Abstract
Background:
Health plans and risk-bearing provider organizations seek information sources to inform proactive interventions for patients at risk of adverse health events. Interventions should take into account the strong relationship between social context and health. This retrospective cohort study of a Medicare Advantage population examined whether a change in self-reported health-related quality of life (HRQOL) signals a subsequent change in healthcare needs.
Methods:
A retrospective longitudinal analysis of administrative claims data was conducted for participants in a Medicare Advantage plan with prescription drug coverage (MAPD) who responded to 2 administrations of the Centers for Disease Control and Prevention 4-item Healthy Days survey within 6–18 months during 2015–2018 Changes in HRQOL, as measured by the Healthy Days instrument, were compared with changes in utilization and costs, which were considered to be a reflection of change in healthcare needs.
Results:
A total of 48,841 individuals met inclusion criteria. Declining HRQOL was followed by increases in utilization and costs. An adjusted analysis showed that every additional unhealthy day reported one year after baseline was accompanied by an $8 increase in monthly healthcare costs in the subsequent six months for the average patient.
Conclusions:
Declining HRQOL signaled subsequent increases in healthcare needs and utilization.
Implications:
Findings suggest that HRQOL assessments in general, and the Healthy Days instrument in particular, could serve as a leading indicator of the need for interventions designed to mitigate poor health outcomes and rising healthcare costs.
Level of evidence:
III.
Keywords: Healthy days, Medicare advantage, Health-related quality of life, Healthcare utilization
1. Introduction
U.S. healthcare expenditures grew 4.6% in 2018 and accounted for 17.7% of the gross domestic product,1 largely due to increasingly prevalent chronic disease.2,3 Both chronic disease and expenditures can be negatively impacted by upstream social determinants of health (SDOH).4–9 Self-reported health-related quality of life (HRQOL) reflects unmet health-related social needs (HRSN), which are the individually experienced consequences of SDOH.10–15 A practical means of anticipating a change in individuals’ healthcare needs that reflects both disease and social context might enable health plans and providers to intervene and mitigate adverse health outcomes.
In the present study, two items from the 4-item version of the Centers for Disease Control and Prevention (CDC) Healthy Days survey provided a global measure of HRQOL quantified as the number of self-perceived unhealthy days.16 Prior cross-sectional research has shown greater numbers of reported unhealthy days to be associated with numerous clinical, quality and economic measures.17–22 Other cross-sectional studies have demonstrated an association between more unhealthy days and more HRSNs.23–25
Longitudinal studies have linked global self-reported health to future mortality rates in older adults.26,27 However, no published study has explored the longitudinal relationship between HRQOL and comprehensive measures of future health other than mortality. This study aimed to determine whether a change in self-reported HRQOL is followed by a change in overall healthcare utilization and costs in a Medicare Advantage population.
2. Methods
2.1. Data source and study population
An Advarra institutional review board granted a waiver of informed consent for this retrospective cohort study using administrative claims data from a national Medicare Advantage company. Study inclusion criteria were enrollment in a Medicare Advantage plan with prescription drug coverage (MAPD), response to two plan-administered Healthy Days surveys within 6–18 months 2015–2018, and continuous enrollment at least 6 months prior to Survey 1 and 3–6 months following Survey 2. Patients diagnosed with end-stage renal disease or entering hospice care during the study period were excluded.
Since 2015, the organization has routinely administered the 4-item Healthy Days survey via voice-activated technology telephone calls to random samples of Medicaid and Medicare Advantage participants June through December.23,28 Bold Goal Markets, which are metropolitan targeted as part of a population health strategy, are oversampled. In these areas, partnerships with local organizations address chronic conditions and HRSN. If an individual responded to more than two routine Healthy Days surveys during the study time frame, the first two surveys were included as Surveys 1 and 2.
2.2. Measures
The main outcome measure was change in overall per member per month (PMPM) costs, comparing costs following Survey 2 with costs prior to Survey 1. Costs were measured in terms of PMPM adjusted for varying follow-up times, which ranged from three to six months following Survey 2. Costs included all allowable charges. Costs for services provided under capitated payment arrangements were assigned the median value for non-capitated fee-for-service claims matched by procedure and payment level. Costs were adjusted to 2018 dollars using the Medical Consumer Price Index. Also measured were component costs for different types of utilization: inpatient stays, emergency department (ED) visits, primary care physician (PCP) visits, other outpatient services, other medical services (those provided outside medical facilities, eg, home health services), and prescription drug fills. The occurrence during the follow-up period of an inpatient admission, ED visit or PCP visit was assessed. Cost and utilization were considered surrogate measures of healthcare needs.
