Abstract
Introduction
Sepsis is a life-threatening condition that poses a globally high mortality rate. Identifying risk factors is crucial. Insulin resistance and the TYG index, associated with metabolic disorders, may play a role. This study explores their correlation with mortality in non-diabetic septic patients.
Methods
This retrospective cohort study used data from the MIMIC-IV (version 2.1) database, which includes over 50,000 ICU admissions from 2008 to 2019 at Beth Israel Deaconess Medical Center in Boston. We included adult patients with sepsis who were admitted to the intensive care unit in the study. The primary outcome was to evaluate the ability of TYG to predict death at 28-day of hospital admission in patients with sepsis.
Results
The study included 2213 patients with sepsis, among whom 549 (24.8%) died within 28 days of hospital admission. We observed a non-linear association between TYG and the risk of mortality. Compared to the reference group (lower TYG subgroup), the 28-day mortality increased in the higher TYG subgroup, with a fully adjusted hazard ratio of 2.68 (95% CI: 2.14 to 3.36). The area under the curve (AUC) for TYG was 67.7%, higher than for triglycerides alone (AUC = 64.1%), blood glucose (AUC = 62.4%), and GCS (AUC = 63.6%), and comparable to SOFA (AUC = 69.3%). The final subgroup analysis showed no significant interaction between TYG and each subgroup except for the COPD subgroup (interaction P-values: 0.076–0.548).
Conclusion
In our study, TYG can be used as an independent predictor for all-cause mortality due to sepsis within 28 days of hospitalization.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-09711-4.
Keywords: Sepsis, Triglyceride, Glucose, Mortality, Prognosis, Intensive care unit
Introduction
Sepsis is a potentially fatal organ damage caused by insufficient response to infection, accompanied by pathophysiological abnormalities and metabolic disorder [1–3]. It is one of the leading causes of death in worldwide and the mortality rate is as high as 10–50% based on age and disease severity [4]. The early identification and intervention of sepsis risk factors have become the focal points and challenges of research [5]. Previous studies have shown that the poor prognosis of sepsis is often associated with the severity of the disease [6, 7]. Moreover, some studies have found that lipid metabolism disorders and blood glucose levels also seem to be associated with the poor prognosis of sepsis. However, the existing research evidence regarding the risk factors of sepsis is limited and insufficient.
Insulin resistance (IR), a compensatory response caused by the decrease of insulin action due to various reasons, has been proven to be associated with the release of inflammatory factors and metabolic disorders. Numerous studies have indicated that IR is associated with the prognosis of pathological processes such as cardiovascular and cerebrovascular diseases and other diseases [8–10]. Triglyceride Glucose (TYG) index, a simple and effective biomarker for IR, has highly correlation with the gold standard for evaluating IR–hyperinsulinaemic–euglycaemic clamp [11]. Former studies have proven that TYG index is involved in metabolic disorders and inflammatory response, and may be a favorable indicator for predicting the poor prognosis or death of cardiovascular and cerebrovascular diseases and critical diseases [12, 13]. Additionally, some studies have found that the TYG index has a better predictive effect on non-diabetes.
Presently, the correlation between the TYG index and the poor prognosis of sepsis lacked strong and sufficient research evidence to support. Whether the TYG index can be a emerging and effective indicator for predicting the risk of death in sepsis to guide treatment remains to be verified. Therefore, this study aims to explore the correlation between mortality in non-diabetic patients with sepsis and the TYG index, as well as to compare the predictive efficacy of the TYG index with different indicators for predicting patient mortality.
Materials and methods
Database and study population
This retrospective cohort study utilized the fourth-generation Medical Information Mart for Intensive Care (MIMIC-IV version 2.1) database. This is a longitudinal single-center database that contains data on more than 50,000 ICU admissions from 2008 to 2019 at Beth Israel Deaconess Medical Center in Boston. To obtain access, the author, Sen Zhang, completed an online training course and exam (certification number: 51383956). The use of the MIMIC-IV database was approved by the institutional review board of both Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. The data is anonymized, and therefore the requirement for informed consent was waived.
Sepsis was diagnosed using the Sepsis-3 criteria: specifically, if patients presented with documented or suspected infection and a change of > 2 points in the total Sequential Organ Failure Assessment (SOFA) score, they were considered as having sepsis. We selected patients from MIMIC-IV with diagnosis of sepsis as the study population. Exclusion criteria were: (1) patients with the Sequential Organ Failure Assessment (SOFA) < 2, (2) patients with any type of diabetes, (3) patients without ICU records or with multiple ICU admissions, (4) patients with ICU length of stay < 24 h, (5) patients under 18 years of age, (6) patients with missing triglyceride or glucose values. Figure S1 displays the patient selection flowchart.
Definition of TYG index
The TYG index is calculated as TYG = Ln [fasting triglycerides (mg/dL) × fasting glucose (mg/ dL)/2] [11].
Data extraction
We utilized the PostgreSQL tool (v.16, PostgreSQL Global Development Group, Berkley, California, USA) to extract data. Using structured query language (SQL), we extracted patients' diagnostic information based on International Classification of Diseases (ICD) codes, either ICD-9 or ICD-10. Relevant variables were extracted within 24 h of patient admission, including: (1) baseline characteristics: age, gender, race, the use of statin medications and insulin; (2) comorbidities: myocardial infarction, cerebrovascular disease, chronic obstructive pulmonary disease, hypertension, liver disease, kidney disease and malignancy; (3) vital signs: heart rate, systolic blood pressure, diastolic blood pressure, mean arterial pressure and mean respiratory rate; (4) disease severity scores: Sequential Organ Failure Assessment (SOFA) score and Glasgow Coma Scale (GCS); (5) laboratory parameters: hematocrit, hemoglobin, white blood cell count, monocyte, platelets, blood urea nitrogen (BUN), creatinine, albumin, alanine transaminase (ALT), aspartate aminotransferase (AST), glucose, bicarbonate, sodium, potassium, aniongap, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglyceride; (6) hospitalization and ICU data: admission and discharge times, ICU admission and discharge times, and date and time of death. The patient's 28-day mortality rate was calculated based on hospitalization time and follow-up data as outcome data. Mortality rates were calculated based on the time of first diagnosis of sepsis in MIMIC-IV 2.1 and date of death outside the hospital. All measured parameters used in this study were the average values obtained within 24 h of ICU admission.
