Abstract
Background
Eczema (atopic dermatitis) is the most burdensome skin condition worldwide and cannot currently be prevented or cured. Topical anti‐inflammatory treatments are used to control eczema symptoms, but there is uncertainty about the relative effectiveness and safety of different topical anti‐inflammatory treatments.
Objectives
To compare and rank the efficacy and safety of topical anti‐inflammatory treatments for people with eczema using a network meta‐analysis.
Search methods
We searched the Cochrane Skin Specialised Register, CENTRAL, MEDLINE, Embase and trial registries on 29 June 2023, and checked the reference lists of included studies.
Selection criteria
We included within‐participant or between‐participant randomised controlled trials (RCTs) in people of any age with eczema of any severity, but excluded trials in clinically infected eczema, seborrhoeic eczema, contact eczema, or hand eczema. We included topical anti‐inflammatory treatments used for at least one week, compared with another anti‐inflammatory treatment, no treatment, or vehicle/placebo. Vehicle is a 'carrier system' for an active pharmaceutical substance, which may also be used on its own as an emollient for dry skin. We excluded trials of topical antibiotics used alone, complementary therapies, emollients used alone, phototherapy, wet wraps, and systemic treatments.
Data collection and analysis
We used standard Cochrane methods. Primary outcomes were patient‐reported eczema symptoms, clinician‐reported eczema signs and investigator global assessment. Secondary outcomes were health‐related quality of life, long‐term control of eczema, withdrawal from treatment/study, and local adverse effects (application‐site reactions, pigmentation changes and skin thinning/atrophy were identified as important concerns through patient and public involvement). We used CINeMA to quantify our confidence in the evidence for each outcome.
Main results
We included 291 studies involving 45,846 participants with the full spectrum of eczema severity, mainly conducted in high‐income countries in secondary care settings. Most studies included adults, with only 31 studies limited to children aged < 12 years. Studies usually included male and female participants, multiple ethnic groups but predominantly white populations. Most studies were industry‐funded (68%) or did not report their funding sources/details. Treatment duration and trial participation were a median of 21 and 28 days (ranging from 7 days to 5 years), respectively. Interventions used were topical corticosteroids (TCS) (172), topical calcineurin inhibitors (TCI) (134), phosphodiesterase‐4 (PDE‐4) inhibitors (55), janus kinase (JAK) inhibitors (30), aryl hydrocarbon receptor activators (10), or other topical agents (21). Comparators included vehicle (170) or other anti‐inflammatory treatments. The risk of bias was high in 242 of the 272 (89.0%) trials contributing to data analyses, most commonly due to concerns about selective reporting. Network meta‐analysis (NMA) was only possible for short‐term outcomes.
Patient‐reported symptoms
NMA of 40 trials (6482 participants) reporting patient‐reported symptoms as a binary outcome ranked tacrolimus 0.1% (OR 6.27, 95% CI 1.19 to 32.98), potent TCS (OR 5.99, 95% CI 2.83 to 12.69), and ruxolitinib 1.5% (OR 5.64, 95% CI 1.26 to 25.25) as the most effective, all with low confidence. Mild TCS, roflumilast 0.15%, and crisaborole 2% were the least effective. Class‐level sensitivity analysis found potent/very potent TCS had similar effectiveness to potent TCI and was more effective than mild TCI and PDE‐4 inhibitors.
NMA of 29 trials (3839 participants) reporting patient‐reported symptoms as a continuous outcome ranked very potent TCS (SMD ‐1.99, 95% CI ‐3.25 to ‐0.73; low confidence) and tacrolimus 0.03% (SMD ‐1.57, 95% CI –2.42 to ‐0.72; moderate confidence) the highest. Direct information for tacrolimus 0.03% was based on one trial of 60 participants at high risk of bias. Roflumilast 0.15%, delgocitinib 0.25% or 0.5%, and tapinarof 1% were the least effective. Class‐level sensitivity analysis found potent/very potent TCS had similar effectiveness to potent TCI and JAK inhibitors and mild/moderate TCS was less effective than mild TCI.
A further 50 trials (9636 participants) reported patient‐reported symptoms as a continuous outcome but could not be included in NMA.
Clinician‐reported signs
NMA of 32 trials (4121 participants) reported clinician signs as a binary outcome and ranked potent TCS (OR 8.15, 95% CI 4.99, 13.57), tacrolimus 0.1% (OR 8.06, 95% CI 3.30, 19.67), ruxolitinib 1.5% (OR 7.72, 95% CI 4.92, 12.10), and delgocitinib 0.5% (OR 7.61, 95% CI 3.72, 15.58) as most effective, all with moderate confidence. Mild TCS, roflumilast 0.15%, crisaborole 2%, and tapinarof 1% were the least effective. Class‐level sensitivity analysis found potent/very potent TCS more effective than potent TCI, mild TCI, JAK inhibitors, PDE‐4 inhibitors; and mild TCS and PDE‐4 inhibitors had similar effectiveness.
NMA of 49 trials (5261 participants) reported clinician signs as a continuous outcome and ranked tacrolimus 0.03% (SMD ‐2.69, 95% CI ‐3.36, ‐2.02) and very potent TCS (SMD ‐1.87, 95% CI ‐2.69, ‐1.05) as most effective, both with moderate confidence; roflumilast 0.15%, difamilast 0.3% and tapinarof 1% were ranked as least effective. Direct information for tacrolimus 0.03% was based on one trial in 60 participants with a high risk of bias. For some sensitivity analyses, potent TCS, tacrolimus 0.1%, ruxolitinib 1.5%, delgocitinib 0.5% and delgocitinib 0.25% became some of the most effective treatments. Class‐level analysis found potent/very potent TCS had similar effectiveness to potent TCI and JAK inhibitors, and moderate/mild TCS was more effective than mild TCI.
A further 100 trials (22,814 participants) reported clinician signs as a continuous outcome but could not be included in NMA.
Investigator Global Assessment
NMA of 140 trials (23,383 participants) reported IGA as a binary outcome and ranked ruxolitinib 1.5% (OR 9.34, 95% CI 4.8, 18.18), delgocitinib 0.5% (OR 10.08, 95% CI 2.65, 38.37), delgocitinib 0.25% (OR 6.87, 95% CI 1.79, 26.33), very potent TCS (OR 8.34, 95% CI 4.73, 14.67), potent TCS (OR 5.00, 95% CI 3.80, 6.58), and tacrolimus 0.1% (OR 5.06, 95% CI 3.59, 7.13) as most effective, all with moderate confidence. Mild TCS, crisaborole 2%, pimecrolimus 1%, roflumilast 0.15%, difamilast 0.3% and 1%, and tacrolimus 0.03% were the least effective. In a sensitivity analysis of low risk of bias information (12 trials, 1639 participants), potent TCS, delgocitinib 0.5% and delgocitinib 0.25% were most effective, and pimecrolimus 1%, roflumilast 0.15%, difamilast 1% and difamilast 0.3% least effective. Class‐level sensitivity analysis found potent/very potent TCS had similar effectiveness to potent TCI and JAK inhibitors and were more effective than PDE‐4 inhibitors; mild/moderate TCS were less effective than potent TCI and had similar effectiveness to mild TCI.
Longer‐term outcomes over 6 to 12 months showed a possible increase in effectiveness for pimecrolimus 1% versus vehicle (4 trials, 2218 participants) in a pairwise meta‐analysis, and greater treatment success with mild/moderate TCS than pimecrolimus 1% (based on 1 trial of 2045 participants).
Local adverse effects
NMA of 83 trials (18,992 participants, 2424 events) reporting application‐site reactions ranked tacrolimus 0.1% (OR 2.2, 95% CI 1.53, 3.17; moderate confidence), crisaborole 2% (OR 2.12, 95% CI 1.18, 3.81; high confidence), tacrolimus 0.03% (OR 1.51, 95%CI 1.10, 2.09; low confidence), and pimecrolimus 1% (OR 1.44, 95% CI 1.01, 2.04; low confidence) as most likely to cause site reactions. Very potent, potent, moderate, and mild TCS were least likely to cause site reactions.
NMA of eight trials (1786 participants, 3 events) reporting pigmentation changes found no evidence for increased pigmentation changes with TCS and crisaborole 2%, with low confidence for mild, moderate or potent TCS and moderate confidence for crisaborole 2%.
NMA of 25 trials (3691 participants, 36 events) reporting skin thinning found no evidence for increased skin thinning with short‐term (median 3 weeks, range 1‐16 weeks) use of mild TCS (OR 0.72, 95% CI 0.12, 4.31), moderate TCS (OR 0.91, 95% CI 0.16, 5.33), potent TCS (OR 0.96, 95% CI 0.21, 4.43) or very potent TCS (OR 0.88, 95% CI 0.31, 2.49), all with low confidence. Longer‐term outcomes over 6 to 60 months showed increased skin thinning with mild to potent TCS versus TCI (3 trials, 4069 participants, 6 events with TCS).
Authors' conclusions
Potent TCS, JAK inhibitors and tacrolimus 0.1% were consistently ranked as amongst the most effective topical anti‐inflammatory treatments for eczema and PDE‐4 inhibitors as amongst the least effective. Mild TCS and tapinarof 1% were ranked amongst the least effective treatments in three of five efficacy networks. TCI and crisaborole 2% were ranked most likely to cause local application‐site reactions and TCS least likely. We found no evidence for increased skin thinning with short‐term TCS but an increase with longer‐term TCS.
Keywords: Adult; Child; Female; Humans; Administration, Topical; Adrenal Cortex Hormones; Adrenal Cortex Hormones/administration & dosage; Adrenal Cortex Hormones/therapeutic use; Anti-Inflammatory Agents; Anti-Inflammatory Agents/administration & dosage; Anti-Inflammatory Agents/therapeutic use; Bias; Eczema; Eczema/drug therapy; Emollients; Emollients/therapeutic use; Quality of Life; Randomized Controlled Trials as Topic
Plain language summary
Comparing skin treatments for eczema
Key messages:
° Strong corticosteroids, JAK inhibitors and tacrolimus 0.1% (all drugs that suppress the immune system) are consistently effective at reducing signs and symptoms of eczema.
° Unwanted effects, such as burning and stinging, are more likely with tacrolimus, pimecrolimus and crisaborole and less likely with corticosteroids; other unwanted effects, such as skin thinning, are only likely when strong corticosteroids are used long term.
° Given uncertainty about long‐term effectiveness and safety, other factors such as availability, cost and priorities, should be considered.
What did we want to find out?
Eczema (atopic dermatitis) is a common inflammatory skin condition with no cure. We compared various skin‐applied (topical) anti‐inflammatory treatments used to reduce eczema symptoms. We wanted to find the most effective and also the safest topical anti‐inflammatory treatments for people with eczema.
What did we do?
We included randomised trials for people with eczema of all ages and severities. Topical treatments had to be used for at least a week and were compared with other anti‐inflammatory treatments or none. We excluded trials of contact dermatitis or hand eczema, and excluded trials of antibiotics, complementary therapies, moisturisers alone, phototherapy, wet wraps, or treatments which are taken as a tablet, syrup or injection.
We looked at different types of evidence of effectiveness: patient‐reported symptoms of eczema, clinician‐reported signs of eczema, and side effects. Our analysis followed standard Cochrane procedures, and we used a tool called CiNEMA to rate our confidence in the evidence.
What did we find?
We analysed 291 studies involving 45,846 people with eczema of different severities. Most of the studies took place in wealthier countries and focused on adults. Only 31 studies focused on children under 12 years. They assessed treatments like corticosteroid creams and calcineurin inhibitors. The studies lasted between seven days and five years and most studies were funded by companies who make anti‐inflammatory treatments for eczema.
Potent corticosteroids, Janus kinase inhibitors such as ruxolitinib 1.5%, and tacrolimus 0.1% were consistently effective at reducing signs and symptoms of eczema. Phosphodiesterase‐4 inhibitors such as crisaborole 2% were amongst the least effective. Side effects such as burning and stinging were more likely with tacrolimus, pimecrolimus and crisaborole and less likely with corticosteroids. There was no evidence of increased skin thinning with short‐term (16 weeks or fewer) use of any treatment, including once or twice daily potent corticosteroids. We found that longer‐term (over 16 weeks) use of corticosteroids may increase skin thinning. Skin thinning occurred in about 1 in 300 people who used mild, moderate or potent corticosteroids for six months to five years.
Our confidence in the evidence varied across the different types of measurements and different treatments. That depended on things like the size and number of trials of the different treatments, how different the results were between the trials, and whether we judged the results had been reported fairly.
What are the limitations of the evidence?
There was not enough information on the long‐term effectiveness and safety of topical anti‐inflammatory treatments. This review should be seen as a guide to the range of treatments that work best and are safest for short‐term control for most people with eczema. Product availability, cost and individual priorities vary, so there is no single treatment that is best for everyone.
How up to date is this evidence?
The evidence is up to date to June 2023.
Summary of findings
Summary of findings 1. Summary of findings: Patient‐reported symptoms of eczema (binary).
|
Patient or population: participants with a clinical diagnosis of eczema Interventions: topical anti‐inflammatory treatments Comparator (reference): vehicle Outcome: patient‐reported symptoms of eczema (binary), short‐term (1 to 16 weeks after initiation of treatment) Setting(s): secondary care | |||||||
| Treatment | Direct evidence (from 40 RCTs, 6482 participants) | Relative effect OR (95% CI) | Anticipated response rate with control | Anticipated response rate with treatment | Difference (95% CI) | SUCRA | CINeMA |
| TCS potent | 6 RCTs, 699 participants | 5.99 (2.83, 12.69) | 309 per 1000 | 729 per 1000 | 420 per 1000 (250 to 541) | 0.75 | Low ["Within‐study bias","Heterogeneity"] |
| Tacrolimus 0.1% | Indirect only | 6.27 (1.19, 32.98) | 309 per 1000 | 738 per 1000 | 429 per 1000 (39 to 627) | 0.74 | Low ["Within‐study bias","Heterogeneity"] |
| Ruxolitinib 1.5% | 2 RCTs, 465 participants | 5.64 (1.26, 25.25) | 309 per 1000 | 716 per 1000 | 407 per 1000 (51 to 609) | 0.71 | Low ["Within‐study bias","Heterogeneity"] |
| TCS very potent | 1 RCT, 61 participants | 5.08 (0.51, 50.76) | 309 per 1000 | 695 per 1000 | 386 per 1000 (‐124 to 648) | 0.65 | Low ["Within‐study bias","Imprecision"] |
| Tacrolimus 0.03% | Indirect only | 4.56 (0.49, 42.48) | 309 per 1000 | 671 per 1000 | 362 per 1000 (‐130 to 641) | 0.63 | Low ["Within‐study bias","Imprecision"] |
| Pimecrolimus 1% | 10 RCTs, 1712 participants | 3.59 (1.84, 7.01) | 309 per 1000 | 617 per 1000 | 308 per 1000 (142 to 449) | 0.58 | Low ["Within‐study bias","Heterogeneity"] |
| Tapinarof 1% | 1 RCT, 77 participants | 3.13 (0.33, 29.34) | 309 per 1000 | 583 per 1000 | 274 per 1000 (‐180 to 620) | 0.52 | Low ["Within‐study bias","Imprecision"] |
| TCS moderate | 5 RCTs, 1426 participants | 2.62 (1.18, 5.81) | 309 per 1000 | 540 per 1000 | 231 per 1000 (37 to 413) | 0.47 | Low ["Within‐study bias","Heterogeneity"] |
| Crisaborole 2% | 1 RCT, 63 participants | 1.15 (0.17, 7.71) | 309 per 1000 | 341 per 1000 | 32 per 1000 (‐238 to 466) | 0.27 | Low ["Within‐study bias","Imprecision"] |
| Roflumilast 0.15% | 1 RCT, 81 participants | 1.03 (0.12, 9.23) | 309 per 1000 | 316 per 1000 | 7 per 1000 (‐260 to 496) | 0.26 | Moderate ["Within‐study bias","Imprecision"] |
| TCS mild | 2 RCTs, 80 participants | 1.35 (0.51, 3.53) | 309 per 1000 | 376 per 1000 | 67 per 1000 (‐122 to 303) | 0.25 | Low ["Within‐study bias","Indirectness","Imprecision"] |
Abbreviations: CI: Confidence Interval CINEMA: Confidence in Network Meta‐analysis OR: Odds ratio RCT: Randomised controlled trial SUCRA: Surface area under the cumulative ranking curve TCS: Topical corticosteroids
Summary of findings 2. Summary of findings: Patient‐reported symptoms of eczema (continuous).
|
Patient or population: participants with a clinical diagnosis of eczema Interventions: topical anti‐inflammatory treatments Comparator (reference): vehicle Outcome: Patient‐reported symptoms of eczema (continuous), short‐term (1 to 16 weeks after initiation of treatment) using Patient Oriented Eczema Measure (POEM), a 28‐point symptom score with better eczema indicated as a lower score and a minimally important change of 3. Setting(s): secondary care | |||||||
| Treatment | Direct evidence (from 29 RCTs, 3839 participants) | Relative effect SMD (95% CI) | Anticipated POEM with control | Anticipated POEM with treatment | Difference (95% CI) | SUCRA | CINeMA |
| TCS very potent | indirect only | ‐1.99 (‐3.25, ‐0.3) | 11.88 | 1.09 | ‐10.8 (‐17.62 to ‐3.97) | 0.94 | Low ["Within‐study bias","Incoherence"] |
| Tacrolimus 0.03% | 1 RCT, 60 participants | ‐1.57 (‐2.42, ‐0.72) | 11.88 | 3.39 | ‐8.5 (‐13.08 to ‐3.91) | 0.88 | Moderate ["Within‐study bias"] |
| TCS potent | 2 RCTs, 102 participants | ‐1.37 (‐2.04, ‐0.69) | 11.88 | 4.49 | ‐7.4 (‐11.07 to ‐3.72) | 0.83 | Very low ["Within‐study bias","Heterogeneity", "Incoherence"] |
| Ruxolitinib 1.5% | 3 RCTs, 662 participants | ‐1.12 (‐1.71, ‐0.53) | 11.88 | 5.81 | ‐6.08 (‐9.26 to ‐2.89) | 0.72 | Moderate ["Within‐study bias"] |
| Tacrolimus 0.03% or 0.1% | 1 RCT, 42 participants | ‐1.09 (‐2.28, 0.1) | 11.88 | 6 | ‐5.88 (‐12.32 to 0.56) | 0.66 | Very low ["Within‐study bias","Heterogeneity", "Incoherence"] |
| TCS mild | 2 RCTs, 86 participants | ‐.93 (‐1.56, ‐0.3) | 11.88 | 6.87 | ‐5.02 (‐8.43 to ‐1.6) | 0.63 | Moderate ["Within‐study bias", "Heterogeneity"] |
| TCS moderate | indirect only | ‐0.94 (‐1.85, ‐0.02) | 11.88 | 6.8 | ‐5.08 (‐10.03 to ‐0.13) | 0.62 | Moderate ["Within‐study bias"] |
| Crisaborole 2% | 1 RCT, 78 participants | ‐0.69 (‐1.79, 0.4) | 11.88 | 8.12 | ‐3.76 (‐9.69 to 2.17) | 0.50 | Low ["Within‐study bias", "Indirectness"," Imprecision", "Heterogeneity", "Incoherence"] |
| Difamilast 1% | 4 RCTs, 577 participants | ‐0.65 (‐1.19, ‐0.11) | 11.88 | 8.34 | ‐3.54 (‐6.47 to ‐.62) | 0.49 | Low ["Within‐study bias", "Heterogeneity"] |
| Tacrolimus 0.1% | indirect only | ‐0.65 (‐2.03, 0.74) | 11.88 | 8.38 | ‐3.51 (‐11.01 to 4) | 0.48 | Very low ["Within‐study bias", "Imprecision", "Incoherence"] |
| Pimecrolimus 1% | 2 RCTs, 146 participants | ‐0.53 (‐1.31, 0.25) | 11.88 | 9 | ‐2.89 (‐7.12 to 1.34) | 0.43 | Very low ["Within‐study bias", "Imprecision", "Heterogeneity", "Incoherence"] |
| Difamilast 0.3% | 3 RCTs, 270 participants | ‐0.43 (‐1.04, 0.18) | 11.88 | 9.56 | ‐2.33 (‐5.65 to 0.99) | 0.35 | Low ["Within‐study bias","Imprecision", "Heterogeneity"] |
| Delgocitinib 0.5% | 2 RCTs, 227 participants | ‐0.41 (‐1.14, 0.33) | 11.88 | 9.69 | ‐2.19 (‐6.17 to 1.79) | 0.34 | Low ["Within‐study bias","Imprecision", "Heterogeneity"] |
| Tapinarof 1% | 1 RCT, 163 participants | ‐0.36 (‐1.4, 0.68) | 11.88 | 9.94 | ‐1.95 (‐7.59 to 3.69) | 0.33 | Very low ["Within‐study bias","Imprecision", "Incoherence"] |
| Delgocitinib 0.25% | 2 RCTs, 206 participants | ‐0.21 (‐.94, 0.53) | 11.88 | 10.77 | ‐1.11 (‐5.09 to 2.86) | 0.25 | Low ["Within‐study bias", "Imprecision"] |
| Roflumilast 0.15% | 1 RCT, 88 participants | ‐0.06 (‐1.14, 1.02) | 11.88 | 11.57 | ‐0.32 (‐6.16 to 5.53) | 0.22 | Low ["Within‐study bias","Imprecision", "Incoherence"] |
Abbreviations: CI: Confidence Interval CINEMA: Confidence in Network Meta‐analysis POEM: Patient oriented eczema measure
RCT: Randomised controlled trial SMD: Standardised mean difference
SUCRA: Surface area under the cumulative ranking curve TCS: Topical corticosteroids
Summary of findings 3. Summary of findings: Clinician‐reported signs of eczema (binary).
| Treatment | Direct evidence (from 32 RCTs, 4121 participants) | Relative effect OR (95% CI) | Anticipated response rate with control | Anticipated response rate with treatment | Difference (95% CI) | SUCRA | CINeMA |
|
Patient or population: participants with clinical diagnosis of eczema Interventions: topical anti‐inflammatory treatments Comparator (reference): vehicle Outcome: clinician reported signs (binary); short‐term (1 to 16 weeks after initiation of treatment) Setting(s): secondary care, inpatient and outpatient, private and public practice | |||||||
| TCS potent | 4 RCTs, 341 participants | 8.15 (4.9, 13.57) | 191 per 1000 | 658 per 1000 | 467 per 1000 (345 to 571) | 0.87 | Moderate ["Within‐study bias"] |
| Tacrolimus 0.1% | 1 RCT, 61 participants | 8.06 (3.3, 19.67) | 191 per 1000 | 655 per 1000 | 464 per 1000 (247 to 632) | 0.84 | Moderate ["Within‐study bias"] |
| Ruxolitinib 1.5% | 3 RCTs, 879 participants | 7.72 (4.92, 12.1) | 191 per 1000 | 645 per 1000 | 454 per 1000 (346 to 550) | 0.84 | Moderate ["Within‐study bias"] |
| Delgocitinib 0.5% | 3 RCTs, 323 participants | 7.61 (3.72, 15.58) | 191 per 1000 | 642 per 1000 | 451 per 1000 (276 to 595) | 0.83 | Moderate ["Within‐study bias"] |
| Difamilast 1% | 2 RCTs, 532 participants | 5.42 (3.06, 9.58) | 191 per 1000 | 561 per 1000 | 370 per 1000 (228 to 502) | 0.67 | Moderate ["Within‐study bias"] |
| Delgocitinib 0.25% | 3 RCTs, 306 participants | 5.26 (2.55, 10.87) | 191 per 1000 | 554 per 1000 | 363 per 1000 (185 to 528) | 0.65 | Moderate ["Within‐study bias"] |
| TCS moderate | 1 RCT, 37 participants | 5.22 (2.55, 10.67) | 191 per 1000 | 552 per 1000 | 361 per 1000 (185 to 525) | 0.64 | Moderate ["Within‐study bias"] |
| Pimecrolimus 1% | 5 RCTs, 750 participants | 3.65 (2.4, 5.57) | 191 per 1000 | 463 per 1000 | 272 per 1000 (170 to 377) | 0.48 | Moderate ["Within‐study bias"] |
| Difamilast 0.3% | 1 RCT, 166 participants | 3.22 (1.45, 7.13) | 191 per 1000 | 431 per 1000 | 240 per 1000 (64 to 436) | 0.42 | High ["Within‐study bias"] |
| Crisaborole 2% | 2 RCTs, 169 participants | 2.98 (1.42, 6.26) | 191 per 1000 | 413 per 1000 | 222 per 1000 (60 to 405) | 0.39 | High [No concerns] |
| Roflumilast 0.15% | 1 RCT, 89 participants | 2.43 (0.88, 6.7) | 191 per 1000 | 364 per 1000 | 173 per 1000 (‐19 to 422) | 0.33 | Very Low ["Within‐study bias","Imprecision","Heterogeneity"] |
| Tapinarof 1% | 1 RCT, 126 participants | 2.45 (1, 6.02) | 191 per 1000 | 366 per 1000 | 175 per 1000 (‐1 to 396) | 0.32 | Low ["Within‐study bias","Imprecision","Heterogeneity"] |
| TCS mild | 1 RCT, 44 participants | 2.22 (0.74, 6.64) | 191 per 1000 | 343 per 1000 | 152 per 1000 (‐42 to 419) | 0.28 | Low ["Within‐study bias","Imprecision"] |
Abbreviations: CI: Confidence Interval CINEMA: Confidence in Network Meta‐analysis OR: Odds ratio RCT: Randomised controlled trial SUCRA: Surface area under the cumulative ranking curve TCS: Topical corticosteroids
Summary of findings 4. Summary of findings: Clinician‐reported signs of eczema (continuous).
|
Patients or population: participants with clinical diagnosis of eczema Interventions: topical anti‐inflammatory treatments Comparator (reference): vehicle Outcome: clinician‐reported signs of eczema (continuous), short‐term (1 to 16 weeks after initiation of treatment) Settings: secondary care, inpatient and outpatient | |||||||
| Treatment | Direct evidence (from 49 RCTs, 5261 participants) | Relative effect SMD (95%CI) | Anticipated EASI with control | Anticipated EASI with treatment | Difference (95% CI) | SUCRA | CINeMA |
| Tacrolimus 0.03% | 1 RCT, 60 participants | ‐2.69 (‐3.36, ‐2.02) | 8.66 | 0 | ‐15.8 (‐19.72 to ‐11.87) | 0.997 | Moderate ["Within‐study bias"] |
| TCS very potent | indirect only | ‐1.87 (‐2.69, ‐1.05) | 8.66 | 0 | ‐10.98 (‐15.82 to ‐6.15) | 0.93 | Moderate ["Within‐study bias"] |
| TCS mild | 2 RCTs, 54 participants | ‐1.12 (‐1.58, ‐0.65) | 8.66 | 2.1 | ‐6.56 (‐9.30 to ‐3.81) | 0.75 | Moderate ["Within‐study bias"] |
| Ruxolitinib 1.5% | 3 RCTs, 783 participants | ‐1.08 (‐1.45, ‐0.70) | 8.66 | 2.35 | ‐6.31 (‐8.53 to ‐4.10) | 0.73 | Moderate ["Within‐study bias"] |
| Delgocitinib 0.5% | 3 RCTs, 323 participants | ‐0.99 (‐1.4, ‐0.59) | 8.66 | 2.82 | ‐5.84 (‐8.21 to ‐3.46) | 0.68 | Moderate ["Within‐study bias"] |
| TCS potent | 1 RCT, 84 participants | ‐0.95 (‐1.35, ‐0.55) | 8.66 | 3.07 | ‐5.59 (‐7.92 to ‐3.26) | 0.64 | Moderate ["Within‐study bias"] |
| Tacrolimus 0.1% | 2 RCTs, 86 participants | ‐0.94 (‐1.41, ‐0.47) | 8.66 | 3.17 | ‐5.49 (‐8.25 to ‐2.73) | 0.63 | Moderate ["Within‐study bias","Incoherence"] |
| Pimecrolimus 1% | 4 RCTs, 401 participants | ‐0.83 (‐1.19, ‐0.48) | 8.66 | 3.78 | ‐4.88 (‐6.98 to ‐2.79) | 0.55 | Low ["Within‐study bias","Incoherence"] |
| Crisaborole 2% | 3 RCTs, 311 participants | ‐0.73 (‐1.17, ‐0.3) | 8.66 | 4.35 | ‐4.31 (‐6.84 to ‐1.79) | 0.48 | Moderate ["Within‐study bias","Indirectness","Heterogeneity"] |
| Difamilast 1% | 4 RCTs, 577 participants | ‐0.72 (‐1.08, ‐0.35) | 8.66 | 4.44 | ‐4.22 (‐6.36 to ‐2.08) | 0.47 | Moderate ["Within‐study bias"] |
| Delgocitinib 0.25% | 3 RCTs, 306 participants | ‐0.73 (‐1.13, ‐0.32) | 8.66 | 4.4 | ‐4.26 (‐6.62 to ‐1.9) | 0.47 | Moderate ["Within‐study bias","Heterogeneity"] |
| TCS moderate | 2 RCTs, 168 participants | ‐0.72 (‐1.14, ‐0.3) | 8.66 | 4.42 | ‐4.24 (‐6.70 to ‐1.77) | 0.46 | Moderate ["Within‐study bias","Heterogeneity"] |
| Tapinarof 1% | 1 RCT, 126 participants | ‐0.64 (‐1.35, 0.07) | 8.66 | 4.91 | ‐3.75 (‐7.91 to 0.42) | 0.42 | Low ["Within‐study bias","Imprecision","Heterogeneity"] |
| Roflumilast 0.15% | 1 RCT, 88 participants | ‐0.42 (‐1.16, 0.33) | 8.66 | 6.22 | ‐2.44 (‐6.79 to 1.92) | 0.31 | Moderate ["Within‐study bias","Imprecision"] |
| Difamilast 0.3% | 3 RCTs, 270 participants | ‐0.47 (‐0.89, ‐0.05) | 8.66 | 5.88 | ‐2.78 (‐5.25 to ‐0.32) | 0.31 | Low ["Within‐study bias","Heterogeneity"] |
Abbreviations: CI: Confidence Interval CINEMA: Confidence in Network Meta‐analysis EASI: Eczema area and severity index
RCT: Randomised controlled trial SMD: Standardised mean difference
SUCRA: Surface area under the cumulative ranking curve TCS: Topical corticosteroids
Summary of findings 5. Summary of findings: Investigator Global Assessment (binary).
|
Patient or population: participants with clinical diagnosis of eczema Interventions: topical anti‐inflammatory treatments Comparator (reference): vehicle Outcome: IGA (binary); short‐term (1 to 16 weeks after initiation of treatment) Settings: secondary care, inpatient and outpatient, private and public practice | |||||||
| Treatment |
Direct evidence (from 140 RCTs, 23,383 participants) |
Relative effect (95% CI) | Anticipated response rate in control | Anticipated response rate with treatment | Difference (95% CI) | SUCRA | CINeMA |
| Ruxolitinib 1.5% | 3 RCTs, 879 participants | 9.34 (4.8, 18.18) | 256 per 1000 | 762 per 1000 | 506 per 1000 (367 to 606) | 0.86 | Moderate ["Within‐study bias"] |
| TCS very potent | 2 RCTs, 438 participants | 8.34 (4.73, 14.67) | 256 per 1000 | 741 per 1000 | 485 per 1000 (364 to 579) | 0.84 | Moderate ["Within‐study bias"] |
| Delgocitinib 0.5% | 2 RCTs, 165 participants | 10.08 (2.65, 38.37) | 256 per 1000 | 776 per 1000 | 520 per 1000 (221 to 674) | 0.83 | Moderate ["Within‐study bias"] |
| Delgocitinib 0.25% | 2 RCTs, 169 participants | 6.87 (1.79, 26.33) | 256 per 1000 | 702 per 1000 | 446 per 1000 (126 to 645) | 0.73 | Moderate ["Within‐study bias"] |
| Tacrolimus 0.1% | 10 RCTs, 1718 participants | 5.06 (3.59, 7.13) | 256 per 1000 | 635 per 1000 | 379 per 1000 (297 to 454) | 0.68 | Moderate ["Within‐study bias"] |
| TCS potent | 16 RCTs, 1708 participants | 5 (3.8, 6.58) | 256 per 1000 | 632 per 1000 | 376 per 1000 (310 to 438) | 0.67 | Moderate ["Within‐study bias"] |
| TCS moderate | 8 RCTs, 1335 participants | 4.46 (3.19, 6.24) | 256 per 1000 | 605 per 1000 | 349 per 1000 (267 to 426) | 0.62 | Moderate ["Within‐study bias"] |
| Tapinarof 1% | 3 RCTs, 262 participants | 3.68 (1.73, 7.82) | 256 per 1000 | 558 per 1000 | 302 per 1000 (117 to 473) | 0.53 | Moderate ["Within‐study bias"] |
| Difamilast 1% | 6 RCTs, 927 participants | 3.45 (1.97, 6.02) | 256 per 1000 | 542 per 1000 | 286 per 1000 (148 to 418) | 0.51 | Moderate ["Within‐study bias"] |
| Tacrolimus 0.03% | 10 RCTs, 2576 participants | 3.53 (2.6, 4.8) | 256 per 1000 | 548 per 1000 | 292 per 1000 (216 to 367) | 0.51 | Moderate ["Within‐study bias"] |
| Roflumilast 0.15% | 1 RCT, 89 participants | 2.43 (0.65, 9.01) | 256 per 1000 | 454 per 1000 | 198 per 1000 (‐73 to 500) | 0.39 | Moderate ["Within‐study bias","Imprecision"] |
| Difamilast 0.3% | 5 RCTs, 558 participants | 2.56 (1.37, 4.78) | 256 per 1000 | 468 per 1000 | 212 per 1000 (65 to 366) | 0.38 | Moderate ["Within‐study bias","Heterogeneity"] |
| Pimecrolimus 1% | 17 RCTs, 4064 participants | 2.39 (1.78, 3.21) | 256 per 1000 | 451 per 1000 | 195 per 1000 (123 to 269) | 0.35 | Moderate ["Within‐study bias","Heterogeneity"] |
| Crisaborole 2% | 5 RCTs, 1725 participants | 2.14 (1.22, 3.76) | 256 per 1000 | 424 per 1000 | 168 per 1000 (40 to 308) | 0.32 | Low ["Within‐study bias","Heterogeneity"] |
| TCS mild | 1 RCT, 46 participants | 1.38 (0.94, 2.02) | 256 per 1000 | 321 per 1000 | 65 per 1000 (‐12 to 154) | 0.17 | Low ["Within‐study bias","Imprecision","Heterogeneity","Incoherence"] |
Abbreviations: CI: Confidence Interval CINEMA: Confidence in Network Meta‐analysis RCT: Randomised controlled trial SUCRA: Surface area under the cumulative ranking curve TCS: Topical corticosteroids
Summary of findings 6. Summary of findings: Local adverse events (application‐site reactions).
|
Patient or population: participants with a clinical diagnosis of eczema Interventions: topical anti‐inflammatory treatments Comparator (reference): vehicle Outcome: application‐site reactions, tolerability events, burning, stinging, irritation (binary); short‐term (within 16 weeks of treatment initiation) Setting(s): secondary care | |||||||
| Treatment | Direct evidence (from 83 RCTs, 18,992 participants) | Relative effect (95% CI) | Anticipated event rate in control | Anticipated event rate with treatment | Difference (95% CI) | SUCRA | CINeMA rating |
| Tacrolimus 0.1% | 5 RCTs, 2364 participants | 2.09 (1.46, 3) | 80 per 1000 | 154 per 1000 | 74 per 1000 (33 to 126) | 0.83 | Moderate ["Within‐study bias"] |
| Crisaborole 2% | 4 RCTs, 1247 participants | 2.11 (1.19, 3.76) | 80 per 1000 | 155 per 1000 | 75 per 1000 (14 to 166) | 0.82 | High ["Heterogeneity"] |
| Pimecrolimus 1% | 15 RCTs, 2482 participants | 1.49 (1.05, 2.12) | 80 per 1000 | 114 per 1000 | 35 per 1000 (4 to 75) | 0.71 | Low ["Within‐study bias","Heterogeneity"] |
| Tacrolimus 0.03% | 8 RCTs, 3470 participants | 1.49 (1.08, 2.04) | 80 per 1000 | 114 per 1000 | 34 per 1000 (6 to 70) | 0.71 | Low ["Within‐study bias","Heterogeneity"] |
| Tapinarof 1% | 1 RCT, 163 participants | 1.01 (0.06, 17.91) | 80 per 1000 | 81 per 1000 | 1 per 1000 (‐75 to 528) | 0.57 | Low ["Within‐study bias","Imprecision"] |
| TCS mild | 1 RCT, 768 participants | 0.51 (0.3, 0.85) | 80 per 1000 | 42 per 1000 | ‐38 per 1000 (‐54 to ‐11) | 0.38 | Moderate ["Within‐study bias","Heterogeneity"] |
| TCS moderate | 3 RCTs, 670 participants | 0.49 (0.25, 0.93) | 80 per 1000 | 40 per 1000 | ‐39 per 1000 (‐58 to ‐5) | 0.37 | Moderate ["Within‐study bias","Heterogeneity"] |
| Roflumilast 0.15% | 1 RCT, 90 participants | 0.33 (0.01, 8.84) | 80 per 1000 | 27 per 1000 | ‐52 per 1000 (‐79 to 354) | 0.32 | Moderate ["Within‐study bias","Imprecision"] |
| TCS potent | 7 RCTs, 1149 participants | 0.35 (0.22, 0.55) | 80 per 1000 | 29 per 1000 | ‐51 per 1000 (‐61 to ‐34) | 0.26 | Moderate ["Within‐study bias"] |
| TCS very potent | 3 RCTs, 492 participants | 0.33 (0.13, 0.81) | 80 per 1000 | 27 per 1000 | ‐52 per 1000 (‐69 to ‐14) | 0.25 | Low ["Within‐study bias","Incoherence"] |
Abbreviations: CI: Confidence Interval CINEMA: Confidence in Network Meta‐analysis RCT: Randomised controlled trial SUCRA: Surface area under the cumulative ranking curve TCS: Topical corticosteroids
Summary of findings 7. Summary of findings: Local adverse events (pigmentation).
|
Patient or population: participants with a clinical diagnosis of eczema Interventions: topical anti‐inflammatory treatments Comparator (reference): vehicle Outcome: pigmentation changes, colour change, hypopigmentation or hyperpigmentation; short‐term (within 4 weeks of treatment initiation) Setting(s): secondary care | |||||||
| Treatment | Direct evidence (from 8 RCTs, 1786 participants) | Relative effect OR (95% CI) | Anticipated event rate with control | Anticipated event rate with treatment | Difference (95% CI) | SUCRA | CINeMA |
| Crisaborole 2% | 1 RCT, 754 participants | 1.51 (0.06, 37.21) | 10 per 10,000 | 15 per 10,000 | 5 per 1000 (‐9 to 349) | 0.60 | Moderate ["Imprecision"] |
| TCS moderate | indirect only | 0.75 (0.02, 30.42) | 10 per 10,000 | 7 per 10,000 | ‐3 per 1000 (‐10 to 285) | 0.49 | Low ["Within‐study bias","Indirectness","Imprecision"] |
| TCS potent | 1 RCT, 30 participants | 0.75 (0.03, 19.23) | 10 per 10,000 | 7 per 10,000 | ‐3 per 1000 (‐10 to 179) | 0.49 | Low ["Within‐study bias","Indirectness","Imprecision"] |
| TCS mild | 1 RCT, 768 participants | 0.56 (0.02, 14.02) | 10 per 10,000 | 6 per 10,000 | ‐4 per 1000 (‐10 to 128) | 0.39 | Low ["Within‐study bias","Imprecision"] |
There were no pigmentation events in any placebo/vehicle arms included in our analysis. Therefore, we used the British National Formulary to estimate a control event rate (British National Formulary). The British National Formulary classifies depigmentation as a rare or very rare event, occurring in fewer than 1 in 1000 people treated with topical steroids. We use this hypothetical incidence rate of 0.1% to calculate potential differences in incidence between control and treatment.
Abbreviations: CI: Confidence Interval CINEMA: Confidence in Network Meta‐analysis OR: Odds ratio RCT: Randomised controlled trial SUCRA: Surface area under the cumulative ranking curve TCS: Topical corticosteroids
Summary of findings 8. Summary of findings: Local adverse events (skin thinning and related signs).
|
Patient or population: participants with a clinical diagnosis of eczema Interventions: topical anti‐inflammatory treatments Comparator (reference): vehicle Outcome: skin thinning, atrophy, striae or telangiectasia; short‐term (within 6 weeks of treatment initiation) Setting(s): secondary care | |||||||
| Treatment | Direct evidence (from 25 RCTs, 3691 participants) | Relative effect (95% CI) | Anticipated event rate with control | Anticipated event rate with treatment | Difference (95% CI) | SUCRA | CINeMA |
| TCS potent | 3 RCTs, 578 participants | 0.96 (0.21, 4.43) | 7 per 1000 | 7 per 1000 | 0 per 1000 (‐6 to 24) | 0.57 | Low ["Within‐study bias","Imprecision"] |
| TCS moderate | 1 RCT, 51 participants | 0.91 (0.16, 5.33) | 7 per 1000 | 7 per 1000 | 0 per 1000 (‐6 to 30) | 0.54 | Low ["Within‐study bias","Imprecision"] |
| TCS very potent | 3 RCTs, 919 participants | 0.88 (0.31, 2.49) | 7 per 1000 | 6 per 1000 | ‐1 per 1000 (‐5 to 11) | 0.52 | Low ["Within‐study bias","Imprecision"] |
| Tacrolimus 0.1% | indirect only | 0.88 (0.01, 60.36) | 7 per 1000 | 6 per 1000 | ‐1 per 1000 (‐7 to 299) | 0.51 | Low ["Within‐study bias","Imprecision"] |
| TCS mild | 1 RCT, 768 participants | 0.72 (0.12, 4.31) | 7 per 1000 | 5 per 1000 | ‐2 per 1000 (‐6 to 23) | 0.44 | Low ["Within‐study bias","Imprecision"] |
| Pimecrolimus 1% | 1 RCT, 38 participants | 0.15 (0.01, 1.59) | 7 per 1000 | 1 per 1000 | ‐6 per 1000 (‐7 to 4) | 0.10 | Low ["Within‐study bias","Imprecision"] |
Abbreviations: CI: Confidence Interval CINEMA: Confidence in Network Meta‐analysis RCT: Randomised controlled trial SUCRA: Surface area under the cumulative ranking curve TCS: Topical corticosteroids
Summary of findings 9. Summary of findings: Withdrawals due to adverse events.
|
Patient or population: participants with a clinical diagnosis of eczema Interventions: topical anti‐inflammatory treatments Comparator (reference): vehicle Outcome: withdrawal from treatment or trial, clearly due to an adverse effect of the intervention; short‐term (within 8 weeks of treatment initiation) Setting(s): secondary care | |||||||
| Treatment | Direct evidence (from 11 RCTs, 2404 participants) | Relative effect OR (95% CI) | Anticipated withdrawal rate with control | Anticipated withdrawal rate with treatment | Difference (95% CI) | SUCRA | CINeMA |
| Tacrolimus 0.1% | 1 RCT, 62 participants | 3.31 (0.13, 84.33) | 10 per 1000 | 33 per 1000 | 23 per 1000 (‐9 to 457) | 0.75 | Low ["Within‐study bias","Imprecision"] |
| Tacrolimus 0.03% | indirect only | 1.92 (0.13, 29.09) | 10 per 1000 | 20 per 1000 | 10 per 1000 (‐9 to 222) | 0.65 | Low ["Within‐study bias","Imprecision"] |
| TCS moderate | 2 RCTs, 776 participants | 1.55 (0.24, 10.19) | 10 per 1000 | 16 per 1000 | 6 per 1000 (‐8 to 86) | 0.61 | Low ["Within‐study bias","Imprecision"] |
| Delgocitinib 0.5% | 1 RCT, 97 participants | 1.51 (0.06, 38.15) | 10 per 1000 | 16 per 1000 | 6 per 1000 (‐10 to 274) | 0.56 | Low ["Within‐study bias","Imprecision"] |
| Delgocitinib 0.25% | 1 RCT, 101 participants | 1.42 (0.06, 35.9) | 10 per 1000 | 15 per 1000 | 5 per 1000 (‐10 to 262) | 0.56 | Low ["Within‐study bias","Imprecision"] |
| TCS mild | indirect only | 0.61 (0.05, 7.94) | 10 per 1000 | 6 per 1000 | ‐4 per 1000 (‐10 to 66) | 0.35 | Low ["Within‐study bias","Imprecision"] |
| TCS potent | 1 RCT, 30 participants | 0.39 (0.04, 4.24) | 10 per 1000 | 4 per 1000 | ‐6 per 1000 (‐10 to 32) | 0.23 | Low ["Within‐study bias","Indirectness","Imprecision"] |
Abbreviations: CI: Confidence Interval CINEMA: Confidence in Network Meta‐analysis OR: Odds ratio RCT: Randomised controlled trial SUCRA: Surface area under the cumulative ranking curve TCS: Topical corticosteroids
Background
Description of the condition
Eczema, also called atopic dermatitis or atopic eczema, is a common and burdensome skin disease. Eczema is most common in early life, affecting up to 20% of infants and about 6% of school‐age children and adolescents and 5% of adults worldwide (De Lusignan 2021; Hay 2014; Langan 2023; Williams 2008). It is a chronic, fluctuating condition that varies in severity. In mild eczema, individuals may have only occasional localised patches of inflamed skin that cause minimal symptoms, but severe eczema causes extensive erythema, excoriations and chronic skin changes such as lichenification.
Eczema can have a substantial impact on quality of life and psychosocial domains (social, academic, and occupational) through persistent itch and the stigma associated with having visibly affected skin (Carroll 2005; Chamlin 2004; Drucker 2017; Lewis‐Jones 2006). The impact on quality of life can exceed that reported in other chronic conditions, such as asthma and diabetes, especially when eczema is severe or affects readily visible areas (Beattie 2006; Drucker 2017; Kemp 2003; Vittrup 2023). Eczema also causes an economic burden and, in the USA alone, the direct costs of eczema are estimated as over 1 billion US dollars per year (Drucker 2017). Costs which affect people with eczema and their families include buying moisturisers, washing products or special clothing and taking time off work to care for a child with eczema (Carroll 2005).
Description of the intervention
While eczema often improves during early childhood or puberty, a significant proportion of individuals still require treatment in adult life (Abuabara 2018), and in others, eczema does not start until adulthood. Eczema is managed with long‐term treatments. For the majority of people, treatment is topical. Emollients (bland moisturisers) are a mainstay of eczema treatment, but predominantly have a preventative role through hydrating the skin and improving the skin barrier; they are inadequate to reduce inflammation, except in very mild cases. To treat actively inflamed areas, topical anti‐inflammatory agents are needed (NICE 2007).
Topical corticosteroids (TCS) are the first‐line topical anti‐inflammatory treatment for eczema (NICE 2007; Wollenberg 2020). TCS are typically applied once or twice daily to actively inflamed areas of skin for seven to 14 days, or until resolution. Prolonged, continuous use of TCS is not usually advised. However, 'weekend therapy' (i.e. the proactive regular twice‐weekly application of treatment) is used, and can improve long‐term control in people with frequent flares (Williams 2004). TCS preparations can be classified into different potency groups based on vasoconstriction assays (Eichenfield 2014), and different potencies are used for treatment depending on the individual's age, site of application, and disease severity. Antimicrobials and salicylic acid may also be added to TCS preparations. TCS agents have a very well‐established safety profile as they have been used extensively since the 1950s. Despite this, research suggests that many people with eczema and their carers have a number of concerns about TCS (Teasdale 2021), including skin thinning (Charman 2000). Although such adverse effects are typically associated with inappropriate use of more potent TCS agents (Charman 2000; Hajar 2015), people with eczema and their carers also have concerns when using mildly potent agents (Bos 2019; Charman 2000). These concerns can have important implications for treatment adherence and, consequently, disease control.
Topical calcineurin inhibitors (TCI) have been available since 2000. Current TCI agents in use include tacrolimus 0.03% ointment, tacrolimus 0.1% ointment, and pimecrolimus 1% cream. Tacrolimus 0.03% ointment and pimecrolimus 1% cream are indicated for use in individuals aged two years or older, and tacrolimus 0.1% ointment is approved in those aged 16 years or more (Wollenberg 2020). However, TCI are commonly used in children aged under two years, and this is supported by guideline recommendations (Sidbury 2023; Wollenberg 2020). American Academy of Dermatology guidelines strongly recommend the use of TCI or TCS for managing eczema (Sidbury 2023). The European Task Force on Atopic Dermatitis (ETFAD) recommends first‐line use of TCI at sensitive sites (with a preference for pimecrolimus in mild eczema and tacrolimus in moderate and severe eczema) and for long‐term topical treatment (Wollenberg 2020). The National Institute for Health and Care Excellence (NICE) states that TCI are an option for the second‐line treatment of moderate‐to‐severe eczema in individuals aged two years or over “that has not been controlled by TCS or where there is a serious risk of important adverse effects from further TCS use, particularly irreversible skin atrophy” (NICE 2007).
While TCI are often promoted as being ‘steroid‐sparing’, there is limited evidence about their relative safety compared with TCS. In a 2016 review, more than two‐thirds of all TCI studies had not used any active comparator (Wilkes 2016), and in the only long‐term head‐to‐head trial comparing TCI against mild potency TCS, these agents were similarly safe and effective (Sigurgeirsson 2015). A 2015 Cochrane Review found that serious adverse events were rare for both TCI and TCS (Cury Martins 2015). The most common adverse effects of TCI were local tolerability issues, such as burning or stinging on application (Cury Martins 2015). A black‐box warning for theoretical carcinogenicity also exists for TCI, based upon postmarketing case reports of skin cancer and lymphoma. This risk of carcinogenicity is controversial as observational data have suggested no increased risk of keratinocyte carcinoma (Asgari 2020), lymphoma (in vitiligo patients; Ju 2021), and cancer overall (Paller 2020). Although one meta‐analysis reported a small increased risk of lymphoma Lam 2021, that was not confirmed in a more recent and comprehensive systematic review (Devasenapathy 2023).
TCI agents cost more than TCS per gram, and prescription costs of TCI may be difficult to justify in the absence of comparative data showing improved efficacy or safety compared with TCS. Expenditure on topical treatments for eczema may rise further as new, more costly topical anti‐inflammatory treatments come to market (Table 10). A topical phosphodiesterase‐4 (PDE‐4) inhibitor, crisaborole, was licenced in the USA in 2016 and in the European Union in 2020. More topical agents are likely to be approved soon, including janus kinase (JAK) inhibitors, such as ruxolitinib, tofacitinib and delgocitinib (Bissonnette 2016; Dhillon 2020), and aryl hydrocarbon receptor activators, such as tapinarof (Paller 2021). Reported reasons for developing new topical treatments include existing treatments having local tolerability issues, restrictions for use on sensitive skin areas, or insufficient efficacy. However, trials of new topical therapeutics have generally made few direct comparisons to available treatments to allow efficacy and safety to be compared, so it is difficult to determine whether new topical treatments meet this envisioned treatment need. Establishing whether newly approved agents are more effective or safer than TCS and TCI will be important for clinicians and people with eczema to make informed treatment decisions and to use limited healthcare resources most effectively.
1. Licenced interventions: cost in local currency (per 100 g).
| Licenced drug 1 | United Kingdom2 | United States3 | Japan6 |
| Topical corticosteroids (TCS) | |||
| Mild TCS (e.g. hydrocortisone acetate 1%) |
£5.86 | $17.18 | ‐ |
| Moderate TCS (e.g. clobetasone butyrate 0.05%) |
£5.44 | ‐ | ¥1670.00 |
| Potent TCS (e.g. betamethasone valerate 0.1%) |
£3.54 | $8.89 | ¥2020.00 |
| Very potent TCS (e.g. clobetasol proprionate 0.05%) |
£7.90 | $22.80 | ¥1740.00 |
| Topical calcineurin inhibitors (TCI) | |||
| pimecrolimus 1% | £59.07 | $234.53 | ‐ |
| tacrolimus 0.03% | £70.92 | $226.1 | ¥8380.00 |
| tacrolimus 0.1% | £47.93 | $241.86 | ¥7470.00 |
| Topical janus kinase (JAK) inhbitors | |||
| ruxolitinib 1.5% | ‐ | $1596.174 | ‐ |
| delgocitinib 0.25% | ‐ | ‐ | ¥13,930.00 |
| delgocitinib 0.5% | ‐ | ‐ | ¥14,490.00 |
| Topical phosphodiesterase IV (PDE‐4) inhibitors | |||
| crisaborole 2% | ‐ | $230.00 | ‐ |
| difamilast 1% | ‐ | ‐ | ¥15,210.00 |
| difamilast 0.3% | ‐ | ‐ | ¥14,200.00 |
| Topical aryl hydrocarbon receptor (AHR) activators | |||
| tapinarof 1% | ‐ | $2447.005 | ‐ |
1 Delgocitinib 0.25%/0.5% and difamilast 0.3%/1% are approved in Japan; tapinarof 1% is approved for psoriasis
2 Costs obtained from British National Formulary Drugs A to Z (tariffs)
3 Costs obtained from Pharmacoeconomic Review Report: Crisaborole unless otherwise stated
4 Cost for 60‐100 g obtained from Opzelura®
5 Cost obtained from Drugs.com Vtama prices
6 Costs obtained from KEGG MEDICUS
How the intervention might work
The discussed agents are all topical anti‐inflammatory agents that reduce pro‐inflammatory cytokine production. These cytokines are molecules secreted by cells of the immune system which escalate immune responses, so reducing them leads to improved appearance of the skin and reduced itch. Topical agents also have favourable adverse effect profiles compared to systemic immunosuppressants and do not need regular blood tests for monitoring. Topical agents are thus a cornerstone of eczema treatment.
TCS have a broad anti‐inflammatory action. They bind to glucocorticoid intracellular receptors and reduce levels of cytokines such as tumour necrosis factor (TNF), interleukin (IL) 1, IL‐2 and interferon (IFN)‐γ (Ahluwalia 1998). TCS also cause vasoconstriction (reduced blood flow) in the affected skin (Altura 1966). At sensitive sites such as the face, neck, and groin, TCS are typically limited to mild or moderate potency to reduce the risk of skin thinning.
TCI inhibit the enzyme calcineurin, which has the effect of decreasing T‐cell proliferation and the production of cytokines including IL‐2, IL‐3, IL‐4, IL‐12, TNF, and IFN‐γ (Cury Martins 2015). They have also been demonstrated to affect mast cell activation and decrease the number and co‐stimulatory ability of dendritic cells in the epidermis (Eichenfield 2014).
PDE‐4 inhibitors inhibit the enzyme PDE‐4, which leads to a decrease in cyclic adenosine monophosphate (cAMP) levels (Zebda 2018). PDE‐4 activity is increased in circulating inflammatory cells of people with eczema, and inhibition of PDE‐4 in monocytes in vitro leads to reduced release of pro‐inflammatory cytokines (Paller 2016).
JAK inhibitors inhibit JAK‐STAT signalling. There are 4 JAKs ‐ JAK1, JAK2, JAK3, and tyrosine kinase (TYK) ‐ which are associated with type I and type II cytokine receptors. JAK is activated when these cytokine receptors are bound by their ligands, which comprise more than 60 cytokines and growth factors (Howell 2019). JAK then activates small molecules in the cytoplasm, which move to the nucleus and bind to DNA in order to alter gene transcription. These small molecules in the cytoplasm are called STATs due to their function ('Signal Transducers and Activator of Transcription') and speed of action ('stat' effect) (Stark 2012). Inhibition of the pathway can have anti‐inflammatory effects. For example, at least 90% of IL‐2 receptor signalling is JAK‐dependent (Schwartz 2017).
The aryl hydrocarbon receptor is a cytosolic ligand‐activated transcription factor that can be activated by various different molecules. It is widely expressed in the skin and plays an important role as a critical regulator of innate and adaptive immune responses, impacting the balance of Th17 and regulatory T cells (Smith 2017) and modulating gene expression important for skin barrier proteins, such as filaggrin, hornerin, and involucrin (Smith 2017). Activation of aryl hydrocarbon receptor is thus a target for new drugs, such as tapinarof (Smith 2017), and is also one of the modes of action of coal tar, which can be used in inflammatory skin diseases.
Why it is important to do this review
A Cochrane Skin prioritisation process in 2017, which included the involvement of people with eczema, identified ‘network meta‐analysis of topical anti‐inflammatory treatments for treating eczema’ as a key priority for a Cochrane Skin review (Presley 2019). This research question was prioritised because there is currently inadequate evidence to guide treatment decisions for topical anti‐inflammatory agents in eczema. Most new topical therapies are initially compared against vehicle (a carrier system for an active treatment, which can also be used on its own as an emollient). This makes it difficult for healthcare professionals and people with eczema to make a judgement on how the new treatment compares with existing active treatments in terms of benefits and potential harms and cost (Williams 2021). The relative absence of reliable comparative clinical trial data means that treatment decisions when using topical anti‐inflammatory products for eczema are sometimes not evidence‐based.
Defining the most effective and safest topical treatments would ensure that resources are best allocated in eczema management. Commencing safe, effective topical therapy may also reduce the need for repeat primary care visits and secondary care referrals resulting from uncontrolled disease, an inability to start a certain medication, or a lack of confidence in the safety profile of certain medications.
To our knowledge, there are no large, well‐conducted and transparently reported platform trials with multiple topical anti‐inflammatory treatments to compare efficacy and safety. A well‐conducted network meta‐analysis (NMA) is therefore the best method available to make direct and indirect comparisons between the many treatments available for eczema. Our review is especially timely due to the large number of new therapeutics emerging in this area. Without undertaking this planned work, there will likely be confusion surrounding the best topical treatment for eczema, and potentially an associated increase in prescription costs without a positive impact on patient outcomes.
Objectives
To compare and rank the efficacy and safety of topical anti‐inflammatory treatments for people with eczema using a network meta‐analysis.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), where individuals or groups are randomised, were included. Trials that did not state that they were randomised, along with trials that were explicitly quasi‐randomised or non‐randomised designs, were excluded. The first phase of cross‐over trials (due to the risk of carry‐over effects to subsequent periods), cluster‐randomised trials and within‐participant trials were included. Trials where participants might be equally likely to receive alternative treatments, so that the transitivity assumption is not violated, were also included.
Types of participants
Trials of participants with a clinical diagnosis of eczema (i.e. atopic eczema or atopic dermatitis; Johanssen 2004) established using diagnostic criteria for eczema (or a modified version of such criteria) or diagnosis by a healthcare professional were included. Participants with clinically infected eczema were not included. There were no restrictions on age, sex, ethnicity, or disease severity.
Specific forms of eczema that were not atopic eczema, such as irritant contact eczema, allergic contact eczema, hand eczema, discoid eczema, asteatotic eczema, frictional eczema, stasis eczema, photosensitive eczema, and seborrhoeic eczema were excluded. Where trials included both eligible and non‐eligible participants (e.g. where some participants have eczema, but others have irritant contact eczema), only trials where data on eligible participants with eczema were available separately were included.
Types of interventions
The intervention is topical anti‐inflammatory treatments. The following interventions were included.
Topical corticosteroids (TCS), including combinations with antimicrobials (with the caveat that studies where eligibility is limited to clinically infected eczema will not be included) or salicylic acid. TCS were grouped by potency (i.e. mild, moderate, potent, and very potent);
Topical calcineurin inhibitors (TCI), such as tacrolimus and pimecrolimus;
Topical phosphodiesterase IV (PDE‐4) inhibitors, such as crisaborole;
Topical Janus kinase (JAK) inhibitors, such as ruxolitinib or delgocitinib;
Topical aryl hydrocarbon receptor (AHR) activators, such as tapinarof;
Any other well‐characterised novel anti‐inflammatory treatments.
In general, eligible interventions are listed in the British National Formulary (BNF) as suitable products for treating eczema, but products not included in the BNF were included if they were clinically relevant. To ensure our review was relevant and focused, trials of historical topical therapies that are no longer used worldwide for managing eczema (such as coal tar), were not included, except for trials of discontinued TCS, for which the potency of the TCS preparation can be inferred.
As topical anti‐inflammatories were assessed, trials assessing: emollients alone, topical antibiotics alone, complementary therapies, topical probiotics, bandaging/wet wraps, systemic treatments, lasers, and phototherapy were not included. Trials where systemic anti‐inflammatory treatments such as dupilumab, methotrexate, ciclosporin, corticosteroids, or phototherapy were used in combination with topical anti‐inflammatory agents were not included. These were excluded because the primary mechanism of action is not anti‐inflammatory, or because the treatment is not topically applied. Trials where participants in both intervention and comparator groups were using oral antihistamines were excluded, as these are not thought to be effective treatments for eczema.
In general, standard, licenced treatment regimens, such as once‐ or twice‐daily application of topical anti‐inflammatory cream, gel, lotion, or ointment to affected skin, were considered. Once‐ or twice‐daily frequency of application and variations in duration of treatment were pooled for meta‐analysis. Trials where anti‐inflammatory treatments are used differently to standard licenced recommendations (e.g., initiated at less than once per day or more than twice per day for TCS and TCI) or for durations of less than one week were excluded. Treatment regimens which start with a standard regimen as per licenced recommendations, but then reduce to less frequent application after the initial daily treatment period, were still included in the review, but the variations in treatment regimens used may impact the Confidence In Network Meta‐Analysis (CINeMA) certainty of evidence assessments.
Trials using the following comparators: different topical anti‐inflammatory treatments, placebo/vehicle/emollient, or no treatment were included. Vehicle is a 'carrier system' for an active pharmaceutical substance, which may also be used on its own as an emollient for dry skin. The primary focus of this review was the NMA since Cochrane Reviews and other systematic reviews are already available for direct comparisons of topical anti‐inflammatory treatments versus placebo or no treatment. Trials where the comparator was the same topical treatment but with a different method of application were excluded, as this has been evaluated for TCS in a separate Cochrane Review (Lax 2022). In the NMA, a single control node of 'placebo/vehicle/emollient' was used. 'No treatment' will be included as a separate node, but where trials using 'no treatment' allow emollient in both arms of the trial, the control group was included in the 'placebo/vehicle/emollient' node.
Classifications of potencies of TCS vary between different countries. To standardise potency classifications of TCS in our study, a classification we established as part of a separate Cochrane Review (Lax 2022) was used (mild, moderate, potent, or very potent ‐ see Table 11). In brief, we prioritised the potency classification used in the BNF, followed by the WHO Classification 2018; and, if neither source assigned a potency, relevant guidelines, advice from clinicians in the relevant country, and available trial documents were considered. For TCI, different nodes were assigned for different potencies of tacrolimus and pimecrolimus. For other drug classes, a similar approach was taken, unless the potency classification of different concentrations of the same pharmacological agent was not known to differ. Sensitivity analysis was undertaken where an alternative TCS potency classification was used, and where we used a more pooled approach to potency classification.
2. Classification of topical corticosteroid potency.
| Drug Name | Strength | Preparation | Generation | Potency | Source | Notes |
| alclometasone dipropionate | 0.05% | ointment | no | moderate | British National Formulary | |
| alclometasone dipropionate | 0.05% | cream | no | moderate | British National Formulary | |
| alclometasone dipropionate | ‐ | ‐ | no | moderate | inferred from British National Formulary | 0.05% is moderate ‐ no other strengths in the classification |
| betamethasone 17‐valerate | 0.1% | ointment | no | potent | British National Formulary | |
| betamethasone dipropionate | 0.05% | cream | no | potent | British National Formulary | |
| betamethasone dipropionate | 0.05% | ointment | no | potent | British National Formulary | |
| betamethasone dipropionate | ‐ | cream | no | potent | inferred from British National Formulary | 0.05% is potent. |
| betamethasone valerate | ‐ | ‐ | no | potent | inferred from British National Formulary | Assumed a standard preparation unless specified; therefore potent. |
| betamethasone valerate | 0.1% | cream | no | potent | British National Formulary | |
| betamethasone valerate | 0.12% | ointment | no | potent | British National Formulary | |
| betamethasone valerate | 0.1% | fatty ointment | no | potent | British National Formulary | Other preparations are potent at this strength. |
| betamethasone valerate | ‐ | cream | no | potent | inferred from British National Formulary | Although 0.025% is moderate, assumed no dilution from the standard unless specified; therefore potent. |
| betamethasone valerate | ‐ | ointment | no | potent | inferred from British National Formulary | Although 0.025% is moderate, assumed no dilution from the standard unless specified; therefore potent |
| clobetasol propionate | 0.05% | cream | no | very potent | British National Formulary | |
| clobetasol propionate | 0.05% | ointment | no | very potent | British National Formulary | |
| clobetasone | 0.05% | cream | no | moderate | European Directorate for the Quality of Medicines | Not listed in any other charts without the salt; assumed moderate |
| clobetasone 17‐butyrate | 0.05% | lotion | no | moderate | inferred from British National Formulary | Lotion not listed; therefore assumed moderate as for other preparations |
| clobetasone butyrate | 0.05% | cream | no | moderate | British National Formulary | |
| clobetasone butyrate | 0.05% | ointment | no | moderate | British National Formulary | |
| clobetasone butyrate | ‐ | cream | no | moderate | inferred from British National Formulary | No strength given so assumed moderate unless specified |
| clocortolone pivalate | 0.1% | cream | no | moderate | European Directorate for the Quality of Medicines | In National Psoriasis Foundation (USA) moderate as 0.1% cream. Clocortolone is moderate in European Directorate for the Quality of Medicines without strength or salt. |
| desonide | 0.05% | ‐ | no | mild | WHO Classification 2018 | Assumed cream formulation |
| desonide | 0.05% | cream | no | mild | WHO Classification 2018 | |
| desonide | 0.1% | micronized cream | no | mild | WHO Classification 2018 | Assumed as cream formulation; therefore mild |
| desonide | 0.1% | ointment | no | moderate | inferred from Resource Clinical (USA) | Information for 0.05%, therefore, moderate as it is a higher strength ointment |
| desonide | 0.05% | lotion | no | mild | inferred from WHO Classification 2018 | Assuming mild as for the cream |
| desonide | 0.05% | ointment | no | moderate | Resource Clinical (USA) | Ointment appears in one of the USA charts only as moderate. |
| desonide | 0.1% | cream | no | moderate | inferred from WHO Classification 2018 | Assuming moderate to be consistent with ointment |
| diflorasone diacetate | 0.05% | cream | no | moderate | WHO Classification 2018 | |
| diflorasone diacetate | 0.05% | ointment | no | very potent | WHO Classification 2018 | |
| diflucortolone valerate | 0.1% | ointment | no | potent | British National Formulary | |
| diflucortolone valerate | 0.1% | water/oil emulsion | no | potent | inferred from British National Formulary | Assumed potent as for the ointment |
| difluorocortolone valerianate | 0.1% | cream | no | potent | inferred from British National Formulary | Assumed as for diflucortolone valerate 0.1% cream, which is potent |
| fluclorolone acetonide | ‐ | cream | no | potent | European Directorate for the Quality of Medicines | Fluclorolone unspecified is potent, but no salt, preparation or% given |
| fluclorolone acetonide | ‐ | ointment | no | potent | European Directorate for the Quality of Medicines | Fluclorolone unspecified is potent, but no salt, preparation or% given |
| flumethasone pivalate | 0.02% | cream | no | mild | Kim 2015 | Not listed in any of the other charts |
| flumethasone pivalate | 0.2% | ointment | no | moderate | European Directorate for the Quality of Medicines | 10 times strength; European Directorate for the Quality of Medicines states moderate where no strength is specified. |
| fluocinolone acetonide | 0.025% | cream | no | potent | British National Formulary | |
| fluocinolone acetonide | 0.01% | cream | no | moderate | British Association of Dermatologists 2015 | |
| fluocinolone acetonide | 0.025% | ointment | no | potent | British National Formulary | |
| fluocinonide | 0.1% | cream | no | very potent | National Psoriasis Foundation (USA) | |
| fluocinonide | 0.05% | cream | no | potent | British National Formulary | |
| fluocinonide | ‐ | Non‐aqueous synthetic base | no | potent | inferred from British National Formulary | No strengths given, but 0.05% potent in British National Formulary for cream and ointment preparations |
| fluocortin butylester | 0.75% | cream | no | mild | Kim 2015 | Not listed in any of the other charts |
| fluocortolone | 0.2% | ‐ | no | moderate | inferred from British National Formulary | Assumed moderate, as for 0.25% |
| fluocortolone | 0.5% | ointment | no | moderate | inferred from British National Formulary | Assumed moderate, as for 0.25% |
| fluocortolone/fluocortolone caproate | 0.25% | water/oil emulsion | no | moderate | inferred from British National Formulary | |
| fluocortolone/fluocortolone caproate | 0.25% | ointment | no | moderate | British National Formulary | |
| fluprednidene‐21‐acetate | 0.1% | cream | no | moderate | inferred from European Directorate for the Quality of Medicines | Fluprednidene in European Directorate for the Quality of Medicines as moderate (no% given) |
| flurandrenolone acetonide | 0.05% | ointment | no | moderate | WHO Classification 2018 | Assumed to be the same as flurandrenolide and fludroxycortide |
| fluticasone propionate | 0.005% | ointment | yes | potent | British National Formulary | |
| fluticasone propionate | 0.05% | cream | yes | potent | British National Formulary | |
| GW870086X | 2% | cream | NA | NA | NA | Novel corticosteroid |
| GW870086X | 0.2% | cream | NA | NA | NA | Novel corticosteroid |
| halcinonide | 0.1% | cream | no | potent | WHO Classification 2018 | |
| halobetasol propionate | 0.05% | cream | no | very potent | Resource Clinical (USA) | Not listed in any of the other charts |
| halometasone | 0.05% | cream | no | potent | European Directorate for the Quality of Medicines | In European Directorate for the Quality of Medicines as potent but no% given or preparation and not in any other charts listed |
| hydrocortisone | 1% | cream | no | mild | British National Formulary | |
| hydrocortisone | 1% | fatty cream | no | mild | inferred from British National Formulary | Assumed as for a standard preparation; therefore mild |
| hydrocortisone | 1% | ‐ | no | mild | British National Formulary | |
| hydrocortisone | ‐ | cream | no | mild | inferred from British National Formulary | Assumed a standard strength; therefore mild |
| hydrocortisone | 1% | ointment | no | mild | British National Formulary | |
| hydrocortisone | 2.5% | ointment | no | mild | British National Formulary | |
| hydrocortisone | 0.5% | cream | no | mild | British National Formulary | |
| hydrocortisone | 0.5% | hydrophilic ointment | no | mild | inferred from British National Formulary | Assumed as for a standard 0.5% ointment; therefore mild |
| hydrocortisone 17‐butyrate | 0.1% | cream | no | potent | British National Formulary | |
| hydrocortisone 17‐butyrate | 0.1% | fatty cream | no | potent | inferred from British National Formulary | Assumed potent as for ointment and cream |
| hydrocortisone 17‐butyrate | 0.1% | lotion | no | potent | inferred from British National Formulary | Assumed potent as for ointment and cream |
| hydrocortisone 17‐butyrate | 0.1% | ointment | no | potent | British National Formulary | |
| hydrocortisone acetate | 1% | ointment | no | mild | WHO Classification 2018 | Assumed mild in WHO Classification 2018 as for the cream |
| hydrocortisone buteprate | 0.1% | fatty cream | no | moderate | European Directorate for the Quality of Medicines | Hydrocortisone 17‐butyrate, 21‐propionate |
| hydrocortisone butyrate | ‐ | ‐ | no | potent | inferred from British National Formulary | No strength or preparation given, so assuming potent as for standard 0.1% cream or ointment |
| hydrocortisone valerate | 0.2% | cream | no | moderate | WHO Classification 2018 | |
| hydrocortisone valerate | 0.2% | ointment | no | moderate | WHO Classification 2018 | |
| methylprednisolone aceponate | 0.1% | cream | no | potent | European Directorate for the Quality of Medicines | |
| methylprednisolone aceponate | 0.1% | ointment | no | potent | European Directorate for the Quality of Medicines | |
| methylprednisolone aceponate | 0.1% | fatty ointment | no | potent | Australian Pharmaceutical Benefits Scheme 2022 | |
| methylprednisolone aceponate | 0.1% | lotion | no | potent | Australian Pharmaceutical Benefits Scheme 2022 | Only chart, where it is listed as a lotion. |
| methylprednisolone aceponate | ‐ | cream | no | potent | European Directorate for the Quality of Medicines | Assumed 0.1% and therefore potent |
| mometasone furoate | 0.1% | ointment | yes | potent | British National Formulary | |
| mometasone furoate | 0.1% | cream | yes | potent | British National Formulary | |
| mometasone furoate | 0.1% | fatty cream | yes | potent | British National Formulary | |
| mometasone furoate | ‐ | cream | yes | potent | inferred from British National Formulary | Assumed 0.1%; therefore potent |
| prednicarbate | 0.25% | cream | no | moderate | Kim 2015 | |
| prednicarbate | 0.25% | ointment | no | moderate | Kim 2015 | |
| prednicarbate | ‐ | ointment | no | moderate | National Psoriasis Foundation (USA) | No% given ‐ in National Psoriasis Foundation (USA) chart as prednicarbate 0.1% cream (Dermatop) as moderate potency |
| prednisolone 17‐valerate 21‐acetate | 0.30% | ‐ | no | moderate | Kim 2015 | |
| tralonide | 0.025% | ointment | no | potent | Scherrer 1974 | Discussed with other old potent preparation, and it is fluorinated; therefore, we assumed potent. |
| triamcinolone | 0.1% | cream | no | potent | inferred from British National Formulary | Assumed triamcinolone acetonide and, as for the ointment |
| triamcinolone | 0.1% | ointment | no | potent | inferred from British National Formulary | Assumed this is triamcinolone acetonide; therefore, the ointment is potent. |
| triamcinolone acetonide | 0.1% | ointment | no | potent | British National Formulary |
NA: not applicable
Types of outcome measures
Outcomes were identified by the Harmonizing Outcome Measures for Eczema (HOME) initiative as the core outcome domains for eczema treatment trials (Williams 2022).
Outcomes were divided into short‐term (1 to 16 weeks after initiation of treatment) and longer‐term (> 16 weeks after initiation of treatment). For outcomes measured at multiple time points, the time point closest to 12 weeks for short‐term and closest to one year for longer‐term outcomes were evaluated. If there were equidistant assessments of the same outcome in the same trial, for example, at eight weeks and 16 weeks, the time point with the lowest risk of bias information was used; if this was equal, the time point with the most complete data was used; and if the time points were equal, the longer outcome was used, since patient and public involvement has indicated an interest in longer‐term outcomes where they are available.
Primary outcomes
There are two co‐primary outcomes:
-
Patient‐reported symptoms of eczema. Data were extracted based on the following instruments, in decreasing priority order:
Patient‐Oriented Eczema Measure (POEM) ‐ based on consensus in the HOME initiative (Spuls 2017);
Itch measured using an instrument recommended by HOME – currently the Peak Pruritus‐Numerical Rating Scales (PP‐NRS; Yosipovitch 2019);
Patient‐Oriented SCORAD (PO‐SCORAD; Stalder 2011);
Itch measured using other instruments such as the Leuven Itch Scale (LIS; Haest 2011), Itch Severity Scale (ISS; Majeski 2007), or Visual Analogous Scales (VAS);
Self Administered Eczema Area and Severity Index (SA‐EASI; Housman 2002);
Patients’ Global Assessment (PGA; Farina 2011);
Atopic Dermatitis Assessment Measure (ADAM; Charman 1999);
Any other instruments.
-
Clinician‐reported signs of eczema. Data were extracted based on the following instruments, in decreasing priority order:
Eczema Area and Severity Index (EASI; Hanifin 2001) ‐ based on consensus in the HOME initiative (Schmitt 2014);
Scoring Atopic Dermatitis (SCORAD; Kunz 1997);
Six Area, Six Sign Atopic Dermatitis (SASSAD) severity score (Charman 2002);
Body Surface Area (BSA) affected;
Any other instruments.
Investigator Global Assessment (IGA) was considered separately from other clinician‐reported signs of eczema, since the construct of IGA as a single global assessment differs from other measures of eczema signs. We are also conscious of the emphasis that some regulatory authorities place on IGA as an outcome separate from other measures of eczema signs. Therefore, IGA data were always extracted, where reported, in addition to other measures of clinician‐reported signs of eczema.
For the NMA, the IGA score was dichotomised (i.e. converted the reported score for each participant into a binary score of clear or almost clear eczema versus moderate or no improvement, measured either alone or as part of a composite measure). That means, for an individual trial, IGA was reported as the number of participants achieving the threshold closest to clear or almost clear eczema. Where there was significant heterogeneity in the NMA of IGA, the impact of these choices was explored, e.g. composite versus non‐composite measures of IGA, as one possible source of heterogeneity in a sensitivity analysis. The characterisation of IGA eczema severity was recorded and the following hierarchy of preferred measures was used:
IGA score indicating clear or almost clear eczema (e.g. a score of 0 or 1) or a comparable construct;
IGA score which uses a composite of clear or almost clear eczema together with a single measure of another aspect of IGA, for example, a specific level of BSA affected or a specific level of improvement/change (such as a 2‐point reduction in IGA score);
IGA severity of eczema measured in another binary way, which is not comparable to ‘clear or almost clear’ versus other;
IGA response of eczema reported as a binary measure;
IGA severity of eczema reported as a continuous measure;
IGA response of eczema reported as a continuous measure.
Secondary outcomes
There are three secondary outcomes.
-
Health‐related quality of life. Data were extracted based on the following instruments, in decreasing priority order:
Dermatology Life Quality Index (DLQI; Finlay 1994), including Children’s DLQI; CDLQI) and Infants' Dermatitis Quality of Life Index (IDQOL);
Quality of Life Index for Atopic Dermatitis (QoLIAD; Whalley 2004);
Skindex (Chren 2012);
Any other disease‐specific health‐related quality of life instrument.
-
Long‐term control of eczema. Data were extracted based on the following instruments, in decreasing priority order:
Recap of Eczema Control (RECAP; Howells 2020);
Atopic Dermatitis Control Tool (ADCT; Pariser 2020);
Any other instruments.
-
Local adverse effects, namely:
Tolerability events (e.g. application‐site reactions/stinging);
Occurrence of cosmetic effects, including pigmentation changes and skin thinning/atrophy (identified to be of particular concern to people with eczema in relation to TCS through patient and public involvement in protocol development);
Withdrawal from treatment or study due to adverse effects of the intervention.
Search methods for identification of studies
All relevant RCTs, regardless of language or publication status (published, unpublished, in press, or in progress) were eligible for inclusion. We did not perform a separate search for adverse effects of the target interventions. We considered data on adverse effects from the included studies only.
Electronic searches
The Cochrane Skin Information Specialist (Liz Doney) searched the following databases, initially up to 8 March 2022, and this was updated by one review author (BC) up to 29 June 2023 for relevant trials with no restriction by date using strategies based on the draft strategy for MEDLINE in our published protocol (Steele 2022):
The Cochrane Skin Specialised Register 2021 via the Cochrane Register of Studies (CRS‐Web) using the search strategy in Appendix 1;
The Cochrane Central Register of Controlled Trials (CENTRAL) 2023, Issue 2 in the Cochrane Library using the strategy in Appendix 2;
MEDLINE via Ovid (from 1946 onwards) using the strategy in Appendix 3; and
Embase via Ovid (from 1974 onwards) using the strategy in Appendix 4.
Trial registers
The Cochrane Skin Information Specialist also searched the trial registers listed below on 8 March 2022 and Bridget Candy updated this on 19 January 2023 and again on 29 June 2023:
ClinicalTrials.gov (www.clinicaltrials.gov), using the search strategy in Appendix 5;
World Health Organization (WHO) International Clinical Trials Registry (ICTRP) search platform (www.who.int/trialsearch), using the search strategy in Appendix 6.
Searching other resources
Searching reference lists
We examined selected bibliographies of included studies and any relevant systematic reviews identified for further references to potentially eligible studies.
Correspondence with trialists
In general, trial authors or sponsors of missing data were not contacted, but this was carried out in cases of suspected fraud and in cases where key data points, such as standard deviations, were missing from the published reports and could not be reliably estimated.
Errata and retractions
The Cochrane Skin Information Specialist ran a specific search to identify errata or retractions related to our included studies, and we examined any relevant retraction statements and errata that were retrieved.
Data collection and analysis
Cochrane’s Screen4Me workflow, available on the Cochrane Information Specialist’s portal, was used to help assess the search results (Marshall 2018; Noel‐Storr 2020; Noel‐Storr 2021; Thomas 2021). Screen4Me comprises three components, of which two were used:
Known assessments – a service that matches records in the search results to records that have already been screened in Cochrane Crowd and have been labelled as 'an RCT' or as 'not an RCT';
The RCT classifier – a machine learning model that distinguishes RCTs from non‐RCTs.
For clarity, the unit of interest for this review is the study. Multiple reports of a single study (full papers, conference abstracts, posters, etc.) were grouped under a single study reference identification.
Selection of studies
Two authors (of SL, BC, CR, LS, or RB) independently screened all titles and abstracts using Covidence to determine whether studies met our inclusion criteria, and all full texts were then examined to confirm suitability for inclusion. Disagreements were resolved by discussion with an experienced systematic review author (RB or AD).
We followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) for Network Meta‐Analyses (PRISMA‐NMA) extension and we summarised in a PRISMA flow diagram the number of eligible studies identified (Hutton 2015). This included the number of studies identified but excluded as ineligible, the total number eligible for inclusion in the qualitative synthesis, and the number included in the analysis. Reasons for exclusion were recorded.
Data extraction and management
A standardised data extraction form was used to extract from published reports the desired outcome data detailed under Types of outcome measures and Characteristics of included studies. Data were extracted from published reports and clinical trials registries. We did not access clinical study reports from regulators such as the US Food and Drug Administration. The data extraction form was piloted prior to initiation of formal data extraction. Data was extracted from the following descriptors, baseline characteristics, and effect modifiers.
Author and year of publication;
Methods: study design, sample size, duration of trial participation;
Participants: geographical location, community or healthcare setting, inclusion criteria and exclusion criteria, number of individuals randomised, number of withdrawals, baseline characteristics (age, gender, ethnicity, duration of eczema, eczema severity);
Interventions: name of interventions and comparators, frequency, duration, concurrent treatment, site treated (face included/excluded);
Outcomes: primary and secondary outcomes specified and collected, time points reported;
Notes: original language of publication, sponsorship/funding for trial, trial registration, dates trial conducted, notable conflicts of interest of trial authors.
One team member (of SJL, BC, CR, RP, EA, MD, or MF) conducted the data extraction and another team member (SJL or RB) checked it. Disagreements about data extraction or study characteristics were resolved by discussion at a weekly team meeting or by reference to another member of the study team as needed (RB or AD). Two authors (EV or SC) conducted data analyses using Stata version 17 network and mvmeta package and all authors contributed to entering findings into the Cochrane RevMan Web computer software (RevMan Web 2022), interpreting data and exploring findings through planned or post hoc sensitivity analyses.
The characteristics of each included study are summarised in Characteristics of included studies, and the approach to managing missing data is detailed in Dealing with missing data.
Assessment of risk of bias in included studies
The RoB 2 tool was used to evaluate the risk of bias in included study outcomes (Sterne 2019). This allows the risk of bias to be evaluated ‘per outcome’ rather than just ‘per study’. Focus was on the outcomes to be included in the Summary of findings tables, and the latest versions of the Microsoft Word templates for parallel‐group trials, the first phase of cross‐over trials, and cluster‐randomised trials, were used, as appropriate (from www.riskofbias.info). Signalling questions and algorithms in the RoB 2 tools and associated guidance were used to make judgements about the risk of bias for each domain. The answers to the full signalling questions are available on an online repository at the time of full review publication. The RoB 2 tool assesses the following domains.
Bias arising from the randomisation process;
Bias due to deviations from intended interventions – here we will be assessing the effect of assignment, and not the effect of adherence;
Bias due to missing outcome data;
Bias in measurement of the outcome;
Bias in the selection of the reported result.
One team member (of SJL, BC, CR, RP, EA, MD, or MF) assessed the risk of bias and another team member (SJL or RB) checked the assessments. Disagreements about risk of bias assessments were resolved by discussion at a weekly team meeting or by reference to another member of the study team as needed (AD). To reach an overall risk of bias judgement for a specific outcome, the following criteria were used.
Low risk of bias ‐ all domains considered low risk for the specific result;
Some concerns ‐ some concerns have been raised in at least one domain for the specific result, but no domains are considered at high risk of bias;
High risk of bias ‐ at least one domain is considered high risk for the specific result or there are some concerns for at least 2 domains.
Measures of treatment effect
Pairwise and network meta‐analyses
Continuous outcomes
For continuous variables, if all studies used the same scale, the mean difference (MD) with an associated 95% confidence interval (CI) was reported using this scale. However, if studies used different scales to measure the continuous outcome (e.g. some studies used EASI and others used SCORAD), which was anticipated, a standardised mean difference (SMD) and the associated 95% CI were used. These were re‐expressed as recommended core outcome measures for eczema trials i.e. POEM and EASI, according to the guidance regarding Cohen’s effect sizes in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2022). The control group scores were mean scores from studies in the same NMA without a high risk of bias. It is not possible to include both change from baseline and follow‐up scores within the same meta‐analysis if the meta‐analysis is reporting SMDs. Therefore, the focus was on follow‐up outcome data rather than change from baseline. Any obvious baseline differences were noted and incorporated into the RoB2 assessment.
Binary outcomes
For binary outcomes, odds ratios (OR) with 95% CI were calculated. If there were outcomes with zero events or 100% of events in all arms, they were shown on the relevant forest plot but excluded from the statistical meta‐analysis, as these outcomes do not provide any information on relative effects. Where studies contributed zero events or 100% of events in at least one but not all arms, a continuity correction of 0.5 was used (Sweeting 2004).
Outcomes with continuous and binary data
It is not possible to include both continuous and binary data within the same meta‐analysis. For the outcome ‘eczema signs’, both continuous and binary data were extracted as many studies reported either or both measures; these were analysed in separate pairwise and network meta‐analyses. As most studies measuring IGA reported binary data representing treatment successes, these were extracted in preference to continuous data. For other outcomes, such as ‘eczema symptoms’ or ‘quality of life’, only binary outcome data were extracted for trials where no continuous outcome data were reported that were suitable for data extraction.
Additional considerations for network meta‐analyses
The NMA enables relative treatment effects and relative treatment rankings to be derived from the extracted data.
Unit of analysis issues
In parallel‐group RCTs, the unit of analysis is the participant. For trials with differences in the level at which randomisation occurred (e.g. cross‐over trials, cluster‐randomised trials), guidance from the CochraneHandbook was used to consider the potential impact of this when conducting our analysis (Higgins 2021a).
Cluster‐randomised trials
We planned to include cluster‐randomised trials, but none were identified.
Cross‐over trials
Cross‐over trials were included, but data were only included from the first phase of the trial due to the risk of carry‐over effects in subsequent periods.
Within‐participant trials
Within‐participant trials were included but, due to concerns about these trials, a sensitivity analysis was performed excluding these trial designs (see Sensitivity analysis). When included, paired data were extracted from within‐participant trials where the unit of analysis was the separate body part. For paired data from studies with no suspicion of contamination across intervention sites, analysis was performed using the generic inverse‐variance method after accounting for within‐participant variability (Higgins 2021b). If paired data were not available, attempts were made to estimate the paired data using methods and guidance from the Cochrane Handbook (Higgins 2021a), and correct the variance using the Becker‐Balagtas method (Elbourne 2002). An intraclass correlation coefficient of 0.5 was assumed in all calculations. Findings from within‐participant trials were presented with variance‐adjusted denominators, and denominators were labelled as 'participants' rather than 'sides'. For outcomes which affected the whole body (e.g. systemic adverse events), data were not extracted from within‐participant trials as it was not possible to determine which treatment caused the event.
Multiple intervention groups
Trials assessing multiple interventions in different arms were included, after judging whether multiple interventions were eligible for inclusion in pairwise meta‐analysis. For pairwise meta‐analysis, we planned to split treatment arms into two or more groups to avoid double‐counting of participants if required (i.e. if more than one treatment comparison was eligible for a trial per analysis). The nature of multi‐arm trials was taken into account by the Stata network and mvmeta commands for network meta‐analyses.
Further details can be found in Appendix 7.
Dealing with missing data
For continuous outcomes, means and standard deviations were required for meta‐analysis. Where the standard deviation was missing, an attempt was made to calculate the value from the standard error of the mean difference as reported from 95% CIs or P values (Higgins 2021b). Where data were reported as interquartile ranges or ranges, the Cochrane Handbook guidance was followed for estimating standard errors where the underlying data appeared likely to be normally distributed. In general, trial authors or sponsors of missing data were not contacted, but contact was made with one author team to confirm trial eligibility, detailed in the acknowledgements section.
Where numerical data were unavailable and data reported in figures or graphs needed to be extracted, the WebPlotDigitizer tool was used to extract the data (Rohatgi 2021).
For both continuous and binary data, in primary analyses and in any sensitivity analyses, the intention‐to‐treat (ITT) principle was followed in the analyses by including data from all participants included in the trials who had outcome data available, irrespective of their compliance with the assigned intervention. Hence, all randomised participants who had data available were included in the analysis, which required measuring all participants’ outcomes. Where outcomes for some participants were missing, we used available case analysis data based on all participants with outcome data available. Where the total number of participants analysed was missing, denominators were imputed in a sensitivity analysis using numbers randomised (see below). The proportion of missing data and reasons for missingness contributed to the RoB 2 evaluation.
Assessment of heterogeneity
Conceptual (clinical and methodological) and statistical heterogeneity was explored between the included studies. Clinical heterogeneity was explored by assessing differences between studies in the characteristics of included participants, interventions, and outcomes. Methodological variability included assessments of differences in study design and risk of bias between trials. Statistical heterogeneity was quantified within pairwise analyses using the I2 statistic, considering the thresholds defined in the CochraneHandbook for interpretation (Deeks 2021):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Where there was substantial or considerable heterogeneity (I2 ≥ 50%), possible sources for heterogeneity were explored including, where appropriate, sensitivity analyses excluding any studies identified as outliers. Heterogeneity was also explored by other sensitivity analyses and subgroup analyses for potential effect modifiers (see Sensitivity analysis and Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
To assess reporting bias, funnel plots were produced. These investigate the relation between study effect size and its precision. The premise is that small studies are more likely to remain unpublished if their results are non‐significant or unfavourable, whereas larger studies get published regardless, leading to funnel‐plot asymmetry (Ioannidis 2007). Where validity conditions were met (i.e. low heterogeneity, 10 or more studies, at least one study with significant results, and a ratio of the maximal to minimal variance across studies greater than four (Ioannidis 2007)), funnel plots were produced and used for visual inspection and Egger's funnel plot asymmetry tests to assess for possible reporting bias at the outcome level. For network meta‐analyses, comparison‐adjusted funnel plots were produced using the netfunnel command in Stata.
Our search strategy also encompassed clinical trial registries, so trials that should have published results but had not done so would be identified, which would aid in our understanding of reporting bias at the whole trial level.
Data synthesis
Network meta‐analysis
For all outcomes, where feasible, a full NMA was used to explore all direct and indirect comparisons. The primary NMA was planned to only include those outcome data rated overall as having low risk of bias or some concerns (i.e. excluding data at high risk of bias). However, NMA for low/some concerns risk of bias data was not possible for most outcomes, due to high risk of bias for most information. We thus made a post hoc decision to analyse all available data, rather than just low/some concerns risk of bias data, for the primary analysis.
Networks were produced for the following outcomes.
Clinician‐assessed signs of eczema (continuous, short‐term);
Clinician‐assessed signs of eczema (binary – using instruments other than IGA, short‐term);
Clinician‐assessed signs of eczema (binary ‐ using IGA categorised as clear/almost clear versus not, short‐term);
Patient‐reported symptoms of eczema (binary, short‐term);
Patient‐reported symptoms of eczema (continuous, short‐term);
Safety ‐ application‐site reactions (binary), pigmentation changes (binary), skin thinning/atrophy (binary), withdrawals due to adverse events (binary), all short‐term.
No networks were possible for the HOME‐recommended core outcome domains, long‐term control or HRQoL. It was not possible to produce networks for long‐term assessment of any outcomes.
Interventions which included a combination of topical anti‐inflammatory and additional product(s) that are unlikely to have a direct anti‐inflammatory effect, such as topical antibiotics, were included in the relevant node with the topical anti‐inflammatory alone.
The NMA was performed using a random‐effects model in Stata with the mvmeta command within the network suite of commands for NMA (White 2015) and the Stata commands for graphing, ranking and reporting network results (Chaimani 2013). A frequentist framework was used. For the whole network, heterogeneity was quantified using the heterogeneity parameter Tau, which is the estimated standard deviation of underlying effects across studies. Contour‐enhanced comparison‐adjusted funnel plots were used to evaluate reporting bias for the NMA (Chaimani 2013). To rank the efficacy for all treatments, Surface Under the Cumulative Ranking (SUCRA) values were used, together with corresponding treatment effect CIs, to indicate the uncertainty of rankings (Salanti 2011). Rank probabilities of all the groups were estimated by estimating the SUCRA values of each group, ranging from zero to one, where larger SUCRA values indicate a better outcome.
Assessment of transitivity and consistency in the network meta‐analysis
The treatment network was described and presented graphically. This determined whether NMA was feasible, as severe imbalance in the amount of evidence for each intervention may affect the power and reliability of the overall analysis. The geometry of the network described the number of included interventions by node size and the extent to which there were trials comparing different pairs of interventions by edge width (Ioannidis 2009; Mills 2013). Considerations of heterogeneity and coherence were also important in determining whether synthesising the results across trials in an NMA was justifiable.
The transitivity assumption is that there are no important differences between trials making different comparisons other than the treatments being compared. As such, estimates of an intervention's effect from indirect comparisons should be similar to those generated from direct comparisons. To assess similarities between trials, heterogeneity was assessed as well as the distribution of effect modifiers (baseline eczema severity, treatment duration). Consistency/coherence was assessed between evidence sources (i.e. that the treatment effects from direct and indirect evidence are equivalent); both global and local approaches were used.
To evaluate local consistency, the standard node‐splitting approach was used (also known as SIDE: Separating Indirect from Direct Evidence) and implemented via Stata’s sidesplit command, which is part of the network command suite. This allows the estimates given for each pairwise comparison to be split into direct and indirect components, and the difference between these components can be assessed for incoherence (White 2015).
To evaluate global design inconsistency within the whole network, the 'design by treatment interaction' model (Veroniki 2013) was used, which estimates a global Chi2 statistic for network coherence. Statistical significance was set at 5%. When interpreting incoherence tests, the inconsistency factors were considered as well as their uncertainty and the impact on clinical implications, based on visual inspection of the 95% CIs of direct and indirect odds ratios and the range of equivalence. Inconsistency factors were eczema severity, age, risk of bias, method of outcome assessment (HOME‐approved versus not HOME‐approved), treatment duration, and trial design (within‐ versus between‐participant comparison).
Pairwise meta‐analysis
The primary focus of this systematic review was NMA, so the extracted data were only used to create pairwise comparison meta‐analyses where NMA was not possible, for example, for long‐term outcomes. We also described narratively any extracted data which were unsuitable for meta‐analysis. We did not make adjustments for multiple testing, and results were interpreted in totality with the emphasis on estimated intervention effects rather than significance tests.
Pairwise meta‐analysis was performed for each comparison with a random‐effects model using the DerSimonian and Laird method (DerSimonian 1986). This adjusts for heterogeneity between studies, which is likely. For dichotomous data, where results are estimated for individual studies with low numbers of events (< 10 in total) or where the total sample size is < 30 participants and a risk ratio is used, the proportion of events in each group together with a P value from a Fisher’s Exact test was reported.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses for each pairwise and network meta‐analysis (all outcomes) were undertaken according to the following characteristics, where reported in sufficient trials:
Site of treatment: studies that included facial application versus studies that did not;
Disease severity: moderate/severe versus mild/moderate disease;
Age: < 12 years versus ≥ 12 years.
How best to treat sensitive sites such as facial skin is of interest to people with eczema and healthcare professionals because of concerns over adverse effects of topical treatments. Milder or more severe eczema, or eczema in different age groups, may also respond differently to different treatments, and the trade‐off of treatment benefits versus harms may be different for these groups.
A statistical test of interaction was used to evaluate the possibility of subgroup effects, and we considered a P value of < 0.05 to indicate a significant interaction. All treatment estimates were accompanied by two‐sided 95% CIs.
Sensitivity analysis
The following sensitivity analyses were undertaken for outcomes included in the 'Summary of findings' tables (see Summary of findings and assessment of the certainty of the evidence).
Information rated as low risk of bias or 'some concerns', by excluding high risk of bias information. We had originally intended for this to be the primary analysis, with a sensitivity analysis that included high risk of bias data. However, due to a paucity of low or some concerns risk of bias information, we reversed this so that the primary analysis was all information regardless of the risk of bias rating; and the risk of bias sensitivity analysis excluded data with high risk of bias.
Using an alternative TCS potency classification system, the United States 7‐point Classification system (1 = super potent, 2 = potent, 3 = upper mid‐strength, 4 = mid‐strength, 5 = lower mid‐strength, 6 = mild, 7 = least potent), expressed as a consolidated 4‐category scale (Bowie 2022).
-
Outcomes reported using the HOME core outcome measurement instruments only. These are currently:
EASI for clinical signs;
POEM or (for individuals old enough to self‐report symptoms) PP‐NRS for symptoms;
DLQI, CDLQI, or IDQoL for quality of life;
RECAP or ADCT for long‐term control.
These additional sensitivity analyses were performed for NMA.
Analysis excluding within‐participant data for the eczema signs and symptoms networks only, due to concerns about the validity of these study designs;
-
Class‐level sensitivity analysis, by condensing treatment nodes:
TCS combined into mild/moderate and potent/very potent (i.e. 2 nodes);
TCI combined into mild (pimecrolimus 1%/tacrolimus 0.03%) and potent (tacrolimus 1%/3%) (i.e. 2 nodes);
Other classes of topical anti‐inflammatories, included as a single node for each class of medication, i.e. one node for PDE‐4 inhibitors, one node for JAK inhibitors, one node for AHR activators.
Post hoc sensitivity analyses included:
Analysis imputing the number randomised, where no denominator was reported;
Analysis of continuous outcome data using alternative correlation coefficients of 0.25 and 0.75 for within‐participant data or where change from baseline was converted to an end of treatment score.
Summary of findings and assessment of the certainty of the evidence
As mentioned in Types of interventions, the primary focus of this Cochrane review was the NMA. To assess confidence in the evidence of the network of interventions, the Confidence in NMA (CINeMA) approach was used (Nikolakopoulou 2020). Full details of this process are listed in Appendix 7. This was undertaken by two review authors (of EV, SC, or RB), with team meetings to resolve any discrepancies, where necessary. CINeMA software was used for CINeMA assessments of the six domains detailed below.
Within‐study bias (majority rule);
Reporting bias;
Indirectness;
Imprecision;
Heterogeneity;
Incoherence.
We based our overall assessments of confidence on the grading in all the six domains of CINeMA. We started at high confidence and dropped the level of confidence by one step for each domain with major concerns, or for more than one domain with some concerns. However, the possibility that single issues might impact multiple domains was taken into account, to avoid inappropriate multiple downgrades for single NMA issues. Therefore, for major concerns with both within‐study bias and reporting bias, we only downgraded once; and for major concerns with both heterogeneity and incoherence, we also only downgraded once.
'Summary of findings' tables for NMA were performed using the approach recommended by Cochrane and GRADE (Yepes‐Nunez 2019), with a cautious approach to the interpretation of rankings due to concerns about the reliability of ranking information in summaries of NMA findings. A separate NMA 'summary of findings' table was created for each of the network outcomes for which a network was possible. CINeMA judgements and 'summary of findings' tables were taken into account in relation to the results in both the main analysis and the sensitivity analyses and any subgroup effects or narrative information identified.
Results
Description of studies
See: Characteristics of included studies, Characteristics of studies awaiting classification, Characteristics of ongoing studies and Characteristics of excluded studies.
Results of the search
Our search of the six electronic databases (see Electronic searches) retrieved 12,630 records. Our citation searching identified 16 additional studies that appeared to meet the inclusion criteria. Our screening of the reference lists of the included publications did not reveal additional RCTs. We therefore had a total of 12,646 records. We screened a total of 5031 records after de‐duplication, and excluded 4188 based on titles and abstracts. We obtained the full‐text reports of the remaining 843 records. We excluded 143 studies (in 151 full‐text reports) (Characteristics of excluded studies). We classified 25 studies as awaiting classification (Characteristics of studies awaiting classification), and we identified 95 ongoing studies (Characteristics of ongoing studies).
We included 291 trials reported in 590 references. Twenty‐three required translation into English from several languages: Chinese (Dou 2006a; Dou 2006b; Liu 2014b); Czech (Fadrhoncova 1982; Salava 2021); Danish (Ludvigsen 1975); French (Craps 1973); German (Busch‐Heidger 1993; Gehring 1996; Harder 1983; Nolting 1991; Schuppli 1983; Ulrich 1991); Italian (Gelmetti 1978; Giannetti 1981; Innocenti 1977; Pala 1982); Japanese (Anonymous 1981a; Asada 1987; JapicCTI‐163372; Otsuki 2003), Korean (Ryu 1997); Portuguese (Van Del Rey 1983); Spanish (Sanabria‐Silva 1991). For eleven of these, the translation was undertaken previously by the author team of another Cochrane review (Lax 2022). For a further description of our screening process, see the study flow diagram (Figure 1).
1.

PRISMA flow chart of search results and study selection
Included studies
Design
Most trials were of parallel design (288/291): between‐participant (206) or within‐participant (82). The other three trials were cross‐over within‐participant (Gradman 2007; Polano 1960; Samman 1962). Fifty‐six had more than two treatment arms. There were no cluster‐randomised trials or factorial trials.
Sample size
Where reported, trials included a total of 45,846 participants (samples ranged from 3 to 2439 participants). See Characteristics of included studies.
Setting
Where settings were reported, 54 of 274 (19.7%) studies were conducted in more than one country; of these, four involved settings in 10 or more countries (Landis 2022; Reitamo 2005; Sigurgeirsson 2005; Wahn 2002).
Using the classification by the World Bank Group for 2023 to 2024 (World Bank Group 2023), we found most trials were conducted in high‐income economies (243): including European countries (107); US (105); Canada (35); Japan (24); Australia (14); South Korea (6); Israel (4); New Zealand (3); Chile (1); Hong Kong (1); Israel (1); Singapore (2); Taiwan (1). Of these, nine were also conducted in upper‐middle income countries: South Africa (6); Argentina (1); Bulgaria (1); Brazil (1); China (2); Colombia (1) Ecuador (1); Guatemala (1); Mexico (1); Peru (1); Russia (1); Turkey (1). Two trials were also conducted in lower‐middle income countries: Morocco and Tunisia (Doss 2009; Doss 2010), and another was also conducted in Venezuela, an unclassified economy (Sigurgeirsson 2015).
Seventeen trials were conducted exclusively in an upper‐middle income country: China (8); Brazil (2); Mexico (4); Thailand (2); Malaya (1). Twelve were conducted exclusively in lower‐middle income countries: Bangladesh (4), India (5), Iran (2), Egypt (1). It was not possible to report or infer where the remaining 19 trials were conducted.
Most trials (189) were reported or assumed, based on the author's descriptions, to be conducted in secondary‐care settings. Four other studies were specifically conducted in private dermatology clinics (Cullen 1971a; Mali 1976; Maloney 1998; Noren 1989). The nature of the study setting was unspecified in the remaining 98 trials.
Interventions
The most common type of treatment (172) was a topical corticosteroid (TCS). Using the British National Formulary (British National Formulary) potency classification followed by the WHO classification (WHO Classification 2018), the TCS most commonly evaluated were potent (130); others were mild (60), moderate (55), very potent (20) or more than one potency.
Topical calcineurin inhibitors (TCI) were evaluated in 134 comparisons: pimecrolimus 1% (56); tacrolimus 0.1% (33), tacrolimus 0.03% (31), or other TCI drug/potency.
Topical phosphodiesterase 4 (PDE‐4) inhibitors were evaluated in 55 comparisons: crisaborole (22); difamilast (14); deoxynortryptoquivalone (9); roflumilast (6); cipamfylline 0.15% (2); PF‐07038124 0.01% (1); or AN2898 1% (1).
Topical janus kinase (JAK) inhibitors were evaluated in 30 comparisons: brepocitinib (6); delgocitinib (15); ruxolitinib (8); tofacitinib 2% (1).
Topical aryl hydrocarbon receptor (AHR) activators were evaluated in 10 comparisons: tapinarof 1% (6); tapinarof 0.5% (4), and five comparisons were a combination of TCS with another anti‐inflammatory.
Twenty‐one further comparisons involved another well‐characterised novel anti‐inflammatory treatment: ammonium trichlorotellurate (AS101) (2); the cyclooxygenase inhibitors bendazac (2), suprofen (1), and ufenamate (2); the protein kinase inhibitor CT 327 (1); the polyunsaturated fatty acid DS107 (2); the G protein‐coupled receptor 19 agonist HY209 (2); the selective glucocorticoid receptor agonist mapracorat (3); the multi‐functional anti‐inflammatory drug MRX‐6 (Celsus Therapeutics 2014) (1); the nitric oxide‐releasing cream SB414 (2); and the nuclear factor of activated T cells inhibitor UR‐1505 (3).
Many comparators/controls were vehicle (170).
The interventions are summarised in Figure 2.
2.

Network of all interventions included in the review
Duration of intervention was stated in 280 trials, with a median of three weeks (ranging from 7 days to 5 years). For most trials (260), the intervention duration was less than 16 weeks. Duration of study participation was stated in 272 trials, with a median of three weeks (ranging from 7 days to 5 years). For most trials (252), the duration of participation was less than 16 weeks.
Participants
Most trials included both younger (aged < 12 years) and older (≥ 12 years) participants (108) (age range 1 month to 70 years), or were restricted to participants aged 12 years or over (111) (range 18 to 85 years). Others were restricted to children up to the age of 12 years (31) (range from 2 months to 12 years). In 41 trials, participant age was not specified.
Two hundred and twelve trials involved both male and female participants, three involved male participants only, and one female only. Seventy‐five studies did not specify whether participants were male or female.
One hundred and ninety‐three studies did not report the ethnicity of participants. Most other trials (76) involved multiple ethnic groups; of these, 67 included participants who were white (where this group often accounted for most participants); other ethnic groups included in trials of multiple ethnic groups were black/African‐American (57), Asian (35), mixed ethnicity (9), Hispanic‐Latino (8) and unknown or other groups (36) including American‐Indian and Pacific Islander. Other trials included only one ethnic group: white (7), Asian (14) (often Japanese), and African‐American (1).
Eighty‐three trials did not report eczema severity in participants at baseline. Ninety‐seven trials only included participants whose eczema was not severe: mild‐to‐moderate eczema (87); mild only (2); moderate only (8). Seventy‐five trials included participants with severe eczema: moderate‐to‐severe (65); moderate‐to‐very‐severe (1); severe only (4); severe‐to‐very‐severe (2); very severe (1); eczema moderate or greater (2). Thirty‐six trials had a broader range of disease severity, covering both non‐severe and non‐mild: mild to severe (7); mild to very severe (4), "multiple categories" (24); up to severe (1).
Most studies did not report the site of eczema (186). Where reported, 49 involved non‐facial eczema, 46 involved both facial and non‐facial, and 10 facial only. Duration of eczema varied widely: in studies where participants both below and over age 12 years were eligible, duration ranged from three months to six years; in studies restricted to participants age 12 years or over, duration ranged from six months to 71 years; in studies with participants aged 12 years or younger, duration ranged from three months to 10 years. Sixty‐four trials did not report the duration of eczema.
Outcomes
One hundred and twenty‐nine trials used an instrument to measure patient‐reported symptoms of eczema; of these, 11 used the HOME‐recommended instrument 'Patient‐Oriented Eczema Measure' (POEM). One hundred and seventy‐seven trials used an instrument(s) to measure clinician‐reported signsof eczema; of these, 93 used the HOME‐recommended instrument 'Eczema Area and Severity Index' (EASI). Twenty‐four trials used an instrument to measure health‐related quality of life; of these, 12 used the Dermatology Life Quality Index (DLQI), six used the Children’s DLQI (CDLQI), and two used the Infants' Dermatitis Quality of Life Index (IDQOL). Two trials reported long‐term control of eczema, neither of which used the HOME‐recommended instrument, Recap of Eczema Control.
One hundred and forty‐eight trials reported adverse events; of these, 28 reported skin atrophy.
Funding sources/conflict of interest
Most trials (199, 68%) were industry‐funded; of these, one was also charity co‐funded. Other trials were supported by government funding (6), university funding (1), or did not specify the funding source (85). Trials which didn't specify a funding source also did not specify whether authors had any conflict of interest (84), or reported no author conflicts of interest (1).
Excluded studies
A total of 143 studies were excluded (see Characteristics of excluded studies). The most common reason for excluding trials was that the study included an ineligible intervention (64). Other reasons were: ineligible comparator (21); same potency TCS (29); did not meet the criteria for eczema diagnosis (18); no relevant outcomes recorded (9); and ineligible study design (2).
Studies awaiting classification
We identified 25 studies as potentially eligible and awaiting classification (Characteristics of studies awaiting classification). They were not included in the review as they lacked sufficient details to establish their eligibility. Most commonly this was related to a lack of detail about the intervention (n = 23); other reasons were lack of clarity about the comparator group (5) or study design (4).
Ongoing studies
We identified 95 ongoing studies that appeared to be eligible but had not yet reported any outcomes (Characteristics of ongoing studies). Ongoing trials evaluated TCI (31) such as tacrolimus and pimecrolimus; PDE‐4 inhibitors (19); JAK inhibitors (12); TCS (12); AHR activators (3), highly selective S1PR1 agonists (3); and other anti‐inflammatories (15).
Risk of bias in included studies
Figure 3 and Figure 4 summarised the ‘Risk of bias’ assessments (by study) for the 272 trials which reported at least one relevant outcome. The risk of bias assessments were similar across outcomes within each trial, so the summary risk of bias assessments represent the outcome with the lowest risk of bias for each study. The overall risk of bias was high for 242 (89.0%), some concerns for 24 (8.8%) and low for 6 (2.2%) trials. Of note, NMA for low risk of bias data was not possible for most outcomes. We thus made a post hoc decision to analyse all available data, rather than just the low risk of bias data, for the primary analyses.
3.

Risk of bias in included studies
4.
Summary of Risk of Bias assessments
Our detailed risk of bias assessments, with consensus responses to the signalling questions of the RoB 2 tool, can be accessed in the Open Science Framework (OSF) repository: https://osf.io/6ujga/?view_only=81e37bc34a514a938764fd610a0cb761.
Domain 1: Randomisation
Two hundred and twenty (80.9%) trials had some concerns either due to lack of detail about the method used to ensure allocation concealment or, for within‐participant studies, due to some potential for contamination. Forty‐seven (17.3%) trials described the method used for generating the allocation sequence and allocation concealment in sufficient detail and were therefore judged as having low risk of bias. Five (1.8%) were judged as at high risk of bias, as the method of sequence generation was not described or was unclear.
Domain 2: Deviations from intended interventions
One hundred and thirty‐six (50.0%) trials were judged as having low risk of bias. Eighty (29.4%) were judged as at high risk of bias, mainly due to poor reporting of the numbers of randomised participants included in analysis; high numbers excluded from analysis for potentially inappropriate reasons such as adverse effects or treatment changes; and lack of clarity around data analysis in studies with high post‐randomisation losses to follow‐up. The remaining 56 (20.6%) trials were judged as having some concerns, due to uncertainty in relation to blinding of participants/investigators and small numbers of participants inappropriately excluded from analysis.
Domain 3: Missing outcome data
One hundred and forty‐three (52.6%) trials were judged as having low risk of bias. Thirty (11.0%) trials had some concerns and 99 (36.4%) were at high risk of bias; mainly due to a high proportion of randomised participants not being accounted for in the analysis, or uncertainty about how many participants were used to generate the results presented.
Domain 4: Measurement of outcome
Two hundred and thirty‐six (86.8%) trials were judged as having low risk of bias, and eight (2.9%) were judged as having some concerns, due to missing information on how the outcome was measured. The remaining 28 (10.3%) trials were judged as having high risk of bias. These were mainly open‐label trials or trials with lack of clarity around blinding procedures, where outcome assessment may have been influenced by knowledge of the intervention received by participants or researchers.
Domain 5: Selection of reported result
Two hundred and forty (88.2%) trials were judged as having some concerns, mainly due to lack of pre‐trial registration or publicly available trial protocols, making it difficult to determine whether the outcomes were presented based on a prespecified plan. Two (0.7%) trials were considered to be at high risk of bias from selective outcome reporting. For these trials, outcomes were reported in a potentially misleading manner; for example, focusing on early time points where later time points were not significantly different between groups. The remaining 30 (11.0%) trials were judged as being at low risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9
The effects of interventions are summarised by outcome. Further details of subgroup and sensitivity analyses and narrative information categorised by outcome and intervention are available in the OSF repository. Summary of findings tables and the text describing NMA findings focus on the interventions that were licenced, or likely soon to be licenced at the time of publication in at least one region, for managing eczema or another inflammatory skin condition. These interventions are: TCS; TCI tacrolimus 0.1% and 0.03%, pimecrolimus 1%; JAK inhibitors ruxolitinib 1.5%, delgocitinib 0.25% and delgocitinib 0.5%; PDE‐4 inhibitors crisaborole 2%, roflumilast 0.15%, difamilast 1% and 0.3%; and AHR activator tapinarof 1%.
Outcomes for combination treatments and other topical anti‐inflammatory treatments which are classified as JAK inhibitors, PDE‐4 inhibitors, or others/not specified are included in the OSF repository but are not summarised in this section. For some outcomes and their subgroup/sensitivity analyses, suitable data for NMA were not available. For brevity, when a pre‐planned NMA, subgroup analysis, or sensitivity analysis was not possible, it has not been commented on below. All available data and analyses are summarised in this section.
Patient‐reported symptoms of eczema (binary)
Network meta‐analysis
Primary NMA (short‐term) included 40 trials (n = 6482) of TCS, TCI, PDE‐4 inhibitors, JAK inhibitors, an AHR activator, and other interventions (Figure 5). NMA showed most topical anti‐inflammatory treatments to be more effective than vehicle (Figure 6). NMA ranked potent TCS (odds ratio (OR) 5.99, 95% confidence interval (CI) 2.83, 12.69), the TCI tacrolimus 0.1% (OR 6.27, 95% CI 1.19, 32.98), and the JAK inhibitor ruxolitinib 1.5% (OR 5.64, 95% CI 1.26, 25.25) as most effective (Table 1). Mild TCS (OR 1.35, 95% CI 0.51, 3.53) and the PDE‐4 inhibitors roflumilast 0.15% (OR 1.03, 95% CI 0.12, 9.23) and crisaborole 2% (OR 1.15, 95% CI 0.17, 7.71) were ranked as least effective. For very potent TCS, mild TCS, tapinarof 1%, crisaborole 2% and roflumilast 0.15%, direct evidence was limited to a single trial with fewer than 100 data points and, for tacrolimus 0.1% and tacrolimus 0.03%, estimates were based on indirect evidence only.
5.

Patient‐reported symptoms of eczema (binary) ‐ primary analysis ‐ network map
6.

Patient‐reported symptoms of eczema (binary) ‐ forest plot of NMA effects (exp(b) = OR) with placebo comparator
There was no significant inconsistency (incoherence) for comparisons in this network (Table 12). However, CIs for effect estimates were wide for most comparisons, and confidence in evidence evaluated using CINeMA was moderate for roflumilast (0.15%) and low for all other interventions. All CINeMA judgements included a downgrade for within‐study bias. Interventions which had wide CIs including both no effect and a clinically significant effect compared with placebo were also downgraded for imprecision (very potent TCS, tacrolimus 0.03%, tapinarof 1%, crisaborole 2%, roflumilast 0.15%, and mild TCS). Potent TCS, tacrolimus 0.1%, ruxolitinib 1.5%, pimecrolimus 1%, and moderate TCS, were downgraded for heterogeneity. Indirectness was an additional concern for mild TCS; however, when considered alongside the extent of within‐study bias and imprecision, a further downgrade was not considered to be needed. A funnel plot of studies with placebo comparators showed no evidence of publication bias (Figure 7).
3. Patient‐reported signs of eczema (binary) NMA ‐ assessment of inconsistency.
| Treatment | Comparator | NMA effect (95% CI) | Direct effect (95% CI) | Indirect effect (95% CI) | Z‐value | P value |
| TCS potent | pimecrolimus 1% | 0.60 (0.24, 1.49) | 0.19 (0.04, 0.87) | 1.06 (0.36, 3.09) | ‐1.81 | 0.071 |
| crisaborole 2% | pimecrolimus 1% | 3.11 (0.45, 21.30) | 1.35 (0.14, 13.12) | 22.69 (0.67, 766.87) | ‐1.32 | 0.189 |
| TCS mild | TCS potent | 4.46 (1.69, 11.76) | 2.52 (0.66, 9.58) | 8.28 (2.05, 33.34) | ‐1.21 | 0.227 |
| TCS mild | placebo/vehicle | 0.74 (0.28, 1.95) | 1.53 (0.25, 9.5) | 0.56 (0.18, 1.75) | 0.91 | 0.361 |
| pimecrolimus 1% | placebo/vehicle | 0.28 (0.14, 0.54) | 0.26 (0.13, 0.52) | 0.73 (0.06, 9.03) | ‐0.77 | 0.440 |
| TCS potent | crisaborole 2% | 0.19 (0.03, 1.33) | 0.12 (0.01, 1.19) | 0.62 (0.02, 23.92) | ‐0.75 | 0.456 |
| TCS moderate | TCS potent | 2.29 (0.88, 5.93) | 4.93 (0.52, 46.59) | 1.93 (0.66, 5.58) | 0.74 | 0.459 |
| crisaborole 2% | placebo/vehicle | 0.87 (0.13, 5.78) | 1.22 (0.12, 12.22) | 0.4 (0.01, 12.77) | 0.52 | 0.601 |
| TCS potent | tacrolimus 0.1% | 1.05 (0.21, 5.22) | 1.46 (0.18, 11.57) | 0.6 (0.04, 8.47) | 0.52 | 0.604 |
| pimecrolimus 1% | tacrolimus 0.1% | 1.75 (0.33, 9.12) | 1.08 (0.09, 12.7) | 2.63 (0.27, 25.77) | ‐0.52 | 0.604 |
| TCS mild | TCS moderate | 1.95 (0.74, 5.14) | 2.31 (0.61, 8.73) | 1.59 (0.37, 6.84) | 0.37 | 0.713 |
| TCS moderate | placebo/vehicle | 0.38 (0.17, 0.85) | 0.36 (0.14, 0.95) | 0.43 (0.1, 1.96) | ‐0.19 | 0.848 |
| TCS potent | cipamfylline 0.15% | 0.27 (0.04, 1.83) | 0.28 (0.03, 2.61) | 0.22 (0, 16.42) | 0.09 | 0.927 |
| cipamfylline 0.15% | placebo/vehicle | 0.63 (0.09, 4.31) | 0.66 (0.07, 6.3) | 0.52 (0.01, 37.12) | 0.09 | 0.927 |
| TCS potent | placebo/vehicle | 0.17 (0.08, 0.35) | 0.16 (0.06, 0.42) | 0.17 (0.05, 0.63) | ‐0.05 | 0.957 |
| TCS potent | pimecrolimus 1% with TCS potent | 0.94 (0.12, 7.56) | 0.94 (0.12, 7.57) | 0.03 (., .) | 0.01 | 0.995 |
| pimecrolimus 1% | tacrolimus 0.03% | 1.27 (0.15, 10.68) | 1.27 (0.15, 10.68) | 0.1 (., .) | 0.00 | 0.997 |
.=inestimable
Abbreviations: CI: Confidence interval NMA: Network meta‐analysis TCS: Topical corticosteroids
7.

Patient‐reported signs of eczema (binary) ‐ funnel plot of study effects with placebo comparators
Subgroup and sensitivity analyses from NMA (short‐term)
NMA at class level (36 trials; n = 6132) ranked potent TCI and potent/very potent TCS as the most effective, with mild/moderate TCS and PDE‐4 inhibitors ranked the least effective. There was no significant inconsistency for comparisons in the class‐level network. Potent TCS had similar effectiveness to potent TCI (OR 0.97, 95% CI 0.21, 4.56) and were more effective than mild TCI (OR 1.77, 95% CI 0.75, 4.14), a difference that was statistically significant for direct evidence but was not seen in the indirect estimate. Potent TCS were more effective than PDE‐4 inhibitors (OR 4.01, 95% CI 1.33, 12.12), based on both direct and indirect evidence. There were no network estimates for TCS versus the AHR activator or JAK inhibitors.
Other subgroup and sensitivity analyses did not identify important differences from the main NMA, and are summarised below.
In the subgroup analysis of trials in people with non‐severe eczema (17 trials; n = 2682), potent TCS and the JAK inhibitor ruxolitinib 1.5% were ranked as most effective, with mild and moderate TCS and the PDE‐4 inhibitors roflumilast 0.15% and crisaborole 2% ranked as the least effective. There was significant inconsistency for three comparisons. For potent TCS versus pimecrolimus 1%, indirect effects suggest similar effectiveness, but direct evidence suggests potent TCS was more effective than pimecrolimus 1%. For potent TCS versus vehicle, indirect effects suggest greater effectiveness of potent TCS compared with vehicle, compared with direct effects, although both estimates supported effectiveness. For crisaborole 2% versus pimecrolimus 1%, indirect effects suggest pimecrolimus 1% was more effective than crisaborole 2%, but direct evidence suggests similar effectiveness.
In a subgroup analysis of studies in people with severe eczema (11 trials; n = 1892), potent TCS and the TCI tacrolimus 0.1% were ranked as most effective, with mild and moderate TCS and the TCI pimecrolimus 1% ranked as least effective. There was no significant inconsistency for comparisons in this network.
In the subgroup analysis of trials in people with eczema over age 12 years (17 trials; n = 2602), potent TCS, the TCI tacrolimus 0.1% and the JAK inhibitor ruxolitinib 1.5% were ranked as most effective, with the PDE‐4 inhibitor roflumilast 0.15% ranked as least effective. There was significant inconsistency for the comparison of potent TCS versus pimecrolimus 1%, where indirect effects suggest similar effectiveness, but direct evidence suggests potent TCS was more effective than pimecrolimus 1%.
In a sensitivity analysis using an alternative classification for TCS potency (42 trials; n = 6571), the JAK inhibitor ruxolitinib 1.5% and potent and very potent TCS were ranked as most effective and mild TCS, PDE‐4 inhibitors roflumilast 0.15% and crisaborole 2% were ranked as least effective. There was significant inconsistency between the comparison of moderate TCS versus pimecrolimus 1%, where indirect effects suggest similar effectiveness, but direct evidence suggests moderate TCS was more effective than pimecrolimus 1%.
In the sensitivity analysis excluding within‐participant studies (32 trials; n = 5978), potent TCS, the TCI tacrolimus 0.1% and the JAK inhibitor ruxolitinib 1.5% ranked as most effective, and PDE‐4 inhibitors roflumilast 0.15% and crisaborole 2% and mild TCS ranked as least effective. There was no significant inconsistency between comparisons in this network.
In sensitivity analysis imputing missing denominators (48 trials; n = 8006), the TCI tacrolimus 0.1%, the JAK inhibitor ruxolitinib 1.5% and potent TCS were ranked as most effective, with mild TCS, PDE‐4 inhibitors crisaborole 2% and roflumilast 0.15% as least effective. There was significant inconsistency between the comparison of potent TCS versus pimecrolimus 1%, where indirect effects suggest similar effectiveness, but direct evidence suggests potent TCS was more effective than pimecrolimus 1%.
In sensitivity analyses with alternative correlation coefficients of 0.25 or 0.75, potent TCS, the TCI tacrolimus 0.1% and the JAK inhibitor ruxolitinib 1.5% were ranked as most effective. Mild TCS and the PDE‐4 inhibitors crisaborole 2% and roflumilast 0.15% were ranked as least effective. As was the case for the primary analysis, there was no significant inconsistency between comparisons in this network.
Narrative information
Eight trials involving more than 3452 participants did not have appropriate data for inclusion in NMA. Trials reported significantly increased treatment response compared with vehicle for mild, potent, or very potent TCS, tacrolimus 0.1%, or crisaborole 2%.
Patient‐reported symptoms of eczema (continuous)
Network meta‐analysis
Primary NMA (short‐term) included 29 trials (n = 3839) of TCS, TCI, PDE‐4 inhibitors, JAK inhibitors, AHR activators, and other interventions (Figure 8). NMA ranked very potent TCS (SMD ‐1.99, 95% CI ‐3.25, ‐0.73), tacrolimus 0.03% (SMD ‐1.57, 95% CI –2.42, ‐0.72), potent TCS (SMD ‐1.37, 95% CI ‐2.04, ‐0.69) and ruxolitinib 1.5% (SMD ‐1.12, 95% CI ‐1.71, ‐0.53) the highest (Figure 9). The JAK inhibitors delgocitinib 0.5% (SMD ‐0.41, 95% CI ‐1.14, 0.33), tapinarof 1% (SMD ‐0.36, 95% CI ‐1.40, 0.68), delgocitinib 0.25% (SMD ‐0.21, 95% CI ‐0.94, 0.53), and roflumilast 0.15% (SMD ‐0.06, 95% CI ‐1.14, 1.02) were ranked lowest. Direct evidence was limited to single trials with fewer than 100 data points for the TCI tacrolimus 0.03%, tacrolimus 0.03% or 0.1% together, and the PDE‐4 inhibitors roflumilast 0.15% and crisaborole 2%. For very potent TCS, moderate TCS, and tacrolimus 0.1%, the estimate was based on indirect evidence only (Table 2).
8.

Patient reported symptoms of eczema (continuous) ‐ primary analysis ‐ network map
9.

Patient reported signs of eczema (continuous) ‐ primary analysis ‐ forest plot of NMA effects with placebo comparator
For all network comparisons, there was no significant difference between direct and indirect estimates (Table 13). Confidence in evidence evaluated using CINeMA was moderate for potent TCS, mild TCS, tacrolimus 0.03%, and ruxolitinib 1.5%, but low or very low for all other licenced interventions. Data for all interventions was downgraded for within‐study bias. Imprecision, heterogeneity, and incoherence were additional concerns about several interventions. Indirectness was also present for crisaborole 2%. A funnel plot of comparisons with placebo showed no evidence of publication bias (Figure 10).
4. Patient‐reported signs of eczema (continuous) NMA ‐ assessment of inconsistency.
| Treatment | Comparator | NMA effect (95% CI) | Direct effect (95% CI) | Indirect effect (95% CI) | Z value | P value |
| placebo/vehicle | ruxolitinib 1.5% | ‐1.10 (‐1.68, ‐0.51) | ‐0.96 (‐1.52, ‐0.39) | ‐3.49 (‐5.98, ‐1) | 1.93 | 0.053 |
| TCS potent | ruxolitinib 1.5% | 0.25 (‐0.54, 1.04) | ‐0.32 (‐1.36, 0.72) | 0.9 (‐0.24, 2.05) | ‐1.55 | 0.121 |
| TCS mild | placebo/vehicle | 0.92 (0.29, 1.55) | 0.59 (‐0.26, 1.44) | 1.32 (0.38, 2.27) | ‐1.14 | 0.253 |
| TCS potent | placebo/vehicle | 1.35 (0.67, 2.03) | 1.68 (0.75, 2.6) | 0.95 (‐0.08, 1.98) | 1.03 | 0.301 |
| TCS mild | tacrolimus 0.03% | ‐0.65 (‐1.46, 0.17) | ‐0.43 (‐1.46, 0.6) | ‐1.03 (‐2.41, 0.34) | 0.69 | 0.491 |
| placebo/vehicle | tacrolimus 0.03% | ‐1.57 (‐2.41, ‐0.72) | ‐1.86 (‐3.04, ‐0.67) | ‐1.25 (‐2.5, ‐0.01) | ‐0.69 | 0.491 |
| TCS mild | TCS potent | ‐0.43 (‐1.10, 0.24) | ‐0.26 (‐1.11, 0.6) | ‐0.76 (‐1.92, 0.4) | 0.68 | 0.496 |
| delgocitinib 0.25% | delgocitinib 0.5% | ‐0.20 (‐1.09, 0.69) | 0 (‐1.14, 1.14) | ‐0.57 (‐2.11, 0.97) | 0.58 | 0.560 |
| delgocitinib 0.5% | placebo/vehicle | 0.40 (‐0.33, 1.14) | 0.46 (‐0.33, 1.25) | ‐0.33 (‐3.28, 2.62) | 0.50 | 0.615 |
| delgocitinib 0.25% | placebo/vehicle | 0.21 (‐0.53, 0.94) | 0.16 (‐0.63, 0.96) | 0.82 (‐2.15, 3.78) | ‐0.42 | 0.676 |
| difamilast 0.3% | difamilast 1% | ‐0.22 (‐0.83, 0.38) | ‐0.22 (‐0.87, 0.44) | ‐0.33 (‐2.79, 2.13) | 0.09 | 0.927 |
| difamilast 0.3% | placebo/vehicle | 0.43 (‐0.18, 1.05) | 0.43 (‐0.24, 1.1) | 0.55 (‐1.91, 3) | ‐0.09 | 0.927 |
| TCS moderate | TCS potent | ‐0.43 (‐1.05, 0.19) | ‐0.43 (‐1.05, 0.19) | ‐2.27 (‐718.44, 713.89) | 0.01 | 0.996 |
| TCS potent | TCS very potent | ‐0.63 (‐1.69, 0.44) | ‐0.63 (‐1.69, 0.44) | 2.07 (‐1229.86, 1234.01) | 0.00 | 0.997 |
| TCS potent | tacrolimus 0.1% | 0.72 (‐0.49, 1.93) | 0.72 (‐0.49, 1.93) | 3.42 (‐1233.69, 1240.53) | 0.00 | 0.997 |
Abbreviations: CI: Confidence interval NMA: Network meta‐analysis TCS: Topical corticosteroids
10.

Patient‐reported signs of eczema (continuous) ‐ primary analysis ‐ funnel plot for studies containing placebo arms and Egger's test result
Subgroup and sensitivity analyses from NMA (short‐term)
NMA at class level (28 trials; n = 3723) ranked potent/very potent TCS, mild TCI and JAK inhibitors as most effective, with mild/moderate TCS and PDE‐4 inhibitors ranked least effective. There was significant inconsistency between the comparison of JAK inhibitors with vehicle, where indirect effects suggest JAK inhibitors were relatively more effective than vehicle, in comparison to the estimates from direct evidence. Potent/very potent TCS had similar effectiveness to potent TCI (SMD 0.44, 95% CI ‐0.48, 1.37) and similar effectiveness to JAK inhibitors (SMD 0.47, 95% CI ‐0.20, 1.14). Mild/moderate TCS were less effective than potent/very potent TCS (SMD ‐0.48, 95% CI ‐0.94, ‐0.02) and had similar effectiveness to mild TCI (SMD ‐0.24, 95% CI ‐0.93, 0.46). There were no network estimates for TCS versus PDE‐4 inhibitors or the AHR activator.
In a subgroup analysis of non‐severe eczema and in sensitivity analysis using HOME‐recommended outcome assessments, tacrolimus 0.03% was no longer ranked as one of the most effective treatments. Subgroup and sensitivity analyses did not identify other important differences from the main NMA, and are summarised below.
In the subgroup analysis of trials in people with non‐severe eczema (12 trials; n = 1508), the JAK inhibitor ruxolitinib 1.5%, potent TCS, and mild TCS were ranked as most effective, with the PDE‐4 inhibitors difamilast 0.3% and 1% and the TCI pimecrolimus 1% ranked as least effective. There was significant inconsistency between three comparisons: potent TCS versus mild TCS, mild TCS versus vehicle, and ruxolitinib 1.5% versus placebo. For potent TCS versus mild TCS and for mild TCS versus vehicle, direct evidence suggests greater superiority than indirect evidence. For ruxolitinib 1.5% versus placebo, indirect evidence suggests greater superiority than direct evidence.
In a subgroup analysis of studies in people aged over 12 years (17 trials; n = 2384), the JAK inhibitor ruxolitinib 1.5% and the TCI pimecrolimus 1% ranked most effective, and potent and moderate TCS were ranked as least effective. There was significant inconsistency between three comparisons: potent TCS versus moderate TCS, moderate TCS versus vehicle, and ruxolitinib 1.5% versus placebo. For potent TCS versus moderate TCS, indirect evidence suggests greater superiority than direct evidence. For ruxolitinib 1.5% versus placebo, and moderate TCS versus placebo, direct evidence suggests greater superiority than indirect evidence.
In a sensitivity analysis using an alternative classification for TCS potency (26 trials; n = 3432), the TCI tacrolimus 0.03% alone or with 0.1%, very potent TCS, and the JAK inhibitor ruxolitinib 1.5% were ranked as most effective and PDE‐4 inhibitors difamilast 0.3%, tacrolimus 0.1% and roflumilast 0.15% were ranked as least effective. There was no significant inconsistency between comparisons in this sensitivity analysis.
In a sensitivity analysis of HOME‐recommended outcome assessments (11 trials; n = 1837), potent TCS and the JAK inhibitor ruxolitinib 1.5% were ranked as most effective and PDE‐4 inhibitors difamilast 0.3% or 1% and the JAK inhibitor delgocitinib 0.5% were ranked as least effective. There was no significant inconsistency between comparisons in this sensitivity analysis.
In a sensitivity analysis excluding within‐participant studies (22 trials; n = 3161), the TCI tacrolimus 0.03% alone or with 0.1%, the JAK inhibitor ruxolitinib 1.5% and potent TCS were ranked as most effective and the PDE‐4 inhibitors roflumilast 0.15% and difamilast 0.3%, TCI pimecrolimus 1% and moderate TCS were ranked as least effective. There was significant inconsistency between three comparisons: potent versus moderate TCS, potent TCS versus vehicle, and moderate TCS versus vehicle. For potent TCS versus moderate TCS and potent TCS versus vehicle, indirect evidence suggests greater superiority than direct evidence. For moderate TCS versus vehicle, direct evidence suggests inferiority of moderate TCS.
In sensitivity analysis imputing missing denominators (35 trials, n = 5193), very potent TCS, the TCI tacrolimus 0.03% alone or with 0.1%, and the JAK inhibitor ruxolitinib 1.5% ranked as most effective. Moderate TCS and the PDE‐4 inhibitors crisaborole 2%, roflumilast 0.15%, and difamilast 0.3% ranked as least effective. There was significant inconsistency between three comparisons: potent versus moderate TCS, potent TCS versus vehicle, and moderate TCS versus vehicle. For potent TCS versus moderate TCS, indirect evidence suggests greater superiority than direct evidence, whereas the reverse was true for potent TCS versus vehicle.
In the sensitivity analyses with alternative correlation coefficients of 0.25 or 0.75, the TCI tacrolimus 0.03% alone or with 0.1%, and the JAK inhibitor ruxolitinib 1.5% were ranked as most effective, consistent with the primary analysis. PDE‐4 inhibitors crisaborole 2%, roflumilast 0.15%, difamilast 0.3% and moderate TCS were ranked as least effective. There was still significant inconsistency between potent versus moderate TCS, potent TCS versus vehicle, and moderate TCS versus vehicle.
In a sensitivity analysis excluding Sikder 2005, very potent or potent TCS and tacrolimus 0.03% or 0.1% were ranked as most effective. Roflumilast 0.15% was ranked as least effective. There was significant inconsistency in the comparison of ruxolitinib 1.5% versus vehicle. Indirect evidence suggests greater superiority than direct evidence. There was no significant inconsistency between other comparisons. This sensitivity analysis was undertaken due to the surprisingly high ranking of tacrolimus 0.03%, which persisted despite the exclusion of Sikder 2005.
In a sensitivity analysis using four‐week outcome data from Raoof 2020 in place of eight‐week outcome data, very potent and potent TCS, tacrolimus 0.03% and 0.1% and ruxolitinib 1.5% were ranked as most effective. Delgocitinib 0.5% and 0.25%, tapinarof 1% and roflumilast 0.15% were ranked as least effective. There was no significant inconsistency in this network. This sensitivity analysis was undertaken due to the shorter duration of potent TCS use (4 weeks) compared with ruxolitinib 1.5% use (8 weeks) in Raoof 2020.
Narrative information
Fifty studies (n = 9636) did not have appropriate data for inclusion in NMA. Studies reported significantly reduced eczema symptoms compared with vehicle for moderate or potent TCS, tacrolimus 0.03% or 0.1%, crisaborole 2%, difamilast 0.3% or 1%, roflumilast 0.15%, delgocitinib 0.25%, 0.5%, 1% or 3%, and tapinarof 0.5% or 1%. In active comparator studies, potent TCS was more effective than mild or moderate TCS, tacrolimus 0.03%, and pimecrolimus 1%; outcomes for comparisons between potent TCS and tacrolimus 0.1% were inconsistent between studies. Moderate TCS, tacrolimus 0.03%, and tacrolimus 0.1% were more effective than pimecrolimus 1%, and there was no difference between crisaborole 2% and pimecrolimus 1%.
Clinician‐reported signs of eczema (binary)
Network meta‐analysis
Primary NMA (short‐term) included 32 trials (n = 4121) of TCS, TCI, PDE‐4 inhibitors, JAK inhibitors, AHR activator and other interventions (Figure 11). NMA showed most topical anti‐inflammatory treatments to be more effective than vehicle (Figure 12). NMA ranked potent TCS (OR 8.15, 95% CI 4.99, 13.57), the TCI tacrolimus 0.1% (OR 8.06, 95% CI 3.30, 19.67), the JAK inhibitors ruxolitinib 1.5% (OR 7.72, 95% CI 4.92, 12.10) and delgocitinib 0.5% (OR 7.61, 95% CI 3.72, 15.58) as most effective (Table 3). Mild TCS (OR 2.22, 95% CI 0.74, 6.64), PDE‐4 inhibitors roflumilast 0.15% (OR 2.43, 95% CI 0.88, 6.70) and crisaborole 2% (OR 2.98, 95% CI 1.42, 6.26), and the AHR activator tapinarof 1% (OR 2.45, 95% CI 1.00, 6.02) were ranked as least effective. For tacrolimus 0.1%, moderate TCS, mild TCS and roflumilast 0.15%, direct evidence was limited to a single trial with fewer than 100 observations.
11.
Clinician‐reported signs of eczema (binary) ‐ network map ‐ primary analysis
12.

Clinician‐reported signs of eczema (binary) ‐ forest plot of NMA effects (exb(b) = OR) compared with placebo ‐ primary analysis
For the comparison of ruxolitinib 1.5% with placebo there was significant inconsistency with indirect evidence suggesting greater effectiveness than direct evidence (Table 14). For all other network comparisons, there was no significant difference between direct and indirect estimates. However, confidence intervals for effect estimates were wide for most comparisons. Confidence in evidence evaluated using CINeMA was moderate or high for most licenced interventions but low for tapinarof 1% and for mild TCS. The main reason for CINeMA downgrades was within‐study bias. For the interventions which did not show a statistically significant improvement compared with placebo (roflumilast 0.15%, tapinarof 1%, mild TCS), there were additional downgrades for imprecision; for roflumilast 0.15% and tapinarof 1%, there were also further downgrades for significant heterogeneity. A funnel plot of comparisons with placebo showed no evidence of publication bias (Figure 13).
5. Clinician‐reported signs of eczema (binary) NMA ‐ assessment of inconsistency.
| Treatment | Comparator | NMA effect (95% CI) | Direct effect (95% CI) | Indirect effect (95% CI) | Z value | P value |
| ruxolitinib 1.5% | placebo/vehicle | 0.13 (0.20, 0.08) | 0.15 (0.22, 0.10) | 0.02 (0.13, 0.00) | 2.06 | 0.039 |
| TCS potent | ruxolitinib 1.5% | 0.95 (0.52, 1.72) | 1.64 (0.72, 3.76) | 0.61 (0.29, 1.3) | 1.73 | 0.084 |
| delgocitinib 0.25% | tacrolimus 0.1% | 1.53 (0.63, 3.72) | 2.36 (0.87, 6.43) | 0.52 (0.11, 2.46) | 1.64 | 0.100 |
| TCS potent | tacrolimus 0.1% | 0.99 (0.39, 2.54) | 0.4 (0.1, 1.69) | 1.85 (0.57, 5.97) | ‐1.61 | 0.107 |
| TCS mild | TCS potent | 3.67 (1.37, 9.83) | 6.65 (1.95, 22.65) | 1.48 (0.33, 6.63) | 1.57 | 0.117 |
| delgocitinib 0.25% | placebo/vehicle | 0.19 (0.09, 0.39) | 0.14 (0.06, 0.32) | 1.13 (0.1, 12.21) | ‐1.53 | 0.127 |
| delgocitinib 0.25% | delgocitinib 0.5% | 1.45 (0.75, 2.79) | 1.68 (0.85, 3.34) | 0.45 (0.07, 3.03) | 1.28 | 0.202 |
| TCS mild | TCS moderate | 2.35 (0.86, 6.47) | 1.29 (0.29, 5.79) | 3.86 (0.99, 15.09) | ‐1.06 | 0.290 |
| TCS moderate | placebo/vehicle | 0.19 (0.09, 0.39) | 0.09 (0.02, 0.49) | 0.22 (0.1, 0.49) | ‐0.93 | 0.350 |
| delgocitinib 0.5% | tacrolimus 0.1% | 1.06 (0.44, 2.54) | 1.31 (0.47, 3.68) | 0.62 (0.12, 3.06) | 0.79 | 0.428 |
| TCS potent | placebo/vehicle | 0.12 (0.07, 0.20) | 0.14 (0.08, 0.26) | 0.09 (0.04, 0.23) | 0.78 | 0.436 |
| tacrolimus 0.1% | placebo/vehicle | 0.12 (0.30, 0.05) | 0.08 (0.36, 0.02) | 0.17 (0.63, 0.05) | ‐0.69 | 0.493 |
| TCS potent | crisaborole 2% | 0.37 (0.17, 0.79) | 0.32 (0.13, 0.79) | 0.52 (0.13, 2.12) | ‐0.58 | 0.560 |
| TCS mild | placebo/vehicle | 0.45 (0.15, 1.35) | 0.70 (0.06, 8.34) | 0.4 (0.11, 1.41) | 0.39 | 0.697 |
| crisaborole 2% | pimecrolimus 1% | 1.23 (0.56, 2.66) | 1.38 (0.5, 3.79) | 1 (0.27, 3.73) | 0.38 | 0.707 |
| difamilast 0.3% | difamilast 1% | 1.68 (0.79, 3.60) | 1.78 (0.74, 4.24) | 1.14 (0.11, 11.61) | 0.35 | 0.725 |
| difamilast 0.3% | placebo/vehicle | 0.31 (0.14, 0.69) | 0.29 (0.11, 0.74) | 0.45 (0.05, 4.22) | ‐0.35 | 0.725 |
| pimecrolimus 1% | placebo/vehicle | 0.27 (0.18, 0.42) | 0.28 (0.18, 0.44) | 0.19 (0.03, 1.41) | 0.35 | 0.730 |
| delgocitinib 0.5% | placebo/vehicle | 0.13 (0.06, 0.27) | 0.14 (0.06, 0.31) | 0.1 (0.01, 1.24) | 0.19 | 0.850 |
| TCS potent | pimecrolimus 1% | 0.45 (0.25, 0.81) | 0.42 (0.16, 1.11) | 0.46 (0.21, 1.03) | ‐0.13 | 0.894 |
| crisaborole 2% | placebo/vehicle | 0.34 (0.16, 0.70) | 0.32 (0.13, 0.79) | 0.36 (0.07, 1.91) | ‐0.10 | 0.918 |
| TCS moderate | TCS potent | 1.56 (0.90, 2.70) | 1.56 (0.84, 2.87) | 1.58 (0.43, 5.87) | ‐0.02 | 0.983 |
Abbreviations: CI: Confidence interval NMA: Network meta‐analysis TCS: Topical corticosteroids
13.

Clinician‐reported signs of eczema (binary) ‐ funnel plot of studies with placebo comparators
Subgroup and sensitivity analyses from NMA
Subgroup and sensitivity analyses did not identify important differences from the main NMA, and are summarised narratively below.
NMA at class level (26 trials, n = 3210) ranked potent TCS and JAK inhibitors as most effective, with mild/moderate TCS, mild or potent TCI, PDE‐4 inhibitors and AHR activators ranked least effective. There was significant inconsistency between comparisons of JAK inhibitors with vehicle or potent TCS, where indirect effects suggest JAK inhibitors were relatively less effective than potent TCS and relatively more effective than vehicle, in comparison to the estimates from direct evidence. Potent TCS were more effective than potent TCI (OR 2.47, 95% CI 0.52, 11.64), based on direct evidence only, and potent TCS were more effective than mild TCI (OR 2.01, 95% CI 1.01, 3.97), based on both direct and indirect evidence. Potent TCS were more effective than JAK inhibitors in indirect evidence only (direct evidence OR 0.60, 95% CI 0.26, 1.40; indirect OR 2.08, 95% CI 0.99, 4.38). Potent TCS were also more effective than PDE‐4 inhibitors (OR 2.05, 95% CI 1.03, 4.10), and mild TCI had similar effectiveness to PDE‐4 inhibitors (OR 1.02, 95% CI 0.53, 1.96) based on both direct and indirect evidence. There was no network estimate for TCS versus AHR activator.
In the subgroup analysis of trials in people with non‐severe eczema (14 trials, n = 1802), moderate or potent TCS, the JAK inhibitor ruxolitinib 1.5% and the PDE‐4 inhibitor difamilast 1% were ranked as most effective, with the PDE‐4 inhibitors roflumilast 0.15% and crisaborole 2% ranked as least effective. There was no significant inconsistency between comparisons in this network.
In a subgroup analysis of studies in people with eczema over the age of 12 years (18 trials, n = 2788), the TCI tacrolimus 0.1% and the JAK inhibitors ruxolitinib 1.5% and delgocitinib 0.5% were ranked as most effective, with the PDE‐4 inhibitor roflumilast 0.15% and the AHR activator tapinarof 1% ranked as least effective. There was no significant inconsistency between comparisons in this network.
In a sensitivity analysis using an alternative classification for TCS potency (34 trials, n = 4312), potent TCS were ranked as most effective, with the TCI tacrolimus 0.1%, JAK inhibitors ruxolitinib 1.5% and delgocitinib 0.5%, and moderate TCS ranked as effective. The PDE‐4 inhibitors roflumilast 0.15%, crisaborole 2% and difamilast 0.3%, and the AHR activator tapinarof 1% ranked as least effective. There was significant inconsistency in the comparison of potent versus moderate TCS, where indirect effects suggest similar effectiveness, but direct evidence suggests potent TCS were more effective than moderate TCS.
In a sensitivity analysis limited to the studies reporting the HOME‐recommended outcome measure EASI (19 trials, n = 3498), potent TCS, the TCI tacrolimus 0.1%, the JAK inhibitors ruxolitinib 1.5%, delgocitinib 0.5% and delgocitinib 0.25% ranked as most effective, and PDE‐4 inhibitors roflumilast 0.15% and crisaborole 2%, and AHR activator tapinarof 1% ranked as least effective. There was no significant inconsistency between comparisons in this network.
In the sensitivity analysis excluding within‐participant trials (25 trials, n = 3737), moderate and potent TCS, the TCI tacrolimus 0.1%, JAK inhibitors ruxolitinib 1.5% and delgocitinib 0.5% ranked as most effective, and PDE‐4 inhibitors roflumilast 0.15% and crisaborole 2%, and AHR activator tapinarof 1% ranked as least effective. There was significant inconsistency in the comparison of TCI tacrolimus 0.1% with potent TCS, where indirect effects suggest tacrolimus 0.1% may be more effective than potent TCS, but direct evidence suggests potent TCS may be more effective than tacrolimus 0.1%.
In a sensitivity analysis imputing missing denominators (35 trials, n = 5559), the TCI tacrolimus 0.1%, JAK inhibitors ruxolitinib 1.5%, delgocitinib 0.5% and delgocitinib 0.25% and potent TCS were ranked as most effective, with mild TCS, PDE‐4 inhibitors crisaborole 2%, roflumilast 0.15% and difamilast 0.3%, and AHR activator tapinarof 1% as least effective. There was no significant inconsistency between comparisons in this network.
In the sensitivity analyses with alternative correlation coefficients of 0.25 or 0.75, potent TCS, the TCI tacrolimus 0.1%, the JAK inhibitors ruxolitinib 1.5% and delgocitinib 0.5% were ranked as most effective. Mild TCS, PDE‐4 inhibitor roflumilast 0.15% and the AHR activator tapinarof 1% were ranked as least effective. As was the case for the primary analysis, for comparison of ruxolitinib 1.5% with placebo, there was significant inconsistency in these networks.
In a sensitivity analysis using 4‐week outcome data from Raoof 2020 in place of eight‐week outcome data, potent TCS, tacrolimus 0.1%, ruxolitinib 1.5% and delgocitinib 0.5% were ranked as most effective. Mild TCS, roflumilast 0.15%, tapinarof 1% and crisaborole 2% were ranked as least effective. There was no significant inconsistency in this network. This sensitivity analysis was undertaken due to the shorter duration of potent TCS use (4 weeks) compared with ruxolitinib 1.5% use (8 weeks) in Raoof 2020.
Narrative information
Six parallel‐group trials in 2550 children and one within‐participant trial in eight children and adults with eczema did not have appropriate data for inclusion in NMA. Trials reported significantly increased treatment response compared with vehicle for pimecrolimus 1%, roflumilast 0.15% and tapinarof 1% and also for tacrolimus 0.1% compared with moderate TCS.
Clinician‐reported signs of eczema (continuous)
Network meta‐analysis
Primary NMA (short‐term) included 49 trials (n = 5261) of TCS, TCI, PDE‐4 inhibitors, JAK inhibitors, AHR activator and other interventions (Figure 14). NMA showed most topical anti‐inflammatory treatments to be more effective than vehicle (49 trials, n = 5261; Figure 15). NMA ranked tacrolimus 0.03% (SMD ‐2.69, 95% CI ‐3.36, ‐2.02) and very potent TCS (SMD ‐1.87, 95% CI ‐2.69, ‐1.05) as most effective (Table 4). PDE‐4 inhibitors roflumilast 0.15% (SMD ‐0.42, 95% CI ‐1.16, 0.33) and difamilast 0.3% (SMD ‐0.47, 95% CI ‐0.89, ‐0.05), and the AHR activator tapinarof 1% (SMD ‐0.64, 95% CI 1.35, 0.07) were ranked as least effective. For tacrolimus 0.03%, potent TCS and roflumilast 0.15%, direct evidence was limited to a single trial with fewer than 100 observations, and for very potent TCS, the estimate was based on indirect evidence only.
14.
Clinician‐reported signs of eczema (continuous) ‐ primary ‐ network map
15.

Clinician‐reported signs of eczema (continuous) ‐ primary ‐ NMA effects with placebo comparator
For the comparison between licenced interventions and vehicle, there was no significant inconsistency (Table 15). However, confidence intervals for effect estimates were wide for most comparisons, and confidence in the evidence evaluated using CINeMA was low (pimecrolimus 1%, tapniarof 1% and difamilast 0.3%) or moderate for all other licenced interventions. The main reasons for CINeMA downgrades were within‐study bias and heterogeneity. A funnel plot of comparisons with placebo showed no evidence of small study effects or publication bias (Figure 16).
6. Clinician‐reported signs of eczema (continuous) NMA ‐ assessment of inconsistency.
| Treatment | Comparator | NMA effect (95% CI) | Direct effect (95% CI) | Indirect effect (95% CI) | Z value | P value |
| UR 1505 1% | tacrolimus 0.1% | ‐0.64 (‐1.60, 0.32) | ‐0.17 (‐1.21, 0.87) | ‐2.54 (‐4.54, ‐0.54) | 2.11 | 0.04 |
| UR 1505 1% | placebo/vehicle | 0.29 (‐0.66, 1.25) | ‐0.16 (‐1.19, 0.87) | 2.22 (0.2, 4.23) | ‐2.11 | 0.04 |
| tacrolimus 0.03% | placebo/vehicle | 2.69 (3.36, 2.02) | 2.16 (3.02, 1.3) | 3.4 (4.38, 2.41) | ‐1.85 | 0.07 |
| TCS mild | tacrolimus 0.03% | ‐1.57 (‐2.24, ‐0.91) | ‐2.1 (‐2.96, ‐1.24) | ‐0.87 (‐1.86, 0.13) | ‐1.85 | 0.07 |
| pimecrolimus 1% | placebo/vehicle | 0.83 (0.48, 1.19) | 0.95 (0.58, 1.32) | 0.05 (‐0.92, 1.01) | 1.72 | 0.09 |
| TCS mild | pimecrolimus 1% | 0.29 (‐0.23, 0.80) | 0.83 (0.03, 1.64) | ‐0.07 (‐0.72, 0.58) | 1.72 | 0.09 |
| tacrolimus 0.1% | placebo/vehicle | 0.94 (1.41, 0.47) | 0.6 (1.22, ‐0.02) | 1.38 (2.1, 0.67) | ‐1.63 | 0.10 |
| AN2898 1% | crisaborole 2% | ‐0.41 (‐1.17, 0.35) | ‐0.15 (‐0.98, 0.68) | ‐1.48 (‐3.14, 0.17) | 1.43 | 0.15 |
| AN2898 1% | placebo/vehicle | 0.33 (‐0.42, 1.07) | 0.12 (‐0.67, 0.91) | 1.46 (‐0.26, 3.18) | ‐1.43 | 0.15 |
| TCS moderate | placebo/vehicle | 0.72 (0.30, 1.14) | 1.01 (0.42, 1.59) | 0.45 (‐0.13, 1.03) | 1.34 | 0.18 |
| TCS moderate | TCS potent | ‐0.23 (‐0.53, 0.07) | ‐0.32 (‐0.64, 0.01) | 0.24 (‐0.51, 1) | ‐1.34 | 0.18 |
| TCS potent | tacrolimus 0.1% | 0.02 (‐0.49, 0.53) | ‐0.31 (‐1.05, 0.43) | 0.33 (‐0.39, 1.05) | ‐1.21 | 0.23 |
| delgocitinib 0.25% | tacrolimus 0.1% | ‐0.21 (‐0.76, 0.34) | ‐0.45 (‐1.2, 0.29) | 0.07 (‐0.73, 0.87) | ‐0.95 | 0.34 |
| ruxolitinib 1.5% | placebo/vehicle | 1.08 (1.45, 0.70) | 1.04 (1.43, 0.65) | 1.67 (3.28, 0.06) | ‐0.74 | 0.46 |
| TCS potent | ruxolitinib 1.5% | ‐0.12 (‐0.61, 0.37) | ‐0.29 (‐1.01, 0.43) | 0.03 (‐0.66, 0.71) | ‐0.62 | 0.53 |
| TCS potent | placebo/vehicle | 0.95 (0.55, 1.35) | 0.75 (0, 1.5) | 1.03 (0.55, 1.5) | ‐0.61 | 0.54 |
| TCS mild | TCS potent | 0.17 (‐0.39, 0.72) | ‐0.08 (‐1.06, 0.89) | 0.29 (‐0.39, 0.96) | ‐0.61 | 0.54 |
| E6005 0.01% | E6005 0.2% | ‐1.49 (‐2.63, ‐0.36) | ‐1.6 (‐2.87, ‐0.33) | ‐1.08 (‐3.45, 1.3) | ‐0.39 | 0.70 |
| E6005 0.01% | placebo/vehicle | ‐1.26 (‐2.39, ‐0.13) | ‐1.16 (‐2.41, 0.08) | ‐1.69 (‐4.1, 0.73) | 0.39 | 0.70 |
| delgocitinib 0.5% | tacrolimus 0.1% | 0.06 (‐0.49, 0.61) | ‐0.02 (‐0.78, 0.74) | 0.15 (‐0.66, 0.97) | ‐0.31 | 0.75 |
| TCS mild | placebo/vehicle | 1.12 (0.65, 1.58) | 1.19 (0.45, 1.93) | 1.06 (0.45, 1.68) | 0.26 | 0.79 |
| difamilast 0.3% | placebo/vehicle | 0.47 (0.05, 0.89) | 0.46 (0.01, 0.91) | 0.65 (‐0.91, 2.2) | ‐0.23 | 0.82 |
| difamilast 0.3% | difamilast 1% | ‐0.24 (‐0.66, 0.17) | ‐0.23 (‐0.67, 0.21) | ‐0.42 (‐1.98, 1.14) | 0.23 | 0.82 |
| delgocitinib 0.5% | placebo/vehicle | 0.99 (0.59, 1.40) | 1.01 (0.57, 1.44) | 0.89 (‐0.43, 2.22) | 0.16 | 0.88 |
| delgocitinib 0.25% | placebo/vehicle | 0.73 (0.32, 1.13) | 0.74 (0.3, 1.17) | 0.63 (‐0.70, 1.96) | 0.15 | 0.88 |
| delgocitinib 0.25% | delgocitinib 0.5% | ‐0.27 (‐0.73, 0.19) | ‐0.27 (‐0.8, 0.26) | ‐0.27 (‐1.29, 0.75) | 0.01 | 0.996 |
| TCS potent | TCS very potent | ‐0.92 (‐1.64, ‐0.20) | ‐0.92 (‐1.64, ‐0.2) | 1 (‐1243.67, 1245.67) | 0.00 | 0.998 |
| TCS potent | azathioprine 4% and TCS potent | ‐0.20 (‐0.97, 0.57) | ‐0.2 (‐0.97, 0.57) | 1.71 (‐1267.08, 1270.51) | 0.00 | 0.998 |
| TCS potent | pimecrolimus 1% with TCS potent | ‐0.02 (‐0.66, 0.62) | ‐0.02 (‐0.66, 0.62) | 1.88 (‐1275.61, 1279.36) | 0.00 | 0.998 |
Abbreviations: CI: Confidence interval NMA: Network meta‐analysis TCS: Topical corticosteroids
16.

Clinician‐reported signs of eczema (continuous) ‐ funnel plot of studies with placebo comparators
Subgroup and sensitivity analyses from NMA
Most subgroup and sensitivity analyses did not rank tacrolimus 0.03% as amongst the most effective treatments. Subgroup and sensitivity analyses did not identify other important differences from the main NMA, and are summarised below.
NMA at class level (48 trials, n = 5057) ranked mild, potent, and very potent TCS and JAK inhibitors as most effective, with PDE‐4 inhibitors and AHR activators ranked as least effective. There was no significant inconsistency between comparisons in this network. Potent/very potent TCS had similar effectiveness to potent TCI (SMD 0.03, 95% CI ‐0.60, 0.65) and mild/moderate TCS were more effective than mild TCI (SMD 0.45, 95% CI ‐0.05, 0.95). Potent or potent/very potent TCS had similar effectiveness to JAK inhibitors (potent TCS, SMD 0.02, 95% CI ‐0.61, 0.65; potent/very potent TCS, SMD 0.04, 95% CI ‐0.49, 0.58). There was no network estimate for TCS versus PDE‐4 inhibitors or TCS versus AHR activators.
In the subgroup analysis of trials in people with non‐facial eczema (15 trials, n = 1184), very potent TCS, pimecrolimus 1%, tacrolimus 0.1% and potent TCS were ranked as most effective, with moderate TCS and the PDE‐4 inhibitors difamilast 0.3%, 1% and crisaborole 2% ranked as least effective. There was significant inconsistency between multiple comparisons in this network involving tacrolimus 0.1%, moderate TCS and potent TCS.
In the subgroup analysis of studies in people with severe eczema (15 trials, n = 1397), very potent TCS was ranked as most effective, followed by mild TCS, pimecrolimus 1% and tacrolimus 0.1%. Moderate TCS and the JAK inhibitor delgocitinib 0.25% or 0.5% were ranked as least effective. There was no significant inconsistency between comparisons in this network.
In the subgroup analysis of trials in people with non‐severe eczema (15 trials, n = 1384), mild TCS, moderate TCS and the JAK inhibitor ruxolitinib 1.5% were most effective. Tacrolimus 0.1% and the PDE4 inhibitors roflumilast 0.15% and difamilast 0.3% were least effective. There was no significant inconsistency between comparisons in this network.
In the subgroup analysis of studies in people aged over 12 years (30 trials, n = 3398), very potent TCS and the JAK inhibitors ruxolitinib 1.5% and delgocitinib 0.5% were most effective. PDE‐4 inhibitors difamilast 0.3%, roflumilast 0.15% and JAK inhibitor delgocitinib 0.25% were ranked as least effective. There was significant inconsistency only in the comparisons of UR 1505 1% with vehicle or tacrolimus 0.1%.
In sensitivity analysis using an alternative classification for TCS potency (44 trials, n = 4781), tacrolimus 0.03%, very potent TCS, potent TCS and ruxolitinib 1.5% were ranked as most effective, with the PDE‐4 inhibitors difamilast 0.3% and 1% and roflumilast 0.15% and the AHR activator tapinarof 1% ranked as least effective. There was significant inconsistency between the comparison of UR 1505 1% and vehicle or tacrolimus 0.1%, and for tacrolimus 0.1% or pimecrolimus 1% versus moderate TCS or vehicle.
In sensitivity analysis limited to the trials reporting the HOME outcome measure EASI (37 trials, n = 5788), ruxolitinib 1.5%, mild TCS were ranked as most effective, and roflumilast 0.15%, difamilast 0.3% and tapinarof 1% as least effective. There was significant inconsistency in the comparison of crisaborole 2% with potent TCS, but no significant consistency for other comparisons in this network.
In sensitivity analysis excluding within‐participant studies (38 trials, n = 6031), tacrolimus 0.03% and 0.1% and ruxolitinib 1.5% were ranked as most effective. PDE‐4 inhibitors roflumilast 0.15%, difamilast 0.3% and AHR activator tapinarof 1% were ranked as least effective. There was no significant inconsistency between comparisons in this network.
In sensitivity analysis imputing missing denominators (61 trials, n = 7413), tacrolimus 0.1% and 0.03%, JAK inhibitors ruxolitinib 1.5% and delgocitinib 0.5% and very potent and potent TCS were ranked as most effective, with mild TCS, PDE‐4 inhibitors crisaborole 2%, roflumilast 0.15% and difamilast 0.3%, and AHR activator tapinarof 1% as least effective. There was significant inconsistency between comparisons of tacrolimus 0.03% with mild TCS and with tacrolimus 0.1%, for potent TCS, for pimecrolimus 1% versus vehicle, and for pimecrolimus 1% versus mild TCS. There was no significant inconsistency between other comparisons in this network.
In sensitivity analyses with alternative correlation coefficients of 0.25 or 0.75, tacrolimus 0.03%, very potent and mild TCS and ruxolitinib 1.5% were ranked as most effective and roflumilast 0.15% and difamilast 0.3% as least effective. There was significant inconsistency between comparisons of UR 1505 1% with vehicle and with tacrolimus 0.1% in these networks, and no significant inconsistency between other comparisons in these networks.
In sensitivity analysis excluding Sikder 2005, very potent, potent or mild TCS, ruxolitinib 1.5% and delgocitinib 0.5% were ranked as most effective. Roflumilast 0.15%, difamilast 0.3% and 1% and tapinarof 1% were ranked as least effective. There was significant inconsistency between comparisons of UR 1505 1% with vehicle and with tacrolimus 0.1% in this network, and no significant inconsistency between other comparisons. This sensitivity analysis was undertaken due to the surprisingly high ranking of tacrolimus 0.03% which was driven by one small study (Sikder 2005).
In sensitivity analysis using four‐week outcome data from Raoof 2020 in place of eight‐week outcome data, tacrolimus 0.03% and very potent TCS were ranked as most effective, followed by mild TCS, ruxolitinib 1.5% and potent TCS. Roflumilast 0.15%, difamilast 0.3% and tapinarof 1% were ranked as least effective. There was significant inconsistency between comparisons of UR 1505 1% with vehicle and with tacrolimus 0.1% in this network, and no significant inconsistency between other comparisons. This sensitivity analysis was undertaken due to the shorter duration of potent TCS use (4 weeks) compared with ruxolitinib 1.5% use (8 weeks) in Raoof 2020.
Narrative information
One hundred studies (n = 22,814) did not have appropriate data for inclusion in NMA. Studies reported significantly reduced eczema signs compared with vehicle for potent or very potent TCS, tacrolimus 0.03% or 0.1%, pimecrolimus 1%, crisaborole 2%, difamilast 0.3% or 1% and tapinarof 1%. Studies reported similar effectiveness of potent TCS and tacrolimus 0.1%, and greater effectiveness of mild/moderate TCS compared with pimecrolimus 1%.
Investigator Global Assessment
Network meta‐analysis
Primary NMA (short‐term) included 140 trials (n = 23,383) of TCS, TCI, PDE‐4 inhibitors, JAK inhibitors, an AHR activator and other interventions (Figure 17). NMA showed most topical anti‐inflammatory treatments to be more effective than vehicle (Figure 18). NMA ranked very potent TCS (OR 8.34, 95% CI 4.73, 14.67), potent TCS (OR 5.00, 95% CI 3.80, 6.58), the JAK inhibitors ruxolitinib 1.5% (OR 9.34, 95% CI 4.8, 18.18), delgocitinib 0.5% (OR 10.08, 95% CI 2.65, 38.37) and delgocitinib 0.25% (OR 6.87, 95% CI 1.79, 26.33), and the TCI tacrolimus 0.1% (OR 5.06, 95% CI 3.59, 7.13) as most effective (Table 5). Mild TCS (OR 1.38, 95% CI 0.94, 2.02), the PDE‐4 inhibitors crisaborole 2% (OR 2.14, 95% CI 1.22, 3.76), roflumilast 0.15% (OR 2.43, 95% CI 0.65, 9.01), difamilast 0.3% (OR 2.56, 95% CI 1.37, 4.78) and difamilast 1% (OR 3.45, 95% CI 1.97, 6.02), and the TCI tacrolimus 0.03% (OR 3.53, 95% CI 2.60, 4.80) and pimecrolimus 1% (OR 2.39, 95% CI 1.78, 3.21) were ranked as least effective. Direct evidence was limited to a single trial with fewer than 100 data points for mild TCS and roflumilast 0.15%.
17.
IGA (binary) ‐ primary analysis ‐ network map
18.

IGA (binary) ‐ primary analysis ‐ forest plot of NMA effects (exp(b) = OR) with placebo comparator
There was significant inconsistency between direct and indirect evidence for several clinically important comparisons (Table 16). For the comparison of moderate TCS versus tacrolimus 0.03%, indirect evidence suggests similar effectiveness, whereas direct evidence suggests moderate TCS was more effective. For mild TCS versus vehicle, indirect evidence suggests mild TCS was more effective, whereas direct evidence suggests similar effectiveness.
7. Investigator Global Assessment (binary) NMA ‐ assessment of inconsistency.
| Treatment | Comparator | NMA effect (95% CI) | Direct effect (95% CI) | Indirect effect (95% CI) | Z value | P value |
| TCS mild | bendazac 3% | 2.99 (0.70, 12.81) | 1 (0.18, 5.49) | 50.66 (3.26, 787.06) | ‐2.38 | 0.017 |
| bendazac 3% | placebo/vehicle | 0.24 (0.06, 1.06) | 0.02 (0, 0.23) | 0.77 (0.13, 4.39) | ‐2.38 | 0.017 |
| TCS moderate | tacrolimus 0.03% | 0.79 (0.52, 1.20) | 0.08 (0.01, 0.61) | 0.87 (0.57, 1.32) | ‐2.25 | 0.025 |
| TCS moderate | tacrolimus 0.03% w TCS moderate | 16.88 (1.57, 181.80) | 7 (0.58, 84.57) | 899.61 (13.48, 60,047.84) | ‐2.25 | 0.025 |
| tacrolimus 0.03% | tacrolimus 0.03% w TCS moderate | 21.32 (1.96, 231.73) | 91 (6.14, 1349.52) | 0.71 (0.02, 31.72) | 2.25 | 0.025 |
| TCS mild | placebo/vehicle | 0.73 (0.50, 1.07) | 3.8 (0.76, 19.1) | 0.66 (0.45, 0.97) | 2.07 | 0.039 |
| TCS very potent | tacrolimus 0.1% | 0.61 (0.32, 1.15) | 0.03 (0, 1.08) | 0.67 (0.35, 1.28) | ‐1.67 | 0.095 |
| TCS moderate | TCS potent | 1.12 (0.82, 1.54) | 1.4 (0.9, 2.17) | 0.88 (0.56, 1.39) | 1.44 | 0.149 |
| TCS moderate | placebo/vehicle | 0.22 (0.16, 0.31) | 0.18 (0.11, 0.28) | 0.28 (0.18, 0.45) | ‐1.41 | 0.16 |
| ruxolitinib 1.5% | placebo/vehicle | 0.11 (0.06, 0.21) | 0.12 (0.06, 0.25) | 0.03 (0.00, 0.33) | 1.11 | 0.268 |
| tapinarof 1% | placebo/vehicle | 0.27 (0.13, 0.58) | 0.31 (0.14, 0.68) | 0.07 (0.00, 0.89) | ‐1.11 | 0.268 |
| TCS potent | pimecrolimus 1% | 0.48 (0.32, 0.71) | 0.26 (0.07, 0.96) | 0.51 (0.34, 0.76) | ‐0.95 | 0.341 |
| TCS potent | crisaborole 2% | 0.43 (0.23, 0.79) | 0.26 (0.07, 0.93) | 0.49 (0.25, 0.98) | ‐0.88 | 0.381 |
| TCS potent | tacrolimus 0.1% | 1.01 (0.69, 1.49) | 1.35 (0.63, 2.92) | 0.92 (0.58, 1.44) | 0.86 | 0.39 |
| TCS potent | ruxolitinib 1.5% | 1.87 (0.93, 3.76) | 2.83 (0.78, 10.25) | 1.55 (0.66, 3.64) | 0.76 | 0.448 |
| tacrolimus 0.1% | tapinarof 1% | 0.73 (0.33, 1.62) | 1.16 (0.27, 4.92) | 0.59 (0.23, 1.55) | 0.76 | 0.448 |
| TCS mild | pimecrolimus 1% | 1.74 (1.09, 2.77) | 3.5 (0.52, 23.39) | 1.66 (1.02, 2.69) | 0.75 | 0.455 |
| TCS mild | TCS potent | 3.63 (2.55, 5.17) | 3.24 (1.99, 5.27) | 4.14 (2.46, 6.98) | ‐0.68 | 0.497 |
| TCS mild | TCS moderate | 3.24 (2.18, 4.81) | 2.75 (1.45, 5.19) | 3.6 (2.18, 5.94) | ‐0.65 | 0.514 |
| crisaborole 2% | placebo/vehicle | 0.47 (0.27, 0.82) | 0.44 (0.24, 0.79) | 0.89 (0.12, 6.63) | ‐0.65 | 0.514 |
| TCS mild | tacrolimus 0.03% | 2.57 (1.68, 3.93) | 3.18 (1.46, 6.91) | 2.34 (1.41, 3.9) | 0.65 | 0.518 |
| TCS mild | tacrolimus 0.1% | 3.68 (2.32, 5.82) | 5.01 (1.66, 15.19) | 3.45 (2.08, 5.73) | 0.6 | 0.547 |
| TCS very potent | placebo/vehicle | 0.12 (0.07, 0.21) | 0.15 (0.06, 0.37) | 0.11 (0.05, 0.22) | 0.56 | 0.574 |
| tacrolimus 0.03% | placebo/vehicle | 0.28 (0.21, 0.38) | 0.26 (0.18, 0.39) | 0.32 (0.19, 0.53) | ‐0.56 | 0.577 |
| delgocitinib 0.5% | tacrolimus 0.1% | 0.50 (0.13, 1.92) | 0.69 (0.11, 4.46) | 0.35 (0.05, 2.45) | 0.49 | 0.623 |
| difamilast 0.3% | difamilast 1% | 1.35 (0.75, 2.40) | 1.31 (0.72, 2.37) | 2.38 (0.19, 30.02) | ‐0.45 | 0.652 |
| difamilast 0.3% | placebo/vehicle | 0.39 (0.21, 0.73) | 0.41 (0.21, 0.78) | 0.22 (0.02, 2.71) | 0.45 | 0.652 |
| TCS potent | cipamfylline 0.15% | 0.29 (0.09, 0.88) | 0.33 (0.09, 1.26) | 0.17 (0.01, 2.04) | 0.45 | 0.653 |
| cipamfylline 0.15% | placebo/vehicle | 0.70 (0.23, 2.15) | 0.82 (0.22, 3.08) | 0.42 (0.04, 5) | 0.45 | 0.653 |
| tacrolimus 0.03% | tacrolimus 0.1% | 1.43 (1.01, 2.04) | 1.38 (0.91, 2.09) | 1.59 (0.79, 3.2) | ‐0.34 | 0.734 |
| delgocitinib 0.25% | tacrolimus 0.1% | 0.74 (0.19, 2.83) | 0.62 (0.1, 3.99) | 0.89 (0.13, 6.32) | ‐0.26 | 0.793 |
| pimecrolimus 1% | tacrolimus 0.03% | 1.48 (0.98, 2.22) | 1.69 (0.5, 5.72) | 1.45 (0.94, 2.25) | 0.23 | 0.818 |
| TCS potent | tacrolimus 0.03% | 0.71 (0.49, 1.01) | 0.75 (0.34, 1.63) | 0.69 (0.46, 1.04) | 0.17 | 0.867 |
| pimecrolimus 1% | placebo/vehicle | 0.42 (0.31, 0.56) | 0.41 (0.3, 0.57) | 0.45 (0.18, 1.11) | ‐0.16 | 0.871 |
| UR 1505 1% | placebo/vehicle | 2.19 (0.34, 14.13) | 2.33 (0.31, 17.64) | 1.66 (0.03, 87.81) | 0.16 | 0.876 |
| UR 1505 1% | tacrolimus 0.1% | 11.10 (1.71, 72.09) | 10 (1.02, 97.99) | 14.09 (0.42, 477.64) | ‐0.16 | 0.876 |
| tacrolimus 0.1% | placebo/vehicle | 0.20 (0.14, 0.28) | 0.19 (0.12, 0.30) | 0.20 (0.12, 0.36) | ‐0.15 | 0.885 |
| delgocitinib 0.25% | placebo/vehicle | 0.15 (0.04, 0.56) | 0.16 (0.03, 0.82) | 0.11 (0, 5.11) | 0.13 | 0.896 |
| delgocitinib 0.5% | placebo/vehicle | 0.10 (0.03, 0.38) | 0.11 (0.02, 0.55) | 0.08 (0, 3.5) | 0.13 | 0.896 |
| crisaborole 2% | pimecrolimus 1% | 1.12 (0.60, 2.08) | 1.03 (0.25, 4.15) | 1.14 (0.56, 2.31) | ‐0.13 | 0.898 |
| TCS potent | placebo/vehicle | 0.20 (0.15, 0.26) | 0.2 (0.14, 0.3) | 0.2 (0.13, 0.29) | 0.08 | 0.935 |
| pimecrolimus 1% | tacrolimus 0.1% | 2.12 (1.36, 3.29) | 2 (0.14, 29.24) | 2.12 (1.36, 3.32) | ‐0.04 | 0.966 |
| TCS potent | TCS very potent | 1.67 (0.96, 2.89) | 1.67 (0.84, 3.33) | 1.65 (0.65, 4.17) | 0.03 | 0.98 |
| TCS potent | pimecrolimus 1% w TCS potent | 0.86 (0.21, 3.44) | 0.86 (0.21, 3.44) | 0.03 (., .) | 0.01 | 0.996 |
| ufenamate 5% | placebo/vehicle | 0.67 (0.13, 3.33) | 0.67 (0.13, 3.33) | 1.09 (., .) | 0 | 0.999 |
| suprofen 1% | ufenamate 5% | 0.92 (0.25, 3.30) | 0.92 (0.25, 3.3) | 2.45 (., .) | 0 | 0.999 |
.=inestimable
Abbreviations: CI: Confidence interval NMA: Network meta‐analysis TCS: Topical corticosteroids
CIs for effect estimates were wide for most comparisons, and confidence in evidence evaluated using CINeMA was low or moderate for most licenced interventions. The main reason for CINeMA downgrades was within‐study bias. Imprecision was a concern for roflumilast 0.15%, but not considered sufficient for a further downgrade in addition to the within‐study bias. Heterogeneity was a concern for crisaborole 2%, difamilast 0.3%, and pimecrolimus 1%, but not sufficient to justify an additional downgrade for difamilast 0.3% and pimecrolimus 1%. Mild TCS had concerns in several CINeMA domains (within‐study bias, imprecision, heterogeneity, and incoherence) which, when taken together, resulted in low confidence. A funnel plot of studies with placebo comparators showed no evidence of publication bias (Figure 19).
19.
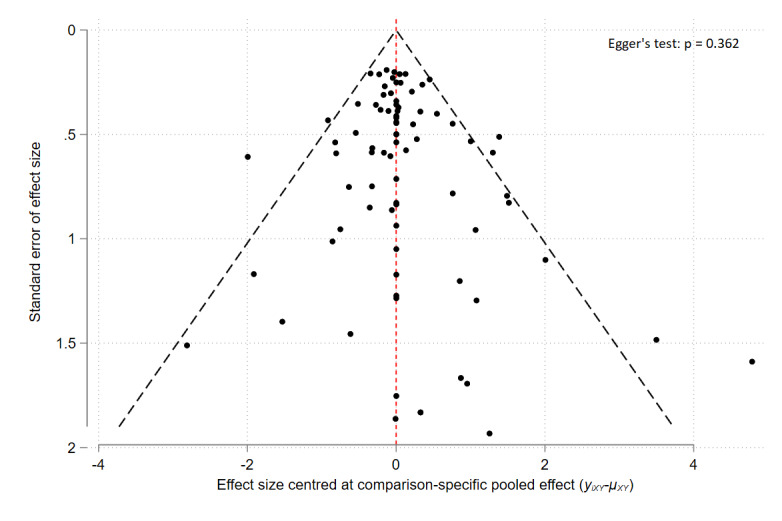
IGA (binary) ‐ primary analysis ‐ funnel plot of studies containing placebo
Longer‐term data over 6 to 12 months follow‐up for this outcome were insufficient for NMA, but were sufficient for a pairwise meta‐analysis of pimecrolimus 1% versus vehicle (Analysis 1.1), showing a possible increase in long‐term IGA response with pimecrolimus 1%. There was significant statistical heterogeneity, with one smaller study in adults reporting a significant difference in favour of pimecrolimus 1% at 24 weeks, and three larger studies in children reporting no significant difference between pimecrolimus 1% and vehicle at six to twelve months. Longer‐term data over 12 months follow‐up were available from one trial comparing pimecrolimus 1% versus mild/moderate TCS, which reported more treatment success with mild/moderate TCS than pimecrolimus 1% (Sigurgeirsson 2015; OR 1.37, 95% 1.12, 1.69; n = 2045). All five trials were funded by the manufacturer of pimecrolimus 1%.
1.1. Analysis.

