Abstract
Objective.
Neutrophil dysregulation and the type I interferon (IFN) axis have been proposed to contribute to premature cardiovascular disease, a leading cause of mortality in patients with systemic lupus erythematosus (SLE). In the present study, we evaluated the ability of anifrolumab, a type I IFN receptor–blocking antibody, to reduce neutrophil extracellular trap (NET) formation and modulate cardiometabolic disease markers in comparison to placebo.
Methods.
Study subjects comprised patients with moderate-to-severe SLE who were enrolled in phase IIb of the MUSE trial (A Phase II, Randomized Study to Evaluate the Efficacy and Safety of MEDI-546 in Subjects with Systemic Lupus Erythematosus), with healthy individuals as controls. Blood samples were collected from SLE patients (n = 305) and healthy controls (n = 10–20) before the initiation of treatment (baseline) and from SLE patients after they had been treated with 300 mg of anifrolumab (n = 99) or placebo (n = 102). Baseline IFN gene signature test status was determined, and the IFN gene signature (21-gene panel) was monitored over time. Serum proteins were measured by multiplex immunoassay or ultrasensitive Simoa assay. NET complexes, cholesterol efflux capacity (CEC), and glycoprotein acetylation (GlycA) and other lipid parameters were assessed in plasma.
Results.
Formation of NET complexes and levels of tumor necrosis factor (TNF) and interleukin-10 (IL-10) were correlated with extent of type I IFN pathway activity. NET complexes and IL-10 levels were up-regulated in SLE patients compared to healthy controls (P < 0.008). The cardiometabolic disease markers CEC and GlycA were also found to be dysregulated in patients with SLE (P < 0.001 versus healthy controls). Type I IFN receptor inhibition with anifrolumab significantly reduced NET complexes and GlycA and improved CEC compared to baseline (P < 0.05) whereas no improvements were seen with placebo. Levels of TNF and IL-10 were reduced with anifrolumab compared to placebo (P < 0.05).
Conclusion.
These data support a key role for type I IFNs in modulating factors contributing to SLE vasculopathy and suggest that inhibition of this pathway could decrease cardiovascular risk in individuals with SLE.
INTRODUCTION
Cardiovascular disease (CVD) resulting from premature atherosclerosis is one of the predominant causes of mortality in systemic lupus erythematosus (SLE) (1). Traditional cardiovascular risk factors cannot fully account for this enhanced CVD (2). Immune dysregulation likely contributes to vascular damage in SLE (3). However, the exact cellular and molecular mechanisms underlying accelerated atherosclerosis in SLE remain unclear.
SLE patients frequently display an elevated type I interferon (IFN) gene signature (IFNGS) (4). Type I IFNs have direct and indirect deleterious effects on the vasculature (5-7) and have been implicated in the induction of insulin resistance (IR) (8). Subclinical markers of CVD have been shown to be associated with type I IFN activity in SLE, independent of Framingham CVD risk factors (6), and the observed concurrence of cardiometabolic manifestations and interferonopathy raises the question of a causal link between type I IFN and CVD (9).
Neutrophil dysregulation has also been implicated in cardiovascular damage in SLE (10-13). Neutrophils release neutrophil extracellular traps (NETs) in a cell death–associated process (14), and this phenomenon has proatherogenic effects (15). Immune complexes containing NET autoantigens induce plasmacytoid dendritic cells and other innate immune cells to aberrantly enhance type I IFN synthesis (16-18). In turn, type I IFNs can prime neutrophils to extrude NET complexes when exposed to SLE autoantibodies (18).
Increased levels of netting neutrophils and NET complexes have been identified in the tissues and blood of SLE patients (11,19,20). Moreover, low-density granulocytes (LDGs), a subset of neutrophils characterized by enhanced NET formation, are elevated in SLE and are associated with vascular inflammation and coronary plaque severity (10-12).
In addition, NETs increase cardiovascular risk through oxidant-generating enzymes, such as myeloperoxidase (MPO), which oxidizes high-density lipoprotein (HDL) (21). HDL oxidation promotes inflammatory responses in macrophages and impairs cholesterol efflux capacity (CEC), as demonstrated by a reduced capacity of plasma HDL to accept cholesterol from macrophages (21,22). Impaired CEC promotes accumulation of lipid-laden macrophages in atherosclerotic lesions, which are a hallmark of atherosclerosis (23).
Current practical CVD assessments (e.g., carotid and coronary plaque quantification) in SLE patients require expensive imaging technology and capture disease at late stages. Therefore, there is interest in the potential of circulating biomarkers to predict CVD risk in SLE (12). Indeed, impaired CEC is associated with CVD risk in the general population (21,22) and with greater non-calcified coronary plaque burden in SLE (12).
A recently described biomarker for cardiometabolic disease and systemic inflammation is glycoprotein acetylation (GlycA), a nuclear magnetic resonance (NMR) signal found on acute-phase response proteins (24). GlycA is a predictor of CVD risk and a measure of subclinical CVD in SLE, rheumatoid arthritis, and psoriasis (25,26).
We hypothesized that type I IFN pathway inhibition could reduce NET formation, immune mediators of endothelial dysfunction, and cardiometabolic disease biomarkers such as CEC and GlycA. We quantified markers of vascular damage in SLE patients before and after treatment with anifrolumab, a human monoclonal antibody specific for the type I IFN receptor IFNAR-1 that potently inhibits the IFNGS (27). These markers were assessed in samples from patients with moderate-to-severe SLE who participated in the phase IIb MUSE trial (A Phase II, Randomized Study to Evaluate the Efficacy and Safety of MEDI-546 in Subjects with Systemic Lupus Erythematosus) of anifrolumab (27).
PATIENTS AND METHODS
Patient population and sample collection.
Details of the randomized, double-blind phase IIb MUSE trial (ClinicalTrials.gov identifier: NCT01438489) and patient population (including patient disposition, inclusion/exclusion criteria, and patient demographic/baseline characteristics) have been previously published (27). In the MUSE trial, blood samples were obtained from adults ages 18 to 65 years who fulfilled at least 4 of the 11 American College of Rheumatology 1997 classification criteria for SLE (28) and who had moderate-to-severe SLE as assessed by SLE Disease Activity Index 2000 (SLEDAI-2K) (29).
Patients were randomized at a 1:1:1 ratio to receive intravenous infusions of 300 mg of anifrolumab (n = 99), 1,000 mg of anifrolumab (n = 104), or placebo (n = 102) every 4 weeks along with standard therapy, with final doses administered at 48 weeks. Plasma/sera from fasting patients were collected on days 0, 169, and 365. IFNGS test status was measured prior to randomization, and oral glucocorticoid tapering was allowed after randomization (27). For healthy donor fasting samples, whole blood was collected after written informed consent was obtained from donors recruited by the MedImmune Blood Donor Program. Healthy donors consisted of MedImmune or AstraZeneca employees, who were anonymously enrolled in the MedImmune Research Specimen Collection Program. The healthy controls were matched to the cohort of SLE patients by age, race, and sex. Analyses were performed using samples from the placebo group and the 300 mg anifrolumab group (the dosage selected for progression to phase III clinical trials), with the exception of baseline assessments of proteomics, complete blood cell count (CBC), and gene signature, where available samples from the full study cohort were assessed.
The present study was conducted in accordance with the Declaration of Helsinki and the International Council for Harmonisation Guidelines for Good Clinical Practice. Independent ethics committee or independent Institutional Review Board (IRB) approvals were obtained (Syneos; IRB no. 201300430), and all patients provided written informed consent in accordance with local requirements.
Measurement of neutrophil numbers.
Blood samples from patients were collected before the initiation of treatment on day 1, and neutrophil numbers were derived from the CBC with the differential cell count.
Analysis of IFNGS test status and expression in SLE patients.
IFNGS test.
An analytically validated 4-gene (IFI27, IFI44, IFI44L, and RSAD2) IFNGS test was conducted in whole blood by quantitative polymerase chain reaction (qPCR) as noted in a previous publication (27) in order to determine IFNGS test status. Patients were categorized into IFNGS test–high and IFNGS test–low groups at baseline using a predetermined ΔCt-based cutoff point in the trough of the bimodal distribution.
