Abstract
Like other alphaherpesviruses, pseudorabies virus (PrV) exhibits restricted gene expression during latency. These latency-associated transcripts (LATs) are derived from the region located within 0.69 to 0.77 map units of the viral genome. However, the presence of such viral RNAs during a productive infection has not been described. Although several transcripts originating between 0.706 to 0.737 map units have been detected in PrV-infected cultured cells, their relationship to the LATs has not been examined. Therefore, to determine if any correlation exists between PrV LAT gene expression in the natural and laboratory systems, transcription from the LAT gene region during lytic infection of cultured neuronal and nonneuronal cells was evaluated. A Northern blot assay using single-stranded RNA probes complementary to the spliced in vivo 8.4-kb largest latency transcript (LLT) detected 1.0-, 2.0-, and 8.0-kb poly(A) RNAs in all PrV-infected cells lines. The 1.0- and 8.0-kb transcripts partially overlapped the first and second exons of the LLT, respectively. In contrast, portions of both LLT exons comprised the 2.0-kb RNA sequence, which lacked the same intron as the LLT. Generation of this transcript began about 243 bp downstream of the LLT initiation site and terminated near the junction of BamHI fragments 8′ and 8. Its synthesis was inhibited by cycloheximide but not by cytosine β-d-arabinofuranoside, which suggests that the 2.0-kb RNA is not an immediate-early gene product. Thus, although the PrV LAT gene is transcriptionally active during a productive infection of cultured cells, the resulting RNAs are distinctive from the LLT.
Porcine herpesvirus 1, or pseudorabies virus (PrV), is classified as a member of the Alphaherpesvirinae subfamily within Herpesviridae (6, 26, 27). This virus produces neurological, respiratory, and reproductive disease (Aujeszky’s disease) in swine, its natural host (1, 6). As with other alphaherpesviruses, a primary PrV infection can progress into latency in its natural host (14, 15, 19). During the PrV latent infection, transcription of the viral genome is restricted to an inverted repeat region in a manner similar to herpes simplex virus type 1 (HSV-1) (2–4, 10, 13, 21, 30). RNAs, termed latency-associated transcripts (LATs), are expressed from the region between 0.69 and 0.77 map units (from BamHI fragments 14 to 8) of the viral genome and are synthesized in the opposite direction relative to IE180 and EP0 gene transcription (10, 21). Several sizes of LATs (8.4, 4.5 to 5.0, 2.0, and 0.95 kb) have been detected in latently infected porcine trigeminal ganglia (8, 10, 20–22). The largest latency transcript (LLT; 8.4 kb) expressed from this region is polyadenylated and spliced to remove a 4.6-kb intron between nucleotides 1510 (within BamHI fragment 14) and 6164 (within BamHI fragment 8′) relative to the transcriptional start site. The first and second exon coding sequences of the LLT are located between BamHI fragments 6-14 and 8′-5, respectively, and thus overlap head to tail with either the EP0 or IE180 gene (Fig. 1). The origins of the other LATs have not been definitively characterized. However, the available data indicate that all of the LATs detected in latently infected porcine trigeminal ganglia are complementary to the 3′ end of the IE180 gene (10, 19–21). Therefore, it is possible that these LATs are smaller processed products from the primary transcript that also gives rise to the LLT.
FIG. 1.
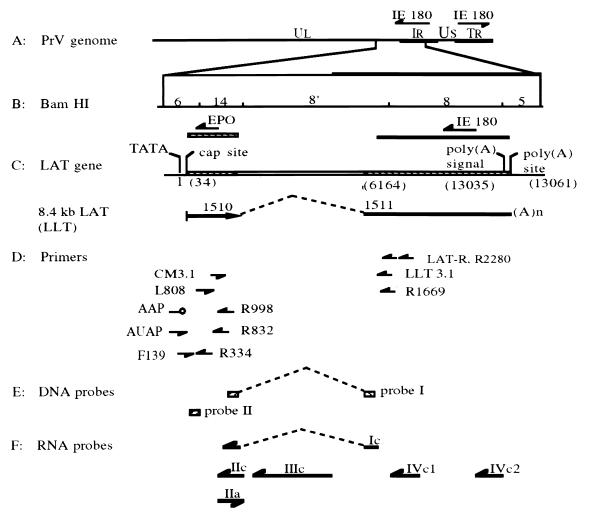
Schematic of the PrV genome with locations of primers used in RT-PCR and 5′ RACE as well as probes used for hybridization relative to the LAT gene region. (A) Diagram of the PrV genome and locations of the two IE180 gene copies, with the arrows showing their direction of transcription. (B) Expanded diagram of BamHI fragments 6, 14, 8′, 8, and 5; locations of the IE180 and EP0 genes, with arrows showing their direction of transcription. (C) Location of the LAT gene in relation to PrV-Be BamHI fragments, direction of its transcription (arrow), intron boundaries (dashed line), map units after intron splicing, and the resultant in vivo spliced transcript (LLT, 8.4-kb LAT). (D) Relative locations and orientations of genomic primers CM3.1, F139, L808, LAT-R, LLT3.1, R1669, R2280, R334, R832, and R998 as well as nongenomic primers AAP and AUAP. (E) Relative locations of DNA probes I and II used in Southern hybridization. (F) Relative positions and orientations of ssRNA probes Ic, IIc, IIIc, IVc1, IVc2, and IIa. Probe Ic extends from first to the second exon over the intron region. All probes are drawn in an approximate scale with respect to their relative location in the PrV genome shown in panel B.
HSV-1 (2–4, 26, 30) and bovine herpesvirus 1 (BHV-1) (18, 23–25) have been shown to produce LATs during infection of cultured mammalian cells; similar expression by PrV has not been demonstrated. Although several RNAs (2.0, 4.4, 8.2, and 9.5 kb) originating primarily from the LAT intron region have been detected during a productive infection of cultured cells (9), a possible correlation(s) between these lytic cycle viral transcripts and LATs has not been determined. Therefore, LAT gene expression during a lytic infection was examined by using reverse transcription (RT)-PCR and Northern hybridization with single-stranded RNA (ssRNA) probes complementary to the in vivo-processed LLT and its intron region. To investigate possible host effects on LAT gene expression, this phenomenon was examined in both neuronal and nonneuronal cell lines.
MATERIALS AND METHODS
Cell lines and virus.
