Abstract

Short peptides are attractive building blocks for the fabrication of self-assembled materials with significant biological, chemical, and physical properties. The microscopic and macroscopic properties of assemblies are usually closely related to the dimensionality of formed hydrogen bond networks. Here, two completely different supramolecular architectures connected by distinct hydrogen bond networks were obtained by simply adding a hydroxyl group to switch from cyclo-tryptophan-alanine (cyclo-WA) to cyclo-tryptophan-serine (cyclo-WS). While hydroxyl-bearing cyclo-WS molecules provided an additional hydrogen bond donor that links to adjacent molecules, forming a rigid three-dimensional network, cyclo-WA arranged into a water-mediated zipper-like structure with a softer two-dimensional layer template. This subtle alteration resulted in a 14-fold enhancement of Young’s modulus values in cyclo-WS compared to cyclo-WA. Both cyclo-dipeptides exhibit biocompatibility, high fluorescence, and piezoelectricity. The demonstrated role of dimensionality of hydrogen bond networks opens new avenues for rational design of materials with precise morphologies and customizable properties for bioelectronic applications.
Short peptides and their bioinspired derivatives serve as promising building blocks for myriad potential applications of supramolecular assemblies at the interface of life sciences and nanotechnology.1−6 Peptides can self-assemble into supramolecular structures and architectures with long-range ordered arrangements governed by noncovalent interactions that can be controlled to embed valuable characteristics, including mechanical, piezoelectric, optical, and electronic properties.7,8 These properties enable diverse applications in the fields of structural materials, optical detection, sensing, and energy harvesting.9−15 For instance, engineering-ordered diphenylalanine microrod arrays with uniform polarization demonstrated a significant piezoelectric constant of 17.9 pm V–1 that produced an open-circuit voltage of up to 1.4 V and achieved a power density of 3.3 nW cm–2 in nanogenerator devices.16 Moreover, encasing γ-glycine films in poly(vinyl alcohol) generated piezoelectricity and mechanical flexibility sufficient for in vivo sensing.17 However, achieving the control and optimization of their assembled structures and properties remains a significant challenge.
The distinctive properties of biomaterials, which enable their diverse applications, are closely related to the supramolecular packing mode.18 In general, the packing mode is influenced by the formation of hydrogen bond networks in terms of their dimensionality.19 For example, in two-dimensional (2D) layered structures, adjacent molecules within layers are bound by specific, directional noncovalent intermolecular interactions, such as dense hydrogen bonds or π–π interaction networks, which confer rigidity and mechanical strength.20 The binding between layers is typically weaker, being generally mediated by nonspecific van der Waals forces between hydrophobic side chains. This dimensional heterogeneity endows the materials with high flexibility, along with in-plane toughness, resulting in robust yet bendable, adaptable micro/nanostructures.20 In contrast, supramolecular architectures with 3D hydrogen bonding networks naturally exhibit higher mechanical strength and stability,21 which makes them suitable deformation-resistant components in electromechanical systems. Consequently, understanding and controlling the dimensionality of hydrogen bond networks in supramolecular arrangements is crucial and fundamental for the full potential of unlocking functional bioderived and bioinspired materials. While layered structures connected by 2D hydrogen bond networks are commonly observed in biological supramolecular architectures, including amino acid and short peptide assemblies, three-dimensional (3D) networks are rarer.22−25 Current studies on the modification of material dimensionality primarily focus on altering of their macroscopic morphologies through the selective use of growth templates, solvents, pH values, and other environmental factors.15,26−30 The potential to unlock diverse peptide material functions through modulating the dimensionality of hydrogen bond networks at the atomic level has often been underexplored. Such comprehension and control require a full understanding the relationship between molecular structures, lattice arrangements, and physical properties. Moreover, hydroxyl groups have been demonstrated to act as hydrogen bond donors, facilitating the formation of dense hydrogen bond networks.22 Inspired by this, we speculate that the addition of hydroxyl groups in short peptide molecules plays an important role in determining the hydrogen bond network dimensionality of supramolecular packing.
Here, we achieve two distinct packing structures of very similar cyclic dipeptides: a 2D water-mediated layered structure and a dry 3D network structure. This was realized through the rational design of functional groups within the engineered peptide structures (Figure 1). The simple insertion of a hydroxyl group provided an additional hydrogen bond donor that connects adjacent peptide molecules, triggering the formation of the zigzag-like 3D supramolecular stacking, as confirmed by X-ray crystallography. In the absence of the hydroxyl group, the molecule coordinated with water molecules to form 2D hydrogen bond layers that were stabilized by only weak dispersive van der Waals forces between diketopiperazine rings in the interlayer stacking. The 2D layered structure with in-plane β-sheet-like hydrogen bonds exhibited flexibility and a higher predicted piezoelectric response. In contrast, the hydroxyl-induced 3D hydrogen bond network demonstrated a mechanical stiffness that was 14-fold higher. Moreover, both assemblies demonstrated excellent thermostability, biocompatibility, and optical properties, making them suitable for imaging, sensing, and energy harvesting in vivo. Our work provides insight into the self-assembly of various dimensionalities of supramolecular packing simply by strategically optimizing the building blocks. By exploring the interplay between lattice arrangements and physical properties, we create new opportunities to generate nanomodified materials with micro and macroscopic mechanical, electrical, and optical properties tailored for diverse potential applications.31,32
Figure 1.

Schematic illustration of modulating the material dimensionality via hydroxyl-directed supramolecular packing of cyclo-dipeptide molecule building blocks.
