Abstract
Atypical lymphoplasmacytic and immunoblastic proliferation (ALPIBP) was first reported in 1984 as characteristic histological findings in lymph nodes associated with autoimmune diseases, but it has not been clearly defined to date. To summarize the histological characteristics and clinical diagnoses associated with ALPIBP, we searched MEDLINE and EMBASE for all peer-reviewed articles using keywords including “atypical lymphoplasmacytic and immunoblastic lymphadenopathy” from their inception to December 27, 2023. We also summarized the courses of three cases with a pathological diagnosis of ALPIBP. Nine articles with 52 cases were included. Among the total of 55 cases, including the three from our institution, the median age of the cases was 63.5 years with a female predominance (69.5%). Lymphadenopathy was generalized in 65.6% and regional in 34.4% of cases. RA (24.4%), SLE (24.4%), and autoimmune hemolytic anemia (20.0%), were common clinical diagnoses. A combination of cytotoxic chemotherapy was used in 15.6% of cases due to the suspicion of malignancy. Nodal T-follicular helper cell lymphoma, angioimmunoblastic type, methotrexate-associated lymphoproliferative disorders, and IgG4-related diseases were listed as important diseases that need to be pathologically differentiated from ALPIBP. This review summarizes the current understanding of the characteristics of ALPIBP. Given that underrecognition of ALPIBP could lead to overdiagnosis of hematological malignancy and unnecessary treatment, increased awareness of the condition in pathologists and clinicians is crucial.
Keywords: systematic review, atypical lymphoplasmacytic and immunoblastic proliferation, IgG4-related disease, angioimmunoblastic T-cell lymphoma
INTRODUCTION
Atypical lymphoplasmacytic and immunoblastic proliferation (ALPIBP) is a term that refers to characteristic histological findings in lymph nodes, often associated with autoimmune diseases. It was first proposed by Koo et al. in 1984.1 Since then, its association has been reported with several conditions, including systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA).2,3
Histologically, ALPIBP needs to be differentiated from malignant lymphoma, Nodal T-follicular helper cell lymphoma, angioimmunoblastic type (nTFHL-AI) in particular (previously known as angioimmunoblastic T-cell lymphoma), methotrexate-associated lymphoproliferative disorders, and IgG4-related diseases (IgG4-RD).4 While ALPIBP is generally considered a benign and reactive condition, it is not well recognized by pathologists and clinicians. Its diagnosis requires careful consideration of histological and genetic findings, in addition to detailed clinical information. To date, no review has been conducted to summarize the clinicopathological presentations of ALPIBP despite the fact that it is a condition occasionally encountered in clinical practice. Herein, we aimed to provide information through case presentations and systematic review.
MATERIALS AND METHODS
Study Design
This is a systematic review conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)5,6 combined with case reports of our own cases. Cases with histological diagnosis of ALPIBP at Okayama University were included.
Search Strategy
We searched MEDLINE and EMBASE for all peer-reviewed articles from their inception to December 27th, 2023. No filters for study design and language were used. A manual screening for additional pertinent articles was done using the reference lists of all articles that met the eligibility criteria. The search strategy involved relevant keywords, including “cranial nerve six,” “abducens nerve,” and “giant cell arteritis”. The search was conducted by two authors (MFN and YN) independently. See Appendix for detailed search terms.
Eligibility Criteria
The criteria for the inclusion of articles are the following:
(1) Peer-reviewed articles describing cases with pathology findings compatible with ALPIBP.
(2) Randomized controlled trials (RCTs), case-control studies, cohort studies (prospective or retrospective), cross-sectional studies, case series, case reports, and conference abstracts.
(3) Adult patients.
The exclusion criteria included the following:
(1) Qualitative studies, review articles, and commentaries.
Study Selection
Articles selected for full-text assessment were assessed independently by MFN and YN using EndNote 20 reference management software. Articles considered eligible were then evaluated in full length with the inclusion and exclusion criteria.
Data Extraction and Definition
A standardized data collection form that followed the PRISMA and Cochrane Collaboration guidelines for systematic reviews was used to obtain the following information from each study: title, name of authors, year of publication, country of origin, study characteristics, target outcome, aims, study and comparative groups, key findings, and limitations. We also statistically analyzed data from existing case reports and case series to identify the clinical characteristics of the included cases.
Case Collection and Immunohistochemical Staining
Three cases of ALPIBP were identified from surgical pathology consultation files from the Department of Pathology of Okayama university. Immunohistochemical staining was performed on an automated Bond Max instrument (Leica Biosystems, Wetzlar, Germany) using the following primary antibodies: CD20 (1:100, L26; DAKO, Carpinteria, USA), CD3 (1:200, LN10; Novocastra, Newcastle, UK), CD5 (1:100, 4C7; Novocastra), CD7(1:100, LP15; Leica Biosystems), CD4(1:50, 1F6; Nitirei, Tokyo, Japan), CD8(1:200, C8/144B; Nitirei), CD10 (1:100, 56C6; Novocastra), CD15 (1:50, Carb- 3; DAKO), CD30 (1:40, Ber- H2; DAKO), CD21(1:20, 1F8; DAKO), PD-1(1:200, NAT; Abcam, Cambridge, UK), ICOS(1:75, SP98; Abcam), CXCL13(1:150, Q53X90; R&D Systems, Minneapolis, USA), IgG4 (1:10000, MC011, The Binding Site, Birmingham, UK); IgG (1:600, RWP49; Novocastra); IL-6(1:100, 10C12; Leica Biosystems), and Ki-67 (1:2500, MIB- 1; DAKO). In situ hybridization was also performed for κ and λ light chains (Leica Biosystems) and EBV- encoded small RNA (EBER1; Novocastra).
Ethical Statement
This study was approved by the Institutional Review Board of Okayama University (protocol number 2007-033), and performed in accordance with the tenets of the Declaration of Helsinki.
