Abstract
Background
Obesity has been linked to arterial stiffness, while no consensus was reached on the association. We aimed to clarify the association of general and central obesity with arterial stiffness by combining observational studies and Mendelian randomization (MR) study.
Methods
Two cross-sectional studies were performed in UK Biobank and Fuqing Cohort, respectively. Two-sample MR study was conducted using summary data of GWASs from GIANT consortium and UK Biobank. General obesity and central obesity were measured using body mass index (BMI) and waist circumference (WC), respectively. Arterial stiffness was measured by arterial stiffness index (ASI) in UK Biobank or branchial-ankle pulse wave velocity (baPWV) in Fuqing Cohort.
Results
Two observational studies found a consistent positive association of BMI and WC with arterial stiffness when adjusting for age, sex, education, smoking, alcohol drinking, physical activity, and LDL cholesterol. However, when additionally adjusting for metabolic traits (i.e., systolic blood pressure, diastolic blood pressure, blood glucose, triglycerides, high-density lipoprotein cholesterol, and WC or BMI), the association with BMI changed to be inverse. As compared to the lowest quintile group, the adjusted ORs across groups of second to fifth quintile were 0.93, 0.90, 0.83, and 0.72 in UK Biobank and 0.88, 0.65, 0.63, and 0.50 in Fuqing Cohort. In contrast, the positive relationship with WC remained stable with the adjusted ORs of 1.23, 1.46, 1.60, and 1.56 in UK Biobank and 1.35, 1.44, 1.77, and 1.64 in Fuqing Cohort. MR analyses provided supportive evidence of the negative association with BMI (OR = 0.97, 95%CI = 0.94–1.00) and the positive association with WC (OR = 1.14, 95%CI = 1.08–1.20).
Conclusions
Observational and genetic analyses provide concordant results that central obesity is independently related to arterial stiffness, while the role of general obesity depends on metabolic status.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-024-03546-1.
Keywords: Obesity, Arterial stiffness, Observational study, Mendelian randomization, Epidemiology
Background
Arterial stiffness is a sensitive predictor of various vascular pathologies, including vascular aging, atherosclerosis, calcification, inflammation, and so on, which has been demonstrated to be strongly related to the incidence and mortality of cardiovascular diseases [1–3]. As a non-invasive marker, it has the potential to be used in early screening and intervention of cardiovascular diseases. Several indices have been used to assess the stiffness of arteries, such as carotid-femoral pulse wave velocity (cfPWV), branchial-ankle PWV (baPWV), and arterial stiffness index (ASI) [4, 5]. The cfPWV is the most commonly used and both baPWV and ASI have been reported to be highly correlated with cfPWV [6–8].
Obesity has mounted to an epidemic level and has been reported to increase the risk of multiple chronic diseases, such as coronary heart disease, stroke, and some types of cancer. Body mass index (BMI) is the most widely used index for general obesity assessment, but incapable of accurately evaluating the regional adiposity [9]. Central adiposity can be better measured by waist circumference (WC), which always coexists with other metabolic disorders, such as hypertension, diabetes, or dyslipidemia, leading to metabolic syndrome [10]. Some studies found discrepancies between general and central obesity in the role of cardiovascular diseases, for example, central obesity was more closely associated with major cardiac events than general obesity [11, 12]. A number of studies have explored the role of obesity in arterial stiffness, some of which investigated the separate effects of general and abdominal obesity on arterial stiffness, while no consensus has been reached on the correlation [12–29]. For example, previous studies have reported a positive, negative, or non-significant association between BMI and arterial stiffness, so as for WC, and most of which had relatively small sample size and rarely further explored the underlying reasons [12–29].
Thus, in this study, we aimed to clarify the association of general obesity measured by BMI and central obesity measured by WC with arterial stiffness in two cross-sectional studies from both European and Asian populations, and further examine the causality via two-sample Mendelian randomization (MR) approach.
Methods
Cross-sectional studies
Study population
We conducted two cross-sectional studies among participants enrolled in baseline survey of UK Biobank Project and Fuqing Cohort Project, respectively. UK Biobank is a large-scale population-based study recruiting more than 500,000 residents in 22 assessment centers across the UK, in which the baseline visit was completed between March 2006 and July 2010 [30]. The baseline survey in both projects included questionnaires, physical measurements, and biological sample collection. Fuqing Cohort is an ongoing project in Fuqing city of China, the baseline survey of which was initiated in 2019 [31].
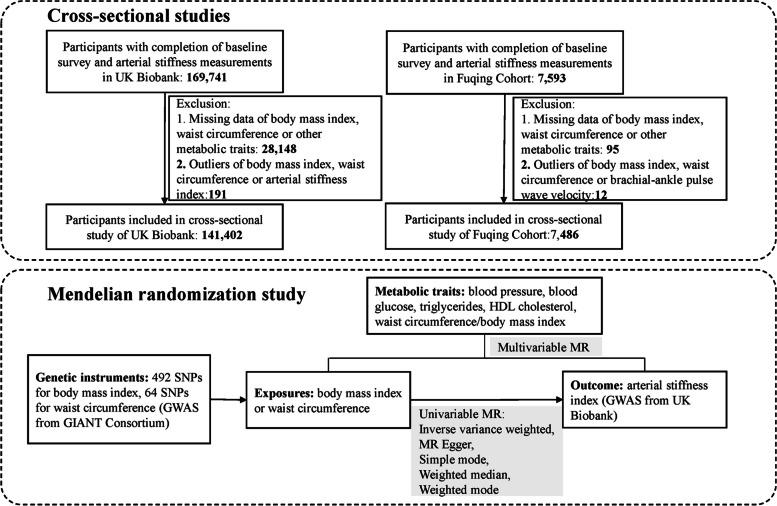
A total of 169,741 and 7593 participants have completed both baseline assessment and arterial stiffness measurements in UK Biobank and Fuqing Cohort (until December 2022), respectively. The exclusion criteria included: (1) participants with missing data of measurements for BMI, WC, or other metabolic traits (blood pressure, blood glucose, HDL cholesterol, and triglycerides); (2) participants with outliers of BMI, WC, or ASI/baPWV, in which mean ± 5 standard deviations were used to establish the upper and lower boundaries of outlier. As shown in the flowchart (Fig. 1), after exclusion, a total of 141,402 and 7486 participants were finally included in two cross-sectional studies, respectively.