The main predictor of interest was change between Survey 1 and Survey 2 in total patient-reported physically and mentally unhealthy days, according to the Healthy Days instrument. The Healthy Days instrument, which has been used to assess quality of life in previously published studies,29–33 was chosen because of its simplicity, global measures, incorporation of both physical and mental health, and demonstrated usefulness for surveying older populations, identifying health disparities and tracking population health.16,28,34 Surveys 1 and 2 consisted of all four Healthy Day core questions (CDC HRQOL-4),16 but only these questions were used in this study: “Now thinking about your physical health, which includes physical illness and injury, for how many days during the past 30 days was your physical health not good?” and “Now thinking about your mental health, which includes stress, depression, and problems with emotions, for how many days during the past 30 days was your mental health not good?” The number of physically unhealthy days and mentally unhealthy days reported in each of the two survey administrations were summed for a possible total of 0 (no unhealthy days) to 60 (30 physically and 30 mentally unhealthy days). The CDC recommends capping total physically and mentally unhealthy days at 30 because responses apply to a 30-day time span.28,35 However, since this study was focused on the impact of a change between surveys, a cap of 30 unhealthy days would not have had sufficient granularity to detect change for patients reporting an uncapped total of close to or more than 30 unhealthy days at baseline. Thus, a 0–60 range was adopted as a severity scale. This approach has been used elsewhere.36 The difference in the total number of unhealthy days reported in Surveys 1 and 2 was used to assess change in HRQOL.
A number of baseline patient characteristics were measured (Table 1) and considered in the adjusted analyses of utilization, which used unhealthy days as a categorical variable.
Table 1.
Characteristics of study participants and the 2016 humana population enrolled in medicare advantage with a drug plan.
| Characteristic |
Proportion or Mean |
|
|---|---|---|
| Study Participantsa | 2016 MAPDa | |
|
| ||
| Total Study Population, N (%) | 48,851 (100) | 2,925,522 (100) |
| Age in Years, Mean (SD) | 74.6 (8.7) | 73.1 (10.1) |
| Female Sex, n(%) | 29,405 (60.2) | 1,642,209 |
| Race, n(%)b | (56.1) | |
| White | 37,013 (75.8) | 2,252,652 (77.0) |
| Black | 9492 (19.4) | 454,334 (15.5) |
| Hispanic | 573 (1.2) | 76,649 (2.6) |
| Asian | 354 (0.7) | 33,936 (1.2) |
| Native American | 47 (0.1) | 5558 (0.2) |
| Other race, unknown race, or missing data | 1379 (2.8) | 102,393 (3.5) |
| Original Eligibility for Medicare Based on Disability, n(%) | 9820 (20.1) | 850,788 (29.1) |
| Dual Eligibility for Medicaid as Well as Medicare | 1091 (2.2) | 22,320 (0.8) |
| Eligibility for Low Income Subsidy, n(%)c | 3007 (6.2) | 247,127 (8.4) |
| Medicare Advantage Product Typed | ||
| Point of Service | 81 (0.2) | – |
| Health Maintenance Organization | 33,470 (68.5) | 1,665,261 (56.9) |
| Local Preferred Provider Organization | 8305 (17.0) | 750,270 (25.6) |
| Private Fee-for-Service | 743 (1.5) | 95,376 (3.3) |
| Regional Preferred Provider Organization | 2827 (5.8) | 414,615 (14.2) |
| Attribution to PCP in Payment Arrangement with Downside Risk, n(%) | 17,844 (36.5) | 745,765 (25.5) |
| Medicare Geographic Division, n(%) | ||
| East North Central | 5956 (12.2) | 403,020 (13.8) |
| West North Central | 2093 (4.3) | 147,026 (5.0) |
| East South Central | 6960 (14.2) | 362,548 (12.4) |
| West South Central | 7722 (15.8) | 430,808 (14.7) |
| Mountain | 4238 (8.7) | 171,919 (5.9) |
| South Atlantic | 21,582 (44.2) | 1,188,141 (40.6) |
| Others (New England, Middle Atlantic, Pacific) | 300, 0.6 | 222,060 (7.6) |
| Rural Residence, n(%)e | 3145 (6.4) | 458,797 (15.7) |
| Bold Goal Market, n(%)f | 16,099 (33.0) | 434,700 (14.9) |
| CCI Score, Mean (SD)g | 4.4 (2.4) | 4.5 (2.4) |
| CCI Score Category, n(%)g | ||
| Low (0–7) | 43,669 (89.4) | 2,601,734 (88.9) |
| Medium (8–12) | 5026 (10.3) | 306,519 (10.5) |
| High (≥13) | 156 (0.3) | 17,269 (0.6) |
| Specific Comorbidities, n(%)h | ||
| Hypertension | 34,395 (70.4) | 1,987,371 (67.9) |
| Diabetes | 16,167 (33.1) | 975,867 (33.4) |
| Cancer | 5056 (10.3) | 278,224 (9.5) |
| COPD | 7223 (14.8) | 454,613 (15.5) |
| CHF | 5082 (10.4) | 323,610 (11.1) |
| Depression | 4534(9.3) | 292,455 (10.0) |
| CAD | 10,875 (22.3) | 661,090 (22.6) |
| Healthcare Costs PMPM, mean (SD) | ||
| Total | $879.3 (1943.9) | – |
| Inpatient | $146.5 (760.2) | – |
| Outpatient | $192.2 (539.5) | – |
| Pharmacy | $256.9 (965.2) | – |
| ≥1 Instance of Utilization, n(%) | ||
| Inpatient Stay | 3080.0 (6.3) | – |
| ED visit | 5059.0 (10.4) | – |
| Outpatient visit | 45,137.0 (92.4) | – |
CAD, coronary artery disease; CCI, Charlson Comorbidity Index; CHF, congestive heart failure; COPD, chronic obstructive lung disease; ED, emergency department; PMPM, per member per month.
