Abstract
Background
The prevalence of metabolic (dysfunction)-associated fatty liver disease (MAFLD) increases together with the epidemic of childhood obesity. An important mechanism in the phenomenon appears to be insulin resistance (IR), the assessment of which in children is problematic. The homeostatic model assessment of IR (HOMA-IR), commonly used for this, is not standardized and appears not to correlate with IR in the pediatric population. Therefore, our study aimed to evaluate potential substitute indices of IR, including the triglyceride-glucose index (TyG), triglyceride to high-density lipoprotein cholesterol ratio (TG/HDL-C), modified TyG indices: TyG-waist circumference (TyG-WC) and TyG-body mass index (TyG-BMI) as surrogate markers of MAFLD in obese children suspected to have liver disease.
Material/Methods
The retrospective study included 264 obese children admitted to the Department to diagnose suspected liver disease. MAFLD was diagnosed according to the International Expert Consensus Statement. Anthropometric measurements and laboratory tests were made and the indices were calculated. Receiver operating characteristics analysis was performed to calculate the power of the indices.
Results
MAFLD was diagnosed in 184 patients (70%). Obese children with MAFLD showed significantly higher activity of liver enzymes and concentration of total cholesterol, TG, WC, and waist-to-hip ratio compared to non-hepatopathic obese controls (n=80). The most important indices in identifying MAFLD were: TyG (AUC=0.641, p<0.001, cut-off =8.41, sensitivity=57.4%, specificity=68.8%), and TG/HDL-C (AUC=0.638, p<0.001, cut-off=2.5, sensitivity=48.6%, specificity=76.3%). TyG-BMI and HOMA-IR were not useful predictors.
Conclusions
TyG and TG/HDL-C can be considered as potential surrogate biomarkers in predicting MAFLD in obese children.
Keywords: Adolescent, Insulin Resistance, Metabolic Syndrome, Non-alcoholic Fatty Liver Disease, Pediatric Obesity
Introduction
The epidemic of childhood obesity results in an increasing prevalence of metabolic (dysfunction) associated fatty liver disease (MAFLD) and MAFLD is regarded as the main cause of chronic liver pathology in children and adolescents [1]. It can also occur in normal-weight individuals, as shown in several publications [2,3], and is considered in the development of the diagnostic criteria of MAFLD, in which overweight or obesity is only 1 of 3 disorders whose coexistence with hepatic steatosis allows the diagnosis of MAFLD to be made [4]. Unfortunately, in this group of patients, hepatic steatosis is often underdiagnosed because, remaining asymptomatic for a long time, it does not trigger screening in the general population.
Insulin resistance (IR) is reported to be one of the important mechanisms involved in metabolic dysfunction’s pathogenesis. In obese or overweight children, a strong association between IR and a higher prevalence of the components of metabolic syndrome and MAFLD has been noted [4–6]. Clinically, IR is a condition in which greater-than-physiological insulin secretion by pancreatic island beta cells is required to maintain normal blood glucose levels. IR is a chronic state of reduced insulin sensitivity of target tissues that plays an essential role in metabolic processes, which leads to systemic hyperinsulinemia [7]. There are multiple organs involved in the pathogenesis of IR, with the skeletal muscle, adipose tissue, and liver playing significant roles. The liver is a very important organ of glucose metabolism, responsible for glucose production and storage, and insulin plays an important role in controlling the activity of key enzymes in these processes [8]. Therefore, early diagnosis of insulin-resistant children and adolescents is very important not only for population-based research but also for clinical practice [9].
It is widely accepted that hyperinsulinemic-euglycemic clamp (HEC) is the criterion standard for measuring insulin action in vivo [10], but it is invasive, time-consuming (requiring 6 or more hours of continuous bedside monitoring and infusion adjustments), expensive, and difficult to apply in everyday clinical practice in children [11]. Moreover, the homeostatic model assessment of insulin resistance (HOMA-IR), which is widely used in adults, does not have standardized values in children and seems not to correlate with IR in this group of patients [4].
