Key Points
Question
Does the connective tissue growth factor inhibitor pamrevlumab decrease the rate of decline in lung function for patients with idiopathic pulmonary fibrosis compared with placebo?
Findings
In this phase 3 randomized clinical trial including 356 adults with idiopathic pulmonary fibrosis, the decline in forced vital capacity at 48 weeks did not differ significantly between patients treated with pamrevlumab (–260 mL) vs placebo (–330 mL).
Meaning
Use of pamrevlumab among patients with idiopathic pulmonary fibrosis did not result in a difference in decline in forced vital capacity from baseline to 48 weeks compared with placebo.
Abstract
Importance
Current treatments for idiopathic pulmonary fibrosis slow the rate of lung function decline, but may be associated with adverse events that affect medication adherence. In phase 2 trials, pamrevlumab (a fully human monoclonal antibody that binds to and inhibits connective tissue growth factor activity) attenuated the progression of idiopathic pulmonary fibrosis without substantial adverse events.
Objective
To assess the efficacy and safety of pamrevlumab for patients with idiopathic pulmonary fibrosis.
Design, Setting, and Participants
Phase 3 randomized clinical trial including 356 patients aged 40 to 85 years with idiopathic pulmonary fibrosis who were not receiving antifibrotic treatment with nintedanib or pirfenidone at enrollment. Patients were recruited from 117 sites in 9 countries between July 18, 2019, and July 29, 2022; the last follow-up encounter occurred on August 28, 2023.
Interventions
Pamrevlumab (30 mg/kg administered intravenously every 3 weeks; n = 181) or placebo (n = 175) for 48 weeks.
Main Outcomes and Measures
The primary outcome was absolute change in forced vital capacity (FVC) from baseline to week 48. There were 5 secondary outcomes (including time to disease progression, which was defined as a decline of ≥10% in predicted FVC or death). The exploratory outcomes included patient-reported symptoms. Adverse events were reported.
Results
Among 356 patients (mean age, 70.5 years; 258 [72.5%] were men; 221 [62.1%] were White), 277 (77.8%) completed the trial. There was no significant between-group difference for absolute change in FVC from baseline to week 48 (least-squares mean, −260 mL [95% CI, −350 to −170 mL] in the pamrevlumab group vs −330 mL [95% CI, −430 to −230 mL] in the placebo group; mean between-group difference, 70 mL [95% CI, −60 to 190 mL], P = .29). There were no significant between-group differences in any of the secondary outcomes or in the patient-reported outcomes. In the pamrevlumab group, there were 160 patients (88.4%) with treatment-related adverse events and 51 patients (28.2%) with serious adverse events vs 151 (86.3%) and 60 (34.3%), respectively, in the placebo group. During the study, 23 patients died in each group (12.7% in the pamrevlumab group vs 13.1% in the placebo group).
Conclusions and Relevance
Among patients with idiopathic pulmonary fibrosis treated with pamrevlumab or placebo, there was no statistically significant between-group difference for the primary outcome of absolute change in FVC from baseline to week 48.
Trial Registration
ClinicalTrials.gov Identifier: NCT03955146
This randomized clinical trial compares the efficacy and safety of pamrevlumab vs placebo for patients with idiopathic pulmonary fibrosis.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a rare interstitial lung disease that is characterized by cough, worsening dyspnea, and fatigue in adults who are usually older than 60 years of age. The worldwide prevalence of IPF is estimated to be 0.33 to 4.51 per 10 000 people and there are 0.09 to 1.30 new cases of IPF per 10 000 people diagnosed each year.1 Patients experience progressive loss of lung function, exercise intolerance, and decreased health-related quality of life, including the inability to perform activities of daily living.2,3 Without treatment, the prognosis of IPF is poor, with a median survival of approximately 3 to 5 years.2,3,4,5
Two antifibrotic drugs (pirfenidone and nintedanib) approved for treating IPF have become the standard of care worldwide.3,6,7,8 In phase 3 trials, antifibrotic treatment was associated with a reduced rate of lung function decline,6,7,8,9 but individual responses were variable and unpredictable, and patients did not experience improvement in symptoms or health-related quality of life.6,7 More effective treatments for IPF are needed10,11,12 because the clinical need has been only modestly met with nintedanib and pirfenidone. These treatments slow the rate of lung function decline, which is measured by forced vital capacity (FVC), but up to 40% of patients may discontinue therapy because of intolerability, including gastrointestinal, hepatic, and dermatologic adverse events.6,7,13,14,15 Furthermore, other assessments of treatment response (such as the diffusing capacity of the lungs for carbon monoxide, quantification of the reduction in lung fibrosis as measured by high-resolution computed tomography imaging, walk test variables, patient-reported outcomes, and health-related quality of life) have not been improved by the currently available antifibrotic treatments.6,7,16
Pamrevlumab is a fully human monoclonal antibody that binds to and inhibits the activity of connective tissue growth factor, a central mediator of tissue remodeling and fibrosis.17,18 Pamrevlumab demonstrated favorable results and good tolerability in phase 2 studies of IPF,19,20 significantly slowing the rate of lung function decline and decreasing the extent of lung fibrosis (as quantified with high-resolution computed tomography imaging), and adverse events were generally mild.
Based on the positive results from the phase 2 trials,19,20 pamrevlumab had the potential to attenuate lung function decline and preserve health-related quality of life. The objective of the ZEPHYRUS-1 trial was to confirm the efficacy and safety of pamrevlumab compared with placebo for patients with IPF.
Methods
Study Design
The current study was a phase 3, double-blind, placebo-controlled, randomized clinical trial conducted at 117 sites in 9 countries worldwide. The trial protocol appears in Supplement 1 and the statistical analysis plan appears in Supplement 2. The study comprised a 6-week screening period, a 48-week treatment period, and a safety assessment period that lasted up to 60 days after the last dose of study drug (eMethods and eFigure 1 in Supplement 3).
Intervention and Randomization
Patients were randomized 1:1 to receive 30 mg/kg of pamrevlumab administered intravenously every 3 weeks or placebo (Figure 1). Randomization was stratified by prior treatment with antifibrotic therapy with pirfenidone, nintedanib, or both (yes or no) and Gender-Age-Pulmonary (GAP) function stage (I, II, or III).21 The GAP function stage is determined via a composite measurement to predict survival (scores are awarded for participant self-reported sex, age, FVC, and diffusing capacity of the lungs for carbon monoxide) and was included to offer a more comprehensive assessment of disease severity than lung function alone. Study visits were planned for day 1 (first dose) and then every 3 weeks through week 48. Pulmonary function tests were conducted during screening, prior to the first dose on day 1, and every 6 to 12 weeks through week 48.