The two groups are not independent and cannot be compared statistically.
Race categories were defined by the Centers for Medicare & Medicaid.
Low Income Subsidy is a federal subsidy to help with Medicare Part D costs.
Product type refers to the type of Medicare Advantage plan, eg, health maintenance organization (HMO) versus preferred provider organization (PPO).
Rural residence was equated with nonmetro areas as defined in the 2013 Rural-Urban Continuum code.
See text for description of Bold Goal Markets.
The CCI score was calculated with data from 12 months prior to Survey 1 according to published methods for use with claims data.37
Comorbidities were determined by data from 24 months prior to Survey 1. Healthcare costs and utilization were determined with data from 6 months prior to Survey 1.
2.3. Statistical analyses
Survey 1 was considered the index event. Preindex costs and utilization were measured for the six months prior to Survey 1. Other baseline characteristics were measured as of the month of the index date or with longer-term historical data. Follow-up costs and utilization were calculated over three to six months, depending on duration of continuous enrollment, following Survey 2.
Follow-up versus preindex PMPM costs were compared descriptively for each unhealthy days change category, with cutoff points for categories determined by the distribution of Survey 2 versus Survey 1 differences. Distribution of changes lent itself to these categories: 11 to 60 fewer unhealthy days (considered to represent substantial improvement in HRQOL), 2 to 10 fewer (modest improvement), no change (1 fewer to 1 additional unhealthy day), 2 to 10 more (modest decrement), and 11 to 60 more (substantial decrement).
An adjusted analysis of utilization was performed with three distinct generalized estimating equation (GEE) models to generate odds ratios (ORs) for inpatient admission, ED visit and PCP visit at three and six months following Survey 2, comparing each unhealthy days change category with no change in unhealthy days. To adjust for observable confounders, covariates representing baseline characteristics were considered for addition to each model, as were interaction terms. Final models were based on univariate tests of confounding, tests for multicollinearity, and backward selection. Variables that did not differ between unhealthy day change categories were not included in the regression models.
PMPM costs at three to six months following Survey 2 were modeled with a two-way fixed effects repeated measures model in which unhealthy days was the main independent variable. In contrast to the adjusted analysis of utilization measures, adjusted analysis of costs treated unhealthy days as a continuous variable representing survey-specific reporting. The other predictors were time, represented by a binary variable for Survey 2 versus Survey 1, and subject (study participant). The variable for time as a fixed effect controlled for observable and nonobservable effects that would vary over time between surveys and that would be common to all study participants. The variable for subject as a fixed effect controlled for time-invariant (permanent) observable and nonobservable differences between study participants, such as age and sex. Inclusion of these fixed effects obviated the need for adding covariates to the models. Given the relatively short interval between surveys (median 12 months), it was assumed that changes in between-subject differences in nonpermanent traits, such as comorbidity or functional status, would be minimal. Distinct models were constructed for overall costs and for each component cost defined by utilization category.
2.4. Assessment of the representativeness of the study cohort
Because MAPD participants living in Bold Goal Markets are oversampled for routine Healthy Days surveys, the representativeness of the cohort of survey respondents identified for this study was assessed through comparison with the total 2016 MAPD population.
3. Results
A total of 48,851 unique, eligible study participants were identified. Table 1 shows the study cohort was very similar to the 2016 MAPD population, with the exception the study cohort was less likely to live in a rural area, which was not surprising since the Bold Goal metropolitan areas were oversampled for this study. The mean and median intervals between Survey 1 and Survey 2 were 12.8 and 12 months. At baseline, (Survey 1) respondents reported an average of 12.5 (SD, 15.7) total unhealthy days, 7.7 (SD, 9.9) physically unhealthy days and 4.8 (SD, 8.4) mentally unhealthy days.
Slightly fewer than one third of participants reported no appreciable change in total unhealthy days. The remaining responses were evenly distributed between decreases in unhealthy days (substantial or moderate improvement in HRQOL) and increases (moderate or substantial decrement, Table 2). Physically unhealthy day totals, declines and increases exceeded those of mentally unhealthy days.
Table 2.
