Abstract
Introduction:
Autonomic dysreflexia (AD) is a potentially life-threatening consequence in high (above T6) spinal cord injury that involves multiple incompletely understood mechanisms. While peripheral arteriolar vasoconstriction, which controls systemic vascular resistance, is documented to be pronounced during AD, the pathophysiological neurovascular junction mechanisms of this vasoconstriction are undefined. One hypothesized mechanism is increased neuronal release of norepinephrine and co-transmitters. We tested this by examining the effects of blockade of pre-synaptic neural release of norepinephrine and co-transmitters on cutaneous vasoconstriction during AD, using a novel non-invasive technique; bretylium (BT) iontophoresis followed by skin blood flow measurements via laser doppler flowmetry (LDF).
Methods:
Bretylium, a sympathetic neuronal blocking agent (blocks release of norepinephrine and co-transmitters) was applied iontophoretically to the skin of a sensate (arm) and insensate (leg) area in 8 males with motor complete tetraplegia. An nearby untreated site served as control (CON). Cutaneous vascular conductance (CVC) was measured (CVC = LDF/mean arterial pressure) at normotension before AD was elicited by bladder stimulation. The percent drop in CVC values from pre-AD vs. AD was compared among BT and CON sites in sensate and insensate areas.
Results:
There was a significant effect of treatment but no significant effect of limb/sensation or interaction of limb × treatment on CVC. The percent drop in CVC between BT and CON treated sites was 25.7±1.75 vs. 39.4±0.87, respectively (P = 0.004).
Conclusion:
Bretylium attenuates, but does not fully abolish vasoconstriction during AD. This suggests release of norepinephrine and cotransmitters from cutaneous sympathetic nerves is involved in cutaneous vasoconstriction during AD.
Keywords: Spinal cord injury, Autonomic dysreflexia, Cutaneous vascular conductance
1. Introduction
Autonomic dysreflexia (AD) is a potentially life-threatening consequence of a spinal cord injury (SCI) at or above the T6 level characterized by abrupt elevations in blood pressure in response to noxious stimuli below the lesion level (Wan and Krassioukov, 2014; Krassioukov, 2009; Eldahan and Rabchevsky, 2018). Most AD episodes are insidious, asymptomatic, yet frequent (Mathias, 2006), i.e., transient rises in SBP >20 mmHg can occur up to 33 times per day (Hubli and Krassioukov, 2014; Dance et al., 2017). It is suspected that repeated unrecognized elevations in blood pressure throughout the day in persons with high SCI contributes to structural alterations in the vasculature, cardiometabolic disease, and impairment of immune responses (West et al., 2013; Phillips et al., 2014; Mironets et al., 2020; Krassioukov et al., 2009). Current treatments of AD can elicit large drops in BP leading to hypotension once the inciting noxious stimuli is removed. AD treatments are based on an incomplete understanding of the underlying neuro-pathophysiology within the arterioles which primarily control vascular resistance and thus blood pressure regulation.
Neurophysiologically, AD is triggered by activation of large diameter afferents and occurs only in persons with injuries at or above the level of T6, which is above the level of sympathetic outflow to the splanchnic vascular bed (Stjernberg et al., 1986; Guttmann and Whitteridge, 1947). Bladder and bowel distension are the most common instigators of AD in persons with SCI. The increase in activity and predominance of C-fiber afferents (normally dormant in a non-neurogenic bladder) (de Groat, 1990; Wiart et al., 1998) post SCI in the wall of the bladder detrusor muscle and colon causes the stretch receptor reflex arc to be hypersensitive (Brown et al., 2018). Post-SCI C-fiber sprouting in the lumbosacral cord amplifies noious afferent input from bowel/bladder distension or another noxious source (e.g.; ingrown toenail) resulting in a hyperexcitable viscerosympathetic or somatosympathetic reflex response, ultimately causing profound peripheral vasoconstriction of splanchnic (Karlsson, 1999), muscle (Brown et al., 2018; Wallin and Stjernberg, 1984), and cutaneous (Brown et al., 2018; Wallin and Stjernberg, 1984) vascular beds (Rabchevsky, 2006; Corbett et al., 1975; Brown et al., 2018; Wallin and Stjernberg, 1984; Macefield et al., 2012). It is likely that the lack of supraspinal excitatory support increases the excitability through neural plasticity and reorganization of neural circuits after SCI (Brown et al., 2018). In this scenario, increased neural release of neurotransmitters would be expected along the entire efferent sympathetic pathways including the spinal preganglionic neurons (SPNs) and the sympathetic nerves to the blood vessels. It is likely that neural plasticity after SCI alters the physiology at the neurovascular junction (West et al., 2013; Laird et al., 2008) to accentuate the VC from SNS reflex stimulation, however the specific pathophysiologic changes in sympathetic neurovascular junctions are poorly understood.
As previously mentioned, arterioles are primarily responsible for controlling vascular resistance and thus blood pressure regulation. As cutaneous arteriolar VC is well documented to be pronounced during AD (Laird et al., 2008; Al Dera and Brock, 2018), we chose this easily accessible vascular bed to test our hypothesis. Four underlying pathological mechanisms causing exaggerated VC have been proposed: 1) increased neuronal release of norepinephrine (NE) and co-transmitters (CT) (e.g.; NPY and ATP); 2) impaired reuptake of NE (uptake-1); 3) post-junctional alpha-receptor hypersensitivity and/or 4) increases in circulating catecholamines (Epinephrine > NE) from adrenal stimulation (Eldahan and Rabchevsky, 2018; Karlsson et al., 1997). None of these mechanisms have been fully proven or is widely accepted, though some have been investigated.
Groothius et al. examined changes in leg vascular resistance during AD (persons with SCI) and cold pressor test (able-bodied) with and without competitive blockade of alpha-adrenergic receptors (via femoral artery infusion of phentolamine) (Groothuis et al., 2010). Phentolamine provides a relatively transient and non-selective post-junctional alpha blockade that antagonizes or reverses the pressor effects of epinephrine (Gould, 1969; Saeed et al., 1982). While the increase in leg vascular resistance was significantly attenuated in persons with SCI with phentolamine, it was completely blocked in persons without SCI (Groothuis et al., 2010). It is not certain if this complete blockade resulted from differing affinity and bioavailablity of phentolamine (less likely) or varying pharmacokinetics in persons with vs. without SCI (more likely). However, their results indicate that leg vascular resistance increases in persons with SCI during AD may not be mediated entirely by alpha receptor activation by neural release of NE. Indeed, it may be that adrenergic co-transmission which would not be altered by phentolamine is also involved in persons with SCI but not in persons without SCI with supraspinal control. We thus tested the role of non-adrenergic nerve cotransmission in AD. If pre-synaptic inhibition of release of NE and CT abolishes the VC during AD, (compared to control sites), this would indicate that not only is the AD induced VC mediated by NE and CT release at the neurovascular junction, but that it also does not involve circulating catecholamines. We hypothesized that AD involves adrenergic co-transmission to contract arteriolar smooth muscle thereby effecting the exaggerated increase in vascular resistance and hence increased blood pressure. (Fig. 1).
Fig. 1.
