Abstract
Background
This study aimed to detect the volatile organic compound (VOC), 3-hydroxy-2-butanone (acetoin) using gas chromatography-ion mobility spectrometry (GC-IMS) in antimicrobial-resistant Klebsiella pneumoniae (K. pneumoniae) carbapenemase (KPC)-producing bacteria.
Material/Methods
Using stromal fluid of blood culture bottles (BacT/ALERT® SA) as the medium, 3-hydroxy-2-butanone (acetoin) released by K. pneumoniae during growth was detected using GC-IMS. The impact of imipenem (IPM) and carbapenemase inhibitors [avibactam sodium or pyridine-2,6-dicarboxylic acid (DPA)] on the emission of 3-hydroxy-2-butanone (acetoin) from various carbapenemase-producing K. pneumoniae was further investigated. Subsequently, VOCal software was used to generate a pseudo-3D plot of 3-hydroxy-2-butanone (acetoin), and the relative peak volumes were exported for data analysis. Standard strains served as references, and the findings were validated with clinical isolates.
Results
The pattern of temporal changes in the 3-hydroxy-2-butanone (acetoin) release from K. pneumoniae in the absence of IPM was consistent with the growth curve. After the IPM addition, carbapenemase-positive strains released significantly higher contents of 3-hydroxy-2-butanone (acetoin) than carbapenemase-negative strains at the late exponential growth phase (T2). Notably, adding avibactam sodium significantly decreased the 3-hydroxy-2-butanone (acetoin) content released from the class A carbapenemase-producing strains as compared to the absence of the carbapenemase inhibitor. Conversely, adding DPA significantly decreased the 3-hydroxy-2-butanone (acetoin) content released from the class B carbapenemase-producing strains (both standard and clinical strains, all P<0.05).
Conclusions
This study demonstrated the potential of 3-hydroxy-2-butanone (acetoin) as a VOC biomarker for detecting carbapenemase-producing K. pneumoniae, as revealed by GC-IMS analysis.
Keywords: Carbapenemase, Imipenem, Ion Mobility Spectrometry, Klebsiella pneumonia, Acetoin
Introduction
Recently, the extensive use of carbapenem antibiotics in clinical practice has led to a concerning rise in carbapenem resistance among Klebsiella pneumoniae (K. pneumoniae). Since the 1990s, the prevalence of carbapenem-resistant K. pneumoniae (CRKP) has progressively increased globally, resulting in high morbidity and mortality rates associated with these infections [1]. Furthermore, the emergence of antimicrobial-resistant K. pneumoniae carbapenemase (KPC)-producing bacteria has become a pressing global issue [2–5].
Carbapenemase production is the main mechanism of resistance to carbapenem antibiotics in CRKP strains, with K. pneumoniae carbapenemase (KPC-type), New Delhi metallo-beta-lactamase (NDM-type) carbapenemase, and oxacillinase-48 (OXA-48-type) carbapenemase being the most prevalent types [6,7]. In China, the main genetic determinant of CRKP is K. pneumoniae carbapenemase-2 (KPC-2), accounting for approximately 70% of cases [8]. Carbapenemases are classified according to the Ambler molecular classification into classes A, B, and D. Class A and D carbapenemases are serine-like enzymes, and class B are metalloenzymes [9]. Notably, antimicrobial drugs exhibited varying in vitro antimicrobial activities against different carbapenemase-producing strains [10]. Metalloenzyme-producing CRKP strains often show sensitivity to aztreonam. Conversely, the broad-spectrum antibiotic ceftazidime-avibactam (CAZ-AVI) possesses robust antimicrobial properties against carbapenemase (serine-like enzymes)-producing CRKP strains but lacks efficacy against metalloenzyme-producing CRKP counterparts. Therefore, the accurate and prompt identification and classification of carbapenemases in CRKP are crucial for the appropriate clinical administration of anti-infective medication.
Currently, the laboratory tests for carbapenemases rely on the modified carbapenem inactivation method (mCIM) and the Carbapenemase Non-Phenotypic (Carba NP) test, as recommended by the Clinical and Laboratory Standards Institute (CLSI) [11, 12]. However, these techniques require routine cultivation of pure colonies, with certain methods also necessitating overnight culture for a minimum of 1-2 days [13]. This delay hinders prompt clinical diagnosis and treatment. Despite developing various carbapenemase detection techniques [14–16], their implementation has been limited due to intricate procedures or high costs.
Recently, researchers have explored the potential of volatile organic compounds (VOCs) for strain identification [17–19], and applying volatile metabolites to antibiotic drug susceptibility testing has gained increasing attention [20-23]. For instance, gas chromatography-mass spectrometry (GC-MS) detection of 3-methyl-1-butanol enabled early identification of carbapenemase-positive CRKP strains [22], while non-targeted GC-MS analysis of VOC changes shows promise in the early CRKP strains identification [20]. Moreover, Smart et al. [23] employed thermal desorption-GC-MS to identify nine compounds that distinguished between cephalexin-sensitive and cephalexin-resistant isolates.
Gas chromatography-ion mobility spectrometry (GC-IMS) has emerged as a cutting-edge detection method in recent years. This innovative technique leverages the principles of chromatographic separation and ionization reactions to detect VOCs. The process begins with the pre-separation through a chromatographic column and is followed by their introduction into an ionization reaction zone by a carrier gas (nitrogen or air). Under the influence of an ion source, the carrier gas molecules and sample molecules undergo a series of ionization and ion-molecule reactions, resulting in the charging of sample molecules and their transformation into molecular ions. These ions are then driven by an electric field into a drift region through periodically opened ion gates, where they are separated and detected based on their varying migration rates due to continuous collisions with counterflowing neutral drift gas molecules [24]. Moreover, GC-IMS integrates the superior separation capabilities of gas chromatography (GC) with the enhanced sensitivity of ion mobility spectrometry (IMS). This innovative method eliminates the necessity for solid-phase microextraction by allowing the analysis of headspace components from solid or liquid samples through direct headspace injection. With a limit of detection reaching the parts per billion by volume (ppbv) level, GC-IMS facilitates both qualitative and quantitative analysis of individual compounds or labelers [25,26].
3-hydroxy-2-butanone (acetoin) emerges as a pivotal physiological metabolite synthesized by a diverse array of microorganisms thriving in glucose-enriched environments or other fermentable carbon sources. The catabolic conversion of this metabolite is primarily mediated by the acetoin dehydrogenase enzyme system (AoDH ES). Detecting acetoin-forming capacity, frequently achieved through the Voges-Proskauer reaction, serves as a valuable tool for classifying microorganisms. In bacteria grown in glucose-containing media, the release of 3-hydroxy-2-butanone (acetoin) is primarily regulated by the coordinated action of two enzymes: α-acetolactate synthase and α-acetolactate decarboxylase. 3-hydroxy-2-butanone (acetoin) plays a crucial role in these microorganisms, with its physiological significance encompassing acidification avoidance, nicotinamide adenine dinucleotide (NAD)/NADH (reduced form of NAD) ratio regulation, and carbon storage facilitation, ultimately maintaining the metabolic homeostasis and adaptability of microbial communities [27,28]. Notably, previous studies have demonstrated that K. pneumoniae releases 3-hydroxy-2-butanone (acetoin) during blood cultures [21,29] and in trypticase soy broth (TSB) [30].