Several studies to date have pointed to the possibility of using alternative IR indicators in the screening of non-alcoholic fatty liver disease (NAFLD) in children [12–16]. They highlighted the advantages of such a diagnostic method as being both easily accessible, also in the primary care setting, and cost-effective. However, the presented results show discrepancies in terms of the efficacy and usefulness of individual indicators and showed the need for continued research on a large group of patients. Moreover, for the European population, studies on a homogeneous group of White children would also be useful. In addition, the studies conducted so far have been based on the diagnosis of NAFLD, which a few years ago changed the terminology and criteria for diagnosis to MAFLD, taking into account, among other things, the importance of IR and other metabolic disorders in the development of this condition, which may have changed the utility of the markers and requires validation.
Hence, among the markers studied to date, we have selected 4 that have been shown to be most effective in predicting NAFLD [12,13,16] and seem to be the simplest, fastest, widely available, and cost-effective, because of their potential greatest value for broadly screening MAFLD in the pediatric population, both in clinical and ambulatory practice. We also compared them with HOMA-IR, the most widely used IR marker.
Therefore, the aim of this study was to evaluate potential substitute indices of IR, including the triglyceride-glucose index (TyG), triglyceride to high-density lipoprotein cholesterol ratio (TG/HDL), modified TyG indices: TyG-waist circumference (TyG-WC), and TyG-body mass index (TyG-BMI), as surrogate markers of MAFLD in overweight/obese children suspected to have liver disease.
Material and Methods
The protocol of the study was approved by the local bioethics committee (APK. 002.373.2023). Due to the study’s retrospective nature, as an analysis of medical records, informed consent from patients was not required. This retrospective study included 264 obese/overweight children.
The following inclusion criteria were applied in the study: children aged 8–18 years, with excessive body weight, hospitalized in the Department of Pediatrics, Gastroenterology, Hepatology, Nutrition, Allergology and Pulmonology, University Children’s Clinical Hospital in Białystok between 2007 and 2022 due to suspected liver disease – hepatomegaly and/or elevated alanine aminotransferase (ALT) activity and/or fatty liver in ultrasound (USG) examination – to extend the diagnosis.
We limited the study group to overweight and obese children because, due to their increased risk of various disorders, such patients are more likely to undergo additional examinations and represent the majority of children diagnosed with hepatic steatosis hospitalized in our Department. This limitation allowed for the creation of a larger and more standardized group, allowing for a reliable comparison.
MAFLD was diagnosed in 184 children (70%) according to the International Expert Consensus Statement [4]. According to them, the diagnosis of MAFLD requires the presence of hepatic steatosis on imaging studies, morphological examination of a liver biopsy, or the finding of abnormal biomarkers in the form of persistently elevated serum ALT activity. In addition, the co-occurrence of 1 of 3 features should be stated: overweight or obesity assessed by BMI value or visceral obesity measured by waist circumference (WC), the finding of a pre-diabetic state or type 2 diabetes mellitus, or the finding of other concomitant metabolic disorders – at least 2 out of 4 symptoms: elevated concentration of triglycerides (TG), reduced high-density lipoprotein cholesterol (HDL) concentration, hypertension, and an abnormal TG to HDL ratio (as a marker of IR).
The control group consisted of non-hepatopathic obese/overweight children, who accounted for 30% (n=80) of the patients included in the clinical analysis.
Children with concomitant liver diseases were excluded from the study: viral hepatitis (type C and B), autoimmune hepatitis, toxic and drug-induced liver injury, selected metabolic liver diseases (alpha-1-antitrypsin deficiency, Wilson’s disease), cystic fibrosis, and celiac disease. To exclude these diseases, a history of the use of drugs and substances with potential hepatotoxic effects was taken with patients and their parents, and the following tests were performed: HBs antigen, anti-HCV antibodies, proteinogram, immunoglobulin (Ig) G, alpha-1 antitrypsin level, ceruloplasmin, tissue transglutaminase IgA antibodies, and sweat chloride test. None of the children included in the study were diagnosed with diabetes mellitus.
