Abstract
Background
Inflammation plays a critical role in tumor development. Inflammatory cell infiltration and inflammatory mediator synthesis cause changes in the tumor microenvironment (TME) in several cancers, especially in intrahepatic cholangiocellular carcinoma (ICC). However, methods to ascertain the inflammatory state of patients using reliable biomarkers are still being explored.
Method
We retrieved the RNA sequencing and somatic mutation analyses results and the clinical characteristics of 244 patients with ICC from published studies. We performed consensus clustering to identify the molecular subtypes associated with inflammation. We compared the prognostic patterns, clinical characteristics, somatic mutation profiles, and immune cell infiltration patterns across inflammatory subtypes. We performed quantitative real-time polymerase chain reaction (qRT-PCR) and immunohistochemistry (IHC) to confirm gene expression. We performed logistic regression analyses to construct a nomogram predicting the inflammatory status of patients with ICC.
Results
Our results confirmed that ICC can be categorized into an inflammation-high subtype (IHS) and an inflammation-low subtype (ILS). Patients from each group had distinct prognosis, clinical characteristics, and TME composition. Patients with ICC in the IHS group showed poorer prognosis owing to the immunosuppressive microenvironment and high frequency of KRAS and TP53 mutations. Cancer-associated fibroblast (CAF)-derived COLEC11 reduced myeloid inflammatory cell infiltration and attenuated inflammatory responses. The results of qRT-PCR and IHC experiments confirmed that COLEC11 expression levels were significantly reduced in tumor tissues compared to those in paracancerous tissues. Patients with ICC in the IHS group were more likely to respond to treatment with immune checkpoint inhibitors (ICIs) owing to their higher tumor mutational burden (TMB) scores, tumor neoantigen burden (TNB) scores, neoantigen counts, and immune checkpoint expression levels. Finally, we developed a nomogram to effectively predict the inflammatory status of patients with ICC based on their clinical characteristics and inflammatory gene expression levels. We evaluated the calibration, discrimination potential, and clinical utility of the nomogram.
Conclusion
The inflammatory response in IHS is primarily induced by myeloid cells. COLEC11 can reduce the infiltration level of this group of cells, and myeloid inflammatory cells may be a novel target for ICC treatment. We developed a novel nomogram that could effectively predict the inflammatory state of patients with ICC, which will be useful for guiding individualized treatment plans.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12967-024-05529-5.
Keywords: Intrahepatic cholangiocellular carcinoma, TME, Inflammation, Neutrophil, Tumor mutational burden
Introduction
Intrahepatic cholangiocellular carcinoma (ICC) is the second-most common primary hepatic malignancy after hepatocellular carcinoma in terms of its incidence, which has increased continuously over the past decade [1]. Radical resection is the only treatment method for patients with ICC. However, ICC is highly malignant, and the resectability rate in patients is only 15-20% owing to the following issues. First, most of the tumors metastasize before the initial diagnosis, and the residual hepatic tissue is insufficient to compensate. Second, because patients typically have a weak physique or poor health, they are unable to tolerate tumor resection procedures [2]. Risk factors for ICC primarily include intrahepatic bile duct stones, viral hepatitis, cirrhosis, and chronic cholangitis, which often lead to chronic inflammation of the liver and bile ducts, thus inducing ICC [3]. Thus, the development of tumors is closely related to inflammation. Therefore, exploring the regulatory mechanisms underlying the development of inflammation-induced tumors can help identify novel drug targets at an early stage, which is of great significance for tumor prevention and treatment.
Inflammation plays a critical role in the induction and progression of tumors. Tumor-related inflammation involves the infiltration of inflammatory cells and the production of inflammatory mediators in tumor tissues, which alter the TME [4]. Acute inflammation can promote the death of cancer cells by activating anti-tumor immune responses [5]. However, chronic inflammation is usually characterized by Th2 type immune responses and the infiltration of immunosuppressive bone marrow cells, which secrete reactive oxygen species, proinflammatory cytokines, chemokines, growth factors, and proangiogenic mediators. These cause tissue damage, epithelial mutations, endothelial dysfunction, angiogenesis, immunosuppression, and matrix remodeling, eventually inducing tumor progression, invasion, metastasis, and drug resistance [6, 7]. Anti-inflammatory therapy has garnered attention owing to its ability to directly inhibit tumor cells and its interventional effects on the TME. The elucidation of the T-cell immunosuppression signal has aided the successful transformation of anti-tumor immune strategies involving the use of ICIs [8]. Although these immunotherapies have achieved good clinical efficacy in various cancers, they exert lasting effects only in a limited number of populations and may be rendered ineffective owing to primary or secondary therapeutic resistance [9–11]. With the frequent occurrence of clinical resistance to ICIs and the gradual decoding of immunosuppression signals, inflammation-targeted strategies are increasingly being applied in combined immunotherapy treatments [12]. However, there exists significant inflammatory heterogeneity among different patients with ICC. Therefore, methods for identifying strong inflammatory subtypes using reliable biomarkers and guiding individualized treatment is an urgent issue that warrants investigation.
In this study, we analyzed the bulk RNA sequencing data, somatic mutation data, and clinical characteristics of 244 patients with ICC. We identified two types of ICC with different inflammatory states based on the expression levels of inflammation-related genes. The two types of inflammatory states in ICC showed significant heterogeneity in immune cell infiltration, somatic mutations, and molecular characteristics. Briefly, the strong inflammatory response group was primarily characterized by the infiltration of proinflammatory neutrophils, macrophages, and Th2 cells, which promoted the formation of an immunosuppressive microenvironment. Among somatic mutations, KRAS and TP53 mutations had a higher frequency in the IHS group, whereas IDH1 mutation had a higher frequency in the ILS group. We verified this using the single-cell RNA sequencing and spatial transcriptome data of patients with ICC. We identified that COLEC11 protein derived from CAF can effectively inhibit the infiltration of myeloid inflammatory cells and suppress inflammation. Finally, we constructed a nomogram based on the clinical characteristics and inflammatory gene expression patterns of patients to effectively determine the inflammatory state of patients with ICC.
