Abstract
Purpose
This study aimed to isolate and characterize palmatine from Fibraurea tinctoria Lour stems, quantify its content, and determine its antioxidant and antidiabetic activities.
Patients and Methods
Palmatine was isolated from the methanol extract of Fibraurea tinctoria Lour stems by silica gel column chromatography. Structural elucidation of the isolated compounds was performed using spectral data analysis and comparison with the literature. High-Performance Liquid Chromatography (HPLC) was used to quantitatively determine palmatine in the crude methanol extract and fractions. The DPPH and non-enzymatic SOD mimic methods were used to assess the antioxidant activity of the methanol extract, fractions, and isolated compounds. The antidiabetic activity was evaluated in silico by the molecular docking method of alpha-glucosidase and DPP-IV enzymes. Palmatine was used as a test ligand and was compared with berberine and its native ligand or standard compounds.
Results
The isolated compound was identified as palmatine. Quantification of palmatine compound by HPLC showed that palmatine was found in the extract and all fractions. In the in vitro antioxidant activity test using the DPPH method, fraction 4 showed the highest activity, with an IC50 value of 91 ppm. In contrast, using the non-enzymatic SOD mimic method, the methanol extract, fraction 5, and isolated compound (palmatine) exhibited very strong antioxidant activity, with IC50 values of 18, 20, and 28 ppm, respectively. The in silico antidiabetic activity of palmatine is thought to have the potential to inhibit these two enzymes.
Conclusion
These results showed that Fibraurea tinctoria Lour stems have potential as an antioxidant and antidiabetic agent. Further research on phytochemical and pharmacological is required to validate the use of this plant species for the treatment of various diseases, especially diabetes mellitus.
Keywords: Fibraurea tinctoria Lour, berberine, palmatine, antioxidant, antidiabetic
Introduction
The prevalence of diabetes mellitus has significantly increased worldwide. Globally, 537 million people have diabetes, which is projected to increase to 643 million by 2030, and this figure is expected to rise by 783 million or approximately 46% by 2045.1 Diabetes mellitus is a chronic metabolic disease characterized by elevated blood glucose levels and abnormalities in the metabolism of carbohydrates, proteins, and fats owing to insufficient insulin production or inadequate insulin activity.2
Oxidative stress and diabetes mellitus have been related in several studies.3,4 Oxidative stress is a condition in which there is an imbalance between the amounts of ROS and antioxidant capacity. ROS mainly encompasses free radicals such as superoxide anion radicals (O2●-), hydroxyl radicals (•OH), and non-free-radical species such as hydrogen peroxide (H2O2) and singlet oxygen (1O2).5 Numerous studies have demonstrated that oxidative stress, mediated mainly by the hyperglycemia-induced generation of free radicals along with a decrease in antioxidant enzyme activity, contributes to the development and progression of diabetes and its complications. Therefore, it became clear that ameliorating oxidative stress through antioxidant treatment may be an effective strategy for improving diabetes and reducing diabetic complications.6–8
Recently, there has been considerable interest in identifying natural antioxidants in plant materials. Extensive studies have been conducted on the antioxidant effects of various substances, including plant-derived antioxidants. It was found that the phytochemical components of medicinal plants exhibit antioxidant activity and synergistic effects with hypoglycemic drugs; thus, they are highly effective in diabetes treatment.9 Antioxidant therapy defends beta cells against oxidative stress-induced apoptosis and preserves the function of beta cells. Previous studies have shown that antioxidants diminish diabetes-related complications and improve insulin sensitivity.10
Fibraurea tinctoria, or what is known as Akar Kuning (Yellow Root) in Kalimantan, is a yellow vine and has long been used by indigenous tribes in Kalimantan for the traditional treatment of various diseases, including diabetes, jaundice, and malaria. The parts often used for treatment are leaves, roots, stems, and bark.11,12 Several studies have been conducted on the pharmacological activity of Fibraurea tinctoria and have shown that this plant has anti-inflammatory,13 anti-malarial,14 anti-microbial,15 and anti-proliferative in cervical, oral, and liver cancers.16,17 According to the taxonomy, this plant belongs to the Menispermaceae family,18 which is known to produce approximately 22 different types of alkaloids. One of them is protoberberine alkaloids, such as berberine, which has been reported to have various pharmacological properties, including antioxidant and antidiabetic properties.19
Berberine, a quaternary ammonium salt from the protoberberine group of isoquinoline alkaloids, is a yellow alkaloid found in several plants.20 Berberine has several derivatives, including palmatine.21 Berberine and palmatine have similar molecular structures, with only a slight difference in substitution of the isoquinoline moiety.22 In contrast to berberine, which has a methylenedioxy moiety at positions C2 and C3 in its tetracyclic structure, palmatine is substituted with four methoxyl groups23 (Figure 1).
Figure 1.
Palmatine and Berberine structure.
Several studies have reported that palmatine has pharmacological effects, including antioxidant and blood glucose regulatory effects.24–27 Palmatine reduced blood glucose levels, increased insulin levels, and improved oxidative stress in STZ-induced diabetic animal models, as indicated by decreased MDA levels and increased enzymatic antioxidant levels.24,26 Palmatine can activate proteins related to antioxidant activity, which protects cells from reactive oxygen species and endoplasmic stress in STZ-induced diabetic animal models.25 These pharmacological effects indicate that palmatine is valuable for preventing and treating certain diseases, including diabetes mellitus and its complications.28 To the best of our knowledge, quantification and evaluation of the antioxidant and antidiabetic activities of palmatine, especially from Fibraurea tinctoria Lour, have not been extensively investigated. This study aimed to isolate, characterize, and determine the palmatine content from the stems of Fibraurea tinctoria Lour and investigate their antioxidant and antidiabetic activities.
Material and Methods
Materials
The plant material was collected in August 2021 from Menua Sadap Village, West Kalimantan Province, Indonesia. It was identified by Mrs. Mukarlina of the Department of Biology, Faculty of Mathematics and Natural Science, Tanjung Pura University, Indonesia (No. 119/A/LB/FMIPA/UNTAN/2021).
Technical-grade solvents were used for extraction and column chromatography after distillation. Silica gel 60 (Merck, 0.063–0.200 mm and 0.200–0.500 mm) was used for column chromatography (CC), whereas precoated silica gel 60 F254 (Merck) was used for thin-layer chromatography (TLC). The DPPH reagent was obtained from Wako (Japan). The reference standard of berberine chloride (purity ≥98.0%, batch No. SLCB1670) was provided by Sigma-Aldrich (St. Louis, MO, USA). Palmatine chloride (purity ≥98.0%, batch No. SLCM3523) was supplied from Merck (Darmstadt, Germany). HPLC-grade methanol and acetonitrile were purchased from Merck (Darmstadt, Germany), whereas trifluoroacetic acid (TFA) was acquired from Sigma-Aldrich (St. Louis, MO, USA).
In silico antidiabetic activity prediction, molecular docking was performed between alpha-glucosidase and dipeptidyl peptidase (DPP-IV) enzymes with palmatine, berberine, and their native ligands or standard compounds. The X-ray crystallographic structures of alpha-glucosidase (PDB ID: 3W37) and DPP-IV (PDB ID: 6B1E), bound with their co-crystallized ligands were obtained from the Protein Data Bank (https://www.rcsb.org/). The 3D structures of berberine and palmatine were retrieved from PubChem (https://pubchem.ncbi.nlm.nih.gov/).
Instrument
The UV-Vis spectra were measured using a Shimadzu UV-1800 ultraviolet-visible spectrophotometer (Shimadzu, Co., Ltd., Kyoto, Japan), and the IR spectra were recorded using a Perkin-Elmer Spectrum 100 FT-IR spectrometer (PerkinElmer, Inc., USA). Meanwhile, mass spectra (MS) were performed on a mass spectrometer Waters Acquity TQ detector using ESI + mode (Waters Corporation, Milford, MA, USA), and NMR spectra were recorded on a JEOL JNM ECZR500 MHz spectrometer (JEOL, Japan) with TMS as an internal standard. HPLC analysis was performed on a Waters Alliance e2695 HPLC system (Waters Corporation, Milford, MA, USA) with column Merck LiChroCART (250 mm x 4.6 mm). The chromatograph was equipped with a UV-Vis 2489 detector (Waters Corporation, Milford, MA, USA). The antioxidant activity test was measured using Biochrom EZ Read 400 Plus Microplate Reader (Biochrom Ltd., Cambridge, UK).
