Abstract
Background
Although the thymus undergoes degeneration with the advancement of age, recent studies have continuously revealed that the thymus possesses the potential for regeneration and may reverse this aging trend. Furthermore, an increasing number of studies indicate an association between thymus function and immunotherapy. Considering that lung cancer patients typically undergo chest computed tomography (CT) scans during treatment, this provides convenient conditions for us to observe thymic remodeling through imaging data. Therefore, exploring the changes in the thymus on CT images is of great significance for understanding its relationship with the efficacy of immunotherapy in non-small cell lung cancer (NSCLC) patients. This study investigated the CT imaging characteristics of thymic density changes in patients with advanced NSCLC after immunotherapy. The primary objective was to determine whether changes in thymic density are predictors of response to immunotherapy in patients with NSCLC.
Methods
A total of 412 patients with advanced NSCLC who underwent immunotherapy were included. Thymic density measurements were taken initially and after immunotherapy, with the annualized change calculated. Comprehensive analysis, including disease progression, survival, and subgroup assessments, was conducted. The primary outcome was overall survival (OS), and the secondary outcomes were progression-free survival (PFS), objective response rate (ORR) and disease control rate (DCR).
Results
The annual change in density of the thymic region ranged from −108 to 108 HU after the initiation of ICIs. Patients were categorized into “loss” or “non-loss” groups (210 vs. 202) based on thymic density changes. Analysis of short-term progression of solid tumors revealed no statistically significant differences in ORR (P=0.55) and DCR (P=0.67) between the two groups. Throughout the entire follow-up period, 41 patients (19.5%) in the “loss” group and 64 patients (31.7%) in the “non-loss” group died. Thymic density reduction was not associated with PFS (P=0.08), but it was positively associated with increased OS (P=0.003). The results were consistent across subgroups.
Conclusions
Thymic density changes were observed in nearly all NSCLC patients undergoing immunotherapy, with decreased density associated with longer OS. These findings suggest a potential association between thymic density changes and immune efficacy in NSCLC immunotherapy.
Keywords: Thymus, immune checkpoint inhibitors (ICIs), non-small cell lung cancer (NSCLC), computed tomography (CT), immunotherapy
Highlight box.
Key findings
• This study found that in non-small cell lung cancer (NSCLC) patients undergoing immunotherapy, there were subtle changes in thymus density in nearly every individual, and patients with decreased thymus density had longer overall survival (OS).
What is known and what is new?
• The thymus has the potential to regenerate, and the trend of degeneration with age can be reversed, with changes in thymic volume being observable through chest computed tomography (CT) scans. Thymus function is associated with immunotherapy.
• The relationship between changes in the thymus on imaging and the efficacy of immunotherapy is not yet clear.
What is the implication, and what should change now?
• A decrease in thymus density may serve as a surrogate marker for thymus function, indicating enhanced thymus and immune system activity. This finding may provide valuable insights for guiding future patient use of immunotherapeutic drugs or enhancing the efficacy of immunotherapy.
Introduction
Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for approximately 85% of all lung cancer cases (1). Despite advancements in treatment modalities including surgical intervention, chemotherapy, and targeted therapy, the overall prognosis for patients with NSCLC remains poor, particularly in advanced-stage disease, with an approximate five-year survival rate of about 20% (2). In recent years, the clinical utilization of immune checkpoint inhibitors (ICIs) in cancer treatment has reached a high level of maturity, with the combination of ICIs and chemotherapy emerging as the predominant first-line approach for managing advanced NSCLC (3).
The thymus, functioning as a primary lymphoid organ, assumes a pivotal role in the generation and development of naive T cells that play a critical role in adaptive immunity (4). ICIs prevent tumor suppression of T cells and restart the tumor immune cycle by binding to immune checkpoints. Thymosin alpha 1 (Tα1) produced by the thymus present in the natural thymus is not only used as a booster of vaccine response, but also widely used in cancer patients and a wide range of infections, and its value in the field of cancer has become a research hotspot today (5). Tα1 is involved in multiple steps of the tumor immune cycle, acting directly on precursor T cells to promote their proliferation and maturation (6). Tα1 acts synergistically with ICIs in the treatment of melanoma, showing better efficacy than monotherapy (7). Guo et al.’s study confirmed that Tα1 as adjuvant immunomodulatory therapy can significantly improve progression-free survival (PFS) and overall survival (OS) in patients with NSCLC after R0 resection, and the absolute OS improvement was greater than 10% at 5 years after surgery (5). The latest research suggests that zoledronic acid (ZA) and Tα1 enhance the immunotherapeutic efficacy in the treatment of patients with immunologically unresponsive prostate cancer by bolstering antitumor immunity (8). Tα1 secreted by the thymus enhances the efficacy of ICIs and adjuvant immunomodulatory therapy, playing a crucial role in bolstering antitumor immunity in cancer treatment. Previous studies have shown that cell-mediated immune system disorders and thymic fat aging may be one of the factors leading to the decline of immunity (9). The thymus, being the largest at puberty, undergoes dynamic changes in early life, particularly during infancy and early childhood, as the T cells it houses migrate throughout the body and experience age-related alterations such as volume reduction and loss of epithelial cells. This process, known as thymus degeneration, is accompanied by a gradual decline in thymus output and function (10,11). It is noteworthy, however, that the rate at which thymic involution occurs exhibits interindividual variation. The conventional perspective holds that the clinical significance of thymus degeneration is deemed to be inconsequential. Nevertheless, mounting evidence suggests that thymus degeneration serves as a prominent marker of T cell senescence, which is concomitant with immune senescence and inflammatory processes (12). The NEJM study highlights the association between thymectomy and higher all-cause mortality and cancer risk, demonstrating that the homeostatic disruption caused by thymectomy is sufficient to adversely affect key health outcomes—strong evidence that the adult thymus still has an important functional role (13).