In summary, our findings elucidate the relationship between inflammation and tumor induction and progression from varying perspectives. We identified novel inflammatory subtypes and effective anti-inflammatory genes in ICC and prepared a nomogram to predict the inflammatory state. Our findings provide an important theoretical basis for the clinical application of anti-inflammatory drugs in combination with immunotherapy.
Materials and methods
Data collection
Two hundred and forty-four patients with ICC were enrolled in the study, and their RNA sequencing, somatic mutation, and matched clinical data were obtained from a study by Dong et al. [13]. In the validation set, the RNA sequencing and corresponding clinical data of 83 patients with ICC were obtained from the GEO database (accession number: GSE89749). Single-cell RNA sequencing data from four ICC tumor tissue samples and three paracancerous tissue samples were also retrieved from the GEO database (accession number: GSE138709). In addition, high-resolution spatial transcriptome data of patients with ICC were obtained from a study by Wu et al. [14]. Tumor and paracancerous tissue samples were obtained from ten patients with ICC who had undergone surgical resection at the Department of Hepatobiliary and Pancreatic Surgery, the First Medical Center of the Chinese People’s Liberation Army General Hospital.
Consensus clustering
Consensus clustering was performed to identify the inflammation-associated molecular subtypes using the “ConcensusClusterPlus” package in R. Briefly, a k-means clustering method was used with 50 iterations (80% of the samples used each time). The optimal number of clusters was determined according to the clustering scores of the cumulative distribution function (CDF) curve, and relative changes in the area under the CDF curve were evaluated. Principal component analysis (PCA), which is commonly used for reducing dimensionality, was used to verify the reliability of the consensus clustering.
Gene set variation analysis (GSVA) and gene set enrichment analysis (GSEA)
GSVA, an unsupervised and non-parametric approach, is used to assess the enrichment of gene sets in correlation with mRNA expression in each sample. Gene sets retrieved from the MSigDB (https://www.gseamsigdb.org/gsea/msigdb/) database were comprehensively scored using the GSVA algorithm. Using c2.cp.kegg.v7.0.symbols.gmt as the reference gene set, the expression matrices of samples from the IHS and ILS were used for GSEA. GSEA 4.0 was used with 1,000 permutations and a screening threshold of FDR < 0.05.
Immune infiltration analysis
To explore the immune characteristics of ICC samples, we used xcell (http://xcell.ucsf.edu/) and determined the relative proportions of immune cell types. The tumor immune dysfunction and rejection (TIDE) (http://tide.dfci.harvard.edu/) algorithm was used to compare the differences in immunotherapy efficacy between the two groups.
Somatic mutation analysis
Somatic mutation data of 244 patients with ICC were analyzed using the maftools function of R software. Mutant genes were visualized using waterfall plots.
Nomogram construction and validation
We first fitted all independent variables using the glm function of R software and calculated the Akaike Information Criterion (AIC) using stepwise logistic regression analysis. We then screened the smallest independent variable with an AIC value for constructing the model. Subsequently, we calculated the partial regression coefficients of each variable included in the model. The positive probability of occurrence of the outcome variable for each patient was calculated using the formula Logit(p) = β0 + β1 * X1 + β2* X2…… βk*Xk. We constructed nomograms according to the degree of contribution (regression coefficient) of each variable to the outcome variable. Based on the findings of logistic regression analysis, scores were assigned to each value level of each risk factor, and each score was then added. Receiver operating characteristic (ROC) curves were prepared to evaluate the predictive ability of the nomogram. Calibration curves were prepared to assess the performance of the nomogram. Decision curve analysis (DCA) was performed to evaluate the clinical practicability of the nomogram. The “rms”, “pROC”, and “rmda” packages of R software were used to generate the nomogram, ROC curve, and calibration curve for performing DCA.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Tumor tissues and adjacent paracancerous tissues from ten patients with ICC who had undergone surgical resection were collected and stored at -80 °C. Total RNA was extracted from these samples using TRIzol reagent and then transcribed into cDNA using a PrimeScript RT kit. The cDNA concentration was measured using SYBR qPCR Master Mix. The 2−ΔΔCt method was used to calculate the relative expression levels of genes, using the glyceraldehyde-3-phosphate dehydrogenase gene (GAPDH) as the normalization control.
Immunohistochemistry (IHC)
ICC tumor tissues and adjacent paracancerous tissues were first fixed with formaldehyde and embedded in paraffin. Then, the paraffin-embedded tissue sections were cut into slices of 4 μm and incubated with Rabbit anti-human COLEC11 primary antibody at 4 °C for 12 h. Following this, the slices were treated with a horseradish peroxidase (HRP)-labeled secondary antibody at room temperature for 1 h. Finally, the slices were stained with DAB and hematoxylin in turns, washed, mounted with an anti-fade reagent, and covered with a cover glass.
Statistical analysis
Statistical analyses were conducted using R software (v4.1.2). Categorical variables in the two groups were compared using the Wilcoxon t-test. Univariate and multivariate Cox regression analyses were performed to investigate the prognostic value of different clinicopathological characteristics. The Kaplan-Meier curve and log-rank test were used to compare the survival differences between the two groups. The Benjamini–Hochberg method was used to adjust the p-value. P < 0.05 was considered to indicate the level of significance.