Fig. 1.
Study flowchart
Assessments of exposures
General obesity and central obesity were measured by BMI and WC, respectively. Standing height was measured using a Seca 202 telescopic height-measuring rod in UK Biobank and using a Tanita BC-601 bioimpedance analyzer in Fuqing Cohort. Weight was measured using a Tanita BC-418 MA body composition analyzer in UK Biobank and a Tanita BC-601 bioimpedance analyzer in Fuqing Cohort. BMI was calculated as the formula of weight/height2 (kg/m2). WC was measured with a non-stretchable sprung tape at the halfway between the lowest rib and the top of iliac crest.
Assessment of outcome
Arterial stiffness was the outcome of this study, assessed by a photoplethysmograph transducer (PulseTrace PCA 2TM, CareFusion, USA) in UK Biobank or baPWV with an automatic waveform analyzer (BP-203 RPE III, OMRON Health Medical (China) Co, Ltd) in Fuqing Cohort [32]. In brief, in UK Biobank, the participant was seated, and removed restrictive clothing from the upper arm; clipping the PulseTrace infrared sensor onto the index finger of dominant hand and measurement taken over 10 to 15 s. The ASI was assessed as the height of the participants divided by the time between the first (systolic) and second (diastolic) wave peaks [33]. In Fuqing Cohort, the measurement was undertaken by trained investigators. Participants wore light clothes and were asked to lie in a supine position; both arms and ankles were wrapped with cuffs, connected to a plethysmographic sensor and oscillometric pressure sensor, and a heartbeat monitor was set on the left edge of the sternum and electrocardiogram electrodes were set on both wrists. The average values of baPWV on the left and right sides were used for analysis. ASI > 13 m/s or BaPWV ≥ 18 m/s is considered indicative of arterial stiffness [34, 35].
Assessment of covariates
In both projects, each participant was interviewed using a structured questionnaire to collect data of socio-demographic information, lifestyle factors, history of disease, and medications. Age was recorded at baseline assessment. Education background was classified as college/university degree, A levels/AS levels/O levels/GCSEs/equivalent, CSEs/equivalent/NVQ/equivalent, other professional qualifications/unknown in UK Biobank; or four categories, corresponding to ≥ 10, 7–9, 1–6, and 0 years of schooling in Fuqing Cohort. Alcohol drinking status was categorized as never, former, and current drinker. Smoking was also categorized as never, former, and current smoker. Physical activity was assessed using the International Physical Activity Questionnaire, recorded as summed metabolic equivalent (MET) minutes per week for all activity and grouped by quartiles [3, 36]. Blood pressure was consecutively measured twice by using blood pressure measuring device (Omron HEM-7015IT in UK Biobank, Omron U30 in Fuqing Cohort), and the average blood pressure of two readings was used in the analysis. Blood samples collected at baseline were used for serum lipids and blood glucose measurement. Serum lipids and blood glucose were measured using a Beckman Coulter clinical chemistry analyzer (AU5800) in UK Biobank or a Toshiba automatic biochemical analyzer (TBA-120FR) in Fuqing Cohort [34, 37]. The altered metabolic traits (i.e., increased blood pressure, increased blood glucose, increased triglycerides, decreased HDL cholesterol, and general obesity by BMI/central obesity by WC) were defined according to the International Diabetes Federation definition [38].
Two-sample Mendelian randomization study
Data source
In two-sample MR study, we obtained single nucleotide polymorphisms (SNPs) strongly associated with body sizes from the GIANT consortium with sample sizes of 681,275 for BMI and 245,746 for WC, respectively [39, 40]. The study outcome was arterial stiffness, measured by ASI, and the GWAS summary data was obtained from UK Biobank comprising 127,121 participants [41]. These GWASs were performed on rank based inverse normal transformed (INT) residuals, so in which the estimate was equivalent to per 1-SD of inverse rank normal transformed continuous traits increase in per additional effect allele. All GWAS studies used in this study were conducted in population of European ancestry. Instrumental variants of metabolic traits were obtained from Global Lipids Genetics Consortium (GLGC) for HDL cholesterol and triglycerides, Meta-Analyses of Glucose and Insulin-related Traits Consortium (MAGIC) for blood glucose, and International Consortium of Blood Pressure (ICBP) for blood pressure, respectively. Details of the data resources are shown in Additional file 1: Table S1.
Genetic instruments selection
In the univariable MR analysis, SNPs strongly associated with exposure at the genomic significance level (P < 5*10−8) were selected as candidate instrumental variables. The SNP with smallest P value retained in the presence of linkage disequilibrium, which was assessed by r2 > 0.001 (clumping window size = 10,000 kb) with genomic data from 1000 Genomes Project as the reference. Available proxies were used as substitutes if SNPs were unavailable in the GWAS dataset of outcome, while SNPs without available proxies and palindromic SNPs were excluded. In multivariable MR analysis, SNPs from the univariable MR analysis and available in GWASs of both exposures were used after linkage disequilibrium clumping by r2 > 0.001 (clumping window size = 10,000 kb) for building instruments.
