Abstract
In 2022, the Australian Government listed the koala as endangered in several states due to habitat destruction, traffic strikes, dog attacks, and Chlamydia pecorum disease. This study evaluates a 10-year assessment of a Major Outer Membrane Protein-based vaccine’s effectiveness against chlamydial disease in wild koalas from Southeast Queensland. Over a decade, 680 koalas were tracked, with five vaccine trials involving 165 koalas. While prior studies only offered up to two years of data, this study’s extended period allowed a thorough evaluation of vaccine efficacy. Results showed that vaccinated koalas had significantly lower disease incidence, with a 64% reduction in chlamydial mortality. This vaccine demonstrated positive impacts on both male and female koalas, highlighting its crucial role in conserving the Australian koala population and mitigating the threats they face.
Subject terms: Vaccines, Clinical microbiology
Introduction
The koala was listed in 2022 by the Australian Government as an endangered species in three out of five states/territories of Australia in which the species naturally occurs (Queensland, New South Wales, and the Australian Capital Territory) due to ongoing and significant population declines1. The decline in these states was attributable to several main factors: Climate change, habitat loss, fragmentation and degradation, traffic strikes, dog attacks and disease. One measure of koala health is through wildlife hospital admissions. Statistics from a Queensland-based study in 2017 identified that only 17% of koalas admitted to wildlife hospitals were released between the years 1997 and 2013 with 16,758 koalas classified as either dead on arrival or euthanised2. The three top reasons for a koala being admitted are infectious disease, motor vehicle trauma and wasting2. Koalas suffer from a wide range of infectious pathogens, including Bordetella, Koala retrovirus (KoRV), Cryptococcus, Gamma herpes virus, and Chlamydia3–6. Of these infections, those involving the intracellular bacterium Chlamydia pecorum are by far of the most concern, as they are highly prevalent (up to 73% in some populations)3,7, and cause premature mortality, and chronic, painful conditions, such as blindness, and reproductive disease7,8.
Chlamydia pecorum infections can result in disease of the conjunctivae, urinary tract, and reproductive tract, with chronic infections resulting in keratoconjunctivitis (blindness), severe cystitis (bladder inflammation), and various lesions leading to reproductive sterility (permanent infertility)3,7. Left untreated, these infections can result in significant pain, discomfort, and premature death. Koalas with severe, untreated Chlamydia infections may have their life expectancy reduced by several years. In the wild, a koala might live for 10-15 years, but a severe infection could reduce this to 5-7 years or less (8).
The current treatment for chlamydiosis is the use of antibiotics (chloramphenicol or doxycycline)9. However, treatment is not without risk of potentially fatal gastro-intestinal dysbiosis. Furthermore, infection and/or treatment do not prevent future infection9,10.
Fortunately, considerable effort over the past 15 years has been focused on the development of a koala Chlamydia vaccine11,12. There have been 14 separate koala vaccine trials conducted within South East Queensland, assessing different antigens, adjuvants, modes of delivery, and immunological responses11,12. During this time, a separate project was also conducted on a specific population of wild koalas being monitored over a 10-year period in South East Queensland’s Moreton Bay region13–16. The project involved the intensive telemetric monitoring and veterinary management of approximately 150 koalas (at any given time) in this region for a 10-year period (2013 – 2023)17. This intensive monitoring also allowed for regular (approximately every 6 months) veterinary examinations of all monitored koalas and subsequent management of any koalas identified with disease. Five separate vaccine trials were conducted during this program, utilising the C. pecorum Major Outer Membrane Protein (MOMP) as the antigenic target with varying adjuvants and dosage regimens. Separately, each of these trials identified that, in wild koalas, a MOMP-based vaccine can elicit a strong anti-Chlamydia systemic and mucosal antibody response that persists for more than two years13,14,16,18. They also identified that, by careful selection of the adjuvant, this can be achieved with a single dose of vaccine in both healthy and diseased animals13,14,16,18 with a disease incidence reduction of 42% and the incidence of infection reduced by 82%13. However, individually, these trials involved small numbers ( < 20) of animals for only six to 24-months, limiting the statistical robustness of results.
