Abstract
Introduction
Annona muricata contains acetogenins, which have shown promising anticancer activity against various cell lines. This study aims to evaluate and compare the anticancer activity of the crude extract of Annona muricata and its nano formulation on Squamous Cell Carcinoma—25 (SCC-25) oral cancer cell lines.
Methods
The crude extract of Annona muricata was prepared using standard extraction techniques, while its nano formulation was synthesized through nanoparticle fabrication methods. Authenticated SCC-25 cell lines were obtained from ATCC and cultured and treated with varying concentrations of both the crude extract and nano formulation. Cell viability assays, apoptosis assays, Cell Cycle assay, ROS, and MMP analysis techniques were employed to assess the anticancer activity and mechanism of action.
Results
In the MTT assay, the Annona formulation treated cells exhibited lower IC50 values compared to the crude extract treated SCC-25 cell lines. In the cell cycle assay, the Annona crude extract induced higher cell cycle arrest in the G1 phase in SCC-25 cell lines compared to the control. The nano formulation of Annona demonstrated significantly higher cell cycle arrest in G1 phase compared to both the control and the Annona crude extract-treated SCC-25 cell lines. The crude extract showed less apoptotic activity in apoptosis assay when compared to control, whereas the Annona formulation exhibited higher late apoptosis compared to the control, indicating the potential anticancer properties of Annona. The mean fluorescent intensity test of SCC-25 oral cancer cells treated with Annona crude extract and Annona formulation showed a significant loss of Mitochondrial membrane potential compared to the control. The percentage of MMP was lower in Annona-treated cells, while the Annona formulation treated cells showed similar results to the control. The mean fluorescent intensity of ROS in SCC-25 oral cancer cells treated with Annona crude extract and Annona formulation showed significantly lower Reactive oxygen species production compared to the control. The percentage of ROS was lower in Annona treated cells compared to the formulation, but the Annona formulation-treated cells showed lower values than the control.
Conclusion
In conclusion, both the crude extract and nano formulation of Annona muricata possess potent anticancer activity against SCC-25 oral cancer cell lines. However, the nano formulation exhibited superior efficacy, suggesting its potential for further development as a therapeutic agent for oral cancer treatment.
Keywords: Annona muricata, Nano formulation, Anticancer activity, SCC-25 cell lines
Introduction
Annona comprises 119 species and is one of the 129 genera in the Annonaceae family. This plant genus contains acetogenins, alkaloids, flavonoids, terpenoids, essential oils, and other chemical classes, according to phytochemical research. The primary components of the Annona genre are acetogenins (ACGs) [1, 2], of which examples have been shown to have a wide range of pharmacological characteristics [3], such as antiviral, anticancer [4, 5], immunosuppressive, pesticidal, antiprotozoal, antibacterial [5], and antimalarial effects.
Because of their intriguing potential as anticancer agents, these acetogenins from the Annona genus are being isolated.
Given the need for alternative and effective anticancer therapies, it is crucial to explore novel drug targets that are highly specific and efficient in inhibiting cancer growth while being non-toxic to healthy cells and affordable for patients. This study aims to assess the potential of Graviola leaf extract (Annona muricata) as a natural anticancer agent, aligning with the aforementioned criteria. While Annona muricata has shown anticancer effects on various cell lines such as Ehrlich ascites carcinoma cells [6], breast cancer cell lines [7], prostate cancer [8] and pancreatic cancer cell lines (FG/COLO357 and CD18/HPAF), research on its effects on oral squamous cell carcinoma (SCC-25) remains limited [9–11], hence prompting this research. To increase the bioavailability, a new nano formulation of the crude extract is also formulated and studied for its anticancer activity.
The Aims of the present study are.
To evaluate the in-vitro anticancer (antiproliferative effects and apoptotic events) properties of graviola crude extract and its Nano formulation
To evaluate the antioxidant activity of crude extract of leaves of Annona muricata on SCC-25 cancer cell lines and their cytokinetic behavior.
To determine cytotoxicity of Graviola and percentage cell inhibition at various phases of cell cycle.
To compare the anti cancer activity of crude extract of graviola leaves with Nano formulation on SCC -25 cell lines.