Thymic density can be visualized on chest computed tomography (CT), but its clinical utility is limited due to the anatomical nature of the thymus. However, recent studies have highlighted a significant underestimation of adult remaining thymus tissue (14). While the correlation between thymus density and thymus function from CT plain scans is still lacking in evidence, the key role of thymus solid components in immunoinflammation may provide insights into future treatment strategies and clinical decision-making. Notably, previous studies have shown a strong association between CT-assessed thymic total fat replacement and depletion of naive CD8+ T cells, suggesting that thymic CT may serve as a clinically relevant marker of immunosenescence (15).
Although it is well-recognized that the thymus undergoes age-related degeneration, emerging evidence suggests that it can regenerate and potentially reverse the degenerative process (16). The question of whether thymus remodeling is evident on CT images is of interest, given the ready availability of chest CT scans for lung cancer patients. We assessed thymus density using chest CT imaging and assessed its density change to explore its relevance to treatment outcomes following immunotherapy in patients with NACLC. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-203/rc).
Methods
Study participants and design
The single-center retrospective cohort study was conducted at Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, from March 2020 to September 2022. A total of 412 patients diagnosed with advanced NSCLC who were undergoing immunotherapy were included in the study. Inclusion criteria for participants were: (I) age over 18 years; (II) a diagnosis of stage III or IV NSCLC according to the NCCN Oncology Clinical Practice Guidelines: NSCLC (17); (III) histologically or cytologically confirmed NSCLC; (IV) receiving at least three cycles of immunotherapy; (V) first diagnosis and complete treatment at the study institution. Exclusion criteria for participants in both groups were: (I) concurrent presence of another type of tumor; (II) previous surgical treatment; (III) thymoma or anterior mediastinal space involvement; (IV) history of sternal trauma and sternotomy; (V) absence of data and medical records; (VI) poor image quality of chest CT scans. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. S054). Written informed consent was waived due to the retrospective nature of this study.
Furthermore, we randomly included 100 patients who had undergone standard chemotherapy as a control group to eliminate the influence of other confounding factors.
Data acquisition and analysis
All CT images were obtained using the Siemens Healthcare scanner, which featured a tube voltage of 120 kVp and automated tube current regulation for optimized image quality. The images were meticulously reconstructed using advanced technology, utilizing a matrix size of 512×512 and a precise collimation of 64 mm × 0.625 mm. The standardized reconstructed slice thickness and interval of 1.5 mm guaranteed consistent and accurate measurements. Furthermore, the Picture Archiving and Communication System (PACS) system is employed for image storage and CT value measurements, with CT values serving as indicators of thymic tissue density, measured in Hounsfield unit (HU) for attenuation. All scans were obtained at lower radiation doses and no contrast agent was used.
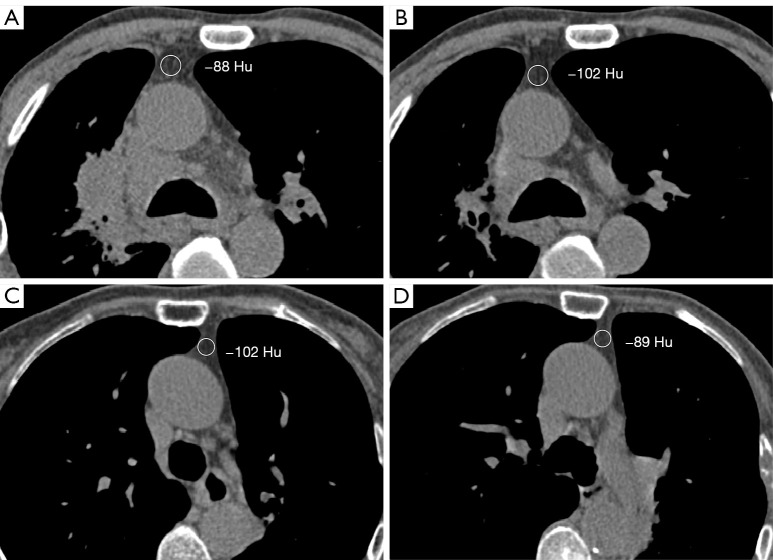
Patient data, including age, gender, demographics, clinical data, laboratory results, and chest CT images, were obtained from institutional case records from the date of admission to discharge or death. We conduct telephone follow-up assessments every six months with patients, focusing primarily on disease progression and mortality information until the patient’s death. The density of the thymic region in the chest CT scan was recorded independently by two radiologists with more than 7 years of experience, as shown in Figure 1. Region of interest (ROI) was selected from the relatively homogeneous areas within the anterior mediastinum to assess the overall CT values. The size of the ROIs was approximately 100 mm2, and their positions were determined by the observers. The first observer assessed the images twice, with measurements taken after a 4-week washout period, while the second observer conducted a single assessment. Neither was aware of the other’s results. Any discrepancies were resolved through consensus, with the final CT values of the thymic region being jointly determined by both observers. The intra- and inter-observer reliability for measurement of thymic density was excellent at 0.96 [95% confidence interval (CI): 0.89–0.99] and 0.97 (95% CI: 0.91–0.99).
Figure 1.
Measurement of thymic density in the anterior mediastinum: (A,B) the first and last chest CT images of the same patient, respectively, who showed an annual decrease in thymic density; (C,D) the first and last chest CT images, respectively, of another patient, who presented with an increase in annualized thymic density. HU, Hounsfield unit; CT, computed tomography.
All areas of interest were delineated on fixed mediastinal Windows (window level =50 HU, window width =500 HU). In each patient, the density difference in the anterior mediastinal thymus region was measured from the two chest CT images with the greatest time interval. This density difference was then divided by the interval in months and multiplied by twelve months to calculate the annualized thymus progression value, as determined by the following formula:
| [1] |
Among them, a is the attenuation/Hounsfield unit measurement by ROI before treatment (HU), b is the attenuation/Hounsfield unit measurement by ROI at the latest follow-up (HU), c is the interval between the two measurements (m), and v is the annualized values of thymic density change (HU).