A 21-gene IFNGS panel (21-IFNGS).
The 21-IFNGS was generated using a 21-gene qPCR assay to measure the extent of type I IFN signaling dysregulation in patients with SLE as previously described (4).
Measurement of NET complexes and LDG gene signature.
NET complexes in patient sera were quantified using capture enzyme-linked immunosorbent assays (ELISAs) that detect complexes of MPO–DNA, neutrophil elastase (NE)–DNA, or citrullinated histone H3 (CitH3)–DNA, as published previously (17). For detection of MPO–DNA, patient samples were incubated overnight on high-binding 96-well ELISA plates with a mouse anti-human MPO antibody (clone 4A4; AbD Serotec) at 4°C in Cell Death Detection kit (Roche) coating buffer. Nonspecific binding sites were blocked in 1% bovine serum albumin, and plasma samples diluted in blocking buffer were incubated overnight at 4°C. After washing, anti-DNA–peroxidase (Roche) detection antibody was incubated for 1.5 hours at room temperature, and 3,3',5,5'-tetramethylbenzidine substrate (Sigma) was added before stopping reagent (Sigma), and absorbance was measured at 450 nm. Similarly, NE–DNA and CitH3–DNA were detected using rabbit anti-human NE (Calbiochem) or rabbit anti-human CitH3 (Abcam ab5103) capture antibodies, respectively, followed by 1-hour incubations with the monoclonal mouse anti–double-stranded DNA primary antibody (Millipore) and anti-mouse IgG horseradish peroxidase-conjugated (Bio-Rad) secondary antibody.
The gene signature of LDGs (12) from patients with SLE was measured as described in the Supplementary Methods (available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41518/abstract).
Serum protein measurements.
Serum samples were stored at −80°C and then shipped to Myriad RBM (Austin, Texas). Serum IFNα, IFNβ, IFNγ, interleukin-10 (IL-10), and tumor necrosis factor (TNF) were quantified using a Simoa immunoassay. All other serum proteins were measured using a Luminex quantitative immunoassay according to standard procedures.
Measurement of CEC.
HDL CEC assays were performed using J774 cells derived from a murine macrophage cell line according to previously published methods (30). Briefly, 3 × 105 J774 cells per well were plated and radiolabeled with 2 μCi of 3H-cholesterol/ml. ATP-binding cassette transporter A1 was up-regulated via incubation for 16 hours with 0.3 mM of 8-(4-chlorophenylthio)-cAMP. Apolipoprotein B–depleted plasma (2.8%) was added to the efflux medium for 4 hours. Liquid scintillation counting was used to quantify the efflux of radioactive cholesterol from the cells. Efflux was calculated as follows: [μCi of 3H-cholesterol in medium containing 2.8% apolipoprotein B–depleted subject plasma – μCi of 3H-cholesterol in plasma-free medium]/[μCi of 3H-cholesterol in medium containing 2.8% apolipoprotein B–depleted pooled control plasma – μCi of 3H-cholesterol in pooled control plasma-free medium].
A CEC defect was identified based on values that were 2 SD below the mean level of CEC in plasma obtained from 5 healthy adult volunteers (Supplementary Figure 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41518/abstract). All assays were performed in duplicate.
GlycA and LipoProfile NMR spectroscopy.
Based on the methods described in the study by Otvos et al (24), plasma samples were adjusted to a density of 1.22 g/ml in sodium bromide and centrifuged at 84,000g for 48 hours at a temperature of 4°C to separate the lipoprotein and protein fractions. The 2 fractions were dialyzed against NMR diluent (50 mM sodium phosphate, 120 mM KCI, 5 mM Na2EDTA, 1 mM CaCl2, pH 7.4) and adjusted for concentration before centrifugal passage through a 10-kd Centricon ultrafilter (Merck Millipore) to yield the desired molecular weight fraction. A standard 0.01-M N-acetylglucosamine sample was prepared in NMR diluent. GlycA and other lipid parameters were measured in plasma by NMR spectroscopy (LabCorp) using the NMR LipoProfile test spectrum (24). Cutoff values to determine the dysregulation of GlycA and lipids were defined as the mean ± 2 SD of values in healthy donors.
Insulin resistance (IR).
IR was measured as described in the Supplementary Methods, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41518/abstract.
Statistical analysis.
Mann-Whitney 2-tailed U tests were used to compare the levels of CEC, GlycA, IL-10, IR, neutrophils, NET complexes, and TNF between SLE patients and healthy donors and between SLE patients who received 300 mg of anifrolumab and SLE patients who received placebo. Spearman’s rank correlation was used to analyze associations of vascular inflammation marker levels, neutrophil numbers, and NET complexes with the 21-IFNGS analyses and IFNα protein levels. Associations of vascular inflammation markers, neutrophil numbers, and NET complexes with IFNGS test status, SLEDAI scores, and Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) scores were analyzed using Welch’s t-test for group comparisons. Changes from baseline for the aforementioned markers following treatment with 300 mg of anifrolumab or placebo were compared using signed rank tests. Statistical analyses were performed using RStudio 1.1.383.
RESULTS
Association of IFNα with a type I IFNGS in SLE.
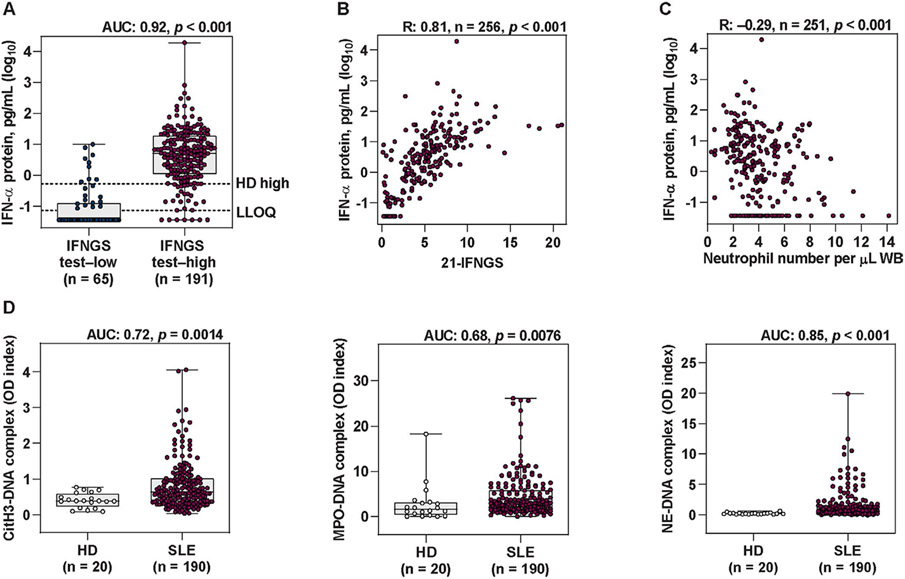
Due to low abundance, the relative contribution of the major type I IFN proteins, IFNα and IFNβ, to atherosclerosis pathogenesis in SLE remains unclear. Using ultrasensitive immunoassays, we analyzed the association of serum protein levels of IFNα and IFNβ with the type I IFNGS at baseline in the MUSE cohort. IFNα was quantifiable in serum samples obtained at baseline from the majority of patients (205 of 256, or 80.1%), including 96.3% (184 of 191) who were IFNGS test–high and 32.3% (21 of 65) who were IFNGS test–low (Figure 1A). In contrast, IFNβ was quantifiable in only 2.0% of patients (5 of 256), indicating that IFNα is the dominant type I IFN protein in circulation in lupus.
