Abstract
Rastrelliger brachysoma (Bleeker, 1851), the short mackerel, is a dietary staple and of significant economic demand in Southeast Asia and Thailand. However, the demand for short mackerel has precipitated an overfishing crisis, leading to a depletion of fish stocks. Overfishing, coupled with parasitism, may result in a decline in the population of R. brachysoma. Digenetic trematode infection is prevalent in marine fish and has a considerable impact on the overall health of the fish. Here, to identify digenetic trematodes infecting R. brachysoma, we aim to determine the identity, prevalence, and intensity of digenean infections in R. brachysoma from the Gulf of Thailand. A total of 194 short mackerel were obtained from Chon Buri Province, where digeneans were isolated and identified. The molecular identity of the digeneans was confirmed using the nuclear 28S rRNA gene. Of the 194 short mackerel, 100% were found to be infected with digeneans, comprising of Lecithocladium, Prodistomum, Opechona, and Aphanurus. Lecithocladium was the most prevalent (98%) and had the highest intensity of infection (37 mean intensity), followed by Prodistomum (75% prevalence and 17 mean intensity). Our study thus presents the first evidence of digeneans infecting the economically important short mackerel from the Gulf of Thailand. The high infection rate of digenetic trematodes may have implications on the health of R. brachysoma, further driving their population decline. These data underscore the importance of safeguarding fisheries resources in the Gulf of Thailand, and downstream conservation efforts are crucial for evidence-based management decisions to safeguard the long-term sustainability of fish resources.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00436-024-08308-9.
Keywords: Thailand, Digenea, Rastrelliger brachysoma, Molecular identification
Introduction
Rastrelliger brachysoma (Bleeker, 1851), commonly known as the short mackerel, stands as a linchpin in Southeast Asian economies, and is celebrated for its affordability and rich protein content (Viboonkit et al. 2023). Its ubiquity as a dietary staple, particularly in Thailand, has a profound economic significance, where it enjoys widespread consumer popularity (Indaryanto et al. 2015a; Koolkalya et al. 2020). Despite its ability to spawn throughout the year (Kongseng et al. 2021; Tint et al. 2020), the short mackerel experiences heightened fishing activities from January to March and June to August, signaling its intricate ecological dynamics within the Gulf of Thailand (Tint et al. 2020). This maritime expanse, particularly around Chon Buri Province, emerges as a focal point for short mackerel aggregation, serving as a vital hub for regional export (Tint et al. 2020).
However, the burgeoning demand for short mackerel has precipitated an overfishing crisis, resulting in a stark depletion of stocks in recent years (SEAFDEC 2018). Nevertheless, amidst this ecological challenge, the insidious impact of parasitism on the overall health and resilience of R. brachysoma populations may be underestimated. For example, the acanthocephalan Corynosoma strumosum was found to be associated with the fertility and population decline of the Caspian sprat (Habibi & Shamsi 2018). Additionally, adult digenetic trematodes, which are prevalent in marine fish, also wield considerable influence on fish health, with their intensity and pathogenicity dictating the severity of the affliction (Abdel-Gaber et al. 2019; Khatoon et al. 2007; Madhavi & Lakshmi 2011; Presswell et al. 2023). Noteworthy among these parasites are hemiurid trematodes such as Lecithocladium invasor and Stomachicola muraenesocis where infection can compromise the overall fitness and reproductive success of fishes (Keser et al. 2007). Lecithocladium invasor was found in the esophagus of acanthurid fishes and can penetrate the esophageal mucosa and induce nodular granulomas (Chambers et al. 2001). Increased host tissue response was also encountered when fish were infected with Stomachicola muraenesocis (Nasira et al. 1998).
While prior studies have delved into nematode infections in marine fish from the Gulf of Thailand (Nuchjangreed et al. 2006; Purivirojkul 2009), a conspicuous gap remains regarding investigating digenean, specifically in R. brachysoma. To date, there have been no reports of helminths infecting R. brachysoma in Thailand. However, parasites have been reported infecting R. brachysoma in other Southeast Asian countries. The acanthocephalan belonging to genus Echinorhynchus was found in R. brachysoma from fish markets in the Philippines (Molina 2021). Additionally, a startlingly high prevalence of the digenean Lecithocladium angustiovum (belonging in family Hemiuridae) infection in R. brachysoma was discovered in Indonesia, underscoring the urgency of similar investigations within the Gulf of Thailand’s waters (Indaryanto et al. 2015b).
