Abstract
Background
The non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) serves as a novel composite lipid indicator for atherosclerosis. However, the association between NHHR and mortality in patients with diabetes or prediabetes remains unclear. Consequently, the objective of this study was to investigate the relationship between NHHR and both all-cause and cardiovascular mortality in US adults with diabetes or prediabetes.
Methods
This study included 12,578 adult participants with diabetes or prediabetes from the US National Health and Nutrition Examination Survey (1999–2018). Mortality outcomes were ascertained by linking to the National Death Index (NDI) record up to December 31, 2019. We employed a weighted multivariate Cox proportional hazards model and restricted cubic splines to assess the associations between NHHR and all-cause and cardiovascular mortality. A segmented Cox proportional hazards model was used for evaluating threshold effects. Furthermore, a competing risks analysis was performed to explore the relationship between NHHR and cardiovascular mortality.
Results
During a median follow-up period of 8.08 years, 2403 participants encountered all-cause mortality, with 662 of them specifically succumbing to cardiovascular mortality. The restricted cubic splines revealed a U-shaped association between NHHR and all-cause mortality, while an L-shaped association was observed for cardiovascular mortality. The analysis of threshold effects revealed that the inflection points for NHHR and all-cause and cardiovascular mortality were 2.72 and 2.83, respectively. Specifically, when the baseline NHHR was below the inflection points, a negative correlation was observed between NHHR and both all-cause mortality (HR: 0.76, 95% CI: 0.68–0.85) and cardiovascular mortality (HR: 0.70, 95% CI: 0.57–0.85). Conversely, when the baseline NHHR exceeded the inflection points, a positive correlation was observed between NHHR and both all-cause mortality (HR: 1.11, 95% CI: 1.06–1.16) and cardiovascular mortality (HR: 1.08, 95% CI: 1.00–1.16).
Conclusions
Among US adults with diabetes or prediabetes, a U-shaped association was observed between NHHR and all-cause mortality, whereas an L-shaped association was identified with cardiovascular mortality. The inflection points for all-cause and cardiovascular mortality were 2.72 and 2.83, respectively.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-024-03536-3.
Keywords: NHHR, Diabetes, Prediabetes, Mortality, Cardiovascular disease, NHANES
Background
Diabetes and its complications are among the leading causes of death and disability worldwide, posing a significant challenge to global public health. The global incidence of diabetes is increasing annually due to factors such as population aging and unhealthy dietary habits [1, 2]. Epidemiological studies have shown that in 2021, there were approximately 529 million individuals worldwide with diabetes, and by 2050, the number of affected individuals is projected to reach 1.31 billion [3]. Moreover, diabetes ranks as the eighth leading cause of death globally, with associated healthcare expenditures reaching a staggering $966 billion, imposing a significant economic burden on healthcare systems [3–5]. Importantly, diabetes is closely associated with several major causes of death worldwide and is a primary risk factor for cardiovascular disease (CVD) [3]. Although cancer is also emerging as a primary cause of death in individuals with diabetes in certain regions, CVD remains the leading cause of mortality among individuals with diabetes [6, 7].
CVD among individuals with diabetes is multifactorial, and controlling cardiovascular risk factors can significantly reduce the incidence of cardiovascular events. Research has demonstrated that atherosclerotic cardiovascular diseases (ASCVDs) caused by factors such as hypertension, high blood glucose, dyslipidemia, obesity, and insulin resistance are the primary causes of mortality in diabetes patients [7–9]. Notably, dyslipidemia plays a pivotal role in the development of ASCVD in individuals with diabetes. Dyslipidemia in diabetes patients is characterized by increased levels of the non-high-density lipoprotein cholesterol (non-HDL-C), including low-density lipoprotein cholesterol (LDL-C), intermediate-density lipoprotein, and very low-density lipoprotein remnants, all of which are contributors to the progression of atherosclerosis [10, 11]. In recent years, the clinical value of non-HDL-C has gained widespread attention and recognition. In 2021, the UK-based National Institute for Health and Care Excellence (NICE) recommended that non-HDL-C be used as the primary target for reducing CVD risk in patients with diabetes, replacing LDL-C. This recommendation emphasizes the significance of non-HDL-C in assessing CVD risk and treatment efficacy [12, 13]. However, a novel composite lipid indicator, the non-HDL-C to high-density lipoprotein cholesterol (HDL-C) ratio (NHHR), has demonstrated promising predictive value in assessing the risk of various diseases, including coronary artery disease, diabetes, abdominal aortic aneurysm, and carotid atherosclerosis [14–17]. Compared to non-HDL-C, the NHHR is a superior and comprehensive indicator that takes into account both the risk factor (non-HDL-C) and the protective factor (HDL-C) in atherosclerosis [18, 19]. However, the prognostic value of NHHR in patients with diabetes remains unclear. To address this gap, we conducted a study utilizing the National Health and Nutrition Examination Survey (NHANES) database. The aim of this research was to investigate the association between NHHR and all-cause and cardiovascular mortality in participants with diabetes or prediabetes, as well as its association with various population characteristics, aiming to provide valuable insights into disease management and prevention strategies.
Methods
Study population and design
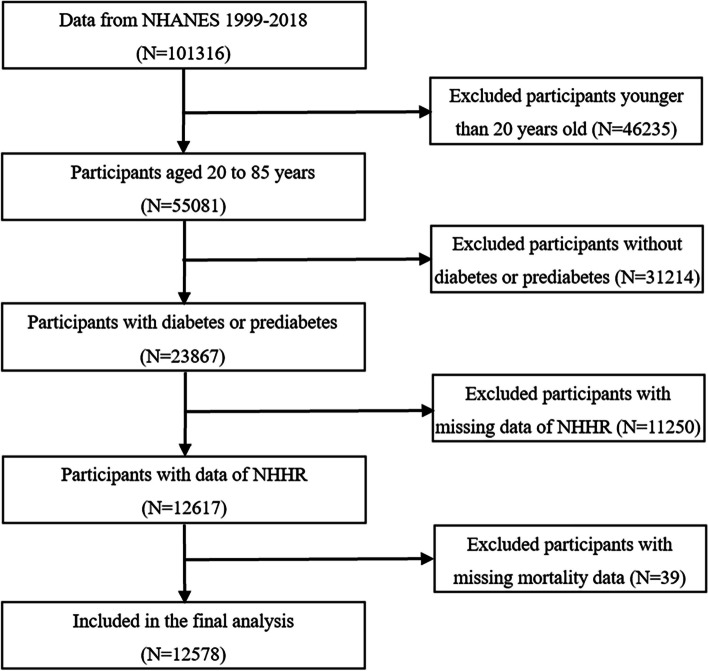
The NHANES is a nationwide survey study designed to assess the nutritional and health status of adults and children in the USA. The survey employed a stratified, complex multistage sampling methodology and incorporates interviews, physical examinations, and laboratory tests [20]. The study protocol was formally approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board, and all participants involved in the study provided informed consent by signing consent forms [21]. This study utilized data from the NHANES database spanning from 1999 to 2018, encompassing a total of 101,316 participants. Initially, participants under the age of 20 were excluded (n = 46,235). Subsequently, participants who did not meet the diagnostic criteria for diabetes or prediabetes according to the 2021 American Diabetes Association guidelines [22] were excluded (n = 31,214). Diabetes was defined as (1) self-reported diabetes, (2) insulin injection or hypoglycemic medication usage, (3) hemoglobin A1c (HbA1c) ≥ 6.5%, (4) fasting blood glucose (FBG) ≥ 7.0 mmol/L, and (5) 2-h postprandial glucose (2hPG) ≥ 11.1 mmol/L. Prediabetes was defined as (1) HbA1c 5.7–6.4%, (2) FBG 5.6–6.9 mmol/L, and (3) 2hPG 7.8–11.0 mmol/L. Subsequently, participants lacking NHHR data were excluded (n = 11,250). Finally, after excluding participants with missing mortality data (n = 39), a total of 12,578 participants were included in this study (Fig. 1).
