Abstract
The cellular stress response system in immune cells plays a crucial role in regulating the development of inflammatory diseases. In response to cellular damage or microbial infection, the assembly of the NLRP3 inflammasome induces pyroptosis and the release of inflammatory cytokines. Meanwhile, Angiogenin (Ang)-mediated transfer RNA-derived small RNAs (tsRNAs) promote cell survival under stressful conditions. While both tsRNAs and inflammasomes are induced under stress conditions, the interplay between these two systems and their implications in regulating inflammatory diseases remains poorly understood. In this study, it was demonstrated that Ang deficiency exacerbated sodium arsenite-induced activation of NLRP3 inflammasome and pyroptosis. Moreover, Ang-induced 5′-tsRNAs inhibited NLRP3 inflammasome activation and pyroptosis. Mechanistically, 5′-tsRNAs recruit DDX3X protein into stress granules (SGs), consequently inhibiting the interaction between DDX3X and NLRP3, thus leading to the suppression of NLRP3 inflammasome activation. Furthermore, in vivo results showed that Ang deficiency led to the downregulation of tsRNAs, ultimately leading to an exacerbation of NLRP3 inflammasome-dependent inflammation, including lipopolysaccharide-induced systemic inflammation and type-2 diabetes-related inflammation. Altogether, our study sheds a new light on the role of Ang-induced 5′-tsRNAs in regulating NLRP3 inflammasome activation via SGs, and highlights tsRNAs as a promising target for the treatment of NLRP3 inflammasome-related diseases.

Subject terms: Cell death and immune response, Inflammasome
Introduction
Excessive activation of the inflammasome is involved in the development of several inflammatory disorders, including atherosclerosis, inflammatory bowel disease, rheumatoid arthritis, sepsis, and type-2 diabetes (T2D) [1, 2]. The inflammasome is a cytosolic multimeric protein complex composed of the nucleotide-binding domain and leucine-rich repeat containing proteins (NLRs) or AIM2, pyrin, adapter protein ASC and Caspase-1 [3]. The NLRP3 inflammasome can be triggered by a variety of stimuli, including various pathogen-associated molecular patterns (PAMPs), such as bacterial, viral and fungal infections; or endogenous damage-associated molecular patterns (DAMPs), including tissue damage, metabolic dysregulation [4]. These stimuli collectively elicited an integrated cellular stress signal. Activation of NLRP3 inflammasome triggers the Caspase-1-dependent maturation and release of several pro-inflammatory cytokines, including interleukin-1β (IL-1β) and IL-18 [5–7]. Caspase-1 can also cleave gasdermin D (GSDMD) to generate GSDMD-NT, which forms plasma membrane pores to induce pyroptosis and release pro-inflammatory cytokines [8]. However, the process by which NLRP3 inflammasome sense cellular stress and the molecules involved in this process has not been fully elucidated.
Transfer RNA-derived small RNAs (tsRNAs), also known as tRNA-derived fragments (tRFs), are a group of novel small non-coding RNAs cleaved from the anticodon loop of mature tRNAs in response to cellular stressors such as infection, tissue damage, and metabolic dysregulation [9–11]. Recent evidence indicates that tsRNAs play a role in multiple biological processes, including regulating translation [12, 13], apoptosis [14], and intergenerational phenotype transmission [15–17]. RNase A family participates in various biological actions, such as regulating intra- or extracellular RNA metabolism, as well as exhibiting antiviral, antibacterial, and antifungal activities, neurotoxicity, promoting cell proliferation, anti-apoptosis, and immunomodulatory abilities [18–20]. Angiogenin (Ang), a member of the RNase A family, cleaves mature tRNAs within the anticodon loops, producing 30–35 nt 5′-tsRNAs (also known as 5′-tRNA halves or tRNA-derived stress-induced RNAs, tiRNAs) and 40–50 nt 3′-tsRNAs [12, 21–24]. During stress, the activation of Ang facilitates the fragmentation of tRNA, resulting in an overabundance of tsRNAs, which may lead to the development of pathophysiological conditions [17, 25]. Increasing evidence indicates that tsRNAs are highly expressed in the immune system, such as hematopoietic and lymphoid tissues, compared with the other tissues [26, 27]. Furthermore, Ang-induced tsRNAs have significant regulatory roles in various pathological conditions, particularly those related to inflammatory diseases [26–32]. Although both tsRNAs and inflammasomes are induced under stress conditions, their interplay and implications for regulating inflammatory diseases are poorly understood.
Stress granules (SGs) are formed under various stress conditions, such as heat shock, oxidative stress, ischemia, and viral infection [33]. It possesses multiple functions, including blocking global translation, affecting biomolecular storage, regulating mRNA triage and expression, influencing cell signaling and apoptosis, and inhibiting viral replication [34, 35]. The dynamic assembly of SGs can be explored as a therapeutic target in multiple diseases. Researchers have proposed that Ang-induced tsRNAs may contribute to the formation of SGs in response to cellular stress. These are non-membrane-bound cytoplasmic structures that contain RNA and RNA-binding proteins (RNPs) [36–38]. Drino et al. reported that the RNA helicase DEAD-box helicase 3 X-linked (DDX3X) bind with tRNAs and related to the formation of SGs [39]. Additionally, DDX3X competitively binds to the NACHT domain of NLRP3, thereby inhibiting inflammasome activation [34, 40–42]. Therefore, DDX3X sits at a crossroad between inflammation and stress adaptation and acts as a live-or-die checkpoint in the stressed cells [34, 43, 44]. Thus, it is important to investigate whether tsRNAs regulate inflammasome activation via SGs assembly.
Sodium arsenite (hereafter referred to as Ar) is a stressor that is known to cause robust tRNA cleavage into tsRNAs via Ang and the subsequent formation of SGs [23, 33, 37, 45]. However, there are conflicting results regarding the impact of Ar on the activation of NLRP3 inflammasome [34, 46–49]. Some studies proposed that Ar can trigger pyroptosis by activating the NLRP3 inflammasome [46, 47, 49], whereas others suggested that Ar may decrease NLRP3 inflammasome activation [34, 50]. Therefore, we utilized Ar to stimulate the accumulation of tsRNAs in bone marrow-derived macrophages (BMDMs). This model was employed to investigate the potential crosstalk between tsRNAs and the NLRP3 inflammasome. We also explored the potential involvement of Ang-induced tsRNAs in the regulation of systemic inflammation induced by lipopolysaccharide (LPS) and inflammation related to T2D. Our work demonstrates that targeting Ang-induced tsRNAs could be a promising strategy for treating NLRP3 inflammasome-related inflammatory diseases.
Results
Ar induces activation of NLRP3 inflammasome and accumulation of tsRNAs
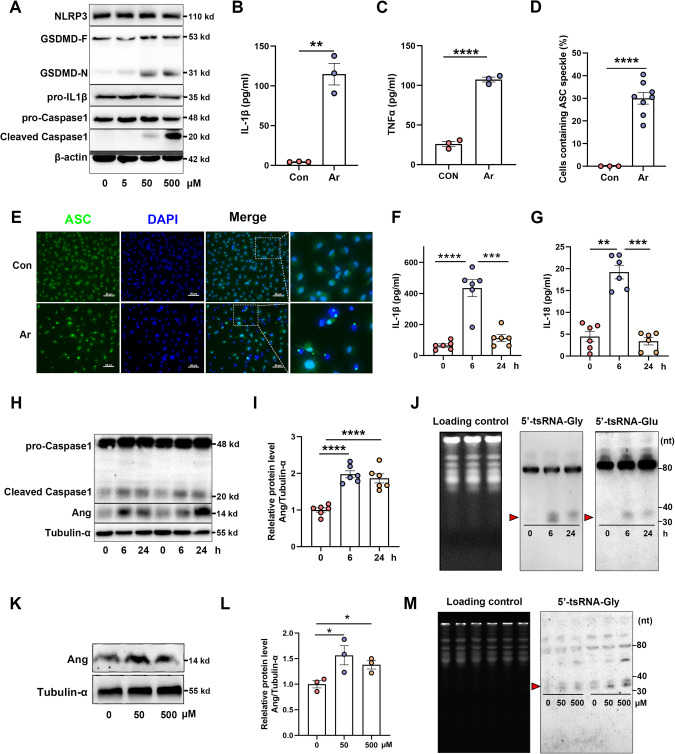
There are conflicting results regarding the activation of the NLRP3 inflammasome by Ar [34, 46, 47, 49, 50]. To investigate the impact of Ar on NLRP3 inflammasome activation, BMDMs were exposed to varying concentrations of Ar. The findings demonstrate that treatment with Ar promotes the cleavage of Caspase-1 and GSDMD (Fig. 1A). Additionally, exposed to Ar increase the secretion of pro-inflammatory cytokines, including IL-1β and TNFα (Fig. 1B, C). NLRP3-dependent, ASC nucleation-induced oligomerization is considered to be a common mechanism of NLRP3 inflammasome activation [51]. We found that Ar-induced the activation of NLRP3 inflammasome resulted in the condensation of ASC, which formed a large cytosolic speck within each cell (Fig. 1D, E). We subsequently verified these phenomena in vivo by intraperitoneally injecting Ar into wild-type mice for 6 and 24 h. Following the treatment, a significant increase in the concentration of serum IL-1β (Fig. 1F) and IL-18 were observed (Fig. 1G). Additionally, a notable elevation in the abundance of cleaved Caspase1 was observed in the liver upon treatment with Ar (Fig. 1H). Taken together, these results indicate that Ar activates the NLRP3 inflammasome both in vivo and in vitro.
