Key Points
Question
Does acetaminophen (paracetamol) improve respiratory, circulatory, or kidney dysfunction in critically ill patients with sepsis, and is acetaminophen more effective in patients with higher plasma cell-free hemoglobin?
Findings
In this randomized clinical trial that included 447 adults, acetaminophen was safe but the number of days alive and free of organ support was not meaningfully different in the acetaminophen arm (20.2 days) vs the placebo arm (19.6 days). There was no significant interaction between cell-free hemoglobin levels and acetaminophen.
Meaning
Intravenous acetaminophen was safe but did not significantly improve days alive and free of organ support in critically ill patients with sepsis.
Abstract
Importance
Acetaminophen (paracetamol) has many pharmacological effects that might be beneficial in sepsis, including inhibition of cell-free hemoglobin-induced oxidation of lipids and other substrates.
Objective
To determine whether acetaminophen increases days alive and free of organ dysfunction in sepsis compared with placebo.
Design, Setting, and Participants
Phase 2b randomized, double-blind, clinical trial conducted from October 2021 to April 2023 with 90-day follow-up. Adults with sepsis and respiratory or circulatory organ dysfunction were enrolled in the emergency department or intensive care unit of 40 US academic hospitals within 36 hours of presentation.
Intervention
Patients were randomized to 1 g of acetaminophen intravenously every 6 hours or placebo for 5 days.
Main Outcome and Measures
The primary end point was days alive and free of organ support (mechanical ventilation, vasopressors, and kidney replacement therapy) to day 28. Treatment effect modification was evaluated for acetaminophen by prerandomization plasma cell-free hemoglobin level higher than 10 mg/dL.
Results
Of 447 patients enrolled (mean age, 64 [SD, 15] years, 51% female, mean Sequential Organ Failure Assessment [SOFA] score, 5.4 [SD, 2.5]), 227 were randomized to acetaminophen and 220 to placebo. Acetaminophen was safe with no difference in liver enzymes, hypotension, or fluid balance between treatment arms. Days alive and free of organ support to day 28 were not meaningfully different for acetaminophen (20.2 days; 95% CI, 18.8 to 21.6) vs placebo (19.6 days; 95% CI, 18.2 to 21.0; P = .56; difference, 0.6; 95% CI, −1.4 to 2.6). Among 15 secondary outcomes, total, respiratory, and coagulation SOFA scores were significantly lower on days 2 through 4 in the acetaminophen arm as was the rate of development of acute respiratory distress syndrome within 7 days (2.2% vs 8.5% acetaminophen vs placebo; P = .01; difference, −6.3; 95% CI, −10.8 to −1.8). There was no significant interaction between cell-free hemoglobin levels and acetaminophen.
Conclusions and Relevance
Intravenous acetaminophen was safe but did not significantly improve days alive and free of organ support in critically ill sepsis patients.
Trial Registration
ClinicalTrials.gov Identifier: NCT04291508
This randomized trial assesses whether acetaminophen would increase the number of days alive and free of organ support to day 28 among critically ill patients with sepsis and respiratory or circulatory organ dysfunction.
Introduction
Acetaminophen (paracetamol) has many pharmacological effects that might be beneficial in sepsis. In addition to analgesia, antipyresis, and cyclooxygenase-2 inhibition,1 acetaminophen is a potent and specific hemoprotein reductant that can block hemoglobin-induced oxidation of lipids and other substrates.2,3 Circulating cell-free hemoglobin levels are elevated in the majority of critically ill patients with sepsis and are independently associated with development of organ dysfunction including acute respiratory distress syndrome (ARDS) and death.4,5,6
In observational studies, receipt of acetaminophen was associated with improved survival in critically ill patients with sepsis with elevated plasma cell-free hemoglobin4 and in a large, critically ill, medical and surgical cohort.7 Acetaminophen is also tested in several, small clinical trials involving patients with sepsis. In the phase 2a Acetaminophen for Reduction of Oxidative Stress in Sepsis trial, which enrolled 40 patients with sepsis, organ dysfunction and plasma cell-free hemoglobin of 10 mg/dL or higher, the administration of enteral acetaminophen reduced plasma biomarkers of lipid peroxidation and improved kidney function compared with placebo.8 In another phase 2a trial, acetaminophen improved kidney function in patients with severe falciparum malaria compared with control.9 However, a large randomized trial of acetaminophen administration for treatment of fever in patients with suspected infection did not show a mortality benefit.10 Taken together, these findings support the need for a larger phase 2b clinical trial of acetaminophen in sepsis.
To address this need, the National Heart, Lung, and Blood Institute (NHLBI) Prevention and Early Treatment of Acute Lung Injury (PETAL) Network undertook the Acetaminophen and Ascorbate in Sepsis: Targeted Therapy to Enhance Recovery (ASTER) platform. This multicenter phase 2b randomized, double-blind, placebo-controlled, platform tested 2 different pharmacological therapies (intravenous vitamin C or intravenous acetaminophen) vs a common placebo in patients with sepsis and respiratory or circulatory organ dysfunction. Only the results of the acetaminophen trial are presented; the vitamin C arm was terminated early and the results will be presented elsewhere. The first objective was to test the hypothesis that acetaminophen would increase the number of days alive and free of organ support to day 28 among critically ill patients with sepsis and respiratory or circulatory organ dysfunction. The second objective was to test for a differential treatment effect of acetaminophen by preenrollment cell-free hemoglobin level to determine the utility of plasma cell-free hemoglobin level as a method for predictive enrichment in future clinical trials of acetaminophen.
Methods
Trial Design and Oversight
This phase 2b multicenter, randomized, double-blind trial of intravenous acetaminophen vs placebo was originally part of a 3-arm platform trial in which participants were randomized 1:1:1 to 1 of 2 treatments (intravenous acetaminophen or vitamin C) or a common placebo. On June 15, 2022, the vitamin C arm of the trial was stopped after enrolling 79 participants due to external clinical trial data for vitamin C.11 The acetaminophen arm of the trial was restarted on August 22, 2022. The trial was conducted under an investigational new drug application with the US Food and Drug Administration and was overseen by an independent data and safety monitoring board appointed by the NHLBI and by a central institutional review board. There was a single interim analysis evaluating efficacy and futility. The NHLBI PETAL Network coordinating center managed and analyzed all trial data. The writing committee drafted the manuscript and vouched for the accuracy and completeness of the data and for the adherence of the trial to the protocol. Written informed consent was obtained from all study subjects or their legally authorized representatives.