Given the preceding justification and the escalating detection rate of CRKP in blood cultures [31,32], this study builds upon previous findings [21]. The previous research demonstrated the potential of GC-IMS for non-targeted analysis in simulated blood cultures, effectively identifying CRKP strains [21]. Therefore, this study aimed to utilize GC-IMS technology to detect a specific VOC biomarker, 3-hydroxy-2-butanone (acetoin), in antimicrobial-resistant KPC-producing bacteria, facilitating early identification of carbapenemase-producing CRKP strains.
Material and Methods
Ethics Statement
In this study, K. pneumoniae isolation was conducted following hospital laboratory protocol without identifiable patient data linked to the samples. Consequently, the Medical Research Ethics Committee of the Second Affiliated Hospital of Nanchang University exempted the study from ethics approval.
Sources of the Strains and Carbapenemase Detection
The standard strains of K. pneumoniae, including ATCC BAA-700603 (carbapenemase-negative), ATCC BAA-1706 (carbapenemase-negative), ATCC BAA-1705 (blaKPC-positive), ATCC BAA-2146 (blaNDM-positive), and ATCC BAA-2524 (blaOXA-48-positive), were obtained from the American Type Culture Collection (ATCC, USA).
Additionally, 69 clinical strains were isolated from the Second Affiliated Hospital of Nanchang University and are currently maintained in the research team’s strain bank [21,22]. Among these, 25 strains were carbapenem-susceptible K. pneumoniae (CSKP) strains, while the remaining 44 strains (CRKP) exhibited resistance to carbapenem. All K. pneumoniae strains (standard and clinical strains) underwent re-testing for antimicrobial susceptibility, interpreted according to the CLSI 2022 [12] guidelines. The mCIM and modified ethylenediaminetetraacetic acid (EDTA)-carbapenem inactivation method (eCIM) [12] were performed again to validate the carbapenemase type, confirmed by polymerase chain reaction (PCR). Moreover, all clinical isolates were evaluated for the presence of carbapenemase-related genes utilizing macrogenomic next-generation sequencing (mNGS) technology, provided by Qiantang Life Science Technology Co. Ltd. (Suzhou, China). Subsequently, all K. pneumoniae strains were stored at −80°C for further analysis.
Bacterial Culture and Sample Preparation
Figure 1 shows the growth curve of K. pneumoniae (ATCC BAA-700603) in blood culture bottle medium (BacT/ALERT® SA; Ref. 259789; Biomérieux, Nürtingen, Germany), with an initial concentration of 107 colony-forming units per milliliter (CFU/mL) bacteria at 37°C and agitation of 200 revolutions per minute (rpm) in a 6 mL volume. The growth curve indicates that K. pneumoniae grew fastest after 3 h of incubation and reached the end of the exponential growth phase at around 5 h.
Figure 1.

The growth curve of K. pneumoniae (ATCC BAA-700603). The free online tool Chiplot (https://www.chiplot.online). K. pneumoniae – Klebsiella pneumoniae; ATCC – American Type Culture Collection.
All experimental strains were inoculated on Columbia blood agar plates and incubated overnight at 37°C. Bacterial suspensions were prepared and added to test tubes containing 6 mL of blood culture bottle medium (initially 107 CFU/mL), capped, and incubated at 37°C with agitation at 200 rpm. Blank culture media served as control. To evaluate the effect of IPM on 3-hydroxy-2-butanone (acetoin) release from K. pneumoniae, a final concentration of 0.25 mg/mL of IPM (Solarbio, China) was added after 3 h (T0) of incubation. The final concentration of IPM in carbapenemase-negative CRKP strains was 16 μg/mL. To assess the impact of carbapenemase inhibitors on 3-hydroxy-2-butanone (acetoin) emission, IPM and avibactam sodium (1 mg/L) [33] or DPA (100 μg/mL) [34] were added after 3 h (T0). The standard strains and clinical strains were processed identically. Experiments were repeated six times for standard strains and in triplicate for clinical strains.
Following the inoculation of standard strains, with or without IPM, into a blood culture bottle medium, 500 μL samples were collected for GC-IMS analysis at various incubation periods: 3h (T0), 4h (T1), 5h (T2), 6h (T3), and 7h (T4). GC-IMS analysis of the standard strain with carbapenemase inhibitors and clinical strains was performed at the T2 time point.
The Measurement of 3-hydroxy-2-butanone (Acetoin) by GC-IMS
The FlavourSpec® GC-IMS equipment (G.A.S., Shandong HaiNeng Scientific Instrument Co., Ltd., Shandong, China) was employed to detect 3-hydroxy-2-butanone (acetoin). The GC-IMS parameters were set as follows: incubation time: 3 min; incubation temperature: 60°C; rotating speed: 500 rpm; injector temperature: 85°C; headspace gas volume: 1 mL; drift tube temperature (T1): 45°C; chromatographic column temperature (T2): 80°C; chromatographic column: MXT-WAX column (high-polar column, 15 m×0.53 mm, 0.1 μm, RESTEK, Bellefonte, PA, USA); carrier and drift gases: nitrogen (N2) of 99.99% purity (Jiangzhu Industrial Co., Ltd., Nanchang, China); ionization source: tritium source; average radiation energy: 5.68 keV; drift tube length: 98 mm; ionization mode: positive ionization; drift tube flow: 150 mL/min; chromatographic column flow: 2 mL/min (0–3 min), 10 mL/min (3–10 min); total analysis time: 10 min.
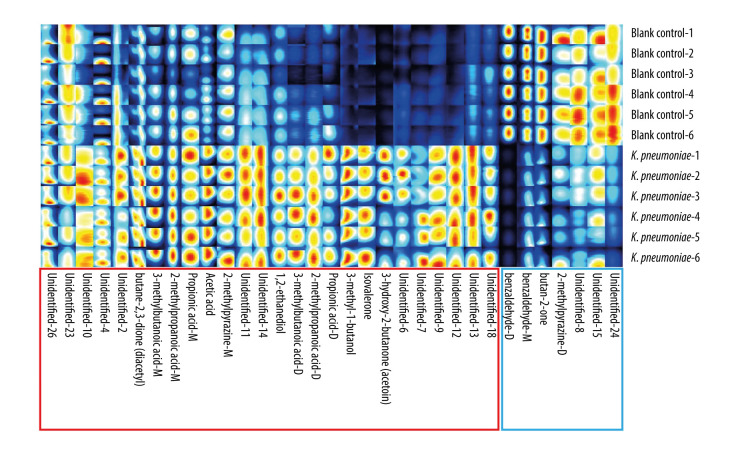
The fingerprint of VOCs emitted by K. pneumoniae (ATCC BAA-700603) after 5 h of incubation in the medium of blood culture bottles (BacT/ALERT® SA) is depicted in Figure 2, and the details of VOCs have been described in previous studies [21]. The VOC fingerprints generated by all the understudied K. pneumoniae strains, following a 5-h incubation period in a blood culture flask substrate, aligned with the pattern displayed in Figure 2 (without IPM addition).
