Abstract
Translation is an elementary cellular process that involves a large number of factors interacting in a concerted fashion with the ribosome. Numerous natural products have emerged that interfere with ribosomal function, such as puromycin, which mimics an aminoacyl tRNA and causes premature chain termination. Here, we introduce a photoswitchable version of puromycin that, in effect, puts translation under optical control. Our compound, termed puroswitch, features a diazocine that allows for reversible and nearly quantitative isomerization and pharmacological modulation. Its synthesis involves a new photoswitchable amino acid building block. Puroswitch shows little activity in the dark and becomes substantially more active and cytotoxic, in a graded fashion, upon irradiation with various wavelengths of visible light. In vitro translation assays confirm that puroswitch inhibits translation with a mechanism similar to that of puromycin itself. Once incorporated into nascent proteins, puroswitch, reacts with standard puromycin antibodies, which allows for tracking de novo protein synthesis using western blots and immunohistochemistry. As a cell-permeable small molecule, puroswitch can be used for nascent proteome profiling in a variety of cell types, including primary mouse neurons. We envision puroswitch as a useful biochemical tool for the optical control of translation and for monitoring newly synthesized proteins in defined locations and at precise time points.
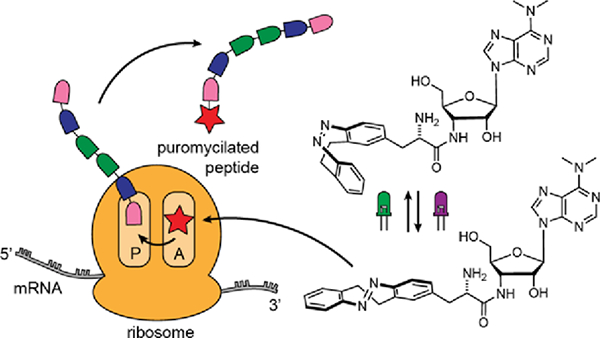
Graphical Abstract
INTRODUCTION
Translation is an essential process in cellular growth, plasticity, and differentiation that is particularly active in cancer progression, viral pathogenesis, and memory formation.1–5 To study translation, many naturally occurring molecules have been employed that inhibit or stall it, such as cycloheximide, anisomycin, or puromycin. Puromycin structurally mimics the 3’ end of an aminoacylated tRNA (aa-tRNA), featuring an amide linkage between its amino acid and nucleoside moieties, instead of the more labile ester linkage of aa-tRNAs.6,7 As such, it exhibits a unique mechanism of action that involves entry into the ribosomal A site and covalent trapping of the nascent polypeptide chain from the P-site. After its incorporation, puromycin cannot be further cleaved by incoming aa-tRNAs, leading to stalling and subsequent premature chain release of the puromycylated peptide.8,9
Puromycin has been extensively used in molecular biology and various assays that involve puromycylation have been reported.10 In SUrface SEnsing of Translation (SUnSET), antibody-coupled detection of puromycylated peptides on the cell surface is used to study translation fluctuations.11 Others include the subcellular localization of translating ribosomes with the ribopuromycylation method (PRM), where puromycylated nascent chains are immobilized on ribosomes by the use of chain elongation inhibitors.12 Structural analogs of puromycin have been developed to further broaden the scope of its applications (Fig. 1A). For example, O-propargyl-puromycin (OPP) features an alkyne group that allows for subsequent enrichment or visualization of puromycylated proteins through copper-catalyzed “click” reaction with an azide conjugated biotin or fluorophore.13
Figure 1.
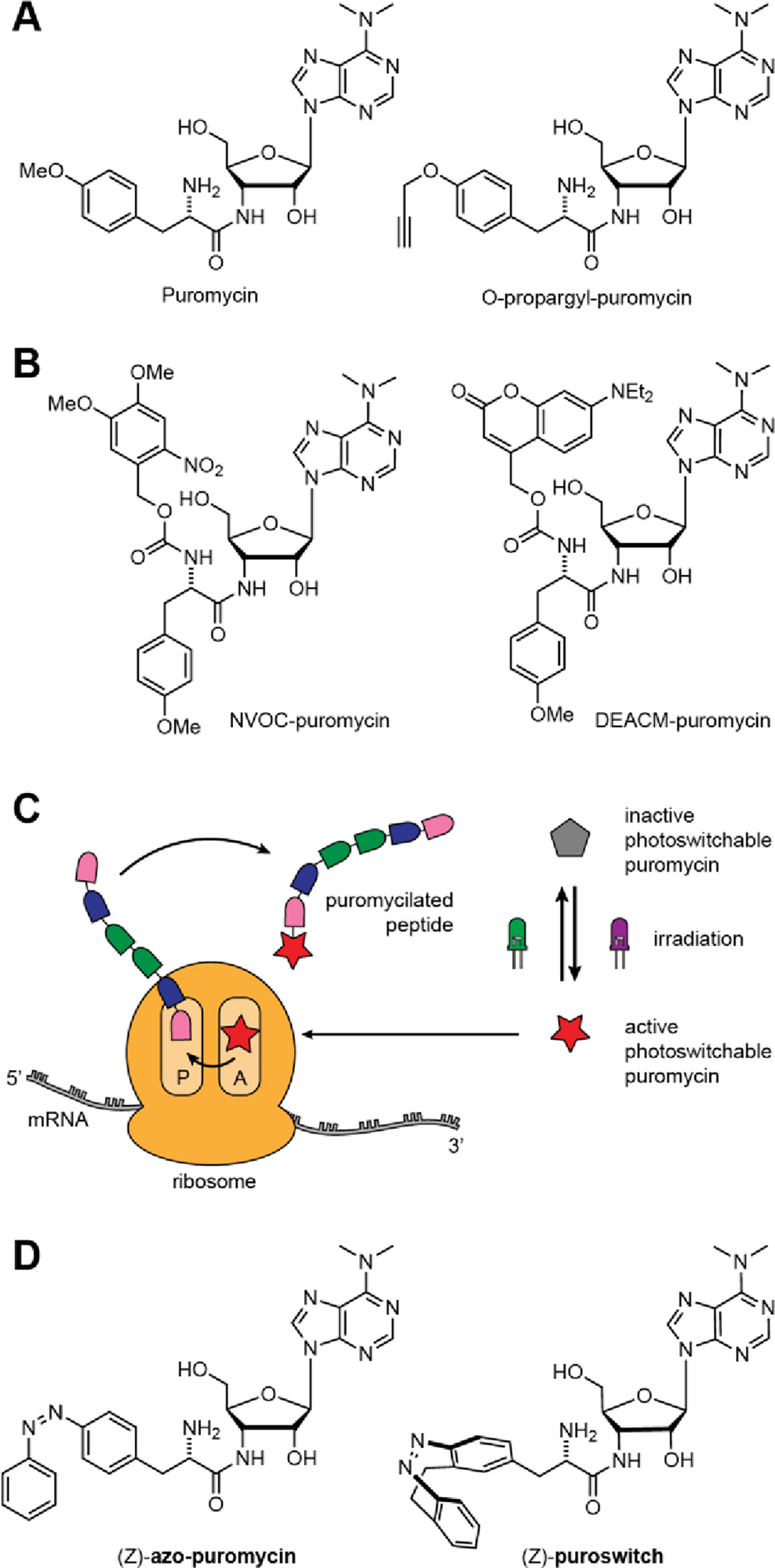
A) Puromycin and functionalized analog O-propargyl-puromycin. B) Photocaged analogs of puromycin. C) Proposed mode of action of photoswitchable puromycin analogs. D) Photoswitchable puromycin analogs synthesized.