Statistical analysis
To analyze the data, we utilized various statistical methods and models. Firstly, we stratified patients based on the 28-day mortality rate of patients with sepsis to describe their baseline characteristics, with continuous variables expressed as median or quartiles and categorical variables expressed as frequency and percentage. Differences between continuous variables were compared using analysis of variance or rank-sum test, while differences between categorical variables were compared using chi-square test or Fisher's exact test. Then, we employed univariate and multivariate analyses to explore the factors influencing 28-day mortality in patients with sepsis. We employed Cox regression models to ascertain the predictive association between the TYG index and the 28-day mortality rate in patients with sepsis, with results presented as hazard ratios (HR) and 95% confidence intervals (CI). In the unadjusted model, no covariates were adjusted; in Model II, adjustments were made for age, gender, and race; in Model III, compared to Model II, further adjustments were made for hemoglobin, white blood cell, platelets, BUN, creatinine, albumin, bicarbonate, potassium, anion gap, chloride, LDL, HDL; Model IV further adjusted for mean heart rate, mean SBP, mean DBP, mean MBP, mean respiratory rate, statin, septic shock, SOFA score, GCS score, and Charlson comorbidity index, based on Model III. We used restricted cubic spline models to test for possible linear associations between TYG levels and 28-day mortality rate. Receiver Operating Characteristic (ROC) analysis was used to assess the predictive ability of triglyceride, glucose, TYG and SOFA for mortality at 28-day of admission, and the sensitivity and specificity of each index, as well as to calculate the Area Under Curve (AUC), and stratified the sample based on the optimal cutoff value for TYG determined by the ROC curve and used Kaplan–Meier to plot survival curves. Additionally, we conducted subgroup analyses to assess whether there were differences in 28-day mortality rate between different subgroups of sepsis based on TYG levels. All analyses were conducted using the statistical software R.4.2 and SPSS 25.0, and P < 0.05 (two-tailed) was considered statistically significant.
Results
Baseline characteristics of the study patients
We extracted data from 2213 eligible patients from the MIMIC-IV database (Figure S1). The median age of the patients was 65.4 years (interquartile range, 53.9–77.7), with an overall mortality rate of 24.8%. There were no significant differences between the two groups of patients in terms of gender. Patients who died within 28 days of admission had higher levels of TYG compared to the surviving patients (9.3 [9.0, 9.7] vs 8.9 [8.5, 9.4], P < 0.001). Additionally, patients who died within 28 days had lower levels of hematocrit, hemoglobin, platelets, albumin, bicarbonate, chloride, HDL, SBP, DBP, MBP, GCS score as well as higher levels of white blood cell, BUN, creatinine, ALT, AST, potassium, aniongap, LDL, heart rate, respiratory rate, SOFA score and Charlson's comorbidity index (all P < 0.05) (Table 1). Notably, the usage of statins among surviving patients was significantly higher than among those who did not survive (44.1% vs 34.6%, P < 0.001).
Table 1.
Baseline characteristics of patients stratified by 28-d mortality
| Variable | Total (n = 2213) | Survivors (n = 1664) | Non-survivors (n = 549) | P value |
|---|---|---|---|---|
| Age,years | 65.4 (53.9,77.7) | 63.9 (52.3,76.6) | 70.7 (58.4,81.9) | < 0.001 |
| Gender, n (%) | 0.161 | |||
| Female | 991 (44.8) | 731(43.9) | 260 (47.4) | |
| Male | 1222 (55.2) | 933(56.1) | 289 (52.6) | |
| Ethnicity, n (%) | < 0.001 | |||
| White | 1506 (68.1) | 1162 (69.8) | 344 ((62.7) | |
| Black | 184 (8.3) | 145 (8.7) | 39 (7.1) | |
