Abstract
Herein, we report that Autographa californica nucleopolyhedrovirus, a member of the Baculoviridae family, is capable of stimulating antiviral activity in mammalian cells. Baculoviruses are not pathogenic to mammalian cells. Nevertheless, live baculovirus is shown here to induce interferons (IFN) from murine and human cell lines and induces in vivo protection of mice from encephalomyocarditis virus infection. Monoclonal antibodies specific for the baculovirus envelope gp67 neutralize baculovirus-dependent IFN production. Moreover, UV treatment of baculovirus eliminates both infectivity and IFN-inducing activity. In contrast, the IFN-inducing activity of the baculovirus was unaffected by DNase or RNase treatment. These data demonstrate that IFN production can be induced in mammalian cells by baculovirus even though the cells fail to serve as a natural host for an active viral infection. Baculoviruses, therefore, provide a novel model in which to study at least one alternative mechanism for IFN induction in mammalian cells.
Viral infection of mammalian cells results in the production of various cytokines, including members of the interferon (IFN) family (23). Double-stranded RNA (dsRNA), produced during the replication of many RNA and DNA viruses, has been shown to induce alpha/beta IFNs (IFN-α/β) in various cell types (23). In fact, the IFN-stimulating ability of dsRNA can be mimicked in vitro by synthetic RNA copolymers, such as poly(I)-poly(C). However, viral replication is not always essential for virus-induced IFN-α/β production, and interaction of viral proteins with the host cell membrane may serve as an alternative stimulus for IFN production (4, 7, 11, 13).
Baculoviruses are a family of enveloped double-stranded DNA viruses that are primarily pathogenic to insects in the order Lepidoptera. The host specificity of baculovirus has been well studied, due to safety concerns with regard to its use as a pesticide and as a protein expression system. Although baculovirus is known to infect over 30 species of Lepidoptera, it does not replicate in other insect cells or in any of the over 35 mammalian cell lines studied (15, 21, 25). Baculovirus does, however, enter mammalian cells, and viral DNA is able to reach the nucleus (9, 21, 25). The species-specific nature of the infection is, in part, dependent on the promoter of the baculovirus which is active only in Lepidoptera. Experimental studies have shown that when an exogenous promoter such as that derived from Rous sarcoma virus or cytomegalovirus is inserted into the baculovirus genome, the modified virus becomes capable of gene expression in non-Lepidoptera cell lines, including various mammalian cells (1, 5, 6, 10).
We report here the novel finding that baculovirus can stimulate IFN production from both human and mouse cells in vitro and in vivo. The IFN-stimulating activity of baculovirus required live virus and was not due to the presence of viral RNA, DNA, or bacterial endotoxin. Since baculovirus does not multiply in mammalian cells, these results support the concept that viral-induced IFN production is not always dependent on viral replication. Yet, the activity is inhibited by antibodies against viral gp67 (a protein required for viral entry into cells) and UV inactivation of the virus. These data suggest that viral-dependent processes in addition to protein-protein interactions at the cell surface are required for IFN induction by baculovirus. Baculovirus thus represents a model to elucidate at least one additional mechanism by which the virus triggers innate immunity in the mammalian host.
MATERIALS AND METHODS
Reagents.
Recombinant cytokines were obtained from the following sources. Murine IFN-γ (1.3 × 107 U/mg) was obtained from Genentech (South San Francisco, Calif.), and murine IFN-γ (7 × 106 U/mg) for in vivo studies was a gift from Laurence Ozmen Hoffmann-La Roche (Basel, Switzerland). Human IFN-αa/d (3.6 × 107 U/mg) was a gift from Michael Brunda Hoffmann-La Roche (Nutley, N.J.). poly(I)-poly(C) was obtained from Sigma (St. Louis, Mo.). Antibodies were obtained from the following sources. Sheep anti-murine IFN-α/β was kindly provided by Ion Gresser (Laboratory of Viral Oncology, Centre National de la Recherche Scientifique, Villejuif, France). Rat monoclonal anti-murine IFN-β was purchased from Yamasa Shoyu Co., Ltd. (Chiba-ken, Japan). Hamster anti-murine IFN-γ (H22) and hamster anti-glutathione S-transferase (PIP-1D7) were produced as described (20). Monoclonal antibody against Autographa californica envelope gp67 (AcV5) was a generous gift from Gary Blissard (Boyce Thompson Institute for Plant Research, Cornell University, Ithaca, N.Y.). Biotinylated donkey anti-sheep immunoglobulin G (IgG) was purchased from Sigma; streptavidin-horseradish peroxidase conjugate was purchased from Zymed Laboratories Inc. (South San Francisco, Calif.). All tissue culture media and media components used in this study were free of endotoxin as tested by Limulus amebocyte lysate assay (BioWhittaker, Walkersville, Md.).
Generation of recombinant murine IFN-β.
Murine IFN-β (6.3 × 107 U/mg) was produced in a baculovirus expression system as follows. The murine IFN-β cDNA was generously provided by Tadastugu Taniguchi (Department of Immunology, University of Tokyo, Tokyo, Japan). A 3′ nine-His tag was added by PCR, and the DNA was subcloned into the baculovirus expression vector PVL 1392 (Pharmingen, San Diego, Calif.). Baculovirus was transfected and amplified in Sf9 cells. Protein was produced by baculovirus infection of Sf9 cells and purified on a Ni-nitrilotriacetic acid agarose column (Qiagen, Hilden, Germany). IFN-β was eluted with 250 mM imidazole (Sigma), and activity was determined by using a cytopathic effect assay (20). The functional activity of purified recombinant IFN-β was neutralized by several different commercially available IFN-β-specific monoclonal antibodies.
Animals.
C57BL/6NCr female mice (4 to 6 weeks old) were purchased from NCI/Charles River Laboratories Inc. (Frederick, Md.). 129×C57BL6 mice were bred in-house at Washington University. CBA/J mice were purchased from Taconic Farms, Inc. (Germantown, N.Y.), and Armenian hamsters were obtained from Cytogen Research and Development (Cambridge, Mass.). The care and experimental use of all animals used in this study were approved by the Animal Care and Use Committees at the respective institutions.
Cells and baculovirus.
