ABSTRACT
Traditionally, successful vaccines rely on specific adaptive immunity by activating lymphocytes with an attenuated pathogen, or pathogen subunit, to elicit heightened responses upon subsequent exposures. However, recent work with Mycobacterium tuberculosis and other pathogens has identified a role for “trained” monocytes in protection through memory-like but non-specific immunity. Here, we used an in vitro co-culture approach to study the potential role of trained macrophages, including lung alveolar macrophages, in immune responses to the Live Vaccine Strain (LVS) of Francisella tularensis. F. tularensis is an intracellular bacterium that replicates within mammalian macrophages and causes respiratory as well as systemic disease. We vaccinated mice with F. tularensis LVS and then obtained lung alveolar macrophages, or derived macrophages from bone marrow. LVS infected and replicated comparably in both types of macrophages, whether naïve or from LVS-vaccinated mice. LVS-infected macrophages were then co-cultured with either naïve splenocytes, splenocytes from mice vaccinated intradermally, or splenocytes from mice vaccinated intravenously. For the first time, we show that immune (but not naïve) splenocytes controlled bacterial replication within alveolar macrophages, similar to previous results using bone marrow-derived macrophage. However, no differences in control of intramacrophage bacterial replication were found between co-cultures with naïve macrophages or macrophages from LVS-vaccinated mice; furthermore, nitric oxide levels and interferon-gamma production in supernatants were largely comparable across all conditions. Thus, in the context of in vitro co-cultures, the data do not support development of trained macrophages in bone marrow or lungs of mice vaccinated with LVS intradermally or intravenously.
IMPORTANCE
The discovery of non-specific “trained immunity” in monocytes has generated substantial excitement. However, to date, training has been studied with relatively few microbes (e.g., Mycobacterium bovis Bacille Calmette-Guérin, a live attenuated intracellular bacterium used as a vaccine) and microbial substances (e.g., LPS), and it remains unclear whether training during infection is common. We previously demonstrated that vaccination of mice with Francisella tularensis Live Vaccine Strain (LVS), another live attenuated intracellular bacterium, protected against challenge with the unrelated bacterium Listeria monocytogenes. The present study therefore tested whether LVS vaccination engenders trained macrophages that contributed to this protection. To do so, we used a previous in vitro co-culture approach with murine bone marrow-derived macrophages to expand and study lung alveolar macrophages. We demonstrated that alveolar macrophages can be productively infected and employed to characterize interactions with LVS-immune lymphocytes. However, we find no evidence that either bone marrow-derived or alveolar macrophages are trained by LVS vaccination.
KEYWORDS: Francisella LVS, macrophages, trained immunity, alveolar
INTRODUCTION
Vaccination targets adaptive immunity mediated by memory T and B lymphocytes, activating the immune system with an attenuated pathogen or a pathogen subunit to elicit faster, stronger responses upon subsequent exposures. Recent work in experimental models as well as clinical studies have suggested that vaccination may also stimulate durable immunological memory in innate immune cells, dubbed “trained immunity.” Trained immunity appears to be mediated by epigenetic, metabolic, and functional reprogramming of innate myeloid and lymphoid immune cells (1, 2). Stimulation of innate immune cells leads to the development of chromatin marks in cellular DNA, such as histone acetylation and methylation. These marks promote the unfolding of chromatin, thus facilitating transcription and expression of genes that regulate production of proinflammatory factors. Residues of these changes remain even after the stimulus has been removed, promoting enhanced gene expression after secondary challenge (3).
A growing body of evidence supports that innate immune memory is induced at both the central and local levels in the bone marrow and tissues, respectively. While earlier studies identified the presence of trained bone marrow progenitors (4, 5), recent findings support the development of trained tissue resident monocytes, including microglia and alveolar macrophages (AMØs), with increased glycolytic metabolism and chemokine production; these responses are functional hallmarks of trained immunity (6–8). For example, Yao et al. (7) demonstrated that an adenovirus infection can induce a lasting memory phenotype in alveolar macrophages, associated with upregulation of MHC II, host defense genes, glycolytic activity, and neutrophil chemokine responses.
The development of trained immunity is further supported by the functional role of “trained” innate cells. Epidemiological studies have shown non-specific protection from live attenuated vaccines, including vaccines against tuberculosis, polio, smallpox, and measles (9). For instance, the tuberculosis vaccine Bacille Calmette-Guérin (BCG) has prompted non-specific protection against human papillomavirus, respiratory syncytial virus, influenza virus, and herpes simplex virus (10). An important example comes from a randomized controlled study in people, which found that administration of BCG protected against experimental infection with an attenuated yellow fever vaccine strain. The reduced viral load was correlated with upregulation of interleukin 1β (IL-1β), a cytokine associated with trained immunity (11). Remarkably, BCG vaccination has also been associated with reductions in morbidity and mortality from malignancies and autoimmune diseases, presumably through induction of innate immune memory mechanisms (1, 2, 12). Moreover, in mice, BCG vaccination has shown protective effects against challenge with Staphylococcus aureus, Candida albicans, Schistosoma mansoni, influenza, hepatitis, and HSV (1, 2, 10, 12).