| Other | 523 (23.6) | 357 (21.5) | 166 (30.2) | |
| Hospital LOS,days | 13.5 (6.9,25.1) | 15.5 (7.7,29.5) | 10.2 (4.8,16.4) | < 0.001 |
| ICU LOS,days | 3.7 (1.8,9.6) | 3.4 (1.8,9.6) | 4.4 (2.0,9.9) | 0.003 |
| Laboratory parameters | ||||
| Hematocrit (%) | 31.1 (26.9,35.9) | 31.4 (27.2,36.0) | 30.3 (26.2,35.2) | 0.005 |
| Hemoglobin (g/dL) | 10.2 (8.8,11.8) | 10.3 (8.9,11.9) | 9.8 (8.4,11.4) | 0.016 |
| White blood cell (K/µL) | 12.7 (8.3,18.6) | 12.3 (8.1,18.1) | 14.1 (9.0,20.0) | 0.010 |
| Platelets (K/uL) | (174.0,113.0,253.5) | 178.0 (118.0,258.5) | 158.0 (92.3,241.0) | 0.013 |
| BUN (mg/dL) | 23.5 (15.0,40.0) | 21.5 (14.0,35.0) | 32.5 (20.5,53.0) | < 0.001 |
| Creatinine (mg/dL) | 1.2 (0.8,1.9) | 1.1 (0.8,1.7) | 1.5 (1.0,2.5) | < 0.001 |
| Albumin (g/dL) | 2.9 (2.4,3.3) | 2.9 (2.5,3.4) | 2.7 (2.2,3.2) | < 0.001 |
| ALT (IU/L) | 32.0 (15.0,101.5) | 29.5 (13.0,87.3) | 42.0 (19.5,124.5) | < 0.001 |
| AST (IU/L) | 69.0 (30.0,267.0) | 66.5 (29.0,265.6) | 82.5(33.5,281.0) | 0.074 |
| Bicarbonate (mEq/L) | 21.0 (18.5,24.0) | 21.5 (18.5,24.0) | 20.0 (16.5,23.0) | < 0.001 |
| Sodium (mEq/L) | 138.0 (135.0,140.5) | 138.0 (135.0,140.5) | 138.0 (133.5,141.5) | 0.984 |
| Potassium (mEq/L) | 4.1 (3.7,4.6) | 4.1 (3.7,4.5) | 4.2 (3.8,4.8) | < 0.001 |
| Chloride (mEq/L) | 104.5 (100.5,108.5) | 104.5 (101.0,108.0) | 103.5 (98.0,108.5) | 0.004 |
| Aniongap(mEq/L) | 15.0 (13.0,17.5) | 14.5 (12.5,17.0) | 16.5 (14.0,20.0) | < 0.001 |
| LDL(mg/dL) | 94.0 (68.0,123.0) | 92.0 (67.5,120.0) | 99.0 (71.0,132.0) | 0.008 |
| HDL(mg/dL) | 49.0 (36.0,63.0) | 50.0 (36.0,64.0) | 45.0 (35.0,61.0) | < 0.001 |
| TYG | 9.0 (8.6,9.5) | 8.9 (8.5,9.4) | 9.3 (9.0,9.7) | < 0.001 |
| Vital signs | ||||
| Mean SBP,mmHg | 108.0(100.7,117.0) | 108.4 (101.2,117.4) | 106.1 (99.0,114.7) | < 0.001 |
| Mean DBP,mmHg | 60.4 (54.2,66.6) | 61.0 (54.8,66.8) | 58.6 (52.2,64.6) | < 0.001 |
| Mean MBP,mmHg | 73.3 (68.1,79.6) | 73.7 (69.0,80.0) | 71.6 (66.4,77.5) | < 0.001 |
| Mean heart rate,bpm | 92.3 (80.0,105.2) | 91.7 (79.5,104.5) | 94.7 (82.1,108.2) | 0.043 |
| Mean respiratory rate, times/min | 21.0 (18.2,24.2) | 20.8 (18.0,24.0) | 21.5 (18.6,25.0) | 0.019 |
| Statin, n (%) | < 0.001 | |||
| NO | 1290 (58.3) | 931 (55.9) | 359 (65.4) | |
| YES | 923 (41.7) | 733 (44.1) | 190 (34.6) | |
| Insulin, n (%) | 0.206 | |||
| NO | 1116 (50.4) | 852 (51.2) | 264 (48.1) | |
| YES | 1097 (49.6) | 812 (48.8) | 285 (51.9) | |
| Septic Shock, n (%) | < 0.001 | |||
| NO | 914 (41.3) | 777 (85.0) | 137 (15.0) | |
| YES | 1299 (58.7) | 887 (68.3) | 412 (31.7) | |
| Score | ||||
| SOFA | 8.0 (4.0,11.0) | 7.0 (4.0,10.0) | 10.0 (7.0,14.0) | < 0.001 |
| GCS | 13.0 (8.0,15.0) | 14.0 (10.0,15.0) | 10.0 (5.0,14.0) | < 0.001 |
| Charlson’s comorbidity index | 5.0 (4.0,7.0) | 5.0 (3.0,7.0) | 7.0 (5.0,9.0) | < 0.001 |
Continuous and ordinal variables were expressed as medians (with interquartile range), and categorical variables as absolute counts (with percentage), LOS Length of Stay, BUN blood urea nitrogen, ALT alanine transaminase, AST aspartate aminotransferase, HDL high-density lipoprotein, LDL low-density lipoprotein, TYG Triglyceride Glucose index, SBP systolic blood pressure, DBP diastolic blood pressure, MBP mean blood pressure, SOFA Sequential Organ Failure Assessment, GCS Glasgow Coma Scale
Restricted cubic splines (RCS) analysis
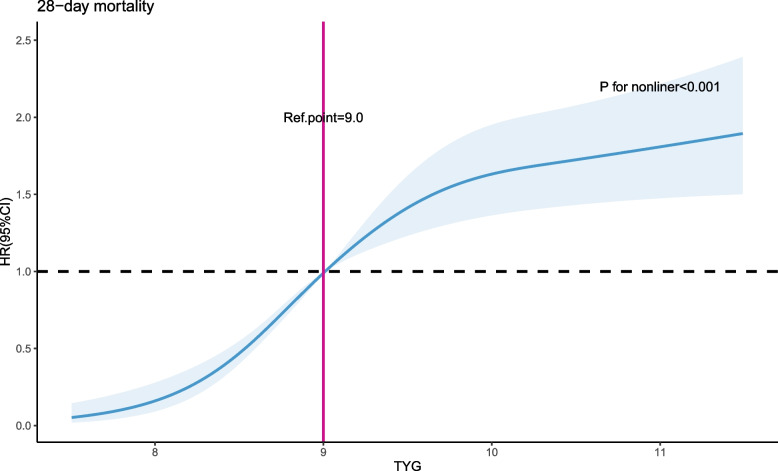
We utilized restricted cubic splines (RCS) to investigate and visualize the relationship between TYG and the 28-day mortality in patients with sepsis. After adjusting for all relevant variables, we observed a nonlinear association between TYG and the 28-day mortality (non-linear P < 0.001, Fig. 1). As TYG levels increase, the risk of 28-day mortality in patients with sepsis initially rises rapidly and then stabilizes. The optimal cutoff value is TYG = 9.0.
