Abstract
This Article shares the proceedings from the August 29th, 2023 (day 1) workshop “Physiologically Based Biopharmaceutics Modeling (PBBM) Best Practices for Drug Product Quality: Regulatory and Industry Perspectives”. The focus of the day was on model parametrization; regulatory authorities from Canada, the USA, Sweden, Belgium, and Norway presented their views on PBBM case studies submitted by industry members of the IQ consortium. The presentations shared key questions raised by regulators during the mock exercise, regarding the PBBM input parameters and their justification. These presentations also shed light on the regulatory assessment processes, content, and format requirements for future PBBM regulatory submissions. In addition, the day 1 breakout presentations and discussions gave the opportunity to share best practices around key questions faced by scientists when parametrizing PBBMs. Key questions included measurement and integration of drug substance solubility for crystalline vs amorphous drugs; impact of excipients on apparent drug solubility/supersaturation; modeling of acid–base reactions at the surface of the dissolving drug; choice of dissolution methods according to the formulation and drug properties with a view to predict the in vivo performance; mechanistic modeling of in vitro product dissolution data to predict in vivo dissolution for various patient populations/species; best practices for characterization of drug precipitation from simple or complex formulations and integration of the data in PBBM; incorporation of drug permeability into PBBM for various routes of uptake and prediction of permeability along the GI tract.
Keywords: PBBM, biopredictive dissolution, permeability, solubility, precipitation, modeling, IVIVC, IVIVR, bioequivalence, CQAs
1. Introduction
The use of physiologically based biopharmaceutics models (PBBMs) to support the understanding of drug product (DP) quality attributes and the setting of clinically relevant specifications for their control is gaining importance, as shown in the growing number of submissions to regulatory authorities around the world and publications on this topic in the scientific community.1
The workshop “Physiologically Based Biopharmaceutics Modeling (PBBM) Best Scientific Practices for Drug Product Quality: Regulatory and Industry Perspectives” sponsored by FDA in collaboration with the University of Maryland Center of Excellence in Regulatory Science and Innovation (M-CERSI) was held on August 29–31, 2023 and facilitated the discussion on PBBM case studies together with specific day hot topics. This paper provides a summary report on Day 1 of this workshop, which focused on considerations for PBBM parametrization. The morning session included a keynote speech from Prof. Jennifer Dressman, the readout from regulatory agencies on the analysis of four submitted PBBM case studies, and a panel discussion focusing on how sponsors parametrized their models with in vitro inputs. During the afternoon session, five parallel breakout (BO) sessions covered the following topics:
Solubility: Best practices for integration of solubility in PBBM
Development of biopredictive dissolution methods: Best practices for data generation as input to PBBM
Methods for integrating dissolution in PBBM: Best practices for modeling dissolution
Precipitation: Best practices for integration of precipitation in PBBM
Permeability: Best practices for integration of permeability in PBBM
2. Morning Presentations
2.1. Introduction to the Workshop. Bhagwant Rege (FDA)
FDA’s Office of Pharmaceutical Quality believes that everyone deserves to have confidence in their next dose of medicine and that pharmaceutical quality ensures the availability, safety, and efficacy of every dose. Biopharmaceutics is the link between DP quality and clinical performance in the patient. Patient centric quality standards (PCQSs) ensure that the DP consistently delivers clinical performance to the patient as described on the label in terms of safety and efficacy over its shelf life and from batch to batch. PCQSs can provide additional flexibility to pharmaceutical manufacturers while maintaining quality by establishing acceptance criteria based on clinical performance rather than process capability or manufacturing process control. PCQSs also avoid under- or overdiscriminating specifications which are not in the patient’s interests. The main obstacle to establishing PCQSs is a weak or often missing link between the in vitro and in vivo performances of the DPs. PBBM can help to overcome this obstacle. PBBM is a subset of Physiologically Based Pharmacokinetic (PBPK) models that are specific for biopharmaceutics applications. PBBM has more than 10 years of regulatory history. PBBM is mechanistic by nature because it integrates physicochemical properties of the drug, drug substance (DS), DP, the formulation composition, the route of administration, and the gastrointestinal (GI) physiology to predict in vivo exposures. PBBM can provide the crucial link between in vitro and in vivo performance of drug products to establish PCQS, which includes the dissolution method and acceptance criteria, dissolution safe space, and specifications for critical bioavailability attributes such as particle size distribution, polymorphism or crystalline content, granule properties, and manufacturing process parameters. PBBM can also provide supportive evidence for biowaivers including the biopharmaceutics classification system (BCS) based biowaivers and additional strength waivers as well as scientific bridging for 505(b)(2) products. FDA has cosponsored two workshops on PBBM in 20172−4 and 2019.5 FDA also published the draft guidance on the use of PBPK analyses for biopharmaceutics applications in 2020.6 Currently, global regulatory acceptance of PBBM has some challenges. They include lack of the prospective PBBM strategy leading to inadequate model input, validation, and biologically implausible optimizations to fit model predictions to clinical data. A primary objective of this workshop was to discuss best practices on PBBM with respect to model input (in vitro and in vivo), model validation, and model applications; discuss new areas of PBBM applications such as generics and modified release (MR) products; and finally explore the areas of agreement between the industry and regulators for the future harmonization efforts.
2.2. Keynote Speech: PBBM: Impact and Future Perspective. Jennifer Dressman
Prof. Jennifer Dressman kicked off the conference with a plenary lecture on the current status of PBBM for various routes of administration. She highlighted that the physiology at the given site of administration should be adequately captured and that release tests must be tailored to the specific site of application, as well as the dosage form applied. Modeling is then required to bring both of these aspects together and translate the results into a prediction of plasma and/or local concentration profiles. For modeling systemic levels, it is highly recommended to start with the disposition kinetics and compare the model against clinical intravenous (IV) data whenever possible.
Probably, the most advanced PBBMs are those for oral drug delivery. Much data exists for the physiology of the GI tract, and quite sophisticated models are already available in the most frequently used software tools. One area in which we could do better is the modeling of GI motility, particularly in the fed state, which may have a large impact on the gastric distribution of the drug and consequently its gastric emptying. In the past few years, there has been a concerted effort across academic institutions to create biopharmaceutical tests which better mimic release from the formulation in the GI tract.7−9 As a result, biorelevant media have largely replaced United State Pharmacopeia (USP) standard buffers as test media in pharmaceutical development. However, the most widely used equipment is still the USP Type 2 (Paddle) apparatus, and it remains to be seen whether other apparatuses can attain the same broad level of acceptance. Likewise, while assessing permeability by running bioavailability studies in animals has been largely replaced by studies in cell lines such as Caco-2 and Madin-Darby canine kidney (MDCK) cells, we still need better models for human permeability.
To build a “digital twin”-based population pharmacokinetic (PK) model, the variability in physiology and its ramifications in terms of inter- and intraindividual variability in release rate and permeability must be taken into account. Efforts to mechanistically model both release from different types of dosage forms and drug permeability are already underway and have achieved some success.10−12 Using ibuprofen as a test compound, creation of a robust in silico model to describe its dissolution under various conditions was demonstrated.12 Further, case examples showcased the joint impact of formulation and food on itraconazole PK and the joint impact of formulation and proton pump inhibitor (PPI) on AstraZeneca development compound PK.13,14
Similar approaches have been used to build PBBMs for other routes of administration. For the dermal route, many different formulation types are available, and the choice of formulation will have a strong impact on the depth of permeation into (and beyond) the skin.15 The challenge lies in tailoring the release studies to the intended site of drug delivery. Like the GI tract, skin physiology is quite well understood, and the next tasks will be to capture changes in skin physiology with body location, patient age, ethnicity, and disease state. Nevertheless, PBBM has already progressed to the point where virtual bioequivalence (VBE) assessments of topical formulations are starting to gain acceptance at the regulatory level.16
For long-acting injectables, PBBMs are used to describe simultaneous release and biodegradation of polymeric vehicles, and there are also some recent advances made in biopharmaceutics evaluation e.g., the Dispersion Releaser.17 In the case of products that are inhaled, biopharmaceutical models include considerations of particle size and shape, with measurements of tissue permeability in the lung frequently being conducted in Calu-3 cells.
In summary, PBBM has really picked up the pace in the past few years, and by 2030, it is likely that we will have reliable PBBM across a range of routes of administration. The advantages of PBBM are self-evident–with the physiological “digital twin” approach, we should be able to predict first-in-human levels better, as well as reduce the number and/or size of studies necessary to identify drug–drug interactions (DDI) and food effect interactions. The impact of PBBM will be more biowaivers based on VBE, application to “beyond the rule of five” drugs, and the reduction or even elimination of animal studies in formulation development, which will culminate in more effective medicines becoming available to patients sooner.
2.3. Case Study 1: A PBBM Based Dissolution Safe-Space for a BCS Class II Drug Substance. Shereeni Veerasingham and Arthur Okumu (HEALTH Canada)
2.3.1. Background
PBBM was utilized to establish a dissolution safe-space for an immediate release (IR) tablet from Amgen containing a BCS Class II drug substance. The drug is a weak base, hydrochloride salt with a pKa of approximately 9. Following oral administration of the tablet, the maximum plasma concentration (Cmax) is achieved in approximately 6 h. Administration of the tablet with food increases the rate and extent of drug absorption, with a greater impact observed with a high-fat meal compared to a low-fat meal. The clinical knowledge space includes tablet variants that were evaluated in clinical bioequivalence studies, including a tablet variant that was found to be nonbioequivalent to the target profile. The nonbioequivalent tablet variant had a significantly slower in vitro dissolution profile than the target profile. PBBM based VBE trials were conducted to determine the in vitro dissolution edge of failure for bioequivalence and establish a dissolution safe-space for the tablet. The question of interest was, can the dissolution specification for the oral tablet be widened and still ensure bioequivalent in vivo performance?
2.3.2. Model Development
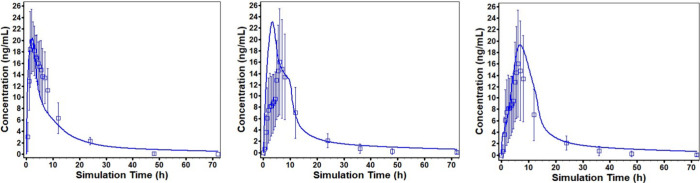
The PBBM used the Advanced Compartmental Absorption and Transit (ACAT) model in GastroPlus (ver. 9.8.3, Simulations Plus Inc., Lancaster, CA). Changes were made to the default ACAT model based on literature research, in vitro data, and clinical observations to optimize simulations for the tablet. The disposition model was developed based on the physicochemical and biopharmaceutical properties and intravenous (IV) and oral PK data from 5 clinical studies. Initial Michaelis–Menten constant (Km) and maximum reaction velocity (Vmax) values for CYP3A4 and CYP1A2 were estimated by ADMET predictor (Simulations Plus Inc., Lancaster, CA). Clearance was determined by optimizing the Km and Vmax values to fit the observed clinical plasma concentration following the IV infusion of the drug at three different doses. During oral absorption model development, the effective permeability (Peff) was fitted to PK data for the oral solution obtained under fasting conditions and verified by comparison of the simulation for fed conditions to observed PK data. In addition, the percentage of fluid in the small intestine and colon were updated to 7.5% and 3%, respectively, to reflect values reported in the literature.18 The PK profile for the oral solution was simulated reasonably well (Figure 1, left panel).
Figure 1.
Simulated concentration versus time profiles (solid line) and observed profiles with %CV (open squares) for oral solution (left panel), initial simulation for the tablet (middle panel), and simulation for the tablet following refinement of the model (right panel).
However, PK simulations for the tablet overpredicted the Cmax and underpredicted the time to the maximum concentration (Tmax) (Figure 1, middle panel). Further model refinement was therefore undertaken, considering that, due to a common ion effect, aqueous solubility of the drug (HCl salt) decreases in the presence of chloride ions. The aqueous solubility of the drug is relatively constant in the range of pH 3.5 to 5.0 and decreases at pH greater than 5.0. The in vivo pH-solubility profile was assumed to vary with formulation (solution or tablet), the volume of water administered with the tablet, and the prandial state. The in vitro and in vivo pH-solubility profiles were calculated using the Henderson–Hasselbalch equation and the estimated in vivo chloride ion concentration at the time of drug administration.
Dissolution was assumed to be controlled by the diffusion of the drug through a stagnant film layer surrounding the dissolving particle as described by Pepin et al., 2019.19 In vitro dissolution rates were fitted to a theoretical product particle size distribution (P-PSD) and were validated by using P-PSD to predict dissolution at different pHs. The predicted dissolution profiles matched the measured profiles at pH 1.3, 2.0, and 4.5. However, at pH 6.8, the P-PSD and bulk pH/solubility overpredicted the dissolution rate. Using surface pH/solubility at pH 6.8 improved the prediction but resulted in a modest underprediction compared with the measured profile. The P-PSD values were used as input to simulate in vivo dissolution for the ACAT model.
Due to the pH profile in the GI tract, supersaturation of the drug can occur, leading to precipitation. A mechanistic model based on classical nucleation theory was used to account for differences in the nucleation and growth rates for the oral solution and the tablet. Further, for the tablet simulations, the pH in the ascending colon was reduced from pH 6.8 to 4.86 based on the pH value obtained from an in vitro experiment. The reduction in pH accounts for microenvironmental pH effects of undissolved drug in the ascending colon, and the longer residence time and low chloride concentration are expected to allow for further drug dissolution and absorption. Simulation for tablet following refinement of the model indicated a good fit to the observed profile (Figure 1, right panel).
2.3.3. Model Validation and Application
Model validation employed data sets that were independent from those used in model development and included a data set for a different formulation. The validation was based on single simulation comparisons to the observed PK profiles from three clinical studies. Additional validation included comparisons of simulations to PK profiles obtained from a food effect study (low-fat and high-fat meals) and a DDI study using ketoconazole as the perpetrator. Prespecified acceptance criteria were met for most studies, except for area under the concentration versus time curve (AUC) in one PK study (Average Fold Error (AFE): 1.35) and Cmax for the low-fat, low-calorie simulation (AFE: 1.27). Overall, the model validation was considered adequate for the intended use of the model to determine a dissolution safe space.
Parameter sensitivity analysis (PSA) identified CYP3A4 metabolism kinetics, small intestine transit times, small intestine and colon fluid volumes, and ascending colon pH as key parameters with an impact on Cmax and exposure, assessed as the AUC.
Prior to model application, the ability of the population simulation to capture the observed intersubject PK variability was evaluated. Parameters identified by the PSA as influential parameters were adjusted to account for intersubject and intrasubject differences. The simulated probability contours of the plasma concentration time profile across 10 population simulation trials mimicked the range of variability observed between subjects in the clinical data set. Conservative criteria for bioequivalence were set with a requirement that all trials (10 out of 10) needed to meet the bioequivalence criteria of the 90% confidence interval of the ratio of the test to reference Cmax and AUC within 80–125%. The ability of VBE trials to simulate observed clinical results was evaluated by using a tablet variant that was not bioequivalent to the target profile. The bioequivalence criteria were not met for 1 of 10 virtual trials, indicating agreement in the conclusions of the virtual trials and clinical studies.
To define a safe-space, theoretical dissolution profiles were generated by altering the Weibull Ph1 fraction (f1). As f1 decreases, dissolution is slower with an increase in P-PSD, and PK simulations display a correspondingly lower Cmax. Simulated PK for the theoretical profiles was then compared to that of the reference tablet in VBE trials. Of note, model complexity and software limitations led to unsuccessful trial simulations for some subjects (simulations did not run to completion). Of 42 virtual subjects included in the trial, only the first 32 completed subjects for the reference formulation and corresponding subject simulation for the test formulation were used for the analysis. For the slowest f1 profile (f1-slow), 1 of 10 virtual trials did not meet bioequivalence criteria, with a Cmax ratio 90% CI < 80%. All f1 profiles faster than f1-slow were bioequivalent to the reference tablet. A dissolution safe space was defined based on the results of the VBE trials and could permit widening of dissolution specifications.
2.3.4. Regulatory Perspective
This PBBM applied a mechanistic approach to in vivo drug pH-solubility profiles with consideration for common chloride ion effects and precipitation. However, the adjusted solubility profiles focused only on the most impacted GI tract regions, i.e., the stomach and colon, to limit the model complexity. As precipitation is a key consideration for this model, experimental data are recommended to support the assumption for regulatory submissions. Validation of the model based on single simulations was considered adequate, but some concerns were noted for the population simulations and VBE trials. Regulators noted that the variability of the virtual subjects for the population simulations was not fully representative of that observed in clinical trials, as probability contours covered the observed variability at a 95% prediction interval in only 5 of 10 trials. Further, virtual trial simulations were unsuccessful for some subjects due to the model complexity and software limitations. The predictive ability of the model for the nonbioequivalent tablet variant was also questioned as 1 out of 10 trials did not meet the bioequivalence criteria. The overall assessment takes into account the model risk, which was considered low per the credibility assessment framework.20 The defined safe-space was considered adequate to permit widening of dissolution specifications, considering a margin of error in view of the simulation results obtained for the nonbioequivalent tablet variant.
2.4. Case Study 2: Justification of Dissolution Specification for Lesinurad. Anders Lindahl (Swedish Medical Products Agency) and Flora Musuamba Tshinanu (Federal Agency for Medicines and Health Products, Belgium)
2.4.1. Background
The modeling work for this product has been described previously in 2016, making this one of the first published PBBM with regulatory implication.21 Lesinurad is a selective uric acid reabsorption inhibitor, administered orally as an IR tablet (Zurampic 200 and 400 mg) for treatment of hyperuricemia associated with gout. Lesinurad, a weak acid with a pKa of 3.2, has low solubility at low pH values, high solubility at pH values above pH 5, and a high intestinal permeability, i.e, BCS 2.21 During the marketing application procedure, an in silico PBBM was submitted to FDA in support of the proposed in vitro specification of Q = 80% in 30 min. The PBBM was not submitted to the European Medicines Agency (EMA) throughout the marketing authorization application (MAA) procedure. Of note, the in vitro dissolution specification limit, Q = 80% at 30 min, was accepted based on the in vitro dissolution of several pivotal batches and two nonbioequivalent batches. In this scenario, where the model is only descriptive and the key decision is taken based on other data, the regulatory impact of the model is considered low. However, the model assessment exercise was performed irrespective of this consideration in the context of the preparation to the workshop, and several issues were identified.
2.4.2. Model Development, Validation, and Application
The modeling platform was GastroPlus (Version 9.0.0, Simulations Plus Inc., Lancaster, CA). Individual PK data was obtained from a clinical bioavailability study, including a 15 min IV infusion microtracer dose of 0.1 mg (14C lesinurad) and an oral dose of 400 mg of lesinurad, in 12 subjects. While IV data were used to estimate disposition parameters (volumes of distribution and clearances), the oral PK profiles obtained in the same subjects at 400 mg dose were used to calculate individual gastric emptying patterns and optimize the individual Peff data. Thus, a top-down data driven approach was used to create individual models with subject-specific gastric emptying rates (lag time) and Peff. From the EMA perspective, a bottom-up approach would have been preferred for characterization of Peff.22 The % default values for standard volume occupation by water in the small intestine and colon (40% and 10%, respectively) were reduced to 7.5% and 2%, respectively, with reference to Schiller et al.18 In vitro dissolution data were fitted to a P-PSD that would match observed in vitro dissolution per batch using the quality control method. The obtained P-PSD was then used as the input in GastroPlus. Moreover, the formulation was switched to a delayed release enteric coated tablet in the model in GastroPlus to ensure no release in the stomach. Finally, to be able to fit the model to the individual PK profiles, it was, according to the modeling report, necessary to reduce the dose for the nonbioequivalent batch in the GastroPlus platform to compensate for the lower PK exposures observed in the clinical study comparing the nonbioequivalent batch to the pivotal batch used in the model building. The dose was reduced to 352 mg in the model instead of the 400 mg that was dosed in the clinical study, and the sponsor concluded that the model could adequately predict the Cmax ratio between the two batches. These could be considered manual manipulations in the context of the bottom-up data driven approach that can be questioned given the limited amount of clinical data available and given the absence of convincing justification in the documentation submitted by the applicant. From an EMA regulatory point of view, this approach would not have been acceptable for higher regulatory impact applications. PSA was performed for each subject and each batch for Peff, P-PSD, and solubility. However, PSA was missing for the formulation switch, change in GI volumes, and gastric emptying time.
The intended scenario was simulated with use of a virtual population (n = 25) based on the subjects included in the model building and a product batch with an in vitro dissolution similar to the suggested specification limit. Between-subject variability was randomly introduced (within the observed ranges) for gastric emptying and gastric pH. However, no within-subject variability was simulated as part of the sensitivity analysis. Predicted intervals from simulated trials were tighter than those observed in clinical studies. The sponsor concludes that bioequivalence is expected for a batch with product specification limit Q = 80% at 30 min, based on the PBBM. This conclusion is not shared by the EMA regulators given the identified caveats of the model. Instead, as mentioned above, the suggested in vitro dissolution specification for drug product was accepted based on the in vitro dissolution of several pivotal batches and two nonbioequivalent batches.
2.4.3. Regulatory Perspective
In summary, the EMA regulators identified issues with uncertainties in Peff and gastric emptying (fitted values), fluid volumes in the GI tract, formulation switch, manually adjusting the dose during model verification, and lower variability in the simulated virtual population compared to in the clinical studies. The model would not have been accepted to justify an extended in vitro dissolution safe space beyond the Q = 80% in 30 min, if this was requested, because it would then be considered a medium to high regulatory impact. In these cases, the described issues would have been considered critical. In order to illustrate the decision-making process from the initial question to the final answer regarding the model acceptance, the EMA regulatory assessors have filled the credibility assessment matrix for the case of lesinurad as shown in Table 1.23,24 At the EMA, filling the credibility matrix is considered good practice in regulatory submissions including modeling and simulation with medium and high regulatory impact applications. In this case, the matrix was filled for lesinurad for an illustrative purpose only.
Table 1. Credibility Matrix for Lesinurad PBBM.
| item | entry |
|---|---|
| Investigational product | Lesinurad (ZURAMPIC) is a selective uric acid reabsorption inhibitor, administered orally as an IR tablet |
| Type of model | ACAT PBBM as implemented in Gastroplus |
| Scientific question(s) of interest | Is a dissolution specification of Q = 80% at 30 min acceptable for lesinurad tablets? |
| Context of use | The objective of the model is to predict the dissolution profiles in vitro and in vivo and related parameters. The modeling package is intended to support the proposed specifications for dissolution and particle size. |
| Comparative in vitro dissolution data for pivotal batches are available to answer the question of interest using the quality control (QC) in vitro dissolution method for drug product. | |
| Model influence | Low |
| Decision consequence | Low |
| Regulatory impact and risk assessment | Low |
| Basis for acceptability of the MIDD approach | No formal qualification of the PBBM platform would be requested given the low regulatory impact |
| Output of model evaluation | Data submitted do not support formal platform qualification. Several issues are identified with the implementation of model building and evaluation and with model predictive performance. |
| Model informed decision | The product dissolution specification (QC) was accepted based on in vitro dissolution data for pivotal batches. If the regulatory impact was higher, the in silico model would not be accepted. |
2.5. Case Study 3: Justification of Formulation Bioequivalence Despite Differences in Dissolution for Acalabrutinib Capsules. Rebecca Moody (FDA)
2.5.1. Background
AstraZeneca submitted a PBBM case study based on publicly available data from several publications on acalabrutinib capsules.19,25,26 Acalabrutinib is a BCS Class II weak diprotic base drug substance formulated as a 100 mg IR capsule for the treatment of adult patients with mantle cell lymphoma who have received at least one prior therapy. The purpose of the submitted PBBM was to evaluate if differences in the in vitro dissolution between two drug product batches had an impact on the in vivo absorption, measured via PK end points. Specifically, during product development, two batches (W026394 and L0505009) had similar dissolution profiles in low pH media (pH 1) but had different dissolution profiles in pH 4.5 Acetate Buffer and FaSSIF media as assessed by the similarity factor (f2). It is noted that both batches were dosed in clinical trials in parallel studies with adequate outcomes.
2.5.2. Model Development, Validation, and Application
In summary, the PBBM strategy involved modeling of individual subject PK data and then validating whether that population was able to reproduce the observed mean Cmax and AUC from several different clinical scenarios. Individual models were constructed via top-down analysis for an 8-subject population for which microdose IV and oral administration capsule PK data were available. In building the oral absorption model, gut Vmax for CYP3A4 was individually fitted based on oral PK profiles, and a subject-specific gastric retention time was added to account for observed lag times. In vitro dissolution was incorporated into the model mechanistically through the P-PSD approach.25
In the discussion of the P-PSD approach, it was noted that an appropriate number of in vitro dissolution data points are useful for fitting (i.e., to capture the full profile), that the fewest number of bins should be used for fitting, and that the prediction ability of the fitted P-PSD needs to be validated in several pH media to be considered acceptable. Ideally, a well-structured framework is in place prior to extracting the P-PSD and identifies the dissolution media to be used for P-PSD extraction (and why), the optimization process for fitting and reducing the number of bins, and the steps for validation. For the acalabrutinib case study, however, a P-PSD was extracted for 4 different drug product batches using different dissolution conditions (i.e., pH 1 for Phase 1 capsules vs pH 6.8 for batches representative of commercial capsules) and the number of bins (i.e., 10) was not fully justified.
For the PSA, several physiological and drug related parameters were varied to assess their impact on acalabrutinib exposure for one subject of the 8-subject population. The one subject was selected to be representative of the population based on their total clearance, volume of distribution, and gut CYP3A4 Vmax. From the PSA, it was clear that there are relevant differences in acalabrutinib exposure (Cmax and AUC) due to several parameters; however, only CYP3A4 Vmax and gastric residence time were incorporated into the model with individual fitting for both parameters. Other parameters, such as Peff, were assumed to be constant across the population without sufficient justification. In addition, the model would benefit from clarity regarding the ranges of the parameters tested and whether they are representative of the ranges expected in the greater population. Addressing the uncertainty regarding input parameters and the potential clinical relevance of those uncertainties to assess the model consequence and reliability would be useful.20
The model was validated by evaluating the accuracy of the 8-subject population in simulating acalabrutinib exposure from 16 different clinical scenarios. The model predicted the Cmax and AUC ratios between test (W026394) and reference (L0505009) batches were close to 1.0, and the 90% confidence intervals were comprised between the bioequivalence (BE) limits of 0.8–1.25.