RESULTS
Case Presentations
Case 1
An 88-year-old Japanese male with a history of diffuse large B cell lymphoma (DLBCL), chronic kidney disease, benign prostate hyperplasia, and bilateral knee osteoarthritis was referred to the hospital due to recurrent purpura and edema on bilateral lower extremities for 13 months. He initially underwent a skin biopsy which suggested leukocytoclastic vasculitis. He was started on betamethasone cream. The laboratory results on admission are summarized in Table 1. He had thrombocytopenia, anti-nuclear antibody (ANA) titer 1:160, hypocomplementemia, and was positive for anti-cardiolipin antibody, but negative for double stranded deoxyribonucleic acid (ds-DNA) antibody and anti-Smith antibody. While his diagnosis was considered likely SLE, he had diffuse lymphadenopathy which raised concerns for DLBCL recurrence. Computed tomography (CT) showed bilateral axillary lymph adenopathy. A positron emission tomography (PET) scan showed FDG-avid lesions in his bilateral neck, axillary, mediastinal, and peri-aortic lymph nodes, with an SUV max of the axillary lymph node of 8.38. Axillary lymph node biopsy revealed vascular proliferation, plasmacytosis, and scattered CD20-positive immunoblasts in the expanded interfollicular area. Immunohistochemistry staining (IHC) showed no aberrant expression of T cell markers (CD3, CD5, CD7), and CD4-positive cells and CD8-positive cells were mixed. There was no significant expression of PD-1 or ICOS. Infiltrating plasma cells were strongly positive for IL-6 staining. The IgG4/IgG-positive cell ratio was approximately 20%. κ and the λ chain in situ hybridization (ISH) indicated bitype. The pathological findings were consistent with ALPIBP. T-cell receptor (TCR) rearrangement was not detected by Polymerase Chain Reaction (PCR).
Table 1. Laboratory values on admission, Case 1.
| Complete blood count | |||
|---|---|---|---|
| WBC | 4.92 (103/uL) | sIL-2R | 2728.7 U/mL |
| Hb | 11.0 g/dL | PR3-ANCA | 0.90 IU/mL |
| HCT | 34.0% | MPO-ANCA | <0.50 IU/mL |
| Plt | 24 (103/uL) | MMP-3 | 87.6 ng/mL |
| Biochemical findings | RF | 1387.4 U/mL | |
| Na | 133 mEq/L | Anti-RNP antibody | 2.61 U/mL |
| K | 4.7 mEq/L | Anti-Smith antibody | 4.37 U/mL |
| Cl | 103 mEq/L | Anti-SS-A antibody | 1.77 U/mL |
| Ca | 8.2 mg/dL | Anti-SS-B antibody | 1.17 U/mL |
| HCO3 | 22.5 mmol/L | Anti-CCP antibody | 0.7 U/mL |
| BUN | 36 mg/dL | CH50 | <10 U/mL |
| Cr | 1.65 mg/dL | IgG | 4920.5 mg/dL |
| Alb | 3.6 mg/dL | IgG4 | 1706.6 mg/dL |
| AST | 35 IU/L | IgA | 122.1 mg/dL |
| ALT | 20 IU/L | IgM | 185.0 mg/dL |
| ALP | 94 IU/L | C3 | 23.8 mg/dL |
| T-Bil | 0.4 mg/dL | C4 | 0.9 mg/dL |
| PT | 10.8 sec | aCL antibody | 20 U/L |
| APTT | 2.7 sec | ANA titer | 1:160 |
| PT-INR | 1.02 | ||
Abbreviations: WBC: white blood cells; Hb: hemoglobin; HCT: hematocrit; Plt: platelet; Na: sodium; K: potassium; Cl: chloride; Ca: calcium; Mg: magnesium; BUN: blood urea nitrogen; Cr: creatinine; Alb: albumin; AST: aspartate aminotransferase; ALT: alanine aminotransferase; ALP: alkaline phosphatase; T-Bil: total bilirubin; PT: prothrombin time; APTT: activated partial thromboplastin time; SIL-2R serum soluble interleukin-2 receptor; PR3-ANCA: proteinase 3 antineutrophil cytoplasmic antibodies; MPO-ANCA: perinuclear anti-neutrophil cytoplasmic antibodies; MMP-3: matrix metallopeptidase 3; RF: rheumatoid factor; aCL: anti-cardiolipin autoantibody; ANA: antinuclear antibody
Case 2
A 74-year-old Japanese female with a history of type 2 diabetes mellitus presented with rashes on her bilateral upper and lower extremities for 3 years. She initially presented at the dermatology clinic due to persistent rashes which were not relieved with epinastine topical creams. Given a skin biopsy suggesting urticarial vasculitis, she was started on prednisone 20 mg per day, which was weaned off over a year. However, she subsequently developed submandibular masses and was hospitalized for further investigation. The laboratory results on admission are summarized in Table 2. In addition to Sjogren’s syndrome, IgG4-related lymphoproliferative disease was also suspected based on IgG 2176 mg/dl and IgG4 637 mg/dl. Based on the 2019 American College of Rheumatology/European League Against Rheumatism classification criteria for IgG4-related disease, the patient did not satisfy the entry criteria for IgG4-RD.7 Also, she did not meet any of the classification criteria of autoimmune diseases. Concerningly, she had persistent and progressive inguinal lymphadenopathy during the hospital course, for which she had an inguinal lymph node biopsy. Pathological analysis revealed expanded interfollicular areas and indistinct follicular structures. Clear cells, characteristic of nTFHL-AI, were not observed. Plasmacytosis and immunoblast-like large lymphocytes (CD20-positive) were observed in the expanded interfollicular areas, as well as hypervascularization. CD3-positive and CD20-positive cells were equally intermingled. PD-1-positive cells were slightly abundant, but CD10 and CXCL13 were not significantly expressed. CD21 staining revealed no follicular dendritic cell proliferation. ISH showed negative EBER and no immunoglobulin light chain restriction. No immunoglobulin heavy chain (IgH) or TCR gene rearrangement was observed. Collectively, the pathological finding was most compatible with ALPIBP in a patient with an undifferentiated autoimmune disease.