We compared the assembly dimensionality of two types of cyclo-dipeptides, selected based on their anticipated supramolecular packing efficiencies determined by their hydrogen bond properties. Hence, we chose cyclo-tryptophan-alanine (cyclo-WA), which lacks hydroxyl groups, and cyclo-tryptophan-serine (cyclo-WS), which has a hydroxyl group on its hydroxymethyl side chain. The cyclo-WA molecules self-assembled into long prismoid-like structures, forming layer-by-layer stacking crystals in a water and methanol solution at room temperature (Figure 2a,b and Figure S1) or in water at −5 °C (Figure S2), as observed using scanning electron microscopy (SEM) and optical microscopy. cyclo-WA crystals obtained through the slow evaporation of a water and methanol mixed solution were larger, likely due to the lower nucleation rate and the availability of ample building blocks. Conversely, the cyclo-WA crystals formed by cooling in water exhibited smaller sizes, due to limited diffusion, which induced a low growth rate. Under the same growth condition, the cyclo-WS molecules self-assembled into distinctly different, half-moon-like structures with smooth surfaces in water at −5 °C (Figure 2c,d and Figure S3). This distinct morphology suggests varying supramolecular architectures resulting from the addition of a hydroxyl group to the molecular building block. The diverse supramolecular packing was confirmed through powder X-ray diffraction (XRD) measurements (Figure S4), revealing distinct diffraction peak positions that indicate variations in the crystal structures. Cyclo-WA crystals obtained in both water–methanol mixtures at room temperature and pure water at −5 °C exhibited the same structure (Figure S4), indicating the diversity of growth approaches. Powder XRD patterns for both cyclo-WA and cyclo-WS assemblies were highly consistent with the simulated XRD patterns from their single crystals (Figure 2e,f), confirming a high phase purity. Notably, the diffraction peaks at 6.7°, 13.5°, 20.2°, 27.0°, and 34.1° corresponded to the (001), (002), (003), (004), and (005) crystal planes of the cyclo-WA crystal, respectively (Figure 2e and table S1). Similarly, the diffraction peaks of the cyclo-WS crystal at 13.0°, 16.2°, 17.4°, 23.4°, and 26.2° corresponded to the (011), (110), (021), (012), and (022) crystal planes (Figure 2f and table S1), respectively, confirming the different supramolecular packing modes.
Figure 2.
(a) SEM image of the cyclo-WA crystal obtained from a water and methanol solution, with the layer-by-layer stacking structure illustrated in the inset panel. (b) Zoomed-in view of the cyclo-WA crystal (marked with a red dotted rectangle in a). (c) SEM image of cyclo-WS crystals obtained from a water, with the smooth continuous 3D surface illustrated in the inset panel. (d) Zoomed-in view of the cyclo-WS crystal (marked with a red dotted rectangle in c). (e, f) XRD patterns of (e) cyclo-WA and (f) cyclo-WS crystals. (g) FTIR spectra of cyclo-WA and cyclo-WS crystals. (h) Raman spectra of cyclo-WA and cyclo-WS crystals. (i) Thermostability of cyclo-WA and cyclo-WS crystals.
Fourier transform infrared spectroscopy (FTIR) measurements confirmed the formation of different hydrogen bond types and patterns in the cyclo-WA and cyclo-WS assemblies (Figure 2g). In cyclo-WA crystals, a peak located at ∼3408 cm–1 indicated the presence of hydrogen-bonded water molecules,33 indicative of ordered crystallographic water molecules trapped in the crystal structure. No water bands were observed in cyclo-WS crystals, and instead, a peak at 3326 cm–1 corresponding to the peptide – OH band was detected,34 indicating the absence of ordered water molecules and the presence of the hydroxyl-functional group. A peak at 1053 cm–1 was attributed to the bending modes of C–OH groups coupled with the C–O stretching.35 In summary, cyclo-WA formed crystals through bridging with water molecules, whereas cyclo-WS provided an additional hydrogen bond donor through the hydroxyl-functional group to directly bind adjacent molecules, resulting in crystal formation. These findings were further supported by Raman measurements (Figure 2h). The peak at 3394 cm–1 was assigned to the H2O bending mode in cyclo-WA crystals, while the peaks at 1198 and 3331 cm–1 reflected the – OH stretching mode in cyclo-WS crystals.36−38
To assess the structural thermostability of the cyclo-WA and cyclo-WS crystals stabilized by the water-mediated and direct hydrogen bond modes, respectively (Figure 1), we performed thermal gravimetric analysis (TGA). As expected, the water molecules of cyclo-WA were lost at ∼101 °C, and the assembly started to degrade at ∼344 °C (Figure 2i). Cyclo-WS displayed similar high thermostability up to ∼308 °C (Figure 2i). The high thermal stability of the crystals may result from supramolecular architectures built from both hydrogen bonding and aromatic interactions.
