ABSTRACT
The relationship between chronic kidney disease (CKD) and cognitive function has received increased attention in recent years. Antibacterial agents (ABs) represent a critical component of therapy regimens in patients with CKD due to increased susceptibility to infections. Following our reviewing work on the neurocognitive impact of long-term medications in patients with CKD, we propose to focus on AB-induced direct and indirect consequences on cognitive function. Patients with CKD are predisposed to adverse drug reactions (ADRs) due to altered drug pharmacokinetics, glomerular filtration decline, and the potential disruption of the blood–brain barrier. ABs have been identified as a major cause of ADRs in vulnerable patient populations. This review examines the direct neurotoxic effects of AB classes (e.g. beta-lactams, fluoroquinolones, aminoglycosides, and metronidazole) on the central nervous system (CNS) in patients with CKD. We will mainly focus on the acute effects on the CNS associated with AB since they are the most extensively studied effects in CKD patients. Moreover, the review describes the modulation of the gut microbiota by ABs, potentially influencing CNS symptoms. The intricate brain–gut–kidney axis emerges as a pivotal focus, revealing the interplay between microbiota alterations induced by ABs and CNS manifestations in patients with CKD. The prevalence of antibiotic-associated encephalopathy in patients with CKD undergoing intravenous AB therapy supports the use of therapeutic drug monitoring for ABs to reduce the number and seriousness of ADRs in this patient population. In conclusion, elucidating AB-induced cognitive effects in patients with CKD demands a comprehensive understanding and tailored therapeutic strategies that account for altered pharmacokinetics and the brain–gut–kidney axis.
Keywords: adverse drug reactions, antibacterial agents, chronic kidney disease, cognitive impairment, drugs
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Patients with chronic kidney disease (CKD) have a high comorbidity burden and are frequently admitted to hospital for the management of an acute illness [1]. Hence, these patients have a high long-term medication load and are frequently exposed to drugs for acute care, including antibacterial agents (ABs). The number of prescription drugs and the complexity of drug management increases as CKD progresses [2]. First, CKD-associated metabolic disturbances (such as the accumulation of uremic toxins) modify drug pharmacokinetics (PK) and pharmacodynamics (PD) (especially for drugs cleared by the kidneys) and therefore require dose adjustments or drug withdrawal to prevent adverse drug reactions (ADRs). Second, drug dose levels may also need to be adjusted in dialyzed patients (see associated review, part 1) [3]. Third, kidney transplant recipients have the most complex drug regimens, with immunosuppressants, prophylactic treatments, and drugs taken for comorbid conditions and complications such as infections [4]. Finally, considering the high number of prescription drugs in CKD patients, the risk of pharmacokinetic and pharmacodynamic interactions with drugs, including AB, is elevated and may lead to an enhancement of drug side effects.
Because of polypharmacy due to high comorbidity burden and complex drug regimens, patients with CKD are prone to ADRs [5]. The incidence of ADRs increases as kidney function deteriorates, as a result of drug-related nephrotoxicity, drug accumulation, and drug interactions [6].
Similarly, CKD is a risk factor for adverse reactions to drugs acting on the central nervous system (CNS). For example, disruption of the blood–brain barrier (BBB) is associated with CKD and may further modify the effectiveness of some drugs by increasing their penetration into the brain parenchyma [3]. In turn, this might induce major CNS-related ADRs. In our previous work, we reviewed the most common medications associated with cognitive impairment (in both the general population and patients with CKD) and described their effects [7]. Most of the reviewed drugs are taken over the long term. However, ABs deserve further attention because they are widely used among patients with CKD and potentially associated with specific CNS toxicity. We will mainly focus on the acute effects on the CNS associated with AB since they are the most extensively studied effects in CKD patients. Furthermore, evidence for chronic effects on cognition in general is scarce. Nevertheless, investigating whether chronic or repeated antibiotic use could be linked to long-term alterations in cognitive function in CKD patients warrants further research. Indeed, a prospective population-based cohort study among 14 542 participants in the Nurses’ Health Study II suggested that the long-term antibiotic use in midlife is associated with small decreases in cognition assessed 7 years later [8]. In the same line, the effects of long-term/recurrent use of AB in childhood on developing cognitive impairment in middle and old age have been evaluated in the UK Biobank Database. The authors demonstrated that the likelihood for the development of cognitive impairment increased by 18% among AB users compared to non-users, independently of factors such as age, sex, educational qualification, ethnicity, income, smoking status, alcohol consumption, body mass index, history of hypertension, and history of diabetes [9]. In addition, the association between dementia incidence and AB use was retrospectively analyzed in a population-based South Korean cohort [10]. The used AB classes included penicillin, cephalosporins, macrolides, fluoroquinolones, sulfonamides, lincosamides, tetracyclines, and vancomycin. The authors showed an increased risk for overall dementia, Alzheimer's disease, and vascular dementia with long-term AB use as well as for overall dementia and Alzheimer's disease with use of more than five AB classes as compared to non-AB users. Results were adjusted for age, sex, body mass index, smoking status, alcohol consumption, physical activity, income, comorbidity burden, fasting blood sugar, systolic blood pressure, total cholesterol, and antidepressant use.
Here, we shall first review the direct neurotoxic effects of different AB classes on the CNS in CKD and then describe how ABs may accentuate CNS symptoms by modulating the gut microbiota.
Antibacterial agents and CKD
Antibacterials (AB) are among the most commonly prescribed medicines worldwide [11]. Patients with CKD are at increased risk for infectious complications due to comorbidities, presence of vascular or peritoneal access as well as immunosuppression secondary to immunosuppressive therapy and to renal dysfunction per se [12–14]. Indeed, both humoral immunity and cellular immunity are affected in CKD, with low immune cell activity and low antibody levels. This immune dysfunction is present at the onset of CKD but intensifies as kidney disease progresses and is most prominent in patients on dialysis [15]. After kidney transplantation, infections constitute a major cause of morbidity and mortality in immunosuppressed patients. Thus, ABs are frequently prescribed to CKD patients for treatment and prophylaxis.
However, AB therapy in patients with CKD has a number of specific aspects to consider: (i) renal excretion of most ABs; (ii) enhanced drug clearance through extracorporeal therapies; (iii) decreased plasma protein binding in the context of hypoalbuminemia and uremia; (iv) the role of tubular secretion to achieve local therapeutic drug levels (e.g. in urinary tract infections); and (v) interactions with chronic therapeutic regimens (e.g. clarithromycin with calcineurin inhibitors, fluoroquinolones with metallic phosphate binders and iron preparations) [16, 17].
ABs have previously been identified as important causes of adverse drug events in vulnerable patient populations [18]. Several classes of AB have neurotoxic effects on the peripheral and central nervous systems, with clinical manifestations such as antibiotic-associated encephalopathy (AAE) [19]. CKD is a known risk factor for the occurrence of AAE, which in turn is regarded as one of the commonest AB-induced ADRs in CKD patients [20, 21]. The overall prevalence of AAE has been estimated at 4.4% in hospitalized end-stage kidney disease (ESKD) patients receiving intravenous AB therapy, but is likely to be underestimated given the difficulty in inferring causality in sepsis and with comorbidities [21]. Distinct clinical phenotypes of AAE have been described with the use of different AB classes (Table 1) [20].
Table 1:
Antimicrobial agents. Mechanisms of neurotoxicity and clinical picture.
| AB class | Mechanism neurotoxicity | Clinical picture |
|---|---|---|
| Beta-lactam | Inhibition of GABA neurotransmission | Increased excitability (high doses) |
| Penicillins | Non-competitive binding to GABA-A receptors | Confusion, disorientation, hallucinations, myoclonus, and convulsions. Coma in high doses. Hoigne's syndrome (acute psychosis associated with IM procaine benzylpenicillin) |
|
Cephalosporins
|
Competitive binding to GABA-A receptors High drug penetration in CNS Enhanced glutaminergic activity |
Confusion, disorientation, hallucinations, myoclonus, and convulsions. Non-convulsive status epilepticus and language dysfunction potentially mimicking stroke. Cefazolin—headache, dizziness, drowsiness, confusion; Cefuroxime (<1%)—chills, headache, dizziness, drowsiness, irritability, trismus; Ceftazidime—seizures; Ceftriaxone (<1%)—chills, headache, dizziness, seizure; Cefepime (very frequent): encephalopathy, aphasia, myoclonus, seizure, non-convulsive status epilepticus 2 to 4 days after initiation. |
|
Carbapenems
|
High tissue penetrance Antagonism of GABA-A receptor binding site Interaction with antiepileptic drugs |
Seizures, encephalopathy, hallucinations. Neuropsychiatric features (altered mental status) 5 to 7 days after initiation Ertapenem—lower seizure risk (small volume of distribution + high protein binding); Meropenem—lower seizure risk. Additionally causes delirium and myoclonic jerking. |
| Fluoroquinolones | Inhibition of GABA neurotransmission (structural similarity to GABA) Interference with NMDA |
Common: confusion, agitation, insomnia, drowsiness. Less common: hallucinations, suicidal ideation and toxic psychosis; dizziness, restlessness, Rare: seizures 2 to 3 days after initiation |
| Sulfonamides | Unknown (proposed deficiency in glutathione, secondary deficiency in dopamine and serotonin) | Apathy, depression, aseptic meningitis, ataxia, chills, headache, insomnia, seizures |
| Macrolides |
Unknown (proposed metabolism involving cytochrome P450 3A4) | <1%: Acute psychosis, vertigo, dizziness, drowsiness, headache. Erythromycin—seizures |
| Metronidazole |
Unknown (proposed interference with thiamin pathway, free radical formation). Usually long-term users | Cerebellar symptoms Altered mental status, seizures, peripheral neuropathies and psychosis. |
| Linezolid |
Non-dose-related, weak nonselective monoamine oxidase inhibitor, leading to inhibition of serotonin metabolism Mitochondrial toxicity due to reduced protein synthesis inhibition of monoamine oxidase. Interaction with anticholinergic medications |
Serotonin syndrome (agitation, confusion, hyperreflexia), delirium. Peripheral (pain, numbness, paresthesia, weakness) and cranial nerve (optical) neuropathies, when used for >27 days |
| Aminoglycosides |
Activation of NMDA receptors | Numbness, seizure, abnormal gait, ataxia, confusion, headache, lethargy, seizure, vertigo, pseudotumor cerebri Ototoxicity |
| Polymyxins |
Unknown, but dose dependent | Oral parestesia (streptomycin), ataxia and visual disturbances. Seizures, confusion, hallucinations, vertigo |
| Isoniazid | Reversible, preventable with pyridoxine supplementation, and dose-related—formation of pyridoxal isonicotinyl hydrazine that leads to competitive inhibition of vitamin B6 action | Peripheral neuropathy (most frequent), paresthesia, sensory impairment, seizures, encephalopathy. Optical neuritis. |
| Ethambutol | Probable formation of pyridoxal isonicotinyl hydrazine that leads to competitive inhibition of vitamin B6 action | Confusion, dizziness, hallucination, headache, peripheral neuritis. Depression and suicidal ideation. Optical neuritis (irreversible blindness reported) |
Role of therapeutic drug monitoring
Therapeutic drug monitoring (TDM) measures the amount of drug in various compartments, such as blood, serum, and/or plasma, and interstitial or other fluids to ensure the amount of drug taken by a patient is safe and effective. TDM as a discipline has evolved from monitoring a few classical anticonvulsants in the 1970s to routine monitoring of a number of drugs in different classes, including AB [22]. The role of TDM as an adjunct to clinical decision making is based on limited data from prospective randomized-controlled studies, as well as on data from systemic reviews and a general consensus on the value of PK/PD surrogacy [7, 23–27]. As discussed in our partner review, CKD-associated changes in drug PK and PD are relevant for dose adjustments and illustrate the use of TDM in the setting of altered PK (Table 2) [3]. The specific TDM targeted ranges are out of scope for this review although some general observations might be useful. Overall, methods in TDM, typically based on liquid chromatography coupled to tandem mass spectrometry, range from single measurements at a specific timepoint (e.g. at trough concentration or maximum concentration for vancomycin or gentamicin) to several measurements at specific timepoints after the administration to comprehensively assess drug exposure by calculating the area under the curve. Based on our clinical experience, there are several key messages to remember before using TDM in daily practice: (i) The preferred TDM approach is proactive (regardless of clinical situation and based on risk factors for altered individual PK) as opposed to reactive (in response to clinical deterioration). (ii) TDM should be performed at the time of drug initiation or changed dosage regimen. (iii) For TDM purposes, a system should be in place assuring compliance with precise timing and handling according to laboratory instructions.