Patient outcomes
Baseline demographics and clinical outcomes of the patients were retrospectively collected. The response to treatment with ICIs was evaluated using RECIST criteria v1.1 (18) based on chest CT scans. OS is defined as the duration from study enrollment to patient death. PFS was defined as the time from the first dose of treatment to either radiographic imaging progression or death, whichever occurred first. The overall response rate (ORR) included patients who achieved a complete response (CR) or partial response (PR). The disease control rate (DCR) included patients who achieved a CR, PR, or stable disease (SD). Progressive disease (PD) encompassed patients who exhibited radiographic evidence of intrapulmonary progression or extrapulmonary spread.
Statistical analysis
Analysis was performed using IBM SPSS Statistics (version 28). Cohen’s weighted Kappa coefficient was used to determine the agreement of thymus scores between internal and internal observers. Descriptive statistics were employed to assess the distribution of variables. Continuous variables were presented as either mean with standard deviation or median with interquartile range (IQR), while categorical variables were summarized as counts and percentages. According to the cut-off value of attenuation/Hounsfield unit measurement by ROI, the patients were divided into the “thymus density non-loss” group and the “thymus density loss” group. we employed the Kaplan-Meier method to perform a time-event analysis of PFS and OS. The log-rank test was utilized to compare the OS and PFS outcomes between the two patient groups. To examine the influence of thymic component loss on patient survival, we employed univariate and multifactorial Cox regression models. In the univariate analysis, parameters with a P value less than 0.10 were chosen for inclusion in the multivariate Cox regression model. Afterwards, we calculated the hazard ratio (HR) along with its corresponding 95% CI.
We conducted subgroup analyses using HR based on various factors, including gender, age (<65 vs. ≥65 years), body mass index (<25 vs. ≥25 kg/m2), hypertension, smoking history, hyperlipidemia, tumor stage, ICI type, neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR). Additionally, we evaluated the presence of interactions within these subgroups. The χ2 test was employed to compare the stratified HRs among these subgroups. All statistical tests were two-tailed. Statistical significance was defined as a p-value less than 0.05.
Results
Patients
By calculation, we found that the density change values in the thymic region followed a normal distribution with a critical value of 0. Therefore, the annualized thymic density progression values were compared with 0—if the value was greater than or equal to 0, it was categorized as “non-loss”, otherwise it was classified as “loss”. Of these, in the “non-loss” group, there were 193 patients with thymic density progression, 35 of whom had previous steroid use. During the measurement of Hounsfield unit values in the thymus area, ROI standard deviations were simultaneously recorded. The median ROI standard deviations at baseline and after treatment were 16.28 (IQR: 13.70, 18.41) and 17.00 (IQR: 13.92, 20.57) respectively, indicating a good uniformity of density within the ROI. We consecutively selected 100 patients who received standard chemotherapy from February to July 2020. Inclusion and exclusion criteria were consistent with the immunotherapy queue, except for the treatment modality. The aim was to evaluate the progression of thymic density to exclude the impact of factors other than ICIs on the results. The baseline characteristics of the ICI group and the standard-chemotherapy cohort (non-ICI group) are summarized in Table S1. In particular, follow-up time did not differ between the two groups (22 vs. 23 months, P=0.45). There was no statistically significant correlation between the change in thymic density and the survival of patients in the standard chemotherapy cohort (P=0.22, Figure S1). Besides, in patients treated with immunotherapy, the annual change in density of the thymic region ranged from −108 to 108 HU. Out of all the enrolled patients, 255 (61.9%) were over 65 years of age, while 157 (38.1%) were under 65 years of age. We also documented the history of steroid use in patients and found no difference between the two groups (P=0.50), with 37 individuals (9%) in the “non-loss” group having a history of steroid use. Notably, 158 participants had a baseline thymic density of less than −100 HU, while only two had a thymic density of more than 0 HU. Interestingly, the ages of these two patients were 47 and 71 years, respectively. Overall, the two groups did not exhibit significant differences in terms of age, sex, cancer type, tumor stage, or basic biochemical indicators (Table 1).
Table 1. Baseline characteristics of the study population stratified according to the presence or absence of thymic density loss (n=412).
| Characteristics | Non-loss | Loss | P value |
|---|---|---|---|
| Patients | 202 | 210 | |
| Gender | 0.18 | ||
| Male | 173 (42.0) | 189 (45.9) | |
| Female | 29 (7.0) | 21 (5.1) | |
| Age (years) | >0.99 | ||
| <65 | 77 (18.7) | 80 (19.4) | |
| ≥65 | 125 (30.3) | 130 (31.6) | |
| Body mass index (kg/m2) | 0.11 | ||
| ≤ median* | 158 (38.3) | 177 (43) | |
| > median | 44 (10.7) | 33 (8) | |
| Total bilirubin (mg/dL) | 11.1 (4.8) | 11.0 (5.6) | 0.91 |
| A/G ratio | 1.5 (1.5) | 1.4 (0.3) | 0.47 |
| Smoking | 107 (26.0) | 117 (28.4) | 0.58 |
| Hypertension | 71 (17.2) | 63 (15.3) | 0.27 |
| Hyperlipidemia | 66 (16.0) | 60 (14.6) | 0.37 |
| History of steroid use | 37 (9.0) | 44 (10.7) | 0.50 |
| Neutrophil to lymphocyte ratio | 0.63 | ||
| ≤2 | 170 (41.3) | 173 (42.0) | |
| >2 | 32 (7.8) | 37 (9.0) | |
| Platelet to lymphocyte ratio | 0.54 | ||
| ≤150 | 130 (31.6) | 129 (31.3) | |
| >150 | 72 (17.5) | 81 (19.7) | |
| Pathological types | 0.87 | ||
| Adenocarcinoma | 92 (22.3) | 97 (23.5) | |
| Squamous carcinoma | 97 (23.5) | 102 (24.8) | |
| Other | 13 (3.2) | 11 (2.7) | |
| Stages | 0.69 | ||
| Stage IV | 146 (35.4) | 148 (35.9) | |
| Stage III | 56 (13.6) | 62 (15) | |
| Type of ICIs | 0.96 | ||
| PD-1 antibody | 185 (44.9) | 192 (46.6) | |
| PD-L1 antibody | 17 (4.1) | 18 (4.4) |
Data are presented as n (%) or mean (standard deviation). *, the median body mass index is 25 kg/m2. Non-loss, patients with no loss of thymic density; Loss, patients with loss of thymic density; A/G ratio, ratio of albumin to globulin; ICIs, immune checkpoint inhibitors; PD-1, programmed cell death-1; PD-L1, programmed cell death-ligand 1.
