Abstract
Aims
Leadless pacemaker therapy was introduced to overcome lead- and pocket-related complications in conventional transvenous pacemaker systems. Implantation via the femoral vein, however, may not always be feasible. The aim of this study was to evaluate leadless pacemaker implantation using a jugular vein approach and compare it to the standard implantation via the femoral vein.
Methods and results
The records of the first consecutive 100 patients undergoing Micra™ leadless pacemaker implantation via the right internal jugular vein from two centres were included in this study. Peri-procedural safety and efficacy of the jugular approach were compared to the first 100 patients using a femoral implantation approach at the University Hospital Zurich. One hundred patients underwent successful implantation of a leadless pacemaker via the internal jugular vein (mean age, 81.18 ± 8.29, 60% males). Mean procedure time was 35.63 ± 10.29 min with a mean fluoroscopy time of 4.66 ± 5.16 min. The device was positioned at the inferior septum in 25 patients, at the high septum in 24 patients, and mid-septum in 51 patients. The mean pacing threshold was 0.56 ± 0.35 V at 0.24 ms pulse width with a sensed amplitude of 10.0 ± 4.4 mV. At follow-up, electrical parameters remained stable in all patients. Compared with femoral implantation, patients undergoing the jugular approach were of similar age and had similar comorbidities. Mean procedure (48.9 ± 21.0 min) and fluoroscopy times (7.7 ± 7.8 min, both P < 0.01) were shorter compared to the femoral approach. Electrical parameters were similar between the two approaches. There were only two complications during jugular veinous implantations (1 pericardial effusion and 1 dislocation), compared to 16 complications using the femoral approach (1 pericardial effusion, 2 femoral artery injuries, and 13 major groin haematomas).
Conclusion
The jugular approach may represent a safe and efficient alternative to femoral implantation of the Micra leadless pacemaker.
Keywords: Leadless pacing, Jugular vein, Pacemaker
Graphical Abstract
Graphical Abstract.
What’s new?
All jugular leadless pacemaker implantations were successful, and there was no bailout transfemoral implantation required.
Electrical parameters were good and remained stable in all patients undergoing a jugular approach.
The jugular approach may represent a safe and efficient alternative to the femoral route for implantation of leadless pacemakers
Introduction
Over the last 10 years, leadless pacing has become an important option for patients requiring pacemaker therapy, particularly to overcome potential transvenous lead- and pocket-related complications.1 Indeed, compared to conventional transvenous devices, device complications may be reduced by >50%, together with a high implantation success rate.2–4 However, leadless pacing also carries potential complications related to the implantation procedure. Pericardial tamponade and complications in the groin area, such as local haematoma, arteriovenous fistula, or arterial pseudoaneurysm, occur with a risk of approximately 1% each.2,3 Moreover, stenosis or a tortuous anatomy of the inferior vena cava (IVC) may hinder the successful implantation of a femoral leadless pacemaker.2,3 Alternative access sites may therefore be desirable to overcome these limitations. The first pilot series of leadless pacemaker implantation via the internal jugular venous route were reported to be safe and effective.5,6 The aim of this analysis was to compare jugular with the standard femoral leadless pacemaker implantation in a large tertiary referral centre.
Methods
Study population
This prospective analysis includes the first 50 consecutively enrolled patients undergoing jugular leadless pacemaker implantation from September 2022 to May 2024 at the University Hospital Zurich and the first 50 enrolled patients undergoing jugular leadless pacemaker implantation from March 2018 to December 2020 at the Haga Teaching Hospital in The Hague. The latter were part of a previous publication on a pilot experience of leadless pacemaker implantation via the jugular route.5 There was no contraindication for these patients to have a standard femoral implantation procedure; as such, a femoral implantation was the predefined bailout strategy in case jugular access or implantation turned out to not be feasible. Follow-up was conducted by assessing all available patient records from patient visits, hospitalizations, and device interrogation and tracings. The interventional and electrical parameters of the jugular approach were compared to the first 100 patients at the University Hospital Zurich who had undergone femoral leadless pacemaker implantation from 2015 to 2019. An access site haematoma was considered major if it resulted in significant clinical impact including prolonged hospitalization, transfusion, and interventional therapy. The study was approved by the local ethics committee of both institutions.
