ABSTRACT
Candida auris, an emerging fungal pathogen, predominately colonizes human skin leading to serious invasive infections in humans. Though it is assumed that skin colonization can lead to invasive infection, dissemination potential of C. auris from skin to internal organs is still unknown. In this study, immunocompetent and immunocompromised mouse models of intradermal skin infection were used to compare the dissemination potential of C. auris to internal organs. Our results suggest that C. auris persists in the skin tissue of both immunocompetent and immunocompromised infected mice even at 30 days post-infection. Furthermore, C. auris can readily disseminate from skin tissue to internal organs such as the spleen and kidney as early as 24 h post-infection and was detected until 30 days post-infection. Taken together, our findings for the first time indicate that murine skin intradermally infected with C. auris can readily disseminate to internal organs and cause invasive infections.
IMPORTANCE
Candida auris is a multi-drug-resistant emerging fungal pathogen colonizes the human skin and causes life-threatening infections. However, whether C. auris can disseminate from the skin to internal organs is unclear. Understanding the dissemination potential of C. auris in both immunocompetent and immunocompromised hosts is necessary to monitor susceptible individuals and to develop novel approaches to prevent and treat this emerging fungal pathogen. Using mouse models of intradermal C. auris skin infection, our findings report a novel observation that mice skin intradermally infected with C. auris can readily disseminate to internal organs leading to systemic disease. These findings help explain the colonization, persistence, and dissemination potential of C. auris in immunocompetent and immunocompromised hosts and reveal that skin infection is a potential source of invasive infection.
KEYWORDS: Candida auris, skin infection, dissemination
OBSERVATION
Candida auris was recently classified as an urgent threat and critical priority fungal pathogen by the US Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) (1–3). Unlike other Candida species, such as Candida albicans which colonize the oral cavity and gastrointestinal tract, C. auris mainly colonizes the human skin leading to nosocomial transmission and systemic infections (4–6). Furthermore, C. auris enters the skin tissue and persists long term in humans and mice (4, 7). Recent findings suggest that C. auris was detected in murine skin tissue even after 4 months of infection and can persist for long term in the skin (4, 8). Though it was assumed that skin colonization can lead to invasive infection, to date the dissemination potential of C. auris from skin to internal organs is not known.
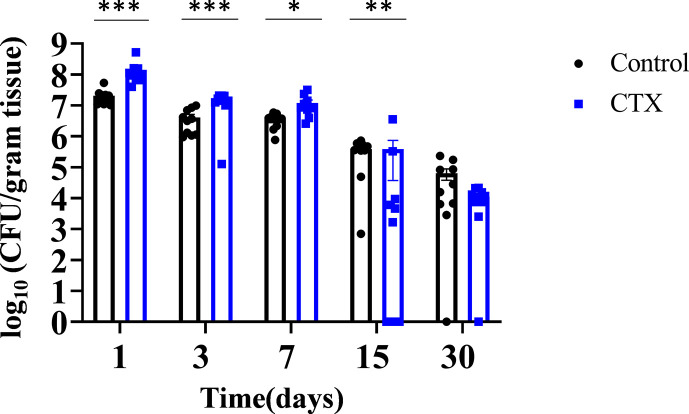
Since immunocompromised patients are highly susceptible to invasive fungal infections including C. auris, we utilized immunocompetent and immunocompromised C57BL/6 mouse models to compare the intradermal fungal load and the dissemination potential of C. auris from skin tissue to internal organs. Immunocompromised mice received cyclophosphamide 3 days prior to infection and subsequent injections once a week throughout the experiment. Untreated and cyclophosphamide-treated immunocompromised mice were intradermally infected with C. auris 0387 South Asian strain. Groups of untreated and immunocompromised infected mice were euthanized 1, 3, 7, 15, and 30 days post-infection to determine the fungal load in skin tissue and in internal organs such as the spleen and kidney. At early time points, 1, 3, and 7 days post-infection, mice treated with cyclophosphamide had a statistically significant higher fungal load in skin tissue (Fig. 1). Following 24 h of infection, the average fungal load in the skin tissue was 7.31 ± 0.66 log10 CFU/gram of tissue for untreated mice and 8.15 ± 0.76 log10 CFU/gram of tissue for the immunocompromised mice. After 3 days post-infection, the average fungal load in skin tissue of untreated mice was 6.61 ± 0.60 log10 CFU/gram of tissue, whereas cyclophosphamide-treated mice average was 7.16 ± 0.63 log10 CFU/gram of tissue (Fig. 1). After 7 days post-infection, the