Single-crystal
XRD characterizations of as-prepared cyclo-WA and
cyclo-WS crystals were carried out to provide insight into the two
distinct hydrogen bond-mediated supramolecular packing modes. Cyclo-WA
and cyclo-WS single crystals were obtained using the same growth condition
to eliminate the influence of temperature and solvent on the assembly
process. The structures revealed the atomic-scale features of the
assemblies both in the absence of the hydroxyl group (cyclo-WA) and
in its presence (cyclo-WS) within the peptide building unit. The crystal
structure of cyclo-WA assemblies was determined to be a monoclinic
space group P21 with cell parameters of
a = 6.2780 Å, b = 7.9007 Å, and c = 13.2593 Å (Table S1 and Figure S5). The asymmetric building
unit consisted of one cyclo-WA and one water molecule (Figure 3a), while the unit cell contained
two peptide molecules and two water molecules (Figure S6). Notably, the water molecule served as a hydrogen
bond donor, facilitating connections between adjacent molecules (Figure 3b). In the crystallographic 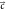 direction, molecules packed into a β-strand-like
arrangement that was stabilized by a pair of hydrogen bonds between
oxygen and nitrogen atoms on adjacent diketopiperazine rings, with
N(H)backbone... C=O (donor··· acceptor)
distances of 3.009 and 3.004 Å, respectively (Figure 3c). The strands were connected
through oxygen atoms that formed bonds with interstitial water molecules,
with an O(H)water... C=O (donor···
acceptor) distance of 2.862 Å (Figure 3c), to form supramolecular β-sheets
that further extended into a single layer with a 2D hydrogen bond
network. In the crystallographic
direction, molecules packed into a β-strand-like
arrangement that was stabilized by a pair of hydrogen bonds between
oxygen and nitrogen atoms on adjacent diketopiperazine rings, with
N(H)backbone... C=O (donor··· acceptor)
distances of 3.009 and 3.004 Å, respectively (Figure 3c). The strands were connected
through oxygen atoms that formed bonds with interstitial water molecules,
with an O(H)water... C=O (donor···
acceptor) distance of 2.862 Å (Figure 3c), to form supramolecular β-sheets
that further extended into a single layer with a 2D hydrogen bond
network. In the crystallographic 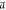 direction, another parallel layer was stabilized
by aromatic interactions of side-chain indole rings and packed in
an interlocked zipper-like orientation, forming a double-layer structure
(Figure 3d). The aromatic
indole rings were arranged in an “edge-to-face” configuration,
with a distance of 3.45 Å between the nearest aromatic indole
rings (Figure S7). Weak van der Waals forces
connected the layers (Figure 3d and Figure S8). Therefore, cyclo-WA,
with its 2D hydrogen bond networks, allows layer-by-layer supramolecular
stacking, resulting in long prismoid-like structures (Figure 2a). This layered structure
differs from that of typical reported peptides, where interlayer molecules
are stabilized primarily by aromatic interactions, without the formation
of 2D hydrogen bond networks.20,39
direction, another parallel layer was stabilized
by aromatic interactions of side-chain indole rings and packed in
an interlocked zipper-like orientation, forming a double-layer structure
(Figure 3d). The aromatic
indole rings were arranged in an “edge-to-face” configuration,
with a distance of 3.45 Å between the nearest aromatic indole
rings (Figure S7). Weak van der Waals forces
connected the layers (Figure 3d and Figure S8). Therefore, cyclo-WA,
with its 2D hydrogen bond networks, allows layer-by-layer supramolecular
stacking, resulting in long prismoid-like structures (Figure 2a). This layered structure
differs from that of typical reported peptides, where interlayer molecules
are stabilized primarily by aromatic interactions, without the formation
of 2D hydrogen bond networks.20,39
Figure 3.
(a) The cyclo-WA molecular asymmetric unit with an ordered water molecule. Color code: green, C; blue, N; red, O, with hydrogens omitted for clarity. (b) Water molecules acting as hydrogen bond donors to bridge the cyclo-WA molecules. (c) The single-layer arrangement of cyclo-WA molecules. (d) The cyclo-WA molecules packing into zipper-like units and creating a 2D layer-by-layer structure. (e) The cyclo-WS molecular asymmetric unit. (f) Hydroxyl groups acting as hydrogen bond donors to connect the cyclo-WS molecules. (g) The cyclo-WS molecules connected in dense hydrogen bond networks. (h) The cyclo-WS molecular arrangement in a 3D zigzag-like network.
Furthermore, the cyclo-WS crystal shared the same
space group (P21) as the cyclo-WA crystal
but had different
cell parameters (a = 6.1773 Å, b = 13.3209 Å, and c = 8.1672
Å) (Table S1 and Figure S9). The asymmetric
building unit comprised a single peptide molecule (Figure 3e), and two peptide molecules
were contained in a unit cell (Figure S10) without water molecules. The serine hydroxyl group served as a
hydrogen bond donor, directly connecting adjacent molecules without
the need for intermediate water molecules (Figure 3f). In the bc plane, one
cyclo-WS molecule was linked to four adjacent molecules by forming
two pairs of hydrogen bonds (Figure 3g). The indole rings were bound to diketopiperazine
rings on the neighboring molecules, with an N(H) ... C=O (donor···
acceptor) distance of 2.873 Å. In the backbone, one oxygen atom
connected to the hydroxyl group on the adjacent molecule, and another
oxygen atom interacted with the nitrogen atom on the next indole ring.
An additional hydrogen bond was formed between the hydroxyl group
and a diketopiperazine ring on an adjacent molecule, with an O(H)hydroxyl... C=O (donor··· acceptor) distance
of 2.729 Å. These molecules aligned along the a-axis and packed in a zigzag-like orientation in the crystallographic 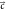 direction, creating a 3D hydrogen bond
network (Figure 3h).
The addition of a hydroxyl group triggered a dramatic transformation
in the supramolecular packing, transitioning from a 2D layered to
a 3D network structure, by providing a hydrogen bond donor to directly
connect adjacent molecules. Therefore, increasing the number of hydrogen
bond sites, such as hydroxyl groups, in molecules promotes the formation
of architectures with 3D hydrogen bond networks. With fewer of these
sites, molecules tend to form crystal structures with 2D or one-dimensional
(1D) hydrogen bond networks.
direction, creating a 3D hydrogen bond
network (Figure 3h).
The addition of a hydroxyl group triggered a dramatic transformation
in the supramolecular packing, transitioning from a 2D layered to
a 3D network structure, by providing a hydrogen bond donor to directly
connect adjacent molecules. Therefore, increasing the number of hydrogen
bond sites, such as hydroxyl groups, in molecules promotes the formation
of architectures with 3D hydrogen bond networks. With fewer of these
sites, molecules tend to form crystal structures with 2D or one-dimensional
(1D) hydrogen bond networks.
The diverse dimensionality of supramolecular packing reflects the difference in hydrogen bond interactions, which can be investigated by Hirshfeld surface analysis, a useful tool for predicting the intermolecular interactions in organic crystals.40−42 In the cyclo-WA dnorm surface, areas denoted in red indicated strong O···H and H···O interactions, resulting from the formation of hydrogen bonds between dipeptide and water molecules (Figure S11a). In contrast, the red regions in the cyclo-WS dnorm surface were not only located near indole and diketopiperazine rings but also in proximity to the hydroxyl group, which serves as an additional hydrogen bond donor (Figure S11b). In addition, the blue area highlighted less polar interactions, including aromatic interactions and van der Waals forces. The detailed contribution of the interactions between O and H atoms to overall interatomic interactions was quantified in 2D fingerprint plots. As shown in Figure S11c and S11d, cyclo-WS, with its 3D networks, exhibited a higher contact between H and O atoms compared to cyclo-WA with 2D layers, suggesting stronger hydrogen bond strength in cyclo-WS.