Four cell lines permissive for a PrV productive infection were used: Crandall-Rees feline kidney (CRFK); porcine kidney (PK-15); Madin-Darby bovine kidney (MDBK); and N1E, a murine neuroblastoma cell line (ATCC C1300; American Type Culture Collection, Rockville, Md.). All four cell lines were maintained in Eagle’s minimum essential medium (MEM) supplemented with 10% calf serum (HyClone Laboratories, Inc., Logan, Utah), penicillin (100 U/ml), streptomycin (100 μg/ml), and gentamicin (0.25 mg/ml) (Sigma, St. Louis, Mo.).
The Becker (Be) strain of PrV was propagated and titrated in CRFK cells maintained in MEM supplemented with 2% calf serum and antibiotics as described above. PrV LAT gene expression during a productive infection was investigated in each cell line infected with 5 to 6 PFU/cell of PrV-Be. The effect of protein synthesis inhibition on PrV LAT gene expression was examined by adding 100 μg/ml of cycloheximide (Sigma) to the inoculum and again in the medium after the adsorption period. To investigate whether PrV LAT gene expression was dependent on DNA synthesis, cytosine β-d-arabinofuranoside (araC; 50 μg/ml; Sigma) was added to the medium after adsorption as described previously (5).
RNA isolation.
Total RNA was isolated from virus-infected or uninfected monolayers (105 cells) by using a Trizol RNA extraction kit (Life Technologies, Inc., Gaithersburg, Md.) according to manufacturer’s instructions. The extracted RNA was suspended in 50 μl of distilled H2O treated with diethyl pyrocarbonate (DEPC; Sigma) and then incubated with 1 U of DNase (RNase free; Promega Corp., Madison, Wis.) in 50 μl of DNase buffer (50 mM Tris [pH 7.2], 10 mM MgCl2, 1 mM dithiothreitol, 5 U of RNase inhibitor) for 30 min at 37°C. Following incubation, the DNase-treated RNA was extracted once with phenol-chloroform (1:1) and then precipitated in 1 volume of 2-propanol at −20°C for at least 1 h. RNA pellets were washed once with cold 75% ethanol, suspended in 1 mM EDTA (DEPC treated, pH 7.5), and stored at −80°C. Poly(A) RNAs were isolated from 0.5 to 1.0 mg of total RNA by using PolyATract isolation systems (Promega) as instructed by the manufacturer. The poly(A) RNA was eluted in DEPC-treated distilled H2O and then stored at −80°C.
Plasmids.
Plasmid pAC38 (provided by A. K. Cheung, National Animal Disease Center, Ames, Iowa) contains 656 bp of the PrV LLT, which consists of a 422-bp upstream and a 234-bp downstream sequence adjacent to the intron splicing sites. Plasmids pB14, pB8′, and pB8 were constructed by inserting the PrV BamHI 14, BamHI 8′, and BamHI 8 fragments, respectively, into the BamHI site of pBluescript SK II (+) (Stratagene, La Jolla, Calif.). Plasmids pBK8′ and pBN8 were constructed by ligating the remainder of pB8′ and pB8 after digestion with KpnI and NotI, respectively.
Primers.
Oligonucleotides were synthesized by the standard phosphoramidite procedure on an automated synthesizer (Operon Technologies, Inc., Alameda, Calif.). The DNA sequence data necessary for primer construction were obtained from the published sequences of PrV genes (GenBank accession no. M57505 [10]). All primer locations relative to the PrV LAT gene region are shown in Fig. 1. Primers R2280, LAT-R, R998, and R832 extend from nucleotides 2280 to 2260 (GTCCTCCTCCTCCTCTGCGT), 1613 to 1597 (TGGTGGGAGGTGGACG), 998 to 978 (TCGATACGCTGTTTGACAT), and 832 to 812 (GGTGCACACGGAGGATCTGA), respectively, on the LAT gene anticoding strand. Primers R1669 (GGTGGGAAGAAGTAGAAGGT) and L808 (CTCCGTCAGATCCTCCGTGT) extend from nucleotides 1669 to 1649 and 808 to 828 on the LAT anticoding and coding strands, respectively. Primers LLT3.1 and CM3.1 extend from nucleotides 1578 to 1558 and 1326 to 1346 on the LAT anticoding and coding strands, respectively (10, 11). Primers R334 (ACTTCGGCTCCCGCTC) and F139 (CAGCCATAGAAGACACCGGG) extend from nucleotides 334 to 318 and 139 to 158 on the LAT anticoding and coding strands, respectively.
RT-PCR.
First-strand cDNA was synthesized from 1 to 3 μg of total or 50 to 100 ng of poly(A) RNA by using 10 pmol of primer LAT-R or R2280 and Superscript reverse transcriptase II (Life Technologies) according to the manufacturer’s recommendations. PCR was performed with a volume of 50 μl consisting of 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, 0.001% (wt/vol) gelatin, 200 μM each deoxynucleoside triphosphate, 0.6 μM each primer (LLT3.1 and CM3.1 or L808 and R1669), 0.25 U of Taq polymerase (Perkin-Elmer, Branchburg, N.J.), and 2 μl of the completed RT reaction mixture. The mixture was subjected to 31 cycles of 94°C for 1 min, 60°C for 45 s, and 72°C for 1 min and then incubated at 72°C for 4 min in a DNA thermal cycler (MJ Research, Inc., Watertown, Mass.). Ten microliters (one-fifth) of each RT-PCR sample was analyzed in an 1.5% agarose gel in TBE buffer (45 mM Tris-borate, [pH 8.0], 1 mM EDTA) and then visualized by UV illumination after staining with ethidium bromide (1 μg/ml). Commercially available øX174 replicative-form DNA/HaeIII fragments (Life Technologies) served as size markers.
5′ RACE.
The 5′ ends of total or poly(A) RNAs were obtained by a 5′ RACE (amplification of 5′ cDNA ends) system as instructed by the manufacturer (Life Technologies). Briefly, the first-strand cDNA was synthesized with gene-specific primers LAT-R or R998 as indicated in Fig. 1. Approximately 1 to 3 μg of total or 500 ng of poly(A) RNA per μl was used as the template in a 25-μl RT reaction. After purification of the first-strand cDNA, its 5′ end was tailed with dCTP by using terminal deoxynucleotidyltransferase. The oligo(dC) cDNA was then amplified with a second gene-specific primer, R832, and the abridged anchor primer (AAP) specific for the 5′ dC tail. The primary PCR products were then reamplified with the heminested gene-specific primer R334 and the abridged universal amplification primer (AUAP) under conditions recommended by the manufacturer. The 5′ RACE products were analyzed as described for the RT-PCR amplimers.