Fig. 1.
A multiple-variable adjusted restricted cubic spline method was employed to depict the relationship between TYG levels and 28-day mortality in patients with sepsis. The association between TYG and 28-day mortality demonstrated a non-linear pattern characterized by an initial rapid increase followed by stabilization. The shaded area represents the 95% CI. HR: hazard ratio; CI: confidence interval; TYG: Triglyceride Glucose index
ROC curve analysis
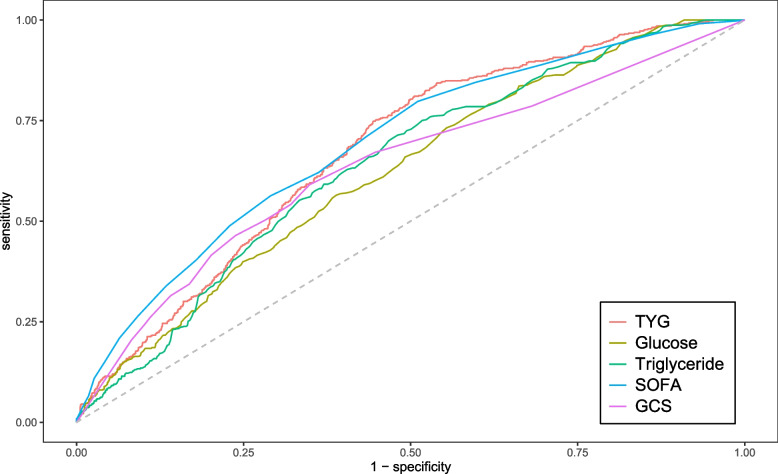
We plotted ROC curves for the four indicators of triglyceride, glucose, TYG and SOFA for predicting all-cause mortality within 28-day of admission in patients with sepsis, and the information in Fig. 2 is listed in Table S1. The AUC of TYG [67.7% (95% CI: 65.2%-70.1%)] was superior to that of triglyceride [64.1% (61.5%-66.6%)], glucose [62.4% (59.8%-64.9%)], and GCS [63.6% (60.9%-66.4%)], and comparable to SOFA [69.3% (66.8%-71.8%)]. Consequently, TYG has a significant predictive advantage. We meanwhile obtained the best cut-off value of 8.9 for TYG, which had a sensitivity of 81.1% and a specificity of 49.5%. We used this as the stratification basis to plot Kaplan–Meier curves for different TYG level subgroups.
Fig. 2.
ROC curves for sepsis patients. TYG: Triglyceride Glucose index; SOFA, Sequential Organ Failure Assessment
Kapla-Meier analysis
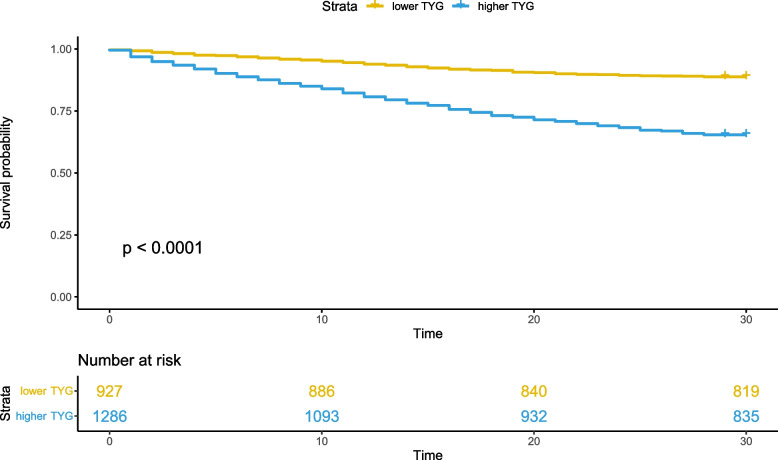
We stratified the overall patient population into low TYG subgroup and high TYG subgroup using the optimal cutoff value obtained from the ROC curve (Fig. 3). It is evident that ischemic stroke patients with higher TYG levels exhibit significantly lower 28-day survival rates compared to those with lower TYG levels (P < 0.001, determined by the log-rank test).
Fig. 3.
Kaplan–Meier survival curve for mortality according to TYG. TYG: Triglyceride Glucose index
Prediction of 28-day mortality in critically ill patients with sepsis based on TYG levels at admission
We conducted univariate Cox regression analysis of 28-day mortality rate in critically ill patients with sepsis (Table S2). In the unadjusted model, higher TYG levels increased the 28-day mortality rate in patients with sepsis (HR 3.53, 95% CI 2.85 to 4.38, P < 0.001) (Table 2). In Model II, after adjusting for age, gender, and race, TYG was predictive of the 28-day mortality rate in patients with sepsis (HR 1.39, 95% CI 1.32 to 1.46, P < 0.001). Compared to the lower TYG level group, higher TYG levels increased the 28-day mortality rate in patients with sepsis (HR 3.67, 95% CI 2.96 to 4.55, P < 0.001). In Model III, further adjustments were made for hemoglobin, white blood cell count, platelet count, blood urea nitrogen, creatinine, albumin, bicarbonate, potassium, aniongap, chloride, low-density lipoprotein, high-density lipoprotein, and TYG remained predictive of the 28-day mortality rate in patients with sepsis (HR 1.24, 95% CI 1.17 to 1.31, P < 0.001). The 28-day mortality rate continued to increase in the higher TYG group (HR 3.11, 95% CI 2.49 to 3.88, P < 0.001). Model IV further adjusted for mean heart rate, mean systolic blood pressure, mean diastolic blood pressure, mean arterial blood pressure, mean respiratory rate, statin use, septic shock, SOFA score, GCS score, and Charlson comorbidity index. TYG remained predictive of the 28-day mortality rate in patients with sepsis (HR 1.24, 95% CI 1.16 to 1.32, P < 0.001), and higher TYG levels also increased the 28-day mortality rate (HR 2.68, 95% CI 2.14 to 3.36, P < 0.001).