The hydrogen bond modes and overall strength of the supramolecular packing affect the mechanical strength of assemblies, which can be evaluated by Young’s modulus and point stiffness measurements.43,44 Using atomic force microscopy (AFM), the tip was scanned over the surface of cyclo-WA and cyclo-WS single crystals with a smooth area of 5 × 5 μm2. We determined Young’s modulus values of cyclo-WA and cyclo-WS crystals (Figure 4a-f and Figure S12–S14) by fitting force–displacement traces collected at different points. The measured planes were the (001) crystal plane for cyclo-WA and cyclo-WS, corresponding to the largest predicted plane, according to the Bravais, Friedel, Donnay, and Harker (BFDH) theory (Figure S15).45,46 The cyclo-WA crystals displayed a relatively low Young’s modulus of ∼3.5 GPa, suggesting mechanical flexibility (Figure 4b). On the other hand, the Young’s modulus of cyclo-WS crystals was much higher at approximately ∼49 GPa (Figure 4e), which is about 14 times greater than that of cyclo-WA. A significant enhancement in the point stiffness was observed from 56.6 N m–1 for cyclo-WA to 407.9 N m–1 for cyclo-WS (Figure 4c,f), indicating a 7× improvement in point stiffness. The dramatic improvement in the mechanical properties can be ascribed to the different packing architectures. The cyclo-WA molecules, connected by 2D hydrogen bond networks, exhibited weaker atomic interactions between layers, while the cyclo-WS molecules, linked by a 3D hydrogen bond network, demonstrated higher mechanical strength.20,21 Our approach demonstrates an efficient method to control the mechanical properties of biomaterials by engineering the building unit through a single site substitution of H → OH to create the desired 3D hydrogen bond network.
Figure 4.
(a-f) Comparison of (a-c) cyclo-WA and (d-f) cyclo-WS. (a, d) Topographic modulus maps. (b, e) The statistical Young’s modulus distributions. (c, f) Statistical point stiffness distributions. (g-i) DFT-predicted (g) dielectric constants where εr represents the average dielectric constant, (h) piezoelectric strain constants and (i) voltage constants for cyclo-WA, and cyclo-WS crystals.
The noncentrosymmetric structures of cyclo-WA and cyclo-WS crystals indicate that they will exhibit some degree of electromechanical response. The size of the piezoelectric response of biomaterials, namely the generation of voltage in response to deformation, is associated with the directionally aligned hydrogen bonds generating net uncompensated supramolecular dipoles.47 The piezoelectric charge constants, piezoelectric strain constants, and piezoelectric voltage constants, as well as the dielectric constants of cyclo-WA and cyclo-WS crystals, were predicted by density functional theory (DFT) calculations (Figure 4g-i and Table S2,S3). The cyclo-WA and cyclo-WS crystals have the same space group, P21, resulting in a similar-shaped piezoelectric tensor, including three out-of-plane and five shear nonzero piezoelectric coefficients. Cyclo-WA exhibited significantly higher out-of-plane and shear piezoelectric charge constants compared to cyclo-WS. Especially, the cyclo-WA crystals exhibited the highest out-of-plane and shear piezoelectric coefficients of d22 = 9.3 pC N1–, and d34 = 11.6 pC N1–, respectively (Figure 4h). In contrast, the cyclo-WS crystals showed smaller piezoelectric responses with an out-of-plane and a shear piezoelectric coefficient of d22 = 4.8 pC N1–, and d34 = 9.4 pC N1–, respectively (Figure 4h). The larger response for cyclo-WA is consistent with the softer layered structure, which facilitates the piezoelectric polarization under the same applied force. The continuous 3D hydrogen bond network observed for cyclo-WS results in high stiffness along the longitudinal direction (Figure 4e,f), accompanied by lower out-of-plane piezoelectricity. Furthermore, a calculated maximum piezoelectric voltage constant (Vmax) value on the order of 0.5 V (Figure 4i) suggests potential applications for the cyclo-dipeptides in implanted actuators, strain sensors, and energy harvesting devices, with predicted g34 of 490.0 mV mN–1 and 425.4 mV mN–1 for cyclo-WA and cyclo-WS, respectively.
To test the potential suitability of the peptides as implanted biomaterials, we investigated the biocompatibility of cyclo-WA and cyclo-WS crystals toward HeLa cells by an MTT-based cell viability assay. This was necessary as the utilization of organic solvents during materials synthesis and crystal growth may reduce the biocompatibility of both natural and bioinspired peptides, which in turn limits the application of peptides as implantable materials in the fields of biomedicine, bioengineering, and energy harvesting in vivo.48 More specifically, it is essential to determine the influence of functional groups, including the – OH site, on the cellular toxicity of the peptides. As displayed in Figure S16, both as-prepared cyclo-dipeptide assemblies showed high biocompatibility, in which the cellular viability of cells grown in the presence of 1 mg mL–1 cyclo-WA and cyclo-WS reached 80% and 82%, respectively after incubating at 37 °C for 24 h (Figure S16). Although the increase in peptide concentration decreased cellular viability, it was still maintained at near 60% for cyclo-WA and more than 60% for cyclo-WS at a high concentration of 10 mg mL–1 (Figure S16). Following the good cell viability (negligible cytotoxicity or no toxicity at all) assays, we examined the in vitro interaction of human cells with cyclo-WA and cyclo-WS crystals after 24 h (Figure S17). We observed very light green fluorescence residing in the cytoplasm (Figure S17), and very high fluorescence in the nucleus (after staining with life cell nucleus dye (Hoechst dye) in the nucleus). Moreover, we noticed that the cell is healthy even after treatment with cyclo-WA and cyclo-WS crystals, which suggests that the above-synthesized crystal is highly biocompatible. The result was further confirmed by the live/dead analysis of cells using live-cell confocal microscopy (Figure S17), indicating the excellent biocompatibility of cyclo-WA and cyclo-WS crystals. For future applications, it would be interesting to evaluate cell viability over a longer time postincubation with cyclo-peptides, extending beyond the standard 24-h live/dead assay used in the current work. Combining the high predicted piezoelectric response (Figure 4h,i) with the measured biocompatibility, the dipeptide crystals show significant potential for implanted electronic device applications.