RNA and DNA probes.
All ssRNA probes were labeled with [α-32P]UTP (400 Ci/mmol; Amersham Corp., Arlington Heights, Ill.) and generated with known specificity as runoff RNA transcripts from recombinant Bluescript plasmids by using an RNA transcription kit (Stratagene). Probes Ic, IIc and IIa, IIIc, IVc1, and IVc2 were derived from sequences in pAC38, pB14, pBK8′, pB8, and pBN8, respectively (Fig. 1). Probes having the “a” or “c” designation are either the same sense as (a) or antisense to (c) the LATs. Probe Ic is homologous to the entire 656 nucleotides of the processed LLT present in pAC38 and was used to detect LAT gene-specific transcripts. Probes IIc and IIa, produced from the complete PrV BamHI 14 fragment, were used for the detection of the LLT first exon coding sequence and the EP0 gene mRNA, respectively. Probe IIIc, which was used to detect the intron sequence spliced from the LLT, was generated from the PrV BamHI 8′ sequence located between an internal KpnI site and the BamHI site adjacent to the BamHI 14 fragment. Probe IVc1 was transcribed from the BamHI 8 sequence between an internal SmaI site and the BamHI site distal to the BamHI 8′ fragment. Probe IVc2 contains the BamHI 8 sequence between an internal NotI site and the BamHI site adjoining the BamHI 5 region. Both probes IVc1 and IVc2 were used for the detection of the LLT second exon coding region.
DNA probe I was derived from the 252-bp PCR product that was amplified from pAC38 with primers CM3.1 and LLT3.1. Primer sequences at both ends were removed neatly by digestion with SmaI and MaeIII, resulting in a 174-bp fragment (Fig. 1), which was used to verify the RT-PCR products made with primer pairs CM3.1 and LLT3.1 or R1669 and L808. DNA probe II was generated from the 200-bp PCR product amplified from the 5′ end of the first exon of the LLT coding sequence, using primers R340 and F139 with PrV genomic DNA as the template (Fig. 1). The sequence representative of primer R340 was removed by HaeIII digestion of the PCR product, and the remaining portion was used as a probe to verify the 5′ RACE products. The DNA probes were gel purified and labeled with [α-32P]dCTP (Amersham Corp.), using a random priming labeling system (Life Technologies).
Northern blot analysis.
Approximately 2 to 5 μg of poly(A) RNA or 15 to 20 μg of total RNA was resolved by electrophoresis in an 1% agarose-formaldehyde gel (2.2 M formaldehyde, 40 mM morpholinepropanesulfonic acid [pH 7.0], 1 mM EDTA, 10 mM sodium acetate). The resolved RNAs were then transferred to a Zeta-Probe membrane (Bio-Rad Laboratories, Richmond, Calif.) in 10× SSC (1.5 M NaCl, 0.15 M sodium citrate [pH 7.0]) overnight. The membrane was rinsed in 2× SSC, air dried, and then exposed in a UV cross-linker unit (Bio-Rad Laboratories) for 50 s. Prehybridization was performed at 60°C for at least 4 h in 50% formamide–5× Denhardt’s solution (0.1% bovine serum albumin, 0.1% polyvinylpyrrolidone, 0.1% Ficoll)–0.5 M NaCl–10 mM Tris-HCl (pH 8.0)–10 mM EDTA–100 μg of denatured sonicated salmon sperm DNA per ml–100 μg of yeast RNA per ml–1% sodium dodecyl sulfate (SDS). Prior to its addition for overnight hybridization at 60°C, the RNA probe was denatured in 50% formamide at 70°C for 5 min. After hybridization, the blot was sequentially washed once in 1× SSC–0.1% SDS at 60°C, once in 1× SSC–0.1% SDS at 68°C, and twice in 0.1× SSC–0.1% SDS at 68°C for 30-min intervals and finally placed on Cronex 4× film (Dupont Corp., Boston, Mass.) at −70°C. The blot was reprobed after removal of the previous probe by boiling the membrane twice for 30 min in 0.1% SDS. RNA sizes were estimated by comparison to their mobilities to those of commercially available RNA markers (Ambion, Inc., Austin, Tex.).
Southern hybridization analysis.
The RT-PCR and 5′ RACE products were verified by hybridization with DNA probes I and II, respectively (Fig. 1). The products were separated in an 1.5% agarose gel and then transferred to a Zeta-Probe membrane in the presence of 0.4 N NaOH for a period of at least 4 h. Conditions for prehybridization and hybridization have been previously described (28).
RT-PCR and 5′ RACE product sequence analysis.
Gel-purified RT-PCR products amplified from total and poly(A) RNA templates were sequenced at the Biotechnology Center (University of Illinois, Urbana) by an automated fluorescent sequencing method. Gel-purified 5′ RACE products were separately cloned into the pCR II Vector (Invitrogen Corp., San Diego, Calif.) according to the manufacturer’s instructions and then sequenced as described above. Alignments to previously published PrV LAT sequences (10) were performed with the BLAST function provided by the National Center for Biotechnology Information (19a).
RESULTS
Expression of lytic cycle viral RNAs from the PrV LAT gene.
