A (which has a low ceiling effect) in Kazui with SDT in Luciano.
5.3. Analysis.

Comparison 5: Cognitive function, Outcome 3: Symbol Digit Tests ‐ unpaired, parallel assessment < 6 months
5.4. Analysis.

Comparison 5: Cognitive function, Outcome 4: Symbol Digit Tests ‐ paired assessment < 12 months
Qualitative cognitive function (short term)
Kazui demonstrated an improvement in the (5‐point) iNPHGS‐cog scale by 0.5 points (95% CI 0.2 to 0.8; P < 0.001; 88 participants) in the unpaired, parallel assessment (Kazui 2015). Their paired assessment of the delayed‐CSF‐shunt group replicated these findings with a 0.4‐point reduction (SD 0.7; 80 participants) (Kazui 2015).
Cognitive function (long term)
No study assessed this outcome.
Urinary function (short term)
Luciano showed a large 22.93 (95% CI 7.7 to 38.1; P < 0.007; 18 participants) improvement in the 36‐point Overactive Bladder Questionnaire short form (OAB‐q SF) in unpaired, parallel assessment of shunt versus initially inactive shunt. The paired assessment of the delayed‐CSF‐shunt group also improved by a similar order of magnitude, but the SD was very large and the sample size small; the findings were compatible with chance assuming no effect of shunting (17.1, SD 24.5; P > 0.05) (Luciano 2023). Kazui also showed an improvement in the (5‐point) iNPHGS‐urine by 0.7 points (95% CI 0.4 to 1.1; P < 0.001; 88 participants) in parallel assessment of shunt versus delayed‐shunt group (Kazui 2015). The paired assessment of the delayed‐shunt group also showed an improvement by 0.6 points (SD −0.9; 38 participants) 12 months following shunt surgery, but they did not describe this comparison statistically (Kazui 2015). Toma found that five of eight participants improved by at least 1 point on their (5‐point) bespoke ordinal continence scale in the immediate active VP‐shunt group compared with only one of the six participants followed up in the control group (compatible with chance, P = 0.14).
Discussion
Summary of main results
This systematic review is the first to assess the efficacy of CSF‐shunting in people with iNPH including only trials that are randomised and controlled. We included four RCTs in the review, only three of which had data that could be meta‐analysed. The total number of participants was low: 135 of 140 enrolled participants received shunt surgery, 88 (65%) with LP shunts and 47 (35%) with VP shunts. Kazui 2015 was a large, unblinded RCT that compared LP‐shunt surgery with controls who had delayed LP‐shunt surgery; the other three RCTs were small and compared active VP‐shunting with controls who underwent VP‐shunt surgery but had delayed active VP‐shunting (Luciano 2023; Tisell 2011; Toma 2016). Kazui was the only study that mandated specific radiological findings of NPH, and was also the only study that did not require participants to have objective improvement following CSF tap test (CSFTT) or external lumbar drain (ELD).
All studies used a 'one‐arm, cross‐over' RCT design, which allowed both unpaired, parallel group assessment and also paired assessment of the group assigned to delayed CSF‐shunting. While the primary goal of the meta‐analysis was the unpaired parallel group assessment, the paired assessment helped add to the certainty of the findings where both assessments concurred. This was clearest with our primary outcome of gait speed (short term) (Analysis 1.1). We had some concerns about risk of bias in one of the three studies in the parallel analysis, and we downgraded the certainty of evidence by one level, as the small sample size resulted in potential imprecision. As such, CSF‐shunting probably improves gait speed in the short term by a moderate amount (Table 1).
For the unpaired parallel assessment of disability (short term), we found that CSF‐shunting reduced disability by a very large effect when measured by number of participants who improved (Analysis 3.1). This was robust to sensitivity analysis removing Kazui, the largest, but also the only unblinded, study. To better understand the magnitude of effect size, we binarised disability into those who were functionally independent or not following CSF‐shunt surgery and again detected a large effect size with an NNTB of only 3.4 (Analysis 3.2; Analysis 3.1). This outcome had a low risk of bias. We downgraded the certainty of evidence by one level, as the overall sample size was small, leading to potential imprecision. CSF‐shunting probably increases the chances of being independent after surgery by a large amount (Table 1).
Kazui was the only study to compare CSF‐shunt surgery with no surgery, and unsurprisingly demonstrated that LP‐shunt surgery was associated with a much higher AE rate three months after surgery (Kazui 2015). There were no deaths; repeated cranial surgery was rare; and these AEs are expected with lumbar‐CSF drainage (Kazui 2015). Given the small sample size, that only one study assessed this outcome in a controlled way, and the lack of assessor blinding in this study, we assessed the certainty of the evidence as very low (Table 1).
Due to study design limitations, we narratively described non‐controlled AEs in the four included studies (Kazui 2015; Luciano 2023; Tisell 2011; Toma 2016). Definitions of AEs varied across studies; Toma and Tisell only documented SAEs and did not comment on the severity or expectedness of AEs. This led to a wide range of reported AEs, and we were only able to compare SAEs between studies (Kazui 2015; Luciano 2023; Tisell 2011; Toma 2016).
Overall, AEs occurred in about half of participants in the year following shunt surgery, and although half of these were considered serious, repeat surgery occurred in a minority, and mortality was low at 3% and was unrelated to shunt surgery. Most AEs that could have been shunt‐related were managed conservatively, and post‐shunt cranial surgery was rare. Headaches were an expected AE and were relatively common after CSF‐shunt surgery, and slightly more common in those with lumbar surgery than with VP‐shunt surgery. Ischaemic strokes were frequent (8% of participants), with the majority occurring in those who had lumbar (non‐cranial) shunt surgery. This is higher than the typical annual risk rate for similar age‐matched controls (typically 0.5% to 1%), especially given that individuals with atrial fibrillation and anticoagulation were largely excluded from the studies assessed, and it was not entirely expected (Akyea 2021; Lavados 2021). Vascular risk factors are a known risk factor in the development of hydrocephalus (Jaraj 2016), but there is a need for further studies to understand if stroke risk is increased in the NPH population.
For the secondary outcome urinary function (short term), two studies detected an improvement with CSF‐shunting, and the third showed a trend towards improvement (Kazui 2015; Luciano 2023; Toma 2016). It was not possible to be certain about the magnitude of effect size, as the scales used were not comparable. One of the studies did not produce any data on urinary outcomes. Two used similar ordinal scales, but one study did not provide IPDs for this outcome necessary to make comparable assessments. It seems likely that CSF‐shunting improves urinary function, even though we were not able to demonstrate this in meta‐analysis.
We did not find a convincing effect of CSF‐shunting on our secondary outcome of cognitive function (short term). Although there was a small positive effect, CIs crossed the null effect, and there was high heterogeneity between studies. We assessed the certainty of the evidence as very low (Table 1). There was a subtle signal of improvement in SDTs with a small effect size, but the sample size was very small (Analysis 5.3). In general, in the four studies assessed, cognitive screening was not done in a uniform manner, and the tools that were most commonly used (MMSE, MoCA) were not designed for frequent repetition or for the condition under study. Assessing cognition in NPH using tests that are sensitive to change in specific cognitive domains that are understood to improve more post‐shunt in iNPH may provide more insight. It should be noted that Toma used long neuropsychological batteries in his study, but did not get many participants with complete data sets, which may suggest that prolonged batteries are unacceptable to the patient population (Toma 2016).