Given the compelling data on BCG’s ability to induce trained immunity, we evaluated the development of trained immunity following administration of another live attenuated bacterial vaccine, Francisella tularensis Live Vaccine Strain (LVS). In the USA, LVS has been studied as an investigational vaccine against tularemia for many years (13). Similar to Mycobacterium tuberculosis, F. tularensis is an intracellular bacterium that infects macrophages. Additionally, immune responses to both F. tularensis and M. tuberculosis are characterized by dominance of Th1 T cell responses. We have used LVS vaccination as a biosafety level 2 model in mice and rats to study the nature of innate and T cell-mediated protective immunity to intracellular bacteria in general (14). Mice and rats are natural hosts for Francisella; both can be vaccinated with LVS by a variety of routes and then survive challenge with virulent F. tularensis by several routes (15, 16). Notably, LVS vaccination of mice also provides some protection against a secondary challenge with Listeria monocytogenes administered soon after vaccination (17). This protection depends on interferon-γ (IFN-γ) (17), but otherwise, the mechanisms involved are unknown.
In previous studies, we have used an in vitro co-culture system to study mechanisms by which immune T cells control the replication of Francisella or M. tuberculosis within infected macrophages. The relevance of the in vitro approach to in vivo host-pathogen interactions has been amply demonstrated by its success in predicted vaccine-induced protection of mice and rats challenged in vivo (18–21). However, to date, only bone marrow-derived macrophages (BMMØs) have been used in this system. Because training can depend on the route of vaccination, here we adapted this in vitro co-culture approach to evaluate control of bacterial replication in alveolar macrophages as well. We then used both methods to study the potential roles of myeloid trained immunity in Francisella infections and to examine whether LVS administered intradermally (i.d.) or intravenously (i.v.) stimulates trained immunity.
RESULTS AND DISCUSSION
Intramacrophage LVS growth and lymphocyte-mediated control of bacterial growth is comparable in BMMØs from naïve or parenterally vaccinated mice
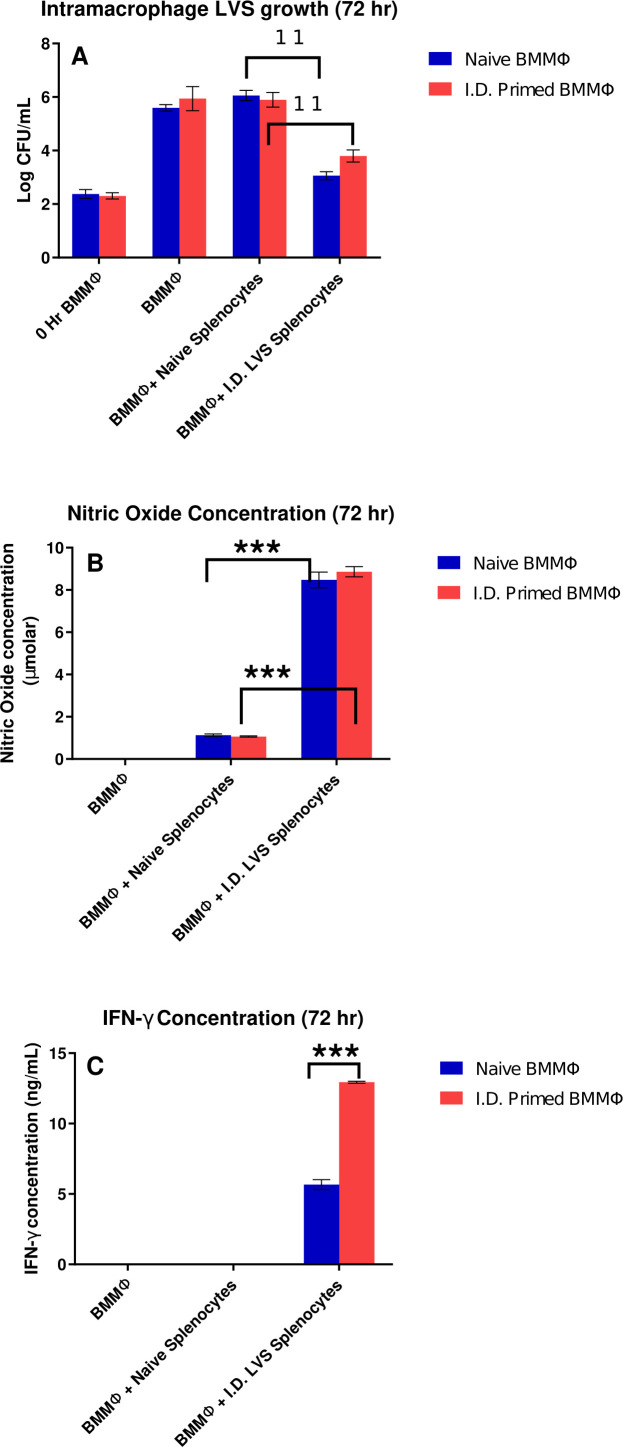
BMMØs from naïve mice and from mice vaccinated with LVS i.d. were infected with LVS, and some LVS-infected BMMØs were co-cultured with naïve or immune splenocytes. After 72 hours, supernatants were recovered, and BMMØs were lysed to determine bacterial CFU. Bacterial uptake at the start of infection (0 hour) and after 72 hours was similar in BMMØs from naïve or vaccinated mice (Fig. 1A). LVS bacterial replication was not controlled in either type of BMMØs when co-cultured with naïve splenocytes. In contrast, intramacrophage bacterial replication was significantly controlled by LVS-immune lymphocytes but was comparable when using BMMØs derived from either naïve mice or mice vaccinated i.d. with LVS (Fig. 1A).