Table 2:
Antimicrobial agents and TDM
| Drug class | Role of TDM for toxicity |
|---|---|
| Aminoglycosides | Ototoxicity [166] AMK: Cmin > 5 mg/l GEN: Cmin > 1 mg/l TOB: Cmin > 1 mg/l |
| Beta-lactams | Neurotoxicity [24, 56, 166] PIP: Cmin > 361.4 mg/l MEM: Cmin > 44.5–64.2 mg/l FLX: Cmin > 125.1 mg/l FEP: Cmin > 20 mg/l, Css > 60 mg/l |
| Fluoroquinolones | General toxicity [166, 167] Unclear |
| Oxazolidinones | Neurotoxicity [168] LZD: Cmin >2 mg/l, >4 weeks treatment duration |
| Polymyxins | Nephrotoxicity [166] COL: Cmin > 2.4 mg/l PMB: AUC24 > 100 |
| Sulfonamides | General toxicity [169] SXT: SMX >150 mg/l |
AMK: amikacin; AUC24–area under the concentration–time curve during a 24-hour period (estimation based on one or several samples taken, measured in mg/l × h); Cmin: minimum steady-state concentration monitoring for intermittent infusions (sample obtained prior to next dose); Css: steady-state concentrations for continuous infusions (sample obtained at any time during infusion); COL: colistin; FEP: cefepime; FLX: flucloxacillin; GEN: gentamycin; LZD: linezolid; MEM: meropenem; PIP: piperacillin; PMB: polymyxin B; SMX: sulfamethoxazole; SXT: co-trimoxazole (trimethoprim/sulfamethoxazole); TOB: tobramycin.
Antibacterials and direct neurotoxic effects
In the following, data on AAE in CKD patients will be presented according to different AB classes.
Beta-lactam-antibiotics
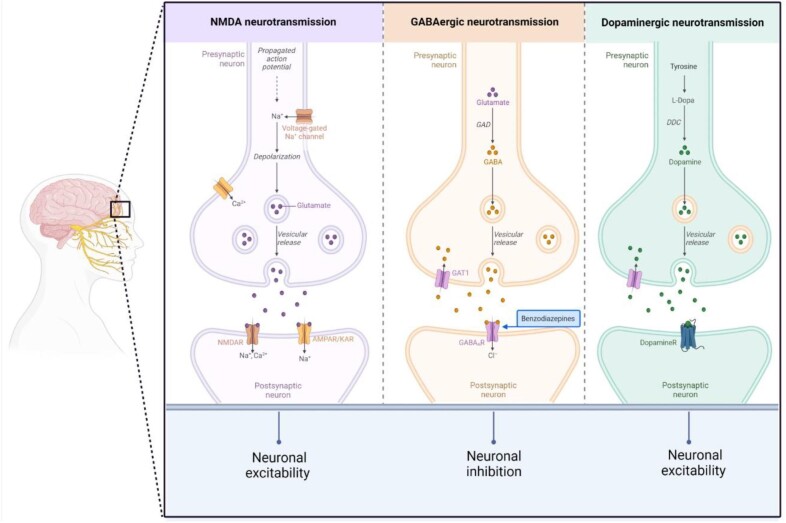
Beta-lactam antibiotics have classically been associated with neurotoxic effects [28]. CNS manifestations are mainly attributed to inhibition of the gamma-aminobutyric acid (GABA)-mediated neurotransmission leading to increased excitability. The interaction is linked to the core beta-lactam ring that is structurally similar to GABA, leading to non-competitive (e.g. penicillins) or competitive (e.g. cephalosporins) binding to the GABA-A receptor [29, 30]. The binding affinity is increased by predominantly basic C-2 side chain structures, e.g. in imipenem as compared with other carbapenems. In addition, inhibition of GABA-mediated neurotransmission also occurs through interaction with the benzodiazepine receptor and decrease in number of benzodiazepine receptors (Fig. 1) [29].
Figure 1:
Central neuro-transmitter systems (created by BioRender.com). NMDA, N-methyl-D-aspartate; NMDAR, N-methyl-D-aspartate receptor; AMPAR/KAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor/kainate receptor; GABA, γ-Aminobutyric acid; GAD, Glutamate decarboxylase; GAT1, GABA transporter 1; GABA A R, γ-Aminobutyric acid-A receptor; DDC, DOPA decarboxylase.
Penicillins
Despite their large therapeutic window and different structural characteristics, virtually all penicillin molecules may exhibit neurotoxic effects at high serum levels [31]. Clinical manifestations include confusion, disorientation, hallucinations, myoclonus, and convulsions with onset within days of starting treatment and potential reversibility after drug cessation. A particular case concerns the acute psychotic picture associated with the intramuscular administration of procaine benzylpenicillin referred to as Hoigne's syndrome, mainly attributed to the procaine component influencing central dopaminergic neurotransmission analogous to other local anesthetics (Fig. 1) [32]. By contrast, chronic effects on cognitive function are not part of the typical clinical picture, although penicillins were among the AB classes associated with dementia risk in the population-based cohort mentioned earlier [10].
In the non-CKD population, penicillin central neurotoxicity has mainly been described in the setting of direct intraventricular application and high-dose-treatment [28, 33–43]. However, impaired renal function represents the chief risk factor and a relevant number of reported cases had preexisting stable CKD or acutely worsening kidney function. Encephalopathy occurred as consequence of failed dose adaptation, but also despite adapted dose regimens [38, 43–49]. In hemodialysis patients, two cases of encephalopathy induced by piperacillin-tazobactam have been reported [50, 51]. Neves et al. describe clinical improvement after two hemodialysis sessions with a switch to a high flux filter. In peritoneal dialysis patients, rapid improvement after one hemodialysis session has been noted, whereas another case showed resolution after drug cessation and with continued peritoneal dialysis [52, 53]. Kidney replacement therapy (continuous in roughly one-third) or creatinine clearance <10 ml/min were predictors of overdosing with oxacillin and cloxacillin in ICU patients with severe infections [43].
Cephalosporins
Cephalosporin-induced neurotoxic effects are well known and tend to occur more frequently with cefepime and ceftazidim [29, 54]. Neurotoxicity might be favored by high drug penetration into the CNS and competitive binding to GABA-A receptors, in contrast to non-competitive binding of penicillins. Clinical symptoms mirror those observed from other beta-lactam AB, but also include non-convulsive status epilepticus and language dysfunction potentially mimicking stroke [20]. Furthermore, cephalosporins were among the AB classes studied in a retrospective cohort analysis, which showed an increased risk for overall dementia in a cumulative and duration-dependent manner [10]. In a retrospective cohort study that included 319 in-hospital patients undergoing TDM for cefepime, the incidence of neurotoxicity was 23.3% and correlated with plasma trough levels. Median onset of symptoms after treatment commenced was 2 days with 81% of patients recovering after drug cessation within 2 days [55]. The overwhelming majority of cases has been described in patients with impaired kidney function [56]. Reports on the effectiveness of hemodialysis as therapy for cefepime-induced neurotoxicity have shown conflicting results [56].
Carbapenems
Despite seizures regarded as the most prominent manifestation of carbapenem-associated neurotoxicity, neuropsychiatric features may also occur with these antibiotics [57]. However, this spectrum does not include chronic effects on cognitive function. In comparison with benzylpenicillin, carbapenems have been shown to exhibit higher neurotoxic potential due to high tissue penetrance that may be potentiated due to specific molecule properties as outlined above [58, 59]. In addition, the interaction with antiepileptic drugs increases the risk for seizure occurrence [60]. Among carbapenems, meropenem exhibits less neurotoxic effects than imipenem or ertapenem [61]. Neurotoxic effects are mainly described in the context of high-dose-treatment, preexisting CNS lesions, as well as renal impairment [62, 63]. However, these effects have been reported even with kidney function-adjusted regimens [48]. For example, among 26 reviewed cases of neurotoxicity associated with the use of ertapenem, 69% had acutely or chronically decreased kidney function [63]. Symptoms occurred between two and ten days and were reversible after drug discontinuation with or without hemodialysis treatment. However, long-lasting effects of up to two weeks have also been reported [64]. In patients undergoing chronic hemodialysis treated with ertapenem, an incidence of 10% for neurotoxic effects was described [65]. Drug elimination through hemodialysis sessions has been reported at 30% and up to 72% using low flux and high flux filters, respectively [64, 66]. In kidney transplant recipients, two reports have suggested an interaction between imipenem/cilastatin and cyclosporine A, leading to increased neurotoxicity [67, 68]. Given its lower neurotoxic potential, meronem may be the preferred carbapenem to use in CKD patients at high risk of neurotoxicity.
Fluoroquinolones
ADRs affecting the CNS are well known among this drug class with an incidence of 1.1%–6.6% reported for ciprofloxacin [69, 70]. Manifestations include a wide range of neurological and psychiatric symptoms with a predominance of psychotic syndromes and a rare occurrence of seizures [71, 72]. In addition, epilepsy and serious cognitive impairment following the use of intravenous levofloxacin injection have been described in a single case. Improvement of the disorder after drug withdrawal was noted, which may be considered a rare adverse effect of levofloxacin [73].