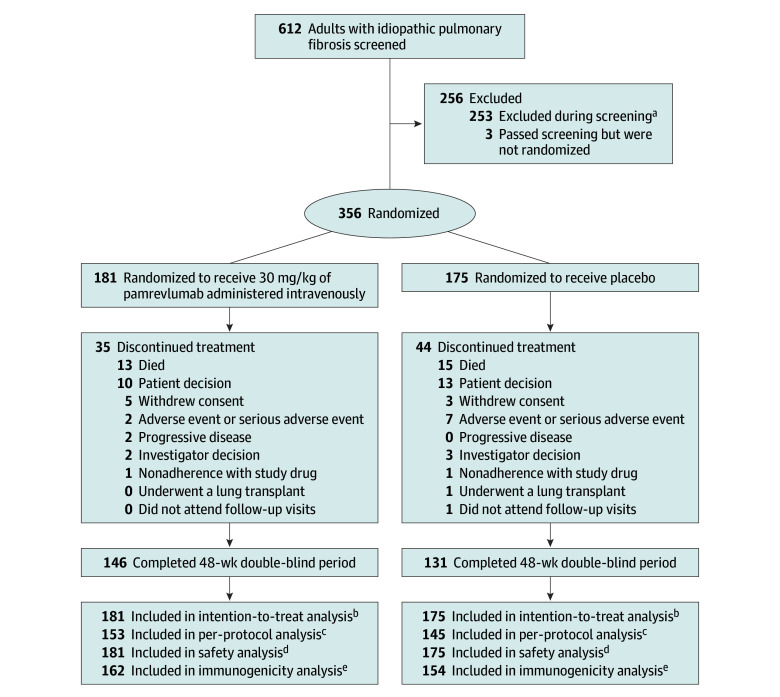
Figure 1. Patient Randomization and Follow-Up.
aThe most common reasons were (1) percent predicted forced vital capacity values did not meet inclusion criteria at screening and on day 1, (2) did not have a guideline-based diagnosis of idiopathic pulmonary fibrosis, (3) did not have a diagnosis of idiopathic pulmonary fibrosis confirmed by high-resolution computed tomography imaging scan, (4) had percent predicted values for diffusing capacity of the lungs for carbon monoxide that did not meet inclusion criteria, and (5) had evidence of significant obstructive lung disease.
bIncluded all randomized patients during the 48-week study.
cIncluded all randomized patients (1) who completed at least 36 weeks of treatment with a pulmonary function test assessment at baseline and at least once after baseline and (2) without major protocol deviations that significantly affected the efficacy analyses.
dIncluded all patients who received at least 1 dose of study drug.
eIncluded all patients who were in the safety population and who had at least 1 evaluable immunogenicity assessment after baseline.
Patients
Patients were eligible if they (1) were aged 40 to 85 years, (2) had received a guideline-defined22 diagnosis of IPF within the preceding 7 years with evidence of parenchymal fibrosis (reticulation) that was 10% or greater to less than 50% and honeycombing within the whole lung of less than 25% on a high-resolution computed tomography imaging scan (confirmed by an independent central reviewer before randomization), (3) had a predicted FVC that was greater than 45% to less than 95%, and (4) had a predicted diffusing capacity of the lungs for carbon monoxide corrected for hemoglobin that was 25% or greater to 90% or less.
Patients receiving either nintedanib or pirfenidone at the time of screening or within 1 week of screening were excluded. Patients could be naive to treatment or may have received an antifibrotic treatment approved by the US Food and Drug Administration that was discontinued due to intolerance to therapy, disease progression, or patient choice. Protocol amendment 2.0 (September 2019), which took effect after 22 patients were enrolled in the study, allowed patients to receive nintedanib, pirfenidone, or both after the start of the study period for worsening respiratory functional status at the discretion of the site investigators.
Key exclusion criteria included significant obstructive lung disease (defined as ratio of forced expiratory volume in first second of expiration to FVC <0.70 or the extent of emphysema was greater than the extent of fibrosis on high-resolution computed tomography) or interstitial lung disease other than IPF, pregnancy, smoking within 3 months of screening, and sustained improvement in IPF severity within the 12 months prior to screening. All inclusion and exclusion criteria appear in the eMethods in Supplement 3.
Race and ethnicity information was collected as specified in the trial protocol (Supplement 1). Participants self-reported race from the provided categories of Asian, Black or African American, or White. Ethnicity was self-reported as Hispanic, Latino, or non-Hispanic.
The study was conducted in accordance with the International Conference on Harmonisation E6 Guideline for Good Clinical Practice, the Declaration of Helsinki, any other applicable regulatory requirements, and institutional review board or independent ethics committee requirements at each site. All patients provided written, informed consent prior to enrollment.
Efficacy Outcomes
The primary outcome was the absolute change in FVC from baseline that was measured every 6 to 12 weeks with pulmonary function tests until week 48. The secondary outcomes included (1) time to disease progression (defined as a decline of ≥10% in predicted FVC or death); (2) time to any component of the clinical composite outcome (adjudicated acute exacerbation of IPF, respiratory hospitalization, or death); (3) change in quantitative lung fibrosis volume (assessed via machine learning) from baseline to week 48; (4) time to first adjudicated acute exacerbation of IPF; (5) time to all-cause mortality; and (6) time to first respiratory hospitalization.
Exploratory Outcomes
The exploratory outcomes included time to composite respiratory hospitalization, a decline of 10% or greater in predicted FVC, or death; change in predicted FVC from baseline to week 48; and change in relative predicted FVC from baseline to week 48. In addition, patient-reported outcomes were assessed by measuring changes from baseline at week 48 for the St George’s Respiratory Questionnaire score, the University of California San Diego–Shortness of Breath Questionnaire score, and the Leicester Cough Questionnaire score.
Safety Analysis
The incidence and severity of treatment-emergent adverse events and treatment-emergent serious adverse events were summarized by treatment group. Clinically significant changes from baseline in vital signs and laboratory tests were identified and summarized. Hypersensitivity, anaphylactic, and immunogenicity reactions were monitored and assessed. All adverse events were adjudicated by a data and safety monitoring board that met approximately every 6 months to review safety data and provide input and feedback to the study sponsor (FibroGen) and the investigators.
Statistical Analysis
We aimed to enroll 340 patients, which would provide 90% power based on a 2-sided α level of .05 for a 2-sample t test to detect a 120-mL difference in FVC from baseline. This difference was chosen to represent the expected approximate 150-mL annual decline observed in the natural course of the disease while accommodating for the likelihood of background therapy.23 The differences in FVC from baseline were compared between the treatment and placebo groups. Additional information appears in the statistical analysis plan in Supplement 2.