Materials and Methods
Extraction of Annona muricata leaf crude extract and preparation of its Nano formulation
The aqueous crude extract of leaves of Annona muricata is obtained from the Department of Nano technology, The Tamil Nādu agricultural university, Coimbatore. The active constituents of Annona were extracted via sonication and incorporated into a nano carrier to prepare a nano formulation containing the extract's bioactive components. This formulation comprised nano colloidal particles of Annona bio actives, with a hydrodynamic diameter of 180 nm. The stability of these nano systems was achieved through steric interactions, facilitated by a combination of surfactants like sorbitan esters. Additionally, surface modification of the colloidal particles was conducted using biopolymers such as collagen to enhance absorption and facilitate translocation within the human system upon oral administration.
Cell Culture
The present study is designed to be conducted on immortalized cell lines (SCC-25) lines purchased from the American Type Culture Collection (ATCC CRL-1628) (RRID: CVCL_1682). The cells were grown in T25 (25 cm2) cell culture flasks coated with polystyrene. The culture medium used was Dulbecco’s modified Eagle’s medium (DMEM). This growth media was supplemented with 10% foetal bovine serum (Sigma-Aldrich, St. Louis, Mo, USA), phenol red (Thermo Scientific, New Delhi, India) and 1% penicillin–streptomycin (Sigma, New Delhi, India) and were maintained at 37ºC in a humidified atmosphere containing 95% air and 5% CO2.
MTT Assay
The initial phase of the study involves evaluating the antioxidant and cytotoxic effects, utilizing the MTT assay, of both the crude extract and its nano formulation on Scc-25 cell lines. This assessment aims to determine cell viability and cellular proliferation by comparing control and test samples, while also establishing the IC50 value for both the crude extract and its nano formulation. Additionally, the nano carrier undergoes separate evaluation via the MTT assay to confirm its lack of potency or cytotoxicity.
The MTT assay employs 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), an in vitro cytotoxicity assay that utilizes colorimetric methods to determine the number of viable cells based on mitochondrial dehydrogenase activity. This principle operates on the premise that mitochondrial dehydrogenase enzymes, producing NADH or NADPH, reduce the colourless tetrazolium salt into a coloured, aqueous-soluble formazan product through the mitochondrial activity of viable cells at 37 °C. The MTT cytotoxicity assay is conducted for both the Annona crude extract and its nano formulation, with IC50 values determined for each.
Cell cycle Assay
The next phase of the study involves examining the cell cycle arrest induced by both the crude extract and nano formulation of Annona on SCC-25 cancer cell lines through flow cytometry, specifically utilizing propidium iodide and DxFlex equipment (Beckman Coulter).
For the cell cycle analysis, 1 × 106 SCC-25 cells are initially seeded in 6-well plates and cultured in DMEM High Glucose supplemented with 10% FBS for 24 h under standard cell culture conditions. Following this incubation period, the cells are treated with either the Annona crude extract, Annona formulation, or left untreated as controls for another 24 h. Subsequently, the trypsinized cells are washed twice with PBS and then suspended in propidium iodide staining solution (containing 50 μg/ml propidium iodide, 200 μg/ml DNase free RNase, 4 mM sodium citrate, 0.1% Triton X-100), followed by a 15 min incubation period in the dark. The experiment is conducted using flow cytometry equipment, specifically DxFlex (Beckman Coulter), to analyze the cell cycle dynamics.
Apoptosis assay: The subsequent part of the study is to determine the apoptotic effects of both the crude extract and nano formulation, along with control samples, on SCC cell lines using flow cytometry with DxFlex (Beckman Coulter). This analysis employs Annexin V conjugated with PE and 7-AAD (7-AminoActinomycin (7-AAD) dual stained MB231 cells). Annexin V-PE binds to phosphatidylserine in cell membranes during early apoptosis, while 7-AAD binds to cellular DNA, indicating late-apoptotic or necrotic cell death. Cells are treated with the IC50 concentration of Annona and its formulation for 24 h, followed by trypsinization and washing with ice-cold PBS. Subsequently, the cells are suspended in 1 × binding buffer at a concentration of 1 × 106 cells/ml. Annexin V-PE and 7-AAD are added to each cell suspension, followed by gentle mixing and a 15 min incubation in the dark at room temperature. After incubation, ice-cold 1 × binding buffer is added, mixed gently, and the percentage of apoptotic cells in the population is determined.