In order to allow for spatiotemporal control of the application of puromycin, photocaged versions of the compound have been introduced by the Schwalbe and Schuman groups (Fig. 1B). In these compounds, the amino acid position is appended with the photolabile groups O-Nitroveratryloxycarbonyl (NVOC) or 7-Diethylamino-4-methylcoumarin (DEACM).14,15 Upon irradiation with UV-A light, puromycin is released. However, photocaging approaches are inherently limited by the irreversible nature of the process where the photocage group, once cleaved, cannot be re-attached. This constraint prevents the spatiotemporal interrogation of processes that occur in specific time intervals as the compound would remain active after release and also diffuse to non-irradiated areas.16 Recently, the Schwalbe group developed a once-reversible caged puromycin analog with two photoresponsive groups that could activate and deactivate the compound with two different wavelengths of light.17 However, the use of UV-light (365 nm) for the uncaging of these compounds is not ideal for in vivo applications as it can have low tissue penetration and prolonged exposure can be damaging to cell cultures and model organisms, making the development of red-shifted tool compounds a desirable goal.18,19
Here we describe the development of a photoswitchable puromycin analog that allows for fully reversible and tunable translation inhibition (Fig. 1C). The compound, which we named puroswitch, can be photoisomerized with two wavelengths of visible light, which results in a large difference in biological activity. This feature makes our photoswitchable compound suitable for biological applications where one seeks to understand the role of protein synthesis with temporal and spatial control.
RESULTS
Design, synthesis, and photophysical characterization of photoswitchable puromycin analogs
Previously published structure-activity relationship studies showed that the amino acid side chain of puromycin can be modulated to a certain degree without losing activity.20,21 In addition, photoswitchable amino acids have been developed for the optical control of peptides and proteins.22,23 We chose azobenzene as our photoswitch as it is known for its fatigue resistance, large and predictable geometrical changes, and well-characterized photothermal properties.24,25 We thought to “azo-extend” the 4-position of puromycin’s amino acid side chain by substituting the methoxy group with an phenyl diazene moiety, yielding azo-puromycin (Fig. 1D).26 Intriguingly, this very molecule had already been reported by the Sisido group as spectroscopic standard.27 To the best of our knowledge, however, it was never evaluated for its biological activity, especially in a light-dependent fashion. Synthesis of the azobenzene analog was achieved through a HATU promoted coupling of the known Fmoc-protected azobenzene amino acid 1 with the commercially available puromycin aminonucleoside 2, followed by protecting group removal (Fig. 2A). The photoswitching and thermal relaxation properties of the compound are shown in the Supporting Information (Fig. S2). The optimal wavelength for switching to the cis isomer was 370 nm and rapid isomerization back to the trans form could be achieved with wavelengths >450 nm.
Figure 2.
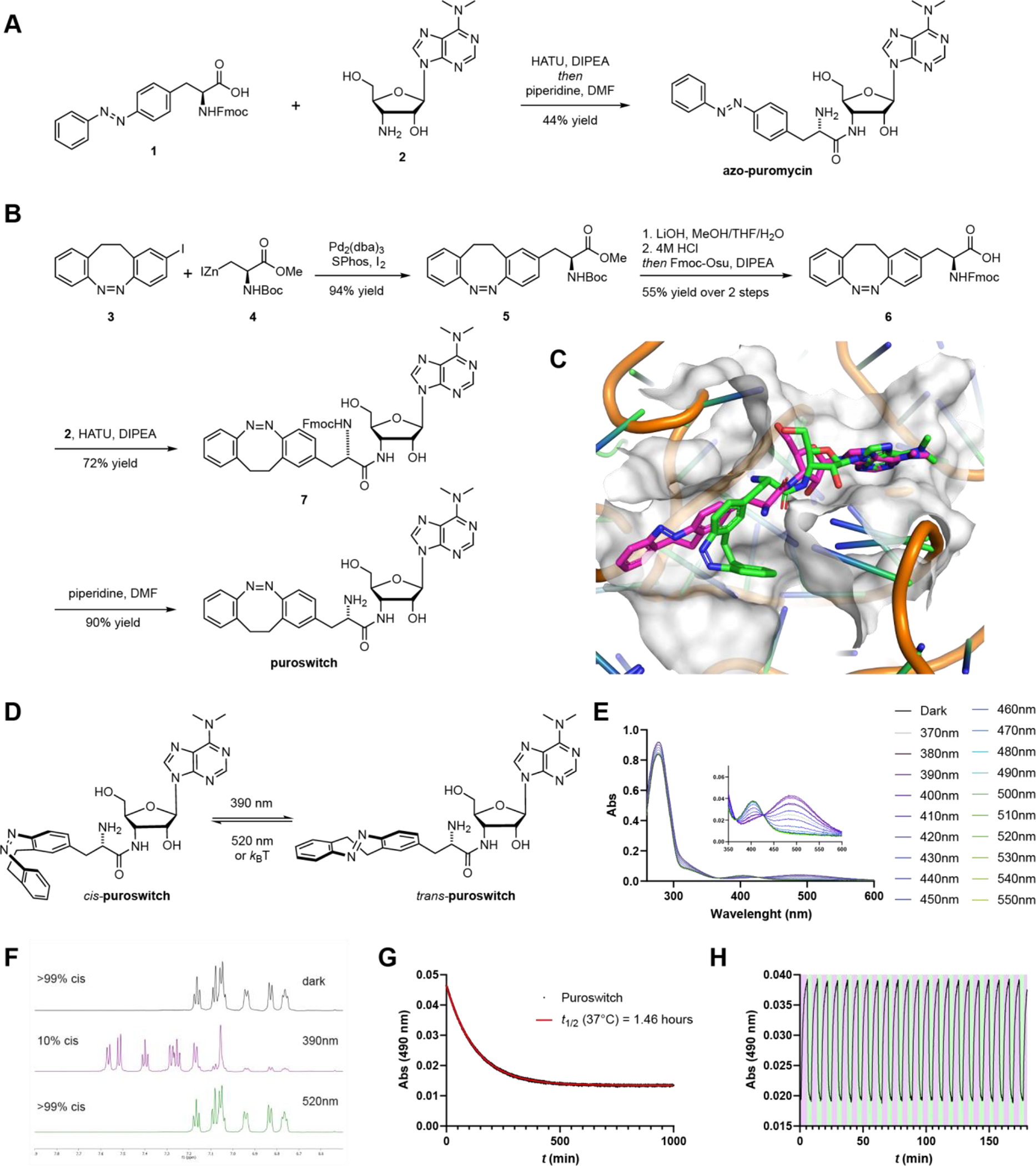