The primary efficacy outcome was assessed using a mixed model for the repeated measures approach with fixed effects for treatment, visit (as a factor), treatment × visit interaction, randomization stratification factors, and covariates (baseline values, sex, age, race, and height). The mixed model for the repeated measures approach was also used for the secondary outcomes of pulmonary function test variables and other continuous variables. A Cox regression model was used for time to event variables.
All assessments collected were considered for analysis regardless of whether the data were collected during treatment or after a patient discontinued treatment. All analyses assumed that any missing data were missing at random unless otherwise stated. Missing start and stop dates for prior and concomitant medications, procedures, nondrug therapies, and adverse events were imputed. The worst observed values after baseline were applied for all death analyses. Any adverse events with a missing relationship were imputed as related. Safety data were summarized descriptively.
All statistical analyses were performed using SAS version 9.3 or higher (SAS Institute Inc). The treatment effects were summarized with point estimates and 95% CIs. All P values were 2-sided and no adjustment was made for multiple comparisons.
Results
Patient Disposition
Between July 2019 and April 2022, 612 patients with IPF were screened for inclusion and 356 were randomized (181 [50.8%] in the pamrevlumab group vs 175 [49.2%] in the placebo group; Figure 1 and Table 1). Among 356 patients (mean age, 70.5 years; 258 [72.5%] were men; 221 [62.1%] were White), 277 (77.8%) completed the trial. All enrolled patients received at least 1 dose of the study drug. The last follow-up encounter occurred on August 28, 2023.
Table 1. Demographics and Baseline Clinical Characteristics.
| Pamrevlumab (n = 181) | Placebo (n = 175) | |
|---|---|---|
| Age group, No. (%) | ||
| ≤64 y | 41 (22.7) | 28 (16.0) |
| 65-74 y | 77 (42.5) | 94 (53.7) |
| ≥75 y | 63 (34.8) | 53 (30.3) |
| Sex, No. (%) | ||
| Male | 132 (72.9) | 126 (72.0) |
| Female | 49 (27.1) | 49 (28.0) |
| Race, No. (%) | ||
| Asian | 63 (34.8) | 69 (39.4) |
| Black or African American | 0 | 1 (0.6) |
| White | 117 (64.6) | 104 (59.4) |
| Othera | 1 (0.6) | 1 (0.6) |
| Hispanic or Latino ethnicity, No. (%) | 54 (29.8) | 44 (25.1) |
| Time since first diagnosis of idiopathic pulmonary fibrosis, median (range), y | 2.1 (0.01-7.3) | 2.1 (0.01-7.2) |
| Tobacco smoking status, No. (%) | ||
| Currently smoke | 0 | 0 |
| Formerly smoked | 128 (70.7) | 110 (62.9) |
| Never smoked | 53 (29.3) | 65 (37.1) |
| Prior therapy for idiopathic pulmonary fibrosis, No. (%) | ||
| Pirfenidone alone | 66 (36.5) | 61 (34.9) |
| Nintedanib alone | 17 (9.4) | 15 (8.6) |
| Pirfenidone and nintedanib | 16 (8.8) | 18 (10.3) |
| Pulmonary function, mean (SD) | ||
| Forced vital capacity, mL | 2367 (618.5) | 2416 (645.3) |
| Predicted forced vital capacity, % | 69.6 (11.6) | 71.8 (11.5) |
| Predicted diffusing capacity of the lungs for carbon monoxide, % | 49.9 (14.1) | 50.0 (14.2) |
| Ratio of FEV1 to forced vital capacity | 0.8 (0.06) | 0.8 (0.06) |
| Gender-Age-Pulmonary function stage, No. (%)b | ||
| I (0-3 points) | 66 (36.5) | 66 (37.7) |
| II (4-5 points) | 105 (58.0) | 96 (54.9) |
| III (6-8 points) | 10 (5.5) | 13 (7.4) |
| Quantitative lung fibrosis volume, mean (SD), mLc | 568.7 (267.98) | 587.7 (306.10) |
| Patient-reported outcomes, mean (SD) | ||
| St George’s Respiratory Questionnaire scored | 50.3 (20.11) | 47.3 (19.81) |
| University of California San Diego–Shortness of Breath Questionnaire scoree | 38.5 (24.7) | 33.1 (24.3) |
| Leicester Cough Questionnaire scoref | 15.1 (4.20) | 15.0 (3.71) |
Abbreviation: FEV1, forced expiratory volume in the first second of expiration.
The patient in the pamrevlumab group listed Native Bolivian as race and the patient in the placebo group listed Hispanic.
This validated, multidimensional, prognostic staging system uses 4 commonly measured clinical and physiological variables (sex, age, and 2 lung physiological variables [forced vital capacity and diffusing capacity of the lungs for carbon monoxide]). The total score ranges from 0 to 8; higher scores indicate worse prognoses. Stage I indicates that the disease may require aggressive symptom management, but not immediate lung transplant. Stage II indicates that lung transplant may be considered based on patient preferences and disease progression. Stage III indicates a need for an immediate listing for lung transplant, palliative care, or both.
This computer-assisted quantitative scoring system measures the degree and progression of lung fibrosis viewed by high-resolution computed tomography. Volumes range from 0 mL to maximum lung volume; greater volumes represent increased fibrosis.
The score range is from 0 to 100; lower scores indicate better health and fewer symptoms. A score between 25 and 50 indicates moderate impairment. A score greater than 50 indicates severe impairment in health-related quality of life and the ability to complete activities of daily living.
The score range is from 0 to 120; lower scores indicate lower severity of shortness of breath. A score between 20 and 60 indicates moderate shortness of breath. A score of 60 or greater indicates severe shortness of breath that would have an effect on daily living and health-related quality of life.
The score range is from 3 to 21; lower scores indicate greater health impairment due to cough. A score between 14 and 17 indicates moderate impairment due to cough. A score greater than 17 indicates severe impairment that would have an effect on daily living and health-related quality of life.
Of enrolled patients, 277 (77.8%) completed 48 weeks (146 [80.7%] in the pamrevlumab group vs 131 [74.9%] in the placebo group). More than half of the patients had previously received approved treatment for IPF with either nintedanib or pirfenidone (Table 1). In the pamrevlumab group, 99 patients (54.7%) had received antifibrotic therapy (66 [36.5%] had received pirfenidone alone, 17 [9.4%] had received nintedanib alone, and 16 [8.8%] had received pirfenidone and nintedanib). In the placebo group, 94 patients (53.7%) had received antifibrotic therapy (61 [34.9%] had received pirfenidone alone, 15 [8.6%] had received nintedanib alone, and 18 [10.3%] had received pirfenidone and nintedanib).