With a 'one‐arm, cross‐over' study design, all participants end the trial in the active (shunted) intervention arm, and as such there are no longer‐term, controlled outcome data. As a result of this limitation, data regarding long‐term outcomes of CSF‐shunt surgery will not be of the highest quality. The lack of long‐term data may also have limited the ability to detect improvement in some outcomes (e.g. cognitive function), which observational studies have shown can take some time to improve (Takeuchi 2019).
Overall completeness and applicability of evidence
The clearest limitation of this meta‐analysis is the low total number of participants having shunt surgery (135). There are previous examples where a large RCT has dispelled the findings of meta‐analyses with limited sample size (Horner 1992; ISIS‐4). As such, further studies with larger participant numbers are needed to increase the certainty of the evidence. Larger RCT studies will also be able to demonstrate treatment effects with a lesser magnitude of effect size, which this meta‐analysis was insufficiently powered to do. We are aware of ongoing trials meeting our criteria that are in progress (NCT05081128). Given the lack of ethnicity data published in the included studies, it is difficult to understand how these findings can be applied to ethnic minority groups who are likely to be underrepresented in these studies.
As only one study assessed LP‐shunt surgery, and the sample size was low in those having VP‐shunt surgery, it was not meaningful to perform subgroup analysis of each specific technique. As the beneficial effects of VP‐shunt surgery and LP‐shunt surgery are thought to be similar in non‐randomised studies, this is probably not a critical issue for decision‐makers when considering the potential benefits of shunt surgery (Hashimoto 2010). As only one study showed benefit of CSF‐shunting in participants with iNPH who did not have any form of CSFTT or ELD, we feel that further studies will need to replicate the findings of Kazui to justify this practice.
A further limitation of the study is that the different study designs used (immediate surgery versus delayed onset, LP versus VP shunts) mean that there is no controlled evidence to understand the frequency and type of AEs experienced with VP‐shunting versus none, or active LP‐shunting versus inactive shunting. This limitation is most obvious for stroke, which seems to occur frequently, but the risk for non‐shunted iNPH populations is unclear. Given that NPH studies are designed primarily to demonstrate the potential benefit of shunting rather than to compare frequencies of AEs, and that the preferred study design in NPH is complex, it seems unlikely that any RCT data will ever be produced to generate randomised, controlled data for AEs. It is likely that the best quality of evidence possible for this outcome will be from non‐randomised, non‐controlled, prospective observational studies of NPH patients. Two studies did not formally document non‐serious AEs, so the event rate may be artificially low for this subset of AEs compared with other studies. We do not think this will affect reports of SAEs, which are likely to be most influential for decision‐makers considering shunt surgery.
The patients studied here were in general a 'pure' iNPH cohort without established dementia or other medical causes for symptoms of Hakim's triad. More work is needed to understand the effect of concurrent neurodegenerative disease and the use of CSF biomarkers to inform shunt‐responsiveness. The current results can be extrapolated to clinical practice for those with iNPH who do not clearly have neurodegenerative disease or multiple medical comorbidities. There were limited data on how those with chronic hydrocephalus becoming symptomatic in adulthood were differentiated from iNPH in the studies assessed. Individuals with this form of hydrocephalus will need to be studied independently of typical iNPH to understand if there is a similar benefit of shunting in this population.
The lack of a standardised urinary function scale compromised our ability to compare all studies, and the lack of IPD limited our ability to understand the magnitude of benefit in improvement in urinary function post‐shunt. More work is needed in this regard to confirm the magnitude of benefit of shunting iNPH on urinary function.
There was also a lack of data regarding qualitative scales for gait and cognitive function, which is a limitation, as such scales can be useful in providing a tangible understanding of the magnitude of benefit for decision‐makers.
We did not detect any quality of life outcome data, which is also a limitation given the importance of this outcome in helping patients make decisions about their care.
Quality of the evidence
We used the GRADE approach to assess the certainty of the evidence (Table 1). We downgraded the certainty of the evidence for two outcomes, gait speed (short term) and patient disability (short term), one level to moderate due to small sample size and potential imprecision (Kazui 2015; Luciano 2023; Toma 2016). We downgraded the certainty of the evidence for qualitative gait function (short term) two levels to low due to small sample size and risk of bias, as there was lack of assessor blinding for one study in the analysis, and one other study did not publish their study protocol or data analysis plan before publication (Kazui 2015; Tisell 2011). We assessed the certainty of evidence for adverse events (short term) and cognitive function (short term) as very low. The evidence was downgraded due to small sample size and lack of assessor blinding. For AEs (short term) there were also study limitations (only one form of surgery evaluated) leading to indirectness, while for cognitive function (short term) there was a high degree of heterogeneity leading to inconsistency.
Based on the findings of this review, CSF‐shunting probably improves gait speed and patient disability in the short term following surgery for iNPH and may also improve qualitative gait function in the short term. The evidence regarding the effect of CSF‐shunting on AEs and cognitive function in the short term following surgery is very uncertain.
Potential biases in the review process
We are confident that the literature search identified all relevant studies, and that we attempted to gain as much published and unpublished data as possible. Despite this, we did not have IPD, which limited our ability to contextualise the effect size (particularly as regards urinary function). We did not have major concerns regarding conflicts of interest in the studies assessed.
Agreements and disagreements with other studies or reviews
This study is an update of the last Cochrane review by Esmonde and colleagues, which did not find any randomised evidence at that time to help guide opinion (Esmonde 2002). This is the first review to our knowledge comparing studies with randomised data on the effect of shunting in iNPH. Our results are generally in keeping with improvement in gait speed and urinary function seen in prospective, non‐randomised studies and meta‐analysis of non‐RCT data (Giordan 2018; Halperin 2015; Hashimoto 2010; Klinge 2012; Toma 2013). We were not able to confirm the improvement in cognition that these studies found. The improvement in disability shown in the current study is novel in a meta‐analysis.
The overall AE rate we discovered of 52% is relatively high when compared to high‐quality observational studies; Klinge and colleagues reported a procedural complication rate of 28%, and Williams and colleagues a rate of 8.8% (Klinge 2012; Williams 2022). Mortality in our study was low and in keeping with the existing literature. Reoperation in this study was 8.8%, which is within the range published in prospective observational studies (3.6% to 15%) (Klinge 2012; Williams 2022).
We detected an ischaemic stroke rate of 8% in the 12 months following shunt surgery for iNPH. This is high and has not previously been reported. It is uncertain if this is a true finding or a spurious result due to relatively low sample size. Further work is necessary to confirm this finding.
Authors' conclusions
Implications for practice.
This systematic review is the first to show a treatment benefit for cerebrospinal fluid (CSF)‐shunting in idiopathic normal pressure hydrocephalus (iNPH) when limiting the analysis to randomised, high‐quality evidence. CSF‐shunting probably offers a large chance of being functionally independent compared with non‐shunting in the short term following surgery. CSF‐shunting probably increases gait speed by a moderate amount compared with non‐shunting in the short term following surgery. Urinary function probably improves with CSF‐shunting in iNPH, but there is uncertainty about the magnitude of benefit. We are very uncertain about improvement in general cognition or executive function with CSF‐shunting in the short term.