Design, synthesis and photophysical characterization of photoswitchable puromycin compounds. A) Synthesis of azo-puromycin. B) Synthesis of puroswitch. C) Docking of puroswitch in the A site of the ribosome. Best pose from the cis (green) and trans (purple) puroswitch isomers are shown. Model is derived from the structure of CC-puromycin bound to the A site of the 50S ribosomal subunit (PDB: 1Q82). D) Isomerization of cis-puroswitch to trans-puroswitch. E) UV-vis spectra of puroswitch (50 μM) in the dark and at different photostationary states in DMSO at r. t. F) 1H-NMR spectra of puroswitch with different PSS’ in DMSO at r.t. G) Thermal relaxation of puroswitch (50 μM) at 37°C in DMSO H) Reversible trans → cis isomerization of puroswitch (50 μM) at 390 (purple):520 (green) nm irradiation in DMSO at r.t.
To assess the light-dependent biological activity of azo-puromycin, we tested its effect on mammalian cells. Puromycin has a well-known cytotoxic and growth inhibitory effect in both prokaryotic and eukaryotic cells, allowing us to test its light-dependent activity in a cell viability assay using our ‘Cell DISCO’ system for pulsed irradiation.28,29 Human embryonic kidney (HEK) 293t cells were treated with increasing concentrations of puromycin and its analog and were either irradiated with 390 nm for 72 hours at 100 ms every 10s or incubated in the dark. As expected, puromycin showed a similar level of cytotoxicity in both light and dark-adapted conditions (Fig. 3A). Azo-puromycin was assayed in similar conditions but with 370 nm light irradiation and exhibited cellular toxicity but was 2-fold less potent than puromycin. Unfortunately, we did not observe light-dependent differences in the toxicity of azo-puromycin (Fig. 3B). This indicated that both photoisomers, despite their different configurations, were active inhibitors of protein synthesis. Moreover, computational docking of both isomers showed that despite the geometric changes in the azobenzene amino acid sidechain, both isomers occupied the puromycin binding pocket of the ribosome in a similar fashion (Fig. S3) Thus, we decided to not further pursue the biological evaluation of this compound.
Figure 3.
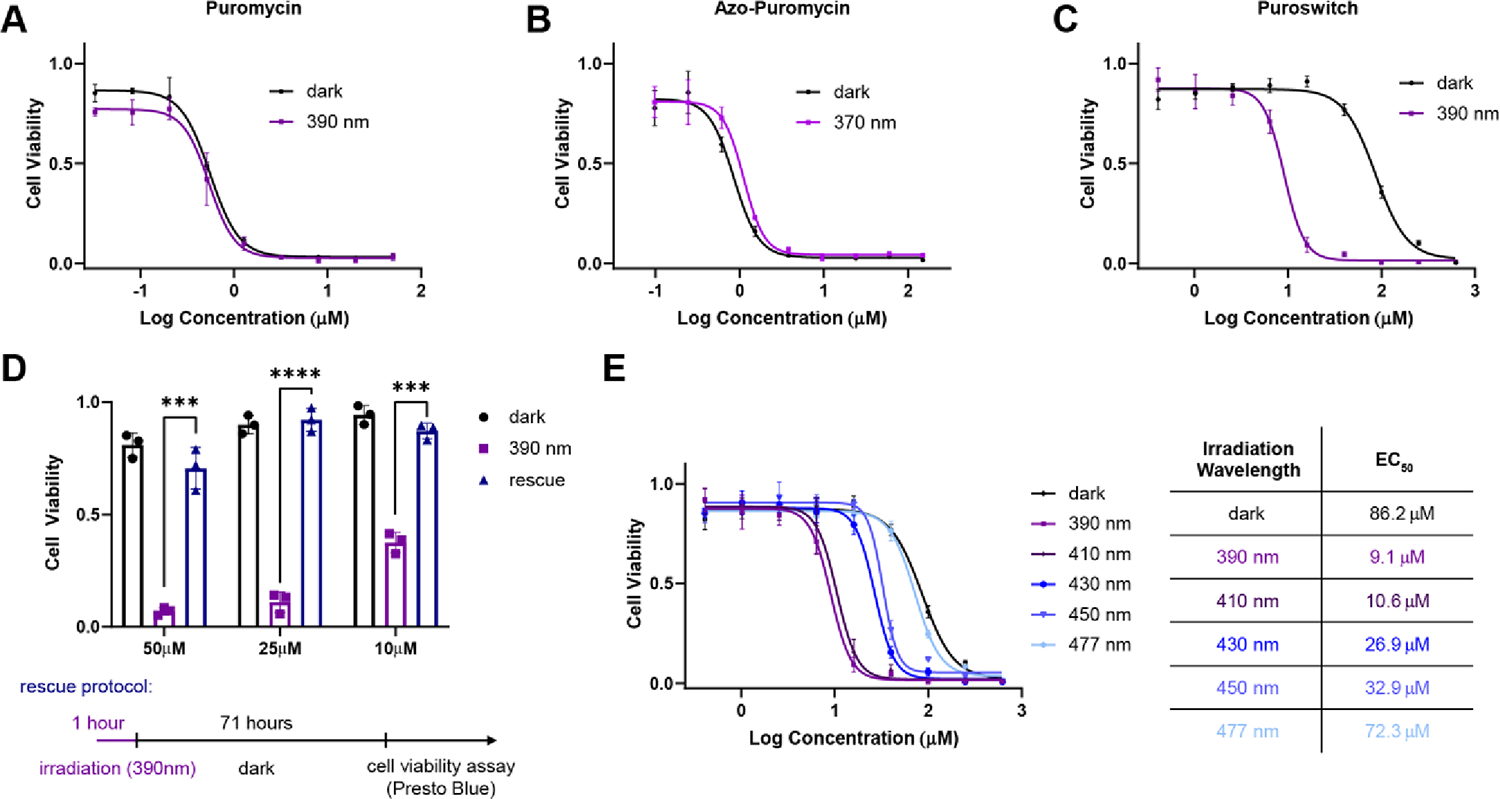
Light-dependent viability of HEK293t cells. A-C) Viability curves for cells treated with different compounds for 72 hours with pulse irradiation (100 ms every 10s) or dark incubation. D) Viability of cells incubated with puroswitch under dark, 390 nm irradiation, or rescue protocol. Error bars represent mean values ± SD. Unpaired t-test were performed (****, p<0.0001; ***, p<0.001). E) Viability curves for cells incubated with puroswitch under pulse irradiation (100 ms every 10s) at different wavelengths of light and table summarizing the EC50 at different PSS.
Inspired by recent success with diazocines in photopharmacology, we next synthesized and evaluated a puromycin derivative that we eventually named “puroswitch” (Fig. 1D).30,31 Diazocines are emerging diaryl diazene photoswitches that, unlike azobenzenes, are more thermodynamically stable in their cis configuration. They can be switched back and forth with visible wavelengths of light around 390 nm and 520 nm and reach good photostationary states of more than 90% of the trans isomer and almost quantitative amounts of the cis isomer.32
A computational docking study of puroswitch revealed that the trans isomer better occupies a binding pocket in the ribosome, closely resembling the binding of puromycin itself, while the cis isomer could not properly inhabit that binding pocket (Fig. 2C; Fig. S3). Additionally, we speculated that the bent geometry of the cis isomer could shield the amine functional group from forming amide bonds with the growing peptide chain and consequently diminishing its effect on translation. Isomerization to the trans isomer would better expose the amine and allow for our puromycin analog to be incorporated into the nascent peptide chain.