Statistical analyses
Cross-sectional studies
Baseline characteristics are presented as frequency and percentage for categorical variables and as mean and standard deviation for continuous variables, and χ2 test or t-test was used to test the difference in baseline characteristics between participants with and without arterial stiffness. Logistic regression was used to explore the association between BMI or WC and arterial stiffness, in which both BMI and WC were modeled as quintiles and the lowest quintile used as reference. Two multivariable models were built, in which model 1 included age, sex, education, smoking, alcohol drinking, physical activity, and low-density lipoprotein (LDL) cholesterol; model 2 additionally adjusted for traits included in the metabolic syndrome (i.e., systolic blood pressure, diastolic blood pressure, blood glucose, triglycerides, high-density lipoprotein (HDL) cholesterol, and WC or BMI). Test for trend was conducted by modeling quintiles as continuous variables. A series of sensitivity analyses were conducted. First, logistic regression with restricted cubic spline was applied to explore the non-linear relationship between BMI or WC and arterial stiffness by including the exposure as a continuous variable. Second, BMI or WC quintile-specific estimated marginal means were reported and compared by using analysis of covariance. Third, multivariable models were built by adding each metabolic trait one by one to adjust for potential confounding variables. Fourth, stratified analysis by the number of altered metabolic traits (< 3 vs ≥ 3) was performed to evaluate how the association between BMI/WC and arterial stiffness differed between the different number of altered metabolic traits. And the product term of exposure and number of altered metabolic traits was generated for assessing the interaction effect between BMI or WC and the cumulative burden of altered metabolic traits in arterial stiffness. Fifth, we explored the relation by other anthropometric indices, including waist-to-hip ratio or waist-to-height ratio.
Two-sample Mendelian randomization study
Inverse variance weighting Mendelian randomization method (IVW-MR) was used as the main method to generate the estimate, in which random effect model was used in the presence of heterogeneity. The other four methods, including MR-Egger, weighted median method (WME), weighted mode, and simple mode, were further applied to assess the robustness of the results. The R2 (proportion of the variance explained by the SNP) and the F-statistics were calculated to assess the strength of each instrument [42]. The heterogeneity or horizontal pleiotropy of instrumental variables was tested by Cochrane Q test or MR-Egger regression intercept term, respectively. Leave-one-out analysis was performed to assess the sensitivity of each IV. Besides, multivariable MR analyses including exposure and each metabolic trait were further performed to explore the role of metabolic traits in the relationship between exposure and outcome.
All analyses were performed in R 4.1.2 or SAS 9.4 and P value of < 0.05 was considered as of statistical significance. Bonferroni correction approach was also applied to take multiple testing into account. There were two cross-sectional studies and two outcomes, we thus adjusted the thresholds of the significance level as P < 0.0125 (calculated as 0.05/(2*2)) in observational studies, and P < 0.025 (calculated as 0.05/2) in MR study with two outcomes.
Results
Cross-sectional association between general or central obesity and arterial stiffness
As shown in Additional file 1: Table S2, a total of 141,402 and 7486 participants were included in cross-sectional studies from UK Biobank and Fuqing Cohort, in which 16,020 (11.33%) and 1427 (19.06%) participants experienced elevated arterial stiffness, respectively. Significant difference was observed in most characteristics between groups, except for alcohol drinking in UK Biobank or sex, alcohol drinking, and physical activity in Fuqing Cohort. Individuals with arterial stiffness were older and had a higher mean value of BMI, WC, blood pressure, fasting glucose, triglycerides, and LDL cholesterol; a lower mean value of HDL cholesterol; and a greater proportion of low education level in both projects.
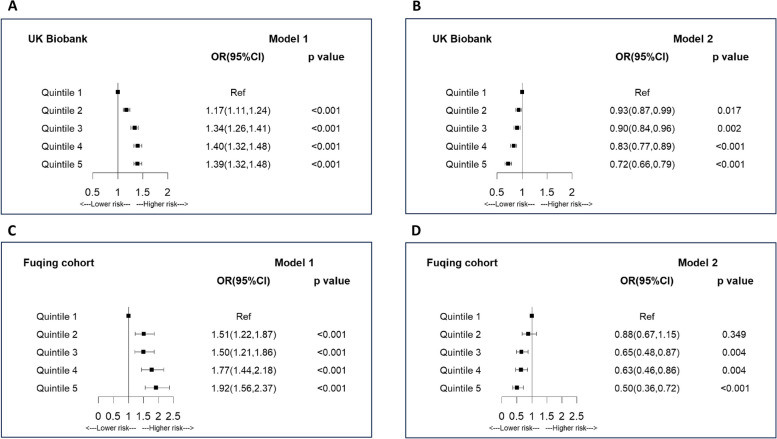
Figure 2 and Additional file 1: Table S3 show the association between general obesity measured by BMI and arterial stiffness. There was evidence for a consistent positive association between BMI and arterial stiffness when adjusting for confounders in both studies. As compared to the lowest quintile group, groups with quintiles 2–5 were significantly associated with elevated arterial stiffness with the adjusted ORs in model 1 of 1.17, 1.34, 1.40, and 1.39 (P-trend < 0.001) in UK Biobank and 1.51, 1.50, 1.77, and 1.92 (P-trend < 0.001) in Fuqing Cohort. However, after additionally adjusting for metabolic traits (i.e., WC, blood pressure, blood glucose, triglycerides, and HDL cholesterol), the observed associations changed to inverse associations with the adjusted ORs in model 2 of 0.93, 0.90, 0.83, and 0.72 (P-trend < 0.001) in UK Biobank and 0.88, 0.65, 0.63, and 0.50 (P-trend < 0.001) in Fuqing Cohort. After Bonferroni correction, these observed associations kept significant. Similar results were observed when using restricted cubic spline regression in both studies (Additional file 2: Fig. S1). Analysis of covariance also reported concordant evidence in both studies that means of ASI or baPWV increased with increasing BMI quintiles in model 1 but declined with increasing BMI quintiles in model 2 (Additional file 2: Fig. S2). Additional file 1: Table S4 shows that waist circumference and blood pressure played a more important role in the association between BMI and arterial stiffness. Stratified analysis after adjustment of all confounders found a negative association in the population with less than three altered metabolic traits, which was not observed among those with ≥ 3 altered metabolic traits (Additional file 1: Table S5). And significant interaction was observed between BMI and number of altered metabolic traits in arterial stiffness (P-interaction < 0.001 in both studies).
Fig. 2.