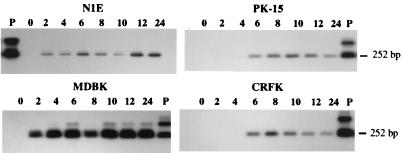
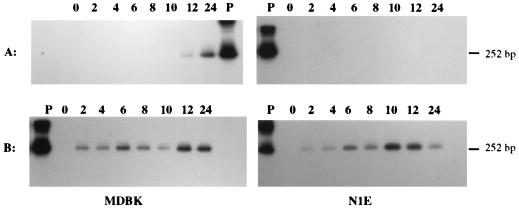
Expression of the PrV LAT gene during a productive infection of cultured cells was first investigated by using RT-PCR. Initially, the cDNAs generated by using primer LAT-R were subjected to PCR with the primer pair CM3.1 and LLT3.1, designed to amplify a 252-bp sequence spanning the intron region of the LLT. When PrV-infected neuronal (N1E) and nonneuronal (MDBK, CRFK, and PK-15) cells were screened for the presence of a spliced transcript, a 252-bp product could be generated from the RNA of N1E and MDBK cells starting at 2 h postinfection (p.i.) and from the RNA of CRFK and PK-15 cells by 6 h p.i. (Fig. 2). Thereafter, a spliced transcript was detected in all four cell lines up to 24 h p.i. Amplification did not occur when the reverse transcriptase was omitted from the RT reaction or with RNA from mock-infected cells (data not shown). The correctly sized amplimers also annealed to DNA probe I, which corresponds to the PrV genomic regions that flank the LLT intron but is positioned internal to the primer binding sites (Fig. 1). Thus, at least a portion of the LAT gene is expressed early in both neuronal and nonneuronal infected cells. Moreover, either transcription of the LAT gene is continuous or the transcript remains stable throughout the in vitro productive infection.
FIG. 2.
Autoradiogram of RT-PCR amplicons generated by using total RNA from PrV-Be-infected cells. The identity of the cell line is indicated above each blot. The products were generated by PCR with primers LLT3.1 and CM3.1 and cDNA synthesized from the total RNA extracted from cells at 0 to 24 h p.i. (as indicated above each lane) with primer LAT-R as templates. The resultant amplicons were resolved in an agarose gel, transferred to a nylon membrane, and then hybridized with 32P-labeled DNA probe I. The positive control (P) is the result of PCR amplification of pAC38 using primers CM3.1 and LLT3.1. The expected size of the amplicon arising from a spliced LAT-like transcript is shown on the right.
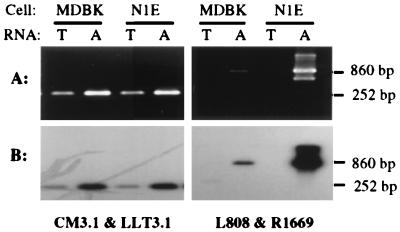
To verify that the transcripts detected in the infected cell lines are spliced like the LLT, total and poly(A) RNAs from PrV-infected N1E and MDBK cells were subjected to RT-PCR using an additional pair of amplification primers (R1669 and L808). In this case, the predicted size of the amplimer would increase from 252 to 860 bp. For the first-strand cDNA synthesis, gene-specific primer R2280 (2,280 nucleotides downstream of the 5′ end of the LLT) was used (Fig. 1). Both a 252-bp and an 860-bp product could be amplified from poly(A) RNA obtained from PrV-infected N1E and MDBK cells at 6 h p.i., using primers pairs CM3.1-LLT 3.1 and R1169-L808, respectively (Fig. 3A). Once again, both products were complementary to DNA probe I (Fig. 3B). Therefore, a transcript that maintained the same polarity and displayed the same splicing pattern as LLT was detected during a productive infection of cultured cells.
FIG. 3.
Electrophoregram and autoradiogram of RT-PCR products derived from total (lanes T) and poly(A) (lanes A) RNAs isolated from PrV-infected N1E and MDBK cells at 6 h p.i. Products were generated by using cDNAs synthesized from RNAs with primer R2280. Primer pairs LLT3.1-CM3.1 and R1669-L808 were used for the specific amplification of a spliced region analogous to that of the LLT. The products were resolved in an 1.5% agarose gel (A), transferred to a nylon membrane, and hybridized with 32P-labeled DNA probe I (B). The anticipated amplicon sizes are shown on the right.
Sequence analysis of the RT-PCR products.
To determine the degree of homology between the spliced lytic cycle viral RNA and the LLT, the 252- and 860-bp RT-PCR products were sequenced. The 252-bp amplimers generated from the total RNA of each infected cell line were found to have 99% homology with the corresponding region of the processed LLT (Fig. 4A). Likewise, the 860-bp amplimer generated from the poly(A) RNA of infected N1E cells had greater than 98% homology with the corresponding region of the LLT (Fig. 4B). The intron boundary in the 252- and 860-bp products is exactly the same as that of the LLT. Thus, both the LLT and some of the viral RNAs present during a productive infection of cultured mammalian cells undergo the same posttranscriptional modification.
FIG. 4.
Sequence alignment of the in vitro PrV LAT RT-PCR product with the reported in vivo cDNA sequence of the PrV LLT (GenBank accession no. M57505 [9]). (A) The sequence sources (in vivo LLT cDNA and the 208-bp RT-PCR products from the corresponding PrV-infected cells) are shown on the left. (B) LAT, the cDNA sequence of the LLT expressed during latency; N1E, the sequence of the 831-bp RT PCR products generated by using poly(A) RNA from PrV-infected N1E cells at 6 h p.i. with primers R2280, R1669, and L808. Dashes, identity to the published sequence; underlined nucleotides, transversions in the PrV-Be strain genome. The juncture between the first and second exons of the processed LLT is between the boldface nucleotides.
As to the discrepancies between the nucleotide sequences, the underlined transversion (A-C) and transition (T-G and G-T) mutations reflect the genetic differences between the Be strain used in this study and the Indiana Funkhauser strain from which the published in vivo LLT was derived (10). In contrast, the unique transition mutation in the LAT-like RNA from virus-infected CRFK cells (Fig. 4A) and the transversion in the 830-bp sequence (Fig. 4B) may have occurred during RT-PCR.
Northern hybridization analysis.