Fig. 1.
Flow chart of the study participants
Assessment of NHHR
Blood lipid parameters are critical components of cardiovascular disease (CVD) laboratory assessments in the NHANES and are essential for assessing CVD risk. These parameters encompass a spectrum of measurements, including total cholesterol (TC), HDL-C, LDL-C, and triglycerides. The associated testing procedures were performed at the mobile examination centers (MECs). After collection, serum samples were processed and then stored. These samples were then dispatched to the University of Minnesota for comprehensive analysis. In our study, we employed the NHHR to evaluate participants’ lipid levels and CVD risk. The NHHR was calculated based on serum TC and HDL-C levels from the NHANES database for the years 1999–2018. Non-HDL-C is defined as TC minus HDL-C, and the NHHR is calculated as the ratio of non-HDL-C to HDL-C [23]. Participants were divided into four groups (Q1, Q2, Q3, and Q4) based on the NHHR quartiles, with the Q1 group serving as the reference group.
Ascertainment of mortality
To ascertain the all-cause and cardiovascular mortality status of the participants in this study, we utilized the public-use linked mortality files provided by the NCHS. These files integrate survey participants’ data with death certificate records from the National Death Index (NDI) using a probabilistic matching algorithm. The follow-up mortality data were updated until December 31, 2019. The specific cause of death was established based on the International Classification of Diseases, Tenth Revision (ICD-10), with cardiovascular deaths including codes I00-I09, I11, I13, and I20-I51 [24]. All-cause mortality refers to the total of all specific cause deaths.
Assessment of covariates
The selection of covariates in this study took into account a range of demographic characteristics and health-related information, including age, sex (male or female), race (Mexican American, non-Hispanic White, non-Hispanic Black, or other), education (less than high school, high school or equivalent, or some college or above), and marital status (married/living with partner, widowed/divorced/separated, or never married). The poverty income ratio (PIR) was divided into the following categories: low income (< 1.3), moderate income (1.3–3.5), and high income (> 3.5). Body mass index (BMI) was classified as normal/underweight (< 25), overweight (25–30), or obese (> 30). Smoking status was categorized as never smoking (smoking fewer than 100 cigarettes in a lifetime), former smoking (smoking more than 100 cigarettes in a lifetime but currently not smoking), or current smoking (smoking more than 100 cigarettes in a lifetime and currently smoking every day). Drinking status was dichotomized as yes or no (defined as whether the individual consumed more than 12 drinks per year). Physical activity was classified as vigorous, moderate, or other. Hypertension was defined as self-reported hypertension, the use of antihypertensive medication, or an average blood pressure of > 130 mmHg or a diastolic pressure of > 80 mmHg. Hyperlipidemia was defined as self-reported high cholesterol level, use of cholesterol-lowering medications, or laboratory examination indicating TC ≥ 200 mg/dl, triglycerides ≥ 150 mg/dl, LDL-C ≥ 130 mg/dl, and HDL < 40 mg/dl (male) or < 50 mg/dl (female). The participants’ medication status is defined as follows: antihypertensive treatment (yes or no), lipid-lowering treatment (yes or no), and antihyperglycemic treatment (only oral medication, only insulin, insulin and oral medication, or untreated).
Statistical analyses
The NHANES survey was conducted using a complex, multistage, probability sampling design to ensure the representativeness of the research results for the US civilian non-institutionalized population. In our study, we incorporated sample weights, stratification, and clustering for analysis purposes. The participants were divided into four groups based on the quartiles (Q1–Q4) of the NHHR. Continuous variables were reported as the means and standard deviations (SDs), while categorical variables were reported as frequencies and percentages. The baseline characteristics of the four participant groups were compared using analysis of the variance (ANOVA) for continuous variables and the chi-square (χ2) test for categorical variables. We established three models to control for confounding factors and used weighted multivariate Cox proportional hazards models to estimate the associations between NHHRs and all-cause and cardiovascular mortality. Model 1 did not include any covariate adjustments. Model 2 was adjusted for age, sex, race, education, marital status, and PIR. Model 3 was further adjusted for BMI, smoking status, drinking status, physical activity, hypertension, hyperlipidemia, antihypertensive treatment, lipid-lowering treatment, and antihyperglycemic treatment. We also conducted a test for multicollinearity among all variables included in the analysis. The variance inflation factor (VIF) for each variable was below 5 (Additional file 1: Table S1), indicating no evidence of significant multicollinearity. Moreover, the linear trend test was performed to assess the trend by designating the median NHHR for each quartile as a continuous variable in the regression model. We employed multiple imputation by chained equations (MICE) to address missing values, thereby minimizing the reduction in sample size caused by the absence of covariate data. The proportions of missing covariates and the methods of imputation are detailed in the Additional File 1: Tables S2.
To investigate the dose–response relationship between NHHR and mortality, we employed Cox proportional hazards regression models incorporating restricted cubic splines (RCSs) for smooth curve fitting. If the RCS indicated a nonlinear association, we identified the inflection point using the “segmented” package based on the likelihood-ratio test and the bootstrap resampling method. Subsequently, we assessed the association between NHHR and mortality using a segmented Cox proportional hazards model across both sides of the inflection point. We performed subgroup analyses stratified by age (< 60 or ≥ 60 years), sex (male or female), race (Mexican American, non-Hispanic White, non-Hispanic Black, or other), smoking status (former, current, or never), drinking status (yes or no), physical activity (vigorous, moderate, or other), BMI (normal/underweight, overweight, or obese), and classification of diabetes (diabetes or prediabetes). Likelihood ratio tests were used to assess the interactions among the subgroups. To evaluate the stability of the models, we performed several sensitivity analyses. Firstly, we excluded participants with missing values for variable to eliminate the potential impact of missing data on the primary outcomes. Secondly, given that cancer is one of the major causes of mortality among diabetic patients and is intimately associated with all-cause mortality, we excluded participants who self-reported a diagnosis of cancer at baseline to mitigate the potential confounding effects. Thirdly, to minimize the potential for reverse causality, we excluded all participants from the first 2 years of follow-up by delaying their entry time. Fourthly, we utilized the Fine and Gray subdistribution hazards model [25] to examine the association between the NHHR quantiles and the risk of cardiovascular mortality, treating non-cardiovascular death as a competing risk. Finally, to mitigate the influence of extreme values, we excluded participants with a NHHR exceeding the mean ± 3 SD. All analyses were performed using R software, version 4.2.1, and a two-sided P-value < 0.05 was considered to indicate statistical significance. The Bonferroni correction threshold to address multiple comparisons and establish statistical significance (α = 0.05/8 = 0.006 for subgroup interaction tests).
Results
Characteristics of the study participants
A total of 12,578 participants were included in this study, with an average age of 53.06 years and a male proportion of 53.9%. The mean NHHR of the participants was calculated to be 3.14 ± 1.47. Table 1 presents the baseline characteristics of the participants according to NHHR quartiles. Compared to participants in the lowest quartile, those with higher NHHRs are often younger, male, and exhibit higher levels of HbA1c and TC. Furthermore, in the higher NHHR quartiles, an increase in the number of participants with obesity, current smoking, alcohol consumption, and physical inactivity has been observed, which suggests potential association between unhealthy lifestyle habits and an elevated NHHR.
