Abstract
Segment 2 of bluetongue virus (BTV) serotype 10, which encodes the outer capsid protein VP2, was tagged with the S-peptide fragment of RNase A and expressed by a recombinant baculovirus. The recombinant protein was subsequently purified to homogeneity by virtue of the S tag, and the oligomeric nature of the purified protein was determined. The data obtained indicated that the majority of the protein forms a dimer and, to a lesser extent, some trimer. The recombinant protein was used to determine various biological functions of VP2. The purified VP2 was shown to have virus hemagglutinin activity and was antigenically indistinguishable from the VP2 of the virion. Whether VP2 is responsible for BTV entry into permissive cells was subsequently assessed by cell surface attachment and internalization studies with an immunofluorescence assay system. The results demonstrated that VP2 alone is responsible for virus entry into mammalian cells. By competition assay, it appeared that both VP2 and the BTV virion attached to the same cell surface molecule(s). The purified VP2 also had a strong affinity for binding to glycophorin A, a sialoglycoprotein component of erythrocytes, indicating that VP2 may be responsible for BTV transmission by the Culicoides vector to vertebrate hosts during blood feeding. Further, by various enzymatic treatments of BTV-permissive L929 cells, preliminary data have been obtained which indicated that the BTV receptor molecule(s) is likely to be a glycoprotein and that either the protein moiety of the glycoprotein or a second protein molecule could also serve as a coreceptor for BTV infection.
Bluetongue virus (BTV) is the prototype virus of the genus Orbivirus, one of the nine genera in the family Reoviridae. Biologically, orbiviruses are distinct from other members of the family. These viruses are transmitted to particular vertebrate species (e.g., sheep, cattle, horses, and deer) by the bite of arthropod vectors (e.g., Culicoides species for BTV) via a blood meal. The specificity and abilities of the viruses to replicate in such distinct hosts raise a number of important scientific questions, in particular relating to host cell entry. The initiation of a virus infection involves virus binding to ligands on the cell surface prior to entry by a number of possible mechanisms (depending on the virus). The proteins exposed on the outer capsid layer of most viruses usually include the virus attachment proteins, which are crucial for the recognition of specific cellular receptors prior to the virus entry into the host cell. BTV is a nonenveloped double-shelled virus consisting of seven proteins and an RNA genome of 10 double-stranded segments (22, 28). The outer capsid is made up of two proteins (VP2 and VP5) and encapsidates the internal core. The surface of this core is a complex network of 260 trimers of a single protein, VP7, which is supported by the next protein layer, made up of 120 molecules of VP3 protein (8, 22). The outer capsid is in direct contact with the VP7 layer of the core (17). The internal region of the core is occupied by three minor proteins, VP1, VP4, and VP6, which are associated with the double-stranded RNA genome of 10 segments (22, 28).
From three-dimensional image reconstruction and cryoelectron microscopic studies, it appears that one of the outer capsid proteins, is a “sail”-shaped spike and is located above 180 of the 260 VP7 trimers (9). The second protein, on the other hand, appears to form 120 globular structures that sit neatly on each of the six-membered rings of VP7. The sail-shaped spikes, project 4 nm beyond the globular molecules, thus making the globular protein less exposed (9). Since VP2 is the principal antigen responsible for BTV serotype specificity and virus neutralization activity and confers protection in sheep against virulent virus challenge, the sail-shaped spike is likely to be the VP2 protein and the less highly exposed globular protein is the VP5 protein (10, 14, 23, 27).
Generally, in most animal viruses, virus attachment to the cell surface requires the recognition of and interaction with specific cellular receptors by a virus attachment protein prior to entry into the host cell. For BTV, of the seven structural proteins, VP2 is the most likely candidate for the virus attachment protein due to its outermost location on the virion, its involvement in serotype-specific virus neutralization and hemagglutination activity, and the fact that it provides protective immunity to animals (4, 6, 20, 23, 26). This view had also been supported by earlier studies which showed that virion particles lacking VP2, but with exposed VP5 and possibly VP7, were incapable of binding to BHK-21 cells, indicating that VP2 may be responsible for cell binding (11). However, to date no direct evidence supporting the role of VP2 in virus entry had been reported. To investigate whether VP2 alone is indeed responsible for BTV entry into the host cells, it is essential to obtain purified VP2 in solution which is antigenically and functionally equivalent to the native viral protein.
In this study, to determine the role of VP2 in virus entry, we have prepared a recombinant tagged VP2 of BTV serotype 10 by using the baculovirus expression system. By virtue of the tag, namely, an S-peptide tag (15), VP2 was purified to homogeneity in a soluble form. The oligomeric nature of the purified protein was determined prior to the functional analysis which showed that the protein formed multimers. To obtain evidence that the purified protein was functionally active, we have assessed the hemagglutination and neutralization activities of the protein. Both activities were retained by the recombinant purified protein. The protein was then used to investigate its host cell binding and internalization activities by an immunofluorescence assay with BTV-permissive and -nonpermissive cell lines. It is also likely that VP2 mediates BTV binding to erythrocytes during BTV transmission via blood meals by the Culicoides vector. Therefore, we also investigated this issue by using purified sialoglycophorin A of erythrocytes in a binding assay, since it has been shown previously that BTV binds erythrocytes via glycophorin A (5).
The specificity of each activity was further confirmed by using purified virion alone or in the presence of VP2 as a competitor. We showed that the purified protein could bind to both glycophorin A and mammalian cells equally well, could be internalized into the permissive cells, and could compete with the virion for each of these activities. The data also show that both VP2 and BTV bound to the same receptor site(s) on the cell surface and that BTV infectivity could be inhibited by glycophorin A. To identify the putative BTV receptor(s), the biochemical nature of the receptor molecule(s) on the mammalian cell surface was partially characterized by using various enzymatic treatments. The data obtained from these enzymatic studies indicated that the BTV receptor molecule(s) is likely to be a glycoprotein and that a second protein molecule could also serve as a coreceptor for BTV infection.
MATERIALS AND METHODS
Cells and viruses.
The cell lines used in this study were mouse fibroblasts (L929), Madin-Darby canine kidney (MDCK) cells, BHK-21 (baby hamster kidney) cells, and Vero (African green monkey kidney) cells. The cells were grown in Leibovitz L-15 medium or in RPMI medium supplemented with HEPES, 1% l-glutamine, and penicillin-streptomycin (GIBCO, Glasgow, United Kingdom), and 10% fetal bovine serum (FBS) and incubated at 37°C. The BTV strain used was the cell culture-adapted U.S. strain BTV serotype 10 (BTV-10; Colorado isolate). The virus was propagated in BHK-21 cells, and its titer was determined by a plaque assay in Vero cells. The BTV particles were purified as described by Mertens et al. (20). The purified virus preparations were concentrated to 30 to 60 μl for binding assays in 96 well-plates by using a Microcon 30 apparatus (Amicon Inc., Lexington, Mass.). The Sf9 insect cell line originating from Spodoptera frugiperda, the fall army worm, was used for the propagation of recombinant baculoviruses. The Autographa californica nuclear polyhedrosis virus (AcNPV) was used in all these studies. The protocols used for the propagation and maintenance of the Sf9 cells were those described by King and Possee (16).
Construction of the RNase S-peptide fusion BTV-VP2 expression vector.
Construction of the transfer vector containing the S-peptide–VP2 (S-VP2) sequences (pAcYM1.SgsTh.10BTV-VP2) is described below. The transfer vector (pAcYM1.BTV10-VP2 [14, 19]) containing VP2 was linearized, and PCR was used to introduce linkers representing XbaI, SpeI, and SmaI restriction enzyme sites at the 5′ end of the 1.7-kbp fragment. This 1.7-kbp product fragment was excised with PstI and BamHI. The forward primer (P1) used was 5′-GGCGAGGATCCAAATATGTCTAGAACTAGTCCCGGGGAGGAATTCGTCATACCAG-3′, and the reverse primer (P2) used was 5′-TCGGCGACACCCTCAGT-3′. The 1.2-kbp fragment was also derived from PstI and BamHI digestions of another aliquot of the transfer vector pAcYM1.BTV10-VP2. The 1.7-kbp PCR product was religated with the 1.2-kbp fragment of VP2 and introduced into a pAcYM1 vector. Paired oligonucleotides of S-peptide (5′-CTAGAAAAGAAACCGCTGCCGCTAAATTTGAAAGACAACACATGGACAGCT-3′ and 3′-TTTTCTTTGGCGACGGGGATTTAAACTTTCTGTTGTGTACCTGTGAGATC-5′) (15), glycine-serine spacer (5′-CTAGAGGTGGCAGTGGGGGAA-3′ and 3′-TCCACCGTCACCCCCTTGATC-5′), and thrombin cleavage site (5′-CTAGTTTGGTTCCCAGAGGCTCGCCC-3′ and 3′-AAACCAAGGGTCTCCGAGCGGG-5′) were annealed and ligated into the specific restriction enzyme sites of the modified 5′ end of the linearized pAcYM1.10BTV-VP2 recombinant vector to produce the plasmid pAcYM1.SgsTh.10BTV-VP2.