Mortality after shunt surgery is rare, and repeated surgery is needed in fewer than one in 10 patients. The apparent high risk of ischaemic stroke seen following shunt surgery was unexpected and needs to be confirmed in studies specifically designed to explore this.
Patients over the age of 60 with a constellation of gait, cognitive, and urinary impairment and hydrocephalus on brain imaging can benefit from CSF‐shunt surgery.
Implications for research.
Patients are tolerant of the 'one‐arm, cross‐over' trial design pioneered in iNPH by Tisell and colleagues.
The number of participants included in this review was low, and there is a need for similar studies to increase the certainty of the findings presented.
Work is needed to standardise the scales used in iNPH clinical trials to facilitate future meta‐analysis. The lack of a standardised approach to assessing urinary symptoms in iNPH is stark, given that it is one of the core clinical symptoms.
Future studies in normal pressure hydrocephalus (NPH) need to provide ethnicity data to ensure that the findings are widely applicable to diverse populations and also to identify undertreated groups. More evidence on the effect of shunting on carer burden and quality of life is clearly needed, as there are very limited randomised controlled trial data for these important outcomes.
Not all participants reviewed in this work were CSF‐shunt responsive, and there is a pressing academic need to investigate the cause of shunt unresponsiveness. Given the lack of specific radiological criteria used in three of the four studies assessed here, and the fact that not all studies required provocative CSF‐testing, more work is needed to understand the minimum radiological criteria needed for shunt responsiveness and the role for provocative testing before shunt surgery.
The participants included in the studies reviewed were not frail or elderly. More evidence is required to understand the effect of frailty, neurodegenerative disease, and hydrocephalus chronicity and severity on shunt responsiveness in iNPH.
More work is also needed on the classification of iNPH and how to manage patients with chronic hydrocephalus that becomes symptomatic rather than those who develop iNPH in adulthood.
History
Protocol first published: Issue 3, 2022
Risk of bias
Acknowledgements
Editorial and peer‐reviewer contributions
The Cochrane Dementia and Cognitive Improvement Group supported the authors in the development of this review.
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Terry J Quinn, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK;
Managing Editor (selected peer reviewers, provided editorial guidance to authors, edited the article): Luisa Fernandez Mauleffinch, Cochrane Central Editorial Service;
Editorial Assistant (conducted editorial policy checks, collated peer‐reviewer comments, and supported the editorial team): Sara Hales‐Brittain, Cochrane Central Editorial Service;
Copy Editor (copy editing and production): Lisa Winer, Cochrane Central Production Service;
Peer reviewers (provided comments and recommended an editorial decision): Emma Axon, Cochrane Methods Support Unit (methods review); Nuala Livingstone, Cochrane Editorial & Methods Department (methods review); Jo Platt, Central Editorial Information Specialist (search review); Brian Duncan (consumer review); Sogha Khawari, MRCS, BMBS, BMedSci (clinical review); Sevil Yasar, MD, PhD, Johns Hopkins University, School of Medicine, Department of Neurology (clinical review); and Jason M Schwalb, MD, FAANS, FACS, FAES, Henry Ford Medical Group, Detroit, MI, USA (clinical review).
The authors would like to personally thank Sue Marcus and Jenny McCleery for their helpful suggestions and guidance and Candida Fenton for help with our literature search.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| 1. CENTRAL (The Cochrane Library) http://crso.cochrane.org/SearchSimple.php (Date of most recent search: 15 February 2023) |
#1 MESH DESCRIPTOR Hydrocephalus, Normal Pressure EXPLODE ALL TREES 47 #2 (normal pressure hydrocephalus):TI,AB,KY 105 #3 NPH:TI,AB,KY 824 #4 iNPH:TI,AB,KY 62 #5 #1 OR #2 OR #3 OR #4 912 #6 MESH DESCRIPTOR Cerebrospinal Fluid Shunts EXPLODE ALL TREES 173 #7 MESH DESCRIPTOR Ventriculoperitoneal Shunt EXPLODE ALL TREES 61 #8 shunt*:TI,AB,KY 3117 #9 #6 OR #7 OR #8 3134 #10 #5 AND #9 90 |
March 2022: 90 Feb 2023: 8 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) (Date of most recent search: 15 February 2023) |
1 Hydrocephalus, Normal Pressure/ 2 normal pressure hydrocephalus.ti,ab. 3 NPH.ti,ab. 4 iNPH.ti,ab. 5 or/1‐4 6 exp Cerebrospinal Fluid Shunts/ 7 Ventriculoperitoneal Shunt/ 8 shunt*.ti,ab. 9 or/6‐8 10 5 and 9 11 randomized controlled trial.pt. 12 controlled clinical trial.pt. 13 randomized.ab. 14 placebo.ab. 15 drug therapy.fs. 16 randomly.ab. 17 trial.ab. 18 groups.ab. 19 or/11‐18 20 exp animals/ not humans.sh. 21 19 not 20 22 10 and 21 |
March 2022: 305 Feb 2023: 18 |
| 3. EMBASE via OVID 1974 to present (Date of most recent search: 15 February 2023) |
1 normotensive hydrocephalus/ 2 normal pressure hydrocephalus.ti,ab. 3 normotensive hydrocephalus.ti,ab. 4 NPH.ti,ab. 5 iNPH.ti,ab. 6 or/1‐5 7 exp cerebrospinal fluid drainage system/ 8 brain ventricle peritoneum shunt/ 9 shunt*.ti,ab. 10 or/7‐9 11 6 and 10 12 randomized controlled trial/ 13 controlled clinical trial/ 14 random$.ti,ab. 15 randomization/ 16 intermethod comparison/ 17 placebo.ti,ab. 18 (compare or compared or comparison).ti. 19 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 20 (open adj label).ti,ab. 21 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 22 double blind procedure/ 23 parallel group$1.ti,ab. 24 (crossover or cross over).ti,ab. 25 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 26 (assigned or allocated).ti,ab. 27 (controlled adj7 (study or design or trial)).ti,ab. 28 (volunteer or volunteers).ti,ab. 29 trial.ti. 30 or/12‐29 31 11 and 30 |
March 2022: 499 Feb 2023: 65 |
| 4. PSYCINFO via OVID (Date of most recent search: 15 February 2023) |
1 exp Hydrocephalus/ 2 normal pressure hydrocephalus.ti,ab. 3 NPH.ti,ab. 4 iNPH.ti,ab. 5 or/1‐4 6 shunt*.ti,ab. 7 5 and 6 8 exp Clinical Trials/ 9 randomly.ab. 10 randomi?ed.ti,ab. 11 placebo.ti,ab. 12 groups.ab. 13 "double‐blind*".ti,ab. 14 "single‐blind*".ti,ab. 15 RCT.ti,ab. 16 or/8‐15 17 7 and 16 |
March 2022: 62 Feb 2023: 2 |
| 5. CINAHL (EBSCOhost) (Date of most recent search: 15 February 2023) |