Fig. 1. Sodium arsenite (Ar) induces activation of NLRP3 inflammasome and accumulation of tsRNAs in vitro and in vivo.
A BMDMs were treated with different doses of Ar (0, 5, 50, 500 μM) for 1 h and cell extracts were analyzed by immunoblotting for NLRP3, GSDMD-F and GSDMD-N, pro-IL-1β, pro-Caspase1 and cleaved Caspase1. Supernatants were analyzed by Enzyme-linked immunosorbent assay (ELISA) for IL-1β (B) and TNFα (C). Data are presented as Mean ± SEM, n = 3, **p < 0.01, ****p < 0.0001. Quantification (D) and representative immunofluorescence images (E) of ASC speck formation of BMDMs stimulated with 50 μM Ar for 1 h. Scale bars, 50 μm. Data are presented as Mean ± SEM, n = 8, ****p < 0.0001. F–J Eight-week-old male mice were injected intraperitoneally with Ar (4 mg/kg BW) for 0, 6, 24 h. Serum were analyzed by ELISA for IL-1β (F) and IL-18 (G). Data are presented as Mean ± SEM, n = 6, **p < 0.01, ***p < 0.001, ****p < 0.0001. H, I Immunoblot analysis of pro-Caspase1, cleaved Caspase1 and Ang in liver. J Northern blot analyses of 5′-tsRNA-Gly and 5′-tsRNA-Glu in the liver after Ar for 0, 6 or 24 h. K, L The relative expression level of Ang in BMDMs with Ar or saline treatment. Data are presented as Mean ± SEM, n = 3, *p < 0.05. M Northern blot analyses of 5′-tsRNA-Gly in BMDMs treatment with Ar or saline for 1 h.
To confirm the effect of Ar on tRNA cleavage, we analyzed the expression of Ang and tsRNAs in both liver tissue and BMDMs, where Ang is known to be highly expressed. As expected, the expression level of Ang was upregulated in liver tissue after Ar treatment (Fig. 1H, I). Previous researches have demonstrated that tRNA-Gly and tRNA-Glu exhibit higher sensitivity towards cleavage mediated by Ang [17, 52]. Here, we found that the levels of 5′-tsRNA-Gly and 5′-tsRNA-Glu were upregulated by Ar treatment in liver (Fig. 1J). Moreover, the expression levels of Ang (Fig. 1K, L), and 5′-tsRNA-Gly (Fig. 1M), were upregulated in BMDMs after Ar treatment. These findings collectively suggest that Ar triggers the upregulation of Ang and accumulation of tsRNAs, both in vivo and in vitro.
Ang-mediated 5′tsRNAs alleviate Ar-induced activation of NLRP3 inflammasome
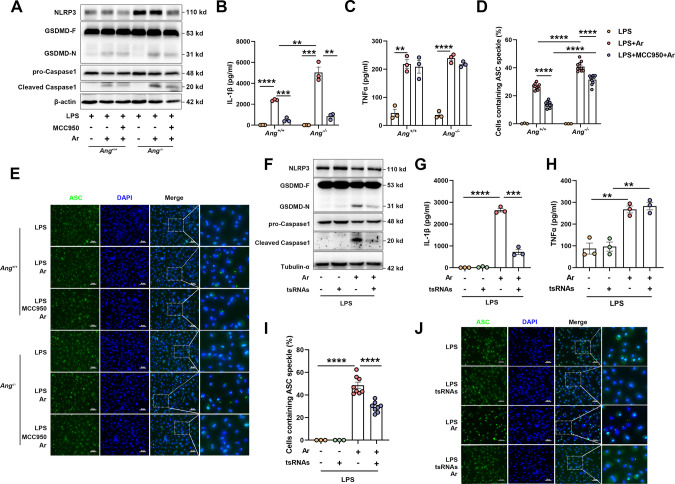
It is known that a deficiency of Ang-induced tsRNAs can exacerbate the development of inflammatory diseases [30, 53, 54]. The BMDMs from Ang+/+ and Ang−/− mice were primed by LPS to stimulate the expression of NLRP3, pro-Caspase1, and pro-IL-1β [55, 56] and then treated with Ar. Ar-treated Ang−/− BMDMs demonstrated elevated activation levels of NLRP3 inflammasome than Ang+/+ BMDMs, as evidenced by the increase of cleaved Caspase1 and GSDMD-N (Fig. 2A). Moreover, the secretion of IL-1β was significantly higher in Ar-treated Ang−/− BMDMs compared with Ang+/+ BMDMs (Fig. 2B). In contrast, we only found a mild effect of Ang deficiency on TNFα production, which is NLRP3 inflammasome independent (Fig. 2C). In addition, the percentage of cells that contained ASC specks were significantly increased in Ar-treated Ang−/− BMDMs compared with Ang+/+ BMDMs (Fig. 2D, E).
Fig. 2. Ang mediated 5′tsRNAs alleviate sodium arsenite (Ar) induced activation of NLRP3 inflammasome.
A–E BMDMs form Ang+/+ and Ang−/− mice were primed by 500 ng/ml LPS for 3 h, followed with 10 μM MCC950 for 1 h, and then treated with 50 μM sodium arsenite (Ar) for 1 h. A Cell extracts were analyzed by immunoblotting for NLRP3, GSDMD-F and GSDMD-N, pro-Caspase1 and cleaved Caspase1. Supernatants were analyzed by ELISA for IL-1β (B) and TNFα (C). Data are presented as Mean ± SEM, n = 3, **p < 0.01, ***p < 0.001, ****p < 0.0001. Quantification (D) and Representative immunofluorescence images (E) of ASC speck formation in BMDMs. Scale bars, 50 μm. Data are presented as Mean ± SEM, n = 10, ****p < 0.0001. F–J BMDMs were transfected with 200 nM total tsRNAs for 2 h, then primed by 500 ng/ml LPS for 4 h and treated with 50 μM Ar for 1 h. F Cell extracts were analyzed by immunoblotting for NLRP3, GSDMD-F and GSDMD-N, pro-Caspase1 and cleaved Caspase1. Supernatants were analyzed by ELISA for IL-1β (G) and TNFα (H). Data are presented as Mean ± SEM, n = 3, **p < 0.01, ***p < 0.001, ****p < 0.0001. Quantification (I) and representative immunofluorescence images (J) of ASC speck formation in BMDMs. Scale bars, 50 μm. Data are presented as Mean ± SEM, n = 8, ****p < 0.0001.
To further investigate the impact of Ang deficiency on the activation of NLRP3 inflammasome, we employed MCC950, a diarylsulfonylurea compound that specifically inhibits NLRP3 inflammasome activation without affecting AIM2, NLRC4, or NLRP1 inflammasomes [56, 57]. MCC950 administration inhibited Ar-induced activation of NLRP3 inflammasome, as evidenced by a decrease in cleaved Caspase1 and GSDMD-N levels (Fig. 2A), and the reduced secretion of IL-1β (Fig. 2B), as well as a suppression in the formation of ASC specks (Fig. 2D, E) in both Ar-treated Ang+/+ and Ang−/− BMDMs. In contrast, MCC950 treatment had a limited effect on TNFα production (Fig. 2C). All results consistently demonstrate that Ang deficiency exacerbates the activation of NLRP3 inflammasome and pyroptosis induced by Ar and the effect can be prevented by a NLRP3 inflammasome inhibitor.
To investigate whether Ang mediated 5′tsRNAs could mitigate the activation of NLRP3 inflammasome, a mixture of the 5′-tsRNAs expressed most abundantly was synthesized, and then transfected into BMDMs using established protocols (Supplementary Table 1 and Supplementary Fig. 1) [36, 58]. Pretreatment with tsRNAs alleviated Ar-induced activation of NLRP3 inflammasome, as indicated by the downregulation of cleaved Caspase1 and GSDMD-N (Fig. 2F). Moreover, the secretion of IL-1β was decreased by tsRNAs transfection (Fig. 2G). In contrast, Ar-induced TNFα secretion was not affected by the addition of tsRNAs (Fig. 2H). Furthermore, tsRNAs pre-treatment significantly decreased the percentage of cells that contained ASC specks compared with cells that were treated with Ar alone (Fig. 2I, J). Overall, these findings suggest that tsRNAs mitigated the activation of NLRP3 inflammasome induced by Ar.