Implantation procedure
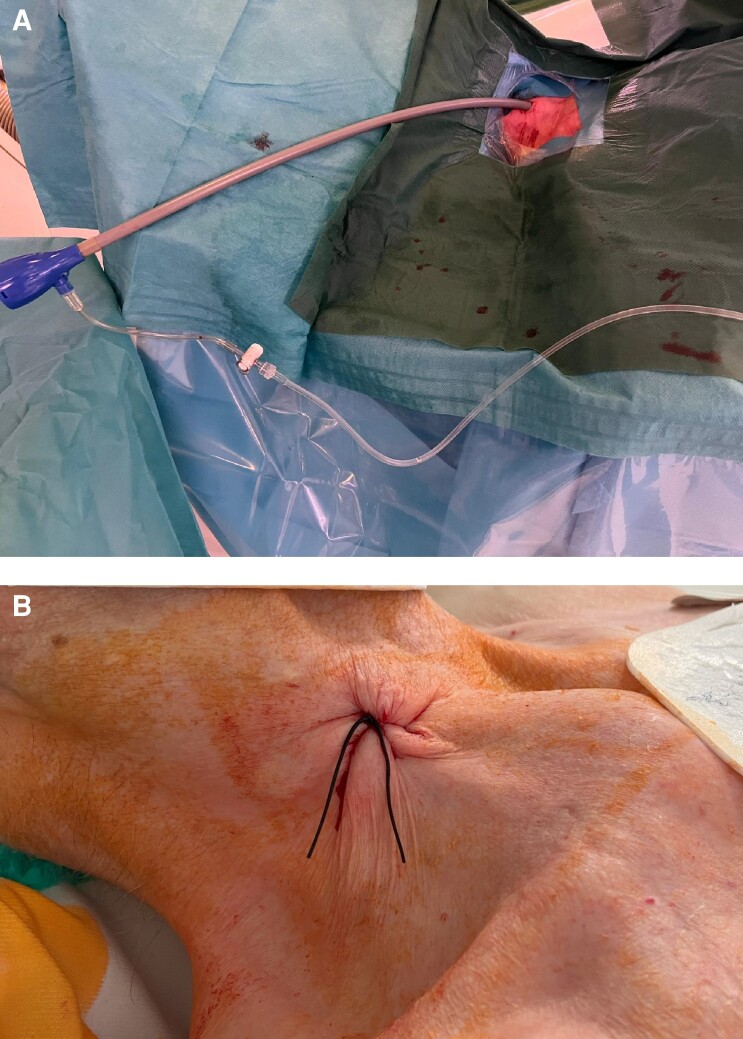
All implantations were performed in an electrophysiology laboratory as described before.6 Routinely, no sedation was applied except an initial bolus of 25–50 µg fentanyl. Peri-operative infection prophylaxis using cefuroxime was administered to all patients. Skin disinfection was performed at the area between the two heads of the sternocleidomastoid muscle and the clavicle. After the patient was draped, 1% lidocaine was injected under the skin using a small subcutaneous needle. Puncture of the right internal jugular vein was guided by ultrasound with a linear probe in all patients. A standard J-wire was introduced and advanced under fluoroscopy guidance into the IVC. A 1 cm skin incision was carefully performed, as for the femoral access, and a standard 9 Fr sheath introduced over the J-wire. The J-wire was then replaced by an Amplatz Super Stiff™ 0.035 in guidewire. The puncture site was predilated using a 20 Fr dilator followed by advancing the 27 Fr introducer sheath (Medtronic, Minneapolis, MN, USA; Figure 1A). The sheath was flushed, connected to a heparinized saline drip, and a bolus of 3000–5000 units of heparin was injected. A sterile table was placed at the right superior site of the patient’s head to facilitate sheath and device handling.