MMP Assay: The next phase of the study aims to detect cell apoptosis by assessing the loss of mitochondrial membrane potential (MMP) induced by both the crude extract and nano formulation of Annona on SCC cell lines. The collapse of mitochondrial membrane potential coincides with the opening of the mitochondrial permeability transition pores, leading to the release of cytochrome C into the cytosol, thereby triggering downstream events in the apoptotic cascade. This assessment will be conducted using Mitotracker Deep Red.
In this analysis, 1 × 106 SCC-25 cells are initially seeded in 6-well plates and cultured in DMEM High Glucose supplemented with 10% FBS for 24 h under standard cell culture conditions. Subsequently, the cells are treated with the Annona crude extract, Annona formulation, or left untreated (serving as controls) for 24 h. Following treatment, the trypsinized cells are washed twice with PBS and then suspended in 1 ml of PBS containing 5 µl of Mitotracker Deep Red (Invitrogen, USA). The cell suspension is then incubated for 15 min in the dark to allow for Mitotracker Deep Red staining of mitochondria. The experiment is conducted using flow cytometry, which enables the quantification of MMP loss in the treated SCC-25 cells. This method allows for the measurement of alterations in mitochondrial membrane potential, which serves as an indicator of apoptosis induction.
ROS Assay: The final phase of the study involves analyzing reactive oxygen species (ROS) levels induced by both the crude extract and nano formulation of Annona on SCC-25 cell lines using 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA). For ROS analysis, 1 × 106 SCC-25 cells are seeded in 6-well plates and cultured in DMEM High Glucose supplemented with 10% FBS for 24 h under standard cell culture conditions. Following this incubation period, the cells are treated with the Annona crude extract, Annona formulation, or left untreated (serving as controls) for another 24 h. After treatment, the trypsinized cells are washed twice with PBS and then suspended in 1 ml of PBS containing 5 µl of DCFH-DA (2′,7′-dichlorodihydrofluorescein diacetate) from Sigma (USA). The cell suspension is then incubated for 15 min in the dark to allow DCFH-DA to penetrate the cells.
DCFH-DA is a cell-permeable probe that is deacetylated by intracellular esterases to form DCFH, which is then oxidized by ROS to form the fluorescent compound dichlorofluorescein (DCF). Therefore, the fluorescence intensity of DCF is directly proportional to the levels of ROS within the cells. The experiment is conducted using flow cytometry, enabling the quantification of ROS levels in the treated SCC-25 cells. This analysis provides insight into the potential oxidative stress induced by the Annona crude extract and nano formulation on the SCC-25 cell lines.
Results
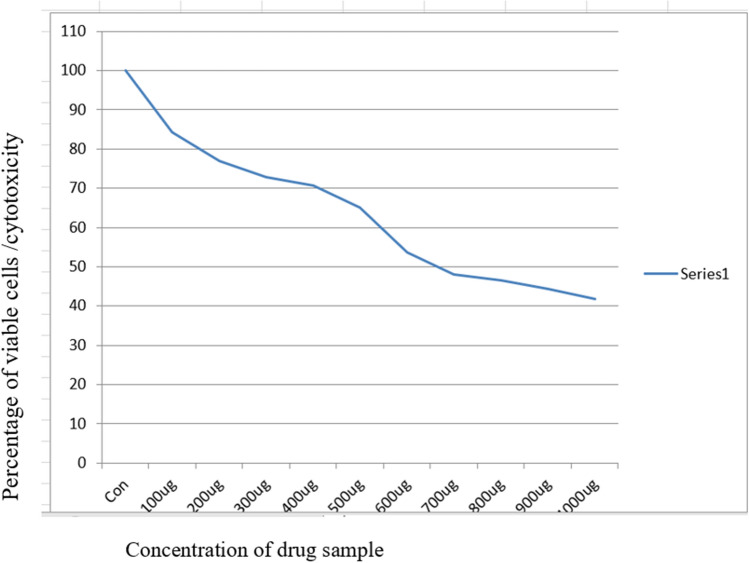
In the MTT cytotoxicity assay, the IC50 values were measured for both the crude extract and Nanoformulation, resulting in 660 and 270 ug/ml respectively (Figs. 1, 2). This indicates that the Annona formulation treated SCC-25 cell lines have lower IC50 values compared to those treated with the crude extract.