It has been reported that Ang-induced tsRNAs inhibit global translation in several cell types [11, 12]. To investigate whether tsRNAs-induced inhibition of NLRP3 inflammasome activation by an arrest of translation, we checked the protein levels of NLRP3, pro-IL-1β (the inactive cytoplasmic precursor of IL-1β), and pro-Caspase1 (the inactive cytoplasmic precursor of Caspase1). The results showed that transfection of tsRNAs did not affect the levels of NLRP3, pro-IL-1β, and pro-Caspase1 (Supplementary Fig. 2). This suggests that tsRNAs-induced inhibition of NLRP3 inflammasome activation is independent of translation.
tsRNAs recruit DDX3X into SGs to alleviate the activation of NLRP3 inflammasome
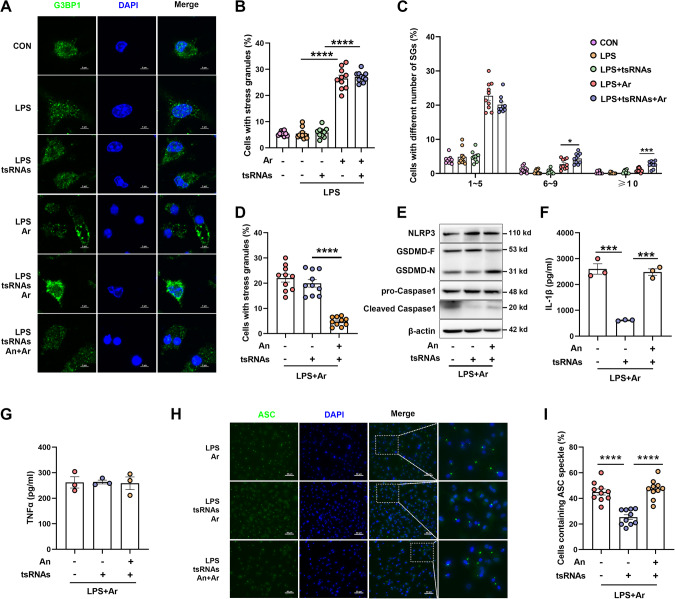
It has been reported that Ang-induced tsRNAs can promote the assembly of stress-induced SGs [36, 37]. However, a recent study indicated that neither physiological nor non-physiological concentrations of tsRNAs were able to induce the formation of SGs [59]. To investigate the role of tsRNAs in the formation of SGs, we transfected a pool of synthesized 5′-tsRNAs into BMDMs and then induced SGs formation by Ar. Specifically, confocal microscopy imaging of G3BP1, a marker of SGs [60], revealed that Ar treatment induced the formation of SGs in LPS-primed BMDMs (Fig. 3A and Supplementary Fig. 3A). Transfection of 5′-tsRNAs into BMDMs did not affect the percentage of cells containing SGs, compared with cells that were treated with Ar alone (Fig. 3A, B). However, the percentage of cells containing 6–10 SGs were increased (Fig. 3C). To confirm whether tsRNAs-mediated inhibition of NLRP3 inflammasome activation is regulated by SGs, we treated BMDMs with anisomycin (An, the inhibitor of SGs [34]) to inhibit Ar-induced SGs assembly (Fig. 3A, D and Supplementary Fig. 3B–E). Inhibition of SGs formation by An agent leads to a decrease in cell viability (Supplementary Fig. 3E), consistent with previous reports that SGs promote cell survival [35, 61]. An treatment reduced SGs assembly and blocked tsRNAs-induced inhibition of NLRP3 inflammasome activation (Fig. 3E), with the secretion of IL-1β but not TNFα in supernatants significantly increased upon treatment with An (Fig. 3F, G). Moreover, co-treatment with An showed a significant increase in the percentage of cells containing ASC specks (Fig. 3H, I). To further confirm the involvement of SGs in tsRNAs-triggered suppression of NLRP3 inflammasome activation, we employed another system of pyroptosis and NLRP3 inflammasome activation by LPS and nigericin (Nig). First, we transfected BMDMs with 5′-tsRNAs and then exposed them to LPS priming, followed by Nig treatment. We observed no significant difference in the cleavage of Caspase1 and GSDMD, secretion of IL-1β and TNFα, or ASC specks formation in BMDMs with or without tsRNAs (Supplementary Fig. 4A–D). To investigate the difference between the two different systems of NLRP3 inflammasome activation, we measured the formation of SGs. No significant difference in the formation of SGs in BMDMs treated with LPS priming, regardless of whether followed by Nig treatment (Supplementary Fig. 4E, F). Taken together, these findings suggest that the assembly of SGs is essential for the inhibition of NLRP3 inflammasome activation mediated by tsRNAs.
Fig. 3. Transfection of 5′tsRNAs alleviate the activation of NLRP3 inflammasome via SGs.
BMDMs were transfected with 200 nM total tsRNAs for 2 h, then primed by 500 ng/ml LPS for 4 h and treated with 50 μM sodium arsenite (Ar) with or without anisomycin (An) for 1 h. A Confocal microscopy imaging of G3BP1. Scale bars, 5 μm. B–D Percentage of SGs-positive cells and quantification of SGs formation in BMDMs stimulated with LPS and Ar with or without An and tsRNAs as indicated. Data are presented as Mean ± SEM, n = 10, *p < 0.05, ***p < 0.001, ****p < 0.0001. E Immunoblot analysis of NLRP3, GSDMD-F and GSDMD-N, pro-Caspase1 and cleaved Caspase1. Supernatants were analyzed by Enzyme-linked immunosorbent assay for IL-1β (F) and TNFα (G). Data are presented as Mean ± SEM, n = 3, ***p < 0.001. Representative immunofluorescence images (H) and quantification of ASC speck formation (I) of BMDMs stimulated with LPS and Ar with or without An and tsRNAs as indicated. Scale bars, 50 μm. Data are presented as Mean ± SEM, n = 10, ****p < 0.0001.
Next, we investigated the mechanism underlying how tsRNAs alleviate the activation of NLRP3 inflammasome through SGs. It has been suggested that DDX3X, an ATP-dependent RNA helicase present in SGs, interacts with NLRP3 and facilitates the activation of inflammasomes [34]. However, the formation of SGs causes DDX3X to be sequestered, thereby inhibiting NLRP3 inflammasome activation [34]. Recent findings indicated that there is a direct association between DDX3X, tRNAs, and tsRNAs [39]. Moreover, Ang-induced tsRNAs can be incorporated into SGs [37]. Based on our findings, we hypothesize that Ang-induced tsRNAs have the capacity to interact with DDX3X, thereby reducing the association between DDX3X and NLRP3. Utilizing super-resolution microscopy, we observed a slight increase in the colocalization of DDX3X and G3BP1 upon transfection of tsRNAs (Fig. 4A). Conversely, transfection of tsRNAs resulted in a decrease of NLRP3 colocalized with DDX3X (Fig. 4B). Additionally, Co-immunoprecipitation (Co-IP) also confirmed that there was an interaction between DDX3X and NLRP3 (Supplementary Fig. 5). Moreover, tsRNAs treatment reduced the binding between DDX3X and NLRP3 (Fig. 4C). To investigate the direct interaction between tsRNAs and DDX3X, we performed RNA immunoprecipitation (RIP) and RT-PCR to confirm their interaction in this system. The results indicated that DDX3X exhibits interaction with 5′tsRNAs, and Ar stimulates enhanced binding of 5′tsRNAs-Gly with DDX3X (Fig. 4D). There is no significant difference in the presence of SGs between Ar-treated Ang+/+ BMDMs and Ang−/− BMDMs (Fig. 4E). Super-resolution microscopy imaging showed that Ar-treated Ang−/− BMDMs resulted in a slight decrease of G3BP1 co-localized with DDX3X (Fig. 4F). Conversely, the co-localization of NLRP3 and DDX3X was increased in Ar-treated Ang−/− BMDMs (Fig. 4G). These findings provide evidence of physical interaction between NLRP3 and DDX3X, supporting the notion that tsRNAs augment the recruitment of DDX3X into SGs and decrease the interaction between DDX3X and NLRP3, thereby mitigating the activation of NLRP3 inflammasome.
Fig. 4. 5′tsRNAs recruit DDX3X into SGs to alleviate the activation of NLRP3 inflammasome.
A–D BMDMs were treated with 200 nM total tsRNAs for 2 h, then primed by 500 ng/ml LPS for 4 h and then treated with 50 μM sodium arsenite (Ar) for 1 h. A Super-resolution microscope imaging of G3BP1 and DDX3X in BMDMs to visualize the subcellular localization. B Super-resolution microscope imaging of NLRP3 and DDX3X in BMDMs to visualize the subcellular localization. Scale bars, 2 μm. C Co-immunoprecipitation (IP) of NLRP3 and DDX3X in BMDMs. The colocalization of DDX3X and G3BP1 or NLRP3 was analyzed by ImageJ software. D RNA Immunoprecipitation (RIP) and RT-PCR experiments to demonstrate the direct interaction between tsRNAs and DDX3X. Representative blots (n = 3). E–G BMDMs form Ang+/+ and Ang−/− mice were stimulated with 500 ng/ml LPS for 4 h and treated with or without 50 μM Ar for 1 h. E Percentage of SGs-positive cells. Data are presented as Mean ± SEM, n = 10. F Super-resolution microscope imaging of G3BP1 and DDX3X to visualize the subcellular localization. G Super-resolution microscope imaging of DDX3X and NLRP3 to visualize the subcellular localization. Scale bars, 2 μm.
Ang deficiency aggravates LPS-induced systemic inflammation
We subsequently explored whether Ang-induced tsRNAs were involved in the regulation of inflammatory responses via inhibiting the activation of NLRP3 inflammasome in vivo. We first investigated the effect of LPS on the hepatic expression of Ang and the production of tsRNAs in mice. Our results showed a noteworthy increase in the expression of Ang after LPS treatment (Fig. 5A–C). Moreover, Ang-induced tsRNAs (5′-tsRNA-Gly and 5′-tsRNA-Glu) were upregulated in LPS-treated mice, whereas their expression levels were reversed upon Ang deletion (Fig. 5D). To further confirm the impact of Ang deficiency on NLRP3 inflammasome activation during LPS-induced systemic inflammation, mice underwent MCC950 treatment for 2 weeks prior to LPS exposure. Subsequently, the concentrations of NLRP3 inflammasome-dependent inflammatory cytokines (IL-1β and IL-18) and NLRP3 inflammasome-independent inflammatory cytokine (TNFα) in the serum were assayed. Our findings showed that Ang deficiency elevated serum IL-1β and IL-18 production (Fig. 5E, F) but had a limited effect on TNFα production (Fig. 5G). In LPS-treated Ang−/− mice, the protein level of cleaved-Caspase1 was higher than Ang+/+ mice in the liver (Fig. 5H). MCC950 treatment inhibited the activation of NLRP3 inflammasome, as evidenced by the decrease of IL-1β and IL-18 (Fig. 5E, F) and cleavage of Caspase1 (Fig. 5H, I). In contrast, MCC950 treatment had a limited effect on TNFα production (Fig. 5G). These results indicate that the absence of Ang-induced tsRNAs enhances the severity of LPS-induced NLRP3 inflammasome-mediated systemic inflammation.