Additional prior and concomitant medication use appears in eTables 1 and 2 in Supplement 3. There were 30 important protocol deviations in the pamrevlumab group (16.6%) vs 26 in the placebo group (14.9%) (eTable 3 in Supplement 3). No important protocol deviations were related to COVID-19. There were 56 patients (30.9%) in the pamrevlumab group who missed 1 or more infusions vs 65 (37.1%) in the placebo group; 27 (14.9%) vs 23 (13.1%), respectively, missed infusions due to COVID-19.
The treatment groups were well balanced in terms of demographics and clinical characteristics (Table 1). In the pamrevlumab group, the mean age was 70.2 years (SD, 7.8 years) and 132 patients (72.9%) were men; the mean baseline FVC was 2367 mL (SD, 619 mL). In the placebo group, the mean age was 70.8 years (SD, 7.0 years) and 126 patients (72.0%) were men; the mean baseline FVC was 2416 mL (SD, 645 mL).
During the study period, antifibrotic treatment was added to the study medication for 35 patients (19.3%) in the pamrevlumab group vs 27 (15.4%) in the placebo group. In the pamrevlumab group, pirfenidone alone was added for 17 patients (9.4%), nintedanib alone was added for 15 (8.3%), and pirfenidone and nintedanib were added for 3 (1.7%). In the placebo group, pirfenidone alone was added for 14 patients (8.0%), nintedanib alone was added for 9 (5.1%), and pirfenidone and nintedanib were added for 4 (2.3%). The time to the addition of antifibrotic treatment was not analyzed.
Primary Efficacy Outcome
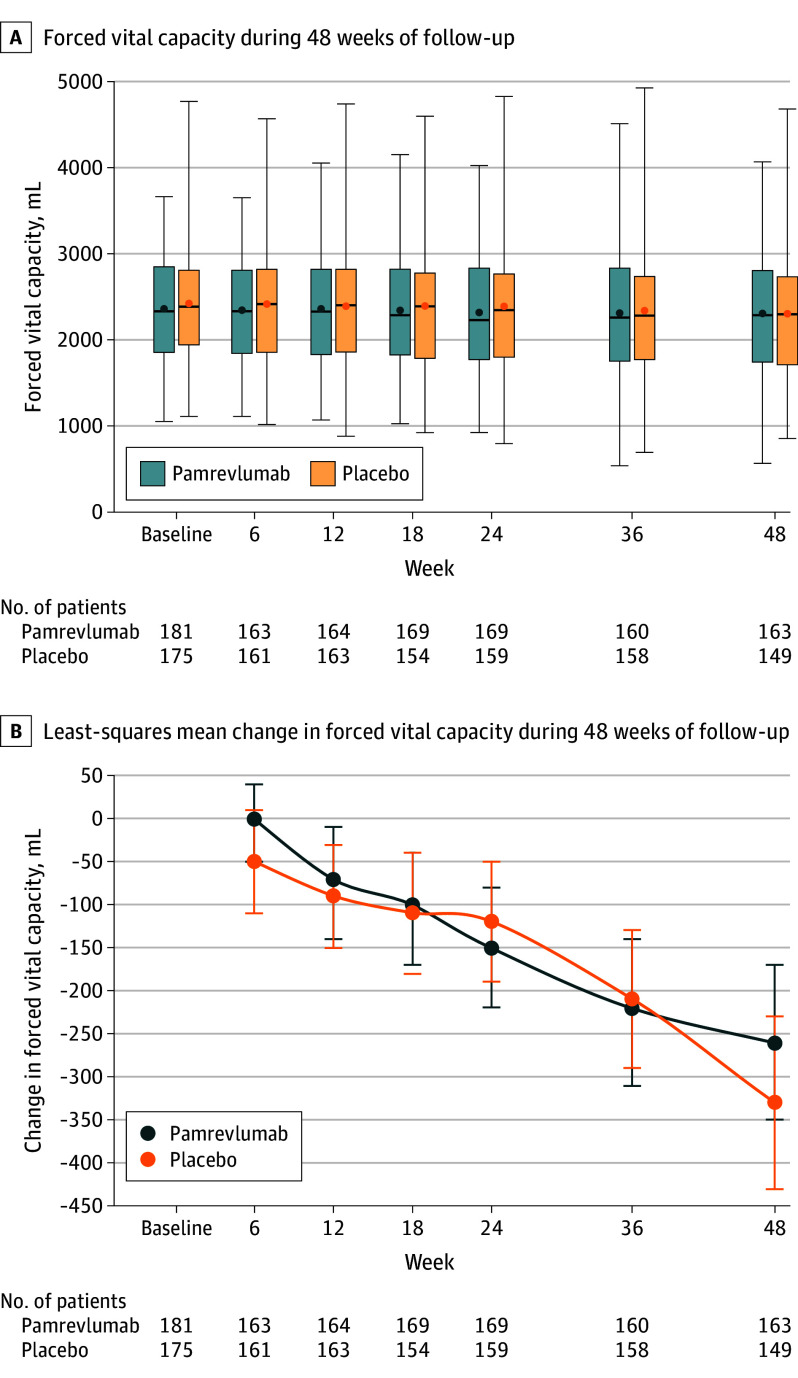
In the pamrevlumab group, absolute FVC decreased from a mean of 2367 mL (SD, 618.5 mL) at baseline to 2310 mL (SD, 692.7 mL) at week 48 vs from 2416 mL (SD, 645.3 mL) at baseline to 2308 mL (SD, 737.5 mL) at week 48 in the placebo group. The least-squares mean decline in FVC was similar in both groups (−260 mL [95% CI, −350 to −170 mL] in the pamrevlumab group vs −330 mL [95% CI, −430 to −230 mL] in the placebo group; mean between-group difference, 70 mL [95% CI, −60 to 190 mL], P = .29) (Figure 2). There were no significant between-group differences in the subgroups stratified by prior use of approved IPF treatment, use of nintedanib or pirfenidone during the study, or GAP function stage (eTable 4 and eFigure 2 in Supplement 3), for the tests of between-group interactions (eTable 5 in Supplement 3), or for the analyses of observed vs imputed data (eTable 6 in Supplement 3).
Figure 2. Change in Forced Vital Capacity From Baseline to Week 48 (Primary Outcome).