2.5.3. Regulatory Perspective
Overall, considering the totality of evidence, the risk of bioinequivalence for drug product batches W026394 and L0505009 due to dissimilar dissolution at high pH (i.e., pH 4.5 and above) was low. However, the application of PBBM for future use is considered limited due to uncertainties. Specifically, questions remain concerning the use of an 8-subject data set as representative of the wider population (without being able to capture within-subject variability) and the selection of fitted parameters without appropriate justification. To support future application of the PBBM, additional data from clinical studies involving DDI could support assumptions regarding CYP3A4 Vmax. It could also be beneficial to incorporate power and sample size calculations based on the observed variabilities from population studies so that the model would have greater utility and wider generalizability.
As a future discussion point for the modeling community, there were concerns and unknown consequences from health authorities on the topic of model multiplicity. There were at least 3 Acalabrutinib PBBMs highlighted in this case study: (1) the GastroPlus model submitted for regulatory approval to the U.S. FDA, (2) the GastroPlus model described in peer-reviewed publications, and (3) the model developed in Simcyp. This adds an additional layer of complexity, as slight differences were noted between each model. Where is the boundary for “fit for purpose”? In an ideal world, would there be one model for one drug product, one model that would be used throughout the drug product’s entire lifecycle for all purposes (e.g., DDIs, postapproval changes, biowaivers, etc.)?
2.6. Case Study 9: A Retrospective Case Study on Fluconazole. Øyvind Holte (Norwegian Medical Products Agency)
2.6.1. Background
The data included in this case study was selected from a wide body of data that exists for fluconazole–different strengths of tablets and capsules, oral solution, and also an intravenous formulation. The results of several clinical PK studies, performed between 1983 and 2019, were available for development and verification of the model. The company investigated whether PBBM could demonstrate bioequivalence between the various drug products despite significantly differing dissolution profiles and whether a validated PBBM approach could provide the ability to establish a dissolution safe space for bioequivalence.
2.6.2. Model Development, Validation, and Application
IV formulation data were used to confirm the clearance and volume of distribution for fluconazole, readily available from the literature. Second, GI absorption of fluconazole was estimated based on the exposure following dosing of oral solutions (two concentrations). Finally, oral solid dose formulations (tablets and hard capsules) were included in the model, supported by the in vitro dissolution performance (Weibull parametrization or the Johnson model–particle size distribution of fluconazole).
A total of 17 simulations were performed to develop the model. Separate data sets were used for model development and model validation.
The model concluded that there is no significant food effect for the oral hard capsules. Likewise, fluconazole PK is not affected by the concomitant intake of antacid. The model was further used to predict the bioequivalence (Cmax and AUC) of a series of oral solid formulations exhibiting a range of in vitro dissolution rates. Compared to a commercial formulation, some of these formulations had dissolution profiles that were clearly not “similar” based on the f2 algorithm. In other words, these dissolution data would typically not be accepted to support a BCS-based biowaiver. The model predicted that some of these formulations were bioequivalent, regardless of an f2 < 50. The formulations with the slowest dissolution rate were predicted by the model to be nonbioequivalent. These results were used to justify a possible widening of the acceptance criteria for the dissolution test.
VBE trials were ultimately performed to replicate the results of the previously conducted PK studies. Furthermore, VBE trials were used to establish the appropriate dissolution criteria, based on virtual batches having dissolution profiles between an unacceptable (slow) batch and the slowest among the acceptable clinical batches. Based on the VBE trials, a suitable acceptance criterion is NLT 80% dissolved in 75 min. This is substantially wider than the current acceptance criterion at 30 min, which is normal for an immediate-release drug product.
2.6.3. Regulatory Perspective
It is acknowledged that for the purpose of this case study all relevant details were not available. The clinical trials were conducted without any intent of supporting PK modeling, and certain drug product details relevant to modeling are not available. Based on the data presented for this case study, the regulators had many questions regarding the conclusions made by the company. There are uncertainties regarding the model’s ability to predict the PK of fluconazole.
VBE trials were performed, based on the model, to recapitulate the observed results from the available BE studies. However, certain assumptions made by the company were in question, and the conclusions made based on the VBE were not the same conclusions as found by the various regulatory authorities. In conclusion, based on the data provided with the case study, the PBBM represents limited value and would probably not be considered sufficient as a substitute for clinical data in a regulatory setting.
The company’s conclusions, which were supported by the model predictions, would normally use a bioequivalence study approach (in the absence of modeling). From a patient safety perspective, future batches of a drug product should not differ significantly from the batches used in a pivotal clinical trial. Therefore, wide dissolution rate acceptance criteria are normally not acceptable. A large batch-to-batch variation could indicate nonbioequivalence. It is acknowledged that, for certain drug products, the in vitro dissolution rate may not be directly related to clinical efficacy and safety, and relatively large differences can be acceptable. PBBM is well suited to support such decisions.
The model development presented with this case study is based on a substantial amount of clinical data–more than what can be expected for a new drug product under development. Still, the data have certain deficiencies. As indicated above, the clinical trials were not planned and conducted with the development of a PBBM in mind.
For example, detailed information regarding the PSD was not available for all of the batches, and this model input parameter was therefore assumed or estimated. Also, the conditions used for dissolution testing were not the same for all of the drug products: A higher paddle rotation speed can lead to a faster dissolution rate. This makes the head-to-head comparison of the various dissolution results and their use as model input difficult. For a bottom-up modeling approach, such uncertainties reduce the credibility of the model predictions. Apparently, no sensitivity analysis was performed during the model development.
Several of the simulations overestimated the Cmax and/or the AUC, and no efforts were made to adjust or correct the initial model based on these observations. Although the model predicted no significant effect of concomitant antacid or food intake, the confidence in such results is reduced by the underlying uncertainty of each model estimation.
In conclusion, it is believed that the presented PBBM would not be accepted as a substitute for BE trials to support a marketing authorization. However, the concerns indicated above would possibly be resolved during an application procedure.
2.7. Panel Discussion
The panel discussion brought together the following regulators from multiple health authorities: Rebecca Moody (FDA), Luiza Borges (ANVISA), Maria Malamatari (MHRA), Øyvind Holte (Norwegian Medical Products Agency), Shereeni Veerasingham (Health Canada), and Shinichi Kijima (PMDA). The moderators were Paul Seo (FDA) and Sumit Arora (Janssen).
The panel members were asked a series of questions regarding model parametrization.
2.7.1. Q1: What Is Your Opinion on the Use of Fitted Parameters versus Generated Data. In Particular, What Level of Fitting/Extrapolation Would Be Acceptable?
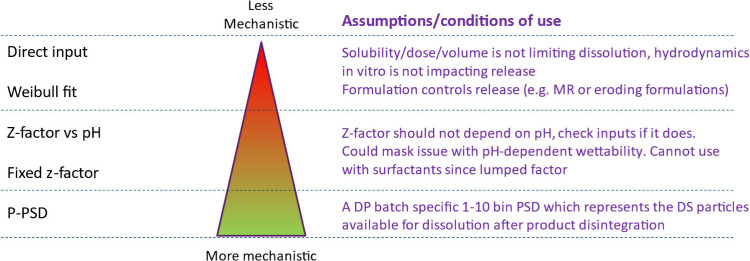
Øyvind Holte (Norwegian MPA) pointed out that, if model input parameters are fitted, it would be useful for them to be constant during model verification and validation where relevant. The model verification would in fact highlight whether the assumptions made or the model parameters that were fitted are correct (or not). For example, when dissolution data are introduced in a PBBM with a mechanistic model such as the Z-factor or P-PSD, the adequacy of the Z-factor or P-PSD should be verified in vitro by checking if the dissolution of the same batch obtained using different methodologies can be adequately predicted. This step should be made on several drug product batches of the same formulation and process to verify the dissolution model adequacy prior to its introduction in the PBBM. The panelists expressed the need for more data to demonstrate how the P-PSD works. Xavier Pepin (Simulations Plus, Inc.) responded that the P-PSD represents the surface of drug substance available in the drug product for dissolution and a measurement of this surface area with an orthogonal technique could be difficult (See Section 3.3). Ultimately, the P-PSD validation in vitro and in vivo in different conditions of the GI tract demonstrates its usability, as was suggested by the panelists.
2.7.2. Q2: How Important Is the Model Contribution to the Regulatory Decision for Quality Aspects of Drug Development, Submission, and Postapproval Changes?
Kuemmel et al.20 have developed a credibility assessment framework applicable to model informed drug development which defines a model influence, i.e., whether there exist additional data to support the question that the model tries to answer, and the decision consequence, i.e., the potential consequences to the patients if the decision supported by the model would be wrong. Both model influence and decision consequences can be used to assess the risk of the PBBM.
Shereeni Veerasingham (HC) stated that there is no current guideline in Canada regarding the development, validation, and use of PBBM. A case-by-case approach is employed, and the totality of the data submitted to support the file application is used to guide the decision.
Luiza Borges (ANVISA) pointed out that, for ANVISA, the PBBM is evaluated in terms of proposed application, development, and validation. The identification of the most influential model parameters is key. The data sets used for model validation are also examined for relevance. Uncertain parameters that are fitted would be expected to be highlighted. Finally, the totality of the relevant data provided for the model application is then considered for the evaluation.
Shinichi Kijima (PMDA) indicated that a few submissions to PMDA were reviewed using a quality decision making process, and PMDA’s cross functional team was involved in those reviews.
Rebecca Moody (FDA) stated that FDA typically reviews submissions of PBBMs with an interdisciplinary approach. The aim of the review is to understand the risks to the patient and what the model indicates in terms of product quality variations. Like other agencies, the totality of the data is considered to support the decision.
Øyvind Holte (Norwegian MPA) indicated that the number of PBBM cases reviewed by EMA is currently less than 5 and that EMA is therefore relatively new to this type of submission. It was also recommended to contact EMA in advance, if the intent of the PBBM is to waive a clinical evaluation, to set respective expectations, to agree on a process, and to organize the right review team.
2.7.3. Q3: What Is the Level of Parameter Justification Expected for a PBBM?
Panelists indicated that, whether parameters originate from experiments or fitted to other sources of data, it is useful for the measuring methods to be standard and well described. Fitting parameters within an acceptable range is not prohibited; however, justification with adequate scientific references would be helpful.
2.7.4. Q4: Are Virtual Bioequivalence Studies Acceptable?
Panelists indicated that they see that the number of VBE studies in PBBM submissions is growing. Since this is a clear direction that industry is taking, the panelists suggested that the populations included in VBE studies should be wider. In addition, the within-subject variability should be present, ideally using mechanistic models and compared to that observed in the clinic as much as possible. The virtual studies would be expected to reproduce the observed variability.
2.7.5. Q5: Are There Any Other Expectations in Terms of the Content and Format for Submitted PBBMs?
Panelists mentioned that visualization of the whole modeling strategy is very important, in addition to the assumptions made and their verification. Panelists also expressed the desire to see the model development history, i.e., why certain changes were made from default values, their magnitude, and how it impacted the model outcome. The industry participants believe that a report template could be useful for both regulators and industry to set expectations for future submissions. It would be important to include some details in each section to describe data expectations, with some examples. A template will be proposed by industry experts as a separate article.
3. Breakout Sessions
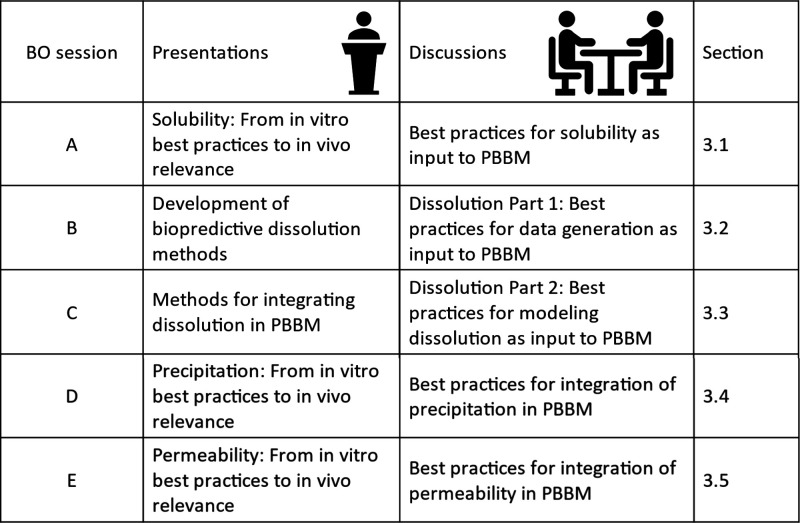
The overview of Day 1 presentations and BO sessions is presented in Figure 2.
Figure 2.
Overview of Day 1 presentations and BO sessions.
3.1. BO Session A - Solubility: From in Vitro Best Practices to in Vivo Relevance
This session began with speaker Deanna Mudie (Lonza) and was led by Evangelos Kotzagiorgis (EMA) and Claire Mackie (Janssen), with Tessa Carducci (Merck & Co., Inc., Rahway, NJ, USA) and Mario Cano-Vega (Amgen) as scribes.
3.1.1. Presentation
Solubility is a fundamental driver of drug bioperformance.27 It is one of the fundamental properties that defines the BCS and is an important input to PBBM.28 Generally, it defines the maximum concentration of a drug in solution (e.g., in GI fluid) at equilibrium or a metastable, supersaturated state. A compound’s solubility is influenced by the interplay between the properties of the drug, the excipients within the formulation, and the GI fluid. This interplay affects the overall bulk solubility along the GI tract and the solid particle surface solubility, as well as solubilization in bile, fats, and formulation components.29,30 Overall, solubility impacts a drug’s oral bioperformance via its influence on properties such as dissolution, precipitation, and maximum concentration in solution, i.e., the driving force for absorption.
3.1.1.1. Case Study 1: Impact of Excipients on Solubility and Dissolution
Deanna Mudie discussed a case study showing how excipients can impact the solubility and dissolution rate of the BCS Class 2 drug substance, belinostat. Belinostat was formulated as three different spray dried amorphous solid dispersions (ASDs) using different dispersion polymers, one enteric (HPMCAS-M) and the other two neutral (PVP K30 and PVP VA64).31 Belinostat amorphous solubility was measured in the absence and presence of these polymers using an in vitro UV solvent shift test.32 When no polymer was present, amorphous solubility exceeded 1800 μg/mL in gastric medium (pH 2 HCl) and 2500 μg/mL in intestinal medium (phosphate buffer at pH 6.5 containing FaSSIF powder). However, in the presence of polymer, the amorphous solubility was depressed at least 2- to 6-fold with the highest depression for PVP VA.31
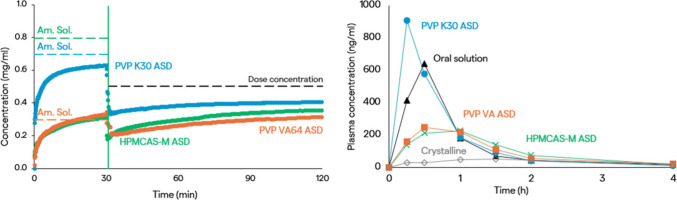
When the extent of dissolution of ASDs was measured in a nonsink dissolution test in intestinal medium, the results matched the amorphous solubility values measured in the UV solvent shift test. However, the results differed when a transfer dissolution test was run with ASDs dissolved in a gastric medium (pH 2 HCl) at a nonsink dose, where concentrated intestinal medium (phosphate buffer at pH 6.5 containing FaSSIF powder) was added after 30 min (Figure 3). In this case, while the PVP VA and PVP K30 ASDs reached the solubilities measured in the solvent shift test, solubility was significantly lower for the ASD made with HPMCAS-M. This was because these ASD particles aggregated in the gastric medium due to the low solubility of HPMCAS-M at acidic pH. In vitro dissolution profiles were incorporated into oral absorption simulations, using the Takano Z-factor method in GastroPlus.33 The HPMCAS-M ASD had the smallest z-factor and the largest calculated effective particle radius, reflecting the particle aggregation observed in the dissolution test. The PVP K30 ASD had the highest z-factor and driving force for dissolution. This mirrors an in vivo study in fasted beagles, where the PVP K30 ASD performed best (Figure 3).31 Furthermore, oral absorption simulations gave a good description of the concentration–time profiles. It was clear that the ASD dispersion polymer impacted the belinostat in vivo performance by attenuating amorphous solubility and driving effective particle size. High belinostat and polymer solubility in gastric medium maximized in vitro dissolution rate and in vivo AUC and Cmax.
Figure 3.
Belinostat in vitro and in vivo performance. Left: Concentrations in an in vitro gastric-to-intestinal transfer dissolution test (solid lines) were calculated with measured amorphous solubilities in gastric media (dashed lines). Right: Plasma concentration–time profiles in fasted beagle dogs (50 mg dose, n = 4).
3.1.1.2. Case Study 2: Impact of Excipients on Solubilization and Permeability
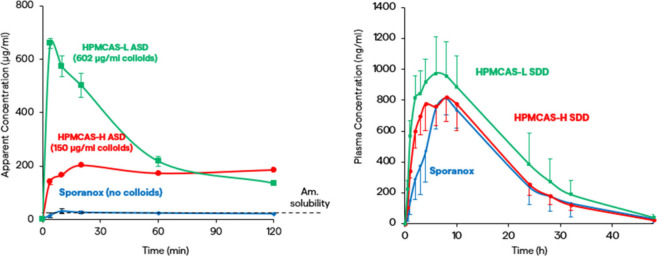
In another example, Deanna Mudie showed how nanosized drug–polymer colloids can increase the driving force for absorption. This example was for itraconazole, a highly lipophilic BCS 2 weak base formulated as spray dried ASDs using different grades of HPMCAS.34 Itraconazole ASDs formed nanosized drug–polymer colloids in the intestinal donor medium of an in vitro membrane flux test, contributing to “dissolved” concentrations above the amorphous solubility (Figure 4).35 Concentration and size of drug–polymer colloids were determined using microcentrifugation, ultracentrifugation, and dynamic light scattering.35 More colloids were produced with the ASD made using hydrophilic HPMCAS-L than with the more hydrophobic HPMCAS-H. The marketed formulation, Sporanox, did not form drug–polymer colloids. Drug–polymer colloids increased the rate of permeation into the acceptor medium of the in vitro membrane flux test with the fastest rate seen for the highest colloid-forming, HPMCAS-L ASD.
Figure 4.
Itraconazole in vitro and in vivo performance. Left: Concentrations in the intestinal donor medium of an in vitro membrane flux test. Right: Plasma concentration–time profiles in fasted rats (50 mg/kg, n = 6).
Faster permeation occurs because absorption of these formulations is limited by the unstirred water layer (UWL) adjacent to the membrane, and drug–polymer colloids increase effective drug diffusivity by acting as “shuttles” and helping to replenish free drug at the membrane surface.34,35 This phenomenon was accounted for in oral absorption simulations by modifying the effective permeability (Peff) in GastroPlus to account for the higher Peff of colloid-forming formulations (Peff, nano).36 When these ASDs were administered to fasted rats, a trend similar to the in vitro experiments was observed, with the highest absorption rates corresponding with the highest colloid concentrations. Absorption simulations captured the concentration–time profiles well (Figure 4).36 However, drug–polymer colloids do not always improve the absorption. Drug–polymer colloids have the potential to improve absorption by increasing effective drug diffusivity when absorption is solubility-permeability-limited and permeation is UWL limited. Also, the colloid concentration must be large compared to the concentration of unbound plus micelle bound drug.35 The influence of drug–polymer colloids on permeation can be predicted by comparing calculated Peff, nano to Peff and running PSAs. For this case study, it was concluded that drug–polymer colloids in excess of amorphous solubility increased the absorption rate of itraconazole ASDs. Drug–polymer colloid concentration can be measured in vitro, and Peff, nano can be used to model the influence on in vivo performance.
3.1.1.3. Case Study 3: Impact of Dissolved Drug on Surface Solubility and Dissolution
Deanna Mudie discussed how dissolved acidic or basic drugs can influence solid particle surface solubility and dissolution rate by modulating the surface pH. This example was for acalabrutinib, a BCS 2 weak base. Acalabrutinib free base shows a 43% reduction in AUC when taken with PPI due to reduced solubility and gastric dissolution at elevated gastric pH. A maleate salt form of acalabrutinib mitigates this effect.37 Surface pH can be estimated in vitro by measuring the pH of a saturated solution of the drug in the relevant medium. Results of measurements of acalabrutinib in HCl or NaOH were shown for an acalabrutinib ASD, the crystalline free base, and the maleate salt form.38 For the crystalline and amorphous free base, the pH of a saturated solution was higher than the starting bulk pH below the highest acalabrutinib pKa, with a larger pH change for the amorphous drug due to its higher intrinsic solubility. On the other hand, a saturated solution of the maleate salt form showed minimal pH change at low pH, but a decrease in slurry/surface pH above pHmax.37
Modeling dissolution rate using bulk rather than surface pH carries a risk of misrepresenting dissolution rate for cases when surface pH differs from bulk medium pH. Surface solubility can be accounted for in oral absorption software by, for example, setting bulk pH equal to surface pH or inputting surface solubility rather than bulk solubility as a function of pH in, e.g., GastroPlus.38
Bottom-up oral absorption predictions of crystalline and amorphous acalabrutinib in fasted beagle dogs treated with either pentagastrin (gastric pH ∼ 1–2) or famotidine (gastric pH ∼ 6–7) provided good in vivo study prediction accuracy (absolute average fold error of AUC0-inf < 1.6).38 However, not accounting for surface pH/solubility only modestly affected the simulations. A 15–20% difference in simulated AUC and Cmax was observed for the crystalline free base in pentagastrin-treated dogs, with no difference for the other simulations. This result is attributed to the rapid dissolution rate and solubility-limited absorption of acalabrutinib at bulk pH 2 and similarity between bulk and surface pH at pH 6. However, Pepin et al. modeled dissolution rate of crystalline acalabrutinib and found that use of bulk instead of surface solubility led to an overall 48% overprediction across the GI pH range, with prediction error highest at bulk pH 4.5 (up to 250%) where a difference between surface and bulk pH is observed and dissolution rate is much slower.19
Deanna Mudie discussed some criteria for predicting when a weakly basic or acidic drug or excipient would tend to modulate surface pH and dissolution. For example, the tendency for pH modulation increases as weak acid pKa decreases or weak base pKa increases, when intrinsic solubility increases, and when buffer capacity decreases.30 Published calculations using inputs such as pKa(s), intrinsic solubility, and buffer properties can be used to predict when surface pH is not equal to bulk pH.19,25 In addition, surface pH changes are most likely to impact oral absorption simulations when dissolution is rate-limiting. PSAs were conducted to determine the sensitivity. For this case study, it was concluded that acalabrutinib can modulate surface pH, and the extent and direction of pH modulation depends on solid form type (e.g., amorphous, crystalline, salt). The extent to which drug surface pH modulation in vitro manifests as changes in AUC and Cmax in vivo and in silico depends on drug, formulation, and fluid properties.
To end the talk, Deanna Mudie concluded that solubility drives oral bioperformance through dissolution, precipitation, and permeation and is influenced by the interplay between the drug, the formulation, and the GI fluids. Importantly, both solubility and bioperformance can be predicted using targeted in vitro tools combined with PBBM.
3.1.2. Discussion
During breakout session A, participants discussed fundamental questions regarding the measurement and utilization of solubility data.
3.1.2.1. Q1: What Specifically Do Bulk and Surface Solubility Measurements Assess and Why Are These Assessments Crucial in the Context of PBPK/PBBM Modeling?
Bulk drug solubility allows the calculation of drug amount dissolved at equilibrium if the volume of the medium is known, and its properties are not altered with time. Conversely, surface solubility is the drug solubility at the drug solid–liquid interface. While bulk solubility influences factors, such as solution-mediated precipitation, surface solubility drives drug dissolution and surface-mediated precipitation. For weakly acidic and basic drugs, surface pH may deviate from bulk pH when there is an acid–base reaction occurring at the drug liquid interface.39,40 Consequently, measuring both bulk and surface solubility evaluations is important to accurately capture dissolution and precipitation rates in PBBMs. The choice of buffer for these measurements was highlighted as a key consideration and should align with the specific region of the GI tract being simulated.
Furthermore, the session discussed the dynamic impact of excipients on the surface and bulk pH. For example, acidulants included in formulations gradually dissolve over time, and the extent of their effect depends on both time and concentration. This comprehensive discussion illuminated the critical role of understanding bulk and surface solubility and the contributing factors in making informed decisions during drug product development.
3.1.2.2. Q2: Which Media (e.g., FaSSIF V1 and V2) Should Be Chosen for Accurate Comparison to the in Vivo Situation, Considering Factors Such as the Presence and Concentration of Bile Salts, Fats in the Stomach, and Buffer pH?
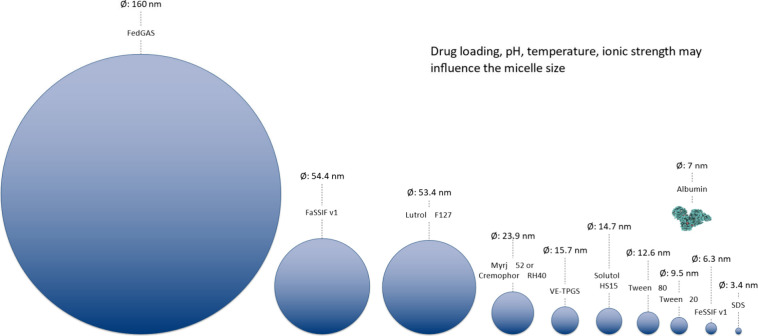
Participants agreed that there is not a one-size-fits-all “best” version of simulated GI media to choose for accurate prediction of in vivo conditions but that each may serve distinct purposes in modeling scenarios.7,41 When measuring drug solubilities across different versions of FaSSIF and aspirated human intestinal fluids, researchers have found solubility values to vary between media.42,43 In addition, no single medium captures the normal variation in these fluids.44 It is important to understand the properties and compositions of different types of simulated media and how they may interact with the drug product of interest to influence solubility, dissolution, and precipitation. For example, fasted state simulated intestinal fluid (FaSSIF) evolved to have a lower buffer capacity when moving from version 1 to version 3. Version 3 incorporates additional bile components (e.g., lecithin hydrolysis products and cholesterol) that are not found in versions 1 or 2.30 Factors such as buffer capacity and buffer species can impact surface solubility for acidic and basic drugs, and the type and concentration of bile components impact solubilization, especially for lipophilic drugs when nonionized at the medium pH.45 Some participants noted that FaSSIF v1 appears to be suitable for BCS classes 1 and 3 compounds, whereas FaSSIF v2 may better capture solubilities of some BCS class 2 and 4 compounds.
