Abstract
This article comprehensively reviews how cerebral hypoxia impacts the physiological state of neurons and dendritic spines through a series of molecular changes, and explores the causal relationship between these changes and neuronal functional impairment. As a severe pathological condition, cerebral hypoxia can significantly alter the morphology and function of neurons and dendritic spines. Specifically, dendritic spines, being the critical structures for neurons to receive information, undergo changes such as a reduction in number and morphological abnormalities under hypoxic conditions. These alterations further affect synaptic function, leading to neurotransmission disorders. This article delves into the roles of molecular pathways like MAPK, AMPA receptors, NMDA receptors, and BDNF in the hypoxia-induced changes in neurons and dendritic spines, and outlines current treatment strategies. Neurons are particularly sensitive to cerebral hypoxia, with their apical dendrites being vulnerable to damage, thereby affecting cognitive function. Additionally, astrocytes and microglia play an indispensable role in protecting neuronal and synaptic structures, regulating their normal functions, and contributing to the repair process following injury. These studies not only contribute to understanding the pathogenesis of related neurological diseases but also provide important insights for developing novel therapeutic strategies. Future research should further focus on the dynamic changes in neurons and dendritic spines under hypoxic conditions and their intrinsic connections with cognitive function.
Keywords: Cerebral hypoxia, Neuron, Dendritic spines
Introduction
Cerebral hypoxia is a condition that profoundly affects brain function and structure, with its impact extending beyond the brain itself to the entire central nervous system (Verma et al. 2024). The brain is a highly oxygen-dependent organ, and when hypoxia occurs, it triggers a series of complex events that ultimately impact the health and survival of neurons, as well as the integrity of dendritic spines (Hours et al. 2023). Dendritic spines, the tiny protrusions on neurons, are responsible for receiving signals from other neurons (Priel et al. 2022). Their morphological and density changes can provide some insights into synaptic function and neuronal connectivity.
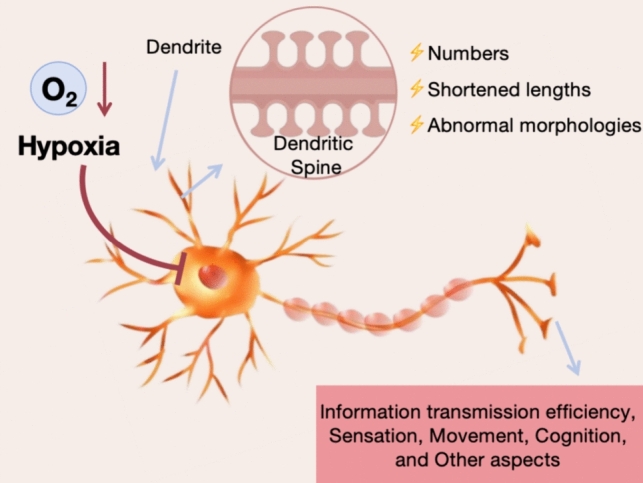
Under hypoxic conditions, abnormal dendritic spine structure becomes a common phenomenon, manifesting as decreased numbers, shortened lengths, and abnormal morphologies (Gao et al. 2021). These structural abnormalities further affect synaptic function, leading to impaired neural signal transmission and disrupted neuron generation and integration, thereby closely linking them to neuropathological processes in various diseases (Taylor et al. 2023a, b). Hippocampal CA1 neurons are particularly sensitive to cerebral hypoxia, exhibiting a delayed and selective pattern of damage. In damaged neurons, the injury first appears in the apical dendrites and gradually spreads throughout the entire neuron, highlighting the fragility of the apical dendrites (Virga et al. 2023; Merino-Serrais et al. 2023). This damage not only affects the structure and function of neurons but also leads to impeded information transmission, further compromising cognitive function (Fig. 1).
Fig. 1.
Structural abnormalities of neuronal dendritic spines under hypoxic conditions and their impact on cognitive function
In this review, we have conducted an in-depth exploration of the molecular mechanisms underlying the changes in neurons and dendritic spines induced by cerebral hypoxia. Specifically, we have emphasized elucidating the causal relationship between the molecular alterations triggered by hypoxia and the physiological impacts on neurons and synapses.
Molecular Mechanisms Underlying Hypoxia-Induced Changes
Hypoxia has profound effects on the brain, particularly on neurons and dendritic spines. The mechanisms underlying these changes are complex and involve multiple molecular pathways. A better understanding of these mechanisms can aid in the development of effective treatment strategies to mitigate the harmful effects of hypoxia and promote neuronal recovery.
MAPK
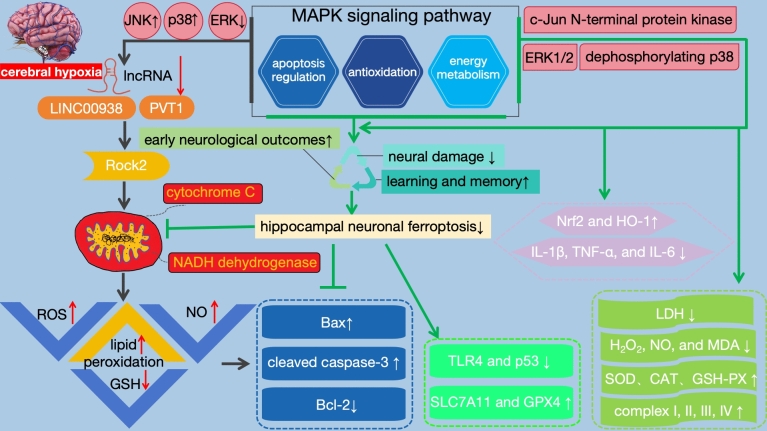
In the complex pathological process of cerebral hypoxia diseases, the MAPK signaling pathway plays a crucial role (Fig. 2). As a central regulator, it not only modulates the apoptosis process of nerve cells but also affects key biological mechanisms such as antioxidant defense systems and energy metabolism, thereby exerting protective effects on nerve cells under cerebral hypoxic conditions. Notably, recent studies have increasingly focused on the regulatory role of lncRNAs in the MAPK pathway. For instance, lncRNAs such as LINC00938 and PVT1 have been found to finely regulate the activity of the MAPK signaling pathway. Under cerebral hypoxic conditions, the expression levels of these lncRNAs often decrease, and this downregulation is closely associated with increased neuronal apoptosis and significantly elevated oxidative stress levels. Specifically, lncRNAs can target and regulate the level of Rock2. When lncRNA expression is downregulated, the activity of Rock2 may be affected, leading to a series of adverse consequences, such as ROS accumulation, increased NO production, accelerated lipid peroxidation, reduced GSH synthesis, and mitochondrial dysfunction (Zhang et al. 2021a, b). These processes not only exacerbate the oxidative stress state of nerve cells but also potentially lead to disorders in cellular energy metabolism, further promoting neuronal apoptosis. Further research has revealed that downregulation of lncRNA expression leads to upregulated expression of Bax and cleaved caspase-3, while the expression of Bcl-2 is suppressed. This change triggers or accelerates the apoptotic process of nerve cells, resulting in the loss of nerve cells and impairment of brain function. Additionally, lncRNAs exert neuroprotective effects by inhibiting the phosphorylation of JNK and p38 MAPK. When lncRNA expression decreases, the phosphorylation levels of JNK and p38 MAPK increase, activating these kinases and promoting neuronal apoptosis. Simultaneously, the inhibitory effect of lncRNAs on the ERK signaling pathway is weakened, further exacerbating neuronal damage (Zhao et al. 2023). Therefore, in-depth research on the regulatory role and mechanisms of lncRNAs in cerebral hypoxia diseases is of significant importance for developing new treatment strategies and drugs.
Fig. 2.
Regulatory role and neuroprotective mechanisms of MAPK signaling pathway in cerebral hypoxic diseases
The activation of the MAPK pathway has demonstrated significant effects in the treatment of cerebral hypoxia injury. This pathway activation significantly alleviates neural damage caused by cerebral hypoxia, improves early neurological outcomes, and has a positive impact on learning and memory functions. The key to this protective mechanism lies in the regulation of multiple crucial biological processes by the MAPK pathway. Firstly, the MAPK pathway exerts its anti-apoptotic effect by regulating the expression of Bcl-2 family proteins. Specifically, it promotes the expression of Bcl-2 while reducing the expression of Bax and caspase-3. This regulatory mechanism helps maintain the survival state of neural cells and reduces the occurrence of apoptosis. Additionally, the MAPK pathway also mitigates inflammatory responses and apoptosis by downregulating the expression of TLR4 and p53. TLR4 is an important inflammatory signaling receptor, while p53 is a crucial regulator of apoptosis. By decreasing the expression of these two factors, the MAPK pathway inhibits the occurrence of inflammatory responses and apoptosis, thereby protecting neural cells from damage. In protecting mitochondria from injury, the MAPK pathway also plays an important role. It enhances the antioxidant capacity of mitochondria by upregulating the expression of SLC7A11 and GPX4 (Zhu et al. 2021). Simultaneously, this pathway inhibits the release of mitochondrial cytochrome C and prevents hippocampal neuronal ferroptosis, further protecting neural cells from injury.
Apart from the aforementioned functions, the MAPK pathway also enhances cellular antioxidant capacity by upregulating the expression of Nrf2 and HO-1. Nrf2 and HO-1 are two crucial antioxidant enzymes that eliminate oxidative stress products within cells, protecting them from oxidative damage. Additionally, the MAPK pathway downregulates the expression levels of inflammatory factors such as IL-1β, TNF-α, and IL-6, reducing the LDH activity and the release of oxidative stress products like H2O2, NO, and MDA caused by hypoxic injury. This enhancement improves the activities of SOD, CAT, GSH-PX, and complexes I, II, III, IV, activates NADH dehydrogenase and cytochrome C oxidase, thus strengthening the cellular antioxidant and anti-inflammatory capabilities (Jing et al. 2022). These actions collectively enhance the antioxidant and anti-inflammatory abilities of cells, contributing to the protection of neural cells from damage.
In terms of enhancing cell viability, the MAPK pathway achieves this by activating extracellular signal-regulated kinases ERK1/2 and c-Jun N-terminal protein kinase, as well as dephosphorylating p38MAPK. The activation and dephosphorylation of these kinases regulate intracellular signaling pathways, promoting cell survival and proliferation. Finally, the MAPK pathway also enhances cellular energy metabolism and antioxidant stress capability by upregulating the expression of PGC-1α and SIRT1. PGC-1α and SIRT1 are two vital regulatory factors that participate in cellular energy metabolism and antioxidant stress responses. By increasing the expression levels of these two factors, the MAPK pathway safeguards cells from oxidative cell toxicity. Studies have confirmed that hydrogen can mitigate ischemic brain injury by regulating the MAPK/HO-1/PGC-1α pathway, providing a new direction for the treatment of related diseases in the future (Wang et al. 2020a, b).
AMPA Receptors
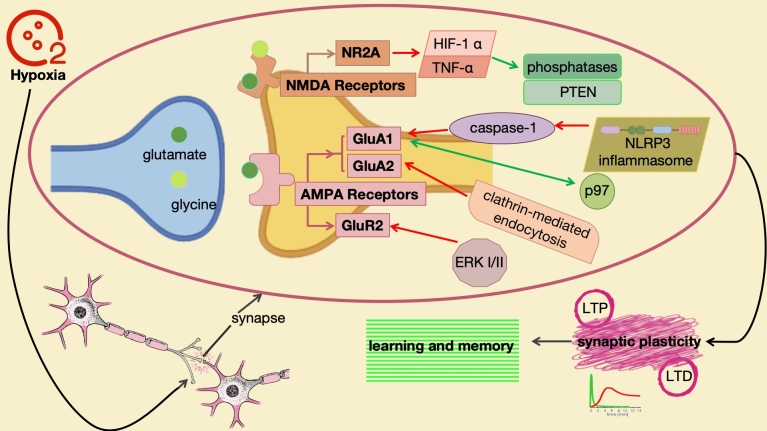
AMPA receptors, as members of the excitatory glutamate receptor family, play a crucial role in the function of the nervous system. These receptors influence neuronal and dendritic spine transmission through their specific subunits, particularly GluA1 and GluA2 (Chen et al. 2020). In neural synapses, AMPA receptors are primarily responsible for rapid excitatory synaptic transmission, where the expression of GluA1 subunit on the postsynaptic membrane is pivotal for maintaining the excitatory state of neurons. However, under pathological conditions of cerebral hypoxia, the balance of the nervous system is disrupted. During this state, the NLRP3 inflammasome is abnormally activated, triggering a cascade reaction of caspase-1. This series of biochemical events leads to a significant reduction in the protein level of GluA1 subunit in AMPA receptors, further affecting the function of neurons and dendritic spines (Roberts et al. 2023). Since the expression of GluA1 on the postsynaptic membrane is crucial for neuronal excitability, its reduced level directly impacts the normal function of neurons, including advanced neural activities such as learning and memory.
Under normal conditions, the expression and localization of GluA1 are precisely regulated by the nervous system. However, in the case of cerebral hypoxia, this regulatory mechanism is disrupted, leading to impaired neuronal function. To address this challenge, researchers have proposed a series of strategies. One approach involves inhibiting the NLRP3 inflammasome signaling pathway to prevent the activation of caspase-1, thereby preventing the reduction of GluA1 levels. Another strategy is to enhance the interaction between p97 and GluA1, which helps restore the expression and localization of GluA1 on the postsynaptic membrane, promoting the recovery of the nervous system. In addition to these strategies, the non-competitive, selective AMPA receptor antagonist Perampanel has also demonstrated a protective effect against hypoxia-induced brain injury. Through pretreatment, Perampanel can reduce the activity of the AMPA glutamate excitatory receptor GluA1 subtype in hippocampal pyramidal neurons, thus activating synaptic plasticity during the critical period of LTP (Heit et al. 2021). This finding provides new insights for the treatment of cerebral ischemia injury. Furthermore, research on the GluA2 subunit of AMPA receptors has also revealed its specific role under cerebral hypoxia conditions. The GluA2 subunit participates in persistent synaptic depression through clathrin-mediated endocytosis. However, compared to the GluA1 subunit, GluA2 exhibits differences in internalization responses and regulatory mechanisms (Chen et al. 2014). These differences provide a deeper understanding of the mechanisms of AMPA receptors under cerebral ischemia conditions.