Association between body mass index and arterial stiffness. A Model 1 in UK Biobank Project; B Model 2 in UK Biobank. C Model 1 in Fuqing Cohort; D Model 2 in Fuqing Cohort; *Model 1 was adjusted for age, sex, education, smoking, alcohol drinking, physical activity, and LDL cholesterol. Model 2 was adjusted for age, sex, education, smoking, alcohol drinking, physical activity, LDL cholesterol, blood pressure, blood glucose, triglycerides, HDL cholesterol, and waist circumference
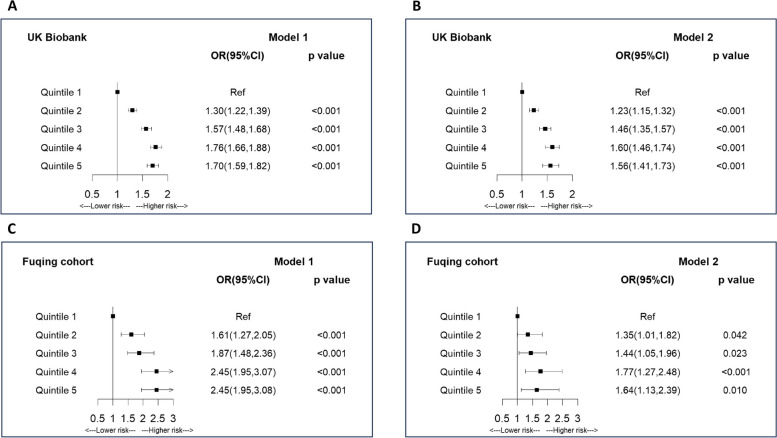
Figure 3 and Additional file 1: Table S3 show the association between central obesity measured by WC and arterial stiffness. When adjusting for confounders in model 1, there was a positive relation between WC and arterial stiffness. As compared to the lowest quintile group, groups with quintiles 2–5 were significantly related to elevated arterial stiffness with the adjusted ORs of 1.30, 1.57, 1.76, and 1.70 (P-trend < 0.001) in UK Biobank and 1.61, 1.87, 2.45, and 2.45 (P-trend < 0.001) in Fuqing Cohort. In contrast to BMI, the observed positive relationship remained stable after additionally adjusting for metabolic traits in model 2 with the adjusted ORs of 1.23, 1.46, 1.60, and 1.56 (P-trend < 0.001) in UK Biobank and 1.35, 1.44, 1.77, and 1.64 (P-trend = 0.009) in Fuqing Cohort. After Bonferroni correction, these observed associations kept significant. Restricted cubic spline regression and analysis of covariance also generated consistent results in terms of the positive relation in two models across both studies (Additional file 2: Figs. S3 and 4). Additional file 1: Table S4 shows that blood pressure played a more pronounced role in the association between WC and arterial stiffness. Stratified analysis after adjustment of all confounders found similar associations of WC with arterial stiffness between groups with < 3 and ≥ 3 altered metabolic traits in UK Biobank, while a stronger but insignificant association in the group with ≥ 3 altered metabolic traits in Fuqing Cohort (Additional file 1: Table S5). And significant interaction was observed between WC and number of altered metabolic traits in arterial stiffness (P-interaction < 0.001 in UK Biobank, P-interaction = 0.019 in Fuqing Cohort). Additional file 1: Table S6 shows similar positive associations when measuring central obesity by waist-to-hip ratio or waist-to-height ratio in both projects.
Fig. 3.
Association between waist circumference and arterial stiffness. A Model 1 in UK Biobank Project; B Model 2 in UK Biobank. C Model 1 in Fuqing Cohort; D Model 2 in Fuqing Cohort; *Model 1 was adjusted for age, sex, education, smoking, alcohol drinking, physical activity, and LDL cholesterol. Model 2 was adjusted for age, sex, education, smoking, alcohol drinking, physical activity, LDL cholesterol, blood pressure, blood glucose, triglycerides, HDL cholesterol, and body mass index
Mendelian randomization between general or central obesity and arterial stiffness
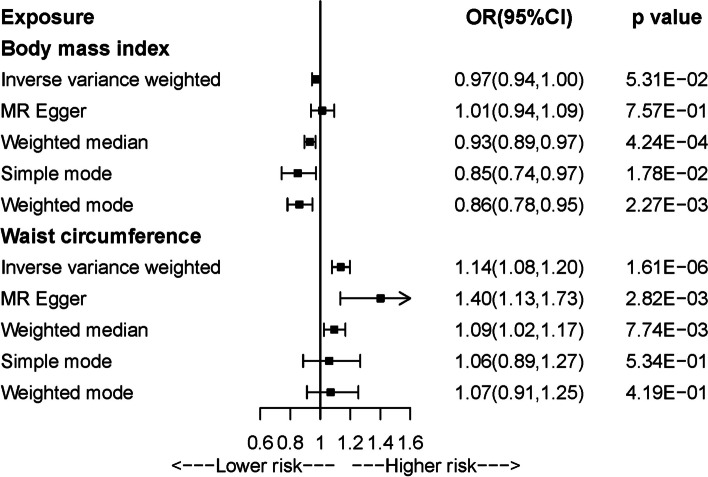
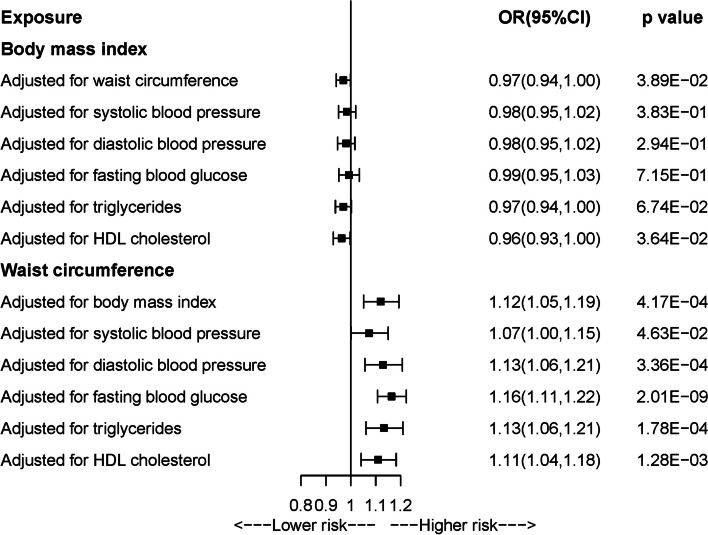
As shown in Additional file 1: Tables S7 and S8, F-statistics for all genetic instrument variants were over 10, indicating minimized weak instrument bias. Figure 4 and Additional file 1: Table S9 present the results from univariable MR analyses of BMI and WC with arterial stiffness. A borderline, negative causal association was observed between BMI and arterial stiffness in IVW-MR analysis (OR = 0.97, 95%CI = 0.94–1.00), which was consistent with the results from the other four MR methods. In Additional file 1: Table S10, sensitivity analysis by using another GWAS for BMI without sample overlap with the outcome generated similar results. Positive relationship between WC and arterial stiffness was concordantly observed across five MR methods, with statistical significance in IVW-MR (OR = 1.14, 95%CI = 1.08–1.20), MR-Egger, and weighted median approaches. After Bonferroni correction, the association of BMI with AS tended to be insignificant while the relation of WC with AS remained significant. Cochrane Q test indicated the presence of heterogeneity, while leave-one-out analysis showed that the direction of observed associations kept stable after leaving each IV (Additional file 2: Figs. S5 and 6). There was no evidence of horizontal pleiotropy. Multivariable MR study showed that the observed association between BMI and arterial stiffness attenuated to null when adjusting for blood pressure, blood glucose, or triglycerides, while the association between WC and arterial stiffness always remained significant after adjustment for any metabolic trait (Fig. 5 and Additional file 1: Table S11).