Comparison 1: Investigator Global Assessment (binary) ‐ long‐term, Outcome 1: Pimecrolimus 1% versus vehicle
Subgroup and sensitivity analyses from NMA
NMA at class level (126 trials, n = 21,414) ranked JAK inhibitors, potent/very potent TCS and potent TCI as most effective, with mild TCI, mild/moderate TCS and PDE‐4 inhibitors ranked least effective. There was significant inconsistency for mild/moderate TCS versus vehicle, where direct effects suggest a greater difference in favour of mild/moderate TCS than indirect evidence. Potent/very potent TCS had similar effectiveness to potent TCI (OR 1.09, 95% CI 0.71, 1.67), and potent/very potent TCS were more effective than mild TCI (OR 1.80, 95% CI 1.27, 2.55). Mild/moderate TCS had similar effectiveness to mild TCI (OR 0.89, 95% CI 0.61, 1.28), and mild/moderate TCS were less effective than potent TCI (OR 0.54, 95% 0.34, 0.84). Potent/very potent TCS were more effective than PDE‐4 inhibitors (OR 2.08, 95% CI 1.30, 3.30), and had similar effectiveness to JAK inhibitors (OR 0.69, 95% CI 0.37, 1.28). There were no network estimates for TCS versus AHR activators.
In other subgroup and sensitivity analyses, mild TCS and/or pimecrolimus 1% were usually ranked amongst the least effective treatments. Subgroup and sensitivity analyses did not identify other important differences from the main NMA, and are summarised below.
In a subgroup analysis of studies in people with non‐facial eczema (21 trials, n = 1873), very potent, potent and moderate TCS were ranked as most effective, with TCI pimecrolimus 1% and mild TCS ranked as least effective. There was significant inconsistency in the comparison of potent versus moderate TCS, where indirect effects suggest potent TCS were less effective, but direct evidence suggests potent TCS were more effective than moderate TCS. There was also significant inconsistency in the comparison of potent TCS versus placebo, where indirect evidence suggests a greater difference in favour of potent TCS compared with direct evidence.
In a subgroup analysis of trials in people with non‐severe eczema (40 trials, n = 7666), the JAK inhibitor ruxolitinib 1.5%, moderate TCS and potent TCS were ranked as most effective with mild TCS and crisaborole 2% ranked as least effective. There was significant inconsistency in the comparison of potent TCS versus pimecrolimus 1%, where indirect effects suggest similar effectiveness, but direct evidence suggests potent TCS were more effective than pimecrolimus 1%.
In the subgroup analysis of studies in people with severe eczema (43 trials, n = 6318), very potent TCS, potent TCS, tacrolimus 0.1% and the JAK inhibitor delgocitinib 0.5% or 0.25% were ranked as most effective, with TCI pimecrolimus 1% and mild TCS ranked as least effective. There was significant inconsistency between direct and indirect evidence for three comparisons. Indirect effects suggest moderate TCS were more effective than potent; direct effects suggest potent TCS were more effective. Indirect effects suggest tacrolimus 0.03% and moderate TCS were similarly effective; direct effects suggest moderate TCS were more effective. Direct effects suggest a greater difference in effectiveness between tacrolimus 0.03% and vehicle than indirect effects.
In the subgroup analysis of trials in people with eczema over the age of 12 years (54 trials, n = 8715), the JAK inhibitor ruxolitinib 1.5% and very potent TCS were ranked as most effective, with the PDE‐4 inhibitors roflumilast 0.15%, difamilast 0.3% and 1%, pimecrolimus 1% and TCI tacrolimus 0.03% ranked as least effective. There was no significant inconsistency between comparisons in this network.
In a subgroup analysis of trials in people with eczema under the age of 12 years (9 trials, n = 3462), potent TCS was ranked as most effective, and mild TCS least effective. There was no significant inconsistency between comparisons in this network.
In a sensitivity analysis limited to outcomes at low risk of bias (12 trials, n = 1639), potent TCS and the JAK inhibitors delgocitinib 0.5% and delgocitinib 0.25% were ranked as most effective, and the TCI pimecrolimus 1%, PDE‐4 inhibitors roflumilast 0.15%, difamilast 1% and difamilast 0.3% ranked as least effective. There was no significant inconsistency between comparisons in this network.
In a sensitivity analysis using an alternative classification for TCS potency (136 trials, n = 22,764), very potent TCS and the JAK inhibitors delgocitinib 0.5% and ruxolitinib 1.5% were ranked as most effective, and mild TCS, PDE‐4 inhibitors roflumilast 0.15%, difamilast 0.3% and crisaborole 2% and TCI pimecrolimus 1% ranked as least effective. There was significant inconsistency between direct and indirect evidence for three clinically relevant comparisons. Indirect effects suggest a greater difference in effectiveness in favour of moderate TCS versus mild TCS, and in favour of mild TCS versus vehicle, than direct effects. Direct effects suggest a greater difference in effectiveness in favour of moderate TCS versus vehicle, than indirect effects.
In a sensitivity analysis excluding within‐participant studies (97 trials, n = 20,303), the JAK inhibitors delgocitinib 0.5% and ruxolitinib 1.5% ranked as most effective, and mild TCS, PDE‐4 inhibitors roflumilast 0.15%, crisaborole 2% and difamilast 0.3% ranked as least effective. There was significant inconsistency between direct and indirect evidence for comparison of tacrolimus 0.03% with moderate TCS. Indirect effects suggest the two interventions were similarly effective, but direct effects suggest moderate TCS was more effective than tacrolimus 0.03%.
In a sensitivity analysis imputing missing denominators (156 trials, n = 27,856), very potent TCS and the JAK inhibitors delgocitinib 0.5% and ruxolitinib 1.5% ranked as most effective, and mild TCS, PDE‐4 inhibitors roflumilast 0.15%, crisaborole 2% and difamilast 0.3% and TCI pimecrolimus 1% ranked as least effective. There was significant inconsistency between direct and indirect evidence for two clinically relevant comparisons. Direct effects suggest greater effectiveness for moderate TCS versus tacrolimus 0.03%, compared with indirect effects. Indirect effects suggest greater effectiveness for mild TCS versus vehicle, compared with direct effects.
In sensitivity analyses with alternative correlation coefficients of 0.25 or 0.75, very potent TCS and JAK inhibitors ruxolitinib 1.5% and delgocitinib 0.5% were ranked as most effective, consistent with the primary analysis. Mild TCS, PDE‐4 inhibitor crisaborole 2% and TCI pimecrolimus 1% were ranked as least effective. There was significant inconsistency in these networks across key comparisons, consistent with the primary analyses.
In a sensitivity analysis using four‐week outcome data from Raoof 2020 in place of eight‐week outcome data, very potent and potent TCS, ruxolitinib 1.5%, delgocitinib 0.5% or 0.25% and tacrolimus 0.1% were ranked as most effective. Mild TCS, crisaborole 2%, pimecrolimus 1% and roflumilast 0.15% were ranked as least effective. There was significant inconsistency between comparisons of mild TCS versus vehicle, where direct effects suggest a greater difference in favour of mild TCS than indirect evidence. There was no significant inconsistency between other comparisons. This sensitivity analysis was undertaken due to the shorter duration of potent TCS use (4 weeks) compared with ruxolitinib 1.5% use (8 weeks) in Raoof 2020.
Narrative information
Twenty‐six trials in more than 3750 participants did not have appropriate data for inclusion in NMA. Trials reported significantly increased treatment response compared with vehicle for mild or potent TCS, tacrolimus 0.03% or 0.1%, pimecrolimus 1%, delgocitinib 0.5%, roflumilast 0.15% and tapinarof 1%, increased treatment response for potent TCS compared with tacrolimus 0.1% and for tacrolimus 0.1% compared with moderate TCS.
Health‐related quality of life (binary)
NMA was not possible for this outcome. Pairwise meta‐analysis was possible for two studies comparing pimecrolimus 1% versus vehicle (Analysis 2.1), showing a possible improvement in health‐related quality of life with pimecrolimus.
2.1. Analysis.

Comparison 2: Health‐related quality of life (continuous) ‐ short‐term, Outcome 1: Pimecrolimus 1% versus vehicle
Narrative information
Twenty‐three other trials (n = 6878) reported this outcome. Trials reported significantly improved health‐related quality of life compared with vehicle for crisaborole 2%, difamilast 1%, tacrolimus 0.03% or 1% and ruxolitinib 1.5%. Findings for pimecrolimus 1% versus vehicle were not consistent, with most trials reporting no difference, but one trial (n = 272) reported improved longer‐term quality of life with pimecrolimus. Active comparator trials suggest potent TCS is more effective than tacrolimus 0.1%.
Long‐term control
NMA was not possible for this outcome. Pairwise meta‐analysis for two trials comparing pimecrolimus 1% versus vehicle over six to 12 months follow‐up (Analysis 3.1) showed a possible improvement in long‐term control with pimecrolimus. There was no significant difference in a trial of children under 12 years old (Kapp 2002), but improved long‐term control with pimecrolimus in a trial of adults (Meurer 2002).
3.1. Analysis.

Comparison 3: Long‐term control of eczema (binary), Outcome 1: Pimecrolimus 1% versus vehicle
Local adverse events: application‐site reactions
Network meta‐analysis
Primary NMA (short‐term) included 83 trials (n = 18,992) of TCS, TCI, PDE‐4 inhibitors, JAK inhibitors, an AHR activator and other interventions (Figure 20). NMA suggests TCI and crisaborole 2% are more likely than vehicle to cause application‐site reactions and TCS less likely (Figure 21). The TCI tacrolimus 0.1% (OR 2.09, 95% CI 1.46, 3.00), tacrolimus 0.03% (OR 1.49, 95% CI 1.08, 2.04), pimecrolimus 1% (OR 1.49, 95% CI 1.05, 2.12), and the PDE‐4 inhibitor crisaborole 2% (OR 2.11, 95% CI 1.19, 3.76) were ranked as most likely to cause local application‐site reactions. NMA ranked TCS as safest: very potent TCS (OR 0.33, 95% CI 0.13, 0.81), potent TCS (OR 0.35, 95% CI 0.22, 0.55), moderate TCS (OR 0.49, 95% CI 0.25, 0.93), and mild TCS (OR 0.51, 95% CI 0.30, 0.85) (Table 6). CIs were wide, including the possibility of no difference between vehicle and tapinarof 1% or roflumilast 0.15%. For roflumilast 0.15%, direct evidence was limited to a single trial with fewer than 100 participants.
20.

Primary analysis ‐ local adverse events ‐ site events ‐ network map
21.

Primary analysis ‐ local adverse events ‐ site events ‐ forest plot of NMA effects (epb(b) = OR) with placebo comparator
For the comparison of very potent TCS with potent TCS, there was significant inconsistency, with indirect evidence suggesting greater risk of application‐site reactions with very potent TCS, but no difference seen in direct evidence (Table 17). For all other network comparisons, there was no significant difference between direct and indirect estimates.
8. Local adverse events (application‐site reactions) NMA ‐ assessment of inconsistency.
| Treatment | Comparator | NMA effect (95% CI) | Direct effect (95% CI) | Indirect effect (95% CI) | Z value | P value |
| TCS potent | TCS very potent | 0.94 (0.42, 2.12) | 0.55 (0.24, 1.3) | 5.35 (1.05, 27.2) | ‐2.42 | 0.016 |
| TCS very potent | placebo/vehicle | 3.07 (1.23, 7.67) | 0.67 (0.11, 4.12) | 4.89 (1.87, 12.76) | ‐1.9 | 0.058 |
| TCS potent | crisaborole 2% | 6.10 (3.00, 12.41) | 34.32 (3.69, 319.37) | 4.84 (2.26, 10.36) | 1.6 | 0.109 |
| tacrolimus 0.03% | tacrolimus 0.1% | 1.41 (1.02, 1.95) | 1.25 (0.87, 1.81) | 2.05 (1.07, 3.91) | ‐1.3 | 0.192 |
| TCS potent | tacrolimus 0.1% | 6.04 (3.82, 9.54) | 8.24 (4.32, 15.72) | 4.59 (2.48, 8.48) | 1.29 | 0.196 |
| crisaborole 2% | pimecrolimus 1% | 0.71 (0.37, 1.35) | 0.27 (0.06, 1.34) | 0.86 (0.43, 1.74) | ‐1.28 | 0.2 |
| TCS very potent | tacrolimus 0.1% | 6.43 (2.61, 15.84) | 1 (0.04, 23.83) | 7.53 (3.02, 18.81) | ‐1.2 | 0.23 |
| pimecrolimus 1% | tacrolimus 0.03% | 1.00 (0.67, 1.47) | 0.67 (0.32, 1.42) | 1.15 (0.73, 1.81) | ‐1.2 | 0.23 |
| TCS moderate | tacrolimus 0.03% | 3.06 (1.59, 5.88) | 12.25 (1.15, 130.94) | 2.73 (1.38, 5.37) | 1.2 | 0.232 |
| TCS moderate | tacrolimus 0.03% w TCS moderate | 0.73 (0.11, 5.02) | 2.15 (0.16, 29.23) | 0.11 (0, 4.32) | 1.2 | 0.232 |
| tacrolimus 0.03% | tacrolimus 0.03% w TCS moderate | 0.24 (0.04, 1.54) | 0.18 (0.03, 1.21) | 3.55 (0.03, 427.74) | ‐1.2 | 0.232 |
| pimecrolimus 1% | placebo/vehicle | 0.67 (0.47, 0.95) | 0.76 (0.49, 1.17) | 0.52 (0.28, 0.97) | 0.98 | 0.329 |
| tacrolimus 0.03% | placebo/vehicle | 0.67 (0.49, 0.93) | 0.61 (0.41, 0.89) | 0.83 (0.49, 1.41) | ‐0.95 | 0.341 |
| crisaborole 2% | placebo/vehicle | 0.47 (0.27, 0.84) | 0.52 (0.28, 0.94) | 0.12 (0.01, 2.32) | 0.94 | 0.35 |
| TCS potent | pimecrolimus 1% | 4.30 (2.57, 7.22) | 7.08 (2.13, 23.5) | 3.86 (2.18, 6.85) | 0.91 | 0.366 |
| TCS mild | tacrolimus 0.03% | 2.93 (1.78, 4.83) | 2.53 (1.37, 4.68) | 3.86 (1.71, 8.71) | ‐0.83 | 0.407 |
| TCS moderate | placebo/vehicle | 2.06 (1.07, 3.95) | 1.28 (.35, 4.73) | 2.4 (1.13, 5.10) | ‐0.82 | 0.413 |
| TCS mild | placebo/vehicle | 1.97 (1.18, 3.30) | 2.84 (1.03, 7.8) | 1.74 (0.95, 3.17) | 0.82 | 0.415 |
| tacrolimus 0.1% | placebo/vehicle | 0.48 (0.33, 0.68) | 0.41 (0.23, 0.71) | 0.54 (0.33, 0.89) | ‐0.75 | 0.454 |
| TCS mild | TCS moderate | 0.96 (0.46, 2.01) | 1.54 (0.35, 6.86) | 0.82 (0.35, 1.92) | 0.72 | 0.473 |
| TCS moderate | TCS potent | 0.71 (0.39, 1.30) | 0.82 (0.39, 1.71) | 0.55 (0.2, 1.54) | 0.61 | 0.545 |
| TCS mild | tacrolimus 0.1% | 4.13 (2.41, 7.09) | 3.35 (1.26, 8.87) | 4.57 (2.37, 8.83) | ‐0.53 | 0.599 |
| TCS mild | TCS potent | 0.68 (0.37, 1.27) | 1 (0.05, 18.9) | 0.67 (0.36, 1.27) | 0.26 | 0.796 |
| TCS potent | placebo/vehicle | 2.88 (1.81, 4.59) | 3.05 (1.31, 7.07) | 2.8 (1.62, 4.85) | 0.17 | 0.867 |
| TCS potent | tacrolimus 0.03% | 4.28 (2.75, 6.67) | 4.45 (2.2, 9.01) | 4.17 (2.34, 7.42) | 0.14 | 0.885 |
| pimecrolimus 1% | tacrolimus 0.1% | 1.40 (0.93, 2.12) | 1.4 (0.66, 2.96) | 1.41 (0.85, 2.34) | ‐0.01 | 0.994 |
| TCS potent | azathioprine 4% and TCS potent | 1.00 (0.12, 8.44) | 1 (0.12, 8.44) | 8.32 (., .) | 0 | 0.997 |
| TCS potent | pimecrolimus 1% w TCS potent | 1.45 (0.21, 9.98) | 1.45 (0.21, 9.98) | 12.08 (., .) | 0 | 0.997 |
| ufenamate 5% | placebo/vehicle | 1.30 (0.27, 6.25) | 1.30 (0.27, 6.25) | 1.51 (., .) | 0 | 1 |
| suprofen 1% | ufenamate 5% | 0.66 (0.09, 4.61) | 0.66 (0.09, 4.61) | 0.91 (., .) | 0 | 1 |
.=inestimable
Abbreviations: CI: Confidence interval NMA: Network meta‐analysis TCS: Topical corticosteroids
Confidence in evidence evaluated using CINeMA was low or moderate for all licenced interventions except crisaborole 2%, which was judged high. The main reasons for CINeMA downgrades were within‐study bias and imprecision. Incoherence was a concern for very potent TCS. Heterogeneity was a concern for crisaborole 2% (although not sufficiently to downgrade), tacrolimus 0.03%, pimecrolimus 1%, moderate TCS and mild TCS. A funnel plot of comparisons with placebo showed no evidence of publication bias (Figure 22).
22.

Primary analysis ‐ local adverse events ‐ site events ‐ funnel plot of studies with placebo comparators and Egger's test results
Longer‐term data for this outcome over 6 to 12 months were insufficient for NMA, but were sufficient for a pairwise meta‐analysis of pimecrolimus 1% versus vehicle (Analysis 4.1), showing no evidence for a long‐term increase in application‐site reactions with pimecrolimus 1%.
4.1. Analysis.

Comparison 4: Application site reactions (binary) ‐ long‐term, Outcome 1: Pimecrolimus 1% versus vehicle
Subgroup and sensitivity analyses from NMA (short‐term)
Subgroup and sensitivity analyses did not identify important differences from the main NMA, and are summarised below.
NMA at class level (75; n = 17,316) ranked JAK inhibitors, TCI and PDE‐4 inhibitors as most likely to cause application‐site reactions and mild/moderate and potent/very potent TCS ranked least likely. There was no significant inconsistency between comparisons in this network. Potent/very potent TCS were less likely to cause application‐site reactions than potent TCI (OR 0.17, 95% CI 0.11, 0.25), mild TCI (OR 0.23, 95% CI 0.16, 0.35) and PDE‐4 inhibitors (OR 0.20, 95% CI 0.10, 0.39). Mild/moderate TCS were less likely to cause application‐site reactions than potent TCI (OR 0.24, 95% CI 0.15, 0.38) or mild TCI (OR 0.34, 95% CI 0.22, 0.51).
In a subgroup analysis for non‐facial use (16; n = 2456), very potent TCS, pimecrolimus 1% and tacrolimus 0.1% and 0.03% were ranked as most likely to cause application‐site reactions and mild, moderate, and potent TCS ranked as least likely. There was no significant inconsistency between comparisons in this network.
In people with non‐severe eczema (36; n = 6950), tacrolimus 0.1%, crisaborole 2% and pimecrolimus 1% were ranked as most likely to cause application‐site reactions and roflumilast 0.15%, mild TCS, moderate TCS, and potent TCS ranked as least likely. There was significant inconsistency in the comparison of potent TCS versus crisaborole 2%.
In people with severe eczema (28; n = 7466), tacrolimus 0.1% and 0.03%, and pimecrolimus 1% were ranked as most likely to cause application‐site reactions and mild, moderate, and potent TCS ranked as least likely. There was no significant inconsistency between comparisons in this network.
In people with eczema over the age of 12 years (38 trials; 8007 participants), tacrolimus 0.1% and 0.03%, and pimecrolimus 1% were ranked as most likely to cause application‐site reactions and mild, moderate, and potent TCS ranked as least likely. There was no significant inconsistency between comparisons in this network.
In a sensitivity analysis limited to outcomes at low risk of bias (20; n = 4939), tacrolimus 0.1% and 0.03%, crisaborole 2% and pimecrolimus 1% were ranked as most likely to cause application‐site reactions and roflumilast 0.15%, mild TCS, moderate TCS, and potent TCS ranked least likely. There was significant inconsistency in the comparison of tacrolimus 0.03% versus vehicle with direct evidence indicating increased risk with tacrolimus 0.03%, but indirect evidence was inconclusive.
In a sensitivity analysis using an alternative classification for TCS potency (79; n = 18,717), tacrolimus 0.1% and 0.03%, crisaborole 2% and pimecrolimus 1% were ranked as most likely to cause application‐site reactions and roflumilast 0.15% and all potencies of TCS ranked least likely. There was significant inconsistency in the comparison of very potent versus moderate TCS.
In a sensitivity analysis excluding within‐participant trials (63; n = 17,623), tacrolimus 0.1%, crisaborole 2%, tacrolimus 0.03% and pimecrolimus 1% were ranked as most likely to cause application‐site reactions and roflumilast 0.15%, mild TCS, moderate TCS, and potent TCS ranked least likely. There was no significant inconsistency between comparisons in this network.
In sensitivity analyses with alternative correlation coefficients of 0.25 or 0.75, (83; n = 18,956), tacrolimus 0.1% and 0.03%, crisaborole 2% and pimecrolimus 1% were ranked as most likely to cause application‐site reactions than vehicle, consistent with the primary analysis. All potencies of TCS ranked least likely.
Narrative information
Twenty‐three trials with at least 3118 participants did not have appropriate data for inclusion in NMA. Trials reported low rates of application‐site reactions with mild, moderate, potent and very potent TCS, and PDE‐4 inhibitors but increased application‐site reactions with tacrolimus 0.03%, 0.1%, and pimecrolimus 1% when compared with vehicle or with TCS.
Local adverse events: pigmentation changes
Network meta‐analysis
Primary NMA (short‐term) included 8 trials (n = 1786, 3 events) of TCS and a PDE‐4 inhibitor (Figure 23). NMA showed that none of the topical anti‐inflammatory treatments had a significant increase in the odds of pigmentation changes compared to vehicle (Figure 24). All CIs were wide, and direct evidence for potent TCS versus vehicle was limited to a single trial with fewer than 100 participants. For moderate TCS versus vehicle, the effect estimate was based on indirect evidence only (Table 7). There was no significant inconsistency between comparisons in this network and there were insufficient trials for assessment of publication bias (Table 18). Confidence in evidence evaluated using CINeMA was low for mild, moderate, and potent TCS, and moderate for crisaborole 2%.
23.
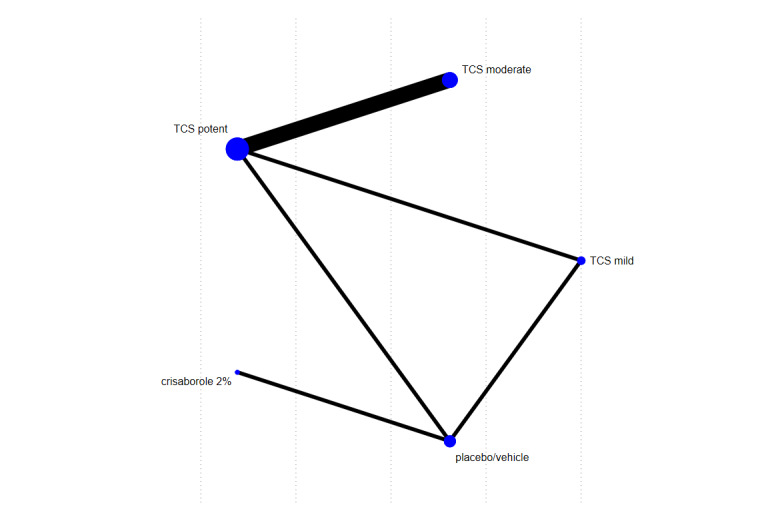
Local adverse events (pigmentation events) ‐ network map ‐ primary analysis
24.

Local adverse events (pigmentation) ‐ forest plot of NMA effects (exp(b) = OR) with placebo comparator
9. Local adverse events (pigmentation) NMA ‐ assessment of inconsistency.
| Treatment | Comparator | NMA effect (95% CI) | Direct effect (95% CI) | Indirect effect (95% CI) | Z value | P value |
| TCS mild | TCS potent | 1.34 (0.05, 34.57) | 1 (0.02, 54.47) | 2.37 (0.01, 633.4) | ‐0.25 | 0.806 |
| TCS mild | placebo/vehicle | 1.79 (0.07, 44.76) | 2.37 (0.05, 119.58) | 1 (0, 282.17) | 0.25 | 0.806 |
| TCS potent | placebo/vehicle | 1.34 (0.05, 34.28) | 1 (0.02, 53.66) | 2.36 (0.01, 639.5) | ‐0.25 | 0.806 |
| TCS moderate | TCS potent | 1.00 (0.17, 5.96) | 1 (0.17, 5.96) | 0.56 (0, 1.01x10269) | 0.00 | 0.999 |
Abbreviations: CI: Confidence interval NMA: Network meta‐analysis TCS: Topical corticosteroids
Subgroup and sensitivity analyses from NMA (short‐term)
Sensitivity analysis by class (8; n = 1786) differed from the primary analysis as mild and moderate TCS were collapsed into a single TCS mild/moderate node. The resulting NMA was consistent with the primary analysis, showing that none of the topical anti‐inflammatory treatments had a significant increase in the odds of pigmentation changes compared to vehicle, and there was no significant inconsistency. Sensitivity analysis by class showed all treatments had a comparable, and very low, risk of pigmentation changes.
Other subgroup and sensitivity analyses did not identify important differences from the main NMA. There were trivial numerical differences from the primary analysis in the subgroup analysis of studies in people with non‐severe eczema (8; n = 1768), as there were only 16 participants with severe eczema removed in the latter.
Narrative information
No narrative information for pigmentation changes was identified.
Local adverse events: skin thinning/atrophy
Network meta‐analysis
Primary NMA (short‐term) included 25 trials (n = 3691, 36 events) of TCS, TCI and a combination therapy (Figure 25). NMA showed that none of the topical anti‐inflammatory treatments had a significant increase in the odds of skin thinning compared to vehicle (Figure 26). NMA ranked mild TCS (OR 0.72, 95% CI 0.12, 4.31) and pimecrolimus 1% (OR 0.15, 95% CI 0.01, 1.59) as safest, with potent TCS (OR 0.96, 95% CI 0.21, 4.43) and moderate TCS (OR 0.91, 95% CI 0.16, 5.33) as least safe (Table 8). All CIs were wide, with direct evidence for moderate TCS, tacrolimus 0.1%, and pimecrolimus 1% limited to single trials with fewer than 100 participants or indirect evidence only. There was no significant inconsistency between comparisons in this network (Table 19). Confidence in evidence evaluated using CINeMA was low for all interventions due to within‐study bias and imprecision. There were insufficient trials for assessment of publication bias.
25.

Local adverse events (atrophy) ‐ network map
26.

Local adverse events (atrophy) ‐ forest plot of NMA effects (exp(b) = OR) with placebo comparator
10. Local adverse events (skin thinning) NMA ‐ assessment of inconsistency.
| Treatment | Comparator | NMA effect (95% CI) | Direct effect (95% CI) | Indirect effect (95% CI) | Z value | P value |
| TCS mild | pimecrolimus 1% | 0.20 (0.03, 1.53) | 0.11 (0.01, 1.04) | 2.22 (0.03, 184.93) | ‐1.19 | 0.23 |
| pimecrolimus 1% | placebo/vehicle | 6.89 (0.63, 75.38) | 1 (0.02, 52.98) | 20.72 (1.03, 414.88) | ‐1.19 | 0.23 |
| TCS mild | TCS potent | 1.34 (0.34, 5.36) | 1.91 (0.34, 10.81) | 0.72 (0.07, 7.2) | 0.67 | 0.50 |
| TCS potent | placebo/vehicle | 1.04 (0.23, 4.78) | 1.58 (0.19, 13.03) | 0.65 (0.07, 5.97) | 0.57 | 0.57 |
| TCS mild | placebo/vehicle | 1.39 (0.23, 8.37) | 2.37 (0.05, 119.58) | 1.21 (0.16, 9.1) | 0.30 | 0.77 |
| TCS mild | TCS moderate | 1.27 (0.26, 6.28) | 1.01 (0.06, 16.47) | 1.43 (0.2, 9.95) | ‐0.20 | 0.84 |
| TCS moderate | placebo/vehicle | 1.09 (0.19, 6.38) | 0.89 (0.02, 46.62) | 1.15 (0.16, 8.24) | ‐0.11 | 0.91 |
| TCS moderate | TCS potent | 1.05 (0.33, 3.31) | 1.02 (0.29, 3.66) | 1.18 (0.09, 16.28) | ‐0.10 | 0.92 |
| TCS potent | TCS very potent | 0.92 (0.16, 5.31) | 1.02 (0.02, 52.12) | 0.9 (0.13, 6.36) | 0.06 | 0.96 |
| TCS very potent | placebo/vehicle | 1.13 (0.40, 3.17) | 1.14 (0.39, 3.3) | 1 (0.01, 71.5) | 0.06 | 0.96 |
| TCS potent | pimecrolimus 1% w TCS potent | 2.91 (0.12, 71.77) | 2.91 (0.12, 71.77) | 3.13 (., .) | 0.00 | 1.00 |
| TCS very potent | tacrolimus 0.1% | 1.00 (0.02, 59.99) | 1 (0.02, 60) | 1.28 (., .) | 0.00 | 1.00 |
.=inestimable
Abbreviations: CI: Confidence interval NMA: Network meta‐analysis TCS: Topical corticosteroids
Longer‐term data over six to 60 months for this outcome were insufficient for NMA but were reported for TCS versus TCI in three studies (Analysis 5.1), showing an increase in long‐term skin thinning with TCS (6 events in 2044 participants with TCS vs. 0 events in 2025 participants with TCI; P = 0.031, Fisher's exact test). The three included trials evaluated potent TCS versus tacrolimus 0.1% over six months follow‐up (Reitamo 2005), moderate TCS versus pimecrolimus 1% over one year follow‐up (Luger 2004), and mild/moderate TCS versus pimecrolimus 1% over five years follow‐up (Sigurgeirsson 2015). The three trials were all funded by TCI manufacturers and included treatment of both facial and non‐facial areas affected by eczema. The study authors did not comment on the reversibility of the skin thinning changes identified, nor did they provide details about the location and nature of the changes identified.
5.1. Analysis.

Comparison 5: Skin thinning (binary) ‐ long‐term, Outcome 1: Topical corticosteroids versus topical calcineurin inhibitors
Subgroup and sensitivity analyses from NMA
Subgroup and sensitivity analyses did not identify important differences from the main NMA, and are summarised below.
NMA at class level (22; n = 3417) ranked a combination as most likely to cause skin thinning compared to vehicle, but this was based on indirect evidence only and with wide confidence intervals, which included no effect for all interventions. There was no significant inconsistency between comparisons in this network. The odds of skin thinning and related signs did not differ between potent/very potent TCS and potent TCI (OR 1.00, 95% CI 0.02, 59.99) or between mild/moderate TCS and mild TCI (OR 5.06, 95% CI 0.69, 37.27) but confidence intervals were wide.
In a subgroup analysis of studies in people with non‐severe eczema (8; n = 1853), pimecrolimus 1%, moderate TCS, mild TCS and potent TCS were not more likely than vehicle to cause skin thinning, but confidence intervals were wide. There was no significant inconsistency between comparisons in this network.
In a subgroup analysis of studies in people with severe eczema (13; n = 1608), very potent and potent TCS were ranked as most likely to cause skin thinning, with mild and moderate TCS ranked as least likely with wide CIs, which included no effect for all interventions. There was no significant inconsistency between comparisons in this network.
In a sensitivity analysis using an alternative classification for TCS potency (19; n = 3312), moderate and potent TCS were ranked as most likely to cause skin thinning and roflumilast 0.15%, pimecrolimus 1% and mild TCS ranked as least likely with wide confidence intervals which included no effect for all interventions. There was no significant inconsistency between comparisons in this network.
In a sensitivity analysis excluding within‐participant studies (19; n = 3547), very potent and potent TCS were ranked as most likely to cause skin thinning and pimecrolimus 1% and mild TCS ranked as least likely with wide confidence intervals which included no effect for all interventions. There was no significant inconsistency between comparisons in this network.
In sensitivity analyses with alternative correlation coefficients of 0.25 or 0.75, 25 trials (n = 3691), findings were similar to the primary analysis.
Narrative information
Two trials (n = 381) of moderate TCS versus vehicle reported short‐term outcome data which could not be included in the NMA. One case of telangiectasia was reported in a moderate TCS treatment arm.
Withdrawals due to adverse events
Network meta‐analysis
Primary NMA (short‐term) included 11 trials (n = 2404) of TCS, TCI, JAK inhibitors, and other interventions (Figure 27). NMA showed that none of the topical anti‐inflammatory treatments had a significant increase in the odds of withdrawal due to adverse effects of the intervention compared to vehicle (Figure 28). NMA ranked potent TCS (OR 0.39, 95% CI 0.04, 4.24) and mild TCS (OR 0.61, 95% CI 0.05, 7.94) as safest and the TCI tacrolimus 0.1% (OR 3.31, 95% CI 0.13, 84.33) and tacrolimus 0.03% (OR 1.92, 95% CI 0.13, 29.09) as least safe; however, CIs were wide and included no effect (Table 9). Direct evidence for all interventions was limited to single trials in fewer than 100 participants or indirect evidence only, except for moderate TCS and delgocitinib 0.25%. There was no significant inconsistency between comparisons in this network (Table 20).
27.

Withdrawal due to AE ‐ network map
28.
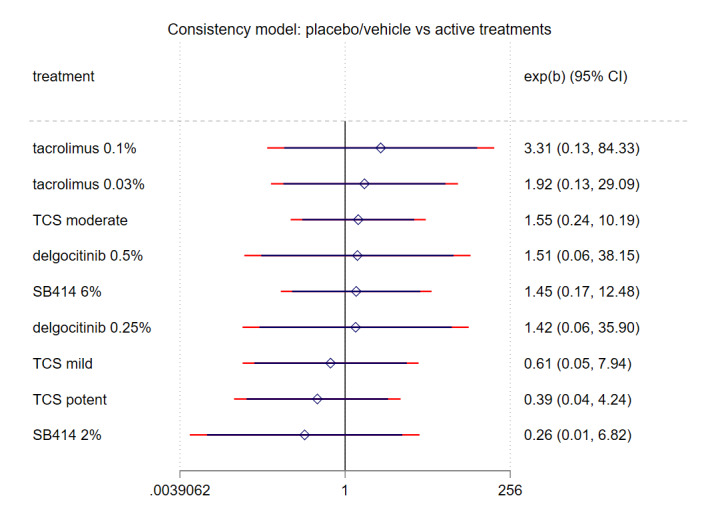
Withdrawal due to AE ‐ forest plot of NMA effects (exp(b) = OR) with placebo comparator
11. Withdrawal from treatment or trial due to adverse effect of intervention ‐ assessment of inconsistency.
| Treatment | Comparator | NMA effect (95% CI) | Direct effect (95% CI) | Indirect effect (95% CI) | Z value | P value |
| TCS mild | TCS moderate | 2.53 (0.22, 28.46) | 6.89 (0.35, 134.75) | 0.35 (0.01, 22.85) | 1.14 | 0.256 |
| TCS moderate | placebo/vehicle | 0.64 (0.10, 4.22) | 1.04 (0.13, 8.09) | 0.05 (0, 5.8) | 1.14 | 0.256 |
| TCS potent | placebo/vehicle | 2.54 (0.24, 27.33) | 1 (0.06, 17.62) | 19.42 (0.28, 1346.21) | ‐1.14 | 0.256 |
| TCS mild | TCS potent | 0.64 (0.09, 4.72) | 0.32 (0.01, 8.24) | 0.98 (0.08, 12.34) | ‐0.53 | 0.597 |
| TCS mild | tacrolimus 0.03% | 3.12 (0.51, 19.26) | 2.5 (0.29, 21.54) | 5.43 (0.18, 163.85) | ‐0.38 | 0.706 |
| TCS potent | tacrolimus 0.03% | 4.87 (0.79, 29.81) | 6.05 (0.71, 51.38) | 2.79 (0.09, 84.86) | 0.38 | 0.706 |
| delgocitinib 0.25% | tacrolimus 0.1% | 2.32 (0.23, 23.25) | 2.69 (0.13, 55.35) | 3.74 (0, 1.78x1088) | 0.00 | 0.997 |
| delgocitinib 0.25% | placebo/vehicle | 0.70 (0.03, 17.72) | 0.41 (0.01, 11.92) | 0.48 (0, 7.41x10100) | 0.00 | 0.999 |
Abbreviations: CI: Confidence interval NMA: Network meta‐analysis TCS: Topical corticosteroids
Confidence in evidence evaluated using CINeMA was low for all licenced interventions in the network. The main reasons for CINeMA downgrades were within‐study bias and imprecision. Indirectness was also a concern for potent TCS. A funnel plot of comparisons with placebo showed no evidence of small study effects or publication bias (Figure 29).
29.