Protons can move along hydrogen bonds and water molecules in crystals, lowering the conductance band gap between valence-occupied levels and unoccupied electronic states.49 To study the electronic properties of the two distinct assemblies, we characterized the electronic band structures and density of states (DOS) properties of cyclo-WA and cyclo-WS crystals using DFT calculations. The cyclo-WA structure with bridging water molecules showed a band gap of 3.21 eV above a Fermi level at 1.21 eV (Figure 5a,b). Cyclo-WS showed a slightly larger band gap (3.45 eV) (Figure S18), which may reflect the absence of water-based proton hopping sites in cyclo-WS.49 The calculated band gaps of cyclo-WA and cyclo-WS of 3.21 and 3.45 eV, respectively, are consistent with the trend and close to systematic slight underestimation50 to the measured values (3.33 eV for cyclo-WA and 3.87 eV for cyclo-WS) obtained from UV–visible absorption spectroscopy (Figure S19). The corresponding wavelengths for cyclo-WA and cyclo-WS are 375 and 366 nm, respectively.
Figure 5.
(a) Calculated band structure of the cyclo-WA crystal. (b) The corresponding projected-to-atoms density of states with the Fermi energy level shifted to zero. The calculated electronic properties of cyclo-WS are shown in Figure S18. (c, d) Microscopy images of (c) cyclo-WA and (d) cyclo-WS crystals in a dark field and in different fluorescence channels, as indicated.
The proton transfer between hydrogen bond donors and acceptors also results in lower electron excitation energies, thereby improving fluorescence.51 Furthermore, supramolecular structures containing aromatic residues can potentially exhibit high fluorescence properties, as the dipolar coupling between the excited states of aromatic residues contributes to fluorescence.50 Based on our measurements (Figure 5c,d), both cyclo-WA and cyclo-WS assemblies emitted blue, cyan, green, and weak yellow fluorescence under UV light excitation. These fluorescence properties may arise from a combination of aromatic interactions, hydrogen bonds, and a red-edge excitation shift.52 However, the assemblies showed variable degrees of fluorescence, with the cyclo-WS assembly effectively invisible by yellow fluorescence (Figure 5d). Fluorescence emission spectra were recorded for cyclo-WA and cyclo-WS assemblies to verify their fluorescent properties. Cyclo-WA crystals emit at approximately 332 and 387 nm in the UV region when excited between 280 and 310 nm and 320–350 nm, respectively (Figure S20a). Additionally, these crystals exhibit blue light emission when excited between 360 and 410 nm (Figure S20a). Conversely, cyclo-WS crystals show emission at about 328 nm with excitation from 280 to 300 nm (Figure S20b). Notably, the emission shifts from the UV to the blue light region as the excitation wavelength increases from 310 to 410 nm (Figure S20b).
In this work, we demonstrated two different dimensionalities of supramolecular packing of cyclo-dipeptides: 2D layered and 3D network structures with distinct macroscale properties, achieved simply by replacing one hydrogen atom with a hydroxyl group to insert a key hydrogen bond moiety. The cyclo-dipeptide molecule lacking hydroxyl groups formed 2D layered structures through connected water-mediated hydrogen bonds, stabilized by zipper-like motifs and weak van der Waals contacts between the layers. In contrast, the addition of a hydroxyl group on the molecule provided a hydrogen bond donor to directly connect adjacent molecules in a water-free zigzag-like 3D hydrogen bond network. As a result, the dimensional, optical, mechanical, piezoelectric, and electronic characteristics can be modulated. Specifically, the 3D network structure demonstrated a 14-fold increase in stiffness compared to the 2D structure. Meanwhile, the water-mediated 2D layered structure, with weak interlayer interactions exhibited a smaller predicted band gap and higher predicted piezoelectricity along the longitudinal direction, whereas the 3D network structure generating a chemically and physically more rigid structure with weaker electromechanical response overall. Moreover, an MTT-based cell viability assay confirmed the good biocompatibility of both cyclo-dipeptide crystals, underscoring their potential for in vivo applications. Our findings provide a base for understanding how diverse supramolecular packing modes embed functionality in 2D layered and 3D network structures, paving the way to programmable dimensionality of hydrogen bond networks at the atomic level and thus programmable properties for a wide range of self-assembled materials.
Acknowledgments
This work was supported in part by the Ministry of Science and Technology of Israel project (grant no. 3-18130) within the China-Israel Cooperative Scientific Research (2022YFE0100800), the ISF- Israel Science Foundation by Grant no. 3246/23, Twin2pipsa - Twinning for excellence in biophysics of protein interactions and self-assembly grant (101079147), Open Foundation of the State Key Laboratory of Fluid Power and Mechatronic Systems (GZKF-202224) and International Partnership Program of the Chinese Academy of Sciences (039GJHZ2023058FN). D.T. and P.A.C. acknowledge support from Science Foundation Ireland (SFI) under award number 12/RC/2275_P2 (SSPC) and supercomputing resources at the SFI/Higher Education Authority Irish Center for High-End Computing (ICHEC).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmaterialslett.4c00665.