A, The boxes represent the IQRs, the horizontal lines within the boxes represent the medians, the error bars extending from the boxes indicate the minimum and maximum values, and the dots represent the means. B, The error bars represent the 95% CIs.
Secondary Efficacy and Exploratory Outcomes
No significant between-group differences were found for any of the secondary efficacy or exploratory outcomes measured at week 48 (Table 2 and Table 3).
Table 2. Secondary Outcomes Representing Disease Progressiona.
| Secondary outcomes | Patients who experienced event | Absolute between-group difference in incident rates, % (95% CI) | Hazard ratio (95% CI) |
P value for between-group difference | |||
|---|---|---|---|---|---|---|---|
| Pamrevlumab (n = 181) | Placebo (n = 175) | ||||||
| No. (%) | Time to event, median (95% CI), wk |
No. (%) | Time to event, median (95% CI), wk |
||||
| Disease progressionb | 49 (27.1) | 54.3 (52.7 to NE) | 56 (32.0) | 50.7 (50.3 to 54.0) | –4.9 (–14.4 to 4.5) | 0.78 (0.52 to 1.15) | .20 |
| Any component of the clinical composite outcomec | 40 (22.1) | 62.7 (59.0 to NE) | 45 (25.7) | NE (57.9 to NE) | –3.6 (–12.5 to 5.2) | 0.84 (0.54 to 1.29) | .43 |
| First acute idiopathic pulmonary fibrosis exacerbation | 18 (9.9) | 62.7 (62.7 to NE) | 15 (8.6) | NE (NE to NE) | 1.4 (–4.6 to 7.4) | 1.13 (0.56 to 2.30) | .72 |
| All-cause mortality | 23 (12.7) | NE (60.6 to NE) | 23 (13.1) | NE (60.1 to NE) | –0.4 (–7.4 to 6.5) | 1.00 (0.56 to 1.79) | >.99 |
| First hospitalization for respiratory reason | 28 (15.5) | 62.7 (59.0 to NE) | 37 (21.1) | NE (57.9 to NE) | –5.7 (–13.7 to 2.3) | 0.68 (0.41 to 1.12) | .13 |
Abbreviation: NE, not evaluable (insufficient number of events occurred to accurately calculate the medians or 95% CIs).
A mixed model for repeated measures using SAS software was used for all statistical analyses.
Defined as a decline of 10% or greater in predicted forced vital capacity or death.
Acute idiopathic pulmonary fibrosis exacerbation, hospitalization for respiratory reason, or death.
Table 3. Exploratory Outcomes Representing Health-Related Quality of Life.
| Exploratory outcomes | Patients with measurement | Between-group difference | ||||
|---|---|---|---|---|---|---|
| Pamrevlumab (n = 181) | Placebo (n = 175) | Absolute difference (95% CI) | P value | |||
| No. (%) | Change in outcome, least-squares mean (SD) |
No (%) | Change in outcome, least-squares mean (SD) |
|||
| Quantitative lung fibrosis volume, mLa | 157 (86.7) | 251.4 (478.14) | 143 (81.7) | 268.0 (465.87) | −16.58 (−103.21 to 70.05) | .71 |
| St George’s Respiratory Questionnaire scoreb | 153 (84.5) | 11.3 (25.47) | 133 (76.0) | 12.1 (24.86) | −0.72 (−5.44 to 3.99) | .76 |
| University of California San Diego–Shortness of Breath Questionnaire scorec | 147 (81.2) | 19.6 (34.51) | 129 (73.7) | 20.5 (34.00) | −0.91 (−7.40 to 5.59) | .78 |
| Leicester Cough Questionnaire scored | 154 (85.1) | −2.4 (6.00) | 137 (78.3) | −2.7 (5.78) | 0.36 (−0.73 to 1.46) | .52 |
This computer-assisted quantitative scoring system measures the degree and progression of lung fibrosis viewed by high-resolution computed tomography. Volumes range from 0 mL to maximum lung volume; greater volumes represent increased fibrosis.
The score range is from 0 to 100; lower scores indicate better health and fewer symptoms. A score between 25 and 50 indicates moderate impairment. A score greater than 50 indicates severe impairment in health-related quality of life and the ability to complete activities of daily living.
The score range is from 0 to 120; lower scores indicate lower severity of shortness of breath. A score between 20 and 60 indicates moderate shortness of breath. A score of 60 or greater indicates severe shortness of breath that would have an effect on daily living and health-related quality of life.
The score range is from 3 to 21; lower scores indicate greater health impairment due to cough. A score between 14 and 17 indicates moderate impairment due to cough. A score greater than 17 indicates severe impairment that would have an effect on daily living and health-related quality of life.
Safety
Adverse events were similar between the groups (Table 4). Of the patients who received pamrevlumab, 160 (88.4%) experienced treatment-emergent adverse events and 51 (28.2%) experienced treatment-emergent serious adverse events; 1 event (0.6%) was determined to be related to the study treatment. Similarly, 151 patients (86.3%) in the placebo group experienced treatment-emergent adverse events and 60 (34.3%) experienced treatment-emergent serious adverse events; no events were determined to be related to the study treatment.
Table 4. Treatment-Emergent Adverse Events (TEAEs).
| No. (%) | ||
|---|---|---|
| Pamrevlumab (n = 181) | Placebo (n = 175) | |
| Any TEAEsa | 160 (88.4) | 151 (86.3) |
| Leading to treatment discontinuation | 1 (0.6) | 7 (4.0) |
| Leading to treatment interruption | 11 (6.1) | 11 (6.3) |
| Related to study treatment | 20 (11.0) | 25 (14.3) |
| Serious TEAEsb | 51 (28.2) | 60 (34.3) |
| Leading to treatment discontinuation | 1 (0.6) | 4 (2.3) |
| Leading to treatment interruption | 4 (2.2) | 9 (5.1) |
| Related to study treatment | 1 (0.6) | 0 |
| Maximum severity of TEAEs | ||
| Mild | 40 (22.1) | 27 (15.4) |
| Moderate | 69 (38.1) | 69 (39.4) |
| Severe | 32 (17.7) | 33 (18.9) |
| Life-threatening | 3 (1.7) | 7 (4.0) |
| Died | 16 (8.8) | 15 (8.6) |
| Most common TEAEs | ||
| Cough | 37 (20.4) | 25 (14.3) |
| Idiopathic pulmonary fibrosis | 28 (15.5) | 30 (17.1) |
| COVID-19 | 23 (12.7) | 17 (9.7) |
| Dyspnea | 19 (10.5) | 16 (9.1) |
| Bronchitis | 14 (7.7) | 14 (8.0) |
| Headache | 13 (7.2) | 12 (6.9) |
| Pneumonia | 13 (7.2) | 14 (8.0) |
| Fatigue | 12 (6.6) | 10 (5.7) |
| Hypertension | 11 (6.1) | 5 (2.9) |
| Upper respiratory tract infection | 11 (6.1) | 6 (3.4) |
| Diarrhea | 10 (5.5) | 11 (6.3) |
| Dizziness | 10 (5.5) | 7 (4.0) |
| Nasopharyngitis | 10 (5.5) | 6 (3.4) |
| Weight loss | 6 (3.3) | 11 (6.3) |
Occurred on or after the first dose of study drug and within 60 days after the last dose.