The orientation of plasmids containing inserts was examined by PCR and restriction enzyme analyses. The orientations and junction sequences were confirmed to be correct and in frame by sequencing by the dideoxy chain termination method (24). Recombinant baculoviruses were obtained by transfecting Sf9 cells with a mixture of a derivative of AcNPV DNA (BacPAK6; Clontech) linearized by Bsu 361 (16) and the constructed pAcYM1.SgsTh.10BTV-VP2 plasmid DNA. The BacPAK6 carries the lacZ gene. The recombinant baculovirus (AcS-10BTV-VP2) was selected by using blue-white screening after the incorporation of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) into the overlay medium. The expression of full-length S-peptide-tagged VP2 (S-VP2) was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by detection by Coomassie blue staining, and the specificity of VP2 was confirmed by both Western blotting and radioimmunoprecipitation with polyclonal BTV-10 antisera and monoclonal antibodies (MAbs) to BTV-10–VP2, respectively.
Propagation, extraction, and purification of S-VP2.
Sf9 cells at 2 × 106 cells/ml cultivated as suspension cultures in TC-100 medium containing 5% FBS and antibiotics were infected with the recombinant baculovirus at a multiplicity of infection (MOI) of 10-25 pfu/cell. After a 48-h incubation at 28°C, the cells were centrifuged at 2,000 × g for 10 min at 4°C and the supernatant was discarded. The cells were washed once with cold phosphate-buffered saline (PBS) and centrifuged as before. Cold TNET (50 mM Tris-HCl [pH 8.0], 0.2 M NaCl, 1 mM EDTA, 1% Triton X-100) buffer containing protease inhibitors (100 μM phenylmethylsulfonyl fluoride, 1 μM aprotinin, 1 μM pepstatin A, 10 μM leupeptin) was added to the cells, which were left for 10 min and then homogenized with a glass Dounce homogenizer at 4°C. The protease inhibitors were added to minimize proteolytic degradation during this initial recovery step. The cells were centrifuged, and the supernatant was harvested. Fresh TNET was added, and the cells were again homogenized and centrifuged. The supernatants were pooled and centrifuged at 10,000 × g at 4°C for 30 min. The soluble extract was harvested.
Purification of baculovirus-expressed S-VP2.
VP2 was purified by the protocol of the S-Tag purification kit (Novagen, Madison, Wis.). Briefly, the S-VP2–baculovirus recombinant protein extracted from the Sf9 cells was bound to S-protein–agarose beads for 30 min at room temperature. The bound S-VP2 and S-protein–agarose complex was washed twice with 10 volumes of TNC (20 mM Tris-HCl [pH 8.4], 150 mM NaCl, 2.5 mM CaCl2) to remove all traces of Triton X-100 subsequent to thrombin cleavage. The S-peptide, glycine-serine spacer, and thrombin cleavage site were then cleaved from VP2 by biotinylated-thrombin digestion in the presence of TNC. The biotinylated thrombin was then removed from the solution with streptavidin-agarose beads. Before the addition of the S-protein–agarose beads to the mixture to capture the biotinylated thrombin, the beads were washed three times in 100 mM sodium phosphate buffer (pH 7.5) to remove any traces of sodium azide which might interfere with biological assays involving the use of live cells. According to the manufacturer, the efficiency of the streptavidin-agarose in capturing the biotinylated thrombin is >99.98%. The supernatant contained soluble recombinant VP2. For use in the various assays, the proteins were concentrated with a Centricon 30 apparatus (Amicon Inc.). The protein concentration was estimated by the Bradford (2) protein assay method.
Analysis of the multimeric nature of VP2.
Preliminary studies on the multimeric nature of the purified 35[S]methionine-radiolabeled VP2 were conducted by analyzing the protein by gel electrophoresis (7%) followed by autoradiography. The protein was resuspended in sample buffer (1% SDS, 15% glycerol, 10 mM Tris-HCl [pH 6.8]) with or without 1% β-mercaptoethanol and with or without heating at 100°C for 2 min. Apparent molecular masses were estimated by using the phosphorylase b cross-linked protein marker (Sigma) in the molecular mass range 97.4, 194.8, 292, 389.6, 487, and 584 kDa and the high-molecular-mass marker (Sigma) in the molecular mass range 29, 45, 66, 97.4, 116, and 205 kDa.
Metabolic labeling of BTV and recombinant baculovirus-infected cells.
To prepare radiolabeled BTV proteins, monolayers of BHK-21 cells in 25-cm2 tissue culture flasks were infected with BTV at a MOI of 5 to 10 and incubated at 37°C for 2 to 3 days or until the first signs of early cytopathic effect was seen. For radiolabeling of VP2, Sf9 cells (106 cells/ml) in 35-mm dishes were infected with recombinant baculovirus AcNPV-VP2 (without the S-tag) or AcS–BTV-10–VP2 at a MOI of 5 to 10 and incubated at 28°C for 36 to 48 h. At the end of infection, the cells were starved of methionine for 30 min and radiolabeled for 2 h with 35[S]methionine. Both BTV-infected BHK-21 cells and recombinant VP2 virus-infected Sf9 cells were washed, scraped into PBS, and centrifuged. Cells were lysed by adding 500 μl of lysis buffer (20 mM sodium phosphate buffer containing 150 mM NaCl [pH 7.5], 1% Triton X-100, and protease inhibitors). The cells were centrifuged at 10,000 × g for 10 min, and the supernatant was harvested.
Preparation of antibodies against the expressed purified VP2.
Monospecific polyclonal antibody (PAb) against the purified VP2 was prepared in mice by standard procedures for antibody production by L. Jones, Institute of Virology and Environmental Microbiology, Oxford. The immunoglobulin fraction of the antisera was purified with the MAb Trap GII system (Pharmacia) as specified by the manufacturer. The protein concentration was estimated by the Bradford (2) protein assay method.
Immunoprecipitation assay.
The immunoprecipitation assay was conducted by the methods of Hussy et al. (12). Antisera used for binding to protein A-Sepharose (Pharmacia, Milton Keynes, United Kingdom) were MAbs 1, 2, and 3 against BTV-10 VP2 (a generous gift from J. McLachlan, University of Georgia), normal rabbit serum, PAb against purified BTV-10, or the antibody against VP2. These were held for 2 h at 4°C. The beads with the immobilized antibodies were incubated with 10 μl (2 μg) of purified 35[S]methionine-labeled VP2 or BTV-infected cell lysates for 16 h at 4°C. The precipitates were washed three times with standard radioimmunoprecipitation assay buffer (RIPA buffer) (PBS with 0.1% SDS, 1% Triton X-100, and 0.5% deoxycholate). The complexes were heated for 10 min in SDS-gel sample buffer prior to separation on SDS-polyacrylamide gels followed by autoradiography.
Plaque reduction assay.
The plaque reduction assay was conducted as described previously (14, 19). Briefly, a 10-fold dilution of the anti-BTV PAb, the monospecific anti-VP2 PAb, or normal preimmune rabbit serum was mixed with 100 PFU of BTV per ml and added to wells containing confluent monolayers of Vero cells. After a 4-h incubation at 37°C, an overlay of 1% (wt/vol) carboxymethyl cellulose in L-15 medium was added and the plates were incubated at 37°C for a further 5 days. Plaques were visualized by staining with 1.5% (wt/vol) crystal violet in 95% (vol/vol) ethanol. The plaque reduction neutralization titers were expressed as the reciprocal of the antiserum dilution that gave a 50% reduction in plaque number.
