Abstract
Background
HHLA2 (human endogenous retrovirus-H long terminal repeat-associating protein 2) represents a recently identified member of the B7 immune checkpoint family, characterized by limited expression in normal tissues but notable overexpression in various cancer types. Nevertheless, the precise function and interaction with immune cells remain poorly understood, particularly in laryngeal squamous cell carcinoma (LSCC). This investigation endeavored to elucidate the biological significance of HHLA2 within the tumor microenvironment of human LSCC tissues and delineate the clinical relevance and functional roles of HHLA2 in LSCC pathogenesis.
Methods
Through multiplexed immunohistochemistry analyses conducted on tissue microarrays sourced from LSCC patients (n = 72), the analysis was executed to assess the expression levels of HHLA2, density and spatial patterns of CD68+HLA-DR+CD163− (M1 macrophages), CTLA-4+CD4+FoxP3+ (CTLA-4+Treg cells), CTLA-4+CD4+FoxP3− (CTLA-4+Tcon cells), exhausted CD8+T cells, and terminally exhausted CD8+T cells in LSCC tissues. Survival analysis was conducted to evaluate the prognostic significance of HHLA2 and these immune checkpoints or immune cell populations, employing COX regression analysis to identify independent prognostic factors.
Results
Kaplan–Meier (K–M) survival curves revealed a significant association between HHLA2 expression and overall survival (OS) in LSCC. Elevated levels of HHLA2 were linked to reduced patient survival, indicating its potential as a prognostic marker (HR: 3.230, 95%CI 0.9205–11.34, P = 0.0067). Notably, increased infiltration of CD68+ cells (total macrophages), STING+CD68+HLA-DR+CD163− (STING+M1 macrophages), CTLA-4+CD4+FoxP3+, CTLA-4+CD4+FoxP3−, PD-1+LAG-3+CD8+T cells, and PD-1+LAG-3+TIM-3+CD8+T cells strongly linked to poorer survival outcomes (P < 0.05). A discernible trend was observed between the levels of these immune cell populations, STING+CD68+ (STING+ total macrophages), CD68+HLA-DR+CD163−, STING+CD68+CD163+HLA-DR− (STING+M2 macrophages), PD-1+LAG-3−CD8+T cells, PD-1+TIM-3+CD8+T cells, and PD-1+LAG-3+TIM-3−CD8+T cells and prognosis. Importantly, multivariate COX analysis identified HHLA2 as an independent predictive factor for OS in LSCC patients (HR = 3.86, 95% CI 1.08–13.80, P = 0.038). This underscored the potential of HHLA2 as a critical marker for predicting patient outcomes in LSCC.
Conclusions
HHLA2 emerged as a detrimental prognostic biomarker for assessing OS in LSCC patients. Relative to other immune checkpoints, HHLA2 exhibited heightened predictive efficacy for the prognosis of LSCC patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-024-03791-6.
Keywords: Laryngeal squamous cell carcinoma, HHLA2, Immune infiltrating cell, Prognosis
Introduction
Laryngeal cancer, the second most common malignancy in the respiratory system after lung cancer, exhibits a high mortality rate and a notably elevated incidence among head-and-neck tumors [1]. Squamous cell carcinoma is the predominant histological subtype [2]. Despite therapeutic advancements leading to a 5-year overall survival (OS) rate approaching 50%, over 40% of patients with laryngeal squamous cell carcinoma (LSCC) are typically diagnosed at an advanced stage [3, 4]. Currently, there is a significant lack of predictive tools to assess the progression of LSCC.
Recent studies have underscored the significant impact of immune checkpoint expression within the tumor microenvironment (TME) on LSCC prognosis [5]. However, despite the widespread adoption of immunotherapy employing immune checkpoint inhibitors (ICIs) across diverse cancer types, including LSCC, response rates remain constrained [6]. Immune checkpoints play a critical role in preserving self-tolerance and modulating the duration and intensity of the physiological immune response in peripheral tissues, encompassing numerous co-stimulatory and co-inhibitory pathways inherent to the immune system [7]. While immune checkpoints serve to combat infections and safeguard the body from harm, dysregulated expression of these checkpoints may foster tumor growth [8, 9]. Combinations of different checkpoints can define T cells or other immune cell subpopulations [10, 11].
The human endogenous retrovirus-H long terminal repeat-associating protein 2 (HHLA2, also known as B7-H7) represents a member of the B7 immunoglobulin superfamily, playing a pivotal role in immune evasion while potentially facilitating cancer cell proliferation [8]. In non-small cell lung cancer (NSCLC), HHLA2 dysfunction has been shown to impede cell proliferation, migration, and invasion via inhibiting EGFR/MAPK/ERK signaling pathway and inhibit M2 polarization via downregulating IL-10 [12]. Notably, in human prostate cancer, HHLA2 exerts a significant influence on the immunosuppressive microenvironment and serves as an independent prognostic indicator [13]. High HHLA2 expression emerges as an independent prognostic factor in hepatocellular carcinoma (HCC), with patients exhibiting elevated HHLA2 levels demonstrating heightened sensitivity and improved responses to chemotherapy and immunotherapy [14]. The OS rate of clear cell renal cell carcinoma (ccRCC) patients with higher HHLA2 expression was significantly poorer than that of patients with lower HHLA2 expression [15]. Similarly, patients with low HHLA2 expression in CD68+ tumor-associated macrophages (TAMs) had significantly better OS than those with high HHLA2 expression in pancreatic cancer tissues [16].
Recent attention has centered on elucidating the immunomodulatory role of HHLA2. Its function as either a co-stimulatory signal instigating T cell activation or a co-inhibitory signal suppressing T cells hinges upon the specific receptor it engages [17–19]. HHLA2 exhibits a co-inhibitory effect on T lymphocyte responses, markedly diminishing the production of cytokines such as IFN-γ, TNF-α, IL-5, IL-10, IL-13, IL-17a, and IL-22 upon incubation with T lymphocytes [20]. Within malignant glioma, the tumor-infiltrating immune cell model underscores a negative correlation between HHLA2 and TAMs, underscoring the significance of the HHLA2-TAM association [21]. Constitutive expression of HHLA2 protein on the surface of human monocytes or macrophages further reinforces its role within the immune milieu [17]. These co-inhibitory ligands within the B7 family interact with receptors of the CD28 family, such as PD-1 and CTLA-4, to attenuate T cell activity [22]. Previous investigations have revealed widespread expression of HHLA2 in PD-L1 negative NSCLC, osteosarcoma, and intrahepatic cholangiocarcinoma patient cohorts, suggesting HHLA2 as a promising target for immunotherapeutic interventions [17, 23, 24]. However, the nexus between HHLA2 and immune checkpoints in LSCC remains uncharted territory.
In our present investigation, we employed multiplexed immunohistochemistry (mIHC) to analyze the expression of HHLA2, tumor-infiltrating lymphocytes (TILs), and macrophage infiltration within LSCC tissue samples. Subsequently, we deliberated upon the implications of the interplay between HHLA2 expression and immune checkpoints on the prognosis of LSCC.