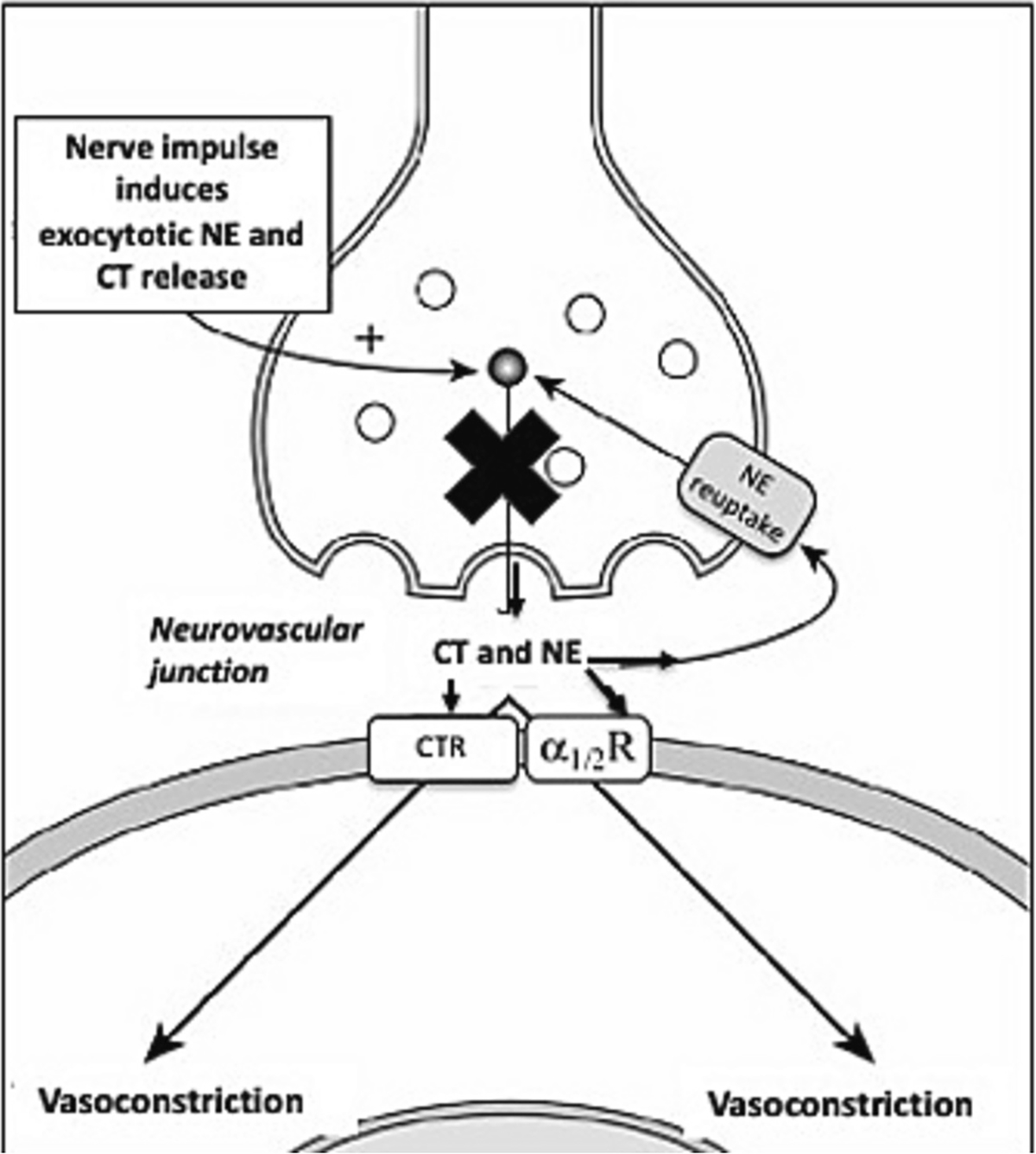
Anatomical schematic of the neurovascular junction. The X indicates the mechanism of action of Bretylium which blocks presynaptic release of NE and CT during neural stimulation.
To test our hypothesis, we studied the cutaneous vasculature as an accessible vascular bed that can be examined in humans safely. We used a non-invasive technique of iontophoresis of bretylium (BT) which pre-synaptically blocks neural release of all NE and CT from noradrenergic nerves locally.(see Fig. 1) (Kellogg Jr. et al., 1989) These new and novel methods have not been previously used to elucidate the pathophysiology of peripheral VC during AD. We tested whether blockade of neuronal release of NE and CT would abolish cutaneous VC responses during AD.
2. Methods
2.1. Participants
8 male persons with motor complete (AIS A or B) SCI at or above the level of T6 with a history of autonomic dysreflexia were enrolled. All human participants gave written informed consent, and protocols were approved by the university-affiliated Institutional Review Board and therefore were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The neurological level of injury (NLI) was determined using the 2019 revised International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) examination, performed by an SCI board-certified physician. Inclusion criteria: 1) chronic SCI >1 year in duration and 2) age 18–65 years old. Exclusion criteria: 1) uncontrolled hypertension 2) history of AD with life-threatening sequela such as stroke, seizure, or myocardial infarction 3) pre-existing diagnosis of cardiovascular disease 4) medications interfering with vasomotor tone (e.g.; alpha and beta agonists/antagonists) 5) active cigarette smoking and 6) neurological illness other than SCI. The demographic and injury characteristics of the male participants are presented in Table 1.
Table 1.
Demographics and injury characteristics of participants.
| Participants with tetraplegia (n = 8) | |
|---|---|
|
| |
| Age (years +/− SD) | 46 ±14 |
| Bladder management method | |
| Intermittent catheterization | n = 6 |
| Indwelling catheter | n = 2 |
| Neurological level of injury (NLI) | C2–7 [C2 (1), C4 (3), C6 (3), C7 (1)] |
| AIS A | 6 |
| AIS B | 2 |
| Time since injury (years +/− SD) | 19 ±13 |
2.2. Study preparation
Participants presented to the laboratory in the morning after an overnight fast.
2.3. Instrumentation and measurements
2.3.1. Iontophoresis
Noradrenergic nerve blockade was achieved by iontophoretic delivery of a solution of 10 mM of BT reconstituted in 2 ml of propylene glycol that was administered for 10 min at 250 μA over a 0.64 cm2 area. This dose has been effective in blocking VC during whole body cold stress in persons without SCI (Kellogg Jr. et al., 1989). Iontophoretic delivery of BT was applied in 2 areas of skin: 1) a sensate area above the NLI (ventral arm) and 2) an insensate area below the NLI (pretibial leg). A nearby (<1 cm medial or lateral at same dermatomal level) untreated site served as control (CON) (Kellogg Jr. et al., 1989).
2.3.2. Data collection
To allow the cutaneous vascular hyperemia from the iontophoretic current to resolve and for skin blood flow to return to baseline, participants rested for 90 min after iontophoresis in the supine position in the hospital bed prior to instrument placement and data collection. All data was collected using the Biopac Data acquisition system. After 90 min of rest, 10 min of continuous blood pressure and heart rate was measured via a finger cuff (Portapres), while skin blood flow was simultaneously measured via laser Doppler flowmetry (LDF). CVC was calculated during the data acquisition by the equation .
2.3.3. Local skin heating
As local skin temperature influences skin blood flow, baseline local skin temperatures were controlled with skin heaters at each LDF site. These local skin temperatures were held at 34 °C for approximately 10–15 min. Once a stable LDF measurement at 34 °C was obtained, local skin heaters were increased to 39 °C, the temperature which optimizes detection of VC responses (Wong and Hollowed, 2017; Taylor et al., 1985). After the LDF response to local heating at 39 °C plateaued, bladder percussion or bladder filling occurred.