Mouse embryo fibroblasts (MEF) were derived from day 12 129×C57BL6 mouse embryos, immortalized by continual passage as described by Todaro and Green (22), and maintained in Dulbecco’s modified Eagle’s medium (BioWhittaker) containing 10% heat-inactivated fetal calf serum (HyClone, Logan, Utah), 4 mM l-glutamine, 1 mM sodium pyruvate, 50 U of penicillin per ml, 50 μg of streptomycin per ml, and 1% nonessential amino acids (D−10+NEAA). Mouse kidney fibroblasts (MKF), derived from 129×C57BL6 mice, were transformed by transfection with pPSVE1-B1, a plasmid encoding the polyoma virus large T antigen as previously described (16), and maintained in D−10+NEAA. Cos-7, BALB/c CL.7, and RAW264.7 cells were purchased from the American Type Culture Collection (ATCC; Rockville, Md.) and routinely grown in D−10+NEAA. Normal human dermal fibroblasts (NHDF) were purchased from the ATCC and maintained in Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum, 4 mM l-glutamine, 1 mM sodium pyruvate, and 5 μg of gentamicin per ml. Cultured spleen cells were obtained from CBA/J mice 3 to 6 months of age and cultured in RPMI (BioWhittaker) with 10% fetal calf serum, 2 mM l-glutamine, 1 mM sodium pyruvate, 50 U of penicillin per ml, 50 μg of streptomycin per ml, and 5× 10−5 M 2-mercaptoethanol (2-ME). IFN-α/β receptor knockout (IFNAR1−/−) primary MEF were derived from day 12 129Sv/Ev mouse embryos and were grown in D−10+NEAA. Sf9 cells were obtained from the ATCC and maintained in Graces media with lactalbumin and yeastolate plus 10% fetal calf serum, 50 μg of gentamicin per ml, 0.02% pluronic F-68 (Gibco, Grand Island, N.Y.), and 2 mM Glutamax (Gibco). A. californica nucleopolyhedrovirus (AcNPV) was obtained from Pharmingen.
Plasmids.
Plasmid p166 B+1−Ac Spe Bgl containing the AcNPV gp64 open reading frame under control of the Orgyia pseudotsugata multinucleocapsid nucleopolyhedrovirus promoter was a generous gift from Gary Blissard.
IFN-stimulating preparation (ISP).
Baculovirus-infected Sf9 cells were grown in spinner flasks. After centrifugation, 1.5 liters of the cultured media was applied directly to a 7-ml (10-mm inner diameter by 88-mm length) POROS HS cation exchange column (PerSeptive Biosystems, Inc., Foster City, Calif.) equilibrated with 0.02 M Bis-Tris (pH 6) containing 0.05 M NaCl and 10% (vol/vol) glycerol (buffer A) attached to a Beckman Gold HPLC System (Beckman Instruments, Fullerton, Calif.). Contaminating proteins were eluted by using a 12-column volume gradient from 0 to 25% buffer B (buffer A with 1 M NaCl). The preparation was eluted from the column with a step gradient to 100% buffer B, and activity was determined by using an in vitro cytopathic effect assay with NHDF and encephalomyocarditis virus (EMCV). Active fractions were pooled, and the preparation was adjusted to a concentration of 200 μg of total protein per ml. This preparation was shown to contain live virus by infection of Sf9 cells.
In vitro cytopathic effect assays.
The ability of ISP to induce an antiviral state in vitro was determined by using a cytopathic effect assay performed essentially as described previously (20). In this assay, cells were seeded in triplicate into 96-well tissue culture plates (MEF and MKF, 104 per well; BALB/c CL.7 cells, 7.5 × 104 per well; primary IFNAR1−/− cells, 104 per well; NHDF, 2 × 104 per well) and incubated with serial dilutions of ISP, IFN-αA/D, IFN-β, IFN-γ, or supernatant or cells (whole or disrupted by repeated freeze-thaw) from baculovirus-infected or uninfected Sf9 cells as indicated. After 24 h, the media were removed, the cells were washed extensively with warm medium, and vesicular stomatitis virus (VSV) was added. In the case of NHDF, EMCV was used. Cell viability was determined 24 to 48 h later by crystal violet staining and quantitated by spectroscopy. Values were plotted as means of the triplicate wells.
Where indicated, an indirect assay approach was taken to determine whether ISP induced IFN expression. In this assay, cells were plated (spleen cells, 2 × 105 per well; RAW264.7 cells, 1.5 × 105 per well) and treated with IFN or ISP as above. After 24 h, the media were removed and placed onto a confluent monolayer of MKF (104 per well), which do not respond to ISP but do respond to IFN-α/β and IFN-γ. After 24 h, the MKF were washed and exposed to VSV. Cell viability was determined as above. In experiments where antibody inhibition was studied, anti-IFN antibodies were added simultaneously with the ISP.
In vivo antiviral activity assays.
The ability of ISP to induce an antiviral state in vivo was determined following EMCV infection, as described previously (19). C57B1/6NCr mice, between 6 to 10 weeks of age, were administered phosphate-buffered saline (PBS), 1 μg of murine IFN-γ, 1 μg of IFN-αA/D, and 0.7 or 7 μg of ISP intraperitoneally (i.p.) at 20 and 1 h prior to a single lethal dose (2 × 104 PFU) of EMCV, given i.p. (n = 10). Hind limb paralysis was monitored for 23 days postinfection, and animals were sacrificed when both hind limbs became paralyzed.
Production of ISP-specific monoclonal antibodies.
A male Armenian hamster was immunized by i.p. injection with 20 μg (100 μl) of the ISP emulsified in complete Freund’s adjuvant and boosted three times with 10 μg (50 μl) of ISP emulsified in incomplete Freund’s adjuvant every 10 days. Thirty days after the last injection, the animal was boosted intravenously with 10 μg (50 μl) of ISP in PBS. Three days later, the spleen was harvested for production of monoclonal antibodies. Hybridomas were prepared as previously described (20) by fusion of immune splenocytes to the hypoxanthine-aminopterin-thymidine-sensitive murine myeloma cell line P3X63Ag8.653 with PEG 1500 (Boehringer Mannheim, Indianapolis, Ind.) at a splenocyte/myeloma cell ratio of 5:1.