Anthropometric measurements, including weight, height, BMI, which was calculated as weight (kg) divided by height squared (m2), and WC, were taken. WC was measured according to the World Health Organization’s (WHO) protocol, taking the measurement approximately halfway between the lower edge of the last palpable rib and the top of the iliac crest. Anthropometric measurements were standardized for age and sex based on WHO centile charts [17]. BMI values greater than 1 standard deviation (SD) according to WHO or waist circumference above the 90th percentile was considered excessive body weight [4]. The evidence of fatty liver was confirmed by liver USG, which was performed by the same radiologist, without knowledge of patients’ clinical data, using a General Electric Voluson E8 equipped with a convex sonde 3–5 Mhz (Boston, USA).
Blood samples were collected by venipuncture after a 12-h fast, followed by immediate centrifugation and freezing at −80°C until further analysis. All laboratory tests, including those performed for the differential diagnosis of other liver diseases, were performed using standard clinical laboratory techniques applied at the Department of Pediatric Laboratory Diagnostics of the Medical University of Białystok Children’s Clinical Hospital. HOMA-IR was evaluated according to the formula described by Matthews et al [18]:
The potential indices of IR: TyG, TG/HDL, and modified TyG indices: TyG-WC and TyG-BMI were calculated according to the formulae [12,19]:
Statistical analysis was conducted using IBM SPSS Statistics 27.0 [20]. To assess the normality of continuous variables, we employed Shapiro-Wilk tests. Given that many variables exhibited significant deviations from normal distribution, we used non-parametric methods in our analyses. As a result, continuous variables are represented as medians along with their 1st–3rd quartiles (Q1–Q3), whereas frequencies and percentages are provided for categorical variables. For comparing continuous variables between groups, we used the Mann-Whitney U test, which does not assume a normal distribution. Similarly, Spearman’s correlations were applied to determine the strength of associations. The chi-squared test was used to assess associations among categorical variables.
Additionally, we performed receiver operating characteristics (ROC) analysis to evaluate the diagnostic capability of selected indices in identifying children with MAFLD. Areas under the curve (AUC) were calculated and benchmarked against a value of 0.5. This threshold signifies a lack of diagnostic ability, equivalent to random chance, on the ROC plot. This step was crucial for assessing the discriminative power of the indices. Optimal cut-off values were identified by maximizing the Youden index [21], a widely recognized method for identifying the optimal cut-off point in ROC analysis, offering a practical balance between sensitivity and specificity. This method effectively pinpoints the most advantageous trade-off between true-positive and false-positive rates.
In multivariable logistic regression analysis, odds ratios (OR) with corresponding 95% confidence intervals (95% CI) were computed for statistically significant variables in group comparison tests and ROC analysis. This enabled us to assess the strength and direction of associations between these significant predictors and the occurrence of MAFLD while adjusting for potential confounders.
Regarding missing data, in each analysis, we employed a complete-case analysis approach by simply excluding cases with any missing data in variables required for a particular analysis. This method ensured that only complete datasets were analyzed, thereby maintaining the integrity and reliability of the statistical evaluations performed. We established statistical significance at the conventionally accepted threshold of P<0.05.
Results
A retrospective analysis of medical documentation was conducted involving 264 overweight/obese children, with a median age 12 years, among which MAFLD was diagnosed in 184 patients (70%). Girls constituted 31.8% of the total group. In the group of patients with MAFLD, girls accounted for 25.5%, while in the control group they represented 46.3%. Obese children with MAFLD showed significantly higher serum activity of liver enzymes and concentration of total cholesterol, TG, WC, and waist-to-hip ratio compared to non-hepatopathy overweight/obese controls (n=80). The demographic data and laboratory measurements of each group are presented in Table 1. All study participants were White.
Table 1.
