Abstract
Atherosclerosis, a chronic systemic inflammatory condition, is implicated in most cardiovascular ischemic events. The pathophysiology of atherosclerosis involves various cell types and associated processes, including endothelial cell activation, monocyte recruitment, smooth muscle cell migration, involvement of macrophages and foam cells, and instability of the extracellular matrix. The process of endothelial-to-mesenchymal transition (EndoMT) has recently emerged as a pivotal process in mediating vascular inflammation associated with atherosclerosis. This transition occurs gradually, with a significant portion of endothelial cells adopting an intermediate state, characterized by a partial loss of endothelial-specific gene expression and the acquisition of “mesenchymal” traits. Consequently, this shift disrupts endothelial cell junctions, increases vascular permeability, and exacerbates inflammation, creating a self-perpetuating cycle that drives atherosclerotic progression. While endothelial cell dysfunction initiates the development of atherosclerosis, autophagy, a cellular catabolic process designed to safeguard cells by recycling intracellular molecules, is believed to exert a significant role in plaque development. Identifying the pathological mechanisms and molecular mediators of EndoMT underpinning endothelial autophagy, may be of clinical relevance. Here, we offer new insights into the underlying biology of atherosclerosis and present potential molecular mechanisms of atherosclerotic resistance and highlight potential therapeutic targets.
Keywords: Atherosclerosis, EndoMT, Autophagy
1. Introduction
Atherosclerosis is a major contributor to global mortality and morbidity. It is now characterized as an inflammatory condition that leads to arterial wall stiffening and thickening as well as plaque development [1]. Despite several hypotheses aimed at explaining the progression of atherosclerosis, a comprehensive understanding of its pathogenesis remains elusive [2-4]. Endothelial dysfunction significantly impacts atherosclerosis progression [5,6]. Endothelium, constituting the inner most layer of blood vessels, serves as a vital regulator of vascular homeostasis [7]. The primary role of endothelium is to create a barrier that governs the exchange of molecules and cells between the blood stream and the vessel wall. Beyond serving as a mechanical and non-thrombogenic barrier safeguarding the vascular wall, the endothelium also functions as a crucial and versatile network that plays a vital role in multiple physiological functions. In response to various chemical and biochemical signals, endothelium releases factors that control vascular tone, smooth muscle cell (SMC) proliferation and migration, adhesion of immune cells, thromboresistance and inflammation within vessels [8]. Over past decades, there has been a gradual unraveling of the diverse functions carried out by the endothelial cells (EC), some of which include immune regulation, endocrine and paracrine signaling, the preservation of redox homeostasis, and their role in influencing vascular tone.
Recently, EndoMT has emerged as a potential mechanism for the pathophysiological progression of atherosclerosis, shifting the focus on cell trans-differentiation and acquisition of new gene expression profiles and concordant shift in morphology and behavior [9]. EndoMT denotes a phenomenon wherein ECs transition into mesenchymal cells. This transformative process involves the acquisition of mesenchymal cell traits, marked by the loss of cell-cell contacts and cell polarity in response to both biochemical and biomechanical stimuli [10]. Interestingly, one of the physiological outcomes of EndoMT in atherosclerosis involves the process of autophagy. Attention is increasingly directed towards the crucial role of autophagic flux in maintaining the integrity of normal blood vessel walls. Moreover, emerging evidence establishes connections between autophagy and various vital physiological processes, including redox homeostasis, lipid metabolism, and the secretion of vasomodulatory substances, influencing the viability and function of ECs. Therefore, strategies aimed at enhancing autophagy in ECs holds promise for the treatment of atherosclerosis. In this review, we have endeavored to explicate the correlation between EndoMT and endothelial autophagy, emphasizing the contribution of this association to the pathogenesis of atherosclerosis. A thorough understanding of these dynamic cellular shifts could potentially pave the way for the development of more specific treatments for atherosclerosis.
2. Exploring the role of EndoMT in atherosclerosis
The development of atherosclerosis is significantly influenced by endothelial dysfunction [5,6]. In healthy individuals, endothelium, which constitutes the cell layer immediately adjacent to the luminal surface of blood vessels, serves as a primary regulator of vascular homeostasis [7]. In atherosclerosis, ECs encounter diverse biochemical and physical stimuli from the bloodstream. Factors such as low-density lipoprotein (LDL), cholesterol, also inflammatory cytokines IL-1β, TNFα, TGFβ and wall shear stress can trigger EndoMT, disrupting EC homeostasis. This, in turn, results in endothelial dysfunction, ultimately contributing to the progression of atherosclerosis.
During this progression, ECs undergo a loss of apical-to-basal membrane polarity and cell-to-cell adhesion. Instead, they adopt a migratory, fibroblast-like phenotype. Moreover, a marked downregulation of EC markers, including platelet endothelial cell adhesion molecule (PECAM), vascular endothelial cadherin (VE-cadherin), vascular endothelial growth factor receptor 2 (VEGFR-2), and endothelial nitric oxide synthase (eNOS) are observed. Simultaneously, mesenchymal markers such as α-smooth muscle actin (α-SMA), neural cadherin (N-cadherin), fibroblast-specific protein 1 (FSP1), fibronectin, vimentin, and type I and type III collagen (ColI/III) are upregulated during EndoMT [11-13]. During the initial phases of EndoMT, documented reductions in intercellular adhesion forces within the endothelial monolayer accompany an observed increase in cellular stiffness [14]. Although EndoMT is a natural physiological process integral to cardiac development during embryogenesis and septate formation, its pathological occurrence has been implicated in the initiation, progression, and stabilization of atherosclerosis plaques, pulmonary arterial hypertension (PAH), fibrosis, pathological neovascularization, mitral valve thickening and cancer [14-22].
Mesenchymal cells assume crucial roles in this disease, contributing to processes such as the secretion of proinflammatory cytokines and growth factors, and the production of collagen and metalloproteinases, which are essential for plaque calcification and the formation of a fibrous cap. In vivo tracking systems for the EC lineage have suggested that ECs undergoing EndoMT significantly contribute to the population of fibroblast-like cells observed in atherosclerotic plaques. This cellular contribution is evidenced by the co-expression of markers specific to both the endothelial and mesenchymal phenotypes. The extent of EndoMT positively correlates with the instability of plaques [11,23]. Matrix metalloproteinases (MMPs) have a significant association with unstable atherosclerotic lesions, and fibroblast-like cells resulting from EndoMT exhibit heightened expression of MMP1, MMP9 and MMP10 compared to regular fibroblasts those are expressed mesenchymal markers vimentin and PDGFR [24,25]. MMPs are instrumental in vascular wall remodeling and the development of atherosclerosis by contributing to inflammatory activation and subsequent endothelial dysfunction [26]. Specifically, the activation of endothelial MMP2 can compromise endothelial integrity and function [26]. Additionally, recruited vascular wall cells leverage MMPs to remodel the surrounding extracellular matrix, influencing the migration, proliferation, and apoptosis of both ECs and vascular smooth muscle cells (VSMCs) [27].
Nevertheless, the pathogenesis of atherosclerosis is intricate, involving endothelial dysfunction, inflammatory responses, oxidative stress, SMC activation, and thrombosis. Notably, endothelial dysfunction has been regarded as the primary factor initiating the cascade leading to atherosclerosis [28,29]. Furthermore, numerous studies have unveiled the interplay between endothelial dysfunction and inflammatory stress in vascular biology. In the context of chronic inflammation, prolonged activation of ECs triggered by dysregulated release of cytokines, such as interleukin 6 (IL-6), TNF-α, IL-1β or an impaired endothelial-dependent immune response, can lead to endothelial dysfunction [30]. Moreover, the NLRP3 inflammasome, beyond its crucial role as an immune response sensor, is implicated in endothelial dysfunction and the pathogenesis of atherosclerosis [31]. NLRP3 inflammasomes govern caspase-1 activation and pro-IL-1β processing in macrophages, initiating inflammatory responses within the vascular wall that contribute to the progression of atherosclerosis [31].
The overexpression of adhesion molecules, including ICAM-1 and VCAM-1, serves as an early indicator of endothelial dysfunction and atherosclerosis [32]. Recent findings indicate that EndoMT plays a pivotal role in the intricate interplay between inflammatory stress and endothelial dysfunction. The prolonged stimulation of ECs by diverse factors, such as pro-inflammatory cytokines and hypoxia, lead to an imbalance in endothelial homeostasis, culminating in EndoMT.
Remarkably, Helmke et.al uncovered in vivo evidence supporting a bidirectional crosstalk between macrophages and the process of EndoMT whereby macrophages within atherosclerosis lesions demonstrate an upregulation of mesothelial markers, thereby promoting EndoMT. Conversely, cells undergoing EndoMT impact the function and phenotypes of macrophages, influencing processes such as lipid uptake [16]. Complementing in vivo and in vitro studies, single cell sequencing technology has been instrumental in advancing our comprehension of the landscape and pathophysiology of human atherosclerotic plaques. A recent study identified 14 cell populations within plaques, including ECs, SMCs, B cells, myeloid cells, and T cells under various cellular activation states [33]. Notably, one subclass of ECs within this study was shown to express SMC markers like alpha-actin 2 (ACTA2), notch receptor 3 (NOTCH3), and myosin heavy chain 11 (MYH11), indicating that this subtype was undergoing EndoMT [33]. This discovery provides further weight to the notion of EC plasticity within atherosclerotic plaques. Collectively, these findings offer strong evidence for the close association between EndoMT and the initiation and development of atherosclerotic plaques.
2.1. Deciphering EndoMT signaling in atherosclerosis: Unraveling the mechanisms
The recognition of EndoMT as a significant contributor to pathologies like atherosclerosis and fibrosis has necessitated further investigation into the underlying molecular mechanisms propelling this process. The initiation of EndoMT can be triggered by various mechanisms and is coordinated by an intricate network of epigenetic regulators, transcription factors, and noncoding RNAs [34-37]. Pathological conditions such as inflammation, oxidative stress, elevated blood sugar, and low shear stress can prompt EndoMT(Fig. 1) [36].
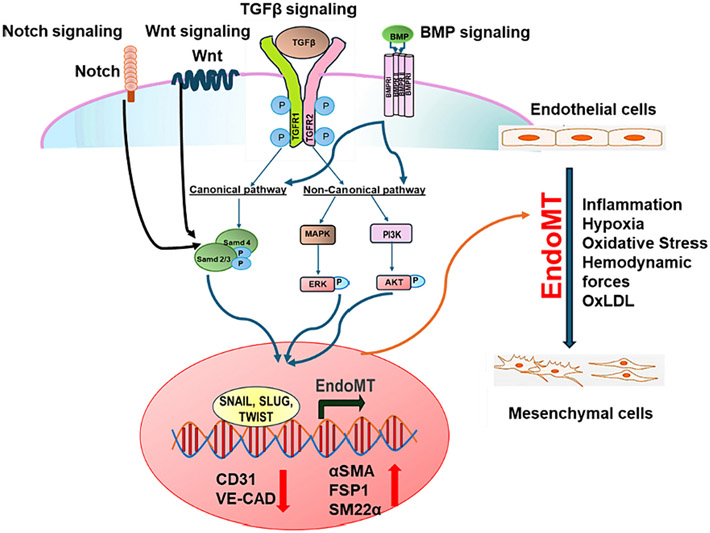
Fig. 1.
The signaling pathway involved in EndoMT in pathogenesis of atherosclerosis. Transcription factors associated with EndoMT related transcription factors such as Snail, Slug, Twist are regulated by upstream signaling including BMP, TGF-β, Wnt, and Notch, which in turn regulate endothelial and mesenchymal gene expression, ultimately inducing EndoMT.
The transforming growth factor-beta (TGF-β) signaling pathway, particularly, the canonical downstream SMAD pathway, has been extensively studied as a critical regulator of EndoMT (Fig. 1) [36]. The TGF-β signaling pathway serves as a canonical modulator of EndoMT, exhibiting crosstalk with other pathways such as fibroblast growth factor (FGF), Notch signaling, and bone morphogenetic protein (BMP) pathways (Fig. 1). In atherosclerosis, the expression of long non-coding RNA H19 is elevated in aortic tissues and correlates with severity of cardiovascular disease [37,38]. H19 upregulates TGFβ receptor 2 (TGFβR2) and TSP1 through the let-7/TET1 pathway, indicating its potential to influence markers associated with EndoMT, including Slug, SM22-α, Vimentin, and fibronectin 1 (FN1) [39]. Furthermore, non-coding RNAs play an essential role in EndoMT. The ensuing discussion expounds on the mechanisms underlying EndoMT in the context of atherosclerosis.
3. Role of TGF-β signaling in EndoMT
The impact of the TGF-β signaling pathway on EndoMT has been thoroughly investigated (Fig. 1). The TGF-β pathway plays crucial functions in both physiological processes such as embryonic development, differentiation, cell growth, cell death, and tissue homeostasis, as well as in pathological processes including auto-immune responses, inflammation, fibrosis, angiogenesis, oncogenesis, and cardiovascular disease [40]. Mammals possess three TGF-β isotypes: TGF-β1, TGF-β2, and TGF-β3. While all three TGF-β isoforms have the capacity to initiate EndoMT, studies in human microvascular and pulmonary progenitor valve endothelial cells have revealed that TGF-β2 is the most potent among these isoforms [41,42]. TGF-β family ligands bind to both type I and type II receptors, leading to the phosphorylation and activation of the transducer small mother against decapentaplegic (SMAD). The activated SMAD is then imported into the nucleus, where it regulates gene transcription [43].
SMADs are a set of proteins activated by serin phosphorylation, categorized into three subgroups based of their functions: Subgroup 1 compromises receptor regulated SMADs (SMAD 1–3, SMAD 5 and SMAD 8), subgroup 2 includes inhibitory SMADs (SMAD 6 and SMAD 7), and subgroup 3 consists of the common SMAD (SMAD 4) [44]. Upon binding of TGF-β with its receptors, the SMAD2/3 complex is recruited and phosphorylated. This phosphorylation event prompts the formation of a SMAD2/3/4 complex, subsequently translocating into the nucleus. The nuclear SMAD2/3/4 complex has the capability to bind with transcription factors and, thereby regulating gene expression [44,45]. In the context of epithelial-to-mesenchymal transition (EMT) or EndoMT, the specific transcription factors that interact with SMAD complexes include SNAIL and SLUG [46,47]. Earlier research indicated that the TGF-β-SMAD-SNAIL/SLUG signaling pathway not only diminishes the expression of endothelial genes, including CD31 and VE-cadherin, but also enhances the expression of mesenchymal genes such as fibroblast activation protein (FAP) and α-SMA [23,48-50].
