Abstract
Background
We aimed to investigate the association between OA and treatment with dementia risk and structural brain abnormalities.
Methods
We recruited a total of 466,460 individuals from the UK Biobank to investigate the impact of OA on the incidence of dementia. Among the total population, there were 63,081 participants diagnosed with OA. We subsequently categorised the OA patients into medication and surgery groups based on treatment routes. Cox regression models explored the associations between OA/OA treatment and dementia risk, with the results represented as hazard ratios (HRs) and 95% confidence intervals (95% CI). Linear regression models assessed the associations of OA/OA therapy with alterations in cortical structure.
Results
During an average of 11.90 (± 1.01) years of follow-up, 5,627 individuals were diagnosed with all-cause dementia (ACD), including 2,438 AD (Alzheimer’s disease), and 1,312 VaD (vascular dementia) cases. Results revealed that OA was associated with the elevated risk of ACD (HR: 1.116; 95% CI: 1.039–1.199) and AD (HR: 1.127; 95% CI: 1.013–1.254). OA therapy lowered the risk of dementia in both medication group (HR: 0.746; 95% CI: 0.652–0.854) and surgery group (HR: 0.841; 95% CI: 0.736–0.960). OA was negatively associated with cortical area, especially precentral, postcentral and temporal regions.
Conclusions
Osteoarthritis increased the likelihood of developing dementia, and had an association with regional brain atrophy. OA treatment lowered the dementia risk. OA is a promising modifiable risk factor for dementia.
Keywords: osteoarthritis, osteoarthritis treatment, dementia, brain structure, older people
Key Points
Osteoarthritis is associated with an increased risk of dementia, especially Alzheimer’s disease.
Medications lower dementia risk, prominently nonsteroidal anti-inflammatory drug and opioids.
Joint replacements reduce dementia risk, notably knee replacement has a stronger effect.
Osteoarthritis causes reduction in cortical areas at baseline.
Introduction
Dementia is an age-related disease characterised by a gradual decline in cognitive capacities [1]. It has imposed heavy burden on patients with their caregivers and the national healthcare system [2]with no effective measures to cure or slow its progression. Hence, management of its modifiable risk factors for prevention is essential [3].
A growing number of studies demonstrated that OA raised the risks of cognitive impairment and dementia [4–6], with a possibility via pathway of local inflammatory cytokines [7, 8]. Long-term exposure to pain in patients with OA has also been found to cause the occurrence of dementia [9–11]. Distinct and effective therapies, principally surgical and medicinal treatments, have been developed for osteoarthritis [12, 13], and possibly to decrease indirectly the rates of subsequent dementia [14]. However, prior studies to investigate whether medication reduced the risk of subsequent dementia in OA patients yielded inconsistent outcomes, some of which were negative [15–17], positive [18, 19] and neutral [20–22]. Considering the improved efficacy after OA treatment [23], so if medication or surgery reverts the heightened risk of dementia caused by osteoarthritis, it may be one of the risky elements for dementia that is susceptible to modification.
Inconsistent structural brain changes after different OA treatments (including surgeries and medications) were observed [24–26], while the mechanisms underlying the associations between OA and brain imaging measures are still unclear [27, 28]. Therefore another intention of this study was to identify the brain regions related to OA and treatment, contributing to a clearer appreciation of the potential mechanisms of dementia.
Utilising the massive sample size and longitudinal tracking over long periods of the UK Biobank (UKB), we systematically and comprehensively examine the associations of OA/OA treatment and dementia subtypes, and brain structures to extensively investigate the hidden linkages. We hypothesise that the risk of dementia is elevated by OA, and would be lowered by OA treatments. We also speculated that OA patients will carry greater risks of structural atrophy in the cortical regions based on previous literature.
Methods
Study population
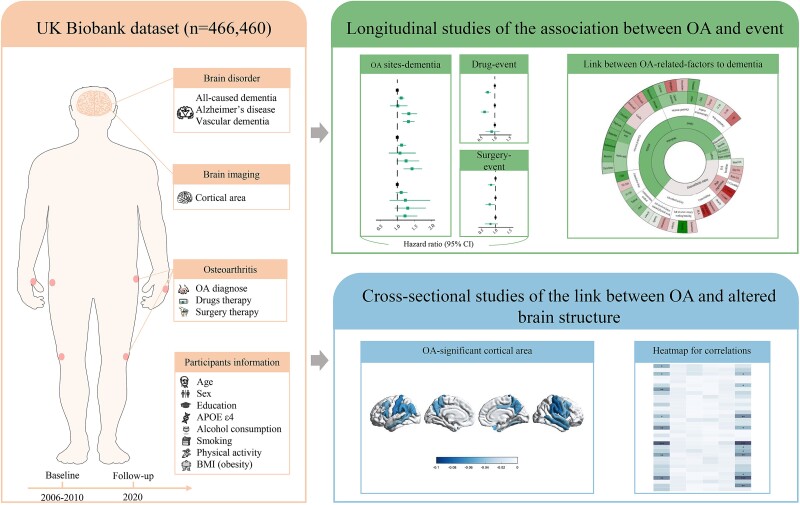
UKB (https://www.ukbiobank.ac.uk/) is a large nationwide prospective cohort study that enrolled 502,494 individuals. We recruited 466,460 participants between 2006 and 2010 from the UKB database and followed up these participants until the onset of dementia or the endpoint date (31 December 2020) (Fig. 1).
Figure 1.
Structural summary of study. The research overview summarises the selection and analysis process of the analysis population. Abbreviations: OA, osteoarthritis; BMI, body mass index.
Exposure to OA
OA was ascertained based on primary care, hospitalisation, self-report, death or hospital inpatient data defined by International Classification of Diseases, 10th edition (ICD-10) codes M15-M19 [29]. We conducted a subgroup analysis by categorising participants into hand OA (ICD-10: M15, M18), knee OA (ICD-10: M17) and hip OA (ICD-10: M16) subgroups. We defined participants without OA at baseline to be controls. Additionally, we took into consideration the influencing factors of OA, such as history of joint injury, manual labor and pain experience, and also explored their associations with dementia risk.