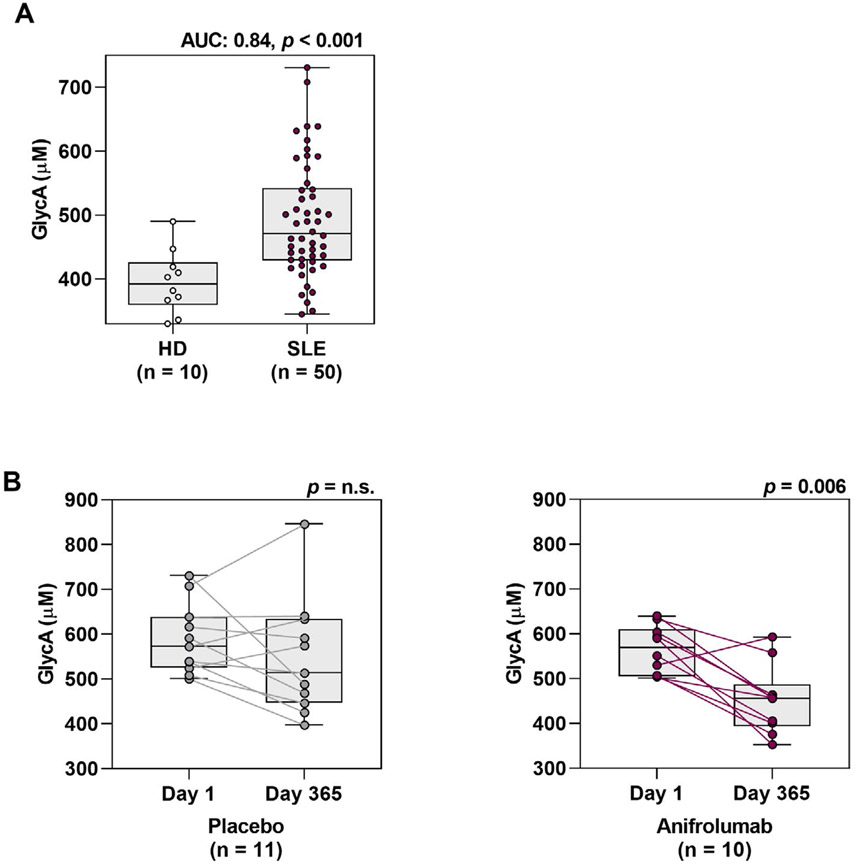
Figure 1.
Elevated type I interferon (IFN) pathway activity and neutrophil extracellular trap (NET) complex levels in patients with systemic lupus erythematosus (SLE). A, Levels of IFNα protein measured by quantitative Simoa immunoassay in type I IFN gene signature (IFNGS) test–low patients and IFNGS test–high patients with moderate-to-severe SLE. Broken lines represent the maximum IFNα level in healthy donors (HD high [0.53 pg/ml]) as well as the lower limit of quantification (LLOQ [0.073 pg/ml]). B, Levels of IFNα protein measured by Simoa immunoassay versus levels of 21-IFNGS expression. C, Number of neutrophils versus levels of IFNα protein measured by Simoa immunoassay. D, Levels of the NET complexes citrullinated histone H3 (CitH3)–DNA, myeloperoxidase (MPO)–DNA, and neutrophil elastase (NE)–DNA measured by capture enzyme-linked immunosorbent assay in healthy donors and in patients with moderate-to-severe SLE. Values in A and D are shown as box plots. Each box represents the 25th and 75th percentiles, lines inside the boxes represent the median, and whiskers represent the ranges. Symbols represent individual subjects. P values were calculated using a Mann-Whitney 2-tailed U test (A and D) or 2-tailed Spearman’s rank correlation (B and C). AUC = area under the curve; WB = whole blood; OD = optical density.
Significantly higher serum IFNα protein concentrations were observed in IFNGS test–high patients compared to IFNGS test–low patients (area under the curve [AUC] 0.92, P < 0.001). Median IFNα protein level in IFNGS test–high patients was greater than the maximum level assessed in healthy donors (Figure 1A). While the 4-gene IFNGS test categorizes patients into 2 distinct subgroups, the 21-IFNGS test generates a continuous 21-IFNGS score (4). Serum IFNα protein levels correlated with the whole blood 21-IFNGS in SLE patients (Spearman’s rank correlation R = 0.81, P < 0.001) (Figure 1B), supporting the theory that there is a direct contribution of IFNα to lupus IFN gene signatures.
Proteins that significantly correlated with both IFNα protein levels and the 21-IFNGS comprised several atherosclerosis- and vascular dysfunction–associated proteins, including TNF, IL-10, angiopoietin 2, vascular cell adhesion molecule 1, macrophage inflammatory protein 3β, monocyte chemotactic protein 1, progranulin, IFNγ–inducible 10-kd protein, and von Willebrand factor (Table 1) (31-36).
Table 1.
Serum analytes showing a correlation with IFNα protein levels and IFN 21-gene signature expression in patients with systemic lupus erythematosus*
| Analyte† | IFNα | 21-IFNGS | ||||||
|---|---|---|---|---|---|---|---|---|
| No. | Rho | P | FDR | No. | Rho | P | FDR | |
| IFN and IFN-inducible cytokines | ||||||||
| 21-IFNGS | 255 | 0.82 | <10−14 | <10−14 | – | – | – | – |
| IFNα | – | – | – | - | 255 | 0.82 | <10−14 | <10−14 |
| MCP-2 | 256 | 0.65 | <10−14 | <10−14 | 301 | 0.67 | <10−14 | <10−14 |
| IP-10 | 256 | 0.63 | <10−14 | <10−14 | 301 | 0.63 | <10−14 | <10−14 |
| BAFF | 256 | 0.62 | <10−14 | <10−14 | 301 | 0.57 | <10−14 | <10−14 |
| MIP-3β | 256 | 0.60 | <10−14 | <10−14 | 301 | 0.63 | <10−14 | <10−14 |
| I-TAC | 256 | 0.59 | <10−14 | <10−14 | 301 | 0.56 | <10−14 | <10−14 |
| BLC | 256 | 0.43 | 3.49 × 10−13 | 2.48 × 10−12 | 301 | 0.43 | 4.44 × 10−15 | 3.61 × 10−14 |
| MCP-1 | 256 | 0.37 | 7.48 × 10−10 | 4.03 × 10−9 | 301 | 0.34 | 1.54 × 10−9 | 7.93 × 10−9 |
| Eotaxin 2 | 256 | −0.23 | 1.88 × 10−4 | 6.21 × 10−4 | 301 | −0.21 | 1.84 × 10−4 | 6.10 × 10−4 |
| Vascular damage/lipid dysregulation | ||||||||
| TNF | 254 | 0.62 | <10−14 | <10−14 | 253 | 0.61 | <10−14 | <10−14 |
| IL-10 | 254 | 0.55 | <10−14 | <10−14 | 253 | 0.51 | <10−14 | <10−14 |
| Angiopoietin 2 | 256 | 0.52 | <10−14 | <10−14 | 301 | 0.47 | <10−14 | <10−14 |
| VCAM-1 | 256 | 0.49 | <10−14 | <10−14 | 301 | 0.47 | <10−14 | <10−14 |
| vWF | 255 | 0.32 | 1.42 × 10−7 | 6.35 × 10−7 | 300 | 0.33 | 6.37 × 10−9 | 3.15 × 10−8 |
| Apo A-I | 50 | −0.33 | 0.021 | 0.044 | 50 | −0.37 | 0.009 | 0.021 |
| Medium HDL particle count | 50 | −0.34 | 0.015 | 0.032 | 50 | −0.36 | 0.010 | 0.023 |
| HDL cholesterol | 50 | −0.37 | 0.009 | 0.020 | 50 | −0.41 | 0.003 | 0.009 |
| Total cholesterol | 50 | −0.39 | 0.005 | 0.013 | 50 | −0.39 | 0.005 | 0.012 |
| HDL in the H3P size range | 50 | −0.50 | 2.07 × 10−4 | 6.81 × 10−4 | 50 | −0.46 | 8.69 × 10−4 | 0.003 |
| Neutrophil dysregulation | ||||||||
| Progranulin | 256 | 0.62 | <10−4 | <10−14 | 301 | 0.57 | <10−14 | <10−14 |
| CitH3–DNA | 185 | 0.23 | 0.001 | 0.004 | 188 | 0.22 | 0.002 | 0.006 |
| MPO–DNA | 185 | 0.19 | 0.008 | 0.020 | 188 | 0.21 | 0.003 | 0.009 |
| Neutrophil number | 251 | −0.29 | 2.13 × 10−6 | 8.89 × 10−6 | 296 | −0.18 | 0.002 | 0.005 |
| Immune dysregulation/other | ||||||||
| β2-microglobulin | 255 | 0.52 | <10−14 | <10−14 | 300 | 0.52 | <10−14 | <10−14 |
| Ficolin 3 | 256 | 0.45 | 3.02 × 10−14 | 2.28 × 10−13 | 301 | 0.48 | <10−14 | <10−14 |
| IL-2 | 253 | 0.43 | 6.89 × 10−13 | 4.75 × 10−12 | 252 | 0.40 | 2.37 × 10−11 | 1.44 × 10−10 |
| IFNγ | 256 | 0.42 | 1.67 × 10−12 | 1.08 × 10−11 | 255 | 0.46 | 1.04 × 10−14 | 8.17 × 10−14 |
| IgE | 256 | 0.37 | 1.22 × 10−9 | 6.40 × 10−9 | 301 | 0.33 | 6.37 × 10−9 | 3.15 × 10−8 |