Comparison of selected clinical, biochemical parameters, and potential insulin resistance indices between the group of children diagnosed with metabolic disfunction-associated fatty liver disease and the control group.
| Parameter | MAFLD group (n=184) median (Q1–Q3) |
Control group (n=80) median (Q1–Q3) |
p |
|---|---|---|---|
| Anthropometric | |||
| Age (years) | 12 (11–15) | 12 (10–15) | NS |
| Male, n (%) | 137 (74.5%) | 43 (53.8%) | <0.01 |
| BMI (kg/m2) | 28.06 (25.70–32.50) | 28.93 (25.54–31.68) | NS |
| WC (cm) | 96 (90–105.75) | 93 (85.50–99.50) | 0.03 |
| HC (cm) | 101 (94–111.25) | 104 (95–112) | <0.01 |
| WHR | 0.95 (0.90–0.99) | 0.92 (0.86–0.97) | <0.01 |
| Biochemical | |||
| ALT (U/l) | 40 (26–64.75) | 20 (15–28.00) | <0.01 |
| AST (U/l) | 30 (24–40.75) | 22 (19–25.75) | <0.01 |
| GGTP (IU/l) | 23 (18–31) | 17 (14–22) | <0.01 |
| Bilirubin (mg/dl) | 0.53 (0.40–0.74) | 0.5 (0.36–0.74) | NS |
| Cholesterol (mg/dl) | 167 (144–189.75) | 152.5 (137.50–177.75) | 0.02 |
| HDL (mg/dl) | 45 (39–51) | 46.5 (40–56.75) | NS |
| LDL (mg/dl) | 98 (78–117.5) | 94 (76–110.75) | NS |
| TG (mg/dl) | 108 (80.25–153.75) | 85.5 (62.25–111) | <0.01 |
| Glucose (mg/dl) | 89 (84–94) | 89 (84–93) | NS |
| Insulin (μIU/ml) | 17.2 (12.6–22.68) | 14.85 (10.78–20.68) | NS |
| Uric acid (mg/dl) | 6 (5.12–6.94) | 5.6 (4.65–6.50) | 0.04 |
| IR indices | |||
| HOMA-IR | 3.7 (2.72–5.10) | 3.3 (2.38–4.52) | NS |
| TyG | 8.5 (8.15–8.82) | 8.29 (7.89–8.54) | <0.01 |
| TyG-BMI | 240.14 (219.89–275.59) | 236.22 (212.52–268.09) | NS |
| TyG-WC | 789.05 (689.11–891.18) | 756.31 (623.71–814.58) | 0.01 |
| TG/HDL | 2.42 (1.62–3.77) | 1.75 (1.21–2.47) | <0.01 |
ALT – alanine aminotransferase; AST – aspartate aminotransferase; BMI – body mass indexl GGTP – gamma-glutamyl transpeptidase; HC – hip circumference; HDL – high density lipoprotein; HOMA-IR – homoeostatic model assessment of insulin resistance; IR – insulin resistance; LDL – low density lipoprotein; MAFLD – metabolic disfunction-associated fatty liver disease; NS – nonsignificant; TG – triglycerides; TG/HDL – triglyceride to high-density lipoprotein cholesterol ratio; TyG – triglyceride-glucose index; TyG-BMI – TyG-body mass index; TyG-WC – TyG-waist circumference; WC – waist circumference; WHR – waist-to-hip ratio.
Insulin Resistance Indices
The ROC analysis, presented in Table 2, was performed to determine which potential surrogates of the IR had the best predictive value for distinguishing patients with MAFLD from those without MAFLD in a group of obese children. The best results were determined for TyG (AUC=0.641; P=0.0001 with 57.4% sensitivity and 68.8% specificity at the cut-off level 8.41) and TG/HDL (AUC=0.638; P=0.001 with 48.6% sensitivity and 76.3% specificity at the cut-off level 2.5). The AUC of the TyG-WC index (cut-off 852.97, sensitivity 32.8%, specificity 88.8%), was statistically significant at 0.597. The overview of the ROC curves is presented in Figure 1.
Table 2.