average fungal burden in the skin tissue of untreated mice was 6.55 ± 0.57 log10 CFU/gram of tissue in comparison with 7.08 ± 0.65 log10 CFU/gram of tissue in cyclophosphamide-treated mice. At 15 days, the fungal burden was 5.59 ± 0.49 log10 CFU/gram of tissue in untreated, infected mice compared to 5.59 ± 0.55 log10 CFU/gram of tissue in the cyclophosphamide-treated mice. At 30 days post-infection, the average fungal burden in the skin tissue of untreated mice was 4.80 ± 0.44 log10 CFU/gram of tissue, and cyclophosphamide-treated mice was 4.05 ± 0.34 log10 CFU/gram of tissue. We observed that cyclophosphamide-treated mice on days 1, 3, 7, and 15 post-infection did exhibit decreased healing when compared to the untreated group. These mice also exhibited visible soft tissue swelling at the infection sites that yielded purulent material on the cut surface. Taken together, C. auris persisted in the skin tissues of mice in both immunocompetent and immunocompromised mice until 30 days post-infection.
Fig 1.
Kinetics of fungal load in mouse skin tissue treated with and without cyclophosphamide. Mice were intradermally infected with C. auris AR0387. The control group received only fungal inoculation and CTX group received fungal inoculation and cyclophosphamide treatment. Each group consisting of 10 mice per group were euthanized on 1, 3, 7, 15 and 30 days post-infection and skin tissue were collected. Tissues were homogenized and plated onto YPD agar containing antibiotics. Data are represented as mean ± SEM for each group. Statistical significance was calculated using unpaired student’s t-test for days 1,3, and 7; Mann-Whitney U-test was used for days 15 and 30. *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 were considered as significant.
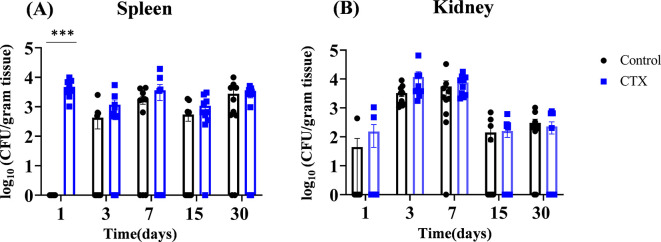
Next, we examined whether C. auris disseminates from skin tissue to internal organs such as the spleen and kidney. Surprisingly, we identified fungal dissemination from intradermal skin to spleen and kidney in both untreated and cyclophosphamide-treated mice after 1, 3, 7, 15, and 30 days post-infection (Fig. 2). The untreated mice did not exhibit detectable levels of fungal burden on day 1 post-infection. The average fungal load in the spleen of untreated mice on 3, 7, 15, and 30 days post-infection was 2.63 ± 0.24 log10, 3.24 ± 0.27 log10, 2.74 ± 0.23 log10, and 3.45 ± 0.30 log10 CFU/gram of tissue. The average fungal load in the spleen of cyclophosphamide-treated mice was 3.67 ± 0.29 log10, 3.07 ± 0.27 log10, 3.55 ± 0.33 log10, 3.04 ± 0.25 log10, and 3.41 ± 0.27 log10 CFU/gram of tissue on 1, 3, 7, 15, and 30 days post-infection, respectively (Fig. 2A). Cyclophosphamide-treated mice showed 3.5 log10 fold higher fungal load than untreated control in spleen. All mice within the cyclophosphamide-treated group exhibited marked splenomegaly at all time points for tissue collection. At 1, 3, 7, 15, and 30 days post-infection, the average fungal load in the kidneys of untreated mice were 1.64 ± 0.16 log10, 3.52 ± 0.29 log10, 3.74 ± 0.35 log10, 2.15 ± 0.18 log10, and 2.49 ± 0.20 log10 CFU/gram of tissue, whereas cyclophosphamide-treated mice had 2.18 ± 0.20 log10, 4.07 ± 0.38 log10, 3.88 ± 0.32 log10, 2.20 ± 0.18 log10, and 2.36 ± 0.20 log10 CFU/gram of tissue. Collectively, our findings suggest that mice infected intradermally infected with C. auris can disseminate to internal organs such as the spleen and kidney and persist until 30 days post-infection. Given that fungal dissemination was observed in mice intradermally infected with C. auris, we examined if mice epicutaneously infected with C. auris can disseminate to internal organs. We used the epicutaneous model utilized by Huang et al. (4) and examined the fungal dissemination in both immunocompetent and immunocompromised mice. Mice were infected topically every other day three times and the fungal burdens in both the spleen and kidneys were measured on 3, 15, and 30 days post-infection. Our results identified that mice epicutaneously infected with C. auris did not observe detectable levels of fungal colonies in the spleen and kidney of both immunocompetent and immunocompromised groups on days 3, 15, and 30 post-infection.
