Abstract
To replicate in vivo, viruses must circumvent cellular antiviral defense mechanisms, including those induced by the interferons (IFNs). Here we demonstrate that simian virus 5 (SV5) blocks IFN signalling in human cells by inhibiting the formation of the IFN-stimulated gene factor 3 and gamma-activated factor transcription complexes that are involved in activating IFN-α/β- and IFN-γ-responsive genes, respectively. SV5 inhibits the formation of these complexes by specifically targeting STAT1, a component common to both transcription complexes, for proteasome-mediated degradation. Expression of the SV5 structural protein V, in the absence of other virus proteins, also inhibited IFN signalling and induced the degradation of STAT1. Following infection with SV5, STAT1 was degraded in the absence of virus protein synthesis and remained undetectable for up to 4 days postinfection. Furthermore, STAT1 was also degraded in IFN-pretreated cells, even though the cells were in an antiviral state. Since pretreatment of cells with IFN delayed but did not prevent virus replication and protein synthesis, these observations suggest that following infection of IFN-pretreated cells, SV5 remains viable within the cells until they eventually go out of the antiviral state.
Virus infection of susceptible host cells activates the transcription of many cellular genes, including the interferons (IFNs), that are involved in antiviral defense, cell growth regulation, and immune activation. IFNs represent a group of cytokines with the unique ability to establish an antiviral state in cells through the expression of many IFN-stimulated genes (ISGs). A number of these ISGs encode intracellular enzymes, the best known of which is a protein kinase (PKR). Although induced by IFN, PKR remains inactive unless cells also produce excess double-stranded RNA (e.g., as a result of viral infection). Activated PKR modifies and inactivates eukaryotic initiation factor 2α, a key component of the eukaryotic translational apparatus, leading to the shutoff of viral protein synthesis (9). IFNs also induce 2′5′ oligoadenylate synthetase (30), which, together with RNase L, results in accelerated RNA degradation and thus also an inhibition of protein synthesis. In addition, IFNs down-regulate the cell cycle (45) and induce a proapoptotic state in cells (3) as well as up-regulate the surface expression of class I major histocompatibility complex (MHC) molecule, thereby enhancing peptide presentation to T cells (27).
The biological activities of IFNs are initiated by the binding of IFN-α/β and IFN-γ to their cognate receptors, which results in the activation of distinct but related signalling pathways. IFN-α/β signal via receptor-associated tyrosine kinases, Jak1 and Tyk2, that phosphorylate and activate the signal transducers and activators of transcription, STAT1 and STAT2. Upon phosphorylation, STAT1 and STAT2 form heterodimers which translocate to the nucleus, where they associate with the DNA-binding protein p48 to form interferon-stimulated gene factor 3 (ISGF3). ISGF3 binds IFN-stimulated response elements (ISREs) to drive the expression of IFN-α/β regulated genes. Similarly, IFN-γ signals via receptor-associated tyrosine kinases, Jak1 and Jak2, mediating phosphorylation and activation of STAT1 but not STAT2. STAT1 homodimers form the active transcription complex, gamma-activated factor (GAF) which bind to gamma-activated sequence (GAS) elements in regulatory regions of IFN-γ-inducible genes. Since STAT1 is also activated by IFN-α/β, the GAS complex can be formed in response to IFN-α/β. Thus, Jak1 and STAT1 are the common components of IFN-α/β and IFN-γ signal transduction pathways (for a review, see reference 43). The STAT1 gene contains multiple exons which encode two forms of STAT1 (37). STAT1α (91 kDa) is 750 amino acids in length; STAT1β (84 kDa) is the product of a differentially spliced mRNA which encodes a protein of 712 amino acids (37, 50). Both forms are known to be phosphorylated on a single residue, Tyr-701, allowing their dimerization and translocation to the nucleus to bind DNA (40, 41). However, STAT1α is the only transcriptionally active form of STAT1 since STAT1β lacks the 38 C-terminal amino acids containing the known transcriptional activation domain (24, 37). For maximal transcriptional activity, STAT1α must also be phosphorylated on Ser-727 (49), a residue that is missing in STAT1β.
For most known IFN-induced antiviral activities, there are examples of virally encoded gene products that antagonize their effects. Viral products that specifically inhibit PKR, block or down-regulate MHC class I expression, stimulate cell division, inhibit apoptosis, or act as decoy MHC-like molecules to prevent NK cell activation have been described (31, 39, 42). To date, there are few examples of viruses inhibiting transcriptional responses to IFNs; certain poxviruses secrete soluble IFN receptor proteins which block the IFN-γ responses (47); similarly, vaccinia virus encodes a soluble IFN-α/β receptor (44). Other viruses have been shown to block transcriptional responses by altering the levels or function of critical components of the signalling pathways. For example, the E1A product of adenovirus has been described as having the ability to block IFN responses by interfering with transcription (19), and Look et al. (20) have shown that the adenovirus E1A protein can directly suppress STAT1 function. It has also been demonstrated that the K9 open reading frame of human herpesvirus 8 can block transcriptional responses to IFN-α/β and IFN-γ (52), and there is evidence that human cytomegalovirus alters Jak1 levels, thereby disrupting the IFN-α/β and IFN-γ signalling pathways (23). It has also been noted that there are decreased levels of STAT1α in cells persistently infected with mumps virus (51).
We have previously demonstrated that the paramyxovirus simian virus 5 (SV5) blocks IFN-α/β signalling in human but not murine cells (6), thereby defining a host cell constraint which may prevent SV5 from crossing species barriers and causing disease in mice. Here we extend these findings by showing that SV5 can also block IFN-γ signalling in human cells and identify both the cellular target and the viral protein responsible for this inhibition.