Hemagglutination and hemagglutination inhibition assays.
Hemagglutination (HA) and hemagglutination inhibition (HI) assays were conducted as described by Van der Walt (26) with a V-bottom microtiter plate. BTV particles were subjected to titer determination in HA tests, and 8 HA units were used for HI assays. The reciprocal of the highest virus dilution showing 50% hemagglutination was considered the HA titer, and the highest serum dilution showing 50% inhibition of hemagglutination was considered as the HI titer.
Immunofluorescence.
Two different cell lines, permissive L929 and nonpermissive mock MDCK cells for BTV growth were used. The cells were grown on coverslips with either 15 μg of VP2, 10 μg of VP5, 30 μg of purified core-like particles (CLPs) (6) or BTV (infected at a MOI of 10). The binding reaction was conducted at 4°C for 1 h, and internalization was carried out at 37°C for another 1 h. For surface membrane immunofluorescence, cells were washed in cold PBS and then fixed in an isotonic solution of 2% paraformaldehyde for 1 min at 4°C. The anti-VP2 and anti-VP5 polyclonal antibodies in PBS containing 1% bovine serum albumin (BSA) were added, and the mixture was incubated at 37°C for another 1 h. For CLP and BTV virion detection, a PAb-BTV was used. The bound antibody was detected with the appropriate fluorescein-conjugated anti-species immunoglobulin. For internal staining, following three washes with PBS containing 1% BSA, the monolayers were permeabilized by 50% methanol–50% acetone solution for 10 s. After thorough washing with PBS containing 1% BSA and incubation with the appropriate primary antibody, detection with the appropriate anti-species immunoglobulin was performed as described for surface membrane immunofluorescence.
Quantitation of internalization.
Internalization of VP2 was quantitated by the methods of White et al. (30). Briefly, L929 or MDCK cells were grown on the 96-well plates, and 30 μl (22.5 μg) of radiolabeled VP2 was added to each well. For each internalization experiment, duplicate wells were used. After a 1-h incubation at 4°C, VP2 was removed, washed with serum-free L-15 medium, and further incubated at 37°C to allow internalization of the bound VP2. The cells were then washed with cold PBS and treated with 500 μg of proteinase K (Boehringer Mannheim Biochemicals, Lewes, United Kingdom) for 30 min at 4°C to remove surface-bound VP2. The cells were washed with PBS–0.1% BSA solubilized in RIPA buffer, and bound radiolabeled VP2 was quantitated by scintillation counting. The average radioactivity counts from the duplicate wells were calculated. To determine the efficiency of proteinase K, a similar control experiment was carried out in parallel. However, in this experiment, both binding and internalization were performed at 4°C. The percentage of radioactivity incorporation was calculated by subtracting the counts obtained from control cells.
Competition for binding sites by BTV virions, CLPs, and VP6 protein.
The binding ability of BTV receptors was determined by a competitive assay. A 10-fold excess of unlabeled purified BTV-10 (600 μg), CLPs (600 μg), or VP6 (15 μg) with 15 μg of radiolabeled VP2 was added to confluent monolayers of L929 cells and incubated for 1 h at 4°C. In earlier studies, we determined that 10 to 15 μg of VP2 was required to saturate 105 L929 cells in a well of a 96-multiwell plate. The mixture was removed, and the cells were washed and lysed. The bound radiolabeled VP2 protein was precipitated with 10% trichloroacetic acid, and radioactivity was determined by a scintillation counter in the presence of a liquid scintillation cocktail (Beckman, Fullerton, Calif.). The percentage of VP2 binding was calculated as follows: actual VP2 binding = (B/A) × 100%, where A (cpm) is the mean of the readings of VP2 binding and B (cpm) is the mean of the readings of VP2 binding plus BTV virions, CLPs, or VP6.
Blocking of binding and replication of BTV by VP2.
VP2 at 5, 30, and 60 μg was added for 30 min at 4°C to duplicate wells (96-well plate) containing monolayers of prechilled (4°C) Vero cells. After this time, the unadsorbed VP2 was removed, the cells were washed twice with serum-free medium, and 25 μl of BTV-10 containing 100 PFU per well was then adsorbed onto the cells for 1 h at room temperature. After this time, the unadsorbed virus was removed and 100 μl of L-15 medium–2% FBS was added to the wells. Procedures to observe plaque formation were performed as described earlier.
Binding of purified proteins to sialoglycophorin A.
The binding of purified VP2 to sialoglycophorin A (Sigma, Poole, United Kingdom) was examined by an indirect enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well plates (Immunlon; Dynex, McLean, Va.) were coated with 1 μg of glycophorin A (Sigma Chemical Co.) per well in carbonate coating buffer (15 mM Na2CO3, 36 mM NaHCO3 [pH 9.6]) and incubated overnight at 4°C. The plates were washed four times with PBS and blocked with 1% skim milk in PBS at 37°C for 1 h. VP2 at a final concentration of 1, 4, 8, 15, and 20 μg per 50 μl of diluting buffer (0.5% skim milk in PBS with 0.05% Tween 20) was added to the plates, and the mixtures were incubated for 1 h at 37°C. As a control, two additional purified recombinant BTV proteins, rVP5 and rVP7, were tested in parallel. The proteins bound to the glycophorin A were detected with anti-BTV PAb by incubation at 37°C for 1 h. After further washings, affinity-purified alkaline phosphatase-labeled anti-species immunoglobulin G was added and the mixture was further incubated at 37°C for 1 h. The assay reaction was developed with the substrate p-nitrophenyl phosphate disodium (pNPP; Sigma) in 100 mM glycine–1 mM MgCl2–1 mM ZnCl2 (pH 10.4). The reaction was left to develop at 37°C for 30 min and then stopped with 3 N NaOH. The optical density was determined at 405 nm.
Inhibition of BTV infectivity by glycophorin A.
Glycophorin A (Sigma) diluted in PBS was added to a standard amount of BTV (50 PFU/well) at final concentrations of 1, 10, 30, and 50 μg/well, and the mixture was incubated for 2 h at 4°C. The glycophorin-BTV mix was then adsorbed onto a monolayer of Vero cells that had been seeded on 24-well plates for 1 h at room temperature. The mixture was removed, and the wells were washed twice with L-15 medium and finally with 500 μl of L-15 medium–2% FBS. Positive virus control wells without glycophorin and wells with cells alone were also included. As negative controls, commercially available laminin and RGD peptides (Sigma) at similar concentrations were also used. The plates were incubated for 5 to 6 days and subjected to plaque reduction procedures as previously described.
To determine whether glycophorin A could also block VP2 binding sites, glycophorin A (25, 50, 100, and 200 μg) was mixed with 15 μg of purified VP2 (200 μl volume) for 2 h at 4°C. These mixtures were each added to confluent Vero cells in a 24-well plate and incubated for 1 h at 4°C to allow binding. The glycophorin A-VP2 complex was then removed, and BTV (50 PFU/ml) was added. Plaque formation was determined as previously described.
Treatment of viable L929 cells with proteases, phospholipases, and sodium periodate.
Monolayers of L929 cells in 96-well plates were washed twice prior to treatment with chymotrypsin (type II, from bovine pancreas), proteinase K (from Tritirachium album), phospholipase C (type 1, from Clostridium perfringens), phospholipase A2 (from bovine pancreas), and neuraminidase (type VIII, from C. perfringens and from Vibrio cholerae). The cells were washed up to four times before being treated with trypsin. The cells were incubated with 40 μl of each of the five enzymes in PBS (pH 7.2) at 37°C for 30 min. They were treated at 4°C for 30 min with sodium periodate prepared in PBS (pH 5.6). After enzymatic treatment, the cells were washed three times with PBS–0.1% BSA and cooled to 4°C, and 30 μl (1 μg) of radiolabeled VP2 was added. The cells were incubated at 4°C for 1 h, lysed with RIPA buffer, and subjected to scintillation counting. The amount of radiolabeled VP2 attached to the cells was determined by measuring the total radioactivity (counts per minute) on the scintillation counter. The counts were compared with those from control wells of untreated cells bound to a similar amount of VP2.
RESULTS
High-level expression and rapid purification of S-VP2.