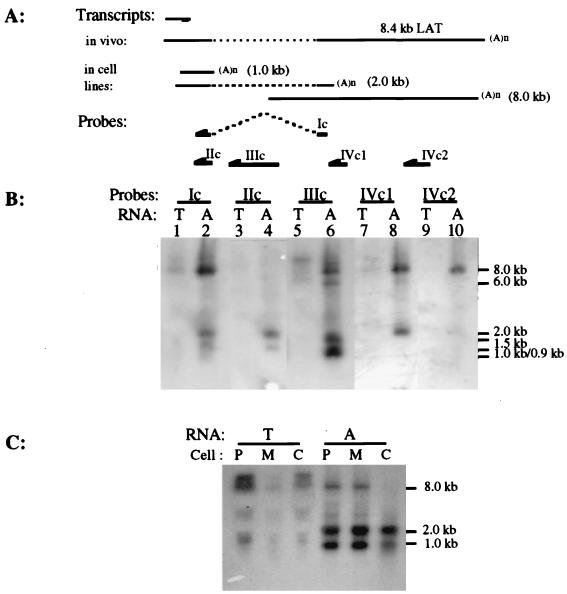
The initial RT-PCR results and sequence comparisons demonstrated that RNAs having undergone splicing identical to that of the LLT could be detected in PrV-infected cells at 6 h p.i. (Fig. 2). To explore a possible relationship between these two types of transcripts, Northern hybridization was performed with total and poly(A) RNAs from PrV-infected neuronal (N1E) and nonneuronal (CRFK, PK-15, and MDBK) cells, using LLT-specific ssRNA probes. When the ssRNA probe Ic, which contains a 422-bp upstream and 234-bp downstream contiguous sequence adjacent to the intron junction in the processed LLT, was used, poly(A) transcripts of approximately 1.0, 2.0, and 8.0 kb in size were detected in infected N1E (Fig. 5B, lane 2), MDBK (Fig. 5C), and PK-15 (Fig. 5C) cells. Since probe Ic is specific for the LLT region that lacks the intervening sequence present in the LAT gene, it is possible that the RNA species detected in this study have homology to only one of the flanking exons in the LLT. To examine this possibility, RNA probes complementary to the first (IIc) or second (IVc1) flanking exon were used in a subsequent hybridization. While a 2.0-kb and 1.0-kb poly(A) RNA species hybridized with probe IIc (Fig. 5B, lane 4), only an 8.0-kb and a 2.0-kb poly(A) RNA annealed to probe IVc1 (Fig. 5B, lane 8). Similar results were obtained in hybridizations of RNAs from PrV-infected nonneuronal cells with probes IIc and IVc1 (data not shown). Therefore, the 1.0- and 8.0-kb poly(A) RNAs transcribed from the LAT gene during a lytic viral infection contain at least a portion of the first and second exons of the LLT, respectively. The 2.0-kb poly(A) RNA appears to be comprised of part of both exons.
FIG. 5.
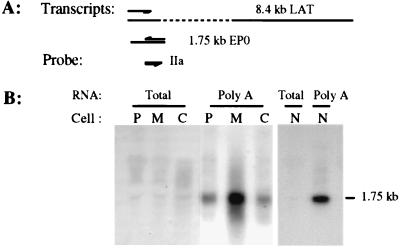
Northern hybridization of total and poly(A) RNAs from PrV-infected cells. (A) Polarity and relationship of probes and transcripts from the LAT gene region during latency (in vivo) and productive infection of cultured cell lines. The arrow above transcripts represents the direction of transcription of the LAT gene. (B) Results of Northern hybridization of total (lanes T) and poly(A) (lanes A) RNAs from PrV-infected neuronal cells at 6 h p.i. RNAs were separated in a 2.2 M formaldehyde–1% agarose gel and then transferred to a nylon membrane. The blot was sequentially hybridized with 32P-labeled ssRNA probes Ic, IIc, IVc1, IIIc, and IVc2. Molecular size markers are indicated on the right. (C) At 6 h p.i., RNAs were isolated from PK-15 (lanes P), CRFK (lanes C), and MDBK (lanes M) cells. The RNAs [total (lanes T) and poly(A) (lanes A)] were separated in a 2.2 M formaldehyde–1% agarose gel and then transferred to a nylon membrane. The blot was hybridized with 32P-labeled ssRNA probe Ic. Molecular size markers are indicated on the right.
Since the 2.0-kb poly(A) RNA annealed to the ssRNA probes complementary to sequences that are separated by a distance of 4.8 kb (BamHI fragments 14 [probe IIc] and 8 [probe IVc1]), this lytic cycle viral RNA must be the processed transcript detected by RT-PCR (Fig. 1). To confirm that this 2.0-kb RNA is the spliced product having an intervening sequence between BamHI fragments 14 and 8 removed, the PrV-infected N1E cellular RNA blot was hybridized with probe IIIc. This probe is specific for the intron region of the unprocessed LLT. As shown in Fig. 5B (lane 6), four poly(A) RNAs (0.9, 1.5, 6.0, and 8.0 kb) having sizes other than 2.0 kb were detected.
Since the 8.0-kb poly(A) RNA identified by probe Ic did not hybridize to probe IIc, it is possible that this transcript starts within the BamHI 8′ fragment downstream of the first exon of the LLT and extends through BamHI fragments 8 and 5. To address this possibility, RNA from PrV-infected N1E cells was hybridized with a probe (IVc2) specific for the region at the end of the BamHI 8 fragment adjoining the BamHI 5 region. As shown in Fig. 5B (lane 10), only the 8.0-kb RNA annealed with this probe. Since this transcript was also complementary to probes representing the antisense regions of BamHI fragments 8 (IVc1 and IVc2) and 8′ (IIIc) but not 14 (IIc) (Fig. 1), it appears that transcription of the 8.0-kb RNA initiates within the LLT intron or the BamHI 8′ fragment and continues into the BamHI 8′ and 5 regions.
To verify that the probes and the detected RNAs were of the described specificity and quality, the RNA blot used above (Fig. 5B) was examined by using ssRNA probe IIa, which is specific for the EP0 gene mRNA. As shown in Fig. 6, only one RNA species of a size corresponding to the EP0 gene transcript (1.75 kb [10]) annealed to this probe.
FIG. 6.
Northern hybridization of total and poly(A) RNAs from PrV-infected cells. RNAs from PK-15 (lanes P), MDBK (lanes M), CRFK (lanes C), and N1E (lanes N) cells were harvested at 6 h p.i., separated in a 2.2 M formaldehyde–1% agarose gel, and then transferred to a nylon membrane. RNAs were hybridized with 32P-labeled ssRNA probe IIa, which is complementary to the EP0 mRNA and has the same polarity as LAT. Molecular size for the EP0 mRNA is indicated on the right.
Determination of the 5′ end of the in vitro 2.0-kb RNA.