The synthesis of puroswitch began with the mono-iodinated diazocine 3 (Fig. 2B), which was obtained in three steps through our previously reported route.30 Subsequent Negishi-Jackson coupling with iodoalanine derived organozinc iodide 4 afforded diazocine 5 in an excellent yield.33 After ester hydrolysis and amide coupling with 2, deprotection of the Boc group and purification of the resulting amino acid proved to be challenging.
Thus, we decided to exchange protecting groups in a one pot reaction by treating the hydrolysis product with 4M HCl, followed by addition of Fmoc-Osu and a base, which gave 6. Finally, amide coupling with 2 and Fmoc deprotection proceeded smoothly to afford puroswitch.
Puroswitch was shown to optimally isomerize to the trans isomer with 390 nm irradiation, where a PSS of 1:9 cis:trans could be achieved. Rapid trans to cis isomerization could be achieved by irradiating with 525 nm light, achieving a PSS of >99:1 cis:trans (Fig. 2D–F). When kept in the dark, the trans enriched diazocine relaxes to the more thermodynamically stable cis form with a thermal half-life of 1.46 hours at 37°C in DMSO (Fig. 2G). This feature is desirable as the compound is relatively bistable but also capable of relaxing to its inactive form in the dark. As usually observed for diazocine photoswitches, many cycles of photochemical isomerization were possible without any noticeable fatigue (Fig. 2H).
Puroswitch exhibits a large difference in bioactivity and enables chromodosing
The light-dependent biological activity of puroswitch in HEK293t cells was assessed using cell viability assays as earlier described. The median effective concentration (EC50) was determined to be 86.2 μM in the dark and 9.1 μM when irradiated with 390 nm, resulting in a 10-fold difference in activity (Fig. 3C). These results indicate that the compound becomes more toxic upon irradiation, which matched our hypothesis that the cis compound could effectively inhibit translation.
Next, we tested the reversibility of our compound. To this end, we designed a rescue assay where HEK293t cells were treated with puroswitch at 50, 25 and 10 μM concentrations and immediately pulse irradiated with 390 nm. After one hour, the samples were incubated in the dark to allow puroswitch to slowly relax to its dark-adapted cis state over 71 hours. Subsequently, cell viability was measured with Presto Blue™ for both set of samples and normalized to that of DMSO treated cells (Fig. 3D). The results showed that samples exposed to irradiation with 390 nm for 72 hours experienced considerable cytotoxicity, while the samples that were allowed to relax back to the cis form after 1 h showed viability similar to those kept in the dark.
Another characteristic property of photoswitches and potential advantage over photocaged alternatives is that the concentration of active species can be titrated with the color of the incident light as the PSS is a function of the wavelength (“chromodosing”).29,34 To illustrate this point, we ran a chromodosing experiment where we incubated HEK293t cells with different concentrations of puroswitch and illuminated the cells with different wavelengths of light. We found that the EC50 of puroswitch could be gradually tuned with the color of incident light (Fig. 3E).