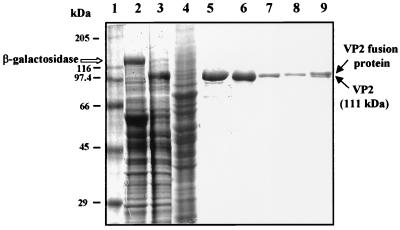
To obtain large quantities of highly purified VP2, we used the baculovirus expression system and coupled the protein to the S-peptide tag. Segment 2 of BTV-10 double-stranded RNA was modified at the 5′-terminus to accommodate the S-peptide. The S-peptide was fused to the VP2 sequence by using a spacer of five glycine-serine residues which formed an extended rigid structure to minimize the interaction between the S-peptide and the VP2. This was followed by a thrombin cleavage site. The fusion protein was subsequently expressed in Sf9 cells by a recombinant baculovirus. Expression was detectable as early as 14 h postinfection and reached a peak by 36 to 48 h (data not shown). The yield of VP2 at 48 h of infection was reasonably high, and generally 2 × 108 cells in 100 ml of culture yielded approximately 1 mg of purified protein. The high-affinity interaction between the S-peptide portion of the fusion protein and immobilized S-protein on agarose beads and the ability of the VP2 to be liberated from the remainder of the fusion protein by cleavage at the thrombin site provide a simple and efficient procedure for the isolation of VP2 in a purified form. Upon cleavage with biotinylated thrombin, about 80 to 90% of the bound VP2 was recovered. The biotinylated thrombin was then efficiently (>99.8%) removed with streptavidin-agarose beads. Subsequent SDS-PAGE analysis of the VP2 showed that it corresponded to an electrophoretically homogenous preparation of a 111-kDa band (Fig. 1).
FIG. 1.
SDS-PAGE analysis (10% polyacrylamide) of the expression and purification of recombinant VP2. Lanes: 1, molecular mass markers; 2, Sf9 cells infected with BacPAK6 (a derivative of AcNPV) that expresses β-galactosidase; 3, Sf9 cell lysates infected with AcS–BTV-10–VP2 recombinant baculovirus; 4, supernatant of cell lysate after binding with the S-protein beads; 5, S-VP2 bound to S-protein–agarose beads; 6, VP2 cleaved from the beads with biotinylated thrombin; 7 and 8; second and third washings from the beads, respectively; 9: S-VP2 left on beads after cleavage with biotinylated thrombin.
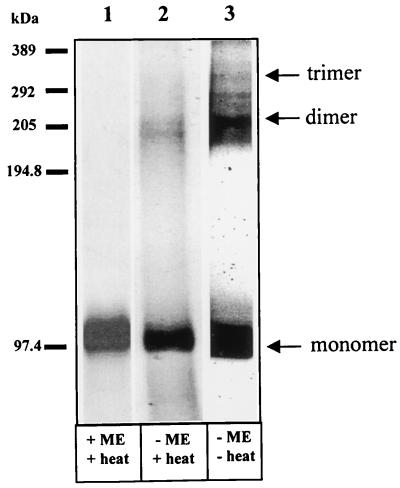
Multimeric nature of VP2.
By cryoelectron microscopy and image analyses of virions and VLPs, VP2 appears to be present as a trimer in the virus particle. Whether VP2 is indeed trimeric and whether the purified protein forms an oligomeric structure have been investigated by using 35[S]methionine-labeled purified VP2 protein. Using PAGE analysis, when VP2 was dissociated in the presence of β-mercaptoethanol and heat treatment, a single band with an apparent molecular mass of 111 kDa was precipitated (Fig. 2, lane 1). However, in the absence of the reducing agent, when the samples were heated, two closely migrated additional bands with apparent molecular masses of 220 and 200 kDa were observed (lane 2), representing the multimeric nature of VP2. The 200-kDa band could represent intermediate fragments of the 220-kDa dimer. In contrast, in the absence of β-mercaptoethanol and heat treatment, three extra bands with apparent molecular masses of 220, 290, and 330 kDa were observed (lane 3). The 220-kDa band was the most prominent of the three and was equivalent to the size of a VP2 dimer. When calculated, approximately one-third of the total amount of VP2 was in the dimeric form. The 330-kDa band, which was equivalent to the VP2 trimer, was sensitive to heat treatment and much less abundant than the dimeric band. Nevertheless, the data clearly demonstrated that the purified protein had the ability to form multimers in solution and that the multimers of VP2 were stabilized by disulfide linkages. The 290-kDa band, between the 220- and 330-kDa bands, was most likely to be the intermediate form of the trimer. Both the monomeric and multimeric forms of VP2 were recognized specifically by the anti-VP2 antibody in Western blot analysis (data not shown).
FIG. 2.
SDS-PAGE of purified VP2 multimer. Purified [35S]methionine-radiolabeled VP2 was analyzed by SDS-PAGE (7% polyacrylamide) in the presence (lane 1, +ME) and absence (lane 2, −ME) of 1% β-mercaptoethanol and with (lanes 1 and 2, +heat) and without (lane 3, −heat) heating. The monomer (110 kDa), dimer (220 kDa), and trimer (330 kDa) are indicated.
The antigenicity of the purified VP2 is indistinguishable from that of BTV-derived native VP2.
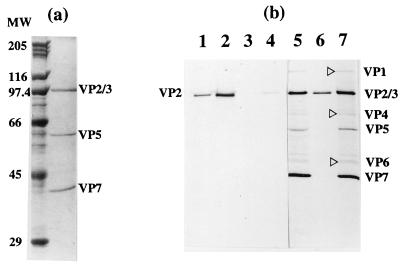
VP2 is the principal antigen that elicits serotype-specific virus-neutralizing antibodies. The neutralizing epitopes on VP2 are predominantly conformation dependent (13, 29). Therefore, conformation-dependent antibodies were used in an immunoprecipitation assay to determine whether the antigenic properties of VP2 were maintained upon purification and indistinguishable from those of native BTV particles. Two types of antibodies, (i) MAbs directed against epitopes on VP2 and (ii) a PAb directed against whole BTV particles (anti-BTV PAb), were used. As shown in Fig. 3b (lanes 1 and 2), the two antibodies immunoprecipitated VP2 with equal efficiencies. The preimmune serum, however, did not immunoprecipitate VP2 (lane 3) as expected. The antigenicity of the purified protein was further assessed by raising antibodies against the purified protein and using this monospecific antiserum for recognition of the BTV proteins. This was determined by immunoprecipitation with BTV-infected cell lysates. The antibody was capable of precipitating native BTV particles from infected cell lysates (Fig. 3b, lane 7) as competently as a conformation-dependent MAb-VP2 did (lane 5), confirming the specificity and authenticity of the purified VP2. For comparison, the SDS-PAGE analysis of purified BTV particles exhibiting the four major proteins, VP2, VP3, VP5, and VP7, is shown in Fig. 3a. To determine the immunogenicity of the recombinant protein, the neutralizing activity of the VP2 antibody was investigated in a BTV plaque reduction assay. The antibody raised against the expressed purified VP2 neutralized BTV virion infectivity and had a significant titer (i.e., 104), indicating the ability of the antibody to recognize neutralizing epitopes on the BTV virion. The data obtained by these studies conclusively demonstrate that the VP2 is antigenically and functionally similar to native VP2.
FIG. 3.
SDS-PAGE analysis (10% polyacrylamide) of BTV and immunoprecipitated VP2. Radiolabeling of the VP2 in Sf9 cells (48 h postinfection) and BTV in BHK-21 cells (15 h postinfection) were carried out for 2 h with [35S]methionine as described in Materials and Methods. (a) Purified BTV particles (8 μg) showing VP2, VP3, VP5, and VP7 detected by Coomassie blue staining. MW, molecular weight in thousands. (b) Immunoprecipitation assay showing the precipitation of radiolabeled purified VP2 by MAb 1 (lane 1), MAb 2 (lane 2), preimmune rabbit serum (lane 3), and anti-BTV PAb at a 1:1,000 dilution (lane 4) and at a 1:200 dilution (lane 6) respectively. The immunoprecipitation of radiolabeled BTV proteins in the supernatant of BTV-infected BHK-21 cells by MAb 1 is shown in lane 5 and that by anti-VP2 PAb is shown in lane 7. The four major proteins and three minor proteins (▹) are indicated.
The purified VP2 agglutinates erythrocytes.