Figure 2.
The fingerprint of VOCs emitted by K. pneumoniae (ATCC BAA-700603) after 5 h incubation in blood culture bottles (BacT/ALERT® SA) medium. Red boxes indicate VOCs emitted by K. pneumoniae, while blue boxes represent VOCs absorbed by K. pneumoniae. VOCal (version 0.1.3), G.A.S., Shandong HaiNeng Scientific Instrument Co., Ltd., Shandong, China. VOCs – volatile organic compounds; K. pneumoniae – Klebsiella pneumoniae; ATCC – American Type Culture Collection; M – monomer; D – dimer.
Using C4-C9 ketones (2-butanone, 2-pentanone, 2-hexanone, 2-heptanone, 2-octanone, 2-nonanone), from Shandong HaiNeng Scientific Instrument Co., Ltd. (Shandong, China), as reference standards for data calibration, 3-hydroxy-2-butanone (acetoin) was identified based on the retention index (RI) and drift time (RIP relative) in the GC-IMS library (NIST library and IMS library) [21, 35]. GC-IMS detection of 3-hydroxy-2-butanone (acetoin) is characterized as follows: Chemical Abstract Service Registry Number (CAS#): C513860; Formula: C4H8O2; Molecular weight (MW): 88.1; RI: 1304.2; Retention time (Rt): 226.606 s; and Drift time (Dt): 1.33401 a.u. (arbitrary units).
Statistical Analysis
The VOC fingerprint emitted by K. pneumoniae and the pseudo-3D plots of 3-hydroxy-2-butanone (acetoin) were generated by VOCal software (version 0.1.3; G.A.S., Shandong HaiNeng Scientific Instrument Co., Ltd., Shandong, China). The relative peak volume values (integrating peak intensities within specific regions after comparing calibrations) of 3-hydroxy-2-butanone (acetoin) were extracted from the VOCal software and exported to Microsoft Excel (Excel for MacOS, 2020) for further analysis. All statistical analyses were performed using R (version 4.2.3; The R Foundation, Vienna, Austria). As the data did not follow a normal distribution, the Mann-Whitney U test was used for comparisons between groups. A P-value less than 0.05 was considered statistically significant (P<0.05). Data visualizations were created using the online tool Chiplot (https://www.chiplot.online) [36].
Results
Effect of IPM on the Release of 3-hydroxy-2-butanone (Acetoin) from Standard Strains of K. pneumoniae
The content of 3-hydroxy-2-butanone (acetoin) released by all standard strains increases progressively over time (T0–T4) without IPM addition (Figure 3A). However, after a 3-h incubation period (T0), IPM addition to the culture medium resulted in a consistent level of 3-hydroxy-2-butanone (acetoin) release by K. pneumoniae ATCC BAA-1706 (carbapenemase-negative), with no further changes in content observed from T0 to T4. In contrast, carbapenemase-positive standard strains displayed a temporal variation in 3-hydroxy-2-butanone (acetoin) release, similar to the same trend without IPM addition (Figure 3B). The content of 3-hydroxy-2-butanone (acetoin) exhibited a pronounced escalation between T1 and T2, aligning with the logarithmic phase of the growth curve (Figure 1). Therefore, subsequent analysis focused mainly on the T2 time point.
Figure 3.
The temporal variation patterns of 3-hydroxy-2-butanone (acetoin) emission by K. pneumoniae (standard strains, T0-T4). (A) The temporal variation patterns of 3-hydroxy-2-butanone (acetoin) emission by K. pneumoniae strains when imipenem was not added. (B) The temporal patterns of 3-hydroxy-2-butanone (acetoin) emitted by K. pneumoniae strains when imipenem was added. The free online tool Chiplot (https://www.chiplot.online). ATCC – American Type Culture Collection.
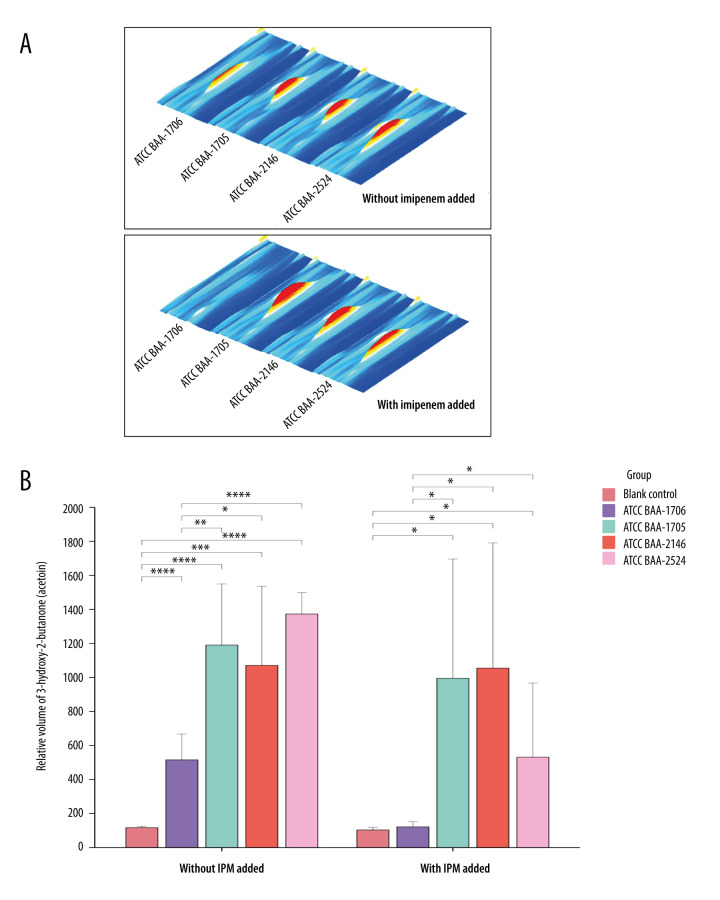
With IPM addition, the pseudo-3D plots illustrating the change of 3-hydroxy-2-butanone (acetoin) in standard strains are shown in Figure 4A (T2). Notably, IPM addition resulted in a significant decrease in 3-hydroxy-2-butanone (acetoin) contents emitted by K. pneumoniae ATCC BAA-1706 compared to its release without IPM. Interestingly, no statistically significant difference was observed in the relative peak volume (semi-quantified) of 3-hydroxy-2-butanone (acetoin) emitted by K. pneumoniae ATCC BAA-1706 at T2 with IPM addition, compared to the blank medium (P>0.05) (Figure 4B).
Figure 4.