Isolation Compound from Extract of Fibraurea tinctoria Lour
All dried samples were pulverized and passed through a 40-mesh sieve before extraction. The air-dried and powdered stems (1.00 kg) were extracted with MeOH at room temperature (4 × 4 L, 24 h each). Subsequently, the solvent was evaporated, and the MeOH extract (22.3 g) was obtained. The MeOH extract was then partitioned by silica gel column chromatography with stepwise elution of n-hexane, EtOAc, and MeOH with a gradient of 50%, successively to yield five fractions (Fr.1–5). The fraction obtained was then evaporated to obtain Fr.1 (0.3092 g), Fr.2 (1.5289 g), Fr.3 (2.2309 g), Fr.4 (9.5288 g) and Fr.5 (9.7322 g). Fr.5 was further separated on a silica gel column chromatography with EtOAc/MeOH at a gradient of 10% to provide eleven fractions (Fr.5.1–11). Fr.5.8 was chromatographed on silica gel with eluent using EtOAc/MeOH at a gradient of 5% to obtain twenty-five fractions (Fr.5.8.1–25). Fr.5.8.21 (0.0611 g) was acidified using 25 mL of 1% hydrochloric acid and then basified with ammonium hydroxide to a pH of 9.5–10. The alkaloids were then extracted with chloroform. The organic phase from Fr.5.8.21 was then subjected to further TLC testing using silica gel 60 F254 with EtOAc: MeOH (1:1, v/v) solvent and one drop of ammonia to obtain the compound 1 (7 mg).
Structure Determination of Isolated Compound
The chemical structure of the isolated compound was established based on spectroscopic data, such as Ultraviolet-Visible (UV-Vis), Infrared (IR), 1D-NMR (1H, 13C, DEPT 135), and 2D-NMR (HMQC, COSY, HMBC) plus Mass Spectroscopy (MS) data. The HMQC and DEPT 135 experiments helped to assign the 13C, while HMBC and COSY established the connectivities of the molecular fragments. The spectra and structure elucidation analyses were further aided by comparing the observed and published 1H and 13C NMR data.
Determination of Palmatine Contents
Chromatographic analysis of palmatine was performed on a Waters Alliance system consisting of an e2695 separation module and a UV-Vis 2489 detector (Waters Corporation, Milford, MA, USA) and equipped with column Merck LiChroCART (250 mm x 4.6 mm). The mobile phase comprised 0.1% trifluoroacetic acid in water (solvent A) and acetonitrile (solvent B). The gradient elution was applied: 0–1 min, 20% B; 1–6 min, 20–30% B; 6–12 min, 30–40% B, 12–12.01 min, 20% B, 12.01–15 min 20% B. The eluent flow rate and injection volume were 1.0 mL/min and 10 µL, respectively. Detection for quantification was performed at 346 nm.
Evaluation of the Antioxidant Activity
The antioxidant activities of extract, fractions, and isolated compound was determined using the DPPH and non-enzymatic SOD mimic methods, following standardized protocols. Briefly, for the DPPH test, the sample solution and methanol were added to the microplate wells. DPPH solution was added to each sample, incubated for 30 min in the dark, and then measured at 517 nm. A blank solution containing DPPH in methanol was prepared. The absorbance value of each concentration variation was recorded, and the IC50 value was calculated.29 For the non-enzymatic SOD mimic method, sample and bioassay reagent solutions were prepared (the riboflavin solution was dissolved in EDTA and NBT solutions and then homogenized and diluted with phosphate buffer). Working solutions I (containing riboflavin) and II (without riboflavin) were prepared. The test solution was prepared in a microplate well and irradiated in a light box for 15 min. The measurements were performed at 550 nm.30
Molecular Docking Simulations
The molecular docking method was used to investigate the prediction of antidiabetic activity in silico. Autodock 4.0 was the set of tools used. The Chimera program was used to separate the alpha-glucosidase and DPP-IV enzymes from their native ligands and stored in the PDB format. Using Autodock 4.0, alpha-glucosidase and DPP-IV free from native ligands were molecularly anchored to berberine and palmatine. To set the grid box and docking area, the receptors and ligands kept in pdbqt format were opened and stored in gpf format. The ligand-receptor binding that was achieved with the help of genetic algorithm parameters was recorded in the dpf format. Using the Autogrid4 and Autodock4 formulas, both files were docked and saved on the command prompt. The conformation with the lowest binding affinity was selected as the best conformation. The interactions between alpha-glucosidase and DPP-IV enzymes with berberine and palmatine were visualized using the BIOVIA Discovery Studio program.
Results
Structural Determination of Isolated Compound
Compound 1 was isolated as a yellow solid with a molecular formula of C21H24NO4 as determined by MS (m/z 352.68 [M-H]+. The IR spectrum showed the presence of H-C-sp2 stretch (3344 cm−1), H-C-sp3 stretch (2849 and 2910 cm−1), benzene stretch (1230 and 1278 cm−1), -CO stretch (1103 cm−1), and -CN stretch (1037 cm−1). Further supporting data from the UV-Vis spectrum showed absorption at 225, 267, 348, and 429 nm together with IR absorption bands, indicating the presence of a diene group, a benzenoid band, an extension of the conjugation of benzene with diene, and the presence of an auxochrome group attached to benzene conjugated with diene, respectively.
The 13C NMR spectrum determined that there are twenty-one carbon signals consisting of four methyls (δC 55.3, 56.3, 55.7, and 61.2), two methylenes (δC 26.5 and 56.0), six methines (δC 108.5, 110.9, 119.9, 123.2, 126.6, and 145.0), and nine quaternary carbons (δC 119.1, 121.9, 128.7, 133.9, 138.4, 144.3, 149.5, 150.6, and 152.4). The 1H NMR spectrum showed a proton signal at δH 8.09 (1H, d, J = 8.5 Hz) and δH 8.00 (1H, d, J = 9 Hz). The coupling constant values of 8.5 and 9 Hz indicated the ortho position of the aromatic ring. Furthermore, there is a shift at δH 3.25 (2H, d, J = 6.5 Hz) and δH 4.91 (2H, d, J = 7 Hz), which indicates that the two protons are adjacent (vicinal hydrogens). The other four protons are at δH 7.63 (1H, s), 7.02 (1H, s), 9.74 (1H, s), and 8.79 (1H, s), indicating a para position. In addition, one proton singlet at δH 9.74 indicated that the corresponding carbon (C-8) was directly attached to the quaternary nitrogen31 (Table 1).
Table 1.
1H and 13C NMR Spectroscopic Data of Compound 1 (CD3O3; 500 Hz; δ in Ppm)
| Position | δH (ΣH, mult, J in Hz) | δC, type |
|---|---|---|
| 1 | 7.63 (1H, s) | 108.5 (CH) |
| 2 | – | 149.5 (C) |
| 3 | – | 150.6 (C) |
| 4 | 7.02 (1H, s) | 110.9 (CH) |
| 4a | – | 128.7 (C) |
| 5 | 3.25 (2H, d, J = 6.5 Hz) | 26.5 (CH2) |
| 6 | 4.91 (2H, d, J = 7 Hz) | 56.0 (CH2) |
| 8 | 9.74 (1H, s) | 145.0 (CH) |
| 8a | – | 121.9 (C) |
| 9 | – | 144.3 (C) |
| 10 | – | 152.4 (C) |
| 11 | 8.09 (1H, d, J = 8.5 Hz) | 126.6 (CH) |
| 12 | 8.00 (1H, d, J = 9 Hz) | 123.2 (CH) |
| 12a | – | 133.9 (C) |
| 13 | 8.79 (1H, s) | 119.9 (CH) |
| 13a | – | 138.4 (C) |
| 13b | – | 119.1 (C) |
| 2-OCH3 | 3.91 (3H, s) | 55.3 (CH3) |
| 3-OCH3 | 4.08 (3H, s) | 56.3 (CH3) |
| 9-OCH3 | 4.19 (3H, s) | 61.2 (CH3) |
| 10-OCH3 | 3.97 (3H, s) | 55.7 (CH3) |
The COSY spectrum showed correlation between H-1 at δH 7.63 (1H, s) with H-4 at δH 7.02 (1H, s), H-11 at δH 8.09 (1H, d, J=8.5 Hz) with H-12 at δH 8.00 (1H, d, J=9 Hz), H-5 at δH 3.25 (2H, d, J=6.5 Hz) with H-6 at δH 4.91 (2H, d, J=7 Hz). The position of 3-OCH3 was confirmed by the HMBC correlation between the proton at δH 4.08 (3H, s, 3-OCH3) and the carbon at δC 150.6 (C-3). In addition, the position of 2-OCH3 was confirmed by the COSY correlation of the proton at δH 3.91 (3H, s, 2-OCH3) with the proton at δH 4.08 (3H, s, 3-OCH3), suggesting that the position of 2-OCH3 is adjacent to 3-OCH3. The position of 9-OCH3 was confirmed by the HMBC correlation between the proton at δH 4.19 (3H, s, 9-OCH3) and the carbon at δC 144.3 (C-9). Moreover, the position of 10-OCH3 was confirmed by the COSY correlation of the proton at δH 3.97 (3H, s, 10-OCH3) with the proton at δH 4.19 (3H, s, 9-OCH3), which suggested that the position of 10-OCH3 is adjacent to 9-OCH3. Based on spectral data analysis and comparison with published papers,32 compound 1 was identified as palmatine, which has a molecular weight of 352 g/mol. The Key COSY and HMBC correlations of compound 1 are shown in Figure 2.