Fig. 5. Ang deficiency aggravates LPS-induced systemic inflammation.
A–D Eight-week-old male mice were injected intraperitoneally with 20 mg/kg LPS or saline (Con) for 4 h. A–C The relative expression level of Ang in the liver. Data are presented as Mean ± SEM, n = 8, ***p < 0.001, ****p < 0.0001. D The abundance of 5′-tsRNA-Gly and 5′-tsRNA-Glu in liver tissue detected by Northern blot. E–I Male Ang+/+ and Ang−/− mice were injected intraperitoneally with MCC950 for 2 weeks and then treated with 20 mg/kg LPS or saline for 4 h. The concertation of IL-1β (E), IL-18 (F), and TNFα (G) in serum were detected by ELISA. Data are presented as Mean ± SEM, n = 6, *p < 0.05, **p < 0.01, ***p < 0.001. H Immunoblot was performed to detect pro-Caspase1 and cleaved Caspase1 in the liver. I The ratio of cleaved Caspase1 to pro-Caspase1. Data are presented as Mean ± SEM, n = 6, *p < 0.05, ***p < 0.001.
Ang deficiency aggravates high-fat diet (HFD)-induced insulin resistance
Increasing evidence suggests that chronic NLRP3-mediated inflammation plays a role in the development of insulin resistance and T2D [62–64]. To determine whether Ang-induced tsRNAs play a role in regulating obesity-related insulin sensitivity by inhibiting NLRP3 inflammasome activation, we subjected Ang+/+ and Ang−/− mice to a high fat diet (HFD) or a normal diet (ND) for a period of 14 weeks. Firstly, we observed that the expression of Ang was upregulated in the liver of HFD mice (Fig. 6A–C). Moreover, Ang-induced tsRNAs (5′-tsRNA-Gly and 5′-tsRNA-Glu) were upregulated in the liver of HFD mice, whereas their expression levels were reversed upon Ang deletion (Fig. 6D). Mice fed a HFD exhibited significant elevations in body weight, serum triglyceride levels, and total cholesterol levels. However, no significant differences were observed between the two genotypes (Supplementary Fig. 6A–C). Subsequently, we aimed to investigate if Ang-induced tsRNAs could enhance obesity-related insulin sensitivity. As anticipated, Ang+/+ mice exhibited glucose intolerance and insulin resistance after being subjected to the HFD treatment. However, the Ang−/−-HFD mice showed significantly higher glucose than other mice, as revealed by glucose tolerance test (GTT) and insulin tolerance test (ITT) assays (Fig. 6E–H). These results suggest that Ang-induced tsRNAs are implicated in the development of glucose intolerance and insulin resistance induced by HFD. Furthermore, to investigate the impact of Ang deficiency on the inflammatory response induced by metabolic stress, tissue culture assays were performed ex vivo. Our findings are consistent with previous research, demonstrating increased levels of IL-1β and TNFα in liver tissue (Fig. 6I, J) as well as in white adipose tissue (WAT, Fig. 6K, L) of Ang+/+-HFD mice compared with Ang+/+-ND mice. In contrast, IL-1β levels in the liver and WAT (Fig. 6I, K) were higher in Ang−/−-HFD mice than Ang+/+-HFD mice, whereas, TNFα demonstrated no significant differences between the two groups (Fig. 6J, L). Collectively, our results indicate that Ang contribute to HFD-induced upregulation of the tsRNAs, activation of the NLRP3 inflammasome, and the development of metabolic disorders.
Fig. 6. Ang deficiency aggravates high-fat diet (HFD)-induced insulin resistance.
Male Ang+/+ and Ang−/− mice were fed with a normal diet (ND) or a high-fat diet (HFD) for 14 weeks. A–C The relative expression level of Ang in the liver. D The abundance of 5′-tsRNA-Gly and 5′-tsRNA-Glu in liver tissue detected by Northern blot. E Blood glucose during glucose tolerance test (GTT). *(red): the difference between Ang+/+-HFD and Ang−/−-HFD, *(black): the difference between Ang+/+-ND and Ang−/−-ND. F Area under the curve (AUC) statistics for (E). Data are presented as Mean ± SEM, n = 8. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. G Blood glucose during insulin tolerance test (ITT). *(red): the difference between Ang+/+-HFD and Ang−/−-HFD. H AUC statistics for (G). Data are presented as Mean ± SEM, n = 8, *p < 0.05, **p < 0.01. Liver (I, J) and white adipose tissue (WAT) (K, L) were isolated and cultured for 24 h, and supernatants were analyzed by ELISA for the release of IL-1β and TNFα. Data are presented as Mean ± SEM, n = 3, *p < 0.05, **p < 0.01. M The levels of NO in serum. Data are presented as Mean ± SEM, n = 8, *p < 0.05, **p < 0.01. N–P HUVECs were exposed to 25 mM D-glucose (HG) or 5 mM D-glucose plus 20 mM mannitol for 24 h. Cells were treated with 10 ng/ml recombinant ANG protein or transfected with 200 nM total tsRNAs. N Supernatants were analyzed by ELISA for IL-1β. Data are presented as Mean ± SEM, n = 3, *p < 0.05, ****p < 0.0001. O Quantification of ASC speck formation in HUVECs. Data are presented as Mean ± SEM, n = 8, ****p < 0.0001. P The levels of NO in supernatants. Data are presented as Mean ± SEM, n = 3, *p < 0.05, ****p < 0.0001.
Endothelial dysfunction caused by high glucose is recognized as an important event in the pathogenesis of diabetes-related vascular complications. Mice fed a HFD demonstrated a significant decrease in nitric oxide (NO) levels, which is a crucial biomarker for endothelial dysfunction (Fig. 6M). Furthermore, Ang−/−-HFD mice exhibited an even more pronounced decrease in NO levels (Fig. 6M). Then, human umbilical vein endothelial cells (HUVECs) were utilized to assess the impact of Ang and tsRNAs on high glucose-induced NLRP3 inflammasome activation and endothelial dysfunction. Both recombinant ANG treatment and tsRNAs transfection downregulated high glucose-induced NLRP3 inflammasome activation in HUVECs, as evidenced by reducing the levels of IL-1β (Fig. 6N) and the formation of ASC speckle (Fig. 6O and Supplementary Fig. 7). Furthermore, both recombinant ANG treatment and tsRNAs transfection reversed high glucose-induced reduction of NO levels in HUVECs (Fig. 6P). These findings demonstrate that both Ang and tsRNAs had a suppressive role in high glucose-induced NLRP3 inflammasome activation and endothelial dysfunction.
Discussion
In recent years, increasing evidence has shown that both Ang and tsRNAs play essential roles in the development of various inflammatory diseases. We show here that Ang-induced tsRNAs are intracellular inhibitors of NLRP3 inflammasome activation, further suggesting their role in controlling inflammatory responses. Mechanistically, tsRNAs recruit the DDX3X protein into SGs, thereby preventing the interaction between DDX3X and NLRP3, which activates the NLRP3 inflammasome (Fig. 7). Additionally, in vivo experiments showed that Ang deficiency resulted in the downregulation of 5′-tsRNAs, leading to the amplification of NLRP3 inflammasome-dependent inflammation, including LPS-induced systemic inflammation and T2D-related inflammatory responses.
Fig. 7. Schematic illustration of how tsRNAs interact with DDX3X to inhibite the activation of NLRP3 inflammasome via stress granules.
Ang-mediated tsRNAs recruit the DDX3X protein into SGs, resulting in the inhibition of the interaction between DDX3X and NLRP3, ultimately leading to the suppression of NLRP3 inflammasome activation.
It is well-established that competition for DDX3X molecules between SGs and the NLRP3 inflammasome is essential in coordinating the activation of innate responses and subsequent cell fate decisions during stress conditions [34, 43, 44]. However, the mechanism underlying the incorporation of DDX3X into SGs remains unclear. Upon stress, the activation of Ang promotes tRNA fragmentation, which subsequently results in excessive production of tsRNAs and potentially modulates pathophysiological processes [17, 25]. Ang-induced tsRNAs have the ability to trigger the formation of SGs, and can subsequently be incorporated into these structures [37]. It has been demonstrated that Ang-induced tsRNAs can form an RNP complex with cytochrome c, which is released from the mitochondria, and that the tsRNA-mediated sequestration of cytochrome c fosters cell survival by preventing apoptosome formation and apoptosis under cellular stress [14]. In the present study, we found that transfection of 5′-tsRNAs into BMDMs did not alter the proportion of cells exhibiting or lacking SGs. It is consistent with a recent report, which suggested that neither physiological nor non-physiological levels of tsRNAs were able to induce the formation of SGs [59]. However, inhibition of Ar-induced SGs assembly reduced tsRNAs-induced inhibition of NLRP3 inflammasome activation. Furthermore, our observations indicate that 5′-tsRNAs fail to suppress Nig-induced NLRP3 inflammasome activation in which absence of SGs formation. Taken together, this suggests that SGs assembly is essential for tsRNAs-mediated inhibition of NLRP3 activation. Recently, Drino et al. reported that the RNA helicase DDX3X binds to tsRNAs and assists in the formation of SGs [39]. Here, we found that tsRNAs treatment reduced the binding between DDX3X and NLRP3 but promoted the co-localization of DDX3X and G3BP1. Moreover, we found a combination of tsRNAs with DDX3X and Ar promoted tsRNAs to bind to DDX3X. This suggests a strong possibility of interaction between tsRNAs and DDX3X during activation of the NLRP3 inflammasome. Our collective findings indicate that tsRNAs are involved in the recruitment of DDX3X into SGs, and the absence of DDX3X hinders the activation of the NLRP3 inflammasome.