Funnel plot of studies with placebo comparator and Eggers test results
Subgroup and sensitivity analyses from NMA (short‐term)
Subgroup and sensitivity analyses did not identify important differences from the main NMA, and are summarised below.
NMA at class level (11; n = 2404) ranked mild and potent TCI as most likely to cause withdrawal and potent/very potent TCS ranked least likely, with wide confidence intervals which included no effect. There was no significant inconsistency between comparisons in this network. Potent/very potent TCS were associated with lower risk of withdrawal than mild TCI (OR 0.18, 95% CI 0.03, 1.07) and mild/moderate TCS were also associated with lower risk of withdrawal than mild TCI (OR 0.37, 95% CI 0.06, 2.19) but with wide confidence intervals which included no effect.
In a sensitivity analysis using an alternative classification for TCS potency (11; n = 2404), tacrolimus 0.1%, tacrolimus 0.03%, and pimecrolimus 1% were ranked as most likely to cause withdrawal and potent and moderate TCS ranked least likely with wide confidence intervals which included no effect. There was no significant inconsistency between comparisons in this network.
In a sensitivity analysis excluding within‐participant studies (9; n = 2278), tacrolimus 0.1%, delgocitinib 0.5%, and delgocitinib 0.25% were ranked as most likely to cause withdrawal and mild and potent TCS ranked least likely with wide confidence intervals which included no effect. There was no significant inconsistency between comparisons in this network.
In sensitivity analyses with alternative correlation coefficients of 0.25 or 0.75, (11; n = 2404), the findings were consistent with the primary analysis and there were no significant inconsistencies for comparisons in this network.
Narrative information
Six trials (n = 1484) did not have appropriate data for inclusion in NMA or pairwise meta‐analysis. Trials reported low rates of withdrawal due to adverse effects of treatment for mild TCS, pimecrolimus 1%, tacrolimus 0.03%, and tacrolimus 0.1%.
Discussion
Summary of main results
This Cochrane review compares topical anti‐inflammatory treatments for eczema. A total of 291 trials and 45,846 participants with eczema were included. The largest NMA synthesising findings on IGA included data from 140 trials (n = 23,383). We identified that treatments from five different classes had the most trial evidence: TCS (n = 172), TCI (n = 134), PDE‐4 inhibitors (n = 55), JAK inhibitors (n = 30), and AhR activators (n = 10). Experimental therapies were also included but our 'Summary of findings' tables focus on treatments that are licenced for use in people with inflammatory skin conditions. The NMA evidence addresses two questions that are relevant for people with eczema, their carers and healthcare practitioners.
1. Which topical anti‐inflammatories are the most effective for treating eczema?
For efficacy, we included two domains from the HOME Core Outcome Set: patient‐reported symptoms and clinician‐reported signs. We also included a binary outcome, IGA, as this enabled us to include a large volume of data from older trials of commonly‐used topical anti‐inflammatory treatments, and is required by key regulators. We considered binary and continuous measurements separately. Potent TCS, JAK inhibitors and tacrolimus 0.1% were consistently ranked as amongst the most effective topical anti‐inflammatory treatments for eczema and PDE‐4 inhibitors as amongst the least effective. Mild TCS and tapinarof 1% were ranked amongst the least effective treatments in three of five efficacy networks. Confidence of findings rated using CiNEMA was low to moderate.
Results from NMAs for different efficacy measures were largely consistent but, for NMA of continuous outcomes (clinician‐reported signs and patient‐reported symptoms), tacrolimus 0.03% and mild TCS were ranked amongst the most effective treatments. This is counter‐intuitive, as these interventions are widely believed to be less effective than more potent interventions in the same class (i.e. tacrolimus 0.1% and potent TCS). We identified a single, small study at high risk of bias which accounted for the high ranking of tacrolimus 0.03% (Sikder 2005) in clinician‐reported signs, but did not appear to account for the high ranking in patient‐reported symptoms. We also note that continuous outcome meta‐analysis can more easily detect potential small study effects than binary outcome measure meta‐analysis (Lin 2020). For continuous efficacy outcomes, only 33 to 40% of trials with relevant information could be included in NMA, due to reporting issues where variance or numbers evaluated were not clearly stated. This compares with 82 to 91% for binary efficacy outcomes, meaning that there was less comprehensive incorporation of available evidence in the continuous outcome networks. In the narrative information for both clinician‐reported signs and patient‐reported symptoms, continuous outcomes accounted for the majority of available trials and trial participants reporting these outcomes. For both continuous outcomes, narrative information suggests that mild TCS and tacrolimus 0.03% are less effective than potent TCS or tacrolimus 0.1%.
There was insufficient evidence to conduct NMA for longer‐term efficacy outcomes (> 16 weeks after treatment initiation).
2. Which topical anti‐inflammatories are the safest for treating eczema?
Local application‐site reactions, pigmentation changes and skin thinning/atrophy were identified as important concerns during patient and public engagement work for this project. NMA identified an increased risk of application‐site reactions (e.g. burning/stinging) relative to vehicle with tacrolimus 0.1% (moderate confidence), crisaborole 2% (high confidence), tacrolimus 0.03% (low confidence), and pimecrolimus 1% (low confidence). For tacrolimus and pimecrolimus, both TCI, burning/stinging is a well‐recognised adverse effect and is often included in information for people with eczema and their carers. Local site reactions may reduce over time, and cooling the medication has been recommended to mitigate this adverse effect (Cury Martins 2015; Pena 2023). Local application‐site reactions were less common with mild, moderate and potent TCS (all moderate confidence) or very potent TCS (low confidence) than vehicle.
Pigmentation changes can mean hypopigmentation (lighter areas of skin) or hyperpigmentation (darker areas of skin) and are a common consequence of eczematous inflammation. NMA did not identify any evidence for increased hypo‐ or hyperpigmentation with mild, moderate or potent TCS (low confidence) or crisaborole 2% (moderate confidence).
Skin thinning is characterised clinically by translucent skin with prominent vasculature (Charman 2003). NMA did not identify any increased risk of skin thinning with mild, moderate, potent or very potent TCS (all low confidence) relative to vehicle during short‐term use (median of 3 weeks). However, when compared to TCI, longer‐term use of mild to potent TCS, over six months to five years, was associated with skin thinning in approximately 1 in 300 people with eczema.
Overall completeness and applicability of evidence
Overall, the results are applicable to people in higher‐income countries: currently available interventions and report short‐term effects on symptoms, signs and local adverse reactions. More studies are needed of people in low or middle‐income countries, reporting long‐term efficacy and safety outcomes.
Participants
Our findings are predominantly based on trials conducted in higher‐income countries. Race or ethnicity was only reported in one‐third of studies; however, when it was recorded, a range of racial/ethnic groups were represented in the trials. Where eczema severity was reported, most trials included people with eczema that was not severe, or with a range of eczema severity. This reflects the general population where most people have mild eczema. Age was reported in most trials, but only about one in ten trials focussed on participants less than 12 years. Eczema prevalence peaks in the first two years of life, often remitting in early childhood, so the low number of paediatric studies is an under‐representation of young children in eczema treatment trials (De Lusignan 2021).
Interventions and comparators
We identified a large number of trials of TCS and TCI, with fewer clinical trials of PDE‐4 inhibitors, JAK inhibitors and tapinarof. This reflects the currently available treatments and the treatments most commonly used to gain control of eczema flares. As some TCS evaluated are no longer marketed, and the availability of individual TCS products differs internationally, we grouped TCS by potency to consolidate nodes and improve generalisability of the findings. There was a relative lack of active comparator trials, especially for pimecrolimus, where there were over 20 trials comparing pimecrolimus 1% to vehicle without another active comparator arm. This is consistent with previous literature highlighting the lack of active comparator trials in topical eczema treatments, and was one of the reasons for conducting this NMA (Wilkes 2016).
Efficacy outcomes
Trials more often reported clinician‐evaluated signs of eczema than patient‐reported symptoms of eczema, and the most commonly reported clinician‐evaluated sign was the IGA. Consistent with a previous systematic review of IGA (Futamura 2016), the scales and construct of reported IGA outcomes varied. We anticipated this variation, so developed a hierarchy of preference for extracting IGA data in our study protocol, such that most IGA data extracted in this review represented 'clear/almost clear' on a 5 or 6 point IGA scale. Trials should focus on patient‐important outcomes such as patient‐reported symptoms, in order to give consumers and healthcare practitioners confidence that treatments are impacting on symptoms. Less than 10% of trials measuring patient‐reported symptoms reported POEM or NRS‐11, which are the HOME‐recommended instruments in this domain. Whilst it is encouraging that 44% of trials had some data on patient‐reported symptoms, trials often relied on a 10‐point visual analogue scale of pruritus. Future trials should ensure that the validated, HOME‐recommended instruments are used for measuring patient‐reported symptoms. Half of trials measuring clinician‐reported signs reported the HOME‐recommended instrument EASI, indicating the now widespread use of validated instruments in this domain. The HOME Core Outcome Set also recommends measuring quality of life and long‐term control of eczema. However, only 8% of trials reported data on quality of life and long‐term control was only reported in two trials, neither using the HOME‐recommended instruments RECAP nor ADCT (Williams 2022). This reflects the relatively new addition of these outcome domains to HOME, and hopefully subsequent trials will measure and report these more consistently.
Most of the included trials were short term and NMA was not possible for longer‐term outcomes. While anti‐inflammatories are frequently used to get control of eczema flares, eczema is often a long‐term condition and long‐term outcomes from intermittent use of anti‐inflammatories are of high interest to people affected by eczema.
Safety outcomes
Adverse events of interest to consumers were not clearly reported in most trials. Application‐site reactions were reported in over one‐third of trials, but pigmentation changes attributed to interventions were reported in only eight trials, and skin thinning in less than 10% of trials. Long‐term safety, and local adverse effects related to facial application of topical anti‐inflammatories were rarely reported. Although study withdrawal was often reported, withdrawal from treatment or study due to an adverse effect of a study intervention was only reported in 17 studies.
Quality of the evidence
CINeMA: Within‐study bias
Almost all information was rated down for within‐study bias, due to significant risk of bias issues in most trial outcomes. The main within‐study bias issues identified were unclear description of allocation concealment; lack of pre‐trial registration or publicly available trial protocol; and reporting issues around participants excluded from analysis or unavailable due to missing outcome data. While some of these issues were more common in older studies, where guidance for reporting clinical trials was less well‐developed, the prevalence of reporting issues in modern trials continues to be high.
CINeMA: Reporting bias
We did not identify significant evidence of study‐level reporting bias through assessment of potential small study effects using funnel plot and Egger's test assessments, or through evaluation of clinical trial registrations listed under ongoing studies. No downgrades were therefore made for this CINeMA domain.
CINeMA: Indirectness
There were concerns about indirectness for binary patient‐reported symptoms (mild TCS), continuous clinician signs (crisaborole) and for pigmentation changes (moderate and potent TCS) but no other downgrades were made for this CINeMA domain. Where most evidence contributing to an effect estimate was derived from within‐participant trials, evidence was downgraded for indirectness.
CINeMA: Imprecision
Downgrading for imprecision was made when the 95% confidence intervals extended beyond the range of equivalence on both sides of the no effect line, so that the estimate was compatible with clinically important effects in both directions. Downgrades for imprecision were common, especially for patient‐reported symptoms, pigmentation changes, atrophy and withdrawal outcomes, where numbers of trials and participants were relatively sparse, leading to wide confidence intervals. Ranges of equivalence were defined using published minimally clinically important differences for specific instruments such as EASI (6.6) and POEM (3.4) (Schram 2012) and OR 0.8 to 1.2 for binary outcomes.
CINeMA: Heterogeneity
Downgrades for heterogeneity were made for at least one intervention in most networks, but were especially common in the patient‐reported symptoms and application‐site reactions networks. These downgrades were made when confidence intervals and prediction intervals led to different conclusions with an important impact on decision‐making.
CINeMA: Incoherence
We used both global (design by treatment interaction) and local (node‐splitting) approaches to evaluate incoherence, the agreement between direct and indirect evidence informing network effect estimates. Downgrades for incoherence were not commonly made, and were most common in the two continuous outcome efficacy networks ‐ patient‐reported symptoms and clinician‐reported signs.
Potential biases in the review process
This was a comprehensive Cochrane systematic review with NMA of topical anti‐inflammatory treatments for eczema. Although the search strategy was comprehensive and was cross‐checked with another recent NMA on this topic (Chu 2023) for completeness, we did not conduct a separate search for adverse effects and the review was limited to randomised controlled trials. Systematic reviews of randomised controlled trials may not be the best way to identify adverse effects of interventions, and observational studies including pharmacovigilance databases have an important role. We also did not contact authors/sponsors of trials to request original data or query methodological details, due to the large number of trials conducted over several decades and the resource limitations of the project team.
Although we extracted data for all concentrations of all anti‐inflammatory treatments, we did not use unlicenced concentrations in analyses, where data for a licenced concentration was available. We also did not evaluate topical treatments without a well‐characterised mechanism of action, and we excluded topical anti‐inflammatory treatments which have either been evaluated elsewhere and found not to be effective for managing eczema (Matterne 2019) or have anti‐inflammatory mechanisms which are not likely to be relevant to the inflammation associated with eczema. Such exclusions may, or may not, importantly influence all effect estimates in each network, and evidence certainty.
As with all systematic reviews, we were constrained by the availability and reporting quality of clinical trials in this field. Concerns about selective reporting were a common issue, highlighted in the risk of bias assessments, and an absence of relevant outcome information often limited opportunities for meta‐analysis. This was most notable for the continuous outcomes, where the majority of trials and participants could not be included in NMA. We did not access clinical study reports from regulators such as the US Food and Drug Administration, which can be a useful source of safety data. While a significant proportion of studies reported EASI scores, core outcome measures recommended by HOME were not commonly reported in other domains. This meant we had to rely on pooled assessments of heterogeneous measures of similar outcomes for primary meta‐analyses. In general, long‐term outcomes, which are of most interest to patients and their carers, were rarely reported. No NMA was possible in this systematic review for long‐term outcomes. The limited longer‐term safety data available for TCS treatments has also been discussed elsewhere (Harvey 2023). The lack of comprehensive long‐term outcome data limits any conclusions that can be drawn about the effectiveness or safety of topical anti‐inflammatory treatments for eczema.
NMA relied on an assumption of transitivity, and it is possible that we failed to identify relevant impacts of transitivity because eczema severity was not reported consistently across studies and a range of eczema severities was included in the trials, from mild to very severe.
Finally, data extraction and risk of bias assessment were not undertaken in duplicate, although all primary data extractions and risk of bias assessments were thoroughly reviewed, checked and revised, where necessary, by a second reviewer. Optimal practice is to have full duplicate data extraction and risk of bias assessments, which we were unable to do due to resource limitations.
Agreements and disagreements with other studies or reviews
Comparison with other NMAs
A previous network meta‐analysis of topical treatments for eczema included evaluation of the safety and effectiveness of topical anti‐inflammatory treatments (Chu 2023). Key similarities and differences between the previous NMA and our work are summarised in Table 21. Search strategies were similar, but there were differences in inclusion criteria, approaches to data extraction and pooling, risk of bias assessment, rating the certainty of evidence, and approaches to summarising overall results. This has led to differences in effectiveness rankings and classification, although both reviews concluded that TCS and potent TCI are amongst the most effective treatments. We also focussed on different safety outcomes in the previous review. For example, local application‐site reactions were prioritised in this review, and overall adverse event rates and infections were prioritised in Chu 2023. This difference is explained by differences in the outcomes of each group's patient and public involvement work. Differences in patient and public priorities for safety may reflect cultural differences between populations surveyed, or differences in awareness of potential issues associated with commonly‐used topical eczema treatments in different settings. The safety outcomes that we focussed on have been highlighted as common concerns in relation to TCS (Li 2017), namely site reactions, pigmentation changes, and skin atrophy. Chu 2023 addressed concerns which are more commonly associated with TCI and JAK inhibitors, such as infection and cancer risk (Agarwal 2022; Chu 2023; Devasenapathy 2023). Of note, Chu 2023 did not explicitly address our prioritised safety outcomes, but they were subsequently addressed during the preparation of a linked guideline (AAAAI/ACAAI Atopic Dermatitis Guideline Panel 2024).
12. Comparison of approach and findings with existing eczema topicals NMA.
| Attribute | Chu 2023 | Our review |
| Inclusion criteria | ||
| Study designs | Excluded within‐participant designs | Included within‐participant designs with sensitivity analyses excluding them |
| Populations | People of any age with eczema of any severity | People of any age with eczema of any severity |
| Included treatments/comparators | Included well‐characterised anti‐inflammatory treatments, antimicrobials, prescription moisturisers, complementary therapies, and novel agents, applied at any dose and any frequency | Included well‐characterised anti‐inflammatory treatments at licensed doses, but excluded antibiotics, moisturisers and complementary therapies |
| TCS classification | 7‐point US classification, with sensitivity analyses using a World Health Organization 4‐class system and a 3‐class system | Classification based on United Kingdom British National Formulary and World Health Organization classifications (Lax 2022), with sensitivity analysis using a 4‐class adaptation of the US classification (Bowie 2022) |
| Treatment regimens included | Included trials inducing remission (use of interventions to control active eczema at randomisation) and trials maintaining remission (use of interventions to prevent future eczema flares after gaining initial control). These two groups of trials were analysed separately. | Restricted to trials with a randomised induction of remission phase, some of which also included longer‐term evaluation of a maintenance‐only phase. Excluded trials of maintenance interventions without an induction of remission phase |
| Outcomes evaluated | ||
| Efficacy outcomes | Guided by the HOME Core Outcome Set and formal guideline prioritisation setting including patient and caregiver partners, where SCORAD was selected as the primary measure for clinician‐reported signs, due to volume of data and measurement properties in mild eczema (Chopra 2017), with the HOME‐recommended instrument EASI as sensitivity analysis. Additional outcomes included itch, sleep disturbance, and eczema flares. | Guided by the HOME Core Outcome Set with the addition of Investigator Global Assessment due to volume of data |
| Safety outcomes | Adverse events evaluated included those leading to discontinuation, and skin infections, which were prioritised through patient and public involvement and literature review. | Specific local adverse effects prioritised through patient and public involvement (application‐site reactions, pigmentation changes and skin thinning) and withdrawal from either treatment or trial due to adverse effects which were clearly attributed to the intervention |
| Methodology | ||
| Analytical philosophy | Bayesian with frequentist sensitivity analysis | Frequentist |
| Approach to pooling | • Different concentrations of same drug pooled for analysis, if there was no evidence of an interaction by concentration. Sensitivity analysis undertaken using separate concentrations • Continuous and binary measures of the same outcome pooled together, with sensitivity analysis excluding binary measures • Data obtained using different continuous and binary instruments were transformed to a measure familiar to clinicians (usually HOME recommended instrument) with sensitivity analysis evaluating alternative summary measures such as standardised mean difference. • Patient‐reported symptoms, sleep disturbance, and itch analysed separately |
• Different concentrations of same drug analysed separately, with summary of findings tables focussed on licenced concentrations • Continuous and binary data analysed separately and independent of each other • Data obtained using different continuous outcome instruments pooled as standardised mean difference and transformed to a HOME recommended instrument for interpretation. Binary outcome data pooled separately, using similar cut‐offs across studies • Patient‐reported symptoms and itch analysed together |
| Assessment of risk of bias | RoB 2.0 | RoB 2.0 |
| Assessment of certainty | GRADE | CiNEMA |
| Approach to summarising findings | Ranking considered point estimates, confidence/credible intervals, and certainty of evidence for each treatment compared to all other treatments, using a minimally contextualised framework (Brignardello‐Petersen 2020). | Ranking using SUCRA, point estimates and confidence/prediction intervals for comparison with vehicle, supported by CiNEMA confidence ratings and active comparison estimates |
| Findings | ||
| Efficacy outcomes | · Pimecrolimus, tacrolimus, and moderate‐potency TCS ranked amongst the most effective · TCS class 1 and 2 (mainly potent/very potent) and tacrolimus 0.1% ranked amongst the most effective for improving eczema signs and itch · PDE‐4 inhibitors ranked as intermediate effectiveness for improving eczema signs, itch, and eczema‐related quality of life |
· Potent TCS, JAK inhibitors and tacrolimus 0.1% ranked as most effective · PDE‐4 inhibitors ranked amongst the least effective |
| Safety outcomes | · Little to no difference in overall adverse events with topical JAK inhibitors, pimecrolimus, and tacrolimus · Moderate‐certainty evidence for mid‐potency TCS as amongst the best in reducing adverse events · Very potent TCS and JAK inhibitors ranked amongst the least likely treatments for discontinuation due to adverse events; TCI and other TCS had intermediate rankings. · Skin thinning was not evaluated in this NMA, but was assessed separately for the linked guideline AAAAI/ACAAI Atopic Dermatitis Guideline Panel 2024 where no important increase in skin thinning with TCS was seen. |
· TCI and crisaborole 2% ranked most likely to cause local application‐site reactions · TCS ranked least likely to cause local application‐site reactions · Potent and mild TCS ranked best in reducing withdrawal due to adverse events and tacrolimus 0.1% and 0.03% were most likely to be associated with withdrawal · No evidence for increased skin thinning with short‐term TCS but small skin thinning risk with long‐term TCS |
| Risk of bias | Domains 1 and 5 of RoB 2 were usually rated as 'low risk'. Missing outcome data were the most common risk of bias issues identified, but subgroup analysis and network meta‐regression showed no credible difference between trials at high versus low risk of bias. | Domains 1 and 5 of RoB 2 were usually rated as 'some concerns'. Selective reporting was the most common risk of bias issue identified, and most studies and outcomes were rated as being at high risk of bias |
| Conflicts of interest | ||
| Project funding | American Academy of Allergy, Asthma & Immunology (AAAAI) and American College of Allergy, Asthma, and Immunology funded this work. The funders declared financial support from pharmaceutical companies who market topical anti‐inflammatory treatments for eczema. The funders contributed to defining the scope of the review, but had no role in the study design, data collection, data analysis, data interpretation, manuscipt drafting or decision to submit for publication. The funders were provided with a copy of the report at the time of submission and were not able to prevent or delay its publication. The independent review team had the ability, but not the obligation, to consider the funders’ feedback. | United Kingdom National Institute for Health and Care Research funded this work. The funder peer reviewed and supported the project but had no role in the design, conduct, interpretation or publication of the report. |
| Author disclosures | Out of 40 authors, 29 (72%) did not have relevant conflicts of interest (including the corresponding and first authors), and 11 authors disclosed relevant conflicts of interest, which related to companies that manufacture or import pimecrolimus, tacrolimus, topical steroids, topical JAK inhibitors, or antibiotics. These 11 authors with potential conflicts provided clinical input but did not participate in any data collection or formal analysis. The non‐conflicted independent academic review group led the systematic review and analyses. Steps were taken to avoid including conflicted authors in decision‐making related to products relevant to their conflicts of interest. | Authors adhered to the Cochrane conflicts of interest policy which requires that at least two‐thirds of authors, the corresponding author, the first author and the funder have no relevant conflict of interest in the three years prior to the project or planned for the year following completion of the project. All authors declared no conflict of interest with companies that manufacture, import or market topical anti‐inflammatory treatments or potential competitor treatments. |
Abbreviations: CINEMA: Confidence in network meta‐analysis EASI: Eczema area and severity index HOME: Harmonising outcome measures for eczema JAK: Janus kinase NMA: Network meta‐analysis PDE‐4: Phosphodiesterase 4 SCORAD: Scoring atopic dermatitis
SUCRA: Surface under the cumulative ranking curve TCI: Topical calcineurin inhibitor TCS: Topical corticosteroid
Both reviews found high‐potency TCS and tacrolimus 0.1% were likely to be amongst the most effective short‐term treatments for improving eczema severity. Chu 2023 also included studies of topical anti‐inflammatory treatments for maintaining control of eczema, without an initial randomised daily treatment regimen. They found that moderate potency TCS and TCI were the most studied and amongst the most effective treatments for maintaining control of eczema.
Pimecrolimus 1% was either mid‐ranked or amongst the least effective treatments in our review, and was similarly classified in Chu 2023 for inducing remission. The short‐term outcome data from both reviews is in agreement that pimecrolimus 1% is a relatively weak topical anti‐inflammatory treatment for induction of remission in people with eczema. Systemic calcineurin inhibitors carry safety concerns around malignancy, especially lymphoma. Malignancy and lymphoma risk associated with TCI were not evaluated in Chu 2023, but have been evaluated elsewhere (Devasenapathy 2023).
We found evidence that JAK inhibitors may be amongst the most effective short‐term treatments for eczema, with low‐to‐moderate confidence. Chu 2023 found moderate‐to‐high‐certainty evidence for topical ruxolitinib and topical delgocitinib JAK inhibitors to have intermediate effectiveness for clinician‐evaluated eczema signs and patient‐reported itch. This small difference in ranking is likely to relate to differences in analytical approaches and the totality of the evidence included in the networks. For example, we limited analyses to licenced potencies of drugs and Chu 2023 pooled different potencies of the same drug if they showed similar treatment effects. The treatment arms with lower potencies of ruxolitinib in general showed slightly lower treatment effects than the 1.5% concentration in the ruxolitinib trials (NCT03745638; NCT03745651; Raoof 2020). A second potential explanation for our slightly higher ranking of ruxolitinib is the potency classification of triamcinolone 0.1% cream, used as an active comparator up to four weeks in Raoof 2020. Triamcinolone 0.1% cream was classified as potent in our primary analysis, but is classified as lower potency in the classifications used by Chu 2023. Triamcinolone 0.1% cream is classified as lower mid‐strength (5) in the 7‐point United States classification system and moderate in the shorter four‐point United States classification. By classifying triamcinolone 0.1% cream as potent, we may have spuriously increased the relative ranking of ruxolitinib, since other TCS classified as potent TCS in the networks may be more potent than triamcinolone 0.1% cream. However, sensitivity analyses using the four‐point US system showed a similar ranking of ruxolitinib as in the primary analysis. Similarly, a sensitivity analysis using four‐week outcome data from Raoof 2020 in place of eight‐week outcome data for these outcomes did not materially change the rankings. This was undertaken because there was a difference in treatment duration between interventions in Raoof 2020, with eight weeks' treatment for ruxolitinib but only four weeks' treatment for triamcinolone. Finally, the two reviews had different approaches to inclusion of data in NMA. We were careful not to include data in NMA unless the denominators were clearly stated, due to the risk of exaggerating selectively reported data by imputing denominators. This may partly explain our inclusion of a lower proportion of the potentially eligible outcome data in NMA compared with Chu 2023, especially for continuous outcome measures. However, we undertook sensitivity analyses imputing missing denominators and both these and the summarised narrative data were consistent with the primary NMA findings. Chu 2023 included a higher proportion of eligible studies and participants in NMA, and also combined continuous and binary measures of the same outcome in each NMA, resulting in larger NMA networks for each comparable outcome. Taken together, these differences are likely to explain the small difference in ruxolitinib rankings between the two NMAs, for comparable outcomes.
TCS had good safety profiles in both reviews, with JAK inhibitors also ranked as likely to reduce discontinuation due to adverse events amongst the short‐term studies in Chu 2023. Systemic JAK inhibitors carry safety concerns around increased risk of serious infection, thrombosis or cytopenias, and overall mortality, which were not evaluated in our review (Samuel 2023). Differences in other safety outcomes are accounted for by a focus on different outcome measures of safety between the two reviews, in turn accounted for by different patient and caregiver priorities identified by each review group. Currently, there are no agreed core outcome measures for adverse events in eczema trials.
Overall, it is reassuring that despite potentially important differences in inclusion criteria, analytical approach and outcome priorities, most findings are consistent between these first two NMAs of topical anti‐inflammatory treatments for eczema.
Comparison with other pairwise meta‐analyses
The increased effectiveness of potent TCS relative to mild TCS has been shown in a previous review comparing different strategies for using TCS to treat eczema; however, there was insufficient evidence that potent TCS were any more or less effective than moderate or very potent TCS, respectively (Lax 2022). The low rate of skin thinning with longer‐term TCS found in the present NMA also confirms the findings of Lax 2022 and a systematic review of the long term safety of topical corticosteroids (Harvey 2023). While skin thinning is a well‐known adverse effect of prolonged, inappropriate/continuous TCS use, the evidence from clinical trials suggests that skin thinning is rare. None of the trials commented on the location or reversibility of identified cases of skin thinning.
In our NMA, the TCI tacrolimus 0.1% ranked favourably across patient‐reported and clinician‐reported outcomes, especially the binary data analyses, with comparable effectiveness to potent TCS relative to vehicle. The findings for pimecrolimus 1% and tacrolimus 0.03% suggest lower effectiveness than potent TCS but similar to mild/moderate TCS. These findings are consistent with a previous systematic review which reported similar effectiveness between TCI and TCS (Broeders 2016). We found TCI were most likely to cause application‐site reactions but did not increase skin thinning or withdrawal rates, which is also consistent with the findings of Broeders 2016.
Two previous systematic reviews reported that topical JAK inhibitors were associated with significant improvements in effectiveness relative to placebo (Arora 2020; Tsai 2021). Tsai and colleagues also found that JAK inhibitors were associated with a significant increase in the number of treatment‐emergent adverse events and adverse events leading to discontinuation. However, the nature of these events was not reported, so we cannot compare those findings to our data focussed on adverse events of particular interest to patients.
A systematic review of the effectiveness and safety of PDE‐4 inhibitors showed that, in mild‐to‐moderate eczema, they had favourable effectiveness compared to vehicle (Yang 2019). In considering the relative effectiveness of all topical anti‐inflammatories for eczema, this NMA ranked PDE‐4 inhibitors, such as crisaborole 2%, as amongst the least effective treatments across outcomes, and less favourable than more conventional treatments such as potent TCS. Whilst there was no difference in withdrawals due to adverse events between PDE‐4 inhibitors and vehicle, there were significantly more treatment‐related events overall, most commonly application‐site reactions. Yang and colleagues concluded that PDE‐4 inhibitors are much safer than TCS and TCI for the treatment of eczema; in contrast, our review found TCI and crisaborole increase application‐site reactions, whereas TCS do not. A second systematic review of the safety of PDE‐4 inhibitors evaluated treatment‐emergent adverse events overall, application‐site pain, application‐site pruritus, and exacerbation of eczema (Martín‐Santiago 2022). PDE‐4 inhibitors did not significantly differ from vehicle for any outcome. The difference in findings may be explained by our approach of evaluating specific treatment‐related local adverse events of interest to patients, taking care to avoid counting events that were not directly related to the intervention.
To our knowledge, there have been no pairwise systematic reviews of tapinarof for people with eczema.
Comparison with guideline recommendations
Our findings are largely in agreement with current guidelines for eczema treatment, when recognising that topical JAK inhibitors are not yet approved for use in Europe. European Guidelines recommend TCS and TCI (Wollenberg 2022); Japanese guidelines recommend TCS, topical tacrolimus, and topical JAK inhibitor (delgocitinib 0.25‐0.5%) (Saeki 2022); and one recent US guideline (AAAAI/ACAAI Atopic Dermatitis Guideline Panel 2024) recommends TCS and TCI. The recent US guideline also conditionally recommends against ruxolitinib 1.5% as first‐line therapy, partly due to low‐certainty class‐level safety concerns, and conditionally recommends crisaborole 2% for mild/moderate eczema for those who prefer not to use TCS or TCI (AAAAI/ACAAI Atopic Dermatitis Guideline Panel 2024). Another recent US guideline, Sidbury 2023, strongly recommends TCS, TCI, topical JAK inhibitors and PDE‐4 inhibitors for managing eczema. In both our NMA and the previous NMA, PDE‐4 inhibitors had a lower efficacy than TCS, TCI, and topical JAK inhibitors.
Authors' conclusions
Implications for practice.
Our Cochrane review identified, with low‐to‐moderate confidence, that the most efficacious topical anti‐inflammatory treatments for eczema are potent and very potent TCS, potent TCI (tacrolimus 0.1%), and a topical JAK inhibitor (ruxolitinib 1.5%).
The choice of topical treatment for eczema involves many nuances specific to individual cases, including body site and severity, and thus our NMA cannot universally recommend a single treatment. Important decision‐making considerations that we did not evaluate in this review include patient and carer preferences, and cost. As mentioned in Description of the intervention, there is a huge difference in cost between TCS and the newer, more expensive topical anti‐inflammatory treatments (Table 10). For example, the cost of TCI is more than ten times greater than potent topical corticosteroids in the UK and the US. Other considerations that might also be important for clinical decision‐making include anatomical site, patient age, and eczema severity and chronicity. For example, the small risk of skin thinning associated with TCS may be much more relevant in areas such as the central face area, where some patients and carers might place a high value on avoiding skin thinning (Li 2017). In contrast, for eczema on the palms, soles, or at eczematous sites that are thickened and lichenified, prolonged TCS treatment may be more acceptable to some patients and carers where there is a need to restore the abnormally thickened skin to a more normal look and feel. Licencing status is also relevant to clinical decision‐making. For example, the TCI pimecrolimus 1% is approved from three months of age, tacrolimus 0.03% from two years of age, and tacrolimus 0.1% from 16 years in Europe (Wollenberg 2022). Approval ages are similar in Canada and the US, except for pimecrolimus 1%, which is approved from 2 years of age only in the US. Topical ruxolitinib 1.5% is approved from age 12 years in the US, but approval is limited to short‐term and non‐continuous chronic treatment of less than 20% of body surface area (US Food and Drug Administration 2021) due to concerns about systemic absorption and consequent adverse effects.
Safety considerations are important for clinical decision‐making and patient/carer preference. The safety profile of TCS is a major patient concern, and this phenomenon has been termed “steroid phobia” or “corticophobia”(Cotter 2022; Li 2017; Nickles 2023). Historically, steroid phobia centred on skin thinning and systemic absorption causing adrenal suppression (Charman 2000) but the most common concern is now reported to be topical steroid withdrawal, also termed topical steroid addiction (Hajar 2015; Nickles 2023; Oulee 2022). Steroid phobia is important because TCS is the most commonly used topical anti‐inflammatory treatment for eczema (Fenske 2022). In this review, we found reassuring evidence that short‐term use of mild to very potent TCS may not be associated with increased skin thinning. Longer‐term TCS use was associated with a small risk of skin thinning, of uncertain clinical significance due to limited reporting details. This is consistent with previous understanding that prolonged continuous application of TCS, especially potent TCS, can cause skin thinning (Charman 2003; Harvey 2023; Olsen 1986). Such studies suggest that skin thinning is initially reversible, but becomes irreversible if TCS use continues (Charman 2003).
A caveat to our safety data is that data were collected in the setting of an RCT, where the amount of medication provided is typically controlled and the time period for evaluating adverse effects is typically short term. RCTs are often underpowered for detecting rare but potentially important adverse effects. We were unable to evaluate the phenomenon of topical corticosteroid withdrawal in this Cochrane review, but this is thought to occur after the prolonged application of moderate‐to‐high potency TCS (Cotter 2022). In one systematic review of more than 1000 participants with topical steroid withdrawal, TCS had been used daily for more than six months on the face or genitalia in more than 90% of participants (Hajar 2015). A recent case series similarly reported that 92% of patients had used mid‐ to high‐potency TCS for more than three months continually (Brookes 2023). The contrast between safety with controlled TCS use in RCTs and the inappropriate treatment regimens associated with adverse effects of TCS highlights a need for clear messaging on appropriate TCS use (Santer 2022; Waldecker 2017). Potency is not clearly understood by many patients, and clear labelling of potency on TCS is not provided with medications, even though many patients believe this would be helpful (Moss 2023).
Other topical therapies may serve as useful adjuncts for eczema, particularly at sensitive sites. TCI do not appear to cause skin thinning, consistent with previous literature (Cury Martins 2015). However, together with crisaborole 2%, we found that TCI increase local application‐site reactions. In some countries, such as the US, both TCI and topical JAK inhibitors also have black box warnings for potential cancer risk (Cury Martins 2015; US Food and Drug Administration 2021). These warnings for topical ruxolitinib 1.5% are partly related to adverse effects of other JAK inhibitors used orally to treat other inflammatory conditions (US Food and Drug Administration 2021a). In some countries, such as Canada, TCI do not carry a warning about cancer risk. We were not able to assess cancer risk in this review. Other reviews which include observational studies have explored cancer risk related to topical anti‐inflammatory treatments. Topical calcineurin inhibitors are associated with increased cancer risk in animal models, and increased lymphoma risk when used orally after organ transplants in humans. Lymphoma risk with TCI was found to be very small in one systematic review (Lam 2021), and a subsequent, more comprehensive systematic review found moderate certainty that TCI for eczema did not cause an important increase in cancer risk, either all‐cause cancer or specific cancers such as lymphoma (Cury Martins 2015; Devasenapathy 2023; Paller 2020; Wollenberg 2022). For topical JAK inhibitors, PDE‐4 inhibitors, and AHR activators, long‐term safety is uncertain due to limited available data, especially for the inappropriately higher, more prolonged dosing than those used outside clinical trials.
Implications for research.
We identified a number of research needs in this field. These include increased transparency and independence, reporting of core outcome instruments, generalisability of study populations, evaluation of long‐term outcomes, consistent adverse event reporting, and active comparator trials.
We identified significant issues of potential bias due to selective reporting and an almost complete absence of clinical trials undertaken by independent groups without a conflict of interest in relation to manufacturers or distributors of topical anti‐inflammatory products. Transparency and independence of clinical trial teams are important ways to optimise the credibility and utility of clinical trials. Information from independent studies would also be valuable regarding long‐term safety issues, such as the risk of skin thinning from TCS use for eczema, and this may be best achieved through prospective cohort studies. To our knowledge, this is the second completed NMA of topical anti‐inflammatory treatments for eczema (Chu 2023). Whilst some degree of replication with another high‐quality review can be useful, updating or replicating this evidence base will only be important when the body of evidence for newer treatments has increased sufficiently, to avoid research duplication and waste. In psoriasis, more than 50 NMAs of systemic treatments have been published (Guelimi 2022). Most of these NMAs were funded by pharmaceutical companies and tended to report in favour of the company product (De Vrieze 2018; Guelimi 2022). Independence and transparency are as important in NMA as in original clinical trials.
We observed that data from many trials could not be included in NMA. Clinical trial participation is an investment of patients’ time and frequently involves randomisation to vehicle, which is associated with inferior response and, consequently, the morbidity associated with skin inflammation. The failure to include trial data in subsequent evidence analyses not only disregards patient contributions and contributes to research waste (Moher 2016), but also impairs the ability of evidence synthesis to accurately guide clinical practice. To maximise trial inclusion in evidence syntheses, trial outcomes should be comprehensively and transparently reported, including reporting of denominators. Trials should also collect outcome data using harmonised, validated measurement tools across the domains important to patients and clinicians, developed for eczema by the HOME initiative (Schmitt 2012; Williams 2022). We found relatively few studies reporting outcome data using HOME‐recommended instruments for patient symptoms or quality of life, and we did not identify any outcome data using the HOME‐recommended instruments for long‐term control. Consistent reporting of HOME outcome measures in clinical trials of topical anti‐inflammatory treatments for eczema will improve opportunities for meta‐analysis in the future.
Generalisability of findings was limited to higher‐income countries, secondary care settings and adult or older child populations. More studies are needed from low‐ or middle‐income countries, in community settings, and by enroling young children, where the burden of eczema is highest. Studies in non‐white ethnic groups would also be useful given the different patterns of eczema, such as follicular lichenification that occur more frequently in people with darker skin.
Our PPI work emphasised the importance of including long‐term outcomes for efficacy and safety, but our review was unable to perform NMA for long‐term outcomes due to a lack of long‐term outcome data. More comprehensive characterisation of long‐term efficacy and safety outcomes is required. Agreed measurement tools for adverse events, including skin thinning and tolerability, would facilitate future analyses (Lax 2022).
NMA is a valuable tool to compare therapies from different trials, but there are limitations (Watt 2019). Head‐to‐head active comparator trials remain the gold‐standard to provide the most reliable comparison of available treatments (Williams 2021). Although placebo‐controlled trials are necessary to establish the efficacy and safety of new agents relative to vehicle, once established across age groups, the use of an appropriate active comparator is important (Williams 2021). Future research should avoid repeated, potentially redundant vehicle comparator trials and develop active comparator trials early in product development (Wilkes 2016).
History
Protocol first published: Issue 7, 2022
Acknowledgements
Acknowledgements from the authors
This project is funded by the National Institute for Health and Care Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number NIHR201993). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
The authors would like to thank Harley Kwok (School of Public Health, LKS Faculty of Medicine, The University of Hong Kong) and Dawn Wong (University College London, MSc graduate) for assistance with screening Chinese texts; Joanne Chalmers from the Centre of Evidence‐Based Dermatology for her contribution to development of this project and the grant application; and Peter Arkwright for clarification about whether randomisation was used in a potentially eligible trial.
We would also like to thank the Centre of Evidence Based Dermatology Patient Panel and Eczema Care Online stakeholders for their valued contributions in defining the scope and prioritising the key outcomes of concern to people with eczema.
Editorial and peer‐reviewer contributions
Cochrane Skin supported the authors in the development of this review. Liz Doney, Cochrane Skin Information Specialist, is acknowledged for developing the search methods and the draft search strategies. The following people conducted the editorial process for this review:
Sign‐off Editor (final editorial decision): Robert Dellavalle, University of Colorado Anschutz Medical Campus
Managing Editor (collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Joey Kwong, Cochrane Central Editorial Service
Editorial Assistant (selected peer reviewers, conducted editorial policy checks, supported editorial team): Jacob Hester, Cochrane Central Editorial Service
Copy Editor (copy‐editing and production): Anne Lethaby, c/o Cochrane Central Production Service
Peer‐reviewers (provided comments and recommended an editorial decision): Robin Guelimi, EpiDermE, Université Paris Est Créteil, Créteil, France (clinical/content review); Andrea Correa‐Pérez, Faculty of Medicine, Universidad Francisco de Vitoria, Madrid, Spain; Hospital Pharmacy and Medical Devices Department, Hospital Central de la Defensa "Gómez‐Ulla", Madrid, Spain (clinical/content review); Malcolm Brewster, Ferry Road Health Centre, East Sussex, UK (consumer review); Steve McDonald, Cochrane Australia (search review); Jen Hilgart, Cochrane Evidence Production and Methods Directorate (methods review)*.
*With contributions from Jo‐Ana Chase and Clare Miles, Cochrane Evidence Production and Methods Directorate
Appendices
Appendix 1. Cochrane Skin Specialised Register via the Cochrane Register of Studies (CRS‐Web) search strategy
1 eczema or neurodermatiti* AND INREGISTER 2 atopic next dermatiti* AND INREGISTER 3 #1 OR #2 4 (topical NEXT (corticosteroid* OR corti‐costeroid* OR steroid* OR glucocorticoid* OR gluco‐corticoid* OR corticoid$)) AND INREGISTER alclometasone OR amcinonide OR beclometasone OR beclomethasone OR betamethasone OR budesonide OR clobetasol OR clobetasone OR clocortolone pivalate OR crisaborole OR deprodone OR desonide OR desoximetasone OR dexamethasone OR diflorasone OR diflucortolone OR difamilast OR fluclorolone OR fludroxycortide OR flumetasone OR flumethasone OR fluocinolone OR fluocinonide OR fluocortin OR fluocortolone OR fluprednidene OR flurandrenolide OR flurandrenolone OR fluticasone OR halcinonide OR halobetasol OR halometasone OR hydrocortisone OR hydro‐cortisone OR cortisol OR masipredone OR methylprednisolone OR methyl‐prednisolone OR mometasone OR phosphodiesterase inhibitor* OR pimecrolimus OR prednicarbate OR prednisolone OR pimecrolimus OR fk506 OR tapinarof OR WBI‐1001 OR tacrolimus OR delgocitinib OR ruxolitinib OR triamcinolone OR ulobetasol AND INREGISTER 5 calcineurin inhibitor* AND INREGISTER 6 JAK inhibitor* or jakinib* or janus kinase inhibitor* AND INREGISTER 7 aryl hydrocarbon receptor* AND INREGISTER 8 AHR activator* AND INREGISTER 9 PDE‐4 or phosphodiesterase AND INREGISTER 10 anti inflammator* or antiinflammator* AND INREGISTER 11 topical* OR cutaneous OR emollient* OR oil OR oils OR ointment* OR cream* OR salve* OR unguent* OR lotion* OR moisturi* AND INREGISTER 12 #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #12 OR #11 13 #3 AND #13
Appendix 2. CENTRAL (Cochrane Library) search strategy
#1 MeSH descriptor: [Eczema] this term only #2 MeSH descriptor: [Dermatitis, Atopic] this term only #3 MeSH descriptor: [Neurodermatitis] this term only #4 eczema*:ti,ab #5 neurodermatiti*:ti,ab #6 (atopic next dermatiti*):ti,ab #7 {or #1‐#6} #8 MeSH descriptor: [Emollients] this term only #9 emollient*:ti,ab #10 moisturi*:ti,ab #11 MeSH descriptor: [Oils] this term only #12 (oil or oils):ti,ab #13 MeSH descriptor: [Ointments] this term only #14 ointment*:ti,ab #15 (cream* or salve* or unguent* or lotion*):ti,ab #16 (topical* next corticosteroid*):ti,ab #17 (topical* next cortico next steroid*):ti,ab #18 (topical* next steroid*):ti,ab #19 (topical* next glucocorticoid*):ti,ab #20 (topical* next gluco next corticoid*):ti,ab #21 (topical* next corticoid*):ti,ab #22 alclometasone dipropionate:ti,ab #23 amcinonide:ti,ab #24 beclometasone dipropionate:ti,ab #25 [mh Betamethasone] #26 betamethasone benzoate:ti,ab #27 Betamethasone butyrate propionate:ti,ab #28 betamethasone dipropionate:ti,ab #29 (betamethasone near valerate):ti,ab #30 [mh Clobetasol] #31 clobetasol:ti,ab #32 clobetasone:ti,ab #33 clocortolone pivalate:ti,ab #34 Deprodone propionate:ti,ab #35 [mh desonide] #36 desonide:ti,ab #37 [mh desoximetasone] #38 desoximetasone:ti,ab #39 diflorasone:ti,ab #40 [mh Diflucortolone] #41 diflucortolone:ti,ab #42 fluclorolone:ti,ab #43 (fludroxycortide or flurandrenolide or flurandrenolone):ti,ab #44 [mh Flurandrenolone] #45 (flumetasone or flumethasone):ti,ab #46 [mh Flumethasone] #47 fluocinolone acetonide:ti,ab #48 [mh "Fluocinolone Acetonide"] #49 [mh Fluocinonide] #50 fluocinonide:ti,ab #51 fluocortin:ti,ab #52 [mh Fluocortolone] #53 fluocortolone:ti,ab #54 fluprednidene:ti,ab #55 [mh Halcinonide] #56 halcinonide:ti,ab #57 (halobetasol or ulobetasol):ti,ab #58 halometasone:ti,ab #59 hydrocortisone butyrate:ti,ab #60 hydrocortisone aceponate:ti,ab #61 hydrocortisone acetate:ti,ab #62 hydrocortisone valerate:ti,ab #63 masipredone hydrochloride:ti,ab #64 prednicarbate:ti,ab #65 Prednisolone valerate acetate:ti,ab #66 triamcinolone:ti,ab #67 [mh Triamcinolone] #68 MeSH descriptor: [Calcineurin Inhibitors] this term only #69 calcineurin inhibitor*:ti,ab #70 [mh tacrolimus] #71 tacrolimus:ti,ab #72 fk506:ti,ab #73 pimecrolimus:ti,ab #74 {or #8‐#73} #75 MeSH descriptor: [Janus Kinase Inhibitors] this term only #76 (JAK inhibitor* or jakinib* or janus kinase inhibitor*):ti,ab #77 #75 or #76 #78 (delgocitinib or ruxolitinib or tofacitinib):ti,ab #79 JTE‐052:ti,ab #80 MeSH descriptor: [Receptors, Aryl Hydrocarbon] this term only #81 aryl hydrocarbon receptor*:ti,ab #82 (AHR next activator$):ti,ab #83 #80 or #81 or #82 #84 (tapinarof or WBI‐1001):ti,ab #85 MeSH descriptor: [Phosphodiesterase 4 Inhibitors] this term only #86 ((PDE‐4 or phosphodiesterase) near/3 inhibitor*):ti,ab #87 #85 or #86 #88 difamilast:ti,ab #89 crisaborole:ti,ab #90 [mh Beclomethasone] #91 beclomethasone:ti,ab #92 budesonide:ti,ab #93 [mh budesonide] #94 [mh cortisone] or cortisone:ti,ab #95 [mh Hydrocortisone] #96 (hydrocortisone or hydro next cortisone or cortisol):ti,ab #97 MeSH descriptor: [Dexamethasone] this term only #98 dexamethasone:ti,ab #99 fluticasone:ti,ab #100 [mh Methylprednisolone] #101 (methylprednisolone next (aceponate or acetate)):ti,ab #102 (methyl next prednisolone next (aceponate or acetate)):ti,ab #103 mometasone:ti,ab #104 [mh Prednisolone] or prednisolone:ti,ab #105 [mh "Adrenal Cortex Hormones"] #106 [mh Glucocorticoids] #107 MeSH descriptor: [Anti‐Inflammatory Agents] this term only #108 (anti inflammator* or antiinflammator*):ti,ab #109 {or #90‐#108} #110 MeSH descriptor: [Administration, Topical] this term only #111 MeSH descriptor: [Administration, Cutaneous] this term only #112 MeSH descriptor: [Emollients] this term only #113 MeSH descriptor: [Oils] this term only #114 MeSH descriptor: [Ointments] this term only #115 (topical* or cutaneous or emollient* or oil or oils or ointment* or cream* or salve* or unguent* or lotion*):ti,ab #116 {or #110‐#115} #117 #77 and #116 #118 #83 and #116 #119 #87 and #116 #120 #109 and #116 #121 #74 or #78 or #79 or #84 or #88 or #89 or #117 or #118 or #119 or #120 #122 #7 and #121 CENTRAL search narrative
Lines 1‐7: disease terms
Lines 8‐15: generic topical treatment terms
Lines 16‐67: steroids usually used in topical form
Lines 68‐74: topical calcineurin inhibitors
Lines 75‐79: JAK inhibitors
Lines 80‐84: aryl hydrocarbon receptor activators
Lines 85‐87: Phosphodiesterase 4 Inhibitors
Lines 88‐109: steroids which can be administered in various ways, including topically, along with broad anti‐inflammatory terms. The terms have been combined with a set of topical terms (lines 110‐116).
Appendix 3. MEDLINE (Ovid) search strategy
Ovid MEDLINE(R) ALL
*Please note: the resulting numbers are for the first search from March 1946 to March 07, 2022.
1 exp Eczema/ or eczema$.ti,ab. 24634 2 Dermatitis, Atopic/ 22443 3 atopic dermatiti$.ti,ab. 23617 4 neurodermatiti$.ti,ab. or Neurodermatitis/ 1767 5 or/1‐4 49529 6 exp Emollients/ 5304 7 emollient$.ti,ab. 1949 8 moisturi$.ti,ab. 2958 9 exp Oils/ or oil.ti,ab. or oils.ti,ab. 206666 10 Ointments/ or ointment$.ti,ab. 19940 11 (cream$ or salve$ or unguent$ or lotion$).ti,ab. 25494 12 (topical$ adj3 (corticosteroid$ or corti‐costeroid$)).ti,ab. 7427 13 (topical$ adj3 steroid$).ti,ab. 6293 14 (topical$ adj3 (glucocorticoid$ or gluco‐corticoid$)).ti,ab. 603 15 (topical$ adj3 corticoid$).ti,ab. 145 16 alclometasone dipropionate.ti,ab. 23 17 amcinonide.ti,ab. 45 18 beclometasone dipropionate.ti,ab. 136 19 Betamethasone/ or betamethasone benzoate.ti,ab. 6178 20 Betamethasone butyrate propionate.ti,ab. 15 21 betamethasone dipropionate.ti,ab. 570 22 (betamethasone adj2 valerate).ti,ab. 693 23 clobetasol.ti,ab. or Clobetasol/ 1946 24 clobetasone.ti,ab. 114 25 clocortolone pivalate.ti,ab. 13 26 Deprodone propionate.ti,ab. 0 27 Desonide/ or desonide.ti,ab. 147 28 desoximetasone.ti,ab. or Desoximetasone/ 117 29 diflorasone.ti,ab. 37 30 Diflucortolone/ or diflucortolone.ti,ab. 138 31 fluclorolone.ti,ab. 9 32 (fludroxycortide or flurandrenolide or flurandrenolone).ti,ab. 69 33 Flurandrenolone/ 133 34 (flumetasone or flumethasone).ti,ab. 181 35 Flumethasone/ 311 36 fluocinolone acetonide.ti,ab. or Fluocinolone Acetonide/ 1690 37 fluocinonide.ti,ab. or Fluocinonide/ 288 38 fluocortin.ti,ab. 44 39 Fluocortolone/ or fluocortolone.ti,ab. 313 40 fluprednidene.ti,ab. 4 41 halcinonide.ti,ab. or Halcinonide/ 90 42 (halobetasol or ulobetasol).ti,ab. 93 43 halometasone.ti,ab. 35 44 hydrocortisone butyrate.ti,ab. 94 45 hydrocortisone aceponate.ti,ab. 25 46 hydrocortisone acetate.ti,ab. 753 47 hydrocortisone valerate.ti,ab. 10 48 ma?ipredone hydrochloride.ti,ab. 1 49 prednicarbate.ti,ab. 103 50 Prednisolone valerate acetate.ti,ab. 1 51 triamcinolone.ti,ab. or Triamcinolone/ 10317 52 Calcineurin Inhibitors/ 4213 53 calcineurin inhibitor$.ti,ab. 7822 54 Tacrolimus/ 17039 55 tacrolimus.ti,ab. 17558 56 fk506.ti,ab. 6261 57 pimecrolimus.ti,ab. 836 58 or/6‐57 310545 59 Janus Kinase Inhibitors/ 938 60 (JAK inhibitor$ or jakinib$ or janus kinase inhibitor$).ti,ab. 2968 61 59 or 60 3292 62 delgocitinib.ti,ab. 31 63 ruxolitinib.ti,ab. 1833 64 tofacitinib.ti,ab. 1868 65 JTE‐052.ti,ab. 9 66 Receptors, Aryl Hydrocarbon/ or aryl hydrocarbon receptor$.ti,ab. or AHR activator$.ti,ab. 8784 67 (tapinarof or WBI‐1001).ti,ab. 39 68 Phosphodiesterase 4 Inhibitors/ 1330 69 ((PDE‐4 or phosphodiesterase) adj3 inhibitor$).ti,ab. 13509 70 68 or 69 14162 71 difamilast.ti,ab. 3 72 crisaborole.ti,ab. 128 73 Beclomethasone/ or beclomethasone.ti,ab. 3908 74 Budesonide/ or budesonide.ti,ab. 6721 75 exp Cortisone/ or cortisone.ti,ab. 23566 76 Hydrocortisone/ or hydrocortisone.ti,ab. or hydro cortisone.ti,ab. or cortisol.ti,ab. 103466 77 Dexamethasone/ or dexamethasone.ti,ab. 76419 78 fluticasone.ti,ab. 4135 79 Methylprednisolone/ 19781 80 (methylprednisolone adj (aceponate or acetate)).ti,ab. 828 81 (methyl adj prednisolone adj (aceponate or acetate)).ti,ab. 63 82 mometasone.ti,ab. 1056 83 Prednisolone/ or prednisolone.ti,ab. 48245 84 exp Adrenal Cortex Hormones/ 413211 85 Glucocorticoids/ 68465 86 Anti‐Inflammatory Agents/ 88468 87 (anti inflammator$ or antiinflammator$).ti,ab. 204631 88 or/73‐87 685830 89 administration, topical/ or administration, cutaneous/ 63523 90 Emollients/ 2039 91 Oils/ 12546 92 Ointments/ 13119 93 (topical$ or cutaneous or emollient$ or oil or oils or ointment$ or cream$ or salve$ or unguent$ or lotion$).ti,ab. 464369 94 or/89‐93 498032 95 88 and 94 38868 96 61 and 94 275 97 66 and 94 307 98 70 and 94 439 99 58 or 62 or 63 or 64 or 65 or 67 or 71 or 72 or 95 or 96 or 97 or 98 331411 100 randomized controlled trial.pt. 560324 101 controlled clinical trial.pt. 94719 102 randomized.ab. 552851 103 placebo.ab. 226123 104 clinical trials as topic.sh. 199418 105 randomly.ab. 377352 106 trial.ti. 257833 107 100 or 101 or 102 or 103 or 104 or 105 or 106 1430930 108 exp animals/ not humans.sh. 4968174 109 107 not 108 1316202 110 5 and 99 and 109 1584 Search narrative
We contacted the North West Medicines Information Centre & National Dental Medicines Information Service (via email 24 November 2021) for advice on new topical medications for eczema that we should search for. They replied (1 December 2021) having run a report in their horizon scanning database suggesting crisaborole, ruxolitinib and delgocitinib. These terms have been incorporated into our search strategy.
Lines 1‐5: disease terms
Lines 6‐11: generic topical treatment terms
Lines 12‐51: steroids usually used in topical form
Lines 52‐57: topical calcineurin inhibitors
Lines 59‐65: JAK inhibitors
Lines 66‐67: aryl hydrocarbon receptor activators
Lines 68‐69: Phosphodiesterase 4 Inhibitors
Lines 71‐87: steroids which can be administered in various ways, including topically, along with broad anti‐inflammatory terms. The terms have been combined with a set of topical terms (lines 89‐93).
Lines 100‐109: Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format, from section 3.6.1 in Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M‐I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, et al (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6. Cochrane, 2019. Available from: www.training.cochrane.org/handbook
Appendix 4. Embase (Ovid) search strategy
*Please note: the resulting numbers are for the first search from March 1946 to 7 March 2022.
1 eczema/ 27367 2 eczema$.ti,ab. 28557 3 atopic dermatitis/ 49614 4 atopic dermatiti$.ti,ab. 37715 5 neurodermatiti$.ti,ab. 764 6 neurodermatitis/ 2515 7 or/1‐6 84646 8 emollient agent/ 6711 9 emollient$.ti,ab. 3530 10 moisturi$.ti,ab. 4828 11 oil/ 18475 12 (oil or oils).ti,ab. 216675 13 ointment/ 12497 14 ointment$.ti,ab. 16975 15 skin cream/ 916 16 salve/ 86 17 lotion/ 2724 18 (cream$ or salve$ or unguent$ or lotion$).ti,ab. 35929 19 (topical$ adj3 (corticosteroid$ or corti‐costeroid$)).ti,ab. 11436 20 (topical$ adj3 steroid$).ti,ab. 10453 21 (topical$ adj3 (glucocorticoid$ or gluco‐corticoid$)).ti,ab. 852 22 (topical$ adj3 corticoid$).ti,ab. 253 23 alclometasone dipropionate/ 314 24 alclometasone dipropionate.ti,ab. 57 25 amcinonide/ 414 26 Betamethasone/ 18205 27 amcinonide.ti,ab. 98 28 beclometasone dipropionate.ti,ab. 316 29 betamethasone benzoate.ti,ab. 11 30 Betamethasone butyrate propionate.ti,ab. 39 31 betamethasone dipropionate/ 2951 32 betamethasone dipropionate.ti,ab. 906 33 (betamethasone adj2 valerate).ti,ab. 913 34 clobetasol.ti,ab. or clobetasol/ 4628 35 clobetasone/ or clobetasone.ti,ab. 411 36 clocortolone pivalate.ti,ab. or clocortolone pivalate/ 105 37 Deprodone propionate.ti,ab. 5 38 desonide/ 1112 39 desonide.ti,ab. 171 40 desoximetasone.ti,ab. or desoximetasone/ 848 41 diflorasone/ or diflorasone.ti,ab. 137 42 diflucortolone/ or Diflucortolone.ti,ab. 289 43 fludroxycortide/ 394 44 (fludroxycortide or flurandrenolide or flurandrenolone).ti,ab. 51 45 fluclorolone/ or fluclorolone.ti,ab. 25 46 flumetasone.ti,ab. or flumetasone/ 417 47 flumethasone.ti,ab. 144 48 fluocinolone acetonide.ti,ab. or fluocinolone acetonide/ 2985 49 fluocinonide/ or fluocinonide.ti,ab. 1762 50 fluocortin/ or fluocortin.ti,ab. 91 51 Fluocortolone.ti,ab. or fluocortolone/ 871 52 fluprednidene/ or fluprednidene.ti,ab. 75 53 halcinonide.ti,ab. or halcinonide/ 601 54 (halobetasol or ulobetasol).ti,ab. 137 55 halometasone.ti,ab. or halometasone/ 189 56 hydrocortisone butyrate/ or hydrocortisone butyrate.ti,ab. 1421 57 hydrocortisone acetate.ti,ab. or hydrocortisone acetate/ 2106 58 hydrocortisone aceponate.ti,ab. or hydrocortisone aceponate/ 80 59 hydrocortisone valerate.ti,ab. or hydrocortisone valerate/ 242 60 ma?ipredone hydrochloride.ti,ab. 1 61 prednicarbate.ti,ab. or prednicarbate/ 800 62 Prednisolone valerate acetate.ti,ab. or prednival acetate/ 87 63 triamcinolone/ or triamcinolone.ti,ab. 22036 64 calcineurin inhibitor/ or pimecrolimus/ or tacrolimus/ 99937 65 calcineurin inhibitor$.ti,ab. 14445 66 tacrolimus.ti,ab. 36148 67 fk506.ti,ab. 7649 68 pimecrolimus.ti,ab. 1174 69 or/8‐63 343283 70 (JAK inhibitor$ or jakinib$ or janus kinase inhibitor$).ti,ab. 6098 71 janus kinase inhibitor/ 4493 72 70 or 71 8074 73 delgocitinib/ or ruxolitinib/ or tofacitinib/ 13263 74 (delgocitinib or ruxolitinib or tofacitinib).ti,ab. 8476 75 JTE‐052.ti,ab. 14 76 aromatic hydrocarbon receptor/ 10247 77 ((aryl adj hydrocarbon adj receptor$) or (AHR adj activator$)).ti,ab. 8834 78 76 or 77 11975 79 tapinarof/ 92 80 (tapinarof or WBI‐1001).ti,ab. 62 81 phosphodiesterase IV inhibitor/ 3132 82 ((PDE‐4 or phosphodiesterase) adj3 inhibitor$).ti,ab. 17782 83 81 or 82 19473 84 difamilast/ 56 85 difamilast.ti,ab. 7 86 crisaborole/ 401 87 crisaborole.ti,ab. 229 88 beclometasone/ 7667 89 beclomethasone.ti,ab. 3981 90 budesonide/ 22847 91 budesonide.ti,ab. 9596 92 cortisone/ 13024 93 cortisone.ti,ab. 8329 94 hydrocortisone/ 133709 95 (hydrocortisone or hydro‐cortisone or cortisol).ti,ab. 98890 96 dexamethasone/ 167924 97 dexamethasone.ti,ab. 85378 98 fluticasone/ 8854 99 fluticasone.ti,ab. 7458 100 methylprednisolone/ 107858 101 methylprednisolone aceponate/ 616 102 (methylprednisolone adj (aceponate or acetate)).ti,ab. 1018 103 methylprednisolone acetate/ 3743 104 (methyl adj prednisolone adj (aceponate or acetate)).ti,ab. 89 105 mometasone furoate/ 5605 106 mometasone.ti,ab. 1869 107 prednisolone/ 136053 108 prednisolone.ti,ab. 42261 109 corticosteroid/ or glucocorticoid/ 344762 110 antiinflammatory agent/ 69060 111 (anti inflammator$ or antiinflammator$).ti,ab. 282957 112 or/88‐111 1091969 113 topical drug administration/ or cutaneous drug administration/ 84802 114 emollient agent/ 6711 115 ointment/ 12497 116 oil/ 18475 117 (topical$ or cutaneous or emollient$ or oil or oils or ointment$ or cream$ or salve$ or unguent$ or lotion$).ti,ab. 602665 118 skin cream/ 916 119 salve/ 86 120 lotion/ 2724 121 or/113‐120 653543 122 72 and 121 551 123 78 and 121 462 124 83 and 121 809 125 112 and 121 70101 126 69 or 73 or 74 or 75 or 79 or 80 or 84 or 85 or 86 or 87 or 122 or 123 or 124 or 125 391529 127 Randomized controlled trial/ 698809 128 Controlled clinical study/ 465161 129 random$.ti,ab. 1762880 130 randomization/ 93238 131 intermethod comparison/ 280636 132 placebo.ti,ab. 337541 133 (open adj label).ti,ab. 95072 134 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 254105 135 double blind procedure/ 192954 136 parallel group$1.ti,ab. 29022 137 (crossover or cross over).ti,ab. 115114 138 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 374454 139 (controlled adj7 (study or design or trial)).ti,ab. 401608 140 trial.ti. 352612 141 or/127‐140 2825646 142 exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 30219903 143 human/ or normal human/ 23345315 144 142 and 143 23345315 145 142 not 144 6874588 146 141 not 145 2527908 147 7 and 126 and 146 2866
Search narrative:
Lines 1‐6: disease terms
Lines 8‐18: generic topical treatment terms
Lines 19‐63: steroids usually used in topical form
Lines 64‐68: topical calcineurin inhibitors
Lines 70‐75: JAK inhibitors
Lines 76‐80: aryl hydrocarbon receptor activators
Lines 81‐82: Phosphodiesterase 4 Inhibitors
Lines 84‐111: steroids which can be administered in various ways, including topically, along with broad anti‐inflammatory terms. The terms have been combined with a set of topical terms (lines 113‐121).
[Lines 127‐146: Based on terms suggested for identifying RCTs in Embase (section 3.6.2) in Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M‐I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, et al (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6. Cochrane, 2019. Available from: www.training.cochrane.org/handbook]
Appendix 5. ClinicalTrials.gov search strategy
Advanced search:
Condition or disease: Eczema OR Atopic Dermatitis Other terms: topical OR topically OR emollient OR emollients OR ointment OR ointments OR cream OR creams OR moisturizer OR moisturizers OR moisturiser OR moisturisers OR oil OR oils OR salve OR salves OR unguent OR unguents OR lotion OR lotions
Applied filters: Interventional studies (Clinical trials) Limited to Phase 2‐4 trials
Appendix 6. WHO ICTRP search strategy
Advanced search: eczema OR atopic dermatitis in the Title topical OR topically OR emollient OR emollients OR ointment OR ointments OR cream OR creams OR moisturizer OR moisturizers OR moisturiser OR moisturisers OR oil OR oils OR salve OR salves OR unguent OR unguents OR lotion OR lotions in the Intervention Recruitment status: All Limited to Phase 2‐4 trials
Appendix 7. Statistical analysis supplement
Additional study inclusion criteria
We included within‐participant trials but, due to concerns about these trials, we performed a sensitivity analysis excluding these trial designs. When included, we extracted paired data from within‐participant trials where the unit of analysis was the separate body part. For paired data from studies with no suspicion of contamination across intervention sites, we performed analysis using the generic inverse‐variance method after accounting for within‐participant variability (Higgins 2021b). If paired data were not available, we attempted to estimate the paired data using methods and guidance from the Cochrane Handbook (Higgins 2021a), and correct the variance using the Becker‐Balagtas method (Elbourne 2002). We assumed an intraclass correlation coefficient of 0.5 in all calculations but undertook post hoc sensitivity analyses, exploring the effect of varying this to 0.25 or 0.75. We did not extract data on outcomes which affect the whole body (e.g. systemic adverse events) from within‐participant trials as it is not possible to determine which treatment caused the event. We didn't identify any cluster‐randomised trials, but had planned for the unit of analysis to be the groups of participants randomised to different interventions. If data from a cluster‐randomised trial had been inappropriately analysed, we would have considered inclusion by adjusting for the study design using intra‐cluster correlation coefficients – either those reported in the study or calculated using data from the study, if appropriate.
Additional information on Confidence in NMA (CINeMA) approach
CINeMA: Within‐study bias
This combines the risk of bias judgements for each contributing study outcome with a quantification of the contribution that each study makes to the relative treatment effect using a percentage contribution matrix. The single risk of bias judgement for each comparison is defined by a majority rule. We summarised these, for each network estimate, as follows.
No concerns;
Some concerns;
Major concerns.
The overall judgement was informed by the sensitivity analysis which included the low risk of bias data, where possible.
CINeMA: Reporting bias
Factors influencing reporting bias were as follows.
Suspected reporting bias, for example, where we were unable to access unpublished data, where data were restricted to early evidence about a new intervention or to industry‐funded trials, or where prior data suggested reporting bias in relation to the interventions studied;
Undetected reporting bias, where prospective trial registries, protocols, and statistical analysis plans were available and suggested close adherence to prospective plans in the published studies, or where empirical evaluation of patterns of results did not indicate small study effects;
Funnel plots were used to assess whether there were any small‐study effects or evidence of reporting bias through asymmetry in the plot.
CINeMA: Indirectness
Each study included in the network was evaluated according to its relevance to the research question, classified into low, moderate, or high indirectness. We made study‐level judgements considering participant, intervention, and outcome characteristics that were likely to be associated with the relative effect of an intervention (i.e. effect‐modifying variables, in our case, baseline eczema disease severity and duration of treatment). We also assessed the relevance of the study design to the research question. We combined these study‐level judgements about indirectness related to effect‐modifying variables with the percentage contribution matrix. Where most of the evidence was derived from studies with significant concerns about indirectness, for example, within‐participant studies, certainty was downgraded for indirectness.
CINeMA: Imprecision
We evaluated imprecision by assessing the range of effects incorporated by the two‐sided 95% CIs. In order to do this, we defined the range of equivalence for each of the networks as below.
A rating of ‘major concerns’ was given for 95% CIs which extended beyond the area of equivalence on the opposite side of the no effect line to the point estimate, so that the estimated treatment effect was compatible with clinically important effects in both directions;
A rating of ‘some concerns’ was given if the CI extended into but not beyond the area of equivalence on the opposite side of the no effect line;
‘No concerns’ was assigned if the CI was entirely on one side of the no‐effect line or entirely within the area of equivalence.
Ranges of equivalence:
For mean difference using specific instruments such as EASI (Hanifin 2001), POEM (Spuls 2017) or SCORAD (Kunz 1997), we used published minimally clinically important differences: 8.2 for SCORAD, 6.6 for EASI and 3.4 for POEM (Schram 2012);
For SMD – a SMD equivalent to a small effect (< 0.2);
For binary measures – odds ratio from 0.8 to 1.2.
CINeMA: Heterogeneity
We calculated Tau2 for each network and compared the CIs and prediction intervals to evaluate heterogeneity, using the ranges of equivalence defined above. Outcomes were as follows.
No concerns where the CIs and prediction intervals led to the same conclusions;
Some concerns where CIs and prediction intervals led to different conclusions with less impact for decision‐making;
Major concerns where heterogeneity was high and likely to have an important impact on decision‐making.
CINeMA: Incoherence
This is a specific form of heterogeneity, the agreement between direct and indirect evidence. We used both global and local approaches to evaluate incoherence.
To evaluate local consistency, we used the standard node‐splitting approach (also known as SIDE: Separating Indirect from Direct Evidence), implemented via Stata’s sidesplit command, which is part of the network command suite. This allowed the estimates given for each pairwise comparison to be split into direct and indirect components, and the difference between these components was assessed for incoherence (White 2015).
To evaluate global design inconsistency (within the whole network), we used the 'design by treatment interaction' model (Veroniki 2013), which estimated a global Chi2 statistic for network coherence. Statistical significance was set at 5%. When interpreting incoherence tests, we considered the inconsistency factors as well as their uncertainty and the impact on clinical implications, based on visual inspection of the 95% CIs of direct and indirect odds ratios and the range of equivalence. Inconsistency factors included eczema severity, age, risk of bias, method of outcome assessment (HOME‐approved versus not HOME‐approved), treatment duration and trial design (within‐ versus between‐participant comparison).
Overall assessments of confidence
Overall assessments of confidence were based on the grading in all six domains of CINeMA. We started at high confidence and dropped the level of confidence by one step for each domain's major concerns, or two domains with some concerns. However, we took account of the possibility that single issues might impact on multiple domains and avoided inappropriate multiple downgrades for single NMA issues.
We produced summary of findings tables for summarising NMA findings using the approach recommended by Cochrane and GRADE (Yepes‐Nunez 2019), but were cautious in our interpretation of rankings due to concerns about the reliability of ranking information in summaries of NMA findings. We created a separate NMA summary of findings table for each of the outcomes for which a network was possible. Overall assessments of effectiveness and safety took into account the results of the main analyses and their CiNEMA ratings, the planned sensitivity analyses, and any subgroup effects, pairwise analyses or narrative information identified.
Summary of findings and assessment of the evidence for pairwise meta‐analyses
The primary focus of this systematic review was the NMA, since Cochrane Reviews and other systematic reviews are already available for direct comparisons of topical anti‐inflammatory treatments versus placebo or no treatment. However, for any primary, subgroup or sensitivity analyses where NMA was not possible, as described below, we undertook pairwise meta‐analyses. Together with summaries of narrative information, these informed our overall conclusions about the relative effectiveness and safety of different topical anti‐inflammatory treatments for eczema.
For summary of findings tables, we included effects of the following licenced topical anti‐inflammatory treatments compared with vehicle or no treatment:
Mild TCS
Moderate TCS
Potent TCS
Very potent TCS
Pimecrolimus 1%
Tacrolimus 0.03%
Tacrolimus 0.1%
Crisaborole 2%
Difamilast 0.3%
Difamilast 1%
Roflumilast 0.15%
Ruxolitinib 1.5%
Delgocitinib 0.5%
Delgocitinib 0.25%
Tapinarof 1%
The outcomes for inclusion in the summary of findings tables, where suitable data for NMA were available, were:
Clinician‐assessed signs of eczema – binary;
Clinician‐assessed signs of eczema ‐ continuous;
Investigator global assessment ‐ binary;
Patient‐reported symptoms of eczema ‐ binary;
Patient‐reported symptoms of eczema ‐ continuous;
Safety – withdrawal from treatment or trial due to adverse events related to the study intervention;
Safety – local application‐site reactions;
Safety ‐ local pigmentation changes;
Safety ‐ local signs of skin thinning/atrophy.
Data and analyses
Comparison 1. Investigator Global Assessment (binary) ‐ long‐term.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Pimecrolimus 1% versus vehicle | 4 | 2218 | Odds Ratio (IV, Random, 95% CI) | 1.51 [0.95, 2.42] |
Comparison 2. Health‐related quality of life (continuous) ‐ short‐term.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Pimecrolimus 1% versus vehicle | 2 | 275 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.46, 0.04] |
Comparison 3. Long‐term control of eczema (binary).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Pimecrolimus 1% versus vehicle | 2 | 442 | Odds Ratio (IV, Random, 95% CI) | 2.22 [0.98, 5.04] |
Comparison 4. Application site reactions (binary) ‐ long‐term.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Pimecrolimus 1% versus vehicle | 3 | 1378 | Odds Ratio (IV, Random, 95% CI) | 1.05 [0.38, 2.91] |
Comparison 5. Skin thinning (binary) ‐ long‐term.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Topical corticosteroids versus topical calcineurin inhibitors | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abbasi 2017.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, single‐centre, parallel study Trial registration number: IRCT2014101419529N1 Country: Iran Outpatient or hospital: asthma and allergy clinic Date trial conducted: November 2014 to December 2015 Duration of trial participation: 14 days Inclusion criteria: · Aged under 15 years of age · Atopic Dermatitis (AD) according to the Hanifin and Rajka criteria · Mild‐to‐moderate AD using Scoring Atopic Dermatic (SCORAD) Index < 50) Exclusion criteria: · Severe AD (SCORAD > 50) · A secondary skin infection · Another skin disease · Immunodeficiency disorder · Used TCS or TCI within the previous 2 weeks · Used systemic corticosteroids or immunosuppressive agents within the previous 4 weeks Additional design details: study has three randomised groups; one aqueous extract of fig fruit was not relevant to this review. |
| Participants | Total number randomised: 39 participants (19 to apply intervention and 20 to apply vehicle) Age: intervention group mean 28.36, assume months (SD 37.25); placebo group 27, assume months (SD 27) Sex: intervention group female 64.2%, male 35.7%; placebo group female 13.3%, male 86.6% Ethnicity, duration of eczema: not reported Severity of eczema: intervention group SCORAD mean 29.53 (SD 13.58); placebo group SCORAD mean 28.48 (SD 10.34) Body site: not reported Number of withdrawals: 10: intervention group lost to follow‐up n = 4, lack of efficacy n = 1; placebo group lost to follow‐up n = 1, lack of efficacy n = 4 Notes: none |
| Interventions | Run‐in details: not reported Intervention: hydrocortisone 1% cream used twice daily for 14 days Concurrent treatment: not reported Other key information: none Comparator: vehicle cream used twice daily for 14 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: to quote: "Subjects were under the additive‐free diets, including: preservatives, colorings, flavor substances, among others." Notes: to quote: "Each child randomly received one of the three products with the specified dose of tip finger unit for his or her age on the skin lesions, twice daily for 2 weeks." |
| Outcomes | · Scoring Atopic Dermatitis Index (SCORAD) · Intensity · Pruiritus (Re‐occurence of cutaneous lesions was reported as a second outcome in trial registration form) |
| Notes | Funding source: Vice Chancellor for Research of Mashhad University of Medical Sciences Declarations of interest: to quote: "The authors declare that they have no conflict of interest". Original language of publication: English Other: Study did not report planned longer term evaluation. To quote: "Given the fact that 15 of the 45 patients were not available for the second phase of follow‐up, this part of the study was not reported completely." |
Abramovits 2010.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: US Outpatient or hospital: 15 centres, sites not reported Date trial conducted: June 2005 to May 2006 Duration of trial participation: 29 days Inclusion criteria: · Clinical diagnosis of stable, mild‐to‐moderate Atopic Dermatitis (AD) according to the Hanifin and Rajka's criteria · Severity of AD according to PGA score of 2 or 3 · A minimum percent surface area involvement of at least 10% body surface area (BSA) Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 264 participants (131 to apply intervention and 133 to apply vehicle) Age: average 7.08 years across groups: hydrocortisone butyrate group 7.37 years; vehicle group 6.8 years Sex: males 57%, females 43% Ethnicity: white 64% with the majority of non‐Hispanic/non‐Latino descent 80% Duration of eczema: not reported Severity of eczema: 39% had mild AD as assessed on PGA scale and 61% moderate Body site: not reported Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: hydrocortisone butyrate 0.1% cream used twice daily for 29 days Concurrent treatment: not reported Other key information: none Comparator: vehicle cream used twice daily for 29 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Overall disease severity using 5‐point Physician’s Global Assessment (PGA) · Lesion severity using Eczema Area and Severity Index (EASI) and percent of BSA involvement · Overall pruritus using 4‐point scale 0 = none to 3 = severe · Adverse events |
| Notes | Funding source: not reported Declarations of interest: to quote: "The authors certify that there are no real or apparent conflicts of interest". Original language of publication: English Other: none |
Adam 1978.
| Study characteristics | |
| Methods | Trial design: randomised, parallel study Trial registration number: not reported Country: Malaya Outpatient or hospital, date trial conducted, duration of trial participation: not reported Inclusion criteria: patients with skin disease because of contact eczema, photosensitivity, discoid eczema, AD or psoriasis Exclusion criteria: not reported Additional design details: AD participants were a subset with some results reported separately. |
| Participants | Total number randomised: 14 participants with AD (8 to apply the intervention and 6 to apply the comparator) Age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: no topical applications for at least two weeks prior to trial Intervention: desoximetasone 0.25% ointment used twice daily for ≥ 30 days · Concurrent treatment: not reported · Other key information: Brand: Esperson Comparator: betamethasone valerate (assumed 0.05%) ointment used twice daily for ≥ 30 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: to quote: "No other treatment was given". Notes: none |
| Outcomes | Response to therapy at up to one month was grouped as: excellent to good, fair and poor. In "Excellent to good" response, the skin condition cleared and remission was maintained throughout their follow‐up. In "Fair" response, both symptoms and signs were less but there was no remission. If skin change remained as it was in the first visit or improvement was minimal, the response was categorised as "Poor". |
| Notes | Funding source: Hoechst Malaysia Sdn. Bhd Declarations of interest: not declared Original language of publication: English Other: none |
Allenby 1981.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, within‐participant study Trial registration number: not reported Country: UK Outpatient or hospital: outpatient dermatology departments in the UK from the list of affiliations Date trial conducted: not reported Duration of trial participation: 7 days (or as near as possible) Inclusion criteria: · Eczema (or psoriasis, but analysed separately) requiring treatment in hospital · Clinically similar bilateral lesions suitable for topical corticosteroids (TCS) treatment Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 66 sides treated (33 to apply the intervention and 33 to apply the comparator) Age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: clobetasone butyrate 0.05% ointment used twice daily for 7 days · Concurrent treatment: not reported · Other key information: proprietary: Eumovate Comparator: hydrocortisone butyrate 0.1% ointment used twice daily for 7 days · Concurrent treatment: not reported · Other key information: proprietary: Locoid Concurrent treatments received alongside both intervention and comparator: not under occlusion Notes: none |
| Outcomes | · Global assessment (rated healed, improved, static or worse) at 7 days or as near as possible · Global preference (did one side respond better than the other) at 7 days or as near as possible |
| Notes | Funding source: not reported Declarations of interest: not declared but one author is affiliated to Glaxo Laboratories LTD, Greenford, Middlesex Original language of publication: English Other: none |
Almeyda 1973.
| Study characteristics | |
| Methods | Trial design: randomised, within‐participant study Trial registration number: not reported Country: UK Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 2 to 3 weeks Inclusion criteria: patients with AD Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 100 sides treated (50 to apply the intervention and 50 to apply the comparator) Age: 9 months to 69 years; n = 28 ≤ 12, n = 22 adults Sex: not reported Ethnicity: not reported Duration of eczema: not reported; however the authors stated that "most […] had already received treatment". Severity of eczema: mild n = 2; moderate n = 32; severe n = 16 Body site: not reported Number of withdrawals: no withdrawals Notes: none |
| Interventions | Run‐in details: not reported Intervention: betamethasone valerate 0.1% cream used twice daily for 14 to 21 days · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone 1% cream used twice daily for 14 to 21 days · Concurrent treatment: not reported · Other key information: stabilised in 10% urea cream Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Disease severity score |
| Notes | Funding source: not reported Declarations of interest: Pharmacia (G.B) Ltd provided urea‐hydrocortisone for the study. Original language of publication: English Other: none |
Almeyda 1974.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: UK Outpatient or hospital, date trial conducted: not reported Duration of trial participation: to quote: "2 weeks, and in a few instances for 4 weeks" Inclusion criteria: patients with AD Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 72 sides treated (36 to apply the intervention and 36 to apply the comparator) Age: to quote: "ages ranged from 18 months to 74 years; fifteen patients were under twelve years". Sex: males n = 18, females n = 18 Ethnicity, duration of eczema: not reported Severity of eczema: to quote: "the original eczema was severe in twenty patients, moderate in six and mild in ten". Body site, number of withdrawals: not reported Notes: to quote: "Five patients also had ichthyosis, and a further four patients also presented dry fissured hand or foot eczema". |
| Interventions | Run‐in details: to quote: "One week before the trial began, the patients were instructed to discontinue all topical treatments". Intervention: urea hydrocortisone 1% powder cream used three times daily for 14 days (28 days for some participants) · Concurrent treatment: not reported · Other key information: none Comparator: betamethasone valerate 0.1% cream used three times daily for 14 days (28 days for some participants) · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · "Clinical condition" assessed as excellent (if eczema had cleared completely), good (if partially cleared), no improvement or deterioration · Patients were asked to comment on the acceptability of the two treatments. |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Anonymous 1981a.
| Study characteristics | |
| Methods | Trial design: randomised, multicentre, within‐participant study Trial registration number: not reported Country: Japan Outpatient or hospital: 16 hospitals Date trial conducted: December 1978 to July 1979 Duration of trial participation: 8 months Inclusion criteria: · 1 or 2 with symmetrical rash · Acute eczema/contact dermatitis with serious papule or tiny blister · AD mainly with xerosis/desquamation Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 128 sides treated (64 to apply the intervention and 64 to apply the comparator) Age, sex, ethnicity, duration of eczema, severity of eczema: body site: not reported Number of withdrawals: 8 Notes: none |
| Interventions | Run‐in details: not reported Intervention: ufenamate 5% ointment used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: TCS were allowed on non‐target areas. Notes: none |
| Outcomes | · Individual signs like itchiness, redness, papula, desquamation, and lichen · Total improvement of the site and left‐right comparison · Total usefulness of the site and left‐right comparison after the trial |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: Japanese Other: none |
Antiga 2011.
| Study characteristics | |
| Methods | Trial design: randomised, parallel study Trial registration number: not reported Country: Italy Outpatient or hospital, date trial conducted: not reported Duration of trial participation: three weeks Inclusion criteria: · Male or female adult, any ethnic group · AD according to the Hanifin and Rajka's criteria · SCORAD score 15 or more Exclusion criteria: · Infectious diseases that require treatment · HIV positivity · Systematic diseases that contraindicate the use of tacrolimus · Clinically‐relevant renal and/or hepatic failure · Pregnancy or lactation · Skin lesions that require treatment and that co‐localise with AD lesions · Cicatricial or pigmentary skin lesions that widely co‐localise with AD lesions · Superinfected AD · Any skin lesion that co‐localises with AD lesions and that can not be treated by tacrolimus, according to the experimenter’s judgement · Known allergy to macrolides or any excipient included in the ointment · Alcohol/drug abuse, psychiatric disturbances or any other condition that, according to the experimenter’s judgement, is able to compromise communication with the experimenter himself. Additional design details: none |
| Participants | Total number randomised: 24 participants Age: mean age 40.9 years, range from 20 to 65 Sex: male n = 11, female n = 13 Ethnicity: not reported Duration of eczema: not reported Severity of eczema: median pre‐treatment SCORAD values were 44.3 (range 27.4 to 57.2; 25 to 75th percentile 35.5 to 50.4) for the tacrolimus group and 40.1 (range 25.4 to 58.3; percentiles 32.1 to 48.4) for the hydrocortisone butyrate group Body site: not reported Number of withdrawals: 3 Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.1% ointment used twice daily for 21 days Concurrent treatment: not reported Other key information: none Comparator: hydrocortisone butyrate 0.1% ointment used twice daily for 21 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Signs and symptoms of AD using SCORAD · Immunohistochemistry to investigate the expression of Toll‐like Receptor (TLR) ‐1, ‐2, ‐4 and ‐9 in the lesional skin |
| Notes | Funding source: Astellas Pharma Inc. Declarations of interest: to quote: "The study was funded by Astellas Pharma Inc". Original language of publication: English Other: none |
Asada 1987.
| Study characteristics | |
| Methods | Trial design: multicentre, within‐participant study Trial registration number: not reported Country: Japan Outpatient or hospital: 11 hospitals Date trial conducted: January 1987 to May 1987 Duration of trial participation: 5 months Inclusion criteria: AD with symmetrical eczema Exclusion criteria: · Requirement for systemic corticosteroids · Had already applied TCS to affected areas Additional design details: none |
| Participants | Total number randomised: 194 sides treated (97 to apply the intervention and 97 to apply the comparator) Age, sex, ethnicity: all were eligible; baseline characteristics not reported Duration of eczema, severity of eczema: not reported Body site: excluding head, hands, feet or genital area Number of withdrawals: 5 Notes: one participant in 5 withdrawals had asymmetric eczema, so was included in the safety analysis, but excluded from the effectiveness analyses. |
| Interventions | Run‐in details: not reported Intervention: suprofen 1% ointment used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: ufenamate 5% ointment used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: oral antihistamines or antiallergic drugs when itching was severe · TCS were allowed in non‐target areas Notes: none |
| Outcomes | · Individual signs such as itchiness, redness, papula/vesicle, erosion, crusta, desquamation, and lichen · Total improvement of the site and left‐right comparison · Total usefulness of the site and left‐right comparison of it after the trial |
| Notes | Funding source: ointments were provided by Kayaku Co. Ltd and Hokuriku Pharmacy Co. Ltd. Declarations of interest: not declared Original language of publication: Japanese Other: none |
Aschoff 2009.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, single‐centre, within‐participant study Trial registration number: NCT00180141 Country: Germany Outpatient or hospital: one health centre in Germany, assume outpatient Date trial conducted: April 2005 to June 2007 Duration of trial participation: 3 weeks Inclusion criteria: · ≥ 18 years of age · AD according to the Hanifin and Rajka's criteria, on at least 1% of skin surface area on both forearms · Atopic Dermatitis Severity Index (ADSI) score of at least 6 (range 0 to 15, with higher score for more severe symptoms) (difference between right and left arm ≤ 1) · IGA of 2 or 3 on both forearms Exclusion criteria: · Participation in a clinical study within the last 4 weeks · Systemic therapy with cytostatics or immunosuppressants within the last 24 weeks · Antibiotic or topical therapy to treat AD in the last 2 weeks · Known allergy to components of pimecrolimus cream 1% · Genetic defects of the epidermal barrier (e.g. Netherton syndrome) · Acute viral infection (e.g. herpes simplex) in the target area · Severe AD (IGA ≥ 4) · Immune incompetence · Skin malignancies · Drug or alcohol abuse · Pregnancy and breastfeeding · Women of childbearing age without reliable contraception Additional design details: none |
| Participants | Total number randomised: 40 sides treated (20 to apply intervention and 20 to apply vehicle) Age: mean 25.7 years (range: 18 to 58) Sex: female n = 11, male n = 9 Ethnicity: not reported Duration of eczema: mean 16.89 years (range: 0.36 to 45.78) Severity of eczema: IGA score 7 mild, 13 moderate Body site: forearm Number of withdrawals: 0 Notes: none |
| Interventions | Run‐in details: none Intervention: pimecrolimus 1% cream used twice daily for 3 weeks Concurrent treatment: not reported Other key information: none Comparator: vehicle cream used twice daily for 3 weeks Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: hydrocortisone‐acetate 1% (once daily) or emollients on all body surface areas but excluding the forearms Notes: none |
| Outcomes | · ADSI at 21 days · Investigator's Global Assessment (IGA) at 21 days · Pruritis using visual analogue scale (VAS) 0 to 10 at 21 days · Microcirculation, skin hydration, transepidermal water loss (TEWL) to 21 days |
| Notes | Funding source: Technische Universität Dresden Declarations of interest: not declared Original language of publication: English Other: none |
Bagatell 1974.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: US Outpatient or hospital, date trial conducted, duration of trial participation: not reported Inclusion criteria: · Common corticosteroid‐responsive dermatoses: AD, neurodermatitis, eczematous dermatitis, psoriasis · Multiple bilateral lesions of similar severity, persistency and aetiology Exclusion criteria: not reported Additional design details: outcomes reported per type of dermatosis |
| Participants | Total number randomised: 1072 sides treated (536 to apply the intervention and 536 to apply the comparator) Age: 75% of patients between 16 and 60 years old Sex: 57% female, 43% male Ethnicity: 96% white Duration of eczema, severity of eczema: not reported Body site: not reported Number of withdrawals: 79/536 did not follow protocol or were lost to follow‐up. It is not clear whether any of those who withdrew had AD. Notes: totals in number randomised were given for the whole study population; number per diagnosis not provided |
| Interventions | Run‐in details: not reported Intervention: halcinonide 0.1% cream used three times daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: betamethasone valerate 1% cream used three times daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: none Notes: use of local or systemic medications such as corticosteroids, antihistamines and antipruritics was prohibited. |
| Outcomes | · Investigator assessment of erythema, oedema, transudation, lichenification, scaling and pruritus at the end of treatment in regard to superiority between treatments and response per side rated as excellent, good, fair or poor · Adverse events (AE) |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Bagatell 1983.
| Study characteristics | |
| Methods | Trial design: Double‐blind, randomised, parallel study Trial registration number: not reported Country: United States Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 21 days Inclusion criteria: · Aged ≥ 12 with AD established for ≥ 1 year (moderate‐severe) · Stable or worsening disease within the last week · TSS ≥ 6 (erythema, induration and pruritus each scored 0 = absent, 1 = slight, 2 = moderate, 3 = severe) · All the considered signs had to be present on the target lesions (face, neck, trunk and extremities; excluding hands and feet). Exclusion criteria: · Pregnancy · Requirement for concomitant topical anti‐inflammatory, systemic steroids, or other therapy that might affect the disease (e.g. tar, tranquillisers, antihistamines) Additional design details: none |
| Participants | Total number randomised: 249 enroled participants (127 to apply the intervention and 122 to apply the comparator). Age: of the 229 who completed the trial: alclometasone group n = 114 mean age 37 years, range 12 to 77; hydrocortisone group n = 115 mean age 36 years, range 12 to 72 Sex: of the 229 who completed the trial: alclometasone group: males n = 43: females n = 71; hydrocortisone group: males n = 39: females n = 76 Ethnicity: of the 229 who completed the trial: alclometasone group: white n = 97; black n = 13; other n = 4; hydrocortisone group: white n = 93; black n = 18; other n = 4 Duration of eczema: alclometasone group: mean 13 years, range 1 to 54; hydrocortisone group: mean 14 years, range 1 to 48 Severity of eczema: % body involvement at baseline: · Alclometasone group: < 25% n = 82, 26 to 50% n = 25, 51 to 75% n = 6, 76 to 100% n = 1; hydrocortisone group: < 25% n = 79; 26 to 50% n = 29; 51 to 75% n = 6; 76 to 100% n = 1 Body site: lesions on the face, neck, trunk and extremities (excluding hands and feet) Number of withdrawals: 20 participants were not included; 18 did not meet protocol requirements, 1 experienced an adverse event in the alclometasone group stated in the first paragraph of the results; however, the safety results stated that 3 participants discontinued because of AE. Notes: none |
| Interventions | Run‐in details: no TCS within the past 2 weeks and no systemic corticosteroid within the past 4 weeks Intervention: alclometasone dipropionate 0.05% cream used three times daily for 21 days · Concurrent treatment: without occlusion · Other key information: none Comparator: hydrocortisone 1% cream used three times daily for 21 days · Concurrent treatment: no occlusion · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Clinical signs/symptoms (erythema, induration and pruritus, each of which were scored from 0 = absent to 3 = severe) · Participants were questioned and examined for adverse events such as irritation, sensitisation, folliculitis, atrophy, or any systemic effect · IGA (1 = cleared (100% clearance of all signs except for residual discolouration), 2 = marked improvement (between 75% and 100% clearance of signs), 3 = moderate improvement (50% to 75% clearance), 4 = slight improvement (clearance of < 50%), 5 = no change, 6 = exacerbation (flare at site) |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: This study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Bangert 2011.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: CASM981C2436 Country: 10 centres: UK n = 1, Germany n = 3, Austria n = 1, US n = 3, France n = 2 Outpatient or hospital: not reported Date trial conducted: April 2004 to June 2005 Duration of trial participation: 5 weeks Inclusion criteria: · Males ≥ 20 years of age · At least a 3‐year history of AD · AD according to the Hanifin and Rajka crtieria, and extrinsic forms of AD were identified with a prick‐test and/or elevated IgE background. · After treatment with TCS, patients were required to have a localised Eczema Area and Severity Index (EASI) score of ≤ 1 prior to entry into the double‐blind treatment period. · At screening, patients were required to have mild‐to‐moderate AD, defined as an IGA of the whole body of 2 (mild) or 3 (moderate); and a localised EASI of 1 to 8, and affecting bilateral arms and/or legs ≥ 10 cm2 Exclusion criteria: · Immunocompromised status, concurrent skin disease at the target site, active skin infections, history of rheumatic fever, prosthetic constituents, poor response to tacrolimus or pimecrolimus or a known hypersensitivity to study medications, serious reaction to anaesthetics, or any other clinical condition that could interfere with study evaluations · Phototherapy, systemic corticosteroids or other systemic therapy known or suspected to have an effect on AD within 4 weeks · Antihistamines within 7 days, or any topical therapy known or suspected to have an effect on AD during their current episode Additional design details: none |
| Participants | Total number randomised: 67 participants (34 to apply the intervention and 33 to apply the vehicle) Age: pimecrolimus group mean age 31.0 years (standard deviation (SD) 11.22), median 27 years, range 20 to 67 years; vehicle group 34.3 years (SD 9.92), median 34 years, range 19 to 65 Sex: 100% male Ethnicity: pimecrolimus group: Caucasian 70.6%, black 26.5%, other 2.9%: vehicle group: Caucasian 69.7%, black 21.3%, other 9.1% Duration of eczema: at least 3 years Severity of eczema: whole body IGA: pimecrolimus group mild 47.1%, moderate 52.6%; in vehicle group mild 36.4%, moderate 63.6% Body site: extent of disease: pimecrolimus group head/neck 64.7%, trunk 70.6%, upper limbs 97.1%, lower limbs 91.2%; in vehicle group head/neck 78.8%, trunk 75.8%, upper limbs 100%, lower limbs 81.8%, whole body 57.6% Number of withdrawals: 4/34 in pimecrolimus group, 14/33 in vehicle group Notes: none |
| Interventions | Run‐in details: an open‐label, 2‐week run‐in period: treatment with TCS for a maximum of 2 weeks until near clearance (EASI = 0 or 1, clear or almost clear) Intervention: pimecrolimus 1% cream used twice daily for 21 days Concurrent treatment: not reported Other key information: none Comparator: vehicle cream used twice daily for 21 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Molecular and cellular profile in skin biopsies and blood samples · Localised EASI, a composite variable of the severity (rated 0‐3) of erythema, infiltration/papulation, excoriations, and lichenification at the target sites as assessed by the investigator · Whole body IGA, 6‐point ordinal scale ranging from 0 = clear to 5 = very severe disease to evaluate the patient’s disease state at the time of the assessment · AE and serious AE with severity and relationship to study drug, and regular assessments of vital signs |
| Notes | Funding source: Novartis Declarations of interest: not declared Original language of publication: English Other: none |
Barba 2003.
| Study characteristics | |
| Methods | Trial design: randomised double‐blind, multicentre, parallel study Trial registration number: not reported Country: Mexico Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 3 weeks Inclusion criteria: · Paediatric patients (aged 3 months to 18 years) with mild‐to‐moderate AD of the face Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 114 participants (76 to apply the intervention and 38 to apply the vehicle cream) Age: 3 months to 18 years Sex, ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐moderate Body site: face Number of withdrawals: 5/76 in the pimecrolimus group and 3/38 in the vehicle group did not complete. No reasons provided Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream used not specified for 21 days Concurrent treatment: not reported Other key information: none Comparator: vehicle cream used not specified for 21 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: none Notes: none |
| Outcomes | · Facial IGA at three weeks · EASI at three weeks · Pruritis at three weeks · Patient assessment of good or complete control at three weeks · AE at three weeks |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: one |
Barba‐Rubio 1976.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, single‐centre, within‐participant study Trial registration number: not reported Country: Mexico Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 14 to 21 days (dependent on response) Inclusion criteria: varied steroid responsive dermatitis, including AD with bilateral lesions of similar severity Exclusion criteria: not reported Additional design details: only characteristics of the subgroup of AD participants were reported. |
| Participants | Total number randomised: 18 sides treated (9 to apply intervention and 9 to apply comparator) Age: not reported per disease group; only overall age range for all types of dermatitis, which was 1 month to 68 years Sex: not reported per disease group Ethnicity, duration of eczema, severity of eczema: not reported Body site: not reported per disease group Number of withdrawals: not reported per disease group Notes: none |
| Interventions | Run‐in details: not reported Intervention: halcinonide 0.1% cream used three times daily for 14 to 21 days Concurrent treatment: neomycin sulfate 0.25% and bystatid 100,000 units per gram Other key information: none Comparator: hydrocortisone 1% cream used three times daily for 14 to 21 days Concurrent treatment: iodochlorhydroxyquin 3% Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Overall evaluation of therapeutic response and comparative response in regard to treatment superiority · Local AE |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Bieber 2007.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre parallel study Trial registration number: EUCTR2004‐002099‐40‐DE Country: Germany, Italy, Spain Outpatient or hospital: 25 centres in Germany, Italy and Spain; not reported if outpatient or hospital Date trial conducted: February to August 2005 Duration of trial participation: 3 weeks Inclusion criteria: · Acute flare of AD according to the IGA score (IGA ≥ 4) ‘severe’ or ‘very severe’ · History of moderate‐to‐severe form of AD for at least one year · Age between 2 and 15 years · Affected BSA: minimum of 5% · Washout periods to be observed before start of study medication: ‐ At least 4 weeks have passed since any use of systemic therapy for AD, any vaccination, local AD therapy using protopic or elidel and since antihistaminic therapy ‐ At least 1 week has passed since local AD therapy using corticosteroids Exclusion criteria: · Indication for systemic therapy of AD · Known sensitivity to advantan, neribas, or protopic or to any excipients of the respective formulations. Known sensitivity to macrolides · Lymphadenopathy · Known immune deficiency, hepatic insufficiency and/or renal insufficiency · Acute herpes simplex, mononucleosis, or mollusca contagiosa infection · Acute and severe impetigo contagiosa (a slight superinfection of eczema is no exclusion criterion) · Severe other viral, bacterial, or fungal skin infection · Acute infestation · Generalised erythroderma, Netherton’s syndrome · Any conditions that compromise the patient's ability to understand the patient information, to give informed consent, to comply with the trial protocol, or to complete the study · Patient is a dependent person, e.g. a relative/family member of the investigator and/or is a member of the investigator’s staff Additional design details: none |
| Participants | Total number randomised: 265 participants (129 in the intervention group and 136 in the comparator group) Age: methylprednisolone group mean 7.8 years (SD 4.2); tacrolimus group 7.5 years (SD 4.2). Age ranged from 2 to 15 with the majority from 2 to 6 years (43.4% in the methylprednisolone group and 47% in the tacrolimus group) Sex: not reported Ethnicity: methylprednisolone group, Caucasian 94.5%, black 2.3%, oriental 2.3% and one other; tacrolimus group, Caucasian 98.5%, black n = 1, oriental n = 1 Duration of eczema: at least one year Severity of eczema: acute severe or very severe flare of AD (IGA 4 or more) Body site: not reported Number of withdrawals: eight participants withdrew, two in methylprednisolone group (one protocol deviation, one lost to follow‐up) and six in the tacrolimus group (four because of AE, one withdrew consent, and one lost to follow‐up) Notes: none |
| Interventions | Run‐in details: washout periods to be observed before start of study medication: ‐ at least 4 weeks have passed since any use of systemic therapy for AD, any vaccination or local AD therapy using protopic or elidel ‐ at least 2 weeks have passed since antihistaminic therapy ‐ at least 1 week has passed since local AD therapy using corticosteroids Intervention: methylprednisolone aceponate 0.1% ointment used twice daily for 21 days Concurrent treatment: not reported Other key information: none Comparator: tacrolimus 0.03% ointment used twice daily for 21 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA score time points over 21 days · EASI score time points over 21 days · BSA affected time points over 21 days · Children’s Dermatology Life Quality Index (CDLQI) (results not provided) · Patient or parent assessment of Itch during last 24 hours and quality of sleep using VAS at end of treatment (21 days) · AE · Medication costs |
| Notes | Funding source: Intendis GmbH, Berlin Declarations of interest: two authors affiliated with commercial sponsor Intendis who manufactured one of the interventions tested in the study Original language of publication: English Other: none |
Binder 1977.
| Study characteristics | |
| Methods | Trial design: randomised, parallel study Trial registration number: not reported Country: USA Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 2 weeks Inclusion criteria: · Eczema/AD · No significant secondary diseases Exclusion criteria: · Received systemic or topical corticosteroid therapy within the past two weeks · Had tuberculosis · Had fungal or viral lesions of the skin · History of hypersensitivity to steroids · Required concomitant medications that might interfere with evaluations · Pregnant or lactating Additional design details: none |
| Participants | Total number randomised: 34 participants (17 to apply clocortolone pivalate and 17 to apply vehicle) Age: mean age 30 years Sex: female n = 20, male n = 9 Ethnicity: not reported Duration of eczema, severity of eczema, body site: not reported Number of withdrawals: five; reasons were that they did not return for evaluation and protocol deviation Notes: none |
| Interventions | Run‐in details: not reported Intervention: clocortolone pivalate 0.1% cream used three times daily for 14 days Concurrent treatment: not reported Other key information: none Comparator: vehicle cream used three times daily for 14 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Index of Observed Clinical Symptomatology (an objective summation of ratings of severity for all observed clinical symptoms, erythema, vesicles and bullae, papules, plaques, scaling crusting, fissures and lichenification, at each evaluation (rated 0 absent, 1 mild, 2 moderate and 3 severe) · AE · The Physician's Rating of Improvement, a subjective evaluation of the response. Rating 0 (worse) to 4 (excellent) |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Bissonnette 2010.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, single‐centre, parallel study Trial registration number: NCT00837551 Country: USA Outpatient or hospital: not reported Date trial conducted: March to October 2008 Duration of trial participation: 35 days (28 days treatment with one week follow‐up) Inclusion criteria: · 18 to 65 years · AD according to Hanifin criteria affecting 1 to 10% BSA excluding face, groin, scalp and genital areas · EASI < 12 and IGA of 2 to 3 · Good general health without any condition that might interfere with the study evaluation · Women of childbearing potential had to have a negative pregnancy test and were required to use adequate contraception throughout the study. Exclusion criteria: · Spontaneously improving or rapidly deteriorating AD · Lesions on only hands and/or feet · Active non‐atopic forms of dermatitis, or skin diseases other than AD · Concomitant medical condition that could put the patient at risk · History of neurological/psychiatric disorders that could affect participation · Systemic immunomodulatory therapies within 12 weeks · Prolonged exposure to ultraviolet radiation (natural/artificial), phototherapy or systemic AD therapy within 4 weeks · Alcohol abuse within 2 years · History of allergy to ingredients of the study cream · Used an investigational drug within 1 month of day 0 or were currently participating in another trial Additional design details: none |
| Participants | Total number randomised: 36 in protocol/37 in paper participants Age, sex, ethnicity, duration of eczema: not reported Severity of eczema: IGA 2 to 3 Body site: facial eczema was excluded. Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention one: tapinarof 0.5% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention two: tapinarof 1% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: placebo/vehicle cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both interventions and comparator: not reported Notes: none |
| Outcomes | · EASI, mean and percentage change from baseline · SCORAD, mean and percentage change from baseline · IGA, mean and percentage change from baseline · BSA, mean and percentage change from baseline · Pruritus using 10 cm VAS, mean and percentage change from baseline · Safety and tolerability assessed by clinical examination, electrocardiography, haematology and clinical chemical analysis, urinalysis, and AE |
| Notes | Funding source: Welichem Biotech Inc Declarations of interest: some authors affiliated to Welichem Biotech Inc (Burnaby, British Columbia, Canada), and Innovaderm Research Inc (Montreal, Quebec, Canada). Welichem Biotech Inc developed tapinarof Original language of publication: English Other: none |
Bissonnette 2012.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre study Trial registration number: NCT01098734 Country: Canada Outpatient or hospital: 5 centres Date trial conducted: November 2009 to July 2010 (patients recruited) Duration of trial participation: 42 days Inclusion criteria: · Male or female aged between 18 and 65 years · Chronic AD for 6 or more months according to the Hanifin's criteria · Minimum of 3% and a maximum of 20% BSA affected with AD Exclusion criteria: · Spontaneously improving or rapidly deteriorating AD · Presence of AD lesions on only hands and/or feet · Allergic contact dermatitis or other non‐atopic forms of dermatitis Additional design details: the study involved two randomised phases and three‐arms. The data from the first randomised phase is presented here. Data from the second phase were not extracted as this would involve double counting of participants who were in the vehicle arm being re‐randomised to active arms. |
| Participants | Total number randomised: 148 participants (50 to apply the intervention and to apply the comparator). Age: tapinarof 0.5% mean 32.2 years (SD 12.4); tapinarof 1% mean 30.9 years (SD 11.6); vehicle: mean 35.4 (SD 13.1) Sex: tapinarof 0.5%: 40% males; tapinarof 1%: 34% males; vehicle: 47% males Ethnicity: not reported Duration of eczema: at least 6 months Severity of eczema: mild‐to‐severe Body site: not reported Number of withdrawals: 10 in total; tapinarof 0.5%: n = 3 (AE n = 2, prohibited medicine n = 1); tapinarof 1%: n = 7 (AE n = 2, withdrew consent n = 3, investigator discretion n = 1, prohibited medicine n = 1) Notes: none |
| Interventions | Run‐in details: to stop all topical AD therapy and any phototherapy, photochemotherapy, or systemic AD therapy within 2 weeks prior to the baseline visit. Prolonged exposure to natural or artificial sources of ultraviolet radiation and systemic immunomodulatory therapies for other conditions were prohibited for 4 weeks prior to the baseline visit. Intervention one: tapinarof 0.5% cream used twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Intervention two: tapinarof 1% cream used twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA score · EASI score · SCORAD which included BSA and pruritus severity score |
| Notes | Funding source: Welichem Biotech Inc. Declarations of interest: five authors declared conflicts as a speaker and/or consultant, and/or advisory board member and ⁄ or investigator and ⁄or have received honoraria and grants from various pharmaceutical companies including Astellas, La Roche‐ Posay, Leo, Galderma and Welichem Biotech Inc. Three authors are full‐time employees of Welichem Biotech Inc. Original language of publication: English Other: none |
Bissonnette 2016.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: NCT02001181 Country: Canada Outpatient or hospital: to quote: 'The study was conducted at five investigator sites in Canada'. Not reported if outpatients or hospital Date trial conducted: December 2013 to September 2014 Duration of trial participation: 4 weeks Inclusion criteria: · Clinical diagnosis of AD according to Hanifin and Rajka criteria ≥ 6 months duration and clinically stable for ≥ 1 month before the study · IGA of 2 or 3 (mild‐to‐moderate) · 2 to 20% of BSA involved the head (excluding scalp), neck, trunk (excluding groin and genitals), or limbs (excluding palms and soles). Exclusion criteria: · Skin conditions/infections · Atopic dermatitis on groin, genitals, palm or soles · Laboratory abnormalities at baseline · Pregnancy, breastfeeding, or of childbearing potential without effective contraception · Unable to washout (topical agents for ≥ 2 weeks, phototherapy or systemic immunosuppressive or cytostatic therapy for ≥ 16 weeks, or corticosteroid therapy (oral or injectable) for ≥ 4 weeks) Additional design details: None |
| Participants | Total number randomised: 69 participants (35 in intervention group and 34 in vehicle group) Age: mean age: overall 31.4 years (SD 10.1), tofacitinib group 32.4 years (SD 9.8), vehicle group 30.4 years (SD: 10.4) Sex: vehicle group female 52.9%, male 47.1%; intervention group female 54.3%, males 45.7% Ethnicity: all participants were described as "non‐Hispanic/Latino": intervention group: white n = 30, black n =1, Asian n = 3, other n = 1; vehicle group: white n = 25, black n =2, Asian n =7 Duration of eczema: not reported Severity of eczema: EASI total score: intervention group mean 5.4 (SD 2.6); vehicle group mean 5.7 (SD 3.1). Number of participants judged mild: intervention group: n = 10; vehicle group: n = 9. Number of participants judged moderate: both intervention and vehicle groups n = 20 Body site: not reported for individuals; head (excluding hair‐bearing scalp), neck, trunk (excluding groin and genitals), or limbs (excluding palms and soles) Number of withdrawals: intervention group n = 1, lost to follow‐up; vehicle group n = 3, 1 withdrawal by participant; 2 AE Notes: none |
| Interventions | Run‐in details: not reported Intervention: tofacitinib 2% ointment used twice daily for 28 days Concurrent treatment: not reported Other key information: none Comparator: vehicle ointment used twice daily for 28 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: non‐medicated emollient and sunscreen were permitted on non‐lesional skin only. Use of any topical product was prohibited on lesional skin, except for shampoos containing tar, salicylic acid or least potent corticosteroid products (namely hydrocortisone ≤ 1% and hydrocortisone acetate ≤ 1%) to be used on lesional hair‐bearing scalp. Medications such as corticosteroid inhalers and intranasal sprays were permitted if on a stable regimen. Notes: none |
| Outcomes | · EASI at baseline and 4 weeks follow‐up · IGA at 4 weeks |
| Notes | Funding source: Pfizer Inc. Declarations of interest: ten authors had declarations of interest; six are employees and shareholders of Pfizer who developed the intervention, four have served on advisory boards, received grants and/or acted as a consultant for Pfizer or another pharmaceutical company. Original language of publication: English Other: none |
Bissonnette 2019.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, single‐centre, within‐participant study Trial registration number: NCT03233529 Country: Canada Outpatient or hospital: not reported Date trial conducted: July 2017 to May 2018 Duration of trial participation: double‐blind trial for 14 days, with a 28‐day open‐label phase where all participants received crisaborole Inclusion criteria: · Aged ≥ 18 years · Active eczema according to the Hanifin and Rajka's criteria · 6 months duration of eczema with stable disease for 1 month or longer · ISGA score of mild (2) or moderate (3) · Percentage of treatable BSA 0.5 ≥ and ≤ 10 · Presence of two target lesions of 3 cm x 3 cm or greater with identical lesional ISGA scores ≥ 3, ≥ 5 cm apart and excluding face, scalp, axilla, genitals, groin area, palms, backs of the hands, or soles Exclusion criteria: · Clinically infected eczema · Previous treatment with any PDE4 inhibitor unless stopped for lack of efficacy Additional design details: none |
| Participants | Total number randomised: 80 sides treated (40 to apply the intervention and 40 to apply the comparator) Age: mean 32.2 years (SD 11.2), range from 18 to 57 Sex: females n = 27: males n = 13 Ethnicity: white: n = 34; black n = 3; Asian n = 3; other n = 0 Duration of eczema: ≥ 6 months Severity of eczema: based on global: mild severity. n = 5; moderate n = 35 Body site: not reported per participant, however, excluding face, scalp, axilla, genitals, groin area, palms, backs of the hands, or soles Number of withdrawals: there were two withdrawals due to worsening of eczema, one in the randomised comparison phase, judged unrelated to the study medication. Notes: baseline target (for trial treatments) lesion characteristics were balanced across treatments. |
| Interventions | Run‐in details: prior to the study, a washout period was required: ≥ 12 weeks or 4 half‐lives for biologicals, ≥ 28 days for oral immunosuppressants, including corticosteroids, or phototherapy, ≥ 14 days for topicals containing urea, corticosteroids, calcineurin inhibitors, prescription skin barrier products, systemic antibiotics, and doxepin, ≥ 7 days for topical antibiotics, antibacterial soaps/topical sodium hypochlorite‐based products, topical or oral antihistamines and ≥ 8 hours for emollients Intervention: crisaborole 2% ointment used twice daily for 14 days Concurrent treatment: not reported. Other key information: each treatment was applied to a target lesion. If the target lesion was less than 100 cm2, the application area included surrounding non‐lesional skin; if the target treatment area was larger than 100 cm2, only 100 cm2 of the lesion was treated. Comparator: vehicle 0% ointment used twice daily for 14 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: emollients were not allowed on target lesions. Notes: none |
| Outcomes | · Investigator assessment of clinical signs/symptoms at day 15 Lesion TSS change from baseline at day 15 · Investigator’s Static Global Assessment (ISGA) score and proportion of patients achieving ISGA success change from baseline at day 8 and 15 · Lesion pruritus numerical rating scale (NRS) score at each visit up to day 15 · Lesion TEWL · Percentage of treatable BSA · AEs · Key skin AD biomarkers |
| Notes | Funding source: supported by Pfizer who manufacture crisaborole Declarations of interest: to quote: "R. Bissonnette is an investigator, consultant, advisory board member, and speaker for and/or receives honoraria from Aquinox Pharma, Antiobix, Asana, Astellas, Brickell Biotech, Dermavant, Dermira, Dignity Sciences, Eli Lilly, Galderma, Glenmar". Original language of publication: English Other: none |
Bleeker 1975.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: Sweden Outpatient or hospital: not reported; however, the author is affiliated to a hospital dermatology department in Sweden. Date trial conducted: not reported Duration of trial participation: 2 weeks Inclusion criteria · Patients with psoriasis or eczema; to quote: "bilateral lesions similar in severity, persistency and aetiology." · Participants were to quote: "selected for their ability to follow instructions carefully regarding application of the medication." Exclusion criteria: not reported Additional design details: results presented separately for the patients with AD |
| Participants | Total number randomised: 54 sides (27 to apply the intervention and 27 to apply the comparator) Age, sex, ethnicity, duration of eczema: not reported Severity of eczema: 11 severe, 16 moderate Body site: not reported Number of withdrawals: none of the participants were reported to have withdrawn. Notes: none |
| Interventions | Run‐in details: not reported Intervention: halcinonide 0.1% cream used twice daily for 14 days Concurrent treatment: not reported Other key information: none Comparator: clobetasol propionate 0.05% cream used twice daily for 14 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · AE · Objective comparative clinical response (erythema, oedema, transudation, lichenification and scaling) · Subjective criteria (pruritus and pain) · Overall clinical response (4‐point scale: 'excellent', 'good', 'fair', 'poor'); this took into account the objective and subjective criteria as well as rapidity, maximum clearance and maintenance of therapeutic response. |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Boguniewicz 1998.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre study Trial registration number: not reported Country: USA Outpatient or hospital: 18 study centres Date trial conducted: not reported Duration of trial participation: 46 days Inclusion criteria: · Children ages 7 to 16 years · Moderate to severe AD according to the Hanifin and Rajka criteria · 5% to 30% BSA involvement · Menstruating female patients had to have a negative pregnancy test result and practice effective birth control Exclusion criteria: requiring anti‐infective drugs Additional design details: none |
| Participants | Total number randomised: 180 participants (43 to apply intervention one, 49 intervention two, 44 intervention three, and 44 the comparator) Age: mean: tacrolimus 0.03% group 10.1 years (SD 2.2); tacrolimus group 0.1% 10.8 years (SD 2.7); tacrolimus arm 0.3% 10.5 years (SD 2.8), placebo group 10.4 years (SD 2.9) Sex: M:F: tacrolimus 0.03% group 18:25; tacrolimus group 0.1% 21:28; tacrolimus group 0.3% 23:21, placebo group 18:26 Ethnicity: tacrolimus 0.03% group, n = 24 white, n = 12 black, n = 7 other; tacrolimus group, 0.1% n = 38 white, n = 10 black, n =1 other; tacrolimus arm 0.3%, n = 32 white, n = 11 black, n = 1 other; placebo group, n = 27 white, n = 14 black, n = 3 other Duration of eczema: mean: tacrolimus 0.03% group 8.1 years (SD 3.5); tacrolimus group 0.1% 7.8 years (SD 3.5), tacrolimus group 0.3% 8.8 years (SD 3.4); placebo group 8.7 years (SD 3.7) Severity of eczema: tacrolimus 0.03% group n = 38 moderate, n = 5 severe; tacrolimus group 0.1% n = 42 moderate, n = 7 severe; tacrolimus group 0.3% n = 39 moderate, n = 5 severe; placebo group n = 32 moderate, n = 12 severe Body site: not reported Number of withdrawals: 29 withdrew; tacrolimus group 0.03% arm n = 1 lack of efficacy, n = 1 non‐compliance; tacrolimus group 0.1% n = 4 non‐compliance, n = 1 AE; tacrolimus group 0.3%, n = 4 AE; placebo group n = 4 lack of efficacy, n = 2 AE, N = 1 non‐compliance Notes: none |
| Interventions | Run‐in details: to quote: "before enrollment, patients had to stop topical and inhaled corticosteroids for 1 week and systemic corticosteroids for 6 weeks. Patients receiving ultraviolet light therapy and immunotherapeutic agents had to stop such therapy at least 1 month before enrollment." Intervention one: tacrolimus 0.03% ointment used twice daily for 22 days · Concurrent treatment: not reported · Other key information: none Intervention two: tacrolimus 0.1% ointment used twice daily for 22 days · Concurrent treatment: not reported · Other key information: none Intervention three: tacrolimus 0.3% ointment used twice daily for 22 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for 22 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Physician’s Global Assessment (PGA) · Modified EASI · Patient's assessment of pruritus · Head and neck total score of physician assessment · Patient assessment of overall treatment effect · AE · Complete blood count and biochemistry panel |
| Notes | Funding source: Fujisawa USA, Inc. Declarations of interest: not declared Original language of publication: English Other: none |
Brautigam 2006.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: not reported Country: Germany Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 24 weeks Inclusion criteria: · Patients with severe AD (IGA score = 4) Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 62 participants (34 to apply the intervention and 28 to apply the comparator) Age, sex, ethnicity, duration of eczema: not reported Severity of eczema: severe (IGA = 4) Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream used as required for 24 weeks Concurrent treatment: not reported Other key information: intervention to quote: "to be initiated at early signs and symptoms of atopic eczema exacerbation and continued until complete resolution" Comparator: vehicle cream used as required for 24 weeks Concurrent treatment: none Other key information: comparator to quote: "to be initiated at early signs and symptoms of atopic eczema exacerbation and continued until complete resolution" Concurrent treatments received alongside both intervention and comparator: prednicarbate 0.25% as required Notes: none |
| Outcomes | · Decrease from baseline of EASI at 24 weeks · Decrease from baseline at 1 week EASI · Pruritis score at one day |
| Notes | Funding source: Novartis Pharma, the developer of pimecrolimus Declarations of interest: one author was an employee of Novartis Pharma. Original language of publication: English Other: none |
Breneman 2005.
| Study characteristics | |
| Methods | Trial design: randomised controlled, multicentre study Trial registration number: not reported Country: US Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 4 weeks Inclusion criteria: · Male or female with stable moderate‐to‐severe AD · DSS of erythema, excoriation and induration/papulation, of at least 6 in the target area · at least 3 of the following: pruritus, flexural lichenification, linearity in adults with a variety of skin lesions, chronic or chronically‐relapsing dermatitis · Personal or family history of atopy (asthma, allergic rhinitis, AD) Exclusion criteria: · Concomitant medical or dermatologic disorders precluding accurate evaluation of AD · Using interfering concomitant therapies · Known sensitivities to any ingredients of the study treatments Additional design details: none |
| Participants | Total number randomised: 229 participants Age: intervention (lotion) mean 39.3 years (SD 19); intervention (emollient) mean 42.4 years (SD 17.8); vehicle group mean 40.7 years (SD 19.4) Sex: intervention group males n = 84, females n = 112; vehicle group males n = 15, females n = 18 Ethnicity: intervention group: white n =136, black n = 39, oriental n = 7, Hispanic n = 10, other n = 4; vehicle group: white n = 23, black n = 8, oriental n = 0, Hispanic n = 2, other n = 0 Duration of eczema: intervention group 1 to 880 months; vehicle group 6 to 429 months Severity of eczema: moderate‐to‐severe eczema was eligible Body site: not reported Number of withdrawals: 23 of which 16 were receiving active treatment and 7 vehicle. Subject request and loss to follow‐up were the major reasons for discontinuation of participants receiving treatment. In the placebo group, one discontinued due to an adverse event. Notes: none |
| Interventions | Run‐in details: specific washout periods were to be respected for topical and systematic AD treatments. Intervention one: clobetasol propionate 0.05% lotion used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Intervention two: clobetasol propionate 0.05% cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle lotion used twice daily for 14 days · Concurrent treatment: not reported · Other key information: one Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Global Severity scale · Dermatologic Sum Score (DSS) · Safety |
| Notes | Funding source: Galderma Medical Affairs, Inc Declarations of interest: authors included employees of Galderma R&D, those receiving honoraries for participating in this trial or project funding from the company. Original language of publication: English Other: Outcome data were more complete at 2 weeks follow‐up than at end of follow‐up (at 4 weeks). |
Busch‐Heidger 1993.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: not reported Country: Germany Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 5 weeks; however, the paper also stated to quote: "treatment lasted for a minimum of 3 days and a maximum of 44 days. The median was between 20 and 21 days for both treatment groups." Inclusion criteria: patients diagnosed with AE Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 75 participants (38 to apply the intervention and 37 to apply the comparator) Age: average of 40 years (range 18 to 82) overall; HC buteprate group median 29.5 years (range 18 to 80); HC 17‐butyrate group median 42 years (range 18 to 82) Sex: overall, male n = 31 and female n = 44; HC buteprate group, male n = 19, female n = 18; HC 17‐butyrate group2, male n = 12, female n = 26 Ethnicity: not reported Duration of eczema: 83.8% had eczema since they were ≤ 10, 16.2% had eczema from ≥ 11 years Severity of eczema, body site: not reported Number of withdrawals: 6 people completed the trial prematurely (but all 75 participants were included in the evaluation). Notes: none |
| Interventions | Run‐in details: the English translation stated, to quote: "all ongoing treatments had been completed for at least 1 day and with corticosteroids 3 days before baseline". Intervention: hydrocortisone butyrate 0.1% fatty cream used twice daily for 5 weeks · Concurrent treatment: not reported · Other key information: brand: 'alfson creSa' Comparator: hydrocortisone buteprate 0.1% fatty cream used twice daily for 5 weeks. · Concurrent treatment: not reported · Other key information: brand: 'pandel creSa' Concurrent treatments received alongside both intervention and comparator: not reported Notes: the frequency of treatment application was reduced to once daily for some participants (unclear which), and ceased upon healing. |
| Outcomes | · Tolerability rated by the participant as very good, good, moderate or bad · Participant assessment of itching with scores 0 = not available, 1 = mild, 2 = moderate, and 3 = severe · Physician assessment of signs and symptoms: erythema, blistering, infiltration, scaling, lichenification, and excoriation as well as itching with scores 0 = not available, 1 = mild, 2 = moderate, and 3 = severe. The sum of scores was the main criterion and 'healing' was defined as blistering, infiltration, excoriation and itching completely disappeared, and the parameters, erythema, scaling and lichenification reaching a maximum score of 1. · Effectiveness rated by physicians as very good, good, moderate or bad · Physician evaluation of tolerability (assumed) · AE |
| Notes | Funding source: not reported Declarations of interest: none declared; however, the authors are affiliated to Medical Dept of a private company, Basotherm Biberach GmbH Original language of publication: German Other: This study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Cahn 1961a.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blinded within‐participant study Trial registration number: not reported Country: not reported Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 1 week Inclusion criteria: · AD and symmetrical skin lesions with equal degree of involvement Exclusion criteria: not reported Additional design details: the paper includes several controlled studies of fluocinolone acetonide cream and additional observational data. In scope for this review is the study of fluocinolone 0.025% (potent TCS) versus placebo (includes 12 double‐blind and 3 unblinded participants) and the study of fluocinolone 0.025% versus hydrocortisone 1% (mild TCS) |
| Participants | Total number randomised: 30 sides treated (15 to apply the intervention and 15 to apply the comparator) Age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: fluocinolone acetonide 0.025% cream used three times daily for 7 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used three times daily for 7 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: to quote: "In six of the patients, treatment was extended to two weeks." |
| Outcomes | · Number of participants with a "great" or "excellent" response · Instances of irritation or allergic reaction |
| Notes | Funding source: Synalar Cream and the other materials used in the study were supplied by Syntex laboratories (manufacturers of Synalar) Declarations of interest: not declared Original language of publication: English Other: none |
Cahn 1961b.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blinded, controlled within‐participant study Trial registration number, country, outpatient or hospital, date trial conducted: not reported Duration of trial participation: 1 week Inclusion criteria: AD (amongst other steroid‐responsive dermatoses) Exclusion criteria: not reported Additional design details: the paper included several controlled trials of fluocinolone acetonide cream and additional observational data. In scope for this review is the trial of fluocinolone 0.025% (potent TCS) versus placebo and the trial of fluocinolone 0.025% versus hydrocortisone 1% (mild TCS) as they are clearly randomised trials. |
| Participants | Total number randomised: 40 sides treated (20 to apply the intervention and 20 to apply the comparator) Age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: fluocinolone acetonide 0.025% cream used three times daily for 7 days · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone 1% cream used three times daily for 7 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Number of participants in whom fluocinolone performed better, hydrocortisone better or both equal · Instances of irritation or allergic reaction |
| Notes | Funding source: Synalar Cream and the other materials used in this paper were supplied by Syntex laboratories (manufacturers of Synalar). Declarations of interest: not declared Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Cai 2023.
| Study characteristics | |
| Methods | Trial design: randomised, parallel controlled study Trial registration number: ChiCTR‐IIR‐17012153 Country: China Outpatient or hospital: 5 centres Date trial conducted: October 2017 to April 2019 Duration of trial participation: 6 weeks Inclusion criteria: · Male, female aged over 18 years · AD (of at least 6 months, BSA 3% to 20%) and IGA of = 3 Exclusion criteria: · Pregnancy, lactation or people who plan to be pregnant · Any other skin diseases that may affect the evaluation, or the presence of scars, foetal plaques or tattoos in the affected area that may affect the evaluation · Lesions confined to the hands and feet · Skin local bacterial, viruses and fungal infections · Using topical anti‐inflammatory drugs within 2 weeks · Infection requiring treatment with systemic antibiotics, antivirals, antiparasitics, antiprotozoals, or antifungals within 4 weeks before the baseline visit · Phototherapy, photo‐chemotherapy within four weeks prior to the baseline visit · Abnormal liver function · Known or suspected hypersensitivity to any of the constituents of the investigational product · Alcohol, drug addiction/dependence · Treatment with other investigational drugs within three months or current participation in another clinical trial; · Those that researchers think are unsuitable for this study Additional design details: none |
| Participants | Total number randomised: 115 participants (29 to apply intervention one, 29 intervention two and intervention three and 28 to apply the comparator) Age: placebo group, mean 42.8 years (SD 14.9); tacrolimus group, mean 35.2 years (SD 13.4); benvitimod 0.5% group, mean 42.6 years (SD 15.6); benvitimod 1% group, mean 39.4 years (SD 17.0) Sex: placebo group, female 53.6%, male 46.4%; tacrolimus group, female 27.6%, male 72.4%; benvitimod 0.5% group, female 55.2%, males 44.8%; benvitimod 1% group, female 37.9%, male 62.1% Ethnicity, duration of eczema: not reported Severity of eczema: IGA score: placebo group, moderate 89.3%, severe 10.7%; tacrolimus group, moderate 79.3%, severe 17.2%, very severe 3.4%; benvitimod 0.5% group, moderate 69%, severe 31%; benvitimod 1% group, moderate 82.8%, severe 17.2% Body site: trunk or limbs Number of withdrawals: 13; placebo group, n = 6, tacrolimus group n = 1, benvitmod 0.5% group, n = 3, benvitimod 1% group, n = 3 Notes: none |
| Interventions | Run‐in details: not reported Intervention one: benvitimod 1% cream used twice daily for 6 weeks · Concurrent treatment: not reported · Other key information: none Intervention two: benvitimod 0.5% cream used twice daily for 6 weeks · Concurrent treatment: not reported · Other key information: none Intervention three: tacrolimus 0.1% cream used twice daily for 6 weeks · Concurrent treatment: not reported · Other key information: none Comparator: Intervention: vehicle cream used twice daily for 6 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: no concomitant use of other AD treatment was allowed during this trial. Notes: none |
| Outcomes | · Percentage of patients with IGA 0 or 1 · Average decline in IGA, BSA, EASI, and pruritus · AE, adverse drug reactions, and serious adverse events and clinical laboratory measurements |
| Notes | Funding source: Beijing Wenfeng Tianji Pharmaceuticals Co., Ltd. Declarations of interest: to quote "none" Original language of publication: English Other: none |
Caproni 2007.
| Study characteristics | |
| Methods | Trial design: randomised, single‐centre, parallel study Trial registration number: not reported Country: Italy Outpatient or hospital: outpatients (a hospital clinic) Date trial conducted: not stated, only "since 2004" Duration of trial participation: 21 days Inclusion criteria: · AD according to Hanifin and Rajka's criteria and requiring treatment · SCORAD ≥ 15 · 18 years and older · Contraception during and until 4 weeks after the study Exclusion criteria: · Infectious diseases requiring treatment, including HIV · Systemic disease for which tacrolimus is contraindicated · Clinically relevant renal/hepatic failure · Pregnancy/lactation · Cicatricial or pigmentary skin lesions that co‐localise with AD lesions, or any skin lesions requiring treatment, including those for which tacrolimus cannot be used (investigator's judgement) · Infected AD · Allergy to macrolides or excipients in the ointment · Alcohol/drug abuse/psychiatric disturbance or any other disorder that may affect communication (investigator's judgement) Additional design details: none |
| Participants | Total number randomised: 20 participants Age: intervention group mean, age 41.6 years, range 23 to 65; comparator group mean, age 33.4 years, range 20 to 63 Sex: male n = 7, female n = 9 Ethnicity, duration of eczema: not reported Severity of eczema: moderate‐to‐severe. Baseline SCORAD: intervention group mean, 43.8 (SD 10.75), range 29 to 60.3; comparator group mean, 46.5 (SD 14.0), range 25.2 to 54.3 Body site: trunk or limbs Number of withdrawals: 4 participants enroled did not complete the study; no reasons were given. Notes: none |
| Interventions | Run‐in details: pharmacological washout for two weeks prior to the study Intervention: tacrolimus 0.1% ointment used twice daily for 21 days Concurrent treatment: not reported Other key information: none Comparator: hydrocortisone butyrate 1% ointment used twice daily for 21 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · SCORAD at 21 days (end of treatment) · AE · Biopsies were taken for immunohistochemical staining using a number of monoclonal antibodies |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
CASM981C1301.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: CASM981C1301 Country: Japan Outpatient or hospital: 31 centres in Japan; not reported if outpatient or hospital Date trial conducted: November 2003 to December 2004 Duration of trial participation: 26 weeks Inclusion criteria: · Aged 2 to < 16 years · Diagnosis of AD according to Williams criteria, affecting ≥ 5 % BSA with IGA ≥ 2 (i.e. at least mild disease) Exclusion criteria: · Phototherapy or systemic therapy which could have affected AD within 4 weeks of the study · Antibiotics, antivirals or antifungals, or other topical therapy which could have affected their AD within 2 weeks of the study · Systemic anti‐allergic drugs (sodium cromoglicate, tranilast or suplatast tosilate) within 7 days of the study · History of malignant disease · Active skin infections or other systemic infections · Other skin conditions that could have affected study evaluations Additional design details: 3‐arm trial where all interventions were directly compared |
| Participants | Total number randomised: 240 participants (83 to apply intervention one; 79 to apply intervention two and 78 to apply the comparator) Age: pimecrolimus 1 % group, mean age 8.0 years (SD 3.6), pimecrolimus 0.6 % group, mean age 7.99 years (SD 3.1), vehicle group, 8.6 years (SD 3.5) Sex: pimecrolimus 1 % group, female n = 36; male n = 47, pimecrolimus 0.6 % group, female n = 42; male n = 37, vehicle group, female n = 42; male n =36 Ethnicity: all participants Asian Duration of eczema: not reported Severity of eczema: baseline IGA: pimecrolimus 1 % group, mild 28.9 %, moderate 63.9 %, severe 7.2 %; pimecrolimus 0.6 % group, mild 35.4 %, moderate 54.4 %, severe 10.1 %; vehicle group, mild 33.3 %, moderate 57.7 %, severe 9 % Body site: not reported Number of withdrawals: pimecrolimus 1 % group, n = 13, pimecrolimus 0.6 % group, n = 5, vehicle group n = 15 Notes: none |
| Interventions | Run‐in details: not reported Intervention one and two: pimecrolimus 1 % or 0.6 % cream used twice daily for 26 weeks Concurrent treatment: not reported Other key information: none Comparator: vehicle cream used twice daily for 26 weeks Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: topical corticosteroids were permitted twice daily as a rescue therapy for severe or very severe eczema: hydrocortisone butyrate on body/limbs; clobetasone butyrate on face/neck; and prednisolone valerate acetate lotion on scalp. After the remission of eczema by the treatment of topical corticosteroids, pimecrolimus cream 1 %, 0.6 % or vehicle was applied again. Notes: none |
| Outcomes | · Reducing and preventing flares · Safety assessments (recording all AE, serious AE, and regular monitoring of haematology, blood chemistry, urinalysis, and physical examinations) |
| Notes | Funding source: Novartis Declarations of interest: not declared Original language of publication: English Other: none |
CASM981C2322.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: ASM981 C2322 Country: China Outpatient or hospital: not reported Date trial conducted: January to August 2004 Duration of trial participation: 4 weeks Inclusion criteria: · Children or adults with mild‐to‐moderate AD · ≥ 5% BSA Exclusion criteria: · Females of childbearing potential without adequate contraception · Pregnancy or breastfeeding · Topical therapy having an effect on AD within 7 days of the trial · Systemic corticosteroid or leukotriene antagonist during 1 month preceding the trial Additional design details: none |
| Participants | Total number randomised: 336 participants (168 to apply the intervention and 168 to apply the comparator) Age: mean: intervention group mean, 21.05 years (SD 13.94); comparator group, 19.07 years (SD 13.33) Sex: intervention group, n = 57 females, n = 111 males; comparator group, n = 70 females, n = 98 males Ethnicity: Asian Duration of eczema: not reported Severity of eczema: mild‐to‐moderate Body site: not reported Number of withdrawals: Intervention group n = 8; comparator group n = 26 Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: none Notes: none |
| Outcomes | · IGA 0 or 1 · IGA (head and neck) · EASI · Pruritus · AE; to quote: "Safety evaluation comprised observation and recording of all adverse events, severe adverse events (and the relationship between severity and the trial drug) and pregnancy. Haematology, blood biochemistry and urinalysis were regularly monitored in the central laboratory. Vital symptoms, physical exam results and body weight were also evaluated." · Withdrawal due to AE |
| Notes | Funding source: Novartis Declarations of interest: not declared Original language of publication: English Other: none |
Cato 2001.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: USA Outpatient or hospital: two sites Date trial conducted: not reported Duration of trial participation: 2 weeks Inclusion criteria: · Chronic AD · Minimal of three stable or worsening AD lesions present for at least a year · A lesion total score of 7 or greater for at least two of the three lesions included in the study (scoring was based on severity of each sign and symptom erythema, induration and pruritus (0 = absent to 6 = markedly severe) Exclusion criteria: · Women nursing or pregnant · Active infection · Receiving systemic therapy or other investigational agents within 2 weeks of study entry Additional design details: the study has three arms of which one is not relevant for this review. It is the same TCS as the other active arm but with the addition of laurocapram whose known mode of action is not as an anti‐inflammatory but as an enhancer of percutaneous penetration of drugs. |
| Participants | Total number randomised: 100 participants (50 to apply the intervention and 50 to apply the comparator) Age: range from 18 to 86 years Sex: not reported Ethnicity: majority Caucasian Duration of eczema: at least one year Severity of eczema, body site: not reported Number of withdrawals: no patients dropped out Notes: none |
| Interventions | Run‐in details: stopped using any anti‐AD medication one week before enrolment Intervention: triamcinolone acetonide 0.05 % cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Disease sign/symptom severity score for erythema, induration, pruritus · Global evaluation of disease status |
| Notes | Funding source: not reported Declarations of interest: author affliations include Durham Pharmaceuticals Ltd. Original language of publication: English Other: none |
Chapman 1979.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind trial, within‐participant study Trial registration number: not reported Country: UK Outpatient or hospital: outpatients Date trial conducted: not reported Duration of trial participation: 3 weeks Inclusion criteria: AD with activity on two sides of the body Exclusion criteria: not reported Additional design details: 16 participants applied ointments to dry skin; 24 applied ointments to wet skin |
| Participants | Total number randomised: 80 sides treated (40 to apply the intervention and 40 to apply the comparator) Age: 2 months to 70 years Sex, ethnicity: not reported Duration of eczema: 2 months to 35 years Severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: hydrocortisone 1 % used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone butyrate 0.1 % ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA · Clinician reported signs · Participant assessment of efficacy |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: 16 participants applied intervention/control to dry skin and 24 to wet/damp skin |
Chapman 2005a.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre study Trial registration number: not reported Country: USA Outpatient or hospital: 37 investigational sites Date trial conducted: September 2001 to October 2002 Duration of trial participation: up to 42 days Inclusion criteria: · Male and female patients had a diagnosis of mild‐to‐moderate AD · 2% to 30% BSA · Aged 2 to 15 years Exclusion criteria: · Skin disease other than AD in the treatment area such as infections and dyspigmentation · Previously used tacrolimus ointment for AD, or had a known hypersensitivity to macrolides or excipients of the ointment. Additional design details: this is one of two identically‐designed, independent, randomised, double‐blind, 6‐week studies; the other is in adults and several outcomes are pooled between the two. |
| Participants | Total number randomised: 618 (pooled between the two trials) participants Age: combined (paediatric and adult study), both treatment groups median 15 years (range 2 to 79) Sex: tacrolimus group, male 41%, female 59%; vehicle group, 41.4%, female 58.6% Ethnicity: tacrolimus group, white 69.7%, African‐American 21.6%, other 8.7%; vehicle group, 70.4%, African‐American group 23.8%, other 5. 9% Duration of eczema: not reported Severity of eczema: IGA,% of patients: mild: tacrolimus group 61%, vehicle group 62.5%; moderate: tacrolimus group 39%, vehicle group 37.5% Body site: not reported Number of withdrawals: tacrolimus group n = 50; vehicle group n = 102 Reasons were lost to follow‐up n = 39, AE n = 32, lack of efficacy n = 38, non‐compliant n = 16, other n = 26 Notes: none |
| Interventions | Run‐in details: the following treatments were prohibited during the study: nonsteroidal immunosuppressants, UV light therapy, systemic and topical corticosteroids, topical antihistamines, topical antimicrobials, and any other medicated topical agent. Non‐medicated topical agents were permitted only in the areas not being treated with study medication. Intranasal or inhaled corticosteroids were permitted if use was restricted to 2 mg/d or less (prednisone equivalent). Systemic antihistamines were permitted only if the patient was taking a stable dose at baseline; this dose could be decreased or discontinued during the study. Sunscreens were permitted throughout the study; use of cosmetics on treatment sites was prohibited. Intervention: tacrolimus 0.03% ointment used twice daily for up to 42 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for up to 42 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: study medication was applied twice daily to the affected area or areas for up to 6 weeks, or until 1 week after the affected area or areas were completely clear, whichever came first. |
| Outcomes | · IGA ·% BSA · Physician’s assessment of individual signs in each of the 4 body regions · Patient’s assessment of Itch · EASI · AE |
| Notes | Funding source: Astellas Pharma US, Inc. Declarations of interest: three authors were full‐time employees of Astellas Pharma US, Inc; other authors have received research support/grants, have been speakers, clinical investigators or consultants for Astellas Pharma US, or Novartis Pharmaceuticals Corp. Original language of publication: English Other: pooled analysis was extracted where data from individual trials were not reported. |
Chapman 2005b.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre study Trial registration number: not reported Country: USA Outpatient or hospital: 37 investigational sites Date trial conducted: September 2001 to October 2002 Duration of trial participation: up to 42 days Inclusion criteria: · Male and female patients with mild‐to‐moderate AD · 2% to 30% BSA · Aged ≥ 16 years Exclusion criteria: · Skin diseases other than AD in the treatment area such as infections and dyspigmentation · Previously used tacrolimus ointment for AD, or had a known hypersensitivity to macrolides or excipients of the ointment Additional design details: this is one of two identically‐designed, independent, randomised, double‐blind, 6‐week studies; the other is in paediatric patients and several outcomes were pooled between the two studies. |
| Participants | Total number randomised: 618 (pooled between the two trials) participants Age: median: combined (children and adult study) in both groups, 15 years (2 to 79) Sex: tacrolimus group, male 41%, female 59 %; vehicle group, male 41.4%, female 58. 6% Ethnicity: tacrolimus group, white 69.7%, African‐American 21.6%, other 8.7%; vehicle group, white 70.4%, African‐American 23.8%, other 5.9% Duration of eczema: not reported Severity of eczema: IGA, tacrolimus group mild 61 %, moderate 39 %; vehicle group mild 62.5 %, moderate 37. 5 % Mild: in tacrolimus group 61%, in vehicle group 62.5%; moderate: in tacrolimus group 39%, in vehicle group, 37.5% Body site: not reported Number of withdrawals: tacrolimus group n = 50; vehicle group n = 102. Reasons were lost to follow up n = 39, AE n = 32, lack of efficacy n = 38, non‐compliant n = 16, other n = 26 Notes: none |
| Interventions | Run‐in details: the following treatments were prohibited during the study: nonsteroidal immunosuppressants, UV light therapy, systemic and topical corticosteroids, topical antihistamines, topical antimicrobials, and any other medicated topical agent. Non‐medicated topical agents were permitted only in the areas not being treated with study medication. Intranasal or inhaled corticosteroids were permitted if use was restricted to 2 mg/d or less (prednisone equivalent). Systemic antihistamines were permitted only if the patient was taking a stable dose at baseline; this dose could be decreased or discontinued (but not increased) during the study. Sunscreens were permitted throughout the study; use of cosmetics on treatment sites was prohibited. Intervention: tacrolimus 0.03% ointment used twice daily for up to 42 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for up to 42 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: study medication was applied twice daily to the affected area or areas for up to 6 weeks, or until 1 week after the affected area or areas were completely clear, whichever came first. |
| Outcomes | · IGA · % BSA · Physician’s assessment of individual signs in each of the 4 body regions · Patient’s assessment of Itch · EASI · AE |
| Notes | Funding source: Astellas Pharma US, Inc. Declarations of interest: three authors were full‐time employees of Astellas Pharma US, Inc; other authors have received research support, grants, been speakers, clinical investigators or consultants for Astellas Pharma US, or Novartis Pharmaceuticals Corp. Original language of publication: English Other: none |
Charney 1975.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number, country, outpatient or hospital, date trial conducted: not reported Duration of trial participation: 3 weeks Inclusion criteria: · AD that was stable or worsening · A sum of ≥ 4 for erythema and one other sign or symptom scored from 0 = none to 4 = most severe Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 30 participants Age, sex, ethnicity, duration of eczema: not reported Severity of eczema: all participants had a sum of ≥ 4 for erythema and one other sign or symptom scored from 0 = none to 4 = most severe. Body site: not reported Number of withdrawals: 2 patients did not return for all follow‐up visits and were excluded from analysed data. It is unclear which groups they were in. An additional 9 participants stopped treatment early because of clearing of their disease or treatment failure. Notes: none |
| Interventions | Run‐in details: not reported Intervention: betamethasone dipropionate 0.05% cream used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: no additional anti‐inflammatory or antipruritic treatments, including topical or systemic corticosteroids, were permitted. Notes: none |
| Outcomes | · Signs and symptoms of erythema, induration, exudation, lichenification, scaling, excoriations, and pruritus were scored from 0 = none to 4 = most severe. · Overall change in clinical status compared to baseline · AE |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Craps 1973.
| Study characteristics | |
| Methods | Trial design: double‐blind, randomised, within‐participant study Trial registration number: not reported Country: not reported; however, the trial authors were affiliated to Sandoz Ltd, Switzerland Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 14 days Inclusion criteria: patients with bilateral eczema, making the following assumptions: · Diagnosed by a medical doctor · All were eczema patients (psoriasis is mentioned in the introduction, but methods suggest eczema patients) Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 100 sides treated (50 to apply the intervention and 50 to apply the comparator) Age, sex, ethnicity, duration of eczema, severity of eczema: not reported Body site: to quote: "extremities" Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: fluocinolone acetonide 0.025% cream used for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: clocortolone pivalate 0.1% cream used for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Severity of signs (erythema, exudation, vesiculation, desquamation, pruritus, lichenification) reported by the clinician (assumed) using a 4‐point scale from 0 = absent to 3 = very severe · AE "systematically investigated and registered" |
| Notes | Funding source: not reported Declarations of interest: none declared; however, trial authors were affiliated to Sandoz Ltd, who manufacture fluocinolone acetonide cream. Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Cullen 1971a.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: US Outpatient or hospital: private dermatology practice Date trial conducted: not reported Duration of trial participation: ≥ 14 days Inclusion criteria: AD (also psoriasis, but those results have not been extracted). The paper stated, to quote: "simultaneous symmetrically‐paired comparison method" from which it can be inferred that patients were judged to have bilateral lesions. AD was categorised as severe and moderate in the results; however, it was not clear whom they had intended to recruit in terms of severity. Exclusion criteria: not reported Additional design details: this trial was reported alongside two other trials; one where a TCS of the same potency was compared to another TCS and one in patients that did not have AD; hence, these have not been extracted for this review. |
| Participants | Total number randomised: 44 sides treated (22 to apply the intervention and 22 to apply the comparator) Age, sex: ethnicity, duration of eczema: not reported Severity of eczema: 8 participants had severe disease at baseline and 14 had moderate disease. Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tralonide 0.005% ointment used for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · AE e.g. uncomfortable burning, changes in pigmentation, folliculitis, skin atrophy, or increased capillary fragility · Objective evaluation through observation of erythema, scaling, vesiculation, oozing, crusting, pustulation, fissuring, lichenification, thickening, and induration and subjective evaluation of pruritus, burning, and pain, judged excellent, good, partial improvement, no improvement, or worse |
| Notes | Funding source: none stated; however, tralonide was supplied by Eli Lilly and Co., Indiana. Declarations of interest: not declared Original language of publication: English Other: none |
Cullen 1971b.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant trial Trial registration number: not reported Country: US Outpatient or hospital: private dermatology practice Date trial conducted: not reported Duration of trial participation: ≥ 14 days Inclusion criteria: AD (also psoriasis, but those results have not been extracted). The paper stated to quote: "simultaneous symmetrically‐paired comparison method" from which it can be inferred that patients were judged to have bilateral lesions. AD was categorised as severe and moderate in the results; however. However, it was not clear whom they had intended to recruit in terms of severity. Exclusion criteria: not reported Additional design details: this trial was reported alongside two other trials; one where a TCS of the same potency was compared to another TCS and one in patients that did not have AD; hence, these have not been extracted for this review. |
| Participants | Total number randomised: 24 sides treated (12 to apply the intervention and 12 to apply the comparator) Age, sex, ethnicity, duration of eczema: not reported Severity of eczema: severe n = 5, moderate n = 7 Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tralonide 0.025% ointment used at unspecified frequency for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: fluorandrenolone acetonide 0.05% ointment used at unspecified frequency for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · AE e.g. uncomfortable burning, changes in pigmentation, folliculitis, skin atrophy, or increased capillary fragility · Objective evaluation of effectiveness of treatments through observation of erythema, scaling, vesiculation, oozing, crusting, pustulation, fissuring, lichenification, thickening, and induration and subjective evaluation of pruritus, burning, and pain, judged excellent, good, partial improvement, no improvement, or worse |
| Notes | Funding source: not reported; however, medication interventions were supplied by Eli Lilly and Co., Indiana. Declarations of interest: not declared Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022 |
Cunliffe 1974.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: UK Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 6 weeks Inclusion criteria: patients with eczema or psoriasis Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 40 sides treated (20 to apply the intervention and 20 to apply the comparator) Age: average: males 30 years (range 4 to 70); females 29 years (range 4 to 76) Sex: male n = 8, female n = 12 Ethnicity, duration of eczema, severity of eczema, body site: not reported Number of withdrawals: there were no withdrawals Notes: none |
| Interventions | Run‐in details: not reported Intervention: fluocinide 0.05% cream used three times daily for 42 days · Concurrent treatment: not reported · Other key information: none Comparator: betamethasone valerate 0.1% cream used three times daily for 42 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Global improvement score · Improvement of individual symptoms of erythema, scaling, weeping and itching · Tolerance |
| Notes | Funding source: not reported Declarations of interest: not declared, but one of the two authors was affiliated with a pharmaceutical company. Original language of publication: English Other: none |
Dahnhardt 2021.
| Study characteristics | |
| Methods | Trial design: randomised, parallel study Trial registration number: not reported Country: Germany Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 12 weeks Inclusion criteria: · Active AD (SCORAD‐values between 10 and 63 [local SCORAD]) · Eczematous lesions on the forearm Exclusion criteria: not reported Additional design details: In the reactive phase, forearm lesions patients were treated with either of the randomised treatments for 10 days. In the subsequent proactive phase, patients applied either of the treatments twice‐weekly for 12 weeks. |
| Participants | Total number randomised: 20 participants Age: 19 to 48 years Sex: male n = 12, female n = 8 Ethnicity, duration of eczema, severity of eczema: not reported Body site: all patients had eczematous lesions on the forearm. It is not clear if they had any elsewhere. Number of withdrawals: four patients (two from each treatment group) withdrew during proactive therapy, thus 16 patients completed the proactive phase. Reasons for withdrawal were exacerbation, colds, spontaneous cat hair allergy and non‐adherence to the treatment plan. Notes: none |
| Interventions | Run‐in details: did not use emollients on the treatment site for at least 3 days prior to participation Intervention: mometasone furoate 0.1% cream used twice daily for 10 days followed by twice‐weekly for 12 weeks · Concurrent treatment: not reported · Other key information: none Comparator: tacrolimus 0.1% ointment used twice daily for 10 days followed by twice‐weekly for 12 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Local SCORAD · Skin capacitance · TEWL · Morphometric analysis |
| Notes | Funding source: not reported Declarations of interest: two authors declared no conflicts of interest. Another is an employee of LEO Pharma GmbH, another was involved in studies from Astellas, LEO Pharma, Novartis, Regeneron and Beiersdorf. Original language of publication: English Other: none |
De Gregorio 1970a.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: Italy Outpatient or hospital: dermatology clinic Date trial conducted: not reported Duration of trial participation: 5 to 12 days Inclusion criteria: · Eczematous eruptions symmetrically distributed · Expected to respond to steroid cream Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 38 sides treated (19 to apply the intervention and 19 to apply the comparator) Age: adults Sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: bendazac 3% cream used twice daily for 5 to 14 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 5 to 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · effect on erythema, oedema, vesiculation, weeping, scaling, infiltration, itching, burning · IGA |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
De Gregorio 1970b.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: Italy Outpatient or hospital: dermatology clinic Date trial conducted: not reported Duration of trial participation: 5 to 12 days Inclusion criteria: · Eczematous eruptions symmetrically distributed · Expected to respond to steroid cream Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 48 sides treated (24 to apply the intervention and 24 to apply the comparator) Age: adults Sex, ethnicity, duration of eczema, severity of eczema, body site: not reported Number of withdrawals: there were no withdrawals. Notes: none |
| Interventions | Run‐in details: not reported Intervention: bendazac 3% cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone 3% cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Overall evaluation as to which studied area per treatment showed greater clinical improvement |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
De Prost 1989.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: France Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 14 days Inclusion criteria: AD according to Hanifin and Rajka criteria and with stable, symmetrical lessions Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 40 sides treated (20 to apply cyclosporin and 20 to apply the vehicle comparator) Age: mean 11.6 years (SD 7.5), range 2 and 29 years Sex, ethnicity, duration of eczema, severity of eczema: not reported Body site: popliteal fossae, cubital fossae, extensor surface of the knees, wrists Number of withdrawals: two, one lost to follow‐up because of leg fracture, and one dropped out as on the 6th day as they experienced severe local irritation with burning sensation on both sides after application Notes: none |
| Interventions | Run‐in details: not reported Intervention: cyclosporin 10% gel used twice daily for 14 days Concurrent treatment: not reported Other key information: none Comparator: vehicle gel used twice daily for 14 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA · Individual signs and symptoms of pruritus, erythema, oozing, crusts, xerosis lichenification |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
De Rie 1991.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: the Netherlands Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 8 days Inclusion criteria: patients with two comparable lesions of AD, each 10‐25 cm2 and ADSI > 7, with ≥ 3 basic features (pruritus, typical presentation, chronic or chronically relapsing, personal or family history of atopy) Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: not clear: 16 sides to apply the intervention. Not clear the number to apply the comparator Age: intervention group range was 3 to 63 years; comparator group not reported Sex: male n = 6, female n = 10 Ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: cyclosporin A 10% gel or ointment used twice daily for 21 days Concurrent treatment: not reported Other key information: none Comparator: vehicle formulation not stated used twice daily for 21 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · ADSI · Blood pressure · Haematology · Biochemistry · Levels of cyclosporin |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: no results are presented for placebo arm |
Del Rosso 2007.
| Study characteristics | |
| Methods | Trial design: randomised, investigator‐blinded, within‐participant study Trial registration number: not reported Country: USA Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 4 weeks Inclusion criteria: adult with AD with symmetric involvement of target areas for evaluation, mainly the extremities Exclusion criteria: · Use of a systemic or topical corticosteroid, topical calcineurin inhibitor, oral antihistamine · Use of any other medications that could affect study results Additional design details: none |
| Participants | Total number randomised: 14 sides treated (7 to apply the intervention and 7 to apply the comparator) Age: 18 to 24 years Sex: male n = 3, female n = 4 Ethnicity, duration of eczema, severity of eczema: not reported Body site: antecubital n = 3, popliteal/legs n = 3, arms/forearms n = 1 Number of withdrawals: not reported Notes: AD participants were a subgroup of the study |
| Interventions | Run‐in details: not reported Intervention: fluocinonide 0.1% cream used once daily for up to 28 days · Concurrent treatment: not reported · Other key information: none Comparator: tacrolimus 0.1% ointment used twice daily for up to 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA · Subject Global Assessment · Pruritus · Safety |
| Notes | Funding source: none stated Declarations of interest: To quote: "Dr. Del Rosso is a consultant, speaker, and/or researcher for Allergan, Coria, Galderma, Graceway, Intendis, Medicis, Onset Therapeutics, Obagi Medical Products, Ortho Dermatology, PharmaDerm, Quinnova, Ranbaxy, SkinMedica, Stiefel, Triax, Unilever, and Warner‐Chilcott. Dr. Bhambri reports no relevant conflicts of interest." Original language of publication: English Other: none |
Del Rosso 2009.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind study Trial registration number: not reported Country: USA Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 2 weeks Inclusion criteria: · Adults with AD · 2 to 10% BSA · Clinically stable for at least one month · Total symptom score ≥ 7 out of 12 based on the investigator’s evaluation (erythema, infiltration/papulation, excoriations, and lichenification of all affected treatable areas scored 0 = none to 3 = severe) · Pruritus score ≥ 1 · IGA ≥ 3 (moderate) · No systemic/dermatological condition that might interfere with the study results or compromise safety Exclusion criteria: · Topical corticosteroids, topical retinoids, or topical calcineurin inhibitors within two weeks of the trial · Systemic corticosteroids, systemic retinoids, or prolonged sun exposure/ultraviolet light therapy within four weeks of the trial Additional design details: none |
| Participants | Total number randomised: 313 participants (211 to apply the intervention and 102 to apply the comparator) Age: TCS once daily group mean 40.9 years (SD 13.0) range 19 to 76; TCS twice daily group 42.9 years (SD 15.7), range 18 to 79; vehicle once daily 43.7 years (SD 16.5), range 18 to 76; vehicle twice daily group 43.7 years (SD 13.0), range 20 to 71 Sex: male n = 136, female n = 173 Ethnicity: Caucasian n = 243, African‐American n = 44, Asian n = 7, Hispanic n = 27, native American n = 2 Duration of eczema: mean: TCS once daily group, 17.2 years (SD 14.6), range 0.1 to 52; TCS twice daily group, 17.8 years (SD 16.8) range 0.9 to 64; vehicle one daily group, 16.8 years (SD 16.5) range 0.9 to 62; vehicle twice daily group, 17.2 years (SD 15.1), range 1.0 to 57) Severity of eczema: people with moderate eczema were eligible. Body site: not reported Number of withdrawals: to quote: "Twenty‐two subjects discontinued the study for the following reasons: AE (n = 5; 1.6%), protocol violation (n = 1; 0.3%), subject’s request (n = 5; 1.6%), lost to follow‐up (n = 9; 2.9%), and other reasons (n = 2; 0.6%)" Notes: none |
| Interventions | Run‐in details: four weeks required for any medication known to affect serum cortisol levels or HPA axis function for subjects undergoing HPA evaluation Intervention: fluocinonide 0.1% cream used once or twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used once or twice daily for 14 days. · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Overall severity on a 5‐point scale · Severity of erythema, infiltration/papulation, excoriations, and lichenification · Pruritus · BSA · AE · HPA axis suppression |
| Notes | Funding source: not reported Declarations of interest: To quote: "Dr. Del Rosso is a consultant, speaker, and/or researcher for Allergan, Coria, Galderma, Graceway, Intendis, Medicis, Onset Therapeutics, Obagi Medical Products, Ortho Dermatology, PharmaDerm, Quinnova, Ranbaxy, SkinMedica, Stiefel, Triax, Unilever, and Warner‐Chilcott. Dr. Bhambri reports no relevant conflicts of interest." Original language of publication: English Other: none |
Dolle 2015.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: NCT01299610, EUCTR2010‐022280‐35‐DE, NCT01381445 Country: Germany Outpatient or hospital: not reported Date trial conducted: December 2010 to April 2011 Duration of trial participation: up to 41 days Inclusion criteria: · Aged 18 to 55 years and a modified SCORAD > 25 with BSA > 5% · 3 comparable, representative index lesions · Willingness to refrain from current active therapy for ≥ 10 days prior to the trial · Normal ECG and liver function tests, BMI 19.0 to 29.0 kg/m2 · Females of non‐childbearing potential · Males with female partners of childbearing potential must also agree to use approved contraception. Exclusion criteria: · Other systemic or active skin disease which might affect the trial results · Receiving other local treatments within 14 days of the trial (other than hydrocortisone 1% up to 3 days prior) or systemic treatments within 28 days of the trial · Disease only on face, feet and/or hands · Scars, moles, tattoos, body piercings, or sunburn in the test area · Complication of AD such as erythroderma or overt bacterial or viral infection · Recent (< 6 months) superficial viral skin infections such as herpes simplex, or varicella · Contact dermatitis, seborrheic dermatitis and/or occupational eczema at sites of AD · Topical or transdermal treatments on or near the site of application within 14 days of the trial · Likelihood of intensive UV exposure during the study (solar or artificial) · Topical treatment with buproprion within 14 days of the trial · History of cutaneous photodisorder and/or allergy to components of test medications · History of skin, hepatic or renal disease or conditions known to interfere with absorption, distribution, metabolism or excretion of drugs · History of diaphoresis/excessive sweating not restricted to palms or face · Hepatitis B or C positive within 3 months of the trial · Current or chronic history of liver disease, or known hepatic or biliary abnormalities · A positive drug/alcohol screen · HIV‐positive · Regular alcohol consumption within 6 months of the study · Receiving an investigational product within 30 days, 5 half‐lives or twice the duration of the biological effect of the investigational product prior to the study · Receiving more than four new chemical entities within 12 months · Use of drugs, including vitamins, herbal and dietary supplements · Where participation in the study would result in donation in excess of 500 mL blood or blood products within 56 days · History of sensitivity to heparin · Consumption of red wine, citrus fruits or juices from 7 days prior to the trial Additional design details: 3‐period incomplete block crossover design; however, elsewhere treatments were stated to be applied concurrently, suggesting a within‐participant design for the purposes of our review |
| Participants | Total number randomised: 75 sides treated Age: mean 36.2 years (SD 16.68) Sex: males n = 19, females n = 6 Ethnicity: all white Duration of eczema: not reported Severity of eczema: mean SCORAD overall 37.2 (SD 7.95) Body site: Index lesions on arms and legs Number of withdrawals: there were no withdrawals. Notes: none |
| Interventions | Run‐in details: See exclusion criteria Intervention one: GW870086X 2% cream used once daily for 21 ± 2 days · Concurrent treatment: none · Other key information: GW870086 is a novel selective corticosteroid, CAS number 827319‐43‐7. It does not have a potency classification. Intervention two: GW870086X 0.2% cream used once daily for 21 ± 2 days · Concurrent treatment: none · Other key information: GW870086 is a novel selective corticosteroid, CAS number 827319‐43‐7. It does not have a potency classification. Intervention three: fluticasone propionate 0.05% cream used once daily for 21 ± 2 days · Concurrent treatment: none · Other key information: none Comparator: vehicle cream used once daily for 21 ± 2 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: Subjects were assigned to 3 out of the 4 possible treatments. Notes: To quote: "For first three days of the trial, participants applied their randomly assigned treatments at the same time of day during the clinic visits and trial personnel supervised to ensure that the correct application procedures were followed. […] Participants applied their treatments at home on Day 4 to 6, Day 8 to 13 and Day 15 to 20." |
| Outcomes | · Pharmacokinetic and pharmacodynamic parameters · Erythema, oedema/papulation, and excoriation scored as 0 = absent, 1 = mild, 2 = moderate, 3 = severe. Reported as an adjusted change from baseline mean, calculated by fitting a mixed‐effects repeated measures model. A negative response indicates an improvement relative to baseline. · Number of IGA responders: 0 = clear to 5 = very severe. The participant was considered a responder if IGA score reduced by 1 grade and improved from baseline by 2 grades. · Safety and tolerability (adverse events, serious adverse events, abnormal haematology, clinical chemistry parameters of potential clinical importance, abnormal electrocardiogram, abnormal vital signs) |
| Notes | Funding source: none reported Declarations of interest: none stated; however, one of the trial authors is affiliated to GlaxoSmithKline, UK Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022 |
Doss 2009.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: Belgium, Finland, France, Morocco and Tunisia Outpatient or hospital: 80 centres Date trial conducted: February 2004 to July 2005 Duration of trial participation: 6 weeks Inclusion criteria: · Males and females of any ethnic group · Moderate‐to‐severe AD according to Rajka and Langeland criteria · Responded inadequately to TCS Exclusion criteria: · Erythroderma or any genetic epidermal barrier defect · Clinical varicella zoster, herpes simplex or HIV infection · Verruca vulgaris, molluscum contagiosum, superinfected eczema, ulcerated lesions, moderate‐to‐severe acne or rosacea · Substance abuse or mental disorder or known hypersensitivity to any ingredient of the study drug preparations Additional design details: patients in whom treatment was considered ineffective were discontinued at day 21. Remaining patients were followed up for an additional 3 weeks; those who had residual persisting lesions at day 21 could continue once‐daily treatment during this period. |
| Participants | Total number randomised: 479 participants (240 to apply tacrolimus) and 239 to apply fluticasone) Age: tacrolimus group, mean age 6.9 years (SD 3.9), age range 2 to 6 years 51%, 7 to 15 years 47.5%, over 15 years 0.8%; fluticasone group, mean age 6.6 years (SD 4.1), age range 2 to 6 years 57.8%, 7 to 15 years 41.8%, over 15 years 0.4% Sex: tacrolimus group, male 48.3%, female 51.7%; fluticasone group, male 47.3%, female 52.7% Ethnicity: tacrolimus group, Caucasian 89%, black 7.2%, other 3.8%; fluticasone group, Caucasian 86.9%, black 8.4%, other 4.7% Duration of eczema: tacrolimus group, 5.6 years (SD 3.9); fluticasone group, 4.9 years (SD 3.8) Severity of eczema: moderate‐to‐severe Body site: all lesions except eyelids Number of withdrawals: tacrolimus group n = 21; fluticasone group n = 20 Notes: during the study, the use of other treatments for AD was not permitted, including topical and systemic corticosteroids, systemic antihistamines, ultraviolet radiation, non‐steroidal immunosuppressants, sleeping pills or tranquillisers, tar‐based topical medications and pimecrolimus. Topical and systemic corticosteroids for the treatment of conditions other than AD were prohibited during the first 3 weeks of the study, but could be used for a maximum of 4 days during the second 3 weeks if absolutely necessary. |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.03% ointment used twice daily for up to 21 days · Concurrent treatment: not reported · Other key information: none Comparator: fluticasone 0.005% ointment used twice daily for up to 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Response rate:% of patients with an improvement of 60% or more in mEASI at week 3 · Modified (m)EASI score · Severity of pruritus · Sleep quality · Global assessment of clinical response by the physician and the patient or parents · AE |
| Notes | Funding source: Astellas Pharma who manufacture tacrolimus Declarations of interest: not declared Original language of publication: English Other: none |
Doss 2010.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: Belgium, Finland, France, Morocco and Tunisia Outpatient or hospital: 80 centres Date trial conducted: February 2004 to July 2005 Duration of trial participation: 6 weeks Inclusion criteria: · Males and females of any ethnic group · Moderate‐to‐severe AD according to Rajka and Langeland criteria (score 4.5 or greater) · Responded inadequately to TCS Exclusion criteria: · Patients with erythroderma or any genetic epidermal barrier defect · Clinical varicella zoster, herpes simplex or HIV infection · Verruca vulgaris, molluscum contagiosum, superinfected eczema, ulcerated lesions, moderate‐to‐severe acne or rosacea · Substance abuse or mental disorder or known hypersensitivity to any ingredient of the study drug preparations Additional design details: participants in whom treatment was considered ineffective were discontinued at day 21. Remaining participants were followed up for an additional 3 weeks; those who had residual persisting lesions at day 21 could continue once‐daily treatment during this period. |
| Participants | Total number randomised: 479 participants (240 to apply the intervention and 239 to apply the comparator) Age: tacrolimus group, mean age 6.9 (SD 3.9), age range 2 to 6 years, 51%, 7 to 15 years 47.5%, over 15 years 0.8%; fluticasone group, mean age 6.6 years (SD 4.1, age range 2 to 6 years 57.8%, 7 to 15 years (41.8%), over 15 years (0.4%) Sex: tacrolimus group, male 48.3%, female, 51.7%; fluticasone group, male 47.3%, female, 52.7% Ethnicity: tacrolimus group, Caucasian 89%, black 7.2%, other 3.8%; fluticasone group, Caucasian 86.9%, black 8.4%, other 4.7% Duration of eczema: tacrolimus group, mean 5.6 years (SD 3.9); fluticasone group, mean 4.9 years (SD 3.8) Severity of eczema: moderate‐to‐severe eczema eligible Body site: all lesions except eyelids Number of withdrawals: tacrolimus group n = 21; fluticasone group n = 20 Notes: the use of other treatments for AD was not permitted, including topical and systemic corticosteroids, systemic antihistamines, ultraviolet radiation, nonsteroidal immunosuppressants, sleeping pills or tranquillisers, tar‐based topical medications and pimecrolimus. Topical and systemic corticosteroids for the treatment of conditions other than AD were prohibited during the first 3 weeks of the study, but could be used for a maximum of 4 days during the second 3 weeks if absolutely necessary. |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.03% ointment used twice daily for up to 21 days · Concurrent treatment: not reported · Other key information: none Comparator: fluticasone 0.005% ointment used twice daily for up to 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · response rate (% of patients with an improvement of 60% or more in mEASI at week 3) · mEASI score · severity of pruritus · sleep quality · global assessment of clinical response by the physician and the patient or parents · AE |
| Notes | Funding source: Astellas Pharma who manufacture tacrolimus Declarations of interest: not declared Original language of publication: English Other: none |
Dou 2006a.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: China Outpatient or hospital: two departments of dermatology Date trial conducted: July 2003 to January 2004 Duration of trial participation: not reported Inclusion criteria: patients with AD Exclusion criteria: not reported Additional design details: the paper reported two separate trials; Dou 2006a = adults, Dou 2006b = children |
| Participants | Total number randomised: 202 participants (67 to apply intervention one, 68 intervention two, and 67 to apply the comparator) Age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention one: tacrolimus 0.03% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Intervention two: tacrolimus 0.1% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | 10‐item Dermatological Quality of Life Index (DLQI) |
| Notes | Funding source: to quote: "Fujisawa Pharmaceutical (China) Co., Ltd. provided assistance for this study; Shanghai Nisshin. Pharmaceutical Development Co., Ltd. manages and counts the data of this observation". Declarations of interest: not declared Original language of publication: Chinese Other: inclusion assessed by native Chinese speaker. Translation of full text via Google translate app |
Dou 2006b.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: China Outpatient or hospital: two departments of Dermatology Date trial conducted: July 2003 to January 2004 Duration of trial participation: not reported Inclusion criteria: patients with AD Exclusion criteria: not reported Additional design details: the paper reported two separate trials; Dou 2006a = adults, Dou 2006b = children |
| Participants | Total number randomised: 104 participants (51 to apply the intervention and 53 to apply the comparator) Age: 5 to 17 years n = 104, intervention n = 51, comparator n = 53; 2 to 4 years n = 21, intervention n = 12; comparator n = 9 Sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.03% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Quality of life using the CDLQI scale |
| Notes | Funding source: Fujisawa Pharmaceutical (China) Co., Ltd. provided assistance for this study; Shanghai Nisshin, Pharmaceutical Development Co., Ltd. manages and counts the data of this observation. Declarations of interest: not declared Original language of publication: Chinese Other: none |
Draelos 2005.
| Study characteristics | |
| Methods | Trial design: randomised, investigator‐blind, multicentre, parallel study Trial registration number: not reported Country: US Outpatient or hospital: 6 centres Date trial conducted: not reported Duration of trial participation: 13 days Inclusion criteria: · Adult with moderate‐to‐severe AD · Affecting at least 30% of the total body surface area Exclusion criteria: · Patients who were pregnant, breastfeeding, or of childbearing potential and not using medically approved contraception · Significant medical condition that might interfere with study evaluations · Phototherapy or systemic therapy within 1 month; pimecrolimus and/or topical tacrolimus within 2 weeks; other topical therapy within 7 days; systemic corticosteroids within 1 month; systemic antibiotics within 2 weeks; or investigational drugs within 8 weeks Additional design details: none |
| Participants | Total number randomised: 37 participants (18 to apply the intervention and 19 to apply the comparator) Age: mean age: pimecrolimus group 40.9 years (SD 14.3); tacrolimus group 42.5 years (SD 15.1) Sex: pimerolimus group 66.7% female; tacrolimus group 89.5% female Ethnicity: pimecrolimus group, oriental 5.6%, black 11.1, white 77.8%, other 5.6%; tacrolimus group, oriental 10.5%, black 15.8%, white 73.7% Duration of eczema: pimerolimus group 25.3 years (SD 16.80); tacrolimus group 28.7 years (SD 16.88) Severity of eczema: pimerolimus group, 55.6% moderate, 33.3% severe, 11.1% very severe; tacrolimus group, 42.1% moderate, 57.9% severe, 0 very severe Body site: head, neck and limbs both groups 100%, in pimerolimus group, trunk 88.9%, in tacrolimus group 100% Number of withdrawals: no withdrawals Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream used twice daily for 13 days · Concurrent treatment: not reported · Other key information: none Comparator: tacrolimus 0.1% ointment used twice daily for 13 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA · Pruritus · Blood samples for drug concentrations · Safety |
| Notes | Funding source: Novartis Pharmaceuticals Corp Declarations of interest: 3 declared conflicts: two are employees of Novartis Pharmaceuticals Corp.; one an employee of Novartis Pharma AG. Original language of publication: English Other: none |
Duke 1983.
| Study characteristics | |
| Methods | Trial design: randomised, investigator‐blind, multicentre, parallel study Trial registration number, country, outpatient or hospital, date trial conducted: not reported Duration of trial participation: three weeks Inclusion criteria: · Aged ≥ 14 years · Three clinical signs (erythema, induration, and pruritus) with severity ≥ 6 on the following scale (0 = absent, 1 = mild, 2 = moderate, 3 = severe) Exclusion criteria: · Pregnancy or breastfeeding · Requirement for other topical or systemic treatment for AD · Systemic treatment within four weeks of the trial · Topical treatment within one week of the trial · TB or viral infections of the skin or systemic fungal infections Additional design details: not reported |
| Participants | Total number randomised: 68 participants Age: 14 to 74 years Sex: both Ethnicity, duration of eczema: not reported Severity of eczema: having three clinical signs (erythema, induration, and pruritus) with a severity score totalling six or more graded on the following scale (0 = absent, 1 = mild, 2 = moderate, 3 = severe) Body site: could be face, neck, trunk and upper and lower extremities, but not on the palms, soles, or scalp Number of withdrawals: three patients were immediate dropouts and not included in any analysis. One clobeasone‐treated patient was excluded from the efficacy analysis because of concomitant use of tetracycline. Notes: not reported |
| Interventions | Run‐in details: not reported Intervention: alclometasone 0.05% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: clobetasone butyrate 0.05% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: no medication other than study preparation. No systemic medication that might alter the course of the disease. Concomitant therapy for other conditions was to be held constant. Tar baths, ultraviolet (UV) light or Grenz ray therapy not to be used. Sun exposure and changes in exercise habits or environment were to be avoided. Notes: none |
| Outcomes | · IGA · Clinical signs · Local AE · Withdrawal due to AE |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: not reported |
Eichenfield 2002a.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blinded, multicentre parallel studies Trial registration number: not reported Country: not reported; however, authors affiliations suggest USA, Canada, and Europe Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 6 weeks Inclusion criteria: · Aged 1 to 17 years · AD according to Williams criteria and affecting ≥ 5% BSA · IGA = 2 or 3 (mild‐to‐moderate disease) · Using stable bland emollient ≥ 7 days before the trial Exclusion criteria: · Pregnancy or breastfeeding · Phototherapy or systemic therapy for atopic dermatitis within 1 month of the trial · Topical therapy within 7 days of the trial · Systemic antibiotics within 2 weeks of the trial · Significant comorbidity Additional design details: this trial is reported as a pooled analysis with Eichenfield 2002b; outcomes are reported here under Eichenfield 2002a. |
| Participants | Total number randomised: 403 (combined total Eichenfield 2002a, b) participants (267 to apply the intervention and 136 to apply the comparator) Age: for Eichenfield 2002a, b combined: · pimecrolimus group: mean age 6.8; aged < 2 years n = 4; aged 2 to < 12 years n = 220; aged 12 to < 18 years n = 43; vehicle group: mean age 6.6; aged < 2 years n = 5; aged 2 to < 12 years; 21 n = 110, aged 12 to < 18 years n = 21 Sex: for Eichenfield 2002a, b combined: · pimecrolimus group: females n = 127, males n = 140; vehicle: females n = 74, males n = 62 Ethnicity: for Eichenfield 2002a and Eichenfield 2002b combined: · pimecrolimus group: Caucasian n = 146, non‐Caucasian n = 121; vehicle: Caucasian n = 66, non‐Caucasian n = 70 Duration of eczema: not reported Severity of eczema: for Eichenfield 2002a, b combined: · pimecrolimus group: mild n = 80, moderate n = 161, severe n = 23, very severe eczema n = 3; vehicle: mild n = 43, moderate n = 78, severe n = 11, very severe eczema n = 4 Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream used twice daily for 6 weeks · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 6 weeks. · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: first application was supervised by the investigator or study nurse. Application was a thin film at the same times of day, 12 hours apart Notes: none |
| Outcomes | · EASI · IGA · Pruritus · Quality of life of parent of children with AD scale (PIQoL‐AD) · AE |
| Notes | Funding source: Novartis Pharmaceuticals Corporation, East Hanover, NJ Declarations of interest: To quote: "Dr Eichenfield: Consultant to Novartis Pharmaceuticals, and Fujisawa Healthcare but does not own any equity stocks. Dr Lucky: Former consultant to Novartis Pharmaceuticals, Fujisawa Healthcare, and other pharmaceutical companies that manufacture corticosteroids for atopic dermatitis. Mr Boguniewicz: Has received clinical research trial grants from Novartis Pharmaceuticals and Fujisawa Healthcare. Dr Langley: QE2 Health Center received a research grant from Novartis Pharmaceuticals and Fujisawa Healthcare. R. Cherill, K. Marshall, C. Bush: Employees of Novartis Pharmaceuticals. Dr Graeber: Employee of Novartis Pharma AG." Original language of publication: English Other: none |
Eichenfield 2002b.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blinded, multicentre, parallel studies Trial registration number: not reported Country: not reported; however, authors affiliations suggest USA, Canada, and Europe Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 6 weeks Inclusion criteria: · Aged 1 to 17 years · AD according to Williams criteria affecting ≥ 5% BSA · IGA 2 or 3 (mild‐to‐moderate disease) · Using stable bland emollient ≥ 7 days before the trial Exclusion criteria: · Pregnancy or breastfeeding · Phototherapy or systemic therapy for atopic dermatitis within 1 month of the trial · Topical therapy within 7 days of the trial · Systemic antibiotics within 2 weeks of the trial · Significant comorbidity Additional design details: this trial is reported as a pooled analysis with Eichenfield 2002a; outcomes are reported under Eichenfield 2002a. |
| Participants | Total number randomised: 403 (combined total Eichenfield 2002a, b) participants (267 to apply the intervention and 136 to apply the comparator). Age: for Eichenfield 2002a, b combined: · pimecrolimus group: mean age 6.8 years; aged < 2 years n = 4; aged 2 to < 12 years n = 220; aged 12 to < 18 years n = 43 · vehicle group: mean age 6.6 years; aged < 2 years n = 5; aged 2 to < 12 years; 21 n = 110, aged 12 to < 18 years n = 21 Sex: for Eichenfield 2002a, b combined: · pimecrolimus group: females n = 127, males n = 140; vehicle: females n = 74, males n = 62 Ethnicity: for Eichenfield 2002a and Eichenfield 2002b combined: · pimecrolimus group: Caucasian n = 146, non‐Caucasian n = 121; vehicle: Caucasian n = 66, non‐Caucasian participants n = 70 Duration of eczema: not reported Severity of eczema: for Eichenfield 2002a, b combined: · pimecrolimus group: mild n = 80, moderate n = 161, severe n = 23, very severe eczema n = 3; vehicle: mild n = 43, moderate n = 78, severe n = 11, very severe eczema n = 4 Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream used twice daily for 6 weeks · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 6 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: the first application was supervised by the investigator or study nurse. Application was a thin film at the same times of day, 12‐hours apart. Notes: none |
| Outcomes | · EASI · IGA · Pruritus · PIQoL‐AD · AE |
| Notes | Funding source: Novartis Pharmaceuticals Corporation, East Hanover, NJ Declarations of interest: To quote: "Dr Eichenfield: Consultant to Novartis Pharmaceuticals, and Fujisawa Healthcare but does not own any equity stocks. Dr Lucky: Former consultant to Novartis Pharmaceuticals, Fujisawa Healthcare, and other pharmaceutical companies that manufacture corticosteroids for atopic dermatitis. Mr Boguniewicz: Has received clinical research trial grants from Novartis Pharmaceuticals and Fujisawa Healthcare. Dr Langley: QE2 Health Center received a research grant from Novartis Pharmaceuticals and Fujisawa Healthcare. R. Cherill, K. Marshall, C. Bush: Employees of Novartis Pharmaceuticals. Dr Graeber: Employee of Novartis Pharma AG." Original language of publication: English Other: none |
Eichenfield 2006a.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: not reported Country: US Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 4 weeks Inclusion criteria: · Aged 3 months or older · Moderate‐to‐severe AD according to Rajka and Langeland criteria Exclusion criteria: used systemic treatment for AD within the previous month and topical treatment within the previous week Additional design details: none |
| Participants | Total number randomised: 220 participants (110 to apply the intervention and 110 to apply the comparator) Age: three months to 16 years Sex, ethnicity, duration of eczema: not reported Severity of eczema: 61/110 intervention, and 56/110 control moderate/severe at baseline. Unclear what severity the remaining participants were Body site: AD was evaluated weekly by the investigator over four areas (head/neck, trunk, upper limbs, lower limbs) Number of withdrawals: 12 from the two studies reported in Eichenfield 2006 study paper, the other study was reported in this review as Eichenfield 2006b. No breakdown in numbers per study Notes: none |
| Interventions | Run‐in details: not reported Intervention: fluticasone propionate 0.05% lotion used once daily for 28 days Concurrent treatment: not reported Other key information: none Comparator: vehicle lotion used once daily for 28 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator; concomitant medications were prohibited unless emollients or considered necessary Notes: none |
| Outcomes | · BSA affected at each site was estimated using the subject’s palm to represent 1% BSA and ‘‘Rule of Nines’’ to approximate overall BSA affected · PGA compared BSA at baseline and end treatment and was scored as 0 (cleared: 100%), 1 (almost cleared: 90‐99%), 2 (marked clearing: 50‐89%), 3 (modest clearing; < 50%), 4 (no change), 5 (exacerbation) · AE |
| Notes | Funding source: GlaxoSmithKline, NJ, USA Declarations of interest: not declared Original language of publication: English Other: none |
Eichenfield 2006b.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind parallel study Trial registration number: not reported Country: US Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 4 weeks Inclusion criteria: · Aged 3 months or older · Moderate‐to‐severe AD according to Rajka and Langeland criteria Exclusion criteria: used systemic treatment for AD within the previous month and topical treatment within the previous week Additional design details: none |
| Participants | Total number randomised: 218 participants (111 to apply fluticasone propionate (the intervention) and 107 to apply the comparator (vehicle)) Age: 3 months to 16 years Sex, ethnicity, duration of eczema: not reported Severity of eczema: inclusion criteria lists moderate‐to‐severe AD Body site: AD was evaluated weekly by the investigator over four areas (head/neck, trunk, upper limbs, lower limbs) Number of withdrawals: 12 from the two studies reported in Eichenfield 2006 study paper, the other study was reported in this review as Eichenfield 2006a. No breakdown in numbers per study Notes: none |
| Interventions | Run‐in details: not reported Intervention: fluticasone propionate 0.0 5% lotion used once daily for 28 days Concurrent treatment: not reported Other key information: none Comparator: vehicle lotion used once daily for 28 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator; concomitant medications were prohibited unless emollients or considered necessary Notes: none |
| Outcomes | · BSA affected · IGA scored as 0 (cleared; 100%), 1 (almost cleared; 90 to 99%), 2 (marked clearing; 50 to 89%), 3 (modest clearing; < 50%), 4 (no change), 5 (exacerbation) · AE |
| Notes | Funding source: GlaxoSmithKline, NJ, USA Declarations of interest: not declared Original language of publication: English Other: none |
El‐Hefnawi 1978.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, single‐centre, within‐participant study Trial registration number: not reported Country: Egypt Outpatient or hospital: Dermatology department of Ain Shams Faculty of Medicine Date trial conducted: not reported Duration of trial participation: 3 weeks maximum (less if cure achieved) Inclusion criteria: bilateral, symmetrical lesions of equal severity. A number of diagnoses were considered; we have only considered the participants with AD for the purposes of this review. Exclusion criteria: secondary infection Additional design details: not reported |
| Participants | Total number randomised: 10 sides treated (5 to apply the intervention and 5 to apply the comparator) Age: ranging in age from 5 months to 65 years with an average age of 35.9 years. Note: this is for the whole cohort, unclear age range for the conditions of interest in this review Sex: males n = 29, females n = 21. Note: this is for the whole cohort, unclear gender range for the conditions of interest in this review. Ethnicity: not reported Duration of eczema: duration prior to initiation of treatment of the episode studied was more than four weeks in forty patients (80%). Note: this is for the whole cohort, unclear duration for the conditions of interest in this review Severity of eczema: On admission, the disease was rated as moderately severe in forty‐five patients and severe in five. Note: this is for the whole cohort, unclear severity for the conditions of interest in this review Body site: face: n = 2, groin: n = 2, legs: n = 1 Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: halcinonide 0.1% ointment used three times daily for 21 days · Concurrent treatment: not reported · Other key information: the ointment also contained neomycin and amphotericin Comparator: hydrocortisone 1% ointment used three times daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator; no concomitant treatment was allowed during the period of the study. Notes: none |
| Outcomes | · Clinician preference · IGA |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Emer 2011.
| Study characteristics | |
| Methods | Trial design: randomised, clinician‐blind, single‐centre, within‐participant study Trial registration number: NCT01177566 Country: USA Outpatient or hospital: outpatient clinic Date trial conducted: September 2009 to March 2010 Duration of trial participation: four weeks Inclusion criteria: · Male and female · Age ≥ 2 years · Mild‐to‐moderate AD for at least one year · Symmetrical target eczematous areas on opposite sides of the body · IGA at least mild · Females of childbearing potential included if they had negative urine pregnancy test at baseline and agreed to adequate birth control Exclusion criteria: · Pregnant, nursing or attempting to conceive · Known hypersensitivity to any study medication · Active skin infections in selected target areas · Requiring use of medications known to alter the course of AD · Use of systemic antibiotics within 14 days · Systemic corticosteroids or immunosuppresssants within 28 days · TCS or other topical modalities within 7 days · Phototherapy within 28 days Additional design details: none |
| Participants | Total number randomised: 40 sides treated (20 to apply the intervention and 20 to apply the vehicle) Age: range 14 to 50 years Sex: males and females Ethnicity: not reported Duration of eczema: to quote: "at least one year" Severity of eczema: mild‐to‐moderate Body site: not reported Number of withdrawals: none Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream used twice daily for 28 days Concurrent treatment: not reported Other key information: none Comparator: vehicle cream used three times daily for 28 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: none Notes: none |
| Outcomes | · Improvement in IGA · Improvement in signs of AD · Improvement in patient self‐assessments · Withdrawals due to AE |
| Notes | Funding source: Ferndale Laboratories Declarations of interest: to quote: "No relevant conflicts of interest to disclose" Original language of publication: English Other: none |
EUCTR2004‐001036‐23‐IT.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: EUCTR2004‐001036‐23‐IT, 2‐CASM981C2442 Country: 28 centres: Australia n = 5, Canada n = 5, France n = 5, Sweden n = 6, Italy n = 7 Outpatient or hospital: not reported Date trial conducted: October 2004 to July 2005 Duration of trial participation: 6 weeks Inclusion criteria: · Aged ≥ 12 years · Mild‐to‐moderate facial AD (facial IGA 2 to 3; excluding ears and neck) · Intolerant of/dependent on TCS · AD according to Hanifin and Rajka criteria · Women of childbearing potential with a negative pregnancy test and using reliable contraception Exclusion criteria: · Pregnancy or breastfeeding · > 30% BSA in addition to facial eczema · Concurrent skin disease in the study area, active skin infections, or other conditions that could have interfered with the evaluation · Immunocompromised or with a history of malignant disease (except treated basal cell carcinoma) · Previous lack of response or hypersensitivity to pimecrolimus cream · Phototherapy or systemic therapy suspected to affect AD within 4 weeks of the trial · Other investigational drugs during or within 8 weeks of the study · Unlikely to comply with therapy Additional design details: none |
| Participants | Total number randomised: 200 participants (101 to apply the intervention and 99 to apply the comparator) Age: intervention group, mean 33.8 years (SD 15.42), range 12 to 78; comparator group, mean 32.7 years (SD 15.80), range 1 to 81 Sex: intervention group, 43.6% male, 56.45% female; comparator group, 33.3% male, 66.7% female Ethnicity: intervention group, 2% black, 5.9% oriental, 88.1% Caucasian and 4% other; comparator group, 1% black, 8.1% oriental, 4% other, 86.9% Caucasian Duration of eczema, severity of eczema: not reported Body site: facial AD does not detail AD anywhere else Number of withdrawals: n = 87, reasons: AE n = 11, unsatisfactory therapeutic effect n = 56, patient’s condition no longer required study drug n = 7, protocol violation n = 3, patient withdrew consent n = 6, lost to follow‐up n = 4, administrative problems, death 0 Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream used twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Facial IGA score of 0 or 1 (clear or almost clear) at the end of a 6‐week period · Severity Index (EASI) score, head and neck EASI score · Pruritus (itch) score · Time to get clearance of facial AD at the end of 6 weeks |
| Notes | Funding source: Novartis Declarations of interest: not declared Original language of publication: English Other: none |
EUCTR2004‐002688‐25‐GB.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: EUCTR2004‐002688‐25‐GB Country: UK Outpatient or hospital: not reported Date trial conducted: from January 2005 Duration of trial participation: 4 weeks Inclusion criteria: · AD according to Williams criteria · Male or female aged ≥ 18 years · Active moderate facial eczema · Pruritus score ≥ 2 · Where further use of topical corticosteroids is clinically inappropriate Exclusion criteria: · Females of childbearing potential · Phototherapy or systemic therapy known or suspected to affect eczema within 1 month Additional design details: none |
| Participants | Total number randomised: 90 participants Age: intervention group: mean age 49 years (SD 21.4); comparator mean 48 years (SD 17.1) Sex: intervention group, male 40%, female 60%; comparator group, male 45.2%, female 54.8% Ethnicity: intervention group, white 97.5%, black 2.5%; comparator group, white 97.6%, black 2.4%. Duration of eczema: not reported Severity of eczema: moderate Body site: facial Number of withdrawals: intervention group n = 7; comparator n = 16 Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Change from baseline in Quality of Life Index ‐ AD (QoLIAD) · Reduction in facial itch and time to get clearance of eczema · Safety and tolerability |
| Notes | Funding source: Novartis Pharmaceuticals UK Ltd Declarations of interest: not declared Original language of publication: English Other: none |
EUCTR2005‐001650‐25‐DE.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: EUCTR2005‐001650‐25‐DE Country: Germany Outpatient or hospital: not reported Date trial conducted: from November 2005 Duration of trial participation: 6 weeks followed by a 6‐week open label phase Inclusion criteria: · AD according to Hanifin and Rajka criteria · Aged 2 to 11 years · Mild to moderate facial AD at screening (facial IGA 2 to 3; face only; excluding the ears and the neck) · Intolerance of, or dependency on, topical corticosteroids · Females of child‐bearing potential must have a negative pregnancy test and use effective contraception Exclusion criteria: · AD ≥ 30% of BSA · Concurrent skin disease, active skin infections, or other conditions that may interfere with evaluation · Immunocompromised or history of malignancy · Previously reported poor, no clinical response or hypersensitivity to topical calcineurin inhibitors such as pimecrolimus and tacrolimus · Phototherapy or systemic therapy known or suspected to affect AD within 4 weeks of the study · Other investigational drugs during or within 8 weeks of the study · Unlikely to comply with therapy · Pregnancy or breastfeeding Additional design details: none |
| Participants | Total number randomised: 200 participants (99 to apply the intervention and 101 to apply the comparator) Age: 2 to 11 years eligible Sex: all eligible Ethnicity: caucasian n = 134, asian n = 64, pacific islander n = 1, other n = 1 Duration of eczema: not reported Severity of eczema: mild to moderate eczema eligible Body site: facial eczema eligible Number of withdrawals: intervention group n =25, comparator group n = 6 Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream twice daily used for 42 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream twice daily used for 42 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Percentage of patients achieving treatment success, defined as a facial IGA score of 0 or 1 (clear or almost clear) during the 6‐week double‐blind phase |
| Notes | Funding source: Novartis Pharma Services AG Declarations of interest: not declared Original language of publication: English Other: none |
EUCTR2009‐012028‐98‐DE.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, within‐participant study Trial registration number: 2009‐012028‐98 Country: Germany Outpatient or hospital: presumed outpatient Date trial conducted: July 2009 to August 2009 Duration of trial participation: 3 weeks Inclusion criteria · Male or female patients with a diagnosis of AD for > 6 months · Active stage AD (measured by IGA‐Score between 1 and 4) · At least 2 comparable areas of stable AD on bilateral symmetric corresponding sides of the extremities of the body (except for the head and genital area) and of at least 10cm2 in area · Modified EASI score of test areas > 6 and at least 60% of defined test area · Age between 18 and 75 years · A patient of childbearing potential agreed to contraceptive methods for the duration of the trial: (a) strict abstinence (exception: male partner with vasectomy ≥ 3 months prior), (b) combined oral, implanted or injectable contraceptives on a stable dose ≥ 3 months prior, (c) intrauterine device inserted ≥ 1 month prior Exclusion criteria · A medical condition that may confound the study assessments or put the patient at a general risk · Any condition other than AD or treatment that may interfere with the barrier skin function or may lead to dermatitis · A condition of the skin in the test area that in the investigators opinion may confound the study assessments or may put the patient at risk · The patient has exposed the test areas to excessive UV radiation or therapy within 1 month prior or is planning intense UV exposure during the study · Very severe AD as measured by the IGA‐Score 5 or, in the judgement of the investigator, an indication for a systemic anti‐inflammatory therapy · An indication for a topic therapy that requires topical treatment anywhere on the body with a corticosteroid more potent than Class II or more than 10% of the BSA or any non‐corticosteroid anti‐inflammatory topical treatment during the study · Administration of any systemic drug indicated to treat atopic dermatitis within 1 month prior to study entry or during the study or systemic administration of antihistamines within 2 weeks prior to study entry and during the study · Administration of any topical treatment in the region of the designated test areas within 2 weeks prior to study entry or any other topical treatment in the region of the designated test areas during the study · Presence or history of a malignant skin disease (other than surgically removed basalioma or sufficiently treated actinic keratosis) · Presence or history of any malignant disease (other than skin malignancy) in the last 10 years · Known adverse reactions of any severity or hypersensitivity to any ingredient of the IMPs · Presence of cutaneous reactions as a result of vaccination or cutaneous manifestation of tuberculosis, of syphilis or of viral infections · Presence of rosacea or perioral dermatitis or bacterial or mycotic dermal infections in the test areas · Immunotherapy prior to and during the study · Vaccination within 6 days prior to enrolment in the study and during the study · Female with a positive urine pregnancy test, is breast‐feeding or is planning to become pregnant or breast‐feed a child during the study · Participation in any other clinical trial within 4 weeks prior or during this trial · Suspected substance‐abuser or is in the opinion of the investigator unreliable or non‐compliant Additional design details: none |