In light of these considerations, our study seeks to bridge this research gap by undertaking an assessment of the prevalence and intensity of digenean trematode infections in short mackerel sourced from the Gulf of Thailand, focusing on Chon Buri Province. We aim to determine the identity of parasitic species infecting R. brachysoma and provide preliminary insights into the infection status and identity of digeneans infecting R. brachysoma from the Gulf of Thailand. The information obtained may be beneficial to safeguard fisheries resources in the region, ensuring their sustainable management in the future.
Materials and methods
Fish sample collection and parasite isolation
A total of 194 short mackerel were obtained from Ang Sila market in Chon Buri Province from May to July 2023. The short mackerels sold at the market were caught off the coast of Ang Sila, from the Gulf of Thailand. All fish were transported back to the Department of Helminthology, Faculty of Tropical Medicine, Bangkok, on ice, where the fish were weighed, measured, and morphologically identified (Supplementary File 1).
The short mackerels were dissected carefully within 48 h of collection, and the internal organs of the gastrointestinal tract (stomach, intestine, and pyloric caeca) were thoroughly examined under a stereomicroscope for the presence of digeneans. The isolated digeneans were sorted to the genus level based on their morphological characters according to the Keys to Trematoda (Bray et al. 2008; Gibson et al. 2002; Jones et al. 2005), and they were then counted and kept in 70% ethanol at − 20 °C for downstream molecular identification.
Data analysis
The data obtained (Supplementary File 1) were incorporated into Microsoft Excel, and the proportion, prevalence, and mean intensity were calculated. The formulas are provided as follows (Bush et al. 1997):
Molecular confirmation
Representative specimens were individually placed into 1.5-ml microcentrifuge tubes and washed thoroughly with sterile distilled water. Total genomic DNA was isolated from each specimen using the Geneaid genomic DNA mini kit (Geneaid Biotech Ltd., Taipei, Taiwan) following the manufacturer’s recommendations.
Polymerase chain reaction (PCR) was conducted in a T100™ thermocycler (Bio-Rad, California, USA) to amplify the partial nuclear 28S ribosomal RNA (rRNA) gene. The primers used (28S-F: 5′-AAGCATATCACTAAGCGG-3′ and 28S-R: 5′-GCTATCCTGAGGGAAACTTCG-3′) and thermocycling conditions applied follow Curran et al. (2011) (Curran et al. 2011). The 1200 base pairs (bp) amplicons were visualized on a 1% agarose gel stained with SYBR™ Safe (Thermo Fisher Scientific, MA, USA). PCR amplicons were sent for Barcode Taq sequencing (Celemics, Seoul, South Korea) performed by a commercial company.
Following sequencing, electropherograms were manually checked using BioEdit 7.0 and aligned using ClustalX 2.1 together with reference sequences obtained from the NCBI database (Thompson et al. 2002; Hall 1999). The reference sequences used are in Supplementary File 2. The aligned sequences were checked in BioEdit 7.0, and phylogenetic analysis using the neighbour-joining (NJ) and maximum likelihood (ML) method was performed with MEGA X (Kumar et al. 2018). The best-fit nucleotide substitution model with 1000 bootstrap iterations for tree topology was selected for the ML method. Echinostoma was used as outgroups to root the phylogenetic trees. The phylogenetic trees were visualized and labeled with FigTree 1.3.1 (Rambuat 2010).
Results
Infection status of Rastrelliger brachysoma with digeneans
Of the 194 R. brachysoma obtained, 100% of them were found to be infected with digeneans. Four genera, consisting of Lecithocladium, Aphanurus, Prodistomum, and Opechona, were found. Of the four genera, infection with Lecithocladium was the most prevalent (98%), followed by Prodistomum (75%), Opechona (34%), then Aphanurus (20%). In terms of the mean intensity of infection, Lecithocladium was the highest at 37 (range from 1 to 344 individuals per infected fish), followed by Prodistomum, Aphanurus, and Opechona. Among the four genera, Lecithocladium comprised the highest proportion (71%), followed by Prodistomum (26%), Opechona (1.7%), and Aphanurus (1.3%). Lecithocladium was found primarily in the stomach of the short mackerel, while Prodistomum was found in the pyloric caeca and intestine. Table 1 presents the prevalence, mean intensity, and proportion observed for the four genera.