| TRAIL-R3 | 256 | −0.22 | 5.11 × 10−4 | 0.002 | 301 | −0.24 | 2.20 × 10−5 | 8.08 × 10−5 |
| IL-1RII | 256 | −0.36 | 4.08 × 10−9 | 2.05 × 10−8 | 301 | −0.29 | 4.09 × 10−7 | 1.80 × 10−6 |
| Leukocyte number | 251 | −0.41 | 7.62 × 10−12 | 47.9 × 10−11 | 296 | −0.33 | 9.53 × 10−9 | 4.63 × 10−8 |
| Lymphocyte number | 251 | −0.48 | 1.33 × 10−15 | 1.09 × 10−14 | 296 | −0.49 | <10−14 | <10−14 |
IFNα = interferon-α; 21-IFNGS = 21-gene type I IFN gene signature; MCP-2 = monocyte chemotactic protein 2; IP-10 = IFNγ-inducible 10-kd protein; MIP-3β = macrophage inflammatory protein 3β; I-TAC =IFN-inducible T cell α chemoattractant; BLC = B lymphocyte chemoattractant; TNF = tumor necrosis factor; IL-10 = interleukin-10; VCAM-1 = vascular cell adhesion molecule 1; vWF = von Willebrand factor; Apo A-I = apolipoprotein A-I; HDL = high-density lipoprotein; CitH3–DNA = citrullinated histone H3–DNA; MPO–DNA = myeloperoxidase–DNA; IL-1RII = IL-1 receptor type II.
Includes analytes measured with a P value of <0.05 and a false discovery rate (FDR) of <0.05.
Modulation of NET levels in SLE by inhibition of type I IFN signaling.
Neutrophil dysregulation has been proposed as promoting vascular disease in SLE (37). To better understand the relationship between type I IFN signaling, neutrophil dysregulation, and associated biologic processes, we first assessed the correlation of IFNα protein levels and the 21-IFNGS with other analytes in sera. These and other serum analyte correlations are reported in Supplementary Table 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41518/abstract. Neutrophil numbers negatively correlated with serum IFNα protein levels in whole blood (R = −0.29, P < 0.001) (Figure 1C) and with the 21-IFNGS, whereas significantly fewer neutrophils were observed in IFNGS test–high patients compared to IFNGS test–low patients. These results support a direct association between neutrophils and multiple measures of type I IFN pathway activity.
Type I IFNs prime NET formation with specific immune complexes, and NETs reciprocally induce IFNα production (17,18). Circulating NET complex levels (CitH3–DNA, MPO–DNA, and NE–DNA) were elevated in SLE patients compared to healthy donors (Figure 1D) and were negatively correlated with neutrophil numbers (Supplementary Table 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41518/abstract). IFNGS test-high patients had a significantly elevated number of CitH3–DNA NET complexes compared to IFNGS test–low patients (P = 0.030), and the numbers of CitH3–DNA complexes were correlated with the 21-IFNGS and IFNα protein levels (Table 1). Similar associations were observed between the levels of other NET complexes (MPO–DNA and NE–DNA) and measures of type I IFN pathway activity (Table 1 and Supplementary Table 1). Thus, increased numbers of circulating NET complexes in SLE are associated with reduced neutrophil numbers and elevated type I IFN activity.
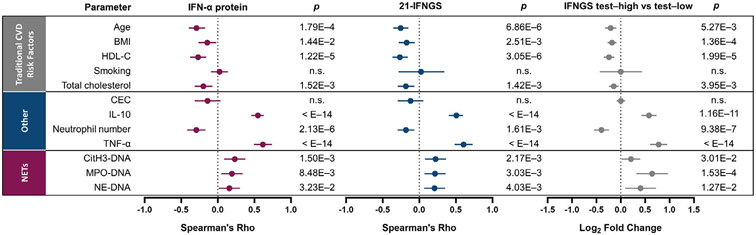
Statistical correlations of the type I IFN pathway with NET complexes and neutrophils are shown in Figure 2. Associations with lupus disease activity are shown in Supplementary Figure 2 and the Supplementary Results, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41518/abstract.
Figure 2.
Association of traditional risk factors for cardiovascular disease (CVD), levels of interleukin-10 (IL-10), number of neutrophils, levels of tumor necrosis factor (TNF), and number of NET complexes with the type I IFN axis in patients with SLE. Age, body mass index (BMI), levels of high-density lipoprotein cholesterol (HDL-C), smoking history, levels of total cholesterol, cholesterol efflux capacity (CEC), levels of IL-10 protein, numbers of neutrophils per microliter of whole blood, TNF protein levels, and levels of circulating NET complexes (CitH3–DNA, MPO–DNA, and NE–DNA) were compared to 3 measures of the type I IFN pathway: IFNα protein levels, the 21-IFNGS, and IFNGS test status (IFNGS test–high versus IFNGS test–low). Spearman’s rank correlation was used to analyze the correlation between the parameters and the 21-IFNGS and IFNα protein levels. Associations of parameters with IFNGS test status was analyzed by Welch’s t-test and was expressed as the log2 fold change. Values are shown as forest plots with 95% confidence intervals. NS = not significant (see Figure 1 for other definitions).
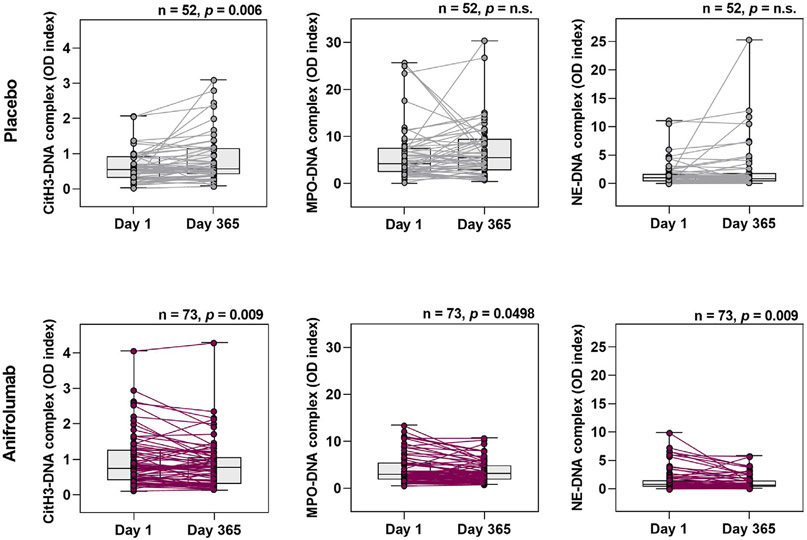
Given the association between NETs and the type I IFN pathway, we quantified circulating NET complexes on days 1 and 365 in patients who received the anti–IFNAR-1 antibody anifrolumab (300 mg) or placebo (Figure 3). Notably, median circulating NET complex levels were significantly decreased on day 365 in patients receiving anifrolumab. Patients who received placebo had increased levels of CitH3–DNA (but not the other NET complexes) on day 365 (P = 0.006 versus day 1). There were no changes in LDG-associated gene signature (12) in patients who received anifrolumab. These results demonstrate that type I IFN pathway inhibition significantly reduced circulating NET complexes without an apparent reduction of gene expression previously associated with LDGs (12). This may indicate that anifrolumab suppressed NET complex formation without altering LDG number.