Patients
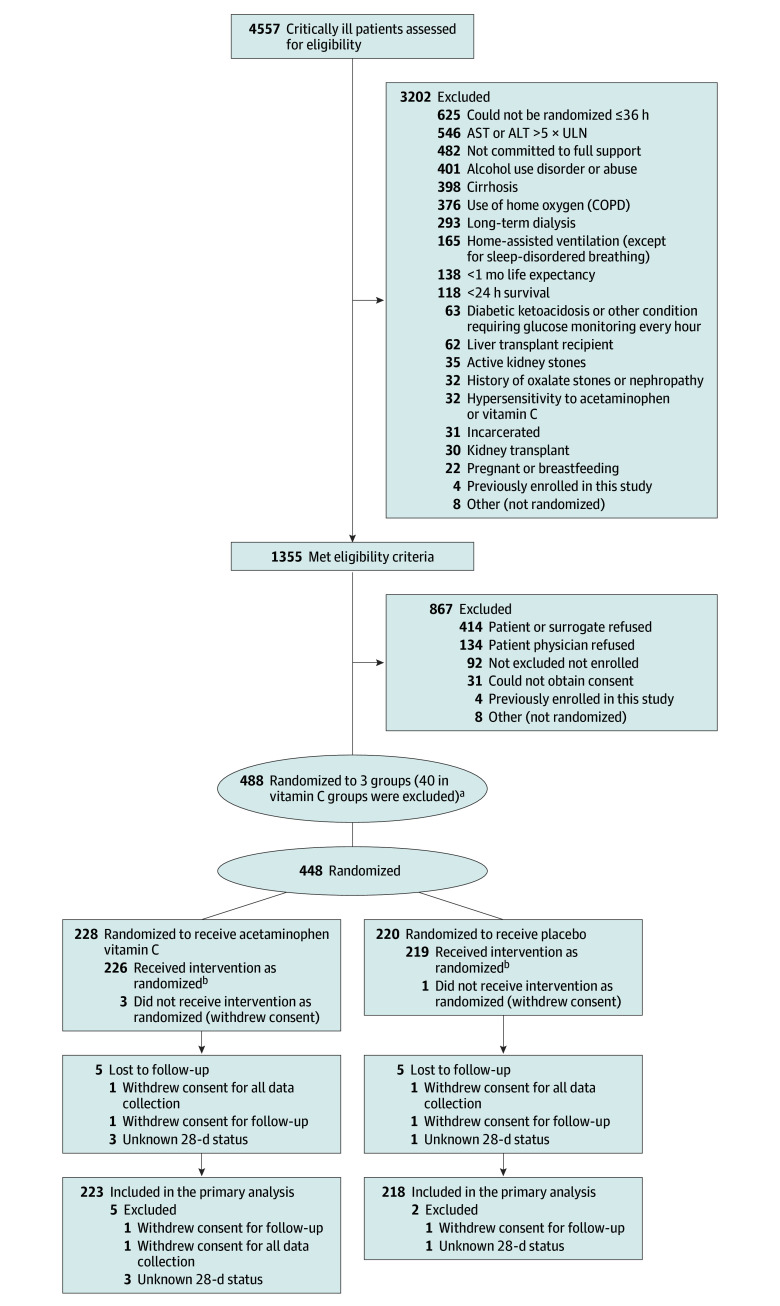
Eligible patients were 18 years or older with sepsis defined as clinical evidence of a known or suspected infection and orders written to administer antibiotics. Eligible patients had either (1) hypotension defined as the need for any vasopressor after administration of at least 1 L of intravenous fluid or (2) respiratory failure defined by mechanical ventilation, noninvasive ventilatory support at any level, or greater than or equal to 6 L/min of supplemental oxygen. Eligible patients could be enrolled if they were admitted (or planned to be admitted) to a study site intensive care unit (ICU) within 36 hours of presentation to the emergency department or presentation to any acute care hospital. A complete list of eligibility criteria is available in the Study Protocol (see Supplement 1). Demographic and clinical data were collected from the medical record for prespecified subgroup analysis, including sex, race, and ethnicity (clinician determined), COVID-19 status, and receipt of acetaminophen between hospital presentation and randomization (Table 1 and Figure 1).
Table 1. Baseline Patient Characteristics.
| Characteristics | No. (%) of patients | |
|---|---|---|
| Acetaminophen (n = 227) | Placebo (n = 220) | |
| Age, mean (SD), y [No.] | 63.9 (15.9) [227] | 64.2 (14.9) [219] |
| Age >75 y | 57 (25.1) | 55 (25.0) |
| Sex | ||
| Male | 115 (50.7) | 106 (48.2) |
| Female | 112 (49.3) | 114 (51.8) |
| Hispanic or Latino, No./total (%) | 31/222 (14.0) | 28/214 (13.1) |
| Race | n = 210 | n = 195 |
| American Indian or Alaska Native | 2 (1.0) | 0 |
| Asian | 6 (2.9) | 12 (6.2) |
| Black | 45 (21.4) | 32 (16.4) |
| Native Hawaiian or Other Pacific Islander | 4 (1.9) | 3 (1.5) |
| White | 153 (72.9) | 148 (75.9) |
| BMI, mean (SD) [No.] | 29.3 (10.2) [227] | 29.0 (9.4) [219] |
| Vasopressors at baseline | 174 (76.7) | 166 (75.5) |
| Assisted ventilation at baseline, No./total (%)a | 99/226 (43.8) | 86/219 (39.3) |
| High-flow nasal oxygen at baseline, No./total (%) | 33/226 (14.6) | 39/219 (17.8) |
| ARDS at baseline, No./total (%) | 41/226 (18.1) | 30/219 (13.7) |
| Time from inclusion to randomization, median (IQR), h [No.] | 9.6 (2.9-19.0) [224] | 9.7 (2.5-19.1) [215] |
| Screening hospital location | ||
| Intensive care unit | 128 (56.4) | 136 (61.8) |
| Emergency department | 95 (41.9) | 82 (37.3) |
| Floor | 2 (0.9) | 0 |
| Other | 2 (0.9) | 1 (0.5) |
| Step-down or intermediate unit | 0 | 1 (0.5) |
| Site of infection | ||
| Pneumonia | 100 (44.1) | 96 (43.6) |
| Urinary tract infection | 37 (16.3) | 52 (23.6) |
| Intraabdominal infection | 24 (10.6) | 19 (8.6) |
| Unknown | 22 (9.7) | 21 (9.5) |
| Skin or soft-tissue infection | 17 (7.5) | 16 (7.3) |
| Other source of infection | 15 (6.6) | 6 (2.7) |
| Influenza or other virus confirmed by testing | 8 (3.5) | 3 (1.4) |
| Central nervous system infection | 3 (1.3) | 4 (1.8) |
| Endocarditis or endovascular infection | 1 (0.4) | 1 (0.5) |
| Vascular catheter-related infection | 0 | 2 (0.9) |
| COVID-19 status ≤3 wk prior to admission | ||
| Positive | 17 (7.5) | 22 (10.0) |
| Negative | 163 (71.8) | 170 (77.3) |
| Unknown | 47 (20.7) | 28 (12.7) |
| Baseline measures | ||
| Creatinine, mean (SD), mg/dL | 1.6 (1.3) | 1.7 (1.3) |
| Outpatient creatinine ≤365 d , mean (SD), mg/dL [No.] | 1.1 (0.8) [164] | 1.2 (1.1) [157] |
| AST, mean (SD), U/L | 45.5 (35.0) | 40.9 (36.4) |
| ALT, mean (SD), U/L | 30.9 (26.6) | 31.4 (34.3) |
| Bilirubin, mean (SD), mg/dL [No.] | 0.9 (0.9) [225] | 0.9 (1.1) [218] |
| Platelets, mean (SD, × 103/μL [No.] | 218.9 (125.9) [226] | 221.1 (133.6) [220] |
| Platelets <50× 103/μL , No./total (%) | 15/226 (6.6) | 10/220 (4.5) |
| Total SOFA score, mean (SD)b | 5.5 (2.5) | 5.2 (2.5) |
| Plasma cell-free hemoglobin, mean (SD), mg/dL [No.] | 24.1 (60.3) [218] | 23.3 (64.9) [212] |
| Plasma cell-free hemoglobin, median (IQR), mg/dL [No.] | 8.3 (4.4-17.0) [218] | 8.5 (4.6-18.6) [212] |
| Prerandomization acetaminophen, No. (%) | 106 (46.7) | 91 (41.4) |
Abbreviations: ALT, alanine aminotransferase; ARDS, acute respiratory distress syndrome; AST, aspartate aminotransferase; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared), COVID-19, coronavirus-associated infectious disease 2019; SOFA, Sequential Organ Failure Assessment.