The changes in 3-hydroxy-2-butanone (acetoin) contents emitted by standard strain at the T2 time point before and after the addition of imipenem. (A) The pseudo-3D plots of 3-hydroxy-2-butanone (acetoin) in each standard strain (comparison between no addition of imipenem and imipenem addition). VOCal (version 0.1.3), G.A.S., Shandong HaiNeng Scientific Instrument Co., Ltd., Shandong, China. (B) Comparison of the relative peak volume (semiquantitative) of 3-hydroxy-2-butanone (acetoin) emitted by standard strains (with imipenem and without imipenem). The free online tool Chiplot (https://www.chiplot.online). The Mann-Whitney U test was used for pairwise comparison. * P<0.05, ** P<0.01, *** P<0.001, and **** P<0.0001. R version 4.2.3, The R Foundation, Vienna, Austria. ATCC – American Type Culture Collection; IPM – imipenem.
Effect of Carbapenemase Inhibitors on the Release of 3-hydroxy-2-butanone (Acetoin) from Standard Strains of K. pneumoniae
The effect of carbapenemase inhibitors on the content of 3-hydroxy-2-butanone (acetoin) emitted by K. pneumoniae was further investigated. Figure 5A shows the pseudo-3D plots illustrating the changes in 3-hydroxy-2-butanone (acetoin) content following the introduction of carbapenemase inhibitors in various standard strains. The combination of IPM and avibactam sodium significantly decreased 3-hydroxy-2-butanone (acetoin) content in K. pneumoniae ATCC BAA-1705 (blaKPC-positive), compared to IPM alone or IPM with DPA (both P<0.05; Figure 5B). Similarly, the combination of IPM and DPA significantly decreased the 3-hydroxy-2-butanone (acetoin) content in K. pneumoniae ATCC BAA-2146 (blaNDM-positive), compared to IPM alone or IPM with avibactam sodium (both P<0.05; Figure 5B). However, compared with the addition of IPM alone, the remaining standard strains showed no notable changes in 3-hydroxy-2-butanone (acetoin) contents after introducing carbapenemase inhibitors (despite P<0.05, but no obvious visual changes observed in the pseudo-3D plots).
Figure 5.
The changes in the content of 3-hydroxy-2-butanone (acetoin) emitted by standard strain at the T2 time point before and after the addition of carbapenemase inhibitors. (A) The pseudo-3D plots of 3-hydroxy-2-butanone (acetoin) contents in standard strains before and after the addition of carbapenase inhibitors. VOCal (version 0.1.3), G.A.S., Shandong HaiNeng Scientific Instrument Co., Ltd., Shandong, China. (B) Comparison of the relative peak volume (semiquantitative) of 3-hydroxy-2-butanone (acetoin) emitted by standard strains before and after the addition of carbapenase inhibitors. The free online tool Chiplot (https://www.chiplot.online). The Mann-Whitney U test was used for pairwise comparison. * P<0.05. R version 4.2.3, The R Foundation, Vienna, Austria. ATCC – American Type Culture Collection; IPM – imipenem; DPA – pyridine-2,6-dicarboxylic acid.
The Potential Application Value of 3-hydroxy-2-butanone (Acetoin) in the Identification of Carbapenemase-Producing K. pneumoniae in Clinical Strains
A validation study utilizing clinical strains was conducted to further elucidate the role of 3-hydroxy-2-butanone (acetoin) in identifying carbapenemase-producing K. pneumoniae. As described in Table 1, among 44 CRKP isolates, the mNGS examination discovered five carbapenemase-negative CRKP strains that were devoid of carbapenemase genes. Subsequently, based on the results of mCIM, eCIM, PCR and mNGS, these CRKP isolates were further classified based on carbapenemase type: KPC-positive CRKP strains (n=20), NDM-positive CRKP strains (n=15), imipenem-hydrolyzing β-lactamase (IMP)-positive CRKP strains (n=4), and carbapenemase-negative CRKP strains (n=5).
Table 1.
Effects of different treatments on 3-hydroxy-2-butanone content (relative peak volume, mean) at the T2 time point for each CRKP clinical isolate.
| N0. | Carbapenem | Carbapenem gene | MIC of IPM (μg/mL) | Treatment | ||
|---|---|---|---|---|---|---|
| IPM | IPM+avibactam-sodium | IPM+DPA | ||||
| CRKP-27 | KPC | bla KPC-2 | ≥16 | 1828.87 | 467.12 | 1282.78 |
| CRKP-41 | KPC | bla KPC-2 | ≥16 | 1677.36 | 214.40 | 1376.25 |
| CRKP-63 | KPC | bla KPC-2 | ≥16 | 1562.70 | 387.01 | 1499.06 |
| CRKP-77 | KPC | bla KPC-2 | ≥16 | 1706.59 | 347.07 | 1536.12 |
| CRKP-237 | KPC | bla KPC-2 | ≥16 | 1030.86 | 367.19 | 839.04 |
| CRKP-249 | KPC | bla KPC-2 | ≥16 | 941.47 | 249.81 | 459.68 |
| CRKP-315 | KPC | bla KPC-2 | ≥16 | 1581.80 | 311.39 | 1396.22 |
| CRKP-324 | KPC | bla KPC-2 | ≥16 | 1756.72 | 341.53 | 1441.28 |
| CRKP-395 | KPC | bla KPC-2 | ≥16 | 1501.83 | 363.56 | 1581.92 |
| CRKP-400 | KPC | bla KPC-2 | ≥16 | 1602.82 | 343.59 | 1444.68 |
| CRKP-438 | KPC | bla KPC-2 | ≥16 | 1523.43 | 123.43 | 1409.86 |
| CRKP-451 | KPC | bla KPC-2 | ≥16 | 1462.53 | 156.75 | 1548.14 |
| CRKP-463 | KPC | bla KPC-2 | ≥16 | 1402.29 | 558.98 | 1650.91 |
| CRKP-472 | KPC | bla KPC-2 | ≥16 | 1714.61 | 381.34 | 1543.71 |
| CRKP-582 | KPC | bla KPC-2 | ≥16 | 1996.89 | 404.08 | 1643.47 |
| CRKP-598 | KPC | bla KPC-2 | ≥16 | 2038.75 | 295.71 | 1477.82 |
| CRKP-601 | KPC | bla KPC-2 | ≥16 | 1543.12 | 474.17 | 1500.05 |
| CRKP-614 | KPC | bla KPC-2 | ≥16 | 1584.56 | 421.57 | 1490.79 |
| CRKP-626 | KPC | bla KPC-2 | ≥16 | 1373.51 | 206.93 | 1674.52 |
| CRKP-634 | KPC | bla KPC-2 | ≥16 | 1654.22 | 261.19 | 1528.93 |
| CRKP-514 | NDM | bla NDM-5 | ≥16 | 1250.96 | 1381.17 | 279.35 |
| CRKP-647 | NDM | bla NDM-5 | ≥8 | 1515.22 | 1646.73 | 496.99 |
| CRKP-816 | NDM | bla NDM-5 | ≥16 | 1663.52 | 1587.01 | 538.98 |
| CRKP-982 | NDM | bla NDM-1 | ≥16 | 2142.75 | 2098.00 | 1111.60 |
| CRKP-1598 | NDM | bla NDM-1 | ≥16 | 1874.23 | 1979.27 | 543.67 |
| CRKP-1780 | NDM | bla NDM-1 | ≥16 | 1763.67 | 1660.79 | 86.19 |
| CRKP-1786 | NDM | bla NDM-1 | 8 | 1241.11 | 1626.78 | 73.56 |
| CRKP-1825 | NDM | bla NDM-5 | 8 | 1472.43 | 1667.14 | 62.94 |
| CRKP-1971 | NDM | bla NDM-5 | ≥16 | 1372.63 | 1619.21 | 65.95 |