2.3.4. Bladder stimulation
AD was successfully stimulated with bladder stimulation via manual bladder percussion for those without indwelling catheters or bladder distension for those with indwelling catheters. Manual bladder percussion was performed the same as previous studies without adverse events (30–50 taps/min over the lower abdomen for 2.5 min) (Wallin and Stjernberg, 1984; Karlsson et al., 1997; Mathias et al., 1976). For those with indwelling catheters, bladder instillation of 60 ml of normal saline with catheter clamping was performed for distension. Bladder stimulation continued until systolic blood pressure (SBP) rose between 20 and 40 mmHg from baseline (definition of AD) (Krassioukov et al., 2009). LDF, blood pressure (SBP, DBP and MAP), heart rate and heart rhythm (EKG) were monitored continuously. Participants then rested supine until SBP returned to baseline levels.
2.3.5. Local skin heating to 45 °C
5–10 min after SBP returned to baseline, the local temperature at all LDF sites was increased to 45 °C and then held constant for 30 min to elicit maximal cutaneous vasodilation (Kellogg Jr. et al., 1993; Minson et al., 2002; Taylor et al., 1984). CVCmax was defined as the average CVC of the final 90 s at skin temperature of 45 °C. Obtaining CVC values at maximal vasodilation for each site allowed for data to be normalized (Kellogg Jr. et al., 1993; Minson, 2010). Table 2 depicts protocol outline.
Table 2.
Timeline of protocol with timing of interventions and data collection.
| Length of procedure (minutes) | 0–10 min | 15–30 min | 2.5 min | 30 min |
|---|---|---|---|---|
|
| ||||
| Local heater temperature | 34 °C | 39 °C | 39 °C | 45 °C |
| AD triggered (bladder stimulation) | bladder stimulation | |||
| Measurements | CVC, HR, MAP | CVC, HR, MAP | CVC, HR, MAP | CVC, HR, MAP |
| Data collection frequency (sec) | q10s | q10s | q10s | q10s |
| CVC data point collected | Final 120s avg | Final 90s avg | Minimum CVC | Final 90s avg |
2.4. Statistical analysis
For each LDF site, CVC was calculated (CVC = LDF/MAP), and normalized to maximal CVC values induced by 45 °C (CVC/CVCmax) (Kellogg Jr. et al., 1993; Minson et al., 2001). CVC measurements were captured every 100 ms and 10 s averages calculated throughout the entire protocol. CVC, HR, SBP and MAP were averaged over the last 120 s at 34 °C to ensure stable/unfluctuating hemodynamics at baseline before interventions of local heating or bladder stimulation. CVC, HR, SBP and MAP were averaged over last 90 s at 39 °C (baseline before AD or “”), during the 150 s of AD (defined as the initial rise of SBP >20 mmHg from baseline and until SBP peaked) and over the final 90 s at 45 °C. The lowest CVC during the 150 s of AD was identified (i.e.; ) to detect the maximum VC during AD. The % drop in CVC was then calculated and compared among BT and CON sites. A 2-way repeated measures ANOVA was conducted to assess the effect of AD on CVC in BT vs. CON and sensate (forearm) vs insensate (leg) sites. In the absence of statistically significant main- and interaction effects of sensation (forearm vs. leg) on CVC, the CVC responses would be grouped together to analyze treatment (BT vs. CON) effects. A one-way repeated measures ANOVA (with Geisser-Greenhouse correction) was conducted to measure the effect of temperature and AD on HR and MAP between 34 °C, 39 °C, 39 °C (AD) and 45 °C conditions. Differences in MAP values were compared to the max MAP during AD (at 39 °C). Differences in HR values were compared to the minimum HR value during AD as well. MAP and HR values at each temperature and during AD were described by means and standard deviation.
3. Results
Iontophoretic administration of bretylium was successful and well tolerated in all subjects.
Six participants used intermittent catheterization so underwent bladder tapping to stimulate AD while 2 persons utilized indwelling catheter and underwent bladder instillation of normal saline with clamping to elicit AD.
Fig. 2 depicts a typical pattern in CVC response during AD from bladder tapping in the arm in BT vs. CON treated sites. In this participant, CVC rose immediately after bladder stimulation/tapping in CON site, but after bladder stimulation stopped CVC dropped much greater in CON than BT treated site. Overall, the change in CVC at the BT site was much less labile during and after AD. This CVC over time tracing represents a typical response.
Fig. 2.
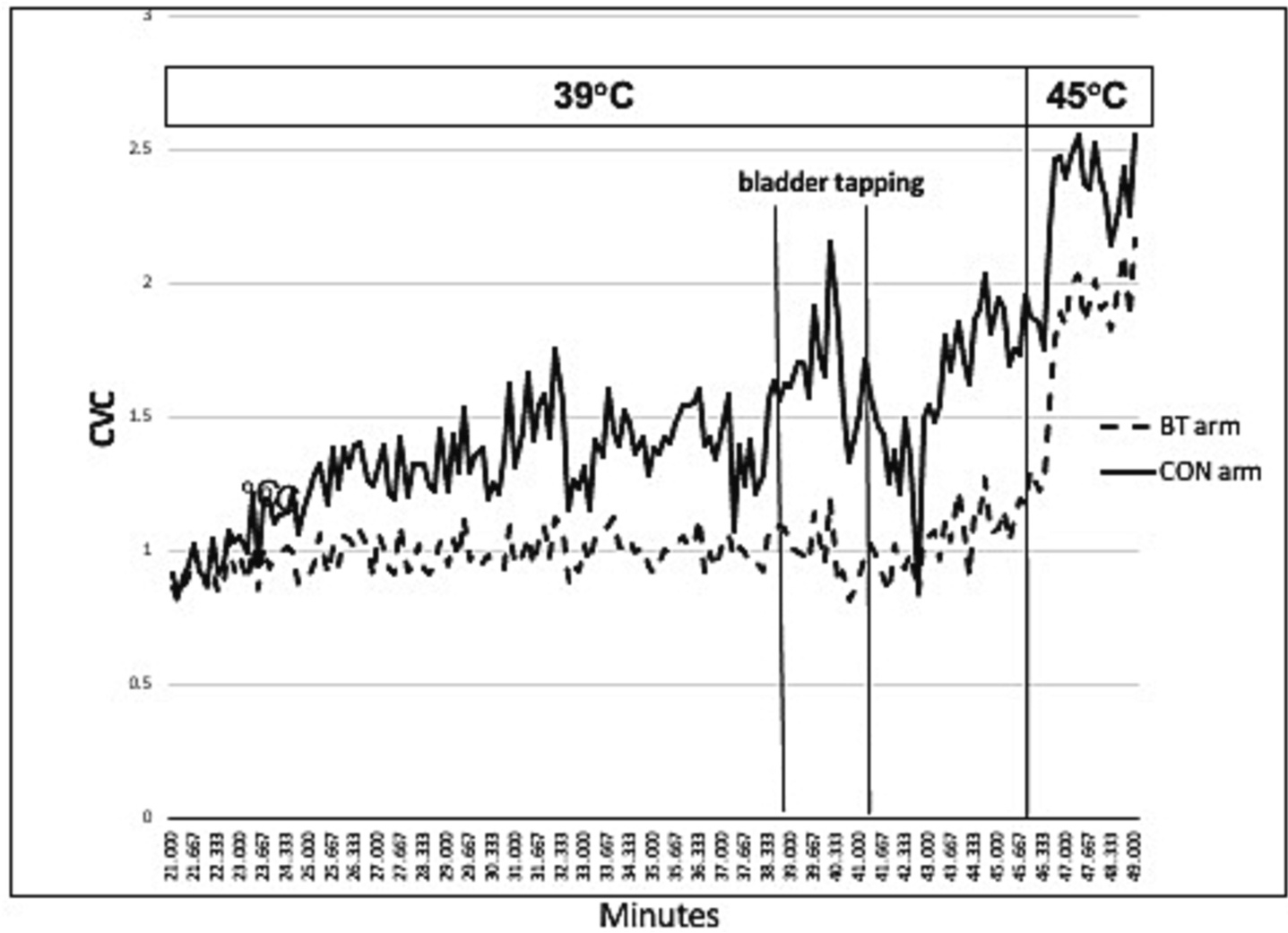
Example of typical CVC response during AD.