Figure 3.
Significantly decreased levels of circulating NET complexes with type I IFN pathway inhibition following 1 year of treatment with anifrolumab. Levels of NET complexes (CitH3–DNA, MPO–DNA, and NE–DNA) were measured by capture enzyme-linked immunosorbent assay on day 1 and day 365 in patients with moderate-to-severe SLE who received 300 mg of the anti–IFNAR-1 antibody anifrolumab or placebo. Values are shown as box plots. Each box represents the 25th and 75th percentiles, lines inside the boxes represent the median, and whiskers represent the ranges. Symbols represent individual subjects. P values were calculated using Wilcoxon’s signed rank test. NS = not significant (see Figure 1 for other definitions).
Modulation of cytokines associated with vascular dysfunction following anifrolumab treatment.
Type I IFN signaling and NET formation have been shown to directly impact the vasculature (6,11), and our study showed that NET complexes were up-regulated in SLE patients (Figure 1). TNF and IL-10 also induce vascular dysfunction (7,38), but their association with type I IFN in SLE is not completely understood. Serum levels of TNF were elevated in IFNGS test–high patients compared to IFNGS test–low patients (P < 0.001) (Supplementary Figure 3, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41518/abstract). The median TNF concentration in IFNGS test–high patients, but not IFNGS test–low patients, was above the range of healthy donors. Levels of TNF, 21-IFNGS, and IFNα protein were observed to be significantly correlated in SLE patients (Figure 2 and Table 1).
IFNGS test–high patients had significantly higher IL-10 levels compared to IFNGS test–low patients (P = 4.4 × 10−10) (Supplementary Figure 3 [http://onlinelibrary.wiley.com/doi/10.1002/art.41518/abstract]). Similar to TNF, serum IL-10 levels correlated with the 21-IFNGS (R = 0.506, P < 10−14) and serum IFNα protein levels (R = 0.549, P < 10−14) (Figure 2 and Table 1). TNF and IL-10 levels were correlated (R = 0.732, P < 10−14) (Supplementary Table 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41518/abstract). Together, these results demonstrate an association between TNF, IL-10, and type I IFN signaling—3 putative key mediators of vascular dysfunction in SLE.
Levels of TNF and IL-10 significantly decreased in IFNGS test–high patients after anifrolumab treatment compared to patients who received placebo at various time points (Supplementary Figure 3). In IFNGS test–high patients, but not in IFNGS test–low patients, IL-10 levels decreased with anifrolumab treatment compared to placebo on day 169 (P = 0.037) and day 365 (P = 0.016), and TNF levels were decreased on day 85 (P = 0.013), day 169 (P = 0.001), and day 365 (P = 0.010).
Modulation of cardiometabolic disease markers by inhibition of type I IFN signaling.
Cholesterol efflux capacity (CEC).
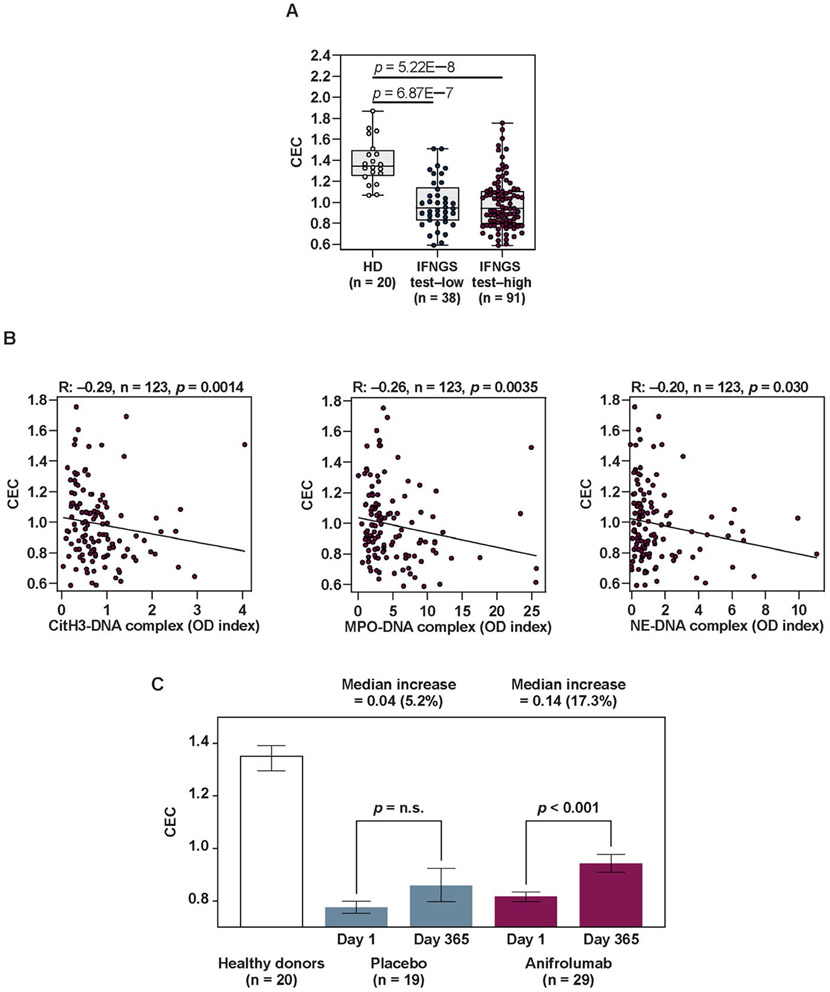
Given that NETs can oxidize HDL and impact CEC (21,22), we assessed the impact of NET inhibition with anifrolumab on these parameters. Baseline CEC values were significantly reduced in SLE patients compared to healthy controls in both IFNGS test–low and IFNGS test–high patients, suggesting proatherogenic dysregulation of HDL function (Figure 4A). Whereas multiple proinflammatory and proatherogenic proteins were associated with the type I IFN pathway (Table 1), we found no association between CEC and IFNGS test status, serum IFNα, or the 21-IFNGS in this cohort (Figure 2 and Supplementary Table 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41518/abstract). In contrast, a significant negative correlation was observed between CEC and circulating NET complex levels (Figure 4B), a finding that was consistent with the observed aberrant HDL function resulting from NET complex–mediated oxidation.
Figure 4.
Correlation of impaired cholesterol efflux capacity (CEC) in SLE patients at baseline with NET complex levels and normalization of CEC following type I IFN–signaling inhibition. A, Levels of CEC in apolipoprotein B (Apo-B)–depleted plasma samples from healthy donors, IFNGS test–low patients with SLE, and IFNGS test–high patients with SLE. Values are shown as box plots. Each box represents the 25th and 75th percentiles, lines inside the boxes represent the median, and whiskers represent the ranges. Symbols represent individual subjects. B, Association between CEC and NET complexes (CitH3–DNA, MPO–DNA, and NE–DNA) assessed in SLE patients using Spearman’s rank correlation. C, Change in CEC levels in Apo-B–depleted plasma samples from IFNGS test–high patients with moderate-to-severe SLE who had defects in CEC at baseline and who were treated with 300 mg of anifrolumab or placebo. CEC defects were defined as levels ≥2 SD below the mean level in healthy donors (mean 0.96). CEC in healthy donors at baseline are shown for reference. Results are the mean ± SEM. P values were calculated using Wilcoxon’s signed rank test. NS = not significant (see Figure 1 for other definitions).