The non-canonical TGF-β pathways play a role in inducing EndoMT by activating various components of the mitogen-activated protein kinase (MAPK) signaling [51,52]. Notably, observations of SMAD-independent activation, particularly through c-Jun-N terminal kinase (JNK) and p38, suggest their potential role in triggering EndoMT [53]. Moreover, there is evidence of cross talk with other pathways like Wnt signaling and TGF-β signaling. Specifically, EndoMT regulated through autophagy has been reported to exhibit cross talk with the TGF-β signaling pathway [54].
Increasing evidence supports a role for the TGF-β signaling pathways in governing cell proliferation, differentiation, adhesion, migration, and apoptosis, not only during embryonic development but also in the pathogenesis of human diseases [55]. The underlying risk factors for atherosclerosis, such as oscillatory shear stress and inflammation induced loss of fibroblast growth factor receptor 1 (FGFR1) expression, can activate TGF-β signaling, thereby contributing EndoMT. Depletion of FGFR1 induces EndoMT through the upregulation of SMC markers and mesenchymal markers, which are also targets of TGF-β [56]. Dong et. al reported that inhibiting FGF signaling by targeting epsins, alongside potentiating TGF-β signaling, restrains EndoMT in atherosclerosis [57].
Epsins are ubiquitously expressed adaptor proteins and chiefly participate in regulating the endocytosis of plasma membrane receptor complexes [58,59]. This process facilitates the degradation of these complexes, influencing EC signaling [60-64]. Through the application of single-cell RNA sequencing and lineage tracing, it has been demonstrated that epsin 1 and epsin 2 are both involved in promoting EndoMT. The absence of endothelial epsins curtails the expression of EndoMT markers and TGF-β signaling both in vitro and in atherosclerotic mice. This association is correlated with the development of smaller lesions in the ApoE−/− mouse model [57]. At a mechanistic level, the deficiency of EC epsins lead to elevated FGFR-1, hindering TGFβ signaling and EndoMT [57]. Epsin directly interacts with ubiquitinated FGFR-1 through their ubiquitin-interacting motif, resulting in the endocytosis and degradation of this receptor complex. Consequently, the administration of a synthetic ubiquitin-interacting-motif-containing peptide, specifically an atheroma ubiquitin-interacting motif peptide inhibitor, significantly mitigates EndoMT and the progression of atherosclerosis [57].
Considering the pivotal role of TGF-β in the regulation of EndoMT, inhibiting TGF-β may be a strategy to reverse this process. The inhibition of EndoMT in mitral valve ECs, both in vivo and in vitro, by Losartan or MEK1/2 inhibitors, possibly through the suppression of non-canonical TGFβ signaling, indicates the potential utilities of these drugs in controlling EndoMT to mitigate the excessive growth and fibrosis observed in the leaflets following myocardial infraction [22,65]. A potential novel treatment strategy for atherosclerosis could involve inhibiting EndoMT by reducing the interaction between epsins and FGFR-1, using a therapeutic peptide. The precise role of TGFβ signaling in atherogenesis or atheroprotection is complex. Toma et al. discussed how TGFβ signaling resistance may enable repair cells to tolerate adverse environments and restore vascular damage. However, unchecked TGFβ resistance can lead to failure in regulating the repair process, resulting in adverse effects on the artery wall. Therefore, elucidating the molecular mechanisms governing vascular repair, including the precise contribution of TGFβ signaling in this process, is essential for developing treatments for occlusive vascular diseases [66]. Although it may be tempting to classify TGFβ as either atheroprotective or atherogenic, it is more likely to play a central role in both normal and pathological vascular repair.
4. Role of the BMP signaling pathway in EndoMT
Bone morphogenetic proteins (BMPs) belong to the TGF-β superfamily of cytokines, originally identified in bone tissue. BMPs exhibit well characterized crosstalk with mechanobiology in the bone marrow. The endothelium is constantly exposed to various mechanical stimuli in the form of wall shear stress induced by blood flow, along with strain and tension resulting from blood pressure and from the surrounding cells and extracellular matrix. The interplay between TGF-β/BMP signaling and these mechanobiological processes are well-understood [67].
To date, over 20 BMPs with diverse functions have been identified [68]. The interaction between BMP ligand and receptors triggers the phosphorylation of SMAD1/5/8, facilitating downstream signaling (Fig. 1) [69]. BMPs interact with two distinct receptors, mediating signal transduction through both SMAD-dependent and SMAD-independent pathways [70]. Activated TGF-β/BMP receptor complexes transmit signals through SMAD transcription factors or elicit various non-canonical responses, including activation of mitogen-activated protein kinases (MAPKs), phosphoinositide-3-kinase (PI3K), and Rho homologous (Rho) GTPase signaling, among others (Fig. 1) [71]. The control of EndoMT is regulated by activin-like kinase (ALK), the receptors for TGFβ/BMP. Ligands like BMP2, BMP4, and TGFβ2 bind to and activate ALK2 and ALK5, triggering Smad1/5/8 and Smad2/3 signaling to induce EndoMT. On the other hand, BMP7 activates ALK2 but not ALK5, leading to the inhibition of EndoMT [72].
Crucially, the generation of reactive oxygen species (ROS) is also essential for BMP6 to govern osteogenic genes, osteogenic differentiation, and calcification [73]. Additionally, the brain and muscle ARNT-like protein-1 (BMAL1) inhibits ROS-induced EndoMT through BMP signaling, therefore inhibiting atherosclerotic plaque progression [74].
5. Role of the Notch signaling pathway in EndoMT
Additional significant pathways relevant to the induction of EndoMT comprise Notch and Wnt/β-catenin signaling (Fig. 1) [75-77]. The Notch and Wnt pathways are vital during cardiac development [78-81]. Microarray studies demonstrated an upregulation in the expression of Notch signaling mediators, including DLL4, Notch3, and Notch4, as well as Wnt signaling mediators FZD2 and FZD8 upon TGF-β activation [82]. Moreover, mice lacking endothelial β-catenin exhibited a significantly diminished TGF-β induced EndoMT [81].
ECs activated by Notch exhibit characteristics of EndoMT, including the downregulation of endothelial markers and the upregulation of mesenchymal markers [83]. Furthermore, Notch activation leads to the overexpression of Slug in ECs, a phenomenon associated with the loss of the endothelial phenotype [84]. Notch signaling contributes to EndoMT independently of or synergistically with TGF-β. TGF-β1 induces the upregulation of various Notch components, including Jagged-1, the receptor Notch-1, N1ICD, recombination signal binding protein J kappa (RBPJK), as well as target genes, hairy enhancer of split-1 (Hes-1) and Hes-5 [85]. In vitro activation of the Notch signaling pathway induces EndoMT by decreasing VE-cadherin expression and promoting α-SMA overexpression. Conversely, inhibition of the Notch signaling pathway through gamma-secretase inhibitors (GSI) mitigates the development of atherosclerotic lesion [86].
6. Role of non-coding RNAs in EndoMT
Non-coding RNAs, such as microRNA (miRNAs), long non-coding (IncRNAs), and circular RNAs (circRNAs), play important roles in regulating the process of EndoMT. Hulshoff et al. have reported an extensive overview detailing the non-coding RNAs implicated in the regulation of EndoMT [35]. MicroRNAs (miRNA), typically ranging from 20 to 40 nucleotides in length, that exert their regulatory effects by binding to the 3’-UTR of target mRNAs, leading to suppression of target mRNAs [87]. mir-374b causes EndoMT through the targeting of MAPK7. MAPK7, reduced in atheroprone hyperplastic regions, is recognized for its inhibitory role in EndoMT. Suppression of mir-374b targets using short hairpin RNAs (shRNAs) resulted in a specific reduction in MAPK7 signaling components.
In ApoE−/− diabetic mice, miR-449a exhibited heightened expression and influenced EndoMT by upregulating the expression of mesenchymal cell markers while diminishing E-cadherin levels. E-cadherin is known to interact with adiponectin receptor 2 (AdipoR2) in lipid rafts. Treatment of ApoE−/− diabetic mice with a miR-449a antagonist resulted in a decrease in atherosclerotic lesions [88]. It is also reported that elevated levels of miR-122 were detected in both ApoE−/− mice and in in vitro EndoMT models. Inhibiting miR-122 in ApoE−/− mice demonstrated a mitigating effect on the advancement of plaque formation. The study also revealed that miR-122 facilitates plaque formation through NPAS3-mediated EndoMT, suggesting its potential role as a novel therapeutic target in the context of atherosclerosis [89].
6.1. Involvement of endothelial cell heterogeneity in EndoMT during atherosclerosis
The introduction of technologies like scRNA seq and other high throughput methods, along with the concurrent advancement of essential bioinformatics tools, have significantly broadened our understanding of the heterogeneity among ECs [90-93]. Before exploring significant findings on EC heterogenicity, as mentioned earlier, it’s important to emphasize that EndoMT in adults typically involves a partial transition.
Utilizing scRNA seq, Tombor et al. [90] demonstrated that after myocardial infraction, in adults mice EndoMT is both restricted and transient. In a separate study, Xu et al. [91] used scRNA seq to present evidence supporting the involvement of EndoMT in aortic valve calcification. In mouse aorta studies, Kalluri et al. [94] employed scRNA seq along with clustering analysis of gene expression. Through clustering analysis of gene expression in aortic cells, 10 distinct populations were identified, representing the primary arterial cell types: Fibroblasts, VSMCs, ECs and immune cells, including monocytes, macrophages, and lymphocytes. Notably, the most significant cellular heterogeneity was observed within the three distinct EC populations.
Gene set enrichment analysis of these EC populations revealed a lymphatic EC cluster and other two populations with a specialization in lipoprotein handling, angiogenesis, and extracellular matrix production. These subpopulations persist and display similar changes in gene expression in response to a Western diet [94]. While a complete atherosclerosis mouse model was not specifically investigated, these endothelial cell subpopulations were persistent in response to feeding mice a Western diet for 8 weeks [94].
Kan et al. [95] conducted another study using scRNA seq on the mouse aorta, revealing results that closely resembled the earlier findings. Their investigation identified three distinct subpopulations of ECs. In this study, unsupervised cluster analysis of transcriptional profiles from 24,001 aortic cells revealed 27 clusters, representing 10 distinct cell types, including endothelial cells, fibroblasts, VSMCs, immune cells (B cells, T cells, macrophages, and dendritic cells), mesothelial cells, pericytes, and neural cells. Following the intake of a high-fat diet (HFD) that contains less glucose and fructose than a Western diet, specific subpopulations of endothelial cells exhibiting lipid transport and angiogenesis capabilities were identified [95].
Additionally, these ECs displayed extensive expressions of contractile genes like Myl9, Tagln, and Acta2. In the HFD group, three major SMC subpopulations exhibited increased expression of extracellular matrix-degradation genes, and a synthetic SMC subcluster showed proportional augmentation, accompanied by an upregulation of proinflammatory genes [95]. Also, it is identified that under HFD conditions, there was an increase in the numbers of aortic-resident macrophages, and the blood-derived macrophages displayed heightened expression of proinflammatory cytokines. This comprehensive study sheds light on the cellular composition of the ascending aorta, providing valuable insights into the roles of different cell types in the development and progression of aortic inflammatory disease.
Further supporting the hypothesis suggesting the susceptibility of specific EC subpopulations to EndoMT, another scRNA seq investigation delineated distinct subpopulations of ECs and EndoMT-derived cells under the influence of oscillatory shear stress [96]. Using a three-layer human coronary artery-on-a-chip model, shear stress levels comparable to those found in atherosclerosis-prone regions of the human arterial tree were applied. Oscillatory shear stress triggered a proinflammatory EndoMT by activating the Notch1/p38 MAPK-NF-κB pathway. This newly identified proinflammatory EndoMT was observed to stimulate SMC proliferation and matrix protein remodeling, primarily facilitated through the secretion of RANTES (regulated upon activation, normal T cell expressed and presumably secreted).
To fully appreciate EC heterogeneity, it is crucial to acknowledge the plasticity of ECs and the diverse mesenchymal fates that can be assumed through EndoMT. Upon induction of EndoMT, ECs predominantly exhibit transitions into phenotypes resembling fibroblasts, myofibroblasts, and SMCs [34,36,47,48,77,97]. ECs undergoing EndoMT have the capacity to generate additional mesenchymal cell types, such as chondrocytes, osteoblasts, and also in mitral valve endothelial cells [98,99].
In this study, it was revealed that this transition occurs through an intermediary mesenchymal stem-like cell during EndoMT [10]. This noteworthy finding diverges from observations in other studies and adds an interesting dimension to our understanding of the diverse outcomes of EndoMT. Employing additional methodologies such as single-cell resolution proteomic experiments, like cytometry by time of flight (CyTOF), or approaches capable of simultaneous RNA and protein detection, such as CITEseq (cellular indexing of transcriptomes and epitopes by sequencing), hold promise for providing further insights into these processes.
6.2. Impact of endothelial autophagy in the initiation of atherosclerosis
Autophagy is a catabolic process crucial for sustaining normal physiological circulation. It is a cellular pathway specialized for the degradation of proteins and organelles in autophagosomes-double membrane vesicles, playing a crucial role in maintaining cellular metabolic homeostasis [100]. It also serves a protective function in cells by facilitating the movement of intracellular cargo and mediating cellular fate determination. The autophagosomes subsequently merge with lysosomes, giving rise to autophagolysosomes, in which a series of degradation processes take place, contributing to the maintenance of normal cellular metabolism [101].
To date, three primary types of autophagy have been demonstrated. Macroautophagy, also referred to as basal autophagy, begins with the formation of phagophores. Basal autophagy plays a crucial role in maintaining vascular homeostasis in ECs [102].
During this process, membranes form the autophagosome, which then fuses with the lysosome, leading to the degradation of large molecules and prevention of protein aggregation [103]. Microautophagy, another type of autophagy, involves the engulfment of cytoplasmic materials through the invagination of the lysosomal membrane. Microautophagy is essential for maintaining membrane integrity, regulating organelle size, and promoting cell survival during nitrogen starvation [104]. On the other hand, chaperone-mediated autophagy facilitates the transportation of cytosolic components across the lysosome with the help of chaperone proteins. [105]. Increased autophagic flux can counteract vascular injuries by enhancing EC functions, thereby impeding disease progression. However, dysregulated autophagy can lead to cell death in various pathological conditions as ischemic hypoxia or oxidative stress.