Investigating solubility in the fed state can be challenging due to the dependence of media composition and resulting drug solubility on meal content.46 In addition, the inclusion of components such as fats in simulated gastric media requires careful preparation and complicated analytical techniques for assessing drug solubility. Nevertheless, gaps in the ability to model drug absorption in the fed state dictate the need to consider the impact of meal components on drug solubility.47 Several types of simulated fed state media, such as FeSSIF, FeSSGF, and FEDGAS (Biorelevant, London, UK) are available for this purpose.46
Considering these findings, the session concluded that it is crucial to deliberate whether customizing the buffer for specific applications or establishing standardized buffers is the most prudent approach. In any case, panelists emphasized the importance of providing precise and comprehensive descriptions when selecting buffers or biorelevant media. Given the limited experience in this field, it becomes imperative to offer supplementary information to facilitate a better understanding of the decisions made and their impact on the model.
3.1.2.3. Q3: When Is the Optimal Time to Measure the Solubility in Human Aspirates?
Measuring drug solubility in human aspirates has not gained widespread adoption due to factors such as availability and cost; however, participants recognized its potential benefits, especially in improving modeling of poorly soluble, nonionizable lipophilic drugs. These drugs often exhibit wide variation in solubility as a function of micelle or vesicle composition, since simulated fluids (e.g., FaSSIF) lack many endogenous, bile- or vesicle-forming components. Participants reached a consensus that the benefit of using aspirated human fluid rather than simulated fluid is probably less important if the drug is ionized in the GI tract. In these cases, pH is the main driver of solubility.45
3.1.2.4. Q4: For Weak Bases, Is There Added Value in Measuring Solubility Across a Broad pH Range, Specifically pH 8–9? If so, Which Media Should Be Considered?
The participants agreed that the pH range over which solubility is measured is an essential factor to consider for weakly basic and weakly acidic drugs. This pH range should cover the GI physiology, i.e., from approximately 1–8. Experimental points should capture multiple degrees of ionization (e.g., 0% ionized, 50% ionized, 90% ionized) depending on the pKa. Measurements at pH values >8 (using NaOH for adjustment) may be needed to capture drug intrinsic solubility for weak bases (i.e., highest basic pKa + 2 pH units). One may also consider determining solubility in purified water and unbuffered media to determine the surface pH of the drug. For salts of weak acids and bases, the measurement of the solubility at and around pHmax is recommended. It was emphasized that researchers should measure the medium pH prior to addition of drug and the pH of the final saturated solution. Both start and final pH values should be reported. The media composition should also be documented since they may comprise common ions with the drug substance, which could depress drug solubility,48 or lead to salt formation which could change the nature of the drug substance.
3.1.2.5. Q5: What Solubility Value Should Be Employed for Release from an Amorphous Solid Dispersion Containing a Polymer?
During the session, participants acknowledged the challenges associated with developing PBBMs for dosage forms containing an amorphous solid dispersion (ASD). When modeling release from ASDs its important to understand whether dissolution is controlled by the drug, the polymer, or the combination of the two.49 When dissolution rate is driven by the drug, the amorphous (i.e., kinetic) solubility in the given medium is likely the appropriate solubility to employ for defining the rate of drug release. However, if the dissolving ASD contains both amorphous and crystalline drugs, then the solubility of the crystalline form in that medium and its impact on drug release may also need to be considered.
When modeling drug precipitation and redissolution of ASDs, the amorphous solubility and solubilities of any crystalline forms to which the amorphous drug may precipitate should be considered. Some ASDs may undergo liquid–liquid phase separation (LLPS) and precipitate to amorphous nanodroplets, which may then redissolve according to the amorphous solubility.50 In other cases, amorphous drug may crystallize, and the solubility of the crystalline form will be an important input to account for drug precipitation and solubility limitations to redissolution along the GI tract.
It was also emphasized by participants that measuring amorphous solubility in the presence of formulation excipients, such as polymers, is critical. For example, ASD polymers can either decrease amorphous solubility or increase it through the formation of drug–polymer colloids.31,35 It is worth highlighting that the impact of these excipients varies as a function of the time and concentration. Participants also noted that, for ASDs, acquiring an in-depth understanding of drug speciation, with a particular focus on detecting drug–polymer colloid formation using different analytical techniques, may be necessary since the presence of these species can impact the driving force for drug permeation.35 These considerations are pivotal for the effective development of PBBMs for ASDs.
In conclusion, the breakout session produced several significant takeaways. Participants in this session recognized the inherent complexity of drug solubility and its substantial influence on the development of PBBMs. The discussion brought to the forefront various critical topics, including distinctions between bulk, surface, thermodynamic, and kinetic solubility as well as points to consider during experimental measurements of these parameters. Given the intricate nature of these phenomena, it is strongly encouraged to include details regarding the rationale behind model development for solubility inputs for regulatory submissions. These should comprise the criteria for selecting and applying specific solubility parameters, choosing appropriate models, defining the experimental conditions for measuring solubility values, and highlighting the theoretical assumptions. Additionally, participants advised conducting parameter sensitivity analyses to ensure a robust and comprehensive understanding of the models utilized in drug product quality assessments.
Important points to consider when measuring bulk and surface solubilities of crystalline and amorphous drugs and formulations are presented in the Supporting Information.
3.2. BO Session B - Dissolution Part 1: Development of a Biopredictive Dissolution Method
This session began with speaker Raimar Loebenberg (University of Alberta) and was led by Paul Seo (FDA) and Nicoletta Fotaki (Bath University), with Ivy Song (Takeda) and Parnali Chatterjee (FDA) as scribes.
3.2.1. Presentation
A typical approach for developing biopredictive dissolution methods for oral drug products is to first classify the molecule of interest according to the BCS and its appropriate subclass depending on the molecule’s functional groups. The next steps involve the choice of dissolution medium and dissolution method and their purpose. For example, a dissolution method used for quality control might be composed of pharmacopeial elements while a biopredictive method can use scientifically relevant setups and media mimicking different GI tract environments (e.g., biorelevant media and the Artificial Stomach and Duodenum (AS&D) apparatus). Another important consideration is the mechanism governing bioavailability by either permeability or dissolution-controlled absorption. If the absorption is permeability-controlled, a minimum dissolution acceptance criterion is desired. Faster dissolution will not change the rate and extent of absorption. This is different if the process is dissolution controlled. Here, any change in drug release will alter the rate of absorption.
Currently, there is unfortunately no universal dissolution medium available that can be used for all drugs. The following examples highlight which media and dissolution methods might be useful in the development of biopredictive dissolution methods.
3.2.1.1. Example 1: Permeability-Controlled Absorption
Etoricoxib is a weak base and is classified as a BCS II drug substance. A study by Okumu et al. showed that, if a transfer model from the acidic stomach conditions into FaSSIF was used, the drug solubility was increased in the simulated intestinal fluid compared to its equilibrium solubility.51 Essentially, a supersaturated drug solution was formed. Then, a flow-through cell combined with a perfusion protocol mimicking the stomach and the different small intestinal segments was used and a dissolution profile was generated. When this profile was used in simulation software, the observed clinical PK data were predicted with a better fit compared to USP type dissolution profiles. Furthermore, a comparison between a solution and the physiologically mimicking flow-through protocol showed that both resulted in superimposable predictions of the PK profiles. The study concluded that, if the drug is fully dissolved in the stomach, it can form a supersaturated solution in the intestine and behaves like a BCS class I drug. Therefore, the AS&D apparatus may be more appropriate for such BCS IIb drug molecules.
3.2.1.2. Example 2: Dissolution-Controlled Absorption
Montelukast sodium is a highly lipophilic drug with acid and basic functional groups. It is a BCS II/IV drug substance. A comparison between dissolution profiles from a USP type 2 apparatus with biorelevant media versus a flow-through protocol using physiologically adapted conditions showed significant differences. In the flow-through cell, the drug release was slower in the first 90 min compared to the USP type test. However, when the data were used in GastroPlus, the flow-through data matched the observed clinical data better than when other dissolution profiles were used as input. An alternative apparatus to the flow-through cell is based on the AS&D apparatus with more compartments.52 This method is also known as in vivo Predictive Dissolution (iPD).53
3.2.1.3. Example 3: Lysosomal Trapping
Lysosomal trapping is a potential mechanism to explain slow availability of lipophilic weak bases that otherwise are expected to rapidly appear in the postabsorptive systemic circulation. Predictability of lysosomal trapping is not well developed, although recent efforts aim to standardize testing for lysosomal trapping.54 Lysosomes are enzyme filled vesicles in the cytoplasm that maintain a low pH inside. A weak base such as dextromethorphan is highly lipophilic at the pH inside of an enterocyte. When the molecule crosses the lipophilic membrane of the lysosome, it finds itself at a much lower pH (4.5–5.5). Here, its hydrophilicity significantly increases due to the drop in pH. Due to this shift in its lipophilic properties, the molecule now needs much longer to exit the lysosome. This is a potential reason it takes more than 16 h for the drug to appear completely in the systemic circulation. Based on simulations, the drug is predicted to completely dissolve in the GI tract and exhibit good permeability. The fraction of the dose absorbed into the enterocytes is about 100% within 2 h. The observed time lapse in the appearance in the systemic circulation is likely due to lysosomal trapping. For drugs such as dextromethorphan, there is a lag time between the fraction of the dose absorbed into the enterocyte and the drug plasma levels. Setting dissolution specifications on the fraction dose absorbed into the enterocyte rather than using drug plasma levels would be beneficial.55 Recently, an artificial lysosomal fluid and a side-by-side diffusion cell method were developed which can be used to screen for the tendency of drugs to be trapped by lysosomes.54
3.2.1.4. Example 4: Enteric Coated Dosage Forms
Literature is full of reports that enteric coated dosage forms are failing in vivo.56 In vitro dissolution testing according to the pharmacopeias uses a two-stage approach in which a dosage form is first tested in acid and then in pH 6.8 phosphate buffer. However, if low buffer capacity carbonate buffer is used instead of phosphate buffer, then the dissolution behavior dramatically changes, and depending on the carbonate concentration, the opening of the enteric coat is delayed. Another in vitro study showed that acidic and basic drugs also impact the delay of the coat opening in the carbonate buffer. Acidic drugs delayed the opening process, while basic drugs increased the coat opening. In low carbonate buffer, the coat opening was much slower compared to phosphate buffer. This was also shown for a failed bioequivalence study of pantoprazole. The dissolutions of the test and reference products were similar in phosphate buffer but differed significantly in carbonate buffer. Thus, carbonate buffers or other surrogates are useful when developing enteric coated dosage forms.57
3.2.1.5. Example 5: Biphasic Dissolution
Biphasic dissolution uses an organic layer on top of an aqueous dissolution medium as a sink for the lipophilic drug molecules. The test can be combined with a flow-through cell. In the present study, low buffer capacity (5 mmol) and low volumes (200 mL) were compared with regular strength phosphate buffer and 900 mL. Test tablets containing ibuprofen, which were made by direct compression or granulation using different excipients, were investigated. The results showed that low buffer capacity and low immersion medium volumes have the best ability to detect differences in the manufacturing processes and formulations. Furthermore, organic sinks could allow for a rebound in aqueous buffer pH after dissolved drugs, which initially caused a drop in the buffer pH due to their acidic nature, partition into the organic layer.58
3.2.1.6. Example 6: Lipid Dissolution
The volume of the lymphatic system is larger than that of the vascular system. However, not much attention is given to this compartment in the context of PBBM. Today, many hydrophobic drugs are formulated into lipid drug delivery systems. Long-chain lipids can increase the lymphatic uptake of hydrophobic drugs. This occurs inside the enterocyte. Here, triglycerides and phospholipids are assembled into chylomicrons. Lipophilic drugs can be loaded into the chylomicrons and exit the enterocyte via the lymphatic pathway. An artificial lymphatic fluid was developed and tested regarding its sensitivity to lymphatic inhibition and enhancement uptake. In a study similar to that of biphasic dissolution, a lymphatic compartment was added to a dissolution vessel. Three commercially available drug products containing terbinafine were tested in a USP type vessel and a flow-through cell.59 The aqueous dissolution of one product was significantly different from that of the other two products. This might be due to excipient differences in the formulations. However, the three products also showed differences in the accumulation of the drug in the lymphatic compartment. This new method is a promising approach to assessing formulations for their lymphatic uptake potential. The model might contribute to in vitro bioequivalence guidelines for lymphotropic formulations.
3.2.1.7. Conclusions
First and foremost, the development of a dissolution method is driven by its purpose. When the development of a biorelevant, biopredictive dissolution method is the goal, the following may be considered: Flow-through cells and transfer-models are useful for dynamic dissolution protocols; small volumes and low buffer concentrations could be considered to mimic the physiological environments in the GI tract; carbonate buffers or suitable surrogates are helpful when evaluating enteric coated formulations; biphasic dissolution is an important tool to mimic the GI environment with dissolution and absorption occurring in parallel; and lipid dissolution is a promising approach to assess excipient effects for lymphotropic drugs.
3.2.2. Discussion
This breakout session expanded and continued the discussions of the Hot Topic B on “Best Practices for Development of Biopredictive Dissolution Methods” as input into PBBM by taking into consideration the following questions.
3.2.2.1. Q1: When Biorelevant Dissolution Methods (e.g., Multicompartmental) Are Necessary, What Is the Best Way to Use These Methods?
Developing a dissolution method should be dependent on its intended use, i.e., whether the method would be used for quality control purposes or for PBBM. For example, for screening for precipitation of weak bases, two-stage tests or transfer models can be useful. Biorelevant dissolution methods mimic biological fluids and physiology and may be developed solely to support PBBM, with no link to the QC dissolution method. In this case, the biopredictive nature of the biorelevant method is verified through the PBBM.2
3.2.2.2. Q2: How Many Different Experimental Conditions Should Be Used for a Single Batch?
There is no fixed number of experimental conditions that should be used to develop a biopredictive dissolution method. However, relevant sets of experiments could be conducted taking into consideration GI physiology, bile salts, buffer capacity, physicochemical properties of the DS, product design, and release mechanisms to develop biopredictive dissolution methods as input for PBBM.
3.2.2.3. Q3: What Are the Pitfalls of Dissolution (e.g., Degradation, Mixture of Polymorphs, and Precipitation) to Be Careful about and How to Deal with It?
Precipitation of drugs is an important consideration in developing a dissolution method. To study the effect of drug precipitation during dissolution testing, transfer experiments are often conducted to estimate the precipitation times as input into PBBM to determine the effect on the bioavailability.
3.2.2.4. Q4: How Do You Separate Artifacts of the Dissolution Test and Its Significance (or Nonsignificance) on in Vivo Response (e.g., Coning Is Often a Dissolution Issue, But Is Minimally a Concern in Vivo)?
Sometimes multiple experiments are conducted to address dissolution artifacts such as coning, cross-linking in capsules, etc.
The use of Apex vessels (previously known as PEAK vessels) to address coning is gaining regulatory acceptance; however, generating as much data as possible early in the product development to address these issues and determine if the developed dissolution method is biopredictive by conducting a PK study is often critical.
3.2.2.5. Q5: How Should Functional Excipient Effects Be Investigated? What Are the Appropriate Methods and How Should Dissolution Methods Be Developed to Evaluate Excipient Effects?
Dissolution methods should take into consideration the effect of key/functional excipients, such as the impact of excipients on bulk vs surface pH. Excipients can alter drug release and absorption; therefore, evaluating the effect of functional excipients early on is crucial. Conducting a pilot in vivo PK study when an important functional excipient is present in the formulation may provide utility when building a dissolution safe space.
3.2.2.6. Q6: Depending on DS and DP Properties, What Level of Variation of Critical Biopharmaceutics Attributes (CBA) Is Needed to Demonstrate Discrimination and a Biopredictive Nature for the Dissolution Method?
Depending on the product design, release mechanism, >10% variations in functional excipients, and process parameters of the final formulation could be used to demonstrate the discriminating ability of the biopredictive/QC dissolution method and their impact on the bioavailability of the drug product (especially for basic drugs that have pH modifiers and enteric coatings).
3.3. BO Session C - Dissolution Part 2: Modeling in Vitro Dissolution Data
This session began with Xavier Pepin (Simulations Plus, Inc.) and was led by Cordula Stillhart (Roche) and Luiza Borges (ANVISA), with Grace Chen (Takeda) and Megerle Scherholz (BMS) as scribes.
3.3.1. Presentation: Methods for Integrating Dissolution
During breakout session C, Xavier Pepin presented a comprehensive overview and description of methods for integrating dissolution profiles into PBBMs, followed by practical considerations on the critical aspects when in vitro dissolution data were used for dissolution model development. This background served as a basis for developing and discussing checklists and a decision tree for the dissolution method selection to support the integration of dissolution data into PBBMs.
There are many ways to integrate dissolution into most PBBM platforms. These methods range from lesser to more mechanistic as shown in Figure 5. For an IR dosage form, using one method over other methods leads to certain assumptions being made regarding the parameters limiting in vivo dissolution.
Figure 5.
Methods to integrate dissolution in a PBBM.
3.3.1.1. Direct Input
The least mechanistic method to integrate dissolution is to use direct input of the in vitro dissolution data into the model. In this case, the assumptions made are that the in vitro dissolution method is representative of the conditions prevailing in vivo, which govern the drug dissolution. In more detail, if such a method is used, one should confirm that neither solubility, drug dose, nor in vivo volume would be limiting the in vivo dissolution, since there are wide differences between the volumes used in vitro and the volumes observed in vivo. In addition, the in vitro hydrodynamics should be representative of in vivo conditions or not impact in vitro release, here again for the same reasons that the in vivo hydrodynamics are different from those in vitro. Such assumptions are reasonable when the drug substance is BCS 1 or BCS 1-like and when the formulation itself is governing the in vitro and in vivo dissolution.
3.3.1.2. Weibull Function
The use of a Weibull function fitted to in vitro dissolution data is also a nonmechanistic approach as the in vivo release depends on time only. Similar assumptions to those supporting the direct input of dissolution data are made when using a Weibull function, although it is preferable to use Weibull over direct input, since the Weibull function provides for a smoother dissolution curve passing through the measured dissolution data. For direct input methods, as the number of time points for measuring dissolution is generally limited, interpolating dissolution data with a linear correlation between measurements may lead to inaccurate predictions of in vitro (and in vivo) dissolution.
3.3.1.3. Z-factor
The use of the Z-factor vs pH profile or constant Z-factor should provide for a more mechanistic model. The Z-factor introduced by Takano et al.33 is a lumped factor which is the ratio of drug diffusion coefficient (D), divided by the product of true density (ρ), radius of the particle (r0), and thickness of the unstirred water layer (h).
| 1 |
It is evident from eq 1 that the Z-factor can also be expressed as the initial drug particle radius in the formulation. It is also evident from this equation that there is only one bin (one particle size) in the Z-factor. Hence, if the observed in vitro dissolution rate shows more than one phase, a single bin may not be enough to adequately characterize the dissolution of the particles comprised in the formulation. Multiple release phases could arise from the presence of extra granular fine drug substance and granulated drug substance or the presence of drug substance particles that wet at different rates.
In theory, there should not be a dependency of Z-factor on pH, as pH governs the drug solubility and is independently considered in the equation proposed by Takano et al. to predict in vitro and in vivo dissolution.33 In addition, the fact that the drug diffusion coefficient is an integral part of the Z-factor definition should lead to caution when employing the Z-factor to fit dissolution data obtained in media comprising surfactants. Indeed, the influence of surfactant micelle size spans an order of magnitude, which would affect the diffusion coefficient of the drug bound to micelles by the same order of magnitude. The size of common micelles summarized from literature data is shown in Figure 6.60−64
Figure 6.
Comparative micelle sizes.
3.3.1.4. P-PSD
The product particle size distribution (P-PSD) was introduced by Pepin et al.19,21,25 where the disappearance of solid drug vs time is expressed as
| 2 |
where  is the drug fraction unbound, Du is the diffusion coefficient of unbound
drug, Db is the diffusion
coefficient of micelle bound drug, A(t) is the available drug surface area at time t, hu(t) is the
unstirred water layer thickness for unbound drug, hb(t) is the unstirred
water layer thickness for micelle bound drug, CS,u is the unbound drug solubility at the
surface of the crystal, and Cu(t) is the unbound drug bulk concentration
at time t. A(0) is the initial drug
substance surface area which can be represented as a 1 to 10 bin spherical
product particle size distribution, the P-PSD. Since the P-PSD can
comprise from 1 to 10 bins, there is enough granularity to fit complex
dissolution profiles including those presenting multiple phases. The
number of bins can be tuned to the observed dissolution data, and
it is recommended to start from the minimum number of bins and increase
the number of bins until there is no difference in the predictive
power across the dissolution data observed. The P-PSD approach can
be applied to all dissolution equations beyond the one presented in eq 2. In fact, in platforms
such as DDDPlus (Simulations Plus), SIVA (Certara), and MoBi (Open
Systems Pharmacology [OSP]), the P-PSD can be fitted to observed dissolution
data. In the above cases, the P-PSD will take the form of a mean spherical
particle radius associated with a distribution across the mean. Only
one mode of distribution is currently available in these platforms.
The equation proposed by Pepin et al. stems from the approach proposed
by Gamsiz et al.;65 however, it assumes
immediate partitioning of drugs to micelles at the surface of the
drug, and different thicknesses of the UWL for free and micelle bound
drug, according to the equation proposed by Pohl et al:66
is the drug fraction unbound, Du is the diffusion coefficient of unbound
drug, Db is the diffusion
coefficient of micelle bound drug, A(t) is the available drug surface area at time t, hu(t) is the
unstirred water layer thickness for unbound drug, hb(t) is the unstirred
water layer thickness for micelle bound drug, CS,u is the unbound drug solubility at the
surface of the crystal, and Cu(t) is the unbound drug bulk concentration
at time t. A(0) is the initial drug
substance surface area which can be represented as a 1 to 10 bin spherical
product particle size distribution, the P-PSD. Since the P-PSD can
comprise from 1 to 10 bins, there is enough granularity to fit complex
dissolution profiles including those presenting multiple phases. The
number of bins can be tuned to the observed dissolution data, and
it is recommended to start from the minimum number of bins and increase
the number of bins until there is no difference in the predictive
power across the dissolution data observed. The P-PSD approach can
be applied to all dissolution equations beyond the one presented in eq 2. In fact, in platforms
such as DDDPlus (Simulations Plus), SIVA (Certara), and MoBi (Open
Systems Pharmacology [OSP]), the P-PSD can be fitted to observed dissolution
data. In the above cases, the P-PSD will take the form of a mean spherical
particle radius associated with a distribution across the mean. Only
one mode of distribution is currently available in these platforms.
The equation proposed by Pepin et al. stems from the approach proposed
by Gamsiz et al.;65 however, it assumes
immediate partitioning of drugs to micelles at the surface of the
drug, and different thicknesses of the UWL for free and micelle bound
drug, according to the equation proposed by Pohl et al:66
| 3 |
A comparison between the use of Z-factor vs the P-PSD approach is presented in Figure 7. The increased predictive performance of the P-PSD approach is related to its ability to differentiate the free and micelle bound drug and also the impact of the micelle size on the diffusion coefficient of micelle bound drugs.
Figure 7.
Comparative prediction of Z-factor and P-PSD fitted on medium without a surfactant to predict dissolution in media with a surfactant. Data generated on acalabrutinib batch L0505009 capsule dissolution.19
The Z-factor and P-PSD approach show similar shape description of the 100 mg acalabrutinib capsule batch L0505009 dissolution profile in phosphate buffer, pH 6.8. If this dissolution data is used to fit the Z-factor and P-PSD, prediction of dissolution of the same batch in media comprising bile salts show the advantage of the P-PSD over the Z-factor (Figure 7). The use of the apparent drug solubility in both tested media with the surfactant and the Z-factor fitted on the medium without the surfactant leads to an overestimation of the observed dissolution rate. The drug will dissolve slower due to the smaller diffusion coefficient of micelle bound drug which is best captured with the P-PSD approach and eq 2. Recently, two additional models for P-PSD were proposed which integrate the fluid velocity in the USP2 dissolution apparatus, the P-PSD HD, and one model predicting drug and excipient sedimentation and cone formation at the bottom of the USP2 vessel, the P-PSD HDC.67 These latter models are important to remove the potential bias coming from formulation sedimentation or to integrate the impact of fluid velocity in USP2, which would be important for large particles or large dosage forms such as eroding tablets or pellets.68,69 The P-PSD concept stems from the fact that the drug substance particle size available for dissolution in the drug product cannot be measured adequately with sizing methods, such as laser diffraction applied to the drug substance (DS PSD). DS PSD is an important quality control of a starting material, but the impact of excipients and manufacturing process conditions on the drug substance area available for dissolution cannot be ignored.
Process: It is well-known that compression forces during dry granulation or tablet manufacture will lead to fragmentation of brittle drug substances and excipients.70 Fragmentation will also affect larger particles at low compression forces and show little effect on smaller particles below a threshold size.71,72 The use of a single Diffusion Layer Model (DLM) scale factor applied to the measured DS PSD to predict the effect of processing parameters on the DS surface area available in a final formulation cannot therefore be sustained theoretically.