Puroswitch enables optical control of translation
To confirm that the cytotoxic effect of puroswitch in cells was caused by translation inhibition, we used a rabbit reticulocyte lysate based in vitro translation assay.35 In this assay, the translation machinery of the cell is assembled in vitro and luciferase mRNA is added to the mixture. The resulting synthesis of the luciferase protein is probed through luminescence. As expected, addition of 10μM of puromycin to the mixture led to an almost negligible luminescence signal (Fig. 4A). Incubation of puroswitch at 30μM concentration in the dark afforded equivalent levels of luminesce to that of our DMSO control. Irradiation of puroswitch with 390 nm light, however, led to significantly reduced levels of luminesce, similar to those observed for puromycin itself. These experiments validate that our compound acts analogously to puromycin, effectively inhibiting translation when irradiated with violet light.
Figure 4.
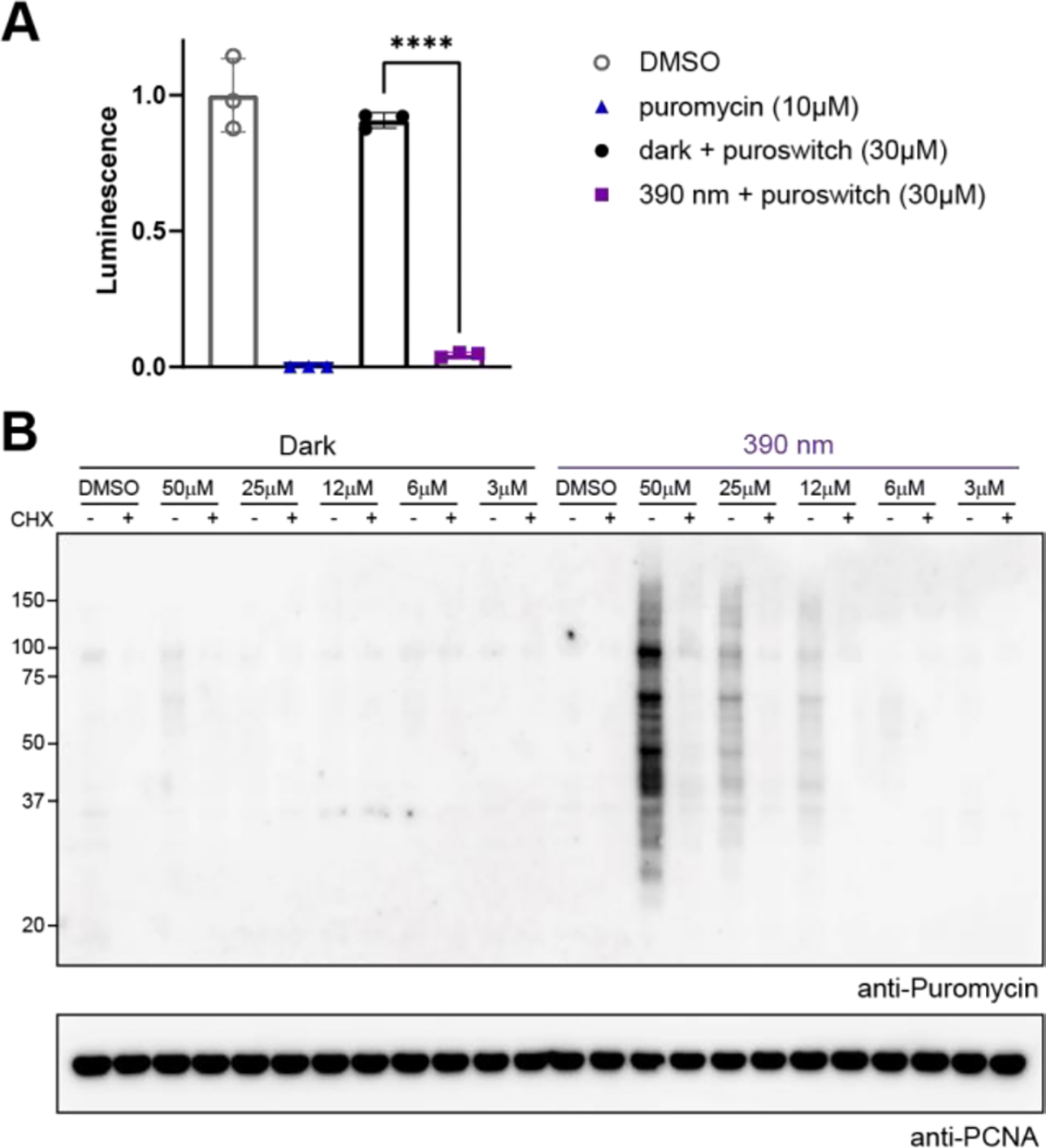
A) Luciferase activity measured for rabbit reticulocyte lysate systems incubated with DMSO, puromycin, or puroswitch. Puroswitch samples were irradiated (395 nm) for 2 minutes or left in the dark. Error bars represent mean values ± SD. Unpaired t-test were performed. (****, p<0.0001) B) Immunoblot analysis after treatment of HEK293t cells with puroswitch for 4 hours in the dark (left) or under irradiation (right) at different concentrations. Cycloheximide (CHX) was also added as a control.
Puroswitch can be used to monitor de novo translation of mRNAs
As a consequence of its mechanism of action, which involves incorporation into nascent polypeptide chains, puromycin can be used to monitor de novo translation using bioorthogonal chemistry or immunofluorescence.11,36–38 Functionalized puromycin analogs that bear handles for biorthogonal chemistry have been used visualization and enrichment through affinity purification.13,39 Puromycin antibodies can bind, albeit usually with lower affinity, to analogs containing chemical modifications in the amino acid side-chain.40 To test if those antibodies could also bind to puroswitch, we treated HEK293t cells with compound at different concentrations. Western blot analysis using puromycin antibodies showed no signal when the cells were incubated in the dark but a clear concentration dependent puromycylation of proteins in the cells that received 390 nm irradiation (Fig. 4B). To ensure that this process is translation dependent, we pre-treated a control group with cycloheximide (CHX), a translation inhibitor. The fact that we observe significant amounts of puromycylated proteins at relatively high molecular weight points to the fact that trans puroswitch is considerably less active than puromycin itself, allowing aminoacyl tRNAs to effectively compete until the ribosome encounters a stop signal and pauses. Taken together, these results show that standard puromycin antibodies bind to our compound and confirm that conjugation of puroswitch to nascent protein chains can be controlled with light.