Table 1.
Prevalence, mean intensity, and proportion of digenean infecting R. brachysoma
| Genus | Number of infected R. brachysoma | Number of individual digenean isolated | Prevalence (%) | Mean intensity (minimum–maximum)a | Proportion (%) |
|---|---|---|---|---|---|
| Lecithocladium | 192 | 7262 | 98 | 37 (1–344) | 71.0 |
| Prodistomum | 147 | 2613 | 75 | 17 (1–122) | 26.0 |
| Opechona | 67 | 183 | 34 | 2 (1–36) | 1.7 |
| Aphanurus | 39 | 134 | 20 | 3 (1–16) | 1.3 |
| Total | 194 | 10,192 |
aThe minimum to maximum number of individual parasite isolated from each infected fish
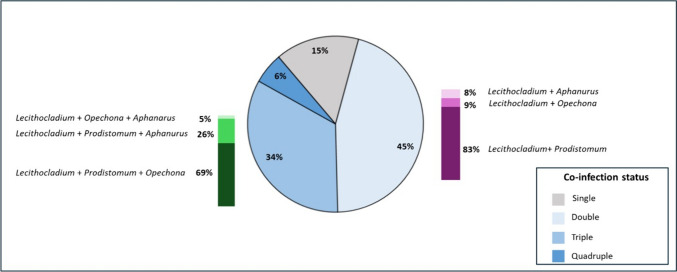
Digenetic infection with more than one genus per fish was also present, where co-infection with two and three genera was common, comprising 45% and 34% of the infected short mackerels, respectively (Fig. 1). Fifteen percent of the infected mackerels had a single infection, while 6% were co-infected with all four genera. Co-infection by Lecithocladium and Prodistomum was the most common of those infected with two genera.
Fig. 1.
Proportion of infected R. brachysoma with single and multiple infection
The pie chart depicts the co-infection status of R. brachysoma presented as percentage of fish with single, double, triple, or quadruple infection with the four digenetic genera. The purple and green stacked bar chart depicts the combination of digenetic genera infecting in R. brachysoma for double and triple infection, respectively.
Molecular confirmation using the nuclear 28S rRNA gene
Using the nuclear 28S rRNA gene, molecular results revealed congruence with the initial morphological identity of the four genera of digeneans isolated from R. brachysoma.
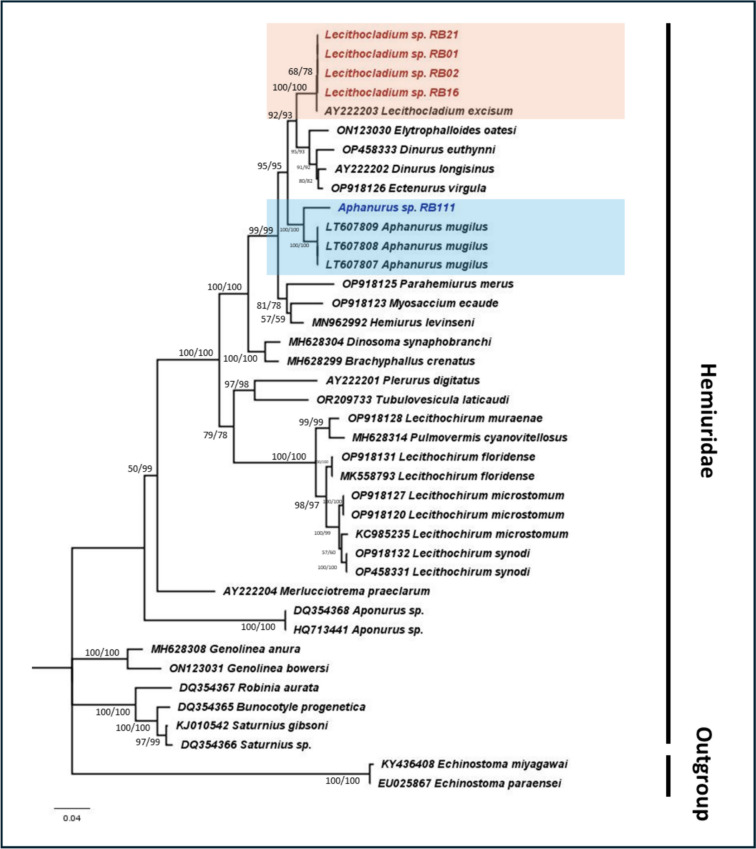
The molecular phylogeny of the family Hemiuridae, consisting of Lecithocladium and Aphanurus, revealed a monophyletic clade for Lecithocladium (our Lecithocladium specimens and Lecithocladium excisum) with strong bootstrap support. Genetic distances between our Lecithocladium specimens and L. excisum were 0.009, while no intraspecies genetic variation was observed within our specimens. Similarly, our Aphanurus specimens formed a monophyletic clade with Aphanurus mugilus, with a genetic distance of 0.041. Figure 2 presents the phylogeny of family Hemiuridae.