Fig 1.
Bacterial growth and lymphocyte-mediated control of in vitro bacterial replication is comparable when using BMMØs from naïve mice or mice vaccinated i.d. with LVS. BMMØs were prepared in 24-well plates from naïve mice or from mice vaccinated with LVS i.d. Confluent macrophages were infected with LVS, and then naïve or immune splenocytes were overlaid on LVS-infected macrophages. Three days later, macrophages were lysed and plated to count recovered CFU/mL (panel A). In panel A, ** denotes a significant difference of P < 0.01 by two-tailed Student’s t-test with equal variance: co-cultures with naïve BMMØs and naïve splenocytes were significantly different from those with naïve BMMØs and splenocytes from i.d. LVS-immune mice; similarly, co-cultures with BMMØs from i.d.-primed mice and naïve splenocytes were significantly different from those with BMMØs from i.d.-primed mice and i.d. LVS-immune splenocytes. Supernatants collected from co-cultures were assessed for levels of nitric oxide (panel B) or for IFN-γ (panel C). Values shown are mean ± SD from triplicate wells of one representative experiment of three experiments of similar design and outcome. In panel C, *** denotes a significant difference of P < 0.001 by two-tailed Student’s t-test with equal variance for the same combinations described in panel A. Differences noted as significant were found in all three replicate experiments.
To further assess potential differences between activities of BMMØs from naïve mice and i.d. LVS-vaccinated mice, supernatants collected from all cultures were assessed for levels of nitric oxide (NO) (Fig. 1B) and IFN-γ (Fig. 1C), both of which regulate intramacrophage bacterial replication. Neither IFN-γ nor NO were detected in supernatants from LVS-infected BMMØs alone. NO levels were similar in supernatants from co-cultures with BMMØs from naïve mice compared to those from i.d. LVS-vaccinated mice. Of note, however, IFN-γ levels were consistently twofold higher in co-cultures with BMMØs from LVS-vaccinated mice (Fig. 1C).
Intramacrophage LVS growth and lymphocyte-mediated control of bacterial growth is comparable in AMØs from naïve or parenterally-vaccinated mice
As noted, to date, previous studies have utilized rodent bone marrow-derived macrophages to quantitate immune lymphocyte-mediated control of intracellular bacterial growth (18–23). Respiratory exposure is an important route of Francisella infection, and tissue resident alveolar macrophages are the first target of infection (24). Therefore, we evaluated macrophages derived from alveolar lavage, including alveolar macrophages obtained from naïve and mice vaccinated i.d. with LVS. Bacterial uptake and replication over 3 days was similar in AMØs from naïve or i.d. LVS-vaccinated mice (Fig. 2). Notably, uptake and growth of LVS over 3 days was similar between AMØs (Fig. 2) and BMMØs (Fig. 1). Further, naïve splenocytes did not inhibit intramacrophage bacterial replication in either type of macrophages. In contrast, splenocytes from mice vaccinated i.d. with LVS significantly controlled LVS growth within AMØs, and control was comparable when using AMØs derived from either naïve mice or mice vaccinated i.d. with LVS (Fig. 2). Thus, the in vitro co-culture approach detects immune lymphocyte-mediated bacterial growth control within this relevant type of macrophages. Supernatants collected from co-cultures were evaluated for NO and IFN-γ levels, but surprisingly concentrations for both were below the limit of detection for all tested conditions (H. Khan, unpublished data; the limit of detection was approximately 0.2 μM for NO and 100 pg/mL for IFN-γ).
Fig 2.
Bacterial growth and lymphocyte-mediated control of in vitro bacterial replication was comparable when using AMØs from naïve mice or mice vaccinated i.d. with LVS. AMØs were prepared in 24-well plates from naïve mice or from mice vaccinated with LVS i.d. Confluent macrophages were infected with LVS, and then naïve splenocytes or splenocytes from mice vaccinated with LVS i.d. were overlaid on LVS-infected macrophages. Three days later, macrophages were lysed and plated on agar to count recovered bacteria. Numbers of bacterial CFU ± SD from triplicate wells of the indicated combinations from one representative experiment of three experiments of similar design and outcome are shown. *** denotes a significant difference of P < 0.001 by two-tailed Student’s t-test with equal variance: co-cultures with naïve AMØs and naïve splenocytes were significantly different from those with naïve AMØs and splenocytes from i.d. LVS-immune mice; similarly, co-cultures with AMØs from i.d.-primed mice and naïve splenocytes were significantly different from those with AMØs from i.d.-primed mice and i.d. LVS-immune splenocytes. Differences noted as significant were found in all three replicate experiments.