SI conversion factors: To convert ALT and AST from U/L to μkat/L, multiply by 0.0167; bilirubin from mg/dL to μmol/L, multiply by 17.04; creatinine from mg/dL to μmol/L, multiply by 88.4.
Assisted ventilation includes mechanical ventilation, noninvasive ventilation, or continuous positive airway pressure of 5 cm of oxygen or more but not high-flow nasal oxygen.
Scores were measured in the respiratory, cardiovascular, hematologic, renal, and coagulation systems, with each organ scored from 0 to 4, resulting in an aggregated score that ranges from 0 to 20, with higher scores indicating greater dysfunction. Twenty is the most severe. The Glasgow Coma Scale score was not included in the SOFA scoring.
Figure 1. Patient Screening, Enrollment, and Follow-up.
Patients could have more than 1 reason for exclusion.
aInitially, 448 patients were randomized to acetaminophen plus vitamin C: 228 to receive acetaminophen active; 220 pooled placebo; 40 vitamin C active. On June 15, 2022, the vitamin C arm of the trial was stopped after enrolling 79 participants due to external clinical trial data for vitamin C (see the Methods section).
bDefined as receiving 1 or more doses of the assigned study drug.
Randomization and Treatments
Patients were randomized using a web-based randomization site. Patients randomized to the acetaminophen arm received acetaminophen at the dose of 1 g in 100 mL diluent (or 15 mg/kg if actual body weight was <50 kg) every 6 hours intravenously for 5 days (20 doses). Patients randomized to placebo received an identical appearing intravenous infusion of 100 mL of 5% dextrose in water every 6 hours for 5 days (20 doses). In both arms, the study drug was discontinued prior to 120 hours, if one of the following occurred, (1) discharge from the study hospital, (2) discharge from the ICU, (3) withdrawal from the study, or (4) death. The serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were monitored on study days 0 and 2 through 5. New measured values of AST or ALT greater than or equal to 10 times the upper limit of normal on any measurement prompted permanent discontinuation of the study drug.
Common Trial Procedures
Standard protocols for low tidal volume ventilation and weaning from mechanical ventilation were protocolized for participants meeting criteria for ARDS.12 Fluid management during shock was unrestricted; balanced crystalloid solution was recommended for fluid resuscitation.13 In patients with ARDS who were not in shock or after shock resolution, a conservative fluid approach was recommended. Guidelines for the management of fever discouraged use of nonsteroidal anti-inflammatory drugs (NSAIDs) or cyclooxygenase 2 inhibitors. No restriction was placed on the use of aspirin in doses of less than or equal to 325 mg/d. The use of open-label acetaminophen was not permitted while receiving the study medication. Physical cooling could be used at the discretion of the clinical team.
End Points
The primary efficacy variable was days alive and free of any organ support (dialysis, assisted ventilation, and vasopressors) to day 28. Fifteen secondary efficacy variables included 28-day ventilator-free, vasopressor-free, new kidney replacement–free, ICU- and hospital-free days; 28-day and 90-day hospital mortality and 90-day all-cause mortality; 28-day duration of ICU stay in survivors and nonsurvivors; initiation of assisted ventilation and kidney replacement therapy to day 28; change in organ–specific and total sepsis–related Sequential Organ Failure Assessment (SOFA) scores between enrollment and study day 7; development of ARDS within 7 days of randomization; change in serum creatinine concentration from enrollment to discharge, death, initiation of dialysis, or 28 days, whichever occurs first; Major Adverse Kidney Events at 28 days (MAKE28), defined as a persistent increase in serum creatinine by 200% from baseline or need for new kidney replacement therapy, or death. To monitor for hepatic injury, safety outcomes included AST, ALT, and bilirubin concentrations measured on study days 0 and 2 through 5, and the Hy Law index of drug-induced liver dysfunction,14,15 defined as postrandomization AST or ALT 3 or more times the upper limit of normal and bilirubin 2 or more times the upper limit of normal on the same calendar day. Because intravenous acetaminophen has been associated with acute decreases in blood pressure,15,16 administration of fluid bolus, new use of vasopressor, or increased dose of vasopressor within 120 minutes of the study drug infusion were recorded. Other safety end points included adverse events, hypersensitivity, or rash. The trough acetaminophen levels after the fifth dose were also measured as a safety end point. Biological end points included change in plasma IL-6, angiopoietin 2, tumor necrosis factor receptor 1 (TNFR1), cell-free DNA, syndecan 1, and cell-free hemoglobin between enrollment (baseline) and days 2 and 3. The definitions of and analytic approach for these end points are outlined in the Protocol and Statistical Analysis Plan in Supplement 1.