Recent human clinical studies and animal experiments have consistently shown that Ang and tsRNAs exhibit anti-inflammatory activities and play a beneficial role in multiple human inflammatory diseases, including inflammatory bowel disease, Parkinson’s disease, and cancers [30, 53, 54]. It is worth noting that the levels of tsRNAs were observed to be dramatically elevated following SARS-CoV-2 infection, particularly in coronavirus disease 2019 (COVID-19) patients with severe symptoms [65]. Additionally, the levels of tsRNAs were upregulated in serum samples from patients with chronic hepatitis B virus infection [10]. Thus, a comprehensive understanding of the underlying anti-inflammatory mechanisms is crucial for the appropriate clinical application of Ang and tsRNAs. Other researchers and we have discovered that LPS can lead to a rapid increase in tsRNA levels in various tissues and body fluids, including serum, spleen, intestine, epididymis, and sperm in mouse and monkey models [10, 17, 66]. Here, we show that Ang deficiency resulted in the downregulation of tsRNAs, thereby upregulating NLRP3 inflammasome-dependent inflammation. Conversely, Ang deficiency had little effect on TNFα production in the serum. Our results highlight that the absence of Ang-induced tsRNAs exacerbates the development of inflammasome-dependent inflammatory diseases.
T2D is a leading cause of morbidity and mortality worldwide and its etiology remains unclear. Several cellular stress response mechanisms have been proposed to explain insulin resistance and islet β-cell dysfunction in T2D, including oxidative stress and endoplasmic reticulum stress [67, 68]. It is noteworthy that each of these cellular stresses is also believed to trigger or be associated with an inflammatory response. Indeed, increasing evidence suggests chronic inflammation is an important pathogenetic factor in the development of insulin resistance and T2D [69, 70]. Numerous SGs have been observed in macrophages and fatty liver tissues of HFD-fed obese mice [71]. Nevertheless, the function of SGs in obesity pathogenesis and associated disorders remains unclear. Additionally, there was an upregulation of tsRNAs abundance in the sperm of mice fed a HFD [15, 72]. In the present study, HFD induced upregulation of Ang and Ang-mediated tsRNAs in the liver of Ang+/+-HFD but not Ang−/−-HFD mice. In addition, liver tissue culture assays indicated that Ang deficiency resulted in the upregulation of NLRP3 inflammasome-dependent inflammation. We suppose that tsRNAs recruit DDX3X into SGs which block the interaction between DDX3X and NLRP3, alleviating the activation of NLRP3 inflammasome. As a result, the Ang−/−-HFD mice showed lower glucose intolerance and insulin resistance. Collectively, these results indicate that Ang-induced tsRNAs are involved in HFD-induced glucose intolerance and insulin resistance via NLRP3 inflammasome during metabolic stress.
Accumulating evidence suggests that inhibiting NLRP3 inflammasome activation has emerged as a crucial therapeutic approach for addressing endothelial dysfunction and subsequent development in diabetic complications [73, 74]. Our findings indicate that Ang deficiency may exacerbate endothelial dysfunction. Both Ang treatment and tsRNAs transfection downregulated high glucose-induced NLRP3 inflammasome activation, as evidenced by reducing the levels of IL-1β and the formation of ASC speckle. Furthermore, both recombinant ANG treatment and tsRNAs transfection reversed high glucose-induced reduction of NO levels in HUVECs. These findings demonstrate that both recombinant ANG treatment and tsRNAs transfection had a suppressive role in endothelial dysfunction. Our findings offer valuable insights into the potential development of new preventive and therapeutic strategies for addressing diabetic complications.
In summary, our study identifies the role of Ang-induced tsRNAs in the regulation of NLRP3 inflammation activation in vitro and in vivo. It is revealed that (1) Ang-induced tsRNAs alleviate the activation of NLRP3 inflammasome; (2) tsRNAs recruit DDX3X into SGs which block the interaction between DDX3X and NLRP3; (3) Ang deficiency results in the downregulation of tsRNAs, which in turn amplifies NLRP3 inflammasome-dependent inflammation, including LPS-induced systemic inflammation and T2D-related inflammation. In light of our findings, targeting Ang-induced tsRNAs might lead to insights and treatments for NLRP3 inflammasome-related diseases.
Materials and methods
Generation of transgenic mouse models and mouse breeding
Wild-type C57BL/6 mice and Ang−/− mice (Strain NO. T011609) were purchased from GemPharmatech (Nanjing, China). Ang−/− mice used CRISPR/Cas9 technology to edit the Ang gene. The Ang gene has 2 transcripts. According to the structure of Ang gene, exon2 of Ang-202 (ENSMUST00000171688.8) transcript is recommended as the knockout region. The region contains all of the coding sequences. Knocking out the region will result in disruption of protein function. The brief process is as follows: sgRNA was transcribed in vitro. Cas9 and sgRNA were microinjected into the fertilized eggs of C57BL/6JGpt mice. Fertilized eggs were transplanted to obtain positive F0 mice which were confirmed by PCR and sequencing. A stable F1 generation mouse model was obtained by mating positive F0 generation mice with C57BL/6JGpt mice. All mice were fed in specific pathogen-free conditions at the Laboratory Animal Center of Nanjing Agricultural University. They accessed food and water ad libitum and were housed in a 12 h light/12 h dark artificial lighting cycle room, the room temperature is 22–25 °C with 40%-60% humidity.
For Ar induced inflammatory model, 8-week-old male mice were injected intraperitoneally with Ar (4 mg/kg BW) (S7400, Sigma). Mice were euthanized after undergoing Ar treatment for 6 and 24 h, and the liver was collected for future analysis. For LPS-induced systemic inflammation, 8-week-old male Ang+/+ and Ang−/− mice were intraperitoneally injected with 10 mg/kg MCC950 (HY-12815A, MedChemExpress) at day 1, 2, 3. After that, the injections were given every other day. Control mice were injected with the same volume (100 μl) of 10% DMSO. All mice were intraperitoneally injected with 20 mg/kg BW LPS (strain O111:B4, L2880, Sigma) at day 14. Mice were euthanized after undergoing LPS treatment for 4 h, and serum was measured by ELISA. For metabolic stress studies, 4-week-old male Ang+/+ and Ang−/− mice were maintained on ND, HFD (60% cal/fat, D12492; Research Diets) for 14 weeks.
Cell culture and stimulation
BMDMs were collected and cultured as described [75]. Six-week-old mice were euthanatized via cervical dislocation. Femurs and tibias were collected in cold PBS. Both ends of the bone were cut and the bone marrow was flushed out into 50 ml centrifugal tube with cold PBS. The cells were then gently blown into a single-cell suspension and passed through a 70 μm sterile strainer. Following centrifuge at 1400 rpm for 5 min, the supernatant was decanted and red blood cells were lysed using Red Blood Cell lysis buffer (C3702, Beyotime) for 5 min. After neutralization with DMEM (319-005-CL, Vicente Biotechnology), bone marrow cells were centrifuged at 1400 rpm for 5 min. After washing twice with cold PBS, cells were resuspended in DMEM containing 20 ng/ml M-CSF (315-02, PeproTech), 10% FBS (10100147c, Gibco), 1% penicillin (100 U/ml) and streptomycin (100 μg/ml) (PS, C0222, Beyotime) and placed into 6 well plates (2 × 106 cells/ml). After 3 days of culture, the medium was completely replaced, and after 2 days, half of the medium was changed. The differentiated BMDMs were then treated with further stimulation on the sixth day. All steps were performed under sterile conditions.
For tsRNAs transfection, BMDMs were transfected with 200 nM total tsRNAs (Supplementary Table 1) by Lipofectamine RNAiMAX transfection reagent (13778150, Invitrogen). After 2 h, BMDMs were primed with 500 ng/ml LPS for 4 h, then treated with 50 μM Ar for 1 h to induce SGs formation. To inhibit SGs formation, 2 μg/ml An (GC11559-10, Glpbio) was added into the LPS-primed BMDMs 20 min before Ar. To inhibit the activation of NLRP3, after LPS priming 3 h, BMDMs were treated with 10 μM MCC950 (HY-12815A, MedChemExpress) for 1 h, and then treated with 50 μM Ar.
HUVECs were maintained in complete endothelial culture medium ECM (1001, Sciencell) supplemented with 1% endothelial cell growth supplement (1052, ScienCell), 5% fetal bovine serum (0025, ScienCell) and 1% penicillin/streptomycin solution (0503, ScienCell) in a humidified atmosphere of 5% CO2 at 37 °C. For high glucose exposure, D-glucose (HY-B0389, MedChemExpress) was added directly in ECM media to obtain a final concentration of 25 mM for 24 h. In addition, 5 mM glucose plus 20 mM mannitol (HY-N0378, MedChemExpress) was used to exclude hyperosmolarity effects. Both HG and hypertonic group were incubated with the presence or absence of 10 ng/ml recombinant ANG protein (HY-P7503, MedChemExpress) or transfected with 200 nM total tsRNAs.