Table 2. Laboratory values on admission, Case 2.
| Complete blood count | |||
|---|---|---|---|
| WBC | 7.42 (103/uL) | PR3-ANCA | 2.5 IU/mL |
| Hb | 14.6 g/dL | MPO-ANCA | 0.9 IU/mL |
| HCT | 43.9% | RF | 275 U/mL |
| Plt | 440 (103/uL) | Anti-RNP antibody | 2.90 U/mL |
| Biochemical findings | Anti-Smith antibody | <0.50 U/mL | |
| Na | 139 mEq/L | Anti-SS-A antibody | 0.89 U/mL |
| K | 4.2 mEq/L | Anti-SS-B antibody | <0.50 U/mL |
| Cl | 102 mEq/L | CH50 | 36 U/mL |
| Ca | 9.7 mg/dL | IgG | 2097 mg/dL |
| BUN | 11.5 mg/dL | IgA | 162 mg/dL |
| Cr | 0.54 mg/dL | IgM | 74.5 mg/dL |
| Alb | 3.6 mg/dL | C3 | 71.5 mg/dL |
| AST | 55 IU/L | C4 | 35 mg/dL |
| ALT | 42 IU/L | aCL antibody | 23 U/L |
| ALP | 88 IU/L | ||
| T-Bil | 0.39 mg/dL | ||
Abbreviations: WBC: white blood cells; Hb: hemoglobin; HCT: hematocrit; Plt: platelet; Na: sodium; K: potassium; Cl: chloride; Ca: calcium; Mg: magnesium; BUN: blood urea nitrogen; Cr: creatinine; Alb: albumin; AST: aspartate aminotransferase; ALT: alanine aminotransferase; ALP: alkaline phosphatase; T-Bil: total bilirubin; PT: prothrombin time; APTT: activated partial thromboplastin time; PR3-ANCA: proteinase 3 antineutrophil cytoplasmic antibodies; MPO-ANCA: perinuclear anti-neutrophil cytoplasmic antibodies; RF: rheumatoid factor; aCL: anti-cardiolipin autoantibody
Case 3
An 85-year-old male was admitted for investigation of the cause of his anemia, sicca, mouth ulcer, and photosensitivity. The laboratory results on admission are summarized in Table 3, which demonstrated hypocomplementemia, thrombocytopenia, hypergammaglobulinemia, and positivity for antiphospholipid antibody. As he had persistent diffuse lymphadenopathy, this raised suspicion of malignant lymphoma. Sampled lymph nodes showed expanded interfollicular areas with hypervascularization and plasmacytosis. Immunoblast-like large cells were also observed. Immunostaining showed separate localization of CD20- and CD3-positive cells, with an admixture of CD4- and CD8-positive cells. There was no significant expression of PD1 or ICOS. Only few lgG4-positive cells were observed (<10/HPF). IL-6 immunostaining showed no significant positivity. The Ki-67 labeling index was low in the interfollicular area. κ and λ chain ISH indicated bitype. Overall, the pathological finding was consistent with ALPIBP likely due to elderly-onset SLE.
Table 3. Laboratory values on admission, Case 3.
| Complete blood count | |||
|---|---|---|---|
| WBC | 4.25 (103/uL) | sIL-2R | 2273 U/mL |
| Hb | 13.4 g/dL | PR3-ANCA | 1.0 IU/mL |
| HCT | 40.5% | MPO-ANCA | <1.0 IU/mL |
| Plt | 60 (103/uL) | Anti-RNP antibody | 2.61 U/mL |
| Biochemical findings | Anti-ds DNA antibody | 10 U/mL | |
| Na | 135 mEq/L | Anti-SS-A antibody | <1.0 U/mL |
| K | 3.9 mEq/L | Anti-SS-B antibody | 1.3 U/mL |
| Cl | 101 mEq/L | CH50 | <12 U/mL |
| Ca | 8.9 mg/dL | IgG | 3612 mg/dL |
| BUN | 23 mg/dL | IgG4 | 236 mg/dL |
| Cr | 0.81 mg/dL | IgA | 342 mg/dL |
| Alb | 3.4 mg/dL | IgM | 492 mg/dL |
| AST | 27 IU/L | C3 | 70 mg/dL |
| ALT | 34 IU/L | C4 | 7 mg/dL |
| ALP | 77 IU/L | aCL antibody | 34 U/L |
| T-Bil | 0.7 mg/dL | ANA titer | 1:40 |
| PT | 87% | Serum protein electrophoresis | |
| APTT | 31.4 sec | Albumin | 40.5% |
| PT-INR | 1.07 | Alpha 1 globulin | 3.1% |
| Alpha 2 globulin | 6.3% | ||
| Beta 1 globulin | 4.3% | ||
| Beta 2 globulin | 4.7% | ||
| Gamma globulin | 41.1% | ||