Measurements
Plasma levels of cell-free hemoglobin, IL-6, angiopoietin 2, TNFR1, cell-free DNA, and syndecan 1 were measured by immunoassay in plasma collected by research staff.
Statistical Analysis
We sought to detect an absolute difference of 2.5 days alive and organ support free in the primary outcome assuming a standard deviation of 8.7 days. With 218 participants in each group, a 2-sample t test had 85% power at a 2-sided α of .05 to detect a significant difference between groups if the true mean difference was 2.5 or more days alive and organ support free. A midstudy interim analysis incorporated preset criteria to stop for efficacy or futility of either arm (see the Protocol in Supplement 1). Participants needed to be free of all 3 components (assisted ventilation, vasopressors, new kidney replacement therapy) to qualify for a day alive and free from organ support.
The primary outcome was compared between the treatment groups using a 2-sample t test with 95% Wald CI. The components of this outcome were reported separately as days free of assisted ventilation, vasopressors, and new kidney replacement therapy in the overall cohort and 28-day all-cause, all-location mortality. The difference in the total number of adverse events between treatment groups was assessed using Poisson regression and the associated 95% Wald CI. For all other outcomes, we reported mean or percentage differences based on 2-sample t tests or χ2 test (Fisher exact tests when specified) with 95% Wald CIs and median differences using a Wilcoxon rank-sum test. Kaplan-Meier method was used to estimate 28-day survival probabilities. Patients were censored on the last day of follow-up, hospital discharge, or death.
Subgroup by treatment interactions for continuous efficacy outcomes was assessed by an analysis of variance model. Subgroup by treatment interactions for binary efficacy outcomes was assessed by a generalized linear model with a logit link function. Both models included a treatment effect, a subgroup effect, and a subgroup-by-treatment interaction.
In prespecified analyses, we assessed the predictive value of baseline cell-free hemoglobin levels higher than 10 mg/dL for identifying patients who had an improvement in the primary end point, the 3 organ support components of the primary end point, 28-day all-cause mortality, and improvement in kidney function as assessed by MAKE28. The cutoff of 10 mg/dL was previously used to enroll patients in a trial of acetaminophen for treatment of sepsis with organ dysfunction8 and is associated with increased mortality in sepsis.4 Baseline cell-free hemoglobin by treatment interactions for continuous efficacy outcomes (primary end point and the organ support components of the primary end point) was assessed by an analysis of variance model. For binary efficacy outcomes (28-day all-cause mortality, improvement in renal function as assessed by MAKE28), we used a generalized linear model with a binary distribution function and logit link function. All models included a treatment effect, a hemoglobin effect, and a hemoglobin by treatment interaction. We also used cubic smoothing splines to estimate the relationship between the treatment effect on 28-day all-cause mortality and the baseline cell-free hemoglobin levels.
All analyses used an intention-to-treat approach and were prespecified in the Statistical Analysis Plan (Supplement 1) unless otherwise noted as post hoc. Safety outcomes are reported for all randomized participants.
All P values are 2-sided (α level ≤. 05). In this exploratory hypothesis-generating phase 2 trial, the a priori statistical analysis plan did not impose multiplicity correction and nominal P values are reported (see Supplement 1). All statistical analyses were conducted using SAS software version 9.4 (SAS Institute Inc) and R (version 4.3.2).
Results
Patients
Between October 13, 2021, and April 2023, 4557 patients were screened for enrollment (Figure 1). A total of 447 patients were enrolled and randomized, 227 to the acetaminophen arm and 220 to the placebo arm. Baseline characteristics and plasma biomarker levels were similar between the 2 groups (Table 1). The most common site of infection was pneumonia (≈44% in each group) and 9% of the cohort had tested positive for SARS-CoV-2 in the 3 weeks prior to enrollment. Overall, 76% of patients were receiving vasopressors at baseline, 42% were receiving assisted ventilation, and 44% had received acetaminophen prior to enrollment. The median time from inclusion to randomization was similar in the 2 arms (median, 9.6 hours; IQR, 2.9-19.0] vs 9.7 hours; IQR, 2.5-19.1).
Treatments
Patients in the acetaminophen arm received a mean (SD) of 12.2 (6.4) doses of acetaminophen compared with 12.8 (6.3) doses of placebo in the placebo arm. The mean (SD) number of missed doses was similar between the 2 arms (1.2 [4.0] doses in acetaminophen the arm vs 0.9 [3.0] doses in the placebo arm). Nonprotocol-specified acetaminophen was administered during the 5-day study period to 8 patients (mean, 1.5 doses) in the acetaminophen arm and to 13 patients (mean, 1.1 doses) in the placebo arm.
Primary Efficacy Outcome
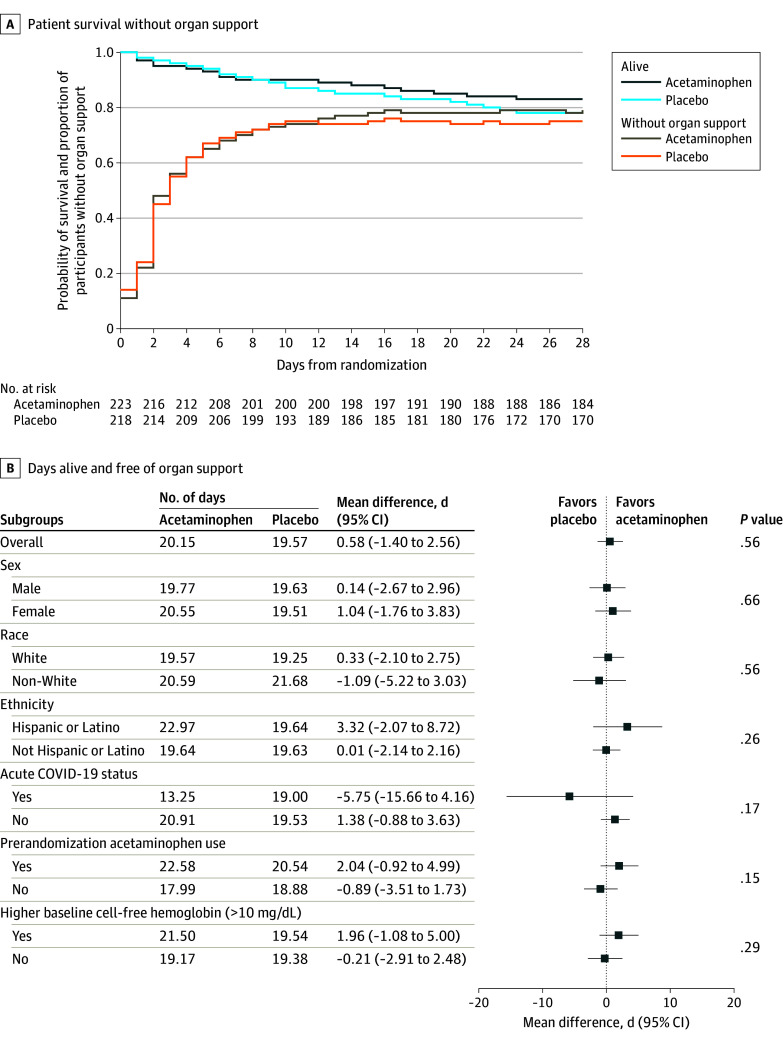
The primary end point of days alive and free of any organ support (dialysis, assisted ventilation, and vasopressors) to day 28 was not different between the acetaminophen arm (20.2 days; 95% CI; 18.8 to 21.6) and the placebo arm (19.6 days; 95% CI, 18.2 to 21.0; P = .56; difference, 0.6; 95% CI, −1.4 to 2.6; Table 2) nor did it differ by subgroup analysis (Figure 2). Twenty-eight day all-cause mortality was 17% in the acetaminophen arm vs 22% in the placebo arm (P = .19).