Dysregulation of autophagy is intricately linked to various health conditions, including cancer, neurodegenerative disease, and age-related diseases such as obesity, diabetes, and cardiovascular disorders [106-108], revealing that autophagy as a dynamic process aids EC adaptation to environmental changes and regulation of their function [109]. The involvement of autophagy in atherosclerosis remains a subject of debate. On one side of the argument, numerous studies have highlighted a protective effect associated with maintenance of basal autophagy in the context of atherosclerosis [110-112]. Throughout progression, from early to advanced stages of atherosclerotic disease, autophagy significantly influences the behavior of ECs, macrophages, and VSMCs; thereby, influencing the trajectory of the lesion. Analysis of mouse and human samples has identified the presence of autophagy markers, such as LC3-II and p62, within plaque cells. This finding suggests impaired or reduced autophagy in the context of atherosclerosis formation [113,114]. The connection between autophagic activity and atherosclerosis suggests that autophagy in the early stages of atheroma development may act as a transient self-defense mechanism of cell-autonomous immunity. However, this self-defense mechanism tends to decline with prolonged lipid oxidation and oxidative stress [115]. As a result, autophagy may constitute a primary protective mechanism within endothelium [116]. Beyond cellular stress, the activation of basal autophagy can be augmented using specific drugs, suggesting that the autophagic machinery represents a potential therapeutic target for a range of diseases (Table 1) [106].
Table 1.
Anti-atherosclerotic effects of various compounds through modulation of EndoMT and autophagy.
| Compound | Functions | Anti-atherosclerotic Effect |
Reference |
|---|---|---|---|
| Simvastatin | Lipid-lowering medication, inhibit endomt, induce KLF4/mir-483 axis in ecs | Suppressing oxidative stress and tgfβ/SMAD signaling | 205,206 |
| RGFP966 | Inhibitor of histone deacetylase 3 (HDAC3), inhibit endomt | Reduce atherosclerotic lesion burden | 207,208 |
| Icariin | Inhibit ox-LDL induced endomt | Protective effect in atherosclerosis | 209 |
| Rapamycin and geniposide | Inhibit endomt via mtor signaling pathway | Improvement in hypertension, a reduction in central arterial stiffness, lower blood pressure in brachial and carotid arteries. | 164,210,211 |
| Everolimus | Rapamycin derivative, mtor inhibitor, autophagy inducer | Anti-atherosclerotic effect, reducing plaque macrophages, enhancing cholesterol efflux, decreasing systemic and local inflammation, inhibiting intra-plaque neovascularization, promoting plaque stability, and diminishing intimal thickening. | 212-215 |
| Resveratrol | Mtor inhibitor, autophagy inducer | Playing protective role against atherosclerosis, reduce arterial stiffness, reduce vascular endothelial dysfunction, anti-oxidative, antiinflammatory, facilitates efferocytosis of apoptotic cells, decreases atherosclerotic plaque size, density, reduces layer thickness and inhibits age related changes | 212,215,216,153 |
| Metformin | Anti-diabetic medication, inhibit mtor through AMPK stimulation, autophagy inducer | Suppress vascular aging, anti-inflammatory effects on endothelial cells, thereby inhibiting atherosclerosis in diabetic patients | 218,219 |
| Trehalose | Mtor suppressor, autophagy inducer by activating TFEB | Restoration of endothelial function by elevating the availability of NO | 220 |
| Statin | Inhibit endomt, mtor suppressor, autophagy inducer in SMC | Enhancement of endothelial function, stabilization of plaques | 221 |
| miR-100 | Autophagy inducer in ecs | Inhibits NF-κβ activity, suppresses the levels of adhesion molecules in ecs, decreases the recruitment of leukocytes, reduces inflammation and atherosclerosis | 222-224 |
Endothelial dysfunction marks the early stages of atherosclerosis. External stimuli, such as shear stress and ox-LDL, intricately modulate the extent and severity of autophagy within the endothelium. Concurrently, the endothelium responds to the lesion initiation process by intricately regulating oxidative stress, inflammatory responses, death signals, and thrombotic factors in a counteractive manner. It is widely recognized that autophagy plays a crucial role in maintaining the proper functioning of ECs [117]. Accumulating evidence suggests that ox-LDL induces EC injury, contributing to atherosclerotic progression. Conversely, the activation of autophagy has been shown to mitigate EC injury induced by ox-LDL, thereby alleviating atherosclerosis [118-120]. Current research indicates that ox-LDL has the ability to trigger EC autophagy [121]. After ox-LDL is taken up, lipids undergo transportation to autophagic vesicles for degradation facilitated by lysosomes. Concurrently, ox-LDL may induce autophagy by triggering endoplasmic reticulum stress [122].
Autophagy in ECs leads to the induction of eNOS expression, subsequently enhancing the accessibility of NO (Fig. 2). This, in turn, reduces oxidative stress and inhibits the production of inflammatory cytokines [123,124]. Like macrophages and SMCs, excessive autophagic activity in ECs can trigger autophagic-induced cell death, contributing to the instability of plaques [124]. Recent evidence demonstrates that inefficient autophagy contributes to the development of atherosclerotic plaques, promoting inflammation, apoptosis, and a senescent phenotype in ECs [125]. However, despite the growing interest in autophagy within various pathophysiological contexts like neurodegeneration, cancer, and cardiac myopathies, its significance in atherosclerosis remains underestimated and overlooked.
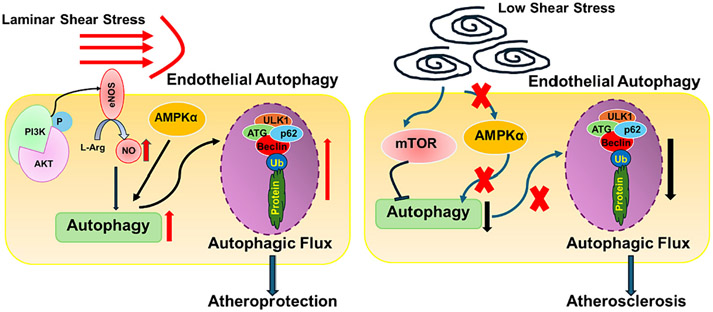
Fig. 2.
A schematic representation demonstrating the response of endothelial autophagy to shear stress and its potential impact on the progression of atherosclerosis. Steady laminar flow can trigger endothelial cells (ECs) to produce nitric oxide (NO) through eNOS activation and stimulate AMPKα, thereby enhancing autophagy levels in ECs, which may help hinder the disease progression. Conversely, low shear stress inhibits AMPKα and activates mTOR pathway, disrupting autophagy and contributing to the development of atherosclerosis.
Additional evidence also indicates that the manipulation of specific microRNAs, particularly miRNA876, in conjunction with an apoptotic agent, could amplify the adverse effects of atherosclerosis on the luminal surface [126]. Moreover, autophagy in ECs governs the secretion of von Willebrand factor, which is an integral constituent of the coagulation process [127]. Zhang et al. illustrated that ox-LDL has the capability to activate autophagy in ECs. Following the uptake of ox-LDL, lipids are conveyed to autophagic vesicles for degradation through lysosomal-mediated processes [127]. Ox-LDL might additionally induce autophagy by provoking endoplasmic reticulum stress. Demonstrating the significance of autophagy in ECs, that transient knockdown of the essential autophagy gene ATG7 led to elevated intracellular levels of intermediate-density lipoprotein (I-LDL) and ox-LDL. This suggests that autophagy plays a crucial role in regulating excess exogenous lipids in ECs [122].
Under high shear stress conditions, EC autophagic fluxes are enhanced, potentially orchestrated by the transcription factors Kruppel-like factor (KLF)2 and KLF4, along with Sirt-1 activating FoxO1 [128,129]. Conversely, low shear stress inhibits AMPKα and activates mTORC1, impeding autophagic flux (Fig. 2). This inhibition ultimately leads to observed cell death, senescence, inflammation, and a predisposition towards atherosclerosis development [128,129]. In an inflammatory environment, ECs suppress the expression of adhesion molecules on their membranes, including PECAM-1 and VE-cadherin, through robust induction of autophagy [130]. This intense autophagy by EC inhibits tissue infiltration resulting from the transendothelial migration of neutrophils, effectively disrupting the potential vicious cycle of subsequent inflammatory responses. It suggests that modulating EC autophagy to inhibit monocyte invasion and infiltration into the subendothelium could serve to temporarily halt the atherogenic process. In summary, endothelial autophagy stands out as a promising tool in addressing endothelial dysfunction. Modulating endothelial autophagy could present a promising avenue for developing a sophisticated treatment for atherosclerosis.
During the intermediate stages of atherosclerosis, autophagy in macrophages plays a pivotal role in suppressing foam cell formation, thereby impeding the progression of atherosclerosis [131]. Autophagy in macrophages is recognized for its crucial protective role in atherosclerosis [132]. Autophagy plays a facilitative role in the degradation process of lipid droplet transport into lysosomes, leading to the efflux of free cholesterol from foam cells and ultimately reducing the formation of foam cells [133].
Consistent with this assertion, suppressing autophagy in macrophages triggers plaque destabilization, leading to the initiation of necrosis through the luminal surface. Conversely, inducing autophagy in macrophages through mTORC1 inhibition contributes to the stabilization of atherosclerosis plaques [134]. Moreover, autophagy exerts an influence on the polarization of macrophages, and the activation of autophagy influences the development of macrophages towards the M2 phenotype, which is characterized by anti-inflammatory properties [135].
In the advances stages of atherosclerosis, the autophagic responses in macrophages are significantly impaired, resulting in the accumulation of lipids, compromised mitochondrial clearance, and the death of macrophages, contributing to the formation of larger necrotic cores [136]. It appears that the activation of C1q/CTRP9, a pro-inflammatory agent during atherosclerotic changes, may initiate the autophagy-related signaling pathway in foamy macrophages, consequently hindering the formation of atherosclerotic lesions in ApoE−/− mice [137,138]. It was reported that the beneficial effects of trehalose administration on autophagy and atherosclerosis involve the induction of lysosomal biogenesis factor TFEB in murine macrophage cells in vivo. These findings support the athero-protective role of autophagic activity in macrophages [139].
Through the modulation of VSMC phenotype, autophagy plays a crucial role in atherosclerosis [140]. A balanced and normal autophagic activity in SMCs is associated with cell survival and contributes to plaque stability. However, excessive autophagic activity can lead to SMC death and subsequent plaque destabilization [141,142]. In the advanced stages of atherosclerosis, the apoptosis of VSMCs, which are the exclusive producers of interstitial collagen fibers within the fibrous cap, inevitably leads to diminished collagen fiber synthesis and thinning of the fibrous cap. Consequently, this process largely influences the vulnerability of the plaque and its propensity to rupture.
The phenotype and function of VSMCs are influenced by autophagy, as evidenced by increased secretion of extracellular matrix and decreased calcification [143]. Recent documentation reveals that inhibiting autophagy through the knockout of Atg7 in SMCs in animal models has detrimental consequences. These include increased senescence, neointima formation and atherogenesis. Furthermore, defective autophagy in Atg7 and ApoE−/− mice have been implicated not only in plaque formation but also in plaque instability and rapture [144]. When Atg7 and ApoE−/− mice were fed a Western diet, there was an observed increase in the number of autophagosomes inside SMCs, suggesting an impaired autophagic response [145]. The autophagy of SMCs may also be regulated by various cytokines, including TNF-α and osteopontin, as well as growth factors such as PDGF [146].PDGF, secreted by several cell types during vascular injury protects against cellular death via activation of autophagy [147].
7. Autophagy and efferocytosis in atherosclerosis
Autophagy literally means “self-eating” and it is a self-protective process. Increasing evidence highlights the significant role of autophagy in suppressing inflammation and apoptosis, while concurrently promoting efferocytosis and cholesterol efflux [141]. The initiation and progression of atherosclerosis depends on three crucial cell types: Macrophages, SMCs, and ECs. Of these, macrophages are the most extensively studied cell-types.
It is commonly acknowledged that apoptosis in macrophages within atherosclerotic lesions, coupled with their impaired efferocytotic function, accelerate plaque necrosis. This process contributes to plaque instability, thrombosis, and ultimately precipitates cardiovascular events [148,149]. The buildup of monocyte-derived macrophages is considered a critical stage in the initiation and evolution of atherosclerotic plaques, with the degree of monocyte accumulation escalating as plaques progress [150]. This accumulation not only diminishes the engrafting function of macrophages within plaques but also exacerbates the impairment of efferocytosis in lesional macrophages due to macrophage apoptosis [151]. Consequently, this sequence of events results in secondary necrosis, further amplifying plaque inflammation [152].
It has been observed that autophagy plays a preventive role in lesional macrophage apoptosis and defective efferocytosis. Using LDL−/− mice, Liu and colleagues revealed that autophagy deficiency increased the overall necrotic area in advanced atherosclerotic plaques and exacerbates the damage induced by oxidative stress [148]. The authors illustrated that the use of resveratrol, a naturally derived phenol, enhanced the efferocytosis of oxLDL-induced apoptotic cells in RAW264.7 cells through the activation of Sirt1-mediated autophagy. This not only demonstrated a protective effect of resveratrol could potentially serve as a novel therapeutic approach for atherosclerotic treatment [153].
Brophy et al. reported that myeloid epsins contribute to atherogenesis by promoting proinflammatory macrophage recruitment and inhibiting efferocytosis, partly through the downregulation of LRP-1. This suggests that targeting epsins in macrophages could represent a novel therapeutic strategy for treating atherosclerosis [154]. We have previously reported the molecular and cellular mechanisms governing efferocytosis in vascular cells, including macrophages and other phagocytic cells. We previously explored the intricate roles of efferocytosis-related molecules in preserving vascular hemostasis and elucidated how impaired efferocytosis contributes to the initiation and advancement of atherosclerotic plaques [155].
It has been demonstrated that atherogenesis is linked to the upregulation of CD47, a crucial anti-phagocytic molecule recognized for conferring resistance to efferocytosis [156,157]. Recent investigations have identified CD47 as a potential target to hinder impaired macrophage efferocytosis within atherosclerotic lesions [158]. CD47, typically found on viable cells, acts as a “do not eat me” signal for efferocytes. It signals through the phagocyte SIRPα receptor protein, preventing engulfment. The expression of CD47 increases as atherosclerotic lesions progress, observed in both human and ApoE−/− mice. CD47 in plaques is specifically localized to dying macrophages, SMCs, and the necrotic core [158]. Furthermore, the administration of antibodies against CD47 in various mouse models significantly decreased atherosclerotic lesion formation compared to controls, resulting in reduced necrotic core formation and fewer apoptotic cells unrelated to macrophages. The elevated expression of CD47 in cells undergoing apoptosis induced by proatherogenic oxidized phospholipids is associated with TNF-α signaling through TNFR1, leading to enhanced transcription via NF-κB activation [158].
Additional studies have indicated that enzyme-triggered primary necrotic death in cells hinders the efficacy of efferocytosis for these dying cells. This inefficiency is likely attributed to abnormal CD47 expression in cells undergoing this form of cell death. The interplay between apoptosis, efferocytosis and autophagy is intricately complex, at times exhibiting contradictions, yet undeniably important in determining the overall fate of the cell. Moreover, this crosstalk serves as a key factor influencing the outcome of death-related pathologies like atherosclerosis, including both its development and treatment.