Table 1.
Baseline characteristics according to NHHR quartiles
| Characteristics | Quartiles of NHHR | |||||
|---|---|---|---|---|---|---|
| Overall | Q1 (< 2.12) | Q2 (2.12–2.91) | Q3 (2.91–3.88) | Q4 (> 3.88) | P value | |
| N(%) | 12,578 | 3267 | 3186 | 3068 | 3057 | |
| Age, years, mean (SD) | 53.05 (16.10) | 55.93 (17.52) | 54.51 (16.17) | 51.97 (15.55) | 49.80 (14.33) | < 0.001 |
| Sex, n(%) | < 0.001 | |||||
| Male | 6732 (53.9) | 1406 (42.3) | 1532 (48.1) | 1766 (57.9) | 2028 (67.3) | |
| Female | 5846 (46.1) | 1861 (57.7) | 1654 (51.9) | 1302 (42.1) | 1029 (32.7) | |
| Race, n (%) | < 0.001 | |||||
| Mexican American | 2337 (8.8) | 428 (6.7) | 556 (8.7) | 646 (9.5) | 707 (10.3) | |
| Non-Hispanic White | 5355 (66.7) | 1336 (65.2) | 1363 (66.3) | 1281 (67.1) | 1375 (68.4) | |
| Non-Hispanic Black | 2523 (11.5) | 905 (16.1) | 669 (12.1) | 552 (10.3) | 397 (7.6) | |
| Other | 2363 (12.9) | 598 (12.1) | 598 (12.8) | 589 (13.1) | 578 (13.6) | |
| Education, n (%) | < 0.001 | |||||
| Less than high school | 3828 (20.4) | 902 (18.0) | 932 (19.2) | 941 (20.7) | 1053 (23.7) | |
| High school grad or equivalent | 2976 (25.7) | 722 (23.4) | 763 (25.6) | 755 (26.7) | 736 (27.0) | |
| Some college or above | 5774 (53.9) | 1643 (58.6) | 1491 (55.2) | 1372 (52.6) | 1268 (49.3) | |
| Marital, n (%) | < 0.001 | |||||
| Married/living with partner | 7875 (66.2) | 1845 (60.9) | 2002 (67.6) | 2000 (67.1) | 2028 (69.3) | |
| Widowed/divorced/separated | 3214 (21.5) | 968 (25.1) | 842 (22.4) | 721 (19.9) | 683 (18.7) | |
| Never married | 1489 (12.2) | 454 (14.0) | 342 (9.9) | 347 (13.0) | 346 (12.0) | |
| PIR, n (%) | < 0.001 | |||||
| Low | 4009 (22.6) | 886 (19.1) | 1011 (22.3) | 991 (22.8) | 1121 (26.2) | |
| Moderate | 4994 (38.3) | 1373 (39.6) | 1254 (38.0) | 1215 (37.8) | 1152 (37.9) | |
| High | 3575 (39.1) | 1008 (41.3) | 921 (39.7) | 862 (39.4) | 784 (35.9) | |
| BMI (kg/m2), n (%) | < 0.001 | |||||
| Normal/underweight | 2720 (20.9) | 1125 (35.2) | 706 (22.1) | 513 (15.2) | 376 (10.9) | |
| Overweight | 4314 (33.9) | 1018 (32.2) | 1087 (33.4) | 1077 (35.1) | 1132 (35.1) | |
| Obese | 5544 (45.2) | 1124 (32.6) | 1393 (44.5) | 1478 (49.7) | 1549 (54.0) | |
| HbA1c, %, mean (SD) | 5.94 (1.13) | 5.84 (0.95) | 5.92 (1.06) | 5.92 (1.08) | 6.07 (1.37) | < 0.001 |
| HDL-C, mmol/L, mean (SD) | 1.33 (0.40) | 1.73 (0.44) | 1.39 (0.27) | 1.20 (0.22) | 0.99 (0.19) | < 0.001 |
| TC, mmol/L, mean (SD) | 5.11 (1.10) | 4.49 (0.93) | 4.86 (0.92) | 5.19 (0.90) | 5.89 (1.12) | < 0.001 |
| Smoking status, n (%) | < 0.001 | |||||
| Never | 6463 (50.4) | 1794 (54.1) | 1719 (53.1) | 1566 (50.2) | 1384 (44.2) | |
| Former | 3695 (29.7) | 971 (31.6) | 918 (28.8) | 917 (29.4) | 889 (29.0) | |
| Current | 2420 (19.9) | 502 (14.3) | 549 (18.2) | 585 (20.4) | 784 (26.7) | |
| Drinking status, n (%) | 0.002 | |||||
| Yes | 8231 (69.9) | 2084 (69.5) | 2005 (67.4) | 1998 (69.5) | 2144 (73.2) | |
| No | 4347 (30.1) | 1183 (30.5) | 1181 (32.6) | 1070 (30.5) | 913 (26.8) | |
| Physical activity, n (%) | 0.034 | |||||
| Vigorous | 2205 (20.7) | 602 (23.1) | 538 (19.7) | 524 (19.3) | 541 (20.6) | |
| Moderate | 3455 (29.9) | 942 (30.5) | 875 (29.7) | 843 (31.0) | 795 (28.4) | |
| Other | 6918 (49.4) | 1723 (46.4) | 1773 (50.5) | 1701 (49.7) | 1721 (51.0) | |
| Hypertension, n (%) | 0.323 | |||||
| Yes | 8185 (61.1) | 2149 (59.3) | 2090 (62.3) | 1967 (61.2) | 1979 (61.7) | |
| No | 4393 (38.9) | 1118 (40.7) | 1096 (37.7) | 1101 (38.8) | 1078 (38.3) | |
| Hyperlipidemia, n (%) | < 0.001 | |||||
| Yes | 9813 (77.9) | 1631 (48.2) | 2310 (71.6) | 2816 (91.6) | 3056 (100.0) | |
| No | 2765 (22.1) | 1636 (51.8) | 876 (28.4) | 252 (8.4) | 1 (0.0) | |
| Antihyperglycemic treatment,n (%) | 0.004 | |||||
| Only oral medication | 1702 (11.3) | 511 (12.3) | 461 (12.7) | 376 (10.1) | 354 (9.9) | |
| Only insulin | 283 (1.8) | 84 (2.3) | 81 (2.0) | 67 (1.6) | 51 (1.2) | |
| Insulin and oral medication | 340 (2.1) | 113 (2.5) | 84 (1.9) | 76 (2.2) | 67 (1.9) | |
| Untreated | 10,253 (84.8) | 2559 (82.9) | 2560 (83.4) | 2549 (86.0) | 2585 (87.0) | |
| Lipid-lowering treatment, n (%) | < 0.001 | |||||
| Yes | 3190 (24.3) | 1077 (32.2) | 890 (27.1) | 691 (21.3) | 532 (16.7) | |
| No | 9388 (75.7) | 2190 (67.8) | 2296 (72.9) | 2377 (78.7) | 2525 (83.3) | |
| Antihypertensive treatment, n (%) | < 0.001 | |||||
| Yes | 4710 (33.9) | 1419 (37.4) | 1293 (37.7) | 1066 (32.5) | 932 (27.9) | |
| No | 7868 (66.1) | 1848 (62.6) | 1893 (62.3) | 2002 (67.5) | 2125 (72.1) | |
| Diabetes, n (%) | 4089 (27.1) | 1095 (27.1) | 1024 (27.8) | 965 (26.3) | 1005(27.3) | 0.748 |
| Prediabetes, n(%) | 8489 (72.9) | 2172 (72.9) | 2162 (72.2) | 2103 (73.7) | 2052 (72.7) | 0.748 |
The data are presented as the mean (SD) or n (%). All estimates were obtained from complex survey designs, analysis of variance or χ2 tests where appropriate
BMI Body mass index, HDL-C High density lipoprotein cholesterol, HbA1c Hemoglobin A1c, PIR Poverty income ratio, SD Standard deviation, TC Total cholesterol
Associations between NHHR and mortality
During a mean follow-up period of 8.08 years, a total of 2403 participant deaths were recorded, of which 662 were attributed to cardiovascular causes. The relationship between NHHR and mortality is presented in Table 2. We employed the weighted multivariate Cox proportional hazards regression model to estimate the independent association between NHHR and the risk of mortality. After adjusting for multiple variables (model 3), the hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause mortality across the lowest to highest quartiles of the NHHR (Q1–Q4) were 1.00 (reference), 0.86 (0.75, 0.99), 0.81 (0.70, 0.93), and 1.05 (0.89, 1.23), respectively. For cardiovascular mortality, the HRs and 95% CIs were 1.00 (reference), 0.76 (0.58, 0.99), 0.78 (0.60, 1.01), and 0.86 (0.65, 1.15), respectively. According to the trend analysis, no significant linear relationships were detected between NHHR and all-cause mortality (P for trend = 0.678) or cardiovascular mortality (P for trend = 0.366).