SV5 is an enveloped virus with a nonsegmented, negative-sense RNA genome. The single-stranded genome encodes eight proteins: the nucleocapsid protein (NP), V protein (V), phosphoprotein (P), matrix protein (M), fusion protein (F), small hydrophobic protein, hemagglutinin-neuraminidase protein, and large polymerase protein (for a review of paramyxoviruses and their replication, see reference 17). P and V are both structural proteins and are encoded by a single gene. They share the same 164 N-terminal amino acids but have unique C termini. The C terminus of V is cysteine rich, binds two atoms of zinc per molecule (28), and is highly conserved among paramyxoviruses. V mRNA is a faithful transcript of the P/V gene, but P mRNA contains two additional nontemplated G residues (46), specifically inserted by the viral RNA polymerase stuttering during transcription of the gene (48), which alters the reading frame of the mRNA. Although it is known that P is part of the virus-encoded polymerase complex, the roles of V in virus replication and pathogenesis are unclear. It appears to be dispensable for virus replication in tissue culture cells, but it is essential for virus pathogenesis in Sendai virus (SeV) (5, 13). The V protein of SV5 has also been shown to bind soluble but not polymeric forms of NP (33), and also to bind to the cellular UV DNA damage binding protein (21). Here we demonstrate that the V, but not the P, protein of SV5 also blocks IFN signalling by targeting STAT1 for proteasome-mediated degradation.
MATERIALS AND METHODS
Cell culture and virus infections.
Human diploid fibroblast 2fTGH cells (29) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (growth medium). SV5 (strain W3 [4]) and recombinant Semliki Forest virus (SFV) infections were performed at a multiplicity of infection (MOI) of 10 in DMEM containing 2% fetal bovine serum (maintenance medium) and in reduced-serum medium (Opti-MEM; Gibco-BRL), respectively. After an adsorption period of 1 to 2 h, the inoculum was removed and replaced with maintenance medium. Cells were treated with either recombinant human IFN-αA/D (rHuIFN-αA/D [34]) or human IFN-γ (catalog no. 80-3348-01; Genzyme Diagnostic) added to maintenance medium at 1,000 IU/ml. For proteasome inhibitor experiments, 2fTGH cells in 6- or 24-well plates were mock infected or infected with SV5 or recSFV in medium containing 0.2% dimethyl sulfoxide (DMSO) that had or had not been supplemented with either 10 μM MG132 or 10 μM lactacystin (catalog no. PI-104; BIOMOL Research Laboratories Inc.). After a 1- to 2-h adsorption period, the medium was removed and replaced with maintenance medium supplemented with 0.2% DMSO or 0.2% DMSO containing 10 μM MG132 or lactacystin, and cells were incubated for a further 6 h. In experiments monitoring IκBα degradation, cells then were or were not treated with tumor necrosis factor alpha (TFN-α) at 10 ng/ml. At 8 h postinfection, (p.i.), the cell monolayers were washed twice in phosphate-buffered saline (PBS) and lysed directly in sodium dodecyl sulfate (SDS)-gel loading buffer (0.05 M Tris-HCl [pH 7.0], 0.2% SDS, 5% 2-mercaptoethanol, 5% glycerol).
Immunoblotting.
At the time of harvest, cells were washed twice in PBS, scraped in SDS-gel loading buffer, and boiled for 5 min. Proteins were separated by electrophoresis through SDS–7.5% polyacrylamide gels, transferred to nitrocellulose membranes, and detected with specific antisera, including a polyclonal anti-STAT1 antibody raised against the N-terminal 194 amino acids, a monoclonal antibody (MAb) reactive to the C terminus of STAT1, and a MAb to the N-terminal region of STAT2 (catalogue no. G16930, S21120, and S21220, respectively; Transduction Laboratories) and polyclonal antibodies to either phosphotyrosine (701) STAT1 or phosphoserine (727) STAT1 (catalogue no. 06-657 or 06-802, respectively; Upstate Biotechnology). Unless otherwise stated, STAT1 was detected with an antibody to the N terminus of STAT1. It should also be noted that antibodies raised against both the N and C termini of STAT1 (G16930 and S21120, respectively) react with STAT1α and STAT1β. IκBα was detected with the MAb 10B (12). All protein-antibody interactions were detected by enhanced chemiluminescence using horseradish peroxidase-conjugated sheep anti-mouse or donkey anti-rabbit immunoglobulin G (Amersham International Ltd., Little Chalfont, United Kingdom).
Plasmid DNAs.
The IFN-α/β-responsive plasmid, p(9-27)4tkΔ(−39)lucter, contained four tandem repeat sequences of the ISRE from the IFN-inducible gene 9-27 fused to the firefly luciferase gene (16); the IFN-γ-responsive plasmid, p(GAS)2tkΔ(−39)lucter, contained a minimal thymidine kinase (TK) promoter and two tandem repeat sequences of the IRF-1 GAS site fused to the luciferase gene (16). pJATlacZ, a plasmid used as a transfection standard, contains a β-galactosidase gene under the control of the rat β-actin promoter (22).
To construct pEF.SV5-P and pEF.SV5-V vectors, SV5 P and V cDNA sequences were obtained from pGEM3 expression plasmids into which the P and V genes had been originally cloned (32). The cDNAs were inserted between the NcoI and XhoI sites of the EF1a promoter vector, pEFlink2 (a kind gift from R. H. Treisman, Imperial Cancer Research Fund).
Transient transfections.
Monolayers of 2fTGH cells grown in 24-well plates to 50 to 70% confluence were transfected with 0.5 μg of DNA and 2 μl of Lipofectamine (Life Technologies Inc.) according to the manufacturer’s instructions. After 16 h, the cells were or were not infected with SV5 and induced with 1,000 IU of rHuIFN-αA/D per ml at 24 h p.i. Four hours after induction by IFN, cells were harvested and assayed for luciferase and β-galactosidase activity as described previously (15). Relative expression levels were calculated by dividing the luciferase values by the β-galactosidase values. The experiments were repeated several times with equivalent results.