| CRKP-B1 | NDM | bla NDM-5 | ≥8 | 2199.14 | 2194.72 | 70.83 |
| CRKP-B17 | NDM | bla NDM-1 | ≥8 | 1806.19 | 1999.71 | 77.01 |
| CRKP-B44 | NDM | bla NDM-1 | 8 | 1370.51 | 1275.86 | 353.18 |
| CRKP-B102 | NDM | bla NDM-1 | 4 | 1539.06 | 1589.66 | 80.11 |
| CRKP-B184 | NDM | bla NDM-5 | ≥8 | 2137.87 | 2173.45 | 90.11 |
| CRKP-B205 | NDM | bla NDM-15 | ≥8 | 2149.60 | 2240.32 | 83.81 |
| CRKP-1228 | IMP | bla IMP-4 | 4 | 1515.97 | 1449.60 | 812.73 |
| CRKP-1748 | IMP | bla IMP-4 | ≥16 | 1513.20 | 1467.71 | 313.02 |
| CRKP-1799 | IMP | bla IMP-4 | 4 | 1822.12 | 1547.69 | 682.82 |
| CRKP-1811 | IMP | bla IMP-4 | ≥16 | 1342.26 | 1315.15 | 812.03 |
| CRKP-681 | – | – | ≥8 | 66.34 | 78.61 | 78.42 |
| CRKP-738 | – | – | ≥2 | 72.69 | 96.60 | 81.89 |
| CRKP-1577 | – | – | 4 | 71.25 | 65.18 | 92.05 |
| CRKP-1864 | – | – | ≥16 | 59.15 | 41.97 | 54.98 |
| CRKP-B216 | – | – | ≥8 | 720.59 | 220.18 | 121.62 |
“−” represents the absence of carbapenemase or carbapenemase gene. IPM – imipenem; DPA – pyridine-2,6-dicarboxylic acid; CRKP – carbapenem-resistant Klebsiella pneumoniae; KPC – Klebsiella pneumoniae-carbapenemases; NDM – New Delhi metallo-β-lactamase; IMP – imipenemase metallo-β-lactamase; MIC – minimum inhibitory concentration.
The addition of IPM resulted in higher 3-hydroxy-2-butanone (acetoin) in the KPC-positive, NDM-positive, and IMP-positive CRKP groups compared to the CSKP group (n=25) (all P<0.05; Figure 6A, 6B). However, the carbapenemase-negative CRKP group did not show a statistically significant difference in 3-hydroxy-2-butanone (acetoin) content when compared to the CSKP group (P>0.05; Figure 6).
Figure 6.
After imipenem addition, changes in the 3-hydroxy-2-butanone (acetoin) content emitted by various carbapenase types of K. pneumoniae strains (clinical isolates) at the T2 time point. (A) The pseudo-3D plots of 3-hydroxy-2-butanone (acetoin) in different carbapenase types of K. pneumoniae strains after the addition of imipenem. VOCal (version 0.1.3), G.A.S., Shandong HaiNeng Scientific Instrument Co., Ltd., Shandong, China. (B) Comparison of the relative peak volume (semiquantitative) of 3-hydroxy-2-butanone (acetoin) emitted by different carbapenase types of K. pneumoniae strains after the imipenem addition. The free online tool Chiplot (https://www.chiplot.online). The Mann-Whitney U test was used for pairwise comparison. **** P<0.0001. R version 4.2.3, The R Foundation, Vienna, Austria. K. pneumoniae – Klebsiella pneumoniae; CSKP – carbapenem-susceptible Klebsiella pneumoniae; CRKP – carbapenem-resistant Klebsiella pneumoniae; KPC – Klebsiella pneumoniae-carbapenemases; NDM – New Delhi metallo-β-lactamase; IMP – imipenemase metallo-β-lactamase.
The addition of carbapenemase inhibitors resulted in pseudo-3D plots similar to those of K. pneumoniae ATCC BAA-1705 for the KPC-positive group and K. pneumoniae ATCC BAA-2146 for the NDM-positive and IPM-positive groups (Figure 7A). Statistical analysis revealed a significant decrease in the relative peak volume of 3-hydroxy-2-butanone (acetoin) in the KPC-positive group with avibactam sodium addition and in the NDM-positive and IMP-positive groups with the DPA addition.
Figure 7.
The changes in 3-hydroxy-2-butanone (acetoin) content emitted by different carbapenase types of K. pneumoniae strains (clinical isolates) at the T2 time point before and after the addition of carbapenase inhibitors. (A) The pseudo-3D plots of 3-hydroxy-2-butanone (acetoin) in different carbapenase types of K. pneumoniae strains before and after the addition of carbapenase inhibitors. VOCal (version 0.1.3), G.A.S., Shandong HaiNeng Scientific Instrument Co., Ltd., Shandong, China. (B) Comparison of the relative peak volume (semiquantitative) of 3-hydroxy-2-butanone (acetoin) emitted by different carbapenase types of K. pneumoniae strains before and after the addition of carbapenase inhibitors. The free online tool Chiplot (https://www.chiplot.online). The Mann-Whitney U test was used for pairwise comparison. * P<0.05, **** P<0.0001. R version 4.2.3, The R Foundation, Vienna, Austria. K. pneumoniae – Klebsiella pneumoniae; CSKP – carbapenem-susceptible Klebsiella pneumoniae; CRKP – carbapenem-resistant Klebsiella pneumoniae; KPC – Klebsiella pneumoniae-carbapenemases; NDM – New Delhi metallo-β-lactamase; IMP – imipenemase metallo-β-lactamase; IPM – imipenem; DPA – pyridine-2,6-dicarboxylic acid.
Finally, Table 1 summarizes the carbapenemase types and genes of the clinical CRKP isolates, the minimum inhibitory concentration (MIC) of IPM, and the GC-IMS detection results of 3-hydroxy-2-butanone (acetoin) released by each CRKP strain under three different treatments. It is noteworthy that the trends of 3-hydroxy-2-butanone (acetoin) content under different treatments for each CRKP strain were consistent with the results above.
Discussion
This study expanded previous research [22] by utilizing a more sensitive GC-IMS assay and blood culture bottles (BacT/ALERT® SA) commonly used in clinical settings as the medium. Additionally, the study investigated temporal variation patterns in the distinctive VOC, 3-hydroxy-2-butanone (acetoin). The findings suggest that 3-hydroxy-2-butanone (acetoin) has the potential as a biomarker for identifying carbapenemase-producing K. pneumoniae, supported by the following pieces of evidence: (1) The temporal release pattern of 3-hydroxy-2-butanone aligns with the observed bacterial growth pattern of K. pneumoniae in blood culture matrix. (2) IPM addition resulted in a significant decrease in 3-hydroxy-2-butanone (acetoin) release by carbapenemase-negative strains during the late exponential growth phase (T2), in comparison to carbapenemase-positive strains (both standard and clinical strains). (3) The addition of carbapenemase inhibitors enabled further identification of carbapenemase phenotypes (class A or B) based on changes in 3-hydroxy-2-butanone (acetoin) content.