Table 2.
HRs (95% CIs) for mortality according to NHHR quartiles
| Quartiles of NHHR | P for trend | ||||
|---|---|---|---|---|---|
| Q1 (< 2.12) | Q2 (2.12–2.91) | Q3 (2.91–3.88) | Q4 (> 3.88) | ||
| All-cause mortality | |||||
| Number of deaths | 658 | 613 | 534 | 598 | |
|
Model 1 HR (95% CI) P value |
1 | 0.83 (0.72,0.95)0.007 | 0.66 (0.57,0.75)0.001 | 0.70 (0.62,0.79)0.001 | 0.001 |
|
Model 2 HR (95% CI) P value |
1 | 0.88 (0.77,1.01)0.074 | 0.79 (0.69,0.89)0.001 | 1.00 (0.87,1.15)0.992 | 0.579 |
|
Model 3 HR (95% CI) P value |
1 | 0.86 (0.75,0.99)0.041 | 0.81 (0.70,0.93)0.004 | 1.05 (0.89,1.23)0.550 | 0.678 |
| CVD mortality | |||||
| Number of deaths | 157 | 181 | 171 | 153 | |
|
Model 1 HR (95% CI) P value |
1 | 0.76 (0.59,0.98)0.038 | 0.70 (0.54,0.91)0.006 | 0.63 (0.48,0.82)0.001 | 0.001 |
|
Model 2 HR (95% CI) P value |
1 | 0.82 (0.63,1.07)0.149 | 0.87 (0.69,1.11)0.266 | 0.95 (0.72,1.25)0.697 | 0.671 |
|
Model 3 HR (95% CI) P value |
1 | 0.76 (0.58,0.99)0.042 | 0.78 (0.60,1.01)0.065 | 0.86 (0.65,1.15)0.319 | 0.366 |
Model 1 was unadjusted
Model 2 was adjusted for sex, age, race, education, marital status, and poverty income ratio
Model 3 was further adjusted for BMI, smoking status, drinking status, physical activity, hypertension, hyperlipidemia, antihyperglycemic treatment, lipid-lowering treatment, and antihypertensive treatment, based on model 2
The results are presented as HRs and 95% CIs
BMI Body mass index, CI Confidence interval, CVD Cardiovascular disease, HR Hazard ratio
Detection of nonlinear relationships
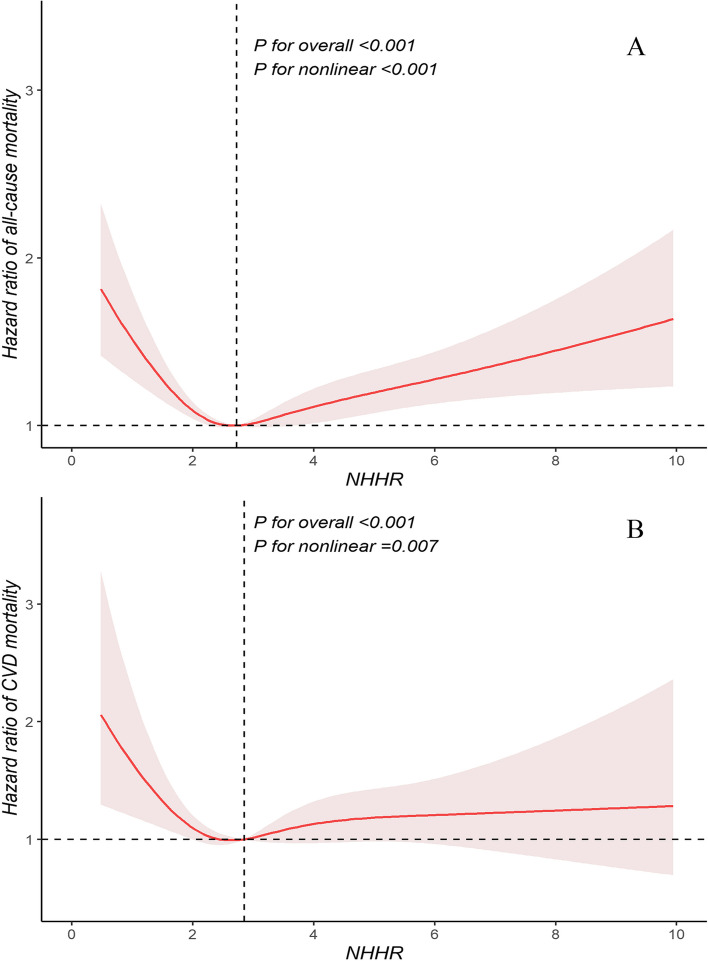
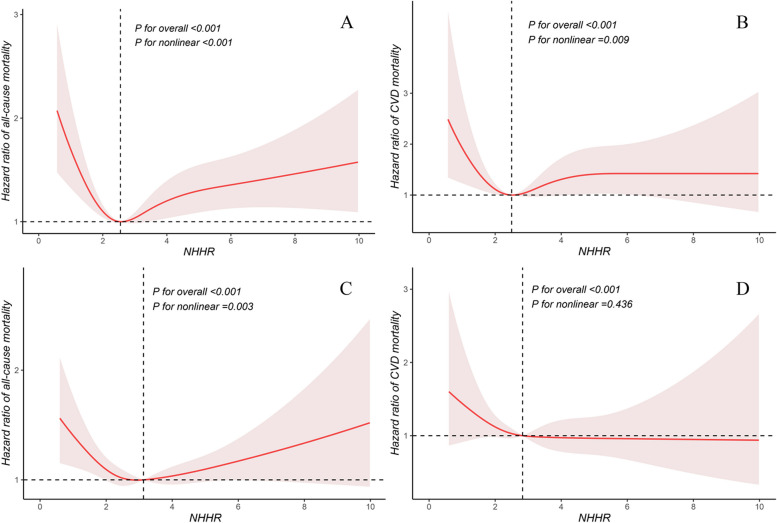
To examine potential nonlinear trends, we employed RCS fitted for Cox proportional hazards models to further investigate the relationship between NHHR and mortality. Notably, we discovered a significant U-shaped association between NHHR and all-cause mortality (Fig. 2A) as well as a significant L-shaped association between NHHR and cardiovascular mortality (Fig. 2B). Utilizing the “segmented” package, we identified that the inflection points for NHHR in relation to the risks of all-cause and cardiovascular mortality were 2.72 and 2.83, respectively. To further investigate these relationships, we employed a segmented Cox proportional hazards model and found that the results of the likelihood ratio tests were statistically significant (Table 3). In particular, below the inflection points, NHHR showed an inverse association with all-cause and cardiovascular mortality. For each unit increase in NHHR, the risk of all-cause mortality decreased by 24% (HR: 0.76, 95% CI: 0.68–0.85, P = 0.001), and the risk of cardiovascular mortality decreased by 30% (HR: 0.70, 95% CI: 0.57–0.85, P = 0.001). Above the inflection points, NHHR showed a positive association with all-cause and cardiovascular mortality. For each unit increase in the NHHR, the risk of all-cause mortality increased by 11% (HR: 1.11, 95% CI: 1.06–1.16, P = 0.001), and the risk of cardiovascular mortality increased by 8% (HR: 1.08, 95% CI: 1.00–1.16, P = 0.027). We also examined the associations between the NHHR and mortality in diabetic and prediabetic populations, respectively (Fig. 3). The results revealed that in participants with diabetes, the NHHR exhibited a U-shaped association with all-cause mortality (P for nonlinear < 0.001) and an L-shaped association with cardiovascular mortality (P for nonlinear = 0.009). For participants with prediabetes, NHHR also demonstrated a U-shaped association with all-cause mortality (P for nonlinear = 0.003) but showed no significant nonlinear correlation with cardiovascular mortality (P for nonlinear = 0.436).