Exposure to OA treatments
Treatment information included records of drug prescriptions and surgery history. We sorted medicine via the Read Codes, British National Formulary codes and Dictionary of Medicines and Devices codes. Surgery was defined as patients underwent joint replacement with OPCS4 code. We defined the participants who received treatment after OA diagnosis as the medication or surgery group and others who never took medication since developed OA as the unmedicated or no-surgery group. The treatment period defined from the start of OA treatments to the end of follow-up. Subsequently, in order to avoid reverse causation and insufficient treatment exposure [30], we further excluded participants who started receiving therapy in 2 years prior to the end point of follow-up.
Dementia outcomes
Diagnosis of dementia is detailed in the Supplementary Methods section. Incident dementia events were diagnosed after enrollment.
Covariates
Covariates are described in the Supplementary Methods.
Neuroimaging data
The magnetic resonance imaging analysed in this study consisted of 66 cortical regions. See the Supplementary Methods for more details.
Statistical analyses
Continuous baseline variables were expressed as mean (standard deviation [SD]) or median (interquartile range), and categorical values were present as number (percentage). We examined these variables using analysis of variance or the Mann–Whitney U test.
Multivariate Cox proportional hazards regression explored the associations between OA/OA treatments and dementia risk, with results presented as hazard ratios (HRs) and 95% confidence interval (95% CIs). Independent analyses firstly adopted a minimal-adjustment Cox model with age and gender as covariates (Model 1). Model 2 additionally made adjustments for APOE ε4, ethnic, education, smoking status, alcohol consumption, BMI and TPA.
To examine whether dementia risk varied with different kinds of outcomes or exposures, we conducted a secondary analysis. To further explore the different effects on dementia risk across different types of OA drugs, we subdivided the medication group into subgroups of nonsteroidal anti-inflammatory drugs (NSAIDs), opioids, glucosamine and corticosteroids. NSAIDs and opioids were further stratified by chemical structure and product name based on the World Health Organization’s the Anatomical Therapeutic Chemical codes. We calculated the cumulative use of NSAIDs and opioids using ‘defined daily doses’, which was divided into four mutually exclusive groups to generating categorical variables over time [31]. Joint replacement is further subdivided mainly into knee replacement and hip replacement.
We used the interactive terms for age, early or late-onset dementia type [31], gender, APOE ε4 status, length of OA, BMI [32] and TPA to assess whether there was stratification effect among the distinct subgroups (P < 0.1). We conducted four sensitivity analyses to test the robustness. More detailed processes were shown in the Supplementary Statistical analyses.
We investigated the association between OA and brain morphometry using a linear regression model. The P values for brain structures were adjusted via the false discovery rate (FDR) correction method. Adjusted significance thresholds were two-sided P < 0.05. Analyses were conducted by using the R survival packages with version 4.1.2.
Results
Participant characteristics
Our study comprised 466,460 participants with a mean age at recruitment of 56.74 (SD 8.08), of whom 254,909 (54.6%) were males. There was a total of 63,081 OA patients, including 4087 (6.5%) with hand OA, 6860 (10.9%) with hip OA and 14,155 (22.4%) with knee OA. After a median follow-up of 11.90 (SD 1.01) years, 5627 developed all-cause dementia [ACD, including 2438 Alzheimer’s disease (AD) and 1312 vascular dementia (VaD)]. In terms of different treatment routes, 17,734 OA patients underwent joint replacement surgeries and 17,856 OA patients received pharmacological treatment. Characteristics of the participants, both clinical and demographic, were shown in Table 1.
Table 1.
Demographic and clinical characteristics according to osteoarthritis conditions
| Variables | Group without osteoarthritis (n = 403,379) | Group with osteoarthritis (n = 63,081) | P value |
|---|---|---|---|
| Age at baseline, years, mean(SD) | 56.12(8.14) | 60.70(6.37) | <0.001 |
| Gender, n (%) | |||
| Female | 186,832(46.3) | 24,719(39.2) | <0.001 |
| Male | 216,547(53.7) | 38,362(60.8) | <0.001 |
| Education, n (%) | |||
| With college degree | 190,014(47.1) | 23,912(37.9) | <0.001 |
| Without college degree | 213,365(52.9) | 39,169(62.1) | <0.001 |
APOE
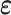 4 carrier, n (%)
4 carrier, n (%) |
|||
| Carrier | 98,511(28.7) | 15,663(28.1) | <0.001 |
| Non-carrier | 244,223(71.3) | 40,147(71.9) | <0.001 |
| Ethnicity, n (%) | |||
| White | 354,391(87.9) | 57,464(91.1) | <0.001 |
| Mixed | 451(0.1) | 80(0.1) | <0.001 |
| Asian | 13,281(3.5) | 1374(2.3) | <0.001 |
| Black | 10,745(2.8) | 1552(2.6) | <0.001 |
| BMI, (kg/m), n (%) | |||
| <18.5 | 2229(0.6) | 176(0.3) | <0.001 |