Table 2. Primary and Secondary Outcomes by Treatment Group.
| Outcomesa | Acetaminophen (n = 227) | Placebo (n = 220) | Difference, Mean (95% CI)b | P value | ||
|---|---|---|---|---|---|---|
| No. | Mean (SD)a | No. | Mean (SD) | |||
| Primary outcome | ||||||
| Days free to day 28 | ||||||
| Organ supportc | 223 | 20.2 (10.6) | 218 | 19.6 (10.6) | 0.6 (−1.4 to 2.6) | .56 |
| Assisted ventilation | 223 | 21.5 (10.2) | 218 | 20.5 (10.6) | 1.1 (−0.9 to 3.0) | .29 |
| Vasopressors | 223 | 22.2 (9.4) | 218 | 21.4 (9.5) | 0.8 (−1.0 to 2.6) | .38 |
| Kidney replacement therapy | 223 | 23.7 (9.0) | 218 | 23.6 (8.7) | 0.1 (−1.5 to 1.8) | .89 |
| 28-d All-cause mortality, No. (%) | 223 | 39 (17.5) | 218 | 49 (22.5) | −5.0 (−12.4 to 2.5) | .19 |
| Secondary outcomes | ||||||
| 28-d Hospital mortality, No. (%) | 224 | 37 (16.5) | 218 | 47 (21.6) | −5.0 (−12.4 to 2.3) | .18 |
| Days free to day 28 | ||||||
| Ventilatord | 223 | 20.9 (11.0) | 218 | 19.4 (11.8) | 1.5 (−0.7 to 3.6) | .18 |
| Vasopressorsd | 223 | 21.4 (10.4) | 218 | 20.0 (11.4) | 1.5 (−0.6 to 3.5) | .16 |
| Kidney replacement therapyd | 223 | 22.4 (11.0) | 218 | 21.5 (11.7) | 0.9 (−1.3 to 3.0) | .42 |
| ICUd | 223 | 19.3 (9.9) | 218 | 18.9 (10.1) | 0.4 (−1.5 to 2.3) | .67 |
| Hospitald | 223 | 11.3 (10.9) | 217 | 11.0 (10.8) | 0.2 (−1.8 to 2.3) | .83 |
| ICU days to day 28 | 222 | 5.3 (6.2) | 217 | 5.1 (6.2) | 0.2 (−0.9 to 1.4) | .70 |
| Initiation of assisted ventilation to day 28, No. (%) | 125 | 21 (16.8) | 132 | 23 (17.4) | −0.6 (−9.8 to 8.6) | .89 |
| Initiation of kidney replacement therapy to day 28, No. (%) | 223 | 22 (9.9) | 218 | 18 (8.3) | 1.6 (−3.7 to 7.0) | .56 |
| Change in SOFA score from baseline to day 7 | 148 | −3.2 (3.3) | 153 | −2.9 (3.1) | −0.2 (−0.9 to 0.5) | .53 |
| 90-d Hospital mortality, No. (%) | 224 | 50 (22.3) | 218 | 54 (24.8) | −2.4 (−10.4 to 5.5) | .54 |
| Development of ARDS within 7 d of randomization, No. (%)e | 183 | 4 (2.2) | 188 | 16 (8.5) | −6.3 (−10.8 to −1.8) | .01 |
| Change in serum creatinine (mg/dL)f | 198 | −0.3 (1.0) | 200 | −0.2 (0.9) | −0.1 (−0.2 to 0.1) | .55 |
| MAKE28, No. (%)g | 221 | 55 (24.9) | 218 | 56 (25.7) | −0.8 (−8.9 to 7.3) | .85 |
| 90-d All-cause all-location mortality, No. (%) | 223 | 58 (26.0) | 218 | 69 (31.7) | −5.6 (−14.1 to 2.8) | .19 |
| Any adverse events, No. of eventsh | 36 | 32 | 4 (−12 to 20) | .63 | ||
Abbreviations: ARDS, acute respiratory distress syndrome; ICU, intensive care unit; MAKE28, Major Adverse Kidney Events at 28 days; SOFA, Sequential Organ Failure Assessment.
SI conversion factor: To convert creatinine from mg/dL to μmol/L, multiply by 88.4.
Unless otherwise indicated, values are mean (SD). The percentage and mean were calculated from the nonmissing records.
Differences are either means (for differences in numbers of days or events or in scores/values) or percentage points (for differences between percents).
Days free of organ support to day 28 are all calendar days without vasopressor use, kidney replacement therapy, and assisted ventilation (free of all 3) to day 28 in survivors and nonsurvivors. Days free of vasopressors, kidney replacement therapy, and assisted ventilation are the number of days free of each of these organ supports individually in survivors and nonsurvivors.
Patients who do not survive for 28 days are assigned zero free days. Patients transferred to another hospital or health care facility were followed up to day 28 to assess these end points.
P value is calculated from Fisher exact test.
Change from enrollment to discharge, death, initiation of dialysis or 28 days, whichever occurs first.
Defined as a persistent increase in serum creatinine by 200% from baseline and need for new kidney replacement therapy, or death.
Participants may have had more than 1 adverse event.
Figure 2. Survival and Days Alive, and Free of Organ Support to Day 28.
A, Survival and the proportion of patients without organ support over the first 28 days after randomization. Organ support included mechanical ventilation, vasopressors, and renal replacement therapy. Probabilities are shown for the 441 patients with complete 28-day follow-up.