S23 S10 AND S22 S22 S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 S21 MH "Single‐Blind Studies" or MH "Double‐Blind Studies" or MH "Triple‐Blind Studies" S20 MH "Crossover Design" S19 MH "Factorial Design" S18 MH "Placebos" S17 MH "Clinical Trials" S16 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" S15 TX crossover OR "cross‐over" S14 AB placebo* S13 TX random* S12 TX trial* S11 TX "latin square" S10 S5 AND S9 S9 S6 OR S7 OR S8 S8 TX shunt* S7 (MH "Ventriculoperitoneal Shunt") S6 (MH "Cerebrospinal Fluid Shunts+") S5 S1 OR S2 OR S3 OR S4 S4 TX iNPH S3 TX NPH S2 TX normal pressure hydrocephalus S1 (MH "Hydrocephalus, Normal Pressure") |
March 2022: 24 Feb 2023: 4 |
| 6. Clarivate Web of Science – core collection (Date of most recent search: 15 February 2023) |
((TS=(normal pressure hydrocephalus OR NPH OR iNPH) AND TS=(Cerebrospinal Fluid Shunts OR Ventriculoperitoneal Shunt OR SHUNT)) AND TS=(randomly OR randomised OR randomized OR "random allocat*" OR RCT OR CCT OR "double blind*" OR "single blind*" OR "double blind*" OR "single blind*" OR trial)) | March 2022: 144 Feb 2023: 10 |
| 7. LILACS (BIREME) (Date of most recent search:15 February 2023) |
normal pressure hydrocephalus OR NPH OR iNPH [Palavras] and Cerebrospinal Fluid Shunts OR Ventriculoperitoneal Shunt OR SHUNT [Palavras] and randomly OR randomised OR randomized OR RCT OR "controlled trial" OR "double blind$" OR placebo [Palavras] | March 2022: 0 Feb 2023: 0 |
| 8. ClinicalTrials.gov (www.clinicaltrials.gov) (Date of most recent search: 15 February 2023) |
normal pressure hydrocephalus OR NPH OR iNPH | Cerebrospinal Fluid Shunts OR Ventriculoperitoneal Shunt OR SHUNT | March 2022: 42 Feb 2023: 3 |
| 9. ICTRP (Date of most recent search: 23 March 2022) |
normal pressure hydrocephalus OR NPH OR iNPH | Cerebrospinal Fluid Shunts OR Ventriculoperitoneal Shunt OR SHUNT | March 2022: 19 Feb 2023: 3 |
| 10. CDCIG specialised register (CRS web) (Date of most recent search: 15 February 2023) |
SHUNT* | March 2022: 28 Feb 2023: 1 |
| TOTAL before de‐duplication | March 2022: 1213 Feb 2023: 114 |
|
| TOTAL after de‐duplication | March 2022: 853 Feb 2023: 89 |
|
Data and analyses
Comparison 1. Gait speed.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Gait speed ‐ unpaired, parallel assessment < 12 months | 3 | 116 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.62 [0.24, 0.99] |
| 1.2 Gait speed ‐ paired assessment < 12 months | 3 | 103 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.68 [0.28, 1.08] |
Comparison 2. Qualitative gait function.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Unpaired parallel assessment < 12 months | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [0.57, 1.23] |
| 2.2 Paired assessment longitudinal < 12 months | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [0.28, 0.92] |
Comparison 3. Patient disability.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Patient disability ‐ number of participants who improved (unpaired, parallel assessment < 12 months) | 3 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.02 [3.61, 22.53] |
| 3.2 Patient disability ‐ functionally independent (unpaired, parallel assessment < 12 months) | 3 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [1.31, 3.31] |
| 3.3 Patient disability ‐ number of participants who improved (paired assessment < 12 months) | 2 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.65, 5.27] |
| 3.4 Patient disability ‐ functionally independent (paired assessment < 12 months) | 2 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.54, 16.53] |
Comparison 4. Adverse events (< 12 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Adverse events < 3 months post‐surgery | 1 | 88 | Odds Ratio (M‐H, Fixed, 95% CI) | 37.58 [4.76, 296.76] |
Comparison 5. Cognitive function.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 General cognitive screening ‐ unpaired, parallel assessment < 6 months | 2 | 104 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.35 [‐0.04, 0.74] |
| 5.2 General cognitive screening ‐ paired assessment < 12 months | 2 | 93 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.01, 0.82] |
| 5.3 Symbol Digit Tests ‐ unpaired, parallel assessment < 6 months | 2 | 87 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.45 [0.03, 0.88] |
| 5.4 Symbol Digit Tests ‐ paired assessment < 12 months | 2 | 79 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.46 [0.01, 0.91] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kazui 2015.
| Study characteristics | |
| Methods |
Study design: 1‐arm, cross‐over randomised controlled trial, assessor blinded Number randomised: 93 (49 immediate LP shunt and 44 postponed LP shunt) Exclusions: 3 from immediate‐shunt group and 2 from delayed‐shunt group did not receive intended intervention. 1 further participant in the delayed group did not receive shunt surgery. Length of follow‐up: 12 months How data were handled: intention‐to‐treat and modified intention‐to‐treat analysis |
| Participants |
Country: Japan Setting: 20 neurosurgical centres Baseline characteristics: Immediate‐shunt group
Delayed‐shunt group
Number of Hakim symptoms: minimum 1 (only 10%), 91% had gait impairment Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention 1: immediate LP shunt Intervention 2: delayed LP shunt after 3 months |
| Outcomes |
Primary outcome: Improvement in disability at 3 months after surgery measured by mRS Secondary outcomes: Improvement in disability at 12 months after surgery Other secondary outcome measures recorded were as follows.
|
| Notes |
Sources of support: Johnson & Johnson and Nihon Medi‐Physics Possible conflicts of interest: HK has received donations for the 15th Japan Congress of Normal Pressure Hydrocephalus from Johnson & Johnson, Nihon Medi‐Physics, Medtronic, Ono Pharmaceutical, Pfizer, and Eisai; research grants from Eisai, Daiichi‐Sankyo, and MSD; and speaker’s honoraria from Johnson & Johnson, Nihon Medi‐Physics, Medtronic, Ono Pharmaceutical, Pfizer, Eisai, Daiichi‐Sankyo, Novartis, Janssen, and Takeda. MM has received donations for the 14th Japan Congress of Normal Pressure Hydrocephalus from Johnson & Johnson, Nihon Medi‐Physics, Medtronic, and Novartis; and speaker’s honoraria from Johnson & Johnson, Nihon Medi‐Physics, Medtronic, Eisai, and Daiichi‐Sankyo. EM has received research grants from Eisai and Fuji Film; consulting fees from Lundbeck; and speaker’s honoraria from Johnson & Johnson, Nihon Medi‐Physics, Medtronic, Eisai, Daiichi‐Sankyo, Janssen, Novartis, and Ono Pharmaceutical. MI has received speaker’s honoraria from Johnson & Johnson and Medtronic. |
Luciano 2023.