7.1. Exploring the interplay: EndoMT and autophagy in atherosclerosis
Vascular ECs possess capabilities in proliferation, adherence, migration, and secretion. Injury to vascular ECs compromises the integrity and barrier function of the endothelium, promoting the deposition of lipids and contributing to the development of atherosclerosis [159]. Earlier investigations have revealed the role of EndoMT in fibrotic aspects of plaque formation and the instability of plaques in the pathological progression of atherosclerosis [14,23]. Given the substantial role of EndoMT in the atherosclerosis process [160], it is crucial to explore novel targets for the prevention and treatment of atherosclerosis, particularly in alleviating vascular endothelial injury.
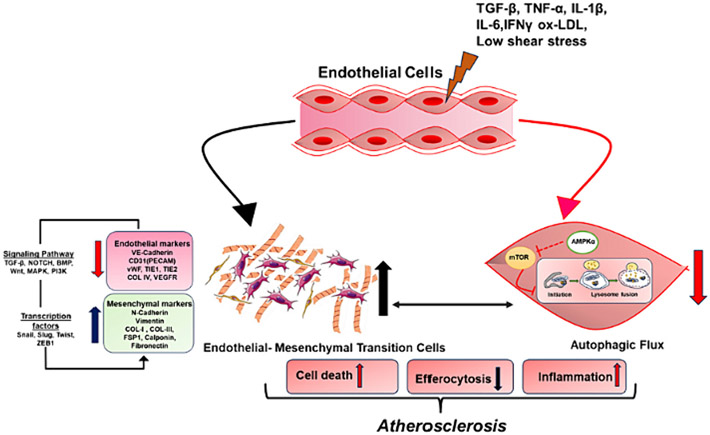
Autophagy is emerging as a primary protective mechanism in endothelium [116]. The dysregulation of autophagy in ECs has been reported to be linked with various pathologic conditions, highlighting the biological significance of autophagy [161-163]. The association between activation of autophagy and the atherosclerotic process suggests that autophagy in early atheroma lesions acts as a transient self-defense mechanism, diminishing over time in the face of prolonged lipid oxidation and oxidative stress [115]. However, the connection between endothelial autophagy and EndoMT remains unexplored, and further investigation is needed to understand the mechanisms underlying autophagy induced EndoMT (Fig. 3).
Fig. 3.
Interplay between endothelial-to-mesenchymal transition (EndoMT) and endothelial autophagy in the pathogenesis of atherosclerosis.
Zou et al. demonstrated that autophagy attenuates EndoMT by promoting Snail degradation in human cardiac microvascular endothelial cells (HCMECs) [164]. In this study, Zou et al. observed simultaneous induction of EndoMT and autophagy by hypoxia in HCMECs. They found that rapamycin, an autophagy enhancer, mitigated EndoMT while promoting angiogenesis. Conversely, agents inhibiting autophagy, such as 3-methylademine (3-MA) and chloroquine (CQ), accelerated the progression of EndoMT, accompanied by a decrease in tube formation under hypoxic conditions. Interestingly, the authors noted that modulating autophagy using rapamycin, 3-MA, or CQ did not influence hypoxia-induced autocrine TGFβ signaling. However, it did alter the expression of Snail protein without affecting Snail mRNA expression. Furthermore, the colocalization of LC3 and Snail suggested that autophagy might mediate Snail degradation under hypoxic conditions in HCMECs. This hypothesis was confirmed through Co-immunoprecipitation revealed the interaction of p62, an autophagy substrate, with Snail, particularly in cells incubated under hypoxia [164].
Wang et al. also showed that autophagy opposes the EndoMT process induced by TGF-β2 by reducing the phosphorylation level of Smad3 [165]. Singh et al. demonstrated that reducing ATG7 expression promotes EndoMT in vitro and increases the expression of key genes involved in TGFβ signaling and fibrosis. They suggest that autophagy could be serve as a significant and innovative pathway connecting EndoMT to organ fibrosis [166]. Zhang et al. showed that AGEs/RAGE-autophagy-EndoMT axis involved in the development of cardiac fibrosis. Knockout of RAGE resulted in the mitigation of cardiac fibrosis by reducing EndoMT regulated by autophagy [97]. In this study, the knockout of RAGE resulted in EndoMT, along with reduced expression of autophagy-related proteins (LC3BII/I and Beclin 1). This intervention alleviated cardiac fibrosis and improved cardiac function in transverse aortic constriction (TAC) mice.
Additionally, inhibitors of autophagy, 3-methylademine (3-MA) and chloroquine (CQ), mitigated both EndoMT and cardiac fibrosis in TAC mice. Notably, the induction of EndoMT by an autophagy inhibitor both in vivo and in vitro [97]. Gao et al. discovered that Sirtuin3 (SIRT3), an NAD-dependent deacetylase, is a key cellular sensor of energy metabolism, controlling EndoMT. The reduction of SIRT3 further triggered the hyperacetylation of endogenous autophagy-regulated gene 5 (ATG5), thereby inhibiting autophagosome maturation and increasing the expression of pyruvate kinase M2 (PKM2) dimer [167]. Moreover, TEPP-46, a selective PKM2 tetramer activator, resulted in decreased lactate levels and a reduction in EndoMT both in vitro and in vivo. Concurrently, inhibiting lactate influx from ECs to VSMCs decreased the expression of synthetic VSMC markers. Transgenic mice with EC-specific overexpression of SIRT3 showed decreased EC transition and partial improvement in vascular fibrosis and collagen accumulation [167].
Hammoutene et al. observed that a deficiency in endothelial autophagy not only contributes to the initiation of liver inflammation, characteristics of endothelial- to- mesenchymal transition, apoptosis, and liver fibrosis during the early phases of non-alcoholic steatohepatitis (NASH) but also supports the progression to more advanced stage of liver fibrosis [168]. Liver sinusoidal endothelial cells (LSECs) from mice deficient endothelial autophagy exhibited increased expression of genes associated with inflammatory pathways. Furthermore, deficiency in autophagy in the LSEC line amplified inflammation (Ccl2, Ccl5, Il6, and VCAM1 expression), features of EndoMT (α-Sma, Tgfb1, Col1a2 expression), and apoptosis (cleaved caspase-3), and perisinusoidal fibrosis. Additionally, mice lacking endothelial autophagy and treated with carbon tetrachloride showed increased perisinusoidal fibrosis [168].
Nivoit et al. showed that endothelial ATG5 plays a pivotal role in the activation of eNOS induced by both fluid shear stress and VEGF [169]. This function regulates vascular tone, tissue perfusion, and adaptive arterial remodeling [169]. Additionally, they showed that endothelial autophagy is essential for maintaining optimal VEGFR2 activity, promoting endothelial recovery following injury, and facilitating neoangiogenesis [169]. Mesenchymal-to-endothelial transition (MEndT) is one of the mechanisms that impacts cardiac fibrosis, playing a pivotal role in cardiac remodeling. Hu et al. discovered that autophagy activation promotes MEndT and increases cytoplasmic and total expression of p53, while decreasing nuclear p53 expression [170]. Furthermore, after nuclear p53 knockout, autophagy promoted MEndT, whereas autophagy inhibited MEndT in p53 overexpressing cells. These findings highlight the role of autophagy in regulating MEndT through nuclear p53 and suggest a new strategy for treating fibrosis diseases [170].
Increasing evidence suggests that Wnt/β-catenin pathways regulate cell proliferation, senescence, and apoptosis [171-173]. Numerous reports highlight that the activation of Wnt/β-catenin serves as a negative regulator of autophagy. For example, there are reports indicating that the activation of Wnt/β-catenin hinders the expression of Beclin1, a pivotal component in autophagic flux [174]. Conversely, documented evidence suggests that the activation of autophagy can suppress Wnt/β-catenin signaling by degrading Dvl [175] or β-catenin [176]. In colorectal tumors, the level of autophagy exhibits an inverse correlation with the activation of Wnt/β-catenin [177]. In glioblastoma, multiple myeloma, and mammary tumors, inhibiting the Wnt/β-catenin pathway was observed to increase the expression of p62, LC3, and Beclin1, consequently enhancing autophagic flux [178-180]. In ovarian cancer, it was demonstrated that the DACT1 protein inhibits Wnt/β-catenin signaling and activates autophagy [181]. On the contrary, in lung cancer, the WIF-1 protein induced autophagy and inhibited Wnt/β-catenin signaling [182]. Additionally, activation of Wnt/β-catenin pathways have been shown to enhance EndoMT [183]. A prior study showed that autophagy precludes HCMECs from undergoing EndoMT [164,184]. Canonical Wnt signaling activity is a distinctive feature of EndoMT [185]. Both autophagy and Wnt/β-catenin pathways play roles in the processes of proliferation, apoptosis, and survival in ECs exposed to either high glucose, hypoxia, or oxidative stress [186].
In a recent study, it was reported that activation of autophagy reduced expression of mesenchymal markers in atherosclerosis models and mitigated endothelial dysfunction [186]. Additionally, the same study revealed that B cell lymphoma 2-associated athanogene (BAG3) protected against EC injury induced by defective autophagy and EndoMT, where EndoMT itself was prompted by defective autophagy. BAG3 has been demonstrated to play a regulatory role in tumor angiogenesis, neurodegenerative diseases, and cardiac diseases. However, its involvement in atherosclerosis is still not well understood.
Diao et al. reported that BAG3 prevents endothelial injury by activating autophagy through the formation of the chaperone-assisted selective autophagy (CASA) complex, thereby contributing to the amelioration of atherosclerosis. The study established a correlation between EndoMT and autophagy and clarified that BAG3 regulates autophagy induced EndoMT by constituting part of the CASA complex [186].
8. Role of TGFβ in regulating EndoMT and autophagy
The TGFβ1 signaling exerts wide-ranging effects that could impact cell growth, differentiation, and the synthesis of extracellular matrix (ECM) proteins [187,188]. Increased levels of TGFβ1 are detected in the heart of rat’s post-myocardial infraction (MI), and this elevation correlates with the phenotypic transition of fibroblasts to myofibroblasts, accompanied by the activation of canonical Smad signaling [189]. Ghavami et al. demonstrated a connection between autophagy and TGFβ1-induced fibrogenesis in human atrial myofibroblasts (hAT-Myofbs) and in a rat model of myocardial infraction (MI) [190]. The authors observed that TGFβ1 enhanced the synthesis of collagen type Iα2 and fibronectin in human atrial myofibroblasts (hATMyofbs), a phenomenon which coincided with an upsurge in autophagic activity in these cells.
Knockdown of ATG7 in hATMyofbs and knockout of ATG5 in mouse embryonic fibroblasts reduced the fibrotic response to TGF-β1 compared to control cells in the experimental setting. The fibrotic response in hATMyofb cells was attenuated by pharmacological inhibition of autophagy using bafilomycin-A1 and 3-methyladenine [190]. The authors also noted elevated levels of protein markers associated with fibrosis, autophagy, and Smad2 phosphorylation in lysates of whole scar tissue. Additionally, they observed the colocalization of punctate LC3B with vimentin, ED-A fibronectin and phosphorylated Smad2 [190].
Dysregulation of TGFβ signaling pathways contributes to the progression of several types of tumors. Suzuki et al. reported that TGFβ induces autophagy in hepatocellular carcinoma cells and mammary carcinoma cells and examined its role in the growth- inhibitory function of TGFβ [191]. The authors demonstrated that TGFβ activates autophagy in specific hepatocellular carcinoma cell lines, leading to cell cycle arrest and apoptosis. In HuH7 human hepatocellular carcinoma cells, TGFβ triggers the accumulation of autophagosomes and conversion of LC3 to its lapidated form, LC3II. The activation of autophagy flux by TGFβ is supported by additional findings showing that TGFβ increases enhances the degradation rate of long-lived proteins and turnover of LC3II [191]. Moreover, TGFβ increases the mRNA expression levels of several autophagy-related genes, including BECN1, ATG5, ATG7, and DAPK. Interestingly, the authors also discovered that TGFβ upregulates certain autophagy-related genes in a Smad4-dependent manner and necessitates new RNA synthesis for inducing autophagy. Therefore, it is suggested that TGFβ could induce autophagy, partially through the Smad pathway and the transcriptional activation of autophagy- related genes [191] (Fig. 4).
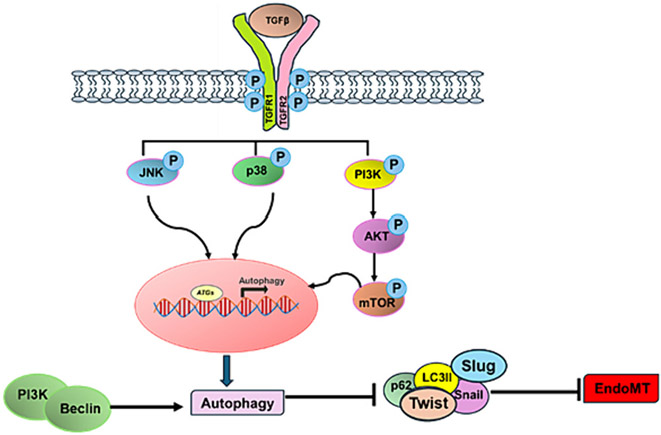
Fig. 4.
A potential mechanism where TGFβ plays a role in the interaction between endothelial autophagy and EndoMT. TGFβ operates as a pivotal player in the intricate interplay between endothelial autophagy and EndoMT. In various disease progressions, TGFβ activates multiple pathways. Autophagy and EndoMT mutually regulate each other. TGFβ stimulates the expression of pro-autophagic genes (ATGs) via the p38 and JNK pathways. Moreover, TGFβ triggers the PI3K-AKT-mTOR pathway, which is associated with promoting EndoMT. Additionally, another potential mechanism involves the autophagic-promoting complex PI3K/BCN1, which facilitates the autophagy-mediated degradation of transcription factors Snail, Slug, and Twist, thereby mitigating EndoMT.
Trelford et al. explored how autophagy, a cellular mechanism for quality control that transports materials to lysosomes, modulates TGFβ signaling pathways involved in promoting epithelial to mesenchymal transition (EMT) and cell migration [192]. By impairing autophagy in non-small cell lung cancer cells using chloroquine, spautin-1, ULK-101, or small interfering RNA (siRNA) targeting autophagy-related genes (ATG5 and ATG7), the authors observed a reduction in TGFβ1-dependent expression of EMT transcription factors and cell markers, as well as diminished stress fiber formation and cell migration [192] Inhibition of autophagy reduces pro-tumorigenic TGFβ signaling by controlling receptor trafficking, leading to decreased phosphorylation of Smad2/Smad3 and diminished nuclear accumulation [192]. TGFβ1 elevates Unc51-like kinase 1 (ULK1) protein levels, triggers AMPK-dependent phosphorylation of ULK1 at serine (S) 555, promotes ULK1 complex formation, and concurrently reduces the activity of mechanistic target of rapamycin(mTOR) on ULK1 [193].