Experiment method, morphology, structure, van der Waals forces, Hirshfield surfaces, and property characterizations including SEM images, single-crystal structural analysis, powder XRD, mechanical properties, DFT calculated results, mechanical, electrical, piezoelectric, and biocompatible property of cyclo-WA and cyclo-WS crystals (PDF)
Crystallographic data (CCDC 2304390) (CIF)
Crystallographic data (CCDC 2304391) (CIF)
Author Contributions
● H.Y. and P.C. contributed equally to this paper. Hui Yuan: Conceptualization, Methodology, Investigation, Methodology, Validation, Visualization, Writing - original draft. Pierre-Andre Cazade: Software, Validation, Writing -original draft. Chengqian Yuan: Validation, Visualization, Writing - Review & Editing. Bin Xue: Methodology, Validation, Writing-original draft. Vijay Bhooshan Kumar: Methodology, Validation, Writing - original draft. Rusen Yang: Validation, Visualization, Writing - Review & Editing. Gal Finkelstein-Zuta: Visualization, Writing - Review & Editing. Lihi Gershon: Methodology, Validation. Maoz Lahav: Methodology, Validation. Sigal Rencus-Lazar: Writing - Review & Editing. Yi Cao: Resources. David Levy: Methodology, Validation. Damien Thompson: Conceptualization, Software, Resources, Writing - Review & Editing. Ehud Gazit: Conceptualization, Validation, Resources, Supervision, Writing - Review & Editing.
The authors declare no competing financial interest.
Supplementary Material
References
- Gazit E. Self-Assembled Peptide Nanostructures: The Design of Molecular Building Blocks and Their Technological Utilization. Chem. Soc. Rev. 2007, 36, 1263–1269. 10.1039/b605536m. [DOI] [PubMed] [Google Scholar]
- Patterson J.; Martino M. M.; Hubbell J. A. Biomimetic Materials in Tissue Engineering. Mater. Today. 2010, 13, 14–22. 10.1016/S1369-7021(10)70013-4. [DOI] [Google Scholar]
- Gilead S.; Gazit E. Self-Organization of Short Peptide Fragments: From Amyloid Fibrils to Nanoscale Supramolecular Assemblies. Supramol. Chem. 2005, 17, 87–92. 10.1080/10610270412331328943. [DOI] [Google Scholar]
- Fleming S.; Ulijn R. V. Design of Nanostructures Based on Aromatic Peptide Amphiphiles. Chem. Soc. Rev. 2014, 43, 8150–8177. 10.1039/C4CS00247D. [DOI] [PubMed] [Google Scholar]
- Michaels T. C. T.; Šarić A.; Curk S.; Bernfur K.; Arosio P.; Meisl G.; Dear A. J.; Cohen S. I. A.; Dobson C. M.; Vendruscolo M.; Linse S.; Knowles T. P. J. Dynamics of Oligomer Populations Formed During the Aggregation of Alzheimer’s Aβ42 Peptide. Nat. Chem. 2020, 12, 445–451. 10.1038/s41557-020-0452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser C. A.; Zhang S. Designer Self-Assembling Peptide Nanofiber Biological Materials. Chem. Soc. Rev. 2010, 39, 2780–2790. 10.1039/b921448h. [DOI] [PubMed] [Google Scholar]
- Yuan C.; Ji W.; Xing R.; Li J.; Gazit E.; Yan X. Hierarchically Oriented Organization in Supramolecular Peptide Crystals. Nat. Rev. Chem. 2019, 3, 567–588. 10.1038/s41570-019-0129-8. [DOI] [Google Scholar]
- Chang R.; Yuan C.; Zhou P.; Xing R.; Yan X. Peptide Self-Assembly: From Ordered to Disordered. Acc. Chem. Res. 2024, 57, 289–301. 10.1021/acs.accounts.3c00592. [DOI] [PubMed] [Google Scholar]
- Berger O.; Adler-Abramovich L.; Levy-Sakin M.; Grunwald A.; Liebes-Peer Y.; Bachar M.; Buzhansky L.; Mossou E.; Forsyth V. T.; Schwartz T.; Ebenstein Y.; Frolow F.; Shimon L. J. W.; Patolsky F.; Gazit E. Light-Emitting Self-Assembled Peptide Nucleic Acids Exhibit Both Stacking Interactions and Watson-Crick Base Pairing. Nat. Nanotechnol. 2015, 10, 353–360. 10.1038/nnano.2015.27. [DOI] [PubMed] [Google Scholar]
- Whaley S. R.; English D. S.; Hu E. L.; Barbara P. F.; Belcher A. M. Selection of Peptides with Semiconductor Binding Specificity for Directed Nanocrystal Assembly. Nature 2000, 405, 665–668. 10.1038/35015043. [DOI] [PubMed] [Google Scholar]
- Lee J. S.; Yoon I.; Kim J.; Ihee H.; Kim B.; Park C. B. Self-Assembly of Semiconducting Photoluminescent Peptide Nanowires in the Vapor Phase. Angew. Chem., Int. Ed. 2011, 50, 1164–1167. 10.1002/anie.201003446. [DOI] [PubMed] [Google Scholar]
- Bera S.; Mondal S.; Xue B.; Shimon L. J. W.; Cao Y.; Gazit E. Rigid Helical-Like Assemblies from a Self-Aggregating Tripeptide. Nat. Mater. 2019, 18, 503–509. 10.1038/s41563-019-0343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen V.; Zhu R.; Jenkins K.; Yang R. Self-Assembly of Diphenylalanine Peptide with Controlled Polarization for Power Generation. Nat. Commun. 2016, 7, 13566. 10.1038/ncomms13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leon Rodriguez L. M.; Hemar Y.; Cornish J.; Brimble M. A. Structure-Mechanical Property Correlations of Hydrogel Forming Β-Sheet Peptides. Chem. Soc. Rev. 2016, 45, 4797–4824. 10.1039/C5CS00941C. [DOI] [PubMed] [Google Scholar]