To investigate whether treatment with anifrolumab could reverse the impairment of CEC in SLE patients (defined as CEC levels that were ≥2 SD below the mean value in healthy donors) (Supplementary Figure 1 [http://onlinelibrary.wiley.com/doi/10.1002/art.41518/abstract]), we measured CEC levels in patients before and after treatment. In IFNGS test–high patients who received anifrolumab treatment, CEC levels were significantly improved (increased 17.3%) on day 365 compared to baseline (P < 0.001) (Figure 4C). In contrast, no significant improvement in CEC levels was observed in IFNGS test–low patients or patients who received placebo. This effect was more pronounced in patients with the greatest CEC impairments at baseline (Supplementary Figure 4, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41518/abstract). CEC was not altered by glucocorticoid tapering alone (Supplementary Figure 5, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.eom/doi/10.1002/art.41518/abstract).
Lipoproteins and traditional CVD risk factors.
We also quantified lipoprotein levels (including particle count and size) by NMR in IFNGS test–high SLE patients. Levels of HDL cholesterol, HDL in the H3P size range, HDL particle count (medium and total), apolipoprotein A-I, and very large triglyceride-rich lipoprotein particles were significantly lower in SLE patients than in healthy controls, whereas levels of medium triglyceride-rich lipoprotein particles were significantly higher in SLE patients compared to healthy controls (Supplementary Table 2, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41518/abstract).
Given that dysregulated HDL levels and function have been associated with CVD in SLE (12,39,40), we assessed the correlation of HDL cholesterol and total cholesterol with IFNα protein, the 21-IFNGS, and IFNGS test status. IFNGS test–high patients had reduced total cholesterol and HDL cholesterol levels compared with IFNGS test–low patients (Figure 2). Total cholesterol and HDL cholesterol levels negatively correlated with the 21-IFNGS and IFNα. Traditional CVD risk factors like body mass index (BMI) and age also had an inverse association with type I IFN measures (Supplementary Results, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41518/abstract). These results indicate that, although IFNGS test–high patients had reduced traditional CVD risk factors (age, BMI, total cholesterol) compared with IFNGS test–low patients, they displayed higher levels of several immune markers of vascular dysfunction and neutrophil dysregulation and lower HDL cholesterol levels, thus supporting the possibility that elevated CVD risk is not predicted by traditional CVD risk factors alone.
To further examine the association between lipoprotein/ lipid parameters and the type I IFN pathway in SLE patients, we evaluated the effect of anifrolumab treatment on lipid parameters that were found to have defects in ≥10 patients at baseline (i.e., values defined as ≥2 SD below the mean value in healthy donors) (Supplementary Table 3, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41518/abstract). In patients with reduced levels of HDL in the H3P size range at baseline, the levels of H3P-sized HDL significantly increased from baseline following treatment with anifrolumab (P = 0.022), whereas no significant change was observed in patients who received placebo. There were no significant, treatment-specific changes in the levels of medium, small, or very small triglyceride-rich lipoprotein particles.
IR status.
As lipoprotein dysregulation and type I IFN signaling have been associated with IR, a CVD risk factor (8,41), we analyzed whether the level of IR was correlated with type I IFN measures at baseline. Analysis of all patients, regardless of IR status, showed no significant difference in the level of IR between IFNGS test–high patients and IFNGS test–low patients. No correlation was observed between level of IR and IFNα levels or the 21-IFNGS (Supplementary Table 1 [http://onlinelibrary.wiley.com/doi/10.1002/art.41518/abstract]). However, among the patients who had early IR (>1.9 on the homeostatic model assessment for IR [HOMA-IR] of which 0.5–1.4 is the optimal range for insulin sensitivity) (42), IFNGS test–high patients had greater IR than IFNGS test–low patients (P = 0.046). There was no reduction in the percentage of IR change from baseline with anifrolumab compared to placebo, even in patients in the upper quartile of IR on the HOMA-IR (≥3.9). Overall, these results do not support a role for type I IFN pathway inhibition in IR modulation in SLE, although there may be value in examining this relationship in a cohort further enriched for patients with varying levels of IR.
Glycoprotein acetylation (GlycA).
GlycA, a marker of cardiometabolic disease assessed by NMR, was previously found to be dysregulated in SLE (26). GlycA levels were significantly elevated in IFNGS test–high patients compared to healthy donors (AUC 0.84, P < 0.001) (Figure 5A). We investigated the effect of anifrolumab treatment on GlycA levels in IFNGS test–high patients who had increased levels of GlycA at baseline. GlycA levels were significantly decreased by day 365 in 10 patients who received anifrolumab treatment (P = 0.006), but not in 11 patients who received placebo (Figure 5B). These results demonstrate that inhibition of the type I IFN signaling pathway in patients with SLE may significantly normalize the levels of GlycA to levels approaching those found in healthy donors.
Figure 5.
Reduction from baseline in the levels of glycoprotein acetylation (GlycA) following type I IFN pathway inhibition in patients with SLE. A, GlycA levels measured at baseline by nuclear magnetic resonance spectroscopy in healthy donors or IFNGS test–high patients with SLE. The AUC is indicated, along with P values calculated by Mann-Whitney U test. B, GlycA levels measured on day 1 and day 365 in IFNGS test–high GlycA-high patients with SLE who were treated with 300 mg of anifrolumab or placebo. GlycA-high was defined as levels ≥2 SD above the mean level in healthy donors (mean 500 μM). P values were calculated using Wilcoxon’s signed rank test. Values are shown as box plots. Each box represents the 25th and 75th percentiles, lines inside the boxes represent the median, and whiskers represent the ranges. Symbols represent individual subjects. NS = not significant (see Figure 1 for other definitions).
DISCUSSION
Significant advances have been made in understanding the underlying mechanisms by which aberrant activation of the type I IFN axis and neutrophil dysregulation drive SLE pathogenesis. Type I IFNs have also been linked to disturbances in vascular function, leading to increased incidence of cardiovascular events in patients with SLE. Based on our previous work, which identified associations between type I IFN signaling, neutrophil dysfunction, and vascular disease in SLE (6,12,16,17), and our current findings, we hypothesize that these dysregulated processes play prominent roles in increased CVD risk in SLE through a wide range of interdependent, downstream, immunopathologic effects.
In patients with moderate-to-severe SLE, we found significant correlations between type I IFN pathway measures and NET complexes, TNF, and IL-10. Some of these variables were directly associated with markers of cardiometabolic dysregulation. Importantly, we found that type I IFN pathway inhibition significantly improved various immunologic and subclinical cardiometabolic parameters implicated in vasculopathy.
The relative contributions of individual type I IFNs in driving downstream immune effects that underlie SLE pathogenesis and the type I IFNGS have been of significant interest. Here, we report the first assessment of the contributions of IFNα and IFNβ to IFNGS test status in the context of an interventional trial in patients with SLE. Previous use of a similar ultrasensitive immunoassay for IFNα identified elevated levels of circulating IFNα protein in plasma from SLE patients compared to controls, and this increase in IFNα levels correlated with the IFN-stimulated gene score (43); however, IFNp was not assessed. We now confirm that IFNα protein has a positive association with IFNGS test status and the 21-IFNGS, whereas serum IFNβ was detected in only a small number of SLE patients. Thus, of the type I IFN proteins, serum IFNα is a major contributor to the IFNGS in SLE. Our results demonstrate a predominant role for IFNα at the systemic level in lupus, although a role for locally produced, tissue-specific IFNβ cannot be ruled out.
Treatment with anifrolumab provided the first opportunity to study the effect of type I IFN receptor inhibition on the levels of TNF protein. In a subset of patients with rheumatoid arthritis, treatment with anti-TNF–blocking antibody significantly elevated type I IFN signaling through a proposed counterbalance of the TNF and type I IFN pathways (44). In SLE, a positive association between TNF and IFNα activity has been described (45). In the present study, serum levels of TNF were significantly lowered with anifrolumab therapy, suggesting that the cross-regulation of TNF and type I IFN signaling pathways is complex and context dependent.