Table 2.
Predictive relationship of TYG with 28-day mortality in cox regression model
| Variable | Model I | Model II | Model III | Model IV | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| TYGa | 1.35 (1.29,1.42) | < 0.001 | 1.39 (1.32, 1.46) | < 0.001 | 1.24 (1.17, 1.31) | < 0.001 | 1.24 (1.16, 1.32) | < 0.001 |
| TYG < 8.9b | Ref | Ref | Ref | Ref | ||||
| TYG ≥ 8.9b | 3.53 (2.85,4.38) | < 0.001 | 3.67 (2.96,4.55) | < 0.001 | 3.11 (2.49,3.88) | < 0.001 | 2.68 (2.14,3.36) | < 0.001 |
Model I: no adjusted
Model II: adjusted for age, gender, race
Model III: adjusted for Model II plus hemoglobin, white blood cell, platelets, BUN, creatinine, albumin, bicarbonate, potassium, aniongap, chloride, LDL, HDL
Model IV: adjusted for Model II and Model III plus mean heart rate, mean SBP, mean DBP, mean MBP, mean respiratory rate, statin, septic shock, SOFA, GCS, Charlson’s comorbidity index
aincluded the continuous variable TYG instead of the categorical variable TYG
bincluded the categorical variable TYG instead of the continuous variable TYG
Subgroup analysis of the relationship between TYG and the 28-day mortality in critically ill patients with Sepsis
To further clarify whether the relationship between TYG and the 28-day mortality rate in critically ill patients with sepsis is influenced by other common comorbidities, subgroup analysis was performed. When the data underwent subgroup analysis based on age, gender, SOFA score, hypertension, cerebrovascular disease, myocardial infarction, heart failure, COPD, and ventilation, Table 3 shows that except for the COPD population (P = 0.003), where an interaction can be observed, there was no significant interaction between TYG and each of the other subgroups. (interaction p-values: 0.076–0.548). This indicates that the relationship between TYG and the 28-day mortality in critically ill patients with sepsis is stable.
Table 3.
Subgroup analyses of association between TYG on admission and 28-d mortality in critically ill patients with sepsis
| Subgroup | HR (95%CI) | p value | p for interaction |
|---|---|---|---|
| Age | 0.371 | ||
| < 65 (n = 1086) | 1.33 (1.24,1.43) | < 0.001 | |
| ≥ 65 (n = 1127) | 1.48 (1.38,1.59) | < 0.001 | |
| Gender | 0.354 | ||
| Female (n = 991) | 1.37 (1.28,1.47) | < 0.001 | |
| Male (n = 1222) | 1.34 (1.24,1.44) | < 0.001 | |
| SOFA | 0.485 | ||
| < 6 (n = 755) | 1.27 (1.18,1.36) | < 0.001 | |
| ≥ 6 (n = 1458) | 1.29 (1.17,1.42) | < 0.001 | |
| Hypertension | 0.542 | ||
| NO (n = 1227) | 1.56(1.35,1.81) | < 0.001 | |
| YES (n = 986) | 1.27(1.20,1.35) | < 0.001 | |
| Cerebrovascular Disease | 0.086 | ||
| NO (n = 1966) | 1.35 (1.28,1.42) | < 0.001 | |
| YES (n = 247) | 1.57 (1.12,2.11) | 0.003 | |
| Myocardial Infarction | 0.076 | ||
| NO (n = 1916) | 1.35 (1.28,1.42) | < 0.001 | |
| YES (n = 297) | 1.79 (1.34,2.41) | < 0.001 | |
| Heart Failure | 0.354 | ||
| NO (n = 1659) | 1.36 (1.29,1.44) | < 0.001 | |
| YES (n = 554) | 1.33 (1.12,1.47) | < 0.001 | |
| COPD | 0.003 | ||
| NO (n = 1695) | 1.33 (1.25,1.40) | < 0.001 | |
| YES (n = 518) | 2.12 (1.72,2.61) | < 0.001 | |
| Ventilator | 0.548 | ||
| NO (n = 307) | 1.39 (1.13,1.70) | 0.002 | |
| YES (n = 1906) | 1.34 (1.27,1.41) | < 0.001 |
SOFA Sequential Organ Failure Assessment, COPD Chronic Obstructive Pulmonary Disease
Discussion
This study is a large retrospective cohort study that explored the correlation between TYG index and mortality of sepsis, providing strong research evidence for biomarkers predicting poor prognosis of sepsis. This study found that TYG index was positively correlated with the 28-day mortality of sepsis. When TYG index > 9.0, the 28-day mortality of sepsis increased with TYG index rising. Even after adjusting for confounding factors, this correlation remained robust after adjusting for confounding factors (such as age, gender, race, hemoglobin, white blood cell, platelets, BUN, creatinine, albumin, bicarbonate, potassium, aniongap, chloride, LDL, HDL, heart rate, respiratory rate, statin, septic shock, SOFA, GCS, Charlson's comorbidity index). Additionally, subgroup analysis showed that the correlation between TYG index and 28-day mortality of sepsis was robust, and TYG index has no interaction with age, gender, hypertension, cerebrovascular disease, myocardial infarction, heart failure and SOFA score. These findings warn that doctors should pay more attention to the identification and intervention of TYG index in sepsis.