Monoclonal antibodies were selected for their ability to inhibit the ISP-induced production of IFN-α/β from RAW264.7 macrophage cells. Serial dilutions of hybridoma supernatants were incubated in 96-well V-bottom plates with 0.2 μg of ISP per ml for 1 h at 37°C in a total volume of 125 μl of D−10+NEAA. This mixture was then added to 1.5 × 105 RAW264.7 cells/well (75-μl volume) in 96-well flat-bottom tissue culture plates. After 8 h at 37°C, 100 μl of the supernatant was removed for IFN enzyme-linked immunosorbent assay (ELISA) (see below).
IFN-α/β ELISA.
Immulon II plates (Dynatech Laboratories, Inc., Chantilly, Va.) were coated with 3 μg of rat monoclonal anti-murine IFN-β per ml of carbonate buffer (pH 9.6) overnight at 4°C. Plates were washed with 0.5 M Tris (pH 7.6) plus 0.2% Triton X-100, and RAW cell-conditioned media was added and incubated overnight at 4°C. The captured IFN was then detected with a 1:1,000 dilution of sheep anti-murine IFN-α/β. Biotinylated anti-sheep antibody was used as a secondary reagent (at 1:1,000), and a streptavidin-horseradish peroxidase conjugate was used as a tertiary reagent (at 1:5,000). The plates were developed by using 1 mM ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] (Boehringer, Mannheim, Germany) in 0.1 M sodium citrate buffer (pH 4.2) plus 0.03% H2O2 and quantitated by spectroscopy at 414 nm.
Immunoprecipitation and Western blotting.
Cultured media from baculovirus-infected or uninfected Sf9 cells (200 μl) or cell lysates from Sf9 cells transiently transfected with the AcNPVgp67 construct were added to 10 μg of purified ISP-6E11 antibody and mixed gently at 4°C. After 2 h, 30 μl of protein A-Sepharose (Pharmacia Biotech, Uppsala, Sweden) was added and samples were mixed for an additional hour. The Sepharose was washed three times with ice-cold PBS, suspended in Laemmli sample buffer with 2-ME, and frozen at −20°C until use. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting were performed with 4 to 20% gradient polyacrylamide gels (Bio-Rad, Hercules, Calif.) unless otherwise indicated. Western blotting was performed as described previously (8). Membranes were blotted with 0.5 μg of biotinylated ISP-6B12 per ml and detected with streptavidin-horseradish peroxidase.
Ultracentrifugation of ISP.
One milliliter of the ISP was ultracentrifuged for 3 h at 100,000 × g at 4°C in an Optima TLX ultracentrifuge (Beckman Instruments). The centrifuged material was divided into three fractions derived from the top third, middle third, and bottom third of the tube. The bottom third was pipetted up and down several times to suspend any sedimented materials.
Demonstration of live baculovirus.
Sf9 cells, growing in log phase, were plated onto 96-well microtiter tissue culture plates (6.5 × 104 cells/well in a 200-μl volume of complete Graces medium) and allowed to adhere for 4 h. Serial dilutions of the ISP were made in complete Graces media in a separate V-bottom microtiter plate. Media were removed from the cells, and 50 μl of the ISP dilutions was incubated with the Sf9 cells for 1 h at 27°C. The ISP was then removed, the cells were washed three times with 200 μl of Graces media at 27°C, and the cells were then cultured in 200 μl of the media for 5 days at 27°C. The media were removed, and the cells were suspended and lysed in 75 μl of 1% Triton X-100–0.1% SDS buffer at 4°C for 20 min. The lysate was cleared by centrifugation for 10 min at 11,750 × g. Twenty microliters of lysate was subjected to SDS-PAGE with 4 to 20% gradient gels in the presence of 2-ME. Evidence of active baculovirus infection was detected by Western blotting, with anti-gp67 (AcV5) hybridoma supernatant (1:1,000).
UV inactivation of the ISP.
The ISP (150 μl) was placed in a sterile tissue culture dish in a laminar flow hood and exposed to shortwave UV light at a distance of 7 cm for 25 min, with mixing after 15 min. Mock-treated ISP was placed in a sterile tissue culture dish in a laminar flow hood and exposed to fluorescent light for 25 min, with mixing after 15 min. Viral viability was assessed by monitoring the capacity of the treated ISP to infect Sf9 cells.
RESULTS
Baculovirus induces antiviral activity in various mammalian cells.
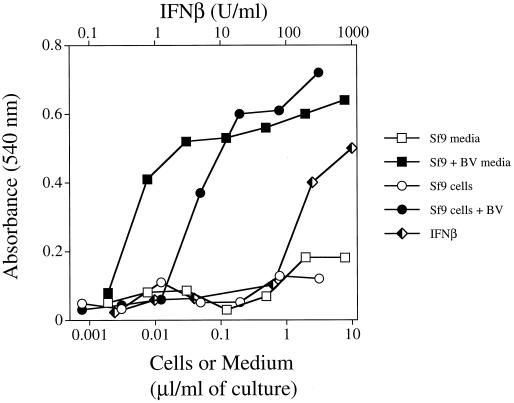
During the purification of a baculovirus-expressed protein, we noted that treatment of MEF with either intact baculovirus-infected Sf9 cells or spent culture media from infected Sf9 cells resulted in profound, dose-dependent protection from VSV infection (Fig. 1). In contrast, MEF were not protected from VSV infection when exposed to either uninfected Sf9 cells or media from uninfected cells.
FIG. 1.
Baculovirus (BV)-infected Sf9 cells and cultured media from infected cells protect MEF from the cytopathic effects of VSV. Baculovirus-infected and uninfected Sf9 cells were grown at 27°C for 1 week. Sf9 cells and cultured media were collected and centrifuged. The cells were suspended in D−10+NEAA at 5 × 106 cells per ml and disrupted by multiple freeze-thaw cycles. Similar results were obtained with baculovirus-infected Sf9 cells that had not undergone freeze-thaw (data not shown). Immortalized MEF were treated with serial dilutions of either baculovirus-infected or uninfected Sf9 cells or cultured media. After 24 h, the cells were washed and treated with VSV. Cells were monitored for cytopathic death and stained with crystal violet at ∼18 h after infection. Each point represents the mean of triplicate wells.