- Yuan H.; Han P.; Tao K.; Liu S.; Gazit E.; Yang R. Piezoelectric Peptide and Metabolite Materials. Research 2019, 2019, 9025939. 10.34133/2019/9025939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R.; Qin Y.; Dai L.; Wang Z. L. Power Generation with Laterally Packaged Piezoelectric Fine Wires. Nat. Nanotechnol. 2009, 4, 34–39. 10.1038/nnano.2008.314. [DOI] [PubMed] [Google Scholar]
- Yang F.; Li J.; Long Y.; Zhang Z.; Wang L.; Sui J.; Dong Y.; Wang Y.; Taylor R.; Ni D.; Cai W.; Wang P.; Hacker T.; Wang X. Wafer-Scale Heterostructured Piezoelectric Bio-Organic Thin Films. Science 2021, 373, 337–342. 10.1126/science.abf2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler-Abramovich L.; Gazit E. The Physical Properties of Supramolecular Peptide Assemblies: From Building Block Association to Technological Applications. Chem. Soc. Rev. 2014, 43, 6881–6893. 10.1039/C4CS00164H. [DOI] [PubMed] [Google Scholar]
- Nonappa; Ikkala O. Hydrogen Bonding Directed Colloidal Self-Assembly of Nanoparticles into 2d Crystals, Capsids, and Supracolloidal Assemblies. Adv. Funct. Mater. 2018, 28, 1704328. 10.1002/adfm.201704328. [DOI] [Google Scholar]
- Adler-Abramovich L.; et al. Bioinspired Flexible and Tough Layered Peptide Crystals. Adv. Mater. 2018, 30, 1704551. 10.1002/adma.201704551. [DOI] [PubMed] [Google Scholar]
- Li D.-L.; Zhang L.; Xu J.-K.; Chen L.-N.; Bao J.-B.; Wang Z.-B. Eco-Friendly Strategy to Improve the Processiblity and Properties of Poly(Vinyl Alcohol) Foams Based on a 3D Hydrogen-Bond Network. Ind. Eng. Chem. Res. 2020, 59, 20011–20021. 10.1021/acs.iecr.0c02910. [DOI] [Google Scholar]
- Bera S.; Mondal S.; Rencus-Lazar S.; Gazit E. Organization of Amino Acids into Layered Supramolecular Secondary Structures. Acc. Chem. Res. 2018, 51, 2187–2197. 10.1021/acs.accounts.8b00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Li Q.; Wu A.; Li J. Co2 Induces Symmetry Breaking in Layered Dipeptide Crystals. Angew. Chem., Int. Ed. Engl. 2023, 62, e202214184 10.1002/anie.202214184. [DOI] [PubMed] [Google Scholar]
- Zelenovskii P. S.; Romanyuk K.; Liberato M. S.; Brandão P.; Ferreira F. F.; Kopyl S.; Mafra L. M.; Alves W. A.; Kholkin A. L. 2D Layered Dipeptide Crystals for Piezoelectric Applications. Adv. Funct. Mater. 2021, 31, 2102524. 10.1002/adfm.202102524. [DOI] [Google Scholar]
- Ganguly R.; Sreenivasulu B.; Vittal J. J. Amino Acid-Containing Reduced Schiff Bases as the Building Blocks for Metallasupramolecular Structures. Coord. Chem. Rev. 2008, 252, 1027–1050. 10.1016/j.ccr.2008.01.005. [DOI] [Google Scholar]
- Bartocci S.; Morbioli I.; Maggini M.; Mba M. Solvent-Tunable Morphology and Emission of Pyrene-Dipeptide Organogels. J. Pept. Sci. 2015, 21, 871–878. 10.1002/psc.2829. [DOI] [PubMed] [Google Scholar]
- Koley P.; Sakurai M.; Aono M. Tunable Morphology from 2D to 3D in the Formation of Hierarchical Architectures from a Self-Assembling Dipeptide: Thermal-Induced Morphological Transition to 1D Nanostructures. J. Mater. Sci. 2015, 50, 3139–3148. 10.1007/s10853-015-8875-6. [DOI] [Google Scholar]
- Sun L.; Fan Z.; Wang Y.; Huang Y.; Schmidt M.; Zhang M. Tunable Synthesis of Self-Assembled Cyclic Peptide Nanotubes and Nanoparticles. Soft Matter. 2015, 11, 3822–3832. 10.1039/C5SM00533G. [DOI] [PubMed] [Google Scholar]
- Tian Y.; Zhang H. V.; Kiick K. L.; Saven J. G.; Pochan D. J. Fabrication of One- and Two-Dimensional Gold Nanoparticle Arrays on Computationally Designed Self-Assembled Peptide Templates. Chem. Mater. 2018, 30, 8510–8520. 10.1021/acs.chemmater.8b03206. [DOI] [Google Scholar]
- Yuan H.; Chen Y.; Lin R.; Tan D.; Zhang J.; Wang Y.; Gazit E.; Ji W.; Yang R. Modified Stranski-Krastanov Growth of Amino Acid Arrays toward Piezoelectric Energy Harvesting. ACS Appl. Mater. Interfaces 2022, 14, 46304–46312. 10.1021/acsami.2c13399. [DOI] [PubMed] [Google Scholar]
- Torricelli F.; Adrahtas D. Z.; Bao Z.; Berggren M.; Biscarini F.; Bonfiglio A.; Bortolotti C. A.; Frisbie C. D.; Macchia E.; Malliaras G. G.; McCulloch I.; Moser M.; Nguyen T.-Q.; Owens R. M.; Salleo A.; Spanu A.; Torsi L. Electrolyte-Gated Transistors for Enhanced Performance Bioelectronics. Nat. Rev. Methods Primers 2021, 1, 66. 10.1038/s43586-021-00065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S. S.; Katz H. E.; Tovar J. D. Solid-State Electrical Applications of Protein and Peptide Based Nanomaterials. Chem. Soc. Rev. 2018, 47, 3640–3658. 10.1039/C7CS00817A. [DOI] [PubMed] [Google Scholar]
- Tomar D.; Rana B.; Jena K. C. The Structure of Water-Dmf Binary Mixtures Probed by Linear and Nonlinear Vibrational Spectroscopy. J. Chem. Phys. 2020, 152, 114707. 10.1063/1.5141757. [DOI] [PubMed] [Google Scholar]