Electrophoretic mobility shift assay (EMSA).
Whole-cell extracts (25) were prepared from mock-infected or SV5-infected cells that had or had not been treated with rHuIFN-αA/D for 1 h prior to harvesting at 24 h p.i. Protein-DNA complexes were formed by incubating 10 μg of protein for 15 min at 30°C with 1 ng of probe (labelled with [a 32P]dATP by filling in the GATC 5′ overhangs with Klenow enzyme on the otherwise double-stranded oligonucleotides 5′ AGGAAATAGAAACTG 3′ [ISRE], or 5′ TGATTTCCCCGAAATG 3′ [GAS]) in a 20-μl reaction mixture containing 20 mM Tris (pH 8.0), 12% glycerol, 2 mM MgCl2, 0.6 mM dithiothreitol, and 375 ng poly(dI-dC) · poly(dI-dC). Complexes were resolved on 6% native polyacrylamide (1:30 bisacrylamide/acrylamide) gels in 0.5× Tris-borate-EDTA, and the dried gels visualized by autoradiography.
RESULTS
SV5 prevents the formation of ISGF3 and GAF complexes in human cells.
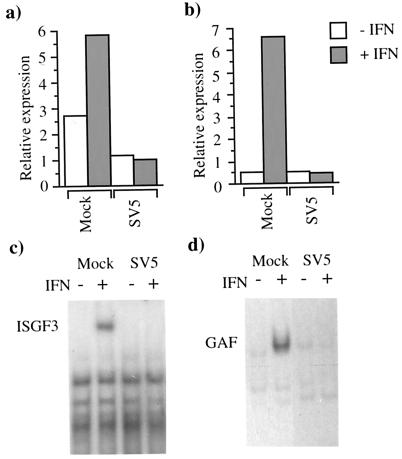
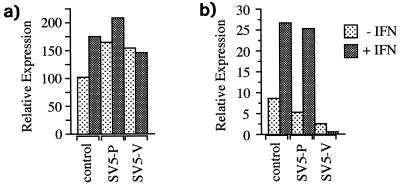
Using luciferase reporter assays, we previously demonstrated that SV5 blocked IFN-α/β signalling in human cells (Fig. 1a) but not murine cells (6). Using a similar approach, we show here that SV5 also blocks IFN-γ signalling in human cells (Fig. 1b) but not in murine cells (data not shown). To determine whether SV5 inhibition of IFN-α/β and IFN-γ signalling in human cells reflected an inability to form the transcription complexes ISGF3 and GAF, EMSAs were performed. In contrast to the induction of ISGF3 and GAF complexes in mock-infected cells treated with IFN, no ISGF3 and GAF complexes were detected in the SV5-infected cell extracts (Fig. 1c and d, respectively). (ISGF3 and GAF complexes were readily detected in SV5-infected murine cells in the presence of IFN [data not shown].)
FIG. 1.
SV5 inhibits IFN-α/β (a) and IFN-γ (b) signalling and the formation of ISGF3 (c) and GAF (d) complexes in 2fTGH cells. Cells were transfected with either the IFN-α/β (a)- or IFN-γ (b)-responsive plasmids together with pJATlacZ and at 16 h posttransfection were either mock infected or infected with SV5. At 24 h p.i. the culture medium was supplemented with rHuIFN-αA/D (a) or IFN-γ (b) or left untreated. Four hours later, luciferase and β-galactosidase activities in the cellular lysates were measured. Luciferase activity, expressed in relative light units, was normalized to β-galactosidase activity. For the EMSAs, cells were mock infected or infected with SV5 for 24 h and then were (+) or were not (−) treated with rHuIFN-αA/D (c) or rHuIFN-γ (d) for 1 h. Extracts were prepared and analyzed by EMSAs using either 32P-labelled ISRE (c) or GAS (d) probe.
Proteasome-mediated degradation of STAT1 in SV5-infected cells.
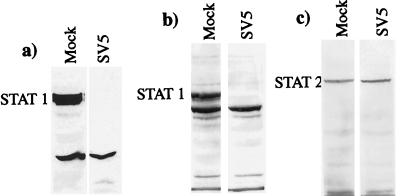
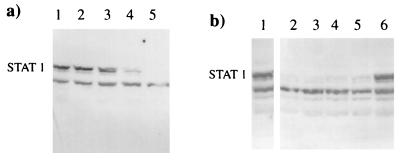
Since STAT1 is the only common component of ISGF3 and GAF complexes, the levels of STAT1 in mock- or SV5-infected human cells were examined by immunoblot blot analysis using antibodies reactive to either the C (Fig. 2a) or N (Fig. 2b) terminus of STAT1. Although STAT1 was readily detectable in mock-infected cells (Fig. 2a and b), no STAT1 was detected in SV5-infected cells (Fig. 2a and b). (It should be noted that both STAT1α and STAT1β were degraded in SV5 infected cells. The lower prominent band in Fig. 2b and subsequent figures is not STAT1β [see the legend to Fig. 2].) Immunoblot analysis using antisera reactive against the phosphotyrosine (701) and phosphoserine (727) forms of STAT1 showed that phosphorylated forms of STAT1 were also degraded in SV5-infected cells (data not shown). In contrast, the levels of STAT2 were comparable in mock- and SV5-infected cells (Fig. 2c).
FIG. 2.
STAT1, but not STAT2, is degraded in SV5-infected human cells. STAT1 and STAT2 were detected by immunoblot analysis in total-cell extracts of mock- or SV5-infected 2fTGH cells (harvested at 20 h p.i.), using antibodies reactive to the C terminus of STAT1 (a) or the N terminus of STAT1 (b) or STAT2 (c). It should be noted that both anti-STAT1 antibodies react with STAT1α and STAT1β (they can be more clearly resolved on lower-percentage SDS-polyacrylamide gels). The lower prominent band, with an estimated molecular mass of 75 kDa, present in both mock-infected and SV5-infected cells (b) has not been identified.