The current statistical analysis combines datasets from this 10-year monitoring period, including vaccination, and identifies an accurate and statistically robust outcome from vaccinating wild koalas with a MOMP-based vaccine.
Results
Animals
A total of 680 koalas (53.1% female) were processed during a 10-year tracking and health monitoring program located within the Moreton Bay region of South East Queensland, Australia. There was a total of 5,228 clinical examinations which occurred between the years 2013 and 2023 with 4,374 examinations occurring as new examinations (removing consecutive examinations during a treatment regime), with the mean age of the koalas at examination being 3.6 years (±2.6 years).
Vaccinations
During this 10-year period, 165 koalas were vaccinated with C. pecorum MOMP in five separate koala vaccine trials. These trials have all since been published, and are detailed in Supplementary tables 1 and 2, including the number of animals included and the variation of MOMP-based vaccination utilised. Although the published findings for these trials only relate to a maximum of 2-years (often shorter duration) of longitudinal data, many of the koalas continued to be monitored until the end of the monitoring program in 2023. This has provided an unprecedented opportunity to assess the effectiveness of a MOMP-based vaccine to protect koalas from chlamydial disease and/or death. For the five separate chlamydial vaccine trials, the analysis focused on each individual vaccine efficacy for incidence of disease and death with disease and identified relatively different findings between vaccines for presence of disease but not death with disease (Supplementary table 2). For each of the individual trials there was a vaccine efficacy difference of more than 76% (between 16.64% and 93.14% effective), however, due to the limited numbers of animals in each trial this analysis is limited. The vaccine-specific efficacy for death with chlamydial disease identified limited variation between trials with all but one trial having no accounts of koala death (Supplementary table 2). The only vaccine trial that had chlamydial disease-related deaths had those deaths occurring at a higher incidence in the vaccinated cohort (0.102) than the unvaccinated cohort (0.081), however the number of animals was too low for meaningful statistical analysis. Furthermore, when these individual cases were examined, it was determined that confounding factors may have played a role in the death in four of five of the koalas. Confounding factors were advanced age (three koalas aged over 10-years) and infection soon after vaccination, presumably before immunisation was established (one death 43-days post vaccination) (Supplementary table 2).
Vaccination of koalas increases protection from chlamydial disease
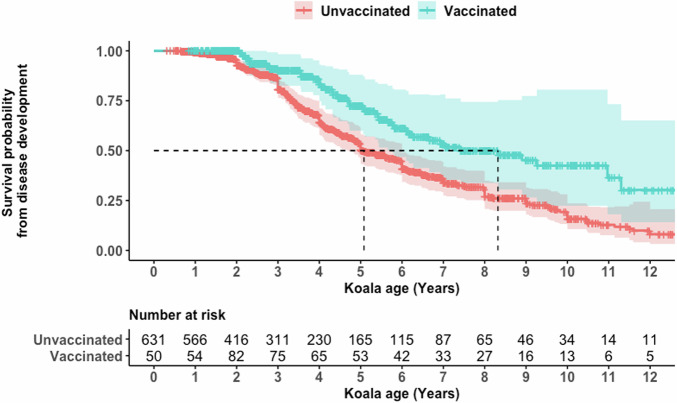
There was an observed reduction in the development of chlamydial disease in vaccinated koalas across their life, compared with unvaccinated koalas. A survival probability analysis (accounting for a time-series of repeated events) identified an approximate 25% increase in the probability of a vaccinated koala not developing signs of chlamydial disease as compared to unvaccinated koalas, which persisted from age two up until the age of 12 years (Fig. 1). The median probability of developing disease was also shifted by more than 3 years, from 5 years old to over 8 years old (Fig. 1).
Fig. 1. Survival probability curve for signs of disease throughout a koala’s life span.
Green represents vaccinated koalas and red represents unvaccinated koalas. The top line plot is the survival probability of a koala being found with signs of disease during its life span. The second table counts down the number at risk for each year of age.
A multivariate cox proportional hazard model identified that only vaccination status had a positive effect on the koala’s survival from developing chlamydial disease (HR 0.55; 95% CI 0.34–0.90; p = 0.02). Neither sex of the koala or the year of the clinical visit were significantly associated (p = 0.9807 and p = 0.5285, respectively) (Table 1).