Fig. 2.
Restricted cubic splines were utilized to evaluate the hypothesis of potential nonlinear relationships between NHHR and all-cause (A) and cardiovascular (B) mortality in participants with diabetes or prediabetes. The NHHRs of 2.72 for A and 2.83 for B were chosen as reference estimates for each hazard ratio (HR). The analysis was adjusted for sex, age, race, education, marital status, poverty income ratio, BMI, smoking status, drinking status, physical activity, hypertension, hyperlipidemia, antihyperglycemic treatment, lipid-lowering treatment, and antihypertensive treatment. The Cox proportional hazards models were fitted, with solid lines representing estimated values and shaded areas representing the corresponding 95% confidence intervals (CIs). The black dashed line represents the location of the inflection point in the curve. BMI, body mass index; CVD, cardiovascular disease
Table 3.
Threshold effect analysis of NHHR on all-cause and CVD mortality in participants with diabetes or prediabetes
| Adjusted HR (95%CI), P value | |
|---|---|
| All-cause mortality | |
| Total | 1.03 (0.99, 1.08) 0.104 |
| Segmented cox proportional hazards model | |
| Inflection point | 2.72 |
| NHHR < 2.72 | 0.76 (0.68, 0.85) 0.001 |
| NHHR ≥ 2.72 | 1.11 (1.06, 1.16) 0.001 |
| P for Log-likelihood ratio | < 0.001 |
| CVD mortality | |
| Total | 0.98 (0.90, 1.06) 0.568 |
| Segmented cox proportional hazards model | |
| Inflection point | 2.83 |
| NHHR < 2.83 | 0.70 (0.57, 0.85) 0.001 |
| NHHR ≥ 2.83 | 1.08 (1.00, 1.16) 0.027 |
| P for Log-likelihood ratio | 0.001 |
The model was adjusted for sex, age, race, education, marital status, poverty income ratio, BMI, smoking status, drinking status, physical activity, hypertension, hyperlipidemia, antihyperglycemic treatment, lipid-lowering treatment, and antihypertensive treatment
BMI Body mass index, CI Confidence interval, CVD Cardiovascular disease, HR Hazard ratio
Fig. 3.
Restricted cubic splines were utilized to evaluate the hypothesis of potential nonlinear relationships between NHHR and all-cause (A) and cardiovascular (B) mortality in participants with diabetes. The NHHRs of 2.55 for A and 2.50 for B were chosen as reference estimates for each hazard ratio (HR). Similarly, restricted cubic splines were utilized to evaluate the hypothesis of potential nonlinear relationships between NHHR and all-cause (C) and cardiovascular (D) mortality in participants with prediabetes. Using NHHR of 3.13 for C and 2.83 for D as reference estimates for each HR, the analysis was adjusted for sex, age, race, education, marital status, poverty income ratio, BMI, smoking status, drinking status, physical activity, hypertension, hyperlipidemia, antihyperglycemic treatment, lipid-lowering treatment, and antihypertensive treatment. The Cox proportional hazards model was used to fit the data, with solid lines representing the estimated values and shaded areas representing the corresponding 95% CIs. The black dashed line represents the location of the inflection point in the curve. BMI, body mass index; CVD, cardiovascular disease
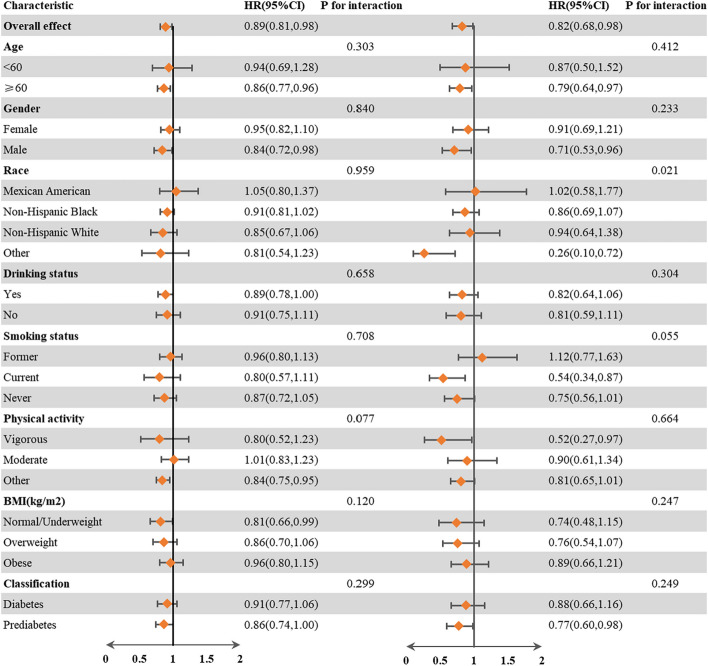
Subgroup analyses
To further elucidate the relationship between NHHR and the risks of all-cause and cardiovascular mortality, we conducted a series of subgroup analyses. In the subgroup analysis (Fig. 4), stratified by sex, age, race, smoking status, drinking status, physical activity, BMI, and diabetes classification, the relationship between NHHR and all-cause and cardiovascular mortality was consistent across all subgroups (P for interaction > 0.006). Notably, no significant interaction was observed between the baseline NHHR and the stratified variables.
Fig. 4.