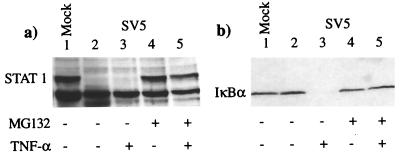
No obvious degradation products of STAT1, such as those that might be expected for a sequence-specific endoprotease such as a caspase (16), were visible in the immunoblots of SV5-infected cells. The failure to observe breakdown intermediates suggested that the proteins were being degraded by a processive protease such as is seen for proteasome-mediated degradation. To test this, the levels of STAT1 were examined in cells treated with the proteasome inhibitors MG132 and lactacystin (potent and structurally unrelated inhibitors of proteasome action [8, 26, 35]). These results (Fig. 3a) clearly showed that in SV5-infected cells, STAT1 was degraded in the absence of MG132 (lanes 2 and 3); however, this effect was blocked by MG132 treatment (lanes 4 and 5). In the same experiment, MG132 also prevented TNF-α-induced degradation of IκBα (Fig. 3b; compare lanes 3 and 5), demonstrating that the inhibitor was effective at blocking proteasome-mediated degradation processes (1, 36). Lactacystin also inhibited the degradation of STAT1 in SV5-infected cells (data not shown).
FIG. 3.
The proteasome inhibitor MG132 blocks degradation of STAT1 in SV5-infected 2fTGH cells and of IκBα in TNF-α-stimulated 2fTGH cells. Mock-infected cells (lanes 1) and SV5-infected cells (lanes 2 to 5) were incubated, from the time of infection, in medium that did (lanes 4 and 5) or did not (lanes 1 to 3) contain the proteasome inhibitor MG132 (10 μM). At 8 h p.i., the medium was (lanes 3 and 5) or was not (lanes 1, 2, and 4) further supplemented with TNF-α; 30 min later, the cells were harvested and the levels of STAT1 (a) and IκBα (b) were estimated by immunoblot analysis.
The V protein of SV5 targets STAT1 for proteasome-mediated degradation.
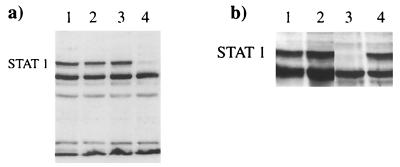
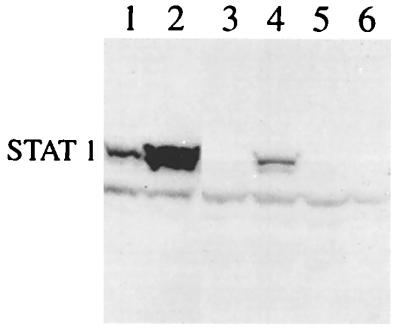
We previously constructed a number of SFV vectors, including those that expressed the fusion (F) or V proteins of SV5. To determine whether either of these proteins might be responsible for the observed degradation of STAT1, we infected cells (at a high MOI) with these viruses or a virus that expressed β-galactosidase and estimated the levels of STAT1 by immunoblot blot analysis (Fig. 4a). Negligible levels of STAT1 were present in cells infected with a recombinant SFV that expressed the V protein of SV5 (recSFV/V; Fig. 4a, lane 4). In contrast, levels of STAT1 similar to that observed in mock-infected cells (Fig. 4, lane 1) were present in cells infected with a recombinant SFV that expressed either the SV5 F protein (recSFV/F) (lane 2) or β-galactosidase (recSFV/lacZ) (lane 3).
FIG. 4.
The V protein of SV5 induces proteasome-mediated degradation of STAT1. (a) 2fTGH cells were mock infected (lane 1) or infected with recSFV/F (lane 2), recSFV/lacZ (lane 3), or recSFV/V (lane 4) for 8 h, and total-cell extracts were probed for STAT1 as previously described. (Immunofluorescence analysis confirmed that >95% of the cells were expressing the appropriate virus proteins at the time of harvest.) (b) 2fTGH cells were mock infected (lanes 1 and 2) or infected with recSFV/V (lanes 3 and 4). The proteasome inhibitor MG132 (10 μM) was (lanes 2 and 4) or was not (lanes 1 and 3) added to the culture medium at the time of infection. At 8 h p.i., cells were harvested and levels of STAT1 were estimated by immunoblot analysis.
To confirm that the mechanism of degradation of STAT1 induced by the V protein of SV5 was the same as that observed in SV5-infected cells, the levels of STAT1 were examined in cells treated with the proteasome inhibitor MG132. These results demonstrated that MG132 inhibited the degradation of STAT1 in cells infected with recSFV/V (Fig. 4b; compare lanes 3 and 4). In the same experiment, no degradation of STAT1 was observed in cells expressing either SV5 F or the β-galactosidase proteins in the presence or absence of the inhibitor (data not shown).
The first 164 N-terminal amino acids are common between the V and P proteins of SV5, but V and P possess unique C termini. It was therefore important to ascertain whether P could also block IFN signalling. To address this question, we cloned the P and V genes into the EF1α promoter vector pEFlink2 and measured the ability of the expressed proteins to block the activation of the IFN-α/β-responsive plasmid. It can clearly be seen from Fig. 5 that expression of P and V had no effect on the TK control promoter (Fig. 5a). However, V, but not P, blocked activation of the IFN-α/β-responsive promoter (Fig. 5b).
FIG. 5.