| Participants | Total number randomised: 100 sides treated (50 to apply the intervention and 50 to apply the comparator) Age: range 18 to 75 years Sex: male and female Ethnicity: not reported Duration of eczema: at least 6 months Severity of eczema: IGA score between 1 to 4 Body site: extremities of the body (except head and groin) Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: none Intervention: prednicarbat 0.25% used twice daily for 21 days Concurrent treatment: not reported Other key information: none Comparator: vehicle used twice daily for 21 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: none Notes: none |
| Outcomes | • Physicians’ assessment of tolerability (folliculitis, bruise (ecchymosis), whitehead (milia), dermal atrophy, telangiectasia, local infections, local allergic reactions before and after application) • Participants’ assessment of tolerability • mEASI • AEs/SAEs (severity, nature and frequency) • PGA of tolerability • IGA of tolerability • Abnormal values obtained during physical exam and vital signs |
| Notes | Funding source: Galen Pharma Declarations of interest: not declared but prednicarbat is manufactured by Galen Pharma Original language of publication: English Other: none |
EUCTR2009‐013792‐22‐FI.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: EUCTR2009‐013792‐22‐FI Country: Finland Germany Outpatient or hospital: not reported Date trial conducted: December 2009 to July 2010 Duration of trial participation: 4 weeks Inclusion criteria: · AD according to Hanifin and Rajka criteria · IGA scored as mild (2) to moderate (3) · Treatment lesions located on the trunk and/or limbs. · Treatment lesions involving 3% to 10% of the total BSA · Either gender between 18 and 65 years · Female patients on hormonal contraceptives according to stufy protocol requirements Exclusion criteria: · Systemic treatment with immunosuppressive drugs or corticosteroids within 6 weeks · Topical treatment with immunomodulators within 2 weeks · TCS from WHO groups II, III or IV within 1 week · Use of topical or systemic antibiotics within 2 weeks · Other topical therapy on the treatment areas areas within 1 week · Change in systemic anti‐histamine therapy within two weeks · PUVA or UVB within 4 weeks · Clinical infection on the treatment area · Known or suspected severe renal insufficiency or severe hepatic disorders · History of an immunocompromised disease · concomitant serious disease which might affect the AD treatment in this trial ·.Planned extensive sun exposure of treatment areas during trial participation · Known or suspected hypersensitivity to component(s) of the investigational product or comparator · Females who are pregnant or are breast feeding. · Females intending to temporarily or permanently stop their hormonal contraceptive regime Additional design details: none |
| Participants | Total number randomised: 183 participants treated (24 to apply intervention one, 25 each to apply intervention two, three and six, 29 intervention four, 30 intervention six and 25 to apply the comparator) Age: 18 to 64 years Sex: both Ethnicity, duration of eczema: not reported Severity of eczema: mild to moderate Body site: trunk and/or limbs Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: Intervention one: LEO 29102 0.03% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention two: LEO 29103 0.1% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention three: LEO 29104 0.3% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention four: LEO 29105 1% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention five: LEO 29106 2.5% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention six: pimecrolimus 1% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI · IGA of disease severity · Symptom free responders · Pruritus · Erythema · Oedema/induration/papulation · Excoriation · lichenification · Time to permanent response |
| Notes | Funding source: Leo Pharma A/S Declarations of interest: not declared Original language of publication: English Other: none |
EUCTR2010‐024279‐14‐CZ.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multi‐centre, parallel study Trial registration number: EUCTR2010‐024279‐14‐CZ Country: 35 centers: Czech Republic n = 4, Germany n = 12, Hungary n = 5, Latvia n = 6, and Poland n = 8 0utpatient or hospital: not reported Date trial conducted, duration of trial participation: not reported Inclusion criteria: · Male or female 18 to 65 years of age · AD according to Hanifin and Rajka Criteria · BSA affected by AD lesions ≥5% at screening and start of treatment · Static IGA score ≥3, corresponding to at least moderate AD at screening and start of treatment · Willingness to avoid excessive exposure of diseased areas to natural or artificial sunlight · Female subjects of non‐childbearing age or using an accepted effective method of contraception Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 197 participants treated (48 to apply intervention one, 50 to apply intervention two, 52 intervention three and 47 to apply the comparator) Age: 18 to 64 years Sex: all Ethnicity, duration of eczema: not reported Severity of eczema: Static Investigator’s Global Assessment score = 3, corresponding to at least moderate AD Body site: Head and neck, upper limbs, trunk and lower limbs Number of withdrawals: 38 Notes: none |
| Interventions | Run‐in details: not reported Intervention one: mapracorat 0.1% ointment used for 28 days · Concurrent treatment: not reported · Other key information: none Intervention two: mapracorat 0.3% ointment used for 28 days · Concurrent treatment: not reported · Other key information: none Intervention three: mapracorat 1% ointment used for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle 0% ointment used for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI · Safety and tolerability · Dose‐response relationship · Systemic exposure |
| Notes | Funding source: Intendis GmbH Declarations of interest: not declared Original language of publication: English Other: none |
EUCTR2011‐001175‐38‐DE.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: EUCTR2011‐001175‐38‐DE Country: Germany Outpatient or hospital: not reported Date trial conducted: February to March 2012 Duration of trial participation: 14 days Inclusion criteria: · Male or female aged 18 years or older · AD according to Hanifin and Rajka criteria, or seborrheic eczema or stasis dermatitis · Two comparable lesional areas of at least 2 cm2, with the clinical condition of atopic eczema or or seborrheic eczema or stasis dermatitis rated mild · The lesions should be located contralaterally for patients with atopic eczema and stasis dermatitis · An erythema score = 1 in both lesional areas · The physical examination of the skin must be without disease findings unless the investigator considers an abnormality to be irrelevant to the outcome of the clinical trial; · female patients of childbearing potential must either be surgically sterile or agree to use a reliable method of contraception or vasectomized partner · written informed consent obtained Exclusion criteria: · Acne, suntan, eczema other than atopic eczema, seborrheic eczema or stasis dermatitis hyper‐ or hypopigmentation or tattoos in the treatment areas · Syphilitic or tuberculous dermatitis, varicella or vaccine reactions · Dark‐skinned persons whose skin color prevents ready assessment of skin reactions · Evidence of drug or alcohol abuse · Pregnancy or nursing · UV‐therapy within 6 weeks before first treatment · Symptoms of a clinically significant illness that may influence the outcome of the trial in the four weeks before and during the trial · Participation in the treatment phase of another clinical trial within the last four weeks prior to the first administration of investigational drug in this clinical trial · Known allergic reactions to components of the investigational product/s · Treatment with systemic or locally acting medications which might counter or influence the trial aim within four weeks before the baseline visit and during the trial · Contraindications according to the summary of product characteristics of Soventol® HydroCort 0.5% and Soventol® HydroCort 0.25% Additional design details: none |
| Participants | Total number randomised: 68 sides (34 sides to apply intervention and 34 the comparator) Age: 18 to 64 years Sex: male or female Ethnicity: excluded: dark‐skinned persons whose skin color prevents ready assessment of skin reactions Duration of eczema: not reported Severity of eczema: mild Body site: not reported Number of withdrawals: 2 Notes: none |
| Interventions | Run‐in details: Intervention: hydrocortisone acetate 0.5% cream three times daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle 0% cream used three times daily for 14 days · Concurrent treatment: not reported · Other key information:none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Area under the Curve (AUC) of baseline corrected erythema scores · Clinical assessment of edema/papulation, oozing/crusting, excoriations, scaling and lichenification using a five‐point scale |
| Notes | Funding source: Medice Arzneimittel Pütter GmbH & CO.KG Declarations of interest: not declaared Original language of publication: English Other: none |
EUCTR2015‐004899‐30‐Outside‐EU/EEA.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multi‐centre, parallel study Trial registration number: EUCTR2015‐004899‐30‐Outside‐EU/EEA Country: China Outpatient or hospital, date trial conducted, duration of trial participation: not reported Inclusion criteria: · Diagnosis of eczema, according to: 1) Erythema, papilla/water blister, lichenification, skin damage with infiltration, 2) unknown reason, recurrent attacks; 3) itching in diseased skin · BSA disease involvement of less than or equal to 10% as assessed by palm method · Must present with moderate and above eczema as defined by a score greater than or equal to 3 using the IGA of eczema severity Exclusion criteria: · Any systemic disorder or active skin disease that would in any way confound interpretation of the study results or subjects who present with scars, moles, tattoos, body piercings, sunburn in the test area which could interfere with the assessment of lesions at screening · Eczema restricted to the face, the feet or the hands only · Any anti‐infectives drug for a current complication of overt bacterial, fungal and viral infection · History of recent (<1 month) active or presence of current superficial skin infections of viral aetiology such as herpes simplex, or varicella · Exposed to: topical agents administered in the diseased skin, including emollient ‐ 1 week; systemic administration of anti‐histamine agents ‐ 2 week; systemic administration of corticosteroid ‐4 week; systemic administration of immunosuppressive drugs ‐ 4 week; UV therapy ‐4 week · Foreseeable intensive UV exposure during the study · History of clinically significant cardiovascular, pulmonary, gastrointestinal, liver, neurological, renal or haematological abnormalities · History of allergy to components of test medications to be used in the study · History of anaphylaxis to food, medications, insect venom, or latex Additional design details: none |
| Participants | Total number randomised: 240 participants (120 to apply the intervention and 120 to apply the comparator) Age: mean age intervention group 42.90 years (SD 12.93); comparator 41.89 years (SD 12.65) Sex: female:males: intervention group 73: 46; comparator group 66: 53 Ethnicity, duration of eczema: not reported Severity of eczema: participants must have BSA disease involvement of less than or equal to 10% as assessed by palm method with moderate and above eczema as defined by a score greater than or equal to 3 using IGA of eczema severity. Body site: not reported Number of withdrawals: intervention group 7 due to lack of efficacy n = 4, voluntary withdrawal n = 2, withdrew for other reason n = 1; comparator group 11 due to withdrawal due to AE n = 1, protocol violation n = 1, lack of efficacy n = 8, withdrew for other reasons n = 1 Notes: none |
| Interventions | Run‐in details: not reported Intervention: clobetasone butyrate 0.05% cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: placebo 0% cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Changes from baseline in EASI score · Changes from baseline pruritus scores (VAS) · Subject‐based assessment score of disease control of pruritus symptoms |
| Notes | Funding source: GlaxoSmithKline Declarations of interest: not declared Original language of publication: English Other: none |
Fadrhoncova 1982.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, single‐centre, within‐participant study Trial registration number: not reported Country: Czech Republic, assumed from the affiliation of the author Outpatient or hospital: hospitalised patients at a dermatology clinic in Prague, assumed from the affiliation of the author Date trial conducted: not reported Duration of trial participation: 4 weeks Inclusion criteria: patients with bilateral and symmetrical eczema on the limbs and torso (not face) Exclusion criteria: · Signs of impetiginisation · Pregnancy · Malignant disease Additional design details: none |
| Participants | Total number randomised: 52 sides treated (26 to apply the intervention and 26 to apply the comparator) Age: range 2 to 66 years Sex: males n = 10: females n = 16 Ethnicity: not reported Duration of eczema: not reported Severity of eczema: the paper gives mean scores on various clinical parameters at baseline, but it is very difficult to interpret Body site: limbs and torso (not face) Number of withdrawals: 2 participants did not tolerate the treatment and were excluded after week 1. The final column of table 2 (n) suggests that there were no further withdrawals Notes: none |
| Interventions | Run‐in details: not reported Intervention: hydrocortisone butyrate 0.1% cream used twice daily for 4 weeks · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone 1% cream used twice daily for 4 weeks. · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: no occlusion. Both participants and nurses were instructed to wash their hands between left and right side applications to prevent contamination Notes: none |
| Outcomes | · Investigator assessment of 10 clinical signs and symptoms (pruritus, lichenification, infiltration, erythema, exudation, amount of crusts, amount of vesicles, amount of papules, amounts of pustules, and exfoliation), scored from 0 = absent to 4 = very severe, reported separately as group means (SD) and as a combined score. Combined score assumed to be the mean value of each sign/symptom multiplied by the number of participants reporting that sign, summed for all signs/symptoms · Participant and physician preference for each treatment over the other · AE |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: Czech; translated for Lax 2022 by Simona Slezáková Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022 |
Fattah 1976.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, single‐centre, within‐particpant study Trial registration number: not reported Country: Egypt Outpatient or hospital: Dermatology Department, Ain‐Shams Faculty of Medicine Date trial conducted: not reported Duration of trial participation: 3 weeks Inclusion criteria: presence of bilateral, symmetrical lesions of similar aetiology and severity Exclusion criteria: secondary bacterial or fungal infections Additional design details: included patients with psoriasis, eczematous dermatitis, neurodermatitis, and AD. Only extracted for the purpose of this review AD data, participant characteristics, bar site, were not reported individually for the AD participants |
| Participants | Total number randomised: 8 sides treated (4 to apply the intervention and 4 to apply the comparator) Age: overall n = 36 aged between 11 to 50 years, n = 14 aged above fifty years. Specific age for AD patients not reported Sex: overall males n = 31, females n = 19. Sex for AD patients not reported. Ethnicity: not reported Duration of eczema: for AD patients not reported. Severity of eczema: for AD patients not reported. Body site: arms n = 2, hands n = 2 Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: at least seven days before the inititation of the study any previous therapy was discontinued Intervention: halcinonide 0.1% cream used three times daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone 1% cream used three times daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Changes in erythema, scaling, oedema, transudation, and lichenification · Participants compared sides in regards to itching, pain and general comfort |
| Notes | Funding source: not reported Declarations of interest: author affiliated to the Squibb Institute, which is likely to be related to Squibb pharmaceutical company who market halcinonide Original language of publication: English Other: not reported |
Fisher 1979.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number, country, outpatient or hospital, date trial conducted: not reported Duration of trial participation: three weeks Inclusion criteria: people with AD Exclusion criteria: · Systemic or intralesional corticosteroids or antimetabolite drugs, if used, had not been employed in the treatment of these patients for more than three months prior to the beginning of the study. · Pregnancy · < 2 years of age Additional design details: none |
| Participants | Total number randomised: 214 sides treated Age: 2 to 73 years Sex: men n = 40, women n = 67 Ethnicity: caucasian n = 48, black n = 58, oriental n = 1 Duration of eczema: 0.7 to 480 months Severity of eczema: mild n = 13, moderate n = 66, severe n = 28 Body site: not reported Number of withdrawals: 13 Notes: none |
| Interventions | Run‐in details: not reported Intervention: fluiconinonide 0.05% cream used three times daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: betamethasone valerate 0.1% cream used three times daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA continuous, 5 point scale at 3 weeks · AE |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Fowler 2007.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: USA Outpatient or hospital: 15 centres ‐ not reported if outpatient or hospital Date trial conducted: not reported Duration of trial participation: 8 days Inclusion criteria · Children and adolescents aged 2 to 17 years · AD according to the Williams et al criteria · Mild to moderate AD (IGA score of 2 or 3) involving at least 5% total BSA with moderate to severe pruritus (baseline pruritus score of 2 or 3) Exclusion criteria · Immunocompromised · A concurrent skin disease that could interfere with evaluations · AD triggered by a known unavoidable allergen or irritant · Had an active viral or bacterial infection Additional design details: none |
| Participants | Total number randomised: 174 participants (86 to apply the intervention and 88 to apply the comparator) Age: intervention group: mean 6.5 years (SD 4.1), range 2 to 17 years, vehicle group: 7.4 years (SD 4.2), range 2 to 17 years Sex: not reported Ethnicity: not reported Duration of eczema: not reported Severity of eczema: intervention group n = 26 (30%) mild disease, n = 60 (70%) moderate; vehicle group n = 31 (36%) had mild disease, n = 56 (64%) moderate Body site: not reported Number of withdrawals: intervention group n = 5: reasons administration problems n = 2, unsatisfactory effect n = 1, lost to follow‐up n = 2, protocol violations n = 0; vehicle group n = 13, reasons: AE n = 1, administration problems n = 6, unsatisfactory effect n = 3, lost to follow‐up n = 0, protocol violations n = 3 Notes: none |
| Interventions | Run‐in details: all patients or their caregivers were required to apply a bland emollient starting at least 3 days prior to randomization Intervention: pimecrolimus 1% cream used twice daily for 7 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle used twice daily for 7 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: To qoute "all subjects or their caregivers were required to apply a bland emollient throughout the 7‐day treatment period" Notes: To qoute "no topical and systemic agents known or thought to have efficacy in treating AD or its associated pruritus, including sedating antihistamines, were permitted during the study" |
| Outcomes | · Time to a 1 point or more pruritus score improvement from baseline. The pruritus severity score consisted of a 4‐point scale used to evaluate the intensity of itching and scratching over the preceding 24‐hour period. Scoring (0 [absent] no pruritus; 1 [mild] occasional slight itching and scratching; 2 [moderate] constant or intermittent itching and scratching that is not disturbing sleep; 3 [severe] bothersome itching and scratching that is disturbing sleep) · Emergent AEs throughout the study · Percentage of subjects at study completion with a 1 point or more IGA score improvement from baseline |
| Notes | Funding source: Novartis Pharmaceuticals Corporation Declarations of interest: two authors were employees of Novartis Pharmaceuticals Corporation. Two other authors had work as a consultant, researcher, annd/or speaker for Novartis Pharmaceuticals Corporation Original language of publication: English Other: none |
Frankel 2011.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, single‐centre, within‐particpant study Trial registration number: NCT01202149 Country: US Outpatient or hospital: outpatient clinic Date trial conducted: March to October 2010 Duration of trial participation: 28 days Inclusion criteria · Males and females age 2 or above · Females of child‐bearing potential must have a negative urine pregnancy test at the baseline visit and agreed to use adequate birth control during the study · Diagnosis of mild to moderate AD for at least one year with symptoms on the extrematies · Has symmetrical target eczematous areas on opposite sides of the body with an IGA of at least 2 or 3 (mild‐moderate severity) for each selected target lesion Exclusion criteria · Females who are pregnant, attempting to conceive, or breastfeeding · Known hypersensitivity to either study drug · AD on > 30% body surface area · Current active skin malignancy or infection · Requiring the use of medications known to alter the course of atopic dermatitis during the study treatment · Received systemic antibiotics within 2 weeks · Using systemic corticosteroids or immunosuppressants within 28 days of the screening visit · Received topical corticosteroids or other topical therapies for AD within 7 days of the screening visit · Using phototherapy (UVB, PUVA) within 28 days of the screening visit · Currently participating in or, with in the previous 28 days, have participated in another study for the treatment of AD Additional design details: none |
| Participants | Total number randomised: 60 sides treated (30 to apply the intervention and 30 to apply the comparator) Age: 4 to 69 years old Sex, ethnicity, duration of eczema, severity of eczema: not reported Body site: inclusion criteria state need for AD on extremities Number of withdrawals: 2, not reported why Notes: none |
| Interventions | Run‐in details: none Intervention: pimecrolimus cream 1% cream used twice daily for 28 days Concurrent treatment: not reported Other key information: none Comparator: ceramide‐hyaluric acid emollient used twice daily for 28 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA · TLSS · Photography · Self‐assessment of itch and treatment preference · Skin atrophy and telangiectasia |
| Notes | Funding source: Onset Dermatologics Declarations of interest: the authors have no relevant financial conflicts of interest to disclose Original language of publication: English Other: none |
Freeman 2006.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: not reported Country: Australia Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 28 days Inclusion criteria: adult with mild to moderate AD Exclusion criteria: not reported Additional design details: the trial has three groups, the third is of TCS with a gold compound which is not eligible for this review |
| Participants | Total number randomised: 56 participants Age: mean age 39 years, range 18 to 81 years Sex: 54% male Ethnicity, duration of eczema: not reported Severity of eczema: one mild, the rest moderare AD according to SCORAD Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: one week run‐in. No further details Intervention: mometasone 0.1% ointment used once daily for unclear duration Concurrent treatment: not reported Other key information: none Comparator: vehicle ointment used once daily for unclear duration. Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Change from baseline SCORAD index at day 21 · QOL measured using SKINDEX · Patient and investigator global assessments |
| Notes | Funding source: Psiron Limited Declarations of interest: not declared Original language of publication: English Other: none |
Fujita 2021.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, within‐participant study Trial registration number: NCT03954158 Country: Japan Outpatient or hospital: outpatient, 3 study sites (from study protocol) Date trial conducted: June to December 2019 Duration of trial participation: 2 weeks Inclusion criteria · Active AD according to Hanifin and Rajka criteria · ≥ 6 months history of AD prior to screening · AD clinically stable for >1 month with an ISGA of 2 (mild) or 3 (moderate) at baseline. · AD lesions on their upper or lower limbs or ventral body trunk · BSA of 1‐30% at baseline Exclusion criteria · Previous history of angioedema, anaphylaxis, sensitivity to any componant of crisaborole ointment · Treatment with any topical or systemic PDE4 inhibitor Additional design details Randomisation was into 4 groups, but has been treated as a single pairwise comparison with subgroup analyses for the purposes of this review |
| Participants | Total number randomised: 162 individual lesions (81 to apply the intervention and 81 to apply the comparator) Age: cohort 1 ≥ 12 years; cohort 2 2 to 11 years Sex: · cohort 1 QD adminstration group male = 9, female = 11); BID male, n = 4, female, n = 17 · cohort 2 QD administration group male = 12; female = 8; BID administration group male, n = 9; female, n = 11 Ethnicity: asian Duration of eczema: ≥ 6 months history of AD Severity of eczema: mild (ISGA of 2) or moderate (ISGA of 3) Body site: AD lesions on upper or lower limbs or ventral body trunk (excluding scalp, genitals and groin area). Target lesions were not located on the face, neck, scalp, axilla, genitals, groin area, palms, dorsal side of hands, dorsal side of body trunk or soles) Number of withdrawals: no withdrawals Notes: none |
| Interventions | Run‐in details: not reported Intervention: crisaborole 2% ointment used once or twice daily for 14 days Concurrent treatment: not reported Other key information: none Comparator: vehicle ointment used once or twice daily for 14 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Change from baseline in TSS · Change from baseline in ISGA · Change from baseline in pruritic assessments in target lesions (peak pruritic NRS) in cohort 1 and the Itch Severity Scale Self‐Report (patients aged 6 to 11 years) and Caregiver‐Reported Itch Severity NRS (patients age 2 to 11 years) in cohort 2 · Safety of both crisaborole regimens, and SAE |
| Notes | Funding source: Pfizer Inc Declarations of interest: three authors were employees of Pfizer R&D Japan; two were stockholders of Pfizer Inc; and one had received research grants from Pfizer R&D Japan Original language of publication: English Other: none |
Furue 2014.
| Study characteristics | |
| Methods | Trial design: randomised, multicentre, parallel study Trial registration number: NCT01461941 Country: Japan Outpatient or hospital: 11 medical institutions in Japan Date trial conducted: not reported Duration of trial participation: 4 weeks Inclusion criteria: · Aged 20 to 64 years · AD according to Japanese Dermatological Association · 5 to 30% BSA Exclusion criteria: · Biological therapy within 6 months of the study · Systemic therapy such as corticosteroids (oral, injection, suppository and inhalant preparations), immunosuppressants, antihistamines/antiallergic agents · Chinese herbal medicines for AD · Phototherapy within 28 days of the study · Very potent topical corticosteroids within 28 days of the study · Other opical analgesic, antipruritic or anti‐inflammatory treatments within 7 days of the study · Active infection · Kaposi’s varicelliform eruption, scabies, molluscum contagiosum, impetigo contagiosum, psoriasis, Netherton’s syndrome or other autoimmune disorders which may affect the pathological evaluation of AD Additional design details: there is an 8 week extension phase of this study but it is open‐label with no control so data not extracted for this phase |
| Participants | Total number randomised: 78 participants (52 to apply the intervention and 26 to apply the comparator) Age: mean (range) intervention: 31.4 years (20 to 53); comparison 30.7 years (20 to 49) Sex: intervention: male n = 29, female n = 23; comparator: male n n = 15, female n = 11 Ethnicity: Japanese Duration of eczema: mean (range) intervention: 22.7 years (0 to 49); comparator: 25.5 years (7 to 45) Severity of eczema: EASI score mean intervention: 9.2 (SD 4.6); comparator 9.4, (SD 5.3) Body site: not reported Number of withdrawals: Intervention n = 5; comparator n = 6 Notes: not reported |
| Interventions | Run‐in details: not reported Intervention: E6005 0.2% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: the concurrent use of the following treatments was prohibited during the entire study period: biological agents such as TNF‐a inhibitors, anti‐IgE antibodies, and anti‐CD20 antibodies; corticosteroid preparations except eye drops and nasal sprays; immunosuppressants; antihistamines/antiallergic agents; Chinese herbal medicines for AD; topical analgesic or antipruritic preparations; and phototherapy. Concomitant use of moisturizers was permitted Notes: none |
| Outcomes | · Local AE · EASI ‐ clinical reported signs continuous · SCORAD · Itch · Withdrawal due to AE |
| Notes | Funding source: Eisai Co., Ltd., Japan Declarations of interest: to qoute: "this study was supported by Eisai Co., Ltd. Y.K., H.A. and S.H. are employees of Eisai Co., Ltd. H.N. received grants and research support from Eisai Co., Ltd. M.F., N.H. and H.N. served as consultants to Eisai Co., Ltd." Original language of publication: English Other: none |
Gehring 1996.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind study Trial registration number: not reported Country: German Outpatient or hospital, date trial conducted: not reported Duration of trial participation: two weeks Inclusion criteria: patients with neurodermatis Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 63 evaluated participants Age: intervention group mean 27 years, range 14 to 57; comparator group mean 30 years, range 13 to 17 Sex: intervention group: males n = 9, females n = 23; comparator group males n = 15, females n = 16 Ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: hydrocortisone 1% ointment used twice daily for 7 days · Concurrent treatment: days 8 to 14 partipants applied emulsion only · Other key information: none Comparator: vehicle emulsion used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Physicans clinical evaluation of redness and roughness of skin · Patient self assessment of skins roughness and itching via a visual analogue scale 1 to 10 · Measurement of skin colour · Laser doppler flowmetry · Transdermal water loss |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: German Other: none |
Gelmetti 1978.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: Italy Outpatient or hospital: Clinica di Dermatologia Pediatrica Date trial conducted: not reported Duration of trial participation: 7 days (assumed) Inclusion criteria: symmetrical and uniform dermatitis (for the purposes of this review only the data from participants with AD was extracted) Exclusion criteria: not reported for AD Additional design details: none |
| Participants | Total number randomised: 20 sides treated (10 to apply the intervention and 10 to apply the comparator) Age: range: 3 months to 10 years Sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: diflucortolone valerate 0.1% cream used twice (or three times) daily for 7 days (assumed) · Concurrent treatment: not reported · Other key information: none Comparator: betamethasone valerate 0.1% cream used twice (or three times) daily for 7 days (assumed) · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: Without occlusion Notes: none |
| Outcomes | Number of participants with regression ≥ 80% · Number judged good (of good, average, without result, worsening) · to qoute: "Side effects" (AE) (not specific enough for inclusion in this review) |
| Notes | Funding source: Roche were involved in the study but detail unclear Declarations of interest: not declared Original language of publication: Italian Other: none |
Gentry 1973.
| Study characteristics | |
| Methods | Trial design: double‐blind, randomised, multicentre, parallel study Trial registration number: not reported Country: USA Outpatient or hospital: hospital outpatient dermatology clinics Date trial conducted: not reported Duration of trial participation: 4 weeks Inclusion criteria: · Adults and children of both sexes · Steroid‐responsive dermatoses (results are reported separately for AD) Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 5 participants (3 to apply the intervention and 2 to apply the comparator) Age: Adults and children (ages unspecified) Sex, ethnicity, duration of eczema, severity of eczema, body site: not reported Number of withdrawals: none Notes: to quote "Comparison of the distribution of age, sex, and diagnoses of patients receiving each preparation indicated adequacy of randomisation" |
| Interventions | Run‐in details: not reported Intervention: fluocinolone acetonide 0.025% cream used twice daily for 4 weeks · Concurrent treatment: not reported · Other key information: none Comparator: desonide 0.05% cream used twice daily for 4 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: participants were encouraged to apply the medication sparingly |
| Outcomes | · IGA (6‐point scale rated as cleared, excellent, good,fair, poor, no effect and exacerbation) · Physician assessment of signs and symptoms: erythema, induration, pruritus and scaling scored 0‐3 · AE · Laboratory tests: blood cell count, liver function tests, renal function tests |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022 |
Gether 2023.
| Study characteristics | |
| Methods | Trial design: randomised, quadruple‐blinded (participant, care provider, investigator, outcomes assessor) parallel study Trial registration number: NCT04114097 Country: Denmark Outpatient or hospital: Department of Dermatology and Allergy Date trial conducted: August 2019 to March 2021 Duration of trial participation: 6 weeks (2 weeks daily treatment phase and then 4 weeks of twice weekly treatment) Inclusion criteria: · Aged 18 to 75 years · AD according to Hanifin and Rajka criteria ≥ 3 years · BMI ≤ 30 kg/m2 · Haemoglobin A1c ≤ 42 mmol/mol · Normal haemoglobin · In general should not be diagnosed with diseases that may affect or be affected by the study/treatment Exclusion criteria: · Diabetes mellitus · Other chronic inflammatory diseases beside AD and non‐treatment demanding rhinitis or asthma · Pregnancy and breastfeeding · Treatment with drugs that might affect glucose metabolism beside TCS within a month · Daily smoker, alcoholic, or drug abuser · Hypersensitivity to protopic or betnovate Additional design details: none |
| Participants | Total number randomised: 36 participants Age: 18 to 75 years Sex: all Ethnicity: not reported Duration of eczema: ≥ 3 years Severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: betamethasone dipropionate 0.1% ointment used twice daily for two weeks then twice weekly for four weeks · Concurrent treatment: a placebo was also administered to this group · Other key information: none Comparator: tacrolimus 0.1% ointment used twice daily for two weeks then twice weekly for four weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Set out to measure relevant outcomes but no relevant outcomes had been reported at the time of the most recent search |
| Notes | Funding source: Leo Pharma Declarations of interest: not declared Original language of publication: English Other: none |
Ghatikar 1974.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, within‐participant study Trial registration number: not reported Country: India Outpatient or hospital: six dematology centres, most participants were outpatients Date trial conducted: not reported Duration of trial participation: up to 14 days Inclusion criteria · Steroid‐responsive dermatological disorder · Bilaterally symmetrical lesions Exclusion criteria: not reported Additional design details: subgroup had AD with some results provided separately |
| Participants | Total number randomised: 6 with AD (on 6 side to apply the intervention and on the other 6 sides to apply the comparator) Age: not reported on who recruited. All ages were selected for inclusion Sex, ethnicity: not reported Duration of eczema: not reported separately for the subgroup those who had AD Severity of eczema, body site: not reported Number of withdrawals: 3/6 withdrew ‐ no details on why Notes: none |
| Interventions | Run‐in details: not reported Intervention: betamethasone benzoate 0.025% cream used three times daily for 14 days Concurrent treatment: not reported Other key information: none Comparator: vehicle used three times daily for 14 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Improvement in skin lesions in terms of oozing, scaling crusting, excoriation, erthyema ‐ to quote: "etc." |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Giannetti 1981.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: Italy Outpatient or hospital: outpatients, assumed Universita di Pavia lnstituto di Clinica Dermatologica from the affiliation of the authors Date trial conducted: not reported Duration of trial participation: 3 weeks Inclusion criteria: patients with AD of mild‐to‐medium severity. The trial also stated that lesions were to be symmetrical and not complicated by microbial or fungal infections. Patients stopped all therapy for up to 15 days. Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 40 sides treated (20 to apply the intervention and 20 to apply the comparator) Age: range from 7 months to 14 years Sex: male n = 10; female n = 10 Ethnicity: not reported Duration of eczema: average 5 years and 4 months (range from 5 months to 13 years) Severity of eczema: not clearly reported Body site: not reported Number of withdrawals: none Notes: none |
| Interventions | Run‐in details: participants stopped all therapy for ≥ 15 days Intervention: hydrocortisone butyrate 0.1% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: proprietary: Locoidon, Brocades Comparator: hydrocortisone acetate 1% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: applied morning and evening under occlusion with a simple bandage. It was stated in the methods that treatment would be suspended in the event of any side effects (AE). |
| Outcomes | · AE including cutaneous atrophy · Assessment of signs and symptoms: erythema, vesiculation, exudation, desquamation, excoriation, lichenification and pruritus, each scored 0 = symptom absent to 3 = severe · IGA (assumed) of improvement relative to baseline: number of sides where complete healing or partial symptom regression occurred were reported. It is assumed that this was the signs and symptoms assessment combined (erythema, vesiculation, exudation, desquamation, excoriation, lichenification and pruritus, each scored 0 = symptom absent to 3 = severe). · Where possible, itching scores should also be noted (it has been assumed this was participant‐assessed because of the nature of the symptoms; stated "where possible", but not clear in report) |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: Italian Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Goh 1999.
| Study characteristics | |
| Methods | Trial design: randomised, third‐party‐blind, within‐participant study Trial registration number: not reported Country: Singapore Outpatient or hospital: National Skin Centre Singapore Date trial conducted: April to October 1994 Duration of trial participation: weeks (follow‐up on day 22) Inclusion criteria: patients with moderate‐severe bilateral chronic eczema on the limbs. Chronic eczema was evidenced with lichenified scaly patches and plaques for at least 6 months. Exclusion criteria: · Pregnancy · Known hypersensitivity to corticosteroids · Presence of skin atrophy (e.g. telangiectasia and/or striae) · Those on systemic steroids within 28 days of entering the trial Additional design details: none |
| Participants | Total number randomised: 60 participants Age: mean age 45.7 years (range 16 to 85) Sex: male n = 25, female n = 33 Ethnicity: not reported Duration of eczema: mean duration of eczema 7.5 years (range 3 to 30 years) Severity of eczema: not clearly reported Body site: bilaterial chronic eczema on limbs Number of withdrawals: 2 participants withdrew from the trial; no reasons were given. Notes: The baseline characteristics were only for the 58 participants who completed the trial. |
| Interventions | Run‐in details: none Intervention: mometasone furoate 0.1% cream used once daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: clobetasol propionate 0.05% cream used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: no medication other than the trial medication was to be applied to the trial area. Notes: cream tubes were dispensed at the beginning of each week of the trial; participants were asked to return used tubes at the next weekly visit. One treatment applied to one limb and one treatment applied to the other limb. |
| Outcomes | · Examination for signs of skin atrophy · Cosmetic acceptability · Participants' assessment of response to treatment (excellent, good, fair, or poor) · Dermatologists' assessment of response to treatment (cleared = 100% improvement; marked ≥ 75% improvement; moderate = 50%‐75% improvement; slight = signs/symptoms of chronic eczema (including erythema, induration, crusting, scaling, excoriation and pruritus); these were scored upon entry into the trial using a severity scale which ranged from 0 = none to 3 = severe). Overall total scores were also calculated. · Side effects (AE) (not specifically mentioned apart from skin atrophy, but mentioned in the results/discussion) |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: This study has previously been extracted by this group; some content is reproduced from Lax 2022 |
Gooderham 2021.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: NCT03916081 Country: USA and Canada Outpatient or hospital: presumed outpatient Date trial conducted: May to November 2019 Duration of trial participation: 4 weeks Inclusion criteria: · Male or female aged 12 years and older · AD for at least 6 months · BSA involvement of at least 2% but no more than 25% · vIGA‐AD (validated Investigator Global Assessment‐Atopic Dermatitis) score of 'mild' ('2') or 'moderate ('3') · EASI score of ≥ 5 · Females of childbearing potential must have a negative serum pregnancy test at screening and, if sexually active, agree to use birth control throughout the trial. · In good health as judged by the investigator, based on medical history, physical examination, and clinical tests Exclusion criteria: · Any serious medical condition or clinically significant physical examination or test abnormality that would prevent study participation or place the subject at significant risk · Evidence of skin conditions other than AD Additional design details: none |
| Participants | Total number randomised: 136 participants (46 to apply intervention one, 45 intervention two and 45 to apply the comparator) Age: ≥ 12 years Sex: male n = 43, female n = 93 Ethnicity: white n = 88, black n = 36, multiple/other n = 12 Duration of eczema: not reported Severity of eczema: mild‐to‐moderate. BSA of at least 2 but no more than 25% EASI score of ≥ 5 at baseline Body site: not reported Number of withdrawals: completion rate was over 90% in all treatment groups. Two patients (one with roflumilast 0.0 5% and one in the vehicle group) discontinued the study drug due to an AE. Notes: none |
| Interventions | Run‐in details: not reported Intervention: Intervention one: roflumilast 0.15% cream used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention two: roflumilast 0.0 5% cream used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI total score change and percent change · EASI‐75 and EASI‐50 score improvement · BSA involvement change · Worse Itch Numerical Rating Score (WI‐NRS) Pruritis Score change and improvement |
| Notes | Funding source: Arcutis Biotherapeutics, Inc. Declarations of interest: 13 authors were investigators, consultants, and/or advisory board members for Arcutis Biotherapeutics, Inc. ZDD has received grant support from Arcutis Biotherapeutics, one author had a patent application relevant to this work, and six authors were employees of Arcutis Biotherapeutics, Inc. Original language of publication: English Other: results are limited to a poster and an abstract |
Gower 2022.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind study Trial registration number: NCT04040192 Country: Australia, United States, Canada, China, Israel, Turkey Outpatient or hospital: not reported (presumed outpatient) Date trial conducted: September 2019 to January 2022 Duration of trial participation: 52 weeks Inclusion criteria: · Male or female · 3 months of age or older · AD according to Hanifin and Rajka criteria · Minimum of 5% BSA affected by AD · ISGA score of mild (2) or moderate (3) Exclusion criteria: has any clinically significant medical disorder, condition, or disease (including active or potentially recurrent non‐AD dermatological conditions and known genetic dermatological conditions that overlap with AD, such as Netherton syndrome) Additional design details: none |
| Participants | Total number randomised: 494 participants Age: 3 months and older Sex, ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐moderate Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: crisaborole 2% ointment used once daily for 52 weeks · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used once daily for 52 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Flare‐free maintenance · Incidence of treatment‐emergent AE · Number of flare‐free days · Number of flares · Pruritis response maintenance · EASI response maintenance · Patient‐reported outcome response maintenance (DQoL and Patient‐Reported Outcomes Measurement Information System (POEM)) · Severity of flares (EASI and ISGA) · Duration of flares |
| Notes | Funding source: Pfizer Declarations of interest: not declared, but Pfizer manufactures crisaborole Original language of publication: English Other: discrepancy in sample size between NCT record (494) and abstract (497) on participant numbers |
Gradman 2007.
| Study characteristics | |
| Methods | Trial design: randomised, investigator‐blind, single‐centre, cross‐over study Trial registration number: not reported Country: Denmark Outpatient or hospital: Children's Clinic, Randers (assumed outpatients) Date trial conducted: February to April 2005 Duration of trial participation: 10 weeks Inclusion criteria: · Children aged 5 to 14 years with eczema according to Hanifin and Rajka criteria · Normal current growth · Tanner stage 1 Exclusion criteria: · Anti‐inflammatory treatment within one week of the study · Concurrent asthma or rhinitis if requiring treatment with inhaled or intranasal glucocorticoids · Other chronic illnesses · Surgery performed within 4 weeks of the study · Fever > 39.5 for > 5 days · Tanner stage 2‐5 Additional design details: none |
| Participants | Total number randomised: 20 participants (20 to apply the intervention and 20 to apply the comparator) Age: mean age 8.1 years (range 5.1 to 12) Sex: female n = 13, male n = 7 Ethnicity: not reported Duration of eczema: mean duration 77 months Severity of eczema: to quote: "at inclusion median EASI score was 4.1 [1.8; 9.5]" Body site: arm, leg and trunk Number of withdrawals: to quote: "a girl withdrew during the second treatment period due to complaints about a burning sensation in the skin lasting many hours after applying tacrolimus. Another girl was lost to follow‐up during the first treatment period". Notes: none |
| Interventions | Run‐in details: there were five periods each of 2 weeks duration. After a run‐in, the children were allocated to treatment with either mometasone furoate or tacrolimus for treatment period 1, followed by a washout period before the children were given the opposite treatment for period 2. Intervention: mometasone furoate 0.1% ointment used twice daily for 14 days Concurrent treatment: not reported Other key information: none Comparator: tacrolimus 0.1% ointment used twice daily for 14 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: throughout the study, emollient was applied twice daily. No other medication was allowed. Notes: none |
| Outcomes | · EASI · Lower leg length · Urine analysis · AE |
| Notes | Funding source: grants from Fujisawa Scandinavia, Aase & Einer Danielsens Fond and Fonden til Faglig Udvikling af Speciallaegepraksis Declarations of interest: not declared Original language of publication: English Other: results were not extracted as the first time period results were not presented separately. |
Griffiths 2002.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, within‐particpant study Trial registration number: not reported Country: 14 centres in Canada, Denmark, The Netherlands and the UK Outpatient or hospital: multicentre Date trial conducted: not reported Duration of trial participation: 14 days Inclusion criteria: · Male or female aged 18 years and over · AD with stable, symmetrical lesions on both arms, with a minimum Total Severity Score (TSS) of 6 for a selected target lesion · Females of childbearing potential had a negative urine pregnancy test and used adequate contraception throughout the study. Exclusion criteria: · Suspected microbial, fungal or viral superinfection, scarring or hyperpigmentation within the target lesions, treatment with systemic antihistamines, antibacterials or topical therapies other than emollients in the previous week Additional design details: this is also a parallel group study as participants were randomised to cipamfylline vs. vehicle and cipamfylline vs. hydrocortisone 17‐butyrate. |
| Participants | Total number randomised: 103 sides treated Age: mean 33.1 years, range from 18 to 61 Sex: male n = 25, female n = 29 Ethnicity, duration of eczema: not reported Severity of eczema: a minimum TSS of 6 for a selected target lesion Body site: arms Number of withdrawals: n = 3 Notes: none |
| Interventions | Run‐in details: not reported Intervention one: cipamfylline (PDE‐4) cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Intervention two: hydrocortisone‐17‐butyratee 0. 1% cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Intervention three: clobetasol propionate 0.05% cream used once daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used once daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: the treatment areas were defined as from the wrist skin crease to the shoulder. No other topical treatments on the arms were allowed. The participants were instructed to wash their hands thoroughly after applying the first cream and before applying the second cream to the other arm. Notes: none |
| Outcomes | · IGA · Patient and clinician‐reported signs · Withdrawal due to AE |
| Notes | Funding source: Leo Pharmaceuticals Declarations of interest: author affiliated to Leo Pharmaceuticals Original language of publication: English Other: none |
Guttman‐Yassky 2017a.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant trial Trial registration number: NCT02376049 Country: Canada, Israel Outpatient or hospital: not reported Date trial conducted: February to July 2015 Duration of trial participation: 2 weeks Inclusion criteria: adults with mild‐to‐moderate AD Exclusion criteria: · Fitzpatrick Skin Type score > 5 · Systemic immunosuppressants in the last 4 weeks · Topical steroids/immunomodulators in the last 2 weeks · Moisturisers in the past 3 days · Participation in other trials within 4 weeks Additional design details: none |
| Participants | Total number randomised: 120 lesions treated (30 in each of the four groups) Age: 33.9 years mean (SD 14.9) Sex: female n = 14, male n = 16 Ethnicity: Asian n = 1, African‐American n = 4, white n = 25 Duration of eczema: mean 29.3 years (SD 16.3) Severity of eczema: mild‐to‐moderate Body site: non‐facial eczema Number of withdrawals: 1 = due to use of prohibited medication Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream used once daily for 14 days · Concurrent treatment: not reported · Other key information: none Intervention two: betamethasone dipropionate 0.05% cream used once daily for 14 days · Concurrent treatment: not reported · Other key information: none Intervention three: clobetasol propionate 0.05% cream used once daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used once daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA · Withdrawal due to AE (not reported by group) · Local AE (not reported by group) |
| Notes | Funding source: Leo Pharma A/S Declarations of interest: three authors have received grant/research support or/and sit on advisory boards and/or act as consultants for pharmaceutical companies; for two, this includes Leo Pharma. Two other authors are employees of Leo Pharma A/S. The remaining authors declared that they had no relevant conflicts of interest. Original language of publication: English Other: none |
Guttman‐Yassky 2019.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre study Trial registration number: not reported Country: US Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 2 weeks Inclusion criteria: adults with mild‐to‐moderate AD Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 48 participants (17 to apply intervention one, 17 intervention two, and 14 to apply the comparator) Age: adult Sex, ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐moderate Body site: not reported Number of withdrawals: 3 withdrew for adverse site reactions Notes: none |
| Interventions | Run‐in details: not reported Intervention one: SB414 2% cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Intervention two: SB414 6% cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI · Target lesion severity score · Itch NRS · AE |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Hamza 2018.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind study Trial registration number: NCT02925793 Country: Canada, US, South Africa Outpatient or hospital: clinic Date trial conducted: December 2016 to July 2018 Duration of trial participation: 10 weeks Inclusion criteria: · Active AD according to Hanifin and Rajka criteria · Mild‐to‐moderate AD defined by an IGA score of 3 or 2 · AD covering a minimum of 5% of the BSA at baseline · Male or female patients aged 18 years and older Exclusion criteria: · Used systemic treatments (other than biologics) that could affect AD within 4 weeks, e.g. retinoids, methotrexate, cyclosporine, hydroxycarbamide (hydroxyurea), azathioprine and oral/injectable corticosteroids. Intranasal corticosteroids for stable medical conditions are allowed. Additional design details: none |
| Participants | Total number randomised: 327 participants (106 to apply the intervention and to apply the comparator) Age: mean age 40.6 years (SD 15.91) Sex: female 57.7%, male 42.3% Ethnicity: American‐Indian or Alaskan native n = 0, African 18.7%, native Hawaiian or other Pacific Islander 1.1%, black or African‐American 22.1%, white 46.9%, unknown 10.7% Duration of eczema, severity of eczema, body site: not reported Number of withdrawals: 82/327 withdrew; 25 in vehicle arm, and 29 and 28 in active arms. 12 withdrawals for adverse events, 12 for lack of efficacy. The most common reason was for "withdrawal by subject". Notes: none |
| Interventions | Run‐in details: Intervention one: DS107 1% cream used twice daily for 56 days · Concurrent treatment: placebo/vehicle · Other key information: none Intervention two: DS107 5% cream used twice daily for 56 days · Concurrent treatment: placebo/vehicle · Other key information: none Comparator: placebo/vehicle used daily for 56 days · Concurrent treatment: · Other key information: Concurrent treatments received alongside both intervention and comparator: emollients could be applied to other areas of dry skin that were not in the defined treatment area. Once enroled, patients were restricted from using any other treatment for AD. Any medication or therapeutic intervention deemed necessary for the patient, and which in the opinion of the investigator did not interfere with the safety and efficacy evaluations, could be continued unless they were included in the prohibited list of medications and therapeutic regimens. Notes: intervention is a CD40 inhibitor which has a direct immune suppressive action. |
| Outcomes | · Pruritus NRS · EASI · IGA |
| Notes | Funding source: DS Biopharma Declarations of interest: not declared Original language of publication: English Other: none |
Handa 1985.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: the lead author was affiliated to the Department of Skin and V.D., Government Medical College, Patiala‐147 001, India. Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 3 weeks Inclusion criteria: a clinical diagnosis (supported by histopathological evidence where needed), of which AD was one of the named conditions with data presented separately. Exclusion criteria: · Pregnancy · Cases of tuberculosis, syphilis, or viral diseases such as vaccinia, variola, and varicella Additional design details: none |
| Participants | Total number randomised: 52 participants completed the trial (unclear if a different number randomised), 7 of which were AD. Sides treated (104 to apply the intervention and 104 to apply the comparator) Age: overall age ranged from 10 to 75 years, but baseline data were not reported separately for AD participants Sex: baseline data were not reported separately for AD participants. Ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: diflucortolone valerate 0.1% ointment used applied 3 times daily for the first 3 days, twice daily for up to 2 weeks, then once daily for the third week · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone acetate 1% ointment used applied 3 times daily for the first 3 days, twice daily for up to 2 weeks, then once daily for the third week · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: no other corticosteroid was given either locally or systemically. Notes: none |
| Outcomes | · IGA (4‐point scale: very good/complete healing, good/distinct improvement, poor/slight improvement, failure/no treatment success) · Systemic effects (not reported in the methods, only in the results) |
| Notes | Funding source: Schering A.G. Berlin/Bergkamen (Division of German Remedies Limited) supplied both ointments Declarations of interest: not declared Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Handa 2022.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: not reported Country: India Outpatient or hospital: paediatric dermatology outpatient clinic Date trial conducted: not reported Duration of trial participation: 6 weeks Inclusion criteria: · Age between 1 and 12 years · Mild‐to‐moderate AD (objective SCORAD up to 40) Exclusion criteria: · History of using systemic corticosteroids, nonsteroidal systemic immunosuppressants, or phototherapy within 4 weeks · History of using topical corticosteroids, TCIs, topical antibiotics, or any medicated topical agent within 1 week Additional design details: all patients received antihistamines and emollients irrespective of their group allocation. |
| Participants | Total number randomised: 50 participants (25 to apply the intervention and 25 to apply the comparator) Age: 1 to 12 years Sex: male n = 25, female n = 25 Ethnicity: not reported Duration of eczema: 25 to 26 months Severity of eczema: mild‐to‐moderate Body site: not reported Number of withdrawals: none reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.1% ointment used twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Comparator: fluticasone 0.05% cream used twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: antihistamines and emollients Notes: none |
| Outcomes | SCORAD |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Hanifin 2001a.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre study Trial registration number: not reported Country: USA Outpatient or hospital: unspecified medical centres Date trial conducted: September 1996 to July 1998 Duration of trial participation: 12 weeks Inclusion criteria: · Males and females ≥ 16 years of age · Moderate‐to‐severe AD according to Hanifin and Rajka; Rajka and Langeland criteria, affecting 10 to 100% BSA · Willing not to use other therapies during the trial, including topical antibiotics Exclusion criteria: · Serious skin disorder, pigmentation, or scarring in affected areas · Infected AD · Systemic disease that would contraindicate the use of tacrolimus or uncontrolled chronic condition · Pregnancy or breastfeeding Additional design details: three studies reported in one paper. Study characteristics reported separately. Where analyses were pooled with Hanifin 2001b ± Hanifin 2001c, pooled outcomes were extracted here and not in the other two tables of study descriptions. |
| Participants | Total number randomised: 304 participants (103 to apply intervention one, 99 to apply intervention two, and 102 to apply the comparator) Age: vehicle group mean 38.6 years (SD 13.8); tacrolimus 0.03% group mean 38.0 years (SD 13.8); tacrolimus 0.1% group mean 39.3 years (SD 13.0) Sex: vehicle group 49% males, 51% females; tacrolimus 0.03% group 39.8% males, 60.2% females; tacrolimus 0.1% group 38.4% males, 61.6% females Ethnicity: vehicle group, 65.7% white; 29.4% African‐American; 4.9% other; tacrolimus 0.03% group, 67.0% white; 27.2% African‐American; 5.9% other; tacrolimus 0.1% group, 66.7% white; 26.3% African‐American; 7.1% other Duration of eczema: not reported Severity of eczema: vehicle group, 48.0% moderate severity, 52% severe; tacrolimus 0.03% group, 52.4% moderate severity, 47.6% severe; tacrolimus 0.1% group, 39.4% moderate severity, 60.6% severe Body site: vehicle group 89.2% facial/neck involvement; tacrolimus 0.03% group 79.6% facial/neck involvement; tacrolimus 0.1% group 79.8% facial/neck involvement Number of withdrawals: vehicle group n = 64; due to AE n = 12, lack of efficacy n = 41, and administrative reasons n = 11; tacrolimus 0.03% group n = 30; due to AE n = 5, lack of efficacy n = 11, and administrative reasons n = 14; tacrolimus 0.1% group n = 28; due to AE n = 7, lack of efficacy n = 10, and administrative reasons n = 11 Notes: none |
| Interventions | Run‐in details: no antimicrobial within 7 days of the trial Intervention one: tacrolimus 0.03% ointment used twice daily for 12 weeks · Concurrent treatment: not reported · Other key information: none Intervention two: tacrolimus 0.1% ointment used twice daily for 12 weeks · Concurrent treatment: not reported · Other key information: none Comparator: vehicle 0% ointment used twice daily for 12 weeks · Concurrent treatment: none · Other key information: not reported Concurrent treatments received alongside both intervention and comparator; all were instructed to apply a thin layer to areas of active disease. Notes: to quote: "Treatment continued until approximately 1 week after complete clearance of atopic dermatitis or up to 12 weeks." |
| Outcomes | · EASI · Clinical signs of atopic dermatitis (erythema, oedema, excoriation, lichenification, oozing, and scaling) rated from 0 = absent to 3 = severe for each of four body regions (head/neck, trunk, upper limbs, and lower limbs). The average values for non‐head/neck areas were presented. · IGA · Patient assessment of pruritus · DLQI · AE |
| Notes | Funding source: Fujisawa Healthcare, Inc. Declarations of interest: To quote: "The authors are on Fujisawa Healthcare’s speakers’ bureau, have received grant/research support, and/or have been consultants for Fujisawa Healthcare, or are employees of Fujisawa Healthcare." Original language of publication: English Other: none |
Hanifin 2001b.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre study Trial registration number: not reported Country: USA Outpatient or hospital: unspecified medical centres Date trial conducted: September 1996 to July 1998 Duration of trial participation: 12 weeks Inclusion criteria: · Males and females ≥ 16 years of age · Moderate‐to‐severe AD according to Hanifin and Rajka; Rajka and Langeland criteria affecting 10 to 100% BSA · Willing not to use other therapies during the trial, including topical antibiotics Exclusion criteria: · Serious skin disorder, pigmentation, or scarring in affected areas · Infected AD · Systemic disease that would contraindicate the use of tacrolimus or uncontrolled chronic condition · Pregnancy or breastfeeding Additional design details: three studies reported in one paper. Study characteristics reported separately. Where analyses were pooled with Hanifin 2001a ± Hanifin 2001c, pooled outcomes were reported in Hanifin 2001a and not in the other two tables of study descriptions. |
| Participants | Total number randomised: 328 participants (108 to apply intervention one, 110 to apply intervention two, and 110 to apply the comparator) Age: vehicle group mean 38.5 years (SD 14.3); tacrolimus 0.03% group mean 37.9 years (SD 13.8); tacrolimus 0.1% group mean 39.2 years (SD 15.8) Sex: vehicle: 40.9% males, 59.1% females, tacrolimus 0.03%: 50% males, 50% females; tacrolimus 0.1%: 42.7% males, 57.3% females Ethnicity: vehicle group, 66.4% white, 24.5% African‐American, 9.1% other; tacrolimus 0.03% group, 69.4% white, 25.0% African‐American, 5.6% other; tacrolimus 0.1% group, 66.4% white, 26.4% African‐American, 7.3% other Duration of eczema: not reported Severity of eczema: vehicle group, 44.5% moderate severity, 55.5% severe; tacrolimus 0.03% group, 36.1% moderate severity, 63.9% severe; tacrolimus 0.1% group, 42.7% moderate severity, 57.3% severe Body site: vehicle group 89.1% had facial/neck involvement; tacrolimus 0.03% group 92.6% had facial/neck involvement; tacrolimus 0.1% group 90.9% had facial/neck involvement Number of withdrawals: vehicle group: n = 81; due to AE n = 14, lack of efficacy n = 54, and administrative reasons n = 13; tacrolimus 0.03% group n = 31; due to AE = 8, lack of efficacy n = 15, and administrative reasons n = 8; tacrolimus 0.1% group n = 24; due to AE n = 4, lack of efficacy n = 8, and administrative reasons n = 12 Notes: none |
| Interventions | Run‐in details: no antimicrobial within 7 days of the trial Intervention one: tacrolimus 0.03% ointment used twice daily for 12 weeks · Concurrent treatment: not reported · Other key information: none Intervention two: tacrolimus 0.1% ointment used twice daily for 12 weeks · Concurrent treatment: not reported · Other key information: none Comparator: vehicle 0% ointment used twice daily for 12 weeks · Concurrent treatment: · Other key information: Concurrent treatments received alongside both intervention and comparator; all were instructed to apply a thin layer to areas of active disease. Notes: to quote: "Treatment continued until approximately 1 week after complete clearance of atopic dermatitis or up to 12 weeks." |
| Outcomes | · EASI · Clinical signs of atopic dermatitis (erythema, oedema, excoriation, lichenification, oozing, and scaling) rated from 0 = absent to 3 = severe for each of four body regions (head/neck, trunk, upper limbs, and lower limbs). The average values for non‐head/neck areas were presented. · IGA · Patient assessment of pruritus · DLQI · AE |
| Notes | Funding source: Fujisawa Healthcare, Inc Declarations of interest: to quote: "The authors are on Fujisawa Healthcare’s speakers’ bureau, have received grant/research support, and/or have been consultants for Fujisawa Healthcare, or are employees of Fujisawa Healthcare." Original language of publication: English Other: none |
Hanifin 2001c.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre study Trial registration number: not reported Country: USA Outpatient or hospital: unspecified medical centres Date trial conducted: August 1997 to June 1998 Duration of trial participation: 12 weeks Inclusion criteria: · Males and females 2 to 15 years of age · Moderate‐to‐severe AD according to Hanifin and Rajka; Rajka and Langeland criteria and affecting 10 to 100% BSA · Willing not to use other therapies during the trial, including topical antibiotics Exclusion criteria: · Serious skin disorder, pigmentation, or scarring in affected areas · Infected AD · Systemic disease that would contraindicate the use of tacrolimus or uncontrolled chronic condition · Pregnancy or breastfeeding Additional design details: three studies reported in one paper. Study characteristics reported separately. Where analyses were pooled with Hanifin 2001a ± Hanifin 2001b, pooled outcomes were reported in Hanifin 2001a and not in the other two tables of study descriptions. |
| Participants | Total number randomised: 351 participants (117 to apply intervention one, 118 to apply intervention two, and 116 to apply the comparator) Age: vehicle group, n = 72 aged 2 to 6 years, and n = 44 aged 7 to 15 years; tacrolimus 0.03% group, n = 74 aged 2 to 6 years, n = 43 aged 7 to 15 years; tacrolimus 0.1% group, n = 69 aged 2 to 6, and n = 49 aged 7 to 15 years Sex: vehicle group male n = 53, female n = 63; tacrolimus 0.03% group male n = 55, female n = 62; tacrolimus 0.1% group male n = 57, female n = 61 Ethnicity: vehicle group, white n = 78, African‐American n = 28, Asian n = 8, other n = 2; tacrolimus 0.03% group, white n = 76, n = 32 African‐American n = 32, Asian n = 7, other n = 0; tacrolimus 0.1% group, white n = 75, African‐American n = 34, Asian n = 6, other n = 3 Duration of eczema: not reported Severity of eczema: vehicle: n = 47 moderate severity, n = 69 severe; tacrolimus 0.03%: n = 45 moderate severity, 72 n = severe; tacrolimus 0.1%: n = 43 moderate severity, n = 75 severe Body site: vehicle group 100% facial/neck involvement; tacrolimus 0.03% group 100% facial/neck involvement; tacrolimus 0.1% group 93% facial/neck involvement Number of withdrawals: vehicle group n = 65 (56.0%), due to application site AE n = 9, other adverse event n = 0, lack of efficacy n = 46, administrative reasons n = 10; tacrolimus 0.03% group n = 23 (19.7%), due to application site AE n = 2, other AE n = 4, lack of efficacy n = 4, administrative reasons n = 13; tacrolimus 0.1% group n = 17 (14.4%), due to application site AE n = 2, other adverse event n = 1, lack of efficacy n = 5, administrative reasons n = 9 Notes: none |
| Interventions | Run‐in details: no antimicrobial within 7 days of the trial. Intervention one: tacrolimus 0.03% ointment used twice daily for 12 weeks · Concurrent treatment: not reported · Other key information: none Intervention two: tacrolimus 0.1% ointment used twice daily for 12 weeks* · Concurrent treatment: not reported · Other key information: none Comparator: vehicle 0% ointment used twice daily for 12 weeks* used twice daily for 12 weeks · Concurrent treatment: · Other key information: Concurrent treatments received alongside both intervention and comparator: all were instructed to apply a thin layer to areas of active disease. Notes: *to quote: "Treatment continued until approximately 1 week after complete clearance of atopic dermatitis or up to 12 weeks." |
| Outcomes | · EASI · Clinical signs of atopic dermatitis (erythema, oedema, excoriation, lichenification, oozing, and scaling) rated from 0 = absent to 3 = severe for each of four body regions (head/neck, trunk, upper limbs, and lower limbs). The average values for non‐head/neck areas were presented. · IGA · Patient assessment of pruritus · DLQI · AE |
| Notes | Funding source: Fujisawa Healthcare, Inc. Declarations of interest: to quote: "The authors are on Fujisawa Healthcare’s speakers’ bureau, have received grant/research support, and/or have been consultants for Fujisawa Healthcare, or are employees of Fujisawa Healthcare." Original language of publication: English Other: none |
Hanifin 2010.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind study followed by an open‐label extension Trial registration number: not reported Country: assumed US as authors were based there Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 36 months Inclusion criteria: aged 3 to 18 months and within 3 months of AD diagnosis Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 1087 participants Age: 3 to 18 months Sex: 62% male Ethnicity: 69% Caucasian Duration of eczema: within 3 months of AD diagnosis Severity of eczema: 92% had mild or moderate AD Body site: number of withdrawals: not reported Notes: less than half (469/1087) of those who entered the trial entered the 36‐month open‐label extension. |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream used twice daily for up to 1095 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle used twice daily for up to 1095 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: to quote: "We used a stepwise treatment approach from emollients for xerosis (Step 1) to study drug for inflammation (Pim vs Control [C] BID, Step 2) to rescue topical corticosteroid (TCS, fluticasone 0.05% cream, Step 3a) to high‐potency TCS and/or oral medication (Steps 3b/4) after 3 days of non‐response at each step". Notes: none |
| Outcomes | · AE, SAE (not reported if local AE) · System organ class affected · Incidence of asthma, food allergy, allergic rhinitis, allergic conjunctivitis |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: the study outcomes were not all fully reported, those that were, are not relevant to this review. Unclear comparator (abstract only) |
Hanifin 2016.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: NCT02068352 Country: USA, Australia, Poland Outpatient or hospital: presumed outpatient Date trial conducted: June 2014 to February 2015 Duration of trial participation: 8 weeks Inclusion criteria: · Aged between 10 and 70 years · AD according to Hanifin and Rajka, and Rajka and Langeland criteria · IGA score of 2 (mild) or 3 (moderate) · At least a 3‐year history of the disease · BSA affected by AD at baseline could not be more than 40%, but had to be at least 5. · The face, neck, and head were not treated under the protocol. · Patients were required to have had a previous positive but inadequate response to 1 or more standard therapies for AD or were currently unable to use a previously successful treatment. Exclusion criteria: · Having received systemic therapy or phototherapy within 28 days · Use of TCS or calcineurin inhibitors within 7 days of study entry Additional design details: none |
| Participants | Total number randomised: 121 participants (41 to apply intervention one, 43 to apply intervention two, and 37 to apply the comparator) Age: 10 to 70 years, mean 34.3 (SD 15.8) Sex: male 40.4%, female 59.5% Ethnicity: white 67.7%, black/African‐American 24.8%, Asian 3.3%, American‐Indian/Alaskan native 0.8%, Pacific Islander 2.5%, unknown 0.8% Duration of eczema: at least 3‐year history Severity of eczema: mild‐to‐moderate Body site: whole body excluding head, neck, face Number of withdrawals: 27, vehicle group n = 9; 0.3% OPA group n = 10; 1% OPA group n = 8 Notes: none |
| Interventions | Run‐in details: none Intervention one: OPA‐15406 0.3% ointment used twice daily for 8 weeks · Concurrent treatment: not reported · Other key information: none Intervention two: OPA‐15406 1% ointment used twice daily for 8 weeks · Concurrent treatment: not reported · Other key information: none Comparator: vehicle 0% ointment used twice daily for 8 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Percentage of participants with success in the IGA score · Change from baseline in overall IGA score · Percentage of participants with AE · Change from baseline in EASI score · Change from baseline in VAS score for pruritus |
| Notes | Funding source: Otsuka Pharmaceuticals and Commercialization, Inc. Declarations of interest: not declared Original language of publication: English Other: none |
Harder 1983.
| Study characteristics | |
| Methods | Trial design: randomised, single‐blind, single‐centre study Trial registration number: not reported Country: Switzerland Outpatient or hospital: Dermatologic Polyclinic of Kantonsspitals Basel Date trial conducted: not reported Duration of trial participation: 3 weeks Inclusion criteria: patients with eczema (acute, subacute and chronic) Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: to quote: "98 patients were included in the study" participants (38 (of 72 "evaluable" participants) to apply the intervention) Age: median female 31.4 years, male 30.5 years Sex: male n = 35, female n = 63 Ethnicity, duration of eczema, severity of eczema: not reported Body site:·fingers, hands, forearms and elbows n = 60, facial n = 8; the remaining cases of eczema were located in the area of the trunk and/or lower limb. Number of withdrawals: of 98 participants included in the trial, 26 were excluded as they: did not come for 2nd consultation n = 7, did not provide important information n = 8, did not use the drug given and the prescribed mode of administration n = 4, or used additional medications potentially n = 7. Notes: none |
| Interventions | Run‐in details: not reported Intervention: diflorasone diacetate 0.05% ointment used once daily for 3 weeks · Concurrent treatment: not reported · Other key information: none Comparator: betamethasone valerate 0.1% ointment used three times daily for 3 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · AE · Overall improvement (−1 = deterioration, 0 = no change, 1 = 1 to 25% improved, 2 = 26 to 50% improved, 3 = 51 to 75% improved, 4 = 76 to 100% improved) · Severity of signs/symptoms (erythema, oedema, lichenification, induration, scaling, excoriation, pruritus and ulceration each scored from 1 = no change, to 4 = serious changes) |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: German Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022 |
Haribhakti 1982.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: India Outpatient or hospital: the author is affiliated to a hospital in Ahmedabad. Date trial conducted: not reported Duration of trial participation: up to 3 weeks Inclusion criteria: children with bilateral eczema Exclusion criteria: · Children with tuberculosis, viral and fungal skin disease · Children requiring treatment with antihistamines, systemic drugs or other drugs that might interfere with the trial medications Additional design details: the methods section stated to quote: "children with infected lesions were included in the study only after treatment with appropriate antibiotics." |
| Participants | Total number randomised: 21 children completed the study; however, the baseline number of males and females suggests 25 were randomised. 50 sides treated (25 to apply the intervention and to apply the comparator) Age: average 2.96 years (standard error (SE) 0.66) Sex: male n = 18, female n = 7 Ethnicity: not reported Duration of eczema: average duration 6.7 months Severity of eczema: average BSA involved 12.5% (SE 1.43). Average pretreatment signs scores were 10.98 (SE 0.694) in the moderate TCS group, and 10.60 (SE 0.648) in the mild TCS group. Body site: To quote: "sites such as face and flexures" Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: clobetasone butyrate cream (Eumovate) used twice daily for 2 weeks; three weeks if necessary · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone cream unspecified used twice daily for 2 weeks; three weeks if necessary · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: without occlusion. The outpatient card also advised on the quantity to be applied. Notes: Standard TCS strengths have been assumed to enable potency classification. |
| Outcomes | · Participant preference · Investigator preference · Local changes suggestive of skin atrophy · Objective parameters (erythema, oedema, papules, vesicles, exudation, crusting, scaling, lichenification/hyperkeratosis, excoriation and others) graded from 0 = absent to 3 = severe · Subjective parameters (pruritus and pain) graded from 0 = absent to 3 = severe · Average absolute and percentage reduction in total scores (assumed to be the sum of the objective and subjective parameters) |
| Notes | Funding source: none reported, however the following is acknowledged, to quote: "I also thank M/s Glaxo Laboratories, Bombay for supplying drugs and for their help in conducting the trial." Declarations of interest: not declared Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Hebert 2006.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blinded, multicentre study Trial registration number: not reported Country: US Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 3 weeks Inclusion criteria: · 18 years or older and of any race · Clinical diagnosis of AD of at least 2 months duration · 2 bilateral symmetrical target lesions Exclusion criteria: · Superinfected eczema · Pregnancy · Psoriasis · Any confounding or topical or systematic medications within 28 days of entering the study or topical corticosteroids within one week of entering the study Additional design details: clinical study in which double‐active (tacrolimus ointment 0.1% on top of desoximetasone ointment 0.25%) and single‐active (tacrolimus ointment 0.1% on top of inert desoximetasone vehicle) |
| Participants | Total number randomised: 164 sides treated (82 to apply the intervention and 82 to apply the comparator) Age: mean 45.9 years (SD 18.0) range from 18 to 85 Sex: 40% male, 60% female Ethnicity: 24% black, 7% Asian, 6% Hispanic, 59% white, other 4% Duration of eczema, severity of eczema, body site: not reported Number of withdrawals: 5 because of noncompliance, protocol violation, or withdrawal due to an adverse event Notes: none |
| Interventions | Run‐in details: not reported Intervention: desoximetasone 0.25% ointment with tacrolimus 0.1% used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: tacrolimus 0.1% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Individual and summary symptoms scores · Safety · PGA · Pruritus |
| Notes | Funding source: Taro Pharmaceuticals USA, Inc. Declarations of interest: four authors are advisors and/or consultants and/or investigators and/or speakers for pharmaceutical companies; for three, this includes Taro Pharmaceuticals USA, Inc. Two other authors are employees and are shareholders of Taro Pharmaceuticals USA. Original language of publication: English Other: none |
Hebert 2007a.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind multicentre, parallel study Trial registration number: not reported Country: US Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 28 days Inclusion criteria: · Stable, mild‐to‐moderate AD according to the Hanifin and Rajka criteria · Severity of AD according to PGA of 2 or 3 · A minimum percent surface area involvement of at least 10% BSA Exclusion criteria: not reported Additional design details: this study (A) is presented alongside another study (B). To quote: "Two phase III studies of slightly different design were conducted. Both trials were randomized, blinded, vehicle‐controlled trials in which neither the subject nor the investigator knew the identity of the assigned test material. Assignment to treatment arms in these studies included a desonide hydrogel to vehicle ratio of 3 to 1 for the first study and a ratio of 2 to 1 for the second study". |
| Participants | Total number randomised: 381 participants (289 to apply the intervention and 92 to apply the vehicle) Age: between 3 months to 18 years of age Sex: inclusion criteria male or female, no further details Ethnicity: of any race Duration of eczema: to quote: "must have an itchy skin in the last 12 months" Severity of eczema: a minimum disease severity characterised by IGSS of mild‐to‐moderate and individual scores of at least mild for both erythema and induration Body site: not reported Number of withdrawals: 27 Notes: none |
| Interventions | Run‐in details: not reported Intervention: desonide 0.05% hydrogel used twice daily for 28 days Concurrent treatment: not reported Other key information: none Comparator: vehicle gel used twice daily for 28 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGSS (Investigators Global Severity Score) · Per cent change from baseline in severity scores for the signs and symptoms of AD · Pruritus severity, and per cent BSA affected by AD · Safety |
| Notes | Funding source: SkinMedica, Inc. Declarations of interest: corresponding author affiliated to SkinMedica Original language of publication: English Other: none |
Hebert 2007b.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind multicentre, parallel study Trial registration number: not reported Country: US Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 28 days Inclusion criteria: · A clinical diagnosis of stable, mild‐to‐moderate AD according to Hanifin and Rajka criteria · Severity of AD according to PGA score of 2 or 3 · A minimum percent surface area involvement of at least 10% BSA Exclusion criteria: not reported Additional design details: To quote: "Two phase III studies of slightly different design were conducted. Both trials were randomized, blinded, vehicle‐controlled trials in which neither the subject nor the investigator knew the identity of the assigned test material. Assignment to treatment arms in these studies included a desonide hydrogel to vehicle ratio of 3 to 1 for the first study and a ratio of 2 to 1 for the second study". |
| Participants | Total number randomised: 201 participants (136 to apply the intervention and 65 to apply the vehicle) Age: between 3 months to 18 years of age Sex: inclusion criteria male or female, no further details Ethnicity: to quote: "of any race" Duration of eczema: to quote: "must have an itchy skin in the last 12 months" Severity of eczema: a minimum disease severity characterised by IGSS of mild‐to‐moderate and individual scores of at least mild for both erythema and induration as defined below (efficacy assessment) Body site: not reported Number of withdrawals: 14 Notes: none |
| Interventions | Run‐in details: not reported Intervention: desonide 0.05% hydrogel (gel) used twice daily for 28 days Concurrent treatment: not reported Other key information: none Comparator: vehicle gel used twice daily for 28 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGSS · Per cent change from baseline in severity scores for the signs and symptoms of AD · Pruritus severity, and the per cent BSA affected by AD · Safety |
| Notes | Funding source: SkinMedica, Inc. Declarations of interest: corresponding author affiliated to commercial funder SkinMedica Original language of publication: English Other: none |
Hebert 2008a.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind study Trial registration number: not stated Country: USA Outpatient or hospital, date trial conducted: not reported Duration of trial participation: four weeks Inclusion criteria: · Age 3 months to 17 years · ISGA score of 2 or 3 out of 5 · Sum of scores for erythema, induration/population, and oozing/crusting (each 0‐4) of more than or equal to 4 · ≥ 5% BSA Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 581 participants Age: 3 months to 17 years Sex, ethnicity, duration of eczema: not reported Severity of eczema: eligibility included an ISGA score of 2 or 3 out of 5; baseline characteristics were not reported. Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: none Intervention: desonide 0.05% foam used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle foam used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Efficacy was evaluated using the ISGA score combined with induration/papulation and erythema scores · Treatment success was defined as an ISGA score of clear or almost clear (0 or 1), a score of 0 or 1 for both erythema and induration/papulation at week 4 (or end of treatment) and a minimum improvement in the ISGA score of 2 grades from baseline to week 4 (or end of treatment). · Local AE |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: data from this trial has been reported in pooled analyses with Hebert 2008b and Hebert 2008c, extracted once here to prevent duplication |
Hebert 2008b.
| Study characteristics | |
| Methods | Trial design: randomised, mulitcentre study Trial registration number: not reported Country: USA Outpatient or hospital: multicentre Date trial conducted: July 2004 to July 2005 (patients enroled) Duration of trial participation: four weeks Inclusion criteria: · Age 3 months to 17 years · AD according to Hanifin and Rajka criteria · ISGA score of 2 or 3 out of 5 (mild‐to‐moderate severity) · Sum of scores for erythema, induration/population, and oozing/crusting (each 0‐4) of more than or equal to 4 · ≥ 25% BSA · Normal cosyntropin stimulation test (serum cortisol > 18.0 g/dL) Exclusion criteria: · Any disease of the HPA axis (e.g. Addison’s disease, Cushing’s syndrome, pituitary tumour) · Known hypersensitivity to any component of study medication, cosyntropin, or natural adrenocorticotropic hormone · Use of any investigational therapy 4 weeks before the study · Systemic therapy or phototherapy for AD within 4 weeks of the study · Topical therapies for AD within 1 week of the study · Use of oestrogens, spironolactone or systemic retinoids within 8 weeks of, and during, the trial · Pregnancy or unwilling to use reliable contraception during the study Additional design details: none |
| Participants | Total number randomised: 81 participants Age: mean 6.7 years (SD 5.1) Sex: females n = 51, males n = 30 Ethnicity: Asian n = 5, African‐American n = 29, white n = 35, Hispanic n = 10, other n = 2 Duration of eczema: not reported Severity of eczema: 74% had moderate disease at baseline Body site: not reported Number of withdrawals: 5 participants did not complete the treatment period, due to lost to follow‐up n = 3, AE n = 1, and disease progression n = 1 Notes: none |
| Interventions | Run‐in details: not reported Intervention: desonide 0.05% foam used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle foam used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · HPA axis suppression · Local AE |
| Notes | Funding source: Stiefel Laboratories Inc. Declarations of interest: to quote: "Disclosure: The principal authors have served as clinical investigators on other studies sponsored by Stiefel Laboratories Inc." Original language of publication: English Other: local safety data is presented only in a pooled analysis with Hebert 2008a and Hebert 2008c, extracted once under Hebert 2008a |
Hebert 2008c.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind study Trial registration number: not reported Country: US Outpatient or hospital, date trial conducted: not reported Duration of trial participation: four weeks Inclusion criteria: · Age 3 months to 17 years · ISGA score of 2 or 3 out of 5 · Sum of scores for erythema, induration/population, and oozing/crusting (each 0‐4) of more than or equal to 4 · ≥ 5% BSA Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 106 participants Age: 3 months to 17 years (from inclusion criteria) Sex, ethnicity, duration of eczema: not reported Severity of eczema: eligibility included an ISGA score of 2 or 3 out of 5; baseline characteristics were not reported. Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: none Intervention: desonide 0.05% foam used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle foam used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Efficacy was evaluated using the ISGA score combined with induration/papulation and erythema scores. · Treatment success was defined as an ISGA score of clear or almost clear (0 or 1), a score of 0 or 1 for both erythema and induration/papulation and a minimum improvement in the ISGA score of 2 grades from baseline · Local AE |
| Notes | Funding source: Stiefel Laboratories Inc. Declarations of interest: to quote: "Disclosure: The principal authors have served as clinical investigators on other studies sponsored by Stiefel Laboratories Inc." Original language of publication: English Other: local safety data is presented only in a pooled analysis with Hebert 2008a and Hebert 2008b, extracted once under Hebert 2008a. |
Ho 2003.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blinded, multicentre, parallel study Trial registration number: not reported Country: Australia, Brazil, Canada, Germany, South Africa, and Spain Outpatient or hospital: 25 centres unspecified Date trial conducted: not reported Duration of trial participation: 6 weeks Inclusion criteria: · Aged 3 to 23 months · Diagnosis of AD · ≥ 5% of total BSA affected and with a baseline IGA of 2 (mild) or 3 (moderate) Exclusion criteria: · Immunocompromised, other concurrent or active skin disease or viral skin infections, or a known hypersensitivity to study drug · Received phototherapy or systemic treatments known to affect AD within the previous month, topical therapy within the previous week, or sedative antihistamines to treat pruritus within the previous week Additional design details: none |
| Participants | Total number randomised: 186 participants (123 to apply the intervention and 63 to apply the comparator) Age: intervention group mean 12.6 months (SD 6.25); comparator group: 12.7 months (SD 6.29) Sex: intervention group: male n = 68, comparator group male n = 34 Ethnicity: intervention group, white n = 65, black n = 16, Asian n = 3, other n = 39; comparator group, white n = 44, black n = 4, Asian n = 1, other n = 14 Duration of eczema: not reported Severity of eczema: based on IGA: intervention group, mild n = 40, moderate n = 83; comparator group, mild n = 21, moderate n = 42 Body site: not reported Number of withdrawals: intervention group n =14, due to unsatisfactory effect n = 8, others not stated; comparator group n = 27, due to unsatisfactory effect n = 26, other not stated Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream used twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 42 days. · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA: 6‐point scale, ranging from 0 (clear) to 5 (very severe disease) · EASI · Severity of pruritus and an assessment of disease control by the subject’s primary caregiver · AE, including monitoring of haematology, blood chemistry, urinalysis, physical examination and vital signs |
| Notes | Funding source: Novartis Pharmaceuticals Corporation Declarations of interest: not declared Original language of publication: English Other: none |
Hoeger 2009.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: NCT00130364 Country: Germany, South Korea and Slovakia Outpatient or hospital: not reported Date trial conducted: August 2005 to August 2006 Duration of trial participation: 6 weeks Inclusion criteria: · Aged 2 to 11 years, with mild‐to‐moderate facial AD (based on the facial IGA at baseline) · Dependent on, or intolerant of, TCS. Intolerance to TCS was defined by either an allergic reaction preventing use of TCS, presence of perioral dermatitis, rosacea, telangiectasia and ⁄or skin atrophy known to have resulted from use of TCS, or eyelid dermatitis associated with raised intraocular pressure ⁄history of glaucoma. Dependence was defined as an unacceptable level of recent use of TCS on the head and neck that, based on the investigator’s judgement and patient’s medical history, may have resulted in AEs. Exclusion criteria: · AD on > 30% of total body surface area · Concurrent skin disease in the study area, active skin infections, or other conditions that might interfere with study evaluations · Immunocompromised patients or patients with a history of malignant disease · Previous treatment with topical pimecrolimus or tacrolimus where there was either poor clinical response or hypersensitivity resulted · Within 4 weeks prior to the start of the trial, had received phototherapy or systemic therapy known or suspected to have an effect on AD, or had received investigational drugs within 8 weeks of the first application of the study drug or planned use of other investigational drugs during the study Additional design details: additional 6 weeks follow‐up, but open‐label |
| Participants | Total number randomised: 200 participants (99 to apply the intervention and 101 to apply the vehicle) Age: 2 to 11 years old. The median age was 5 years in each group. Sex: overall: males 50.5%, females 49.5%; pimecrolimus group: males 43.4%, females 56.6%; vehicle group: males 57.4%, females 42.6% Ethnicity: white: 67%, Asian: 32% Duration of eczema: not reported Severity of eczema: mild‐to‐moderate facial atopic dermatitis. Patients were well‐matched for baseline IGA severity. Body site: facial atopic dermatitis, dependent on or intolerant of topical corticosteroids Number of withdrawals: 6 patients discontinued in the pimecrolimus group (3 for unsatisfactory therapeutic effect, 1 protocol deviation and 2 withdrawal of consent); 25 in the vehicle group (2 for AE, 17 unsatisfactory therapeutic effect, 1 protocol deviation, 3 withdrawal of consent, 2 lost to follow‐up) Notes: none |
| Interventions | Run‐in details: none Intervention: pimecrolimus 1% cream used twice daily for 42 days Concurrent treatment: not reported Other key information: none Comparator: vehicle cream used twice daily for 42 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: emollients Notes: patients stopped using the treatment early if they achieved a facial IGA of 0 (clear). Treatment was to be resumed upon recurrence of first signs and symptoms of atopic dermatitis. Treatment was applied to face, head and neck at the same time each day, 12 hours apart. For treatment of the rest of the body, the study medication was to be applied twice daily, intermittently, on an ‘as‐needed’ basis, i.e. to each affected area of the skin (excluding face, head and neck) until clearance occurred, when treatment stopped. |
| Outcomes | · Treatment success at day 43, as assessed by the facial IGA score (0/1 = clear/almost clear) · Overall EASI, head and neck EASI at day 43 · Pruritus severity score at day 43 · Time to clearance of facial atopic dermatitis (facial IGA score of 0/1) over 6 weeks · Effect on eyelid dermatitis · Amount of study drug used (by weight) · Safety and tolerability after 6 weeks treatment |
| Notes | Funding source: supported by a grant from Novartis Pharma AG Declarations of interest: four authors received research funds and ⁄or fees from Novartis as speakers. Three authors are employees of Novartis. Original language of publication: English Other: none |
Hoetzenecker 2005.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: not reported Country: unspecified Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 3 weeks Inclusion criteria: mild‐to‐moderate AD Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 22 participants (8 each to apply intervention one and two, and 6 to apply the comparator) Age: 18 to 60 years, average 27 years Sex: female n = 17 Ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐moderate Body site: excluding face Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: before study, participants had to stop oral treatment with systemic corticosteroids or other immunosuppressive drugs for 2 weeks, treatment with UV radiation for 4 weeks, and topical application of corticosteroids or other immunosuppressive drugs for 2 weeks, and none of these treatments were allowed during the study. Intervention one: pimecrolimus 1% cream used twice daily for 3 weeks · Concurrent treatment: not reported · Other key information: none Intervention two: betamethasone valerate 0.1% cream used twice daily for 3 weeks · Concurrent treatment: not reported · Other key information: none Comparator: vehicle 0% cream used twice daily for 3 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | EASI |
| Notes | Funding source: Novartis Declarations of interest: one author was a Novartis employee. Original language of publication: English Other: none |
Hofman 2006.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: Australia, Poland, Germany, Iceland, Hungary Outpatient or hospital: 37 centres across Europe and Australia Date trial conducted: not reported Duration of trial participation: 7 months Inclusion criteria: children aged 2 to 11 years diagnosed with atopic dermatitis according to the Hanifin and Rajka criteria Exclusion criteria: not reported Additional design details: the study had a third arm of children who did not have AD. They formed a control group. The primary objective of the study was whether the application of a hydrocortisone regimen or 0.0 3% TAC‐O had any effect on the immune response after vaccination against meningococcal serogroup C disease. |
| Participants | Total number randomised: 250 participants (125 to apply the intervention and 125 to apply the comparator*) Age: intervention group, mean 6 years (SD 3.1) range 2 to 11; comparator group, mean 6.2 years (SD 3.1) range 2 to 11 Sex: intervention group, 43.3% male, 57.7% female; comparator group, 47.9% male, 52.1% female Ethnicity: intervention group, 0.9% black, 1.8% oriental, 95.5% white and 1.8% other; comparator group, 0% black, 1.7% oriental, 95.9% white, other 2.5% Duration of eczema: not reported Severity of eczema: intervention group, 57.7% moderate, 42.3% severe; comparator group, 63.6% moderate, 36.4% severe Body site: not reported Number of withdrawals: more patients in the intervention group discontinued the study prematurely n = 20 (18.0%) compared with those in the comparator group n = 13 (10.7%). Lack of efficacy was the main reason for discontinuation in the hydrocortisone arm n = 8 (7.2%). The main reasons for discontinuation in the tacrolimus group were lack of efficacy n = 3 (2.5%) and ineffectiveness of vaccination n = 3 (2.5%). Notes: *the number of participants was not clear. In the statistical analysis section, it was stated that each of the two treatment groups at randomisation had 125 participants. In the results section on safety, there were 124 participants in one intervention group and 133 in the other group. |
| Interventions | Run‐in details: not reported Intervention: hydrocortisone butyrate 0.1% ointment used twice daily for up to 213 days · Concurrent treatment: 1% hydrocortisone acetate to head and neck for first two weeks of treatment · Other key information: after the first two weeks, hydrocortisone acetate was applied to all affected areas until clearance. Comparator: tacrolimus 0.03% ointment used twice daily for three weeks and then once daily until clearance for up to 213 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · AE · Clinical improvement · Serum bactericidal antibody · Serogroup C‐specific IgG avidity |
| Notes | Funding source: Astellas Pharma Declarations of interest: no further conflicts declared Original language of publication: English Other: none |
Hung 2007.
| Study characteristics | |
| Methods | Trial design: randomised, single‐centre, parallel study Trial registration number: not reported Country: Taiwan Outpatient or hospital: outpatients Date trial conducted: February 2004 to February 2005 Duration of trial participation: 8 weeks Inclusion criteria: AD according to Hanifin and Rajka criteria; moderate‐to‐severe according to Rajka and Langeland criteria Exclusion criteria: · Systemic topical antibiotics or corticosteroids within 4 weeks of the study · Clinical signs of secondary infection Additional design details: not reported |
| Participants | Total number randomised: 60 participants (30 to apply the intervention and 30 to apply the comparator) Age: mean 15.6 years, range from 9 months to 33 years Sex: male n = 26, female n = 34 Ethnicity, duration of eczema: not reported Severity of eczema: SCORAD mean 55.3 (standard error of mean (SEM) 1.9) Body site, number of withdrawals: not reported Notes: not reported |
| Interventions | Run‐in details: not reported Intervention: fluticasone propionate 0.05% cream used twice daily for 8 weeks · Concurrent treatment: not reported · Other key information: none Comparator: tacrolimus 0.03% ointment used twice daily for 8 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator. Participants were instructed to apply the treatment regimen to all affected areas, without occlusion. In those who used fusidic acid cream, it was applied first to all affected areas followed by fluticasone propionate or tacrolimus 20 minutes later. Notes: none |
| Outcomes | · SCORAD · BSA |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Innocenti 1977.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: Italy, assumed by the authors' affiliation Outpatient or hospital: Sondrio Civil Hospital Dermatology Department, assumed by the authors' affiliation Date trial conducted: not reported Duration of trial participation: 1 week Inclusion criteria: patients with bilateral AD where the lesions were distributed symmetrically Exclusion criteria: patients without complete clinical assessment Additional design details: AD participants were a subgroup in the study. |
| Participants | Total number randomised: 3 participants with AD sides treated (3 sides to apply the intervention and 3 sides to apply the comparator) Age, sex: not reported separately for the AD participants Ethnicity, duration of eczema: not reported Severity of eczema: 2 participants had moderate‐severity disease and 1 had high‐severity Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: diflucortolone valerate 0.1% emulsion used twice daily for 7 days · Concurrent treatment: not reported · Other key information: potency inferred based on alternative formulations Comparator: fluocortolone/fluocortolone caproate 0.25% emulsion used twice daily for 7 days · Concurrent treatment: not reported · Other key information: potency inferred based on alternative formulations Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · AE · IGA (treatment effect judged as null, small, good, or excellent) |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: Italian Other: This study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Iraji 2015.
| Study characteristics | |
| Methods | Trial design: randomised, single‐blinded, parallel study Trial registration number: IRCT2014092519292N1 Country: Iran Outpatient or hospital: Skin Diseases and Leishmaniasis Research Center, Isfahan University of Medical Sciences Date trial conducted: from November 2013 Duration of trial participation: 8 weeks Inclusion criteria: · 2 to 18 years with moderate or severe AD · Unresponsive to common treatments after at least 6 months · ≥ 3 items out of each major and minor criteria as described in the protocol Exclusion criteria: · Any laboratory abnormality · Receiving treatment interacting with AZT (one of the intervention drugs is AZT) within 1 month of the trial · History of liver or BM disorders Additional design details: none |
| Participants | Total number randomised: 70 participants (35 to apply the intervention and 35 to apply the comparator) Age: intervention group mean 9.05 years (SD 4.98); comparator group mean 8.68 years (SD 4.65), range from 2 to 18 years Sex: intervention group male n = 17 (48.6%); comparator group n = 18 (51.4%) male Ethnicity: not reported Duration of eczema: at least 6 months Severity of eczema: moderate or severe Body site: non‐facial eczema Number of withdrawals: 3 individuals in the intervention group and two patients in the comparator group were lost to follow‑up due to unknown reasons and were replaced by new participants. Notes: none |
| Interventions | Run‐in details: not reported Intervention: azathioprine 4% and betamethasone valerate 0.05% cream used twice daily for 64 days · Concurrent treatment: not reported · Other key information: none Comparator: betamethasone valerate 0.05% cream used twice daily for 64 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · SCORAD · Local AE |
| Notes | Funding source: Isfahan University of Medical Sciences Declarations of interest: not declared Original language of publication: English Other: none |
JapicCTI‐163372.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: JapicCTI‐163372 Country: Japan Outpatient or hospital: 14 outpatients Date trial conducted: from September 2016 Duration of trial participation: not reported Inclusion criteria: · AD according to Hanifin and Rajka criteria · Male or female · Hospitalisation status: outpatient Age: 15 to 70 years AD for at least 3 years AD affecting ≥ 5% to ≤ 40% of BSA; IGA score of 2 (mild) or 3 (moderate) Exclusion criteria: · AD or contact dermatitis flare‐up · Active viral skin infection · Current or history of malignancy Additional design details: none |
| Participants | Total number randomised: 200 participants (67 to apply intervention one, 67 intervention two and 66 to apply the comparator) Age: mean 30.9 years (SD 9.9), 15 to 18 years n = 3, between 18 and 65 years n = 197 Sex: female n = 70; male n = 130 Ethnicity: Japanese Duration of eczema: at least 3 years Severity of eczema, body site: not reported Number of withdrawals: 55 withdrew: 0.3% group, for AE n =15, clinical decision n = 2, protocol violation n = 1, withdrawal by subject n = 0; 1% group, for AE n = 7, clinical decision n = 2, protocol violation n = 0, withdrawal by subject n = 1; placebo group, for AE n = 15, doctors decision n = 0, protocol decision n = 0, withdrawal by subject n = 4 Notes: none |
| Interventions | Run‐in details: not reported Intervention one: difamilast 0.3% ointment used twice daily for 56 days · Concurrent treatment: not reported · Other key information: none Intervention two: difamilast 1% ointment used twice daily for 56 days · Concurrent treatment: not reported · Other key information: none Comparator: placebo/vehicle used twice daily for 56 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · POEM and affected BSA · IGA · EASI · VAS for pruritus (only for patients aged 7 to 14 years) · Time to response in IGA and VRS · Plasma concentrations of OPA‐15406 and trough concentrations of OPA‐15406 · AE, physical examination, vital signs (including body weight), clinical laboratory values, and 12‐lead ECG |
| Notes | Funding source: Otsuka Pharmaceutical Co., Ltd Declarations of interest: not declared Original language of publication: Japanese Other: none |
Jensen 2009.
| Study characteristics | |
| Methods | Trial design: randomised, within‐participant study Trial registration number: EudraCT‐Nr. 2004‐004824‐11 Country, outpatient or hospital, date trial conducted: not reported Duration of trial participation: 3 weeks Inclusion criteria: · Mild‐to‐moderate AD according to Hanifin and Rajka criteria · Target lesion score of 3 to 8 (on a scale of 0‐12) for both right and left target lesions · Symmetric AD lesions (not differing > 1 point between the right and left sides) affecting the upper limbs by at least 10% Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 30 sides treated (15 to apply the intervention and 15 to apply the comparator) Age, sex, ethnicity, duration of eczema, severity of eczema: not reported Body site: upper limb Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: betamethasone valerate 0.1% cream used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: emollients for the treatment of AD and other non‐pharmaceutical interventions were allowed as normal practice only on non‐target lesions and monitored by the investigator. Notes: none |
| Outcomes | Partial EASI, where only the upper limbs were evaluated, was used to assess disease severity. Each symptom (erythema, infiltration/induration, excoriation, and lichenification) was given a score ranging from 0 (none) to 3 (severe). |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Jensen 2013.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: EudraCT‐No. 2007‐003106‐99 Country, outpatient or hospital, date trial conducted: not reported Duration of trial participation: 3 weeks Inclusion criteria: · Mild‐to‐moderate AD according to Hanifin and Rajka criteria · Target lesion score of 3 to 8 (on a scale of 0‐12) for both right and left target lesions · Symmetric AD lesions (not differing > 1 point between the right and left sides) affecting the upper limbs by at least 10% Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 30 sides treated (15 to apply the intervention and 15 to apply the comparator) Age: 21 to 45 years Sex: female n = 7, male n = 9 Ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐moderate Body site: upper limb Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: triamcinolone acetonide 0.1% cream used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI · Pruritus |
| Notes | Funding source: not reported Declarations of interest: two authors had been supported by grants from a pharmaceutical company, Novartis, and one was a consultant. One was employed by Novartis. Novartis manufactures primecrolimus. Original language of publication: English Other: none |
Jirakova 2015.
| Study characteristics | |
| Methods | Trial design: randomised, single‐centre, parallel study Trial registration number: not reported Country: Czech Republic Outpatient or hospital: Department of Dermatovenerology, Charles University and Hospital Bulovka in Prague; no details whether participants were outpatients or inpatients Date trial conducted: not reported Duration of trial participation: 3 months Inclusion criteria: · Aged 2 to 65 years · Diagnosis of AD with trunk or limb lesions Exclusion criteria: · Systemic treatment of AD (except antihistamines) during the study period · Non‐compliance · Another skin disease or treatment with other topical medications on the examined lesions · History of allergy to the topical treatments used · Clinical signs of bacterial, viral or parasitic skin infection Additional design details: none |
| Participants | Total number randomised: 45 participants Age: mean 21.1 years (SD 13.6), range from 2 to 53 years Sex: male n = 15, female n = 30 Ethnicity, duration of eczema, severity of eczema: not reported Body site: upper limb 90.9% or trunk 9.1% Number of withdrawals: one participant was excluded from the study during the second control visit due to deterioration of AD leading to escalation to cyclosporine. Notes: not reported |
| Interventions | Run‐in details: not reported Intervention: methylprednisolone aceponate cream used once daily for 3 months Concurrent treatment: not reported Other key information: potency assumes standard concentration. Comparator: tacrolimus ointment used twice daily for 3 months Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Study set out to measure relevant outcomes, but did not report any outcome data at the time of the most recent search. |
| Notes | Funding source: supported by the grant from the Czech Ministry of Health IGA NT 13465‐4 Declarations of interest: not reported Original language of publication: English Other: no relevant outcomes |
Jorizzo 1995.
| Study characteristics | |
| Methods | Trial design: randomised, investigator‐blinded, multicentre, parallel study Trial registration number: not reported Country: USA Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 5 weeks for the majority of participants; extended to 6 months for a subgroup of 36/113 Inclusion criteria: · Mild‐to‐moderate AD · Aged 12 years or less Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 113 participants (57 to apply the intervention and 56 to apply the comparator) Age: overall mean 4.8 years, range 10 months to 12 years; intervention group, 47.4%, range 3 years or less, 21.1% 4 to 6 years, 15.8% 7 to 9 years, 15.8% 10 to 12 years; comparator group, 39.3% 3 years, 30.4% 4 to 6 years, 19.6% 7 to 9 years, 10.7% 10 to 12 years Sex: female n = 62, male n = 51 Ethnicity: not reported Duration of eczema: not reported Severity of eczema: summary score* of specific lesions at baseline (read using WebPlotDigitizer): intervention group mean = 8.23; comparator group mean = 8.40 Body site: not reported Number of withdrawals: not clear, 2 participants in the intervention group had no follow‐up data; 90 participants completed randomised phase. Notes: *sum of scores of the following signs: erythema, lichenification, excoriations, oozing and crusting, induration and papules assessed by the physician, and pruritus assessed by participants, guardians or parents. Each was ranked on a scale of 0 to 3, from 0 = none to 3 = severe. To quote the authors: "A baseline comparison of demographics and disease severity revealed no significant differences between treatment groups at the start of the study". |
| Interventions | Run‐in details: no interfering topical medication 14 days before study start or systemic antihistamines 7 days before study start. No systemic corticosteroids 30 days before study start, to quote: "However, the physician could enter a patient into the study before the end of the washout period, if the patient had worsening pruritus that required treatment". Intervention: desonide 0.05% ointment used twice daily for 35 days for the majority; extended to 6 months for a subgroup Concurrent treatment: not reported Other key information: participants used around 4 grams of ointment per day. Comparator: hydrocortisone 1% ointment used twice daily for 35 days for the majority; extended to 6 months for a subgroup Concurrent treatment: not reported Other key information: participants used around 4 grams of ointment per day. Concurrent treatments received alongside both intervention and comparator: non‐medicated soap (Cetaphil) Notes: to quote: "Compliance was monitored by examination of the returned tubes for approximate use, and all tubes were weighed on return to the sponsor. In addition, parents were provided a diary to record any missed doses." |
| Outcomes | · Evaluation of specific lesions by the physician. Erythema, lichenification, excoriations, oozing and crusting, induration, and papules ranked on a scale of 0 to 3. from 0 = none to 3 = severe · Pruritus ranked by participants, guardians or parents on a scale of 0 to 3, from 0 = none to 3 = severe · IGA of improvement of specific lesions (5‐point scale: clear/100% clearance apart from residual discolouration, marked improvement/50%‐74% clearance, slight improvement/< 50% clearance, no change, and exacerbation) · Stinging and burning ranked from 0 = none to 3 = severe · Signs of atrophy (under 8x magnifying lamp) ranked from 0 = none to 3 = severe |
| Notes | Funding source: not reported Declarations of interest: none declared; however three authors were affiliated to Galderma. Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Ju 2013.
| Study characteristics | |
| Methods | Trial design: randomised, single‐blind, multicentre, parallel study Trial registration number: not reported Country: China Outpatient or hospital: four hospitals; unclear if in or outpatients Date trial conducted: June to November 2011 Duration of trial participation: not reported Inclusion criteria: · Male or female with chronic eczema, aged between 18 and 65 years · Skin lesions with a total surface area of not more than 10% · Total score of severity of illness > 12 points at first clinic visit Exclusion criteria: · Another dermatological condition that could interfere with clinical evaluation including bacterial, viral or fungal infection · Skin rash on face and skin folds · Known allergy to any ingredients or structural analogues of tested products · Serious heart, liver, kidney dysfunction or immune dysfunction; neuropsychiatric disorders or severe endocrine diseases · Any systemic treatment, including corticosteroids or immunomodulators therapy for eczema within 4 weeks prior to screening or antihistamine therapy within 2 weeks, as well as topical glucocorticoid steroids or nonsteroidal anti‐inflammatory drugs therapy within 2 weeks · Pregnant or the possibility of pregnancy in women and lactating women Additional design details: not reported |
| Participants | Total number randomised: 250 participants Age: intervention group 45.75 years (SD 12.84); comparator group 44.58 years (SD 13.07) Sex: F/M: intervention group 63/69; comparator group 39/51 Ethnicity: not reported Duration of eczema: intervention group 59.37 months (SD 86.26); comparator group 64.54 months (SD 94.98) Severity of eczema, body site: not reported Number of withdrawals: 28 Notes: not reported |
| Interventions | Run‐in details: not reported Intervention: betamethasone dipropionate 0.05% ointment used once daily for 28 days · Concurrent treatment: calcipotriol · Other key information: not reported Comparator: halometasone 0.05% cream used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: triclosan Notes: none |
| Outcomes | · Comparison of signs and symptoms · Clinical analysis of overall efficacy · Total sign score · AE |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: not reported |
Kang 1998.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind study Trial registration number, country, outpatient or hospital, date trial conducted: not reported Duration of trial participation: up to 3 weeks Inclusion criteria: adults with moderate‐to‐severe eczema (> 75% BSA) Exclusion criteria: not reported Additional design details: the study was stated to have sequential groups; it is unclear if this meant a cross‐over design, or a study where the number of participants was not fixed in advance. |
| Participants | Total number randomised: 26 participants Age: adults were eligible. Sex, ethnicity, duration of eczema: not reported Severity of eczema: people with moderate‐to‐severe eczema were eligible. Body site: not reported Number of withdrawals: to quote: "Five patients (84%) in the vehicle group discontinued the study due to lack of efficacy." No other withdrawals reported Notes: none |
| Interventions | Run‐in details: not reported Intervention one: tacrolimus 0.3% used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Intervention two: tacrolimus 0.1% used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Intervention three: tacrolimus 0.03% used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle 0% used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Number with marked or excellent improvement at 3 weeks · Reduction in affected BSA · Application site reactions · Laboratory profile, including blood concentration of tacrolimus |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Kaplan 1978.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind study Trial registration number: not reported Country: US Outpatient or hospital: outpatients Date trial conducted: not reported Duration of trial participation: 3 weeks Inclusion criteria: typical manifestations of chronic AD and had not used topical medication for 2 weeks Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 60 participants (30 to apply the intervention and 30 to apply the comparator) Age: intervention group mean 21.2 years; comparator group mean age 19.7 years Sex: intervention group, male 31%; comparator group, male 27% Ethnicity: intervention group Duration of eczema: not reported Severity of eczema: moderate as judged by global impression Body site: not reported Number of withdrawals: 2 were withdrawn, both in the hydrocortisone group; one for immediate burning with subsequent drying of the skin and one was lost to follow‐up Notes: none |
| Interventions | Run‐in details: not reported Intervention: hydrocortisone 0.50% ointment used for 21 days Concurrent treatment: not reported Other key information: none Comparator: betamethasone valerate cream used for 21 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Pruritus, erythema, scaling, lichenification, oozing, excoriation, and overall global impression on a six‐point rating scale (0, none; 1, mild; 3, moderate; 5, severe) |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Kapp 2002.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: Belgium, Canada, France, Germany, New Zealand, South Africa, Spain, UK Outpatient or hospital: unclear Date trial conducted: not reported Duration of trial participation: 12 months Inclusion criteria: · AD according to Seymour criteria · AD affecting ≥ 5% of total BSA · IGA score of ≥ 2 Exclusion criteria: · Phototherapy or systemic therapy known or suspected to affect AD ≤ 1 month before the first application of study medication · Topical therapy known or suspected to affect AD ≤ 7 days before the first application of study medication · Systemic antibiotics ≤ 2 weeks before the first application of study medication · Immunocompromised or had a history of malignant disease; had active skin infections; had other infections that required treatment with prohibited medications (i.e. generally medication that could affect a patient’s AD); and had other skin conditions that could affect the evaluation of study treatment. Additional design details: none |
| Participants | Total number randomised: 251 participants (204 to apply the intervention and 47 to apply the comparator) Age: intervention group, mean 12.2 months (range 3 to 23 months); vehicle group, mean 11.8 months (range 2 to 23 months) Sex: intervention group, male 66.7%, female 33.3%; vehicle group, male 60.9%, female 39.1% Ethnicity: white Duration of eczema: not reported Severity of eczema: to quote: "The majority of patients in each group had moderate AD at baseline." EASI score at baseline: pimecrolimus group: mean 12.3 (range 1.2 to 58.0); vehicle group: mean 12.6 (range 1.6 to 45.5) Body site: not reported Number of withdrawals: intervention group: n = 50 did not complete the study (24.5%), of which n = 21 discontinued due to intervention being ineffective; n = 10 lost to follow‐up, n = 19 other reasons for discontinuing; vehicle group, n = 19 did not complete the study (40.4%), of which n = 1 did not receive allocated therapy, n = 15 discontinued due to intervention being ineffective; n = 1 lost to follow‐up, n = 2 other reasons for discontinuing Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream used twice daily for up to 365 days (applied until complete clearance of signs and symptoms) Concurrent treatment: not reported Other key information: none Comparator: vehicle cream used twice daily for up to 365 days (applied until complete clearance of signs and symptoms) Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: emollients used for dry skin. Moderately potent topical corticosteroids allowed for flares not controlled by study medication and were used until clearance or maximum treatment duration allowed by country label. The TCS used were: 0.02% difluprednate cream, 0.1% hydrocortisone butyrate cream, 0.05% clobetasone butyrate cream, 0.02% triamcinolone acetonide cream, and 0.2% hydrocortisone valerate cream Notes: none |
| Outcomes | · Incidence of flares (IGA score was assessed as 4 or 5) of AD at 6 months · IGA score · EASI · Caregivers’ assessments of pruritus and the level of overall disease control. · AE (physical examination and standard haematology, blood chemistry, and urinalysis tests) |
| Notes | Funding source: Novartis Pharma AG, Basel, Switzerland Declarations of interest: not declared Original language of publication: English Other: none |
Kaufmann 2004.
| Study characteristics | |
| Methods | Trial design: randomised, multicentre, parallel study Trial registration number: not reported Country: Germany Outpatient or hospital: 19 centres Date trial conducted: June 2001 to July 2002 Duration of trial participation: 4 weeks Inclusion criteria: · aged 3 to 23 months, with a diagnosis of AD · affecting 5% of the BSA ·a baseline IGA score of 2 (mild disease severity) or greater, based on the degree of erythema and infiltration/population Exclusion criteria: · Patients with insufficient washout periods i.e. one month or less for systemic corticosteroids and phototherapy, less than 2 weeks for antibiotics, and 1 week for topical steroids or other topical therapies that might have an effect on AD · immunocompromised patients, patients with other significant concomitant diseases or those with a known hypersensitivity to the study drug Additional design details: none |
| Participants | Total number randomised: 196 participants (130 to apply the intervention and 66 to apply the comparator) Mean age: intervention group 12.3 years (SD 6.1); comparator group 11.5 years (SD 5.8) Sex: intervention group 62.8% male, 37.2% female; comparator group 71.2% male and 28.8% female Ethnicity: intervention group: black 1.6%, Asian 5.4%, Caucasian 90.7%, other 2.3%; comparator group: black 0%, Asian 6.1%, Caucasian 92.4%, other 1.5% Duration of eczema: not reported Severity of eczema: intervention group, 9.3% mild, 58.1% moderate, 26.4% severe, 6.2% very severe; comparator group, 12.1% mild, 59.1% moderate, 25.8% severe, 3.0% very severe Body site: not reported Number of withdrawals: intervention group n = 13; comparator group n = 25. To quote: "the main reason for early discontinuation was lack of therapeutic effect in five patients (3.8%) in the pimecrolimus group and 23 patients (34.8%) in the vehicle group". Notes: none |
| Interventions | Run‐in details: patients with insufficient washout periods were excluded i.e. 1 month or less for systemic corticosteroids and phototherapy, less than 2 weeks for antibiotics, and 1 week for TCS or other topical therapies that might have an effect on AD Intervention: pimecrolimus 1% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator; emollients were only permitted on areas not treated with study medication. Notes: none |
| Outcomes | · Signs and symptoms using SCORAD · Treatment success using IGA and EASI · Quality of life using PQoL‐AD · Primary carers assessment of disease response, pruritus and sleep loss · Safety |
| Notes | Funding source: Novartis Pharma GmbH Declarations of interest: two authors reported grant research support from various pharmaceutical companies, including Novartis who developed pimecrolimus. Original language of publication: English Other: none |
Kaufmann 2006.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: Germany, Denmark, Canada, Finland and Norway Outpatient or hospital: 25 centres Date trial conducted: not reported Duration of trial participation: 7 days Inclusion criteria: not reported but assumed just adults were recruited Exclusion criteria: · Topical medication for pruritus relief or antibiotic therapy, within 7 days · Systemic medication known to have a sedative effect or to affect pruritus, within 2 weeks · Tacrolimus ointment 0.03% or pimecrolimus cream 1%, within 2 weeks; or phototherapy or any systemic therapy known or suspected to have an effect on AD, within 1 month · Concurrent skin disease · AD triggered by an unavoidable irritant/allergen · Active infection requiring prohibited treatment · Tinea corporis, intertriginous tinea, head lice, scabies, chicken pox or impetigo · History of poor response to topical tacrolimus ointment · Immunocompromised patients, patients with a history of malignant disease, and females who were pregnant, breastfeeding or not using a medically approved method of contraception Additional design details: none |
| Participants | Total number randomised: 198 participants (100 to apply the intervention and 98 to apply the comparator) Age: intervention group, mean 33.1 years (SD 12.08), range 18 to 69; vehicle group, mean 33.8 years (SD 12.70), range 18 to 81 Sex: intervention group, male n = 40 (40%), female n = 60 (60%); vehicle group, male n = 41 (41.8%), female n = 57 (58.2%) Ethnicity: intervention group, Caucasian n = 93 (93%); vehicle group, Caucasian n = 93 (94.9%) Duration of eczema: not reported Severity of eczema: intervention group: 26% mild disease, 74% moderate disease; vehicle group: 33.7% mild disease, 66.3% moderate disease (based on IGA score) Body site: not reported Number of withdrawals: 100/100 participants completed treatment in the pimecrolimus group; 87/98 completed treatment in the vehicle group. Notes: none |
| Interventions | Run‐in details: all patients were treated with bland emollient for 3 days Intervention: pimecrolimus 1% cream used twice daily for 7 days Concurrent treatment: not reported Other key information: none Comparator: vehicle cream used twice daily for 7 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator; concomitant use of bland emollients was permitted in both groups. Notes: none |
| Outcomes | · Time taken to achieve pruritus improvement of ≥ 1 point on a 4‐point pruritus severity scale, compared with baseline · Percentage of patients achieving pruritus improvement of ≥ 1 point compared with baseline (pruritus response), within 48 and/or 72 hours · Percentage of patients with a pruritus response within 4, 5, 6 and 7 days’ treatment · Percentage of patients with a pruritus severity score of 0 or 1 for treatment days 2, 3, 4, 5, 6 and 7, regardless of baseline scores · Percentage of patients with an IGA score improvement of ≥ 1 point at study completion (the end of the DB phase), compared with baseline |
| Notes | Funding source: sponsored by a research grant from Novartis Pharma AG, Basel, Switzerland Declarations of interest: not declared Original language of publication: English Other: none |
Kempers 2004.
| Study characteristics | |
| Methods | Trial design: randomised, multicentre, parallel study Trial registration number: not reported Country: USA Outpatient or hospital: 17 dermatology clinics and two allergy/immunology centres Date trial conducted: not reported Duration of trial participation: 6 weeks, with further uncontrolled 20 week extension Inclusion criteria: aged 2 to 17 years with moderate AD, i.e. with an IGA score of 3 Exclusion criteria: · Phototherapy within 1 month · Topical therapy within 7 days · Systemic corticosteroids within 1 month · Systemic antibiotics within 2 weeks Additional design details: none |
| Participants | Total number randomised: 141 participants (71 to apply the intervention and 70 to apply the comparator) Age: intervention group, 82% 2 to 12 years, 18% 13 to 17 years, mean age 8.1 years (SD 4.49); comparator group, 86% 2 to 12 years, 14% 13 to 17 years, mean age 7.8 years (SD 3.97) Sex: intervention group, male 44%, female 56%; comparator group, male 44%, female 56% Ethnicity: intervention group, black 18%, Asian 4%, white 63%, other 14%; comparator group, black 20%, Asian 6%, white 44%, other 6% Duration of eczema: intervention group, mean duration 79.5 months (SD 52.56); comparator group, 79 months (SD 40.15) Severity of eczema: intervention group, 99% moderate, 1% severe; comparator group, 99% moderate, 1% severe Body site: intervention group, head/neck 80%, trunk 78%, upper limbs 97%, lower limbs 94%; comparator group, head/neck 74%, trunk 80%, upper limbs 99%, lower limbs 99% Number of withdrawals: n = 13 Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream used twice daily until complete clearance of disease (IGA score of 0) or up to 42 days · Concurrent treatment: not reported · Other key information: none Comparator: tacrolimus 0.03% ointment used twice daily until complete clearance of disease (IGA score of 0) or up to 42 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: use of emollients (not containing ingredients such as alpha‐hydroxy or fruit acids, urea, or vitamins A, D, or E) supplied by the study centre was permitted for non‐lesional areas only Notes: none |
| Outcomes | · Patient assessment of local tolerability (ASRs) and formulation attributes · Incidence of local ASRs experienced with the two treatments · IGA · Patient evaluation of pruritus severity · Other signs and symptoms of AD (oozing/crusting, hyperpigmentation, hypopigmentation, dry skin/xerosis) · Percentage of total BSA affected by AD · AE and safety evaluations based on laboratory parameters (e.g. haematology, blood chemistry, and urinalysis) and vital signs |
| Notes | Funding source: Novartis Pharmaceuticals Corporation Declarations of interest: seven authors have received grant/research support from various pharmaceutical companies, two of which are consultants for Novartis. Three of the authors are employees of Novartis Pharmaceuticals Corporation. Novartis developed pimecrolimus. Original language of publication: English Other: none |
Kim 2012.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: not reported Country: Korea Outpatient or hospital: authors from Chungbuk National University Hospital Date trial conducted: not reported Duration of trial participation: up to 4 weeks Inclusion criteria: children with facial eczema in spite of using hydrocortisone lotion 1% or more potent TCS over 2 weeks Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 30 participants (15 to apply the intervention and 15 to apply the comparator) Age: aged 3 to 23 months Sex, ethnicity, duration of eczema, severity of eczema: not reported Body site: facial eczema Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: hydrocortisone lotion 1% or more potent TCS over 2 weeks Intervention: pimecrolimus 1% cream used twice daily for up to 14 days · Concurrent treatment: not reported · Other key information: none Comparator: desonide 0.05% cream used twice daily for up to 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: treatment used until clearance of facial AD or for a maximum of 4 weeks |
| Outcomes | · Overall EASI · Skin atrophy and telangiectasia were assessed by dermoscopy. |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: results suggest IGA was used (clear or almost clear) but there was nothing in the methods about IGA. |
Kimball 2008.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: US Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 2 weeks Inclusion criteria: · Aged 12 or over · Moderate‐to‐severe AD Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 377 participants (251 to apply the intervention and 126 to apply the comparator) Age: the percentage of adolescents (age 12 to 18 years) enroled was 27% Sex, ethnicity, duration of eczema: not reported Severity of eczema: most participants (87%) had a baseline ISGA score of 3 (moderate) Body site: not reported Number of withdrawals: 8% terminated the study early due to: AE: intervention group n = 1, comparator group n = 3; participant non‐compliance: intervention group n = 1, comparator group n = 1; disease progression: intervention group n = 1, comparator group n = 7; participant request: intervention group n = 2, comparator group n = 5; and other: intervention group n = 7, comparator group n = 4 Notes: none |
| Interventions | Run‐in details: not reported Intervention: clobetasol propionate 0.05% foam used twice daily for 14 days Concurrent treatment: not reported Other key information: none Comparator: placebo/vehicle used twice daily for 14 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Number clear or almost clear with improvement ≥ 2 · AE |
| Notes | Funding source: Stiefel Laboratories, Inc. Declarations of interest: two authors had served as clinical investigators on other studies sponsored by Stiefel Laboratories, Inc, and two authors were employees of Stiefel Laboratories, Inc. Original language of publication: English Other: none |
Kirkup 2003a.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: 16 centres in 5 countries in Europe and South Africa (assumed from acknowledgements) Outpatient or hospital: clinic attendance Date trial conducted: not reported Duration of trial participation: up to 18 weeks (1 to 2‐week run‐in phase, 2 to 4‐week acute phase, 3‐month maintenance phase, follow‐up 2 weeks post‐trial) Inclusion criteria: · Children aged 2 to 14 years · Experiencing a flare of moderate‐to‐severe AD with a total AD score of ≥ 6 Exclusion criteria: · Signs of skin infection or the dermatitis was severe enough to warrant hospital admission · Use of potent or very potent TCS or systemic treatment for skin disease during the previous three weeks · Oral or parenteral corticosteroids within the previous 12 months · History of adverse response to corticosteroids · Concomitant serious or unstable disease · Participation in another clinical trial within the previous four weeks Additional design details: participants were randomised once at the end of the run‐in period. The randomised part of these trials was also in 2 treatment phases, acute phase (twice daily TCS) and then maintenance (TCS applied at first sign of the flare). |
| Participants | Total number randomised 137 participants (70 to apply the intervention and 67 to apply the comparator) Age: in both groups, the mean age 8 years (SD 3) range 4 to 14. Missing for one participant in the intervention group Sex: intervention group: female n = 35, male n = 34 (missing for one participant); comparator group: female n = 38, male = 29 Ethnicity: not reported Duration of eczema: intervention group: median 5 years, range 0 to 14; comparator group: median 6 years, range 0 to 14 Severity of eczema: baseline mean total AD score (see outcomes for definition of 0‐21 scale): intervention group: 11.97 (n = 67), comparator group: 11.82 (n = 67) Body site: intervention group: hands and wrists 31%, front of arms 21%, back of legs 17%, feet and ankles 9%, neck and shoulders 9%, front of legs 7%, back of arms 3%, other 3%; comparator group: hands and wrists 28%, front of arms 24%, back of legs 18% Number of withdrawals: n = 31; intervention group n = 18, comparator group n = 13. Reasons (some participants provided > 1 reason) included: treatment failure (intervention group n = 2; comparator group n = 8), non‐compliance or personal reasons (intervention n = 7, comparator n = 2; early cure (intervention n = 3, comparator n = 1), AE (comparator n = 1), protocol violation (intervention n = 1, comparator n =1), not specified (intervention n = 7, comparator n =1) Notes: none |
| Interventions | Run‐in details: all participants initially applied hydrocortisone 1% cream twice daily to affected areas for 1 to 2 weeks. Intervention: fluticasone propionate 0.05% cream used twice daily for 14 to 28 days until the investigator judged their eczema was stabilised. Participants then entered a 3‐month maintenance phase and applied the trial treatment "as required" (up to twice daily) to affected areas at the first sign of a relapse. Concurrent treatment: not reported Other key information: none Comparator: hydrocortisone 1% cream used twice daily for 14 to 28 days until the investigator judged their eczema was stabilised. Participants then entered a 3‐month maintenance phase and applied the trial treatment "as required" (up to twice daily) to affected areas at the first sign of a relapse. Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: Investigators were permitted to issue tubes of hydrocortisone 1% cream for the face (labelled "face treatment") and emollients as required. Regular inhaled and intranasal corticosteroids were permitted. Notes: none |
| Outcomes | · Total AD score: (maximum 21) = number of body areas affected (out of a possible 12 body areas) + sum of scores for target area (maximum 9 as erythema, excoriation and lichenification were each graded 0‐3) · Physician‐reported "overall assessment of treatment success" ‐ grouped ‘much improved/improved’ or ‘same/worse/much worse’ at end of acute phase · Daytime itch recorded through participant diaries. Mean values over the last 7 days before the end of the acute treatment phase were used in the analysis. Data were grouped as improved (‘better than ever’, ‘better than usual’) or not improved (‘same’, ‘worse than ever’, worse than usual’) · Sleep disturbance through participant diaries · AE · Routine urinalysis, biochemical and haematological screening at enrolment and end of the maintenance phase · Weight of used and unused trial cream tubes · Intensity of rash recorded in participant diaries · Usage of antipruritic or sedative drugs during the maintenance phase · Median time to recurrence of AD. Recurrence defined as an increase of 1.0 in either the number of body areas affected or, in the sum of scores (for erythema, excoriation and lichenification) for the target area. Time to recurrence of AD was calculated from the visit dates for those participants who had a recurrence during the maintenance phase. |
| Notes | Funding source: Glaxo Wellcome R & D, UK (FLT411/412) Declarations of interest: not declared Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Kirkup 2003b.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: 14 centres in 7 countries in Europe and South Africa (assumed from acknowledgements) Outpatient or hospital: clinic attendance Date trial conducted: not reported Duration of trial participation: up to 18 weeks (1 to 2 week run‐in phase, 2 to 4 week acute phase, 3‐month maintenance phase, follow‐up 2 weeks post‐trial) Inclusion criteria: · Children aged 2 to 14 years · Participants experiencing a flare of moderate‐severe AD with a total AD score of ≥ 6 (see outcomes for definition) Exclusion criteria: · Signs of skin infection or the dermatitis was severe enough to warrant hospital admission · Use of potent or very potent TCS · Systemic treatment for skin disease during the previous 3 weeks · Oral or parenteral corticosteroids within the previous 12 months · History of adverse response to corticosteroids · Concomitant serious or unstable disease · Participation in another clinical trial within the previous 4 weeks Additional design details: participants were randomised once at the end of the run‐in period. The randomised part of these trials was also in 2 treatment phases, acute phase (twice daily TCS) and then maintenance (TCS applied at first sign of the flare). |
| Participants | Total number randomised: 128 participants (66 to apply the intervention group and 62 to apply the comparator group) Age: overall mean 8 years (SD 3) Sex: intervention group, female n = 26, male n = 40; comparator group, female n = 33, male = 29 Ethnicity: not reported Duration of eczema: intervention group, median 4 years, range 0 to 14 years; comparator group, median 6 years, range 0 to 14 years Severity of eczema: baseline mean total AD score (see outcomes for definition of 0‐21 scale): intervention group: 10.71 (n = 59); comparator group: 12.11 (n = 54) Body site: intervention group, hands and wrists 18%, front of arms 30%, back of legs 14%, feet and ankles 5%, neck and shoulders 12%, front of legs 6%, back of arms 3%, other 12%; comparator group, hands and wrists 21%, front of arms 19%, back of legs 23% Number of withdrawals: n = 18: intervention group n = 7, comparator group n =11 due to (some participants provided > 1 reason) treatment failure (comparator n = 5); non‐compliance or personal reasons (intervention n = 2, comparator n = 3), AE (intervention n = 1, comparator n = 3), protocol violation (comparator n = 2), not specified (intervention n = 4, comparator n = 4) Notes: none |
| Interventions | Run‐in details: all participants initially applied hydrocortisone 1% cream twice daily to affected areas for 1 to 2 weeks. Intervention: fluticasone propionate 0.05% cream used twice daily for 14 to 28 days until the investigator judged their eczema was stabilised. Participants then entered a 3‐month maintenance phase and applied the trial treatment "as required" (up to twice daily) to affected areas at the first sign of a relapse. Concurrent treatment: not reported Other key information: none Comparator: hydrocortisone butyrate 0.1% cream used twice daily for 14 to 28 days until the investigator judged their eczema was stabilised. Participants then entered a 3‐month maintenance phase and applied the trial treatment "as required" (up to twice daily) to affected areas at the first sign of a relapse. Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: investigators were permitted to issue tubes of hydrocortisone 1% cream for the face (labelled "face treatment") and emollients, as required. Regular inhaled and intranasal corticosteroids were permitted. Notes: none |
| Outcomes | · Total AD score: (maximum 21) = number of body areas affected (out of a possible 12 body areas) + sum of scores for target area (maximum 9 as erythema, excoriation and lichenification were each graded 0‐3) · Physician‐reported "overall assessment of treatment success" grouped ‘much improved/improved’ or ‘same/worse/much worse’ · Daytime itch recorded through participant diaries · Sleep disturbance through participant diaries · AE · Routine urinalysis, biochemical and haematological screening at enrolment and end of the maintenance phase · Weight of used and unused trial cream tubes at end of 3‐month maintenance phase · Intensity of rash recorded in participant diaries. For the diary card data, mean values over the last 7 days before the end of the acute treatment phase were used in the analysis. · Usage of antipruritic or sedative drugs during the maintenance phase · Median time to recurrence of AD. Recurrence defined as an increase of 1.0 in either the number of body areas affected or, in the sum of scores (for erythema, excoriation and lichenification) for the target area. Time to recurrence of AD was calculated from the visit dates for those participants who had a recurrence during the maintenance phase. |
| Notes | Funding source: Glaxo Wellcome R & D, UK (FLT411/412) Declarations of interest: not declared Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Koppes 2016.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, single‐centre, parallel study Trial registration number: NTR 4541 Country: the Netherlands Outpatient or hospital: outpatient clinic at VU University Medical Center, Amsterdam Date trial conducted: recruited between October and December 2014 Duration of trial participation: 6 weeks Inclusion criteria: · AD according to the Hanifin and Raijka criteria · Mild‐to‐moderate AD · Aged 18 to 70 years · At least 2 symmetrical (on either side of the body) skin sites with comparable AD severity Exclusion criteria: · Extensive UV exposure in the last 14 days and/or expected exposure during the study · Skin disease other than AD · Use of antibiotics prior (at least 4 weeks) to the study and/or expected use during the study · Use of systemic immunosuppressing drugs prior (at least 4 weeks) to the study and/or expected use during the study · Severe disorders within the last 6 months Additional design details: participants were randomised to two groups: Cer‐Mg and hydrocortisone (group I) or Cer‐Mg and emollient (group II). The participants then applied Cer‐Mg to one side of the body and hydrocortisone or emoliient to the other side. It isn't clear if this part was randomised; therefore, this study is being treated as a parallel group RCT only. |
| Participants | Total number randomised: 100 participants (50 to apply the intervention and 50 to apply the comparator) Age: intervention group median 28.5 years (range 23 to 51 years); comparator group median 25.0 years (range 21 to 35 years) Sex: intervention group, male n = 16, female n = 32; comparator group, male n = 19, female n = 28 Ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐moderate Body site: not reported Number of withdrawals: five patients were excluded during the study: in comparator group, n = 3 due to allergic reaction to emollient n = 2 and severe worsening of eczema symptoms n = 1; intervention group n = 2, both for non‐compliance with the study protocol Notes: none |
| Interventions | Run‐in details: participants could not use any AD medication for at least 2 weeks prior to participation. Intervention: hydrocortisone acetate 0.01% cream used twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Comparator: emollient cream used twice daily for 42 days · Concurrent treatment: not reported · Other key information: unguentum leniens (also called cold cream, consists of arachis oil (peanut oil), purified water, white beeswax and glyceryl monooleate) Concurrent treatments received alongside both intervention and comparator: not to apply any other product on other lesions, except the study creams Notes: ceramides and magnesium cream on a lesion on one side of the body and simultaneously with hydrocortisone or emollient on a lesion on the contralateral side. Relevant for this review: only hydrocortisone versus emollient comparison. Participants were instructed to apply one fingertip unit (approximately 1 g) and were instructed not to apply cream on the morning of measurements. |
| Outcomes | · SCORAD at 3 and 6 weeks from baseline. A modified local SCORAD was used ‐ the scoring parameters were performed on the investigated skin sites and the body surface area was set to 1%. · Biophysical parameters included transepidermal water loss, skin surface pH and erythema. · Natural moisturising factors in the stratum corneum |
| Notes | Funding source: Omega Pharma, Nazareth, Belgium. Omega Pharma provided study medication free of charge. To quote: "It had no involvement in generating, analysing or processing data, nor in scientific input, and it had no input into the generation of this article". Declarations of interest: one author was reimbursed by Omega Pharma for international conference attendance. Original language of publication: English Other: measurements were performed under the same climate conditions (21°C, controlled humidity) between September and January, by one investigator. |
Krueger 2006.
| Study characteristics | |
| Methods | Trial design: randomised, parallel study Trial registration number: not reported Country: USA and Mexico Outpatient or hospital: 8 centres Date trial conducted: not reported Duration of trial participation: 12 weeks Inclusion criteria: · Adults with moderate‐to‐severe AD according to Hanifin and Rajka and Rajka and Langeland criteria and of at least 3 months duration · Affecting 35% to 75% of BSA not including scalp Exclusion criteria: · Severe defective epidermal barrier function · Any other skin disorder · Clinically infected AD · Used systematic immunosuppressants or immunomodulators within 2 weeks prior · Topical or systematic steroids or topical immunomodulators within one week prior Additional design details: none |
| Participants | Total number randomised: 40 participants (19 to apply the intervention and 21 to apply the comparator) Age: intervention group, mean 42.5 years SD 17.0, range 17‐74; in tacrolimus 0.1% comparator group, mean 39.6 years SD 14.5, range 18‐69 years Sex: intervention group, female 68.4%, male 31.6%; comparator group, female 66.7%, male 33.3% Ethnicity: intervention group, black 21.1%, Asian 0, white 73.7% and other 5.3%; comparator group, black 19%, Asian 9.5%, white 61.9%, other 9.5% Duration of eczema: not reported Severity of eczema: intervention group, 26.3% moderate, 73.7% severe; comparator group, 33.3% moderate, 66.7% severe Body site: not reported Number of withdrawals: 11 withdrew Notes: none |
| Interventions | Run‐in details: the use of any form of tacrolimus or pimercrolimus was prohibited for 2 weeks prior. Intervention: tacrolimus 0.03% ointment used twice daily for 84 days · Concurrent treatment: not reported · Other key information: none Comparator: tacrolimus 0.1% ointment used twice daily for 84 days. · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · BSA · Withdrawals due to AE |
| Notes | Funding source: authors affiliated to Astellas Pharma Declarations of interest: not declared Original language of publication: English Other: none |
Kuokkanen 1987.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: Finland (assumed from author affiliations) Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 3 weeks Inclusion criteria: · White children aged 2 to 10 years · Diagnosed with eczema (bilateral, symmetrical) and otherwise in general good health · To quote: "the severity of erythema, induration, and pruritus at test sites was rated on a 4‐point scale ranging from 0 (absent) to 3 (severe). To be included in the trial, a child had to have (a) each sign or symptom, (b) the rating of the severity of each had to be equal at paired test sites, (c) the ratings at each test site had to total at least 6, and (d) the condition had to be stable or slowly worsening for more than one week." Exclusion criteria: · Evidence of skin atrophy · Hypersensitivity to any component of either trial medication · Use of topical or systemic steroids within the 2 weeks of the trial · Requirement for > 45 gm of medication weekly per test site · Use of any treatment known to affect eczema within a month of the trial · Requirement for medication (topical or systemic) that might affect the course of eczema Additional design details: none |
| Participants | Total number randomised: 37 sides treated (16 applied to the right side (intervention) and 18 applied to the right side (comparator)) Age: randomised to right‐side intervention, mean 6.8 years, range 2.0 to 10.0; randomised to right‐side comparator, mean 7.6 years, range 3.0 to 10.0 Sex: randomised to right‐side intervention, male n = 10, female = 6; randomised to right‐side comparator, male n = 9, female n = 9 Ethnicity: all were white. Duration of eczema: right‐side intervention, mean 4.5 years, range 0.3 to 8; right‐side comparator, mean 6.2 years, range 1 to 10 Severity of eczema: right‐side intervention group: stable n = 5, slowly worsening n = 10, rapidly worsening n = 1; right‐side comparator group, stable n = 6, slowly worsening n = 10, rapidly worsening n = 2 Body site: to quote: "lesions on the arms, legs, or torso were selected as paired test sites in each participant". Number of withdrawals: 3 did not return after the initial visit and were not included. Two received antibiotics during the trial and were evaluated for safety but not comparative efficacy. Eight did not return for follow‐up visits "at the weekly interval" but were still included in efficacy evaluations. Notes: 6 per group, had been treated with treatments previously; 11 had received hydrocortisone lotions or liniments, 3 with emollient creams or ointments. 1 participant also received antihistamine for urticaria for 4 days in the 1st week of treatment. |
| Interventions | Run‐in details: not reported Intervention: alclometasone dipropionate 0.05% ointment used twice daily for 3 weeks · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone 1% ointment used twice daily for 3 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: participants were to apply enough to cover the test site and to gently massage in the medications. They were also instructed to wash their hands carefully between applications. Nothing other than trial treatments could be applied to the test sites. Notes: to quote: "When lesions cleared in less than three weeks, application of the trial medication was to continue but in an area of only 3 cm2 within each test site." |
| Outcomes | · Sum of erythema, induration, and pruritus at paired test sites (0 = absent to 3 = severe) · AE assessed by careful examination and questioning of caregivers at each visit · Visual assessment of test site for signs of cutaneous atrophy; skin thinning, shininess, striae, bruising, telangiectasia, loss of hair, elasticity and normal skin markings and wasting of muscle and subcutaneous fat. Skin thinning, shininess, and striae were graded on a 4‐point scale from 0 = absent to 3 = severe. Telangiectasia was evaluated at a 3 cm2 area by counting visible blood vessels within each test site using a 2 x magnifying lens. · Comparative efficacy (equivalent, alclometasone results better or hydrocortisone results better) · IGA: cleared (100% clearance of monitored signs and symptoms except for residual discolouration); markedly improved (75% to < 100% clearance of monitored signs and symptoms); moderately improved (5 0% to < 75% clearance of monitored signs and symptoms); slightly improved (< 50% clearance of monitored signs and symptoms); unchanged; or exacerbated |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Landis 2022.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: NCT03903822 Country: Australia, Bulgaria, Canada, Denmark, Germany, Hungary, Japan, Latvia, Poland, United States Outpatient or hospital: not reported Date trial conducted: May 2019 to May 2020 Duration of trial participation: 6 weeks Inclusion criteria: · AD for at least 3 months · IGA score of 2 or 3 · EASI score 3 to 21 · BSA 2 to 20% · PP‐NRS of grade 2 or more Exclusion criteria: · Other dermatological diseases · Fitzpatrick > 5 · Clinically abnormal ECG, vital signs, or laboratory values · Infection with HBV, HCV, herpes zoster or tuberculosis Additional design details: 8 interventions were directly compared. |
| Participants | Total number randomised: 292 participants (37 each to apply intervention one, three and six, 36 each to apply intervention two, four and five, and 37 to apply comparator one and 36 comparator two) Age: average per study group: vehicle QD: 39.1 years; 0.1% QD 40.8 years; 0.3% QD 43.4 years; 1% QD 38.4 years; 3% QD 40.5 years; vehicle BID 42.0 years; 0.3% BID 39.4 years; 1% BID: 38.1 years Sex: number female/male per study group: vehicle QD female 20/male 17; 0.1% QD female 19/male 12; 0.3% OD: female 24/male 12; 1% QD female 23/male 14; 3% QD: female 15/ males 21; vehicle BID female 19/male 17; 0.3% BID female 16/male 20; 1% BID female 20/male 17 Ethnicity: per group: vehicle QD Asian n = 4, African‐American n = 8, white n = 24, unknown = 1; 0.1% QD Asian n = 8, African‐American n = 7, white n = 22; 0.3% OD: Asian n = 10, African‐American n = 4, white n = 21, more than one race n = 1; vehicle BID: Asian n = 9, Hawaiian n = 1, African‐American n = 6, white n = 20; 0.3% BID Asian n = 9, African‐American n = 5, white n = 22, 1% BID Asian n = 7, African‐American n = 6, white n = 24 Duration of eczema: AD for at least 3 months eligible Severity of eczema: IGA score 2 or 3 eligible Body site: all areas affected by AD, except for hair‐bearing scalp Number of withdrawals: per group: Vehicle QD n = 3 AE, n = 2 withdrawal by subject, n = 2 refused further treatment, n = 2 other 0.1% QD n = 3 AE, n = 2 physician decision, n = 4 withdrawal by subject, n = 1 other 0.3% OD n = 1 AE, 2 withdrawal by subject 1% QD n = 1 AE, n = 1 pregnancy, n = 1 protocol violation, n = 2 withdrawal by subject 3% QD n = 1 AE, n = 1 lack of efficacy, n = 1 pregnancy, n = 1 withdrawal by subject, n = 1 other Vehicle BID n = 6 AE, n = 1 lack of efficacy, n = 2 withdrawal by subject, n = 1 refused further treatment, n = 1 other 0.3% BID n = 1 AE, n = 1 lost to follow‐up, n = 3 withdrawal by subject 1% BID n = 1 lost to follow‐up, n = 3 withdrawal by subject Notes: none |
| Interventions | Run‐in details: not reported Intervention one: brepocitinib 0.1% cream used once daily for 42 days · Concurrent treatment: not reported · Other key information: none Intervention two: brepocitinib 0.3% cream used once daily for 42 days · Concurrent treatment: not reported · Other key information: none Intervention three: brepocitinib 1% cream used once daily for 42 days · Concurrent treatment: not reported · Other key information: none Intervention four: brepocitinib 3% cream used once daily for 42 days · Concurrent treatment: not reported · Other key information: none Intervention five: brepocitinib 0.3% cream used twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Intervention six: brepocitinib 1% cream used twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Comparator one: vehicle cream used once daily for 42 days · Concurrent treatment: not reported · Other key information: none Comparator two: vehicle cream used twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI · EASI‐75 · IGA · Peak pruritus NRS · Withdrawal due to AE |
| Notes | Funding source: Pfizer Declarations of interest: authors affiliated to Pfizer and independent authors reported conflicts of interest related to Pfizer. Original language of publication: English Other: none |
Lassus 1983.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind study Trial registration number: not reported Country: Finland Outpatient or hospital: assumed to be secondary care in Finland according to the affiliation of the author Date trial conducted: not reported Duration of trial participation: 2 weeks Inclusion criteria: · Patients with an established diagnosis of AD for at least 1 month, stable and worsening over the preceding week · White children · Aged 5 to 11 years · Each of the following 3 signs present: erythema, induration, pruritus; summed severity score of each sign was graded 0 = absent to 3 = severe Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 40 participants (20 to apply the intervention and 20 to apply the comparator) Age: intervention group mean 7.5 years (median 7.0); comparator group mean 8.4 years (median 8.0) Sex: intervention group: male n = 7, female n = 13; comparator group male n = 10, female n = 10 Ethnicity: all white Duration of eczema: both groups mean duration 5.5 years; in intervention group, 12 participants had been diagnosed 1 to 6 years, 8 diagnosed 6 to 10 years; comparator group 7 had been diagnosed 1 to 5 years, 13 diagnosed 6 to 10 years Severity of eczema: intervention group mean TSS pre‐treatment 7.70; comparator group mean 8.05 Body site: not reported Number of withdrawals: there were no withdrawals and all participants were included in the analyses of efficacy and safety Notes: to quote: "The two treatment groups did not differ significantly in age, sex, race, duration of disease, or per cent of body involvement." |
| Interventions | Run‐in details: none Intervention: alclometasone dipropionate 0.05% cream used twice daily for 14 days · Concurrent treatment: none · Other key information: proprietary: Vaderm, Schering, USA Comparator: hydrocortisone butyrate 0.1% cream used twice daily for 14 days · Concurrent treatment: none · Other key information: proprietary: Locoid, Brocades UK Concurrent treatments received alongside both intervention and comparator: none Notes: applied as a thin layer to areas on the face, neck, trunk, and upper and lower extremities. Palms, soles, and scalp were not included. Medication was not to be applied within 3 hours of a trial visit. |
| Outcomes | · Participant‐reported and clinically observed AE · Disease signs (erythema, induration, pruritus) in pre‐selected target areas scored 0 = absent to 3 = severe, reported separately and summed with a percentage improvement · IGA of improvement at treated areas (cleared = 100% clearance of signs and symptoms except for residual discolouration, marked improvement = 76% to 100% clearance, moderate improvement = 50% to 75% clearance, slight improvement = < 50% clearance, no change, exacerbation) |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022 |
Lassus 1984.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: not reported Country: Finland Outpatient or hospital: Department of Dermatology, Helsinki University Central Hospital Date trial conducted: not reported Duration of trial participation: 2 weeks Inclusion criteria: · Disease present for at least 1 month and diagnosed as stable or worsening for more than 1 week · Each child manifested three disease signs of AD (erythema, induration, and pruritus) with a total severity score of 6 or more, when each sign was graded as follows: 0 = absent; 1 = mild; 2 = moderate; 3 = severe Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 43 participants (22 to apply the intervention and 21 to apply the comparator) Age: 5 to 11 years Sex: female n = 27, male n = 16 Ethnicity: white Duration of eczema: present for at least 1 month Severity of eczema: 67% of the patients had up to 25% of the BSA involved with AD. Body site: lesions on the face, neck, trunk, and upper and lower extremities were included as study areas. Number of withdrawals: no withdrawals Notes: none |
| Interventions | Run‐in details: none Intervention: alclometasone dipropionate 0.05% cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: clobetasone butyrate 0.05% cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Disease signs graded in preselected target areas using the scale: 0 = absent; 1 = mild; 2 = moderate; 3 = severe · The investigator globally evaluated improvement in the overall disease condition · AE |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Lawlor 1995.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, single‐centre, parallel study Trial registration number: not reported Country: UK Outpatient or hospital: clinics at St John's Hospital, including the contact dermatitis clinic when patch testing was negative Date trial conducted: not reported Duration of trial participation: 28 days Inclusion criteria: · Mild‐to‐moderate eczema · At least 10% involvement · Included hands, epigastrium, shoulder/thorax, lower leg, thigh/knee, head or neck, abdomen or feet · Age 18 to 60 Exclusion criteria: · Facial eczema, exudative, infected, and widespread (> 50%) · Oral corticosteroids 4 weeks prior · Concomitant serious disease · Female and not on adequate contraception · Severe involvement in either scratch marks, erythema, scaling, lichenification, vesiculation, and hyperkeratosis Additional design details: none |
| Participants | Total number randomised: 51 participants (24 to apply the intervention and 27 to apply the comparator) Age: 17 to 68 years Sex: female or male Ethnicity: not reported Duration of eczema: 0.3 to 28 years Severity of eczema: mild‐to‐moderate Body site: hands, epigastrium, shoulder/thorax, lower leg, thigh/knee, head or neck, abdomen Number of withdrawals: 6 due to, to quote "not making sufficient progress". 5 from control, 1 from treatment. A further 13 had treatment stopped by the doctor because of lack of progress, AE, or infection. Notes: none |
| Interventions | Run‐in details: not reported Intervention: prednicarbate 0.25% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: avoid using soap and use aqueous cream BP as soap substitute. Treatment was discontinued at any visit at which eczema was clear. Notes: none |
| Outcomes | · Clinician‐reported signs: "physical signs of excoriation, erythema, scaling, lichenification and vesiculation recorded on a scale of 1 to 5 (1 = none, 5 = very severe)" · Patient‐reported signs: pruritis on a VAS · AE · IGA (excellent, good, fair, poor, exacerbation) · Patient‐reported signs: patient global evaluation (excellent, good, fair, poor, exacerbation) · Clinician/patient‐reported signs: overall efficacy, tolerability, cosmetic acceptability ‐ investigator and patient assessment |
| Notes | Funding source: not reported Declarations of interest: one author supported by Hoechs Ag, Germany (suppliers of intervention) Original language of publication: English Other: none |
Lebrun‐Vignes 2000.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, single‐centre, parallel study Trial registration number: not reported Country: France Outpatient or hospital: Department of Pediatric Dermatology of the Hôpital de Bordeaux, France Date trial conducted: October 1989 to February 1991 Duration of trial participation: 30 days (15 days treatment, 15 days follow‐up) Inclusion criteria: children < 8 years admitted to hospital with an episode of severe, non‐infected AD Exclusion criteria: · Unconfirmed diagnosis · Bacterial, viral, or fungal superinfection · History of allergy to desonide or betamethasone dipropionate · Systemic treatment with corticosteroids within the preceding month · Topical treatment with corticosteroids within the preceding 2 weeks Additional design details: none |
| Participants | Total number randomised: 29 participants (15 to apply the intervention and 14 to apply the comparator) Age: intervention group mean 13.80 months (SD 13.81); comparator group mean 14.29 months (SD 9.24) Sex: intervention group male n = 9, female n = 6; comparator group 6 male n = 6, female n = 8 Ethnicity: not reported Duration of eczema: not reported Severity of eczema: intervention group mean percentage BSA involved 44.20% (SD 20.15); comparator group 36.86% (SD 26.22). Lesion score intervention group mean 6.87 (SD 3.14); comparator group mean 7.57 (SD 2.28). Assessed for a representative skin area from 0 = absence of improvement to 3 = considerable improvement for erythema, pruritus, discharge, excoriation, and lichenification (max = 15) Body site: not reported Number of withdrawals: 1 participant in the intervention group was lost to follow‐up after the 5th day. The study also stated, to quote: "some information was not available," but there was no further information given. Notes: none |
| Interventions | Run‐in details: not reported Intervention: desonide 0.1% cream used twice daily for 15 days · Concurrent treatment: none · Other key information: proprietary: Locatop, Laboratoire Pierre Fabre Dermatologie Comparator: betamethasone dipropionate 0.05% cream used twice daily for 15 days · Concurrent treatment: none · Other key information: proprietary: Diprosone, Schering‐Plough Concurrent treatments received alongside both intervention and comparator: all participants were asked to use mild soap, emollient, and antiseptic foam solution. If necessary, participants could also receive antihistamines, sedatives or antibiotics. Notes: applied without occlusion whilst admitted to hospital for 5 days, then once daily until day 7, then once on alternate days until day 15. Follow‐up was an additional 15 days. |
| Outcomes | · Percentage BSA involved · Clinical side effects and local and systemic tolerance assessed by investigators and parents/caregivers · Plasma cortisol levels (samples taken between 8 am and 9 am, measured by competitive binding assays) · Number of relapses · Lesion score (most representative area) judged by a physician as 0 = absence of improvement, 1 = slight improvement, 2 = moderate improvement, or 3 = considerable improvement for the following signs: erythema, pruritus, discharge, excoriation, and lichenification (max score 15) |
| Notes | Funding source: not reported Declarations of interest: none declared; however, one of the authors was affiliated to Laboratoires Pierre Fabre. Original language of publication: French Other: translation in English was available. This study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Lebwohl 1999.
| Study characteristics | |
| Methods | Trial design: multicentre, randomised, evaluator‐blind, parallel study Trial registration number: not reported Country: 10 centres in the USA Outpatient or hospital: assumed to be secondary care as the primary author is a dermatologist Date trial conducted: not reported Duration of trial participation: 22 days Inclusion criteria: · Aged 2 to 12 years · Moderate‐to‐severe AD · ≥ 15% total BSA involvement, excluding face and forehead, current exacerbation with a target area ≥ 20 cm2 · A six‐sign/symptom severity score ≥ 8 and ≤ 18 for the target area (erythema, induration/lichenification, scaling/crusting, exudation, excoriation, and pruritus graded from 0 = none to 3 = severe) · Severity ≥ 2 required for erythema and for 1 other sign Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 219 participants (109 to apply the intervention and 110 to apply the comparator) Age: 2 to 12 years were eligible Sex, ethnicity, duration of eczema: not reported Severity of eczema: moderate‐to‐severe based on inclusion criteria Body site: not the face or forehead Number of withdrawals: 43 discontinued the trial early (from the safety population): · Clearance of signs and symptoms (18 in intervention group, 9 in the comparator group) · Treatment failure (0 in intervention group, 1 in comparator group) Notes: none |
| Interventions | Run‐in details: participants had failed to respond to at least 7 consecutive days of topical hydrocortisone treatment ending within a week of enrolment of this trial Intervention: mometasone furoate 0.1% cream used once daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone valerate 0.2% cream used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: no other therapies for AD were permitted. Notes: a target area (not face or forehead) of ≥ 20 cm2 was selected by the investigator for specific evaluation of the effects of treatment on disease signs and symptoms. |
| Outcomes | · Severity in the target area of erythema, induration/lichenification, scaling/crusting, exudation, excoriation, and pruritus graded on a scale from 0 = none to 3 = severe · IGA (cleared = 100% improvement; excellent = 75%–99%; good = 5 0% to 74%; fair = 25% to 49%; poor < 25%; exacerbation = flare‐up) · Treatment‐related atrophy · Application site reactions |
| Notes | Funding source: Schering Plough Inc. Declarations of interest: not declared Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Lee 2014.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind study Trial registration number: not reported Country: South Korea Outpatient or hospital, date trial conducted: not reported Duration of trial participation: up to 4 weeks under double‐blinded, randomised conditions Inclusion criteria: · Presence of AD for greater than 3 months · A lesion covering over 5% of the patient’s body surface area involving the face, head and neck · An IGA score of greater than 2 · Persistent AD lesions despite the use of existing potency class VII or greater TCS for more than two weeks Exclusion criteria: · Inability to receive local formulation treatment due to the presence of an external wound · The presence of other infections, diseases or skin disorders · Treatment with a systemic steroid, systemic immunosuppressant, or oriental herbal medicine within the previous 3 months · General inappropriateness for the study as determined by the clinical trial investigator Additional design details: if clinical improvement was evident within the entire lesion (IGA score ≤ 1) prior to completion of the 4 weeks of treatment, the patients were switched to an open‐label phase. Upon conclusion of the double‐blind phase, a 24‐week extension was initiated to assess long‐term efficacy and safety. |
| Participants | Total number randomised: 55 participants (28 to apply the intervention and 27 to apply the comparator) Age: pimecrolimus group mean 0.7 years (SD 0.3); desonide group mean 0.7 years (SD 0.5) Sex: pimecrolimus group male n = 17, female n = 11; desonide group male n = 20, female n = 7 Ethnicity: not reported Duration of eczema: more than 3 months Severity of eczema: mean IGA scores (95% confidence interval): pimecrolimus group 3.9 (95% CI 3.5 to 4.2); desonide group 3.6 (95% CI 3.2 to 4.0) Body site: a lesion covering over 5% of the patient’s body surface area involving the face, head and neck Number of withdrawals: one patient lost to follow‐up in the pimecrolimus group Notes: none |
| Interventions | Run‐in details: study only included participants who still had persistent AD lesions after a washout period of using existing potency class VII or greater topical steroids for more than two weeks Intervention: pimecrolimus 1% cream used twice daily for up to 28 days · Concurrent treatment: not reported · Other key information: none Comparator: desonide 0.05% cream used twice daily for up to 28 days. · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: none Notes: if clinical improvement was evident within the entire lesion (IGA score # 1) prior to completion of the 4 weeks of treatment, the patients were switched to an open‐label phase. In the open‐label phase, all patients applied the corresponding medication to their lesion based on authorisation and off‐label approval if topical administration was required due to the worsening of lesions. |
| Outcomes | · IGA (0 = clear, 1 = almost clear). Upon achievement of an IGA score of 0 or 1, the patient was switched to the open‐label challenge. · EASI score · Safety assessments consisted of the monitoring and recording of all adverse events as well as dermoscopic assessments of face, head and neck lesions for skin atrophy and telangiectasia · Analysis of endogenous metabolites: haematology, blood chemistry, morning serum cortisol, T cell subset and urinalysis |
| Notes | Funding source: multiple grants including the KFDA, the Bio‐Synergy Research Project of the Ministry of Science, ICT and Future Planning through the National Research Foundation, the National Research Foundation of Korea grant by the Korean government, Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology and BK21 Plus Program Declarations of interest: the authors declared no competing financial interests. Original language of publication: English Other: none |
Leibsohn 1974.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number, country, outpatient or hospital, date trial conducted: not reported Duration of trial participation: maximum study period of 3 weeks Inclusion criteria: bilateral dermatosis lesions of similar severity, persistence and aetiology Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 16 sides treated (8 to apply the intervention and 8 to apply the comparator) Age: range from 8 to 72 years; 94% were adults. Note, this is for the whole study; AD cohort unknown Sex: male n = 44; female n = 46. Note, this is for the whole study; AD cohort unknown Ethnicity, duration of eczema, severity of eczema, body site: not reported Number of withdrawals: two patients failed to return after the first visit. Six patients were evaluated only at the end of week 1, and twenty‐four were evaluated after weeks 1 and 2. Sixty patients were evaluated weekly for the 3‐week treatment period (except for one patient who was not evaluated for week 2). Therapy ceased in eighteen patients when sufficient clinical remission had been obtained before the end of the suggested treatment period. Note, this is for the whole study; AD cohort unknown Notes: none |
| Interventions | Run‐in details: not reported Intervention: halcinonide cream used three times daily for 21 days · Concurrent treatment: not reported · Other key information: 0.1% assumed as not stated Comparator: betamethasone valerate 0.1% cream used three times daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: the participants were instructed to apply to the left‐sided lesions a designated cream containing corticosteroid, liberally and without occlusion, then to wash their hands before applying to the right‐sided lesions, in a similar manner, the other designated cream. The use of other corticosteroids, antihistamines and antipruritics was prohibited during the treatment period. Notes: none |
| Outcomes | · Overall objective comparative clinical responses · Overall evaluation of therapeutic response ‐ IGA |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Leo 2004.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: not reported Country: USA Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 14 days (assuming that it is until end of treatment, as opposed to a follow‐up assessment after end of treatment) Inclusion criteria: children and adolescents (aged 2 to 17 years) with AD Exclusion criteria: treated with any topical medication in the previous 4 weeks. Additional design details: none |
| Participants | Total number randomised: 19 participants (9 to apply the intervention and 10 to apply the comparator) Age: age range from 7 to 17 years; intervention group mean 9.8 years (SD 3.3); comparator group 10.7 years (SD 3.1) Sex: intervention group male n = 6, female n = 3; comparator group male n = 4, female n = 6 Ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐moderate AD Body site: not reported Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: 1‐week washout period using only an over‐the‐counter moisturiser to the affected areas of skin. Participants were instructed to stop all oral antihistamines, TCS, sleep medications, and antibiotics for the duration of the study. Intervention: pimecrolimus 1% cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: to quote: "Subjects were instructed to stop all oral antihistamines, TSC, sleep medications, and antibiotics for the duration of the study". Notes: none |
| Outcomes | · EASI used to characterise the extent of AD · CDLQ · Family’s perception of pruritus and general appearance was obtained from a standard‐point scale |
| Notes | Funding source: Novartis Pharmaceuticals Corporation Declarations of interest: not declared Original language of publication: English Other: none |
Leung 2009.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: USA, Canada and Switzerland Outpatient or hospital: 13 outpatients Date trial conducted: not reported Duration of trial participation: 42 days Inclusion criteria: · Outpatients aged 2 to 50 years · Mild‐to‐moderate AD according to IGA affecting at least 5% BSA · History of corticosteroid insensitivity that was not limited to the hands or feet · Documented colonisation of AD lesions with staphylococcus aureus Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 73 participants (47 to apply the intervention and 26 to apply the comparator) Age: intervention group, mean 18.3 years (SD 12.9), median 16 range 2 to 49); vehicle group, 18.8 years (SD 15.3), median 14.5 (range 2 to 49) Sex: intervention group, male n = 18, female n = 29; vehicle group, male n =14, female n = 12 Ethnicity, duration of eczema: not reported Severity of eczema: IGA, intervention group, mild n = 8, moderate n = 36, severe n = 3; vehicle group, mild n = 3, moderate n = 20, severe n = 3 Body site: not reported Number of withdrawals: intervention group, lost to follow‐up n = 1, discontinued intervention n = 8, due to unsatisfactory therapeutic effect n = 3, AE n = 3, protocol violation n = 1, abnormal test value n = 1; vehicle group, discontinued intervention n = 8, due unsatisfactory therapeutic effect n = 4, AE n = 2, protocol violation n = 1, patient withdrew consent n = 1 Notes: none |
| Interventions | Run‐in details: treatments known or suspected to have an effect on AD had to be stopped at least 4 weeks prior to baseline; systemic retinoids or investigational drugs at least 8 weeks and antibiotics at least 2 weeks Intervention: pimecrolimus 1% cream used twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Staphylococcus aureus colonisation in the four most affected eczematous lesions (the ‘target lesions’), superantigens, and lymphocyte insensitivity to CS · EASI · Overall IGA six‐point ordinal scale: 0, clear; 1, almost clear; 2, mild disease; 3, moderate disease; 4, severe disease; 5, very severe disease · Pruritus score, recording pruritus over the previous 24 hours (0, absent; 1, mild; 2, moderate; 3, severe) as assessed by the investigator in discussion with the subject or caregiver · Patient's AD assessment (0, complete disease control; 1, good disease control; 2, limited disease control; 3, uncontrolled disease) conducted by the subject or the caregiver, which evaluated the subject’s disease state in the 7 days prior to the assessment · IGA score for target lesion, comprising a local global assessment score assessed separately for the four lesions with the highest S. aureus count |
| Notes | Funding source: Novartis Pharmaceuticals Corporation Declarations of interest: all authors had conflicts of interest. These were research funds from Novartis and NIH ⁄ NIAMS, consulting fees from Novartis, and speaking fees from Novartis. Original language of publication: English Other: none |
Levy 1974.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: USA Outpatient or hospital: outpatient Date trial conducted: not reported Duration of trial participation: 21 days Inclusion criteria: comparable, symmetrical areas of steroid‐responsive dermatoses Exclusion criteria: not reported Additional design details: this study also included 25 patients with psoriasis and with miscellaneous corticosteroid responsive dermatoses. |
| Participants | Total number randomised: 44 sides treated (22 to apply the intervention and 22 to apply the comparator) Age: 10 to 67 years Sex: male n = 12, female n = 10 Ethnicity: not reported Duration of eczema: 4 months to 60 years Severity of eczema, body site: not reported Number of withdrawals: 7 patients withdrew but this was not separated by skin condition. Notes: none |
| Interventions | Run‐in details: not reported Intervention: fluocinonide 0.05% FAPG used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle FAPG used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: FAPG is a specially designed base that combines the physical properties of a cream and a gel. |
| Outcomes | · Improvement evaluation based mainly on the appearance of the lesions in the test areas. The categories of scoring were: very good or excellent; moderate improvement; and no improvement or worse. |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Levy 2005.
| Study characteristics | |
| Methods | Trial design: randomised, parallel‐group study Trial registration number, country, outpatient or hospital: not reported, date trial conducted, duration of trial participation: not reported Inclusion criteria: adult with mild‐to‐moderate AD Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 133 participants Age: adult 18 years or above Sex, ethnicity, duration of eczema: not reported Severity of eczema: to quote: "At baseline, 88.7% had mild to moderate atopic dermatitis affecting 2.1 to 66.5% of body surface area". Body site, number of withdrawals: not reported Notes: 133 participants were treated in the trial; it was not clear if this was the number that were randomised. |
| Interventions | Run‐in details: not reported Intervention one: tacrolimus 0.01% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention two: tacrolimus 0.03% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: |
| Outcomes | · Treatment success defined as clear or almost clear evaluation of affected areas · Safety was evaluated based on incidence of adverse experiences, laboratory parameters, and physical examinations. |
| Notes | Funding source: Fujisawa Healthcare, Inc. Declarations of interest: not declared Original language of publication: English Other: |
Liu 2014b.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: not reported Country: China Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 2 weeks Inclusion criteria: · Aged 12 to 65 years · Male or female · Eczema; measured by the palm method (taking the subject's palm area as 1%), the subject's affected skin occupies a BSA ≤ 10%; according to the IGA, it should be moderate (3 points) and above. Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 240 participants (120 to apply the intervention and 120 to apply the comparator) Age: aged 12 to 65 years Sex: intervention group, male n = 46, female n = 73; comparator group, male n = 53, female n = 66 Ethnicity, duration of eczema: not reported Severity of eczema: according to IGA at least moderate; there was no significant difference in the severity of eczema between the two groups (P > 0.05). Body site: not reported Number of withdrawals: n = 18 with a dropout rate of 7.50% Notes: none |
| Interventions | Run‐in details: not reported Intervention: clobetasone butyrate 0.05% cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI · Subject self‐assessment of pruritus symptoms (VAS) · IGA · Safety · Withdrawals due to AE |
| Notes | Funding source: Sino‐US Tianjin SmithKline Pharmaceutical Co., Ltd. Declarations of interest: not declared Original language of publication: Chinese Other: none |
Lucky 1997.
| Study characteristics | |
| Methods | Trial design: randomised, single‐centre, parallel study Trial registration number: not reported Country: US (assumed from affiliations) Outpatient or hospital: outpatient setting (assumed from affiliations) Date trial conducted: not reported Duration of trial participation: 4 weeks Inclusion criteria: children with ≥ 20% BSA affected by AD Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 20 participants (10 to apply the intervention) and 10 to apply the comparator) Age: mean 4.7 years intervention group (range 11 months to 11 years); mean 2.6 years comparator group (13 months to 8 years) Sex: intervention group, male n = 6, female n = 4; comparator group, male n = 7, female n = 3 Ethnicity, duration of eczema: not reported Severity of eczema: average BSA: intervention group, 38.1%, range 20% to 70%); comparator group, 37.1% (range 20% to 80%) Body site: not reported Number of withdrawals: 5; 3 in intervention group, 2 in comparator group Notes: none |
| Interventions | Run‐in details: not reported Intervention: desonide 0.05% ointment twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone 2.5% ointment twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Baseline morning serum cortisol · ACTH test · AE (stated to be measured, but not reported) |
| Notes | Funding source: not reported but all laboratory trials were performed by SmithKline Beecham, Clinical Laboratories Declarations of interest: not declared; however, several authors were affiliated to Galderma Laboratories, Inc, Fort Worth, Texas, who produce the desonide ointment used. Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Ludvigsen 1975.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: Denmark Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 14 days Inclusion criteria: AD with similar lesions Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 50 participants (50 sides to apply the intervention and 50 sides to apply the comparator) Age: range from 6 months to 22 years Sex, ethnicity, duration of eczema, severity of eczema, body site: not reported Number of withdrawals: 4 did not attend follow‐up; reason why not provided Notes: none |
| Interventions | Run‐in details: not reported Intervention: calmuril‐hydrocortisone 1% cream used twice daily for "approx" 14 days · Concurrent treatment: not reported · Other key information: none Comparator: triamcinolone acetonide 0.1% cream used twice daily for "approx" 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · to quote: "morphological exemplification changes" · Itch · Dryness · Patient preference · AE |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: Danish Other: Paper in Danish. Translation via google translation and google app, plus advice of native Danish speaker on results |
Luger 2001.
| Study characteristics | |
| Methods | Trial design: randomised, parallel group Trial registration number: not reported Country: 14 centres in Belgium, Denmark, Finland, Germany, the Netherlands, Norway and the UK Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 3 weeks Inclusion criteria: · Male and female adults (≥ 18 years) · AD according to Hanifin and Rajka criteria and Rajka and Langeland crireria of at least moderate · 5% to 30% of the total BSA Exclusion criteria: · Concomitant medical conditions that could interfere with study evaluations · Pregnancy, breastfeeding, or of child‐bearing potential not using medically approved contraception Additional design details: none |
| Participants | Total number randomised: 260 participants (42 each to apply intervention one, intervention three and intervention five, 46 to apply intervention two, 45 intervention four, and 43 to apply the comparator) Age: mean (range) per group: vehicle group, 33 years (18 to 69); 0.05% group, 33 years (19 to 70); 0.2% group, 30 years (18 to 51); 0.6% group, 28 years (18 to 57); 1% group, 28 (18 to 62); BMV group, 32 years (18 to 71) Sex: females and males Ethnicity: Caucasian n = 250; other n = 10 Duration of eczema: median per group: vehicle 24 years; 0.05% 26 years; 0.2% 23.5 years; 0.6% 22.5 years; 1% 22 years; BVM 25 years Severity of eczema: moderate n = 244, severe n = 16 Body site: non‐facial eczema Number of withdrawals: 61 Notes: none |
| Interventions | Run‐in details: not reported Intervention one: pimecrolimus 0.05% cream used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Intervention two: pimecrolimus 0.2% cream used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Intervention three: pimecrolimus 0.6% cream used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Intervention four: pimecrolimus 1% cream used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Intervention five: betamethasone valerate 0.1% cream used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator; the use of other treatments for AD or corticosteroids for the treatment of asthma was prohibited. Notes: if complete clearance occurred before the end of treatment, study medication was stopped. |
| Outcomes | · EASI · Pruritus · Patient assessment · AE · Withdrawal |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Luger 2004.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre study Trial registration number: not reported Country: 35 centres in nine countries; Belgium, Canada, Denmark, Finland, France, Germany, The Netherlands, Norway and the UK Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 1 year Inclusion criteria: · 18 to 79 years · AD of moderate‐to‐severe disease · Affecting at least 5% of the total BSA Exclusion criteria: · Treatment with phototherapy · Radiation therapy or systemic therapy for AD in the previous month · Treatment with topical therapy (other than tar shampoo on the scalp) not stopped 24 hours before first application of study medication · Malignancy Additional design details: not reported |
| Participants | Total number randomised: 658 participants (328 to apply the intervention and 330 to apply the comparator) Age: 18 to 79 years; intervention group, mean 33.4 years; comparator group, mean 33.5 years Sex: male/female; intervention group 146/182; comparator group 153/177 Ethnicity: intervention: Caucasian n = 294, black n = 6, oriental n = 16, other n = 7, missing n = 5; control: Caucasian n = 293, black n = 15, oriental n = 10, other n = 7, missing n = 5 Duration of eczema: not reported Severity of eczema: mild‐to‐severe (inclusion criteria stated moderate‐to‐severe but table of characteristics listed mild‐to‐severe) Body site: all Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream used twice daily for 1 year · Concurrent treatment: not reported · Other key information: none Comparator: triamcinolone acetonide 0.1% cream used twice daily for 1 year · Concurrent treatment: 1% hydrocortisone acetate cream (for the face, neck and intertriginous areas) · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Local AE · EASI · IGA |
| Notes | Funding source: Novartis Pharma AG Declarations of interest: not declared Original language of publication: English Other: none |
Lupton 1982.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, within‐participant study Trial registration number: not reported Country: US Outpatient or hospital: San Diego, California, St. Louis, Missouri, Greensboro, North Carolina, and Birmingham, Michigan, US Date trial conducted: not reported Duration of trial participation: up to 2 weeks Inclusion criteria: no details but participants had AD Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 466 sides treated (233 to apply the intervention and 233 to apply the comparator) Age: 2 to 67 years (average 19.8 years) Sex: male n = 98, female n = 135 Ethnicity: white n = 185, black n = 42, oriental n = 5, Latin‐American = 1 Duration of eczema: not reported Severity of eczema: mild n = 33 patients, moderately severe n = 128, and severe n = 72 Body site: not reported Number of withdrawals: 19, mostly "as they failed to apply the test preparations properly, or did not continue therapy to the proper end‐point" Notes: none |
| Interventions | Run‐in details: not reported Intervention: halcinonide 0.1% ointment used three times daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used three times daily for 14 days · Concurrent treatment: not reported · Other key information: a plasticised hydrocarbon gel (a polyethylene and mineral oil gel base) with glycols, polyethylene sorbitan tristearate, and butylated hydroxytoluene as a preservative Concurrent treatments received alongside both intervention and comparator; except for four patients, no other local or systemic medications that could affect the dermatitis were prescribed. These four patients also received topical steroids on areas other than the test site (two patients), an oral antibiotic (one patient), and a tranquilliser (one patient) Notes: a maximum treatment period of two weeks was specified, but therapy was discontinued earlier if a complete remission occurred on either side |
| Outcomes | · IGA: excellent (4), good (3), fair (2), or poor (1), based on the rapidity and completeness of the response and taking into consideration the initial severity of the disease · Superiority: the comparative clinical response, a direct comparison of lesion resolution on opposing sides of the body, was assessed weekly taking into consideration such factors as decrease in lesion size, erythema, oedema, transudation, and lichenification. · Occurrence of bacterial or yeast infections or the development of skin atrophy |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Mali 1976.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number, country: not reported Outpatient or hospital: private clinic Date trial conducted: not reported Duration of trial participation: 3 weeks Inclusion criteria: people with steroid‐responsive dermatosis, with eczema data presented separately Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 66 (total cohort included AD, psoriasis, other dermatoses ‐ unclear how many in the total randomised for AD) participants Age, sex, ethnicity, duration of eczema, severity of eczema, body site: not reported Number of withdrawals: 16 because of concomitant systemic corticosteroid therapy, broken code, or insufficient data. (unclear how many of these were AD participants) Notes: none |
| Interventions | Run‐in details: not reported Intervention: betamethasone dipropionate cream used twice daily for 21 days · Concurrent treatment: not reported · Other key information: brand: diprosone Comparator: flumethasone pivalate 0.2% cream used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Patients and physicians combined overall evaluation (extracted into clinical reported signs binary) |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Maloney 1998.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre study Trial registration number: not reported Country: USA Outpatient or hospital: four private dermatology clinics, non‐hospitalised patients Date trial conducted: not reported Duration of trial participation: 4 weeks Inclusion criteria: · non‐hospitalised patients (≥ 12 years old) with moderate‐to‐severe AD covering 2% or more of their BSA · all patients had at least one lesion 2 cm or more in diameter Exclusion criteria: · Immunocompromised, pregnant, or nursing · Skin atrophy, telangiectasia or striae in skin areas to be treated · Had received topical treatments for AD within 1 week prestudy, intramuscular triamcinolone within 6 weeks prestudy, or long‐term systemic corticosteroid usage within 6 months prestudy Additional design details: none |
| Participants | Total number randomised: 81 participants (41 to apply the intervention and 40 to apply the comparator) Age: ≥ 12 years old Sex, ethnicity, duration of eczema: not reported Severity of eczema: moderate‐to‐severe AD covering 2% or more of their BSA Body site: not reported Number of withdrawals: 37 patients (90%) in the intervention group and 24 (60%) in the comparator group completed all 43 days of the study. No withdrawals in the intervention group were for treatment failure, whereas 10 patients (25%) in the comparator group withdrew for this reason. Notes: none |
| Interventions | Run‐in details: not reported Intervention: clobetasol propionate 0.05% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: none Notes: a fingertip unit, equalling approximately 0.5 g in males and 0.43 g in females (enough to cover approximately 2% of the body), was used to measure and apply a thin film of study drug to the affected areas. |
| Outcomes | · Physician’s gross assessment based on the percentage improvement of the target lesion · Changes from baseline in severity scores for six signs/symptoms (erythema, pruritus, induration/papulation, lichenification, erosion/oozing/crusting, and scaling/dryness) and for total signs/symptoms according to the sane severity scoring system · Patients rated their response to treatment as excellent, good, fair, poor, or worse. · Laboratory assessments |
| Notes | Funding source: not reported Declarations of interest: not declared but one author (Jeanine Johnson Brown) is from PharmD Glaxo Dermatology, A Division of Glaxo Wellcome Inc. Original language of publication: English Other: none |
Marten 1980.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: not reported Country: UK Outpatient or hospital: outpatients Date trial conducted: not reported Duration of trial participation: 4 weeks Inclusion criteria: children with moderate‐to‐severe eczema Exclusion criteria: · Receiving systemic corticosteroids · Had infected eczema · Had very limited eczema Additional design details: none |
| Participants | Total number randomised: 20 participants (10 to apply the intervention and 10 to apply the comparator) Age: range from 1.5 to 13 years Sex: females and males Ethnicity: black n = 13, white n = 6, Asian n = 1 Duration of eczema: not reported Severity of eczema: moderate and severe Body site: not reported Number of withdrawals: 3 children were lost to follow‐up; none were withdrawn for clinical reasons. It was unclear to which group they belonged. Notes: none |
| Interventions | Run‐in details: not reported Intervention: hydrocortisone 17‐butyrate 0.1% ointment twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone 1% ointment twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator; no other local application was permitted during the trial. Oral antipruritics were permitted if the same dose as before the trial. Notes: none |
| Outcomes | Itching, assumed to be patient‐assessed |
| Notes | Funding source: Brocades Ltd supplied the study drugs. Declarations of interest: not declared Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Matheson 2008.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: USA Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 29 days Inclusion criteria: · Male and female aged 3 months to less than 18 years · Stable mild‐to‐moderate AD according to Hanifin and Rajka criteria · PGA score of 2 or 3 and at least a 10% BSA involvement · General good health · Agreed to the requirements and restrictions of the study: consistent usage of soaps, moisturisers, lotions, creams, ointments, sunscreens or other skin products; consistent usage of hair products and urine pregnancy test for female subjects of childbearing potential Exclusion criteria: pregnant or lactating subjects, immunocompromised subjects and subjects with extensive disease which could not be reasonably controlled with TCS Additional design details: none |
| Participants | Total number randomised: 284 participants (139 to apply the intervention and 145 to apply the comparator) Age: intervention group, mean 7.31 years, range 0.3 months to 17.8 years; comparison group, mean 6.97, range 0.4 months to 17.6 years Sex: intervention group, 64/139 (46%) male; comparator group, 79/145 (54%) male Ethnicity: intervention group: white n = 66, black/African‐American n = 40, Asian n = 9, American‐Indian or Alaskan native n = 2; comparator group, white n = 32, black/African‐American n = 52, Asian n = 4 Duration of eczema: not reported Severity of eczema: PGA score intervention group, mild n = 65, moderate n = 74; comparator group, mild n = 69, moderate 75, severe n = 1 Body site: not reported Number of withdrawals: intervention group, n = 7 (subject request n = 2, lost to follow‐up n = 5); comparative group, n = 25 (subject request n = 7, lost to follow‐up n = 5, lack of efficacy n = 6, AE n = 5, other n = 2) Notes: none |
| Interventions | Run‐in details: not reported Intervention: hydrocortisone butyrate 0.1% lotion twice daily for 4 weeks · Concurrent treatment: not reported · Other key information: none Comparator: vehicle lotion twice daily for 4 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Overall disease severity using a 5‐point ordinal PGA scale (0 = clear to 4 = severe) · Physician's assessment of severity of individual signs (erythema, induration/papulation, lichenification, excoriation, and oozing/crusting) of the overall body and body region (head/neck, upper limbs, trunk, and lower limbs) using a four‐point ordinal scale (0 = none to 3 = severe) · EASI score · Subject self‐assessment of pruritus (0 = none to 3 = severe) · Physicians' assessment of percent BSA involvement by body region (head/neck, upper limbs, trunk and lower limbs) · Change in IGA · Overall therapeutic success assessed by the investigator and patient · Patient's assessment of severity of pruritus |
| Notes | Funding source: Ferndale Laboratories Declarations of interest: the authors were compensated for performing the clinical study and editorial support for the authors of the manuscript was provided by employees of Therapeutics Inc. and data analysis support was provided by employees of QST Consulting Inc. Original language of publication: English Other: none |
Meenan 1963.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, single‐centre, within‐participant study Trial registration number: not reported Country: Ireland Outpatient or hospital: the Children's Hospital, Dublin, assumed from the author's affiliation. Both outpatients and inpatients were included. Date trial conducted: not reported Duration of trial participation: up to 2 weeks Inclusion criteria: patients with infantile AD, said to be in the 'usual pattern' and with symmetrical and equally severe lesions Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 50 sides treated (25 to apply the intervention and 25 to apply the comparator) Age: average 3 years, 2 months (range from 6 months to 12 years) Sex, ethnicity, duration of eczema: not reported Severity of eczema: not reported; however, it was stated that 22 cases were outpatients and 3 were inpatients. Younger children tended to show "weeping and crusted lesions" and older children tended to be "erythematous, scaly, and sometimes lichenified". Body site: not reported Number of withdrawals: 5 participants were only treated for 11 days and not 2 weeks, but it is unclear why. Notes: none |
| Interventions | Run‐in details: not to use TCS in the 3 weeks preceding the trial Intervention: fluocinolone acetonide 0.01% cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone 1% cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Comparative response assessed by 3 judges: the author, a parent, and an independent judge (medical registrar or nurse). Factors that influenced decision‐making: diminution of scaling, weeping, redness (and mother able to say if scratched 1 side less). Each could assign a point to the side that had a better response than the other. If there was no difference between sides, no point was awarded. The maximum score possible was 3. For intern participants, another nurse replaced the parent at week 2 (or at end of treatment). |
| Notes | Funding source: none reported, however Imperial Chemicals Industries, Ltd. provided the creams. Declarations of interest: not declared Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Meurer 2002.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: Germany Outpatient or hospital: dermatological university hospitals n = 12, dermatological clinic n = 1, dermatology practices n = 3 Date trial conducted: September 1999 to June 2000 Duration of trial participation: 24 weeks Inclusion criteria: · AD affecting at least 5 % of total BSA · IGA score 3 or 4 Exclusion criteria: · PUVA, high dose UVA, systemic therapy with corticosteroids, immunosuppressants or cytostatics in previous 3 months · Topical therapies for AD in previous 2 weeks · Systemic antibiotics in previous 2 weeks · Systemic steroids for indications other than AD in previous month · Pregnancy, lactation, women of childbearing age not using reliable contraception · Need for treatment with potent topical steroids · Severe concurrent allergic diseases · Diseases associated with immunosuppression or malignancy · Presence of skin conditions that could affect evaluation of study treatment · Active skin infections requiring treatment with a prohibited medication or active herpes simplex infections Additional design details: none |
| Participants | Total number randomised: 192 participants (96 to apply the intervention and 96 to apply the comparator) Age: 18 to 69 years Sex: intervention group, male n = 36, female n = 60; comparator group, male n = 41, female n = 55 Ethnicity, duration of eczema: not reported Severity of eczema: moderate‐to‐severe Body site: not reported Number of withdrawals: comparator group 37.5%; intervention group 22.9%. Withdrawals due to unsatisfactory therapeutic effect: comparator group 27.1%; intervention group 15.6% Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1 % cream twice daily for 24 weeks · Concurrent treatment: not reported · Other key information: none Comparator: vehicle twice daily for 24 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: prednicarbate 0.25 % cream was permitted as a rescue therapy for a maximum of 7 days twice daily, followed by a further 7 days once daily. Cetirizine was also permitted (no details). Notes: none |
| Outcomes | · IGA · EASI · Pruritis severity · Patient self‐assessment · Quality of life DLQI · Quality of life QoLIAD · AE |
| Notes | Funding source: Novartis Pharma Declarations of interest: three authors were employees of Novartis Pharma. Original language of publication: English Other: none |
Meurer 2010.
| Study characteristics | |
| Methods | Trial design: randomised, multicentre, parallel study Trial registration number: CASM981C2439 Country: US Outpatient or hospital: 65 centres: Italy n = 10, USA n = 15, South Africa n = 5, Canada n = 7, Germany n = 28 Date trial conducted: May 2005 to January 2007 Duration of trial participation: 4 weeks Inclusion criteria: · Aged 2 or above and under 17 years · Severe AD as defined by a whole body IGA score of 4.0 or greater · Disease affecting 5% or greater of the total BSA, excluding the face Exclusion criteria: patients with abnormal HPA‐axis function Additional design details: a 4‐week randomised treatment period was followed by a 12‐week observational period. |
| Participants | Total number randomised: 376 participants (193 to apply the intervention and 183 to apply the comparator) Age: mean 8.3 years (SD 4.61), age group less than 2 years n = 1, 2 to 12 years n = 287, 13 to 17 years n = 85, median 8 years (range 1 to 17) Sex: 50.7% male, 49.3% female Ethnicity: Asian 8.3%, black 9.4%, white 61.9%, Native American 1.1%, other 19%, Pacific Islander 0.3% Duration of eczema: not reported Severity of eczema: based on IGA, 81.8% had severe disease; 17.2% very severe Body site: not reported Number of withdrawals: 10, 5 in each group. Reasons: AE n = 1, lost to follow‐up n = 4, protocol violation n = 1, subject withdrew consent n = 2, unsatisfactory therapeutic effect n = 2 Notes: none |
| Interventions | Run‐in details: phototherapy and systemic therapies were prohibited for 4 weeks. Topical therapy with pimecrolimus, tacrolimus, tar or corticosteroids were also not allowed for 7 days. Intervention: pimecrolimus 1% cream combined with TCS used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream combined with TCS used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: fluticasone propionate 0.5% (or hydrocortisone acetate cream 1% for face, neck and intertriginous areas, if needed) Notes: none |
| Outcomes | · Incidence rates of AEs . Time to relapse of AD during the observation period . Time to AD treatment success . AD treatment success and improvement . Improvement in extent and severity of AD . Relief of pruritus (success defined as 0 = absent or 1 = mild) . Improvement in health‐related QoL over baseline (measured by the PIQoL‐AD for participants aged 2 to 12 years only) |
| Notes | Funding source: Novaritis Declarations of interest: authors affiliation to Novartis Original language of publication: English Other: none |
Mobacken 1986.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: not reported Country, outpatient or hospital, date trial conducted: not reported Duration of trial participation: 21 days Inclusion criteria: · Aged 3 to 16 years old · Eczema of at least 2 months' duration · Sum of severity scores ≥ 5. ≥ 2 signs/symptoms had to be moderate in severity (physician chose a test site, excluding palms, soles or scalp, for evaluation and graded the severity of erythema, induration, pruritus, scaling and excoriation from 0 = absent to 3 = severe) Exclusion criteria: · Tuberculosis of the skin or viral infections with skin lesions · Systemic therapy for eczema within 4 weeks · Topical therapy within 2 weeks · Requirement for additional topical or systemic medication that could affect the disease course · Participants using occlusive dressings Additional design details: none |
| Participants | Total number randomised: 60 participants (30 to apply the intervention and 30 to apply the comparator) Age: alclometasone group mean 9.1 years, range 3 to 16; hydrocortisone group mean 10 years, range 3 to 16 Sex: alclometasone group, male n = 13, female n = 16; hydrocortisone group, male n = 15, female n = 14 Ethnicity: not reported Duration of eczema: alclometasone group, mean 5.5 years (range: 0.2 to 15); hydrocortisone group, 5.9 years (0.3 to 15) Severity of eczema: mean sign/symptom scores: alclometasone 8.28, hydrocortisone 8.28. Body site: aclometasone group, extremities n = 27, chest n = 1, back n = 1; hydrocortisone group, extremities n = 28, abdomen n = 1 Number of withdrawals: one participant from each group withdrew. One female in the alclometasone group did not return for the final visit due to a fever. One female from the hydrocortisone group returned early for the final visit because the eczema was "almost unchanged". Notes: none |
| Interventions | Run‐in details: none Intervention: alclometasone dipropionate 0.05% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone 1% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator; no treatment was applied at least 3 hours prior to physician evaluation at interim and final visits. No medication other than the test medication was applied to the test site. Tar baths, UV light or Grenz ray therapy or antipruritic medications were not allowed. Notes: thin coat of medication was applied without occlusion. |
| Outcomes | · Severity of signs/symptoms. The physician chose a test site, excluding palms, soles or scalp, for evaluation for each participant. The physician graded the severity of erythema, induration, pruritus, scaling and excoriation at this site as 0 = absent, 1 = mild, 2 = moderate or 3 = severe. · IGA defined as (1) cleared: 100% clearance except for residual discolouration, (2) marked improvement: between 75% and 100% clearance of signs and symptoms, (3) moderate improvement: between 50% and 75% clearance of symptoms, (4) slight improvement: < 50% clearance of signs and symptoms, (5) no change: no detectable improvement form baseline evaluation, and (6) exacerbation: flare at treatment site · AE volunteered by the participant or determined by the physician |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Mowla 2020.
| Study characteristics | |
| Methods | Trial design: randomised study Trial registration number: not reported Country: Bangladesh Outpatient or hospital: department of Dermatology and Venereology, Chittagong Medical College Hospital Date trial conducted: not reported Duration of trial participation: 16 weeks Inclusion criteria: · Aged 2 to 10 years · Mild‐to‐moderate AD involved 50% or less of BSA Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 200 participants Age, sex, ethnicity, duration of eczema, severity of eczema, body site: not reported Number of withdrawals: 24, no reasons provided Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.1% ointment used for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: clobetasone butyrate 0.05% ointment used for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI score · IGA |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Mudaliyar 2020.
| Study characteristics | |
| Methods | Trial design: randomised study Trial registration number: not reported Country: India Outpatient or hospital: the Department of Pharmacology in collaboration with the Department of Dermatology, UPUMS Saifai, Etawah Date trial conducted: not reported Duration of trial participation: 56 days Inclusion criteria: · Age 2 to 16 years of either sex · AD according to the Hanifin‐Rajka criteria Exclusion criteria: · Any form of dermatitis other than AD · Untreated bacterial, fungal or viral skin lesion, or history of allergic reaction in the past to topical medications employed in this study Additional design details: to quote; "we divided our study into 2 phases in order to assess the efficacy and safety of topical treatment in the acute flare‐up phase followed by a 4‐week maintenance phase." |
| Participants | Total number randomised: 37 participants (19 to apply the intervention and 18 to apply the comparator) Age: overall, mean 10.13 years (SD 3.61): fluticasone group, 9.84 years (SD 3.78); tacrolimus group, 10.44 years (SD 3.50) Sex: male:female: fluticasone group 12:7; tacrolimus group 9:9 Ethnicity, duration of eczema: not reported Severity of eczema: according to SCORAD: fluticasone group, mean 38.75 (SD 4.14); tacrolimus, mean 36.60 (SD 4.45) Body site: not reported Number of withdrawals: 2 children from the fluticasone group discontinued the study, as they were satisfied with lesion improvements and did not show interest in continuing the treatment for one additional month. Notes: none |
| Interventions | Run‐in details: not reported Intervention: fluticasone 0.005% ointment used twice daily for 56 days · Concurrent treatment: not reported · Other key information: none Comparator: tacrolimus 0.03% ointment used once daily for 56 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · SCORAD · Subjective items captured by SCORAD (sleepiness, pruritus) · QOL |
| Notes | Funding source: none Declarations of interest: no conflicts Original language of publication: English Other: none |
Munro 1967.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, within‐participant study Trial registration number: not reported Country: UK Outpatient or hospital: outpatients from 3 different centres Date trial conducted: not reported Duration of trial participation: approximately 1 week; to quote: "patients were normally reviewed after 7 days, though some were seen more frequently, and a few at slightly longer intervals". Inclusion criteria: individuals with approximately symmetrical eczema (right and left sides) Exclusion criteria: not reported Additional design details: 43 participants with psoriasis were not extracted as not eligible for this review. |
| Participants | Total number randomised: 132 sides treated (66 to apply the intervention and 66 to apply the comparator) Age, sex: not reported separately for eczema‐only participants Ethnicity, duration of eczema, severity of eczema, body site: not reported Number of withdrawals: 2 participants initially included failed to carry out the instructions given and were excluded; it is not clear if these were eczema or psoriasis participants. Notes: none |
| Interventions | Run‐in details: not reported Intervention: betamethasone valerate 0.10% ointment used twice daily for 7 days · Concurrent treatment: not reported · Other key information: betnovate (soft paraffin and hydrogenated lanolin base) Comparator: fluocortolone/fluocortolone caproate 0.25% ointment used once daily for 7 days · Concurrent treatment: not reported · Other key information: ultralanum plain (base was soft paraffin aqueous emulsion containing 30% water) Concurrent treatments received alongside both intervention and comparator; mild sedatives or antihistamines were administered if indicated. Notes: 29 participants were treated with occlusion with polyethylene film where thought to be necessary; 37 participants were not. |
| Outcomes | · Participant's comparison between the two treatments in improvement in itching or other symptoms (healed, improved, static or worse) · Dermatologist's comparison between the two treatments in improvement in erythema, scaling, induration, and oedema (healed, improved, static or worse) |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Munro 1975.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number, country, outpatient or hospital, date trial conducted, duration of trial participation: not reported Inclusion criteria: participants are stated to have bilateral approximately symmetrical lesions of eczema. Exclusion criteria: not reported Additional design details: this study had three different concentrations of moderate TCS versus placebo extracted as if one pairwise comparison, but with outcome data split by concentration. |