Puroswitch can be incorporated into newly synthesized proteins of primary neurons
Next, we tested the usefulness of puroswitch for measuring and controlling mRNA translation in neurons. To assess this, we cultivated mouse primary neurons until maturation and then exposed them to puroswitch for 1 h, with or without irradiation with 390 nm light. To confirm that the signal seen was indeed a reliable measure of de novo protein synthesis, we ran control experiments in which translation was inhibited with CHX. We found that activation of puroswitch with light greatly increased its incorporation into peptides. This effect was significantly attenuated in the presence of CHX, confirming that the signal seen was due to de novo protein synthesis (Fig. 5). In the dark, we observed incorporation of puroswitch at much lower levels. Again, the signal could be completely abrogated by treatment with CHX.
Figure 5.
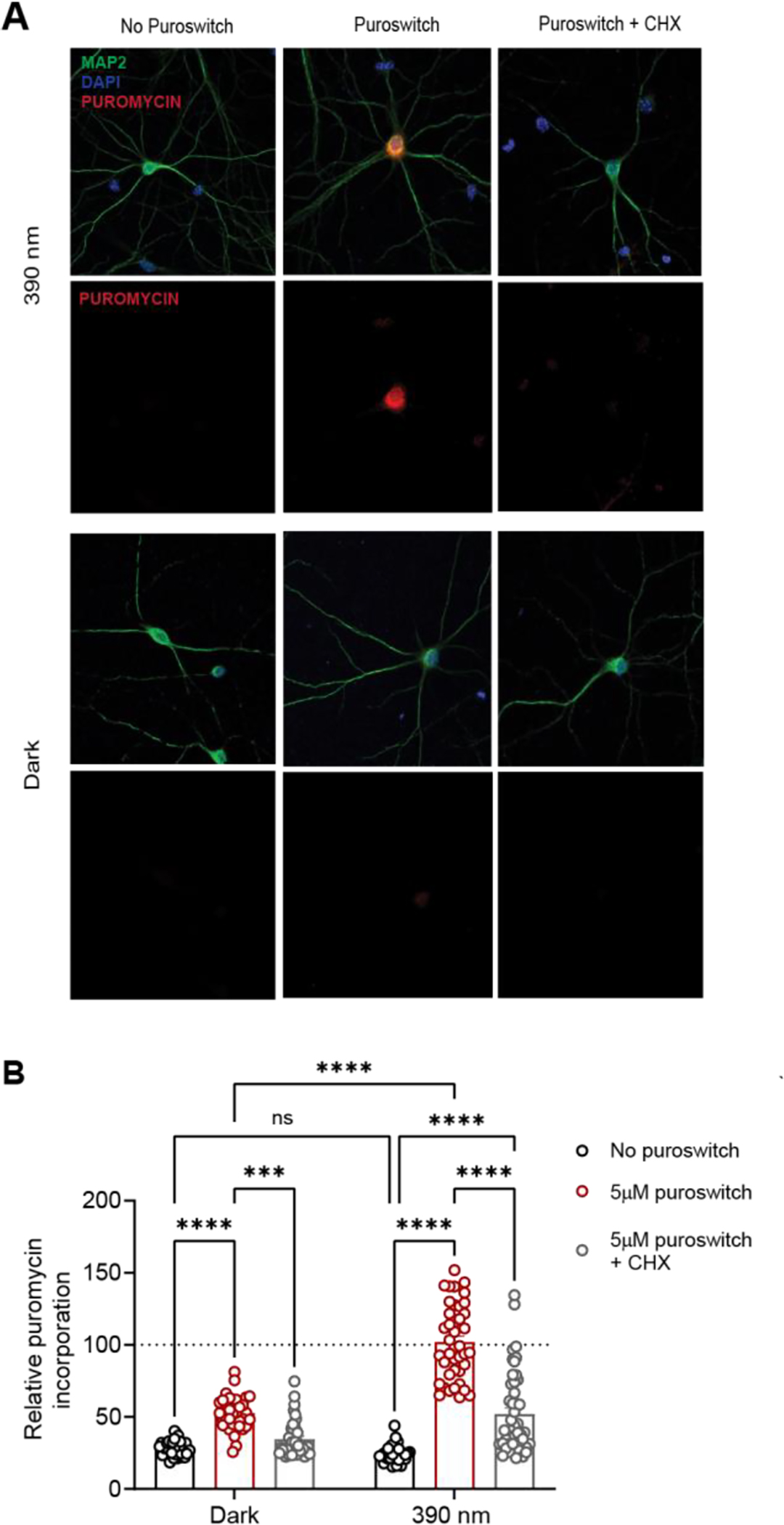
Puroswitch incorporation into newly synthesized proteins of rat primary neurons. A) Microscopic immunocytochemistry images of primary neurons stained with anti-MAP2 antibodies (cyan), DAPI (blue), and anti-puromycin antibodies (red). Neurons were incubated with or without puroswitch (5 μM) for 1 hour with 390 nm irradiation (100 ms every 10s) or in the dark. Cycloheximide (CHX) was also added as an additional control. B) Relative puroswitch incorporation for different compound treatments. Error bars represent mean values ± SEM. Two-way ANOVA with Tukey’s multiple comparisons were performed (****, p<0.0001; ***, p<0.001).
DISCUSSION
Here, we have introduced an analog of puromycin that contains an a diazocine photoswitch and have evaluated its biological effects in a light dependent fashion. Its synthesis involves the photoswitchable amino acid precursors 5 and 6, which should be of broad utility to those interested in photoresponsive peptides and proteins. Buildings blocks of this type are easily accessible by combining the methodology for oxidative cyclization of ethylenedianilines with Negishi-Jackson couplings.30,33 Diazocine side-chain amino acids are complimentary to classical azobenzene containing ones, first introduced by the Goodman group, which have been used to control various biological functions.22–23 To demonstrate that the Fmoc protected building block 6 is compatible with solid phase peptide synthesis, we incorporated into a short heptapeptide (Fig. S4).