We and others have reported previously that the virus HA activity of the BTV virion may be associated with VP2, although no direct evidence had been presented (4, 6, 20, 26). To demonstrate unequivocally that VP2 is indeed the hemagglutinin of BTV and to examine whether the purified protein retained its biological activity, the HA activity of VP2 was assessed by using a direct HA assay system as well as the HI test as described in Materials and Methods. The results obtained from the HA and HI tests demonstrated that VP2 alone is sufficient for HA activity and that it retained complete HA activity in its purified form. The VP2 (15 μg) agglutinated sheep erythrocytes and gave a HA titer of 32 to 64 (Fig. 4A). The buffer TNC, which was used for elution of VP2 and as a negative control, did not hemagglutinate the sheep erythrocytes (Fig. 4B). The HA activity of BTV particles (1.5 × 106 PFU/ml) which gave an HA titer of 16 to 32 is shown in Fig. 4C. The specificity of the HA activity was further confirmed by the inhibitory effect of the anti-VP2 antibody on HA activity. As expected, the HA reaction was indeed specific for VP2, since the VP2 polyclonal serum had pronounced effect on the HA activity of the BTV particles (Fig. 4D). Altogether, our data demonstrated that VP2 is the BTV hemagglutinin protein.
FIG. 4.
HA activity of purified recombinant VP2 and inhibition of BTV HA activity by anti-VP2 antibody. Briefly, 25 μl containing 15 μg of VP2 was serially diluted twofold. A 50-μl volume of 0.25% sheep erythrocytes was then added to the diluted VP2 and incubated for 1 h at 22°C. The HA activity exhibited by purified VP2 gave a titer of 32 to 64 (A); the thrombin cleavage buffer used as negative control did not exhibit any HA (B); and the HA activity of BTV particles (1.5 × 106 PFU/ml) as positive control gave a titer of 16 to 32 (C). The inhibition of HA activity of BTV by anti-VP2 antibody was carried out with twofold serial dilutions of the VP2 antibody and incubation with BTV (∼8 HA units) for 30 min at 22°C before the addition of 50 μl of 0.25% sheep erythrocytes. The HI titer of the monospecific anti-VP2 PAb was 128 (D).
VP2 is responsible for virus internalization.
Of the four major structural proteins of the BTV virion, VP3 protein is the innermost and VP2 is the outermost protein. However, the other outer capsid protein, VP5, and the core surface protein, VP7, are partly exposed. Therefore, to determine which of these proteins plays a primary role in cell attachment and penetration, binding and internalization studies with permissive and nonpermissive cell lines were carried out. For both studies, appropriate antigens together with fluorescence detection were used. In the binding assay, only antigens on the cell surface were probed with antibody, but in the internalization assay, the plasma membranes of the cells were permeabilized to allow detection of antigens within the cells. Cell attachment and penetration experiments were carried out with purified VP2, purified VP5, CLPs (since VP7 is located on the surface), and BTV virions. VP7 appears to play a role in cell attachment to certain hosts (e.g., Culicoides spp. and insect cell lines), and it is thought that it might also play a role in attachment to mammalian cells. Two types of mammalian cell cultures were used, one that is permissive for BTV infection (L929 cells) and one that is nonpermissive (MDCK cells).
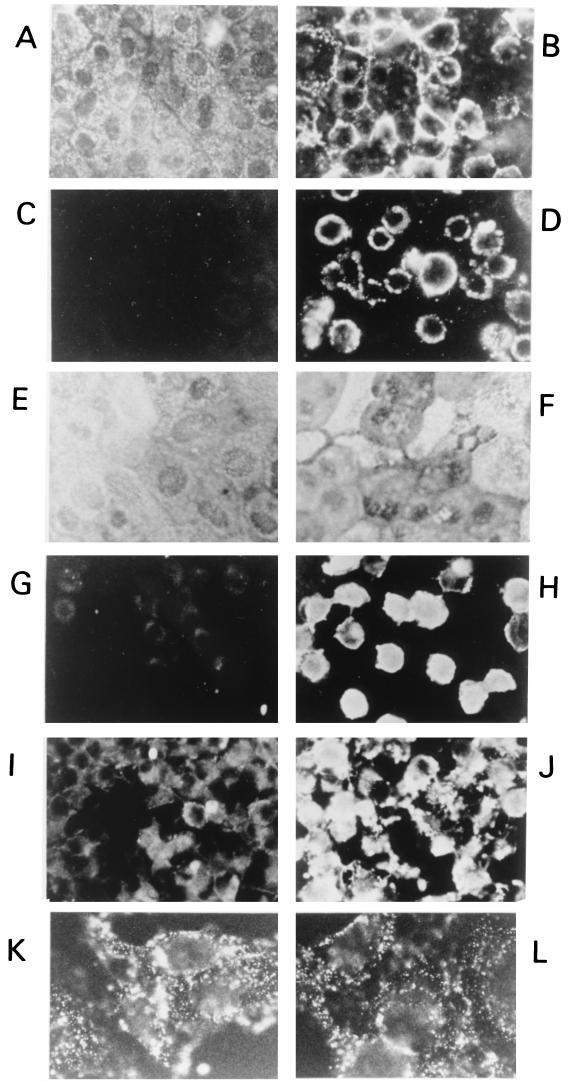
For these studies, we used an immunofluorescence technique combined with appropriate monospecific PAbs and MAbs. In most binding assays performed by the immunofluorescence technique, antigens detected on the surface of cellular plasma membranes were usually observed as a ring of fluorescent signal outlining the cells or as ground-glass-like signals on the cell surface. When the cells were further incubated at 37°C for a further 1 h to allow internalization to occur and by methanol-acetone treatment to permeabilize or remove the plasma membrane layer, the fluorescent signalling pattern indicative of internalization, i.e., homogeneous staining of the cytoplasm, was observed. The fluorescent signals obtained for the binding of VP2 to both MDCK cells (Fig. 5B) and L929 cells (Fig. 5D) were strong and widespread. The signal for internalization of VP2 was, however, more intense for L929 cells (Fig. 5H) than for MDCK cells (Fig. 5F). The signals in L929 cells were also intense when BTV was used in the internalization assay (Fig. 5J). In contrast, a very low level of signal indicating a very low level of internalization was obtained with CLPs that consist of only VP3 and VP7 (Fig. 5I). For VP5, there was intense binding of the protein to the cells (Fig. 5K). However, the ground-glass appearance of the fluorescent signals persisted and intensified even in the cells where VP5 was internalized (Fig. 5L). This morphology could be due to changes on the cells which are probably the result of the cytotoxic effect of VP5.
FIG. 5.
Indirect immunofluorescent staining showing binding to and internalization of MDCK and L929 cells by VP2, VP5, BTV, and CLPs. (A and C) Controls for the binding assays in MDCK and L929 cells in the absence of VP2. (B and D) Binding of VP2 to MDCK and L929 cells. (E and G) Controls for the internalization assay of MDCK and L929 cells. (F and H) Internalization by VP2 of MDCK and L929 cells. (I and J) Internalization of the L929 cells by CLPs and BTV. (K and L) Binding of VP5 to and internalization of VP5 by L929 cells. Magnification, ×736 (A to J) and ×2,944 (K and L).
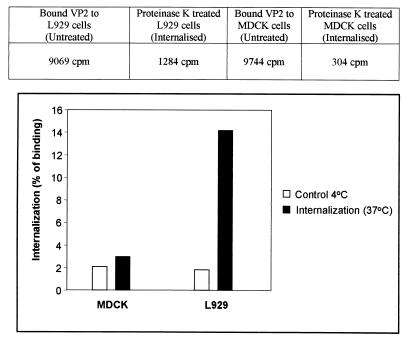
Control binding and internalization assays were carried out without the addition of VP2. No fluorescent signal was obtained for binding with either MDCK cells (Fig. 5A) or L929 cells (Fig. 5C). Similarly, as expected, no signal was achieved in these cells (Fig. 5E and G) for internalization procedures without VP2 and VP5. In the binding and internalization assay described above, there was moderate to strong binding and internalization of VP2 by both the MDCK and L929 cells. The quantity of VP2 internalization within each type of cells was further assessed by using 35[S]methionine-labeled VP2. Prebound radiolabeled VP2 (held at 4°C) was allowed to enter cells by incubation at 37°C for 1 h, to initiate the entry of the bound VP2. Any remaining surface-bound VP2 was then removed by proteinase K digestion. More than 95% of surface-bound VP2 was removed. The cells were then washed, lysed, and subjected to scintillation counting. Control experiments to demonstrate the effectiveness of proteinase K removal of bound VP2 were carried out at 4°C. The results obtained clearly demonstrated that the level of VP2 internalization into L929 cells was much greater than in MDCK cells (12% of bound VP2 was internalized by the cells compared to only 1% by MDCK cells) (Fig. 6). The final percentage was obtained after subtracting the control from the total internalization. These results indicate that on its own VP2 can enter permissive cells and also indicate that there are specific cellular receptors on the L929 cells which mediate the entry of VP2.
