Abstract
Our previous reports indicated that Epstein-Barr virus (EBV) contributes to the malignant phenotype and resistance to apoptosis in Burkitt’s lymphoma (BL) cell line Akata (N. Shimizu, A. Tanabe-Tochikura, Y. Kuroiwa, and K. Takada, J. Virol. 68:6069–6073, 1994; J. Komano, M. Sugiura, and K. Takada, J. Virol. 72:9150–9156, 1998). Here we report that the EBV-encoded small RNAs (EBERs) are responsible for these phenotypes. Transfection of the EBER genes into EBV-negative Akata clones restored the capacity for growth in soft agar, tumorigenicity in SCID mice, resistance to apoptotic inducers, and upregulated expression of bcl-2 oncoprotein that were originally retained in parental EBV-positive Akata cells and lost in EBV-negative subclones. This is the first report which provides evidence that virus-encoded RNAs (EBERs) have oncogenic functions in BL cells.
Epstein-Barr virus (EBV) was demonstrated to be present in more than 90% of endemic Burkitt’s lymphoma (BL) cases in equatorial Africa and New Guinea and in about 10% of sporadic BL cases in the western hemisphere (reviewed in reference 23). The important feature of the BL cell is the juxtaposition of the c-myc locus to the immunoglobulin H enhancer through a reciprocal translocation [t(8;14)] and, more rarely, to the Igκ locus [t(2;8)] or the Igλ locus [t(8;22)]. This feature led to the hypothesis that this translocation resulted in constitutive activation of the c-myc gene, which seemed to be a key step in the initiation of BL (12). However, the role of EBV in the genesis of BL is still an open question. To gain insight into this problem, we have investigated the pathogenic role of EBV in BL cell line Akata. The Akata cell line is an EBV-positive BL cell line derived from a Japanese patient and possessing chromosomal translocation t(8;14) (29). It retains type I latency even in long-term cultures, in which EBV nuclear antigen (EBNA) 1, EBV-encoded RNAs (EBERs, specifically EBER-1 and EBER-2), latent membrane protein (LMP) 2A, and BARF0 are expressed (13). In most BL cell lines, latency is converted from type I to type III in serial cultures, in which cells express all the EBNAs (1, 2, 3a, 3b, 3c, and LP), LMPs 1, 2A, and 2B), EBERs, and BARF0 (reviewed in reference 1). EBV-immortalized B-cell lines (lymphoblastoid cell lines) also have type III latency (24).
Hitherto, it has been impossible to demonstrate that the presence of EBV with type I latency has any biological effects in BL cells. Akata cells enabled us to resolve this problem. EBV-negative cell clones can be isolated from parental Akata cells, since EBV DNA has been spontaneously lost during cultivation (27). Type I latency was restored by EBV reinfection in EBV-negative Akata cells. We successfully established a system to confirm whether any phenotypic differences in cells were due to EBV. This system compared two pairs of cell clones: (i) EBV-positive and -negative cell clones obtained from an EBV-positive Akata cell clone and (ii) neomycin resistance gene (neoR)-transfected and EBV-reinfected cell clones obtained from an EBV-negative Akata cell clone by use of recombinant EBV carrying neoR. Using this system, we verified that, in Akata cells, EBV was necessary for the malignant phenotype, resistance to apoptosis, and upregulated expression of bcl-2 oncoprotein (13, 27). These were the first reports that provided direct evidence that the presence of EBV had an oncogenic role and that type I latency conferred resistance to apoptosis in vitro. We also demonstrated that EBNA-1 was not responsible for these phenotypes in Akata cells (13). Similar results were also reported by Ruf et al. (25).
In this report, we focused upon the possible involvement of EBERs in the malignant phenotype and resistance to apoptosis in Akata cells. EBER-1 and EBER-2 are small nuclear RNAs transcribed by the RNA polymerase III system; they are, respectively, 166 and 172 nucleotides long (7). Being abundant, EBER-1 has often been targeted as a probe for in situ hybridization studies to identify the presence of EBV (5). With an EBER deletion recombinant virus, it was demonstrated that EBERs were not necessary for the B-cell-immortalizing function of EBV (28). To date, the biological functions of EBERs remain unclear.