DS Particle Aggregation: Aggregation of primary particles in the DS is another factor that can induce a strong bias to predicting the DS surface available for dissolution. Loose or strong aggregates can form in a drug substance because of material properties, manufacturing process, or storage. Laser diffraction methods would typically size an aggregate of primary particles as one large particle with low surface to volume ratio, leading to an under-estimation of the drug surface area available for dissolution, as easily demonstrated by comparing laser diffraction predicted powder surface area to BET specific surface area for various batches of drug substances showing various levels of aggregation.25
Shape: The shape of particles will also influence the difference between laser diffraction predicted size and surface area measured with an orthogonal technique such as BET specific surface area.73 Laser diffraction techniques, which project a volume equivalent sphere for each particle, will introduce a bias to the measurements the further away the particle is from a spherical morphology.74
Wettability: Finally, the DS particle size cannot predict the impact of the drug substance wetting ability on the dissolution rate. Kim et al. have shown that dry coating the surface of drug crystals with a hydrophilic or hydrophobic material can influence aggregation of particles up to a certain surface coverage and also influence drug dissolution through the alteration of the surface energy of the drug, which would change how water can wet the drug surface.75 The correlation between drug wettability and dissolution has been reported in the literature,76 and the formulation scientists frequently employ wetting agents as excipients to improve the wettability of drugs in final formulations. The sensitivity of the dissolution rate to drug wettability is especially pronounced for small particles. For example, nanosizing technologies require the presence of surfactants to achieve the desired size and suspension stability, i.e., preventing aggregation and reducing speed of Ostwald ripening.77
For all of the reasons highlighted above, the size of DS particles measured prior to processing the DS into the final formulation is rarely a good predictor of the drug substance area available for dissolution. There may be rare exceptions to this rule, for example, if the formulation is a suspension or if the formulation is dry but comprises wettable amorphous spray dried drug particles encapsulated with low energy processes. The effect of formulation excipients and processing parameters should be integrated into the mechanistic modeling approaches of drug product dissolution. The P-PSD or Z-factors can serve this purpose.
3.3.2. Discussion
The discussion was centered around 5 key questions.
3.3.2.1. Q1: What Is the Appropriate Dissolution Model for an IR Formulation?
A recent review by Anand et al.1 showed that direct input, Weibull function, Z-factor, or P-PSD approaches were widely applied methods for integrating dissolution in PBBM. Mechanistic approaches like the Z-factor or the P-PSD were mostly used for low-solubility products, and mechanistic methods were applied in 60% of the 27 case studies.
The advantages of mechanistic dissolution models over Weibull functions is that the between- and within-subject variability in terms of in vivo dissolution during population modeling can be captured in a more relevant way. Instead of applying random variation of dissolution (as can be achieved with a Weibull function), mechanistic models will rely on variation in system parameters (e.g., volumes, pH, transit times, composition in bile salts) to recalculate a different in vivo dissolution for the drug product for each simulation. This will warrant closer to reality in vivo dissolution compared to random variations. Also, the use of mechanistic models is the only option when the model is to be used to predict the impact of prandial state, pH related DDI, or in vivo dissolution across different populations, all situations where the GI physiological changes may profoundly affect in vivo dissolution rate and make it deviate from the dissolution rate measured in vitro. The criteria to select a dissolution method should therefore be driven by the understanding of the drug product release mechanism and the limitations to in vitro and in vivo dissolution, the impact of manufacturing process and formulation on dissolution, and how well this can be simulated with a given approach. For mechanistic models, it is recommended to generate dissolution data with the same batch in several media/conditions to be able to verify the choice of model and prediction performance in vitro prior to integration of the batch specific data (Z-factor or P-PSD) in the model. Ideally, to perform the fitting of dissolution data to extract the Z-factor or P-PSD, the method chosen would be discriminative, and the batch dissolution would show an adequate profile with possibly full dissolution in the medium considered. Practically, this would correspond to picking a dissolution method where most measured data comprise between 20% and 80% drug dissolved. Typically, a 1×-dissolution method described by Kuiper,2 where the drug dose divided by the dissolution volume nears the drug solubility in the dissolution medium, ensures maximal discrimination while allowing full dissolution. Using only one method to fit a mechanistic dissolution model over using all dissolution methods simultaneously is optimal, as the integration of nondiscriminating methods may lead to bias in the batch specific Z-factor or P-PSD determination.26 Based on the strengths and limitations of each individual dissolution modeling method presented during breakout session C, a decision tree for dissolution model selection was discussed with the audience. The proposed decision tree provides considerations for developing a dissolution model depending on the disintegration properties of the dosage form, the occurrence of coning or sedimentation during dissolution testing, and the sensitivity of the dissolution rate toward changes in agitation conditions, volume, dose, and pH, as well as the presence of surfactant in the dissolution medium. The proposed decision tree is tailored to oral IR dosage forms and presents a clear description of the modeling assumptions to be considered when selecting a dissolution model. There was general agreement from the attendees that such a decision tree for dissolution model selection provides a valuable tool for both biopharmaceutics modelers in the pharmaceutical industry as well as for regulators when reviewing submitted PBBM cases (Figure 8).
Figure 8.
Presented decision tree to choose a dissolution model for introduction into PBBM.
3.3.2.2. Q2: What Are the Input Parameters Required to Mechanistically Evaluate the in Vitro Dissolution Data?
When developing a mechanistic dissolution model, the availability of high-quality input data for model parametrization should be a priority. This includes the availability of a sufficient number of in vitro dissolution profiles collected under relevant experimental conditions depending on the intended purpose of the model. For example, if the PBBM aims at predicting a pH-related DDI, then the dissolution model may need to be developed and validated using in vitro data generated under various pH conditions. Defining the experimental parameters describing the dissolution setup is prudent for each corresponding dissolution data set, and for dissolution media including surfactants, the properties of the micellar system should also be adequately characterized. Table 2 presents a list of suggested data to collect and could serve as a checklist in the context of the dissolution model development.
Table 2. List of Data to Collect for Dissolution Model Development.
| input data | comments |
|---|---|
| Dissolution data on at least 3 independent methods using the same batch | The dissolution conditions should be adapted to the DS or DP properties and the intended use of the model. For example, neutral compounds may require dissolution profiles with a range of doses, volumes, and presence of surfactants. Ionizable drugs may require media with different physiologically relevant pH conditions. If the model is intended to predict food effects, dissolution in FaSSIF and FeSSIF is helpful, while if the model should predict DDIs with acid reducing agents, testing in acidic and neutral pH conditions would be logical. If a dissolution method is discarded, a justification would be useful. |
| Parameters describing in vitro dissolution conditions | |
| Medium volume | |
| Medium composition | |
| Medium pH | |
| Temperature | |
| Apparatus type | |
| Agitation speed | |
| Drug dose | |
| Drug solubility in the dissolution medium | The surface solubility should be measured or computed. If there is a rapid phase change, e.g., salt disproportionation to a free base, the free base surface solubility at the pH of medium and using the buffers of the medium should be characterized. |
| Parameters describing dissolution media with surfactants | |
| Drug solubility in the blank dissolution medium (without surfactants) | |
| Concentration of micelles | |
| Drug affinity to the surfactant system | Ideally, the apparent drug solubility (Sapp) is measured in the buffer of interest with increasing surfactant concentrations ([S]) above the critical micelle concentration (CMC). The slope of the linear portion (Sapp vs [S]) describes the drug affinity to the surfactant system. Some quantitative structure activity relationship (QSAR) models can be developed to predict the affinity for specific systems based on literature data. |
| CMC of the surfactant in the dissolution medium | Literature data could be valuable. Care is needed with regards to the effect of pH and temperature on the CMC of certain surfactants. |
| Size of the micelles | Literature data of the pure surfactant micelles could be useful, but ideally the micelles size is measured in presence of the drug. For this purpose, at the end of the dissolution experiment, the medium is centrifuged, and the supernatant is analyzed using dynamic light scattering methods. This is important for drugs which have surface active properties and can form mixed micelles with bile salts or artificial surfactants. An average hydrodynamic radius is needed. |
In addition to the in vitro data that are generated for direct input into the dissolution model, there might be a need to generate supplementary data to support some specific modeling assumptions or to mechanistically explain some anomalies. For example, if the slow dissolution in pure aqueous systems is attributed to poor drug wettability, this hypothesis may be strengthened by the generation of in vitro dissolution data, including a surfactant. Similarly, if in vitro dissolution is slow, presumably due to poor tablet disintegration, the hypothesis may be further supported by the generation of in vitro dissolution profiles of the pure DS or of drug product intermediates (granules or final blend prior to tablet compression). Such mechanistic investigations may not directly feed into the model but provide key information to increase the confidence in the selected model parameters and modeling assumptions.
3.3.2.3. Q3: What Are the Criteria and Acceptable Thresholds for in Vitro Dissolution Model Validation?
If more than one mechanistic modeling method may be applicable, the calculation of model performance indicators such as the average fold error (AFE) and absolute average fold error (AAFE) can provide rationale for method choice. Ultimately, the prediction performances of various dissolution modeling methods in the PBBM could also be compared. Examples of dissolution modeling fitting and impact on PBBM prediction are also shared. The outcome can be found in the Supporting Information.
3.3.2.4. Q4: Which Are the Factors to Be Considered When Modeling Dissolution?
Prior to the integration of dissolution data into a PBBM, a critical assessment of the quality and relevance of the experimental dissolution data may be useful. In this context, there are several factors to pay attention to, as summarized below.
Agitation: The impact of agitation should be considered when choosing an integration method. All models are derived from the Noyes-Whitney equation78 (i.e., Johnson,79,80 Wang-Flanagan,81 Takano,33 Gamsiz,65 Pepin,19 or Salehi,82) and rely on the definition of the UWL thickness around dissolving particles. The UWL thickness is a function of fluid velocity around the dissolving particle in the dissolution medium (in vitro and in vivo). When the fluid velocity tends to zero, the thickness of the UWL tends to the radius of the spherical particle; as an approximation, the UWL thickness is equal to the particle radius up to an upper limit of 30 μm, which is supported by simulations and experiments performed in the literature.83,84 Also, this hypothesis fits with the low fluid velocity typically measured in vivo throughout the GI tract, where the average velocity is in the range of 1–2 cm/s, with transient peak velocities of more than 15 cm/s.85−87
For particle sizes larger than 30 μm, the UWL thickness typically depends on the agitation as shown for example by Scholz et al.69 When a significant impact of agitation on the dissolution rate is shown, the in vitro dissolution model should accommodate the impact of hydrodynamics.
Surface pH and Surface Solubility: When the drug shows acidic or basic moieties, depending on the pH and composition of the aqueous dissolution medium, an acid–base reaction can happen locally at the surface of the dissolving drug particles, without necessarily affecting the bulk pH. This reaction will change the pH within the UWL. The maximal changes are observed at the surface of the drug. This phenomenon was described theoretically and experimentally in the literature for weak acids, bases, and their salts thanks to the work of Higuchi et al.,88,89 Mooney et al.,90,91 and Serajuddin et al.92,93 Since the drug surface solubility drives the dissolution rate, it is imperative to consider the drug surface solubility to mechanistically model in vitro and in vivo dissolution rates.25,40,94,95 If there is a rapid phase change, such as salt disproportionation to the free base, then the free base surface solubility at the medium pH should be determined.
Surface pH, also known as microenvironmental pH, is driven by the drug substance but can also be largely influenced by excipients added to the formulation,96,97 and excipients should be considered when analyzing dissolution data. Formulation composition should always be known so as to evaluate potential interactions between the drug and excipients during dissolution but also in the solid state, as these reactions can also lead to polymorphic transitions.98
Chemical Degradation: Chemical degradation can happen during dissolution and impact the amount of drug that is dissolved. A typical example is that of rifampicin dissolution in presence of or without isoniazid.99 The presence of bell shape dissolution curves or the existence of a dissolution plateau less than that of the theoretical batch assay could indicate the potential for in vitro degradation. The degradation rate should be measured in a separate experiment with solubilized drug by measuring the drug concentration over time in the dissolution medium.100 If degradation is confirmed, it can be integrated into the model (in vitro and in vivo) to account for a better fit of in vitro dissolution and amount of drug available for in vivo absorption.101
Physical Degradation: Bell shapes or plateaus during dissolution may also demonstrate (beyond the lack of enough solubility or medium volume to dissolve the full drug dose) that a polymorphic drug transition happens or that there is a polymorphic impurity in the drug substance. For example, the mixture of different polymorphic forms with different solubility values will lead to a variation in the rate and extent of dissolution.102 Precipitation from an amorphous to a crystalline form, or from a salt/cocrystal to its free form, will lead to a change in dissolution rate or even to complete stop of drug dissolution if the precipitation occurs on the surface of the drug product.103,104 The presence of cosolvents or polymers can also change the rate and extent of surface precipitation,105 and, where relevant, such excipients should be considered critical to the product performance.106
Drug Product Disintegration: The impact of capsule opening,107,108 or tablet disintegration,109 on the dissolution profile has been widely presented in the literature. Since dissolution models assume that all the drug particles are available at time zero for dissolution, the disintegration time or capsule opening time should be removed from the observed dissolution data prior to fitting the dissolution rate. This can be achieved by subtracting the time needed for drug release from the observed dissolution time. If possible, models for capsule opening and tablet disintegration should be fitted to in vitro data and applied to in vivo data.110 It is also known that in vivo capsule opening,111 or in vivo tablet disintegration,112,113 takes longer than the time observed during USP disintegration testing and would impact gastric residence in vivo.114
Method Artificial Effects: In addition to the intrinsic properties of the drug substance and drug product described above, the in vitro dissolution performance may be affected by artificial effects in the in vitro dissolution setup, which may not necessarily have relevance for in vivo dissolution. Such effects include in vitro sedimentation or coning and the interaction with components of the dissolution medium. In vitro sedimentation introduces a bias to the dissolution rate and extent and should be corrected prior to PBBM introduction. The solubility product of ionizable compounds in the presence of specific buffer salts and/or surfactants should be carefully considered (e.g., formation of less soluble lauryl sulfate salts in the presence of SLS or reduced hydration of Eudragit RS in the presence of chloride ions in the dissolution medium).115 In summary, a robust understanding of the experimental dissolution data is required to ensure the development of a meaningful dissolution model able to capture the in vivo performance in a mechanistic manner. To facilitate this process, the critical aspects to consider are summarized in Table 3, which may serve as a checklist in the context of in vitro data evaluation for the dissolution model development.
Table 3. Presented Checklist for in Vitro Data Evaluation Prior to Dissolution Model Development.
| checklist question | answer |
|---|---|
| Is there a risk for chemical degradation of the drug substance in solution? | □ Yes □ No |
| Is there a risk for physical degradation, such as | |
| polymorphic form change | □ Yes □ No |
| precipitation of crystalline form from an amorphous state | □ Yes □ No |
| precipitation of the free form from salt/cocrystal | □ Yes □ No |
| other | □ Yes □ No |
| Is dissolution incomplete (less than assay value) under sink conditions? | □ Yes □ No |
| If yes, an investigation of the root cause should be provided. | |
| Is the dissolution rate influenced by the presence of certain excipients (e.g., cyclodextrins, ionic interaction with croscarmellose sodium)? | □ Yes □ No |
| If yes, it needs to be carefully assessed whether the interaction has any in vivo relevance and whether this effect can be captured mechanistically in the dissolution model. | |
| Is the DS or DP wettability poor in aqueous media? | □ Yes □ No |
| Is there a risk for interaction with medium components? (e.g., ionic interaction with buffer species or surfactants) | □ Yes □ No |
| Is sedimentation or coning observed during in vitro dissolution? | □ Yes □ No |
| Is the solubility at the DS or DP surface expected to be different from the bulk solubility? | □ Yes □ No |
| If yes, the surface solubility should be measured or computed. | |
| What is the mechanism of release and is it compatible with the erosion of particles from their outside surface? | □ Yes □ No |
| If yes, the approaches such as Z-Factor or P-PSD can be applied | |
| Is there a delay to drug release due to capsule opening or tablet disintegration? | □ Yes □ No |
| If yes, the capsule opening/disintegration should be mechanistically modeled or the time for drug release should be removed from the measured dissolution time point prior to fitting dissolution. |
3.3.2.5. Q5: What Is the Appropriate Quality and Quantity of Data to Be Generated to Allow Dissolution Model Validation?
The quality of data is defined by the evaluation of potential factors to consider which may introduce a bias to the dissolution measurement as shown in the check-list for in vitro data evaluation prior to dissolution model development (Table 3), leading to the list of necessary input parameters needed for dissolution modeling (Table 2). In terms of quantity, there is no definite number at this stage, but it seems that n = 3 different conditions covering the physiological pH range could be sufficient. Care should be taken to obtain adequate release profiles in each dissolution method (see Q1) and to favor dissolution methods where the main component/parameter in the dissolution medium/method influencing drug product dissolution is integrated. For example, for large particles or extended-release matrixes, dissolution data with different agitation rates often provide insight into the release mechanism. For drug substances that are sensitive to pH, covering the physiological pH range is typical. Finally, for drugs that are sensitive to the presence of surfactants in the medium, a comparison of dissolution profiles with synthetic and natural occurring surfactants is warranted.
3.4. BO Session D - Precipitation: From in Vitro Best Practices to in Vivo Relevance
This session began with speaker Christian Wagner (Merck Healthcare KGaA, Darmstadt, Germany) and was led by Poonam Delvadia (FDA) and Mark McAllister (Pfizer), with André Dallmann (Bayer) and Elizabeth Gray (FDA) as scribes.
3.4.1. Presentation: To Precipitate or Not to Precipitate, That Is the Question!
Loosely adapted from Shakespeare’s Hamlet,116 pharmaceutical scientists have been asking this question for decades, because drug precipitation in the small intestine can affect the rate and/or extent of oral drug absorption. This, in turn, can contribute to PK variability and can jeopardize the efficacy of an orally administered drug. Thus, there is a huge need for predictive tools to assess the impact of potential drug precipitation on the absorption of orally administered drugs.5,117
Drug precipitation typically occurs from a supersaturated state, i.e., when the solubility of the drug exceeds its thermodynamic solubility. Weakly basic drugs are especially susceptible to drug precipitation because their solubility is markedly higher in the (fasted) stomach than in the small intestine. Upon gastric emptying of dissolved drug into the small intestine, the drug’s solubility drops, and molecule clusters form, grow, and precipitate once a critical cluster size is reached (nucleation and growth theory). Besides weakly basic drugs, supersaturating formulations such as ASDs and self-(micro) emulsifying drug delivery systems (S(M)EDDSs) can also be subject to intestinal drug precipitation. Whether or not a drug precipitates thus depends on several drug, formulation, and physiological factors. In any case, the driver of drug precipitation is the reduction of free energy in the system.118−120
Its complex nature underlines the need for tools that reliably predict luminal drug precipitation, allowing for the translation of results from the lab (in vitro) into the clinics (in vivo) via PBBM tools (in silico). During recent years, various in vitro precipitation assays have been developed.121 These assays can be applied throughout the development cycle of a drug, i.e., from early research through life-cycle management. The commonality of most of the in vitro assays is that they strive to simulate physiological conditions by transferring a drug solution or suspension from an artificial stomach (donor) into an artificial small intestine (acceptor) compartment.122 The concentration of dissolved drug can be measured by various techniques, such as liquid chromatography or in-line UV–vis.123−125 On the one hand, small-scale assays are typically used to investigate the precipitation behavior of the drug in a typical preformulation setting, i.e., using small quantities of the drug substance121,126−128 On the other hand, large scale models typically use physiologically relevant gastric and intestinal fluid volumes, which allows for performance-testing of formulations.122,129−132 More advanced models, which aim at simulating the interplay between drug precipitation and absorption, have also been published.129,133−135
Of note, a drug can precipitate as crystalline or amorphous form(s), which, in turn, can impact the rate and extent of redissolution of the precipitate. Likewise, the particle size of the precipitate can also impact its redissolution kinetics.117,118 A well-known example of amorphous precipitation is gefitinib, which was shown to precipitate in an amorphous state and then slowly recrystallize.136 Whenever possible, characterizing the solid state of the precipitated drug, testing for redissolution, and adapting the PBBM accordingly would be a viable approach.
Despite significant advances during the past 20 years, all in vitro systems to predict drug precipitation remain highly artificial, as they are not capable of reflecting the complex nature of human anatomy and physiology in its totality. The comparably high number of in vitro precipitation assays described in the literature indicates a lack of harmonization/standardization, especially since the selection of a suitable in vitro precipitation model seems to be a case-by-case decision, depending on the drug and formulation properties. A “universal” in vitro model capable of simulating luminal drug precipitation for a wide variety of compounds and at various conditions (dose, prandial, or disease state, formulation) would increase confidence in in vitro-based precipitation predictions.
In addition to in vitro precipitation assays, luminal sampling from volunteers or clinical PK data can also be used to deduce whether a drug may be prone to precipitation.47,137,138 For example, if PK data from a well-designed single ascending dose study indicate linearity in relevant PK parameters such as AUC, Cmax, and elimination (no flip-flop kinetics), the impact of precipitation on drug absorption becomes unlikely. In contrast, nonlinear AUC or Cmax, or a pronounced shift in tmax, may indicate nonlinear absorption, potentially deriving from solubility/dissolution limitations and/or drug precipitation. Time-dependent effects, nonlinear clearance mechanisms, disease state (healthy volunteer vs patients), changes in dose and/or formulation, and other confounding factors should be taken into consideration when deducing precipitation characteristics from clinical PK data. In contrast to in vitro data, mechanistic insights into the precipitation process cannot be gained from in vivo data because in vivo data typically do not give mechanistic insights into drug precipitation. Therefore, parameter identification remains a potential issue when precipitation characteristics are deduced from clinical data.
To translate insights from drug precipitation into a meaningful prediction and potentially extrapolate to untested scenarios, the results from an in vitro precipitation study (including solid state and redissolution characterization of the precipitate) or a clinical PK trial (including luminal aspiration studies) can be used to inform a PBBM.5,47,117,130,139−148 This translational, integrative approach permits the prediction of luminal drug precipitation at various doses and prandial states and for different formulations. Commercially available PBBM tools typically offer two possibilities of applying precipitation kinetics to the simulations, i.e., by applying a simplistic precipitation rate constant or time, combined with supersaturation, or by applying a mechanistic nucleation and growth model.141,145,147,148 The latter approach allows for the mechanistic simulation of drug precipitation by fitting nucleation and growth parameters to in vitro or in vivo data. From a scientific perspective, in vitro precipitation setups should be suited to extract nucleation and growth parameters for use as input for a PBBM. However, the low number of publications describing the application of software built-in mechanistic precipitation tools indicates that the advantage of applying these tools as part of a commercially available PBBM suite still needs to be demonstrated.
“To precipitate, or not to precipitate” – this question remains unanswered, at least partly. As has been discussed in the scientific community previously,117 the results of this workshop also revealed that our currently available in vitro tools to predict drug precipitation are often lacking “universal” predictive power, because there is no in vitro tool currently available, which is capable of predicting drug precipitation (or the lack thereof) for a wide variety of drugs and formulations.
Likewise, there are still significant knowledge gaps, for example, with respect to our understanding of the impact of GI hydrodynamics and transit rates (including the “Magenstraße”), distribution of fluid pockets, impact of intestinal mucus, and transporter effects on luminal drug precipitation. Understanding these properties would aid in developing improved in vitro precipitation setups and more predictive PBBM tools. PBBM tools should benefit from ongoing advances in scientific research and constantly be updated with state-of-the-art knowledge.
Despite significant improvements during the past decades in terms of in vitro methodology to test for drug precipitation, computational and software capabilities to model it, and knowledge about the anatomy and physiology of the human GI tract (which, beside the drug properties itself, affect the rate and extent of drug precipitation), predicting drug precipitation is still associated with a high degree of uncertainty, especially for drugs with impaired absorption.
For this purpose, a decision tree on how to test for drug precipitation and apply it to a PBBM was presented during the workshop (Figure 9). The decision tree is adapted based on recommendations from a previous publication and reflects the general workflow applied to precipitation predictions in PBBMs in one of the IQ working group’s member companies (Merck Healthcare KGaA, Darmstadt, Germany).47
Figure 9.
Presented decision tree on how to test for drug precipitation and apply it to a PBBM. An explanation for “high quality human PK data” can be found in the main text. SAD Single ascending dose study (dose escalation study).
As clinical PK data are thought to provide the highest evidence on impaired drug absorption, evoked by, e.g., drug precipitation, the starting point of the decision tree is the question of the availability of clinical PK data.
The left side of the decision tree (“no clinical data available”) describes bottom-up in vitro methods to deduce precipitation parameters for the PBBM input. Given the lack of a “universal” precipitation assay, the decision tree does not recommend using a particular in vitro assay to predict drug precipitation. Instead, it leaves the discretion of the biopharmaceutical scientist to decide on a suitable assay. One key element of the decision tree is the recommendation to apply precipitation scenarios to the PBBM. For example, the modeler could apply a “no versus a moderate precipitation scenario” (in vitro setup indicates no or very modest precipitation) or a “moderate versus a high precipitation scenario” (in vitro setup indicates precipitation). This approach mitigates the uncertainties associated with many in vitro precipitation assays, particularly their tendency to overpredict drug precipitation.
The right side (“clinical data available”) describes a top-down method for deducing precipitation kinetics, i.e., the analysis of clinical PK data. The key to reliably deduce precipitation parameters is the availability of high-quality PK data, e.g., from a dose escalation study, which would ideally be conducted in healthy volunteers. Other confounding factors, such as nonlinear clearance mechanisms or time-dependent effects, should be excluded. One drawback of the top-down approach is the lack of parameter identification (e.g., individual impact of drug dissolution, precipitation, and redissolution on the PK profile); i.e., this approach is a nonmechanistic one.
The decision tree presented herein considers the above-mentioned uncertainties around the in vitro and in silico prediction of drug precipitation. It can be flexibly adapted based on specific needs and can be refined continuously based on future scientific advancements. Therefore, the decision tree should be understood as a practical tool rather than a strict “operating procedure”.
3.4.2. Discussion
After the presentation, the audience was guided by Mark and Poonam to discuss the five highlighted questions below.
3.4.2.1. Q1: Which Limitations of Commonly Used in Vitro Precipitation Assays Based on Transfer Methodology Can Be Addressed by an Improved Experimental Design?