Intramacrophage LVS growth and lymphocyte-mediated control of bacterial growth is comparable in BMMØs from naïve or systemically-vaccinated mice
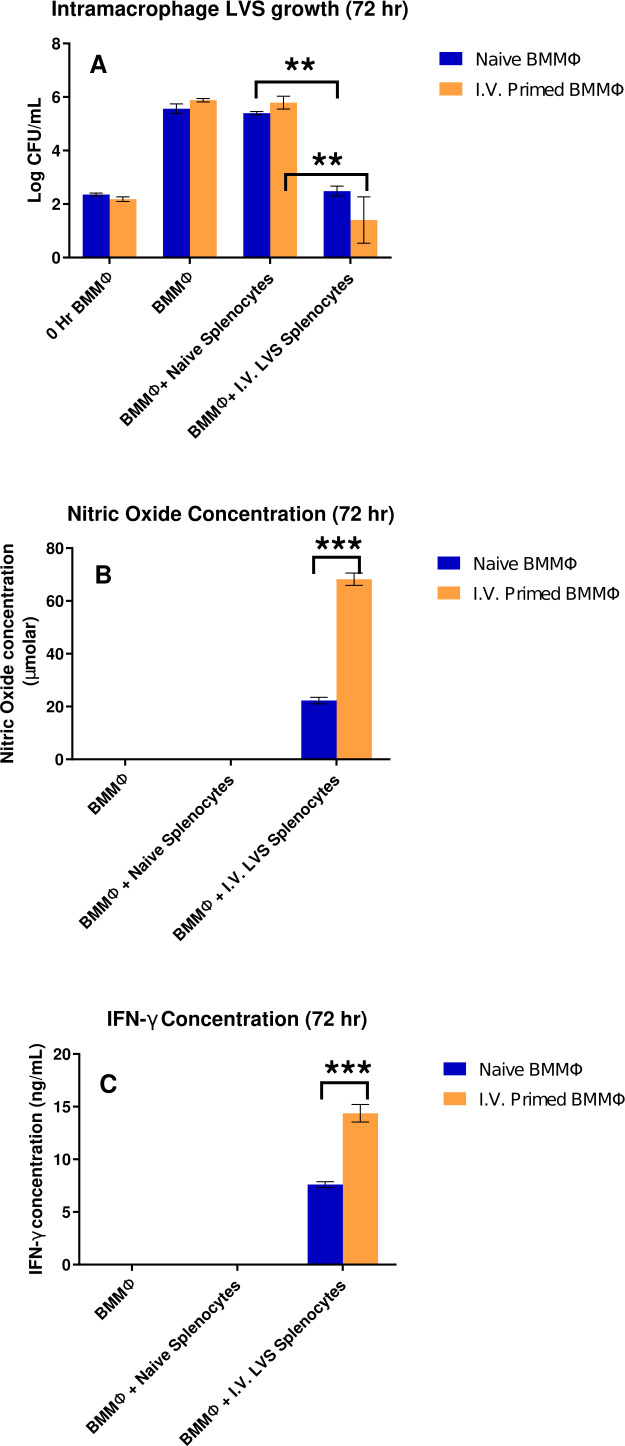
Previous studies demonstrated that intravenous BCG vaccination stimulated greater degrees of trained immunity compared to intradermal administration (25–27). We performed experiments of similar design using mice vaccinated with LVS i.v. Similar to results obtained when using lymphocytes from i.d. vaccinated mice, bacterial uptake, bacterial replication, and lymphocyte-mediated control of in vitro bacterial replication were all comparable when using BMMØs from naïve mice or mice vaccinated i.v. with LVS. Bacterial replication was not controlled in co-cultures with naïve splenocytes, and immune lymphocyte-mediated control was significant compared to co-cultures with naïve splenocytes (Fig. 3A). However, NO production (Fig. 3B) as well as IFN-γ levels (Fig. 3C) in co-cultures were consistently two fold higher in co-cultures using BMMØs from mice vaccinated with LVS i.v. These observations are similar to that seen for IFN-γ levels in co-cultures with BMMØs from LVS-vaccinated mice (Fig. 1C). The biological significance of these relatively subtle in vitro differences is not clear, particularly in light of the lack of differences in control of bacterial growth (Fig. 1A and 3A). Taken together, we do not consider these small in vitro differences to be evidence of macrophage training during LVS vaccination.
Fig 3.
Lymphocyte-mediated control of in vitro bacterial replication was comparable when using BMMØs from naïve mice or mice vaccinated i.v. with LVS. BMMØs were prepared in 24-well plates from naïve mice or from mice vaccinated with LVS i.v. Confluent macrophages were infected with LVS, and then naïve splenocytes or splenocytes from mice vaccinated with LVS i.v. were overlaid on LVS-infected macrophages. Three days later, macrophages were lysed and plated to count recovered CFU/mL (panel A). In panel A, ** denotes a significant difference of P < 0.01 by two-tailed Student’s t-test with equal variance: co-cultures with naïve BMMØs and naïve splenocytes were significantly different from those with naïve BMMØs and splenocytes from i.v. LVS-immune mice; similarly, co-cultures with BMMØs from i.v.-primed mice and naïve splenocytes were significantly different from those with BMMØs from i.v.-primed mice and i.v. LVS-immune splenocytes. Supernatants collected from co-cultures were assessed for levels of nitric oxide (panel B) or for IFN-γ (panel C). Values shown are mean ± SD from triplicate wells of one representative experiment of three experiments of similar design and outcome. In panels B and C, *** denotes a significant difference of P < 0.001 by two-tailed Student’s t-test with equal variance for the same combinations described in panel A. Differences noted as significant were found in all three replicate experiments.