Results
Two inflammation-based subtypes identified using consensus clustering
First, we identified the inflammation-related gene set (IRGS, Additional file 2: Table 1) using the inflammatory response signaling pathway in Hallmarker. The results of enrichment analysis showed that the IRGS was primarily enriched in the inflammatory response, cytokine-cytokine receptor interaction, and regulation of leukocyte migration signaling pathways (Fig. 1A). We performed consensus clustering to identify clusters based on ICC inflammation. After k-means clustering, we identified two groups that showed different patterns of inflammatory gene expression (Fig. 1B and C, Additional file 3: Table 2). The expression levels of inflammatory genes in the different clusters differed significantly. Cluster 1 (C1) had higher expression levels of inflammatory genes, whereas Cluster 2 (C2) had lower levels (Fig. 1D). The ssGSEA method was used to quantify the inflammatory response score of each patient with ICC. The results confirmed that patients with ICC in group C1 had a higher inflammatory response score than patients in group C2 (Fig. 1E, P < 0.001). Therefore, we defined C1 as the IHS and C2 as the ILS. We performed PCA to compare the differences in transcriptional patterns between different inflammatory subgroups. Interestingly, patients from C1 and C2 were widely separated from each other (Fig. 1F), which confirmed the different landscapes between IHS and ILS. Based on the protein expression level, patients with ICC could also be stratified into the IHS and ILS groups (Additional file 1: Figure S1A-S1B). To further confirm our findings, we validated them using an external independent dataset (GSE89749) comprising the mRNA expression data of 83 patients with ICC. Patients with ICC could be stratified as IHS and ILS in the GSE89749 dataset as well (Additional file 1: Figure S1C–S1D).
Fig. 1.
Identification of three inflammatory subtypes in ICC. (A) Results of enrichment analysis of inflammatory response-related genes. (B) Delta area curve of consensus clustering. (C) Heatmap depicting the consensus clustering solution (k = 2) for 222 genes in 244 samples. (D) Heatmap showing the expression of 222 inflammatory response-related genes. (E) Violin plots indicating the differences among these subtypes. (F) Plots showing the results of principal component analysis
Different inflammatory groups exhibit different prognostic and clinical characteristics
Evidence from previous studies has confirmed that cytokines produced in response to chronic inflammation can induce abnormal inflammatory signaling pathways by inducing gene mutations. These can alter the expression and transformation of oncogenes and tumor suppressor genes, thereby inhibiting apoptosis and inducing angiogenesis. In our study, patients with ICC in the IHS group had poor prognosis and the shortest overall survival (HR = 2.75, 95% CI (1.84, 4.10), P = 2.9e − 07) (Fig. 2A). In contrast, patients with ICC in the ILS group showed better prognosis and longer overall survival. Consistent results were observed at the protein expression level (Fig. 2B). We further compared the clinical characteristics between the IHS and ILS groups. Interestingly, patients in the IHS group showed biliary tract stone disease, an advanced TNM stage, perineural invasion, distant metastasis, regional lymph node metastasis, TP53 mutation, and KRAS mutation. Conversely, patients in the ILS group showed IDH1/2 mutation (Fig. 2C). This finding suggests that in ICC, the inflammatory response is associated with tumor invasion and oncogene mutations. We further compared the effects of inflammatory responses in different clinical characteristic subgroups. We observed significant differences in the overall survival between patients with ICC in the two subgroups for intrahepatic metastasis, HBV status (0: negative; 1: positive), vascular invasion, regional lymph node metastasis, TNM stage, CEA (> 5 µg/L: high), γ-GT (> 50 U/L: high), and KRAS mutation (Fig. 2D). In the IHS group, patients with ICC along with intrahepatic metastasis, an HBV-positive status, vascular invasion, regional lymph node metastasis, TNM stage (III + IV), a high CEA, a high γ-GT level, and KRAS mutations had a poor prognosis. This result suggested that inflammatory responses combined with clinical characteristics can be effective for the accurate stratification of patients with ICC and can be used as a reliable indicator for predicting their overall survival.
Fig. 2.
Prognostic and clinical characteristics of different inflammatory subtypes. (A) Survival analysis results for the consistent clustering of mRNA expression data. (B) Survival analysis results for the consistent clustering of protein expression data. (C) Percentage differences in clinical characteristics between the IHS and ILS groups. (D) Differences in survival in inflammatory subtypes across clinical subgroups
Notably, in the IHS group, HBV-positive patients had a longer overall survival than HBV-negative patients, but this was not the case in the ILS group. This suggests that in the IHS group, patients with ICC with HBV infection-induced inflammation had better prognosis than non-HBV patients. Moreover, in the entire cohort, patients with ICC with HBV had a longer overall survival, which is consistent with the findings of Zhang et al. [15]. We hypothesize that this phenomenon may be attributed to the activation of systemic immune responses in patients with long-term HBV infection, which suppresses ICC progression.
The type of TME differs based on the inflammatory subgroup
Considering the strong influence of inflammation on immune cell infiltration, we compared the differences in the TME between the IHS and ILS groups. Briefly, compared with the ILS group, the IHS group had greater immune and microenvironment scores, whereas the converse was true for the stromal scores (Fig. 3A). This result suggested that the IHS group was associated with greater immune cell infiltration. Subsequently, we compared the differences in the immune cell infiltration levels between the two groups. Specifically, the abundance of CD4 + cells, B cells, CD8 + killer cells, Th2 cells, and immunosuppressive cells (neutrophils, iDCs, and M2-type macrophages) was significantly higher in the IHS group, whereas the abundance CD8 + naive T cells, plasma cells, fibroblasts, and Th1 cells was lower (Fig. 3B). The IHS group had higher myeloid inflammation scores, indicating that the inflammatory response in ICC was primarily induced by myeloid cells (neutrophils and proinflammatory macrophages) and altered the level of immune cell infiltration in the TME (Fig. 3C). Interestingly, the infiltration of immune cells and immunosuppressive cells in the TME was greater in the IHS group, and the cytotoxicity and exhaution scores were significantly higher than those in the ILS group (Fig. 3D and E). This indicates that the immune cells in the IHS group were in a state of immunosuppression, which also explains the poor prognosis of patients with ICC in the IHS group. In addition, the extracellular matrix remodeling and angiogenesis components were also significantly enriched in the IHS group (Fig. 3F). Fibroblast infiltration was significantly higher in the ILS group (Fig. 3B)and was associated with a better prognosis (Fig. 3G and H). This suggests that fibroblasts may be involved in the resistance to myeloid-cell-induced inflammatory responses. The results of correlation analysis showed that fibroblast infiltration in the TME exhibited significant negative correlation with neutrophil and macrophage infiltration (Fig. 3I) but showed positive correlation with CD8 + central memory T cells (Tcm) infiltration.