Abbreviations: WBC: white blood cells; Hb: hemoglobin; HCT: hematocrit; Plt: platelet; Na: sodium; K: potassium; Cl: chloride; Ca: calcium; Mg: magnesium; BUN: blood urea nitrogen; Cr: creatinine; Alb: albumin; AST: aspartate aminotransferase; ALT: alanine aminotransferase; ALP: alkaline phosphatase; T-Bil: total bilirubin; PT: prothrombin time; APTT: activated partial thromboplastin time; SIL-2R serum soluble interleukin-2 receptor; PR3-ANCA: proteinase 3 antineutrophil cytoplasmic antibodies; MPO-ANCA: perinuclear anti-neutrophil cytoplasmic antibodies; MMP-3: matrix metallopeptidase 3; anti-dsDNA: anti-double stranded DNA; aCL: anti-cardiolipin autoantibody; ANA: antinuclear antibody
Search Results and Study Selection
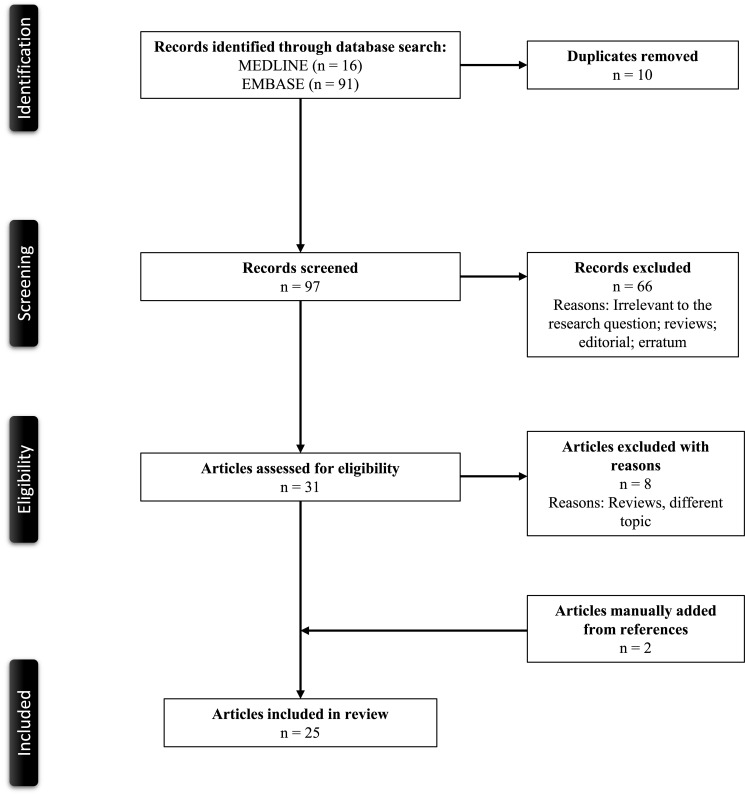
Figure 1 demonstrates a PRISMA flow diagram summarizing the identification, screening, eligibility, and inclusion and exclusion processes of the studies involved. The initial MEDLINE and EMBASE databases review yielded seven and eight articles, respectively. Seven duplicate studies were removed. Eight articles were screened based on their relevance and article type. One article was excluded as it only included non-ALPIBP cases,4 and they were then evaluated for full-text review for study inclusion per our eligibility criteria. Two articles were added from manual screening of references. As a result, nine articles were included in the review.1–3, 8–13
Fig. 1.
PRISMA flowchart of the search strategy
Clinical Characteristics
Table 4 presents the baseline demographics, diagnostic findings, and chief clinical features of the individual cases, including the three cases from our institution (n = 52). The median age of the cases was 63.5 years (interquartile range (IQR) 49.3–72.5) and they were predominantly female (70.6%). Among the cases that clearly described the distribution of lymphadenopathy, 62.1% had generalized lymphadenopathy, while 37.9% had regional lymphadenopathy that raised concerns for malignancy. The enlarged lymph nodes described in the literature were relatively small, usually measuring about 1.0–1.5 cm in maximum diameter.
Table 4. Baseline demographics, laboratory findings, and chief features of the 52 cases.
| Data available (%) * | Median (IQR) | |
|---|---|---|
| Age (years) | 40/52 (76.9) | 63.5 (49.3–72.5) |
| Sex | 51/52 (98.1) | |
| Male | 15/51 (29.4) | |
| Female | 36/51 (70.6) | |
| Past medical history | 19/52 (36.5) | |
| RA | 4/19 (21.1) | |
| SLE | 4/19 (21.1) | |
| SjS | 3/19 (15.8) | |
| Others | 8/19 (42.2) | |
| Lymphadenopathy | 29/52 (55.8) | |
| Regional | 11/29 (37.9) | |
| Generalized | 18/29 (62.1) | |
| Clinical diagnosis | 42/52 (80.8) | |
| RA | 11/42 (26.2) | |
| SLE | 11/42 (26.2) | |
| AIHA | 9/42 (21.4) | |
| SjS | 2/42 (4.8) | |
| Cryoglobulinemia | 2/42 (4.8) | |
| Drug-induced (Sulfasalazine, ST) | 2/42 (4.8) | |
| Others | 5/42 (11.9) | |
| Initial treatment | 30/52 (57.7) | |
| Corticosteroid monotherapy | 14/30 (46.7) | |
| Cyclophosphamide, vincristine, and prednisone | 5/30 (16.7) | |
| Corticosteroid with non-steroidal immunosuppressant | 3/30 (10.0) | |
| Others | 8/30 (26.7) |
Abbreviations: AIHA: autoimmune hemolytic anemia; SjS: Sjögren syndrome; SLE: systemic lupus erythematosus; RA: rheumatoid arthritis; Trimethoprim/sulfamethoxazole
ALPIBP most commonly occurred along with RA (26.2%), SLE (26.2%), and autoimmune hemolytic anemia (20.0%). The therapeutic interventions were described for 30/52 (57.7%) of the cases. While 46.7% received corticosteroid monotherapy that aligned with the clinical diagnosis of autoimmune diseases, 16.7% received a combination of cytotoxic chemotherapy due to the suspicion of malignancy. While 16/50 (30.8%) died during the follow-up periods, the preliminary causes of deaths were mainly pneumonia, heart failure, or other types of infection, except for one case described by Koo et al. who died from heart failure but was found to have malignant lymphoma on autopsy.