Materials and methods
Participants
A total of 72 patients diagnosed with LSCC who underwent tumor resection at the Third Affiliated Hospital of Soochow University between 2010 and 2021 were enrolled in the study. The inclusion criteria stipulated the absence of serious underlying comorbidities, immunological diseases, or other concomitant malignant tumors besides pathologically confirmed LSCC. All patients underwent either LSCC resection or biopsy, with histopathological diagnoses conducted by two qualified physicians. Regular postoperative follow-up was carried out either at the clinic or via telephone communication. Overall survival (OS) was defined as the time elapsed from surgical pathology confirmation to death or from surgery to the last observation for surviving patients. All samples were anonymized in accordance with local ethical guidelines, adhering to the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from all participants, and the study protocol received approval from the Review Board of the Third Affiliated Hospital of Soochow University (2024-024). To minimize the risk of sampling errors, our team constructed the tissue microarrays at our hospital. We also enlisted the expertise of two professional pathologists who collaboratively examined the slides and selected cancerous tissues from the tumor center for sampling.
mIHC
The mIHC procedure was conducted using the Opal 7-color fluorescent IHC kit (Catalog No. NEL811001KT, PerkinElmer, USA) in conjunction with automated quantitative analysis (PerkinElmer, USA), following the manufacturer’s instructions. Briefly, the expression and immunolocalization of HHLA2 and immune infiltrating cells in the tissue microarrays (TMAs) of LSCC were detected for characterization. Additionally, 4′,6-diamidino-2-phenylindole (DAPI) was utilized for nucleus identification, while cytokeratin (CK) was employed for epithelial cancer staining. The primary antibodies utilized in the immunostaining included HHLA2, CD68, CD163, HLA-DR, STING, CD4, FoxP3, CTLA-4, CD8, PD-1, LAG-3, and TIM-3 (Supplementary Table 1). Subsequently, six slides of TMAs were incubated with HRP-conjugated secondary antibodies (PerkinElmer, USA) in an Opal working solution (PerkinElmer, USA), followed by mounting with ProLong Diamond Antifade Reagent containing DAPI (Thermo fisher, USA). The Tissue FAXS system (Tissue Gnostics Asia Pacific Limited, Austria) was then utilized for panoramic multispectral scanning of the TMA slides. The acquired images were processed using Strata Quest analysis software (Version No. 7.0.1.165, Tissue Gnostics Asia Pacific Limited, Austria) as described previously. DAPI staining was selected to generate a binary mask of all viable cells in the image, and subsequently, the numbers of HHLA2 and immune infiltrating cells were counted and recorded.
Assessment of HHLA2 staining on cancer cells
The staining score for HHLA2 was assessed utilizing the H-score scoring method. This method calculates the H-score as follows: H-score = (percentage of unstained cells × 0) + (percentage of weakly stained cells × 1) + (percentage of moderately stained cells × 2) + (percentage of strongly stained cells × 3). Subsequently, the obtained H-scores were utilized for subsequent statistical analyses [25, 26].
Statistical analyses
We calculated the cutoff value of the data using the surv_cutpoint package in R software (Version 4.3.3). Samples below the cutoff value were classified as the low expression or infiltration group, while those above were classified as the high expression or infiltration group. Correlation analysis was performed using the Spearman correlation coefficient, with a significance threshold set at P < 0.05.
Results
Clinical characteristics of LSCC patients
The study encompassed the analysis of 72 samples obtained from patients diagnosed with LSCC, comprising 70 males (97.2%) and 2 females (2.8%). Age was delineated utilizing a cutoff value of 72, while tumor size was stratified based on a cutoff value of 2.7 cm. In accordance with the eighth American Joint Committee on Cancer (AJCC) staging system, 38 patients were categorized as stages I + II, whereas 34 patients were classified as stages III + IV. Comprehensive clinical details are outlined in Table 1.
Table 1.
Correlation between infiltration levels of immune cells and clinicopathological parameters of LSCC patients
| Clinical parameters | Sum | CD68+ infiltration level |
χ2 | P value | CD4+FoxP3+ infiltration level |
χ2 | P value | CTLA-4+CD4+FoxP3+ | χ2 | P value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | ||||||||
| Gender | |||||||||||||
| Male | 70 | 61 | 9 | 1.000a | 22 | 48 | 0.00 | 1.00b | 29 | 41 | 0.182a | ||
| Female | 2 | 2 | 0 | 1 | 1 | 2 | 0 | ||||||
| Age | |||||||||||||
| < 72 | 59 | 51 | 8 | 0.01 | 0.908b | 16 | 43 | 3.50 | 0.061 | 25 | 34 | 0.06 | 0.803 |
| ≥ 72 | 13 | 12 | 1 | 7 | 6 | 6 | 7 | ||||||
| Tumor size (cm) | |||||||||||||
| < 2.7 | 51 | 49 | 2 | 9.23 | 0.002b | 20 | 31 | 4.25 | 0.039b | 22 | 29 | 0.00 | 0.983 |
| ≥ 2.7 | 21 | 14 | 7 | 3 | 18 | 9 | 12 | ||||||
| TNM stage | |||||||||||||
| I + II | 38 | 36 | 2 | 2.58 | 0.108b | 17 | 21 | 6.06 | 0.014 | 18 | 20 | 0.611 | 0.435 |
| III + IV | 34 | 27 | 7 | 6 | 28 | 13 | 21 | ||||||
| T stage | |||||||||||||
| I + II | 42 | 39 | 3 | 1.60 | 0.206b | 18 | 24 | 5.522 | 0.019 | 20 | 22 | 0.86 | 0.355 |
| III + IV | 30 | 24 | 6 | 5 | 25 | 11 | 19 | ||||||
| N stage | |||||||||||||
| 0 | 60 | 55 | 5 | 3.66 | 0.056b | 21 | 39 | 0.315a | 26 | 34 | 0.01 | 0.915 | |
| I + II | 12 | 8 | 4 | 2 | 10 | 5 | 7 | ||||||
| Pathological grade | |||||||||||||
| I | 10 | 10 | 0 | 1.69 | 0.474a | 6 | 4 | 3.93 | 0.149a | 5 | 5 | 0.24 | 0.886 |
| II | 40 | 35 | 5 | 11 | 29 | 17 | 23 | ||||||
| III | 22 | 18 | 4 | 6 | 16 | 9 | 13 | ||||||
| Location | |||||||||||||
| Supraglottic | 19 | 13 | 6 | 14.2 | 0.001a | 4 | 15 | 1.86 | 0.455a | 4 | 15 | 5.434 | 0.055a |
| Glottic | 48 | 47 | 1 | 18 | 30 | 25 | 23 | ||||||
| Subglottic | 5 | 3 | 2 | 1 | 4 | 2 | 3 | ||||||
*P < 0.05: Statistically significant difference
Pa Fisher's exact test
Pb continuous corrected Chi-square test
Effect of HHLA2 expression on patient prognosis and tumor proliferation
In the mIHC analysis, HHLA2 and Ki67 staining were conducted to ascertain their expression patterns. Among the 72 samples examined, positive HHLA2 staining exhibited enrichment within the tumor region of LSCC tissues relative to non-tumor regions (Fig. 1A). Subsequent exploration aimed to ascertain the potential correlation between the percentage of HHLA2+ cells and patient survival. Notably, heightened levels of HHLA2 were linked with diminished OS across the cohort of 72 patients (HR = 3.23, 95% CI 0.9205–11.34, P = 0.0067, Fig. 1B). Additionally, a negative association emerged between Ki67+ expression in tumors and patient OS (HR = 4.122, 95% CI 0.0775–21.92, P = 0.0022, Fig. 1C), with Ki67 serving as a marker of tumor proliferation. Enhanced Ki67 expression correlated with accelerated tumor proliferation and shortened patient survival.
Fig. 1.
Characterization of the expression, localization, and prognostic values of HHLA2 and Ki67 in human LSCC tissues. An enlarged subsection of the core was highlighted, showing each of the individual markers in the composite image after spectral unmixing, together with the DAPI nuclear marker (pseudo-colored gray-blue) and the autofluorescence signal (pseudo-colored black). A. The expressions of HHLA2 (membrane, pseudo-colored green) and Ki67 (nuclear, pseudo-colored blue) in adjacent normal tissues of HCC were identified by CK (membrane, pseudo-colored purple), together with the DAPI nuclear marker (pseudo-colored gray-blue), The ratio of the main chart to the sub-chart is 1:11. B, C. Kaplan–Meier survival analysis was performed to predict the prognostic values of HHLA2+ and Ki67+ expression in human LSCC tissues. D. Kaplan–Meier survival analysis was performed to predict the prognostic value of HHLA2+ combined with Ki67+ in LSCC tissues. E. Correlation analysis was performed to predict the relationship between the expression of HHLA2 and Ki67. *P < 0.05, **P < 0.01, *** P < 0.001, ****P < 0.0001
Moreover, comparative analysis was conducted to elucidate the prognostic implications of different combinations of HHLA2 and Ki67 expression on epithelial cells. Results revealed that patients characterized by low co-expression of HHLA2 and Ki67 demonstrated significantly superior OS compared to counterparts with high co-expression of HHLA2 and Ki67 (P < 0.0001, Fig. 1D). Furthermore, patients exhibiting high co-expression of HHLA2 and Ki67 displayed reduced OS relative to those with elevated HHLA2 expression but low Ki67 expression (P < 0.05, Fig. 1D). Noteworthy is the significant positive correlation observed between HHLA2 and Ki67 expression in patients diagnosed with LSCC (R = 0.31, P = 0.0086, Fig. 1E).