Fig. 3.
Differences in the immune landscapes among different inflammatory subgroups. (A) Differences in the immune, microenvironment, and stromal scores between the IHS and ILS groups. (B) Differences in immune cell infiltration between the IHS and ILS groups. (C) Differences in the myeloid inflammation scores between the IHS and ILS groups. (D) Differences in cytotoxicity scores between the IHS and ILS groups. (E) Differences in inhibitory scores between the IHS and ILS groups. (F) Differences in extracellular matrix remodeling and angiogenesis scores between the IHS and ILS groups. (G) Impact of fibroblast infiltration on the prognosis of patients with ICC. (H) Survival differences among different fibroblast infiltration subgroups in the inflammatory groups. (I) Results of the correlation analysis of fibroblast and immune cell infiltration proportions. (J) Differences in the COLEC11 mRNA and protein expression levels in different inflammatory subgroups. (K) Correlation analysis of the expression levels of COLEC11 and other immunity-related genes. (L) Effects of COLEC11 expression on prognosis in different inflammatory subgroups. (K) Correlation analysis of the expression levels of COLEC11 and immunity-related genes in the GSE89749 dataset
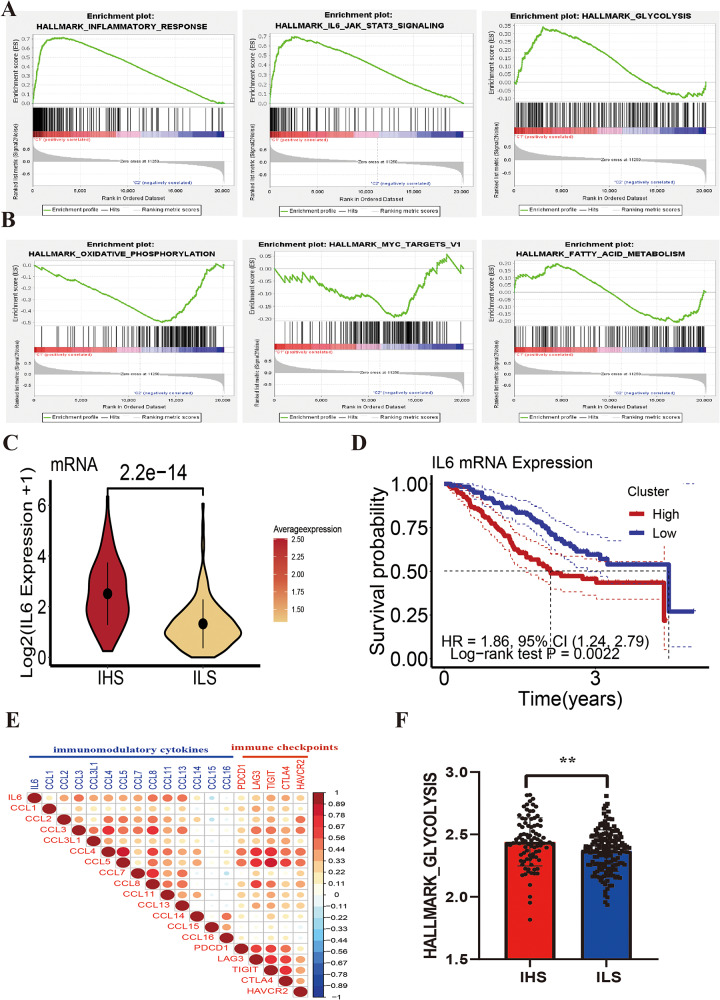
Collectin-11, a newly discovered soluble C-type lectin, is a pattern recognition molecule of the innate immune system. It plays a unique role in host defense, embryonic development, and acute inflammation [16]. At the single-cell level, COLEC11 is primarily secreted by fibroblasts (Additional file 1: Figure S2A-S2B). The expression level of COLEC11 was significantly higher in the ILS group, both at the mRNA and protein levels (Fig. 3J). In addition, the expression level of COLEC11 was negatively correlated with the level of neutrophil and macrophage infiltration. It also showed significant negative correlation with the expression of the myeloid cell-derived inflammatory factor IL1B and neutrophil marker genes (S100A8, S100A9, and S100A12) but showed positive correlation with the expression of T-cell cytotoxicity genes (GZMA, CCR5, and CCL5) (Fig. 3K). Patients with ICC with high COLEC11 mRNA or protein expression had a better prognosis (Additional file 1: Figure S2C-S2D). After stratification based on the inflammatory response, patients with ICC with high COLEC11 expression in the ILS group showed a better prognosis, whereas in the IHS group, the same was observed in patients with low COLEC11 expression (Fig. 3L). This suggests that fibroblast-derived COLEC11 can reduce the infiltration of inflammatory myeloid cells, alleviate inflammatory responses, and exert anti-tumor effects. Similarly, we observed consistent results in an independent external validation set (GSE89749) (Fig. 3M, Additional file 1: Figure S2E), which confirmed our hypothesis.