Here, we present evidence that EBERs play an oncogenic role in Akata cells. This is the first report which provides evidence that virus-encoded RNAs (EBERs) have oncogenic functions in BL cells.
MATERIALS AND METHODS
Cell culture.
The Akata cell line of BL origin was maintained in RPMI 1640 medium (Sigma) supplemented with 10% fetal bovine serum (FBS) (Gibco), 40 U of penicillin per ml, and 50 μg of streptomycin per ml at 37°C in a 5% CO2 humidified atmosphere.
Plasmid construction.
neoR driven by the simian virus 40 promoter was derived from pcDNA3 (Invitrogen). EBER-1 and EBER-2 open reading frames (ORFs) are located at 6,628 to 6,796 bp and at 6,958 to 7,129 bp, respectively, on the EcoRI K fragment of Akata EBV DNA, which corresponds to the EcoRI J fragment of B95-8 EBV DNA. pEK contained the EcoRI K fragment of Akata EBV DNA and neoR. pEKS10 contained 10 tandem repeats of the SacI-EcoRI subfragment (6,297 to 7,325 bp) from the EcoRI K fragment of Akata EBV DNA and neoR. At each end of the SacI-EcoRI subfragment from the EcoRI K fragment, BglII or BamHI sites were added and concatenated in a direction-restricted fashion.
Transfection and cloning.
Plasmids were introduced into Akata cells by the electroporation method as described previously (13). For cloning cells, 104 cells were transferred to each well of a flat-bottom 96-well plate (Falcon) with 200 μl of medium containing 700 μg of G418 (Gibco) per ml 2 days after transfection. Half of the medium was changed every 5 days until colonies emerged.
EBV infection.
Preparation of the virus solution was described previously (10). Cells (5 × 106) were suspended in 2 ml of 1:2-diluted EBV solution (Akata strain with the neoR gene) for 1 h at room temperature with continuous mild mixing. Then, they were washed three times and cultured for 2 days. Selection and cloning procedures are described above.
Northern blot analysis.
Total RNA was extracted from Akata cells with Trizol reagent (Gibco) according to the manufacturer’s instructions. Samples (1 to 2 μg per lane) were electrophoresed in 2% agarose–formaldehyde gels, transferred to Hybond N+ nylon membranes (Amersham), fixed with alkali, prehybridized, hybridized with a probe, and washed under stringent conditions (15 mM NaCl, 1.5 mM sodium citrate, 0.1% sodium dodecyl sulfate). The probe for EBERs was an EcoRI K fragment, so that both EBER-1 and EBER-2 transcripts were detected. Fifty nanograms of DNA was radiolabeled with a Ready-Prime labeling kit (Amersham).
RT-PCR.
Reverse transcriptase PCR (RT-PCR) was performed as described previously (13). Briefly, total RNA was extracted from Akata cells with Trizol reagent (Gibco). RNA was solubilized in distilled water and treated with DNase I (Gibco). The primers used for EBER-1 were AGG ACC TAC GCT GCC CTA GA (5′ sequence) and AAA ACA TGC GGA CCA CCA GC (3′ sequence); the product was 166 bp (30). After reverse transcription, 100 ng of the cDNA was subjected to PCR in the presence of 5 U of Taq polymerase (Gibco) in a total volume of 50 ml with a model 2400 Thermal Cycler (Perkin-Elmer) for 12 cycles. The amplified products were electrophoresed in 2% agarose gels and stained with ethidium bromide.
Soft-agar colony assay.
Wells of a six-well plate (Falcon) were covered with 0.5% SeaPlaque agarose (FMC, Rockland, Maine) containing RPMI 1640 medium and 12% FBS. Onto this base, 104 cells in 0.4% SeaPlaque agarose containing RPMI 1640 medium and 12% FBS were embedded. After 2 to 3 weeks of incubation, colonies that contained more than 100 cells were counted.