Table 1.
Time-varying cox proportional hazards regression model results for vaccination, sex, and year of clinical observation for a koala’s survivability from chlamydial disease
| coef | exp(coef) | se(coef) | z | p* | 0.95 CI | |
|---|---|---|---|---|---|---|
| Vaccination | -0.59 | 0.55 | 0.17 | -2.40 | 0.02 | 0.34 - 0.90 |
| Sex | 0.00 | 1.00 | 0.12 | -0.02 | 0.98 | 0.69 - 1.44 |
| Year of observation | -0.03 | 0.97 | 0.02 | -0.63 | 0.53 | 0.90 - 1.06 |
*Significant values assessed as <0.05.
Vaccination protects koalas from death with chlamydial disease
In total, 680 koalas were monitored over the 10-year period between 2013 and 2023 within the Moreton Bay project. During this time 110 koalas were found dead or died while in care (either through euthanasia, natural causes, injuries sustained in the wild or disease). Significantly fewer vaccinated koalas died during the 10-year period than unvaccinated koalas: 9.7% (16/165) versus 14.9% (94/631), respectively (Table 2). This trend continued when analysis was limited to only koalas that died with signs of chlamydial disease: 3.0% (5/165) vaccinated versus 8.5% (54/631) unvaccinated (Table 2). This indicates a vaccine efficacy of 64.7% in koalas for prevention of death associated with signs of chlamydial disease.
Table 2.
Total number of koalas monitored during the 10-year MBRL project and the number of koala deaths that occurred
| Total Koalas | Total deaths | Died with Disease | ||||
|---|---|---|---|---|---|---|
| Number of koalas | All koalas | Number of koalas | Each group | Number of koalas | Each group | |
| Unvaccinated | 631 | 79.30% | 94 | 14.90% | 54 | 8.50% |
| Vaccinated | 165 | 20.70% | 16 | 9.70% | 5 | 3.00% |
| Total* | 796 | 110 | 59 | |||
*Total includes 116 koalas that were unvaccinated initially then were vaccinated so are counted twice.
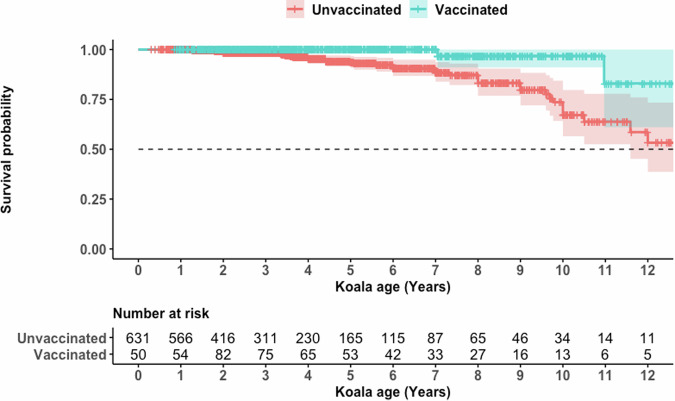
This reduction in death with chlamydial disease also persisted for the life of the vaccinated koalas, with vaccinated koalas protected from dying with disease for >12 years of age (Fig. 2).
Fig. 2. Survival probability curve for death with disease throughout a koala’s life span.
Green represents vaccinated koalas and red represents unvaccinated koalas. The top line plot is the survival probability of a koala dying with signs of disease with shaded areas indicating 95% confidence intervals and the dashed horizontal line indicating the median survival. The second table counts down the number of occurrences for each year of age.
Similar to preceding analyses, a mixed-effects Cox proportional hazards regression model identified that only vaccination status had a protective effect against death of a koala presenting with chlamydial disease (HR 0.26; 95% CI 0.09–0.79; p = 0.02). Neither sex or year of clinical observation had a significant effect (p = 0.1057 and 0.401, respectively) (Table 3).
Table 3.