Both the canonical Smad4 pathway and the non-canonical TGFβ activated kinase1/tumor necrosis factor receptor-associated factor 6/P38 mitogen-activated protein kinase (TAK1-TRAF6-P38 MAPK) pathways play crucial roles in TGFβ1-induced autophagy. Specifically, the TAK-TRAF6-P38 MAPK was found to be vital for the downregulation of mTOR S2448 phosphorylation, ULK1 S555 phosphorylation, and autophagosome formation [193]. Although silencing Smad4 using siRNA did not affect mTOR-dependent ULK1 S757 phosphorylation, it did lead to a reduction in AMPK-dependent ULK1 S555 phosphorylation and autophagosome formation. Additionally, both Smad4 silencing and inhibition of the TAK1-TRAF6-P38 MAPK pathway resulted in decreased colocalization of autophagosomes with lysosomes in the presence of TGFβ [193].
Takagaki et al. revealed an intriguing link where autophagy deficiencies in ECs led to IL6-dependent EndoMT and subsequent organ fibrosis, accompanied by metabolic abnormalities in mice. Inhibition of autophagy, either through a specific inhibitor or siRNA targeting ATG5 in HMVECs, induced EndoMT. Elevated IL6 levels were observed in the culture medium of ATG5 siRNA-transfected HMVECs compared to the control group, and neutralizing IL6 with a specific antibody completely prevented EndoMT in ATG5 siRNA-transfected HMVECs [194]. It was observed that endothelial-specific atg5 knockout mice (Atg5 Endo; Cdh5-Cre Atg5 flox/flox mice) exhibited both kidney and heart fibrosis associated with EndoMT compared to controls. Plasma IL6 levels were elevated in Atg5 Endo mice compared to controls, and fibrosis was accelerated in HFD-treated Atg5 Endo Mice. Neutralization of IL6 with a specific antibody alleviated EndoMT and fibrosis in HFD-fed Atg5 Endo mice, leading to the improvement of metabolic abnormalities [194].
Cerebral cavernous malformation (CCM) is a congenital cerebrovascular disorder, impacting around 0.3–0.5% of the population. It is marked by enlarged and leaky capillaries, increasing the risk of seizures, focal neurological impairments, and potentially fatal intracerebral hemorrhages. Marchi et al. proposed that defective autophagy is closely associated with EndoMT, a pivotal process contributing to the progression of CCM [195]. The deletion of the KRIT1, one of the three major genes mutated in CCMs, significantly inhibits autophagy, resulting in the abnormal buildup of the autophagy adaptor p62/SQSTM1, impaired quality control mechanisms, and heightened intracellular stress. Loss of KRIT1 function triggers activation of the mTOR-ULK1 pathway, a key regulator of autophagy, and treatment with mTOR inhibitors partially restores some of the molecular and cellular abnormalities linked to CCM [195]. Similarly, defective autophagy is also observed in CCM2-silenced human ECs, as well as in cells and tissues from an endothelial-specific CCM3-knockout mouse model, and in human CCM lesions [195].
9. Role of mTOR in regulating EndoMT
Autophagy has emerged as a potentially crucial factor in regulating EndoMT by reducing TGF-β2-induced EndoMT [196]. Additionally, autophagy activation has been demonstrated to diminish Snail expression by reducing Smad3 phosphorylation levels, thereby counteracting EndoMT [165]. Moreover, the pharmacological inhibition of mTOR has been linked to autophagy activation and a reduction in EndoMT, indicating a direct association between mTOR-mediated autophagy inhibition and EndoMT [197]. The mTOR pathway exerts precise control over the various stages of autophagy. In instances of starvation, where energy production is compromised, the activation of AMPK and subsequent inhibition of mTOR result in the stimulation of autophagy. It was demonstrated that mTOR phosphorylation played a role in EndoMT, and in vitro studies using cultured rat pulmonary artery ECs revealed that BMP-7 suppressed hypoxia-induced phosphorylation of mTORC1. These collective findings highlight BMP-7 as a potent antagonist of hypoxia-induced EndoMT in pulmonary artery ECs, with its mechanism of action involving the mTORC1 signaling pathway [198].
Bleomycin (BLM) is recognized as a gentle and efficacious sclerosant, extensively employed in the management of vascular malformations (VMs) [199]. BLM is also a widely used inducer of fibrosis and is frequently employed to establish models of pulmonary fibrosis [200,201]. Remarkably, a recent study revealed distinct morphological changes in pulmonary ECs within a BLM- induced pulmonary model, which were subsequently identified as EndoMT [202]. Zhang et al. demonstrated that sustained exposure to BLM triggered alterations in ECs resembling EndoMT, with the transformation influenced by the EMT-associated transcription factor Slug in an Akt/ mTOR pathway-dependent manner [203].
He et al. elucidated the underlying mechanism involving the long-coding RNA maternally expressed gene 3 (MEG3) and DNA methyl-transferase 1 (DNMT1) in EndoMT associated with diabetic retinopathy (DR) [204]. Using a rat model induced by streptozotocin (STZ) injection and a high-glucose -induced cell model, they found that DNMT1 facilitated MEG3 promoter methylation, suppressing MEG3 expression by recruiting methyltransferase. This process activated the PI3K/Akt/mTOR signaling pathway, thereby accelerating EndoMT in DR [204].
10. Therapeutic implications
Considering the impact of EndoMT on regulating atherosclerosis, targeting EndoMT disruption could present a therapeutic avenue for addressing this condition. In fact, certain compounds and clinical medications may exhibit protective effects against atherosclerosis by inhibiting EndoMT (Table 1). Similar to EndoMT, autophagy undergoes upregulation in numerous cardiovascular diseases, exhibiting both protective and detrimental effects depending on the context and the specific disease. Therefore, pharmacological modulation of autophagy emerges as an innovative therapeutic strategy to prevent or mitigate myocardial damage during various diseases.
EndoMT is a multifaceted process influenced by numerous factors, including signaling pathways and non-coding RNAs. Despite this complexity, there remains a shortage of effective drugs capable of reversing EndoMT. Looking ahead, the utilization of single-cell/high-throughput sequencing technology may offer valuable insights into identifying EndoMT-associated targets for the treatment of atherosclerosis.
Simvastatin, a lipid-lowering medication used clinically, was found to inhibit EndoMT. Research by Lai et al. revealed that simvastatin can impede EndoMT through the upregulation of the KLF4/miR-483 axis in HUVEC [205]. Furthermore, simvastatin attenuated 1-Palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine (POVC)-induced EndoMT by suppressing oxidative stress and TGFβ/SMAD signaling, suggesting its potential therapeutic application in addressing atherosclerosis [206]. RGFP966, an inhibitor of histone deacetylase 3 (HDAC3), is a significant regulator of cardiovascular diseases and has been identified as upregulated in atherosclerotic disease [207]. It demonstrates the ability to reduce atherosclerotic lesion burden by inhibiting EndoMT in the aortic root [208]. On the other hand, icariin, a compound derived from epimedium, effectively inhibits ox-LDL-induced EndoMT through the H19/miR-148b-3p/ELF5 (E74-like factor 5) pathway. By inducing H19 overexpression, icariin attenuates the EndoMT process, thereby exerting a protective effect in atherosclerosis [209]. Rapamycin and geniposide, both known as autophagy enhancers, were found to mitigate EndoMT via mTOR signaling pathway. This mechanism was validated in vitro using HUVECs and cardiac microvascular ECs, as well as in vivo in bleomycin mouse model of tissue fibrosis [164,210].
Fundamental research and numerous clinical trials have focused on targeting the autophagic process to treat atherosclerosis. Based on these studies, various autophagy stimulators have demonstrated effectiveness in mitigating atherosclerosis. Therefore, stimulation of autophagy holds promise as a treatment strategy for atherosclerosis, and targeted therapies directed at autophagy may be effective in this context (Table 1). The therapeutic significance of mTOR inhibitors in various diseases is well-documented. In human kidney transplant patients, rapamycin, an mTOR inhibitor, demonstrated cardioprotective benefits. The treatment led to an improvement in hypertension, a reduction in central arterial stiffness, and lowered blood pressure in brachial and carotid arteries [211].
Everolimus, a rapamycin derivative and mTOR inhibitor, is among the most extensively researched and well-known inducers of autophagy. These agents manifest a variety of anti-atherosclerotic effects, such as reducing plaque macrophages, enhancing cholesterol efflux, decreasing systemic and local inflammation, inhibiting intra-plaque neovascularization, promoting plaque stability, and diminishing intimal thickening [212-214]. The increased clearance of toxic materials by Everolimus, through mTOR inhibition and subsequent induction of autophagy, has shown promise [215]. However, these encouraging findings are somewhat overshadowed by its adverse effects on blood lipid and glucose levels, making combined therapy with statins or metformin desirable.
Resveratrol, another mTOR inhibitor and autophagy inducer, possesses anti-oxidative and anti-inflammatory properties, playing a protective role against various diseases such as atherosclerosis. It facilitates the efferocytosis of apoptotic cells, decreases atherosclerotic plaque size and density, reduces layer thickness and inhibits age-related changes [212,215,216]. A recent in vitro study further confirmed that resveratrol promotes the efferocytosis of apoptotic cells by activating autophagy [153]. Resveratrol exhibits vascular benefits by mitigating arterial stiffness and vascular endothelial dysfunction. It achieves this by enhancing NO-mediated vasorelaxation, which in turn reduces vascular oxidative stress and inflammation while suppressing endothelial apoptosis [217].
Metformin, an anti-diabetic medication, activates autophagy and inhibits mTOR through AMPK stimulation. It has been shown to reduce vascular complications in diabetic patients and suppress vascular aging, thereby inhibiting atherosclerosis in diabetic individuals [218,219]. Recent findings indicate that metformin’s anti-inflammatory effects on ECs are autophagy-dependent, although further research is needed to fully understand whether metformin’s cardiovascular benefits are directly linked to autophagy activation. Trehalose induces an increase in autophagic flux by activating TFEB, a process normally suppressed by mTOR. This mechanism contributes to the restoration of endothelial function by elevating the availability of NO in the arteries of elderly mice [220].
Statins, renowned for their pleiotropic effects including anti-inflammatory properties, enhancement of EC function, and stabilization of plaques, have been shown to trigger autophagy in SMCs by inhibiting the mTOR pathway [221].
MiR-100 reduces inflammation and atherosclerosis by promoting endothelial autophagy. An elevated expression of miR-100 enhances autophagic flux, thereby reducing NF-κB activity and suppressing levels of adhesion molecules in ECs, leading to decreased recruitment of leukocytes. RAPTOR, a component of mTORC1, is targeted by miR-100 in the endothelium [222]. Ultimately, caloric restriction and endurance exercise offer advantages in combating cardiac aging by inducing autophagy through the suppression of mTOR [223]. In the vascular context, it mitigates wall thickening and vascular stiffness, and enhances eNOS expression and bioavailability, thereby, restoring endothelial function [224].
In conclusion, while autophagy frequently serves as a mechanism for cell survival, excessive induction of autophagy could lead to autophagic cell death. Therefore, it is crucial to accurately determine and target the therapeutic window for modulating autophagy. Finally, we anticipated that future research would provide novel drugs or drug combinations that effectively harness the beneficial outcomes of autophagy induction and inhibition of EndoMT in atherosclerosis and various other vascular conditions.
10.1. Concluding remarks and future perspectives
In this review, we have expounded the interplay between EndoMT and endothelial autophagy in the context of atherosclerosis. ECs, as the innermost layer of blood vessels, are the initial targets in the onset of atherosclerosis. Disrupted shear stress and elevated lipid levels in the blood exert an overwhelming burden on the endothelium, triggering its activation and instigating the pathological progression of atherosclerosis.
Over the past two decades, our understanding of EndoMT has evolved from a theoretical conception to a well-characterized biological process. Moreover, recent fundamental studies have elucidated the role of autophagy in endothelium. These studies demonstrate that endothelial autophagy is fundamentally cytoprotective and plays a regulatory role in response to blood flow and stress. An even less investigated aspect of endothelial autophagy relates to its role in crucial aspects of endothelial cell plasticity. In this context, impaired autophagy results in a notable loss of endothelial cell markers, a characteristic typically associated with EndoMT. The link between autophagy and EndoMT has been ascertained in the progression of cardiovascular disease [225]. A recent study employing scRNA-seq revealed significant upregulation of autophagy-associated genes in a subset of EC undergoing endothelial-to-hematopoietic transition [226], emphasizing the pivotal role of autophagy in regulating EC plasticity.
Although the significance of vascular inflammation in atherosclerosis has long been acknowledged, the factors facilitating its progression and enhancing its therapeutic resistance have scarcely been explored. Moreover, treatments centered on lipid lowering therapies or singlecytokine inhibition, at best exhibit a slowing effect but do not halt or reverse the disease. The recognition of EndoMT-induced autophagy as the central mechanism governing ongoing vessel wall inflammation holds the potential to reveal novel therapeutic avenues. The efficient endothelial-specific suppression of these signaling cascades not only appear to inhibit vessel wall inflammation and impede the growth of atherosclerotic plaques but also induces significant regression of mature atherosclerotic lesions in mouse models.
Acknowledgements
This work was supported by NIH R01 grants (HL162367, HL141853, HL156362, HL158097, HL093242, and HL146134) to H.C., D-Z.W. and J.S., and a NIH K99 grant (HL171947) to K.C. as well as an American Heart Association (AHA) Career Development Award (24CDA1272397) to B.S.