| 18.5–24.9 | 134,627(33.6) | 13,551(21.6) | <0.001 |
| 25–29.9 | 171,210(42.7) | 25,783(41.2) | <0.001 |
| ≥30 | 92,823(23.2) | 23,134(36.9) | <0.001 |
| Smoking status, n (%) | |||
| Never | 220,190(54.9) | 31,242(49.9) | <0.001 |
| Previous | 137,307(34.2) | 25,172(40.2) | <0.001 |
| Current | 43,562(10.9) | 6231(9.9) | <0.001 |
| Alcohol drinking status, n (%) | |||
| Never | 17,600(4.4) | 3379(5.4) | <0.001 |
| Previous | 14,036(3.5) | 3206(5.1) | <0.001 |
| Current | 370,441(92.1) | 56,277(89.5) | <0.001 |
| Depressive status, n (%) | |||
| Yes | 90,853(23.7) | 15,673(26.3) | <0.001 |
| No | 293,212(76.3) | 44,002(73.7) | <0.001 |
| Total physical activity, n (%) | |||
| <600 | 92,651(24.3) | 16,478(27.9) | <0.001 |
| 600–2,999 | 183,409(48.1) | 26,026(44.1) | <0.001 |
| ≥3,000 | 105,555(27.7) | 16,460(27.9) | <0.001 |
| Dementia types, n (%) | |||
| All-caused dementia | |||
| Yes | 4330(1.1) | 1297(2.1) | <0.001 |
| No | 399,049(98.9) | 61,784(97.9) | <0.001 |
| Alzheimer’s disease | |||
| Yes | 1862(0.5) | 576(0.9) | <0.001 |
| No | 399,049(98.9) | 61,784(97.9) | <0.001 |
| Vascular dementia | |||
| Yes | 998(0.2) | 314(0.5) | <0.001 |
| No | 399,049(98.9) | 61,784(97.9) | <0.001 |
| Osteoarthritis types, n (%) | |||
| Hand OA | NAa | 4087(6.5) | <0.001 |
| Hip OA | NAa | 6860(10.9) | <0.001 |
| Knee OA | NAa | 14,155(22.4) | <0.001 |
| Only hand OA | NAa | 1,527(2.4) | <0.001 |
| Only hip OA | NAa | 2,845(4.5) | <0.001 |
| Only knee OA | NAa | 7,346(11.6) | <0.001 |
| Hand hip OA | NAa | 95(0.2) | <0.001 |
| Hand knee OA | NAa | 201(0.3) | <0.001 |
| Hip knee OA | NAa | 253(0.4) | <0.001 |
| Hand hip knee OA | NAa | 23(0.0) | <0.001 |
| Osteoarthritis duration, years, mean (SD) | NAa | 17.55(9.38) | <0.001 |
| Treatment | |||
| Drug therapy, n (%) | |||
| No | NAa | 34,033(54.0) | <0.001 |
| Yes | NAa | 17,823(28.3) | <0.001 |
| NSAID | NAa | 11,445(18.1) | <0.001 |
| Opioid | NAa | 12,431(19.7) | <0.001 |
| Glucosamine | NAa | 1291(0.2) | <0.001 |
| Corticosteroid | NAa | 4792(7.6) | <0.001 |
| Surgery therapy, n (%) | |||
| No | NAa | 44,973(71.3) | <0.001 |
| Yes | NAa | 17,708(28.1) | <0.001 |
| Hip surgery | NAa | 7,617(12.1) | <0.001 |
| Knee surgery | NAa | 8,672(13.7) | <0.001 |
Data are presented as n (%) and mean (SD). The P values are derived using Student’s t test, Mann–Whitney U test or 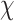 2 test among diagnosed with OA or without OA group.
2 test among diagnosed with OA or without OA group.
Abbreviations: BMI, body mass index; APOEε4, apolipoprotein E4; TPA, total physical activities; SD, standardised deviation; NSAID, nonsteroidal anti-inflammatory drug; NA, not applicable.
The untreated group and treated group were only classified in participants with osteoarthritis.
Associations between OA and dementia risk
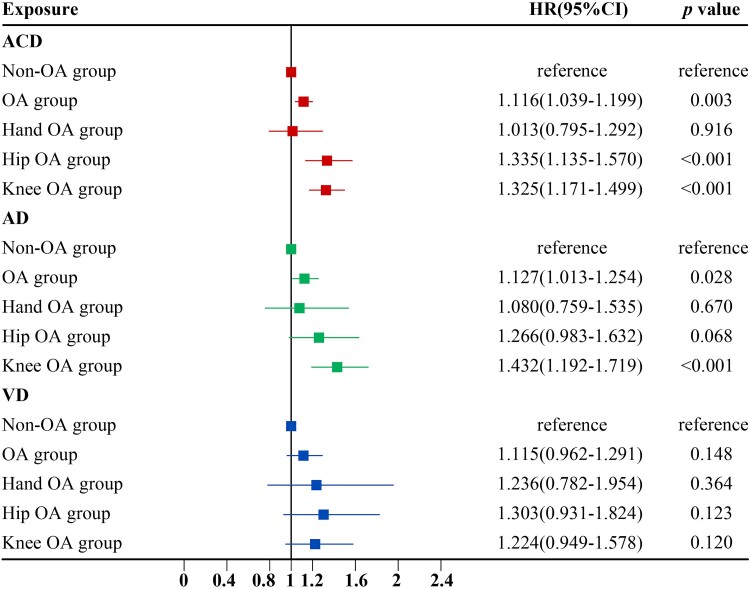
At baseline, 63,081 participants were reported with a diagnosis of OA, of which 1,297 developed ACD during the follow-up. Multivariable Cox proportional hazards models revealed OA was significantly associated with elevated risk of ACD (HR: 1.116; 95% CI: 1.039–1.199) and AD (HR: 1.127; 95% CI: 1.013–1.254) (Fig. 2). Concerning the different sites, we found that knee OA significantly elevated the risk of ACD and AD, and hip OA markedly added the elevated ACD risk (Supplementary Table 1). To account for the cumulative effect of the number of OA sites, we further subdivided the different sites into joints [33]. When only one joint was affected, single knee OA and single hip OA were associated with a meaningful risk of dementia. When multiple joints were simultaneously involved, knee and hip double joint OA was associated with dementia (HR: 2.197; 95%CI: 1.180–4.091; Supplementary Table 1). This is consistent with the results of the primary analysis.
Figure 2.
Association of OA and OA treatment with incident dementia during follow-up in the fully-adjusted model. The red, green and blue squares represent the HR of osteoarthritis to ACD, AD and VD in three outcomes, where the non-OA group is used as reference. The red, blue and green horizontal lines indicate the corresponding 95% CIs around the HRs. HRs were calculated using Cox proportional hazards regression analysis after adjustments for age, sex, ethnic, education, BMI, TPA, smoking status, alcohol status and APOE ε4 status. Abbreviations: ACD, all-cause dementia; AD, Alzheimer’s disease; VD, vascular dementia; HR: hazard ratio; BMI, body mass index; APOE, apolipoprotein E; TPA, total physical activities.
The observed associations were more pronounced in older, male, early-onset dementia, APOE ε4 carrier, OA duration >5 years, BMI ≥ 30 and TPA ≥ 3000 subgroups (Supplementary Tables 3–9). The sensitivity analysis by excluding participants with OA diagnosed from self-reported sources and inflammatory arthritis showed the associations of OA with ACD and AD became more pronounced (Supplementary Tables 10 and 11). Even considering potential selection bias and the competing risk of all-cause mortality, the results remained robust (Supplementary Tables 12 and 13).