GluR2, an important subtype of AMPA receptors, plays a crucial role in neural signal transmission and synaptic function regulation. Specifically, under cerebral hypoxia conditions, the activity of GluR2 in AMPA receptors is significantly enhanced. This augmentation not only directly affects synaptic plasticity, referring to the efficiency and flexibility of information transmission between neurons, but also further impacts the normal function of neurons. Specifically, the increased activity of GluR2 may lead to excessive excitation of synaptic transmission, interfering with the normal electrical activity of neurons and even causing neuronal damage. Additionally, the enhanced activity of GluR2 is closely related to the activation of oxidative stress. Oxidative stress is a physiological response generated by cells in response to external stress, but excessive oxidative stress can lead to cell damage. Under cerebral hypoxia conditions, the excessive activation of GluR2 may exacerbate this process, further aggravating neuronal injury. Meanwhile, the increase in ERK I/II proteins is considered one of the downstream effects of GluR2 activity enhancement (Cui et al. 2020). ERK I/II are key enzymes involved in various cellular functions, including cell proliferation, differentiation, and apoptosis. In the nervous system, ERK I/II also participates in many important biological processes. However, when GluR2 is excessively active under cerebral hypoxia conditions, it may lead to abnormal activation of ERK I/II, further affecting the normal function of neurons.
However, these seemingly adverse biological processes are not irreversible. Recent studies have shown that certain natural compounds and drugs possess the ability to regulate these processes. For instance, the intervention of icariin can effectively modulate the GluR2/ERK I/II pathway, thus mitigating the impact of cerebral hypoxia on GluR2, protecting neurons, and improving cognitive function (Guo et al. 2020). Apart from icariin, other drugs also demonstrate similar potential. For example, fingolimod, an anti-inflammatory and neuroprotective agent, can restore the imbalance in gene expression of excitatory and inhibitory receptors, including key receptors such as the AMPA receptor GluR2 subunit, NMDA receptor NR2A subunit, and GABA receptor γ2 subunit (Hajipour et al. 2023). These findings provide strong support for the therapeutic potential of icariin and fingolimod in diseases such as cerebral ischemia.
NMDA Receptors
Like AMPA receptors, NMDA receptors are also excitatory glutamate receptors that play a pivotal role in neuronal communication, synaptic plasticity, and advanced neural activities such as learning and memory (Fig. 3). Specifically, under cerebral hypoxia conditions, the functional changes of NMDA receptors and their impacts on related biological processes have become a focal point of research (Devereaux et al. 2023). During cerebral hypoxia, glutamatergic drive during respiration significantly increases. This change not only reflects the self-regulatory mechanism of the nervous system in response to hypoxic stress, but also reveals the close connection between the glutamatergic system and respiratory neural plasticity. However, with the enhancement of glutamatergic drive, there is a decreasing trend in respiratory neural plasticity, further suggesting the potential threat of cerebral hypoxia to neural system function. Research has found that cerebral hypoxia not only upregulates the GluR2 subunit of AMPA receptors but also affects the NR2A subunit of NMDA receptors. This impact is not limited to the expression levels of receptor subunits but also involves related signaling pathways and molecular mechanisms. For instance, cerebral hypoxia can lead to elevated levels of HIF-1α and TNF-α, while reducing the levels of phosphatases and PTEN (Wang et al. 2021). These changes not only reveal the direct impact of cerebral hypoxia on NMDA receptor function but also suggest its potential role in neurological diseases.
Fig. 3.
Functional and regulatory mechanisms of AMPA and NMDA receptors under cerebral hypoxia conditions
NMDA receptors play a significant role in the regulation of synaptic plasticity and sensitivity to redox status (Arias-Cavieres et al. 2021). Synaptic plasticity serves as the foundation for learning and memory in the nervous system, and NMDA receptors are crucial participants in this process. NMDA receptor-dependent LTP and LTD are two crucial manifestations of synaptic plasticity (Yao et al. 2021). However, it is noteworthy that cerebral hypoxia can inhibit both forms of plasticity (Bueschke et al. 2023). This inhibition not only affects the efficiency of neuronal information transmission but may also adversely impact learning and memory functions. Furthermore, NMDA receptors are highly sensitive to changes in redox status. Under cerebral hypoxia conditions, the redox balance within the nervous system is disrupted, leading to the generation of oxidative stress. This oxidative stress not only affects the normal function of neurons but may also have a direct impact on NMDA receptors. For instance, cerebral hypoxia can lead to a reduction in the expression of GluN1, GluN2A, and GluN2B subunits in the hippocampus (Zhang et al. 2023a, b, c). These subunits are the primary components of NMDA receptors, and their reduction not only affects the function of NMDA receptors but may further exacerbate neuronal damage (Zhang et al. 2023a, b, c).
The roles and characteristics of NMDA receptors and AMPA receptors in the nervous system exhibit significant differences (Hofmann et al. 2024). These disparities are not only reflected in their activation mechanisms, but also include their sensitivity to redox status and the roles they play in synaptic plasticity. These unique characteristics enable these two receptors to play distinct roles in the normal functioning of neurons as well as in pathological states.
First, from the perspective of activation mechanisms, there are significant differences between AMPA receptors and NMDA receptors. AMPA receptors primarily respond rapidly to glutamate, rapidly opening ion channels within milliseconds to mediate fast excitatory synaptic transmission. This rapid response makes AMPA receptors play a crucial role in neural information transmission. In contrast, NMDA receptors require the combined action of glutamate and glycine for activation. More specifically, NMDA receptors are also influenced by membrane potential depolarization, only being fully activated and participating in slow excitatory synaptic transmission when the postsynaptic neuron depolarizes to a certain extent. This activation mechanism enables NMDA receptors to play a more complex and intricate role in neural information transmission (Yıldızhan and Nazıroğlu 2023).
Secondly, in terms of sensitivity to redox status, NMDA receptors are more sensitive compared to AMPA receptors. Redox status is an essential parameter of the intracellular environment, crucial for maintaining the normal function of neurons. Under pathological conditions such as cerebral hypoxia, when the redox status is imbalanced, the function of NMDA receptors is more vulnerable to impairment. Such impairment may lead to changes in synaptic plasticity and a decline in cognitive function. In contrast, although AMPA receptors may also be influenced by redox status, their sensitivity is lower, enabling them to maintain a certain level of function even during redox imbalance.
In terms of synaptic plasticity, AMPA receptors and NMDA receptors also play distinct roles. AMPA receptors primarily participate in basic synaptic transmission, ensuring the basic communication function between neurons. In contrast, NMDA receptors are more involved in synaptic plasticity, such as LTP and LTD. These processes are crucial for learning and memory, affecting neuronal connectivity and information processing capabilities by altering the strength and efficiency of synapses. Therefore, NMDA receptors play an indispensable role in the learning and memory functions of the nervous system.
BDNF
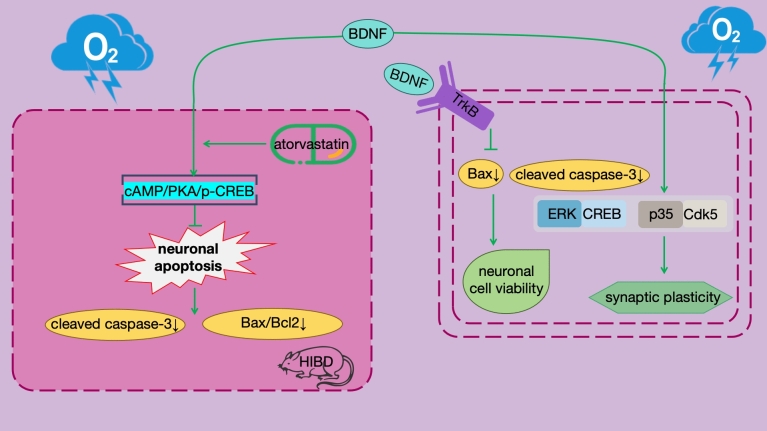
BDNF plays a pivotal role in HIBD. BDNF, a protein widely present in the nervous system, is crucial for the growth, development, differentiation, and survival of neurons. Specifically in HIBD, BDNF exerts profound effects on neuronal apoptosis by regulating the specific signaling pathway, namely the cAMP/PKA/p-CREB signaling pathway (Fig. 4). cAMP, as a second messenger, transmits signals within the cell, activating PKA. The activated PKA then phosphorylates CREB, converting it into p-CREB, which initiates a series of gene expressions related to neuronal survival. In this process, BDNF, as a key member of these genes, is regulated by p-CREB. When BDNF expression increases, it can inhibit neuronal apoptosis through a series of downstream effects. Experimental studies have shown that in the HIBD rat model, activating the BDNF pathway can significantly reduce the degree of cerebral infarction and improve learning and memory function. This effect is closely related to the downregulation of neuronal apoptosis-related indicators such as cleaved caspase-3 and Bax/Bcl-2 expression by BDNF. However, when cAMP or BDNF is inhibited, its anti-apoptotic effect is significantly reduced. This further underscores the importance of BDNF in the neuroprotection of HIBD. Therefore, strategies targeting the regulation of the BDNF pathway have become potential approaches for the treatment of HIBD.
Fig. 4.
Neuroprotective effects of BDNF regulation in HIBD
In recent years, the application of atorvastatin in the treatment of HIBD has attracted widespread attention. Atorvastatin, a commonly used lipid-lowering drug, has been found to possess neuroprotective effects. By activating the cAMP/PKA/p-CREB/BDNF pathway, atorvastatin can inhibit neuronal apoptosis, providing new ideas and methods for the treatment of HIBD (Yu et al. 2022). Furthermore, studies have reported the role of silenced long non-coding antisense RNA BDNF and overexpressed BDNF-engineered mesenchymal stem cells in reducing the severity of HIBD. These studies not only offer new insights into the mechanisms of BDNF in neuroprotection but also provide strong support for the development of novel therapeutic strategies (Qiao et al 2020; Ahn et al. 2021).
BDNF has long been a focus of attention in the field of neuroscience, and its crucial role in HIBD has been widely recognized. However, as research progresses, we are gradually discovering that BDNF also plays a pivotal role in a wider range of hypoxia-related neurological diseases. In complex environments such as acute normobaric hypoxia, neurological damage caused by COVID-19, and high-altitude hypoxia, BDNF actively participates in the protection of neurons and dendritic spines as well as the regulation of functional recovery. These findings provide new insights into our understanding of the protective mechanisms of BDNF in neurological diseases (Fan et al. 2023).
Under cerebral hypoxia conditions, the expression levels of BDNF and its receptor TrkB are significantly increased, a change crucial for the survival and functional recovery of neurons (Asgarzadeh et al. 2022). The mechanism underlying this increase in expression may be attributed to BDNF's ability to inhibit the expression of apoptosis-related proteins such as Bax and cleaved caspase-3, effectively reducing neuronal apoptosis and enhancing cell viability (Zhai et al. 2022). This process serves as an important self-protection mechanism of the nervous system in response to hypoxic stress. In addition to directly inhibiting the expression of apoptosis-related proteins, BDNF also regulates neuronal synaptic plasticity and cognitive function by influencing the activity of downstream signaling pathways such as ERK/CREB and p35/Cdk5 (Tao et al. 2022). The activation of these signaling pathways is significant for neuronal growth, development, and repair, contributing to the functional recovery of the nervous system in a hypoxic environment. However, it is worth noting that although BDNF expression levels increase under hypoxic conditions, its neuroprotective effects may not always be directly correlated with its elevated levels. For instance, in studies on acute normobaric hypoxia, no significant association was found between elevated BDNF levels and decreased executive function in young males (Chroboczek et al. 2022). This finding suggests that the mechanisms and effects of BDNF may vary under different hypoxic environments and disease conditions, requiring further in-depth research.
Furthermore, recent studies have revealed that regulatory factors such as miR-126 and miR-210 play significant roles in BDNF-mediated neuroprotection. These regulatory factors influence the expression and activity of BDNF, thereby modulating neuronal survival and function. For example, when EPC-EXs and NPCs-EXs are combined with miR-126 and miR-210 overexpression, they can mimic the neuroprotective effects of BDNF and exert protective effects on neurons through the Nox2/ROS/TrkB signaling pathway (Xu et al. 2023a, b). This discovery further emphasizes the central role of BDNF in neuronal protection and provides new insights for developing novel therapeutic approaches.
Overall, MAPK, AMPA receptors, NMDA receptors, and BDNF play crucial neuroprotective roles in hypoxia-related neurological diseases by regulating neuronal survival, functional recovery, and synaptic plasticity. However, the specific mechanisms and effects may vary depending on the hypoxic environment and disease conditions. Therefore, further exploration is needed in future studies to delve into the specific mechanisms and therapeutic effects of MAPK, AMPA receptors, NMDA receptors, and BDNF under different hypoxic environments and disease settings.
Cerebral Hypoxia and Neuronal Function
Following cerebral hypoxic injury, neuronal structures undergo a series of complex changes, with particularly notable losses of dendrites and their spinous structures (Fişek et al. 2023). Dendrites serve as crucial sites for neurons to receive information, and their structural integrity is essential for neural signal transmission (Wang et al. 2023a, b, c, d). Changes in dendritic structure caused by hypoxic injury not only affect the normal function of neurons but also lead to dysfunction in sensation, movement, and cognition, among other aspects (Wang et al. 2023a, b, c, d). Therefore, exploring effective methods to promote the recovery of dendritic structure, particularly the regeneration and stabilization of dendritic spines, after hypoxic injury holds significant academic and practical value for neural function recovery.
The Harmful Effects of Cerebral Hypoxia on Neurons
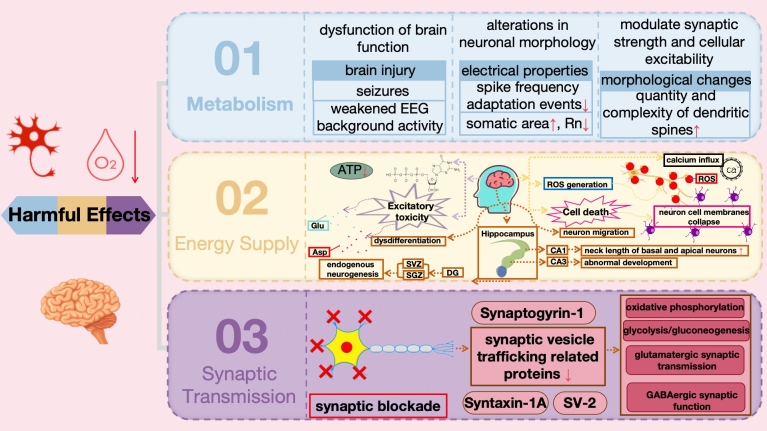
The brain, as the most energy-consuming organ, is extremely sensitive to energy homeostasis in terms of neuronal growth (Vinopal et al. 2023). Under pathological conditions such as stroke, neurons suffer apoptosis and necrosis due to insufficient blood supply (Nakamura et al. 2023). Hypoxia, a state triggered by various conditions including stroke, heart attack, respiratory failure, and high-altitude exposure, profoundly affects neuronal function (Hou et al. 2023). Neurons, also known as nerve cells, are the fundamental units of the nervous system's structure and function, interconnecting to form neural pathways for the transmission of electrical or chemical signals (Jékely 2021). Cerebral hypoxia impacts neuronal metabolism, energy supply, and synaptic transmission (Fig. 5) (Luo et al. 2023; Hu et al. 2023; Skórkowska et al. 2024).
Fig. 5.