Two-way repeated measures ANOVA showed that the percent drop in CVC between BT and CON sites (i.e., 25.7±1.75 vs. 39.4 +/− 0.87; Fig. 3) was statistically significant (F [[1, 7] = 17.38, p = 0.004). The percent drop in CVC (flow per unit pressure) was BT: 26.9 +/− 9.77 vs. CON: 38.8+/− 13.3 in the arms and BT: 24.46±19.46 vs. CON: 40.03 +/− 12.74 in the legs. Neither the effect of site/sensation (arm vs leg) (F [1, 7] = 0.007), P = 0.94) nor the interaction between site/sensation vs. BT and CON treatment (F [1, 7] = 0.15), P = 0.71) were statistically significant.
Fig. 3.
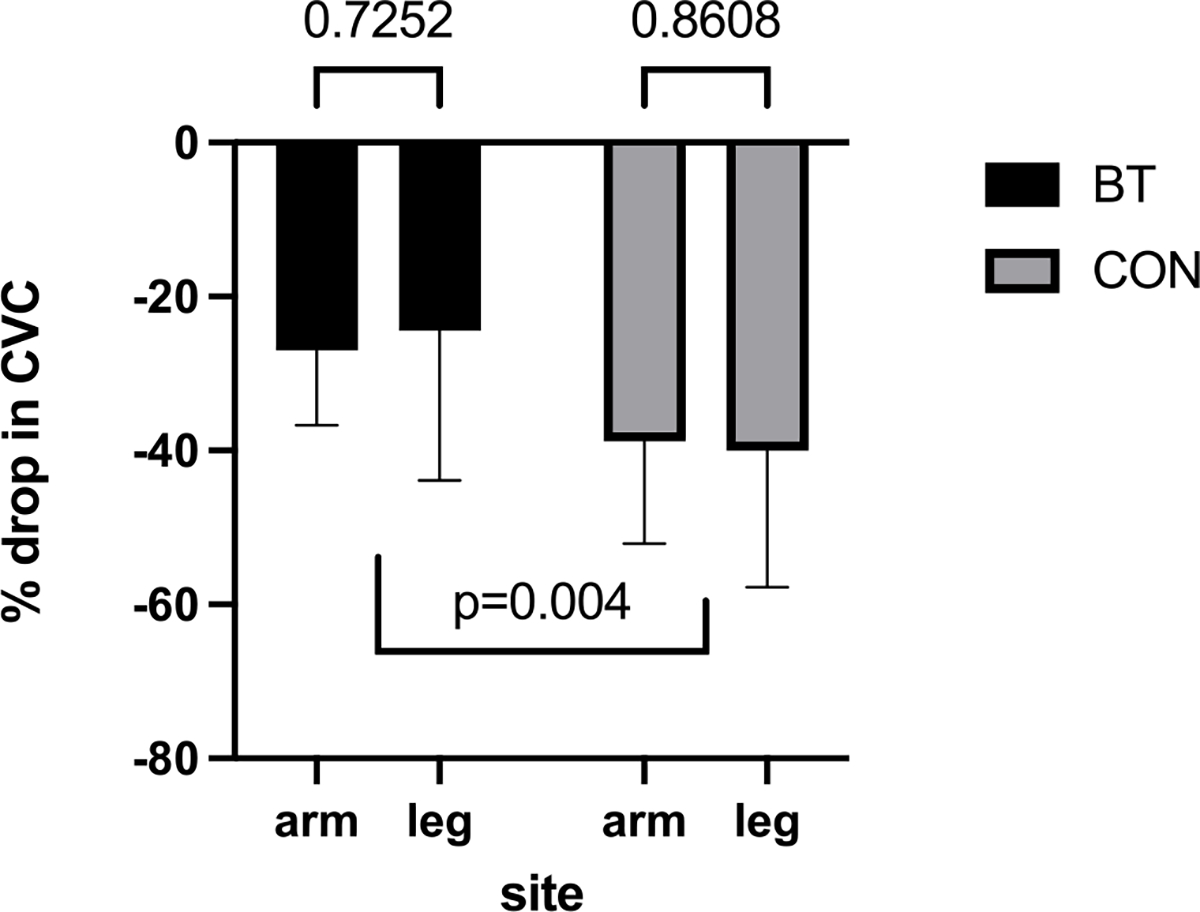
Percent drop in CVC during AD in bretylium treated vs. control sites.
MAP at baseline (34 °C) ranged from 57 to 87 mmHg with a mean of 69 +/− 10.5 mmHg (Fig. 4A). At 39 °C, MAP ranged from 55 to 99 mmHg with a mean of 78 +/− 14.1 mmHg. The difference between mean MAP at 34 °C vs. 39 °C was not statistically significant (P = 0.42). After AD induction at 39 °C, MAP (averaged over 150 s/2.5 min) ranged from 72 to 139 with a mean of 103 +/− 22 mmHg, and the maximum MAP during AD ranged from 78 to 163 mmHg with mean of 116±28 mmHg. One-way repeated measures ANOVA showed a statistically significant effects of condition (i.e., temperature +/− AD) on MAP (F [2.328, 16.29] = 30.8, P < 0.0001). Tukey’s multiple comparisons test showed a statistically significant difference in MAP at 39 °C before AD compared to avg. MAP during AD at 39 °C (p = 0.0084) and max MAP (p = 0.0024) during AD.
Fig. 4.