Fig 2.
Kinetics of fungal load in internal organs from infected mice treated with and without cyclophosphamide. Groups of C. auris AR0387-infected mice from untreated (Control) and cyclophosphamide-treated group (CTX) were euthanized on 1, 3, 7, 15, and 30 days post-infection and internal organs such as the spleen and kidney were collected. (A) Spleen and (B) kidney were homogenized and plated onto YPD agar containing antibiotics to determine the fungal load. 10 mice per group per time point were used. Data are represented as mean ± SEM for each group. Statistical significance was calculated using the Mann-Whitney U test, excluding day 1 for spleen data; this data set exhibited normal distribution, and significance was evaluated using an unpaired t-test with Welch’s approximation. ***P ≤ 0.001 was considered as significant.
Taken together, our findings suggest that C. auris persists in the skin tissue of untreated and cyclophosphamide-treated mice and can readily disseminate to internal organs such as the spleen and kidney. Although there is no overall trend in fungal colonization and dissemination potential of C. auris between untreated and cyclophosphamide-treated mice, our findings for the first time indicate that C. auris intradermal infection can disseminate to internal organs and potentially lead to invasive systemic infections in humans. Similar to systemic mouse models using intraperitoneal and intravenous route of C. auris infection that disseminate to kidneys (9, 10), our results suggest that mice intradermally infected with C. auris can readily disseminate to internal organs even 1 day post-infection. Moreover, the model presented by Xin et al. also suggested no statistically significant difference in overall fungal burden in the kidneys of immunocompetent and immunocompromised C57BL/6 mice 2 days post-infection (10) which agrees with our findings. Furthermore, our findings suggest that intradermal but not epicutaneous infection leads to C. auris dissemination to internal organs. These results suggest that tissue trauma and (or) wound infection may potentially lead to C. auris dissemination to internal organs.
Considering that C. auris preferentially colonizes human skin, this mouse model is a clinically relevant infection model to study the pathogenesis of this emerging fungal pathogen. Furthermore, this mouse model could be useful to test the effect of local and systemic antifungal therapeutics. Previous findings using the mouse epicutaneous infection model suggest that skin colonization is different among the four major clades of C. auris (4). Future studies using different clades and clinical isolates of C. auris are important to understand the dissemination potential and persistence of various clades in internal organs. We used cyclophosphamide as it is a commonly used immunosuppressive drug in cancer patients and individuals undergoing transplantation (11). However, it is critical to examine the effect of other immunosuppressive drugs in C. auris skin colonization, intradermal infection, and dissemination to internal organs. Collectively, our findings report a novel observation that intradermal infection of C. auris leads to systemic infection.