Intramacrophage LVS growth and lymphocyte-mediated control of bacterial growth is comparable in AMØs from naïve or parenterally-vaccinated mice
Finally, we studied outcomes in co-culture assays using alveolar macrophages obtained from either naïve mice or mice vaccinated with LVS i.v. Replication of bacteria was similar when using AMØs from naïve mice or mice vaccinated i.v. with LVS. As seen with the BMMØs, naïve splenocytes did not inhibit intramacrophage bacterial replication in AMØs co-cultured with naïve splenocytes. In contrast, AMØs co-cultured with splenocytes from mice vaccinated i.v. significantly controlled the intramacrophage LVS growth, and control was comparable when AMØs were obtained from naïve mice or i.v.-vaccinated mice (Fig. 4). Supernatants collected from co-cultures were evaluated for NO and IFN-γ levels. However, similar to results using BMMØs, the concentrations were below the limit of detection for all tested conditions (Khan, unpublished data).
Fig 4.
Lymphocyte-mediated control of in vitro bacterial replication was comparable when using AMØs from naïve mice or mice vaccinated i.v. with LVS. AMØs were prepared in 96-well plates from naïve mice or from mice vaccinated with LVS i.v. Confluent macrophages were infected with LVS, and then naïve splenocytes or splenocytes from mice vaccinated with LVS i.v. were overlaid on LVS-infected macrophages. Three days later, macrophages were lysed and plated to count recovered bacteria. Numbers of bacterial CFU ± SD from triplicate wells of the indicated combinations from one representative experiment of three experiments of similar design and outcome are shown. ** denotes a significant difference of P < 0.01 by two-tailed Student’s t-test with equal variance: co-cultures with naïve AMØs and naïve splenocytes were significantly different from those with naïve AMØs and splenocytes from i.v. LVS-immune mice; similarly, co-cultures with AMØs from i.v.-primed mice and naïve splenocytes were significantly different from those with AMØs from i.v.-primed mice and i.v. LVS-immune splenocytes. Differences noted as significant were found in all three replicate experiments.
Despite having similar biology, our findings demonstrate that not all live attenuated intracellular bacterial vaccines readily induce trained monocytes or macrophages. Of note, the prototype training example, Mycobacterium bovis BCG, is highly inflammatory, sufficient to effectively treat bladder cancer (28). LVS is not particularly inflammatory, and its LPS has minimal activity (29–31). That said, LVS vaccination activated sufficient responses, including IFN-γ production, to transiently provide nonspecific protection against Listeria challenge (17). The present results suggest that this outcome is not likely to be mediated by trained immunity.
The results shown here, and the interpretations, are limited by reliance on in vitro approaches that focused on bacterial replication. We did not evaluate epigenetic signatures, another hallmark of “trained” immunity, due to lack of functional evidence. In general, the findings do not rule out the development of trained myeloid cells in response to LVS vaccination. These studies were designed to evaluate the possible development of monocyte training by LVS vaccination in order to consider further in vivo evaluations. The latter could include studies in mice lacking a presumed critical component of training, such as hypoxia-inducible factor 1-α (32). Nonetheless, in light of the results shown here and the appropriate interest in limiting the use of experimental animals, it is difficult to justify further animal-intensive studies.
Importantly, these studies further lay the groundwork for future studies of interactions between alveolar macrophages and immune lymphocytes, demonstrating that the in vitro co-culture approach readily detects immune functions relevant to vaccine-induced bacterial control. The unexpected observation of strong control that was not accompanied by detectable NO or IFN-γ production will be an important topic for future studies. For the present, the results shown here collectively support the conclusion that intramacrophage Francisella growth as well as lymphocyte-mediated control of in vitro Francisella LVS replication are comparable when using macrophages derived from the bone marrow or the lungs of naïve mice or mice vaccinated with LVS either parenterally or systemically. Therefore, in the context of the in vitro co-culture system, the data to date do not support substantial development of trained macrophages in LVS-vaccinated mice.
MATERIALS AND METHODS
Experimental animals
Six- to 14-week-old male C57BL/6J mice from Jackson Laboratories (Bar Harbor, ME, USA) were housed in microisolator cages. All mice were fed ad libitum with autoclaved food and water. Within each experiment, all animals were age matched. Infection studies included frequent observations and observed humane endpoints. At the indicated time points or at the end of a study, animals were euthanized with carbon dioxide inhalation in a euthanasia chamber where carbon dioxide was introduced at the rate of at least 20% of the chamber volume per minute, or by ketamine-xylazine overdose where indicated. No animals were subjected to anesthesia. No illness or death due to experimental infections was expected in these studies, but any mice that unexpectedly exhibited more than 20% weight loss, or that were unable to ambulate sufficiently to obtain water or food, were humanely sacrificed by carbon dioxide inhalation.
Immunizations
For each experiment, mice were vaccinated either i.d. or i.v. For i.d. injections, mice were given 1 × 104 LVS in 100 µL phosphate buffered saline (PBS) at the base of the tail. Intravenous injections were administered into the lateral tail vein at a dose of 1 × 102 LVS in 200 µL PBS. Actual vaccination doses were confirmed retrospectively by plating the inoculum onto Mueller Hinton (MH) plates. At 8 weeks after vaccination, mice were humanely euthanized and cells from the lungs, bone marrow, or spleens were harvested for in vitro assays.