Puroswitch builds on and transcends existing approaches to control and monitor translation with caged puromycin derivatives. Photoswitchable probes can be reversibly activated and deactivated and have favorable ON kinetics. Puroswitch is stable over many switching cycles and does not generate additional species that can be light-absorbent or toxic. Additionally, diazocine photoswitches have high quantum yields and require low-light intensities in the visible range to isomerize. Puroswitch could be effectively isomerized in vitro and in cell culture with standard LED’s (<10 mW/cm2). Finally, the activity of puroswitch can be titrated with the color of incident light. This process, which we term chromodosing, allows for the precise control over the concentration of the active species, a feature that can be difficult to achieve with conventional pharmacology or caged ligands.
Puroswitch can be used as a reliable tool for nascent proteome profiling in cells as complex and sensitive as neurons. Neurons are fast-responsive cells, and localized changes in synaptic proteome composition are thought to be necessary for a wide range of processes, such as synapse formation, synaptic pruning, and memory consolidation.41 The features of puroswitch are well matched with these processes providing a suitable tool to study de novo translation in specified locations and at precise time points. We believe that puroswitch will empower the development of more complex puromycin-based assays and generate new insights into the time course and location of cellular translation.
Supplementary Material
ACKNOWLEDGMENT
T.K. is supported by the NYU MacCracken Fellowship. J.M. thanks the National Cancer Institute (NCI) for a F99/K00 award (K00CA253758). This research was supported by the National Institutes of Health (NIH) grant NS122316 and the NYU Grossmann School of Medicine.
Footnotes
ASSOCIATED CONTENT
Supporting Information
Experimental procedures and characterization data; photophysical characterization; computational docking; synthetic recommendations; computational data (PDF)
Puroswitch docking structures (PDB)
REFERENCES
- (1).Kristensen AR; Gsponer J; Foster LJ Protein Synthesis Rate Is the Predominant Regulator of Protein Expression during Differentiation. Mol. Syst. Biol. 2013, 9, 689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Laham-Karam N; Pinto GP; Poso A; Kokkonen P Transcription and Translation Inhibitors in Cancer Treatment. Front. Chem. 2020, 8, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Jaafar ZA; Kieft JS Viral RNA Structure-Based Strategies to Manipulate Translation. Nat. Rev. Microbiol. 2019, 17, 110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Richter JD; Klann E Making Synaptic Plasticity and Memory Last: Mechanisms of Translational Regulation. Genes Dev. 2009, 23, 1–11. [DOI] [PubMed] [Google Scholar]
- (5).Costa-Mattioli M; Sossin WS; Klann E; Sonenberg N Translational Control of Long-Lasting Synaptic Plasticity and Memory. Neuron 2009, 61, 10–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Tercero JA; Espinosa JC; Lacalle RA; Jiménez A The Biosynthetic Pathway of the Aminonucleoside Antibiotic Puromycin, as Deduced from the Molecular Analysis of the Pur Cluster of Streptomyces Alboniger(∗). J. Biol. Chem. 1996, 271, 1579–1590. [DOI] [PubMed] [Google Scholar]
- (7).Yarmolinsky MB; Haba GLDL Inhibition by Puromycin of Amino Acid Incorporation into Protein*. Proc. Natl. Acad. Sci. 1959, 45, 1721–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Nathans D Puromycin Inhibition of Protein Synthesis: Incorporation of Puromycin into Peptide Chains. Proc. Natl. Acad. Sci. 1964, 51, 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Semenkov Yu.; Shapkina, T.; Makhno, V.; Kirillov, S. Puromycin Reaction for the A Site-Bound Peptidyl-TRNA. FEBS Lett. 1992, 296, 207–210. [DOI] [PubMed] [Google Scholar]
- (10).Aviner R The Science of Puromycin: From Studies of Ribosome Function to Applications in Biotechnology. Comput. Struct. Biotechnol. J. 2020, 18, 1074–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Schmidt EK; Clavarino G; Ceppi M; Pierre P SUnSET, a Nonradioactive Method to Monitor Protein Synthesis. Nat. Methods 2009, 6, 275–277. [DOI] [PubMed] [Google Scholar]
- (12).David A; Dolan BP; Hickman HD; Knowlton JJ; Clavarino G; Pierre P; Bennink JR; Yewdell JW Nuclear Translation Visualized by Ribosome-Bound Nascent Chain Puromycylation. J. Cell Biol. 2012, 197, 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Liu J; Xu Y; Stoleru D; Salic A Imaging Protein Synthesis in Cells and Tissues with an Alkyne Analog of Puromycin. Proc. Natl. Acad. Sci. 2012, 109, 413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Buhr F; Kohl-Landgraf J; tom Dieck S; Hanus C; Chatterjee D; Hegelein A; Schuman EM; Wachtveitl J; Schwalbe H Design of Photocaged Puromycin for Nascent Polypeptide Release and Spatiotemporal Monitoring of Translation. Angew. Chem. Int. Ed. 2015, 54, 3717–3721. [DOI] [PubMed] [Google Scholar]
- (15).Elamri I; Heumüller M; Herzig L-M; Stirnal E; Wachtveitl J; Schuman EM; Schwalbe H A New Photocaged Puromycin for an Efficient Labeling of Newly Translated Proteins in Living Neurons. ChemBioChem 2018, 19, 2458–2464. [DOI] [PubMed] [Google Scholar]