Correlation between HHLA2 expression and immune cell infiltration in the TME
Further analysis was conducted to investigate the relationship between HHLA2 expression and CD4+T cell subsets infiltration in LSCC tissue samples (Fig. 2A). It was observed that HHLA2 expression exhibited a significant correlation with the infiltration of various immune cells, including CTLA-4+CD4+FoxP3+ and CTLA-4+CD4+FoxP3− (P < 0.05, Fig. 2B,C). Interestingly, although we did not observe a significant correlation between HHLA2 expression and the infiltration of M1-type macrophages (CD68+HLA-DR+CD163−) or M2-type macrophages (CD68+CD163+HLA-DR−), our analysis revealed that HHLA2 was more closely associated with M1-type macrophages compared to M2-type macrophages (Supplementary Fig. 1A–C).
Fig. 2.
Characterization of the expressions, localizations, and correlation of HHLA2 and immune checkpoint in human LSCC tissues. An enlarged subsection of the tissue core was highlighted, showing each of the individual markers in the composite image after spectral unmixing, together with the DAPI nuclear marker (pseudo-colored gray-blue) and the autofluorescence signal (pseudo-colored black). A. The expressions of HHLA2 (membrane, pseudo-colored green) and immune checkpoint in adjacent normal tissues of LSCC were identified by CK (membrane, pseudo-colored purple), together with the DAPI nuclear marker (pseudo-colored gray-blue). The ratio of the main chart to the sub-chart is 1:9. B, C. Correlation analysis was performed using the Spearman R to predict the relationship between HHLA2 expression and immune cells infiltration
The impact of immune cell infiltrations in the TME on patient prognosis
Macrophage polarization plays an essential role in the TME. Activation of STING-related pathways has been associated with macrophage polarization toward an M1 phenotype, and STING agonists have demonstrated the ability to reprogram M2-like pro-tumor macrophages into an M1-like anti-tumor state in a macrophage STING-dependent manner [27, 28]. The expression of STING serves as an indicator of macrophage activation. Regulatory T cells (Treg cells, CD4+FoxP3+), known for their role in suppressing anti-tumor immune responses, also play a role in curbing aberrant immune responses against self-antigens. CTLA-4, a potent co-inhibitory molecule, is constitutively expressed by conventional T cells (Tcon cells, CD4+FoxP3−) and by Treg cells following activation. Treatment with anti-CTLA-4 antibodies has been shown to attenuate the suppressive function of effector Treg cells or induce their elimination [29]. Exhausted CD8+T cells (CD8+Tex), expressing exhaustion markers such as PD-1, LAG-3, and TIM-3, can be categorized into pre-exhausted T cells (PD-1+CD8+) that respond to checkpoint blockade therapy and terminally exhausted T cells (PD-1+LAG-3+TIM-3+CD8+) that do not respond to such therapy [30].
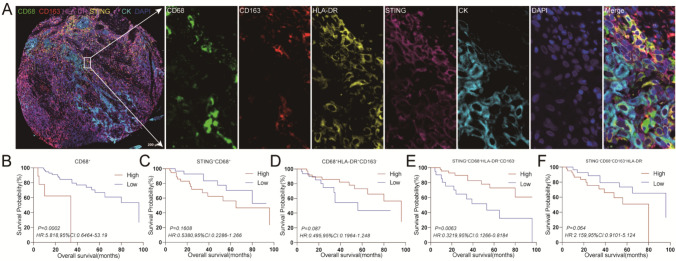
To further elucidate the impact of immune cell infiltration in the TME on patient prognosis, the infiltration of various immune cell subsets was analyzed in TMAs (Fig. 3A, 4A, 5A). Elevated levels of macrophages and certain subsets of tumor-infiltrating T cells were associated with reduced OS in the cohort of 72 patients, including CD68+ (Fig. 3B), STING+CD68+HLA-DR+CD163− (Fig. 3E), CTLA-4+CD4+FoxP3+ (Fig. 4B), CTLA-4+CD4+FoxP3− (Fig. 4C), PD-1+LAG-3+CD8+ (Fig. 5B), and PD-1+LAG-3+TIM-3+CD8+ (Fig. 5E) (P < 0.05). Furthermore, these immune cell subsets, including STING+CD68+ (Fig. 3C), CD68+HLA-DR+CD163− (Fig. 3D), STING+CD68+CD163+HLA-DR− (Fig. 3F), PD-1+LAG-3−CD8+ (Fig. 5C), and PD-1+TIM-3+CD8+ (Fig. 5D), as well as PD-1+LAG-3+TIM-3−CD8+ (Fig. 5F), displayed a decreasing trend in patient survival time with higher infiltration levels of above-mentioned cell subsets.
Fig. 3.
Characterization of the expressions, localizations, and prognostic value of immune checkpoint of macrophages in human LSCC tissues. An enlarged subsection of the core was highlighted, showing each of the individual markers in the composite image after spectral unmixing, together with the DAPI nuclear marker (pseudo-colored gray-blue) and the autofluorescence signal (pseudo-colored black). A. The expressions of immune checkpoint in adjacent normal tissues of LSCC were identified by CK (membrane, pseudo-colored blue), together with the DAPI nuclear marker (pseudo-colored gray-blue), The ratio of the main chart to the sub-chart is 1:9. B-F. Kaplan–Meier survival analysis was performed to predict macrophages and subtypes of macrophages in human LSCC tissues
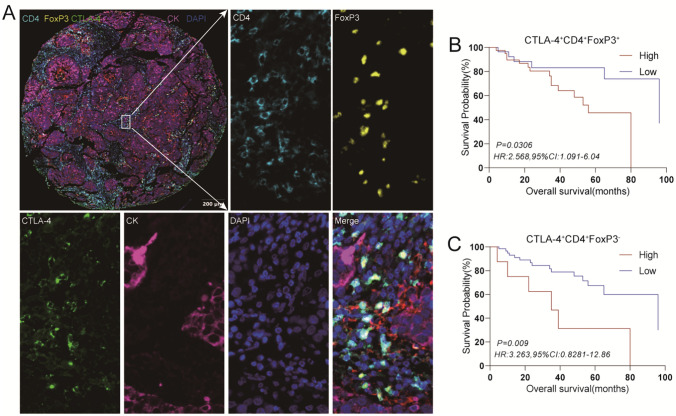
Fig. 4.
Characterization of the expressions, localizations, and prognostic value of immune checkpoint of CD4+ T cells in human LSCC tissues. An enlarged subsection of the core was highlighted, showing each of the individual markers in the composite image after spectral unmixing, together with the DAPI nuclear marker (pseudo-colored gray-blue) and the autofluorescence signal (pseudo-colored black), The ratio of the main chart to the sub-chart is 1:10. A. The expressions of immune checkpoint in adjacent normal tissues of LSCC were identified by CK (membrane, pseudo-colored purple), together with the DAPI nuclear marker (pseudo-colored gray-blue). B, C. Kaplan–Meier survival analysis was performed to predict the prognostic values of CD4+T cell subtypes in human LSCC tissues
Fig. 5.