| Study characteristics | |
| Methods |
Study design: 1‐arm, cross‐over randomised controlled trial, assessor and participant blinded Number randomised: 18 (9 active VP shunt, 9 initially inactive VP shunt) Exclusions: all participants received intended immediate intervention. 2 participants became medically unwell during study (1 gallbladder necrosis, 1 contralateral stroke). Length of follow‐up: 12 months How data were handled: intention‐to‐treat |
| Participants |
Country: United States, Canada, Sweden Setting: multicentre Baseline characteristics: Immediate‐shunt group
Delayed‐shunt group
Number of Hakim symptoms: gait + 1 other Inclusion criteria:
Exclusion criteria:
Entry modification:
|
| Interventions |
Intervention 1: active VP shunt Intervention 2: initially inactive VP shunt changed to active at 4 months |
| Outcomes |
Primary outcome: Improvement in gait velocity measured by 10 MWT Secondary outcomes: MoCA; Symbol Digit Modalities Test; Overactive Bladder Questionnaire, short form; Beck Depression Inventory, Second Edition; Lawton Activities of Daily Living/Independent Activities of Daily Living Questionnaire; and mRS scores |
| Notes |
Sources of support: this study was supported by Integra LifeSciences, without control of study design, data collection, analysis, or publication. Other support and consultation were provided by the Hydrocephalus Association through the Adult Hydrocephalus Clinical Research Network, the National Center for Advancing Translational Sciences (Grant U24TR001609 in support of the Johns Hopkins‐Tufts Trial Innovation Center) for consultation on recruitment, and the National Institute on Aging (U19‐AG033655) for initial protocol review. Neurosurgery administrative support was also provided to the Johns Hopkins Neurosurgery CSF Disorders Center through George Berry and William Lickle. Possible conflicts of interest: Dr Holubkov is on the Data Safety Monitoring Board Service for Pfizer Inc. Dr Moghekar is a non‐paid member of the Medical Advisory Board at the nonprofit Hydrocephalus Association. Dr Hamilton has been paid by Integra Canada and Integra International for general educational lectures at professional meetings not related to the contents of this paper, and received a one‐time consultant fee under USD 2000 from CereVasc Inc, for whom he is a current member of the Data Safety Monitoring Board. |
Tisell 2011.
| Study characteristics | |
| Methods |
Study design: 1‐arm, cross‐over randomised controlled trial, assessor and participant blinded Number randomised: 14 (7 per group) Exclusions: none Length of follow‐up: 6‐month formative with 5‐year remote How data were handled: intention‐to‐treat |
| Participants |
Country: Sweden Setting: single centre Baseline characteristics: Immediate‐shunt group
Delayed‐shunt group
Overall, mean participant age was 75 years, and 36% of participants were female. Number of Hakim symptoms: n/a Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention 1: active VP shunt Intervention 2: initially inactive VP shunt changed to active at 3 months |
| Outcomes |
Primary outcome: Composite scores of cognition: Bingley Memory Test, Rey Auditory Verbal Learning Test, Grooved Pegboard Test, Stroop Test, Reaction Time Test Composite scores of gait: Time to sit, 10 MWT, 6 flight stair escalation time, steps to turn 180°, 3‐metre TUG walk time and step count |
| Notes |
Sources of support: this study was supported by unrestricted grants from the Edit Jacobson Foundation, the John and Britt Wennerstrom Foundation, and the Pfizer Foundation for clinical neurological research and the Per‐Olof Ahl Foundatino for research on vascular diseases of the brain. Possible conflicts of interest: none stated. |
Toma 2016.
| Study characteristics | |
| Methods |
Study design: 1‐arm, cross‐over randomised controlled trial, assessor and participant blinded Number randomised: 15 Exclusions: all participants had the intended intervention. 1 withdrew consent following surgery in the inactive group. Length of follow‐up: 12 months How data were handled: modified intention‐to‐treat |
| Participants |
Country: United Kingdom Setting: single centre Baseline characteristics: Immediate‐shunt group
Delayed‐shunt group
Number of Hakim symptoms: gait + 1 other Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention 1: active VP shunt Intervention 2: initially inactive VP shunt changed to active at 3 months |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Notes |
Sources of support: nil Possible conflicts of interest: Toma PhD funded by B Braun. |
10 MWT: 10‐metre walk test CSF: cerebrospinal fluid CSFTT: cerebrospinal fluid tap test CT: computed tomography FAB: frontal assessment battery iNPH: idiopathic normal pressure hydrocephalus iNPHGS: idiopathic normal pressure hydrocephalus grading scale LP: lumboperitoneal MMSE: Mini‐Mental State Examination MoCA: Montreal Cognitive Assessment MRI: magnetic resonance imaging mRS: modified Rankin Scale n/a: not available NPH: normal pressure hydrocephalus SD: standard deviation SSS‐WAIS‐III: Symbol Search subset of Wechsler Adult Intelligence Scale, Third Edition TMT‐A: trail making test A TUG: timed up‐and‐go test VP: ventriculoperitoneal
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Saehle 2014 | The control group had the shunt setting gradually changed, so it was not set to a true on or off setting. |
Characteristics of studies awaiting classification [ordered by study ID]
NCT01798641.
| Methods | Cross‐over, placebo‐controlled randomised controlled trial |
| Participants | People with iNPH with gait impairment |
| Interventions | Active VP versus inactive VP shunt |
| Outcomes | Gait speed (TUG score), Tinetti gait scale, Kubo normal pressure hydrocephalus scale, Keifer scale |
| Notes | Completed in 2018 but not yet published |
iNPH: idiopathic normal pressure hydrocephalus TUG: timed up‐and‐go test VP: ventriculoperitoneal
Characteristics of ongoing studies [ordered by study ID]
NCT05081128.
| Study name | PENS |
| Methods | Double‐blind, parallel randomised controlled trial |
| Participants | Age ≥ 60 years; diagnosis of iNPH and recommendation for shunt surgery |
| Interventions | Active ventriculoperitoneal shunt |
| Outcomes | Gait velocity, disability, cognition as assessed by the Montreal Cognitive Assessment (MoCA), bladder control as assessed by the Overactive Bladder Questionnaire, short form (OAB‐q SF) |
| Starting date | 18 May 2022 |
| Contact information | Jessica Wollett, penstrial@jh.edu |
| Notes |
iNPH: idiopathic normal pressure hydrocephalus
Differences between protocol and review
We did not expect to find multiple semi‐ordinal scales that measured disability, but we were able to compare these scales by binarising the data to above and below meaningful thresholds, which allowed us to measure the magnitude of effect size that would not have been possible otherwise. We were not able to perform a 'paired' assessment of this outcome in this way, but we did perform a paired 'better or not' comparison of disability to ensure that the direction of effect was not affected by our 'unpairing' of the data.
In our protocol we defined 'short‐term' outcomes as those being measured less than six months post‐cerebrospinal fluid (CSF)‐shunt insertion. We unexpectedly found that paired data were available in groups that had delayed CSF‐shunting between eight and nine months post‐shunt for the outcomes gait speed, disability, and cognitive function. While it seems inappropriate to compare three‐ to four‐month post‐shunt data with eight‐ to nine‐month post‐shunt data, it does not feel correct to consider eight‐ to nine‐month data sets as 'long term', as this is not what patients would consider a long‐term effect of shunting. As such, we revised our definition of short term to less than 12 months. We did not directly compare eight‐ to nine‐month post‐CSF‐shunt data with the three‐ to four‐month post‐CSF‐shunt data from parallel‐group assessments. This was consistent with our prior analysis plan.
We did not intend to analyse cognitive tests other than general screening tools such as the Mini–Mental State Examination (MMSE). We found some studies that did compare similar subdomain cognitive scales, and felt it would be inappropriate to ignore these data.
In two studies, adverse events were described in detail in narrative form but were not formally categorised as 'serious adverse events' and 'non‐serious adverse events', as in the other two studies. In this case, we retrospectively categorised adverse events using criteria that were used to define adverse events in the other studies (ICH 1994).