Western blot
Tissue samples (30 mg) were homogenized using 1 ml lysis buffer (P0013B, Beyotime), which contains 1× protease inhibitor (K1007, APExBIO) and 1× Phosphatase inhibitor (K1015, APExBIO). After centrifuging at 4 °C, 12,000 × g for 10 min, supernatant was collected. For cells, we added 50 μl lysis buffer for BMDMs per 6-well plate. Protein content was determined using the BCA protein assay kit (P0012S, Beyotime) according to the manufacturer’s protocol. Protein samples were separated using 6%-12% SDS polyacrylamide gels and transferred onto polyvinylidene fluoride membrane (IPVH00010, Millipore) at 100 V for 1.5 h. Then the membranes were incubated with 5% milk for 2 h at room temperature. After that, membranes were incubated with the primary antibodies at 4 °C, with gentle shaking overnight. The primary antibodies used were rabbit anti-NLRP3 (1:1000, #15101, Cell Signaling Technology), mouse anti-Caspase1 (1:1000, AG-20B-0042, AdipoGen), rabbit anti-GSDMD (1:1000, ab219800, Abcam), rabbit anti-IL-1β (1:1000, ab254360, Abcam), mouse anti-Angiogenin antibody (1:100, sc-74528, Santa Cruz), mouse anti-DDX3X antibody (1:100, sc-365768, Santa Cruz), rabbit anti-Tubulinα (1:1000, AC007, ABClonal), rabbit β-actin (1:50,000, AC026, ABClonal). Images were captured using the VersaDoc 4000MP system (Bio-Rad, Hercules, CA) and the band density was analyzed with ImageJ.
ELISA
Supernatants from cell culture or tissue culture as well as serum were assayed for mouse IL-1β (EK201B, MULTI SCIENCE), IL-18 (CSB-E04609m, CUSA BIO), TNFα (CSB-E04741m, CUSA BIO) and human IL-1β (KE00021, proteintech) according to manufacturer’s instructions.
Immunofluorescence
BMDMs were fixed in 4% paraformaldehyde for 10 min at room temperature, then incubated with 0.3% Triton X-100 membrane permeabilization solution (TBST) for 1 h at room temperature. After 10 min in blocking buffer (5% bovine serum albumin in TBST) at room temperature, BMDMs were subsequently incubated with primary antibody overnight at 4 °C. The antibody reagents were rabbit anti-ASC antibody (1:800, #67824, Cell Signaling Technology), rabbit anti-G3BP1 antibody (1:600, 13057-2-AP, Proteintech), rabbit anti-NLRP3 antibody (1:200, 15101, Cell Signaling Technology) or mouse anti-DDX3X antibody (1:100, sc-365768, Santa Cruz) overnight at 4 °C. The next day, incubation was done at 37 °C for 30 min, then washed 3 times with TBST, and later incubated with Goat anti-Rabbit IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 488 (1:500, A-11008, Invitrogen), CoraLite594-conjugated Goat AntiMouse IgG (H + L) (1:200, SA00013-3, proteintech) secondary antibody for 1 h at 37 °C. Washing was repeated thrice with TBST and then incubated with DAPI (C1005, Beyotime) for 5 min. For ASC specks, samples were imaged through a fluorescence microscope (DMI6000 B, Leica). For SGs, samples were imaged through a SP5 confocal microscope (A1 HD25, Nikon). For colocalization of DDX3X and G3BP1 or NLRP3, samples were imaged through a super-resolution microscope (N-SIM, Nikon). The colocalization of DDX3X and G3BP1 or NLRP3 was analyzed by ImageJ software [76].
Northern blot
Total RNA was extracted from mouse tissues or BMDMs using TRIzol reagents (TSP401, Tsingke). RNA was separated by 15% urea-PAGE gel, stained with TS-GelRed (1:10,000, TSJ002, TSINGKE), and immediately imaged and transferred to nylon membranes (FFN13, Beyotime), then crosslinked for 2 h at 80 °C. Membranes were pre-hybridized with ULTRAhyb® Ultrasensitive Hybridization Buffer (AM8670, Invitrogen) for at least 1 h at 42 °C. The membranes were incubated with 16 nM DIG-labeled oligonucleotide probes overnight (12–16 h) at 42 °C. The probes were synthesized by GENEWIZ, Inc. as shown in Supplementary Table 2. The membranes were washed twice with a low stringent buffer (2× SSC with 0.1% (wt/vol) SDS) at 42 °C for 15 min each time, then washed twice with a high stringent buffer (0.1× SSC with 0.1% (wt/vol) SDS) for 5 min each time, finally rinsed in washing buffer (1× SSC) for 10 min. Following the washes, the membranes were transferred into 1× blocking buffer (REF: 11096176001, Roche) and incubated at room temperature for 2 h, after that, the DIG antibody, Anti-Digoxigenin-AP Fab fragments (REF: 11093274910, Roach) was added into the blocking buffer at a ratio of 1:10,000 and incubated for half an hour at room temperature. The membranes were then washed four times in DIG washing buffer (1× maleic acid buffer, 0.3% Tween-20) for 15 min each time, rinsed in DIG detection buffer (0.1 M Tris-HCl, 0.1 M NaCl, pH 9.5) for 5 min, and then incubated with CSPD ready-to-use reagent (REF: 11755633001, Roach). The membranes were incubated with the CSPD reagent at 37 °C for 15 min in the dark. Images were captured using the VersaDoc 4000MP system (Bio-Rad, Hercules, CA).
Co-immunoprecipitation (Co-IP)
Whole-cell extracts were lysed in IP buffer (P0013, Beyotime), which contained 1× protease inhibitor (K1007, APExBIO) and 1× Phosphatase inhibitor (K1015, APExBIO). Protein concentration was detected and diluted to a final concentration of 1000 ng/μl after ultrasonication. Firstly, the supernatants were incubated with protein G Plus-Agarose Immunoprecipitation reagent (sc-2003, Santa Cruz) for 2 h to remove unless specifically bound proteins. The Plus-Agarose Immunoprecipitation reagent was removed and incubated with NLRP3 antibodies (1:200, Cell Signaling Technology) overnight at 4 °C. The next day, protein G Plus-Agarose Immunoprecipitation reagent was added to the supernatants overnight at 4 °C. Afterwards, all beads were washed three times with PBS which contained 1× protease inhibitor and 1× Phosphatase inhibitor. Then 2× loading buffer was added to each sample and boiled at 100 °C for 5 min followed by western blotting.
RNA immunoprecipitation (RIP)
To examine the tsRNA: DDX3X interaction in vivo, BMDMs were grown in 10 cm dish as previous described. We added 50 μM Ar in the medium for 1 h, and harvested and washed twice with ice-cold PBS. The following step was used BersinBio TM RNA Immunoprecipitation (RIP) Kit (Bes5101, BersinBio) as the manufacturer’s instruction.
RT‐PCR for small RNAs
Reverse Transcription for tsRNAs were performed using Servicebio ®miRNA First Strand cDNA Synthesis Kit (Tail method) (G3334-25, Servicebio) following the manufacturer’s instruction. Briefly, 200 ng of RNA was isolated from the DDX3X-IPs, and subsequently polyadenylated, and then converted to cDNAs with a unique adaptor in the presence of reverse transcriptase, and the cDNAs were amplified with specific small RNA primer in combination with the universal adaptor to examine the expression of a particular small RNA. The components of PCR mixtures were: 2× GoTaq® Green Master Mix (12.5 μl), forward primer (0.5 μl, 10 μM) and universal primer (0.5 μl, 10 μM), cDNA (1 μl) and double‐distilled water (10.5 μl). For equal loading of each sample. The conditions used for PCR were as follows: 95 °C for 10 min, followed by 23 (Glu) or 28 (Gly) cycles at 95 °C for 15 s, 60 °C for 15 s and 72 °C for 15 s. Primers were shown in Supplementary Table 3. The PCR products were analyzed by electrophoresis on 4% agarose gels stained with TS-GelRed (TSJ002, Tsingke).
Blood glucose examination during GTT and ITT
For GTT, mice were injected intraperitoneally with glucose (2 g/kg body weight) after 12 h fasting. For ITT, mice were intraperitoneally injected with insulin (0.75 IU/kg, 12584-586, Aladdin) after 6 h fasting. Blood samples were collected from the tail vein before glucose or insulin injection (0 min) and at 15, 30, 60, and 120 min afterward. Blood glucose was immediately determined by Accu-Chek glucose meters (ACCUCHEK Active Blood Glucose Meter, Roche) at different time points.
Tissue culture
The isolated liver and WAT tissues were washed twice in PBS, minced into fine pieces, and cultured in 12-well plates (0.5 g/well) in M199 medium (P012, Nanjing Jiancheng Bioengineering Institute) supplemented with 5% FBS, penicillin and streptomycin. After 24 h, culture supernatants were analyzed by ELISA kit for IL-1β and TNFα.
qRT-PCR
Total RNA was extracted the same as before [17]. The mRNA level of Ang was assessed using qRT-PCR. The primers for amplifying mouse Ang were purchased from Beijing Tsingke Biotech Co., Ltd. Primer sequence were shown in Supplementary Table 3.
Determination of total NO (NO3−/NO2−) concentration
NO concentration was determined using an indirect method based on measurement of nitrite concentration according to Griess’s reaction. NO levels were determined with a test kit (S0023, Beyotime) by colorimetric method.