The V protein of SV5, but not P, blocks activation of an IFN-α/β-responsive promoter in human cells. 2fTGH cells were transfected with a mixture of plasmids that contained the luciferase gene under the control of either the herpes simplex virus TK promoter (a) or an IFN-α/β-responsive promoter (b; 0.1 μg), together with 0.3 μg of pEFlink2 (control), pEF.SV5-P (expressing the SV5 P protein), or pEF.SV5-V (expressing the SV5 V protein). Also included in the transfection mix was 0.1 μg of plasmid pJATlacZ, in which the lacZ gene is under the control of the rat β-actin promoter. At 40 h posttransfection, the culture medium was (+) or was not (−) supplemented with IFN. Four hours later, luciferase and β-galactosidase activities in cellular lysates were measured. Luciferase activity, expressed in relative light units, was normalized to β-galactosidase activity.
Infecting virus can induce the degradation of STAT1 in the absence of virus protein synthesis.
Since the V protein is a structural component of the SV5 virion, it was of interest to determine how quickly STAT1 disappeared following SV5 infection and whether virus protein synthesis was required. In a time course experiment following infection of cells at a high MOI (10 PFU/cell), there was a significant reduction in the amount of STAT1 by 4 h p.i. and complete loss of STAT1 by 8 h p.i. (Fig. 6a, lanes 4 and 5, respectively). In contrast, STAT2 levels remained constant throughout the experiment (data not shown). Furthermore, immunofluorescence analysis and immunoprecipitation of 35S-labelled polypeptides showed that little virus protein synthesis had occurred by 4 h p.i. (data not shown), suggesting that STAT1 might be degraded in the absence of virus protein synthesis. To determine whether this was the case, cells were infected with SV5 that had been inactivated by UV light such that the virus could no longer synthesize detectable amounts of virus proteins. These results demonstrated that UV-inactivated virus also induced the degradation of STAT1 (data not shown; see also Fig. 6b).
FIG. 6.
STAT1 was rapidly degraded in SV5-infected cells in the absence of virus protein synthesis. (a) Total-cell extracts of 2fTGH cells that were mock infected (lane 1) or infected with SV5 for 1, 2, 4, or 8 h (lanes 2 to 5, respectively) were probed for STAT1 by immunoblot analysis. (b) 2fTGH cells were mock infected (lane 1) or infected with UV-inactivated SV5 (lanes 2 to 6). At 6 (lanes 1 and 2) and 24, 48, 72, and 96 (lanes 3 to 6, respectively) h p.i. total cell extracts were probed for STAT1 by immunoblot analysis. (At no time throughout the latter experiment were cells positive for virus antigens, as judged by immunofluorescence.)
To determine how long it took cells to recover normal levels of STAT1 in the absence of virus protein synthesis, cells were infected with UV-inactivated virus and harvested at various times p.i., and levels of STAT1 were estimated (Fig. 6b). At 3 days p.i., STAT1 protein levels remained negligible (Fig. 6b, lane 5), and it took up to 4 days before substantial levels of STAT1 could be detected (Fig. 6b, lane 6).
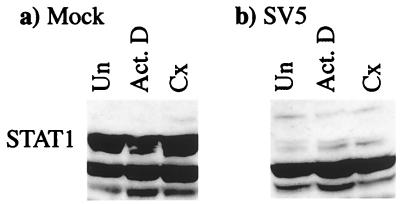
The finding that virus (and cellular) transcription and protein synthesis were not required for the degradation of STAT1 in SV5-infected cells was confirmed by using actinomycin D and cycloheximide, inhibitors of transcription and protein synthesis, respectively. The levels of STAT1 in untreated mock-infected cells (Fig. 7a) were similar to that in mock-infected cells treated with either actinomycin D or cycloheximide for 8 h (Fig. 7a), thereby demonstrating that there is a slow turnover of STAT1 in uninfected cells. In contrast, STAT1 was rapidly degraded in SV5-infected cells that had or had not been treated with actinomycin D or cycloheximide throughout the infection (Fig. 7b).
FIG. 7.
Inhibition of transcription and protein synthesis did not prevent the degradation of STAT1 in SV5-infected cells. 2fTGH cells were untreated (Un) or treated with actinomycin D (Act. D; 10 μg/ml) or cycloheximide (Cx; 50 μg/ml) 1 h prior to being mock infected or infected with SV5. Actinomycin D or cycloheximide (as appropriate) was also present throughout infection period. Total-cell extracts were made at 6 h p.i., and the levels of STAT1 were detected by immunoblot analysis.
STAT1 is degraded in IFN-pretreated cells by infecting virus.
Pretreatment of cells with IFN delays the onset of virus protein synthesis until between 24 and 48 h p.i., compared to about 8 h p.i. in untreated cells (6). One explanation for this delay would be that pretreatment of cells with IFN induced an antiviral state which efficiently inhibited virus replication. However, in the absence of continued IFN signalling (due to the degradation of STAT1 by infecting virus), the antiviral state in these cells was not maintained, thus permitting the infecting virus to eventually replicate. Consistent with this model, infection with SV5 induced the degradation of STAT1 in IFN-pretreated cells. It can be seen from Fig. 8 that IFN stimulated STAT1 expression (the STAT1 gene is under the control of an IFN-responsive promoter), as the levels were significantly higher in mock-infected cells pretreated with IFN than in untreated cells (Fig. 8; compare lanes 1 and 2). Nevertheless, in IFN-pretreated cells that had been infected with SV5, only small amounts of STAT1 were detected at 6 h p.i., and no STAT1 was detected at 24 h p.i. (Fig. 8, lanes 4 and 6, respectively).
FIG. 8.
STAT1 was degraded in IFN-pretreated cells. 2fTGH cells were (lanes 2, 4, and 6) or were not (1, 3, and 5) treated with rHuIFN-αA/D for 24 h prior to mock infection (lanes 1 and 2) or infection with SV5 (lanes 3 to 6). Total-cell extracts were made from cells harvested at either 6 (lanes 1 to 4) or 24 (lanes 5 and 6) h p.i., and levels of STAT1 were detected by immunoblot analysis.