Fig. 1.
Shows determination of IC50 value of the crude extract of Annona by MTT assay and IC50 value was 660 ug/ml
Fig. 2.
Shows determination of IC50 values of Nano formulation of Annona on SCC-25 cell lines by MTT assay which was found to be 270 ug/ ml. Annona formulation has lower IC 50 values compared to crude extract treated SCC-25 cell lines
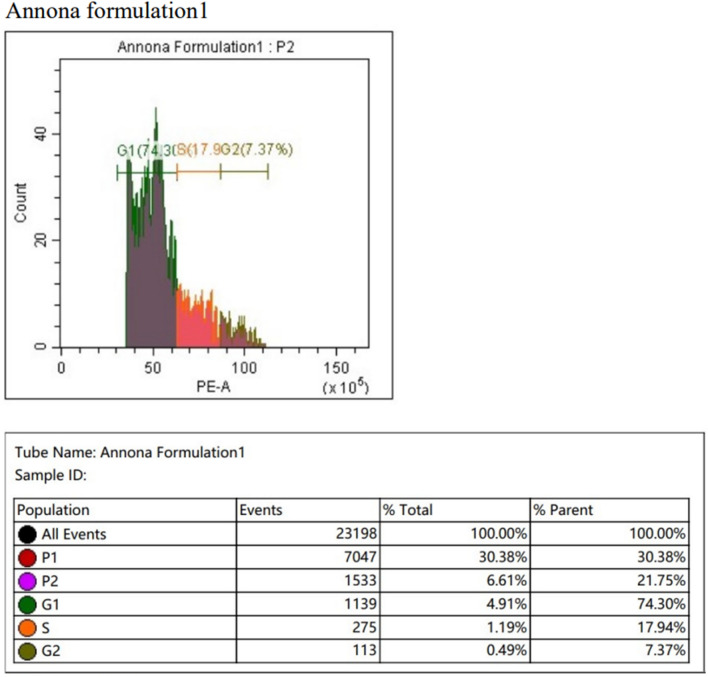
In the cell cycle assay conducted via flow cytometry, the Annona crude extract induced higher cell cycle arrest in the G1 phase compared to the control. Conversely, the Annona formulation exhibited significantly higher activity of G1 phase cell cycle arrest compared to both the control and the Annona crude extract treated SCC-25 cell lines (Figs. 3, 4 and 5).
Fig. 3.
Shows cell cycle arrest produced by the control SCC 25 cancer cell lines by flow cytometry (Cell cycle analysis) using propiodine iodine
Fig. 4.
Shows cell cycle arrest produced by the crude extract of Annona on SCC 25 cancer cell lines by flow cytometry (Cell cycle analysis) using propiodine iodine
Fig. 5.
Shows cell cycle arrest produced by the Nano formulation of Annona on SCC 25 cancer cell lines by flow cytometry (Cell cycle analysis) using propiodine iodine
The flow cytometry study aimed at detecting apoptosis using Annexin V conjugated with PE 7AAD revealed that the Annona crude extract exhibited less apoptotic activity than the control (Figs. 6, 7). However, the Annona formulation demonstrated higher late apoptosis compared to the control (Figs. 6, 8).
Fig. 6.
Shows apoptotic effects of control SCC cell lines by flow cytometry using Annexin V conjugated with PE 7AAD
Fig. 7.
Shows apoptotic effects of crude extract on SCC cell lines by flow cytometry using Annexin V conjugated with PE 7AAD
Fig. 8.