Fig. 2.
Maximum likelihood (GTR G + I) phylogeny of family Hemiuridae based on the partial nuclear 28S rRNA gene sequences as genetic marker
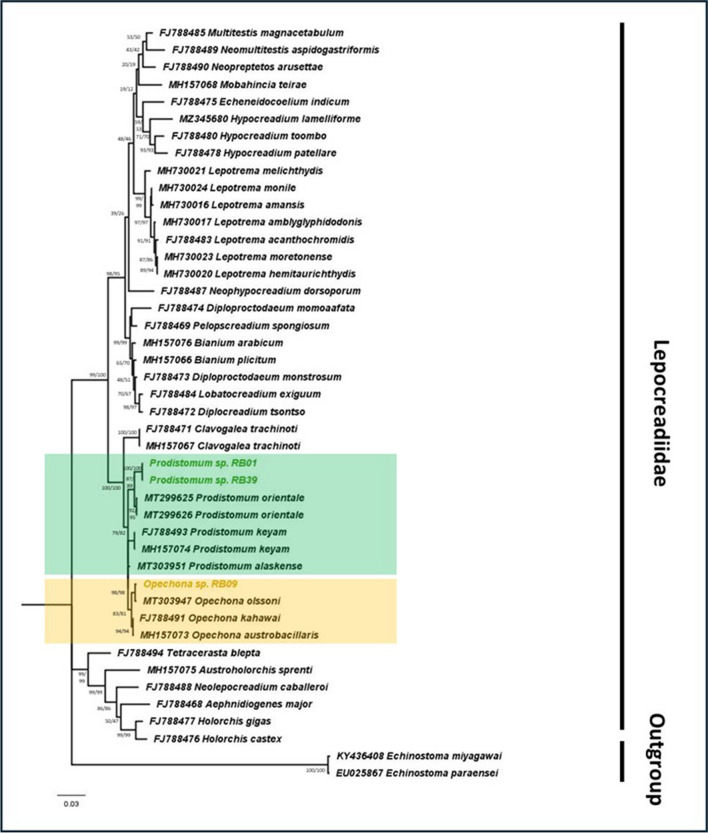
The molecular phylogeny of the family Lepocreadiidae, consisting of Prodistomum and Opechona, also supported the congruence of their morphological identity (Fig. 3). The phylogeny revealed a close relationship between our Prodistomum specimens and reference Prodistomum orientale, in which this monophyletic group formed a sister clade to Prodistomum keyam. The genetic distance between our Prodistomum specimens and P. orientale was 0.009, and no intraspecies variation was observed among our Prodistomum specimens. Similarly, our Opechona specimen clustered together with Opechona olssoni, where the genus Opechona was revealed to be monophyletic. The genetic distance between our Opechona specimen and O. olssoni was 0.001. Additionally, the sister group relationship between the two genera (Prodistomum and Opechona) was observed with the 28S rRNA gene phylogeny.
Fig. 3.
Maximum likelihood (GTR G + I) phylogeny of family Lepocreadiidae based on the partial nuclear 28S rRNA gene sequences as genetic marker
The numbers at the nodes indicate bootstrap (BS) support obtained through 1000 replications (ML/NJ). NCBI sequences are shown with their accession numbers. The representative sequences for Lecithocladium and Aphanurus are highlighted in “red” and “blue,” respectively.
The numbers at the nodes indicate bootstrap (BS) support obtained through 1000 replications (ML/NJ). NCBI sequences are shown with their accession numbers. The representative sequences for Prodistomum and Opechona are highlighted in “green” and “yellow,” respectively.