While the proinflammatory effects of TNF are well understood, less is known about the role of IL-10 in SLE vascular disease. We have previously described the impairment of endothelial cell differentiation by IL-10 (7). In SLE, type I IFN can impart proinflammatory functions on IL-10, a canonical antiinflammatory cytokine in other diseases (46). As serum IL-10 levels were also decreased with anifrolumab, results suggest that type I IFN pathway inhibition down-regulates 2 cytokines that may contribute to vascular dysfunction.
We observed that lower levels of neutrophils in SLE samples, which were previously observed to rapidly correct with anifrolumab treatment during the MUSE trial (47), correlated with levels of circulating NET complexes, consistent with enhanced cell death through NET formation. NET complex levels may predict worsening of SLE disease and have been shown to be associated with endothelial cell activation and cardiovascular comorbidity or arterial thrombosis (20). Extracellular release of oxidizing enzymes within NET complexes, such as MPO, can directly damage the vasculature and oxidize HDL, impairing its antiatherogenic functions (21). We found a significant correlation between levels of circulating NET complexes and impaired CEC resulting from the proatherogenic oxidized HDL. In our study, type I IFN pathway inhibition with anifrolumab significantly normalized NET complex levels and CEC, with limited effect on other lipid parameters and no detectable effect on IR. These findings are especially noteworthy from a mechanistic perspective given that statins do not systematically improve CEC in CVD (22). Our results suggest that NET complex formation and the proatherogenic effects of HDL are modulated by type I IFNs, supporting previous findings that type I IFN primes SLE neutrophils to produce NET complexes (18). As NET complexes also induce aberrant type I IFN production by plasmacytoid dendritic cells, our results support a mechanism by which type I IFNs and NET complexes may form a self-amplifying pathogenic loop (16-18).
Similar to the observed normalization of CEC, we found that type I IFN pathway inhibition decreased GlycA levels in SLE patients. GlycA was previously associated with neutrophil gene networks, and the major protein contributors to the GlycA NMR signal (α1-acid glycoprotein and haptoglobin) can be synthesized and secreted from neutrophil granules (48), indicating that type I IFN pathway inhibition improved a measure of neutrophil effector function. Additionally, multiple immunomodulatory analytes associated with atherosclerosis correlated with type I IFN measures and were previously shown to normalize toward baseline levels with anifrolumab treatment (47). Together, these findings support a model where vascular damage occurs through multiple mechanisms driven by the downstream effects of unabated IFNα-mediated inflammation.
Growing evidence suggests that abrogating chronic inflammation may be beneficial for reducing CVD risk. In the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study, neutralization of IL-1β resulted in a decreased number of adverse cardiac events, independent of lipid levels (49,50). Whether other chronic inflammation pathways drive CVD, especially in autoimmune diseases, is an important question. We have provided the first foundational evidence in a clinical SLE setting that type I IFN–mediated inflammation may play a similar role in driving CVD progression, thereby paving the way for future studies to understand the potential benefit for SLE patients.
A limitation of this study is that impaired CEC and altered levels of GlycA are suggestive of the presence of subclinical vascular disease, rather than being direct clinical markers of CVD. While we have demonstrated that blocking type I IFN diminishes proatherogenic markers, further work is needed to show that inhibition of this pathway results in clinical improvement of SLE vascular dysfunction and of adverse cardiovascular events. However, CEC and GlycA are associated with signs of clinical CVD (coronary disease and plaque burden) (22,26) and represent accessible serum biomarkers that may enable earlier CVD detection in patients with SLE.
Direct measurements of aortic vascular inflammation in patients with CVD can be obtained with positron emission tomography/computed tomography and can be used as risk markers for CVD progression (12). Short-term changes in arterial inflammation are associated with long-term disease progression of atherosclerosis, validating the benefit of measuring subclinical early stage biomarkers to assess CVD risk (51). However, these advanced imaging techniques are not accessible to all patients. Furthermore, studying CVD risk in SLE using traditional approaches has low feasibility given disease incidence/prevalence and the relatively young age at which lupus is typically diagnosed. Using the identification of meaningful CVD risk biomarkers in the selection of patients for intervention is a promising approach to address such challenges. Our findings demonstrate that several subclinical markers with proposed physiologic links to SLE-associated CVD risk (enhanced NET formation, impaired CEC, and elevated GlycA levels) are significantly modulated by type I IFN pathway inhibition. Further studies are needed to determine how these subclinical markers, or other proatherogenic analytes assessed in this study, are associated with CVD progression in SLE and other autoimmune diseases. If such associations are established, these biomarkers have the potential to be practical tools to facilitate earlier CVD detection and improved symptom monitoring of CVD risk and vasculopathy.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the patients who provided samples for this research.
Supported by AstraZeneca (Gaithersburg, Maryland). AstraZeneca and the National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH have a Collaborative Research and Development Agreement related to this work.
ROLE OF THE STUDY SPONSOR
This study was sponsored by AstraZeneca. All authors interpreted the data, critically reviewed the manuscript for important intellectual content, approved the final draft, and agreed to its submission. Medical writing support was funded by AstraZeneca and provided by Rosie Butler, PhD, of JK Associates, Inc., a member of Fishawack Health. Authors Casey, Smith, L. Wang, and Illei were employees of AstraZeneca and authors Sinibaldi, Yu, S. Wang, and White are current employees of AstraZeneca.
Footnotes
ClinicalTrials.gov identifier: NCT01438489.
Data underlying the findings described in this article may be obtained in accordance with the AstraZeneca data sharing policy (https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure).
ADDITIONAL DISCLOSURES
Author Casey is a current employee of Regeneron Pharmaceuticals, and authors Smith, L. Wang, and Illei are current employees of Viela Bio.