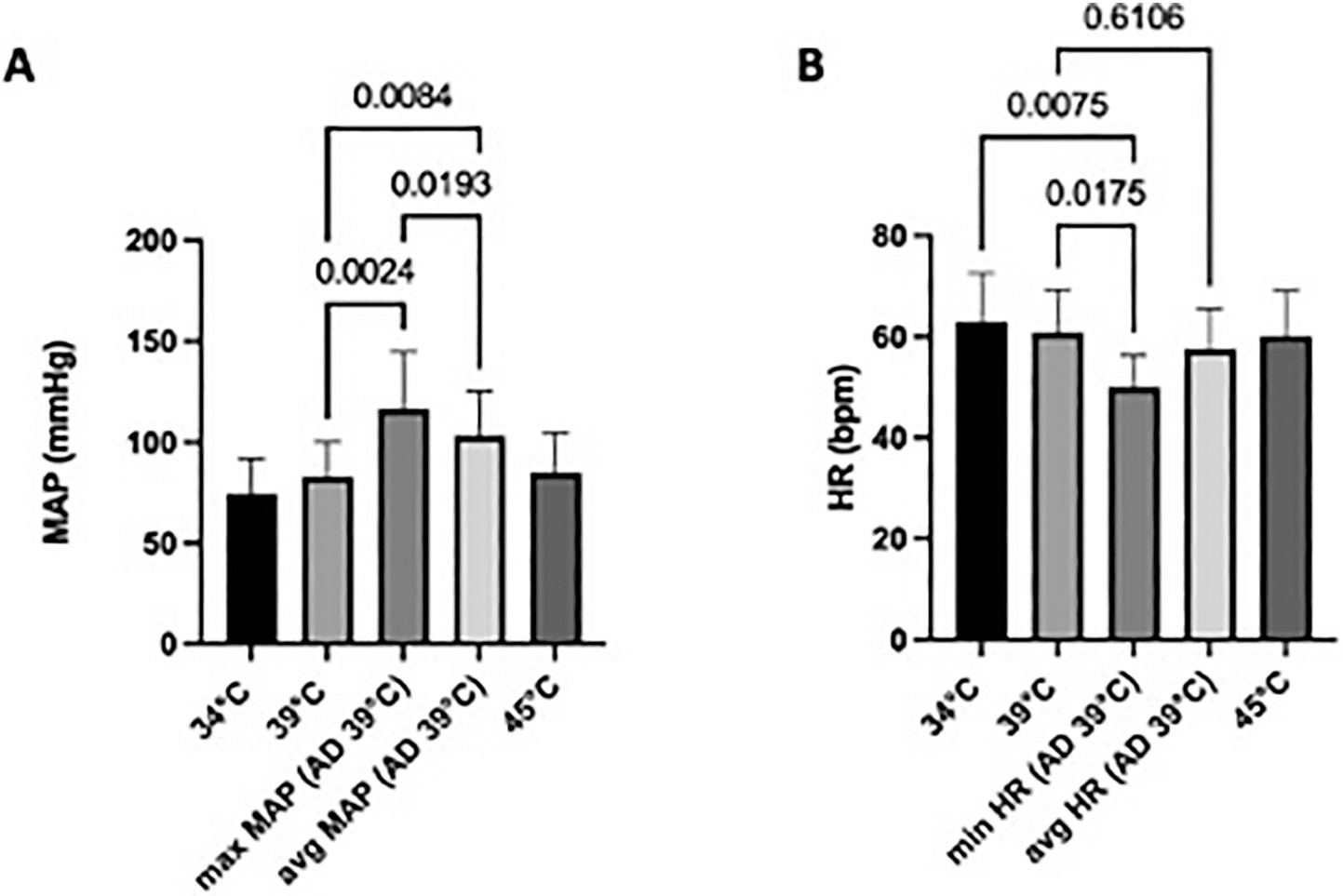
A: MAP (mmHg) and B: HR (bpm) changes over time.
HR at baseline (34 °C) ranged from 44 to 74 with a mean of 62+/− 9 BPM (Fig. 4B). At 39 °C, HR ranged from 47 to 74 with a mean of 60±8 BPM. There was no significant difference in mean HR at 34 °C vs. 39 °C (p = 0.49). During AD induction at 39 °C, HR (averaged over 150 s/2.5 min) ranged from 47 to 70 with a mean of 57±8 BPM. The minimum HR during AD ranged from 43 to 60 BPM with mean of 50±6 BPM. One-way repeated measures ANOVA showed a statistically significant impact effect of condition (i.e., temperature +/− AD) on HR (F [1.787, 12.51] = 14.58; p = 0.0007). Tukey’s multiple comparisons test showed significant differences in minimum HR during AD when compared to HR at 34 °C, 39 °C before AD (p = 0.0075 and p = 0.0175, respectively), however, average HR before AD at 39 °C and during AD (39 °C) was not statistically different.
4. Discussion
We investigated if the prejunctional neural release of NE and CT (e.g.; ATP, galanin, and NPY) was responsible for arteriolar VC during AD using a novel technique, iontophoretic delivery of bretylium to block prejunctional release of both NE and all CT during AD. Our results showed that prejunctional BT blockade attenuated VC during AD but did not abolish the response. This clearly demonstrates that prejunctional release of transmitters from noradrenergic nerves participates in the arteriolar VC during AD. Furthermore, as BT abolition of neurotransmitter release did not totally abolish AD-induced VC, our results indicate that another mechanism, perhaps circulating catecholamines, effects a part of AD-induced VC. Our results, combined with those of Groothius et al., suggest that prejunctional release of co-transmitters could effect a portion of AD induced VC; however additional studies with a post-junctional antagonist (e.g.; phentolamine or yohimbine) will be needed to verify this. We will briefly review evidence for a few other mechanisms effecting VC during AD reported in the literature, and suggest how our findings relate to them.
4.1. Neural release of NE
In able-bodied persons, after neural release into the synaptic cleft, a substantial proportion of NE is cleared by neuronal re-uptake (Fig. 1), thus only a small fraction (10–20 %) spills over into the venous drainage of an organ to enter the plasma (Esler et al., 1990). At rest, lower resting sympathetic neural activity and lower resting circulating NE in persons with SCI (compared to AB controls) have both been well documented (Wallin and Stjernberg, 1984; Schmid et al., 1985; Schmid et al., 1998). On the other hand, variable circulating plasma catecholamine levels have been reported in persons with SCI during AD (Karlsson et al., 1997; Mathias et al., 1976; Krum et al., 1992). Some have reported unchanged or slightly increased levels, while chronic SCI rodent models have shown that epinephrine increased five-fold and norepinephrine increased one and a half fold during AD (Leman et al., 2000). Krum et al. showed that AD (induced by bladder distension) of 9 persons with tetraplegia caused no significant changes in plasma levels of norepinephrine, renin, aldosterone, vasopressin, arginine, or atrial natriuretic peptide (Krum et al., 1992). Interestingly, Mathias et al. found that plasma NE during AD was slightly increased but remained significantly lower than resting NE levels in uninjured controls (Mathias et al., 1976). While animal and human results vary for multiple possible reasons including, 1. technical complexity in measuring circulating NE that is cleared rapidly or 2. variable levels and autonomic completeness in SCI study participants; it appears in humans, that neural release of NE is not significantly elevated from baseline during AD and thus plays a small role in the rise in BP during AD. Despite equivocal data on NE rise during AD, peripheral vascular hypersensitivity responses during AD are consistently documented and could effect an exaggerated VC response to low circulating catecholamine levels (Wallin and Stjernberg, 1984; Sharif and Hou, 2017). This implicates possible pre (neural release of CT) and post synaptic changes (e.g.; alpha receptor hypersensitivity and impaired NE reuptake) in AD pathophysiology.