Figure 2.
Key 1H-1H COSY and HMBC correlations in compound 1.
Determination of Palmatine Contents
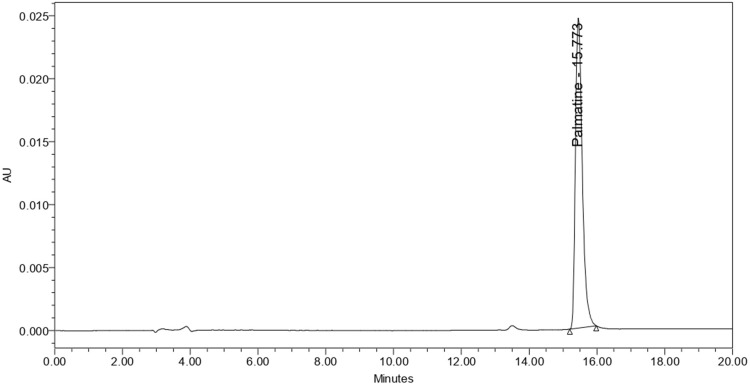
HPLC analysis of palmatine using RP-C18 stationary phase and gradient elution with 0.1% trifluoroacetic acid in water (solvent A) and acetonitrile (solvent B). The absorbance at 346 nm was used to characterize the chromatogram and quantify palmatine content. The retention time of the standard palmatine chloride was 15.773 min, and the HPLC chromatogram is shown in Figure 3.
Figure 3.
HPLC chromatogram of standard palmatine chloride at 8 µg/mL.
The formulas LOD = 3.3σ/S and LOQ = 10σ/S, where σ and S represent the standard deviation of the response and the slope of the calibration curve, respectively, were used to compute the limits of detection (LOD) and limit of quantification (LOQ). The lowest concentration of the analyte in a sample that can be detected using the developed method is known as the limit of detection. On the other hand, the lowest concentration that can be measured in the operating conditions is represented by the limit of quantification.33 Table 2 shows the palmatine retention time data, calculated calibration curve, linearity, LOD, and LOQ.
Table 2.
Retention Time, Calibration Curves, Linearity, LOD, and LOQ HPLC Analysis for Palmatine
| Compound | Retention time | Calibration Curve | r2 | LOD (µg/mL) | LOQ (µg/mL) |
|---|---|---|---|---|---|
| Palmatine | 15.773 | y = 42890x + 743.9 | 0.9998 | 0.1683 | 0.5101 |
Afterwards, the stems of Fibraurea tinctoria Lour were subjected to extraction using methanol and fractionated using n-hexane, ethyl acetate, and methanol with a gradient of 50% to obtain five fractions (fractions 1–5). The extract and fractions were analyzed using HPLC to determine the palmatine content.
Quantification of palmatine compounds by HPLC in extract and fractions from the stems of Fibraurea tinctoria Lour showed that palmatine was found in the extract and all fractions. In the methanol extract, the palmatine content was 1.5398%. On the other hand, the palmatine content was determined to be a high amount at 2.8523% in fraction 5, and the lowest content of palmatine was detected in fraction 1 at 0.0027%, as seen in Table 3.
Table 3.
Palmatine Contents in Fibraurea tinctoria Lour
| Samples | Yield (%) |
Palmatine Contents | |
|---|---|---|---|
| mg/g | % | ||
| MeOH extract | 2.23 | 15.3983 | 1.5398 |
| Fraction 1 | 0.04 | 0.0269 | 0.0027 |
| Fraction 2 | 0.15 | 0.0343 | 0.0034 |
| Fraction 3 | 0.22 | 0.0667 | 0.0067 |
| Fraction 4 | 0.95 | 0.0756 | 0.0076 |
| Fraction 5 | 0.97 | 28.5229 | 2.8523 |
Evaluation of the Antioxidant Activity
The extract, fractions, and the isolated compound from Fibraurea tinctoria Lour were examined for their antioxidant activity using the DPPH and non-enzymatic SOD mimic (mSOD) methods, and Table 4 presents the results. A substance is categorized as a very strong (IC50 value <50 ppm), strong (IC50 value <50-100 ppm), moderate (IC50 value <101-150 ppm), and weak (IC50 value >150 ppm) antioxidant, according to Molyneux (2004).34
Table 4.
Antioxidant Activity of Fibraurea tinctoria Lour
| Extract/Fractions/Compound | IC50 (ppm) | |
|---|---|---|
| DPPH method | Non-enzymatic SOD mimic method | |
| MeOH extract | 113 | 18 |
| Fraction 1 | 46,057 | > 15,000 |
| Fraction 2 | 1,113 | 545 |
| Fraction 3 | 212 | 210 |
| Fraction 4 | 91 | 60 |
| Fraction 5 | 516 | 20 |
| Compound 1 (palmatine) | 1,835 | 28 |
| Berberine chloride (standard) | 5,881 | 12 |
| Quercetin (standard) | 4 | 5 |
In an in vitro antioxidant activity test using the DPPH method, fraction 4 showed the highest activity, with an IC50 value of 91 ppm. In contrast, using the mSOD method, the methanol extract and fraction 5 showed very strong antioxidant activity, with IC50 values of 18 and 20 ppm, respectively. The isolated compound (palmatine) showed very strong antioxidant activity using the mSOD method, with an IC50 value of 28 ppm. In contrast, the DPPH method showed very weak antioxidant activity, with an IC50 value of 1,835 ppm.
In this study, berberine and quercetin were used as standards. Berberine is a well-known protoberberine alkaloid widely used in treating diabetes mellitus and is reported to have antioxidant activity.11 Meanwhile, quercetin is a major plant flavonoid known as a potent antioxidant.35 Berberine showed very strong antioxidant activity when measured using the mSOD method, with an IC50 of 12 ppm, but showed very weak antioxidant activity using the DPPH method. In contrast, quercetin showed very strong antioxidant activity in the DPPH and mSOD methods, with IC50 values of 4 and 5 ppm, respectively.
Molecular Docking Simulations
The prediction of antidiabetic activity was evaluated in silico using molecular docking. A docking study was performed to predict the mode of interaction between the identified compounds with alpha-glucosidase and DPP-IV enzymes. The binding interactions of palmatine were compared with those of berberine and its native ligands or standard compounds. The docking results of the compounds are presented in Table 5. The lower or more negative value of the binding energy indicates a higher binding affinity within the active site of the respective protein target.
Table 5.
Antidiabetic Activity Prediction
| Receptor | Ligand | Binding energy (Kcal/mol) | Hydrogen bond |
|---|---|---|---|
| Alpha-glucosidase enzyme (3W37) | Acarbose | −8.0 | Arg552, His626, Asp568, Asp232, Asp469 |
| Berberine | −6.8 | Arg552, Lys506 | |
| Palmatine | −6.1 | Arg552, Asp568 | |
| DPP-IV enzyme (6B1E) | Vildagliptin | −6.4 | Tyr547, Ser630 |
| Sitagliptin | −8.6 | Gln553, Tyr666, Glu206, Phe357 | |
| Berberine | −7.4 | Tyr547, Tyr585, Tyr662, Gln553 | |
| Palmatine | −7.1 | Tyr547, Tyr585, Lys554, Cys551 |
In silico molecular docking of palmatine, berberine, and its native ligands or standard compounds (acarbose, vildagliptin, and sitagliptin) was performed on the active site of the alpha-glucosidase (PDB ID 3W37) and DPP-IV (PDB ID 6B1E) enzymes. The docking studies results of palmatine, berberine, and the standard for alpha-glucosidase (acarbose) showed binding affinity of −6.1, −6.8, and −8.0 kcal/mol, while those for DPP-IV (vildagliptin and sitagliptin) were −7.1, −7.4, −6.4, and −8.6, respectively.