Subgroup analysis of the associations between NHHR and all-cause and cardiovascular mortality. The reference NHHRs for all-cause mortality were NHHRs < 2.72, while for cardiovascular mortality, the reference was NHHRs < 2.83. The model was adjusted for sex, age, race, education, marital status, poverty income ratio, BMI, smoking status, drinking status, physical activity, hypertension, hyperlipidemia, antihyperglycemic treatment, lipid-lowering treatment, and antihypertensive treatment. BMI, body mass index; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio
Sensitivity analysis
In the sensitivity analysis, after excluding participants with missing covariate values at baseline, a total of 10,438 participants were included in the study. The results showed that the associations between NHHR and all-cause and cardiovascular mortality remained relatively stable (Additional file 1: Table S3). Excluding participants who self-reported cancer at baseline, the final study population comprised 11,109 individuals. After adjusting for all confounding factors, the association between NHHR and mortality was found to be largely consistent with the primary analysis (Additional file 1: Table S4). After excluding participants during the first 2 years of follow-up, the study sample consisted of 11,510 participants. Similar associations between NHHR and all-cause and cardiovascular mortality were observed (Additional file 1: Table S5). In the Fine and Gray competing risks models, the relationship between NHHR and cardiovascular mortality was found to be robust (Additional file 1: Table S6). After excluding extreme values (mean ± 3 SD) of the NHHR, a total of 12,447 participants were included in the study. After adjusting for a range of potential confounding factors, the associations between NHHR and both all-cause and cardiovascular mortality persisted (Additional file 1: Table S7).
Discussion
To the best of our knowledge, our study is the first prospective cohort study to reveal the association between NHHR and the risk of all-cause and cardiovascular mortality in participants with diabetes or prediabetes. Our findings indicate a U-shaped association between NHHR and all-cause mortality as well as an L-shaped association with cardiovascular mortality among participants with diabetes or prediabetes. The threshold effect analysis results reveal that the NHHR has inflection points at 2.72 and 2.83 for the risks of all-cause and cardiovascular mortality, respectively. Our study underscores the significance of the NHHR as a valuable clinical prognostic indicator for both all-cause and cardiovascular mortality in patients with diabetes or prediabetes. Furthermore, it holds immense importance in disease risk stratification and prognosis.
The NHHR is a novel lipid ratio indicator that is cost-effective and easily obtainable, and previous studies have revealed its clinical predictive value in various diseases. A longitudinal cohort study by Sheng et al. [14] involving 15,464 participants demonstrated that the NHHR is a superior indicator for predicting diabetes risk compared to conventional lipid markers, with an inflection point at approximately 2.74. This finding is essentially in agreement with the results of our study. You et al. [26] reported a positive correlation between NHHR and coronary artery disease, noting that an elevated NHHR is associated with an increased risk of acute coronary syndrome. Similarly, Mao et al. [17] demonstrated that the NHHR serves as an independent predictor for severe coronary artery disease and major adverse cardiovascular events and is associated with the prognosis of patients with non-ST-segment elevation myocardial infarction. These studies indirectly support the conclusions of our research. Additionally, other studies have revealed a relationship between NHHR and depression as well as suicidal ideation in adults, shedding light on the connection between lipid metabolism and mental health [23, 27].
Lipid profiles provide crucial information about lipid metabolism and an individual’s health status and can be used to predict the risk of certain diseases [28]. Although the biological mechanisms linking NHHR to mortality remain unclear, several studies have suggested a potential association between NHHR and atherosclerosis [14, 29]. Atherosclerosis in patients with diabetes is associated with several pathological mechanisms, including diabetes dyslipidemia, hyperglycemia, and insulin resistance, all of which play important roles in the development of atherosclerosis and are associated with adverse CVD outcomes [30, 31]. Specifically, insulin resistance in diabetic patients leads to an increase in the production of very LDL (VLDL) particles by the liver. The combined effect of these VLDL particles with intestinal chylomicron remnants results in hypertriglyceridemia, which in turn induces an increase in the number of small dense LDL (sd-LDL) particles. As sd-LDL particles undergo glycosylation and oxidation by free radicals, this process enhances vascular endothelial permeability and promotes macrophage aggregation, thus contributing to the gradual formation of atherosclerotic plaques [11, 32]. When atherosclerotic plaques are eroded or rupture, triggering thrombotic events that can ultimately precipitate acute cardiovascular events, resulting in patient mortality. Consequently, dyslipidemia is a critical link in both the development of atherosclerosis and the adverse prognosis in diabetic patients [30]. Diabetic dyslipidemia is characterized by elevated levels of non-HDL-C, including LDL-C and other components, which contribute to the development of atherosclerosis and thus render it a superior predictor of ASCVD [33–35]. Conversely, HDL-C is known to exert anti-inflammatory, antioxidant, and anti-atherogenic effects, displaying a negative correlation with the incidence of ASCVD [36, 37]. The NHHR incorporates all lipid-related information relevant to both atherogenic and anti-atherogenic processes, offering a more comprehensive depiction of their balance. It serves as a more reflective indicator of a patient’s lipid health status.
Our study uncovered a negative correlation between NHHR and age, as well as HDL-C at baseline, whereas a positive correlation was noted between NHHR, HbA1c, and TC. This finding consistent with the research of Hong et al. [38], which suggests that NHHR may serve as a cardiovascular risk factor and is associated with adverse outcomes in individuals. Our research revealed a U-shaped association between NHHR and all-cause mortality and an L-shaped association with cardiovascular mortality. The inflection points for these association were identified at NHHR values of 2.72 and 2.83, respectively. Below the inflection points, for every unit increase in NHHR, the all-cause and cardiovascular mortality was found to decrease by 24% and 30%, respectively. Conversely, above the inflection points, a unit increase in NHHR was associated with an increase in all-cause and cardiovascular mortality by 11% and 8%, respectively. Therefore, for patients with diabetes or prediabetes, maintaining the NHHR within the range of approximately 2.7 to 2.8 may significantly minimize the risk of adverse outcomes. Multiple studies have partially supported this conclusion. Several studies [39–41] have shown a U-shaped relationship between non-HDL-C and all-cause and cardiovascular mortality, observed across populations of hypertensive participants, those with chronic kidney disease, and males not receiving statin therapy. Furthermore, two prospective cohort studies have shown a U-shaped relationship between HDL-C levels and all-cause mortality [42]. The findings of these studies suggest that both HDL and non-HDL-C levels should be maintained within a reasonable range. Notably, our study also demonstrates that both excessively high and excessively low NHHRs are associated with an increased risk of mortality. When the NHHR levels are excessively high, the risk of patient mortality increases, which is attributed to the accelerated development of atherosclerosis associated with extremely high non-HDL-C levels, leading to an elevated risk of mortality [43]. We also found that excessively low NHHRs can lead to a significant increase in the risk of mortality. Possible mechanisms for this observation are as follows: (1) an excessively low NHHR is often associated with low levels of TC. Lower levels of TC have been associated with poorer health conditions [44] or a reduction in cholesterol levels due to frailty and illness, which may potentially increase the risk of mortality [45, 46]. (2) An excessively low NHHR may also be attributed to extreme high levels of HDL-C, which are typically caused by genetic variations and can exacerbate the risk of coronary heart disease and mortality[39, 42]. However, the specific mechanisms underlying the association between NHHR and the risk of diabetes-related mortality remain to be further investigated.
We further examined the populations with diabetes and prediabetes separately. The results revealed a U-shaped association between NHHR and all-cause mortality in participants with diabetes, while an L-shaped association was observed with cardiovascular mortality. In participants with prediabetes, the NHHR was also associated with all-cause mortality in a U-shaped manner but showed a linear correlation with cardiovascular mortality. The aforementioned discrepancies may be attributed to the relatively small number of participants experiencing cardiovascular mortality in the study. The sensitivity analysis results indicated that the relationships between the NHHR and both all-cause and cardiovascular mortality remained relatively stable.