Discussion
We confirmed the presence of adult digeneans belonging to four distinct genera, highlighting the prevalence and intensity of infection within this fish population. Additionally, all the short mackerel obtained were infected with digeneans. The identity of these digeneans and their respective prevalence provides a crucial foundation regarding the infection status to understand parasitic infections in R. brachysoma, underlining the need for targeted research and management strategies to mitigate their impact.
Among the four genera identified, Lecithocladium emerged as the most prevalent, exhibiting the highest intensity of infection. This finding aligns with previous research conducted in Thailand, where Lecithocladium cristatum was isolated and identified from the black pomfret fish (Parastromateus niger) in markets in Bangkok (Oopkaew et al. 2020). The high prevalence of Lecithocladium (72%) in another fish species demonstrates the potential for cross-species transmission of digenean parasites within marine ecosystems, posing a significant threat to the health and sustainability of multiple fish species. While the presence of adult digeneans in marine fish does not directly impact human health, parasitic infections can have cascading effects on the health, quality, and market value of the fish caught. Moreover, as fishes caught from the Gulf of Thailand are transported to markets in various provinces in Thailand, the knowledge of parasitic infection in R. brachysoma may have an impact on consumer preferences, further affecting their market value of R. brachysoma. Thus, effective fisheries management and disease surveillance programs are crucial to mitigate the impact of parasitic infection in fishes (Cribb et al. 2002; Khatoon et al. 2007).
Furthermore, the pathological effects of Lecithocladium infection on the gastrointestinal tract of marine fish have been documented globally. Pathological changes, including severe inflammation, tissue atrophy, and lysis, have been observed in fish species such as Naso vlamingii infected with L. invasor and Lecithocladium chingi (Chambers et al. 2001). Similarly, observations of gastrointestinal tract damage due to Lecithocladium and Bucephalus infection in the orange-spotted trevally (Carangoides bajad) from the Red Sea highlight the broad geographic distribution and ecological impact of these parasitic infections (Bakhraibah & Bin Dohaish 2023). These findings underscore the urgent need for comprehensive surveillance and management efforts to mitigate the spread and impact of digenean parasites on marine ecosystems worldwide.
Given the high value and economic importance of short mackerel for countries bordering the Gulf of Thailand, collaborative efforts have been made to mitigate the decline of the short mackerel population (Tint et al. 2020). These efforts include the transboundary promotion of collaborative fishing management and data collection regarding the short mackerel population. Also, refugia sites were demarcated in Trat Province, aiming to mitigate the impact of short mackerel overfishing (Munprasit et al. 2022). By providing the first evidence of digenetic trematode infection in R. brachysoma, we underscore the importance of sustainably protecting this valuable fish species, as population decline may be further exacerbated by parasitic infection and overfishing. Additionally, the partial 28S rRNA gene sequences of the four genera are provided, which may facilitate future research in enhancing genetic information available for digenetic trematodes in marine fishes.
Conclusion
In conclusion, our study presents the first evidence of digeneans infecting economically important short mackerel from the Gulf of Thailand in Chon Buri Province. This information holds significance for future management strategies to protect fish resources in Thailand. However, as only one genetic marker was utilized in this study, incorporating additional genetic markers from different loci and genetic markers with better taxonomic resolution may aid in species-level identification of isolated digeneans for future investigations. Furthermore, future sampling of short mackerels from various localities along the Gulf of Thailand has the potential to expand our understanding of the parasitic infection status of R. brachysoma in Thailand. These efforts are crucial for informing evidence-based management decisions and safeguarding the long-term sustainability of marine ecosystems and fisheries resources.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We wish to acknowledge the Department of Helminthology, Faculty of Tropical Medicine, Mahidol University for technical support. Portions of this manuscript were generated with the assistance of OpenAI's ChatGPT language model (https://chatgpt.com) and subsequently reviewed by the authors.
Author contribution
W.P. and U.T. conceptualized the study; C.K., A.C., S.S., N.R., W.P., U.T. performed the methodology and formal analysis; C.K. wrote the original draft; A.C., S.S., N.R., W.P., U.T. edited and reviewed the draft. All authors read and approved the final manuscript.
Funding
Open access funding provided by Mahidol University
Data availability
All data generated during this study are included in the published article and its additional files. The newly generated sequences were deposited in the GenBank database under the accession numbers PP768936 – PP768943.