Figure 1.
(A) Entry site of the introducer sheath at the patient’s neck. (B) Puncture suture after removal of the sheath and delivery catheter. The access site is closed with a modified figure-of-eight stitch.
The delivery tool was advanced until the distal end of the sheath was positioned in the lower right atrium and the latter retracted back to the level of the superior vena cava (SVC). As for the femoral approach, a 15–30° right anterior oblique orientation is chosen, and the flection button on the delivery catheter is oriented downwards, which will direct the curve of the catheter towards the tricuspid annulus. Then, flexion is applied to direct the delivery catheter towards the centre of the tricuspid annulus, which is facilitated by the more anterior location of the SVC (as compared to the IVC) in combination with the natural curve of the delivery tool. In order to confirm coaxial alignment with the centre of the tricuspid annulus, a 45° left anterior oblique position is used to adjust the anterior/posterior orientation of the delivery system.
As for the femoral approach, the goal was to place the device at the septal area of the right ventricle (RV). The natural curve of the delivery catheter frequently directs the device into a high septal/right ventricular outflow tract position. However, the course within the vascular system straightens the delivery tool, and (particularly also in comparison to the femoral approach) it is easily possible to reach nearly all regions of the RV septum (high/mid/low) via the superior approach. Optimal location of the device was confirmed by injecting contrast dye. If a satisfactory position was found, the device was deployed, and electrical parameters were measured (a pacing threshold of <1 V at 0.24 ms and a sensed R-wave amplitude of >5 mV were considered adequate). Proper fixation of the leadless pacemaker was verified with the pull and hold test. If at least two out of four tines were engaged with the myocardium, the tether was cut and the device released. The delivery tool and the sheath were removed. The puncture site was closed with a modified figure-of-eight stitch (Figure 1B). Immediately after closing the puncture site, the interventional table was tilted into a shallow reversed Trendelenburg position to reduce venous pressure at the neck and to avoid the risk of bleeding.
Femoral implantation followed standard operating procedures as previously described.7 Of note, during the first years, ultrasound was not routinely used for Micra™ implantation via the femoral approach at our center.
Statistics
Categorical variables are reported as counts (percentage) and were compared and assessed through contingency tables and using a χ2 or Fisher’s exact test, as appropriate. Continuous variables are reported as mean± standard deviation. Distribution of normality was assessed with the Shapiro–Wilk test. Student’s t-test and Mann–Whitney U test were used for comparison of the continuous variables which were normally and not normally distributed, respectively. A two-sided P value of <0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics (Version 23).
Results
Jugular implantation
Patient characteristics are summarized in Table 1. Mean age was 81.18 ± 8.29 years, and 60% (n = 60) were male. The primary indication for pacemaker implantation was atrioventricular (AV) block in 39% (n = 39) of patients followed by tachy–brady syndrome or planned AV node ablation in 32 patients with permanent atrial fibrillation and 29 patients with sick sinus/pre-automatic pauses. There were no differences in the type of cardiomyopathy or number of patients with RV and/or right atrium (RA) dilation.
Table 1.
Baseline characteristics
| Jugular approach | Femoral approach | P value | |
|---|---|---|---|
| Age (years) | 81.18 ± 8.29 | 81.65 ± 6.22 | 0.649 |
| Left ventricular ejection fraction | 54.89 ± 733 | 53.68 ± 11.79 | 0.569 |
| Comorbidities | |||
| Atrial fibrillation | 73 (73%) | 79 (79%) | 0.317 |
| Chronic coronary artery disease | 36 (36%) | 34 (34%) | 0.768 |
| Valvular disease (severe AS, AI, MI, MS) | 43 (43%) | 27 (27%) | 0.027 |
| Chronic kidney disease | 37 (37%) | 67 (67%) | 0.001 |
| Chronic obstructive pulmonary disease | 13 (13%) | 7 (7%) | 0.258 |
| Peripheral artery disease | 18 (18%) | 19 (19%) | 0.857 |
| Diabetes mellitus | 24 (24%) | 18 (18%) | 0.302 |
| Cancer | 14 (14%) | 16 (16%) | 0.843 |
| Previous stroke | 20 (20%) | 15 (15%) | 0.358 |
Abbreviations: AS, aortic valve stenosis; AI, aortic valve insufficiency; MI, mitral valve insufficiency; MS, mitral valve stenosis.