| Participants | Total number randomised: 409 participants in the results table; unclear how many sides were randomised Age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: clobetasone butyrate 0.01%, 0.05% cream · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone 1% cream used (unspecified frequency) for (unspecified duration) · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator; occlusive dressings were not used. Notes: none |
| Outcomes | · Number of participants in which moderate was deemed better, mild better or both deemed equal · 'Untoward' effects were reported in the discussion, but it was unclear which subsection of participants it related to. |
| Notes | Funding source: not reported Declarations of interest: one author is affiliated with Glaxo Laboratories LTD. Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. A second trial included in this paper was not in scope for our review as it was not clearly randomised |
Murrell 2007.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: NCT00121316 Country: USA, Canada, France and Switzerland Outpatient or hospital: not reported Date trial conducted: October 2004 to July 2005 (including 6‐week open‐label phase) Duration of trial participation: up to 42 days in RCT Inclusion criteria: · Aged 12 years or over with AD according to Hanifin and Rajka criteria · Mild‐to‐moderate facial AD (IGA of 2 or 3) · Dependent on, or intolerant of, TCS defined by either a cutaneous reaction preventing use of TCS, or an AE resulting from TCS therapy Exclusion criteria: · AD on > 30% of total BSA in addition to facial eczema · Concurrent skin disease in the study area; active skin infections, or other conditions that might interfere with evaluation · Immunocompromised patients or patients with a history of malignant disease (except treated basal cell carcinoma) · Poor clinical responders or those hypersensitive to pimecrolimus cream · Received phototherapy or systemic therapy known or suspected to have an effect on AD within 4 weeks prior to the start of the trial Additional design details: if participants' condition was resolved before 42 days, they left the trial. |
| Participants | Total number randomised: 200 participants (101 to apply the intervention and 99 to apply the comparator) Age: intervention group, mean 33.8 years (SD 15.42), range 12 to 78; vehicle group, mean 32.7 years (SD 15.80), range 12 to 81) Sex: overall: females:males 61.5: 38.5%; vehicle group, females 66.7%; intervention group, females 56.4% (not statistically significant between groups) Ethnicity: intervention group, 88.1% white, 2% black, 5.9% oriental, 4% other; vehicle group, 86.9% white, 1% black, 8% oriental, 4% other Duration of eczema: not reported Severity of eczema: facial IGA score, intervention group, mild 42.6%, moderate 57.4%; vehicle group, mild 35.4%, moderate 64.6% Body site: Facial AD with AD on 30% or less of TBA Number of withdrawals: intervention group, 26.7%: because of AE 5%, unsatisfactory therapeutic effect 11.9%, patient's condition no longer required study drug 5.9%, protocol violation 1%, patient withdrew consent 1%, lost to follow‐up 2%, administrative problems, death, other 0%; vehicle group, 60.6%: because of AEs 6.1%, unsatisfactory therapeutic effect 44.4%, patient's condition no longer required study drug 1%, protocol violation 2%, patient withdrew consent 5.1%, lost to follow‐up 2%, administrative problems, death, other 0% Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream twice daily for 42 days · Concurrent treatment: not reported · Other key information: treatment until total clearance of their facial AD (IGA = 0) or for a maximum of 6 weeks Comparator: vehicle cream twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Facial IGA score assessed at week 6. Treatment success was defined as a facial IGA of 0 or 1 indicating clearance or almost clearance of AD. · Overall EASI, head and neck EASI score · Pruritus score. A four‐point score recording pruritus over the previous 24 hours (0, absent; 1, mild; 2, moderate; 3, severe) as assessed by the investigator in discussion with the subject or caregiver · Patients achieving at least a 60% reduction from baseline in overall EASI or head and neck EASI score at week 6 were defined as responders. · Clinical evaluation of skin atrophy and telangiectasia on the face was performed using dermatoscopy. Atrophy and telangiectasia were evaluated using a five‐point scale according to Frosch 1981. · Eyelid dermatitis score ranging from 0 to 15 represented the sum of the scores for each of the five clinical signs (erythema, lichenification, pruritus, scaling ⁄ dryness, oozing ⁄ crusting) each graded from 0 (clear ⁄ absent) to 3 (severe) · Head and neck pruritus score ranging from 0 (no pruritus) to 3 (severe pruritus) |
| Notes | Funding source: not reported Declarations of interest: two authors were employed by Novartis. Original language of publication: English Other: none |
Nakagawa 1997.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre study Trial registration number: not reported Country: Japan Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 21 days Inclusion criteria: adults with AD Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 212 participants Age: adult Sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention one: tacrolimus 0.03% ointment used for 21 days · Concurrent treatment: not reported · Other key information: none Intervention two: tacrolimus 0.1% ointment used for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Intensities of erythema, papules, lichenification and pruritus · AE |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Nakagawa 1998.
| Study characteristics | |
| Methods | Trial design: randomised study Trial registration number: not reported Country: Japan (assumed) Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 7 weeks Inclusion criteria: AD with face and neck lesions Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: not reported Age, sex, ethnicity, duration of eczema, severity of eczema: not reported Body site: face and neck Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.1% ointment · Concurrent treatment: not reported · Other key information: none Comparator: alclometasone dipropionate 0.1% ointment · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Number cleared or markedly improved |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Nakagawa 2018a.
| Study characteristics | |
| Methods | Trial design: randomised, parallel study Trial registration number: JapicCTI‐152887 Country: Japan Outpatient or hospital: 38 medical institutions in Japan Date trial conducted: May 2015 onwards Duration of trial participation: 4 weeks Inclusion criteria: · Japanese patients aged 16 to 65 years · Diagnosis of moderate‐to‐severe AD according to criteria of the Japanese Dermatological Association · mEASI score ≥ 10 · IGA of ≥ 3 · BSA 10 to 30% Exclusion criteria: · Biologics within 24 weeks of the study · Mild‐to‐potent corticosteroids, tacrolimus, or anti‐inflammatory drugs within 7 days · Very potent corticosteroids, immunosuppressants and live vaccines within 28 days · Active infection at the application site · Tuberculosis, hepatitis, HIV infection Additional design details: to quote: "this study was not under double‐blind conditions in the JTE‐052 and vehicle groups because the appearance of each strength of the JTE‐052 ointments and vehicle ointment was different. However, to keep the investigators and patients blinded concerning the study treatment, the following methods were performed: the designated site personnel other than the investigators dispensed the study drugs and collected them from patients, and neither investigators nor patients were informed about the appearance of the ointments. The tacrolimus group was under open‐label conditions; neither investigators nor patients were blinded concerning the study treatment, as this group was included in the study as a reference group for exploratory comparison with the JTE‐052 groups." |
| Participants | Total number randomised: 327 participants (69 to apply intervention one, 65 each intervention two and four, 66 intervention three, 30 intervention five, and 32 to apply the comparator) Age: mean for each group: vehicle: 31.6 years, 0.25%: 31.5 years, 0.5%: 29.5 years, 1%: 28.6 years, 3%: 32.3 years; tacrolimus: 33.1 Sex: per group: vehicle, male n = 19, female n = 12; 0.25% male n = 48, female n = 21; 0.5% male n = 39, female n = 26; 1% male n = 48, female n = 18: 3% male n =4 2, female n = 23; tacrolimus male n = 23, female n = 14 Ethnicity: all participants were Japanese Duration of eczema: average per group: vehicle 25 years; 0.25% 23.4 years; 0.5% 20.9 years; 1% 20.8 years; 3% 25.2 years; tacrolimus 23.0 years Severity of eczema: per group: vehicle: moderate n = 26, severe n = 5; 0.2 5% moderate n = 61, severe n = 8; 0.5% moderate n = 61, severe n = 4; 1% moderate n =5 6; severe n = 10; 3% moderate n = 56, severe n = 9; tacrolimus: moderate n = 12, severe n = 4 Body site: unspecified; however, both facial IGA and IGA were mentioned. Number of withdrawals: per group: vehicle, n = 3 due to worsening of AD, n = 1 withdrawal by patient, n = 2 lost to follow‐up; 0.25%, n = 1 AE, n = 1 worsening of AD, n = 1 lost to follow up; 0.5%, 1 AE, n = 1 pregnancy, 1%, n = 1 AE, 1 worsening of AD, n = 1 withdrawal by patient; 3%, 1 lost to follow‐up Notes: none |
| Interventions | Run‐in details: not reported Intervention one: delgocitinib 0.25% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention two: delgocitinib 0.5% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention three: delgocitinib 1% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention four: delgocitinib 3% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention five: tacrolimus 0.1% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI · EASI‐75 · IGA binary · IGA continuous · PP‐NRS · Withdrawal due to AE |
| Notes | Funding source: Japan Tobacco Inc. Declarations of interest: one author was a consultant and/or has received research grants and/or honoraria from Japan Tobacco Inc., LEO Pharma and Maruho Co. Ltd; another has received honoraria from Japan Tobacco Inc. and two were employees of Japan Tobacco Inc. Original language of publication: English Other: none |
Nakagawa 2018b.
| Study characteristics | |
| Methods | Trial design: randomised, parallel study Trial registration number: not reported Country: Japan Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 8 days Inclusion criteria: adults with AD Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 20 participants (8 to apply intervention one, 8 intervention two, and 4 TO apply the comparator) Age: mean: vehicle group: 24.8 years (SD 5.6) range 21 to 33; 1% group: 26.5 years (SD 5.4) range 21 to 33; 3% group: 30 years (SD 8.2) range 21 to 43 Sex: male n = 18, female n = 2 Ethnicity: all participants were Japanese. Duration of eczema: mean duration: vehicle group, 18.8 years (SD 10.8); 1% group, 20.3 years (SD 9.2); 3% group, 26.4 years (SD 7.3) Severity of eczema: vehicle: moderate n = 14; severe n = 6, 0, based on EASI and IGA scores Body site: not reported Number of withdrawals: one due to personal reasons (in 3% group) Notes: none |
| Interventions | Run‐in details: not reported Intervention one: delgocitinib 1% ointment used twice daily for 7 days · Concurrent treatment: not reported · Other key information: none Intervention two: delgocitinib 3% ointment used twice daily for 7 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for 7 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator; rescue medication of hydrocortisone butyrate ointment 0.1% could be used for the treatment of worsening of AD. Notes: 1% and 3% JTE‐052 ointments were applied in the morning and the evening with a 12‐hour interval in a stepwise approach with two different dose groups. 5 g of JTE‐052 was applied to the areas affected by inflammatory eczema, excluding the head, neck, palm, sole, groin and genitals. The application area had to be the same throughout the study and JTE‐052 ointment was removed in the morning on day 8. The study did not report that this was undertaken in the same way for the placebo group, but we assumed that it was. |
| Outcomes | · Safety · Efficacy using IGA, EASI and pruritus · |
| Notes | Funding source: Japan Tobacco Inc. Declarations of interest: one author was a consultant and/or has received research grants and/or honoraria from Japan Tobacco Inc., LEO Pharma and Maruho Co. Ltd; another has received honoraria from Japan Tobacco Inc and two were employees of Japan Tobacco Inc. Original language of publication: English Other: none |
Nakagawa 2019.
| Study characteristics | |
| Methods | Trial design: randomised study Trial registration number: JapicCTI‐173553 Country: Japan Outpatient or hospital: 21 medical institutions Date trial conducted: not reported Duration of trial participation: 4 weeks Inclusion criteria: · Aged 2 to 15 years · AD and EASI score ≥ 5 · IGA score ≥ 2 (mild) · 5 to 30% BSA Exclusion criteria: · Active infection · History of tuberculosis · Hepatitis B/C · History or presence of a malignant tumour · Corticosteroid 28 days before baseline · Weak corticosteroids or tacrolimus 7 days before baseline Additional design details: none |
| Participants | Total number randomised: 103 participants (34 each to apply the intervention one and two, and 35 to apply the comparator) Age: 2 to 15 years Sex: per group: vehicle: female n = 17, male n = 18; 0.25%: female n = 12, male n = 22; 0.5%: female n = 16, male n = 18 Ethnicity: not reported Duration of eczema: average duration of AD in years per group: vehicle, 6.4; 0.25%, 6.1; 0.5%, 6.6 Severity of eczema: IGA ≥ 2 Body site: not reported Number of withdrawals: per group: vehicle: n = 2 AE, n = 2 worsening of AD, n = 1 withdrawal by patient; 0.25%: no withdrawals; 0.5%: no withdrawals Notes: none |
| Interventions | Run‐in details: not reported Intervention one: delgocitinib 0.25% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention two: delgocitinib 0.5% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI · EASI‐75 · IGA · Itch |
| Notes | Funding source: Japan Tobacco Declarations of interest: authors reported conflicts of interest relating to Japan Tobacco. Original language of publication: English Other: none |
Nakagawa 2020.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind study Trial registration number: JapicCTI‐173554 Country: Japan Outpatient or hospital: 24 medical institutions in Japan Date trial conducted: not reported Duration of trial participation: 4 weeks Inclusion criteria: · Japanese patients ≥ 16 years · AD according to Japanese Dermatological Association · mEASI score of 10 or greater · IGA score of 3 (moderate) or 4 (severe) · Affecting 10 to 30% BSA Exclusion criteria: · Active infections, hepatitis or HIV infection · Biologic and strongest corticosteroids within 24 weeks of the trial · Medium and weak corticosteroids within 7 days of the trial Additional design details: none |
| Participants | Total number randomised: 158 participants (106 to apply the intervention and 52 to apply the comparator) Age: mean 31.7 years; delgocitinib group, 31.4 years; vehicle group, 32.3 years Sex: delgocitinib group, female 39.6%, male 60.4%; vehicle group, female 34.6%, male 65.4% Ethnicity: not reported Duration of eczema: mean 24.8 years; delgocitinib group, 24.7 years, vehicle group, 24.8 years Severity of eczema: IGA score of 3 (moderate 69%) or 4 (severe 31%) Body site: not reported Number of withdrawals: vehicle group, n = 2 worsening of AD, n = 1 withdrawal by patient; delgoocitinib group, no withdrawals Notes: vehicle group, 20 shifted during study to part 2 of the research programme due to worsening AD; delgocitinib group, 8 shifted to part 2 of the research programme due to worsening AD |
| Interventions | Run‐in details: not reported Intervention: delgocitinib 0.5% ointment used twice daily for 28 days Concurrent treatment: not reported Other key information: none Comparator: vehicle ointment used twice daily for 28 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI · EASI‐75 · IGA continuous · PP‐NRS |
| Notes | Funding source: Japan Tobacco Inc. Declarations of interest: three authors received consulting and/or speaker honoraria and/or were members of an advisory board and/or received research grants from various pharmaceutical companies including study funders. Two other authors were employees of Japan Tobacco Inc. Original language of publication: English Other: none |
Nakagawa 2021.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: JapicCTI‐184064 Country: Japan Outpatient or hospital: 23 medical institutions in Japan, not reported if outpatient or hospital Date trial conducted: not reported Duration of trial participation: 4 weeks Inclusion criteria: · AD diagnosis according to Japanese Dermatological Association · mEASI score ≥ 5 · IGA score of 2 (mild), 3 (moderate) or 4 (severe) · 5‐30% BSA Exclusion criteria: · Active infections, hepatitis or HIV infection · Biologic and strongest corticosteroids within 24 weeks of the trial · Medium and weak corticosteroids within 7 days of the trial Additional design details: 52‐week extension period (part 2) where all participants received the intervention treatment |
| Participants | Total number randomised: 137 participants (69 to apply the intervention and 68 to apply the comparator) Age: delgocitinib group, 2 to 6 years 37.7%, 7 to 11 years 40.6%, 12 to 15 years 21.7%; vehicle group, 2 to 6 years 39.7%, 7 to 11 years 36.8%, 12 to 15 years 23.5% Sex: delgocitinib group, female 43.5%, male 56.5%; vehicle group, female 54.4%, male 45.6% Ethnicity: not reported Duration of eczema: delgocitinib group, average duration 5.8 years; vehicle group, average duration 6.2 years Severity of eczema: IGA score of 2 (mild), 3 (moderate) or 4 (severe) on a scale of 0‐5 Body site: not reported Number of withdrawals: vehicle group, 1 due to protocol deviation; delgocitinib group, no withdrawals Notes: none |
| Interventions | Run‐in details: not reported Intervention: delgocitinib 0.25% ointment twice daily for 28 days Concurrent treatment: not reported Other key information: none Comparator: vehicle ointment twice daily for 28 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI · EASI‐75 · IGA binary · PP‐NRS continuous |
| Notes | Funding source: Funded by Japan Tobacco Inc and Torii Pharmaceutical Co Declarations of interest: most of the authors received consulting fees from Japan Tobacco Inc. Original language of publication: English Other: none |
Natarajan 1974.
| Study characteristics | |
| Methods | Trial design: randomised, multicentre, double‐blind study Trial registration number: not reported Country: India Outpatient or hospital: attending the department of Dermatology, Government Stanley Hospital Date trial conducted, duration of trial participation: not reported Inclusion criteria: · Ambulatory male or female · Aged 12 to 75 years · Eczematous conditions (AD, contact dermatitis, lichen simplex chronicus, and eczematous dermatosses of hands and feet) Skin involvement of less than 27% Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 3 participants (1 to apply the intervention and 2 to apply the comparator) Age: no detail per type of dermatosis Sex: not reported for eczema only Ethnicity, duration of eczema: not reported Severity of eczema: not reported for eczema only Body site: not reported Number of withdrawals: 0 Notes: in total, 100 participants were included, of which 3 had AD. |
| Interventions | Run‐in details: not reported Intervention: triamcinolone acetonide 0.1% ointment used twice daily for 7 days · Concurrent treatment: not reported · Other key information: none Comparator: desoximetasone 0.25% ointment used twice daily for 7 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator; bathing was permitted before the application of ointments in the morning. Notes: none |
| Outcomes | · Itching cleared · Lichenification cleared · Global improvement · Local AE |
| Notes | Funding source: Hoechst Pharmaceuticals Limited supplied the drug. Declarations of interest: not declared Original language of publication: English Other: none |
NCT00810862.
| Study characteristics | |
| Methods | Trial design: randomised, single‐blind, within‐participant study Trial registration number: NCT00810862 Country: not reported Outpatient or hospital: not reported Date trial conducted: November 2006 to June 2008 Duration of trial participation: three weeks Inclusion criteria: · African‐American children aged 2 to 17 years · Mild‐to‐moderate AD Exclusion criteria: · mEASI < 3 · Allergy to elidel or components · Oral steroids, immunosuppressive agents, cytostatics or phototherapy within 4 weeks · Continuous or noncontinuous use of pimecrolimus or tacrolimus for > 11 months within 2 weeks · Active skin infections · Immunocompromised · History of skin cancer or lymphoma · Hypopigmentation in study areas · Pregnancy or breastfeeding Additional design details: none |
| Participants | Total number randomised: 36 sides treated (18 to apply the intervention and 18 to apply the comparator) Age: 2 to 17 years Sex: male or female Ethnicity: African‐American Duration of eczema: not reported Severity of eczema: mild‐to‐moderate Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream twice daily for 21 days Concurrent treatment: not reported Other key information: none Comparator: vehicle cream twice daily for 21 days Concurrent treatment: not reported Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · mEASI · Modified IGA · Hypopigmentation |
| Notes | Funding source: Children's Hospital of Michigan Declarations of interest: not declared Original language of publication: English Other: early termination of study due to lack of enrolment |
NCT00927212.
| Study characteristics | |
| Methods | Trial design: randomised, quadruple‐blinded (participant, care provider, investigator, outcomes assessor), single‐centre, parallel study Trial registration number: NCT00927212 Country: Israel Outpatient or hospital: Dermatology Department, Sheba Medical Center, Ramat‐Gan Date trial conducted: June 2009 to September 2010 Duration of trial participation: 10 weeks (6‐week treatment phase and 4‐week follow‐up phase) Inclusion criteria: · Diagnosis of AD ≥ 6 months prior to the study · BSA ≤ 20% · Male and female ≥ 18 · "Adequate" general health Exclusion criteria: · Pregnancy or breastfeeding · Concomitant dermatologic or medical condition(s) which may interfere with the patient's response evaluation · Evidence of infection in the target areas · Known sensitivity to any drug component · Immunocompromised patients · Concomitant medications such as: ‐ topical corticosteroid within 2 weeks of the study ‐ systemic steroids and immunosuppressants within 1 month of the study ‐ systemic anti‐histamines and antibiotics within 2 weeks of the study ‐ phototherapy within 4 weeks of the study ‐ anticipated exaggerated exposure to sunlight during the treatment period and 4 weeks prior Additional design details: the study was terminated early, to quote: "for 'sponsors' considerations" |
| Participants | Total number randomised: 5 participants Age: ≥ 18 years Sex: male or female Ethnicity: not reported Duration of eczema: ≥ 6 months Severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: aS101 2% ointment twice daily for 6 weeks · Concurrent treatment: not reported · Other key information: none Comparator: aS101 4% ointment twice daily for 6 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Modified SCORAD · Remission period |
| Notes | Funding source: BioMAS Ltd Declarations of interest: not declared Original language of publication: English Other: unclear if the intervention was an anti‐inflammatory |
NCT00996008.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: NCT00996008 Country: Switzerland Outpatient or hospital: 5 hospitals Date trial conducted: November 2009 to October 2010 Duration of trial participation: 14 days Inclusion criteria: · Males and females > 18 years · Mild‐to‐moderate active AD (EASI 2 or 3; visible eczema, erythema and pruritus) · ≥ four symmetrical target lesions at inclusion Exclusion criteria: · If female of childbearing potential: not using an adequate and appropriate form of contraception such as oral contraceptives; intra‐uterine device; contraceptive injection, implant or patch · Pregnancy, breastfeeding, or intention to become pregnant during the study period · Allergy to any component of the study medications · Topical corticosteroids or other topical treatments for AD within 2 weeks of the study · Systemic treatment for atopic dermatitis within 4 weeks of the study · Presence of major medical illness requiring systemic therapy, including cancers · Clinical diagnosis of bacterial skin infection · Clinically relevant ECG abnormality or significant abnormal clinical laboratory tests · Any investigational drug or clinical study within three months of the study · History of drug, alcohol or other substance abuse or other factors limiting the ability to comply with this protocol Additional design details: terminated due to slow recruitment before target enrolment was achieved |
| Participants | Total number randomised: 15 participants Age: adult patients were eligible Sex, ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐moderate severity eczema was eligible Body site, number of withdrawals: not reported Notes: study terminated early due to poor recruitment |
| Interventions | Run‐in details: not reported Intervention: CT 327 cream twice daily for 14 days · Concurrent treatment: not reported · Other key information: TrkA Kinase Inhibitor Comparator: vehicle cream twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Improvements from baseline in modified EASI · Proportion of subjects with modified EASI‐75 |
| Notes | Funding source: Creabilis SA Declarations of interest: not declared Original language of publication: English Other: No outcome data reported |
NCT01037881.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre study Trial registration number: NCT01037881/EudraCT Number 2009‐013792‐22/LEO 29102‐C21 Country: Canada, Finland, Germany Outpatient or hospital: not reported Date trial conducted: December 2009 to June 2010 Duration of trial participation: 4 weeks Inclusion criteria: · AD according to Hanifin and Rajka criteria · IGA assessment scored as mild (2) to moderate (3) · Treatment lesions located on the trunk and limbs and involving 3% to 10% of the total BSA · Either gender between 18 years and 65 years of age Exclusion criteria: · Systemic treatment with immunosuppressive drugs or corticosteroids within 6 weeks · Topical treatment with immunomodulators within 2 weeks · Topical treatment with corticosteroids from WHO groups II, III or IV within 1 week · Use of topical or systemic antibiotics within 2 weeks · PUVA or UVB therapy within 4 weeks · Clinical infection (viral, fungal or bacterial) in the treatment area · Known or suspected severe renal insufficiency or hepatic disorders · History of an immunocompromised disease (e.g. lymphoma, HIV, Wiskott‐Aldrich Syndrome) · Concomitant serious disease which might affect the AD treatment in this trial · Females who are pregnant or are breastfeeding · Females intending to temporarily or permanently stop their hormonal contraceptive regimen during and up to one month post‐study termination visit Additional design details: none |
| Participants | Total number randomised: 183 participants (24 to apply intervention one, 25 intervention two, three and six, 29 intervention four, 30 intervention five, and 25 to apply the comparator) Age: mean per group: vehicle 39.1 years (SD 14.0); LEO29102 0.0003% 36.2 years (SD 14.4); LEO29102 0.001% 34.9 years (SD 11.8); LE029102 0.00 3% 38.3 years (SD 12.6); LE029102 0.10% 32.0 years (SD 10.0); LE029102 0.25% 35.5 years (SD 13.7); primecrolimus 29.9 years (SD 8.8) Sex: female:male% per group: vehicle 44.0:56.0; LEO29102 0.0003% 33.3:66.7; LEO29102 0.001% 40.0:60.0; LE029102 0.003% 16.0:84.0; LE029102 0.10% 51.7:48.3; LE029102 0.25% 43.3:56.7; primecrolimus 36.0:64.0 Ethnicity, duration of eczema, severity of eczema: not reported Body site: not reported Number of withdrawals: n = 20 Notes: none |
| Interventions | Run‐in details: not reported Intervention one: LEO29102 0.0003% cream used twice daily for up to 28 days · Concurrent treatment: not reported · Other key information: none Intervention two: LEO29102 0.001% cream used twice daily for up to 28 days · Concurrent treatment: not reported · Other key information: none Intervention three: LEO29102 0.003% cream used twice daily for up to 28 days · Concurrent treatment: not reported · Other key information: none Intervention four: LEO29102 0.1% cream used twice daily for up to 28 days · Concurrent treatment: not reported · Other key information: none Intervention five: LEO29102 0.25% cream used twice daily for up to 28 days · Concurrent treatment: not reported · Other key information: none Intervention six: pimecrolimus 1% cream used twice daily for up to 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for up to 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI Score · Number of participants that were symptom‐free responders at follow‐up using IGA · Participants' assessment of pruritus on trunk and limbs · Participants' overall assessment of disease severity · Application site reactions |
| Notes | Funding source: Leo Pharma Declarations of interest: not declared Original language of publication: English Other: unclear if the intervention is an anti‐inflammatory |
NCT01053247.
| Study characteristics | |
| Methods | Trial design: randomised, quadruple‐blind, multicentre, parallel study Trial registration number: NCT01053247 Country: US and Central America Outpatient or hospital: not reported Date trial conducted: January 2008 to August 2009 Duration of trial participation: 2 weeks Inclusion criteria: · AD, otherwise in good health · Percent BSA minimum requirements (unspecified) · ≥ 18 years old Exclusion criteria: · Pregnancy, breastfeeding, or planning a pregnancy during the study · Any other systemic or dermatological disorders Additional design details: this study compared a branded versus generic tacrolimus 0.1% ointment to placebo. For the purposes of this study, the trial was treated as a pairwise comparison of tacrolimus 0.1% ointment versus placebo, the data from both tacrolimus arms being pooled together. |
| Participants | Total number randomised: 793 participants (529 to apply the interventions and 264 to apply the comparator) Age: mean: unbranded intervention group 43.0 years (SD 16.29); branded intervention group 43.7 years (SD 16.74); vehicle group 43.3 years (SD 17.14) Sex: females:males: unbranded intervention group 148:121; branded intervention group 158:102; vehicle group 161:103 Ethnicity, duration of eczema, severity of eczema, body site: not reported Number of withdrawals: intervention groups n = 39, participant withdrawal n = 13, withdrawal because of lack of efficacy n = 1, protocol violation n = 3, lost to follow‐up n = 13, AE n = 9; vehicle group n = 28, participant withdrawal n = 15, lack of efficacy n = 1, protocol violation n = 2, lost to follow‐up n = 7, AE n = 3 Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.1% ointment twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA success · Change in clinical signs and symptoms compared to baseline · Change in pruritus compared to baseline · Change in% BSA compared to baseline · Local AE ‐ application site reaction · Withdrawal due to AE |
| Notes | Funding source: Fougera Pharmaceuticals Inc Declarations of interest: not declared Original language of publication: English Other: none |
NCT01139450.
| Study characteristics | |
| Methods | Trial design: randomised, quadruple‐blind, multicentre, parallel study Trial registration number: NCT01139450 Country: US and Central America Outpatient or hospital: not reported Date trial conducted: January 2009 to August 2009 Duration of trial participation: 4 weeks Inclusion criteria: · AD, otherwise in good health · Percent BSA minimum requirements (unspecified) · ≥ 8 years old Exclusion criteria: · Pregnancy, breastfeeding, or planning a pregnancy during the study · Any other systemic or dermatological disorders Additional design details: this study compared a branded versus generic tacrolimus 0.03% ointment to placebo. For the purposes of this study, the trial was treated as a pairwise comparison of tacrolimus 0.03% ointment versus placebo, the data from both tacrolimus arms being pooled together. |
| Participants | Total number randomised: 899 participants (599 to apply the interventions and 300 to apply the comparator) Age: mean age, unbranded intervention group, 27.74 years (SD18.1); branded intervention group, 28.8 years (SD 18.99); vehicle group, 27.6 years (SD 17.34) Sex: females:males, unbranded intervention group, 186:116; branded intervention group, 194:103; vehicle group, 186:114 Ethnicity, duration of eczema, severity of eczema, body site: not reported Number of withdrawals: intervention groups: n = 52, due to participant withdrawal n = 17, withdrawal because of lack of efficacy n = 4, protocol violation n = 11, lost to follow‐up n = 16, study‐reported AE n = 4; vehicle group n = 38, due to participant withdrawal n = 13, withdrawal because of lack of efficacy n = 8, protocol violation n = 2, lost to follow‐up n = 11, study‐reported AE n = 3, administrative n = 1 Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.03% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA success · Change in clinical signs and symptoms · Change in pruritus · Change in% BSA · Local AE |
| Notes | Funding source: Fougera Pharmaceuticals Inc Declarations of interest: not declared Original language of publication: English Other: none |
NCT01702181.
| Study characteristics | |
| Methods | Trial design: randomised, quadruple‐blinded (participant, care provider, investigator, outcomes assessor), parallel study Trial registration number: NCT01702181 Country: USA Outpatient or hospital: not reported Date trial conducted: August 2012 to December 2013 Duration of trial participation: 28 days Inclusion criteria: · Adult 18 to 65 years of age · AD affecting ≥ 5% BSA for part 1 and ≥ 10% BSA for part 2 (excluding face, neck, and head) · Has a positive but inadequate response to standard AD therapy including topical steroids or phototherapy Exclusion criteria: use of systemic or topical therapy for contact or atopic dermatitis within 30 days of the trial Additional design details: the phrase "sequential dose cohorts" was used, but no detail was given. |
| Participants | Total number randomised: 92 participants Age: 18 to 65 years Sex: all Ethnicity, duration of eczema, severity of eczema: not reported Body site: excluding face, neck and head Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention one: difamilast 0.3% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention two: difamilast 1% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention three: difamilast 3% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: tacrolimus 0.1% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Number of subjects with AE, and% of clinically relevant changes from baseline in Draize Scale, physical examinations, vital signs, laboratory assessments, and electrocardiograms · Measurement of drug levels in the blood · IGA |
| Notes | Funding source: Otsuka Pharmaceutical Development & Commercialization, Inc. Declarations of interest: not declared Original language of publication: English Other: none |
NCT01856764.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: NCT01856764 Country: Germany Outpatient or hospital: not reported Date trial conducted: June 2013 to March 2014 Duration of trial participation: 14 days Inclusion criteria: · Male or female between 18 and 65 years of age with AD according to Hanifin and Rajka's criteria · Lesional skin areas of moderate severity characterised by modified local SCORAD of at least 4, with an erythema score ≥ 2, and confirmed by a qualified investigator · An index/target lesion of moderate severity approximately 20 cm2, suitable for topical treatment · Male who is nonsterilised and sexually active with a female partner of childbearing potential, agrees to use adequate contraception from signing of informed consent throughout the duration of the study and for 12 weeks after last dose. · Female of childbearing potential who is sexually active with a nonsterilised male partner, agrees to use routinely adequate contraception from signing of informed consent throughout the duration of the study and for 12 weeks after last dose. Exclusion criteria: · Received any investigational compound within 30 days, or within 5 half‐lives of the compound · History of AD unresponsive or poorly responsive to topical treatments · History of hypersensitivity to PDE‐4 inhibitors or to drugs of similar chemical classes or any inactive ingredients of the trial medication · Clinically significant abnormal haematological parameters of haemoglobin, haematocrit or erythrocytes · History or current clinically relevant allergy or idiosyncrasy to drugs or food · Had extensive exposure to ultraviolet light in the 4 weeks prior to first application of study medication, including tanning and sun beds or is intending to have such exposure during the study treatment · Evidence of oozing of target lesion · Current skin complications for which treatment with anti‐infectives are indicated · Systemic treatment with corticosteroids or retinoids for studied condition within 3 months · Topical or transdermal treatments on or near the intended site of application within 4 weeks · Treatment with systemic/locally acting medications/procedures that might counter or influence the study aim within 4 weeks · Used emollients/moisturisers on areas to be treated within 24 hours prior to the first application of study medication · Used biologics at any time for treatment of AD · Clinically significant illness that may influence the outcome of the trial from 4 weeks before day 1 through trial completion · Significant abnormal laboratory test that would compromise the participant's ability to safely complete the trial · Significant medical condition and/or conditions that would interfere with the treatment, safety or compliance with the protocol. · History of infection with hepatitis B, hepatitis C, or human immunodeficiency virus or a history of alcoholism or illicit drug abuse within 5 years prior to study · If a female is pregnant, nursing or planning a pregnancy during the study period · History of cancer that has not been in remission for at least 5 years prior to the first dose of study medication. This criterion did not apply to those participants with successfully resected basal cell or stage I squamous cell carcinoma of the skin. · Chronic or clinically relevant acute infection or a history of depression associated with suicidal ideation and behaviour · Haemodynamically significant cardiac arrhythmias or heart valve deformations Additional design details: the phrase "sequential dose cohorts" was used, but no detail was given. |
| Participants | Total number randomised: 40 participants (20 to apply the intervention and 20 to apply the comparator) Age: adult, mean 34.6 years (SD 10.66) Sex: male n = 20, female n = 20 Ethnicity: white Duration of eczema: not reported Severity of eczema: moderate severity Body site: not reported Number of withdrawals: no withdrawals in either group Notes: none |
| Interventions | Run‐in details: not reported Intervention: roflumilast 0.5% cream used twice daily for 15 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 15 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Change in modified SCORAD · Change in TEWL values · Change in participants' assessment of pruritus |
| Notes | Funding source: AstraZeneca Declarations of interest: not declared Original language of publication: English Other: none |
NCT02031445.
| Study characteristics | |
| Methods | Trial design: randomised, quadruple‐blinded (participant, care provider, investigator, outcomes assessor), multicentre, parallel study Trial registration number: NCT02031445 Country: Israel Outpatient or hospital: several dermatology clinics Date trial conducted: August 2014 to March 2015 Duration of trial participation: 4 weeks (assumed) Inclusion criteria: · ≥ 2 and ≤ 17 years of age, of any race or ethnicity · Mild‐to‐moderate AD, IGA score of 2 or 3 according to Hanifin and Rajka, Rothe 1980 criteria, ≥ 3 months prior · ≥ 5% total body surface area Exclusion criteria: · TSC within 7 days of the study · Systemic corticosteroids, topical calcineurin inhibitors, phototherapy or immunosuppressive therapy within 14 days · Requirement for systemic treatment of AD · Systemic anti‐infective or antibiotic treatment within 14 days of the study · Clinical conditions other than atopic dermatitis that may interfere with the evaluation · Secondary infection of atopic dermatitis within the target area or open skin infections in any area · Pregnancy or breastfeeding · History of sensitivity or to any component of the study medication · History of severe anxiety and/or depression; or suicide attempt · HIV · Laboratory result outside the normal range that is clinically relevant · Participation in any other trial within 6 weeks of the study · Chronic condition(s) which are unstable or uncontrolled · Use of non‐sedating antihistamines during or within 7 days of the study · Drug or alcohol abuse, mental dysfunction, or other condition limiting the subject's ability to be compliant with study requirements Additional design details: none |
| Participants | Total number randomised: 73 participants Age: ≥ 2 and ≤ 17 years of age Sex: any Ethnicity: any Duration of eczema: ≥ 3 months Severity of eczema: IGA score of 2 or 3 corresponding to mild/moderate Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: MRX‐6 2% cream used for 28 days (assumed) · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA · AE |
| Notes | Funding source: Celsus Therapeutics PLC Declarations of interest: not declared Original language of publication: English Other: terminated as interim analysis showed lack of efficacy |
NCT02118766.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: NCT02118766 Country: US Outpatient or hospital: not reported (presumed outpatient) Date trial conducted: March 2014 to April 2015 Duration of trial participation: 28 days Inclusion criteria: · Males or females 2 years and older · AD according to Hanifin and Rajk criteria · AD involvement ≥ 5% treatable% BSA (excluding the scalp) · ISGA score of mild (2) or moderate (3) · All female subjects of childbearing potential must use acceptable methods of contraception from the screening visit continuously until 30 days after stopping study drug. Exclusion criteria: · As determined by the study doctor, a medical history that may interfere with study objectives · Unstable AD or any consistent requirement for high potency topical corticosteroids · History of use of biologic therapy (including intravenous immunoglobulin) · Recent or anticipated concomitant use of systemic or topical therapies that might alter the course of AD · Recent or current participation in another research study · Females who are breastfeeding, pregnant, or with plans to get pregnant during the participation in the study · Participation in a previous AN2728 clinical trial Additional design details: identifical study to NCT02118792 except the number recruited was slightly different |
| Participants | Total number randomised: 763 participants (507 to apply the intervention and 256 to apply the comparator) Age: 2 to 6 years n = 240, 7 to 11 years n = 228, 12 to 17 years n = 188, 18 or over n = 103 Sex: male n = 427, female n = 332 Ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐moderate Body site: except scalp and venous access area Number of withdrawals: 61; due to AE n = 9, withdrawal by subject n = 9, lost to follow‐up n = 9, withdrawal by parent/guardian n = 30, other n = 4 Notes: none |
| Interventions | Run‐in details: not reported Intervention: crisaborole 2% ointment twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Percentage of participants who achieved treatment success based on ISGA · Treatment‐emergent AE and SAE · Clinically significant change in vital signs · Clinically significant change in laboratory values · Percentage of participants' ISGA score of clear (0) or almost clear (1) · Time to achieve treatment success based on ISGA · Change in signs of AD · Time to improvement in pruritus · Change in DLQI Score |
| Notes | Funding source: Pfizer Declarations of interest: not declared Original language of publication: English Other: none |
NCT02118792.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: NCT02118792 Country: US Outpatient or hospital: not reported (presumed outpatient) Date trial conducted: March 2014 to April 2015 Duration of trial participation: 28 days Inclusion criteria: · Males or females 2 years and older · AD according to Hanifin and Rajk criteria · AD involvement ≥ 5% treatable BSA (excluding the scalp) · ISGA score of mild (2) or moderate (3) · All female subjects of childbearing potential must use acceptable methods of contraception from the screening visit continuously until 30 days after stopping study drug. Exclusion criteria: · As determined by the study doctor, a medical history that may interfere with study objectives · Unstable AD or any consistent requirement for high potency TCS · History of use of biologic therapy (including intravenous immunoglobulin) · Recent or anticipated concomitant use of systemic or topical therapies that might alter the course of AD · Recent or current participation in another research study · Females who are breastfeeding, pregnant, or with plans to get pregnant during the participation in the study · Participation in a previous AN2728 clinical trial Additional design details: identical study to NCT02118766 except the number recruited was slightly different |
| Participants | Total number randomised: 764 participants (514 to apply the intervention and 250 to apply the comparator) Age: 2 to 11 years n = 474, 12 to 17 years n = 183, 18 or over n = 106 Sex: male n = 420, female n = 343 Ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐moderate Body site: except scalp and venous access area Number of withdrawals: n = 61 reasons: AE n = 9, withdrawal by subject n = 9, lost to follow‐up n = 8, withdrawal by parent/guardian n = 34, other n = 7, randomised but not treated n = 1 Notes: none |
| Interventions | Run‐in details: not reported Intervention: crisaborole 2% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Percentage of participants who achieved treatment success based on ISGA · Number of participants with treatment‐emergent AEs and SAEs · Number of participants with clinically significant change in vital signs · Number of participants with clinically significant change in laboratory values · Number of participants with local tolerability symptoms · Percentage of participants with an ISGA score of clear (0) or almost clear (1) · Time to achieve treatment success based on ISGA · Change in signs of AD · Time to improvement in pruritus · Change in DLQI |
| Notes | Funding source: Pfizer Declarations of interest: not declared Original language of publication: English Other: Luger 2022 have published data from this trial and NCT02118766 in March 2022. |
NCT02376049.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind (participant, investigator), within‐participant study Trial registration number: NCT02376049 Country: Canada and Israel Outpatient or hospital: not reported Date trial conducted: February 2015 to July 2015 Duration of trial participation: 14 days Inclusion criteria: · Mild‐to‐moderate AD · Four comparable target areas with TSS of ≥ 5, ≥ 2 cm apart · Difference in TSS ≤ 2 between the target areas · Erythema score ≥ 2 between the target areas Exclusion criteria: · Investigator's opinion · Fitzpatrick skin type > 5 · Topical treatment with prohibited medications on the target areas · Systemic treatment, phototherapy, or emollients within a prohibited time frame Additional design details: none |
| Participants | Total number randomised: 30 sides treated Age: 18 years and older Sex: all Ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐moderate Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention one: pimecrolimus 1% cream used once daily for 14 days · Concurrent treatment: not reported · Other key information: none Intervention two: betamethasone dipropionate 0.05% cream used once daily for 14 days · Concurrent treatment: not reported · Other key information: none Intervention three: clobetasol propionate 0.05% cream used once daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used once daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | TSS change |
| Notes | Funding source: LEO Pharma Declarations of interest: not declared Original language of publication: English Other: none |
NCT02595073.
| Study characteristics | |
| Methods | Trial design: randomised, quadruple‐blinded (participant, care provider, investigator, outcome assessor) parallel study Trial registration number: NCT02595073 Country: USA Outpatient or hospital: not reported Date trial conducted: September 2015 to January 2017 Duration of trial participation: 28 days Inclusion criteria: · Male and non‐pregnant females · Age 6 months or older · Moderate‐to‐severe AD Exclusion criteria: participant lacks stable diagnosis of AD or has been diagnosed with mild AD Additional design details: none |
| Participants | Total number randomised: 124 participants (61 to apply the intervention and 63 to apply the comparator) Age: intervention group, mean 43.5 years (SD 18.8); comparator group, mean 41.7 years (SD 18.5) Sex: intervention group: female n = 36, male n = 25; comparator group: female n = 32, male n = 31 Ethnicity: intervention group, Hispanic or Latino: n = 25, not Hispanic or Latino n = 36; comparator group, Hispanic or Latino: n = 31, not Hispanic or Latino: n = 32 Duration of eczema: not reported Severity of eczema: EASI mean, intervention group, 18.8 (SD 4.4); comparator group, 18.6 (SD 3.6) Body site: not reported Number of withdrawals: 3 Notes: none |
| Interventions | Run‐in details: not reported Intervention: desoximetasone used at unspecified frequency for 28 days · Concurrent treatment: not reported · Other key information: 0.25% assumed Comparator: vehicle used unspecified frequency for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA clinical success · BSA change in baseline · AE |
| Notes | Funding source: Taro Pharmaceuticals USA Declarations of interest: not declared Original language of publication: English Other: none |
NCT02950922.
| Study characteristics | |
| Methods | Trial design: randomised, quadruple‐blinded (participant, care provider, investigator, outcomes assessor), parallel study Trial registration number: NCT02950922 Country: Canada and USA Outpatient or hospital: outpatient clinic Date trial conducted: November 2016 to July 2017 Duration of trial participation: 28 days Inclusion criteria: · Males and females with AD according to Hanifin and Rajka criteria · Adults 18 to 70 years or adolescents 12 to 17 years · AD covering ≥ 3% and < 40% of the BSA and with an IGA of 2 or 3 (mild‐to‐moderate). Scalp, palms and soles were excluded from the BSA calculation: participants with mild disease (IGA = 2) will be limited to approximately 25% of total enrolment. · Minimum EASI score of 7 · Females of childbearing potential and male subjects who are engaging in sexual activity that could lead to pregnancy must use adequate birth control methods while on study and for 2 weeks after stopping study drugs. · AD present for at least 12 months according to the patient/caregiver and stable disease for at least 1 month according to the patient/caregiver Exclusion criteria: · Hepatitis B surface antigen or Hepatitis C antibody · HIV antibody · Kaposi's varicelliform eruption, scabies, molluscum contagiosum, impetigo, psoriasis, connective tissue disorder, or Netherton's syndrome, or any other disease which could have an effect on study evaluations · Use of prohibited medication: biological products that might have significantly affected the evaluation of AD condition within 6 months of the start and during the study · Where participants in the study donated blood or blood products > 500 mL within a 56‐day period · Pregnancy or breastfeeding · History of sensitivity or contraindications to the study medications · Treatment with an investigational product within the following time period prior to the first dosing day in the current study: 30 days or 5 half‐lives/twice the duration of effect of the investigational product · Current or history of cancer within 5 years (except fully excised skin basal cell carcinoma, squamous cell carcinoma or carcinoma in situ of the cervix) · Active infection that required oral or intravenous administration of antibiotics, antifungal or antiviral agents within 7 days of the study · Advanced disease or abnormal laboratory test values that could affect the safety of the subject or the study · Significant hepatic, renal, respiratory, endocrine, haematologic, neurologic, psychiatric, or cardiovascular system abnormalities or laboratory abnormality that could affect the safety of the subject or the study · Excessive sun exposure or use of tanning booths within 28 days of the trial or unwillingness to minimise such exposure during the study Additional design details: none |
| Participants | Total number randomised: 157 participants Age: 12 to 70 years Sex: all Ethnicity: not reported Duration of eczema: 1 year Severity of eczema: mild‐to‐moderate Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention one: E6005 0.2% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention two: E6005 0.5% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for 28 days · Concurrent treatment: not reported Concurrent treatments received alongside both intervention and comparator: if a participant used prohibited medications (see exclusion criteria) during study period, withdrawal from the study was at the discretion of the investigator and medical monitor. Notes: none |
| Outcomes | · AE · Laboratory values, vital signs, ECG · Plasma concentrations of E6005 and M11 metabolite |
| Notes | Funding source: Dermavant Sciences GmbH Declarations of interest: not declared Original language of publication: English Other: none |
NCT03386032.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: NCT03386032 Country: USA Outpatient or hospital: not reported Date trial conducted: November 2017 to August 2018 Duration of trial participation: 9 weeks including an 8‐week treatment phase Inclusion criteria: · Otherwise healthy, male or female, aged 12 to 65 years old · Diagnosis of moderate‐to‐severe AD (SCORAD ≥ 25) Exclusion criteria: · Involvement in another clinical trial within the past two weeks · Diagnosed with or treated for cancer within the past 5 years · Requirement for topical or systemic medications that could affect their AD during the study period (except inhaled steroids and/or stable antihistamines for asthma or allergies) · Used systemic treatments within the past 30 days or 5 half‐lives · Known hypersensitivity to corticosteroid creams or other ingredients of the investigational product · Diagnosis with any oat allergies or derivatives · Active infections or antibiotics in the past 7 days · Presence of physical attributes or skin conditions that might interfere with the clear visual or instrumental assessments · Diagnosis of an immunologic or infectious disease which could place the subject at risk or interfere with the accuracy of the study results · Employment by the sponsor company or clinical testing site · Diabetes · Active or chronic allergic reaction evidenced by eosinophils > 0.3 X 109/L on screening · Planned visit to a sunny climate, tanning booths or other UV sources during the study · Pregnancy, lactation, or planning to become pregnant in the next 6 months Additional design details: none |
| Participants | Total number randomised: 65 participants (11 to apply the intervention and 18 to apply the comparator) Age: intervention group, mean 42 years (SD 15.4); comparator group, mean 39.6 years (SD 15.3) Sex: intervention group, 63.6% female; comparator group, 83.3% female Ethnicity: intervention group, 54.5% black, 36.4% white, 9.1% more than one race; comparator group, 50% black, 50% white Duration of eczema: not reported Severity of eczema: SCORAD scores: intervention group, mean 45.33 (SD 4.33); comparator group, 52.21 (SD 3.36) Body site: not reported Number of withdrawals: 7 all in the comparator group due to lack of effect n = 2, AE = 2, lost to follow‐up n = 3 Notes: none |
| Interventions | Run‐in details: one week "washout phase" Intervention: desonide 0.05% cream · Concurrent treatment: placebo cream applied topically, twice daily, for 8 weeks, once in the morning and once in the evening, to atopic dermatitis lesions and whole body as body moisturiser · Other key information: in the trial the TCS was one of two active interventions. The other active intervention was not specified and data have not been extracted for that arm. Comparator: vehicle cream used twice daily for 8 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · SCORAD of participants with moderate AD · SCORAD of participants with severe AD |
| Notes | Funding source: Procter and Gamble Declarations of interest: not declared Original language of publication: English Other: none |
NCT03539601.
| Study characteristics | |
| Methods | Trial design: randomised, assessor‐blind, multicentre, parallel study Trial registration number: NCT03539601 Country: USA, Germany, Italy, Poland, Sweden, Switzerland, UK Outpatient or hospital: presumed outpatient Date trial conducted: April 2018 to December 2020 Duration of trial participation: 4 weeks Inclusion criteria: · Male or female 2 years and older · AD according to the Hanifin and Rajka criteria · AD involvement of > 5% treatable% BSA (excluding the scalp) · ISGA score of mild (2) or moderate (3) (excluding the scalp) · Female of childbearing potential who had a negative urine pregnancy test at the screening visit and negative urine pregnancy test at the baseline visit prior to randomisation Exclusion criteria: any clinically significant medical disorder, condition, or disease (including active or potentially recurrent non‐AD dermatological conditions and known genetic dermatological conditions that overlap with AD) Additional design details: none |
| Participants | Total number randomised: 237 participants (59 to apply intervention one, 71 intervention two, 47 intervention three, and 60 to apply the comparator) Age: 2 years to adult Sex: male 40.9%; female 59.1% Ethnicity: Hispanic or Latino 9.4%; not Hispanic or Latino 90.6% Duration of eczema: not reported Severity of eczema: mild‐to‐moderate AD Body site: total body excluding scalp Number of withdrawals: 20 Notes: none |
| Interventions | Run‐in details: not reported Intervention one: crisaborole 2% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention two: hydrocortisone butyrate 0.1% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention three: primecrolimus 1% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Per cent change in EASI score · Number of participants with AEs · Number of participants with clinically significant changes in vital signs · Number with clinically significant abnormal laboratory parameters · Number who achieved success in ISGA · Time to Improvement in EASI score · Change in% BSA · Change pruritus (NRS) in participants aged greater than or equal to 12 years · Change in participant‐reported itch severity scale in participants aged 6 to 11 years · Change in observer‐reported itch severity scale in participants aged less than 6 years · Change in DLQI or CDLQI · Change in Dermatitis Family Impact Questionnaire (DFI) in participants aged 2 to 17 |
| Notes | Funding source: Pfizer Declarations of interest: not declared Original language of publication: English Other: trial was terminated prior to completion. The reason cited quote: "business reasons only and not related to any safety concerns regarding crisaborole" |
NCT03645057.
| Study characteristics | |
| Methods | Trial design: randomised, single‐centre, parallel study Trial registration number: NCT03645057 Country: USA Outpatient or hospital: outpatient (assumed) Date trial conducted: February 2019 to August 2021 Duration of trial participation: 12 weeks Inclusion criteria: · Male and female aged between 2 and 15 years · ≤ moderate AD or eczema (ISGA 2 or 3 and ≥ 3% BSA, excluding scalp) · If taking or prescribed antihistamines, must be on stable dose of antihistamines. If taking topical steroids, must be on stable dose of topical steroid. If taking tacroliumus, crisaborole, or other topical steroid‐sparring medications are eligible for this study if he/she agrees to a two‐week “washout period” prior to starting study procedures. If taking systemic anti‐inflammatory therapy, must be on a stable dose of the systemic anti‐inflammatory medication for at least six weeks prior to enrolment, and it must be anticipated that this dose would not change during participation in this study, unless deemed medically necessary. Exclusion criteria: · Paediatric participant < 2 years old or > 15 years old · A diagnosis of another skin disease · No caregiver participation Additional design details: none |
| Participants | Total number randomised: 46 participants (23 to apply the intervention and 23 to apply the comparator) Age: 2 to 15 years old Sex: male crisaborole group n = 8; tacrolimus group n = 12; female crisaborole group n = 15, tacrolimus group n = 11 Ethnicity: Hispanic/Latino n = 6, Asian n = 3, black/African‐American n = 11, white n = 32, more than one race n = 1 Duration of eczema: not reported Severity of eczema: moderate or less severity Body site: area score is recorded for four regions of the body (head and neck, trunk including genital area, upper limbs, and lower limbs including buttocks) Number of withdrawals: one dyad (i.e. 1 child patient with 1 caregiver) was lost to follow‐up after randomisation (crisaborole arm) but before baseline assessment. Notes: none |
| Interventions | Run‐in details: two week washout of tacrolimus or crisaborole, or other steroid‐sparring medication prior to randomisation Intervention: crisaborole 2% ointment used twice daily for 84 days · Concurrent treatment: not reported · Other key information: none Comparator: tacrolimus 0.03% ointment used twice daily for 84 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · PROMIS Paediatric Itch Short‐Form, Pain Interference‐Pediatric · CDLQI · Children's Sleep Habits Questionnaire · EASI |
| Notes | Funding source: not reported (sponser University of Rochester) Declarations of interest: not declared Original language of publication: English Other: There were 47 patient/caregiver dyads (i.e. 94 participants total = 47 child patients with 47 caregivers). The assessments extracted were from the children (n = 47) enroled in the study. |
NCT03725722.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: NCT03725722 Country: Australia, Canada, US Outpatient or hospital: Leo Pharma investigational sites Date trial conducted: December 2018 to May 2020 Duration of trial participation: 8 weeks Inclusion criteria: · Age ≥ 18 years · AD according to Hanifin and Rajka criteria and ≥ 1 year previously · 5‐50% BSA · Severity graded mild‐to‐severe (vIGA‐AD ≥ 2) Exclusion criteria: · AD lesions on scalp · Other active dermatological conditions including contact dermatitis · Tanning beds or phototherapy within 4 weeks of the study · Systemic immunosuppressive or corticosteroids within 4 weeks of the study · 3 or more bleach baths any week within 4 weeks of the study · Topical corticosteroids, calcineurin inhibitors, PDE‐4 inhibitors, or oral antibiotics within 2 weeks of the study · Change in systemic antihistamine therapy within 2 weeks of the study · Live vaccines within 4 weeks of the study · Biologics within 6 months or 5 half‐lives of the study · Active skin infection within 1 week of the study · Clinically significant infection within 4 weeks of the study Additional design details: none |
| Participants | Total number randomised: 251 participants (49 to apply intervention one, 50 intervention two, 50 intervention three, 52 intervention four, and 50 to apply the comparator) Age: mean: delgocitinib 0.1%, 43.8 years (SD 16.1); delgocitinib 0.3%, 38.5 years (SD 16.6); delgocitinib 0.8%, 42.0 years (SD 17.4); delgocitinib 2%,: 37.4 years (SD 14.7); vehicle, 41.3 years (SD 19.6) Sex: delgocitinib 0.1%, females n = 34, males n = 15; delgocitinib 0.3%, females n = 33, males n = 17; delgocitinib 0.8%, females n = 32, males n = 18; delgocitinib 2%, females n = 37, males n = 15; vehicle, females n = 36, 14 males n = 14 Ethnicity: delgocitinib 0.1%, white n = 27, black or African‐American n = 3, Asian n = 12, other n = 7; delgocitinib 0.3%, white n = 28, black or African‐American n = 11, Asian n = 9, other n = 2; delgocitinib 0.8%, white n = 23, black or African‐American n = 5, Asian n = 19; delgocitinib 2%, white = 28, black or African‐American = 4, Asian = 15, Hawaiian = 0, American‐Indian = 1, other = 4; vehicle, white = 26, black or African‐American = 8, Asian = 14, Hawaiian = 1, American‐Indian = 0, other = 1 Duration of eczema: AD ≥ 1 year eligible Severity of eczema: mild‐to‐severe eligible Body site: not the scalp; otherwise unspecified Number of withdrawals: delgocitinib 0.1%, lack of efficacy n =2, AE n = 2, withdrawal by subject, n = 4, lost to follow‐up n = 3; delgocitinib 0.3%, lack of efficacy n = 1, AE n = 2, withdrawal by subject, n = 1, lost to follow‐up n = 1, COVID19 n = 1; delgocitinib 0.8%, lack of efficacy, n = 2, AE n = 1, lost to follow‐up n = 2, personal reason n = 1; delgocitinib 2%, withdrawal by subject n = 4, lost to follow‐up n = 2; vehicle, lack of efficacy n = 2, AE n = 6, withdrawal by subject n = 4, lost to follow‐up n = 1, personal reason n = 1 Notes: none |
| Interventions | Run‐in details: not reported Intervention one: delgocitinib 0.1% cream used twice daily for 8 weeks · Concurrent treatment: not reported · Other key information: none Intervention two: delgocitinib 0.3% cream used twice daily for 8 weeks · Concurrent treatment: not reported · Other key information: none Intervention three: delgocitinib 0.8% cream used twice daily for 8 weeks · Concurrent treatment: not reported · Other key information: none Intervention four: delgocitinib 2% cream used twice daily for 8 weeks · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 8 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI, EASI‐75 · IGA · Withdrawals due to AE, local AE |
| Notes | Funding source: LEO Pharma Declarations of interest: not declared Original language of publication: English Other: none |
NCT03745638.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, randomised, multicentre, parallel study Trial registration number: NCT03745638 Country: Canada, France, Germany, Hungary, Italy, Poland, US Outpatient or hospital: 79 study locations listed in the protocol Date trial conducted: December 2018 to December 2020 Duration of trial participation: vehicle control period: 8 weeks Inclusion criteria: · Males and females ages 12 or over · AD according to Hanifin and Rajka criteria, ≥ 2 years prior to the study · IGA score of 2‐3 · Willingness to discontinue agents used to treat AD from screening to follow‐up · ≥ 1 ‘target lesion’ not located on hands, feet or genitalia · Avoidance of pregnancy or fathering of children Exclusion criteria: · Unstable AD within 4 weeks of the study · Immunocompromised, infection requiring antibiotics within 2 weeks of the study, other skin disorder or other types of eczema · Any other serious illness · Use of the following treatments within their respective washout period (12 weeks for biological agents, 4 weeks for corticosteroids and immunosuppressives, 2 weeks for antihistamines, 1 week for topical AD treatments) · Previous use of JAK inhibitors · UV light therapy within 2 weeks of the study · HIV positivity · Abnormal liver function tests · Pregnancy · Alcoholism or drug addiction within 1 year of the study Additional design details: this was a 3‐arm trial where all interventions were directly compared. |
| Participants | Total number randomised: 631 participants (253 to apply the intervention one, 252 to apply intervention two and 126 to apply the comparator) Age: mean overall 35.2 years (SD 18.5); ruxolitinib 1.5% group, 33.7 years (SD 17.15); ruxolitinib 0.75% group, 36.8 years (SD 19.0); vehicle group, 35.2 years (SD 18.11) Sex: total females 62%; ruxolitinib 1.5% group, 62. 5%; ruxolitinib 0.75%, 61.1%; vehicle group, 62.7% Ethnicity: American‐Indian or Alaskan native: ruxolitinib group 1.5% n = 0, ruxolitinib 0.75% group, n = 2, vehicle group, n = 0; Asian: ruxolitinib 1.5% group, n = 14, ruxolitinib 0.75% group, n = 10, vehicle group, n = 8; Native Hawaiian or other Pacific Islander: ruxolitinib 1.5% group n = 0, ruxolitinib 0.75% group n = 3, vehicle group n = 0; black or African‐American: ruxolitinib 1.5% group = 56, ruxolitinib 0.75% group: n = 55, vehicle group n = 29; white: ruxolitinib 1.5% group n = 177, ruxolitinib 0.75% group n = 173, vehicle group n = 85; other: ruxolitinib 1.5% group n = 6, ruxolitinib 0.75% group n = 9, vehicle group n = 4; Hispanic or Latino: ruxolitinib 1.5% group n = 0, ruxolitinib 0.75% group n = 30, vehicle group n = 21 Duration of eczema: ≥ 2 years Severity of eczema: IGA score of 2 or 3 eligible Body site: not hands, feet, or genitalia Number of withdrawals: vehicle group n = 31, ruxolitinib 1.5%group n = 28, ruxolitinib 0.75% group n = 30 Notes: none |
| Interventions | Run‐in details: not reported Intervention one: ruxolitinib 1.5% cream used twice daily for 8 weeks · Concurrent treatment: not reported · Other key information: none Intervention two: ruxolitinib 0.75% cream used twice daily for 8 weeks · Concurrent treatment: not reported · Other key information: none Comparator: vehicle 0% used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both interventions and comparator: not reported Notes: none |
| Outcomes | · EASI improvement · IGA · POEM used to measure sleep, quality of life · Itch measured via NRS · SCORAD · Change in AD afflicted% BSA · Change in DLQI · Change in CDLQI · PGIC · Change in EuroQuality of Life Five Dimensions (EQ‐5D‐5L) VAS · AEs · Change in Work Productivity and Activity Impairment Questionnaire: Specific Health Problem (WPAI‐SH version 2.0) · Plasma concentrations of rucolitinib |
| Notes | Funding source: Incyte Corporation Declarations of interest: multiple authors declared payments from Incyte Corporation and other relevant companies. Original language of publication: English Other: none |
NCT03745651.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: NCT03745651 Country: Bulgaria, Canada, Czech Republic, Germany, Poland, Spain, US Outpatient or hospital: not reported Date trial conducted: December 2018 to November 2020 Duration of trial participation: 8 weeks (vehicle control period) Inclusion criteria: · Adolescents aged 12 to 17 years, and men and women aged ≥ 18 years · Diagnosis of AD according to Hanifin and Rajka criteria · AD duration ≥ 2 years · IGA score of 2 to 3 · BSA (excluding scalp) 3 to 20% · Agreement to discontinue all agents used to treat AD from screening through the final follow‐up visit · ≥ 1 representative target lesion, ≥ 10 cm2 (excluding hands, feet, or genitalia) · Willingness to avoid pregnancy or fathering of children Exclusion criteria: · Unstable AD · Concurrent conditions and history of other diseases (immunocompromised, chronic or acute infections, acute bacterial or fungal skin infection, other skin disorders, presence of AD lesions on only the hands and feet, other types of eczema) · Any other serious illness or medical, physical or psychiatric conditions · Use of treatments without washout period ‐ biologic agents, corticosteroids, antihistamines, topical treatments for AD (see Run‐in section) · Previously use of JAK inhibitors · Abnormal liver function tests · UV therapy within 2 weeks of the study · HIV‐positive · Pregnancy or breastfeeding · Alcoholism or drug addiction Additional design details: 3‐arm trial where all interventions were directly compared |
| Participants | Total number randomised: 618 participants (246 to apply the intervention one, 248 to apply intervention two and 124 to apply the comparator) Age: mean overall: 36.4 years (SD 18.38); ruxolitinib 1.5% group, 35.9 years (SD 18.01); ruxolitnib 0.75% group, 35.8 years (18.45); vehicle group, 38.9 years (SD 18.90) Sex: female overall n = 380 (61.5%; ruxolitinib 1.5% group, n = 150 (61%); ruxolitinib 0.75% group, n =150 (60.5%); vehicle group, n = 80 (64.5%) Ethnicity: Hispanic/Latino: ruxolitinib 1.5% group n = 30, ruxolitinib 0.75% group n = 31, placebo group n = 17; white: ruxolitinib 1.5% group n = 178, ruxolitinib 0.75% group n = 174, placebo group n = 85; black/African‐American: ruxolitinib 1.5% group n = 57, ruxolitinib 0.75% group n = 63, vehicle group n = 32; Asian: ruxolitinib 1.5% group n = 6, ruxolitinib 0.75% group n = 6, vehicle group n = 2; American‐Indian/Alaskan native: ruxolitinib 1.5% group n =1, ruxolitinib 0.75% group n = 0, placebo group n = 0; Native Hawaiian/Pacific Islander: ruxolitinib 1.5% group n = 0, ruxolitinib 0.75% group n = 0, placebo group n = 2; other: ruxolitinib 1.5% group n = 4, ruxolitinib 0.75% group n = 5, placebo group n = 3 Duration of eczema: ≥ 2 years Severity of eczema: IGA score of 2 or 3 at baseline Body site: presence of AD lesions must involve classical areas such as the face or the folds Number of withdrawals: ruxolitinib 1.5% group n = 22, ruxolitinib 0.75% group n = 39, placebo group n = 19 Notes: none |
| Interventions | Run‐in details: · Biologic agents within 12 weeks of the study or 5 half‐lives · Systemic corticosteroids or adrenocorticotropic hormone analogs, cyclosporin, methotrexate, azathioprine, or other systemic immunosuppressive or immunomodulating agents within 4 weeks of the study · Immunisations and sedating antihistamines, unless nonsedating or long‐term stable regimen within 2 weeks of the study · Other topical treatments for AD (other than bland emollients) within 1 week of the study. Diluted sodium hypochlorite "bleach" baths are permitted if ≤ 2 per week and frequency is consistent throughout the study. · Current treatment or treatment within 30 days or 5 half‐lives with another investigational medication or current enrolment in another trial Intervention one: ruxolitinib 1.5% cream used twice daily for 8 weeks used twice daily for 8 weeks · Concurrent treatment: not reported · Other key information: none Intervention two: ruxolitinib 0.75% cream used twice daily for 8 weeks Comparator: vehicle cream used twice daily for 8 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both interventions and comparator: not reported Notes: none |
| Outcomes | · EASI score, including percentage who achieved EASI‐75 · Percentage who achieved Investigator's Global Assessment ‐ Treatment Success (IGA‐TS) Percentage with a ≥ 4‐point improvement in itch NRS · Percentage with a clinically meaningful (≥ 6‐point) improvement in PROMIS short form ‐ Sleep · Per cent change in SCORAD · Change in AD afflicted% BSA · Change in POEM, DLQI and CDLQI · Mean PGIC · Change in EQ‐5D‐5L VAS · Change in WPAI‐SHP version 2.0 · Plasma concentrations of ruxolitinib · AE |
| Notes | Funding source: Incyte Corporation Declarations of interest: multiple authors declared payments from Incyte Corporation and other relevant companies Original language of publication: English Other: none |
NCT04360187.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: NCT04360187 Country: China, Japan, Korea, Outpatient or hospital: outpatient clinic Date trial conducted: July 2020 to September 2021 Duration of trial participation: 28 days Inclusion criteria: · 2 years or older · AD according to the Hanifin and Rajka criteria · AD involvement of > 5% treatable BSA (excluding scalp) · ISGA score of mild (2) or moderate (3) (excluding scalp) · Female subjects of childbearing age with a negative pregnancy test at screening Exclusion criteria: · Any clinically significant medical disorder, condition or disease (including active or potentially recurrent non‐AD dermatological conditions and known genetic dermatological conditions that overlap with AD) · Unstable AD or any consistent requirement for high/strong potency or very high/very strong topical corticosteroids to manage AD signs and symptoms · History of angioedema or anaphylaxis · Significant active systemic or localised infection, including infected AD · Received any of the prohibited medications/therapies that may alter the course of AD without the required minimum washout · History of cancer within 5 years or having undergone treatment for cancer · Known sensitivity to the investigational product Additional design details: none |
| Participants | Total number randomised: 391 participants (260 to apply the intervention and 131 to apply the comparator) Age: 2 to 11 years n = 192: vehicle group n = 96, intervention group n = 123; 12 to 17 years n = 40: vehicle group n = 15, intervention group n = 25; > 18 years n = 159: vehicle group n = 47, intervention group n = 112 Sex: male n = 205: vehicle group n = 67, intervention group n = 138; female n = 186: vehicle group n = 64, intervention group n = 122 Ethnicity: Asian Duration of eczema: not reported Severity of eczema: ISGA score of mild (2) or moderate (3) Body site: all treatable AD‐involved areas (excluding the scalp) Number of withdrawals: n = 38: vehicle group n = 23, intervention group n = 15 Notes: none |
| Interventions | Run‐in details: participation in other studies involving investigational drugs within 30 days prior to study entry and/or during study participation Intervention: crisaborole 2% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI · Treatment‐emergent (TE)AE/SAE · Clinical laboratory parameters · Significant changes in vital signs · ISGA · Peak pruritus NRS · Percent BSA · EASI · Itch severity scale · DLQI · CDLQI · The Infants' Dermatitis Quality of Life Index (IDQOL) · POEM |
| Notes | Funding source: Pfizer Declarations of interest: not declared Original language of publication: English Other: none |
NCT04530643.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind (participant, investigator), parallel study Trial registration number: NCT04530643 Country: Korea Outpatient or hospital: hospital settings Date trial conducted: August 2020 to September 2021 Duration of trial participation: 4 weeks (assumed from outcomes) Inclusion criteria: · Males and females ≥ 19 years · Clinical diagnosis of AD according to Hanifin and Rajka criteria · IGA of 2 or 3 · BSA 5 to 40% Exclusion criteria: · Clinically significant hypersensitivity reactions to drugs containing taurodeoxycholate, aspirin, antibiotics, etc. · Clinically significant presence or history of liver, kidney, respiratory, endocrine, neurologic, haematologic, mental, haemorrhagic, cardiovascular diseases · Systemic infection · Asthma · Oral (assumed) steroids, oral antibiotics, body photochemotherapy, or immunosuppressive drugs within 4 weeks of the trial · Topical steroids or antibiotics within 2 weeks of the trial · Taking prohibited medication · Creatinine > twice the upper limit of normal · AST/ALT > twice the upper limit of normal · Participating in other clinical trials or bioequivalence studies within 6 months of the trial · HIV infection or seropositivity, positive/undeterminable HBsAg, HBcAb, or hepatitis C virus antibody · Skin diseases or conditions that may interfere with clinical trial evaluation · Malignant tumour within 5 years · Requirement for treatment with topical drugs containing ceramide, hyaluronic acid, urea or filaggrin · Drinking or substance abuse within 2 years · Positive urine drug screening tests i.e. amphetamine, barbiturate, benzodiazepine, cannabinoid, cocaine, opiate, cotinine · Pregnancy, breastfeeding, or considering pregnancy during the study Additional design details: none |
| Participants | Total number randomised: 80 participants Age: ≥ 19 years eligible Sex: all Ethnicity, duration of eczema: not reported Severity of eczema: IGA of 2 or 3 at baseline visit Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention one: HY209 0.3% gel used for 28 days · Concurrent treatment: not reported · Other key information: GPCR19 agonist Intervention two: HY209 0.5% gel used for 28 days · Concurrent treatment: not reported · Other key information: GPCR19 agonist Comparator: placebo used for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI · IGA · Pruritus |
| Notes | Funding source: Shaperon Declarations of interest: not declared Original language of publication: English Other: none |
NCT04664153.
| Study characteristics | |
| Methods | Trial design: randomised, quadruple‐blinded (participant, care provider, investigator, outcome assessor), parallel study Trial registration number: NCT04664153 Country: Australia, Canada, Poland, US Outpatient or hospital: not reported Date trial conducted: December 2020 to August 2021 Duration of trial participation: 6 weeks Inclusion criteria: · AD for at least 3 months; IGA score of 2 (mild), or 3 (moderate) and covering 5% to 20% of BSA · Plaque psoriasis for at least 6 months; PGA score of 2 (mild), or 3 (moderate) and covering 5% to 15% (inclusive) of BSA Exclusion criteria: · Presence of skin comorbidities that would interfere with study assessment or response to treatment · Current or recent history of severe, progressive, or uncontrolled renal, hepatic, haematological, gastrointestinal, metabolic, endocrine, pulmonary, cardiovascular, or neurological disease · Other medical or psychiatric conditions including recent (within the past year) or active suicidal ideation/behaviour or laboratory abnormality that may increase the risk of study participation or, in the investigator's judgement, make the participant inappropriate for the study · Systemic infection · Asthma · Oral (assumed) steroids, oral antibiotics, body photochemotherapy, or immunosuppressive drugs within 4 weeks of the trial · Topical steroids or antibiotics within 2 weeks of the trial · Taking prohibited medication · Creatinine > twice the upper limit of normal · AST/ALT > twice the upper limit of normal · Participating in other clinical trials or bioequivalence studies within 6 months of the trial · HIV infection or seropositivity, positive/undeterminable HBsAg, HBcAb, or hepatitis C virus antibody · Skin diseases or conditions that may interfere with clinical trial evaluation · Malignant tumour within 5 years · Requirement for treatment with topical drugs containing ceramide, hyaluronic acid, urea or filaggrin · Drinking or substance abuse within 2 years · Positive urine drug screening tests for amphetamine, barbiturate, benzodiazepine, cannabinoid, cocaine, opiate, cotinine · Pregnancy, breastfeeding, or considering pregnancy during the study Additional design details: data extracted for AD participants only |
| Participants | Total number randomised: 70 participants Age: 18 to 70 years Sex: male or female Ethnicity: Hispanic or Latino comparator group: n = 8, intervention group n = 11; white, black or African‐American/Asian comparator group: n = 25, n = 8, n = 1; intervention group n = 29, n = 5, n = 2 Duration of eczema: at least 3 months Severity of eczema: mild or moderate Body site: not reported Number of withdrawals: intervention n = 2; comparator group n = 3 Notes: none |
| Interventions | Run‐in details: not reported Intervention one: PF‐07038124 0.01% ointment used daily for 28 days used · Concurrent treatment: not reported · Other key information: GPCR19 agonist Intervention two: HY209 0.5% gel used daily for 28 days used · Concurrent treatment: not reported · Other key information: GPCR19 agonist Comparator: placebo used daily for 28 days used · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI · IGA · Pruritus patient‐assessed · AE · Withdrawal due to AE |
| Notes | Funding source: Pfizer Declarations of interest: not declared Original language of publication: English Other: none |
NCT04773587.
| Study characteristics | |
| Methods | Trial design: randomised double‐blind study Trial registration number: NCT04773587 Country: US, Canada Outpatient or hospital: not reported Date trial conducted: from January 2021 Duration of trial participation: 4 weeks Inclusion criteria: · Male or female, aged 6 years and older · AD 6 months duration (3 months for children) · Stable disease for the past 4 weeks with no significant flares · Females of childbearing potential must have a negative serum pregnancy test and a negative urine pregnancy. In addition, those sexually active must agree to use at least one form of a highly effective or barrier method of contraception throughout the trial. · In good health Exclusion criteria: · Any serious medical condition or clinically significant abnormality that would prevent study participation · Unstable AD or any consistent requirement for high potency topical steroids · Unwilling to refrain from prolonged sun exposure and from using a tanning bed or other artificial light emitting devices for 4 weeks prior to baseline/day 1 and during the study · Previous treatment with ARQ‐151 · History of chronic alcohol or drug abuse within 6 months prior to screening Additional design details: not reported |
| Participants | Total number randomised, age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: roflumilast 0.15% cream used once daily for 28 days · Concurrent treatment: not reported · Other key information: Comparator: 0% cream used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA Success · EASI ·Itch |
| Notes | Funding source: Arcutis Biotherapeutics, Inc Declarations of interest: not declared Original language of publication: English Other: none |
NCT04773600.
| Study characteristics | |
| Methods | Trial design: randomised, triple‐blinded, multicentre study Trial registration number: NCT04773600 Country: US, Canada Outpatient or hospital: 53 study locations Date trial conducted: February 2021 to September 2022 Duration of trial participation: 4 weeks Inclusion criteria: · Males and females, aged 6 years and older · AD 6 months duration (3 months for children). Stable disease for the past 4 weeks with no significant flares · Females of childbearing potential must have a negative serum pregnancy test. In addition, sexually active FOCBP must agree to use at least one form of a highly effective or barrier method of contraception throughout the trial. · In good health Exclusion criteria: · Any serious medical condition or clinically significant abnormality or any consistent requirement for high potency topical steroids · Unwilling to refrain from prolonged sun exposure and from using a tanning bed or other artificial light emitting devices (LEDs) for 4 weeks prior · Females who are pregnant, wishing to become pregnant during the study, or are breastfeeding · Previous treatment with ARQ‐151 · A history of chronic alcohol or drug abuse within 6 months Additional design details: none |
| Participants | Total number randomised: 683 participants Age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: n.r Notes: this was an identical trial to NCT04773587; the only difference was study location. |
| Interventions | Run‐in details: not reported Intervention: ARQ‐151 (roflumilast) 0.15% cream used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: 0% cream used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA success, defined as a vIGA‐AD score of 'clear' or 'almost clear' and a 2‐grade improvement from baseline · Reduction in EASI · WI‐NRS score (itch) · vIGA‐AD |
| Notes | Funding source: Arcutis Biotherapeutics, Inc Declarations of interest: not declated Original language of publication: English Other: none |
NCT05014568.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind study Trial registration number: NCT05014568 Country: US and Canada Outpatient or hospital: not reported Date trial conducted: from 2021 Duration of trial participation: 8 weeks Inclusion criteria: · Male or female aged 2 and above with AD · AD ≥ 5% and ≤ 35% of the BSA · vIGA‐AD score of ≥ 3 · An EASI score of ≥ 6 at screening and baseline · AD present for at least 6 months for ages 6 years old and above or 3 months for ages 2 to 5 years old · Female of childbearing potential who are engaging in sexual activity that could lead to pregnancy should use acceptable birth control methods and must not be pregnant. Exclusion criteria: · Immunocompromised · Chronic or acute systemic or superficial infection requiring treatment with systemic antibacterials or antifungals · Laboratory values outside defined criteria · Current or chronic history of liver disease OR of cancer within 5 years except for adequately treated cutaneous basal cell carcinoma, squamous cell carcinoma or carcinoma in situ of the cervix · History of or ongoing serious illness or medical, physical, or psychiatric condition(s) that may interfere with the subject's participation in the study · Pregnant or lactating females · History of sensitivity to the study medications, or a history of drug or other allergy · Previous known participation in a clinical study with tapinarof Additional design details: none |
| Participants | Total number randomised: 407 Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: 30‐day screening period ‐ no details on what this entailed Intervention: tapinarof 1% cream used once daily for 56 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used once daily for 56 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA · EASI · BSA · Pruritus score |
| Notes | Funding source: Dermavant Sciences GmbH Declarations of interest: not declared Original language of publication: English Other: none |
NCT05032859.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind study Trial registration number: NCT05032859 Country: USA Outpatient or hospital: not reported Date trial conducted: from 2021 Duration of trial participation: 8 weeks Inclusion criteria: · Male and female aged 2 and above with AD · AD covering ≥ 5% and ≤ 35% of the BSA · vIGA‐AD score of ≥ 3 · EASI score of ≥6 · AD present for at least 6 months for ages 6 years old and above or 3 months for ages 2 to 5 years old · Female of childbearing potential who are engaging in sexual activity that could lead to pregnancy should use acceptable birth control methods. · Must not be pregnant Exclusion criteria: · Immunocompromised · Chronic or acute systemic or superficial infection requiring treatment with systemic antibacterials or antifungals within one week prior to baseline visit · Significant dermatological or inflammatory condition other than AD that would make it difficult to interpret data or assessments during the study · Laboratory values outside defined criteria · Current or chronic history of liver disease or cancer within 5 years except for adequately treated cutaneous basal cell carcinoma, squamous cell carcinoma or carcinoma in situ of the cervix · History of or ongoing serious illness or medical, physical, or psychiatric condition(s) that may interfere with the subject's participation in the study · Pregnant or lactating females · History of sensitivity to the study medications or a history of drug or other allergy · Previous participation in a clinical study with tapinarof Additional design details: none |
| Participants | Total number randomised: 406 Withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tapinarof 1% cream used once daily · Concurrent treatment: not reported · Other key information: none Comparator: 0% cream used once daily · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · vIGA‐AD score of clear or almost clear (0 or 1) · ≥ 75% improvement in EASI · Mean change in% BSA affected · Assessment of percent body surface area as an estimate of the percentage of total involved skin with AD · Proportion of subjects with ≥ 90% improvement in EASI · EASI scoring system |
| Notes | Funding source: Dermavant Sciences GmbH Declarations of interest: none declared Original language of publication: English Other: none |
Nemoto 2016.
| Study characteristics | |
| Methods | Trial design: randomised multicentre study Trial registration number: NCT02094235 Country: Japan Outpatient or hospital: outpatients Date trial conducted: participants enroled between January and November 2014 Duration of trial participation: 14 days Inclusion criteria: · Male or female outpatients aged 2 to 15 years · Mild‐to‐moderate AD according to the Japanese Dermatological Association · ≥ one evaluable skin lesion (typical AD lesions measuring 25–100 cm2 in the trunk or extremities with at least one moderate or severe symptom of erythema, infiltration/papulation, excoriation or lichenification) Exclusion criteria: · History of biological therapy within 6 months before the study drug application · Very strong or strongest TCS within 28 days · Weak, medium or strong TCS, or TCI within 7 days (except hydrocortisone butyrate preparations for treatment of areas where the study drug could not be applied) · Oral anti‐allergics (sodium cromoglycate, oxatomide, suplatast tosilate or tranilast) within 14 days · Active infection, Kaposi’s varicelliform eruption, scabies, molluscum contagiosum, impetigo contagiosa, psoriasis, Netherton’s syndrome or other autoimmune disorders, which may affect study evaluations Additional design details: none |
| Participants | Total number randomised: 62 participants (10 to apply intervention one, 32 intervention two and 20 vehicle) Age: E6005 0.05% group mean 8.8 years, range 5 to 15; E6005 0.2% group 8.2 years, range 2 to 14; placebo group mean 8.3 years, range 2 to 15 Sex: E6005 0.05% group male 60%, female 40%; E6005 0.2% group male 34.4%, female 65.6%; placebo male 60%, female 40% Ethnicity: Japanese 100% Duration of eczema: not reported Severity of eczema: E6005 0.05% group mean severity score 10.1 (SD 1.2); E6005 0.2% group mean 9.7 (SD 1.6); placebo group mean 10.3 (SD 2.0) Body site: evaluable skin lesions on trunk or extremities were eligible Number of withdrawals: to quote: "one subject receiving treatment with vehicle voluntarily discontinued the study". Notes: none |
| Interventions | Run‐in details: not reported Intervention: E6005 0.05% ointment used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Intervention: E6005 0.20% ointment used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: Notes: none |
| Outcomes | · AE · Safety assessments via clinical laboratory tests, vital signs, ECG · Pharmacokinetic profiles · Severity scores · IGA · Itching |
| Notes | Funding source: Eisai Co., Ltd Declarations of interest: seven authors served as either consultants to or employees of Eisai Co., Ltd. Original language of publication: English Other: none |
Neumann 1971.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐study Trial registration number: not reported Country: Mexico Outpatient or hospital: outpatients Date trial conducted, duration of trial participation: not reported Inclusion criteria: · Bilateral eczematous lesions in AD, contact dermatitis, seborrhoeic dermatitis, diaper rash, nummular eczema · Either sex or any age Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 63 participants Age: not reported Sex: male or female Ethnicity, duration of eczema, severity of eczema, body site: not reported Number of withdrawals: there were no withdrawals. Notes: not reported |
| Interventions | Run‐in details: not reported Intervention one: betamethasone‐17 valerate 0.1% cream used once daily for 15 days · Concurrent treatment: not reported · Other key information: none Intervention two: flucinonide 0.05% cream used once daily for 15 days · Concurrent treatment: not reported · Other key information: none Comparator: placebo used once daily for 15 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Percentage showing complete healing |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Neumann 2008.
| Study characteristics | |
| Methods | Trial design: randomised, single‐centre, parallel study Trial registration number: not reported Country: Germany Outpatient or hospital: a daycare centre as well as the AD outpatient service of the Department of Dermatology, University Medical Center Freiburg, Germany Date trial conducted: not reported Duration of trial participation: minimuml 146 days, maximum 623 days, with a mean of 335 days Inclusion criteria: · Moderately severe AD · Age at least 16 years Exclusion criteria: · Pregnancy · Phototherapy (high‐dose UVA therapy prohibited) · Systemic corticosteroid/cyclosporine A in the 4 weeks preceding the start of observation · Participation in studies · Known sensitivity towards relevant ingredients of the ointments · Age < 16 years · Observation period < 6 months or < 5 visits Additional design details: none |
| Participants | Total number randomised: 50 participants Age: intervention group: mean 37.6 years, range 17.5 to 64.7, comparator group: mean 36.3 years, range 17 to 73.3 Sex: male:female, intervention group 9:11; comparator group 8:12 Ethnicity: not reported Duration of eczema: intervention group, mean 17.5 years, range 0.6 to 35.4; comparator group, mean 19.3 years, range 0.3 to 37.7 Severity of eczema: intervention group, mean 14.35, range 1 to 29; comparator group, mean 17.4, range 5 to 34 Body site: not reported Number of withdrawals: n = 10 Notes: not reported |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.1% ointment used once to twice daily (depending on condition of the skin) for minimal observation period was 146 days, maximum 623 days, with a mean of 335 days (11.1 months. · Concurrent treatment: not reported · Other key information: none Comparator: standard therapy group of TCS class, I to III German Classification according to Niedner, used once to twice daily (depending on condition of the skin) for minimal observation period was 146 days, maximum 623 days, with a mean of 335 days (11.1 months. · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI |
| Notes | Funding source: Astellas Company (formerly Fujisawa) Declarations of interest: not declared Original language of publication: English Other: none |
Nolting 1991.
| Study characteristics | |
| Methods | Trial design: randomised, single‐blind, multicentre, parallel study Trial registration number: not reported Country: Germany Outpatient or hospital: multiple centres Date trial conducted: not reported Duration of trial participation: 3 weeks Inclusion criteria: · Children 2 to 12 years in good general condition · Confirmed diagnosis of medium‐to‐severe AD, assessed using the following criteria; erythema, infiltration and itching were used and their expression was determined on the basis of a scale: 0 = not available, 1 = light, 2 = moderate, 3 = severe. If necessary, half values could be used. The maximum output was therefore possible at 9 points. Patients with at least 2 points per symptom, and a total of 6 points were included in the trial. Exclusion criteria: · Known hypersensitivity to trial medications · Taking local or systemic medications that affect adrenal cortical function or AD, e.g. tar, UV light, antiproliferative medications · Treated with systemic corticosteroids within 4 weeks or TCS within 7 days of trial enrolment · Skin atrophy at baseline Additional design details: It was stated that results were documented after 1 and 2 weeks of treatment, but also that this was done on the 14th or 21st day. |
| Participants | Total number randomised: 67 participants (33 to apply the intervention and 34 to apply the comparator) Age: mometasone group, average 6.6 years; prednicarbate group, average 6.4 years Sex: mometasone group, male n = 17, female n = 16; prednicarbate group, male n =16, female n =18 Ethnicity: not reported Duration of eczema: mometasone group, 3.8 years; prednicarbate group, 4.1 years Severity of eczema: mometasone group, 1 to 25% BSA involved n = 14, 26 to 50% n = 10, 51 to 75% n = 7, and 2 had 75 to 100% n = 2; prednicarbate group, 1% to 25% BSA n = 16, 26 to 50% involved n = 9, 51 to 75% involved n = 7, 75 to 100% n = 2 Body site, number of withdrawals: not reported Notes: the authors reported no differences between groups for age, duration, initial severity, gender, disease severity, localisation and extent of the disease. |
| Interventions | Run‐in details: not reported Intervention: mometasone furoate 0.1% cream used once daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: prednicarbate 0.2 5% cream used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: none Notes: applied in a thin layer |
| Outcomes | · Laboratory tests such as full blood count, haemoglobin, haematocrit, AST, ALT, urea, total bilirubin, alkaline phosphatase, creatinine and urinary status · Local AE · Physician and participant/parent evaluation of efficacy and cosmetic acceptance of the test medication · IGA (assumed): 1 = healed (100% healed except for residual post‐inflammatory hyperpigmentation of the skin); 2 = marked improvement (75 to 99% improvement); 3 = moderate improvement (50 to 74% improvement); 4 = slight improvement (< 5% improvement); 5 = no change (no significant change compared to the admission trial); 6 = exacerbation · Severity score (erythema, infiltration and itching each scored 0 to 3 with 0 = not available to 3 = severe). If necessary, half values could be used. |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: German Other: study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Noren 1989.
| Study characteristics | |
| Methods | Trial design: randomised, parallel study Trial registration number: not reported Country: Sweden (assumed) Outpatient or hospital: participants recruited through a private dermatology clinic, assumed by the authors' affiliations. Date trial conducted: not reported Duration of trial participation: 5 weeks; 1‐week pretreatment phase, 4‐weeks active treatment Inclusion criteria: ≥ 16 years of age with moderate‐to‐severe AD attending a private dermatology clinic Exclusion criteria: · Serious psychiatric disease · Infected eczema Additional design details: none |
| Participants | Total number randomised: 45 (21 (11 participants potent, 10 participants potent plus habit reversal) to apply intervention, and 24 (11 participants mild only, 13 participants mild plus habit reversal) to apply the comparator) Age: mean 24.8 years, range from 16 to 46 Sex: male n = 16, female n = 29 Ethnicity, duration of eczema: not reported Severity of eczema: baseline total improvement score was approximately 2.28 (extracted using WebPlotDigitizer) from an assessment of dryness, scaling, erythema and infiltration graded from 0 = nil to 3 = severe. Body site: not reported Number of withdrawals: 2 participants withdrew; no reason was given, and it was not clear to which group they belonged. Notes: none |
| Interventions | Run‐in details: not reported Intervention: betamethasone valerate (unspecified concentration) cream used twice daily for 4 weeks · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone (unspecified) used twice daily for 4 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: all participants were instructed on how to apply the medication in the same manner. Notes: the habit reversal intervention received by some participants in each group involved: when becoming aware of the desire to scratch, participants were asked to clench their fists for 30 seconds. If the itch did not resolve, then they were asked to press a finger nail or pinch the itching spot. Participants were also encouraged to practice this at least twice daily. |
| Outcomes | · Frequency of scratching was reported on the record form by the participant using a hand counter daily. · Dryness, scaling, erythema, and infiltration, were each graded from 0 = nil to 3 = severe. |
| Notes | Funding source: not reported Declarations of interest: none declared Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Ohba 2016.
| Study characteristics | |
| Methods | Trial design: randomised, investigator‐blinded, study Trial registration number: NCT01179880 Country: Japan Outpatient or hospital: not reported Date trial conducted: September 2010 to December 2012 Duration of trial participation: 11 days Inclusion criteria: · Japanese adult male patients with AD according to guidelines for management of atopic dermatitis by the Japanese Dermatological Association · Evaluable typical eczema on the back · Age 20 to less than 65 Exclusion criteria: · Eye symptoms (e.g. cataract, retinal detachment), Kaposi varicelliform eruption, and molluscum contagiosum or impetigo contagiosa · Had or have a severe allergy such as anaphylactic shock, anaphylactic reaction and anaphylactoid reaction or allergy Additional design details: multiple‐dose descending study. In each group, participants were randomised (at a ratio of 4:1) to receive an application of either 5 g of E6005 ointment (0.01% or 0.03% or 0.1% or 0.2%) or 5 g of vehicle ointment. |
| Participants | Total number randomised: 40 participants (8 in each group to apply the interventions and 8 to apply the comparator) Age: mean 26.3 years (SD 4.2) Sex: male only Ethnicity: Japanese Duration of eczema: mean 17.86 years (SD 8.71) Severity of eczema: mild n = 10, moderate n = 22, severe n = 6, very severe n = 2 Body site: target site posterior trunk Number of withdrawals: one patient in the E6005 0.03% group was withdrawn on day 6 because of an adverse event. One patient in the vehicle group was withdrawn on day 6 because of progression of AD. Notes: none |
| Interventions | Run‐in details: participants were allowed to use external steroids and tacrolimus ointment 8 or more days before baseline and bland emollients free of medicinal properties throughout the study period. Intervention one: E6005 0.01% used once on day 1, twice daily on days 2 to 9, and once on day 10 for 8 days · Concurrent treatment: not reported · Other key information: none Intervention two: E6005 0.03% used once on day 1, twice daily on days 2 to 9, and once on day 10 for 8 days · Concurrent treatment: not reported · Other key information: none Intervention three: E6005 0.1% used once on day 1, twice daily on days 2 to 9, and once on day 10 for 8 days · Concurrent treatment: not reported · Other key information: none Intervention four: E6005 0.2% used once on day 1, twice daily on days 2 to 9, and once on day 10 for 8 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used once on day 1, twice daily on days 2 to 9, and once on day 10 for 8 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Severity score according to SCORAD · EASI · TWL of the targeted lesion, total immunoglobulin E, CCL17 (thymus and activation‐regulated chemokine), eosinophil counts and lactate dehydrogenase |
| Notes | Funding source: Eisai Co, Ltd Declarations of interest: four authors were employed by Eisai Co. Ltd., which holds patents on E6005 (US7939540, EP1992622, JP4778550). Eisai Co, Ltd. was involved in the design and execution of the studies; data collection, analysis and interpretation; and preparation, review and approval of the manuscript before submission. Three authors disclosed no conflicts of interest. Original language of publication: English Other: none |
Ono 2020.
| Study characteristics | |
| Methods | Trial design: randomised, single‐centre, parallel study Trial registration number: not reported Country: Japan Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 36 days Inclusion criteria: · Male or female aged 20 to 55 years with AD according to the Hanifin and Rajka criteria · mild‐to‐moderate AD · percentage of treatable BSA of 25 or more Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 12 participants (10 to apply the intervention and 2 to apply the comparator) Age: mean 33.1 years (SD 11.24) Sex: all male Ethnicity: Asian: Japanese Duration of eczema: not reported Severity of eczema: 83.3% of participants had moderate ISGA score, the rest mild Body site: not reported Number of withdrawals: one in either group, both for a TEAE Notes: none |
| Interventions | Run‐in details: to quote: "a washout period for AD treatments which prohibited use of weak potency topical corticosteroids within 3 days, medium potency topical corticosteroids within 7 days, strong potency topical corticosteroids or calcineurin inhibitors within 14 days, and systemic (oral/injectable) corticosteroids 28 days prior to the first investigational product application" Intervention: crisaborole 2% ointment used twice daily for 8 days · Concurrent treatment: not reported · Other key information: none Comparator: placebo/ointment used twice daily for 8 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · incidence of TEAE · Plasma pharmacokinetic profile |
| Notes | Funding source: not reported Declarations of interest: two authors have received research grants from Pfizer R&D Japan, and three authors are employees of Pfizer. Original language of publication: English Other: none |
Onumah 2013.
| Study characteristics | |
| Methods | Trial design: randomised, parallel study Trial registration number: not reported Country: USA Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 12 weeks Inclusion criteria: · Moderate AD with 10 to 25% body surface area involvement · Failed to respond to first‐line treatments such as TCS Exclusion criteria: · History of known hypersensitivity or idiosyncratic reaction to any components of the study drug · Females of childbearing age who are pregnant, breastfeeding, or not using a reliable method of birth control · Uncontrolled chronic disease or an underlying medical condition that precludes study participation based on investigator discretion · Participated in a drug study within one month of the baseline visit · A skin disease at presentation which could interfere with the diagnosis or evaluation of atopic dermatitis · History of drug or alcohol abuse within the past 5 years, or known non‐compliance with medical treatment or unreliability Additional design details: none |
| Participants | Total number randomised: 20 participants (10 to apply the intervention and 10 to apply the comparator) Age: not reported Sex: pimecrolimus group, male 40%, female 60%; tacrolimus group, 50% male, 50% female Ethnicity: pimecrolimus group, black 60%, white 35%, other 5%; tacrolimus group, black 50%, white 50% Duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: no washout was required for this study since efficacy was not a primary end point. Intervention: pimecrolimus 1% cream used twice daily for 84 days · Concurrent treatment: not reported · Other key information: none Comparator: tacrolimus 1% ointment used twice daily for 84 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Patient product preference · Patient global assessment · Rating of symptoms of pruritus, stinging, burning and pain · DLQI · IGA · BSA |
| Notes | Funding source: not reported Declarations of interest: one author has served as either advisor, investigator, consultant or speaker for Galderma, Allergan, Bayer, Promius, Puracap, Quinnova, Stiefel/GSK, Taro, and Valeant. Another has not disclosed any relevant conflict of interest. Original language of publication: English Other: none |
Otsuki 2003.
| Study characteristics | |
| Methods | Trial design: randomised, parallel study Trial registration number: not reported Country: Japan Outpatient or hospital: 28 hospitals and 5 dermatology clinics Date trial conducted: July 2000 to March 2001 Duration of trial participation: 9 months Inclusion criteria: · Aged 2 to 15 years old · Moderate‐to‐severe AD · Moderate or severe rashes existed on their trunk, arm or leg Exclusion criteria: · Other dermatological diseases on the evaluation site · Phototherapy (ex PUVA), systemic corticosteroids, or systemic immunomodulator within 4 weeks · Drug sensitivity · Current or past history of malignancy Additional design details: none |
| Participants | Total number randomised: 221 participants (75 to apply intervention one, 71 intervention two and 75 to apply the comparator) Age: 2 to 15 years were eligible Sex: all eligible Ethnicity, duration of eczema: not reported Severity of eczema: moderate‐to‐severe was eligible; baseline characteristics not reported Body site: trunk, upper limb or lower limb Number of withdrawals: 43; vehicle group n = 32, 0.03% tacrolimus group n = 11, 0.1% tacrolimus group n = 0 Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.03% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Intervention: tacrolimus 0.1% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: systemic antihistamines or anti‐allergics as needed Notes: amount of application was according to body weight: < 20kg: 1 g/dose, 20 to 30 kg: 2 g/dose, 30 to 40 kg: 3 g/dose, 40 to 50 kg: 4g/dose, > 50 kg: 5 g/dose |
| Outcomes | · Improvement score in individual signs (1‐6) and BSA score on evaluated site and on whole applied site · Change in total individual signs on evaluated site · Change in BSA on evaluated site · Change in itch |
| Notes | Funding source: not reported Declarations of interest: not declared in the manuscript, but this was a clinical trial of Protopic® Original language of publication: Japanese Other: none |
Pala 1982.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind study Trial registration number: not reported Country: Italy Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 3 weeks Inclusion criteria: symmetrical, non‐infectious, eczematous dermatitis Exclusion criteria: pregnancy Additional design details: none |
| Participants | Total number randomised: 60 sides treated (30 to apply the intervention and 30 to apply the comparator) Age: mean 45.8 years (range 24 to 60) Sex: females n = 17, males n = 13 Ethnicity: not reported Duration of eczema: ranged from one month to 6 years Severity of eczema, body site: not reported Number of withdrawals: 3 out of 30, 2 because of AE of 'irritation' Notes: none |
| Interventions | Run‐in details: to quote: "an off‐therapy period of at least 2 weeks was scheduled prior to study admission". Intervention: hydrocortisone butyrate 0.1% cream used three times daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: halcinonide 0.1% cream used three times daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Severity of symptoms |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: Italian Other: Google translation was used to translate from Italian to English. |
Paller 2005a.
| Study characteristics | |
| Methods | Trial design: randomised, investigator‐blind study Trial registration number: NCT00667160 Country: USA Outpatient or hospital: outpatient (assumed) Date trial conducted: December 2002 to November 2003 Duration of trial participation: up to 6 weeks Inclusion criteria: · AD according to Hanifin and Rajka criteria, at least mild using the IGADA and involving at least 5% of BSA, to a maximum of 20% · Negative pregnancy test and agreement to use effective contraception · 2 to 16 years of age Exclusion criteria: · Another skin disorder in the areas to be treated · Extensive scarring or pigmented lesions in the areas to be treated that might interfere with measurements · Clinically infected AD · Likely to require systemic corticosteroids or intranasal or inhaled corticosteroids for an off‐label indication or at higher doses than the maximum labelled dosing for the drug · Known hypersensitivity to macrolides or any excipient of either study medication · Chronic condition which is either not stable or not well controlled · Pregnancy or breastfeeding Additional design details: to quote: "If any individual lesion cleared, treatment applications continued in these areas for 1 additional week and thereafter the cleared area(s) was excluded from further treatment, while the remaining lesions continued to be treated; and if all treated areas completely cleared before the week 6 visit, treatment continued in all areas for 1 additional week, followed by an end of study (EOS) evaluation." |
| Participants | Total number randomised: 426 participants (209 to apply the intervention and 217 to apply the comparator) Age: tacrolimus group: 6.5 years (SD 3.8); pimecrolimus group: 6.3 years (SD 3.7) Sex: tacrolimus group 52.9% female; pimecrolimus group 57.1% female Ethnicity: tacrolimus group, white 46.2%; African‐American 26.4%, other 27.4%; pimecrolimus group, white 42.4%, African‐American 28.6%, other 29.0% Duration of eczema: not reported Severity of eczema: all participants had mild IGADA and 5‐20% BSA Body site: not reported Number of withdrawals: 103; tacrolimus group n = 47, pimecrolimus group n = 56 Notes: none |
| Interventions | Run‐in details: up to 4‐week washout depending on prior treatments for eczema Intervention: tacrolimus 0.03% ointment used twice daily for 6 weeks · Concurrent treatment: not reported · Other key information: none Comparator: pimecrolimus 1% cream used twice daily for 6 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: use of sunscreens was permitted; however, non‐medicated topical treatments such as emollients were not permitted on areas where the study medications were being used. Notes: to quote: "Study medication was applied twice daily to the affected area(s) for up to 6 weeks or until 1 week after the affected area(s) was completely cleared, whichever came first." |
| Outcomes | · Change in EASI · IGADA · Patient evaluation of itch VAS · BSA · Safety and AE |
| Notes | Funding source: Astellas Pharma Inc. Declarations of interest: a number of authors are employed by Astellas Pharma or Novartis Pharmaceuticals Corp. Other authors reported research support and/or grants from Astellas or Novartis, and some have been funded speakers. Original language of publication: English Other: reported in the same paper as Paller 2005b; Paller 2005c. |
Paller 2005b.
| Study characteristics | |
| Methods | Trial design: randomised, investigator‐blind study Trial registration number: NCT00666159 Country: USA Outpatient or hospital: outpatient (assumed) Date trial conducted: December 2002 to May 2003 Duration of trial participation: up to 6 weeks Inclusion criteria: · AD according to Hanifin and Rajka criteria, at least mild using the IGADA and involving at least 5% of BSA · Negative pregnancy test and agreement to use effective contraception · 2 to 16 years of age Exclusion criteria: · Another skin disorder in the areas to be treated · Extensive scarring or pigmented lesions in the areas to be treated that might interfere with measurements · Clinically infected atopic dermatitis · Likely to require systemic corticosteroids or intranasal or inhaled corticosteroids for an off‐label indication or at higher doses than the maximum labelled dosing for the drug · Known hypersensitivity to macrolides or any excipient of either study medication · Chronic condition which is either not stable or not well controlled · Pregnancy or breastfeeding Additional design details: to quote: "If any individual lesion cleared, treatment applications continued in these areas for 1 additional week and thereafter the cleared area(s) was excluded from further treatment, while the remaining lesions continued to be treated; and if all treated areas completely cleared before the week 6 visit, treatment continued in all areas for 1 additional week, followed by an end of study evaluation." |
| Participants | Total number randomised: 226 participants (112 to apply the intervention and 114 to apply the comparator) Age: tacrolimus group 6.3 years (SD 3.9); pimecrolimus group 6.5 years (SD 3.9) Sex: tacrolimus group 49.1% female; pimecrolimus group 44.2% female Ethnicity: tacrolimus group, white 38.4%; African‐American 41.1%, other 20.5%; pimecrolimus group, white 39.8%, African‐American 39.8%, other 20.4% Duration of eczema: not reported Severity of eczema: mild tacrolimus group 0.9%, pimecrolimus group 1.8%; moderate tacrolimus group 81.1%, pimecrolimus group 75.2%; severe tacrolimus group 16.2%, pimecrolimus 21.2%; very severe tacrolimus 1.8%, pimecrolimus 1.8% Body site: not reported Number of withdrawals: 79: tacrolimus n = 36; pimecrolimus n = 43 Notes: none |
| Interventions | Run‐in details: up to 4‐week washout depending on prior treatments for eczema Intervention: tacrolimus 0.1% ointment used twice daily for 6 weeks · Concurrent treatment: none reported · Other key information: none Comparator: pimecrolimus 1% cream used twice daily for 6 weeks · Concurrent treatment: none reported. · Other key information: none Concurrent treatments received alongside both intervention and comparator; use of sunscreens was permitted; however, non‐medicated topical treatments such as emollients were not permitted on areas where the study medications were being used. Notes: to quote: "Study medication was applied twice daily to the affected area(s) for up to 6 weeks or until 1 week after the affected area(s) was completely cleared, whichever came first." |
| Outcomes | · Change in EASI · IGADA · Patient evaluation of itch VAS · BSA · Safety and AE |
| Notes | Funding source: Astellas Pharma Inc. Declarations of interest: a number of authors were employed by Astellas Pharma or Novartis Pharmaceuticals Corp. Other authors reported research support and/or grants from Astellas or Novartis, and some have been funded speakers. Original language of publication: English Other: reported in the same paper as Paller 2005a; Paller 2005c |
Paller 2005c.
| Study characteristics | |
| Methods | Trial design: randomised, investigator‐blind study Trial registration number: NCT00666302 Country: USA, Canada Outpatient or hospital: outpatient (assumed) Date trial conducted: October 2002 to November 2003 Duration of trial participation: up to 6 weeks Inclusion criteria: · AD according to Hanifin and Rajka criteria, and at least mild using the IGADA and involving at least 5% of BSA · Negative pregnancy test and agreement to use effective contraception · ≥ 16 years of age Exclusion criteria: · Another skin disorder in the areas to be treated · Extensive scarring or pigmented lesions in the areas to be treated that might interfere with measurements · Clinically infected atopic dermatitis · Likely to require systemic corticosteroids or intranasal or inhaled corticosteroids for an off‐label indication or at higher doses than the maximum labelled dosing for the drug · Known hypersensitivity to macrolides or any excipient of either study medication · Chronic condition which is either not stable or not well controlled · Pregnancy or breastfeeding Additional design details: to quote: "If any individual lesion cleared, treatment applications continued in these areas for 1 additional week and thereafter the cleared area(s) was excluded from further treatment, while the remaining lesions continued to be treated; and if all treated areas completely cleared before the week 6 visit, treatment continued in all areas for 1 additional week, followed by an end of study (EOS) evaluation." |
| Participants | Total number randomised: 413 participants (210 to apply the intervention and 203 to apply the comparator) Age: tacrolimus group 39.6 years (SD 15.5); pimecrolimus group 38.5 years (SD 14.0) Sex: tacrolimus 59% female; pimecrolimus 61.6% female Ethnicity: tacrolimus, white 43.3%, African‐American 39.5%, other 16.3%; pimecrolimus, white 47.3%, African‐American 36.5%, other 17.1% Duration of eczema: not reported Severity of eczema: mild: tacrolimus group 32.9%, pimecrolimus group 31.0%; moderate: tacrolimus group 46.7%, pimecrolimus group 44.3%; severe: tacrolimus group 15.7%, pimecrolimus group 19.2%; very severe: tacrolimus group 4.8%, pimecrolimus group 5.4% Body site: not reported Number of withdrawals: 90; tacrolimus n = 42; pimecrolimus n = 48 Notes: none |
| Interventions | Run‐in details: up to 4‐week washout depending on prior treatments for eczema Intervention: tacrolimus 0.1% ointment used twice daily for 6 weeks · Concurrent treatment: none reported · Other key information: none Comparator: pimecrolimus 1% cream used twice daily for 6 weeks · Concurrent treatment: none reported. · Other key information: none Concurrent treatments received alongside both intervention and comparator: use of sunscreens was permitted; however, non‐medicated topical treatments such as emollients were not permitted on areas where the study medications were being used. Notes: to quote: "study medication was applied twice daily to the affected area(s) for up to 6 weeks or until 1 week after the affected area(s) was completely cleared, whichever came first." |
| Outcomes | · Change in EASI · IGADA · Patient evaluation of itch VAS · BSA · Safety and AE |
| Notes | Funding source: Astellas Pharma Inc Declarations of interest: a number of authors were employed by Astellas Pharma or Novartis Pharmaceuticals Corp. Other authors reported research support and/or grants from Astellas or Novartis, and some have been funded speakers. Original language of publication: English Other: reported in the same paper as Paller 2005a; Paller 2005b |
Paller 2019.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre study Trial registration number: NCT02564055/GlaxoSmithKline study 203121 Country: USA, Canada, Japan Outpatient or hospital: 53 sites Date trial conducted: December 2015 to January 2017 Duration of trial participation: 16 weeks Inclusion criteria: · Male or female aged 12 to 65 years with a clinical diagnosis of AD · BSA involvement of 5% to 35% (excluding the scalp) · IGA score 3 or greater Exclusion criteria: · Unstable AD · Concurrent or a history of serious illness · Immunocompromised · Infections requiring treatment · Other skin disorders Additional design details: 6‐arm trial, involved three comparators and two different dose schedules |
| Participants | Total number randomised: 247 participants (81 to apply intervention one, 84 intervention two and 82 to apply the comparator) Age, sex, ethnicity, duration of eczema, severity of eczema, body site: not reported Number of withdrawals: 56 in total Notes: none |
| Interventions | Run‐in details: prohibited concomitant medications, products with required washout Intervention one: tapinarof 1% cream used once or twice daily for 84 days · Concurrent treatment: not reported · Other key information: none Intervention two: tapinarof 0.5% cream used once or twice daily for 84 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used once or twice daily for 84 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Proportion of patients with an IGA score of clear or almost clear · Improvement in IGA · Improvement in EASI · Mean change in weekly average of daily pruritus NRS score based on the Daily Signs and Symptoms Severity Diary · Mean change in percentage of BSA affected · Patients’ impression of severity of AD symptoms, assessed on a scale ranging from 1 (mild) to 4 (very severe) · POEM was used to assess pruritus, sleep disturbance, skin bleeding, skin weeping/oozing, cracked skin, flaking skin, and dry/rough skin. · AE and tolerability |
| Notes | Funding source: Dermavant Sciences Inc. Declarations of interest: five authors have had involvement with pharmaceutical companies. These were included as investigators/consultants/speakers/advisory board members and/or receiving research grants and/or honoraria as an investigator for various pharmaceutical companies. Two other authors are employees with stocks of the company that funded the study. Original language of publication: English Other: none |
Patzelt‐Wenczler 2000.
| Study characteristics | |
| Methods | Trial design: randomised, partial double‐blind, single‐centre, within‐participant study Trial registration number: not reported Country: Germany Outpatient or hospital: one centre; no further details Date trial conducted: not reported Duration of trial participation: 2 weeks Inclusion criteria: · Moderate AD on both arms · With pruritis, lichenification of the joint flexures and chronic or chronically recurrent course Exclusion criteria: not reported Additional design details: only comparison groups TSC and placebo are relevant and reported here. The third group involved Kamillosan (containing a herb ‐ chamoile) only. All participants received kamillosan. They were either randomised to apply kamillosan to left or right arm with the opposite arms as either hydrocortisone or placebo. The study is described as partially blinded as Kamiiosan differed in appearance and smell from the other two comparator groups. |
| Participants | Total number randomised: 72 sides treated (36 to apply the intervention and 36 to apply the comparator) Age: 45.5 years on average Sex, ethnicity, duration of eczema: not reported Severity of eczema: a sum score of pruritis, erythema and desquamation of 3 to 7 (score range 0 to 9) distal on both arms Body site: left and right arms were sites for application of test treatments. Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: hydrocortisone 0.5% cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Score and sum score of pruritus, erythema and desquamation · Assessment of oedema, vesicles, papules/pustules, lichenification, excoriation and fissures · AE |
| Notes | Funding source: not reported Declarations of interest: one of the two authors was an employee of ASTA Medica AG (a Pharma company). Original language of publication: English Other: none |
Polano 1960.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, single‐centre, crossover study Trial registration number: not reported Country: the Netherlands Outpatient or hospital: dermatological department, Municipal Hospital, The Hague (assumed by authors' affiliations) Date trial conducted: not reported Duration of trial participation: approximately 4 weeks Inclusion criteria: · Long‐standing neurodermitis · Unresponsive to standard treatment Exclusion criteria: secondary infection Additional design details: cross‐over of four treatments with results not presented by time period, so outcomes could not be extracted |
| Participants | Total number randomised: unclear; at least 22 were enroled Age, sex: ethnicity: not reported Duration of eczema: quoted to be "of long standing" Severity of eczema, body site: not reported Number of withdrawals: ≥ 8 failed to complete treatment. Notes: none |
| Interventions | Run‐in details: to quote: "each test treatment was given consecutively according to randomised sequence without washout". Intervention one: hydrocortisone 1% ointment used for 7 days · Concurrent treatment: not reported · Other key information: none Intervention two: prednisolone 0.5% ointment used for 7 days · Concurrent treatment: not reported · Other key information: none Intervention three: prednisolone 0.5% ointment used for 7 days · Concurrent treatment: 0.5% neomycin · Other key information: none Comparator: vehicle/placebo ointment used for 7 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside interventions and comparator: not reported Notes: participants returned to clinic to receive the next intervention when treatment was used up or within a week. |
| Outcomes | Itching, lichenification, infiltration, redness, weeping, crusting and apparent secondary infection scored from 0 = absence to 5 = maximum development |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: data on outcomes not reported as results for the first period were not provided separately. |
Portnoy 1969.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, half‐sided Trial registration number: not reported Country: UK Outpatient or hospital: outpatients from the Manchester and Salford Hospital for Skin Diseases, UK according to the affiliation of the author Date trial conducted: not reported Duration of trial participation: 1 week Inclusion criteria: outpatients with bilateral, symmetrical eczema Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 78 sides treated (39 to apply the intervention and 39 to apply the comparator). Age, sex, ethnicity, duration of eczema, severity of eczema, body site: not reported Number of withdrawals: 1 participant dropped out ("defaulted"); it was not clear if this was an eczema or psoriasis participant. Notes: none |
| Interventions | Run‐in details: not reported Intervention: fluocortolone 0.2% used twice daily for 7 days · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone 1.0% used twice daily for 7 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside interventions and comparator: not reported Notes: to quote "In order to reduce the risk of mixture of the preparations, applicators labelled "left" and "right" were supplied." "The preparations used were 1% hydrocortisone in a base composed of liquid paraffin, soft paraffin, cetyl alcohol, lauryl sulphate and propylene glycol and 0.1% fluocortolone trimethyl acetate and 0.1% fluocortolone caproate, in a base containing white wax, lanoline, mineral oil, white paraffin, amphocerin K and demineralized water". |
| Outcomes | · Preference for 1 steroid over the other (N.B. the paper stated "only where the patient agreed with the trialist reading the result was a definitive positive finding recorded".) |
| Notes | Funding source: not reported; however, Schering A. G. Berlin were acknowledged as having provided materials and contributed to statistical analysis. Declarations of interest: not declared Original language of publication: English Other: this trial included patients with eczema and psoriasis; only data for eczema participants were extracted. This study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Prado de Oliveira 2002.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, single‐centre study Trial registration number: not reported Country: Brazil Outpatient or hospital: Dermatology Department of the Hospital das Clinicas, Faculty of Medicine, University of São Paulo, Brazil Date trial conducted: not reported Duration of trial participation: last visit was 42 days; however, the paper stated that there was a mean number of treatment days: mometasone 26.8, desonide 25.7, and that the duration of treatment varied from 7 to 42 days. Inclusion criteria: · Children with AD involving 6% of the BSA aged 2‐12 years of age · Of any race or sex · Without other clinically significant disease · Minimum total score* of 8 from the clinical variables and 2 for the erythema variable (*erythema, lichenification, desquamation, excoriation and pruritus) were assessed. These were scored 0 ‐ absent: no evident sign/symptom; 1 ‐ slight: sign/symptom present, though ill‐defined and easily tolerated; 2 ‐ moderate; sign/symptom present, well‐defined and uncomfortable, although still tolerable; 3 ‐ intense: sign/symptom difficult to tolerate, interfering in the daily activities and/or during sleep Exclusion criteria: · Abnormal results from laboratory examinations performed during baseline visits · Previous serious disease · Immunosuppression · Evidence of cutaneous atrophy in the target area of treatment · Use of TCS or any other therapies for dermatitis in the 7 days prior to the beginning of the trial · Use of emollients in the 2 days prior to the trial · History of previous hypersensitivity to desonide or mometasone furoate · Use of any other medication in the 15 days before the beginning of the trial that could have clinical effect on the course of dermatitis · Use of systemic corticosteroid in the 28 days prior to the beginning of the trial · Use of antibiotics 1 week before beginning the trial Additional design details: none |
| Participants | Total number randomised: 25 participants (13 to apply the intervention and 12 to apply the comparator) Age: intervention group, mean 7.2 years (SD 2.7), range 3 to 11 years; comparator group, mean 4.8 years (SD 3.1), range 2 to 12 years Sex: not reported Ethnicity: intervention group, white n = 8, black n = 0, mixed n = 4; comparator group, white n = 4, black n = 1, mixed n = 7 Duration of eczema: not reported; however, the text stated "several previous attempts at treatment were mentioned for AD, of which the most frequent were antihistamines, antibiotics, and topical corticoids, all with recurrence of the picture". Severity of eczema: mean sum of total scores intervention group 9.1; comparator group 8.7 Body site: not reported Number of withdrawals: one participant in the desonide group interrupted treatment due to bronchopneumonia. All others completed the trial. Notes: none |
| Interventions | Run‐in details: none Intervention: mometasone furoate 0.1% cream used once daily for up to 42 days · Concurrent treatment: not reported · Other key information: none Comparator: desonide 0.05% cream used once daily for up to 42 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: during the trial, participants were not allowed to use antibiotics, antihistamines, topical emollients, other corticosteroids or any other drug shown in clinic or laboratory to be associated with hepatotoxicity or that could induce an increase in hepatic enzymes. Notes: treatments applied after a bath |
| Outcomes | · IGA: improvement of signs and symptoms · Signs of cutaneous atrophy: thinning of the skin, striae, shiny skin, telangiectasia, loss of elasticity, loss of normal lines on the cutaneous surface · Tolerance to the drugs was evaluated according to the participant's complaints, whether spontaneous or prompted by questions, and whether these could be related to the treatment. · Laboratory tests including total blood count, glycaemia, TGO and TGP at baseline and day 42. TGO and TGP assumed to be aspartate (AST) and alanine transaminases (ALT) (Spanish translation of synonyms) · Heart rate, blood pressure and respiratory rate in sitting and standing positions · Body surface involved based on evaluation of the palm of the participant's right hand, which is equal to 1% of the total body surface · Clinical severity scores for variables such as erythema, lichenification, desquamation, excoriation and pruritus |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Rafanelli 1993.
| Study characteristics | |
| Methods | Trial design: randomised, to quote: "third‐party blind", single‐centre, parallel study Trial registration number: not reported Country: Italy Outpatient or hospital: Dermatological Division Hospital "S. Maria delle Croci", Ravenna Date trial conducted: not reported Duration of trial participation: 21 days Inclusion criteria: · Children with AD, otherwise in good general health · Presence in a target area: erythema, induration and pruritus · Total severity score ≥ 6 according to a scale from 0 = none to 3 = severe (presumably scored for each sign, but this was not stated) · Stable disease or disease that worsened for > a week · Had not received corticosteroids either topically in the week before the trial or systemically in the 4 weeks before the trial · Did not show signs of atrophy on physical examination · Were not known to be hypersensitive to the drugs or to the components of the formulations Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 60 participants (30 to apply the intervention and 30 to apply the comparator) Age: intervention group, mean 7.7 years (SD 3.2) range 2 to 12; comparator group, 6.8 years (SD 3.1) range 2 to 12 years Sex: male n = 12, female n = 18 Ethnicity: not reported Duration of eczema: intervention group, mean 26.7 months (SD 22.2) range 1 to 72; comparator group, 16.4 months (SD 15.7) range 1 to 8) Severity of eczema: mean total signs and symptoms score: intervention group, 7.8 (SD 1.1); comparator group, 7.2 (SD 0.9), sum of scores 0 = none to 3 = severe for each of erythema, induration, and pruritus Body site: 17/30 in each group had target areas on the arms/forearms. Other sites were shoulders, chest, abdomen, buttocks, back, hands, legs, and feet. Number of withdrawals: none; to quote: "all patients completed the study". Notes: no medication that might interfere with the drugs under investigation was allowed; all other medications given during the trial were recorded. |
| Interventions | Run‐in details: not reported Intervention: mometasone furoate 0.1% cream used once daily for 21 days · Concurrent treatment: all other medications used by the participants during the trial were recorded. · Other key information: none Comparator: clobetasone 0.05% cream used twice daily for 21 days · Concurrent treatment: all other medications used by the participants during the trial were recorded. · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Laboratory tests, including blood cortisol · Total and individual signs and symptoms scores from 0 = none to 3 = severe, for erythema, induration, and pruritus · Safety evaluation involving questioning the participant/parents about side effects and a skin examination for alterations or atrophy · Physician global evaluation according to the scale 1 = cleared (disappearance of all symptoms); 2 = marked improvement (> 75% clearance of symptoms); 3 = moderate improvement (50‐75% clearance of symptoms); 4 = slight improvement (< 50% clearance of symptoms); 5 = no change (no improvement from baseline); 6 = exacerbation (worsening at evaluated sites) · Parents' evaluation of efficacy (1 = excellent, 2 = good, 3 = fair, 4 = poor) |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Rahman 2008.
| Study characteristics | |
| Methods | Trial design: randomised study Trial registration number: not reported Country: Bangladesh Outpatient or hospital: Dermatology and Venereology Department Date trial conducted: not reported Duration of trial participation: 3 weeks Inclusion criteria: not reported, but title indicated the population had AD Exclusion criteria: to quote: "In the case of females, special attention was given regarding menstrual history and use of contraceptives. A general physical examination and necessary investigations were done to rule out any systemic illness." Additional design details: none |
| Participants | Total number randomised: 60 participants (30 to apply the intervention and 30 to apply the comparator). Age: overall age range: 2 to 45 years, intervention: median 7.0 years (SD 1.83), comparator: median 8.5 years (SD 1.44) Sex: intervention, 60% males: 40% females; comparator, 50% males: 50% females Ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.03% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: vaseline used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | ·% BSA affected · Individual signs of AD · SCORAD · Pruritus · AE (query and physical examination) |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Rahman 2015.
| Study characteristics | |
| Methods | Trial design: randomised study Trial registration number: not reported Country: Bangladesh Outpatient or hospital: Department of Dermatology and Venereology Date trial conducted: March 2012 to 2013 Duration of trial participation: 6 weeks Inclusion criteria: · Aged 2 to 10 years · Intermittent or persistent symptoms of AD according to Hanifin‐Rajka criteria · AD for at least one year Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 60 participants (30 to apply the intervention and 30 to apply the comparator) Age: 2 to 10 years. Mean age tacrolimus group, 5.97 years (SD 2.25); hydrocortisone group, 5.4 years (SD 2.66) Sex: 53.3% male, 46.7% female Ethnicity: not reported Duration of eczema: at least a year Severity of eczema, body site: not reported Number of withdrawals: there were no withdrawals. Notes: none |
| Interventions | Run‐in details: washout phase of 2 weeks Intervention: tacrolimus 0.03% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone acetate 1% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: no concurrent treatments were allowed during the study. Notes: none |
| Outcomes | · EASI · AE · Evaluation of erythema, oedema/induration/papulation, excoriation, oozing/weeping/crusting, scale and lichenification |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: not reported Other: none |
Rajka 1986.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: Norway Outpatient or hospital: outpatients Date trial conducted: not reported Duration of trial participation: 4 weeks Inclusion criteria: · Symmetrical, bilateral AD · Aged > 16 years of age · Global severity of moderate Exclusion criteria: · Participants with a primary bacterial or viral skin infection (e.g. erysipelas, tuberculosis, syphilis, varicella, vaccinia, herpes zoster, herpes simplex) · Secondarily infected dermatosis · Malignant disease · Pregnant and lactating, and women of childbearing age not taking adequate precautions to avoid pregnancy · Requiring systemic steroids or who had used glucocorticoids within the preceding 2 weeks Additional design details: none |
| Participants | Total number randomised: to quote: "30 patients were admitted to be randomised"; however, also mentioned: "drop‐outs had to be replaced"; 60 sides treated (30 to apply the intervention and 30 to apply the comparator) Age: mean age 28.5 years (SD 11.6) range from 17 to 63 Sex: male n = 10, female n = 20 Ethnicity: not reported Duration of eczema: mean duration 16.7 years (SD 8.4) range from 2 to 40 Severity of eczema: baseline assessment of global severity of skin symptoms: intervention group, mean 2.8 (SD 0.4) n = 30; comparator group, mean 2.8 (SD 0.4) n = 30 Body site: not reported Number of withdrawals: unclear Notes: none |
| Interventions | Run‐in details: not reported Intervention: hydrocortisone butyrate 0.1% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: brand: locoid® Comparator: desonide 0.1% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: brand: apolar® Concurrent treatments received alongside both intervention and comparator: not reported Notes: applied for 4 weeks or to quote: " until complete clearance of the lesions of the involved side had occurred, whichever was the shortest." Use of occlusive dressings was not permitted. Participants were asked to wash their hands between applications to avoid contamination. |
| Outcomes | · AE (spontaneously reported) · Participant preference for cosmetic acceptability · Severity of erythema, induration and scaling were assessed according to Fredriksson, Lassus and Salde (1983). Skin lesions on both sides of the body were individually rated. · IGA improvement and the severity of skin symptoms on both sides (5‐point scale: 0 = none to 4 = very severe). Only 1 skin lesion or area on each treated side |
| Notes | Funding source: not reported Declarations of interest: not declared, but one author affiliated to Research and Development, Gist‐brocades Original language of publication: English Other: study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Ramelet 1982.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, study Trial registration number: not reported Country: Switzerland Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 14 days Inclusion criteria: · Clearly defined resistant (defined as being present for over a year) AD · Male or female · Erythema, induration and scaling needed to be present and each sign had to be at least moderately severe. Exclusion criteria: not reported Additional design details: study included both AD and psoriasis patients. Results presented only for AD patients |
| Participants | Total number randomised: 12 participants (6 to apply the intervention and 6 to apply the comparator) Age: intervention group, mean 33 years, range 18 to 71; comparator group, mean 26 years, range 18 to 56 Sex: intervention group, male n = 3, female n = 3; comparator group, male n = 4, female n = 2 Ethnicity: not reported Duration of eczema: intervention group, mean 11 years, range 3 to 30 years; control group, mean duration 21 years, range 5 to 51 Severity of eczema body site: not reported Number of withdrawals: one participant withdrew from the comparator group because of treatment failure. No withdrawals from the other group Notes: AD was exacerbating in all patients. |
| Interventions | Run‐in details: not reported Intervention: betamethasone dipropionate 0.05% ointment used twice daily for 14 days · Concurrent treatment: not reported · Other key information: brand: Diprolene Comparator: diflucortolone 0.3% ointment used twice daily for 14 days · Concurrent treatment: not reported · Other key information: brand: Neriforte Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Erythema, induration, scaling · Overall therapeutic efficacy · Tolerance and safety |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Ramsay 1996.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: not reported Country: Canada Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 2 weeks Inclusion criteria: · Male and female patients · Older than 3 years · Mild‐to‐moderate degrees of AD · AD considered amenable to treatment with a mild topical steroid Exclusion criteria: systemic steroids, systemic or topical antibiotics or other effective topical therapy within the previous 7 days Additional design details: none |
| Participants | Total number randomised: 68 participants Age: ≥ 3 years Sex, ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐moderate Body site: not reported Number of withdrawals: 3/68 failed to return after randomisation. Between follow‐up visits, 4 withdrew (1 defaulted, 2 had an unacceptable treatment response and 1 had an unacceptable AE). None were in the intervention group. Notes: none |
| Interventions | Run‐in details: not reported Intervention: hydrocortisone acetate 1% cream used for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: no treatment used for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: fusidic acid 2% Notes: both groups took fusidic acid, one in combination with the TCS treatment. |
| Outcomes | · 4‐point sign/symptom scale, used for each of the parameters of erythema, scaling, oedema, itching, serous discharge, and crusting · Overall severity of the lesions was assessed by the investigator and the patient. · Overall clinical response assessed by the investigator · Microbiological assessment |
| Notes | Funding source: Leo Laboratories Canada Ltd. Declarations of interest: not declared Original language of publication: English Other: this is the second study in the published paper. The first was not eligible. |
Raoof 2020.
| Study characteristics | |
| Methods | Trial design: randomised, triple‐blinded (participant, investigator, assessor), parallel study Trial registration number: NCT03011892 Country: Canada and USA Outpatient or hospital: 52 study locations Date trial conducted: January 2017 to March 2018 Duration of trial participation: 8 weeks Inclusion criteria: · AD according to Hanifin and Rajka criteria ≥ 2 years before the study · IGA score of 2‐3 · 3‐20% BSA · Willing to discontinue all agents used to treat AD Exclusion criteria: · Active infections · Topical treatments within 2 weeks of the study · Systemic immunosuppressive drugs within 4 weeks of the study · Other dermatological diseases (e.g. psoriasis) · History of other diseases with treatment that could complicate assessments · Cytopenias · Severely impaired liver function · Potent systemic cytochrome inhibitors or fluconazole within 2 weeks of the study · Previously used JAK inhibitors Additional design details: 6‐arm trial where all interventions were directly compared |
| Participants | Total number randomised: 307 participants Age: mean: vehicle group 34.8 years (SD 15.04); triamcinolone group 38.5 years (SD 16.19); ruxolitinib 0.15% group 38.4 years (SD 13.83); ruxolitinib 0.5% group 38.6 years (SD 13.73); ruxolitinib 1.5% QD group 39.1 years (SD 14.54); ruxolitinib 1.5% BD group: 39.0 years (SD 15.72) Sex: vehicle group female 61.5%; triamcinolone group female 54.9%; ruxlitinib 0.15% group female 51%; ruxlitinib 0.5% group female 52.9%; ruxlitinib 1.5% QD group female 59.6%; ruxlitinib 1.5% BD group female 48% Ethnicity: vehicle group, white n = 27, black or African‐American n = 15, Asian n = 8, Hawaiian. n = 1, other n =1; triamcinolone group, white n = 28, black/African‐American n = 13, Asian n = 8, other n = 2; ruxlitinib 0.15% group, white n = 27, black/African‐American n = 17, Asian n = 5, other n = 2; ruxlitinib 0.5% group, white n = 33, black/African‐American n = 10, Asian n = 8; ruxlitinib 1.5% QD group, white n = 24, black/African‐American n =17, Asian n = 10, other n = 1; ruxlitinib 1.5% BD group, white n = 33, black/African‐American n = 13, Asian n = 2 Duration of eczema: at least 2 years Severity of eczema: vehicle group, mild n = 15, moderate n = 35; triamcinolone group, mild n = 18, moderate n = 33; ruxolitinib 0.15% group, mild n = 16, moderate n = 35; ruxolitinib 0.5% group, mild n = 17, moderate n = 34; ruxolitinib 1.5% QD group, mild n = 15, moderate n = 36; ruxolitinib 1.5% BD group, mild n = 14, moderate n = 36 Body site: facial involvement: vehicle group n = 21; triamcinolone group n = 21; ruxolitinib 0.15% group n = 19; ruxolitinib 0.5% group n = 17; ruxolitinib 1.5% QD group n = 20; ruxolitinib 1.5% BD group n = 18 Number of withdrawals: vehicle group, lack of efficacy n = 1, other n = 1, physician decision n = 1, protocol violation n = 1, withdrawal by participant n = 7; triamcinolone, AE n = 1, lost to follow‐up n = 3, other n = 1, withdrawal by participant t n = 5; ruxolitinib 0.15% group, AE n = 1, lost to follow‐up n = 2, 1 other n = 1, physician decision n = 1, withdrawal by participant n = 1; ruxolitinib 0.5% group, lost to follow‐up n = 5, withdrawal by subject n = 2; ruxolitinib 1.5% QD group, other n =1, pregnancy n = 1, withdrawal by subject n = 4, randomised but not treated n = 1; ruxolitinib 1.5% BD group, lost to follow‐up n = 2, withdrawal by subject n = 3 Notes: none |
| Interventions | Run‐in details: not reported Intervention one: triamcinolone 0.1% cream used twice daily for 28 days followed by 28 days of vehicle · Concurrent treatment: not reported · Other key information: none Intervention two: ruxolitinib 0.15% cream used twice daily for 56 days · Concurrent treatment: not reported · Other key information: none Intervention three: ruxolitinib 0.5% cream used twice daily for 56 days · Concurrent treatment: not reported · Other key information: none Intervention four: ruxolitinib 1.5% cream used once daily for 56 days · Concurrent treatment: not reported · Other key information: none Intervention five: ruxolitinib 1.5% cream used twice daily for 56 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 56 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI · EASI 50 · IGA · PP‐NRS · Withdrawals due to AE |
| Notes | Funding source: Incyte Corporation Declarations of interest: one author has served as a consultant and sat on advisory boards for various pharmaceutical companies. Original language of publication: English Other: none |
Reitamo 2002.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: 8 European countries Outpatient or hospital: 27 centres, unspecified Date trial conducted: not reported Duration of trial participation: 3 weeks Inclusion criteria: · Males and females aged 16 to 70 years · AD according to Hanifin and Rajka criteria and moderate‐to‐severe according to Rajka and Langeland criteria · ≥ 5% BSA Exclusion criteria: a serious skin disorder other than AD that required treatment Additional design details: none |
| Participants | Total number randomised: 570 participants (193 to apply intervention one, 191 intervention two and 186 to apply the comparator) Age: mean age hydrocortisone butyrate 0.1% group 30.8 years (SD 10.3); tacrolimus group 0.03% group 31.1 years (SD 10.3); tacrolimus 0.1% group 32.4 (SD 11.4) Sex: male:female: hydrocortisone butyrate group 46.8:53.2; tacrolimus 0.03% group 43.5:56.5; in tacrolimus 0.1% group 42.9:57.1 Ethnicity: not reported Duration of eczema: median duration: hydrocortisone butyrate 0.1% group 24 years; tacrolimus group 0.03% 23 years; tacrolimus 0.1% group 25 years Severity of eczema: moderate/severe AD: hydrocortisone butyrate 0.1% group mean 44.6/55.4; tacrolimus 0.03% group 46.1/53.9; tacrolimus 0.1% group 50.8/49.2 Body site: hydrocortisone butyrate 0.1% group: head and neck 95.7%, upper limbs 0%, trunk 91.4%; tacrolimus 0.1% group: head and neck 95.8%, upper limbs 99.5%, trunk 90.1%, lower limbs 85.23%; tacrolimus 0.03% group: head and neck 93.3%, upper limbs 98.4%, trunk 90.2%, lower limbs 88.1% Number of withdrawals: tacrolimus group 0.1% n = 22 (AE n = 78, withdrew consent n = 6, noncompliance or lost to follow‐up n = 4, prohibited therapy n = 3, lack of efficacy n = 1); tacrolimus group 0.03% n = 22 (AE n = 7, withdrew consent n = 6, noncompliance or lost to follow‐up n = 3, prohibited therapy n = 4, lack of efficacy n = 2); hydrocortisone butyrate group n = 17 (AE n = 3, withdrawal of consent n = 4, noncompliance or lost to follow‐up n = 6, prohibited therapy n = 2, lack of efficacy n = 2) Notes: none |
| Interventions | Run‐in details: prohibited therapies comprised topical and systemic corticosteroids, antihistamines and antimicrobials, coal tar, topical nonsteroidal anti‐inflammatory drugs, nonsteroidal immunosuppressants, UV light treatments, hypnotics and sedatives, and other investigational drugs. Washout periods for these therapies ranged from 5 days to 6 weeks. Inhaled or intranasal corticosteroids were restricted to 1 mg/d. Intervention one: tacrolimus 0.10% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Intervention two: tacrolimus 0.03% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone 0.1% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: Notes: none |
| Outcomes | · Investigator‐rated erythema, oedema‐induration‐papulation, excoriations, and lichenification on a scale of 0 to 3 · Total BSA affected by AD for 4 body regions (head and neck, trunk, upper limbs, and lower limbs) · Patients assessment of the intensity of itching · mEASI · Clinical improvement in the physician’s global evaluation of clinical response · AE |
| Notes | Funding source: not reported Declarations of interest: one of the two authors was from ASTA Medica AG (a pharma company). Original language of publication: English Other: none |
Reitamo 2004.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre study Trial registration number: not reported Country: Finland, UK, The Netherlands, France, Hungary Outpatient or hospital: 42 centres Date trial conducted: not reported Duration of trial participation: 5 weeks Inclusion criteria: · Male and female patients aged 2 to 15 years · AD according to Hanifin and Rajka criteria · Moderate‐to‐severe AD according to Rajka and Langeland criteria · 5 to 100% of the total BSA Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 624 participants (417 to apply the intervention and 207 to apply the comparator) Age: mean: hydrocortisone acetate 1% group 7.2 years (SD 4.1); tacrolimus 0.03% once daily group 6.7 years (SD 3.9); tacrolimus 0.0 3% twice daily group 6.9 years (SD 4.2) Sex: hydrocortisone acetate 1% group, male 51.7%, female 48.3%; tacrolimus 0.03% once daily group, male 48.3%, female 51.7%; tacrolimus 0.03% twice daily group, male 45.2%, female 54.8% Ethnicity: ‐ Caucasian: hydrocortisone acetate 1% group n = 179; tacrolimus 0.03% once daily group n = 172: tacrolimus 0.03% twice daily group n = 172 ‐ Black: hydrocortisone acetate 1% group n = 9; tacrolimus 0.03% once daily group n = 9; tacrolimus 0.03% twice daily group n = 6 ‐ Oriental: hydrocortisone acetate 1% group n = 6; tacrolimus 0.03% once daily group n = 10; tacrolimus 0.03% twice daily group n = 13 ‐ Other: hydrocortisone acetate 1% group n = 13; tacrolimus 0.03% once daily group n = 16; tacrolimus 0.03% once daily group n = 19 Duration of eczema: mean: hydrocortisone acetate 1% group; 6.3 years (SD 4.0); tacrolimus 0.03% once daily group 5.7 years (SD 3.8); tacrolimus 0.03% once daily group 6.1 years (SD 4.0) Severity of eczema: ‐ Mild: hydrocortisone acetate 1% group n = 0; tacrolimus 0.03% once daily group n = 0; tacrolimus 0.03% twice daily group n = 1 ‐ Moderate: hydrocortisone acetate 1% group n = 93; tacrolimus 0.03% once daily group n = 108; tacrolimus 0.03% twice daily n =111 ‐ Severe: hydrocortisone acetate 1% group n = 114; tacrolimus 0.03% once daily group n = 99; tacrolimus 0.03% twice daily group n = 98 Body site: not reported Number of withdrawals: lack of efficacy: hydrocortisone acetate 1% group n = 17; tacrolimus 0.03% once daily group n = 8; tacrolimus 0.03% twice daily group n = 4 ‐ AE: hydrocortisone acetate 1% group n = 6; tacrolimus 0.03% once daily group n = 3; tacrolimus 0.03% twice daily group n = 8 ‐ Prohibited therapy: hydrocortisone acetate 1% group n = 1; tacrolimus 0.03% once daily group n = 5; tacrolimus 0.0 3% twice daily group n = 1 ‐ Withdrawal of consent: hydrocortisone acetate 1% n = 11; tacrolimus 0.03% once daily n = 6; tacrolimus 0.0 3% twice daily n = 4 ‐ Other: hydrocortisone acetate 1% n = 6; tacrolimus 0.03% once daily n = 4; tacrolimus 0.03% twice daily n = 4 Notes: none |
| Interventions | Run‐in details: prohibited therapies during the study included topical or systemic corticosteroids, antimicrobials and antihistamines, coal tar, topical nonsteroidal anti‐inflammatory drugs, UV treatments, hypnotics and sedatives, and systemic immunosuppressive agents. The washout phase for these therapies ranged from a minimum of 5 days to a maximum of 6 weeks prior to the start of the study. The washout period for systemic corticosteroids and nonsteroidal immunosuppressants was 4 weeks. Inhaled or intranasal corticosteroids were limited to 1 mg per day. Intervention: tacrolimus 0.03% ointment used once or twice daily for 21 days · Concurrent treatment: to ensure blinding, in the TCI once daily group participants also used a vehicle control as the second application. · Other key information: none Comparator: hydrocortisone acetate 1% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator; bath oil and non‐medicated emollients were permitted. Notes: none |
| Outcomes | · mEASI · EASI score · mEASI‐60 · IGA · Patient’s assessment of global response · Physician’s assessment of affected BSA · Quality of sleep · Safety |
| Notes | Funding source: Fujisawa GmbH, Munich, Germany Declarations of interest: not declared Original language of publication: English Other: none |
Reitamo 2005.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre study Trial registration number: not reported Country: 12 European countries, including Finland, France, Denmark, Germany, Italy, the Netherlands Outpatient or hospital: 57 centres in 12 European countries Date trial conducted: November 2000 (first patient, first visit) to May 2002 (last patient, last visit) Duration of trial participation: 6 months Inclusion criteria: · male or female aged 18 years or older · AD based according to Hanifin and Rajka criteria · moderate‐to‐severe AD as defined by the scoring system of Rajka and Langeland Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 972 participants (487 to apply the intervention and 485 to apply the comparator) Age: mean: intervention group 32.1 years (SD 11.6); comparator group 32.9 years (SD 12.0) Sex: intervention group male 46.2%, female 53.8%; comparator group male 46.2%, female 53.8% Ethnicity: not reported Duration of eczema: intervention group, mean 26.1 years (SD 13.1), range 0 to 72; comparator group, mean 24.9 years (SD 13.7), range 0 to 84 Severity of eczema: intervention group: moderate 58.8%, severe 41.2%; comparator group: moderate 56.1%, severe 43.9% Body site: head, neck, trunk and extremities Number of withdrawals: TCS group 42.1% discontinued the study; tacrolimus group 25.6% patients discontinued Notes: none |
| Interventions | Run‐in details: prohibited therapies during the study included topical corticosteroids for the treatment of AD, systemic corticosteroids, systemic antimicrobials, sedating antihistamines, coal tar, ultraviolet (UV) radiation treatments, hypnotics and sedatives. Intervention: tacrolimus 0.1% ointment used twice daily for up to 182 days · Concurrent treatment: none reported · Other key information: patients applied either a thin layer of 0.1% tacrolimus ointment twice daily to all affected body areas. This differed from the comparator arm. Comparator: hydrocortisone acetate 0.1% ointment used twice daily for up to 182 days · Concurrent treatment: not reported · Other key information: patients applied 1% hydrocortisone acetate ointment twice daily to the head and neck and 0.1% hydrocortisone butyrate ointment twice daily to the trunk and extremities. This differed from the tacrolimus arm. Concurrent treatments received alongside both intervention and comparator; bath oil was permitted and patients were allowed to use a non‐medicated emollient 2 hours after application of the study ointment. Notes: after clearance, the lesions were to be treated for an additional 7 days. In the event of flare, ointment application was to resume twice daily until the lesion cleared. |
| Outcomes | · Response rate at month 3, defined as the proportion of patients with at least 60% improvement in the mEASI · Physician’s global evaluation of clinical response · Patient’s assessment of global response · Physician’s assessment of individual signs · Affected total BSA · Patient’s assessment of itch and quality of sleep · Number of days on treatment as a percentage of days in the study ·Safety assessments of the monitoring of AE and vital signs as well as clinical laboratory evaluations |
| Notes | Funding source: the authors received study grants from Fujisawa GmbH, Munich, Germany, to perform the study. Declarations of interest: not declared Original language of publication: English Other: none |
Rosenberg 1971.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind within‐participant study Trial registration number: not reported Country: USA Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 14 days Inclusion criteria: bilateral and symmetrical lesions of AD (amongst other dermatoses not in scope for this review) Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 8 sides treated (4 to apply the intervention and 4 to apply the comparator) Age: 10, 18, 19 and 48 years Sex: female Ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: fluocinonide 0.0 5% cream four times daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: betamethasone valerate 0.1% cream four times daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator; to quote: "No systemically administered corticosteroids or other topical or systemic therapy were used." Notes: none |
| Outcomes | · IGA of excellent, moderate, or no response (2,1, or 0) · Superior clinical response |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Rossi 2002.
| Study characteristics | |
| Methods | Trial design: randomised, investigator‐blinded, parallel study Trial registration number: not reported Country: UK, France, and Germany, assumed from the list of affiliations Outpatient or hospital: Secondary care, assumed from the list of affiliations Date trial conducted: not reported Duration of trial participation: 3 weeks Inclusion criteria: not reported; however, title stated AD Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 152 enrolled; unclear how many were randomised participants Age, sex: ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: flucortolone 0.5% ointment used at unspecified frequency for up to 3 weeks · Concurrent treatment: not reported · Other key information: none Comparator: desonide 0.05% lotion used at unspecified frequency for up to 3 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Efficacy: sum of scores for erythema, infiltration/papulation, excoriation, lichenification and oozing/crusting · Pruritus · Safety |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Roth 1978a.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: USA Outpatient or hospita, date trial conducted: not reported Duration of trial participation: 4 weeks or until clearance Inclusion criteria: bilateral lesions of chronic AD, primarily on the limbs Exclusion criteria: pregnancy Additional design details: three eligible trials were reported in the same publication; described here as Roth 1978a; Roth 1978b; Roth 1978c. |
| Participants | Total number randomised: 58 sides treated (29 to apply the intervention and 29 to apply the comparator) Age, sex: not reported separately for each trial Ethnicity: not reported Duration of eczema: mean 12.1 years Severity of eczema: not reported Body site: to quote: "primarily on the limbs"; otherwise unspecified Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: participants receiving systemic or topical steroids discontinued at least 2 weeks preceding the trial. Participants receiving anti‐metabolites were discontinued at least 3 months before. Intervention: hydrocortisone valerate 0.2% cream used three times daily for 4 weeks · Concurrent treatment: not reported · Other key information: brand: Westcort Comparator: hydrocortisone 1% cream used three times daily for 4 weeks · Concurrent treatment: not reported · Other key information: brand: Cortdome Concurrent treatments received alongside both intervention and comparator: some medications were permitted during the trial (e.g. antihistamines for allergic rhinitis, insulin for diabetes, antibiotics, tranquillisers), with the dosage kept at pre‐trial levels or changed only as therapeutically necessary. However, no anti‐metabolites, steroids (systemic or topical) were permitted. Notes: participants were asked to wash their hands between applications, and to apply the cream marked 'left' with the right hand, and the cream marked 'right' with the left hand. Plastic gloves were provided for the participants to wear on the hand applying the medication if there were lesions on the hands being treated. |
| Outcomes | · Assessment of signs/symptoms: pruritus, erythema, scaling, excoriation, and lichenification evaluated on a 10‐point scale, with cleared = 0 for all symptoms · Participant reports of AE · Participant judgement of which side had responded better · Overall judgement of which side had responded better · Overall judgement defined as cleared, excellent, good, no effect or worse |
| Notes | Funding source: not reported Declarations of interest: not declared; however, one of the authors stated Westwood Pharmaceuticals Inc, Buffalo, New York as their affiliation. Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Roth 1978b.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: USA Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 4 weeks or until clearance Inclusion criteria: patients with bilateral lesions of chronic AD, primarily on the limbs Exclusion criteria: pregnancy Additional design details: three eligible trials are reported in the same publication described here as Roth 1978a; Roth 1978b; Roth 1978c. |
| Participants | Total number randomised: 38 sides treated (19 to apply the intervention and 19 to apply the comparator) Age, sex: not reported separately for each trial Ethnicity: not reported Duration of eczema: mean 7.2 years Severity of eczema: not reported Body site: to quote: "primarily on the limbs"; otherwise unspecified Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: participants receiving systemic or topical steroids discontinued at least 2 weeks preceding the trial. Participants receiving anti‐metabolites were discontinued at least 3 months before. Intervention: hydrocortisone valerate 0.2% cream used three times daily for 4 weeks · Concurrent treatment: not reported · Other key information: brand: Westcort Comparator: betamethasone valerate 0.1% cream used three times daily for 4 weeks · Concurrent treatment: not reported · Other key information: brand: Valisone Concurrent treatments received alongside both intervention and comparator: some medications were permitted during the trial (e.g. antihistamines for allergic rhinitis, insulin for diabetes, antibiotics, tranquillisers), with the dosage kept at pre‐trial levels or changed only as therapeutically necessary. However, no anti‐metabolites, steroids (systemic or topical) were permitted. Notes: participants were asked to wash their hands between applications, and to apply the cream marked 'left' with the right hand, and the cream marked 'right' with the left hand. Plastic gloves were provided for the participants to wear on the hand applying the medication if there were lesions on the hands being treated. |
| Outcomes | · Participant report of AE · IGA (unclear if 4‐ or 8‐point scale was reported in the results) |
| Notes | Funding source: not reported Declarations of interest: none declared; however, one of the authors stated Westwood Pharmaceuticals Inc, Buffalo, New York as their affiliation. Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Roth 1978c.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: USA Outpatient or hospital: to quote: "patient population''; one author was affiliated to the Department of Dermatology, University of California, School of Medicine, San Francisco, California. Date trial conducted: not reported Duration of trial participation: 4 weeks or until clearance Inclusion criteria: bilateral lesions of chronic AD, primarily on the limbs Exclusion criteria: pregnancy Additional design details: three eligible trials are reported in the same publication, described here as Roth 1978a; Roth 1978b; Roth 1978c. |
| Participants | Total number randomised: 40 sides treated (20 to apply the intervention and 20 to apply the comparator) Age, sex: not reported separately for each trial Ethnicity, duration of eczema, severity of eczema: not reported Body site: to quote: "Primarily on the limbs"; otherwise unspecified Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: participants receiving systemic or topical steroids discontinued at least 2 weeks preceding the trial. Participants receiving anti‐metabolites were discontinued at least 3 months before. Intervention: hydrocortisone valerate 0.2% cream used three times daily for 14 days · Concurrent treatment: not reported · Other key information: brand: Westcort Comparator: placebo cream used three times daily (assumed) for 14 days · Concurrent treatment: not reported · Other key information: brand: unspecified Concurrent treatments received alongside both intervention and comparator: some medications were permitted during the trial (e.g. antihistamines for allergic rhinitis, insulin for diabetes, antibiotics, tranquillisers), with the dosage kept at pre‐trial levels or changed only as therapeutically necessary. No anti‐metabolites, steroids (systemic or topical) were permitted. Notes: participants were asked to wash their hands between applications, and to apply the cream marked 'left' with the right hand, and the cream marked 'right' with the left hand. Plastic gloves were provided for the participants to wear on the hand applying the medication if there were lesions on the hands being treated. |
| Outcomes | · Participant report of AE · IGA (unclear if 4‐ or 8‐point scale is reported in the results) |
| Notes | Funding source: not reported Declarations of interest: not declared; however, one of the authors stated Westwood Pharmaceuticals Inc, Buffalo, New York as their affiliation. Original language of publication: English Other: the betamethasone valerate‐controlled study has previously been extracted by this group, and Roth and colleagues stated that the same methodology was used for the placebo‐controlled trial; therefore, some content is reproduced from Lax 2022. |
Rustin 2002.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: Finland, The Netherlands, United Kingdom, Germany, France, and USA Outpatient or hospital: 27 centres Date trial conducted: not reported Duration of trial participation: 5 weeks Inclusion criteria: · Male or female · 2 to 15 years old · AD according to Hanifin and Rajka criteria and moderate‐to‐severe according to Rajka and Langeland criteria · 5 to 60% BSA Exclusion criteria: · Serious skin disorder other than AD that required treatment · History of eczema herpeticum Additional design details: none |
| Participants | Total number randomised: 560 participants (185 to apply intervention one, 189 intervention two and 186 to apply the comparator) Age: 2 to 15 years: hydrocortisone acetate 1% group mean 7.2 years (SD 4.0); tacrolimus 0.03% group mean 7.6 years (SD 4.4); tacrolimus 0.1% group 7.2 years (SD 3.9) Sex: male:female: hydrocortisone acetate 1% group, 51.4:48.6; tacrolimus 0.03% group 40.2:59.8; tacrolimus 0.1% group 51.6:48.4 Ethnicity:% white: hydrocortisone acetate 1% group 81.1; tacrolimus 0.03% group 74.1; tacrolimus 0.1% group 77.4 Duration of eczema: median of current episode: hydrocortisone acetate 1% group 10.9 months; tacrolimus 0.03% group 6.4 months; tacrolimus 0.1% group 6.2 months Severity of eczema: moderate‐severe AD: hydrocortisone acetate 1% group 51.4/48.6; tacrolimus 0.03% group 60.8/39.2; tacrolimus 0.1% group 54.3/45.7 Body site: hydrocortisone acetate 1% group, head and neck 86.5%, upper limbs 98.9%, trunk 83.8%, lower limbs 95. 1%; tacrolimus 0.03% group, head and neck 86.8%, upper limbs 98.9%, trunk 75.7%, lower limbs 95.8%; tacrolimus 0.1% group, head and neck 88.2% Number of withdrawals: hydrocortisone acetate 1% group n = 10.8; tacrolimus 0.03% group n =11.1; tacrolimus 0.1% group n = 7.0 Notes: none |
| Interventions | Run‐in details: prohibited therapies during the study were topical and systemic corticosteroids, antimicrobials and antihistamines, coal tar, topical nonsteroidal anti‐inflammatory drugs, nonsteroidal immunosuppressants, UV light treatments, hypnotics and sedatives, and other investigational drugs. Washout periods for these therapies ranged from 5 days to 6 weeks. Intervention one: hydrocortisone acetate 1% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Intervention two: tacrolimus 0.03% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Intervention three: tacrolimus 0.1% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: treatment consisted of a thin layer of ointment applied twice daily to areas of actively diseased skin. Treatment of cleared lesions was to be stopped 7 days after clearance. Inhaled or intranasal corticosteroids were limited to 1 mg/d. Notes: none |
| Outcomes | · Investigator‐assessed erythema, oedema‐induration‐papulation, excoriations, and lichenification · BSA · Patient‐assessed intensity of itching · mEASI · IGA · AE |
| Notes | Funding source: Fujisawa GmbH, Munich, Germany Declarations of interest: not declared, but study was funded by Fujisawa GambH. Original language of publication: English Other: none |
Ruzicka 1997.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre study Trial registration number: not reported Country: Germany, Hungary, the Netherlands, Poland, UK, Denmark, France, Italy, Finland Outpatient or hospital: 16 centres Date trial conducted: not reported Duration of trial participation: 5 weeks Inclusion criteria: · Male or female · 13 to 60 years of age · Moderate‐to‐severe AD according to the criteria of Rajka and Langeland · Symptomatic area of at least 200 cm2 of skin on the trunk or extremities or both · No evidence of hypersensitivity to the ointment base Exclusion criteria: received any therapy for AD, other than emollients or antihistamines, within three weeks before the start of the washout phase Additional design details: |
| Participants | Total number randomised: 215 participants (54 to apply intervention one and two, 53 to apply intervention three and 54 to apply the comparator) Age: mean: tacrolimus 03% group 30 years (SD 12); tacrolimus 0.1% group 28 years (SD 9); tacrolimus 0.3% group 27 years (SD 10); vehicle group 29 years (SD 1) Sex: female: tacrolimus 0.03% group 52%; tacrolimus 0.1% group 59%; tacrolimus 0.3% group 63%; vehicle group 52% Ethnicity: white tacrolimus 0.03% group 96%; tacrolimus 0.1% group 94%; tacrolimus 0.3% group 94%; vehicle group 98% Duration of eczema, severity of eczema: not reported Body site: the affected area could be noncontiguous and could include the trunk, extremities, face, and neck, but at least 200 cm2 had to be on the trunk or extremities Number of withdrawals: tacrolimus 0.03% group 13%, because of use of prohibited therapy 4%, AE 2%, other 7%; tacrolimus 0.1% group 13%, because of prohibited therapy 0%, AE 7%, other 6%; tacrolimus 0.3% group 14%, because of use of prohibited therapy 6%, adverse event 0%, other 2%; vehicle group 39%, because of use of prohibited therapy 24%, AE 9%, other 6% Notes: none |
| Interventions | Run‐in details: one week washout phase Intervention one: tacrolimus 0.03% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Intervention one: tacrolimus 0.1% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Intervention one: tacrolimus 0.3% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: no concurrent treatment, other than emollients or bath oil, was allowed during the study. Explicitly prohibited were other experimental treatments, tranquillisers and sleeping pills, and systemic, topical, or inhaled corticosteroids, antihistamines, and antimicrobial drugs. Notes: |
| Outcomes | · Overall assessment of the condition of the treated area by investigator and patient · Change in the sum of the scores for erythema, oedema, and pruritus in the treated area from baseline to the completion of treatment |
| Notes | Funding source: Fujisawa, Munich, Germany Declarations of interest: not declared Original language of publication: English Other: one |
Ryu 1997.
| Study characteristics | |
| Methods | Trial design: randomised, single‐blinded, study Trial registration number: not reported Country: South Korea Outpatient or hospital: Department of Dermatology at Kangnam St. Mary's Hospital, Seoul Date trial conducted: April to June 1997 Duration of trial participation: 2 weeks Inclusion criteria: · Aged ≥ 3 years · Mild‐to‐moderate AD according to Hanifin and Rajka criteria · > 5% BSA involved with moderate or more severe disease (we have assumed that the discrepancy between "mild and moderate" and "moderate or more severe disease" is that the former is a global assessment and the latter is local) · Had not received systemic steroid treatment or radiation treatment within the past month or topical steroids in the past week Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 24 participants (12 to apply the intervention and 12 to apply the comparator) Age, sex, ethnicity, duration of eczema: not reported Severity of eczema: BSA: intervention group 47%, comparator group 29%. 8 symptoms (erythema, oedema/vesicles/crusting, scaling, excoriation, lichenification, pig‐/depigmentation, pruritus, loss of sleep) were scored according to 4 categories with 0 being absent Body site: not reported Number of withdrawals: not reported; however, one participant in the comparator group was not included in the analysis and the one participant in this group stopped application due to worsening of involved area despite treatment. Notes: not clear whether the signs/symptoms were assessed by participants and doctors (though it seems from the lists of signs/symptoms that input would have been required from participants (i.e. loss of sleep) and doctors (i.e. lichenification) |
| Interventions | Run‐in details: none Intervention: mometasone furoate 0.1% cream used once daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: fluocortin butylester 0.75% cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: participants were advised not to use 'exterior use' makeup or other local topically applied medications except for toner and lotion. Notes: Applied thinly to involved area |
| Outcomes | · BSA · Participant subjective satisfaction with treatment (excellent, good, poor, exacerbation) · Signs/symptoms (erythema, oedema/vesicles/crust, scales, excoriation, lichenification, pig‐/depigmentation, pruritus, loss of sleep) scored from 0 = absent to 3 = severe in the most severe region ("target area") and over the whole body involved · Assessment in comparison to baseline scores as excellent (76 to 100% decrease), good (51 to 75% decrease), poor (0 to 50% decrease), and exacerbation at baseline and days 3, 8 and 14. Assumed to be an IGA but it may be linked to the signs score · AE including skin atrophy |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: Korean Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Saeki 2019.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: NCT02914548 Country: Japan Outpatient or hospital: outpatient Date trial conducted: September 2016 to June 2017 Duration of trial participation: screening 2 to 30 days, treatment 8 weeks, post‐treatment observation 2 weeks Inclusion criteria: · Male or female · Outpatient · Age 15 to 70 years · AD based according to Hanifin and Rajka criteria · AD for at least 3 years and affecting ≥ 5% to ≤ 0% of BSA · IGA score of 2 (mild) or 3 (moderate) Exclusion criteria: · Pregnant, possibly pregnant, or breastfeeding, who desire to become pregnant or to have their partner become pregnant during the trial period and up until 30 days after the final administration of IMP, or who are unable to either remain abstinent or employ at least two of the specified birth control methods during the trial period and up until 30 days after the final administration of IMP · Clinically significant complication or history of any of the following: · Cardiac disease, endocrinologic disease, pulmonary disease, neurologic disease, psychiatric disease, hepatic disease, renal disease, haematologic disease, immunologic or immunocompromised disease · AD or contact dermatitis flare‐up, within 28 days prior · Concurrent or history of skin disease other than AD · Active viral skin infection or clinically infected AD · Current or history of malignancy within the previous 5 years, except for those in whom basal‐cell skin carcinoma or squamous‐cell skin carcinoma was judged to have been cured · Current or history of recurrent bacterial infection resulting in hospitalisation or requiring intravenous antibiotic treatment within the past 2 years · Abnormal haematology or serum chemistry · Clinically significant blood pressure or pulse rate findings · Unable to stop using topical corticosteroids, topical immunomodulators, topical retinoids and topical antihistamine from 7 days prior · Unable to stop using systemic corticosteroids, systemic immunomodulators, systemic antimetabolites, systemic retinoids and biologics from 28 days prior · Unable to stop treatment with ultraviolet light A, narrowband ultraviolet B, or ultraviolet light B from 28 days prior · Unable to stop using systemic antihistamines, sodium cromoglicate, tranilast, or suplatast tosilate from 7 days prior · Known hypersensitivity (including history) to any drugs (prescription, OTC, etc.) or any ingredient of OPA‐15406 ointment · Used any other investigational drug within 4 months prior to the baseline examination or who are scheduled to participate in any other clinical trial · Never been treated with a prescription medication for AD or who are satisfied with their current AD treatment regimen Additional design details: none |
| Participants | Total number randomised: 200 participants Age: 15 to 75 years Sex: male 65%, female 35% Ethnicity: Japanese (100%) Duration of eczema: vehicle group 25.2 years, 0.3% group 25.1 years, 1% group 24.4 years Severity of eczema: IGA score of 2 or 3 Body site: not reported Number of withdrawals: vehicle group n = 20; 0.3% group n = 21; 31.3%, 1% group n = 14 Notes: none |
| Interventions | Run‐in details: not reported Intervention one: difamilast 0.3% ointment used twice daily for 8 weeks · Concurrent treatment: not reported · Other key information: none Intervention two: difamilast 1% ointment used twice daily for 8 weeks · Concurrent treatment: not reported · Other key information: none Comparator: placebo/vehicle ointment used twice daily for 8 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · AEs · Responder rate IGA · Change in EASI score · Change in VAS for pruritus score · Change in Verbal Rating Scale (VRS) for pruritus score · Change in POEM score · Change in percentage affected BSA · Mean (SD) OPA‐15406 plasma trough concentrations |
| Notes | Funding source: Otsuka Pharmaceutical Co., Ltd. Declarations of interest: two authors had received consultation fees from Otsuka Ltd and the other three authors were employees of Otsuka Ltd. Original language of publication: English Other: none |
Saeki 2020.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: NCT03018691 Country: Japan Outpatient or hospital: outpatient Date trial conducted: January to June 2017 Duration of trial participation: 28 days Inclusion criteria: · Age 2 to 14 years · AD according to the Hanifin and Rajka criteria · AD affecting ≥ 5% to ≤ 40% of BSA · IGA score of 2 (mild) or 3 (moderate) Exclusion criteria: · Judged by the investigator or subinvestigator to be problematic in withdrawing blood · Unable to stop using topical corticosteroids, topical immunomodulators, topical retinoids and topical antihistamine from 7 days prior · Unable to stop using systemic corticosteroids, systemic immunomodulators, systemic antimetabolites, systemic retinoids, and biologics from 28 days prior · Unable to stop treatment with ultraviolet A, narrowband ultraviolet B, and ultraviolet B from 28 days prior · Unable to continue in the trial without changing the dosage and administration of systemic antihistamines, sodium cromoglicate, tranilast, or suplatast tosilate from 7 days prior Additional design details: none |
| Participants | Total number randomised: 73 participants Age: 2 to 14 years Sex: male 71.2%; female 28.8% Ethnicity: Asian (100%) Duration of eczema: vehicle: 7.2+/‐3.3; 0.3% group: 7.5+/‐3.9; 1% group: 7.2+/‐3.3 Severity of eczema: IGA score of 2 or 3 at baseline Body site: not reported Number of withdrawals: vehicle group n = 7, 0.3% group n = 2, 1% group n = 1 Notes: none |
| Interventions | Run‐in details: not reported Intervention one: difamilast 0.3% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention two: difamilast 1% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: placebo/vehicle ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · AEs · IGA at week 4 · Change in EASI Score · Change VAS for pruritus score · Change in VRS for pruritus score · Change in POEM score · Change in percentage affected BSA · Mean (SD) OPA‐15406 plasma trough concentrations |
| Notes | Funding source: Otsuka Pharmaceutical Co., Ltd. Declarations of interest: three authors have received fees for consultation from Otsuka Pharmaceutical Co., Ltd. Original language of publication: English Other: none |
Saeki 2022a.
| Study characteristics | |
| Methods | Trial design: randomised, triple‐blind, parallel study Trial registration number: JapicCTI‐194700 and NCT03911401 Country: Japan Outpatient or hospital: outpatients Date trial conducted: May to December 2019 Duration of trial participation: 4 weeks Inclusion criteria: · Male or female aged 2 to 14 years · AD diagnosed according to the diagnostic criteria of the Japanese Dermatological Association · AD for at least 3 years · IGA score of mild‐to‐moderate · BSA of 5 to 40% (excluding scalp) Exclusion criteria: · AD or contact dermatitis flare‐up within 28 days · Received one of several therapies prior to the study (e.g. UV therapy, systemic or topical corticosteroids) · Unable to continue without changing regimen of systemic antihistamines, sodium cromoglicate, tranilast or suplatast tosilate Additional design details: none |
| Participants | Total number randomised: 251 participants (83 to apply intervention one, 85 intervention two and 83 to apply the comparator) Age: range from 2 to 14 years Sex: male or female Ethnicity: Japanese Duration of eczema: difamilast 0.3% group 5 years (SD 3.2); difamilast 1% group 5.2 years (SD 3.0); vehicle group 5.5 years (SD 2.8) Severity of eczema: mild‐to‐moderate Body site: none facial eczema Number of withdrawals: difamilast 0.3% group n = 7, difamilast 1% group n = 9, vehicle group n = 24 Notes: none |
| Interventions | Run‐in details: not reported Intervention one: difamilast 0.3% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention two: difamilast 1% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: placebo/vehicle ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA ·EASI ·POEM ·Withdrawal due to AE |
| Notes | Funding source: Otsuka Pharmaceutical Co Ltd. Declarations of interest: none declared Original language of publication: English Other: none |
Saeki 2022b.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre study Trial registration number: NCT03908970 Country: Japan Outpatient or hospital: 30 investigational sites Date trial conducted: March 2019 to December 2019 Duration of trial participation: none Inclusion criteria: · Male or female aged 15 to 70 years · AD diagnosed according to the diagnostic criteria of the Japanese Dermatological Association · AD for at least 3 years · IGA score of mild‐to‐moderate · BSA of 5 to 40% (excluding scalp) Exclusion criteria: · Pregnant, possibly pregnant, or breastfeeding, who desire to become pregnant or to have their partner become pregnant or who are unable to either remain abstinent or employ at least two of the specified birth control methods · AD or contact dermatitis flare‐up defined as a rapid intensification of AD, within 28 days prior to the baseline examination · Concurrent or history of skin disease other than AD and who are judged inappropriate for assessment of AD in the present trial · Active or clinical signs of viral skin infection · Current or history of malignancy within the previous 5 years · Current or history of recurrent bacterial infection resulting in hospitalisation or requiring intravenous antibiotic treatment within the past 2 years · Clinically significant complication or history of: cardiac disease, endocrinologic disease, pulmonary disease, neurologic disease, psychiatric disease, hepatic disease, renal disease, haematologic disease, immunologic or immunocompromised disease · Other major disease or other severe uncontrolled condition · Abnormal haematology or serum chemistry · Clinically abnormal blood pressure or pulse rate · Unable to stop allergen immunotherapy (or desensitisation therapy) from 3 months prior · Unable to stop treatment with ultraviolet A, narrowband ultraviolet B, and ultraviolet B from 28 days prior · Unable to stop using systemic corticosteroids, immunosuppressants, antimetabolites, or retinoids, and biologics from 28 days prior · Unable to stop using very strong or higher potency topical corticosteroids for skin (excluding scalp) · Unable to continue in the trial without changing the dosage and administration of systemic antihistamines, sodium cromoglycate, tranilast, or suplatast tosilate from 7 days prior · Hypersensitivity (including history) to any drugs or any ingredient of study drug ointment · Used any other investigational drug within 4 months · Does not respond to treatment with existing topical drugs for AD Additional design details: none |
| Participants | Total number randomised: 364 participants (182 to apply the intervention and 182 to apply the comparator) Age: mean: intervention group 31.7 years (SD 10.9); comparator group 32.1 years (SD 10.6) Sex: intervention group 55.5% male; comparator group 52.7% male Ethnicity: not reported Duration of eczema: intervention group 24.6 years (SD 11.3); comparator group 25 years (SD 11.1) Severity of eczema: intervention group: mild 6.6%, moderate 76.4%, severe 17%, very severe 0%; comparator group: mild 5.5%, moderate 76.9%, severe 16.5%, very severe 1.1% Body site: not reported Number of withdrawals: 63: intervention group 9.3%; comparator group 25.8% Notes: none |
| Interventions | Run‐in details: not reported Intervention: difamilast 1% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: placebo/vehicle ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA · EASI · Verbal rating scale for pruritus · POEM · Skindex‐16 score · BSA · Safety |
| Notes | Funding source: Otsuka Pharmaceutical Co Ltd. Declarations of interest: One author has received consultation fees from Otsuka Pharmaceutical Co, Ltd. Several authors are employees of Otsuka Pharmaceutical Co, Ltd. Original language of publication: English Other: none |
Salava 2021.
| Study characteristics | |
| Methods | Trial design: randomised, single‐centre, parallel study Trial registration number: not reported Country: Finland Outpatient or hospital: paediatric unit of Helsinki University Hospital, Skin and Allergy Hospital, Helsinki Date trial conducted: not reported Duration of trial participation: one week washout period with a 36‐month follow‐up Inclusion criteria: · Aged 1 to 3 years · Moderate‐to‐severe AD according to Rajka and Langeland severity score 4.5 to 7.5 (moderate) or 8 to 9 (severe) Exclusion criteria: · Recurrent infections e.g. otitis, pneumonia and skin infections · Signs of immune deficiency or chronic diseases · Continuous medications including inhaled corticosteroids Additional design details: none |
| Participants | Total number randomised: 152 participants (75 to apply the intervention and 77 to apply the comparator) Age: mean 1.43 years; intervention group mean 1.44 years; comparator group: mean 1.42 years Sex: male n = 79, female n = 73; intervention group male n = 44, female n = 31; comparator group: male n = 35, female n = 34 Ethnicity, duration of eczema: not reported Severity of eczema: according to Rajka and Langeland criteria: moderate n = 82, severe eczema n = 70; intervention group: moderate n = 41, severe eczema n = 34; comparator group: moderate n = 41, severe eczema n = 36 Body site: not reported Number of withdrawals: 27: intervention group n = 12,; comparator group n = 15, to quote: "any withdrawals were mostly due to noncompliance or to the family moving away." Notes: none |
| Interventions | Run‐in details: to quote: "1‐week washout period" Intervention: hydrocortisone acetate 1% cream twice daily for 3 to 7 days or until clearance, then twice‐weekly · Concurrent treatment: not reported · Other key information: treatment could be escalated to hydrocortisone butyrate 0.1% cream if needed Comparator: tacrolimus 0.03% ointment twice daily for 3 to 7 days or until clearance, then twice‐weekly · Concurrent treatment: not reported · Other key information: treatment could be escalated to tacrolimus 0.1% ointment if needed Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Safety including infection rate and growth parameters (height and weight) · EASI · Vaccination responses and other safety relevant laboratory values · Skin‐related and non‐skin‐related infections were noted retrospectively through information from parents/caregivers. Type and severity could not be verified. (assessed at baseline, week 1, months 1, 3, 6, 9 and 12, and then 6 monthly up to 36 months) |
| Notes | Funding source: to quote: "The work was supported by: The Pediatric Research Foundation, Helsinki University Hospital Research Fund, Sigrid Juselius Foundation, Päivikki and Sakari Sohlberg Foundation, Finnish Dermatological Society, Allergy Research Foundation, Väinö and Laina Kivi Foundation, Orion Research Foundation, Ida Montin Foundation, Orion Pharma Finland and Astellas Pharma. The sponsors had no influence on the study". Declarations of interest: not declared Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Salavec 2004.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind study Trial registration number: not reported Country: Czech Republic (assumed from the affiliations of the authors) Outpatient or hospital: hospital clinics (assumed from the affiliations of the authors) Date trial conducted: not reported Duration of trial participation: 12 months Inclusion criteria: children with mild‐to‐moderate AD Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 40 participants (27 to apply the intervention and 13 to apply the comparator) Age: average 8.67 years (range 2 to 17) Sex, ethnicity, duration of eczema: not reported Severity of eczema: 22 monitored patients had moderate eczema; mild eczema was also eligible Body site: not reported Number of withdrawals: to quote: "3 patients in each group of monitored patients did not complete the clinical trial lasting 12 months." Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream used twice daily for 12 months · Concurrent treatment: not reported · Other key information: brand: Elidel Comparator: vehicle cream used twice daily for 12 months · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: participants were also expected to use emollient, and hydrocortisone butyrate (Locoid) was used for a week in the event of a worsening of the eczema. Notes: none |
| Outcomes | · EASI · Pruritus · Time to/incidence of flares · AE |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: Czech Other: none |
Samman 1962.
| Study characteristics | |
| Methods | Trial design: randomised, cross‐over study Trial registration number: not reported Country: UK Outpatient or hospital: Department of Dermatology, Westminster Hospital, London Date trial conducted, duration of trial participation: not reported Inclusion criteria: some form of eczema or dermatitis Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 30 sides treated (15 to apply the intervention and 15 to apply the comparator) Age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: out of 31 patients who entered the trial, 15 were given both preparations. It is not clear what the other participants received. Quote: "It was not possible to give both preparations to every patient, either because the lesions had entirely or almost entirely subsided, or because the response to the first preparation was of such a nature that it was not considered wise to use the other apparently identical material." |
| Interventions | Run‐in details: not reported Intervention: fluocinolone acetonide 0.025% cream · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone 1% cream used · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Patient preference |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Sanabria‐Silva 1991.
| Study characteristics | |
| Methods | Trial design: randomised, parallel study Trial registration number: not reported Country: Mexico Outpatient or hospital: outpatients; National Institute of Paediatrics Date trial conducted: May to December 1985 Duration of trial participation: 4 weeks of treatment followed by 10 days of post‐treatment follow‐up Inclusion criteria: · Age 3 to 12 years · AD diagnosed by a doctor from the Dermatology Department · Lesions on > 30% of the body surface · The percentage affected was calculated based on the rule of 9s. Exclusion criteria: · Under 3 years old (due to absorption being greater in this age group) · Over 12 years old (due to AD being less common in adolescence) · Secondary infection · Hypersensitivity to corticosteroids · Concurrent systemic illness · Treated with topical or systemic steroids 4 weeks before the trial Additional design details: none |
| Participants | Total number randomised: 45 participants (15 to apply intervention one, 15 intervention two and 15 to apply the comparator) Age: 28 pre‐school children: 17 school children Sex: females n = 14, males n = 31 Ethnicity: not reported Duration of eczema: not reported Severity of eczema: potent TCS group (15): 8 participants with 30 to 50% BSA affected, 6 with 51 to 70%, and 1 with > 70%; placebo group (15): 9 participants with 30 to 50% BSA affected, 6 with 51 to 70%; mild TCS group: not reported Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: betamethasone dipropionate 0.0 5% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention: hydrocortisone 1% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: placebo 0% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: parents/carers were instructed at baseline on how to apply the medications. Rules of care were also recommended; these included: use of emollients when the skin appears dry; other medication was prohibited; bathing daily if possible; use of cotton underwear Notes: none |
| Outcomes | · to quote: "Extensiveness of lesions" (noting percentage of skin affected, presence or absence of erythema, lichenification and scabs); and photographs · Number of participants relapsing · Number of participants experiencing rebound · AE |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: Spanish Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Savin 1976.
| Study characteristics | |
| Methods | Trial design: randomised double‐blind study Trial registration number: not reported Country: US Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 3 weeks Inclusion criteria: · AD or psoriasis of moderate‐to‐very severe · AD of discrete confluent oedematous papules which were intensely pruritic in the classical anatomical distribution and becoming lichenified from rubbing Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 27 participants Age, sex, ethnicity, duration of eczema: not reported Severity of eczema: 26 moderate, one very severe Body site: not reported Number of withdrawals: at least 5. Unclear what reasons for withdrawal were Notes: none |
| Interventions | Run‐in details: all patients stopped topical medication one week prior and systematic medication three weeks prior. Diuretics and digitalis were continued prior to and through the study. Intervention: betamethasone dipropionate 0.05% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone 1% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: the participants were instructed to bathe daily with ordinary soap and to use no other topicals like emollient or bath oils. Notes: none |
| Outcomes | · Clinical effectiveness rated as excellent, good, fair or poor · Total symptom score |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Schachner 2005.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre study Trial registration number: not reported Country: USA Outpatient or hospital: 18 study sites Date trial conducted: September 2001 to November 2002 Duration of trial participation: 6 weeks Inclusion criteria: · 2 to 15 years of age · Mild or moderate AD according to Hanifin and Rajka criteria · 2 to 30% BSA Exclusion criteria: · Additional skin disorder in the target area · Clinically infected AD · Known hypersensitivity to macrolides or excipients of the ointment · Previous use of tacrolimus ointment for AD · Pregnancy or breastfeeding Additional design details: none |
| Participants | Total number randomised: 317 participants (158 to apply the intervention and 159 to apply the comparator) Age: mean: intervention group 6.7 years (SD 4.0); comparator group 7.0 years (SD 4.1) Sex: intervention group male n = 47, female n = 53; comparator group male n = 47, female n = 53 Ethnicity: intervention group, white n= 65, black n = 23, Asian n = 6, other n = 5; comparator group, white n = 71, black n = 23, Asian n = 4, other n = 1 Duration of eczema: not reported Severity of eczema: intervention group: mild 61%, moderate 39%; comparator group: mild 60%, moderate 40% Body site: intervention group 59% head and neck involvement; comparator group 54% head and neck involvement Number of withdrawals: intervention group n = 29; lost to follow‐up n =10, AE n = 7, lack of efficacy n = 4, other n = 8; comparator group n = 61; lost to follow‐up n = 14, AE n = 12, lack of efficacy n = 20, other n = 15 Notes: none |
| Interventions | Run‐in details: to quote: "specific prestudy and concomitant therapy restrictions"; these were unspecified. Intervention: tacrolimus 0.03% ointment used twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: to quote: "thin coating [of study medications] at least 2 hours before bathing for up to 6 weeks by patients or caregivers. Treatment was continued for an additional week after any individual lesions cleared. Thereafter, the cleared area(s) was excluded from additional treatment, while all remaining lesions continued to be treated. If all treated areas completely cleared before the week‐6 visit, treatment continued in all areas for 1 additional week and was followed by an end‐of‐study visit. No treatments continued beyond 6 weeks." · Nonsteroidal immunosuppressants, other investigational drugs, systemic corticosteroids, UV light therapy, as well as concomitant topical medications were not allowed within up to 4 weeks of the trial and during the treatment period. · Intranasal or inhaled corticosteroids were permitted for FDA‐approved indications and not exceeding maximal approved doses. · Sunscreen and nonmedicated emollients were permitted on untreated areas. · Cosmetics were prohibited on treated areas. · Oral antihistamines were permitted if the participant was on a stable dose at baseline; dosage could be decreased or discontinued during the study. Notes: none |
| Outcomes | · Percentage with IGADA “clear” or “almost clear” · EASI · Patient assessment of itch (VAS) · Withdrawal · Local AE |
| Notes | Funding source: Astellas Pharma US, Inc. Declarations of interest: To quote: "Ms Shull and Dr Jaracz are full‐time employees of Astellas Pharma US, Inc. All other authors served as principal investigators in this study. Drs Schachner and Boguniewicz have received research support/grants and lecture honoraria from Astella". Original language of publication: English Other: none |
Schuppli 1983.
| Study characteristics | |
| Methods | Trial design: randomised, multicentre study Trial registration number: not reported Country: Austria and Switzerland Outpatient or hospital, date trial conducted, duration of trial participation: not reported Inclusion criteria: · Acute eczematic dermatosis of various origins · Adult male or female Exclusion criteria: not reported Additional design details: AD was a subgroup of the study sample with results provided separately. |
| Participants | Total number randomised: 26 participants Age, sex: not reported for AD subgroup separately Ethnicity: not reported Duration of eczema, severity of eczema: not reported for AD subgroup separately Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: halometasone 0.05% cream used twice daily for 20 days · Concurrent treatment: not reported · Other key information: none Comparator: betamethasone dipropionate 0.05% cream used twice daily for 20 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: no other treatment of eczematic dermatosis was allowed during the trial period. Notes: none |
| Outcomes | AE, investigator therapeutic effect |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: German Other: none |
Sears 1997.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: USA Outpatient or hospital: assumed outpatient Date trial conducted: not reported Duration of trial participation: 14 days Inclusion criteria: disease severity determined by the total score of 7 signs (infiltration, scaling, erythema, lichenification, vesicles, papules, and excoriation) evaluated on a 4‐point scale (0 = absent, 1 = mild, 2 = moderate, 3 = severe). Total score of > 6 required for randomisation Exclusion criteria: · Concurrent diagnosis of an acute systemic illness, including convalescence, severe hepatic disease, or severe renal impairment · Suspected viral, bacterial, or fungal infection of the skin areas selected for treatment · Systemic treatment within the month preceding the trial · Concomitant systemic treatment that might affect the clinical status of the lesions · Known hypersensitivity to or intolerance for topical corticosteroids or any of the ingredients of the study preparations · Pregnant or lactating women or women of childbearing potential who were not using effective contraception Additional design details: none |
| Participants | Total number randomised: 194 participants (121 to apply the intervention and 73 to apply the comparator) Age: average 37.7 years (range 17 to 76) Sex: female n = 107; male = 87 Ethnicity: not reported Duration of eczema: treatment group 10.8 years (SD 12.7); placebo group 11.6 years (SD 15 years) Severity of eczema: treatment group, mild = 18.3%, moderate = 69.2%, severe = 12.5%; placebo group, mild = 15.1%, moderate = 63%, severe = 21.9% Body site: treatment areas of approximately 15% of body surface on the basis of location, size and severity. Treatment areas did not include the face, hands, groin or axillae. Number of withdrawals: 26 withdrawals, 15 treatment group; 11 placebo group Notes: none |
| Interventions | Run‐in details: use of the following medications was a disqualification: topical corticosteroids within 2 weeks of the study; retinoids, intralesional or repository corticosteroids, or long‐acting antihistamines (half‐life > 24 hours) within 4 weeks of the study; or any investigational drug within 1 month of the study Intervention: hydrocortisone buteprate 0.1% cream used once daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used once daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Mean lesion score (7‐point signs score) · Investigator rating of overall improvement at each efficacy level · % patients receiving good/excellent efficacy · Patient evaluation of cosmetic qualities of treatment · Safety and tolerability assessment |
| Notes | Funding source: Savage Laboratories, Melville, New York Declarations of interest: not declared Original language of publication: English Other: none |
Sefton 1983a.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: USA Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 14 days Inclusion criteria: · With bilateral, symmetrical lesions of stable, chronic AD · Mild‐to‐moderate severity disease Exclusion criteria: · Experiencing an acute flare or a rebound effect from prior treatment · Atypical AD, also those with to quote: "a generalised exfoliative or vesicular, exudative stage" · Disease only on hands and feet · Known sensitivity to components of the trial medication · Secondary infection · Pregnancy Additional design details: none |
| Participants | Total number randomised: 150 sides treated (75 to apply the intervention and 75 to apply the comparator) Age: not reported for this subgroup Sex: not reported for this trial alone, however all were eligible Ethnicity, duration of eczema: not reported for this subgroup Severity of eczema: not reported for this subgroup alone; mild and moderate severity cases were eligible. Body site: not reported Number of withdrawals: not reported for this subgroup alone; of 145 randomised to the three active comparator trials, 131 were evaluated. 14 were excluded owing to protocol deviations or missed follow‐up visits. Notes: none |
| Interventions | Run‐in details: participants did not take TCSs in the 2 weeks preceding the trial, or parenteral steroids in the 4 weeks preceding. Intervention: hydrocortisone valerate 0.2% ointment used twice daily for 14 days. · Concurrent treatment: none · Other key information: brand: Westcort, Westwood Pharmaceuticals, Inc. Comparator: betamethasone valerate 0.1% ointment used twice daily for 14 days · Concurrent treatment: none · Other key information: brand: Valisone, Schering Corp Concurrent treatments received alongside both intervention and comparator: written and oral instructions were given to all participants. Other topical medications and concurrent systemic corticosteroids were prohibited during the trial. Notes: none |
| Outcomes | · AE (all written comments from case report forms) · Investigator assessment of signs from 'none'/'clear' to most severe (100): pruritus (paper explicitly stated investigator‐assessed), erythema, scaling, papulation, lichenification, vesiculation · IGA 0 = clear to 100 = most severe |
| Notes | Funding source: not reported, however the trial authors are affiliated to Bristol‐Myers Pharmaceutical Research and Development Division, Buffalo, New York. Declarations of interest: not declared Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. There were some reporting differences between the two reports of this trial, e.g. the number of withdrawals differed slightly. |
Sefton 1983b.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: USA Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 14 days Inclusion criteria: · With bilateral, symmetrical lesions of stable, chronic AD · Mild‐to‐moderate‐severity disease Exclusion criteria: · Experiencing an acute flare or a rebound effect from prior treatment · Atypical AD, also those with "a generalised exfoliative or vesicular, exudative stage" · Disease only on hands and feet · Known sensitivity to components of the trial medication · Secondary infection · Pregnancy Additional design details: none |
| Participants | Total number randomised: 78 sides treated (39 to apply the intervention and 39 to apply the comparator) Age: not reported for AD alone Sex: not reported for AD alone; however all were eligible. Ethnicity, duration of eczema: not reported for this trial alone Severity of eczema: not reported for AD alone; mild and moderate severity cases were eligible. Body site: not reported Number of withdrawals: not reported for this trial alone; of 145 randomised to the three active comparator trials, 131 were evaluated. 14 were excluded owing to protocol deviations or missed follow‐up visits. Notes: none |
| Interventions | Run‐in details: participants did not take TCSs in the 2 weeks preceding the trial, or parenteral steroids in the 4 weeks preceding. Intervention: hydrocortisone valerate 0.2% ointment used twice daily for 14 days · Concurrent treatment: none · Other key information: brand: Westcort, Westwood Pharmaceuticals, Inc. Comparator: triamcinolone acetonide 0.1% ointment used twice daily for 14 days · Concurrent treatment: none · Other key information: brand: Kenalog, E. R. Squibb & Sons, Inc Concurrent treatments received alongside both intervention and comparator: written and oral instructions were given to all participants. Other topical medications and concurrent systemic corticosteroids were prohibited during the trial. Notes: none |
| Outcomes | · AE (all written comments from case report forms) · Investigator assessment of signs from 'none'/'clear' to most severe (100): pruritus (paper explicitly stated investigator‐assessed), erythema, scaling, papulation, lichenification, vesiculation · IGA (0 = clear, 100 = most severe) |
| Notes | Funding source: not reported, however, the trial authors are affiliated to Bristol‐Myers Pharmaceutical Research and Development Division, Buffalo, New York. Declarations of interest: none declared; as above. Original language of publication: English Other: This study has previously been extracted by this group; some content is reproduced from Lax 2022. There were some reporting differences between the two reports of this trial, e.g. the number of withdrawals differed slightly. |
Sefton 1983c.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: USA Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 14 days Inclusion criteria: · Has bilateral, symmetrical lesions of stable, chronic AD · Mild‐to‐moderate‐severity disease Exclusion criteria: · Experiencing an acute flare or a rebound effect from prior treatment · Atypical AD; also those with "a generalised exfoliative or vesicular, exudative stage" · Disease only on hands and feet · Known sensitivity to components of the trial medication · Secondary infection · Pregnancy Additional design details: none |
| Participants | Total number randomised: 62 sides treated (31 to apply the intervention and 31 to apply the comparator) Age: not reported for this trial alone Sex: not reported for this trial alone; however, all were eligible. Ethnicity: not reported for this trial alone Duration of eczema: not reported for this trial alone Severity of eczema: not reported for this trial alone; mild and moderate severity cases were eligible. Body site: not reported Number of withdrawals: not reported for this trial alone; of 145 randomised to the three active comparator trials, 131 were evaluated. 14 were excluded owing to protocol deviations or missed follow‐up visits. Notes: none |
| Interventions | Run‐in details: participants did not take TCSs in the 2 weeks preceding the trial, or parenteral steroids in the 4 weeks preceding. Intervention: hydrocortisone valerate 0.2% ointment used twice daily for 14 days. · Concurrent treatment: none · Other key information: brand: Westcort, Westwood Pharmaceuticals, Inc. Comparator: fluocinolone acetonide 0.025% ointment used twice daily for 14 days · Concurrent treatment: none · Other key information: Brand: Synalar, Syntex Laboratories, Inc. Concurrent treatments received alongside both intervention and comparator: other topical medications and concurrent systemic corticosteroids were prohibited during the trial. Notes: none |
| Outcomes | · AE: all written comments from case report forms · Investigator assessment of signs from 'none'/'clear' to most severe (100): pruritus (paper explicitly stated investigator‐assessed), erythema, scaling, papulation, lichenification, vesiculation · IGA (0 = clear 100 = most severe) |
| Notes | Funding source: not reported; however, the trial authors are affiliated to Bristol‐Myers Pharmaceutical Research and Development Division, Buffalo, New York. Declarations of interest: none declared; as above Original language of publication: English Other: This study has previously been extracted by this group; some content is reproduced from Lax 2022. There were some reporting differences between the two reports of this trial, e.g. the number of withdrawals differed slightly. |
Sefton 1983d.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant trial Trial registration number: not reported Country: USA Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 14 days Inclusion criteria: · Has bilateral, symmetrical lesions of stable, chronic AD · Mild‐to‐moderate‐severity disease · Aged ≥ 12 years Exclusion criteria: · Experiencing an acute flare or a rebound effect from prior treatment · Atypical AD; also those with "a generalised exfoliative or vesicular, exudative stage" · Disease only on hands and feet · Known sensitivity to components of the trial medication · Secondary infection · Pregnancy Additional design details: none |
| Participants | Total number randomised: 128 sides treated (64 to apply the intervention and 64 to apply the comparator) Age: ≥ 12 years eligible Sex: not reported for this trial alone; however all were eligible. Ethnicity, duration of eczema: not reported for this trial alone Severity of eczema: not reported for this trial alone; mild and moderate severity cases were eligible. Body site: not reported Number of withdrawals: not reported for this trial alone; of 145 randomised to the three active comparator trials, 131 were evaluated. 14 were excluded owing to protocol deviations or missed follow‐up visits. Notes: none |
| Interventions | Run‐in details: participants did not take TCSs in the 2 weeks preceding the trial, or parenteral steroids in the 4 weeks preceding. Intervention: hydrocortisone valerate 0.2% ointment used twice daily for 14 days · Concurrent treatment: not reported · Other key information: brand: Westcort, Westwood Pharmaceuticals, Inc. Comparator: vehicle ointment used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: written and oral instructions were given to all participants. Other topical medications and concurrent systemic corticosteroids were prohibited during the trial. Notes: none |
| Outcomes | · AE: all written comments from case report forms · Investigator assessment of signs from 'none'/'clear' to most severe (100): pruritus (paper explicitly stated investigator‐assessed), erythema, scaling, papulation, lichenification, vesiculation · IGA (0 = clear 100 = most severe) |
| Notes | Funding source: none reported; however, the trial authors are affiliated to Bristol‐Myers Pharmaceutical Research and Development Division, Buffalo, New York. Declarations of interest: none declared; as above Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Siegfried 2006.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: CASM981CUS04 Country: USA Outpatient or hospital: 35 sites Date trial conducted: October 2001 to November 2002 Duration of trial participation: up to 24 weeks Inclusion criteria: · Male or female aged 3 months to 11 years · Mild‐to‐severe AD, as determined by an IGA score of 2 (mild), 3 (moderate) or 4 (severe) · AD affecting 5% total BSA based on rule of 9s, on a stable dose of an allowed bland emollient for at least 1 week at baseline, and who were outpatients at baseline · AD for at least one year with symptoms on arms, trunk, and/or legs. · AD using diagnostic criteria of Sampson 1990 in children younger than 2 years of age and diagnostic criteria of Williams 1994 in children 2 years of age and older · Disease must be stable or slowly worsening. Exclusion criteria: · Female patients of childbearing potential were excluded if they were pregnant, breastfeeding, or not practising a medically approved method of contraception during and up to at least 4 weeks after the end of treatment. · Use of any systemic therapy within 1 month of study start, any topical therapy other than a low‐potency to mid‐potency topical corticosteroid within 7 days of study start, being immunocompromised, or having any active infection or concurrent skin condition that would interfere with evaluation Additional design details: the trial involved a treatment algorithm of up to 3 stages of care escalation, to quote: "[1] subjects in both treatment groups applied a bland emollient (e.g. petroleum jelly, EucerinH cream, CurelH cream) to areas of dry skin twice daily. [2] At the first sign of AD, subjects applied the study drug or vehicle twice daily to eczematous sites until complete resolution of the skin inflammation. [3] If AD had not improved or severe AD occurred (i.e. IGAw4 was considered a major flare), the evening pimecrolimus dose or vehicle was replaced with a mid‐potency topical CS. The flare regimen was continued until all signs and symptoms of AD resolved or for a maximum of 3 weeks prior to resuming the treatment algorithm". |