Fig. 4.
Univariable Mendelian randomization analysis of body mass index and waist circumference with arterial stiffness
Fig. 5.
Multivariable Mendelian randomization analysis of body mass index and waist circumference with arterial stiffness
Discussion
In the present study, results from observational studies in both European and Asian populations demonstrated that central obesity measured by WC was independently associated with elevated arterial stiffness, and the causality of which was supported via MR analysis. However, the role of general obesity measured by BMI was dependent on metabolic status, in which the positive relationship attenuated to null when adjusting for blood pressure or WC, even changed to a negative association after adjustment for all other metabolic phenotypes. Besides, MR analysis also suggested a negative association between BMI and arterial stiffness. These findings provided observational and genetic evidence that central obesity is an independent predictor of arterial stiffness, but the impact of general obesity is dependent on metabolic status, indicating that appropriate overweight free of abdominal visceral adiposity and metabolic disorders (i.e., metabolically healthy obesity) might benefit for arterial stiffness.
With the development of economic level, the prevalence of obesity is continuously growing. Although obesity has been recognized as a risk factor of numerous diseases, emerging data suggested that body fat distribution might play a more pronounced role in the risk of chronic diseases [43]. BMI is the most handy and common metric to measure general obesity. Several indices have been used to assess central obesity, such as WC, WHR, and WHtR, in which WC is the most commonly used [10]. A number of studies have investigated the association of obesity with arterial stiffness but generated mixed results. For example, cross-sectional studies in NHANES of 5309 healthy participants and in 1442 Chinese obese and overweight adults found a positive association of obesity with arterial stiffness regardless of being measured by BMI, WC, or other related indices [16, 28]. However, some observational studies found discrepancies between general and abdominal obesity in relation to arterial stiffness. Three cross-sectional studies in European or Japanese populations with sample sizes of 305, 1031, and 2789, respectively, found that central obesity measured by WC or WHR was significantly related to arterial stiffness, while no evidence for the association between BMI and arterial stiffness [19, 23, 25]. Another three cross-sectional studies including 1662 individuals in Brazil, 697 participants in Korea, and 794 older adults in China found a negative association between BMI and arterial stiffness [27, 29, 44]. Some studies suggested that the role of obesity in arterial stiffness might be affected by metabolic status. For example, a cohort study consisted of 1190 Chinese participants reported that the association of increased BMI and arterial stiffness was predominantly mediated through blood pressure, and another cross-sectional study also indicated that metabolically unhealthy individuals defined as individuals having two or more of the metabolic syndrome components suffered increased risk of arterial stiffness and BMI was positively associated with arterial stiffness only in metabolically healthy individuals, in which WC was not included in multivariable model [15, 26]. Regarding central obesity, the Brazilian study found a positive association between WC and arterial stiffness but depending on blood pressure, while the study in Korea reported a negative relation between central obesity and arterial stiffness in subjects with metabolic syndrome [20, 27]. These inconsistent results may be related to relatively small sample size and difference in covariates included in multivariable models.
In this study, we thus performed two large-scale cross-sectional studies from European and Asian populations to clarify the association, which yielded concordant results regarding the discrepancies between general and central obesity in arterial stiffness. A positive association of both general and central obesity was observed with arterial stiffness if not including metabolic phenotypes as covariates. However, the observed association with BMI changed to be inverse after additional adjustment for metabolic phenotypes, while the association between central obesity and arterial stiffness kept stable when considering metabolic phenotypes. MR analyses provided additional supportive genetic evidence of the negative association with BMI and positive association with WC. These findings suggest that general obesity may not be an independent risk factor of arterial stiffness but affect the occurrence of arterial stiffness via related metabolic sequelae. Besides, central obesity has previously been demonstrated to be more closely related to metabolic impairment than general obesity [45]. The role of central obesity in developing metabolic syndrome has been described as early as in 1991, which is now a criterion for metabolic syndrome diagnosis [21, 45]. All these pieces of evidence indicate that obesity-related metabolic impairments played a predominated role in the arterial stiffness than obesity itself, which may provide some clues for the phenomenon of “obesity paradox” in cardiovascular outcomes.