Effect
Throughout the entire follow-up period, 41 patients (19.5%) in the “loss” group and 64 patients (31.7%) in the “non-loss” group died. Analysis of the short-term progression of solid tumors showed that ORR (P=0.55) and DCR (P=0.67) were not statistically different between the two groups (Table S2). Furthermore, short-term immunotherapy response can be categorized into two groups based on outcome: responders (CR and PR) and non-responders (SD and PD). Logistic regression was employed to analyze the correlation between thymic density changes and immunotherapy response, and it was found that there was no correlation between them (Table S3, OR =1.13 95% CI: 0.77–1.66, P=0.55). Demographic, clinical, and cancer-related variables were included in univariate Cox proportional hazards models (Tables 2,3). Among them, the ratio of albumin to globulin (A/G ratio) and stage showed a correlation with prognosis in univariate analysis of PFS. Multivariate analysis showed that lower A/G ratio (HR =0.62 95% CI: 0.40–0.97, P=0.036), higher tumor stage (HR =2.30 95% CI: 1.59–3.31, P<0.001), and no loss of thymic density (HR =1.33 95% CI: 1.01–1.74, P=0.042) were associated with a higher risk of disease progression. the multivariate regression analysis suggested that age (HR =1.80 95% CI: 1.21–2.67, P=0.004), stages (HR =2.23 95% CI: 1.31–3.79, P=0.003) and thymus density with non-loss (HR =1.68 95% CI: 1.13–2.50, P=0.010) were independent predictors of OS.
Table 2. Effect of altered thymic density and prognostic factors on progression-free survival after immune checkpoint inhibitor therapy in univariate and multivariate Cox regression models (n=412).
| Parameter | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Gender | |||||
| Male | Reference | ||||
| Female | 1.17 (0.79, 1.74) | 0.44 | |||
| Age (years) | |||||
| <65 | Reference | ||||
| ≥65 | 1.07 (0.81, 1.41) | 0.63 | |||
| Body mass index (kg/m2) | |||||
| < median* | Reference | Reference | |||
| ≥ median | 0.74 (0.51, 1.07) | 0.11 | 0.77 (0.53, 1.12) | 0.18 | |
| Total bilirubin (mg/dL) | 1.00 (0.97, 1.03) | 0.89 | |||
| A/G ratio | 0.61 (0.39, 0.95) | 0.03 | 0.62 (0.40, 0.97) | 0.04 | |
| Smoking | |||||
| No | Reference | ||||
| Yes | 0.85 (0.65, 1.11) | 0.24 | |||
| Hypertension | |||||
| No | Reference | ||||
| Yes | 1.13 (0.85, 1.49) | 0.41 | |||
| Hyperlipidemia | |||||
| No | Reference | ||||
| Yes | 0.89 (0.66, 1.19) | 0.43 | |||
| Neutrophil to lymphocyte ratio | |||||
| ≤2 | Reference | Reference | |||
| >2 | 1.45 (0.98, 2.16) | 0.07 | 0.96 (0.62, 1.47) | 0.84 | |
| Platelet to lymphocyte ratio | |||||
| ≤150 | Reference | Reference | |||
| >150 | 1.68 (1.25, 2.26) | <0.001 | 1.36 (0.99, 1.88) | 0.059 | |
| Pathological types | |||||
| Adenocarcinoma | Reference | ||||
| Squamous carcinoma | 0.74 (0.56, 0.98) | 0.04 | |||
| Other | 0.80 (0.45, 1.43) | 0.45 | |||
| Stages | |||||
| Stage III | Reference | Reference | |||
| Stage IV | 2.42 (1.69, 3.45) | <0.001 | 2.30 (1.59, 3.31) | <0.001 | |
| Type of ICIs | |||||
| PD-1 antibody | Reference | ||||
| PD-L1 antibody | 1.21 (0.76, 1.91) | 0.42 | |||
| Group | |||||
| Loss | Reference | Reference | |||
| Non-loss | 1.27 (0.97, 1.66) | 0.08 | 1.33 (1.01, 1.74) | 0.042 | |
*, the median body mass index is 25 kg/m2. Non-loss, patients with no loss of thymic density; Loss, patients with loss of thymic density; CI, confidence interval; A/G ratio, ratio of albumin to globulin; ICIs, immune checkpoint inhibitors; PD-1, programmed cell death-1; PD-L1, programmed cell death-ligand 1.