All devices were successfully implanted using the jugular approach. Throughout the procedure, including access site dilation and sheath advancement into the right atrium, none of the patients reported discomfort or vagal reactions. The mean procedure time was 42.42 ± 18.36 min, with a mean fluoroscopy time of 4.66 ± 5.1 min. The device was positioned at the inferior septum in 25, at the mid-septal septum in 50, and at the high septum in 25 patients. In 70 patients, a sufficient device position was obtained at the first attempt, in 17 at the second, in 7 at the third, in 3 at the fourth, in 3 at the sixth, and in 1 patient in the eighth attempt. The mean final pacing threshold recorded at the end of the procedure was 0.56 ± 0.35 V with a pulse width of 0.24 ms. Ventricular sensing was measured at 10.0 ± 4.4 mV, and impedance at 772.32 ± 218.55 ohm (refer to Table 1 and Figure 2).
Figure 2.
Device parameters [RV (A) sensing, (B) impedance, and (C) threshold] at implantation, Day 1 and Day 14.
One pericardial effusion and one device dislocation occurred during the peri-interventional period. On the first day post-implantation, electrical parameters remained stable in all patients, with an average pacing threshold of 0.59 ± 0.52 V at 0.24 ms and a sensed amplitude of 11.39 ± 4.38 mV (Table 1 and Figure 2). Also at follow-up, all measured device parameters remained stable without significant changes (Table 1 and Figure 2).
Comparison with femoral implantation
Compared with femoral implantation, patients undergoing jugular transvenous pacemaker implantation were of similar age and had similar comorbidities. Atrial fibrillation (AF) was more common in the transfemoral group reflecting the indication spectrum of leadless pacemaker implantation during that period of time. Mean procedure and fluoroscopy times as well as dose were significantly shorter in the jugular compared to the femoral approach (procedure time 48.9 ± 21.0 min and fluoroscopy duration 7.7 ± 7.8 min, P < 0.01; Table 1). There were only small and clinically not meaningful differences in electrical parameters between the two approaches, both at implantation and during follow-up (Tables 2 and 3). There were only 2 major complications in the jugular group compared to 16 using the femoral approach (2 arterial injuries, 1 pericardial effusion, and 13 major haematomas).
Table 2.
Procedural and electrical parameters in patients undergoing jugular and femoral approach of leadless pacemaker implantation
| Jugular approach | Femoral approach | P value | |
|---|---|---|---|
| Final position | |||
| Infero-septal | 25 (25%) | 9 (9%) | <0.001 |
| Mid-septal | 50 (50%) | 89 (89%) | |
| High septal | 25 (25%) | 2 (2%) | |
| Procedure time (min) | 42.42 ± 18.36 min | 48.94 ± 21.04 | 0.02 |
| Fluoroscopy time (min) | 4.66 ± 51.52 | 7.65 ± 7.82 | 0.001 |
| Fluoroscopy dose (cG × cm2) | 828.68 ± 1264.53 | 842.83 ± 1006.30 | 0.930 |
| Final threshold (V @0.24 ms) | 0.56 ± 0.35 | 0.54 ± 0.31 | 0.722 |
| Final sensing (mV) | 10.0 ± 4.4 | 9.88 ± 4.42 | 0.799 |
| Final impedance (ohm) | 772.32 ± 218.55 | 705.84 ± 142.32 | 0.011 |
Table 3.