Declarations
Ethics approval
Ethical clearance was provided by the Animal Care and Use Committee, Faculty of Tropical Medicine, Mahidol University, Bangkok (No. FTM-ACUC 022/2023E).
Consent to participate and publish
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdel-Gaber R, Fouad D, Ataya F, Morsy K, Maher S (2019) Morphological identification and molecular characterization of 18S rDNA of two hemiurid trematodes (Lecithocladium cristatum Rudolphi, 1819 and Lecithocladium parviovum Yamaguti, 1953) infecting the greater lizardfish Saurida tumbil (Pisces: Synodontidae) inhabiting the Red Sea. Gene 683:243–252. 10.1016/j.gene.2018.10.041 10.1016/j.gene.2018.10.041 [DOI] [PubMed] [Google Scholar]
- Bakhraibah A, Bin Dohaish A (2023) Histopathological changes caused by parasites in Carangoides bajad fish in the Red Sea, Jeddah. Bull Univ Agric Sci Vet Med Cluj Napoca 80:16–26 [Google Scholar]
- Bray RA, Gibson DI, Jones A (2008) Keys to Trematoda:, vol 3. CABI Publishing [Google Scholar]
- Bush A, Lafferty K, Lotz J, Shostak W (1997) Parasitology meets ecology on its own terms. J Parasitol 83:575–583 10.2307/3284227 [DOI] [PubMed] [Google Scholar]
- Chambers CB, Carlisle MS, Dove ADM, Cribb TH (2001) A description of Lecithocladium invasor n.sp. (Digenea: Hemiuridae) and the pathology associated with two species of Hemiuridae in acanthurid fish. Parasitol Res 87:666–673. 10.1007/s004360100421 10.1007/s004360100421 [DOI] [PubMed] [Google Scholar]
- Cribb TH, Chisholm LA, Bray RA (2002) Diversity in the Monogenea and Digenea: does lifestyle matter? Int J Parasitol 32:321–328. 10.1016/S0020-7519(01)00333-2 10.1016/S0020-7519(01)00333-2 [DOI] [PubMed] [Google Scholar]
- Curran SS, Tkach VV, Overstreet RM (2011) Phylogenetic affinities of Auriculostoma (Digenea: Allocreadiidae), with descriptions of two new species from Peru. J Parasitol 97:661–670. 10.1645/GE-2641.1 10.1645/GE-2641.1 [DOI] [PubMed] [Google Scholar]
- Gibson DI, Jones A, Bray RA (2002) Keys to Trematoda:, vol 1. CABI Publishing [Google Scholar]
- Hall TA (1999) BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98 [Google Scholar]
- Habibi F, Shamsi S (2018) Preliminary report of occurrence of Corynosoma spp. (Acanthocephala: Polymorphidae) in Southern Caspian sprat (Clupeonella grimmi). Parasitol Res 117:3327–3331 10.1007/s00436-018-6012-6 [DOI] [PubMed] [Google Scholar]
- Indaryanto F, Imai H, Wardiatno Y (2015) Genetic variation of short body mackerel, Rastrelliger brachysoma of Jawa Island, Indonesia based on mtDNA control region sequences. AACL Bioflux 8:648–655 [Google Scholar]
- Indaryanto F, Abdullah MF, Wardiatno Y, Tiuria R, Imai H (2015) A description of Lecithocladium angustiovum (Digenea: Hemiuridae) in short mackerel, Rastrelliger brachysoma (Scombridae), of Indonesia. Trop Life Sci Res 26:31–40 [PMC free article] [PubMed] [Google Scholar]
- Jones A, Bray RA, Gibson DI (2005) Keys to Trematoda:, vol 2. CABI Publishing [Google Scholar]
- Keser R, Bray RA, Oguz MC, Celen S, Erdogan S, Doguturk S, Aklanoglu G, Marti B (2007) Helminth parasites of digestive tract of some teleost fish caught in the Dardanelles at Canakkale, Turkey. Helminthologia 44:217–221. 10.2478/s11687-007-0035-3 10.2478/s11687-007-0035-3 [DOI] [Google Scholar]
- Khatoon N, Nazia AK, Bilqees FM, Rizwana AG (2007) Effects of trematode infection on the intestinal epithelium of Pomadasys olivaceum (Day, 1875). Int J Biol Biotech 4:245–248 [Google Scholar]
- Koolkalya S, Trueman C, Sawudee A, Jutagate T (2020) Determination of short mackerel Rastrelliger brachysoma (Bleeker, 1851) stocks in the Gulf of Thailand using otolith microchemistry. AFS 33:249–257 [Google Scholar]