EBER-1 in situ hybridization.
EBER-1 was detected with a digoxigenin-labeled oligonucleotide probe complementary to the EBER-1 sequence (5′-AGA CAC CGT CCT CAC CAC CCG GGA CTT GTA-3′) (5). A sense probe against EBER-1 was used for negative control studies.
Apoptosis assay.
Cells in the log phase were incubated for 16 h in the presence of 20 μg of cycloheximide (CHX) (Wako, Osaka, Japan) per ml, for 40 h with 1 mM glucocorticoid (Pharmacia or Upjohn), or for 65 h in a 100% CO2-saturated humidified atmosphere (hypoxic stress). The viability of cells was quantified by an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (Cell Titer 96; Promega).
Western blot analysis.
Western blot analysis was performed as described previously (27). For immunostaining, blots were incubated with mouse anti-human bcl-2 monoclonal antibody bcl-2/100 (Pharmingen).
RESULTS
EBER expression in EK- and EKS10-transfected Akata cells.
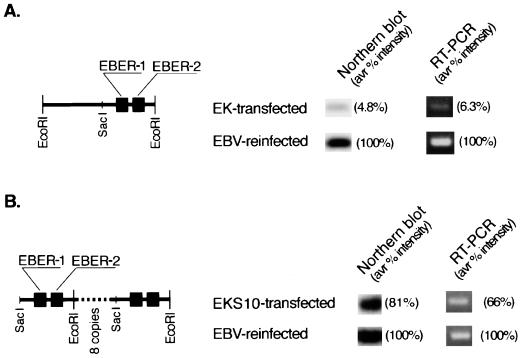
We constructed a plasmid, pEK, that carried a single copy of EBER and neoR. We isolated G418-resistant clones by transfecting pEK into an EBV-negative Akata cell clone. Compared with the level of EBERs in EBV-reinfected cell clones, the average signal intensities for EBERs in four EK-transfected cell clones were 4.8% by Northern blot analysis and 6.3% by RT-PCR, even though clones expressing the highest levels were analyzed (Fig. 1A). To obtain higher levels of EBER expression in transfected cells, we constructed a plasmid, pEKS10, that contained 10 tandem repeats of the SacI-EcoRI subfragment from the EcoRI K fragment of Akata EBV DNA. We isolated G418-resistant clones by transfecting pEKS10 into an EBV-negative Akata cell clone. Compared with the level in EBV-reinfected cell clones, the average levels of EBER expression in four EKS10-transfected cell clones were 81% by Northern blot analysis and 66% by RT-PCR (Fig. 1B). It was difficult to obtain levels of EBER expression in transfected cells equivalent to those in EBV-reinfected cells. The amount of the EBER-1 transcript was approximately twofold higher than that of the EBER-2 transcript in EBV-positive, EBV-reinfected, and EBER-transfected Akata cell clones by a semiquantitative RT-PCR assay.
FIG. 1.
Relative EBER expression in EK- and EKS10-transfected Akata cells compared with EBV-reinfected Akata cells. For each EBER transfectant derived from different EBV-negative clones, more than 50 cell clones were examined for EBER expression, and 4 to 6 clones with the highest levels of EBER expression were chosen for further studies. Representative results are shown. Four EBER-transfected cell clones from an EBV-negative Akata cell clone were mixed and subjected to quantitative assays for EBER. (A) Schematic drawing of the EcoRI K fragment of Akata EBV DNA (left) and relative EBER expression in EK-transfected cells (right). (B) Schematic drawing of EKS10 (left) and relative EBER expression in EKS10-transfected cells (right). avr, average.
Growth characteristics of EK- and EKS10-transfected Akata cells.