Table 3. Effect of altered thymic density and prognostic factors on overall survival after immune checkpoint inhibitor therapy in univariate and multivariate Cox regression models (n=412).
| Parameter | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | ||
| Gender | |||||
| Male | Reference | ||||
| Female | 0.84 (0.45, 1.56) | 0.57 | |||
| Age (years) | |||||
| <65 | Reference | Reference | |||
| ≥65 | 1.91 (1.30, 2.80) | <0.001 | 1.80 (1.21, 2.67) | 0.004 | |
| Body mass index (kg/m2) | |||||
| < median* | Reference | ||||
| ≥ median | 0.76 (0.45, 1.30) | 0.32 | |||
| Total bilirubin (mg/dL) | 1.02 (0.99, 1.06) | 0.25 | |||
| A/G ratio | 0.58 (0.31, 1.09) | 0.09 | 0.64 (0.33, 1.24) | 0.19 | |
| Smoking | |||||
| No | Reference | ||||
| Yes | 1.08 (0.74, 1.59) | 0.68 | |||
| Hypertension | |||||
| No | Reference | Reference | |||
| Yes | 1.68 (1.14, 2.47) | 0.01 | 1.37 (0.91, 2.05) | 0.13 | |
| Hyperlipidemia | |||||
| No | Reference | ||||
| Yes | 0.97 (0.64, 1.46) | 0.87 | |||
| Neutrophil to lymphocyte ratio | |||||
| ≤2 | Reference | ||||
| >2 | 1.52 (0.85, 2.72) | 0.16 | |||
| Platelet to lymphocyte ratio | |||||
| ≤150 | Reference | Reference | |||
| >150 | 1.61 (1.05, 2.47) | 0.03 | 1.30 (0.83, 2.03) | 0.25 | |
| Pathological types | |||||
| Adenocarcinoma | Reference | ||||
| Squamous carcinoma | 0.80 (0.54, 1.18) | 0.26 | |||
| Other | 0.70 (0.28, 1.75) | 0.45 | |||
| Stages | |||||
| Stage III | Reference | Reference | |||
| Stage IV | 2.26 (1.34, 3.79) | 0.002 | 2.23 (1.31, 3.79) | 0.003 | |
| Type of ICIs | |||||
| PD-1 antibody | Reference | ||||
| PD-L1 antibody | 1.03 (0.52, 2.04) | 0.94 | |||
| Group | |||||
| Loss | Reference | Reference | |||
| Non-loss | 1.79 (1.21, 2.65) | 0.004 | 1.68 (1.13, 2.50) | 0.010 | |
*, the median body mass index is 25 kg/m2. Non-loss, patients with no loss of thymic density; Loss, patients with loss of thymic density; CI, confidence interval; A/G ratio, ratio of albumin to globulin; ICIs, immune checkpoint inhibitors; PD-1, programmed cell death-1; PD-L1, programmed cell death-ligand 1.
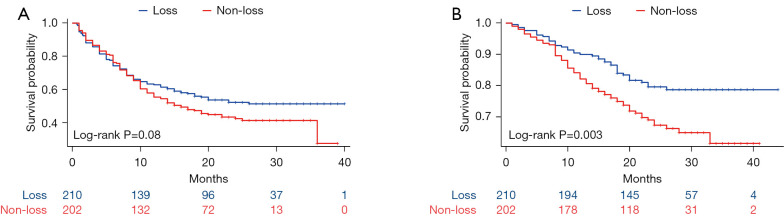
The presence or absence of thymic density loss did not show a significant difference in PFS (P=0.08) between the groups. However, a significant disparity in OS (P=0.003) (Figure 2). These findings suggest that patients with thymic density loss may have a prolonged survival. Because of the short follow-up, we could not obtain the median survival time.
Figure 2.
Survival curves of progression-free survival (A) and overall survival (B) stratified by thymic density changes following immunotherapy in non-small cell lung cancer.
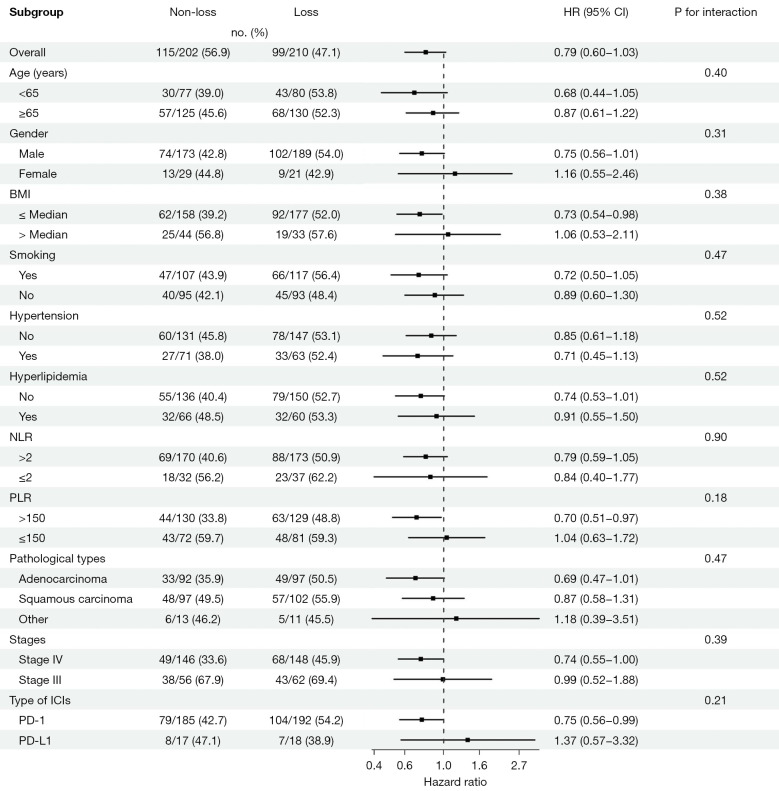
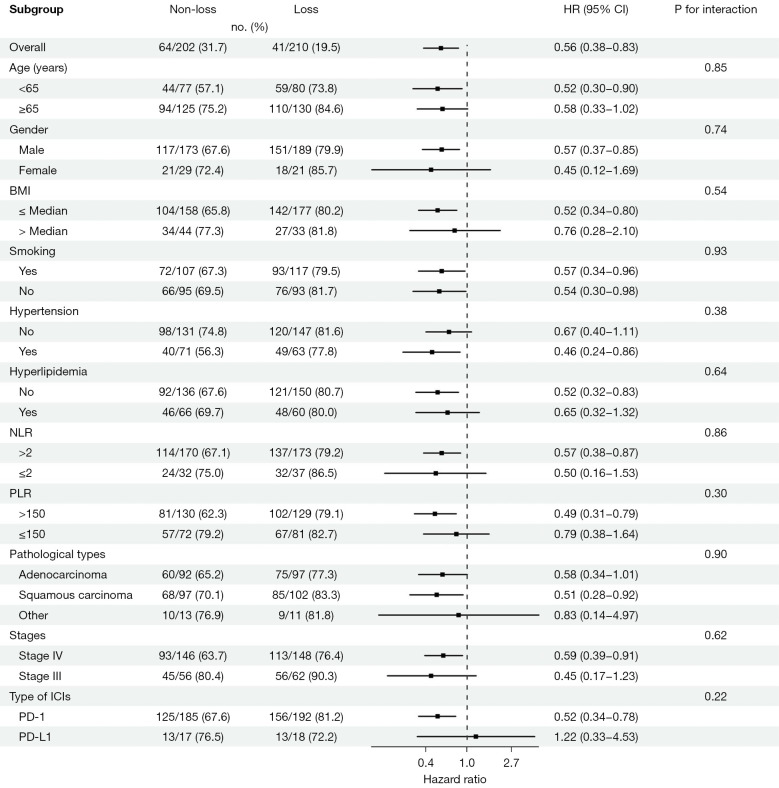
The findings from our subgroup analysis revealed that the risk of PFS was elevated in the “non-loss” group compared to the “loss” group among subgroups characterized by a BMI below the median (median =25 kg/m2), a PLR greater than 150, or treatment with programmed cell death-1 (PD-1) antibodies (Figure 3). In the subgroups comprising patients over the age of 65 years, males, those with a BMI below the median, hypertension, the absence of hyperlipidemia, elevated NLR and PLR, squamous cell carcinoma, stage IV lung cancer, and treatment with PD-1 antibodies, the “non-loss” group had a higher risk of mortality (Figure 4). In the subgroup analysis both of PFS and OS, we observed that none of the P values for interaction reached statistical significance.
Figure 3.
Forest plot of hazard ratios and confidence intervals for progression-free survival in non-small cell lung cancer patients following immunotherapy. HR, hazard ratio; CI, confidence interval; BMI, body mass index; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; ICIs, immune checkpoint inhibitors; PD-1, programmed cell death-1; PD-L1, programmed cell death-ligand 1.
Figure 4.
Forest plot of hazard ratios and confidence intervals for overall survival in non-small cell lung cancer patients following immunotherapy. HR, hazard ratio; CI, confidence interval; BMI, body mass index; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; ICIs, immune checkpoint inhibitors; PD-1, programmed cell death-1; PD-L1, programmed cell death-ligand 1.
Discussion