Reported unhealthy days.
| Survey 1 | Survey 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| Change Category | Patients, N(%) | UHD, mean (SD) | UHD, mean (SD) | Change in UHD, (SD) | ||||||
|
|
|
|
||||||||
| Total | P-UHD | M-UHD | Total | P-UHD | M-UHD | Total | P-UHD | M-UHD | ||
|
| ||||||||||
| Overall | 48,851 | 12.51 | 7.75 | 4.76 | 12.59 | 7.75 | 4.84 | 0.084 | 0.002 | 0.08 |
| (100) | (15.69) | (9.89) | (8.45) | (15.67) | (9.81) | (8.44) | (15.98) | (10.98) | (8.90) | |
| 11–60 fewer (substantial improvement) | 7980 | 33.95 | 20.74 | 13.21 | 9.23 | 5.64 | 3.59 | − 24.72 | − 15.10 | − 9.62 |
| (16.3) | (13.44) | (8.86) | (11.73) | (10.40) | (7.15) | (6.05) | (11.34) | (9.97) | (10.74) | |
| 2–10 fewer (modest improvement) | 8753 | 14.28 | 9.06 | 5.22 | 9.03 | 5.52 | 3.51 | − 5.25 | − 3.53 | − 1.7 |
| (17.9) | (13.42) | (8.47) | (7.74) | (12.61) | (7.94) | (6.68) | (2.70) | (4.76) | (4.52) | |
| No Change (− 1 to +1) | 14,960 | 4.11 | 2.60 | 1.52 | 4.12 | 2.60 | 1.52 | 0.002 | 0.003 | − 0.001 |
| (30.6) | (10.76) | (6.75) | (5.28) | (10.76) | (6.71) | (5.23) | (0.48) | (2.30) | (2.28) | |
| 2–10 more (modest decrement) | 9057 | 8.75 | 5.37 | 3.38 | 13.98 | 8.83 | 5.17 | 5.23 | 3.45 | 1.78 |
| (18.5) | (12.56) | (7.90) | (6.64) | (13.36) | (8.36) | (7.61) | (2.72) | (4.66) | (4.42) | |
| 11–60 more (significant decrement) | 8101 | 9.16 | 5.71 | 3.45 | 33.83 | 20.55 | 13.28 | 24.67 | 14.84 | 9.83 |
| (16.6) | (10.44) | (7.31) | (6.01) | (13.40) | (8.75) | (11.62) | (11.23) | (9.98) | (10.75) | |
UHD, unhealthy day; P-UHD, physically unhealthy days; M-UHD, mentally unhealthy days; SD, standard deviation.
Overall unadjusted costs increased from $879 PMPM preindex to $1056 PMPM during follow-up. PMPM costs slightly declined (by $44) for the substantial HRQOL improvement subgroup and slightly increased (by $55) for the modest improvement subgroup but substantially increased in both decrement subgroups (by $312 PMPM, modest decrement; by $373 PMPM, substantial decrement). PMPM costs also increased by $175 in the no change subgroup. (Data are not shown for the change subgroups.)
Adjusted utilization patterns, comparing each change group to the no change subgroup, differed between the HRQOL improvement subgroups and the decrement subgroups (Table 3). The two improvement subgroups had significantly smaller odds, relative to the no change group, of an inpatient admission or ED visit at both 3 months (range, 25 to 48% less likely, depending on outcome measure and change category) and six months (29–41% less likely). The odds ratios (ORs) for PCP visits suggested a relatively greater likelihood with improving HRQOL, but results were significant only at three months (10% to 14% more likely).
Table 3.
Adjusted relative likelihood of inpatient visits, emergency department visits and primary care physician visits, HRQOL change versus No change.
| Change Category | Inpatient Visits, | ED Visits, | PCP Visits, | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Adjusted OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) | ||||
|
|
|
|
||||
| 3 months | 6 months | 3 months | 6 months | 3 months | 6 months | |
|
|
|
|
|
|
|
|
| No Change (−1 to +1) | Ref | Ref | Ref | Ref | Ref | Ref |
|
| ||||||
| 11–60 fewer (substantial improvement) | 0.5 | 0.59 | 0.67 | 0.68 | 1.14 | 1.10 |
| (0.41–0.66) | (0.48–0.71) | (0.57–0.78) | (0.60–0.78) | (1.05–1.25) | (0.99–1.23) | |
| 2–10 fewer (modest improvement) | 0.61 | 0.63 | 0.75 | 0.71 | 1.10 | 1.10 |
| (0.49–0.77) | (0.52–0.76) | (0.65–0.87) | (0.63–0.81) | (1.01–1.20) | (0.99–1.22) | |
| 2–10 more (modest decrement) | 1.15 | 1.16 | 0.92 | 0.85 | 1.05 | 1.11 |
| (0.93–1.41) | (0.96–1.38) | (0.79–1.06) | (0.75–0.97) | (0.97–1.14) | (1.00–1.23) | |
| 11–60 more (substantial decrement) | 1.14 | 1.14 | 0.92 | 0.86 | 1.04 | 1.03 |
| (0.91–1.40) | (0.94–1.37) | (0.79–1.06) | (0.76–0.98) | (0.96–1.14) | (0.92–1.15) | |
ED, emergency department; OR, odds ratio; PCP, primary care physician.