Harmful effects of cerebral hypoxia on neurons
Under hypoxic conditions, neurons may adjust their metabolic pathways, inducing abnormal brain function, such as brain injury including seizures and weakened EEG background activity (Primiani et al. 2023). Moreover, hypoxia can also induce various cellular morphological changes, thereby reshaping neuronal excitability and function. The complexity of dendrites has a significant impact on the electrical properties of cells. For example, in pyramidal cells, the high complexity of dendrites is associated with changes in neuronal activity, manifesting as a reduction in spike frequency adaptation events. Additionally, the increase in somatic area is associated with a decrease in neuronal Rn (Qiao et al. 2023). Furthermore, the increase in dendritic complexity and the number of nodes is closely related to the formation of dendritic spines, which are special compartments of excitatory synapses that can regulate synaptic strength and thus increase cellular excitability. Some scholars have confirmed that the effects of continuous hypoxia on rectus abdominis motor neurons are mainly manifested as hyperexcitability and morphological changes in neurons (da Silva et al. 2019). Specifically, continuous hypoxia enhances neuronal synaptic strength, mainly reflected in discharge frequency and EPSCs, and induces tonic active neuronal morphological changes, particularly an increase in the number and complexity of dendritic spines. Such changes help neurons maintain appropriate respiratory drive under hypoxic conditions. Other studies have shown that after brief hypoxia in newborn rats, the development of dendritic and dendritic spines in brain neurons is affected, leading to abnormal synaptic activity and memory impairment. This research provides a new perspective for exploring the effects of cerebral hypoxia and neuronal plasticity (Tang et al. 2020).
Under hypoxic conditions, the transition from oxygen-dependent aerobic metabolism to anaerobic metabolism to generate energy affects the ionic balance and excitability of neurons, resulting in the inhibition of neuronal discharge activities and ATP production (Ban et al. 2024). As the primary energy source for neurons, ATP depletion leads to the loss of cell membrane ion gradients, subsequently triggering cellular dysfunction and death. Additionally, hypoxia triggers the release of excitatory neurotransmitters (such as glutamate and aspartate), potentially inducing excitotoxicity and exacerbating neuronal damage (Poli et al. 2024). Hypoxic brain injury initiates a series of complex cascade reactions, with the initial disruption of electrophysiological activities within the brain. Subsequent calcium influx induces calcium-dependent excitotoxicity, accompanied by the production of large amounts of ROS, ultimately leading to cell membrane disruption and cell death (Lou et al. 2024). Studies have shown that hypoxic brain injury significantly promotes endogenous neurogenesis in the SVZ and SGZ of the hippocampal DG, as well as increases the neck length of basal and apical neurons in the hippocampal CA1 region and leads to abnormal development in the CA3 region. These regions are the main gathering places for NPCs in the adult brain. However, despite the migration of newly generated neurons to ischemic lesions such as the striatum and granular layer of the hippocampus to replace damaged neurons, the proportion of them differentiating into mature neurons is quite limited. This suggests that hypoxia not only directly damages neurons but also poses significant obstacles to the regeneration and differentiation processes of neurons. Therefore, the relationship between hypoxia and neuronal function is not only reflected in direct damage effects but also lies in its profound impact on the mechanisms of neuronal regeneration and differentiation.
Additionally, hypoxia can lead to alterations in synaptic transmission and impairments in neuronal communication (Zhang et al. 2024). Brain hypoxic injury significantly disrupts spontaneous synaptic activities in neurons, particularly in hippocampal CA1 pyramidal cells, where a short-term "synaptic blockade" phenomenon is observed, causing downregulation of proteins related to synaptic vesicle trafficking, such as Syntaxin-1A, Synaptogyrin-1, and SV-2. Besides the interference with synaptic vesicle cycling, this injury can profoundly impact various biological processes including oxidative phosphorylation, glycolysis/gluconeogenesis, glutamatergic synaptic transmission, and GABAergic synaptic function (Li et al. 2023a, b). These changes manifest as reduced amplitude and frequency of miniature EPSCs in hippocampal CA1 neurons, weakened glutamatergic and GABAergic receptor currents, decreased cofilin-1, hindered cytoskeletal rearrangement, and impaired neurotransmitter transmission. These alterations have profound effects on the behavioral and cognitive functions of the nervous system (Lippman-Bell et al. 2021). Hypoxic injury may also lead to modifications of NMDA receptors in the brain, affecting signal transduction from BLA neurons to IL via presynaptic TrkB receptors, resulting in morphological changes in the developing brain, such as reduced dendritic length in the hippocampus, severe impairments in spatial memory, decreased brain growth, ventriculomegaly, cortical generation impairment, and reduced volumes of subcortical white matter, corpus callosum, and cortex. Additionally, there is downregulation of Nogo-A, an inhibitor of myelin-associated axonal regeneration (Morin et al. 2021; Sanchez-Bezanilla et al. 2020).
The Beneficial Effects of Cerebral Hypoxia on Neurons
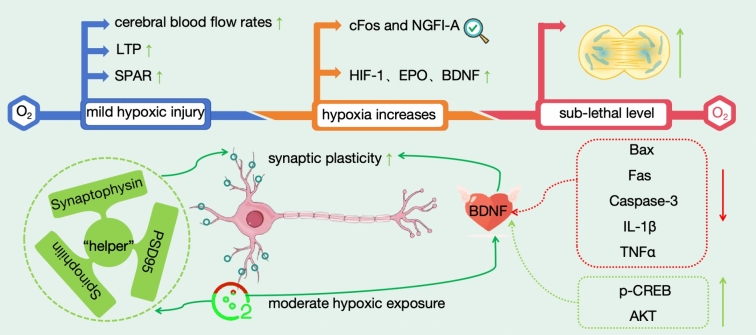
Although hypoxic injury has deleterious effects, moderate hypoxic exposure can induce neuronal protection and cell survival, promote neural plasticity, and complement synaptic plasticity (Fig. 6). "Moderate hypoxic exposure" refers to exposing cells or tissues to a relatively hypoxic environment for a specific duration, in which the level of oxygen deprivation is moderate enough to induce cellular protection and survival without causing fatal damage. This state encompasses both the degree and the duration of the hypoxic exposure. Brief hypoxia, as a preconditioning stimulus, can enhance neuronal synaptic plasticity, significantly influencing synaptic plasticity-related proteins such as Synaptophysin, Spinophilin, and PSD95. This maintains normal neural network excitability, enhances the survival rate of specific neuronal populations, and improves the tolerance of GABAergic neurons to hypoxia, thereby promoting cellular adaptation and survival (Lechner et al. 2021). Studies have shown that under hypoxic conditions in the brain, overexpression of BDNF significantly enhances the effects of brief hypoxic preconditioning, boosts the tolerance of GABAergic neurons to hypoxia, and significantly increases the expression of anti-apoptotic genes such as Stat3, Socs3, and Bcl-xl. This enhances cerebrovasodilation and reduces blood viscosity. Additionally, BDNF overexpression can reduce the expression of pro-apoptotic and pro-inflammatory genes such as Bax, caspase-3, Fas, IL-1β, and TNFα, and increase the levels of AKT and CREB phosphorylation (Turovskaya et al. 2020).
Fig. 6.
Beneficial effects of cerebral hypoxia on neurons
When the brain sustains mild hypoxic injury, it initiates a series of neuroprotective mechanisms. For instance, by upregulating cerebral blood flow rates, the brain ensures adequate oxygen and nutrient supply to neurons, maintaining their normal functions (Liu et al. 2021). Furthermore, this hypoxic stimulus can enhance learning and memory capabilities by potentiating LTP—a phenomenon of synaptic transmission enhancement. Numerous genes regulate LTP, including Akt1, Arc, Bdnf, Creb1, Gria1, Grin2a, Grin2b, and Mapk1. The mRNA expression levels of these LTP-regulating genes are crucial for memory and learning processes (Sanchez-Brualla et al. 2023). Additionally, SPAR, which is associated with dendritic spines, is activated, aiding in the modulation of neuronal morphology and function, thereby strengthening neuronal resilience (Ying et al. 2023).
As the severity of hypoxia increases, the brain further adjusts its response mechanisms. At this stage, the brain is capable of correcting the abnormal expression of certain genes, such as cFos and NGFI-A, which can lead to neuropathological outcomes during severe hypoxia. By rectifying these aberrant expressions, the brain mitigates the damage to neurons caused by hypoxia and maintains their normal function (Yang et al. 2024a, b, c). Additionally, moderate hypoxia may induce the expression of genes associated with neuronal survival, protection, and transportation, such as HIF-1, EPO, and BDNF. The expression of these genes aids neurons in better adapting and surviving in hypoxic environments (Richter et al. 2022). Research has confirmed that HIF-1α-induced upregulation of the m6A reader IGF2BP1 promotes the recovery of peripheral nerve injury by enhancing the stability of SLC7A11 mRNA (An et al. 2023).
When hypoxia reaches a sub-lethal level, the brain employs more extreme measures to safeguard neurons from apoptosis or necrosis. In such circumstances, the brain enhances neuronal viability by promoting cellular mitotic activity (Chao et al. 2020). Mitosis is the process of cells replicating themselves, and by increasing mitotic activity, the brain can facilitate neuronal regeneration and repair, thereby averting cell apoptosis or necrosis (Sandhu et al. 2022). This protective mechanism serves as the brain's final defense line under extreme hypoxic conditions (D'aes et al. 2023).
The Role of Astrocytes and Microglia Under Hypoxic Conditions
Under hypoxic conditions, the stability and recovery capability of the nervous system are severely challenged, wherein astrocytes and microglia play crucial roles (Xin et al. 2023). In this complex physiological environment, astrocytes and microglia, with their unique biological characteristics and functions, are indispensable in protecting neuronal and synaptic structures, regulating their functions, and participating in the repair process after injury.
Firstly, as significant support cells in the nervous system, astrocytes exhibit neuroprotective properties in hypoxic environments. They buffer toxic substances released by neurons, such as excitatory amino acids and K+, and utilize Na+-dependent glutamate transporters (e.g., GLAST and GLT-1) on the cell membrane to remove glutamate from the extracellular space, significantly reducing the excitatory toxicity of glutamate outside the cell (Hsu et al. 2022). This provides robust protection for neurons, effectively preventing them from further damage. Additionally, astrocytes secrete protective neurotrophic factors, such as BDNF, which have significant effects on neuronal survival and synaptic function (Zhang et al. 2021a, b). However, it is noteworthy that under extreme hypoxic conditions, astrocytes may undergo transformation, releasing toxic cytokines that damage neurons, depending on the severity and duration of hypoxia. Studies have shown that regulating the activation state of astrocytes can delay the onset of seizures and respiratory arrest induced by hypoxia, further emphasizing their crucial role under hypoxic conditions.
Concurrently, microglia also demonstrate unique value under hypoxic conditions (Yu et al. 2024). As immune cells in the central nervous system, microglia play a crucial role in maintaining vascular integrity. In hypoxic environments, the absence of microglia disrupts the vascular coupling of astrocytes, leading to increased vascular leakage and affecting the neuronal environment. Furthermore, microglia regulate immune responses to influence neuronal survival and function, although the specific mechanisms are not fully understood. After hypoxia, microglia may participate in neuronal protection and regeneration processes, promoting neuronal survival and regeneration by clearing damaged neurons and synaptic debris. Additionally, microglia secrete various growth factors, such as NGF and BDNF, which are significant for promoting neuronal survival and regeneration, providing a favorable environment for neuronal survival (Zhou et al. 2020).
In summary, astrocytes and microglia play important protective and regulatory roles in neuronal and synaptic structures under hypoxic conditions. They alleviate hypoxia-induced neuronal damage and promote neuronal survival and regeneration through their unique mechanisms. Therefore, in the research and treatment of hypoxia-related neurological diseases, we must fully recognize the significant roles and potential value of these two glial cells to provide new ideas and methods for disease prevention and treatment.
Impact on Dendritic Spines
The impact of hypoxia on dendritic spines is profound and complex, altering not only their morphology, number, density, and plasticity but also the structure and function of neurons, thereby exerting adverse effects on the normal operation of the brain. Therefore, a thorough investigation of the effects of hypoxia on dendritic spines and their underlying mechanisms holds significant importance for understanding the pathogenesis of neurocognitive disorders and other neurological diseases, as well as for developing effective treatment strategies.
The Developmental Impact of Hypoxia on Dendritic Spines
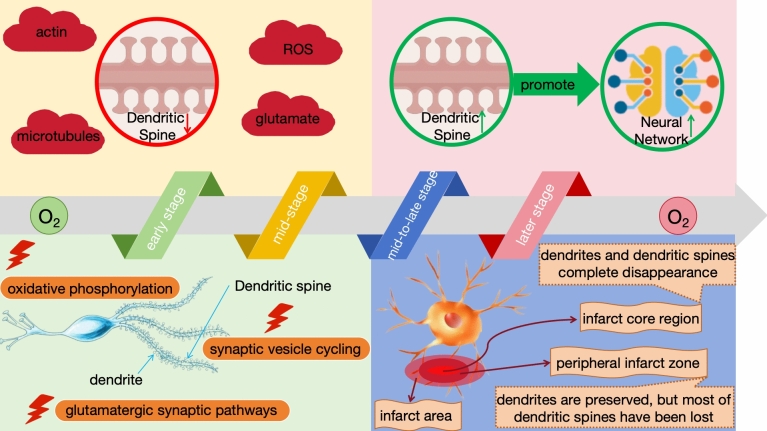
Recent studies have demonstrated that hypoxic environments significantly impact the morphology, number, and density of dendritic spines, while the adaptive adjustments of dendritic spines may also contribute to the recovery and remodeling of neural networks (Kang et al. 2020). Under hypoxic conditions in the brain, dendritic spines undergo a series of morphological changes, thereby affecting their density and directly or indirectly influencing neuronal functions such as information transmission efficiency and cognitive abilities (Zhuravin et al. 2019a, b). Hypoxia, as a common physiological and pathological state, has a pronounced impact on the development of dendritic spines (Fig. 7) (Zhuravin et al. 2019a, b).
Fig. 7.
Effects of hypoxia on dendritic spine development
During the early stages of hypoxia, subtle swelling occurs in dendritic shafts, manifesting as a beaded-like alteration that persists. Concurrently, the length and width of dendritic spines gradually increase to accommodate the neural signaling demands in a hypoxic environment. This compensatory mechanism aims to enhance synaptic transmission efficiency in response to hypoxia (Turlova et al. 2023). However, excessive spine elongation and compensation may lead to the formation of abnormal neural circuits. These changes are closely associated with abnormalities in oxidative phosphorylation, synaptic vesicle cycling, and glutamatergic synaptic pathways. The accumulation of glutamate within cells is further linked to neurocognitive dysfunction, such as cognitive impairment (Sanmarco et al. 2023).