Several molecules have been shown to cross the BBB despite non-lipophilic properties [74]. Neurotoxic reactions are ascribed to inhibition of GABA neurotransmission given the structural similarity to GABA with displacement of GABA from its receptor [75]. However, interference with N-methyl-D-aspartate (NMDA) neurotransmission has also been demonstrated in vitro (Fig. 1) [76]. In addition, drug interactions with xanthine derivates and non-steroidal anti-inflammatory drugs can potentially increase neurotoxicity [75]. Additional risk factors for the development include high dosage and renal impairment [77]. Among 63 reviewed cases of quinolone-associated AAE, 22% had underlying kidney impairment [20]. Few cases of quinolone neurotoxicity have been reported in patients on hemodialysis and peritoneal dialysis, some but not all following overdosing [78–82].
Sulfonamides
Sulfonamide-induced neurotoxic effects have been recognized early after the discovery of this AB class with acute psychosis as a main manifestation [83]. However, sulfonamides were included in a retrospective cohort analysis showing an increased risk with cumulative or long-term AB use [10]. The incidence of this ADR has been estimated up to 23.5% in HIV-infected patients, but it might be difficult to evaluate given the comorbid condition and frequent co-medication with steroids in the setting of Pneumocystis jiroveci pneumonia [84]. Despite high penetration into the CNS, the mechanisms underlying these effects are unknown [85]. Deficiency in glutathione as well as reduced tetrahydrobiopterin synthesis with secondary deficiency in dopamine and serotonin have been proposed [86, 87]. Neurotoxic effects occur in a dose-dependent manner [84]. No impaired renal function was noted in a cohort of HIV-infected patients developing acute psychosis under treatment with co-trimoxazol [84]. In another retrospective cohort of 20 kidney transplant recipients treated for Pneumocystis jiroveci pneumonia, four patients developed acute psychosis that resolved within 24 hours after discontinuing the drug [88].
Macrolides
The main neurotoxic manifestation induced by macrolide AB is an acute psychosis-like syndrome; however, CNS effects may include dizziness, vertigo, insomnia, tinnitus, confusion, and disorientation [20, 89]. In addition, macrolides were one of the seven classes of ABs analyzed in a population-based cohort showing that AB exposure may increase the risk for dementia in a cumulative duration-dependent manner [10]. In an early small cohort of elderly patients treated with clarithromycin, side effects affecting the CNS occurred in more than half of the patients [90]. The pathophysiological substrate of these neuropsychiatric effects is currently not known [91]. However, given the metabolism involving cytochrome P450 3A4, the co-administration of other molecules metabolized through this pathway may lead to increased risk for neurotoxicity [89]. Importantly, CYP 3A4 activity has been reported to be reduced in uremia and to be partly restored after hemodialysis treatment [92]. However, among 54 and 38 reviewed cases of macrolide- and clarithromycin-induced neurotoxicity, only four and two cases had preexisting kidney impairment, respectively [20, 89]. Several cases of clarithromycin-induced hallucinations in peritoneal dialysis patients have been published so far, in one case despite the use of kidney-adapted dosing regimens [93–95]. On the other hand, a neuroprotective potential of erythromycin has been suggested. Thus, in a mouse model for Alzheimer's disease, erythromycin showed high anti-amyloid effects [96]. Furthermore, in a clinical randomized-controlled trial, perioperative use of erythromycin led to improved cognitive performance after coronary artery bypass grafting surgery compared to standard of care therapy [97]. However, although a candidate drug for exploring the potential to prevent cognitive decline and progression to dementia based on benefits to synaptic impairment, erythromycin was excluded as it passes the BBB only if used in high doses potentially causing hematological complications [98].
Metronidazole
Metronidazole neurotoxicity produces a distinctive clinical phenotype. In an incidental cohort of 336 425 elderly patients treated with metronidazole for the first time, 0.2% developed CNS symptoms within 100 days of therapy start [99]. The clinical picture is characterized by encephalopathy with cerebellar symptoms and typical MR imaging signs occurring with a latency of weeks after treatment begins, rather than within days as described for other AB [20, 100, 101]. However, neuropsychiatric patterns including altered mental status and seizures, as well as peripheral neuropathies, psychosis, and rapid, reversible cognitive decline, can also occur [101–104]. Time to resolution typically is longer than that described for other AB and might be incomplete in rare cases [20, 101, 102]. Despite metronidazole readily crossing the BBB, the mechanism by which it exerts its neurotoxic effects is unknown [85]. Among other hypotheses, interference with the thiamine pathway and free radical formation has been discussed [105–107]. At least 15% of the 136 patients collected by Sorensen et al. exhibiting metronidazole-induced neurotoxicity had preexisting hepatic dysfunction, whereas 7% had CKD [101]. Conflicting data exist concerning the acute and cumulative dose-dependency of metronidazole neurotoxicity, which has been suggested by some authors, based on single case reports, but has not been confirmed by others reporting larger cohorts [99, 101, 108, 109]. Indeed, such a finding could be of relevance in the immunosuppressed and aged CKD patient population. Data on patients undergoing dialysis treatment are scarce. Hemodialysis has been used in the context of metronidazole-induced neurotoxicity and accidental overdose in hemodialysis patients, respectively [110, 111]. In kidney transplant recipients, metronidazole-induced neurotoxicity has been reported after variable treatment duration and with complete resolution of symptoms after drug discontinuation [112, 113].
Linezolid
Besides more common neurotoxic effects, including peripheral and cranial nerve neuropathies and serotonin syndromes, very few data exist on the potential occurrence of linezolid-induced encephalopathy [114–116]. Symptoms in these cases resolved after drug discontinuation. In contrast, no long-term effects on cognition have been described to our knowledge. Linezolid penetrates the BBB [85]. One postulated mechanism of injury is mitochondrial toxicity due to reduced protein synthesis [117]. In addition, inhibition of monoamine oxidase by linezolid might explain some of the CNS effects, including ‘serotonin syndrome’, delirium and interaction with anticholinergic medications [118]. However, comorbid conditions, including electrolyte abnormalities and alcoholism, as well as co-medications do not allow for any firm conclusion. In only one case was reduced kidney function reported [114].
Aminoglycosides
Neurotoxic effects associated with the use of aminoglycosides are known to affect the cochlea, neuromuscular and autonomic transmission, as well as the peripheral nervous system. However, very limited data suggest the occurrence of encephalopathy after exposure to gentamicin with ultrastructural evidence of lysosomal pathology, whereas distinctive reproducible lesions of the brain stem have also been reported in an animal study after high-dose intracisternal gentamicin administration [119, 120]. However, no chronic effects on cognitive functions have been described. As demonstrated for ototoxicity, activation of NMDA receptors has been shown to be involved with direct intrastriatal application of neomycin [121].
Polymyxins
Polymyxins such as colistin are known to exhibit neurotoxic effects comprising neuromuscular blockade (after intramuscular administration), paresthesia, ataxia, and visual disturbances [122]. However, the occurrence of seizures, confusion, and hallucinations, as well as severe encephalopathy, have also been reported [122–124]. By contrast, to our knowledge, no lasting effects on cognitive functions have been reported. CNS effects are thought to be facilitated by the lipophilic structure of polymyxins [125]. However, the exact mechanism for central neurotoxicity is still unknown. Kidney impairment has not been described as a risk factor for the occurrence of CNS neurotoxicity [122]. Polymyxin use was nevertheless associated with a high frequency of overall neurotoxic events in a cohort of 213 kidney transplant recipients with mainly paresthesiae, but also hallucinations in 3.4% [126].
Antimycobacterials
Active tuberculosis, caused by the Mycobacterium tuberculosis bacteria, remains one of the main infectious causes of death worldwide, with variable geographical prevalence. Recommended treatment for active tuberculosis relies on quadritherapy with isoniazid, rifampicin, pyrazinamide, and ethambutol [127]. CKD is known to be associated with a higher prevalence of tuberculosis, but very few data are available in CKD patients on the frequency of prescription and tolerance of antimycobacterials [128].
Isoniazid
Isoniazid is the main antimycobacterial associated with neurotoxicity and causes peripheral neuropathy. Described effects on the CNS mainly include encephalopathy; however, in a prospective cohort of 100 patients receiving isoniazid for treatment of latent tuberculosis, nine suffered from cognitive impairment of unspecified duration [129]. An American study assessed the cognitive function of 25 adolescents who received isoniazid for at least 6 months before the treatment, during, and after its cessation and did not find significant impact on attentionnal function [130]. Isoniazid neurotoxicity is mainly due to altered metabolism of pyridoxine (vitamin B6). High-dose pyridoxine supplementation is recommended if signs of isoniazid-induced peripheral neuropathy appear. Some case reports and case series suggested increased isoniazid neurotoxicity in ESKD due to altered pyridoxine metabolism, resulting in severe deficiency in pyridoxal phosphate (active form of pyridoxine), as well as an important removal of pyridoxal phosphate by renal replacement therapies, including hemodialysis and peritoneal dialysis [131, 132]. Despite the rarer occurrence of isoniazid neurotoxicity affecting the CNS, CNS toxicity seems to be more frequent in CKD patients and cases of encephalopathy (presenting mainly as consciousness disorders, seizures, or cerebellitis) were reported in these patients [133, 134]. It is therefore recommended to prescribe pyridoxine supplementation to CKD patients taking isoniazid (minimum dosage 100 mg/day) for the duration of treatment to prevent its neurotoxicity.
Ethambutol
Optic neuropathy is a common complication of ethambutol treatment and can occur early following the initiation of ethambutol. Patients will usually present bilateral central visual acuity loss and dyschromatopsia [135]. However, rare cases of ethambutol-induced psychosis and confusion have also been reported [136–138]. No association has been reported between ethambutol and chronic cognitive dysfunction. Pathophysiology of ethambutol-induced neurotoxicity is poorly understood, but is suggested to be related to the metal chelating effect of ethambutol through two putative mechanisms: copper chelation disrupting mitochondrial metabolism and zinc chelation inhibiting lysosomal activation [135]. CKD patients are exposed to higher concentrations of ethambutol and are considered at higher risk of ethambutol-induced optic neuropathy, although the evidence is weak [132, 139]. Of note, concomitant administration of isoniazid might increase the risk of ethambutol-induced optic neuropathy.