Research has shown that K. pneumoniae can release 3-hydroxy-2-butanone (acetoin) during its growth process [29,30]. As a fermentation bacterium, K. pneumoniae phosphorylates extracellular glucose and other monosaccharides into glucose 6-phosphate through the phosphotransferase system (PTS) [37,38]. This glucose 6-phosphate is then utilized by the bacteria through the glycolytic pathway, producing pyruvate, which is further metabolized to generate acetolactate. The enzyme α-acetolactate decarboxylase ultimately converts acetolactate to 3-hydroxy-2-butanone (acetoin) [27,30,39]. Although the metabolic mechanism of 3-hydroxy-2-butanone (acetoin) in K. pneumoniae requires further investigation, the findings of this study demonstrated that IPM significantly reduced 3-hydroxy-2-butanone (acetoin) release from CSKP as compared to the absence of IPM. IPM probably exerts its inhibitory effect on bacterial cell wall synthesis, resulting in bacterial cell wall defects, subsequent bacterium expansion, and cell lysis due to the alteration of bacterial cytoplasmic osmotic pressure, ultimately causing bacterial death [40]. Apparently, CSKP strains were killed by IPM, and their growth metabolism ceased, resulting in significantly reduced release of 3-hydroxy-2-butanone (acetoin), which is closely related to the growth metabolism of K. pneumoniae, consistent with previous research [20].
Moreover, avibactam, a newly developed β-lactamase inhibitor, possesses a non-lactam structural scaffold and lacks substantial antimicrobial efficacy independently. However, it can effectively inhibit class A (including ESBLs and KPCs), class C, and some class D β-lactamases [41]. Notably, adding avibactam sodium significantly reduced 3-hydroxy-2-butanone (acetoin) release in class A carbapenemase-producing K. pneumoniae but had no significant effect on other strains compared to no addition of avibactam sodium. Previous studies have demonstrated the inhibitory impact of avibactam sodium on OXA-48 carbapenemase [42,43], but our findings did not replicate this, possibly attributed to a low concentration of avibactam sodium. Further investigation is needed to ascertain the underlying cause. Meanwhile, DPA, a novel metallo-β-lactamase effective against class B carbapenemases [44], selectively hindered 3-hydroxy-2-butanone (acetoin) release in K. pneumoniae strains producing class B carbapenemases (NDM- and IMP-positive strains), as observed in this study.
The findings from the previous study [22] indicated that 3-methyl-1-butanol has the potential to serve as a biomarker for the detection of carbapenemase-producing K. pneumoniae in TSB. However, further analysis using pseudo-3D plots and semiquantitative analysis in this study revealed that alteration in 3-hydroxy-2-butanone (acetoin) exhibited greater specificity. Consequently, 3-methyl-1-butanol was not subjected to further investigation in this study. In fact, this discrepancy can be attributed to variations in the culture substrates and assay methods employed. Meanwhile, one of the previous studies [21] successfully identified CRKP strains by analyzing changes in K. pneumoniae-associated VOCs under different treatments, but this approach was non-targeted and could not identify characteristic VOCs. Therefore, the present study improves upon this approach and simplifies the detection of carbapenemase-producing K. pneumoniae by utilizing the characteristic alterations of 3-hydroxy-2-butanone (acetoin) as an entry point.
Unfortunately, the limited diversity of clinical strains in our collection, predominantly KPC-positive CRKP strains, restricted the complete validation of current findings, necessitating a cautious interpretation of results. However, epidemiological data revealed that blaKPC-2 emerged as the prevailing carbapenemase gene in China, accounting for up to 94% of the clinical CRKP isolates [31], consistent with the regional epidemiological data [22]. Notably, the utilization of pseudo-3D plots and semiquantitative analysis of the content of 3-hydroxy-2-butanone (acetoin) in this study facilitated the accurate identification of CRKP strains that produce class A or B carbapenemases, providing a valuable reference for future investigations.
Another point of concern was the identification of carbapenemase-negative CRKP. These strains cannot produce carbapenemases and owe their carbapenem resistance to the absence of membrane pore proteins OmpK35 and OmpK36, combined with the expression of extended-spectrum β-lactamase enzymes (blaCTX-M, blaSHV, and blaAmpC) or high expression of efflux pumps [45]. Moreover, the non-uniform minimum inhibitory concentrations (MIC) of these strains, despite their IPM resistance, hindered the distinction between CSKP and carbapenemase-negative CRKP based on 3-hydroxy-2-butanone (acetoin) changes, even at a reduced imipenem concentration of 16 μg/mL. Therefore, future studies require a more comprehensive investigation into the optimal imipenem concentration to address this challenge.
The present study has several strengths: (1) Commercial blood culture bottles were used as the medium to enhance clinical applicability, providing a new strategy for early identification of carbapenemase-producing CRKP bacteria, unlike traditional media such as TSB [20,22]. (2) GC-IMS offers superior trace detection capabilities, achieving detection limits as low as nanograms or even picograms [46], and offers advantages over GC-MS, including reduced detection time, no pre-treatment requirements, and rapid sample detection (within 13 min in our study), thus facilitating batch detection. GC-IMS also employs fingerprinting and pseudo-3D plots for intuitive visualization of VOC disparities. (3) The study demonstrates the potential of 3-hydroxy-2-butanone (acetoin) for identifying carbapenemase-producing CRKP in the stromal fluid of blood culture bottles, using standard strains as references and clinical strains for validation, highlighting its significance in this context.
Limitations
This study has certain limitations. First, the impact of blood addition on 3-hydroxy-2-butanone (acetoin) release remains uncertain, consistent with the previous studies [30] indicating that blood addition has no influence on the VOC content produced by K. pneumoniae in blood-free media. This phenomenon warrants further research to understand its impact on VOC contents. Second, the exclusive use of clinical strains from a single medical center limits the generalizability of our method, highlighting the need for larger sample sizes and diverse carbapenemases to reinforce our research outcomes. Third, the GC-IMS assay technique, while valuable, requires specialized instrumentation and expertise and incurs higher costs, making it less accessible in the medical field. Fourth, detecting carbapenemase-positive strains based on the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) method is recommended by the CLSI guidelines [12]. Regrettably, this study did not employ this method because: 1) Although MALDI-TOF MS technology can rapidly detect the types of carbapenemases, its disadvantage lies in the fact that there are currently only a limited number of studies focusing on specific carbapenemases, and there is no unified and feasible methodology [47]. 2) Although MALDI-TOF MS technology has established a relatively comprehensive database for microbial identification, the existing databases may still be incomplete or have accuracy issues when detecting specific strains or resistance patterns. This may lead to misdiagnosis or missed detection of carbapenemase-producing strains. Instead, this research proposed GC-IMS as a new strategy for detecting carbapenemase-positive CRKP strains by identifying changes in 3-hydroxy-2-butanone (acetoin). This method complements the MALDI-TOF MS-based method and aims to improve the speed and accuracy of detecting carbapenemase-positive CRKP strains. We believe that combining both methods can effectively combat the spread of drug-resistant strains, ensuring patient health and safety. Lastly, further evaluation is needed to confirm the specificity of 3-hydroxy-2-butanone (acetoin) for K. pneumoniae; however, utilizing a fingerprint, as depicted in Figure 2, will aid in differentiating K. pneumoniae from other bacterial species.