Contributions of authors
Chris Carswell wrote the protocol and manuscript. Chris Carswell, Anastasia Gontsarova, Kevin Tsang, Ron Pearce, Davina Richardson, Hilary Watt, and Abi Methley all contributed to study design and subsequent manuscript editing. Ron Pearce, Anastasia Gontsarova, and Chris Carswell performed risk of bias assessments. Ron Pearce, Anastasia Gontsarova, Chris Carswell, and Abi Methley performed abstract screening. Ron Pearce, Anastasia Gontsarova, and Chris Carswell performed in‐depth study review and data extraction. Hilary Watt reviewed statistical analyses.
Sources of support
Internal sources
-
Imperial College London, UK
C Carswell is a Senior Clinical fellow at Imperial College and benefits from the research environment and facilities.
External sources
-
Medical Research Council (MRC), UK
C Carswell holds an MRC Clinical Academic Research Partnership award which funds dedicated time to investigate normal pressure hydrocephalus.
-
National Institute for Health and Care Research (NIHR), UK
Cochrane Review Group infrastructure This project was supported by the NIHR via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement Group until 31 March 2023. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Declarations of interest
Ron Pearce declares no conflicts of interest.
Anastasia Gontsarova is an independent contractor‐consultant with Biogen.
Davina Richardson declares no conflicts of interest.
Abi Methley is the owner of Innovative Clinical Psychology Solutions Ltd, a private clinical psychology practice incorporated in 2021 that provides services for clients with neurological conditions. She was employed in an NHS neuropsychology service until 2022.
Hilary Clare Watt is an independent contractor‐consultant with the Medical Research Council.
Kevin Tsang declares no conflicts of interest.
Chris Carswell declares no conflicts of interest.
New
References
References to studies included in this review
Kazui 2015 {published and unpublished data}
- Kazui H, Miyajima M, Mori E, Ishikawa M, SINPHONI-2 Investigators. Lumboperitoneal shunt surgery for idiopathic normal pressure hydrocephalus (SINPHONI-2): an open-label randomised trial. The Lancet. Neurology 2015;14(6):585-94. [DOI] [PubMed] [Google Scholar]
Luciano 2023 {published and unpublished data}
- Luciano M, Holubkov R, Williams MA, Malm J, Nagel S, Moghekar A, et al. Placebo-controlled effectiveness of idiopathic normal pressure hydrocephalus shunting: a randomized pilot trial. Neurosurgery 2023;92(3):481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tisell 2011 {published data only}
- Tisell M, Tullberg M, Hellström P, Edsbagge M, Högfeldt M, Wikkelsö C. Shunt surgery in patients with hydrocephalus and white matter changes. Journal of Neurosurgery 2011;114(5):1432-8. [DOI] [PubMed] [Google Scholar]
Toma 2016 {unpublished data only}
- Toma AK, Watkins LD. Surgical management of idiopathic normal pressure hydrocephalus: a trial of a trial. British Journal of Neurosurgery 2016;30(6):605. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Saehle 2014 {published data only}
- Saehle T, Farahmand D, Eide PK, Tisell M, Wikkelsö C. A randomized controlled dual-center trial on shunt complications in idiopathic normal-pressure hydrocephalus treated with gradually reduced or "fixed" pressure valve settings. Journal of Neurosurgery 2014;121(5):1257-63. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT01798641 {unpublished data only}
- NCT01798641. A randomized cross-over study for normal pressure hydrocephalus (ARCS NPH) [Efficacy of shunt surgery in normal pressure hydrocephalus: a randomized cross-over study]. clinicaltrials.gov/study/NCT01798641 (first received 26 February 2013).
References to ongoing studies
NCT05081128 {published data only}
- NCT05081128. A placebo-controlled efficacy in iNPH shunting (PENS) trial [Efficacy in iNPH shunting (PENS) trial (PENS)]. clinicaltrials.gov/study/NCT05081128 (first received 18 October 2021).
Additional references
Adams 1965
- Adams RD, Fisher CM, Hakim S, Ojemann RG, Sweet WH. Symptomatic occult hydrocephalus with "normal" cerebrospinal-fluid pressure. A treatable syndrome. New England Journal of Medicine 1965;273:117-26. [DOI] [PubMed] [Google Scholar]
Akyea 2021
- Akyea RK, Vinogradova Y, Qureshi N, Patel RS, Kontopantelis E, Ntaios G, et al. Sex, age, and socioeconomic differences in non-fatal stroke incidence and subsequent major adverse outcomes. Stroke 2021;52(2):396-405. [DOI: 10.1161/STROKEAHA.120.031659] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andersson 2019
- Andersson J, Rosell M, Kockum K, Lilja-Lund O, Söderström L, Laurell K. Prevalence of idiopathic normal pressure hydrocephalus: a prospective, population-based study. PLOS ONE 2019;14(5):e0217705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Badhiwala 2015
- Badhiwala JH, Nassiri F, Alhazzani W, Selim MH, Farrokhyar F, Spears J, et al. Endovascular thrombectomy for acute ischemic stroke: a meta-analysis. JAMA 2015;314(17):1832-43. [DOI] [PubMed] [Google Scholar]
Bahar‐Fuchs 2019
- Bahar‐Fuchs A, Martyr A, Goh AM, Sabates J, Clare L. Cognitive training for people with mild to moderate dementia. Cochrane Database of Systematic Reviews 2019, Issue 3. Art. No: CD013069. [DOI: 10.1002/14651858.CD013069.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Banizs 2005
- Banizs B, Pike MM, Millican CL, Ferguson WB, Komlosi P, Sheetz J, et al. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development (Cambridge, England) 2005;132(23):5329-39. [DOI] [PubMed] [Google Scholar]
Bluett 2023
- Bluett B, Ash E, Farheen A, Fasano A, Krauss JK, Maranzano A, et al. Clinical features of idiopathic normal pressure hydrocephalus: critical review of objective findings. Movement Disorders Clinical Practice 2023;10(1):9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bradley 2015
- Bradley WG. CSF flow in the brain in the context of normal pressure hydrocephalus. American Journal of Neuroradiology 2015;36(5):831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Børgesen 1982
- Børgesen SE, Gjerris FL. The predictive value of conductance to outflow of CSF in normal pressure hydrocephalus. Brain: A Journal of Neurology 1982;105(Pt 1):65-86. [DOI] [PubMed] [Google Scholar]
Cabral 2011
- Cabral D, Beach TG, Vedders L, Sue LI, Jacobson S, Myers K, et al. Frequency of Alzheimer's disease pathology at autopsy in patients with clinical normal pressure hydrocephalus. Alzheimer's & Dementia 2011;7(5):509-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carswell 2024
- Carswell C. Consensus outcome of RoB signalling questions. Shunting for idiopathic normal pressure hydrocephalus; 2024. zenodo.org/records/10590681.
Covidence [Computer program]
- Covidence. Version accessed 16 May 2024. Melbourne, Australia: Veritas Health Innovation, 2024. Available at covidence.org.