In the two decrement subgroups, no significant associations between change in unhealthy days and inpatient stays during the follow-up period were detected, although point estimates were consistent with a greater likelihood relative to the no change subgroup. Like the improvement subgroups, the decrement subgroups had fewer ED visits (nonsignificant at three months; 14% to 15% less likely at six months) and slightly more PCP visits (nonsignificant) relative to no change.
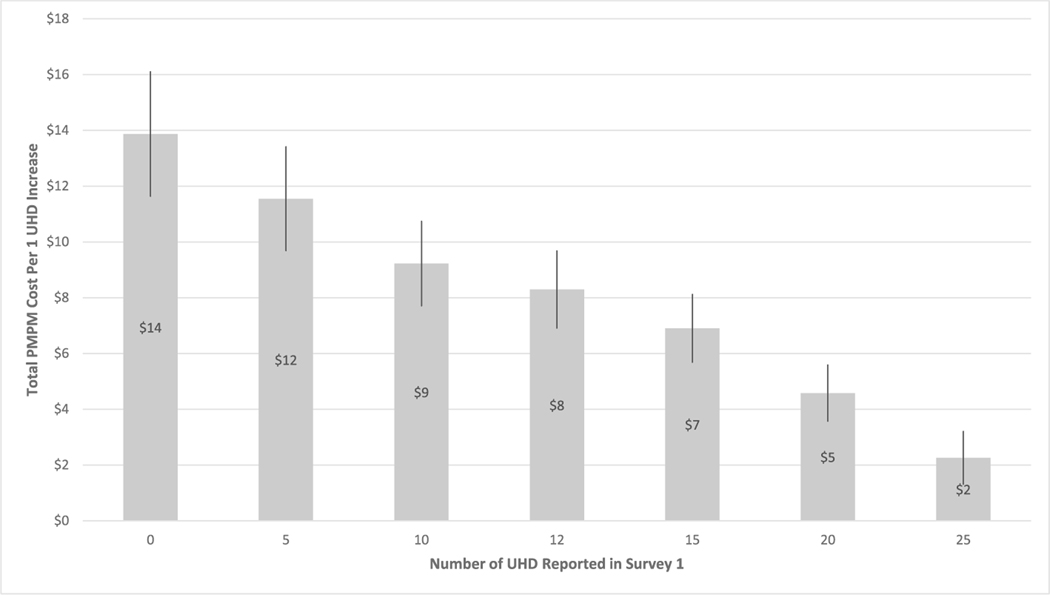
Adjusted analysis of overall PMPM costs, the key outcome measure, showed an $8 PMPM increase ($48 increase over six months) per single unhealthy day increase between Survey 1 and Survey 2. Models for the component costs showed PMPM increases in inpatient costs ($4), ED visit costs (<$1), PCP visit costs (<$1), outpatient costs ($1), other medical costs ($3) and pharmacy costs ($1). These adjusted cost results pertain only to individuals with the average of 12 unhealthy days at baseline. Fig. 1 presents estimated overall PMPM increases in costs for varying numbers of baseline unhealthy days.
Fig. 1.
Adjusted Additional Per Member Per Month (PMPM) Costs for Each 1-Day Increase in Unhealthy Days (UHD) by Baseline Number of Unhealthy Days aa Vertical lines represent 95% confidence intervals.
4. Discussion
In a national population of patients enrolled in Medicare Advantage health plans, a decline in unhealthy days (improvement in HRQOL) over approximately one year was followed by a decrease or only a slight increase in costs, while an increase in unhealthy days (worsening HRQOL) was followed by substantial increases in costs. Costs also increased in the subgroup reporting no appreciable change in unhealthy days, consistent with the increase in costs for the overall study population. Overall, cost increases in the study population are possibly due to an aging study population, disease progression and/or trends in the healthcare system.
An adjusted analysis showed that among individuals initially reporting an average of 12 unhealthy days, for every additional reported unhealthy day total allowable costs over the next six months increased by an average $8 per month (Fig. 1), a $48 average six-month increase in costs per individual. This estimate is based on a severity scale of 0–60 total unhealthy days, rather than the conventional cap at 30 days. Limitations to the conventional 30-day cap have been discussed by others [28].
Although it may be obvious that a change in self-reported health is related to future healthcare needs and associated costs, few studies have documented the potential role of HRQOL as a signal of changing health. Furthermore, no other studies have explored this potential using Healthy Days measures to track HRQOL.