B, The primary outcome was days alive and free of organ support, including mechanical ventilation, vasopressors, and renal replacement therapy to day 28. Non-White racial groups are plotted together due to small numbers in individual racial groups. The P value for overall between-group difference was derived by t test and the P values for subgroup interaction by analysis of variance.
Secondary Outcomes
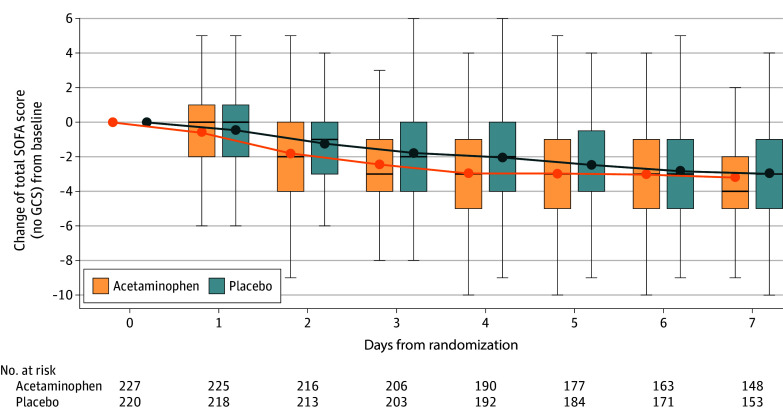
Fifteen secondary outcomes were analyzed (Table 2). Hospital mortality at 90-day and all-cause mortality at 90-day were not significantly different between treatment arms. In the acetaminophen arm, improvements in total SOFA scores and SOFA coagulation component scores were significantly greater on study days 2 through 4 (Figure 3 and eFigure 1 in Supplement 2) and improvements in SOFA respiratory scores were significantly greater on study days 1 through 4. The number of patients developing ARDS was significantly lower in the acetaminophen arm compared with placebo (2.2% vs 8.5%; P = .01). Other secondary clinical end points were not significantly different between arms (Table 2). The change in plasma levels of IL-6, angiopoietin-2, TNFR1, cell-free DNA, and syndecan 1 from baseline to day 3 was not significantly different in the acetaminophen arm compared with placebo (eTable 1 in Supplement 2).
Figure 3. Change of Total Sequential Organ Failure Assessment (SOFA) Scores From Enrollment to Day 7 by Treatment Arm.
Data are reported as mean (dots), median (bars), IQR (boxes), and values falling within 1.5 × IQR (whiskers). Changes in total SOFA score from enrollment were compared each day by t test. P values for days 2 through 4 are P < .05. GCS indicates Glasgow Coma Scale.
Safety Outcomes
Baseline and daily AST, ALT, and bilirubin measurements did not differ between the 2 treatment groups (eTable 2 in Supplement 2). The number of patients who developed a new increase in AST or ALT to greater than 10 times the upper limit of normal was low and similar in the 2 groups (4% in the acetaminophen arm and 3% in the placebo arm). The number of patients developing new indices of liver dysfunction by Hy Law was also low (2% in the acetaminophen group and 1% in the placebo group). There was 1 allergic reaction in the placebo arm. The percentage of patients requiring a fluid bolus or initiation or increase of a vasopressor after a dose of the study drug was similar in the acetaminophen arm (40%) compared with the placebo arm (41%, P = .87). Daily and total fluid balance over the first 7 days was similar between the 2 arms (eTable 3 in Supplement 2). Trough acetaminophen levels were measured in the acetaminophen arm after at least 5 doses and no levels were higher than the Rumack-Matthew line17 of potential hepatotoxicity. There were no differences in adverse events when compared by treatment arm (eTable 4A-4C in Supplement 2).
Predictive Enrichment by Plasma Cell-Free Hemoglobin Levels
At baseline, the median cell-free hemoglobin was 8.3 mg/dL (IQR, 4.4-17.0) in the acetaminophen group and 8.5 mg/dL (IQR, 4.6-18.6) in the placebo group. When stratified by a prespecified threshold (10 mg/dL), there were no significant interactions of the acetaminophen treatment effect and baseline plasma cell-free hemoglobin levels for the primary outcome, its components, and MAKE28 (Table 3) comparing patients higher than (>10 mg/dL, n = 188) vs lower than (≤10 mg/dL, n = 239, Table 3; eTable 5 in Supplement 2).
Table 3. Primary Outcomes, Major Adverse Kidney Events at 28 Days (MAKE28) and Adverse Events by Treatment Group in Higher (>10 mg/dL) and Lower (≤10 mg/dL) Baseline Cell-Free Hemoglobin Level Groups.
| Outcomesa | High baseline cell-free hemoglobin (n = 190) | Low baseline cell-free hemoglobin (n = 240) | High vs low baseline cell-free hemoglobin | |||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) [No.] | Difference (95% CI) | Mean (SD) [No.] | Difference (95% CI) | Difference in differences (95% CI) | P value | |||
| Acetaminophen | Placebo | Acetaminophen | Placebo | |||||
| Days free to day 28 | ||||||||
| Organ support | 21.5 (10.2) [92] | 19.5 (10.6) [96] | 2.0 (−1.1 to 5.0) | 19.2 (10.9) [124] | 19.4 (10.6) [115] | −0.2 (−2.9 to 2.5) | 2.2 (−1.9 to 6.2) | .29 |
| Assisted ventilation | 22.7 (9.8) [92] | 20.2 (10.8) [96] | 2.6 (−0.4 to 5.5) | 20.6 (10.5) [124] | 20.5 (10.5) [115] | 0.1 (−2.6 to 2.7) | 2.5 (−1.5 to 6.5) | .22 |
| Vasopressors | 23.8 (8.1) [92] | 21.0 (9.9) [96] | 2.8 (0.1 to 5.5) | 21.1 (10.2) [124] | 21.3 (9.4) [115] | −0.2 (−2.6 to 2.2) | 3.0 (−0.6 to 6.6) | .11 |
| Kidney replacement therapy | 24.5 (8.7) [92] | 23.7 (8.7) [96] | 0.7 (−1.8 to 3.3) | 23.1 (9.3) [124] | 23.2 (8.8) [115] | −0.1 (−2.4 to 2.2) | 0.8 (−2.6 to 4.2) | .64 |
| All-cause mortality at 28 d, No. (%) | 11 (12.0) [92] | 20 (20.8) [96] | −8.9 (−19.4 to 1.6) | 27 (21.8) [124] | 29 (25.2) [115] | −3.4 (−14.2 to 7.3) | −5.4 (−20.5 to 9.6) | .48 |
| MAKE28, No. (%) | 20 (22.0) [91] | 22 (22.9) [96] | −0.9 (−12.9 to 11.0) | 33 (26.8) [123] | 34 (29.6) [115] | −2.7 (−14.2 to 8.7) | 1.8 (−14.8 to 18.3) | .83 |
| Any adverse events, No. of eventsb | 14 | 12 | 2 (−8 to 12) | 19 | 20 | −1 (−13 to 11) | ||
Abbreviation: MAKE28, Major Adverse Kidney Events at 28 days.