Bacteria and growth conditions
Francisella tularensis LVS (ATCC 29684) was used to grow a working stock as described in detail previously (18). Briefly, F. tularensis LVS was streaked on MH plates (33); a single colony was then isolated and grown to mid-log phase in supplemented MH broth prior to freezing aliquots at −80°C. The subsequent working stock was quality controlled as previously described (15).
Culture of LVS-infected macrophages and co-culture with splenocytes from LVS-vaccinated animals
Mouse BMMØs were cultured and infected with LVS as described previously (18). Briefly, BMMØs were cultured in complete Dulbecco's modified Eagle medium (DMEM with 10% heat-inactivated fetal calf serum, 0.2 mM L-glutamine, 0.1 mM non-essential amino acids, 10 mM HEPES buffer, 1 mM sodium pyruvate, and 1 mM sodium bicarbonate) and macrophage colony stimulating factor (M-CSF) in 24-well plates. After 7 days, triplicate confluent macrophage monolayers were infected for 2 hours with F. tularensis LVS at a multiplicity of infection (MOI) of 1:50 (bacterium to BMMØ ratio). Monolayers were then washed, treated with 50 µg/mL gentamicin for 45 minutes, and then washed again with antibiotic-free medium. To measure uptake at 0 hours, some macrophages were lysed with water and plated for bacterial counts. Single-cell suspensions of splenocytes from vaccinated or naïve mice were added to LVS-infected macrophages at a concentration of 5 × 106 cells/well. After 72 hours, supernatants were collected and stored at −80°C for further analyses, and the remaining macrophages were lysed and plated for bacterial counts.
Mouse AMØs were prepared through bronchial alveolar lavage (BAL), cultured, and infected with LVS as described above for BMMØs with minor revisions. Briefly, for the BAL, mice were euthanized through an intraperitoneal injection of a ketamine-xylazine cocktail at 0.8 mL per mouse. BAL was collected via insertion of a cannula through a 20G × 1 1/4-inch needle. The cannula was used to flush cells from the lungs with cold wash buffer (PBS with 2% fetal bovine serum and 0.5 mM EDTA). The cells were then pelleted and re-suspended in complete media (DMEM with 10% heat-inactivated fetal calf serum, 0.2 mM L-glutamine, 0.1 mM non-essential amino acids, 10 mM HEPES buffer, 1 mM sodium pyruvate, and 55 mM 2-β-mercaptoethanol) with 2.5 µg/mL amphotericin B and 10 µg/mL ampicillin for 1 hour on ice. After the incubation, cells were washed again and re-suspended in antibiotic-free media. Cells were cultured in media in 96-well plates at 1.25 × 105 cells/well. The next day, triplicate confluent macrophage monolayers were infected for 2 hours with F. tularensis LVS at an MOI of 2:1 (bacteria to AMØ ratio). Monolayers were then washed, treated with 50 µg/mL gentamicin for 45 minutes, and then washed again with antibiotic-free medium. Single-cell suspensions of splenocytes from vaccinated or naïve mice were added to LVS-infected macrophages at a concentration of 2.5 × 105 cells/well. After 72 hours, macrophages were lysed and plated for bacterial counts.
Cytokine and nitric oxide determination in co-culture supernatants
Supernatants recovered from co-cultures were assayed for mouse IFN-γ and nitric oxide. Mouse IFN-γ was quantitated using a sandwich enzyme linked immunosorbent assay, according to the manufacturer’s instructions (BD Pharmingen, San Jose, CA, USA). Cytokine concentrations were determined by comparing unknown values to a standard curve of recombinant mouse IFN-γ protein (BD Biosciences) at known values. The limit of detection for IFN-γ was estimated as 90 pg/mL. Nitric oxide levels were measured using the Griess reaction and quantified by comparison to serially diluted NaNO2 as a standard. The limit of detection for NO was estimated as 0.5 µM.
Statistical analyses
The significance of differences between the indicated samples was assessed using GraphPad Prism, applying a two-tailed Student’s t-test as described in the figure legends, with a P-value of <0.05 considered significant.
ACKNOWLEDGMENTS
We are grateful to our colleagues Mustafa Akkoyunlu, Roberto De Pascalis, and Sherry Kurtz for thoughtful and helpful reviews of the manuscript.
Contributor Information
Karen L. Elkins, Email: karen.elkins@fda.hhs.gov.
Jose Martinez-Navio, University of Miami, Miami, Florida, USA.
ETHICS APPROVAL
All experiments were performed under protocols approved by the Animal Care and Use Committee (ACUC) of CBER/FDA (Animal Study Protocol #1993-03). These protocols meet the standards for humane animal care and use set by the Guide for the Care and Use of Laboratory Animals and PHS policy.