There are several limitations in this study. Firstly, as an observational study, we cannot establish a causal relationship between NHHR and mortality. Secondly, this study only assessed the prognostic value of the baseline NHHR and did not investigate the association between changes in NHHR over time and mortality. Thirdly, we did not adjust for diabetes classification or disease duration, which may introduce potential biases. Fourthly, the study population consisted primarily of the general population in the US, so caution should be exercised when extrapolating the findings to other ethnicities. Finally, the proportion of patients with CVD outcomes in the study population was small, potentially limiting the statistical power to detect differences between groups.
Conclusions
The findings of this study demonstrate that the NHHR serves as a valuable predictive indicator for all-cause and cardiovascular mortality in participants with diabetes or prediabetes. In our nationally representative sample of adults with diabetes or prediabetes in the US, we observed a U-shaped association between NHHR and all-cause mortality as well as an L-shaped association with cardiovascular mortality. Routine monitoring of NHHR may contribute to the evaluation of mortality risk and prognosis in participants with diabetes or prediabetes.
Supplementary Information
Additional file 1: Table S1. Assessment of multicollinearity among independent variables. Table S2. The proportion of missing covariates and imputation methods. Table S3. HRsfor mortality according to NHHR quartiles after excluding participants with any missing covariate values at baseline. Table S4. HRsfor mortality according to NHHR quartiles after excluding participants with self-reported cancer at baseline. Table S5. HRsfor mortality according to NHHR quartiles after excluding participants from the first 2 years of follow-up. Table S6. Association between NHHR and cardiovascular mortality using Fine & Gray competing risks models. Table S7. HRsfor mortality according to NHHR quartiles after excluding extreme valuesof NHHR.
Acknowledgements
We sincerely express our gratitude to the participants and investigators of the NHANES study for their invaluable contributions, which have provided significant support and assistance to our research endeavors.
Abbreviations
- ASCVD
Atherosclerotic cardiovascular disease
- BMI
Body mass index
- CI
Confidence intervals
- CVD
Cardiovascular disease
- DM
Diabetes mellitus
- FBG
Fasting blood glucose
- HbA1c
Hemoglobin A1c
- HDL-C
High-density lipoprotein cholesterol
- HR
Hazard ratios
- LDL-C
Low-density lipoprotein cholesterol
- MECs
Mobile examination centers
- MICE
Multiple imputation by chained equations
- NCHS
National Center for Health Statistics
- NDI
National death index
- NHHR
Non-high density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio
- Non-HDL-C
Non-high density lipoprotein cholesterol
- PG
Postprandial glucose
- RCS
Restricted cubic spline
- Sd-LDL
Small dense low-density lipoprotein
- TC
Total cholesterol
- VIF
Variance inflation factor
- VLDL
Very low-density lipoprotein
Authors’ contributions
The study was conceived by BY, ML, and RG, who were responsible for performing the data analysis and manuscript writing. TZ and ZY extracted the data from the official NHANES website. HZ contributed to the revision and review of the manuscript. AG and XF conducted a repeat analysis of the data and verified the results. All authors read and approved the final manuscript.
Funding
This study was supported by the Innovation Fund of the China Academy of Chinese Medical Sciences (CI2021A04701) and the National Key Research and Development Program of China (2021YFF 0901404).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The NHANES protocol was approved by the Institutional Review Board of the National Center for Health Statistics (https://www.cdc.gov/nchs/nhanes/irba98.htm). All participants provided written informed consent.
Consent for publication
All authors have agreed to the submission and publication of this research.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Binyang Yu and Min Li contributed equally to this study.
References
- 1.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. 10.1038/nrendo.2017.151 [DOI] [PubMed] [Google Scholar]
- 2.Gregg E, Buckley J, Ali MK, Davies J, Flood D, Mehta R, et al. Improving health outcomes of people with diabetes mellitus: global target setting to reduce the burden of diabetes mellitus by 2030. Lancet. 2023;401(10384):1302. 10.1016/S0140-6736(23)00001-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402(10397):203–34. 10.1016/S0140-6736(23)01301-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiels MS, Haque AT, Berrington de González A, Freedman ND. Leading causes of death in the US during the COVID-19 pandemic, March 2020 to October 2021. JAMA Intern Med. 2022;182(8):883–6. 10.1001/jamainternmed.2022.2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. 10.1016/j.diabres.2021.109119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22. 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang M, Sperrin M, Rutter MK, Renehan AG. Cancer is becoming the leading cause of death in diabetes. Lancet. 2023;401(10391):1849. 10.1016/S0140-6736(23)00445-2 [DOI] [PubMed] [Google Scholar]
- 8.Joseph JJ, Deedwania P, Acharya T, Aguilar D, Bhatt DL, Chyun DA, et al. Comprehensive management of cardiovascular risk factors for adults with type 2 diabetes: a scientific statement from the American Heart Association. Circulation. 2022;145(9):e722–59. 10.1161/CIR.0000000000001040 [DOI] [PubMed] [Google Scholar]
- 9.Wang CCL, Hess CN, Hiatt WR, Goldfine AB. Atherosclerotic cardiovascular disease and heart failure in type 2 diabetes – mechanisms, management, and clinical considerations. Circulation. 2016;133(24):2459. 10.1161/CIRCULATIONAHA.116.022194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodkinson A, Tsimpida D, Kontopantelis E, Rutter MK, Mamas MA, Panagioti M. Comparative effectiveness of statins on non-high density lipoprotein cholesterol in people with diabetes and at risk of cardiovascular disease: systematic review and network meta-analysis. BMJ. 2022;376:e067731. 10.1136/bmj-2021-067731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta M, Tummala R, Ghosh RK, Blumenthal C, Philip K, Bandyopadhyay D, et al. An update on pharmacotherapies in diabetic dyslipidemia. Prog Cardiovasc Dis. 2019;62(4):334–41. 10.1016/j.pcad.2019.07.006 [DOI] [PubMed] [Google Scholar]
- 12.Emerging Risk Factors Collaboration, Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993–2000. [DOI] [PMC free article] [PubMed]
- 13.National Institute for Health and Care Excellence. CKS. Lipid modification - CVD prevention. Available from: https://cks.nice.org.uk/topics/lipid-modification-cvd-prevention/. Accessed 28 Jan 2024.
- 14.Sheng G, Liu D, Kuang M, Zhong Y, Zhang S, Zou Y. Utility of non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio in evaluating incident diabetes risk. Diabetes Metab Syndr Obes. 2022;15:1677–86. 10.2147/DMSO.S355980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin G, Tu J, Zhang C, Tang X, Luo L, Wu J, et al. The value of the apoB/apoAΙ ratio and the non-HDL-C/HDL-C ratio in predicting carotid atherosclerosis among Chinese individuals with metabolic syndrome: a cross-sectional study. Lipids Health Dis. 2015;14:24. 10.1186/s12944-015-0023-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin W, Luo S, Li W, Liu J, Zhou T, Yang F, et al. Association between the non-HDL-cholesterol to HDL- cholesterol ratio and abdominal aortic aneurysm from a Chinese screening program. Lipids Health Dis. 2023;22(1):187. 10.1186/s12944-023-01939-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao Q, Zhao J, Zhao X. Association of non-HDL-C-to-HDL-C ratio with coronary lesions and its prognostic performance in first-onset NSTEMI. Biomark Med. 2023;17(1):29–39. 10.2217/bmm-2022-0548 [DOI] [PubMed] [Google Scholar]
- 18.Ouimet M, Barrett TJ, Fisher EA. HDL and reverse cholesterol transport. Circ Res. 2019;124(10):1505–18. 10.1161/CIRCRESAHA.119.312617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feig JE, Hewing B, Smith JD, Hazen SL, Fisher EA. High-density lipoprotein and atherosclerosis regression: evidence from preclinical and clinical studies. Circ Res. 2014;114(1):205–13. 10.1161/CIRCRESAHA.114.300760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen T-C, Clark J, Riddles MK, Mohadjer LK, Fakhouri THI. National Health and Nutrition Examination Survey, 2015–2018: sample design and estimation procedures. Vital Health Stat. 2020;2(184):1–35. [PubMed]
- 21.NHANES - National Health and Nutrition Examination Survey Homepage. Available from: https://www.cdc.gov/nchs/nhanes/index.htm. Accessed 28 Jan 2024.