During the mid-stage of hypoxia, dendrites in the core infarct area undergo severe damage, manifesting as notable fragmentation and degeneration. This results in a significant reduction in the integrity and quantity of dendritic spines. Such damage may indicate a weakening of synaptic connections, profoundly affecting neural signal transmission and further disrupting neural network functions (Xu et al. 2022). The underlying mechanisms of this damage are closely related to complex factors such as energy metabolism dysfunction, enhanced oxidative stress, and the accumulation of excitatory amino acids triggered by hypoxia (Kelley et al. 2022). For instance, functional abnormalities in actin (which is externally regulated by secretory cues, contact-mediated factors, and neuronal activity) and microtubules (with Sox11, N-cadherin, Sema3E, and Rho GTPases serving as transcriptional factors) can exacerbate dendritic damage. Additionally, the increase in ROS and the overactivation of glutamate may lead to the atrophy or even disappearance of dendritic spines (Holland et al. 2024).
During the mid-to-late stage of hypoxia, different patterns of dendritic changes are observed in the infarct core region and its surrounding areas (Xu et al. 2023a, b). Specifically, dendrites in the infarct core region suffer severe and irreversible damage, leading to their complete disappearance. This phenomenon is likely closely related to severe energy metabolism dysfunction and enhanced oxidative stress in the core region (Mira et al. 2020). In the peripheral infarct zone, located around the infarct core, dendrites are preserved, but most of their dendritic spines have been lost (Giusti et al. 2019). This significantly reduces the number of synaptic connections, severely compromising neural signal transmission (Claiborne et al. 2024). Such damage may be associated with relative insufficiency in energy supply and persistent oxidative stress in this region (Harbin et al. 2023). In contrast, dendritic spines in regions far from the infarct boundary exhibit relative stability in length and density. This may be attributed to the smaller impact of hypoxia on neurons in these areas, resulting in relatively mild energy metabolism and oxidative stress responses (Dromard et al. 2021). Consequently, dendritic spines in these regions are able to maintain a certain number and structure, thereby preserving relatively normal neural signal transmission function (He et al. 2022).
In the later stage of hypoxia, the evolution of dendritic spines accelerates, undergoing a remarkable regenerative process. Over time, the number and density of dendritic spines gradually return to pre-hypoxic levels. This recovery is not only reflected in quantity, but also in the integrity of structure and function (Tang et al. 2023a, b). Stable dendritic spines significantly enhance the efficiency of neuronal communication by establishing new synaptic connections (Dubey et al. 2024). Notably, the formation and enlargement of dendritic spines play a pivotal role in this process, not only indicating the enhancement of synaptic connections, but also serving as a key mechanism for neurons to adapt to and repair hypoxic damage (Acuña-Hinrichsen et al. 2021). With the increase in the number and size of dendritic spines, the signal transmission capacity of neurons is significantly improved, and the function of neural networks gradually recovers (Gellner et al. 2022). Concurrently, dendrites themselves undergo significant remodeling, exhibiting more complex morphologies and parallel alignments with newly formed blood vessels. This facilitates the exchange of substances and energy supply between dendrites and blood vessels, further promoting the recovery and regenerative process of neurons (Yamauchi et al. 2022).
The Mechanism of Action of Postsynaptic Markers on Dendritic Spines
Dendritic spines, tiny yet crucial protrusions on neurons, exhibit diverse morphologies, including slender, mushroom, stubby, and filopodia types. They are responsible for receiving neural signals from axons and play a pivotal role in information transmission and processing within neural networks (Rasia-Filho et al. 2023). These different types of dendritic spines may exhibit distinct dynamic changes and adaptability in response to external stimuli and internal signals. Mushroom-shaped dendritic spines are characterized by their large head structures, enabling them to receive synaptic connections from multiple axons. Therefore, in response to external stimuli, mushroom-shaped dendritic spines can simultaneously integrate signals from multiple axons, increasing the diversity and complexity of signals. This characteristic makes mushroom-shaped dendritic spines play a significant role in information processing, learning, and memory. Slender dendritic spines, due to their elongated structure, enable the formation of longer connections between neurons. This feature makes them adept at long-distance transmission of neural signals. In response to external stimuli or internal signals, slender dendritic spines can quickly transmit signals from axons to dendrites or from dendrites to the cell body, achieving efficient signal transmission. Stubby dendritic spines have relatively short and stout structures, allowing them to form stable connections between neurons. In response to external stimuli, stubby dendritic spines can rapidly transmit signals to the cell body, and due to their structural stability, they can maintain connections for extended periods, ensuring stable signal transmission. Furthermore, stubby dendritic spines may also participate in local signal processing and regulation in neurons. Filopodia-type dendritic spines exhibit elongated, filamentous structures, enabling them to form extensive neural network connections. In response to external stimuli, filopodia-type dendritic spines can rapidly propagate signals throughout the network, achieving rapid signal diffusion and sharing. Additionally, due to their structural flexibility, filopodia-type dendritic spines can undergo morphological changes to adapt to different signal transmission requirements.
Specifically, dendritic spines involve critical biological processes such as the regulation of excitatory receptor-mediated currents, the conduction of postsynaptic potentials, and the diffusion of intracellular calcium ions from the spine head to the dendrite (Fernholz et al. 2024). Due to their high plasticity, dendritic spines can undergo adaptive changes in response to environmental stimuli, such as excitatory toxicity, LTP, or LTD, thereby enabling fine-tuning of neural network functions. These adaptive changes are crucial for the normal function and plasticity of the nervous system (Pérez-Acuña et al. 2023). Research has confirmed that immature and elongated dendritic spines are closely associated with brain hypoxic diseases (Taylor et al. 2023a, b). That is, under hypoxic conditions, the normal development of dendritic spines is severely disrupted, leading to the formation of unstable synapses with immature dendritic spines. These synapses lack complete postsynaptic markers, such as PSD-95 and Gephyrin, resulting in impaired neural function (Fok et al. 2024).
PSD95, as the core scaffold protein in the excitatory PSD region, is crucial for maintaining the structure and function of mature excitatory glutamatergic synaptic dendritic spines (Wang et al. 2023a, b, c, d). Under hypoxic conditions in the brain, the expression of PSD95 is significantly downregulated due to hypoxia, leading to adverse effects on the morphology of dendritic spines and cognitive function (Zhang et al. 2022a, b). Cirbp, a cold shock protein, can regulate the post-transcription of PSD95 by binding to its 3'-UTR region. In the hippocampus, overexpression of Cirbp can attenuate the negative impact of hypobaric hypoxia on PSD95 expression, thereby alleviating dendritic spine injury and cognitive developmental delay caused by hypoxia. Studies have shown that the Cirbp-PSD95 axis plays a pivotal role in protecting neurons from hypoxia-induced morphological abnormalities and cognitive defects in hippocampal dendritic spines (Zhou et al. 2021). Further investigation of this mechanism is expected to provide new strategies for the treatment of neurodegenerative diseases such as cerebral ischemia.
Gephyrin, similar to PSD95, is also a postsynaptic protein that occupies a central position in the excitatory PSD of neurons and is an indispensable component of the postsynaptic membrane (Li et al. 2024). Gephyrin closely collaborates with scaffold proteins such as PSD-95 to maintain the structural stability and functional integrity of synapses (Marcos et al. 2022). Generally speaking, brain hypoxia does not affect brain weight, brain morphology, body weight, the number of neuronal cells, or neuroglial activation. However, it can lead to increased spontaneous neurotransmitter release, which impairs coordinated synaptic activities and results in abnormal spontaneous synaptic activities (Zhang et al. 2022a, b). Hypoxia can directly interfere with the expression levels and spatial distribution of Gephyrin, thereby affecting the structure and function of dendritic spines. This is mainly manifested as a decrease in the density of dendritic spines in DG granule cells, an increase in dendritic diameter, a reduction in dendritic length, and a decrease in the width of the stratum lucidum in the hippocampal CA3 region (Rosenberg et al. 2023). Particularly, when the expression of Gephyrin is downregulated, the stability of the postsynaptic membrane is compromised, synaptic transmission efficiency is reduced, and the morphological development and plasticity of dendritic spines are disrupted. This poses a serious challenge to neuronal information transmission and cognitive function (Moreno-Jiménez et al. 2023).
Moreover, NL1, as one of the critical postsynaptic proteins, plays a vital role in regulating the structure and function of neural synapses (Zhang and Zhang. 2023). Studies have shown that NL1 can affect the efficiency of neural signal transmission by increasing the number of glutamatergic synapses (Sell et al. 2024). Additionally, NL1 can tightly bind to PSD95 and interact directly with NMDA receptors, thereby precisely regulating the transmission process of excitatory synapses (Luo et al. 2020). The two major classes of molecules, NRXs and NLs, play pivotal roles in synapse formation and stability (Ducrot et al. 2023). However, under hypoxic pathological conditions, the expression levels of NRXs decrease, while the expression of NLs increases (Yin et al. 2020). Such changes in expression patterns disrupt the original balance between inhibitory and excitatory synaptic transmission, profoundly affecting the normal function of the nervous system (Feller et al. 2023). Notably, hypoxia not only affects the expression of NRXs and NLs but also reduces the levels of PSD95, with this impact being particularly significant in the hippocampus, a brain region closely associated with advanced neural activities such as learning and memory (Wang et al. 2020a, b).
The Impact of Hypoxia on Dendritic Spine Plasticity
Hypoxia not only significantly impacts the morphology, number, and density of dendritic spines but also impairs their plasticity—the ability of dendritic spines to adjust their morphology and function in hypoxic environments, crucial for learning and memory (Fu et al. 2024). Actin exists in dendrites in two forms: G-actin and F-actin. The latter is essential for the formation, stabilization, and regulation of dendritic spines through interactions with ABPs and postsynaptic signaling molecules such as acetylcholine, glutamic acid, glycine, and GABA. Hypoxia disrupts actin dynamics, upsetting the balance of interactions between F-actin and ABPs, thereby affecting the structural stability of dendritic spines (Guo et al. 2019). This alteration weakens the mechanisms of dendritic spine formation and maintenance, and interferes with their regulatory processes (Li et al. 2021).
As the plasticity of dendritic spines underlies LTP—a crucial molecular and cellular model related to learning and memory, its impairment directly leads to the blockade of LTP, concurrently reducing AP discharge and increasing the current of sAHP channels (McClendon et al. 2019). When LTP signals are triggered, CaMKII is activated and dissociates from F-actin, resulting in decreased stability of F-actin (Tullis and Bayer 2023). Simultaneously, actin depolymerizing factor/cofilin (ADF/Cofilin) enters and severs F-actin, initiating its breakdown (Dinet and Michelot, 2023). Additionally, the decreased concentration of proteins that stabilize F-actin (e.g., Drebrin, gelsolin, villin, thymosin β4, profilin, cortactin, adducin, and α-actin) further promotes the decomposition of F-actin (Greve et al. 2024).
Once F-actin is severed, its new free ends promote the polymerization of G-actin, leading to the formation of new F-actin (Appalabhotla et al. 2023). Additionally, Aip1 and Arp2/3 complex enter the spines and bind to F-actin, promoting the branching of F-actin and resulting in a more complex F-actin structure, which contributes to the enlargement of dendritic spines (Gonzalez Rodriguez et al. 2023). Furthermore, cerebral ischemia further affects the regulation of ABPs by disrupting calcium signaling (e.g., CaM, calpains, NFAT) and intracellular pathways (e.g., cAMP-PKA pathway and MAPK pathway), exacerbating the reduction in dendritic spine plasticity (Schneider et al. 2021). Extensive research confirms that cerebral ischemia comprehensively impairs dendritic spine plasticity by affecting actin dynamics, postsynaptic signaling molecule interactions, and LTP-related mechanisms, thereby exerting profound effects on the brain's learning and memory functions.
Post-hypoxic Consequences on Structural Synaptic Changes
In recent years, with the in-depth study of neurobiological mechanisms under hypoxic conditions, we have gradually recognized the significant impact of hypoxia on the morphology, number, and density of dendritic spines, as well as its profound influence on synaptic structural changes. As the crucial nodes for information transmission between neurons, synaptic structural changes are directly linked to the function and efficiency of neural networks.
During the initial stages of hypoxia, the compensatory elongation and broadening of dendritic spines not only adapt to the needs of neural signal transmission in hypoxic environments but also trigger changes in synaptic structure. Specifically, the presynaptic membrane releases increased neurotransmitters to compensate for the decreased synaptic transmission efficiency caused by hypoxia (Turlova et al. 2023). Meanwhile, the number of receptors on the postsynaptic membrane may also change to adapt to the altered neurotransmitter concentrations. This adaptive adjustment of synaptic structure helps maintain the stability of neural networks and promotes neuronal survival.
However, as hypoxia persists, synaptic structural changes gradually become negative. During the middle stages of hypoxia, severe damage to dendrites in the core infarct area leads to the weakening and disruption of synaptic connections. This damage not only reduces the number of synapses but also alters their morphology and distribution. Some synapses may disappear completely due to the fragmentation and degeneration of dendrites, while others may lose function due to the atrophy of dendritic spines (Holland et al. 2024). This destruction of synaptic structure has profound effects on neural signal transmission, further exacerbating neurological dysfunction.
In the later stages of hypoxia, despite irreversible damage to dendrites in the core infarct area, the synaptic structure in the peri-infarct zone exhibits a degree of plasticity. Although most dendritic spines are lost, the remaining synapses may compensate for the reduction in numbers by enlarging and enhancing their functions (Claiborne et al. 2024). This plasticity of synaptic structure provides potential for neural network recovery but also poses new challenges. Excessive enlargement and enhancement of synapses may lead to the formation of abnormal neural circuits, triggering a series of neurological dysfunctions.
Finally, during the post-hypoxic period, as dendritic spines regenerate and remodel, the synaptic structure gradually returns to normal levels. Stable dendritic spines significantly enhance the efficiency of information transmission between neurons by establishing new synaptic connections. This restoration of synaptic structure is not only reflected in quantity but also in structural and functional integrity (Dubey et al. 2024). Newly formed synapses exhibit higher transmission efficiency and stronger plasticity, contributing to the recovery and remodeling of neural networks.
In conclusion, the impact of hypoxia on synaptic structure is a complex and dynamic process. From initial compensatory adjustments to middle-stage structural damage and later-stage recovery and remodeling, the changes in synaptic structure are closely related to the function and efficiency of neural networks. Therefore, in future research, we need to further explore the mechanisms of synaptic structural changes under hypoxic conditions to better understand the effects of hypoxia on the nervous system and provide new ideas and methods for the prevention and treatment of related diseases.