Mixed-effects Cox proportional hazard regression model results for vaccination, sex, and year of observation for a koala’s survivability from chlamydial-related death
| coef | exp(coef) | se(coef) | z | p | 0.95 CI | |
|---|---|---|---|---|---|---|
| Vaccination | -1.35 | 0.26 | 0.62 | -2.38 | 0.02 | 0.09 - 0.79 |
| Sex | -0.65 | 0.52 | 0.40 | -1.62 | 0.11 | 0.24 - 1.15 |
| Year of observation | -0.06 | 0.95 | 0.07 | -0.84 | 0.40 | 0.83 - 1.08 |
Discussion
The use of vaccines to reduce the transmission of infectious agents has been employed in humans for over 600 years, with the aim of reducing disease burden over the patient’s lifetime19. However, vaccines for wildlife are designed with different goals and are tested against distinct criteria20. There have been many wildlife vaccines developed against a range of bacteria and viruses that are utilised to either protect endangered species or limit spill-over to farmed animals or humans21. Constraints associated with endangered species may limit researchers’ ability to conduct target host effectiveness studies, especially challenge trials. Furthermore, wild animals can be challenging to locate, capture, and vaccinate on a single occasion, with multi-dose vaccine regimens presenting even more challenges. It is also often impractical to achieve high vaccine coverage in wild populations, necessitating careful consideration in vaccine design20. These challenges have resulted in many imperfect vaccines being developed and implemented in vaccine strategies, where protection from disease, but not infection, has been achieved. This reduced protection has been theorised to result in increased transmission of virulent pathogens by increasing shedding time when the animal would normally have died from the disease22. Nevertheless, despite these complexities, wild animal vaccines have been successfully developed and utilised for many years, although many in the absence of controlled effectiveness studies20. Some examples of wild animal vaccines include rabies vaccines for Ethiopian Wolves, African Wild Dogs, European Red Foxes, North American Coyotes and North American Racoons (27).
The development of various koala chlamydial vaccines has now spanned 15-years, with many previous trials confirming their safety and induction of a Chlamydia-specific immune response11,12. However, assessing vaccine effectiveness has posed significant challenges due to the complexity and cost of intensive management of wild koala populations, including the cost of repeat captures, inaccessible habitats, and the extended timeframes required.
This current analysis comprehensively evaluates C. pecorum MOMP-based vaccine effectiveness across a large population of wild koalas over multiple generations, through regular screening over a 10-year period. The results demonstrate that a MOMP-based vaccine for koalas can protect individuals from both developing chlamydial disease and, crucially, from dying due to chlamydial disease. This study stands as the largest and longest-ever conducted on koalas, conclusively confirming the significant positive impact of a MOMP-based chlamydial vaccine. Specifically, this analysis reveals that vaccination extended the age at which disease will affect 50% of the population by three years, crucially during koala breeding ages, from 5 years to 8 years. The vaccine was also shown to decrease the likelihood of a koala dying from chlamydial disease by 64.7% (decrease from 8.5% to 3%). This also correlates with an improvement in a koala’s survival probability for death due to chlamydial disease, with a 12% decrease in survival probability at 10 years of age if unvaccinated.
This analysis underscores that chlamydial vaccination reduces the risk of both developing and dying from chlamydial disease in vaccinated koalas. However, the primary goal of a koala chlamydial vaccine is to aid in the recovery of a declining population. To this end, two modelling studies have previously been reported to assess the level of effectiveness required to reverse the trajectory of a declining koala population23,24. Rhodes et al.24 focused on a single koala population in South East Queensland, Australia, which had experienced a 64% decline over the previous decade. They quantified mortality rates due to dog attacks, traffic strikes, and chlamydial disease and found that a 38.9% reduction in mortality from all three factors would be necessary to stabilise the population. Interestingly, when each factor was assessed individually, only disease emerged as a single factor capable of population stabilisation, with a reduction of 58.7% (though some uncertainty was noted due to credible 95% intervals)24. Nevertheless, the study recommended management plans addressing all threats as the most robust strategy. The modelling by Rhodes et al. suggests that the current chlamydial vaccine described in this study, could effectively stabilise a similar declining koala population if similar levels of vaccine coverage were achieved (20%), due to the 65% reduction in chlamydial mortality in vaccinated koalas. However, it is worth noting that this modelling dataset is specific to a single population and does not account for environmental stochasticity.