Discussion
Diabetes mellitus is currently the most common health problem worldwide.1 Since oxidative stress is known to play a role in the pathogenesis of diabetes and its complications, antioxidant therapies have potential value in its treatment. Currently, research related to drug discovery in diabetes not only focuses on insulin-centric targets but also includes glucose-centric strategies, such as the antioxidant protection of beta cells. Most medicinal plants with antidiabetic properties possess antioxidant activity.36 Several studies have shown that antioxidants deliver promising results as a useful complementary therapeutic approach for the treatment of diabetes and for reducing complications.7,8,37
Fibraurea tinctoria is a traditional medicine used by native tribes of Kalimantan to treat diabetes mellitus.12 Palmatine is one of the main bioactive compounds present in several representatives of different botanical genders, such as Fibraurea spp., Coptis spp., Phellodendron spp., Corydalis spp., Berberis spp., Papaver spp., Enantia spp., and others.38 Palmatine has been reported to have several pharmacological properties, including antidiabetic and antioxidant properties.23,28,38
In this study, palmatine was isolated from natural sources using silica gel column chromatography, and its chemical structure was established based on spectral data, such as UV-Vis, IR, NMR, and MS, and compared with previously published papers. The analysis of palmatine content was performed using the HPLC method. No quantification of palmatine in Fibraurea tinctoria Lour has been performed to date. According to this study, the methanol extract from the stems of Fibraurea tinctoria Lour contained a significant quantity of palmatine (15.3983 mg/g). Determination of the palmatine content in fractions showed that as the polarity of the solvent increased, the palmatine content also increased. Among the five fractions, fraction 5 had the highest palmatine content (28.5229 mg/g). The results of this study indicate that the stem extract composition is based on alkaloids and that palmatine is one of the main constituents. A thorough HPLC-based quantification of palmatine and berberine from Coptis chinensis was published by Li et al.39 In their study, this plant was also found to contain protoberberine alkaloids, with palmatine and berberine contents of 16.2412 and 57.3066 mg/g, respectively. Based on this study, our results showed a slightly lower palmatine content than Coptis chinensis.
This study tested the antioxidant activity using DPPH and mSOD methods. The DPPH method is widely used to evaluate the antioxidant activity of a sample by examining its ability to remove the free radicals originating from DPPH. The single electron of the nitrogen atom in DPPH was reduced to the corresponding hydrazine by removing the hydrogen atom from the antioxidant.40 Apart from the DPPH method, the mSOD method can be used to evaluate the antioxidant activity. To test mSOD activity in vitro, xanthine oxidase, which is usually used in enzymatic SOD testing as a source of superoxide anion species (O2●-), was replaced with riboflavin via non-enzymatic photoreduction and nitroblue tetrazolium (NBT) as an indicator. The activity of mSOD was demonstrated by its ability to inhibit the reduction of active redox indicators by O2●- species to obtain an IC50 value measured at λmax 560 nm.41
The antioxidant activity of plants is closely related to their secondary metabolite contents. Phenolic compounds are the primary antioxidant-active compounds found in plants. This is because of their aromatic ring, which makes it possible to relocate and stabilize the unpaired electrons in their structure and facilitates the donation of electrons and hydrogen atoms from their hydroxyl groups.42 However, in addition to phenolic compounds, other natural compounds with promising antioxidant properties, such as alkaloids, have received little attention.
Alkaloids are secondary metabolites containing one or more nitrogen atoms and are generally found in a heterocyclic ring structure. Previous studies have shown that the alkaloids have been studied as potential antioxidants. The antioxidant activity of alkaloids depends on their structures.43,44 The high antioxidant activity of isoquinoline alkaloids is due to the presence of hydroxyl groups (donating hydrogen atoms) in the skeleton of the alkaloid compounds, the presence of N-containing groups, and the availability of H atoms. Furthermore, the group’s position and degree of methylation can affect the antiradical activity of these alkaloids.45 Another study conducted by Yin et al showed that the presence of aryl hydroxyl/phenolic groups or vicinal triol systems in diterpenoid alkaloids is responsible for their antiradical activity.46
The antioxidant activity of aporphine alkaloids increases when the N-acetyl group at the N6 position is changed to an N-methylsulfonyl group,47 whereas in non-phenolic aporphine, which does not have an -OH group, stabilization of the benzylic C-6a radical with a nitrogen lone pair strengthens its antioxidant properties.48 The occurrence of double bonds, two secondary amines, and glucose residues in indole alkaloid structures is responsible for their ability to protect against ROS.49 In imidazole alkaloids, the acid-base equilibrium plays an important role in the free radical scavenging activity. These deprotonated alkaloid species exhibited free radical-scavenging activity.50 Research conducted by Yoon et al showed that quinoline alkaloids with two aromatic OH groups at positions 3 and 8 exhibit strong antioxidant activity. In contrast, the O-substituted quinolines at positions 3 and 4 showed very weak antioxidant activity.51 Piperidine showed strong antioxidant activity owing to its oxidizable SH group.52
There have been conflicting findings from several previous investigations on the antioxidant capacity of palmatine. Research conducted by Chaves et al showed that palmatine isolated from Guatteria friesiana showed very strong activity against DPPH radicals, with an EC50 value of 3.48 μg/mL.53 A similar research by Okechukwu et al demonstrated that palmatine obtained from Coscinium fenestratum could inhibit free radicals produced by DPPH.54 In a study by Mridula et al, palmatine showed very strong antioxidant activity against DPPH radicals with an IC50 value of 45.4 μM.27 In contrast, Jang et al revealed that palmatine isolated from Coptis chinensis exhibits weak or inactive antioxidant activity against DPPH radicals.55
Based on the in vitro antioxidant activity, the DPPH assay results showed that the extract and fractions of Fibraurea tinctoria Lour showed potential as antioxidants. On the other hand, the palmatine compound isolated from Fibraurea tinctoria Lour showed very weak or inactive antioxidant activity against DPPH radicals, with an IC50 value >1000 ppm. However, in this study, the isolated compound, palmatine, showed a higher IC50 value than the berberine standard.
In the mSOD method, the methanol extract, fraction 5, isolated compound (palmatine) from the stems of Fibraurea tinctoria Lour and the berberine standard showed very strong antioxidant activity, with IC50 values <50 ppm. Superoxide dismutase (SOD) is an intracellular antioxidant enzyme responsible for regulating the basal levels of oxidative stress arising from mitochondrial and cytosolic superoxide (O2•-) production. This enzyme is believed to be the key element in the first line of antioxidant defense in the cell and is one of the diabetic biomarkers that is highly desirable to measure its function in studies.56 Apart from SOD, other antioxidant enzymes, such as catalase (CAT) and glutathione peroxidase (GPx), play an important role in fighting free radicals. Glutathione (GSH) is a substrate required for GPx antioxidant action. Collectively, these enzymes work together to defend the cells against oxidative stress.57
In diabetes mellitus, there is a significant decrease in antioxidant enzyme activity and a significant increase in malondialdehyde (MDA), a lipid peroxidation product commonly known as a marker of oxidative stress.58,59 Palmatine has been reported to reduce MDA levels and increase enzymatic antioxidant activities.24,26,27,60–66 Palmatine (10 mg/kg for six weeks) reduced blood glucose levels and increased insulin levels in STZ-induced diabetic rats. These changes are related to reduced oxidative stress, which is characterized by reduced MDA levels and increased SOD enzyme activity.26 A similar study showed that palmatine at a dose of 2 mg/kg for 90 days improved oxidative stress in STZ-induced diabetes mellitus animal models, as indicated by reduced MDA levels and increased SOD, CAT, and GSH activities.24 Palmatine can activate proteins related to antioxidant activity, which protects cells from ROS and endoplasmic stress in STZ-induced diabetes mellitus animal models.25
The strong antioxidant activity of palmatine inhibits the formation of free radicals, which play an important role in glucose auto-oxidation and non-enzymatic protein glycation, thereby weakening the antioxidant defense system and insulin resistance. In addition, palmatine shows the ability to reduce and inhibit lipid peroxidation and chelate metal ions, which are reported to be effective in inhibiting the formation of advanced glycation end products (AGEs) that play a role in the pathological complications of diabetes mellitus. Palmatine can react with the carbonyl groups of reducing sugars and dicarbonyl intermediate compounds, thereby blocking their conversion to AGEs. The anti-glycation activity of palmatine is attributed to its antioxidant properties and ability to capture reactive carbonyls. The ability of palmatine to react with carbonyls is the primary mechanism underlying the inhibition of protein glycation.27
Palmatine stabilizes the free radicals via the nitrogen atom of the isoquinoline moiety. SAR studies of palmatine have shown that it contains positively charged nitrogen, which is structurally unstable. The presence of conjugate double bonds and the influence of the substituent on the benzene ring, namely methoxy, push electrons to the nitrogen atom to stabilize the charge. Apart from the resonance or mesomeric effect, the induction effect of the electron-pushing group, namely, the methoxy group, directs the phi electrons to move toward the nitrogen atom. This transfer of phi electrons can potentially cause homolytic bond breakage or the formation of nitrogen radicals. During homolytic bond breakage, one radical electron is donated to the ROS or RNS radical, thereby stabilizing the ROS/RNS radical. The palmatine radicals formed can stabilize themselves by the mesomeric effect and induction of the benzene ring and benzene substituents so that they are not harmful to the body.