| Participants | Total number randomised: 275 participants (183 to apply the intervention and 92 to apply the comparator) Age: intervention group: mean 59 months (SD 38.06), range 3 to 140; comparator group: mean 61.8 months (SD 40.9), range 3‐143 Sex: intervention group male 54.1%; comparator group male 55.4% Ethnicity: intervention group: white 50.8%, black 23%, oriental 4.9%, other 21%; comparator group: white 55.4%, black 31.5%, oriental 5.4%, other 23.9% Duration of eczema: not reported Severity of eczema: mild‐to‐severe: intervention group mean IGA 2.9 (0 = clear to 5 very severe), vehicle group 2.9. In both groups, the mean pruritus severity score was 1.9 (0 = absent, 3 = severe) and total BSA affected 29%. Body site: not reported Number of withdrawals: intervention group, lost to follow‐up 7.7%, unsatisfactory therapeutic effect 3.8%, patient withdrawal consent 4.4%, AE 2.2%; comparator group, lost to follow‐up 6.5%, unsatisfactory therapeutic effect 14.1%, patient withdrawal of consent 4.3%, AE 3.3% Notes: none |
| Interventions | Run‐in details: 1‐month washout for systemic anti‐inflammatory agents or phototherapy; a 1‐week washout for all topical agents other than low‐ to mid‐potency CSs; and a 2‐week washout for all systemic antibiotics Intervention: pimecrolimus 1% cream used twice daily for 24 weeks · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 24 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: to quote: "subjects in both treatment groups applied pimecrolimus or vehicle to eczematous sites twice daily (bid) and a bland emollient, such as petroleum jelly, EucerinH cream, or CurelH cream, to areas of dry skin". Notes: none |
| Outcomes | Pruritus VAS, IGA, EASI and QoL |
| Notes | Funding source: Novartis Pharmaceuticals Corporation Declarations of interest: two authors were affiliated to Novartis. Original language of publication: English Other: none |
Sigurgeirsson 2005.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: ASM981C2315 Country: 44 centres in 10 countries: Belgium n = 2, Canada n = 5, Finland n = 4, France n = 5, Iceland n = 4, Norway n = 2, Poland n = 3, Spain n = 3, Sweden n = 8, and UK n = 8 Outpatient or hospital: not reported Date trial conducted: Sept 2003 to July 2004 Duration of trial participation: 26 weeks Inclusion criteria: · Children and adolescents 2 to 17 years of age · History of mild‐to‐moderate AD requiring treatment with TCS, pimecrolimus or tacrolimus at least twice in the 6 months preceding randomisation · At least one treatment event occurring within 3 months of randomisation · Clear or almost clear of disease by IGA = 1 Exclusion criteria: · Medical history, and concomitant illnesses and treatments that could interfere with study evaluations · Inadequate response to tacrolimus or pimecrolimus; immunocompromised status, concurrent skin disease that could interfere with study evaluation; active bacterial, viral, or fungal infections; head lice or scabies; or known hypersensitivity to any ingredient of the study medications Additional design details: none |
| Participants | Total number randomised: 521 participants (256 to apply the intervention and 265 to apply the comparator) Age: 2 to 17 years Sex: f:m: intervention group 147:109; comparator group 161:104 Ethnicity: number intervention:comparator: white 244:242, black 1:2, Asian 6:14, other 5:7 Duration of eczema: at least 6 months Severity of eczema: mild‐to‐moderate requiring treatment with TCS, pimecrolimus or tacrolimus at least twice in the 6 months preceding randomisation, and with at least one treatment event occurring within 3 months of randomisation Body site: not reported Number of withdrawals: intervention:comparator: withdrawn 24:56, due to AE 1:6, due to lack of efficacy 8:23 Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream twice daily for 26 weeks · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream twice daily for 26 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: in the event of worsening of AD after at least 3 days of study treatment use and after investigator confirmation of flare, a medium potency TCS, or alternative TCS for sensitive body areas, was allowed. Notes: none |
| Outcomes | · Flare‐free days reflected the number of days on study without TCS use (per patient diary). · Flares per patient ranked according to the number of flares they experienced during the trial. · Time to first flare and the duration of TCS treatment required per flare were also assessed as secondary efficacy parameters. Since there were a sufficient number of flares after the initial one, the time to subsequent flares was also assessed. · Safety assessments consisted of monitoring and recording all AEs, serious AEs and pregnancies, vital signs and areas of disease involvement. |
| Notes | Funding source: Novartis Declarations of interest: not declared Original language of publication: English Other: none |
Sigurgeirsson 2015.
| Study characteristics | |
| Methods | Trial design: randomised, multicentre, parallel study Trial registration number: NCT00120523 Country: countries of investigators: Canada, Iceland, Spain, South Africa, Portugal, Sweden, Argentina, Ecuador, Colombia, Chile, Guatemala, Peru, Venezuela, USA, Hungary, Turkey, Czech Republic, China, Taiwan, Hong Kong, Singapore, Germany, The Netherlands, Greece Outpatient or hospital: 31 hospital settings, assumed to be outpatients Date trial conducted: enrolment from April 2004 to October 2005 Duration of trial participation: five years Inclusion criteria: · Aged 3 to up to 12 months · Infant eczema diagnosis according to Seymour criteria · 5% or more of the total body surface area affected · IGA 2 to 3 (range: 0 to 5), indicating mild‐to‐moderate disease Exclusion criteria: · Using systemic corticosteroids, immunosuppressants, cytostatic drugs, or phototherapy within 4 weeks · Using topical tacrolimus or pimecrolimus ointment or PIM within 2 weeks · Using topical therapy e.g. TCS within 3 days · Immunocompromised patients or patients with a history of malignant disease · Active acute viral skin infection · Clinically infected eczema · Failure to thrive or developmental abnormalities · Known hypersensitivity to any ingredient of the study medications · Clinical conditions that could interfere with the evaluation (investigator's discretion) Additional design details: none |
| Participants | Total number randomised: 2439 participants (1229 to apply the intervention and 1210 to apply the comparator) Age: TCS group: mean 7.1 months (SD: 2.7); TCI group: mean 7.1 months (SD: 2.7) Sex: TCS group: males 61.2%; TCI group males 61.7% Ethnicity: TCS group: Caucasian 58.8%; black 6.6%; Asian 9.7%; other 24.9%; TCI group: Caucasian 61.1%; black 5.5%; Asian 9.9%; other 23.6% Duration of eczema: not reported Severity of eczema: TCS group, mild 46.0%; moderate 53.8%; severe 0.2%; very severe 0.1%; TCI group, mild 47.3%; moderate 52.7% Body site: participants had overall and facial IGA assessments. Number of withdrawals: 339 did not complete the study because of AE = 12, lack of effect = 13, protocol violation = 11, consent withdrawal = 166, lost to follow‐up = 130, administrative = 6, death = 1 Notes: none |
| Interventions | Run‐in details: see exclusion criteria Intervention: low‐moderate potency TCS (investigator's discretion) used at first signs/symptoms of eczema until eczema clearance or according to manufacturer's instructions and reinitiated at the occurrence of first signs and symptoms of AD flares · Concurrent treatment: not reported · Other key information: none Comparator: pimecrolimus 1% cream used twice daily at first signs/symptoms of eczema until eczema clearance or according to manufacturer's instructions and reinitiated at the occurrence of first signs and symptoms of AD flares · Concurrent treatment: In the event of a flare, TCI swapped to TCS, then a clinic visit was scheduled if the condition worsened. · Other key information: none Concurrent treatments received alongside both intervention and comparator: if dry skin, or first signs/symptoms of eczema, bland emollient twice daily · If suspected infection, systemic/topical antibiotics/antivirals/antifungals with TCS at investigator's discretion Notes: none |
| Outcomes | · AE · Height and weight · Effect on the developing immune system · Treatment success rate (IGA score of 0 = clear or 1 = almost clear) for the whole body and face · BSA Involved · PIQoL‐AD up to five years in some centres · Blood pressure and pulse raised |
| Notes | Funding source: from Novartis Pharmaceuticals Corporation and Meda Pharma GmbH & Co. KG Declarations of interest: to quote: "Dr Paller has consulted for Valeant. The other authors have no financial relationships relevant to this article to disclose". "Dr Sigurgeirsson has received research grants from or lectured/consulted for Novartis". Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Harvey 2023. |
Sikder 2005.
| Study characteristics | |
| Methods | Trial design: randomised, multicentre study Trial registration number: not reported Country: Bangladesh Outpatient or hospital: 3 centres in Dhaka Date trial conducted: October 2004 to February 2005 Duration of trial participation: 6 weeks Inclusion criteria: · 7 to 15 years (older children) of either sex with a diagnosis of AD according to the criteria of Hanifin and Rajka · AD severity grading of moderate‐to‐severe · Disease involvement of at least 5% but not more than 50% of the total BSA Exclusion criteria: · Having a serious skin disease other than AD that required treatment · History of eczema herpeticum · Had received topical treatment for AD within 2 weeks and/or systemic drug for AD within 4 weeks Additional design details: none |
| Participants | Total number randomised: 45 participants (15 each to apply intervention one, two and the comparator) Age: 7 to 9 years n = 16, 10 to 12 years n = 17, 13 to 15 years n = 12 Sex: male 56%, female 44% Ethnicity: not reported Duration of eczema: current episode of AD less than 6 months 66.7%, greater than 6 months 33.3% Severity of eczema: not reported Body site: head and neck 53%, upper limb 93.3%, trunk 82.2%, lower limb 73.3% Number of withdrawals: 4/45, to quote: "Three patients lost during treatment; of them two received 0.03% tacrolimus ointment and one received 0.05% clobetasone butyrate. Another dropout observed at the end of follow‐up who received combination regimens." Notes: none |
| Interventions | Run‐in details: not reported Intervention one: tacrolimus 0.03% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention two: clobetasone butyrate 0.05% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: tacrolimus 0.03% with clobetasone butyrate 0.05% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: a thin layer of ointment and/or cream was applied twice daily to areas of actively diseased skin. All other topical and systemic drugs used in AD were prohibited; only bath oil and nonmedicated emollients were allowed. Inhaled or intranasal corticosteroids, if being used, were limited to 1 mg per day. Notes: none |
| Outcomes | · % BSA · IGA · Intensity of itching · mEASI · AE |
| Notes | Funding source: Square Pharmaceuticals Ltd provided intervention drugs. Declarations of interest: not declared Original language of publication: English Other: none |
Smith 2005.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: UK Outpatient or hospital, date trial conducted, duration of trial participation: not reported Inclusion criteria: adult with mild‐to‐moderate AD Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 137 participants (71 to apply the intervention and 66 to apply the comparator) Age, sex, ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐moderare Body site: not reported Number of withdrawals: 55% of participants in the intervention group and 61% of participants in comparator group did not complete the study. Notes: none |
| Interventions | Run‐in details: patients managed their own treatment according to self‐assessment of their AD. They received pimecrolimus cream 1% twice daily as acute therapy until remission (satisfactory clearance) occurred and were then randomised 1:1 to either pimecrolimus or vehicle daily for 2 weeks following complete clearance. If relapse occurred, acute treatment with pimecrolimus twice daily was recommenced. Intervention: pimecrolimus cream 1% cream used once daily for 14 days · Concurrent treatment: not reported. TCS were not allowed. · Other key information: none Comparator: vehicle cream used once daily for 14 days. · Concurrent treatment: TCS were not permitted. · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Number of study days spent in remission, which was evaluated using the Wilcoxon rank‐sum test, at the two‐sided 5% significance level · Signs, symptoms and severity of AD, including EASI · Use of emollients |
| Notes | Funding source: Novartis Pharmaceuticals Corporation Declarations of interest: not declared Original language of publication: English Other: none |
Smitt 1993.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind study Trial registration number: not reported Country: the Netherlands Outpatient or hospital: outpatients department of Paediatric Dermatology Date trial conducted, duration of trial participation: not reported Inclusion criteria: children with severe AD according to Hanifin and Rajka criteria and for ≥ one year Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 40 participants (20 to apply the intervention and 20 to apply the comparator) Age: alclometasone group, mean 5.1 years (range 1 to 15); triamcinolone acetonide group, mean 3 years (range 1 to 11) Sex, ethnicity: not reported Duration of eczema: ≥ one year Severity of eczema: described as severe in abstract Body site: alclometasone group test sites were face, neck, head n = 3 participants and extremities n = 17; triamcinolone acetonide group sites were face, neck, head n = 6 and extremities n = 14. Number of withdrawals: unclear for 13 as not all measurements were taken. It is not clear how many of these remained in the study. Four were treatment failures and one dropped out. Reasons for the other eight could be refusual of assessment, but it was not clear. Notes: none |
| Interventions | Run‐in details: former TCS were discontinued two weeks before the study Intervention: triamcinolone acetonide 0.1% cream used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: alclometasone dipropionate 0.05% cream used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: use of bath oil, white petrolatum and antihistamines (only when already used before the trial) were continued as long as necessary. Notes: none |
| Outcomes | · Severity of signs and symptoms · Plasma cortisol levels |
| Notes | Funding source: Essex (Nederland) BV, a subsidiary of Schering Plough Corporation USA Declarations of interest: not declared Original language of publication: English Other: none |
Sparkes 1974.
| Study characteristics | |
| Methods | Trial design: randomised, multi‐armed, within‐participant study Trial registration number: not reported Country: UK, Sweden, Finland and Belgium Outpatient or hospital: most participants were outpatients. Date trial conducted: not reported Duration of trial participation: 7 days Inclusion criteria: bilateral lesions of eczema where there was minimal difference in severity between sides Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 188 (visual approximation) sides treated Age, sex, ethnicity, duration and severity of eczema, body site: not reported Number of withdrawals: not reported for eczema participants alone Notes for withdrawals: number reported was 1150, but this was inclusive of psoriasis and eczema participants. There was 1 withdrawal out of 1150 owing to worsening of disease; unclear if a psoriasis or eczema participant |
| Interventions | Run‐in details: not reported Intervention one: clobetasol propionate 0.05% cream used twice daily (more at investigator's discretion) for 7 days · Concurrent treatment: not reported · Other key information: none Intervention two: clobetasol propionate 0.05% ointment used twice daily (more at investigator's discretion) for 7 days · Concurrent treatment: not reported · Other key information: none Comparator one: betamethasone valerate 0.12% cream used twice daily (more at investigator's discretion) for 7 days · Concurrent treatment: not reported · Other key information: brand: Betnovate, standard concentration assumed as not stated in the paper Comparator two: fluclorolone acetonide 0.025% cream used twice daily (more at investigator's discretion) for 7 days · Concurrent treatment: not reported · Other key information: not reported Comparator three: flucinonide 0.05% FAPG used twice daily (more at investigator's discretion) for 7 days · Concurrent treatment: not reported · Other key information: brand: Metosyn. Standard concentration assumed as not stated in the paper Comparator four: betamethasone valerate 0.12% ointment used twice daily (more at investigator's discretion) for 7 days · Concurrent treatment: not reported · Other key information: brand: Betnovate. Standard concentration assumed as not stated in the paper. Comparator five: fluclorolone acetonide 0.025% ointment used twice daily (more at investigator's discretion) for 7 days · Concurrent treatment: not reported · Other key information: not reported Concurrent treatments received alongside both intervention and comparator: with or without polythene occlusion. All participants were asked to wash their hands between treatment applications. Notes: For the fluclorolone acetonide comparators, the brand Topilar is used with standard concentration assumed as not stated in the paper, and potency inferred from classification of 0.2%. It is unclear who were randomised to which comparisons and how many eczema patients were treated. All that is known is that all participants received a clobetasol propionate preparation. *FAPG" said to have the properties of both a cream and an ointment (Portney 1972). Visual approximation from published figure of number evaluated in each group. |
| Outcomes | · AE were reported, and many participants were explicitly asked about sensation on application at up to day 7; however, the discussion stated that they were not looked for. · Investigator preference for which side gave the best response · IGA: clinician (assumed) rated the lesions on a 4‐point scale as 'healed', 'improved', 'static' or 'worse' (not reported) · Participant assessment (equal response, preference for clobetasol, preference for other steroid) |
| Notes | Funding source: not reported; however, trial authors were affiliated to Glaxo Laboratories Ltd. Declarations of interest: not declared Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Spergel 2007.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, within‐patient study Trial registration number: NCT00119158 Country: US Outpatient or hospital: four academic centres Date trial conducted: October 2004 to June 2005 Duration of trial participation: 2 weeks Inclusion criteria: · Age 2 to 65 years · AD according to the American Academy of Dermatology Consensus Conference (2001) · At least two lesions of AD on symmetrical part of the body (same location for each side of the body), of severe intensity (m‐EASI is at least 7 on each site, with erythema of at least 3 (severe) and papulation/infiltration of at least 3 (severe)) and similar severity (m‐EASI does not differ from more than 2 points on both sides) · Female is able to enter and participate in this study if she is of: Non‐childbearing potential or childbearing potential, has a negative pregnancy test (urine) at the screen visit and agrees to an adequate method of birth control throughout the study (which may, at the investigator's discretion, include abstinence) Exclusion criteria: · Active skin infection or other skin disorders · History of immune deficiencies or history of malignant disease · Patients with moderate‐to‐severe lichenification at the target areas (i.e. score 2 or 3) · Active cutaneous bacterial, viral or fungal infections Additional design details: none |
| Participants | Total number randomised: 94 sides treated (47 to apply the intervention and 47 to apply the comparator) Age: 2 to 65 years Sex: male n = 18 Ethnicity, duration of eczema: not reported Severity of eczema: severe Body site: extremities Number of withdrawals: two participants were excluded from analysis: one patient used oral steroids for an acute exacerbation of asthma and the second participant was noncompliant with the treatment regimen. Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream used once daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used once daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: fluticasone propionate cream 0.05%. Participants had to stop topical medications 1 week and systemic therapies 4 weeks prior to the start of the study. During the study, concomitant topical or systemic therapies for AD were prohibited. Notes: none |
| Outcomes | · EASI · IGA · Patient assessed signs ‐ not reported |
| Notes | Funding source: Novartis Declarations of interest: not declared but author affiliation to Novartis Original language of publication: English Other: none |
Spergel 2015.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: NCT00124709 Country: US Outpatient or hospital: 35 clinical locations Date trial conducted: October 2003 to November 2008 Duration of trial participation: up to 3 years for RCT phase. Mean duration 2.8 years Inclusion criteria: · Age 3 to 18 months · Diagnosis of AD · Family history of atopy · At least mild AD at baseline (IGA greater or equal to 2) · Clinical evidence of AD for no longer than 3 months Exclusion criteria: · Diagnosis of or substantial clinical evidence for food or other allergies at baseline · Receiving topical tacrolimus or any topical agent with a possible effect on AD within 7 days, daily treatment with antihistamines, or other systemic therapy within 1 month before first study drug application. Additional design details: none |
| Participants | Total number randomised: 1091 participants (546 to apply the intervention and 545 to apply the comparator) Age: intervention group, mean 7.1 months (SD 3.76), range less than 3 months to 18 months; comparator group, mean 7.4 months (SD 4.06), range less than 3 months to 18 months Sex: intervention group: female n = 194, male n = 349; comparator group: female n = 218, male n = 326 Ethnicity: not reported Duration of eczema: recent‐onset (3 months or less) Severity of eczema, body site: not reported Number of withdrawals: 527: intervention group, randomised but did not take medication n = 3, AE n = 3, lack of efficacy n = 5, protocol violation n = 3, withdrawal by subject n = 127, lost to follow‐up n = 114; comparator group, randomised but did not take medication = 1, adverse event = 0, lack of efficacy = 10, protocol violation = 5, withdrawal by subject = 136, lost to follow‐up = 120 Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus cream 1% cream used twice daily · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice weekly (consecutive) · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: rescue therapy with topical corticosteroid (TCS; fluticasone propionate 0.05% cream; referred to as treatment step 3a) was permitted if 3 days of study medication led to no improvement, with investigators making decisions until week 14 and caregivers thereafter (training provided at the randomisation visit). Daily application of emollients on healthy and inflamed skin was encouraged. Notes: All participants entered a dose escalation scheme. All patients received emollient only during treatment step 1. During treatment step 2, patients were randomised 1:1 to twice‐daily pimecrolimus 1% or vehicle‐only cream. Patients received add‐on once‐daily TCS in treatment step 3a (medium strength) or treatment step 3b (potent). With severe disease exacerbations, an oral agent was used in treatment step 4. |
| Outcomes | · Proportion of disease‐free days · Effect of early use of pimecrolimus cream 1% in reducing the incidence of asthma at 6 years of age Note: The results for this efficacy variable were not reported due to early termination of the study. · Long‐term safety in infants and young children Note: The results of this secondary outcome were not reported due to early termination of the study. · Incidence of allergic rhinitis, allergic conjunctivitis and food allergies Note: The results at six years were not reported due to early termination of the study. · Corticosteroid and pimecrolimus drug use · AD remission time · Patient/caregiver quality of life · EASI score · TBSA affected · Pruritus and erythema scores · IGA scores |
| Notes | Funding source: Novartis Declarations of interest: authors declared conflicts of interest with Novartis and Astellas which are potentially relevant to this trial. Original language of publication: English Other: none |
Stalder 1994.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: not reported Country: France Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 14 days Inclusion criteria: · Male and female children · AD according to Hanifin and Rajka criteria Exclusion criteria: · Topical or oral antibiotics, topical antiseptics or topical corticosteroids for at least 8 days before trial · With evidence of clinical infection requiring antibiotic therapy Additional design details: none |
| Participants | Total number randomised: 40 participants Age: mean 40 months, range 4.5 months to 15 years Sex: female n = 18, male n = 22 Ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐moderate Body site: not reported Number of withdrawals: 1 of 40 could not be evaluated; reason not stated Notes: none |
| Interventions | Run‐in details: not reported Intervention: desonide cream (concentration unclear) used once daily for 7 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle used once daily for 7 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Global score on the extent and severity of lesions · Skin dryness, pruritus and sleep using an analogue scale · Local lesion score (elbow or knee flexural areas). Score based on erythema, oozing, squamous lesions, crusts, lichenification and excoriation, score 0 = none to 3 = severe · Bacteriological sample on bacteria flora on skin lesions (elbow or knee flexural areas) |
| Notes | Funding source: not reported Declarations of interest: authors affiliated to Pierre Fabre Laboratories Original language of publication: English Other: none |
Stein‐Gold 2013.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, within‐participant trial Trial registration number: NCT01602341 Country: USA and Australia Outpatient or hospital: outpatient (assumed) Date trial conducted: August 2012 to January 2013 Duration of trial participation: 29 days Inclusion criteria: · Male or female 12 to 17 years of age, inclusive · AD according to the criteria of Hanifin and Rajka · BSA of AD involvement ≤ 35% · Presence of two comparable target lesions · Females of childbearing potential must use a highly effective method of birth control. Males with partners of childbearing potential should inform them of their participation in this clinical study and use a highly effective method of birth control during the study. Exclusion criteria: · Significant confounding conditions as assessed by study doctor · Unstable or actively infected AD · Active or potentially recurrent dermatologic condition other than atopic dermatitis in the target lesion area that may confound evaluation · History or evidence of allergies requiring acute or chronic treatment (except seasonal allergic rhinitis) · Concurrent or recent use of certain topical or systemic medications or phototherapy without a sufficient washout period · Treatment for any type of cancer (except squamous cell carcinoma, basal cell carcinoma, or carcinoma in situ of the skin, curatively treated with cryosurgery or surgical excision only) within the last 5 years · Current pregnancy or lactation, or intent to become pregnant during the study · Known sensitivity to any of the components of the study drug Additional design details: none |
| Participants | Total number randomised: 172 sides treated (86 to apply the intervention and 86 to apply the comparator) Age: 12 to 17 years Sex: male n = 34; female n = 52 Ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐moderate Body site: not reported Number of withdrawals: none Notes: none |
| Interventions | Run‐in details: not reported Intervention: crisaborole 2% ointment used once or twice daily for 29 days · Concurrent treatment: not reported · Other key information: none Comparator: crisaborole 0.5% ointment used once or twice daily for 29 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Improvement in ADSI scores · Number of participants with clinically significant changes in vital signs and in laboratory abnormalities · Treatment‐emergent AEs, SAEs · Local tolerability symptoms |
| Notes | Funding source: Pfizer Declarations of interest: not declared Original language of publication: English Other: none |
Sudilovsky 1981.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number, country, outpatient or hospital, date trial conducted: not reported Duration of trial participation: 3 weeks Inclusion criteria: · AD (or psoriasis vulgaris; results presented separately) · Bilateral lesions of similar severity and chronicity · Not received corticosteroid medication for at least 1 week preceding Exclusion criteria: has a previous history of poor response to TCSs Additional design details: if complete remission was obtained in < 3 weeks, treatment could be stopped. |
| Participants | Total number randomised: 116 sides treated (58 to apply the intervention and 58 to apply the comparator) Age, sex: not reported separately for AD population Ethnicity, duration of eczema, severity of eczema, body site: not reported Number of withdrawals: withdrawals were not explicitly reported, but outcome data were unavailable for 4/58 participants at the end of treatment. Notes: none |
| Interventions | Run‐in details: all participants received induction (1 to 4 weeks). They were treated with topical tacrolimus (of 0.03% for patients<16 years old and of 0.1% otherwise) and emollients twice daily in addition to their usual topical corticosteroid treatment. Intervention: tacrolimus 0.03% for patients <16 years and 0.1% otherwise used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle used twice daily for 28 days · Concurrent treatment: not reported · Other key information: emollient Concurrent treatments received alongside both intervention and comparator: topical corticosteroid treatment (maximum use, 10 g/week) Notes: none |
| Outcomes | · Patient‐assessed pruritus · SCORAD |
| Notes | Funding source: Ministry of Health, Labour and Welfare, Japan Declarations of interest: not declared Original language of publication: English Other: none |
Takeuchi 2012.
| Study characteristics | |
| Methods | Trial design: randomised, open‐label, multicentre study Trial registration number: not reported Country: Japan Outpatient or hospital: 8 medical centres/universities in Japan Date trial conducted: not reported Duration of trial participation: 4 weeks induction (not randomised), 4 weeks therapy (randomised) Inclusion criteria: · Has AD and >10 years old · VAS‐itch scores (max = 100) were 30 to 80. · For four weeks, all the patients were treated with topical tacrolimus (of 0.03% for patients<16 years old and of 0.1% otherwise) and emollients twice daily in addition to their usual topical corticosteroid treatment (maximum use, 10 g/week), and change of VAS‐itch score was examined. Patients who showed a reduced VAS‐itch score by>20 points were considered to show relief from pruritis, while only such induction therapy responders proceeded into maintenance treatment. Exclusion criteria: · VAS‐itch scores<30 or>80 · Treated with orally administered corticosteroids, cyclosporine, or antihistamines within two weeks prior to the registration Additional design details: not reported |
| Participants | Total number randomised: 70 participants (35 to apply the intervention and 35 to apply the comparator) Age: intervention group, mean 30.5 years (SD 13.2); comparator group, mean 30.8 years (SD 11.9) Sex: M:F: intervention group: 17:18; comparator group: 20:15 Ethnicity, duration of eczema, severity of eczema, body site: not reported Number of withdrawals: 1 dropped out during the randomised phase of the study maintenance therapy. The participant was in the comparator group and no reasons were given for dropping out. Notes: not reported |
| Interventions | Run‐in details: all the patients received induction (1 to 4 weeks). All were treated with topical tacrolimus (of 0.03% for patients<16 years old and of 0.1% otherwise) and emollients twice daily in addition to their usual topical corticosteroid treatment. Intervention: tacrolimus 0.03% for patients<16 years and 0.1% otherwise used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle used twice daily for 28 days · Concurrent treatment: not reported · Other key information: emollient Concurrent treatments received alongside both intervention and comparator: topical corticosteroid treatment (maximum use, 10 g/week) Notes: none |
| Outcomes | · Patient‐assessed pruritus · SCORAD |
| Notes | Funding source: Ministry of Health, Labour and Welfare, Japan Declarations of interest: not declared Original language of publication: English Other: none |
Thaci 2005.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, within‐participant study Trial registration number: not reported Country: Germany, Switzerland Outpatient or hospital, date trial conducted: not reported Duration of trial participation: two weeks Inclusion criteria: adult with AD with at least 10% involvement of each upper limb (excluding hands) Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 111 sides treated Age, sex, ethnicity, duration of eczema, severity of eczema: not reported Body site: upper limb excluding hands Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus cream 1% ointment used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · TLS assessed using the partial EASI · Composite variable evaluating the severity of the clinical signs of erythema, infiltration/papulation, excoriations, and lichenification (range 0 to 12) · Itching/scratching at the target lesion sites in the 24 hours prior to the assessment was assessed by the patient using a 4‐point (0 to 3) score. |
| Notes | Funding source: Novartis Pharma AG Declarations of interest: not declared; two authors affiliated to Novartis Original language of publication: English Other: none |
Tharp 1996.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number, country: not reported Outpatient or hospital: to quote: "study centers" Date trial conducted: August to December 1999 Duration of trial participation: 4 weeks Inclusion criteria: · 12 years or older · Established diagnosis of eczema Exclusion criteria: · Lesions of the scalp, face, axillae, and groin were not chosen as the target lesion. · Prescribed medications with associated washout periods · Interfering disease states · Sensitivity to ingredients of the study medication or other optical or systemic steroid therapy · Acute self‐limited eczema or whose eczema is likely to improve spontaneously Additional design details: the study has 3 group comparisons; for the purposes of this review, we have combined the groups that evaluated the treatment drug as either once or twice a day. |
| Participants | Total number randomised: 238 participants (158 to apply the intervention and 80 to apply the comparator) Age: intervention group, mean 38 years, range 14 to 82; comparator group, mean 36 years, range 12 to 87 Sex: M:F, intervention group: 104:54 comparator group: 56:24 Ethnicity: white n = 162, black n = 37, Asian n = 15, other n = 24 Duration of eczema: median 11 years, range from 0 to 71 years Severity of eczema: n = 152 worsening, n = 85 stable moderate‐to‐severe Body site: lesions of the scalp, face, axillae, and groin were not chosen as the target lesion. Number of withdrawals: 55: intervention group n = 33, comparator group n = 22 Notes: none |
| Interventions | Run‐in details: not reported Intervention: fluticasone propionate 0.05% cream used once daily or twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: occlusive dressings were not used. No other treatments or medications for eczema were to be used. Notes: none |
| Outcomes | · IGA binary · Patients' subjective assessment · Clinician‐reported signs (continuous) · AE |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Torok 2003.
| Study characteristics | |
| Methods | Trial design: randomised, investigator‐blind study Trial registration number: not reported Country: US Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 21 days Inclusion criteria: · Male or female aged 16 to 65 years · AD for at least 6 months and affecting between 5% and 20% of the body (excluding the face) · DSS of at least 5 for the target area to be evaluated (approximately 30 to 50 cm2, excluding the face) Exclusion criteria: · Had underlying disease · Other dermatologic conditions that required systemic therapy or use of a topical agent Additional design details: none |
| Participants | Total number randomised: 57 participants (19 each to apply intervention one, two and the comparator) Age: equivalent numbers of patients were enroled in each 10‐year age cohort (between 16 and 65 years); 88% of patients were younger than 50 years and 60% were younger than 40 years Sex: 38.6% male, 61.4% female Ethnicity: white 94.7%, black 3.5%, other 1.8% Duration of eczema: at least 6 months Severity of eczema, body site: not reported Number of withdrawals: there were no withdrawals. Notes: none |
| Interventions | Run‐in details: not reported Intervention one: tacrolimus 0.1% cream with clocortolone pivalate 0.1% used twice daily for 21 days · Concurrent treatment: not reported · Other key information: treated according to the approved labelling for each product Intervention two: clocortolone pivalate 0.1% used twice daily for 21 days · Concurrent treatment: not reported · Other key information: participants randomised to this treatment group applied both products twice a day, with at least one application after bathing. TCS was applied first, and TCI was applied 15 minutes later. Additional applications of TCS (more than twice a day) were allowed if the cleanser or lotion did not reduce skin irritation adequately at the site of TCI treatment. Comparator: tacrolimus 0.1% cream used twice daily for 21 days · Concurrent treatment: not reported · Other key information: treated according to the approved labelling for each product Concurrent treatments received alongside both intervention and comparator: participants were instructed in the daily use of a mild cleanser (Cetaphil® Gentle Skin Cleanser) and moisturiser (Cetaphil Moisturizing Lotion). They were not permitted to treat the skin of the face, scalp, or groin. Notes: none |
| Outcomes | · Physicians and patients evaluation of signs and symptoms · Dermatologic Sum Score · Global Severity · Global Improvement |
| Notes | Funding source: not reported Declarations of interest: authors declared consultancy payments and are affiliated to the following companies, Health Point, and Topical Solutions Ltd. Original language of publication: English Other: none |
Traulsen 1997a.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind study Trial registration number: not reported Country: Denmark Outpatient or hospital: four Nordic centres Date trial conducted, duration of trial participation: not reported Inclusion criteria: · At least 12 years of age · Suffering from AD with two affected symmetrical skin areas with comparable activity Exclusion criteria: · Pregnant or nursing women · Clinical signs of infection of the selected skin areas · Treated with systemic corticosteroid within 1 month, with topical steroids within 1 week before the baseline assessment or with another treatment which might have affected the clinical status of the eczema Additional design details: paper presented two studies, one on cream (Traulsen 1997a) and one on ointment (Traulsen 1997b) |
| Participants | Total number randomised: 192 sides treated (86 to apply the intervention and 86 to apply the comparator) Age: mean 26.4 years, range 13 to 59 years Sex: male n = 32, female n = 51 Ethnicity, duration of eczema: not reported Severity of eczema: to quote: "The overall severity was recorded as mild in 9 patients, moderate in 41, severe in 30 and very severe in 3. The disease was recorded as ’improving’ at the time of entry in 3 patients, ’stable’ in 31 and ’worsening’ in 43". Body site: not reported Number of withdrawals: 3; did not fit inclusion criteria n = 1, lost to follow‐up n = 2 Notes: none |
| Interventions | Run‐in details: not reported Intervention: hydrocortisone propionate 0.1% cream used once daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: betamethasone valerate 0.1% cream used once daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: locobase cream was used as the inactive preparation for non‐affected skin areas and for morning application to affected areas not selected for evaluation. Notes: none |
| Outcomes | · Sum scores for the five symptoms, erythema, scaling, vesiculation, papules and pruritus (and individual) · Investigator overall evaluation of the clinical effects · Patient preference regarding efficacy and cosmetic properties |
| Notes | Funding source: Nycomed Pharma (Basoderm a/s) and Taisho Inc. Declarations of interest: not declared Original language of publication: English Other: none |
Traulsen 1997b.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind study Trial registration number: not reported Country: Denmark Outpatient or hospital: 3 Nordic centres Date trial conducted: not reported Duration of trial participation: 14 days Inclusion criteria: · At least 12 years of age · Suffering from AD with two affected symmetrical skin areas with comparable activity Exclusion criteria: · Pregnant or nursing women, patients · Clinical signs of infection of the selected skin areas · Treated with systemic corticosteroid within 1 month, with topical steroids within 1 week before the baseline assessment or with another treatment which might have affected the clinical status of the eczema Additional design details: paper presented two studies, one on cream (Traulsen 1997a) and one on ointment (Traulsen 1997b). |
| Participants | Total number randomised: 164 sides treated (82 to apply the intervention and 82 to apply the comparator) Age: mean 23.3 years, range from 14 to 40 years Sex: male n = 31, female n = 49 Ethnicity, duration of eczema: not reported Severity of eczema: to quote: "The overall severity of atopic dermatitis was found to be moderate in 43 patients, severe in 23 patients and very severe in 11 patients. The disease at entry was ‘improving’ in 1, ’stable’ in 36 and ’worsening’ in 43 patients". Body site: not reported Number of withdrawals: 2; did not fit inclusion criteria n = 1, lost to follow‐up n = 1 Notes: none |
| Interventions | Run‐in details: not reported Intervention: hydrocortisone propionate 0.1% ointment used once daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: betamethasone valerate 0.1% ointment used once daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: locobase cream was used as the inactive preparation for non‐affected skin areas and for morning application to affected areas not selected for evaluation. Notes: none |
| Outcomes | · Sum scores for the five symptoms: erythema, scaling, vesiculation, papules and pruritus · Investigator overall evaluation of the clinical effects · Patient preference regarding efficacy and cosmetic properties |
| Notes | Funding source: Nycomed Pharma (Basoderm a/s) and Taisho Inc. Declarations of interest: not reported Original language of publication: English Other: none |
Turnbull 1975.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: New Zealand Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 14 days Inclusion criteria: · Has steroid‐responsive dermatoses · Similar bilaterial lesions Exclusion criteria: not reported Additional design details: study included participants with a range of dermatoses. |
| Participants | Total number randomised: 14 sides treated (7 to apply the intervention and 7 to apply the comparator) Age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: halcinonide 0.1% cream used three times daily for 7 days · Concurrent treatment: not reported · Other key information: none Comparator: betamethasone valerate 0.1% cream used three times daily for 7 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Decrease in erythema, oedema, transudation, lichenification and scaling · Pain relief and pruritus · Overall therapeutic response |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Udompataikul 2011.
| Study characteristics | |
| Methods | Trial design: randomised, investigator‐blind study Trial registration number: not reported Country: Thailand Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 6 weeks Inclusion criteria: · Children aged between 2 and 15 years · Mild‐to‐moderate AD according to Hanifin and Rajka criteria · Skin lesions on both flexural areas of the body Exclusion criteria: skin infections Additional design details: none |
| Participants | Total number randomised: 60 sides treated (30 to apply the intervention and 30 to apply the comparator) Age: mean age 5.8 years, range from 2 months to 10 years Sex: male n = 14, female n = 12 (completed study) Ethnicity, duration of eczema, severity of eczema: not reported Body site: lesions on flexural parts of the body Number of withdrawals: 4, to quote: "One patient was excluded because of bacterial folliculitis that occurred while following the protocol, and three patients dropped out because of incomplete following up". Notes: none |
| Interventions | Run‐in details: A washout period of up to 4 weeks was required for patients taking oral medications, and up to 2 weeks for patients receiving topical medications. Intervention: hydrocortisone 1% lotion used twice daily for 28 days · Concurrent treatment: not reported · Other key information: for a further 14 days, participants were given a placebo cream to use. This was so they could assess relapse rate. Comparator: vehicle lotion used twice daily for 42 days · Concurrent treatment: In addition, the placebo lotion included Licochalcone A. This is a derivative from liquorice, which is thought to have antiinflammatory properties. We did not include it as an active intervention as the focus of this review was not on herbal treatments. · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · SCORAD · Excoriation, lichenification, dryness, pruritus · Relapse rate · Patient satisfaction |
| Notes | Funding source: not reported Declarations of interest: to quote: "no conflicts of interest" Original language of publication: English Other: none |
Ulrich 1991.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind study Trial registration number: not reported Country: Germany from the affiliations of the authors Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 14 days Inclusion criteria: · Has acute episodes of AD up to 40% (10% in infants) of their total BSA · Able to have topical treatment only Exclusion criteria: · Pregnancy and breastfeeding · Secondary infection · Concomitant tuberculosis, syphilitic or viral infections · Accompanying diseases such as diabetes mellitus, leukaemia, parasitic manifestations, hypogammaglobulinaemia, perioral dermatitis, rosacea or vaccination reactions · Concomitant treatment with another topical preparation other than the trial medication · Concomitant systemic therapy with antihistamines, immunosuppressants, corticosteroids or ACTH · Known hypersensitivity reactions of the skin compared to the active ingredients or excipients Additional design details: none |
| Participants | Total number randomised: 165 participants (81 to apply the intervention and 84 to apply the comparator) Age: average 26 years (range 8 months to 63 years). "The two treatment groups were comparable in terms of […] age". Sex: male n = 88, female n = 77. "The two treatment groups were comparable in terms of […] sex". Ethnicity: not reported Duration of eczema: The two treatment groups were comparable in terms of […] duration of the current episode. Severity of eczema: intervention group, 24 involvement up to 9% BSA, 37 10% to 20%, and 20 had 21% to 40%; 5 were judged to have mild disease, 33 were moderate, and 43 were severe; comparator group, 84 involvement up to 9% BSA, 39 10% to 20%, and 13 had 21% to 40%; 2 were judged to have mild disease, 45 were moderate, and 37 were severe. Body site: not reported Number of withdrawals: no withdrawals Notes: extraction from English translation |
| Interventions | Run‐in details: not reported Intervention: halometasone 0.05% cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: there is a discrepancy between the German and English summary on the strength of the halometasone used. The English summary stated 0.5%, German consistently stated 0.05%. We have assumed 0.05% as this is a standard Sicorten preparation. Comparator: prednicarbate 0.25% cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: applied without occlusion Notes: none |
| Outcomes | · Clinical efficacy, collected by the doctor, assessed on a 5‐point scale with the scores: 1 = healing, 2 = definite improvement, 3 = moderate improvement, 4 = mild or no improvement, 5 = worsening · Severity of the disease and the onset of the illness, as indicated by the 1st signs or symptoms of recovery · AE |
| Notes | Funding source: not reported Declarations of interest: not declared; however, one of the authors is affiliated to Zyma GmbH, Munich. Original language of publication: German Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Van Del Rey 1983.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: not reported Country: assumed to be Brazil from the affiliations of the authors Outpatient or hospital: assumed to be a secondary care setting from the affiliations of the authors Date trial conducted: not reported Duration of trial participation: 3 weeks Inclusion criteria: · Any race or sex > 12 years old · Diagnosed with AD > 1 year ago · Erythema, induration and pruritus all present and, when scored from 0 = absent to 3 = severe, the sum of the scores was at least 6 · Stable disease (resistant to common treatments) or worsened > 1 week ago Exclusion criteria: · Pregnancy · Requirement for other topical steroidal anti‐inflammatory drugs in addition to trial medication · Requirement for systemic treatment for any other reason Additional design details: none |
| Participants | Total number randomised: 30 participants (15 to apply the intervention and 15 to apply the comparator) Age: < 18: alclometasone group n = 2, hydrocortisone butyrate group n = 1, total = 3 18 to 30 years: alclometasone group = 7, hydrocortisone butyrate group = 11, total = 18 31 to 40 years: alclometasone group = 4, hydrocortisone butyrate group = 2, total = 6 Sex: males n = 13, females n = 16 overall (baseline data not available for 1 individual): alclometasone group, male n = 7, female n = 8; hydrocortisone butyrate group, male n = 6, female n = 8 Ethnicity: black: hydrocortisone butyrate group n = 2, alclometasone group n = 1, total = 3; white: hydrocortisone butyrate group n = 12, alclometasone group n = 13, total = 25; others: hydrocortisone butyrate group n = 0, alclometasone group n = 1, total = 1 Duration of eczema: 1 to 5 years n = 26, 6 to 10 years n = 2, > 10 years n = 1: alclometasone group 1 to 5 years n = 14, 6 to 10 years n = 1; hydrocortisone group 1 to 5 years n = 12, 6‐10 years n = 1, > 10 years n = 1 Severity of eczema: (assumed) sum of scores for erythema, induration and pruritus: alclometasone group, mean 7.20 (SE 0.31); hydrocortisone butyrate group, mean 7.14 (SE 0.29) Body site: not reported Number of withdrawals: 1 participant excluded retrospectively as the primary diagnosis was seborrhoeic dermatitis. Notes: none |
| Interventions | Run‐in details: not reported Intervention: hydrocortisone butyrate (unspecified) used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: alclometasone dipropionate (unspecified) used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: we have assumed standard strengths of these TCS in order to classify their potency as they were not clearly given in the paper. |
| Outcomes | · Participant assessment of efficacy · Clinical observation for AE · Investigator assessment of clinical signs including erythema, induration and pruritus (assumed although translation is not certain about exact signs) scored on a 4‐point scale from 0 = absent to 3 = severe · IGA: 1 = eliminated (100%, only residual discolouration remaining), 2 = 'sensitive' improvement (75%‐ < 100% elimination of the signs and symptoms being monitored), 3 = moderate improvement (50%‐ < 75% elimination), 4 = small improvement (< 50% elimination), 5 = no change (no visible improvement) |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: Portuguese Other: This study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Van Leent 1998.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: not reported Country: the Netherlands, Switzerland Outpatient or hospital: outpatient clinic Date trial conducted: not reported Duration of trial participation: 35 days Inclusion criteria: · AD according to the Hanifin and Rajka criteria, with at least 1% BSA affected on both arms Exclusion criteria: · Receiving radiation therapy, systemic therapy with cytostatics, or immunosuppressive drugs within 24 weeks · Receiving phototherapy or systemic therapy for AD within 1 month · Receiving antibiotics or topical therapy for AD within 2 weeks before randomisation (however, the once‐daily use of 1% hydrocortisone acetate was allowed on all lesions except for the test sides selected for the study, and emollients were allowed to be used liberally but not on the test sides) · Taking antihistamines within 1 week before randomisation; and acute skin infection (superinfection) Additional design details: none |
| Participants | Total number randomised: 68 sides treated (34 to apply the intervention and 34 to apply the comparator) Age: mean age: twice daily group 35.75 years (SD 13.65); once daily group 29.11 years (1SD 3.21) Sex: male:female number: twice‐daily group 9:7; once daily group 7:11 Ethnicity, duration of eczema: not reported Severity of eczema: mean ADSI: twice‐daily group ‐ side treated with pimecrolimus 8.06 (SD 1.39), side treated with placebo 8.13 (1.20); once‐daily group ‐ side treated with pimecrolimus 7.72 (SD 1.23), side treated with placebo 7.78 (1.26) Body site: arms Number of withdrawals: 7 patients discontinued participation during the treatment phase; for exacerbation of the AD on untreated or placebo‐treated sides (n = 4), skin infection on the placebo‐treated n = 3 Notes: the ADSI score had to be at least 6, and the severity of the 2 sides was not allowed to differ by more than 1 point. In addition, the 2 sides had to be symmetrical. |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus cream 1% cream used once or twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used once or twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · At all visits, 5 symptoms of AD (pruritus, erythema, exudation, excoriation, and lichenification) were scored on a 4‐point (range, 0 to 3) scale. The sum of the 5 symptoms was defined as the ADSI score (range, 0 to 15). · A modification of the standardised grading according to Hanifin. Scoring was performed separately for the 2 treated areas. |
| Notes | Funding source: Novartis Pharma AG Declarations of interest: not declared Original language of publication: English Other: none |
Vanderploeg 1976.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, placebo‐controlled, parallel‐group study Trial registration number: not reported Country: USA (assumed from author's affiliation) Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 21 days Inclusion criteria: moderate‐to‐severe AD Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 36 participants Age: intervention group: median 22 years (range 2 to 63); comparator group: median 16.5 years (range 3 to 50) Sex: intervention group: male n = 6, female n = 11; comparator group: male n = 4, female n = 12 Ethnicity: intervention group: white n = 14, black n = 3; comparator group: white n = 15, black n = 1 Duration of eczema: not reported Severity of eczema: mean total symptom score at baseline (scale, erythema, pruritus, thickness of lesions, and crusting scored from 0 = none to 4 = very severe): intervention group: 11.4; comparator group: 11.2 Body site: not reported Number of withdrawals: 3 (unclear which group) Notes: none |
| Interventions | Run‐in details: no systemic corticosteroids ≥ 3 months prior to the study · No topical steroids for ≥ 1 week prior to the study Intervention: betamethasone dipropionate 0.05% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Mean total symptom score (scale, erythema, pruritus, thickness of lesions, and crusting scored from 0 = none to 4 = very severe) · Physicians overall evaluation of treatment response (excellent = 75%‐100% improvement) · AE, pruritus |
| Notes | Funding source: not reported Declarations of interest: none declared Original language of publication: English Other: none |
Veien 1984.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, within‐participant study Trial registration number: not reported Country: Denmark (assumed from the authors affiliations) Outpatient or hospital: outpatients from dermatology clinics (assumed from the authors affiliations) Date trial conducted: not reported Duration of trial participation: 4 weeks Inclusion criteria: · Chronic, symmetrical and bilateral AD · Aged < 10 years · Either sex Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 80 sides treated (40 to apply the intervention and 40 to apply the comparator) Age: mean 4.1 years (SD 2.9), range from 10 months to 10 years Sex: male n = 23, female n = 17 Ethnicity: not reported Duration of eczema: not reported (other than "chronic") Severity of eczema: moderate n = 18 participants, severe n = 21, very severe n = 1. Mean score 2.6 (SD 0.6) based on a 5‐point scale from 0 = none to 4 = very severe Body site: not reported Number of withdrawals: one participant missed the 2‐week assessment, otherwise no dropouts reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: hydrocortisone butyrate 0.1% cream used twice daily for 28 days or until clearance · Concurrent treatment: not reported · Other key information: brand: Locoid Comparator: hydrocortisone 1% cream used twice daily for 28 days or until clearance. · Concurrent treatment: not reported · Other key information: brand: Uniderm; considered to be an "advanced base formulation" Concurrent treatments received alongside both intervention and comparator: to prevent contamination, all participants' parents/carers were told to wash their hands before applying each treatment. Treatments were applied without occlusion. Notes: none |
| Outcomes | · AE (spontaneously reported) · Number completely healed (with no relapse) · Participant/parent preferences rated as −2 = very much worse, −1 = worse, 0 = the same, 1 = better, 2 = very much better · IGA using a 5‐point scale (0 = none, 1 = slight, 2 = moderate, 3 = severe, 4 = very severe); therapeutic results rated as moderate for a 1‐point improvement, good for a 2‐point improvement and excellent for a 3‐point improvement |
| Notes | Funding source: none stated; however, one author is affiliated to Gist‐brocades, The Netherlands. Declarations of interest: not declared; however, one author is affiliated to Gist‐brocades, The Netherlands. Original language of publication: English Other: study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Verboom 2002.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country, outpatient or hospital, date trial conducted, duration of trial participation: not reported Inclusion criteria: children with AD Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 733 participants Age: children Sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: treated with corticosteroiods (unspecified) Notes: none |
| Outcomes | · IGA · QoL · Cost‐effectiveness |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: Englsih Other: none |
Vernon 1991.
| Study characteristics | |
| Methods | Trial design: randomised, blind, multicentre, parallel study Trial registration number: none Country: US Outpatient or hospital: two settings, to quote: "Departments of Dermatology and Pediatrics, University of Rochester School of Medicine and Dentistry, and the Departments of Dermatology and Pediatrics, University of Colorado School of Medicine", assumed from the authors' affiliations Date trial conducted: not reported Duration of trial participation: seven weeks: 6 weeks treatment, followed by assessment 1 week after the end of treatment Inclusion criteria: · Children with AD aged between 6 months and 12 years · Initial BSA involved ≥ 15% · Target area scoring ≥ 8/15 in severity based on the sum of 5 signs and symptoms: erythema, lichenification, skin surface disruption i.e. crusting and scaling, excoriation, and pruritus, each scored between 0 = none and 3 = severe. The erythema score was required to be ≥ 2. · Acceptable values for blood cell count, blood electrolytes, glucose, liver enzymes, triglycerides, cholesterol, and plasma cortisol Exclusion criteria: · Receiving systemic steroids within 28 days of enrolment · Using TCS within 7 days of enrolment Additional design details: at the end of the third week, participants whose dermatitis had cleared or shown no improvement were dropped from the trial. |
| Participants | Total number randomised: 48 participants (24 to apply the intervention and 24 to apply the comparator) Age: 7 months to < 2 years n = 12, 2 to 6 years n = 22, > 6 years to 12 years n = 14 Sex, ethnicity, duration of eczema: not reported Severity of eczema: mean target area score 11.3 in the mometasone group, 11.7 in the HC group based on the sum of 5 signs and symptoms: erythema, lichenification, skin surface disruption, excoriation, and pruritus Body site: 39/48 had sites on extremities. Number of withdrawals: 15 participants in each group completed the trial early, owing to clearing of their dermatitis (median duration 3 weeks). Five in the HC group discontinued: lack of response (3), flare‐up of asthma and need for steroids (1) and lost to follow‐up (1); mometasone group: 1 discontinued, infection of scalp Notes: none |
| Interventions | Run‐in details: antihistamines and emollients stopped 2 days before the start of the trial Intervention: mometasone furoate 0.1% cream used twice daily for up to 6 weeks · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone 1% cream used twice daily for up to 6 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: The amount of medication used each week was weighed and recorded. Instructions for application were given by an unblinded investigator. Bathing was not permitted for ≥ 8 hours after application, and occlusive dressings were not allowed. The participants' usual bathing routine and soaps were to be continued unchanged throughout the trial, and any food/environmental allergens that the participant had avoided prior to the trial were to be avoided. |
| Outcomes | · AEs · Morning plasma cortisol levels and other laboratory tests including complete blood cell count, blood electrolytes, glucose, liver enzymes · Global evaluations of improvement: 1 = cleared (100% clearance of signs and symptoms), 2 = marked improvement (≥ 75% clearance), 3 = moderate improvement (≥ 50% clearance), 4 = slight improvement · Per cent BSA involvement · Prespecified target lesion severity score: sum of 5 signs and symptoms: erythema, lichenification, skin surface disruption i.e. crusting and scaling, excoriation, and pruritus, each scored between 0 = none and 3 = severe · Parents' evaluation of efficacy of treatment |
| Notes | Funding source: Schering‐Plough Research, Kenilworth, N.J. Declarations of interest: not declared Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Vives 2015.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, single‐centre, within‐participant study Trial registration number: EudraCT 2007‐002550‐27 Country: France Outpatient or hospital: one investigational centre Date trial conducted: recruitment September 2007 to April 2008 (to avoid major seasonal influence on signs and symptoms) Duration of trial participation: 28 days Inclusion criteria: · Men and women aged 18 to 65 years with Fitzpatrick skin phototype I to IV · AD according to the UK Working Party’s Diagnostic Criteria for AD · AD lesions on at least 2 symmetrical topographic areas (arms, legs, or trunk) · Mild‐to‐moderate disease severity (SCORing AD value between 8 and 30) · Sum of the size of each of the areas selected could not exceed 20% of the body. · Women with childbearing potential were required to be using an effective means of contraception Exclusion criteria: · Using any systemic or topical therapy for AD within the previous 8 weeks or 2 weeks · Used anti‐inflammatory drugs, antihistamine drugs, or antibiotic agents during the previous week, immunosuppressant and/or corticosteroid drugs during the previous week, immunosuppressant and/or corticosteroid drugs during the previous month, or systemic retinoid drugs during the previous 6 months Additional design details: failure to recruit the target population after 8 months led to termination of the trial when 28 patients had been recruited. |
| Participants | Total number randomised: 56 sides treated Age: mean 28 years (range from 19 to 56 years) Sex: 43% male; 57% female Ethnicity, duration of eczema, severity of eczema: not reported Body site: AD lesions treated were in topographic areas (arms, legs, or trunk) Number of withdrawals: 3 were excluded for major protocol deviations (antihistamines intake n = 1, missing applications and visits n = 2) Notes: participants were randomised in blocks of 6 with an equal probability to 1 of 6 treatment groups. |
| Interventions | Run‐in details: not reported Intervention one: UR‐1505 0.50% ointment used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention two: UR‐1505 1% ointment used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention three: UR‐1505 2% ointment used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention four: tacrolimus 0.10% ointment used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA · EASI · Local tolerability assessed by clinicians for erythema, oedema, skin dryness, desquamation and roughness · Functional and physical signs of tightness, stinging, itching, warmth/burning and roughness were assesssed by patients. · Blood and urine safety · AE |
| Notes | Funding source: Palau Pharma SA Declarations of interest: three authors were employees of Palau Pharma S.A. at the time that the study was conducted. Another author was an employee of Statelis, a company outsourced by Pharmascan for data management and other support activities. Original language of publication: English Other: none |
Wahn 2002.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: 13 countries Outpatient or hospital: 53 centres Date trial conducted: July to December 1999 Duration of trial participation: 1 year Inclusion criteria: · Aged 2 to 17 years · AD according to Wiliams and colleagues criteria · AD affecting at least 5% BSA · IGA more than or equal to 2 Exclusion criteria: · Received phototherapy or systemic therapy known or suspected to affect AD up to 1 month before first application of study medication · Topical therapy known or suspected to affect AD up to 7 days before · Systemic antibiotics up to 2 weeks before · Infections requiring treatment with prohibited medications · Skin conditions which would affect evaluation Additional design details: none |
| Participants | Total number randomised: 713 participants (476 to apply the intervention and 237 to apply the comparator) Age: mean: intervention group: 8 years (range 1 to 17); comparator group: 7.9 years (range 2 to 17) Sex: intervention: male 47.3%, female 52.7%; comparator group: male 47.3%, female 52.7% Ethnicity, duration of eczema: not reported Severity of eczema: IGA 1‐5 Body site: not reported Number of withdrawals: intervention group: 150 discontinued; comparator group: 122 discontinued Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream used twice daily for 1 year · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used for 1 year · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: emollients to treat dry skin. Second‐line moderately potent topical corticosteroids allowed for flares (0.02% difluprednate cream, 0.25% prednicarbate cream, 0.1% hydrocortisone butyrate cream, 0.05% clobetason butyrate cream, 0.02% triamcinolone acetonide cream, 0.2% hydrocortisone valerate cream) Notes: none |
| Outcomes | · IGA · EASI · Safety assessments |
| Notes | Funding source: Novartis Declarations of interest: one author has acted as a consultant to Novartis and to other manufacturers of therapies for AD, three were employees of Novartis at the time of the study and a fifth had been funded by Novartis for a research fellowship. Original language of publication: English Other: none |
Wananukul 2013.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, within‐participant study Trial registration number: not reported Country: Thailand Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 4 weeks Inclusion criteria: children with mild‐to‐moderate AD Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 110 sides treated (55 to apply the intervention and 55 to apply the comparator) Age: 3 months to 14 years Sex, ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐moderate Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: hydrocortisone 1% other formulation used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: moisturiser with licochalcone (a type of natural phenol that can be extracted from the liquorice plant), 0% other formulations used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · SCORAD · TEWL |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Whalley 2002.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: not reported Country: UK Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 12 months Inclusion criteria: children aged 2 months to 2 years Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: at least 155 participants (126 to apply the intervention and 29 to apply the comparator) Age: 2 months to 2 years Sex, ethnicity, duration of eczema, severity of eczema: not reported, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream used for 1 year · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | PIQoL‐AD: quality of life of parents of children with AD CLDQI: to be completed by the parents of children aged 0 to 8 years with AD |
| Notes | Funding source: not reported Declarations of interest: not declared, but authors affiliated to Galen Research, Manchester, UK; and Novartis Pharma AG, Basel, Switzerland Original language of publication: English Other: none |
Wolkerstorfer 1998.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: not reported Country: not reported but the trial authors were from the Netherlands Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 7 weeks (1 week run‐in period, up to 4 weeks treatment, 2 weeks follow‐up) Inclusion criteria: · Moderately active AD · Aged 3 to 8 years Exclusion criteria: use of systemic treatment for AD in the month preceding the trial Additional design details: none |
| Participants | Total number randomised: 22 participants (12 to apply the intervention and 10 to apply the comparator) Age: of the 21 completing the trial, (assumed mean, SD) fluticasone propionate group, mean age 4.9 years (SD 1.7); clobetasone butyrate group, mean 4.1 years (SD 1.1) Sex: fluticasone propionate group, male n = 4, female n = 8; clobetasone butyrate group, male n = 7, female n = 2 Ethnicity, duration of eczema: not reported Severity of eczema: SCORAD (assumed mean, SD): fluticasone propionate group, mean 29 (SD 6.2); clobetasone butyrate group mean 32 (SD 5.6) Body site: not reported Number of withdrawals: one participant withdrew from the clobetasone group because of varicella. Notes: not reported |
| Interventions | Run‐in details: one‐week washout with only emollient, hydrocortisone acetate 1%, and antihistamine if required Intervention: fluticasone propionate 0.05% cream used once daily for 28 days · Concurrent treatment: vehicle cream was applied in the morning, active treatment in the evening · Other key information: none Comparator: clobetasone butyrate 0.0 5% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: during the trial, all children used the same basic skin care (emollient, bath oil). Notes: treatment was stopped if ObjSCORAD decreased to below 9 (clinically healed), or after 4 weeks. During a 2‐week follow‐up period, only basic skin care with no anti‐inflammatories was permitted. |
| Outcomes | · Objective SCORAD (modified consensus of the European Task Force on Atopic Dermatitis) · AE (reported in methods but no results given) |
| Notes | Funding source: not reported Declarations of interest: not declared, but one author was affiliated with Glaxo Wellcome. Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Woods 2011.
| Study characteristics | |
| Methods | Trial design: randomised, single‐centre study Trial registration number: NCT00819507 Country: USA Outpatient or hospital: outpatient university‐based dermatology clinic Date trial conducted: January 2009 to June 2010 Duration of trial participation: 2 weeks Inclusion criteria: · 12 years of age or older with AD according to Hanifin‐Rajka criteria · IGA of moderate, severe, or very severe · Failed to achieve adequate disease control despite appropriate topical or systemic therapy · A candidate, according to the principal investigator, for a super‐potent topical steroid course Exclusion criteria: · Active skin infection · Hypersensitivity to any ingredients in fluocinonide 0.1% cream · Previous use of super‐potent topical steroids within two weeks of starting study (class I steroid) · Current use of systemic therapy, unless on stable doses for at least three months Additional design details: none |
| Participants | Total number randomised: 50 sides treated (25 to apply the intervention and 25 to apply the comparator) Age: mean 39 years (range 17 to 67) Sex: female n = 14, male n = 11 Ethnicity: Hispanic n = 1, mixed n = 1, Asian n = 2, African‐American = 2, Caucasian n = 19 Duration of eczema: not reported Severity of eczema: moderate‐to‐severe AD according to Hanifin‐Rajka criteria Body site: target areas could not be chosen from the hands, feet, face or genital area. Number of withdrawals: there were no withdrawals. Notes: none |
| Interventions | Run‐in details: there was no washout period required for use of class II or less potent topical steroids, representing a typical clinical scenario in which patients use progressively more potent topical steroids to achieve disease control. Intervention: fluocinonide 0.1% cream used once daily for 14 days or until clear · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used once daily for 14 days or until clear · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: systemic therapy (including phototherapy) for AD was allowed only if the subject was on stable doses for at least three months. Topical antipruritics or topical anti‐inflammatory medications were not permitted during the study, but required no washout. The use of emollients (only Cetaphil™ cream, Aquaphor™, plastibase, or petrolatum) was permitted during the study, but not for eight hours prior to measurements. The use of antibiotics (topical or oral) or antihistamines was permitted during the study. Participants continued all other oral medications needed for stable medical problems. Notes: none |
| Outcomes | · Basal trans‐epidermal water loss in acute lesional skin · Change in skin capacitance · EASI score · Pruritus VAS · Quality of life · IGA |
| Notes | Funding source: Medicis Pharmaceutical Corporation Declarations of interest: three authors had no relevant conflicts of interest to disclose. Another author received a research grant from Medicis Pharmaceuticals for this project, and has served as consultant to and received honorariums from various pharmaceutical companies. Original language of publication: English Other: none |
Wortzel 1975a.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: USA (assumed from author affiliation) Outpatient or hospital: multiple outpatient centres Date trial conducted: not reported Duration of trial participation: 22 days Inclusion criteria: AD and psoriasis patients (data only extracted for AD patients) Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 128 participants (66 to apply the intervention and 62 to apply the comparator) Age, sex, ethnicity, duration of eczema: not reported Severity of eczema: not reported, although it was stated that these were not hospitalised because of the severity of their condition. Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: betamethasone dipropionate 0.05% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: brand: Diprosone Comparator: hydrocortisone 1% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: brand: unspecified Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Investigator's opinion of drug effects · Overall therapeutic response, assumed to be assessed by the physician: 5‐point scale (excellent, good, fair, poor, exacerbation) · AE |
| Notes | Funding source: test treatment was supplied by Schering Corporation, Bloomsfield, N. J. when it was not generally available. Declarations of interest: not declared, apart from the above Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Wortzel 1975b.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: USA (assumed from author affiliation) Outpatient or hospital: multiple outpatient centres Date trial conducted: not reported Duration of trial participation: 22 days Inclusion criteria: AD and psoriasis patients (data only extracted for AD patients) Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 54 participants (24 to apply the intervention and 30 to apply the comparator) Age, sex, ethnicity, duration of eczema: not reported Severity of eczema: not reported, although it was stated that these were not hospitalised because of the severity of their condition Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: betamethasone dipropionate 0.05% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: brand: Diprosone Comparator: vehicle ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: brand: unspecified Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Investigator's opinion of drug effects · Overall therapeutic response, assumed to be assessed by the physician: 5‐point scale (excellent, good, fair, poor, exacerbation) · AE |
| Notes | Funding source: test treatment was supplied by Schering Corporation, Bloomsfield, N. J. when it was not generally available. Declarations of interest: not declared, apart from the above Original language of publication: English Other: none |
Wortzel 1975c.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: USA (assumed from author affiliation) Outpatient or hospital: multiple outpatient centres Date trial conducted: not reported Duration of trial participation: 22 days Inclusion criteria: AD and psoriasis patients (data only extracted for AD patients) Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 6 participants (2 to apply the intervention and 4 to apply the comparator) Age, sex, ethnicity, duration of eczema: not reported Severity of eczema: very severe; these patients had been hospitalised because of the severity of their condition. Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: betamethasone dipropionate 0.05% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: brand: Diprosone Comparator: vehicle ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: brand: unspecified Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Investigator's opinion of drug effects · Overall therapeutic response, assumed to be assessed by the physician: 5‐point scale (excellent, good, fair, poor, exacerbation) · AE |
| Notes | Funding source: test treatment was supplied by Schering Corporation, Bloomsfield, N. J. when it was not generally available. Declarations of interest: not declared apart from the above Original language of publication: English Other: none |
Wu 2013.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: China Outpatient or hospital: author affiliation to a Department of Dermatology Date trial conducted: not reported Duration of trial participation: 40 days (10 day treatment phase and 30 day follow‐up) Inclusion criteria: infantile acute or subacute facial eczema according to AA Dermatology Consensus Conference on Pediatric Atopic Dermatitis Exclusion criteria: · Renal impairment, liver disease, abnormal liver function tests, haematological abnormalities, or autoimmune disease other than eczema · Clinical signs of infection · Topical and systemic treatments with antibiotics, corticosteroids, antihistamines, immunosuppressive agents and traditional Chinese herbal medicine within the previous 2 weeks Additional design details: The group including LXA4 treatment was not included on the basis that it is a fatty acid similar to a leukotriene according to https://cdn.caymanchem.com/cdn/insert/10033.pdf. |
| Participants | Total number randomised: 40 participants (20 to apply the intervention and 20 to apply the comparator) Age: mean 4.2 months, range from 1 to 12 months Sex: intervention group 6/19 female; comparator group 7/17 female Ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐severe Body site: acute and subacute facial eczema Number of withdrawals: n = 4, comparator group n = 3 as parent caregiver refused ineffective treatment; intervention group n = 1 because of use of concomitant medication not permitted in the trial Notes: none |
| Interventions | Run‐in details: not reported Intervention: mometasone furoate 0.1% cream used twice daily for 10 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 10 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · The investigator and the patient’s caregiver severity ratings of erythema, papulation, vesiculation, scaling, lichenification and pruritus from 0 = absent to 4 = extremely severe · IGA even‐point scale modified from EASI where 0 = completely clear to 6 = worse · IDQOL, evaluated by the patient’s caregiver · AEs, physical examination and laboratory investigations |
| Notes | Funding source: medical emphasis grant from the government of Jiangsu province Declarations of interest: not declared Original language of publication: English Other: none |
Yasuda 1976.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, within participant, multicentre study Trial registration number: not reported Country: Japan Outpatient or hospital: 29 Dermatology Departments Date trial conducted: not reported Duration of trial participation: 7 days Inclusion criteria: people with AD, acute eczematous dermatitis or psoriasis vulgaris Exclusion criteria: not reported Additional design details: reports on three pairwise within‐participant comparisons between hydrocortisone butyrate and one of three controls. For the purposes of this review, we have extracted the two comparisons; the third is excluded as it is a comparison of two TCS of the same potency. Only the data on eczema patients were extracted. |
| Participants | Total number randomised: unclear, 144 patients with AD were 'scheduled', of which data from 105 was evaluated. Age, sex, ethnicity, duration of eczema, severity of eczema, body site: not reported Number of withdrawals: unclear, 105 were evaluated, 7 dropped out, and 32 were not evaluated. Reasons were not provided. Notes: none |
| Interventions | Run‐in details: not reported Intervention: hydrocortisone butyrate 0.1% ointment used twice daily for 7 days · Concurrent treatment: not reported · Other key information: none Comparator one: hydrocortisone acetate 1% ointment used twice daily for 7 days · Concurrent treatment: not reported · Other key information: none Comparator two: vehicle ointment used twice daily for 7 days · Concurrent treatment: not reported · Other key information: brand: Plastibase Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Clinician preference (effectiveness) |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
Yawalkar 1991.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: not reported Country: Germany Outpatient or hospital: to quote: "performed by dermatologists" Date trial conducted: not reported Duration of trial participation: 12 to 17 days Inclusion criteria: · AD (non‐infected, acute, severe exacerbation) · Age ≥ 15 years · Affecting up to 20% of total BSA and suitable for topical therapy Exclusion criteria: · Age < 15 years · Pregnancy or breastfeeding · Secondary microbial infection of lesions · Concomitant tuberculosis, syphilitic or viral infections · Diabetes mellitus · Leukaemia · Parasitic infestations · Perioral dermatitis Additional design details: none |
| Participants | Total number randomised: unclear; the results from 117 are relevant to this review. Age, sex, ethnicity, duration of eczema: not reported Severity of eczema: moderate‐to‐severe Body site: not reported Number of withdrawals: 16 participants were excluded for protocol violations or non‐compliance with the planned treatment schedule (unclear how many of these were from the included comparison). Notes: To quote: "The treatment groups were comparable with regard to the number of participants, age, sex, duration of present attack, and severity and extent of the disease." |
| Interventions | Run‐in details: not reported Intervention: halobetasol propionate 0.05% cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: brand: Ultravate Comparator: betamethasone dipropionate 0.0 5% cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: without occlusion Notes: none |
| Outcomes | · Disease severity (4‐point scale) · AE · Participant‐reported cosmetic acceptability and ease of application · IGA of therapeutic effect (4‐point scale: 1 = healed, 2 = marked improvement, 3 = moderate improvement, 4 = slight/no improvement) · Onset of therapeutic action |
| Notes | Funding source: an educational grant from Westwood‐Squibb Pharmaceuticals Declarations of interest: not declared in addition to the funder, but 2 employees from Ciba‐Geigy Limited (now Novartis) were acknowledged for their roles in organising the trial and commenting on the manuscript Original language of publication: English Other: this study has previously been extracted by this group; some content is reproduced from Lax 2022. |
Zane 2013.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, vehicle‐controlled, within‐participant study Trial registration number: NCT01301508 Country: Australia Outpatient or hospital: outpatient (assumed) Date trial conducted: May to November 2011 Duration of trial participation: 6 weeks Inclusion criteria: · AD that has been clinically stable for ≥ 1 month · BSA of AD involvement ≤ 35%, excluding involvement of the face, scalp, and groin · Presence of two comparable target lesions · Females of childbearing potential must use at least one highly effective method of birth control. Males with partners of childbearing potential should inform them of their participation in this clinical study and use highly effective methods of birth control during the study. Exclusion criteria: · Concurrent or recent use of certain topical or systemic medications or phototherapy without a sufficient washout period · Active or potentially recurrent dermatologic condition other than AD in the target lesion area that may confound evaluation Additional design details: this trial is nested within a parallel‐assignment to one of two intervention‐comparator pairs. We have treated them as two separate within‐participant trials for the purposes of this review analysis. |
| Participants | Total number randomised: 92 sides treated (intervention comparator pair one ‐ 25 to apply intervention one and 25 to apply the comparator; intervention comparator pair two ‐ 21 to apply intervention two and 21 to apply the comparator) Age: 18 to 75 years Sex: male n = 10, female n = 11 Ethnicity: not reported Duration of eczema: clinically stable for ≥ 1 month Severity of eczema: mild‐to‐moderate Body site: trunk, upper extremities or lower extremities Number of withdrawals: 1 withdrawal Notes: none |
| Interventions | Run‐in details: not reported Intervention one: AN2898 1% ointment used twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Intervention two: AN2898 2% ointment used twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · ADSI score · Number of participants with treatment‐emergent AEs and SAEs |
| Notes | Funding source: Pfizer Declarations of interest: not declared Original language of publication: English Other: none |
Zuberbier 2008.
| Study characteristics | |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: not reported Country: Germany Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 24 weeks Inclusion criteria: · Age 2 to 17 years · Active AD of all degrees of severity Exclusion criteria: · If skin condition did not improve significantly under treatment with prednicarbate for a maximum of 21 days · Had received topical corticosteroids within 7 days, or phototherapy or systemic corticosteroids/immunosuppressants within 1 month prior to entry · Children with active acute viral infection or children who were immunocompromised Additional design details: none |
| Participants | Total number randomised: 184 participants Age: 2 to 17 years Sex: males n = 65, females n = 75 Ethnicity: Caucasian n = 130, black n = 2, oriental n = 7, other n = 1 Duration of eczema: not reported Severity of eczema: IGA score: 1 n = 41, 2 n = 44, 3 n = 5 Body site: all participants analysed had facial involvement. Number of withdrawals: 22 participants withdrew, 21.9% from the comparator group and 10.5% from the intervention group Notes: none |
| Interventions | Run‐in details: treated for at least 7 days with prednicarbate 0.25% cream Intervention: pimecrolimus 1% cream used twice daily for 24 weeks. Apply intervention "as long as signs of AD were present". · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 24 weeks. Apply intervention "as long as signs of AD were present". · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: concomitant use of emollients was allowed when needed. In the event of a flare (unacceptable severity of itching/scratching or onset of oozing), treatment with study medication was replaced by treatment with prednicarbate 0.25% cream. As soon as the flare was controlled (marked reduction in itching/scratching and no oozing), the treatment with topical steroids was discontinued and the study medication reinitiated. Notes: none |
| Outcomes | · AE · EASI score at end of study (a score between 0 and 72; it is calculated by taking into account the extent and the severity of the clinical signs, erythema, infiltration/papulation, excoriation and lichenification) · The Patient’s Overall Self‐Assessment score included pruritus, sleep disturbances and general disease control of the patients · CDLQI and the Parents’ Index of Quality of Life‐Atopic Dermatitis |
| Notes | Funding source: Novartis Declarations of interest: not declared Original language of publication: English Other: none |
ACTH: adrenocorticotropic hormone AD: atopic dermatitis ADCT: Atopic Dermatitis Control Tool ADSI: Atopic Dermatitis Severity Index AE: adverse events ALT: alanine transaminase ASRs: Patient assessment of local tolerability AST: aspartate transaminase AZT: azidothymidine BID: twice a day BMI: body mass index BSA: body surface area CDLQI: Children’s Dermatology Life Quality Index DFI: Dermatitis Family Impact Questionnaire DLQI: Dermatology Life Quality Index DQoL: the Dermatology Quality of Life Scale DSS: dermatologic sum score EASI: Eczema Area and Severity Index EASI‐75: improvement of at least 75% in lesion extent and severity EASI‐50 score: improvement of at least 50% in lesion extent and severity ECG: electrocardiogram EQ‐5D‐5L: EuroQuality of Life Five Dimensions FDA: US Food and Drug Administration FOCBP: females of child‐bearing potential HBcAb: hepatitis B blood tests HBsAg: hepatitis B surface antigen HCV: hepatitis C virus HIV: human immunodeficiency viruses HPA: hypothalamic‐pituitary‐adrenal IDQOL: Infants' Dermatitis Quality of Life Index IGA: Investigator's Global Assessment IGADA: Investigator's Global Atopic Dermatitis Assessment IGAL: Investigator Global Assessment IGA‐TS: Investigator's Global Assessment ‐ Treatment Success IgE: immunoglobulin E IGSS: Investigators Global Severity Score ISGA: Investigator’s Static Global Assessment ‐ a 5‐point scale mEASI(50): number of participants with at least 50% improvement in modified EASI NRS: numerical rating scale PDE‐4 inhibitors: phosphodiesterase‐4 inhibitors PGA: Physician’s Global Assessment PGIC: Patient Global Impression of Change PIQoL‐AD: Quality of life of parent(s) of children with AD scale POEM: Patient‐Reported Outcomes Measurement Information System PROMIS: Patient‐Reported Outcomes Measurement Information System PUVA: psoralen plus ultraviolet‐A radiation therapy QD: once a day QoL: quality of life QoLIAD: Quality of Life Index ‐ AD RECAP: Recap of atopic eczema questionaire SAE: serious adverse event(s) SA‐EASI: self‐administered EASI SASSAD: six area, six sign atopic dermatitis severity score SCORAD: Scoring Atopic Dermatitis Index SD: standard deviation SE: standard error SEM: standard error of mean TCI: topical calcineurin inhibitors TCS: topical corticosteroids TEAE: treatment‐emergent adverse event(s) TEWL: transepidermal water loss TLR: Toll‐like Receptor TLS: target lesion scores TLSS: Target Lesion Symptoms Score TSS: Total Severity Score/Total Sign Score UV: ultraviolet UVA: ultraviolet A therapy vIGA‐AD: validated Investigator Global Assessment‐Atopic Dermatitis VAS: visual analogue scale VRS: verbal rating scale WHO: World Health Organisation WI‐NRS: Worst‐Itch Numerical Rating Scale WPAI‐SHP: Work Productivity and Activity Impairment Questionnaire: Specific Health Problem
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdel‐Aal 1976 | Ineligible comparator |
| Afshar 2013 | No relevant outcomes measured/recorded (immunohistochemical analysis) |
| Ahumada 1982 | Ineligible intervention |
| Alex 2018 | Ineligible intervention |
| Alexander 1973 | Does not meet the criteria for eczema diagnosis |
| Alexopoulos 2023 | Ineligible intervention |
| Amerio 1998 | Same potency TCS |
| Anonymous 2018 | Ineligible intervention |
| Arico 1976 | Same potency TCS |
| Arkwright 2007 | Ineligible study design |
| Asada 1990 | Same potency TCS |
| Ashurst 1970 | Does not meet the criteria for eczema diagnosis |
| Ashurst 1972 | Does not meet the criteria for eczema diagnosis |
| August 1985 | Does not meet the criteria for eczema diagnosis |
| Aussems 1972 | Ineligible intervention |
| Belliboni 1964 | Ineligible intervention |
| Berberian 1999 | Ineligible intervention |
| Bergoend 1982 | Does not meet the criteria for eczema diagnosis |
| Berth‐Jones 2003 | Ineligible intervention |
| Bieber 2021 | Ineligible intervention |
| Binder 1972 | Ineligible intervention |
| Bircher 2002 | Ineligible intervention |
| Bleehen 1995 | Ineligible comparator |
| Bloudek 2023 | No relevant outcomes measured/recorded |
| Boguniewicz 2008 | Ineligible intervention |
| Breneman 2006 | Ineligible intervention |
| Buckley 1964 | Same potency TCS |
| Bureau 1963 | Ineligible intervention |
| Camacho 1996 | Same potency TCS |
| Caproni 2006 | No relevant outcomes measured/recorded (immunohistochemical analysis) |
| Chiarenza 1982 | Does not meet the criteria for eczema diagnosis |
| ChiCTR2000030216 | Ineligible intervention |
| ChiCTR2000030246 | Ineligible comparator |
| Colpas 2010 | Ineligible comparator |
| Cranswick 2006 | No relevant outcomes measured/recorded (immunohistochemical analysis) |
| Danby 2014 | No relevant outcomes measured/recorded |
| De Waure 2013 | Does not meet the criteria for eczema diagnosis |
| Desmons 1977 | Ineligible comparator |
| Dhar Smalakar 1999 | Ineligible comparator |
| Doherty 1979 | Same potency TCS |
| Drake 1999 | Ineligible intervention |
| Durand 1975 | Ineligible intervention |
| Elgart 1978 | Same potency TCS |
| EUCTR2005‐002803‐18‐LV | Ineligible comparator |
| EUCTR2009‐010498‐19‐FR | Ineligible intervention |
| EUCTR2009‐012194‐35‐DE | Ineligible intervention |
| EUCTR2009‐017407‐28‐DE | Ineligible comparator |
| Gip 1982 | Same potency TCS |
| GP Medical Research 1967 | Ineligible intervention |
| GP Research Group | Does not meet the criteria for eczema diagnosis |
| Grejs 1978 | Does not meet the criteria for eczema diagnosis |
| Guillot 1983 | Same potency TCS |
| Guttman‐Yassky 2017b | Ineligible intervention |
| Hamada 1996 | Ineligible intervention |
| Haneke 1992 | Same potency TCS |
| Iraji 2022 | Ineligible comparator |
| IRCT2016102323235N5 | Ineligible intervention |
| Kamiya 2022 | Ineligible comparator |
| Kanof 1975 | Ineligible intervention |
| Kassis 1982 | Same potency TCS |
| Katsambas 1973 | Ineligible intervention |
| Kim 2013 | Same potency TCS |
| Kircik 2009 | Ineligible intervention |
| Kwon 2019 | Ineligible intervention |
| Lecomte 2003 | No relevant outcomes measured/recorded (cost‐effectiveness analysis) |
| Lee 2021 | Ineligible intervention |
| Leeming 1974 | Same potency TCS |
| Lessard 1980 | Same potency TCS |
| Liu 2000 | Ineligible comparator |
| Liu 2008 | Ineligible intervention |
| Liu 2018 | Ineligible intervention |
| Liu 2022 | No relevant outcomes measured/recorded |
| Londono 1976 | Ineligible intervention |
| Luderschmidt 1981 | Does not meet the criteria for eczema diagnosis |
| Lundell 1974 | Same potency TCS |
| Lundell 1975 | Does not meet the criteria for eczema diagnosis |
| Mackey 1974 | Ineligible comparator |
| Mackey 1977 | Does not meet the criteria for eczema diagnosis |
| Manusov 1974 | Ineligible intervention |
| Marchesi 1994 | Ineligible study design |
| Methaderm Study Group 1989 | Same potency TCS |
| Miki 1990 | Same potency TCS |
| Morley 1976 | Same potency TCS |
| Munro 1977 | Same potency TCS |
| NCT00106496 | Ineligible intervention |
| NCT00185510 | Ineligible intervention |
| NCT00480610 | Ineligible intervention |
| NCT00568412 | Ineligible comparator |
| NCT00616538 | Ineligible comparator |
| NCT00828412 | Ineligible comparator |
| NCT00919763 | Ineligible intervention |
| NCT01119313 | Ineligible comparator |
| NCT01429701 | Ineligible comparator |
| NCT01850849 | Ineligible intervention |
| NCT02426359 | Ineligible intervention |
| NCT02576938 | Ineligible intervention |
| NCT02582177 | Does not meet the criteria for eczema diagnosis |
| NCT03059693 | Ineligible intervention |
| NCT03627767 | Ineligible intervention |
| NCT03796676 | Ineligible intervention |
| NCT03859986 | Ineligible intervention |
| NCT03911401 | Ineligible intervention |
| NCT04490109 | Ineligible intervention |
| NCT05018806 | Ineligible intervention |
| NCT05318300 | Ineligible intervention |
| NCT05792826 | Ineligible intervention |
| Okoshi 2022 | Ineligible intervention |
| Park 2022 | Ineligible intervention |
| Pellanda 2005 | Ineligible intervention |
| Queille 1984 | Ineligible intervention |
| Reidhav 1996 | Same potency TCS |
| Reyes 1977 | Ineligible intervention |
| Rodriguez 1977 | Same potency TCS |
| Ronnstad 2023 | Ineligible intervention |
| Roth 1973a | Ineligible comparator |
| Roth 1973b | Ineligible intervention |
| Sandstrom 2006 | Ineligible comparator |
| Schmidt 1987 | Does not meet the criteria for eczema diagnosis |
| Schneider 1977 | Does not meet the criteria for eczema diagnosis |
| Schuttelaar 2003 | Ineligible comparator |
| Sefton 1983e | Same potency TCS |
| Snyder 2022 | Ineligible intervention |
| Stahle 1965 | Does not meet the criteria for eczema diagnosis |
| Stahle 1965a | Does not meet the criteria for eczema diagnosis |
| Strick 1975 | Ineligible intervention |
| Sudilovsky 1975 | Same potency TCS |
| Suresh 2017 | Ineligible comparator |
| Takemura 1988 | Same potency TCS |
| TCTR20220210004 | Same potency TCS |
| TCTR20220313002 | Ineligible intervention |
| Thaci 2010 | Ineligible intervention |
| Toda 1983 | Ineligible intervention |
| Ueda 1989 | Same potency TCS |
| Van der Meer 1997a | Ineligible intervention |
| Van Halewijn 2023 | No relevant outcomes measured/recorded |
| Van Zuiden 1978 | Same potency TCS |
| Venier 1982 | Same potency TCS |
| Wang 1995 | Does not meet the criteria for eczema diagnosis |
| Wang 2003 | Same potency TCS |
| Weitgasser 1972a | Ineligible intervention |
| Wilson 1973 | Does not meet the criteria for eczema diagnosis |
| Xhauflaire‐Uhoda 2007 | No relevant outcomes measured/recorded (skin barrier analysis) |
| Zirwas 2017 | Ineligible intervention |
TCS: topical corticosteroids
Characteristics of studies awaiting classification [ordered by study ID]
Bos 2002.
| Methods | Trial design: double‐blind study Trial registration number, country, outpatient or hospital, date trial conducted, duration of trial participation: 3 weeks Additional design details: study awaiting classification as unclear if randomised Inclusion criteria: children with AD Exclusion criteria: not reported |
| Participants | Total number randomised: 624 participants Age: 2 to 6 years Sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention one: tacrolimus 0.03% ointment used twice daily · Concurrent treatment: not reported · Other key information: none Intervention two: hydrocortisone acetate 1.% ointment used twice daily · Concurrent treatment: not reported · Other key information: Comparator: not reported · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: |
| Outcomes | · Modified EASI · Physicians global evaluation · AE |
| Notes | Funding source: not reported Declarations of interest: not reported Original language of publication: English Other: results provided but not extracted as unclear if the trial is a randomised study |
ChiCTR2100043963.
| Methods | Trial design: parallel study Trial registration number: ChiCTR2100043963 Country: China Outpatient or hospital: not reported Date trial conducted: from March 2021 Duration of trial participation: not reported Inclusion criteria: · Patients meeting the diagnostic criteria for AD according to Hanifin‐Rajka criteria · Chronic or chronically relapsing course · Age from 2 to 45 years, male or female, Chinese Han population. 3.0 < SCORAD < 50 points Exclusion criteria: · Combined with other skin diseases, such as psoriasis, vitiligo, lupus erythematosus · Local infection at the lesion site · Autoimmune diseases, such as rheumatoid arthritis · A history of severe systemic disease or malignant tumour · A history of systemic use of anti‐inflammatory drugs within 1 month; or a history of systemic use of antibiotics and antifungal drugs within 1 month; or a history of topical use of corticosteroids, calcineurin and other anti‐inflammatory drugs within 2 weeks; or a history of topical antibiotics or antifungal topical medication within 2 weeks · Participated in other clinical trials in the past month Additional design details: none |
| Participants | Total number randomised: not reported Age: 2 to 45 years Sex: both Ethnicity: Chinese Han Duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: Propionate 0.05% cream · Concurrent treatment: not reported · Other key information: none Comparator: Placebo 0.% cream · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI · VAS · IGA · SCORAD |
| Notes | Funding source: Scientific Research Innovation Program of Shanghai Education Commission Declarations of interest: not declared Original language of publication: English Other: Note: trial registration lists intervention as "topical application of 0.05% propionate cream"; therefore, insufficient information to classify potency. |
ChiCTR2100044339.
| Methods | Trial design: randomised, parallel study Trial registration number: ChiCTR2100044339 Country: China Outpatient or hospital: Shaanxi Traditional Chinese Medicine Hospital ‐ Tertiary Date trial conducted: from April 2021 Duration of trial participation: not reported Inclusion criteria: · Diagnosis of AD according to Hanifin and Rajka criteria · SCORAD score ≤ 40 · Aged between 7 and 60 years · Participant or guardian has signed the informed consent form. Exclusion criteria: · Severe primary diseases, such as cardiovascular disease, respiratory diseases, liver and kidney disease, blood system diseases, tumours and mental illness · Known experimental drug allergies · Abnormal clinical values · Pregnancy and breastfeeding or planning to get pregnant within 3 months · In the range of at least 4 weeks using systemic immunomodulatory or immunosuppressive agents. In the range of at least 2 weeks using systemic or local antibiotics. In the range of at least 2 weeks using antihistamines. In the range of at least 2 weeks using PUVA, UVA or UVB. · In the range of at least 2 weeks of local application of tacrolimus or glucocorticoids. · Participating in other clinical trials or have participated in other clinical trials within 1 month. · Other reasons the researchers considered unsuitable for inclusion. Additional design details: none |
| Participants | Total number randomised: not reported Age: 7 to 60 years Sex: both Ethnicity, duration of eczema: not reported Severity of eczema: SCORAD score ≤ 40 Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: Taxisan · Concurrent treatment: not reported · Other key information: none Comparator: vaseline · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Scoring Atopic Dermatitis Index · VAS · IGA · DLQI · Colonisation of Staphylococcus aureus |
| Notes | Funding source: Xi'an Municipal Bureau of Science and Technology Declarations of interest: not declared Original language of publication: English Other: unclear if the intervention was an anti‐inflammatory |
ChiCTR2100053644.
| Methods | Trial design: randomised, blinded, single‐centre, parallel study Trial registration number: ChiCTR2100053644 2021 Country: China Outpatient or hospital: University of Hong Kong‐Shenzhen Hospital Date trial conducted: from December 2021 Duration of trial participation: 28 days Inclusion criteria: · 6 to 65 years, no gender limit · AD according to Hanifin and Rajka criteria, history ≥ 6 months · IGA 2 to 3 points · AD lesions involved (excluding scalp lesions, but including facial lesions) 3%‐20% of the BSA · Female subjects with fertility and male subjects who have not undergone vasectomy should take effective contraceptive measures. Exclusion criteria: · AD skin lesion area infected · History of angioedema or allergic reactions · Other active inflammatory skin diseases · Systemic or topical PDE‐4 inhibitors in the past · Clinically significant active systemic infections · ALT or AST > 2 times the upper limit of normal, or renal function Cr > upper limit of normal · Unwilling to restrict their excessive ultraviolet exposure · Received biological treatment within 12 weeks or 5 half‐lives · Used within 4 weeks: systemic glucocorticoids and/or immunosuppressants; or sunbathing, phototherapy or systemic use for the purpose of treating AD of Chinese medicine or natural medicine · Used within 2 weeks: topical use of glucocorticoids or topical calcineurin inhibitors; or topical use of Chinese medicines or natural medicines for the purpose of treating AD; or other topical treatments · Used topical antimicrobial agents within 1 week · Severe central nervous system, cardiovascular, respiratory, liver, kidney, gastrointestinal, urinary, endocrine, or blood system diseases · Severe mental illness, or other conditions that affect research compliance, which may interfere with the implementation of clinical trials · History of malignant tumours · History of severe allergies to external skin preparations · Long‐term drug abuse or alcohol abuse history within 6 months · Female suspected of being pregnant, lactating, or preparing to become pregnant during the trial · Participated in other drug/device clinical research within 3 months before randomisation · Females who have reproductive potential but are unwilling or unable to use a reliable contraceptive Additional design details: none |
| Participants | Total number randomised: not reported Age: 6 to 65 years Sex: both Ethnicity: not reported Duration of eczema: at least 6 months Severity of eczema: mild‐to‐moderate Body site: excluding scalp but not face Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: cliborrol ointment used for 28 days · Concurrent treatment: not reported · Other key information: none Comparator, concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: desonide cream Notes: none |
| Outcomes | · EASI · BSA · IGA · Skin Quality of Life Index questionnaire |
| Notes | Funding source: Pfizer Declarations of interest: not declared Original language of publication: English Other: unclear if the intervention was an anti‐inflammatory |
EUCTR2004‐004052‐39‐DK.
| Methods | Trial design: randomised, double‐blinded, cross‐over study Trial registration number: EUCTR2004‐004052‐39‐DK Country: Denmark Outpatient or hospital: not reported Date trial conducted: complete 2005, date started not provided Duration of trial participation: not reported Inclusion criteria: male or female child or adolescent with AD Exclusion criteria: · Puberty development: Tanner stage II ‐ V · Reversal of any anti‐inflammatory treatment for AD one week before visit 1 · Concomitant bronchial asthma and allergic rhinoconjunctivitis requiring treatment with glucocorticoids · Endocrinological diseases – including growth disorders – or other chronic diseases · Major operative interventions 4 weeks before and during the examination · High‐fever illness of more than 3 days duration Additional design details: none |
| Participants | Total number randomised, sex, ethnicity, duration of eczema, severity of eczema, body site: number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.1% ointment · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone‐17‐butyrate 0.1% ointment · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Lower leg growth |
| Notes | Funding source: Fujisawa Scandinavia AB Declarations of interest: not declared Original language of publication: English Other: unclear if trial has taken place |
EUCTR2005‐002471‐32‐GB.
| Methods | Trial design: randomised, double‐blind multicentre study Trial registration number: EUCTR2005‐002471‐32‐GB Country: UK Outpatient or hospital, date trial conducted, duration of trial participation: not reported Inclusion criteria: · AD according to Hanifin and Rajka crieria · Mild‐to‐moderate AD defined by a SASSAD score of 10 to 30 · Either sex aged 8 years and over · Willing and able to provide written informed consent, after being informed of all the pertinent aspects of the trial. In the case of patients aged under 16 years of age, parental or guardian consent must be obtained in addition to patient assent. · Willing/able to limit bathing/showering to a maximum of once daily, without additional bath emollient · Willing/able to use a standard aqueous cream for washing · Agree to minimise their alcohol consumption throughout the study, with a limit of 14 units per week Exclusion criteria: · Acutely infected eczema, or areas where the lesions are crusted, weeping or pustular · Severe eczema on their face or eyes, ano‐genital area or severe broken skin or infected skin · Received antibiotic therapy in the previous 2 weeks · Received significant topical or systemic therapy likely to interfere with the study in the previous 2 weeks · Using, or having used in the past month, any significant concomitant medication which might affect their eczema · Significant concurrent illness which might affect their eczema · History of allergic, hypersensitivity or idiosyncratic reaction to any of the active ingredients or any components of the study medications · Female patients who are pregnant, breastfeeding, or sexually active and not using reliable contraception and/or not prepared to do so for the duration of the trial · Participated in a clinical trial involving a drug within 30 days · History of alcohol/drug abuse Additional design details: 3‐armed trial of which only one comparison is relevant. Little detail was provided on the trial, including whether it was ever completed. |
| Participants | Total number randomised, sex, ethnicity, duration of eczema, severity of eczema, body site: number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: hydrocortisone acetate 1% cream · Concurrent treatment: not reported · Other key information: none Comparator: 0% cream · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Reduction in the signs of eczema as determined by the Investigator’s SASSAD rating scale at 4 weeks compared to baseline |
| Notes | Funding source: Stiefel Laboratories Maidenhead Ltd (commonly known as Stiefel International R&D) Declarations of interest: not declared Original language of publication: English Other: unclear if trial has taken place |
EUCTR2007‐002182‐12‐GB.
| Methods | Trial design: randomised, single‐blind, single‐centre parallel study Trial registration number: EUCTR2007‐002182‐12‐GB 2007 Country: UK Outpatient or hospital, date trial conducted, duration of trial participation: not reported Inclusion criteria: · Mild‐to‐moderate AD according to Hanifin and Rajka criteria and Rajka and Langeland grading system (score of 3 to 7.5) · IGA score of 2 or 3 · Either sex aged 2 to 65 years · Sexually active females of childbearing potential participating in the study must use a medically acceptable form of contraception (which includes oral contraception, injectable or implantable methods, intrauterine devices, or properly used barrier contraception). A female of childbearing potential is defined as one who is biologically capable of becoming pregnant. Exclusion criteria: · Severe AD who require systemic or very‐potent topical steroid treatment · Facial AD · Acutely infected AD, or areas where the lesions are crusted, weeping, or pustular · Female subjects who are pregnant or lactating · Currently receiving or who have received during the 14 days before study entry, topical or systemic therapy likely to interfere with the study · Participated, currently participating or who plan to participate in any other research study involving an investigational product or device at any time during and after the 4 weeks before study entry · Any significant concurrent illness including psoriasis, rosacea, acne vulgaris, peri‐oral dermatitis and primary skin lesions caused by infection with fungi or bacteria · History of skin disease, other than AD, or allergy, likely to interfere with the study · Past medical history of hepatic, renal, cardiac, pulmonary, digestive, haematological, neurological, locomotor, cancer or psychiatric disease, which, in the opinion of the Investigator would compromise the safety of the subject or affect the outcome of the study · Known sensitivity to the test product ingredients Additional design details: none |
| Participants | Total number randomised, age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: W0153 · Concurrent treatment: not reported · Other key information: none Comparator: betamethasone‐17‐valerate 0.122% cream · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Difference in time to the next AD flare (days) between the treatment groups · IGA (six‐point scale) at visits 1 to 6 inclusive · Overall rating of response to study investigational product by participant (five‐point scale) at visits 4, 5 and 6 · Change in the signs measured by SASSAD as determined by the Investigator’s SASSAD rating scale at weeks 4, 8 and 12 post‐clearance as compared to baseline |
| Notes | Funding source: Stiefel Laboratories Declarations of interest: not declared Original language of publication: English Other: unclear if the intervention is an anti‐inflammatory |
EUCTR2007‐007789‐39‐FR.
| Methods | Trial design: randomised, double‐blind, multicentre, within‐participant study Trial registration number: EUCTR2007‐007789‐39‐FR 2008 Country: France Outpatient or hospital: not reported Date trial conducted: 2008 Duration of trial participation: 4 weeks Inclusion criteria: · Males and females of non‐childbearing potential, over 18 years of age · Two symmetric plaques of atopic dermatitis on each side of the body of similar severity Exclusion criteria: not reported Additional design details: the study has results but was classified as awaiting classification as the status of CD4802 0.1% ointment is not clear. The study was prematurely halted because of AE. |
| Participants | Total number randomised: 24 sides treated (12 to apply the intervention and 12 to apply the comparator) Age: mean 36.8 years (SD 13.6), range from 18 to 56 years Sex: male n = 12, female n = 0 Ethnicity: Caucasian 75%, 8.3% each of black, Asian and other Duration of eczema, severity of eczema, body site: not reported Number of withdrawals: 0 Notes: none |
| Interventions | Run‐in details: not reported Intervention: CD4802 0.1% ointment used once daily for 28 days · Concurrent treatment: not reported · Other key information: the study is awaiting classification as intervention mode of action is unknown. Comparator: 0% (placebo) ointment used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Sum of erythema, excoriation, oedema/papulation, oozing/crusting and lichenification of target areas, individual scores of erythema, excoriation, oedema/papulation, oozing/crusting and lichenification of target areas (on a 0 to 3 scale) · Pruritus (on a 0 to 100 mm VAS) and clearing score · Subject’s and Investigator’s preference at day 29 (left versus right side) · AE at each visit ‐ vital signs ‐ Physical examination |
| Notes | Funding source: Galderma R&D Declarations of interest: not reported Original language of publication: English Other: the study was prematurely halted. To quote: "Due to the occurrence of AEs of irritation and/or worsening of eczema on only one side with aggravation of different signs of scoring, in seven subjects out of 12, an interim statistical analysis was performed on the first 12 subjects completed. This analysis aimed at providing preliminary results in order to reach a decision about the relevance of including the last eight patients as planned initially in the protocol; thus, the sponsor assumed that the data already collected were to be sufficient to justify stopping the study at 12 subjects for futility. The interim t‐statistic for AUC (TSS) was in favour of the vehicle; the probability of final significance for n = 20 was virtually zero. There was no value in continuing the study. The trial was stopped with the 12 subjects already completed. Concerning the safety, throughout the study, 10 (83.3%) subjects reported 18 AEs. Ten AEs were related to the tested product CD4802 0.1%, among which 9 were dermatologic AEs and one was increased calciuria.Out of 9 dermatologic related AEs (among 8 subjects, 66.7%) observed for CD4802 0.1%, 6 were “flare of eczema” or “flare of eczema or irritation” on target lesion, 2 were depigmentation of elbow fold and one was an irritation on target lesion. All these dermatological related AEs (9) were reported as mild to moderate in severity and did not lead to treatment discontinuation. They all resolved with no residual effects." Results of trial are provided but it was unclear if the intervention is eligible. |
EUCTR2011‐000917‐38‐DE.
| Methods | Trial design: randomised, double‐blind, multicenter, study Trial registration number: EUCTR2011‐000917‐38‐DE 2011/NCT01428297 Country: US, Germany, France, Netherlands Outpatient or hospital: 17 centres in the United States, Germany, France, and Netherlands Date trial conducted: May 2011 to February 2014 Duration of trial participation: 4 weeks Inclusion criteria: · Male and female, 18 to 65 years · Presence of AD confirmed by itchy skin condition in the past 12 months (must have) Plus three or more of the following: ‐ history of involvement of skin creases ‐ personal history of asthma or hay fever ‐ history of generally dry skin in the past year ‐ onset before age of 2 years ‐ visible flexural dermatitis · Diagnosis of at least moderate AD by the IGA and a minimum target area (right or left) situated on the forearm including the antecubital fossa with a corresponding baseline TLSS Exclusion criteria: · History of hypersensitivity to any of the study drugs or to drugs of similar chemical classes or history of serious allergic reaction · History of abnormal skin reactivity to UV light. Unusual exposure to UV light in the previous 3 weeks of study start, including tanning and sun beds · Pregnant or lactating women · Women of childbearing potential must use highly effective contraception. · Use of topical prescription treatment for eczema within 1 week prior to initial dosing of TCS · Recent previous treatment with systemic treatment including phototherapy. A washout period will be required for such patients to be eligible to participate in the trial. Additional design details: the study involved 3 cohorts of which only one was relevant to this review ("Healthy volunteers for Part 1, subjects with AD for Part 2 and subjects with Netherton Syndrome (NS) for Part 3") |
| Participants | Total number randomised: 49 participants (25 to apply the intervention and 24 to apply the comparator) Age: mean 35 years (SD 13.1), range from 18 to 64 Sex: 49% male, 51% female Ethnicity: 80% Caucasian, 10% black, 6% Asian, 4% other Duration of eczema, severity of eczema, body site: not reported Number of withdrawals: 1 Notes: |
| Interventions | Run‐in details: not reported Intervention: BPR277 1.% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: intervention of unknown mode Comparator: 0.% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · AE · TLSS was assessed each week for the treatment areas. |
| Notes | Funding source: Novartis Pharma AG Declarations of interest: not declared Original language of publication: English Other: results of the study are available but not extracted as we did not know the intervention's mode of action. Unclear if the intervention is an anti‐inflammatory |
Guo 2000.
| Methods | Trial design: controlled study Trial registration number, country, outpatient or hospital, date trial conducted, duration of trial participation: not reported Inclusion criteria: patients with eczematous dermatitis, neurodermatitis or AD Exclusion criteria: not reported Additional design details: unable to obtain abstract/paper. Extraction based on title. Unclear if relevant so classed as awaiting classification |
| Participants | Total number randomised, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: laikede cream · Concurrent treatment: not reported · Other key information: unclear mechanism of action of intervention Comparator: mometasone furoate 0.1% cream · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Not reported |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Notes: unclear if the intervention is an anti‐inflammatory |
Gutgesell 1998.
| Methods | Trial design: double‐blind, within‐participant study Trial registration number, country, outpatient or hospital, date trial conducted, duration of trial participation: not reported Inclusion criteria: adults with severe longstanding AD Exclusion criteria: not reported Additional design details: study awaiting classification as unclear if randomised |
| Participants | Total number randomised: number reported but not clear if randomised Age: 22 to 36 years Sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.1% ointment used once or twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone 3.% ointment used once or twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: the area of treatment did not exceed 20% of the whole BSA. Notes: none |
| Outcomes | · Modified SCORAD · Pruritus · AE |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: unclear study design (abstract only) |
Hanifin 2002.
| Methods | Trial design: two‐armed study Trial registration number: not reported Country: US, Canada, Germany, France Outpatient or hospital, date trial conducted, duration of trial participation: not reported Inclusion criteria: paediatric patients Exclusion criteria: not reported Additional design details: study design unclear, so labelled as awaiting classification |
| Participants | Total number randomised: not reported Age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: it did not state clearly if the paediatric patents had AD. |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus cream · Concurrent treatment: not reported · Other key information: none Comparator: 0.% cream · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Application‐site burning |
| Notes | Funding source: Novartis Pharmaceuticals Inc, NJ, US Declarations of interest: not declared Original language of publication: English Other: pooled analysis where original trials could not be identified |
JapicCTI‐205315.
| Methods | Trial design: randomised, single‐blind, single‐centre, within‐participant study Trial registration number: JapicCTI‐205315 Country: Japan Outpatient or hospital: not reported Date trial conducted: June 2020 to August 2020 Duration of trial participation: not reported Inclusion criteria: AD Exclusion criteria: · Malignancy · Serious allergy, allergy to tacrolimus or cutaneous hypersensitivity to topical drugs · Diseases considered inappropriate for study participation, such as serious cardiac, hepatic, renal, pulmonary, or haematologic diseases · Severe renal dysfunction, severe hyperkalemia or associated symptoms · Ulcer or erosion at the application site · Ichthyosis‐like erythroderma (Netherton syndrome, etc.) · Skin infections at the application site · Diseases that affect the evaluation of heat sensation, pain and pruritus (except for atopic dermatitis) Additional design details: No results provided and unclear what the control is |
| Participants | Total number randomised: sides treated not reported (number not reported to apply the intervention and number not reported to apply the comparator). Age: ≥ 20 years Sex: all eligible Ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus hydrate 0.1% ointment used once daily · Concurrent treatment: not reported · Other key information: none Comparator: not reported · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Skin irritation · Pain score · Heat score · Itch score |
| Notes | Funding source: Maruho Co., Ltd. Declarations of interest: not declared Original language of publication: English Other: Unclear comparator |
JPRN‐jRCT2031210012.
| Methods | Trial design: randomised, active‐controlled, single‐blind, parallel study Trial registration number: jRCT2031210012 Country: Japan Outpatient or hospital: not reported Date trial conducted: April 2021 onwards Duration of trial participation: not reported Inclusion criteria: · Diagnosis of AD · Aged ≥ 20 years Exclusion criteria: · Malignancy · Serious allergy, allergy to tacrolimus or cutaneous hypersensitivity to topical drugs · Diseases considered inappropriate for study participation, such as serious cardiac, hepatic, renal, pulmonary, or haematologic diseases · Severe renal dysfunction, severe hyperkalemia or associated symptoms · Ulcer or erosion at the application site · Ichthyosis‐like erythroderma · Skin infections at the application site Additional design details: none |
| Participants | Total number randomised: not reported Age: ≥ 20 years Sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.1% ointment used once daily · Concurrent treatment: not reported · Other key information: M1151C2 appears to be tacrolimus Comparator: unspecified active control · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Not reported |
| Notes | Funding source: Maruho Co., Ltd. Declarations of interest: not declared Original language of publication: English Other: this may be linked to JapicCTI‐205315 |
Koller 2023.
| Methods | Trial design: randomised, double‐blind study Trial registration number, country, outpatient or hospital, date trial conducted, duration of trial participation: not reported Inclusion criteria: people with AD Exclusion criteria: not reported Additional design details: to quote: "first in‐human phase 2 study". Limited details as presented only as a conference abstract. Awaiting classification as unclear mechanism of action |
| Participants | Total number randomised: 41 participants Age: 2 to 66 years; n = 18, < 12 years; n = 10, 12 to 17 years Sex, ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐moderate Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: AB‐ 101A 40% hydrogel · Concurrent treatment: not reported · Other key information: none Comparator: vehicle · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: to quote: "AB‐101a is a novel, first‐in‐class complex drug from a single plant source. AB‐101a's multiple compounds provide multiple mechanisms of actions including anti‐inflammatory, anti‐pruritic, and antibacterial activity, indicating AB‐101a should be effective in AD". |
| Outcomes | · Per cent with 1‐point decrease in IGA score · EASI · BSA · Secondary Infection Rating Scale · Itch |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: awaiting classification as unclear mechanism of action |
Liu 2014a.
| Methods | Trial design: randomised, multicentre study Trial registration number, country, outpatient or hospital, date trial conducted: not reported Duration of trial participation: 6 months Inclusion criteria: paediatric patients with moderate‐to‐severe AD Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 60 participants Age: "paediatric" Sex, ethnicity, duration of eczema: not reported Severity of eczema: moderate‐to‐severe Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.03% ointment twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle for 42 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | EASI |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: awaiting classification as unclear comparator |
NCT00392067.
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: NCT00392067 Country: Canada, Denmark, Finland, UK Outpatient or hospital: at least two hospitals Date trial conducted: October 2006 to July 2007 Duration of trial participation: 3 weeks Inclusion criteria: · Males between 18 and 50 years · AD according to Hanifin and Rajka criteria · Score of erythema, induration/papulation, excoriation and lichenification, 4/5 clinical signs in the Total Severity Score ≥ moderate involvement, i.e. severity ≥ 2 · Treatment area appropriate for topical treatment · Attending outpatient clinic or private practice of a dermatologist Exclusion criteria: · Systemic treatment with immunosuppressive drugs or corticosteroids within 4 weeks of the study · PUVA or UVB therapy within 4 weeks of the study · Topical treatment with immunomodulators or corticosteroids from WHO groups III or IV within 2 weeks · Other topical therapy on the treatment area (except hydrocortisone cream 1% on head and neck lesions, and emollient on the entire body) within 1 week of the study · Use of any other kind of treatment for AD during the study except investigational product on trunk and limbs lesions, hydrocortisone cream 1% on head and neck lesions, and emollient on the entire body · Use of antihistamines · Exfoliative erythrodermia · Clinical infection in the treatment area · Planned exposure to amount of sun or ultraviolet light during the study · Known or suspected hypersensitivity to any component of the investigational product · Known or suspected severe renal insufficiency or severe hepatic disorders · Known, history of, or suspected abnormality of calcium homeostasis associated with clinically significant hypercalcaemia · History of cancer including skin cancer · History of an immunocompromised disease · Treatment with any non‐marketed drug within 4 weeks of the study Additional design details: none |
| Participants | Total number randomised: 74 participants (23 to apply intervention one, 26 intervention two and 25 to apply the comparator) Age: 18 to 50 years Sex: males Ethnicity, duration of eczema: not reported Severity of eczema: at least moderate severity Body site: trunk and limbs Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: to quote: "Prior to randomisation (Visit 1) a washout period was to be completed if the patient was treated, or had recently been treated with atopic dermatitis treatments or other relevant medication, as defined by the exclusion criteria." Intervention: calcipotriol 50 mcg/g and LEO 80122 0.6 mg/g cream used once daily for 21 days · Concurrent treatment: not reported · Other key information: none Intervention: calcipotriol 15 mcg/g and LEO 80122 0.2 mg/g cream used once daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle 0% cream used once daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: use of antihistamines or any other kind of treatment for atopic dermatitis (other than study treatment) was excluded during the study period. Notes: none |
| Outcomes | · IGA · Clinician‐reported signs · EASI · Patient overall assessment · Safety · Patients with controlled disease · Withdrawal due to AE Data points not reported: none of the secondary efficacy response criteria (IGA, EASI, patients' overall assessment of disease severity, patients' assessment of pruritus and patients with controlled disease) demonstrated statistical significance or a tendency to efficacy for either of the active treatments. |
| Notes | Funding source: LEO Pharma Declarations of interest: not declared Original language of publication: English Other: awaiting classification because of unclear mechanism of action of intervention |
NCT00932074.
| Methods | Trial design: randomised, controlled, parallel, triple‐blind, study Trial registration number: NCT00932074 Country: US Outpatient or hospital: not reported Date trial conducted: July 2009 to June 2010 Duration of trial participation: not reported Inclusion criteria: · Male or female aged 18 years to 64 years · AD meeting the Hanifin and Rajka criteria for AD that involves both the head/neck and the torso/limbs, and affects 5 to 30% of the subject's total BSA as determined using the rule of nines · IGSS of 3 (moderate) or 4 (severe) in the selected treatment area on the torso/limbs, and a baseline IGSS of 2 (mild), 3 (moderate) or 4 (severe) in the selected treatment area on the head/neck · Female of childbearing potential has a negative pregnancy test and will be using an effective method of contraception during the study Exclusion criteria: · Female who is pregnant, nursing an infant, or planning a pregnancy during the study period · Oozing/crusting score of 3 (moderate) or greater at the selected treatment area on the head/neck or torso/limbs · A concurrent skin condition that could interfere with assessment of treatment · Treatment with any investigational drug or device within 30 days or 5 half‐lives (whichever is longer) prior to the baseline visit, or concurrent participation in another clinical trial with an investigational drug or device Additional design details: none |
| Participants | Total number randomised: 58 participants Age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: study results not provided |
| Interventions | Run‐in details: not reported Intervention one: KP413 3% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: KP413 ‐ classed as an anti‐inflammatory whose mechanism of action is as an immunomodulator (Adis Insight Springer) Intervention two: KP413 1% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: 0.% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGSS time frame: 4 weeks · Plasma levels of KP‐413 · Signs and symptoms of AD, time frame: 4 weeks · Subject's NRS of Pruritus Score, time frame: 4 weeks · Per cent of AD‐affected BSA, time frame: 4 weeks |
| Notes | Funding source: Kaken Pharmaceutical Dow Pharmaceutical Sciences Declarations of interest: not reported Original language of publication: English Other: unclear if the intervention is an anti‐inflammatory |
NCT01781663.
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: NCT01781663 Country: Spain, Israel Outpatient or hospital, date trial conducted, duration of trial participation: not reported Inclusion criteria: · Male or female between 2 and 12 years of age · Moderate AD (SCORAD < 40) that is amenable to treatment · AD meets Hanifin's criteria (at least 3 basic features and at least 3 minor features) · AD is, in the opinion of the investigator, stable for the past 7 days · Subject's parents are able to apply the study product twice a day for a consecutive period of 42 days · Subject's parents agree that the subject will not change his/her lifestyle during the study period (including: regular body hygiene product (soap), the number of baths and showers per day, the laundry detergent and fabric softener used to wash clothes) · Subject's parents agree to use only the test product during the study period. Exclusion criteria: · Another dermatological disease/condition that could interfere with the clinical evaluation, including infected AD lesions · Previous history of allergy to cosmetic products or to any of the ingredients included in the tested formulations · Received a topical or a systemic immune‐modulator for the treatment of AD within 14 days · Underwent phototherapy within 28 days · Expected to be extensively exposed to the sun during the trial · Underwent any experimental treatment within 14 days Additional design details: none |
| Participants | Total number randomised: not reported Age: between 2 and 12 years Sex: male and female Ethnicity, duration of eczema: not reported Severity of eczema: moderate Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: KAM2904 face cream, KAM3008 body lotion · Concurrent treatment: not reported · Other key information: none Comparator: petrolatum‐based moisturiser · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: awaiting classification as unclear if intervention is eligible |
| Outcomes | · Change in SCORAD · Change in EASI · Change in the scoring of individual symptoms of atopic dermatitis · Trend in the change of SCORAD ·Trend in the change of EASI · Trend in the change of individual symptoms of atopic dermatitis · Safety will be measured by the number and severity of device‐related AEs. |
| Notes | Funding source: Kamedis Ltd Declarations of interest: not declared Original language of publication: English Other: unclear if the intervention is an anti‐inflammatory |
NCT03089229.
| Methods | Trial design: randomised, double‐blind, study Trial registration number: NCT03089229 Country: India Outpatient or hospital: presumed outpatient Date trial conducted: March to July 2017 Duration of trial participation: 12 weeks Inclusion criteria: · Moderate‐to‐severe AD as determined by PGA ≥ 3 and SCORAD > 25 · Male or female, aged 12 to 65 years Exclusion criteria: · Currently participating or has participated in another interventional clinical study at this or any other facility in the past 3 months · Currently or has been diagnosed or treated for cancer in the past 5 years · Requires any topical or systemic medications that could affect the course of their AD during the study period (except inhaled steroids and/or stable antihistamines for asthma or allergies) · Hypersensitivity to any corticosteroid creams · Active infections or has used antibiotics in the past 7 days · Physical attributes or skin conditions that might interfere with clear visual assessments · Immunologic or infectious disease which could place the subject at risk or interfere with the accuracy of the study results. · Immunosuppressant drugs or immunotherapy within the past 30 days or 5 half‐lives · Dependent on oral medication for any skin disease/condition or could not, in the opinion of the investigator, tolerate the restriction of discontinuing the medicine as required in this study · Currently pregnant or lactating or planning to become pregnant in the next 6 months ·Any other condition or factor the investigator or their duly assigned representative believes may affect the ability of the subject to complete the study or the interpretation of the results Additional design details: none |
| Participants | Total number randomised: 30 participants Age: 12 to 65 years Sex: male and female Ethnicity, duration of eczema: not reported Severity of eczema: moderate‐to‐severe Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: 1 week wash‐in period prior to treatment Intervention: HAT01H cream used twice daily for 91 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle 0.% cream used twice daily for 91 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Change in SCORAD score · Proportion of patients achieving a PGA score of 0 or 1 · Incidence of treatment‐emergent AEs |
| Notes | Funding source: Haus Bioceuticals Declarations of interest: industry‐funded Original language of publication: English Other: unclear if the intervention is an anti‐inflammatory |
NCT03134352.
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: NCT03134352 Country: China Outpatient or hospital: presumed outpatient Date trial conducted: April 2017 to September 2018 Duration of trial participation: 21 days Inclusion criteria: · Male or female between 18 and 65 years of age · Diagnosis of subacute eczema (Zhao 2001 ‐ authors do not provide complete reference) · BSA disease involvement between 3 to 10% as assessed by palm method · IGA score of 2 or 3 · Skin lesions should be on the trunk or extremities, without palms/soles, face/scalp, and vulvar areas involved. · Based on Tradional Chinese Medicine theory (Zheng 2002 ‐ authors do not provide complete reference), patients with TCM "damp‐heat" symptoms to be included. Main symptoms: erythema, pruritus, papules with less exudation; secondary symptoms: mild infiltration, excoriations, crusting, papulovesicle, blister, irritability, thirst, yellowish urine and dry stool; tongue and pulse analysis: red tongue with yellow or yellowish coating · Generally in good health Exclusion criteria: · History of any systemic disorders or active skin diseases (e.g. psoriasis) that would in any way confound interpretation of the study results · Current complication of overt bacterial, fungal or viral infection for which treatment with anti‐infectives is indicated · History of hepatic and kidney function insufficiency · QT interval corrected according to Bazett's formula or QT interval corrected according to Fridericia's formula ≥ 450 msec; or QTc ≥ 480 msec in subjects with bundle branch block · History or examination verified by physical and screening of clinically significant cardiovascular, pulmonary, gastrointestinal, liver, renal, haematological, neurological, abnormalities and psychological disorders which will interfere with the efficacy and/or safety of the individual subject · History of allergy to any component of test medications to be used in the study · Exposed to therapy within the set time frame: ‐ systemic administration of antihistamine agents for 1 week or corticosteroids for 4 weeks or immunosuppressive drugs for 4 weeks or any TCM drugs for 2 weeks ‐ topical corticosteroid agents administered in the diseased skin for 1 week or immunosuppressive drugs administered in the diseased skin for 1 week or any TCM drugs for 1 week ‐ UV therapy for 4 weeks · History of alcohol or drug abuse · Pregnant women, women who are breastfeeding, or sexually active women of childbearing potential who are not practising an acceptable method of birth control, as determined by the investigator. An acceptable method of birth control must be used during the entire study by sexually active women of childbearing potential. · Has received an investigational drug or participated in any other research trial within 30 days · EASI score reached 2 and above for lichenification and/or oedema Additional design details: none |
| Participants | Total number randomised: 290 participants Age: 18 to 65 years Sex: male and female Ethnicity, duration of eczema: not reported Severity of eczema: BSA disease involvement between 3 to 10% (inclusive) as assessed by palm method; IGA score of 2 or 3 Body site: trunk and extremities Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: 1 week Intervention one: ZL‐3101 5% ointment used once daily · Concurrent treatment: not reported · Other key information: none Intervention two: ZL‐3101 5% ointment used twice daily · Concurrent treatment: not reported · Other key information: none Comparator: placebo ointment used twice daily · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: use of inhaled/intranasal steroids is permitted prior to and during the conduct of the study if already being used by the subject. Notes: none |
| Outcomes | EASI score changes from baseline to day 21 |
| Notes | Funding source: Zai Lab Pty. Ltd. Declarations of interest: industry‐funded Original language of publication: English Other: unclear if the intervention is an anti‐inflammatory |
NCT04753034.
| Methods | Trial design: randomised, double‐blind study Trial registration number: NCT04753034 Country: USA Outpatient or hospital: presumed outpatient Date trial conducted: January to May 2021 Duration of trial participation: 28 days Inclusion criteria: · Adolescent or adult subjects aged 12 to 65 years · Overall IGA score of 2 (mild) or 3 (moderate) at baseline on a 5‐point IGA Exclusion criteria: AD with known hypersensitivity to excipients of TER‐101 ointment or immunocompromised Additional design details: none |
| Participants | Total number randomised: 63 participants (31 to apply the intervention and 32 to apply the comparator) Age: 12 to 65 years Sex: male n = 18, female n = 45 Ethnicity: white n = 36, Asian n = 7, black n = 17, other n = 1, more than one race n = 2 Duration of eczema: not reported Severity of eczema: mild‐to‐moderate, overall IGA score of 2 or 3 at baseline on a 5‐point scale Body site: treatment areas will exclude the scalp, nose, mouth and eyes Number of withdrawals: vehicle group n = 1, TER‐101 group n = 2 Notes: none |
| Interventions | Run‐in details: not reported Intervention: TER‐101 2.75% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Per cent change in EASI · Changes in EASI · Change in IGA · Changes in itch · Tolerability |
| Notes | Funding source: Teres Bio, Inc. Declarations of interest: industry‐funded Original language of publication: English Other: unclear if the intervention is an anti‐inflammatory |
Ruzicka 2002b.
| Methods | Trial design: double‐blind study Trial registration number, country, outpatient or hospital, date trial conducted, duration of trial participation: not reported Inclusion criteria: aged 2 to 15 years with moderate‐to‐severe AD Exclusion criteria: not reported Additional design details: none |
| Participants | Participants aged 2 to 15 years with moderate‐to‐severe AD Total number randomised: not reported Age: 12 to 15 years Sex, ethnicity, duration of eczema: not reported Severity of eczema: moderate‐to‐severe AD Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.03% ointment for three weeks · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone acetate ointment for three weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Tacrolimus blood concentration |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: unclear study design (abstract only) |
Szybejko‐Machaj 2002.
| Methods | Trial design: clinical study Trial registration number, country, outpatient or hospital, date trial conducted, duration of trial participation: not reported Inclusion criteria, exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 30 participated Age: 6 to 14 years Sex, ethnicity, duration of eczema, severity of eczema: moderate‐to‐severe AD, body site, number of withdrawals: not reported Notes: details in abstract were limited, so unable to assess eligibility |
| Interventions | Run‐in details: not reported Intervention: pentoxifylline · Concurrent treatment: not reported · Other key information: none Comparator: without pentoxifylline · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: details were limited, so unable to assess eligibility |
| Outcomes | SCORAD |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: unclear study design (abstract only) |
Wang 2014.
| Methods | Trial design: randomised controlled trial Trial registration number: not reported Country: China Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 4 weeks Inclusion criteria: · 18 to 65 years · Diagnostic criteria for chronic eczema · BSA < 10% Exclusion criteria: · Systemic anti‐eczema treatment within 4 weeks · Disease affected the folds of the face, vulva, armpits, groin, "etc." · Allergic to a certain ingredient or similar ingredients of the preparation · Pregnant and breastfeeding women · Parathyroid disease, abnormal calcium metabolism or hypercalcaemia, Cushing's syndrome, diabetes, severe heart, liver, kidney disease, immunodeficiency, malignant tumour, eczematous tumours or mentally ill Additional design details: none |
| Participants | Total number randomised: 100 participants (50 to apply the intervention and 50 to apply the comparator) Age: intervention group, average 38.32 years, range 18 to 63; comparator group, average 38.04 years, range 18 to 65 Sex: intervention group, female n = 24 and male n = 24; comparator group, female n = 26 and male n = 21 Ethnicity: not reported Duration of eczema: intervention group, average 2.94 years, range 6 months to 11 years; comparator group, average 2.96 years, range 6 months to 12 years Severity of eczema, body site: not reported Number of withdrawals: intervention group n = 2; comparator group n = 3 withdrew after randomisation but before treatment Notes: none |
| Interventions | Run‐in details: not reported Intervention: betamethasone (presented as (trade name Defubao, 15 g/stick, containing 504 mg/g calcipotriol and betamethasone 0.5 mg/g) ointment used once daily for 28 days · Concurrent treatment: calcipotriol · Other key information: information on concentration not reported Comparator: halometasone 0.05% ointment used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI · Overall improvement · AEs |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: Chinese Other: paper in Chinese, with English abstract. Google translation app was used to translate the main text. Insufficient information to classify the potency of the intervention |
AD: atopic dermatitis AE: adverse events ALT: analine transaminase AST: aspartate transaminase AUC: area under the curve BSA: body surface area cr: controlled release DLQI: Dermatology Life Quality Index EASI: Ezema Area and Severity Index IGA: Investigators Global Assessment IGSS: Investigators Global Severity Score NRS: numerical rating scale PDE‐4: phosphodiesterase‐4 inhibitors PGA: Patient Global Assessment PUV: psoralen ultraviolet therapy QT(c): corrected QT interval SCORAD: SCORing Atopic Dermatitis scale SASSAD: Six areas, six signs of atopic dermatitis TCM: traditional Chinese medicine TLSS: Target Lesions Symptom Score TSS: Total Sign Score UV: ultraviolet UVA: ultraviolet A therapy VAS: Visual Analogue Scale
Note: the number randomised per intervention group, and concentration, frequency and duration of interventions are noted only if information was provided in the study.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12616000294459.
| Study name | A phase I, safety, tolerability and efficacy study of topical AKP‐11 administration to participants with atopic dermatitis |
| Methods | Trial design: randomised study Trial registration number: ACTRN12616000294459/U1111‐1180‐2112 Country: Australia Outpatient or hospital: not reported Date trial conducted: March 2016 to January 2017 Duration of trial participation: 35 days Inclusion criteria: · Male or female aged 18 to 65 years · AD according to the criteria of Hanifin and Rajka · Mild‐to‐moderate AD and equal to 10% of BSA · L‐EASI score of equal to 1 for each of the signs at an appropriate target site · BMI between 18.0 and 40.0 kg/m2 · Female participants of childbearing potential with negative pregnancy test at screening and negative urine pregnancy test at day 1, AND; ‐ Agrees to abstinence ‐ OR agrees to use condoms plus one other acceptable form of contraception ‐ OR has only same‐sex partners, when this is her preferred and usual lifestyle ‐ OR has a vasectomised partner, which should be the sole partner for that participant · Male participants with female partners of childbearing potential must agree to abstinence or to use condoms plus partner use of an acceptable contraceptive · Negative test for Human Immunodeficiency Virus (HIV), Hepatitis B and C · Negative drug screening test (drugs of abuse; creatinine control, testing for amphetamines, barbiturates, cocaine, opiates, cannabinoids and benzodiazepines) result (urine test) Exclusion criteria: · Skin condition other than AD · History of allergy and/ or hypersensitivity to any of the stated ingredients of the formulations · Smoked more than 10 cigarettes a day in the last 12 months · Treatment with any of the following within 4 weeks: systemic retinoids; immunosuppressant agents; phototherapy or photochemotherapy; high‐potency topical corticosteroids; “alternative medicine” treatments; or sun exposure or tanning bed use, or any other therapy that, in the opinion of the investigator, could modify disease activity · Topical treatment within 2 weeks prior to commencement of study treatment and for the duration of the study, including: moderate‐potency topical corticosteroids; topical retinoids; or keratolytics, coal tar and dithranol or any other topical that, in the opinion of the investigator, could modify disease activity · Received any investigational research agent or therapeutic biologic within 30 days or 5 half‐lives · Received an investigational vaccine within 6 months, a live attenuated vaccine within 60 days or a registered vaccine within 30 days · Drug or alcohol abuse within 6 months · Clinical signs of active infection and/or high temperature · Anticipate surgery within the trial period or history of major surgery within 3 months · A depot injection or an implant of any drug within 3 months, except for a contraceptive implant · Evidence of current or previous clinically significant neurological, endocrinal, cardiovascular, pulmonary, haematological, malignant, immunologic, psychiatric, metabolic or other uncontrolled systemic disease, or finding of the medical examination, including any other condition Additional design details: study completed but results not available |
| Participants | Total number randomised, age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: 30 mg of AKP‐11 used twice daily for up to 4 weeks or one week post‐complete clearance · Concurrent treatment: not reported · Other key information: twice daily as needed Comparator: placebo ointment used twice daily for up to 4 weeks or one week post‐complete clearance · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: AKP‐11 is a highly selective S1PR1 agonist. |
| Outcomes | · Safety · Change in: EASI/L‐EASI using 6 or 4‐point scale; SCORAD, pruritus score using VAS scale |
| Starting date | March 2016 |
| Contact information | Skin & Cancer Foundation
Level 1, 80 Drummond Street
Carlton VIC 3053 pfoley@skincancer.asn.au |
| Notes | Funding source: Akaal Pharma PTY Ltd. Declarations of interest: commercial sector/industry Original language of publication: English Other: none |
ACTRN12617000763347.
| Study name | A phase II, safety, tolerability and efficacy study of topical AKP‐11 administration to participants with atopic dermatitis |
| Methods | Trial design: randomised study Trial registration number: ACTRN12617000763347/CT‐2017‐CTN‐01676‐1 v1 Country: Australia Outpatient or hospital: not reported Date trial conducted: July to November 2017 Duration of trial participation: days Inclusion criteria: · Male or female aged 18 to 65 years · AD according to the American Academy of Dermatology and stable for at least two weeks · Mild‐to‐severe AD with less than or equal to 20% of BSA; other than hair bearing scalp, palms of hands, soles and feet and genitals · Baseline Investigator’s Static Global Assessment (ISGA) score of mild (2) to severe (4) · BMI between 18 and 45.0 kg/m2 · Female of non‐childbearing potential · Female of childbearing potential with negative urine pregnancy test at screening and negative urine pregnancy test at day 1, AND; ‐ Agree to abstinence for the duration of the study if this is in line with the usual and preferred lifestyle; OR agree to use condoms plus one other acceptable form of contraception; i.e. intra‐uterine device, hormonal contraception or a female diaphragm, from screening until 4 weeks after dosing with study drug; OR has only same‐sex partners; OR has a vasectomised partner, which should be the sole partner for that participant · Male participants with female partners of childbearing potential must agree to abstinence if this is in line with the usual lifestyle, or to use condoms plus partner use of an acceptable contraceptive for the duration of the study · Negative test results for Human Immunodeficiency Virus (HIV), hepatitis B and hepatitis C at the time of screening · Negative drug screening test (drugs of abuse; creatinine control, testing for amphetamines, barbiturates, cocaine, opiates, cannabinoids and benzodiazepines) result (urine test) · A 12‐lead ECG at screening that, in the opinion of the investigator, has no abnormalities that compromise the subject’s safety in this study Exclusion criteria: · A skin condition other than AD · Treatment with any of the following within 4 weeks prior and for the duration of the study: systemic retinoids; systemic immunosuppressant agents; phototherapy or photochemotherapy; “alternative medicine” treatments; or deliberate abnormal sun exposure or tanning bed use, or any other therapy that in the opinion of the investigator could modify disease activity · Topical treatment within 2 weeks and for the duration of the study to the area to be treated with the study ointment · Received any investigational research agent or therapeutic biologic within 30 days or 5 half‐lives (whichever is longer) prior to the first dose of investigational product · Received an investigational vaccine within 6 months prior to baseline, or a live attenuated vaccine within 60 days prior to baseline, or intend to have a live vaccination during the course of the study (killed/inactive vaccines are allowed) · Clinical signs of active infection and/or a temperature of above 38.0 degrees at the time of screening. Study entry may be deferred at the discretion of the Principal Investigator. · Anticipate surgery within the trial period or history of major surgery within 3 months of screening · A depot injection or an implant of any drug within 3 months, except for a contraceptive implant · Evidence of current or previous clinically significant neurological, endocrinal, cardiovascular, pulmonary, haematological, malignant, immunologic, psychiatric, metabolic or other uncontrolled systemic disease, or finding of the medical examination, including any other condition that, in the opinion of the investigator, would compromise the safety of the participant or interfere with assessment of endpoints or unsuitable for enrolment or impact on the quality of the data Additional design details: study terminated after recruiting 7 participants, to quote: "upon a request of a licensee, who will conduct phase II trials". The "data collected is being analysed" and a report was to be prepared. |
| Participants | Total number randomised: 7 Age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: AKP‐11 30 mg ointment used twice daily for up to 28 days · Concurrent treatment: not reported · Other key information: none Comparator: placebo ointment twice daily for up to 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: AKP‐11 is a highly selective S1PR1 agonist. The allocated treatment area was equal or less than 3% of treatable BSA. During each application, the entire content of the study ointment will be applied thinly on the allocated AD area/s (~1.5 mg/ cm2). After the study ointment has been applied in a uniform layer, the area will be thoroughly rubbed for approximately one minute. |
| Outcomes | Time to improvement and success in ISGA as two grades or greater improvement from baseline Safety endpoints: AE, vital signs, physical examination |
| Starting date | 2017 |
| Contact information | Richard.Walsh@sa.gov.au/gurmit.gill@latrobe.edu.au. Akaal Pharma Pty Ltd. Chemistry Department Thomas Cherry Building # 301E La Trobe University Bundoora, VIC ‐ 3083 |
| Notes | Funding source: Akaal Pharma PTY Ltd. Declarations of interest: commercial sector/industry Original language of publication: English Other: none |
Bos 2000.
| Study name | Long‐term safety and efficacy of SDZ ASM 981 cream in adult patients with atopic dermatitis |
| Methods | Trial design: randomised, double‐blind, single centre study Trial registration number: not reported Country: Switzerland, eight other European countries and Canada Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 1 year Inclusion criteria: AD according to Hanifin and Rajka criteria Exclusion criteria: not reported Additional design details: to quote: "SDZ ASM 981", which is 1% pimecrolimus cream |
| Participants | Total number randomised: 658 Age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1% cream used twice daily as needed · Concurrent treatment: not reported · Other key information: not reported Comparator: triamcinolone acetonide 0.1% cream for trunk and extremities. Hydrocortisone 1% cream for face and neck · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA, EASI, local AE |
| Starting date | Not reported |
| Contact information | Not reported |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: conference abstract of protocol, no results reported |
ChiCTR‐IPR‐17013807.
| Study name | Efficacy and safety of calcineurin inhibitors compared with that of glucocorticoid for the treatment of atopic dermatitis |
| Methods | Trial design: randomised parallel study Trial registration number: ChiCTR‐IPR‐17013807 Country: China Outpatient or hospital: outpatients Date trial conducted, duration of trial participation: not reported Inclusion criteria: outpatients with AD aged 14 to 80 years Exclusion criteria: · Serious uncontrolled chronic liver, kidney, heart, blood system, diabetes and lung disease · History of malignant tumour · Autoimmune disease or immunodeficiency disease · Women who are pregnant, breastfeeding or using inappropriate contraception measures · Involved with other study drug Additional design details: none |
| Participants | Total number randomised: not reported Age: 14 to 80 years Sex: both Ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: eloson cream · Concurrent treatment: not reported · Other key information: none Comparator: tacrolimus 0.1% ointment · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI · BSA · Self evaluation of pruritus |
| Starting date | Not reported |
| Contact information | Not reported |
| Notes | Funding source: Shanghai Sixth People's Hospital Declarations of interest: not declared Original language of publication: Chinese Other: none |
ChiCTR‐TRC‐09000738.
| Study name | Clinical trial of tacrolimus as a therapeutic agent for atopic dermatitis in Chinese children: objective assessment of clinical efficacy in itch reduction and immunomodulatory effects |
| Methods | Trial design: randomised, parallel study Trial registration number: ChiCTR‐TRC‐09000738 Country: Hong Kong Outpatient or hospital: not reported Date trial conducted: from December 2005 Duration of trial participation: 2 to 4 weeks Inclusion criteria: · Eczema · Aged 2 to 15 years Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 100 (target) participants (50 (target) to apply the intervention and 50 (target) to apply the comparator) Age: 2 to 15 years Sex: both Ethnicity, duration of eczema, severity of eczema, body site: number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus for 14 to 28 days · Concurrent treatment: not reported · Other key information: none Comparator: placebo · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | SCORAD |
| Starting date | 2005 |
| Contact information | Kam Iun Hon, phone 852 26322859 |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
ChiCTR‐TRC‐12002479.
| Study name | 0.03% tacrolimus ointment long‐term intermittent maintain treatment of children with moderate to severe atopic dermatitis multicenter, open, randomized, controlled study |
| Methods | Trial design: randomised, multicentre, parallel study Trial registration number: ChiCTR‐TRC‐12002479 Country: China Outpatient or hospital: not reported Date trial conducted: 2012 to not reported Duration of trial participation: 6 months Inclusion criteria: First stage: · Moderate‐to‐severe AD according to Williams and Rajka and Langeland criteria · Aged 2‐15 years old, male or female · When AD recurred, to visit the hospital within 3 days Second stage: · Completed the first phase · IGA ≤ 2 after the first stage treatment Exclusion criteria: · Known tacrolimus ointment allergy patients · AD skin lesion area has serious skin infections · Serious liver and kidney disease, blood system disease, autoimmune disease, chronic severe infections, diabetes, mental illness, taking drugs, alcoholic · Malignant tumour or other issue that may affect the correct evaluation of the curative effect of serious illnesses · Local use of glucocorticoid hormone or other immunosuppressive drugs withdrawal time less than 1 week · Systemic application of corticosteroids and other immunosuppressive drug withdrawal time less than 4 weeks Additional design details: details were not clear on the two‐stage phasing of the trial; in particular, whether participants remain on same treatment schedule and the timing of the two phases. |
| Participants | Total number randomised: not reported Age: 2 to 15 years Sex: both Ethnicity, duration of eczema, severity of eczema, body site: number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.03% ointment twice weekly for 182 days · Concurrent treatment: not reported · Other key information: none Comparator: 'conventional recurrence treatment' · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · First recurrence · The numbers with second stage recurrence · The severity of the disease of the second stage recurrence · The time of duration of the second stage recurrence · Pruritus score duration of the second stage recurrence · Total tacrolimus use amount of the second stage |
| Starting date | 2012 |
| Contact information | Not reported |
| Notes | Funding source: Astellas Pharma China Inc. Declarations of interest: not declared Original language of publication: English Other: none |
ChiCTR‐TRC‐12002591.
| Study name | Optimization of light, moderate to severe atopic dermatitis treatment strategies of the stabilization period: multicenter, open, randomized controlled study |
| Methods | Trial design: randomised, parallel study Trial registration number: ChiCTR‐TRC‐12002591 Country: China Outpatient or hospital: not reported Date trial conducted: from September 2012 Duration of trial participation: not reported Inclusion criteria: · Mild‐to‐moderate AD according to Williams criteria · Males and females aged 2 to 70 years · Referred to the hospital within 2 days of flare Exclusion criteria: · Known allergy to the study drug or its ingredients · Serious infections of the skin in the areas affected by AD · Severe liver and kidney diseases, blood diseases, autoimmune diseases, chronic severe infections, diabetes, mental illness, drug and alcohol abuse · Malignancy or other serious diseases that may affect evaluations · Topical corticosteroids or other immunosuppressant within a week of the trial · Systemic corticosteroids and other immunosuppressive agents within two weeks of the trial · Participation in another trial within a month of the present trial Additional design details: none |
| Participants | Total number randomised: not reported Age: 2 to 70 years Sex: all eligible Ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐moderate Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus ointment · Concurrent treatment: not reported · Other key information: none Comparator: fluticasone propionate cream · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Unclear |
| Starting date | Not reported |
| Contact information | Yun‐fei Cai, cyf_epi@163.com |
| Notes | Funding source: to quote: "Health industry funding for research and special projects" Declarations of interest: not declared Original language of publication: English Other: none |
ChiCTR2100054661.
| Study name | Comparative study on the efficacy and safety of 0.03% tacrolimus ointment and 2% criborol ointment in the treatment of moderate atopic dermatitis in children |
| Methods | Trial design: randomised, parallel study Trial registration number: ChiCTR2100054661 Country: China Outpatient or hospital, date trial conducted: not reported Duration of trial participation: not reported Inclusion criteria: · Aged 2 to 12 years; gender is not limited · Moderate AD according to the Williams criteria Exclusion criteria: · Allergic to 0.03% tacrolimus ointment or 2% criborole ointment or have contraindications · Local rash combined with viral, bacterial or fungal infection · Severe liver and kidney disease, blood system disease, autoimmune disease, chronic severe infection, diabetes, mental illness or malignant tumour and other serious diseases that may affect the correct evaluation of curative effect · Withdrawal time of topical corticosteroids is less than 4 weeks and/or withdrawal time of systemic corticosteroids and other immunosuppressants is less than 12 weeks · Other reasons considered inappropriate by the researcher, such as mental illness, communication disorder Additional design details: none |
| Participants | Total number randomised, age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: 0.03% tacrolimus ointment · Concurrent treatment: not reported · Other key information: none Comparator: 2% criborol ointment · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Local EASI · Local Atopic Dermatitis Severity Score · Local Investigator Overall Score · Local VAS pruritus score |
| Starting date | 31/12/21 |
| Contact information | He Chun hechuncd@163.com |
| Notes | Funding source: Chinese Association of Rehabilitation Medicine Declarations of interest: not declared Original language of publication: English Other: none |
ChiCTR2200059419.
| Study name | A randomized, double‐blind, multicenter, placebo‐controlled phase III clinical study to evaluate the efficacy and safety of benvitimod cream in the treatment of mild to moderate atopic dermatitis |
| Methods | Trial design: randomised, double‐blind study Trial registration number: ChiCTR2200059419 Country: China Outpatient or hospital: not reported Date trial conducted: May 2022 Duration of trial participation: 8 weeks Inclusion criteria: · 18 years old or older, any gender · AD of more than 6 months and according to the criteria of the 2020 edition of Chinese Guidelines for the Diagnosis and Treatment of AD · EASI ≤ 21, percentage of BSA ≤ 20% · Overall score IGA ≥ 3 Exclusion criteria: · Skin lesions limited to the head, neck, hands and feet · Liver function tests not within normal parameters · Cardiovascular, respiratory, gastrointestinal, liver, kidney, blood, nervous or psychological diseases, and the condition is unstable or not well controlled · Any systemic diseases or other active skin diseases or markings that may affect the evaluation of test results · Malignant tumour · Severe concomitant diseases, may need to be given systemic hormone therapy or other interventions, which affect research participation or require frequent active monitoring · Skin local bacterial, viral and fungal infections · Mental illness, or other reasons that may interfere with participating in the experiment · Known to be allergic to any component of the test drug · Severe hypersensitivity to food, drugs, insect venom or rubber · Women who are pregnant, lactating, or planning to become pregnant · Alcoholism, drug abuse and known drug dependence · Therapies used before enrolment ‐ topical drug therapy within 2 weeks ‐ Systemic immunotherapy within 4 weeks ‐ Biological agents for AD within 4 weeks (or 5 half‐lives, whichever is longer) ‐ Ultraviolet therapy and photochemotherapy within 4 weeks ‐ Participation in clinical trials of other drugs or medical devices within 4 weeks Additional design details: none |
| Participants | Total number randomised, age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: benvitimod cream · Concurrent treatment: not reported · Other key information: none Comparator: placebo · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Proportion (of participants) with IGA of 0 or 1 and a decrease of ≥ 2 points score at week 8 · Proportion with recurrence and the time of first recurrence of 1.0% benvitimod cream · Proportion of participants with recurrence who achieved IGA = 0 or 1 · Incidence of TEAE and SAE · Percentage decline in EASI · Proportion of participants with ≥ 50%/75%/ 90% improvement in EASI score · Proportion of participants with ≥ 3 score improvement in pruritus (VAS) · Overall EASI improvement rate and its changes · Average IGA decrease · Average DLQI decrease · Average BSA decrease and changes with time · Average pruritus VAS decrease and changes with time · Number of recurrences in recurrence participants · Proportion of participants with ≥ 50% improvement in EASI score after retreatment 8 weeks in recurrence participants · Incidence of AE/ADR at each visit during medication · Incidence of clinical abnormalities |
| Starting date | May 2022 |
| Contact information | Shen Juan, shenjuan@tianjipharma.com.cn or Xue Jiajun, xuejiajun@tianjipharma.com.cn; both affiliated with Guangdong Zhonghao Pharmaceutical Co., Ltd. |
| Notes | Funding source: to quote: "primary sponsor Peking University People's Hospital" Declarations of interest: not declared, but contacts are affiliated with Guangdong Zhonghao Pharmaceutical Co., Ltd. Original language of publication: Chinese Other: none |
ChiCTR2200060584.
| Study name | A randomized controlled study comparing the efficacy and safety of 2% crisaborole ointment versus 1% pimemolimus cream in the treatment of mild to moderate atopic dermatitis in children aged 2 years and older |
| Methods | Trial design: randomised, parallel study Trial registration number: ChiCTR2200060584 Country: China Outpatient or hospital, date trial conducted: not reported Duration of trial participation: not reported Inclusion criteria: · Male or female aged 2 to 17 years · AD according to the criteria of Hanifin and Rajka · ISGA score of mild or moderate (excluding scalp) with a BSA affected greater than 5% Exclusion criteria: · Active or potentially relapsing non‐AD skin disease and skin disease genetic overlap with AD or suffering with severe systemic disease or disease with an injurious immune system · AD of unstable clinical condition or always requires high‐potency or ultra‐high topical glucocorticoids to control disease signs and symptoms · History of angioedema or hypersensitivity reactions · Significant active systemic or local infection · Received prohibited drugs/treatments that may change the course of AD and has not performed the required minimum elution or is expected to use prohibited drugs/treatments · The time of surgery or medical procedure of patients overlaps with the period of the clinical study. · History of cancer or received any types of cancer treatment within the last 5 years (except for squamous cell carcinoma, basal cell carcinoma or carcinoma in situ of the skin that clinical cured by cryosurgery or surgical resection) · Known allergies to any component of the study drugs · Suffering from other acute or chronic medical or psychiatric diseases · Pregnant or lactating female individuals; the female individuals that have reproductive potential but are unwilling or unable to use an appropriate contraceptive method specified in this study protocol throughout the study period and at least 28 days after the last use of the study drug Additional design details: none |
| Participants | Total number randomised, age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: 2% crisaborole ointment used twice a day · Concurrent treatment: not reported · Other key information: none Comparator: 1% pimemolimus cream used twice a day · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Percentage of patients with ISGA success/improvement · Percentage change in EASI · Change from baseline in NRS scores · Change BSA affected · Local ISGA ‐ facial success over time · Patient‐reported itch severity scale scores for participants 6 years and older and younger than 12 years · Observer‐reported changes in itch severity scale scores over time from baseline for participants younger than 6 years · DLQI/CDLQI/IDQoL · The Dermatitis Family Impact Questionnaire, DFI |
| Starting date | 2022 |
| Contact information | Ping Li liping20081110@126.com |
| Notes | Funding source: Chinese National Health Association Declarations of interest: not declared Original language of publication: Chinese Other: none |
CTRI/2012/05/002636.
| Study name | A randomized, double‐blind, placebo‐controlled, three‐arm, parallel assignment, multi‐centre, therapeutic equivalence study of two tacrolimus 0.1% topical ointment formulations in adult patients with moderate to severe atopic dermatitis |
| Methods | Trial design: randomised, double‐blinded, parallel study Trial registration number: CTRI/2012/05/002636 Country: India, Poland Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 6 weeks Inclusion criteria: · Male or non‐pregnant, non‐lactating female of any ethnic group, 18 to 70 years of age · AD according to Hanifin and Rajka criteria · Moderate‐to‐severe AD according to Rajka and Langeland criteria · Non‐immunocompromised adults who have failed to respond adequately to other topical prescription treatments for AD, or when those treatments are not advisable in the opinion of the Investigator · Last application of medicated topical agents, intake of systemic antihistamines, intranasal or inhaled corticosteroids (> 1 mg/day) for more than 7 days · Last application of tacrolimus ointment, intake of systemic corticosteroids and nonsteroidal immunosuppressants minimum 3 weeks prior to randomisation Exclusion criteria: · Newly diagnosed or treated AD · Mild or very severe AD that requires systemic therapy · Undergoing UV treatments for AD · Clinically infected AD at the baseline visit Additional design details: none |
| Participants | Total number randomised: not reported Age: 18 to 70 years Sex: both Ethnicity, duration of eczema: not reported Severity of eczema: moderate‐to‐severe Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus monohydrate 0.1% ointment used twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Intervention: protopic (tacrolimus) 0.1% ointment used twice daily for 41 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for 41 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Change from baseline in EASI score to day 56 · Percentage of patients with 60% improvement in EASI |
| Starting date | Not reported |
| Contact information | Project manager, Sheejith K. sheejith@lambda‐cro.com |
| Notes | Funding source: Accord Healthcare Ltd. Declarations of interest: not declared Original language of publication: English Other: recruiting |
CTRI/2018/11/016407.
| Study name | A randomized, double‐blind, placebo‐controlled, three arms, parallel‐group, multiple‐site bioequivalence study with clinical endpoints |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: CTRI/2018/11/016407 Country: India Outpatient or hospital: multiple hospitals or research centres Date trial conducted: from November 2018 Duration of trial participation: 15 days plus an additional 7 days follow‐up after the last application of study treatment (assumed) Inclusion criteria: · Non‐immunocompromised male or female, ≥ 18 years · Confirmed diagnosis of AD for ≥ 3 months · Clinical diagnosis of moderate‐to‐severe AD that has not responded adequately to other topical prescription treatments, or for whom those treatments are not advisable by the investigator · IGA at least moderate (3 or 4) and SCORAD > 15 · ≥ 20% BSA at baseline · Subjects of childbearing potential must use adequate contraception throughout and female subjects must not be/likely to be pregnant or lactating, with a negative serum pregnancy test at screening and negative urine pregnancy test at randomisation. Exclusion criteria: · Active cutaneous bacterial or viral infection in any treatment area at baseline · Sunburn, extensive scarring, or pigmented lesion(s) in any treatment area at baseline, if likely to interfere with evaluations · History of confounding skin conditions · Pregnancy and breastfeeding · History or presence of Netherton’s Syndrome, immunological deficiencies or diseases, HIV, diabetes, malignancy, serious active recurrent infection, clinically significant severe renal insufficiency or severe hepatic disorders · Use within one month of oral or intravenous corticosteroids, phototherapy, PUVA, tanning booths, non‐prescription UV light sources, immunomodulators or immunosuppressive therapies, interferon, cytotoxic drugs, tacrolimus, or pimecrolimus · Use within 14 days of systemic antibiotics, calcipotriene or other vitamin D preparations, or retinoids · Use within 7 days prior to antihistamines, topical antibiotics, topical corticosteroids or other topical drug products · Use within 24 hours of any topical product in the areas to be treated · Known allergy or hypersensitivity to tacrolimus · Unwillingness to minimise or avoid natural and artificial sunlight exposure · Clinically significant unstable medical disorders, life‐threatening diseases, or current malignancies · Positive for hepatitis B surface antigen, hepatitis C antibody or HIV antibody or any other condition, that might compromise subject safety or affect study results · Abnormal baseline findings considered by the investigator to indicate conditions that might affect study endpoints and/or might increase the risk to the participant Additional design details: trial was terminated early; unclear if results for 13 recruited were reported |
| Participants | Total number randomised: 501 (target) Age: ≥ 18 years Sex: all Ethnicity, duration of eczema: not reported Severity of eczema: IGA at least moderate (3 or 4) and SCORAD > 15 Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.1% ointment used twice daily for unclear duration · Concurrent treatment: not reported · Other key information: includes both the Protopic and Encube Ethicals Private Ltd's tacrolimus group Comparator: vehicle ointment used twice daily for unclear duration · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Proportion of the per protocol population in each treatment group with treatment success at day 15 ± 2 (IGA of 0 = clear or 1 = almost clear within all treatment areas) · Change in severity score of erythema, induration//papulation, lichenification, and pruritus · SCORAD score |
| Starting date | Not reported |
| Contact information | Sushant Dhabekar, project manager, sushant.dhabekar@accutestglobal.com |
| Notes | Funding source: Encube Ethicals Private Limited Declarations of interest: not declared Original language of publication: English Other: none |
CTRI/2022/07/043740.
| Study name | A clinical trial to study the effects of two drugs, tofacitinib 2% w/w ointment and 0.1% hydrocortisone butyrate ointment in patients suffering from dermatitis |
| Methods | Trial design: randomised, parallel study Trial registration number: CTRI/2022/07/043740 Country: India Outpatient or hospital: not reported Date trial conducted: not yet recruiting Duration of trial participation: 2 weeks Inclusion criteria: · 18 to 60 years old with clinically stable AD according to Hanifin and Rajka criteria · PGA score of 2 (mild) or 3 (moderate), AD covering at least 20% of total BSA Exclusion criteria: · Active forms of other dermatitides/eczematous conditions; evidence of skin conditions that would interfere with AD evaluation or treatment response · AD on the groin or genitals; evidence of active, latent or inadequately treated Mycobacterium tuberculosis infection · Hepatitis B/C or HIV infection; history of disseminated or recurrent herpes zoster infection; infection requiring hospitalisation < 6 months prior to the study · Infection requiring oral or topical antimicrobial therapy < 2 weeks prior to the study · History of lymphoproliferative disorders or malignancies (except adequately treated or excised non‐metastatic basal cell or squamous cell skin cancer or cervical carcinoma in situ) or previous treatment with tofacitinib · not able to wash out treatments for AD Additional design details: none |
| Participants | Total number randomised: n.r. Age: 18 to 60 years old Sex, ethnicity: not reported Duration of eczema: minimum 6 months Severity of eczema: mild‐to‐moderate Body site: non‐facial eczema Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tofacitinib 2.% ointment used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone butyrate 0.1% ointment used twice daily · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI · PGA · BSA · Itch Severity item |
| Starting date | July 2022 |
| Contact information | kapil@inquestbp.com. Mr Kapil Pandya |
| Notes | Funding source: Lyka Labs Limited Declarations of interest: not declared Original language of publication: English Other: none |
CTRI/2022/07/044136.
| Study name | A multicenter, prospective, randomized, open‐label, two‐arm, parallel‐group, phase III, active controlled, non‐inferiority, comparative study to assess efficacy and safety of tofacitinib ointment 2% w/w to pimecrolimus cream 1% w/w in patients with mild to moderate atopic dermatitis |
| Methods | Trial design: randomised, multicentre, parallel study Trial registration number: CTRI/2022/07/044136 Country: India Outpatient or hospital: multicentre, mostly hospitals, medical schools, care homes Date trial conducted: from July 2022 Duration of trial participation: 30 days Inclusion criteria: · Male or female, 12 to 60 years · Diagnosis of AD according to Hanifin and Rajka criteria · Mild‐to‐moderate AD (vIGA‐AD score of 2 or 3) · AD involvement of at least 5% treatable BSA (excluding the scalp) and up to and including 20% of total BSA at day 1 using Mostellar formulae · For female subjects: evidence of post‐menopause or, for females of childbearing age, negative serum or urine pregnancy test prior to randomisation · Females of childbearing age must agree to use highly effective contraceptive measures. Exclusion criteria: · Experiencing or has a history of other concomitant skin conditions · Clinically unstable AD or having consistent requirement for either oral, or parenteral corticosteroids to manage AD ·History of eczema herpeticum within 12 months, and/or a history of 2 or more episodes of eczema herpeticum in the past · Immunocompromised · History of herpes zoster infection · Skin infection or any other infection that requires treatment, or is currently being treated, with topical or systemic antibiotics · Clinically significant medical disorder, condition, or disease or clinically significant physical examination finding at screening visit that may interfere with study objectives/safety of the subjects · History of TB or living with contacts having TB in last 1 year · History of lymphoproliferative disorders or malignancies (except adequately treated or excised nonmetastatic basal cell or squamous cell skin cancer or cervical carcinoma in situ) · Already treated with phototherapy, systemic immunosuppressants, cytostatic drugs, systemic corticosteroid, oral Janus kinase inhibitor, monoclonal antibody, leukotriene antagonist, systemic antibiotics, or herbal medications with unknown properties or known beneficial effects for AD within 1 month · Topical therapy (corticosteroids, tars, antihistamines, antibiotic creams, phosphodiesterase 4 [PDE4] inhibitors, retinoids or benzoyl peroxide products [BPO], antibacterial medications or antibacterial products included in soaps, bleach baths, or topical sodium hypochlorite‐based products) within 14 days · Use of other investigational products used for the treatment of AD within 2 months · Allergic to tofacitinib or any macrolide or pimecrolimus or any excipients or any component of test/reference formulation · HIV‐positive, hepatitis‐B & C‐positive · Pregnant, lactating women and those planning to get pregnant · Participated in other clinical trials within 3 months or not considered suitable for this trial by the researchers Additional design details: none |
| Participants | Total number randomised: not reported Age: 12 to 60 years Sex: both Ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐moderate Body site: excluding scalp Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tofacitinib 2.% ointment used twice daily for 30 days · Concurrent treatment: not reported · Other key information: none Comparator: pimecrolimus 1.% cream used twice daily for 30 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Efficacy, safety, and tolerability. Methods of assessment unspecified |
| Starting date | Not reported |
| Contact information | Dr Hemant Zaveri, hemant_zaveri@intaspharma.com |
| Notes | Funding source: Intas Pharmaceuticals Ltd. Declarations of interest: not declared Original language of publication: English Other: none |
CTRI/2022/08/045019.
| Study name | A randomized, double‐blind, vehicle‐controlled, three arm, parallel group, multi‐centric, clinical study to establish the therapeutic equivalence of tacrolimus 0.03% topical ointment of Intas Pharmaceuticals Limited with protopic® 0.03% topical ointment in patients with moderate to severe atopic dermatitis |
| Methods | Trial design: randomised, parallel study Trial registration number: CTRI/2022/08/045019 Country: India Outpatient or hospital: not reported Date trial conducted: not yet recruiting Duration of trial participation: 4 weeks Inclusion criteria: · Male or female 18 years or more · Medically stable · AD for at least 3 months and moderate‐to‐severe according to Hanifin and Rajka criteria and affected area at least 10% BSA · vIGA of disease severity of at least moderate · Failed to respond adequately to other topical prescription treatments · A female participant not pregnant or breastfeeding Exclusion criteria: · History of clinically significant physical or mental conditions · Known allergies, hypersensitivity, or intolerance to tacrolimus topical ointment · Contraindications to the use of tacrolimus 0.03% topical ointment per product monograph of Protopic®. · History or current evidence of positive hepatitis B surface antigen · History or current evidence of human immunodeficiency virus infection · Lymphoma, leukaemia, or any malignancy within the past 5 years except for basal cell or squamous epithelial carcinomas of the skin that have been resected with no evidence of metastatic disease for 3 years · Clinically significant severe renal insufficiency or severe hepatic disorders · Active cutaneous bacterial or viral infection in any treatment area at baseline requiring oral antimicrobials within 14 days and topical antimicrobials within 7 days · History of confounding skin conditions · A known case of genetic epidermal barrier defect · History or known case of congenital or acquired immunodeficiencies · Presence of serious active infection or recurrent infection within the last 14 days Additional design details: none |
| Participants | Total number randomised: not reported Age: 18 years+ Sex: any Ethnicity: not reported Duration of eczema: at least 3 months Severity of eczema: at least moderate Body site: not reported Number of withdrawals: not yet recruiting Notes: none |
| Interventions | Run‐in details: n.r. Intervention: tacrolimus 0.03% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: includes both tacrolimus and protopic arms Comparator: vehicle · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA · Individual signs and symptoms of AD (i.e. erythema, induration/papulation, lichenification and pruritus) |
| Starting date | Not reported |
| Contact information | Prashant Modi, prashantmodi@lambda‐cro.com |
| Notes | Funding source: Intas Pharmaceuticals Limited, India Declarations of interest: not declared Original language of publication: English Other: none |
CTRI/2022/08/045020.
| Study name | A study to establish the therapeutic equivalence of tacrolimus 0.1 % topical ointment in adult patients with moderate to severe atopic dermatitis |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: CTRI/2022/08/045020 Country: India Outpatient or hospital: mostly hospitals, one clinic, one medical school Date trial conducted: not yet recruiting Duration of trial participation: 2 weeks Inclusion criteria: · Male or female aged 18 years or more. · Medically stable on the basis of physical examination, medical history, vital signs and clinical laboratory tests · AD for at least 3 months · Clinical diagnosis of moderate‐to‐severe AD according to Hanifin and Rajka criteria and affected area of involvement at least 10 % BSA · A validated vIGA of disease severity of at least moderate · Failed to respond adequately to other topical prescription treatments for AD · A female participant who is not pregnant or breastfeeding, not of childbearing potential or using a contraceptive method that is highly effective Exclusion criteria: · Medical history of serious physical or mental condition · Known allergies, hypersensitivity, or intolerance to tacrolimus topical ointment · Contraindications to the use of tacrolimus 0.1 % topical ointment · Medical history or current evidence of positive hepatitis B, C surface antigen or current evidence of positive hepatitis C antibody · Medical history or current evidence of human immunodeficiency virus · Lymphoma, leukaemia, or any malignancy within the past 5 years except for basal cell or squamous epithelial carcinomas of the skin that have been resected with no evidence of metastatic disease for 3 years; carcinoma in situ of the cervix; or malignancy, which is considered cured with minimal risk of recurrence. · Clinically significant severe renal insufficiency or severe hepatic disorders as judged by the investigator · Active cutaneous bacterial or viral infection in any treatment area requiring oral antimicrobials within 14 days and topical antimicrobials within 7 days · Sunburn, extensive scarring, or pigmented lesion(s) in any treatment area · History of confounding skin conditions · A known case of genetic epidermal barrier defect · History or known case of congenital or acquired immunodeficiencies · Presence of serious active infection or recurrent infection · Use of following within 30 days ‐ Oral or intravenous corticosteroids, UVA/UVB therapy, PUVA (psoralen plus ultraviolet A) therapy, Tanning booths, Nonprescription UV light sources, Immunomodulators or immunosuppressive therapies, Interferon, Cytotoxic drugs, Tacrolimus, Pimecrolimus · Use within 14 days of systemic antibiotics, calcipotriene or other vitamin D preparation, retinoids · Use within 7 days of antihistamines, topical antibiotics, topical corticosteroids Additional design details: none |
| Participants | Total number randomised: not reported Age: 18 years+ Sex: both Ethnicity: not reported Duration of eczema: at least 3 months Severity of eczema: at least moderate Body site: not reported Number of withdrawals: not yet recruiting Notes: none |
| Interventions | Run‐in details: Intervention: tacrolimus 0.1 % ointment used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA · Incidence and severity of AE |
| Starting date | Not reported |
| Contact information | Prashant Modi, prashantmodi@lambda‐cro.com |
| Notes | Funding source: Intas Pharmaceuticals Limited Declarations of interest: not declared Original language of publication: English Other: None |
CTRI/2022/12/047916.
| Study name | An investigator initiated, randomized, placebo and active controlled, evaluator blinded study to assess the efficacy and tolerability of pimecrolimus lotion 0.8% (ENZ001) in the treatment of target lesions in patients with moderate atopic dermatitis |
| Methods | Trial design: randomised study Trial registration number: CTRI/2022/12/047916 Country: India Outpatient or hospital: not reported Date trial conducted: from December 2022 Duration of trial participation: 29 days Inclusion criteria: · Non‐immunocompromised, male and/or non‐pregnant, or non‐lactating female patient aged between 12 and 65 years (inclusive) · Diagnosed AD for at least 6 months and currently in the active stage · Moderate AD on both sides · Two symmetrical, comparable in severity and size, left/right treatment Target Lesions (TL) on arms, legs, or torso · Each TL with a TSS of at least 7 and TL should measure between 10 cm² and 100 cm² · Both TLs have similar severity (max 1 point difference in TSS), and of similar area · If female of childbearing potential, willing to use an acceptable form of birth control during the study · Normally active and in good health by medical history and physical examination Exclusion criteria: · Female who is pregnant, breastfeeding, or who plans to become pregnant during the study period · Active cutaneous bacterial or viral infection in any treatment area · Non‐AD lesion, skin disease, or infection in the target lesions that could interfere with the study assessments · Sunburn, extensive scarring, or pigmented lesion(s) in any treatment area · History of confounding skin conditions · History or presence of Nethertonâs Syndrome, immunological deficiencies or diseases, HIV, diabetes, malignancy, serious active or recurrent infection, clinically significant severe renal insufficiency or severe hepatic disorders · Use within 28 days of systemic antibiotics/calcipotriene or other vitamin D preparations/retinoids/corticosteroids (note: use of intranasal/inhaled or ophthalmic corticosteroids for stable medical conditions are allowed) or systemic immunosuppressant · Use within 7 days of: topical or antihistamines, topical antibiotics, topical corticosteroids/immunomodulators or other topical drug products such as antibacterial soaps, or topical sodium hypochlorite‐based products · Use within 24 hours prior to baseline of any topical product in the areas to be treated · Known allergy or hypersensitivity to pimecrolimus or any other component of the test product or reference product · Engaged in activities that involve excessive or prolonged exposure to sunlight or not willing to minimise or avoid natural and artificial sunlight exposure during treatment · Other acute or chronic medical or psychiatric conditions · Signs and symptoms suggestive of COVID‐19 infection Additional design details: none |
| Participants | Total number randomised, age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 0.8% lotion used twice a day for 28 days · Concurrent treatment: not reported · Other key information: none Comparator one: elidel 1.% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator two: vehicle for pimecrolimus lotion 0.8% used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Efficacy, safety and local tolerability (no further details given on measures) |
| Starting date | Not reported |
| Contact information | Dr Ankesh Barnwal, abarnwal@cliantha.com, Cliantha Research Limited |
| Notes | Funding source: Encube Ethicals Pvt. Ltd. Declarations of interest: not declared Original language of publication: English Other: none |
CTRI/2023/05/052282.
| Study name | A double‐blind, randomized, placebo‐controlled, three‐arm, parallel‐group, multi‐centre clinical study to evaluate the safety and clinical endpoint bioequivalence of tacrolimus ointment 0.1% w/w of JAMP Pharma Corporation, Canada and Protopic® tacrolimus ointment, 0.1% (w/w) of LEO Pharma Inc., Thornhill, Ontario, L3T 7W8 and compare other active treatments to a placebo control in the treatment of non‐immunocompromised patients with moderate to severe atopic dermatitis |
| Methods | Trial design: randomised, double‐blind study Trial registration number: CTRI/2023/05/052282 Country: India Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 15 days Inclusion criteria: · Non‐immunocompromised male or female, 12 years of age or greater, diagnosis of moderate‐to‐severe AD for at least 3 months with greater than or equal to 20% BSA affected according to Hanifin and Rajka criteria · At least three of the following: itching, chronic relapsing course, typical morphology and distribution of lesions, or familial and/or personal history of other atopic disorders · IGA score of at least moderate, greater than or equal to 3 · AD for which the use of alternative, conventional therapies are deemed inadvisable because of potential risks, or are not adequately responsive or are intolerant to other topical prescription treatments for AD or alternative, conventional therapies · Female participants with childbearing potential should have a negative urine pregnancy test at screening and should be willing to use an acceptable form of birth control during the study. . Agree to concomitant therapy restrictions during the study, including discontinuation of non‐medicated topical agents such as creams, lotions, and emollients (to treatment area); topical antihistamines; topical antimicrobials; topical antifungals; topical or systemic corticosteroids; light treatments; non‐steroid immunosuppressants; and other investigational drugs, in good health, as confirmed by medical history and physical examination, and free from any clinically significant disease, other than AD, that might interfere with the study evaluations Exclusion criteria: · Females who are pregnant, nursing, or planning a pregnancy · Clinically infected AD · A skin disorder other than AD or history or presence of skin disorders that may interfere with the study evaluations · Has sunburn, pigmentation, extensive scarring, or pigmented lesions in the proposed treatment areas which could interfere with the rating of efficacy parameters · Known or suspected history or presence of a clinically significant systemic disease, life‐threatening disease, or current malignancies, serious active or recurrent infection or clinically significant severe renal insufficiency or severe hepatic disorders or any other significant medical condition likely to compromise participation, or to place the patient at risk · Treated with photo anti‐psoriatic therapies/drugs, UVA/UVB therapy, psoralen plus ultraviolet A therapy, tanning booths, non‐prescription UV light sources, immunomodulators or immunosuppressive therapies, interferon, cytotoxic drugs or pimecrolimus within 1 month · Taken systemic corticosteroids within the past 1 month; treated with any marketed or investigational biologic treatment for psoriasis or atopic disease within the past three months or five half‐lives of the biologic, whichever is longer · Treated within 14 days of systemic antibiotics, calcipotriene or other vitamin D preparations, or retinoids; applied topical antimicrobials, topical or oral antihistamines, topical corticosteroids, topical antifungals or other medicated topical agents to the affected areas within the past seven days · Applied any topical agents in the areas to be treated within the previous 24 hours · Currently using calcium channel blockers and/or cimetidine which are CPY3A inhibitors · Known hypersensitivity to macrolides or any excipient of the ointment · Not willing to avoid constant sun exposure and the use of tanning booths or other UV light sources · Consumes excessive amounts of alcohol, abuse drugs, or has any condition that would compromise compliance, in the investigator’s opinion, with the protocol · Treated with an investigational drug or investigational device within 30 days · History of difficulty in accessing veins to draw blood Additional design details: none |
| Participants | Total number randomised, age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention one: tacrolimus ointment, 0.1% (w/w) of LEO Pharma used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Intervention two: tacrolimus ointment, 0.1% (w/w) of JAMP Pharma Corporation used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: placebo 0.% ointment used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Treatment success (IGSA) Change in severity of erythema, induration/papulation, lichenification and pruritus |
| Starting date | 2023 |
| Contact information | Dr Shendkar Sonal Mahadev, Lifepoint Multispeciality Hospital, 3rd floor, Clinical Research Department, 145/1 Mumbai Bangalore Highway, Near Hotel Sayaji Wakad Pune 411057 Maharashtra, Pune MAHARASHTRA 411057 India |
| Notes | Funding source: JAMP Pharma Corporation, Quebec, J4B 5H3, Canada Declarations of interest: not declared Original language of publication: English Other: none |
CTRI/2023/06/054208.
| Study name | A multicenter, randomized, double vlind, placebo‐controlled, two‐arm, phase II clinical study to evaluate safety, tolerability and efficacy of topical 1% AKP‐11 in atopic dermatitis participants |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: CTRI/2023/06/054208 Country: India Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 28 days Inclusion criteria: · Aged between 16 and 65 years of either sex with AD according to Hanifin and Rajika criteria · Mild‐to‐moderate AD as per SCORAD and EASI · With at least one AD spot with baseline score of pruritus as high‐moderate to severe itch (VAS ≥ 6 cm) with average pruritus of (VAS ≥ 6 cm) over 3 consecutive days Exclusion criteria: · History of allergy/ hypersensitivity to any of the ingredients of the treatments · Any skin condition other than AD, that, in the opinion of the investigator, could interfere with the trial medication · Suspected COVID‐19 symptoms · Topical treatment within 2 weeks that, in the opinion of the investigator, could modify disease activity Additional design details: none |
| Participants | Total number randomised, age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: AKP‐11 1% ointment, three times daily for 1 to 7 days and twice daily for 7 to 14 days · Concurrent treatment: not reported · Other key information: none Comparator: placebo 0.% ointment, three times daily for 1 to 7 days and twice daily for 7 to 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: AKP‐11 is a highly selective S1PR1 agonist. |
| Outcomes | · Percentage change in SCORAD · Percentage change in EASI · Proportion of participants with an improvement of pruritus on VAS scale · Proportion of participants with an IGA success defined as ≥ 2 points improvement · Safety of AKP‐11 ointment |
| Starting date | 2023 |
| Contact information | Dr Perumal M, perumd88@gmail.com |
| Notes | Funding source: Akaal Pharma Pty Ltd. Declarations of interest: not declared Original language of publication: English Other: none |
EUCTR2004‐001644‐80‐HU.
| Study name | Clinical study on tacrolimus ointment over the long term |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: EUCTR2004‐001644‐80‐HU Country: Hungary Outpatient or hospital, date trial conducted, duration of trial participation: not reported Inclusion criteria: · Male or female of any ethnicity · Aged 2 to 15 years with mild‐to‐severe AD according to Rajka/Langeland criteria (score ≥ 3) · Female of childbearing potential must use contraception. Exclusion criteria: · Genetic epidermal barrier defect · Pregnancy or breastfeeding · Clinically significant skin infection in the affected area · Known hypersensitivity to macrolides or any excipient of the ointment · Participation in any other drug trial within 28 days of the trial or during the trial · Substance abuse, psychiatric disorder or condition · HIV seropositivity Additional design details: none |
| Participants | Total number randomised: not reported Age: 2 to 15 years Sex: any Ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐severe Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: pre‐study restrictions: · No topical corticosteroids, topical immunomodulators (except for intranasal/inhaled corticosteroids) for 3 days prior to open‐label phase · No systemic corticosteroids (for the treatment of AD only) for 5 days prior to open‐label Intervention: tacrolimus 0.03% ointment · Concurrent treatment: not reported · Other key information: none Comparator: placebo ointment · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Number of flares requiring a substantial therapeutic intervention: treatment of > 7 days scheduled relative to exacerbation day 1 visit and an IGA 3‐5 at exacerbation day 1 visit |
| Starting date | Not reported |
| Contact information | Not reported |
| Notes | Funding source: Fujisawa GmbH Declarations of interest: not declared Original language of publication: English Other: recruitment may be ongoing or finished |
EUCTR2004‐002477‐23‐BE.
| Study name | Comparative, multicentre, randomised, double‐blind study to assess the efficacy of tacrolimus 0.1% ointment versus fluticasone 0.005% ointment in adult patients suffering from moderate to severe atopic dermatitis and presenting with so‐called "red face" lesions of the head and neck |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: EUCTR2004‐002477‐23‐BE Country: Belgium and Finland Outpatient or hospital, date trial conducted, duration of trial participation: not reported Inclusion criteria: · Male or female of any ethnicity · Age ≥ 16 years · Moderate‐to‐severe AD · Female of childbearing age using effective contraception · Complying with the following criteria for therapeutic washout prior to the study: topical corticosteroids or antibiotics (3 days); systemic corticosteroids for the treatment of atopic dermatitis or antihistamines (5 days); nonsteroidal immunosuppressants (cyclosporin, methotrexate), tacrolimus ointment or pimecrolimus (2 weeks); UVA/UVB therapy or other study drugs (4 weeks); tacrolimus ointment + pimecrolimus (2 weeks) Exclusion criteria: · Genetic epidermal barrier defect · Seborrhoeic dermatitis or contact dermatitis affecting the "face", or any other facial erythema of non‐atopic origin · Pregnancy or breastfeeding · Presenting with a clinical infection due to the VZV virus, HSV1‐2 viruses, verruca vulgaris or molluscum contagiosum · Superinfected eczema · Known hypersensitivity to macrolides or to any other excipient in the tacrolimus 0.1% ointment or fluticasone 0.00 5% ointment preparations · Ulcerated lesions · Moderate‐to‐severe acne with rosacea · Participation in another clinical study or who have participated in another clinical study within 28 days of the study · Substance abuse or any mental disorder · HIV seropositivity Additional design details: none |
| Participants | Total number randomised: not reported Age: ≥ 16 years eligible Sex: all eligible Ethnicity: any eligible Duration of eczema: not reported Severity of eczema: moderate‐to‐severe Body site: head and neck Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.1% ointment used (unspecified frequency) for 21 days (assumed based on outcomes) · Concurrent treatment: not reported · Other key information: none Comparator: fluticasone propionate 0.005% ointment for 21 days (assumed from outcomes) · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Modified local EASI, calculated solely for the "face" · Percentage of patients with an improvement of ≥ 60% local mEASI score |
| Starting date | Not reported |
| Contact information | Not reported |
| Notes | Funding source: Fujisawa GmbH Declarations of interest: not declared Original language of publication: English Other: none |
EUCTR2004‐002478‐47‐BE.
| Study name | Comparative, multicentre, randomised, double‐blind study to assess the efficacy of tacrolimus 0.03% ointment versus fluticasone 0.005% ointment in children aged 2 years or over suffering from moderate to severe atopic dermatitis |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: EUCTR2004‐002478‐47‐BE Country: Belgium and France? Outpatient or hospital, date trial conducted, duration of trial participation: not reported Inclusion criteria: · Male or female of any racial origin · Aged 2 to 15 years with moderate‐to‐severe AD according to Rajka & Langeland criteria (score = 4.5) that has not responded sufficiently to conventional therapies · Females of childbearing age using effective contraceptive measures Exclusion criteria: · Genetic epidermal barrier defect, such as Netherton's syndrome, or erythroderma · Pregnancy or breastfeeding · Clinical infection due to the VZV virus, HSV1‐2 viruses, verruca vulgaris or molluscum contagiosum · Superinfected eczema · Known hypersensitivity to one of the agents contained in either study treatment · Ulcerated lesions · Moderate‐to‐severe acne with rosacea · Participation in another clinical study during or within 28 days prior to this study · Any type of substance abuse or any mental disorder/psychological state which may interfere with follow‐up · HIV positivity Additional design details: none |
| Participants | Total number randomised: 460 (target) participants Age: 2 to 15 years Sex: females and males Ethnicity, duration of eczema: not reported Severity of eczema: moderate‐to‐severe Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: therapeutic washout of: · Topical corticosteroids 3 days prior · Systemic corticosteroids for the treatment of atopic dermatitis 5 days prior · Nonsteroidal immunosuppressants 2 weeks prior Intervention: tacrolimus 0.03% ointment for 21 days (assumed) · Concurrent treatment: not reported · Other key information: protopic FK506 Comparator: fluticasone propionate 0.005% ointment used for 21 days (assumed) · Concurrent treatment: not reported · Other key information: brand name: Cutivate Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Number of patients presenting with a 60% improvement in mEASI score |
| Starting date | Not reported |
| Contact information | Not reported |
| Notes | Funding source: Fujisawa GmbH Declarations of interest: not declared Original language of publication: English Other: Clinical trial report, no results reported |
EUCTR2004‐004824‐11‐DE.
| Study name | Role for pimecrolimus in restoring skin barrier function and normalizing epidermal lipid content and differentiation in atopic epidermis: a randomized, intra‐patient, double‐blind study in adults with atopic dermatitis treated with 1% pimecrolimus cream and 0.1% triamcinolone acetonide cream as treatment control twice daily for 3 weeks |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: EUCTR2004‐004824‐11‐DE Country: Germany Outpatient or hospital: not reported Date trial conducted: April 2005 to date not reported Duration of trial participation: 3 weeks Inclusion criteria: adults with AD of mild‐to‐moderate severity Exclusion criteria: pregnant, nursing, immunocompromised or presenting signs of skin atrophy Additional design details: none |
| Participants | Total number randomised: not reported Age: ≥ 18 years eligible Sex: female or male Ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐moderate eligible Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus 1.% cream used twice daily for 21 days · Concurrent treatment: not reported · Other key information: elidel Comparator: betamethasone valerate (with neomycin) 0.1% cream used twice daily for 21 days · Concurrent treatment: not reported · Other key information: betnesol V‐Creme Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Transepidermal water loss · Examination of epidermal cell proliferation and cell differentiation |
| Starting date | Not reported |
| Contact information | Not reported |
| Notes | Funding source: Universitätshautklinik Kiel Declarations of interest: none declared Original language of publication: English Other: none |
EUCTR2011‐006236‐23‐PL.
| Study name | A randomised, double‐blind, placebo‐controlled, three‐arm, parallel assignment, multi‐centre, therapeutic equivalence study of two tacrolimus 0.1% topical ointment formulations in adult patients with moderate to severe atopic dermatitis |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: EUCTR2011‐006236‐23‐PL Country: India, Poland Outpatient or hospital: not reported Date trial conducted: from June 2012 Duration of trial participation: not reported Inclusion criteria: · Male or non‐pregnant, non‐lactating female of any ethnic group, 18 to 70 years of age · AD according to Hanifin and Rajka diagnostic criteria · Moderate‐to‐severe AD (i.e. a score of at least 4.5) according to Rajka and Langeland criteria · Non‐immunocompromised adults who have failed to respond adequately to other topical prescription treatments for AD, or when those treatments are not advisable in the opinion of the investigator · Last application of medicated topical agents, intake of systemic antihistamines, intranasal or inhaled corticosteroids (> 1 mg/day) should be a minimum of 7 days prior to randomisation. · Last application of tacrolimus ointment, intake of systemic corticosteroids and nonsteroidal immunosuppressants should be a minimum of 3 weeks prior to randomisation. · Both male and female patients of childbearing potential must practise adequate contraception and females must not be pregnant or lactating and must have a negative serum pregnancy test at screening and negative urine pregnancy test at randomisation. Exclusion criteria: · Newly diagnosed or treatment naïve · Mild or very severe AD that requires systemic therapy · Need of undergoing UV treatment for AD · Clinically infected AD · Any dermatological condition other than AD, scar/wound/tattoo at the application site or in its close vicinity that may interfere with the evaluation · History of allergy or hypersensitivity to tacrolimus or any of the ointment excipients, pimecrolimus, any macrolides · History or known case of congenital or acquired immunodeficiencies, which in the investigator’s opinion would contraindicate the use of immunosuppressants, including but not limited to human immunodeficiency virus infection and cancer · Known case of genetic epidermal barrier defect such as Netherton’s syndrome or generalised erythroderma · Known case of Cushing’s syndrome. · Hepatic failure Additional design details: none |
| Participants | Total number randomised: not reported Age: 18 to 64 years Sex: all Ethnicity: not reported Duration of eczema: "newly diagnosed" participants excluded Severity of eczema: moderate‐to‐severe Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.1% ointment used for 42 days · Concurrent treatment: not reported · Other key information: two types of tacrolimus 0.1% ointment compared to placebo Comparator: vehicle 0.% ointment used for 42 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | EASI, AE |
| Starting date | Not reported |
| Contact information | ezyto@lambda‐cro.com, clinical trial management |
| Notes | Funding source: Accord Healthcare Ltd, Intas Pharmaceuticals Ltd. Declarations of interest: not declared Original language of publication: English Other: none |
EUCTR2013‐000715‐25‐DE.
| Study name | A multi‐center, randomised, double‐blind, phase II trial with intraindividual comparison to assess superiority of soventol hydrocort 1% cream versus vehicle on lesional skin in patients with mild atopic eczema, seborrheic eczema or stasis dermatitis and to assess safety of Soventol HydroCort 1% cream |
| Methods | Trial design: randomised, double‐blind study Trial registration number: EUCTR2013‐000715‐25‐DE Country: Germany Outpatient or hospital: not reported Date trial conducted: from July 2013 Duration of trial participation: not reported Inclusion criteria: • Male or female aged 18 years or older • AD according to Hanifin and Rajka criteria, or seborrhoeic eczema or stasis dermatitis • Two comparable lesional areas of at least 2 cm2, with the clinical condition of AD or seborrhoeic eczema or stasis dermatitis rated mild • Lesions located contralaterally for patients with AD and stasis dermatitis • An erythema score = 1 in both lesional areas • Female of childbearing potential must either be surgically sterile or agree to use a reliable method of contraception, sexual abstinence or vasectomised partner. Exclusion criteria: · Acne, suntan, eczema other than AD, seborrhoeic eczema or stasis dermatitis, massive hyper‐ or hypopigmentation or tattoos in the treatment areas · Syphilitic or tuberculous dermatitis, varicella or vaccine reactions · Dark‐skinned persons whose skin colour prevents ready assessment of skin reactions · Drug or alcohol abuse · Pregnancy or nursing · UV‐therapy within 6 weeks · Symptoms of a clinically significant illness that may influence the outcome of the trial · Known allergic reactions to components of the investigational product/s · Treatment with systemic or locally acting medications which might counter or influence the trial aim within four weeks before the baseline visit and during the trial · Contraindications according to the summary of product characteristics of Soventol® HydroCort 0.5% and Linolacort® Hydro 1.0% · Institutionalised because of legal or regulatory order Additional design details: none |
| Participants | Total number randomised: not reported Age: 18 to 64 years Sex: all Ethnicity: excluded to quote: "dark‐skinned persons whose skin color prevents ready assessment of skin reactions" Duration of eczema: not reported Severity of eczema: mild Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: hydrocortisone 1.% cream · Concurrent treatment: not reported · Other key information: none Comparator: vehicle 0.% cream · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | IMP versus vehicle with respect to the AUC of baseline corrected erythema scores · Clinical assessment of oedema/papulation, oozing/crusting, excoriations, scaling and lichenification |
| Starting date | Not reported |
| Contact information | betina.vanfuerden@bioskin.de |
| Notes | Funding source: MEDICE Arzneimittel Pütter GmbH & CO.KG Declarations of interest: not declared Original language of publication: English Other: none |
EUCTR2014‐005593‐11‐AT.
| Study name | Diacerein for the treatment of atopic dermatitis |
| Methods | Trial design: randomised, double‐blind study Trial registration number: EUCTR2014‐005593‐11‐AT 2015 Country: Austria Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 4 weeks Inclusion criteria: · Minimum age of 18 years · AD mild‐to‐medium severity (SCORAD < 50 points) according to Hanifin and Rajka criteria · Presence of lesional skin changes of mild‐to‐medium, characterised by the modified, local SCORAD of at least 4 · Presence of mild‐to‐medium degree lesions on at least 3% of the BSA suitable for topical therapy Exclusion criteria: · Known or suspected intolerances against diacerein, auxiliaries of the investigational product (especially tartrazine) or substances with similar structure · Known or suspected intolerances against the cream base · Pregnancy or lactation · Use of topical, anti‐pruritic therapy of antibiotic therapy at the treated body areas, 7 days before the beginning of the trial · Systemic therapy with known sedative or anti‐pruritic effect 2 weeks before the beginning of the trial · Topical application of tacrolimus cream 0.03% or pimecrolimus cream 1% two weeks before the beginning of the trial on the treated body areas · Phototherapy or systemic therapy with known effect on AD one month before the trial · Clinically super infected atopic dermatitis at the treated skin area · Further skin diseases, apart from AD, at the treated skin areas · Other relevant diseases (e.g. acute infections, active tumours) · Severe hepatic impairment or immunosuppression Additional design details: none |