The design of in vitro precipitation assays should be based on the intended application and what data are required; for example, is the assay being used to perform formulation ranking or for informing PBBM input? There was a debate around the criticality of integrating a permeability-like component within the in vitro precipitation assay, particularly for compounds with high permeability. As a general concept, it was suggested that the thoughtful inclusion of a well-designed permeability component (absorption compartment) in the in vitro dissolution assay would be expected to help with generating more accurate quantitative predictions and rank orders for formulations. However, it was also recognized that the practical limitations for modifying in vitro assays to accurately simulate in vivo permeability were significant. Biphasic dissolution assays that are designed in a two-stage manner (e.g., addition of the lipid phase and pH shift after 30 min to reflect the transfer from stomach to the upper intestine) were also considered by some participants as an improved method.
It is also important to understand what the solid state of the precipitant is for modeling. The particle size (distribution) of the precipitate(s) should ideally be measured in vitro so that it can be included in a PBBM, along with the measurement of pH values and whether they have changed to account for these inputs in the model. It was suggested that precipitated material be isolated and dissolution measured to accurately characterize the redissolution performance.
It was also suggested that two-phasic and/or transfer computational models can be used as a good approach when attempting to correlate in vitro and in vivo supersaturation concentrations.
Another member in the audience from industry stated that different methodologies are used based on whether they are looking at the drug product or the drug substance. The totality of data obtained from different in vitro experiments should then be considered. Though it is always difficult to incorporate a permeability component with in vitro systems, a complex model with an absorptive component has been helpful.
The audience seemed to agree that how you present a drug to an absorptive surface area in vitro is very important because in vitro modeling can overestimate concentrations at which precipitation occurs. For many compounds in developmental stages, though early precipitation data may have raised a red flag, usually those early precipitation risks are not as limiting as predicted by in vitro data; therefore, should we consider permeability to be a saver for some drugs that precipitate? This again stresses the importance of including an absorption compartment in the in vitro dissolution assay.
Ultimately, while there are many different transfer models used to measure the rate of precipitation, there is not a one size fits all approach, as the complexity of the assay required depends upon the question (e.g., drug precipitation propensity, impact of formulation, etc.) that we are asking.
3.4.2.2. Q2: Can We Identify the Class of Compounds for Which the Need to Integrate a Permeation-Like Process in the Precipitation Assay Is Essential for Accurate Estimation of Precipitation, and What Are the Recommended Experimental Options for This?
It was suggested to build a data set of molecules across the range of physicochemical space to define supersaturation and precipitation performance that could be used in verifying models. It was noted that a number of compounds had been studied during the IMI OrBiTo project and a recent review that summarizes the available human data from intubation for a large number of molecules could be a useful starting point for such a database.149
3.4.2.3. Q3: What Are the Options/Best Practices for Characterizing (Or Predicting) Precipitated Material Attributes (Form, Particle Size, and Solubility) for Accurate Input to PBBM?
Initially, an attendee in the audience stated that prior to looking into the software capabilities samples should be collected so that the solid state of the precipitate and its particle size can be determined and measured. Though many agreed, based on the responses from industry, this is not a common practice. Some industry representatives reported that precipitated material attributes are nowadays increasingly characterized, but concerns were raised about whether enough precipitated material could be obtained for analysis.
However, drugs may precipitate as amorphous forms, which are known to exhibit higher solubility, or as crystalline forms that exhibit lower solubility. An example of gefitinib was discussed and shows that gefitinib precipitates in an amorphous form that converts to a crystalline form.136 This example underscores the importance of understanding the solid-state characteristics for modeling. Nevertheless, the question remains: What is the best approach (mechanistic or descriptive) given that there is no standard practice?
Further discussion centered around redissolution, which can be used to back-calculate particle size. It was stated that this approach is easier than measuring the particle size.
A series of experiments conducted with posaconazole were also discussed, as in vitro experiments using the transfer assay showed an aggregate structure that was not crystalline or amorphous.150,151 More specifically, the obtained phase-separated species appeared to be metastable, reaching a plateau above the thermodynamic solubility but below the supersaturated state. The attributes of this phase-separated species could not be further elucidated. This observation challenges the current practice of in vitro to in vivo translation; can we assume from these studies that what happens in vitro translates to in vivo? As in vivo particles do not grow in an isolated medium, they might have attributes different from those of precipitates isolated from in vitro experiments. There was also some discussion about overpredicting precipitation, as ketoconazole precipitates strongly in vitro, but in vivo, it was determined that only about 10% of the dose precipitated. It was again stressed that a curated set of case examples with well understood in vivo behavior would be helpful to define parameters that need to be better characterized in vitro.
3.4.2.4. Q4: What Are the Best Practices for Modeling Precipitation under Physiologically Relevant Luminal Conditions–First Order Fixed Rate Constant/Mechanistic Nucleation and Growth Predictions in Dynamic pH/Fluid Volumes?
The first approach brought up was a bottom-up approach, in which the kinetics observed in the in vitro experiment are modeled. Subsequently, the dissolution–precipitation model is integrated in a PBBM framework via IVIVE to simulate the behavior in vivo. This approach was preferred over a top-down approach, where precipitation kinetics are fitted to observed PK data.
From a physical and mechanistic modeling perspective, it was considered valuable to separate processes involved in dissolution and precipitation from each other, measure them individually, and then combine all of the individual mechanisms in a model to obtain an improved outcome.
A question arose regarding whether anyone has used the emptying half-life in modeling and then investigated variability? Similarly, it was emphasized that physiological variability needs to be accounted for in addition to the variability associated with the pharmaceutical performance of the delivery system in the PBBM. Given the extreme interindividual variability in parameters related to precipitation, population simulations will likely cover the whole range of precipitation constants. Norvir (ritonavir formulated as ASD tablet) was given as an example where interindividual variability should be considered.152
Additionally, in the case of a precipitation risk, consideration should be given to mitigate this risk through the use of precipitation inhibitors or by using a salt of the drug. The latter option might be an alternative to more complex bioenhancement systems like ASD formulations. One answer referenced tacrolimus (an ASD) in which the precipitation risk was mitigated through formulation; however, it should always come down to an understanding of the biopharmaceutics risk.
3.4.2.5. Q5: How Can Precipitation from Supersaturating Delivery Systems, Such as ASDs, Be Modeled? What Options Are Available to Account for Complex Speciation, Including Liquid–liquid Phase-Separated Nanodroplets?
This is particularly challenging and something that requires further work due to the complexities that arise with the presence of polymer and surfactants, for example, which make prediction difficult. Mass transfer models should account for the mixed speciation of the drug. The consensus in the room was that it needs to be guided by the accurate in vitro performance of a supersaturating system.
3.5. BO Session E - Permeability: From in Vitro Best Practices to in Vivo Relevance
This session began with speaker Hans Lennernäs (Uppsala University) and was led by Christer Tannergren (AstraZeneca) and Rodrigo Cristofoletti (University of Florida), with Xiaojun Ren (Novartis) and Eleftheria Tsakalozou (FDA) as scribes.
3.5.1. Presentation
3.5.1.1. Introduction
By understanding the permeability of a drug candidate in the GI tract, medicinal chemists and biopharmaceutical scientists are expected to be able to design efficacious and safe drug compounds. These new drug compounds together with improved knowledge of regional intestinal permeability will also allow them to optimize and develop pharmaceutical formulations with high oral bioavailability and less intra- and interindividual variability and to better control of the plasma concentration–time effect relationship.
The investigation and optimization of intestinal permeability are among other key factors, such as potency, efficacy, and drug–drug interactions, that are crucial in the drug discovery and development processes of oral pharmaceutical products. Permeability plays a key role in determining the rate and extent of intestinal absorption of a drug. If a drug has poor permeability (BCS class III or IV), it may not be effectively transported into the bloodstream and could have a limited and highly variable therapeutic response. On the other hand, if a drug has high permeability and a poor pH-dependent solubility (BCS class II), the low and erratic rate and extent of absorption may be overcome with a sophisticated and innovative formulation design, such as ASD. This allows for the development of oral products with less variable plasma PK and more effective doses, which can improve patient compliance and overall treatment outcomes.153,154
Determining the intestinal permeability of drug candidates has significantly contributed to reducing the attrition rates of drugs in development. Previously, about 40% of drug candidates were discarded due to poor ADME (absorption, distribution, metabolism, and excretion) properties. However, by focusing on understanding and optimizing permeability, this attrition rate was reduced to around 10%. The limited permeability observed 2–3 decades ago can be attributed to the fact that, during that time, a significant number of drug candidates targeted extracellular sites, and membrane permeation was not considered a crucial aspect of pharmacological discovery efforts.155−157 Recent advancements in drug discovery and medicinal and biological chemistry have expanded the possibilities for developing oral drugs that were previously considered to have unfavorable physicochemical properties. These new modalities, with physicochemical properties beyond the rule of five, have opened up a broader range of options for formulating drugs that can be effectively absorbed across the intestinal barriers.158−161 In addition, considering the permeability along the human GI tract is an essential step in the innovation and development of oral pharmaceutical products featuring new modalities and challenging physicochemical properties.162−165
3.5.1.2. Intestinal Permeability Models and Approaches
Overall, the intestinal barrier is a complex system that plays a crucial role in maintaining a delicate balance between absorption and protection. It acts as a physical and immunological barrier to prevent the invasion of pathogens and the absorption of toxic substances. The small intestine, with its unique architecture and cell composition, is the major site of nutrient and drug absorption in the body. Intestinal mucosa is a dynamic physiological barrier that receives and reacts to neuroendocrine signals to maintain a harmonious interplay between absorptive permeability, protective barrier functions, and secretory functions.166 Regional differences along the GI tract, such as between the small and large intestine, can have significant implications for pharmaceutical development. It is important to consider these biopharmaceutical and physiological factors in the design of drugs to ensure their optimal delivery, absorption, and effectiveness.
The intestinal epithelium, the fastest renewing tissue in human, is made up of multiple cell types with a microenvironment consisting of a dynamic multiparametric and three-dimensional (3D) architecture, making it particularly challenging to recreate in vitro.166 The intestinal tissue is organized in finger-like protrusions called villi and invaginations called crypts. Intestinal organoids, also known as enteroids, colonoids, or “mini-guts”, are three-dimensional structures derived from stem cells that recapitulate the architecture and function of the intestine.167,168 Furthermore, combined recent advances in cellular biology and microfabrication technologies have led to the development of various bioengineered systems to model and provide more in vivo relevant investigations of the intestinal mucosal physiology and pathophysiology. These microfabricated in vitro models may constitute an alternative to current approaches for screening and biopharmaceutics evaluation, as well as provide insights into fundamental mechanisms governing intestinal homeostasis and pathologies.167,169
It is important to evaluate drug substance solubility, as drugs must be dissolved prior to transport across the intestinal barriers. The mass transfer (J) of dissolved drug molecules across semipermeable intestinal barriers is strongly affected by the nature and functions of the intestinal mucosal barrier and especially epithelial barrier. Different transport mechanisms can be involved in the process, and more than one mechanism may be employed for a single drug molecule. The net permeation process for a drug occurs via passive transcellular (lipoidal) and paracellular diffusion and/or carrier-mediated transport in both the absorptive and secretory (efflux) directions to various extents. To accurately determine the permeability of a drug, it is necessary to quantify the concentration of the drug adjacent to the intestinal membrane. This depends on the local distribution model applied in the various permeability models.170,171
A variety of in silico, in vitro, and in vivo permeability models are used in biopharmaceutical studies during all parts of the drug discovery/development process to predict and characterize human drug absorption.172−174 The selected intestinal permeability model will need to reflect the intended use of the permeability estimate at different stages of the drug development process. Permeability models comprise simple simulations and in vitro systems with high-throughput capacity, which are typically used in early drug development to sort compounds.
More complex models involving animals, humans, and/or PBBM are employed in the later stages of nonclinical or early clinical drug development. This is particularly crucial when more in vivo relevant predictions are essential for successful translational science and product development. For instance, it is obvious that regional permeability data plays a pivotal role in shaping decisions regarding the choice and design of modified release dosage forms.28,162,163
Human fraction dose absorbed (fa) and measured jejunum permeability can be thought of as potential prediction gold standards.175−178 Intestinal catheters have been used for decades in physiology, nutrition, microbiology, PK, and biopharmaceutic research. Studies involving catheters of different lengths and sizes have significantly increased the knowledge regarding the function and regulation of various processes of the human GI tract. The gold-standard permeability values are those that are determined with GI devices after local single dose administration or perfusion of a certain intestinal segment.165 A review has compiled historical human intestinal Peff values of 80 substances from 61 clinical trials performed in all parts of the human intestinal tract. The investigated substances include drugs, monosaccharaides, amino acids, dipeptides, vitamins, steroids, bile acids, ions, fatty acids, and water. It is well-known that intestinal catheters that are intended to be placed in the more distal small intestine or even proximal colon are challenging to biopharmaceutical researchers and clinicians.162−165
Single-pass perfusion of a certain region of rat intestine (in situ) is the best characterized and most thoroughly validated animal model for investigations of small and large intestinal permeability.179 A high correlation between human and rat small intestine (R2 = 0.8–0.95) was observed for drug intestinal permeability with both carrier-mediated absorption and passive diffusion mechanisms. Moderate correlation between the two species was also found for the expression levels of transporters in the duodenum, which provides evidence of a similarity in the molecular mechanisms of drug absorption. Transport properties (permeability) for different compounds were also highly correlated between rat and human when using rat intestinal specimens in the Ussing chamber model. In contrast, no correlation between rat and human intestine was found for the expression of metabolizing enzymes, which may adequately account for the well-established difference in drug metabolism and oral bioavailability in the two species.179−181
3.5.1.3. Immediate and Modified Release in the Design of the Oral Dosage Form
Design and development of the most appropriate oral dosage form depend on biopharmaceutical properties, terminal half-life (i.e., dosing rate), and plasma exposure effect relationship for the drug. The fraction dose absorbed (fa) needs to be synchronized to intestinal permeability, dissolution rate, and regional intestinal transit for the final design of the dosage form.
The small intestine is the major site of nutrient and drug absorption in the body, which is established with a characteristic 3D architecture and cell composition. It is recognized that regional differences exist along the GI tract regarding barrier functions, neuroendocrine processes, and immunological effects, which have a major impact on pharmaceutical development. Interestingly, a larger surface area of the intestinal lining is at a higher risk of being highly exposed by digestive enzymes, potential toxic xenobiotics, and luminal microbiota. Thus, it might be that mammals try to find an optimal balance between protection and service by having a small surface area that prevents extensive uptake and epithelial exposure to luminal content and simultaneously provides a large enough mucosal surface for optimal digestion and nutrient absorption.
Quantitative geometrical data of the human GI system vary considerably, especially the surface area enlargement of the intestine due to folds, villi, and microvilli. The inner surface of the small intestine is grossly enlarged by folds, villi, and microvilli, and the large intestine mucosa does not have folds comparable to those of the plicae circularis, except in the rectum. It is claimed that the total surface area of the intestinal mucosa is about the size of a tennis court (260–300 m2) with a reported value of 0.3 m2 for the large intestine.182 It has also been claimed that the major part of orally administered drugs are absorbed in the jejunum/ileum, as those account for 99% of the total absorption surface.183 However, according to Fändriks and Helander in 2014 the small intestine represents about 92–93% of the total intestinal surface area, which leaves some surface area in the large intestine for drug absorption from oral modified release formulations.182
3.5.1.4. Intestinal Transport Across Intestinal Barrier
The permeation of a dissolved drug molecule across semipermeable biological barriers is dependent on the molecular properties of the drug, transport mechanism(s), drug concentration, and the nature and conditions of the barrier. The transport mechanisms for a drug molecule may include passive lipoidal and paracellular diffusion and/or carrier-mediated (CM) transport in both the absorptive and excretive directions.182 Recently, the CM transport route has been proposed to be the universal transport mechanism, with no impact from passive lipoidal diffusion.184 However, Hans Lennernäs indicated that the experimental evidence for this transporter-only theory is weak, and the opposing view that there is a coexistence between CM and passive transport processes is more probable.182,185
CM transporters are primarily important for the absorptive transport of water-soluble nutrients, such as glucose, vitamins, and amino acids, where they enable uptake from, for instance, the intestinal lumen into the bloodstream. However, this transport mechanism might be important for some drug compounds, such as levodopa and valacyclovir, but is in general considered as relatively rare.186,187
An investigational drug having a (net) in vitro efflux ratio (ER) higher than 2 is classified as an efflux transporter substrate, when any pH difference is considered in the applied in vitro model (e.g., Caco-2 cells or transfected cells overexpressing P-gp).188,189 Rhodamine 123, digoxin, vinblastine, paclitaxel, and quinidine are often used as probe substrates for demonstrating the presence of the P-gp transporter. The ER for vinblastine, digoxin, cimetidine, and quinidine were 4.25, 5.41, 1.79, and 5.85, respectively.163 Despite being classified as an efflux transporter substrate, their fraction dose absorbed is 65% for cimetidine and >80% for the other three drugs. This again demonstrates that drugs with an identified ER higher than 2 need to be investigated in vivo since it has often been shown that there is no or limited in vivo P-gp efflux effect on the extent of absorption.163,174 Paclitaxel has been reported to be a P-gp substrate and in recent in vitro (Caco-2 model) and in vivo PK studies in rats by using the specific P-gp and Breast Cancer Resistance Protein (BCRP) inhibitor encequidar.190,191 Altogether these studies support that P-gp might have a quantitative effect when efflux ratio is extensive. However, the role of an efflux substrate remains unclear in many cases. For instance, a selective estrogen receptor degrader-antagonist was reported to have a high efflux (ER > 30), which was saturable and decreased significantly at concentrations at and above 30 μM (i.e., ER was <15 at concentrations ≥30 μM).192 The solubility was high in aqueous media (>900 μM), and the candidate had a high fraction absorbed in all species examined (fa ≥ 50–100%). Despite being a drug candidate with a high ER, it had favorable physicochemical properties that resulted in good oral bioavailability in several preclinical species and potent in vivo activity in a mouse xenograft model.192
The regional differences between the colon and the small intestine regarding the expression of efflux transporters and the tight junction may potentially also affect the rate and extent of colon absorption as well as the prediction performance in this investigation.193 However, it has previously been concluded that there is no indication that efflux-mediated transport limits colon absorption, which suggests that it is likely the intrinsic passive permeability that is the major determinant of the membrane transport in the colon.162,163 This is further supported by recently established correlations between in vitro permeability and human colon absorption, where the in vitro assays mainly measure the passive drug transport.162,163 Furthermore, as the main source for the estimated permeability in this investigation was the Caco-2 model, which is of colonic origin, it is likely that the well-known effect of narrower tight junctions in the colon was appropriately accounted for in the predictions.
3.5.1.5. Conclusions
Regional human intestinal permeability was identified as one important factor for future intestinal permeability determinations in both in vitro and in vivo models. Especially human regional intestinal permeability is of importance for the validation of existing and improved bioengineered in vitro intestinal transport models.
Determinations of in vivo colon permeability are of special urgency but are very difficult in humans. Novel GI capsule systems, GI devices with external control, and capsules connected to long GI-tube methodologies are useful in those projects.
In vitro intestinal Papp-values in the Ussing and 2D cell monolayer models need scaling and adjustment prior to use in PBBM.
The choice of permeability model is important for the assessment of the effect of pharmaceutical excipients. Caco-2 cell monolayers have been shown to often overpredict the potential in vivo effects of pharmaceutical excipients, and this higher sensitivity is explained by the given multiple differences between the simple Caco-2 monolayer and human in vivo intestine with its additional features like its mucus layer and full neuroendocrine feedback systems.173,193−195
Future intestinal organoids and 3D bioengineered intestinal models might exhibit morphological and physiological features that resemble those of native intestinal mucosa. These more complex in vitro systems are promising but require extensive evaluation and validation prior to use in rational drug discovery and development and for regulatory decision-making.
Encequidar and elacridar may be very useful tools to assess the effect of intestinal efflux mediated by P-gp and/or BCRP on the rate and extent of intestinal absorption.
Biopharmaceutics has an exciting future with the development of novel GI devices for assessment in humans and animals, bioengineered in vitro systems mimicking in vivo, advanced modeling with molecular dynamic simulation and artificial neural network (ANN) in drug discovery, and extended use of more accurate PBBMs in all part of drug development. Model and knowledge development to predict the effective permeability of new and interesting challenging drug candidates beyond Lipinski’s rule of 5 with a molar mass above 700 and Log D > 5 will be an important part for any future successful drug development.158−160 These novel ANN simulation tools for oral drugs may also be applied before synthesis and even potentially allow for optimization of relevant physicochemical properties of new molecules of interest.155,196
3.5.2. Discussion
The main objective of this part of the session was to discuss best practices for the integration of permeability in PBBM.
3.5.2.1. Q1: What Are the Available Methods to Estimate Jejunal Peff and What Is the Rank Order between the Methods with Regard to Confidence in the Peff Estimation?
The majority of the attendees stated that they use MDCK or Caco-2 cell systems to estimate jejunal Peff. PAMPA may be used at early stages of drug development according to the session participants. An in-house calibration curve is normally used for the in vitro to in vivo permeability extrapolation. A few participants used built-in calibration curves from commercially available software, such as GastroPlus or Simcyp. It was stated that, when a calibration curve is used, it should cover low, moderate, and high permeability compounds. To reduce interstudy or interlaboratory variability, a calibrator, or a compound with known in vivo permeability, is often utilized. On rare occasions, QSAR models have been used directly to estimate Peff. Finally, the participants shared that oral solution PK data can be used to optimize Peff.
It was anecdotally agreed that the experimentally obtained measurements of Peff from in vitro assays are a measure of passive permeability. When there is a need for characterizing protein-mediated transport, transfected cell lines may be used. While for high passive permeability compounds, the impact of protein-mediated efflux may be limited, it is important to characterize the impact of efflux transporters for low passive permeability compounds, understanding the variability of experimentally obtained Vmax or Km. For lipophilic compounds or to address food effect, biorelevant media may be used.
The value of the in situ permeability in a rat model was discussed in terms of challenges in extrapolation or experimental variability. Most regulators shared that Caco-2 data are most commonly reported in regulatory applications. Canadian and European regulatory agencies indicated that well-controlled in situ data may be accepted.
Differences in how passive Peff and transporter kinetics are integrated into various software need to be considered. There was an agreement that the Caco-2 cell model performs well for high permeability compounds. It is important though to cross check across a variety of data sets and Peff measurements collected using different methodologies.
3.5.2.2. Q2: Confidence in Peff Estimation – Low vs High Permeability Compounds?
Most participants agreed that there is a high degree of confidence in the estimated Peff for high permeability compounds, while the confidence in the estimated Peff for low to moderate permeability compounds was lower. Although no conclusions were made during the discussion regarding a cutoff value between high and low Peff, a Peff of 1.34 × 10–4cm/s, corresponding to the measured human jejunal Peff of metoprolol and a fraction absorbed in humans of 90%,197 has been used previously for this purpose. Similarly, minoxidil, with an observed human fraction absorbed of 85%, can be applied as a divider between high and low permeability. The group also acknowledged that the extensive interlaboratory variability in the measured in vitro permeability is a factor playing a role in the credibility of the final estimates of the human Peff, especially for low permeability compounds. Therefore, a reference data set for high and low permeability marker compounds established within each lab is beneficial.
3.5.2.3. Q3: How Do We Use in Vitro Permeability Data Generated in Biorelevant Media as Input?
Biorelevant media such as FaSSIF and FeSSIF may improve the solubility of some compounds in the apical chamber, but micelle entrapment/binding may bias estimation of apparent permeability (Papp) across monolayers. For example, Caco-2 Papp of lipophilic compounds like danazol is inversely proportional to the concentration of bile salt in the donor chamber,198 whereas Papp of more hydrophilic compounds was insensitive to the bile salts concentration.198 Careful consideration should be exercised when using Papp data obtained in biorelevant media as input since it may represent a mixture of micelle-entrapment and permeability. Measuring free concentration in the donor chamber of the Transwell system or modeling drug-micelle binding and Papp simultaneously may be helpful, but further studies are needed to access the benefits of either approach. Finally, when biorelevant media are used, pH in the mucus layer in vivo needs to be taken into consideration. Mucus pH approximates the upper gut pH. Therefore, considering the mucus layer pH and the composition of the lipids in the mucus in vivo versus in vitro may be key to more reliable estimations of Peff.
3.5.2.4. Q4: Papp–Peff Correlation vs Fitting Peff to Observed Data–When to Do What?
Several methodologies have emerged throughout the years to calculate gut permeability (effective permeability, Peff) for orally administered drug products. Some of these methodologies, such as the Caco-2 in vitro system, have been initially developed to select candidates or inform “go-no go” decisions based on their permeability characteristics or to assess the need for in vivo testing. It was agreed that novel technologies such as PBBM and experimental data have been leveraged to generate in vivo predictions of the permeability in virtual populations. Accumulating knowledge in the field indicates that for high permeability compounds the Caco-2 in vitro approach appears to be of high confidence. In the absence of data collected in a Caco-2 in vitro system, a mathematical model (such as PBBM) may leverage appropriate clinical PK data sets, e.g., for a nonprecipitating oral solution to derive (estimate) a Peff value. The challenge with this approach is the type of observed data that is utilized for predicting (“fitting”) this parameter, which may include individual or mean PK profile data from an oral solution or any other dosage form for which drug release from the dosage form, and not permeation through the gut epithelium, is the rate limiting step. The use of individual level PK data may result in inflating the intersubject variability incorporated into an in silico model, while the use of an oral dosage form, other than oral solution, may lead to a parameter model identifiability issue.
As such, leveraging in vitro permeability data collected in a Caco-2 system toward an initial “bottom-up” approach for Peff is advisable. Confirming the calculated Peff using informative clinical PK data is necessary. In the case where Caco-2 data do not result in satisfactory predictions, it may be acceptable to perform parameter optimization on Peff within the developed PBBM compared with the available clinical PK data. Gut metabolism, particularly relevant for high extraction drugs, was identified as a complicating factor for Peff characterization in the PBBM during the discussion. To handle model identifiability, for PBBM development purposes, applying an in vitro-in vivo extrapolation to inform a “bottom-up” approach in which gut metabolism is mechanistically predicted was suggested. Knowledge on the relative contribution of the gut metabolism toward the overall metabolism (clearance) was identified as critical toward accurately capturing the gut extraction ratio in a PBBM. It is expected that this recommended workflow will perform better for highly permeable compounds compared to low permeability compounds for which additional challenges may need to be addressed.