Therapeutic Strategies
Cerebral hypoxia exerts significant regulatory effects on the morphology and function of neurons and dendritic spines. Currently, researchers are dedicated to developing drugs that can intervene in hypoxia-induced changes, aiming to protect the integrity of neurons and dendritic spines and provide novel therapeutic strategies for the treatment of cerebral hypoxia.
| Molecular Mechanism | Drug/Technology | Cerebral hypoxia disease | Model | In vivo/In vitro | Regulatory Factors | References |
|---|---|---|---|---|---|---|
| MAPK | IHT | AD | 8-Month-old APP/PS1 mice + PAM model | In vivo + In vitro | The activation of TFEB induced by IHT is associated with the inhibition of the AKT-MAPK-mTOR pathway | Wang et al. (2023a, b, c, d) |
| HP-BMSCs | Cardiac arrest can result in cerebral ischemia–reperfusion injury and poor neurological outcomes | a cardiac arrest rat model | In vivo | HP-BMSCs attenuate brain injury by reducing the expression of HMGB1, TLR4, NF-κB p65, p38 MAPK, and JNK in the cerebral cortex | Tang et al. (2023a, b) | |
| Intermittent fasting along with hydroalcoholic extract of Centella-asiatica | ischemic stroke | sub-acute hypoxia-induced ischemic stroke in adult zebrafish | In vivo | Subacute hypoxia can promote behavioral changes, free radical production, and alterations in brain tissue oxidative stress status (SOD, GSH-Px, and LPO) in zebrafish, accompanied by mitochondrial dysfunction (complexes I, II, and IV), neuroinflammation (IL-10, IL-1β, and TNF-α), and alterations in signaling molecules (AMPK, MAPK, GSK-3β, Nrf2) | Bindal et al. (2024) | |
| Sanpian decoction | cerebral ischemia–reperfusion injury | the rat model of MCAO/R | In vivo | Sanpian decoction upregulates SIRT1 expression, downregulates p-ERK/ERK and HIF-1α levels, increases cerebral blood flow, improves neurological function, and reduces neuronal apoptosis | Yang et al. (2024a, b, c) | |
| rhEPO | TBI and delayed hypoxemia | murine model of TBI and delayed hypoxemia | In vivo | rhEPO enhances neural regeneration and repair after cerebral ischemia by activating the MAPK/CREB signaling pathway, aiming to affect neurogenesis, neuroprotection, and synaptic density following cerebral ischemia | Celorrio and Friess (2023), Celorrio et al. (2022) | |
| Naoluoxintong formula and its split prescriptions | cerebral ischemia–reperfusion | MCAO/R rats with QDBS | In vivo | Naoluoxintong formula and its split prescriptions effectively promote cerebrovascular regeneration in a rat model of cerebral ischemia reperfusion by significantly inhibiting the activity of p38 MAPK and effectively activating a series of factors closely related to angiogenesis, including VEGFA, VEGFR2, CD31, Ang1, Ang2, and Tie2 | Xiao et al. (2023) | |
| Hydrogen Gas | Neonatal HIE | a rat model of neonatal HIBI + OGD/R nerve growth factor-differentiated PC12 cells |
In vivo + In vitro |
Hydrogen attenuates hypoxic-ischemic brain injury in neonatal rats by regulating the MAPK/HO-1/PGC-1α pathway. Hydrogen activates MAPKs, leading to the induction of HO-1 expression. Subsequently, HO-1 upregulates the expression of PGC-1α and SIRT1, thereby enhancing cellular antioxidant defense capabilities and mitigating brain injury | Wang et al. (2020a, b) | |
| AMPA receptors | Ampakines | repeated hypoxic episodes | adult male Sprague–Dawley rats | In vivo | Ampakine regulates AMPA receptors through allosteric modulation, affecting the efficiency and intensity of synaptic transmission, thereby enhancing the promoting effect of hypoxia on diaphragmatic movement | Thakre and Fuller (2024) |
| two PHDIs, JNJ-4204193 and roxadustat | acute ischemic stroke | isolated rat hippocampal slices | In vitro | APHDIs regulate synaptic transmission and plasticity by influencing the quantity, trafficking, and expression of the GluA2 subunit of AMPA receptors, thereby exerting neuroprotective effects during ischemic stress | Moreton et al. (2023) | |
| Taxifolin | ischemic stroke | hippocampal cell cultures after 40 min of OGD/R | In vitro | Taxifolin potentially enhances the function of AMPA receptors by increasing the expression of genes encoding AMPA receptor subunits, thereby affecting neuronal signal transmission and excitability, while also reducing the expression of pro-oxidant enzyme NOS and pro-inflammatory cytokine IL-1β | Turovskaya et al. (2019) | |
| Icariin | neonatal epilepsy | hypoxia-induced neonatal epilepsy rats | In vivo | Icariin protects against neuronal damage and improves cognitive function in neonatal epileptic rats induced by hypoxia through modulation of the AMPA receptor GluR2/ERK I/II pathway | Guo et al. (2020) | |
| Fingolimod | neonatal epilepsy | hypoxia-induced neonatal seizure pups | In vivo | Fingolimod may affect memory function and synaptic transmission by regulating the expression or function of AMPA receptors, particularly their GluR2 subunit | Hajipour et al. (2023) | |
| NMDA receptors | Telaprevir | Ischemic Stroke | ischemic stroke mice | In vivo | Terawer alleviates cerebral ischemic injury by affecting NMDA receptors (particularly the GluN2B subunit) and inhibiting MALT1, thereby improving neural function in mice | Zhang et al. (2023a, b, c) |
| Carbamathione | stroke | PC-12 cell cultures as a cell-based model and BCAO for stroke | In vivo + In vitro | Carbamathione attenuated NMDA-mediated glutamate currents, resulting in the activation of the AKT signaling pathway. This led to an increase in the expression of cell survival biomarkers such as Hsp 27, P-AKT, and Bcl-2, as well as a decrease in the expression of cell death markers like Beclin 1, Bax, and cleaved caspase-3 | Modi et al. (2023) | |
| brain machine interface techniques | neurocognitive disorders | mice | In vivo | Training mice through brain machine interface techniques to enhance their low gamma power in local field potentials led to an increase in the transcriptional level of NMDA receptors | Shi et al. (2024) | |
| Amantadine | brain injury | SH-SY5Y and HEK293 cells | In vitro | Amantadine alleviates hypoxia-induced mitochondrial oxidative neurotoxicity, apoptosis, and inflammation by regulating NMDA receptors to reduce Ca2 + influx, and inhibiting TRPM2 and TRPV4 channels | Öcal et al. (2022) | |
| esketamine and buprenorphine | panic disorder | male Wistar rats exposed to acute hypoxia | In vivo | Esketamine and buprenorphine exhibited similar anti-panic effects in acute hypoxic rats, with esketamine potentially acting through antagonism of NMDA receptors, while the effects of buprenorphine were primarily related to its interaction with opioid receptors | Maraschin et al. (2022) | |
| Fingolimod | neonatal epilepsy | hypoxia-induced neonatal seizure pups | In vivo | Fingolimod may exert neuroprotective effects by regulating the expression or function of NMDA receptors, particularly the NR2A subunit, through mechanisms such as modulating calcium influx and altering receptor phosphorylation states | Hajipour et al. (2023) | |
| BDNF | Asiatic acid | Prenatal hypoxia | intrauterine hypoxia-exposed zebrafish | In vivo | Asiatic acid may exert neuroprotective effects by increasing the expression of BDNF, which plays a crucial role in the growth, differentiation, and survival of neurons | Ariani et al. (2023) |
| Sub-dose anesthetics combined with chloride | Cerebral ischemia-hypoxia | CCH model | In vivo | Sub-dose anesthetics combined with chloride regulators can significantly reduce hypoxic injury, improve cognitive function, decrease intracellular chloride accumulation, reduce cell death, restore the compensatory effect of GABA, and increase the expression of BDNF | Yang et al. (2024a, b, c) | |
| Pterostilbene | chronic intermittent hypoxia | CIH mouse model | In vivo | Pterostilbene alleviates chronic intermittent hypoxia-induced oxidative stress injury in neural cells by upregulating BDNF expression, modulating immune responses (increasing the levels of anti-inflammatory Th2 cells and Treg cells while decreasing the levels of proinflammatory Th1 cells and Th17 cells), and inhibiting glial cell activation through the p-ERK signaling pathway | Liu et al. (2023) | |
| Hypoxic Preconditioning | hypoxia/ischemia injury | ICR mice | In vivo | Hypoxic Preconditioning downregulates the expression of DNMT3A and DNMT3B, resulting in decreased DNA methylation levels in the BDNF gene promoter region. This reduction in methylation levels leads to upregulation of BDNF expression. The upregulated BDNF further activates the BDNF/TrkB signaling pathway, exerting positive effects on neuronal growth, differentiation, and function, ultimately promoting learning and memory capabilities in mice | Zhang et al. (2023a, b, c) | |
| A new peptide, VD11 | spinal cord injury |
PC12 cells subjected to hypoxia + rats with spinal cord injury |
In vitro + In vivo | VD11 promotes the secretion and expression of BDNF, upregulating its levels in injured spinal cords. BDNF subsequently binds to its receptors, activating downstream signaling pathways (such as AMPK and AKT signaling pathways), thereby promoting neuronal growth, differentiation, and survival. Ultimately, this leads to improved structural and functional recovery following spinal cord injury | Li et al. (2023a, b) | |
| Dexmedetomidine | HIBD in neonates | HIBD was induced in postnatal day 7 rats | In vivo | Dexmedetomidine upregulates the expression of BDNF, subsequently activating its receptor TrkB and downstream CREB signaling pathway. This series of signal transduction processes promotes hippocampal neurogenesis and affects the polarization of astrocytes, thereby alleviating neuronal damage and cognitive dysfunction caused by HIBD | Chen et al. (2024) |
Summary and Outlook
The molecular changes induced by cerebral hypoxia and their impacts on the physiology of neurons and dendritic spines represent a complex yet crucial research field. Dendritic spines, as delicate protrusions on neurons, serve as crucial sites for information reception and processing, and the synaptic structures formed on them are the core hubs for information transmission between neurons. Under hypoxic pathological conditions, the metabolism and function of neurons are profoundly affected, leading to significant alterations in synaptic structure and function. Moreover, under hypoxic conditions, astrocytes and microglia play a protective, regulatory, and reparative role in neuronal and synaptic structures. By delving into the causal relationships of these changes, we can gain a deeper understanding of the pathogenesis of related neurological diseases and provide strong support for the development of novel therapeutic strategies. Future research should further focus on the dynamic changes of neurons and dendritic spines under hypoxic conditions and their intrinsic connections with cognitive function, aiming to provide new breakthroughs in the prevention and treatment of related neurological diseases. (The complete abbreviations are shown in Table 1).
Table 1.
List of abbreviations (according to alphabetical order)
| Abbreviations | Full name |
|---|---|
| ABPs | Actin-Binding Proteins |
| AD | Alzheimer's disease |
| AMPA | α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| AP | Action potential |
| ATP | Adenosine triphosphate |
| BCAO | Bilateral carotid artery occlusion |
| BDNF | Brain-derived neurotrophic factor |
| BLA | Basolateral amygdala |
| CaM | Calmodulin |
| CaMKII | Calcium/calmodulin-dependent protein kinase II |
| CCH | Chronic cerebral hypoxia |
| CIH | Chronic intermittent hypoxia |
| Cirbp | Cold inducible RNA-binding protein |
| DG | Dentate gyrus |
| DNMT | DNA methyltransferase |
| EEG | Electroencephalogram |
| EPC | Endothelial progenitor cell |
| EPO | Erythropoietin |
| EPSCs | Excitatory postsynaptic currents |
| EXs | Exosomes |
| F-actin | Fibros actin |
| GABA | γ-aminobutyric acid |
| G-actin | Globular actin |
| GLAST | Glutamate-aspartate transporter |
| GLT-1 | Glial glutamate transporter-1 |
| GSH | Glutathione |
| HIBD | Hypoxic-ischemic brain damage |
| HIBI | Hypoxic-ischemic brain injury |
| HIE | Hypoxic-ischemic encephalopathy |
| HIF-1 | Hypoxia inducible factor-1 |
| HP-BMSCs | Hypoxic preconditioned BMSCs |
| IHT | Intermittent hypoxia therapy |
| IL | Infralimbic area |
| lncRNA | Long non-coding RNA |
| LTD | Long-term depression |
| LTP | Long-term potentiation |
| MALT1 | Mucosa-associated lymphoid tissue lymphoma translocation protein 1 |
| MAPK | Mitogen-activated protein kinase |
| MCAO/R | Middle cerebral artery occlusion/reperfusion |
| NFAT | Nuclear Factor of Activated T Cells |
| NGF | Nerve Growth Factor |
| NL1 | Neuroligin-1 |
| NLs | Neuroligins |
| NMDA | N-Methyl-d-aspartic acid |
| NO | Nitric oxide |
| NPCs | Neural progenitor cells |
| NR2A | N-methyl-d-aspartate receptor 2A subunit |
| NRXs | Neurexins |
| OGD/R | Oxygen–glucose deprivation/reperfusion |
| PHDIs | Prolyl-4-hydroxylase domain enzyme inhibitors |
| PSD | Postsynaptic density |
| PTEN | Phosphatase and tensin homologue |
| PVT1 | Plasmacytoma variant translocation 1 |
| QDBS | Qi deficiency with blood stasis |
| rhEPO | Recombinant human EPO |
| Rn | Cell input resistance |
| ROS | Reactive oxygen species |
| sAHP | Slow afterhyperpolarization |
| SGZ | Subgranular zone |
| SIRT1 | Sirtuin 1 |
| SPAR | Spine-associated Rap-specific GTPase |
| SVZ | Subventricular zone |
| TBI | Traumatic brain injurie |
| TFEB | The transcription factor EB |
Author contributions
Chao Cui, Youzhen Wei, and Qingbin Ni wrote the main manuscript text, Xue Jiang and Yumei Wang prepared figures 1–4, Chao Li and Zhaochen Lin prepared figures 5–7. All authors reviewed the manuscript.
Funding
The Project Supported by Chinese Medicine Science and Technology Project of Shandong Province (No. M-2022082) and Shandong Provincial Natural Science Foundation (No. ZR2021MH025) and Tai’an Science and Technology Innovation Development Project (No. 2023NS153).
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Competing interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Youzhen Wei, Email: wei-youzhen@163.com.
Qingbin Ni, Email: niqingbin0538@126.com.