Sensitivity, specificity, cut-off points, and area under the curve (AUC) of the assessed indices.
| Index | AUC | SE | 95% CI (AUC) | p | Cut-off | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|
| HOMA-IR | 0.56 | 0.038 | (0.485–0.635) | 0.1164 (NS) | >3.52 | 56.8% | 56.3% |
| TyG | 0.641 | 0.035 | (0.572–0.711) | 0.0001 | >8.41 | 57.4% | 68.8% |
| TyG-BMI | 0.549 | 0.039 | (0.473–0.625) | 0.2104 (NS) | >275.71 | 25.1% | 88.8% |
| TyG-WC | 0.597 | 0.036 | (0.526–0.668) | 0.0074 | >852.97 | 32.8% | 88.8% |
| TG/HDL | 0.638 | 0.036 | (0.568–0.709) | 0.0001 | >2.5 | 48.6% | 76.3% |
AUC – area under the curve; CI – confidence interval; HOMA-IR – homoeostatic model assessment of insulin resistance; NS – non-significant; SE – standard error; TG/HDL – triglyceride to high-density lipoprotein cholesterol ratio; TyG – triglyceride-glucose index; TyG-BMI – TyG-body mass index; TyG-WC – TyG-waist circumference.
Figure 1.
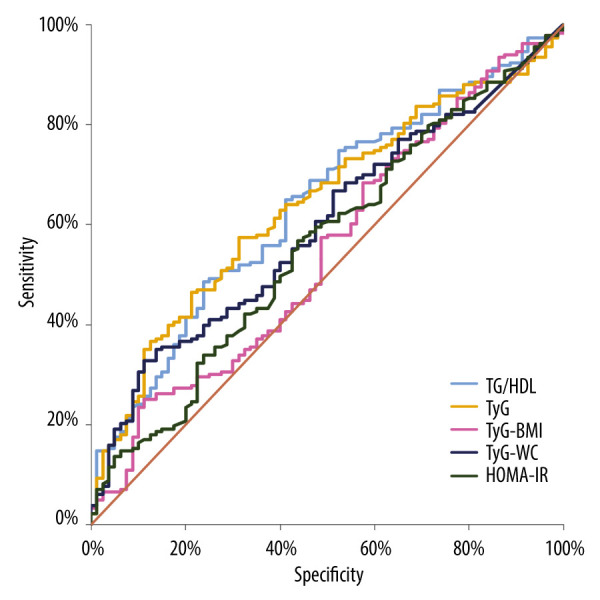
Overview of the ROC curves. HOMA-IR- Homoeostatic model assessment of insulin resistance, TG/HDL- triglyceride to high-density lipoprotein cholesterol ratio, TyG- triglyceride-glucose index, TyG-BMI- TyG-body mass index, TyG-WC- TyG-waist circumference. The figure was created in Microsoft Excel, version 2021.
Logistic Regression
The multivariate logistic regression model adjusted for age, sex, and BMI was created to see which potential IR indices might be associated with an increased likelihood of MAFLD diagnosis in obese children. HOMA-IR, TyG, TyG-BMI, and TG/HDL seemed to be statistically significant factors with the best predictive value for TyG (OR=2.225, 95% CI 1.304–3.796; P=0.003) (Table 3).
Table 3.
Odds ratios for metabolic dysfunction-associated fatty liver disease.
| Parameter | OR | p | 95% CI |
|---|---|---|---|
| HOMA-IR | 1.158 | 0.043 | 1.005–1.334 |
| TyG | 2.225 | 0.003 | 1.304–3.796 |
| TyG-BMI | 1.028 | 0.003 | 1.009–1.048 |
| TyG-WC | 1.000 | NS | 1.000–1.001 |
| TG/HDL | 1.382 | 0.001 | 1.137–1.680 |
CI – confidence interval; HOMA-IR – homoeostatic model assessment of insulin resistance; NS – nonsignificant; OR – odds ratio; TG/HDL – triglyceride to high-density lipoprotein cholesterol ratio; TyG – triglyceride-glucose index; TyG-BMI – TyG-body mass index; TyG-WC – TyG-waist circumference.