Rifampicin
Interestingly, experimental murine studies suggested that rifampicin might have neuroprotective effects. Rifampicin decreased apoptosis and increased neuron viability in in vitro models of neurotoxicity and decreased the neurotoxicity and aggregation of amyloid beta protein in rat pheochromocytoma cells. [140, 141]. In addition, several experimental studies found that rifampicin improved cognitive performance in models of cognitive impairment in rodents [142]. In humans, the potential beneficial effect of rifampicin is still debated. A randomized-controlled study evaluating daily doxycycline + rifampicine versus placebo in patients with probable Alzheimer's disease found an improvement in cognitive decline in the treated group [143]. However, the subsequent randomized-controlled DARAD trial did not find any effect of rifampicin on cognition in patients with Alzheimer's disease [144]. However, no clinical data have evaluated whether rifampicin might mitigate neurotoxicity induced by isoniazid and ethambutol. An experimental study found that prenatal exposition to ethambutol, isoniazid, and rifampicin could induce cognitive dysfunction in rats [145].
Antibacterials and effects on gut microbiota
Microbiota in CKD
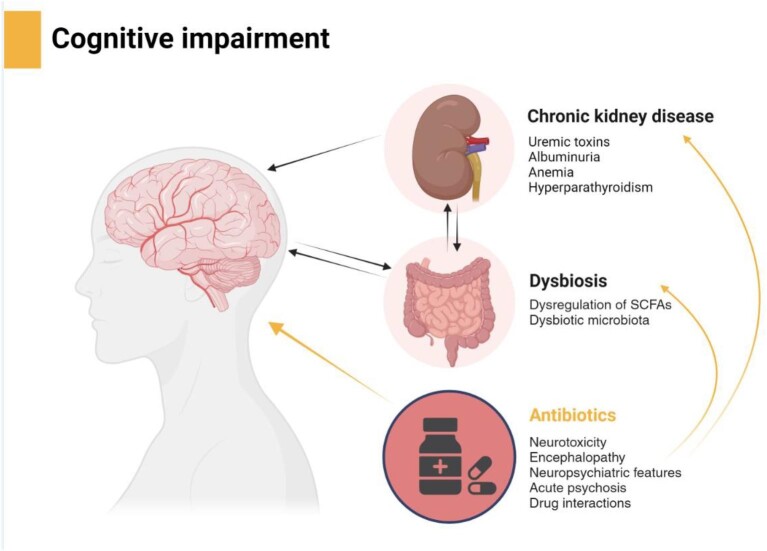
The composition of bacteria, fungi, archaea, and viruses colonizing the GI tract is collectively referred to as gut microbiota. It forms a complex and mutually beneficial relationship with the host, and its composition is considered to play an important role for the maintenance of the host homeostasis, as well as for the development of certain diseases [146]. Since several years, CKD has been associated with dysbiosis, an imbalanced intestinal microbial community with quantitative and qualitative changes in the composition and metabolic activities of the gut microbiota [147]. It is thought that in CKD increasing urea concentrations lead to alterations in the intestinal flora that can increase production of gut-derived toxins [148]. Likewise, changes in microbiota composition and structure produce excessive amounts of uremic toxins and less reno-protective metabolites [149]. Thus, in pathological states, interactions between the kidneys and gut microbiota are bidirectional and current management of dysbiosis in CKD should be considered as a novel focus for the management of CKD (Fig. 2).
Figure 2:
Interaction between antibacterials, microbiota, and kidney function contributing to cognitive impairment (created by BioRender.com). SCFAs, short-chain fatty acids.
It was demonstrated that healthy gut microbiota has reno-protective roles. Short-chain fatty acids (SCFA) are products of microbial fermentation that protect tubular cells against oxidative stress and biogenesis of mitochondria. They can also reduce ischemia-reperfusion kidney injury and inflammation, as well as infiltration of immune and apoptotic cells in the injured kidneys of mice [150–152]. However, in CKD, dysregulation of SCFAs and their receptors has been reported, and CKD-linked dysbiosis increased the microbiota-derived uremic toxins, trimethylamine-N-oxide, and activation of aryl hydrocarbon receptors [153, 154]. In patients with stage 3–4 CKD there is also an increase in aerobic and a decrease in anaerobic bacteria [155]. In rats with CKD induced by 5/6 nephrectomy, abundance of bacterial taxa differs significantly compared with controls and the serum levels of uremic toxins in these animals correlated with the abundance of Clostridia- and bacteroidia-affiliated species in the gut microbiota [156, 157].
Antibacterials and Microbiota
Prescribed ABs are considered a major risk factor for alteration of gut microbiota composition, diversity, and abundance. Even short-term AB treatment can shift the gut microbiota to a long-term dysbiotic state. The post-antibiotic dysbiosis includes loss of bacterial diversity and reduced colonization resistance against pathogens. Furthermore, one of the major concerns of AB use is the long-term alterations of the healthy gut microbiota and horizontal transfer of resistance genes that could result in accumulation of bacteria with multidrug resistance genes. Unfortunately, CKD patients are frequently exposed to ABs for curative or prophylactic indications. Thus, the already altered composition and function of gut microbiota are further worsened by AB treatment.
A systematic review of AB-induced changes in human gut microbiota from published data between 1979 until 2017 revealed that dysbiosis rapidly develops under AB administration. After AB treatment, composition of the gut microbiota generally returns to similar pretreatment state within several weeks, but not in all cases [158]. For example, prescribed amoxicillin induced an increase in Enterobacteria, a decrease or no change in anaerobic bacteria, Lactobacillus and Bifidobacteria, as well as an increase in Bacterioides and overgrowth of Candida in some cases. Amoxicillin with clavulanic acid induced an increase in aerobic gram-positive cocci and Enterobacteria, including Escherichia coli, a decrease in Lactobacillus and Bifidobacteria, as well as overgrowth of Clostridium. This review from a UK primary care setting suggests that once treatment has been stopped, gut bacteria are capable of recovery to their pretreatment state. However, the fact that the microbiota may not have fully recovered suggests that some ABs have a persistent effect on certain bacterial species.
Antibacterials, microbiota, and effects on the CNS
The association of long-term AB use with cognitive function is controversial. A prospective, population-based cohort study was conducted on >14 000 women to investigate whether at least 2 months of AB exposure is associated with cognitive scores [8]. It was concluded that long-term ABs use in midlife was associated with a small decrease in cognition assessed seven years later. Assessment included global cognition, learning and working memory, psychomotor speed, and attention. On the other hand, short-term AB use can be linked to extreme neurological changes such as encephalopathy or psychosis that have been attributed to direct neurotoxic effects (see previously). However, at present it is unclear whether AB effects on microbiota composition play an additional role in these cases.
Conversely, psychotropic agents do have an effect on gut microbiota and prolonged exposure to such agents is often associated with marked gastrointestinal changes, including altered food intake, bowel motility, gastric emptying, and transit time. In addition, mounting evidence suggests that physical and mental disturbances lead to changes in gastrointestinal motility in both animals and humans. In the experimental model of depressive-like behavior in rats, treatment with oxytocin exerted anxiolytic and antidepressant effects, but also induced a strong shift in microbiota composition. The magnitude of that shift was associated with behavioral tests scores [159]. Furthermore, this study showed that a specific bacterial genus, Mogibacterum, which increased in abundance, was associated with low-anxiety behavior in rats.
Therefore, it might be concluded that the relation between gut and CNS is bidirectional and that drugs affecting CNS affect gut microbiota, while AB when affecting gut microbiota have effects on CNS and cognitive functions (Fig. 2).
Antibacterials and the brain-gut-kidney axis
The term ‘brain–gut–kidney axis’ was introduced by Yang in a review on the relationship between gut microbiota and hypertension in CKD [160]. However, considering CKD patients and the negative impact of ABs on gut microbiota that may lead to, or at least add to, cognitive decline, we should think about the brain-gut-kidney axis as an important pathogenic in patients with kidney disease.
In addition, several risk factors may contribute to cognitive impairment such as cardiovascular disease, inflammation, and head injury, but other CKD-related factors such as anemia, hyperparathyroidism, uremic toxins, or albuminuria may also contribute [161, 162].
Finally, kidney dysfunction and uremia have implications for the BBB and gut–blood barrier through reduced expression of tight-junction proteins in both barriers. In addition, gut-derived uremic toxins are associated with increased inflammation and oxidative stress that may be associated with anemia and mineral metabolism disorders. Kidney-dependent gut dysbiosis is also linked to decreased SCFAs, barrier damage, and Th17 polarization. Taken together, these factors affect brain and cognitive functions. Furthermore, dysregulation of the tryptophan kynurenine pathway of CKD might also be associated with the cognitive impairment as part of the brain-gut-kidney axis [160, 163].
Where do AB-dependent microbiota changes or dysbiosis stand in relation to other factors? It seems particularly challenging to distinguish what comes first and how original disruption of cognitive functioning by ABs is associated with its effects on the gut microbiota already changed by CKD. More studies should be conducted analyzing the impact of prescribed ABs on cognitive impairment in CKD patients. Moreover, therapeutic approaches to AB prescribing involving prebiotic, probiotic, and symbiotic supplementation CKD patients should be considered to restore gut microbiota balance.
CONCLUSION
In conclusion, AB dosing in CKD patients involves regimen adaptations taking into account the altered pharmacokinetics. Moreover, caution is required to detect acute changes in kidney function in these patients in the clinical setting of infection. In addition, the AB drug mechanism of action (time- vs concentration-dependent), therapeutic window, severity of infection, microbial sensitivity, infection site, and host immunodeficiency have all to be considered to ensure adequate dosing and avoid under-dosing [164]. In particular, and in most cases, the administration of loading doses in the normal range is possible [165]. To avoid ADRs affecting the CNS, AB dose levels must be measured and adjusted using TDM.
Acknowledgements
The authors would like to thank Prof. Giovambattista Capasso, acting chair of Cognitive Decline in Nephro-Neurology: European Cooperative Target (CONNECT) Action and members of COST Action for their support.
Contributor Information
Sophie Liabeuf, Pharmacoepidemiology Unit, Department of Clinical Pharmacology, Amiens University Medical Center, Amiens, France; MP3CV Laboratory, EA7517, Jules Verne University of Picardie, Amiens, France.
Gaye Hafez, Department of Pharmacology, Faculty of Pharmacy, Altinbas University, Istanbul, Turkey.
Vesna Pešić, Faculty of Pharmacy, University of Belgrade, Belgrade, Serbia.
Goce Spasovski, Department of Nephrology, Clinical Centre “Mother Theresa”, Saints Cyril and Methodius University, Skopje, North Macedonia.
Mickaël Bobot, Aix-Marseille University, Department of Nephrology, AP-HM, La Conception Hospital, Marseille, France; C2VN Laboratory, Inserm 1263, INRAE 1260, Aix-Marseille University, Marseille, France.
Romaldas Mačiulaitis, Department of Nephrology, Lithuanian University of Health Sciences, Kaunas, Lithuania; Institute of Physiology and Pharmacology, Faculty of Medicines, Lithuanian University of Health Sciences, Kaunas, Lithuania.
Inga Arune Bumblyte, Department of Nephrology, Lithuanian University of Health Sciences, Kaunas, Lithuania.