| Participants | Total number randomised, age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: diacerein, IL1 inhibitor · Concurrent treatment: not reported · Other key information: none Comparator: placebo cream · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Reduction of local pruritus · Improvement of the skin by 30% according to the modified SCORAD · Improvement in quality of life · Determination of Il‐1ß levels in patient serum |
| Starting date | Not reported |
| Contact information | v.wally@salk.at, Scientific Associate |
| Notes | Funding source: University Hospital of Dermatology, Paracelsus Medical University Declarations of interest: not declared Original language of publication: English/German Other: unclear if the trial has taken place |
EUCTR2016‐003013‐96‐DK.
| Study name | A randomised, blinded, placebo‐controlled, single centre pilot study to evaluate the safety and efficacy of AVX001 3% ointment (NG) administered topically once daily to patients with mild, moderate AD |
| Methods | Trial design: randomised, double‐blind study Trial registration number: EUCTR2016‐003013‐96‐DK Country: Denmark Outpatient or hospital: not reported Date trial conducted: From October 2016 Duration of trial participation: 6 weeks Inclusion criteria: · Caucasian male and female aged 16 years or more with AD according to Hanifin‐Rajka criteria and with any degree of severity based on SCORAD · AD affecting symmetrical anatomic sites · Target lesions with active dermatitis more than 3 cm in diameter and with at least two of the signs present: erythema, infiltration and scaliness, in each test site. Symmetrical target areas, defined as approximately the size of a palm when distributing ointment equivalent to a full fingertip, should, according to the investigator’s spontaneous clinical judgement, be comparable in disease activity. · Physical examination of the skin must be without abnormal findings other than AD unless the investigator considers an abnormality to be irrelevant to the outcome of the clinical trial. Exclusion criteria: · Any condition that, in the opinion of the investigator, could interfere with clinical assessments · Any permanent or transient conditon within a 4‐week period prior to dosing that may interfere with the subjects’ safety or ability to participate, and any condition that, according to investigator’s evaluation, may confound or invalidate clinical assessments and recordings · Females must either be of non‐childbearing potential or post‐menopausal or agree to use a reliable method of contraception. · Topical treatment of the selected target lesions with topical steroids, topical calcineurin inhibitors, anti‐bacterials or antihistamines 2 weeks prior to dosing · Systemic long‐term treatments, such as azathioprine, are allowed provided the dose of such a drug is not changed during the study. If changed, the investigator shall decide if the change is small and unlikely to influence the study outcome or, alternatively, of a magnitude which is likely to be a clinically significant influence to target areas. · Phototherapy within 4 weeks prior to dosing or during the study treatment phase and extensive sun exposure during the study and 1 week prior to baseline evaluation · Use of emollients on the TLs within 3 days prior to dosing and during the study treatment phase · Known or suspected hypersensitivity to component(s) of the investigational product(s) · Females who are pregnant or trying to fall pregnant during the study as well as females that are breastfeeding Additional design details: none |
| Participants | Total number randomised: not reported Age: age 16 or more Sex: both Ethnicity: Caucasian Duration of eczema: not reported Severity of eczema: any Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: AVX001 0.% ointment used once daily for 42 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used once daily for 42 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Number experiencing local skin reactions and any other local AEs at the treated sites · Assessment of systemic safety based on reported SAE, AE, laboratory data, vital signs and physical examination · Physician’s Global Assessment |
| Starting date | Not reported |
| Contact information | peter@damsbo.net |
| Notes | Funding source: Avexxin AS Declarations of interest: not declared Original language of publication: English Other: none |
EUCTR2018‐004370‐96‐DK.
| Study name | The effects of topical corticosteroid use on insulin sensitivity and bone turnover |
| Methods | Trial design: randomised, double‐blind controlled study Trial registration number: EUCTR2018‐004370‐96‐DK Country: Denmark Outpatient or hospital: outpatient clinic at Department of Dermatology and Allergy, Herlev‐ Gentofte Hospital, other Departments of Dermatology, including private clinics in the region of Copenhagen and Zealand Date trial conducted: February 2019 to March 2021 Duration of trial participation: six weeks Inclusion criteria: BMI < 30, AD at least 3 years Exclusion criteria: No prediabetes or diabetes, no other chronic inflammatory diseases (including but not limited to rheumatoid arthritis, inflammatory bowel disease etc.) besides AD and non‐treatment demanding rhinitis or asthma Additional design details: preliminary results only available |
| Participants | Total number randomised: 36 participants (18 to apply the intervention and 18 to apply the comparator) Age: median 26 years, interquartile range: Q1 23 to Q3 37 years Sex: female n = 19, male n = 17 Ethnicity: not reported Duration of eczema: of at least 3 years Severity of eczema, body site: not reported Number of withdrawals: n = 1, from tacrolimus group; reason not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: betamethasone 17‐valerate 0.1% ointment used daily for 2 weeks and twice‐weekly for 4 weeks for 42 days · Concurrent treatment: not reported · Other key information: none Comparator: tacrolimus 0.1% ointment used daily for 2 weeks and twice‐weekly for 4 weeks for 42 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Insulin sensitivity · Bone turnover · AE |
| Starting date | Not reported |
| Contact information | Department of Dermatology, Gentofte Hospital, lise.gether.01@regionh.dk |
| Notes | Funding source: Gentofte Hospital Declarations of interest: not declared Original language of publication: English Other: data results preliminary only |
EUCTR2019‐002686‐35‐DE.
| Study name | Clinical study to compare two cutaneous ointments with the active substance methylprednisolone aceponate 0.1 % and one cutaneous ointment without active substance for patients with atopic dermatitis |
| Methods | Trial design: randomised, double‐blind study Trial registration number: EUCTR2016‐003013‐96‐DK Country: Germany Outpatient or hospital: not reported Date trial conducted: from October 2016 Duration of trial participation: 6 weeks Inclusion criteria: · Adults and children/adolescents of both sexes of at least 6 years of age · Acute flare of AD according to IGA score 2 (mild) or 3 (moderate) · Affected BSA at least 10% and not more than 40% · For women of childbearing potential: established highly efficient contraceptive method during the whole study · For all female patients of childbearing potential: urine pregnancy test with negative result prior to study start · AD affecting symmetrical anatomic sites with disease severity mild, moderate and severe AD · Target lesions with active dermatitis more than 3 cm in diameter and with at least two of the signs present: erythema, infiltration and scaliness, in each test site. Symmetrical target areas, defined as approximately the size of a palm when distributing ointment equivalent to a full fingertip, should, according to the investigator’s spontaneous clinical judgement, be comparable in disease activity. · Physical examination of the skin must be without abnormal findings other than AD unless the investigator considers an abnormality to be irrelevant to the outcome of the clinical trial Exclusion criteria: · Any systemic treatment of atopic dermatitis within the last 4 weeks prior to study inclusion · Any topical treatment or physical therapy of AD in the test area 2 weeks prior to study inclusion · Presence of tuberculous or syphilitic processes in the treatment area · Presence of viral infections, rosacea, perioral dermatitis, ulcera, acne vulgaris, atrophic skin diseases and vaccination skin reactions in the area to be treated · Presence of bacterial and/or mycotic skin diseases in the treatment area · Current diagnosis of glaucoma or cataract · Known intolerance or hypersensitivity against methylprednisolone aceponate or any of the other ingredients in the study medication · Sunburn, extensive scarring, or pigmented lesion(s) in any treatment area, which would interfere with evaluations · Severe acute or chronic concomitant disease with severe impairment of the general condition · Other concomitant diseases which may ‐ taking the present knowledge into account ‐ influence the parameters evaluated in the study in a way that an objective evaluation would be impossible or influence the methods of measurement used in this study or the resulting data · Women with existing or intended pregnancy or during lactation Additional design details: there are two active groups of the same TCS with the same potency recorded here as one group. |
| Participants | Total number randomised, age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention one: methylprednisolone aceponate 0.1 % cream used · Concurrent treatment: not reported · Other key information: none Comparator: placebo cream · Concurrent treatment: not reported · Other key information: Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI score · Per cent change of the total affected BSA · Change in IGA · Evaluation of overall therapeutic success by the investigator and patient · Patient's assessment of severity of pruritus |
| Starting date | Not reported |
| Contact information | Clinicaltrials.Dermapharm@dermapharm.com |
| Notes | Funding source: Dermapharm AG Declarations of interest: not declared Original language of publication: English Other: none |
Hanifin 2006.
| Study name | A novel topical nuclear factor kappa‐B decoy |
| Methods | Trial design: randomised, double‐blind study Trial registration number: not reported Country: US Outpatient or hospital: 8 US centres Date trial conducted, duration of trial participation: not reported Inclusion criteria: · 18 to 65 years old · PGA score equivalent to a diagnosis of mild‐to‐moderate AD Exclusion criteria: · Used any topical or systemic treatment for AD within 1 week or 4 weeks of day 1, respectively · Met any other exclusion criteria Additional design details: detail limited in short abstract |
| Participants | Total number randomised, age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention one: nuclear factor kappa‐B decoy 0.25% used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Intervention two: nuclear factor kappa‐B decoy 0.5% used twice daily for 21 days · Concurrent treatment: not reported · Other key information: Intervention three: 0.% used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI · Itch · Symptom severity of target area · Safety assessment including laboratory tests, vital signs, ecgm physical examination, application site burning and adverse events · IGA |
| Starting date | not reported |
| Contact information | not reported |
| Notes | Funding source: Corgentech Inc Declarations of interest: not declared Original language of publication: English Other: none |
IRCT20091012002581N6.
| Study name | Comparison of efficacy of tacrolimus 0.03% ointment with mometason ointment in treatment of pediatric atopic dermatitis |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: IRCT20091012002581N6 Country: Iran Outpatient or hospital, date trial conducted, duration of trial participation: not reported Inclusion criteria: · Aged 2 to 10 years · AD lesion ≤ 5 cm · Mild‐to‐moderate limb lesions Exclusion criteria: · Concomitant skin conditions · Infected skin lesions Additional design details: none |
| Participants | Total number randomised: not reported Age: 2 to 10 years eligible Sex, ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐moderate eligible Body site: limb lesions Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.03% ointment used twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Comparator: mometasone ointment used twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · AD lesion severity index · Size of AD lesions · Patient symptoms · Safety |
| Starting date | Not reported |
| Contact information | Drherizchi@yahoo.com, Dr Hamide Herizchi Ghadim |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: none |
ISRCTN27411543.
| Study name | A randomised double‐blind cross‐over trial comparing betamethasone valerate and tacrolimus ointment for the treatment of moderate to severe atopic eczema |
| Methods | Trial design: randomised, double‐blinded, cross‐over study Trial registration number: ISRCTN27411543 Country: UK Outpatient or hospital: London hospital Date trial conducted: 2003 to 2005 Duration of trial participation: not reported Inclusion criteria: moderate‐to‐severe AD Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised, age, sex, ethnicity, duration of eczema: not reported Severity of eczema: moderate‐to‐severe Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: betamethasone valerate · Concurrent treatment: not reported · Other key information: none Comparator: tacrolimus · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · SCORAD score · Ointment weight · AE |
| Starting date | 2003 |
| Contact information | R. C. H. Yu, Dermatology Department, Middlesex Hospital, Mortimer Street, W1N 8AA London, United Kingdom Telephone: +44 (0)20 7380 9224 |
| Notes | Funding source: UK government Declarations of interest: not reported Original language of publication: English Other: none |
JapicCTI‐121946.
| Study name | A phase 2a study of M520102 in atopic dermatitis patients |
| Methods | Trial design: randomised, investigator‐blind, multicentre, parallel study Trial registration number: JapicCTI‐121946 Country: Japan Outpatient or hospital: outpatients Date trial conducted, duration of trial participation: not reported Inclusion criteria: AD either sex, aged between 16 and 63 years Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised, age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: M520102 (phosphodiesterase 4 inhibitor) used twice daily · Concurrent treatment: not reported · Other key information: none Comparator: placebo used twice daily · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Eczema area and severity, pruritus |
| Starting date | Not reported |
| Contact information | ctinfo@mii.maruho.co.jp |
| Notes | Funding source: Maruho Co., Ltd. Declarations of interest: not reported Original language of publication: Japanese Other: none |
JapicCTI‐173484.
| Study name | A multicenter, randomized, double‐blind, vehicle‐controlled, parallel‐group trial to assess the safety and efficacy of 0.3% and 1% OPA‐15406 ointments when administered for 4 weeks in pediatric patients with atopic dermatitis |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: JapicCTI‐173484 Country: Japan Outpatient or hospital, date trial conducted, duration of trial participation: 8 weeks Inclusion criteria: · AD according to Hanifin and Rajka criteria · Either sex, aged between 2 and 14 years Exclusion criteria: · AD or contact dermatitis flare‐up defined as a sudden intensification of A · Active viral skin infection · Current or history of malignancy Additional design details: none |
| Participants | Total number randomised, age, sex: not reported Ethnicity: Japanese Duration of eczema, severity of eczema, body site, affected areas (excluding scalp), number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: OPA‐15406 Ointment (0.3%, 1%) twice daily · Concurrent treatment: not reported · Other key information: none Comparator: placebo twice daily · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · The number and percentage of subjects experiencing AEs · IGA, EASI and plasma concentration of OPA‐15406 |
| Starting date | Not reported |
| Contact information | Otsuka Pharmaceutical Co., LTD. Drug Information Center |
| Notes | Funding source: Otsuka Pharmaceutical Co., Ltd. Declarations of interest: not declared Original language of publication: Japanese Other: none |
JPRN‐jRCT2031210255.
| Study name | Phase 3 clinical study of JTE‐061 Cream ‐ randomized vehicle‐controlled study (part 1) and extension study (part 2) in patients with atopic dermatitis |
| Methods | Trial design: randomised study Trial registration number: JRCT2031210255 Country: Japan Outpatient or hospital: outpatients Date trial conducted: from September 2021 Duration of trial participation: 8 weeks Inclusion criteria: · Japanese patients aged ≥ 12 years · AD according to criteria of the Japanese Dermatological Association · IGA, EASI, and% BSA meeting study criteria Exclusion criteria: · Significant dermatological or inflammatory condition that, in the investigator's opinion, would complicate assessments during the trial · Current acute bacterial, fungal, or viral skin infection within 1 week of the trial · Use prohibited therapy within a certain period · Serious concomitant disease(s) · Current cancer or within 5 years of the trial Additional design details: none |
| Participants | Total number randomised: 210 (target) participants Age: ≥ 12 years old Sex: either Ethnicity: Japanese Duration of eczema, severity of eczema: not reported Body site: all affected areas (excluding scalp) Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tapinarof 1.% cream used once daily for 8 weeks · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used once daily for 8 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Proportion with IGA 0 = clear or 1 = almost clear with a minimum 2‐grade improvement from baseline |
| Starting date | Not reported |
| Contact information | clinicaltrials‐info‐r@jt.com |
| Notes | Funding source: Nemoto Takanori Declarations of interest: not declared Original language of publication: Japanese Other: none |
JPRN‐UMIN000006955.
| Study name | The effect of proactive therapy with tacrolimus and betamethasone butyrate on atopic dermatitis: randomized placebo‐controlled study |
| Methods | Trial design: randomised, parallel study Trial registration number: JPRN‐UMIN000006955 Country: Japan Outpatient or hospital: not reported Date trial conducted: from 2011 Duration of trial participation: not reported Inclusion criteria: not reported Exclusion criteria: · Intolerance or discouraged to use tacrolimus or steroid ointment · Pregnant woman Additional design details: none |
| Participants | Total number randomised: not reported Age: 20 years plus Sex: both Ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: |
| Interventions | Run‐in details: not reported Intervention one: tacrolimus · Concurrent treatment: not reported · Other key information: none Intervention two: betamethasone butyrate · Concurrent treatment: not reported · Other key information: none Comparator: vaseline · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · SCORAD (severity score) · IGA · Safety · Serum cortisol · Serum TARC · DLQI · Intensity of itch · History of usage of external medicine · AE |
| Starting date | 2011 |
| Contact information | h‐murota@derma.med.osaka‐u.ac.jp |
| Notes | Funding source: "self‐funded" Declarations of interest: not declared Original language of publication: English Other: unclear if licenced use of the interventions |
JPRN‐UMIN000022212.
| Study name | To confirm the clinical efficacy and safety of topical steroid cream (PVA‐N11) in the treatment of atopic dermatitis |
| Methods | Trial design: randomised study Trial registration number: JPRN‐UMIN000022212 Country: Japan Outpatient or hospital: not reported Date trial conducted: 2016 ‐ unspecified Duration of trial participation: 2 weeks Inclusion criteria: AD with mild or moderate rash on test site (both arms) Exclusion criteria: · Severe skin disorders on test site (both arms) and deemed inappropriate to participate in this study by the principal investigator or sub‐investigators · Any complications which affect the results of this study · Those who might use any external‐use medicines, anti‐allergy drugs, or immunosuppressants · Pregnant or lactating patients. Patients with suspicion of pregnancy Additional design details: none |
| Participants | Total number randomised: not reported Age: 16 to 49 years Sex: any Ethnicity, duration of eczema: not reported Severity of eczema: mild or moderate Body site: arms Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: PVA‐N11 used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: to quote: "active comparator" used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Severity of AD · TWL, water content of the stratum corneum, erythema and pigmentation, and skin surface topography · QoL |
| Starting date | 2016 |
| Contact information | m.abe@dermalabo.co.jp |
| Notes | Funding source: Kao Corporation Declarations of interest: not declared Original language of publication: English Other: none |
NCT00120302.
| Study name | Quality of life study in adults with facial eczema |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: NCT00120302 Country: UK Outpatient or hospital: not reported Date trial conducted: not clear but conducted before January 2008 Duration of trial participation: 28 days Inclusion criteria: · Males and females aged 18 years or older · Active moderate facial AD at baseline (facial IGA score of 3), within 3 days of a deterioration in symptoms that necessitates further treatment · AD according to criteria by Williams and colleagues 1994: itchy skin condition plus 3 or more of the following 5 items listed below: ‐ History of involvement of skin creases ‐ History of asthma or hay fever ‐ History of general dry skin in the last year ‐ Visible flexural oedema ‐ Onset under the age of 2 years · Pruritus score of 2 or above at baseline · Patients in whom further use of TCS is clinically inappropriate due to: ‐ burning, stinging, allergic reaction or other adverse event that prevents the patient from using topical corticosteroids to successfully treat an AD flare on the face ‐ presence of rosacea, telangiectasia, skin atrophy or glaucoma as a result of topical corticosteroid usage on the face ‐ presence of AD on the eyelids or patients where previous treatment has been unsatisfactory and who would prefer to try an alternative treatment option Exclusion criteria: · Females of childbearing potential: ‐ pregnant or breastfeeding ‐ menstruating, capable of becoming pregnant, and not practising a medically approved method of contraception during and up to at least 4 weeks after the end of study treatment. A negative pregnancy test for all females of childbearing potential is required at the screening visit. 'Medically approved' contraception may include abstinence at the discretion of the investigator. · At baseline and throughout the study, all patients: ‐ who have received phototherapy or systemic therapy ‐ who have received systemic corticosteroids within 1 month. Patients on a stable maintenance dose of inhaled corticosteroids may participate ‐ known or suspected contact allergic dermatitis ‐ received systemic antibiotics within 2 weeks prior to visit 1 ‐ used oral or topical antihistamines for the treatment of pruritus within 2 weeks ‐ applied topical therapy within 2 weeks ‐ used potent or very potent TCS within 4 weeks ‐ immunocompromised or have a history of malignant disease ‐ history of poor or no clinical response, or hypersensitivity to topical pimecrolimus cream 1% ‐ have concurrent skin disease of such severity in the study area that it could interfere with the evaluation. ‐ have active bacterial, viral or fungal infections ‐ received any investigational drugs within 8 weeks of visit 1, or plan to use any other investigational drugs during the course of this study ‐ have abuse problems, mental dysfunction or other factors limiting their ability to cooperate fully in study‐related procedures Additional design details: none |
| Participants | Total number randomised: 76 participants Age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus cream 1.% cream used for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: 0.% cream used for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Change in QoL · Between‐treatment comparison of IGA |
| Starting date | 2005 |
| Contact information | Novartis Pharmaceuticals UK |
| Notes | Funding source: Novartis Declarations of interest: not declared Original language of publication: English Other: none |
NCT00121381.
| Study name | Pimecrolimus cream 1% plus topical corticosteroid in patients (2‐17 years of age) with severe atopic dermatitis |
| Methods | Trial design: randomised, double‐blinded, parallel study Trial registration number: NCT00121381 Country: US Outpatient or hospital: not reported Date trial conducted: May 2005 to January 2007 Duration of trial participation: not reported Inclusion criteria: · Severe AD · 5% of BSA affected Exclusion criteria: · Concurrent skin diseases (infections) · Immunocompromised · Recent phototherapy or systemic therapy Additional design details: none |
| Participants | Total number randomised: 400 participants Age: 2 to 17 years Sex: all Ethnicity, duration of eczema: not reported Severity of eczema: severe Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: none Intervention: pimecrolimus 1% cream and unspecified topical corticosteroid twice daily · Concurrent treatment: none · Other key information: none Comparator: vehicle cream and unspecified topical corticosteroid used twice daily · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: topical corticosteroid twice daily is included in the description of the intervention and comparator. Notes: none |
| Outcomes | · Incidence of AE · Time to relapse of AD · Time to treatment success according to IGA · Efficacy measured by IGA |
| Starting date | 2005 |
| Contact information | Not reported |
| Notes | Funding source: Novartis Declarations of interest: not declared Original language of publication: English Other: none |
NCT00125333.
| Study name | Topical NF‐kappaB decoy in the treatment of atopic dermatitis |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: NCT00125333 Country: USA Outpatient or hospital: not reported Date trial conducted: March 2005 to November 2005 Duration of trial participation: not reported Inclusion criteria: · Aged 18 to 65 years · Mild‐to‐moderate AD · If receiving antihistamines, are on a stabilised dose, and expect to maintain this dose throughout the study · Females or males of reproductive potential who are compliant in using adequate birth control or are females or males not of reproductive potential Exclusion criteria: · Concomitant dermatologic or medical condition(s) which may interfere with the investigator's ability to evaluate the subject's response to the study drug · immunocompromised status · Any clinically significant abnormal clinical laboratory test · History of malignancy not in remission for at least 5 years excluding basal cell carcinoma and non‐periorificial squamous cell carcinoma of the skin · An active intercurrent infection · Applied any topical medication or herbal preparation to the area selected for treatment within 1 week; have used any systemic antibiotic within 1 week; have used any systemic treatment for atopic dermatitis within 4 weeks; have used an investigational drug for any reason within 4 weeks; have used intranasal and/or inhaled corticosteroids at doses > 2 mg prednisone or equivalent per day within 4 weeks; or have used immunosuppressive or immunomodulating drugs such as etanercept, alefacept, or infliximab within 16 weeks · History of hypersensitivity or allergic reactions to parabens or any other ingredient in the vehicle formulation· · If female, are pregnant or lactating, or intend to become pregnant during the study period · If male, have a female partner who is pregnant or lactating, or intends to become pregnant during the study period Additional design details: none |
| Participants | Total number randomised: approximately 75 Age: 18 to 65 years Sex: male and female Ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐moderate Body site: excluding face, hands, feet, scalp or groin Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: have applied any topical medication (including corticosteroid, calcineurin inhibitor, topical H1 and H2 antihistamines, topical antimicrobials, other medicated topical agents) or herbal preparation to the area selected for treatment within 1 week Intervention: NF‐kappaB Decoy 0.% used twice daily · Concurrent treatment: not reported · Other key information: none Comparator: placebo · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Safety and tolerability |
| Starting date | 2005 |
| Contact information | Not reported |
| Notes | Funding source: Anesiva, Inc. Declarations of interest: not declared Original language of publication: English Other: none |
NCT00354510.
| Study name | Topical GW842470X in adult patients with moderate atopic dermatitis |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: NCT00354510 Country: Germany and The Netherlands Outpatient or hospital: outpatient (assumed) Date trial conducted: from March 2006 Duration of trial participation: 21 days Inclusion criteria: · Moderate AD (IGA = 3) · > 5% BSA · Female patients of childbearing potential must use appropriate contraception Exclusion criteria: · Active skin disease other than AD · Systemic treatment for AD or other topical or transdermal treatments within 14 days prior to the trial · Topical treatment with tar, any corticosteroid, topical immunomodulators or oral treatment with any corticosteroids within 10 days prior to the trial and/or oral antihistamines within 5 days Additional design details: none |
| Participants | Total number randomised: 190 participants Age: 18 to 65 years Sex: all Ethnicity, duration of eczema: not reported Severity of eczema: moderate Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: GW842470X 3% cream used for 21 days. · Concurrent treatment: not reported · Other key information: none Comparator: placebo · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI · SCORAD · IGA · Pruritus and sleep loss · Safety and tolerability |
| Starting date | 2006 |
| Contact information | Not reported |
| Notes | Funding source: GlaxoSmithKline Declarations of interest: not declared Original language of publication: English Other: Clinical trial registration. Recruitment status: Complete. No results posted |
NCT00356642.
| Study name | Study of single and ten day repeat atopical applications of GW842470X cream on the skin of patients with atopic dermatitis |
| Methods | Trial design: randomised, single‐blind, parallel study Trial registration number: NCT00356642 Country: Germany Outpatient or hospital: outpatient (assumed) Date trial conducted: June to December 2005 Duration of trial participation: 22 days Inclusion criteria: · AD (moderate‐to‐severe), but otherwise healthy · BMI 18.5 to 29.9 m2 · At least 2 index lesions and BSA involvement > 10% Exclusion criteria: · Systemic treatment for atopic dermatitis or other topical or transdermal treatments within 14 days prior to the study · Topical treatment with tar or any treatment with corticosteroids within 14 days prior to the study (except 1% hydrocortisone) · Systemic or active skin disease other than atopic dermatitis Additional design details: none |
| Participants | Total number randomised: 45 participants Age: 18 Years to 67 years Sex: all Ethnicity, duration of eczema: not reported Severity of eczema: BSA > 10% Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: 14‐day washout of current treatment Intervention: GW842470X (100 to 1000 mg) cream used for 22 days · Concurrent treatment: not reported · Other key information: none Comparator: placebo used for 22 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · SCORAD · BSA determination · Clinical photography · Number of subjects with abnormal physical examination, blood pressure, heart rate, 12‐lead ECG assessment, lead II cardiac monitoring, or clinical laboratory tests · AUC · Cmax · Time to max concentrate |
| Starting date | 2005 |
| Contact information | Not reported |
| Notes | Funding source: GlaxoSmithKline Declarations of interest: not declared Original language of publication: English Other: Clinical trial registration, recruitment status complete, no results posted |
NCT00510003.
| Study name | Assessment of pruritus improvement with pimecrolimus treatment on the areas affected by mild‐to‐moderate AD, in patients 2 to 11 years |
| Methods | Trial design: randomised, double‐blind, multicentred study Trial registration number: NCT00510003 Country: Spain Outpatient or hospital: not reported Date trial conducted: from December 2004 Duration of trial participation: 21 days Inclusion criteria: · Males and females aged between 2 and 12 years · Outpatients with diagnosis of AD · AD affecting ≥ 5% of total BSA and IGA score of 2 (mild) or 3 (moderate) · Pruritus (itching) severity assessment score of 2 (moderate) or 3 (severe) Exclusion criteria: · Being breastfed by women receiving systemic or prohibited medication · Children with known hypersensitivity to study medication · Children who received phototherapy or systemic therapy (immunosuppressants, cytostatics) known to affect AD within the previous month or topical therapy, systemic corticosteroids, systemic antibiotics or leukotriene antagonists within the previous week · Immunocompromised children or children with a history of malignant disease · Children who received investigational drugs within 8 weeks prior to first application of study medication · Children who had concurrent skin disease or active bacterial, viral or fungal infection Additional design details: none |
| Participants | Total number randomised: 117 participants Age: 2 to 11 years old Sex, ethnicity, duration of eczema, severity of eczema, body site: number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus cream 1.% cream used for 15 days · Concurrent treatment: not reported · Other key information: none Comparator: 0.% cream used for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Change in pruritus VAS · Reduction ≥ 50% in pruritus VAS |
| Starting date | 2004 |
| Contact information | Not reported |
| Notes | Funding source: Novartis Declarations of interest: not declared Original language of publication: English Other: none |
NCT00689832.
| Study name | Protopic ointment in children [with] atopic eczema |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: NCT00689832 Country: France Outpatient or hospital: not reported Date trial conducted: February 2004 to August 2005 Duration of trial participation: 6 weeks Inclusion criteria: · Children 2 to 15 years with moderate‐to‐severe AD according to Rajka & Langeland criteria (≥ 4.5) that had not responded sufficiently to conventional therapies · Female patients of childbearing age: effective contraception throughout the study and for four weeks after the study · Therapeutic washout for AD treatments Exclusion criteria: · Genetic epidermal barrier defect · Pregnancy or breastfeeding · Clinical infection due to the VZV virus, HSV1‐2 viruses, verruca vulgaris or molluscum contagiosum · Superinfected eczema · Known hypersensitivity to macrolides or any other excipient or agent in either ointment · Ulcerated lesions · Moderate‐to‐severe acne or rosacea · Substance abuse or mental disorder/psychological state which, in the investigator's opinion, might interfere with follow‐up · Known HIV positivity Additional design details: none |
| Participants | Total number randomised: 487 participants Age: 2 to 15 years Sex: All Ethnicity, duration of eczema: not reported Severity of eczema: moderate‐to‐severe according to Rajka & Langeland criteria (≥ 4.5) Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: none Intervention: tacrolimus 0.03% ointment used twice daily until clearance, for a maximum of 3 weeks and then, on residual lesions, once daily for up to 3 further weeks for 42 days · Concurrent treatment: not reported · Other key information: none Comparator: fluticasone 0.005% ointment used twice daily until clearance, for a maximum of 3 weeks and then, on residual lesions, once daily for up to 3 further weeks for 42 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Percentage of patients presenting at the week 3 visit with an improvement of at least 60% in their modified EASI from baseline · mEASI and EASI scores at each visit and percentage change from baseline · Global assessment of clinical response |
| Starting date | 2004 |
| Contact information | Not reported |
| Notes | Funding source: Astellas Pharma Inc. Declarations of interest: not declared Original language of publication: English Other: none |
NCT00690105.
| Study name | Protopic ointment in adult [with] atopic eczema of the face |
| Methods | Trial registration number: NCT00690105 Country: France Outpatient or hospital: assumed secondary care Date trial conducted: February 2004 to July 2005 Duration of trial participation: up to 6 weeks Inclusion criteria: · Moderate‐to‐severe AD according to Rajka & Langeland criteria · Lesions to the head and neck defined as 'red face' or 'facial eczema': erythema affecting at least 10% of the surface of the head, neck, chest, nape of neck, due to long‐term AD · ≥ 2 flare‐ups of 'facial' eczema during the past 12 months where conventional treatment had proved ineffective or poorly tolerated · Female patients of childbearing age using effective contraception during the study and for four weeks after · Therapeutic washout for atopic dermatitis treatments Exclusion criteria: · Genetic epidermal barrier defect, such as Netherton's syndrome, or suffering from erythroderma · Seborrhoeic dermatitis or contact dermatitis affecting the 'face', or any other facial erythema of non‐atopic origin · Clinical infection due to the VZV virus, HSV1‐2 viruses, verruca vulgaris or molluscum contagiosum · Superinfected eczema · Known hypersensitivity to macrolides or to any other excipient in tacrolimus 0.1% ointment · Known hypersensitivity to one of the agents contained in the fluticasone 0.005% ointment preparation · Ulcerated lesions · Moderate‐to‐severe acne or rosacea · Participation in another clinical study during or within 28 days prior to the study · Any type of substance abuse or any mental disorder/psychological state which, in the investigator's opinion, might interfere with the patient's follow‐up · Serologically‐proven HIV positivity Additional design details: none |
| Participants | Total number randomised: 577 participants Age: adults Sex, ethnicity: not reported Duration of eczema: at least 12 months Severity of eczema: moderate‐to‐severe Body site: facial dermatitis (except eyelids) Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.1% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: fluticasone 0.005% ointment used twice daily for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: to quote: "Treatments were to be applied twice daily over all lesions on the face (except for the eyelids) until clearance, for a maximum of 3 weeks and then, in case of uncleared residual lesions, once daily for up to 3 further weeks." |
| Outcomes | · Percentage of participants with ≥ 60% improvement in mEASI · Percentage of participants no longer presenting with 'facial' erythema · Assessment of facial pruritus · IGA of clinical response on the 'face' |
| Starting date | 2004 |
| Contact information | Not reported |
| Notes | Funding source: Astellas Pharma Inc. Declarations of interest: not declared Original language of publication: English Other: none |
NCT00833079.
| Study name | Bioequivalence of two tacrolimus 0.1% topical ointment formulations in patients with atopic dermatitis |
| Methods | Trial design: randomised, quadruple‐blinded, parallel study Trial registration number: NCT00833079 Country, outpatient or hospital: not reported Date trial conducted: October 2008 to February 2010 Duration of trial participation: 2 weeks Inclusion criteria: · Male or non‐pregnant, non‐lactating female, ≥ 18 years · Unresponsive to therapies such as topical corticosteroids, or, in the investigators opinion, such first‐line therapy would be deemed inadvisable · If female and of childbearing potential, using a reliable method of contraception during the study · AD according to Hanifin and Rajka criteria, moderate severity or severe (IGSA) · BSA ≥ 20% · EASI ≥ 15 Exclusion criteria: · Clinically infected AD · Any additional dermatological condition that, in the Investigator's opinion, may interfere with study evaluations · Pregnancy or breastfeeding · History of allergy or sensitivity to tacrolimus, pimecrolimus, or any macrolides such as clindamycin or erythromycin · Diagnosis or history or any disease, which, in the investigators opinion, would contraindicate the use of immunosuppressants · Use of nonsteroidal immunosuppressants · Regular use of intranasal or inhaled corticosteroids, greater than the equivalent of 2 mg of prednisone/day, within 14 days of the study · Use of non‐sedating histamines for 7 days prior to and during the study Additional design details: none |
| Participants | Total number randomised: 500 participants Age: ≥ 18 years Sex: all Ethnicity, duration of eczema: not reported Severity of eczema: IGSA of 3 (moderate) or 4 (severe) Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.1% ointment used twice daily for 14 days · Concurrent treatment: not reported · Other key information: two different types of tacrolimus used in this study combined into one arm for the purposes of our review. One is manufactured by Taro; one Protopic. Comparator: vehicle ointment used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Number of participants with ISGA score of 0 or 1 ·% change in BSA, in EASI AND in ISGA · Safety and adverse AE profile |
| Starting date | 2008 |
| Contact information | Not reported |
| Notes | Funding source: Taro Pharmaceuticals USA Declarations of interest: not declared Original language of publication: English Other: none |
NCT00944632.
| Study name | Dose escalation of different concentrations of ZK 245186 in atopic dermatitis |
| Methods | Trial design: randomised, quadruple (participant, care provider, investigator, outcomes assessor), blind, parallel study Trial registration number: NCT00944632 Country: South Africa Outpatient or hospital: not reported Date trial conducted: July 2009 to September 2010 Duration of trial participation: 4 weeks Inclusion criteria: · AD according to Hanifin and Rajka criteria · Mild‐to‐moderate AD · Washout periods for systemic and topical treatments for AD · Females must use effective contraception. Exclusion criteria: · Pregnant or lactating women · Conditions that, in the opinion of the investigator, may pose a risk or interfere with the evaluation of the study · Wide‐spread AD requiring systemic treatment · Immunocompromised · Skin diseases other than AD in the treatment area Additional design details: none |
| Participants | Total number randomised: 64 participants Age: 18 to 55 years Sex: all Ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐moderate Body site, number of withdrawals: not reported Notes: |
| Interventions | Run‐in details: not reported Intervention: ZK 245186 0.01% ointment used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention: 0.1% ointment used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention: 0.% ointment used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle 0.03% ointment used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Assessment of the severity of AD at target lesion, based on clinical signs of inflammation · IGA |
| Starting date | 2009 |
| Contact information | Not reported |
| Notes | Funding source: Bayer Declarations of interest: not declared Original language of publication: English Other: none |
NCT00946478.
| Study name | Effect of pimecrolimus cream on cathelicidin levels in subjects with eczema |
| Methods | Trial design: randomised, double‐blind study Trial registration number: NCT00946478 Country: US Outpatient or hospital, date trial conducted, duration of trial participation: not reported Inclusion criteria: · Age 18 to 70 years · Target lesion IGA ≥ 2 · Target IGA = 0 (for non‐lesional site) · Male or female of any race and ethnicity · Chronic AD for more than one year duration · Of childbearing potential; must be willing to practice effective birth control during the study Exclusion criteria: · Patients ≥ 18 years of age with only AD of the face · Women of childbearing potential not using the contraception method(s) specified in this study · Severe medical condition(s) that, in the view of the investigator, prohibits participation in the study · Hypersensitivity to pimecrolimus cream or any excipient of the cream · A skin disorder in addition to dermatitis in the areas to be treated · Netherton's syndrome or other genodermatoses that result in a defective epidermal barrier · Pregnant or nursing females · Immunocompromised patient, or with a history of active or malignant disease (excluding non‐melanoma skin cancer) · History of psychiatric disease or history of alcohol or drug abuse that would interfere with the ability to comply with the study protocol · Significant concurrent medical condition(s) at screening that, in the view of the investigator, prohibits participation in the study · Active viral or fungal skin infections at the target areas · Use of any oral or topical antibiotic during the study and up to one week prior to entering the study · Use of any local therapy for AD less than one week prior to screening · Use of any systemic immunosuppressive therapy for AD fewer than four weeks prior to screening Additional design details: none |
| Participants | Total number randomised: 40 participants (20 to apply the intervention and 20 to apply the comparator) Age: 18 to 65 years: intervention group mean 26.2 years (SD 8.4); vehicle group 28.6 years (SD 9.6) Sex: intervention group: male n = 9, female n = 7, unknown n = 4; vehicle group: male n = 6, female n = 7, unknown n = 7 Ethnicity, duration of eczema, severity of eczema, body site: not reported Number of withdrawals: no withdrawals Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus cream 1.% cream used twice daily for up to 21 days · Concurrent treatment: not reported · Other key information: Comparator: 0.% cream used twice daily for up to 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Cathelicidin mRNA expression levels · AE |
| Starting date | Not reported |
| Contact information | Not reported |
| Notes | Funding source: Novartis Pharmaceuticals Corporation and University of California, San Diego Declarations of interest: not declared Original language of publication: English Other: none |
NCT00980135.
| Study name | Effect of pimecrolimus cream on cathelicidin levels in subjects with eczema |
| Methods | Trial design: randomised parallel Trial registration number: NCT00980135 Country: Germany Outpatient or hospital: not reported Date trial conducted: November 2009 to May 2010 Duration of trial participation: 29 days Inclusion criteria: · Male or female Caucasians · Aged between 18 and 65 years · Mild AD presenting a scoring AD (SCORAD) rating between 3 and 25 · Skin type I‐IV according to Fitzpatrick · Acute AD symptoms in each assessment areas (local SCORAD ≥ 3 and ≤ 12) · Acute symptom of pruritus Exclusion criteria: · Any other skin disease at the test area that would interfere with the clinical assessment in the opinion of the investigator · Moles, tattoos, strong pigmentation, or scars at the test area that would interfere with the clinical assessment · Regular intake of antiphlogistic drugs · Any condition or treatment which might influence the trial within 14 days prior to screening as well as during the trial (exception: topical treatment of AD lesions other than the test areas (for example, face) with low potency steroids restricted to small areas) · UV‐therapy or the use of solarium within 30 days before screening as well as during the trial · Any alternative treatment for AD within 30 days before screening as well as during the trial Additional design details: none |
| Participants | Total number randomised: 40 participants Age: 18 to 65 years Sex: male or female Ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Total number randomised: 40 participants Age: 18 to 65 years Sex: male or female Ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Outcomes | · Efficacy rate versus comparator and untreated skin · Local side effects on the skin [ · Local SCORAD |
| Starting date | 2009 |
| Contact information | Not reported |
| Notes | Funding source: Bayer Declarations of interest: not reported Original language of publication: English Other: none |
NCT01228513.
| Study name | Efficacy and safety of different concentrations of ZK245186 in atopic dermatitis |
| Methods | Trial design: randomised, quadruple (participant, care provider, investigator, outcomes assessor), parallel study Trial registration number: NCT01228513 Country: Japan, US Outpatient or hospital: not reported Date trial conducted: November 2010 to July 2011 Duration of trial participation: 4 weeks Inclusion criteria: · AD according to Hanifin and Rajka criteria · BSA affected by AD at or less than 15% at start of treatment Exclusion criteria: · Pregnancy and breastfeeding · Conditions that may pose a threat to the patient or affect the outcome of the study · Wide‐spread atopic dermatitis (AD) requiring systemic treatment · Immunocompromised conditions · At least 2 weeks after local AD treatment and treatment with systemic antibiotics · At least 1 month after systemic AD treatment Additional design details: none |
| Participants | Total number randomised: 263 participants Age: 18 to 55 years Sex: all Ethnicity, duration of eczema: not reported Severity of eczema: BSA 15% or less Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention one: ZK245186 0.01% ointment used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention two: ZK245186 0.03% ointment used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention three: ZK245186 0.1% ointment used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle 0.% ointment used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI · Self‐assessment of pruritus |
| Starting date | 2010 |
| Contact information | Not reported |
| Notes | Funding source: Bayer Declarations of interest: not declared Original language of publication: English Other: none |
NCT01359787.
| Study name | Efficacy and safety of different concentrations of mapracorat ointment over 4 weeks in atopic dermatitis |
| Methods | Trial design: randomised, quadruple (participant, care provider, investigator, outcomes assessor), blind, parallel study Trial registration number: NCT01359787 Country: Germany Outpatient or hospital: not reported Date trial conducted: May to September 2011 Duration of trial participation: 4 weeks Inclusion criteria: · AD according to Hanifin and Rajka criteria · Willingness to avoid excessive exposure of diseased areas to natural or artificial sunlight Exclusion criteria: · Pregnancy and breastfeeding · Clinically relevant disease, which could interfere with the study conduct or the evaluation and interpretation of the study results · Clinically manifested immunosuppressive disorder or known history of malignant disease · History of relevant drug and/or food allergies Additional design details: none |
| Participants | Total number randomised: 197 participants Age: 18 to 65 years Sex: all Ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention one: mapracorat 0.01 % ointment used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention two: mapracorat 0.03 % ointment used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention three: mapracorat 0.1 % ointment used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI · Self‐assessed pruritus ‐ VAS |
| Starting date | 2011 |
| Contact information | Not reported |
| Notes | Funding source: Bayer Declarations of interest: not declared Original language of publication: English Other: none |
NCT01567995.
| Study name | Randomised, double‐blind, placebo‐controlled study of topical clobetasone butyrate 0.05% cream in subjects with eczema for two weeks to evaluate the efficacy and safety |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: NCT01567995 Country: China Outpatient or hospital: not reported Date trial conducted: February 2008 to February 2009 Duration of trial participation: 2 weeks Inclusion criteria: · Diagnosis of eczema, according to: 1) erythema, papilla/water blister, lichenification, skin damage with infiltration, 2) unknown reason, recurrent attacks; 3) itching in diseased skin · BSA disease involvement of less than or equal to 10% as assessed by palm method · Present with moderate and above eczema as defined by a score greater than or equal to 3 using the IGA of eczema severity Exclusion criteria: · Any systemic disorder or active skin disease that would in any way confound interpretation of the study results or subjects who present with scars, moles, tattoos, body piercings, sunburn in the test area which could interfere with the assessment of lesions at screening · Eczema restricted to the face, the feet or the hands only · Any anti‐infective drug for a current complication of overt bacterial, fungal and viral infection · History of recent (< 1 month) active or presence of current superficial skin infections of viral aetiology such as herpes simplex, or varicella · Exposure to therapy within the set time frame of: topical agents administered to the diseased skin, including emollient ‐ 1 week; systemic administration of antihistamine agents ‐ 2 weeks; systemic administration of corticosteroids ‐ 4 weeks; systemic administration of immunosuppressive drugs ‐ 4 weeks; UV therapy ‐ 4 weeks · Foreseeable intensive UV exposure during the study. Subjects must not be exposed to intense direct sunlight for long periods, and must not use skin tanning devices for the duration of the study. · History of clinically significant cardiovascular, pulmonary, gastrointestinal, liver, neurological, renal or haematological abnormalities · History of allergy to components of test medications · History of anaphylaxis to food, medications, insect venom, or latex Additional design details: none |
| Participants | Total number randomised: 240 participants Age: 12 to 65 years Sex, ethnicity, duration of eczema: not reported Severity of eczema: moderate‐to‐severe eczema defined by IGA score greater than or equal to 3 Body site: not reported Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: clobetasone butyrate 0.05% cream for 14 days · Concurrent treatment: not reported · Other key information: Comparator: 0.% used for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Change from baseline in EASI score · Reduction percentage of EASI score after 7 days of treatment |
| Starting date | 2008 |
| Contact information | Not reported |
| Notes | Funding source: GlaxoSmithKline Declarations of interest: not declared Original language of publication: English Other: none |
NCT01993420.
| Study name | A safety and efficacy of DRM02 in subjects with atopic dermatitis |
| Methods | Trial design: randomised, parallel‐group, triple‐masked (participant, investigator, outcome assessor), placebo‐controlled phase 2 trial Trial registration number: NCT01993420 Country: Canada Outpatient or hospital: not reported Date trial conducted: October 2013 to February 2014 Duration of trial participation: 6 weeks Inclusion criteria: · Male or female 18 to 70 years old · Clinical diagnosis of stable AD with two lesions of similar size and an identical EASI score of 5‐9 at the target lesion · Male or non‐pregnant, non‐lactating females Exclusion criteria: · Significant infection at the target lesion · Prior or concomitant use of systemic therapies for AD within the past 4 weeks · Prior or concomitant use of topical treatments for AD within 10 cm of the target lesion within the past 4 weeks · Prior or concomitant use of oral retinoids for AD within the last 6 months · Use of biologics for AD within the past 3 months, or 5 half‐lives (whichever is longer) · Poor skin condition within 5 cm of the target lesion · Current drug or alcohol abusers; have a history of immunodeficiency disease; or are at poor medical risk because of other systemic diseases or active uncontrolled infections · Unstable medical condition or a medical condition not adequately controlled with standard medical therapy · Participating in an experimental therapy study or who have received experimental therapy within 30 days · Clinically significant laboratory value at screening Additional design details: none |
| Participants | Total number randomised: 21 participants Age: 18 to 70 years Sex: either Ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: DRM02 0.25% gel used twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle gel used twice daily for 42 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Physician's lesion assessment for treatment effect · Change in physician's lesion assessment · Physician's lesion assessment dichotomised into "success" and "failure" · Severity of target lesion EASI |
| Starting date | 2013 |
| Contact information | None |
| Notes | Funding source: Dermira, Inc. Declarations of interest: none declared Original language of publication: English Other: none |
NCT02079688.
| Study name | Efficacy, safety, tolerability, pharmacokinetics and pharmacodynamics study of the topical formulation SB011 applied to lesional skin in patients with atopic eczema |
| Methods | Trial design: randomised, double‐blind (investigator, outcomes assessor), parallel study Trial registration number: NCT02079688 Country: Germany Outpatient or hospital: Division for Immunodermatology and Allergy Research Clinic for Dermatology, Allergy and Venereology, Hannover Medical School Date trial conducted: February 2014 to November 2016 Duration of trial participation: 15 days Inclusion criteria: · Caucasian male or female aged 18 to 69 years · Smoking ≤ 10 cigarettes/day · AD according to Hanifin and Rajkaa criteria · SCORAD 20‐50 (mild‐to‐moderate severity) · Two comparable lesional areas of approximately 50 cm2 each on the arms, legs, chest, stomach or neck, clinical condition of AD defined by a modified local SCORAD of 7‐10 with 2 parameters (erythema and one another) ≥ 2 · Increased total IgE, increased specific IgE of ≥ 1 of the sx1 allergens with CAP classification II [> 0.7 KU/L] · TEWL in lesional areas ≥ 12 g/m²h · Except for AD, asthma‐like AD or allergic rhinitis assessed as healthy based on a screening examination · If of childbearing potential, both male and female participants must agree to use two methods of contraception. Exclusion criteria: · History of allergic reactions to any component of the IMP · Presence of clinically significant diseases other than asthma or atopic diseases which may influence the results of the trial · Inherent or acquired immune deficiency, immune deficiency as a consequence of drug use · Immune‐mediated diseases · Suntan, hyperpigmentation or tattoos in the test fields · Skin colour that may affect assessment of skin reactions · Systemic bacterial, mycotic, or severe viral systemic infections or other severe systemic disease · Resting heart rate < 50 and > 100 bpm, systolic blood pressure < 100 and > 150 mmHg, diastolic blood pressure < 60 and > 95 mmHg · UV therapy within 6 weeks · History or current evidence of a malignant tumour · Clinically relevant abnormalities in serology, clinical, chemical, haematological, or in any other laboratory variables · Chronic or acute infections · Pregnancy or breastfeeding · Secondary infections on the lesions to be treated · History of previous administration of SB011 or any other registered or investigational oligonucleotide‐based drug · History or presence of alcohol or drug abuse, consumption of alcohol within 48 h before administration of IMPs and during the trial · Use of any medication except allowed concomitant medication within 2 weeks · Treatment with known cytochrome P450 enzyme inducing or inhibiting agents within 30 days of the trial · Consumption of grapefruit, grapefruit juice within 14 days · Requirement for skin care products in the treatment area(s) · Use of skin care products with antiseptic components during the last four weeks or antiseptic textiles with contact with the target lesions · Proneness to orthostatic dysregulation, fainting, or blackouts Additional design details: none |
| Participants | Total number randomised: 25 participants Age: 18 to 69 Years Sex: all Ethnicity: Caucasian Duration of eczema: ≥ 12 weeks Severity of eczema: SCORAD 20‐50 Body site: arms, legs, chest, stomach or neck Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: SB011, 2% (water/oil/water) emulsion of hgd40, twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: 0.% cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · SCORAD · Pruritus (self‐assessed; 10‐point rating scale) · Subjective efficacy assessment · Adverse events |
| Starting date | 2014 |
| Contact information | Not reported |
| Notes | Funding source: Sterna Biologicals GmbH & Co. KG Declarations of interest: not declared Original language of publication: English Other: none |
NCT02219633.
| Study name | Effect of LEO 39652 cream in adults with mild to moderate Atopic Dermatitis |
| Methods | Trial design: randomised, parallel study Trial registration number: NCT02219633 Country: Germany Outpatient or hospital: not reported Date trial conducted: July 2014 to January 2015 Duration of trial participation: 22 days Inclusion criteria: · AD according to Hanifin and Rajka criteria and with mild‐to‐moderate disease severity (IGA 2 or 3) · Two symmetrical and comparable entire treatment areas, on the same body region (left and right part) · In good health · Female of childbearing potential and male subjects must be willing to consent to using highly effective methods of contraception Exclusion criteria: · Any condition in the treatment areas that, in the opinion of the investigator, could interfere with clinical assessments, e.g. acne, infection, rash (other than atopic dermatitis), sunburn, hyper‐or hypopigmentation, scars · Dark‐skinned persons (i.e. skin type IV to VI according to Fitzpatrick classification system) whose skin colour prevents reliable clinical assessments · Any permanent (or transient within 28 days prior to dosing) disease (in particular cardiac disease such as heart failure or history of myocardial infarction) that may interfere with the subjects' safe participation in the trial, with the subjects' ability to participate in the trial or with the clinical assessments · Congenital or acquired immunodeficiencies or in subjects on therapy that causes immunosuppression Additional design details: |
| Participants | Total number randomised: 30 participants Age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: Leo 39652 0.% cream used for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: 0.% cream used for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Total sign score at end of treatment · Investigator's treatment area assessment of disease severity · Participants treatment area assessment of disease severity and assessment of itching · Transepidermal water loss |
| Starting date | 2014 |
| Contact information | Not reported |
| Notes | Funding source: LEO Pharma Declarations of interest: not declared Original language of publication: English Other: unclear if the intervention is an anti‐inflammatory |
NCT02496546.
| Study name | An explorative trial evaluating the effect of LEO 32731 cream in adults with mild to moderate atopic dermatitis |
| Methods | Trial design: randomised, parallel study Trial registration number: NCT02496546 Country: Germany Outpatient or hospital: Proinnovera GmbH, Center of Dermatology Excellence, Münster Date trial conducted: July 2015 to March 2016 Duration of trial participation: 3 weeks Inclusion criteria: · Males ≥ 18 years · AD according to Hanifin and Rajka criteria and with mild‐to‐moderate disease severity (IGA 2 or 3) · Two symmetrically located and comparable target areas of 20 to 50 cm2 each, i.e. on the same body region (left and right part) · TSS ≥ 5 on both target areas with difference < 2 between them, sign score for erythema ≥ 2 on both target areas, and difference in investigator's assessment of severity ≤ 1 between them Exclusion criteria: Any other condition in the treatment areas that could interfere with clinical assessments, e.g. acne, infection, rash, sunburn, hyper‐ or hypopigmentation, or scars Additional design details: none |
| Participants | Total number randomised: 30 participants Age: not reported Sex: male Ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: orismilast 2.% cream used for 21 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used for 21 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Individual sign scores (4‐point scale) of erythema, oedema/papulation, oozing/crusting, excoriations, lichenification and dryness for each area · Total sign score: sum of individual sign scores · Investigator's assessment of disease severity |
| Starting date | 2015 |
| Contact information | Not reported |
| Notes | Funding source: LEO Pharma Declarations of interest: not declared Original language of publication: English Other: unclear if the intervention is an anti‐inflammatory |
NCT02601703.
| Study name | Tacrolimus ointment, 0.1% in the treatment of moderate to severe atopic dermatitis |
| Methods | Trial design: randomised, triple‐blinded (participant, investigator, outcome assessor), multicentre, parallel study Trial registration number: NCT02601703 Country: US Outpatient or hospital: multiple unspecified locations Date trial conducted: August 2015 to October 2016 Duration of trial participation: 15 days Inclusion criteria: · Non‐immunocompromised male or non‐pregnant, non‐lactating female, ≥ 18 years old · AD according to Hanifin and Rajka criteria and for at least 3 months · IGA of 3‐4 (moderate‐severe) · BSA ≥ 20% · Treated with a bland emollient for at least 7 days Exclusion criteria: · Active cutaneous bacterial or viral infection in any treatment area · Sunburn, extensive scarring, or pigmented lesion(s) in any treatment area · History of confounding skin conditions, e.g. psoriasis, rosacea, erythroderma, or ichthyosis · History or presence of Netherton's Syndrome, immunological deficiencies or diseases, HIV, diabetes, malignancy, serious active or recurrent infection, clinically significant severe renal insufficiency or severe hepatic disorders · Allergy or hypersensitivity to tacrolimus or any other component of the study products Additional design details: the study compared a branded versus generic tacrolimus 0.1% ointment to placebo. For the purposes of this study, the trial was treated as a pairwise comparison of tacrolimus 0.1% ointment versus placebo; the data from both tacrolimus arms being pooled together |
| Participants | Total number randomised: 1110 participants Age: ≥ 18 years Sex: All Ethnicity: not reported Duration of eczema: at least 3 months Severity of eczema: IGA score of 3‐4 (moderate‐severe). Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.1% ointment · Concurrent treatment: not reported · Other key information: none Comparator: placebo ointment · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA of 0 or 1 within all treatment areas · Change in signs and symptoms from baseline (erythema, induration/papulation, lichenification and pruritus) |
| Starting date | 2015 |
| Contact information | Not reported |
| Notes | Funding source: Glenmark Pharmaceuticals Ltd. India Declarations of interest: none declared Original language of publication: English Other: none |
NCT02732314.
| Study name | Efficacy and tolerability of new topical formulations in subjects with atopic dermatitis |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: NCT02732314 Country: US Outpatient or hospital: not reported Date trial conducted: March 2016 to June 2016 Duration of trial participation: 84 days Inclusion criteria: · Generally healthy, male or female, 12 to 65 years old · Moderate or greater AD as determined by the Physician's Global Assessment (PGA of 3 or 4) Exclusion criteria: · Currently participating or has participated in another interventional clinical study at this or any other facility in the past 2 weeks · Currently or has been diagnosed or treated for cancer in the past 5 years · Requires any topical or systemic medications that could affect the course of their AD during the study period (except inhaled steroids and/or stable antihistamines for asthma or allergies) · A known hypersensitivity to any corticosteroid creams · Known sensitivity to epinephrine, xylocaine or topical antibiotics · A wound‐healing or blood‐clotting abnormality · Active infections or has used antibiotics in the past 7 days · Physical attributes or skin conditions that might interfere with the clear visual or instrumental assessments · Immunologic or infectious disease which could place the subject at risk or interfere with the accuracy of the study results · Used any immunosuppressant drugs or immunotherapy within the past 30 days or 5 half‐lives · Diabetic · Dependent on oral medication for any skin disease/condition or could not, in the opinion of the investigator, tolerate the restriction of discontinuing the medicine as required in this study · Currently pregnant or lactating or planning to become pregnant in the next 6 months · History of keloid formation following skin injury · Routinely taking anticoagulant medications · Any other condition or factor the investigator or their duly assigned representative believes may affect the ability of the subject to complete the study or the interpretation of the results Additional design details: none |
| Participants | Total number randomised: 53 participants Age, sex, ethnicity, duration of eczema: not reported Severity of eczema: with AD SCORAD >16 Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: One week washout Intervention: desonide 0.0 5% cream used twice daily for 84 days · Concurrent treatment: not reported · Other key information: none Comparator: placebo cream 0.% cream used twice daily for 84 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: throughout the treatment phase, the subjects were required to use only the provided bar soap for bathing and showering and to apply their test product twice per day, once in the morning and once in the evening. Notes: none |
| Outcomes | SCORAD |
| Starting date | 2016 |
| Contact information | Not reported |
| Notes | Funding source: Procter & Gamble Beauty Declarations of interest: not declared Original language of publication: English Other: none |
NCT02865356.
| Study name | Topical solution for the treatment of atopic dermatitis (CYCLATOP) |
| Methods | Trial design: randomised, double‐blind, multicentre study Trial registration number: NCT02865356 Country: Spain Outpatient or hospital: not reported Date trial conducted: September 2016 to April 2017 Duration of trial participation: unclear Inclusion criteria: · Male or female aged between 2 and 75 years · AD diagnosis according to the Hanifin and Rajka criteria with one eczema outbreak at the screening and baseline · Presence of at least two lesional areas. These areas should be at the left and right side of the body and occupying a BSA in each side between 0.1% and 10% of the BSA, with IGA score of the two sites not differing by more than 1 point. · Mild‐to‐moderate disease severity of AD defined by an IGA score of 2 or 3 at baseline (IGA scale from 0 to 4) · Total BSA of AD involvement ≤ 10% on each side of the body (≤ 20% maximum) · Normal weight as defined by a Quetelet Index · General medical condition, in the investigator's opinion, which does not interfere with the assessments and the completion of the trial Exclusion criteria: · Female of childbearing potential without the use of effective birth control methods, or not willing to continue practising these birth control methods for at least 30 days after the end of the treatment period · Pregnant woman or with a positive pregnancy test or breastfeeding at baseline · Any condition in the lesions that, in the opinion of the investigator, could interfere with clinical assessments, e.g. acne, infection, rash other than AD, sunburn, scars, hairy or tattooed area · Receiving phototherapy or systemic therapy for AD within 4 weeks · Receiving antibiotics, topical therapy for AD within 2 weeks · Taking antihistamines within 1 week · Receiving radiation therapy, systemic therapy with cytostatics or biological therapy within 24 weeks, or with previous history of malignancy (excluding basal cell carcinoma) · Any acute skin infection · Confirmed hypertension, renal disease or serious infections at screening. Currently active allergy such as, but not limited to, drug allergy, food allergy or hay fever · Previously demonstrated clinically significant allergy or hypersensitivity to any of the excipients of the trial medication administered in this trial · An investigational drug trial within 30 days · Any condition which, in the opinion of the investigator, could compromise the patient safety or compliance with trial procedures · Received or are planning to receive any vaccination within 28 days Additional design details: none |
| Participants | Total number randomised: 36 sides treated Age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: cyclosporine 5.% other formulation used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: 0.% other formulation used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Change in EASI score of the treated body area · Change in IGA score |
| Starting date | 2017 |
| Contact information | Not reported |
| Notes | Funding source: Spherium Biomed Declarations of interest: not declared Original language of publication: English Other: none |
NCT02949960.
| Study name | DMT210 topical gel in the treatment of atopic dermatitis |
| Methods | Trial design: randomised, quadruple‐blind (participant, care provider, investigator, outcome assessor), parallel study Trial registration number: NCT02949960 Country: US Outpatient or hospital: not reported Date trial conducted: October 2016 to July 2017 Duration of trial participation: 28 days Inclusion criteria: · Male or female 12 years or older · Chronic, stable AD for ≥ 3 months, with 5 to 35% (inclusive) BSA · Presence of two similar target lesions; one lesion within each of the two treatment areas Exclusion criteria: Use of topical therapies for AD within the treatment areas within 2 weeks of the study Additional design details: none |
| Participants | Total number randomised: 19 participants Age: ≥ 12 years Sex: all Ethnicity: not reported Duration of eczema: ≥ 3 months Severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: DMT210 5.% gel used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle gel used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · ADSI score of each target lesion · Change in individual signs and symptoms of each target lesion from baseline · IGA of the treatment area · AE |
| Starting date | 2016 |
| Contact information | Not reported |
| Notes | Funding source: Dermata Therapeutics Declarations of interest: not declared Original language of publication: English Other: none |
NCT03002571.
| Study name | Efficacy and safety of IDP‐124 lotion |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: NCT03002571 Country: USA Outpatient or hospital: not reported Date trial conducted: April 2018 to April 2020 Duration of trial participation: not reported Inclusion criteria: · Moderate‐to‐severe AD · Male or female ≥ 2 years old · Non‐immunocompromised and failed to respond to other topical treatment for AD or for whom they are not advisable Exclusion criteria: · Pregnancy, planning a pregnancy (females), or breastfeeding · Active clinical bacterial or viral infection in any treatment area · Sunburn, extensive scarring, or pigmented lesion(s) in any treatment area likely to interfere with evaluations · Confounding skin conditions, e.g. psoriasis, rosacea, erythroderma, or ichthyosis (other than ichthyosis vulgaris) Additional design details: none |
| Participants | Total number randomised: 338 participants Age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: pimecrolimus lotion for 6 weeks · Concurrent treatment: not reported · Other key information: IDP‐124 is pimecrolimus Comparator: vehicle lotion for 6 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | IGA |
| Starting date | 2018 |
| Contact information | Not reported |
| Notes | Funding source: Bausch Health Americas, Inc. Declarations of interest: not declared Original language of publication: English Other: none |
NCT03058783.
| Study name | Efficacy and safety of IDP‐124 lotion for the treatment of moderate to severe atopic dermatitis in pediatric and adult subjects |
| Methods | Trial design: randomised, quadruple‐blinded study Trial registration number: NCT03058783 Country: US Outpatient or hospital: not reported Date trial conducted: August 2018 to May 2020 Duration of trial participation: 6 weeks Inclusion criteria: Male or female at least 2 years of age and older with moderate‐to‐severe AD Exclusion criteria: · Females who are pregnant, breastfeeding, or who wish to become pregnant during the study period · Active cutaneous bacterial or viral infection in any treatment area · Sunburn, extensive scarring, or pigmented lesion(s) in any treatment area · History of confounding skin conditions, e.g. psoriasis, rosacea, erythroderma, or ichthyosis (other than ichthyosis vulgaris) · History or presence of: ‐ basal cell carcinoma of skin, effectively treated more than 2 years ago ‐ carcinoma of cervix, effectively treated more than 5 years ago ‐ immunological deficiencies or diseases, HIV, or serious recurrent infection ‐ clinically significant severe renal insufficiency or severe hepatic disorders · Current or recent serious infection · Non‐immunocompromised who failed to respond adequately to other topical prescription treatment for AD or for whom those treatments are not advisable Additional design details: none |
| Participants | Total number randomised: 328 participants Age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: IDP‐124 · Concurrent treatment: not reported · Other key information: IDP is of unknown mode of action Comparator: 0.% lotion · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Per cent of subjects with treatment success at week 6 |
| Starting date | 2018 |
| Contact information | Not reported |
| Notes | Funding source: Bausch Health Americas, Inc. Declarations of interest: the study director is from Valent Pharmaceuticals Original language of publication: English Other: none |
NCT03394677.
| Study name | Study of RVT‐501 topical ointment in pediatric patients with atopic dermatitis |
| Methods | Trial design: randomised, double‐blind, multicentre study Trial registration number: NCT03394677 Country: USA and Canada Outpatient or hospital: not reported Date trial conducted: January to July 2018 Duration of trial participation: 4 weeks Inclusion criteria: · Male or female aged 2 to 17 years · AD according to Hanifin and Rajka criteria · 5‐40% BSA (excluding scalp, palms, and soles) · IGA of 2‐3 (mild‐moderate severity) · Females of childbearing potential must have a negative pregnancy test, and both female and male patients must use an accepted birth control method during the study and for 2 weeks after. · Stable disease for at least 1 month Exclusion criteria: · Positive hepatitis B surface antigen, hepatitis C antibody, or HIV antibody test · Normal alanine aminotransferase, aspartate aminotransferase, and total bilirubin levels · Kaposi's varicelliform eruption, scabies, molluscum contagiosum, impetigo, psoriasis, severe acne, connective tissue disorder, or Netherton's syndrome, or any other disease that could impact study evaluations · Systemic or topical corticosteroids, immunosuppressants, excessive sun exposure, tanning booth, or phototherapy including psoralen and ultraviolet A therapy, herbal medicines, unless specifically approved by the sponsor, crisaborole or other topical PDE4 inhibitor, tacrolimus and pimecrolimus, antibiotics, antifungal or antivirus medications, antihistamines (except loratadine, fexofenadine hydrochloride, and cetirizine hydrochloride) · Pregnancy or breastfeeding · History of or risk of sensitivity to the study medications · Received an investigational product within the following time period prior to the study: 30 days, 5 half‐lives, or twice the duration of the biological effect of the investigational product (whichever is longer) · History of cancer within the last 5 years · Active infection in AD · Pruritus due to conditions other than AD · Advanced disease or recent abnormal laboratory test values that could affect the safety of the patient or the implementation of this study · History of serious hypersensitivity to PDE4 inhibitors · Evidence of significant hepatic, renal, respiratory, endocrine, haematologic, neurologic, psychiatric, or cardiovascular system abnormalities or laboratory abnormalities that will affect the health of the patient or interfere with interpretation of the results Additional design details: none |
| Participants | Total number randomised: 110 participants Age: 2 to 17 years Sex: male or female Ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐moderate (5 to 40% BSA) and an IGA of 2 or 3 at baseline Body site: not reported Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: E6005 0.5% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA · EASI · Peak Pruritis Numeric Rating Scale · BSA · Frequency and severity of adverse events · Plasma concentrations of E6005/RVT‐501 and M11 metabolite |
| Starting date | 2018 |
| Contact information | Not reported |
| Notes | Funding source: Dermavant Science GmbH Declarations of interest: not declared Original language of publication: English Other: none |
NCT03832010.
| Study name | Steroid‐reducing effects of Crisaborole |
| Methods | Trial design: randomised, triple‐blinded study Trial registration number: NCT03832010 Country: US Outpatient or hospital: not reported Date trial conducted: December 2019 to February 2024 Duration of trial participation: not reported Inclusion criteria: · Children aged 2 or older (< 18) · Diagnosed with AD mild‐to‐moderate on the IGA Exclusion criteria: · Known allergy to a constituent of the studied products · AD severe on the IGA scale · Medical problems which interfere with completion of protocols in this study · Pregnant or lactating females Additional design details: none |
| Participants | Total number randomised, age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention one: crisaborole 2.% · Concurrent treatment: not reported · Other key information: none Intervention two: vehicle 0.% · Concurrent treatment: not reported · Other key information: none Intervention three: emolient 0.% · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: hydrocortisone for affected areas with eczema on face, armpits or groin; triamcinolone for affected areas on the body excluding face, armpits or groin; moisturise all over the body with Aquaphor Notes: The trial had three arms where the formulations of the treatments per arm were not clear and also whether all participants per arm received, in addition to the concurrent treatments, the comparator treatments. |
| Outcomes | · Steroid usage · Eczema severity using SCORAD · Quality of life · Severity of itching |
| Starting date | 2019 |
| Contact information | Not reported |
| Notes | Funding source: Johns Hopkins University, Pfizer Declarations of interest: not declared Original language of publication: English Other: none |
NCT03868098.
| Study name | Different application rates of crisaborole ointment 2% in adults with mild to moderate atopic dermatitis |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: NCT03868098 Country: Canada Outpatient or hospital: not reported Date trial conducted: May 2019 to November 2020 Duration of trial participation: 15 days Inclusion criteria: · Confirmed diagnosis of active AD with at least a 6‐month history prior to screening and that has been clinically stable for 1 month · Has four application areas of 3 cm in diameter with a TSS of ≥ 5 and TAA of moderate at day 1. The application areas must not be located on the face, scalp, axillae, groin, genitals, hands, and feet. The application areas should be ≥ 1 cm apart. Exclusion criteria: · Female who is breastfeeding, pregnant, or who is planning to become pregnant during the study · Clinically infected AD · Fitzpatrick's Skin Phototype ≥ 5 · Known to have immune deficiency or is immunocompromised · History of cancer or lymphoproliferative disease within 5 years prior · Major surgery within 8 weeks prior to day 1 or a major surgery planned during the study · Any clinically significant medical condition that would, in the opinion of the investigator, put the subject at undue risk or interfere with interpretation of study results · Known history of chronic infectious disease · Known lack of efficacy with crisaborole · Known or suspected allergy to crisaborole · Known history of clinically significant drug or alcohol abuse in the last year Additional design details: Participant will receive 4 treatments: the 3 different application rates of the active treatment and the vehicle |
| Participants | Total number randomised: 124 sides treated (93 to apply the intervention and 31 to apply the comparator) Age: 18 years or older Sex: all eligible Ethnicity: not reported Duration of eczema: at least 6 months Severity of eczema: TSS of ≥ 5 and TAA of moderate Body site: the application areas must not be located on the face, scalp, axillae, groin, genitals, hands, and feet. The application areas should be ≥ 1 cm apart. Number of withdrawals: not reported Notes: |
| Interventions | Run‐in details: clinically stable AD for at least 1 month Intervention: crisaborole 2.% ointment used once daily for 15 days · Concurrent treatment: not reported · Other key information: the summary stated that application would occur once daily for 2 weeks but the purpose of the study was to look at 3 different application rates. These were not reported. Comparator: placebo ointment 0.% ointment used once daily for 15 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Total sign score · Target area assessment |
| Starting date | 2019 |
| Contact information | Not reported |
| Notes | Funding source: Innovaderm Research Inc. Declarations of interest: Industry‐funded Original language of publication: English Other: none |
NCT04008784.
| Study name | Improvement of short term outcome of mild to moderate atopic dermatitis using a combination of crisaborole and a concomitant topical corticosteroid over a 8 week period |
| Methods | Trial design: randomised, open‐label, single‐centre, parallel study Trial registration number: NCT04008784 Country: USA Outpatient or hospital: clinical research centre Date trial conducted: September 2019 to September 2020 Duration of trial participation: 8 weeks Inclusion criteria: · Male and female patients aged 2 to 79 years of age with confirmed diagnosis of AD · AD that has been clinically stable for ≥ 1 month · Total BSA of AD involvement ≤ 35%, excluding involvement of the scalp · Patient or patient's parent(s)/legal representative/guardian must be willing and able to apply study medications as directed, comply with study instructions, and commit to attending all visits. · Females of childbearing potential must use at least one highly effective method of birth control. Males with partners of childbearing potential should inform them of their participation in this clinical study and use highly effective methods of birth control during the study. Exclusion criteria: · Concurrent or recent use of certain topical or systemic medications or phototherapy without a sufficient washout period · Active or potentially recurrent dermatologic condition other than AD in the target lesion area that may confound evaluation · Significant confounding conditions · History or evidence of allergies requiring acute or chronic treatment · Pregnancy or lactation · History of sensitivity to the study medications, or components there of or a history of drug or other allergy · Active infection in AD areas requiring antibiotics, antifungals, or antiviral agents within 7 days · Pruritus due to conditions other than AD · History of and/or concurrent condition of serious hypersensitivity to PDE4 inhibitors · Use of any prohibited medication · Visible skin disease or damaged skin at the application site · Psoriasis and/or active AD/eczema · Not willing to refrain from using any topical/systemic analgesics such as aspirin · Pregnant, plan to become pregnant during the study, or are breastfeeding a child · Using medication which, in the opinion of the investigative personnel, will interfere with the study results, including anti‐inflammatory medications · Any known sensitivity to adhesives · Received treatment for any type of internal cancer within 5 years prior to study entry; or have a history of, or are currently being treated for skin cancer · Unstable AD or any consistent requirement for high‐potency topical corticosteroids to manage AD signs and symptoms · Any clinically significant medical disorder, condition, or disease or clinically significant physical examination Additional design details: none |
| Participants | Total number randomised: 16 participants (8 to apply the intervention and 8 to apply the comparator) Age: not reported Sex: male and female Ethnicity: not reported Duration of eczema: clinically stable for at least a month Severity of eczema: mild‐to‐moderate (BSA < 35% excluding the scalp) Body site: not reported Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: Washout period of up to 4 weeks prior to baseline Intervention: crisaborole 2.% ointment twice daily for 56 days · Concurrent treatment: not reported · Other key information: none Comparator: crisaborole 2.% ointment with triamcinolone acetonide 0.1% twice daily for 56 days · Concurrent treatment: not reported · Other key information: crisaborole 2% plus triamcinolone acetonide 0.1% ointment for the first 2 weeks, then crisaborole 2% alone for the following 6 weeks Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA · Improvement in patient itching |
| Starting date | 2019 |
| Contact information | Not reported |
| Notes | Funding source: Pfizer Declarations of interest: not declared Original language of publication: English Other: none |
NCT04194814.
| Study name | Skin bioMARkers for atopic eczema therapy evaluation |
| Methods | Trial design: randomised, observer‐blind study Trial registration number: NCT04194814 Country: UK Outpatient or hospital: dedicated skin barrier research suite Date trial conducted: November 2020 to September 2021 Duration of trial participation: 4 weeks Inclusion criteria: · AD defined according to the UK working party diagnostic criteria · Male or female aged 18 to 65 years old Exclusion criteria: · Known allergy/hypersensitivity to any of the excipients of the trial preparations · Acne, suntan, birthmarks, multiple nevi, tattoos, blemishes or dense body hair that obstruct the test areas · Pregnant female participants; breastfeeding female participants; and female participants of childbearing potential who are unwilling or unable to use a highly effective method of contraception as outlined in this protocol for the duration of the study and for at least 28 days after the last dose of investigational product · Use of any topical product on the test areas within 7 days, including cosmetic moisturisers and sunscreen · Used a tanning bed within 28 days of baseline · Used any medication that could interfere with the trial aim prior to the start of the study Additional design details: none |
| Participants | Total number randomised: 74 sides treated (37 to apply the intervention and 37 to apply the comparator) Age: 18 to 65 years Sex: male or female Ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: no topical product within 7 days of baseline Intervention: crisaborole 2.% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: betamethasone valerate 0.1% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Epidermal thickness · Erythema · TEWL ‐ skin barrier function · TEWL ‐ after tape‐stripping · Skin dryness · Natural moisturising factor |
| Starting date | 2020 |
| Contact information | Not reported |
| Notes | Funding source: Sheffield Teaching Hospitals NHS Foundation Trust Declarations of interest: none declared Original language of publication: English Other: none |
NCT04313400.
| Study name | Topically applied AMTX‐100 CF for adult patients with mild to moderate atopic dermatitis |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: NCT04313400 Country: USA Outpatient or hospital: unspecified investigational sites Date trial conducted: not reported Duration of trial participation: 28 days Inclusion criteria: · Male or female subjects ≥ 18 years · If female and fertile, a negative urine pregnancy test · Willing/able to comply with study requirements, including using an acceptable method of contraception if female and fertile · Physician‐diagnosed AD (Eichenfield‐revised criteria of Hannifin and Rajka), ≥ 6 months prior to the study · vIGA‐AD™ = 2 or 3 (mild‐to‐moderate) · EASI < 23 · BSA 5 to 70% (excluding the scalp, face, eyes, eyelids, neck, hands, palms, feet, groin, genitals or the axillae) appropriate for topical treatment Exclusion criteria: · Pregnant or breastfeeding women, or planning for pregnancy within 6 months · Women postpartum ≤ 3 months · Planned major surgical intervention within the study period · History of drug or alcohol abuse · Participation in a clinical trial within 3 months, or more than two clinical trials within 12 months · Concurrent or recent use of prescription moisturisers, topical steroids, topical immunosuppressive/immunomodulative drugs, topical vitamin D3 derivative, topical retinoids, anthralin, coal tar or salicylic acid within 14 days · Severe AD (vIGA‐AD™ > 3) · Any systemic treatments, immunotherapy, biologics or phototherapy for AD within 12 months or during the trial · Use of prohibited medications and procedures · Unstable AD or consistent requirement for high‐potency topical corticosteroids · Significant active systemic or localised infection, including infected AD · Regular use of a tanning booth/parlour within 4 weeks · Previous use of AMTX‐100 CF · Other medical or psychological conditions that may contraindicate participation or interfere with study evaluation · Concurrent contact dermatitis or history of anaphylaxis Additional design details: whilst authors described their study as a cross‐over, they described the second phase as a parallel‐group trial for the purposes of our review. |
| Participants | Total number randomised: not reported Age: ≥ 18 years eligible Sex: females and males both eligible Ethnicity: not reported Duration of eczema: at least 6 months Severity of eczema: mild‐to=moderate Body site: excluding the scalp, face, eyes, eyelids, neck, hands, palms, feet, groin, genitals or the axillae Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: none Intervention: AMTX‐100 0.11% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention: AMTX‐100 0.33% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention: AMTX‐100 1.1% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle 0.% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Number of subjects with vIGA‐AD™ of 0 (clear) or 1 (almost clear) and a reduction of ≥ 2 points |
| Starting date | 2020 |
| Contact information | Not reported |
| Notes | Funding source: Amytrx Therapeutics, Inc. Declarations of interest: not declared Original language of publication: English Other: none |
NCT04435392.
| Study name | Jaktinib hydrochloride cream for atopic dermatitis |
| Methods | Trial design: double‐blind randomised trial with two parts: (1) dose increasing; (2) dose extension Trial registration number: NCT04435392 Country: China Outpatient or hospital: not reported Date trial conducted: October 2020 onwards Duration of trial participation: not reported Inclusion criteria: · Aged 18 to 65 · Gender not restricted · Clinical diagnosis of AD · IGA of 2 (mild) or 3 (moderate) · BSA affected 10 to 20% Exclusion criteria: · Skin conditions/infections · Laboratory abnormalities · Females who are pregnant, breastfeeding, or are of childbearing potential without effective contraception · Other conditions (investigator's discretion) Additional design details: To quote: "In the dose‐increasing part, a multicenter, dose‐increasing, randomized, double‐blind, placebo‐parallel control design was adopted. Subjects were enrolled into different dose groups. Subjects in each group were observed for 4 weeks after administration". |
| Participants | Total number randomised: not reported Age: 18 to 65 years eligible Sex: not restricted Ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: jaktinib 0.5% cream used twice daily for 56 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 56 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: not reported |
| Outcomes | Number clear or almost clear (IGA) |
| Starting date | 2020 |
| Contact information | Not reported |
| Notes | Funding source: Suzhou Zelgen Biopharmaceuticals Co., Ltd. Declarations of interest: not declared Original language of publication: English Other: none |
NCT04598269.
| Study name | Study of ATI‐1777 in adult patients with moderate or severe atopic dermatitis |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: NCT04598269 Country: USA Outpatient or hospital: Aclaris investigational sites Date trial conducted: September 2020 to April 2021 Duration of trial participation: 6 weeks Inclusion criteria: · AD according to Hanifin and Rajka criteria · Males and females aged 18 to 65 years · Negative pregnancy test and 2 forms of effective contraception · 6‐month history of AD and no significant flares within the preceding 4 weeks · ≥ 1 representative lesion of 3 cm, not located on hands, feet or genitalia · Moderate or severe IGA (score 3 or 4) · 3‐20% BSA · Willing to refrain from moisturisers, emollients, sunscreen, and sun exposure throughout · Willing to refrain from washing, swimming, or strenuous exercise leading to sweating for 6 hours after application of study medications · Good general health Exclusion criteria: · Unstable or refractory AD · EASI > 48 · Symptoms associated with AD therapy · Skin disease or infected AD · Use of treatments within washout period (see Run‐in) · Previous failure to respond to JAK inhibitors · Current use of oral antihistamine · Medical marijuana unless stable dose for ≥ 14 days preceding the study · Clinically significant laboratory or electrocardiogram abnormalities · Active or latent infections · History of serious local skin infection (cellulitis, abscess) · Current (or history of) severe, progressive, or uncontrolled immunologic, hepatic, gastrointestinal, pulmonary, cardiovascular, genitourinary (renal), haematological, neurologic or cerebral disorders, infectious disease or coagulation disorders, or systemic or cutaneous malignancy and/or lymphoproliferative disease within the last 5 years · Known significant exposure to an individual with a confirmed diagnosis of COVID‐19 during the screening period · Herpes zoster or CMV infection within 2 months of the study, or individuals experiencing ≥ 4 outbreaks of herpes simplex virus per year · Known allergy to any ingredients in the study drug · Pregnancy and breastfeeding · Major surgery within 3 months of the study · HIV‐positive Additional design details: 4‐week treatment period with an additional 2‐week post‐treatment follow‐up phase |
| Participants | Total number randomised: 50 participants Age: 18 to 65 years old Sex, ethnicity: not reported Duration of eczema: ≥ 6 months Severity of eczema: moderate or severe (IGA score 3 or 4) Body site: representative lesion should not be on hands, feet or genitalia; the protocol inclusion criteria also stated BSA should not include scalp and face Number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: Systemic antibiotics, Janus kinase (JAK) inhibitors (systemic and topical), non‐biologic immunosuppressants, cytostatic agents, or phototherapy within 4 weeks of the study · Systemic corticosteroids within 2 weeks of the study (intranasal, inhaled) Intervention: ATI‐1777 2% solution used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle solution used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Not reported |
| Starting date | 2020 |
| Contact information | Not reported |
| Notes | Funding source: Aclaris Theraputics Inc. Declarations of interest: not declared Original language of publication: English Other: none |
NCT04615962.
| Study name | Topical cream SNG100 for treatment in moderate atopic dermatitis subjects |
| Methods | Trial design: randomised, triple‐blinded, parallel study Trial registration number: NCT04615962 Country: Israel Outpatient or hospital, date trial conducted, duration of trial participation: not reported Inclusion criteria: · Male or female 6 to 19 years old with moderate AD confirmed by a dermatologist · IGA of 3 · Severity of AD defined as moderate by SCORAD range of 26‐50 and EASI range of 7.1‐21 points · Women of childbearing potential must use a proper contraception method. Exclusion criteria: · Medical history that may interfere with study objectives · AD lesions that occur only on the face and scalp · Secondary infection with bacteria, fungi, or virus · Recent or current participation in another research study · Females who are breastfeeding, pregnant, or with plans to get pregnant during the participation in the study · Prior wound, tattoo, pigmentation or infection in the treated area Additional design details: none |
| Participants | Total number randomised, age, sex, ethnicity: duration of eczema, severity of eczema, body site: number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention one: SNG100 used for 14 days · Concurrent treatment: not reported · Other key information: concentration not stated. "A combination of low potency steroid and skin barrier repair agent" Intervention two: hydrocortisone acetate 1.% cream used for 14 days · Concurrent treatment: not reported · Other key information: none Intervention three: mometasone furoate cream used for 14 days · Concurrent treatment: not reported · Other key information: concentration not stated: "Mometasone is a medium‐strength corticosteroid". Notes: none |
| Outcomes | · Safety and tolerability as measured by completion of a full prescribed treatment course, treatment interruptions, SAE and AE · Usability · Change from baseline in the SCORAD index · EASI Score · Itch Numeric Rate |
| Starting date | 2020 |
| Contact information | Not reported |
| Notes | Funding source: Seanergy Dermatology Ltd. Declarations of interest: not declared Original language of publication: English Other: none |
NCT04717310.
| Study name | Evaluate the efficacy and safety of topical SHR0302 ointment in patients with mild to moderate atopic dermatitis |
| Methods | Trial design: randomised, quadruple‐blinded, multicentre, parallel study Trial registration number: NCT04717310 Country: China Outpatient or hospital: multiple hospital sites Date trial conducted: ongoing Duration of trial participation: not reported Inclusion criteria: · Males or females aged ≥ 18 and ≤ 75 years · Mild‐to‐moderate AD according to Hanifin and Rajka criteria with duration ≥ 6 months · Women of childbearing potential, and all men, willing to use effective contraception Exclusion criteria: · Other active skin disease, skin infections, or diseases that cause skin itching · Allergy to topical drugs · Serious concomitant illness requiring systemic corticosteroids, frequent monitoring, or otherwise interfere with participation · History of mental illness such as anxiety and depression · Serious and uncontrolled disease that may raise concerns about safety, compliance, study evaluations, or require prohibited drugs · HIV, hepatitis B, hepatitis C, or syphilis‐related laboratory test positive · Malignancy or a history of malignancy, except for fully treated or resected skin from non‐metastatic basal cell carcinoma or squamous cell carcinoma · Pregnant or breastfeeding female subjects, or subjects able to father children or of childbearing potential unwilling/unable to use effective contraception Additional design details: none |
| Participants | Total number randomised, age, sex, ethnicity, duration of eczema: severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention one: ivarmacitinib 2.% ointment used twice daily for 56 days · Concurrent treatment: not reported · Other key information: none Intervention two: ivarmacitinib 0.5% ointment used twice daily for 56 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle 0.% ointment used twice daily for 56 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Percentage change from baseline EASI at week 8 |
| Starting date | December 2020 |
| Contact information | lingyu.guo@reistonebio.com |
| Notes | Funding source: Reistone Biopharma Company Limited Declarations of interest: not declared Original language of publication: English Other: none |
NCT04737304.
| Study name | Study with BEN2293 in patients with mild to moderate atopic dermatitis |
| Methods | Trial design: randomised, double‐blind study Trial registration number: NCT04737304 Country: UK Outpatient or hospital: not reported Date trial conducted: from 2020 Duration of trial participation: not reported Inclusion criteria: · 18 to 65 years · Male or female with mild‐to‐moderate AD (based on vIGA) free from other clinically significant illness or disease that may adversely affect the safety of the patient or the integrity of the study as determined by medical history, physical examination, laboratory safety and other assessments · AD for at least 6 months · Previous or current successful treatment with topical corticosteroids · A vIGA score of 2 (mild) to 3 (moderate) Exclusion criteria: · AD of such severity that the patient could not comply with the demands of the study · Any skin tattoo, scar, cuts, bruises, or other skin damage, including excessive UV exposure, at the possible IMP application sites · AD lesions affecting > 3% untreatable areas (face, scalp, genitals, palms of hands or soles of feet) · Have concomitant skin disease or infection or presence of skin comorbidities in the study area to be dosed that may interfere with study assessments · Excessively hirsute in areas of skin to be dosed with study ointment · Unwilling to stop hair removal by any means to skin areas to be dosed with study ointment for 2 weeks prior to day ‐1 and throughout the duration of the study · Participated in a clinical study and has received medication or a new chemical entity within 3 months Additional design details: none |
| Participants | Total number randomised, age, sex, ethnicity, duration of eczema: severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: BEN2293 a Pan‐trk antagonist 0.25% or 1% ointment · Concurrent treatment: not reported · Other key information: none Comparator: 0% ointment · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Safety and tolerability · Laboratory investigation of excretion and clearance of investigative drug · Itch reduction · EASI score · vIGA score · EQ‐5D score |
| Starting date | October 2020 |
| Contact information | Not reported |
| Notes | Funding source: BenevolentAI Bio Declarations of interest: not declared Original language of publication: English Other: none |
NCT04845620.
| Study name | Trial of PDE4 inhibition with roflumilast for the management of atopic dermatitis |
| Methods | Trial design: randomised, double‐blind, multicentre study Trial registration number: NCT04845620, EUCTR2021‐006903‐14‐PL Country: Canada, Dominican Republic, Poland, US Outpatient or hospital: clinical sites Date trial conducted: ongoing Duration of trial participation: 5 weeks Inclusion criteria: · Male or female, aged 2 to 5 years · AS ≥ 6 weeks · Stable disease for the past 4 weeks with no significant flares · In good health Exclusion criteria: · Serious medical condition or clinically significant abnormality · Consistent requirement for high‐potency topical steroids · Unwilling to refrain from prolonged sun exposure for 4 weeks prior · Previous treatment with study medication Additional design details: none |
| Participants | Total number randomised, age, sex, ethnicity, duration of and severity of eczema: body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: roflumilast 0.05% cream used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used once daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Number of treatment successes (vIGA‐AD clear/almost clear and a 2‐grade improvement from baseline) · EASI‐75 |
| Starting date | April 2021 |
| Contact information | medinfo@arcutis.com |
| Notes | Funding source: Arcutis Biotherapeutics, Inc. Declarations of interest: not declared Original language of publication: English Other: none |
NCT04921969.
| Study name | A study to assess the efficacy and safety of ruxolitinib cream in children with atopic dermatitis |
| Methods | Trial design: randomised, double‐blind study Trial registration number: NCT04921969 Country: US and Canada Outpatient or hospital: not reported Date trial conducted: from 2021 Duration of trial participation: 8 weeks Inclusion criteria: · AD according to Hanifin and Rajka criteria and duration of at least 3 months · IGA score of 2 to 3 ·% BSA (excluding scalp) of AD involvement of 3% to 20% · For children aged 6 years to < 12 years, baseline itch NRS score ≥ 4 ·Participants/guardians agree to discontinue all agents used by the participant to treat AD · At least 1 target lesion that measures at least 5 cm2 and representative of the participant's disease state but not located on the hands, feet, or genitalia · Willingness to avoid pregnancy or fathering a child for the duration of study participation Exclusion criteria: · Unstable course of AD over the previous 4 weeks · Concurrent conditions and history as follows: ‐ Immunocompromised ‐ Chronic or acute infection requiring treatment with systemic antibiotics, antivirals, antiparasitics, antiprotozoals, or antifungals within 2 weeks ‐ Active acute bacterial, fungal, or viral skin infection within 1 week ‐ Any other concomitant skin disorder, pigmentation, or extensive scarring that may interfere with the evaluation of AD lesions or compromise participant safety ‐ Presence of AD lesions only on the hands or feet, without prior history of involvement of other classic areas, such as the face or the flexural folds ‐ Chronic asthma requiring more than 880 µg of inhaled budesonide or equivalent high dose of other inhaled corticosteroids ‐ Any serious illness or medical, physical, or psychiatric condition(s) that would interfere with full participation in the study, including administration of study drug and attending required study visits; pose a significant risk to the participant; or interfere with interpretation of study data · Use of any of the following treatments within the indicated washout period before the baseline visit: ‐ 5 half‐lives or 12 weeks, whichever is longer ‐ biologic agents ‐ 4 weeks ‐ systemic corticosteroids or adrenocorticotropic hormone analogues, cyclosporin, methotrexate, azathioprine, or other systemic immunosuppressive or immunomodulating agents ‐ 2 weeks ‐ immunisations with activated vaccines; sedating antihistamines unless on a long‐term stable regimen ‐ 1 week ‐ use of topical treatments for AD. Diluted sodium hypochlorite "bleach" baths are allowed as long as they do not exceed 2 baths per week. · Participants who have previously received JAK inhibitors, systemic or topical. Ultraviolet light therapy or prolonged exposure to natural or artificial sources of UV radiation within 2 weeks prior to the baseline visit and/or intention to have such exposure during the study, which is thought by the investigator to potentially impact the participant's AD · Positive serology test results at screening for HIV antibody · Current treatment or treatment within 30 days or 5 half‐lives (whichever is longer) with another investigational medication or current enrolment in another investigational drug protocol Additional design details: none |
| Participants | Total number randomised, age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: ruxolitnib 1.5% cream used twice daily for at least 56 days · Concurrent treatment: not reported · Other key information: none Comparator: 0.% cream used twice daily for 56 days · Concurrent treatment: not reported · Other key information: participants who completed week 8 assessments with no additional safety concerns will continue into the 44‐week long‐term safety period with those initially randomised to vehicle cream re‐randomised (1:1) in a blinded manner to 1 of the 2 active treatments. Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA‐ treatment success · EASI score · Itch improvement · Treatment‐emergent AE |
| Starting date | 2021 |
| Contact information | Not reported |
| Notes | Funding source: Incyte Corporation Declarations of interest: not declared Original language of publication: English Other: none |
NCT04992546.
| Study name | Study of the safety, tolerability, and pharmacokinetics of topically administered PRN473 (SAR444727) in patients with mild to moderate atopic dermatitis |
| Methods | Trial design: randomised, double‐blind, within‐participant study Trial registration number: NCT04992546 Country: USA and Canada Outpatient or hospital: not reported Date trial conducted: ongoing Duration of trial participation: 13 weeks Inclusion criteria: · Males and females aged 18 to 70 years with mild to moderate AD ≥ 6 months · Stable disease for the 4 weeks and no significant flares in AD · vIGA‐AD score of moderate or mild · ≥ 1% and no more than 14% BSA (excluding scalp, palms, soles and genitals) · ≥ 2 target lesions ≥ 100 cm2 with a difference no greater than 1 in lesion TSS and ≥ 5 cm apart on trunk or upper extremities (excluding genitals and palms) · If female with childbearing potential, a negative pregnancy test, and agreement to practice effective contraception · If male, agreement to use effective contraception with female partners of childbearing potential · Good health Exclusion criteria: · Failed 2 or more prior systemic treatments for AD · Live or attenuated vaccine within 12 weeks · Unstable AD or consistent requirement for high‐potency topical steroids · Active systemic or localised bacterial, viral, fungal, and helminth infection within 30 days · Unwilling to refrain from prolonged sun exposure or artificial light emitting devices within 4 weeks · Other skin conditions · Known genetic dermatological conditions that overlap with AD · Previous use of a BTK inhibitor · Pregnancy, planning a pregnancy, or breastfeeding · Undergoing, or planning to undergo patch testing or food challenges · Major surgery within 4 weeks · Drug abuse or regular alcohol consumption within 6 months Additional design details: none |
| Participants | Total number randomised, age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: up to a 5‐week screening period Intervention: atuzabrutinib NS gel used (unclear frequency) for 42 days · Concurrent treatment: not reported · Other key information: intervention frequency is described as multiple doses for 42 days. Comparator: vehicle gel used (unclear frequency) for 42 days · Concurrent treatment: not reported · Other key information: comparator frequency is described as multiple doses for 14 days, then atuzabrutinib for 28 days. Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | Application‐site reactions |
| Starting date | August 2021 |
| Contact information | Not reported |
| Notes | Funding source: Principia Biopharma, a Sanofi Company Declarations of interest: not declared Original language of publication: English Other: none |
NCT05127421.
| Study name | Ruxolitinib cream in participants with facial and/or neck atopic dermatitis Involvement |
| Methods | Trial design: randomised, double‐blind, study Trial registration number: NCT05127421 Country: USA Outpatient or hospital: not reported Date trial conducted: ongoing Duration of trial participation: not reported Inclusion criteria: · Adolescents and adults · AD with duration ≥ 6 months · Overall and a face and/or neck IGA score of 2 or 3 · ≥ 0.5% BSA on the face and/or neck · ≤ 20% BSA (face and/or neck plus other body areas) · Willingness to avoid pregnancy or fathering children Exclusion criteria: · Unstable AD over the previous 4 weeks · Immunocompromised, chronic or acute infection requiring systemic treatments, acute skin infection, concomitant skin conditions including other types of eczema, and chronic asthma requiring high dose of inhaled corticosteroids · Serious illness or medical, physical, or psychiatric condition · Previous treatment with JAK inhibitors · Pregnancy, planning a pregnancy, or breastfeeding · Laboratory values outside defined criteria Additional design details: none |
| Participants | Total number randomised: not reported Age: adolescents and adults eligible Sex, ethnicity: not reported Duration of eczema: ≥ 6 months eligible Severity of eczema, body site: number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: ruxolitinib 1.5% cream used twice daily for 8 weeks (assumed). · Concurrent treatment: not reported · Other key information: none Comparator: vehicle cream used twice daily for 8 weeks (assumed) · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI · AE |
| Starting date | November 2021 |
| Contact information | medinfo@incyte.com |
| Notes | Funding source: Incyte Corporation Declarations of interest: not declared Original language of publication: English Other: none |
NCT05200403.
| Study name | Improvement in scratch behavior and sleep In patients with atopic dermatitis |
| Methods | Trial design: randomised, single‐blind study Trial registration number: NCT05200403 Country: USA Outpatient or hospital: not reported Date trial conducted: ongoing Duration of trial participation: not reported Inclusion criteria: Children: · Male or female participants aged between ≥ 3 months of age and < 5 years · Demonstrated fluency in English (as age appropriate) · AD according to Hanifin and Rajka criteria · ≥ 5% and < 40% BSA, excluding the scalp · IGA of mild or moderate · EASI ≥ 3 · Minimum Observer Reported Itch Assessment score of 2 Adult caregiver: · Primary caregiver aged ≥ 18 and ≤ 75 years · IGA 0 or 1 of AD at the screening visit, and no diagnosis of AD Exclusion criteria: Children: · Clinically significant medical disorder that precludes participation in study activities · Use of systemic corticosteroids or immunosuppressive agents within 28 days · Use of topical AD treatment within 7 days · Past crisaborole treatment · Planned surgical or medical procedure during study participation · Documented non‐AD related insomnia, sleep apnoea or other sleep‐related disorders · Childhood Asthma Control Test (ages 4 to 5 years) < score 20 · Significant eczema where the wrist or ankle devices need to be worn · History of angioedema or anaphylaxis · Significant active systemic or localised infection · Planned surgical or medical procedure during study participation · Cardiac pacemakers, electronic pumps or other implanted medical devices · AD lesions on the fingers or hands or within 2 cm of the mouth Adult caregiver: · Clinically significant medical disorder that precludes participation in study activities · History of regular alcohol consumption exceeding 7 drinks/week for females or 14 drinks/week for males within 6 months and during the trial · Current shift worker or travelling across more than two time zones within 14 days and/or during the study · Planned surgical or medical procedure during study participation · Investigational site staff members involved in the study and their family members, or supervised by the investigator · Allergy to polyurethane resin, skin nickel allergy, silicone, and/or adhesives · Pregnancy · Primary caregiver or sharing the same domicile as another child in the study Additional design details: none |
| Participants | Total number randomised, age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: crisaborole 2.% ointment used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle ointment used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: no nappy rash creams, lotions, ointments, powders, etc. on AD lesions Notes: none |
| Outcomes | · Number and duration of children's nighttime scratching episodes (accelerometry) · Observer Reported Itch Assessment (ORIA) |
| Starting date | July 2022 |
| Contact information | Madisen Wicker, MS mk09wick@bu.edu |
| Notes | Funding source: Pfizer Declarations of interest: not reported Original language of publication: English Other: none |
NCT05324618.
| Study name | Tacrolimus versus hydrocortisone in atopic dermatitis |
| Methods | Trial design: randomised, double‐blind study Trial registration number: NCT05324618 Country: Egypt Outpatient or hospital: not reported Date trial conducted: from 2022 Duration of trial participation: at least 4 months Inclusion criteria: · Male or female, 2 to 16 years old · AD according to Hanifin and Rajka criteria Exclusion criteria: · Serious skin disorder other than AD · Taking systemic corticosteroids or anti‐inflammatory medications Additional design details: none |
| Participants | Total number randomised, age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: ongoing study |
| Interventions | Run‐in details: not reported Intervention: tacrolimus used twice daily for 120 days · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone used twice daily for 120 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Serum level of different inflammatory cytokines · Dermatitis severity scale using the mEASI score · Incidence rate of burning and sting sensation · Degree of erythema using EASI · Assessment of the incidence of AE |
| Starting date | 2022 |
| Contact information | Amal A. Mohamed, Prof. amalahmedhcp@yahoo.com |
| Notes | Funding source: sponsors and collaborators: Ain Shams University, National Hepatology & Tropical Medicine Research Institute Declarations of interest: not declared Original language of publication: English Other: none |
NCT05326672.
| Study name | Phase III clinical study of benvitimod cream in the treatment of mild to moderate atopic dermatitis |
| Methods | Trial design: randomised, double‐blind, multicentre study Trial registration number: NCT05326672 Country: China Outpatient or hospital: settings not clear Date trial conducted: from 2022 Duration of trial participation: 8 weeks Inclusion criteria: · Age ≥ 18 years · Diagnosis of AD, course of disease ≥ 6 months, EASI ≤ 21 and 3% ≤ BSA ≤ 20% · IGA ≥ 3 Exclusion criteria: · Skin lesions limited to head, neck, hands and feet · Abnormal laboratory measurements · Obvious cardiovascular, respiratory, gastrointestinal, liver, kidney, blood, neurological and psychological diseases that are unstable or not well controlled · Any systemic disease or other active skin disease that may affect the evaluation of the study results, or participants have scar, freckle, tattoo in the affected area that may affect the evaluation of skin lesions · Malignant neoplasms · Severe comorbid conditions requiring systematic hormone therapy or other interventions, affect study participation or require frequent active monitoring · Skin infection with local bacteria, viruses and fungi · Mental illness or other reasons may interfere with participation in the study · Known to be allergic to any of the components of the drug · Severe hypersensitivity to food, drugs, insect venom, rubber · Pregnant, breastfeeding, or planning to become pregnant · Alcohol, drug abuse and known drug dependence · Prior to enrolment, the following treatments were used within the specified time period: ‐ External medication used within 2 weeks ‐ Systemic immunotherapy used within 4 weeks ‐ Received biologics for atopic dermatitis ‐ Received UV therapy and photochemotherapy within 4 weeks Additional design details: none |
| Participants | Total number randomised, age, sex: not reported Ethnicity: not reported, but study based in China Duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tapinarof (benvitimod) 1.% cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: 0.% cream used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Proportion of participants with IGA of 0 (complete removal) or 1 (nearly complete removal) and a decrease of ≥ 2 points score from baseline to week 8 · Percentage decline in EASI score to week 8 |
| Starting date | 2022 |
| Contact information | Jianzhong Zhang rmzjz@126.com |
| Notes | Funding source: Peking University People's Hospital, Zhonghao Pharmaceutical Declarations of interest: not declared Original language of publication: English Other: none |
NCT05375955.
| Study name | A study to learn about the study medicine (PF‐07038124) in patients with mild to moderate atopic dermatitis or mild to severe plaque psoriasis |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: NCT05375955 Country: US, Canada, Japan, UK Outpatient or hospital: 32 sites Date trial conducted: from 2022 Duration of trial participation: 12 weeks Inclusion criteria for AD: · Male or female aged 12 years or older · AD for at least 3 months · IGA score of 2 (mild), or 3 (moderate) · AD covering 5% and up to 40% of BSA · A PP‐NRS average score of ≥ 2 Exclusion criteria: · Presence of skin comorbidities that would interfere with study assessment or response to treatment · Psychiatric condition · Current or recent history of severe, progressive, or uncontrolled disease · History of systemic, chronic or acute skin infection requiring hospitalisation, and parenteral antimicrobial therapy · Recent, significant trauma or major surgery · History of cancer or have undergone treatment for any type of cancer, except for adequately treated or excised non‐metastatic basal cell or squamous cell cancer of the skin or cervical carcinoma in situ with no evidence of recurrence · History of angioedema or anaphylaxis to topical products or known sensitivity to any of the components of the investigational products · Use of any prohibited concomitant medication(s) · Previous administration with an investigational drug within 30 days or 5 half‐lives preceding the first dose of study intervention · Participants with abnormal laboratory measurements · Clinically relevant abnormal electrocardiogram Additional design details: unclear if this is a 2‐armed or 4‐armed trial and if all results are separate for participants with AD |
| Participants | Total number randomised, age, sex, ethnicity: not reported Duration of eczema: at least 3 months Severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: PF 07038124 0.01, 0.03 or 0.06% ointment for 84 days · Concurrent treatment: not reported · Other key information: unclear if dose‐escalating infers this is undertaken in one arm Comparator: vehicle 0.% ointment used for 84 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · IGA score of clear (0) or almost clear (1) · Proportion of participants achieving ≥ 75% improvement in EASI‐7 · Proportion of participants having ≥ 4 points of reduction from baseline in weekly averages of PP‐NRS · Per cent CFB in affected BSA · Incidence of AE and SAEs · Number of participants with clinically significant changes in vital signs · Number of participants with clinically significant changes in electrocardiogram and in laboratory abnormalities · Incidence of increase in local tolerability |
| Starting date | September 2022 |
| Contact information | ClinicalTrials.gov_Inquiries@pfizer.com |
| Notes | Funding source: Pfizer Declarations of interest: not declared Original language of publication: English Other: none |
NCT05432596.
| Study name | Study of ATI‐1777 in patients 12 to 65 years old with moderate or severe atopic dermatitis |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: NCT05432596 Country: US Outpatient or hospital: multiple sites Date trial conducted: 2022 ongoing Duration of trial participation: 4 weeks Inclusion criteria: · Male or non‐pregnant, non‐nursing female 12 to 65 years old · At least a 6‐month history of AD and no significant AD flares for the 4 weeks prior Exclusion criteria: · Unstable course of AD · Concomitant skin disease or clinically infected AD or presence of other skin disease in the area to be dosed that may interfere with study assessments · Pregnant, nursing, or planning to become pregnant during the study Additional design details: none |
| Participants | Total number randomised, age, sex, ethnicity: not reported Duration of eczema: at least 6 months Severity of eczema, body site: not reported Number of withdrawals: ongoing study Notes: none |
| Interventions | Run‐in details: not reported Intervention one: ATI‐1777 2.% used twice or once daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention two: ATI‐1777 1.% used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention three: ATI‐1777 0.5% used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: placebo/vehicle used twice or once for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: 6‐armed study; the two arms of intervention frequency, once or twice daily, have been combined. |
| Outcomes | · Percentage change from baseline in EASI score at week 4 |
| Starting date | May 2022 |
| Contact information | Marco Cardillo, clinicaloperations@aclaristx.com |
| Notes | Funding source: Aclaris Therapeutics, Inc. Declarations of interest: not declared Original language of publication: English Other: none |
NCT05487963.
| Study name | Tolerability and effectiveness of CGB‐500 topical ointment, 1% tofacitinib, for the treatment of atopic dermatitis |
| Methods | Trial design: randomised parallel double‐blind study Trial registration number: NCT05487963 Country: not reported Outpatient or hospital: outpatient Date trial conducted: from 2022 Duration of trial participation: 8 weeks Inclusion criteria: · Outpatient, male or female of any race, 18 years of age or older. Females of childbearing potential must have a negative urine pregnancy test and practice a reliable method of contraception. · AD according to Hanifin and Rajka criteria for at least 6 months, that has been stable for ≥ 3 months · IGA score of 2 (mild) or 3 (moderate) · EASI score of 1.1 to 12.0 (i.e. mild or moderate) · AD on the arms and other body parts covering at least 2% of total BSA and up to and including 12% of total BSA · 1 to 3 target lesions of total surface area of 15 to 30 cm2, located on the body part visible to the subject · Agree to use only low‐potency corticosteroid (up to 1% hydrocortisone) topical cream or ointment for the other affected parts of the body that are not being treated with the investigational product in the study, and at least 5 cm away from the treated area · In general good health · A peak pruritus NRS score of ≥ 30 on the scale of 0 to 100 Exclusion criteria: · Pregnant, breastfeeding, or of childbearing potential and not practising reliable birth control · Known hypersensitivity or previous allergic reaction to any constituent of the Investigational Product · Any transient flushing syndrome · Severe or unstable AD · Skin infections that can interfere with reliable AD assessments · Basal cell carcinoma within 6 months · History of confounding skin conditions, immunological deficiencies or diseases, HIV, diabetes, malignancy, serious active or recurrent infection, clinically significant severe renal insufficiency, or severe hepatic disorders · Use within 1 month of: 1) oral or intravenous corticosteroids, 2) UVA/UVB therapy, 3) PUVA therapy, 4) tanning booths, 5) non‐prescription UV light sources, 6) immunomodulators or immunosuppressive therapies, 7) interferon, 8) cytotoxic drugs, 9) crisaborole, 10) pimecrolimus, or 11) injectable biologics · Use within 14 days of: 1) systemic antibiotics, 2) systemic Janus Kinase products, 3) calcipotriene or other vitamin D preparations, or 4) retinoids · Use within 7 days of: 1) antihistamines, 2) topical antibiotics, 3) topical corticosteroids or 4) other topical drug products · Use within 24 hours prior of any topical product in the areas to be treated · Known allergy or hypersensitivity to tofacitinib or any other component of the Investigational Product (i.e. essential oils, choline, phosphatidylcholine, glycerol, propylene glycol, polyethylene glycol) · Uncontrolled systemic disease · Foreseen unprotected and intense/excessive UV exposure during the study · Scheduled or planned surgical procedures · Medical or psychiatric conditions, or a personal situation, that may increase the risk associated with study participation or may interfere with interpretation of study results or subject compliance and, in the opinion of the Principal Investigator, makes the subject inappropriate for study entry · Clinically significant alcohol or drug abuse, or history of poor cooperation or unreliability Additional design details: none |
| Participants | Total number randomised: not reported Age: aged 18 or over Sex: either sex Ethnicity: not reported Duration of eczema: at least 6 months Severity of eczema: mild‐to‐moderate Body site: on arms and other parts of the body Number of withdrawals: not reported Notes: none |
| Interventions | Intervention: ATI‐1777 1.% other formulation used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none |
| Outcomes | · Frequency of TEAEs · Per cent change in EASI score at week 8 · Percentage of subjects who achieve a ≥ 75% improvement from baseline in lesional EASI score |
| Starting date | September 2022 |
| Contact information | Not reported |
| Notes | Funding source: CAGE Bio Inc. Declarations of interest: not declared Original language of publication: English Other: none |
NCT05579899.
| Study name | Safety and efficacy study of EVO101 topical cream in AD |
| Methods | Trial design: randomised, triple‐blinded, parallel study Trial registration number: NCT05579899 Country: United States Outpatient or hospital: not reported Date trial conducted: from September 2022 Duration of trial participation: 8 weeks Inclusion criteria: · Males or non‐pregnant, non‐lactating females, aged 18 years or older · AD for at least 1 year · IGA score of 2 or 3 · BSA of AD involvement of 4 to 12% · EASI of 5 to 20 Exclusion criteria: · Significant AD flare within 4 weeks · Use of biologic therapy within 12 weeks · Regular use of tanning booth within 4 weeks · Skin condition that could interfere with study assessments Additional design details: none |
| Participants | Total number randomised, age, sex, ethnicity: not reported Duration of eczema: at least 1 year Severity of eczema: IGA 2 or 3 Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: Intervention: EVO101 cream used twice daily for 56 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle 0.% cream used twice daily for 56 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI · IGA · BSA · Pruritus ‐ NRS |
| Starting date | September 2022 |
| Contact information | janice.drew@evommune.com |
| Notes | Funding source: Evommune, Inc. Declarations of interest: not declared Original language of publication: English Other: none |
NCT05607901.
| Study name | A comparative clinical trial evaluating the effect and safety of tacrolimus versus hydrocortisone |
| Methods | Trial design: randomised, double‐blind parallel study Trial registration number: NCT05607901 Country: Egypt Outpatient or hospital: not reported Date trial conducted: from 2022 Duration of trial participation: not reported Inclusion criteria: AD, 2 to 16 years of age and male or female Exclusion criteria: other skin disorders of serious type and who are taking systemic corticosteroids or anti‐inflammatory medications Additional design details: none |
| Participants | Total number randomised: not reported Age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: tacrolimus 0.03% ointment used twice daily for 120 days · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone 1.% ointment used twice daily for 120 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Inflammatory cytokine levels in the blood |
| Starting date | October 2022 |
| Contact information | Not reported |
| Notes | Funding source: Tanta University Declarations of interest: not declared Original language of publication: English Other: none |
NCT05608343.
| Study name | To assess the efficacy of difamilast ointment in mild to moderate atopic dermatitis |
| Methods | Trial design: randomised, double‐blind, multicentre study Trial registration number: NCT05608343 Country: US Outpatient or hospital: 40 sites Date trial conducted: not reported Duration of trial participation: 4 weeks Inclusion criteria: · Male or female ≥ 2 years of age · AD based according to the American Academy of Dermatology criteria · AD for at least 6 months and with involvement ≥ 5% to ≤ 40% of treatable BSA excluding scalp · AD severity of mild (2) or moderate (3) as measured by the IGA of AD Severity score Exclusion criteria: · Pregnant or breastfeeding or who plan to become pregnant during the study and through 90 days after the last dose of study drug · History of unstable AD · Active or acute skin infection and/or clinically infected AD · Greater than mild depression and suicidal ideation · Known history of alcohol abuse or illicit drugs within 6 months Additional design details: none |
| Participants | Total number randomised, age, sex: ethnicity: not reported Duration of eczema: at least 6 months Severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: difamilast 1.% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: 0.% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Success on the 5‐point IGA at day 29 |
| Starting date | October 2022 |
| Contact information | Not reported |
| Notes | Funding source: Acrotech Biopharma Inc. Declarations of interest: not declared Original language of publication: English Other: none |
NCT05650320.
| Study name | To demonstrate the superiority of IMP (0.3% and 1% OPA‐15406 ointment) versus the vehicle in pediatric patients with AD |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: NCT05650320 Country: China Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 4 weeks Inclusion criteria: · Male or female, aged 2 to 14 years · AD according to Hanifin and Rajka criteria · AD affecting ≥ 5% to ≤ 40% of BSA (excluding scalp) Exclusion criteria: · AD or contact dermatitis rapid deterioration, within 28 days Additional design details: none |
| Participants | Total number randomised: not reported Age: 2 to 14 years Sex: either Ethnicity, duration of eczema, severity of eczema, body site: number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: OPA‐15406 (Difamilast) 0.3% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention: OPA‐15406 (Difamilast) 1.% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: 0.% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Success rate in IGA · Success rate in EASI |
| Starting date | Not reported |
| Contact information | Zhijing Chang, changzhijing@cn.otsuka.com |
| Notes | Funding source: Otsuka Beijing Research Institute Declarations of interest: not declared Original language of publication: English Other: none |
NCT05667623.
| Study name | A comparison trial to demonstrate the superiority of 1% OPA‐15406 ointment to the vehicle in adult AD subjects |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: NCT05667623 Country: China, Korea (not reported if North or South) Outpatient or hospital: not reported Date trial conducted: not reported Duration of trial participation: 4 weeks Inclusion criteria: · Outpatient · 15 to 70 years old, male or female · AD according to Hanifin and Rajka criteria · History of AD for at least 3 years · AD affecting ≥ 5% to ≤ 40% of BSA (excluding scalp) · IGA score of 2 or 3 Exclusion criteria: · Pregnant, possibly pregnant, or breastfeeding, who desire to become pregnant or to have their partner become pregnant during the trial period and up until 30 days after the final administration of IMP, or who are unable to either remain abstinent or employ at least two specified birth control methods · AD or contact dermatitis rapid deterioration within 28 days · Concurrent or history of skin disease other than AD and who are judged inappropriate for assessment of AD in the present trial · Active viral skin infection or clinical signs of such infection · A current/history of malignancy within the previous 5 years · A current/history of recurrent bacterial infection resulting in hospitalisation or requiring intravenous antibiotic treatment within the past 2 years · Clinically significant complication or history of any serious disorders that the investigator judges would prevent safe conduct of the trial or impact efficacy assessment of the IMP, for example cardiac disease, endocrinologic disease, pulmonary disease · Any abnormal haematology or serum chemistry results at screening visit · Judged by the investigator to have a clinically significant abnormal blood pressure or pulse rate at the screening and baseline visits · Unable to stop allergen immunotherapy (or desensitisation therapy) from 3 months · Unable to stop treatment with ultraviolet A, narrowband ultraviolet B, and ultraviolet B from 28 days prior to the baseline examination or to stop using systemic corticosteroids, systemic immunosuppressants, systemic antimetabolites, systemic retinoid, and biologics from 28 days prior to the baseline visit · Unable to stop using topical corticosteroids for skin (excluding scalp) categorised as very strong potency from 21 days prior to the baseline visit · Unable to stop using topical corticosteroids for skin (excluding scalp) categorised as strong potency 7 days prior to the baseline visit. However, intra‐ocular, intra‐nasal, intra‐auricular, and inhaled corticosteroids may be considered if the investigator judges that their use will not impact assessment of the affected area or participants are unable to stop using topical corticosteroids for skin (excluding scalp) categorised as low or medium potency · Hypersensitivity to any drugs or any ingredient of OPA‐15406 ointment · Participated in previous trials for OPA‐15406 and have been administered the IMP · Used any other investigational drug within 4 months prior or who are scheduled to participate in any other clinical trial during the trial period · Never been treated with medication for AD or who are satisfied with their current AD treatment or not responding at all to treatment with existing topical drugs for AD Additional design details: none |
| Participants | Total number randomised, age, sex, ethnicity: not reported Duration of eczema: at least 3 years Severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: OPA‐15406 (Difamilast) 1.% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: 0.% ointment used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Success rate and change from baseline in IGA at week 4 · Success rate in EASI |
| Starting date | 2022 |
| Contact information | Jianzhong Zhang, rmzjz@126.com |
| Notes | Funding source: Otsuka Beijing Research Institute Declarations of interest: not declared Original language of publication: English Other: none |
NCT05729074.
| Study name | A randomized, placebo/active controlled, single/multiple dosing, dose escalation clinical phase 1a/b trial to evaluate the safety, tolerability and pharmacokinetic properties of IN‐A002 ointment in healthy adult male volunteers and mild to moderate atopic dermatitis patients |
| Methods | Trial design: randomised study Trial registration number: NCT05729074 Country: South Korea Outpatient or hospital: not reported Date trial conducted: from February 2023 Duration of trial participation: 28 days Inclusion criteria: · Adult male aged between 19 and 65 years · BMI between 18.0 and 30.0 kg/m2 · AD according to Hanifin and Rajka criteria; between 5% to 30% BSA involvement · AD diagnosed by EASI score of mild or moderate Exclusion criteria: · Prior/current history of clinically significant skin disorders other than AD, hepatic, renal, neurological, psychiatric, respiratory, endocrine, haematological, malignancy, urogenital, cardiovascular, digestive, or musculoskeletal disease · Clinically significant systemic or topical skin infection · Taken systemic corticosteroids or immunosuppressive agents that can affect AD‐related signs and symptoms within 4 weeks; taken systemic anti‐inflammatory or immunomodulatory drugs and systemic antibiotics that can affect AD‐related signs and symptoms within 2 weeks prior; received UV light therapy to mitigate AD‐related symptoms; expected to take the above drugs or therapy within the study duration · Treatment with oral antihistamines or topical drugs that can affect AD‐related signs and symptoms prior to the expected initial application date or within 5 life‐lives, or expected to take the above drugs within the study duration · Subjects whose hair is in the drug application site from 2 weeks prior to the expected initial application date to PSV · Hypersensitivity to the drugs, including Janus Kinase inhibitors and excipients, and other drugs, or have a history of clinically significant hypersensitivity · Suspected acute viral or bacterial infection within 2 weeks prior, or fever at the time of screening · Has clinically significant findings on 12‐lead ECG at screening · Abnormalities in clinical laboratory tests at the time of screening · Received live vaccines within 3 months prior, or plan to receive live vaccines · History of drug abuse, or who have a positive urine test for drugs of abuse · Abnormal vital signs at screening · Taken prescription drugs or oriental medicines that may affect the characteristics of the investigational product within 2 weeks prior or have taken OTC drugs or vitamin preparations within 10 days prior · Participated in other clinical trials within 6 months prior · Donated whole blood within 2 months prior or received blood transfusion within 1 month or who cannot continuously abstain from blood donation · History of regular alcohol consumption within 6 months prior or who cannot continuously abstain from alcohol consumption · History of average use of 10 cigarettes daily within 3 months prior and who cannot cease smoking · Consumed grapefruit‐containing food from 48 hours prior to the expected initial application date to PSV, or who cannot avoid taking grapefruit‐containing food · Consumed caffeine‐containing food from 24 hours prior to the expected initial application date to the last blood sampling point, or subjects who cannot avoid drinking caffeine‐containing food · Participating in regular strenuous exercise greater than daily physical activity from 48 hours prior to the expected initial application date to PSV, or subjects who cannot abstain from strenuous exercise during this period · Subjects or spouses (or partners) who have a plan for pregnancy or are unable to use a highly effective method of contraception, barrier method over a period from the informed consent to 90 days after the last dose of investigational product Additional design details: none |
| Participants | Total number randomised, age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention one: IN‐A002 ointment 1% used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention two: IN‐A002 ointment 3% used twice daily for 28 days · Concurrent treatment: not reported · Other key information: Comparator: Pimecrolimus 1% cream used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: IN‐A002 is a JAK inhibitor. |
| Outcomes | · AE · Physical examination results classified as normal, not clinically significant, or clinically significant by the judgement of the investigator · Clinical laboratory test · Topical Reaction Assessment: the numerical scores on the findings of skin reactions (erythema, papulae, oedema, bulla, blister) after single/multiple dosing of topical IN‐A002 ointment · Each subject will subjectively evaluate the skin irritation intensity (pain) from the range of 1 to 10 using NRS. · Changes in EASI score · IGA changes in score · Changes in Itch Questionnaire: NRS for pruritus, NRS for sleep disturbance, duration of itching · DLQI |
| Starting date | February 2023 |
| Contact information | Haerim Jang haerim.jang@inno‐n.com |
| Notes | Funding source: HK inno.N Corporation Declarations of interest: not declared Original language of publication: English Other: none |
NCT05843422.
| Study name | A first‐in‐human study of QY211 gel in adult subjects |
| Methods | Trial design: randomised, double‐blind, parallel study Trial registration number: NCT05843422 Country: China Outpatient or hospital: not reported Date trial conducted: from March 2023 Duration of trial participation: Inclusion criteria: · Male or female aged between 18 and 65 · AD according to Hanifin‐Rajka criteria and for at least 6 months · IGA score of mild or moderate · Total area of AD lesions 5% to 20% BSA Exclusion criteria: · With or suspected active tuberculosis, latent untreated/incompletely cured tuberculosis, or mycobacterium tuberculosis infection as judged by the investigator and/or infectious disease specialist · Hepatitis B surface antigen positive or hepatitis C virus antibody‐positive; or HIV antibody or treponema pallidum antibody‐positive · Any clinical symptoms of bacterial, viral, parasitic or fungal infection requiring treatment · Previous disseminated herpes zoster, or disseminated herpes simplex or recurrent (≥ 2 attacks) local herpes zoster · History of mental illness or genetic history of mental illness or epilepsy, the use of antipsychotic drugs, sedative drugs · History of other connective tissue diseases, or severe cardiovascular, liver, kidney, digestive tract, nerve, skin and other diseases, or patients with malignant tumours, systemic sex hormone therapy or other interventions may be required, and participation in this study may be at risk as judged by the investigator · Non‐immune factors (such as hyperlipidaemia, diabetes, thyroid disease) · Previously suffered from lymphatic diseases, or have signs or symptoms of lymphoproliferative diseases · Suffering from other skin diseases that affect the evaluation of the test results, or the presence of large tattoos, birthmarks, skin scars and other conditions in the skin lesions · Known immunodeficiency disease or first‐degree relatives with hereditary immunodeficiency disease · Cerebral haemorrhage or cerebral infarction within 1 year · Previous thromboembolism or other high‐risk groups prone to thromboembolism · Previous or planned organ transplantation · Clinical or laboratory tests judged by the investigator as abnormal with clinical significance · History of alcoholism or drug abuse within 6 months · Participated in any investigational drug 4 weeks (or 5 half‐lives, whichever is longer) before screening or participated in any medical device clinical trial within 3 months · Non‐physiological blood loss ≥ 400 mL within 3 months before screening, or plan to donate blood during the study or within 1 month after the end of the study · Severe cardiac abnormalities · Systemic or local use of JAK inhibitors within 3 months prior · Major surgical procedures or serious trauma within 8 weeks or anticipation of major surgical procedures during the trial, and all surgical or major trauma‐related AEs had not recovered before screening · Received biological therapy for AD within 8 weeks (or 5 elimination half‐lives, whichever is longer) · Received live (attenuated) vaccine within 4 weeks, or planned to receive live (attenuated) vaccine during treatment and within 4 weeks after the last use of investigational product · Used long‐acting anticoagulants or need to continue anticoagulant therapy (except aspirin ≤ 100 mg/day) within 4 weeks · Receiving systemic therapy known or likely to affect AD within 4 weeks (or 5 half‐lives, whichever is longer), phototherapy or receiving antihistamines within 2 weeks · Received topical drugs known or likely to affect AD within 2 weeks · Use of topical antibiotics, antibacterial soap, sodium hypochlorite in the target application area within 7 days or use of emollients not provided by the sponsor or designated by the investigator within 12 hours · Allergic constitution or suspected allergy to the active ingredients or excipients of the investigational drug · Pregnant women, lactating women, or patients refuse to voluntarily take effective contraceptive measures during the screening period until 6 months after the end of the last dose · Will not avoid prolonged exposure to natural or artificial ultraviolet radiation Additional design details: trial is in two populations; the details here are only for AD participants |
| Participants | Total number randomised, age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention one: QY211 0.1% gel · Concurrent treatment: not reported · Other key information: none Intervention two: QY211 0.3% gel · Concurrent treatment: not reported · Other key information: none Intervention three: QY211 0.8% gel · Concurrent treatment: not reported · Other key information: none Intervention four: QY211 1.5% gel · Concurrent treatment: not reported · Other key information: none Comparator: placebo · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: QY211 is a JAK inhibitor. |
| Outcomes | · Number of participants with AE/SAE · Number of participants with clinical laboratory abnormalities · Number of participants with clinically significant changes in physical examination · Number of participants with clinically significant treatment‐emergent electrocardiogram (ECG) · Rate of change in EASI · Proportion of patients with at least a 2‐point improvement in IGA score |
| Starting date | March 2023 |
| Contact information | Jing Zhang, Ph.D, M.D zhangj_fudan@163.com |
| Notes | Funding source: E‐nitiate Biopharmaceuticals (Hangzhou) Co., Ltd. Declarations of interest: not declared Original language of publication: English Other: none |
NCT05870865.
| Study name | A study to evaluate the anti‐pruritic effectiveness of ASN008 in adults with mild to moderate atopic dermatitis |
| Methods | Trial design: randomised, double‐blind, multicentre, parallel study Trial registration number: NCT05870865 Country: US Outpatient or hospital: 5 sites Date trial conducted: not reported Duration of trial participation: up to 12 weeks Inclusion criteria: · Male or female 18 years or older · Diagnosis of mild‐to‐moderate AD for at least 12 months with no significant disease flares for at least 4 weeks · vIGAscore of 2 or 3 · BSA affected by AD ≤ 20% · Peak pruritus NRS ≥ 7 · Body mass index (BMI) ≤ 40 kg/m2 · Willingness to avoid pregnancy or fathering children Exclusion criteria: · Any female who is breastfeeding, pregnant, or who is planning to become pregnant during the trial · Active infection requiring treatment, including skin infections · History of skin disease or presence of a skin condition that, in the opinion of the investigator, would interfere with trial assessments · Any clinically significant medical or psychiatric condition or physical/laboratory/ECG/vital signs abnormality that would, in the opinion of the investigator, put the participant at undue risk or interfere with interpretation of trial results · Use of any of the following treatments within the indicated washout period before day 1: ‐ Doxepin, hydroxyzine, or diphenhydramine use within 1 week prior ‐ Optical product containing urea or any antihistamine within 1 week prior ‐ Use of systemic antibiotic within 2 weeks, or topical antibiotics within 1 week prior ‐ AD topical medication use within 2 weeks prior ‐ Psoralen‐UVA or UVB phototherapy or excimer laser within 4 weeks prior ‐ Systemic medication use including biologics that could affect AD less than 6 weeks prior · Known hypersensitivity to ASN008 or its excipients Additional design details: none |
| Participants | Total number randomised, age, sex, ethnicity, duration of eczema, severity of eczema, body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: during the 28‐day screening period, all treatments for AD and/or itch will be stopped to allow for washout, as applicable and according to eligibility requirements. Consistent daily or twice daily use of a non‐prescription emollient is required at least 7 days prior to day 1. Intervention one: ASN008 gel 1.25% used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention two: ASN008 gel 2.5% used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Intervention three: ASN008 gel 5% used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle gel used twice daily for 28 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: consistent daily or twice daily use of a non‐prescription emollient is required throughout the trial until the follow‐up (week 8). No other products, including, but not limited to, topical corticosteroids, calcineurin inhibitors, biologics, or Janus Kinase (JAK) inhibitors (topical or oral) may be used during the trial. Notes: ASN008 is a sodium channel blocker. |
| Outcomes | · Per cent change of 7‐day average daily peak pruritus NRS · Pruritus response defined as a 7‐day average of daily peak pruritus NRS reduction greater than or equal to 4 points · Change and per cent change in the EASI score · Change in total BSA · Change from baseline in POEM · Pharmacokinetics · TEAE |
| Starting date | May 2023 |
| Contact information | soothe.eczema@trialspark.com |
| Notes | Funding source: TrialSpark Declarations of interest: not declared Original language of publication: English Other: none |
Piscitelli 2018.
| Study name | Cerdulatinib (DMVT‐502), a novel, topical dual Janus kinase/spleen tyrosine kinase inhibitor, impact on the cellular and molecular cutaneous signature in patients with atopic dermatitis |
| Methods | Trial design: parallel study Trial registration number: not reported Country: US (assumed from the affiliations of the authors) Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 14 days Inclusion criteria: AD ≤ 10% BSA involved Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: unclear Age: cerdulatinib group median age 28.5 years; vehicle/placebo group not reported Sex, ethnicity, duration of eczema: not reported Severity of eczema: ≤ 10% BSA Body site: not reported Number of withdrawals: to quote: "no safety‐related withdrawals" Notes: none |
| Interventions | Run‐in details: not reported Intervention: cerdulatinib 0.4% gel used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Comparator: vehicle 0.% gel used twice daily for 14 days · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · EASI · Treatment‐related adverse events |
| Starting date | Not reported |
| Contact information | Not reported |
| Notes | Funding source: not reported Declarations of interest: not declared;, however, authors' affiliations include Dermavant Science, Inc., Enzyvant, Inc., and Immunovant, Inc. Original language of publication: English Other: none |
Rhemus 2006.
| Study name | A double‐blind, placebo‐controlled study of a natural topical anti‐inflammatory for treatment of atopic dermatitis and psoriasis |
| Methods | Trial design: randomised, double‐blind study Trial registration number: not reported Country: US Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 8 weeks Inclusion criteria: · Mild‐to‐moderate AD · Contralateral plaques Exclusion criteria: not reported Additional design details: to quote: "Subjects were randomized into 1 of 4 groups. Each group received the active cream, which was applied to one plaque and either hydrocortisone 1% or placebo to apply to the contralateral plaque". |
| Participants | Total number randomised, age, sex, ethnicity, duration of eczema, severity of eczema, body site: not reported Number of withdrawals: no results Notes: none |
| Interventions | Run‐in details: not reported Intervention: hydrocortisone 1.% cream · Concurrent treatment: not reported · Other key information: in the trial, TCS is one of two comparators; the other is a placebo. Comparator: vehicle/placebo · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | To quote: "Standard quantitative and qualitative assessments of atopic dermatitis were performed at baseline visit, week 2, and week 4". |
| Starting date | Not reported |
| Contact information | Not reported |
| Notes | Funding source: Nu Skin Enterprises Declarations of interest: not declared Original language of publication: English Other: none |
Schlesinger 2020.
| Study name | Short‐term outcome of mild to moderate atopic dermatitis using a combination treatment of crisaborole ointment 2% and a concomitant topical cortiscosteroid |
| Methods | Trial design: randomised study Trial registration number: not reported Country: USA Outpatient or hospital, date trial conducted: not reported Duration of trial participation: 8 weeks Inclusion criteria: · Mild‐to‐moderate AD · Aged 2 to 79 years Exclusion criteria: not reported Additional design details: none |
| Participants | Total number randomised: 16 Age: 2 to 79 years Sex, ethnicity, duration of eczema: not reported Severity of eczema: mild‐to‐moderate Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: crisaborole 2% with triamcinolone acetonide 0.1% ointment used at unspecified frequency for 8 weeks · Concurrent treatment: not reported · Other key information: none Comparator: crisaborole 2.% ointment used at unspecified frequency for 8 weeks · Concurrent treatment: not reported · Other key information: none Concurrent treatments received alongside both intervention and comparator: not reported Notes: none |
| Outcomes | · Percentage with IGA 0 = clear or 1 = or almost clear with a > 2 grade improvement from baseline · Reduction in ADSI · Improvement in SCORAD · Reduction in pruritus |
| Starting date | Not reported |
| Contact information | Not reported |
| Notes | Funding source: Pfizer Declarations of interest: industry‐funded Original language of publication: English Other: none |
Van Halewijn 2018.
| Study name | Different potencies of topical steroids for children with eczema: protocol for an observational cohort study with embedded randomized controlled trial |
| Methods | Trial design: randomised, parallel study Trial registration number: EUCTR2017‐001525‐40‐NL NTR6679 Country: the Netherlands Outpatient or hospital: primary care Date trial conducted: not reported Duration of trial participation: 24 weeks Inclusion criteria: · AD according to ICPC‐code S87/S88 or prescription for topical treatment of eczema · Aged 12 weeks to 18 years · Visited the GP for AD or received repeated prescription for AD in previous 12 months · A need to intensify topical treatment from patients' and/or parents'‐point of view · TIS‐score ≥ 3 and < 6 Exclusion criteria: · As determined by the GP (e.g. family problems) · Currently under treatment by a dermatologist · No access to internet (necessary to fill in weekly online questionnaire) · Previous AE with any of the medications or hypersensitivity to corticosteroids · Use of TCS within 2 weeks · > 50% of body affected · Other skin disorders hampering assessment of eczema · Pregnancy or lactation · Untreated skin infections caused by a bacterium, virus, fungal, or parasite or incurable wounds or ulcerative skin disorders · Ichthyoses, acne vulgaris, rosacea, juvenile plantar dermatosis, skin atrophy, skin lesions · Nappy (diaper) rash · Perianal and genital itching · AD on eyelid(s) Additional design details: none |
| Participants | Total number randomised: not reported Age: 12 weeks to 18 years Sex, ethnicity, duration of eczema: not reported Severity of eczema: TIS‐score ≥ 3 and < 6 eligible Body site, number of withdrawals: not reported Notes: none |
| Interventions | Run‐in details: not reported Intervention: fluticasone propionate cream 0.05% or ointment 0.005% used (unspecified frequency) for treatment of a flare within a 24‐week period · Concurrent treatment: not reported · Other key information: none Comparator: hydrocortisone 1% cream used once daily for treatment of a flare within a 24‐week period. · Concurrent treatment: if not improved with mild TCS within 1–2 weeks, triamcinolone acetonide 0.1% cream once daily will be prescribed. If there is no improvement in turn within 1 to 2 weeks, fluticasone propionate cream 0.05% cream will be prescribed once daily. · Other key information: none Concurrent treatments received alongside both intervention and comparator: All children advised to use emollients daily Notes: Children aged > 2 years follow a predefined weaning of scheme when symptoms have improved. Children < 2 years will be reassessed after 1 to 2 weeks. |
| Outcomes | · Changes in POEM · EASI · Quality of life with IDQoL or CDLQI depending on age · Local AE (e.g. painful application, telangiectasia, atrophy, hypopigmentation) |
| Starting date | Around 2017 |
| Contact information | k.vanhalewijn@erasmusmc.nl |
| Notes | Funding source: not reported Declarations of interest: not declared Original language of publication: English Other: study has previously been extracted by this group; some content is reproduced from Lax 2022. |
ADR: adverse drug reactions ADSI: Atopic Dermatitis Severity Index AE: adverse events BMI: body mass index BSA: body surface area CDLQI: Children's Dermatology Life Quality Index DLQI: Dermatology Life Quality Index EASI: Eczema Area and Severity Index HIV: Human Immunodeficiency Virus IDQoL: Infants' Dermatitis Quality of Life Index IGA: Investigators Global Assessment ISGA: Investigator’s Static Global Assessment L‐EASI: local‐EASI mEASI: modified EASI QoL: quality of life QoLIAD: Quality of Life Index ‐ AD SAE: serious adverse events SCORAD: Scoring of Atopic Dermatitis TEAEs: treatment‐emergent adverse events VAS: visual analogue scale
Note: the number randomised per intervention group, and concentration, frequency and duration of interventions are noted only if information was provided in the study.
Differences between protocol and review
The protocol stated that risk‐of‐bias assessments would be performed in duplicate but, due to resource limitations, these were done by one data extractor and checked by another. One reviewer (of SJL, BC, CMR, RP, EA, MD, or MF) produced both the initial data extraction and risk‐of‐bias assessments. All were subsequently checked for accuracy and consistency by an independent reviewer (SJL or RB for data extraction; RB for risk‐of‐bias assessments).
The protocol stated that treatment combinations including co‐interventions would not be included as nodes in the network. However, these were included in networks where the combination treatment was a combination of two or more eligible interventions, and were included as a separate treatment node to the individual interventions, e.g. Hebert 2006.
The protocol stated the primary analyses would exclude studies judged at high risk of bias, with a sensitivity analysis including all available numerical data. As most data were judged at high risk, excluding them meant that network meta‐analysis was not possible for most outcomes and interventions of clinical interest, resulting in an uninformative review. We therefore made a post hoc decision to analyse all available data for the primary analysis, with a sensitivity analysis excluding data at high risk of bias. The single NMA for non‐high risk of bias data is presented as a sensitivity analysis in the review, for the outcome IGA binary.
The protocol stated (i) pairwise meta‐analysis would be performed for all study data suitable for meta‐analysis and (ii) for all outcomes, where feasible, we would undertake a full network meta‐analysis to explore all direct and indirect comparisons. Due to the large number of studies and treatment nodes and to avoid duplication with direct comparisons in the network meta‐analysis, pairwise meta‐analysis was only performed where study data was suitable for pooling, but insufficient for network meta‐analysis to be feasible.
As stated in the protocol, we were most interested in standard, licenced treatment regimens. Whilst unlicenced concentrations were eligible for the review and data were extracted, we excluded unlicenced treatment regimens from network meta‐analysis for those treatments with both licenced and unlicenced concentrations, since the unlicenced concentrations are not likely to be relevant for clinical practice. This decision was taken in order to reduce the complexity of the networks and rationalise the number of treatment nodes.
Within the network meta‐analysis, we planned to quantify the contribution that each study makes to the relative treatment effect using a percentage contribution matrix. However, due to the large number of studies and treatment nodes, it was not possible to produce this in the currently available software in Stata.
To be eligible for meta‐analysis, a study had to report the number of participants included in the analysis, which was missing for a larger number of trials than expected. An additional post hoc sensitivity analysis was conducted using the number randomised for those trials.
Post hoc sensitivity analyses were also undertaken using alternative correction factors of 0.25 and 0.75 for network meta‐analyses where a correction factor was assumed to account for correlations in within‐participant studies.
Network meta‐analysis was undertaken for key local adverse events of application‐site reaction, pigmentation changes and skin thinning. These were identified as important outcomes after submission of the protocol for publication through patient and public involvement feedback and focus groups.
We did not include league tables as planned (Table 3 in protocol) because these were difficult to read due to the large number of interventions.
Contributions of authors
SL and RB co‐ordinated contributions from the co‐authors and wrote the final draft of the review.
SL, BC, CR, LS, and RB screened papers against eligibility criteria.
SL, BC, CR, RP, EA, MD, MF, and RB extracted data for the review.
SL, BC, CR, RP, EA, MD, MF, and RB assessed the risk of bias in included studies.
EV, BC and SC entered data into RevMan.
EV, SL, BC, LS, CR, BS, EA, AR, DC, MF, MS, HW, SC, AD and RB analysed and interpreted data.
AR was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes were relevant to consumers.
EV, BS, EA, and SC were the statistical and methodological co‐authors.
LS, DC, MF, MS, HW, AD and RB were the clinical co‐authors.
Disclaimer
This project is funded by the National Institute for Health and Care Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number NIHR201993). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. This project was also supported by the National Institute for Health and Care Research via Cochrane Infrastructure funding to Cochrane Skin. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
Sources of support
Internal sources
-
Nil, Other
No internal sources of support
External sources
-
The National Institute for Health and Care Research (NIHR), UK
The NIHR, UK, is the largest single funder of Cochrane Skin
-
The National Institute for Health and Care Research (NIHR), UK
Research for Patient Benefit Grant (NIHR201993)
Declarations of interest
Stephanie J Lax: NIHR (grants 201993 and RP‐PG‐0216‐20007); previously published evidence syntheses and knowledge mobilisation work on the subject which are referenced fully in the review: Lax 2022; Harvey 2023; Cowdell 2023; previously published an editorial on one of the included studies: Landis 2022
Eleanor Van Vogt: none known
Bridget Candy: none known
Lloyd Steele: none known
Clare Reynolds: none known
Beth Stuart: none known
Roses Parker: no relevant interests; Commissioning Editor, The Cochrane Collaboration; not involved in the commissioning of this review and work on this review conducted prior to beginning the role as Commissioning Editor
Emma Axon: no relevant interests; employed by Cochrane as an Evidence Synthesis Methodology Editor within the Cochrane Methods Support Unit
Amanda Roberts: none known
Megan Doyle: Imperial College London (conference); University of Nottingham Medical Student
Derek Chu: no relevant interests; author on AAAAI/ACAAI Atopic Dermatitis topical treatments systematic review and guideline; allergist‐immunologist, remunerated through government funds
Masaki Futamura: Maruho, Otsuka Pharmaceutical, Torii Pharmaceutical (speaking engagement, end April 2019); paediatric allergist, National Hospital Organization, Nagoya Medical Center; Director, Japanese Society of Pediatric Allergy and Clinical Immunology
Miriam Santer: no relevant interests; published blog in BMJ about topical treatments for eczema; General Practitioner within the NHS at Denmark Road Medical Centre, Bournemouth, UK; member of the board of the NIHR School for Primary Care Research. I was previously a subpanel member for the NIHR Programme Grants for Applied Research 2018‐2023.
Hywel C Williams: no relevant interests; investigator on the following trial published in the BMJ 19 years ago: Thomas KS, Armstrong S, Avery A, Po AL, O'Neill C, Young S, Williams HC. Randomised controlled trial of short bursts of a potent topical corticosteroid versus prolonged use of a mild preparation for children with mild or moderate atopic eczema. BMJ. 2002;324(7340):768; doi: 10.1136/bmj.324.7340.768.
Suzie Cro: NIHR advanced fellowship
Aaron M Drucker: no relevant interests; published Drucker 2020 and author of Canadian Dermatology Today; dermatologist at Women's College Hospital; Vice Chair of Scientific and Medical Advisory Committee and has received research grants from the National Eczema Association (non‐profit). Consultant for Canadian Association for Drugs and Technology in Health; Editor of Cochrane Skin
Robert J Boyle: no relevant interests; paediatric allergist and helps manage eczema in children and young people. The organisations that I work for are Imperial Healthcare NHS Trust and as a self‐employed paediatric allergist at HCA Healthcare and Sterling Health; Joint Co‐ordinating Editor, Cochrane Skin (2018‐2023), Senior Editor for Children and Families (2018‐2021), Senior Editor for Mental Health and Neuroscience (2020‐2021), Deputy Co‐ordinating Editor, Cochrane Skin (2023‐current); receives fees for editorial work from Wiley and the British Society for Allergy and Clinical Immunology, and has received fees for expert witness work in relation to allergy.
EA, RB, HW, RP, and AD have had editorial roles at Cochrane, but were not involved in the editorial process or decision‐making for this review.
New
References
References to studies included in this review
Abbasi 2017 {published data only}
- Abbasi S, Kamalinejad M, Babaie D, Shams S, Sadre Z, Gheysarif M, et al. A new topical treatment of atopic dermatitis in pediatric patients based on Ficus carica L. (Fig): a randomized, placebo-controlled clinical trial. Complementary Therapies in Medicine 2017;35:85-91. [DOI] [PubMed] [Google Scholar]
- IRCT2014101419529N1. Fig fruit extract cream and hydrocortisone ointment in atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=IRCT2014101419529N1 (first recieved 2015).
Abramovits 2010 {published data only}
- Abramovits W, Oquendo M. Hydrocortisone butyrate 0.1% lipocream in pediatric patients with atopic dermatitis. Skinmed 2010;8(2):72-9. [PubMed] [Google Scholar]
Adam 1978 {published data only}
- Adam BA. An open clinical trial comparing (paired comparisons) desoximetasone with betamethasone 17-valerate. Medical Journal of Malaysia 1978;32(4):302-3. [PubMed] [Google Scholar]
Allenby 1981 {published data only}
- Allenby CF, Sparkes CG. Halogenation and topical corticosteroids: a comparison between the 17-butyrate esters of hydrocortisone and clobetasone in ointment bases. British Journal of Dermatology 1981;104(2):179-83. [DOI] [PubMed] [Google Scholar]
Almeyda 1973 {published data only}
- Almeyda J, Fry L. Controlled trial of the treatment of atopic eczema with a urea-hydrocortisone preparation versus betamethasone 17-valerat. British Journal of Dermatology 1973;88(5):493-5. [DOI] [PubMed] [Google Scholar]
Almeyda 1974 {published data only}
- Almeyda J, Burt BW. Double-blind controlled study of treatment of atopic eczema with a preparation of hydrocortisone in a new drug delivery system versus betamethasone 17-valerate. British Journal of Dermatology 1974;91(5):579-83. [DOI] [PubMed] [Google Scholar]
Anonymous 1981a {published data only}
- Anonymous. Usefulness of HF-264 ointment in the treatment of acute eczema, contact dermatitis and atopic dermatitis. Results of paired comparative study by the double blind technique. Nishinihon Journal of Dermatology 1981;43(3):474‐81. [Google Scholar]
Antiga 2011 {published data only}
- Antiga E, Volpi W, Torchia D, Fabbri P, Caproni M. Effects of tacrolimus ointment on toll-like receptors in atopic dermatitis. Clinical and Experimental Dermatology 2011;36(3):235-41. [DOI] [PubMed] [Google Scholar]
Asada 1987 {published data only}
- Asada Y. Clinical evaluation of suprofen ointment in atopic patients: paired comparative studies with ufenamate ointment. Yakuri to Chiryo 1987;15(11):573-83. [Google Scholar]
Aschoff 2009 {published data only}
- Aschoff R, Schwanebeck U, Brautigam M, Meurer M. Skin physiological parameters confirm the therapeutic efficacy of pimecrolimus cream 1% in patients with mild-to-moderate atopic dermatitis. Experimental Dermatology 2009;18(1):24-9. [DOI] [PubMed] [Google Scholar]
- NCT00180141. Elidel-study: Elidel in patients with atopic dermatitis. clinicaltrials.gov/ct2/show/NCT00180141 (first received 16 September 2005).
Bagatell 1974 {published data only}
- Bagatell FK. Halcinonide: a new potent topical anti-inflammatory drug. Cutis; Cutaneous Medicine for the Practitioner 1974;14(3):459-62. [Google Scholar]
Bagatell 1983 {published data only}
- Bagatell FK, Barkoff JR, Cohen HJ, Lasser AE, McCormick GE, Rex IH. A multicenter comparison of alclometasone dipropionate cream 0.05% and hydrocortisone cream 1.0% in the treatment of atopic dermatitis. Current Therapeutic Research, Clinical and Experimental 1983;33(1):46-52. [Google Scholar]
Bangert 2011 {published data only}
- Bangert C, Strober BE, Cork M, Ortonne JP, Luger T, Bieber T, et al. Clinical and cytological effects of pimecrolimus cream 1% after resolution of active atopic dermatitis lesions by topical corticosteroids: a randomized controlled trial. Dermatology (Basel, Switzerland) 2011;222(1):36-48. [DOI] [PubMed] [Google Scholar]
- CASM981C2436. A multicenter, 5-week, randomized, double-blind, placebo controlled, parallel group study exploring the effects of pimecrolimus cream 1% (twice daily application) vs. placebo control (twice daily application) on the molecular and cellular profile of adults. https://www.novctrd.com/ctrdweb/trialresult/trialresults/pdf?trialResultId=2168 (first received 15 April 2004).
- NCT00117377. Effects of pimecrolimus cream 1% on the molecular and cellular profile of adult male patients with atopic dermatitis. clinicaltrials.gov/show/NCT00117377 (first received 6 July 2005).
Barba 2003 {published data only}
- Barba JF, Beirana A, Cohen V, Dominguez L, Duran C, Leon G, et al. Pimecrolimus cream 1% is effective, well tolerated and safe in infants/children with atopic eczema of the face. Journal of the European Academy of Dermatology and Venereology : JEADV 2003;17(Suppl 3):182. [Google Scholar]
Barba‐Rubio 1976 {published data only}
- Barba-Rubio J. Clinical evaluation of a new halcinonide-antifungal combination. Current Therapeutic Research, Clinical and Experimental 1976;20(5):655-60. [PubMed] [Google Scholar]
Bieber 2007 {published data only}
- Bieber T, Vick K, Folster-Holst R, Belloni-Fortina A, Stadtler G, Worm M, et al. Efficacy and safety of methylprednisolone aceponate ointment 0.1% compared to tacrolimus 0.03% in children and adolescents with an acute flare of severe atopic dermatitis. Allergy 2007;62(2):184-9. [DOI] [PubMed] [Google Scholar]
- EUCTR2004-002099-40-DE. Double-blind, randomized, reference-controlled, multicenter, parallel-group study to compare the efficacy and safety of Advantan ointment once daily with Protopic 0.03% ointment twice daily over max. 3 weeks in 250 children and adolescents with atopic dermatitis - Advantan vs. Protopic in children. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2004-002099-40-DE (first received 19 May 2005).
Binder 1977 {published data only}
- Binder RH. Clinical study of clocortolone pivalate in the treatment of eczema/atopic dermatitis. Current Therapeutic Research - Clinical and Experimental 1977;21(6):796-801. [Google Scholar]
Bissonnette 2010 {published data only}
- Bissonnette R, Chen G, Bolduc C, Maari C, Lyle M, Tang L, et al. Efficacy and safety of topical WBI-1001 in the treatment of atopic dermatitis: results from a phase 2A, randomized, placebo-controlled clinical trial. Archives of Dermatology 2010;146(4):446-9. [DOI] [PubMed] [Google Scholar]
- Bissonnette R, Lyle M, Wanggui Y, Li B, Chen G, Tang L, et al. Safety and efficacy of a non-steroidal anti-inflammatory agent (WBI-1001) as a novel topical treatment for psoriasis and atopic dermatitis. Journal of Investigative Dermatology 2009;129(Suppl 1):S58. [Google Scholar]
- Bissonnette R, Maari C, Bolduc C, Chen G, Tang L. A substituted trans-stilbene derivative as a novel topical treatment for atopic dermatitis: results from a randomized, placebo-controlled clinical trial. Journal of the American Academy of Dermatology 2010;62(3 Suppl):AB9. [Google Scholar]
- NCT00837551. Phase IIa study of WBI-1001 cream for atopic dermatitis. clinicaltrials.gov/show/NCT00837551 (first received 5 February 2009).
Bissonnette 2012 {published data only}
- Bissonnette R, Poulin Y, Zhou Y, Tan J, Hong HC, Webster J, et al. Efficacy and safety of topical WBI-1001 in patients with mild to severe atopic dermatitis: results from a 12-week, multicentre, randomized, placebo-controlled double-blind trial. British Journal of Dermatology 2012;166(4):853-60. [DOI] [PubMed] [Google Scholar]
- NCT01098734. Non-steroid, atopic dermatitis phase IIb 12-week trial; topical WBI-1001 cream. clinicaltrials.gov/show/NCT01098734 (first received 5 April 2010).
Bissonnette 2016 {published data only}
- Bissonnette R, Papp KA, Poulin Y, Gooderham M, Raman M, Mallbris L, et al. Topical tofacitinib for atopic dermatitis: a phase IIa randomized trial. British Journal of Dermatology 2016;175(5):902-11. [DOI] [PubMed] [Google Scholar]
- NCT02001181. Tofacitinib ointment for atopic dermatitis (atopic eczema). clinicaltrials.gov/show/NCT02001181 (first received 4 December 2013).
- Purohit VS, Ports WC, Wang C, Riley S. Systemic tofacitinib concentrations in adult patients with atopic dermatitis treated with 2% tofacitinib ointment and application to pediatric study planning. Journal of Clinical Pharmacology 2019;59(6):811‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bissonnette 2019 {published data only}
- Bissonnette R, Guttman-Yassky E, Werth JL, Zang C, Cha A, Vlahos B, et al. Pruritus response and skin biomarkers of atopic dermatitis with crisaborole vs. vehicle in patients with mild-to-moderate atopic dermatitis. British Journal of Dermatology 2020;183(4):e104. [Google Scholar]
- Bissonnette R, Pavel AB, Diaz A, Werth JL, Zang C, Vranic I, et al. Crisaborole and atopic dermatitis skin biomarkers: an intrapatient randomized trial. Journal of Allergy and Clinical Immunology 2019;144(5):1274‐89. [DOI] [PubMed] [Google Scholar]
- Bissonnette R, Ports WC, Zang C, Vranic I, Werth JL, Purohit VS, et al. Early improvement in pruritus and reduction in clinical signs in a phase 2a mechanism of action study of crisaborole in mild to moderate atopic dermatitis. Experimental Dermatology 2018;27(S2):49‐50. [Google Scholar]
- Guttman-Yassky E, Pavel AB, Diaz A, Werth JL, Zang C, Vranic I, et al. Changes in skin biomarkers following crisaborole treatment correlate with clinical improvement in atopic dermatitis. Journal of the Dermatology Nurses' Association 2020;12(2):85-8. [Google Scholar]
- Guttman-Yassky E, Pavel AB, Diaz A, Werth JL, Zang C, Vranic I, et al. Improvement in skin inflammation and barrier function biomarkers with crisaborole treatment in atopic dermatitis (AD). Journal of Investigative Dermatology 2019;139(5):S97. [Google Scholar]
- Kim M, Del Duca E, Cheng J, Carroll B, Facheris P, Estrada Y, et al. Crisaborole reverses dysregulation of the mild to moderate atopic dermatitis proteome towards nonlesional and normal skin. Journal of the American Academy of Dermatology 2023;89:283-92. [DOI] [PubMed] [Google Scholar]
- NCT03233529. Crisaborole ointment 2% skin biomarker biopsy study in atopic dermatitis. clinicaltrials.gov/ct2/show/NCT03233529 (first received 28 July 2017).
Bleeker 1975 {published data only}
- Bleeker J. Double-blind comparison between two new topical corticosteroids, halcinonide 0.1% and clobetasol propionate cream 0.05%. Current Medical Research and Opinion 1975;3(4):225-8. [DOI] [PubMed] [Google Scholar]
Boguniewicz 1998 {published data only}
- Boguniewicz M, Fiedler VC, Raimer S, Lawrence ID, Leung DY, Hanifin, JM, Pediatric Tacrolimus Study Group. A randomized, vehicle-controlled trial of tacrolimus ointment for treatment of atopic dermatitis in children. Journal of Allergy and Clinical Immunology 1998;102(4 Pt 1):637-44. [DOI] [PubMed] [Google Scholar]
Brautigam 2006 {published data only}
- Brautigam M, Meurer M. Steroid-sparing potential of pimecrolimus cream 1% in the long-term management of severe atopic eczema in adults. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB83. [Google Scholar]
Breneman 2005 {published data only}
- Breneman D, Fleischer AB Jr, Kaplan D, Lebwohl M, Miller B, Pariser D, et al. Clobetasol propionate 0.05% lotion in the treatment of moderate to severe atopic dermatitis: a randomized evaluation versus clobetasol propionate emollient cream. Journal of Drugs in Dermatology 2005;4(3):330-6. [PubMed] [Google Scholar]
- Breneman D, Kraus S, Lebwohl M, Millder B, Foley V. Clobetasol propionate 0.5% is equivalent as lotion or emollient cream in atopic dermatitis. Annales de Dermatologie et de Venereologie 2002;129(Suppl 1 Pt 2):1S409. [Google Scholar]
Busch‐Heidger 1993 {published data only}
- Busch-Heidger B, Hevert F, Rozman T. Modern corticosteroid in a modern base. Hydrocortisone buteprate (HBP) in a new base in the treatment of atopic dermatitis. Aktuelle Dermatologie 1993;19(12):360-3. [Google Scholar]
Cahn 1961a {published data only}
- Cahn MM, Levy EJ. Fluocinolone acetonide, a new topical corticosteroid: clinical and pharmacologic evaluation. Journal of New Drugs 1961;1(6):262-7. [PubMed] [Google Scholar]
Cahn 1961b {published data only}
- Cahn MM, Levy EJ. Fluocinolone acetonide, a new topical corticosteroid: clinical and pharmacologic evaluation. Journal of New Drugs 1961;1(6):262-7. [PubMed] [Google Scholar]
Cai 2023 {published data only}
- Cai L, Zhao Y, Zheng M, Zhang F, Sun Q, Liu Q, et al. A multi-center, double-blind, randomized, placebo- and positive- controlled phase II clinical study of benvitimod for the treatment of atopic dermatitis. Chinese Medical Journal 2023;136(2):251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ChiCTR-IIR-17012153. Multi-centre, randomised, double-blind, placebo- and comparator-controlled trial on benvitimod cream in patients with atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR-IIR-17012153 (first received 27 July 2017).
Caproni 2007 {published data only}
- Caproni M, Torchia D, Antiga E, Terranova M, Volpi W, Del Bianco E, et al. The comparative effects of tacrolimus and hydrocortisone in adult atopic dermatitis: an immunohistochemical study. British Journal of Dermatology 2007;156(2):312-9. [DOI] [PubMed] [Google Scholar]
CASM981C1301 {published data only}
- CASM981C1301. Confirmatory study in pediatric patients with atopic dermatitis. https://www.novctrd.com/ctrdweb/trialresult/trialresults/pdf?trialResultId=1997 (first received 14 November 2003).
- Liu L, Wen W, Dou X, Wang B, Ma D, Li H, et al. Pimecrolimus cream 1% for the treatment of atopic dermatitis in Chinese children and adults: multicenter, randomized, double-blind, parallel-group, vehicle-controlled trial. Chinese Journal of Dermatology 2007;40(1):34-7. [Google Scholar]
CASM981C2322 {published data only}
- CASM981C2322. A 4-week, randomized, multicenter, double-blind, vehicle-controlled, parallel-group clinical trial to evaluate the efficacy and safety of pimecrolimus cream 1% in the short-term treatment of patients with mild to moderate atopic dermatitis (eczema). www.novctrd.com/ctrdweb/trialresult/trialresults/pdf?trialResultId=1936 (first received 18 January 2004).
Cato 2001 {published data only}
- Cato A, Swinehart JM, Griffin EI, Sutton L, Kaplan AS. Azone enhances clinical effectiveness of an optimized formulation of triamcinolone acetonide in atopic dermatitis. International Journal of Dermatology 2001;40(3):232-6. [DOI] [PubMed] [Google Scholar]
Chapman 1979 {published data only}
- Chapman RS. Treatment of atopic dermatitis. Practitioner 1979;223(1337):713-6. [PubMed] [Google Scholar]
Chapman 2005a {published data only}
- Chapman MS, Schachner LA, Breneman D, Boguniewicz M, Gold MH, Shull T, et al. Tacrolimus ointment 0.03% shows efficacy and safety in pediatric and adult patients with mild to moderate atopic dermatitis. Journal of the American Academy of Dermatology 2005;53(2 Suppl 2):S177-85. [DOI] [PubMed] [Google Scholar]
Chapman 2005b {published data only}
- Chapman MS, Schachner LA, Breneman D, Boguniewicz M, Gold MH, Shull T, et al. Tacrolimus ointment 0.03% shows efficacy and safety in pediatric and adult patients with mild to moderate atopic dermatitis. Journal of the American Academy of Dermatology 2005;53(2 Suppl 2):S177-85. [DOI] [PubMed] [Google Scholar]
Charney 1975 {published data only}
- Charney P, Leibsohn E. Atopic dermatitis: treatment with topical betamethasone dipropionate. Cutis; Cutaneous Medicine for the Practitioner 1975;16(4):739-41. [Google Scholar]
Craps 1973 {published data only}
- Craps L, Ferner U. Methods of clinical trial of a local corticoid. Archives Belges de Dermatologie et de Syphiligraphie 1972;28(2):97-110. [PubMed] [Google Scholar]
- Craps L, Ferner U. Procedures for the clinical investigation of a topical corticosteroid. Acta Dermato-Venereologica, Supplementum 1973;53:13-23. [PubMed] [Google Scholar]
Cullen 1971a {published data only}
- Cullen SI. Clinical evaluation of tralonide ointment, a new topical steroid. Current Therapeutic Research: Clinical and Experimental 1971;9:595-601. [PubMed] [Google Scholar]
Cullen 1971b {published data only}
- Cullen SI. Clinical evaluation of tralonide ointment, a new topical steroid. Current Therapeutic Research: Clinical and Experimental 1971;9:595-601. [PubMed] [Google Scholar]
Cunliffe 1974 {published data only}
- Cunliffe WJ, Nicholls JT. A comparison of metosyn and betnovate in the treatment of eczema and psoriasis. Journal of International Medical Research 1974;2(1):71-5. [Google Scholar]
Dahnhardt 2021 {published data only}
- Dahnhardt D, Bastian M, Dahnhardt-Pfeiffer S, Buchner M, Folster-Holst R. Comparing the effects of proactive treatment with tacrolimus ointment and mometasone furoate on the epidermal barrier structure and ceramide levels of patients with atopic dermatitis. Journal of Dermatological Treatment 2021;32(7):721-9. [DOI] [PubMed] [Google Scholar]
De Gregorio 1970a {published data only}
- De Gregorio M, Meneghelli PL. Clinical evaluation of bendazac (AF-983). A non-steroid topical anti-inflammatory agent. Acta Dermato-Venereologica 1970;50(6):466-8. [PubMed] [Google Scholar]
De Gregorio 1970b {published data only}
- De Gregorio M, Meneghelli PL. Clinical evaluation of bendazac (AF-983). A non-steroid topical anti-inflammatory agent. Acta Dermato-Venereologica 1970;50(6):466-8. [PubMed] [Google Scholar]
Del Rosso 2007 {published data only}
- Del Rosso JQ, Conte ET. An investigator-blinded evaluation of fluocinonide 0.1% cream in the treatment of atopic dermatitis and psoriasis vulgaris. Cosmetic Dermatology 2007;20(9):545-52. [Google Scholar]
Del Rosso 2009 {published data only}
- Del Rosso J. Daily application of fluocinonide 0.1% cream for the treatment of atopic dermatitis. Journal of the American Academy of Dermatology 2010;62(3 Suppl):AB46. [PMC free article] [PubMed] [Google Scholar]
- Del Rosso JQ, Bhambri S. Daily application of fluocinonide 0.1% cream for the treatment of atopic dermatitis. Journal of Clinical and Aesthetic Dermatology 2009;2(9):24-32. [PMC free article] [PubMed] [Google Scholar]
De Prost 1989 {published data only}
- De Prost Y, Bodemer C, Teillac D. Double-blind randomized placebo-controlled trial of local cyclosporine in atopic dermatitis letter. Archives of Dermatology 1989;125(4):570. [DOI] [PubMed] [Google Scholar]
- De Prost Y, Bodemer C, Teillac D. Randomised double-blind placebo-controlled trial of local cyclosporin in atopic dermatitis. Acta Dermato-Venereologica 1989;144(Suppl):136-8. [DOI] [PubMed] [Google Scholar]
De Rie 1991 {published data only}
- De Rie MA, Meinardi MM, Bos JD. Lack of efficacy of topical cyclosporin A in atopic dermatitis and allergic contact dermatitis. Acta Dermato-Venereologica 1991;71(5):452-4. [PubMed] [Google Scholar]
Dolle 2015 {published data only}
- Dolle S, Hielscher N, Bareille PJ, Hardes K, Robertson J, Worm M. Clinical efficacy and tolerability of a novel selective corticosteroid in atopic dermatitis - two randomised controlled trials. Skin Pharmacology and Physiology 2015;28(3):159-66. [DOI] [PubMed] [Google Scholar]
- EUCTR2010-022280-35-DE. A randomised, double-blind, placebo-controlled study of topical GW870086X formulation in subjects with moderate or severe atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2010-022280-35-DE (first received 15 September 2010).
- NCT01299610. A study to test the effect of 2 different doses of topical GW870086X on atopic dermatitis also including a postive control and a placebo. clinicaltrials.gov/ct2/show/NCT01299610 (first received 18 February 2011).
- NCT01381445. A study assessing GW870086's potential to cause skin thinning. https://clinicaltrials.gov/show/NCT01381445 (first received 27 June 2011).
Doss 2009 {published data only}
- Doss N, Reitamo S, Dubertret L, Fekete GL, Kamoun MR, Lahfa M, et al. Superiority of tacrolimus 0.1% ointment compared with fluticasone 0.005% in adults with moderate to severe atopic dermatitis of the face: results from a randomized, double-blind trial. British Journal of Dermatology 2009;161(2):427-34. [DOI] [PubMed] [Google Scholar]
Doss 2010 {published data only}
- Doss N, Kamoun MR, Dubertret L, Cambazard F, Remitz A, Lahfa M, et al. Efficacy of tacrolimus 0.03% ointment as second-line treatment for children with moderate-to-severe atopic dermatitis: evidence from a randomized, double-blind non-inferiority trial vs. fluticasone 0.005% ointment. Pediatric Allergy and Immunology 2010;21(2 Pt 1):321-9. [DOI] [PubMed] [Google Scholar]
Dou 2006a {published data only}
- Dou X, Liu LL, Xie ZQ, Chen LJ, Li L, Feng SY, et al. The impact of tacrolimus ointment on health-related quality of life of Chinese adult and pediatric patients with atopic dermatitis [Chinese]. Journal of Clinical Dermatology 2006;35(1):50-2. [Google Scholar]
Dou 2006b {published data only}
- Dou X, Liu LL, Xie ZQ, Chen LJ, Li L, Feng SY, et al. The impact of tacrolimus ointment on health-related quality of life of Chinese adult and pediatric patients with atopic dermatitis [Chinese]. Journal of Clinical Dermatology 2006;35(1):50-2. [Google Scholar]
Draelos 2005 {published data only}
- Draelos Z, Nayak A, Pariser D, Shupack JL, Chon K, Abrams B, et al. Pharmacokinetics of topical calcineurin inhibitors in adult atopic dermatitis: a randomized, investigator-blind comparison. Journal of the American Academy of Dermatology 2005;53(4):602-9. [DOI] [PubMed] [Google Scholar]
Duke 1983 {published data only}
- Duke EE, Maddin S, Aggerwal A. Alclometasone dipropionate in atopic dermatitis: a clinical study. Current Therapeutic Research, Clinical and Experimental 1983;33(5):769-74. [Google Scholar]
Eichenfield 2002a {published data only}
- Barbier N, Paul C, Luger T, Allen R, De Prost Y, Papp K, et al. Validation of the eczema area and severity index for atopic dermatitis in a cohort of 1550 patients from the pimecrolimus cream 1% randomized controlled clinical trials programme. British Journal of Dermatology 2004;150(1):96‐102. [DOI] [PubMed] [Google Scholar]
- Boguniewicz M, Eichenfield L, Honig P, Langley R, Lucky A, Paller A, et al. Pimecrolimus (ElidelA, SDZ ASM 981) cream 1% is safe in the long term management of atopic eczema. Journal of the European Academy of Dermatology and Venereology : JEADV 2001;15(S2):110. [Google Scholar]
- Boguniewicz M, Eichenfield L, Ling M, Cherill R. Pimecrolimus (SDZ ASM 981) cream 1% provides significant and rapid relief of pruritus in pediatric patients with moderate to severe atopic dermatitis. Journal of Investigative Dermatology 2002;119(1):348. [Google Scholar]
- Eichenfield LF, Lucky AW, Boguniewicz M, Langley RG, Cherill R, Marshall K, et al. Safety and efficacy of pimecrolimus (ASM 981) cream 1% in the treatment of mild and moderate atopic dermatitis in children and adolescents. Journal of the American Academy of Dermatology 2002;46(4):495-504. [DOI] [PubMed] [Google Scholar]
- Eichenfield LF, Lucky AW, Langley RG, Lynde C, Kaufmann R, Todd G, et al. Use of pimecrolimus cream 1% (Elidel) in the treatment of atopic dermatitis in infants and children: the effects of ethnic origin and baseline disease severity on treatment outcome. International Journal of Dermatology 2005;44(1):70-5. [DOI] [PubMed] [Google Scholar]
- Langley RG, Eichenfield LF, Lucky AW, Boguniewicz M, Barbier N, Cherill R. Sustained efficacy and safety of pimecrolimus cream 1% when used long-term (up to 26 weeks) to treat children with atopic dermatitis. Pediatric Dermatology 2008;25(3):301-7. [DOI] [PubMed] [Google Scholar]
- Lucky A, Ho V, Boguniewicz M, Gupta A, Kaufmann R, Ling M, et al. Pimecrolimus (SDZ ASM 981) cream is effective in patients aged 3 months to 17 years with atopic dermatitis. Annales de Dermatologie et de Venereologie 2002;129(Suppl 1 Pt 2):1S417. [Google Scholar]
- Pariser D, Paller A, Langley R, Paul C. Efficacy and local tolerability of pimecrolimus cream 1% in the treatment of atopic dermatitis in the face/neck region of pediatric subjects. Journal of Investigative Dermatology 2002;119(1):348. [Google Scholar]
- Whalley D, Huels J, McKenna SP, Van Assche D. The benefit of pimecrolimus (Elidel, SDZ ASM 981) on parents' quality of life in the treatment of pediatric atopic dermatitis. Pediatrics 2002;110(6):1133-6. [DOI] [PubMed] [Google Scholar]
- Whalley D, McKenna S, Huels J, Van Assche D. The benefit of pimecrolimus (ElidelA, SDZ ASM 981) on quality of life in the treatment of mild-to-moderate paediatric atopic eczema. Journal of the European Academy of Dermatology and Venereology : JEADV 2001;15(S2):112. [DOI] [PubMed] [Google Scholar]
Eichenfield 2002b {published data only}
- Eichenfield LF, Lucky AW, Boguniewicz M, Langley RG, Cherill R, Marshall K, et al. Safety and efficacy of pimecrolimus (ASM 981) cream 1% in the treatment of mild and moderate atopic dermatitis in children and adolescents. Journal of the American Academy of Dermatology 2002;46(4):495-504. [DOI] [PubMed] [Google Scholar]
Eichenfield 2006a {published data only}
- Eichenfield LF, Miller BH, Cutivate Lotion Study Group. Two randomized, double-blind, placebo-controlled studies of fluticasone propionate lotion 0.05% for the treatment of atopic dermatitis in subjects from 3 months of age. Journal of the American Academy of Dermatology 2006;54(4):715-7. [DOI] [PubMed] [Google Scholar]
Eichenfield 2006b {published data only}
- Eichenfield LF, Miller BH, Cutivate Lotion Study Group. Two randomized, double-blind, placebo-controlled studies of fluticasone propionate lotion 0.05% for the treatment of atopic dermatitis in subjects from 3 months of age. Journal of the American Academy of Dermatology 2006;54(4):715-7. [DOI] [PubMed] [Google Scholar]
El‐Hefnawi 1978 {published data only}
- El-Hefnawi H, El-Shiemy S, Paris R, Tadros SS. Double-blind paired comparison clinical trial of halcinonide and hydrocortisone. Cutis; Cutaneous Medicine for the Practitioner 1978;22(1):97-9. [PubMed] [Google Scholar]
Emer 2011 {published data only}
- Emer JJ, Frankel A, Sohn A, Lebwohl M. A bilateral comparison study of pimecrolimus cream 1% and a topical medical device cream in the treatment of patients with atopic dermatitis. Journal of Drugs in Dermatology 2011;10(7):735-43. [PubMed] [Google Scholar]
- NCT01177566. Pimecrolimus cream 1% (Elidel®) and medicated device cream (EletoneTM) in the treatment and maintenance of atopic dermatitis. clinicaltrials.gov/show/NCT01177566 (first received 9 August 2010).
EUCTR2004‐001036‐23‐IT {published data only}
- EUCTR2004-001036-23-IT. A 12 week multicenter study consisting of a 6 week double blind, randomized, vehicle controlled, parallel group phase, followed by a 6 week open label phase, to assess the safety and efficacy of Elidel Cream 1% in mild to moderate head and neck atopic dermatitis of patients intolerant of topical corticosteroids. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2004-001036-23-IT (first received 29 December 2004).
EUCTR2004‐002688‐25‐GB {published data only}
- EUCTR2004-002688-25-GB. A 4-week, randomized, multicenter, parallel-group, placebo-controlled study to investigate the effect of Elidel (pimecrolimus) cream 1% on the Quality of Life (QoL) of patients with moderate facial Atopic Eczema (AE), in whom previous treatment, including appropriate use of topical corticosteroids (TCS), has been unsatisfactory. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2004-002688-25-GB (first received 27 June 2005).
EUCTR2005‐001650‐25‐DE {published data only}
- EUCTR2005-001650-25-DE. A 12 week multicenter study consisting of a 6 week double blind, randomized, vehicle controlled, parallel group phase, followed by a 6 week open label phase, to assess the efficacy and safety of Elidel® Cream 1% in mild to moderate facial atopic dermatitis of patients (2 to 11 years of age, inclusive) intolerant of, or dependent on, topical corticosteroids. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2005-001650-25-DE (first received 12 August 2005).
EUCTR2009‐012028‐98‐DE {published data only}
- EUCTR2009-012028-98-DE. A placebo-controlled, multicentre, double-blinded, intra-individual comparison to gain evidence of the safety, tolerability and efficacy of prednicarbat cream and ointment in patients with active atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2009-012028-98-DE (first received 4 May 2009).
EUCTR2009‐013792‐22‐FI {published data only}
- EUCTR2009-013792-22-FI. A phase 2, proof of concept and dose finding study, investigating treatment efficacy of LEO 29102 cream (2.5 mg/g, 1.0 mg/g, 0.3 mg/g, 0.1 mg/g, 0.03 mg/g), LEO 29102 cream vehicle, and Elidel (Pimecrolimus) cream 10 mg/g, after cutaneous administration twice daily for 4 weeks. An international, multi-centre, prospective, randomised, double-blind, 7 arm, vehicle-controlled, parallel group study [LEO 29102 cream in the treatment of atopic dermatitis]. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2009-013792-22-FI (first received 8 October 2009).
EUCTR2010‐024279‐14‐CZ {published data only}
- EUCTR2010-024279-14-CZ. Double-blind, randomized, vehicle-controlled, multicenter, multinational, parallel-group study of the efficacy and safety of mapracorat ointment in concentrations of 0.01%, 0.03% and 0.1% over max. 4 weeks in subjects with Atopic Dermatitis (AD) - efficacy and safety of different concentrations of mapracorat in AD. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2010-024279-14-CZ (first received 4 February 2011).
EUCTR2011‐001175‐38‐DE {published data only}
- EUCTR2011-001175-38-DE. Comparison of defined skin parameters in two comparable lesional skin areas after topical treatment with Soventol HydroCort 0.5 % Cremogel or placebo. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2011-001175-38-DE (first received 4 November 2011).
EUCTR2015‐004899‐30‐Outside‐EU/EEA {published data only}
- EUCTR2015-004899-30-Outside-EU/EEA. Study into the safety and efficacy of eczema cream clobetasone butyrate. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2015-004899-30-Outside-EU/EEA (first received 21 December 2016).
Fadrhoncova 1982 {published data only}
- Fadrhoncova A. Comparative clinical trial with 1% hydrocortisone and 0.1% hydrocortisone butyrate cream in non-infected eczema. Cesko-Slovenska Dermatologie 1982;57(6):309-15. [Google Scholar]
Fattah 1976 {published data only}
- Fattah AA, El-Shiemy S, Faris R, Tadros SS. A comparative clinical evaluation of a new topical steroid 'halcinonide' and hydrocortisone in steroid-responsive dermatoses. Journal of International Medical Research 1976;4(4):228-31. [DOI] [PubMed] [Google Scholar]
Fisher 1979 {published data only}
- Fisher M, Kelly AP. Multicenter trial of fluocinonide in an emollient cream base. International Journal of Dermatology 1979;18(8):660-4. [DOI] [PubMed] [Google Scholar]
Fowler 2007 {published data only}
- Fowler J, Johnson A, Chen M, Abrams K. Improvement in pruritus in children with atopic dermatitis using pimecrolimus cream 1%. Cutis; Cutaneous Medicine for the Practitioner 2007;79(1):65-72. [PubMed] [Google Scholar]
Frankel 2011 {published data only}
- Frankel A, Sohn A, Emer J, Lebwohl M. Bilateral comparison study of pimecrolimus cream 1% and a ceramide-hyaluronic acid based emollient foam in the treatment of patients with atopic dermatitis. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB59. [PubMed] [Google Scholar]
- Frankel A, Sohn A, Patel RV, Lebwohl M. Bilateral comparison study of pimecrolimus cream 1% and a ceramide-hyaluronic acid emollient foam in the treatment of patients with atopic dermatitis. Journal of Drugs in Dermatology 2011;10(6):666-72. [PubMed] [Google Scholar]
- NCT01202149. Bilateral comparison study of Elidel 1% and hylatopic plus emollient foam for the treatment of subjects with atopic dermatitis. clinicaltrials.gov/show/NCT01202149 (first received 15 September 2010).
Freeman 2006 {published data only}
- Freeman S, Day R, Williams K, Liauw W. A new treatment for atopic dermatitis: a randomized double-blind placebo-controlled study. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB3. [Google Scholar]
Fujita 2021 {published data only}
- Fujita K, Yagi M, Moriwaki S, Yoshida M, Graham D. A phase 2b, randomized, double-blind, multicenter, vehicle-controlled study to assess the efficacy and safety of two crisaborole regimens in Japanese patients aged 2 years and older with mild-to-moderate atopic dermatitis. Journal of Dermatology 2021;48(11):1640‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03954158. Study to assess efficacy and safety of two regimens of crisaborole ointment 2% in Japanese participants aged ≥ 2 years with mild to moderate atopic dermatitis. clinicaltrials.gov/show/NCT03954158 (first received 17 May 2019).
Furue 2014 {published data only}
- Furue M, Kitahara Y, Akama H, Hojo S, Hayashi N, Nakagawa H. Safety and efficacy of topical E6005, a phosphodiesterase 4 inhibitor, in Japanese adult patients with atopic dermatitis: results of a randomized, vehicle-controlled, multicenter clinical trial. Journal of Dermatology 2014;41(7):577‐85. [DOI] [PubMed] [Google Scholar]
- Kitahara Y, Hojo S, Nomoto M, Onozuka D, Furue M, Hagihara A. Pharmacokinetic disposition of topical phosphodiesterase-4 inhibitor E6005 in patients with atopic dermatitis. Journal of Dermatological Treatment 2019;30(5):466‐70. [DOI] [PubMed] [Google Scholar]
- NCT01461941. A phase 2 study of E6005 in patients with atopic dermatitis. clinicaltrials.gov/show/NCT01461941 (first received 28 October 2011).
Gehring 1996 {published data only}
- Gehring W, Gloor M. Treatment of the atopic dermatitis with a water-in-oil emulsion with or without the addition of hydrocortisone - results of a controlled double-blind randomized study using clinical evaluation and bioengineering methods. Zeitschrift fur Hautkrankheiten 1996;71(7):554-60. [Google Scholar]
Gelmetti 1978 {published data only}
- Gelmetti C. Double blind comparison of diflucortolone valerate and betamethasone valerate. Giornale Italiano di Dermatologia/Minerva Dermatologica 1978;113(5):307-9. [Google Scholar]
Gentry 1973 {published data only}
- Gentry WC, Rosenberg EW, Goltz RW. A clinical evaluation of 0.05% desonide cream. A new nonfluorinated topical corticosteroid. Archives of Dermatology 1973;107(6):870-1. [PubMed] [Google Scholar]
Gether 2023 {published data only}
- Gether L, Storgaard H, Kezic S, Jakasa I, Hartmann B, Skov-Jeppesen K, et al. Effects of topical corticosteroid versus tacrolimus on insulin sensitivity and bone homeostasis in adults with atopic dermatitis: a randomized controlled study. Allergy 2023;78:1964-79. [DOI] [PubMed] [Google Scholar]
- NCT04114097. The effects of topical corticosteroid use on insulin sensitivity and bone turnover. clinicaltrials.gov/show/NCT04114097 (first received 3 October 2019).
Ghatikar 1974 {published data only}
- Ghatikar KN. A multicentric trial of a new steroid, betamethasone benzoate, in common dermatological disorders. Current Therapeutic Research, Clinical and Experimental 1974;16(6):585-92. [PubMed] [Google Scholar]
Giannetti 1981 {published data only}
- Giannetti A, Laria G, Seidenari S. Hydrocortisone-17-butyrate (0.1 percent) versus hydrocortisone acetate (1 percent) in atopic dermatitis. Controlled double-blind study of 20 children. Minerva Pediatrica 1981;33(12):597-600. [PubMed] [Google Scholar]
Goh 1999 {published data only}
- Goh CL, Lim JT, Leow YH, Ang CB, Kohar YM. The therapeutic efficacy of mometasone furoate cream 0.1% applied once daily vs clobetasol propionate cream 0.05% applied twice daily in chronic eczema. Singapore Medical Journal 1999;40(5):341-4. [PubMed] [Google Scholar]
Gooderham 2021 {published data only}
- Gooderham M, Kircik L, Zirwas M, Lee M, Kempers S, Draelos Z, El al. The safety and efficacy of roflumilast cream 0.15% and 0.05% in patients with atopic dermatitis: randomized, double-blind, phase 2 proof of concept study. Journal of Clinical and Aesthetic Dermatology 2021;14:S15-S16. [DOI] [PubMed] [Google Scholar]
- Gooderham M, Kircik L, Zirwas M, Lee M, Kempers SE, Draelos Z, et al. The safety and efficacy of roflumilast cream 0.15% and 0.05% in atopic dermatitis: phase II proof-of-concept study. Journal of Clinical and Aesthetic Dermatology 2021;14(5S1):S15‐S16. [Google Scholar]
- Gooderham MJ, Kircik LH, Zirwas M, Lee M, Kempers SE, Draelos ZD, et al. The safety and efficacy of roflumilast cream 0.15% and 0.05% in atopic dermatitis: phase II proof-of-concept study. British Journal of Dermatology 2021;184(3):e69. [Google Scholar]
- NCT03916081. Safety and efficacy of ARQ-151 cream in adolescents and adults with atopic dermatitis. clinicaltrials.gov/show/NCT03916081 (first received 16 April 2019).
Gower 2022 {published data only}
- Eichenfield LF, Gower RG, Xu JH, Alam MS, Su JC, Myers DE, et al. Once-daily crisaborole ointment, 2%, as a long-term maintenance treatment in patients aged >= 3 months with mild-to-moderate atopic dermatitis: a 52-week clinical study. American Journal of Clinical Dermatology 2023;24:623-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower R, Xu JH, Alam MS, Su JC, Myers DE, Sanders P, et al. 288 Once-daily crisaborole as a long-term maintenance treatment in patients with mild-to-moderate atopic dermatitis: a 52-week clinical trial. British Journal of Dermatology 2023;188(Suppl 2):ljac140.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower R, Xu JH, Alam MS, Su JC, Myers DE, Sanders P, et al. Once-daily crisaborole as a long-term maintenance treatment in participants with mild-to-moderate atopic dermatitis: a 52-week clinical trial. Pediatric Dermatology 2022;39(5):791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04040192. A study to evaluate long-term maintenance treatment with once daily crisaborole ointment 2% in pediatric and adult participants with mild-to-moderate atopic dermatitis. clinicaltrials.gov/show/NCT04040192 (first received 31 July 2019).
Gradman 2007 {published data only}
- Gradman J, Wolthers OD. Short-term growth in children with eczema during treatment with topical mometasone furoate and tacrolimus. Acta Paediatrica 2007;96(8):1233-7. [DOI] [PubMed] [Google Scholar]
- NCT00236106. Short term growth in children with atopic dermatitis. clinicaltrials.gov/show/NCT00236106 (first received 12 October 2005).
Griffiths 2002 {published data only}
- Griffiths CE, Van Leent EJ, Gilbert M, Traulsen J. Randomised comparison of the type 4 phosphodiesterase inhibitor cipamfylline cream, cream vehicle and hydrocortisone 17-butyrate cream in atopic dermatitis. British Journal of Dermatology 2001;145(Suppl 59):46-7. [DOI] [PubMed] [Google Scholar]
- Griffiths CE, Van Leent EJ, Gilbert M, Traulsen J, Cipamyflline Study Group. Randomized comparison of the type 4 phosphodiesterase inhibitor cipamfylline cream, cream vehicle and hydrocortisone 17-butyrate cream for the treatment of atopic dermatitis. British Journal of Dermatology 2002;147(2):299-307. [DOI] [PubMed] [Google Scholar]
Guttman‐Yassky 2017a {published data only}
- Guttman-Yassky E, Suarez-Farinas M, Ungar B, Oliva M, Suprun M, Todd D, et al. A model to evaluate intra-patient differential effects of topical agents in atopic dermatitis. Journal of Investigative Dermatology (Conference: 2016 Annual Meeting of the Society for Investigative Dermatology) 2016;136:S96. [Google Scholar]
- Guttman-Yassky E, Ungar B, Malik K, Dickstein D, Suprun M, Estrada YD, et al. Molecular signatures order the potency of topically applied anti-inflammatory drugs in patients with atopic dermatitis. Journal of Allergy and Clinical Immunology 2017;140(4):1032-42. [DOI] [PubMed] [Google Scholar]
Guttman‐Yassky 2019 {published data only}
- Guttman-Yassky E, Pavel AB, Durham T, Maeda-Chubachi T. Topical nitric oxide releasing therapy with 2% SB414 cream downregulated major gene expressions in patients with atopic dermatitis. Journal of Investigative Dermatology 2019;139(5 Suppl):S101. [Google Scholar]
- Maeda-Chubachi T, Durham T, Schleicher S, Rich P, Guttman-Yassky E. A topical nitric oxide-releasing cream SB414: results of a phase 1b double-blind, randomized, vehicle-controlled study in patients with mild-to-moderate atopic dermatitis. Experimental Dermatology 2018;27(S2):14. [Google Scholar]
Hamza 2018 {published data only}
- Hamza M, Lebwohl M, Thaci D, Weissbach M, Coughlan D, Guttman-Yassky E, et al. Topical DS107 for the treatment of mild-to-moderate atopic dermatitis: results from ADVANTAGE, an 8 week phase 2b randomized, double-blind, vehicle-controlled study. Experimental Dermatology 2018;27:37‐8. [Google Scholar]
- NCT02925793. Safety and efficacy study of topically applied DS107 cream in mild to moderate atopic dermatitis patients. clinicaltrials.gov/show/NCT02925793 (first received 6 October 2016).
Handa 1985 {published data only}
- Handa F, Puri KP. A randomized double blind study of ointment containing diflucortolone valerate and hydrocortisone acetate. Indian Journal of Dermatology, Venereology and Leprology 1985;51(5):261-2. [PubMed] [Google Scholar]
Handa 2022 {published data only}
- Handa S, Chandrasegaran A, Kanwar AJ, Mahajan R. Comparing the effectiveness of topical fluticasone 0.05% cream versus topical tacrolimus 0.1% ointment in pediatric atopic dermatitis: a randomized controlled trial. Indian Journal of Paediatric Dermatology 2022;23(2):111-5. [Google Scholar]
Hanifin 2001a {published data only}
- Beltrani VS. Tacrolimus ointment: advancing the treatment of atopic dermatitis. Current Allergy and Asthma Reports 2001;1(4):307-8. [DOI] [PubMed] [Google Scholar]
- Drake L, Prendergast M, Maher R, Breneman D, Korman N, Satoi Y, et al. The impact of tacrolimus ointment on health-related quality of life of adult and pediatric patients with atopic dermatitis. Journal of the American Academy of Dermatology 2001;44(Suppl 1):S65-72. [DOI] [PubMed] [Google Scholar]
- Fleischer AB Jr, Ling M, Eichenfield L, Satoi Y, Jaracz E, Rico MJ, et al. Tacrolimus ointment for the treatment of atopic dermatitis is not associated with an increase in cutaneous infections. Journal of the American Academy of Dermatology 2002;47(4):562-70. [DOI] [PubMed] [Google Scholar]
- Hanifin JM, Ling MR, Langley R, Breneman D, Rafal E. Tacrolimus ointment for the treatment of atopic dermatitis in adult patients: part I, efficacy. Journal of the American Academy of Dermatology 2001;44(Suppl 1):S28-38. [DOI] [PubMed] [Google Scholar]
- Kang S, Paller A, Soter N, Satoi Y, Rico MJ, Hanifin JM. Safe treatment of head/neck AD with tacrolimus ointment. Journal of Dermatological Treatment 2003;14(2):86-94. [DOI] [PubMed] [Google Scholar]
- Paller A, Eichenfield LF, Leung DY, Stewart D, Appell M. A 12-week study of tacrolimus ointment for the treatment of atopic dermatitis in pediatric patients. Journal of the American Academy of Dermatology 2001;44(Suppl 1):S47-57. [DOI] [PubMed] [Google Scholar]
- Soter NA, Fleischer AB, Webster GF, Monroe E, Lawrence I. Tacrolimus ointment for the treatment of atopic dermatitis in adult patients: part II, safety. Journal of the American Academy of Dermatology 2001;44(1 Suppl):S39-46. [DOI] [PubMed] [Google Scholar]
Hanifin 2001b {published data only}
- Paller A, Eichenfield LF, Leung DY, Stewart D, Appell M. A 12-week study of tacrolimus ointment for the treatment of atopic dermatitis in pediatric patients. Journal of the American Academy of Dermatology 2001;Vol. 44(Suppl 1):S47-57. [DOI] [PubMed] [Google Scholar]
Hanifin 2001c {published data only}
- Paller A, Eichenfield LF, Leung DY, Stewart D, Appell M. A 12-week study of tacrolimus ointment for the treatment of atopic dermatitis in pediatric patients. Journal of the American Academy of Dermatology 2001;Vol. 44(Suppl 1):s47-s57. [DOI] [PubMed] [Google Scholar]
Hanifin 2010 {published data only}
- Hanifin JM, Boguniewicz M, Eichenfield LF, Schneider LC, Paller AS, Preston JA, et al. A long-term study of safety and allergic comorbidity development in a randomized trial of pimecrolimus cream in infants with atopic dermatitis. Journal of Investigative Dermatology 2010;130(Suppl 1):S55. [Google Scholar]
Hanifin 2016 {published data only}
- EUCTR2013-003899-12-PL. Protocol 271-12-205: a Phase 2 multi-center, randomized, double-blind, vehicle-controlled, three-arm, parallel group study to assess the safety, tolerability, and efficacy of topical OPA-15406 ointment, in subjects with mild/moderate atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2013-003899-12-PL (first received 19 August 2014).
- Hanifin J, Shideler S, Eichenfield L, Secci A, Smith A, Kornyeyeva E. To report efficacy results from an 8-week phase 2 study treating adolescents and adults with mild to moderate atopic dermatitis treated with 0.3% and 1% OPA-15406 ointment. Acta Dermato-Venereologica 2015;95(7):886. [Google Scholar]
- Hanifin JM, Ellis CN, Frieden IJ, Folster-Holst R, Stein Gold LF, Secci A, et al. OPA-15406, a novel, topical, nonsteroidal, selective phosphodiesterase-4 (PDE4) inhibitor, in the treatment of adult and adolescent patients with mild to moderate atopic dermatitis (AD): a phase-II randomized, double-blind, placebo-controlled study. Journal of the American Academy of Dermatology 2016;75(2):297-305. [DOI] [PubMed] [Google Scholar]
- NCT02068352. A study to evaluate the effectiveness and safety of a topical OPA-15406 ointment to treat subjects with atopic dermatitis. clinicaltrials.gov/show/NCT02068352 (first received 21 February 2014).
Harder 1983 {published data only}
- Harder F, Rufli T. Therapy of eczema. Once daily use of diflorasone diacetate in comparison to thrice daily use of betamethasone-17-valerate. Schweizerische Rundschau fur Medizin Praxis 1983;72(39):1240-2. [PubMed] [Google Scholar]
Haribhakti 1982 {published data only}
- Haribhakti BP. Comparative study of clobetasone butyrate cream (Eumovate) and hydrocortisone cream in children with eczema. Indian Journal of Dermatology, Venereology and Leprology 1982;48(6):344-7. [PubMed] [Google Scholar]
Hebert 2006 {published data only}
- Hebert AA, Koo J, Fowler J, Berman B, Rosenberg C, Levitt J. Desoximetasone 0.25% and tacrolimus 0.1% ointments versus tacrolimus alone in the treatment of atopic dermatitis. Cutis; Cutaneous Medicine for the Practitioner 2006;78(5):357-63. [PubMed] [Google Scholar]
Hebert 2007a {published data only}
- Hebert AA, Cook-Bolden FE, Basu S, Calvarese B, Trancik RJ, Desonide Hydrogel Study Group. Safety and efficacy of desonide hydrogel 0.05% in pediatric subjects with atopic dermatitis. Journal of Drugs in Dermatology 2007;6(2):175-81. [PubMed] [Google Scholar]
Hebert 2007b {published data only}
- Hebert AA, Cook-Bolden FE, Basu S, Calvarese B, Trancik RJ, Desonide Hydrogel Study Group. Safety and efficacy of desonide hydrogel 0.05% in pediatric subjects with atopic dermatitis. Journal of Drugs in Dermatology 2007;6(2):175-81. [PubMed] [Google Scholar]
Hebert 2008a {published data only}
- Friedlander SF, Loss R, Schlessinger J, Potts A. A phase 3 double-blind, randomized, vehicle-controlled study of desonide foam in pediatric and adolescent patients with mild to moderate atopic dermatitis. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB84. [Google Scholar]
- Hebert AA. Desonide foam 0.05%: safety in children as young as 3 months. Journal of the American Academy of Dermatology 2008;59(2):334‐40. [DOI] [PubMed] [Google Scholar]
Hebert 2008b {published data only}
- Hebert AA. Desonide foam 0.05%: safety in children as young as 3 months. Journal of the American Academy of Dermatology 2008;59(2):334‐40. [DOI] [PubMed] [Google Scholar]
Hebert 2008c {published data only}
- Hebert AA. Desonide foam 0.05%: safety in children as young as 3 months. Journal of the American Academy of Dermatology 2008;59(2):334‐40. [DOI] [PubMed] [Google Scholar]
Ho 2003 {published data only}
- Eichenfield LF, Lucky AW, Langley RG, Lynde C, Kaufmann R, Todd G, et al. Use of pimecrolimus cream 1% (Elidel) in the treatment of atopic dermatitis in infants and children: the effects of ethnic origin and baseline disease severity on treatment outcome. International Journal of Dermatology 2005;44(1):70-5. [DOI] [PubMed] [Google Scholar]
- Ho V, Halbert A, Takaoka R, Kauffman R, Todd G, Vanaclocha F, et al. Pimecrolimus (Elidel®, SDZ ASM 981) cream 1% is effective and safe in infants aged 3-23 months with atopic eczema. Journal of the European Academy of Dermatology and Venereology : JEADV 2001;15(Suppl s2):110. [Google Scholar]
- Ho V, Hedgecock S, Bush C, Marshall K, Thurston M, Graeber M. SDZ ASM 981 cream 1% is efficacious and safe in infants aged 3-23 months with atopic dermatitis. Journal of Investigative Dermatology 2001;117(2):532. [Google Scholar]
- Ho VC, Gupta A, Kaufmann R, Todd G, Vanaclocha F, Takaoka R, et al. Safety and efficacy of nonsteroid pimecrolimus cream 1% in the treatment of atopic dermatitis in infants. Journal of Pediatrics 2003;142(2):155-62. [DOI] [PubMed] [Google Scholar]
- Whalley D, Huels J, McKenna SP, Van Assche D. The benefit of pimecrolimus (Elidel, SDZ ASM 981) on parents' quality of life in the treatment of pediatric atopic dermatitis. Pediatrics 2002;110(6):1133-6. [DOI] [PubMed] [Google Scholar]
Hoeger 2009 {published data only}
- Hoeger PH, Lee KH, Jautova J, Wohlrab J, Guettner A, Mizutani G, et al. The treatment of facial atopic dermatitis in children who are intolerant of, or dependent on, topical corticosteroids: a randomized, controlled clinical trial. British Journal of Dermatology 2009;160(2):415-22. [DOI] [PubMed] [Google Scholar]
- NCT00130364. Efficacy and safety of pimecrolimus cream 1% in patients (2 to 11 years old) with mild to moderate facial atopic dermatitis. clinicaltrials.gov/show/NCT00130364 (first received 15 August 2005).
Hoetzenecker 2005 {published data only}
- Hoetzenecker W, Ecker R, Kopp T, Stuetz A, Stingl G, Elbe-Buerger A. Pimecrolimus selectively targets T cells in skin lesions of patients with atopic dermatitis. Journal of Investigative Dermatology 2005;124(4 Suppl):A42. [Google Scholar]
- Hoetzenecker W, Ecker R, Kopp T, Stuetz A, Stingl G, Elbe-Burger A. Pimecrolimus leads to an apoptosis-induced depletion of T cells but not Langerhans cells in patients with atopic dermatitis. Journal of Allergy and Clinical Immunology 2005;115(6):1276‐83. [DOI] [PubMed] [Google Scholar]