In addition to changes in healthcare costs, we found changes in unhealthy days were associated with changes in utilization patterns during the follow-up period. The likelihood of an ED visit or inpatient stay was less for the HRQOL improvement subgroups relative to the no change subgroup. The likelihood of a PCP visit was greater for HRQOL improvement versus no change, but only three-month results in the substantial improvement group were significant. In contrast to the improvement subgroups, the decrement subgroups showed a nonsignificant a higher likelihood of inpatient stays when compared with no change in HRQOL. Like the improvement subgroups, the decrement subgroups had fewer ED visits (significant at six months) and slightly more PCP visits (nonsignificant) relative to no change. According to organizational practice,38,39 health plan care coordination programs would more likely have been offered to individuals with worsening HRQOL, which might explain why ED visits would decrease and PCP visits increase in the decrement subgroups. Overall, utilization results were consistent with the main finding the increase in total healthcare expenditures between Surveys 1 and 2 was greater in the decrement subgroups compared with the improvement subgroups.
Previous claims-based research reported an estimated $15.64 PMPM increase per unhealthy day, using a cross-sectional rather than longitudinal design, a less comprehensive approach to controlling for confounders, and a total unhealthy day cap of 30 rather than 60.17 Given the smaller denominator, it is not surprising that the previous estimate ($16 per unhealthy day) was larger than the estimate reported here ($8 per unhealthy day). The previous overall estimate cannot be directly compared with the present study’s estimate, which pertains specifically to individuals reporting 12 unhealthy days at baseline.
Our study findings imply that a change in reported unhealthy days, measured on a 0–60 severity scale, may be a leading indicator of impending changes in healthcare delivery needs. The 4-item survey is easily-administered digitally23,28 and could be useful in population health management. Results could inform a threshold increase in unhealthy days that would flag potential candidates for outreach efforts. In previous research, population interventions to address chronic disease or health status have resulted in changes in reported unhealthy days.29,40–42 Thus, the result of such intervention could conceivably be a restoration of HRQOL.
The strength of a self-report tool like Healthy Days for monitoring signs of a change in health status is that it not only captures clinical health but also reflects the individual’s social context.23,24 Since social factors are not captured in claims or medical records, this simple tool enables a much more comprehensive approach to risk stratification.
Further research is needed to more precisely assess when changes in unhealthy days occur relative to changes in healthcare utilization, how both unhealthy days and costs change relative to clinical health, the relative contribution of physically and mentally unhealthy days to future utilization, and the impact of early interventions triggered by changes in reported unhealthy days. Several issues related to Healthy Days responses merit further investigation: use of the 0–60 severity scale instead of the conventional cap of 30 unhealthy days, counting patterns for days of both physical and mental poor health, distribution of responses by age, and the frequency of more than 30 reported total unhealthy days.
4.1. Limitations
This study was subjected to several limitations, the first being the impossibility of randomized exposure assignment. The observational design precludes conclusions of causality between changes in HRQOL and future costs or utilization. However, the observed temporal relationship between an increase in reported unhealthy days followed by an increase in costs contributes to the possibility of a causal association, especially since each patient served as his or her own historical control. Since time of year of survey administration and the interval between surveys varied, seasonal effects might have confounded study results or influenced the magnitude of observed cost increases. However, the most surveys were administered in December and the mean (median) interval between surveys was 12.8 (12) months. Thus, surveys were generally administered at the same time of year and the interval between initial and second surveys was reasonably uniform. The 60-day severity scale rather than the conventional 30-day cap on total unhealthy days might have resulted in overestimation of unhealthy days compared to results in other studies.29–33 However, capping total unhealthy days at 30 would not fully reflect the dual effects of simultaneously perceived physical and mental poor health. The observation of an approximately normal distribution of change in unhealthy days and the nonnegligible proportion of patients with more than 30 total unhealthy days at baseline (13%) support the utility of the 0 to 60 scale. Adjusted analysis of costs assumed that differences in nonpermanent participant characteristics (eg, comorbidities or functional status) would not change substantially between surveys. Results might be biased (direction unknown) if this assumption did not hold true. The relatively short follow-up period (up to six months) may have resulted in underestimation of the increase in costs that can be expected to follow an increase in unhealthy days. Lastly, our results might not be generalizable to other populations of patients enrolled in Medicare Advantage health plans.
5. Conclusions
In this longitudinal study, changes in reported unhealthy days between two administrations of the four-item Healthy Days survey were followed by changes in overall allowable costs that were generally in the same direction. These findings suggest HRQOL assessments in general, and the Healthy Days instrument in particular, could be used as a leading indicator of subsequent changes in healthcare needs and utilization and thus signal the need for interventions designed to mitigate poor health outcomes and rising healthcare costs.
Acknowledgements
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for- profit sectors. The authors wish to acknowledge the contributions of Teresa Rogstad, MPH, who helped organize study data and drafted this manuscript, and the analytic support provided by Patrick Racsa, MS.
Financial disclosures
Ms. Drzayich Antol reports employment and equity holdings with Humana.
Dr. Hagan reports employment and equity holdings with Humana.
Ms. Nguyen reports employment with Humana.
Dr. Li reports employment with Humana.
Mr. Haugh reports employment with Humana.