The second koala modelling study, published in 2014 by Craig et al., specifically assessed varying chlamydial vaccination efficacies as a management strategy for koalas23. This study simulated koala populations of 500 animals and predicted that by vaccinating 10% of the population annually for five years, the population trajectory could be altered, potentially increasing to as many as 1,000 animals within 15 years23. This model also predicted the population effects of the vaccine at varying efficacies and coverage, allowing for comparisons with the current analysis. As this study only assessed vaccine protection from disease (not infection), this was utilised for comparison, assuming no protection from infection. This indicates that at 52% efficacy, a vaccine targeting females in only 10% of the original population could have a 30% chance of reversing population decline in 10 years under pessimistic boosting assumptions. However, under optimistic boosting assumptions (assuming boosting can increase immunity up to 100% and mating with an infected koala acts as a boost for a previously vaccinated koala), this likelihood could increase to 60%. Nonetheless, it is essential to recognise that this model does not account for changes in other threats, which may not accurately reflect the Moreton Bay koala population.
Another important factor to consider is the possibility of increasing virulent strain transmission, as theorised22. The current study indicates that there was a decrease across the entire population (vaccinated and unvaccinated) in the development of chlamydial disease and death with signs of chlamydial disease. It is also important to note that hyper-virulent strains of Chlamydia have not been identified, and also, these theories focus on very high transmission rates within enclosed environments and are not specific to Chlamydia22. Koalas live in open habitats, predominantly a single koala to each tree, with Chlamydia considered as a sexually transmitted infection, and as such has a very low transmission rate.
A recurring theme is that any koala management plan aiming to have a positive impact on declining populations should involve multiple strategies25, as such addressing traffic, wild and domestic dogs, chlamydial disease, and, most importantly, habitat retention and restoration. This current study demonstrates that a koala Chlamydia vaccine utilising MOMP as the target antigen, effectively reduces the chance of a koala developing signs of chlamydial disease during the breeding age and reduces chlamydial-associated deaths by 65%. Moreover, it suggests that, while the current version of the vaccine may have lower efficacy than desired, when implemented in conjunction with multiple strategies, it can reverse population declines, a conclusion supported by several modelling studies. Future research should focus on developing koala management plans that include vaccination to study the impact on koala population growth and stability.
Methods
Moreton Bay koala monitoring program
During the 10-year period between 2013 and 2023, a koala management program was conducted as part of two large-scale development projects in South East Queensland (the Moreton Bay Rail project and the Mill at Moreton Bay project), Australia. This program involved the capture, monitoring and clinical management of 680 wild koalas, of which a proportion was monitored telemetrically for up to 10-years. Koalas were subjected to standardised, comprehensive clinical examinations by koala-experienced veterinarians, approximately every six months during the period of their monitoring (or more frequently if there were health or welfare concerns). Over 5,228 clinical evaluations were performed under anaesthesia, where disease and infection were monitored, treated as necessary, and recorded, as described previously17.
Clinical record details
Clinical records (comprising a total of 5228 veterinary examinations) from the processing of 680 Moreton Bay koalas between 2013 and 2023 were collated. Data sets used in the analysis included the date of examination, koala name, identification number, sex, age at examination, clinical signs of disease during clinical examination (ocular and urogenital), body condition score, cause of death (chlamydial or other), date vaccinated, and which version of the vaccine was utilised.
Signs of ocular disease included the presence of ocular discharge, the appearance of the cornea, conjunctivae, and skin around the eyes. Signs of urogenital disease included appearance of the cloaca, tail and rump, dirty tail score (including separate staining and wetness scores), dysuria, urine sediment cytology, and ultrasonographic assessment of the bladder, prostate (males), upper reproductive tract (females), ovaries (females), kidneys and ureters.