The chemicals used in this study were purchased from the following vendors: agar (BP1423-500, Fisher Bioreagents, Pittsburgh, PA USA), ampicillin (14417 Cayman Chemicals, Ann Arbor, MI USA), cyclophosphamide (CTX) (239785, Sigma Aldrich, St. Louis, MO USA), isoflurane (Dechra, Fort Worth, TX USA), phosphate buffer solution (PBS 10×) (BP3994, Fisher Bioreagents), sodium chloride (BPB58-212, Fisher Bioreagents, Pittsburgh PA USA), streptomycin (100556, MP Biomedicals, Santa Ana, CA USA), and yeast peptone dextrose (YPD) (242810, BD Difco, Franklin Lakes, NJ USA). Supplies used for this study were obtained from the following vendors: 1 mL syringes (14-826-87, BD Syringes, Fisher Scientific), 10 mL syringes (302995, BD Difco), 27 g hypodermic needles (NEZ27114, Air-Tite, VA USA), U-bottom 96-well plate (229190, Cell Treat, Pepperell, MA USA), 0.2 μm syringe filter (13-1001-06, Fisher Scientific, Pittsburgh, PA USA), veterinary trimmer (Wahl, Kent Scientific, Torrington, CT USA), chemical depilatory cream (Nair, Ewing, NJ USA), and cotton-tipped applicators (19-062-715 Puritan Medical Products, Guilford, ME USA).
C57BL/6J male and female mice from The Jackson Laboratory ranging from 6 to 8 weeks of age, housed at 28°C−29°C, 50% humidity, standard rodent diet (Teklad 2018C) and 12-h light/dark cycles. Groups of mice were infected with C. auris as described previously (8). One group from C. auris-infected mice received weekly injections of cyclophosphamide. Animal use was approved by the Institutional Animal Care and Use Committee (IACUC) at Purdue University. Candida auris South Asian clade strain 0387 was obtained from CDC AR Isolate Bank, USA. This strain was cultured and enriched using YPD medium at 37°C. CFU was determined using measured optical density (OD) to achieve an average of 2 × 107 CFU; this suspension was used for infection at a dose of 100 µL per mouse. Mice were anesthetized with isoflurane and maintained via nosecone. Right dorsal abdominal wall was shaved and received application of depilatory cream (Nair) for 10 seconds. The skin received two additional rinses of water, blotted dry, and skin wiped clean using alcohol. A sterile 1″ 27 g needle was used to inject 100 µL of the C. auris isolate intradermally. Mice were placed into a clean cage in lateral recumbency for recovery.
Mice from the same strain, mixed sexes, and housing conditions as listed above were grouped into two test groups, epicutaneously infected and epicutaneously infected + CTX-treated cages. A group of 5 mice in each group was assigned a designed time point at 3, 15, and 30 days. Mice in the infected + CTX groups did receive the same CTX dose intervals as described above. C. auris South Asian clade strain 0387 inoculates were prepared in the same fashion as described above. The OD was measured, and a fungal load of 1 × 109 CFU per mouse was then applied to the mouse skin using cotton-tipped applicators; mouse skin was prepared as described above 3 days prior to initial application to allow the skin to heal. The fungal suspension was applied every other day interval for three infection days; days post-infection were counted after the last infection day.
Cyclophosphamide 60 mg was dissolved in 3 mL of 0.9% sterile NaCl to make a 20 mg/mL solution. Once dissolved, this solution was passed through a 0.22 μm filter to produce a sterile solution, which was used no longer than 5 days per manufacturer’s instructions. The experimental group of mice received 200 mg/mL (intraperitoneal route) 3 days prior to infection with C. auris, and each experimental timepoint group received weekly injections, alternating left and right abdominal wall starting 7 days post-infection. Prior to weekly injections, each mouse was weighed, and doses were calculated at 150 mg/mL. Injections of cyclophosphamide were delivered using tuberculin syringes with 27 g needles. Mice within the cyclophosphamide groups were maintained in autoclaved cages, autoclaved water/food, and received weekly cage changes.
Groups of mice were euthanized via CO2 followed by cervical dislocation at the specified time points of 1, 3, 7, 15, and 30 days post-infection. Following euthanasia, skin tissues, spleen, and kidney were collected. Skin tissue was homogenized for 2 minutes, and internal organs were homogenized for 1 minute each in 3 mL of PBS. Homogenized suspensions were plated onto YPD agar plates with 100 μg/mL of streptomycin and 100 μg/mL of ampicillin as described before (8). Plates were cultured at 37°C for 24 h and the colonies were counted to determine the fungal load.