Sepsis is a fatal disease, accompanied by metabolic disorders and inflammatory storms. Relevant studies focus on finding novel biomarkers with high sensitivity and specificity for the diagnosis of sepsis and development of new therapies [14]. Studies have indicated that sepsis will promote the glycolysis process, and the increasing glucose will facilitate viral replication or inflammatory storms, thereby aggravating the severity of infection [15–21]. Moreover, studies have suggested that there is a J-type relationship between blood glucose and mortality in non-diabetic patients with sepsis, and the risk of death will increase with the increase of glucose [22]. Currently, the relationship between blood lipids and poor prognosis of sepsis remains inconclusive. Some studies have found that the release of cytokines such as bacterial endotoxin from sepsis will elicit dramatic responses in the host, stimulating the release of plasma catecholamines and glucocorticoids to elevate triglyceride-rich lipoprotein [23–25], which in turn increases lipid stores to fuel the host response to infection and regulate the acute phase response [26–29]. However, the binding of lipoprotein to lipoprotein needs to be inserted into endotoxin lipid A, which in turn triggers the release of inflammatory mediators and leads to a decrease in host responses to infection [30, 31]. Studies have shown that the mortality of sepsis is related to the decrease of total cholesterol, HDL and LDL, and has no significant correlation with triglyceride. However, Cetinkaya et al. suggested that although the mechanism remains unclear, high triglycerides can predict the risk of death in sepsis. This study found that TYG index was associated with 28-day mortality of sepsis, which provided a new idea for the management of blood glucose and lipid in patients.
IR reflects the host poor response to insulin and metabolic abnormalities in many critically ill patients have been suggested to be associated with IR. Additionally, IR involved in various inflammatory processes and can predict the occurrence of poor prognosis of diseases such as myocardial infarction, heart failure, stroke and cancer. Some studies indicated that IR is not only a disease-specific response, but a universal response to critical illness [32]. TYG index, a biomarker similar to the efficacy of the gold standard for evaluating IR–hyperinsulinaemic–euglycaemic clamp, has also been found to be associated with metabolic disorders such as hyperlipidemia, hyperglycemia and obesity, as well as the occurrence of cardiovascular and cerebrovascular diseases such as atherosclerosis and stroke [33–35]. Lin et al. found that the TYG index had a greater predictive power for incident metabolic syndrome than neutrophils [36]. Liao et al. suggested that TYG index is a reliable risk predictor of all-cause mortality in ICU patients [37]. Zhang et al. also indicated that TYG index is a potential predictor of mortality in critically ill stroke [38]. This study obtained the correlation between TYG index and the mortality of sepsis, which provided a supplement for the study of the correlation between the TYG index and the poor prognosis of critically ill diseases. Presently, the reasons why TYG index increases the mortality of sepsis may be as follows: Firstly, IR is closely related to fibrinolytic system disorder, endothelial dysfunction, blood–brain barrier damage and oxidative stress, which will aggravate sepsis-related infections and destroy the host response to infection [39–41]. Secondly, IR is involved in metabolic disorders, which will further aggravate the occurrence of infection due to the release of cytokines and inflammatory factors. Finally, IR is associated with the occurrence of cardiovascular and cerebrovascular diseases and organ dysfunction, combined with hyperglycemia and hyperlipidemia, which will increase the severity of infection and lead to cellular acidosis and oxidative stress to destroy the host immune barrier [42].
The optimal cutoff value based on the ROC curve in this study was 9.0, which was roughly similar to the cutoff value in previous studies. Moreover, we found that serum triglyceride and glucose, as components of the TYG index, were inferior to the TYG index in predicting 28-day mortality of sepsis. The TYG index is a simple, robust and effective predictor of 28-day mortality in sepsis. Therefore, more attention should be paid on TYG index in sepsis, which accompanied by severe metabolic disorders and inflammatory storms. Correcting this index as soon as possible will reduce the incidence of 28-day mortality.
Strengths and limitations
Our study has several advantages. Firstly, to our knowledge, there have been few studies investigating the relationship between TYG and short-term prognosis in patients with sepsis admitted to the ICU. Our study found a nonlinear relationship between TYG and the 28-day all-cause mortality rate in patients with sepsis. This provides a potential theoretical basis for clinicians to develop treatment plans. Additionally, TYG has several advantages, such as simplicity, ease of access, speed, non-invasiveness, and high accuracy. In our subgroup analysis, TYG demonstrated a stable relationship with mortality rate.
This study has some limitations. Firstly, our study is a single-center retrospective study based on electronic medical records, which may lead to inevitable selection bias. Secondly, due to our exclusion criteria, the proportion of excluded variables in the subjects with missing data may lead to bias in the relationship. Lastly, Due to limitations in our study design, we only analyzed the relationship between the initial TYG measurement upon admission and the prognosis. We did not assess the dynamic relationship between TYG and prognosis over time. Therefore, further verification of the predictive performance of TYG requires multicenter and prospective studies.
Conclusion
In our study, TYG can be used as an independent predictor for all-cause mortality due to sepsis within 28 days of hospitalization. Its prognostic value is superior to using triglycerides and glucose alone and is even comparable to that of SOFA. In addition, due to the advantages of easy access, it can help clinicians make decisions in the future.
Supplementary Information
Supplementary Material 1: Figure S1: Flowchart of subject screening; Table S1: Information of ROC curves in Figure 2; Table S2: Univariate Cox retrospective analysis of 28-day mortality in patients with sepsis.