Associations between influencing factors of OA and dementia risk
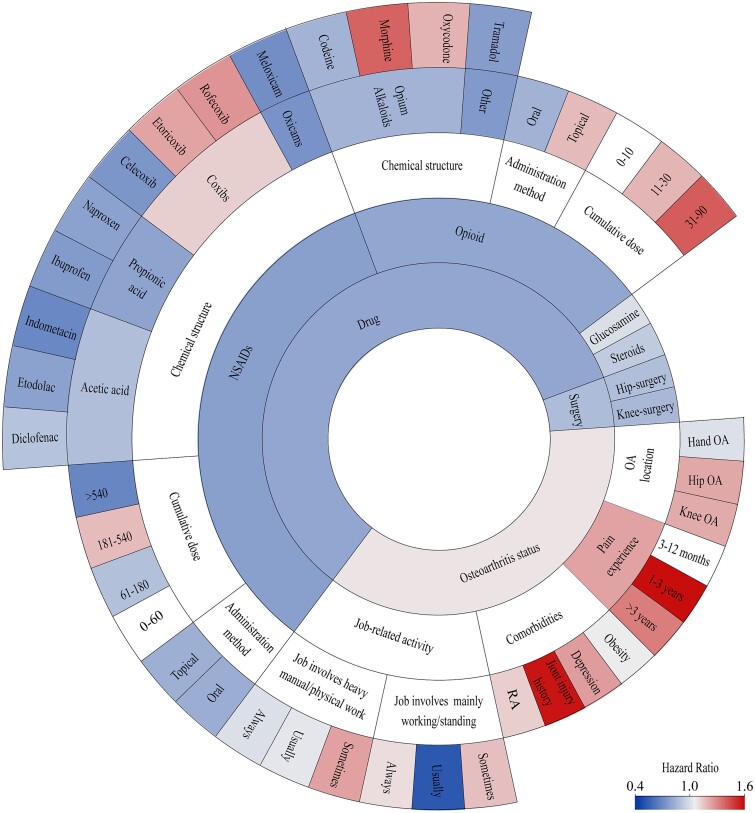
Many factors influence the relationship between OA and dementia, so we have performed a lot of exploratory analyses (see the grey semi-circle named ‘Osteoarthritic status’ in Fig. 3). OA patients with depression (HR: 1.382) and joint injury history (HR: 1.985) showed an elevated risk of dementia. OA patients with obesity showed no significant differences in dementia risk than those without. No disparity of dementia risk was observed between OA patients with and without chronic pain and various pain durations (Supplementary Table 14). Job involving mainly walking/standing or heavy manual/physical work reportedly among OA patients did not elevate the risk of dementia.
Figure 3.
Association between different aspects of osteoarthritis and dementia. Association between different factors of osteoarthritis and dementia risk. The colour of the circle represents the magnitude of HRs, derived from fully adjusted Cox models (adjusted for age, sex, ethnic, education, BMI, TPA, smoking status, alcohol status and APOE ε4 status). Abbreviations: BMI, body mass index; APOE, apolipoprotein E; TPA, total physical activities; RA, rheumatoid arthritis; NSAID, nonsteroidal anti-inflammatory drug.
Associations between OA medications and dementia risk
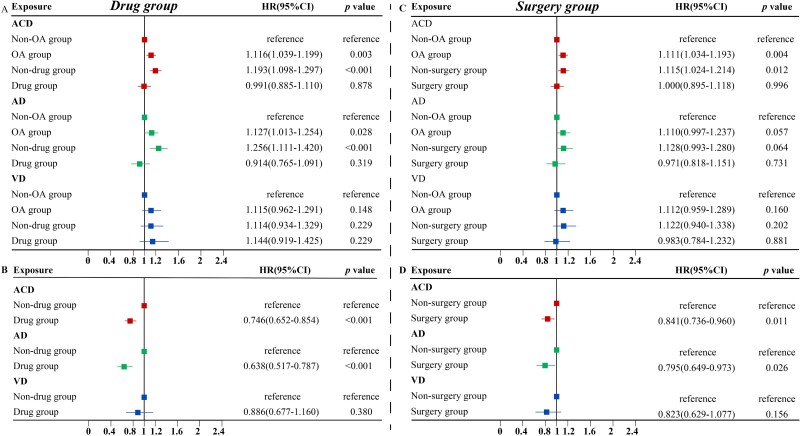
A total of 17,856 individuals with OA received medication. No significant differences in the dementia risk between medication group and healthy controls (Fig. 4A). The protective effect of medication on dementia risk was stronger after excluding 33 patients who started taking medication within two years before the outcome event (HR: 0.746; 95% CI: 0.652–0.854) (Fig. 4B).
Figure 4.
Association of osteoarthritis treatment with incident dementia during follow-up in the fully-adjusted model. (A) displays the association between medication taking and dementia in OA patients compared to those without OA. (B) demonstrates among OA patients, the incidence of dementia in drug group compared to the non-drug group. (C) shows the association between undergoing surgery and dementia in patients with OA compared to non-OA. The plot 4D illustrates among OA patients, the risk of dementia in operative group compared to the non-surgery group. Abbreviations: OA, osteoarthritis; ACD, all-cause dementia; AD, Alzheimer’s disease; VD, vascular dementia; HR: hazard ratio.
In subgroup analyses, the stronger protective effects of OA medications against dementia can be seen in older, late-onset dementia, male and APOE ε4 carrier subgroups. Analyses of dementia subtypes revealed a significant association between OA medications and the risk of AD (Supplementary Table 15). The results of several sensitivity analyses remained robust. (Supplementary Tables 10, 11 and 13).
Oral medications offer stronger protection against dementia (HR: 0.747; 95% CI:0.649–0.859) (Supplementary Table 15). The protective role of NSAIDs and opioid was stronger than that of glucosamine and intra-articular steroid hormone injections. In the subgroups of NSAIDs, propionates (chemical structure) were protective against dementia risk, and ibuprofen and naproxen (product name) within the propionate group were significant. Ibuprofen was associated with AD risk, not VD (Supplementary Table 16). For opioid, protective effects against ACD and AD were observed for codeine and tramadol in the grouping of product name (Supplementary Table 17). Different cumulative exposure subgroups for oral NSAIDs and opioids were not statistically associated with dementia risk and its subtypes (Supplementary Figs 1 and 2).