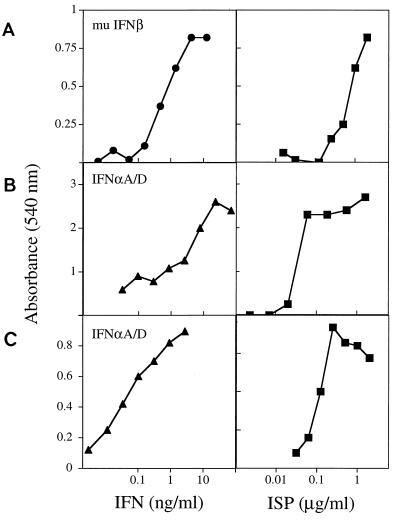
The active component in the cultured media was enriched by cation exchange high-pressure liquid chromatography. The enriched preparation protected murine MEF and the BALB/c fibroblast line CL.7 from the cytopathic effects of VSV (Fig. 2A and B) and also conferred protection to the human NHDF line from infection with EMCV (Fig. 2C). In all cases, the effects of the preparation were comparable to that of exogenously added IFN-α or IFN-β These data suggested either that the factor produced by baculovirus or baculovirus-infected insect cells was capable of directly protecting mammalian cells from viral infection or that it worked through an indirect mechanism, possibly by inducing IFN-α/β production.
FIG. 2.
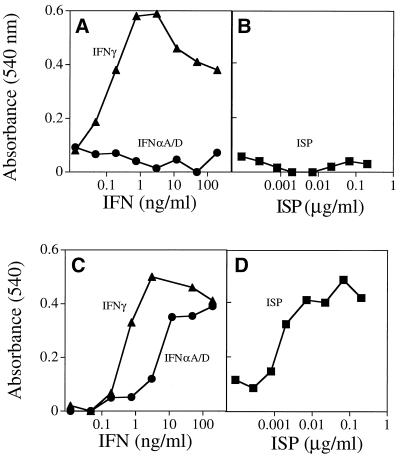
The ISP induces antiviral activity in various mammalian cells. MEF (104) (A), BALB/c CL.7 cells (7.5 × 104) (B), and NHDF (2 × 104) (C) were plated in 96-well tissue culture dishes and treated with serial dilutions of either IFN-αA/D, IFN-β, or ISP for 24 h. The cells were washed and then treated with either VSV (MEF and BALB/c CL.7) or EMCV (NHDF). Cells were monitored for viability by uptake of crystal violet ∼18 h after infection. Each point represents the mean of triplicate wells. mu, murine.
Baculovirus-induced antiviral activity acts indirectly, via IFN-α/β induction.
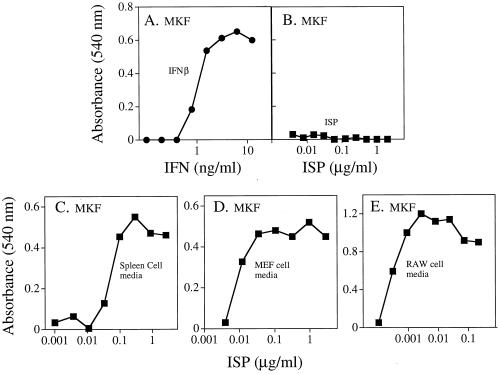
To address the mechanism of action of the baculovirus-induced antiviral activity, we first sought to determine whether it manifested its antiviral effects on cells directly or via the induction of IFN. This study was made possible by the identification of unresponsive cell lines that remained susceptible to VSV infection following exposure to the baculovirus preparation. Specifically, we determined that murine L929 cells, simian Cos-7 cells (data not shown), and a transformed MKF line were unresponsive to the antiviral promoting effects of the baculovirus preparation. MKF treated with IFN-β were protected from subsequent infection with VSV (Fig. 3A). In contrast, MKF were not protected when treated with doses of the preparation that were effective in conferring complete protection to MEF against VSV infection (Fig. 3B). If, however, MKF were exposed instead to media derived from normal murine splenocytes, MEF, or RAW264.7 cells that had been treated with the baculovirus preparation, the MKF cultures were protected from the cytopathic effects of the virus (Fig. 3C to E). These results suggested that the baculovirus preparation functioned indirectly by inducing a soluble antiviral agent.
FIG. 3.
The ISP effects on mammalian cells are indirect. MKF were plated in 96-well tissue culture dishes (104 cells/well) and treated with serial dilutions of IFN-β, ISP preparation, or the cultured media from primary murine spleen, MEF, or RAW264.7 cells which had been treated with serial dilutions of the ISP preparation for 24 h. The MKF were washed and then treated with VSV. Cells were monitored for viability as described. Each point represents the mean of triplicate wells.
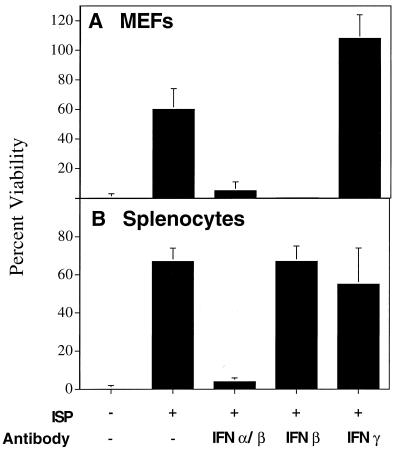
To determine if the baculovirus-induced antiviral agent was IFN, MEF or primary murine spleen cells were treated with 0.5 μg of the preparation per ml in the presence or absence of antibodies that neutralized either all murine IFN-α/β, murine IFN-β only, or murine IFN-γ only. After stimulation, the treated MEF were washed and tested for susceptibility to infection with VSV (Fig. 4A). In addition, the conditioned media from the primary splenocyte cultures were tested for their ability to render MKF resistant to VSV infection (Fig. 4B).
FIG. 4.
The ISP acts via the induction of IFN-α/β. MEF and primary murine spleen cells were plated in 96-well tissue culture dishes and treated with 0.5 μg of ISP/ml in the presence or absence of either polyclonal antibodies specific for all murine IFN-α/β (diluted 1:16), anti-murine IFN-β (150 μg/ml), or anti-murine IFN-γ (150 μg/ml) for 24 h. (A) MEF were washed and treated directly with VSV. (B) The conditioned media from the spleen cells were transferred to MKF for 24 h prior to the addition of VSV. Cells were monitored for cytopathic effect and stained with crystal violet ∼18 h after infection. Each bar represents the mean of triplicate wells ± the standard deviation.