Discussion
Our results show that the simple biochemical markers of IR – TyG, TG/HDL, and modified TyG-WC – appear to be useful in differentiating children with MAFLD from those without liver impairment in an obese pediatric population. However, the AUC for TyG-WC is below 0.6, making this biomarker the least likely to be useful in differentiating between this group of children, despite the statistically significant difference [22]. The TyG index emphasizes the close relationship between IR and MAFLD, as the insulin contained in its formula promotes adipocyte maturation and fatty acid uptake from circulating lipoproteins, while hypertriglyceridemia stimulates the transport of free fatty acids to the liver and increases hepatic glucose production, resulting in the development of organ steatosis [12]. Some studies have described the greater efficacy of modified TyG over TyG in predicting NAFLD [12,14]. This has been explained by the improvement of the efficiency of the index through correlation with parameters of obesity, which is an independent risk factor for the development of hepatic steatosis (HS). However, Song et al found a large discrepancy in the prevalence of overweight and obesity between the study group, in which the majority of patients were overweight, and the control group with a predominance of normal-weight subjects, which may significantly bias the results of biomarkers validated by anthropometric measurements [12]. The TG/HDL ratio assesses the severity of the lipid disorders characteristic of IR, which include hypertriglyceridemia and reduced HDL concentration [23]. Previous studies have shown its significant clinical utility in differentiating children with IR [19,23]. Studies in adult patient populations have also shown its possible use in the diagnosis of NAFLD [15], but to the best of our knowledge such studies in children are lacking. With a growing understanding of the strong association of HS with the metabolic syndrome spectrum, which led to the change in nomenclature from NAFLD to MAFLD in 2021 [4], the usefulness of the aforementioned indices in diagnosis is increasingly emphasized.
The results obtained in this research suggest the superiority of the evaluated new potential IR indices over HOMA-IR in the assessment of MAFLD in children. This also seems to confirm the position of Eslam et al, who recommend the use of the TG/HDL ratio as a marker of IR – one of the features of metabolic abnormalities in the diagnosis of MAFLD in children aged 2–15 years – as opposed to the HOMA-IR used in adults and adolescents over 16 years old [4,23].
The good diagnostic value of the examined indices was assessed using ROC analysis and a logistic regression model. Based on these, we found that the TyG and TG/HDL indices had the best AUC, suggesting their superior utility in differentiating children with and without MAFLD, compared to the other indices evaluated – modified TyG-WC, TyG-BMI, and HOMA-IR.
Although HOMA-IR is a recognized indicator of insulin resistance (IR) in adults, in our study HOMA-IR did not differentiate well between children with and without MAFLD. Similar conclusions about the limited diagnostic value of this indicator, especially in the pediatric population, which lacks standardized reference values, have been raised by researchers before [6,24]. However, due to limited availability and the difficulty of performing HEC, HOMA-IR is widely used for the diagnosis of IR, but it is also used as a reference in some studies to assess the utility of other indices, which may cause inaccurate results [19,25,26]. Opposite results regarding the utility of HOMA-IR in predicting the occurrence of NAFLD were shown, among others, in the research of Ye et al on a group of Chinese children [16]. They showed a statistically significant difference in HOMA-IR levels in a group of children with NAFLD and a control group; ROC analysis for HOMA-IR was not performed. However, it was a study on a small group of patients with widely varying BMIs, with a significantly higher percentage of overweight or obese patients in the group of children with NAFLD than in the non-NAFLD group (62% vs 19%). Also, researchers from Greece, who evaluated the utility of HOMA-IR in assessing the risk of fatty liver in a group of adolescent girls with polycystic ovary syndrome, demonstrated its efficacy, but the study group was small and the results needed to be confirmed on a larger population [27]. The discrepancies described indicate the need for a cautious approach to the use of HOMA-IR as an indicator of MAFLD in the pediatric population and require further evaluation on a larger and more ethnically diverse group, as well as a constant search for better markers.
The present study has some limitations. First of all, it was conducted as a retrospective analysis of patients’ medical records, but this allowed for the formation of a large study group. Despite the large size, it should be noted that this was a single-center group and was not ethnically diverse, making it difficult to apply the results to the general population. In addition, due to the retrospective nature of the study, the sex distribution was unequal in the study group, with a male predominance, which may be a confounding factor in interpretation of the results. The inclusion criteria for the study were overweight/obesity and suspected liver disease, which is also a limitation in predicting the utility of the indices in normal-weight children or children without signs of liver pathology. The children also did not have a HEC performed, which is an accepted criterion standard for IR measurement. Other factors influencing the development of MAFLD, such as nutrition, physical activity, and puberty, were not considered in our study.