The percentage and mean were calculated from the nonmissing records.
Participants may have had more than 1 adverse event.
In exploratory analyses, the change in total and component SOFA scores in high and low hemoglobin groups by treatment group were not meaningfully different (eFigure 2 in Supplement 2). Among patients with baseline cell-free hemoglobin higher than 10 mg/dL, the 28-day all-cause mortality was 12% in the acetaminophen group compared with 21% in the placebo group (P = .10, eFigure 3A in Supplement 2). The difference in mortality was 3% (95% CI, −14% to 7%) in patients with cell-free hemoglobin of 10 mg/dL or lower (P = .53; interaction P = .48). Patients with higher cell-free hemoglobin levels treated with acetaminophen had lower rates of initiation of assisted ventilation compared with placebo (8% vs 23%, interaction P = .02; eTable 6 in Supplement 2). The effect of baseline cell-free hemoglobin level as a continuous variable on 28-day all-cause mortality by treatment arm was tested post hoc in a nonlinear model (eFigure 3B in Supplement 2). Estimated treatment effects of acetaminophen increased as cell-free hemoglobin levels increased, although the confidence bands overlapped.
Discussion
In patients with sepsis and respiratory or circulatory organ dysfunction, treatment with intravenous acetaminophen for 5 days did not improve the primary end point of days alive and free of any organ support (dialysis, assisted ventilation, and vasopressors) to day 28 compared with placebo. There were no significant differences in 28-day or 90-day mortality. Among the 15 secondary outcomes, patients treated with acetaminophen were less likely to develop ARDS and had better total SOFA scores on days 1 through 4. Importantly, intravenous acetaminophen administered every 6 hours for 5 days in this critically ill patient population was safe, with no difference in biomarkers of hepatic injury, systemic hypotension, or other adverse events compared with the placebo group.
These data extend prior evidence from smaller trials of acetaminophen in sepsis. For example, although acetaminophen improved measures of kidney function in sepsis and severe falciparum malaria,8,18 there was no benefit of acetaminophen across a variety of kidney end points, including changes in creatinine levels, need for kidney replacement therapy, and MAKE28 in the current trial. Acetaminophen did have a beneficial effect on several measures of respiratory dysfunction, including new development of ARDS, respiratory SOFA scores on study days 1 through 4, and initiation of assisted ventilation (in the group with higher cell-free hemoglobin). These findings are consistent with prior studies in the isolated perfused human lung showing that acetaminophen can block injurious effects of cell-free hemoglobin on alveolar-capillary barrier dysfunction.19,20 This protective effect is mediated through the hemoprotein reductant activity of acetaminophen that inhibits redox cycling of cell-free hemoglobin thereby limiting its potential to oxidize lipids2,21 and other substrates.
To test whether elevated plasma cell-free hemoglobin might serve as a biomarker for predictive enrichment in a future trial, we carried out a prespecified analysis to test for a differential treatment effect of acetaminophen in patients with plasma cell-free hemoglobin levels higher than 10 mg/dL at enrollment. Only 44% of patients in the current study had cell-free hemoglobin higher than 10 mg/dL, in contrast to prior single-center and observational studies, where 80% to 90% of patients had levels higher than 10 mg/dL4,6,8 using different hemoglobin assays. There was no significant interaction between hemoglobin levels and the primary outcome. However, an exploratory post hoc analysis of 28-day mortality by treatment arm compared with baseline cell-free hemoglobin as a continuous variable suggested that patients with the highest cell-free hemoglobin levels might have a greater treatment effect; this is an important area for future study.
Limitations
This study has several limitations. First, although this trial was of moderate size for a phase 2 hypothesis-generating study, there was limited power to examine for heterogeneity of treatment effect. Second, because of the phase 2 exploratory nature of the trial, no adjustment for multiplicity was imposed, which limits the interpretation of the multiple secondary outcomes. Third, although cell-free hemoglobin levels were measured at baseline, no prospective testing was available at the point of care to evaluate cell-free hemoglobin for predictive enrichment during the trial. Fourth, the study drug was only dosed while patients remained in the ICU, in part out of concern for potential hemodynamic effects of intravenous acetaminophen. For this reason, patients received, on average, only 12 doses of the study drug rather than the planned 20 doses.
Conclusions
Treatment of critically ill patients with sepsis with either respiratory or circulatory organ dysfunction with 1 g of acetaminophen intravenously every 6 hours for 5 days was safe but did not significantly improve the primary outcome of days alive and free of organ support to day 28, including mechanical ventilation, vasopressors, and kidney replacement therapy.
Section Editor: Christopher Seymour, MD, Associate Editor, JAMA (christopher.seymour@jamanetwork.org).