3.5.2.5. Q5: When Can Permeability Input into PBBM Be Based on Passive Permeability Alone, and When Is There a Need to Account for Uptake/Efflux Transporter Mediated Transport?
Inclusion of transporter effects into an in silico model should be data driven. The decision should be based on the experimental results. Nonlinearity in clinical studies could be due to a transporter effect. Further exploration of the extent of the impact may be warranted. A well-controlled modeling and simulation approach may be accepted by regulatory agencies to investigate the impact of a transporter.188,199 A clinical DDI study for transporter inhibition may eventually become warranted.
3.5.2.6. Q6: What Is the Best Practice to Account for Uptake/Efflux Transporter Mediated Transport?
When a transporter effect on the clinical outcome for an orally administered drug is suspected, the extent of the transporter involvement on oral absorption and specifically gut permeability should be thoroughly and systematically investigated. Studies using in vitro and animal models have sometimes been used to determine the need for further in vivo studies in humans. The activity of the transporter protein can be characterized across a dose range of the victim drug and in the presence of well-established transporter activity modifiers within the context of in vitro or in vivo studies exploring potential drug–drug interactions and their clinical impact.
These types of studies provide reliable estimates for parameters describing the saturable component of the absorption process governed by transporter proteins (Michaelis–Menten kinetics). These parameters include but are not limited to Ki (inhibition constant), KI (inhibitor concentration causing half-maximal inactivation), kinact (maximal inactivation rate constant), Km (Michaelis–Menten constant), Jmax (maximal flux rate), and Vmax (maximal rate). Depending on the implementation of the saturable absorption process in a mechanistic PBBM, these parameters may serve as model inputs. With the application of validated, for their intended purpose, in vitro-in vivo extrapolations embedded into PBBMs, population predictions in virtual healthy subjects or patients may be generated. The session participants acknowledged the challenge associated with determining appropriate model inputs for the Vmax parameter, most probably because the in vitro collected Vmax values are typically highly dependent on the in vitro system utilized for data collection.
Additional considerations regarding the regional expression of transporter proteins across the GI tract and the relative expression of these proteins are expected to inform key decisions on the development and validation of PBBMs that incorporate gut transporters. Guidelines and relevant literature are abundantly available for efflux transporters such as P glycoprotein (P-gp) and BCRP. These transporter proteins have been documented to limit bioavailability for orally administered drug substances by pumping them back into the gut lumen after they enter the enterocytes. However, there is a significant knowledge gap regarding uptake gut transporters and their relative contribution to oral absorption, which renders their incorporation into mechanistic in silico models challenging.
3.5.2.7. Q7: What Is the Confidence in Using the Estimated Jejunal Peff to Define the Peff in the Other Compartments?
Based on available experimental data, there is low confidence in using the estimated jejunal Peff to define Peff in the other intestinal compartments. The relative values used for Peff in the jejunum versus colon may be extremely important when modeling ER and MR products.
For low permeability compounds, jejunal Peff is considered to be higher than Peff in the colon.162,163 This reflects the current general understanding within the community.
Commercially available software currently utilizes the same value for Peff in both the jejunum and colon. This value is corrected for effective surface area corresponding to the different gut segments. In the absence of observed data, the group agreed that the correction is necessary but may be an overly simplistic approach. The attendees agreed that it is challenging to understand how the effective surface area in the gut/different regions is estimated and acknowledged that potential “pockets” in the gut are not considered.
3.5.2.8. Q8: How Can Colon Peff Be Estimated?
Experimentally, a colon Peff can be obtained with local administration of the compounds of interest using either intubation or telemetric capsule techniques. Indirectly, when utilizing a modeling approach, the group shared that they would vary the Peff value used as the model input to get the clearance of the observed data. This is essentially a method where modeling fitting is involved.
4. Conclusions and Future Directions
This workshop represented the culmination of a year-long collaborative effort between industry and regulatory authorities and was overall successful in its effort to advance PBBM for oral products. The morning session focused on the regulatory agency discussion of PBBM case studies submitted by industry members of the IQ consortium and provided insights into the regulatory assessment process and some clarity regarding what is looked-for in PBBM regulatory submissions. The afternoon sessions discussed best practices, decision trees, and checklists, which will be useful for future submissions of PBBM regarding the measurement of key input parameters such as drug solubility, drug product dissolution, precipitation, and permeability. In addition, breakout sessions also discussed best practices around how these measurements should be undertaken for various drug and formulation types and how the measured values should be modeled mechanistically and integrated in the PBBMs. There are remaining gaps in our knowledge and limitations to directly translate in vitro data to predict in vivo drug substance or drug product performance, and the breakout session discussions also covered these gaps and proposed, where relevant and feasible, practical approaches to cover these gaps based on preclinical or clinical measured data. Overall, sound model parametrization and explanation thereof are key to the success of PBBM and their acceptance by regulatory agencies, and the requirements for measurements and integration of these parameters should be shared across the scientific community. The decision trees, checklists, and subject matter expert advice presented in this paper and Supporting Information can be understood as practical tools to foster scientific discussion and to continue efforts toward harmonization on best practices for PBBM.
Acknowledgments
The meeting organizers are extremely grateful to Keiasia Robinson and Dana Hammell (University of Maryland, Baltimore) and the FDA Office of Regulatory Science and Innovation (ORSI) for significantly contributing to the success of the workshop. This research was supported by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) as part of a financial assistance award 5U01FD005946 totaling $5,000 with 100% funded by FDA/HHS. The collaboration work was supported by the IQ consortium. The authors would like to acknowledge the collaborative effort of regulators who participated in the PBBM case studies discussions and the open and constructive way they shared their views on the scientific approach and data submitted. In parallel, the authors would like to thank the IQ consortium company members and for this day Amgen, AstraZeneca, and Pfizer for taking the time to develop PBBMs around relevant biopharmaceutical questions, prepare the submissions, and make this exercise possible. Finally, the authors would like to thank Simulations Plus, Inc. and Certara for sharing their advice and training when requested to use their respective modeling platforms.
Glossary
Abbreviations
- 505(b)(2)
A 505(b)(2) application is a new drug application (NDA) described in section 505(b)(2) of the US Federal Food, Drug and Cosmetic Act. It is submitted under section 505(b)(1) of the Act and approved under section 505(c) of the Act
- ACAT
Absorption Compartmental and Transit
- ADMET
ADMET predictor (GastroPlus)
- AAFE
Absolute Average Fold Error
- AFE
Average Fold Error
- ASD
Amorphous Solid Dispersion
- AS&D
Artificial Stomach and Duodenum apparatus
- BCS
Biopharmaceutics Classification System
- BCRP
Breast Cancer Resistance Protein
- BE
Bioequivalence or Bioequivalent
- BO
BreakOut
- CBA
Critical Biopharmaceutics Attribute
- CDER
Center for Drug Evaluation and Research
- CERSI
Center of Excellence in Regulatory Science and Innovation
- Cmax
Maximum Plasma Concentration
- CQA
Critical Quality Attribute
- DDI
Drug–Drug Interactions
- DS
Drug Substance
- DOE
Design Of Experiments
- EMA
European Medicines Agency
- ER
Efflux Ratio
- EU
European Union
- FaSSIF
Fasted State Simulated Intestinal Fluid
- FDA
United States Food and Drug Administration
- GI
Gastrointestinal
- GIS
Gastrointestinal Simulator
- HHS
Health and Human Services
- HIF
Human Intestinal Fluid
- HPMCAS
Hydroxypropylmethylcellulose Acetate Succinate
- HPMCAS-H
Hydroxypropylmethylcellulose Acetate Succinate with a ratio of >1 between acetate and succinate substituents
- HPMCAS-L
Hydroxypropylmethylcellulose Acetate Succinate with a ratio of <1 between acetate and succinate substituents
- HPMCAS-M
Hydroxypropylmethylcellulose Acetate Succinate with a ratio of 1 between acetate and succinate substituents
- IMI
Innovative Medicine Initiative
- IMMC
Interdigestive Migrating Myoelectric Complex
- IR
Immediate Release
- IV
Intravenous
- IVIVC
In vitro In vivo Correlation
- IVIVR
In vitro In vivo Relationship
- Km
Michaelis–Menten constant, the drug concentration at which the reaction rate is at half-maximum
- LLPS
Liquid–Liquid Phase Separation
- MAA
Marketing Authorization Applications
- M-CERSI
Maryland Center of Excellence in Regulatory Science and Innovation
- MDCK
Madin-Darby Canine Kidney
- MR
Modified Release
- MRI
Magnetic Resonance Imaging
- OrBiTo
Oral Biopharmaceutics Tools (IMI consortium)
- PAMPA
Parallel Artificial Membrane Permeability Assay
- PBBM(s)
Physiologically Based Biopharmaceutics Model(s)
- PBBM
Physiologically Based Biopharmaceutics Modeling
- PBPK
Physiologically Based Pharmacokinetics
- PCQS
Patient Centric Quality Standard
- Peff
Effective human Jejunal Permeability
- PepT1
Peptide Transporter 1
- PK
Pharmacokinetics
- PPI
Proton Pump Inhibitor
- P-PSD
Product Particle Size Distribution
- PSA
Parameter Sensitivity Analysis
- PSD
Particle Size Distribution
- PVP K30
Polyvinylpyrrolidone with a relative viscosity to water of about 30 at 1% w/v aqueous solution (K-value)
- PVP VA64
poly(vinylpyrrolidone-co-vinyl acetate). 6:4 linear random copolymer of N-vinylpyrrolidone and vinyl acetate
- QC
Quality Control
- QSAR
Quantitative Structure Activity Relationship
- SBIA
Small Business and Industry Assistance
- SLS
Sodium Lauryl Sulfate
- S(M)EDDS
Self-(Micro) Emulsifying Drug Delivery System
- SR
Solubilization Ratio
- SUPAC
Scale-Up and Post Approval Changes
- Tmax
Time to maximum concentration
- USP
United States Pharmacopeia
- UV
Ultraviolet
- UWL
Unstirred Water Layer
- VBE
Virtual Bioequivalence
- VIS
Visible
- Vmax
Maximum rate of enzyme mediated metabolism or transporter-mediated efflux or uptake
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.molpharmaceut.4c00526.
(1) Points to consider when measuring drug solubility and (2) the comparison of dissolution models taking acalabrutinib capsules as a model solid oral dosage form (PDF)
Author Present Address
◆ Simulations Plus Inc., 42505 10th Street West, Lancaster, CA, USA
This article reflects the views of the individual authors and should not be construed to represent the views, policies or nomenclature of their organizations, including ANVISA, FAMHP, EMA, FDA, Health Canada, MHRA, Norwegian Medical Products Agency, Swedish Medical Products Agency, and PMDA.
The authors declare the following competing financial interest(s): X. Pepin, S. Arora, M. Cano-Vega, T. Carducci, G. Chen, A. Dallmann, T. Heimbach, C. Mackie, M. McAllister, A. Mitra, D. Mudie, X. Ren, G. Rullo, M. Scherholz, I. Song, C. Stillhart, S. Suarez-Sharp, C. Tannergren, and C. Wagner are employees of their respective companies and have ownership, options, and/or interests in their respective stock.
Special Issue
Published as part of Molecular Pharmaceuticsvirtual special issue “2023 PBBM Workshop for Drug Product Quality”.
Supplementary Material
References
- Anand O.; Pepin X. J. H.; Kolhatkar V.; Seo P. The Use of Physiologically Based Pharmacokinetic Analyses—in Biopharmaceutics Applications -Regulatory and Industry Perspectives. Pharm. Res. 2022, 39, 1681. 10.1007/s11095-022-03280-4. [DOI] [PubMed] [Google Scholar]
- Abend A.; Heimbach T.; Cohen M.; Kesisoglou F.; Pepin X.; Suarez-Sharp S. Dissolution and Translational Modeling Strategies Enabling Patient-Centric Drug Product Development: the M-CERSI Workshop Summary Report. AAPS journal 2018, 20 (3), 60. 10.1208/s12248-018-0213-x. [DOI] [PubMed] [Google Scholar]
- Suarez-Sharp S.; Cohen M.; Kesisoglou F.; Abend A.; Marroum P.; Delvadia P.; Kotzagiorgis E.; Li M.; Nordmark A.; Bandi N. Applications of Clinically Relevant Dissolution Testing: Workshop Summary Report. AAPS Journal 2018, 20 (6), 93. 10.1208/s12248-018-0252-3. [DOI] [PubMed] [Google Scholar]
- Heimbach T.; Suarez-Sharp S.; Kakhi M.; Holmstock N.; Olivares-Morales A.; Pepin X.; Sjögren E.; Tsakalozou E.; Seo P.; Li M. Dissolution and Translational Modeling Strategies Toward Establishing an In Vitro-In Vivo Link—a Workshop Summary Report. AAPS Journal 2019, 21 (2), 29. 10.1208/s12248-019-0298-x. [DOI] [PubMed] [Google Scholar]
- Pepin X. J. H.; Parrott N.; Dressman J.; Delvadia P.; Mitra A.; Zhang X.; Babiskin A.; Kolhatkar V.; Suarez-Sharp S. Current State and Future Expectations of Translational Modeling Strategies to Support Drug Product Development, Manufacturing Changes and Controls: A Workshop Summary Report. J. Pharm. Sci. 2021, 110, 555–566. 10.1016/j.xphs.2020.04.021. [DOI] [PubMed] [Google Scholar]
- FDA . The Use of Physiologically Based Pharmacokinetic Analyses — Biopharmaceutics Applications for Oral Drug Product Development, Manufacturing Changes, and Controls; CDER, 2020. [Google Scholar]
- Markopoulos C.; Andreas C. J.; Vertzoni M.; Dressman J.; Reppas C. In-vitro simulation of luminal conditions for evaluation of performance of oral drug products: Choosing the appropriate test media. Eur. J. Pharm. Biopharm. 2015, 93, 173–182. 10.1016/j.ejpb.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Reppas C.; Kuentz M.; Bauer-Brandl A.; Carlert S.; Dallmann A.; Dietrich S.; Dressman J.; Ejskjaer L.; Frechen S.; Guidetti M.; et al. Leveraging the use of in vitro and computational methods to support the development of enabling oral drug products: An InPharma commentary. European Journal of Pharmaceutical Sciences 2023, 188, 106505. 10.1016/j.ejps.2023.106505. [DOI] [PubMed] [Google Scholar]
- Krollik K.; Lehmann A.; Wagner C.; Kaidas J.; Kubas H.; Weitschies W. The effect of buffer species on biorelevant dissolution and precipitation assays - Comparison of phosphate and bicarbonate buffer. Eur. J. Pharm. Biopharm. 2022, 171, 90–101. 10.1016/j.ejpb.2021.09.009. [DOI] [PubMed] [Google Scholar]
- Cristofoletti R.; Dressman J. B. Dissolution Methods to Increasing Discriminatory Power of In Vitro Dissolution Testing for Ibuprofen Free Acid and Its Salts. J. Pharm. Sci. 2017, 106 (1), 92–99. 10.1016/j.xphs.2016.06.001. [DOI] [PubMed] [Google Scholar]
- Cristofoletti R.; Dressman J. B. FaSSIF-V3, but not compendial media, appropriately detects differences in the peak and extent of exposure between reference and test formulations of ibuprofen. Eur. J. Pharm. Biopharm 2016, 105, 134–140. 10.1016/j.ejpb.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Cristofoletti R.; Hens B.; Patel N.; Esteban V. V.; Schmidt S.; Dressman J. Integrating Drug- and Formulation-Related Properties With Gastrointestinal Tract Variability Using a Product-Specific Particle Size Approach: Case Example Ibuprofen. J. Pharm. Sci. 2019, 108 (12), 3842–3847. 10.1016/j.xphs.2019.09.012. [DOI] [PubMed] [Google Scholar]
- Segregur D.; Flanagan T.; Mann J.; Moir A.; Karlsson E. M.; Hoch M.; Carlile D.; Sayah-Jeanne S.; Dressman J. Impact of Acid-Reducing Agents on Gastrointestinal Physiology and Design of Biorelevant Dissolution Tests to Reflect These Changes. J. Pharm. Sci. 2019, 108 (11), 3461–3477. 10.1016/j.xphs.2019.06.021. [DOI] [PubMed] [Google Scholar]
- Segregur D.; Mann J.; Moir A.; Karlsson E. M.; Dressman J. Prediction of plasma profiles of a weakly basic drug after administration of omeprazole using PBPK modeling. European Journal of Pharmaceutical Sciences 2021, 158, 105656. 10.1016/j.ejps.2020.105656. [DOI] [PubMed] [Google Scholar]
- Patel N.; Clarke J. F.; Salem F.; Abdulla T.; Martins F.; Arora S.; Tsakalozou E.; Hodgkinson A.; Arjmandi-Tash O.; Cristea S.; et al. Multi-phase multi-layer mechanistic dermal absorption (MPML MechDermA) model to predict local and systemic exposure of drug products applied on skin. CPT: Pharmacometrics & Systems Pharmacology 2022, 11 (8), 1060–1084. 10.1002/psp4.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakalozou E.; Babiskin A.; Zhao L. Physiologically-based pharmacokinetic modeling to support bioequivalence and approval of generic products: A case for diclofenac sodium topical gel, 1%. CPT: Pharmacometrics & Systems Pharmacology 2021, 10 (5), 399–411. 10.1002/psp4.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janas C.; Mast M. P.; Kirsamer L.; Angioni C.; Gao F.; Mäntele W.; Dressman J.; Wacker M. G. The dispersion releaser technology is an effective method for testing drug release from nanosized drug carriers. Eur. J. Pharm. Biopharm 2017, 115, 73–83. 10.1016/j.ejpb.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Schiller C.; Frohlich C. P.; Giessmann T.; Siegmund W.; Monnikes H.; Hosten N.; Weitschies W. Intestinal fluid volumes and transit of dosage forms as assessed by magnetic resonance imaging. Aliment Pharmacol Ther 2005, 22 (10), 971–979. 10.1111/j.1365-2036.2005.02683.x. [DOI] [PubMed] [Google Scholar]
- Pepin X. J. H.; Sanderson N. J.; Blanazs A.; Grover S.; Ingallinera T. G.; Mann J. C. Bridging in vitro dissolution and in vivo exposure for acalabrutinib. Part I. Mechanistic modelling of drug product dissolution to derive a P-PSD for PBPK model input. Eur. J. Pharm. Biopharm. 2019, 142, 421–434. 10.1016/j.ejpb.2019.07.014. [DOI] [PubMed] [Google Scholar]
- Kuemmel C.; Yang Y.; Zhang X.; Florian J.; Zhu H.; Tegenge M.; Huang S.-M.; Wang Y.; Morrison T.; Zineh I. Consideration of a Credibility Assessment Framework in Model-Informed Drug Development: Potential Application to Physiologically-Based Pharmacokinetic Modeling and Simulation. CPT: Pharmacometrics & Systems Pharmacology 2020, 9 (1), 21–28. 10.1002/psp4.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepin X. J.; Flanagan T. R.; Holt D. J.; Eidelman A.; Treacy D.; Rowlings C. E. Justification of Drug Product Dissolution Rate and Drug Substance Particle Size Specifications Based on Absorption PBPK Modeling for Lesinurad Immediate Release Tablets. Mol. Pharmaceutics 2016, 13 (9), 3256–3269. 10.1021/acs.molpharmaceut.6b00497. [DOI] [PubMed] [Google Scholar]
- EMA . Guideline on the reporting of physiologically based pharmacokinetic (PBPK) modelling and simulation; 2018. [Google Scholar]
- Musuamba F. T.; Skottheim Rusten I.; Lesage R.; Russo G.; Bursi R.; Emili L.; Wangorsch G.; Manolis E.; Karlsson K. E.; Kulesza A.; et al. Scientific and regulatory evaluation of mechanistic in silico drug and disease models in drug development: Building model credibility. CPT: Pharmacometrics & Systems Pharmacology 2021, 10 (8), 804–825. 10.1002/psp4.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musuamba F. T.; Bursi R.; Manolis E.; Karlsson K.; Kulesza A.; Courcelles E.; Boissel J.-P.; Lesage R.; Crozatier C.; Voisin E. M.; et al. Verifying and Validating Quantitative Systems Pharmacology and In Silico Models in Drug Development: Current Needs, Gaps, and Challenges. CPT: Pharmacometrics & Systems Pharmacology 2020, 9 (4), 195–197. 10.1002/psp4.12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepin X. J. H.; Moir A. J.; Mann J. C.; Sanderson N. J.; Barker R.; Meehan E.; Plumb A. P.; Bailey G. R.; Murphy D. S.; Krejsa C. M.; et al. Bridging in vitro dissolution and in vivo exposure for acalabrutinib. Part II. A mechanistic PBPK model for IR formulation comparison, proton pump inhibitor drug interactions, and administration with acidic juices. Eur. J. Pharm. Biopharm. 2019, 142, 435–448. 10.1016/j.ejpb.2019.07.011. [DOI] [PubMed] [Google Scholar]
- Zhou D.; Chen B.; Sharma S.; Tang W.; Pepin X. Physiologically Based Absorption Modelling to Explore the Formulation and Gastric pH Changes on the Pharmacokinetics of Acalabrutinib. Pharm. Res. 2023, 40, 375. 10.1007/s11095-022-03268-0. [DOI] [PubMed] [Google Scholar]
- Amidon G. E.; Hawley M. Oral Bioperformance and 21st Century Dissolution. Mol. Pharmaceutics 2010, 7 (5), 1361–1361. 10.1021/mp100275b. [DOI] [PubMed] [Google Scholar]
- Amidon G. L.; Lennernäs H.; Shah V. P.; Crison J. R. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12 (3), 413–420. 10.1023/A:1016212804288. [DOI] [PubMed] [Google Scholar]
- Fagerberg J. H.; Bergström C. A. Intestinal solubility and absorption of poorly water soluble compounds: predictions, challenges and solutions. Ther Deliv 2015, 6 (8), 935–959. 10.4155/tde.15.45. [DOI] [PubMed] [Google Scholar]
- Mudie D. M.; Samiei N.; Marshall D. J.; Amidon G. E.; Bergström C. A. S. Selection of In Vivo Predictive Dissolution Media Using Drug Substance and Physiological Properties. Aaps j 2020, 22 (2), 34. 10.1208/s12248-020-0417-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A.; Yates I.; Mudie D.; Pivette P.; Goodwin A.; Sarmiento A.; Winter M.; Morgen M.; Vodak D. Mechanistic Study of Belinostat Oral Absorption From Spray-Dried Dispersions. J. Pharm. Sci. 2019, 108 (1), 326–336. 10.1016/j.xphs.2018.09.031. [DOI] [PubMed] [Google Scholar]
- Almeida e Sousa L.; Reutzel-Edens S. M.; Stephenson G. A.; Taylor L. S. Assessment of the Amorphous “Solubility” of a Group of Diverse Drugs Using New Experimental and Theoretical Approaches. Mol. Pharmaceutics 2015, 12 (2), 484–495. 10.1021/mp500571m. [DOI] [PubMed] [Google Scholar]