Shows apoptotic effects of nano formulation on SCC cell lines by flow cytometry using Annexin V conjugated with PE 7AAD
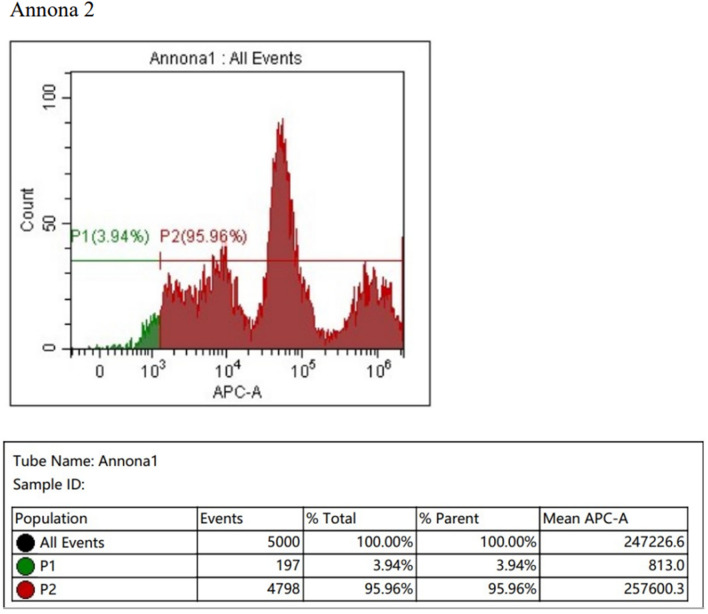
In the apoptosis assay detecting cell apoptosis, by measuring the loss of mitochondrial membrane potential (MMP) using Mitotracker Deep Red (Fig. 9), both the Annona crude extract (Fig. 10) and formulation treated SCC-25 oral cancer cells (Fig. 11) showed a significant loss of MMP compared to the control (Fig. 9). The percentage of MMP was lower in cells treated with Annona, while the Annona formulation showed similar results to the control (Figs. 12 and 13).
Fig. 9.
Shows cell apoptosis by measuring the loss of mitochondrial membrane potential (MMP) in control SCC cells using Mitotracker Deep Red
Fig. 10.
Shows cell apoptosis by measuring the loss of the mitochondrial membrane potential (MMP) by crude extract of Annona treated SCC cell lines using Mitotracker Deep Red
Fig. 11.
Shows cell apoptosis by measuring the loss of the mitochondrial membrane potential (MMP) by nano formulation of Annona treated SCC cell lines using Mitotracker Deep Red
Fig. 12.

Shows Percentage of MMP in Annona and Annona formulation
Fig. 13.

Shows Mean Florescent Intensity of MMP
In the ROS analysis to detect reactive oxygen species using 2,7 dichlorofluorofluorescein diacetate for both crude extract and nano formulation on SCC 25 cell lines (Fig. 14), the mean fluorescent intensity of ROS in Annona crude extract (Fig. 15) and formulation treated SCC-25 oral cancer cells (Fig. 16) was significantly lower compared to the control (Fig. 14). The percentage of ROS was lower in cells treated with Annona compared to the formulation, but the Annona formulation treated cells showed lower values than the control. Generally, high ROS production is associated with aggressive cancer progression. The results of the percentage of ROS and mean fluorescent intensity are exhibited in Figs. 17 and 18.
Fig. 14.
Shows ROS analysis to detect reactive oxygen species for control SCC 25 cell lines using 2,7 di chlorofluoro acetic acid
Fig. 15.
Shows ROS analysis to detect reactive oxygen species for Annona crude extract treated SCC 25 cell lines using 2,7 di chlorofluoro acetic acid
Fig. 16.
Shows ROS analysis to detect reactive oxygen species for Annona Nano formulation treated SCC 25 cell lines using 2,7 di chlorofluoro acetic acid
Fig. 17.

Shows Percentage of ROS in Annona and Annona formulation
Fig. 18.