4.2. Alpha receptor activation
Since clinically, AD appears to be an exaggerated VC sympathetic response, it has been assumed to be alpha receptor mediated, therefore alpha-adrenoreceptor blockers appear to be reasonable treatments for AD. However, after SCI, the number of alpha and beta receptors in insensate skin drop significantly with the greatest decline occurring in alpha receptors in persons with tetraplegia (Rodriguez et al., 1986). This may explain why animal model studies demonstrate that blocking alpha receptors pharmacologically (with agents of unknown affinity and bioavailability) is only partially effective at attenuating the rise in MAP during AD (Santajuliana et al., 1995). Similarly, in the human clinical setting, alpha blockers have not been shown to be consistently effective during AD (Krassioukov et al., 2009; Krum et al., 1992; Lindan et al., 1985). It is important to note that alpha-adrenoreceptor blockade does not impact the vasomotor response to sympathetic 1) beta receptors or the 2) co-transmitters such as adenosine triphosphate (ATP) and neuropeptide Y (NPY) (Mathias, 1991).
To that end, able-bodied humans during cold stress have shown alpha and beta receptor blockade does not fully block cutaneous vasomotor responses, suggesting sympathetic cotransmitter involvement (Stephens et al., 2001). More specifically, it has been delineated that adrenoreceptor activation only effects 60 % of VC during SNS reflex activity, while 40 % may be attributable to other sympathetic VC neurotransmitters co-released with NE such as NPY (Stephens et al., 2004; Simmons et al., 2011) and ATP (Lang et al., 2017). Neuroplasticity after SCI may indeed alter the responses to alpha, beta and co-transmitter receptor activation, however further investigation is needed. In summary, alpha receptor hypersensitivity alone does not explain the peripheral vascular hypersensitivity after SCI.
4.3. NE reuptake
Brock et al. compared vascular sensitivity (via isometric contractions of the mesenteric artery) between 1) phenylephrine (alpha 1 adrenoreceptor agonist), 2) phenylephrine with desmethylimipramine (NE reuptake inhibitor) and 3) methoxamine (alpha receptor agonist that is not a substrate for neuronal reuptake) in T4 spinalized rats (Brock et al., 2006). Sensitivity to phenylephrine was enhanced in the arteries of the rats with SCI compared to non spinalized controls, however this difference from controls was abolished by the NE reuptake inhibition with desmethylimipramine. Sensitivity to the alpha receptor agonist methoxamine that is NOT a substrate for neuronal reuptake was similar among the groups. While the affinity and bioavailability of all 3 agents tested by Brock is unknown, the findings suggest impaired NE reuptake is responsible for enhanced neuronal responses after SCI. This preclinical data that suggests impaired NE reuptake is only study of its kind and needs to be tested in humans. From a clinical perspective, this investigation is important as many medications for pain and depression (e.g.; tricyclic antidepressants and Serotonin and norepinephrine reuptake inhibitors) block neural reuptake. If NE reuptake is impaired in persons with SCI related AD, these drugs could potentiate or lower the threshold for AD.
4.4. Sympathetic neural co-transmission
While NE is the primary classical neurotransmitter involved in sympathetic noradrenergic vasoconstrictor neurons, it is colocalized in perivascular nerves with the sympathetic neural cotransmitters, neuropeptide Y (NPY), galanin and ATP (Stephens et al., 2001; Wallengren, 1997). The effect of cotransmitters on cutaneous vasculature of able bodied humans was studied during whole body cold stress by Stephens et al. (Stephens et al., 2001) Despite a confirmed cutaneous blockade of NE with yohimbine (alpha receptor blockade) and propranolol (beta receptor blockade), significant reflex cutaneous VC still occurs in cold stressed humans. Furthermore, NPY antagonism attenuates reflex cutaneous VC in able-bodied humans (Stephens et al., 2004). While the effect of cotransmitters in VC in able bodied humans during cold stress is known, the role of cotransmitters in autonomic dysreflexia in persons with SCI requires elucidation beyond our present understanding.
Since alpha blockade has consistently been shown to be insufficient at blocking peripheral VC during AD and that neural release of NE is not in large magnitude (thus blockade would likely not impact VC), we sought to investigate Groothius’ hypothesis that CT were also involved in VC during AD. As an aside, we attempted to replicate the results of Groothius et al. however we were unable to successfully deliver phentolamine with iontophoresis due to the agents short half-life (Gould, 1969). Bretylium blocks neural release of all sympathetic neurotransmitters and we have successfully delivered it via iontophoresis in able-bodied humans to fully block VC during cold stress for several hours (Kellogg Jr. et al., 1989). Utilizing exactly the same pharmacological intervention for this study, we aimed to block VC during AD in persons with SCI. We found that with bladder stimulation (whether by tapping or bladder distension with saline) in persons with SCI, CVC in arms (sensate) and legs (insensate) dropped from baseline immediately when SBP rose above 20 mmHg from baseline (definition of AD). Interestingly, the degree of sensation (sensate arm vs. insensate leg) did not make a difference in the CVC responses. This could be explained by the level of innervation of the sympathetic control of the vessels of the arms and legs being below the neurological level of injury in all participants with tetraplegia (Wecht et al., 2021). In some participants, CVC dropped during bladder stimulation but in others the CVC drop occurred immediately after bladder stimulation. CVC changes in BT vs. CON sites, demonstrated that bretylium significantly attenuated the VC response during AD, however, it did not abolish it, as it does in cold stress with able-bodied humans (Kellogg Jr. et al., 1989). Of note, while BT does block all prejunctional neurotransmitter release, it also leaves post junctional receptors unaltered, thus post junctional receptor activation by circulating catecholamines (if elevated) could play a role in AD induced VC. Whether our findings are due to an incomplete blockade (higher dose of BT needed) vs. impaired NE reuptake in setting of an incomplete bretylium blockade vs. hyperresponsiveness to circulating catecholamines vs. another pathophysiological processes is unknown. Nevertheless, it is clear that neural release of NE and CT is at minimum partially responsible for the peripheral VC during AD.
Future studies should combine treatments and attempt to quantify the extent of VC during AD from 1) neural release of NE and CT (with confirmation of BT blockade) and 2) blockade of alpha 1 and 2 receptors (yohimbine) 3) blockade of alpha and beta receptors (with yohimbine and propranolol) and 4) blockade of NE reuptake. Future studies using these protocols in animals may be more valuable as animal models allow for a more highly specific manipulation of ganglionic neurons synapsing with blood vessels. Whether in humans or animals, these protocols could elucidate a more comprehensive understanding of the complex neurovascular pathophysiology of VC during AD (Stephens et al., 2001).
4.5. Changes in MAP and HR during AD
As anticipated, mean MAP during AD significantly rose compared to pre-AD values of any temperature (34 °C or 39 °C). This is consistent with previously reported literature and demonstrates that MAP and CVC responses were consistent with what is typically expected during autonomic dysreflexia incited by bladder distension (Allen and Leslie, 2023). In addition, minimum HR during AD was significantly different than HR in non-AD conditions at all other temperature conditions (34 °C, 39 °C or 45 °C) This finding is consistent with a baroreflex response to drop HR via vagus stimulation during AD, since vagus nerve remains intact after cervical SCI (Allen and Leslie, 2023). These cardiovascular changes (rise in MAP with fall in HR) during bladder distension in this sample of 8 persons with sensorimotor complete (AIS A and B) cervical SCI are consistent with autonomic dysreflexia by definition.