Dr. Radmacher reports employment with Humana.
Dr. Renda reports employment and equity holdings with Humana.
Dr. Shrank reports employment and equity holdings with Humana, and serving as a Director at GetWellNetwork.
Dr. Greenlund has no financial disclosures.
Dr. Thomas has no financial disclosures. Dr. Hacker has no financial disclosures.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Authors Dana Drzayich Antol, Angela Hagan, Hannah Nguyen, Yong Li, Gil Haugh, Michael Radmacher, Karen Worley, Andrew Renda, and William Shrank disclose employment with Humana, Inc., and Dana Drzayich Antol, Angela Hagan, Andrew Renda, and William Shrank disclose equity holdings with Humana. William Shrank also discloses serves as a Director at GetWellNetwork. No financial disclosures were reported by Kurt Greenlund, Craig Thomas or Karen Hacker.
References
- 1.Centers for Medicare & Medicaid Services. National health expenditure data. Historical. Page last modified december 17, 2019. Available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical. Accessed June 12, 2020. [Google Scholar]
- 2.Centers for Disease Control and Prevention. National center for chronic disease prevention and health promotion. Chronic diseases in America. Page Last Reviewed October 23, 2019. Available at: https://www.cdc.gov/chronicdisease/resources/infographic/chronicdiseases.htm. Accessed June 12, 2020. [Google Scholar]
- 3.Raghupathi W, Raghupathi V. An empirical study of chronic diseases in the United States: a visual analytics approach. Int J Environ Res Publ Health. 2018;15(3):431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agrawal SK, Shrank WH. Clinical and social risk adjustment - reconsidering distinctions. N Engl J Med. 2020;382:1581–1583. [DOI] [PubMed] [Google Scholar]
- 5.Dean EB, French MT, Mortensen K. Food insecurity, health care utilization, and health care expenditures. Health Serv Res. 2020;(55 Suppl2):883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Federico MJ, McFarlane 2nd AE, Szefler SJ, Abrams EM. The impact of social determinants of health on children with asthma. J Allergy Clin Immunol Pract. 2020;8: 1808–1814. [DOI] [PubMed] [Google Scholar]
- 7.Parekh TM, Cherrington AL, Bhatia S, et al. The association of low income and high stress with acute care use in COPD patients. Chronic Obstr Pulm Dis. 2020;7:107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wick JY. Social determinants of health: how environmental factors affect health. Sr Care Pharm. 2020;35:56–67. [DOI] [PubMed] [Google Scholar]
- 9.de Lima S, Cesse EAP, de Albuquerque M. Social determinants of death among the elderly: a systematic literature review. Rev Bras Epidemiol. 2014;17(Suppl 2): 178–193. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen AL, McNeil CJ, Han SD, Rhodes SD. Risk and protective factors for health-related quality of life among persons aging with HIV. AIDS Care. 2018;30:518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sampson EL, Bulpitt CJ, Fletcher AE. Survival of community-dwelling older people: the effect of cognitive impairment and social engagement. J Am Geriatr Soc. 2009;57: 985–991. [DOI] [PubMed] [Google Scholar]
- 12.Bowling A, Grundy E. Differentials in mortality up to 20 years after baseline interview among older people in East London and Essex. Age Ageing. 2009;38:51–55. [DOI] [PubMed] [Google Scholar]
- 13.Fernandes SG, Rodrigues AM, Nunes C, et al. Food insecurity in older adults: results from the epidemiology of chronic diseases cohort study 3. Front Med. 2018;5:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kihlström L, Burris M, Dobbins J, et al. Food insecurity and health-related quality of life: a cross-sectional analysis of older adults in Florida. U.S. Ecol Food Nutr 2019;58: 45–65. [DOI] [PubMed] [Google Scholar]
- 15.Frongillo EA, Nguyen HT, Smith MD, Coleman-Jensen A. Food insecurity is associated with subjective well-being among individuals from 138 countries in the 2014 Gallup World Poll. J Nutr. 2017;147:680–687. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Health-related quality of life measures. Available at: https://www.cdc.gov/hrqol/hrqol14_measure.htm; 2020. Accessed July 14, 2020.