Consistent with the data already presented, the baculovirus preparation blocked viral infection in MEF (Fig. 4A). However, protection was ablated if the cultures contained neutralizing antibodies specific for IFN-α/β or IFN-β alone but not when the cultures contained a murine IFN-γ-specific monoclonal antibody (Fig. 4A). Also as expected, the transfer of culture media from primary spleen cells treated with the baculovirus preparation protected MKF from VSV infection (Fig. 4B). However, the addition of neutralizing antibodies against IFN-α/β to the culture medium blocked the transferred antiviral activity. In contrast, IFN-β-specific antibodies did not ablate the antiviral action of the culture medium that was transferred from stimulated splenocytes to MKF. This finding is consistent with the fact that stimulated spleen cells produce predominantly IFN-α, while stimulated fibroblasts produce predominantly IFN-β. Addition of IFN-γ-specific antibodies to spleen cells treated with the baculovirus preparation resulted in a slight reduction of antiviral activity, suggesting that IFN-γ can also be induced by the preparation if the proper cell type is used. In both the direct and indirect assay systems, addition of species-matched nonspecific antibodies had no effect (data not shown).
As a final test to determine whether the baculovirus preparation functions by inducing IFN production in cells, we examined whether the preparation was capable of inducing antiviral activity in fibroblasts derived from mice lacking the IFNAR1 chain of the IFN-α/β receptor (IFNAR1−/− cells). As expected, cells lacking the IFN-α/β receptor were protected from viral infection only when exposed to IFN-γ and not IFN-αA/D (Fig. 5A). Importantly, the baculovirus preparation was unable to directly protect IFNAR1−/− cells from viral lysis (Fig. 5B). In contrast, culture media from IFNAR1−/− fibroblasts treated with the baculovirus preparation were capable of transferring viral protection to MKF (Fig. 5D) comparable to IFN-γ or IFN-αA/D (Fig. 5C). Thus, taken together these data demonstrate that the baculovirus preparation contains an activity that induces IFN production from mammalian cells. For this reason, the activity contained in the baculovirus preparation was given the functional name interferon-stimulating preparation.
FIG. 5.
The ISP does not require the IFN-α/β receptor for induction of IFN-α/β but does require it for the antiviral effects mediated by this cytokine. Primary IFN-α/β IFNAR1−/− fibroblasts were treated for 24 h with serial dilutions of either IFN-γ, IFN-αA/D (A), or the ISP (B). The media were then removed and placed onto MKF for 24 h (C and D, respectively). Both the IFNAR1−/− cells and MKF were treated with VSV. Cells were monitored for cell survival and stained with crystal violet at ∼18 h after infection. Each point represents the mean of triplicate wells.
ISP protects mice from lethal EMCV infection.
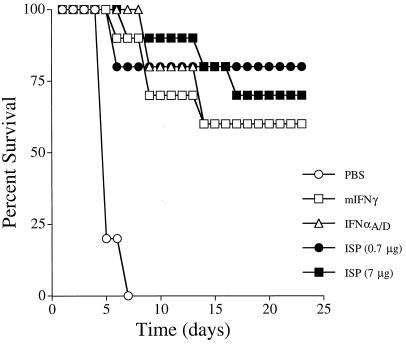
To determine whether the ISP produced antiviral effects under physiologic conditions, the capacity of the ISP to protect mice from in vivo infection with EMCV was compared to that of purified preparations of murine IFN-γ or human IFN-αA/D. In the absence of a viral challenge, neither murine IFN-γ, IFN-αA/D, or the ISP affected survival (data not shown). All EMCV-infected mice that were pretreated with PBS died within 7 days (Fig. 6). In contrast, 60% (6 of 10) of mice treated with murine IFN-γ or IFN-αA/D survived the EMCV challenge. Similarly, 70% (7 of 10) of mice treated with 7 μg of ISP and 80% (8 of 10) of mice treated with 0.7 μg of the ISP were protected from EMCV. Thus, the ISP not only is active on mammalian cells when tested in vitro but also functions to protect mice from viral infection when administered systemically, in vivo.
FIG. 6.
The ISP protects mice from lethal EMCV infection. Groups of 10 mice were injected i.p. with PBS, 1 μg of murine IFN-γ, 1 μg of IFN-αA/D, or one of two concentrations of ISP (0.7 or 7 μg) 20 and 1 h prior to a single lethal dose of EMCV (2 × 104 PFU). Hind limb paralysis was monitored for 23 days postinfection.
Monoclonal antibodies raised against ISP neutralize its IFN-inducing activity and recognize baculovirus gp67.
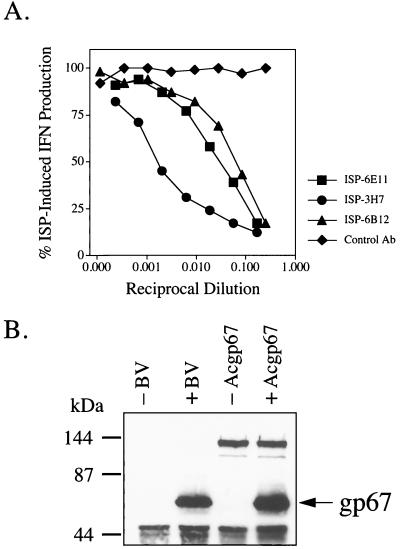
Monoclonal antibodies were prepared from an ISP-immunized Armenian hamster. Neutralizing antibodies were selected based on their ability to inhibit ISP-induced IFN-α/β production from RAW264.7 macrophage cells. Three of these antibodies were selected based on their ability to inhibit ISP-induced IFN production (Fig. 7A) and were used in subsequent experiments. Two of the antibodies (ISP-6E11 and ISP-6B12) were of the IgG class and one (ISP-3H7) was of the IgM class. All of these antibodies were shown by Western blotting and immunoprecipitation to recognize a 67-kDa protein under reducing conditions (data for ISP-6E11 and ISP-6B12 is shown in Fig. 7B). Using one of the antibodies, ISP-6E11, the 67-kDa protein was immunoprecipitated and sequenced (sequence obtained, AEHXNXQMKTXPY). Subsequent BLAST analysis identified the 67-kDa protein as the baculovirus envelope gp67 (sequence, AEHCNAQMKTGPY; accession no. P17501). The specificity of those monoclonal antibodies was further confirmed by showing that ISP-6E11 can immunoprecipitate recombinant gp67 transiently expressed in Sf9 cells (Fig. 7B). Taken together, these data show that antibodies against gp67 are capable of neutralizing the IFN-stimulating activity of the baculovirus preparation.