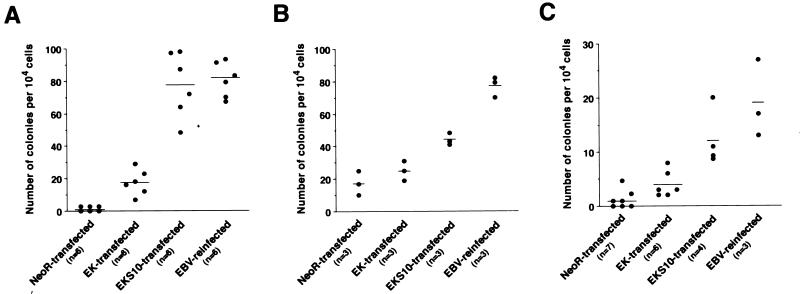
The growth characteristics of EK- and EKS10-transfected Akata cell clones were compared with those of EBV-reinfected cell clones and neoR-transfected cell clones. First, we performed a soft-agar colony assay using cell clones derived from three independent EBV-negative clones (Fig. 2). EBV-reinfected cell clones formed colonies, but neoR-transfected cell clones scarcely did. The absolute number of colonies differed among experiments, presumably due to clonal variation. EK-transfected cell clones formed a few colonies, whereas EKS10-transfected cell clones formed significantly more colonies than neoR-transfected cell clones but formed fewer colonies than EBV-reinfected cell clones (Fig. 2). Compared with data for EBV-reinfected cell clones, the restoration rates for colony production in soft agar in three independent experiments were 21, 13, and 17% (average, 17%) for EK-transfected cell clones and 95, 57, and 63% (average, 72%) for EKS10-transfected cell clones. As the level of EBER expression went up, more cells formed colonies in soft agar.
FIG. 2.
Colony production in soft agar for neoR-transfected, EK-transfected, EKS10-transfected, and EBV-reinfected cells. The individual panels show independent experiments with different EBV-negative Akata cell clones. Each dot represents the number of visible colonies emerging per 104 cells. Horizontal bars represent the mean values for each group. The differences between neoR-transfected and EKS10-transfected cell clones were significant (P, <0.01), as determined by the Mann-Whitney U test.
Second, we examined whether EKS10-transfected cells were tumorigenic in SCID mice. In three separate experiments, tumors developed from EKS10-transfected cells and from EBV-reinfected cells but not from neoR-transfected cells (Table 1). The tumors derived from EKS10-transfected Akata cells showed a typical lymphoma pattern with intensive blood vessel infiltration (Fig. 3). In an in situ hybridization study, almost all the tumor cells were positive for EBER-1 expression.
TABLE 1.
Tumorigenicity of EKS10-transfected Akata cells in SCID mice
| Cells | No. of mice with tumors in exptc:
|
||
|---|---|---|---|
| 1a | 2a | 3b | |
| EBV-positive clones | 3/4 | Not done | 3/3 |
| EBV-negative clones | 0/4 | Not done | 0/3 |
| EBV-reinfected clones | 2/4 | 2/4 | 8/9 (2/3, 3/3, 3/3) |
| EKS10-transfected clones | 1/5 | 1/5 | 7/15 (1/3, 2/3, 2/3, 1/3, 1/3) |
| neoR-transfected clones | 0/4 | 0/4 | 0/15 (0/3, 0/3, 0/3, 0/3, 0/3) |
Six cell clones transfected with neoR or EKS10 or six EBV-reinfected clones derived from an EBV-negative Akata cell clone were pooled, and 107 cells were inoculated into the thigh subcutis of 6-week-old male SCID mice (FOX CHASE C.B-17/Icr-scid Jcl; Clea, Tokyo, Japan).
Five cell clones transfected with neoR or EKS10 or three EBV-reinfected clones derived from an EBV-negative Akata cell clone (1.5 × 107 cells each) were individually inoculated into 4-week-old male SCID mice.
Mice were sacrificed 8 weeks after inoculation, and the developed tumors were measured. The tumors ranged in size from 0.8 to 4.5 cm.
FIG. 3.