Craven 2016
- Craven C, Asif H, Farrukh A, Somavilla F, Toma AK, Watkins L. Case series of ventriculopleural shunts in adults: a single-center experience. Journal of Neurosurgery 2016;126(6):2010-6. [DOI] [PubMed] [Google Scholar]
Deeks 2021
- Deeks J, Higgins J, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Esmonde 2002
- Esmonde T, Cooke S. Shunting for normal pressure hydrocephalus (NPH). Cochrane Database of Systematic Reviews 2002, Issue 3. Art. No: CD003157. [DOI: 10.1002/14651858.CD003157] [DOI] [PMC free article] [PubMed] [Google Scholar]
Espay 2017
- Espay AJ, Da Prat GA, Dwivedi AK, Rodriguez-Porcel F, Vaughan JE, Rosso M, et al. Deconstructing normal pressure hydrocephalus: ventriculomegaly as early sign of neurodegeneration. Annals of Neurology 2017;82(4):503-13. [DOI] [PubMed] [Google Scholar]
Fernández‐Méndez 2019
- Fernández-Méndez R, Richards HK, Seeley HM, Pickard JD, Joannides AJ. Current epidemiology of cerebrospinal fluid shunt surgery in the UK and Ireland (2004-2013). Journal of Neurology, Neurosurgery & Psychiatry 2019;90(7):747-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giordan 2018
- Giordan E, Palandri G, Lanzino G, Murad MH, Elder BD. Outcomes and complications of different surgical treatments for idiopathic normal pressure hydrocephalus: a systematic review and meta-analysis. Journal of Neurosurgery 2018;131(4):1-13. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 15 May 2024. Hamilton (ON): McMaster University (developed by Evidence Prime), 2024. Available at gradepro.org.
Hakim 1965
- Hakim S, Adams RD. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics. Journal of the Neurological Sciences 1965;2(4):307-27. [DOI] [PubMed] [Google Scholar]
Hakim 1976
- Hakim S, Venegas JG, Burton JD. The physics of the cranial cavity, hydrocephalus and normal pressure hydrocephalus: mechanical interpretation and mathematical model. Surgical Neurology 1976;5(3):187-210. [PubMed] [Google Scholar]
Halperin 2015
- Halperin JJ, Kurlan R, Schwalb JM, Cusimano MD, Gronseth G, Gloss D. Practice guideline: idiopathic normal pressure hydrocephalus: response to shunting and predictors of response: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology 2015;85(23):2063-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hashimoto 2010
- Hashimoto M, Ishikawa M, Mori E, Kuwana N. Diagnosis of idiopathic normal pressure hydrocephalus is supported by MRI-based scheme: a prospective cohort study. Cerebrospinal Fluid Research 2010;7:1-18. [DOI: 10.1186/1743-8454-7-18] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hebb 2001
- Hebb AO, Cusimano MD. Idiopathic normal pressure hydrocephalus: a systematic review of diagnosis and outcome. Neurosurgery 2001;49(5):1166-86. [DOI] [PubMed] [Google Scholar]
Hellström 2012
- Hellström P, Klinge P, Tans J, Wikkelsø C. A new scale for assessment of severity and outcome in iNPH. Acta Neurologica Scandinavica 2012;126(4):229-37. [DOI] [PubMed] [Google Scholar]
Higgins 2021
- Higgins J, Li T, Deeks J. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Higgins 2023
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook.
Horner 1992
- Horner SM. Efficacy of intravenous magnesium in acute myocardial infarction in reducing arrhythmias and mortality. Meta-analysis of magnesium in acute myocardial infarction. Circulation 1992;86(3):774-9. [DOI] [PubMed] [Google Scholar]
Hudson 2019
- Hudson M, Nowak C, Garling RJ, Harris C. Comorbidity of diabetes mellitus in idiopathic normal pressure hydrocephalus: a systematic literature review. Fluids Barriers CNS 2019;16(1):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
ICH 1994
- Tietje C, Brouder A. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. In: Tietje C, Brouder A, editors(s). Handbook of Transnational Economic Governance Regimes. Brill | Nijhoff, 2010:1041-53. [Google Scholar]
Ishikawa 2004
- Ishikawa M, Guideline Committee for Idiopathic Normal Pressure Hydrocephalus, Japanese Society of Normal Pressure Hydrocephalus. Clinical guidelines for idiopathic normal pressure hydrocephalus. Neurologia Medico-Chirurgica (Tokyo) 2004;44(4):222-3. [DOI: 10.2176/nmc.44.222] [DOI] [PubMed] [Google Scholar]
ISIS‐4
- ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. ISIS-4: a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. Lancet 1995;345(8951):669-85. [PubMed] [Google Scholar]
Jaraj 2016
- Jaraj D, Agerskov S, Rabiei K, Marlow T, Jensen C, Guo X, et al. Vascular factors in suspected normal pressure hydrocephalus. Neurology 2016;86(7):592-9. [DOI: 10.1212/WNL.0000000000002369] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaraj 2017
- Jaraj D, Rabiei K, Marlow T, Jensen C, Skoog I, Wikkelsø C. Estimated ventricle size using Evans index: reference values from a population-based sample. European Journal of Neurology 2017;24(3):468-74. [DOI] [PubMed] [Google Scholar]
Kimihira 2020
- Kimihira L, Iseki C, Takahashi Y, Sato H, Kato H, Kazui H, et al. A multi-center, prospective study on the progression rate of asymptomatic ventriculomegaly with features of idiopathic normal pressure hydrocephalus on magnetic resonance imaging to idiopathic normal pressure hydrocephalus. Journal of the Neurological Sciences 2020;419:1-5. [DOI] [PubMed] [Google Scholar]
Kitagaki 1998
- Kitagaki H, Mori E, Ishii K, Yamaji S, Hirono N, Imamura T. CSF spaces in idiopathic normal pressure hydrocephalus: morphology and volumetry. AJNR. American Journal of Neuroradiology 1998;19(7):1277-84. [PMC free article] [PubMed] [Google Scholar]
Klinge 2012
- Klinge P, Hellström P, Tans J, Wikkelsø C, European iNPH Multicentre Study Group. One-year outcome in the European multicentre study on iNPH. Acta Neurologica Scandinavica 2012;126(3):145-53. [DOI: 10.1111/j.1600-0404.2012.01676.x] [DOI] [PubMed] [Google Scholar]
Kockum 2018
- Kockum K, Lilja-Lund O, Larsson E -M, Rosell M, Söderström L, Virhammar J, et al. The idiopathic normal-pressure hydrocephalus radscale: a radiological scale for structured evaluation. European Journal of Neurology 2018;25(3):569-76. [DOI] [PubMed] [Google Scholar]
Kockum 2020
- Kockum K, Virhammar J, Riklund K, Soderstrom L, Larsson E-M, Laurell K. Diagnostic accuracy of the iNPH radscale in idiopathic normal pressure hydrocephalus. PLOS ONE 2020;15(4):e0232275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lam 1997
- Lam CH, Villemure JG. Comparison between ventriculoatrial and ventriculoperitoneal shunting in the adult population. British Journal of Neurosurgery 1997;11(1):43-8. [DOI: 10.1080/02688699746681] [DOI] [PubMed] [Google Scholar]
Lavados 2021
- Lavados PM, Hoffmeister L, Moraga AM, Vejar A, Vidal C, Gajardo C, et al. Incidence, risk factors, prognosis, and health-related quality of life after stroke in a low-resource community in Chile (ÑANDU): a prospective population-based study. Lancet Global Health 2021;9(3):e340-51. [DOI: 10.1016/S2214-109X(20)30470-8] [DOI] [PubMed] [Google Scholar]