Trial Protocol and Statistical Analysis Plan
Collaborators
eTable 1. Plasma biomarker levels at baseline and study day 2 and day 3 by treatment group
eTable 2. Daily AST, ALT and bilirubin levels in acetaminophen and matched placebo
eTable 3. Fluid balance (ml) by study day
eTable 4a. Adverse events by organ system and severity in acetaminophen and matched placebo
eTable 4b. Listings of all adverse events in acetaminophen group
eTable 5. Comparison of baseline patient characteristics between the higher and lower cell-free hemoglobin groups
eTable 6. Secondary outcomes by treatment in higher (>10 mg/dL) and lower ≤10 mg/dL baseline cell-free hemoglobin level groups
eFigure 1. Change in individual components Sequential Organ Failure Assessment (SOFA) scores from enrollment to day 7 by treatment arm
eFigure 2a. Total and component SOFA scores from enrollment to day 7 by treatment arm in the higher cell-free hemoglobin group (≥10 mg/dL
eFigure 2b. Total and component SOFA scores from enrollment to day 7 by treatment arm in the lower cell-free hemoglobin group (≤10 mg/dL)
eFigure 3a. 28-day all-cause mortality categorized by cell-free hemoglobin level
eFigure 3b. Non-linear relationship between cell-free hemoglobin and 28-day all-cause mortality by treatment arm
Nonauthor Collaborators. The National Heart, Lung, and Blood Institute Prevention and Early Treatment of Acute Lung Injury Clinical Trials Network
Data Sharing Statement
References
- 1.Hinz B, Cheremina O, Brune K. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J. 2008;22(2):383-390. doi: 10.1096/fj.07-8506com [DOI] [PubMed] [Google Scholar]
- 2.Boutaud O, Moore KP, Reeder BJ, et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci U S A. 2010;107(6):2699-2704. doi: 10.1073/pnas.0910174107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boutaud O, Roberts LJ II. Mechanism-based therapeutic approaches to rhabdomyolysis-induced renal failure. Free Radic Biol Med. 2011;51(5):1062-1067. doi: 10.1016/j.freeradbiomed.2010.10.704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janz DR, Bastarache JA, Peterson JF, et al. Association between cell-free hemoglobin, acetaminophen, and mortality in patients with sepsis: an observational study. Crit Care Med. 2013;41(3):784-790. doi: 10.1097/CCM.0b013e3182741a54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adamzik M, Hamburger T, Petrat F, Peters J, de Groot H, Hartmann M. Free hemoglobin concentration in severe sepsis: methods of measurement and prediction of outcome. Crit Care. 2012;16(4):R125. doi: 10.1186/cc11425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerchberger VE, Bastarache JA, Shaver CM, et al. Haptoglobin-2 variant increases susceptibility to acute respiratory distress syndrome during sepsis. JCI Insight. 2019;4(21):e131206. doi: 10.1172/jci.insight.131206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki S, Eastwood GM, Bailey M, et al. Paracetamol therapy and outcome of critically ill patients: a multicenter retrospective observational study. Crit Care. 2015;19(1):162. doi: 10.1186/s13054-015-0865-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janz DR, Bastarache JA, Rice TW, et al. ; Acetaminophen for the Reduction of Oxidative Injury in Severe Sepsis Study Group . Randomized, placebo-controlled trial of acetaminophen for the reduction of oxidative injury in severe sepsis: the Acetaminophen for the Reduction of Oxidative Injury in Severe Sepsis trial. Crit Care Med. 2015;43(3):534-541. doi: 10.1097/CCM.0000000000000718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plewes K, Kingston HWF, Ghose A, et al. Acetaminophen as a renoprotective adjunctive treatment in patients with severe and moderately severe falciparum malaria: a randomized, controlled, open-label trial. Clin Infect Dis. 2018;67(7):991-999. doi: 10.1093/cid/ciy213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young P, Saxena M, Bellomo R, et al. ; HEAT Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group . Acetaminophen for fever in critically ill patients with suspected infection. N Engl J Med. 2015;373(23):2215-2224. doi: 10.1056/NEJMoa1508375 [DOI] [PubMed] [Google Scholar]
- 11.Lamontagne F, Masse MH, Menard J, et al. ; LOVIT Investigators and the Canadian Critical Care Trials Group . Intravenous vitamin C in adults with sepsis in the intensive care unit. N Engl J Med. 2022;386(25):2387-2398. doi: 10.1056/NEJMoa2200644 [DOI] [PubMed] [Google Scholar]
- 12.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A; Acute Respiratory Distress Syndrome Network . Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301-1308. doi: 10.1056/NEJM200005043421801 [DOI] [PubMed] [Google Scholar]
- 13.Semler MW, Self WH, Wanderer JP, et al. ; SMART Investigators and the Pragmatic Critical Care Research Group . Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378(9):829-839. doi: 10.1056/NEJMoa1711584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reuben A. Hy’s law. Hepatology. 2004;39(2):574-578. doi: 10.1002/hep.20081 [DOI] [PubMed] [Google Scholar]
- 15.Temple R. Hy’s law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf. 2006;15(4):241-243. doi: 10.1002/pds.1211 [DOI] [PubMed] [Google Scholar]
- 16.Schell-Chaple HM, Liu KD, Matthay MA, Sessler DI, Puntillo KA. Effects of IV acetaminophen on core body temperature and hemodynamic responses in febrile critically ill adults: a randomized controlled trial. Crit Care Med. 2017;45(7):1199-1207. doi: 10.1097/CCM.0000000000002340 [DOI] [PubMed] [Google Scholar]
- 17.Rumack BH, Matthew H. Acetaminophen poisoning and toxicity. Pediatrics. 1975;55(6):871-876. doi: 10.1542/peds.55.6.871 [DOI] [PubMed] [Google Scholar]
- 18.Cooper DJ, Grigg MJ, Plewes K, et al. The effect of regularly dosed acetaminophen versus no acetaminophen on renal function in Plasmodium Knowlesi Malaria (PACKNOW): a randomised controlled trial. Clin Infect Dis. 2022;75(8):1379-1388. doi: 10.1093/cid/ciac152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaver CM, Wickersham N, McNeil JB, et al. ; Lung Transplant Outcomes Group (LTOG) . Cell-free hemoglobin promotes primary graft dysfunction through oxidative lung endothelial injury. JCI Insight. 2018;3(2):e98546. doi: 10.1172/jci.insight.98546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaver CM, Upchurch CP, Janz DR, et al. Cell-free hemoglobin: a novel mediator of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2016;310(6):L532-41. doi: 10.1152/ajplung.00155.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plewes K, Kingston HWF, Ghose A, et al. Cell-free hemoglobin mediated oxidative stress is associated with acute kidney injury and renal replacement therapy in severe falciparum malaria: an observational study. BMC Infect Dis. 2017;17(1):313. doi: 10.1186/s12879-017-2373-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
Collaborators
eTable 1. Plasma biomarker levels at baseline and study day 2 and day 3 by treatment group
eTable 2. Daily AST, ALT and bilirubin levels in acetaminophen and matched placebo
eTable 3. Fluid balance (ml) by study day
eTable 4a. Adverse events by organ system and severity in acetaminophen and matched placebo
eTable 4b. Listings of all adverse events in acetaminophen group
eTable 5. Comparison of baseline patient characteristics between the higher and lower cell-free hemoglobin groups
eTable 6. Secondary outcomes by treatment in higher (>10 mg/dL) and lower ≤10 mg/dL baseline cell-free hemoglobin level groups
eFigure 1. Change in individual components Sequential Organ Failure Assessment (SOFA) scores from enrollment to day 7 by treatment arm
eFigure 2a. Total and component SOFA scores from enrollment to day 7 by treatment arm in the higher cell-free hemoglobin group (≥10 mg/dL
eFigure 2b. Total and component SOFA scores from enrollment to day 7 by treatment arm in the lower cell-free hemoglobin group (≤10 mg/dL)
eFigure 3a. 28-day all-cause mortality categorized by cell-free hemoglobin level
eFigure 3b. Non-linear relationship between cell-free hemoglobin and 28-day all-cause mortality by treatment arm
Nonauthor Collaborators. The National Heart, Lung, and Blood Institute Prevention and Early Treatment of Acute Lung Injury Clinical Trials Network
Data Sharing Statement