Footnotes
CRediT authorship contribution statement
Bandana Singh: Writing – original draft. Kui Cui: Writing – review & editing. Shahram Eisa-Beygi: Writing – review & editing. Bo Zhu: Writing – review & editing. Douglas B. Cowan: Writing – review & editing. Jinjun Shi: Writing – review & editing. Da-Zhi Wang: Writing – review & editing, Funding acquisition. Zhenguo Liu: Writing – review & editing, Funding acquisition. Joyce Bishoff: Writing – review & editing. Hong Chen: Writing – review & editing, Writing – original draft, Resources, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- [1].Libby P, et al. , Atherosclerosis, Nat. Rev. Dis. Primers 5 (1) (2019) 56. [DOI] [PubMed] [Google Scholar]
- [2].Zhu Y, et al. , Research Progress on the relationship between atherosclerosis and inflammation, Biomolecules 8 (3) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ahotupa M, Oxidized lipoprotein lipids and atherosclerosis, Free Radic. Res 51 (4) (2017) 439–447. [DOI] [PubMed] [Google Scholar]
- [4].Gistera A, Hansson GK, The immunology of atherosclerosis, Nat. Rev. Nephrol 13 (6) (2017) 368–380. [DOI] [PubMed] [Google Scholar]
- [5].Mudau M, et al. , Endothelial dysfunction: the early predictor of atherosclerosis, Cardiovasc. J. Afr 23 (4) (2012) 222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bonetti PO, Lerman LO, Lerman A, Endothelial dysfunction: a marker of atherosclerotic risk, Arterioscler. Thromb. Vasc. Biol 23 (2) (2003) 168–175. [DOI] [PubMed] [Google Scholar]
- [7].Davignon J, Ganz P, Role of endothelial dysfunction in atherosclerosis, Circulation 109 (23 Suppl 1) (2004) p. III27–32. [DOI] [PubMed] [Google Scholar]
- [8].Deanfield JE, Halcox JP, Rabelink TJ, Endothelial function and dysfunction: testing and clinical relevance, Circulation 115 (10) (2007) 1285–1295. [DOI] [PubMed] [Google Scholar]
- [9].Wesseling M, et al. , The morphological and molecular mechanisms of epithelial/endothelial-to-mesenchymal transition and its involvement in atherosclerosis, Vascul. Pharmacol 106 (2018) 1–8. [DOI] [PubMed] [Google Scholar]
- [10].Moonen JR, et al. , Endothelial progenitor cells give rise to pro-angiogenic smooth muscle-like progeny, Cardiovasc. Res 86 (3) (2010) 506–515. [DOI] [PubMed] [Google Scholar]
- [11].Clere N, Renault S, Corre I, Endothelial-to-mesenchymal transition in Cancer, Front. Cell Dev. Biol 8 (2020) 747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Piera-Velazquez S, Jimenez SA, Endothelial to mesenchymal transition: role in physiology and in the pathogenesis of human diseases, Physiol. Rev 99 (2) (2019) 1281–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jiang Y, et al. , TGF-beta1-induced SMAD2/3/4 activation promotes RELM-beta transcription to modulate the endothelium-mesenchymal transition in human endothelial cells, Int. J. Biochem. Cell Biol 105 (2018) 52–60. [DOI] [PubMed] [Google Scholar]
- [14].Chen PY, et al. , Endothelial-to-mesenchymal transition drives atherosclerosis progression, J. Clin. Invest 125 (12) (2015) 4514–4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sancho A, et al. , A new strategy to measure intercellular adhesion forces in mature cell-cell contacts, Sci. Rep 7 (2017) 46152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Helmke A, et al. , Endothelial-to-mesenchymal transition shapes the atherosclerotic plaque and modulates macrophage function, FASEB J. 33 (2) (2019) 2278–2289. [DOI] [PubMed] [Google Scholar]
- [17].Qin W, et al. , Endothelial to mesenchymal transition contributes to nicotine-induced atherosclerosis, Theranostics 10 (12) (2020) 5276–5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ranchoux B, et al. , Endothelial-to-mesenchymal transition in pulmonary hypertension, Circulation 131 (11) (2015) 1006–1018. [DOI] [PubMed] [Google Scholar]
- [19].Li Z, et al. , MKL1 promotes endothelial-to-mesenchymal transition and liver fibrosis by activating TWIST1 transcription, Cell Death Dis. 10 (12) (2019) 899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jiao K, et al. , 27-hydroxycholesterol-induced EndMT acts via STAT3 signaling to promote breast cancer cell migration by altering the tumor microenvironment, Cancer Biol. Med 17 (1) (2020) 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ubil E, et al. , Mesenchymal-endothelial transition contributes to cardiac neovascularization, Nature 514 (7524) (2014) 585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bartko PE, et al. , Effect of losartan on mitral valve changes after myocardial infarction, J. Am. Coll. Cardiol 70 (10) (2017) 1232–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Evrard SM, et al. , Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability, Nat. Commun 7 (2016) 11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schneider F, et al. , Matrix-metalloproteinase-14 deficiency in bone-marrow-derived cells promotes collagen accumulation in mouse atherosclerotic plaques, Circulation 117 (7) (2008) 931–939. [DOI] [PubMed] [Google Scholar]
- [25].Furman C, et al. , Rosuvastatin reduces MMP-7 secretion by human monocyte-derived macrophages: potential relevance to atherosclerotic plaque stability, Atherosclerosis 174 (1) (2004) 93–98. [DOI] [PubMed] [Google Scholar]
- [26].Carmona-Rivera C, et al. , Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2, Ann. Rheum. Dis 74 (7) (2015) 1417–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Amato B, et al. , Adult vascular wall resident multipotent vascular stem cells, matrix metalloproteinases, and arterial aneurysms, Stem Cells Int. 2015 (2015) 434962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wu MY, et al. , New insights into the role of inflammation in the pathogenesis of atherosclerosis, Int. J. Mol. Sci 18 (10) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Varghese JF, Patel R, Yadav UCS, Novel insights in the metabolic syndrome-induced oxidative stress and inflammation-mediated atherosclerosis, Curr. Cardiol. Rev 14 (1) (2018) 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Perez L, et al. , Endothelial-to-mesenchymal transition: cytokine-mediated pathways that determine endothelial fibrosis under inflammatory conditions, Cytokine Growth Factor Rev. 33 (2017) 41–54. [DOI] [PubMed] [Google Scholar]
- [31].Karasawa T, Takahashi M, Role of NLRP3 Inflammasomes in atherosclerosis, J. Atheroscler. Thromb 24 (5) (2017) 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Habas K, Shang L, Alterations in intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) in human endothelial cells, Tissue Cell 54 (2018) 139–143. [DOI] [PubMed] [Google Scholar]
- [33].Depuydt MAC, et al. , Microanatomy of the human atherosclerotic plaque by single-cell transcriptomics, Circ. Res 127 (11) (2020) 1437–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kovacic JC, et al. , Endothelial to mesenchymal transition in cardiovascular disease: JACC state-of-the-art review, J. Am. Coll. Cardiol 73 (2) (2019) 190–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hulshoff MS, et al. , Non-coding RNA in endothelial-to-mesenchymal transition, Cardiovasc. Res 115 (12) (2019) 1716–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kovacic JC, et al. , Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: from cardiovascular development to disease, Circulation 125 (14) (2012) 1795–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yang Y, et al. , Silencing of long non-coding RNA H19 downregulates CTCF to protect against atherosclerosis by upregulating PKD1 expression in ApoE knockout mice, Aging (Albany NY) 11 (22) (2019) 10016–10030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gao W, et al. , Association of polymorphisms in long non-coding RNA H19 with coronary artery disease risk in a Chinese population, Mutat. Res 772 (2015) 15–22. [DOI] [PubMed] [Google Scholar]
- [39].Cao T, et al. , H19/TET1 axis promotes TGF-beta signaling linked to endothelial-to-mesenchymal transition, FASEB J. 34 (6) (2020) 8625–8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Massague J, TGFbeta signalling in context, Nat. Rev. Mol. Cell Biol 13 (10) (2012) 616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sabbineni H, Verma A, Somanath PR, Isoform-specific effects of transforming growth factor beta on endothelial-to-mesenchymal transition, J. Cell. Physiol 233 (11) (2018) 8418–8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Paruchuri S, et al. , Human pulmonary valve progenitor cells exhibit endothelial/mesenchymal plasticity in response to vascular endothelial growth factor-a and transforming growth factor-beta2, Circ. Res 99 (8) (2006) 861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hata A, Chen YG, TGF-beta signaling from receptors to Smads, Cold Spring Harb Perspect Biol vol. 8 (9) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Attisano L, Wrana JL, Smads as transcriptional co-modulators, Curr. Opin. Cell Biol 12 (2) (2000) 235–243. [DOI] [PubMed] [Google Scholar]
- [45].Zhu H, et al. , A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation, Nature 400 (6745) (1999) 687–693. [DOI] [PubMed] [Google Scholar]
- [46].Kokudo T, et al. , Snail is required for TGFbeta-induced endothelial-mesenchymal transition of embryonic stem cell-derived endothelial cells, J. Cell Sci 121 (Pt 20) (2008) 3317–3324. [DOI] [PubMed] [Google Scholar]
- [47].Cooley BC, et al. , TGF-beta signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling, Sci. Transl. Med 6 (227) (2014) 227ra34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zeisberg EM, et al. , Endothelial-to-mesenchymal transition contributes to cardiac fibrosis, Nat. Med 13 (8) (2007) 952–961. [DOI] [PubMed] [Google Scholar]
- [49].Bai J, et al. , Netrin-1 attenuates the progression of renal dysfunction by blocking endothelial-to-mesenchymal transition in the 5/6 nephrectomy rat model, BMC Nephrol. 17 (1) (2016) 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Fleenor BS, et al. , Replicative aging induces endothelial to mesenchymal transition in human aortic endothelial cells: potential role of inflammation, J. Vasc. Res 49 (1) (2012) 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Song S, et al. , The role of PDGF-B/TGF-beta1/neprilysin network in regulating endothelial-to-mesenchymal transition in pulmonary artery remodeling, Cell. Signal 28 (10) (2016) 1489–1501. [DOI] [PubMed] [Google Scholar]
- [52].Yu L, Hebert MC, Zhang YE, TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses, EMBO J. 21 (14) (2002) 3749–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Medici D, Potenta S, Kalluri R, Transforming growth factor-beta2 promotes Snail-mediated endothelial-mesenchymal transition through convergence of Smad-dependent and Smad-independent signalling, Biochem. J 437 (3) (2011) 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sasaki N, Itakura Y, Toyoda M, Rapamycin promotes endothelial-mesenchymal transition during stress-induced premature senescence through the activation of autophagy, Cell Commun. Signal 18 (1) (2020) 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Jia S, Meng A, TGFbeta family signaling and development, Development 148 (2021) 5. [DOI] [PubMed] [Google Scholar]
- [56].Chen PY, et al. , Fibroblast growth factor receptor 1 is a key inhibitor of TGFbeta signaling in the endothelium, Sci. Signal 7 (344) (2014) ra90. [DOI] [PubMed] [Google Scholar]
- [57].Dong Y, et al. , Targeting Epsins to inhibit fibroblast growth factor signaling while potentiating transforming growth factor-beta signaling constrains endothelial-to-mesenchymal transition in atherosclerosis, Circulation 147 (8) (2023) 669–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Chen H, et al. , Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis, Nature 394 (6695) (1998) 793–797. [DOI] [PubMed] [Google Scholar]
- [59].Ford MG, et al. , Curvature of clathrin-coated pits driven by epsin, Nature 419 (6905) (2002) 361–366. [DOI] [PubMed] [Google Scholar]
- [60].Dong Y, et al. , Motif mimetic of epsin perturbs tumor growth and metastasis, J. Clin. Invest 125 (12) (2015) 4349–4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Liu X, et al. , Temporal and spatial regulation of epsin abundance and VEGFR3 signaling are required for lymphatic valve formation and function, Sci. Signal 7 (347) (2014) ra97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Pasula S, et al. , Endothelial epsin deficiency decreases tumor growth by enhancing VEGF signaling, J. Clin. Invest 122 (12) (2012) 4424–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Tessneer KL, et al. , Genetic reduction of vascular endothelial growth factor receptor 2 rescues aberrant angiogenesis caused by epsin deficiency, Arterioscler. Thromb. Vasc. Biol 34 (2) (2014) 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Rahman HNA, et al. , Selective targeting of a novel Epsin-VEGFR2 interaction promotes VEGF-mediated angiogenesis, Circ. Res 118 (6) (2016) 957–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wylie-Sears J, Levine RA, Bischoff J, Losartan inhibits endothelial-to-mesenchymal transformation in mitral valve endothelial cells by blocking transforming growth factor-beta-induced phosphorylation of ERK, Biochem. Biophys. Res. Commun 446 (4) (2014) 870–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Toma I, McCaffrey TA, Transforming growth factor-beta and atherosclerosis: interwoven atherogenic and atheroprotective aspects, Cell Tissue Res. 347 (1) (2012) 155–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hiepen C, Mendez PL, Knaus P, It takes two to tango: endothelial TGFbeta/BMP signaling crosstalk with Mechanobiology, Cells 9 (9) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sampath TK, Reddi AH, Discovery of bone morphogenetic proteins - a historical perspective, Bone 140 (2020) 115548. [DOI] [PubMed] [Google Scholar]
- [69].Katagiri T, Watabe T, Bone morphogenetic proteins, Cold Spring Harb Perspect Biol 8 (6) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Miyazono K, Maeda S, Imamura T, BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk, Cytokine Growth Factor Rev. 16 (3) (2005) 251–263. [DOI] [PubMed] [Google Scholar]
- [71].Miranda MZ, et al. , TGF-beta1 regulates the expression and transcriptional activity of TAZ protein via a Smad3-independent, myocardin-related transcription factor-mediated mechanism, J. Biol. Chem 292 (36) (2017) 14902–14920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Medici D, Kalluri R, Endothelial-mesenchymal transition and its contribution to the emergence of stem cell phenotype, Semin. Cancer Biol 22 (5–6) (2012) 379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Yung LM, et al. , Bone morphogenetic protein 6 and oxidized low-density lipoprotein synergistically recruit osteogenic differentiation in endothelial cells, Cardiovasc. Res 108 (2) (2015) 278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Zhu M, et al. , BMAL1 suppresses ROS-induced endothelial-to-mesenchymal transition and atherosclerosis plaque progression via BMP signaling, Am J Transl Res 10 (10) (2018) 3150–3161. [PMC free article] [PubMed] [Google Scholar]
- [75].Chang AC, et al. , Notch initiates the endothelial-to-mesenchymal transition in the atrioventricular canal through autocrine activation of soluble guanylyl cyclase, Dev. Cell 21 (2) (2011) 288–300. [DOI] [PubMed] [Google Scholar]