There were several strengths in this study. First, the observational study and MR study have specific strengths and limitations, enabling to complement each other to some extent. Second, two large-scale observational studies from European and Asian populations were conducted that can provide more statistical power to detect associations than previous studies and validation in different populations. Third, several MR methods were applied to examine the causality for the observed association and generated similar results, contributing to ensuring the robustness of results. Fourth, the extensive data collected in UK Biobank and Fuqing Cohort allowed us for comprehensive adjustments of potential confounders and explored the role of metabolic phenotypes in the associations. Nevertheless, several limitations should be noted. First, the ASI and baPWV were used to measure the arterial stiffness in UK Biobank and Fuqing Cohort, respectively, instead of the cfPWV, which is regarded as the gold standard. However, the validity and accuracy of ASI and baPWV have been demonstrated in previous studies as compared to cfPWV. Besides, analyses using ASI and baPWV yielded concordant results, supporting the validity of both indices in turn and suggesting the reliability of the results. Future large-scale population study using cfPWV as measurement is needed to confirm these findings. Second, the possibility of residual bias cannot be completely ruled out, such as measurement error for WC. However, similar results from both observational studies, and again similar results when using waist-to-hip ratio and waist-to-height ratio as alternative exposure variables, suggested the robustness of the findings and possibly small influence of bias due to measurement error for WC. Third, there were over 50% overlapping sample between GWASs from BMI and ASI, which may lead to around 5% relative bias [46]. However, we have performed sensitivity analysis by using another GWAS data source for BMI which has no sample overlap with the outcome. The similar results suggest the robustness of the study findings. Fourth, GWASs used in this MR study were obtained from European populations, and further testing is needed in other ethnic populations.
Conclusions
Observational and genetic analyses provide concordant results that central obesity is an independent risk factor of arterial stiffness, while the role of general obesity depends on metabolic status.
Supplementary Information
Additional file 1: Table S1. Information of GWAS summary data. Table S2. Characteristics of the study population between groups with and without arterial stiffness in cross-sectional studies. Table S3. Association of body mass index or waist circumference with arterial stiffness. Table S4. Association of body mass index or waist circumference with arterial stiffness in models additionally adjusted for each metabolic trait. Table S5. Stratification and interaction analysis of body mass index or waist circumference with arterial stiffness by number of altered metabolic traits. Table S6. Association of waist-to-hip ratio or waist-to-height with arterial stiffness. Table S7. Genetic variants for body mass index. Table S8. Genetic variants for waist circumference. Table S9. Mendelian randomization of body mass index or waist circumference with arterial stiffness. Table S10. Sensitivity analysis for mendelian randomization of body mass index or waist circumference with arterial stiffness. Table S11. Multivariable mendelian randomization of body mass index or waist circumference with arterial stiffness.
Additional file 2: Fig. S1. Association between body mass index and arterial stiffness via restricted cubic spline regression. Fig. S2. Body mass index quintile-specific estimated marginal means of brachial-ankle pulse wave velocity (Ba-PWV) or arterial stiffness index (ASI) via analysis of covariance. Fig. S3. Association between waist circumference and arterial stiffness via restricted cubic spline regression. Fig. S4. Waist circumference quintile-specific estimated marginal means of brachial-ankle pulse wave velocity (Ba-PWV) or arterial stiffness index (ASI) via analysis of covariance. Fig. S5. MR leave-one-out sensitivity analysis for body mass index on arterial stiffness. Fig. S6. MR leave-one-out sensitivity analysis for waist circumference on arterial stiffness.
Acknowledgements
We thank the participants and investigators who contributed to the Fuqing cohort, UK Biobank, GIANT Consortium, Global Lipids Genetics Consortium, ICBP consortium, MAGIC consortium.
Abbreviations
- ASI
Arterial stiffness index
- baPWV
Branchial-ankle pulse wave velocity
- BMI
Body mass index
- cfPWV
Carotid-femoral pulse wave velocity
- CI
Confidence interval
- LDL
Low-density lipoprotein
- HDL
High-density lipoprotein
- WC
Waist circumference
Authors’ contributions
All authors were responsible for the study concept and design. WH, WY, HH, and LC obtained funding. ZG1, QL, XL, WX, ZG3, ZZ, ZQ and SD attended field investigation. WH, ZG1, SD and WQ participated in the pre-processing of the datasets. WH and ZG2 did the statistical analysis and drafted the manuscript. WH, WY, HH, LC and ZG2 critically reviewed and improved the drafts of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Major Scientific Research Program for Young and Middle-aged Health Professionals of Fujian Province, China (2023ZQNZD01020044), National Natural Science Foundation of China (82304213), and Start-up Fund for high-level talents of Fujian Medical University (XRCZX2021026) to Dr. Wuqing Huang; Fujian Province central guide local science and technology development fund project (2023L3009) to Dr. Huashan Hong; Government of Fuqing city [grant number: 2019B003], Department of Science and Technology of Fujian [grant number: 2019L3006, 2019Y9021], and Start-up Fund for high-level talents of Fujian Medical University (XRCZX2017035, XRCZX2020034, XRCZX2023030) to Dr. Weimin Ye; National Natural Science Foundation of China (82241209), Major Science and Technology Project of Fujian Provincial Health Commission (2022ZD01004), and Fujian Provincial Special Reserve Talents Fund (2021–25) to Dr. Liangwan Chen; and Start-up Fund for high-level talents of Fujian Medical University (XCRZX2023005) to Dr. Shanshan Du. The funder had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Availability of data and materials
Genetic summary statistics and analytic code will be made available upon reasonable request to the corresponding author (ywm@fjmu.edu.cn).
Declarations
Ethics approval and consent to participate
UK Biobank study has been approved by the North West Multi-Centre Research Ethics Committee (REC reference: 21/NW/0157). Fuqing Cohort study has been approved by the Ethics Review Committee of Fujian Medical University (approval number [2017–07], [2020–58], [2021–109], and [2022–85]). All participants in UK Biobank and Fuqing Cohort have signed informed consent. Mendelian randomization study is an analysis of publicly available summary data that does not require ethical approval.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wuqing Huang, Zhaojing Gan, Ziting Gao and Qiaofen Lin Share the first authorship.
Liangwan Chen, Huashan Hong and Weimin Ye Share the senior authorship.
Contributor Information
Liangwan Chen, Email: chenliangwan@fjmu.edu.cn.
Huashan Hong, Email: 15959159898@163.com.
Weimin Ye, Email: ywm@fjmu.edu.cn.