Conclusions
In conclusion, GC-IMS was successfully utilized to identify carbapenemase-producing K. pneumoniae through changes in 3-hydroxy-2-butanone (acetoin) content after IPM addition. Simultaneously, the inclusion of carbapenemase inhibitors enabled the differentiation between class A and B carbapenemases. However, further research is warranted to confirm these findings.
Acknowledgments
The authors would like to thank Home for Researchers (www.home-for-researchers.com) for English language editing services. We also acknowledge engineer Liqiang Zhao for helping with GC-IMS data analysis.
Footnotes
Conflict of interest: None declared
Declaration of Figures’ Authenticity: All figures submitted are original creations and have not been previously published or duplicated, either in whole or in part. They appear in this work for the first time.
Financial support: This work was supported by the National Natural Science Foundation of China (82060391), the Postgraduate Innovation Special Foundation of Jiangxi Province (YC2023-B093), the Natural Science Foundation of Jiangxi Province (20202BAB216021), the Medical Health Science and Technology Project of Jiangxi Provincial Health Commission (20201034 and 202130412), and the Chinese Medical Science and Technology Research Projects of Jiangxi Provincial Administration of Traditional Chinese Medicine (2023Z030).
References
- 1.Chang D, Sharma L, Dela Cruz CS, et al. Clinical epidemiology, risk factors, and control strategies of Klebsiella pneumoniae Infection. Front Microbiol. 2021;12:750662. doi: 10.3389/fmicb.2021.750662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen LF, Anderson DJ, Paterson DL. Overview of the epidemiology and the threat of Klebsiella pneumoniae carbapenemases (KPC) resistance. Infect Drug Resist. 2012;5:133–41. doi: 10.2147/IDR.S26613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinkac LM, White R, D’Souza R, et al. Emergence of New Delhi metallo-beta-lactamase (NDM-5) in Klebsiella quasipneumoniae from neonates in a Nigerian hospital. mSphere. 2019;4(2):e00685–18.. doi: 10.1128/mSphere.00685-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang Y, Zhong Q, Chen Y, et al. Ceftazidime/avibactam, polymyxin or tigecycline as a rescue strategy for the treatment of carbapenem-resistant Klebsiella pneumoniae in bloodstream infection: A retrospective cohort study. Infect Drug Resist. 2023;16:2963–71. doi: 10.2147/IDR.S409506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Logan LK, Weinstein RA. The epidemiology of carbapenem-resistant Enterobacteriaceae: The impact and evolution of a global menace. J Infect Dis. 2017;215(Suppl 1):S28–S36. doi: 10.1093/infdis/jiw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Oliveira DMP, Forde BM, Kidd TJ, et al. Antimicrobial resistance in ESKAPE pathogens. Clin Microbiol Rev. 2020;33(3):e00181–19. doi: 10.1128/CMR.00181-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tompkins K, van Duin D. Treatment for carbapenem-resistant Enterobacterales infections: Recent advances and future directions. Eur J Clin Microbiol Infect Dis. 2021;40(10):2053–68. doi: 10.1007/s10096-021-04296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu J, Jiang X, Zhao L, et al. An outbreak of ST859-K19 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese Teaching hospital. mSystems. 2022;7(3):e0129721. doi: 10.1128/msystems.01297-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu D, Yan Z, Cai C, et al. Comparison of the NG-Test Carba 5, Colloidal Gold Immunoassay (CGI) test, and Xpert Carba-R for the rapid detection of carbapenemases in carbapenemase-producing organisms. Antibiotics (Basel) 2023;12(2):300. doi: 10.3390/antibiotics12020300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brennan-Krohn T, Manetsch R, O’Doherty GA, et al. New strategies and structural considerations in development of therapeutics for carbapenem-resistant Enterobacteriaceae. Transl Res. 2020;220:14–32. doi: 10.1016/j.trsl.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen N, Li G, Si Y, et al. Evaluation of LAMP assay using phenotypic tests and PCR for detection of blaKPC gene among clinical samples. J Clin Lab Anal. 2022;36(4):e24310. doi: 10.1002/jcla.24310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. CLSI Document M100-S32. Clinical and Laboratory Standards Institute; Wayne, PA: 2022. Performance Standards for Antimicrobial Susceptibility Testing; 22th Informational Supplement. Available from: https://clsi.org/ [Google Scholar]
- 13.Sherwin R, Winters ME, Vilke GM, et al. Does early and appropriate antibiotic administration improve mortality in Emergency Department patients with severe sepsis or septic shock? J Emerg Med. 2017;53(4):588–95. doi: 10.1016/j.jemermed.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Feng Y, Palanisami A, Kuriakose J, et al. Novel rapid test for detecting carbapenemase. Emerg Infect Dis. 2020;26(4):793–95. doi: 10.3201/eid2604.181655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sattler J, Brunke A, Hamprecht A. Evaluation of CARBA PAcE, a novel rapid test for detection of carbapenemase-producing Enterobacterales. J Med Microbiol. 2021;70(2):001290. doi: 10.1099/jmm.0.001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teethaisong Y, Nakouti I, Evans K, et al. Nitro-Carba test, a novel and simple chromogenic phenotypic method for rapid screening of carbapenemase-producing Enterobacteriaceae. J Glob Antimicrob Resist. 2019;18:22–25. doi: 10.1016/j.jgar.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Zhong Q, Cheng F, Liang J, et al. Profiles of volatile indole emitted by Escherichia coli based on CDI-MS. Sci Rep. 2019;9(1):13139. doi: 10.1038/s41598-019-49436-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ratiu IA, Ligor T, Bocos-Bintintan V, et al. Mass spectrometric techniques for the analysis of volatile organic compounds emitted from bacteria. Bioanalysis. 2017;9(14):1069–92. doi: 10.4155/bio-2017-0051. [DOI] [PubMed] [Google Scholar]