Characterization of the expressions, localizations, and prognostic value of immune checkpoint of CD8+T cells in human LSCC tissues. An enlarged subsection of the core was highlighted, showing each of the individual markers in the composite image after spectral unmixing, together with the DAPI nuclear marker (pseudo-colored gray-blue) and the autofluorescence signal (pseudo-colored black). A. The expressions of immune checkpoints in adjacent normal tissues of LSCC were identified by CK (membrane, pseudo-colored purple), together with the DAPI nuclear marker (pseudo-colored gray-blue). The ratio of the main chart to the sub-chart is 1:11. B-F. Kaplan–Meier survival analysis was performed to predict the prognostic values of CD8+T cell subtype infiltration in human LSCC tissues
M1-type macrophages are known for their pro-inflammatory and anti-tumor properties, whereas M2-type macrophages exhibit anti-inflammatory characteristics and tend to promote tumor progression. In our present study, patients displaying high infiltration levels of M1-type macrophages demonstrated a tendency toward prolonged survival compared to those with low infiltration levels, as indicated by the survival curves. Notably, patients with elevated infiltration of activated M1-type macrophages (STING+CD68+HLA-DR+CD163−, STING+M1 macrophages) exhibited significantly extended OS compared to individuals with low infiltration levels (Fig. 3E). Conversely, although no significant difference was observed in the infiltration of M2-type macrophages concerning patient survival time (Supplementary Fig. 2A), those with heightened infiltration of activated M2-type macrophages (STING+CD68+CD163+HLA-DR−, STING+M2 macrophages) generally experienced shorter survival times relative to their low infiltration counterparts (Fig. 3F). These findings underscored the considerable enhancement of macrophage biological functions facilitated by STING activation.
Regarding Treg cells and Tcon cells, their infiltration levels did not exert a significant influence on patient survival (Supplementary Fig. 2B). However, patients characterized by elevated infiltration of CTLA-4+CD4+FoxP3+ or CTLA-4+CD4+FoxP3− demonstrated significantly shortened survival times compared to those with low infiltration levels (Fig. 4B, C). This suggested that the expression of the CTLA-4 immune checkpoint might represent a pivotal factor influencing the prognosis of LSCC patients. Notably, the infiltration of CD8+T cells did not yield a significant effect on patient prognosis (Supplementary Fig. 2C). However, the presence of terminally exhausted CD8+T cells, including PD-1+LAG-3+CD8+ and PD-1+LAG-3+TIM-3+CD8+, significantly impacted patient survival time (Fig. 5B, E). This highlighted the role of exhausted CD8+T cells as another crucial determinant affecting patient prognosis.
The impact of different combinations of HHLA2 expression and immune cell infiltration on patient prognosis
HHLA2 expression not only facilitates tumor progression but also exerts regulatory effects on the tumor immune microenvironment. Consequently, we delved into the prognostic implications of various combinations of HHLA2 expression and immune cell profiles within tumors. Our findings revealed that patients exhibiting high expression of HHLA2 and increased infiltration of specific macrophage subpopulations (CD68+, STING+CD68+, and CD68+CD163+HLA-DR−) experienced markedly diminished OS compared to those with low HHLA2 expression and decreased specific macrophage subpopulations infiltration levels (P < 0.001, Supplementary Fig. 3A). Intriguingly, individuals characterized by low HHLA2 expression and high M1 macrophage infiltration demonstrated superior OS compared to counterparts with high HHLA2 expression and low M1 macrophage infiltration (P < 0.05, Fig. 6A). Furthermore, OS was significantly enhanced in patients with low HHLA2 expression and high activated M1 macrophage infiltration in contrast to those with high HHLA2 expression and low activated M1 macrophage infiltration (P < 0.0001, Fig. 6A), suggesting a potential augmentation of M1 macrophage function following STING activation. However, this phenomenon was not observed in activated CD68+ cells or even activated M2 macrophages (Fig. 6A, Supplementary Fig. 3A).
Fig. 6.
Kaplan–Meier survival analysis was performed to predict the prognostic values of HHLA2+ combined with immune infiltrating cells in human LSCC tissues. Patients were stratified according to the expression of HHLA2+ and density of immune infiltrating cells. A, B, and C. Cutoffs for expression or infiltration were defined with the Cutoff Finder method. *P < 0.05, **P < 0.01, *** P < 0.001, **** P < 0.0001
Although no significant correlation was detected between CD4+T cells infiltration and patient survival time in TMAs, patients with low HHLA2 expression and high CD4+T cells infiltration exhibited improved OS relative to those with high HHLA2 expression and low CD4+T cells infiltration (P < 0.01, Supplementary Fig. 3B). Similarly, individuals with high expression of HHLA2+ and high infiltration of CD4+FoxP3+ cells experienced significantly worse OS compared to those with low HHLA2+ expression and high infiltration of CD4+FoxP3+, or low presence levels of both (P < 0.05, Fig. 6B). Patients with elevated HHLA2 expression and high infiltration of CTLA-4+CD4+FoxP3+ or CTLA-4+CD4+FoxP3− subsets also demonstrated markedly shorter survival times relative to those with low presence levels of both (P < 0.01, Fig. 6B). These results indicated that while the infiltration of the CD4+T cells subpopulation might not exert a significant effect on the survival of LSCC patients independently, the simultaneous combination with HHLA2 expression led to a significant alteration in patients’ survival time.
In our current study, we found that the infiltration of CD8+T cells did not have a significant effect on predicting patients’ OS. However, patients with low HHLA2+ expression combined with concomitant high CD8+T cells infiltration exhibited significantly prolonged survival compared to those with high levels of both factors (P < 0.001, Supplementary Fig. 4A). Notably, individuals characterized by low HHLA2 expression coupled with low infiltration levels of CD8+Tex cells, including PD-1+LAG-3+CD8+, PD-1−LAG-3+CD8+, PD-1−LAG-3−CD8+, PD-1+TIM-3+CD8+, PD-1+LAG-3+TIM-3+CD8+, PD-1+LAG-3+TIM-3− CD8+, and PD-1+LAG-3−TIM-3−CD8+, demonstrated significantly prolonged survival compared to those with high expression levels of both factors (P < 0.05, Fig. 6C and Supplementary Fig. 4B). This trend was particularly pronounced in patients with high HHLA2 expression combined with high infiltration levels of terminally differentiated CD8+Tex cells (PD-1+LAG-3+CD8+, PD-1+TIM-3+CD8+, PD-1+LAG-3+TIM-3+CD8+, P < 0.001, Fig. 6C). Furthermore, patients exhibiting low expression levels of HHLA2+ and low infiltration levels of CD8+Tex cells (including PD-1+LAG-3+CD8+, PD-1−LAG-3+CD8+, PD-1−LAG-3−CD8+, PD-1+TIM-3+CD8+, PD-1+LAG-3+TIM-3+CD8+, PD-1+LAG-3+TIM-3−CD8+, and PD-1+LAG-3−TIM-3−CD8+) experienced prolonged OS compared to those with high expression levels of HHLA2 and low infiltration levels of CD8+Tex cells (P < 0.05, Fig. 6C and Supplementary Fig. 4B). Moreover, patients with low HHLA2 expression and high infiltration levels of these CD8+ T cell subtypes (PD-1+LAG-3−CD8+, PD-1−LAG-3−CD8+, PD-1−TIM-3+CD8+, and PD-1+LAG-3−TIM-3+CD8+) exhibited improved OS compared to patients with high HHLA2 expression and low infiltration levels of these CD8+ cell subtypes (P < 0.05, Supplementary Fig. 4B). These findings suggested a potential mutual synergy between HHLA2 and exhausted CD8+T cells. However, further investigation is warranted to elucidate specific underlying mechanisms.
Correlation between the expression of HHLA2, tumor-infiltrating immune cells, and clinical parameters
Utilizing TMA technology and multilabel immunofluorescence, we scrutinized the expression and immunolocalization patterns of HHLA2 in 72 LSCC cancer tissues alongside various immune cell types. In the Chi-square test, the infiltration of CD68+, CTLA-4+CD4+FoxP3+, PD-1+LAG-3+CD8+, and PD-1+LAG-3+TIM-3−CD8+cells was associated with tumor location, while CD68+ was linked with tumor size. Additionally, HHLA2+, CD4+FoxP3+, PD-1+LAG-3+CD8+, and PD-1+LAG-3+TIM3−CD8+ cells showed associations with the TNM stage (Tables 1, 2). These findings suggested that LSCC patients with elevated HHLA2 expression were predisposed to advanced stages and metastasis.