- Kongseng S, Phoonsawat R, Wanchana W, Swatdipong A (2021) Genetic mixed-stock analysis of short mackerel, Rastrelliger brachysoma, catches in the Gulf of Thailand: evidence of transboundary migration of the commercially important fish. Fish Res 235:105823 10.1016/j.fishres.2020.105823 [DOI] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. 10.1093/molbev/msy096 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavi R, Lakshmi T (2011) Metazoan parasites of the Indian mackerel, Rastrelliger kanagurta (Scombridae) of Visakhapatnam coast, Bay of Bengal. J Parasit Dis 35:66–74. 10.1007/s12639-011-0028-5 10.1007/s12639-011-0028-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina R (2021) Parasite survey in Rastrelliger brachysoma (short mackerel) from selected fish markets in Zamboanga City, Philippines. JJBS 14:413–416 10.54319/jjbs/140305 [DOI] [Google Scholar]
- Munprasit R, Nootmorn P, Loychuen K (2022) Fish refugia profile for short mackerel in Trat Province, Thailand. In: Establishment and operation of a regional system of fisheries refugia in the South China Sea and Gulf of Thailand. Department of Fisheries, Bangkok, Thailand
- Nasira K, Bilqees FM, Sadia H, Khatoon N, Huma S (1998) Histopathological changes caused by trematode Stomachicola muraenesocis (Yamaguti 1934) in the stomach of fish Muraenesox cinereus (Forsk 1775). Proc Pak Congr Zool 18:181–186 [Google Scholar]
- Nuchjangreed C, Hamzah Z, Suntornthiticharoen P, Sorosjinda-Nuntawarasilp P (2006) Anisakids in marine fish from the coast of Chon Buri Province, Thailand. Southeast Asian J Trop Med Public Health 37(Suppl 3):35–39 [PubMed] [Google Scholar]
- Oopkaew L, Kaewphentmadan S, Bunprasert W, Chontananarth T (2020) First report, prevalence, and molecular identification of Lecithocladium cristatum in the black pomfret fish, Parastromateus niger (Family: Carangidae). Microsc Microanal Res 33:24–27 [Google Scholar]
- Presswell B, Bennett J, Chai X, Poulin R (2023) Physical evidence of direct antagonistic interactions between trematodes in the host gut: the kiss of death? Parasitol Res 122:1953–1957. 10.1007/s00436-023-07883-7 10.1007/s00436-023-07883-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purivirojkul W (2009) An investigation of larval ascaridoid nematodes in some marine fish from the Gulf of Thailand. Kasetsart J (Nat. Sci) 43:85–92 [Google Scholar]
- Rambuat A (2010) FigTree v1.3.1. Institute of evolutionary biology, university of Edinburgh, Edingurgh. https://tree.bio.ed.ac.uk/software/figtree/. Accessed 27 Mar 2024
- SEAFDEC (2018) Fishery Statistical Bulletin of Southeast Asia 2016. Southeast Asian Fisheries Development Center, Bangkok, Thailand [Google Scholar]
- Thompson JD, Gibson TJ, Higgins D (2002) Multiple sequence alignment using ClustalW and ClustalX. Curr Protoc Bioinformatics Chapter 2:Unit 2.3. 10.1002/0471250953.bi0203s00 [DOI] [PubMed]
- Tint KK, Ngin K, Sapari A, Souliphone K, Suwannapoom S, Viron JG, Thanh VTP (2020) Enhancing the management of the Indo-Pacific mackerel resources in the Gulf of Thailand: a synthesis. Fish People 18(1):14–19 [Google Scholar]
- Viboonkit K, Boonmee W, Thotsagotphairee C, Tavitchasri P, Kanloung T (2023) Stock identification of short mackerel (Rastrelliger brachysoma) in the upper and middle Gulf of Thailand by morphological characters. Curr Appl Sci 24:e0255758 10.55003/cast.2023.255758 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated during this study are included in the published article and its additional files. The newly generated sequences were deposited in the GenBank database under the accession numbers PP768936 – PP768943.