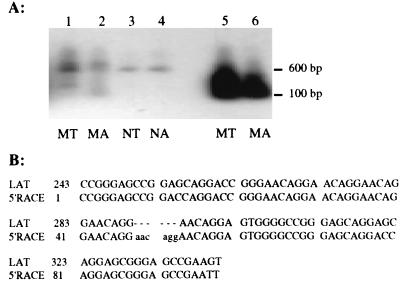
To define the 5′ end of the 2.0 kb RNA expressed during infection of cultured cells, 5′ RACE was used. Total and poly(A) RNAs isolated from PrV-infected N1E and MDBK cells at 6 h p.i. were analyzed. Primers R998 and LAT-R, complementary to regions of the first or second exon of LLT, respectively, were used separately for first-strand cDNA synthesis. Regardless of which cDNA served as the template, an approximately 600- or 100-bp product was generated with primer pair R832-AAP or AUAP-R334 (heminested primer), respectively (Fig. 7A). The larger amplimer was produced from both poly(A) and total RNAs from infected N1E and MDBK cells but could be visualized only after hybridization with DNA probe II, which is specific for the region of the PrV genome located between the binding sites of primers R334 and R832 (Fig. 7A, lanes 1 to 4). However, the 100-bp 5′ RACE product could be readily observed when the primary PCR product was reamplified with AUAP and the heminested primer R334. This smaller amplimer has about 98% sequence homology with the region extending between nucleotides 243 and 334 of the PrV-Be LLT. Therefore, synthesis of the 2.0-kb RNA appears to initiate about 243 bp downstream of the start site of the LLT (Fig. 7B).
FIG. 7.
Autoradiogram and sequence analysis of 5′ RACE product generated by using RNAs from PrV-infected N1E and MDBK cells. The cDNA for 5′ RACE analysis was generated by using total RNA (MDBK [MT] and N1E [NT]) or poly(A) RNA (MDBK [MA] and N1E [NA]) from infected cells 6 h p.i. with primer R998 (for MT and NT) or LAT-R (for MA and NA). (A) Autoradiogram of 5′ RACE products amplified only with primers R832 and AAP (lanes 1 to 4) or subsequently with primers R334 and AUAP (lanes 5 to 8) and then hybridized to DNA probe II. (B) Sequence alignment of the 100-bp 5′ RACE product with the reported cDNA sequence of the PrV LLT (GenBank accession no. M57505 [9]). 5′ RACE, product generated by using total RNA from infected N1E cells with primers AUAP and R334; LAT, published cDNA sequence of the LLT. Nucleotides in lowercase are insertions that occurred in the PrV-Be genome. The underlined nucleotides represent the genetic differences between PrV-Be and the published in vivo PrV-Funkhauser LLT cDNA sequence.
Determination of alpha or beta gene origin of the 2.0-kb lytic cycle viral RNA.
To investigate the effects of protein synthesis inhibition on the transcription of the 2.0-kb spliced lytic cycle viral RNA, N1E and MDBK cells were infected with PrV-Be in the presence of cycloheximide and then maintained in MEM containing cycloheximide. Production of the 2.0-kb RNA was monitored between 2 and 24 h p.i. by RT-PCR with primers LAT-R, CM3.1, and LLT3.1. Amplification of a specific product from the total RNA isolated from infected N1E cells between 2 and 24 h p.i. or from MDBK cells before 12 h p.i. was not observed (Fig. 8A). Presumably, its presence in MDBK cells after 12 h p.i. was due to an exhaustion of the cycloheximide. Therefore, generation of this spliced transcript during a productive infection requires protein synthesis, indicating that it does not originate from an alpha (immediate-early) gene.
FIG. 8.
Autoradiograms of the LAT RT PCR amplicons generated by using total RNA from PrV-infected cells treated with cycloheximide or araC. Virus-infected MDBK and N1E cells were treated with either cycloheximide (A) or araC (B) prior to total RNA extraction at 0 to 24 h p.i. (as indicated above each blot). RT-PCR products were synthesized from total RNA by using primers LAT-R (RT) and CM3.1 plus LLT3.1 (PCR). The products were resolved in an 1.5% agarose gel, transferred to a nylon membrane, and then hybridized with 32P-labeled DNA probe I. The positive control (P) is the PCR product obtained with pAC38 and primers CM3.1 and LLT3.1. The expected size of the amplicon arising from a spliced LAT-like transcript is shown on the right.
The effect of the DNA synthesis inhibitor, araC, on the transcription of the 2.0-kb RNA also was examined in both PrV-infected MDBK and N1E cells. In this case, total RNA from araC-treated, virus-infected cells was screened for the presence of a spliced transcript as described above. As shown in Fig. 8B, a product of the correct size (252 bp) could be amplified from RNA isolated after 2 h p.i. from either infected cell line but not from uninfected cells. Thus, the spliced 2.0-kb RNA encoded by the LAT gene is not dependent on viral DNA synthesis and therefore is probably a beta (early) gene transcript.
DISCUSSION
Expression of the PrV LAT gene during a productive infection of cultured cells has not been previously documented. Although it has been reported that RNAs with the same polarity as the LATs were produced from the PrV BamHI 8′ region, an association between these lytic cycle viral transcripts and the RNAs expressed during in vivo latency was not established (9). Our study demonstrated that the PrV LAT gene is actively transcribed during a productive infection of various mammalian cell lines. At least three lytic cycle viral poly(A) RNAs originate from that portion of the LAT gene extending from the BamHI 14 to 8 region of the PrV genome. They are difficult to detect unless first resolved on the basis of polyadenylation (Fig. 5). Of these poly(A) RNA species, only the 2.0-kb one was determined to have the same intron removed as the LLT. Moreover, apparently the entire nucleotide sequence of the 2.0-kb RNA is contained within that of the LLT. Since its transcriptional start site is about 243 bp downstream of the one for the LLT, synthesis of the 2.0-kb RNA may be regulated by the closely positioned LAP2 promoter, located about 40 to 50 nucleotides upstream of the predicted start site (10, 12). This conclusion is supported by our LAT promoter studies in which removal of the LAP1 promoter abolished LLT production but did not alter expression of the 2.0-kb RNA (unpublished data). Moreover, it should be noted that the analogous herpes simplex virus LAP2 promoter is a LAT gene transcriptional regulatory element (7). Although the PrV-encoded 2.0-kb RNA species could be detected as early as 2 h p.i. in infected cell cultures, inhibition of its synthesis by cycloheximide but not by araC indicated that its source is a beta class gene.