4.6. Limitations
There are three main limitations: 1) Two of the six persons required bladder instillation of 60 ml of normal saline with clamping to distend the bladder and thus elicit AD. Thus, the bladder pressures of those with (n = 2) and without instillation (n = 6) was likely different adding variability in the afferent stimuli eliciting AD. 2) While BT does not block receptors in the synaptic cleft, if does act as a competitive antagonist of NE reuptake. Indeed, BT enters prejunctional noradrenergic nerve terminals through the NE reuptake transporter to cause blockade of noradrenergic neurotransmitter release, thus BT can reduce NE clearance from the cleft by the NE reuptake transporter. This can magnify the effects of circulating NE or epinephrine (if present). Given that BT does not block receptors but does block the NE reuptake transporter, our finding that despite BTs effects on neurotransmitter release, it is likely that circulating catecholamines also participate in the VC induced by AD. 3) Our dose of BT could have been insufficient to completely block NE and cotransmitter release in SCI persons although it does so in AB persons (Kellogg Jr. et al., 1989).
5. Conclusion
In this study, we investigated the impact of blockade of prejunctional neural release of NE and CT at cutaneous neurovascular junctions, bretylium partially blocked VC in cutaneous arterioles during autonomic dysreflexia triggered by bladder irritation (tapping or distension). This indicates that arteriolar VC causing elevation in blood pressure during autonomic dysreflexia is partially mediated by neural release of norepinephrine and cotransmitters.
Acknowledgements
This study was supported with the facilities and resources at the South Texas Veteran’s Health Care System, San Antonio, TX.
Funding
This case report was funded by a SPIRE Award (# RX003585-01A1) from the Department of Veterans Affairs’ Office of Rehabilitation and Research Development.
Footnotes
Declaration of competing interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethics approvals
All human protocols were approved by the university affiliated Institutional Review Board and therefore were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Veterans Affairs local Research Development office approved all protocols in this study. All participants provided written informed consent to participate.
CRediT authorship contribution statement
Michelle Trbovich: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. Yubo Wu: Data curation, Investigation, Methodology, Project administration, Resources, Software, Writing – review & editing. Terry Romo: Data curation, Investigation, Methodology, Project administration, Resources, Writing – review & editing. Wouker Koek: Data curation, Formal analysis, Methodology, Validation, Writing – review & editing. Dean Kellogg: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Data availability
Data will be made available on request.
References
- Al Dera H, Brock JA, 2018. Changes in sympathetic neurovascular function following spinal cord injury. Auton. Neurosci. 209, 25–36. [DOI] [PubMed] [Google Scholar]
- Allen KJ, Leslie SW, 2023. Autonomic dysreflexia. In: StatPearls. Treasure Island (FL) Ineligible Companies. Disclosure: Stephen Leslie Declares no Relevant Financial Relationships With Ineligible Companies. [Google Scholar]
- Brock JA, Yeoh M, McLachlan EM, 2006. Enhanced neurally evoked responses and inhibition of norepinephrine reuptake in rat mesenteric arteries after spinal transection. Am. J. Physiol. Heart Circ. Physiol. 290 (1), H398–H405. [DOI] [PubMed] [Google Scholar]
- Brown R, Burton AR, Macefield VG, 2018. Autonomic dysreflexia: Somatosympathetic and viscerosympathetic vasoconstrictor responses to innocuous and noxious sensory stimulation below lesion in human spinal cord injury. Auton. Neurosci. 209, 71–78. [DOI] [PubMed] [Google Scholar]
- Corbett JL, Debarge O, Frankel HL, Mathias C, 1975. Cardiovascular responses in tetraplegic man to muscle spasm, bladder percussion and head-up tilt. Clin. Exp. Pharmacol. Physiol. Suppl 2, 189–193. [PubMed] [Google Scholar]
- Dance DL, Chopra A, Campbell K, Ditor DS, Hassouna M, Craven BC, 2017. Exploring daily blood pressure fluctuations and cardiovascular risk among individuals with motor complete spinal cord injury: a pilot study. J. Spinal Cord Med. 40 (4), 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldahan KC, Rabchevsky AG, 2018. Autonomic dysreflexia after spinal cord injury: systemic pathophysiology and methods of management. Auton. Neurosci. 209, 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler M, Jennings G, Lambert G, Meredith I, Horne M, Eisenhofer G, 1990. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiol. Rev. 70 (4), 963–985. [DOI] [PubMed] [Google Scholar]
- Gould L, 1969. Appraisal and reappraisal of cardiac therapy. Edited by DeGraff Arthur C., Lyon Alan F., and Phentolamine Julian Frieden.. Am. Heart J. 78 (2), 276–278. [DOI] [PubMed] [Google Scholar]
- de Groat WC, 1990. Central neural control of the lower urinary tract. CIBA Found. Symp. 151, 27–44 (discussion 44–56). [DOI] [PubMed] [Google Scholar]
- Groothuis JT, Rongen GA, Deinum J, et al. , 2010. Sympathetic nonadrenergic transmission contributes to autonomic dysreflexia in spinal cord-injured individuals. Hypertension 55 (3), 636–643. [DOI] [PubMed] [Google Scholar]
- Guttmann L, Whitteridge D, 1947. Effects of bladder distension on autonomic mechanisms after spinal cord injuries. Brain 70 (Pt 4), 361–404. [DOI] [PubMed] [Google Scholar]
- Hubli M, Krassioukov AV, 2014. Ambulatory blood pressure monitoring in spinal cord injury: clinical practicability. J. Neurotrauma 31 (9), 789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson AK, 1999. Autonomic dysreflexia. Spinal Cord 37 (6), 383–391. [DOI] [PubMed] [Google Scholar]
- Karlsson AK, Elam M, Friberg P, Sullivan L, Attvall S, Lonnroth P, 1997. Peripheral afferent stimulation of decentralized sympathetic neurons activates lipolysis in spinal cord-injured subjects. Metabolism 46 (12), 1465–1469. [DOI] [PubMed] [Google Scholar]
- Kellogg DL Jr., Johnson JM, Kosiba WA, 1989. Selective abolition of adrenergic vasoconstrictor responses in skin by local iontophoresis of bretylium. Am. J. Phys. 257 (5 Pt 2), H1599–H1606. [DOI] [PubMed] [Google Scholar]
- Kellogg DL Jr., Johnson JM, Kenney WL, Pergola PE, Kosiba WA, 1993. Mechanisms of control of skin blood flow during prolonged exercise in humans. Am. J. Physiol. Heart Circ. Physiol. 265, H562–H568. [DOI] [PubMed] [Google Scholar]
- Krassioukov A, 2009. Autonomic function following cervical spinal cord injury. Respir. Physiol. Neurobiol. 169 (2), 157–164. [DOI] [PubMed] [Google Scholar]
- Krassioukov A, Warburton DE, Teasell R, Eng JJ, 2009. Spinal Cord Injury Rehabilitation Evidence Research T. A systematic review of the management of autonomic dysreflexia after spinal cord injury. Arch. Phys. Med. Rehabil. 90 (4), 682–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krum H, Louis WJ, Brown DJ, Clarke SJ, Fleming JA, Howes LG, 1992. Cardiovascular and vasoactive hormone responses to bladder distension in spinal and normal man. Paraplegia 30 (5), 348–354. [DOI] [PubMed] [Google Scholar]