Electrical parameters at follow-up
| Jugular approach | Femoral approach | P value | |
|---|---|---|---|
| Threshold Day 1 (V @0.24 ms) | 0.59 ± 0.52 | 0.54 ± 0.35 | 0.446 |
| Sensing Day 1 (mV) | 10.88 ± 4.23 | 10.77 ± 4.35 | 0.865 |
| Impedance Day 1 (ohm) | 695.73 ± 205.55 | 662.87 ± 131.37 | 0.188 |
| Threshold Day 14 (V @0.24 ms) | 0.61 ± 0.44 | 0.61 ± 0.41 | 0.963 |
| Sensing Day 14 (mV) | 11.39 ± 4.38 | 11.67 ± 4.3 | 0.771 |
| Impedance Day 14 (ohm) | 611.08 ± 143.67 | 605.62 ± 96.74 | 0.675 |
Discussion
The aim of this analysis was to evaluate the feasibility and safety of a jugular approach for leadless pacemaker implantation and compare it to the standard femoral approach. Our data indicate that this access site may be as safe and effective with similarly excellent electrical values as well as shorter procedural- and fluoroscopy times.
Jugular implantation procedure
Jugular leadless pacemaker implantation has only recently been introduced into clinical practice.5 Previous pilot studies showed no puncture site–related complications such as haematoma, arteriovenous fistula, or arterial aneurysm, which are some of the most encountered complications with the femoral approach.5,6 One potential reason is that ultrasound guidance was commonly utilized during implantation procedures to puncture the internal jugular vein. Visualization of the puncture site is known to reduce potential complications when inserting central venous lines as well as in electrophysiological procedures.8–11 This may be even more important for the use of large-bore sheaths in the jugular vein due to the close proximity of the carotid artery and potentially severe consequences in case of inadvertent arterial laceration. Additionally, following jugular implantation, patients are advised to sit up immediately after the procedure. This not only offers greater convenience, especially for the elderly, but also notably decreases venous pressure at the neck level, reducing the risk of local haematoma. The lack of a femoral access allows for immediate patient mobilization hence potentially enabling outpatient implantation procedures as previously demonstrated in AF ablation.12
Another benefit is the more direct and shorter path into the right atrium compared to the femoral vein. Occasionally, discomfort and vagal responses occur when advancing the straight 27 Fr sheath through the more tortuous femoral and iliac veins due to mechanical stretching, which was not observed in our patients undergoing jugular implantation.2
In all patients, the device was positioned at the RV septum, deliberately avoiding an apical placement. The SVC’s more anterior position relative to the IVC, combined with the natural shape of the delivery catheter, facilitates better and easier angulation towards the ventricular septum (Figure 3). In the majority of cases, only one attempt was required to achieve a stable and electrically favourable position at the ventricular septum. The one patient who required eight deployments suffered from cardiac amyloidosis, which complicated the implantation procedure. The primary reason for the high number of deployments was unsatisfactory pacing thresholds. However, the shorter and more direct cranial route to the RV also results in a higher translation of forward pressure to the distal tip of the delivery catheter when advancing the device. While only about 10% of the forward pressure directly translates to the end of the delivery catheter in the femoral approach, the more direct force transmission during the jugular approach may potentially increase the risk of RV perforation, especially in an apical position. Although neither our cohort nor a previous pilot study reported such events,5 it remains crucial to be vigilant and utilize different fluoroscopy angulations during the implantation procedure to minimize this risk. Whether currently available risk predictors are also applicable for the jugular approach needs to be clarified in further studies.13
Figure 3.
More anterior access into the right atrium from superior. Abbreviations: IVC, inferior vena cava; LAO, left anterior oblique; RV, right ventricle; SVC, superior vena cava.