Histological Findings
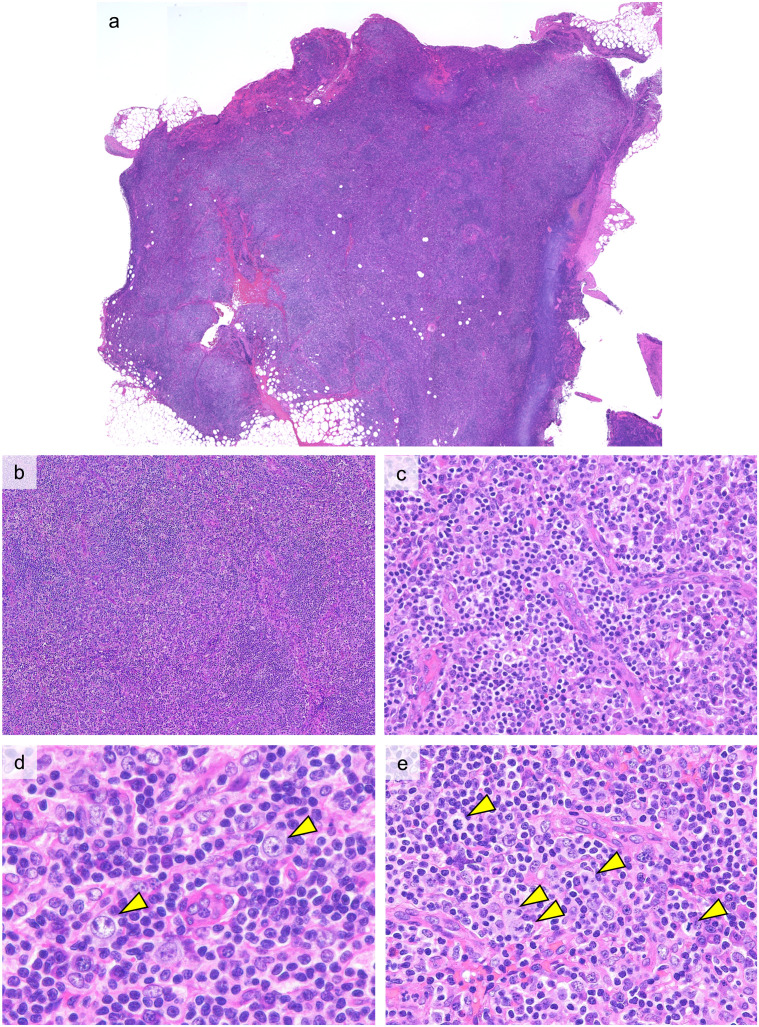
Figure 2 shows representative histopathological findings of ALPIBP (Case 1). The architecture of the lymph nodes is obscure with an expansion of the interfollicular areas observed (Figure 2a, 2b). Proliferation of small blood vessels with plump endothelial cells is evident in expanded interfollicular areas (Figure 2c). Infiltration of a diverse range of cells is seen in the interfollicular areas, including small-to-medium lymphocytes, mature plasma cells, immunoblasts, eosinophils, and histiocytes (Figure 2d). The immunoblasts with large and distinct nucleoli may resemble Hodgkin cells, but typical Reed-Sternberg cells are not identified in ALPIBP. Mitotic figures are relatively easily seen (Figure 2e). Medium-to-large lymphocytes with pale cytoplasm and distinct cell membranes, commonly known as clear cells, are not observed.
Fig. 2.
Histopathological features of ALPIBP (Case 1: H&E staining)
a, b: The follicular structure is obscured and the interfollicular area is expanded.
c: Vascular proliferation with plump endothelial cells is seen in the expanded interfollicular area.
d: Immunoblasts with large nuclei and distinct nucleoli (arrow heads) are observed with a background of small lymphocytes and plasma cells.
e: Mitotic figures (arrow heads) are easily observed.
Immunohistochemical and Genetic Findings
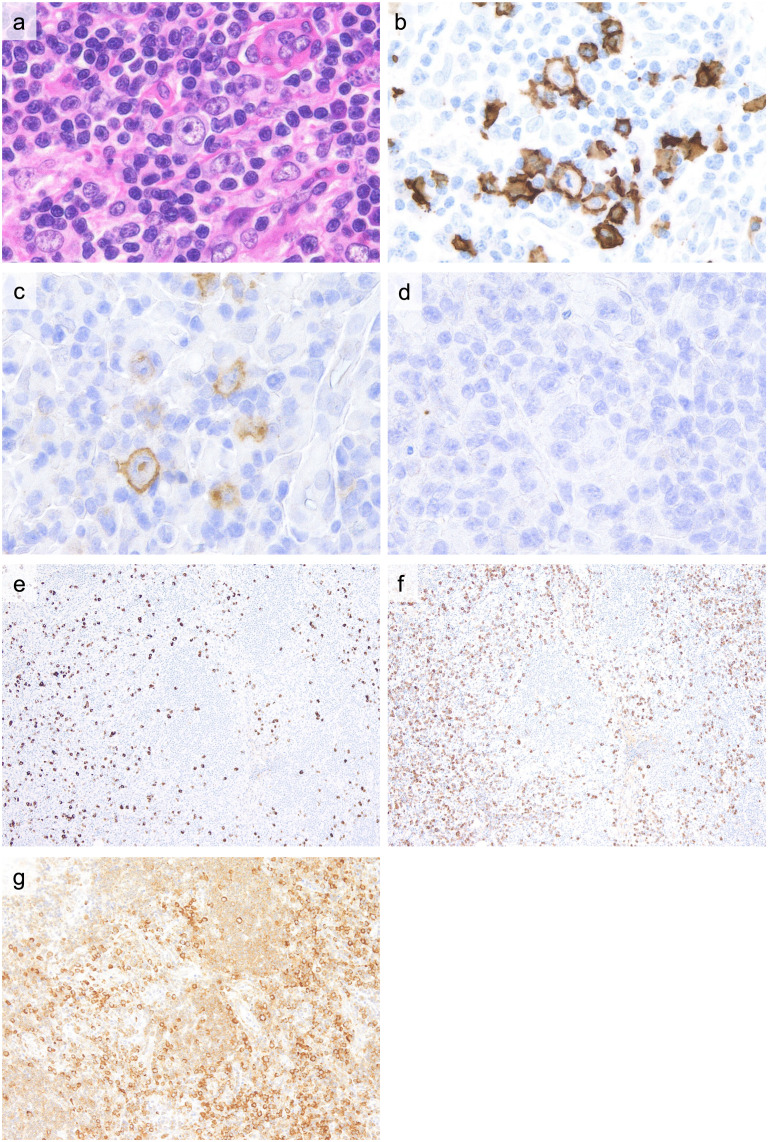
Representative immunohistochemical findings of ALPIBP (Case 1) are summarized in Figure 3. CD3- and CD20-positive cells show a segregated distribution. CD4- and CD8-positive cells are intermingled, and light chain restriction is not seen in infiltrating plasma cells or B cells. Immunoblasts exhibit B-cell characteristics (Figure 3a, b) and may be CD30-positive (Figure 3c), but are usually CD15-negative (Figure 3d). Epstein-Barr virus (EBV) -positive cells are not observed. As ALPIBP can be seen in autoimmune diseases leading to a rise in IL-6, polyclonal hypergammaglobulinemia and the subsequent increase in the number of IgG4-positive cells in tissues is noted. Compared to IgG4-RD, the IgG4/IgG-positive cell ratio usually remains below 40% (Figure 3e, f). Follicular B-cells are weakly positive, and infiltrating plasma cells are strongly positive for IL-6 staining (Figure 3g). No immunoglobulin heavy chain or T-cell receptor gene rearrangement is observed.