The antidiabetic activity was evaluated in silico by molecular docking. This method is one of the most well-known and effective structure-based in silico methods for predicting the interactions between ligands and protein targets, similar to lock-and-key ideas. This process generally begins by predicting the molecular orientation of a ligand in a receptor and then estimating its complementarity using a scoring function.67 The benefit of using in silico approaches is that they can investigate target structures as potential active sites to provide candidate molecules with information regarding hydrogen interactions and binding affinities. Additionally, this makes the research cost-effective and time-efficient.68
Two enzymes, alpha-glucosidase and DPP-IV, which are known to play significant roles in diabetes mellitus, were used in this study. Alpha-glucosidase, the main carbohydrate digestive enzyme, is found at the brush border of the small intestine. The conversion of starch and disaccharides to glucose is catalyzed by this enzyme. The inhibition of alpha-glucosidase slows carbohydrate digestion and prevents postprandial hyperglycemia, which is a major contributor to chronic diabetes-associated complications.69 DPP-IV enzyme rapidly degrades glucagon like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). This major hormone plays a significant role in maintaining blood glucose levels by promoting insulin secretion. DPP-IV inhibitors block GLP-1 and GIP degradation and thus play a significant role in regulating glucose homeostasis.70
The molecular docking study on the alpha-glucosidase enzyme showed that the standard compound, acarbose, has the highest binding affinity value (−8.0 kcal/mol), followed by berberine (−6.8 kcal/mol) and palmatine (−6.1 kcal/mol). This high affinity is due to hydrogen bond interactions between the ligand and active site residues of the receptor. Both berberine and palmatine showed good interactions with alpha-glucosidase, although their binding affinities were lower than that of acarbose. Berberine forms two hydrogen interactions with the amino acid residues Arg552 and Lys506 (Figure 4B). The proton from Arg552 showed a hydrogen interaction with the ether oxygen of berberine at carbon number 2. Meanwhile, the proton from Lys506 showed a hydrogen interaction with the methoxy oxygen of berberine at carbon number 10. In addition, two hydrophobic interactions were observed between Trp432 with the aromatic ring and aliphatic carbon. Similarly, palmatine formed two hydrogen interactions with the amino acid residues Arg552 and Asp568 (Figure 4C). The proton of Arg552 showed a hydrogen interaction with the methoxy oxygen of palmatine at carbon number 2. In addition, the proton of Asp568 interacted with the methoxy hydrogen of palmatine at carbon number 2. Furthermore, palmatine showed six hydrophobic interactions with Trp329, Trp432, Phe601, and Ala234. Three hydrophobic interactions formed between Trp432, Phe601, and Ala234 with aromatic rings; one hydrophobic interaction formed between Trp432 and the methoxy hydrogen on carbon number 3; and two hydrophobic interactions formed between Trp329 and Trp432 with aliphatic carbon number 5.
Figure 4.
(A) 2D and 3D images of docked conformations of alpha-glucosidase enzyme and acarbose. (B) 2D and 3D images of docked conformations of alpha-glucosidase enzyme and berberine. (C) 2D and 3D images of docked conformations of alpha-glucosidase enzyme and palmatine.
The standard compound, acarbose, showed seven hydrogen interactions with amino acid residues Arg552, His626, Asp568, Asp232, and Asp469 (Figure 4A). The oxygen in acarbose showed a hydrogen interaction with His626 of alpha-glucosidase. The proton of Arg552 showed two hydrogen interactions with the oxygen in acarbose on carbon numbers 2 and 3’. Two other hydrogen interactions were formed between the nitrogen amine and hydrogen hydroxy groups in acarbose with Asp232. Furthermore, two hydrogen interactions were formed between Asp568 and Asp469 with hydrogen amine and nitrogen amine, respectively. In addition, acarbose showed three hydrophobic interactions between Trp329, Trp432, and Phe601 with hydrogen from the aliphatic carbon.
The docking results with the DPP-IV enzyme showed that both berberine and palmatine have good interactions with the DPP-IV enzyme where the binding affinity values of berberine (−7.4 kcal/mol) and palmatine (−7.1 kcal/mol) are higher compared to standard compound, vildagliptin (−6.4 kcal/mol), but lower than sitagliptin (−8.6 kcal/mol). Berberine forms four hydrogen interactions with the amino acid residues Tyr585, Tyr547, Tyr662, and Gln553 (Figure 5C). The proton from Tyr585 showed a hydrogen interaction with the methoxy oxygen of berberine at carbon number 9. The proton from Tyr547 showed a hydrogen interaction with the ether oxygen of berberine at carbon number 2. Two other hydrogen interactions were formed between Tyr662 and Gln553 with the ether carbon and methoxy proton at carbon number 10, respectively. In addition to forming hydrogen interactions, berberine exhibits five hydrophobic interactions. Three hydrophobic interactions were formed between Phe357 and Tyr666 with the aromatic ring of berberine and two hydrophobic interactions were formed between Phe357 with hydrogen from the aliphatic carbon. Similarly, palmatine formed four hydrogen interactions with the amino acid residues Lys554, Tyr547, Cys551, and Tyr585 (Figure 5D). The proton from Lys554 showed a hydrogen interaction with the methoxy oxygen on carbon number 2. Meanwhile, three hydrogen interactions were formed between Tyr547, Cys551, and Tyr585 with the methoxy hydrogen on carbon numbers 9 and 10. Palmatine also exhibits four hydrophobic interactions. Two hydrophobic interactions were formed between Tyr547 with the aromatic ring of palmatine and two hydrophobic interactions were formed between Tyr547 with hydrogen from the aliphatic carbon.
Figure 5.
(A) 2D and 3D images of docked conformations of DPP-IV enzyme and vildagliptin. (B) 2D and 3D images of docked conformations of DPP-IV enzyme and sitagliptin. (C) 2D and 3D images of docked conformations of DPP-IV enzyme and berberine. (D) 2D and 3D images of docked conformations of DPP-IV enzyme and palmatine.
Vildagliptin and sitagliptin were used as standard compounds. Sitagliptin exhibited higher binding affinity than vildagliptin. Vildagliptin showed two hydrogen interactions with amino acid residues Ser630 and Tyr547 (Figure 5A). The proton of Ser630 showed a hydrogen interaction with the carbonyl oxygen of vildagliptin and the oxygen of Tyr547 showed a hydrogen interaction with the amine proton of vildagliptin. In addition, vildagliptin showed three hydrophobic interactions between Tyr547, Trp629, and Tyr666 with aliphatic carbon. On the other hand, the other standard compound, sitagliptin, formed five hydrogen interactions with the amino acid residues Gln553, Tyr666, Glu206, and Phe357 (Figure 5B). Three hydrogen interactions were observed between Gln553 and Tyr666 with two fluorine groups. Meanwhile, two other hydrogen interactions were formed between Glu206 with an aliphatic carbon and Phe357 with an amine hydrogen. Additionally, three hydrophobic interactions were observed between Tyr547 with the phi electrons on the aromatic ring and the amide ring and Tyr666 with aliphatic carbon.
Several in vitro tests of palmatine against alpha-glucosidase have been performed and have shown different results. Research conducted by Tang et al showed that palmatine isolated from Rhizoma coptidis was found to possess potent alpha-glucosidase inhibitory (IC50 2.13 mg/mL) in relation to acarbose (IC50 4.98 mg/mL).71 In contrast, palmatine (IC50 9.39 µM) demonstrated lower inhibitory activity toward alpha-glucosidase compared with acarbose (IC50 1.31 µM).72 Likewise, the alpha-glucosidase inhibitory activity assay results showed that the inhibitory ability of berberine (IC50 0.511 g/L) was lower than that of acarbose (IC50 0.379 g/L).73
The in silico findings of this study strengthen the previously reported in vitro evaluation of alpha-glucosidase inhibitory activity.72,73 Theoretically, inhibitory ligands have the potential to deactivate enzymes by occupying their active sites and hindering access to the active pocket.74 Palmatine and berberine were able to occupy the active pocket of alpha-glucosidase, although they were still unable to match the standard acarbose. Molecular docking of alpha-glucosidase revealed that palmatine has almost the same binding affinity as berberine, but palmatine has more hydrophobic interactions than berberine. Hydrophobic bonds are known to greatly contribute to the conformational stability of alpha-glucosidase, so they can be the main key in directing its pharmacological properties.75,76
Furthermore, molecular docking of DPP-IV protein revealed that neither palmatine nor berberine could surpass sitagliptin as a standard. These docking results aligned with previously reported in vitro and in vivo evaluations of DPP-IV inhibitory activity, which showed that palmatine and berberine weakly inhibited DPP-IV compared with the standard drug sitagliptin.72,77,78 However, in this study, it was found that both palmatine and berberine had better binding affinities than the standard vildagliptin. Palmatine and berberine had more hydrogen and hydrophobic interactions than vildagliptin did. Vildagliptin is a potent, selective, competitive, and reversible DPP-IV inhibitor widely used in various countries. Vildagliptin blocks DPP-IV through substrate-like binding to the active site of the enzyme for an extended time, and compared with other DPP-IV inhibitors, only vildagliptin has been shown to block the inactivation of GLP-1 and GIP between meals and overnight.79,80 Vildagliptin has demonstrated efficacy as a single drug and has shown a synergistic effect, which can enhance efficacy and cause few adverse reactions when combined with other antidiabetic drugs or insulin.81–84
Overall, molecular docking plays a key role in drug development, allowing researchers to understand the complex molecular interactions and design more effective and potent compounds. Although the results of this in silico study indicate that palmatine has not shown satisfactory results as an antidiabetic by molecular docking, this observation provides valuable insight into the potential of this compound. From this analysis, it can be concluded that further research using molecular dynamic simulations is needed to understand the accurate and comprehensive mechanisms and interactions between these compounds and their target proteins.