- [76].Fischer A, et al. , The notch target genes Hey1 and Hey2 are required for embryonic vascular development, Genes Dev. 18 (8) (2004) 901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Li L, et al. , C3a and C5a receptor antagonists ameliorate endothelial-myofibroblast transition via the Wnt/beta-catenin signaling pathway in diabetic kidney disease, Metabolism 64 (5) (2015) 597–610. [DOI] [PubMed] [Google Scholar]
- [78].Luxan G, et al. , Endocardial notch signaling in cardiac development and disease, Circ. Res 118 (1) (2016) e1–e18. [DOI] [PubMed] [Google Scholar]
- [79].Bosada FM, et al. , Wnt/beta-catenin signaling enables developmental transitions during valvulogenesis, Development 143 (6) (2016) 1041–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Alfieri CM, et al. , Wnt signaling in heart valve development and osteogenic gene induction, Dev. Biol 338 (2) (2010) 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Liebner S, et al. , Beta-catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse, J. Cell Biol 166 (3) (2004) 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Pinto MT, et al. , Endothelial cells from different anatomical origin have distinct responses during SNAIL/TGF-beta2-mediated endothelial-mesenchymal transition, Am. J. Transl. Res 10 (12) (2018) 4065–4081. [PMC free article] [PubMed] [Google Scholar]
- [83].Noseda M, et al. , Notch activation results in phenotypic and functional changes consistent with endothelial-to-mesenchymal transformation, Circ. Res 94 (7) (2004) 910–917. [DOI] [PubMed] [Google Scholar]
- [84].Niessen K, et al. , Slug is a direct notch target required for initiation of cardiac cushion cellularization, J. Cell Biol 182 (2) (2008) 315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Tian D, et al. , Protective effect of rapamycin on endothelial-to-mesenchymal transition in HUVECs through the notch signaling pathway, Vascul. Pharmacol 113 (2019) 20–26. [DOI] [PubMed] [Google Scholar]
- [86].Lin QQ, et al. , Roles of notch signaling pathway and endothelial-mesenchymal transition in vascular endothelial dysfunction and atherosclerosis, Eur. Rev. Med. Pharmacol. Sci 22 (19) (2018) 6485–6491. [DOI] [PubMed] [Google Scholar]
- [87].Lee RC, Feinbaum RL, Ambros V, The C. Elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14, Cell 75 (5) (1993) 843–854. [DOI] [PubMed] [Google Scholar]
- [88].Jiang L, et al. , miR-449a induces EndMT, promotes the development of atherosclerosis by targeting the interaction between AdipoR2 and E-cadherin in lipid rafts, Biomed. Pharmacother 109 (2019) 2293–2304. [DOI] [PubMed] [Google Scholar]
- [89].Wu X, et al. , Inhibition of miR-122 reduced atherosclerotic lesion formation by regulating NPAS3-mediated endothelial to mesenchymal transition, Life Sci. 265 (2021) 118816. [DOI] [PubMed] [Google Scholar]
- [90].Tombor LS, et al. , Single cell sequencing reveals endothelial plasticity with transient mesenchymal activation after myocardial infarction, Nat. Commun 12 (1) (2021) 681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Xu K, et al. , Cell-type transcriptome atlas of human aortic valves reveal cell heterogeneity and endothelial to mesenchymal transition involved in calcific aortic valve disease, Arterioscler. Thromb. Vasc. Biol 40 (12) (2020) 2910–2921. [DOI] [PubMed] [Google Scholar]
- [92].Kalucka J, et al. , Single-cell transcriptome atlas of murine endothelial cells, Cell 180 (4) (2020) 764–779 e20. [DOI] [PubMed] [Google Scholar]
- [93].Rohlenova K, et al. , Single-cell RNA sequencing maps endothelial metabolic plasticity in pathological angiogenesis, Cell Metab. 31 (4) (2020) 862–877 el4. [DOI] [PubMed] [Google Scholar]
- [94].Kalluri AS, et al. , Single-cell analysis of the Normal mouse aorta reveals functionally distinct endothelial cell populations, Circulation 140 (2) (2019) 147–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Kan H, et al. , Single-cell transcriptome analysis reveals cellular heterogeneity in the ascending aortas of normal and high-fat diet-fed mice, Exp. Mol. Med 53 (9) (2021) 1379–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Zhao P, et al. , Single-cell RNA-seq reveals a critical role of novel pro-inflammatory EndMT in mediating adverse remodeling in coronary artery-on-a-chip, Sci. Adv 7 (34) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Zhang L, et al. , Knockout RAGE alleviates cardiac fibrosis through repressing endothelial-to-mesenchymal transition (EndMT) mediated by autophagy, Cell Death Dis. 12 (5) (2021) 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Medici D, et al. , Conversion of vascular endothelial cells into multipotent stem-like cells, Nat. Med 16 (12) (2010) 1400–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Wylie-Sears J, et al. , Mitral valve endothelial cells with osteogenic differentiation potential, Arterioscler. Thromb. Vasc. Biol 31 (3) (2011) 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Mizushima N, et al. , Autophagy fights disease through cellular self-digestion, Nature 451 (7182) (2008) 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Yau JW, et al. , Endothelial-specific deletion of autophagy-related 7 (ATG7) attenuates arterial thrombosis in mice, J. Thorac. Cardiovasc. Surg 154 (3) (2017) 978–988 e1. [DOI] [PubMed] [Google Scholar]
- [102].Mizushima N, Komatsu M, Autophagy: renovation of cells and tissues, Cell 147 (4) (2011) 728–741. [DOI] [PubMed] [Google Scholar]
- [103].Feng Y, et al. , The machinery of macroautophagy, Cell Res. 24 (1) (2014) 24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Schuck S, Microautophagy - distinct molecular mechanisms handle cargoes of many sizes, J. Cell Sci 133 (17) (2020). [DOI] [PubMed] [Google Scholar]
- [105].Kaushik S, Cuervo AM, The coming of age of chaperone-mediated autophagy, Nat. Rev. Mol. Cell Biol 19 (6) (2018) 365–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Rubinsztein DC, Codogno P, Levine B, Autophagy modulation as a potential therapeutic target for diverse diseases, Nat. Rev. Drug Discov 11 (9) (2012) 709–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Kobayashi S, Liang Q, Autophagy and mitophagy in diabetic cardiomyopathy, Biochim. Biophys. Acta 1852 (2) (2015) 252–261. [DOI] [PubMed] [Google Scholar]
- [108].Kitada M, et al. , Regulating autophagy as a therapeutic target for diabetic nephropathy, Curr. Diab. Rep 17 (7) (2017) 53. [DOI] [PubMed] [Google Scholar]
- [109].Tsukada M, Ohsumi Y, Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae, FEBS Lett. 333 (1–2) (1993) 169–174. [DOI] [PubMed] [Google Scholar]
- [110].Kim KH, Lee MS, Autophagy–a key player in cellular and body metabolism, Nat. Rev. Endocrinol 10 (6) (2014) 322–337. [DOI] [PubMed] [Google Scholar]
- [111].Nussenzweig SC, Verma S, Finkel T, The role of autophagy in vascular biology, Circ. Res 116 (3) (2015) 480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Kim J, Lim YM, Lee MS, The role of autophagy in systemic metabolism and human-type diabetes, Mol. Cells 41 (1) (2018) 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Sergin I, et al. , Inclusion bodies enriched for p62 and polyubiquitinated proteins in macrophages protect against atherosclerosis, Sci. Signal 9 (409) (2016) ra2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Liu H, et al. , Autophagy in atherosclerosis: a phenomenon found in human carotid atherosclerotic plaques, Chin Med J (Engl) 128 (1) (2015) 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Li W, et al. , Autophagy dysfunction and regulatory cystatin C in macrophage death of atherosclerosis, J. Cell. Mol. Med 20 (9) (2016) 1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Kluge MA, Fetterman JL, Vita JA, Mitochondria and endothelial function, Circ. Res 112 (8) (2013) 1171–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].De Meyer GR, et al. , Autophagy in vascular disease, Circ. Res 116 (3) (2015) 468–479. [DOI] [PubMed] [Google Scholar]
- [118].Wang Y, et al. , Paeoniflorin attenuates oxidized low-density lipoprotein-induced apoptosis and adhesion molecule expression by autophagy enhancement in human umbilical vein endothelial cells, J. Cell. Biochem 120 (6) (2019) 9291–9299. [DOI] [PubMed] [Google Scholar]
- [119].Yang Q, et al. , Disocin prevents postmenopausal atherosclerosis in ovariectomized LDLR−/− mice through a PGC-1alpha/ERalpha pathway leading to promotion of autophagy and inhibition of oxidative stress, inflammation and apoptosis, Pharmacol. Res 148 (2019) 104414. [DOI] [PubMed] [Google Scholar]
- [120].Zhou X, et al. , Resveratrol attenuates endothelial oxidative injury by inducing autophagy via the activation of transcription factor EB, Nutr. Metab. (Lond.) 16 (2019) 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Zhang YL, et al. , The autophagy-lysosome pathway: a novel mechanism involved in the processing of oxidized LDL in human vascular endothelial cells, Biochem. Biophys. Res. Commun 394 (2) (2010) 377–382. [DOI] [PubMed] [Google Scholar]
- [122].Torisu K, et al. , Intact endothelial autophagy is required to maintain vascular lipid homeostasis, Aging Cell 15 (1) (2016) 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].LaRocca TJ, et al. , Translational evidence that impaired autophagy contributes to arterial ageing, J. Physiol 590 (14) (2012) 3305–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Bharath LP, et al. , Impairment of autophagy in endothelial cells prevents shear-stress-induced increases in nitric oxide bioavailability, Can. J. Physiol. Pharmacol 92 (7) (2014) 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Vion AC, et al. , Autophagy is required for endothelial cell alignment and atheroprotection under physiological blood flow, Proc. Natl. Acad. Sci. U. S. A 114 (41) (2017) E8675–E8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Xu K, Liu P, Zhao Y, Upregulation of microRNA-876 induces endothelial cell apoptosis by suppressing Bcl-xl in development of atherosclerosis, Cell. Physiol. Biochem 42 (4) (2017) 1540–1549. [DOI] [PubMed] [Google Scholar]
- [127].Torisu T, et al. , Autophagy regulates endothelial cell processing, maturation and secretion of von Willebrand factor, Nat. Med 19 (10) (2013) 1281–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Guixe-Muntet S, et al. , Cross-talk between autophagy and KLF2 determines endothelial cell phenotype and microvascular function in acute liver injury, J. Hepatol 66 (1) (2017) 86–94. [DOI] [PubMed] [Google Scholar]
- [129].Liu J, et al. , Shear stress regulates endothelial cell autophagy via redox regulation and Sirt1 expression, Cell Death Dis. 6 (7) (2015) e1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Reglero-Real N, et al. , Autophagy modulates endothelial junctions to restrain neutrophil diapedesis during inflammation, Immunity 54 (9) (2021) 1989–2004 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Sergin I, Razani B, Self-eating in the plaque: what macrophage autophagy reveals about atherosclerosis, Trends Endocrinol. Metab 25 (5) (2014) 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Shao BZ, et al. , The roles of macrophage autophagy in atherosclerosis, Acta Pharmacol. Sin 37 (2) (2016) 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Chen SG, et al. , Ibrolipim increases ABCA1/G1 expression by the LXRalpha signaling pathway in THP-1 macrophage-derived foam cells, Acta Pharmacol. Sin 31 (10) (2010) 1343–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Tian F, Yu BL, Hu JR, mTOR mediates the cross-talk of macrophage polarization and autophagy in atherosclerosis, Int. J. Cardiol 177 (1) (2014) 144–145. [DOI] [PubMed] [Google Scholar]
- [135].Yang Y, et al. , Non-lethal sonodynamic therapy facilitates the M1-to-M2 transition in advanced atherosclerotic plaques via activating the ROS-AMPK-mTORC1-autophagy pathway, Redox Biol. 32 (2020) 101501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Zhang J, et al. , Contradictory regulation of macrophages on atherosclerosis based on polarization, death and autophagy, Life Sci. 276 (2021) 118957. [DOI] [PubMed] [Google Scholar]
- [137].Li J, et al. , CTRP9 enhances carotid plaque stability by reducing pro-inflammatory cytokines in macrophages, Biochem. Biophys. Res. Commun 458 (4) (2015) 890–895. [DOI] [PubMed] [Google Scholar]
- [138].Zhang L, et al. , C1q/TNF-related protein 9 inhibits THP-1 macrophage foam cell formation by enhancing autophagy, J. Cardiovasc. Pharmacol 72 (4) (2018) 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Sergin I, et al. , Exploiting macrophage autophagy-lysosomal biogenesis as a therapy for atherosclerosis, Nat. Commun 8 (2017) 15750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Salabei JK, Hill BG, Implications of autophagy for vascular smooth muscle cell function and plasticity, Free Radic. Biol. Med 65 (2013) 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Ouimet M, et al. , Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase, Cell Metab. 13 (6) (2011) 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Alizadeh J, et al. , Simultaneous detection of autophagy and epithelial to mesenchymal transition in the non-small cell lung Cancer cells, Methods Mol. Biol 1854 (2019) 87–103. [DOI] [PubMed] [Google Scholar]
- [143].De Meyer GR, Martinet W, Autophagy in the cardiovascular system, Biochim. Biophys. Acta 1793 (9) (2009) 1485–1495. [DOI] [PubMed] [Google Scholar]
- [144].Grootaert MOJ, et al. , Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis, Cardiovasc. Res 114 (4) (2018) 622–634. [DOI] [PubMed] [Google Scholar]
- [145].Grootaert MO, et al. , Defective autophagy in vascular smooth muscle cells accelerates senescence and promotes neointima formation and atherogenesis, Autophagy 11 (11) (2015) 2014–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Martinet W, De Meyer GR, Autophagy in atherosclerosis: a cell survival and death phenomenon with therapeutic potential, Circ. Res 104 (3) (2009) 304–317. [DOI] [PubMed] [Google Scholar]
- [147].Jia G, et al. , Insulin-like growth factor-1 and TNF-alpha regulate autophagy through c-Jun N-terminal kinase and Akt pathways in human atherosclerotic vascular smooth cells, Immunol. Cell Biol 84 (5) (2006) 448–454. [DOI] [PubMed] [Google Scholar]
- [148].Liao X, et al. , Macrophage autophagy plays a protective role in advanced atherosclerosis, Cell Metab. 15 (4) (2012) 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Qian YN, et al. , Adhesion molecule CD146 and its soluble form correlate well with carotid atherosclerosis and plaque instability, CNS Neurosci. Ther 20 (5) (2014) 438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Liu YR, Chen JJ, Dai M, Paeonol protects rat vascular endothelial cells from ox-LDL-induced injury in vitro via downregulating microRNA-21 expression and TNF-alpha release, Acta Pharmacol. Sin 35 (4) (2014) 483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Li Y, et al. , An association study on ADAM10 promoter polymorphisms and atherosclerotic cerebral infarction in a Chinese population, CNS Neurosci. Ther 19 (10) (2013) 785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Brown JD, et al. , NF-kappaB directs dynamic super enhancer formation in inflammation and atherogenesis, Mol. Cell 56 (2) (2014) 219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Liu B, et al. , Enhancement in efferocytosis of oxidized low-density lipoprotein-induced apoptotic RAW264.7 cells through Sirt1-mediated autophagy, Int. J. Mol. Med 33 (3) (2014) 523–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Brophy ML, et al. , Myeloid-specific deletion of Epsins 1 and 2 reduces atherosclerosis by preventing LRP-1 downregulation, Circ. Res 124 (4) (2019) e6–e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Singh B, et al. , Defective efferocytosis of vascular cells in heart disease, Front Cardiovasc Med 9 (2022) 1031293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Oldenborg PA, et al. , Role of CD47 as a marker of self on red blood cells, Science 288 (5473) (2000) 2051–2054. [DOI] [PubMed] [Google Scholar]