Any adverse event or suspected adverse reaction that resulted in any of the following outcomes: death, a life-threatening adverse event, an inpatient hospitalization or prolongation of existing hospitalization, a persistent or significant incapacity or substantial disruption in the ability to conduct normal life functions, a congenital anomaly or birth defect, and any event considered medically important but not meeting the above criteria and may have required a medical or surgical intervention.
Most of the adverse events reported were mild in severity (40 events [22.1%] in the pamrevlumab group vs 27 [15.4%] in the placebo group) or moderate (69 [38.1%] in the pamrevlumab group vs 69 [39.4%] in the placebo group). Most of the common adverse events were consistent with the known clinical course of IPF. Twenty-three patients died in each group (12.7% in the pamrevlumab group vs 13.1% in the placebo group). Of these 46 deaths, 31 (8.7%) occurred within the 60 days after the last dose of study drug (16 [8.8%] in the pamrevlumab group vs 15 [8.6%] in the placebo group) and 15 (4.2%) occurred beyond 60 days after the last dose (7 [3.9%] in the pamrevlumab group vs 8 [4.6%] in the placebo group). No deaths were deemed to be related to the study drug.
At baseline, 2 patients (1.2%) in the pamrevlumab group and none in the placebo group had antidrug antibodies. At the last assessment (28 days after the last dose of study drug), no patients in the pamrevlumab group and 1 patient (0.6%) in the placebo group had treatment-emergent antidrug antibodies.
Discussion
An intravenous dose of pamrevlumab (30 mg/kg every 3 weeks) for 48 weeks did not significantly decrease the rate of decline in FVC compared with placebo in patients with IPF. This study did not meet its primary outcome. Pamrevlumab also did not show significant benefit in any of the secondary or exploratory outcomes. Patients treated with pamrevlumab had no increased frequency of adverse events compared with placebo, and the adverse event profile in the current study was consistent with published reports.19,20 Based on the results of the current study, the planned open-label extension was terminated as well as its companion trial (ZEPHYRUS-2; NCT04419558), which was ongoing.
A decline in FVC represents disease progression and is the most common outcome used in clinical trials of IPF.24,25 Based on phase 2 study results, pamrevlumab was expected to result in less lung function decline and slower disease progression (as measured by FVC) compared with placebo. In the current study, patients in the pamrevlumab group experienced a decrease in FVC of 260 mL compared with 330 mL in the placebo group. Even though the decrease in FVC in the pamrevlumab group was numerically less than in the placebo group, the difference was not significant. The change in the pamrevlumab group is consistent with the loss of lung function observed in previous placebo-controlled phase 2 trials, though the difference was significant in those studies.19,20
Although the reasons why the current study failed to replicate prior results of phase 2 trials of pamrevlumab are unclear, the following factors may have played a role in the observed rate of disease progression. First, the phase 2 studies included smaller cohorts of patients (n = 9019 and n = 10320 vs n = 356 in the current trial). Second, the patients included in the current trial had more lung function impairment (as measured by FVC, diffusing capacity of the lungs for carbon monoxide, and GAP stage), more severe symptoms at baseline (as measured by the St George’s Respiratory Questionnaire and the University of California San Diego–Shortness of Breath Questionnaire), and a longer interval from diagnosis of IPF and study enrollment than patients in the previous studies.19,20 Third, the patients in the current trial were allowed to begin treatment with pirfenidone or nintedanib during the study period if the rate of FVC declined, whereas no additional antifibrotic treatment was allowed in the phase 2 studies19,20 (the studies were placebo-controlled throughout their durations).
Because the COVID-19 pandemic was unforeseen, an analysis of the outcomes for patients affected by COVID-19 as a prespecified subgroup was not possible. During the course of the current study, no COVID-19–related trial protocol deviations were reported and a similar percentage of patients in each treatment group reported COVID-19 as a treatment-emergent adverse event or as a reason for missed treatments. However, patients were allowed to receive infusions of the study drug at home via a qualified home health care service, which likely improved adherence and willingness to continue treatment during the pandemic.
Several phase 2 and 3 studies of IPF and related lung diseases conducted during the COVID-19 pandemic were terminated early due to lack of enrollment or futility.26,27,28,29,30 In contrast to the ISABELA 1 and 2 trials, which were phase 3, randomized clinical trials,27 the current trial did not find an increase in mortality with use of pamrevlumab. The ISABELA 1 and 2 trials,27 which included 1306 patients with IPF, reported increased mortality with ziritaxestat (an autotaxin inhibitor that showed safety and efficacy in phase 2 trials) compared with placebo; both trials were terminated early. The differences observed between the phase 2 and phase 3 trials serve as a reminder that it is important that patient cohorts enrolled in phase 2 and phase 3 trials should be similar and reflect the general population of IPF.
Because of the high mortality rates associated with IPF, new treatment options are urgently needed. Considering the ethical need to allow approved antifibrotic medications as an evolved standard of care for patients with IPF, the expectation of slowed disease progression (as defined by the rate of FVC decline) alone may be insufficient to capture all clinically meaningful changes in patients with IPF.31 Treatment response in future trials of IPF should consider measuring multiple outcomes (eg, change in FVC, respiratory hospitalizations, mortality, and patient-reported outcomes) to assess treatment efficacy according to how the patient feels, functions, and survives.23,24,32
The current trial had several strengths, including its study design as a randomized clinical trial that included a large and diverse population of patients with IPF. In addition, attrition was low (most patients completed the entire 48-week study period and had available data for analysis). Another strength was the use of machine learning to quantify imaging of fibrotic tissue as a prespecified outcome. Furthermore, a mixed model for repeated measures approach was used in the data analysis that mitigates the effect of missing data. The analyses aligned with US Food and Drug Administration recommendations that advocate use of patient-reported and quality-of-life outcomes in addition to objective measures of disease progression in patients with IPF.33,34,35,36
Limitations
The results of this study should be interpreted in the context of its limitations. First, the prespecified protocol allowance for the site investigators to add antifibrotic treatments to pamrevlumab during the study period may have affected the changes in disease progression.