In addition, it is important to note that existing research does not specifically discuss the antioxidant potential of palmatine, especially from the Fibraurea tinctoria Lour. Diabetes mellitus is closely associated with oxidative stress. Therefore, it is important to understand the potential of this compound not only as an antidiabetic agent but also as an antioxidant. Finally, the results obtained from this computational study can provide a solid foundation for future in vitro tests and will assist experimental researchers in validating the antidiabetic activity related to the antioxidant properties of these compounds.
Conclusion
In the present study, palmatine was successfully isolated from Fibraurea tinctoria Lour. Analysis of the palmatine content showed that the stems of Fibraurea tinctoria Lour contained a significant quantity of palmatine. In vitro antioxidant tests showed that Fibraurea tinctoria Lour and its isolated compound (palmatine) possessed potent antioxidant activity. An in silico study showed that palmatine has potential as an antidiabetic agent by inhibiting alpha-glucosidase and DPP-IV enzymes. Considering that oxidative stress plays a key role in the pathogenesis of diabetes and its complications, the antioxidant properties of palmatine add value to its antidiabetic properties. Further research on phytochemicals and pharmacology is required to validate the use of this plant species in the treatment of various diseases, particularly diabetes mellitus.
Acknowledgments
The authors express their gratitude to Universitas Padjadjaran for providing support to all research facilities, the Ministry of Education, Culture, Research and Technology of the Republic of Indonesia for financial support under the PDD grants with contract no. 3018/UN6.3.1/PT.00/2023, and The Indonesia Endowment Fund for Education or Lembaga Pengelola Dana Pendidikan (LPDP), Indonesia (202108211607388).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.IDF. IDF Diabetes Atlas 10th; 2021. Available from: https://diabetesatlas.org/. Accessed July 19, 2024.
- 2.ADA. Standards of Medical Care in Diabetes — 2020. Diabetes Care. 2020;43:S1–S212. [DOI] [PubMed] [Google Scholar]
- 3.Ighodaro OM. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed Pharmacother. 2018;108:656–662. doi: 10.1016/j.biopha.2018.09.058 [DOI] [PubMed] [Google Scholar]
- 4.Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol. 2019;11(3):45–63. [PMC free article] [PubMed] [Google Scholar]
- 5.Sies H. Oxidative stress: concept and some practical aspects. Antioxidants. 2020;9(9):1–6. doi: 10.3390/antiox9090852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang P, Li T, Wu X, Nice EC, Huang C, Zhang Y. Oxidative stress and diabetes: antioxidative strategies. Front Med. 2020;14(5):583–600. doi: 10.1007/s11684-019-0729-1 [DOI] [PubMed] [Google Scholar]
- 7.Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov. 2021;20(9):689–709. doi: 10.1038/s41573-021-00233-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darenskaya MA, Kolesnikova LI, Kolesnikov SI. Oxidative Stress: Pathogenetic role in diabetes mellitus and its complications and therapeutic approaches to correction. Bull Exp Biol Med. 2021;171(2):179–189. doi: 10.1007/s10517-021-05191-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahimi-Madiseh M, Malekpour-Tehrani A, Bahmani M, Rafieian-Kopaei M. The research and development on the antioxidants in prevention of diabetic complications. Asian Pac J Trop Med. 2016;9(9):825–831. doi: 10.1016/j.apjtm.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 10.Rajendiran D, Packirisamy S, Gunasekaran K. A review on role of antioxidants in diabetes. Asian J Pharm Clin Res. 2018;11(2):48–53. doi: 10.22159/ajpcr.2018.v11i2.23241 [DOI] [Google Scholar]
- 11.Purwaningsih I, Maksum IP, Sumiarsa D, Sriwidodo S. A review of fibraurea tinctoria and its component, berberine, as an antidiabetic and antioxidant. Molecules. 2023;28(3):1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sulandjari N, Dewi WS. Asosiasi Akar Kuning (Fibraurea tinctoria Lour) dengan tumbuhan berpotensi obat di Samboja, Kalimantan Timur. J Hutan Trop. 2016;4(3):232–239. [Google Scholar]
- 13.Su C, Chen Y, Liou M, Tsai H, Chang W, Wu T. Anti-inflammatory activities of furanoditerpenoids and other constituents from Fibraurea tinctoria. Bioorg Med Chem. 2008;16(21):9603–9609. doi: 10.1016/j.bmc.2008.09.023 [DOI] [PubMed] [Google Scholar]
- 14.Nguyen-Pouplin J, Tran H, Tran H, et al. Antimalarial and cytotoxic activities of ethnopharmacologically selected medicinal plants from South Vietnam. J Ethnopharmacol. 2007;109(3):417–427. doi: 10.1016/j.jep.2006.08.011 [DOI] [PubMed] [Google Scholar]
- 15.Galappathie S, Palombo EA, Yeo TC, et al. Comparative antimicrobial activity of South East Asian plants used in Bornean folkloric medicine. J Herbs Med. 2014;4(2):96–105. doi: 10.1016/j.hermed.2014.03.001 [DOI] [Google Scholar]
- 16.Manosroi A, Akazawa H, Pattamapun K, Akihisa T, Manosroi W, Manosroi J. Potent anti-proliferative effects against oral and cervical cancers of Thai medicinal plants selected from the Thai / Lanna medicinal plant recipe database “MANOSROI III” Potent anti-proliferative effects against oral and cervical cancers of Thai medici. Pharm Biol. 2015;53(7):1075–1081. doi: 10.3109/13880209.2014.959613 [DOI] [PubMed] [Google Scholar]
- 17.Manosroi A, Akazawa H, Kitdamrongtham W, Akihisa T, Manosroi W, Manosroi J. Potent antiproliferative effect on liver cancer of medicinal plants selected from the Thai / Lanna medicinal plant recipe database “MANOSROI III”. Evid Based Comp Altern Med. 2015;2015:1–11. doi: 10.1155/2015/397181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.TPL. The plant list. version 1.1; 2013. Available from: http://www.theplantlist.org/. Accessed July 19, 2024.