Vaccine versions included in analysis
During the years 2013 to 2023, four similar vaccine versions were trialled in this population of koalas, all targeting the C. pecorum MOMP. The first version included a triple-dose vaccine of full recombinant MOMP (derived from koala strains with MOMP genotypes A, F and G) adjuvanted with immune-stimulating complex (ISC) administered to 43 koalas. The second version included a single-dose vaccine of full recombinant MOMP (MOMPs A, F and G) adjuvanted with a triple adjuvant (Tri-adj) containing Poly I:C, IDR-1002 peptide and poly[di(sodiumcarboxylatoethylphenoxy)phosphazene] (PCEP), administered to 95 koalas. The third and fourth versions of the vaccine included four short peptides, 20 amino acids in length, representing four conserved domains of C. pecorum MOMP combined with the Tri-adj as a single-dose vaccine. These two versions (three and four) also varied with the fourth also containing a recombinant Koala retrovirus (KoRV) protein as another antigen. The third version was administered to 23 koalas and the fourth to 22 koalas. All trials included unvaccinated control animals, 116 of which were vaccinated in a subsequent study. All unvaccinated koalas were included in the analysis up until they were vaccinated at which point they were included in the vaccinated cohort. All initial trial findings, and further details for each of these studies have been previously published13–16,18,26, a summary table is also included (Supplementary table 1).
Animal ethics
All trials included in this retrospective analysis were approved by the Animal ethics Committee at the University of the Sunshine Coast (UniSC) or Queensland University of Technology (QUT) depending on the specific trial. Approval numbers include for UniSC ANA1380 and ANS1857 and QUT 1200000122 and 0700000845. Furthermore, all trials were conducted under Queensland Government Scientific Purposes Permits, (WISP11532912, WA0020117), Australian government, department of Agriculture in-vivo use permits (approval number 2020/007) and were performed under the conditions set out in the Australian Pesticides and Veterinary Medicines Authority permit number PER7250.
Statistical analysis
The analysis focuses on the most critical measures of protection: reduction in evidence of clinical chlamydial disease and death of koalas with chlamydial disease. To assess the effect of the vaccine to protect koalas from death with chlamydial disease and development of disease, a survival analysis was performed to determine the combined effects of vaccination, year of observation and sex, utilising koala age as the base value, utilising the statistical analysis platform R studio and the ‘survival’ package27,28. The time-varying survival analyses were used to account for dynamic changes in the survival probabilities over the course of the study period.
Furthermore, a mixed-effects Cox regression model was utilised to assess the combined effects of vaccination, year of observation and sex, using koala age as the base value, utilising the statistical analysis platform R studio and the ‘coxme’ package27,29. This method allows for the incorporation of both fixed and random effects, thereby capturing individual-level variations and potential clustering within the data.
Both the Cox regression and survival probability assessments were performed utilising the repeated measures, time series approach as described previously30.
Supplementary information
Acknowledgements
This work is based on 10 years of monitoring data and four previously published immunological assessments of vaccination. Thanks to the many researchers, students and veterinarians that have contributed to all previous studies or works related to the Moreton Bay koala project, specifically, Prof Ken Beagley, Dr Amy Robbins, Dr Philipa McKay, Dr Marion Desclozeaux, Dr Shahneaz Ali Khan, Dr Adam Polkinghorne, Dr Courtney Waugh, Jo Loader, Dr Bonnie Quigley, Dr Sharon Nyari and Dr Deidré de Villiers. The authors also thank the many groups that have supported the overall koala disease and vaccine research, including the Queensland Government, the Moreton Bay Rail project team, the Queensland Department of Environment and Heritage Protection, the NSW Office of Planning, Industry and Environment, Moreton Bay Regional Council, Gold Coast City Council, Redland City Council, Gympie Regional Council, NSW Koala Strategy, Friends of the Koala-Lismore, Koala Action Inc, Royal Society of Queensland, Endeavour Veterinary Ecology, Australia Zoo Wildlife Hospital, Lone Pine Koala Sanctuary, Currumbin Wildlife Sanctuary, Wildlife HQ Zoo, Dreamworld QLD, VIDO-Canada, The Sandy Michell Legacy Fund, as well as numerous anonymous donors. Peter Timms secured funding through the Australian Research Council (ARC, Linkage Scheme LP1202000051) and the NSW Planning, Industry and Environment grants part of the NSW Koala Strategy for the vaccine trials used in this analysis. This project received significant support from the Queensland Government (Department of Transport and Main Roads), particularly the Moreton Bay project team and Endeavour Veterinary Ecology (EVE). JH and JG were employed by EVE.