Authors’ contributions
SZ participated in data collection, processing, and analysis, writing of the article, literature search and screening. NC, TF, LW participated in the formatting of the article, and LM participated in the supervision and modification of the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grant from the public, commercial, or not-for-profit funding agencies.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study utilized the MIMIC-IV database, which received approval from the Institutional Review Boards of Beth Israel Deaconess Medical Center and the Massachusetts Institute of Technology in Boston, Massachusetts. It involved a retrospective analysis, and the database does not contain protected health information. Therefore, the Institutional Review Board of the First Hospital of Shanxi Medical University waived the requirements for an ethical approval statement and informed consent. All methods in this study were conducted in accordance with relevant guidelines and regulations, including the Helsinki Declaration.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sen Zhang and Tianhua Fan contributed equally to this work.
References
- 1.Abe T, Yamakawa K, Ogura H, Kushimoto S, Saitoh D, Fujishima S, et al. Epidemiology of sepsis and septic shock in intensive care units between sepsis-2 and sepsis-3 populations: sepsis prognostication in intensive care unit and emergency room (SPICE-ICU). J Intensive Care. 2020;8:44. 10.1186/s40560-020-00465-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleischmann C, Scherag A, Adhikari NKJ, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259–72. 10.1164/rccm.201504-0781OC [DOI] [PubMed] [Google Scholar]
- 3.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markwart R, Saito H, Harder T, Tomczyk S, Cassini A, Fleischmann-Struzek C, et al. Epidemiology and burden of sepsis acquired in hospitals and intensive care units: a systematic review and meta-analysis. Intensive Care Med. 2020;46(8):1536–51. 10.1007/s00134-020-06106-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cetinkaya A, Erden A, Avci D, Karagoz H, Karahan S, Basak M, et al. Is hypertriglyceridemia a prognostic factor in sepsis? Ther Clin Risk Manag. 2014;10:147–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gong Q, Zhang P, Wang J, An Y, Gregg EW, Li H, et al. Changes in mortality in people with IGT before and after the onset of diabetes during the 23-year follow-up of the Da Qing Diabetes prevention study. Diabetes Care. 2016;39(9):1550–5. 10.2337/dc16-0429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang X, Wu T, Chen Q, Jiang J, Jiang Y, Ruan Y, et al. Serum proteomics reveals disorder of lipoprotein metabolism in sepsis. Life Sci Alliance. 2021;4(10):e202101091. 10.26508/lsa.202101091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter SJ, Garvey WT. Insulin action and insulin resistance: diseases involving defects in insulin receptors, signal transduction, and the glucose transport effector system. Am J Med. 1998;105(4):331–45. 10.1016/S0002-9343(98)00300-3 [DOI] [PubMed] [Google Scholar]
- 9.Carlson GL. Insulin resistance and glucose-induced thermogenesis in critical illness. Proc Nutr Soc. 2001;60(3):381–8. 10.1079/PNS200193 [DOI] [PubMed] [Google Scholar]
- 10.Di Pino A, DeFronzo RA. Insulin resistance and atherosclerosis: implications for insulin-sensitizing agents. Endocr Rev. 2019;40(6):1447–67. 10.1210/er.2018-00141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol. 2014;20(13):146. 10.1186/s12933-014-0146-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alizargar J, Bai CH, Hsieh NC, Wu SFV. Use of the triglyceride-glucose index (TyG) in cardiovascular disease patients. Cardiovasc Diabetol. 2020;19(1):8. 10.1186/s12933-019-0982-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiu H, Tsai HJ, Huang JC, Wu PY, Hsu WH, Lee MY, et al. Associations between triglyceride-glucose index and micro- and macro-angiopathies in type 2 diabetes mellitus. Nutrients. 2020;12(2):328. 10.3390/nu12020328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vincent JL, Marshall JC, Namendys-Silva SA, François B, Martin-Loeches I, Lipman J, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2(5):380–6. 10.1016/S2213-2600(14)70061-X [DOI] [PubMed] [Google Scholar]
- 15.Shen TJ, Chen CL, Tsai TT, Jhan MK, Bai CH, Yen YC, et al. Hyperglycemia exacerbates dengue virus infection by facilitating poly(A)-binding protein-mediated viral translation. JCI Insight. 2022;7(21):e142805. 10.1172/jci.insight.142805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Modenesi RF, Pena FM, de Faria CAC, Carvalho RV, de Souza NRM, da Soares JS, et al. Influence on prognosis and prevalence of stress hyperglycemia in a cohort of patients with acute coronary syndrome. Rev Bras Ter Intensiva. 2012;24(4):352–6. 10.1590/S0103-507X2012000400010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vermeer SE, Sandee W, Algra A, Koudstaal PJ, Kappelle LJ, Dippel DWJ, et al. Impaired glucose tolerance increases stroke risk in nondiabetic patients with transient ischemic attack or minor ischemic stroke. Stroke. 2006;37(6):1413–7. 10.1161/01.STR.0000221766.73692.0b [DOI] [PubMed] [Google Scholar]
- 18.Singer M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence. 2014;5(1):66–72. 10.4161/viru.26907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez EL, Lagunoff M. Viral activation of cellular metabolism. Virology. 2015;479–480:609–18. 10.1016/j.virol.2015.02.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou L, He R, Fang P, Li M, Yu H, Wang Q, et al. Hepatitis B virus rigs the cellular metabolome to avoid innate immune recognition. Nat Commun. 2021;12(1):98. 10.1038/s41467-020-20316-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang P, Pan S, Yuan S, Shang Y, Shu H. Abnormal glucose metabolism in virus associated sepsis. Front Cell Infect Microbiol. 2023;13:1120769. 10.3389/fcimb.2023.1120769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W, Chen W, Liu Y, Li L, Li S, Tan J, et al. Blood glucose levels and mortality in patients with sepsis: dose-response analysis of observational studies. J Intensive Care Med. 2021;36(2):182–90. 10.1177/0885066619889322 [DOI] [PubMed] [Google Scholar]