- Takano R.; Sugano K.; Higashida A.; Hayashi Y.; Machida M.; Aso Y.; Yamashita S. Oral absorption of poorly water-soluble drugs: computer simulation of fraction absorbed in humans from a miniscale dissolution test. Pharm. Res. 2006, 23 (6), 1144–1156. 10.1007/s11095-006-0162-4. [DOI] [PubMed] [Google Scholar]
- Stewart A. M.; Grass M. E.; Brodeur T. J.; Goodwin A. K.; Morgen M. M.; Friesen D. T.; Vodak D. T. Impact of Drug-Rich Colloids of Itraconazole and HPMCAS on Membrane Flux in Vitro and Oral Bioavailability in Rats. Mol. Pharmaceutics 2017, 14 (7), 2437–2449. 10.1021/acs.molpharmaceut.7b00338. [DOI] [PubMed] [Google Scholar]
- Stewart A. M.; Grass M. E.; Mudie D. M.; Morgen M. M.; Friesen D. T.; Vodak D. T. Development of a Biorelevant, Material-Sparing Membrane Flux Test for Rapid Screening of Bioavailability-Enhancing Drug Product Formulations. Mol. Pharmaceutics 2017, 14 (6), 2032–2046. 10.1021/acs.molpharmaceut.7b00121. [DOI] [PubMed] [Google Scholar]
- Stewart A. M.; Grass M. E. Practical Approach to Modeling the Impact of Amorphous Drug Nanoparticles on the Oral Absorption of Poorly Soluble Drugs. Mol. Pharmaceutics 2020, 17 (1), 180–189. 10.1021/acs.molpharmaceut.9b00889. [DOI] [PubMed] [Google Scholar]
- Pepin X.; McAlpine V.; Moir A.; Mann J. Acalabrutinib Maleate Tablets: The Physiologically Based Biopharmaceutics Model behind the Drug Product Dissolution Specification. Mol. Pharm. 2023, 20, 2181. 10.1021/acs.molpharmaceut.3c00005. [DOI] [PubMed] [Google Scholar]
- Mudie D. M.; Stewart A. M.; Rosales J. A.; Adam M. S.; Morgen M. M.; Vodak D. T. In Vitro-In Silico Tools for Streamlined Development of Acalabrutinib Amorphous Solid Dispersion Tablets. Pharmaceutics 2021, 13 (8), 1257. 10.3390/pharmaceutics13081257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uekusa T.; Oki J.; Omori M.; Watanabe D.; Inoue D.; Sugano K. Effect of buffer capacity on dissolution and supersaturation profiles of pioglitazone hydrochloride. Journal of Drug Delivery Science and Technology 2020, 55, 101492. 10.1016/j.jddst.2019.101492. [DOI] [Google Scholar]
- Casalini T.; Mann J.; Pepin X. Predicting Surface pH in Unbuffered Conditions for Acids, Bases, and Their Salts - A Review of Modeling Approaches and Their Performance. Mol. Pharmaceutics 2023, 10.1021/acs.molpharmaceut.3c00661. [DOI] [PubMed] [Google Scholar]
- Bou-Chacra N.; Melo K. J. C.; Morales I. A. C.; Stippler E. S.; Kesisoglou F.; Yazdanian M.; Löbenberg R. Evolution of Choice of Solubility and Dissolution Media After Two Decades of Biopharmaceutical Classification System. Aaps j 2017, 19 (4), 989–1001. 10.1208/s12248-017-0085-5. [DOI] [PubMed] [Google Scholar]
- Söderlind E.; Karlsson E. M.; Carlsson A.; Kong R.; Lenz A.; Lindborg S.; Sheng J. J. Simulating fasted human intestinal fluids: understanding the roles of lecithin and bile acids. Mol. Pharmaceutics 2010, 7 (5), 1498–1507. 10.1021/mp100144v. [DOI] [PubMed] [Google Scholar]
- Fuchs A.; Leigh M.; Kloefer B.; Dressman J. B. Advances in the design of fasted state simulating intestinal fluids: FaSSIF-V3. Eur. J. Pharm. Biopharm. 2015, 94, 229–240. 10.1016/j.ejpb.2015.05.015. [DOI] [PubMed] [Google Scholar]
- de la Cruz-Moreno M. P.; Montejo C.; Aguilar-Ros A.; Dewe W.; Beck B.; Stappaerts J.; Tack J.; Augustijns P. Exploring drug solubility in fasted human intestinal fluid aspirates: Impact of inter-individual variability, sampling site and dilution. Int. J. Pharm. 2017, 528 (1), 471–484. 10.1016/j.ijpharm.2017.05.072. [DOI] [PubMed] [Google Scholar]
- Abuhassan Q.; Khadra I.; Pyper K.; Augustijns P.; Brouwers J.; Halbert G. W. Structured solubility behaviour in bioequivalent fasted simulated intestinal fluids. Eur. J. Pharm. Biopharm. 2022, 176, 108–121. 10.1016/j.ejpb.2022.05.010. [DOI] [PubMed] [Google Scholar]
- Baxevanis F.; Kuiper J.; Fotaki N. Fed-state gastric media and drug analysis techniques: Current status and points to consider. Eur. J. Pharm. Biopharm 2016, 107, 234–248. 10.1016/j.ejpb.2016.07.013. [DOI] [PubMed] [Google Scholar]
- Wagner C.; Kesisoglou F.; Pepin X. J. H.; Parrott N.; Emami Riedmaier A. Use of Physiologically Based Pharmacokinetic Modeling for Predicting Drug-Food Interactions: Recommendations for Improving Predictive Performance of Low Confidence Food Effect Models. AAPS Journal 2021, 23 (4), 85. 10.1208/s12248-021-00601-0. [DOI] [PubMed] [Google Scholar]
- Li S.; Doyle P.; Metz S.; Royce A. E.; Serajuddin A. T. Effect of chloride ion on dissolution of different salt forms of haloperidol, a model basic drug. Journal of pharmaceutical sciences 2005, 94 (10), 2224–2231. 10.1002/jps.20440. [DOI] [PubMed] [Google Scholar]
- Saboo S.; Mugheirbi N. A.; Zemlyanov D. Y.; Kestur U. S.; Taylor L. S. Congruent release of drug and polymer: A ″sweet spot″ in the dissolution of amorphous solid dispersions. J. Controlled Release 2019, 298, 68–82. 10.1016/j.jconrel.2019.01.039. [DOI] [PubMed] [Google Scholar]
- Ilevbare G. A.; Taylor L. S. Liquid-Liquid Phase Separation in Highly Supersaturated Aqueous Solutions of Poorly Water-Soluble Drugs: Implications for Solubility Enhancing Formulations. Cryst. Growth Des. 2013, 13 (4), 1497–1509. 10.1021/cg301679h. [DOI] [Google Scholar]
- Okumu A.; DiMaso M.; Lobenberg R. Computer simulations using GastroPlus to justify a biowaiver for etoricoxib solid oral drug products. Eur. J. Pharm. Biopharm 2009, 72 (1), 91–98. 10.1016/j.ejpb.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Okumu A.; DiMaso M.; Lobenberg R. Dynamic dissolution testing to establish in vitro/in vivo correlations for montelukast sodium, a poorly soluble drug. Pharm. Res. 2008, 25 (12), 2778–2785. 10.1007/s11095-008-9642-z. [DOI] [PubMed] [Google Scholar]
- Sinko P. J.; Martin A. N.. Martin’s Physical pharmacy and pharmaceutical sciences: physical chemical and biopharmaceutical principles in the pharmaceutical sciences, Eighth ed.; Wolters Kluwer Health Baltimore, 2023. [Google Scholar]
- Yousef M.; Le T. S.; Zuo J.; Park C.; Chacra N. B.; Davies N. M.; Löbenberg R. Sub-cellular sequestration of alkaline drugs in lysosomes: new insights for pharmaceutical development of lysosomal fluid. Res. Pharm. Sci. 2023, 18 (1), 1–15. 10.4103/1735-5362.363591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger M. B.; Macwan J. S.; Sarfraz M.; Almukainzi M.; Löbenberg R. The Irrelevance of In Vitro Dissolution in Setting Product Specifications for Drugs Like Dextromethorphan That are Subject to Lysosomal Trapping. J. Pharm. Sci. 2019, 108 (1), 268–278. 10.1016/j.xphs.2018.09.036. [DOI] [PubMed] [Google Scholar]
- Amaral Silva D.; Gomes Davanço M.; Davies N. M.; Krämer J.; de Oliveira Carvalho P.; Löbenberg R. Physiologically relevant dissolution conditions towards improved in vitro - in vivo relationship - A case study with enteric coated pantoprazole tablets. Int. J. Pharm. 2021, 605, 120857. 10.1016/j.ijpharm.2021.120857. [DOI] [PubMed] [Google Scholar]
- Amaral Silva D.; Davies N. M.; Doschak M. R.; Al-Gousous J.; Bou-Chacra N.; Löbenberg R. Mechanistic understanding of underperforming enteric coated products: Opportunities to add clinical relevance to the dissolution test. J. Controlled Release 2020, 325, 323–334. 10.1016/j.jconrel.2020.06.031. [DOI] [PubMed] [Google Scholar]
- Amaral Silva D.; Al-Gousous J.; Davies N. M.; Bou Chacra N.; Webster G. K.; Lipka E.; Amidon G. L.; Löbenberg R. Biphasic Dissolution as an Exploratory Method during Early Drug Product Development. Pharmaceutics 2020, 12 (5), 420. 10.3390/pharmaceutics12050420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef M.; Park C.; Henostroza M.; Bou Chacra N.; Davies N. M.; Löbenberg R. Development of a Novel In Vitro Model to Study Lymphatic Uptake of Drugs via Artificial Chylomicrons. Pharmaceutics 2023, 15 (11), 2532. 10.3390/pharmaceutics15112532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayem J.; Zhang Z.; Tomlinson A.; Zarraga I. E.; Wagner N. J.; Liu Y. Micellar Morphology of Polysorbate 20 and 80 and Their Ester Fractions in Solution via Small-Angle Neutron Scattering. J. Pharm. Sci. 2020, 109 (4), 1498–1508. 10.1016/j.xphs.2019.12.016. [DOI] [PubMed] [Google Scholar]
- Okazaki A.; Mano T.; Sugano K. Theoretical dissolution model of poly-disperse drug particles in biorelevant media. Journal of pharmaceutical sciences 2008, 97 (5), 1843–1852. 10.1002/jps.21070. [DOI] [PubMed] [Google Scholar]
- Hammouda B. Temperature Effect on the Nanostructure of SDS Micelles in Water. Journal of research of the National Institute of Standards and Technology 2013, 118, 151–167. 10.6028/jres.118.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S.; Catron N. D.; Zhu A. D.; Lipari J. M.; Wu J.; Gao Y.; Zhang G. G. Z. Predictive Modeling of Micellar Solubilization by Single and Mixed Nonionic Surfactants. J. Pharm. Sci. 2018, 107 (8), 2079–2090. 10.1016/j.xphs.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Leigh M. L. S.; Dos Santos V. R.. Biorelevant composition, WO2020201779A1, 2020.
- Gamsiz E. D.; Ashtikar M.; Crison J.; Woltosz W.; Bolger M. B.; Carrier R. L. Predicting the effect of fed-state intestinal contents on drug dissolution. Pharm. Res. 2010, 27 (12), 2646–2656. 10.1007/s11095-010-0264-x. [DOI] [PubMed] [Google Scholar]
- Pohl P.; Saparov S. M.; Antonenko Y. N. The Size of the Unstirred Layer as a Function of the Solute Diffusion Coefficient. Biophys. J. 1998, 75 (3), 1403–1409. 10.1016/S0006-3495(98)74058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepin X.; Goetschy M.; Abrahmsén-Alami S. Mechanistic models for USP2 dissolution apparatus, including fluid hydrodynamics and sedimentation. J. Pharm. Sci. 2022, 111, 185. 10.1016/j.xphs.2021.10.006. [DOI] [PubMed] [Google Scholar]
- Pepin X. J. H.; Hammarberg M.; Mattinson A.; Moir A. Physiologically Based Biopharmaceutics Model for Selumetinib Food Effect Investigation and Capsule Dissolution Safe Space - Part I: Adults. Pharm. Res. 2023, 40, 387. 10.1007/s11095-022-03339-2. [DOI] [PubMed] [Google Scholar]
- Scholz A.; Kostewicz E.; Abrahamsson B.; Dressman J. B. Can the USP paddle method be used to represent in-vivo hydrodynamics?. J. Pharm. Pharmacol 2010, 55 (4), 443–451. 10.1211/002235702946. [DOI] [PubMed] [Google Scholar]
- Skelbæk-Pedersen A. L.; Vilhelmsen T. K.; Wallaert V.; Rantanen J. Investigation of the effects of particle size on fragmentation during tableting. Int. J. Pharm. 2020, 576, 118985. 10.1016/j.ijpharm.2019.118985. [DOI] [PubMed] [Google Scholar]
- Skelbæk-Pedersen A. L.; Anuschek M.; Vilhelmsen T. K.; Rantanen J.; Zeitler J. A. Non-destructive quantification of fragmentation within tablets after compression from scattering analysis of terahertz transmission measurements. Int. J. Pharm. 2020, 588, 119769. 10.1016/j.ijpharm.2020.119769. [DOI] [PubMed] [Google Scholar]
- Skelbæk-Pedersen A.; Vilhelmsen T.; Wallaert V.; Rantanen J. Quantification of Fragmentation of Pharmaceutical Materials After Tableting. Journal of pharmaceutical sciences 2019, 108 (3), 1246–1253. 10.1016/j.xphs.2018.10.040. [DOI] [PubMed] [Google Scholar]
- Alderete N. M.; Villagran Zaccardi Y.; Coelho Dos Santos G. S.; De Belie N.. Particle size distribution and specific surface area of SCM’s compared through experimental techniques. In Int. RILEM Conference on Materials, Systems and Structures in Civil Engineering 2016-Segment on Concrete with Supplementary Cementitious Materials; RILEM, 2016; pp 61–70. [Google Scholar]
- de Albuquerque I.; Mazzotti M.; Ochsenbein D. R.; Morari M. Effect of needle-like crystal shape on measured particle size distributions. AIChE J. 2016, 62 (9), 2974–2985. 10.1002/aic.15270. [DOI] [Google Scholar]
- Kim S.; Bilgili E.; Davé R. N. Impact of altered hydrophobicity and reduced agglomeration on dissolution of micronized poorly water-soluble drug powders after dry coating. Int. J. Pharm. 2021, 606, 120853. 10.1016/j.ijpharm.2021.120853. [DOI] [PubMed] [Google Scholar]
- Lippold B. C.; Ohm A. Correlation between wettability and dissolution rate of pharmaceutical powders. Int. J. Pharm. 1986, 28 (1), 67–74. 10.1016/0378-5173(86)90148-1. [DOI] [Google Scholar]
- Li J.; Wang Z.; Zhang H.; Gao J.; Zheng A. Progress in the development of stabilization strategies for nanocrystal preparations. Drug Deliv 2021, 28 (1), 19–36. 10.1080/10717544.2020.1856224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokoumetzidis A.; Macheras P. A century of dissolution research: from Noyes and Whitney to the biopharmaceutics classification system. Int. J. Pharm. 2006, 321 (1–2), 1–11. 10.1016/j.ijpharm.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Hintz R.; Johnson K. The effect of particle size distribution on dissolution rate and oral absorption. Int. J. Pharm. 1989, 51 (1), 9–17. 10.1016/0378-5173(89)90069-0. [DOI] [Google Scholar]
- Hintz R. J.; Johnson K. C. The effect of particle size distribution on dissolution rate and oral absorption. Int. J. Pharm. 1989, 51 (1), 9–17. 10.1016/0378-5173(89)90069-0. [DOI] [Google Scholar]
- Wang J.; Flanagan D. R. General solution for diffusion-controlled dissolution of spherical particles. 1. Theory. J. Pharm. Sci. 1999, 88 (7), 731–738. 10.1021/js980236p. [DOI] [PubMed] [Google Scholar]
- Salehi N.; Al-Gousous J.; Mudie D. M.; Amidon G. L.; Ziff R. M.; Amidon G. E. Hierarchical Mass Transfer Analysis of Drug Particle Dissolution, Highlighting the Hydrodynamics, pH, Particle Size, and Buffer Effects for the Dissolution of Ionizable and Nonionizable Drugs in a Compendial Dissolution Vessel. Mol. Pharmaceutics 2020, 17 (10), 3870–3884. 10.1021/acs.molpharmaceut.0c00614. [DOI] [PubMed] [Google Scholar]
- Armenante P. M.; Kirwan D. J. Mass transfer to microparticles in agitated systems. Chem. Eng. Sci. 1989, 44 (12), 2781–2796. 10.1016/0009-2509(89)85088-2. [DOI] [Google Scholar]
- Sugano K. Aqueous Boundary Layers Related to Oral Absorption of a Drug: From Dissolution of a Drug to Carrier Mediated Transport and Intestinal Wall Metabolism. Mol. Pharmaceutics 2010, 7 (5), 1362–1373. 10.1021/mp1001119. [DOI] [PubMed] [Google Scholar]
- Pal A.; Indireshkumar K.; Schwizer W.; Abrahamsson B.; Fried M.; Brasseur J. G. Gastric flow and mixing studied using computer simulation. Proc. Biol. Sci. 2004, 271 (1557), 2587–2594. 10.1098/rspb.2004.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitschies W.; Kosch O.; Monnikes H.; Trahms L. Magnetic Marker Monitoring: An application of biomagnetic measurement instrumentation and principles for the determination of the gastrointestinal behavior of magnetically marked solid dosage forms. Adv. Drug Deliv Rev. 2005, 57 (8), 1210–1222. 10.1016/j.addr.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Worsøe J.; Fynne L.; Gregersen T.; Schlageter V.; Christensen L. A; Dahlerup J. F; Rijkhoff N. J.; Laurberg S.; Krogh K. Gastric transit and small intestinal transit time and motility assessed by a magnet tracking system. BMC Gastroenterol 2011, 11, 145. 10.1186/1471-230X-11-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi W. I.; Parrott E. L.; Wurster D. E.; Higuchi T. Investigation of Drug Release from Solids II. Theoretical and Experimental Study of Influences of Bases and Buffers on Rates of Dissolution of Acidic Solids. journal of the American Pharmaceutical Association. American Pharmaceutical Association 1958, 47 (5), 376–383. 10.1002/jps.3030470522. [DOI] [PubMed] [Google Scholar]
- Higuchi W. I.; Nelson E.; Wagner J. G. Solubility and Dissolution Rates in Reactive Media. journal of Pharmaceutical Sciences 1964, 53 (3), 333–335. 10.1002/jps.2600530321. [DOI] [PubMed] [Google Scholar]
- Mooney K. G.; Mintun M. A.; Himmelstein K. J.; Stella V. J. Dissolution Kinetics of Carboxylic Acids I: Effect of pH Under Unbuffered Conditions. J. Pharm. Sci. 1981, 70 (1), 13–22. 10.1002/jps.2600700103. [DOI] [PubMed] [Google Scholar]
- Mooney K. G.; Mintun M. A.; Himmelstein K. J.; Stella V. J. Dissolution Kinetics of Carboxylic Acids II: Effect of Buffers. J. Pharm. Sci. 1981, 70 (1), 22–32. 10.1002/jps.2600700104. [DOI] [PubMed] [Google Scholar]
- Serajuddin A. T. M.; Jarowski C. I. Effect of Diffusion Layer pH and Solubility on the Dissolution Rate of Pharmaceutical Bases and their Hydrochloride Salts I: Phenazopyridine. J. Pharm. Sci. 1985, 74 (2), 142–147. 10.1002/jps.2600740208. [DOI] [PubMed] [Google Scholar]
- Serajuddin A. T. M.; Jarowski C. I. Effect of Diffusion Layer pH and Solubility on the Dissolution Rate of Pharmaceutical Acids and Their Sodium Salts II: Salicylic Acid, Theophylline, and Benzoic Acid. J. Pharm. Sci. 1985, 74 (2), 148–154. 10.1002/jps.2600740209. [DOI] [PubMed] [Google Scholar]
- Cristofoletti R.; Patel N.; Dressman J. B. Assessment of Bioequivalence of Weak Base Formulations Under Various Dosing Conditions Using Physiologically Based Pharmacokinetic Simulations in Virtual Populations. Case Examples: Ketoconazole and Posaconazole. J. Pharm. Sci. 2017, 106 (2), 560–569. 10.1016/j.xphs.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Hens B.; Seegobin N.; Bermejo M.; Tsume Y.; Clear N.; McAllister M.; Amidon G. E.; Amidon G. L. Dissolution Challenges Associated with the Surface pH of Drug Particles: Integration into Mechanistic Oral Absorption Modeling. AAPS Journal 2022, 24 (1), 17. 10.1208/s12248-021-00663-0. [DOI] [PubMed] [Google Scholar]
- Badawy S. I. F.; Hussain M. A. Microenvironmental pH modulation in solid dosage forms. J. Pharm. Sci. 2007, 96 (5), 948–959. 10.1002/jps.20932. [DOI] [PubMed] [Google Scholar]
- Pudipeddi M.; Zannou E. A.; Vasanthavada M.; Dontabhaktuni A.; Royce A. E.; Joshi Y. M.; Serajuddin A. T. M. Measurement of Surface pH of Pharmaceutical Solids: A Critical Evaluation of Indicator Dye-Sorption Method and its Comparison With Slurry pH Method. J. Pharm. Sci. 2008, 97 (5), 1831–1842. 10.1002/jps.21052. [DOI] [PubMed] [Google Scholar]
- John C. T.; Xu W.; Lupton L. K.; Harmon P. A. Formulating weakly basic HCl salts: relative ability of common excipients to induce disproportionation and the unique deleterious effects of magnesium stearate. Pharm. Res. 2013, 30 (6), 1628–1641. 10.1007/s11095-013-1002-y. [DOI] [PubMed] [Google Scholar]
- Shishoo C. J.; Shah S. A.; Rathod I. S.; Savale S. S.; Kotecha J. S.; Shah P. B. Stability of rifampicin in dissolution medium in presence of isoniazid. Int. J. Pharm. 1999, 190 (1), 109–123. 10.1016/S0378-5173(99)00286-0. [DOI] [PubMed] [Google Scholar]
- Bhangare D.; Rajput N.; Jadav T.; Sahu A. K.; Tekade R. K.; Sengupta P. Systematic strategies for degradation kinetic study of pharmaceuticals: an issue of utmost importance concerning current stability analysis practices. Journal of Analytical Science and Technology 2022, 13 (1), 7. 10.1186/s40543-022-00317-6. [DOI] [Google Scholar]
- Vinarov Z.; Tistaert C.; Bevernage J.; Bohets H.; Augustijns P. Enzymatic prodrug degradation in the fasted and fed small intestine: In vitro studies and interindividual variability in human aspirates. Int. J. Pharm. 2024, 649, 123654. 10.1016/j.ijpharm.2023.123654. [DOI] [PubMed] [Google Scholar]
- Dharani S.; Barakh Ali S. F.; Afrooz H.; Khan M. A.; Rahman Z. Development and Validation of a Discriminatory Dissolution Method for Rifaximin Products. J. Pharm. Sci. 2019, 108 (6), 2112–2118. 10.1016/j.xphs.2019.01.020. [DOI] [PubMed] [Google Scholar]
- Badawy S. I.; Gray D. B.; Zhao F.; Sun D.; Schuster A. E.; Hussain M. A. Formulation of solid dosage forms to overcome gastric pH interaction of the factor Xa inhibitor, BMS-561389. Pharm. Res. 2006, 23 (5), 989–996. 10.1007/s11095-006-9899-z. [DOI] [PubMed] [Google Scholar]
- Hawley M.; Morozowich W. Modifying the Diffusion Layer of Soluble Salts of Poorly Soluble Basic Drugs To Improve Dissolution Performance. Mol. Pharmaceutics 2010, 7 (5), 1441–1449. 10.1021/mp100117k. [DOI] [PubMed] [Google Scholar]
- Guo M.; Wang K.; Qiao N.; Fábián L.; Sadiq G.; Li M. Insight into Flufenamic Acid Cocrystal Dissolution in the Presence of a Polymer in Solution: from Single Crystal to Powder Dissolution. Mol. Pharmaceutics 2017, 14 (12), 4583–4596. 10.1021/acs.molpharmaceut.7b00712. [DOI] [PubMed] [Google Scholar]
- Deshpande T. M.; Shi H.; Pietryka J.; Hoag S. W.; Medek A. Investigation of Polymer/Surfactant Interactions and Their Impact on Itraconazole Solubility and Precipitation Kinetics for Developing Spray-Dried Amorphous Solid Dispersions. Mol. Pharmaceutics 2018, 15 (3), 962–974. 10.1021/acs.molpharmaceut.7b00902. [DOI] [PubMed] [Google Scholar]
- Chiwele I.; Jones B. E.; Podczeck F. The shell dissolution of various empty hard capsules. Chem. Pharm. Bull. (Tokyo) 2000, 48 (7), 951–956. 10.1248/cpb.48.951. [DOI] [PubMed] [Google Scholar]
- Weigert G. G.; Ineu A. P.; Gomes P. Evaluation of hard gelatin capsules and hydroxypropyl methylcellulose containing ampicillin. Química Nova 2012, 35, 286. 10.1590/S0100-40422012000200010. [DOI] [Google Scholar]
- Radwan A.; Amidon G. L.; Langguth P. Mechanistic investigation of food effect on disintegration and dissolution of BCS class III compound solid formulations: the importance of viscosity: MECHANISM OF FOOD EFFECT FOR BCS CLASS III PRODUCT. Biopharmaceutics & Drug Disposition 2012, 33 (7), 403–416. 10.1002/bdd.1798. [DOI] [PubMed] [Google Scholar]
- Markl D.; Zeitler J. A. A Review of Disintegration Mechanisms and Measurement Techniques. Pharm. Res. 2017, 34 (5), 890–917. 10.1007/s11095-017-2129-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rump A.; Weiss F. N.; Schulz L.; Kromrey M.-L.; Scheuch E.; Tzvetkov M. V.; White T.; Durkee S.; Judge K. W.; Jannin V.; et al. The Effect of Capsule-in-Capsule Combinations on In Vivo Disintegration in Human Volunteers: A Combined Imaging and Salivary Tracer Study. Pharmaceutics 2021, 13 (12), 2002. 10.3390/pharmaceutics13122002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholt F.; Di Meo C.; Sloth S.; Müllertz A.; Berthelsen R. Direct visualizing of paracetamol immediate release tablet disintegration in vivo and in vitro. Eur. J. Pharm. Biopharm. 2022, 180, 63–70. 10.1016/j.ejpb.2022.09.007. [DOI] [PubMed] [Google Scholar]
- Steinberg W. H.; Frey G. H.; Masci J. N.; Hutchins H. H. Method for determining in vivo tablet disintegration. J. Pharm. Sci. 1965, 54 (5), 747–752. 10.1002/jps.2600540518. [DOI] [PubMed] [Google Scholar]
- Kelly K.; O’Mahony B.; Lindsay B.; Jones T.; Grattan T. J.; Rostami-Hodjegan A.; Stevens H. N. E.; Wilson C. G. Comparison of the Rates of Disintegration, Gastric Emptying, and Drug Absorption Following Administration of a New and a Conventional Paracetamol Formulation, Using γ Scintigraphy. Pharm. Res. 2003, 20 (10), 1668–1673. 10.1023/A:1026155822121. [DOI] [PubMed] [Google Scholar]
- Pepin X.Use of IVIVc and IVIVe to support formulation development - Industrial case studies. In AAPS meeting - 4th November 2014, San Diego, USA, 2014.