Histological examination of tumors derived from EKS10-transfected Akata cells in SCID mice. Adjacent sections from paraffin-embedded blocks were stained by hematoxylin-eosin or subjected to an in situ hybridization study. Typical lymphoma histology was observed (left panel; magnification, ×400). The EBER-1 in situ hybridization study revealed that almost all the tumor cells were positive for EBER-1 expression (middle panel; magnification, ×400). Dark signals representing the presence of EBER-1 transcripts were specifically present in the nuclei of tumor cells. A negative control for the EBER-1 in situ hybridization study is also shown (right panel; magnification, ×400).
Resistance to apoptosis of EK- and EKS10-transfected Akata cells.
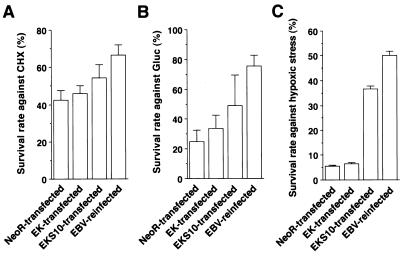
To test whether the expression of EBERs restored resistance to apoptosis, we performed an apoptosis assay using a set of cell clones. Apoptosis was induced by exposing cells to inducers such as CHX, glucocorticoid, and hypoxic stress, and surviving cells were measured by an MTT assay (Fig. 4). The survival rate (%SR) was the highest in EBV-reinfected cells and the lowest in neoR-transfected cells. The difference in %SR between neoR-transfected cells and EK-transfected cells was not significant. In contrast, the %SR of EKS10-transfected cells was significantly higher than that of neoR-transfected cells for all stimuli. Compared with data for EBV-reinfected cells, the restoration rates for EK-transfected cells were 16, 18, and 2.2% (average, 12%) against CHX, glucocorticoid, and hypoxic stress, respectively, and those for EKS10-transfected cell were 50, 48, and 70% (average, 56%), respectively. These results illustrated that the more that EBERs were expressed in cells, the greater the resistance to apoptosis of the cells.
FIG. 4.
Resistance to apoptosis of neoR-transfected, EK-transfected, EKS10-transfected, and EBV-reinfected cells tested with CHX (A), glucocorticoid (Gluc) (B), and hypoxic stress (C). The bars show the means ± standard deviations for six clones. The data are typical results from three independent experiments. As determined by a t test analysis, the differences between mean values for neoR- and EKS10-transfected cells were significant at P values of <0.01 (CHX), <0.05 (Gluc), and <0.001 (hypoxic stress).
EBERs upregulate the expression of bcl-2 oncoprotein.
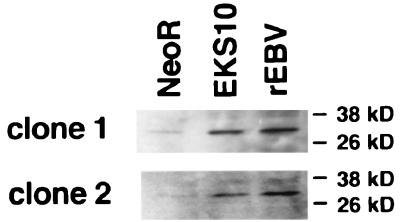
We next examined whether the upregulation of bcl-2 oncoprotein had been restored in EKS10-transfected cells. The level of bcl-2 protein expression in EBV-reinfected cells was found to be higher than that in neoR-transfected cells (Fig. 5). bcl-2 expression in EKS10-transfected cells was higher than that in neoR-transfected cells. We could not find significant upregulation of bcl-2 protein in EK-transfected cells. Finally, the average restoration rate for bcl-2 expression in EKS10-transfected cells was estimated to be 65% by densitometric analysis in several experiments.
FIG. 5.
Expression of bcl-2 protein in neoR-transfected, EKS10-transfected, and EBV-reinfected (rEBV) cells derived from two independent EBV-negative Akata cell clones. The data are typical results from several experiments.
DISCUSSION
In the transfection study, we demonstrated that EBER-expressing Akata cell clones restored the malignant phenotype, resistance to apoptosis, and upregulated expression of bcl-2 protein to levels comparable to the restoration rates for EBER expression in EBV-reinfected cell clones. Thus, it was clear that EBERs were responsible for the malignant phenotype and resistance to apoptosis in Akata cells. Many RNAs are known to have catalytic functions; however, there has been no report describing an oncogenic RNA. This is the first paper that provides evidence that RNA polymerase III-transcribed virus-encoded small RNAs affect the malignant phenotype and resistance to apoptosis.