REFERENCES
- 1. Netea MG, Joosten LAB, Latz E, Mills KHG, Natoli G, Stunnenberg HG, O’Neill LAJ, Xavier RJ. 2016. Trained immunity: a program of innate immune memory in health and disease. Science 352:aaf1098. doi: 10.1126/science.aaf1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, Joosten LAB, van der Meer JWM, Mhlanga MM, Mulder WJM, Riksen NP, Schlitzer A, Schultze JL, Stabell Benn C, Sun JC, Xavier RJ, Latz E. 2020. Defining trained immunity and its role in health and disease. Nat Rev Immunol 20:375–388. doi: 10.1038/s41577-020-0285-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fanucchi S, Fok ET, Dalla E, Shibayama Y, Börner K, Chang EY, Stoychev S, Imakaev M, Grimm D, Wang KC, Li G, Sung WK, Mhlanga MM. 2019. Immune genes are primed for robust transcription by proximal long noncoding RNAs located in nuclear compartments. Nat Genet 51:138–150. doi: 10.1038/s41588-018-0298-2 [DOI] [PubMed] [Google Scholar]
- 4. Kaufmann E, Sanz J, Dunn JL, Khan N, Mendonça LE, Pacis A, Tzelepis F, Pernet E, Dumaine A, Grenier JC, Mailhot-Léonard F, Ahmed E, Belle J, Besla R, Mazer B, King IL, Nijnik A, Robbins CS, Barreiro LB, Divangahi M. 2018. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 172:176–190. doi: 10.1016/j.cell.2017.12.031 [DOI] [PubMed] [Google Scholar]
- 5. Mitroulis I, Ruppova K, Wang B, Chen L-S, Grzybek M, Grinenko T, Eugster A, Troullinaki M, Palladini A, Kourtzelis I, et al. 2018. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 172:147–161. doi: 10.1016/j.cell.2017.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wendeln AC, Degenhardt K, Kaurani L, Gertig M, Ulas T, Jain G, Wagner J, Häsler LM, Wild K, Skodras A, Blank T, Staszewski O, Datta M, Centeno TP, Capece V, Islam MR, Kerimoglu C, Staufenbiel M, Schultze JL, Beyer M, Prinz M, Jucker M, Fischer A, Neher JJ. 2018. Innate immune memory in the brain shapes neurological disease hallmarks. Nature 556:332–338. doi: 10.1038/s41586-018-0023-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yao Y, Jeyanathan M, Haddadi S, Barra NG, Vaseghi-Shanjani M, Damjanovic D, Lai R, Afkhami S, Chen Y, Dvorkin-Gheva A, Robbins CS, Schertzer JD, Xing Z. 2018. Induction of autonomous memory alveolar macrophages requires T cell help and is critical to trained immunity. Cell 175:1634–1650. doi: 10.1016/j.cell.2018.09.042 [DOI] [PubMed] [Google Scholar]
- 8. Zahalka S, Starkl P, Watzenboeck ML, Farhat A, Radhouani M, Deckert F, Hladik A, Lakovits K, Oberndorfer F, Lassnig C, Strobl B, Klavins K, Matsushita M, Sanin DE, Grzes KM, Pearce EJ, Gorki AD, Knapp S. 2022. Trained immunity of alveolar macrophages requires metabolic rewiring and type 1 interferon signaling. Mucosal Immunol 15:896–907. doi: 10.1038/s41385-022-00528-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aaby P, Benn CS. 2019. Developing the concept of beneficial non-specific effect of live vaccines with epidemiological studies. Clin Microbiol Infect 25:1459–1467. doi: 10.1016/j.cmi.2019.08.011 [DOI] [PubMed] [Google Scholar]
- 10. Moorlag S, Arts RJW, van Crevel R, Netea MG. 2019. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect 25:1473–1478. doi: 10.1016/j.cmi.2019.04.020 [DOI] [PubMed] [Google Scholar]
- 11. Arts RJW, Moorlag S, Novakovic B, Li Y, Wang S-Y, Oosting M, Kumar V, Xavier RJ, Wijmenga C, Joosten LAB, Reusken C, Benn CS, Aaby P, Koopmans MP, Stunnenberg HG, van Crevel R, Netea MG. 2018. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe 23:89–100. doi: 10.1016/j.chom.2017.12.010 [DOI] [PubMed] [Google Scholar]
- 12. Covián C, Fernández-Fierro A, Retamal-Díaz A, Díaz FE, Vasquez AE, Lay MK, Riedel CA, González PA, Bueno SM, Kalergis AM. 2019. BCG-induced cross-protection and development of trained immunity: implication for vaccine design. Front Immunol 10:2806. doi: 10.3389/fimmu.2019.02806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Elkins KL, Kurtz SL, De Pascalis R. 2016. Progress, challenges, and opportunities in Francisella vaccine development. Expert Rev Vaccines 15:1183–1196. doi: 10.1586/14760584.2016.1170601 [DOI] [PubMed] [Google Scholar]
- 14. Elkins KL, Cowley SC, Bosio CM. 2003. Innate and adaptive immune responses to an intracellular bacterium, Francisella tularensis Live Vaccine Strain. Microbes Infect 5:135–142. doi: 10.1016/s1286-4579(02)00084-9 [DOI] [PubMed] [Google Scholar]
- 15. Conlan JW, Chen W, Bosio CM, Cowley SC, Elkins KL. 2011. Infection of mice with Francisella as an immunological model. Curr Protoc Immunol Chapter 19:Unit. doi: 10.1002/0471142735.im1914s93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu TH, Zsemlye JL, Statom GL, Hutt JA, Schrader RM, Scrymgeour AA, Lyons CR. 2009. Vaccination of Fischer 344 rats against pulmonary infections by Francisella tularensis type A strains. Vaccine 27:4684–4693. doi: 10.1016/j.vaccine.2009.05.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elkins KL, MacIntyre AT, Rhinehart-Jones TR. 1998. Nonspecific early protective immunity in Francisella and Listeria infections can be dependent on lymphocytes. Infect Immun 66:3467–3469. doi: 10.1128/IAI.66.7.3467-3469.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elkins KL, Cowley SC, Conlan JW. 2011. Measurement of macrophage-mediated killing of intracellular bacteria, including Francisella and Mycobacteria. Curr Protoc Immunol Chapter 14:Unit14. doi: 10.1002/0471142735.im1425s93 [DOI] [PubMed] [Google Scholar]