DISCUSSION
The potential effectiveness of the IFN response, if not blocked, can be judged by the rapid switch-off of SV5 protein synthesis in murine cells following the induction of IFN (SV5 does not block IFN signalling in murine cells [6]). In contrast, human cells infected with SV5 can no longer respond to IFN and thereby switch off virus protein synthesis once it has been established (6). In this report, we show that SV5 specifically targets STAT1 for proteasome-mediated degradation in human (but not murine) cells, thereby blocking both type I and type II IFN signalling by inhibiting the formation of the ISGF3 and GAF transcription complexes. We also demonstrate that SV5 can induce the degradation of STAT1 in human cells that have entered an antiviral state, and this may be of significance in terms of virus pathogenesis. Presumably, in the absence of continued signalling (due to a loss of STAT1), cells cannot remain in an antiviral state and suppress virus replication indefinitely. Thus there must be competition between intracellular virus remaining viable and cells resynthesizing sufficient levels of STAT1 to be able to respond to IFN and thus maintain their antiviral state.
There are clearly many potential advantages for SV5 in blocking both type I and type II IFN signalling. For example, in addition to blocking the autocrine effect of IFN-α/β secreted from infected cells, SV5-infected cells would be insensitive to both IFN-α and IFN-γ released by activated leukocytes and T cells. Furthermore, as IFN can act as a leukocyte-activating cytokine, increasing NK cell activity and acting as a growth factor for memory CD8+ T cells, in the event of SV5 infection of these cells, cellular immune responses may be adversely affected. The fact that SV5 can lead to the degradation of STAT1 in the absence of virus protein synthesis may compound this situation. Furthermore, since V is a structural protein associated with the nucleocapsids of the virion (∼350 molecules per virion [28]), defective virus particles may also contribute to any effects observed.
Several properties have been ascribed to V, including its interaction with both viral NP (33) and cellular (21) proteins. However, it is clear that the interaction of V with NP is not required for either its ability to block IFN signalling or the targeting of STAT1 for proteasome-mediated degradation. Thus, it remains likely that V is a multifunctional protein with a number of independent roles in SV5 replication and pathogenesis. Whether the V protein of other members of the Paramyxovirinae family play exactly the same roles as that performed by the V protein of SV5 remains to be established but seems unlikely. Thus, while this report was under review, Garcin et al. (9a) reported that it was the C proteins of SeV, and not V, that counteract the IFN-induced anti-viral state (SV5 and other rubulaviruses do not encode the C proteins). Since we had previously reported that SeV also blocks IFN signalling (6), it is probable that the molecular mechanisms employed by SeV and SV5 to block the IFN signalling differ in detail. Indeed, it remains to be elucidated exactly how the V protein of SV5 targets STAT1 for proteasome-mediated degradation. V may interact directly with STAT1 and thereby somehow target it for ubiquitination and degradation. Alternatively, V may activate a normal cellular pathway involved in STAT1 turnover. In this respect, it is of note that Kim and Maniatis (14) reported that activated (phosphorylated) STAT1 is degraded by a ubiquitin-proteasome pathway. However, there is some debate as to whether dephosphorylation/nuclear import mechanisms are more important mechanisms of down-regulation of STAT1 than proteolytic degradation (10, 18). The key import mechanisms are more important mechanisms of down-regulation of STAT1 than proteolytic degradation (10, 18). The key difference in the observations reported here is that both phosphorylated and nonphosphorylated forms of STAT1 are degraded in SV5-infected cells.
There are other examples of viruses targeting cellular proteins for proteasome-mediated degradation. The E6 and E7 proteins of human papillomaviruses target p53 and pRB, respectively, for proteasome-mediated degradation (2, 11, 38). Disruption of nuclear structures known as ND10, or PML nuclear bodies, that have been implicated in a number of cellular processes (e.g., response to stress and IFNs, oncogenesis, and viral infection) also occurs during herpes simplex virus infection by a proteasome-dependent process (7). Thus, the targeting of important cellular control proteins for proteasome-mediated degradation may be a general mechanism employed by viruses to usurp cellular pathways.
ACKNOWLEDGMENTS
L. Didcock is indebted to the MRC for a Ph.D. studentship and the Wellcome Trust for current support. S. Goodbourn is a recipient of a Wellcome Trust University award, and D. F. Young is supported by the BBSRC.
We thank Ron Hay (University of St. Andrews) for reagents and helpful advice.