There was a good correlation between the rate of restoration of EBER expression and the biological activity of EBV-reinfected cells. This result clearly illustrates that EBERs contribute to the malignant phenotype and resistance to apoptosis. All the restoration rates for biological phenotypes estimated from individual experiments were somewhat lower than the levels of expression of EBERs in EKS10-transfected cells. The reason for this result is probably that there was a gradual loss of EBER expression in EKS10-transfected cells during cultivation. RT-PCR revealed that EBER-1 expression in EKS10-transfected cells at 14 weeks after transfection was 60% that at 6 weeks (data not shown). The reason for this result is largely unknown; however, it is often seen in stable transformants.
More interestingly, how could EBERs (RNAs) contribute to malignant phenotypes and apoptosis resistance? EBERs were reported to bind to some cellular proteins, La (16), EAP/L22 (31, 32), and PKR (6). Above all, the association of EBERs with PKR is noteworthy. PKR, previously described as an interferon-induced protein kinase, is induced by interferons and activated by double-stranded RNAs to phosphorylate eIF-2α, a translation initiation factor (18). This phosphorylation results in the blockage of protein synthesis, through which interferons are thought to exert their antiviral effects. EBERs were shown to bind to PKR and block the activation of PKR as well as adenovirus VAI and VAII RNAs, which might result in the enhancement of protein synthesis of general mRNAs (26). The dominant negative form of PKR has been shown to predispose cells to malignant transformation or even initiate it (14, 19). In addition, GCN4, a yeast homologue of PKR, is known to regulate specific gene expression via translational control (1). Since the levels of expression of β-actin, c-myc, bax, and PKR in EBV-positive Akata cells were equal to those in EBV-negative Akata cells (data not shown), whereas the expression of bcl-2 protein was specifically upregulated in EBV-positive Akata cells, it is possible that the inactivation of PKR by EBERs enhances the translation of bcl-2 mRNA more efficiently than that of other mRNAs to confer the malignant phenotype and resistance to apoptosis.
However, it remains unclear whether the effects of EBERs are mediated via upregulation of bcl-2 protein. bcl-2 acts in synergy with the c-myc oncogene in tumor progression, this idea was suggested by clinical investigations indicating that the activation of both c-myc and bcl-2 may have conferred an aggressive clinical outcome in lymphoma cases (4, 11, 21). This idea was clearly demonstrated in a transgenic mouse study in which bcl-2 c-myc transgenic mice exhibited accelerated lymphomagenesis (9, 17). In mammalian cells, deregulated expression of c-myc has been shown to contribute not only to tumorigenesis (15) but also to the induction of apoptosis in various cell lines, including BL cell lines (2, 8, 20). The mechanism of synergy between bcl-2 and c-myc seems to be that bcl-2 protects cells from c-myc-induced apoptosis (3, 22, 33). Like Akata cells (29), all BL cells possess a chromosomal translocation involving the c-myc locus and resulting in constitutive activation of the c-myc gene (12). Therefore, BL cells were thought to be predisposed to c-myc-induced apoptosis. Our data suggest that EBV infection would upregulate the expression of bcl-2 protein to protect cells from c-myc-induced apoptosis and to allow c-myc to exert its oncogenic functions. In this way, bcl-2 might cooperate with c-myc in the development of BL.
ACKNOWLEDGMENTS
We thank Tsuyoshi Takeda for EBER staining and Kumi Adachi for technical assistance.
This work was supported by grants-in-aid from the Ministry of Education, Science, Sports, and Culture, Japan, and from the Princess Takamatsu Research Fund.