Author contributions
S.P. and P.T. conceptualised the project. S.P. performed all analyses and performed initial drafts. J.H. and J.G. led the koala captures, monitoring, and data recording. M.J. and J.W. assisted with data analysis, and AM assisted with statistical comparisons. P.T. secured funding and contributed to the analysis. All authors reviewed and approved the final manuscript.
Data availability
All data is available within the manuscript and supplementary files.
Competing interests
Julien Grosmaire is a non-executive director on the board of Wildlife Health Australia. As the agreed national coordinating body for wildlife health in Australia, the organisation may produce guidelines on national wildlife health matters including management of chlamydia in koalas which may inform government implementation. All other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41541-024-00938-5.
References
- 1.Government A. Phascolarctos cinereus (combined populations of Qld, NSW and the ACT). Database. 2022. Species Profile and Threats Database. 12-2-2022. Accessed 12-05-2022. https://www.environment.gov.au/cgi-bin/sprat/public/publicspecies.pl?taxon_id=85104.
- 2.Gonzalez-Astudillo, V., Allavena, R., McKinnon, A., Larkin, R. & Henning, J. Decline causes of Koalas in South East Queensland, Australia: a 17-year retrospective study of mortality and morbidity. Sci. Rep.7, 42587 (2017). /02/20 2017. 10.1038/srep42587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quigley, B. L. & Timms, P. Helping koalas battle disease - Recent advances in Chlamydia and koala retrovirus (KoRV) disease understanding and treatment in koalas. Review. FEMS Microbiol. Rev.44, 583–605 (2020). 10.1093/femsre/fuaa024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nyari, S. et al. Epidemiology of chlamydial infection and disease in a free-ranging koala (Phascolarctos cinereus) population. PLoS One12, e0190114 (2017). 10.1371/journal.pone.0190114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stalder, K. et al. Prevalence and Clinical Significance of Herpesvirus Infection in Populations of Australian Marsupials. PLOS ONE10, e0133807 (2015). 10.1371/journal.pone.0133807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vitali S. D. et al. National Koala Disease Risk Analysis Report V 1.2. National Koala Disease Risk Analysis Report V 12 2023.
- 7.Jelocnik M., Gillett A., Hanger J. & Polkinghorne A. Chlamydiosis in koalas. In: Vogelnest L., Portas T., Vogelnest L., Portas T., eds Current Therapy in Medicine of Australian Mammals. C S I R O Publishing; 2019:495-505.
- 8.Polkinghorne, A., Hanger, J. & Timms, P. Recent advances in understanding the biology, epidemiology and control of chlamydial infections in koalas. Review. Vet. Microbiol.165, 214–223 (2013). 10.1016/j.vetmic.2013.02.026 [DOI] [PubMed] [Google Scholar]
- 9.Robbins, A., Loader, J., Timms, P. & Hanger, J. Optimising the short and long-term clinical outcomes for koalas (Phascolarctos cinereus) during treatment for chlamydial infection and disease. PLoS One13, e0209679 (2018). 10.1371/journal.pone.0209679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillett A. & Hanger J. Koala. In: Vogelnest L., Portas T., Vogelnest L., Portas T., eds Current Therapy in Medicine of Australian Mammals. C S I R O Publishing; 2019:463-487.