- 22.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2020;44(Supplement_1):S15-33. 10.2337/dc21-S002 [DOI] [PubMed] [Google Scholar]
- 23.Qing G, Deng W, Zhou Y, Zheng L, Wang Y, Wei B. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and suicidal ideation in adults: a population-based study in the United States. Lipids Health Dis. 2024;23(1):17. 10.1186/s12944-024-02012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.International statistical classification of diseases and related health problems. Available from: https://iris.who.int/handle/10665/246208. Accessed 3 Feb 2024.
- 25.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601–9. 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.You J, Wang Z, Lu G, Chen Z. Association between the non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio and the risk of coronary artery disease. Biomed Res Int. 2020;2020:7146028. 10.1155/2020/7146028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qi X, Wang S, Huang Q, Chen X, Qiu L, Ouyang K, et al. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and risk of depression among US adults: a cross-sectional NHANES study. J Affect Disord. 2024;344:451–7. 10.1016/j.jad.2023.10.064 [DOI] [PubMed] [Google Scholar]
- 28.Di Angelantonio E, Gao P, Pennells L, Kaptoge S, Caslake M, Thompson A, et al. Lipid-related markers and cardiovascular disease prediction. JAMA. 2012;307(23):2499–506. [DOI] [PMC free article] [PubMed]
- 29.Kim SW, Jee JH, Kim HJ, Jin S-M, Suh S, Bae JC, et al. Non-HDL-cholesterol/HDL-cholesterol is a better predictor of metabolic syndrome and insulin resistance than apolipoprotein B/apolipoprotein A1. Int J Cardiol. 2013;168(3):2678–83. 10.1016/j.ijcard.2013.03.027 [DOI] [PubMed] [Google Scholar]
- 30.Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122. 10.1186/s12933-018-0762-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN. The diabetes mellitus–atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci. 2020;21(5): 1835. 10.3390/ijms21051835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kothari V, Bornfeldt KE. A new treatment strategy for diabetic dyslipidemia? Diabetes. 2020;69(10):2061–3. 10.2337/dbi20-0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: developed by the task force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). Rev Esp Cardiol. 2021;42(34):3227–337. [DOI] [PubMed] [Google Scholar]
- 34.Pencina KM, Thanassoulis G, Wilkins JT, Vasan RS, Navar AM, Peterson ED, et al. Trajectories of non-HDL cholesterol across midlife: implications for cardiovascular prevention. J Am Coll Cardiol. 2019;74(1):70–9. 10.1016/j.jacc.2019.04.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raja V, Aguiar C, Alsayed N, Chibber YS, ElBadawi H, Ezhov M, et al. Non-HDL-cholesterol in dyslipidemia: review of the state-of-the-art literature and outlook. Atherosclerosis. 2023;383:117312. 10.1016/j.atherosclerosis.2023.117312 [DOI] [PubMed] [Google Scholar]
- 36.Endo Y, Fujita M, Ikewaki K. HDL functions—current status and future perspectives. Biomolecules. 2023;13(1): 105. 10.3390/biom13010105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xepapadaki E, Nikdima I, Sagiadinou EC, Zvintzou E, Kypreos KE. HDL and type 2 diabetes: the chicken or the egg? Diabetologia. 2021;64(9):1917–26. 10.1007/s00125-021-05509-0 [DOI] [PubMed] [Google Scholar]
- 38.Hong H, He Y, Gong Z, Feng J, Qu Y. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and kidney stones: a cross-sectional study. Lipids Health Dis. 2024;23(1):102. 10.1186/s12944-024-02089-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng R-X, Xu J-P, Kong Y-J, Tan J-W, Guo L-H, Zhang M-Z. U-shaped relationship of non-HDL cholesterol with all-cause and cardiovascular mortality in men without statin therapy. Front Cardiovasc Med. 2022;9:903481. 10.3389/fcvm.2022.903481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng Q, Liu X-C, Chen C-L, Huang Y-Q, Feng Y-Q, Chen J-Y. The U-shaped association of non-high-density lipoprotein cholesterol levels with all-cause and cardiovascular mortality among patients with hypertension. Front Cardiovasc Med. 2021;8:707701. 10.3389/fcvm.2021.707701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiu H, Wu P-Y, Huang J-C, Tu H-P, Lin M-Y, Chen S-C, et al. There is a U shaped association between non high density lipoprotein cholesterol with overall and cardiovascular mortality in chronic kidney disease stage 3–5. Sci Rep. 2020;10(1):12749. 10.1038/s41598-020-69794-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madsen CM, Varbo A, Nordestgaard BG. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J. 2017;38(32):2478–86. 10.1093/eurheartj/ehx163 [DOI] [PubMed] [Google Scholar]
- 43.Liao P, Zeng R, Zhao X, Guo L, Zhang M. Prognostic value of non-high-density lipoprotein cholesterol for mortality in patients with coronary heart disease: a systematic review and meta-analysis. Int J Cardiol. 2017;227:950–5. 10.1016/j.ijcard.2016.10.106 [DOI] [PubMed] [Google Scholar]
- 44.Tuikkala P, Hartikainen S, Korhonen MJ, Lavikainen P, Kettunen R, Sulkava R, et al. Serum total cholesterol levels and all-cause mortality in a home-dwelling elderly population: a six-year follow-up. Scand J Prim Health Care. 2010;28(2):121–7. 10.3109/02813432.2010.487371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacobs D, Blackburn H, Higgins M, Reed D, Iso H, McMillan G, et al. Report of the conference on low blood cholesterol: mortality associations. Circulation. 1992;86(3):1046–60. 10.1161/01.CIR.86.3.1046 [DOI] [PubMed] [Google Scholar]
- 46.Johannesen CDL, Langsted A, Mortensen MB, Nordestgaard BG. Association between low density lipoprotein and all cause and cause specific mortality in Denmark: prospective cohort study. BMJ. 2020;371:m4266. 10.1136/bmj.m4266 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Assessment of multicollinearity among independent variables. Table S2. The proportion of missing covariates and imputation methods. Table S3. HRsfor mortality according to NHHR quartiles after excluding participants with any missing covariate values at baseline. Table S4. HRsfor mortality according to NHHR quartiles after excluding participants with self-reported cancer at baseline. Table S5. HRsfor mortality according to NHHR quartiles after excluding participants from the first 2 years of follow-up. Table S6. Association between NHHR and cardiovascular mortality using Fine & Gray competing risks models. Table S7. HRsfor mortality according to NHHR quartiles after excluding extreme valuesof NHHR.
Data Availability Statement
No datasets were generated or analysed during the current study.