- 19.Barbosa-Filho JM, Da-Cunha EVL, Gray AI. Alkaloids of the Menispermaceae. Alkaloids Chem Biol. 2000;54:1–190. [Google Scholar]
- 20.Utami AR, Maksum IP, Deawati Y. Berberine and Its Study as an Antidiabetic Compound. Biology. 2023;12(7). doi: 10.3390/biology12070973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vennerstrom JL, Lovelace JK, Waits VB, Hanson WL, Klayman DL. Berberine derivatives as antileishmanial drugs. Antimicrob Agents Chemother. 1990;34(5):918–921. doi: 10.1128/AAC.34.5.918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grabarska A, Wróblewska-łuczka P, Kukula-Koch W, et al. Palmatine, a bioactive protoberberine alkaloid isolated from berberis cretica, inhibits the growth of human estrogen receptor-positive breast cancer cells and acts synergistically and additively with doxorubicin. Molecules. 2021;26(20). doi: 10.3390/molecules26206253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ekeuku SO, Pang KL, Chin KY. Palmatine as an agent against metabolic syndrome and its related complications: a review. Drug Des Devel Ther. 2020;14:4963–4974. doi: 10.2147/DDDT.S280520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogechi Ekeuku S, Nwabueze Okechukwu P, Akyirem Akowoah G, Swee Sen T, Namatama Siyumbwa S, Ruth Anisah Froemming G. Plasma Glucose Lowering Activity of Palmatine and its Effect on Liver, Kidney and Antioxidant Enzymes Parameters in STZ Induced Diabetic Rat Model. Curr Bioact Compd. 2015;11(4):256–263. doi: 10.2174/1573407212666151105185802 [DOI] [Google Scholar]
- 25.Okechukwu PN, Ekeuku SO, Chan HK, Eluri K, Froemming GRA. Palmatine inhibits up-regulation of GRP78 and CALR protein in an STZ-induced diabetic rat model. Curr Pharm Biotechnol. 2021;22(2):288–298. [DOI] [PubMed] [Google Scholar]
- 26.Pakseresht Z, Norouzi P, Hojati V, Moghaddam Kalalian H. Effect of Palmatine Hydrochloride on Oxidative Stress in Streptozotocin–Induced Diabetic Rats. J Adv Med Biomed Res. 2016;24(107):119–129. [Google Scholar]
- 27.Mridula S, Masroor WS, Xavier M, et al. Antioxidant and anti-advanced glycation end products formation properties of palmatine. J Pharm Pharmacogn Res. 2021;9(3):366–378. doi: 10.56499/jppres20.940_9.3.366 [DOI] [Google Scholar]
- 28.Long J, Song J, Zhong L, Liao Y, Liu L, Li X. Palmatine: a review of its pharmacology, toxicity and pharmacokinetics. Biochimie. 2019;162:176–184. doi: 10.1016/j.biochi.2019.04.008 [DOI] [PubMed] [Google Scholar]
- 29.Sungthong B, Srichaikul B. Antioxidant activities, acute toxicity and chemical profiling of torch ginger (Etlingera elatior Jack) inflorescent extract. Pharmacogn J. 2018;10(5):979–982. doi: 10.5530/pj.2018.5.166 [DOI] [Google Scholar]
- 30.Deawati Y, Onggo D, Mulyani I, et al. Metode Non-Enzimatik Riboflavin-Nitrobluetetrazolium (Rb-NBT) Sebagai Teknik Penentuan Aktivitas Penangkal Radikal Bebas Anion Superoksida pada Kompleks Mangan(III)-Salen. Chim Nat Acta. 2018;6(1):12–18. [Google Scholar]
- 31.Keawpradub N, Dej-adisai S, Yuenyongsawad S. Antioxidant and cytotoxic activities of Thai medicinal plants named Khaminkhruea: arcangelisia flava, Coscinium blumeanum and Fibraurea tinctoria. Songklanakarin J Sci Technol. 2005;27(2):455–467. [Google Scholar]
- 32.Malebo HM, Wenzler T, Cal M, et al. Anti-protozoal activity of aporphine and protoberberine alkaloids from Annickia kummeriae. BMC Complement Altern Med. 2013;13(48):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.B-G J, Kang K-H, Yang MH. Development and validation of a HPLC–PDA method for the simultaneous determination of berberine, palmatine, geniposide, and paeoniflorin in Haedoksamul-tang. Appl Sci. 2020;10(16):5482. [Google Scholar]
- 34.Molyneux P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol. 2004;26(2):211–219. [Google Scholar]
- 35.Lesjak M, Beara I, Simin N, et al. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J Funct Foods. 2018;40:68–75. doi: 10.1016/j.jff.2017.10.047 [DOI] [Google Scholar]
- 36.Robertson RP. Antioxidant drugs for treating beta-cell oxidative stress in type 2 diabetes: glucose-centric versus insulin-centric therapy. Discov Med. 2010;9(45):132–137. [PubMed] [Google Scholar]
- 37.Nasri H, Shirzad H, Baradaran A, Rafieian-Kopaei M. Antioxidant plants and diabetes mellitus. J Res Med Sci. 2015;20(5). doi: 10.4103/1735-1995.163977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarabasz D, Kukula-Koch W. Palmatine: a review of pharmacological properties and pharmacokinetics. Phyther Res. 2020;34(1):33–50. doi: 10.1002/ptr.6504 [DOI] [PubMed] [Google Scholar]
- 39.Li L, Zhang D, Wang Y, Liu F, Xu Y, Bao H. Effective extraction of palmatine and berberine from Coptis chinensis by deep eutectic solvents-based ultrasound-assisted extraction. J Anal Methods Chem. 2021;2021(1):9970338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gulcin İ, Alwasel SH. DPPH Radical Scavenging Assay. Processes. 2023;11(8):1–20. doi: 10.3390/pr11082248 [DOI] [Google Scholar]
- 41.Deawati Y, Onggo D, Mulyani I, Hastiawan I, Kurnia D. Activity of Superoxide Dismutase Mimic of [Mn(SALEN)OAc] Complex Compound Non-Enzymatically In Vitro Through Riboflavin Photoreduction. Molekul. 2017;12(1):61–69. doi: 10.20884/1.jm.2017.12.1.294 [DOI] [Google Scholar]
- 42.Chaves N, Santiago A, Alías JC. Quantification of the antioxidant activity of plant extracts: analysis of sensitivity and hierarchization based on the method used. Antioxidants. 2020;9(1). doi: 10.3390/antiox9010076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macáková K, Afonso R, Saso L, Mladěnka P. The influence of alkaloids on oxidative stress and related signaling pathways. Free Radic Biol Med. 2019;134:429–444. doi: 10.1016/j.freeradbiomed.2019.01.026 [DOI] [PubMed] [Google Scholar]
- 44.Atpadkar PP, Gopavaram S, Chaudhary S. Natural–product–inspired bioactive alkaloids agglomerated with potential antioxidant activity: recent advancements on structure-activity relationship studies and future perspectives. Vitamin hormon. 2023;121:355–393. [DOI] [PubMed] [Google Scholar]
- 45.Khamtache-Abderrahim S, Lequart-Pillon M, Gontier E, et al. Isoquinoline alkaloid fractions of Fumaria officinalis: Characterization and evaluation of their antioxidant and antibacterial activities. Ind Crops Prod. 2016;94:1001–1008. [Google Scholar]
- 46.Yin TP, Cai L, Xing Y, et al. Alkaloids with antioxidant activities from Aconitum handelianum. J Asian Nat Prod Res. 2016;18(6):603–610. doi: 10.1080/10286020.2015.1114473 [DOI] [PubMed] [Google Scholar]
- 47.Sharma V, Jaiswal PK, Kumar S, et al. Discovery of Aporphine analogues as potential antiplatelet and antioxidant agents: design, synthesis, structure–activity relationships, biological evaluations, and in silico molecular docking studies. ChemMedChem. 2018;13(17):1817–1832. [DOI] [PubMed] [Google Scholar]
- 48.Muthna D, Cmielova J, Tomsik P, Rezacova M. Boldine and related aporphines: from antioxidant to antiproliferative properties. Nat Prod Commun. 2013;8(12):1797–1800. doi: 10.1177/1934578x1300801235 [DOI] [PubMed] [Google Scholar]
- 49.Fragoso V, Do Nascimento NC, Moura DJ, et al. Antioxidant and antimutagenic properties of the monoterpene indole alkaloid psychollatine and the crude foliar extract of Psychotria umbellata Vell. Toxicol Vitr. 2008;22(3):559–566. [DOI] [PubMed] [Google Scholar]
- 50.Pérez-González A, García-Hernández E, Chigo-Anota E. The antioxidant capacity of an imidazole alkaloids family through single-electron transfer reactions. J Mol Model. 2020;26:1–8. [DOI] [PubMed] [Google Scholar]
- 51.Yoon M-A, Jeong T-S, Park D-S, et al. Antioxidant effects of quinoline alkaloids and 2, 4-di-tert-butylphenol isolated from Scolopendra subspinipes. Biol Pharm Bull. 2006;29(4):735–739. [DOI] [PubMed] [Google Scholar]
- 52.Alexidis AN, Rekka EA, Demopoulos VJ, Kourounakis PN. Novel 1, 4 substituted piperidine derivatives. Synthesis and correlation of antioxidant activity with structure and lipophilicity. J Pharm Pharmacol. 1995;47(2):131–137. [DOI] [PubMed] [Google Scholar]