- 11.Phillips, S., Quigley, B. L. & Timms, P. Seventy years of Chlamydia vaccine research - Limitations of the past and directions for the future. Review. Front. Microbiol.10, 70 (2019). 10.3389/fmicb.2019.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quigley, B. L. & Timms, P. The koala immune response to chlamydial infection and vaccine development—advancing our immunological understanding. Review. Animals11, 380 (2021). 10.3390/ani11020380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desclozeaux, M. et al. Immunization of a wild koala population with a recombinant Chlamydia pecorum Major Outer Membrane Protein (MOMP) or Polymorphic Membrane Protein (PMP) based vaccine: New insights into immune response, protection and clearance. PLoS ONE12, e0178786 (2017). 10.1371/journal.pone.0178786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan, S. A. et al. Humoral immune responses in koalas (Phascolarctos cinereus) either naturally infected with Chlamydia pecorum or following administration of a recombinant chlamydial major outer membrane protein vaccine. Vaccine34, 775–782 (2016). 10.1016/j.vaccine.2015.12.050 [DOI] [PubMed] [Google Scholar]
- 15.Quigley, B. L. et al. Reduction of Chlamydia pecorum and Koala Retrovirus subtype B expression in wild koalas vaccinated with novel peptide and peptide/recombinant protein formulations. Vaccine: X14, 100329 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waugh, C. et al. A prototype recombinant-protein based Chlamydia pecorum vaccine results in reduced chlamydial burden and less clinical disease in free-ranging koalas (Phascolarctos cinereus). PLoS ONE11, e0146934 (2016). 10.1371/journal.pone.0146934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robbins, A., Hanger, J., Jelocnik, M., Quigley, B. L. & Timms, P. Longitudinal study of wild koalas (Phascolarctos cinereus) reveals chlamydial disease progression in two thirds of infected animals. Sci. Rep.9, 13194 (2019). 10.1038/s41598-019-49382-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan, S. A. et al. Antibody and cytokine responses of koalas (Phascolarctos cinereus) vaccinated with Recombinant Chlamydial Major Outer Membrane Protein (MOMP) with two different adjuvants. PLoS ONE11, e0156094 (2016). 10.1371/journal.pone.0156094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. A brief history of vaccines World health organisation. 2023. https://www.who.int/news-room/spotlight/history-of-vaccination/a-brief-history-of-vaccination.
- 20.Knight-Jones, T. J. D., Edmond, K., Gubbins, S. & Paton, D. J. Veterinary and human vaccine evaluation methods. Review. Proc. R. Soc. B: Biol. Sci.281, 20132839 (2014). 10.1098/rspb.2013.2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Guidelines for Management of Disease in Free-ranging Australian Wildlife, (2020).
- 22.Barnett, K. M. & Civitello, D. J. Ecological and Evolutionary Challenges for Wildlife Vaccination. Trends Parasitol.36, 970–978 (2020). 10.1016/j.pt.2020.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Craig, A. P. et al. A 5-year Chlamydia vaccination programme could reverse disease-related koala population decline: Predictions from a mathematical model using field data. Vaccine32, 4163–4170 (2014). 10.1016/j.vaccine.2014.05.049 [DOI] [PubMed] [Google Scholar]
- 24.Rhodes, J. R. et al. Using integrated population modelling to quantify the implications of multiple threatening processes for a rapidly declining population. Biol. Conserv.144, 1081–1088 (2011). 10.1016/j.biocon.2010.12.027 [DOI] [Google Scholar]
- 25.Beyer, H. L. et al. Management of multiple threats achieves meaningful koala conservation outcomes. J. Appl. Ecol.55, 1966–1975 (2018). 10.1111/1365-2664.13127 [DOI] [Google Scholar]
- 26.Kollipara, A. et al. Vaccination of healthy and diseased koalas (Phascolarctos cinereus) with a Chlamydia pecorum multi-subunit vaccine: Evaluation of immunity and pathology. Vaccine30, 1875–1885 (2012). 10.1016/j.vaccine.2011.12.125 [DOI] [PubMed] [Google Scholar]
- 27.R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2018. https://www.R-project.org/.
- 28.ggsurvfit: Flexible Time-to-Event Figures. 2023. https://github.com/pharmaverse/ggsurvfit.
- 29.Therneau T. M., & Grambsch P. M. The Cox Model. Modeling Survival Data: Extending the Cox Model. Springer Link.
- 30.Therneau T., Crowson C., & Atkinson E. Using Time Dependent Covariates and Time Dependent Coe cients in the Cox Model. 2023. https://cran.r-project.org/web/packages/survival/vignettes/timedep.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data is available within the manuscript and supplementary files.