Associations between OA surgeries and dementia risk
Among the baseline OA participants, 17,734 underwent surgeries who had no evidently elevated risk of dementia during the follow-up compared with non-OA controls (Fig. 4C). Within the OA participants, after excluding the 26 who had surgery within 2 years prior to the endpoint, we found the protective role of OA surgeries against dementia remained significant (HR: 0.841; 95% CI: 0.736–0.960) (Fig. 4D).
The protective associations between OA surgeries and dementia risk were still significant in older, male, TPA ≥ 3,000 and BMI ≥30 subgroups (Supplementary Tables 3, 5, 8 and 9). As for dementia subtypes, OA surgeries showed significant associations with the risk of AD (HR: 0.795; 95% CI: 0.649–0.973). In subgroup analyses of surgical procedures, only knee replacement was significantly associated with dementia risk after correction for multiple factors by model 2 (HR: 0.825; 95% CI: 0.695–0.979) (Supplementary Table 18). Yet, no statistically significant association between surgery and dementia risk could be seen in several sensitivity analyses (Supplementary Tables 10, 11 and 13).
Associations between OA/OA treatments and brain structures
The data on structural brain changes are based on the population that underwent magnetic resonance imaging (MRI) brain examinations, of which there were 3,956 in OA patients, 293 in the surgical population and 1,197 in the medication population. Cortical atrophy was observed in OA patients, including the reduced areas of the postcentral gyrus, right precentral gyrus, caudal middle frontal, left inferior parietal lobule, right temporal lobe and left middle temporal lobe, right posterior cingulate gyrus and the precuneus (Supplementary Fig. 3A). The above associations remained significant after FDR correction (Supplementary Fig. 3B). After FDR correction, the cortical atrophy observed in OA participants were not attenuated after OA treatments (Supplementary Table 19).
Discussion
This research demonstrates that OA/OA treatment were associated with the altered risk of dementia. OA conferred a 11.6% higher risk of dementia, whereas OA treatment (surgeries and medications) lowered a 15.9–25.4% dementia risk. Notably, NSAIDs and opioids had significant protective effects on dementia. Besides, OA was related to reduced grey matter area to a large extent. Collectively, our findings indicated that OA might be a risky factor for dementia, and this risk could be reversed through OA treatments.
There are several possible mechanisms underlying the association between OA and risk of dementia. OA could release pro-inflammatory factors into the blood stream, leading to brain inflammation and subsequently contributing to higher risks of cognitive impairment and dementia [7, 34, 35]. Animal experiments observed increased neuroinflammation and aggravated AD pathology in mice by constructing an OA model [34]. An American retrospective study suggested patients with both OA and chronic pain elevated risk of dementia versus those with OA alone [5]. In addition, dementia can be attributed to depressive symptoms among OA patients [36, 37], and we found a 38.2% higher risk of dementia when OA was combined with depression. Obesity might elevate the likelihood of developing dementia among OA patients [38]. Our study revealed that the risk of dementia increased by 11.4% in patients with both OA and obesity than patients with OA alone. Overuse, improper posture, and mechanical loads are recognised to contribute to the initiation and progression of OA [32–40], while we found work intensity did not significantly influence the associations between OA and dementia risk.
NSAIDs suppresses inflammatory response by inhibiting cyclooxygenase, which thus alleviate cognitive decline [41, 42]. We found that the dementia risk fell 23.5% in OA patients taking NSAIDs than those who did not. Topical/oral opioids and NSAIDs comprise the first-line pharmacological therapies for OA [43, 44]. Various types of previous studies have pointed out that ibuprofen [45], naproxen [46] and tramadol [47] lowered the risk of dementia, and we achieved the same results. It is worth noting that all of these medications are associated with pain relief. However, there is no data in the UKB database to clearly and objectively quantify pain scales and accurately record changes in pain fluctuations among patients. Further animal experiments are therefore needed to explore the mechanisms by which more detailed and accurate drugs reduce the risk of dementia. We found no evidence of an association between glucosamine and dementia, which was in line with a previous prospective cohort study based on the UKB database [48]. Our study serves as the first to explore the association between intra-articular steroid injections and dementia risk, yielding no statistically significant association. In addition, previous studies have indicated that paracetamol is less useful for OA [49, 50]. Considering the large base of people taking it, we supplemented the analysis of the association between paracetamol-only and dementia risk while this analysis was not included in the main one. Results suggested that no statistically significance was observed for the effect of paracetamol on dementia risk in OA patients (Supplementary Table 20). In conclusion, our study is the first to restrict our participants to OA patients, and we also explored whether these OA medications could reverse the dementia risk elevated by OA. Joint replacement could improve joint movement and relieve the symptoms of OA [13], and we discovered a dramatic fall in dementia risk among OA patients who underwent OA surgeries. Furthermore, previous studies have failed to compare OA treatment group with non-OA controls. Thus, they could not figure out the independent role of OA treatment in dementia [51]. By including a healthy control group, our study design is a better way to estimate the effectiveness of OA therapy on dementia risk. Grotle et al. discovered a significant dose–response association between obesity (BMI > 30 kg/m2) and the risk of knee OA rather than hip OA [52]. In OA patients with a BMI ≥ 30 kg/m2, knee replacement surgery exerted stronger protection against dementia than hip replacement surgery. However, in this study, we extracted information on obesity from BMI with the recording time was not further clarified. We were unable to calculate the fluctuation of BMI before and after OA/OA treatment. This shortcoming awaits future UKB databases to refine the time of weight recording and calculation of the associated rate of change, or additional databases to refine this information. We also found that OA medications reduced the risk of dementia only in APOE ε4-positive participants, which provided new insights into dementia prevention among APOE ε4 carriers. Women prevail in OA patients [53] and the adults in UKB database has a large proportion of females, although we ended up with more males (54.6%) in our final analysis. We had no selection bias in the inclusion process. To account for this phenomenon, we corrected for the covariate of gender and stratified by sex and found that the male effect was still significant.