Ana Carina Ferreira, Nephrology Department, Centro Hospitalar e Universitário de Lisboa Central, Lisbon, Portugal; Universidade Nova de Lisboa-Faculdade de Ciências Médicas-Nephology, Lisbon, Portugal.
Ana Farinha, Department of Nephrology, Hospital de Vila Franca de Xira, Lisbon, Portugal.
Jolanta Malyszko, Department of Nephrology, Dialysis and Internal Medicine, Medical University of Warsaw, Warsaw, Poland.
Marion Pépin, Department of Geriatrics, Ambroise Paré University Medical Center, APHP, Boulogne-Billancourt, France; Paris-Saclay University, UVSQ, Inserm, Clinical Epidemiology Team, Centre de Recherche en Epidémiologie et Santé des Populations (CESP), Villejuif, France.
Ziad A Massy, Paris-Saclay University, UVSQ, Inserm, Clinical Epidemiology Team, Centre de Recherche en Epidémiologie et Santé des Populations (CESP), Villejuif, France; Department of Nephrology, Ambroise Paré University Medical Center, APHP, Paris, France.
Robert Unwin, Department of Renal Medicine, Royal Free Hospital, University College London, London, UK.
Giovambattista Capasso, Department of Translantional Medical Sciences, University of Campania Luigi Vanvitelli, Naples, Italy; Biogem Research Institute, Ariano Irpino, Italy.
Laila-Yasmin Mani, Department of Nephrology and Hypertension, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
CONNECT Action (Cognitive Decline in Nephro-Neurology European Cooperative Target):
Giovambattista Capasso, Alexandre Andrade, Mustafa Arici, Maie Bachmann, Matthew Bailey, Michelangela Barbieri, Mickaël Bobot, Annette Bruchfeld, Inga Bumblyte, Antonello Calcutta, Giovanna Capolongo, Sol Carriazo, Michele Ceccarelli, Adrian Constantin Covic, Ananya De, Pilar Delgado, Nicole Endlich, Matthias Endres, Fabrizio Esposito, Michele Farisco, Quentin Faucher, Ana Carina Ferreira, Andreja Figurek, Denis Fouque, Casper Franssen, Ivo Fridolin, Sebastian Frische, Liliana Garneata, Loreto Gesualdo, Konstantinos Giannakou, Olivier Godefroy, Aleksandra Golenia, Dimitrios Goumenos, Agnė Gryguc, Eugenio Gutiérrez Jiménez, Gaye Hafez, Ewout Hoorn, Pedro Henrique Imenez Silva, Raafiah Izhar, Dearbhla Kelly, Shelli Kesler, Aleksandra Klimkowicz-Mrowiec, Samuel Knauss, Justina Kurganaite, Hélène Levassort, Sophie Liabeuf, Jolanta Malyszko, Laila-Yasmin Mani, Gianvito Martino, Ziad Massy, Christopher Mayer, Armida Mucci, Alma Mutevelic-Turkovic, Rikke Nielsen, Dorothea Nitsch, Alberto Ortiz, Vasileios Panagiotopoulos, Giuseppe Paolisso, Bojana Pejušković, Marion Pepin, Alessandra Perna, Andrea Perrottelli, Vesna Pešić, Pasquale Pezzella, Merita Rroji (Molla), Ivan Rychlík, Giorgos Sakkas, Mariadelina Simeoni, Maria José Soler Romeo, Goce Spasovski, Ana Starčević, Gioacchino Tedeschi, Francesco Trevisani, Robert Unwin, Evgueniy Vazelov, Carsten Alexander Wagner, Franca Wagner, Christoph Wanner, Andrzej Wiecek, Hong Xu, Miriam Zacchia, Lefteris Zacharia, Irene Zecchino, Carmine Zoccali, Francesco Mattace Raso, Karl Hans Endlich, Norberto Perico, Giuseppe Remuzzi, Francesco Trepiccione, Mark Okusa, Vincenzo Di Marzo, Peter Blankestijn, Kai-Uwe Eckardt, and Maximilian Konig
FUNDING
This article is published as financially supported by the Horizon EU COST Action CA19127-Cognitive Decline in Nephro-Neurology: European Cooperative Target (CONNECT).
AUTHORS’ CONTRIBUTIONS
L.-Y.M, S.L., and G.H. were responsible for the research idea. L.-Y.M. was responsible for the writing and the supervision of review writing. L.-Y.M., V.P., G.S., M.B., R.M., I.A.B., M.P., and Z.A.M. contributed to writing parts of the manuscript. R.M., I.A.B., A.C.F, A.F., and J.M. contributed to table preparation. G.H. and S.L. contributed to figure preparation. All authors critically revised the manuscript. All authors reviewed and approved the manuscript for publication.
DATA AVAILABILITY STATEMENT
No new data were generated or analyzed in support of this research.
CONFLICT OF INTEREST STATEMENT
Robert Unwin is currently working as a Chief Scientist (Kidney Diseases) in Translational Science and Experimental Medicine, Early CVRM (Cardiovascular, Renal and Metabolism), BioPharmaceutical R&D, AstraZeneca, Cambridge, UK. Other authors declare no conflicts of interest related to this work.
REFERENCES
- 1. Kalantar-Zadeh K, Jafar TH, Nitsch D et al. Chronic kidney disease. Lancet 2021;398:786–802. 10.1016/S0140-6736(21)00519-5 [DOI] [PubMed] [Google Scholar]
- 2. Laville SM, Metzger M, Stengel B et al. Evaluation of the adequacy of drug prescriptions in patients with chronic kidney disease: results from the CKD-REIN cohort. Br J Clin Pharmacol 2018;84:2811–23. 10.1111/bcp.13738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liabeuf S, Pešić V, Spasovski G et al. Drugs with a negative impact on cognitive function (part 1): chronic kidney disease as a risk factor. Clin Kidney J 2023;16:2365–77. 10.1093/ckj/sfad241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marienne J, Laville SM, Caillard P et al. Evaluation of changes over time in the drug burden and medication regimen complexity in ESRD patients before and after renal transplantation. Kidney Int Rep 2021;6:128–37. 10.1016/j.ekir.2020.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liabeuf S, Laville M. Drug prescription in patients with chronic kidney disease: a true challenge. Nephrol Dial Transplant 2021;36:385–6. 10.1093/ndt/gfaa164 [DOI] [PubMed] [Google Scholar]
- 6. Laville SM, Gras-Champel V, Moragny J et al. Adverse drug reactions in patients with CKD. Clin J Am Soc Nephrol 2020;15:1090–102. 10.2215/CJN.01030120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hafez G, Malyszko J, Golenia A et al. Drugs with a negative impact on cognitive functions (part 2): drug classes to consider while prescribing in CKD patients. Clin Kidney J 2023;16:2378–92. 10.1093/ckj/sfad239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mehta RS, Lochhead P, Wang Y et al. Association of midlife antibiotic use with subsequent cognitive function in women. PLoS ONE 2022;17:e0264649. 10.1371/journal.pone.0264649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu Z, Wei S, Chen X et al. The effect of long-term or repeated use of antibiotics in children and adolescents on cognitive impairment in middle-aged and older person(s) adults: a cohort study. Front Aging Neurosci 2022;14:1–9. 10.3389/fnagi.2022.833365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim M, Park SJ, Choi S et al. Association between antibiotics and dementia risk: a retrospective cohort study. Front Pharmacol 2022;13:1–12. 10.3389/fphar.2022.888333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Boeckel TP, Gandra S, Ashok A et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014;14:742–50. 10.1016/S1473-3099(14)70780-7 [DOI] [PubMed] [Google Scholar]
- 12. Naqvi SB, Collins AJ. Infectious complications in chronic kidney disease. Adv Chronic Kidney Dis 2006;13:199–204. 10.1053/j.ackd.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 13. Mcdonald HI, Thomas SL, Nitsch D. Chronic kidney disease as a risk factor for acute community-acquired infections in high-income countries: a systematic review. BMJ open 2014;4:e004100. 10.1136/bmjopen-2013-004100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vanholder R, Van Biesen W. Incidence of infectious morbidity and mortality in dialysis patients. Blood Purif 2002;20:477–80. 10.1159/000063556 [DOI] [PubMed] [Google Scholar]
- 15. Johnson DW, Fleming SJ. The use of vaccines in renal failure. Clin Pharmacokinet 1992;22:434–46. 10.2165/00003088-199222060-00003 [DOI] [PubMed] [Google Scholar]
- 16. St Peter WL, Redic-Kill KA, Halstenson CE. Clinical pharmacokinetics of antibiotics in patients with impaired renal function. Clin Pharmacokinet 1992;22:169–210. 10.2165/00003088-199222030-00002 [DOI] [PubMed] [Google Scholar]
- 17. Eyler RF, Shvets K. Clinical Pharmacology of Antibiotics. Clin J Am Soc Nephrol 2019;14:1080–90. 10.2215/CJN.08140718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gurwitz JH, Field TS, Harrold LR et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA: J Am Med Assoc 2003;289:1107–16. 10.1001/jama.289.9.1107 [DOI] [PubMed] [Google Scholar]
- 19. Bhattacharyya S, Darby R, Berkowitz AL. Antibiotic-induced neurotoxicity. Curr Infect Dis Rep 2014;16:448. 10.1007/s11908-014-0448-3 [DOI] [PubMed] [Google Scholar]
- 20. Bhattacharyya S, Darby RR, Raibagkar P et al. Antibiotic-associated encephalopathy. Neurology 2016;86:963–71. 10.1212/WNL.0000000000002455 [DOI] [PubMed] [Google Scholar]
- 21. Huang Q, Li J, Huang N et al. Clinical characteristics and outcomes of antibiotic-associated encephalopathy in patients with end-stage kidney disease. Ren Fail 2022;44:1708–16. 10.1080/0886022X.2022.2134025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dasgupta A. Therapeutic Drug Monitoring: Newer Drugs and Biomarkers. Elsevier: USA, 2012;1–578 [Google Scholar]
- 23. Ensom MHH, Burton ME A critical assessment of the outcomes of therapeutic drug monitoring. In: ME, B. (ed.) Applied Pharmacokinetics and Pharmacodynamics. Principles of Therapeutic Drug Monitoring Lippincott Williams & Wilkins: Philadelphia USA, 2006; pp839–47. [Google Scholar]