Second, the population included had a longer duration of IPF and more advanced disease than the phase 2 trials of pamrevlumab. Third, the desired absolute change of 10% or greater in FVC as a stand-alone outcome should be interpreted with caution in the context of concomitant antifibrotic use.
Conclusions
Among patients with IPF treated with pamrevlumab or placebo, there was no statistically significant between-group difference for the primary outcome of absolute change in FVC from baseline to week 48.
Educational Objective: To identify the key insights or developments described in this article.
-
Why do the authors suggest that the current standard treatments (the antifibrotic drugs pirfenidone and nintedanib) for idiopathic pulmonary fibrosis are relatively ineffective?
The requirement for daily intravenous infusion makes for a costly and inconvenient approach that few patients can tolerate for long.
Up to 40% of patients discontinue therapy because of gastrointestinal, hepatic, and dermatologic adverse effects.
Even though patients experience improved quality of life on these medications, the approach does not alter the rate of lung function decline.
-
What was the result for the primary outcome of change in forced vital capacity (FVC) at 48 weeks?
The mean decline in FVC was similar in both the pamrevlumab group and the placebo group.
Neither the pamrevlumab group nor the placebo group experienced the anticipated decline in FVC.
The pamrevlumab group experienced statistically and clinically significant slowing of FVC decline throughout the treatment period.
-
Why do the authors suggest this phase 3 trial failed to replicate findings from a prior phase 2 trial of pamrevlumab?
Despite efforts to emphasize social distancing and other COVID-19 precautions, the rates of COVID-19 infection differed markedly between the pamrevlumab group and the placebo group.
Increased mortality with pamrevlumab in this trial obscured any potential benefit observed in prior smaller trials.
Participants in this trial had more severe symptoms at baseline and more lung function impairment than participants in prior trials.
Trial protocol
Statistical analysis plan
eMethods
eFigure 1. ZEPHYRUS-1 Study Schema
eFigure 2. Change from Baseline in Forced Vital Capacity (mL): Subgroup Analyses
eTable 1. Prior Medications in the Main Study Cohort
eTable 2. Concomitant Medications for the Main Study Cohort
eTable 3. Important Protocol Deviations by Treatment
eTable 4. Change from Baseline in Forced Vital Capacity (mL): Subgroup Analyses
eTable 5. Change from Baseline in Forced Vital Capacity (mL) at Week 48: Interactions Between Stratification Groups
eTable 6. Change from Baseline in Forced Vital Capacity (mL) at Week 48: Observed vs. Imputed Data
eReference
Nonauthor collaborators
Data sharing statement
References
- 1.Maher TM, Bendstrup E, Dron L, et al. Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir Res. 2021;22(1):197. doi: 10.1186/s12931-021-01791-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghu G, Collard HR, Egan JJ, et al. ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis . An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788-824. doi: 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Podolanczuk AJ, Thomson CC, Remy-Jardin M, et al. Idiopathic pulmonary fibrosis: state of the art for 2023. Eur Respir J. 2023;61(4):2200957. doi: 10.1183/13993003.00957-2022 [DOI] [PubMed] [Google Scholar]
- 4.de Andrade JA, Neely ML, Hellkamp AS, et al. ; IPF-PRO Registry investigators . Effect of antifibrotic therapy on survival in patients with idiopathic pulmonary fibrosis. Clin Ther. 2023;45(4):306-315. doi: 10.1016/j.clinthera.2023.03.003 [DOI] [PubMed] [Google Scholar]
- 5.Behr J, Prasse A, Wirtz H, et al. Survival and course of lung function in the presence or absence of antifibrotic treatment in patients with idiopathic pulmonary fibrosis: long-term results of the INSIGHTS-IPF registry. Eur Respir J. 2020;56(2):1902279. doi: 10.1183/13993003.02279-2019 [DOI] [PubMed] [Google Scholar]
- 6.King TE Jr, Bradford WZ, Castro-Bernardini S, et al. ; ASCEND Study Group . A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083-2092. doi: 10.1056/NEJMoa1402582 [DOI] [PubMed] [Google Scholar]
- 7.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071-2082. doi: 10.1056/NEJMoa1402584 [DOI] [PubMed] [Google Scholar]
- 8.Raghu G, Rochwerg B, Zhang Y, et al. ; American Thoracic Society; European Respiratory Society; Japanese Respiratory Society; Latin American Thoracic Association . An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis: an update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192(2):e3-e19. doi: 10.1164/rccm.201506-1063ST [DOI] [PubMed] [Google Scholar]
- 9.Cameli P, Refini RM, Bergantini L, et al. Long-term follow-up of patients with idiopathic pulmonary fibrosis treated with pirfenidone or nintedanib: a real-life comparison study. Front Mol Biosci. 2020;7:581828. doi: 10.3389/fmolb.2020.581828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koudstaal T, Wijsenbeek MS. Idiopathic pulmonary fibrosis. Presse Med. 2023;52(3):104166. doi: 10.1016/j.lpm.2023.104166 [DOI] [PubMed] [Google Scholar]
- 11.Rajesh R, Atallah R, Bärnthaler T. Dysregulation of metabolic pathways in pulmonary fibrosis. Pharmacol Ther. 2023;246:108436. doi: 10.1016/j.pharmthera.2023.108436 [DOI] [PubMed] [Google Scholar]
- 12.Trachalaki A, Sultana N, Wells AU. An update on current and emerging drug treatments for idiopathic pulmonary fibrosis. Expert Opin Pharmacother. 2023;24(10):1125-1142. doi: 10.1080/14656566.2023.2213436 [DOI] [PubMed] [Google Scholar]