FIG. 7.
Monoclonal antibodies against the ISP neutralize its IFN-inducing activity and recognize gp67. (A) Serial dilutions of hybridoma supernatants were incubated in 96-well V-bottom plates with 0.2 μg of ISP per ml for 1 h at 37°C in a total volume of 125 μl of D−10+NEAA. This mixture was then added to 1.5 × 105 RAW264.7 cells/well (75-μl volume) in 96-well flat-bottom plates. After 8 h at 37°C, 100 μl of the supernatant was removed for IFN-α/β ELISA. The control antibody (Ab) used is a hamster monoclonal antibody which reacts with bacterial glutathione S-transferase. (B) Cultured media from Sf9 cells infected with baculovirus (BV) or control media from uninfected cells (200 μl) or cell lysates from Sf9 cells transiently transfected with the AcNPV gp67 construct were immunoprecipitated with 10 μg of purified ISP-6E11. Immunoprecipitates were subjected to 10% SDS-PAGE in the presence of 2% 2-ME and transferred to nitrocellulose. Membranes were blotted with biotinylated ISP-6B12 (0.5 μg/ml).
IFN-inducing activity in the ISP is not due to the presence of dsRNA, DNA, or lipopolysaccharide.
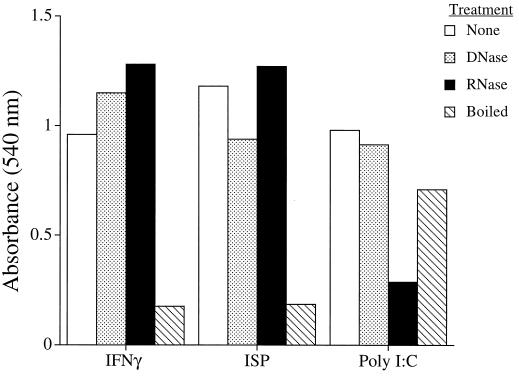
Because dsRNA is known to induce IFN-α/β (23), it was important to determine if baculovirus-derived RNA contributed to the ISP’s ability to stimulate IFN production. As expected, the antiviral activity of IFN-γ was unaffected following treatment with RNase or DNase but was completely destroyed after boiling for 15 min (Fig. 8). In contrast, the antiviral actions of poly(I)-poly(C) were destroyed by RNase treatment but remained unaffected by either DNase or boiling. When subjected to the same treatments, the antiviral activity of the ISP was unaffected by DNase or RNase but was destroyed upon boiling for 15 min. In addition, activity was lost when the ISP was held at pH 2.2 overnight at 4°C (data not shown). These data thus indicate that the IFN-inducing activity of the ISP is neither dsRNA nor DNA. Since the ISP was free of endotoxin (<0.01 endotoxin unit/ml) and was sensitive to heat and pH, the activity cannot be ascribed to lipopolysaccharide contamination of the preparation.
FIG. 8.
The activity of the ISP is not due to dsRNA. Murine IFN-γ (100 U), ISP (2 μg), or poly(I)-poly(C) (5 μg) were used untreated or subjected to digestion for 60 min at room temperature with either 50 U of DNase, 12.5 U of RNase, or 15 min of boiling. These treated compounds were added to primary murine spleen cells for 24 h. The cultured media were then transferred to MKF (which are not responsive to the ISP) for 24 h prior to the addition of VSV. Cells were monitored for cell survival and stained with crystal violet ∼18 h after infection. Each bar represents the mean of triplicate wells.
IFN-inducing activity in the ISP is manifested by live baculovirus.
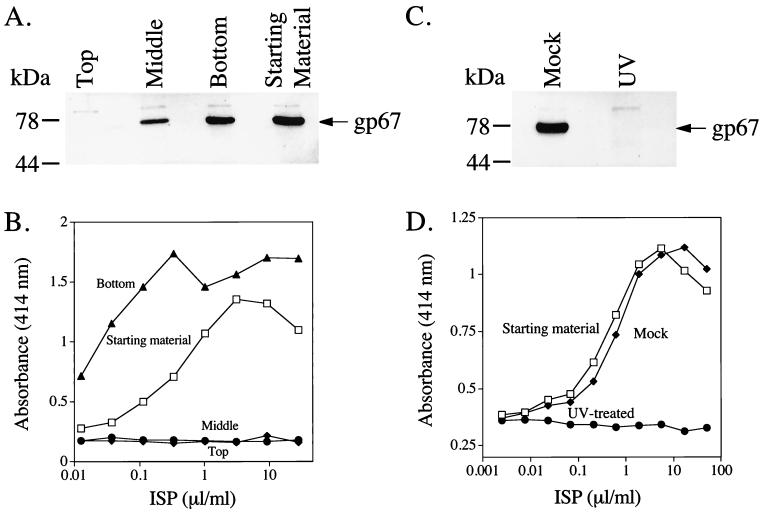
We next sought to determine if intact viral particles were involved in the IFN-stimulating activity of the ISP. Prior to manipulation, the ISP was capable of infecting Sf9 cells (Fig. 9A), as evidenced by synthesis of gp67, and capable of stimulating IFN production from RAW264.7 cells (Fig. 9B). The ISP was subjected to ultracentrifugation for 3 h, under conditions previously shown to sediment viral particles (12). The top third, middle third, and bottom third of the centrifuged preparation were collected and tested for the presence of infectious virus and for IFN-stimulating activity. The top fraction contained no infectious material (i.e., it failed to effect gp67 synthesis) (Fig. 9A) and lacked the ability to stimulate IFN from RAW264.7 cells (Fig. 9B). The middle fraction contained a small amount of infectious material but did not stimulate IFN from RAW264.7 cells. In contrast, the sedimented fraction contained the majority of the Sf9 infectious material and also contained all of the IFN-stimulating activity. This fraction, in fact, showed a log higher specific activity. Moreover, addition of commercially available purified AcMNPV (Pharmingen) to MEF induced a similar type and magnitude of antiviral activity (data not shown). These data demonstrate that the IFN-stimulating activity of the ISP is associated with infectious baculovirus particles.