- 24. Pai Mangalore R, Peel TN, Udy AA et al. The clinical application of beta-lactam antibiotic therapeutic drug monitoring in the critical care setting. J Antimicrob Chemother 2023;78:2395–405. 10.1093/jac/dkad223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Whipple JK, Ausman RK, Franson T et al. Effect of individualized pharmacokinetic dosing on patient outcome. Crit Care Med 1991;19:1480–5. 10.1097/00003246-199112000-00007 [DOI] [PubMed] [Google Scholar]
- 26. Pai Mangalore R, Ashok A, Lee SJ et al. Beta-lactam antibiotic therapeutic drug monitoring in critically ill patients: a systematic review and meta-analysis. Clin Infect Dis 2022;75:1848–60. 10.1093/cid/ciac506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schumacher GE, Barr JT. Therapeutic drug monitoring: do the improved outcomes justify the costs? Clin Pharmacokinet 2001;40:405–9. 10.2165/00003088-200140060-00002 [DOI] [PubMed] [Google Scholar]
- 28. Walker AE, Johnson HC, Kollros JJ. Penicillin convulsions; the convulsive effects of penicillin applied to the cerebral cortex of monkey and man. Surg Gynecol Obstet 1945;81:692–701. [PubMed] [Google Scholar]
- 29. Chow KM, Hui AC, Szeto CC. Neurotoxicity induced by beta-lactam antibiotics: from bench to bedside. Eur J Clin Microbiol Infect Dis 2005;24:649–53. 10.1007/s10096-005-0021-y [DOI] [PubMed] [Google Scholar]
- 30. Sugimoto M, Fukami S, Kayakiri H et al. The beta-lactam antibiotics, penicillin-G and cefoselis have different mechanisms and sites of action at GABA(A) receptors. Br J Pharmacol 2002;135:427–32. 10.1038/sj.bjp.0704496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Manian FA, Stone WJ, Alford RH. Adverse antibiotic effects associated with renal insufficiency. Rev Infect Dis 1990;12:236–49. 10.1093/clinids/12.2.236 [DOI] [PubMed] [Google Scholar]
- 32. Hoigne R, Schoch K., [Anaphylactic shock and acute nonallergic reactions following procaine-penicillin]. Schweiz Med Wochenschr 1959;89:1350–6. [PubMed] [Google Scholar]
- 33. Johnson HC. intraventricular penicillin: a note of warning. JAMA: J Am Med Assoc 1945;127:217–9. 10.1001/jama.1945.92860040001007 [DOI] [Google Scholar]
- 34. Reuling JR. Intrathecal penicillin. J Am Med Assoc 1947;134:16–8. 10.1001/jama.1947.02880180018004 [DOI] [PubMed] [Google Scholar]
- 35. Weinstein L, Lerner PI, Chew WH. Clinical and bacteriologic studies of the effect of “massive” doses of penicillin G on infections caused by Gram-negative bacilli. N Engl J Med 1964;271:525–33. 10.1056/NEJM196409102711101 [DOI] [PubMed] [Google Scholar]
- 36. Mitchell JH. Ampicillin intolerance. Br Med J (Clin Res Ed) 1965;1:251. 10.1136/bmj.1.5429.251-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oldstone MBA, Nelson E. Central nervous system manifestations of penicillin toxicity in man. Neurology 1966;16:693–700. 10.1212/WNL.16.7.693 [DOI] [PubMed] [Google Scholar]
- 38. Conway N, Beck E, Somerville J. Penicillin encephalopathy. Postgrad Med J 1968;44:891–7. 10.1136/pgmj.44.518.891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Raichle ME, Kutt H, Louis S et al. Neurotoxicity of intravenously administered penicillin G. Arch Neurol 1971;25:232–9. 10.1001/archneur.1971.00490030058006 [DOI] [PubMed] [Google Scholar]
- 40. Park-Matsumoto YC, Tazawa T. Piperacillin-induced encephalopathy. J Neurol Sci 1996;140:141–2. 10.1016/0022-510X(96)00119-0 [DOI] [PubMed] [Google Scholar]
- 41. Macknin ML. Behavioral changes after amoxicillin-clavulanate. Pediatr Infect Dis J 1987;6:873–4. 10.1097/00006454-198709000-00024 [DOI] [PubMed] [Google Scholar]
- 42. Bell CL, Watson B, Waring WS. Acute psychosis caused by co-amoxiclav. BMJ 2008;337:a2117. 10.1136/bmj.a2117 [DOI] [PubMed] [Google Scholar]
- 43. Neuville M, El-Helali N, Magalhaes E et al. Systematic overdosing of oxa- and cloxacillin in severe infections treated in ICU: risk factors and side effects. Ann Intensive Care 2017;7:34. 10.1186/s13613-017-0255-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bloomer HA. Penicillin-induced encephalopathy in uremic patients. JAMA: J Am Med Assoc 1967;200:121–3. 10.1001/jama.1967.03120150077011 [DOI] [PubMed] [Google Scholar]
- 45. Malone AJ Jr., Field S, Rosman J et al. Neurotoxic reaction to oxacillin. N Engl J Med 1977;296:453. 10.1056/NEJM197702242960817 [DOI] [PubMed] [Google Scholar]
- 46. Trunet P, Bouvier AM, Otterbein G et al. , [Penicillin-induced encephalopathy]. Nouv Presse Med 1982;11:1781–4. [PubMed] [Google Scholar]
- 47. Kallay M, Tabechian H, Riley G et al. Neurotoxicity due to ticarcillin in patient with renal failure. Lancet 1979;1:608–9. 10.1016/S0140-6736(79)91032-8 [DOI] [PubMed] [Google Scholar]
- 48. Lemaire-Hurtel AS, Gras-Champel V, Hary L et al. , [Recommended dosage adaptation based on renal function is not always sufficient to avoid betalactam antibiotics side effects]. Nephrologie & Therapeutique 2009;5:144–8. [DOI] [PubMed] [Google Scholar]
- 49. Huang W-T, Hsu Y-J, Chu P-L et al. Neurotoxicity associated with standard doses of piperacillin in an elderly patient with renal failure. Infection 2009;37:374–6. 10.1007/s15010-009-8373-3 [DOI] [PubMed] [Google Scholar]
- 50. Bassilios N, Restoux A, Vincent F et al. Piperacillin/tazobactam inducing seizures in a hemodialysed patient. Clin Nephrol 2002;58:327–8. 10.5414/CNP58327 [DOI] [PubMed] [Google Scholar]
- 51. Neves PDMM, Freitas FM, Kojima CA et al. Piperacillin/tazobactam-induced neurotoxicity in a hemodialysis patient: a case report. Hemodial Int 2015;19:143–5. 10.1111/hdi.12194 [DOI] [PubMed] [Google Scholar]
- 52. Tong MKH, Siu Y-P, Yung C-Y et al. Piperacillin/tazobactam-induced acute delirium in a peritoneal dialysis patient. Nephrol Dialysis Transpl 2004;19:1341. 10.1093/ndt/gfh048 [DOI] [PubMed] [Google Scholar]
- 53. Lin C-S, Cheng C-J, Lin S-H et al. Piperacillin/tazobactam-induced seizure rapidly reversed by high flux hemodialysis in a patient on peritoneal dialysis. Am J Med Sci 2007;333:181–4. 10.1097/MAJ.0b013e31803195e7 [DOI] [PubMed] [Google Scholar]
- 54. Grill MF, Maganti R. Cephalosporin-induced neurotoxicity: clinical manifestations, potential pathogenic mechanisms, and the role of electroencephalographic monitoring. Ann Pharmacother 2008;42:1843–50. 10.1345/aph.1L307 [DOI] [PubMed] [Google Scholar]
- 55. Boschung-Pasquier L, Atkinson A, Kastner LK et al. Cefepime neurotoxicity: thresholds and risk factors. A retrospective cohort study. Clin Microbiol Infect 2020;26:333–9. 10.1016/j.cmi.2019.06.028 [DOI] [PubMed] [Google Scholar]
- 56. Mani L‐Y, Kissling S, Viceic D et al. Intermittent hemodialysis treatment in cefepime-induced neurotoxicity: case report, pharmacokinetic modeling, and review of the literature. Hemodial Int 2015;19:333–43. 10.1111/hdi.12198 [DOI] [PubMed] [Google Scholar]
- 57. Oo Y, Packham D, Yau W et al. Ertapenem-associated psychosis and encephalopathy. Intern Med J 2014;44:817–9. 10.1111/imj.12504 [DOI] [PubMed] [Google Scholar]
- 58. Schliamser SE, Broholm K-A, Liljedahl A-L et al. Comparative neurotoxicity of benzylpenicillin, imipenem/cilastatin and FCE 22101, a new injectible penem. J Antimicrob Chemother 1988;22:687–95. 10.1093/jac/22.5.687 [DOI] [PubMed] [Google Scholar]
- 59. Sunagawa M, Matsumura H, Sumita Y et al. Structural features resulting in convulsive activity of carbapenem compounds: effect of C-2 side chain. J Antibiot (Tokyo) 1995;48:408–16. 10.7164/antibiotics.48.408 [DOI] [PubMed] [Google Scholar]
- 60. Carnovale C, Pozzi M, Mazhar F et al. Interactions between antiepileptic and antibiotic drugs: a systematic review and meta-analysis with dosing implications. Clin Pharmacokinet 2019;58:875–86. 10.1007/s40262-018-0720-z [DOI] [PubMed] [Google Scholar]
- 61. Norrby SR. Neurotoxicity of carbapenem antibiotics: consequences for their use in bacterial meningitis. J Antimicrob Chemother 2000;45:5–7. 10.1093/jac/45.1.510629006 [Google Scholar]
- 62. Calandra G, Lydick E, Carrigan J et al. Factors predisposing to seizures in seriously ill infected patients receiving antibiotics: experience with imipenem/cilastatin. Am J Med 1988;84:911–8. 10.1016/0002-9343(88)90071-X [DOI] [PubMed] [Google Scholar]
- 63. Deshayes S, Coquerel A, Verdon R. Neurological adverse effects attributable to beta-lactam antibiotics: a literature review. Drug Saf 2017;40:1171–98. 10.1007/s40264-017-0578-2 [DOI] [PubMed] [Google Scholar]
- 64. Wen M-J, Sung C-C, Chau T et al. Acute prolonged neurotoxicity associated with recommended doses of ertapenem in 2 patients with advanced renal failure. Clin Nephrol 2013;80:474–8. 10.5414/CN107247 [DOI] [PubMed] [Google Scholar]
- 65. El Nekidy WS, Elrefaei H, St. John TJL et al. Ertapenem neurotoxicity in hemodialysis patients-safe and effective dosing is still needed: a retrospective study and literature review. Ann Pharmacother 2021;55:52–58. 10.1177/1060028020938059 [DOI] [PubMed] [Google Scholar]
- 66. Mistry GC, Majumdar AK, Swan S et al. Pharmacokinetics of ertapenem in patients with varying degrees of renal insufficiency and in patients on hemodialysis. J Clin Pharmacol 2006;46:1128–38. 10.1177/0091270006291839 [DOI] [PubMed] [Google Scholar]
- 67. Bösmüller C, Steurer W, Königsrainer A et al. Increased risk of central nervous system toxicity in patients treated with ciclosporin and imipenem/cilastatin. Nephron 1991;58:362–4. 10.1159/000186453 [DOI] [PubMed] [Google Scholar]