Table 2.
Correlation between immune cells infiltration levels, HHLA2 expression level, and clinicopathological parameters of LSCC patients
| Clinical parameter | Sum | PD-1+LAG-3+CD8+ infiltration level |
χ2 | P value | PD-1+LAG-3+TIM-3−CD8+ infiltration level |
χ2 | P value | HHLA2+ expression level | χ2 | P value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | ||||||||
| Gender | |||||||||||||
| Male | 70 | 35 | 35 | 0.493a | 37 | 33 | 0.496a | 59 | 11 | 1.00a | |||
| Female | 2 | 2 | 0 | 2 | 0 | 2 | 0 | ||||||
| Age | |||||||||||||
| < 72 | 59 | 27 | 32 | 2.99 | 0.084b | 29 | 30 | 3.31 | 0.069b | 50 | 9 | 0.00 | 1.00b |
| ≥ 72 | 13 | 10 | 3 | 10 | 3 | 11 | 2 | ||||||
| Tumor size | |||||||||||||
| < 2.7 | 51 | 28 | 23 | 0.86 | 0.353 | 30 | 21 | 1.53 | 0.217 | 44 | 17 | 0.04 | 0.83b |
| ≥ 2.7 | 21 | 9 | 12 | 9 | 12 | 17 | 4 | ||||||
| TNM stage | |||||||||||||
| I + II | 38 | 25 | 13 | 6.68 | 0.010 | 26 | 12 | 6.586 | 0.010 | 35 | 3 | 0.065a | |
| III + IV | 34 | 12 | 22 | 13 | 21 | 26 | 8 | ||||||
| T stage | |||||||||||||
| I + II | 42 | 28 | 14 | 9.42 | 0.002 | 28 | 14 | 6.34 | 0.012 | 38 | 4 | 1.62 | 0.203b |
| III + IV | 30 | 9 | 21 | 11 | 19 | 23 | 7 | ||||||
| N stage | |||||||||||||
| 0 | 60 | 32 | 28 | 0.55 | 0.460 | 34 | 26 | 0.91 | 0.341 | 50 | 10 | 0.086 | 0.770b |
| I + II | 12 | 5 | 7 | 5 | 7 | 11 | 1 | ||||||
| Pathological grade | |||||||||||||
| I | 10 | 8 | 2 | 3.74 | 0.162a | 7 | 3 | 1.18 | 0.608a | 8 | 2 | 0.88 | 0.724a |
| II | 40 | 19 | 21 | 21 | 19 | 35 | 5 | ||||||
| III | 22 | 10 | 12 | 11 | 11 | 18 | 4 | ||||||
| Location | |||||||||||||
| Supraglottic | 19 | 4 | 15 | 9.60 | 0.005a | 5 | 14 | 8.07 | 0.013a | 15 | 4 | 3.90 | 0.108a |
| Glottic | 48 | 30 | 18 | 31 | 17 | 43 | 5 | ||||||
| Subglottic | 5 | 3 | 2 | 3 | 2 | 3 | 2 | ||||||
*P < 0.05 Statistically significant difference
Pa Fisher's exact test was used
Pb Continuous corrected Chi-square test
Cox model analysis based on the expression of HHLA2 and immune infiltrating cells in tumor epithelial cells
Following adjustments for age, tumor size, TNM stage, pathological stage, tumor location, HHLA2 expression levels, the infiltration levels of immune cell subsets (including CD68+, CD68+CD163+HLA-DR−, STING+CD68+CD163+HLA-DR−, CD4+, CD4+FoxP3+, CD8+, PD-1+LAG-3+CD8+, PD-1−LAG-3+CD8+, PD-1+TIM-3+CD8+, and PD-1+LAG-3+TIM-3+CD8+), and other pertinent factors as delineated in Table 3, Cox modeling outcomes revealed that patients exhibiting high HHLA2 expression faced a significantly heightened risk of mortality compared to their counterparts with low HHLA2 expression (HR = 3.86, 95% CI 1.08–13.80, P = 0.038). This underscored the notion that HHLA2 expression stood as an independent prognostic risk factor for LSCC.
Table 3.
Univariate analysis and Cox regression analysis of factors influencing overall survival in LSCC patients
| Clinical parameters | Unfavorable/favorable factor | Monofactor analysis | Proportional hazards model | ||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P Value | ||
| Age | > 72/ ≤ 72 | 0.58(0.21–1.60) | 0.36 | 0.90(0.17–4.79) | 0.90 |
| Tumor size(cm) | > 2.7/ ≤ 2.7 | 2.56(0.92–7.12) | 0.02* | 2.37(0.57–9.86) | 0.24 |
| TNM stage | III + IV/I + II | 3.90(1.62–9.37) | 0.002* | 4.16(650.74–23.54) | 0.11 |
| Pathological grade | III/I + II | 0.55(0.19–1.58) | 0.28 | 0.67(0.25–1.79) | 0.43 |
| Location | Supraglottic + subglottic/glottic | 2.65 (0.89–7.91) | 0.02* | 2.79(0.79–9.90) | 0.11 |
| HHLA2+ | Low/high | 3.23(0.92–11.34) | 0.006* | 3.86(1.08- 13.80) | 0.038* |
| CD68+ | Low/high | 5.82(0.64–53.19) | 0.0002* | 7.13(1.06–47.91) | 0.043* |
| CD68+CD163+HLA-DR− | Low/high | 1.43(0.47–4.37) | 0.47 | 1.81(0.42–7.77) | 0.43 |
| STING+CD68+CD163+HLA-DR− | Low/high | 2.16(0.91–5.12) | 0.06 | 3.98(0.65–24.36) | 0.14 |
| CD4+ | Low/high | 0.56(0.23–1.34) | 0.23 | 0.29(0.07–1.20) | 0.09 |
| CD4+FoxP3+ | Low/high | 1.35(0.56–3.22) | 0.51 | 0.39(0.07–2.11) | 0.27 |
| CD8+ | High/low | 0.75(0.32–1.78) | 0.52 | 0.64(0.17–2.48) | 0.52 |
| PD-1+LAG-3+CD8+ | Low/high | 2.63(1.06–6.50) | 0.015* | 2.63(0.58–11.89) | 0.21 |
| PD-1−LAG-3+CD8+ | Low/high | 1.42(0.47–4.32) | 0.47 | 1.65(0.39–7.03) | 0.49 |
| PD-1+TIM-3+CD8+ | Low/high | 2.30(0.62–8.61) | 0.09 | 1.35(0.21–8.56) | 0.75 |
| PD-1+LAG-3+TIM-3+CD8+ | Low/high | 2.68(0.75–9.57) | 0.03* | 1.67(0.32–8.67) | 0.54 |
*P < 0.05 statistically significant difference
Discussion
HHLA2, a significant member of the B7 family, exhibits expression across various malignancies, encompassing gastric cancer, lung cancer, liver cancer, kidney cancer, and bladder cancer [31–36]. Within these tumors, HHLA2 assumes a pivotal role as a negative co-stimulatory molecule, orchestrating tumor proliferation and immune evasion [37]. Predominantly located on the cell membrane and within the cytoplasm of tumor cells, HHLA2’s influence may vary across tumors due to the dynamic TME during tumor progression [9]. In the context of solid tumors, HHLA2’s functionality hinges upon its interaction with antagonistic receptors, thereby exerting either co-stimulatory or co-inhibitory effects [38]. The transmembrane immunoglobulin structural domain 2 (TMIGD2, CD28H), primarily expressed on resting or naive T cells, facilitates HHLA2’s co-stimulatory role upon interaction, activating T cells via the AKT pathway [39]. Conversely, upon interaction with KIR3DL3 (killer cell Ig-like receptor, three lg domains, and long cytoplasmic) expressed on activated T cells, HHLA2 instigates a co-suppressive effect [40]. The HHLA2/KIR3DL3 axis promotes CD8+T cell exhaustion, drives macrophage polarization toward a pro-tumor M2 phenotype, and suppresses both innate and adaptive tumor immune responses. Consequently, its activity has been implicated as a prognostic biomarker indicating poor outcomes in cancer patients [12]. Notably, the expression of the HHLA2/KIR3DL3 axis in T cells serves to negatively modulate T cell function, preventing overreaction and conferring immune privilege, particularly during processes like pregnancy. However, tumor cells may exploit this negative feedback mechanism to evade immune surveillance [41].