Collected data were statistically evaluated using a Mann-Whitney U-test. However, three data sets from skin samples (days 1, 3, and 7) and one data set from the spleen sample (day 1) were found to exhibit normal distribution; statistical significance was evaluated using unpaired student’s t-tests with Welch’s approximation. P values of ≤ 0.05 were considered significant. Graphical data were generated using Prism GraftPad software, and data present the median as well as standard error of the mean between immunosuppressed and immunocompetent C. auris groups.
ACKNOWLEDGMENTS
This study was supported by NIAID (1R01AI177604 to S.T.).
K.A.T. performed the experiments, analyzed data, and prepared figures. A.D. assisted in mouse experiments. K.A.T. and S.T. wrote the manuscript. All authors contributed and approved the final version of the manuscript.
Contributor Information
Shankar Thangamani, Email: sthangam@purdue.edu.
Sudha Chaturvedi, Mycology Laboratory, Wadsworth Center, Albany, New York, USA.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.00127-24.
Fig. S1.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Ahmad S, Alfouzan W. 2021. Candida auris: epidemiology, diagnosis, pathogenesis, antifungal susceptibility, and infection control measures to combat the spread of infections in healthcare facilities. Microorganisms 9:807. doi: 10.3390/microorganisms9040807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO fungal priority pathogens list to guide research, development and public health action. 2022. Geneva: World Health Organization. https://www.who.int/publications/i/item/9789240060241. [Google Scholar]
- 3. Kadri SS. 2020. Key takeaways from the U.S. CDC’s 2019 antibiotic resistance threats report for frontline providers. Crit Care Med 48:939–945. doi: 10.1097/CCM.0000000000004371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang X, Hurabielle C, Drummond RA, Bouladoux N, Desai JV, Sim CK, Belkaid Y, Lionakis MS, Segre JA. 2021. Murine model of colonization with fungal pathogen Candida auris to explore skin tropism, host risk factors and therapeutic strategies. Cell Host Microbe 29:210–221. doi: 10.1016/j.chom.2020.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shastri PS, Shankarnarayan SA, Oberoi J, Rudramurthy SM, Wattal C, Chakrabarti A. 2020. Candida auris candidaemia in an intensive care unit - prospective observational study to evaluate epidemiology, risk factors, and outcome. J Crit Care 57:42–48. doi: 10.1016/j.jcrc.2020.01.004 [DOI] [PubMed] [Google Scholar]
- 6. Proctor DM, Drummond RA, Lionakis MS, Segre JA. 2023. One population, multiple lifestyles: commensalism and pathogenesis in the human mycobiome. Cell Host Microbe 31:539–553. doi: 10.1016/j.chom.2023.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sparber F, De Gregorio C, Steckholzer S, Ferreira FM, Dolowschiak T, Ruchti F, Kirchner FR, Mertens S, Prinz I, Joller N, Buch T, Glatz M, Sallusto F, LeibundGut-Landmann S. 2019. The skin commensal yeast Malassezia triggers a type 17 response that coordinates anti-fungal immunity and exacerbates skin inflammation. Cell Host Microbe 25:389–403. doi: 10.1016/j.chom.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 8. Datta A, Das D, Nett JE, Vyas JM, Lionakis MS, Thangamani S. 2023. Differential skin immune responses in mice intradermally infected with Candida auris and Candida albicans. Microbiol Spectr 11:e0221523. doi: 10.1128/spectrum.02215-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vila T, Montelongo-Jauregui D, Ahmed H, Puthran T, Sultan AS, Jabra-Rizk MA. 2020. Comparative evaluations of the pathogenesis of Candida auris phenotypes and Candida albicans using clinically relevant murine models of infections. mSphere 5:e00760-20. doi: 10.1128/mSphere.00760-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xin H, Mohiuddin F, Tran J, Adams A, Eberle K. 2019. Experimental mouse models of disseminated Candida auris infection. mSphere 4:e00339-19. doi: 10.1128/mSphere.00339-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bolaños-Meade J, Hamadani M, Wu J, Al Malki MM, Martens MJ, Runaas L, Elmariah H, Rezvani AR, Gooptu M, Larkin KT, et al. 2023. Post-transplantation cyclophosphamide-based graft-versus-host disease prophylaxis. N Engl J Med 388:2338–2348. doi: 10.1056/NEJMoa2215943 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1.