FIG. 9.
The ISP requires live baculovirus for its IFN-stimulating activity. (A) One milliliter of the ISP was subjected to ultracentrifugation at 100,000 × g for 3 h at 4°C. The ISP was removed in three fractions: top, middle, and bottom. These fractions were then used to infect Sf9 cells. Sf9 cells (6.5 × 104/well) were exposed to the ISP fractions for 1 h and then washed thoroughly. After 5 days at 27°C, the media were removed and infected cells were lysed. Cleared lysates (20 μl) were separated on SDS–4 to 20% polyacrylamide gels and evidence of active baculovirus infection was detected by Western blotting with the AcV5 antibody specific for gp67. (B) Portions of the fractions were also added to 1.5 × 105 RAW264.7 cells/well (75-μl volume) in 96-well flat-bottom plates. After 8 h at 37°C, 100 μl of the supernatant was removed for IFN-α/β ELISA. (C) The ISP (125 μl) was placed in a sterile petri dish in a laminar flow hood and subjected to either shortwave UV light for 25 min at a distance of 7 cm or subjected to normal fluorescent light for the same period of time. The two preparations were then used to infect Sf9 cells (C) as above or treat RAW cells for IFN production (D) as above.
To determine if the IFN-stimulating activity of the ISP required live virus or merely required intact viral particles, the baculovirus in the ISP was killed by brief exposure to shortwave UV light. UV-treated baculovirus failed to infect Sf9 cells (Fig. 9C). Following UV treatment, the baculovirus was also unable to stimulate IFN production from RAW264.7 cells (Fig. 9D). In contrast, mock-treated baculovirus stimulated IFN production at concentrations similar to that of untreated virus. Taken together, these data demonstrate that the IFN-stimulating activity in the ISP is due to the presence of live baculovirus.
DISCUSSION
In this report, we make the novel observation that live baculovirus imparts antiviral activity to mammalian cells, both in vitro and in vivo. Our data support the idea that viral replication is not necessary for IFN induction. However, we also show that IFN induction requires more than an interaction between the envelope proteins of the virus and receptors at the responding cell membrane.
The mechanisms by which viruses interact with a host cell and initiate IFN production are not well understood. Although dsRNA has been implicated in this process (23), numerous reports have indicated that inactivated viral particles and purified viral proteins are also capable of inducing IFN-α/β (2–4, 7, 11, 13, 14). These results suggest that protein-protein interactions at the host cell membrane can induce cytokine synthesis. In this report, we demonstrate that none of these mechanisms appear to be sufficient in this system. RNase and DNase treatment of the baculovirus preparation had no effect on its antiviral activity, indicating that double stranded nucleic acids are not responsible for IFN-α/β induction. In addition, UV-inactivated baculovirus was not capable of inducing IFN-α/β from RAW264.7 cells. This is in contrast to reports that UV-inactivated Sendai virus or gluteraldehyde-fixed cells infected with herpes simplex virus type 1, coronavirus, or dengue virus are capable of inducing IFN-α synthesis (4, 7, 11, 13). These data suggest that there is a unique process involved in the baculovirus stimulation of mammalian IFNs that requires live virus.
Antibodies which neutralize the IFN-stimulating ability of the baculovirus preparation were shown to recognize the major envelope glycoprotein known as gp67 or gp64 (18, 24). Infectivity of the budded form of baculovirus is dependent on this protein, as it is required for the penetration of virus into cells by adsorptive endocytosis and ultimately propagation of the virus (18, 24). Furthermore, antibodies against gp67 or gp64 have been shown to inhibit membrane fusion activity of baculovirus (17). Our neutralization studies show that gp67 is involved in the ability of baculovirus to stimulate IFN-α/β. However, overexpression of recombinant gp67 in Sf9 cells failed to induce the antiviral activity that is observed in Sf9 cells infected with intact live baculovirus (data not shown). Moreover, UV-killed virus also lacked IFN-inducing activity. Together, these data show that although gp67 is required for baculovirus-dependent IFN induction in mammalian cells, it is not sufficient. Thus IFN induction cannot simply be ascribed to the binding of gp67 to cell surface receptors.
The host cell specificity of baculovirus has been widely studied (15, 21, 25). Although baculovirus enters mammalian cells and viral DNA is able to reach the nucleus, the virus does not replicate (9, 21, 25). Our data demonstrate that baculovirus induces IFN-α/β in some but not all mammalian cultured cell lines. In addition, the baculovirus preparation is able to prevent death in mice challenged with a lethal dose of EMCV. It is unclear why a virus which does not replicate in mammalian cells would have such a potent effect on mammalian cell systems. More work will be needed before this issue can be clarified.
Our findings were serendipitous and stemmed from the analysis of a baculovirus-expressed protein. Because baculovirus is widely utilized as a protein expression vehicle, caution should be used in the purification process that no live virus contaminates the protein preparation. This is particularly relevant because preliminary observations indicate that the baculovirus preparation is also capable of stimulating a variety of other inflammatory cytokines in mammalian cells in addition to IFN-α/β (data not shown). Thus, it is critical to insure the absence of live baculovirus in preparations of bioactive proteins, especially those that regulate immune responses.
The mechanism for the observed baculovirus-induced IFN expression in mammalian cells remains unclear. It is possible that live baculovirus enters mammalian cells via gp67 and, once internalized, directly stimulates the expression of IFN-α/β. The results presented here lend support to a model in which viral replication is not required for IFN induction. However, some other cellular interaction is necessary and it requires live virus. It is therefore possible that live baculovirus can be used to stimulate innate and adaptive immune responses in a nonspecific manner. It also may serve as a model to study alternative pathways of viral-induced IFN induction.
ACKNOWLEDGMENTS
We thank H. Skip Virgin (Washington University) for helpful comments. We also thank David Parmelee and Reiner Gentz (Human Genome Sciences) for their efforts on protein purification.
This work was supported by a grant from the NCI (CA43059).