- 19. De Pascalis R, Frey B, Rice HM, Bhargava V, Wu TH, Peterson RL, Conlan JW, Sjöstedt A, Elkins KL. 2022. Working correlates of protection predict SchuS4-derived-vaccine candidates with improved efficacy against an intracellular bacterium, Francisella tularensis. NPJ Vaccines 7:95. doi: 10.1038/s41541-022-00506-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Pascalis R, Mittereder L, Kennett NJ, Elkins KL. 2016. Activities of murine peripheral blood lymphocytes provide immune correlates that predict Francisella vaccine efficacy. Infect Immun 84:1054–1061. doi: 10.1128/IAI.01348-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Pascalis R, Chou AY, Bosio CM, Huang C-Y, Follmann DA, Elkins KL. 2012. Development of functional and molecular correlates of vaccine-induced protection for a model intracellular pathogen, F. tularensis LVS. PLoS Pathog 8:e1002494. doi: 10.1371/journal.ppat.1002494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bosio CM, Elkins KL. 2001. Susceptibility to secondary Francisella tularensis LVS infection in B cell deficient mice is associated with neutrophilia but not with defects in specific T cell mediated immunity. Infect Immun 69:194–203. doi: 10.1128/IAI.69.1.194-203.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Collazo CM, Meierovics AI, De Pascalis R, Wu TH, Lyons CR, Elkins KL. 2009. T cells from lungs and livers of Francisella tularensis-immune mice control the growth of intracellular bacteria. Infect Immun 77:2010–2021. doi: 10.1128/IAI.01322-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hall JD, Woolard MD, Gunn BM, Craven RR, Taft-Benz S, Frelinger JA, Kawula TH. 2008. Infected-host-cell repertoire and cellular response in the lung following inhalation of Francisella tularensis Schu S4, LVS, or U112. Infect Immun 76:5843–5852. doi: 10.1128/IAI.01176-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Darrah PA, Zeppa JJ, Maiello P, Hackney JA, Wadsworth MH, Hughes TK, Pokkali S, Swanson PA, Grant NL, Rodgers MA, Kamath M, Causgrove CM, Laddy DJ, Bonavia A, Casimiro D, Lin PL, Klein E, White AG, Scanga CA, Shalek AK, Roederer M, Flynn JL, Seder RA. 2020. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature 577:95–102. doi: 10.1038/s41586-019-1817-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Verreck FAW, Tchilian EZ, Vervenne RAW, Sombroek CC, Kondova I, Eissen OA, Sommandas V, van der Werff NM, Verschoor E, Braskamp G, Bakker J, Langermans JAM, Heidt PJ, Ottenhoff THM, van Kralingen KW, Thomas AW, Beverley PCL, Kocken CHM. 2017. Variable BCG efficacy in rhesus populations: pulmonary BCG provides protection where standard intra-dermal vaccination fails. Tuberculosis (Edinb) 104:46–57. doi: 10.1016/j.tube.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 27. Sharpe S, White A, Sarfas C, Sibley L, Gleeson F, McIntyre A, Basaraba R, Clark S, Hall G, Rayner E, Williams A, Marsh PD, Dennis M. 2016. Alternative BCG delivery strategies improve protection against Mycobacterium tuberculosis in non-human primates: protection associated with mycobacterial antigen-specific CD4 effector memory T-cell populations. Tuberculosis (Edinb) 101:174–190. doi: 10.1016/j.tube.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Redelman-Sidi G, Glickman MS, Bochner BH. 2014. The mechanism of action of BCG therapy for bladder cancer--a current perspective. Nat Rev Urol 11:153–162. doi: 10.1038/nrurol.2014.15 [DOI] [PubMed] [Google Scholar]
- 29. Sandström G, Sjöstedt A, Johansson T, Kuoppa K, Williams JC. 1992. Immunogenicity and toxicity of lipopolysaccharide from Francisella tularensis LVS. FEMS Microbiol Immunol 5:201–210. doi: 10.1111/j.1574-6968.1992.tb05902.x [DOI] [PubMed] [Google Scholar]
- 30. Gunn JS, Ernst RK. 2007. The structure and function of Francisella lipopolysaccharide. Ann N Y Acad Sci 1105:202–218. doi: 10.1196/annals.1409.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cowley SC, Myltseva SV, Nano FE. 1996. Phase variation in Francisella tularensis affecting intracellular growth, lipopolysaccharide antigenicity, and nitric oxide production. Mol Microbiol 20:867–874. doi: 10.1111/j.1365-2958.1996.tb02524.x [DOI] [PubMed] [Google Scholar]
- 32. Cheng S-C, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JHA, Rao NA, Aghajanirefah A, et al. 2014. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345:1250684. doi: 10.1126/science.1250684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fortier AH, Slayter MV, Ziemba R, Meltzer MS, Nacy CA. 1991. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect Immun 59:2922–2928. doi: 10.1128/iai.59.9.2922-2928.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]