REFERENCES
- 1.Abastado J P, Miller P F, Jackson B M, Hinnebusch A G. Suppression of ribosomal reinitiation at upstream open reading frames in amino acid-starved cells forms the basis for GCN4 translational control. Mol Cell Biol. 1991;11:486–496. doi: 10.1128/mcb.11.1.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Askew D S, Ashmun R A, Simmons B C, Cleveland J L. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991;6:1915–1922. [PubMed] [Google Scholar]
- 3.Bissonnette R P, Echeverri F, Mahboubi A, Green D R. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature. 1992;359:552–554. doi: 10.1038/359552a0. [DOI] [PubMed] [Google Scholar]
- 4.Brito-Babapulle V, Crawford A, Khokhar T, Laffan M, Matutes E, Fairhead S, Catovsky D. Translocations t(14;18) and t(8;14) with rearranged bcl-2 and c-myc in a case presenting as B-ALL (L3) Leukemia. 1991;5:83–87. [PubMed] [Google Scholar]
- 5.Chang K L, Chen Y Y, Shibata D, Weiss L M. Description of an in situ hybridization methodology for detection of Epstein-Barr virus RNA in paraffin-embedded tissues, with a survey of normal and neoplastic tissues. Diagn Mol Pathol. 1992;1:246–255. [PubMed] [Google Scholar]
- 6.Clarke P A, Schwemmle M, Schickinger J, Hilse K, Clemens M J, Abastado J P, Miller P F, Jackson B M, Hinnebusch A G. Binding of Epstein-Barr virus small RNA EBER-1 to the double-stranded RNA-activated protein kinase DAI. Nucleic Acids Res. 1991;19:243–248. doi: 10.1093/nar/19.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke P A, Sharp N A, Clemens M J. Expression of genes for the Epstein-Barr virus small RNAs EBER-1 and EBER-2 in Daudi Burkitt’s lymphoma cells: effects of interferon treatment. J Gen Virol. 1992;73:3169–3175. doi: 10.1099/0022-1317-73-12-3169. [DOI] [PubMed] [Google Scholar]
- 8.Evan G I, Wyllie A H, Gilbert C S, Littlewood T D, Land H, Brooks M, Waters C M, Penn L Z, Hancock D C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 9.Fanidi A, Harrington E A, Evan G I. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature. 1992;359:554–556. doi: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- 10.Imai S, Nishikawa J, Takada K. Cell-to-cell contact as an efficient mode of Epstein-Barr virus infection of diverse human epithelial cells. J Virol. 1998;72:4371–4378. doi: 10.1128/jvi.72.5.4371-4378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karsan A, Gascoyne R D, Coupland R W, Shepherd J D, Phillips G L, Horsman D E. Combination of t(14;18) and a Burkitt’s type translocation in B-cell malignancies. Leukemia Lymphoma. 1993;10:433–441. doi: 10.3109/10428199309148200. [DOI] [PubMed] [Google Scholar]
- 12.Klein G. The role of gene dosage and genetic transpositions in carcinogenesis. Nature. 1981;294:313–318. doi: 10.1038/294313a0. [DOI] [PubMed] [Google Scholar]
- 13.Komano J, Sugiura M, Takada K. Epstein-Barr virus contributes to the malignant phenotype and to apoptosis resistance in Burkitt’s lymphoma cell line Akata. J Virol. 1998;72:9150–9156. doi: 10.1128/jvi.72.11.9150-9156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koromilas A E, Roy S, Barber G N, Katze M G, Sonenberg N. Malignant transformation by a mutant of the IFN-inducible dsRNA-dependent protein kinase. Science. 1992;257:1685–1689. doi: 10.1126/science.1382315. [DOI] [PubMed] [Google Scholar]
- 15.Land H, Parada L F, Weinberg R A. Cellular oncogenes and multistep carcinogenesis. Science. 1983;222:771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- 16.Lerner M R, Andrews N C, Miller G, Steitz J A. Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc Natl Acad Sci USA. 1981;78:805–809. doi: 10.1073/pnas.78.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marin M C, Hsu B, Stephens L C, Brisbay S, McDonnell T J. The functional basis of c-myc and bcl-2 complementation during multistep lymphomagenesis in vivo. Exp Cell Res. 1995;217:240–247. doi: 10.1006/excr.1995.1083. [DOI] [PubMed] [Google Scholar]