- Shakespeare W.The tragedy of Hamlet, Prince of Denmark; The Folio Society: London, 1954; p 1600. [Google Scholar]
- Pepin X.; Dressman J.; Parrott N.; Delvadia P.; Mitra A.; Zhang X.; Babiskin A.; Kolhatkar V.; Seo P.; Taylor L.; et al. In Vitro Biopredictive Methods: A Workshop Summary Report. Journal of pharmaceutical sciences 2021, 110, 567–583. 10.1016/j.xphs.2020.09.021. [DOI] [PubMed] [Google Scholar]
- Taylor L. S.; Zhang G. G. Z. Physical chemistry of supersaturated solutions and implications for oral absorption. Adv. Drug Delivery Rev. 2016, 101, 122–142. 10.1016/j.addr.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Lee A. Y.; Erdemir D.; Myerson A. S.. Crystals and Crystal Growth. In Handbook of Industrial Crystallization, 3 ed.; Lee A. Y., Myerson A. S., Erdemir D., Eds.; Cambridge University Press, 2019; pp 32–75. [Google Scholar]
- Moffitt Schall J.; Myerson A. S.. Solutions and Solution Properties. In Handbook of Industrial Crystallization, 3 ed.; Lee A. Y., Myerson A. S., Erdemir D., Eds.; Cambridge University Press, 2019; pp 1–31. [Google Scholar]
- O’Dwyer P. J.; Litou C.; Box K. J.; Dressman J. B.; Kostewicz E. S.; Kuentz M.; Reppas C. In vitro methods to assess drug precipitation in the fasted small intestine - a PEARRL review. J. Pharm. Pharmacol. 2019, 71, 536–556. 10.1111/jphp.12951. [DOI] [PubMed] [Google Scholar]
- Kostewicz E. S.; Wunderlich M.; Brauns U.; Becker R.; Bock T.; Dressman J. B. Predicting the precipitation of poorly soluble weak bases upon entry in the small intestine. J. Pharm. Pharmacol 2010, 56 (1), 43–51. 10.1211/0022357022511. [DOI] [PubMed] [Google Scholar]
- Jede C.; Wagner C.; Kubas H.; Weber C.; Weitschies W. In-line derivative spectroscopy as a promising application to a small-scale in vitro transfer model in biorelevant supersaturation and precipitation testing. J. Pharm. Pharmacol 2018, 70 (10), 1315–1323. 10.1111/jphp.12991. [DOI] [PubMed] [Google Scholar]
- Krollik K.; Lehmann A.; Wagner C.; Kaidas J.; Bülhoff J.; Kubas H.; Weitschies W. Increasing the Robustness of Biopharmaceutical Precipitation Assays - Part II: Recommendations on the use of FaSSIF. J. Pharm. Sci. 2022, 111 (1), 155–163. 10.1016/j.xphs.2021.08.026. [DOI] [PubMed] [Google Scholar]
- Lehmann A.; Krollik K.; Beran K.; Hirtreiter C.; Kubas H.; Wagner C. Increasing the Robustness of Biopharmaceutical Precipitation Assays - Part I: Derivative UV Spectrophotometric Method Development for in-line Measurements. J. Pharm. Sci. 2022, 111 (1), 146–154. 10.1016/j.xphs.2021.08.025. [DOI] [PubMed] [Google Scholar]
- Jede C.; Wagner C.; Kubas H.; Weber C.; Weigandt M.; Koziolek M.; Weitschies W. Automated small-scale in vitro transfer model as screening tool for the prediction of in vivo-dissolution and precipitation of poorly solubles. International journal of pharmaceutics 2019, 556, 150–158. 10.1016/j.ijpharm.2018.12.013. [DOI] [PubMed] [Google Scholar]
- O’Dwyer P. J.; Imanidis G.; Box K. J.; Reppas C. On the Usefulness of Two Small-Scale In Vitro Setups in the Evaluation of Luminal Precipitation of Lipophilic Weak Bases in Early Formulation Development. Pharmaceutics 2020, 12 (3), 272. 10.3390/pharmaceutics12030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S.; Buchanan N. L.; Buchanan C. M. Miniaturized Transfer Models to Predict the Precipitation of Poorly Soluble Weak Bases upon Entry into the Small Intestine. AAPS PharmSciTech 2012, 13 (4), 1230–1235. 10.1208/s12249-012-9851-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourentas A.; Vertzoni M.; Barmpatsalou V.; Augustijns P.; Beato S.; Butler J.; Holm R.; Ouwerkerk N.; Rosenberg J.; Tajiri T. The BioGIT System: a Valuable In Vitro Tool to Assess the Impact of Dose and Formulation on Early Exposure to Low Solubility Drugs After Oral Administration. AAPS Journal 2018, 20 (4), 71. 10.1208/s12248-018-0231-8. [DOI] [PubMed] [Google Scholar]
- Berlin M.; Przyklenk K. H.; Richtberg A.; Baumann W.; Dressman J. B. Prediction of oral absorption of cinnarizine-a highly supersaturating poorly soluble weak base with borderline permeability. Eur. J. Pharm. Biopharm 2014, 88 (3), 795–806. 10.1016/j.ejpb.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Fiolka T.; Dressman J. Development, current applications and future roles of biorelevant two-stage in vitro testing in drug development. J. Pharm. Pharmacol 2018, 70 (3), 335–348. 10.1111/jphp.12875. [DOI] [PubMed] [Google Scholar]
- Annisa V.; Teuku Nanda Saifullah S.; Agung Endro N. Biorelevant dissolution models to assess precipitation of weak base drug. International Journal of Research in Pharmaceutical Sciences 2020, 11 (SPL4), 2165–2172. 10.26452/ijrps.v11iSPL4.4438. [DOI] [Google Scholar]
- Jede C.; Henze L. J.; Meiners K.; Bogdahn M.; Wedel M.; van Axel V. Development and Application of a Dissolution-Transfer-Partitioning System (DTPS) for Biopharmaceutical Drug Characterization. Pharmaceutics 2023, 15 (4), 1069. 10.3390/pharmaceutics15041069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostantini C.; Spilioti E.; Bevernage J.; Ceulemans J.; Hansmann S.; Hellemans K.; Jede C.; Kourentas A.; Reggane M.; Shah L.; et al. Usefulness of the BioGIT system in screening for differences in early exposure in the fasted state on an a priori basis. Int. J. Pharm. 2023, 634, 122670. 10.1016/j.ijpharm.2023.122670. [DOI] [PubMed] [Google Scholar]
- Kostantini C.; Spilioti E.; Bevernage J.; Ceulemans J.; Hansmann S.; Hellemans K.; Jede C.; Kourentas A.; Reggane M.; Shah L.; et al. Screening for Differences in Early Exposure in the Fasted State with in Vitro Methodologies can be Challenging: Experience with the BioGIT System. J. Pharm. Sci. 2023, 112 (8), 2240–2248. 10.1016/j.xphs.2023.03.004. [DOI] [PubMed] [Google Scholar]
- Jede C.; Wagner C.; Kubas H.; Weber C.; Weigandt M.; Koziolek M.; Weitschies W. Application of an automated small-scale in vitro transfer model to predict in vivo precipitation inhibition. Int. J. Pharm. 2019, 565, 458–471. 10.1016/j.ijpharm.2019.05.028. [DOI] [PubMed] [Google Scholar]
- Hens B.; Brouwers J.; Corsetti M.; Augustijns P. Supersaturation and Precipitation of Posaconazole upon Entry in the Upper Small Intestine in Humans. J. Pharm. Sci. 2016, 105 (9), 2677. 10.1002/jps.24690. [DOI] [PubMed] [Google Scholar]
- Rubbens J.; Brouwers J.; Tack J.; Augustijns P. Gastrointestinal dissolution, supersaturation and precipitation of the weak base indinavir in healthy volunteers. Eur. J. Pharm. Biopharm. 2016, 109, 122–129. 10.1016/j.ejpb.2016.09.014. [DOI] [PubMed] [Google Scholar]
- Kambayashi A.; Yasuji T.; Dressman J. B. Prediction of the precipitation profiles of weak base drugs in the small intestine using a simplified transfer (″dumping″) model coupled with in silico modeling and simulation approach. Eur. J. Pharm. Biopharm 2016, 103, 95–103. 10.1016/j.ejpb.2016.03.020. [DOI] [PubMed] [Google Scholar]
- Wagner C.; Jantratid E.; Kesisoglou F.; Vertzoni M.; Reppas C.; Dressman J. B. Predicting the oral absorption of a poorly soluble, poorly permeable weak base using biorelevant dissolution and transfer model tests coupled with a physiologically based pharmacokinetic model. Eur. J. Pharm. Biopharm 2012, 82 (1), 127–138. 10.1016/j.ejpb.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Carlert S.; Palsson A.; Hanisch G.; von Corswant C.; Nilsson C.; Lindfors L.; Lennernas H.; Abrahamsson B. Predicting intestinal precipitation-a case example for a basic BCS class II drug. Pharm. Res. 2010, 27 (10), 2119–2130. 10.1007/s11095-010-0213-8. [DOI] [PubMed] [Google Scholar]
- Franco Y. L.; Da Silva L.; Charbe N.; Kinvig H.; Kim S.; Cristofoletti R. Integrating Forward and Reverse Translation in PBPK Modeling to Predict Food Effect on Oral Absorption of Weakly Basic Drugs. Pharm. Res. 2023, 40 (2), 405–418. 10.1007/s11095-023-03478-0. [DOI] [PubMed] [Google Scholar]
- Hens B.; Pathak S. M.; Mitra A.; Patel N.; Liu B.; Patel S.; Jamei M.; Brouwers J.; Augustijns P.; Turner D. B. In Silico Modeling Approach for the Evaluation of Gastrointestinal Dissolution, Supersaturation, and Precipitation of Posaconazole. Mol. Pharmaceutics 2017, 14 (12), 4321–4333. 10.1021/acs.molpharmaceut.7b00396. [DOI] [PubMed] [Google Scholar]
- Hens B.; Bolger M. B. Application of a Dynamic Fluid and pH Model to Simulate Intraluminal and Systemic Concentrations of a Weak Base in GastroPlus. J. Pharm. Sci. 2019, 108 (1), 305–315. 10.1016/j.xphs.2018.10.041. [DOI] [PubMed] [Google Scholar]
- Kesharwani S. S.; Ibrahim F. A Combined In-Vitro and GastroPlus® Modeling to Study the Effect of Intestinal Precipitation on Cinnarizine Plasma Profile in a Fasted State. AAPS PharmSciTech 2023, 24 (5), 121. 10.1208/s12249-023-02577-w. [DOI] [PubMed] [Google Scholar]
- Matsumura N.; Ono A.; Akiyama Y.; Fujita T.; Sugano K. Bottom-Up Physiologically Based Oral Absorption Modeling of Free Weak Base Drugs. Pharmaceutics 2020, 12 (9), 844. 10.3390/pharmaceutics12090844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statelova M.; Vertzoni M.; Kourentas A. Simulation of Intraluminal Performance of Lipophilic Weak Bases in Fasted Healthy Adults Using DDDPlus(TM). Aaps j 2022, 24 (5), 89. 10.1208/s12248-022-00737-7. [DOI] [PubMed] [Google Scholar]
- Jamei M.; Turner D.; Yang J.; Neuhoff S.; Polak S.; Rostami-Hodjegan A.; Tucker G. Population-Based Mechanistic Prediction of Oral Drug Absorption. AAPS Journal 2009, 11 (2), 225–237. 10.1208/s12248-009-9099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustijns P.; Vertzoni M.; Reppas C.; Langguth P.; Lennernäs H.; Abrahamsson B.; Hasler W. L.; Baker J. R.; Vanuytsel T.; Tack J.; et al. Unraveling the behavior of oral drug products inside the human gastrointestinal tract using the aspiration technique: History, methodology and applications. European Journal of Pharmaceutical Sciences 2020, 155, 105517. 10.1016/j.ejps.2020.105517. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Wang S.; Wang S.; Liu C.; Su C.; Hageman M.; Hussain M.; Haskell R.; Stefanski K.; Qian F. Sodium Lauryl Sulfate Competitively Interacts with HPMC-AS and Consequently Reduces Oral Bioavailability of Posaconazole/HPMC-AS Amorphous Solid Dispersion. Mol. Pharmaceutics 2016, 13 (8), 2787–2795. 10.1021/acs.molpharmaceut.6b00391. [DOI] [PubMed] [Google Scholar]
- Holzem F. L.; Weck A.; Schaffland J. P.; Stillhart C.; Klein S.; Bauer-Brandl A.; Brandl M. Biopredictive capability assessment of two dissolution/permeation assays, μFLUX and PermeaLoop, using supersaturating formulations of Posaconazole. European Journal of Pharmaceutical Sciences 2022, 176, 106260. 10.1016/j.ejps.2022.106260. [DOI] [PubMed] [Google Scholar]
- Arora S.; Pansari A.; Kilford P. J.; Jamei M.; Turner D. B.; Gardner I. A Mechanistic Absorption and Disposition Model of Ritonavir to Predict Exposure and Drug-Drug Interaction Potential of CYP3A4/5 and CYP2D6 Substrates. European Journal of Drug Metabolism and Pharmacokinetics 2022, 47 (4), 483–495. 10.1007/s13318-022-00765-w. [DOI] [PubMed] [Google Scholar]
- Larfors G.; Andersson P.; Jesson G.; Liljebris C.; Brisander M.; Lennernäs H.; Stenke L. Despite warnings, co-medication with proton pump inhibitors and dasatinib is common in chronic myeloid leukemia, but XS004, a novel oral dasatinib formulation, provides reduced pH-dependence, minimizing undesirable drug-drug interactions. Eur. J. Haematol 2023, 111 (4), 644–654. 10.1111/ejh.14059. [DOI] [PubMed] [Google Scholar]
- Sharma S.; Pepin X.; Burri H.; Zheng L.; Kuptsova-Clarkson N.; de Jong A.; Yu T.; MacArthur H. L.; Majewski M.; Byrd J. C. Bioequivalence and Relative Bioavailability Studies to Assess a New Acalabrutinib Formulation That Enables Coadministration With Proton-Pump Inhibitors. Clinical pharmacology in drug development 2022, 11 (11), 1294. 10.1002/cpdd.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipot C. Predictions from First-Principles of Membrane Permeability to Small Molecules: How Useful Are They in Practice?. J. Chem. Inf. Model. 2023, 63 (15), 4533–4544. 10.1021/acs.jcim.3c00686. [DOI] [PubMed] [Google Scholar]
- Kola I.; Landis J. Can the pharmaceutical industry reduce attrition rates?. Nat. Rev. Drug Discovery 2004, 3 (8), 711–716. 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- Lipinski C. A.; Lombardo F.; Dominy B. W.; Feeney P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv Rev. 2001, 46 (1–3), 3–26. 10.1016/S0169-409X(00)00129-0. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Tandon I.; Heelan W.; Wang Y.; Tang W.; Hu Q. Proteolysis-targeting chimera (PROTAC) delivery system: advancing protein degraders towards clinical translation. Chem. Soc. Rev. 2022, 51 (13), 5330–5350. 10.1039/D1CS00762A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGoey D. A.; Chen H.-J.; Cox P. B.; Wendt M. D. Beyond the Rule of 5: Lessons Learned from AbbVie’s Drugs and Compound Collection. J. Med. Chem. 2018, 61 (7), 2636–2651. 10.1021/acs.jmedchem.7b00717. [DOI] [PubMed] [Google Scholar]
- O’Donovan D. H.; De Fusco C.; Kuhnke L.; Reichel A. Trends in Molecular Properties, Bioavailability, and Permeability across the Bayer Compound Collection. J. Med. Chem. 2023, 66 (4), 2347–2360. 10.1021/acs.jmedchem.2c01577. [DOI] [PubMed] [Google Scholar]
- Hornberger K. R.; Araujo E. M. V. Physicochemical Property Determinants of Oral Absorption for PROTAC Protein Degraders. J. Med. Chem. 2023, 66 (12), 8281–8287. 10.1021/acs.jmedchem.3c00740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannergren C.; Bergendal A.; Lennernäs H.; Abrahamsson B. Toward an Increased Understanding of the Barriers to Colonic Drug Absorption in Humans: Implications for Early Controlled Release Candidate Assessment. Mol. Pharmaceutics 2009, 6 (1), 60–73. 10.1021/mp800261a. [DOI] [PubMed] [Google Scholar]
- Sjöberg A.; Lutz M.; Tannergren C.; Wingolf C.; Borde A.; Ungell A.-L. Comprehensive study on regional human intestinal permeability and prediction of fraction absorbed of drugs using the Ussing chamber technique. European Journal of Pharmaceutical Sciences 2013, 48 (1–2), 166–180. 10.1016/j.ejps.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Dahlgren D.; Roos C.; Lundqvist A.; Abrahamsson B.; Tannergren C.; Hellstrom P. M.; Sjogren E.; Lennernas H. Regional Intestinal Permeability of Three Model Drugs in Human. Mol. Pharmaceutics 2016, 13 (9), 3013–3021. 10.1021/acs.molpharmaceut.6b00514. [DOI] [PubMed] [Google Scholar]
- Dahlgren D.; Roos C.; Sjögren E.; Lennernäs H. Direct In Vivo Human Intestinal Permeability (Peff) Determined with Different Clinical Perfusion and Intubation Methods. J. Pharm. Sci. 2015, 104 (9), 2702–2726. 10.1002/jps.24258. [DOI] [PubMed] [Google Scholar]
- Steinert R. E.; Feinle-Bisset C.; Asarian L.; Horowitz M.; Beglinger C.; Geary N. Ghrelin, CCK, GLP-1, and PYY(3–36): Secretory Controls and Physiological Roles in Eating and Glycemia in Health, Obesity, and After RYGB. Physiol Rev. 2017, 97 (1), 411–463. 10.1152/physrev.00031.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoultz I.; Keita A. V. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells 2020, 9 (8), 1909. 10.3390/cells9081909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K.; Kawai S.; Fujii E.; Yano M.; Miyayama T.; Nakano K.; Terao K.; Suzuki M. Development of rat duodenal monolayer model with effective barrier function from rat organoids for ADME assay. Sci. Rep. 2023, 13 (1), 12130. 10.1038/s41598-023-39425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creff J.; Malaquin L.; Besson A. In vitro models of intestinal epithelium: Toward bioengineered systems. J. Tissue Eng. 2021, 12, 204173142098520. 10.1177/2041731420985202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennernäs H.; Lee I. D.; Fagerholm U.; Amidon G. L. A residence-time distribution analysis of the hydrodynamics within the intestine in man during a regional single-pass perfusion with Loc-I-Gut: in-vivo permeability estimation. J. Pharm. Pharmacol 2011, 49 (7), 682–686. 10.1111/j.2042-7158.1997.tb06092.x. [DOI] [PubMed] [Google Scholar]
- Roos C.; Westergren J.; Dahlgren D.; Lennernäs H.; Sjögren E. Mechanistic modelling of intestinal drug absorption-The in vivo effects of nanoparticles, hydrodynamics, and colloidal structures. Eur. J. Pharm. Biopharm. 2018, 133, 70–76. 10.1016/j.ejpb.2018.10.006. [DOI] [PubMed] [Google Scholar]
- Sugano K. Lost in modelling and simulation?. ADMET and DMPK 2021, 9 (2), 75–109. 10.5599/admet.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano K.; Terada K. Rate- and Extent-Limiting Factors of Oral Drug Absorption: Theory and Applications. J. Pharm. Sci. 2015, 104 (9), 2777–2788. 10.1002/jps.24391. [DOI] [PubMed] [Google Scholar]
- Tannergren C.; Jadhav H.; Eckernäs E.; Fagerberg J.; Augustijns P.; Sjögren E. Physiologically based biopharmaceutics modeling of regional and colon absorption in humans. Eur. J. Pharm. Biopharm 2023, 186, 144–159. 10.1016/j.ejpb.2023.03.013. [DOI] [PubMed] [Google Scholar]
- Chen M. L.; Amidon G. L.; Benet L. Z.; Lennernas H.; Yu L. X. The BCS, BDDCS, and regulatory guidances. Pharm. Res. 2011, 28 (7), 1774–1778. 10.1007/s11095-011-0438-1. [DOI] [PubMed] [Google Scholar]
- Lennernäs H.; Ahrenstedt O.; Hällgren R.; Knutson L.; Ryde M.; Paalzow L. K. Regional jejunal perfusion, a new in vivo approach to study oral drug absorption in man. Pharm. Res. 1992, 9 (10), 1243–1251. 10.1023/A:1015888813741. [DOI] [PubMed] [Google Scholar]
- Mehta M.; Polli J. E.; Seo P.; Bhoopathy S.; Berginc K.; Kristan K.; Cook J.; Dressman J. B.; Mandula H.; Munshi U.; et al. Drug Permeability - Best Practices for Biopharmaceutics Classification System (BCS)-Based Biowaivers: A workshop Summary Report. J. Pharm. Sci. 2023, 112 (7), 1749–1762. 10.1016/j.xphs.2023.04.016. [DOI] [PubMed] [Google Scholar]
- Roffey S. J.; Obach R. S.; Gedge J. I.; Smith D. A. What is the objective of the mass balance study? A retrospective analysis of data in animal and human excretion studies employing radiolabeled drugs. Drug Metab Rev. 2007, 39 (1), 17–43. 10.1080/03602530600952172. [DOI] [PubMed] [Google Scholar]
- Dubbelboer I. R.; Dahlgren D.; Sjögren E.; Lennernäs H. Rat intestinal drug permeability: A status report and summary of repeated determinations. Eur. J. Pharm. Biopharm 2019, 142, 364–376. 10.1016/j.ejpb.2019.07.005. [DOI] [PubMed] [Google Scholar]
- Cao X.; Gibbs S. T.; Fang L.; Miller H. A.; Landowski C. P.; Shin H.-C.; Lennernas H.; Zhong Y.; Amidon G. L.; Yu L. X.; et al. Why is it Challenging to Predict Intestinal Drug Absorption and Oral Bioavailability in Human Using Rat Model. Pharm. Res. 2006, 23 (8), 1675–1686. 10.1007/s11095-006-9041-2. [DOI] [PubMed] [Google Scholar]
- Fagerholm U.; Johansson M.; Lennernäs H. Comparison between permeability coefficients in rat and human jejunum. Pharm. Res. 1996, 13 (9), 1336–1342. 10.1023/A:1016065715308. [DOI] [PubMed] [Google Scholar]
- Helander H. F.; Fändriks L. Surface area of the digestive tract - revisited. Scand J. Gastroenterol 2014, 49 (6), 681–689. 10.3109/00365521.2014.898326. [DOI] [PubMed] [Google Scholar]
- Avdeef A. Transport Model. Absorption and Drug Development 2012, 12–30. 10.1002/9781118286067.ch2. [DOI] [Google Scholar]
- Dobson P. D.; Kell D. B. Carrier-mediated cellular uptake of pharmaceutical drugs: an exception or the rule?. Nat. Rev. Drug Discovery 2008, 7 (3), 205–220. 10.1038/nrd2438. [DOI] [PubMed] [Google Scholar]
- Di L.; Artursson P.; Avdeef A.; Benet L. Z.; Houston J. B.; Kansy M.; Kerns E. H.; Lennernäs H.; Smith D. A.; Sugano K. The Critical Role of Passive Permeability in Designing Successful Drugs. ChemMedChem. 2020, 15 (20), 1862–1874. 10.1002/cmdc.202000419. [DOI] [PubMed] [Google Scholar]
- Han H. K.; Oh D. M.; Amidon G. L. Cellular uptake mechanism of amino acid ester prodrugs in Caco-2/hPEPT1 cells overexpressing a human peptide transporter. Pharm. Res. 1998, 15 (9), 1382–1386. 10.1023/A:1011945420235. [DOI] [PubMed] [Google Scholar]
- Lennernäs H.; Nilsson D.; Aquilonius S. M.; Ahrenstedt O.; Knutson L.; Paalzow L. K. The effect of L-leucine on the absorption of levodopa, studied by regional jejunal perfusion in man. Br. J. Clin. Pharmacol. 1993, 35 (3), 243–250. 10.1111/j.1365-2125.1993.tb05691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDER In Vitro Drug Interaction Studies — Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions Guidance for Industry; CDER, 2020. [Google Scholar]
- EMA . ICH M9 guideline on biopharmaceutics classification system-based biowaivers; 2020. [DOI] [PubMed] [Google Scholar]
- Chu J.; Panfen E.; Wang L.; Marino A.; Chen X. Q.; Fancher R. M.; Landage R.; Patil O.; Desai S. D.; Shah D.; et al. Evaluation of Encequidar as An Intestinal P-gp and BCRP Specific Inhibitor to Assess the Role of Intestinal P-gp and BCRP in Drug-Drug Interactions. Pharm. Res. 2023, 40, 2567. 10.1007/s11095-023-03563-4. [DOI] [PubMed] [Google Scholar]
- Kono Y.; Kawahara I.; Shinozaki K.; Nomura I.; Marutani H.; Yamamoto A.; Fujita T. Characterization of P-Glycoprotein Inhibitors for Evaluating the Effect of P-Glycoprotein on the Intestinal Absorption of Drugs. Pharmaceutics 2021, 13 (3), 388. 10.3390/pharmaceutics13030388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. S.; Stead D.; Barlaam B.; Breed J.; Carbajo R. J.; Chiarparin E.; Cureton N.; Davey P. R. J.; Fisher D. I.; Gangl E. T.; et al. Discovery of a Potent and Orally Bioavailable Zwitterionic Series of Selective Estrogen Receptor Degrader-Antagonists. J. Med. Chem. 2023, 66 (4), 2918–2945. 10.1021/acs.jmedchem.2c01964. [DOI] [PubMed] [Google Scholar]
- Fricker G.; Drewe J.; Huwyler J.; Gutmann H.; Beglinger C. Relevance of p-glycoprotein for the enteral absorption of cyclosporin A: in vitro-in vivo correlation. Br. J. Pharmacol. 1996, 118 (7), 1841–1847. 10.1111/j.1476-5381.1996.tb15612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren D.; Sjöblom M.; Lennernäs H. Intestinal absorption-modifying excipients: A current update on preclinical in vivo evaluations. Eur. J. Pharm. Biopharm 2019, 142, 411–420. 10.1016/j.ejpb.2019.07.013. [DOI] [PubMed] [Google Scholar]
- Dahlgren D.; Lennernäs H. Intestinal Permeability and Drug Absorption: Predictive Experimental, Computational and In Vivo Approaches. Pharmaceutics 2019, 11 (8), 411. 10.3390/pharmaceutics11080411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda W. Permeability across lipid membranes. Biochim. Biophys. Acta 2016, 1858 (10), 2254–2265. 10.1016/j.bbamem.2016.03.032. [DOI] [PubMed] [Google Scholar]
- Lennernäs H. Intestinal permeability and its relevance for absorption and elimination. Xenobiotica 2007, 37 (10–11), 1015–1051. 10.1080/00498250701704819. [DOI] [PubMed] [Google Scholar]
- Markopoulos C.; Thoenen F.; Preisig D.; Symillides M.; Vertzoni M.; Parrott N.; Reppas C.; Imanidis G. Biorelevant media for transport experiments in the Caco-2 model to evaluate drug absorption in the fasted and the fed state and their usefulness. European journal of pharmaceutics and biopharmaceutics: official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V 2014, 86 (3), 438–448. 10.1016/j.ejpb.2013.10.017. [DOI] [PubMed] [Google Scholar]
- CDER Clinical Drug Interaction Studies — Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions Guidance for Industry; CDER, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.