Statistical analysis
All analyses were performed with GraphPad Prism 8 (GraphPad Software). Data were presented as mean with standard error of the mean (SEM). The difference between two groups was assessed using unpaired two‐tailed Student’s t test or Mann–Whitney test for non‐normally distributed data. The difference between variables was assessed by one‐way ANOVA with Dunnett’s multiple comparisons test or Kruskal–Wallis with Dunn’s multiple comparisons test for nan-normally distributed data. Equality of survivor function was determined by log‐rank test. P‐value results were denoted by asterisks in the figures (*p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001). The detailed statistical analysis and replicate numbers for each experiment were described in the figure legends, and the exact p value can be found in the corresponding source data file.
Supplementary information
Author contributions
JC contributed to conceptualization, investigation, formal analysis, validation, visualization, writing—original draft, review, and editing. CL contributed to investigation, formal analysis and methodology. SL contributed to investigation and formal analysis. MT contributed to investigation and validation. YS contributed to investigation and validation. XS contributed to investigation and validation. MY contributed to investigation and validation. BH contributed to conceptualization, resources, writing—review and editing, supervision, project administration, and funding acquisition.
Funding
This work was supported by the National Natural Science Foundation of China (32072807, 32372965), the National Key Research and Development Program of China (2022YFD1300405), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Ethical approval
Animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Nanjing Agricultural University. All experiments followed the “Guidelines on Ethical Treatment of Experimental Animals” (2006) No. 398 set by the Ministry of Science and Technology, China, and Regulation regarding the Management and Treatment of Experimental Animals” (2008) No. 45 set by the Jiangsu Provincial People’s Government.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41418-024-01311-8.
References
- 1.Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010;327:296–300. 10.1126/science.1184003 [DOI] [PubMed] [Google Scholar]
- 2.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–86. 10.1038/nature10759 [DOI] [PubMed] [Google Scholar]
- 3.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–87. 10.1038/nm.3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–89. 10.1038/s41577-019-0165-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J, Nunez G. The NLRP3 inflammasome: activation and regulation. Trends Biochem Sci. 2023;48:331–44. 10.1016/j.tibs.2022.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coll RC, Schroder K, Pelegrin P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol Sci. 2022;43:653–68. 10.1016/j.tips.2022.04.003 [DOI] [PubMed] [Google Scholar]
- 7.Sharma BR, Kanneganti TD. NLRP3 inflammasome in cancer and metabolic diseases. Nat Immunol. 2021;22:550–59. 10.1038/s41590-021-00886-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Man SM, Kanneganti TD. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat Rev Immunol. 2016;16:7–21. 10.1038/nri.2015.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson DM, Parker R. Stressing out over tRNA cleavage. Cell. 2009;138:215–19. 10.1016/j.cell.2009.07.001 [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Zhang Y, Shi J, Zhang H, Cao Z, Gao X, et al. Identification and characterization of an ancient class of small RNAs enriched in serum associating with active infection. J Mol Cell Biol. 2014;6:172–74. 10.1093/jmcb/mjt052 [DOI] [PubMed] [Google Scholar]
- 11.Chen Q, Zhang X, Shi J, Yan M, Zhou T. Origins and evolving functionalities of tRNA-derived small RNAs. Trends Biochem Sci. 2021;46:790–804. 10.1016/j.tibs.2021.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivanov P, Emara MM, Villen J, Gygi SP, Anderson P. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol Cell. 2011;43:613–23. 10.1016/j.molcel.2011.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fricker R, Brogli R, Luidalepp H, Wyss L, Fasnacht M, Joss O, et al. A tRNA half modulates translation as stress response in Trypanosoma brucei. Nat Commun. 2019;10:118. 10.1038/s41467-018-07949-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saikia M, Jobava R, Parisien M, Putnam A, Krokowski D, Gao XH, et al. Angiogenin-cleaved tRNA halves interact with cytochrome c, protecting cells from apoptosis during osmotic stress. Mol Cell Biol. 2014;34:2450–63. 10.1128/MCB.00136-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi J, et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science. 2016;351:397–400. 10.1126/science.aad7977 [DOI] [PubMed] [Google Scholar]
- 16.Sarker G, Sun W, Rosenkranz D, Pelczar P, Opitz L, Efthymiou V, et al. Maternal overnutrition programs hedonic and metabolic phenotypes across generations through sperm tsRNAs. Proc Natl Acad Sci USA. 2019;116:10547–56. 10.1073/pnas.1820810116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Ren L, Sun X, Zhang Z, Liu J, Xin Y, et al. Angiogenin mediates paternal inflammation-induced metabolic disorders in offspring through sperm tsRNAs. Nat Commun. 2021;12:6673. 10.1038/s41467-021-26909-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu HY, Chen CY, Hung YF, Lin HR, Chao HW, Shih PY, et al. RNase A promotes proliferation of neuronal progenitor cells via an ERK-dependent pathway. Front Mol Neurosci. 2018;11:428. 10.3389/fnmol.2018.00428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg HF. RNase A ribonucleases and host defense: an evolving story. J Leukoc Biol. 2008;83:1079–87. 10.1189/jlb.1107725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun D, Han C, Sheng J. The role of human ribonuclease A family in health and diseases: a systematic review. iScience. 2022;25:105284. 10.1016/j.isci.2022.105284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goncalves KA, Silberstein L, Li S, Severe N, Hu MG, Yang H, et al. Angiogenin promotes hematopoietic regeneration by dichotomously regulating quiescence of stem and progenitor cells. Cell. 2016;166:894–906. 10.1016/j.cell.2016.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu H, Feng J, Liu Q, Sun F, Tie Y, Zhu J, et al. Stress induces tRNA cleavage by angiogenin in mammalian cells. FEBS Lett. 2009;583:437–42. 10.1016/j.febslet.2008.12.043 [DOI] [PubMed] [Google Scholar]
- 23.Yamasaki S, Ivanov P, Hu GF, Anderson P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J Cell Biol. 2009;185:35–42. 10.1083/jcb.200811106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Luo J, Zhou H, Liao JY, Ma LM, Chen YQ, et al. Stress-induced tRNA-derived RNAs: a novel class of small RNAs in the primitive eukaryote Giardia lamblia. Nucleic Acids Res. 2008;36:6048–55. 10.1093/nar/gkn596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheng J, Xu Z. Three decades of research on angiogenin: a review and perspective. Acta Biochim Biophys Sin. 2016;48:399–410. 10.1093/abbs/gmv131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhahbi JM, Spindler SR, Atamna H, Yamakawa A, Boffelli D, Mote P, et al. 5′ tRNA halves are present as abundant complexes in serum, concentrated in blood cells, and modulated by aging and calorie restriction. BMC Genomics. 2013;14:298. 10.1186/1471-2164-14-298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dhahbi JM. 5′ tRNA Halves: the next generation of immune signaling molecules. Front Immunol. 2015;6:74. 10.3389/fimmu.2015.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin Y, Cai J, Huang D, Zhou B, Luo Z, Yu S, et al. Effects of dexmedetomidine on the expression profile of tsRNAs in LPS-induced acute lung injury. J Clin Lab Anal. 2022;36:e24115. 10.1002/jcla.24115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dou R, Zhang X, Xu X, Wang P, Yan B. Mesenchymal stem cell exosomal tsRNA-21109 alleviate systemic lupus erythematosus by inhibiting macrophage M1 polarization. Mol Immunol. 2021;139:106–14. 10.1016/j.molimm.2021.08.015 [DOI] [PubMed] [Google Scholar]
- 30.Bai R, Sun D, Chen M, Shi X, Luo L, Yao Z, et al. Myeloid cells protect intestinal epithelial barrier integrity through the angiogenin/plexin-B2 axis. EMBO J. 2020;39:e103325. 10.15252/embj.2019103325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen L, Liao T, Chen Q, Lei Y, Wang L, Gu H, et al. tRNA-derived small RNA, 5′tiRNA-Gly-CCC, promotes skeletal muscle regeneration through the inflammatory response. J Cachexia Sarcopenia Muscle. 2023;14:1033–45. 10.1002/jcsm.13187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gong J, Wang P, Liu JC, Li J, Zeng QX, Yang C, et al. Integrative analysis of small RNA and mRNA expression profiles identifies signatures associated with chronic epididymitis. Front Immunol. 2022;13:883803. 10.3389/fimmu.2022.883803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Protter DSW, Parker R. Principles and properties of stress granules. Trends Cell Biol. 2016;26:668–79. 10.1016/j.tcb.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samir P, Kesavardhana S, Patmore DM, Gingras S, Malireddi RKS, Karki R, et al. DDX3X acts as a live-or-die checkpoint in stressed cells by regulating NLRP3 inflammasome. Nature. 2019;573:590–94. 10.1038/s41586-019-1551-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White JP, Lloyd RE. Regulation of stress granules in virus systems. Trends Microbiol. 2012;20:175–83. 10.1016/j.tim.2012.