The 2.0-kb lytic cycle viral RNA can be considered to be either a special product made only during a productive infection or a homolog to the one made during latency. Although clear distinction between these two possibilities awaits sequencing of the in vivo spliced 2.0-kb LAT, its similarity in size to the lytic cycle viral RNA may not be simply fortuitous. A consensus polyadenylation signal (AATAAA) is found to be located about 170 bp upstream of the junction between the BamHI 8′ and 8 fragments. This position is near the predicted termination site for both the in vivo 2.0-kb LAT (8) and the lytic cycle viral 2.0-kb transcript described in this study. Thus, at least these two RNAs may have a common 3′ terminus.
Based on the polyadenylation status of the 2.0-kb lytic cycle viral RNA, this transcript could be translated. In this regard, the RNA has two potential open reading frames (ORFs) capable of encoding a 20-kDa (ORF1, 163 amino acids) and a 47-kDa (ORF2, 393 amino acids) protein. The postulated protein encoded by ORF1 has 44% homology to the apoptosis repressor ARC (17), 36% homology to a protein kinase C substrate (29), and 30% homology to a serine/threonine protein kinase (16). In contrast, homology of the predicted ORF2 protein with any known proteins cannot be established. It would be interesting to determine if either putative protein is actually made during an in vivo productive or latent infection.
Two other lytic cycle viral poly(A) RNAs transcribed from the LAT gene region were also determined to be partially colinear with the LLT. The first, an 1.0-kb RNA, seems to overlap only the first exon sequence of the LLT, since it was complementary only to those ssRNA probes (Ic and IIc) which are specific for this region (Fig. 1). The second, an 8.0-kb RNA species, is also different from the LLT since (i) it hybridizes with a ssRNA probe (IIIc) specific for the intron sequence within the Bam HI 8′ fragment and (ii) it did not anneal with probe IIc, which is specific for the 3′ half of the first exon as well as the first 200 nucleotides of the intron. Thus, since this transcript was also complementary to ssRNA probes IVc1 and Ivc2 with specificity for either the 5′ or 3′ end of the BamHI 8 fragment, it is reasonable to conclude that transcription of this RNA initiates within the BamHI 8′ region and contains most of the LLT second exon coding sequence. Based on the apparent weak hybridization signal obtained between probe Ic and an 8.0-kb RNA derived from infected CRFK cells, production of this 8.0-kb species seems to be relatively low in this cell line (Fig. 5C).
In addition to the three viral lytic cycle RNA products detected by the ssRNA probes specific for the LLT, three poly(A) RNA species (approximately 0.9, 1.5, and 6.0 kb) were found to be transcribed from the LAT gene within the BamHI 8′ region. They could be detected by hybridization with a ssRNA probe (IIIc) specific for the intron region of the LLT but not by probes IIc and IVc2, which are complementary to the first and second LAT exons, respectively. Therefore, they either have different initiation sites or are alternative splicing products from transcripts giving rise to the 2.0-kb lytic cycle viral RNA. Furthermore, they appear to be different from the previously documented BamHI 8′ and 8 fragment-derived RNAs (2.0, 4.4, 8.2, and 9.5 kb) in PrV-infected MDBK cells (9). The presence of those transcripts was detected by ssRNA and double-stranded DNA probes derived from the second exon coding sequence of LLT. It was shown that a 220-nucleotide ssRNA probe (representing the 3′ end of the BamHI 8′ fragment) and a 1,250-nucleotide ssRNA probe (comprised of 550 nucleotides from the 3′ end of the BamHI 8′ fragment and 700 bases of the 5′ end of the BamHI 8 region) could anneal to similar-sized viral RNA products in an S1 nuclease protection assay (9). However, a ssRNA probe derived from the entire BamHI 8′ fragment could protect only 550 to 1,000 nucleotides of RNAs obtained from infected MDBK cells (9). Thus, the previously described 2.0- and 8.2-kb BamHI 8′-derived RNAs may be homologues of the 2.0- and 8.0-kb transcripts detected in this study.
A 4.0- and 5.0-kb poly(A) RNAs (Fig. 5C) and an apparently nonpolyadenylated 9.5-kb RNA (Fig. 5B, lanes 2 and 7; Fig. 5C) were detected in PrV-infected PK-15 and MDBK cells by hybridization with probe Ic. The inability to always detect the largest transcript may be attributed to variability in its relative amount. The 4.0-kb transcript may be homologous to the 4.4-kb BamHI 8′-derived RNA detected in MDBK cells by Cheung (9). The nature of the other two transcripts is unknown, but these three appear to be preferentially expressed in nonneuronal cell lines.
In conclusion, the PrV LAT gene region is far from being quiescent during a lytic infection of cultured mammalian cells. Its activity during an in vivo productive infection of the natural host awaits examination. Uncovering the nature of LAT gene expression during both a productive and a latent infection of the natural host may aid in understanding the mechanism by which herpesvirus latency is established.
ACKNOWLEDGMENTS
We are grateful to W. M. Schnitzlein, M. H. Vodkin, and D. C. Bloom for helpful discussions and review of the manuscript. We also thank A. K. Cheung for providing plasmid pAC38.
This work was supported in part by a grant from the USDA Animal Health and Disease (ILLU-70-0989) and in part by a grant from the University of Illinois Campus Research Board, log no. 95126.