Fig. 3.
Immunohistochemical findings of ALPIBP (Case 1)
a (H&E): Immunoblasts with a distinct nucleolus.
b (CD20 staining), c (CD30 staining), d (CD15 staining): Immunoblasts typically exhibit CD20 positivity, with occasional CD30 positivity. They are negative for CD15.
e (IgG4 staining), f (IgG staining): While there is an increased number of IgG4-positive cells, the IgG4/IgG-positive cell ratio remains below 40%.
g (IL-6 staining): IL-6 staining was weakly positive for follicular B cells and widely positive for interfollicular plasma cells.
DISCUSSION
In the present study, we reported three cases with pathological findings of ALPIBP, combined with a thorough literature review for evidence regarding the entity. This is the first study to clarify detailed clinical presentations and pathological findings related to ALPIBP. The present results suggest that ALPIBP is a histologically defined term often seen in diverse autoimmune diseases or medication-induced hypersensitivity. Given that the underrecognition of ALPIBP among pathologists and clinicians could lead to overtreatment as malignancy, increased awareness of this condition along with associated clinical diagnoses is crucial.
The present study confirmed that ALPIBP is characterized by a diverse cellular infiltration, including B-cell immunoblasts and plasma cells. Additionally, clinical symptoms of the patients tended to be similar to those of autoimmune diseases, such as fever, fatigue, and fluid retention. Thus, it is crucial to differentiate ALPIBP from various lymphomas and lymphoproliferative disorders.
Differential Diagnoses
1. Nodal T-follicular helper cell lymphoma, angioimmunoblastic type (nTFHL-AI)
nTFHL-AI predominantly affects middle-aged to elderly males and presents with generalized lymphadenopathy, frequently accompanied by autoimmune disease-like symptoms such as fever, weight loss, edema, rash, arthritis, and polyclonal hypergammaglobulinemia. Although rare, cases of nTFHL-AI arising in patients with autoimmune diseases like RA have been reported.14 Histologically, nTFHL-AI may resemble ALPIBP.15–17 Although atrophic follicles similar to reactive follicular hyperplasia can still be observed in nTFHL-AI, they are usually associated with near-total destruction of lymph node architecture, and clusters of tumor T-cells with pale cytoplasm, known as clear cells, are not observed in ALPIBP. Immunostaining should be employed for their differentiation; nTFHL-AI typically shows CD4 positivity and CD8 negativity, partial loss of pan-T-cell markers (CD2, CD3, CD5, CD7), and significant expression of at least two or three T-follicular helper cell markers (CD10, CXCL13, ICOS, BCL6, PD-1). The TFH phenotype is defined as the presence of at least two TFH markers (CD10, PD-1, ICOS, CXCL13, BCL6) in addition to CD4.18 The presence of EBER-positive B cells and TCR gene rearrangement, which support the diagnosis of nTFHL-AI, should also be checked.19 RHOA and IDH2 mutations are also present in the neoplastic TFH cell population and may be helpful for nTFHL-AI diagnosis.
2. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)-related Lymphadenopathy
DRESS-related lymphadenopathy is a condition characterized by lymph node enlargement due to hypersensitivity to medications. Histologically, it involves the expansion of the paracortical region, with infiltration of a diverse array of inflammatory cells, including B lymphoblasts, lymphocytes, and plasma cells, as well as angiogenesis into this area. It necessitates differentiation from ALPIBP due to similarities, such as typically being EBER-negative and the absence of IgH and TCR rearrangements. A histological distinction for DRESS-related lymphadenopathy is that there is usually more abundant eosinophil infiltration compared to ALPIBP. Clinically, a history of using certain medications, especially anticonvulsants known for their high frequency of causing such reactions (notably phenytoin and carbamazepine), and improvement of symptoms upon cessation of these causative medications are critical for diagnosis. DRESS-related lymphadenopathy often presents with peripheral blood eosinophilia and atypical lymphocytes.20,21 It is also associated with fever and pruritic rashes. However, it should be noted that these symptoms are not specific and can also occur in autoimmune diseases associated with ALPIBP.