REFERENCES
- 1.Alkalay I, Yaron A, Hatzubai A, Orian A, Ciechanover A, Ben-Neriah Y. Stimulation-dependent IκBα phosphorylation marks the NF-κB inhibitor for degradation via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1995;92:10599–10603. doi: 10.1073/pnas.92.23.10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyer S N, Wazer D E, Band V. E7 protein of human papillomavirus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 1996;56:4620–4624. [PubMed] [Google Scholar]
- 3.Chin Y E, Kitagawa M, Kuida K, Flavell R A, Fu X Y. Activation of the STAT signaling pathway can cause expression of caspase 1 and apoptosis. Mol Cell Biol. 1997;17:5328–5337. doi: 10.1128/mcb.17.9.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choppin P W. Multiplication of a myxovirus (SV5) with minimal cytopathic effects and without interference. Virology. 1964;23:224–233. doi: 10.1016/0042-6822(64)90286-7. [DOI] [PubMed] [Google Scholar]
- 5.Delenda C, Hausmann S, Garcin D, Kolakofsky D. Normal cellular replication of Sendai virus without the trans-frame, nonstructural V protein. Virology. 1997;228:55–62. doi: 10.1006/viro.1996.8354. [DOI] [PubMed] [Google Scholar]
- 6.Didcock L, Young D F, Goodbourn S, Randall R E. Sendai virus and simian virus 5 block activation of interferon-responsive genes: importance for virus pathogenesis. J Virol. 1999;73:3125–3133. doi: 10.1128/jvi.73.4.3125-3133.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everett R D, Freeman P, Saitoh H, Dasso M, Orr A, Kathoria M, Parkinson J. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J Virol. 1998;72:6581–6591. doi: 10.1128/jvi.72.8.6581-6591.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenteany G, Standaert R F, Lane W S, Choi S, Corey E J, Schreiber S L. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 9.Gale M, Jr, Katze M G. Molecular mechanisms of interferon resistance mediated by viral-directed inhibition of PKR, the interferon induced protein kinase. Pharmacol Ther. 1998;78:29–46. doi: 10.1016/s0163-7258(97)00165-4. [DOI] [PubMed] [Google Scholar]
- 9a.Garcin D, Latorre P, Kolakofsky D. Sendai virus C proteins counteract the interferon-mediated induction of an antiviral state. J Virol. 1999;73:6559–6565. doi: 10.1128/jvi.73.8.6559-6565.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haspel R L, Salditt-Georgoeff M, Darnell J E., Jr The rapid inactivation of nuclear tyrosine phosphorylated Stat1 depends upon a protein tyrosine phosphatase. EMBO J. 1996;15:6262–6268. [PMC free article] [PubMed] [Google Scholar]
- 11.Huibregste J M, Scheffner M, Howley P M. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus type 16 or 18. EMBO J. 1991;10:4129–4135. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaffray E, Wood K M, Hay R T. Domain organization of IκBα and sites of interaction with NF-κB p65. Mol Cell Biol. 1995;15:2166–2172. doi: 10.1128/mcb.15.4.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato A, Kiyotani K, Sakai Y, Yoshida T, Nagai Y. The paramyxovirus, Sendai virus, V protein encodes a luxury function required for viral pathogenesis. EMBO J. 1997;16:578–587. doi: 10.1093/emboj/16.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim T K, Maniatis T. Regulation of interferon-gamma-activated STAT1 by the ubiquitin-proteasome pathway. Science. 1996;273:1717–1719. doi: 10.1126/science.273.5282.1717. [DOI] [PubMed] [Google Scholar]
- 15.King P, Goodbourn S. The β-interferon promoter responds to priming through multiple independent regulatory elements. J Biol Chem. 1994;269:30609–30615. [PubMed] [Google Scholar]
- 16.King P, Goodbourn S. STAT1 is inactivated by a caspase. J Biol Chem. 1998;273:8699–8704. doi: 10.1074/jbc.273.15.8699. [DOI] [PubMed] [Google Scholar]
- 17.Lamb R A, Kolakofsky D. Paramyxoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. New York, N.Y: Raven Press; 1996. pp. 1177–1204. [Google Scholar]
- 18.Lee C-K, Bluyssen H A R, Levy D E. Regulation of interferon-α responsiveness by the duration of Janus kinase activity. J Biol Chem. 1997;272:21872–21877. doi: 10.1074/jbc.272.35.21872. [DOI] [PubMed] [Google Scholar]
- 19.Leonard G T, Sen G C. Effects of adenovirus E1A protein on interferon-signalling. Virology. 1996;224:25–33. doi: 10.1006/viro.1996.0503. [DOI] [PubMed] [Google Scholar]
- 20.Look D C, Roswit W T, Frick A G, Gris-Alevy Y, Dickhaus D M, Walter M J, Holtzman M J. Direct suppression of Stat1 function during adenoviral infection. Immunity. 1998;9:871–880. doi: 10.1016/s1074-7613(00)80652-4. [DOI] [PubMed] [Google Scholar]
- 21.Lin G Y, Paterson R G, Richardson C D, Lamb R A. The V protein of the paramyxovirus SV5 interacts with damage-specific DNA binding protein. Virology. 1998;249:189–200. doi: 10.1006/viro.1998.9317. [DOI] [PubMed] [Google Scholar]
- 22.Masson N, Ellis M, Goodbourn S, Lee K A W. Cyclic-AMP response-binding protein and the catalytic subunit of protein kinase A are present in F9 embryonal carcinoma cells but are unable to activate the somatostatin promoter. Mol Cell Biol. 1992;12:1096–1102. doi: 10.1128/mcb.12.3.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller D M, Rahill B M, Lairmore J M, Durbin J E, Waldman J W, Sedmak D D. Human cytomegalovirus inhibits major histocompatibility complex class II expression by disruption of the Jak/Stat pathway. J Exp Med. 1998;187:675–683. doi: 10.1084/jem.187.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller M, Laxton C, Briscoe J, Schindler C, Improta T, Darnell J E, Jr, Stark G R, Kerr I M. Complementation of a mutant cell line: central role of the 91 kDa polypeptide of ISGF3 in the interferon-alpha and -gamma signal transduction pathways. EMBO J. 1993;12:4221–4228. doi: 10.1002/j.1460-2075.1993.tb06106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palombella V J, Maniatis T. Inducible processing of interferon regulatory factor-2. Mol Cell Biol. 1992;12:3325–3336. doi: 10.1128/mcb.12.8.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palombella V J, Rando O J, Goldberg A L, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-κB1 precursor protein and the activation of NF-κB. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 27.Pamer E, Cresswell P. Mechanisms of MHC class I-restricted antigen processing. Annu Rev Immunol. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- 28.Paterson R G, Leser G P, Shaughnessy M A, Lamb R A. The paramyxovirus SV5 V protein binds two atoms of zinc and is a structural component of virions. Virology. 1995;208:121–131. doi: 10.1006/viro.1995.1135. [DOI] [PubMed] [Google Scholar]