Cerebral atrophy assessed on structural MRI has been considered as an effective marker of dementia [54]. Long-term OA pain can lead to decreased quality of life [53], less exercise and poor sleep, which may cause progressive cortical thinning [55, 56]. Chronic pain was associated with brain structural alterations in temporal lobe regions [57, 58]. Temporal cortex is sensitive to AD-related pathological and cognitive changes [59], since superior temporal regions and meso-temporal regions are involved in cognitive domains, including speech perception [60], motion processing [61] and episodic memory [62]. We detected marked reduction in the areas of right and left middle temporal lobes and right superior temporal lobes in OA patients. Previous fMRI studies showed that the activities in motor and somatosensory cortices (precentral and postcentral cortices) were mainly observed in the evoked pain condition [9, 63]. A latest study found that OA was related to speeding up Aβ accumulation and more Aβ and tau deposition in precentral and postcentral cortices [11]. Consistent with the above findings, we found that the areas of postcentral and right precentral cortices in the right and left hemispheres were reduced in the OA patients. The precuneus and posterior cingulate cortex are closely associated with the AD-targeted default mode network [64, 65], and the posterior cingulate cortex connects with regions involved in emotion, executive control and memory [66]. In conclusion, the negative associations between OA and the areas of specific cortical regions indict that OA may cause structural abnormalities in these regions to enhance dementia risk [67].
There are several advantages of our study. First, it is the first comprehensive and systematic assessment of the associations of OA and its treatments with dementia and brain structures in a longitudinal cohort. Second, we utilised computerised pharmacy data to capture drug use throughout the follow-up, subsequently we can characterise medication use throughout the study to trap elaborate medication expenditure. Third, using comprehensive questionnaires and physical assessments, we took a wide range of important confounders into consideration in the analysis, including sociodemographic and lifestyle factors.
This study has several limitations. First, we lacked the detailed data about OA severity (e.g. the Kellgren–Lawrence grades and the evolution of pain levels). The dynamic changes in OA severity might influence our findings on the associations between OA and dementia risk. Second, Foot OA is also one of the more common subtypes of OA [68]. Yet in baseline recruitment of our OA population based on ICD-10 codes, we were unable to be specific to foot OA. This paucity of data relies on further refinement of the database or may be explored in other databases in the future. Third, since the imaging data were from cross-sectional studies, it is beyond our ability to conclude the causalities and temporal relationships between OA and changes in brain structures. Nonetheless, their cross-sectional associations we observed added complementary support to this longitudinal analysis.
In conclusion, our study suggested that OA was associated with increased risk of dementia and atrophic brain structures. However, OA treatments (surgeries and medications) could reverse this risk among OA patients. Therefore, OA should be regarded as a changeable risk factor in the prevention and management of dementia.
Supplementary Material
Acknowledgements
This study is conducted under application number 19542 for UK Biobank Resource. The authors gratefully thank all the participants and professionals contributing to the UK Biobank.
Contributor Information
Rong Guo, Department of Neurology, Qingdao Municipal Hospital, Qingdao University, Qingdao, China.
Ya-Nan Ou, Department of Neurology, Qingdao Municipal Hospital, Qingdao University, Qingdao, China.
Li-Yun Ma, Department of Neurology, Qingdao Municipal Hospital, Qingdao University, Qingdao, China.
Lian Tang, Department of Neurology, Qingdao Municipal Hospital, Qingdao University, Qingdao, China.
Liu Yang, Department of Neurology and Institute of Neurology, Huashan Hospital, State Key Laboratory of Medical Neurobiology and MOE Frontiers Center for Brain Science, Shanghai Medical College, Fudan University, National Center for Neurological Disorders, Shanghai, China.
Jian-Feng Feng, Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China; Key Laboratory of Computational Neuroscience and Brain-Inspired Intelligence (Fudan University), Ministry of Education, Shanghai, China; Fudan ISTBI—ZJNU Algorithm Centre for Brain-Inspired Intelligence, Zhejiang Normal University, Jinhua, China; MOE Frontiers Center for Brain Science, Fudan University, Shanghai, China; Zhangjiang Fudan International Innovation Center, Shanghai, China.
Wei Cheng, Department of Neurology and Institute of Neurology, Huashan Hospital, State Key Laboratory of Medical Neurobiology and MOE Frontiers Center for Brain Science, Shanghai Medical College, Fudan University, National Center for Neurological Disorders, Shanghai, China; Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China; Key Laboratory of Computational Neuroscience and Brain-Inspired Intelligence (Fudan University), Ministry of Education, Shanghai, China; Fudan ISTBI—ZJNU Algorithm Centre for Brain-Inspired Intelligence, Zhejiang Normal University, Jinhua, China.
Lan Tan, Department of Neurology, Qingdao Municipal Hospital, Qingdao University, Qingdao, China.
Jin-Tai Yu, Department of Neurology and Institute of Neurology, Huashan Hospital, State Key Laboratory of Medical Neurobiology and MOE Frontiers Center for Brain Science, Shanghai Medical College, Fudan University, National Center for Neurological Disorders, Shanghai, China.
Glossary
OA, osteoarthritis; ACD, all-caused dementia; AD, Alzheimer’s disease; VaD, vascular dementia; TPA, total physical activity; BMI, body mass index; UKB, UK Biobank; ICD-10, International Classification of Diseases, 10th edition; OPCS4, Office of Population Censuses and Surveys Classification of Interventions and Procedures; SD, standard deviation; 95%CI, 95% confidence interval; NSAIDs, nonsteroidal anti-inflammatory drugs; FDR, false discovery rate; MRI, magnetic resonance imaging.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
This study was supported by grants from the Science and Technology Innovation 2030 Major Projects (2022ZD0211600), the National Natural Science Foundation of China (82071201, 81971032, 92249305, 82071997), Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX01), Research Start-up Fund of Hua Shan Hospital (2022QD002), Excellence 2025 Talent Cultivation Program at Fudan University (3030277001), ZHANG JIANG LAB, Tian Qiao and Chrissy Chen Institute, Shanghai Rising-Star Program (21QA1408700) and the State Key Laboratory of Neurobiology and Frontiers Center for Brain Science of Ministry of Education, Fudan University. The funding sources had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and the decision to submit the manuscript for publication.