- 19.Cox CD, Parker J. Use of 2-aminoacetophenone production in identification of Pseudomonas aeruginosa. J Clin Microbiol. 1979;9(4):479–84. doi: 10.1128/jcm.9.4.479-484.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filipiak W, Zuchowska K, Marszalek M, et al. GC-MS profiling of volatile metabolites produced by Klebsiella pneumoniae. Front Mol Biosci. 2022;9:1019290. doi: 10.3389/fmolb.2022.1019290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li F, Zheng Y, Zhao C, et al. GC-IMS facilitates identification of carbapenem-resistant Klebsiella pneumoniae in simulated blood cultures. AMB Express. 2024;14(1):40. doi: 10.1186/s13568-024-01708-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo H, Hang Y, Zhu H, et al. Rapid identification of carbapenemase-producing Klebsiella pneumoniae using headspace solid-phase microextraction combined with gas chromatography-mass spectrometry. Infect Drug Resist. 2023;16:2601–9. doi: 10.2147/IDR.S404742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smart A, de Lacy Costello B, White P, et al. Sniffing out resistance – rapid identification of urinary tract infection-causing bacteria and their antibiotic susceptibility using volatile metabolite profiles. J Pharm Biomed Anal. 2019;167:59–65. doi: 10.1016/j.jpba.2019.01.044. [DOI] [PubMed] [Google Scholar]
- 24.Kanu AB, Hill HH., Jr Ion mobility spectrometry detection for gas chromatography. J Chromatogr A. 2008;1177(1):12–27. doi: 10.1016/j.chroma.2007.10.110. [DOI] [PubMed] [Google Scholar]
- 25.Nazareth J, Pan D, Kim JW, et al. Discriminatory ability of gas chromatography-ion mobility spectrometry to identify patients hospitalized with COVID-19 and predict prognosis. Open Forum Infect Dis. 2022;9(11):ofac509. doi: 10.1093/ofid/ofac509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Speckbacher V, Zeilinger S, Zimmermann S, et al. Monitoring the volatile language of fungi using gas chromatography-ion mobility spectrometry. Anal Bioanal Chem. 2021;413(11):3055–67. doi: 10.1007/s00216-021-03242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao Z, Xu P. Acetoin metabolism in bacteria. Crit Rev Microbiol. 2007;33(2):127–40. doi: 10.1080/10408410701364604. [DOI] [PubMed] [Google Scholar]
- 28.Lopez JM, Thoms B, Rehbein H. Acetoin degradation in Bacillus subtilis by direct oxidative cleavage. Eur J Biochem. 1975;57(2):425–30. doi: 10.1111/j.1432-1033.1975.tb02317.x. [DOI] [PubMed] [Google Scholar]
- 29.Kiviranta H, Tuomainen A, Reiman M, et al. Qualitative identification of volatile metabolites from two fungi and three bacteria species cultivated on two media. Cent Eur J Public Health. 1998;6(4):296–99. [PubMed] [Google Scholar]
- 30.Rees CA, Smolinska A, Hill JE. The volatile metabolome of Klebsiella pneumoniae in human blood. J Breath Res. 2016;10(2):027101. doi: 10.1088/1752-7155/10/2/027101. [DOI] [PubMed] [Google Scholar]
- 31.Wang M, Earley M, Chen L, et al. Clinical outcomes and bacterial characteristics of carbapenem-resistant Klebsiella pneumoniae complex among patients from different global regions (CRACKLE-2): A prospective, multicentre, cohort study. Lancet Infect Dis. 2022;22(3):401–12. doi: 10.1016/S1473-3099(21)00399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen YB, Ji JR, Liu ZY, et al. [BRICS report of 2021: The distribution and antimicrobial resistance profile of clinical bacterial isolates from bloodstream infection in China]]. Chin J Clin Infect Dis. 2023;16(1):33–47. [in Chinese] [Google Scholar]
- 33.Sy SKB, Zhuang L, Sy S, et al. Clinical pharmacokinetics and pharmacodynamics of ceftazidime-avibactam combination: A model-informed strategy for its clinical development. Clin Pharmacokinet. 2019;58(5):545–64. doi: 10.1007/s40262-018-0705-y. [DOI] [PubMed] [Google Scholar]
- 34.Chen AY, Thomas PW, Stewart AC, et al. Dipicolinic acid derivatives as inhibitors of New Delhi metallo-beta-lactamase-1. J Med Chem. 2017;60(17):7267–83. doi: 10.1021/acs.jmedchem.7b00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Euler M, Perl T, Eickel I, et al. Blood culture headspace gas analysis enables early detection of Escherichia coli bacteremia in an animal model of sepsis. Antibiotics (Basel) 2022;11(8):992. doi: 10.3390/antibiotics11080992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Y, Li X, Yang X, et al. Genome-wide identification and molecular characterization of the AP2/ERF superfamily members in sand pear (Pyrus pyrifolia) BMC Genomics. 2023;24(1):32. doi: 10.1186/s12864-022-09104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kotrba P, Inui M, Yukawa H. Bacterial phosphotransferase system (PTS) in carbohydrate uptake and control of carbon metabolism. J Biosci Bioeng. 2001;92(6):502–17. doi: 10.1263/jbb.92.502. [DOI] [PubMed] [Google Scholar]
- 38.Lengeler JW, Jahreis K. Bacterial PEP-dependent carbohydrate: phosphotransferase systems couple sensing and global control mechanisms. Contrib Microbiol. 2009;16:65–87. doi: 10.1159/000219373. [DOI] [PubMed] [Google Scholar]
- 39.Chen C, Wei D, Shi J, et al. Mechanism of 2,3-butanediol stereoisomer formation in Klebsiella pneumoniae. Appl Microbiol Biotechnol. 2014;98(10):4603–13. doi: 10.1007/s00253-014-5526-9. [DOI] [PubMed] [Google Scholar]
- 40.Kiratisin P, Keel RA, Nicolau DP. Pharmacodynamic profiling of doripenem, imipenem and meropenem against prevalent Gram-negative organisms in the Asia-Pacific region. Int J Antimicrob Agents. 2013;41(1):47–51. doi: 10.1016/j.ijantimicag.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Coleman K, Levasseur P, Girard AM, et al. Activities of ceftazidime and avibactam against beta-lactamase-producing Enterobacteriaceae in a hollow-fiber pharmacodynamic model. Antimicrob Agents Chemother. 2014;58(6):3366–72. doi: 10.1128/AAC.00080-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Livermore DM, Mushtaq S, Warner M, et al. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2011;55(1):390–94. doi: 10.1128/AAC.00756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shirley M. Ceftazidime-Avibactam: A review in the treatment of serious gram-negative bacterial infections. Drugs. 2018;78(6):675–92. doi: 10.1007/s40265-018-0902-x. [DOI] [PubMed] [Google Scholar]
- 44.Hinchliffe P, Tanner CA, Krismanich AP, et al. Structural and kinetic studies of the potent inhibition of metallo-beta-lactamases by 6-phosphonomethylpyridine-2-carboxylates. Biochemistry. 2018;57(12):1880–92. doi: 10.1021/acs.biochem.7b01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durante-Mangoni E, Andini R, Zampino R. Management of carbapenem-resistant Enterobacteriaceae infections. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2019;25(8):943–50. doi: 10.1016/j.cmi.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 46.Zhang L, Shuai Q, Li P, et al. Ion mobility spectrometry fingerprints: A rapid detection technology for adulteration of sesame oil. Food Chem. 2016;192:60–66. doi: 10.1016/j.foodchem.2015.06.096. [DOI] [PubMed] [Google Scholar]
- 47.Yu H, Xu XS, Li M, et al. [Expert consensus statement on laboratory detection and clinical report of carbapenemase among Enterobacterales (second edition)]. Chin J Infect Chemother. 2022;22(4):463–74. [in Chinese] [Google Scholar]