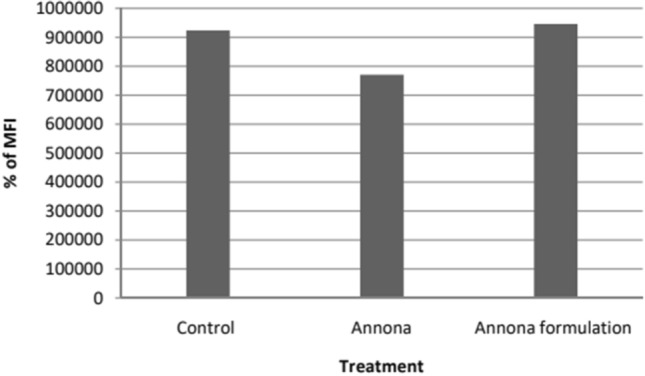
Shows Mean Florescent Intensity of ROS in Annona and Annona formulation
Discussion
The findings of our study on the cytotoxic, cell cycle arresting, apoptotic, and ROS-modulating effects of Annona crude extract and its nano formulation on SCC-25 oral cancer cells align with existing literature on the anticancer properties of Annona species [9–11]. Studies investigating various Annona species have reported similar outcomes, supporting the potential of these plants as sources of anticancer agents.
Previous research has demonstrated the cytotoxicity of Annona extracts against various cancer cell lines, including oral cancer cells [9–11]. Our results corroborate these findings, indicating the cytotoxic effects of both the crude extract and nano formulation on SCC-25 cells, with the nano formulation exhibiting enhanced potency, as evidenced by lower IC50 values.
Furthermore, several studies have highlighted the ability of Annona extracts to induce cell cycle arrest and apoptosis in cancer cells [12, 13]. Consistent with these reports, our study revealed G1 phase cell cycle arrest and apoptotic activity induced by both the crude extract and nano formulation. Notably, the nano formulation demonstrated superior activity in inducing G1 phase arrest and late apoptosis compared to the crude extract, suggesting its potential as a more potent anticancer agent.
Moreover, Annona extracts have been shown to modulate oxidative stress levels in cancer cells by reducing ROS production and enhancing antioxidant defenses [14, 15]. Our findings align with these observations, indicating reduced ROS levels in SCC-25 cells treated with both the crude extract and nano formulation. The nano formulation exhibited lower ROS levels compared to the crude extract, further highlighting its potential antioxidative properties.
In comparison with other herbs studied for their anticancer potential, Annona extracts demonstrate promising efficacy. While numerous herbs have shown anticancer activity through various mechanisms [16], including cytotoxicity, cell cycle regulation, apoptosis induction, and ROS modulation, Annona stands out for its multifaceted approach to cancer therapy [3, 4, 17–20]. The superior cytotoxic, cell cycle arresting, apoptotic, and ROS-modulating effects of the nano formulation further underscore its potential as a potent anticancer agent [21–23] compared to other herbal extracts.
Overall, the results suggest that the nano formulation of Annona extract holds promise as a potent anti-cancer agent, exhibiting superior cytotoxic, cell cycle arresting, apoptotic, and ROS-modulating effects compared to the crude extract. Further investigation into the underlying molecular mechanisms and in vivo efficacy of the nano formulation is warranted to validate its therapeutic potential for oral cancer treatment.
Conclusion
In conclusion, our study underscores the promising potential of the nano formulation of Annona extract as a potent anti-cancer agent against SCC-25 oral cancer cells [21, 23]. The observed cytotoxic, cell cycle arresting, apoptotic, and ROS-modulating effects of both the crude extract and nano formulation are consistent with existing literature on the anticancer properties of Annona species [3, 4, 17–20].
Notably, the nano formulation demonstrates enhanced potency compared to the crude extract, as evidenced by lower IC50 values, superior induction of G1 phase arrest and late apoptosis, and reduced ROS levels. These findings highlight the potential of nano-formulated Annona extract as a comprehensive approach to cancer therapy, offering superior efficacy compared to conventional herbal extracts.
Further research is warranted to elucidate the underlying molecular mechanisms and validate the in vivo efficacy of the nano formulation. Ultimately, our study contributes valuable insights into the potential of Annona extract as a promising anticancer agent for the treatment of oral cancer.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Dr M Rekha and Dr T Dineshkumar, The first draft of the manuscript was written by Dr M Rekha and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Conceptualization: Dr M Rekha. Methodology: Dr M Rekha, Dr Bhuminathan.S and Dr Lakshmanan. A. Formal analysis and investigation: Dr M Rekha and Dr T Dinesh Kumar. Writing—original draft preparation: Dr M Rekha. Writing—review and editing: Dr M Rekha, Dr Bhuminathan S, Dr T Dinesh Kumar and Dr Lakshmanan A.