References
- Acuña-Hinrichsen F, Covarrubias-Pinto A, Ishizuka Y, Stolzenbach MF, Martin C, Salazar P, Castro MA, Bramham CR, Otth C (2021) Herpes simplex virus type 1 neuronal infection triggers the disassembly of key structural components of dendritic spines. Front Cell Neurosci 15:580717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn SY, Sung DK, Chang YS, Sung SI, Kim YE, Kim HJ, Lee SM, Park WS (2021) BDNF-overexpressing engineered mesenchymal stem cells enhances their therapeutic efficacy against severe neonatal hypoxic ischemic brain injury. Int J Mol Sci 22(21):11395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S, Shi J, Huang J, Li Z, Feng M, Cao G (2023) HIF-1α-induced upregulation of m6A reader IGF2BP1 facilitates peripheral nerve injury recovery by enhancing SLC7A11 mRNA stabilization. In Vitro Cell Dev Biol Anim 59(8):596–605 [DOI] [PubMed] [Google Scholar]
- Appalabhotla R, Butler MT, Bear JE, Haugh JM (2023) G-actin diffusion is insufficient to achieve F-actin assembly in fast-treadmilling protrusions. Biophys J 122(18):3816–3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariani A, Khotimah H, Nurdiana N, Rahayu M (2023) Asiatic acid increased locomotor and head width by inducing brain-derivedneurotrophic factor in intrauterine hypoxia-exposed zebrafish. Open Vet J 13(10):1326–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Cavieres A, Fonteh A, Castro-Rivera CI, Garcia AJ 3rd (2021) Intermittent Hypoxia causes targeted disruption to NMDA receptor dependent synaptic plasticity in area CA1 of the hippocampus. Exp Neurol 344:113808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgarzadeh A, Fouladi N, Asghariazar V, Sarabi SF, Khiavi HA, Mahmoudi M, Safarzadeh E (2022) Serum brain-derived neurotrophic factor (BDNF) in COVID-19 patients and its association with the COVID-19 manifestations. J Mol Neurosci 72(9):1820–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban W, Jiang X, Lv L, Jiao Y, Huang J, Yang Z, You Y (2024) Illustrate the distribution and metabolic regulatory effects of pterostilbene in cerebral ischemia-reperfusion rat brain by mass spectrometry imaging and spatial metabolomics. Talanta 266(Pt 2):125060 [DOI] [PubMed] [Google Scholar]
- Bindal P, Roy K, Sarkar B, Rana N, Kapil L, Singh C, Singh A (2024) Intermittent fasting along with hydroalcoholic extract of Centella-asiatica ameliorates sub-acute hypoxia-induced ischemic stroke in adult zebrafish. Comp Biochem Physiol C 279:109871 [DOI] [PubMed] [Google Scholar]
- Bueschke N, Amaral-Silva L, Hu M, Alvarez A, Santin JM (2023) Plasticity in the functional properties of NMDA receptors improves network stability during severe energy stress. bioRxiv 16:188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celorrio M, Friess SH (2023) Chemogenetic inhibition of amygdala excitatory neurons impairs rhEPO-enhanced contextual fear memory after TBI. Neurosci Lett 804:137216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celorrio M, Rhodes J, Shumilov K, Moritz J, Xiao S, Anabayan I, Sauerbeck A, Kummer T, Friess S (2022) Recombinant human erythropoietin induces neuroprotection, activates MAPK/CREB pathway, and rescues fear memory after traumatic brain injury with delayed hypoxemia in mice. Brain Res 1795:148074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao AC, Chen CH, Wu MH, Hou BY, Yang DI (2020) Roles of Id1/HIF-1 and CDK5/HIF-1 in cell cycle reentry induced by amyloid-beta peptide in post-mitotic cortical neuron. Biochim Biophys Acta Mol Cell Res 4:118628 [DOI] [PubMed] [Google Scholar]
- Chen X, Chen A, Wei J, Huang Y, Deng J, Chen P, Yan Y, Lin M, Chen L, Zhang J, Huang Z, Zeng X, Gong C, Zheng X (2024) Dexmedetomidine alleviates cognitive impairment by promoting hippocampal neurogenesis via BDNF/TrkB/CREB signaling pathway in hypoxic-ischemic neonatal rats. CNS Neurosci Ther 30(1):e14486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Li X, Xiong Q, Du Y, Luo M, Yi L, Pang Y, Shi X, Wang YT, Dong Z (2020) Inhibiting NLRP3 inflammasome signaling pathway promotes neurological recovery following hypoxic-ischemic brain damage by increasing p97-mediated surface GluA1-containing AMPA receptors. J Transl Med 21(1):567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Xiong C, Pancyr C, Stockwell J, Walz W, Cayabyab FS (2014) Prolonged adenosine A1 receptor activation in hypoxia and pial vessel disruption focal cortical ischemia facilitates clathrin-mediated AMPA receptor endocytosis and long-lasting synaptic inhibition in rat hippocampal CA3-CA1 synapses: differential regulation of GluA2 and GluA1 subunits by p38 MAPK and JNK. J Neurosci 34(29):9621–9643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chroboczek M, Kujach S, Łuszczyk M, Grzywacz T, Soya H, Laskowski R (2022) Acute normobaric hypoxia lowers executive functions among young men despite increase of BDNF concentration. Int J Environ Res Public Health 19(17):10802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claiborne N, Anisimova M, Zito K (2024) Activity-dependent stabilization of nascent dendritic spines requires nonenzymatic CaMKIIalpha function. J Neurosci 44(2):e1393222023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Jin X, Choi DJ, Choi JY, Kim HS, Hwang DH, Kim BG (2020) Axonal degeneration in an in vitro model of ischemic white matter injury. Neurobiol Dis 134:104672 [DOI] [PubMed] [Google Scholar]
- da Silva MP, Moraes DJA, Bonagamba LGH, Mecawi AS, Varanda WA, Machado BH (2019) Hyperexcitability and plasticity induced by sustained hypoxia on rectus abdominis motoneurons. J Physiol 597(7):1935–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’aes T, Marlier Q, Verteneuil S, Quatresooz P, Vandenbosch R, Malgrange B (2023) Re-evaluating the relevance of the oxygen-glucose deprivation model in ischemic stroke: the example of Cdk inhibition. Int J Mol Sci 24(8):7009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereaux MEM, Chiasson S, Brennan KF, Pamenter ME (2023) The glutamatergic drive to breathe is reduced in severe but not moderate hypoxia in Damaraland mole-rats. J Exp Biol 226(19):246185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinet C, Michelot A (2023) pH gradients guide ADF/cofilin isoforms in pollen tubes. J Cell Biol 222(11):e202310012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromard Y, Arango-Lievano M, Fontanaud P, Tricaud N, Jeanneteau F (2021) Dual imaging of dendritic spines and mitochondria in vivo reveals hotspots of plasticity and metabolic adaptation to stress. Neurobiol Stress 15:100402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey V, Dixit AB, Tripathi M, Sarat Chandra P, Banerjee J (2024) Quantification of neuronal dendritic spine density and lengths of apical and basal dendrites in temporal lobe structures using golgi-cox staining. Methods Mol Biol 2761:57–66 [DOI] [PubMed] [Google Scholar]
- Ducrot C, de Carvalho G, Delignat-Lavaud B, Delmas CVL, Halder P, Giguère N, Pacelli C, Mukherjee S, Bourque MJ, Parent M, Chen LY, Trudeau LE (2023) Conditional deletion of neurexins dysregulates neurotransmission from dopamine neurons. Elife 12:e87902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Chen D, Wang N, Su R, Li H, Ma H, Gao F (2023) Negative relationship between brain-derived neurotrophic factor (BDNF) and attention: a possible elevation in BDNF level among high-altitude migrants. Front Neurol 14:1144959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller B, Fallon A, Luo W, Nguyen PT, Shlaifer I, Lee AK, Chofflet N, Yi N, Khaled H, Karkout S, Bourgault S, Durcan TM, Takahashi H (2023) Alpha-synuclein preformed fibrils bind to beta-neurexins and impair beta-neurexin-mediated presynaptic organization. Cells 12(7):1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernholz MHP, Guggiana Nilo DA, Bonhoeffer T, Kist AM (2024) DeepD3, an open framework for automated quantification of dendritic spines. PLoS Comput Biol 20(2):e1011774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fişek M, Herrmann D, Egea-Weiss A, Cloves M, Bauer L, Lee TY, Russell LE, Häusser M (2023) Cortico-cortical feedback engages active dendrites in visual cortex. Nature 617(7962):769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fok AHK, Huang Y, So BWL, Zheng Q, Tse CSC, Li X, Wong KK, Huang J, Lai KO, Lai CSW (2024) KIF5B plays important roles in dendritic spine plasticity and dendriticlocalization of PSD95 and FMRP in the mouse cortex in vivo. Cell Rep 43(3):113906 [DOI] [PubMed] [Google Scholar]
- Fu C, Tang H, Liu L, Huang Y, Zhou H, Huang S, Peng T, Zeng P, Yang X, He L, Xu K (2024) Constraint-induced movement therapy promotes myelin remodeling and motor function by mediating Sox2/Fyn signals in rats with hemiplegic cerebral palsy. Phys Ther 1:11 [DOI] [PubMed] [Google Scholar]
- Gao QS, Zhang YH, Xue H, Wu ZY, Li C, Zhao P (2021) Brief inhalation of sevoflurane can reduce glial scar formation after hypoxic-ischemic brain injury in neonatal rats. Neural Regen Res 16(6):1052–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellner AK, Sitter A, Rackiewicz M, Sylvester M, Philipsen A, Zimmer A, Stein V (2022) Stress vulnerability shapes disruption of motor cortical neuroplasticity. Transl Psychiatry 12(1):91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giusti L, Molinaro A, Alessandrì MG, Boldrini C, Ciregia F, Lacerenza S, Ronci M, Urbani A, Cioni G, Mazzoni MR, Pizzorusso T, Lucacchini A, Baroncelli L (2019) Brain mitochondrial proteome alteration driven by creatine deficiency suggests novel therapeutic venues for creatine deficiency syndromes. Neuroscience 409:276–289 [DOI] [PubMed] [Google Scholar]
- Gonzalez Rodriguez S, Wirshing ACE, Goodman AL, Goode BL (2023) Cytosolic concentrations of actin binding proteins and the implications for in vivo F-actin turnover. J Cell Biol 222(12):e202306036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve JN, Marquardt A, Heiringhoff R, Reindl T, Thiel C, Di Donato N, Taft MH, Manstein DJ (2024) The non-muscle actinopathy-associated mutation E334Q in cytoskeletal gamma-actin perturbs interaction of actin filaments with myosin and ADF/cofilinfamily proteins. Elife 12:93013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CY, Xiong TQ, Tan BH, Gui Y, Ye N, Li SL, Li YC (2019) The temporal and spatial changes of actin cytoskeleton in the hippocampal CA1 neurons following transient global ischemia. Brain Res 1720:146297 [DOI] [PubMed] [Google Scholar]
- Guo Y, Cai Y, Zhang X (2020) Icariin ameliorates the cognitive function in an epilepsy neonatal rat model by blocking the GluR2/ERK I/II pathway. Folia Neuropathol 58(3):245–252 [DOI] [PubMed] [Google Scholar]
- Hajipour S, Khombi Shooshtari M, Farbood Y, Ali Mard S, Sarkaki A, Moradi Chameh H, Sistani Karampour N, Ghafouri S (2023) Fingolimod administration following hypoxia induced neonatal seizure can restore impaired long-term potentiation and memory performance in adult rats. Neuroscience 519:107–119 [DOI] [PubMed] [Google Scholar]
- Harbin NH, Lustberg DJ, Hurst C, Pare J, Crotty KM, Waters AL, Yeligar SM, Smith Y, Seyfried NT, Weinshenker D, Hepler JR (2023) RGS14 limits seizure-induced mitochondrial oxidative stress and pathology in hippocampus. Neurobiol Dis 181:106128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G, Wang XY, Jia Z, Zhou Z (2022) Characterizing neurotrophic factor-induced synaptic growth in primary mouse neuronal cultures. STAR Protoc 3(1):101112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heit BS, Dykas P, Chu A, Sane A, Larson J (2021) Synaptic and network contributions to anoxic depolarization in mouse hippocampal slices. Neuroscience 461:102–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann GC, de Barcellos G, Filho P, Khodadadi F, Ostrowski D, Kline DD, Hasser EM (2024) Vagotomy blunts cardiorespiratory responses to vagal afferent stimulation via pre- and postsynaptic effects in the nucleus tractus solitarii. J Physiol 602(6):1147–1174 [DOI] [PubMed] [Google Scholar]
- Holland ED, Miller HL, Millette MM, Taylor RJ, Drucker GL, Dent EW (2024) A methodology for specific disruption of microtubules in dendritic spines. BioRxiv 4:24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou P, Fang J, Liu Z, Shi Y, Agostini M, Bernassola F, Bove P, Candi E, Rovella V, Sica G, Sun Q, Wang Y, Scimeca M, Federici M, Mauriello A, Melino G (2023) Macrophage polarization and metabolism in atherosclerosis. Cell Death Dis 14(10):691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hours CM, Gil S, Gressens P (2023) Molecular and cellular insights: a focus on glycans and the HNK1 epitope in autism spectrum disorder. Int J Mol Sci 24(20):15139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PC, Lan YJ, Chen CC, Lee LY, Chen WP, Wang YC, Lee YH (2022) Erinacine A attenuates glutamate transporter 1 downregulation and protects against ischemic brain injury. Life Sci 306:120833 [DOI] [PubMed] [Google Scholar]
- Hu H, Wu S, Lee TJ, Gusdon AM, Liu Y, Choi HA, Ren XS (2023) A novel specific aptamer targets cerebrovascular endothelial cells after ischemic stroke. Sci Rep 13(1):9990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jékely G (2021) The chemical brain hypothesis for the origin of nervous systems. Philos Trans R Soc Lond B 376(1821):20190761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing L, Huang Y, Ma H, Wang H (2022) A novel nitronyl nitroxide radical HPN-C(6) attenuates brain damage in an acute hypobaric hypoxia mouse model through inhibition of the oxidative stress. Neurosci Lett 782:136650 [DOI] [PubMed] [Google Scholar]