The thymus plays a crucial role in the immune system, as it possesses the ability to transform hematopoietic stem cells into mature T cells (19). In recent years, it has been suggested that immunosenescence, rather than the gradual accumulation of mutations, is the main reason for the increase in cancer incidence with age in many cancers (20). The “immunosurveillance hypothesis” suggests that a weakened immune system increases the chances of cancer cell survival (21). As the thymus undergoes degeneration, this situation is more likely to occur, leading to a decline in the body’s ability to fight tumor cells. However, there is currently limited research on the relationship between the thymus and immunotherapy. During our clinical practice, we serendipitously observed subtle variations in thymic density—either a decrease or increase—on chest CT scans. This intriguing finding led us to speculate that changes in thymic volume or density may have a potential correlation with the efficacy of immunotherapy. Furthermore, lung cancer patients undergo multiple chest CT scans during the course of their disease treatment, providing a readily available means to assess thymic density (22) Additionally, lung cancer patients represent a large population predominantly treated with immunotherapy, making them an ideal cohort for our study. To the best of our knowledge, this study is the first to investigate the association between thymic density loss, as evaluated on surface chest CT scans, and the response to immunotherapy in patients with NSCLC. Moreover, our study benefits from a substantial sample size, enhancing the robustness of our findings.
Our study results indicate that patients with reduced Hounsfield unit values in the thymus region after immunotherapy exhibit better OS, whereas there were no differences in immunotherapy response, ORR, DCR and PFS between the two groups. In addition, no heterogeneity was found in the subgroup analyses of PFS and OS between the two groups. Some immunotherapy trials have also yielded similar conclusions (23). PFS, ORR and DCR have been implemented as early clinical endpoints and are widely used for the assessment of antitumor therapies (24,25), but the association between short-term efficacy and long-term efficacy has not been confirmed. Among the population that benefits from immunotherapy, they have not achieved significant short-term efficacy but have shown benefit in long-term efficacy. This result may support the long-tailed effect of immunotherapy, with the observation that the longer the duration of immunotherapy, the greater the benefit for patients (26).
In the predominantly elderly NSCLC population, the thymus appears to have regressed completely, manifesting as a dense low-density area. Thymic degeneration is considered to be an important cause of immune aging. Thymic degeneration can cause heterogeneous changes in the CD4+T cell population, leading to decreased immunity, and increasing the risk of infection, autoimmune diseases and tumors (27). However, the discovery of intrathymic stem cells seems to overturn the view that thymic atrophy is irreversible (28). In other words, it is still possible for the density of thymus to become higher in old age. Numerous studies have been conducted to explore methods for thymus regeneration aiming at enhancing individual immunity (29). These research findings suggest that thymic involution may lead to impaired immune function and poorer prognosis in cancer treatment. However, the density of the thymus is not static, and no studies have thoroughly investigated the relationship between the dynamic changes in thymic density and the efficacy of cancer immunotherapy. Patients with reduced thymic density exhibit longer survival, and we offer two possible explanations for this finding. First, the decrease in thymic density could be attributed to the release of substances involved in the composition of the thymus, or to the migration of T cells from the thymus to the bloodstream and tumor cells, leading to a decrease in density as observed in imaging studies. Second, the decrease in thymic density following immunotherapy could be a rapid response of the thymus to the stimulation of immunotherapy drugs, suggesting enhanced thymic activity. During immunotherapy, the dynamic changes in thymic density may provide more information about the mechanisms of immunotherapy than the thymic density before treatment.