FIG. 6.
Internalization of VP2 in BTV-permissive L929 cells. L929 and nonpermissive MDCK cells were grown as monolayers and prechilled at 4°C, and 22.5 μg of radiolabeled VP2 was added. The cells were incubated at 4°C for 1 h and then transferred to 37°C for an additional 1 h to allow VP2 internalization. They were then treated with proteinase K (500 μg/ml) for 30 min at 4°C and solubilized in lysis buffer. The cell-associated radioactivity was determined by liquid scintillation counting. Results (triplicate wells) are expressed as the percentage of VP2 that bound to the cells. The percentage that internalized MDCK cells was (304/9,744) × 100 = 3%, and the percentage that internalized L929 cells was (1,284/9,069) = 14%. The proteinase K treatment (control, 4°C) degraded >95% of VP2.
These studies show that VP2 could mediate the entry of BTV virions into cells. CLPs with VP7 on the surface were not internalized by the L929 cells. VP5 was also found not to enter the L929 cells. However, the BTV virions were internalized as expected. The data directly show that VP2 mediates cell entry.
The binding of VP2 is competed for by BTV but not by VP6 and CLPs that lack the virion outer capsid proteins.
To examine whether VP2 binds to a specific site(s) on the cell surface and whether such a site(s) is shared by native BTV and purified VP2, a competition assay for receptors was undertaken with purified BTV virions, CLPs, and VP6. This assay would also determine whether VP2 is antigenically indistinguishable from BTV particles. The specificity of radiolabeled VP2 binding to the L929 cells was monitored in the presence of a 10-fold excess of unlabeled BTV and CLPs. The amount of virus added to the cells was estimated based on the amount of VP2 (approximately 25%) present in the total mass of the virion. Therefore, for 15 μg of VP2, which was used for the binding experiment, 600 μg of virus (equivalent to a 10-fold excess of purified VP2) was been used. As expected, unlabeled BTV was able to block ∼80% of VP2 binding to the cells, suggesting that the virus attachment site was conserved in the VP2 molecules (Fig. 7). The CLPs (∼600 μg) and BTV-10–VP6 (15 μg) protein blocked 10 and ∼20%, respectively, of the binding of VP2. The binding of VP2 is therefore efficiently competed for by BTV but not by CLPs that lack the virion outer capsid proteins or by VP6 (an irrelevant protein for binding), indicating that recombinant pure VP2 is indistinguishable from native VP2 on the virion and therefore has the same receptors.
FIG. 7.
Competition of binding of radiolabeled VP2 with BTV virions, CLPs, and VP6 in L929 cells. Radiolabeled VP2 at 15 μg/well was added in duplicate, in the presence of a 10-fold excess of BTV and CLPs, to L929 monolayer cells for 1 h at 4°C. The mixture was removed, the cells were washed and solubilized in lysis buffer, and bound radioactivity was determined by liquid scintillation. Cells bound to radiolabeled VP2 in the absence of BTV, CLPs, and VP6 were used as controls.
Blocking of BTV infectivity by VP2.
The specificity of binding of the VP2 and its ability to compete with virus infection were further confirmed by a different study. In this study, low to high concentrations of purified VP2 were bound (at 4°C) to the BTV-permissive (L929) cell surface in a 24-well plate. The VP2 was then removed, infected with a fixed amount of BTV (100 PFU/well), and allowed to bind for 1 h at 4°C. BTV virions that bound to the cell and were subsequently internalized (incubation at 37°C), permitting virus replication, were assessed by a plaque reduction neutralization assay. At a VP2 concentration of 5 μg/well, only about 5 to 10% of BTV infectivity was blocked; however, at 30 and 60 μg of VP2, BTV replication was completely inhibited (data not shown). The data obtained clearly demonstrated that VP2 could block BTV entry and confirmed the specificity of binding of VP2 to the cellular receptors used by the virus.
VP2 attaches to sialoglycophorin A of erythrocytes.
BTV binds to sheep and human blood erythrocytes (5). That report also suggested that BTV is likely to bind the NeuAcα2-6GalNAc residues on the O-linked oligosaccharides of glycophorin A of erythrocytes. Glycophorin A, a sialoglycoprotein which is distributed abundantly on human erythrocytes, is a highly glycosylated integral transmembrane protein with terminal α2-3Gal-linked and α2-6GalNAc-linked sialic acid residues. About 60% of the total molecular mass of glycophorin A (78,500 Da) is due to the carbohydrate side chains (3, 7).
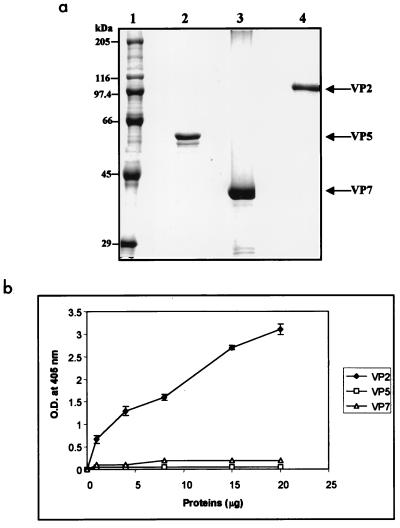
The BTV outer capsid is formed by VP2 and VP5; however, it is not clear whether only VP2 and/or VP5 or possibly the exposed region of VP7 is responsible for binding to the glycophorin A. To investigate this, each recombinant protein was prepared and the purity of each was assessed by SDS-PAGE (Fig. 8a). To determine the protein involved in the attachment to the glycophorin A, an ELISA-based assay system was developed. In this assay system, purified VP2, VP5, or VP7 was added to the glycophorin A-coated plates and the binding of each protein to glycophorin A was measured as described in Materials and Methods. As shown in Fig. 8b, neither VP5 nor VP7 bound glycophorin A. In contrast, VP2 bound glycophorin A strongly, indicating that although VP5 and VP7 are expressed on the BTV virion, only VP2 possesses a binding domain for glycophorin A.
FIG. 8.
Binding of VP2, VP5, and VP7 to glycophorin A. (a) SDS-polyacrylamide gel showing purified VP2, VP5, and VP7 used for binding to glycophorin A. (b) Binding of purified VP2, VP5, and VP7 to glycophorin A in an ELISA. Purified proteins at a final concentration of 1, 4, 8, 15, and 20 μg in 50 μl were added to a fixed amount (1 μg) of glycophorin A (in triplicate wells of a 96-well ELISA plate) and incubated at 37°C for 1 h. The proteins bound to the glycophorin A were detected with an anti-BTV PAb. The primary antibody was detected by using an appropriate affinity-purified alkaline phosphatase-labeled anti-species immunoglobulin G antibody with pNpp as the substrate. The absorbance was read at 405 nm (O.D. at 405 nm), and the means for the triplicate wells are shown.
BTV infectivity in mammalian cells is blocked by glycophorin.
Since the sialic acid-containing serine-linked oligosaccharide residue on glycophorin A was recognized as the structure for BTV binding on erythrocytes, it is possible that a similar structure(s) on mammalian cells also acts as a receptor or as a cellular binding moiety for BTV. To investigate this, the effect of glycophorin A on BTV binding and subsequent infectivity in Vero cells was examined. The infectivity of BTV in Vero cells was determined by the reduction in the number of plaques obtained by using a number of infectious particles (50 PFU/well) in the presence of various concentrations of glycophorin. Plaque numbers were scored in comparison to the control (BTV infection in the absence of glycophorin A). A significant reduction (40%) in plaque number was observed even at the lowest dilution (1 μg) of glycophorin A, indicating that glycophorin A was able to block BTV binding, thus inhibiting replication (Fig. 9). Glycophorin A at 50 μg/100 μl of medium completely blocked BTV attachment to the Vero cells. In contrast, when other cell attachment proteins and peptides such as laminin and RGD peptides were used as controls, 90% plaque formation in comparison to the control was obtained.