Comparison with femoral leadless pacemaker implantation
Mean fluoroscopy times for jugular implantations were shorter than in previous studies using femoral access14–16 and also shorter in comparison to the first hundred femoral leadless pacemaker implantations at our institution. This difference can partly be explained by the shorter and straighter route to the heart as well as by the easier angulation towards the RV septum. This notwithstanding, it may also at least partially reflect the fact that operators were already experienced in standard implantation of leadless pacemakers.17
During a follow-up of 14 days, all pacing parameters remained stable or improved slightly, which is in line with previous published data of patients undergoing femoral leadless pacemaker implantation3 and consistent with our femoral comparator cohort.
We observes two complications in patients undergoing jugular implantation, while a total of 16 major complications were observed in the first 100 femoral implant procedures. The most likely explanation of this difference may be the extensive experience of leadless pacemaker implantation which was present at the time of first attempting jugular implantation at the University Hospital Zurich. Moreover, the routine use of ultrasound guided venous puncture—which is strongly recommended in all implantations, and indispensable for jugular puncture—was not used in the early phase of femoral Micra implantation and has greatly helped avoid vascular complications since. It is tempting to speculate, however, that also the route of access itself may have contributed to a lower complication rate. This is fuelled by the fact that the first 50 cases from The Hague Center represent the first ever leadless pacemaker implantations at this centre. Further studies are necessary to substantiate these findings.
Limitations
Although consecutive patients deemed eligible for jugular implantation were analysed, a certain degree of selection bias cannot be excluded in this prospective observational analysis. Furthermore, our data stem from high-volume tertiary referral centres with extensive experience in both leadless pacemaker implantation and management of large-bore sheaths from the jugular vein. As such, they may not be transferable to other healthcare settings; indeed, extensive training in both leadless pacemaker implantation and jugular vein puncture and large-bore sheath handling is strongly recommended before embarking onto jugular vein leadless pacemaker implantation. Finally, comparison was made to a historical cohort of first 100 transfemoral implantations.
Conclusion
Our data indicate that jugular transvenous leadless pacemaker implantation is a safe and effective alternative to the standard femoral approach, with similar if not better peri-procedural safety and long-term electrical outcomes. This notwithstanding, specific care needs to be taken when using this route of implantation, and further studies are required to confirm the safety of this approach outside large volume centres.
Contributor Information
Nadine Molitor, Department of Cardiology, University Heart Center, University Hospital Zurich, Raemistrasse 100, 8091 Zurich, Switzerland.
Shmaila Saleem-Talib, Department of Cardiology, Haga Teaching Hospital, The Hague, The Netherlands.
Hemanth Ramanna, Department of Cardiology, Haga Teaching Hospital, The Hague, The Netherlands; The Hague University of Applied Sciences, The Hague, The Netherlands.
Daniel Hofer, Department of Cardiology, Triemlispial, Zurich, Switzerland.
Alexander Breitenstein, Department of Cardiology, University Heart Center, University Hospital Zurich, Raemistrasse 100, 8091 Zurich, Switzerland.
Jan Steffel, HeartClinic, Hirslanden Clinic, Zurich, Switzerland.
Funding
None declared.
Data availability
Data are available on reasonable request. On request and associated need, our data are available, while our utmost intention is to protect our patient’s privacy.