Validation of results using IHC and spatial transcriptome data
To further confirm our findings, we collected clinical samples from 10 patients with pathologically confirmed ICC. The qRT-PCR results confirmed COLEC11 overexpression in the adjacent tissues (Fig. 4A). The IHC results were also similar (Fig. 4B). This suggested that the tumor tissues indirectly promoted tumor cell migration and invasion by reducing the COLEC11 expression level. The analysis of ICC spatial transcriptome data showed that the COLEC11-positive region had fewer neutrophils (Fig. 4C). Concurrently, we also observed greater T and B cell infiltration around fibroblasts (Fig. 4C). This finding confirmed that fibroblast-derived COLEC11 reduced inflammatory myeloid cell infiltration.
Fig. 4.
COLEC11 is expressed at low levels in tumor tissues. (A) qRT-PCR findings confirmed the low COLECI1 expression levels in tumor tissues in patients with ICC. (B) IHC confirmed the low expression of COLECI1 in the tumor tissues of patients with ICC. (C) The results of spatial transcriptome data analysis confirmed that COLEC11 could reduce neutrophil infiltration and increase immune killer cell infiltration
Molecular characteristics of different inflammatory subtypes
To further explore the molecular mechanisms underlying the inflammatory characteristics, we performed GSEA using IHS and ILS data. The IHS group was primarily enriched in INFLAMMATORY_RESPONSE, IL6_JAK_STAT3_SIGNALING, NEUTROPHIL_DEGRANULATION, and GLYCOLYSIS signaling pathways (Fig. 5A, Additional file 1: Figure S3A). The ILS group was primarily enriched in OXIDATIVE_PHOSPHORYLATION, MYC_TARGETS_V1, and FATTY_ACID_METABOLISM signaling pathways (Fig. 5B). This indicated the significant functional heterogeneity between the two groups. The IHS groups was significantly enriched in signaling pathways related to tumor progression, such as hypoxia and negative immune regulation signaling pathways (Additional file 1: Figure S3B-S3C). The IHS group was also significantly enriched in NEUTROPHIL_DEGRANULATION and IL6_JAK_STAT3_SIGNALING, suggesting that neutrophil infiltration in the TME and the activation of IL6_JAK_STAT3_SIGNALING play an important role in inflammatory responses. Findings from previous studies have confirmed that the STAT3 signaling pathway activated by IL6 is the primary pathway of inflammation in cancer. It is often activated in malignant cells and plays a key role in regulating the transcription of key genes related to inflammation in the TME [17]. In our study, IL6 was significantly overexpressed in the IHS group (Fig. 5C), and patients with ICC with IL6 overexpression had worse prognosis (HR = 1.86, 95% CI (1.24, 2.79), P = 0.0022) (Fig. 5D). The results of the correlation analysis showed that the IL6 expression level was significantly positively correlated with the expression of immunomodulatory cytokines (CCL2, CCL3, CCL4, and CCL8) and immune checkpoint proteins (PDCD1, LAG3, TIGIT, CTLA4, and HAVCR2) (Fig. 5E). These results suggest that IL6 plays a pro-inflammatory role while promoting immunosuppression. Interestingly, the IHS group was dominated by pathways related to glucose metabolism, whereas the ILS group was dominated by pathways related to fatty acid metabolism (Fig. 5A, B and F), exhibiting two distinct metabolic patterns. Therefore, we speculate that the inflammatory response in the TME enhances glucose metabolism and inhibits fatty acid metabolism. Overall, these findings suggest that in patients with strong inflammatory subtypes, an immunosuppressive microenvironment characterized by the upregulation of immunomodulatory cytokines, expression of immune checkpoints, and infiltration of immunosuppressive cells may be established, which may eventually lead to a poor prognosis.
Fig. 5.
Estimation of anticancer immune activity among inflammatory subtypes. (A) The GSEA shows the signaling pathways enriched in the IHS. (B) GSEA shows the signaling pathways enriched in ILS. (C) Differences in the IL6 expression levels among different inflammatory groups. (D) Effects of IL6 expression on the prognosis of patients with ICC. (E) Correlation between the expression of IL6 and immunity-related genes. (F) Differences among the glycolysis metabolism scores of different inflammatory groups
Different mutation landscapes in different inflammatory subtypes
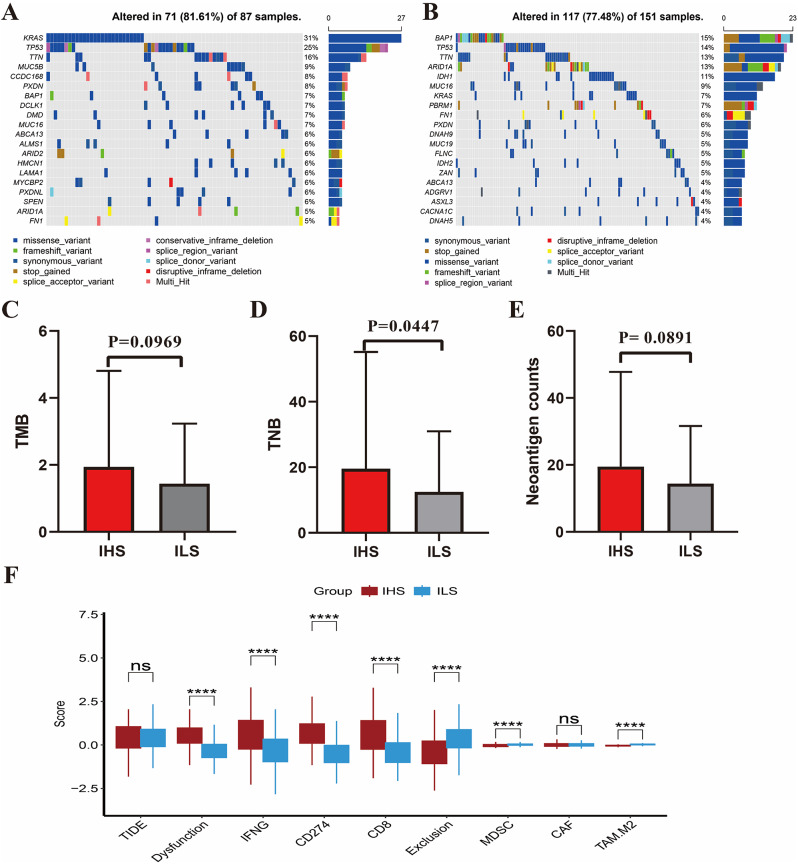
The somatic mutation patterns differed among the inflammatory subtypes. In the IHS group, the frequency of somatic mutations was 81.61%, with KRAS (31%) and TP53 (25%) mutations being predominant (Fig. 6A). In the ILS group, the frequency of somatic mutations was 77.48%, with BAP1 (15%) and TP53 (14%) mutations being predominant (Fig. 6B). In addition, the TMB score, TNB score, and neoantigen counts were higher in the IHS group than in the ILS group (Fig. 6C and E). This finding suggests that the IHS group may have a greater response rate to immunotherapy. Subsequently, the TIDE algorithm was applied to assess the response of patients to immunotherapy. We found that patients with ICC in the IHS group had higher dysfunction, IFNG, CD274, and CD8 scores than patients in the ILS group but had lower exclusion scores (Fig. 6F). This suggests that although the IHS group had a high abundance of infiltrating T cells, most of the cells were dysfunctional. Given that the IHS group had greater immune cell infiltration and tumor immunogenicity and higher expression levels of immune checkpoint genes than the ILS group, patients with ICC in the IHS group were better candidates for ICI therapy. Furthermore, given the higher IDH1 mutation frequency in patients with ICC in the ILS group (11%) (Fig. 6B), patients with a weak inflammatory response may be more sensitive to IDH1-targeted agents, such as ivosidenib.