References
- 1.Ohkuma T, Ninomiya T, Tomiyama H, Kario K, Hoshide S, Kita Y, et al. Brachial-ankle pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta-analysis. Hypertension (Dallas, Tex : 1979). 2017;69(6):1045–52. [DOI] [PubMed] [Google Scholar]
- 2.Heffernan KS, Jae SY, Loprinzi PD. Association between estimated pulse wave velocity and mortality in U.S. adults. J Am Col Cardiol. 2020;75(15):1862–4. [DOI] [PubMed] [Google Scholar]
- 3.Lyle AN, Raaz U. Killing me unsoftly: causes and mechanisms of arterial stiffness. Arterioscler Thromb Vasc Biol. 2017;37(2):e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munakata M. Brachial-ankle pulse wave velocity: background, method, and clinical evidence. Pulse (Basel). 2016;3(3–4):195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baier D, Teren A, Wirkner K, Loeffler M, Scholz M. Parameters of pulse wave velocity: determinants and reference values assessed in the population-based study LIFE-Adult. Clin Res Cardiol. 2018;107(11):1050–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodman RJ, Kingwell BA, Beilin LJ, Hamilton SE, Dart AM, Watts GF. Assessment of central and peripheral arterial stiffness: studies indicating the need to use a combination of techniques. Am J Hypertens. 2005;18(2 Pt 1):249–60. [DOI] [PubMed] [Google Scholar]
- 7.Millasseau SC, Kelly RP, Ritter JM, Chowienczyk PJ. Determination of age-related increases in large artery stiffness by digital pulse contour analysis. Clin Sci (London, England : 1979). 2002;103(4):371–7. [DOI] [PubMed] [Google Scholar]
- 8.Cheng YB, Li Y, Sheng CS, Huang QF, Wang JG. Quantification of the interrelationship between brachial-ankle and carotid-femoral pulse wave velocity in a workplace population. Pulse (Basel). 2016;3(3–4):253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romero-Corral A, Somers VK, Sierra-Johnson J, Jensen MD, Thomas RJ, Squires RW, et al. Diagnostic performance of body mass index to detect obesity in patients with coronary artery disease. Eur Heart J. 2007;28(17):2087–93. [DOI] [PubMed] [Google Scholar]
- 10.Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, et al. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16(3):177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi D, Choi S, Son JS, Oh SW, Park SM. Impact of discrepancies in general and abdominal obesity on major adverse cardiac events. J Am Heart Assoc. 2019;8(18):e013471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wohlfahrt P, Redfield MM, Lopez-Jimenez F, Melenovsky V, Kane GC, Rodeheffer RJ, et al. Impact of general and central adiposity on ventricular-arterial aging in women and men. JACC Heart failure. 2014;2(5):489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koenen M, Hill MA, Cohen P, Sowers JR. Obesity, adipose tissue and vascular dysfunction. Circ Res. 2021;128(7):951–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corden B, Keenan NG, de Marvao AS, Dawes TJ, Decesare A, Diamond T, et al. Body fat is associated with reduced aortic stiffness until middle age. Hypertension (Dallas, Tex : 1979). 2013;61(6):1322–7. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Yan Y, Yang X, Li S, Bazzano L, He J, et al. Long-term burden of higher body mass index and adult arterial stiffness are linked predominantly through elevated blood pressure. Hypertension (Dallas, Tex : 1979). 2019;73(1):229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li G, Yao T, Wu XW, Cao Z, Tu YC, Ma Y, et al. Novel and traditional anthropometric indices for identifying arterial stiffness in overweight and obese adults. Clin Nutr (Edinburgh, Scotland). 2020;39(3):893–900. [DOI] [PubMed] [Google Scholar]
- 17.Cote AT, Phillips AA, Harris KC, Sandor GG, Panagiotopoulos C, Devlin AM. Obesity and arterial stiffness in children: systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2015;35(4):1038–44. [DOI] [PubMed] [Google Scholar]
- 18.Brunner EJ, Shipley MJ, Ahmadi-Abhari S, Tabak AG, McEniery CM, Wilkinson IB, et al. Adiposity, obesity, and arterial aging: longitudinal study of aortic stiffness in the Whitehall II cohort. Hypertension (Dallas, Tex : 1979). 2015;66(2):294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wohlfahrt P, Somers VK, Cifkova R, Filipovsky J, Seidlerova J, Krajcoviechova A, et al. Relationship between measures of central and general adiposity with aortic stiffness in the general population. Atherosclerosis. 2014;235(2):625–31. [DOI] [PubMed] [Google Scholar]
- 20.Won KB, Chang HJ, Niinuma H, Niwa K, Jeon K, Cho IJ, et al. Inverse association between central obesity and arterial stiffness in Korean subjects with metabolic syndrome: a cross-sectional cohort study. Diabetol Metab Syndr. 2015;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Q, Huang QX, Zeng HL, Ma S, Lin HD, Xia MF, et al. Prediction of metabolic disorders using NMR-based metabolomics: the Shanghai Changfeng Study. Phenomics (Cham, Switzerland). 2021;1(4):186–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strasser B, Arvandi M, Pasha EP, Haley AP, Stanforth P, Tanaka H. Abdominal obesity is associated with arterial stiffness in middle-aged adults. Nutr Metab Cardiovasc Dis. 2015;25(5):495–502. [DOI] [PubMed] [Google Scholar]
- 23.Recio-Rodriguez JI, Gomez-Marcos MA, Patino-Alonso MC, Agudo-Conde C, Rodriguez-Sanchez E, Garcia-Ortiz L. Abdominal obesity vs general obesity for identifying arterial stiffness, subclinical atherosclerosis and wave reflection in healthy, diabetics and hypertensive. BMC Cardiovasc Disord. 2012;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chau K, Girerd N, Bozec E, Ferreira JP, Duarte K, Nazare JA, et al. Association between abdominal adiposity and 20-year subsequent aortic stiffness in an initially healthy population-based cohort. J Hypertens. 2018;36(10):2077–84. [DOI] [PubMed] [Google Scholar]
- 25.Ishida A, Taira H, Shinzato T, Ohya Y. Association between visceral fat mass and arterial stiffness among community-based screening participants. Hypertens Res. 2023;46(11):2488–96. [DOI] [PubMed] [Google Scholar]