- 29.Pellegrini S, Shearer J J, Kerr I M, Stark G R. Use of a selectable marker regulated by alpha interferon to obtain mutations in the signaling pathway. Mol Cell Biol. 1989;9:4605–4612. doi: 10.1128/mcb.9.11.4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Player M R, Torrence P F. The 2-5A system: modulation of viral and cellular processes through acceleration of RNA degradation. Pharmacol Ther. 1998;78:55–113. doi: 10.1016/S0163-7258(97)00167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ploegh H L. Viral strategies of immune evasion. Science. 1998;280:248–253. doi: 10.1126/science.280.5361.248. [DOI] [PubMed] [Google Scholar]
- 32.Precious B, Young D F, Bermingham A, Fearns R, Ryan M, Randall R E. Inducible expression of the P, V and NP genes of the paramyxovirus simian virus 5 in cell lines and an examination of NP-P and NP-V interactions. J Virol. 1995;69:8001–8010. doi: 10.1128/jvi.69.12.8001-8010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Randall R E, Bermingham A. NP:P and NP:V interactions of the paramyxovirus simian virus 5 examined using a novel protein:protein capture assay. Virology. 1996;224:121–129. doi: 10.1006/viro.1996.0513. [DOI] [PubMed] [Google Scholar]
- 34.Rehberg E, Kelder B, Hoal E G, Pestka S. Specific molecular activities of recombinant and hybrid interferons. J Biol Chem. 1982;257:11497–11502. [PubMed] [Google Scholar]
- 35.Rock K L, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg A F. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 36.Roff M, Thompson J, Rodriguez M S, Jacque J M, Arenzana-Seisdedos F, Hay R T. Role of IκBα ubiquitination in signal-induced activation of NF-κB in vivo. J Biol Chem. 1996;271:7844–7850. doi: 10.1074/jbc.271.13.7844. [DOI] [PubMed] [Google Scholar]
- 37.Schindler C, Fu X-Y, Improta T, Aebersold R, Darnell J E., Jr Proteins of transcription factor ISGF-3: one gene encodes the 91 and 84 kDa ISGF-3 proteins that are activated by interferon-α. Proc Natl Acad Sci USA. 1992;89:7836–7839. doi: 10.1073/pnas.89.16.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheffner M, Werness B A, Huibregste J M, Levine A J, Howley P M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 39.Seow H F. Pathogen interactions with cytokines and host defence: an overview. Vet Immunol Immunopathol. 1998;63:139–148. doi: 10.1016/s0165-2427(98)00090-7. [DOI] [PubMed] [Google Scholar]
- 40.Shuai K, Schindler C, Prezioso V R, Darnell J E., Jr Activation of transcription by IFN-γ: tyrosine phosphorylation of a 91 kDa DNA binding protein. Science. 1992;259:1808–1812. doi: 10.1126/science.1281555. [DOI] [PubMed] [Google Scholar]
- 41.Shuai K, Stark G R, Kerr I M, Darnell J E., Jr A single phosphotyrosine residue of Stat91 required for gene activation by interferon-γ. Science. 1993;261:1744–1746. doi: 10.1126/science.7690989. [DOI] [PubMed] [Google Scholar]
- 42.Smith G L. Virus strategies for evasion of the host response to infection. Trends Microbiol. 1994;2:81–88. doi: 10.1016/0966-842x(94)90539-8. [DOI] [PubMed] [Google Scholar]
- 43.Stark G R, Kerr I M, Williams B R G, Silverman R H, Schreiber R D. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 44.Symons J A, Alcami A, Smith G L. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell. 1995;81:551–560. doi: 10.1016/0092-8674(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 45.Thomas N S, Pizzey A R, Tiwari S, Williams C D, Yang J. p130, p107, and pRb are differentially regulated in proliferating cells and during cell cycle arrest by alpha-interferon. J Biol Chem. 1998;273:23659–23667. doi: 10.1074/jbc.273.37.23659. [DOI] [PubMed] [Google Scholar]
- 46.Thomas S M, Lamb R A, Paterson R G. Two mRNAs that differ by two nontemplated nucleotides encode the amino coterminal proteins P and V of the paramyxovirus SV5. Cell. 1988;54:891–902. doi: 10.1016/S0092-8674(88)91285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Upton C, Mossman K, McFadden G. Encoding of a homolog of the IFN-gamma receptor by myxoma virus. Science. 1992;258:1369–1372. doi: 10.1126/science.1455233. [DOI] [PubMed] [Google Scholar]
- 48.Vidal S, Curran J, Kolakofsky D. A stuttering model for paramyxovirus P mRNA editing. EMBO J. 1990;9:2017–2022. doi: 10.1002/j.1460-2075.1990.tb08330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wen Z, Zhong Z, Darnell J E., Jr Maximal activation of transcription of Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 50.Yan R, Sajjad Q, Zhong Z, Wen Z, Darnell J E., Jr The genomic structure of the STAT genes: multiple exons in coincident sites in Stat1 and Stat2. Nucleic Acids Res. 1995;23:459–463. doi: 10.1093/nar/23.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yokosawa N, Kubota T, Fujii N. Poor induction of interferon induced 2′,5′-oligoadenylate synthetase (2-5 AS) in cells persistently infected with mumps virus is caused by decrease of STAT-1 alpha. Arch Virol. 1998;143:1985–1992. doi: 10.1007/s007050050434. [DOI] [PubMed] [Google Scholar]
- 52.Zimring J C, Goodbourn S, Offerman M K. Human herpesvirus 8 encodes an interferon regulatory factor (IRF) homolog that represses IRF-1 mediated transcription. J Virol. 1998;72:701–707. doi: 10.1128/jvi.72.1.701-707.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]