FIG. 9.
Inhibition of BTV infectivity by glycophorin A. By using a plaque reduction test, the ability of various amounts of glycophorin A (1, 10, 30, and 50 μg) to block 50 PFU of BTV per ml was assayed. The mean number of plaques from duplicate wells was counted, and the percentage of plaques inhibited in comparison to the virus control wells was plotted.
To further confirm this, a second competition experiment was designed. In this experiment, a fixed amount of purified VP2 (30 μg) was incubated with various amounts (25, 50, 100, and 200 μg) of glycophorin A and the VP2-glycophorin A complexes were tested for their ability to block BTV binding and infectivity in Vero cells. The data obtained from VP2 binding studies (see above) demonstrated that VP2 at 30 μg was able to block BTV binding and thus to block infectivity. In this study, glycophorin A at 100 and 200 μg was able to block binding sites of VP2, rendering the VP2 incapable of binding to host cellular receptors, i.e., the sialoglycoprotein. The VP2 which has interacted with the glycophorin A therefore could bind further to cellular surface attachment sites. BTV virions were therefore able to bind and replicate in the cells and thus form at least 80 to 90% of the plaques found for the control. In contrast, small amounts (at 25 and 50 μg) of glycophorin A bound to VP2 resulted in substantial blockage of BTV binding, resulting in the appearance of only 10 to 25% of the number of plaques found for the control. The data obtained in this study implies that glycophorin A or similar sialoglycoprotein molecules could be the binding site/receptor(s) on mammalian cells to mediate viral entry and replication of BTV.
Sodium periodate, neuraminidase, and proteases abrogate the binding of VP2 to L929 cells.
To characterize the biochemical nature (e.g., protein, lipid, or carbohydrate) of the BTV/VP2 binding component(s) on the cell surface, cells were treated with various enzymes (Table 1) prior to the VP2 binding assay. To ensure that enzyme treatments did not affect the viability of the cells, each enzyme was assessed for its toxicity on cells at various concentrations, so that only the maximum concentration of each enzyme which gave 98 to 100% viability of the cells but presumably still could affect the putative BTV receptor molecule(s) was used to treat the cells prior to each binding assay. Treated cells were subsequently tested for their ability to bind to [35S]methionine-labeled VP2. In each case, untreated cells were also included for the VP2 binding assay. The results of the virus binding to pretreated cells were expressed as a percentage of VP2 binding to untreated control cells (Table 1). Three different proteases, trypsin, chymotrypsin, and proteinase K, were used. The data indicated that trypsin, which cleaves arginine and lysine on the N-terminal side of the scissile bond, had no effect on VP2 binding. However, chymotrypsin and proteinase K, which are more nonspecific in their cleavage sites (e.g., chymotrypsin for aromatic residues), and proteinase K (both aromatic and hydrophobic residues) (1) had reduced the ability of the cells to bind the radiolabeled VP2 by 55 and 44%, respectively.
TABLE 1.
Effect of enzymatic digestion and periodate treatment of L929 cells on the specific attachment of [35S]methionine labeled purified VP2
| Enzyme (concn)a | Inhibition of binding of radiolabeled purified VP2 (%) |
|---|---|
| Trypsin (20 μg/ml) | 0 |
| Chymotrypsin (45 μg/ml) | 55 |
| Proteinase K (0.3 μg/ml) | 44 |
| Phospholipase A2 (2 mg/ml) | 0 |
| Phospholipase C (25 mU/ml) | 0 |
| Neuraminidase (C. perfringens) (5 U/ml) | 0 |
| Neuraminidase (V. cholerae) (5 U/ml) | 25 |
| Sodium m-periodate (0.1 mM) | 50 |
Before binding with the VP2, the toxicity of the enzymes for the cell line L929 was determined by incubating the cells with a range of concentrations of the enzymes. The maximum concentration of enzymes that gave 98 to 100% viability of the cells was used to treat the cells for the binding assay. Cell viability was determined by the trypan blue exclusion method and light microscopy to detect any changes in morphology by comparison with untreated cells.
All the proteases used are serine proteases, and since two of them have an effect on the host cell binding to the VP2, other types of proteases were not used. Treatment with neuraminidase, which removes the terminal amino acid residues from complex carbohydrates reduced VP2 binding by only 25%. Similarly, binding of VP2 was clearly not dependent on phospholipids or, more specifically, the glycosylphosphatidylinositol-linked moieties on the cell surface as demonstrated by treatment of the cells with phospholipases A2 and C. However, treatment of the cells with sodium periodate (a strong oxidizer of carbohydrates) resulted in at least 50% reduction in VP2 binding, indicating that the BTV binding molecule at the cell surface is likely to be rich in carbohydrate moieties. Collectively, these findings suggest that the essential biochemical nature of the binding sites of BTV is a sialic acid-containing glycoprotein and that the second protein molecule(s) may also act as a BTV receptor(s).
DISCUSSION
A recombinant tagged VP2 was produced and successfully purified to high levels. This has enabled us to investigate the functional and biological activities of VP2 in the absence of other viral proteins. The purified VP2 was immunoprecipitated with conformation-dependent neutralizing anti-VP2 MAbs and by an anti-BTV Pab. The purified VP2 was also able to hemagglutinate erythrocytes, yielding direct evidence that VP2 is indeed a hemagglutinin protein. The soluble purified VP2 formed trimers, although the dimeric form appeared to be more stable than the trimer. The multimeric formation correlates well with HA activity, which is generally associated with multimers of proteins. Normally with a multivalent virus, at least two erythrocytes are bound by each virion to produce agglutination (18). The neutralizing and HA activities of VP2 are specific, since the monospecific PAb produced against the purified VP2 was able to immunoprecipitate BTV particles from infected cell lysates, to neutralize BTV particles, and to inhibit HA by BTV particles. Collectively, all these criteria indicated that the purified VP2 was appropriately folded and that the integrity of the neutralizing epitopes and HA domains were preserved.
Many previous reports supported the neutralization and HA formation of VP2; however, in this report we have obtained evidence demonstrating for the first time that VP2 is the protein responsible for entry of BTV into the host cell. We have presented data that VP2 alone is sufficient for virus entry into the BTV-permissive L929 cells, although it binds equally well to the BTV nonpermissive MDCK cells. Such binding could be due to nonspecific binding of VP2, and it could be argued that the nonspecific binding is a necessary step where the virus binds to probably a more abundant or accessible structure for initial attachment, prior to the specific secondary binding to cell receptors for viral entry. This has been supported by a significant difference in VP2 internalization by L929 cells compared to the much lower internalization by MDCK cells. Evidently, the binding of VP2 to the L929 cells was specific, resulting in receptor-mediated endocytosis. BTV-derived core particles lacking the outer capsid proteins have been reported to bind to vertebrate cells at a low level (21). VP7 of BTV is a very hydrophobic protein and binds to any cells easily; however, in this study, VP7 in CLPs was able to mediate binding but not entry of the CLPs into the cells. The outer capsid protein VP5 bound strongly to L929 cells but was not internalized by the cells. In addition, VP5 was cytotoxic to the cells and was not able to saturate the cells (25). Since only VP2 and BTV were internalized by the mammalian cells, we therefore provide evidence that VP2 is the viral protein mediating entry of BTV into cells.
In this study, we also showed that VP2 was able to block BTV infectivity completely and that BTV competed with VP2 for binding sites on the L929 cells. These results demonstrated that the recombinant VP2 is antigenically indistinguishable from the VP2 of BTV and binds to the same cellular receptors as the virus particles do. Since excess glycophorin A was able to block BTV entry completely, this may be the cellular receptor for BTV. Interestingly, VP2 is the only viral protein which binds glycophorin A, supporting our assertions that it is the viral “entry protein.”