- 53.Chaves SKM, Afzal MI, Islam MT, et al. Palmatine antioxidant and anti-acetylcholinesterase activities: a pre-clinical assessment. Cell Mol Biol. 2020;66(4):54–59. doi: 10.14715/cmb/2020.66.4.9 [DOI] [PubMed] [Google Scholar]
- 54.Okechukwu PN, Ekeuku SO, Sharma M, Fromming GRA. Palmatine Identified from Malaysian Local Plant Possess In vitro and In vivo Antidiabetic and Antioxidant Effect on High‐Fat Diet‐Induced Diabetic Rat Model. FASEB J. 2019;33(S1):504–506. [Google Scholar]
- 55.Jang MH, Kim HY, Kang KS, Yokozawa T, Park JH. Hydroxyl radical scavenging activities of isoquinoline alkaloids isolated from Coptis chinensis. Arch Pharm Res. 2009;32(3):341–345. doi: 10.1007/s12272-009-1305-z [DOI] [PubMed] [Google Scholar]
- 56.Trist BG, Hilton JB, Hare DJ, Crouch PJ, Double KL. Superoxide Dismutase 1 in Health and Disease: how a Frontline Antioxidant Becomes Neurotoxic. Angew Chem Int Educ. 2021;60(17):9215–9246. doi: 10.1002/anie.202000451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stephenie S, Chang YP, Gnanasekaran A, Esa NM, Gnanaraj C. An insight on superoxide dismutase (SOD) from plants for mammalian health enhancement. J Funct Foods. 2020;68(November 2019):103917. doi: 10.1016/j.jff.2020.103917 [DOI] [Google Scholar]
- 58.Jamuna Rani A, Mythili SV. Study on total antioxidant status in relation to oxidative stress in type 2 diabetes mellitus. J Clin Diagn Res. 2014;8(3):108–110. doi: 10.7860/JCDR/2014/7603.4121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mahboob M, Rahman MF, Grover P. Serum lipid peroxidation and antioxidant enzyme levels in male and female diabetic patients. Singapore Med J. 2005;46(7):322–324. [PubMed] [Google Scholar]
- 60.Cheng JJ, Ma XD, Ai GX, et al. Palmatine Protects Against MSU-Induced Gouty Arthritis via Regulating the NF-κB/NLRP3 and Nrf2 Pathways. Drug Des Devel Ther. 2022;16(July):2119–2132. doi: 10.2147/DDDT.S356307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hao M, Jiao K. Palmatine Alleviates Acute Myocardial Infarction Through Activating pAMPK/Nrf2 Signaling Pathway in Mouse Model. Rev Bras Farmacogn. 2022;32(4):627–635. doi: 10.1007/s43450-022-00288-0 [DOI] [Google Scholar]
- 62.Tang C, Hong J, Hu C, et al. Palmatine Protects against Cerebral Ischemia/Reperfusion Injury by Activation of the AMPK/Nrf2 Pathway. Oxid Med Cell Longev. 2021;2021(6660193):1–12. doi: 10.1155/2021/6660193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng J, Ma X, Zhang H, et al. 8-Oxypalmatine, a novel oxidative metabolite of palmatine, exhibits superior anti-colitis effect via regulating Nrf2 and NLRP3 inflammasome. Biomed Pharmacother. 2022;153:113335. doi: 10.1016/j.biopha.2022.113335 [DOI] [PubMed] [Google Scholar]
- 64.Cheng D, Liu P, Wang Z. Palmatine attenuates the doxorubicin-induced inflammatory response, oxidative damage and cardiomyocyte apoptosis. Int Immunopharmacol. 2022;106(January):108583. doi: 10.1016/j.intimp.2022.108583 [DOI] [PubMed] [Google Scholar]
- 65.Kim YM, Ha YM, Jin YC, et al. Palmatine from Coptidis rhizoma reduces ischemia-reperfusion-mediated acute myocardial injury in the rat. Food Chem Toxicol. 2009;47(8):2097–2102. doi: 10.1016/j.fct.2009.05.031 [DOI] [PubMed] [Google Scholar]
- 66.Ali H, Dixit S. Extraction optimization of Tinospora cordifolia and assessment of the anticancer activity of its alkaloid palmatine. Sci World J. 2013;2013(376216). doi: 10.1155/2013/376216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pinzi L, Rastelli G. Molecular docking: Shifting paradigms in drug discovery. Int J Mol Sci. 2019;20(18). doi: 10.3390/ijms20184331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saikia S, Bordoloi M. Molecular Docking: Challenges, Advances and its Use in Drug Discovery Perspective. Curr Drug Targets. 2018;20(5):501–521. doi: 10.2174/1389450119666181022153016 [DOI] [PubMed] [Google Scholar]
- 69.Zafar M, Khan H, Rauf A, Khan A, Lodhi MA. In silico study of alkaloids as α-glucosidase inhibitors: hope for the discovery of effective lead compounds. Front Endocrinol. 2016;7(DEC):1–17. doi: 10.3389/fendo.2016.00153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ram H, Kumar P, Purohit A, et al. Improvements in HOMA indices and pancreatic endocrinal tissues in type 2-diabetic rats by DPP-4 inhibition and antioxidant potential of an ethanol fruit extract of Withania coagulans. Nutr Metab. 2021;18(1):1–17. doi: 10.1186/s12986-021-00547-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tang Y, Li S, Li S, et al. Screening and isolating potential α-glucosidase inhibitors from Rhizoma Coptidis by ultrafiltration LC-PDA-ESI/MS combined with high-speed countercurrent chromatography and reverse-phase medium-pressure liquid chromatography. Med Chem Res. 2017;26:3384–3394. [Google Scholar]
- 72.Okechukwu P, Sharma M, Tan WH, et al. In-vitro anti-diabetic activity and in-silico studies of binding energies of palmatine with alpha-amylase, alpha-glucosidase and DPP-IV enzymes. Pharmacia. 2020;67(4):363–371. [Google Scholar]
- 73.Li ZQ, Zuo DY, Qie XD, Qi H, Zhao MQ, Wu YL. Berberine acutely inhibits the digestion of maltose in the intestine. J Ethnopharmacol. 2012;142(2):474–480. doi: 10.1016/j.jep.2012.05.022 [DOI] [PubMed] [Google Scholar]
- 74.Copeland RA. Enzymes: A Practical Introduction to Structure, Mechanism, and Data Analysis. John Wiley & Sons; 2023. [Google Scholar]
- 75.Tang H, Ma F, Zhao D, Xue Z. Exploring the effect of salvianolic acid C on α-glucosidase: inhibition kinetics, interaction mechanism and molecular modelling methods. Process Biochem. 2019;78:178–188. [Google Scholar]
- 76.Di Stefano E, Oliviero T, Udenigwe CC. Functional significance and structure–activity relationship of food-derived α-glucosidase inhibitors. Curr Opin Food Sci. 2018;20:7–12. [Google Scholar]
- 77.Zhao Z, Ma R, Ma Y, et al. Discovery of Nine Dipeptidyl Peptidase-4 Inhibitors from Coptis chinensis Using Virtual Screening, Bioactivity Evaluation, and Binding Studies. Molecules. 2024;29(10):2304. doi: 10.3390/molecules29102304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berger JP, SinhaRoy R, Pocai A, et al. A comparative study of the binding properties, dipeptidyl peptidase‐4 (DPP ‐4) inhibitory activity and glucose‐lowering efficacy of the DPP‐4 inhibitors alogliptin, linagliptin, saxagliptin, sitagliptin and vildagliptin in mice. Endocrinol Diabetes Metab. 2018;1(1):1–8. doi: 10.1002/edm2.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ahrén B, Schweizer A, Dejager S, Villhauer EB, Dunning BE, Foley JE. Mechanisms of action of the dipeptidyl peptidase‐4 inhibitor vildagliptin in humans. Diabetes Obes Metab. 2011;13(9):775–783. [DOI] [PubMed] [Google Scholar]
- 80.Foley JE. Insights Into GLP-1 and GIP Actions Emerging From Vildagliptin Mechanism Studies in Man. Front Endocrinol. 2019;10(November):1–7. doi: 10.3389/fendo.2019.00780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mohan V, Zargar A, Chawla M, et al. Efficacy of a combination of metformin and vildagliptin in comparison to metformin alone in type 2 diabetes mellitus: a multicentre, retrospective, real-world evidence study. Diabetes Metab Syndr Obes. 2021;14:2925–2933. doi: 10.2147/DMSO.S315227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sicras-Mainar A, Navarro-Artieda R. Use of metformin and vildagliptin for treatment of type 2 diabetes in the elderly. Drug Des Devel Ther. 2014;8:811–817. doi: 10.2147/DDDT.S65327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shimodaira M, Niwa T, Nakajima K, Kobayashi M. Beneficial effects of vildagliptin on metabolic parameters in patients with type 2 diabetes. Endocrine, Metab Immune Disord Targets Formerly Curr Drug Targets-Immune. Endocr Metab Disord. 2015;15(3):223–228. [DOI] [PubMed] [Google Scholar]
- 84.Hayashi T, Murayama H, Shinfuku Y, Taniguchi T, Tsumiyama I, Oyama N. Safety and efficacy of vildagliptin: 52-week post-marketing surveillance of Japanese patients with type 2 diabetes in combination with other oral antidiabetics and insulin. Expert Opin Pharmacother. 2020;21(1):121–130. doi: 10.1080/14656566.2019.1685500 [DOI] [PubMed] [Google Scholar]