Hofman 2006 {published data only}
- Hofman T, Cranswick N, Kuna P, Boznanski A, Latos T, Gold M, et al. Tacrolimus ointment does not affect the immediate response to vaccination, the generation of immune memory, or humoral and cell-mediated immunity in children. Archives of Disease in Childhood 2006;91(11):905‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman T. Tacrolimus ointment application does not affect the immediate response to vaccination, the generation of immune memory, or humoral and cell-mediated immunity in children. Journal of the European Academy of Dermatology and Venereology : JEADV 2005;19(Suppl 2):411-2. [Google Scholar]
- Murrell DF. Tacrolimus ointment does not affect the immediate response to vaccination, the generation of immune memory, or humoral and cell-mediated immunity in children. Australasian Journal of Dermatology 2006;47(Suppl 1):A48-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hung 2007 {published data only}
- Hung SH, Lin YT, Chu CY, Lee CC, Liang TC, Yang YH, et al. Staphylococcus colonization in atopic dermatitis treated with fluticasone or tacrolimus with or without antibiotics. Annals of Allergy, Asthma & Immunology 2007;98(1):51-6. [DOI] [PubMed] [Google Scholar]
Innocenti 1977 {published data only}
- Innocenti A, Webb F. Evaluation of diflucortolone valerianate, a new corticosteroid for topical use in dermatoses with acute and chronic course. Comparison with fluocortolone caproate. Chronica Dermatologica 1977;8(5):721-31. [Google Scholar]
Iraji 2015 {published data only}
- IRCT2014092519292N1. Efficacy of topical azathioprine-betamethasone cream with betamethasone cream in cutaneous eczema. trialsearch.who.int/Trial2.aspx?TrialID=IRCT2014092519292N1 (first received 14 November 2014).
- Iraji F, Farhadi S, Faghihi G, Mokhtari F, Basiri A, Jafari-Koshki T, et al. Efficacy of topical azathioprine and betamethasone versus betamethasone-only emollient cream in 2-18 years old patients with moderate-to-severe atopic dermatitis: a randomized controlled trial. Advanced Biomedical Research 2015;4:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
JapicCTI‐163372 {published data only}
- JapicCTI-163372. A multi-center, randomized, double-blind, vehicle-controlled, parallel-group comparison trial to assess the efficacy and safety of OPA-15406 ointment in patients with atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-JapicCTI-163372 (first received 8 September 2016).
Jensen 2009 {published data only}
- EUCTR2007-003106-99-DE. Pimecrolimus and epidermal barrier function: role for pimecrolimus in restoring skin barrier function and normalizing epidermal lipid content and differentiation in atopic epidermis: a randomized, intra-patient, double-blind (right/left arm) study in adults with atopic dermatitis treated with 1% pimecrolimus cream and 0.1% triamcinolone acetonide cream as treatment control twice daily for 3 weeks. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2007-003106-99-DE (first received 17 August 2007).
- Jensen JM, Pfeiffer S, Witt M, Brautigam M, Neumann C, Weichenthal M, et al. Different effects of pimecrolimus and betamethasone on the skin barrier in patients with atopic dermatitis. Journal of Allergy and Clinical Immunology 2009;123(5):1124-33 (Erratum appears in Journal of Allergy and Clinical Immunology 2009 Nov;124(5):1038). [DOI] [PubMed] [Google Scholar]
- Jensen JM, Pfeiffer S, Witt M, Brautigam M, Neumann C, Weichenthal M, et al. Different effects of pimecrolimus and betamethasone on the skin barrier in patients with atopic dermatitis. Journal of Allergy and Clinical Immunology 2009;124(3 Suppl 2):R19‐28. [DOI] [PubMed] [Google Scholar]
- NCT01132079. Pimecrolimus and epidermal barrier function. clinicaltrials.gov/show/NCT01132079 (first received 27 May 2010).
- Proksch E, Jensen JM, Elias P, Folster-Holst R. Substantial improvement of the skin barrier in atopic dermatitis after treatment with pimecrolimus but not with betamethasone. Journal of the American Academy of Dermatology 2007;56(2):AB3. [Google Scholar]
Jensen 2013 {published data only}
- Jensen JM, Weppner M, Dahnhardt-Pfeiffer S, Folster-Holst R, Proksch E. Pimecrolimus cream treatment repairs skin barrier structure more effectively than triamcinolone acetonide (TCA) cream treatment in atopic dermatitis. Experimental Dermatology 2010;19(2):196. [Google Scholar]
- Jensen JM, Weppner M, Dahnhardt-Pfeiffer S, Neumann C, Brautigam M, Schwarz T, et al. Effects of pimecrolimus compared with triamcinolone acetonide cream on skin barrier structure in atopic dermatitis: a randomized, double-blind, right-left arm trial. Acta Dermato-Venereologica 2013;93(5):515-9. [DOI] [PubMed] [Google Scholar]
Jirakova 2015 {published data only}
- Jirakova A, Rob F, Secnikova Z, Koblova K, Dzambova M, Rajska L, et al. Topical corticosteroids but not calcineurin inhibitors induced atrophy after four weeks. Journal of Biological Regulators and Homeostatic Agents 2015;29(3):701‐6. [PubMed] [Google Scholar]
Jorizzo 1995 {published data only}
- Jorizzo J, Levy M, Lucky A, Shavin J, Goldberg G, Dunlap F, et al. Multicenter trial for long-term safety and efficacy comparison of 0.05% desonide and 1% hydrocortisone ointments in the treatment of atopic dermatitis in pediatric patients. Journal of the American Academy of Dermatology 1995;33(1):74-7. [DOI] [PubMed] [Google Scholar]
Ju 2013 {published data only}
- Ju M, Xu A, Duan Y, Wen H, Li T, Xu L, et al. Study of calcipotriol betamethasone ointment in the treatment of patients with refractory chronic eczema. Asian Journal of Pharmaceutical and Clinical Research 2013;6(Suppl 5):34-40. [Google Scholar]
Kang 1998 {published data only}
- Kang S. Tacrolimus ointment for adults with moderate to severe atopic dermatitis: a dose escalation study. Journal of Investigative Dermatology 1998;110(4):681. [Google Scholar]
Kaplan 1978 {published data only}
- Kaplan RJ, Daman L, Rosenberg WE, Feigenbaum S. Topical use of caffeine with hydrocortisone in the treatment of atopic dermatitis. Archives of Dermatology 1978;114:60-2. [PubMed] [Google Scholar]
Kapp 2002 {published data only}
- Kapp A, Bingham A, De Moor A, De Prost Y, Papp K, Potter PC, et al. Pimecrolimus (Elidel, SDZ ASM 981) cream 1%: a new approach to long-term management of atopic eczema in infants. Journal of the European Academy of Dermatology and Venereology : JEADV 2001;15(Suppl s2):111. [Google Scholar]
- Kapp A, Papp K, Bingham A, Folster-Holst R, Ortonne JP, Potter P, et al. Pimecrolimus (SDZ ASM 981) cream: a new treatment strategy for the long-term management of atopic dermatitis in infants. Annales de Dermatologie et de Venereologie 2002;129(Suppl 1 Pt 2):1S412. [Google Scholar]
- Kapp A, Papp K, Bingham A, Folster-Holst R, Ortonne JP, Potter PC, et al. Long-term management of atopic dermatitis in infants with topical pimecrolimus, a nonsteroid anti-inflammatory drug. Journal of Allergy and Clinical Immunology 2002;110(2):277-84. [DOI] [PubMed] [Google Scholar]
- Kapp A, Papp K, Gulliver W, Goertz HP. Treatment with pimecrolimus cream 1% is corticosteroid sparing in infants with atopic dermatitis. Journal of Investigative Dermatology 2002;119(1):350. [Google Scholar]
- Kaufmann R, Goodfield M, Kapp A, Gourmala N. Pimecrolimus cream 1% provides long-term disease management in children with severe atopic dermatitis. Journal of Investigative Dermatology 2002;119(1):350. [Google Scholar]
Kaufmann 2004 {published data only}
- Breuer K, Braeutigam M, Kapp A, Werfel T. Influence of pimecrolimus cream 1% on different morphological signs of eczema in infants with atopic dermatitis. Dermatology 2004;209(4):314-20. [DOI] [PubMed] [Google Scholar]
- Kaufmann R, Folster-Holst R, Hoger P, Thaci D, Loffler H, Staab D, et al. Onset of action of pimecrolimus cream 1% in the treatment of atopic eczema in infants. Journal of Allergy and Clinical Immunology 2004;114(5):1183-8. [DOI] [PubMed] [Google Scholar]
- Staab D, Kaufmann R, Brautigam M, Wahn U, CASM981CDE04-Study Group. Treatment of infants with atopic eczema with pimecrolimus cream 1% improves parents' quality of life: a multicenter, randomized trial. Pediatric Allergy and Immunology 2005;16(6):527-33. [DOI] [PubMed] [Google Scholar]
- Thaci D, Folster-Holst R, Hoger P, Kaufmann R, Brautigam M, Weidinger G, et al. Pimecrolimus cream 1% is effective in the treatment of atopic eczema in infants, irrespective of clinical score. Journal of the European Academy of Dermatology and Venereology : JEADV 2003;17(Suppl 3):P2.37. [Google Scholar]
Kaufmann 2006 {published data only}
- Kaufmann R, Bieber T, Helgesen AL, Andersen BL, Luger T, Poulin Y, et al. Onset of pruritus relief with pimecrolimus cream 1% in adult patients with atopic dermatitis: a randomized trial. Allergy 2006;61(3):375-81. [DOI] [PubMed] [Google Scholar]
Kempers 2004 {published data only}
- Kempers S, Boguniewicz M, Carter E, Jarrat M, Pariser D, Stewart D. Comparison of pimecrolimus cream 1% and tacrolimus ointment 0.03% in paediatric patients with atopic eczema. Journal of the European Academy of Dermatology and Venereology : JEADV 2003;17(Suppl 3):183-4. [Google Scholar]
- Kempers S, Boguniewicz M, Carter E, Jarratt M, Pariser D, Stewart D, et al. A randomized investigator-blinded study comparing pimecrolimus cream 1% with tacrolimus ointment 0.03% in the treatment of pediatric patients with moderate atopic dermatitis. Journal of the American Academy of Dermatology 2004;51(4):515-25. [DOI] [PubMed] [Google Scholar]
Kim 2012 {published data only}
- Kim M, Woo S. The efficacy and safety of pimecrolimus in children less than 2 years old: a randomised, controlled clinical trial. Allergy 2012;67:634. [Google Scholar]
Kimball 2008 {published data only}
- Kimball AB, Gold, MH, Zib B, Davis MW, Clobetasol Propionate Emulsion Formulation Foam Phase III Clinical Study Group. Clobetasol propionate emulsion formulation foam 0.05%: review of phase II open-label and phase III randomized controlled trials in steroid-responsive dermatoses in adults and adolescents. Journal of the American Academy of Dermatology 2008;59:448-54. [DOI] [PubMed] [Google Scholar]
Kirkup 2003a {published data only}
- Kirkup ME, Birchall NM, Weinberg EG, Helm K, Kennedy CT. Acute and maintenance treatment of atopic dermatitis in children - two comparative studies with fluticasone propionate (0.05%) cream. Journal of Dermatological Treatment 2003;14(3):141-8. [DOI] [PubMed] [Google Scholar]
Kirkup 2003b {published data only}
- Kirkup ME, Birchall NM, Weinberg EG, Helm K, Kennedy CT. Acute and maintenance treatment of atopic dermatitis in children - two comparative studies with fluticasone propionate (0.05%) cream. Journal of Dermatological Treatment 2003;14(3):141-8. [DOI] [PubMed] [Google Scholar]
Koppes 2016 {published data only}
- Koppes SA, Charles F, Lammers L, Frings-Dresen M, Kezic S, Rustemeyer T. Efficacy of a cream containing ceramides and magnesium in the treatment of mild to moderate atopic dermatitis: a randomized, double-blind, emollient- and hydrocortisone-controlled trial. Acta Dermato-Venereologica 2016;96(7):948-53. [DOI] [PubMed] [Google Scholar]
Krueger 2006 {published data only}
- Krueger G, Eichenfield L, Holum ME, Keirns J. Tacrolimus pharmacokinetics in adult and pediatric atopic dermatitis patients following topical application of tacrolimus ointment. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB88. [Google Scholar]
- Krueger GG, Eichenfield L, Goodman JJ, Krafchik BR, Carlin CS, Pang ML, et al. Pharmacokinetics of tacrolimus following topical application of tacrolimus ointment in adult and pediatric patients with moderate to severe atopic dermatitis. Journal of Drugs in Dermatology 2007;6(2):185-93. [PubMed] [Google Scholar]
Kuokkanen 1987 {published data only}
- Kuokkanen K, Sillantaka I. Alclometasone dipropionate 0.05% vs hydrocortisone 1.0%: potential to induce cutaneous atrophy in children. Clinical Therapeutics 1987;9(2):223-31. [PubMed] [Google Scholar]
Landis 2022 {published data only}
- EUCTR2018-003050-24-PL. A phase 2b, multicenter, randomized, double-blind, vehicle controlled, parallel group dose ranging study to assess efficacy, safety, tolerability and pharmacokinetics of PF-06700841 cream in participants with mild or moderate atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2018-003050-24-PL (first received 10 June 2019).
- Landis MN, Arya M, Smith S, Draelos Z, Usdan L, Tarabar S, et al. Efficacy and safety of topical brepocitinib for the treatment of mild-to-moderate atopic dermatitis: a phase IIb, randomized, double-blind, vehicle-controlled, dose-ranging and parallel-group study. British Journal of Dermatology 2022;187(6):878-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03903822. Dose ranging study to assess efficacy, safety, tolerability and pharmacokinetics of PF-06700841 topical cream in participants with mild or moderate atopic dermatitis. clinicaltrials.gov/show/NCT03903822 (first received 4 April 2019).
Lassus 1983 {published data only}
- Lassus A. Clinical comparison of alclometasone dipropionate cream 0.05% with hydrocortisone butyrate cream 0.1% in the treatment of atopic dermatitis in children. Journal of International Medical Research 1983;11(5):315-9. [DOI] [PubMed] [Google Scholar]
Lassus 1984 {published data only}
- Lassus A. Alclometasone dipropionate cream 0.05% versus clobetasone butyrate cream 0.05%. A controlled clinical comparison in the treatment of atopic dermatitis in children. International Journal of Dermatology 1984;23(8):565-6. [DOI] [PubMed] [Google Scholar]
Lawlor 1995 {published data only}
- Lawlor F, Black AK, Greaves M. Prednicarbate 0.25% ointment in the treatment of atopic dermatitis: a vehicle-controlled double-blind study. Journal of Dermatological Treatment 1995;6(4):233-5. [Google Scholar]
Lebrun‐Vignes 2000 {published data only}
- Lebrun-Vignes B, Legrain V, Amoric JC, Taieb A. Comparative study of efficacy and effect on plasma cortisol levels of micronized desonide cream 0.1 p. 100 versus betamethasone dipropionate cream 0.05 p. 100 in the treatment of childhood atopic dermatitis. Annales de Dermatologie et de Venereologie 2000;127(6-7):590-5. [PubMed] [Google Scholar]
Lebwohl 1999 {published data only}
- Lebwohl M. A comparison of once-daily application of mometasone furoate 0.1% cream compared with twice-daily hydrocortisone valerate 0.2% cream in pediatric atopic dermatitis patients who failed to respond to hydrocortisone. International Journal of Dermatology 1999;38(8):604‐6. [DOI] [PubMed] [Google Scholar]
Lee 2014 {published data only}
- Lee SJ, Woo SI, Ahn SH, Lim DK, Hong JY, Park JH, et al. Functional interpretation of metabolomics data as a new method for predicting long-term side effects: treatment of atopic dermatitis in infants. Scientific Reports 2014;4:7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leibsohn 1974 {published data only}
- Leibsohn E, Bagatell FK. Halcinonide in the treatment of corticosteroid responsive dermatoses. British Journal of Dermatology 1974;90(4):435-40. [DOI] [PubMed] [Google Scholar]
Leo 2004 {published data only}
- Leo HL, Bender BG, Leung SB, Tran ZV, Leung DY. Effect of pimecrolimus cream 1% on skin condition and sleep disturbance in children with atopic dermatitis. Journal of Allergy and Clinical Immunology 2004;114(3):691-3. [DOI] [PubMed] [Google Scholar]
- Leo HL, Bender BG, Leung SB, Tran ZV, Leung DY. The effect of elidel 1% cream on skin condition and sleep parameters in children with mild to moderate atopic dermatitis (AD). Journal of Allergy and Clinical Immunology 2004;113(2 Suppl):S334. [DOI] [PubMed] [Google Scholar]
Leung 2009 {published data only}
- CASM981C2402. An exploratory, randomized, double-blind, vehicle-controlled, multicenter, parallel dose study investigating the use of pimecrolimus cream 1% in mild to moderate atopic dermatitis patients who demonstrate a clinical insensitivity to topical glucocorticoid. www.novctrd.com/ctrdweb/trialresult/trialresults/pdf?trialResultId=1453 (first received 25 September 2002).
- Leung DY, Hanifin JM, Pariser DM, Barber KA, Langley RG, Schlievert PM, et al. Effects of pimecrolimus cream 1% in the treatment of patients with atopic dermatitis who demonstrate a clinical insensitivity to topical corticosteroids: a randomized, multicentre vehicle-controlled trial. British Journal of Dermatology 2009;161(2):435-43. [DOI] [PubMed] [Google Scholar]
Levy 1974 {published data only}
- Levy J, Highly Jr FM. Double blind trial of a new topical corticosteroid in a US naval hospital. Military Medicine 1974;139(9):728-30. [Google Scholar]
Levy 2005 {published data only}
- Levy AL, Sheehan M, Roberts R. Tacrolimus cream 0.03% is safe and effective in the treatment of mild to moderate atopic dermatitis in adults. Journal of Allergy and Clinical Immunology 2005;115(2 Suppl):S103. [Google Scholar]
Liu 2014b {published data only}
- Liu LL, Gong Y, Zhang JZ, Cal L, Sun QN, Ma DL, et al. A multi-center, randomized, double-blind, parallel, vehicle controlled study on the moderate to severe eczema patients with the treatment of clobetasone butyrate cream. Journal of Clinical Dermatology 2014;43(7):402‐5. [Google Scholar]
Lucky 1997 {published data only}
- Lucky AW, Grote GD, Williams JL, Tuley MR, Czernielewski JM, Dolak TM, et al. Effect of desonide ointment, 0.05%, on the hypothalamic-pituitary-adrenal axis of children with atopic dermatitis. Cutis; Cutaneous Medicine for the Practitioner 1997;59(3):151-3. [PubMed] [Google Scholar]
Ludvigsen 1975 {published data only}
- Ludvigsen KE, Gadborg E. Treatment of Besnier's prurigo with calmuril-hydrocortisone 1% cream and triamcinolone acetonide 0,1% cream. A controlled clinical study. Ugeskrift for Laeger 1975;137(19):1062-4. [PubMed] [Google Scholar]
Luger 2001 {published data only}
- Luger T, Van Leent EJ, Graeber M, Hedgecock S, Thurston M, Kandra A, et al. SDZ ASM 981: an emerging safe and effective treatment for atopic dermatitis. British Journal of Dermatology 2001;144(4):788-94. [DOI] [PubMed] [Google Scholar]
- Van Leent E, Graeber M, Hedgecock S, Thurston M, Spuis P, Box J. SDZ ASM 981: a emerging new standard for the treatment of atopic dermatitis. Journal of the European Academy of Dermatology and Venereology : JEADV 1998;11(Suppl 2):S198. [Google Scholar]
Luger 2004 {published data only}
- Luger TA, Lahfa M, Folster-Holst R, Gulliver WP, Allen R, Molloy S, et al. Long-term safety and tolerability of pimecrolimus cream 1% and topical corticosteroids in adults with moderate to severe atopic dermatitis. Journal of Dermatological Treatment 2004;15(3):169-78. [DOI] [PubMed] [Google Scholar]
Lupton 1982 {published data only}
- Lupton ES, Abbrecht MM, Brandon ML. Short-term topical corticosteroid therapy (halcinonide ointment) in the management of atopic dermatitis. Cutis; Cutaneous Medicine for the Practitioner 1982;30(5):671-5. [PubMed] [Google Scholar]
Mali 1976 {published data only}
- Mali JW. An evaluation of betamethasone dipropionate (Diprosone) versus Locacorten 0.02% cream. Dermatologica 1976;153(3):177-8. [DOI] [PubMed] [Google Scholar]
Maloney 1998 {published data only}
- Maloney JM, Morman MR, Stewart DM, Tharp MD, Brown JJ, Rajagopalan R. Clobetasol propionate emollient 0.05% in the treatment of atopic dermatitis. International Journal of Dermatology 1998;37(2):142-4. [DOI] [PubMed] [Google Scholar]
Marten 1980 {published data only}
- Marten RH, Byrne JP, Peiris S, Butler J, Keenan J. Study of the effects of hydrocortisone and hydrocortisone 17-butyrate ointments on plasma ACTH levels and synacthen responses in children with eczema. Dermatologica 1980;160(4):261-9. [DOI] [PubMed] [Google Scholar]
Matheson 2008 {published data only}
- Matheson R, Kempers S, Breneman D, Draelos Z, Johnson CE, Loss R, et al. Hydrocortisone butyrate 0.1% lotion in the treatment of atopic dermatitis in pediatric subjects. Journal of Drugs in Dermatology 2008;7(3):266-71. [PubMed] [Google Scholar]
Meenan 1963 {published data only}
- Meenan FO. The treatment of infantile eczema with fluocinolone acetonide cream. Journal of the Irish Medical Association 1963;52:75-6. [PubMed] [Google Scholar]
Meurer 2002 {published data only}
- Meurer M, Fartasch M, Albrecht G, Vogt T, Worm M, Ruzicka T, et al. Long-term efficacy and safety of pimecrolimus cream 1% in adults with moderate atopic dermatitis. Dermatology (Basel, Switzerland) 2004;208(4):365-72. [DOI] [PubMed] [Google Scholar]
- Meurer M, Folster-Holst R, Wozel G, Weidinger G, Junger M, Brautigam M. Pimecrolimus cream 1% provides rapid reduction of pruritus in adults with moderate atopic eczema. Journal of the European Academy of Dermatology and Venereology : JEADV 2002;16(Suppl S1):132. [Google Scholar]
- Meurer M, Folster-Holst R, Wozel G, Weidinger G, Junger M, Brautigam M. Pimecrolimus cream 1% provides significant and rapid relief of pruritus and improves disease control and quality of life in atopic dermatitis in adults. Journal of Investigative Dermatology 2002;119(1):350. [DOI] [PubMed] [Google Scholar]
- Meurer M, Folster-Holst R, Wozel G, Weidinger G, Junger M, Brautigam M, CASM-DE-01 study group. Pimecrolimus cream in the long-term management of atopic dermatitis in adults: a six-month study. Dermatology (Basel, Switzerland) 2002;205(3):271-7. [DOI] [PubMed] [Google Scholar]
- Meurer M. Pimecrolimus (SDZ ASM 981) cream improves disease control and quality of life in the long-term management of atopic dermatitis in adults. Annales de Dermatologie et de Venereologie 2002;129:IC1244. [Google Scholar]
- Meurer M. Sparing corticosteroids in adults with atopic eczema. Journal of the European Academy of Dermatology and Venereology : JEADV 2002;16(Suppl S1):96. [Google Scholar]
Meurer 2010 {published data only}
- EUCTR2005-000839-17-DE. A 16-week, randomized, multi-center, parallel-group, pimecrolimus-blinded, controlled study (4-week treatment period followed by 12-week observational period) to evaluate the safety of concomitant use of ASM 981 (pimecrolimus) cream 1% (BID) plus topical corticosteroid (BID) for the treatment of severe atopic dermatitis in patients 2 to 17 years of age. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2005-000839-17-DE (first received 9 May 2006).
- Eichenfield LF, Meurer M, Hultsch T, Ho V. Concomitant use of topical corticosteroids and pimecrolimus cream 1% in the treatment of severe pediatric atopic dermatitis has a safety profile comparable to topical corticosteroids alone. Journal of the American Academy of Dermatology 2009;60(3 Suppl 1):AB69. [Google Scholar]
- Meurer M, Eichenfield LF, Ho V, Potter PC, Werfel T, Hultsch T. Addition of pimecrolimus cream 1% to a topical corticosteroid treatment regimen in paediatric patients with severe atopic dermatitis: a randomized, double-blind trial. Journal of Dermatological Treatment 2010;21(3):157-66. [DOI] [PubMed] [Google Scholar]
Mobacken 1986 {published data only}
- Mobacken H, Hersle K. Alclometasone dipropionate ointment 0.05% versus hydrocortisone ointment 1.0% in children with eczema. Acta Therapeutica 1986;12:269-78. [Google Scholar]
Mowla 2020 {published data only}
- Das SP, Mowla MR, Barua DP, Jahangir SM, Khan I. Efficacy and safety of 0.1% tacrolimus ointment versus 0.05% clobetasone butyrate ointment in childhood atopic dermatitis. Forum Dermatologicum 2020;6(2):33-9. [Google Scholar]
- Mowla MR, Das Sanjoy SP. Efficacy and safety of 0.1% tacrolimus ointment versus 0.05% clobetasone butyrate ointment in childhood atopic dermatitis. Journal of the Dermatology Nurses' Association 2020;12(2):85-8. [Google Scholar]
Mudaliyar 2020 {published data only}
- Mudaliyar VR, Pathak A, Dixit A, Kumar SS. An open-label prospective study to compare the efficacy and safety of topical fluticasone versus tacrolimus in the proactive treatment of atopic dermatitis. Dermatology Practical & Conceptual 2020;10(4):e2020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Munro 1967 {published data only}
- Munro DD, Wilson HT, Rhodes EL. Comparison of betamethasone 17-valerate ointment with fluocortolone and fluocortolone caproate ointment. British Medical Journal 1967;4(5574):275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Munro 1975 {published data only}
- Munro DD, Wilson L. Clobetasone butyrate, a new topical corticosteroid: clinical activity and effects on pituitary-adrenal axis function and model of epidermal atrophy. British Medical Journal 1975;3:626-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murrell 2007 {published data only}
- CASM981C2442. A 12 week multicenter study consisting of a 6 week double blind, randomized, vehicle controlled, parallel group phase, followed by a 6 week open label phase, to assess the safety and efficacy of pimecrolimus cream 1% in mild to moderate head and neck atopic dermatitis of patients intolerant of topical corticosteroid. www.novctrd.com/ctrdweb/trialresult/trialresults/pdf?trialResultId=2214 (first received 19 October 2004).
- Murrell DF, Calvieri S, Ortonne JP, Ho VC, Weise-Riccardi S, Barbier N, et al. A randomized controlled trial of pimecrolimus cream 1% in adolescents and adults with head and neck atopic dermatitis and intolerant of, or dependent on, topical corticosteroids. British Journal of Dermatology 2007;157(5):954-9. [DOI] [PubMed] [Google Scholar]
- NCT00121316. Safety and efficacy of pimecrolimus cream 1% in mild to moderate head and neck atopic dermatitis (AD) patients. clinicaltrials.gov/show/NCT00121316 (first received 21 July 2005).
- Weise-Riccardi S, Calvieri S, Ortonne J, Murrell DF, Ho VC, Emady-Azar S, et al. Randomized vehicle-controlled trial of pimecrolimus cream 1 % in adult patients with mild to moderate head and neck atopic dermatitis intolerant of, or dependent on TCS. Journal of Investigative Dermatology 2006;126(Suppl 1):46. [Google Scholar]
- Weise-Riccardi S, Murrell DF, Ho VC, Ortonne JP, Paul C. Randomized vehicle-controlled trial of pimecrolimus cream 1% in adult patients with mild to moderate head and neck atopic dermatitis intolerant of, or dependent on, topical corticosteroids. British Journal of Dermatology 2006;155(Suppl 1):43-4. [DOI] [PubMed] [Google Scholar]
Nakagawa 1997 {published data only}
- Nakagawa H. FK506 (Tacrolimus) ointment for atopic dermatitis-results of a double-blind, optimal dose finding study in a multicenter trial Japanese FK506 ointment study group. Australasian Journal of Dermatology 1997;38(Suppl 2):62. [Google Scholar]
Nakagawa 1998 {published data only}
- Nakagawa HF. Comparative study of FK506 (Tacrolimus) ointment vs alclometasone dipropionate ointment in atopic dermatitis (face and neck lesions). Journal of Investigative Dermatology 1998;110(4):683. [Google Scholar]
Nakagawa 2018a {published data only}
- JapicCTI-152887. Phase II clinical study of JTE-052 - dose finding study in patients with atopic dermatitis. www.clinicaltrials.jp/cti-user/trial/ShowDirect.jsp?japicId=JapicCTI-152887 (first received 28 April 2015).
- Nakagawa H, Nemoto O, Igarashi A, Nagata T. Efficacy and safety of topical JTE-052, a Janus kinase inhibitor, in Japanese adult patients with moderate-to-severe atopic dermatitis: a phase II, multicentre, randomized, vehicle-controlled clinical study. British Journal of Dermatology 2018;178(2):424-32. [DOI] [PubMed] [Google Scholar]
Nakagawa 2018b {published data only}
- Nakagawa H, Nemoto O, Yamada H, Nagata T, Ninomiya N. Phase 1 studies to assess the safety, tolerability and pharmacokinetics of JTE-052 (a novel Janus kinase inhibitor) ointment in Japanese healthy volunteers and patients with atopic dermatitis. Journal of Dermatology 2018;45:701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakagawa 2019 {published data only}
- JapicCTI-173553. A phase II study of JTE-052 ointment in pediatric patients with atopic dermatitis. www.clinicaltrials.jp/cti-user/trial/ShowDirect.jsp?japicId=JapicCTI-173553 (first received 31 March 2017).
- Nakagawa H, Nemoto O, Igarashi A, Saeki H, Kabashima K, Oda M. Efficacy and safety of topical delgocitinib (JTE-052), janus kinase inhibitor, in Japanese pediatric patients with atopic dermatitis: a phase II, randomized, double-blind, vehicle-controlled study. Journal of the American Academy of Dermatology 2019;81(4 Suppl 1):AB53. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Nemoto O, Igarashi A, Saeki H, Oda M, Kabashima K, et al. Phase 2 clinical study of delgocitinib ointment in pediatric patients with atopic dermatitis. Journal of Allergy and Clinical Immunology 2019;144(6):1575‐83. [DOI] [PubMed] [Google Scholar]
Nakagawa 2020 {published data only}
- JapicCTI-173554. Phase III clinical study of JTE-052 - randomized controlled and long-term extension study in patients with atopic dermatitis. www.clinicaltrials.jp/cti-user/trial/ShowDirect.jsp?japicId=JapicCTI-173554 (first received 31 March 2017).
- Nakagawa H, Nemoto O, Igarashi A, Saeki H, Kaino H, Nagata T. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. Journal of the American Academy of Dermatology 2020;82(4):823‐31 (Correction in Journal of the American Academy of Dermatology 2021; 85(4):1069). [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Nemoto O, Igarashi A, Saeki H, Kaino H. Efficacy and safety of topical delgocitinib (JTE-052), janus kinase inhibitor, in Japanese adult patients with moderate-to-severe atopic dermatitis: a phase III, randomized, double-blind, vehicle-controlled study. Journal of the American Academy of Dermatology 2019;81(4 Suppl 1):AB199. [Google Scholar]
Nakagawa 2021 {published data only}
- JapicCTI-184064. Phase III study of JTE-052 ointment in pediatric patients with atopic dermatitis. www.clinicaltrials.jp/cti-user/trial/ShowDirect.jsp?japicId=JapicCTI-184064 (first received 14 August 2018).
- Nakagawa H, Nemoto O, Igarashi A, Saeki H, Kabashima K, Oda M, et al. Delgocitinib ointment in pediatric patients with atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and a subsequent open-label, long-term study. Journal of the American Academy of Dermatology 2021;85(4):854‐62. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Nemoto O, Igarashi A, Saeki H, Kabashima K, Oda M, et al. Delgocitinib ointment in pediatric patients with atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. Journal of the American Academy of Dermatology 2021;85(3):AB52. [DOI] [PubMed] [Google Scholar]
Natarajan 1974 {published data only}
- Natarajan M. A comparative clinical trial of desoxymethasone in the treatment of eczematous conditions. Indian Practitioner 1974;27(11):525-32. [Google Scholar]
NCT00810862 {published data only}
- NCT00810862. Study of pimecrolimus treatment for atopic dermatitis of African American children. clinicaltrials.gov/show/NCT00810862 (first received 8 December 2008).
NCT00927212 {published data only}
- NCT00927212. Topical application of AS101 for the treatment of atopic dermatitis. clinicaltrials.gov/ct2/show/NCT00927212 (first received 24 June 2009).
NCT00996008 {published data only}
- NCT00996008. CT 327 in the treatment of atopic dermatitis. clinicaltrials.gov/show/NCT00996008 (first received 16 October 2009).
NCT01037881 {published data only}
- NCT01037881. LEO 29102 cream in the treatment of atopic dermatitis. clinicaltrials.gov/show/NCT01037881 (first received 23 December 2009).
NCT01053247 {published data only}
- NCT01053247. Study of 0416 ointment in the treatment of atopic dermatitis. clinicaltrials.gov/show/NCT01053247 (first received 21 January 2010).
NCT01139450 {published data only}
- NCT01139450. Study of 0417 ointment in the treatment of atopic dermatitis. clinicaltrials.gov/show/NCT01139450 (first received 8 June 2010).
NCT01702181 {published data only}
- NCT01702181. A safety study to evaluate the use and effectiveness of a topical ointment to treat adults with atopic dermatitis. clinicaltrials.gov/show/NCT01702181 (first received 5 October 2012).
NCT01856764 {published data only}
- EUCTR2012-003000-12-DE. A human study to investigate the positive and negative effects of ointments containing roflumilast in patients suffering from a chronic inflammatory skin rash. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2012-003000-12-DE (first received 14 August 2012).
- NCT01856764. Topical roflumilast in adults with atopic dermatitis. clinicaltrials.gov/show/NCT01856764 (first received 17 May 2013).
NCT02031445 {published data only}
- NCT02031445. Double-blind, trial to evaluate the safety and efficacy of MRX-6 cream 2%. clinicaltrials.gov/show/NCT02031445 (first received 9 January 2014).
NCT02118766 {published data only}
- Eichenfield L, Call R, Forsha D, Fowler J, Hebert A, Spellman M, et al. Long-term safety of crisaborole in children and adults with mild-to-moderate atopic dermatitis. Pediatric Dermatology 2016;1:S46-S47. [DOI] [PubMed] [Google Scholar]
- Eichenfield L, Call R, Forsha D, Fowler J, Hebert A, Spellman M, et al. Long-term safety of crisaborole ointment in children, adolescents, and adults with mild to moderate atopic dermatitis (abstract). Pediatric Dermatology (Conference: 13th World Congress of Pediatric Dermatology, United States) 2017;34:S32‐S33. [DOI] [PubMed] [Google Scholar]
- Eichenfield LF, Yosipovitch G, Stein Gold LF, Kalabis M, Zang C, Vlahos B, et al. Improvement in disease severity and pruritus outcomes with crisaborole ointment, 2%, by baseline atopic dermatitis severity in children and adolescents with mild-to-moderate atopic dermatitis. Pediatric Dermatology 2020;37(6):1030‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler J, Sidbury R, Zaenglein A, Pariser D, Cook-Bolden F. Crisaborole ointment improves global atopic dermatitis severity: pooled results from two phase 3 clinical trials. Journal of the American Academy of Dermatology 2017;76(6):AB86. [Google Scholar]
- Fowler J, Sidbury R, Zaenglein A, Pariser D, Cook-Bolden F. Crisaborole ointment improves global atopic dermatitis severity: pooled results from two phase 3 clinical trials. Pediatric Dermatology 2017;34(S1):S33. [Google Scholar]
- Fowler J, Sidbury R, Zaenglein AL, Pariser DM, Cook-Bolden FE. Crisaborole ointment improves global atopic dermatitis severity: pooled results from two phase 3 clinical trials. Journal of Investigative Dermatology (Conference: 47th Annual Meeting of the European Society for Dermatological Research, ESDR 2017. Austria) 2017;137(10 Supplement 2):S193. [Google Scholar]
- Fowler J, Sugarman J, Sher L, Zang C, Werth JL, Myers DE, et al. Impact of crisaborole on sleep outcomes in pediatric patients with mild-to-moderate atopic dermatitis and their families. Pediatric Dermatology 2021;38(5):1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler J, Sugarman J, Sher L, Zang C, Werth JL, Myers DE, et al. Impact of crisaborole on sleep outcomes in pediatric patients with mild-to-moderate atopic dermatitis. Dermatology and Therapy 2023;13(4):951-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler J, Sugarman J, Sher L, Zang C, Werth JL, Myers DE, et al. Improvements in sleep outcomes for crisaborole-treated pediatric patients with mild-to-moderate atopic dermatitis and their families. Pediatrics (2021 National Conference and Exhibition Abstract) 2022;149:262. [Google Scholar]
- Fowler JF, Sidbury R, Zaenglein AL, Pariser DM, Cook-Bolden FE. Crisaborole ointment improves global atopic dermatitis severity: pooled results from two phase 3 clinical trials. Australasian Journal of Dermatology (Conference: 51st Annual Scientific Meeting of the Australasian College of Dermatologists. Australia) 2018;59(Supplement 1):66. [Google Scholar]
- Hebert AA, Eichenfield LF, Lebwohl MG, Paller AS, Simpson EL, Tom WL, et al. A novel nonsteroidal, topical anti-inflammatory, phosphodiesterase 4 inhibitor, crisaborole ointment, reduced pruritus and signs of atopic dermatitis in 2 phase 3 studies in children and adults. Pediatrics 2018;141(1):251. [Google Scholar]
- Lio P, Cork J, Blaiss S, Kessel A, Cantrell WC, Werth L, et al. Efficacy and safety of crisaborole in patients with mild-to-moderate atopic dermatitis with and without comorbid allergies or asthma. British Journal of Dermatology 2021;185(3):e110. [DOI] [PubMed] [Google Scholar]
- Lio PA, Spergel JM, Cork MJ, Blaiss MS, Kessel A, Cantrell WC, et al. 26304 Efficacy and safety of crisaborole in patients with mild-to-moderate atopic dermatitis with and without comorbid allergies or asthma. Journal of the American Academy of Dermatology 2021;85(3):AB97. [DOI] [PubMed] [Google Scholar]
- NCT02118766. Safety and efficacy of AN2728 topical ointment, 2% in children, adolescents, and adults (ages 2 years and older) with atopic dermatitis. clinicaltrials.gov/show/NCT02118766 (first received 21 April 2014).
- NCT02118766. Safety and efficacy of AN2728 topical ointment, 2% in children, adolescents, and adults (ages 2 years and older) with atopic dermatitis. https://www.clinicaltrials.gov/study/NCT02118766 (date received April 2014).
- Paller A, Tom W, Lebwohl MM, Blumenthal RL, Boguniewicz M, Eichenfield LS, et al. Crisaborole topical ointment, 2%: a novel, nonsteroidal, topical anti-inflammatory, phosphodiesterase 4 inhibitor: results from two phase 3 studies treating children and adult patients with mild to moderate atopic dermatitis. Journal of the American Academy of Dermatology. 2016;74(5 Suppl 1):AB86. [Google Scholar]
- Paller AS, Luger TA, Hebert AA, Zaenglein AL, Silverberg JI, Tallman AM, et al. Crisaborole ointment improves global atopic dermatitis severity across patients with varying baseline characteristics: pooled results from two phase III trials (abstract). British Journal of Dermatology (Conference: 10th George Rajka International Symposium on Atopic Dermatitis. Netherlands) 2018;179:e39‐e40. [Google Scholar]
- Paller AS, Tom WL, Lebwohl MG, Blumenthal RL, Boguniewicz M, Call RS, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. Journal of the American Academy of Dermatology 2016;75(3):e6.494-503. [DOI] [PubMed] [Google Scholar]
- Paller AS, Tom WL, Lebwohl MG, Blumenthal RL, Boguniewicz M, Call RS, et al. Phase 3 studies treating children and adult patientswithmild tomoderate atopic dermatitis with crisaborole ointment, a novel, nonsteroidal, topical anti-inflammatory, phosphodiesterase 4 inhibitor. Pediatric Dermatology 2016;33(6):705. [Google Scholar]
- Paller AS, Tom WL, Lebwohl MG, Blumenthal RL, Boguniewicz M, Call RS, et al. Results from two phase 3 studies in children and adults with mildto-moderate atopic dermatitis treated with crisaborole topical ointment, 2%, a novel nonsteroidal, topical anti-inflammatory, phosphodiesterase 4 inhibitor. Journal of Clinical and Aesthetic Dermatology 2016;9(5):S10‐11. [Google Scholar]
- Paller AS, Tom WL, Lebwohl MG, Boguniewicz M, Eichenfield LF, Forsha DW, et al. Phase 3 studies treating children and adult patients with mild to moderate atopic dermatitis with crisaborole ointment, a novel, nonsteroidal, topical antiinflammatory, phosphodiesterase 4 inhibitor. Pediatrics 2018;141(1):275. [Google Scholar]
- Paller AS, Yosipovitch G, Tom WL, Lebwohl M, Boguniewicz M, Call RS, et al. Crisaborole ointment effect on global atopic dermatitis severity and early relief in pruritus: pooled results from two phase 3 clinical trials (abstract). Pediatrics 2018;142:814. [Google Scholar]
- Silverberg JI, Tallman AM, Ports WC, Gerber RA, Tan H, Zielinski MA. Evaluating the efficacy of crisaborole using the atopic dermatitis severity index and percentage of affected body surface area. Acta Dermato-Venereologica 2020;100(13):adv00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E, Paller A, Boguniewicz M, Eichenfield L, Feldman S, Silverberg J, et al. Crisaborole demonstrates improvement in quality of life in patients with mild to moderate atopic dermatitis. Experimental Dermatology 2016;25:28‐9. [Google Scholar]
- Simpson EL, Paller AS, Boguniewicz M, Eichenfield LF, Feldman SR, Silverberg JI, et al. Crisaborole ointment improves quality of life of patients with mild to moderate atopic dermatitis and their families. Dermatology and Therapy 2018;8(4):605‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EL, Tom WL, Bushmakin AG, Cappelleri JC, Yosipovitch G, Stander S, et al. Relationship among treatment, pruritus, Investigator’s Static Global Assessment, and Quality of Life in patients with atopic dermatitis. Dermatology and Therapy 2021;11(2):587‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spergel J, Blaiss M, Lio P, Kessel A, Takiya L, Werth J, et al. Efficacy and safety of crisaborole in patients with mild-to-moderate atopic dermatitis with comorbid allergic rhinitis or asthma. Journal of Allergy and Clinical Immunology 2021;147(2):AB28. [DOI] [PubMed] [Google Scholar]
- Spergel J, Blaiss M, Lio P, Kessel A, Takiya L, Werth J, et al. Efficacy of crisaborole in patients with mild-to-moderate atopic dermatitis with and without comorbid allergic rhinitis. Annals of Allergy, Asthma and Immunology 2020;125(5):S46. [DOI] [PubMed] [Google Scholar]
- Stander S, Yosipovitch G, Bushmakin AG, Cappelleri JC, Luger T, Tom WL, et al. Examining the association between pruritus and quality of life in patients with atopic dermatitis treated with crisaborole. Journal of the European Academy of Dermatology and Venereology : JEADV 2019;33(9):1742‐6. [DOI] [PubMed] [Google Scholar]
- Stein-Gold L, Shi V, Vlahos B, Sanders P, Zang C, Cha, et al. Impact of crisaborole in patients with mild-to-moderate atopic dermatitis who received prior treatment. British Journal of Dermatology 2022;187(3):e118-e119. [Google Scholar]
- Ständer P, Yosipovitch G, Bushmakin A, Cappelleri J, Luger T, Tom W, et al. Relationship between pruritus and quality of life in patients with atopic dermatitis treated with crisaborole. Journal of the American Academy of Dermatology 2018;79(3):AB244. [DOI] [PubMed] [Google Scholar]
- Thyssen JP, Zang C, Neary MP, Bushmakin AG, Cappelleri JC, Cha A, et al. Evaluating the severity of atopic dermatitis in children and adults: mapping of Investigator's Static Global Assessment to Eczema Area and Severity Index in phase III studies of crisaborole 2%. British Journal of Dermatology 2021;184(3):e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zane LT, Paller AS, Tom WL, Lebwohl MG, Blumenthal RL, Boguniewicz M, et al. Two phase 3 study results of children and adults with mild-to-moderate atopic dermatitis treated with crisaborole topical ointment, 2%, a novel, nonsteroidal, topical, anti-inflammatory, phosphodiesterase 4 inhibitor. Journal of Immunology (Baltimore, Md. : 1950) 2016;196(1 Suppl):191.26. [Google Scholar]
NCT02118792 {published data only}
- Boguniewicz M, Paller AS, Tom WL, Lebwohl MG, Blumenthal RL, Call RS, et al. Efficacy and safety of crisaborole topical ointment, 2%, a novel, nonsteroidal, topical, anti-inflammatory, phosphodiesterase inhibitor in 2 phase 3 studies in children and adults with mild to-moderate atopic dermatitis. Journal of Allergy and Clinical Immunology 2016;137(2 Suppl):AB397. [Google Scholar]
- Hebert A, Eichenfield L, Lebwohl M, Paller A, Simpson E, Tom W, et al. Reduced pruritus and signs of atopic dermatitis in phase 3 trials with a nonsteroidal topical phosphodiesterase inhibitor, crisaborole. Pediatric Dermatology 2016;33(S1):S46. [Google Scholar]
- Hebert AA, Eichenfield L, Lebwohl MG, Paller AS, Simpson EL, Tom WL, et al. Reduced pruritus and signs of atopic dermatitis in phase 3 trials with a nonsteroidal topical phosphodiesterase inhibitor, crisaborole. Journal of Investigative Dermatology 2016;136(5 Suppl. 1):S49. [Google Scholar]
- NCT02118792. Safety and efficacy of AN2728 topical ointment, 2% in children, adolescents, and adults (aged 2 years and older) with atopic dermatitis. clinicaltrials.gov/show/NCT02118792 (first received 21 April 2014).
- Paller A, Tom W, Call R, Eichenfield L, Forsha D, Rees W, et al. Crisaborole topical ointment 2%, a novel, nonsteroidal, topical, anti-inflammatory, phosphodiesterase inhibitor, in 2 phase 3 studies in children and adults with mild-to-moderate atopic dermatitis. Journal of Managed Care and Speciality Pharmacy 2016;22(4‐A Suppl):S99‐S100. [Google Scholar]
- Paller A, Tom W, Lebwohl M, Blumenthal R, Boguniewicz M, Call R, et al. Two phase 3 studies in atopic dermatitis with crisaborole, the novel, nonsteroidal topical phosphodiesterase 4 inhibitor. Pediatric Dermatology 2016;33(S1):S19. [Google Scholar]
- Paller AS, Tom WL, Call RS, Eichenfield L, Forsha D, Rees W, et al. A novel, nonsteroidal, topical, anti-inflammatory, phosphodiesterase inhibitor, crisaborole topical ointment, 2%, in two phase 3 studies in children and adults with mild to moderate atopic dermatitis. Journal of Investigative Dermatology 2016;136(9 Suppl 2):S166. [Google Scholar]
- Paller AS, Tom WL, Call RS, Eichenfield L, Forsha D, Rees WC, et al. Phase 3 studies in atopic dermatitis with the novel, nonsteroidal topical phosphodiesterase 4 inhibitor, crisaborole. Journal of Investigative Dermatology 2016;136(5 Suppl. 1):S50. [Google Scholar]
- Stein-Gold L, Spelman L, Spellman M, Hughes M, Zane L. Safety and efficacy of AN2728 topical ointment, 2% and 0.5% in adolescents with atopic dermatitis. Journal of Investigative Dermatology 2014;134(Suppl 2):S36. [Google Scholar]
- Stein-Gold L, Zane L, Spelman L, Spellman M, Hughes M. Safety and efficacy of AN2728 topical ointment, 2% and 0.5%, in a phase II dose-ranging study of adolescents with mild to moderate atopic dermatitis. Journal of the American Academy of Dermatology 2014;70(5 Suppl. 1):AB65. [Google Scholar]
- Yosipovitch G, Gold LF, Lebwohl MG, Silverberg JI, Tallman AM, Zane LT. Early relief of pruritus in atopic dermatitis with crisaborole ointment, a non-steroidal, phosphodiesterase 4 inhibitor. Acta Dermato-Venereologica 2018;98(5):484-9. [DOI] [PubMed] [Google Scholar]
- Zane LT, Hebert AA, Eichenfield LF, Lebwohl MG, Paller AS, Simpson EL, et al. Crisaborole topical ointment, 2%, a novel nonsteroidal, anti-inflammatory, topical, phosphodiesterase 4 inhibitor, reduced pruritus and signs of atopic dermatitis in 2 phase 3 studies in children and adults with mild-to-moderate atopic dermatitis. Journal of Immunology (Baltimore, Md. : 1950) 2016;196(1 Suppl):191.27. [Google Scholar]
NCT02376049 {published data only}
- NCT02376049. A clinical trial to compare topical agents in adults with mild to moderate atopic dermatitis (AD). clinicaltrials.gov/show/NCT02376049 (first received 3 March 2015).
NCT02595073 {published data only}
- NCT02595073. Clinical study to evaluate the efficacy and safety of desoximetasone (DSXS) with atopic dermatitis. clinicaltrials.gov/ct2/show/NCT02595073 (first received 3 November 2015).
NCT02950922 {published data only}
- NCT02950922. Study of RVT-501 in adult and adolescent subjects with atopic dermatitis. clinicaltrials.gov/ct2/show/NCT02950922 (first received 1 November 2016).
NCT03386032 {published data only}
- NCT03386032. 8-week atopic dermatitis (AD) treatment study. clinicaltrials.gov/show/NCT03386032 (first received 29 December 2017).
NCT03539601 {published data only}
- EUCTR2018-001043-31-SE. A phase 3b/4 study of the efficacy, safety, and local tolerability of crisaborole ointment, 2% in pediatric and adult subjects (ages 2 years and older) with mild to moderate atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2018-001043-31-SE (first received 10 September 2018).
- NCT03539601. A study of crisaborole ointment 2%; crisaborole vehicle; TCS and TCI in subjects aged ≥ 2 years, with mild-moderate AD. clinicaltrials.gov/show/NCT03539601 (first received 29 May 2018).
NCT03645057 {published data only}
- NCT03645057. ASPIRE: PROs & caregiver burden in children with atopic dermatitis. clinicaltrials.gov/show/NCT03645057 (first received 24 August 2018).
- Wieser J, Chen A, Lee G, Baughman L, Pope EM, Franco A, et al. 388 Impact of crisaborole & tacrolimus 0.03% on patient-reported outcomes and caregiver burden in children with atopic dermatitis. Journal of Investigative Dermatology 2022;142(8):S66. [Google Scholar]
NCT03725722 {published data only}
- NCT03725722. Dose-ranging trial to evaluate delgocitinib cream 1, 3, 8, and 20 mg/g compared to delgocitinib cream vehicle over an 8-week treatment period in adult subjects with atopic dermatitis. clinicaltrials.gov/show/NCT03725722 (first received 31 October 2018).
NCT03745638 {published data only}
- Blauvelt A, Eichenfield LF, Kuligowski ME, Venturanza ME, Sun K, Silverberg JI. Efficacy of ruxolitinib cream among patients with atopic dermatitis based on previous medication history: pooled results from two phase 3 studies. Journal of the American Academy of Dermatology 2021;85(3):AB148. [Google Scholar]
- Blauvelt A, Eichenfield LF, Kuligowski ME, Venturanza ME, Sun K, Silverberg JI. Long-term safety and disease control with ruxolitinib cream in patients with atopic dermatitis based on previous medication history: pooled results from two phase III studies. British Journal of Dermatology 2021;185(3):e136‐e137. [Google Scholar]
- Blauvelt A, Kircik L, Papp KA, Simpson EL, Silverberg JI, Kim BS, et al. Rapid pruritus reduction with ruxolitinib cream treatment in patients with atopic dermatitis. Journal of the European Academy of Dermatology and Venereology : JEADV 2023;37(1):137-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauvelt A, Szepietowski JC, Papp K, Simpson E, Silverberg JI, Kim BS, et al. Itch-free state in patients with atopic dermatitis treated with ruxolitinib cream. Journal of Investigative Dermatology 2021;141(5 Suppl):S55. [DOI] [PubMed] [Google Scholar]
- Blauvelt A, Szepietowski JC, Papp K, Simpson E, Silverberg JI, Kim BS, et al. Ruxolitinib cream rapidly decreases skin pain in atopic dermatitis. Journal of Investigative Dermatology 2021;141(5):S57. [Google Scholar]
- Blauvelt A, Szepietowski JC, Papp K, Simpson E, Sturm D, Kallender H, et al. 307 High-threshold responses to ruxolitinib cream in adolescents and adults with atopic dermatitis. Journal of Investigative Dermatology 2022;142(8 Supplement):S52. [Google Scholar]
- Blauvelt A, Szepietowski JC, Papp K, Simpson EL, Silverberg JI, Kim BS, et al. Itch-free state in patients with atopic dermatitis treated with ruxolitinib cream: a pooled analysis from two randomized phase 3 studies. Journal of the American Academy of Dermatology 2022;14:14. [DOI] [PubMed] [Google Scholar]
- Blauvelt A, Szepietowski JC, Papp K, Simpson EL, Silverberg JI, Kim BS, et al. Ruxolitinib cream rapidly decreases pruritus in atopic dermatitis: pooled results from two phase 3 studies. Journal of the American Academy of Dermatology 2021;85(3):AB121. [Google Scholar]
- Bloudek L, Eichenfield LF, Silverberg JI, Joish VN, Lofland JH, Sun K, et al. Impact of ruxolitinib cream on work productivity and activity impairment and associated indirect costs in patients with atopic dermatitis: pooled results from two phase III studies. American Journal of Clinical Dermatology 2022;20:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenfield L, Augustin M, Armstrong A, Venturanza M, Kallender H, Gao J, et al. Effects of ruxolitinib cream on work productivity and activity impairment in patients with atopic dermatitis: 52-week pooled results from two phase 3 studies. Journal of Managed Care and Speciality Pharmacy 2022;28(10):S97. [Google Scholar]
- Eichenfield LF, Blauvelt A, Kircik L, Venturanza ME, Ren H, Simpson EL. 35163 Ruxolitinib cream provided progressive improvement in patients with atopic dermatitis who did not achieve Investigator's Global Assessment treatment success at week 8: pooled results from 2 phase 3 studies. Journal of the American Academy of Dermatology 2022;87(3 Supplement):AB202. [Google Scholar]
- Eichenfield LF, Silverberg JI, Kuligowski ME, Venturanza ME, Sun K, Augustin M. Effects of ruxolitinib cream on work productivity and activity impairment in patients with atopic dermatitis: pooled results from two phase 3 studies. Journal of the American Academy of Dermatology 2021;85(3):AB179. [Google Scholar]
- Eichenfield LF, Simpson EL, Papp K, Szepietowski JC, Kircik L, Toth D, et al. Long-term safety and disease control of ruxolitinib cream among adolescents with atopic dermatitis: results from two phase 3 studies. Pediatric Dermatology 2021;38(5):1433‐4. [DOI] [PubMed] [Google Scholar]
- Eichenfield LF, Simpson EL, Siegfried EC, Kuligowski ME, Venturanza ME, Sun K, et al. 27633 Efficacy and safety of ruxolitinib cream among adolescents with atopic dermatitis: pooled results from two phase 3 studies. Journal of the American Academy of Dermatology 2021;85(3):AB158. [DOI] [PubMed] [Google Scholar]
- Eichenfield LF, Simpson EL, Siegfried EC, Kuligowski ME, Venturanza ME, Sun K, et al. Efficacy and safety of ruxolitinib cream among adolescents with atopic dermatitis: pooled results from two phase 3 studies. Pediatric Dermatology 2021;38(5):1424. [Google Scholar]
- Eichenfield LF, Stein Gold LF, Brar KK, Chiesa Fuxench ZC, Silverberg JI, Venturanza ME, et al. 275 Effects of ruxolitinib cream on sleep and quality of life over 52 weeks in black patients with atopic dermatitis. Journal of Investigative Dermatology 2022;142(8 Supplement):S47. [Google Scholar]
- Eichenfield LF, Stein Gold LF, Brar KK, Chiesa Fuxench ZC, Silverberg JI, Venturanza ME, et al. 287 Effects of ruxolitinib cream on pruritus in black patients with atopic dermatitis. Journal of Investigative Dermatology 2022;142(8 Supplement):S49. [Google Scholar]
- Eichenfield LF, Stein Gold LF, Chiesa Fuxench ZC, Venturanza ME, Ren H, Brar KK. 34794 Long-term safety and disease control of ruxolitinib cream among Black or African American patients with atopic dermatitis: pooled results from 2 phase 3 studies. Journal of the American Academy of Dermatology 2022;87(3 Supplement):AB180. [Google Scholar]
- Liu H, Gong X, Smith SH. LB991 Clinical serum biomarker profiling in TRuE-AD1 and TRuE-AD2 studies after 8 weeks of treatment with ruxolitinib cream. Journal of Investigative Dermatology 2022;142(8):B27. [Google Scholar]
- NCT03745638. Topical ruxolitinib evaluation in atopic dermatitis study 1 (TRuE AD1) - an efficacy and safety study of ruxolitinib cream in adolescents and adults with atopic dermatitis. clinicaltrials.gov/show/NCT03745638 (first received 19 November 2018).
- Papp K, Szepietowski JC, Kircik L, Toth D, Eichenfield LF, Forman SB, et al. 208 Long-term safety and disease control with ruxolitinib cream in atopic dermatitis: results from two phase 3 studies. Journal of Investigative Dermatology 2021;141(10):S184. [DOI] [PubMed] [Google Scholar]
- Papp K, Szepietowski JC, Kircik L, Toth D, Eichenfield LF, Forman SB, et al. 27716 Efficacy of ruxolitinib cream for the treatment of atopic dermatitis by baseline clinical characteristics: pooled subgroup analysis from two randomized phase 3 studies. Journal of the American Academy of Dermatology 2021;85(3):AB160. [DOI] [PubMed] [Google Scholar]
- Papp K, Szepietowski JC, Kircik L, Toth D, Eichenfield LF, Forman SB, et al. Efficacy of ruxolitinib cream for the treatment of atopic dermatitis by baseline patient demographics: pooled subgroup analysis from two randomized phase 3 studies. Journal of the American Academy of Dermatology 2021;85(3):AB149. [DOI] [PubMed] [Google Scholar]
- Papp K, Szepietowski JC, Kircik L, Toth D, Eichenfield LF, Forman SB, et al. Long-term safety and disease control with ruxolitinib cream in atopic dermatitis: pooled results from two phase 3 studies. British Journal of Dermatology 2022;186(4):e167. [DOI] [PubMed] [Google Scholar]
- Papp K, Szepietowski JC, Kircik L, Toth D, Eichenfield LF, Forman SB, et al. Long-term safety and disease control with ruxolitinib cream in atopic dermatitis: results from two phase 3 studies. Journal of the American Academy of Dermatology 2022;26:26. [DOI] [PubMed] [Google Scholar]
- Papp K, Szepietowski JC, Kircik L, Toth D, Eichenfield LF, Forman SB, et al. Long-term safety and disease control with ruxolitinib cream in atopic dermatitis: results from two phase 3 studies. Journal of the American Academy of Dermatology 88;5:1008-16. [DOI] [PubMed] [Google Scholar]
- Papp K, Szepietowski JC, Kircik L, Toth D, Eichenfield LF, Forman SB, et al. Long-term safety and disease control with ruxolitinib cream in atopic dermatitis: results from two phase III studies. British Journal of Dermatology 2021;185(3):e134‐e135. [DOI] [PubMed] [Google Scholar]
- Papp K, Szepietowski JC, Kircik L, Toth D, Eichenfield LF, Leung DY, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. Journal of the American Academy of Dermatology 2021;85(4):863-72. [DOI] [PubMed] [Google Scholar]
- Papp K, Szepietowski JC, Kircik L, Toth D, Kuligowski ME, Venturanza M, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from two phase III randomized double-blind studies. British Journal of Dermatology 2020;183(4):e116‐e117. [DOI] [PubMed] [Google Scholar]
- Papp K, Szepietowski JC, Kircik L, Toth D, Kuligowski ME, Venturanza ME, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: pooled analysis of two phase III, randomized, double-blind studies. British Journal of Dermatology 2021;184(3):e62‐e63. [DOI] [PubMed] [Google Scholar]
- Papp K, Szepietowski JC, Kircik L, Toth D, Kuligowski ME, Venturanza ME, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: pooled analysis of two phase III, randomized, double-blind studies. Journal of Clinical and Aesthetic Dermatology 2021;14(5 Suppl 1):S12‐S13. [DOI] [PubMed] [Google Scholar]
- Simpson E, Bissonnette R, Kuligowski ME, Venturanza ME, Sun K, Silverberg JI. Effects of ruxolitinib cream in patients with atopic dermatitis with head and/or neck involvement. Journal of Investigative Dermatology 2021;141(5):S55. [Google Scholar]
- Simpson E, Boguniewicz M, Thacj D, Misery L, Armstrong A, Silverberg JI, et al. Effects of ruxolitinib cream on pruritus and sleep in atopic dermatitis: 52-week pooled results from two Phase 3 studies. Journal of Clinical and Aesthetic Dermatology 2022;15(4 Suppl 1):S11. [Google Scholar]
- Simpson E, Lee M, Brar K, Kuligowski M, Venturanza M, Sun K, et al. Disease characteristics and burden in patients with atopic dermatitis: insights from two phase 3 studies of ruxolitnib cream. Journal of Allergy and Clinical Immunology 2021;147(2):AB35. [Google Scholar]
- Simpson E, Lee M, Brar KK, Kuligowski ME, Venturanza ME, Sun K, et al. Efficacy of ruxolitinib cream in adults and adolescents with atopic comorbidities. Journal of Investigative Dermatology 2021;141(5):S58. [Google Scholar]
- Simpson EL, Augustin M, Thaci D, Misery L, Silverberg JI, Armstrong AW, et al. Patient-reported outcomes of ruxolitinib cream for the treatment of atopic dermatitis: pooled results from two phase 3 studies. Journal of the American Academy of Dermatology 2021;85(3):AB178. [Google Scholar]
- Simpson EL, Bissonnette R, Stein Gold LF. 34587 Efficacy of ruxolitinib cream for the treatment of atopic dermatitis by anatomic region: pooled analysis from two randomized phase 3 studies. Journal of the American Academy of Dermatology 2022;87(3 Supplement):AB53. [Google Scholar]
- Simpson EL, Boguniewicz M, Thaci D, Misery L, Armstrong AW, Silverberg JI, et al. Effects of ruxolitinib cream on pruritus and sleep in atopic dermatitis: 52-week pooled results from two phase III studies. British Journal of Dermatology 2022;186(4):e166-e167. [Google Scholar]
- Simpson EL, Boguniewicz M, Thaci D, Misery L, Silverberg JI, Beck LA, et al. Effect of ruxolitinib cream on sleep disturbance and sleep impairment: pooled analysis from two randomized phase 3 studies. Journal of the American Academy of Dermatology 2021;85(3):AB121. [Google Scholar]
- Simpson EL, Kircik L, Blauvelt A, Kuligowski ME, Venturanza ME, Sun K, et al. Effects of ruxolitinib cream in patients with atopic dermatitis with baseline body surface area ≥ 10% and Eczema Area and Severity Index score ≥ 16: pooled results from two phase 3 studies. Journal of the American Academy of Dermatology 2021;85(3):AB157. [Google Scholar]
- Simpson EL, Kircik L, Blauvelt A, Kuligowski ME, Venturanza ME, Sun K, et al. Efficacy of ruxolitinib cream in patients with atopic dermatitis who demonstrated partial responses: pooled analysis from two randomized phase 3 studies. Journal of the American Academy of Dermatology 2021;85(3):AB50. [Google Scholar]
- Simpson EL, Kircik L, Blauvelt A, Kuligowski ME, Venturanza ME, Sun K, et al. Long-term safety and disease control with ruxolitinib cream in patients with more severe atopic dermatitis: pooled results from two phase III studies. British Journal of Dermatology 2021;185(3):e135‐e136. [Google Scholar]
- Simpson EL, Silverberg JI, Armstrong AW, Fuxench ZC, Venturanza ME, Kallender H, et al. Effects of ruxolitinib cream on patient-reported quality of life in atopic dermatitis: 52-week pooled results from two phase III studies. British Journal of Dermatology 2022;187(3):e122-e123. [Google Scholar]
- Szepietowski JC, Eichenfield LF, Silverberg JI, Blauvelt A, Papp K, Kircik L, et al. Efficacy of ruxolitinib cream on pruritus in adolescents with atopic dermatitis: pooled results from two phase 3 studies. Pediatric Dermatology 2021;38(5):1424. [Google Scholar]
NCT03745651 {published data only}
- EUCTR2018-003713-18-ES. Efficacy and safety study of ruxolitinib cream followed by a long term safety period in adolescents and adults with atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2018-003713-18-ES (first received 19 June 2019).
- NCT03745651. TRuE AD2 - an efficacy and safety study of ruxolitinib cream in adolescents and adults with atopic dermatitis. clinicaltrials.gov/show/NCT03745651 (first received 19 November 2018).
NCT04360187 {published data only}
- Ma L, Zhang L, Kobayashi M, Tao X, Qian Q, Cheng H, et al. Efficacy and safety of crisaborole ointment in Chinese and Japanese patients aged ≥ 2 years with mild-to-moderate atopic dermatitis. Journal of Dermatology 2020;50:847-55. [DOI] [PubMed] [Google Scholar]
- NCT04360187. Crisaborole for Asian subjects (≥ 2 years of age) with mild to moderate atopic dermatitis. clinicaltrials.gov/show/NCT04360187 (first received 24 April 2020).
NCT04530643 {published data only}
- NCT04530643. A phase II study of HY209 gel for atopic dermatitis patients (Shaperon). clinicaltrials.gov/show/NCT04530643 (first received 28 August 2020).
NCT04664153 {published data only}
- EUCTR2020-003977-23-PL. A study of the efficacy, safety, tolerability and pharmacokinetics of PF07038124 ointment in participants with eczema or psoriasis. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2020-003977-23-PL (date received 2020).
- NCT04664153. Study to assess efficacy, safety, tolerability and pharmacokinetics of PF-07038124 ointment in participants with atopic dermatitis or plaque psoriasis. clinicaltrials.gov/show/NCT04664153 (first received 11 December 2020).
NCT04773587 {published data only}
- NCT04773587. Trial of PDE4 inhibition with roflumilast for the management of atopic dermatitis. clinicaltrials.gov/show/NCT04773587 (first received 26 February 2021).
- Simpson E, Eichenfield LF, Gooderham M, Gonzalez M, Hebert AA, Papp KA, et al. CO136 Efficacy and safety of roflumilast cream 0.15% in adults and children aged 6 and older with mild to moderate atopic dermatitis in two phase 3 Integument trials. Value in Health 2023;26:S40. [Google Scholar]
NCT04773600 {published data only}
- EUCTR2021-006884-67-PL. A phase 3, 4-week, parallel group, double blind, vehicle-controlled study of the safety and efficacy of ARQ-151 cream 0.15% administered daily in subjects with atopic dermatitis. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2021-006884-67-PL (first received 27/05/2022).
- NCT04773600. Trial of PDE4 inhibition with roflumilast for the management of atopic dermatitis (INTEGUMENT-II). clinicaltrials.gov/show/NCT04773600 (first received 26 February 2021).
NCT05014568 {published data only}
- Eichenfield LF, Silverberg JI, Bissonnette R, Tallman AM, Brown PM, Rubenstein DS, et al. Tapinarof cream 1% once daily for the treatment of moderate to severe atopic dermatitis in children and adults: the pivotal phase 3 ADORING clinical program. Journal of Clinical and Aesthetic Dermatology 2022;15(4 Suppl 1):S15. [Google Scholar]
- Eichenfield LF, Silverberg JI, Bissonnette R, Tallman AM, Brown PM, Rubenstein DS, et al. Tapinarof cream 1% once daily for the treatment of moderate to severe atopic dermatitis in children and adults: the pivotal phase 3 ADORING clinical program. Pediatric Dermatology 2021;38(5):1452. [Google Scholar]
- Eichenfield LF, Silverberg JI, Bissonnette R, Tallman AM, Brown PM, Rubenstein DS, et al. Tapinarof cream 1% once daily for the treatment of moderate-to-severe atopic dermatitis in children and adults: the pivotal phase III ADORING clinical program. British Journal of Dermatology 2022;186(4):e136. [Google Scholar]
- Eichenfield LF, Silverberg JI, Bissonnette R, Tallman AM, Brown PM, Rubenstein DS, et al. Tapinarof cream 1% once daily for the treatment of moderate-to-severe atopic dermatitis in children and adults: the pivotal phase III ADORING clinical programme. British Journal of Dermatology 2021;185(3):e127-e128. [Google Scholar]
- Emma Andrus (Ed). ADORING 1 study reveals promising results for tapinarof in atopic dermatitis. https://www.dermatologytimes.com/view/adoring-1-study-reveals-promising-results-for-tapinarof-in-atopic-dermatitis (first received 18 July 2023) (accessed prior to 02/07/2024).
- NCT05014568. Tapinarof for the treatment of atopic dermatitis in children and adults. clinicaltrials.gov/show/NCT05014568 (first received 20 August 2021).
NCT05032859 {published data only}
- Dermavant Sciences. New positive topline results of tapinarof cream for AD down to 2 years. https://www.dermatologytimes.com/view/new-positive-topline-results-of-tapinarof-cream-for-ad-down-to-2-years (first received 18 July 2023) (accessed prior to 02/07/2024).
- NCT05032859. Tapinarof for the treatment of atopic dermatitis in children and adults (DMVT-505-3102). clinicaltrials.gov/show/NCT05032859 (first received 2 September 2021).
Nemoto 2016 {published data only}
- NCT02094235. A phase 1/2 study of E6005 in pediatric subjects with atopic dermatitis. clinicaltrials.gov/show/NCT02094235 (first received 21 March 2014).
- Nemoto O, Hayashi N, Kitahara Y, Furue M, Hojo S, Nomoto M, et al. Effect of topical phosphodiesterase 4 inhibitor E6005 on Japanese children with atopic dermatitis: results from a randomized, vehicle-controlled exploratory trial. Journal of Dermatology 2016;43(8):881‐7. [DOI] [PubMed] [Google Scholar]
Neumann 1971 {published data only}
- Neumann L. Efficacy of two fluorinated topical steroids in the treatment of common eczemas, on a once a day treatment schedule. Acta Dermato-Venereologica Supplementum 1971;52:74-6. [PubMed] [Google Scholar]
Neumann 2008 {published data only}
- Neumann E, Amtage D, Bruckner-Tuderman L, Mockenhaupt M. A single-center open-label long-term comparison of tacrolimus ointment and topical corticosteroids for treatment of atopic dermatitis. Journal of the German Society of Dermatology 2008;6(7):548-53. [DOI] [PubMed] [Google Scholar]
Nolting 1991 {published data only}
- Nolting S, Glowania HJ, Grunder K, Jablonski K, Donhauser G, Stalleicken D. Therapy of atopical eczema in children. Results of a controlled study with the topical corticosteroid preparations Mometason and Prednikarbat. Der Kinderarzt 1991;22(10):1689-97. [Google Scholar]
Noren 1989 {published data only}
- Noren P, Melin L. The effect of combined topical steroids and habit-reversal treatment in patients with atopic dermatitis. British Journal of Dermatology 1989;121(3):359-66. [DOI] [PubMed] [Google Scholar]
Ohba 2016 {published data only}
- NCT01179880. A study of E6005 in Japanese patients with atopic dermatitis. https://clinicaltrials.gov/show/NCT01179880 (accessed 05 October 2022).
- Ohba F, Matsuki S, Imayama S, Matsuguma K, Hojo S, Nomoto M, et al. Efficacy of a novel phosphodiesterase inhibitor, E6005, in patients with atopic dermatitis: an investigator-blinded, vehicle-controlled study. Journal of Dermatological Treatment 2016;27(5):467-72. [DOI] [PubMed] [Google Scholar]
- Ohba F, Nomoto M, Hojo S, Akama H. Safety, tolerability and pharmacokinetics of a novel phosphodiesterase inhibitor, E6005 ointment, in healthy volunteers and in patients with atopic dermatitis. Journal of Dermatological Treatment 2016;27(3):241-6. [DOI] [PubMed] [Google Scholar]
Ono 2020 {published data only}
- Ono R, Yagi M, Shoji A, Fujita K, Yoshida M, Ports WC, et al. Phase 1 study of crisaborole in Japanese healthy volunteers and patients with atopic dermatitis. Journal of Dermatology 2020;47:25-32. [DOI] [PubMed] [Google Scholar]
Onumah 2013 {published data only}
- Onumah N, Kircik L. Pimecrolimus cream and tacrolimus ointment in the treatment of atopic dermatitis: a pilot study on patient preference. Journal of Drugs in Dermatology 2013;12(10):1145-8. [PubMed] [Google Scholar]
Otsuki 2003 {published data only}
- Ohtsuki M. Tacrolimus ointment is effective and safe in Japanese atopic dermatitis children. Annales de Dermatologie et de Vénéréologie 2002;129(Suppl 1):P0242. [Google Scholar]
- Otsuki M, Kawashima M, Shibata Y, Nakagawa H, Harada S. Efficacy and safety of FK506 (tacrolimus) ointment in children with atopic dermatitis-phase III double-blinded comparison with vehicle ointment. Journal of Clinical Therapeutics & Medicines (Rinsho Iyaku) 2003;19(6):569-95. [Google Scholar]
Pala 1982 {published data only}
- Pala S. Double-blind studies on hydrocortisone 17-butyrate and halcinonide in the topical treatment of non-infectious eczematous dermatitis. Annali Italiani di Dermatologia Clinica e Sperimentale 1982;36(3):301‐6. [Google Scholar]
Paller 2005a {published data only}
- Abramovits W, Fleischer AB, Jaracz E, Breneman D. Adult patients with moderate atopic dermatitis: tacrolimus ointment versus pimecrolimus cream. Journal of Drugs in Dermatology 2008;7(12):1153-8. [PubMed] [Google Scholar]
- NCT00667160. Comparison study between Protopic (tacrolimus ointment) and Elidel (pimecrolimus cream) in pediatric patients with mild atopic dermatitis. clinicaltrials.gov/show/NCT00667160 (first received 25 April 2008).
- Paller AS, Antaya R, Duarte AM, Kirsner R, Schneider L. Tacrolimus ointment is more effective than pimecrolimus cream with a similar safety profile in pediatric patients with atopic dermatitis (abstract). Pediatric Dermatology (10th World Congress on Pediatric Dermatology) 2004;21(3):338. [Google Scholar]
- Paller AS, Lebwohl M, Fleischer AB, Antaya R, Langley RG, Kirsner RS, et al. Tacrolimus ointment is more effective than pimecrolimus cream with a similar safety profile in the treatment of atopic dermatitis: results from 3 randomized, comparative studies. Journal of the American Academy of Dermatology 2005;52(5):810-22. [DOI] [PubMed] [Google Scholar]
Paller 2005b {published data only}
- NCT00666159. Comparison study between Protopic (tacrolimus ointment) and Elidel (pimecrolimus cream) in treating pediatric patients with atopic dermatitis. clinicaltrials.gov/show/NCT00666159 (first received 24 April 2008).
- Paller AS, Lebwohl M, Fleischer AB, Antaya R, Langley RG, Kirsner RS, et al. Tacrolimus ointment is more effective than pimecrolimus cream with a similar safety profile in the treatment of atopic dermatitis: results from 3 randomized, comparative studies. Journal of the American Academy of Dermatology 2005;52(5):810-22. [DOI] [PubMed] [Google Scholar]
Paller 2005c {published data only}
- Fleischer AB Jr, Abramovits W, Breneman D, Jaracz E, US/Canada Tacrolimus Ointment Study Group. Tacrolimus ointment is more effective than pimecrolimus cream in adult patients with moderate to very severe atopic dermatitis. Journal of Dermatological Treatment 2007;18(3):151-7. [DOI] [PubMed] [Google Scholar]
- Kirsner RS, Heffernan MP, Antaya R. Safety and efficacy of tacrolimus ointment versus pimecrolimus cream in the treatment of patients with atopic dermatitis previously treated with corticosteroids. Acta Dermato-Venereologica 2010;90(1):58-64. [DOI] [PubMed] [Google Scholar]
- NCT00666302. A comparison study between Protopic (tacrolimus) ointment and Elidel (pimecrolimus) cream in treating subjects with atopic dermatitis. clinicaltrials.gov/show/NCT00666302 (first received 24 April 2008).
- Paller AS, Lebwohl M, Fleischer AB, Antaya R, Langley RG, Kirsner RS, et al. Tacrolimus ointment is more effective than pimecrolimus cream with a similar safety profile in the treatment of atopic dermatitis: results from 3 randomized, comparative studies. Journal of the American Academy of Dermatology 2005;52(5):810-22. [DOI] [PubMed] [Google Scholar]
Paller 2019 {published data only}
- NCT02564055. A dose-finding study of GSK2894512 cream in subjects with atopic dermatitis (AD). clinicaltrials.gov/show/NCT02564055 (first received 30 September 2015).
- Paller A, Rubenstein D, Tallman A. Treatment of atopic dermatitis with tapinarof cream: secondary outcomes from a phase 2b, randomized parallel-group study. Journal of the American Academy of Dermatology 2019;81(4 Supplement 1):AB140. [Google Scholar]
- Paller A, Rubenstein D, Tallman AM. Treatment of atopic dermatitis with tapinarof cream: secondary outcomes from a phase IIb randomized parallel-group study. British Journal of Dermatology 2020;183(4):e115‐6. [Google Scholar]
- Paller AS, Stein Gold L, Soung J, Tallman AM, Rubenstein DS, Gooderham M. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. Journal of the American Academy of Dermatology 2021;84(3):632‐8. [DOI] [PubMed] [Google Scholar]
- Paller AS, Stein Gold L, Tallman AM, Rubenstein D. Patient-reported outcomes in subjects with atopic dermatitis treated with tapinarof cream: results from a Phase IIb, randomized, parallel-group study. Journal of Clinical and Aesthetic Dermatology 2019;12(5):S16. [Google Scholar]
- Peppers J, Paller AS, Maeda-Chubachi T, Wu S, Robbins K, Gallagher K, et al. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. Journal of the American Academy of Dermatology 2019;80(1):e3.89‐98. [DOI] [PubMed] [Google Scholar]
Patzelt‐Wenczler 2000 {published data only}
- Patzelt-Wenczler R, Ponce-Poschl E. Proof of efficacy of Kamillosan(R) cream in atopic eczema. European Journal of Medical Research 2000;5(4):171-5. [PubMed] [Google Scholar]
Polano 1960 {published data only}
- Polano MK, De Vries HR, Delver A. Analysis of the results obtained in the treatment of atopic dermatitis with corticosteroid and neomycin containing ointments. Dermatologica 1960;120:191-9. [DOI] [PubMed] [Google Scholar]
Portnoy 1969 {published data only}
- Portnoy B. Drug trial: a comparison between the topical efficacy of 1 percent hydrocortisone and 0.2 percent fluocortolone. Australasian Journal of Dermatology 1969;10(3):183-4. [DOI] [PubMed] [Google Scholar]
Prado de Oliveira 2002 {published data only}
- Prado de Oliveira ZN, Cuce LC, Arnone M. Comparative evaluation of efficacy, tolerability and safety of 0.1% topical momethasone furoate and 0.05% desonide in the treatment of childhood atopic dermatitis. Anais Brasileiros de Dermatologia 2002;77(1):25-33. [Google Scholar]
Rafanelli 1993 {published data only}
- Rafanelli A, Rafanelli S, Stanganelli I, Marchesi E. Mometasone furoate in the treatment of atopic dermatitis in children. Journal of the European Academy of Dermatology and Venereology : JEADV 1993;2(3):225-30. [Google Scholar]
Rahman 2008 {published data only}
- Rahman MF, Rashid MM, Sikder AU, Akhtar N, Banu LA, Wahab MA, et al. Efficacy of topical tacrolimus in atopic dermatitis. Journal of Pakistan Association of Dermatologists 2008;18(2):84-92. [Google Scholar]
Rahman 2015 {published data only}
- Rahman MF, Nandi AK, Kabir S, Kamal M, Basher MS, Banu LA. Topical tacrolimus versus hydrocortisone on atopic dermatitis in paediatric patients: a randomized controlled trial. Mymensingh Medical Journal 2015;24(3):457-63. [PubMed] [Google Scholar]
Rajka 1986 {published data only}
- Rajka G, Verjans HL. Hydrocortisone 17-butyrate (Locoid) 0.1% fatty cream versus desonide (Apolar) 0.1% ointment in the treatment of patients suffering from atopic dermatitis. Journal of International Medical Research 1986;14(2):85-90. [DOI] [PubMed] [Google Scholar]
Ramelet 1982 {published data only}
- Ramelet AA, Mauracher E. Treatment of resistant steroid-responsive dermatoses. A comparison of Diprolene and Neriforte. Clinical Trials Journal 1982;19(5):298-307. [Google Scholar]
Ramsay 1996 {published data only}
- Ramsay CA, Savoie JM, Gilbert M, Gidon M, Kidson P. The treatment of atopic dermatitis with topical fusidic acid and hydrocortisone acetate. Journal of the European Academy of Dermatology and Venereology. 1996;7(Suppl 1):S15-S22. [Google Scholar]
Raoof 2020 {published data only}
- Kim BS, Howell MD, Sun K, Papp K, Nasir A, Kuligowski ME. Treatment of atopic dermatitis with ruxolitinib cream (JAK1/JAK2 inhibitor) or triamcinolone cream. Journal of Allergy and Clinical Immunology 2020;145(2):572‐82. [DOI] [PubMed] [Google Scholar]
- Kim BS, Sofen HL, Kuligowski ME, Venturanza M, Sun K, Toth D. Effects of ruxolitinib cream on pruritus and quality of life in adult patients with atopic dermatitis: results from a phase 2, randomized, dose-ranging, vehicle-and active-controlled study. Journal of the American Academy of Dermatology 2019;81(4 Supplement 1):AB198. [DOI] [PubMed] [Google Scholar]
- Kim BS, Sun K, Papp K, Venturanza M, Nasir A, Kuligowski ME. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study. Journal of the American Academy of Dermatology 2020;82(6):1305‐13. [DOI] [PubMed] [Google Scholar]
- NCT03011892. A study to evaluate the safety and efficacy of INCB018424 phosphate cream applied topically to adults with atopic dermatitis. clinicaltrials.gov/show/NCT03011892 (first received 5 January 2017).
- Owens S, Liu H, Sun K, Venturanza M, Kuligowski M, Howell M. Ruxolitinib cream significantly modulates inflammatory profiles of atopic dermatitis patients. Journal of Investigative Dermatology 2019;139(5 Supplement):S173. [Google Scholar]
- Owens S, Sun K, Jones H, Kuligowski M, Howell MD. Association between an itch-free state in atopic dermatitis treated with ruxolitinib cream and systemic inflammatory mediators. British Journal of Dermatology 2020;183(4):e104. [Google Scholar]
- Raoof T, Kircik L, Kuligowski M, Venturanza M, Sun K, Tan J. 12-week efficacy and safety data of ruxolitinib cream in adult patients with atopic dermatitis: results from a phase 2 study. Journal of the Dermatology Nurses' Association 2020;12(2):85-8. [Google Scholar]
Reitamo 2002 {published data only}
- Reitamo S, Rustin M, Ruzicka T, Cambazard F, Kalimo K, Friedmann PS, et al. Efficacy and safety of tacrolimus ointment compared with that of hydrocortisone butyrate ointment in adult patients with atopic dermatitis. Journal of Allergy and Clinical Immunology 2002;109(3):547-55. [DOI] [PubMed] [Google Scholar]
Reitamo 2004 {published data only}
- Bos J, Harper J, Reitamo S, Rustin M, Tacrolimus Ointment Group European. Tacrolimus ointment twice a day is more effective than once daily application or standard corticosteroid therapy in children with atopic dermatitis. Journal of the European Academy of Dermatology and Venereology : JEADV 2002;16(Suppl s1):137. [Google Scholar]
- Reitamo S, Harper J, Bos JD, Cambazard F, Bruijnzeel-Koomen C, Valk P, et al. 0.03% tacrolimus ointment applied once or twice daily is more efficacious than 1% hydrocortisone acetate in children with moderate to severe atopic dermatitis: results of a randomized double-blind controlled trial. British Journal of Dermatology 2004;150(3):554‐62. [DOI] [PubMed] [Google Scholar]
- Reitamo S. Tacrolimus ointment 0.03% (protopic) twice a day is more effective than once a day or conventional therapy in children with moderate to severe AD. Annales de Dermatologie et de Venereologie 2002;129(Suppl 1 Pt 1):1S247. [Google Scholar]
Reitamo 2005 {published data only}
- Mandelin J, Remitz A, Virtanen H, Reitamo S. One-year treatment with 0.1% tacrolimus ointment versus a corticosteroid regimen in adults with moderate to severe atopic dermatitis: a randomized, double-blind, comparative trial. Acta Dermato-Venereologica 2010;90(2):170-4. [DOI] [PubMed] [Google Scholar]
- Poole CD, Chambers C, Allsopp R, Currie CJ. Changes in health related utility among adults with atopic dermatitis treated with tacrolimus ointment compared to a standard corticosteroid regimen. Value in Health 2009;12(3):A77. [Google Scholar]
- Poole CD, Chambers C, Allsopp R, Currie CJ. Quality of life and health-related utility analysis of adults with moderate and severe atopic dermatitis treated with tacrolimus ointment vs. topical corticosteroids. Journal of the European Academy of Dermatology and Venereology : JEADV 2010;24(6):674-8. [DOI] [PubMed] [Google Scholar]
- Reitamo S, Ortonne JP, Sand C, Cambazard F, Bieber T, Folster-Holst R, et al. A multicentre, randomized, double-blind, controlled study of long-term treatment with 0.1% tacrolimus ointment in adults with moderate to severe atopic dermatitis. British Journal of Dermatology 2005;152(6):1282-9. [DOI] [PubMed] [Google Scholar]
- Reitamo S. 0.1% tacrolimus ointment twice daily is an effective treatment for adults with moderate or severe atopic dermatitis. Journal of the European Academy of Dermatology and Venereology : JEADV 2003;17(Suppl 3):308. [Google Scholar]
- Reitamo S. Superior efficacy of 0.1% tacrolimus ointment in treating adults with moderate to severe atopic dermatitis. Journal of the European Academy of Dermatology and Venereology : JEADV 2003;17(Suppl 3):156. [Google Scholar]
- Reitamo SS, Ortonne JP, Petersenz CS. Efficacy and safety of tacrolimus ointment compared with hydrocortisone treatment in adults with moderate-to- severe atopic dermatitis. Journal of the European Academy of Dermatology and Venereology : JEADV 2002;16(Suppl s1):5. [Google Scholar]
Rosenberg 1971 {published data only}
- Rosenberg EW. Fluocinonide. Preliminary evaluation of a new topically applied corticosteroid. Archives of Dermatology 1971;104(6):632-3. [DOI] [PubMed] [Google Scholar]
Rossi 2002 {published data only}
- Rossi AM. Efficacy and safety comparison of desonide 0.05% lotion versus fluocortolone 0.5% ointment in atopic dermatitis. Annales de Dermatologie et de Venereologie 2002;129:P0252. [Google Scholar]
Roth 1978a {published data only}
- Roth HL, Brown EP. Hydrocortisone valerate. Double-blind comparison with two other topical steroids. Cutis; Cutaneous Medicine for the Practitioner 1978;21(5):695-8. [PubMed] [Google Scholar]
Roth 1978b {published data only}
- Roth HL, Brown EP. Hydrocortisone valerate. Double-blind comparison with two other topical steroids. Cutis; Cutaneous Medicine for the Practitioner 1978;21(5):695-8. [PubMed] [Google Scholar]
Roth 1978c {published data only}
- Roth HL, Brown EP. Hydrocortisone valerate. Double-blind comparison with two other topical steroids. Cutis; Cutaneous Medicine for the Practitioner 1978;21(5):695-8. [PubMed] [Google Scholar]
Rustin 2002 {published data only}
- Reitamo S, Van Leent EJ, Ho V, Harper J, Ruzicka T, Kalimo K, et al. Efficacy and safety of tacrolimus ointment compared with that of hydrocortisone acetate ointment in children with atopic dermatitis. Journal of Allergy and Clinical Immunology 2002;109(3):539-46. [DOI] [PubMed] [Google Scholar]
- Rustin M. Tacrolimus ointment (protopic) shows superior efficacy and comparable safety in a short-term comparison versus corticosteroids in children with AD. Annales de Dermatologie et de Venereologie 2002;129(Suppl 1 Pt 2):1S421. [Google Scholar]
- Rustin M. Tacrolimus ointment shows greater efficacy than corticosteroids in the short term treatment of atopic dermatitis in children. Journal of the European Academy of Dermatology and Venereology : JEADV 2002;16(Suppl 1):P2‐42. [Google Scholar]
Ruzicka 1997 {published data only}
- Bieber T. Improvement of atopic dermatitis with tacrolimus ointment (0.03, 0.1 and 0.3%) in a dose-response study with placebo. Australasian Journal of Dermatology 1997;38(Suppl 2):233-4. [Google Scholar]
- Ruzicka T, Bieber T, Schopf E, Rubins A, Dobozy A, Bos JD, et al, European Tacrolimus Multicenter Atopic Dermatitis Study Group. A short-term trial of tacrolimus ointment for atopic dermatitis. New England Journal of Medicine 1997;337(12):816-21. [DOI] [PubMed] [Google Scholar]
Ryu 1997 {published data only}
- Ryu YS, Choi SW, Kim TY, Kim HO, Kim CW. Comparison of the safety and efficacy of mometasone furoate cream 0.1% and fluocortin butylester cream 0.75% in the treatment of atopic dermatitis. Journal of Korean Society for Clinical Pharmacology and Therapeutics 1997;5(2):205-11. [Google Scholar]
Saeki 2019 {published data only}
- NCT02914548. Study of OPA-15406 ointment in patients with atopic dermatitis. clinicaltrials.gov/show/NCT02914548 (first received 26 September 2016).
- Saeki H, Kawashima M, Sugaya S, Oshiden K, Tsubouchi H. Efficacy and safety of topical OPA-15406, a new phosphodiesterase 4 inhibitor, in Japanese patients with atopic dermatitis for 8A weeks: a phase 2, randomized, double-blind, placebo-controlled study. Journal of Dermatology 2019;46(8):672‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saeki 2020 {published data only}
- NCT03018691. Phase 2 study of OPA-15406 ointment in pediatric patients with atopic dermatitis. clinicaltrials.gov/show/NCT03018691 (first received 12 January 2017).
- Saeki H, Baba N, Oshiden K, Abe Y, Tsubouchi H. Phase 2, randomized, double-blind, placebo-controlled, 4-week study to evaluate the safety and efficacy of OPA- 15406 (difamilast), a new topical selective phosphodiesterase type-4 inhibitor, in Japanese pediatric patients aged 2-14 years with atopic dermatitis. Journal of Dermatology 2020;47(1):17‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saeki 2022a {published data only}
- JapicCTI-194700. Comparison trial of OPA-15406 ointment in pediatric patients with atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-JapicCTI-194700 (first received 5 April 2019).
- NCT03911401. Comparison trial of OPA-15406 ointment in pediatric patients with atopic dermatitis. https://clinicaltrials.gov/show/NCT03911401 (first received 04/09/2019).
- Saeki H, Baba N, Ito K, Yokota D, Tsubouchi H. Difamilast, a selective phosphodiesterase 4 inhibitor, ointment in paediatric patients with atopic dermatitis: a phase III randomized double-blind, vehicle-controlled trial. British Journal of Dermatology 2022;186(1):40-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Hide M, Tsuchiya T, Ito K. Difamilast a topical PDE4 inhibitor: phase 3 trials in Japanese adult and pediatric patients with atopic dermatitis. Journal of the American Academy of Dermatology 2021;85(3):AB67. [Google Scholar]
Saeki 2022b {published data only}
- JapicCTI-194678. Comparison trial of OPA-15406 ointment in adult patients with atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-JapicCTI-194678 (first received 22 March 2019).
- NCT03908970. Comparison trial of OPA-15406 ointment in adult patients with atopic dermatitis syndrome. clinicaltrials.gov/show/NCT03908970 (first received 9 April 2019).
- Saeki H, Ito K, Yokota D, Tsubouchi H. Difamilast ointment in adult patients with atopic dermatitis: a phase 3 randomized, double-blind, vehicle-controlled trial. Journal of the American Academy of Dermatology 2022;86(3):607-14. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Hide M, Tsuchiya T, Ito K. Difamilast, a topical PDE4 inhibitor: phase 3 trials in Japanese adult and pediatric patients with atopic dermatitis. Journal of the American Academy of Dermatology 2021;85(3):AB67. [Google Scholar]
Salava 2021 {published data only}
- Ahola MT, Perala MM, Pelkonen A, Makela M, Remitz AM. Does early effective topical treatment of atopic dermatitis in toddlers with either corticosteroids or tacrolimus affect early sensitization? British Journal of Dermatology 2018;179(1):e47‐e48. [Google Scholar]
- Antti A, Salava A, Perala M, Pelkonen AS, Makela MJ, Remitz A. Are infants and toddlers with moderate-to-severe atopic dermatitis undertreated? Experiences of a Finnish tertiary care hospital. Acta Dermato-Venereologica 2021;101(1):1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perala M, Ahola M, Mikkola T, Pelkonen AS, Remitz A, Makela MJ. Young children with moderate-to-severe atopic dermatitis can be treated safely and effectively with either topical tacrolimus or mild corticosteroids. Acta Paediatrica 2020;109(3):550‐6. [DOI] [PubMed] [Google Scholar]
- Perala M, Salava A, Malmberg P, Pelkonen AS, Makela MJ, Remitz A. Topical tacrolimus and corticosteroids in childhood moderate-to-severe atopic dermatitis with impact on airways: a long-term randomized open-label study. Clinical and Experimental Dermatology 2023;48(6):660-6. [DOI] [PubMed] [Google Scholar]
- Salava A, Perälä MA, Pelkonen A, Mäkelä M, Remitz A. Safety of tacrolimus 0.03% and 0.1% ointments in young children with atopic dermatitis: a 36-month follow-up study. Clinical and Experimental Dermatology 2022;47(5):889-902. [DOI] [PubMed] [Google Scholar]
Salavec 2004 {published data only}
- Salavec M, Buckova H. First experiences with 1% pimecrolimus cream therapy in prevention of atopic eczema flares in children (Czechoslovakian). Cesko-Slovenska Dermatologie 2004;79(1):3-7. [Google Scholar]
Samman 1962 {published data only}
- Samman PD, Beer WE. Fluocinolone acetonide. A new steroid preparation for topical use. British Journal of Dermatology 1962;74(3):96-7. [DOI] [PubMed] [Google Scholar]
Sanabria‐Silva 1991 {published data only}
- Sanabria-Silva E, Laterza AM, Tamayo L, Ruiz-Maldonado R. Evaluation of rebound phenomenon in children with atopic dermatitis treated with topical corticosteroids. Dermatologia: Revista Mexicana 1991;35(2):84-9. [Google Scholar]
Savin 1976 {published data only}
- Savin RC. Betamethasone dipropionate in psoriasis and atopic dermatitis. Connecticut Medicine 1976;40(1):5-7. [PubMed] [Google Scholar]
Schachner 2005 {published data only}
- Schachner LA, Lamerson C, Sheehan MP, Boguniewicz M, Mosser J, Raimer S, et al. Tacrolimus ointment 0.03% is safe and effective for the treatment of mild to moderate atopic dermatitis in pediatric patients: results from a randomized, double-blind, vehicle-controlled study. Pediatrics 2005;116(3):e334-42. [DOI] [PubMed] [Google Scholar]
Schuppli 1983 {published data only}
- Schuppli R, Dressler H, Yawalkar SJ, Weirich EG. Comparative clinical trial of a new trihalogenated dermatocorticoid (halometasone) versus betamethasone dipropionate (German). Zeitschrift fur Hautkrankheiten 1983;58(4):230-7. [PubMed] [Google Scholar]
Sears 1997 {published data only}
- Sears HW, Bailer JW, Yeadon A. Efficacy and safety of hydrocortisone buteprate 0.1% cream in patients with atopic dermatitis. Clinical Therapeutics 1997;19(4):710-9. [DOI] [PubMed] [Google Scholar]
Sefton 1983a {published data only}
- Sefton J, Kyriakopoulos AA. Comparative efficacy of hydrocortisone valerate 0.2 percent ointment in the treatment of atopic dermatitis. Cutis; Cutaneous Medicine for the Practitioner 1983;32(1):89-91, 94. [PubMed] [Google Scholar]
- Sefton J, Loder JS, Kyriakopoulos AA. Clinical evaluation of hydrocortisone valerate 0.2% ointment. Clinical Therapeutics 1984;6(3):282-93. [PubMed] [Google Scholar]
Sefton 1983b {published data only}
- Sefton J, Kyriakopoulos AA. Comparative efficacy of hydrocortisone valerate 0.2 percent ointment in the treatment of atopic dermatitis. Cutis; Cutaneous Medicine for the Practitioner 1983;32(1):89-91, 94. [PubMed] [Google Scholar]
- Sefton J, Loder JS, Kyriakopoulos AA. Clinical evaluation of hydrocortisone valerate 0.2% ointment. Clinical Therapeutics 1984;6(3):282-93. [PubMed] [Google Scholar]
Sefton 1983c {published data only}
- Sefton J, Kyriakopoulos AA. Comparative efficacy of hydrocortisone valerate 0.2 percent ointment in the treatment of atopic dermatitis. Cutis; Cutaneous Medicine for the Practitioner 1983;32(1):89-91, 94. [PubMed] [Google Scholar]
- Sefton J, Loder JS, Kyriakopoulos AA. Clinical evaluation of hydrocortisone valerate 0.2% ointment. Clinical Therapeutics 1984;6(3):282-93. [PubMed] [Google Scholar]
Sefton 1983d {published data only}
- Sefton J, Kyriakopoulos AA. Comparative efficacy of hydrocortisone valerate 0.2 percent ointment in the treatment of atopic dermatitis. Cutis; Cutaneous Medicine for the Practitioner 1983;32(1):89-91, 94. [PubMed] [Google Scholar]
- Sefton J, Loder JS, Kyriakopoulos AA. Clinical evaluation of hydrocortisone valerate 0.2% ointment. Clinical Therapeutics 1984;6(3):282-93. [PubMed] [Google Scholar]
Siegfried 2006 {published data only}
- CASM981CUS04. A 6-month, randomized, multicenter, parallel-group, double blind, vehicle-controlled study to evaluate the efficacy and safety of pimecrolimus cream 1% twice daily vs standard of care in the management of mild to severe atopic dermatitis in children 3 months to 11 years. www.novctrd.com/ctrdweb/trialresult/trialresults/pdf?trialResultId=1463 (first received 5 October 2001).
- Siegfried E, Korman N, Molina C, Kianifard F, Abrams K. Safety and efficacy of early intervention with pimecrolimus cream 1% combined with corticosteroids for major flares in infants and children with atopic dermatitis. Journal of Dermatological Treatment 2006;17(3):143-50. [DOI] [PubMed] [Google Scholar]
- Siegfried EC, Olin JT. Pimecrolimus cream 1% rapidly reduces the extent and severity of atopic dermatitis in infants and children, including head-and-neck involvement: post hoc analysis of a 6-month, randomized, vehicle-controlled trial. Journal of Investigative Dermatology 2012;132(Suppl 1):S93. [Google Scholar]
Sigurgeirsson 2005 {published data only}
- CASM981C2316. A 26-week, randomized, multicenter, parallel-group, double-blind, vehicle-controlled study to evaluate the incidence of atopic dermatitis flares when pimecrolimus cream 1% is used at the first signs and/or symptoms of atopic dermatitis and its safety and tolerability in adults 18 years of age and older. https://www.novctrd.com/ctrdweb/trialresult/trialresults/pdf?trialResultId=1954 (first received 29 October 2003).
- Sigurgeirsson B, Ho V, Ferrandiz C, Kowalski ML, Velander H, Andriano K, et al. Effective management of atopic eczema with early intervention: results from a randomized vehicle-controlled trial of pimecrolimus cream 1% in children and adolescents. Journal of the European Academy of Dermatology and Venereology : JEADV 2005;19(Suppl 2):2. [Google Scholar]
- Sigurgeirsson B, Ho V, Ferrándiz C, Andriano K, Grinienko A, Jimenez P, Pimecrolimus 1% cream in (Paediatric) Eczema: Prevention of Progression Multi-Centre Investigator Study Group. Effectiveness and safety of a prevention-of-flare-progression strategy with pimecrolimus cream 1% in the management of paediatric atopic dermatitis. Journal of the European Academy of Dermatology and Venereology : JEADV 2008;22:1290-301. [DOI] [PubMed] [Google Scholar]
Sigurgeirsson 2015 {published data only}
- Bishop M, Vukovic-Wysocki I, Qaqundah P, Poulin Y. A 5-year randomized study to investigate the safety of pimecrolimus cream 1% in the treatment of mild-to-moderate atopic dermatitis in infants: clinical safety. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB56. [Google Scholar]
- Johnson AD, Boguniewicz M, Garcia AN, Lin T, Olin JT. Efficacy and safety of pimecrolimus cream 1% in infants with mild-to-moderate atopic dermatitis: a randomized 5-year study. Pediatric Dermatology 2012;29(5):679-80. [Google Scholar]
- NCT00120523. 5-year safety study of pimecrolimus cream 1% in infants 3-12 months of age with mild to moderate atopic dermatitis. clinicaltrials.gov/show/NCT00120523 (first received 18 July 2005).
- Nieto A, For P. Efficacy and safety of pimecrolimus cream 1% in infants with mild-to-moderate atopic dermatitis: a randomised 5-year study. Allergy 2012;67(S96):16-7. [Google Scholar]
- Poulin Y, Kaoukhov A, Johnson A, Bishop M. A 5-year randomized study to investigate the safety of pimecrolimus cream 1% in the treatment of mild-to-moderate atopic dermatitis in infants: immunologic parameters. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB58. [Google Scholar]
- Sigurgeirsson B, Boznanski A, Todd G, Vertruyen A, Schuttelaar ML, Zhu X, et al. Safety and efficacy of pimecrolimus in atopic dermatitis: a 5-year randomized trial. Pediatrics 2015;135(4):597-606. [DOI] [PubMed] [Google Scholar]
- Sigurgeirsson B, Luger T. A 5-year randomized trial on the safety and efficacy of pimecrolimus in atopic dermatitis: a critical appraisal - author response. British Journal of Dermatology 2017;177(4):1006. [DOI] [PubMed] [Google Scholar]
Sikder 2005 {published data only}
- Sikder Md AU, Al Mamun S, Khan RM, Chowdhury AH, Khan HM, Hoque MM. Topical 0.03% tacrolimus ointment, 0.05% clobetasone butyrate cream alone and their combination in older children with atopic dermatitis - an open randomized comparative study. Journal of Pakistan Association of Dermatologists 2005;15(4):304-12. [Google Scholar]
Smith 2005 {published data only}
- Smith CH, Ormerod AD. Pimecrolimus cream 1% once-daily maintenance therapy for the prevention of relapse in mild to moderate atopic dermatitis in adults. British Journal of Dermatology 2005;153(Suppl 1):37. [Google Scholar]
Smitt 1993 {published data only}
- Smitt JH, Winterberg DH, Oosting J. Treatment of atopic dermatitis with topical corticosteroids in children. Efficacy and systemic effects of triamcinolone acetonide and alclometasone dipropionate. European Journal of Dermatology 1993;3(7):549‐52. [Google Scholar]
Sparkes 1974 {published data only}
- Sparkes CG, Wilson L. The clinical evaluation of a new topical corticosteroid, clobetasol propionate. An international controlled trial. British Journal of Dermatology 1974;90(2):197-203. [DOI] [PubMed] [Google Scholar]
Spergel 2007 {published data only}
- NCT00119158. Combination therapy for atopic dermatitis. clinicaltrials.gov/show/NCT00119158 (first received 13 July 2005).
- Spergel JM, Boguniewicz M, Paller AS, Hebert AA, Gallagher PR, McCormick C, et al. Addition of topical pimecrolimus to once-daily mid-potent steroid confers no short-term therapeutic benefit in the treatment of severe atopic dermatitis; a randomized controlled trial. British Journal of Dermatology 2007;157(2):378-81. [DOI] [PubMed] [Google Scholar]
Spergel 2015 {published data only}
- NCT00124709. Safety and efficacy of pimecrolimus cream 1% in atopic disease modification. clinicaltrials.gov/show/NCT00124709 (first received 28 July 2005).
- Schneider L, Hanifin J, Boguniewicz M, Eichenfield LF, Spergel JM, Dakovic R, et al. Study of the atopic march: development of atopic comorbidities. Pediatric Dermatology 2016;33(4):388-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spergel JM, Boguniewicz M, Schneider L, Hanifin JM, Paller AS, Eichenfield LF. Food allergy in infants with atopic dermatitis: limitations of food-specific IgE measurements. Pediatrics 2015;136(6):e1530‐8. [DOI] [PubMed] [Google Scholar]
Stalder 1994 {published data only}
- Stalder JF, Fleury M, Sourisse M, Rostin M, Pheline F, Litoux P. Local steroid therapy and bacterial skin flora in atopic dermatitis. British Journal of Dermatology 1994;131(4):536-40. [DOI] [PubMed] [Google Scholar]
Stein‐Gold 2013 {published data only}
- NCT01602341. Efficacy and safety of AN2728 topical ointment to treat adolescents with atopic dermatitis. clinicaltrials.gov/show/NCT01602341 (first received 21 May 2012).
- Stein-Gold L, Spelman L, Spellman M, Hughes M, Zane LT. Safety and efficacy of AN2728 topical ointment, 2% and 0.5%, in a phase 2 dose-ranging study of adolescents with mild-to-moderate atopic dermatitis. Australasian Journal of Dermatology 2013;54:16. [PubMed] [Google Scholar]
- Stein-Gold L, Spelman L, Spellman MC, Hughes M, Zane LT. Safety and efficacy of AN2728 topical ointment, 2% and 0.5%, in a phase 2 dose-ranging study of adolescents with mild-to-moderate atopic dermatitis. Journal of the German Society of Dermatology 2014;12:14. [PubMed] [Google Scholar]
Sudilovsky 1981 {published data only}
- Sudilovsky A, Muir JG, Bocobo FC. A comparison of single and multiple applications of halcinonide cream. International Journal of Dermatology 1981;20(9):609-13. [DOI] [PubMed] [Google Scholar]
Takeuchi 2012 {published data only}
- Takeuchi S, Saeki H, Tokunaga S, Sugaya M, Ohmatsu H, Tsunemi Y, et al. A randomized, open-label, multicenter trial of topical tacrolimus for the treatment of pruritis in patients with atopic dermatitis. Annals of Dermatology 2012;24(2):144-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi S, Saeki H, Tokunaga S, Torii H, Nakamura K, Soma Y, et al. A randomized, multicenter trial of topical tacrolimus for treatment of pruritus in patients with atopic dermatitis. Acta Dermato-Venereologica 2009;89:704. [Google Scholar]
Thaci 2005 {published data only}
- Thaci D, Hengge UR, Goertz HP, Rossi AB. Treatment effects of 1% pimecrolimus ointment formulations in adult patients with atopic dermatitis. Journal of the European Academy of Dermatology and Venereology : JEADV 2005;19(Suppl 2):218. [Google Scholar]
Tharp 1996 {published data only}
- Tharp MD. A comparison of twice-daily and once-daily administration of fluticasone propionate cream, 0.05%, in the treatment of eczema. Cutis; Cutaneous Medicine for the Practitioner 1996;57(Suppl 2):19-26. [PubMed] [Google Scholar]
Torok 2003 {published data only}
- Anonymous. Combination atopic dermatitis therapy reduces adverse effects. Formulary (Cleveland, Ohio) 2004;39(5):245-6. [Google Scholar]
- Torok HM, Maas-Irslinger R, Slayton RM. Clocortolone pivalate cream 0.1% used concomitantly with tacrolimus ointment 0.1% in atopic dermatitis. Cutis; Cutaneous Medicine for the Practitioner 2003;72(2):161-6. [PubMed] [Google Scholar]
Traulsen 1997a {published data only}
- Traulsen J. Hydrocortisone buteprate versus betamethasone valerate for once-daily treatment of atopic dermatitis. Journal of Dermatological Treatment 1997;8(2):109-14. [Google Scholar]
Traulsen 1997b {published data only}
- Traulsen J. Hydrocortisone buteprate versus betamethasone valerate for once-daily treatment of atopic dermatitis. Journal of Dermatological Treatment 1997;8(2):109-14. [Google Scholar]
Turnbull 1975 {published data only}
- Turnbull BC. Clinical comparison of halacinonide and betamethasone valerate in common dermatoses. Australasian Journal of Dermatology 1975;16(1):17-9. [DOI] [PubMed] [Google Scholar]
Udompataikul 2011 {published data only}
- Udompataikul M, Srisatwaja W. Comparative trial of moisturizer containing licochalcone A vs. hydrocortisone lotion in the treatment of childhood atopic dermatitis: a pilot study. Journal of the European Academy of Dermatology and Venereology : JEADV 2011;25(6):660-5. [DOI] [PubMed] [Google Scholar]
Ulrich 1991 {published data only}
- Ulrich R, Andresen I. Treatment of acute episodes of atopic dermatitis. Double-blind comparative study with 0.05% halometasone cream versus 0.25% prednicarbate cream. Fortschritte der Medizin 1991;109(36):741-4. [PubMed] [Google Scholar]
Van Del Rey 1983 {published data only}
- Van Del Rey ML, Geller M, Azulay RD. Double-blind study on the efficacy and safety of alclomethasone cream in the treatment of atopic dermatitis. Anais Brasileiros de Dermatologia 1983;58:177-80. [Google Scholar]
Vanderploeg 1976 {published data only}
- Vanderploeg DE. Betamethasone dipropionate ointment in the treatment of psoriasis and atopic dermatitis: a double-blind study. Southern Medical Journal 1976;69(7):862-3. [DOI] [PubMed] [Google Scholar]
Van Leent 1998 {published data only}
- Van Leent E. Topical treatment with the ascomycin macrolactam SDZ ASM 981 is effective in atopic dermatitis. Journal of the European Academy of Dermatology and Venereology : JEADV 1998;11(Suppl 2):S197. [Google Scholar]
- Van Leent E. Topical treatment with the macrolactam SDZ ASM 981 is effective in atopic dermatitis. Australasian Journal of Dermatology 1997;38(Suppl 2):234. [Google Scholar]
- Van Leent EJ, Graber M, Thurston M, Wagenaar A, Spuls PI, Bos JD. Effectiveness of the ascomycin macrolactam SDZ ASM 981 in the topical treatment of atopic dermatitis. Archives of Dermatology 1998;134(7):805-9. [DOI] [PubMed] [Google Scholar]
Veien 1984 {published data only}
- Veien NK, Hattel T, Justesen O, Norholm A, Verjans HL. Hydrocortisone 17-butyrate (Locoid) 0.1% cream versus hydrocortisone (Uniderm) 1% cream in the treatment of children suffering from atopic dermatitis. Journal of International Medical Research 1984;12(5):310-3. [DOI] [PubMed] [Google Scholar]
Verboom 2002 {published data only}
- Verboom P. The cost effectiveness of pimecrolimus (SDZ ASM 981) topical cream 1% and corticosteroids in the treatment of atopic dermatitis. Annales de Dermatologie et de Venereologie 2002;129:P0279. [Google Scholar]
Vernon 1991 {published data only}
- Vernon HJ, Lane AT, Weston W. Comparison of mometasone furoate 0.1% cream and hydrocortisone 1.0% cream in the treatment of childhood atopic dermatitis. Journal of the American Academy of Dermatology 1991;24(4):603-7. [DOI] [PubMed] [Google Scholar]
Vives 2015 {published data only}
- EUCTR2007-002550-27-FR. Double-blind, randomised, active and placebo controlled study to assess the clinical efficacy, skin tolerability and pharmacological activity of a new topical compound (UR-1505 0.5%, 1% and 2%) in patients with mild to moderate atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2007-002550-27-FR (first received 7 June 2007).
- Vives R, Pontes C, Sarasa M, Millier A. Safety and activity of UR-1505 in atopic dermatitis: a randomized, double=blind phase II exploratory trial. Clinical Therapeutics 2015;37(9):1955-65. [DOI] [PubMed] [Google Scholar]
Wahn 2002 {published data only}
- De Prost Y, Wahn U, Multicentre Investigator Group. Pimecrolimus (Elidel®, SDZ ASM 981) cream 1% reduces the need for topical corticosteroids to treat atopic eczema in children. Journal of the European Academy of Dermatology and Venereology : JEADV 2001;15(Suppl s2):110-1. [Google Scholar]
- Ellis CN, Kahler KH, Grueger J, Chang J. Cost effectiveness of management of mild-to-moderate atopic dermatitis with 1% pimecrolimus cream in children and adolescents 2-17 years of age. American Journal of Clinical Dermatology 2006;7(2):133‐9. [DOI] [PubMed] [Google Scholar]
- Goodfield M, Kaufmann R, Bos J, Wahn U, De Prost Y, Manjra A, et al. Pimecrolimus (SDZ ASM 981) cream 1% can be safely used in conjunction with topical corticosteroids: results of a long term paediatric study. Annales de Dermatologie et de Venereologie 2002;129(Suppl 1 Pt 2):1S410. [Google Scholar]
- McKenna SP, Whalley D, De Prost Y, Staab D, Huels J, Paul CF, et al. Treatment of paediatric atopic dermatitis with pimecrolimus (Elidel®, SDZ ASM 981): impact on quality of life and health-related quality of life. Journal of the European Academy of Dermatology and Venereology : JEADV 2006;20(3):248‐54. [DOI] [PubMed] [Google Scholar]
- Papp K, Staab D, Harper J, Potter P, Puig L, Ortonne JP, et al. Effect of pimecrolimus cream 1% on the long-term course of pediatric atopic dermatitis. International Journal of Dermatology 2004;43(12):978-83. [DOI] [PubMed] [Google Scholar]
- Wahn U, Bos JD, Goodfield M, Caputo R, Papp K, Manjra A, et al. Efficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in children. Pediatrics 2002;110(1 Pt 1):e2. [DOI] [PubMed] [Google Scholar]
- Wahn U, Molloy S, Graeber M, Thurston M, Cherill R, De Prost Y. SDZ ASM 981 cream 1%: a new approach to long-term management of atopic dermatitis. Journal of Investigative Dermatology 2001;117(2):533. [Google Scholar]
Wananukul 2013 {published data only}
- Wananukul S, Chatproedprai S, Chunharas A, Limpongsanuruk W, Singalavanija S, Chantorn R. Comparison study of moisturiser containing licochalcone A and 1% hydrocortisone in the treatment of childhood atopic dermatitis. Allergy 2012;67(Suppl 96):634. [PubMed] [Google Scholar]
- Wananukul S, Chatproedprai S, Chunharas A, Limpongsanuruk W, Singalavanija S, Nitiyarom R, et al. Randomized, double-blind, split-side, comparison study of moisturizer containing licochalcone A and 1% hydrocortisone in the treatment of childhood atopic dermatitis. Chotmaihet Thangphaet [Journal of the Medical Association of Thailand] 2013;96(9):1135-42. [PubMed] [Google Scholar]
Whalley 2002 {published data only}
- Whalley D, McKenna S, Huels J, Van Assche D. Pimecrolimus (SDZ ASM 981) in the treatment of paediatric atopic eczema: benefit to quality of life and health-related quality of life. Annales de Dermatologie et de Venereologie 2002;129(Suppl 1 Pt 2):1S426. [Google Scholar]
- Whalley D. Pimecrolimus (SDZ ASM 981) in the treatment of atopic eczema: benefit to quality of life among infants. In: 20th World Congress of Dermatology. 2002:P0283.
- Whalley D. Pimecrolimus (SDZ ASM 981) in the treatment of paediatric atopic eczema: benefit to quality of life and health-related quality of life. In: 20th World Congress of Dermatology. 2002:P0284.
Wolkerstorfer 1998 {published data only}
- Wolkerstorfer A, Strobos MA, Glazenburg EJ, Mulder PG, Oranje AP. Fluticasone propionate 0.05% cream once daily versus clobetasone butyrate 0.05% cream twice daily in children with atopic dermatitis. Journal of the American Academy of Dermatology 1998;39(2 Pt 1):226-31. [DOI] [PubMed] [Google Scholar]
Woods 2011 {published data only}
- NCT00819507. VANOS cream and skin barrier function. clinicaltrials.gov/ct2/show/NCT00819507 (first received 9 January 2009).
- Woods MT, Brown PA, Baig-Lewis SF, Simpson EL. Effects of a novel formulation of fluocinonide 0.1% cream on skin barrier function in atopic dermatitis. Journal of Drugs in Dermatology 2011;10(2):171-6. [PMC free article] [PubMed] [Google Scholar]
Wortzel 1975a {published data only}
- Wortzel MH. A new corticosteroid for moderate/severe dermatoses. Clinical Medicine 1975;82(3):23-6. [Google Scholar]
Wortzel 1975b {published data only}
- Wortzel MH. A new corticosteroid for moderate/severe dermatoses. Clinical Medicine 1975;82(3):23-6. [Google Scholar]
Wortzel 1975c {published data only}
- Wortzel MH. A new corticosteroid for moderate/severe dermatoses. Clinical Medicine 1975;82(3):23-6. [Google Scholar]
Wu 2013 {published data only}
- Wu SH, Chen XQ, Liu B, Wu HJ, Dong L. Efficacy and safety of 15(R/S)-methyl-lipoxin A4 in topical treatment of infantile eczema. British Journal of Dermatology 2013;168(1):172-8. [DOI] [PubMed] [Google Scholar]
Yasuda 1976 {published data only}
- Yasuda T. Clinical experiences with hydrocortisone 17-butyrate. Dermatologica 1976;152(Suppl 1):221-9. [DOI] [PubMed] [Google Scholar]
Yawalkar 1991 {published data only}
- Yawalkar SJ, Schwerzmann L. Double-blind, comparative clinical trials with halobetasol propionate cream in patients with atopic dermatitis. Journal of the American Academy of Dermatology 1991;25(6 Pt 2):1163-6. [DOI] [PubMed] [Google Scholar]
Zane 2013 {published data only}
- Murrell DF, Gebauer K, Spelman L, Zane LT. Crisaborole topical ointment, 2% in adults with atopic dermatitis: a phase 2a, vehicle-controlled, proof-of-concept study. Journal of Drugs in Dermatology 2015;14(10):1108‐12. [PubMed] [Google Scholar]
- NCT01301508. Efficacy and safety of AN2898 and AN2728 topical ointments to treat mild-to-moderate atopic dermatitis. clinicaltrials.gov/show/NCT01301508 (first received 23 February 2011).
- Paller A, Yosipovitch G, Wynnis T, Lebwohl M, Boguniewicz M, Call R, et al. Crisaborole ointment improves global atopic dermatitis severity and provides early relief in pruritus: pooled results from two phase 3 clinical trials. Journal of the American Pharmacists Association 2017;57(3):not reported. [Google Scholar]
- Zane L, Gogoleva T, Heerinckx F, Jermano J. AN2728 and AN2898 ointments demonstrate safety and efficacy in a bilateral study of atopic dermatitis. Journal of Dermatological Science 2013;69(2):e34. [Google Scholar]
- Zane LT, Gebauer KA, Spelman L, Murrell DF, Varigos GA, Heerinckx FA, et al. Boron-based AN2728 and AN2898 ointments demonstrate safety and efficacy in a phase 2A bilateral study of mild-to-moderate atopic dermatitis. Wound Repair and Regeneration 2012;20(5):A69. [Google Scholar]
- Zane LT, Gogoleva T, Heerinckx FA, Jermano JA. Safety and efficacy of AN2728 and AN2898 ointments in a phase 2a bilateral study of mild to-moderate atopic dermatitis. Journal of Investigative Dermatology 2012;132:S90. [Google Scholar]
Zuberbier 2008 {published data only}
- CASM981CDE10. A 24-week, randomized, multicenter, parallel-group, double-blind, vehicle-controlled study on pimecrolimus cream 1% assessing the steroid-sparing effect in the long term management of pediatric patients with severe atopic dermatitis. www.novctrd.com/ctrdweb/trialresult/trialresults/pdf?trialResultId=1956 (first received 1 October 2003).
- NCT00351052. Steroid-sparing effect with pimecrolimus in pediatric patients with atopic dermatitis. clinicaltrials.gov/show/NCT00351052 (first received 12 July 2006).
- Zuberbier T, Brautigam M. Long-term management of facial atopic eczema with pimecrolimus cream 1% in paediatric patients with mild to moderate disease. Journal of the European Academy of Dermatology and Venereology : JEADV 2008;22(6):718‐21. [DOI] [PubMed] [Google Scholar]
- Zuberbier T, Heinzerling L, Bieber T, Schauer U, Klebs S, Brautigam M. Steroid-sparing effect of pimecrolimus cream 1% in children with severe atopic dermatitis. Dermatology (Basel, Switzerland) 2007;215(4):325-30. [DOI] [PubMed] [Google Scholar]
- Zuberbier T, Luger T, Thaci D, Werfel T, Kerscher M, Klebs S. Pimecrolimus cream 1% in the long term management of severe pediatric atopic eczema. Journal of the European Academy of Dermatology and Venereology : JEADV 2005;19(Suppl 2):1. [Google Scholar]
References to studies excluded from this review
Abdel‐Aal 1976 {published data only}
- Abdel-Aal H, Abdel-Fattah A, El Shiemy S, Faris R, Tadros SS. A double-blind comparison of a new combination (halcinonide neomycin amphotericin) and active controls in cutaneous candidiasis and steroid responsive dermatoses. Journal of International Medical Research 1976;4(4):232-6. [DOI] [PubMed] [Google Scholar]
Afshar 2013 {published data only}
- Afshar M, Kotol P, Miller J, Gallo R, Hata T. The effect of pimecrolimus on innate immunity in subjects with atopic dermatitis: a double-blind, randomized, vehicle-controlled study. British Journal of Dermatology 2013;168(2):426‐8. [DOI] [PubMed] [Google Scholar]
Ahumada 1982 {published data only}
- Padilla MA, Stark FB. Comparative double blind study of two anti-eczema creams. Investigacion Medica Internacional 1982;9(1):36‐42. [Google Scholar]
Alex 2018 {published data only}
- Alex P, Sachdev M, Centola C, Shilpakar R, Williams S, Ramnane M, et al. Efficacy and safety of HAT1, a novel topical therapeutic: results from randomized, double-blind, placebo-controlled clinical trials in psoriasis and atopic dermatitis. Journal of the American Academy of Dermatology 2018;79(3):AB120. [Google Scholar]
Alexander 1973 {published data only}
- Alexander S, Lyne C. A preliminary clinical trial of hydrocortisone-17-butyrate. British Journal of Clinical Practice 1973;27(5):177-9. [PubMed] [Google Scholar]
Alexopoulos 2023 {published data only}
- Alexopoulos A, Dakoutrou M, Nasi L, Thanopoulou I, Kakourou T, Kontara L, et al. A randomized, observer-blind, vehicle-control, multi-center clinical investigation for assessing the efficacy and tolerability of a 1% ectoine and hyaluronic acid 0.1%-containing medical device in pediatric patients with mild-to-moderate atopic dermatitis. Pediatric Dermatology 2023;40(1):78-83. [DOI] [PubMed] [Google Scholar]
Amerio 1998 {published data only}
- Amerio PL, Biggio P, Bossi G, Cainelli T, Cappugi P, Cerimele D, et al. Mometasone furoate 0.1% once a day in allergic contact dermatitis and in atopic dermatitis: controlled study versus betamethasone valerate. Dermatologia Clinica 1998;18(4):255‐60. [Google Scholar]
Anonymous 2018 {published data only}
- Anonymous. Abstractband der DDG kompakt und praxisnah. Journal of the German Society of Dermatology (Conference: DDG KOMPAKT und PRAXISNAH, 2018) 2018;16(Supplement 1):not reported. [Google Scholar]
Arico 1976 {published data only}
- Arico M. A new topical corticosteroid: controlled clinical study in dermatological patients. Giornale Italiano di Dermatologia/Minerva Dermatologica 1976;111(10):585-91. [Google Scholar]
Arkwright 2007 {published data only}
- Arkwright PD, Gillespie MC, Ewing CI, David TJ. Blinded side-to-side comparison of topical corticosteroid and tacrolimus ointment in children with moderate to severe atopic dermatitis. Clinical and Experimental Dermatology 2007;32(2):145-7. [DOI] [PubMed] [Google Scholar]
- ISRCTN65507338. Side by side comparison of 0.03% tacrolimus ointment (Protopic) versus usual topical steroid treatment in children with atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=ISRCTN65507338 (date first received 12/09/2003).
Asada 1990 {published data only}
- Asada Y, Sugai T. A well-controlled comparative study for the clinical evaluation of new topical corticosteroid difluprednate (DFBA, MYSER) and dexamethasone dipropionate (DDP, METHADERM). Skin Research 1990;32(6):824-32. [Google Scholar]
Ashurst 1970 {published data only}
- Ashurst PJ, Caldwell IW, Champion RH, Hall-Smith SP, Milne JA. Beclomethasone dipropionate compared with betamethasone-17-valerate in the treatment of inflammatory dermatoses. British Journal of Clinical Practice 1970;24(1):45-7. [PubMed] [Google Scholar]
Ashurst 1972 {published data only}
- Ashurst PJ. Hydrocortisone 17-butyrate, a new synthetic topical corticosteroid. British Journal of Clinical Practice 1972;26(6):263-6. [PubMed] [Google Scholar]
August 1985 {published data only}
- August PJ, Platt NE, Wiseman RA. A test of the half-side comparative design in the clinical evaluation of topical steroids, using diflucortolone and betamethasone creams. British Journal of Clinical Practice 1985;39(10):383-7. [PubMed] [Google Scholar]
Aussems 1972 {published data only}
- Aussems J, Gillesberger W, Van Neer FC, Van Zuiden E. A trial comparing two corticoid preparations for the treatment of eczematous skin disorders. Arzneimittel-Forschung 1972;22(12):2075-8. [PubMed] [Google Scholar]
Belliboni 1964 {published data only}
- Belliboni N. Clinical trial of an ointment containing corticosteroids associated with nitrofurazone and vitamins A and D. Hospital 1964;65:351-7. [PubMed] [Google Scholar]
Berberian 1999 {published data only}
- Berberian BJ, Breneman DL, Drake LA, Gratton D, Raimir SS, Phillips S, et al. The addition of topical doxepin to corticosteroid therapy: an improved treatment regimen for atopic dermatitis. International Journal of Dermatology 1999;38(2):145-8. [DOI] [PubMed] [Google Scholar]
Bergoend 1982 {published data only}
- Bergoend H. Double-blind comparison of hydrocortisone butyrate and halcinonide. Gazette Medicale de France 1982;89(36):4477-8. [Google Scholar]
Berth‐Jones 2003 {published data only}
- Berth-Jones J, Damstra RJ, Golsch S, Livden JK, Van Hooteghem O, Allegra F, et al. Twice weekly fluticasone propionate added to emollient maintenance treatment to reduce risk of relapse in atopic dermatitis: randomised, double-blind, parallel group study. BMJ (Clinical Research Ed.) 2003;326(7403):1367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bieber 2021 {published data only}
- Bieber T, Reich K, Paul C, Tsunemi Y, Augustin M, Lacour JP, et al. Efficacy and safety of baricitinib in combination with topical corticosteroids in moderate-to-severe atopic dermatitis patients with cyclosporine failure, intolerance, or contraindication: 52-week results from the Breeze-Ad 4 trial. Acta Dermato-Venereologica 2021;101(Suppl 221):63. [Google Scholar]
Binder 1972 {published data only}
- Binder R, McCleary J. Comparison of fluocinonide in a double-blind study with betamethasone valerate. Current Therapeutic Research, Clinical and Experimental 1972;14(1):35-8. [PubMed] [Google Scholar]
Bircher 2002 {published data only}
- Bircher AJ, Niederer M, Hohl Ch, Surber Ch. Stealth triamcinolone acetonide in a phytocosmetic cream. British Journal of Dermatology 2002;146(3):531-2. [DOI] [PubMed] [Google Scholar]
Bleehen 1995 {published data only}
- Bleehen SS, Chu AC, Hamann I, Holden C, Hunter JA, Marks R. Fluticasone propionate 0.05% cream in the treatment of atopic eczema: a multicentre study comparing once-daily treatment and once-daily vehicle cream application versus twice-daily treatment. British Journal of Dermatology 1995;133(4):592-7. [DOI] [PubMed] [Google Scholar]
Bloudek 2023 {published data only}
- Bloudek L, Eichenfield LF, Silverberg JI, Joish VN, Lofland JH, Sun K, et al. Impact of ruxolitinib cream on work productivity and activity impairment and associated indirect costs in patients with atopic dermatitis: pooled results from two phase III studies. American Journal of Clinical Dermatology 2023;24(1):109-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenfield L, Augustin M, Armstrong A, Venturanza M, Kallender H, Gao J, et al. Effects of ruxolitinib cream on work productivity and activity impairment in patients with atopic dermatitis: 52-week pooled results from two phase 3 studies. Journal of Managed Care and Speciality Pharmacy 2022;28(10 A Suppl):S97. [Google Scholar]
- Eichenfield LF, Augustin M, Armstrong AW, Venturanza ME, Kallender H, Gao J, et al. Effects of ruxolitinib cream on work productivity and activity impairment in patients with atopic dermatitis: 52-week pooled results from two phase III studies. British Journal of Dermatology 2022;187(3):e126-e127. [Google Scholar]
Boguniewicz 2008 {published data only}
- Boguniewicz M, Eichenfield L, Hebert A, Jarratt M. A multicenter, randomized, vehicle-controlled clinical study to examine the efficacy and safety of MAS063DP nonsteroidal cream in the management of mild to moderate atopic dermatitis in infants and children. Journal of the American Academy of Dermatology 2007;56(2):AB69. [Google Scholar]
- Boguniewicz M, Zeichner JA, Eichenfield LF, Hebert AA, Jarratt M, Lucky AW, et al. MAS063DP is effective monotherapy for mild to moderate atopic dermatitis in infants and children: a multicenter, randomized, vehicle-controlled study. Journal of Pediatrics 2008;152(6):854-9. [DOI] [PubMed] [Google Scholar]
Breneman 2006 {published data only}
- Breneman D, Fleischer A, Abramovits W, Farber H. Tacrolimus ointment with and without topical steroids in patients with moderate to severe atopic dermatitis. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB88. [Google Scholar]
Buckley 1964 {published data only}
- Buckley DB. Double-blind comparative trial of betamethasone 17-valerate and fluocinolone acetonide ointments. Journal of the Irish Medical Association 1964;55:45-7. [PubMed] [Google Scholar]
Bureau 1963 {published data only}
- Bureau Y, Barriere H, Litoux P, Bureau L. Trial of treatment of several dermatoses with betamethasone. Bulletin de la Societe Francaise de Dermatologie et de Syphiligraphie 1963;70:330-4. [PubMed] [Google Scholar]
Camacho 1996 {published data only}
- Camacho F, Garcia Bravo B, Diaz Perez JL, Aguirre A, Arnau C, Garcia Barbal J, et al. A comparative intraindividual double-blind assay between prednicarbate and fluocortolone in the management of atopic dermatitis (Spanish). Actas Dermo-Sifiliograficas 1996;87(1-2):59-63. [Google Scholar]
Caproni 2006 {published data only}
- Caproni M, Torchia D, Antiga E, Volpi W, Fabbri P. Expression of adhesion molecules in atopic dermatitis is reduced by tacrolimus, but not by hydrocortisone butyrate: a randomized immunohistochemical study. Clinical and Experimental Dermatology 2006;31(6):813-7. [DOI] [PubMed] [Google Scholar]
Chiarenza 1982 {published data only}
- Chiarenza A. Results of the use of diflorasone diacetate ointment in dermatology. Giornale Italiano di Dermatologia e Venereologia 1982;117(3):XIX-XXII. [PubMed] [Google Scholar]
ChiCTR2000030216 {published data only}
- ChiCTR2000030216. Clinical study of CAPCS gel in the treatment of atopic dermatitis. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR2000030216 (date first received 25/02/2020).
ChiCTR2000030246 {published data only}
- ChiCTR2000030246. Clinical research of treatment of atopic dermatitis with FuRui moisturizing cream combining with glucocorticoid topical. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR2000030246 (date first received 26/02/2020).
Colpas 2010 {published data only}
- Colpas PT, Miyashiro CA, Schalka S, Neto AG. Comparative study of non-inferiority of a moisturizer cream versus 1% hydrocortisone cream in children with atopic dermatitis. In: 24th World Congress of Dermatology, conference abstract (https://www.wcd2019milan-dl.org/abstract-book/assets/html/abstracts/05-atopic-eczema-dermatitis.html). (accessed 22/07/24).
Cranswick 2006 {published data only}
- Cranswick N. Vaccination response is equivalent in children with atopic dermatitis treated with 0.03% tacrolimus ointment or a hydrocortisone ointment regimen. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB84. [Google Scholar]
Danby 2014 {published data only}
- Danby S, Brown K, Higgs-Bayliss T, Chittock J, Cork M. The effect of a complex emollient cream containing 5% urea, ceramide 3 and lactic acid, compared with a simple emollient, on the skin barrier in older people with dry skin. British Journal of Dermatology 2014;1:57-8. [Google Scholar]
Desmons 1977 {published data only}
- Desmons F, Defrenne C. Clinical and biological trial of difluprednate in cream and gel forms in pediatric dermatology. Lille Medical 1977;22(9 Suppl 2):743-5. [PubMed] [Google Scholar]
De Waure 2013 {published data only}
- De Waure C, Cadeddu C, Venditti A, Barcella A, Bigardi A, Masci S, et al. Non steroid treatment for eczema: results from a controlled and randomized study. Giornale Italiano di Dermatologia e Venereologia 2013;148(5):471-7. [PubMed] [Google Scholar]
Dhar Smalakar 1999 {published data only}
- Dhar Smalakar S. Evaluation of the efficacy of topical doxepin, alone or in combination with topical corticosteroid, in the management of atopic dermatitis. Indian Journal of Dermatology 1999;44(2):58‐60. [Google Scholar]
Doherty 1979 {published data only}
- Doherty JC, MacRae KD, Platt NE. Treatment of chronic eczemas. The comparative efficacy of two creams. Practitioner 1979;222(1330):561-3. [PubMed] [Google Scholar]
Drake 1999 {published data only}
- Drake LA, Cohen L, Gillies R, Flood JG, Riordan AT, Phillips SB, et al. Pharmacokinetics of doxepin in subjects with pruritic atopic dermatitis. Journal of the American Academy of Dermatology 1999;41(2I (2 Pt 1)):209-14. [DOI] [PubMed] [Google Scholar]
Durand 1975 {published data only}
- Durand JR. Clinical trial of a mixture of neomycin, bacitracin, natamycin and dexamethasone. Mediterranee Medicale 1975;3(56):71-4. [Google Scholar]
Elgart 1978 {published data only}
- Elgart ML, Hall JH, Clement ER, Bodian EL. Multicenter comparison of fluocinolone acetonide in an emollient cream base with betamethasone valerate cream. Cutis; Cutaneous Medicine for the Practitioner 1978;22(3):344-6. [Google Scholar]
EUCTR2005‐002803‐18‐LV {published data only}
- EUCTR2005-002803-18-LV. Randomised, placebo-controlled, double-blind, efficacy study of the emollient V0034CR in addition to a moderately potent corticosteroid in the acute phase of treatment of atopic dermatitis in infants. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2005-002803-18-LV (first received 14 December 2005).
EUCTR2009‐010498‐19‐FR {published data only}
- EUCTR2009-010498-19-FR. A multicenter, randomized, intra-individual, double blind, vehicle-controlled study to evaluate the efficacy and safety of CD2027 ointment 9 µg/g applied twice daily over 4 weeks in the treatment of target lesions in adults subjects with atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2009-010498-19-FR (first received 15 May 2009).
EUCTR2009‐012194‐35‐DE {published data only}
- EUCTR2009-012194-35-DE. A single-center, randomized, controlled, observer-blind, phase IV study to develop the atopic localized eczema regression test (ALERT) using marketed topical corticosteroid formulations of different strength and the calcineurin inhibitors pimecrolimus (Elidel®) in patients with a pre-disposition for atopic dermatitis - atopic localized eczema regression test (ALERT). trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2009-012194-35-DE (first received 15 October 2009).
EUCTR2009‐017407‐28‐DE {published data only}
- EUCTR2009-017407-28-DE. A phase II, single-center, randomized, controlled, double-blind study to assess effects on skin conditions and patient reported outcome of a topical formulation containing LAS41002 on lesional skin in patients with atopic eczema - AD. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2009-017407-28-DE (first received 18 December 2009).
Gip 1982 {published data only}
- Gip L. A multi-centre evaluation of locoid and celestona valerate creams in the treatment of patients suffering from non-infected eczema. Current Therapeutic Research - Clinical and Experimental 1982;31(1):14‐9. [Google Scholar]
GP Medical Research 1967 {published data only}
- GP Medical Research Unit. Treatment of eczemas and infected eczemas. British Journal of Clinical Practice 1967;21(10):505-7. [PubMed] [Google Scholar]
GP Research Group {published data only}
- General Practitioner Research Group. Topical corticosteroids in eczema. A comparative trial. Practitioner 1981;225(1353):410-1. [PubMed] [Google Scholar]
Grejs 1978 {published data only}
- Grejs J. Comparative trial of diflucortolon-21-valerate cream and hydrocortisone-17-butyrate cream in skin diseases. Therapie der Gegenwart 1978;117(12):1930-7. [PubMed] [Google Scholar]
Guillot 1983 {published data only}
- Guillot CF, Pizarro RM. Comparative double-blind study between hydrocortisone 17-butyrate vs. betamethasone 17-valerate in the treatment of no infected dermatosis. Archivos Argentinos de Dermatologia 1983;33(3):179-83. [Google Scholar]
Guttman‐Yassky 2017b {published data only}
- Guttman-Yasky E, Yosipovitch G, Murrell D, Hanifin J. Crisaborole ointment provides early relief of pruritus in two phase 3 clinical trials in patients with mild or moderate atopic dermatitis. Acta Dermato-Venereologica 2017;97(8):1015‐6. [Google Scholar]
- Guttman-Yassky E, Yosipovitch G, Murrell D, Hanifin J. Crisaborole ointment provides early relief of pruritus in two phase 3 clinical trials in patients with mild or moderate atopic dermatitis. Journal of Investigative Dermatology 2017;137(10 Suppl 2):S194. [Google Scholar]
- Guttman-Yassky E, Yosipovitch G, Murrell D, Hanifin J. Crisaborole ointment provides early relief of pruritus in two phase 3 clinical trials in patients with mild or moderate atopic dermatitis. Pediatric Dermatology 2017;34(S1):S33-S34. [Google Scholar]
- Guttman-Yassky E, Yosipovitch G, Murrell D, Hanifin J. Crisaborole ointment provides early relief of pruritus regardless of baseline disease severity in two phase 3 clinical trials in patients with mild or moderate AD. Journal of the American Academy of Dermatology 2017;76(6):AB86. [Google Scholar]
Hamada 1996 {published data only}
- Hamada T, Ishii M, Nakagawa K, Kobayashi H, Kitajima J, Chanoki M, et al. Evaluation of the clinical effect of terfenadine in patients with atopic dermatitis. A comparison of strong corticosteroid therapy to mild topical corticosteroid combined with terfenadine administration therapy. Skin Research 1996;38(1):97-103. [Google Scholar]
Haneke 1992 {published data only}
- Haneke E. The treatment of atopic dermatitis with methylprednisolone aceponate (MPA), a new topical corticosteroid. Journal of Dermatological Treatment 1992;3(Suppl 2):13-5. [Google Scholar]
Iraji 2022 {published data only}
- Iraji F, Makhmalzadeh BS, Abedini M, Aghaei A, Siahpoush A. Effect of herbal cream containing fumaria officinalis and silymarin for treatment of eczema: a randomized double-blind controlled clinical trial. Avicenna Journal of Phytomedicine 2022;12(2):155-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
IRCT2016102323235N5 {published data only}
- IRCT2016102323235N5. The impact of Althaea officinalis formulation on controlling the symptoms of atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=IRCT2016102323235N5 (first received 21 December 2016).
Kamiya 2022 {published data only}
- Kamiya K, Saeki H, Tokura Y, Yoshihara S, Sugai J, Ohtsuki M. Proactive versus rank-down topical corticosteroid therapy for maintenance of remission in pediatric atopic dermatitis: a randomized, open-label, active-controlled, parallel-group study (Anticipate study). Journal of Clinical Medicine 2022;11(21):6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanof 1975 {published data only}
- Kanof NB, Rosenberg EW. Fluocinide gel in atopic dermatitis. Cutis; Cutaneous Medicine for the Practitioner 1975;16(2):324-6. [Google Scholar]
Kassis 1982 {published data only}
- Kassis V, Sondergaard J. Local treatment of non-infected bilateral eczema with Locoid (TM) and Celestoderm (TM): a double-blind comparison. Current Therapeutic Research, Clinical and Experimental 1982;31(3 II):446‐50. [Google Scholar]
Katsambas 1973 {published data only}
- Katsambas A, Vareltzidis A. Therapeutic action of a new non-steroid drug (bufexamac) in the treatment of inflammatory dermatologic diseases. Double-blind clinical study. Bruxelles Medical 1973;53(8):463‐8. [PubMed] [Google Scholar]
Kim 2013 {published data only}
- Kim DH, Lee HJ, Park CW, Kim KH, Lee KH, Ro BI, et al. The clinical efficacy of mometason furoate in multi-lamellar emulsion for eczema: a double-blinded crossover study. Annals of Dermatology 2013;25(1):17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kircik 2009 {published data only}
- Kircik L. Skin barrier function and hydration effects of hydrolipid cream alone and in combination in the treatment of mild to moderate atopic dermatitis: investigator-blinded, split-body, pilot study results. Journal of the American Academy of Dermatology 2009;60(3 Suppl 1):AB67. [Google Scholar]
Kwon 2019 {published data only}
- Kwon SH, Lim CJ, Jung J, Kim HJ, Park K, Shin JW, et al. The effect of autophagy-enhancing peptide in moisturizer on atopic dermatitis: a randomized controlled trial. Journal of Dermatological Treatment 2019;30(6):558‐64. [DOI] [PubMed] [Google Scholar]
Lecomte 2003 {published data only}
- Lecomte P, Lambert J. Pharmacoeconomic evaluation of pimecrolimus in long-term management of atopic dermatitis in Belgium. Journal of the European Academy of Dermatology and Venereology 2003;17(Suppl 3):176. [Google Scholar]
Lee 2021 {published data only}
- Lee YW, Won CH, Jung K, Nam HJ, Choi G, Park YH, et al. Efficacy and safety of pac-14028 cream, a novel, topical, nonsteroidal, selective trpv1 antagonist in patients with mild to moderate atopic dermatitis: a phase iib randomized trial. Acta Dermato-Venereologica 2021;101(Suppl 221):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leeming 1974 {published data only}
- Leeming JA, Bor S. Treatment of psoriasis and other steroid responsive dermatoses. Trial of a new topical corticosteroid, betamethasone dipropionate (Diprosone). Clinical Trials Journal 1974;11(1):18-20. [Google Scholar]
Lessard 1980 {published data only}
- Lessard R, Labelle JL. Evaluation of desoximetasone 0.25% emollient cream in atopic dermatitis. Current Therapeutic Research - Clinical and Experimental 1980;27(2):247-54. [Google Scholar]
Liu 2000 {published data only}
- Liu T, Shong KM, Zhang HS, Zhang XL, Zhang ZL, Mao Y, et al. A randomised controlled trial of prednisone versus kenacomb compound cream in the treatment of dermatitis and eczema (Chinese). Journal of Clinical Dermatology 2000;29(2):104-5. [Google Scholar]
Liu 2008 {published data only}
- Liu SP, Huang XL, Fan CY. Effect observation of eczema treated with combined traditional Chinese medicine for external use with cetirizine and hydrocortisone butyrate. Southern China Journal of Dermato-Venereology 2008;15(3):161‐2. [Google Scholar]
Liu 2018 {published data only}
- Liu L, Ong G. A randomized, open-label study to evaluate an intermittent dosing regimen of fluticasone propionate 0.05% cream in combination with regular emollient skin care in reducing the risk of relapse in pediatric patients with stabilized atopic dermatitis. Journal of Dermatological Treatment 2018;29(5):501-9. [DOI: 10.1080/09546634.2017.1401211] [DOI] [PubMed] [Google Scholar]
Liu 2022 {published data only}
- Liu H, Gong X, Smith SH. LB991 Clinical serum biomarker profiling in TRuE-AD1 and TRuE-AD2 studies after 8 weeks of treatment with ruxolitinib cream. Journal of Investigative Dermatology 2022;142(8 Suppl):B27. [Google Scholar]
Londono 1976 {published data only}
- Londono F. Halcinonide-neomycin-nystatin in the treatment of dermatosis and cutaneous candidiasis [Halcinonido-neomicina-nistanina en el tratamiento de dermatosis y candidiasis cutanea]. Medicina Cutanea Ibero-Latino-Americana 1976;4(6):463-7. [PubMed] [Google Scholar]
Luderschmidt 1981 {published data only}
- Luderschmidt C, Neubert U. On the treatment of eczema in practice. Combination therapy with econazole nitrate and triamcinolone acetonide. Zeitschrift fur Allgemeinmedizin 1981;57(28):1929‐34. [PubMed] [Google Scholar]
Lundell 1974 {published data only}
- Lundell ER, Koch E. A new corticosteroid preparation tested in a double-blind trial. Zeitschrift fur Allgemeinmedizin 1974;50(10):463-6. [PubMed] [Google Scholar]
Lundell 1975 {published data only}
- Lundell E. A double-blind trial of a new topical steroid formulation containing desoximetasone against fluocinolonacetonid cream. Zeitschrift fur Hautkrankheiten 1975;Suppl 2:17-9. [PubMed] [Google Scholar]
Mackey 1974 {published data only}
- Mackey JP. A double-blind clinical study of bufexamac and fluocinolone acetonide in dermatitis. Journal of the Irish Medical Association 1974;67(8):214-6. [PubMed] [Google Scholar]
Mackey 1977 {published data only}
- Mackey JP. Diflucortolone valerate and fluocinolone acetonide in eczema: a double-blind trial. Pharmatherapeutica 1977;1(7):462‐5. [Google Scholar]
Manusov 1974 {published data only}
- Manusov P, Van NF, Vinks P, Van ZE. A double-blind trial with two corticoid preparations (skin salves) in eczematous skin complaints. Folha Medica 1974;69(5):581‐3. [Google Scholar]
Marchesi 1994 {published data only}
- Marchesi E, Rozzoni M, Pini P, Cainelli T. Comparative study of mometasone furoate and betamethasone dipropionate in the treatment of atopic dermatitis. Giornale Italiano di Dermatologia e Venereologia 1994;129(1-2):9‐21. [Google Scholar]
Methaderm Study Group 1989 {published data only}
- Methaderm Study Group. Clinical effectiveness of topical 0.1% dexamethasone 17,21-dipropionate on lichenoid eczematous dermatitis. A comparison between 0.1% dexamethasone 17,21-dipropionate and 0.1% diflucortolone valerate under randomized comparative study. Skin Research 1989;31(5):705‐13. [Google Scholar]
Miki 1990 {published data only}
- Miki Y. Clinical study of methaderm cream on psoriasis, chronic eczema and atopic dermatitis: comparative study with 0.1% diflcortolone 21-valerate universal cream. Yakuri to Chiryo (Japanese Pharmacology and Therapeutics) 1990;18(12):5027‐37. [Google Scholar]
Morley 1976 {published data only}
- Morley N, Fry L, Walker S. Clinical evaluation of clobetasone butyrate in the treatment of children with atopic eczema, and its effect on plasma corticosteroid levels. Current Medical Research and Opinion 1976;4(3):223-8. [DOI] [PubMed] [Google Scholar]
Munro 1977 {published data only}
- Munro DD, Robinson TW, Du Vivier AW, France DM, Clayton R, Sparkes CG. Betamethasone valerate ointment compared with fluocinonide FAPG. Archives of Dermatology 1977;113(5):599-601. [PubMed] [Google Scholar]
NCT00106496 {published data only}
- NCT00106496. A multi-center study of short and long-term use of protopic ointment in patients with atopic dermatitis. clinicaltrials.gov/show/NCT00106496 (first received 28 March 2005).
NCT00185510 {published data only}
- NCT00185510. Efficacy and safety study of advantan for maintenance treatment of atopic dermatitis. clinicaltrials.gov/ct2/show/NCT00185510 (first received 16 September 2005).
NCT00480610 {published data only}
- NCT00480610. Treatment and control of atopic dermatitis with 0.1% tacrolimus ointment. clinicaltrials.gov/show/NCT00480610 (first received 31 May 2007).
NCT00568412 {published data only}
- NCT00568412. A clinical study in children and adolescents with mild to moderate atopic dermatitis. clinicaltrials.gov/show/NCT00568412 (first received 6 December 2007).
NCT00616538 {published data only}
- NCT00616538. Epiceram™ device versus mid-strength topical steroid (fluticasone propionate 0.05%) for treatment of atopic dermatitis. clinicaltrials.gov/show/NCT00616538 (first received 15 April 2008).
NCT00828412 {published data only}
- NCT00828412. Comparison of the efficacy and safety of two topical creams for pediatric atopic dermatitis. clinicaltrials.gov/show/NCT00828412 (first received 26 January 2009).
NCT00919763 {published data only}
- NCT00919763. Efficacy and safety study of CD2027 ointment 3 mcg/g twice daily treatment for adults with at least moderate atopic dermatitis. clinicaltrials.gov/show/NCT00919763 (first received 12 June 2009).
NCT01119313 {published data only}
- NCT01119313. Study to investigate skin conditions and patient assessment of LAS 41002 in the treatment of atopic eczema. clinicaltrials.gov/ct2/show/NCT01119313 (first received 7 May 2010).
NCT01429701 {published data only}
- NCT01429701. Effectiveness of polymyxin B sulphate + prednisolone + benzocaine + clioquinol in acute and sub-acute dermatitis eczematous. clinicaltrials.gov/show/NCT01429701 (first received 7 September 2011).
NCT01850849 {published data only}
- NCT01850849. Safety, tolerability and pharmacokinetics in AD subjects and healthy subjects of cutaneous application of LEO 39652 cream. clinicaltrials.gov/show/NCT01850849 (first received 10 May 2013).
NCT02426359 {published data only}
- NCT02426359. Safety and efficacy study of Q301 in moderate to severe atopic dermatitis patients. clinicaltrials.gov/show/NCT02426359 (first received 24 April 2015).
NCT02576938 {published data only}
- NCT02576938. A study of baricitinib (LY3009104) in participants with moderate-to-severe atopic dermatitis. clinicaltrials.gov/show/NCT02576938 (first received 15 April 2015).
NCT02582177 {published data only}
- NCT02582177. Effectiveness study of ketoconazole and betamethasone to treat fungal infection and dermatophytosis. clinicaltrials.gov/show/NCT02582177 (first received 21 October 2015).
NCT03059693 {published data only}
- Alex P, Sachdev M, Centola C, Shilpakar R, Williams S, Ramnane M, et al. Efficacy and safety of HAT1, a novel topical therapeutic: results from randomized, double-blind, placebo-controlled clinical trials in psoriasis and atopic dermatitis. Journal of the American Academy of Dermatology 2018;79(3):AB120. [Google Scholar]
- NCT03059693. Efficacy and tolerability of HAT1 in patients with moderate to severe atopic dermatitis. clinicaltrials.gov/show/NCT03059693 (first received 23 February 2017).
NCT03627767 {published data only}
- NCT03627767. Study to investigate efficacy and safety of PF-04965842 in subjects aged 12 years and over with moderate to severe atopic dermatitis with the option of rescue treatment in flaring subjects. clinicaltrials.gov/ct2/show/NCT03627767 (first received 13 August 2018).
NCT03796676 {published data only}
- NCT03796676. JAK1 inhibitor with medicated topical therapy in adolescents with atopic dermatitis (JADE TEEN). clinicaltrials.gov/ct2/show/NCT03796676 (first received 8 January 2019).
NCT03859986 {published data only}
- NCT03859986. Study in subjects with moderate atopic dermatitis. clinicaltrials.gov/ct2/show/NCT03859986 (first received 1 March 2019).
NCT03911401 {published data only}
- NCT05149313. A study of lebrikizumab in combination with topical corticosteroids in participants having atopic dermatitis (AD) that are not adequately controlled or non-eligible for cyclosporine. https://www.clinicaltrials.gov/study/NCT05149313 (date received 2022).
NCT04490109 {published data only}
- NCT04490109. B244 topical spray for the treatment of pruritus in adults with a history of atopic dermatitis. https://clinicaltrials.gov/study/NCT04490109 (first received 2021).
NCT05018806 {published data only}
- NCT05018806. Proof of concept study of rilzabrutinib in adult patients with moderate-to-severe atopic dermatitis. https://clinicaltrials.gov/show/NCT05018806 (date first received 19/08/2021).
NCT05318300 {published data only}
- NCT05318300. Evaluation of an adapted formula on atopic dermatitis. https://clinicaltrials.gov/ct2/show/NCT05318300 (date first received 2023).
NCT05792826 {published data only}
- NCT05792826. Clinical study of BSZY cream intervention to relieve atopic facial dermatitis. https://www.clinicaltrials.gov/ct2/show/NCT05792826 (date first received 2023).
Okoshi 2022 {published data only}
- Okoshi K, Kinugasa Y, Ito S, Kume T, Seki T, Nishizaka T, et al. Efficacy of pseudo-ceramide-containing steroid lamellar cream in patients with mild to moderate atopic dermatitis: a randomized, double-blind study. Dermatology and Therapy 2022;12:1823-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2022 {published data only}
- Park CW, Kim BJ, Lee YW, Won C, Park CO, Chung BY, et al. Asivatrep, a TRPV1 antagonist, for the topical treatment of atopic dermatitis: phase 3, randomized, vehicle-controlled study (CAPTAIN-AD). Journal of Allergy and Clinical Immunology 2022;149(4):1340-7. [DOI] [PubMed] [Google Scholar]
Pellanda 2005 {published data only}
- Pellanda C, Weber M, Bircher A, Surber C. Low-dose triamcinolone acetonide in the phytocosmetic lichtena reduces inflammation in mild to moderate atopic dermatitis. Dermatology (Basel, Switzerland) 2005;211(4):338-40. [DOI] [PubMed] [Google Scholar]
Queille 1984 {published data only}
- Queille C, Pommarede R, Saurat JH. Efficacy versus systemic effects of six topical steroids in the treatment of atopic dermatitis of childhood. Pediatric Dermatology 1984;1(3):246-53. [DOI] [PubMed] [Google Scholar]
Reidhav 1996 {published data only}
- Reidhav I, Svensson A. Betamethasone valerate versus mometasone furoate cream once daily in atopic dermatitis. Journal of Dermatological Treatment 1996;7(2):87-8. [Google Scholar]
Reyes 1977 {published data only}
- Reyes JP. Topical treatment of inflammatory dermatoses and cutaneous candidiasis with a new corticosteroid antibiotic combination: halcinonide neomycin amphotericin. British Journal of Clinical Practice 1977;31(4):33-5. [PubMed] [Google Scholar]
Rodriguez 1977 {published data only}
- Rodriguez Moreno T, Rodriguez Canas T, Mestre JM. Topical treatment of eczema with flupamesone (Flutenal): a double blind comparison with betamethasone 17 valerate (method of simultaneous symmetrical paired comparisons). Current Therapeutic Research - Clinical and Experimental 1977;21(2):183-6. [PubMed] [Google Scholar]
Ronnstad 2023 {published data only}
- Ronnstad AT, Bay L, Ruge IF, Halling AS, Fritz BG, Jakasa I, et al. Defining the temporal relationship between the skin microbiome, immune response and skin barrier function during flare and resolution of atopic dermatitis: protocol of a Danish intervention study. BMJ Open 2023;13(2):e068395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roth 1973a {published data only}
- Roth HL, Gellin GA. Atopic dermatitis: treatment with a urea-corticosteroid cream. Cutis; Cutaneous Medicine for the Practitioner 1973;11(2):237-9. [Google Scholar]
Roth 1973b {published data only}
- Roth H, McCleary J. Double blind trial of fluocinonide cream in psoriasis and atopic dermatitis. Clinical Medicine 1973b;80(5):16-9. [Google Scholar]
Sandstrom 2006 {published data only}
- Sandstrom Falk MH, Sarnhult T, Hedner T, Faergemann J. Treatment of atopic dermatitis with 1% hydrocortisone and 25% pentane-1,5-diol: effect on Staphylococcus aureus. Acta Dermato-Venereologica 2006;86(4):372-3. [DOI] [PubMed] [Google Scholar]
Schmidt 1987 {published data only}
- Schmidt H, Hjorth N, Holm P. Domoprednate, a new nonhalogenated topical steroid: comparison to hydrocortisone butyrate. Dermatologica 1987;175(3):145-7. [DOI] [PubMed] [Google Scholar]
Schneider 1977 {published data only}
- Schneider C, Koch E. Double blind trial of an ointment containing fluprednylidene acetate plus salicylic acid against salicylic acid alone. Die Therapiewoche 1977;27(47):8610-4. [Google Scholar]
Schuttelaar 2003 {published data only}
- Schuttelaar M. The addition of tetracyclin to triamcinolon ointment in atopic dermatitis: a randomized controlled trial. Journal of the European Academy of Dermatology and Venereology : JEADV 2003;17(Suppl 3):155‐6. [Google Scholar]
Sefton 1983e {published data only}
- Sefton J, Galen WK, Nesbitt LT, Landow RK. Comparative efficacy of hydrocortisone valerate 0.2% cream and triamcinolone acetonide 0.1% cream in the treatment of atopic dermatitis. Current Therapeutic Research, Clinical and Experimental 1983;34(2 I):341‐4. [Google Scholar]
Snyder 2022 {published data only}
- Snyder S, Collins A, Cline A, Bashyam A, Unrue E, Feldman S, et al. Measuring and improving adherence to crisaborole 2% ointment in patients with atopic dermatitis. Journal of Drugs in Dermatology 2022;21(10):1043-8. [DOI] [PubMed] [Google Scholar]
Stahle 1965 {published data only}
- Stahle IO, Webb JG. Comparison of topical fluocinolone and tumenol prednisolone: a double-blind trial. Medical Journal of Australia 1965;2(10):418-20. [PubMed] [Google Scholar]
Stahle 1965a {published data only}
- Stahle IO, Webb JG. A comparative trial of full and half strength betamethasone 17-valerate cream. Australian Journal of Dermatology 1965;8(2):142-5. [PubMed] [Google Scholar]
Strick 1975 {published data only}
- Strick S. Comparison of two active topical preparations in psoriasis and atopic dermatitis. Cutis; Cutaneous Medicine for the Practitioner 1975;16(3):579-83. [Google Scholar]
Sudilovsky 1975 {published data only}
- Sudilovsky A, Clewe TH. Comparative efficacy of halcinonide and fluocinonide creams in psoriasis and eszematous dermatoses. Journal of Clinical Pharmacology 1975;15(11-12):779-84. [DOI] [PubMed] [Google Scholar]
Suresh 2017 {published data only}
- Suresh SH, Shenoy M, Bhat R, Dn B, Jv M, Km S, et al. A comparative study of safety and efficacy of tacrolimus topical ointment (biocon's formulation) versus protopic topical ointment (astellas pharma) in children and adults with atopic dermatitis. Journal of Investigative Dermatology 2017;137(10 Suppl 2):S200. [Google Scholar]
Takemura 1988 {published data only}
- Takemura T. Clinical study of methaderm ointment on atopic dermatitis: comparative study with 0.1% diflucortone valerate ointment. Kiso to Rinsho (the Clinical Report) 1988;22(10):3203‐10. [Google Scholar]
TCTR20220210004 {published data only}
- TCTR20220210004. A study of therapeutic equivalence of mometasone cream and elomet cream in eczema. https://www.thaiclinicaltrials.org/show/TCTR20220210004 (date first received 2022).
TCTR20220313002 {published data only}
- TCTR20220313002. The effectiveness of tiptajo cream for treatment of mild to moderate dermatitis. https://trialsearch.who.int/Trial2.aspx?TrialID=TCTR20220313002 (date first received 2022).
Thaci 2010 {published data only}
- Thaci D, Chambers C, Sidhu M, Dorsch B, Ehlken B, Fuchs S. Twice-weekly treatment with tacrolimus 0.03% ointment in children with atopic dermatitis: clinical efficacy and economic impact over 12 months. Journal of the European Academy of Dermatology and Venereology : JEADV 2010;24(9):1040‐6. [DOI] [PubMed] [Google Scholar]
Toda 1983 {published data only}
- Toda K. Clinical study of new moco KOWA ointment in eczematous skin diseases: well controlled study in comparison with 0.06% betamethasone 17-valerate ointment. Yakuri to Chiryo (Japanese Pharmacology and Therapeutics) 1983;11(3):983-91. [Google Scholar]
Ueda 1989 {published data only}
- Ueda H, Hayakawa R, Matsunaga K. Well-controlled comparative study and open clinical study of 0.05% hydrocortisone-17-butyrate ointment (TS-1020). Skin Research 1989;31(2):248-67. [Google Scholar]
Van der Meer 1997a {published data only}
- Van der Meer JB, Glazenburg EJ, Muldeer PG, Eggnick HF, Coenraads PJ. Efficacy and safety of fluticasone propionate ointment in the long-term treatment of atopic dermatitis. Australasian Journal of Dermatology 1997;38(Suppl 2):235. [Google Scholar]
Van Halewijn 2023 {published data only}
- Van Halewijn K, Van der Most F, Bohnen A, Pasmans S, Bindels P, Elshout G. Atopic dermatitis in children in primary care: baseline characteristics, medication use and severity measures in the Rotterdam Eczema Study. Allergy 2023;78(Suppl 111):375. [DOI] [PubMed] [Google Scholar]
Van Zuiden 1978 {published data only}
- Van Zuiden E. Diflucortolone valerianate and fluocinolone acetonide in eczema and dermatitis. Interindividual randomized double-blind study with 124 patients. Therapie der Gegenwart 1978;117(8):1248-64. [PubMed] [Google Scholar]
Venier 1982 {published data only}
- Venier A, Carnevali P, Alessandrini A, Urbani S. Controlled clinical trial of a topical formulation of fluocortolone (as privalate and caproate esters) plus chlorquinaldol. Clinica Europea 1982;21(5):932-9. [Google Scholar]
Wang 1995 {published data only}
- Wang JB, Jin HZ, Liu YH. Clinical observation on the efficacy of fluticasone propionate cream and ointment in the treatment of eczema. Chinese Journal of Dermatology 1995;28(2):128-9. [Google Scholar]
Wang 2003 {published data only}
- Wang JB, Liu YH, Zhao G, Guo ZP, Tu YT, Maurice G. Hydrocortisone aceponate lipophilic cream has similar efficacy and safety to hydrocortisone butyrate lipocream in the treatment of endogenous eczema. Journal of the European Academy of Dermatology and Venereology : JEADV 2003;17(Suppl 3):347. [Google Scholar]
Weitgasser 1972a {published data only}
- Weitgasser H. Experiences with a dexamethasone-nandrolonedekanoate creme. Wiener Medizinische Wochenschrift (1946) 1972;122(14):196‐8. [PubMed] [Google Scholar]
Wilson 1973 {published data only}
- Wilson L. Betamethasone 17-valerate ointment in a lanolin-free base. Current Medical Research and Opinion 1973;1(4):228-32. [DOI] [PubMed] [Google Scholar]
Xhauflaire‐Uhoda 2007 {published data only}
- Xhauflaire-Uhoda E, Thirion L, Pierard-Franchimont C, Pierard GE. Comparative effect of tacrolimus and betamethasone valerate on the passive sustainable hydration of the stratum corneum in atopic dermatitis. Dermatology (Basel, Switzerland) 2007;214(4):328‐32. [DOI] [PubMed] [Google Scholar]
Zirwas 2017 {published data only}
- Zirwas MJ, Barkovic S. Anti-pruritis efficacy of itch relift lotion and cream in patients with atopic history: comparison with hydroxortisone cream. Journal of Drugs in Dermatology 2017;16(3):243‐7. [PubMed] [Google Scholar]
References to studies awaiting assessment
Bos 2002 {published data only}
- Bos J. Tacrolimus ointment (protopic) 0.03% twice daily as the therapy of choice in young paediatric patients (2-6 years) with moderate to severe AD. Annales de Dermatologie et de Venereologie 2002;129(Suppl 1 Pt 2):1S408. [Google Scholar]
ChiCTR2100043963 {published data only}
- ChiCTR2100043963. The role and application of skin microbiota and its metabolites in atopic dermatitis. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2100043963 (first received 06 March 2021).
ChiCTR2100044339 {published data only}
- ChiCTR2100044339. Experimental study on the effect of taxisan on colonization of Staphylococcus aureus in skin lesions of atopic dermatitis in vivo and in vitro. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2100044339 (first received 16 March 2021).
ChiCTR2100053644 {published data only}
- ChiCTR2100053644. A single-center, randomized, blinded, desonide cream-controlled phase IV to evaluate the safety and efficacy of 2% cliborrol ointment combined with desonide cream for sequential use in patients with mild to moderate atopic dermatitis clinical research. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2100053644 (first received 26 November 2021).
EUCTR2004‐004052‐39‐DK {published data only}
- EUCTR2004-004052-39-DK. Short-term growth in children with atopic dermatitis treated with tacrolimus and hydrocortisone-17-butyrate [Korttidsvækst hos børn med atopisk dermatitis behandlet med tacrolimus og hydrokortison-17-butyrat]. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2004-004052-39-DK (first received 28 October 2004).
EUCTR2005‐002471‐32‐GB {published data only}
- EUCTR2005-002471-32-GB. A phase II, single-centre, investigator-blind, parallel group clinical trial to investigate the efficacy and safety of 1% ciclopirox olamine (Batrafen® cream), compared to 1% hydrocortisone acetate (HC45® cream), compared to a topical emollient (E45® cream), in the treatment of patients with mild to moderate atopic eczema. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2005-002471-32-GB (first received 1 July 2005).
EUCTR2007‐002182‐12‐GB {published data only}
- EUCTR2007-002182-12-GB. A single center, single-blind, parallel group clinical trial to investigate the efficacy and safety of W0153 vs. standard moisturizer in the maintenance of patients with atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2007-002182-12-GB (first received 30 May 2007).
EUCTR2007‐007789‐39‐FR {published data only}
- EUCTR2007-007789-39-FR. Efficacy and safety comparison of CD4802 0.1% ointment versus vehicle in the treatment of target lesions in adults with atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2007-007789-39-FR (first received 17 March 2008).
EUCTR2011‐000917‐38‐DE {published data only}
- EUCTR2011-000917-38-DE. CBPR277X2101 A first-in-human study to evaluate safety and tolerability of repeated topical administrations of BPR277 ointment in healthy volunteers, and safety, tolerability, and preliminary efficacy of multiple topical administrations of BPR277 in patients with atopic dermatitis and Netherton syndrome. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2011-000917-38-DE (first received 18 July 2011).
- NCT01428297. A study evaluating the safety and efficacy of topical BPR277 for the treatment of atopic dermatitis and netherton syndrome. https://clinicaltrials.gov/show/NCT01428297 (first received 2011).
Guo 2000 {published data only}
- Guo ZP, Zhou GP. A clinical controlled trial of laikede cream versus 0.1% eloson cream in the treatment of dermatitis and eczema. Journal of Clinical Dermatology 2000;29(2):109-10. [Google Scholar]
Gutgesell 1998 {published data only}
- Gutgesell C, Jung T, Reich K, Junghans V, Bohn M, Neumann C. Double-blind hydrocortisone-controlled tacrolimus ointment for atopic dermatitis. Journal of Investigative Dermatology 1998;110(4):681. [Google Scholar]
Hanifin 2002 {published data only}
- Hanifin J, Ho V, Kaufmann R, Kapp A, Honig P, Koo J, et al. Pimecrolimus (SDZ ASM 981) cream: good tolerability in paediatric patients. Annales de Dermatologie et de Venereologie 2002;129(Suppl 1 Pt 2):1S411. [Google Scholar]
JapicCTI‐205315 {published data only}
- JapicCTI-205315. A pharmacological study of tacrolimus ointment. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-JapicCTI-205315 (first received 5 June 2020).
JPRN‐jRCT2031210012 {published data only}
- JPRN-jRCT2031210012. A pharmacological study of M1151C2. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-jRCT2031210012 (first received 6 April 2021).
Koller 2023 {published data only}
- Koller N, Spelman L, Benson M, Bhoyrul B, Lee J, Abdulla EA, et al. Phase 2 trial of novel topical drug AB-101a for treatment of mild to moderate atopic dermatitis in children and adults. Pediatric Dermatology 2023;40(Supplement 2):892023. [Google Scholar]
Liu 2014a {published data only}
- Liu L, Zhu X, Zhao Z, Wang S, Ma L, Gu H, et al. Intermittent proactive maintenance treatment with 0.03% tacrolimus ointment in pediatric patients with moderate to severe atopic dermatitis: results from a multicenter, open-label, randomized, controlled study. Journal of Dermatology 2014;41:10. [Google Scholar]
NCT00392067 {published data only}
- EUCTR2006-001472-20-DK. LEO19123 cream in the treatment of atopic dermatitis. A phase II, proof of concept study, testing once daily use of two dose-combinations of LEO19123 cream (calcipotriol and LEO80122) in the treatment of atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2006-001472-20-DK (first received 14 July 2006).
- NCT00392067. Clinical Study Report: LEO 19123 cream in the treatment of atopic dermatitis. https://clinicaltrials.gov/study/NCT00392067 (date first received 24/10/2006).
- NCT00392067. LEO19123 cream in the treatment of atopic dermatitis. clinicaltrials.gov/show/NCT00392067 (first received 25 October 2006).
NCT00932074 {published data only}
- NCT00932074. A study to determine the safety, tolerability and efficacy of KP-413 in subjects with atopic dermatitis (AD). clinicaltrials.gov/ct2/show/NCT00932074 (first received 2 July 2009).
NCT01781663 {published data only}
- NCT01781663. Efficacy of KAM2904 face cream and KAM3008 body lotion treatment in children with atopic dermatitis (AD). clinicaltrials.gov/show/NCT01781663 (first received 1 February 2013).
NCT03089229 {published data only}
- CTRI/2017/05/008636. To evaluate the effectiveness of MetaDerm HAT01H cream in atopic dermatitis. https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2017/05/008636 (first received 23 May 2017).
- NCT03089229. A study to evaluate the efficacy and safety of HAT01H in atopic dermatitis. clinicaltrials.gov/show/NCT03089229 (first received 24 March 2017).
NCT03134352 {published data only}
- NCT03134352. Study to evaluate the efficacy and safety of ZL-3101 in subjects with subacute eczema. clinicaltrials.gov/ct2/show/NCT03134352 (first received 28 April 2017).
NCT04753034 {published data only}
- NCT04753034. Study of TER-101 topical ointment in subjects with atopic dermatitis. clinicaltrials.gov/show/NCT04753034 (first received 12 February 2021).
Ruzicka 2002b {published data only}
- Ruzicka T. Paediatric patients with moderate to severe atopic dermatitis treated with tacrolimus ointment 0.03% show minimal systemic exposure. Annales de Dermatologie et de Venereologie 2002;129(Suppl 1 Pt 2):1S421. [Google Scholar]
Szybejko‐Machaj 2002 {published data only}
- Szybejko-Machaj G, Kubicka W, Kolodziej T, Bialynicki-Birula R. Pentoxifylline therapy of acute atopic dermatitis. Journal of the European Academy of Dermatology and Venereology: JEADV 2002;16(Suppl s1):132. [Google Scholar]
Wang 2014 {published data only}
- Wang F, Cao G-L, Qi S-L, Zhang X-T, Zhang X-Q. Clinical observation of calcipotriol betamethasone ointment in the treatment of chronic eczema. Journal of Clinical Dermatology 2014;43(3):179-81. [Google Scholar]
References to ongoing studies
ACTRN12616000294459 {published data only}
- ACTRN12616000294459. A phase I, safety, tolerability and efficacy study of topical AKP-11 administration to participants with atopic dermatitis. https://anzctr.org.au/Trial/Registration/TrialReview.aspx?id=370241 (first received 06/07/2023).
ACTRN12617000763347 {published data only}
- ACTRN12617000763347. A phase II, safety, tolerability and efficacy study of topical AKP-11 administration to participants with atopic dermatitis. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=372955 (date first received 17/05/2017).
Bos 2000 {published data only}
- Bos JD, Meyer K, De Vries HJ, Molloy S, Kandra A, Graeber M. Long-term safety and efficacy of SDZ ASM 981 cream in adult patients with atopic dermatitis. British Journal of Dermatology 2000;143(Suppl 57):34. [Google Scholar]
ChiCTR2100054661 {published data only}
- ChiCTR2100054661. Comparative study on the efficacy and safety of 0.03% tacrolimus ointment and 2% criborol ointment in the treatment of moderate atopic dermatitis in children. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2100054661 (date first received 23/12/2021).
ChiCTR2200059419 {published data only}
- ChiCTR2200059419. Phase III Clinical Study of Benvitimod Cream in the Treatment of Mild to Moderate Atopic Dermatitis. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2200059419 (date first received 29/04/2022).
ChiCTR2200060584 {published data only}
- ChiCTR2200060584. A randomized controlled study comparing the efficacy and safety of 2% crisaborole ointment versus 1% pimemollimus cream in the treatment of mild to moderate atopic dermatitis in children aged 2 years and older. https://www.chictr.org.cn/showprojEN.html?proj=164592 (first received 05 June 2022).
ChiCTR‐IPR‐17013807 {published data only}
- ChiCTR-IPR-17013807. Efficacy and safety of calcineurin inhibitors compared with that of glucocorticoid for the treatment of atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR-IPR-17013807 (first received 10 December 2017).
ChiCTR‐TRC‐09000738 {published data only}
- ChiCTR-TRC-09000738. A clinical trial of tacrolimus as a therapeutic agent for atopic dermatitis in Chinese children: objective assessment of clinical efficacy in itch reduction and immunomodulatory effects. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR-TRC-09000738 (first received 4 May 2010).
ChiCTR‐TRC‐12002479 {published data only}
- ChiCTR-TRC-12002479. 0.03% Tacrolimus ointment long-term intermittent maintain treatment of children with moderate to severe atopic dermatitis multicenter, open, randomized, controlled study. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR-TRC-12002479 (first received 21 September 2014).
ChiCTR‐TRC‐12002591 {published data only}
- ChiCTR-TRC-12002591. Optimization of light, moderate to severe atopic dermatitis treatment strategies of the stabilization period: multicenter, open, randomized controlled study. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR-TRC-12002591 (first received 16 September 2012).
CTRI/2012/05/002636 {published data only}
- CTRI/2012/05/002636. A study to evaluate effectiveness of tacrolimus 0.1% topical ointment in the patients of the allergic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2012/05/002636 (first received 9 May 2012).
CTRI/2018/11/016407 {published data only}
- CTRI/2018/11/016407. A randomized, double-blind, placebo-controlled, three arms, parallel-group, multiple-site bioequivalence study with clinical endpoints. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2018/11/016407 (first received 22 November 2018).
CTRI/2022/07/043740 {published data only}
- CTRI/2022/07/043740. A clinical trial to study the effects of two drugs, tofacitinib 2% w/w ointment and 0.1% hydrocortisone butyrate ointment in patients suffering from dermatitis. https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2022/07/043740 (first received 05 July 2022).
CTRI/2022/07/044136 {published data only}
- CTRI/2022/07/044136. Effect of tofacitinib ointment 2% w/w compared to pimecrolimus cream 1% w/w in patients with mild to moderate atopic dermatitis (AD). https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2022/07/044136 (first received 19 July 2022).
CTRI/2022/08/045019 {published data only}
- CTRI/2022/08/045019. A randomized, double-blind, vehicle-controlled, three arm, parallel group, multi-centric, clinical study to establish the therapeutic equivalence of tacrolimus 0.03% topical ointment of Intas Pharmaceuticals Limited with protopic® 0.03% topical ointment in patients with moderate to severe atopic dermatitis. https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2022/08/045019 (first received on 29 August 2022).
CTRI/2022/08/045020 {published data only}
- CTRI/2022/08/045020. A study to establish the therapeutic equivalence of tacrolimus 0.1 % topical ointment in adult patients with moderate to severe atopic dermatitis. https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2022/08/045020 (first received 29 August 2022).
CTRI/2022/12/047916 {published data only}
- CTRI/2022/12/047916. An investigator initiated, randomized, placebo and active controlled, evaluator blinded study to assess the efficacy and tolerability of pimecrolimus lotion 0.8% (ENZ001) in the treatment of target lesions in patients with moderate atopic dermatitis. https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2022/12/047916 (first received 6/12/2022).
CTRI/2023/05/052282 {published data only}
- CTRI/2023/05/052282. A double-blind, randomized, placebo-controlled, three-arm, parallel-group, multi-centre clinical study to evaluate the safety and clinical endpoint bioequivalence of tacrolimus ointment 0.1% w/w of JAMP Pharma Corporation, Canada and protopic® tacrolimus ointment, 0.1% (w/w) of LEO Pharma Inc., Thornhill, Ontario, L3T 7W8 and compare both active treatments to a placebo control in the treatment of non-immunocompromised patients with moderate to severe atopic dermatitis. https://ctri.nic.in/Clinicaltrials/advsearch.php (first received May 2023).
CTRI/2023/06/054208 {published data only}
- CTRI/2023/06/054208. Study to evaluate safety, efficacy of topical 1% AKP-11 in atopic dermatitis participants. https://ctri.nic.in/Clinicaltrials/pubview2.php (first received 2023).
EUCTR2004‐001644‐80‐HU {published data only}
- EUCTR2004-001644-80-HU. Clinical study on tacrolimus ointment over the long-term “Control Study - Children” Revised version of FG-506-06-39 - Control Study - Children. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2004-001644-80-HU (first received 3 August 2004).
EUCTR2004‐002477‐23‐BE {published data only}
- EUCTR2004-002477-23-BE. Comparative, multicentre, randomised, double-blind study to assess the efficacy of tacrolimus 0.1% ointment versus fluticasone 0.005% ointment in adult patients suffering from moderate to severe atopic dermatitis and presenting with so-called "red face" lesions of the head and neck. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2004-002477-23-BE (first received 11 July 2005).
EUCTR2004‐002478‐47‐BE {published data only}
- EUCTR2004-002478-47-BE. Comparative, multicentre, randomised, double-blind study to assess the efficacy of tacrolimus 0.03% ointment versus fluticasone 0.005% ointment in children aged 2 years or over suffering from moderate to severe atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2004-002478-47-BE (first received 6 July 2005).
EUCTR2004‐004824‐11‐DE {published data only}
- EUCTR2004-004824-11-DE. Role for pimecrolimus in restoring skin barrier function and normalizing epidermal lipid content and differentiation in atopic epidermis: a randomized, intra-patient, double-blind (right/left arm) study in adults with atopic dermatitis treated with 1% pimecrolimus cream and 0.1 % betamethasone cream as treatment control twice daily for 3 weeks. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2004-004824-11-DE (first received 4 October 2005).
EUCTR2011‐006236‐23‐PL {published data only}
- EUCTR2011-006236-23-PL. A randomized, double-blind, placebo-controlled, three-arm, parallel assignment, multi-centre, therapeutic equivalence study of two tacrolimus 0.1% topical ointment formulations in adult patients with moderate to severe atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2011-006236-23-PL (first received 17 April 2012).
EUCTR2013‐000715‐25‐DE {published data only}
- EUCTR2013-000715-25-DE. Comparison of defined skin parameters in two comparable lesional skin areas after topical treatment with Soventol HydroCort 1% cream or vehicle. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2013-000715-25-DE (first received 26 April 2013).
EUCTR2014‐005593‐11‐AT {published data only}
- EUCTR2014-005593-11-AT. Diacerein for the treatment of atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2014-005593-11-AT (first received 13 January 2015).
EUCTR2016‐003013‐96‐DK {published data only}
- EUCTR2016-003013-96-DK. Avexxin (AVX001) 3% ointment (NG) in atopic dermatitis - safety and efficacy study. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2016-003013-96-DK (first received 27 July 2016).
EUCTR2018‐004370‐96‐DK {published data only}
- EUCTR2018-004370-96-DK. The effects of topical corticosteroid use on insulin sensitivity and bone turnover. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2018-004370-96-DK (first received 17 December 2018).
EUCTR2019‐002686‐35‐DE {published data only}
- EUCTR2019-002686-35-DE. Clinical study to compare two cutaneous ointments with the active substance methylprednisolone aceponate 0.1% and one cutaneous ointment without active substance for patients with atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2019-002686-35-DE (first received 9 June 2021).
Hanifin 2006 {published data only}
- Hanifin J. A novel topical nuclear factor kappa-B decoy: results from a phase I/II trial in atopic dermatitis. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB4. [Google Scholar]
IRCT20091012002581N6 {published data only}
- IRCT20091012002581N6. Comparison of efficacy of tacrolimus 0.03% ointment with mometason ointment in treatment of pediatric atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=IRCT20091012002581N6 (first received 25 November 2019).
ISRCTN27411543 {published data only}
- ISRCTN27411543. A randomised double-blinded cross-over trial comparing betamethasone valerate and tacrolimus ointment for the treatment of moderate to severe atopic eczema. trialsearch.who.int/Trial2.aspx?TrialID=ISRCTN27411543 (first received 30 September 2004).
JapicCTI‐121946 {published data only}
- JapicCTI-121946. A phase 2a study of M520102 in atopic dermatitis patients. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-JapicCTI-121946 (first received 4 September 2012).
JapicCTI‐173484 {published data only}
- JapicCTI-173484. A multicenter, randomized, double-blind, vehicle-controlled, parallel-group trial to assess the safety and efficacy of 0.3% and 1% OPA-15406 ointments when administered for 4 weeks in pediatric patients with atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-JapicCTI-173484 (first received 6 January 2017).
JPRN‐jRCT2031210255 {published data only}
- JPRN-jRCT2031210255. Phase 3 clinical study of JTE-061 cream - randomized vehicle-controlled study (Part 1) and extension study (Part 2) in patients with atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-jRCT2031210255 (first received 18 August 2021).
JPRN‐UMIN000006955 {published data only}
- JPRN-UMIN000006955. The effect of proactive therapy with tacrolimus and betamethasone butyrate on atopic dermatitis: randomized placebo-controlled study. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000006955 (first received 26 December 2011).
JPRN‐UMIN000022212 {published data only}
- JPRN-UMIN000022212. A clinical trial of the effects of topical steroid cream (PVA-N11) on atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000022212 (first received 6 May 2016).
NCT00120302 {published data only}
- NCT00120302. Quality of life study in adults with facial eczema. clinicaltrials.gov/show/NCT00120302 (first received 15 July 2005).
NCT00121381 {published data only}
- NCT00121381. Pimecrolimus cream 1% plus topical corticosteroid in patients (2-17 years of age) with severe atopic dermatitis. clinicaltrials.gov/show/NCT00121381 (first received 21 July 2005).
NCT00125333 {published data only}
- NCT00125333. Topical NF-kappaB decoy in the treatment of atopic dermatitis. clinicaltrials.gov/show/NCT00125333 (first received 1 August 2005).
NCT00354510 {published data only}
- EUCTR2005-005430-12-GB. Randomised, double-blind, placebo-controlled study of topical GW842470X formulation in adult patients with moderate atopic dermatitis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2005-005430-12-GB (first received 10 March 2006).
- NCT00354510. Topical GW842470X in adults patients with moderate atopic dermatitis. clinicaltrials.gov/show/NCT00354510 (first received 20 July 2006).
NCT00356642 {published data only}
- NCT00356642. Study of single and ten day repeat atopical applications of GW842470X cream on the skin of patients with atopic dermatitis. clinicaltrials.gov/show/NCT00356642 (first received 26 July 2006).
NCT00510003 {published data only}
- NCT00510003. Assessment of pruritus improvement with pimecrolimus treatment on the areas affected by mild-to-moderate AD, in patients 2- to 11- year-old. clinicaltrials.gov/show/NCT00510003 (first received 1 August 2007).
NCT00689832 {published data only}
- NCT00689832. Protopic ointment in children [with] atopic eczema. clinicaltrials.gov/show/NCT00689832 (first received 4 June 2008).
NCT00690105 {published data only}
- NCT00690105. Protopic ointment in adult atopic eczema of the face. clinicaltrials.gov/show/NCT00690105 (first received 4 June 2008).
NCT00833079 {published data only}
- NCT00833079. Bioequivalence of two tacrolimus 0.1% topical ointment formulations in patients with atopic dermatitis. clinicaltrials.gov/show/NCT00833079 (first received 30 January 2009).
NCT00944632 {published data only}
- NCT00944632. Dose escalation of different concentrations of ZK 245186 in atopic dermatitis. clinicaltrials.gov/show/NCT00944632 (first received 23 July 2009).
NCT00946478 {published data only}
- NCT00946478. Effect of pimecrolimus cream on cathelicidin levels in subjects with eczema. clinicaltrials.gov/show/NCT00946478 (first received 27 July 2009).
NCT00980135 {published data only}
- NCT00980135. Sinecort pilot efficacy study. clinicaltrials.gov/show/NCT00980135 (first received 18 September 2009).
NCT01228513 {published data only}
- NCT01228513. Efficacy and safety of different concentrations of ZK245186 in atopic dermatitis (AD). clinicaltrials.gov/ct2/show/NCT01228513 (first received 26 October 2010).
NCT01359787 {published data only}
- NCT01359787. Efficacy and safety of different concentrations of mapracorat ointment over 4 weeks in atopic dermatitis (AD). clinicaltrials.gov/show/NCT01359787 (first received 25 May 2011).
NCT01567995 {published data only}
- NCT01567995. Randomised, double-blind, placebo-controlled study of topical clobetasone butyrate 0.05% cream in subjects with eczema for two weeks to evaluate the efficacy and safety. clinicaltrials.gov/show/NCT01567995 (first received 2 April 2012).
NCT01993420 {published data only}
- NCT01993420. A safety and efficacy of DRM02 in subjects with atopic dermatitis. clinicaltrials.gov/ct2/show/NCT01993420 (first received 25 November 2013).
NCT02079688 {published data only}
- NCT02079688. Efficacy, safety, tolerability, pharmacokinetics and pharmacodynamics study of the topical formulation SB011 applied to lesional skin in patients with atopic eczema. clinicaltrials.gov/show/NCT02079688 (first received 6 March 2014).
NCT02219633 {published data only}
- NCT02219633. Effect of LEO 39652 cream in adults with mild to moderate atopic dermatitis (AD). clinicaltrials.gov/show/NCT02219633 (first received 19 August 2014).
NCT02496546 {published data only}
- NCT02496546. An explorative trial evaluating the effect of LEO 32731 cream in adults with mild to moderate atopic dermatitis. clinicaltrials.gov/show/NCT02496546 (first received 14 July 2015).
NCT02601703 {published data only}
- NCT02601703. To study generic tacrolimus ointment, 0.1% in the treatment of moderate to severe atopic dermatitis (inflammation of skin: itchy, red, swollen, and cracked skin). clinicaltrials.gov/show/NCT02601703 (first received 10 November 2015).
NCT02732314 {published data only}
- NCT02732314. Efficacy and tolerability of new topical formulations in subjects with atopic dermatitis. clinicaltrials.gov/show/NCT02732314 (first received 8 April 2016).
NCT02865356 {published data only}
- NCT02865356. Topical solution for the treatment of atopic dermatitis (CYCLATOP). clinicaltrials.gov/ct2/show/NCT02865356 (first received 12 August 2016).
NCT02949960 {published data only}
- NCT02949960. DMT210 topical gel in the treatment of atopic dermatitis. clinicaltrials.gov/show/nct02949960 (first received 31 October 2016).
NCT03002571 {published data only}
- NCT03002571. Efficacy and safety of IDP-124 lotion. clinicaltrials.gov/show/NCT03002571 (first received 23 December 2016).
NCT03058783 {published data only}
- NCT03058783. Efficacy and safety of IDP-124 lotion for the treatment of moderate to severe atopic dermatitis in pediatric and adult subjects. clinicaltrials.gov/show/NCT03058783 (first received 23 February 2017).
NCT03394677 {published data only}
- NCT03394677. Study of RVT-501 topical ointment in pediatric patients with atopic dermatitis. clinicaltrials.gov/show/NCT03394677 (first received 9 January 2018).
NCT03832010 {published data only}
- NCT03832010. Steroid-reducing effects of crisaborole. clinicaltrials.gov/show/NCT03832010 (first received 6 February 2019).
NCT03868098 {published data only}
- NCT03868098. Different application rates of crisaborole ointment 2% in adults with mild to moderate atopic dermatitis. clinicaltrials.gov/ct2/show/NCT03868098 (first received 8 March 2019).
NCT04008784 {published data only}
- NCT04008784. Improvement of short term outcome of mild to moderate atopic dermatitis using a combination of crisaborole and a concomitant topical corticosteroid over a 8 week period. clinicaltrials.gov/show/NCT04008784 (first received 5 July 2019).
NCT04194814 {published data only}
- NCT04194814. Skin bioMARkers for atopic eczema therapy evaluation (SMART). clinicaltrials.gov/show/NCT04194814 (first received 11 December 2019).
NCT04313400 {published data only}
- NCT04313400. Topically applied AMTX-100 CF for adult patients with mild to moderate atopic dermatitis. clinicaltrials.gov/show/NCT04313400 (first received 18 March 2020).
NCT04435392 {published data only}
- NCT04435392. Jaktinib hydrochloride cream for atopic dermatitis. clinicaltrials.gov/show/NCT04435392 (first received 17 June 2020).
NCT04598269 {published data only}
- NCT04598269. Study of ATI-1777 in adult patients with moderate or severe atopic dermatitis. clinicaltrials.gov/ct2/show/NCT04598269 (first received 22 October 2020).
NCT04615962 {published data only}
- NCT04615962. Topical cream SNG100 for treatment in moderate atopic dermatitis subjects. clinicaltrials.gov/show/NCT04615962 (first received 4 November 2020).
NCT04717310 {published data only}
- NCT04717310. Evaluate the efficacy and safety of topical SHR0302 ointment in patients with mild-to-moderate atopic dermatitis. clinicaltrials.gov/show/NCT04717310 (first received 22 January 2021).
NCT04737304 {published data only}
- EUCTR2020-003143-28-GB. A first-in-human trial to investigate how safe, tolerable, effective and how the body handles BEN2293 in patients who have mild to moderate eczema. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2020-003143-28-GB (first received 6 August 2020).
- NCT04737304. A first-in-human PoC study with BEN2293 in patients with mild to moderate atopic dermatitis. clinicaltrials.gov/ct2/show/NCT04737304 (first received 3 February 2020).
NCT04845620 {published data only}
- EUCTR2021-006903-14-PL. A phase 3, 4-week, parallel group, double blind, vehicle-controlled study of the safety and efficacy of ARQ-151 cream 0.05% administered QD in subjects with atopic dermatitis. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2021-006903-14-PL (first received 2021).
- NCT04845620. Trial of PDE4 inhibition with roflumilast for the management of atopic dermatitis (integument-PED). clinicaltrials.gov/show/NCT04845620 (first received 15 April 2021).
NCT04921969 {published data only}
- NCT04921969. A study to assess the efficacy and safety of ruxolitinib cream in children with atopic dermatitis (TRuE-AD3). clinicaltrials.gov/show/NCT04921969 (first received 10 June 2021).
NCT04992546 {published data only}
- NCT04992546. Phase 2a study of the safety, tolerability, and pharmacokinetics of topically administered PRN473 (SAR444727) in patients with mild to moderate atopic dermatitis. clinicaltrials.gov/ct2/show/NCT04992546 (first received 5 August 2021).
NCT05127421 {published data only}
- NCT05127421. Ruxolitinib cream in participants with facial and/or neck atopic dermatitis involvement. clinicaltrials.gov/show/NCT05127421 (first received 19 November 2021).
NCT05200403 {published data only}
- NCT05200403. Improvement In scratch behavior and sleep in patients with atopic dermatitis. clinicaltrials.gov/show/NCT05200403 (first received 20 January 2022).
NCT05324618 {published data only}
- NCT05324618. Tacrolimus versus hydrocortisone in atopic dermatitis. https://clinicaltrials.gov/study/NCT05324618 (first received 16 January 2022).
NCT05326672 {published data only}
- NCT05326672. Phase III clinical study of benvitimod cream in the treatment of mild to moderate atopic dermatitis. https://clinicaltrials.gov/study/NCT05326672 (first received 06 April 2022).
NCT05375955 {published data only}
- NCT05375955. A study to learn about the study medicine (PF-07038124) in patients with mild to moderate atopic dermatitis or mild to severe plaque psoriasis. https://clinicaltrials.gov/study/NCT05375955 (first received 11 May 2022).
NCT05432596 {published data only}
- NCT05432596. Study of ATI-1777 in patients 12 to 65 years old with mild to severe atopic dermatitis. https://clinicaltrials.gov/study/NCT05432596 (first received 01 June 2022).
NCT05487963 {published data only}
- NCT05487963. Tolerability and effectiveness of CGB-500 topical ointment, 1% tofacitinib, for the treatment of atopic dermatitis. https://clinicaltrials.gov/study/NCT05487963 (first received 01 August 2022).
NCT05579899 {published data only}
- NCT05579899. Safety and efficacy study of EVO101 topical cream in atopic dermatitis. https://clinicaltrials.gov/study/NCT05579899 (first received 11 October 2022).
NCT05607901 {published data only}
- NCT05607901. A comparative clinical trial evaluating the effect and safety of tacrolimus versus hydrocortisone. https://clinicaltrials.gov/study/NCT05607901 (first received 28 October 2022).
NCT05608343 {published data only}
- NCT05608343. A phase 3, randomized, double-blind, vehicle-controlled, multicenter study to assess the efficacy and safety of difamilast ointment 1% in children, adolescents, and adults with mild to moderate atopic dermatitis. https://clinicaltrials.gov/study/NCT05608343 (first received 01 November 2022).
NCT05650320 {published data only}
- NCT05650320. To demonstrate the superiority of IMP (0.3% and 1% OPA-15406 ointment) versus the vehicle in pediatric patients with AD. https://clinicaltrials.gov/study/NCT05650320 (first received 06 December 2022).
NCT05667623 {published data only}
- NCT05667623. To demonstrate the superiority of IMP (1% OPA-15406 ointment) to the vehicle in adult patients with AD. https://clinicaltrials.gov/study/NCT05667623 (first received 02 December 2022).
NCT05729074 {published data only}
- NCT05729074. A study to evaluate the safety, tolerability and pharmacokinetic properties of IN-A002 ointment in healthy adult male volunteers and mild to moderate atopic dermatitis patients. https://clinicaltrials.gov/study/NCT05729074 (first received 25/01/2023).
NCT05843422 {published data only}
- NCT05843422. A first-in-human study of QY211 gel in adult subjects. https://classic.clinicaltrials.gov/ct2/show/NCT05843422 (first received 24/04/2023).
NCT05870865 {published data only}
- NCT05870865. A study to evaluate the anti-pruritic effectiveness of ASN008 in adults with mild to moderate atopic dermatitis. https://ichgcp.net/clinical-trials-registry/NCT05870865 (first received 30/03/2023).
Piscitelli 2018 {published data only}
- Piscitelli S, Lee J, McHale K, Collins J, Gillmor D, Tabolt G, et al. Cerdulatinib (DMVT-502), a novel, topical dual Janus kinase/spleen tyrosine kinase inhibitor, improves the cellular and molecular cutaneous signature in patients with atopic dermatitis. Experimental Dermatology 2018;27:44‐5. [Google Scholar]
Rhemus 2006 {published data only}
- Rhemus W, Kim J, Kern D, Murase J. A double-blind, placebo-controlled study of a natural topical anti-inflammatory for treatment of atopic dermatitis and psoriasis. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB210. [Google Scholar]
Schlesinger 2020 {published data only}
- Schlesinger T, Nduaka C, Elstrom T. Improvement of short-term outcome of mild to moderate atopic dermatitis using a combination treatment of crisaborole ointment 2% and a concomitant topical cortiscosteroid over a 8 week period. Journal of the American Academy of Dermatology 2020;83(6):AB211. [Google Scholar]
Van Halewijn 2018 {published data only}
- EUCTR2017-001525-40-NL. Effectiveness of different strengths of hormone cream: providing evidence for a better treatment strategy in children with eczema in general practice. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2017-001525-40-NL (first received 30 May 2017).
- Halewijn K, Van Der Most F, Bohnen A, Pasmans S, Bindels P, Elshout G. Atopic dermatitis in children in the general population: baseline characteristics, medication use, and severity measures in the Rotterdam Eczema study. Allergy 2023;78(Supplement 111):375. [DOI] [PubMed] [Google Scholar]
- NTR6679. Effectiveness of different strengths of topical steroids in children with eczema in general practice. trialsearch.who.int/Trial2.aspx?TrialID=NTR6679 (first received 30 August 2017).
- Van Halewijn KF, Bohnen AM, Van Den Berg PJ, Pasmans Sgma, Bindels PJ, Elshout G. Different potencies of topical steroids for children with eczema: protocol for an observational cohort study with embedded randomized controlled trial. British Journal of Dermatology 2018;179(1):e55. [Google Scholar]
- Van Halewijn KF, Bohnen AM, Van den Berg PJ, Pasmans SGMA, Bindels PJE, Elshout G. Different potencies of topical corticosteroids for a better treatment strategy in children with atopic dermatitis (the Rotterdam Eczema study): protocol for an observational cohort study with an embedded randomised open-label controlled trial. BMJ Open 2019;9(6):e027239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
AAAAI/ACAAI Atopic Dermatitis Guideline Panel 2024
- Chu DK, Schneider L, Asiniwasis RN, Boguniewicz M, De Benedetto A, et al, AAAAI/ACAAI JTF Atopic Dermatitis Guideline Panel. Atopic dermatitis (eczema) guidelines: 2023 american academy of allergy, asthma and immunology/american college of allergy, asthma and immunology joint task force on practice parameters: GRADE- and institute of medicine-based recommendations. Annals of Allergy, Asthma and Immunology 2024;132(3):274-312. [DOI: 10.1016/j.anai.2023.11.009] [DOI] [PubMed] [Google Scholar]
Abuabara 2018
- Abuabara K, Yu AM, Okhovat JP, Allen IE, Langan SM. The prevalence of atopic dermatitis beyond childhood: a systematic review and meta-analysis of longitudinal studies. Allergy 2018;73(3):696-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Agarwal 2022
- Agarwal A, Chen L, Capozza K, Roberts A, Golden DB, Shaker MS, et al. Trustworthy patient-centered guidelines: insights from atopic dermatitis and a proposal for the future. Journal of Allergy and Clinical Immunology 2022;10(11):2875-7. [DOI: 10.1016/j.jaip.2022.06.017] [DOI] [PubMed] [Google Scholar]
Ahluwalia 1998
- Ahluwalia A. Topical glucocorticoids and the skin - mechanisms of action: an update. Mediators Inflammation 1998;7(3):183-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Altura 1966
- Altura BM. Role of glucocorticoids in local regulation of blood flow. American Journal of Physiology 1966;211(6):1393-7. [DOI: 10.1152/ajplegacy.1966.211.6.1393] [PMID: ] [DOI] [PubMed] [Google Scholar]
Arora 2020
- Arora CJ, Khattak FA, Yousafzai MT, Ibitoye BM, Shumack S. The effectiveness of Janus kinase inhibitors in treating atopic dermatitis: a systematic review and meta-analysis. Dermatologic Therapy 2020;33:e13685. [DOI] [PubMed] [Google Scholar]
Asgari 2020
- Asgari MM, Tsai AL, Avalos L, Sokil M, Quesenberry CP. Association between topical calcineurin inhibitor use and keratinocyte carcinoma risk among adults with atopic dermatitis. JAMA Dermatology 2020;156(10):1066-73. [DOI: 10.1001/jamadermatol.2020.2240] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Australian Pharmaceutical Benefits Scheme 2022
- Australian Goverment Department of Health. Pharmaceutical Benefits Scheme. www.pbs.gov.au/browse/body-system?depth=4&codes=d07aa#d07aa (accessed 15 December 2022).
Beattie 2006
- Beattie PE, Lewis-Jones MS. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. British Journal of Dermatology 2006;155(1):145-51. [DOI] [PubMed] [Google Scholar]
Bissonnette 2016
- Bissonnette R, Papp KA, Poulin Y, Gooderham M, Raman M, Mallbris L, et al. Topical tofacitinib for atopic dermatitis: a phase IIa randomized trial. British Journal of Dermatology 2016;175(5):902-11. [DOI] [PubMed] [Google Scholar]
Bos 2019
- Bos B, Antonescu I, Osinga H, Veenje S, De Jong K, De Vries TW. Corticosteroid phobia (corticophobia) in parents of young children with atopic dermatitis and their health care providers. Pediatric Dermatology 2019;36(1):100-4. [DOI] [PubMed] [Google Scholar]
Bowie 2022
- Bowie AC, Tadrous M, Egeberg A, Harvey J, et al. Agreement and correlation between different topical corticosteroid potency classification systems. JAMA Dermatology 2022;158(7):796-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brignardello‐Petersen 2020
- Brignardello-Petersen R, Forez ID, Izcovich A, Santesso N, Hazelwood G, Alhazanni W, et al. GRADE approach to drawing conclusions from a network meta-analysis using a minimally contextualised framework. BMJ (Clinical Research Ed.) 2020;371:m3900. [DOI: 10.1136/bmj.m3900] [DOI] [PubMed] [Google Scholar]
British Association of Dermatologists 2015
- British Association of Dermatologists. British Association of Dermatologists’ information on topical corticosteroids – established and alternative proprietary names, potency, and discontinuation 2015. www.bad.org.uk (accessed 15 July 2022).
British National Formulary
- BMJ Group & Pharmaceutical Press. British National Formulary. bnf.nice.org.uk/ (accessed prior to 4 July 2024).
Broeders 2016
- Broeders JA, Ahmed Ali U, Fischer G. Systematic review and meta-analysis of randomized clinical trials (RCTs) comparing topical calcineurin inhibitors with topical corticosteroids for atopic dermatitis: a 15-year experience. Journal of the American Academy of Dermatology 2016;75(2):410-9. [DOI] [PubMed] [Google Scholar]
Brookes 2023
- Brookes TS, Barlow R, Mohandas P, Bewley A. Topical steroid withdrawal: an emerging clinical problem [Topical steroid withdrawal: an emerging clinical problem]. Clinical and Experimental Dermatology 2023;48(9):1007-11. [DOI: 10.1093/ced/llad161] [DOI] [PubMed] [Google Scholar]
Carroll 2005
- Carroll CL, Balkrishnan R, Feldman SR, Fleischer AB, Manuel JC. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatric Dermatology 2005;22(3):192-9. [DOI] [PubMed] [Google Scholar]
Celsus Therapeutics 2014
- Celsus enrols first patient in MRX-6 Phase II trial for paediatric atopic dermatitis. https://www.clinicaltrialsarena.com/news/newscelsus-enrols-first-patient-in-mrx-6-phase-ii-trial-for-paediatric-atopic-dermatitis-4338741/ 2014 (accessed 20 December 2023).
Chaimani 2013
- Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PloS One 2013;8:e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chamlin 2004
- Chamlin SL, Frieden IJ, Williams ML, Chren MM. Effects of atopic dermatitis on young American children and their families. Pediatrics 2004;114(3):607-11. [DOI] [PubMed] [Google Scholar]
Charman 1999
- Charman DP, Varigos GA. Grades of severity and the validation of an atopic dermatitis assessment measure (ADAM). Journal of Outcome Measures 1999;3(2):162-75. [PubMed] [Google Scholar]
Charman 2000
- Charman CR, Morris AD, Williams HC. Topical corticosteroid phobia in patients with atopic eczema. British Journal of Dermatology 2000;142(5):931-6. [DOI: 10.1046/j.1365-2133.2000.03473.x] [DOI] [PubMed] [Google Scholar]
Charman 2002
- Charman CR, Venn AJ, Williams HC. Reliability testing of the six area, six sign atopic dermatitis severity score. British Journal of Dermatology 2002;146(6):1057-60. [DOI] [PubMed] [Google Scholar]
Charman 2003
- Charman C, Williams H. The use of corticosteroids and corticosteroid phobia in atopic dermatitis. Clinical Dermatology 2003;21(3):193-200. [DOI: 10.1016/s0738-081x(02)00368-1] [DOI] [PubMed] [Google Scholar]
Chopra 2017
- Chopra R, Vakharia PP, Sacotte R, Patel N, Immaneni S, White T, et al. Relationship between EASI and SCORAD severity assessments for atopic dermatitis. Journal of Allergy and Clinical Immunology 2017;140(6):1708-10. [DOI: 10.1016/j.jaci.2017.04.052] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chren 2012
- Chren MM. The Skindex instruments to measure the effects of skin disease on quality of life. Dermatology Clinics 2012;30(2):231-6, xiii. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chu 2023
- Chu DK, Chu AW, Rayner DG, Guyatt GH, Yepes-Nunez JJ, Gomez-Escobar L, et al. Topical treatments for atopic dermatitis (eczema): systematic review and network meta-analysis of randomized trials. Journal of Allergy and Clinical Immunology 2023;152(6):1493-519. [DOI: 10.1016/j.jaci.2023.08.030] [DOI] [PubMed] [Google Scholar]
Cochrane Information Specialist’s portal
- Cochrane Community. Searching: Conducting: Screen4Me. community.cochrane.org/organizational-info/resources/resources-groups/information-specialists-portal/searching-conducting (accessed 01 April 2022).
Cochrane Skin Specialised Register 2021
- Cochrane Skin. More about the Cochrane Skin Specialised Register. skin.cochrane.org/search-methods (accessed 25 November 2021).
Cotter 2022
- Cotter C, Burton T, Proctor A, Moss C, Flohr C. Topical steroid withdrawal syndrome: time to bridge the gap. British Journal of Dermatology 2022;187(5):780-1. [DOI: 10.1111/bjd.21770] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version (accessed after 29 June 2022). Melbourne, Australia: Veritas Health Innovation, 2023. Available at covidence.org.
Cowdell 2023
- Cowdell F, Lax S, Van Onselen J, Pendleton R. Can co-created knowledge mobilisation interventions alter and enhance mindlines to improve childhood eczema care? A UK-based Social Impact Framework evaluation. BMJ Open 2023;13:e065557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cury Martins 2015
- Cury Martins J, Martins C, Aoki V, Gois AF, Ishii HA, Da Silva EM. Topical tacrolimus for atopic dermatitis. Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No: CD009864. [DOI: 10.1002/14651858.CD009864.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Lusignan 2021
- De Lusignan S, Alexander H, Broderick C, Dennis J, McGovern A, Feeney C, et al. The epidemiology of eczema in children and adults in England: a population-based study using primary care data. Clinical and Experimental Allergy 2021;51(3):471-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Vrieze 2018
- De Vrieze J. The metawars. Science 2018;361(6408):1184-8. [DOI] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI: 10.1016/0197-2456(86)90046-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Devasenapathy 2023
- Devasenapathy N, Chu A, Wong M, Srivastava A, Ceccacci R, Lin C, et al, Group Aaaai Acaai Joint Task Force on Practice Parameters for Atopic Dermatitis Guideline Development. Cancer risk with topical calcineurin inhibitors, pimecrolimus and tacrolimus, for atopic dermatitis: a systematic review and meta-analysis. Lancet Child Adolescent Health 2023;7(1):13-25. [DOI: 10.1016/S2352-4642(22)00283-8] [DOI] [PubMed] [Google Scholar]
Dhillon 2020
- Dhillon S. Delgocitinib: first approval. Drugs 2020;80(6):609-15. [DOI] [PubMed] [Google Scholar]
Drucker 2017
- Drucker AM, Wang AR, Li WQ, Sevetson E, Block JK, Qureshi AA. The burden of atopic dermatitis: summary of a report for the National Eczema Association. Journal of Investigative Dermatology 2017;137(1):26-30. [DOI] [PubMed] [Google Scholar]
Drucker 2020
- Drucker AM, Tadrous M. Topical calcineurin inhibitors and skin cancer - another piece of the puzzle. JAMA Dermatology 2020;156(10):1053-4. [DOI] [PubMed] [Google Scholar]
Drugs.com Vtama prices
- Vtama Prices, Coupons and Patient Assistance Programs. https://www.drugs.com/price-guide/vtama# (accessed 12 December 2023).
Eichenfield 2014
- Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. Journal of the American Academy of Dermatology 2014;71(1):116-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31:140-9. [DOI] [PubMed] [Google Scholar]
European Directorate for the Quality of Medicines
- European Directorate for the Quality of Medicines committee of experts. CD-P-PH/PHO - Classification of Medicines as Regards their Supply. https://www.edqm.eu/en/classification-of-medicines-as-regards-their-supply (accessed 15 July 2022).
Farina 2011
- Farina S, Gisondi P, Zanoni M, Pace M, Rizzoli L, Baldo E, et al. Balneotherapy for atopic dermatitis in children at Comano spa in Trentino, Italy. Journal of Dermatological Treatment 2011;22(6):366-71. [DOI] [PubMed] [Google Scholar]
Fenske 2022
- Fenske C, Boytsov N, Guo J, Dawson Z. Prescription market share and treatment patterns in atopic dermatitis: a retrospective observational study using US insurance claims. Advances in Therapy 2022;39(5):2052-64. [DOI: 10.1007/s12325-022-02071-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Finlay 1994
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) - a simple practical measure for routine clinical use. Clinical and Experimental Dermatology 1994;19(3):210-6. [DOI] [PubMed] [Google Scholar]
Frosch 1981
- Frosch PJ, Behrenbeck EM, Frosch K, Macher E. The Duhring chamber assay for corticosteroid atrophy. British Journal of Dermatology 1981;104:57-65. [DOI] [PubMed] [Google Scholar]
Futamura 2016
- Futamura M, Leshem YA, Thomas KS, Nankervis H, Williams HC, Simpson EL. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: many options, no standards. Journal of the American Academy of Dermatology 2016;74(2):288-94. [DOI: 10.1016/j.jaad.2015.09.062] [DOI] [PubMed] [Google Scholar]
Guelimi 2022
- Guelimi R, Afach S, Regnaux JP, Bettuzzi T, Chaby G, Sbidian E, et al. Overlapping network meta-analyses on psoriasis systemic treatments, an overview: quantity does not make quality. British Journal of Dermatology 2022;187(1):29-41. [DOI: 10.1111/bjd.20908] [DOI] [PubMed] [Google Scholar]
Haest 2011
- Haest C, Casaer MP, Daems A, De Vos B, Vermeersch E, Morren MA, et al. Measurement of itching: validation of the Leuven Itch Scale. Burns 2011;37(6):939-50. [DOI] [PubMed] [Google Scholar]
Hajar 2015
- Hajar T, Leshem YA, Hanifin JM, Nedorost ST, Lio PA, Paller AS, et al. A systematic review of topical corticosteroid withdrawal ("steroid addiction") in patients with atopic dermatitis and other dermatoses. Journal of the American Academy of Dermatology 2015;72(3):541-9 e2. [DOI: 10.1016/j.jaad.2014.11.024] [DOI] [PubMed] [Google Scholar]
Hanifin 2001
- Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M, EASI Evaluator Group. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. Experimental Dermatology 2001;10(1):11-8. [DOI] [PubMed] [Google Scholar]
Harvey 2023
- Harvey J, Lax SJ, Lowe A, Santer M, Lawton S, Langan SM, et al. The long-term safety of topical corticosteroids in atopic dermatitis: a systematic review. Skin Health and Disease 2023;3(5):e268. [DOI: 10.1002/ski2.268] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hay 2014
- Hay RJ, Johns NE, Williams HC, Bolliger IW, Dellavalle RP, Margolis DJ, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. Journal of Investigative Dermatology 2014;134(6):1527-34. [DOI] [PubMed] [Google Scholar]
Higgins 2021a
- Higgins JP, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Higgins 2021b
- Higgins JP, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Housman 2002
- Housman TS, Patel MJ, Camacho F, Feldman SR, Fleischer AB, Balkrishnan R. Use of the Self-Administered Eczema Area and Severity Index by parent caregivers: results of a validation study. British Journal of Dermatology 2002;147(6):1192-8. [DOI] [PubMed] [Google Scholar]
Howell 2019
- Howell MD, Kuo FI, Smith PA. Targeting the Janus Kinase family in autoimmune skin diseases. Frontiers of Immunology 2019;10:2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Howells 2020
- Howells LM, Chalmers JR, Gran S, Ahmed A, Apfelbacher C, Burton T, et al. Development and initial testing of a new instrument to measure the experience of eczema control in adults and children: recap of atopic eczema (RECAP). British Journal of Dermatology 2020;183(3):524-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutton 2015
- Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Annals of Internal Medicine 2015;162(11):777-84. [DOI] [PubMed] [Google Scholar]
Ioannidis 2007
- Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. Canadian Medical Association Journal 2007;176(8):1091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ioannidis 2009
- Ioannidis JP. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. Canadian Medical Association Journal 2009;181(8):488-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johanssen 2004
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. Journal of Allergy and Clinical Immunology 2004;113(5):832-6. [DOI: 10.1016/j.jaci.2003.12.591] [DOI] [PubMed] [Google Scholar]
Ju 2021
- Ju HJ, Han JH, Kim MS, Lee SH, Shin JW, Choi M, et al. The long-term risk of lymphoma and skin cancer did not increase after topical calcineurin inhibitor use and phototherapy in a cohort of 25,694 patients with vitiligo. Journal of the American Academy of Dermatology 2021;84(6):1619-27. [DOI] [PubMed] [Google Scholar]
KEGG MEDICUS
- KEGG (Kyoto Encyclopedia of Genes and Genomes) MEDICUS: Drug information search. https://www.kegg.jp/kegg/medicus/ (accessed 12 December 2023).
Kemp 2003
- Kemp AS. Cost of illness of atopic dermatitis in children: a societal perspective. PharmacoEconomics 2003;21(2):105-13. [DOI] [PubMed] [Google Scholar]
Kim 2015
- Kim JE, Kim HJ, Lew BL, Lee KH, Hong SP, Jang YH, et al. Consensus Guidelines for the treatment of atopic dermatitis in Korea (Part 1): general management and topical treatment. Annals of Dermatology 2015;27(5):563-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kunz 1997
- Kunz B, Oranje AP, Labreze L, Stalder JF, Ring J, Taieb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology (Basel, Switzerland) 1997;195(1):10-9. [DOI] [PubMed] [Google Scholar]
Lam 2021
- Lam M, Zhu JW, Tadrous M, Drucker AM. Association between topical calcineurin inhibitor use and risk of cancer, including lymphoma, keratinocyte carcinoma, and melanoma: a systematic review and meta-analysis. JAMA Dermatology 2021;157(5):549-58. [DOI: 10.1001/jamadermatol.2021.0345] [DOI] [PMC free article] [PubMed] [Google Scholar]
Langan 2023
- Langan SM, Mulick AR, Rutter CE, Silverwood R. Trends in eczema prevalence in children and adolescents: a Global Asthma Network Phase I study. Clinical and Experimental Allergy 2023;53:Article 3. [Google Scholar]
Lax 2022
- Lax SJ, Harvey J, Axon E, Howells L, Santer M, Ridd MJ, et al. Strategies for using topical corticosteroids in children and adults with eczema. Cochrane Database of Systematic Reviews 2022, Issue 3. Art. No: CD013356. [DOI: 10.1002/14651858.CD013356.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis‐Jones 2006
- Lewis-Jones S. Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. International Journal of Clinical Practice 2006;60(8):984-92. [DOI] [PubMed] [Google Scholar]
Li 2017
- Li AW, Yin ES, Antaya RJ. Topical corticosteroid phobia in atopic dermatitis: a systematic review. JAMA Dermatology 2017;153(10):1036-42. [DOI: 10.1001/jamadermatol.2017.2437] [DOI] [PubMed] [Google Scholar]
Lin 2020
- Lin L, Shi L, Chu H, Murad MH. The magnitude of small-study effects in the Cochrane Database of Systematic Reviews: an empirical study of nearly 30 000 meta-analyses. BMJ Evidence-Based Medicine 2020;25(1):27-32. [DOI: 10.1136/bmjebm-2019-111191] [DOI] [PMC free article] [PubMed] [Google Scholar]
Luger 2022
- Luger TA, Hebert AA, Zaenglein AL, Silverberg JI, Tan H, Ports WC, Zielinski MA. Subgroup Analysis of Crisaborole for Mild-to-Moderate Atopic Dermatitis in Children Aged 2 to < 18 Years. Paediatr Drugs 2022;24:175-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Majeski 2007
- Majeski CJ, Johnson JA, Davison SN, Lauzon CJ. Itch Severity Scale: a self-report instrument for the measurement of pruritus severity. British Journal of Dermatology 2007;156(4):667-73. [DOI] [PubMed] [Google Scholar]
Marshall 2018
- MarshallI J, Noel-Storr AH, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying randomized controlled trials: an evaluation and practitioner’s guide. Research Synthesis Methods 2018;9(4):602-14. [DOI: 10.1002/jrsm.1287] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martín‐Santiago 2022
- Martín-Santiago A, Puig S, Arumi D, Rebollo Laserna FJ. Safety profile and tolerability of topical phosphodiesterase 4 inhibitors for the treatment of atopic dermatitis: a systematic review and meta-analysis. Current Therapeutic Research 2022;96:100679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matterne 2019
- Matterne U, Bohmer MM, Weisshaar E, Jupiter A, Carter B, Apfelbacher CJ. Oral H1 antihistamines as 'add-on' therapy to topical treatment for eczema. Cochrane Database of Systematic Reviews 2019, Issue 1. Art. No: CD012167. [DOI: 10.1002/14651858.CD012167.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mills 2013
- Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta-analysis. BMJ (Clinical Research Ed.) 2013;346:f2914. [DOI] [PubMed] [Google Scholar]
Moher 2016
- Moher D, Glasziou P, Chalmers I, Nasser M, Bossuyt PM, Korevaar DA, et al. Increasing value and reducing waste in biomedical research: who's listening? Lancet 2016;387(10027):1573-86. [DOI: 10.1016/S0140-6736(15)00307-4] [DOI] [PubMed] [Google Scholar]
Moss 2023
- Moss C, Haider Z, Proctor A. Do people with eczema and their carers understand topical steroid potency? Results of two surveys. Clinical and Experimental Dermatology 2023;49(3):267-70. [DOI: 10.1093/ced/llad372] [DOI] [PubMed] [Google Scholar]
National Psoriasis Foundation (USA)
- National Psoriasis Foundation topical steroid potency chart. www.psoriasis.org/potency-chart (accessed October 2018).
NICE 2007
- National Institute for Health and Care Excellence (NICE). Atopic eczema in under 12s: diagnosis and management [CG57]. Available at www.nice.org.uk/guidance/cg57 (accessed prior to 4 July 2024).
Nickles 2023
- Nickles MA, Coale AT, Henderson WJ, Brown KE, Morrell DS, Nieman EL. Steroid phobia on social media platforms. Pediatric Dermatology 2023;40(3):479-82. [DOI: 10.1111/pde.15269] [DOI] [PubMed] [Google Scholar]
Nikolakopoulou 2020
- Nikolakopoulou A, Higgins JP, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, et al. CINeMA: An approach for assessing confidence in the results of a network meta-analysis. PLoS Medicine 2020;17(4):e1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Noel‐Storr 2020
- Noel-Storr AH, Dooley G, Wisniewski S, Glanville J, Thomas J, Cox S, et al. Cochrane Centralised Search Service showed high sensitivity identifying randomized controlled trials: a retrospective analysis. Journal of Clinical Epidemiology 2020;127:142-50. [DOI: 10.1016/j.jclinepi.2020.08.008] [DOI] [PubMed] [Google Scholar]
Noel‐Storr 2021
- Noel-Storr A, Dooley G, Elliott J, Steele E, Shemilt I, Mavergames C, et al. An evaluation of Cochrane Crowd found that crowdsourcing produced accurate results in identifying randomized trials. Journal of Clinical Epidemiology 2021;133:130-9. [DOI: 10.1016/j.jclinepi.2021.01.006] [DOI] [PubMed] [Google Scholar]
Olsen 1986
- Olsen EA, Cornell RC. Topical clobetasol-17-propionate: review of its clinical efficacy and safety. Journal of the American Academy of Dermatology 1986;15(2 Pt 1):246-55. [DOI: 10.1016/s0190-9622(86)70164-3] [DOI] [PubMed] [Google Scholar]
Opzelura®
- Opzelura® (ruxolitinib) cream 1.5%. https://www.opzelura.com/atopic-dermatitis/cost (accessed 12 December 2023).
Oulee 2022
- Oulee A, Ivanic M, Norden A, Javadi SS, Wu JJ. Atopic dermatitis on TikTok: a cross-sectional study. Clinical and Experimental Dermatology 2022;47(11):2036-7. [DOI: 10.1111/ced.15322] [DOI] [PubMed] [Google Scholar]
Paller 2016
- Paller AS, Tom WL, Lebwohl MG, Blumenthal RL, Boguniewicz M, Call RS, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. Journal of the American Academy of Dermatology 2016;75(3):494-503 e6. [DOI] [PubMed] [Google Scholar]
Paller 2020
- Paller AS, Folster-Holst R, Chen SC, Diepgen TL, Elmets C, Margolis DJ, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. Journal of the American Academy of Dermatology 2020;83(2):375-81. [DOI: 10.1016/j.jaad.2020.03.075] [DOI] [PubMed] [Google Scholar]
Paller 2021
- Paller AS, Stein Gold L, Soung J, Tallman AM, Rubenstein DS, Gooderham M. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. Journal of the American Academy of Dermatology 2021;84(3):632-8. [DOI] [PubMed] [Google Scholar]
Pariser 2020
- Pariser DM, Simpson EL, Gadkari A, Bieber T, Margolis DJ, Brown M, et al. Evaluating patient-perceived control of atopic dermatitis: design, validation, and scoring of the Atopic Dermatitis Control Tool (ADCT). Current Medical Research Opinion 2020;36(3):367-76. [DOI] [PubMed] [Google Scholar]
Pena 2023
- Pena J, Zameza PA, Pixley JN, Remitz A, Feldman SR. A comparison of topical corticosteroids and topical calcineurin inhibitors for the treatment of atopic dermatitis. Journal of Allergy and Clinical Immunology in Practice 2023;11(5):1347-59. [DOI: 10.1016/j.jaip.2023.03.022] [DOI] [PubMed] [Google Scholar]
Pharmacoeconomic Review Report: Crisaborole
- Canadian Agency for Drugs and Technologies in Health. Pharmacoeconomic Review Report: Crisaborole ointment, 2% (Eucrisa): (Pfizer Canada Inc.): Indication: For topical treatment of mild-to-moderate atopic dermatitis in patients two years of age and older. https://www.ncbi.nlm.nih.gov/books/NBK542517/ 2019 (accessed prior to 4 July 2024);Appendix 1, Cost Comparison:not reported. [PubMed]
Portney 1972
- Portney B, Sarkany I. Metosyn’ in psoriasis and eczema. British Journal of Dermatology 1972;86:310. [DOI] [PubMed] [Google Scholar]
Presley 2019
- Presley C, Rundle CW, Kolodziejczyk T, Andrews S, Shumaker P, Anand P, et al. Prioritization of Cochrane systematic reviews. British Journal of Dermatology 2019;181(6):1303-4. [DOI: 10.1111/bjd.18180] [DOI] [PubMed] [Google Scholar]
Resource Clinical (USA)
- Resource clinical topical corticosteroids potency classification. www.resourceclinical.com/clinical-tables/topical-corticosteroids-potency-USA-classification.pdf (accessed October 2018).
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). RevMan Web 2022, Version 4.10.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Rohatgi 2021 [Computer program]
- WebPlotDigitizer. Rohatgi Ankit. WebPlotDigitizer, 2021. Available from automeris.io/WebPlotDigitizer/.
Saeki 2022
- Saeki H, Ohya Y, Furuta J, Arakawa H, Ichiyama S, Katsunuma T, et al. Executive summary: Japanese guidelines for atopic dermatitis (ADGL) 2021. Allergology International 2022;71(4):448-58. [DOI: 10.1016/j.alit.2022.06.009] [DOI] [PubMed] [Google Scholar]
Salanti 2011
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64:163-71. [DOI] [PubMed] [Google Scholar]
Sampson 1990
- Sampson H. Pathogenesis of eczema. Clin Exp Allergy 1990;20:459-67. [DOI] [PubMed] [Google Scholar]
Samuel 2023
- Samuel C, Cornman H, Kambala A, Kwatra SG. A review on the safety of using JAK inhibitors in dermatology: clinical and laboratory monitoring. Dermatology and Therapy (Heidelb) 2023;13(3):729-49. [DOI: 10.1007/s13555-023-00892-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Santer 2022
- Santer M, Muller I, Becque T, Stuart B, Hooper J, Steele M, et al. Eczema Care Online behavioural interventions to support self-care for children and young people: two independent, pragmatic, randomised controlled trials. British Medical Journal 2022;379:e072007. [DOI: 10.1136/bmj-2022-072007] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scherrer 1974
- Scherrer RA, Whitehouse MW. Anti-Inflammatory Agents. Elsevier Inc, 1974. [Google Scholar]
Schmitt 2012
- Schmitt J, Spuls P, Boers M, Thomas K, Chalmers J, Roekevisch E, et al. Towards global consensus on outcome measures for atopic eczema research: results of the HOME II meeting. Allergy 2012;67(9):1111-7. [DOI: 10.1111/j.1398-9995.2012.02874.x] [DOI] [PubMed] [Google Scholar]
Schmitt 2014
- Schmitt J, Spuls PI, Thomas KS, Simpson E, Furue M, Deckert S, et al. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. Journal of Allergy and Clinical Immunology 2014;134(4):800-7. [DOI] [PubMed] [Google Scholar]
Schram 2012
- Schram ME, Spuls PI, Leeflang MM, Lindeboom R, Bos JD, Schmitt J. EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy 2012;67:99-106. [DOI] [PubMed] [Google Scholar]
Schwartz 2017
- Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O'Shea JJ. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nature Reviews Drug Discovery 2017;16(12):843-62. [DOI] [PubMed] [Google Scholar]
Schünemann 2022
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook/archive/v6.3.
Sidbury 2023
- Sidbury R, Alikhan A, Bercovitch L, Cohen DE, Darr JM, Drucker AM, et al. Guidelines of care for the management of atopic dermatitis in adults with topical therapies [Guidelines of care for the management of atopic dermatitis in adults with topical therapies]. Journal of the American Academy of Dermatology 2023;89(1):e1-e20. [DOI: 10.1016/j.jaad.2022.12.029] [DOI] [PubMed] [Google Scholar]
Sigurgeirsson 2015
- Sigurgeirsson B, Boznanski A, Todd G, Vertruyen A, Schuttelaar ML, Zhu X, et al. Safety and efficacy of pimecrolimus in atopic dermatitis: a 5-year randomized trial. Pediatrics 2015;135(4):597-606. [DOI: 10.1542/peds.2014-1990] [DOI] [PubMed] [Google Scholar]
Smith 2017
- Smith SH, Jayawickreme C, Rickard DJ, Nicodeme E, Bui T, Simmons C, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. Journal of Investigative Dermatology 2017;137(10):2110-9. [DOI] [PubMed] [Google Scholar]
Spuls 2017
- Spuls PI, Gerbens LA, Simpson E, Apfelbacher CJ, Chalmers JR, Thomas KS, et al. Patient-Oriented Eczema Measure (POEM), a core instrument to measure symptoms in clinical trials: a Harmonising Outcome Measures for Eczema (HOME) statement. British Journal of Dermatology 2017;176(4):979-84. [DOI] [PubMed] [Google Scholar]
Stalder 2011
- Stalder JF, Barbarot S, Wollenberg A, Holm EA, De Raeve L, Seidenari S, et al. Patient-Oriented SCORAD (PO-SCORAD): a new self-assessment scale in atopic dermatitis validated in Europe. Allergy 2011;66(8):1114-21. [DOI] [PubMed] [Google Scholar]
Stark 2012
- Stark GR, Darnell JE. The JAK-STAT pathway at twenty. Immunity 2012;36(4):503-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical Research Ed.) 2019;366:4898. [DOI] [PubMed] [Google Scholar]
Sweeting 2004
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Statistics in Medicine 2004;23(9):1351-75. [DOI] [PubMed] [Google Scholar]
Teasdale 2021
- Teasdale E, Muller I, Sivyer K, Ghio D, Greenwell K, Wilczynska S, et al. Views and experiences of managing eczema: systematic review and thematic synthesis of qualitative studies. British Journal of Dermatology 2021;184(4):627-37. [DOI] [PubMed] [Google Scholar]
Thomas 2021
- Thomas J, McDonald S, Noel-Storr A, Shemilt I, Elliott J, Mavergames C, et al. Machine learning reduced workload with minimal risk of missing studies: development and evaluation of a randomized controlled trial classifier for Cochrane Reviews. Journal of Clinical Epidemiology 2021;133:140-51. [DOI: 10.1016/j.jclinepi.2020.11.003] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsai 2021
- Tsai HR, Lu JW, Chen LY, Chen TL. Application of Janus kinase inhibitors in atopic dermatitis: an updated systematic review and meta-analysis of clinical trials. Journal of Personalized Medicine 2021;11(4):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
US Food and Drug Administration 2021
- US Food and Drug Administration. OPZELURA™ (ruxolitinib) cream, for topical use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215309s001lbl.pdf 2021 (accessed prior to 4 July 2024).
US Food and Drug Administration 2021a
- FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death 2021 (accessed prior to 4 July 2024).
Veroniki 2013
- Veroniki AA, Vasiliadis HS, Higgins JP, Salanti G. Evaluation of inconsistency in networks of interventions. International Journal of Epidemiology 2013;42(1):332-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vittrup 2023
- Vittrup I, Andersen YM, Skov L, Wu JJ, Agner T, Thomsen SF, et al. The association between atopic dermatitis, cognitive function and school performance in children and young adults. British Journal of Dermatology 2023;188(3):341-9. [DOI: 10.1093/bjd/ljac058] [DOI] [PubMed] [Google Scholar]
Waldecker 2017
- Waldecker A, Malpass A, King A, Ridd MJ. Written action plans for children with long-term conditions: a systematic review and synthesis of qualitative data. Health Expectations 2018;21(3):585-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watt 2019
- Watt J, Tricco AC, Straus S, Veroniki AA, Naglie G, Drucker AM. Research techniques made simple: network meta-analysis. Journal of Investigative Dermatology 2019;139(1):4-12 e1. [DOI: 10.1016/j.jid.2018.10.028] [DOI] [PubMed] [Google Scholar]
Whalley 2004
- Whalley D, McKenna SP, Dewar AL, Erdman RA, Kohlmann T, Niero M, et al. A new instrument for assessing quality of life in atopic dermatitis: international development of the Quality of Life Index for Atopic Dermatitis (QoLIAD). British Journal of Dermatology 2004;150(2):274-83. [DOI] [PubMed] [Google Scholar]
White 2015
- White IR. Network meta-analysis. Stata Journal 2015;15:951-85. [Google Scholar]
WHO Classification 2018
- World Health Organization. WHO model prescribing information: drugs used in skin diseases: classification of topical corticosteroids. apps.who.int/iris/handle/10665/41975 (accessed October 2018).
Wilkes 2016
- Wilkes SR, Nankervis H, Tavernier E, Maruani A, Williams HC. How clinically relevant are treatment comparisons of topical calcineurin inhibitor trials for atopic eczema? Journal of Investigative Dermatology 2016;136(10):1944-9. [DOI] [PubMed] [Google Scholar]
Williams 1994
- Williams HC, Burney PG, Pembroke AC, Hay RJ. The U.K.Working Party’s Diagnostic Criteria for Atopic Dermatitis. III. Independent hospital validation. Br J Dermatol 1994;131:406-16. [DOI] [PubMed] [Google Scholar]
Williams 2004
- Williams HC. Twice-weekly topical corticosteroid therapy may reduce atopic dermatitis relapses. Archives of Dermatology 2004;140(9):1151-2. [DOI] [PubMed] [Google Scholar]
Williams 2008
- Williams H, Stewart A, Von Mutius E, Cookson W, Anderson HR, International Study of Asthma, Allergies in Childhood Phase One, Three Study Groups. Is eczema really on the increase worldwide? Journal of Allergy and Clinical Immunology 2008;121(4):947-54 e15. [DOI] [PubMed] [Google Scholar]
Williams 2021
- Williams HC. New topical treatments for atopic dermatitis: active comparators are needed. Journal of the American Academy of Dermatology 2021;85(4):1065-6. [DOI: 10.1016/j.jaad.2021.06.002] [DOI] [PubMed] [Google Scholar]
Williams 2022
- Williams HC, Schmitt J, Thomas KS, Spuls PI, Simpson EL, Apfelbacher CJ, Home Initiative. The HOME Core outcome set for clinical trials of atopic dermatitis. Journal of Allergy and Clinical Immunology 2022;149(6):1899-911. [DOI: 10.1016/j.jaci.2022.03.017] [DOI] [PubMed] [Google Scholar]
Wollenberg 2020
- Wollenberg A, Christen-Zach S, Taieb A, Paul C, Thyssen JP, Bruin-Weller M, et al. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. Journal of the European Academy of Dermatology and Venereology : JEADV 2020;34(12):2717-44. [DOI] [PubMed] [Google Scholar]
Wollenberg 2022
- Wollenberg A, Kinberger M, Arents B, Aszodi N, Avila Valle G, Barbarot S, et al. European guideline (EuroGuiDerm) on atopic eczema - part II: non-systemic treatments and treatment recommendations for special AE patient populations. Journal of the European Academy of Dermatology and Venereology : JEADV 2022;36(11):1904-26. [DOI: 10.1111/jdv.18429] [DOI] [PubMed] [Google Scholar]
World Bank Group 2023
- World Bank Group country classifications by income level for FY24 (July 1, 2023-June 30, 2024). https://blogs.worldbank.org/opendata/new-world-bank-group-country-classifications-income-level-fy24 (accessed prior to 4 July 2024).
Yang 2019
- Yang H, Wang J, Zhang X, Zhang Y, Qin Z, Wang H, et al. Application of topical phosphodiesterase 4 inhibitors in mild to moderate atopic dermatitis: a systematic review and meta-analysis. JAMA Dermatology 2019;155(5):585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yepes‐Nunez 2019
- Yepes-Nunez JJ, Li SA, Guyatt G, Jack SM, Brozek JL, Beyene J, et al. Development of the summary of findings table for network meta-analysis. Journal of Clinical Epidemiology 2019;115:1-13. [DOI] [PubMed] [Google Scholar]
Yosipovitch 2019
- Yosipovitch G, Reaney M, Mastey V, Eckert L, Abbe A, Nelson L, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. British Journal of Dermatology 2019;181(4):761-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zebda 2018
- Zebda R, Paller AS. Phosphodiesterase 4 inhibitors. Journal of the American Academy of Dermatology 2018;78(3 Suppl 1):S43-S52. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Steele 2022
- Steele L, Stuart B, Axon E, Lax SJ, Harvey J, Roberts A, et al. Topical anti-inflammatory treatments for eczema: network meta-analysis. Cochrane Database of Systematic Reviews 2022, Issue 7. Art. No: CD015064. [DOI: 10.1002/14651858.CD015064] [DOI] [PMC free article] [PubMed] [Google Scholar]