REFERENCES
- 1.Baskerville A, McFerran J B, Dow C. Aujeszky’s disease in pigs. Vet Bull. 1973;43:465–480. [Google Scholar]
- 2.Bennett J L, Gilden D H. The molecular genetics of herpes simplex virus latency and pathogenesis: a puzzle with many pieces still missing. J Neurovirol. 1996;2:225–229. doi: 10.3109/13550289609146884. [DOI] [PubMed] [Google Scholar]
- 3.Black D L. Finding splice sites within a wilderness of RNA. RNA. 1995;1:763–771. [PMC free article] [PubMed] [Google Scholar]
- 4.Block T M, Hill J M. The latency associated transcript (LAT) of herpes simplex virus: still no end in sight. J Neurovirol. 1997;3:313–321. doi: 10.3109/13550289709030745. [DOI] [PubMed] [Google Scholar]
- 5.Bratanich A C, Jones J. Localization of cis-acting sequences in the latency-related promoter of bovine herpesvirus 1 which are regulated by neuronal cell type factors and immediate-early genes. J Virol. 1992;66:6099–6106. doi: 10.1128/jvi.66.10.6099-6106.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown F. The classification and nomenclature of viruses: summary of results of meeting of the international committee on taxonomy of viruses in edmonton, Canada 1987. Intervirology. 1989;30:181–186. doi: 10.1159/000150091. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Schmidt M C, Goins W F, Glorioso J C. Two herpes simplex virus type 1 latency-acting promoters differ in their contributions to latency-associated transcript expression during lytic and latent infections. J Virol. 1995;69:7899–7908. doi: 10.1128/jvi.69.12.7899-7908.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung A K. Detection of pseudorabies virus transcripts in trigeminal ganglia of latently infected swine. J Virol. 1989;63:2908–2913. doi: 10.1128/jvi.63.7.2908-2913.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung A K. The BamHI J fragment (0.76 to 0.737 map units) of pseudorabies virus is transcriptionally active during viral infection. J Virol. 1990;64:977–983. doi: 10.1128/jvi.64.3.977-983.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung A K. Cloning of the latency gene and the early protein O gene of pseudorabies virus. J Virol. 1991;65:5260–5271. doi: 10.1128/jvi.65.10.5260-5271.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung A K. Detection of the large latency transcript of pseudorabies virus by RNA-PCR and its potential in diagnosis. J Vet Diagn Investig. 1994;6:483–486. doi: 10.1177/104063879400600414. [DOI] [PubMed] [Google Scholar]
- 12.Cheung A K, Smith T A. Analysis of the latency-associated transcript/UL-3.5 gene cluster promoter complex of pseudorabies virus. Arch Virol. 1999;144:381–391. doi: 10.1007/s007050050511. [DOI] [PubMed] [Google Scholar]
- 13.Enquist L W. Infection of the mammalian system by pseudorabies virus (PRV) Semin Virol. 1994;5:221–231. [Google Scholar]
- 14.Gutekunst D E. Latent pseudorabies virus infection in swine detected by RNA-DNA hybridization. Am J Vet Res. 1979;40:1568–1572. [PubMed] [Google Scholar]
- 15.Gutkunst D E, Pirtle E C, Miller L D, Stewart W C. Isolation of pseudorabies virus from trigeminal ganglia of a latently sow. Am J Vet Res. 1980;41:1315–1316. [PubMed] [Google Scholar]
- 16.Leung-Tack P, Audonnet J C, Riviere M. The complete DNA sequence and genetic organization of the short unique region (Us) of the bovine herpesvirus type 1 (ST strain) Virology. 1994;199:409–421. doi: 10.1006/viro.1994.1139. [DOI] [PubMed] [Google Scholar]
- 17.Koseki T, Inohara N, Chen S, Nunez G. ARC, an inhibitor of apoptosis expressed in skeletal muscle an heart that interacts selectively with caspases. Proc Natl Acad Sci USA. 1998;95:5156–5160. doi: 10.1073/pnas.95.9.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kutish G, Mainprize T, Rock D L. Characterization of the latency-related transcriptionally active region of the bovine herpesvirus 1 genome. J Virol. 1990;64:5730–5737. doi: 10.1128/jvi.64.12.5730-5737.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lokensgard J R, Thawley D G, Molitor T W. Pseudorabies virus latency: restricted transcription. Arch Virol. 1990;110:129–136. doi: 10.1007/BF01310709. [DOI] [PubMed] [Google Scholar]
- 19a.National Center for Biotechnology Information. 17 May 1999, revision date. BLAST. [Online.] http://www.ncbi.nlm.nih.gov/Blast. National Center for Biotechnology Information, National Institutes of Health, Bethesda, Md. [4 June 1999, last date accessed.]
- 20.Priola S A, Gustafson D P, Wagner E K, Stevens J G. A major portion of the latent pseudorabies virus genome is transcribed in trigeminal ganglia of pigs. J Virol. 1990;64:4755–4760. doi: 10.1128/jvi.64.10.4755-4760.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Priola S A, Stevens J G. The 5′ and 3′ limits of transcription in the pseudorabies virus latency associated transcription unit. Virology. 1991;182:852–856. doi: 10.1016/0042-6822(91)90628-o. [DOI] [PubMed] [Google Scholar]
- 22.Rock D L, Hagemoser W A, Osorio F A, McAllister H A. Transcription from the pseudorabies virus genome during latent infection. Arch Virol. 1988;98:99–106. doi: 10.1007/BF01321010. [DOI] [PubMed] [Google Scholar]
- 23.Rock D L, Hagesmoser W A, Osorio F A, Reed D E. Detection of bovine herpesvirus type 1 RNA in trigeminal ganglia of latently infected rabbits by in situ hybridization. J Gen Virol. 1986;67:2515–2520. doi: 10.1099/0022-1317-67-11-2515. [DOI] [PubMed] [Google Scholar]
- 24.Rock D L, Beam S L, Mayfield J E. Mapping bovine herpesvirus type 1 latency-related RNA in trigeminal ganglia of latently infected rabbits by in situ hybridization. J Virol. 1987;61:3827–3831. doi: 10.1128/jvi.61.12.3827-3831.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rock D L. Latent infection with bovine herpesvirus type 1. Semin Virol. 1994;5:233–240. [Google Scholar]
- 26.Roizman B. Herpesviridae. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. 3rd ed. 1996. pp. 2221–2230. Lippinocott-Raven Publishers, Philadelphia, Pa. [Google Scholar]
- 27.Roizman B, Denrosiers L, Fleckenstein B, Lopez C, Minson A C, Studdert M J. The family herpesviridae: an update. Arch Virol. 1992;123:425–449. doi: 10.1007/BF01317276. [DOI] [PubMed] [Google Scholar]
- 28.Scherba G, Jin L, Schnitzlein W M, Vodkin M H. Differential polymerase chain reaction for detection of wild-type and a vaccine strain of Aujeszky’s disease. J Virol Methods. 1992;38:131–144. doi: 10.1016/0166-0934(92)90176-e. [DOI] [PubMed] [Google Scholar]
- 29.Stumpo D J, Graff J M, Albert K A, Greengard P, Blackshear P J. Molecular cloning, characterization, and expression of a cDNA encoding the ‘80- to 87-kDa’ myristoylated alanine-rich C kinase substrate: a major cellular substrate for protein kinase C. Proc Natl Acad Sci USA. 1989;86:4012–4016. doi: 10.1073/pnas.86.11.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner E K, Bloom D C. Experimental investigation of herpes simplex virus latency. Clin Microbiol Rev. 1997;10:419–443. doi: 10.1128/cmr.10.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]