In contrast to the well-established CD28/CTLA-4/B7-1/B7-2 pathways, the TMIGD2/KIR3DL3/HHLA2 pathways stand out with notable differences from other members of the B7/CD28 family [42]. HHLA2’s involvement in tumor immune evasion is facilitated by its binding to receptors on T lymphocytes, thereby fostering the proliferation and cytokine production of CD4+T cells and CD8+T cells [18]. Noteworthy, co-expression of HHLA2 and PD-L1 has been linked to poorer survival outcomes in patients with ccRCC [43]. Furthermore, investigations reveal an inverse correlation between HHLA2 and CTLA-4 or LAG-3, alongside a positive correlation with the co-stimulatory molecule CD160 in malignant glioma [21]. Some studies posit that the HHLA2 immune checkpoint mirrors the dynamics of the B7-CD28/CTLA-4 pathway, where the B7-CD28 interaction elicits stimulatory effects, while the B7-CTLA-4 interaction exerts inhibitory effects [40].
In our present study, HHLA2 expression was significantly associated with the proliferation of laryngeal squamous cell carcinoma and elevated HHLA2 expression emerged as an adverse prognostic factor for LSCC patients. Furthermore, HHLA2 expression demonstrated positive associations with CTLA-4+CD4+FoxP3+and CTLA-4+CD4+FoxP3− cells; this is the first study to find a positive correlation between HHLA2 and CTLA-4+CD4+FoxP3+ or CTLA-4+CD4+FoxP3− in LSCC. Interestingly, we also found that HHLA2 expression was more closely associated with M1-type macrophages relative to M2-type macrophages. It suggested that HHLA2 might exert its influence on the prognosis of LSCC patients by modulating immune checkpoints on macrophage cells, Treg cells, and Tcon cells. HHLA2 may affect CTLA-4 immune checkpoint expression in Tcon cells or Treg cells by initiating the PI3K/AKT/NF-κB pathway via binding to TMIGD2 [44]. Nevertheless, elucidating the precise mechanisms warrants further investigation. The immune microenvironment within tumors plays a pivotal role in tumor progression [45]. Studies have demonstrated that downregulation of the cyclic GMP-AMP synthase-stimulating factor interferon gene (STING) facilitates the polarization of TAMs into pro-inflammatory subtypes and induces apoptosis in gastric cancer cells [46]. In our investigation, patients exhibiting high infiltration levels of activated M1-type macrophages experienced prolonged survival compared to those with low infiltration levels. Conversely, patients with elevated infiltration of activated M2-type macrophages demonstrated poorer survival outcomes than their counterparts with lower expression levels. This underscored the potential role of STING pathway activation in bolstering the functional capacities of both M1 and M2 macrophage subtypes.
CD4+FoxP3+Treg cells have been implicated in the promotion of cancer development and progression through the inhibition of effector T cells (Teff). These Treg cells exhibit the capability to generate substantial quantities of transforming growth factor-beta (TGF-β), which, in turn, triggers apoptosis in CD8+T cells by fostering the transformation of fibroblasts into cancer-associated fibroblasts (CAF), consequently facilitating tumor immune evasion from cytotoxic T cells [47]. Exhausted T cells are characterized by the heightened expression of multiple suppressor molecules on their surface, including PD-1, CD163, TIM-3, CTLA-4, LAG-3, and CD244. Generally, the increased co-expression of these suppressor receptors correlates with the severity of T cell dysfunction [48]. In our present investigation, we observed that the presence of Treg cells alone did not exert a significant impact on the prognosis of LSCC patients. However, patients exhibiting elevated infiltration levels of CTLA-4+Treg cells displayed markedly poorer prognoses. Intriguingly, the infiltration of CTLA-4+Treg exhibited a correlation with the anatomical site of the tumor. Furthermore, we noted a correlation between the tumor site and the infiltration of PD-1+LAG-3+CD8+ and PD-1+LAG-3+TIM-3+CD8+ T cell populations. It is well-established that supraglottic and subglottic laryngeal cancers exhibit lower 5-year survival rates in comparison with glottic laryngeal cancers and are more predisposed to metastasis and recurrence [49]. We hypothesized that exhausted T cells might tend to aggregate more prominently in supraglottic and subglottic laryngeal cancer; however, this supposition necessitates further substantiation through additional investigations.
The present study investigated the expression of HHLA2 in conjunction with macrophages, CD4+T cells, and CD8+T cells, as well as their respective subclusters. It was observed that patients demonstrating high levels of HHLA2 expression alongside concurrent high infiltration of exhausted T cells exhibited significantly poorer prognoses compared to patients characterized by other combinations. This implied that patients might experience improved prognostic outcomes if both HHLA2 and immune checkpoints were concomitantly targeted rather than individually blocked. Nevertheless, further clinical trials are imperative to corroborate this supposition. Notably, the expression of HHLA2 was found to correlate with the TNM stage of patients, emerging as a superior predictor in multifactorial analyses relative to other immune cells. Moreover, HHLA2 emerged as an independent prognostic factor for predicting outcomes in LSCC patients. Inhibition of HHLA2 expression might lead to a more favorable prognosis compared to targeting other immune checkpoints.
In summary, the up-regulation of HHLA2 was identified in LSCC tissues, exerting a significant impact on patient prognosis. Within the TME of LSCC, HHLA2 appeared to modulate patient prognosis through interactions with immune cells. Our findings suggested that HHLA2 might become a promising prognostic biomarker and a novel target in LSCC immunotherapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 2 Supplementary Figure 1. Characterization of the expressions, localizations, and correlation of HHLA2 and immune checkpoint in human LSCC tissues. An enlarged subsection of the core was highlighted, showing each of the individual markers in the composite image after spectral unmixing, together with the DAPI nuclear marker (pseudo-colored gray-blue) and the autofluorescence signal (pseudo-colored black). A. The expressions of HHLA2 (membrane, pseudo-colored green) and immune checkpoint in adjacent normal tissues of LSCC were identified by CK (membrane, pseudo-colored purple), together with the DAPI nuclear marker (pseudo-colored gray-blue), The ratio of the main chart to the sub-chart is 1:10. B, C. Correlation analysis was performed using the Spearman R to predict the relationship between HHLA2 expression and immune cells infiltration. (TIF 9083 kb)
Supplementary file 3 Supplementary Figure 2. Prognostic values of infiltrating immune cells in human LSCC tissues. A, B, and C. Kaplan–Meier survival analysis was performed to predict the prognostic values of immune infiltrating cells in human LSCC tissues. (TIF 10810 kb)
Supplementary file 4 Supplementary Figure 3. Prognostic values of HHLA2 combined with CD4+T subtypes in human LSCC tissues. A, B. Kaplan–Meier survival analysis was performed to predict the prognostic values of HHLA2+ combined with macrophage subtypes or CD4+T subtypes in human LSCC tissues. *P<0.05, **P< 0.01, *** P< 0.001, **** P< 0.0001. (TIF 10097 kb)
Supplementary file 5 Supplementary Figure 4. Prognostic value of HHLA2 combined with CD8+T subtypes in human LSCC tissues. A, B. Kaplan–Meier survival analysis was performed to predict the prognostic values of HHLA2+ combined with CD8+T subtypes in human LSCC tissues. *P<0.05, **P< 0.01, *** P< 0.001. (TIF 15136 kb)
Author Contribution
Wenjing Li, Changping Wu, and Lujun Chen were contributed methodology; Wenjing Li and Jianqing You were involved in validation; Yi Liu was performed formal analysis; Junjun Chen was done investigation; Haixiang Xue was attributed data curation; Xiao Zheng did writing original draft preparation; Wenjing Li was done writing—review and editing; and Changping Wu and Lujun Chen was involved in supervision. All authors read and approved the final manuscript.