- Espinosa-Andrews H.; Sandoval-Castilla O.; Vázquez-Torres H.; Vernon-Carter E. J.; Lobato-Calleros C. Determination of the Gum Arabic-Chitosan Interactions by Fourier Transform Infrared Spectroscopy and Characterization of the Microstructure and Rheological Features of Their Coacervates. Carbohydr. Polym. 2010, 79, 541–546. 10.1016/j.carbpol.2009.08.040. [DOI] [Google Scholar]
- Liu M. J.; Wang Z.; Wu R. C.; Sun S. Q.; Wu Q. Y. Monitoring All-Trans-Retinoic Acid-Induced Differentiation of Human Acute Promyelocytic Leukemia Nb4 Cells by Fourier-Transform Infrared Spectroscopy. Leukemia 2003, 17, 1670–1674. 10.1038/sj.leu.2403019. [DOI] [PubMed] [Google Scholar]
- Sajan D.; Joe I. H.; Jayakumar V. S. Nir-Ft Raman, Ft-Ir and Surface-Enhanced Raman Scattering Spectra of Organic Nonlinear Optic Material: P-Hydroxy Acetophenone. J. Raman Spectrosc. 2006, 37, 508–519. 10.1002/jrs.1424. [DOI] [Google Scholar]
- Kolesov B. Raman Investigation of H2O Molecule and Hydroxyl Groups in the Channels of Hemimorphite. Am. Mineral. 2006, 91, 1355–1362. 10.2138/am.2006.2179. [DOI] [Google Scholar]
- Bondesson L.; Mikkelsen K. V.; Luo Y.; Garberg P.; Ågren H. Hydrogen Bonding Effects on Infrared and Raman Spectra of Drug Molecules. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2007, 66, 213–224. 10.1016/j.saa.2006.02.045. [DOI] [PubMed] [Google Scholar]
- Yan X.; Su Y.; Li J.; Früh J.; Möhwald H. Uniaxially Oriented Peptide Crystals for Active Optical Waveguiding. Angew. Chem., Int. Ed. 2011, 50, 11186–11191. 10.1002/anie.201103941. [DOI] [PubMed] [Google Scholar]
- Spackman P. R.; Turner M. J.; McKinnon J. J.; Wolff S. K.; Grimwood D. J.; Jayatilaka D.; Spackman M. A. Crystalexplorer: A Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. 10.1107/S1600576721002910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spackman M. A.; Jayatilaka D. Hirshfeld Surface Analysis. CrystEngComm. 2009, 11, 19–32. 10.1039/B818330A. [DOI] [Google Scholar]
- McKinnon J. J.; Fabbiani F. P. A.; Spackman M. A. Comparison of Polymorphic Molecular Crystal Structures through Hirshfeld Surface. Cryst. Growth Des. 2007, 7, 755–769. 10.1021/cg060773k. [DOI] [Google Scholar]
- Azuri I.; Meirzadeh E.; Ehre D.; Cohen S. R.; Rappe A. M.; Lahav M.; Lubomirsky I.; Kronik L. Unusually Large Young’s Moduli of Amino Acid Molecular Crystals. Angew. Chem., Int. Ed. Engl. 2015, 54, 13566–13570. 10.1002/anie.201505813. [DOI] [PubMed] [Google Scholar]
- Yuan H.; Xue B.; Yang D.; Rencus-Lazar S.; Cao Y.; Gazit E.; Tan D.; Yang R. Rational Design of Biological Crystals with Enhanced Physical Properties by Hydrogen Bonding Interactions. Research 2023, 6, 0046. 10.34133/research.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza S.; Miroshnyk I.; Heinämäki J.; Antikainen O.; Rantanen J.; Vuorela P.; Vuorela H.; Yliruusi J. Crystal Morphology Engineering of Pharmaceutical Solids: Tabletting Performance Enhancement. AAPS PharmSciTechnol. 2009, 10, 113–119. 10.1208/s12249-009-9187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H.; Zhang J.; Rencus-Lazar S.; Ren Z.; Lin R.; Gazit E.; Yang R. The Engineering of Molecular Packing in Amino Acid Crystals for the Enhanced Triboelectric Effect. Nano Energy 2023, 110, 108375. 10.1016/j.nanoen.2023.108375. [DOI] [Google Scholar]
- Farrar D.; Ren K.; Cheng D.; Kim S.; Moon W.; Wilson W. L.; West J. E.; Yu S. M. Permanent Polarity and Piezoelectricity of Electrospun Α-Helical Poly(Α-Amino Acid) Fibers. Adv. Mater. 2011, 23, 3954–3958. 10.1002/adma.201101733. [DOI] [PubMed] [Google Scholar]
- Davoodbasha M. A.; Saravanakumar K.; Abdulkader A. M.; Lee S.-Y.; Kim J.-W. Synthesis of Biocompatible Cellulose-Coated Nanoceria with Ph-Dependent Antioxidant Property. ACS Appl. Bio Mater. 2019, 2, 1792–1801. 10.1021/acsabm.8b00647. [DOI] [PubMed] [Google Scholar]
- Andrade-Filho T.; Ferreira F. F.; Alves W. A.; Rocha A. R. The Effects of Water Molecules on the Electronic and Structural Properties of Peptide Nanotubes. Phys. Chem. Chem. Phys. 2013, 15, 7555–7559. 10.1039/c3cp43952f. [DOI] [PubMed] [Google Scholar]
- Borlido P.; Schmidt J.; Huran A. W.; Tran F.; Marques M. A. L.; Botti S. Exchange-Correlation Functionals for Band Gaps of Solids: Benchmark, Reparametrization and Machine Learning. Npj Comput. Mater. 2020, 6, 96. 10.1038/s41524-020-00360-0. [DOI] [Google Scholar]
- Pinotsi D.; Grisanti L.; Mahou P.; Gebauer R.; Kaminski C. F.; Hassanali A.; Kaminski Schierle G. S. Proton Transfer and Structure-Specific Fluorescence in Hydrogen Bond-Rich Protein Structures. J. Am. Chem. Soc. 2016, 138, 3046–3057. 10.1021/jacs.5b11012. [DOI] [PubMed] [Google Scholar]
- Berger O.; et al. Light-Emitting Self-Assembled Peptide Nucleic Acids Exhibit Both Stacking Interactions and Watson-Crick Base Pairing. Nat. Nanotechnol. 2015, 10, 353–360. 10.1038/nnano.2015.27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