- 13.Takehara K, Koga Y, Hachisu Y, et al. Differential discontinuation profiles between pirfenidone and nintedanib in patients with idiopathic pulmonary fibrosis. Cells. 2022;11(1):143. doi: 10.3390/cells11010143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corral M, DeYoung K, Kong AM. Treatment patterns, healthcare resource utilization, and costs among patients with idiopathic pulmonary fibrosis treated with antifibrotic medications in US-based commercial and Medicare supplemental claims databases: a retrospective cohort study. BMC Pulm Med. 2020;20(1):188. doi: 10.1186/s12890-020-01224-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogura T, Inoue Y, Azuma A, et al. Real-world safety and tolerability of nintedanib in patients with idiopathic pulmonary fibrosis: interim report of a post-marketing surveillance in Japan. Adv Ther. 2023;40(4):1474-1493. doi: 10.1007/s12325-022-02411-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maher TM, Strek ME. Antifibrotic therapy for idiopathic pulmonary fibrosis: time to treat. Respir Res. 2019;20(1):205. doi: 10.1186/s12931-019-1161-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipson KE, Wong C, Teng Y, Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012;5(suppl 1):S24. doi: 10.1186/1755-1536-5-S1-S24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saito S, Deskin B, Rehan M, et al. Novel mediators of idiopathic pulmonary fibrosis. Clin Sci (Lond). 2022;136(16):1229-1240. doi: 10.1042/CS20210878 [DOI] [PubMed] [Google Scholar]
- 19.Raghu G, Scholand MB, de Andrade J, et al. FG-3019 anti-connective tissue growth factor monoclonal antibody: results of an open-label clinical trial in idiopathic pulmonary fibrosis. Eur Respir J. 2016;47(5):1481-1491. doi: 10.1183/13993003.01030-2015 [DOI] [PubMed] [Google Scholar]
- 20.Richeldi L, Fernández Pérez ER, Costabel U, et al. Pamrevlumab, an anti-connective tissue growth factor therapy, for idiopathic pulmonary fibrosis (PRAISE): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2020;8(1):25-33. doi: 10.1016/S2213-2600(19)30262-0 [DOI] [PubMed] [Google Scholar]
- 21.Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156(10):684-691. doi: 10.7326/0003-4819-156-10-201205150-00004 [DOI] [PubMed] [Google Scholar]
- 22.Raghu G, Remy-Jardin M, Myers JL, et al. ; American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society . Diagnosis of idiopathic pulmonary fibrosis: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44-e68. doi: 10.1164/rccm.201807-1255ST [DOI] [PubMed] [Google Scholar]
- 23.Raghu G. Idiopathic pulmonary fibrosis: lessons from clinical trials over the past 25 years. Eur Respir J. 2017;50(4):1701209. doi: 10.1183/13993003.01209-2017 [DOI] [PubMed] [Google Scholar]
- 24.Raghu G, Collard HR, Anstrom KJ, et al. Idiopathic pulmonary fibrosis: clinically meaningful primary endpoints in phase 3 clinical trials. Am J Respir Crit Care Med. 2012;185(10):1044-1048. doi: 10.1164/rccm.201201-0006PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karimi-Shah BA, Chowdhury BA. Forced vital capacity in idiopathic pulmonary fibrosis–FDA review of pirfenidone and nintedanib. N Engl J Med. 2015;372(13):1189-1191. doi: 10.1056/NEJMp1500526 [DOI] [PubMed] [Google Scholar]
- 26.Armstrong M. Feb 2 Quick Takes: Roche’s pipeline cuts include IPF therapy from Promedior deal. Published February 2, 2024. Accessed May 1, 2024. https://www.biocentury.com/article/646715/feb-2-quick-takes-roche-s-pipeline-cuts-include-ipf-therapy-from-promedior-deal
- 27.Maher TM, Ford P, Brown KK, et al. ; ISABELA 1 and 2 Investigators . Ziritaxestat, a novel autotaxin inhibitor, and lung function in idiopathic pulmonary fibrosis: the ISABELA 1 and 2 randomized clinical trials. JAMA. 2023;329(18):1567-1578. doi: 10.1001/jama.2023.5355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ClinicalTrials.gov . An efficacy and safety study of BG00011 in participants with idiopathic pulmonary fibrosis. Published December 11, 2020. Accessed January 5, 2024. https://clinicaltrials.gov/study/NCT03573505
- 29.ClinicalTrials.gov . A phase 2b study of inhaled RVT-1601 for the treatment of persistent cough in IPF (SCENIC). Published June 11, 2020. Accessed January 5, 2024. https://clinicaltrials.gov/study/NCT03864328
- 30.Solomon JJ, Danoff SK, Woodhead FA, et al. ; TRAIL1 Network Investigators . Safety, tolerability, and efficacy of pirfenidone in patients with rheumatoid arthritis-associated interstitial lung disease: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet Respir Med. 2023;11(1):87-96. doi: 10.1016/S2213-2600(22)00260-0 [DOI] [PubMed] [Google Scholar]
- 31.Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2022;205(9):e18-e47. doi: 10.1164/rccm.202202-0399ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raghu G, Ghazipura M, Fleming TR, et al. Meaningful endpoints for idiopathic pulmonary fibrosis (IPF) clinical trials: emphasis on ‘feels, functions, survives’: report of a collaborative discussion in a symposium with direct engagement from representatives of patients, investigators, the National Institutes of Health, a patient advocacy organization, and a regulatory agency. Am J Respir Crit Care Med. 2024;209(6):647-669. doi: 10.1164/rccm.202312-2213SO [DOI] [PubMed] [Google Scholar]
- 33.Guidance for industry on patient-reported outcome measures: use in medical product development to support labeling claims; availability. Fed Regist. 2009;74(235):65132-65133. [Google Scholar]
- 34.US Food and Drug Administration . Guidance for industry and FDA staff: qualification process for drug development tools. Published November 2020. Accessed January 5, 2024. https://www.fda.gov/media/133511/download
- 35.Swigris J, Cutts K, Male N, Baldwin M, Rohr KB, Bushnell DM. The Living with Pulmonary Fibrosis questionnaire in progressive fibrosing interstitial lung disease. ERJ Open Res. 2021;7(2):00145-02020. doi: 10.1183/23120541.00145-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swigris JJ, Bushnell DM, Rohr K, Mueller H, Baldwin M, Inoue Y. Responsiveness and meaningful change thresholds of the Living with Pulmonary Fibrosis (L-PF) questionnaire dyspnoea and cough scores in patients with progressive fibrosing interstitial lung diseases. BMJ Open Respir Res. 2022;9(1):e001167. doi: 10.1136/bmjresp-2021-001167 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eMethods
eFigure 1. ZEPHYRUS-1 Study Schema
eFigure 2. Change from Baseline in Forced Vital Capacity (mL): Subgroup Analyses
eTable 1. Prior Medications in the Main Study Cohort
eTable 2. Concomitant Medications for the Main Study Cohort
eTable 3. Important Protocol Deviations by Treatment
eTable 4. Change from Baseline in Forced Vital Capacity (mL): Subgroup Analyses
eTable 5. Change from Baseline in Forced Vital Capacity (mL) at Week 48: Interactions Between Stratification Groups
eTable 6. Change from Baseline in Forced Vital Capacity (mL) at Week 48: Observed vs. Imputed Data
eReference
Nonauthor collaborators
Data sharing statement