- (16).Link KH; Shi Y; Koh JT Light Activated Recombination. J. Am. Chem. Soc. 2005, 127, 13088–13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Elamri I; Abdellaoui C; Bains JK; Hohmann KF; Gande SL; Stirnal E; Wachtveitl J; Schwalbe H Wavelength-Selective Uncaging of Two Different Photoresponsive Groups on One Effector Molecule for Light-Controlled Activation and Deactivation. J. Am. Chem. Soc. 2021, 143, 10596–10603. [DOI] [PubMed] [Google Scholar]
- (18).Lerch MM; Hansen MJ; van Dam GM; Szymanski W; Feringa BL Emerging Targets in Photopharmacology. Angew. Chem. Int. Ed. 2016, 55, 10978–10999. [DOI] [PubMed] [Google Scholar]
- (19).Silva JM; Silva E; Reis RL Light-Triggered Release of Photocaged Therapeutics - Where Are We Now? J. Controlled Release 2019, 298, 154–176. [DOI] [PubMed] [Google Scholar]
- (20).Nathans D; Neidle A Structural Requirements for Puromycin Inhibition of Protein Synthesis. Nature 1963, 197, 1076–1077. [DOI] [PubMed] [Google Scholar]
- (21).Symons RH; Harris RJ; Clarke LP; Wheldrake JF; Elliott WH Structural Requirements for Inhibition of Polyphenylalanine Synthesis by Aminoacyl and Nucleotidyl Analogues of Puromycin. Biochim. Biophys. Acta BBA - Nucleic Acids Protein Synth. 1969, 179, 248–250. [DOI] [PubMed] [Google Scholar]
- (22).Goodman M; Kossoy A Conformational Aspects of Polypeptide Structure. XIX. Azoaromatic Side-Chain Effects. J. Am. Chem. Soc. 1966, 88, 5010–5015. [Google Scholar]
- (23).Albert L; Vázquez O Photoswitchable Peptides for Spatiotemporal Control of Biological Functions. Chem. Commun. 2019, 55, 10192–10213. [DOI] [PubMed] [Google Scholar]
- (24).Hartley GS The Cis-Form of Azobenzene. Nature 1937, 140, 281–281. [Google Scholar]
- (25).Beharry AA; Woolley GA Azobenzene Photoswitches for Biomolecules. Chem. Soc. Rev. 2011, 40, 4422–4437. [DOI] [PubMed] [Google Scholar]
- (26).Broichhagen J; Frank JA; Trauner D A Roadmap to Success in Photopharmacology. Acc. Chem. Res. 2015, 48, 1947–1960. [DOI] [PubMed] [Google Scholar]
- (27).Niinomi T; Sisido M Synthesis of 2′(3′)-O-Aminoacyl-PdCpA Carrying a Photofunctional Nonnatural Amino Acid. Chem. Lett. 1993, 22, 1305–1308. [Google Scholar]
- (28).De La Luna S; Ortín J Pac Gene as Efficient Dominant Marker and Reporter Gene in Mammalian Cells. J. Methods Enzymol. 1992, 16, 376–385. [DOI] [PubMed] [Google Scholar]
- (29).Borowiak M; Nahaboo W; Reynders M; Nekolla K; Jalinot P; Hasserodt J; Rehberg M; Delattre M; Zahler S; Vollmar A; Trauner D; Thorn-Seshold O Photoswitchable Inhibitors of Microtubule Dynamics Optically Control Mitosis and Cell Death. Cell 2015, 162, 403–411. [DOI] [PubMed] [Google Scholar]
- (30).Maier MS; Hüll K; Reynders M; Matsuura BS; Leippe P; Ko T; Schäffer L; Trauner D Oxidative Approach Enables Efficient Access to Cyclic Azobenzenes. J. Am. Chem. Soc. 2019, 141, 17295–17304. [DOI] [PubMed] [Google Scholar]
- (31).Trads JB; Hüll K; Matsuura BS; Laprell L; Fehrentz T; Görldt N; Kozek KA; Weaver CD; Klöcker N; Barber DM; Trauner D Sign Inversion in Photopharmacology: Incorporation of Cyclic Azobenzenes in Photoswitchable Potassium Channel Blockers and Openers. Angew. Chem. Int. Ed. 2019, 58, 15421–15428. [DOI] [PubMed] [Google Scholar]
- (32).Siewertsen R; Neumann H; Buchheim-Stehn B; Herges R; Näther C; Renth F; Temps F Highly Efficient Reversible Z−E Photoisomerization of a Bridged Azobenzene with Visible Light through Resolved S1(Nπ*) Absorption Bands. J. Am. Chem. Soc. 2009, 131, 15594–15595. [DOI] [PubMed] [Google Scholar]
- (33).Ross AJ; Lang HL; Jackson RFW Much Improved Conditions for the Negishi Cross-Coupling of Iodoalanine Derived Zinc Reagents with Aryl Halides. J. Org. Chem. 2010, 75, 245–248. [DOI] [PubMed] [Google Scholar]
- (34).Hüll K; Morstein J; Trauner D In Vivo Photopharmacology. Chem. Rev. 2018, 118, 10710–10747. [DOI] [PubMed] [Google Scholar]
- (35).Pelham HRB; Jackson RJ An Efficient MRNA-Dependent Translation System from Reticulocyte Lysates. European Journal of Biochemistry 1976, 67, 247–256. [DOI] [PubMed] [Google Scholar]
- (36).Hansen WJ; Lingappa VR; Welch WJ Complex Environment of Nascent Polypeptide Chains. J. Biol. Chem. 1994, 269, 26610–26613. [PubMed] [Google Scholar]
- (37).Miyamoto-Sato E; Nemoto N; Kobayashi K; Yanagawa H Specific Bonding of Puromycin to Full-Length Protein at the C-Terminus. Nucleic Acids Res. 2000, 28, 1176–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Nemoto N; Miyamoto-Sato E; Yanagawa H Fluorescence Labeling of the C-Terminus of Proteins with a Puromycin Analogue in Cell-Free Translation Systems. FEBS Lett. 1999, 462, 43–46. [DOI] [PubMed] [Google Scholar]
- (39).Uchiyama J; Ishihama Y; Imami K Quantitative Nascent Proteome Profiling by Dual-Pulse Labelling with O-Propargyl-Puromycin and Stable Isotope-Labelled Amino Acids. J. Biochem. (Tokyo) 2021, 169, 227–236. [DOI] [PubMed] [Google Scholar]
- (40).Ge J; Zhang C-W; Ng XW; Peng B; Pan S; Du S; Wang D; Li L; Lim K-L; Wohland T; Yao SQ Puromycin Analogues Capable of Multiplexed Imaging and Profiling of Protein Synthesis and Dynamics in Live Cells and Neurons. Angew. Chem. Int. Ed. 2016, 55, 4933–4937. [DOI] [PubMed] [Google Scholar]
- (41).Oliveira MM; Klann E EIF2-Dependent Translation Initiation: Memory Consolidation and Disruption in Alzheimer’s Disease. Semin. Cell Dev. Biol. 2022, 125, 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