- Kang JJ, Fung ML, Zhang K, Lam CS, Wu SX, Huang XF, Yang SJ, Wong-Riley MTT, Liu YY (2020) Chronic intermittent hypoxia alters the dendritic mitochondrial structure and activity in the pre-Botzinger complex of rats. FASEB J 34(11):14588–14601 [DOI] [PubMed] [Google Scholar]
- Kelley C, Newton AJH, Hrabetova S, McDougal RA, Lytton WW (2022) Multiscale computer modeling of spreading depolarization in brain slices. eNeuro 9(4):10082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner CR, McNally MA, St Pierre M, Felling RJ, Northington FJ, Stafstrom CE, Chavez-Valdez R (2021) Sex specific correlation between GABAergic disruption in the dorsal hippocampus and flurothyl seizure susceptibility after neonatal hypoxic-ischemic brain injury. Neurobiol Dis 148:105222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Miramontes TG, Czopka T, Monk KR (2024) Synaptic input and Ca2+ activity in zebrafish oligodendrocyte precursor cells contribute to myelin sheath formation. Nat Neurosci 27(2):219–231 [DOI] [PubMed] [Google Scholar]
- Li N, Gao Y, Zhang Y, Deng Y (2023a) An integrated multi-level analysis reveals learning-memory deficits and synaptic dysfunction in the rat model exposure to austere environment. J Proteomics 279:104887 [DOI] [PubMed] [Google Scholar]
- Li N, Chen K, Bai J, Geng Z, Tang Y, Hou Y, Fan F, Ai X, Hu Y, Meng X, Wang X, Zhang Y (2021) Tibetan medicine Duoxuekang ameliorates hypobaric hypoxia-induced braininjury in mice by restoration of cerebrovascular function. J Ethnopharmacol 270:113629 [DOI] [PubMed] [Google Scholar]
- Li SS, Zhang BY, Yin SG, Wei ZQ, Liu NX, Li YL, Wang SY, Shi YH, Zhao J, Wang LJ, Zhang Y, Sun J, Wang Y, Yang XW (2023b) A new peptide, VD11, promotes structural and functional recovery after spinal cord injury. Neural Regen Res 18(10):2260–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman-Bell JJ, Handy M, Nieder CG, Getzfread M, Jensen FE (2021) Altered hippocampal dendritic spine maturation after hypoxia-induced seizures in neonatal rats. Mol Cell Neurosci 113:103629 [DOI] [PubMed] [Google Scholar]
- Liu D, Jiang D, Tekes A, Kulikowicz E, Martin LJ, Lee JK, Liu P, Qin Q (2021) Multi-parametric evaluation of cerebral hemodynamics in neonatal piglets using non-contrast-enhanced magnetic resonance imaging methods. J Magn Reson Imaging 54(4):1053–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Zhou P, Zhang X, Zhao D, Chen H, Hu K (2023) Pterostilbene mediates glial and immune responses to alleviate chronic intermittent hypoxia-induced oxidative stress in nerve cells. PLoS ONE 18(6):e0286686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou H, Yao J, Zhang Y, Wu X, Sun L, Wang Y, Cong D (2024) Potential effect of acupuncture on mitochondrial biogenesis, energymetabolism and oxidation stress in MCAO rat via PGC-1alpha/NRF1/TFAM pathway. J Stroke Cerebrovasc Dis 10:107636 [DOI] [PubMed] [Google Scholar]
- Luo J, Tan JM, Nithianantharajah J (2020) A molecular insight into the dissociable regulation of associative learning and motivation by the synaptic protein neuroligin-1. BMC Biol 18(1):118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q, Zheng J, Fan B, Liu J, Liao W, Zhang X (2023) Enriched environment attenuates ferroptosis after cerebralischemia/reperfusion injury by regulating iron metabolism. Brain Res Bull 203:110778 [DOI] [PubMed] [Google Scholar]
- Maraschin JC, Frias AT, Hernandes PM, Batistela MF, Martinez LM, Joca SRL, Graeff FG, Audi EA, Spera de Andrade TGC, Zangrossi H Jr (2022) Antipanic-like effect of esketamine and buprenorphine in rats exposed to acute hypoxia. Behav Brain Res 418:113651 [DOI] [PubMed] [Google Scholar]
- Marcos A, Ballesteros-Yáñez I, Castillo-Sarmiento CA, Pardo F, Roura-Martínez D, Muñoz-Rodríguez JR, Higuera-Matas A, Ambrosio E (2022) The interactions of alcohol and cocaine regulate the expression of genes involved in the GABAergic, glutamatergic and endocannabinoid systems of male and female rats. Neuropharmacology 206:108937 [DOI] [PubMed] [Google Scholar]
- McClendon E, Wang K, Degener-O’Brien K, Hagen MW, Gong X, Nguyen T, Wu WW, Maylie J, Back SA (2019) Transient hypoxemia disrupts anatomical and functional maturation of preterm fetal ovine CA1 pyramidal neurons. J Neurosci 39(40):7853–7871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino-Serrais P, Plaza-Alonso S, Hellal F, Valero-Freitag S, Kastanauskaite A, Plesnila N, DeFelipe J (2023) Structural changes of CA1 pyramidal neurons after stroke in the contralesional hippocampus. Brain Pathol 27:e13222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira RG, Lira M, Quintanilla RA, Cerpa W (2020) Alcohol consumption during adolescence alters the hippocampal response to traumatic brain injury. Biochem Biophys Res Commun 528(3):514–519 [DOI] [PubMed] [Google Scholar]
- Modi JP, Shen W, Menzie-Suderam J, Xu H, Lin CH, Tao R, Prentice HM, Schloss J, Wu JY (2023) The role of NMDA receptor partial antagonist, carbamathione, as a therapeutic agent for transient global ischemia. Biomedicines 11(7):1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Jiménez EP, Flor-García M, Hernández-Vivanco A, Terreros-Roncal J, Rodríguez-Moreno CB, Toni N, Méndez P, Llorens-Martín M (2023) GSK-3β orchestrates the inhibitory innervation of adult-born dentate granule cells in vivo. Cell Mol Life Sci 80(8):225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreton N, Puzio M, McCormack J, O’Connor JJ (2023) The effects of prolyl hydroxylase inhibition during and post, hypoxia, oxygen glucose deprivation and oxidative stress, in isolated rat hippocampal slices. Brain Res Bull 205:110822 [DOI] [PubMed] [Google Scholar]
- Morin A, Poitras M, Plamondon H (2021) Global cerebral ischemia in adolescent male Long Evans rats: Effects of vanillic acid supplementation on stress response, emotionality, and visuospatial memory. Behav Brain Res 412:113403 [DOI] [PubMed] [Google Scholar]
- Nakamura A, Sakai S, Taketomi Y, Tsuyama J, Miki Y, Hara Y, Arai N, Sugiura Y, Kawaji H, Murakami M, Shichita T (2023) PLA2G2E-mediated lipid metabolism triggers brain-autonomous neural repair after ischemic stroke. Neuron 111(19):2995-3010.e9 [DOI] [PubMed] [Google Scholar]
- Öcal Ö, Coşar A, Nazıroğlu M (2022) Amantadine attenuated hypoxia-induced mitochondrial oxidative neurotoxicity, apoptosis, and inflammation via the inhibition of TRPM2 and TRPV4 channels. Mol Neurobiol 59(6):3703–3720 [DOI] [PubMed] [Google Scholar]
- Pérez-Acuña D, Shin SJ, Rhee KH, Kim SJ, Lee SJ (2023) α-Synuclein propagation leads to synaptic abnormalities in the cortex through microglial synapse phagocytosis. Mol Brain 16(1):72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli S, Mbroh J, Baron JC, Singhal AB, Strbian D, Molina C, Lemmens R, Turc G, Mikulik R, Michel P, Tatlisumak T, Audebert HJ, Dichgans M, Veltkamp R, Hüsing J, Graessner H, Fiehler J, Montaner J, Adeyemi AK, Althaus K, Arenillas JF, Bender B, Benedikt F, Broocks G, Burghaus I, Cardona P, Deb-Chatterji M, Cviková M, Defreyne L, De Herdt V, Detante O, Ernemann U, Flottmann F, García Guillamón L, Glauch M, Gomez-Exposito A, Gory B, Sylvie Grand S, Haršány M, Hauser TK, Heck O, Hemelsoet D, Hennersdorf F, Hoppe J, Kalmbach P, Kellert L, Köhrmann M, Kowarik M, Lara-Rodríguez B, Legris L, Lindig T, Luntz S, Lusk J, Mac Grory B, Manger A, Martinez-Majander N, Mengel A, Meyne J, Müller S, Mundiyanapurath S, Naggara O, Nedeltchev K, Nguyen TN, Nilsson MA, Obadia M, Poli K, Purrucker JC, Räty S, Richard S, Richter H, Schilte C, Schlemm E, Stöhr L, Stolte B, Sykora M, Thomalla G, Tomppo L, van Horn N, Zeller J, Ziemann U, Zuern CS, Härtig F, Tuennerhoff J, PROOF investigators, (2024) Penumbral Rescue by normobaric O = O administration in patients with ischemic stroke and target mismatch proFile (PROOF): study protocol of a phase IIb trial. Int J Stroke 19(1):120–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priel A, Dai XQ, Chen XZ, Scarinci N, Cantero MDR, Cantiello HF (2022) Electrical recordings from dendritic spines of adult mouse hippocampus and effect of the actin cytoskeleton. Front Mol Neurosci 15:769725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primiani CT, Lee JK, O’Brien CE, Chen MW, Perin J, Kulikowicz E, Santos P, Adams S, Lester B, Rivera-Diaz N, Olberding V, Niedzwiecki MV, Ritzl EK, Habela CW, Liu X, Yang ZJ, Koehler RC, Martin LJ (2023) Hypothermic protection in neocortex is topographic and laminar, seizure unmitigating, and partially rescues neurons depleted of RNA splicing protein Rbfox3/NeuN in neonatal hypoxic-ischemic male piglets. Cells 12(20):2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J, Ma H, Chen M, Bai J (2023) Vitamin D alleviates neuronal injury in cerebral ischemia-reperfusion via enhancing the Nrf2/HO-1 antioxidant pathway to counteract NLRP3-mediated pyroptosis. J Neuropathol Exp Neurol 82(8):722–733 [DOI] [PubMed] [Google Scholar]
- Qiao LX, Zhao RB, Wu MF, Zhu LH, Xia ZK (2020) Silencing of long non-coding antisense RNA brain-derived neurotrophic factorattenuates hypoxia/ischemia-induced neonatal brain injury. Int J Mol Med 46(2):653–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasia-Filho AA, Calcagnotto ME, von Bohlen und Halbach O (2023) Introduction: what are dendritic spines? Adv Neurobiol 34:1–68 [DOI] [PubMed] [Google Scholar]
- Richter AE, Bekkering-Bauer I, Verkaik-Schakel RN, Leeuwerke M, Tanis JC, Bilardo CM, Kooi EMW, Scherjon SA, Bos AF, Plösch T (2022) Altered neurodevelopmental DNA methylation status after fetal growth restriction with brain-sparing. J Dev Orig Health Dis 13(3):378–389 [DOI] [PubMed] [Google Scholar]
- Roberts NS, Handy MJ, Ito Y, Hashimoto K, Jensen FE, Talos DM (2023) Anti-seizure efficacy of perampanel in two established rodent models of early-life epilepsy. Epilepsy Behav 143:109194 [DOI] [PubMed] [Google Scholar]
- Rosenberg EC, Chamberland S, Bazelot M, Nebet ER, Wang X, McKenzie S, Jain S, Greenhill S, Wilson M, Marley N, Salah A, Bailey S, Patra PH, Rose R, Chenouard N, Sun SED, Jones D, Buzsáki G, Devinsky O, Woodhall G, Scharfman HE, Whalley BJ, Tsien RW (2023) Cannabidiol modulates excitatory-inhibitory ratio to counter hippocampal hyperactivity. Neuron 111(8):1282-1300.e8 [DOI] [PubMed] [Google Scholar]
- Sanchez-Brualla I, Ghosh A, Gibatova VA, Quinlan S, Witherspoon E, Vicini S, Forcelli PA (2023) Phenobarbital does not worsen outcomes of neonatal hypoxia on hippocampal LTP on rats. Front Neurol 14:1295934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Bezanilla S, Åberg ND, Crock P, Walker FR, Nilsson M, Isgaard J, Ong LK (2020) Growth hormone promotes motor function after experimental stroke and enhances recovery-promoting mechanisms within the peri-infarct area. Int J Mol Sci 21(2):606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu JK, Sodja C, Ribecco-Lutkiewicz M, Wu YT, Ma YS, Wei YH, Sikorska M (2022) Effects of tolerance-induced preconditioning on mitochondrial biogenesis in undifferentiated and differentiated neuronal cells. Front Biosci (landmark Ed) 27(4):115 [DOI] [PubMed] [Google Scholar]
- Sanmarco LM, Rone JM, Polonio CM, Fernandez Lahore G, Giovannoni F, Ferrara K, Gutierrez-Vazquez C, Li N, Sokolovska A, Plasencia A, Faust Akl C, Nanda P, Heck ES, Li Z, Lee HG, Chao CC, Rejano-Gordillo CM, Fonseca-Castro PH, Illouz T, Linnerbauer M, Kenison JE, Barilla RM, Farrenkopf D, Stevens NA, Piester G, Chung EN, Dailey L, Kuchroo VK, Hava D, Wheeler MA, Clish C, Nowarski R, Balsa E, Lora JM, Quintana FJ (2023) Lactate limits CNS autoimmunity by stabilizing HIF-1alpha in dendritic cells. Nature 620(7975):881–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Duong TA, Metz I, Winkelmeier J, Hübner CA, Endesfelder U, Rust MB (2021) Mutual functional dependence of cyclase-associated protein 1 (CAP1) and cofilin1 in neuronal actin dynamics and growth cone function. Prog Neurobiol 202:102050 [DOI] [PubMed] [Google Scholar]
- Sell GL, Barrow SL, McAllister AK (2024) Glutamate signaling and neuroligin/neurexin adhesion play opposing roles that are mediated by major histocompatibility complex I molecules in cortical synapse formation. bioRxiv 7:20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Zhang C, Chen JF, Yao Z (2024) Enhancement of low gamma oscillations by volitional conditioning of local field potential in the primary motor and visual cortex of mice. Cereb Cortex 34(2):bhae051 [DOI] [PubMed] [Google Scholar]
- Skórkowska A, Krzyżanowska W, Bystrowska B, Torregrossa R, Whiteman M, Pomierny B, Budziszewska B (2024) The hydrogen sulfide donor AP39 reduces glutamate-mediated excitotoxicity in a rat model of brain ischemia. Neuroscience 539:86–102 [DOI] [PubMed] [Google Scholar]
- Tang J, Chen Y, Li J, Yan S, Wang Z, Deng X, Feng K, Zhang Y, Chen C, Geng H, Wang Y, Wang L (2023a) 14, 15-EET alleviates neurological impairment through maintaining mitochondrial dynamics equilibrium via AMPK/SIRT1/FoxO1 signal pathways in mice with cerebral ischemia reperfusion. CNS Neurosci Ther 29(9):2583–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Xin X, O’Connor M, Zhang N, Lai B, Man HY, Xie Y, Wei Y (2020) Transient sublethal hypoxia in neonatal rats causes reduced dendritic spines, aberrant synaptic plasticity, and impairments in memory. J Neurosci Res 98(8):1588–1604 [DOI] [PubMed] [Google Scholar]