Funding
No funding was received for conducting this study.
Declarations
Conflict of interest
The authors declare they have no financial interests.
Ethical approval
Though it is exempted from review as it is a study on cell lines, the study is presented and approved by RVS Institutional Ethics Committee.
Informed consent
Not relevant.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.El Sayed KA et al (2016) Polyketide natural products, acetogenins from graviola (Annona muricata L), its biochemical, cytotoxic activity and various analyses through computational and bio programming methods. Curr Pharm Des 22(34):5204–5210 10.2174/1381612822666160531163144 [DOI] [PubMed] [Google Scholar]
- 2.Rady I et al (2018) Anticancer properties of graviola (Annona muricata): a comprehensive mechanistic review. Oxid Med Cell Longev 1826170:1–39 10.1155/2018/1826170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Velázquez-Aponte RA, Cassé C (2020) Antiproliferative properties of ethanolic and aqueous graviola leaf extracts on tongue squamous cell carcinoma cell line-25. J Med Case Rep Rev 3(8):749–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ioannis P et al (2015) Graviola: a systematic review on its anticancer properties. Am J Cancer Prev 3(6):128–131 [Google Scholar]
- 5.Nugraha AS, Damayanti YD, Wangchuk P, Keller PA (2019) Anti-infective and anti-cancer properties of the annona species: their ethnomedicinal uses, alkaloid diversity, and pharmacological activities. Molecules 24(23):4419. 10.3390/molecules24234419 10.3390/molecules24234419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagarathna PKM et al (2019) Screening of anticancer and anti-oxidant activity of leaves of Annona reticulata on EAC induced solid tumor. IJPSR 10(11):4868–4880 [Google Scholar]
- 7.Daddiouaissaa D et al (2019) Antiproliferative activity of ionic liquid-graviola fruit extract against human breast cancer (MCF-7) cell lines using flow cytometry techniques. J Ethnopharmacol 236:466–473 10.1016/j.jep.2019.03.003 [DOI] [PubMed] [Google Scholar]
- 8.Yang C et al (2015) Synergistic interactions among flavonoids and acetogenins in Graviola (Annona muricata) leaves confer protection against prostate cancer. Carcinogenesis 36(6):656–665 10.1093/carcin/bgv046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magadi VP, Ravi V, Arpitha A, Litha KK, Manjunath K (2015) Evaluation of cytotoxicity of aqueous extract of Graviola leaves on squamous cell carcinoma cell-25 cell lines by 3-(4,5-dimethylthiazol-2-Yl) -2,5-diphenyltetrazolium bromide assay and determination of percentage of cell inhibition at G2M phase of cell cycle by flow cytometry: An in vitro study. Contemp Clin Dent 6(4):529. 10.4103/0976-237X.169863 10.4103/0976-237X.169863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mary SJ, Veeravarmal V, Thankappan P, Angelin D, Franklin R, Girish KL (2023) Evaluation of the cytotoxic, anti-proliferative, anti-metastatic and pro-apoptotic effect of aqueous leaf extract of Annona muricata on oral tongue squamous cell carcinoma cell line (SCC-15): an in vitro study. J Oral Maxillofac Pathol. 27(3):469–475. 10.4103/jomfp.jomfp_299_23 10.4103/jomfp.jomfp_299_23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charak S, Sharma M, Porte SM (2021) In vitro evaluation of anticancer activity (efficacy) of a novel ayurvedic polyherbal formulation on human oral carcinoma cell line SCC-40. South Asian J Cancer 00:1–2. 10.1055/s-0041-1735335 10.1055/s-0041-1735335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pieme A, Kumar S, Sylviane D, Moukette B, Boyom F, Jeanne N, Saxena A (2014) Antiproliferative activity and induction of apoptosis by Annona muricata (Annonaceae) extract on human cancer cells. BMC Complement Altern Med 14:516. 10.1186/1472-6882-14-516 10.1186/1472-6882-14-516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pardhasaradhi BV, Reddy M, Ali AM, Kumari AL, Khar A (2004) Antitumour activity of Annona squamosa seed extracts is through the generation of free radicals and induction of apoptosis. Indian J Biochem Biophys 41(4):167–172 [PubMed] [Google Scholar]