- 17.Cordier T, Slabaugh SL, Havens E, et al. A health plan’s investigation of Healthy Days and chronic conditions. Am J Manag Care. 2017;23:e323–e330. [PubMed] [Google Scholar]
- 18.Havens E, Slabaugh SL, Helmick CG, et al. Comorbid arthritis is associated with lower health-related quality of life in older adults with other chronic conditions, United States, 2013–2014. Prev Chronic Dis. 2017;14:E60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen HY, Baumgardner DJ, Rice JP. Health-related quality of life among adults with multiple chronic conditions in the United States, Behavioral Risk Factor Surveillance System. Prev Chronic Dis. 2007;2011(8):A09. [PMC free article] [PubMed] [Google Scholar]
- 20.Drzayich Antol D, Waldman Casebeer A, Khoury R, et al. The relationship between comorbidity medication adherence and health related quality of life among patients with cancer. J Patient Rep Outcomes. 2018;2:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dwyer-Lindgren L, Mackenbach JP, van Lenthe FJ, Mokdad AH. Self-reported general health, physical distress, mental distress, and activity limitation by US county, 1995–2012. Popul Health Metrics. 2017;15:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Treviño LA, Cordier T, Renda A, Gresky D, Ruble M. Impact of antidepressant medication adherence on health-related quality of life among older adults with newly diagnosed major depressive disorder. Poster Presentation at: American Psychhiatri Association Annual Meeting. May 20–24, 2017. San Diego, CA. Available at: https://docushare-web.apps.cf.humana.com/Marketing/docushare-app?file=3114657. Accessed May 26, 2020. [Google Scholar]
- 23.Cordier T, Song Y, Cambon J, et al. A bold goal: more healthy days through improved community health. Popul Health Manag. 2018;21:202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lubetkin EI, Jia H. Burden of disease associated with lower levels of income among US adults aged 65 and older. BMJ Open. 2017;7, e013720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S, Xiang W. Income gradient in health-related quality of life - the role of social networking time. Int J Equity Health. 2019;18:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giltay EJ, Vollaard AM, Kromhout D. Self-rated health and physician-rated health as independent predictors of mortality in elderly men. Age Ageing. 2012;41:165–171. [DOI] [PubMed] [Google Scholar]
- 27.Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav. 1997;38:21–37. [PubMed] [Google Scholar]
- 28.Slabaugh SL, Shah M, Zack M, et al. Leveraging health-related quality of life in population health management: the case for healthy days. Popul Health Manag. 2017; 20:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cole S, Zbikowski SM, Renda A, Wallace A, Dobbins JM, Bogard M. Examining changes in Healthy Days after health coaching. Am J Health Promot. 2019;33: 774–777. [DOI] [PubMed] [Google Scholar]
- 30.Grubbs KM, Cheney AM, Fortney JC, et al. The role of gender in moderating treatment outcome in collaborative care for anxiety. Psychiatr Serv. 2015;66: 265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayes DK, Greenlund KJ, Denny CH, Neyer JR, Croft JB, Keenan NL. Racial/ethnic and socioeconomic disparities in health-related quality of life among people with coronary heart disease, 2007. Prev Chronic Dis. 2011;8:A78. [PMC free article] [PubMed] [Google Scholar]
- 32.Huang DL, Chan KC, Young BA. Poor oral health and quality of life in older U.S. adults with diabetes mellitus. J Am Geriatr Soc. 2013;61:1782–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz CE, Zhang J, Stucky BD, Michael W, Rapkin BD. Is the link between socioeconomic status and resilience mediated by reserve-building activities: mediation analysis of web-based cross-sectional data from chronic medical illness patient panels. BMJ Open. 2019;9, e025602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moriarty DG, Zack MM, Kobau R. The Centers for Disease Control and Prevention’s Healthy Days measures - population tracking of perceived physical and mental health over time. Health Qual Life Outcome. 2003;1:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. Measuring Healthy Days. Population Assessment of Health-Related Quality of Life; November 2000. Available at: https://www.cdc.gov/hrqol/pdfs/mhd.pdf. Accessed June 5, 2020.
- 36.Havens E, Pena J, Slabaugh SL, Cordier T, Renda A, Gopal V. Exploring the relationships between health-related quality of life and health conditions, costs, resource utilization and quality measures. Podium Presentation at: International Society for Quality of Life Research 22nd Annual Conference. October 21–24, 2015. Vancouver, British Columbia. Available at: https://docushare-web.apps.cf.humana.com/Marketing/docushare-app?file=2789059. Accessed November 4, 2020. [Google Scholar]
- 37.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43: 1130–1139. doi: 1110.1097/1101.mlr.0000182534.0000119832.0000182583. [DOI] [PubMed] [Google Scholar]
- 38.Humana. Support at home. https://www.humana.com/manage-your-health/home-and-community-support/support-at-home; 2021. Accessed January 5, 2021.
- 39.Centers for Medicare & Medicaid Services. Special needs plans. Available at: https://www.cms.gov/Medicare/Health-Plans/SpecialNeedsPlans. Accessed January 5, 2021.
- 40.Arija V, Villalobos F, Pedret R, et al. Physical activity, cardiovascular health, quality of life and blood pressure control in hypertensive subjects: randomized clinical trial. Health Qual Life Outcome. 2018;16:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kell KP, Rula EY. Increasing exercise frequency is associated with health and quality-of-life benefits for older adults. Qual Life Res. 2019;28:3267–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nambi GS, Inbasekaran D, Khuman R, Devi S, Shanmugananth Jagannathan K. Changes in pain intensity and health related quality of life with Iyengar yoga in nonspecific chronic low back pain: a randomized controlled study. Int J Yoga. 2014;7: 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]