- 18.Meurs E, Chong K, Galabru J, Thomas N S, Kerr I M, Williams B R, Hovanessian A G. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990;62:379–390. doi: 10.1016/0092-8674(90)90374-n. [DOI] [PubMed] [Google Scholar]
- 19.Meurs E F, Galabru J, Barber G N, Katze M G, Hovanessian A G. Tumor suppressor function of the interferon-induced double-stranded RNA-activated protein kinase. Proc Natl Acad Sci USA. 1993;90:232–236. doi: 10.1073/pnas.90.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milner A E, Grand R J, Waters C M, Gregory C D. Apoptosis in Burkitt lymphoma cells is driven by c-myc. Oncogene. 1993;8:3385–3391. [PubMed] [Google Scholar]
- 21.Mohammad R M, Mohamed A N, Smith M R, Jawadi N S, al-Katib A. A unique EBV-negative low-grade lymphoma line (WSU-FSCCL) exhibiting both t(14;18) and t(8;11) Cancer Genet Cytogenet. 1993;70:62–67. doi: 10.1016/0165-4608(93)90132-6. [DOI] [PubMed] [Google Scholar]
- 22.Oltvai Z N, Milliman C L, Korsmeyer S J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 23.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2397–2446. [Google Scholar]
- 24.Rowe M, Rowe D T, Gregory C D, Young L S, Farrell P J, Rupani H, Rickinson A B. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt’s lymphoma cells. EMBO J. 1987;6:2743–2751. doi: 10.1002/j.1460-2075.1987.tb02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruf I K, Rhyne P W, Yang H, Borza C M, Hutt-Fletcher L M, Cleveland J L, Sample J T. Epstein-Barr virus regulates c-MYC, apoptosis, and tumorigenicity in Burkitt lymphoma. Mol Cell Biol. 1999;19:1651–1660. doi: 10.1128/mcb.19.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharp T V, Schwemmle M, Jeffrey I, Laing K, Mellor H, Proud C G, Hilse K, Clemens M J. Comparative analysis of the regulation of the interferon-inducible protein kinase PKR by Epstein-Barr virus RNAs EBER-1 and EBER-2 and adenovirus VAI RNA. Nucleic Acids Res. 1993;21:4483–4490. doi: 10.1093/nar/21.19.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimizu N, Tanabe-Tochikura A, Kuroiwa Y, Takada K. Isolation of Epstein-Barr virus (EBV)-negative cell clones from the EBV-positive Burkitt’s lymphoma (BL) line Akata: malignant phenotypes of BL cells are dependent on EBV. J Virol. 1994;68:6069–6073. doi: 10.1128/jvi.68.9.6069-6073.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swaminathan S, Tomkinson B, Kieff E. Recombinant Epstein-Barr virus with small RNA (EBER) genes deleted transforms lymphocytes and replicates in vitro. Proc Natl Acad Sci USA. 1991;88:1546–1550. doi: 10.1073/pnas.88.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takada K, Horinouchi K, Ono Y, Aya T, Osato T, Takahashi M, Hayasaka S. An Epstein-Barr virus-producer line Akata: establishment of the cell line and analysis of viral DNA. Virus Genes. 1991;5:147–156. doi: 10.1007/BF00571929. [DOI] [PubMed] [Google Scholar]
- 30.Tierney R J, Steven N, Young L S, Rickinson A B. Epstein-Barr virus latency in blood mononuclear cells: analysis of viral gene transcription during primary infection and in the carrier state. J Virol. 1994;68:7374–7385. doi: 10.1128/jvi.68.11.7374-7385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toczyski D P, Matera A G, Ward D C, Steitz J A. The Epstein-Barr virus (EBV) small RNA EBER1 binds and relocalizes ribosomal protein L22 in EBV-infected human B lymphocytes. Proc Natl Acad Sci USA. 1994;91:3463–3467. doi: 10.1073/pnas.91.8.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toczyski D P, Steitz J A. EAP, a highly conserved cellular protein associated with Epstein-Barr virus small RNAs (EBERs) EMBO J. 1991;10:459–466. doi: 10.1002/j.1460-2075.1991.tb07968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaux D L, Cory S, Adams J M. bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]