- Tang X, Ke J, Chen F, Lin Q, You Y, Zheng N, Gong Z, Han X, Zhuang Y, Chen F (2023b) Hypoxic preconditioned mesenchymal stem cells ameliorate rat brain injury after cardiopulmonary resuscitation by suppressing neuronal pyroptosis. J Cell Mol Med 27(13):1836–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao W, Zhang X, Ding J, Yu S, Ge P, Han J, Luo X, Cui W, Chen J (2022) The effect of propofol on hypoxia- and TNF-alpha-mediated BDNF/TrkB pathway dysregulation in primary rat hippocampal neurons. CNS Neurosci Ther 28(5):761–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KR, Barron T, Hui A, Spitzer A, Yalçin B, Ivec AE, Geraghty AC, Hartmann GG, Arzt M, Gillespie SM, Kim YS, Maleki Jahan S, Zhang H, Shamardani K, Su M, Ni L, Du PP, Woo PJ, Silva-Torres A, Venkatesh HS, Mancusi R, Ponnuswami A, Mulinyawe S, Keough MB, Chau I, Aziz-Bose R, Tirosh I, Suvà ML, Monje M (2023a) Glioma synapses recruit mechanisms of adaptive plasticity. Nature 623(7986):366–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SR, Kobayashi M, Vilella A, Tiwari D, Zolboot N, Du JX, Spencer KR, Hartzell A, Girgiss C, Abaci YT, Shao Y, De Sanctis C, Bellenchi GC, Darnell RB, Gross C, Zoli M, Berg DK, Lippi G (2023b) MicroRNA-218 instructs proper assembly of hippocampal networks. Elife 12:e82729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakre PP, Fuller DD (2024) Pattern sensitivity of ampakine-hypoxia interactions for evoking phrenic motor facilitation in anesthetized rat. J Neurophysiol 131(2):216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullis JE, Bayer KU (2023) Distinct synaptic pools of DAPK1 differentially regulate activity-dependent synaptic CaMKII accumulation. Science 26(5):106723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlova E, Ji D, Deurloo M, Wong R, Fleig A, Horgen FD, Sun HS, Feng ZP (2023) Hypoxia-induced neurite outgrowth involves regulation through TRPM7. Mol Neurobiol 60(2):836–850 [DOI] [PubMed] [Google Scholar]
- Turovskaya MV, Gaidin SG, Mal’tseva VN, Zinchenko VP, Turovsky EA (2019) Taxifolin protects neurons against ischemic injury in vitro via the activation of antioxidant systems and signal transduction pathways of GABAergic neurons. Mol Cell Neurosci 96:10–24 [DOI] [PubMed] [Google Scholar]
- Turovskaya MV, Gaidin SG, Vedunova MV, Babaev AA, Turovsky EA (2020) BDNF overexpression enhances the preconditioning effect of brief episodes of hypoxia, promoting survival of GABAergic neurons. Neurosci Bull 36(7):733–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma K, Amitabh PDN, Reddy MPK, Kohli E (2024) Kynurenines dynamics in the periphery and central nervous system steers behavioral deficits in rats under hypobaric hypoxia. ACS Chem Neurosci 5:78 [DOI] [PubMed] [Google Scholar]
- Vinopal S, Dupraz S, Alfadil E, Pietralla T, Bendre S, Stiess M, Falk S, Camargo Ortega G, Maghelli N, Tolić IM, Smejkal J, Götz M, Bradke F (2023) Centrosomal microtubule nucleation regulates radial migration of projection neurons independently of polarization in the developing brain. Neuron 111(8):1241-1263.e16 [DOI] [PubMed] [Google Scholar]
- Virga DM, Hamilton S, Osei B, Morgan A, Zamponi E, Park NJ, Hewitt VL, Zhang D, Gonzalez KC, Bloss E, Polleux F, Lewis TL Jr (2023) Activity-dependent subcellular compartmentalization of dendritic mitochondria structure in CA1 pyramidal neurons. bioRxiv 26:233 [Google Scholar]
- Wang D, Gao N, Zhou T, Zhang Q, Wang J, Li A (2020a) Effect of neuroligin1 and neurexin1 on the colonic motility in a mouse model of neuronal intestinal dysplasia. Gastroenterol Res Pract 2020:9818652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Daniszewski M, Hao MM, Hernández D, Pébay A, Gleeson PA, Fourriere L (2023a) Organelle mapping in dendrites of human iPSC-derived neurons reveals dynamic functional dendritic Golgi structures. Cell Rep 42(7):112709 [DOI] [PubMed] [Google Scholar]
- Wang P, Zhao M, Chen Z, Wu G, Fujino M, Zhang C, Zhou W, Zhao M, Hirano SI, Li XK, Zhao L (2020b) Hydrogen gas attenuates hypoxic-ischemic brain injury via regulation of the MAPK/HO-1/PGC-1a pathway in neonatal rats. Oxid Med Cell Longev 2020:6978784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Tang J, Zhong M, Chen J, Li T, Dai Y (2021) HIF-1 alpha may play a role in late pregnancy hypoxia-induced autism-like behaviors in offspring rats. Behav Brain Res 411:113373 [DOI] [PubMed] [Google Scholar]
- Wang X, Xie Y, Chen G, Lu Y, Wang D, Zhu L (2023b) Intermittent hypoxia therapy ameliorates beta-amyloid pathology via TFEB-mediated autophagy in murine Alzheimer’s disease. J Neuroinflamm 20(1):240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Xie Y, Niu Y, Wan B, Lu Y, Luo Q, Zhu L (2023c) CX3CL1/CX3CR1 signal mediates M1-type microglia and accelerates high-altitude-induced forgetting. Front Cell Neurosci 17:1189348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, Zhou YN, Jiang L, Wang S, Zhu L, Zhang SS, Yang H, He Q, Liu L, Xie YH, Liang X, Tang J, Chao FL, Tang Y (2023d) Long-term voluntary exercise inhibited AGE/RAGE and microglial activation and reduced the loss of dendritic spines in the hippocampi of APP/PS1 transgenic mice. Exp Neurol 363:114371 [DOI] [PubMed] [Google Scholar]
- Xiao S, Lina W, Jianpeng HU, Limiao Z, Jin W (2023) Effect of Naoluoxintong formula and its split prescriptions on cerebral vascular regeneration in rats with the cerebral ischemia-reperfusion. J Tradit Chin Med 43(6):1140–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin W, Pan Y, Wei W, Tatenhorst L, Graf I, Popa-Wagner A, Gerner ST, Huber S, Kilic E, Hermann DM, Bähr M, Huttner HB, Doeppner TR (2023) Preconditioned extracellular vesicles from hypoxic microglia reduce poststroke AQP4 depolarization, disturbed cerebrospinal fluid flow, astrogliosis, and neuroinflammation. Theranostics 13(12):4197–4216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Han L, Wang Y, Deng D, Ding Y, Zhao S, Zhang Q, Ma L, Chen X (2023a) Prolonged anesthesia induces neuroinflammation and complement-mediated microglial synaptic elimination involved in neurocognitive dysfunction and anxiety-like behaviors. BMC Med 21(1):7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Li Q, Ke Y, Yung WH (2022) Chronic intermittent hypoxia-induced aberrant neural activities in the hippocampus of male rats revealed by long-term in vivo recording. Front Cell Neurosci 15:784045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Zhang H, Li J, Chen Y, Zhong W, Chen Y, Ma X (2023b) Combination of EPC-EXs and NPC-EXs with miR-126 and miR-210overexpression produces better therapeutic effects on ischemic stroke by protecting neurons through the Nox2/ROS and BDNF/TrkB pathways. Exp Neurol 359:114235 [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Okamoto S, Takahashi M, Koike M, Furuta T, Hioki H (2022) A tissue clearing method for neuronal imaging from mesoscopic to microscopic scales. J vis Exp 183:88 [DOI] [PubMed] [Google Scholar]
- Yang C, Wang Y, Li Y, Wang X, Hua W, Yang Z (2024a) Wang H (2024) Sub-dose anesthetics combined with chloride regulators protect the brainagainst chronic ischemia-hypoxia injury. CNS Neurosci Ther 30(2):e14379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Liu X, Zhou Y, Du L, Fu Y, Luo Y, Zhang W, Feng Z, Ge J (2024b) Mei Z (2024) Sanpian decoction ameliorates cerebral ischemia-reperfusion injury by regulating SIRT1/ERK/HIF-1α pathway through in silico analysis and experimental validation. J Ethnopharmacol 318(Pt A):116898 [DOI] [PubMed] [Google Scholar]
- Yang T, Wang Z, Li J, Shan F, Huang QY (2024c) Cerebral lactate participates in hypoxia-induced anapyrexia through its receptor g protein-coupled receptor 81. Neuroscience 536:119–130 [DOI] [PubMed] [Google Scholar]
- Yao H, Liu W, Liao H, Sheng T, Chen P, Zhou H, Pan Y, Xie J, Zhang Q, Zou Z, Chen Z (2021) Geniposide attenuates postischemic long-term potentiation via GluN2A. Pak J Pharm Sci 34(3):909–914 [PubMed] [Google Scholar]
- Yıldızhan K, Nazıroğlu M (2023) NMDA receptor activation stimulates hypoxia-induced TRPM2 channel activation, mitochondrial oxidative stress, and apoptosis in neuronal cell line: modular role of memantine. Brain Res 1803:148232 [DOI] [PubMed] [Google Scholar]
- Yin S, Bai X, Xin D, Li T, Chu X, Ke H, Han M, Chen W, Li X, Wang Z (2020) Neuroprotective effects ofthe sonic hedgehog signaling pathway in ischemic injury through promotion of synaptic and neuronal health. Neural Plast 2020:8815195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying XM, Lyu LJ, Zhang HY, Pan YS, Li SL, Li XM, Ye X, Yang C, He LL (2023) Correlation analysis of Cobb angle and linear spinous process angle in adolescent idiopathic scoliosis. Zhongguo Gu Shang 36(10):949–953 [DOI] [PubMed] [Google Scholar]
- Yu L, Huang L, Zhao Y, Liu S, Zhou R, Yue Y, Sun H, Su X, Liu Q, Li S, Ying J, Zhao F, Qu Y (2024) Atorvastatin promotes pro/anti-inflammatory phenotypic transformation of microglia via wnt/beta-catenin pathway in hypoxic-ischemic neonatal rats. Mol Neurobiol 61(6):3559–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Liu S, Zhou R, Sun H, Su X, Liu Q, Li S, Ying J, Zhao F, Mu D, Qu Y (2022) Atorvastatin inhibits neuronal apoptosis via activating cAMP/PKA/p-CREB/BDNFpathway in hypoxic-ischemic neonatal rats. FASEB J 36(4):e22263 [DOI] [PubMed] [Google Scholar]
- Zhang D, Ruan J, Peng S, Li J, Hu X, Zhang Y, Zhang T, Ge Y, Zhu Z, Xiao X, Zhu Y, Li X, Li T, Zhou L, Gao Q, Zheng G, Zhao B, Li X, Zhu Y, Wu J, Li W, Zhao J, Ge WP, Xu T, Jia JM (2024) Synaptic-like transmission between neural axons and arteriolar smooth muscle cells drives cerebral neurovascular coupling. Nat Neurosci 27(2):232–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Li M, Liang J, Li M, Sun X (2021a) Long non-coding RNA PVT1 inhibits miR-30c-5p to upregulate Rock2 to Modulate cerebral ischemia/reperfusion injury through MAPK signaling pathway activation. Mol Neurobiol 58(11):6032–6048 [DOI] [PubMed] [Google Scholar]
- Zhang S, Fu W, Jia X, Bade R, Liu X, Xie Y, Xie W, Jiang S, Shao G (2023a) Hypoxic preconditioning modulates BDNF and Its signaling through DNA methylation to promote learning and memory in mice. ACS Chem Neurosci 14(12):2320–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Zhang M (2023) Correction to: NL-1 promotes PINK1-parkin-mediated mitophagy through MitoNEET inhibition in subarachnoid hemorrhage. Neurochem Res 89:78 [DOI] [PubMed] [Google Scholar]
- Zhang W, Chen Y, Qin J, Lu J, Fan Y, Shi Z, Song X, Li C, Zhao T (2022a) Prolonged sevoflurane exposure causes abnormal synapse development and dysregulates beta-neurexin and neuroligins in the hippocampus in neonatal rats. J Affect Disord 312:22–29 [DOI] [PubMed] [Google Scholar]
- Zhang X, Peng KZ, Xu SL, Wu MX, Sun HJ, Zhao J, Yang S, Liu SJ, Lia CY, Zhang XM (2023b) The GluN2B-containing NMDA receptor alleviates neuronal apoptosis in neonatal hypoxic-ischemic encephalopathy by activating PI3K-Akt-CREB signaling pathwa. Physiol Res 72(5):669–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XLN, Yang XK, Wu CY, Yuan Y (2021b) Gastrodin exerts protective effects in reactive TNC1 astrocytes via regulation of the Notch signaling pathway. Ann Transl Med 9(24):1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tan BH, Wu S, Wu CH, Suo JL, Gui Y, Zhou CM, Li YC (2022b) Different changes in pre- and postsynaptic components in the hippocampal CA1 subfield after transient global cerebral ischemia. Brain Struct Funct 227(1):345–360 [DOI] [PubMed] [Google Scholar]
- Zhang YY, Peng JJ, Chen D, Liu HQ, Yao BF, Peng J, Luo XJ (2023c) Telaprevir improves memory and cognition in mice suffering ischemic stroke via targeting MALT1-mediated calcium overload and necroptosis. ACS Chem Neurosci 14(17):3113–3124 [DOI] [PubMed] [Google Scholar]
- Zhai Y, Liu BG, Mo XN, Zou M, Mei XP, Chen W, Huang GD, Wu L (2022) Gingerol ameliorates neuronal damage induced by hypoxia-reoxygenation via the miR-210/brain-derived neurotrophic factor axis. Kaohsiung J Med Sci 38(4):367–377 [DOI] [PubMed] [Google Scholar]
- Zhao J, Le M, Li J, Huang Q, Chen H, Zhang W, Mao H, Sun Q, Li A, Zhao Y, Yu L, Yi M, Wang J, Li X, Zhang G, Ma J, Dong X (2023) LINC00938 alleviates hypoxia ischemia encephalopathy induced neonatal braininjury by regulating oxidative stress and inhibiting JNK/p38 MAPK signaling pathway. Exp Neurol 367:114449 [DOI] [PubMed] [Google Scholar]
- Zhou D, Ji L, Chen Y (2020) TSPO Modulates IL-4-Induced Microglia/Macrophage M2 Polarization via PPAR-gamma Pathway. J Mol Neurosci 70(4):542–549 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Lu H, Liu Y, Zhao Z, Zhang Q, Xue C, Zou Y, Cao Z, Luo W (2021) Cirbp-PSD95 axis protects against hypobaric hypoxia-induced aberrant morphology of hippocampal dendritic spines and cognitive deficits. Mol Brain 14(1):129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu K, Zhu X, Sun S, Yang W, Liu S, Tang Z, Zhang R, Li J, Shen T, Hei M (2021) Inhibition of TLR4 prevents hippocampal hypoxic-ischemic injury by regulating ferroptosis in neonatal rats. Exp Neurol 345:113828 [DOI] [PubMed] [Google Scholar]
- Zhuravin IA, Dubrovskaya NM, Vasilev DS, Kozlova DI, Kochkina EG, Tumanova NL, Nalivaeva NN (2019a) Regulation of neprilysin activity and cognitive functions in rats after prenatal hypoxia. Neurochem Res 44(6):1387–1398 [DOI] [PubMed] [Google Scholar]
- Zhuravin IA, Dubrovskaya NM, Vasilev DS, Postnikova TY, Zaitsev AV (2019b) Prenatal hypoxia produces memory deficits associated with impairment of long-term synaptic plasticity in young rats. Neurobiol Learn Mem 164:107066 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.