Data availability
No datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lujun Chen, Email: chenlujun@suda.edu.cn.
Changping Wu, Email: wcpjjt@163.com.
References
- 1.Wang X, Tian L, Li Y (2021) RBM15 facilitates laryngeal squamous cell carcinoma progression by regulating TMBIM6 stability through IGF2BP3 dependent. J Exp Clin Cancer Res 40(1):1–18. 10.1186/s13046-021-01871-4 10.1186/s13046-021-01871-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavaliere M, Bisogno A, Scarpa A et al (2021) Biomarkers of laryngeal squamous cell carcinoma: a review. Ann Diagn Pathol 54:151787. 10.1016/j.anndiagpath.2021.151787 10.1016/j.anndiagpath.2021.151787 [DOI] [PubMed] [Google Scholar]
- 3.Cossu AM, Mosca L, Zappavigna S et al (2019) Long Non-coding RNAs as important biomarkers in laryngeal cancer and other head and neck tumours. Int J Mol Sci 20(14):3444. 10.3390/ijms20143444 10.3390/ijms20143444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koontongkaew S (2013) The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J Cancer 4(1):66–83. 10.7150/jca.5112 10.7150/jca.5112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horozoglu C, Sonmez D, Demirkol S et al (2021) Potential role of immune cell genetic variants associated with tumor microenvironment response in laryngeal squamous cell carcinoma (LSCC) in terms of clinicopathological features. Pathol Res Pract 228:153665. 10.1016/j.prp.2021.153665 10.1016/j.prp.2021.153665 [DOI] [PubMed] [Google Scholar]
- 6.Franz L, Alessandrini L, Calvanese L (2021) Angiogenesis, programmed death ligand 1 (PD-L1) and immune microenvironment association in laryngeal carcinoma. Pathology 53(7):844–851. 10.1016/j.pathol.2021.02.007 10.1016/j.pathol.2021.02.007 [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q, Vignali Dario AA (2016) Co-stimulatory and co-inhibitory pathways in autoimmunity. Immunity 44(5):1034–1051. 10.1016/j.immuni.2016.04.017 10.1016/j.immuni.2016.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janakiram M, Chinai JM, Zhao A et al (2015) HHLA2 and TMIGD2: new immunotherapeutic targets of the B7 and CD28 families. OncoImmunology. 4(8):e1026534. 10.1080/2162402x.2015.1026534 10.1080/2162402x.2015.1026534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janakiram M, Chinai JM, Fineberg S et al (2015) Expression, clinical significance, and receptor identification of the newest B7 family member HHLA2 protein. Clin Cancer Res 21(10):2359–2366. 10.1158/1078-0432.Ccr-14-1495 10.1158/1078-0432.Ccr-14-1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Leun AM, Thommen DS, Schumacher TN (2020) CD8+ T cell states in human cancer: insights from single-cell analysis. Nat Rev Cancer 20(4):218–232. 10.1038/s41568-019-0235-4 10.1038/s41568-019-0235-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan Z, Chen X, Zuo J et al (2023) Comprehensive analysis of scRNA-Seq and bulk RNA-Seq reveals dynamic changes in the tumor immune microenvironment of bladder cancer and establishes a prognostic model. J Transl Med. 10.1186/s12967-023-04056-z 10.1186/s12967-023-04056-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun W, Li S, Tang G et al (2021) HHLA2 deficiency inhibits non-small cell lung cancer progression and THP-1 macrophage M2 polarization. Cancer Med 10(15):5256–5269. 10.1002/cam4.4081 10.1002/cam4.4081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Q, Li K, Lai Y et al (2021) B7 score and T cell infiltration stratify immune status in prostate cancer. J ImmunoTher Cancer 9(8):e002455. 10.1136/jitc-2021-002455 10.1136/jitc-2021-002455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding L, Yu Q, Yang S et al (2022) Comprehensive analysis of HHLA2 as a prognostic biomarker and its association with immune infiltrates in hepatocellular carcinoma. Front Immunol 13:831101. 10.3389/fimmu.2022.831101 10.3389/fimmu.2022.831101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Zhu D, Feng J et al (2019) Overexpression of HHLA2 in human clear cell renal cell carcinoma is significantly associated with poor survival of the patients. Cancer Cell Int 9(1):1–12. 10.1186/s12935-019-0813-2 10.1186/s12935-019-0813-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu Y, Chen J, Liu Y et al (2022) Prognostic values of B7-H3, B7-H4, and HHLA2 expression in human pancreatic cancer tissues based on mIHC and spatial distribution analysis. Pathol Res Pract. 10.1016/j.prp.2022.153911 10.1016/j.prp.2022.153911 [DOI] [PubMed] [Google Scholar]
- 17.Zhao R, Chinai JM, Buhl S et al (2013) HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T-cell function. Proc Natl Acad Sci 110(24):9879–9884. 10.1073/pnas.1303524110 10.1073/pnas.1303524110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Y, Yao S, Iliopoulou BP et al (2013) B7–H5 costimulates human T cells via CD28H. Nat Commun 4(1):1–12. 10.1038/ncomms3043 10.1038/ncomms3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rieder SA, Wang J, White N et al (2020) B7–H7 (HHLA2) inhibits T-cell activation and proliferation in the presence of TCR and CD28 signaling. Cell Mol Immunol 18(6):1503–1511. 10.1038/s41423-020-0361-7 10.1038/s41423-020-0361-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolandi N, Derakhshani A, Hemmat N et al (2021) The positive and negative immunoregulatory role of B7 family: promising novel targets in gastric cancer treatment. Int J Mol Sci 22(19):10719. 10.3390/ijms221910719 10.3390/ijms221910719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi Y, Deng G, Xu P et al (2019) HHLA2 is a novel prognostic predictor and potential therapeutic target in malignant glioma. Oncol Rep. 10.3892/or.2019.7343 10.3892/or.2019.7343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng H, Janakiram M, Borczuk A et al (2017) HHLA2, a new immune checkpoint member of the B7 family, is widely expressed in human lung cancer and associated with EGFR mutational status. Clin Cancer Res 23(3):825–832. 10.1158/1078-0432.Ccr-15-3071 10.1158/1078-0432.Ccr-15-3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng H, Borczuk A, Janakiram M et al (2018) Wide expression and significance of alternative immune checkpoint molecules, B7x and HHLA2, in PD-L1–negative human lung cancers. Clin Cancer Res 24(8):1954–1964. 10.1158/1078-0432.Ccr-17-2924 10.1158/1078-0432.Ccr-17-2924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jing C-Y, Fu Y-P, Yi Y et al (2019) HHLA2 in intrahepatic cholangiocarcinoma: an immune checkpoint with prognostic significance and wider expression compared with PD-L1. J ImmunoTher Cancer 7(1):1–11. 10.1186/s40425-019-0554-8 10.1186/s40425-019-0554-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L, Huang H, Huang Z et al (2023) Prognostic values of tissue-resident CD8+T cells in human hepatocellular carcinoma and intrahepatic cholangiocarcinoma. World J Surg Oncol 21(1):124. 10.1186/s12957-023-03009-6 10.1186/s12957-023-03009-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L-j, Zheng X, Shen Y-p et al (2012) Higher numbers of T-bet+ intratumoral lymphoid cells correlate with better survival in gastric cancer. Cancer Immunol Immunother 62(3):553–561. 10.1007/s00262-012-1358-6 10.1007/s00262-012-1358-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Q, Bergholz JS, Ding L et al (2022) STING agonism reprograms tumor-associated macrophages and overcomes resistance to PARP inhibition in BRCA1-deficient models of breast cancer. Nat Commun 13(1):3022. 10.1038/s41467-022-30568-1 10.1038/s41467-022-30568-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni J, Guo T, Zhou Y et al (2023) STING signaling activation modulates macrophage polarization via CCL2 in radiation-induced lung injury. J Transl Med 21(1):590. 10.1186/s12967-023-04446-3 10.1186/s12967-023-04446-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka A, Sakaguchi S (2016) Regulatory T cells in cancer immunotherapy. Cell Res 27(1):109–118. 10.1038/cr.2016.151 10.1038/cr.2016.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ando M, Ito M, Srirat T et al (2019) Memory T cell, exhaustion, and tumor immunity. Immunol Med 43(1):1–9. 10.1080/25785826.2019.1698261 10.1080/25785826.2019.1698261 [DOI] [PubMed] [Google Scholar]