Fig. 6.
Somatic mutational landscapes in different inflammatory groups. (A) A waterfall plot depicting mutated genes in the IHS group. (B) A waterfall plot depicting mutated genes in the ILS group. (C) Differences among the TMB scores of different inflammatory groups. (D) Differences among the TNB scores of different inflammatory groups. (E) Differences among the neoantigen counts of different inflammatory groups. (F) Differences in TIDE scores and the expression of immune-checkpoint-related genes between the different inflammatory groups
Prediction of inflammatory subtypes based on clinical characteristics combined with inflammatory gene expression patterns
To better predict the inflammatory state of patients with ICC, we used an ROC curve to calculate the AUC values of 219 inflammatory genes. CCRL2 and GNA15 had greater AUC values (0.891 and 0.859, respectively) (Fig. 7A). Thus, we selected the expression data of these two genes, combined with their clinical characteristics, to construct a prediction model. First, 244 patients with ICC were randomly divided into training and validation sets at a ratio of 6:4. To increase the universality and reliability of the prediction model, we fitted the expression levels of CCRL2 and GNA15 with clinical characteristics in the training set and performed stepwise regression analysis to screen the best variables for constructing the model. The AIC was the lowest (80.82) when the tumor diameter, ALB level, and CCRL2 and GNA15 expression levels were included in the model. Subsequently, we calculated the partial regression coefficients of each variable and constructed the following logistic regression equation: Logit(p) = 1.4217 + -0.1322 * ALB level + -0.2953 * tumor diameter + 0.8561 * CCRL2 expression level + 0.3332 * GNA15 expression level. To determine the accuracy of the logistic regression equation for predicting the inflammatory state of patients with ICC more intuitively, we constructed a nomogram based on the ALB level, tumor diameter, and CCRL2 and GNA15 expression levels in the training set (Fig. 7B). In the nomogram, the CCRL2 expression level accounted for approximately 100 points, the GNA15 expression level accounted for approximately 56.6 points, tumor diameter accounted for approximately 8.8 points, and the ALB level accounted for approximately 8.4 points. The individual scores of each risk factor were added, and the probability corresponding to the total score was the probability of eliciting a strong inflammatory response. According to the internal validation results, the c-index of the nomogram was 0.962. The nomogram ROC curve showed good predictive ability in the training set, with an area under the curve (AUC) of 0.962 (95% CI: 0.935–0.988) for predicting the IHS (Fig. 7C). The calibration curve showed that the predicted probability of the IHS in the training set was in good agreement with the actual probability of the IHS (Fig. 7D). Good predictive ability and calibration were observed in the validation set (Fig. 7E and F), with an AUC of 0.882 (95% CI: 0.810–0.953). Although the ROC curve and calibration curve can be used to evaluate the predictive ability and calibration potential of the nomogram, they cannot be used to evaluate the clinical applicability of the prediction model. Therefore, we conducted a decision curve analysis (DCA). The decision curve showed that the probability of using the nomogram to predict the IHS was more favorable than other factors in the test and validation sets (Fig. 7G and H), indicating the clinical value of the nomogram.
Fig. 7.
Construction of a model for predicting the inflammatory state of patients with ICC. (A) ROC scores of inflammation-related genes for predicting a strong inflammation status in patients with ICC. (B) A nomogram for predicting the strong inflammatory state in patients with ICC. (C) In the training set, the ROC curve helped evaluate the predictive efficacy of the nomogram for the inflammatory state of patients with ICC. (D) In the training set, the calibration curve was used to evaluate the predictive efficacy of the nomogram for the inflammatory state of patients with ICC. (E) In the test set, the ROC curve was used to evaluate the predictive efficacy of the nomogram for the inflammatory state of patients with ICC. (F) In the test set, the calibration curve was used to evaluate the predictive efficacy of the nomogram for the inflammatory state of patients with ICC. (G) In the training set, the decision curve analysis (DCA) results indicated that the nomogram had substantial clinical value. (H) In the test set, the DCA results indicated that the nomogram had substantial clinical value
In general, a nomogram constructed using clinical characteristics combined with the expression level of inflammatory genes can be used to effectively determine the inflammatory state of patients with ICC and predict prognosis. This makes the nomogram a valuable tool for guiding the clinical treatment of ICC.