- 23.Givalois L, Dornand J, Mekaouche M, Solier MD, Bristow AF, Ixart G, et al. Temporal cascade of plasma level surges in ACTH, corticosterone, and cytokines in endotoxin-challenged rats. Am J Physiol. 1994;267(1 Pt 2):R164-170. [DOI] [PubMed] [Google Scholar]
- 24.Ong JM, Saffari B, Simsolo RB, Kern PA. Epinephrine inhibits lipoprotein lipase gene expression in rat adipocytes through multiple steps in posttranscriptional processing. Mol Endocrinol. 1992;6(1):61–9. [DOI] [PubMed] [Google Scholar]
- 25.Bagby GJ, Corll CB, Martinez RR. Triacylglycerol kinetics in endotoxic rats with suppressed lipoprotein lipase activity. Am J Physiol. 1987;253(1 Pt 1):E59-64. [DOI] [PubMed] [Google Scholar]
- 26.Harris HW, Gosnell JE, Kumwenda ZL. The lipemia of sepsis: triglyceride-rich lipoproteins as agents of innate immunity. J Endotoxin Res. 2000;6(6):421–30. [PubMed] [Google Scholar]
- 27.Zinellu A, Paliogiannis P, Fois AG, Solidoro P, Carru C, Mangoni AA. Cholesterol and triglyceride concentrations, COVID-19 severity, and mortality: a systematic review and meta-analysis with meta-regression. Front Public Health. 2021;9: 705916. 10.3389/fpubh.2021.705916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka S, Couret D, Tran-Dinh A, Duranteau J, Montravers P, Schwendeman A, et al. High-density lipoproteins during sepsis: from bench to bedside. Crit Care. 2020;24(1):134. 10.1186/s13054-020-02860-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hofmaenner DA, Arina P, Kleyman A, Page Black L, Salomao R, Tanaka S, et al. Association between hypocholesterolemia and mortality in critically Ill patients with sepsis: a systematic review and meta-analysis. Crit Care Explor. 2023;5(2): e0860. 10.1097/CCE.0000000000000860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Netea MG, Demacker PN, Kullberg BJ, Jacobs LE, Verver-Jansen TJ, Boerman OC, et al. Bacterial lipopolysaccharide binds and stimulates cytokine-producing cells before neutralization by endogenous lipoproteins can occur. Cytokine. 1998;10(10):766–72. 10.1006/cyto.1998.0364 [DOI] [PubMed] [Google Scholar]
- 31.Harris HW, Grunfeld C, Feingold KR, Rapp JH. Human very low density lipoproteins and chylomicrons can protect against endotoxin-induced death in mice. J Clin Invest. 1990;86(3):696–702. 10.1172/JCI114765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zauner A, Nimmerrichter P, Anderwald C, Bischof M, Schiefermeier M, Ratheiser K, et al. Severity of insulin resistance in critically ill medical patients. Metabolism. 2007;56(1):1–5. 10.1016/j.metabol.2006.08.014 [DOI] [PubMed] [Google Scholar]
- 33.Huang R, Wang Z, Chen J, Bao X, Xu N, Guo S, et al. Prognostic value of triglyceride glucose (TyG) index in patients with acute decompensated heart failure. Cardiovasc Diabetol. 2022;21(1):88. 10.1186/s12933-022-01507-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu XC, He GD, Lo K, Huang YQ, Feng YQ. The triglyceride-glucose index, an insulin resistance marker, was non-linear associated with all-cause and cardiovascular mortality in the general population. Front Cardiovasc Med. 2020;7:628109. 10.3389/fcvm.2020.628109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson AEW, Kramer AA, Clifford GD. A new severity of illness scale using a subset of acute physiology and chronic health evaluation data elements shows comparable predictive accuracy. Crit Care Med. 2013;41(7):1711–8. 10.1097/CCM.0b013e31828a24fe [DOI] [PubMed] [Google Scholar]
- 36.Lin HY, Zhang XJ, Liu YM, Geng LY, Guan LY, Li XH. Comparison of the triglyceride glucose index and blood leukocyte indices as predictors of metabolic syndrome in healthy Chinese population. Sci Rep. 2021;11(1):10036. 10.1038/s41598-021-89494-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao Y, Zhang R, Shi S, Zhao Y, He Y, Liao L, et al. Triglyceride-glucose index linked to all-cause mortality in critically ill patients: a cohort of 3026 patients. Cardiovasc Diabetol. 2022;21(1):128. 10.1186/s12933-022-01563-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang B, Liu L, Ruan H, Zhu Q, Yu D, Yang Y, et al. Triglyceride-glucose index linked to hospital mortality in critically Ill stroke: an observational multicentre study on eICU database. Front Med (Lausanne). 2020;7:591036. 10.3389/fmed.2020.591036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Presterl E, Staudinger T, Pettermann M, Lassnigg A, Burgmann H, Winkler S, et al. Cytokine profile and correlation to the APACHE III and MPM II scores in patients with sepsis. Am J Respir Crit Care Med. 1997;156(3 Pt 1):825–32. 10.1164/ajrccm.156.3.9607131 [DOI] [PubMed] [Google Scholar]
- 40.Alessi MC, Juhan-Vague I. Metabolic syndrome, haemostasis and thrombosis. Thromb Haemost. 2008;99(6):995–1000. 10.1160/TH07-11-0682 [DOI] [PubMed] [Google Scholar]
- 41.Muniyappa R, Sowers JR. Role of insulin resistance in endothelial dysfunction. Rev Endocr Metab Disord. 2013;14(1):5–12. 10.1007/s11154-012-9229-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–67. 10.1056/NEJMoa011300 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Figure S1: Flowchart of subject screening; Table S1: Information of ROC curves in Figure 2; Table S2: Univariate Cox retrospective analysis of 28-day mortality in patients with sepsis.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.