REFERENCES
- 1.Nossent J, Cikes N, Kiss E, Marchesoni A, Nassonova V, Mosca M, et al. Current causes of death in systemic lupus erythematosus in Europe, 2000–2004: relation to disease activity and damage accrual. Lupus 2007;16:309–17. [DOI] [PubMed] [Google Scholar]
- 2.Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du Berger R, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum 2001;44:2331–7. [DOI] [PubMed] [Google Scholar]
- 3.Kahlenberg JM, Kaplan MJ. The interplay of inflammation and cardiovascular disease in systemic lupus erythematosus [review]. Arthritis Res Ther 2011;13:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao Y, Higgs BW, Morehouse C, de los Reyes M, Trigona W, Brohawn P, et al. Development of potential pharmacodynamic and diagnostic markers for anti-IFN-α monoclonal antibody trials in systemic lupus erythematosus. Hum Genomics Proteomics 2009;2009:374312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denny MF, Thacker S, Mehta H, Somers EC, Dodick T, Barrat FJ, et al. Interferon-α promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis. Blood 2007;110:2907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somers EC, Zhao W, Lewis EE, Wang L, Wing JJ, Sundaram B, et al. Type I interferons are associated with subclinical markers of cardiovascular disease in a cohort of systemic lupus erythematosus patients. PLoS One 2012;7:e37000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cates AM, Holden VI, Myers EM, Smith CK, Kaplan MJ, Kahlenberg JM. Interleukin 10 hampers endothelial cell differentiation and enhances the effects of interferon α on lupus endothelial cell progenitors. Rheumatology (Oxford) 2015;54:1114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koivisto VA, Pelkonen R, Cantell K. Effect of interferon on glucose tolerance and insulin sensitivity. Diabetes 1989;38:641–7. [DOI] [PubMed] [Google Scholar]
- 9.Chen HJ, Tas SW, de Winther MP. Type-I interferons in atherosclerosis. J Exp Med 2020;217:e20190459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denny MF, Yalavarthi S, Zhao W, Thacker SG, Anderson M, Sandy AR, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol 2010;184:3284–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol 2011;187:538–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlucci PM, Purmalek MM, Dey AK, Temesgen-Oyelakin Y, Sakhardande S, Joshi AA, et al. Neutrophil subsets and their gene signature associate with vascular inflammation and coronary atherosclerosis in lupus. JCI Insight 2018;3:99276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmona-Rivera C, Zhao W, Yalavarthi S, Kaplan MJ. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann Rheum Dis 2015;74:1417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science 2004;303:1532–5. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Carmona-Rivera C, Moore E, Seto NL, Knight JS, Pryor M, et al. Myeloid-specific deletion of peptidylarginine deiminase 4 mitigates atherosclerosis. Front Immunol 2018;9:1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med 2011;3:73ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, de Ravin SS, Smith CK, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med 2016;22:146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med 2011;3:73ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marder W, Knight JS, Kaplan MJ, Somers EC, Zhang X, O’Dell AA, et al. Placental histology and neutrophil extracellular traps in lupus and pre-eclampsia pregnancies. Lupus Sci Med 2016;3:e000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore S, Juo HH, Nielsen CT, Tyden H, Bengtsson AA, Lood C. Neutrophil extracellular traps identify patients at risk of increased disease activity and cardiovascular comorbidity in systemic lupus erythematosus. J Rheumatol 2019;47:1652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith CK, Vivekanandan-Giri A, Tang C, Knight JS, Mathew A, Padilla RL, et al. Neutrophil extracellular trap–derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol 2014;66:2532–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 2011;364:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu XH, Fu YC, Zhang DW, Yin K, Tang CK. Foam cells in atherosclerosis. Clin Chim Acta 2013;424:245–52. [DOI] [PubMed] [Google Scholar]
- 24.Otvos JD, Shalaurova I, Wolak-Dinsmore J, Connelly MA, Mackey RH, Stein JH, et al. GlycA: a composite nuclear magnetic resonance biomarker of systemic inflammation. Clin Chem 2015;61:714–23. [DOI] [PubMed] [Google Scholar]
- 25.Connelly MA, Otvos JD, Shalaurova I, Playford MP, Mehta NN. GlycA, a novel biomarker of systemic inflammation and cardiovascular disease risk [review], J Transl Med 2017;15:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purmalek MM, Carlucci PM, Dey AK, Sampson M, Temesgen-Oyelakin Y, Sakhardande S, et al. Association of lipoprotein subfractions and glycoprotein acetylation with coronary plaque burden in SLE. Lupus Sci Med 2019;6:e000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furie R, Khamashta M, Merrill JT, Werth VP, Kalunian K, Brohawn P, et al. Anifrolumab, an anti-interferon-α receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis Rheumatol 2017;69:376–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus [letter]. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 29.Gladman DD, Ibanez D, Urowitz MB. Systemic Lupus Erythematosus Disease Activity Index 2000. J Rheumatol 2002;29:288–91. [PubMed] [Google Scholar]
- 30.Mehta NN, Li R, Krishnamoorthy P, Yu Y, Farver W, Rodrigues A, et al. Abnormal lipoprotein particles and cholesterol efflux capacity in patients with psoriasis. Atherosclerosis 2012;224:218–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akhavanpoor M, Gleissner CA, Gorbatsch S, Doesch AO, Akhavanpoor H, Wangler S, et al. CCL19 and CCL21 modulate the inflammatory milieu in atherosclerotic lesions. Drug Des Devel Ther 2014;8:2359–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grote K, Drexler H, Schieffer B. Renin-angiotensin system and atherosclerosis [editorial], Nephrol Dial Transplant 2004;19:770–3. [DOI] [PubMed] [Google Scholar]
- 33.Kojima Y, Ono K, Inoue K, Takagi Y, Kikuta K, Nishimura M, et al. Progranulin expression in advanced human atherosclerotic plaque. Atherosclerosis 2009;206:102–8. [DOI] [PubMed] [Google Scholar]
- 34.Ley K, Huo Y. VCAM-1 is critical in atherosclerosis. J Clin Invest 2001;107:1209–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voloshyna I, Littlefield MJ, Reiss AB. Atherosclerosis and interferon-y: new insights and therapeutic targets. Trends Cardiovasc Med 2014;24:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu MD, Atkinson TM, Lindner JR. Platelets and von Willebrand factor in atherogenesis. Blood 2017;129:1415–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaplan MJ. Neutrophils in the pathogenesis and manifestations of SLE [review], Nat Rev Rheumatol 2011;7:691–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chia S, Qadan M, Newton R, Ludlam CA, Fox KA, Newby DE. Intra-arterial tumor necrosis factor-α impairs endothelium-dependent vasodilatation and stimulates local tissue plasminogen activator release in humans. Arterioscler Thromb Vasc Biol 2003;23:695–701. [DOI] [PubMed] [Google Scholar]
- 39.Svenungsson E, Jensen-Urstad K, Heimburger M, Silveira A, Hamsten A, de Faire U, et al. Risk factors for cardiovascular disease in systemic lupus erythematosus. Circulation 2001;104:1887–93. [DOI] [PubMed] [Google Scholar]
- 40.Smith CK, Seto NL, Vivekanandan-Giri A, Yuan W, Playford MP, Manna Z, et al. Lupus high-density lipoprotein induces proinflammatory responses in macrophages by binding lectin-like oxidized low-density lipoprotein receptor 1 and failing to promote activating transcription factor 3 activity. Ann Rheum Dis 2017;76:602–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krssak M, Petersen KF, Dresner A, DiPietro L, Vogel SM, Rothman DL, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 1999;42:113–6. [DOI] [PubMed] [Google Scholar]
- 42.The Blood Code. HOMA-IR: what it is & why you should know yours. September 2016. URL: www.thebloodcode.com/homa-ir-know/. [Google Scholar]
- 43.Mathian A, Mouries-Martin S, Dorgham K, Devilliers H, Barnabei L, Salah EB, et al. Monitoring disease activity in systemic lupus erythematosus with single-molecule array digital enzyme-linked immunosorbent assay quantification of serum interferon-α. Arthritis Rheumatol 2019;71:756–65. [DOI] [PubMed] [Google Scholar]
- 44.Cantaert T, Baeten D, Tak PP, van Baarsen LG. Type I IFN and TNFα cross-regulation in immune-mediated inflammatory disease: basic concepts and clinical relevance [review]. Arthritis Res Ther 2010;12:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weckerle CE, Mangale D, Franek BS, Kelly JA, Kumabe M, James JA, et al. Large-scale analysis of tumor necrosis factor α levels in systemic lupus erythematosus. Arthritis Rheum 2012;64:2947–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharif MN, Tassiulas I, Flu Y, Mecklenbrauker I, Tarakhovsky A, Ivashkiv LB. IFN-α priming results in a gain of proinflammatory function by IL-10: implications for systemic lupus erythematosus pathogenesis. J Immunol 2004;172:6476–81. [DOI] [PubMed] [Google Scholar]
- 47.Casey KA, Guo X, Smith MA, Wang S, Sinibaldi D, Sanjuan MA, et al. Type I interferon receptor blockade with anifrolumab corrects innate and adaptive immune perturbations of SLE. Lupus Sci Med 2018;5:e000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ritchie SC, Wurtz P, Nath AP, Abraham G, Flavulinna AS, Fearnley LG, et al. The biomarker GlycA is associated with chronic inflammation and predicts long-term risk of severe infection. Cell Syst 2015;1:293–301. [DOI] [PubMed] [Google Scholar]
- 49.Sliva J, Charalambous C, Bultas J, Karetova D. A new strategy for the treatment of atherothrombosis: inhibition of inflammation [review]. Physiol Res 2019;68:S17–30. [DOI] [PubMed] [Google Scholar]
- 50.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic aisease. N Engl J Med 2017;377:1119–31. [DOI] [PubMed] [Google Scholar]
- 51.Joseph P, Ishai A, Mani V, Kallend D, Rudd JH, Fayad ZA, et al. Short-term changes in arterial inflammation predict long-term changes in atherosclerosis progression. Eur J Nucl Med Mol Imaging 2017;44:141–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.