Rebound thymic hyperplasia has been observed in different types of malignant tumors and different treatments (30,31), suggesting that changes in thymic density may not be uncommon and independent of tumor type and treatment modality. Deniz et al. investigated the overall impact of rebound thymic hyperplasia in patients with NSCLC undergoing systemic chemotherapy. The median time for rebound thymic hyperplasia was 4.5 months (2–7 months) after examination. The OS of patients with rebound thymic hyperplasia (20.04 months; 95% CI: 4.79–35.29) was longer than that of patients without rebound thymic hyperplasia (10.05 months; 95% CI: 8.74–11.36; P=0.049) (32). In contrast, immunotherapy has shown contrasting results, which is an intriguing phenomenon that may be related to the different pharmacological mechanisms of chemotherapy drugs and immunotherapeutic agents. It is recognized that PD-1 is expressed in T cells, B cells, and macrophages, while its ligand, PD-L1, is present in tumors and normal tissues, including the thymus. We hypothesize that in immunotherapy, increased thymic activity is associated with the efficacy of the treatment, and the changes in thymic density can, to some extent, reflect the degree of thymic activity. Currently, there is a lack of sufficient evidence to explain the specific mechanisms underlying these imaging findings, thus further research is needed to elucidate the role of the thymus in immunotherapy. Our study may potentially provide some inspiration for future mechanistic research in this direction.
The A/G ratio has been demonstrated as an independent prognostic factor for predicting mortality risk in patients undergoing immunotherapy (33,34), In our multifactorial analysis of patients, we also found that the A/G ratio might serve as a predictive factor for PFS, consistent with the aforementioned findings. This could be attributed to the fact that albumin is an indicator of nutritional status, liver function, and chronic inflammation, and is closely associated with malnutrition and severe liver impairment, which may lead to poor prognosis of tumors (35). However, the A/G ratio did not demonstrate a significant predictive value in OS analysis, possibly due to the limited sample size.
Our study has several limitations. Firstly, our study is a single-center retrospective study, which may introduce information bias and has certain limitations. For example, we were unable to determine the patterns of attenuation/Hounsfield unit measurement by ROI changes in patients before treatment, which could be a potential confounding factor. Due to the limitations of our retrospective study design, we did not investigate the subpopulations of lymphocytes in patients, which could provide a more comprehensive assessment of their immune status. Subgroup analysis of lymphocyte subsets could potentially offer a more direct reflection of patients’ immune function (36). Concomitantly, information regarding immune-related adverse events was not available, precluding the determination of any potential associations between changes in thymic density and the occurrence of such events. This is a notable omission, as elucidating the relationship between thymic density and immune-related adverse events could provide valuable insights into the dynamics of the immune system and its implications for patient health. Furthermore, information related to other treatments such as radiotherapy and other severe systemic stress factors that may be associated with increased thymic rebound could not be collected. Some studies have suggested that thymic morphology may also serve as a factor revealing thymic function. However, our study primarily focused on changes in thymic density, while the variations in thymic morphology lack a consensus and are difficult to identify on imaging images. Therefore, we did not investigate the changes in thymic morphology. Additionally, our study only targeted patients with NSCLC, and it is expected that future multi-center, multi-cancer randomized controlled trials will be conducted to validate the conclusions drawn from this study.
Overall, our study adds to a growing body of evidence for the role of the thymus in immune function and highlights the potential clinical value of regular chest CT scans in evaluating the efficacy of immunotherapy. Understanding the relationship between changes in thymus density and treatment outcomes in NSCLC patients receiving immunotherapy may have important implications for personalizing treatment approaches and improving patient outcomes.
Conclusions
Decreased thymic density is associated with increased OS in NSCLC patients treated with ICIs. This reduction in thymic density could potentially serve as a surrogate marker for thymic function, indicating heightened thymic and immune system activity. This finding could provide valuable insights for guiding the administration of immune drugs to patients or enhancing the efficacy of immunotherapy in the future.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors extend their sincere gratitude to all individuals who participated in this project and provided assistance, particularly to Liling Li for her help in statistics.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. S054). Written informed consent was waived due to the retrospective nature of this study.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-203/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-203/coif). The authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-203/dss
References
- 1.Li C, Lei S, Ding L, et al. Global burden and trends of lung cancer incidence and mortality. Chin Med J (Engl) 2023;136:1583-90. 10.1097/CM9.0000000000002529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023-75. 10.1016/S0140-6736(17)33326-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reck M, Remon J, Hellmann MD. First-Line Immunotherapy for Non-Small-Cell Lung Cancer. J Clin Oncol 2022;40:586-97. 10.1200/JCO.21.01497 [DOI] [PubMed] [Google Scholar]