References
- 1. Livingston G, Huntley J, Sommerlad Aet al. . Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020; 396: 413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1211–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Winblad B, Amouyel P, Andrieu Set al. . Defeating Alzheimer's disease and other dementias: a priority for European science and society. Lancet Neurol 2016; 15: 455–532. [DOI] [PubMed] [Google Scholar]
- 4. Huang SW, Wang WT, Chou LCet al. . Osteoarthritis increases the risk of dementia: a nationwide cohort study in Taiwan. Sci Rep 2015; 5: 10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Innes KE, Sambamoorthi U. The association of osteoarthritis and related pain burden to incident Alzheimer's disease and related dementias: a retrospective cohort study of U.S. medicare beneficiaries. J Alzheimers Dis 2020; 75: 789–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Innes KE, Sambamoorthi U. The association of perceived memory loss with osteoarthritis and related joint pain in a large appalachian population. Pain Med 2018; 19: 1340–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guerne PA, Carson DA, Lotz M. IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J Immunol 1990; 144: 499–505. [PubMed] [Google Scholar]
- 8. Mengshol JA, Vincenti MP, Brinckerhoff CE. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum 2000; 43: 801–11. [DOI] [PubMed] [Google Scholar]
- 9. Baliki MN, Geha PY, Jabakhanji Ret al. . A preliminary fMRI study of analgesic treatment in chronic back pain and knee osteoarthritis. Mol Pain 2008; 25: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tanner JJ, Hanchate S, Price CCet al. . Relationships between chronic pain stage, cognition, temporal lobe cortex, and sociodemographic variables. J Alzheimers Dis 2021; 80: 1539–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Du J, Li A, Shi Det al. . Association of APOE4, osteoarthritis, β-amyloid, and tau accumulation in primary motor and somatosensory regions in Alzheimer disease. Neurology 2023; 101: e40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dziedzic KS, Healey EL, Porcheret Met al. . Implementing core NICE guidelines for osteoarthritis in primary care with a model consultation (MOSAICS): a cluster randomised controlled trial. Osteoarthr Cartil 2018; 26: 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brignardello-Petersen R, Guyatt GH, Buchbinder Ret al. . Knee arthroscopy versus conservative management in patients with degenerative knee disease: a systematic review. BMJ Open 2017; 7: e016114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang S-W, Wang W-T, Chou L-Cet al. . Osteoarthritis increases the risk of dementia: a nationwide cohort study in Taiwan. Sci Rep 2015; 18: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Breitner JC, Haneuse SJ, Walker Ret al. . Risk of dementia and AD with prior exposure to NSAIDs in an elderly community-based cohort. Neurology 2009; 72: 1899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aisen PS, Schafer KA, Grundman Met al. . Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA 2003; 289: 2819–26. [DOI] [PubMed] [Google Scholar]
- 17. Levine SZ, Rotstein A, Goldberg Yet al. . Opioid exposure and the risk of dementia: a national cohort study. Am J Geriatr Psychiatry 2023; 31: 315–23. [DOI] [PubMed] [Google Scholar]
- 18. Zhang C, Wang Y, Wang Det al. . NSAID exposure and risk of Alzheimer's disease: an updated meta-analysis from cohort studies. Front Aging Neurosci 2018; 10: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oh G, Abner EL, Fardo DWet al. . Patterns and predictors of chronic opioid use in older adults: a retrospective cohort study. PloS One 2019; 14: e0210341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lyketsos CG, Breitner JC, Green RCet al. . Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology 2007; 68: 1800–8. [DOI] [PubMed] [Google Scholar]
- 21. Thal LJ, Ferris SH, Kirby Let al. . A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology 2005; 30: 1204–15. [DOI] [PubMed] [Google Scholar]
- 22. Fink HA, Jutkowitz E, McCarten JRet al. . Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: A systematic review. Ann Intern Med 2018; 168: 39–51. [DOI] [PubMed] [Google Scholar]
- 23. Clement ND, MacDonald D, Burnett R. Primary total knee replacement in patients with mental disability improves their mental health and knee function: a prospective study. Bone Joint J 2013; 95–B: 360–6. [DOI] [PubMed] [Google Scholar]
- 24. Lewis GN, Parker RS, Sharma S et al. . Structural brain alterations before and after total knee arthroplasty: a longitudinal assessment. Pain Med 2018; 19: 2166–76. [DOI] [PubMed] [Google Scholar]
- 25. Lan F, Lin G, Cao G et al. . Altered intrinsic brain activity and functional connectivity before and after knee arthroplasty in the elderly: a resting-state fMRI study. Front Neurol 2020; 11: 556028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Younger JW, Chu LF, D'Arcy NT et al. . Prescription opioid analgesics rapidly change the human brain. Pain 2011; 152: 1803–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Soni A, Wanigasekera V, Mezue M et al. . Central sensitization in knee osteoarthritis: relating presurgical brainstem neuroimaging and PainDETECT-based patient stratification to arthroplasty outcome. Arthritis Rheumatol 2019; 71: 550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schaible HG.. Mechanisms of chronic pain in osteoarthritis. Curr Rheumatol Rep 2012; 14: 549–56. [DOI] [PubMed] [Google Scholar]
- 29. Prieto-Alhambra D, Judge A, Javaid MKet al. . Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis 2014; 73: 1659–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Horwitz RI, Feinstein AR.. The problem of “protopathic bias” in case-control studies. Am J Med 1980; 68: 255–8. [DOI] [PubMed] [Google Scholar]
- 31. Stewart WF, Kawas C, Corrada M et al. . Risk of Alzheimer's disease and duration of NSAID use. Neurology 1997; 48: 626–32. [DOI] [PubMed] [Google Scholar]
- 32. Foley S, Ding C, Cicuttini F et al. . Physical activity and knee structural change: a longitudinal study using MRI. Med Sci Sports Exerc 2007; 39: 426–34. [DOI] [PubMed] [Google Scholar]
- 33. Zeng C, Dubreuil M, LaRochelle MR et al. . Association of tramadol with all-cause mortality among patients with osteoarthritis. Jama 2019; 321: 969–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kyrkanides S, Tallents RH, Miller JN et al. . Osteoarthritis accelerates and exacerbates Alzheimer's disease pathology in mice. J Neuroinflammation 2011; 8: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McGeer PL, McGeer EG.. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev 1995; 21: 195–218. [DOI] [PubMed] [Google Scholar]