- 31.Lin G, Ye H, Wang J et al (2019) Immune checkpoint human endogenous retrovirus-H long terminal repeat-associating protein 2 is upregulated and independently predicts unfavorable prognosis in bladder urothelial carcinoma. Nephron 141(4):256–264. 10.1159/000495887 10.1159/000495887 [DOI] [PubMed] [Google Scholar]
- 32.Janakiram M, Shah UA, Liu W et al (2017) The third group of the B7-CD28 immune checkpoint family: HHLA2, TMIGD2, B7x, and B7–H3. Immunol Rev 276(1):26–39. 10.1111/imr.12521 10.1111/imr.12521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei L, Tang L, Chang H et al (2019) HHLA2 overexpression is a novel biomarker of malignant status and poor prognosis in gastric cancer. Hum Cell 33(1):116–122. 10.1007/s13577-019-00280-2 10.1007/s13577-019-00280-2 [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Hu R, Li X et al (2020) B7–H4 and HHLA2, members of B7 family, are aberrantly expressed in EGFR mutated lung adenocarcinoma. Pathol Res Pract 216(10):153134. 10.1016/j.prp.2020.153134 10.1016/j.prp.2020.153134 [DOI] [PubMed] [Google Scholar]
- 35.Wang R, Guo H, Tang X et al (2021) Interferon gamma-induced interferon regulatory factor 1 activates transcription of HHLA2 and induces immune escape of hepatocellular carcinoma cells. Inflammation 45(1):308–330. 10.1007/s10753-021-01547-3 10.1007/s10753-021-01547-3 [DOI] [PubMed] [Google Scholar]
- 36.Chen D, Chen W, Xu Y et al (2019) Upregulated immune checkpoint HHLA2 in clear cell renal cell carcinoma: a novel prognostic biomarker and potential therapeutic target. J Med Genet 56(1):43–49. 10.1136/jmedgenet-2018-105454 10.1136/jmedgenet-2018-105454 [DOI] [PubMed] [Google Scholar]
- 37.Xiao Y, Freeman GJ (2015) A New B7:CD28 family checkpoint target for cancer immunotherapy: HHLA2. Clin Cancer Res 21(10):2201–2203. 10.1158/1078-0432.Ccr-14-2658 10.1158/1078-0432.Ccr-14-2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Q, Wang J, Chen W et al (2019) B7–H5/CD28H is a co-stimulatory pathway and correlates with improved prognosis in pancreatic ductal adenocarcinoma. Cancer Sci 110(2):530–539. 10.1111/cas.13914 10.1111/cas.13914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ni L, Dong C (2017) New B7 family checkpoints in human cancers. Mol Cancer Ther 16(7):1203–1211. 10.1158/1535-7163.Mct-16-0761 10.1158/1535-7163.Mct-16-0761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhatt RS, Berjis A, Konge JC et al (2021) KIR3DL3 is an inhibitory receptor for HHLA2 that mediates an alternative immunoinhibitory pathway to PD1. Cancer Immunol Res 9(2):156–169. 10.1158/2326-6066.Cir-20-0315 10.1158/2326-6066.Cir-20-0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Lv C, Yu Y et al (2023) KIR3DL3-HHLA2 and TMIGD2-HHLA2 pathways: the dual role of HHLA2 in immune responses and its potential therapeutic approach for cancer immunotherapy. J Adv Res 47:137–150. 10.1016/j.jare.2022.07.013 10.1016/j.jare.2022.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pulanco MC, Madsen AT, Tanwar A et al (2023) Recent advancements in the B7/CD28 immune checkpoint families: new biology and clinical therapeutic strategies. Cell Mol Immunol 20(7):694–713. 10.1038/s41423-023-01019-8 10.1038/s41423-023-01019-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei Y, Ren X, Galbo PM et al (2021) KIR3DL3-HHLA2 is a human immunosuppressive pathway and a therapeutic target. Sci Immunol 6(61):eabf9792. 10.1126/sciimmunol.abf9792 10.1126/sciimmunol.abf9792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burke KP, Chaudhri A, Freeman GJ et al (2024) The B7:CD28 family and friends: unraveling coinhibitory interactions. Immunity 57(2):223–244. 10.1016/j.immuni.2024.01.013 10.1016/j.immuni.2024.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siminzar P, Tohidkia MR, Eppard E et al (2022) Recent trends in diagnostic biomarkers of tumor microenvironment. Mol Imag Biol 25(3):464–482. 10.1007/s11307-022-01795-1 10.1007/s11307-022-01795-1 [DOI] [PubMed] [Google Scholar]
- 46.Miao L, Qi J, Zhao Q et al (2020) Targeting the STING pathway in tumor-associated macrophages regulates innate immune sensing of gastric cancer cells. Theranostics 10(2):498–515. 10.7150/thno.37745 10.7150/thno.37745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Göschl L, Scheinecker C, Bonelli M (2019) Treg cells in autoimmunity: from identification to Treg-based therapies. Semin Immunopathol 41(3):301–314. 10.1007/s00281-019-00741-8 10.1007/s00281-019-00741-8 [DOI] [PubMed] [Google Scholar]
- 48.Dong C (2021) Cytokine regulation and function in T cells. Annu Rev Immunol 39(1):51–76. 10.1146/annurev-immunol-061020-053702 10.1146/annurev-immunol-061020-053702 [DOI] [PubMed] [Google Scholar]
- 49.Hinerman RW, Mendenhall WM, Amdur RJ et al (2002) Early laryngeal cancer. Curr Treat Options Oncol 3(1):3–9. 10.1007/s11864-002-0036-x 10.1007/s11864-002-0036-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file 2 Supplementary Figure 1. Characterization of the expressions, localizations, and correlation of HHLA2 and immune checkpoint in human LSCC tissues. An enlarged subsection of the core was highlighted, showing each of the individual markers in the composite image after spectral unmixing, together with the DAPI nuclear marker (pseudo-colored gray-blue) and the autofluorescence signal (pseudo-colored black). A. The expressions of HHLA2 (membrane, pseudo-colored green) and immune checkpoint in adjacent normal tissues of LSCC were identified by CK (membrane, pseudo-colored purple), together with the DAPI nuclear marker (pseudo-colored gray-blue), The ratio of the main chart to the sub-chart is 1:10. B, C. Correlation analysis was performed using the Spearman R to predict the relationship between HHLA2 expression and immune cells infiltration. (TIF 9083 kb)
Supplementary file 3 Supplementary Figure 2. Prognostic values of infiltrating immune cells in human LSCC tissues. A, B, and C. Kaplan–Meier survival analysis was performed to predict the prognostic values of immune infiltrating cells in human LSCC tissues. (TIF 10810 kb)
Supplementary file 4 Supplementary Figure 3. Prognostic values of HHLA2 combined with CD4+T subtypes in human LSCC tissues. A, B. Kaplan–Meier survival analysis was performed to predict the prognostic values of HHLA2+ combined with macrophage subtypes or CD4+T subtypes in human LSCC tissues. *P<0.05, **P< 0.01, *** P< 0.001, **** P< 0.0001. (TIF 10097 kb)
Supplementary file 5 Supplementary Figure 4. Prognostic value of HHLA2 combined with CD8+T subtypes in human LSCC tissues. A, B. Kaplan–Meier survival analysis was performed to predict the prognostic values of HHLA2+ combined with CD8+T subtypes in human LSCC tissues. *P<0.05, **P< 0.01, *** P< 0.001. (TIF 15136 kb)
Data Availability Statement
No datasets were generated or analyzed during the current study.