Discussion
The development of next-generation sequencing technology, molecular typing based on gene expression and mutation, proteomics, and other technologies has deepened our understanding of ICC pathogenesis. However, the mechanism underlying inflammation in the TME in ICC, the regulation of inflammatory mediators present on immune cells, and the relationship between somatic mutations remain unclear. In the current study, we integrated bulk RNA sequencing, protein sequencing, somatic mutation, single-cell sequencing, and spatial transcriptome data of patients with ICC and classified patients according to their inflammatory response. We also analyzed the differences in the prognosis, TME, and somatic mutation landscape of patients with ICC exhibiting different inflammatory subtypes and elucidated the important role of inflammatory mediators in remodeling the TME. Our results show that ICC can be classified as IHS and ILS, which are characterized by distinct clinicopathological characteristics, prognosis, TME, and somatic mutation patterns. In addition, we also developed and validated a model to evaluate the inflammatory state of patients with ICC. This model has a strong evaluation ability and provides an important basis for the individualized precision treatment of patients with ICC.
The TME is a highly structured ecosystem with various immune cells, cancer-associated fibroblasts (CAFs), endothelial cells, and extracellular matrix (ECM) components [7, 18], which play important roles in tumor induction and progression. We found that the inflammatory responses in ICC were induced by infiltrating myeloid cells, and we observed significant heterogeneity between the IHS and ILS subtypes with respect to the types of immune cells. Generally, patients with IHS had a poor prognosis and showed greater infiltration of immunosuppressive cells (e.g., neutrophils, iDCs, and M2-type macrophages). In contrast, patients with ILS had more tumor-killing cells (e.g., CD8 + naive T-cells, plasma cells, and Th1 cells) infiltrating the TME, indicating that an immunoactive microenvironment is associated with the most favorable clinical prognosis. Interestingly, significant fibroblast infiltration was observed in the ILS group, suggesting that fibroblasts may be involved in the anti-inflammatory response. Among them, COLEC11 is an important protein secreted by fibroblasts, and its expression level showed significant negative correlation with myeloid-derived inflammatory factors (IL1B, S100A8, S100A9, and S100A12) but significant positive correlation with immune killer genes (GZMA, CCR5, and CCL5). Spatial transcriptome results confirmed that the positive region of COLEC11 was primarily positioned around fibroblasts, where fewer neutrophils and more T and B cells were present. This was consistent with the results of previous studies that showed that COLEC11 inhibited APC activation and resisted infection [19, 20]. These results suggest that fibroblast-derived COLEC11 plays a distinct role in resisting myeloid cell-induced inflammatory responses and chemotactic lymphocytes, and the specific underlying mechanism needs to be investigated further.
With a better understanding of the TME and immune escape pathways, ICIs have been developed rapidly in tumor therapy, and encouraging results have been observed in various solid tumors (such as advanced HCC, non-small cell lung cancer, and metastatic melanoma) [21–24]. The application of ICIs has helped make novel breakthroughs in tumor therapy. However, the efficacy of immunotherapy varies considerably among different patients, and only some patients can benefit from it. Effective markers for predicting the treatment responses of patients with tumors are yet to be identified. The success of immune checkpoint blockade therapy is related to multiple factors, namely the immunogenicity of tumors, number and function of tumor-infiltrating T cells, and expression of immune checkpoint. Per our analysis, patients with ICC with high levels of inflammation, more immunosuppressive cells infiltrating the TME, and higher expression levels of immune checkpoint may be sensitive to current ICIs therapies. Therefore, the nomogram we constructed, combined with clinical characteristics of patients, can indirectly indicate the immunosuppressive state of patients with ICC. This can help clinicians screen potential ICIs-responsive populations. However, the association between inflammatory subtypes and the potential outcomes of ICC immunotherapy need to be verified further in vitro or in vivo. This limitation should be considered when interpreting our results.
Conclusions
In conclusion, we established a new ICC classification system based on the inflammatory subtypes of tumors, which yielded meaningful results in the evaluation of patient prognosis and the TME.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
All authors would like to acknowledge the use of GEO datasets as a data source in this study.
Author contributions
BG, YW, and XZ designed and drafted the paper. HJ participated in the collection of specimens. XL prepared the figures. FH, SL, and CL reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
No Funding.
Data availability
Data availability gene expression profiles, clinical information, and mutation data of ICC used in this study were obtained from a study conducted by Dong et al. [13]. GSE89749 and GSE138709 were retrieved from the GEO database.
Declarations
Ethical approval and consent to participate
Written informed consent was obtained from each patient for the use of patient samples, and the study was approved by the First Medical Center of the General Hospital of the Chinese People’s Liberation Army (S2018-111-01). The study was performed in accordance with the recommendations of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Biao Gao ,Yafei Wang and Xianzhou Zhang have contributed equally to this work.
Contributor Information
Feng Han, Email: hf1007@sohu.com.
Chonghui Li, Email: lichonghui@301hospital.com.cn.
Shichun Lu, Email: lushichun@301hospital.com.cn.