- 68. Zazgornik J, Schein W, Heimberger K et al. Potentiation of neurotoxic side effects by coadministration of imipenem to cyclosporine therapy in a kidney transplant recipient—synergism of side effects or drug interaction? Clin Nephrol 1986;26:265–6. [PubMed] [Google Scholar]
- 69. Ball P, Tillotson G. Tolerability of fluoroquinolone antibiotics. Past, present and future. Drug Saf 1995;13:343–58. 10.2165/00002018-199513060-00004 [DOI] [PubMed] [Google Scholar]
- 70. Heyd A, Haverstock D. Retrospective analysis of the safety profile of oral and intravenous ciprofloxacin in a geriatric population. Clin Ther 2000;22:1239–50. 10.1016/S0149-2918(00)83066-0 [DOI] [PubMed] [Google Scholar]
- 71. Tomé AM, Filipe A. Quinolones: review of psychiatric and neurological adverse reactions. Drug Saf 2011;34:465–88. 10.2165/11587280-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 72. Doussau De Bazignan A, Thiessard F, Miremont-Salamé G et al. , [Psychiatric adverse effects of fluoroquinolone: review of cases from the French pharmacologic surveillance database]. Rev Med Interne 2006;27:448–52. 10.1016/j.revmed.2006.02.003 [DOI] [PubMed] [Google Scholar]
- 73. Su Z, Zhang G, Shen Y et al. One case report of epilepsy and rapidly progressive cognitive impairment after levofloxacin treatment. BMC Psychiatry 2023;23:918. 10.1186/s12888-023-05425-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. De Sarro A, Zappalá M, Chimirri A et al. Quinolones potentiate cefazolin-induced seizures in DBA/2 mice. Antimicrob Agents Chemother 1993;37:1497–503. 10.1128/AAC.37.7.1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sarro A, Sarro G. Adverse reactions to fluoroquinolones. an overview on mechanistic aspects. Curr Med Chem 2001;8:371–84. 10.2174/0929867013373435 [DOI] [PubMed] [Google Scholar]
- 76. Schmuck G, SchüRmann A, SchlüTer G. Determination of the excitatory potencies of fluoroquinolones in the central nervous system by an in vitro model. Antimicrob Agents Chemother 1998;42:1831–6. 10.1128/AAC.42.7.1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang LR, Wang Y-M, Chen B-Y et al. Neurotoxicity and toxicokinetics of norfloxacin in conscious rats. Acta Pharmacol Sin 2003;24:605–9. [PubMed] [Google Scholar]
- 78. Zhang J, Huang C, Li H et al. Antibiotic-induced neurotoxicity in dialysis patients: a retrospective study. Ren Fail 2013;35:901–5. 10.3109/0886022X.2013.794684 [DOI] [PubMed] [Google Scholar]
- 79. Bagon J. Neuropsychiatric complications following quinolone overdose in renal failure. Nephrol Dialysis Transplant 1999;14:1337. 10.1093/ndt/14.5.1337a [DOI] [PubMed] [Google Scholar]
- 80. Tattevin P. Confusion and general seizures following ciprofloxacin administration. Nephrol Dialysis Transplant 1998;13:2712–3. 10.1093/ndt/13.10.2712 [DOI] [PubMed] [Google Scholar]
- 81. Idrees N, Almeqdadi M, Balakrishnan VS et al. Hemodialysis for treatment of levofloxacin-induced neurotoxicity. Hemodial Int 2019;23:E40–5. 10.1111/hdi.12687 [DOI] [PubMed] [Google Scholar]
- 82. Lizarraga KJ, Lopez MR, Singer C. Reversible craniocervical dystonia associated with levofloxacin. J Clin Mov Disord 2015;2:10. 10.1186/s40734-015-0021-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Little SC. Nervous and mental effects of the sulfonamides. J Am Med Assoc 1942;119:467–74. 10.1001/jama.1942.02830230001001 [DOI] [Google Scholar]
- 84. Lee K-Y, Huang C-H, Tang H-J et al. Acute psychosis related to use of trimethoprim/sulfamethoxazole in the treatment of HIV-infected patients with Pneumocystis jirovecii pneumonia: a multicentre, retrospective study. J Antimicrob Chemother 2012;67:2749–54. 10.1093/jac/dks283 [DOI] [PubMed] [Google Scholar]
- 85. Nau R, SöRgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev 2010;23:858–83. 10.1128/CMR.00007-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rieder MJ, Uetrecht J, Shear NH et al. Synthesis and in vitro toxicity of hydroxylamine metabolites of sulfonamides. J Pharmacol Exp Ther 1988;244:724–8. [PubMed] [Google Scholar]
- 87. Haruki H, Pedersen MG, Gorska KI et al. Tetrahydrobiopterin biosynthesis as an off-target of sulfa drugs. Science 2013;340:987–91. 10.1126/science.1232972 [DOI] [PubMed] [Google Scholar]
- 88. Walker LE, Thomas S, Mcbride C et al. , ‘Septrin psychosis’ among renal transplant patients with Pneumocystis jirovecii pneumonia. J Antimicrob Chemother 2011;66:1117–9. 10.1093/jac/dkr050 [DOI] [PubMed] [Google Scholar]
- 89. Bandettini Di Poggio M, Anfosso S, Audenino D et al. Clarithromycin-induced neurotoxicity in adults. J Clin Neurosci 2011;18:313–8. 10.1016/j.jocn.2010.08.014 [DOI] [PubMed] [Google Scholar]
- 90. Wallace RJ, Brown BA, Griffith DE. Drug intolerance to high-dose clarithromycin among elderly patients. Diagnostic Microbiology and Infectious Disease 1993;16:215–21. 10.1016/0732-8893(93)90112-K [DOI] [PubMed] [Google Scholar]
- 91. Abouesh A, Stone C, Hobbs WR. Antimicrobial-induced mania (antibiomania): a review of spontaneous reports. J Clin Psychopharmacol 2002;22:71–81. 10.1097/00004714-200202000-00012 [DOI] [PubMed] [Google Scholar]
- 92. Leblond F, Guévin C, Demers C et al. Downregulation of hepatic cytochrome P450 in chronic renal failure. J Am Soc Nephrol: JASN 2001;12:326–32. 10.1681/ASN.V122326 [DOI] [PubMed] [Google Scholar]
- 93. Steinman MA, Steinman TI. Clarithromycin-associated visual hallucinations in a patient with chronic renal failure on continuous ambulatory peritoneal dialysis. Am J Kidney Dis 1996;27:143–6. 10.1016/S0272-6386(96)90043-8 [DOI] [PubMed] [Google Scholar]
- 94. Tse K-C, Li F-K, Tang S et al. Delusion of worm infestation associated with clarithromycin in a patient on peritoneal dialysis. Perit Dial Int 2001;21:415–6. 10.1177/089686080102100416 [DOI] [PubMed] [Google Scholar]
- 95. Ma TK-W, Chow K-M, Choy ASM et al. Clinical manifestation of macrolide antibiotic toxicity in CKD and dialysis patients. Clin Kidney J 2014;7:507–12. 10.1093/ckj/sfu098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tucker S, Ahl M, Cho H-H et al. RNA therapeutics directed to the non coding regions of APP mRNA, in vivo anti-amyloid efficacy of paroxetine, erythromycin, and N-acetyl cysteine. Curr Alzheimer Res 2006;3:221–7. 10.2174/156720506777632835 [DOI] [PubMed] [Google Scholar]
- 97. Thomaidou E, Argiriadou H, Vretzakis G et al. Perioperative use of erythromycin reduces cognitive decline after coronary artery bypass grafting surgery: a pilot study. Clin Neuropharmacol 2017;40:195–200. 10.1097/WNF.0000000000000238 [DOI] [PubMed] [Google Scholar]
- 98. Fessel J. The potential for one drug, administered at the earliest preclinical stage, to prevent the subsequent decline of cognition that eventuates in dementia. Alzheimers Dement (N Y) 2020;6:e12084. 10.1002/trc2.12084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Daneman N, Cheng Y, Gomes T et al. Metronidazole-associated neurologic events: a nested case-control study. Clin Infect Dis 2021;72:2095–100. 10.1093/cid/ciaa395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Patel L, Batchala P, Almardawi R et al. Acute metronidazole-induced neurotoxicity: an update on MRI findings. Clin Radiol 2020;75:202–8. 10.1016/j.crad.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 101. Sørensen CG, Karlsson WK, Amin FM et al. Metronidazole-induced encephalopathy: a systematic review. J Neurol 2020;267:1–13. 10.1007/s00415-018-9147-6 [DOI] [PubMed] [Google Scholar]
- 102. Kuriyama A, Jackson JL, Doi A et al. Metronidazole-induced central nervous system toxicity: a systematic review. Clin Neuropharmacol 2011;34:241–7. 10.1097/WNF.0b013e3182334b35 [DOI] [PubMed] [Google Scholar]
- 103. Schreiber W, Spernal J. Metronidazole-induced psychotic disorder. Am J Psychiatry 1997;154:1170–1. 10.1176/ajp.154.8.1170b [DOI] [PubMed] [Google Scholar]
- 104. Papathanasiou A, Zouvelou V, Kyriazi S et al. Metronidazole-induced reversible encephalopathy in a patient with facioscapulohumeral muscular dystrophy. Clin Neuroradiol 2013;23:217–9. 10.1007/s00062-012-0169-7 [DOI] [PubMed] [Google Scholar]
- 105. Alston TA, Abeles RH. Enzymatic conversion of the antibiotic metronidazole to an analog of thiamine. Arch Biochem Biophys 1987;257:357–62. 10.1016/0003-9861(87)90577-7 [DOI] [PubMed] [Google Scholar]
- 106. Zuccoli G, Pipitone N, Santa Cruz D. Metronidazole-induced and Wernicke encephalopathy: two different entities sharing the same metabolic pathway? Am J Neuroradiol 2008;29 E84; author reply E85. 10.3174/ajnr.A1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Rao DN, Mason RP. Generation of nitro radical anions of some 5-nitrofurans, 2- and 5-nitroimidazoles by norepinephrine, dopamine, and serotonin. A possible mechanism for neurotoxicity caused by nitroheterocyclic drugs. J Biol Chem 1987;262:11731–6. 10.1016/S0021-9258(18)60872-5 [DOI] [PubMed] [Google Scholar]
- 108. Schentag JJ, Ziemniak JA, Greco JM et al. Mental confusion in a patient treated with metronidazole—a concentration-related effect? Pharmacotherapy 1982;2:384–7. 10.1002/j.1875-9114.1982.tb03216.x [DOI] [PubMed] [Google Scholar]
- 109. Halloran TJ. Convulsions associated with high cumulative doses of metronidazole. Drug Intell Clin Pharm 1982;16:409. 10.1177/106002808201600511 [DOI] [PubMed] [Google Scholar]
- 110. Mimura Y, Yahiro M, Masumoto M et al. The pharmacokinetics of oral metronidazole in patients with metronidazole-induced encephalopathy undergoing maintenance hemodialysis. Hemodial Int 2020;24:528–33. 10.1111/hdi.12857 [DOI] [PubMed] [Google Scholar]