- [157].Chao MP, Majeti R, Weissman IL, Programmed cell removal: a new obstacle in the road to developing cancer, Nat. Rev. Cancer 12 (1) (2011) 58–67. [DOI] [PubMed] [Google Scholar]
- [158].Kojima Y, et al. , CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis, Nature 536 (7614) (2016) 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Zhang Y, et al. , MicroRNA-26a prevents endothelial cell apoptosis by directly targeting TRPC6 in the setting of atherosclerosis, Sci. Rep 5 (2015) 9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Souilhol C, et al. , Endothelial-mesenchymal transition in atherosclerosis, Cardiovasc. Res 114 (4) (2018) 565–577. [DOI] [PubMed] [Google Scholar]
- [161].Gonzalez CD, et al. , The emerging role of autophagy in the pathophysiology of diabetes mellitus, Autophagy 7 (1) (2011) 2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Singh R, et al. , Autophagy regulates lipid metabolism, Nature 458 (7242) (2009) 1131–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Yang L, et al. , Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance, Cell Metab. 11 (6) (2010) 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Zou J, et al. , Autophagy attenuates endothelial-to-mesenchymal transition by promoting Snail degradation in human cardiac microvascular endothelial cells, Biosci. Rep 37 (5) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Wang J, et al. , Autophagy regulates endothelial-mesenchymal transition by decreasing the phosphorylation level of Smad3, Biochem. Biophys. Res. Commun 487 (3) (2017) 740–747. [DOI] [PubMed] [Google Scholar]
- [166].Singh KK, et al. , The essential autophagy gene ATG7 modulates organ fibrosis via regulation of endothelial-to-mesenchymal transition, J. Biol. Chem 290 (5) (2015) 2547–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Gao J, et al. , Sirtuin 3 governs autophagy-dependent glycolysis during angiotensin II-induced endothelial-to-mesenchymal transition, FASEB J. 34 (12) (2020) 16645–16661. [DOI] [PubMed] [Google Scholar]
- [168].Hammoutene A, et al. , A defect in endothelial autophagy occurs in patients with non-alcoholic steatohepatitis and promotes inflammation and fibrosis, J. Hepatol 72 (3) (2020) 528–538. [DOI] [PubMed] [Google Scholar]
- [169].Nivoit P, et al. , Autophagy protein 5 controls flow-dependent endothelial functions, Cell. Mol. Life Sci 80 (8) (2023) 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Hu J, et al. , Autophagy modulates mesenchymal-to-endothelial transition via p53, Aging (Albany NY) 12 (21) (2020) 22112–22121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Xia W, et al. , Long noncoding RNA-p21 modulates cellular senescence via the Wnt/beta-catenin signaling pathway in mesenchymal stem cells, Mol. Med. Rep 16 (5) (2017) 7039–7047. [DOI] [PubMed] [Google Scholar]
- [172].Wang JH, et al. , GOLPH3 promotes cell proliferation and tumorigenicity in esophageal squamous cell carcinoma via mTOR and Wnt/beta-catenin signal activation, Mol. Med. Rep 16 (5) (2017) 7138–7144. [DOI] [PubMed] [Google Scholar]
- [173].Huang Y, et al. , miR-218 promoted the apoptosis of human ovarian carcinoma cells via suppression of the WNT/beta-catenin signaling pathway, Mol. Biol. (Mosk) 51 (4) (2017) 629–636. [DOI] [PubMed] [Google Scholar]
- [174].Tao H, et al. , Wnt/beta-catenin signaling pathway activation reverses gemcitabine resistance by attenuating Beclin1-mediated autophagy in the MG63 human osteosarcoma cell line, Mol. Med. Rep 16 (2) (2017) 1701–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Gao C, et al. , Autophagy negatively regulates Wnt signalling by promoting Dishevelled degradation, Nat. Cell Biol 12 (8) (2010) 781–790. [DOI] [PubMed] [Google Scholar]
- [176].Petherick KJ, et al. , Autolysosomal beta-catenin degradation regulates Wnt-autophagy-p62 crosstalk, EMBO J. 32 (13) (2013) 1903–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Panda PK, et al. , Abrus agglutinin stimulates BMP-2-dependent differentiation through autophagic degradation of beta-catenin in colon cancer stem cells, Mol. Carcinog 57 (5) (2018) 664–677. [DOI] [PubMed] [Google Scholar]
- [178].Cicchini M, et al. , Autophagy regulator BECN1 suppresses mammary tumorigenesis driven by WNT1 activation and following parity, Autophagy 10 (11) (2014) 2036–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Su N, Wang P, Li Y, Role of Wnt/beta-catenin pathway in inducing autophagy and apoptosis in multiple myeloma cells, Oncol. Lett 12 (6) (2016) 4623–4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Nager M, et al. , Inhibition of WNT-CTNNB1 signaling upregulates SQSTM1 and sensitizes glioblastoma cells to autophagy blockers, Autophagy 14 (4) (2018) 619–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Li RN, et al. , DACT1 overexpression in type I ovarian cancer inhibits malignant expansion and cis-platinum resistance by modulating canonical Wnt signalling and autophagy, Sci. Rep 7 (1) (2017) 9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Luo X, et al. , Wnt inhibitory factor-1-mediated autophagy inhibits Wnt/beta-catenin signaling by downregulating dishevelled-2 expression in non-small cell lung cancer cells, Int. J. Oncol 53 (2) (2018) 904–914. [DOI] [PubMed] [Google Scholar]
- [183].Aisagbonhi O, et al. , Experimental myocardial infarction triggers canonical Wnt signaling and endothelial-to-mesenchymal transition, Dis. Model. Mech 4 (4) (2011) 469–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Liu Y, et al. , RUNX3 modulates hypoxia-induced endothelial-to-mesenchymal transition of human cardiac microvascular endothelial cells, Int. J. Mol. Med 40 (1) (2017) 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Brade T, Manner J, Kuhl M, The role of Wnt signalling in cardiac development and tissue remodelling in the mature heart, Cardiovasc. Res 72 (2) (2006) 198–209. [DOI] [PubMed] [Google Scholar]
- [186].Diao H, et al. , BAG3 alleviates atherosclerosis by inhibiting endothelial-to-mesenchymal transition via autophagy activation, Genes (Basel) 13 (8) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Brand T, Schneider MD, Transforming growth factor-beta signal transduction, Circ. Res 78 (2) (1996) 173–179. [DOI] [PubMed] [Google Scholar]
- [188].Kingsley DM, The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms, Genes Dev. 8 (2) (1994) 133–146. [DOI] [PubMed] [Google Scholar]
- [189].Hao J, et al. , Interaction between angiotensin II and Smad proteins in fibroblasts in failing heart and in vitro, Am. J. Physiol. Heart Circ. Physiol 279 (6) (2000) H3020–H3030. [DOI] [PubMed] [Google Scholar]
- [190].Ghavami S, et al. , Autophagy is a regulator of TGF-beta1-induced fibrogenesis in primary human atrial myofibroblasts, Cell Death Dis. 6 (3) (2015) e1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Suzuki HI, Kiyono K, Miyazono K, Regulation of autophagy by transforming growth factor-beta (TGF-beta) signaling, Autophagy 6 (5) (2010) 645–647. [DOI] [PubMed] [Google Scholar]
- [192].Trelford CB, Di Guglielmo GM, Autophagy regulates transforming growth factor beta signaling and receptor trafficking, Biochim Biophys Acta Mol Cell Res 1869 (9) (2022) 119284. [DOI] [PubMed] [Google Scholar]
- [193].Trelford CB, Di Guglielmo GM, Canonical and non-canonical TGFbeta signaling activate autophagy in an ULK1-dependent Manner, Front. Cell Dev. Biol 9 (2021) 712124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Takagaki Y, et al. , Endothelial autophagy deficiency induces IL6 - dependent endothelial mesenchymal transition and organ fibrosis, Autophagy 16 (10) (2020) 1905–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Marchi S, et al. , Defective autophagy is a key feature of cerebral cavernous malformations, EMBO Mol. Med 7 (11) (2015) 1403–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Grassi G, et al. , Autophagy regulates hepatocyte identity and epithelial-to-mesenchymal and mesenchymal-to-epithelial transitions promoting Snail degradation, Cell Death Dis. 6 (9) (2015) e1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Marchi S, et al. , Beyond multiple mechanisms and a unique drug: defective autophagy as pivotal player in cerebral cavernous malformation pathogenesis and implications for targeted therapies, Rare Dis 4 (1) (2016) e1142640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Zhang H, et al. , Bone morphogenetic protein-7 inhibits endothelial-mesenchymal transition in pulmonary artery endothelial cell under hypoxia, J. Cell. Physiol 233 (5) (2018) 4077–4090. [DOI] [PubMed] [Google Scholar]
- [199].Muir T, et al. , Intralesional bleomycin injection (IBI) treatment for haemangiomas and congenital vascular malformations, Pediatr. Surg. Int 19 (12) (2004) 766–773. [DOI] [PubMed] [Google Scholar]
- [200].Harrison JH Jr., Lazo JS, High dose continuous infusion of bleomycin in mice: a new model for drug-induced pulmonary fibrosis, J. Pharmacol. Exp. Ther 243 (3) (1987) 1185–1194. [PubMed] [Google Scholar]
- [201].Mouratis MA, Aidinis V, Modeling pulmonary fibrosis with bleomycin, Curr. Opin. Pulm. Med 17 (5) (2011) 355–361. [DOI] [PubMed] [Google Scholar]
- [202].Hashimoto N, et al. , Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis, Am. J. Respir. Cell Mol. Biol 43 (2) (2010) 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Zhang W, et al. , Bleomycin induces endothelial mesenchymal transition through activation of mTOR pathway: a possible mechanism contributing to the sclerotherapy of venous malformations, Br. J. Pharmacol 170 (6) (2013) 1210–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].He Y, et al. , DNMT1-mediated lncRNA MEG3 methylation accelerates endothelial-mesenchymal transition in diabetic retinopathy through the PI3K/Akt/mTOR signaling pathway, Am. J. Physiol. Endocrinol. Metab 320 (3) (2021) E598–E608. [DOI] [PubMed] [Google Scholar]
- [205].Lai B, et al. , Atheroprone flow enhances the endothelial-to-mesenchymal transition, Am. J. Physiol. Heart Circ. Physiol 315 (5) (2018) H1293–H1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Li Y, et al. , Simvastatin inhibits POVPC-mediated induction of endothelial-to-mesenchymal cell transition, J. Lipid Res 62 (2021) 100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Hoeksema MA, et al. , Targeting macrophage histone deacetylase 3 stabilizes atherosclerotic lesions, EMBO Mol. Med 6 (9) (2014) 1124–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Chen L, et al. , HDAC3 inhibitor suppresses endothelial-to-mesenchymal transition via modulating inflammatory response in atherosclerosis, Biochem. Pharmacol 192 (2021) 114716. [DOI] [PubMed] [Google Scholar]
- [209].Liu S, et al. , Icariin attenuates endothelial-mesenchymal transition via H19/miR-148b-3p/ELF5 in ox-LDL-stimulated HUVECs, Mol Ther Nucleic Acids 23 (2021) 464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Qi Q, et al. , Geniposide inhibited endothelial-mesenchymal transition via the mTOR signaling pathway in a bleomycin-induced scleroderma mouse model, Am J Transl Res 9 (3) (2017) 1025–1036. [PMC free article] [PubMed] [Google Scholar]
- [211].Johnson RW, et al. , Sirolimus allows early cyclosporine withdrawal in renal transplantation resulting in improved renal function and lower blood pressure, Transplantation 72 (5) (2001) 777–786. [DOI] [PubMed] [Google Scholar]
- [212].Kurdi A, De Meyer GR, Martinet W, Potential therapeutic effects of mTOR inhibition in atherosclerosis, Br. J. Clin. Pharmacol 82 (5) (2016) 1267–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Liu Y, Levine B, Autosis and autophagic cell death: the dark side of autophagy, Cell Death Differ. 22 (3) (2015) 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Liu Y, et al. , Autosis is a Na+,K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia, Proc. Natl. Acad. Sci. U. S. A 110 (51) (2013) 20364–20371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Sarkar S, et al. , Small molecules enhance autophagy and reduce toxicity in Huntington’s disease models, Nat. Chem. Biol 3 (6) (2007) 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Watanabe T, et al. , Tissue characterization of progressive cardiac allograft vasculopathy in patients with everolimus therapy compared with donor-transmitted atherosclerosis assessed using serial intravascular imaging: a case report, Transplant. Proc 46 (7) (2014) 2456–2461. [DOI] [PubMed] [Google Scholar]
- [217].Pearson KJ, et al. , Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span, Cell Metab. 8 (2) (2008) 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Anfossi G, et al. , The cardiovascular effects of metformin: further reasons to consider an old drug as a cornerstone in the therapy of type 2 diabetes mellitus, Curr. Vasc. Pharmacol 8 (3) (2010) 327–337. [DOI] [PubMed] [Google Scholar]
- [219].Forouzandeh F, et al. , Metformin beyond diabetes: pleiotropic benefits of metformin in attenuation of atherosclerosis, J. Am. Heart Assoc 3 (6) (2014) e001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Sarkar S, et al. , Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein, J. Biol. Chem 282 (8) (2007) 5641–5652. [DOI] [PubMed] [Google Scholar]
- [221].Liu D, et al. , Atorvastatin protects vascular smooth muscle cells from TGF-beta1-stimulated calcification by inducing autophagy via suppression of the beta-catenin pathway, Cell. Physiol. Biochem 33 (1) (2014) 129–141. [DOI] [PubMed] [Google Scholar]
- [222].Pankratz F, et al. , MicroRNA-100 suppresses chronic vascular inflammation by stimulation of endothelial autophagy, Circ. Res 122 (3) (2018) 417–432. [DOI] [PubMed] [Google Scholar]
- [223].Zhang Y, Sowers JR, Ren J, Targeting autophagy in obesity: from pathophysiology to management, Nat. Rev. Endocrinol 14 (6) (2018) 356–376. [DOI] [PubMed] [Google Scholar]
- [224].Donato AJ, et al. , Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice, Aging Cell 12 (5) (2013) 772–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Huang Q, et al. , Endothelial to mesenchymal transition: an insight in atherosclerosis, Front Cardiovasc Med 8 (2021) 734550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Hu Y, et al. , Single-cell RNA sequencing highlights transcription activity of autophagy-related genes during hematopoietic stem cell formation in mouse embryos, Autophagy 13 (4) (2017) 770–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.