A preliminary study was set up to biochemically characterize the nature of BTV receptors on L929 cells. VP2 binding was found to be moderately sensitive to proteolytic digestion and periodate treatment of the cellular membranes, suggesting that BTV interacted with a protein and/or a carbohydrate moiety of a glycoprotein, respectively. If we assumed that the receptor is a sialoglycoprotein, the proteases might cleave the exoplasmic domain of the protein, which supports the oligosaccharides, or the enzymes might act on a completely different set of protein independent of the sialoglycoprotein (Fig. 10). Sialic acid had been reported to be the receptor for BTV binding on erythrocytes, and in this study, there is evidence that sialic acid may also be the receptor on cellular membranes, although the extent of sialic acid abrogation after neuraminidase treatment was low. More intensive studies must be carried out to identify the BTV cellular receptors. However, with the availability of the purified VP2, further work on identifying the regions of VP2 responsible for the binding of glycophorin A and internalization of cellular plasma membranes are currently being conducted in our laboratory.
FIG. 10.
Schematic showing a sialoglycoprotein and a protein. Black arrows show the possible proteolytic digestions of the sialoglycoprotein protein backbone or of a totally different protein.
ACKNOWLEDGMENTS
We thank Jose Costas-Martinez for his support during the initial period of the work and Geoff Sutton and Christophe Wirblich for their critical comments and suggestions. We are also grateful to Linda Jones for the production of polyclonal antibodies.
This work was partially funded by BBSRC. Sharifah S. Hassan was supported by a studentship from the Public Services Department, Malaysia.
REFERENCES
- 1.Beynon R J, Bond J S. The Schecter and Berger nomenclature for protease subsites. In: Beynon R J, Bond J S, editors. Proteolytic enzymes, a practical approach. Oxford, United Kingdom: IRL Press; 1989. pp. 231–240. [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Challou N, Goormaghtigh E, Cabiaux V, Conrath K, Ruysschaert J M. Sequence and structure of the membrane-associated peptide of glycophorin A. Biochemistry. 1994;33:6902–6910. doi: 10.1021/bi00188a020. [DOI] [PubMed] [Google Scholar]
- 4.Cowley J A, Gorman B M. Genetic reassortants for identification of the genome segment coding for the bluetongue virus hemagglutinin. J Virol. 1987;61:2304–2306. doi: 10.1128/jvi.61.7.2304-2306.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eaton B T, Crameri G S. The site of bluetongue virus attachment to glycophorins from a number of animal erythrocytes. J Gen Virol. 1989;70:3347–3353. doi: 10.1099/0022-1317-70-12-3347. [DOI] [PubMed] [Google Scholar]
- 6.French T J, Marshall J, Roy P. Assembly of double-shelled, virus-like particles of bluetongue virus by the simultaneous expression of four structural proteins. J Virol. 1990;64:5695–5700. doi: 10.1128/jvi.64.12.5695-5700.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furthmayr H, Marchesi V T. Subunit structure of human erythrocyte glycophorin A. Biochemistry. 1976;15:1137. doi: 10.1021/bi00650a028. [DOI] [PubMed] [Google Scholar]
- 8.Grimes J, Basak A K, Roy P, Stuart D. The crystal structure of bluetongue virus VP7. Nature. 1995;373:167–170. doi: 10.1038/373167a0. [DOI] [PubMed] [Google Scholar]
- 9.Hewat E A, Booth T F, Roy P. Structure of bluetongue virus particles by cryo-electron microscopy. J Struct Biol. 1992;109:61–69. doi: 10.1016/1047-8477(92)90068-l. [DOI] [PubMed] [Google Scholar]
- 10.Huismans H, Erasmus B J. Identification of the serotype-specific and group specific antigens of bluetongue virus. Onderstepoort J Vet Res. 1983;48:51–58. [PubMed] [Google Scholar]
- 11.Huismans H, Van der Walt N T, Cloete M, Erasmus B J. The biochemical and immunological characterization of bluetongue virus outer capsid polypeptides. In: Compans R W, Bishop D H L, editors. Double stranded RNA viruses. New York, N.Y: Elsevier; 1983. pp. 165–172. [Google Scholar]
- 12.Hussy P, Schmid G, Mous J, Jacobsen H. Purification and in vitro-phospholabeling of secretory envelope proteins E1 and E2 of hepatitis C virus expressed in insect cells. Virus Res. 1996;45:45–57. doi: 10.1016/0168-1702(96)01365-2. [DOI] [PubMed] [Google Scholar]
- 13.Hwang G Y, Li J K K. Identification and localization of a serotypic neutralization determinant on the VP2 protein of bluetongue virus 13. Virology. 1993;195:859–862. doi: 10.1006/viro.1993.1445. [DOI] [PubMed] [Google Scholar]
- 14.Inumaru S, Roy P. Production and characterization of the neutralization antigen VP2 of bluetongue virus serotype 10 using a baculovirus expression vector. Virology. 1987;157:472–479. doi: 10.1016/0042-6822(87)90289-3. [DOI] [PubMed] [Google Scholar]
- 15.Kim J S, Raines R T. Ribonuclease S-peptide as a carrier in fusion proteins. Protein Sci. 1993;2:348–356. doi: 10.1002/pro.5560020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King L A, Possee R D. The baculovirus expression system. A laboratory guide. London, United Kingdom: Chapman & Hall, Ltd.; 1992. [Google Scholar]
- 17.Liu H M, Booth T F, Roy P. Interactions between bluetongue virus core and capsid proteins translated in vitro. J Gen Virol. 1992;73:2577–2584. doi: 10.1099/0022-1317-73-10-2577. [DOI] [PubMed] [Google Scholar]
- 18.Mackow E R, Barnett J W, Chan H, Greenberg H B. The rhesus rotavirus outer capsid protein VP4 functions as a hemagglutinin and is antigenically conserved when expressed by a baculovirus recombinant. J Virol. 1989;63:1661–1668. doi: 10.1128/jvi.63.4.1661-1668.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall J J, Roy P. High level expression of the two outer capsid proteins of bluetongue virus serotype 10: their relationship with the neutralization of virus infection. Virus Res. 1990;15:189–195. doi: 10.1016/0168-1702(90)90027-9. [DOI] [PubMed] [Google Scholar]
- 20.Mertens P P C, Burroughs J N, Anderson J. Purification and properties of virus particles, infectious sub-viral particles and cores of bluetongue virus serotypes 1 and 4. Virology. 1987;157:375–386. doi: 10.1016/0042-6822(87)90280-7. [DOI] [PubMed] [Google Scholar]
- 21.Mertens P P, Burroughs J N, Walton A, Wellby M P, Fu H, O’Hara R S, Brookes S M, Mellor P S. Enhanced infectivity of modified bluetongue virus particles for two insect cell lines and for two Culicoides vector species. Virology. 1996;217:582–593. doi: 10.1006/viro.1996.0153. [DOI] [PubMed] [Google Scholar]
- 22.Prasad B V V, Yamaguchi S, Roy P. Three dimensional structure of single-shelled bluetongue virus. J Virol. 1992;66:2135–2134. doi: 10.1128/jvi.66.4.2135-2142.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy P, Urakawa T, Van Dijk A A, Erasmus B J. Recombinant virus vaccine for bluetongue disease in sheep. J Virol. 1990;64:1998–2003. doi: 10.1128/jvi.64.5.1998-2003.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharifah, S. H., W. F. Wirblich, and P. Roy. Unpublished data.
- 26.Van der Walt N T. A haemagglutination and haemagglutination inhibition test for bluetongue virus. Onderstepoort J Vet Res. 1980;47:113–117. [PubMed] [Google Scholar]
- 27.Van-Dijk A A, Huismans H. The in vitro activation and further characterization of the bluetongue virus-associated transcriptase. Virology. 1980;104:347–56. doi: 10.1016/0042-6822(80)90339-6. [DOI] [PubMed] [Google Scholar]
- 28.Verwoerd D W, Els H J, De Villiers, Ethel-Michele, Huismans H. Structure of the bluetongue virus capsid. J Virol. 1972;10:783–794. doi: 10.1128/jvi.10.4.783-794.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White J R, Eaton B T. Conformation of the VP2 protein of bluetongue virus (BTV) determines the involvement in virus neutralization of highly conserved epitopes within the BTV serogroup. J Gen Virol. 1990;71:1325–1332. doi: 10.1099/0022-1317-71-6-1325. [DOI] [PubMed] [Google Scholar]
- 30.White L J, Ball J M, Hardy M E, Tanaka T N, Kitamoto N, Estes M K. Attachment and entry of recombinant Norwalk virus capsids to cultured human and animal cell lines. J Virol. 1996;70:6589–6597. doi: 10.1128/jvi.70.10.6589-6597.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]