Despite these limitations, we evaluated 4 potential surrogate indices of IR, comparing them to well-known and widely used HOMA-IR. We demonstrated the association of MAFLD with insulin sensitivity disorders and dyslipidemia, which are components of the metabolic syndrome. However, the above results still need further validation in larger groups of patients, since, as mentioned above, the problem of obesity, IR, and MAFLD in the pediatric population is constantly growing.
Conclusions
Our findings suggest that TyG and TG/HDL-C can be considered as potential surrogate biomarkers in predicting MAFLD in obese children. Their efficacy appears to be better than the commonly used HOMA-IR, which did not provide a useful assessment of MAFLD risk in our group. It seems that the use of these indices could effectively, in a simple and low-cost way, help in screening the population of overweight children for MAFLD, which would perhaps increase the early detection of this disorder, enabling implementation of appropriate management. However, further prospective studies in larger groups of patients are necessary.
Acknowledgements
The study was previously presented as a poster at the 55th Annual Meeting of ESPGHAN, 17-20.05.2023, Vienna, and the abstract was published in the Abstract Book (ESPGHAN 55th Annual Meeting Abstracts. J Pediatr Gastroenterol Nutr. 2023;76(S1 Suppl. 1): 881).
Footnotes
Conflict of interest: None declared
Declaration of Figures’ Authenticity: All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: The research of the authors was supported by a grant from the Medical University of Białystok, Poland
References
- 1.Le Garf S, Nègre V, Anty R, Gual P. Metabolic fatty liver disease in children: A growing public health problem. Biomedicines. 2021;9(12):1915. doi: 10.3390/biomedicines9121915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang AY, Dhaliwal J, Mouzaki M. Lean non-alcoholic fatty liver disease. Clin Nutr. 2019;38(3):975–81. doi: 10.1016/j.clnu.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Zdanowicz K, Białokoz-Kalinowska I, Lebensztejn DM. Non-alcoholic fatty liver disease in non-obese children. Hong Kong Med J. 2020;26(5):459–62. doi: 10.12809/hkmj198361. [DOI] [PubMed] [Google Scholar]
- 4.Eslam M, Alkhouri N, Vajro P, et al. Defining paediatric metabolic (dysfunction)-associated fatty liver disease: An international expert consensus statement. Lancet Gastroenterol Hepatol. 2021;6(10):864–73. doi: 10.1016/S2468-1253(21)00183-7. [DOI] [PubMed] [Google Scholar]
- 5.Sakurai Y, Kubota N, Yamauchi T, Kadowaki T. Role of insulin resistance in MAFLD. Int J Mol Sci. 2021;22(8):4156. doi: 10.3390/ijms22084156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz B, Jacobs DR, Moran A, Steinberger J, et al. Measurement of insulin sensitivity in children: comparison between the euglycemic-hyperinsulinemic clamp and surrogate measures. Diabetes Care. 2008;31(4):783–88. doi: 10.2337/dc07-1376. [DOI] [PubMed] [Google Scholar]
- 7.Tagi VM, Giannini C, Chiarelli F. Insulin resistance in children. Front Endocrinol (Lausanne) 2019;10:342. doi: 10.3389/fendo.2019.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebensztejn DM, Flisiak-Jackiewicz M, Białokoz-Kalinowska I, et al. Hepatokines and non-alcoholic fatty liver disease. Acta Biochim Pol. 2016;63(3):459–67. doi: 10.18388/abp.2016_1252. [DOI] [PubMed] [Google Scholar]
- 9.Giannini C, Caprio S. Islet function in obese adolescents. Diabetes Obes Metab. 2012;14(Suppl 3):40–45. doi: 10.1111/j.1463-1326.2012.01643.x. [DOI] [PubMed] [Google Scholar]