REFERENCES
- 1.Boyce F M, Bucher N L. Baculovirus-mediated gene transfer into mammalian cells. Proc Natl Acad Sci USA. 1996;93:2348–2352. doi: 10.1073/pnas.93.6.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capobianchi M R, Ameglio F, Cordiali Fei P, Castilletti C, Mercuri F, Fais S, Dianzani F. Coordinate induction of interferon α and γ by recombinant HIV-1 glycoprotein 120. AIDS Res Hum Retroviruses. 1993;9:957–962. doi: 10.1089/aid.1993.9.957. [DOI] [PubMed] [Google Scholar]
- 3.Capobianchi M R, Ankel H, Ameglio F, Paganelli R, Pizzoli P M, Dianzani F. Recombinant glycoprotein 120 of human immunodeficiency virus is a potent interferon inducer. AIDS Res Hum Retroviruses. 1992;8:575–579. doi: 10.1089/aid.1992.8.575. [DOI] [PubMed] [Google Scholar]
- 4.Capobianchi M R, Facchini J, DiMarco P, Antonelli G, Dianzani F. Induction of alpha interferon by membrane interaction between viral surface and peripheral blood mononuclear cells. Proc Soc Exp Biol Med. 1985;178:551–556. doi: 10.3181/00379727-178-42041. [DOI] [PubMed] [Google Scholar]
- 5.Carbonell L F, Klowden M J, Miller L K. Baculovirus-mediated expression of bacterial genes in dipteran and mammalian cells. J Virol. 1985;56:153–160. doi: 10.1128/jvi.56.1.153-160.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbonell L F, Miller L K. Baculovirus interaction with nontarget organisms: a virus-borne reporter gene is not expressed in two mammalian cell lines. Appl Environ Microbiol. 1987;53:1412–1417. doi: 10.1128/aem.53.7.1412-1417.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charley B, Laude H. Induction of alpha interferon by transmissable gastroenteritis coronavirus: role of transmembrane glycoprotein E1. J Virol. 1988;62:8–11. doi: 10.1128/jvi.62.1.8-11.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenlund A C, Morales M O, Viviano B L, Yan H, Krolewski J, Schreiber R D. Stat recruitment by tyrosine-phosphorylated cytokine receptors: an ordered reversible affinity-driven process. Immunity. 1995;2:677–687. doi: 10.1016/1074-7613(95)90012-8. [DOI] [PubMed] [Google Scholar]
- 9.Groner A, Granagos R R, Burand J P. Interaction of Autographa californica nuclear polyhedrosis virus with two non-permissive cell lines. Intervirology. 1984;21:203–209. doi: 10.1159/000149522. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann C, Sandig V, Jennings G, Rudolph M, Schlag P, Strauss M. Efficient gene transfer into human hepatocytes by baculovirus vectors. Proc Natl Acad Sci USA. 1995;92:10099–10103. doi: 10.1073/pnas.92.22.10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito Y, Nishiyama Y, Shimokata K, Nagata I, Takeyama H, Kunii A. The mechanism of interferon induction in mouse spleen cells stimulated with HVJ. Virology. 1978;88:128–137. doi: 10.1016/0042-6822(78)90116-2. [DOI] [PubMed] [Google Scholar]
- 12.Kapadia S B, Molina H, Van Berkel V, Speck S H, Virgin H W., IV Murine gammaherpesvirus 68 encodes a functional regulator of complement activation. J Virol. 1999;73:7658–7670. doi: 10.1128/jvi.73.9.7658-7670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurane I, Meager A, Ennis F A. Induction of interferon alpha and gamma from human lymphocytes by dengue virus-infected cells. J Gen Virol. 1986;67:1653–1661. doi: 10.1099/0022-1317-67-8-1653. [DOI] [PubMed] [Google Scholar]
- 14.Laude H, Gelfi J, Lavenant L, Charley B. Single amino acid changes in the viral glycoprotein M affect induction of alpha interferon by the corona virus transmissible gastroenteritis virus. J Virol. 1992;66:743–749. doi: 10.1128/jvi.66.2.743-749.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McIntosh A H, Shamy R. Biological studies of a baculovirus in a mammalian cell line. Intervirology. 1980;13:331–341. doi: 10.1159/000149143. [DOI] [PubMed] [Google Scholar]
- 16.Meraz M A, White J M, Sheehan K C F, Bach E, Rodig S J, Dighe A S, Kaplan D H, Riley J K, Greenlund A C, Campbell D, Carver-Moore K, DuBois R N, Clark R, Aguet M, Schreiber R D. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 17.Monsma S A, Blissard G W. Identification of a membrane fusion domain and an oligomerization domain in the baculovirus GP64 envelope fusion protein. J Virol. 1995;69:2583–2595. doi: 10.1128/jvi.69.4.2583-2595.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monsma S A, Oomens A G P, Blissard G W. The GP64 envelope fusion protein is an essential baculovirus protein required for cell-to-cell transmission of infection. J Virol. 1996;70:4607–4616. doi: 10.1128/jvi.70.7.4607-4616.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozmen L, Gribaudo G, Fountoulakis M, Gentz R, Landolfo S, Garotta G. Mouse soluble IFNg receptor as IFNγ inhibitor: distribution, antigenicity, and activity after injection in mice. J Immunol. 1993;150:2698–2705. [PubMed] [Google Scholar]
- 20.Schreiber R D, Hicks L J, Celada A, Buchmeier N A, Gray P W. Monoclonal antibodies to murine γ-interferon which differentially modulate macrophage activation and antiviral activity. J Immunol. 1985;134:1609–1618. [PubMed] [Google Scholar]
- 21.Tjia S T, Zu Altenschildesche G M, Doerfler W. Autographa californica nuclear polyhedrosis virus (AcNPV) DNA does not persist in mass cultures of mammalian cells. Virology. 1983;125:107–117. doi: 10.1016/0042-6822(83)90067-3. [DOI] [PubMed] [Google Scholar]
- 22.Todaro G J, Green H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established cell lines. J Cell Biol. 1963;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vilcek J, Sen G C. Interferons and other Cytokines. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 375–399. [Google Scholar]
- 24.Volkman L E. The 64K envelope protein of budded Autographa californica nuclear polyhedrosis virus. Curr Top Microbiol Immunol. 1986;131:103–118. doi: 10.1007/978-3-642-71589-1_6. [DOI] [PubMed] [Google Scholar]
- 25.Volkman L E, Goldsmith P A. In vitro survey of Autographa californica nuclear polyhedrosis virus interaction with nontarget vertebrate host cells. Appl Environ Microbiol. 1983;45:1085–1093. doi: 10.1128/aem.45.3.1085-1093.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]