3. Adult-onset Still’s Disease (AOSD)
The pathological landscape of AOSD includes several reported patterns.22 Notably the exuberant immunoblastic reaction pattern, which is characterized by hyperplasia of the paracortex and proliferation of large, atypical immunoblasts, is difficult to differentiate from ALPIBP based solely on pathological findings. The diagnosis relies on the presence of clinical criteria for AOSD, such as high fevers exceeding 39°C, arthralgia, typical rashes, and increased leukocytes and neutrophils, leaving the final judgment to the clinicians.
4. Methotrexate-associated lymphoproliferative disorders (MTX-LPD)
MTX-LPD, now defined as a subset of LPDs arising in immune deficiency/dysregulation, can occur in those on methotrexate, exhibits various histological appearances, and is also an important pathological mimicker of ALPIBP.23 Polymorphic-type MTX-LPD, characterized by an expanded interfollicular area with small-to-medium lymphocytes, plasma cells, and immunoblasts resembling Hodgkin cells and expressing B-cell phenotype, can resemble ALPIBP. However, up to 80% of cases of MTX-LPD are EBER-positive by in situ hybridization and there is a history of methotrexate use.24 Clinicians may need to recognize that EBV infection can occasionally present with symptoms similar to autoimmune diseases in older patients.25 EBV-related lymph node lesions may exhibit follicular hyperplasia with hyperplastic germinal centers and a diverse cellular infiltration in interfollicular areas, including small-to-medium-sized lymphocytes, immunoblasts, plasma cells, and eosinophils, with vascular proliferation, resembling ALPIBP. It needs to be noted that EBER positivity does not reject the diagnosis of ALPIBP, as background small lymphocytes can be EBER-positive in cases of autoimmune diseases with immune dysregulation. Similar to MTX-LPD, EBER in situ hybridization can be helpful in differentiating ALPIBP from these lesions. Immunohistochemical staining and detailed clinical history taking are essential to discriminate the three entities.
5. IgG4-related lymphadenopathy
The other histological differential diagnosis of ALPIBP is IgG4-RD. There are multiple types of IgG4-related lymphadenopathy, and Type 3 exhibits a mixture of lymphoplasmacytic cells, immunoblasts, and eosinophils in the expanded interfollicular areas, similar to ALPIBP.26 In cases with ALPIBP, patients could have increased inflammatory cytokines including interleukin-6, which lead to polyclonal hypergammaglobulinemia and a subsequent increase in IgG4-positive cells.11,27 To differentiate ALPIBP from IgG4-RD, comprehensive assessment of the absolute number of IgG4-positive cells, the IgG4/IgG-positive cell ratio, the distribution of extranodal lesions characteristic of IgG4-RD, and the presence or absence of non-specific clinical findings like fever or elevated inflammatory markers, may be crucial.28
There are several limitations of this study that should be discussed. First, given the lack of awareness and the rarity, there is a limited number of studies, and the studies included have a small number of patients. Also, for statistical case analysis, we only included data from well-documented existing case reports and case series to identify the clinical characteristics of the included cases with the level of detail required for in-depth investigation. Nevertheless, to our knowledge, this is the first systematic review to investigate the detailed characteristics of ALPIBP with a focus on relevant differential diagnoses.
In conclusion, for clinicians, increased awareness of ALPIBP as a lymph node finding associated with autoimmune diseases may be crucial to prevent unnecessary diagnostic biopsies and overtreatment. The present findings may pave the way for the concept of ALPIBP to become more widely recognized among both pathologists and clinicians. As more cases are accumulated, it could lead to a deeper understanding of the clinical characteristics of ALPIBP, as well as the frequency of the manifestation of autoimmune diseases following a provisional diagnosis of ALPIBP. When pathologists encounter cases presenting with the pathological features of ALPIBP without a clear diagnosis of autoimmune disease, feedback to clinicians regarding the suspected association with autoimmune diseases may be crucial. Furthermore, subclassification of ALPIBP based on the presence or absence of an autoimmune disease diagnosis at the time of pathological diagnosis, as well as factors including medication history, and accumulating knowledge about the clinical course and outcomes, will contribute to a better understanding of the condition. At this point, for pathologists, given that ALPIBP needs to be differentiated from malignant lymphoma, lymphoproliferative disorders, or IgG4-RD, which requires long-term therapeutic interventions, recognition of the characteristic immunohistochemical findings of each condition is warranted.
Supplemental Materials and Methods
ACKNOWLEDGMENTS
None.
INSTITUTIONAL REVIEW BOARD STATEMENT
Not applicable.
INFORMED CONSENT STATEMENT
Not applicable.
DATA AVAILABILITY STATEMENT
The data presented in this study are available on request from the corresponding author.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest in association with the present study.
FUNDING
None.