- Laird AS, Finch AM, Waite PM, Carrive P, 2008. Peripheral changes above and below injury level lead to prolonged vascular responses following high spinal cord injury. Am. J. Physiol. Heart Circ. Physiol. 294 (2), H785–H792. [DOI] [PubMed] [Google Scholar]
- Lang JA, Krajek AC, Smaller KA, 2017. Evidence for a functional vasoconstrictor role for ATP in the human cutaneous microvasculature. Exp. Physiol. 102 (6), 684–693. [DOI] [PubMed] [Google Scholar]
- Leman S, Bernet F, Sequeira H, 2000. Autonomic dysreflexia increases plasma adrenaline level in the chronic spinal cord-injured rat. Neurosci. Lett. 286 (3), 159–162. [DOI] [PubMed] [Google Scholar]
- Lindan R, Leffler EJ, Kedia KR, 1985. A comparison of the efficacy of an alpha-I-adrenergic blocker in the slow calcium channel blocker in the control of autonomic dysreflexia. Paraplegia 23 (1), 34–38. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Burton AR, Brown R, 2012. Somatosympathetic vasoconstrictor reflexes in human spinal cord injury: responses to innocuous and noxious sensory stimulation below lesion. Front. Physiol. 3, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias CJ, 1991. Role of sympathetic efferent nerves in blood pressure regulation and in hypertension. Hypertension 18(5 Suppl):III22–30. [DOI] [PubMed] [Google Scholar]
- Mathias CJ, 2006. Orthostatic hypotension and paroxysmal hypertension in humans with high spinal cord injury. Prog. Brain Res. 152, 231–243. [DOI] [PubMed] [Google Scholar]
- Mathias CJ, Christensen NJ, Corbett JL, Frankel HL, Spalding JM, 1976. Plasma catecholamines during paroxysmal neurogenic hypertension in quadriplegic man. Circ. Res. 39 (2), 204–208. [DOI] [PubMed] [Google Scholar]
- Minson CT, 2010. Thermal provocation to evaluate microvascular reactivity in human skin. J. Appl. Physiol. 109, 1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson CT, Berry LT, Joyner MJ, 2001. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J. Appl. Physiol. 91, 1619–1626. [DOI] [PubMed] [Google Scholar]
- Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW, 2002. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J. Appl. Physiol. 93, 1644–1649. [DOI] [PubMed] [Google Scholar]
- Mironets E, Fischer R, Bracchi-Ricard V, et al. , 2020. Attenuating neurogenic sympathetic hyperreflexia robustly improves antibacterial immunity after chronic spinal cord injury. J. Neurosci. 40 (2), 478–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AA, Krassioukov AV, Ainslie PN, Cote AT, Warburton DE, 2014. Increased central arterial stiffness explains baroreflex dysfunction in spinal cord injury. J. Neurotrauma 31 (12), 1122–1128. [DOI] [PubMed] [Google Scholar]
- Rabchevsky AG, 2006. Segmental organization of spinal reflexes mediating autonomic dysreflexia after spinal cord injury. Prog. Brain Res. 152, 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez GP, Claus-Walker J, Kent MC, Stal S, 1986. Adrenergic receptors in insensitive skin of spinal cord injured patients. Arch. Phys. Med. Rehabil. 67 (3), 177–180. [DOI] [PubMed] [Google Scholar]
- Saeed M, Sommer O, Holtz J, Bassenge E, 1982. Alpha-adrenoceptor blockade by phentolamine causes beta-adrenergic vasodilation by increased catecholamine release due to presynaptic alpha-blockade. J. Cardiovasc. Pharmacol. 4 (1), 44–52. [DOI] [PubMed] [Google Scholar]
- Santajuliana D, Zukowska-Grojec Z, Osborn JW, 1995. Contribution of alpha- and beta-adrenoceptors and neuropeptide-Y to autonomic dysreflexia. Clin. Auton. Res. 5 (2), 91–97. [DOI] [PubMed] [Google Scholar]
- Schmid A, Huonker M, Barturen JM, et al. , 1985. Catecholamines, heart rate, and oxygen uptake during exercise in persons with spinal cord injury. J. Appl. Physiol. 1998;85 (2), 635–641. [DOI] [PubMed] [Google Scholar]
- Schmid A, Huonker M, Stahl F, et al. , 1998. Free plasma catecholamines in spinal cord injured persons with different injury levels at rest and during exercise. J. Auton. Nerv. Syst. 68 (1–2), 96–100. [DOI] [PubMed] [Google Scholar]
- Sharif H, Hou S, 2017. Autonomic dysreflexia: a cardiovascular disorder following spinal cord injury. Neural Regen. Res. 12 (9), 1390–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons GH, Fieger SM, Wong BJ, Minson CT, Halliwill JR, 2011. No effect of systemic isocapnic hypoxia on alpha-adrenergic vasoconstrictor responsiveness in human skin. Acta Physiol (Oxford) 201 (3), 339–347. [DOI] [PubMed] [Google Scholar]
- Stephens DP, Aoki K, Kosiba WA, Johnson JM, 2001. Nonnoradrenergic mechanism of reflex cutaneous vasoconstriction in men. Am. J. Physiol. Heart Circ. Physiol. 280 (4), H1496–H1504. [DOI] [PubMed] [Google Scholar]
- Stephens DP, Saad AR, Bennett LA, Kosiba WA, Johnson JM, 2004. Neuropeptide Y antagonism reduces reflex cutaneous vasoconstriction in humans. Am. J. Physiol. Heart Circ. Physiol. 287 (3), H1404–H1409. [DOI] [PubMed] [Google Scholar]
- Stjernberg L, Blumberg H, Wallin BG, 1986. Sympathetic activity in man after spinal cord injury. Outflow to muscle below the lesion. Brain 109 (Pt 4), 695–715. [DOI] [PubMed] [Google Scholar]
- Taylor WF, Johnson JM, O’Leary D, Park MK, 1984. Effect of high local temperature on reflex cutaneous vasodilation. J. Appl. Physiol. 57, 191–196. [DOI] [PubMed] [Google Scholar]
- Taylor WF, Johnson JM, Kosiba WA, Kwan CM, 1985. Graded cutaneous vascular responses to dynamic leg exercise. J. Appl. Physiol. 1988;64 (5), 1803–1809. [DOI] [PubMed] [Google Scholar]
- Wallengren J, 1997. Vasoactive peptides in the skin. J. Investig. Dermatol. Symp. Proc. 2 (1), 49–55. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Stjernberg L, 1984. Sympathetic activity in man after spinal cord injury. Outflow to skin below the lesion. Brain 107 (Pt 1), 183–198. [DOI] [PubMed] [Google Scholar]
- Wan D, Krassioukov AV, 2014. Life-threatening outcomes associated with autonomic dysreflexia: a clinical review. J. Spinal Cord Med. 37 (1), 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wecht JM, Krassioukov AV, Alexander M, et al. , 2021. International Standards to document Autonomic Function following SCI (ISAFSCI): second edition. Top. Spinal Cord Inj Rehabil. 27 (2), 23–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West CR, Alyahya A, Laher I, Krassioukov A, 2013. Peripheral vascular function in spinal cord injury: a systematic review. Spinal Cord 51 (1), 10–19. [DOI] [PubMed] [Google Scholar]
- Wiart L, Joseph PA, Petit H, et al. , 1998. The effects of capsaicin on the neurogenic hyperreflexic detrusor. A double blind placebo controlled study in patients with spinal cord disease. Preliminary results. Spinal Cord 36 (2), 95–99. [DOI] [PubMed] [Google Scholar]
- Wong BJ, Hollowed CG, 2017. Current concepts of active vasodilation in human skin. Temperature (Austin) 4 (1), 41–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