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emara MM, Ivanov P, Hickman T, Dawra N, Tisdale S, Kedersha N, et al. Angiogenin-induced tRNA-derived stress-induced RNAs promote stress-induced stress granule assembly. J Biol Chem. 2010;285:10959–68. 10.1074/jbc.M109.077560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lyons SM, Achorn C, Kedersha NL, Anderson PJ, Ivanov P. YB-1 regulates tiRNA-induced stress granule formation but not translational repression. Nucleic Acids Res. 2016;44:6949–60. 10.1093/nar/gkw418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jain S, Wheeler JR, Walters RW, Agrawal A, Barsic A, Parker R. ATPase-modulated stress granules contain a diverse proteome and substructure. Cell. 2016;164:487–98. 10.1016/j.cell.2015.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drino A, König L, Capitanchik C, Sanadgol N, Janisiw E, Rappol T, et al. Identification of RNA helicases with unwinding activity on angiogenin-processed tRNAs. Nucleic Acids Res. 2023;51:1326–52. 10.1093/nar/gkad033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng D, Guo L, Liu J, Song Y, Ma X, Hu H, et al. DDX3X deficiency alleviates LPS-induced H9c2 cardiomyocytes pyroptosis by suppressing activation of NLRP3 inflammasome. Exp Ther Med. 2021;22:1389. 10.3892/etm.2021.10825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, Zhang F, Xu H, Yang H, Shao M, Xu S, et al. TLR4 aggravates microglial pyroptosis by promoting DDX3X-mediated NLRP3 inflammasome activation via JAK2/STAT1 pathway after spinal cord injury. Clin Transl Med. 2022;12:e894. 10.1002/ctm2.894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kienes I, Bauer S, Gottschild C, Mirza N, Pfannstiel J, Schröder M, et al. DDX3X Links NLRP11 to the regulation of type I interferon responses and NLRP3 inflammasome activation. Front Immunol. 2021;12:653883. 10.3389/fimmu.2021.653883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fox D, Man SM. DDX3X: stressing the NLRP3 inflammasome. Cell Res. 2019;29:969–70. 10.1038/s41422-019-0250-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kesavardhana S, Samir P, Zheng M, Malireddi RKS, Karki R, Sharma BR, et al. DDX3X coordinates host defense against influenza virus by activating the NLRP3 inflammasome and type I interferon response. J Biol Chem. 2021;296:100579. 10.1016/j.jbc.2021.100579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu S, Chen Y, Ren Y, Zhou J, Ren J, Lee I, et al. A tRNA-derived RNA fragment plays an important role in the mechanism of arsenite-induced cellular responses. Sci Rep. 2018;8:16838. 10.1038/s41598-018-34899-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu Y, Zhang J, Yao X, Qiu T, Jiang L, Wang N, et al. Ubiquitinated gasdermin D mediates arsenic-induced pyroptosis and hepatic insulin resistance in rat liver. Food Chem Toxicol. 2022;160:112771. 10.1016/j.fct.2021.112771 [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Qiu T, Jiang L, Wang N, Zhu Y, Yan R, et al. NLRP3 inflammasome blocked the glycolytic pathway via targeting to PKLR in arsenic-induced hepatic insulin resistance. Ecotoxicol Environ Saf. 2021;223:112590. 10.1016/j.ecoenv.2021.112590 [DOI] [PubMed] [Google Scholar]
- 48.Jia X, Qiu T, Yao X, Jiang L, Wang N, Wei S, et al. Arsenic induces hepatic insulin resistance via mtROS-NLRP3 inflammasome pathway. J Hazard Mater. 2020;399:123034. 10.1016/j.jhazmat.2020.123034 [DOI] [PubMed] [Google Scholar]
- 49.Zhong G, Wan F, Lan J, Jiang X, Wu S, Pan J, et al. Arsenic exposure induces intestinal barrier damage and consequent activation of gut-liver axis leading to inflammation and pyroptosis of liver in ducks. Sci Total Environ. 2021;788:147780. 10.1016/j.scitotenv.2021.147780 [DOI] [PubMed] [Google Scholar]
- 50.Ahn H, Kim J, Kang SG, Yoon SI, Ko HJ, Kim PH, et al. Mercury and arsenic attenuate canonical and non-canonical NLRP3 inflammasome activation. Sci Rep. 2018;8:13659. 10.1038/s41598-018-31717-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156:1193–206. 10.1016/j.cell.2014.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Su Z, Kuscu C, Malik A, Shibata E, Dutta A. Angiogenin generates specific stress-induced tRNA halves and is not involved in tRF-3-mediated gene silencing. J Biol Chem. 2019;294:16930–41. 10.1074/jbc.RA119.009272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prehn JHM, Jirström E. Angiogenin and tRNA fragments in Parkinson’s disease and neurodegeneration. Acta Pharmacol Sin. 2020;41:442–46. 10.1038/s41401-020-0375-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Honda S, Loher P, Shigematsu M, Palazzo JP, Suzuki R, Imoto I, et al. Sex hormone-dependent tRNA halves enhance cell proliferation in breast and prostate cancers. Proc Natl Acad Sci USA. 2015;112:E3816–25. 10.1073/pnas.1510077112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horvat JC, Kim RY, Weaver N, Augood C, Brown AC, Donovan C, et al. Characterization and inhibition of inflammasome responses in severe and non-severe asthma. Respir Res. 2023;24:303. 10.1186/s12931-023-02603-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–55. 10.1038/nm.3806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu D, Chen Y, Sun Y, Gao Q, Li H, Yang Z, et al. Target of MCC950 in inhibition of NLRP3 inflammasome activation: a literature review. Inflammation. 2019;43:17–23. 10.1007/s10753-019-01098-8 [DOI] [PubMed] [Google Scholar]
- 58.Shi J, Zhang Y, Tan D, Zhang X, Yan M, Zhang Y, et al. PANDORA-seq expands the repertoire of regulatory small RNAs by overcoming RNA modifications. Nat Cell Biol. 2021;23:424–36. 10.1038/s41556-021-00652-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanadgol N, König L, Drino A, Jovic M, Schaefer MR. Experimental paradigms revisited: oxidative stress-induced tRNA fragmentation does not correlate with stress granule formation but is associated with delayed cell death. Nucleic Acids Res. 2022;50:6919–37. 10.1093/nar/gkac495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang P, Mathieu C, Kolaitis RM, Zhang P, Messing J, Yurtsever U, et al. G3BP1 is a tunable switch that triggers phase separation to assemble stress granules. Cell. 2020;181:325–45.e28. 10.1016/j.cell.2020.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marcelo A, Koppenol R, de Almeida LP, Matos CA, Nóbrega C. Stress granules, RNA-binding proteins and polyglutamine diseases: too much aggregation? Cell Death Dis. 2021;12:592. 10.1038/s41419-021-03873-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Villarroya F, Cereijo R, Gavaldà-Navarro A, Villarroya J, Giralt M. Inflammation of brown/beige adipose tissues in obesity and metabolic disease. J Intern Med. 2018;284:492–504. 10.1111/joim.12803 [DOI] [PubMed] [Google Scholar]
- 63.Ying W, Fu W, Lee YS, Olefsky JM. The role of macrophages in obesity-associated islet inflammation and beta-cell abnormalities. Nat Rev Endocrinol. 2020;16:81–90. 10.1038/s41574-019-0286-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127:1–4. 10.1172/JCI92035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu X, Wen YZ, Huang ZL, Shen X, Wang JH, Luo YH, et al. SARS-CoV-2 causes a significant stress response mediated by small RNAs in the blood of COVID-19 patients. Mol Ther Nucleic Acids. 2022;27:751–62. 10.1016/j.omtn.2021.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chu C, Yu L, Wu B, Ma L, Gou LT, He M, et al. A sequence of 28S rRNA-derived small RNAs is enriched in mature sperm and various somatic tissues and possibly associates with inflammation. J Mol Cell Biol. 2017;9:256–59. 10.1093/jmcb/mjx016 [DOI] [PubMed] [Google Scholar]
- 67.Harding HP, Ron D. Endoplasmic reticulum stress and the development of diabetes: a review. Diabetes. 2002;51:S455–61. 10.2337/diabetes.51.2007.S455 [DOI] [PubMed] [Google Scholar]
- 68.Robertson RP, Harmon J, Tran PO, Poitout V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53:S119–24. 10.2337/diabetes.53.2007.S119 [DOI] [PubMed] [Google Scholar]
- 69.Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D, et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci USA. 2011;108:15324–29. 10.1073/pnas.1100255108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–88. 10.1038/nm.2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bai Y, Dong Z, Shang Q, Zhao H, Wang L, Guo C, et al. Pdcd4 is involved in the formation of stress granule in response to oxidized low-density lipoprotein or high-fat diet. PLoS ONE. 2016;11:e0159568. 10.1371/journal.pone.0159568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y, Zhang X, Shi J, Tuorto F, Li X, Liu Y, et al. Dnmt2 mediates intergenerational transmission of paternally acquired metabolic disorders through sperm small non-coding RNAs. Nat Cell Biol. 2018;20:535–40. 10.1038/s41556-018-0087-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bai B, Yang Y, Wang Q, Li M, Tian C, Liu Y, et al. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 2020;11:776. 10.1038/s41419-020-02985-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gora IM, Ciechanowska A, Ladyzynski P. NLRP3 inflammasome at the interface of inflammation, endothelial dysfunction, and type 2 diabetes. Cells. 2021;10:314. 10.3390/cells10020314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Volchuk A, Ye A, Chi L, Steinberg BE, Goldenberg NM. Indirect regulation of HMGB1 release by gasdermin D. Nat Commun. 2020;11:4561. 10.1038/s41467-020-18443-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Diao F, Jiang C, Sun Y, Gao Y, Bai J, Nauwynck H, et al. Porcine reproductive and respiratory syndrome virus infection triggers autophagy via ER stress-induced calcium signaling to facilitate virus replication. PLoS Pathog. 2023;19:e1011295. 10.1371/journal.ppat.1011295 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.