References
- 1.Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma[J]. J Hepatol. 2014;60(6):1268–89. 10.1016/j.jhep.2014.01.021. 10.1016/j.jhep.2014.01.021 [DOI] [PubMed] [Google Scholar]
- 2.Shen WF, Zhong W, Xu F, et al. Clinicopathological and prognostic analysis of 429 patients with intrahepatic cholangiocarcinoma[J]. World J Gastroenterol. 2009;15(47):5976–82. 10.3748/wjg.15.5976. 10.3748/wjg.15.5976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Massarweh NN, El-Serag HB. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma[J]. Cancer Control. 2017;24(3):1145164509. 10.1177/1073274817729245. 10.1177/1073274817729245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantovani A, Cancer. Inflaming metastasis[J]. Nature. 2009;457(7225):36–7. 10.1038/457036b. 10.1038/457036b [DOI] [PubMed] [Google Scholar]
- 5.Landskron G, De la Fuente M, Thuwajit P et al. Chronic inflammation and cytokines in the tumor microenvironment[J]. J Immunol Res, 2014,2014:149185. 10.1155/2014/149185 [DOI] [PMC free article] [PubMed]
- 6.Zhao H, Wu L, Yan G, et al. Inflammation and tumor progression: signaling pathways and targeted intervention[J]. Signal Transduct Target Ther. 2021;6(1):263. 10.1038/s41392-021-00658-5. 10.1038/s41392-021-00658-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Visser KE, Joyce JA. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth[J]. Cancer Cell. 2023;41(3):374–403. 10.1016/j.ccell.2023.02.016. 10.1016/j.ccell.2023.02.016 [DOI] [PubMed] [Google Scholar]
- 8.Galon J, Bruni D. Tumor Immunology and Tumor Evolution: intertwined Histories[J]. Immunity. 2020;52(1):55–81. 10.1016/j.immuni.2019.12.018. 10.1016/j.immuni.2019.12.018 [DOI] [PubMed] [Google Scholar]
- 9.Darvin P, Toor SM, Sasidharan NV, et al. Immune checkpoint inhibitors: recent progress and potential biomarkers[J]. Exp Mol Med. 2018;50(12):1–11. 10.1038/s12276-018-0191-1. 10.1038/s12276-018-0191-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shieh KR, Huang A, Xu Y. Response to Immune checkpoint inhibitor treatment in Advanced Cervical Cancer and Biomarker Study[J]. Front Med (Lausanne). 2021;8:669587. 10.3389/fmed.2021.669587. 10.3389/fmed.2021.669587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haslam A, Prasad V. Estimation of the percentage of US patients with Cancer who are eligible for and respond to checkpoint inhibitor immunotherapy Drugs[J]. JAMA Netw Open. 2019;2(5):e192535. 10.1001/jamanetworkopen.2019.2535. 10.1001/jamanetworkopen.2019.2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou J, Karin M, Sun B. Targeting cancer-promoting inflammation - have anti-inflammatory therapies come of age?[J]. Nat Rev Clin Oncol. 2021;18(5):261–79. 10.1038/s41571-020-00459-9. 10.1038/s41571-020-00459-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong L, Lu D, Chen R, et al. Proteogenomic characterization identifies clinically relevant subgroups of intrahepatic cholangiocarcinoma[J]. Cancer Cell. 2022;40(1):70–87. 10.1016/j.ccell.2021.12.006. 10.1016/j.ccell.2021.12.006 [DOI] [PubMed] [Google Scholar]
- 14.Wu R, Guo W, Qiu X, et al. Comprehensive analysis of spatial architecture in primary liver cancer[J]. Sci Adv. 2021;7(51):g3750. 10.1126/sciadv.abg3750. 10.1126/sciadv.abg3750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Cai JQ, Zhao JJ, et al. Impact of hepatitis B virus infection on outcome following resection for intrahepatic cholangiocarcinoma[J]. J Surg Oncol. 2010;101(3):233–8. 10.1002/jso.21488. 10.1002/jso.21488 [DOI] [PubMed] [Google Scholar]
- 16.Wu W, Liu C, Farrar CA, et al. Collectin-11 promotes the development of renal Tubulointerstitial Fibrosis[J]. J Am Soc Nephrol. 2018;29(1):168–81. 10.1681/ASN.2017050544. 10.1681/ASN.2017050544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones SA, Jenkins BJ. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer[J]. Nat Rev Immunol. 2018;18(12):773–89. 10.1038/s41577-018-0066-7. 10.1038/s41577-018-0066-7 [DOI] [PubMed] [Google Scholar]
- 18.Boelaars K, van Kooyk Y. Targeting myeloid cells for cancer immunotherapy: Siglec-7/9/10/15 and their ligands[J]. Trends Cancer. 2023. 10.1016/j.trecan.2023.11.009. 10.1016/j.trecan.2023.11.009 [DOI] [PubMed] [Google Scholar]
- 19.Wang N, Wu W, Qiang C, et al. Protective role of Collectin 11 in a mouse model of rheumatoid Arthritis[J]. Arthritis Rheumatol. 2021;73(8):1430–40. 10.1002/art.41696. 10.1002/art.41696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu KY, Cao B, Chen WB, et al. Collectin 11 has a pivotal role in host defense against kidney and bladder infection in mice[J]. Kidney Int. 2023. 10.1016/j.kint.2023.11.031. 10.1016/j.kint.2023.11.031 [DOI] [PubMed] [Google Scholar]
- 21.Sharma P, Goswami S, Raychaudhuri D, et al. Immune checkpoint therapy-current perspectives and future directions[J]. Cell. 2023;186(8):1652–69. 10.1016/j.cell.2023.03.006. 10.1016/j.cell.2023.03.006 [DOI] [PubMed] [Google Scholar]
- 22.Serritella AV, Shenoy NK. Nivolumab Plus Ipilimumab vs Nivolumab alone in Advanced Cancers Other Than Melanoma: a Meta-Analysis[J]. JAMA Oncol. 2023;9(10):1441–6. 10.1001/jamaoncol.2023.3295. 10.1001/jamaoncol.2023.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial[J]. Lancet. 2017;389(10088):2492–502. 10.1016/S0140-6736(17)31046-2. 10.1016/S0140-6736(17)31046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribas A, Hamid O, Daud A, et al. Association of Pembrolizumab with Tumor Response and Survival among patients with Advanced Melanoma[J]. JAMA. 2016;315(15):1600–9. 10.1001/jama.2016.4059. 10.1001/jama.2016.4059 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data availability gene expression profiles, clinical information, and mutation data of ICC used in this study were obtained from a study conducted by Dong et al. [13]. GSE89749 and GSE138709 were retrieved from the GEO database.