- 26.Guo W, Jia J, Zhan M, Li X, Zhu W, Lu J, et al. Association of metabolically unhealthy non-obese and metabolically healthy obese individuals with arterial stiffness and 10-year cardiovascular disease risk: a cross-sectional study in Chinese adults. Nutr J. 2023;22(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodrigues SL, Baldo MP, Lani L, Nogueira L, Mill JG, Sa CR. Body mass index is not independently associated with increased aortic stiffness in a Brazilian population. Am J Hypertens. 2012;25(10):1064–9. [DOI] [PubMed] [Google Scholar]
- 28.Huang Y, Hu Y, Bao B. Relationship of body mass index and visceral fat area combination with arterial stiffness and cardiovascular risk in cardiovascular disease-free people: NHANES (2011–2018). Endocr Connect. 2023;12(11):e230291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sang Y, Wu X, Miao J, Cao M, Ruan L, Zhang C. Determinants of brachial-ankle pulse wave velocity and vascular aging in healthy older subjects. Med Sci Monit. 2020;26:e923112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer LJ. UK Biobank: bank on it. Lancet (London, England). 2007;369(9578):1980–2. [DOI] [PubMed] [Google Scholar]
- 31.Su Q, Chen H, Du S, Dai Y, Chen C, He T, et al. Association Between Serum Bilirubin, Lipid Levels, and Prevalence of Femoral and Carotid Atherosclerosis: A Population-Based Cross-Sectional Study. Arterioscler Thromb Vasc Biol. 2023;43(1):136-45. 10.1161/ATVBAHA.122.318086. [DOI] [PubMed]
- 32.Lu Y, Pechlaner R, Cai J, Yuan H, Huang Z, Yang G, et al. Trajectories of age-related arterial stiffness in Chinese men and women. J Am Coll Cardiol. 2020;75(8):870–80. [DOI] [PubMed] [Google Scholar]
- 33.Dregan A, Rayner L, Davis KAS, Bakolis I, Arias de la Torre J, Das-Munshi J, et al. Associations between depression, arterial stiffness, and metabolic syndrome among adults in the UK Biobank population study: a mediation analysis. JAMA Psychiatry. 2020;77(6):598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng M, Zhang X, Chen S, Song Y, Zhao Q, Gao X, et al. Arterial stiffness preceding diabetes: a longitudinal study. Circ Res. 2020;127(12):1491–8. [DOI] [PubMed] [Google Scholar]
- 35.Naidu MU, Reddy BM, Yashmaina S, Patnaik AN, Rani PU. Validity and reproducibility of arterial pulse wave velocity measurement using new device with oscillometric technique: a pilot study. Biomed Eng Online. 2005;4:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498-504. [DOI] [PubMed] [Google Scholar]
- 37.Elliott P, Peakman TC. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol. 2008;37(2):234–44. [DOI] [PubMed] [Google Scholar]
- 38.Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diab Care. 2005;28(11):2745–9. [DOI] [PubMed] [Google Scholar]
- 39.Yengo L, Sidorenko J, Kemper KE, Zheng Z, Wood AR, Weedon MN, et al. Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum Mol Genet. 2018;27(20):3641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Mägi R, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518(7538):187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fung K, Ramírez J, Warren HR, Aung N, Lee AM, Tzanis E, et al. Genome-wide association study identifies loci for arterial stiffness index in 127,121 UK Biobank participants. Sci Rep. 2019;9(1):9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papadimitriou N, Dimou N, Tsilidis KK, Banbury B, Martin RM, Lewis SJ, et al. Physical activity and risks of breast and colorectal cancer: a Mendelian randomisation analysis. Nat Commun. 2020;11(1):597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Després JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28(6):1039–49. [DOI] [PubMed] [Google Scholar]
- 44.Shin JY, Lee HR, Lee DC. Increased arterial stiffness in healthy subjects with high-normal glucose levels and in subjects with pre-diabetes. Cardiovasc Diabetol. 2011;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith U. Abdominal obesity: a marker of ectopic fat accumulation. J Clin Investig. 2015;125(5):1790–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol. 2016;40(7):597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Information of GWAS summary data. Table S2. Characteristics of the study population between groups with and without arterial stiffness in cross-sectional studies. Table S3. Association of body mass index or waist circumference with arterial stiffness. Table S4. Association of body mass index or waist circumference with arterial stiffness in models additionally adjusted for each metabolic trait. Table S5. Stratification and interaction analysis of body mass index or waist circumference with arterial stiffness by number of altered metabolic traits. Table S6. Association of waist-to-hip ratio or waist-to-height with arterial stiffness. Table S7. Genetic variants for body mass index. Table S8. Genetic variants for waist circumference. Table S9. Mendelian randomization of body mass index or waist circumference with arterial stiffness. Table S10. Sensitivity analysis for mendelian randomization of body mass index or waist circumference with arterial stiffness. Table S11. Multivariable mendelian randomization of body mass index or waist circumference with arterial stiffness.
Additional file 2: Fig. S1. Association between body mass index and arterial stiffness via restricted cubic spline regression. Fig. S2. Body mass index quintile-specific estimated marginal means of brachial-ankle pulse wave velocity (Ba-PWV) or arterial stiffness index (ASI) via analysis of covariance. Fig. S3. Association between waist circumference and arterial stiffness via restricted cubic spline regression. Fig. S4. Waist circumference quintile-specific estimated marginal means of brachial-ankle pulse wave velocity (Ba-PWV) or arterial stiffness index (ASI) via analysis of covariance. Fig. S5. MR leave-one-out sensitivity analysis for body mass index on arterial stiffness. Fig. S6. MR leave-one-out sensitivity analysis for waist circumference on arterial stiffness.
Data Availability Statement
Genetic summary statistics and analytic code will be made available upon reasonable request to the corresponding author (ywm@fjmu.edu.cn).