Louvi 2011
- Louvi A, Grove EA. Cilia in the CNS: the quiet organelle claims center stage. Neuron 2011;69(6):1046-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Macki 2020
- Macki M, Mahajan A, Shatz R, Air EL, Novikova M, Fakih M, et al. Prevalence of alternative diagnoses and implications for management in idiopathic normal pressure hydrocephalus patients. Neurosurgery 2020;87(5):999-1007. [DOI] [PubMed] [Google Scholar]
Malm 2006
- Malm J, Eklund A. Idiopathic normal pressure hydrocephalus. Practical Neurology 2006;6(1):14-27. [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mollan 2018
- Mollan SP, Hornby C, Mitchell J, Sinclair AJ. Evaluation and management of adult idiopathic intracranial hypertension. Practical Neurology 2018;18(6):485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Momjian 2004
- Momjian S, Owler BK, Czosnyka Z, Czosnyka M, Pena A, Pickard JD. Pattern of white matter regional cerebral blood flow and autoregulation in normal pressure hydrocephalus. Brain: A Journal of Neurology 2004;127(Pt 5):965-72. [DOI] [PubMed] [Google Scholar]
Morimoto 2019
- Morimoto Y, Yoshida S, Kinoshita A, Satoh C, Mishima H, Yamaguchi N, et al. Nonsense mutation in CFAP43 causes normal-pressure hydrocephalus with ciliary abnormalities. Neurology 2019;92(20):e2364-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Müller‐Schmitz 2020
- Müller‐Schmitz K, Krasavina‐Loka N, Yardimci T, Lipka T, Kolman AG, Robbers S, et al. Normal pressure hydrocephalus associated with Alzheimer's disease. Annals of Neurology 2020;88(4):703-11. [DOI] [PubMed] [Google Scholar]
Nakajima 2021
- Nakajima M, Yamada S, Miyajima M, Ishii K, Kuriyama N, Kazui H, et al. Guidelines for management of idiopathic normal pressure hydrocephalus (third edition): endorsed by the Japanese Society of Normal Pressure Hydrocephalus. Neurologia Medico-Chirurgica 2021;61(2):63-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Narita 2016
- Narita W, Nishio Y, Baba T, Iizuka O, Ishihara T, Matsuda M, et al. High-convexity tightness predicts the shunt response in idiopathic normal pressure hydrocephalus. American Journal of Neuroradiology 2016;37(10):1831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ogino 2006
- Ogino A, Kazui H, Miyoshi N, Hashimoto M, Ohkawa S, Tokunaga H, et al. Cognitive impairment in patients with idiopathic normal pressure hydrocephalus. Dementia and Geriatric Cognitive Disorders 2006;21(2):113-9. [DOI] [PubMed] [Google Scholar]
Ohara 2020
- Ohara M, Hattori T, Yokota T. Progressive supranuclear palsy often develops idiopathic normal pressure hydrocephalus-like magnetic resonance imaging features. European Journal of Neurology 2020;27(10):1930-6. [DOI] [PubMed] [Google Scholar]
Relkin 2005
- Relkin N, Marmarou A, Klinge P, Bergsneider M, Black PMcL. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery 2005;57(3 Suppl):S4-16; discussion ii-v. [DOI] [PubMed] [Google Scholar]
RevMan 2024 [Computer program]
- Review Manager (RevMan). Version 7.10.3. The Cochrane Collaboration, 2024. Available at revman.cochrane.org.
Ringstad 2017
- Ringstad G, Vatnehol SA, Eide PK. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain 2017;140(10):2691-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Román 2018
- Román GC, Verma AK, Zhang YJ, Fung SH. Idiopathic normal-pressure hydrocephalus and obstructive sleep apnea are frequently associated: a prospective cohort study. Journal of the Neurological Sciences 2018;395:164-8. [DOI] [PubMed] [Google Scholar]
Sakakibara 2008
- Sakakibara R, Kanda T, Sekido T, Uchiyama T, Awa Y, Ito T, et al. Mechanism of bladder dysfunction in idiopathic normal pressure hydrocephalus. Neurourology and Urodynamics: Official Journal of the International Continence Society 2008;27(6):507-10. [DOI] [PubMed] [Google Scholar]
Saper 2017
- Saper CB. Is there even such a thing as "Idiopathic normal pressure hydrocephalus"? Annals of Neurology 2017;82(4):514-5. [DOI] [PubMed] [Google Scholar]
Schünemann 2021a
- Schünemann H, Higgins J, Vist G, Glasziou P, Akl E, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Schünemann 2021b
- Schünemann H, Vist G, Higgins J, Santesso N, Deeks J, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Silverberg 2003
- Silverberg GD, Mayo M, Saul T, Rubenstein E, McGuire D. Alzheimer's disease, normal-pressure hydrocephalus, and senescent changes in CSF circulatory physiology: a hypothesis. The Lancet. Neurology 2003;2(8):506-11. [DOI] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
Takeuchi 2019
- Takeuchi T, Yajima K. Long-term 4 years follow-up study of 482 patients who underwent shunting for idiopathic normal pressure hydrocephalus - course of symptoms and shunt efficacy rates compared by age group. Neurologia Medico-Chirurgica 2019;59(7):281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toma 2013
- Toma AK, Papadopoulos MC, Stapleton S, Kitchen ND, Watkins LD. Systematic review of the outcome of shunt surgery in idiopathic normal-pressure hydrocephalus. Acta Neurochirurgica 2013;155(10):1977-80. [DOI] [PubMed] [Google Scholar]
Vanhala 2019
- Vanhala V, Junkkari A, Korhonen VE, Kurki MI, Hiltunen M, Rauramaa T, et al. Prevalence of schizophrenia in idiopathic normal pressure hydrocephalus. Neurosurgery 2019;84(4):883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2022
- Williams MA, Nagel SJ, Golomb J, Jensen H, Dasher NA, Holubkov R, et al. Safety and effectiveness of the assessment and treatment of idiopathic normal pressure hydrocephalus in the Adult Hydrocephalus Clinical Research Network. Journal of Neurosurgery 2022;137(5):1-13. [DOI] [PubMed] [Google Scholar]
Yang 2021
- Yang HW, Lee S, Yang D, Dai H, Zhang Y, Han L, et al. Deletions in CWH43 cause idiopathic normal pressure hydrocephalus. EMBO Molecular Medicine 2021;13(3):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yasar 2017
- Yasar S, Jusue-Torres I, Lu J, Robison J, Patel MA, Crain B, et al. Alzheimer's disease pathology and shunt surgery outcome in normal pressure hydrocephalus. PLOS ONE 2017;12(8):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zaccaria 2020
- Zaccaria V, Bacigalupo I, Gervasi G, Canevelli M, Corbo M, Vanacore N, et al. A systematic review on the epidemiology of normal pressure hydrocephalus. Acta Neurologica Scandinavica 2020;141(2):101-14. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gontsarova 2022
- Gontsarova A, Richardson D, Methley AM, Tsang K, Pearce R, Carswell C. Shunting for idiopathic normal pressure hydrocephalus. Cochrane Database of Systematic Reviews 2022, Issue 3. Art. No: CD014923. [DOI: 10.1002/14651858.CD014923] [DOI] [PMC free article] [PubMed] [Google Scholar]