- 4.James KD, Jenkinson WE, Anderson G. Non-Epithelial Stromal Cells in Thymus Development and Function. Front Immunol 2021;12:634367. 10.3389/fimmu.2021.634367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo CL, Mei JD, Jia YL, et al. Impact of thymosin α1 as an immunomodulatory therapy on long-term survival of non-small cell lung cancer patients after R0 resection: a propensity score-matched analysis. Chin Med J (Engl) 2021;134:2700-9. 10.1097/CM9.0000000000001819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giacomini E, Severa M, Cruciani M, et al. Dual effect of Thymosin α 1 on human monocyte-derived dendritic cell in vitro stimulated with viral and bacterial toll-like receptor agonists. Expert Opin Biol Ther 2015;15 Suppl 1:S59-70. 10.1517/14712598.2015.1019460 [DOI] [PubMed] [Google Scholar]
- 7.Danielli R, Cisternino F, Giannarelli D, et al. Long-term follow up of metastatic melanoma patients treated with Thymosin alpha-1: investigating immune checkpoints synergy. Expert Opin Biol Ther 2018;18:77-83. 10.1080/14712598.2018.1494717 [DOI] [PubMed] [Google Scholar]
- 8.Wang S, Huang M, Chen M, et al. Zoledronic acid and thymosin α1 elicit antitumor immunity against prostate cancer by enhancing tumor inflammation and cytotoxic T cells. J Immunother Cancer 2023;11:e006381. 10.1136/jitc-2022-006381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ackman JB, Kovacina B, Carter BW, et al. Sex difference in normal thymic appearance in adults 20-30 years of age. Radiology 2013;268:245-53. 10.1148/radiol.13121104 [DOI] [PubMed] [Google Scholar]
- 10.Kumar BV, Connors TJ, Farber DL, Human T. Cell Development, Localization, and Function throughout Life. Immunity 2018;48:202-13. 10.1016/j.immuni.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li YR, Zúñiga-Pflücker JC. Thymus aging and immune reconstitution, progresses and challenges. Semin Immunol 2023;70:101837. 10.1016/j.smim.2023.101837 [DOI] [PubMed] [Google Scholar]
- 12.Mittelbrunn M, Kroemer G. Hallmarks of T cell aging. Nat Immunol 2021;22:687-98. 10.1038/s41590-021-00927-z [DOI] [PubMed] [Google Scholar]
- 13.Kooshesh KA, Foy BH, Sykes DB, et al. Health Consequences of Thymus Removal in Adults. N Engl J Med 2023;389:406-17. 10.1056/NEJMoa2302892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Araki T, Nishino M, Gao W, et al. Normal thymus in adults: appearance on CT and associations with age, sex, BMI and smoking. Eur Radiol 2016;26:15-24. 10.1007/s00330-015-3796-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandstedt M, Chung RWS, Skoglund C, et al. Complete fatty degeneration of thymus associates with male sex, obesity and loss of circulating naïve CD8+ T cells in a Swedish middle-aged population. Immun Ageing 2023;20:45. 10.1186/s12979-023-00371-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lynch HE, Goldberg GL, Chidgey A, et al. Thymic involution and immune reconstitution. Trends Immunol 2009;30:366-73. 10.1016/j.it.2009.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:497-530. 10.6004/jnccn.2022.0025 [DOI] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 19.Miller JF. The golden anniversary of the thymus. Nat Rev Immunol 2011;11:489-95. 10.1038/nri2993 [DOI] [PubMed] [Google Scholar]
- 20.Palmer S, Albergante L, Blackburn CC, et al. Thymic involution and rising disease incidence with age. Proc Natl Acad Sci U S A 2018;115:1883-8. 10.1073/pnas.1714478115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dadi S, Chhangawala S, Whitlock BM, et al. Cancer Immunosurveillance by Tissue-Resident Innate Lymphoid Cells and Innate-like T Cells. Cell 2016;164:365-77. 10.1016/j.cell.2016.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drabkin MJ, Meyer JI, Kanth N, et al. Age-stratified Patterns of Thymic Involution on Multidetector CT. J Thorac Imaging 2018;33:409-16. 10.1097/RTI.0000000000000349 [DOI] [PubMed] [Google Scholar]
- 23.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merino M, Kasamon Y, Theoret M, et al. Irreconcilable Differences: The Divorce Between Response Rates, Progression-Free Survival, and Overall Survival. J Clin Oncol 2023;41:2706-12. 10.1200/JCO.23.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. 10.1016/S0140-6736(18)32409-7 [DOI] [PubMed] [Google Scholar]
- 26.Gettinger S, Horn L, Jackman D, et al. Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: Results From the CA209-003 Study. J Clin Oncol 2018;36:1675-84. 10.1200/JCO.2017.77.0412 [DOI] [PubMed] [Google Scholar]
- 27.Thapa P, Farber DL. The Role of the Thymus in the Immune Response. Thorac Surg Clin 2019;29:123-31. 10.1016/j.thorsurg.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ragazzini R, Boeing S, Zanieri L, et al. Defining the identity and the niches of epithelial stem cells with highly pleiotropic multilineage potency in the human thymus. Dev Cell 2023;58:2428-2446.e9. 10.1016/j.devcel.2023.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wertheimer T, Velardi E, Tsai J, et al. Production of BMP4 by endothelial cells is crucial for endogenous thymic regeneration. Sci Immunol 2018;3:eaal2736. 10.1126/sciimmunol.aal2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nasseri F, Eftekhari F. Clinical and radiologic review of the normal and abnormal thymus: pearls and pitfalls. Radiographics 2010;30:413-28. 10.1148/rg.302095131 [DOI] [PubMed] [Google Scholar]
- 31.Chen CH, Hsiao CC, Chen YC, et al. Rebound Thymic Hyperplasia after Chemotherapy in Children with Lymphoma. Pediatr Neonatol 2017;58:151-7. 10.1016/j.pedneo.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 32.Deniz S, Susam S, Aksel N, et al. Effect of Rebound Thymic Hyperplasia on Survival in Chemotherapy-Treated Lung Cancer. Turk Thorac J 2020;21:303-7. 10.5152/TurkThoracJ.2020.18163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guven DC, Aktepe OH, Aksun MS, et al. The association between albumin-globulin ratio (AGR) and survival in patients treated with immune checkpoint inhibitors. Cancer Biomark 2022;34:189-99. 10.3233/CBM-210349 [DOI] [PubMed] [Google Scholar]
- 34.Nakanishi Y, Masuda T, Yamaguchi K, et al. Albumin-globulin ratio is a predictive biomarker of antitumor effect of anti-PD-1 antibody in patients with non-small cell lung cancer. Int J Clin Oncol 2020;25:74-81. 10.1007/s10147-019-01539-2 [DOI] [PubMed] [Google Scholar]
- 35.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 2010;9:69. 10.1186/1475-2891-9-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garrido-Rodríguez V, Herrero-Fernández I, Castro MJ, et al. Immunological features beyond CD4/CD8 ratio values in older individuals. Aging (Albany NY) 2021;13:13443-59. 10.18632/aging.203109 [DOI] [PMC free article] [PubMed] [Google Scholar]