References
- 1. Defaye P, Biffi M, El-Chami M, Boveda S, Glikson M, Piccini J et al. Cardiac pacing and lead devices management: 25 years of research at EP Europace journal. Europace 2023;25:euad202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reynolds D, Duray GZ, Omar R, Soejima K, Neuzil P, Zhang S et al. A leadless intracardiac transcatheter pacing system. N Engl J Med 2016;374:533–41. [DOI] [PubMed] [Google Scholar]
- 3. Duray GZ, Ritter P, El-Chami M, Narasimhan C, Omar R, Tolosana JM et al. Long-term performance of a transcatheter pacing system: 12-month results from the Micra transcatheter pacing study. Hear Rhythm 2017;14:702–9. [DOI] [PubMed] [Google Scholar]
- 4. Piccini JP, Stromberg K, Jackson KP, Laager V, Duray GZ, El-Chami M et al. Long-term outcomes in leadless Micra transcatheter pacemakers with elevated thresholds at implantation: results from the Micra transcatheter pacing system global clinical trial. Hear Rhythm 2017;14:685–91. [DOI] [PubMed] [Google Scholar]
- 5. Saleem-Talib S, van Driel VJ, Nikolic T, van Wessel H, Louman H, Borleffs CJW et al. The jugular approach for leadless pacing: a novel and safe alternative. Pacing Clin Electrophysiol 2022;45:1248–54. [DOI] [PubMed] [Google Scholar]
- 6. Molitor N, Breitenstein A. Leadless pacemaker implantation via the internal jugular vein. Cardiovasc Med 2023;26:194–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roberts PR, Clementy N, Al Samadi F, Garweg C, Martinez-Sande JL, Iacopino S et al. A leadless pacemaker in the real-world setting: the Micra transcatheter pacing system post-approval registry. Hear Rhythm 2017;14:1375–9. [DOI] [PubMed] [Google Scholar]
- 8. Boveda S, Higuera L, Longacre C, Wolff C, Wherry K, Stromberg K et al. Two-year outcomes of leadless vs. transvenous single-chamber ventricular pacemaker in high-risk subgroups. Europace 2023;25:1041–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sobolev M, Shiloh AL, Di Biase L, Slovut DP. Ultrasound-guided cannulation of the femoral vein in electrophysiological procedures: a systematic review and meta-analysis. Europace 2017;19:850–5. [DOI] [PubMed] [Google Scholar]
- 10. Teichgräber UK, Benter T, Gebel M, Manns MP. A sonographically guided technique for central venous access. Am J Roentgenol 1997;169:731–3. [DOI] [PubMed] [Google Scholar]
- 11. Randolph AG, Cook DJ, Gonzales CA, Pribble CG. Ultrasound guidance for placement of central venous catheters. Crit Care Med 1996;24:2053–8. [DOI] [PubMed] [Google Scholar]
- 12. Espinosa T, Farrus A, Venturas M, Cano A, Vazquez-Calvo S, Pujol-Lopez M et al. Same-day discharge after atrial fibrillation ablation under a nurse-coordinated standardized protocol. Europace 2024;26:euae083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Piccini JP, Cunnane R, Steffel J, El-Chami MF, Reynolds D, Roberts PR et al. Development and validation of a risk score for predicting pericardial effusion in patients undergoing leadless pacemaker implantation: experience with the Micra transcatheter pacemaker. Europace 2022;24:1119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bongiorni MG, Della Tommasina V, Barletta V, Di Cori A, Rogani S, Viani S et al. Feasibility and long-term effectiveness of a non-apical Micra pacemaker implantation in a referral centre for lead extraction. Europace 2019;21:114–20. [DOI] [PubMed] [Google Scholar]
- 15. Valiton V, Graf D, Pruvot E, Carroz P, Fromer M, Bisch L et al. Leadless pacing using the transcatheter pacing system (Micra TPS) in the real world: initial Swiss experience from the Romandie region. Europace 2019;21:275–80. [DOI] [PubMed] [Google Scholar]
- 16. El-Chami MF, Al-Samadi F, Clementy N, Garweg C, Martinez-Sande JL, Piccini JP et al. Updated performance of the Micra transcatheter pacemaker in the real-world setting: a comparison to the investigational study and a transvenous historical control. Hear Rhythm 2018;15:1800–7. [DOI] [PubMed] [Google Scholar]
- 17. Haeberlin A, Kozhuharov N, Knecht S, Tanner H, Schaer B, Noti F et al. Leadless pacemaker implantation quality: importance of the operator’s experience. Europace 2020;22:939–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request. On request and associated need, our data are available, while our utmost intention is to protect our patient’s privacy.