REFERENCES
- 1.Koo CH, Nathwani BN, Winberg CD, Hill LR, Rappaport H. Atypical lymphoplasmacytic and immunoblastic proliferation in lymph nodes of patients with autoimmune disease (autoimmune-disease-associated lymphadenopathy). Medicine (Baltimore). 1984; 63: 274-290. [DOI] [PubMed] [Google Scholar]
- 2.Kojima M, Motoori T, Matsuda H, et al. Atypical lymphoplasmacytic and immunoblastic proliferation from systemic lupus erythematosus. A case report. Pathol Res Pract. 2005; 201: 531-535. [DOI] [PubMed] [Google Scholar]
- 3.Kojima M, Motoori T, Hosomura Y, et al. Atypical lymphoplasmacytic and immunoblastic proliferation from rheumatoid arthritis: a case report. Pathol Res Pract. 2006; 202: 51-54. [DOI] [PubMed] [Google Scholar]
- 4.Sato Y, Kojima M, Takata K, et al. Systemic IgG4-related lymphadenopathy: a clinical and pathologic comparison to multicentric Castleman’s disease. Mod Pathol. 2009; 22: 589-599. [DOI] [PubMed] [Google Scholar]
- 5.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018; 169: 467-473. [DOI] [PubMed] [Google Scholar]
- 6.McGowan J, Straus S, Moher D, et al. Reporting scoping reviews-PRISMA ScR extension. J Clin Epidemiol. 2020; 123: 177-179. [DOI] [PubMed] [Google Scholar]
- 7.Wallace ZS, Naden RP, Chari S, et al. The 2019 American College of Rheumatology/European League Against Rheumatism Classification Criteria for IgG4‐Related Disease. Arthritis Rheumatol. 2020; 72: 7-19. [DOI] [PubMed] [Google Scholar]
- 8.Blanco R, McLaren B, Davis B, Steele P, Smith R. Systemic lupus erythematosus-associated lymphoproliferative disorder: report of a case and discussion in light of the literature. Hum Pathol. 1997; 28: 980-985. [DOI] [PubMed] [Google Scholar]
- 9.Kojima M, Motoori T, Nakamura S. Benign, atypical and malignant lymphoproliferative disorders in rheumatoid arthritis patients. Biomed Pharmacother. 2006; 60: 663-672. [DOI] [PubMed] [Google Scholar]
- 10.Kojima M, Motoori T, Asano S, Nakamura S. Histological diversity of reactive and atypical proliferative lymph node lesions in systemic lupus erythematosus patients. Pathol Res Pract. 2007; 203: 423-431. [DOI] [PubMed] [Google Scholar]
- 11.Kojima M, Nakamura N, Tsukamoto N, et al. Atypical lymphoplasmacytic and immunoblastic proliferation of autoimmune disease: clinicopathologic and immunohistochemical study of 9 cases. J Clin Exp Hematop. 2010; 50: 113-119. [DOI] [PubMed] [Google Scholar]
- 12.Nakazato Y, Tsuchida S, Takada-Owada A, et al. Castleman disease and mimickers: Clinicopathological findings of atypical lymphoproliferative disorders associated with autoimmune disease. J Clin Exp Hematop. 2022; 62: 119-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kojima M, Nakamura S, Yamane Y, Tanaka H, Masawa N. Autoimmune disease-associated lymphadenopathy from dermatomyositis. A case report. Pathol Res Pract. 2003; 199: 691-694. [DOI] [PubMed] [Google Scholar]
- 14.Kojima M, Itoh H, Shimizu K, et al. Malignant lymphoma in patients with systemic rheumatic disease (rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis, and dermatomyositis): a clinicopathologic study of 24 Japanese cases. Int J Surg Pathol. 2006; 14: 43-48. [DOI] [PubMed] [Google Scholar]
- 15.Ree HJ, Kadin ME, Kikuchi M, et al. Angioimmunoblastic lymphoma (AILD-type T-cell lymphoma) with hyperplastic germinal centers. Am J Surg Pathol. 1998; 22: 643-655. [DOI] [PubMed] [Google Scholar]
- 16.Attygalle AD, Kyriakou C, Dupuis J, et al. Histologic evolution of angioimmunoblastic T-cell lymphoma in consecutive biopsies: clinical correlation and insights into natural history and disease progression. Am J Surg Pathol. 2007; 31: 1077-1088. [DOI] [PubMed] [Google Scholar]
- 17.Cheuk W, Yuen HKL, Chu SYY, et al. Lymphadenopathy of IgG4-related sclerosing disease. Am J Surg Pathol. 2008; 32: 671-681. [DOI] [PubMed] [Google Scholar]
- 18.Cree IA. The WHO Classification of Haematolymphoid Tumours. Leukemia. 2022; 36: 1701-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiss LM, Jaffe ES, Liu XF, et al. Detection and localization of Epstein-Barr viral genomes in angioimmunoblastic lymphadenopathy and angioimmunoblastic lymphadenopathy-like lymphoma. Blood. 1992; 79: 1789-1795. [PubMed] [Google Scholar]
- 20.Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (Drug Rash with Eosinophilia and Systemic Symptoms: DRESS). Semin Cutan Med Surg. 1996; 15: 250-257. [DOI] [PubMed] [Google Scholar]
- 21.Kim DH, Koh YI. Comparison of diagnostic criteria and determination of prognostic factors for drug reaction with eosinophilia and systemic symptoms syndrome. Allergy Asthma Immunol Res. 2014; 6: 216-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeon YK, Paik JH, Park SS, et al. Spectrum of lymph node pathology in adult onset Still’s disease; analysis of 12 patients with one follow up biopsy. J Clin Pathol. 2004; 57: 1052-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kojima M, Itoh H, Hirabayashi K, et al. Methtrexate-associated lymphoproliferative disorders. A clinicopathological study of 13 Japanese cases. Pathol Res Pract. 2006; 202: 679-685. [DOI] [PubMed] [Google Scholar]
- 24.Gion Y, Iwaki N, Takata K, et al. Clinicopathological analysis of methotrexate‐associated lymphoproliferative disorders: Comparison of diffuse large B‐cell lymphoma and classical Hodgkin lymphoma types. Cancer Sci. 2017; 108: 1271-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kojima M, Sugiura I, Itoh H, et al. Histological varieties of Epstein-Barr virus-related lymph node lesion resembling autoimmune disease-like clinicopathological findings in middle-aged and elderly patients: a study of six cases. Pathol Res Pract. 2006; 202: 609-615. [DOI] [PubMed] [Google Scholar]
- 26.Sato Y, Yoshino T. IgG4-Related Lymphadenopathy. Int J Rheumatol. 2012; 2012: 572539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawano Y, Noma T, Yata J. Regulation of human IgG subclass production by cytokines. IFN-gamma and IL-6 act antagonistically in the induction of human IgG1 but additively in the induction of IgG2. J Immunol. 1994; 153: 4948-4958. [PubMed] [Google Scholar]
- 28.Satou A, Notohara K, Zen Y, et al. Clinicopathological differential diagnosis of IgG4‐related disease: A historical overview and a proposal of the criteria for excluding mimickers of IgG4‐related disease. Pathol Int. 2020; 70: 391-402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.