- 14.Torres MP, Rachagani S, Purohit V, Pandey P, Joshi S, Moore ED et al (2012) Graviola: a novel promising natural-derived drug that inhibits tumorigenicity and metastasis of pancreatic cancer cells in vitro and in vivo through altering cell metabolism. Cancer Lett 323:29–40 10.1016/j.canlet.2012.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gavamukulya Y, Abou-Elella F, Wamunyokoli F, AEL-Shemy H (2014) Phytochemical screening, anti-oxidant activity and in vitro anticancer potential of ethanolic and water leaves extracts of Annona muricata (Graviola). Asian Pac J Trop Med 7:S355–S363 10.1016/S1995-7645(14)60258-3 [DOI] [PubMed] [Google Scholar]
- 16.Jafari S, Saeidnia S, Abdollahi M (2014) Role of natural phenolic compounds in cancer chemoprevention via regulation of the cell cycle. Curr Pharm Biotechnol 15(4):409–421. 10.2174/1389201015666140813124832 10.2174/1389201015666140813124832 [DOI] [PubMed] [Google Scholar]
- 17.Wahab SMA, Jantan I, Haque MA, Arshad L (2018) Exploring the leaves of Annona muricata L. as a source of potential anti-inflammatory and anticancer agents. Frontiers Pharmacol. 10.3389/fphar.2018.00661 10.3389/fphar.2018.00661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moghadamtousi SZ, Fadaeinasab M, Nikzad S, Mohan G, Ali HM, Kadir HA (2015) Annona muricata (Annonaceae): a review of its traditional uses, isolated acetogenins and biological activities. Int J Mol Sci 16(7):15625–15658. 10.3390/ijms160715625.PMID:26184167;PMCID:PMC4519917 10.3390/ijms160715625.PMID:26184167;PMCID:PMC4519917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ilango S, Sahoo DK, Paital B, Kathirvel K, Gabriel JI, Subramaniam K, Jayachandran P, Dash RK, Hati AK, Behera TR, Mishra P, Nirmaladevi R (2022) A review on Annona muricata and its anticancer activity. Cancers (Basel) 14(18):4539. 10.3390/cancers14184539.PMID:36139697;PMCID:PMC9497149 10.3390/cancers14184539.PMID:36139697;PMCID:PMC9497149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balkrishna A, Dabhade NR, Singh A et al (2023) Anticancer acumens of three Annona species: a proportional review. J Cancer Res Clin Oncol 149:6693–6702. 10.1007/s00432-022-04306-5 10.1007/s00432-022-04306-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.González-Pedroza M, Argueta-Figueroa L, García-Contreras R, Jiménez-Martínez Y, Martínez-Martínez E, Navarro-Marchal S, Marchal J, Morales-Luckie R, Boulaiz H (2021) Silver nanoparticles from Annona muricata peel and leaf extracts as a potential potent, biocompatible and low cost antitumor tool. Nanomaterials 11(5):1273. 10.3390/nano11051273 10.3390/nano11051273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taneja N, Alam A, Patnaik RS, Taneja T, Gupta S, Sunil MK (2021) Understanding nanotechnology in the treatment of oral cancer: a comprehensive review. Crit Rev™ Therapeutic Drug Carr Syst 38(6):1–48. 10.1615/CritRevTherDrugCarrierSyst.2021036437 10.1615/CritRevTherDrugCarrierSyst.2021036437 [DOI] [PubMed] [Google Scholar]
- 23.Gavamukulya Y, Maina EN, Meroka AM, El-Shemy HA, Magoma G, Wamunyokoli F (2019) In search of new anticancer drugs: data for cytotoxic activities of green synthesized silver nanoparticles from ethanolic extracts of fruits and leaves of Annona muricata and 5-Fluorouracil against HeLa, PC3 and PNT1A cell lines. Data Brief 28(26):104442. 10.1016/j.dib.2019.104442.PMID:31528676;PMCID:PMC6743010 10.1016/j.dib.2019.104442.PMID:31528676;PMCID:PMC6743010 [DOI] [PMC free article] [PubMed] [Google Scholar]