- 36. Shimura Y, Kurosawa H, Tsuchiya M et al. . Serum interleukin 6 levels are associated with depressive state of the patients with knee osteoarthritis irrespective of disease severity. Clin Rheumatol 2017; 36: 2781–7. [DOI] [PubMed] [Google Scholar]
- 37. Yang L, Deng YT, Leng Y et al. . Depression, depression treatments, and risk of incident dementia: a prospective cohort study of 354,313 participants. Biol Psychiatry 2023; 93: 802–9. [DOI] [PubMed] [Google Scholar]
- 38. Whitmer RA, Gunderson EP, Barrett-Connor E et al. . Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ 2005; 330: 1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Verbeek J, Mischke C, Robinson R et al. . Occupational exposure to knee loading and the risk of osteoarthritis of the knee: a systematic review and a dose-response meta-analysis. SAF Health Work 2017; 8: 130–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lievense A, Bierma-Zeinstra S, Verhagen A et al. . Influence of work on the development of osteoarthritis of the hip: a systematic review. J Rheumatol 2001; 28: 2520–8. [PubMed] [Google Scholar]
- 41. Lehmann JM, Lenhard JM, Oliver BB et al. . Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem 1997; 272: 3406–10. [DOI] [PubMed] [Google Scholar]
- 42. Ricote M, Li AC, Willson TM et al. . The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 1998; 391: 79–82. [DOI] [PubMed] [Google Scholar]
- 43. Kolasinski SL, Neogi T, Hochberg MC et al. . 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2020; 72: 149–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bannuru RR, Osani MC, Vaysbrot EE et al. . OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil 2019; 27: 1578–89. [DOI] [PubMed] [Google Scholar]
- 45. Pasinetti GM.. From epidemiology to therapeutic trials with anti-inflammatory drugs in Alzheimer's disease: the role of NSAIDs and cyclooxygenase in beta-amyloidosis and clinical dementia. J Alzheimers Dis 2002; 4: 435–45. [DOI] [PubMed] [Google Scholar]
- 46. Imbimbo BP, Solfrizzi V, Panza F.. Are NSAIDs useful to treat Alzheimer's disease or mild cognitive impairment? Front Aging Neurosci 2010; 2. 10.3389/fnagi.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dhull DK, Kumar A.. Tramadol ameliorates behavioural, biochemical, mitochondrial and histological alterations in ICV-STZ-induced sporadic dementia of Alzheimer's type in rats. Inflammopharmacology 2018; 26: 925–38. [DOI] [PubMed] [Google Scholar]
- 48. Ai B, Chen L, Cai M et al. . No associations between glucosamine supplementation and dementia or Parkinson's disease: findings from a large prospective cohort study. J Gerontol A Biol Sci Med Sci 2023; 9. 10.1093/gerona/glad123. [DOI] [PubMed] [Google Scholar]
- 49. Bannuru RR, Schmid CH, Kent DM et al. . Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med 2015; 162: 46–54. [DOI] [PubMed] [Google Scholar]
- 50. Zhu X, Wu D, Sang L et al. . Comparative effectiveness of glucosamine, chondroitin, acetaminophen or celecoxib for the treatment of knee and/or hip osteoarthritis: a network meta-analysis. Clin Exp Rheumatol 2018; 36: 595–602. [PubMed] [Google Scholar]
- 51. Ma LZ, Zhang YR, Li YZ et al. . Cataract, cataract surgery, and risk of incident dementia: a prospective cohort study of 300,823 participants. Biol Psychiatry 2023; 93: 810–9. [DOI] [PubMed] [Google Scholar]
- 52. Grotle M, Hagen KB, Natvig B et al. . Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord 2008; 9: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Courties A, Sellam J.. Osteoarthritis and type 2 diabetes mellitus: What are the links? Diabetes Res Clin Pract 2016; 122: 198–206. [DOI] [PubMed] [Google Scholar]
- 54. McKhann GM, Knopman DS, Chertkow H et al. . The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011; 7: 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Alshuft HM, Condon LA, Dineen RA et al. . Cerebral cortical thickness in chronic pain due to knee osteoarthritis: the effect of pain duration and pain sensitization. PloS One 2016; 11: e0161687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cruz-Almeida Y, Fillingim RB, Riley JL III et al. . Chronic pain is associated with a brain aging biomarker in community-dwelling older adults. Pain 2019; 160: 1119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Magon S, Sprenger T, Otti A et al. . Cortical thickness alterations in chronic pain disorder: an exploratory MRI study. Psychosom Med 2018; 80: 592–8. [DOI] [PubMed] [Google Scholar]
- 58. Schwedt TJ, Berisha V, Chong CD.. Temporal lobe cortical thickness correlations differentiate the migraine brain from the healthy brain. PloS One 2015; 10: e0116687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jack CR Jr, Wiste HJ, Weigand SD et al. . Different definitions of neurodegeneration produce similar amyloid/neurodegeneration biomarker group findings. Brain 2015; 138: 3747–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hickok G, Poeppel D.. The cortical organization of speech processing. Nat Rev Neurosci 2007; 8: 393–402. [DOI] [PubMed] [Google Scholar]
- 61. Beauchamp MS.. The social mysteries of the superior temporal sulcus. Trends Cogn Sci 2015; 19: 489–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Squire LR, Stark CE, Clark RE.. The medial temporal lobe. Annu Rev Neurosci 2004; 27: 279–306. [DOI] [PubMed] [Google Scholar]
- 63. Liao X, Mao C, Wang Y et al. . Brain gray matter alterations in Chinese patients with chronic knee osteoarthritis pain based on voxel-based morphometry. Medicine (Baltimore) 2018; 97: e0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cauda F, Palermo S, Costa T et al. . Gray matter alterations in chronic pain: a network-oriented meta-analytic approach. Neuroimage Clin 2014; 4: 676–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Smallwood J, Bernhardt BC, Leech R et al. . The default mode network in cognition: a topographical perspective. Nat Rev Neurosci 2021; 22: 503–13. [DOI] [PubMed] [Google Scholar]
- 66. Deng YT, Kuo K, Wu BS et al. . Associations of resting heart rate with incident dementia, cognition, and brain structure: a prospective cohort study of UK Biobank. Alzheimers Res Ther 2022; 14: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wu L, Wang X, Ye Y et al. . Association of osteoarthritis with changes in structural neuroimaging markers over time among non-demented older adults. Front Aging Neurosci 2021; 13: 664443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Flowers P, Nelson AE, Hannan MT et al. . Foot osteoarthritis frequency and associated factors in a community-based cross-sectional study of white and african american adults. Arthritis Care Res (Hoboken) 2021; 73: 1784–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.