- 10.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–23. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 11.Carreau AM, Xie D, Garcia-Reyes Y, et al. Good agreement between hyperinsulinemic-euglycemic clamp and 2 hours oral minimal model assessed insulin sensitivity in adolescents. Pediatr Diabetes. 2020;21(7):1159–68. doi: 10.1111/pedi.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song K, Lee HW, Choi HS, et al. Comparison of the modified TyG indices and other parameters to predict non-alcoholic fatty liver disease in youth. Biology (Basel) 2022;11(5):685. doi: 10.3390/biology11050685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simental-Mendía LE, Ortega-Pacheco CJ, García-Guerrero E, Sicsik-Aragón MA, et al. The triglycerides and glucose index is strongly associated with hepatic steatosis in children with overweight or obesity. Eur J Pediatr. 2021;180(6):1755–60. doi: 10.1007/s00431-021-03951-1. [DOI] [PubMed] [Google Scholar]
- 14.Song K, Park G, Lee HS, et al. Comparison of the triglyceride glucose index and modified triglyceride glucose indices to predict nonalcoholic fatty liver disease in youths. J Pediatr. 2022;242:79–85.e1. doi: 10.1016/j.jpeds.2021.11.042. [DOI] [PubMed] [Google Scholar]
- 15.Wang R, Dai L, Zhong Y, Xie G. Usefulness of the triglyceride glucose-body mass index in evaluating nonalcoholic fatty liver disease: Insights from a general population. Lipids Health Dis. 2021;20(1):77. doi: 10.1186/s12944-021-01506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye X, Li J, Wang H, Wu J. Pentraxin 3 and the TyG index as two novel markers to diagnose NAFLD in children. Dis Markers. 2021;2021:8833287. doi: 10.1155/2021/8833287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Onis M. 4.1 The WHO child growth standards. World Rev Nutr Diet. 2015;113:278–94. doi: 10.1159/000360352. [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–19. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Park JM, Lee JY, Dong JJ, et al. Association between the triglyceride to high-density lipoprotein cholesterol ratio and insulin resistance in Korean adolescents: A nationwide population-based study. J Pediatr Endocrinol Metab. 2016;29(11):1259–65. doi: 10.1515/jpem-2016-0244. [DOI] [PubMed] [Google Scholar]
- 20.IBM Corp. Released 2020. Version IBM SPSS Statistics for Windows, Version 27.0. IBM Corp; [Google Scholar]
- 21.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Šimundić AM. Measures of diagnostic accuracy: Basic definitions. EJIFCC. 2009;19(4):203–11. [PMC free article] [PubMed] [Google Scholar]
- 23.Giannini C, Santoro N, Caprio S, et al. The triglyceride-to-HDL cholesterol ratio: Association with insulin resistance in obese youths of different ethnic backgrounds. Diabetes Care. 2011;34(8):1869–74. doi: 10.2337/dc10-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dundar C, Terzi O, Arslan HN. Comparison of the ability of HOMA-IR, VAI, and TyG indexes to predict metabolic syndrome in children with obesity: A cross-sectional study. BMC Pediatr. 2023;23(1):74. doi: 10.1186/s12887-023-03892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dikaiakou E, Vlachopapadopoulou EA, Paschou SA, et al. Triglycerides-glucose (TyG) index is a sensitive marker of insulin resistance in Greek children and adolescents. Endocrine. 2020;70(1):58–64. doi: 10.1007/s12020-020-02374-6. [DOI] [PubMed] [Google Scholar]
- 26.Reckziegel MB, Nepomuceno P, Machado T, et al. The triglyceride-glucose index as an indicator of insulin resistance and cardiometabolic risk in Brazilian adolescents. Arch Endocrinol Metab. 2023;67(2):153–61. doi: 10.20945/2359-3997000000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giannouli A, Efthymiou V, Konidari M, et al. The burden of non-alcoholic fatty liver disease in adolescents with polycystic ovary syndrome: A case-control study. J Clin Med. 2023;12(2):557. doi: 10.3390/jcm12020557. [DOI] [PMC free article] [PubMed] [Google Scholar]