- 111. Burda AM, Fischbein CB, Howe T et al. Hemodialysis clearance of metronidazole following overdose. Ann Pharmacother 2005;39:1366. 10.1345/aph.1G052 [DOI] [PubMed] [Google Scholar]
- 112. Dogra P, Bhatt A, Agarwal S et al. Short-course metronidazole-induced reversible acute neurotoxicity in a renal transplant recipient. Saudi J Kidney Dis Transplant 2018;29:1511–4. 10.4103/1319-2442.248315 [DOI] [PubMed] [Google Scholar]
- 113. Graves TD, Condon M, Loucaidou M et al. Reversible metronidazole-induced cerebellar toxicity in a multiple transplant recipient. J Neurol Sci 2009;285:238–40. 10.1016/j.jns.2009.06.011 [DOI] [PubMed] [Google Scholar]
- 114. Fletcher J, Aykroyd LE, Feucht EC et al. Early onset probable linezolid-induced encephalopathy. J Neurol 2010;257:433–5. 10.1007/s00415-009-5340-y [DOI] [PubMed] [Google Scholar]
- 115. Ferry T, Ponceau B, Simon M et al. Possibly linezolid-induced peripheral and central neurotoxicity: report of four cases. Infection 2005;33:151–4. 10.1007/s15010-005-4057-9 [DOI] [PubMed] [Google Scholar]
- 116. Serio RN. Acute delirium associated with combined diphenhydramine and linezolid use. Ann Pharmacother 2004;38:62–5. 10.1345/aph.1D018 [DOI] [PubMed] [Google Scholar]
- 117. Soriano A, Miró O, Mensa J. Mitochondrial toxicity associated with linezolid. N Engl J Med 2005;353:2305–6. 10.1056/NEJM200511243532123 [DOI] [PubMed] [Google Scholar]
- 118. Moellering RC. Linezolid: the first oxazolidinone antimicrobial. Ann Intern Med 2003;138:135–42. 10.7326/0003-4819-138-2-200301210-00015 [DOI] [PubMed] [Google Scholar]
- 119. Bischoff A, Meier C, Roth F., [Gentamicin neurotoxicity (polyneuropathy—encephalopathy)]. Schweiz Med Wochenschr 1977;107:3–8. [PubMed] [Google Scholar]
- 120. Watanabe I, Hodges GR, Dworzack DL et al. Neurotoxicity of intrathecal gentamicin: a case report and experimental study. Ann Neurol 1978;4:564–72. 10.1002/ana.410040618 [DOI] [PubMed] [Google Scholar]
- 121. Segal JA, Harris BD, Kustova Y et al. Aminoglycoside neurotoxicity involves NMDA receptor activation. Brain Res 1999;815:270–7. 10.1016/S0006-8993(98)01123-8 [DOI] [PubMed] [Google Scholar]
- 122. Falagas M, Kasiakou S. Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit Care 2006;10:R27. 10.1186/cc3995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Wadia S, Tran B. Colistin-mediated neurotoxicity. BMJ Case Rep 2014;2014:1–2. 10.1136/bcr-2014-205332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Nigam A, Kumari A, Jain R et al. Colistin neurotoxicity: revisited. BMJ Case Rep 2015;2015:1–2. 10.1136/bcr-2015-210787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kunin CM, Bugg A. Binding of polymyxin antibiotics to tissues: the major determinant of distribution and persistence in the body. J Infect Dis 1971;124:394–400. 10.1093/infdis/124.4.394 [DOI] [PubMed] [Google Scholar]
- 126. Zhou Y, Li Y, Xie X et al. Higher incidence of neurotoxicity and skin hyperpigmentation in renal transplant patients treated with polymyxin B. Br J Clin Pharmacol 2022;88:4742–50. 10.1111/bcp.15384 [DOI] [PubMed] [Google Scholar]
- 127.World Health Organization. WHO Consolidated Guidelines On Tuberculosis: Module 4: Treatment—Drug-Susceptible Tuberculosis Treatment. Geneva: WHO, 2022. [PubMed] [Google Scholar]
- 128. Romanowski K, Clark EG, Levin A et al. Tuberculosis and chronic kidney disease: an emerging global syndemic. Kidney Int 2016;90:34–40. 10.1016/j.kint.2016.01.034 [DOI] [PubMed] [Google Scholar]
- 129. Denholm J, Mcbryde E, Eisen D et al. Adverse effects of isoniazid preventative therapy for latent tuberculosis infection: a prospective cohort study. Drug Healthc Patient Saf 2014;6:145–9. 10.2147/DHPS.S68837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Anderson D, Anderson V, Pentland L et al. Attentional function in secondary school students receiving isoniazid prophylaxis for tuberculosis infection. Epidemiol Infect 2000;124:97–101. 10.1017/S0950268899003362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Constantinescu SM, Buysschaert B, Haufroid V et al. Chronic dialysis, NAT2 polymorphisms, and the risk of isoniazid-induced encephalopathy—case report and literature review. BMC Nephrol 2017;18:282. 10.1186/s12882-017-0703-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Si M, Li H, Chen Y et al. Ethambutol and isoniazid induced severe neurotoxicity in a patient undergoing continuous ambulatory peritoneal dialysis. BMJ Case Rep 2018;2018: 1–5. 10.1136/bcr-2017-223187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Siskind MS, Thienemann D, Kirlin L. Isoniazid-induced neurotoxicity in chronic dialysis patients: report of three cases and a review of the literature. Nephron 2008;64:303–6. 10.1159/000187331 [DOI] [PubMed] [Google Scholar]
- 134. Peter P, John M. Isoniazid-induced cerebellitis: a disguised presentation. Singapore Med J 2014;55:e17–9. 10.11622/smedj.2013188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Chamberlain PD, Sadaka A, Berry S et al. Ethambutol optic neuropathy. Curr Opin Ophthalmol 2017;28:545–51. 10.1097/ICU.0000000000000416 [DOI] [PubMed] [Google Scholar]
- 136. Martin SJ, Bowden FJ. Ethambutol toxicity manifesting as acute onset psychosis. Int J STD AIDS 2007;18:287–8. 10.1258/095646207780658863 [DOI] [PubMed] [Google Scholar]
- 137. Prasad R, Garg R, Verma S. Isoniazid- and ethambutol-induced psychosis. Ann Thorac Med 2008;3:149–51. 10.4103/1817-1737.43083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Hsu CW, Chu KA, Lu T et al. Ethambutol-induced psychosis: a case report. Zhonghua Yi Xue Za Zhi (Taipei) 1999;62:724–7. [PubMed] [Google Scholar]
- 139. Fang J‐T, Chen Y‐C, Chang M‐Y. Ethambutol-induced optic neuritis in patients with end stage renal disease on hemodialysis: two case reports and literature review. Ren Fail 2004;26:189–93. 10.1081/JDI-120038521 [DOI] [PubMed] [Google Scholar]
- 140. Oida Y, Kitaichi K, Nakayama H et al. Rifampicin attenuates the MPTP-induced neurotoxicity in mouse brain. Brain Res 2006;1082:196–204. 10.1016/j.brainres.2006.01.116 [DOI] [PubMed] [Google Scholar]
- 141. Tomiyama T, Asano S, Suwa Y et al. Rifampicin prevents the aggregation and neurotoxicity of amyloid beta protein in vitro. Biochem Biophys Res Commun 1994;204:76–83. 10.1006/bbrc.1994.2428 [DOI] [PubMed] [Google Scholar]
- 142. Yulug B, Hanoglu L, Ozansoy M et al. Therapeutic role of rifampicin in Alzheimer's disease. Psychiatry Clin Neurosci 2018;72:152–9. 10.1111/pcn.12637 [DOI] [PubMed] [Google Scholar]
- 143. Loeb MB, Molloy DW, Smieja M et al. A randomized, controlled trial of doxycycline and rifampin for patients with Alzheimer's disease. J Am Geriatr Soc 2004;52:381–7. 10.1111/j.1532-5415.2004.52109.x [DOI] [PubMed] [Google Scholar]
- 144. Molloy DW, Standish TI, Zhou Q et al. A multicenter, blinded, randomized, factorial controlled trial of doxycycline and rifampin for treatment of Alzheimer's disease: the DARAD trial. Int J Geriatr Psychiatry 2013;28:463–70. 10.1002/gps.3846 [DOI] [PubMed] [Google Scholar]
- 145. Bharathi KN, Natesh TS, Ashwitha Reddy A. Prenatal exposure to anti-tubercular drugs and postnatal effect on growth, development and cognitive ability in rats. Prog Neuropsychopharmacol Biol Psychiatry 2012;37:203–9. 10.1016/j.pnpbp.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 146. Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J 2017;474:1823–36. 10.1042/BCJ20160510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Rysz J, Franczyk B, Ławiński J et al. The impact of CKD on uremic toxins and gut microbiota. Toxins 2021;13:252. 10.3390/toxins13040252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Koppe L, Nyam E, Vivot K et al. Urea impairs β cell glycolysis and insulin secretion in chronic kidney disease. J Clin Invest 2016;126:3598–612. 10.1172/JCI86181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Koppe L, Fouque D, Soulage CO. The role of gut microbiota and diet on uremic retention solutes production in the context of chronic kidney disease. Toxins 2018;10:155. 10.3390/toxins10040155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Vinolo MAR, Rodrigues HG, Nachbar RT et al. Regulation of inflammation by short chain fatty acids. Nutrients 2011;3:858–76. 10.3390/nu3100858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Felizardo RJF, Castoldi A, Andrade‐Oliveira V et al. The microbiota and chronic kidney diseases: a double-edged sword. Clin Transl Immunology 2016;5:e86. 10.1038/cti.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Andrade-Oliveira V, Amano MT, Correa-Costa M et al. Gut bacteria products prevent AKI induced by ischemia-reperfusion. J Am Soc Nephrol 2015;26:1877–88. 10.1681/ASN.2014030288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Yang J, Li Q, Henning SM et al. Effects of prebiotic fiber xylooligosaccharide in adenine-induced nephropathy in mice. Mol Nutr Food Res 2018;62:e1800014. 10.1002/mnfr.201800014 [DOI] [PubMed] [Google Scholar]
- 154. Hsu C-N, Tain Y-L. Chronic kidney disease and gut microbiota: what is their connection in early life? Int J Mol Sci 2022;23: 1–17. 10.3390/ijms23073954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Ranganathan N, Friedman EA, Tam P et al. Probiotic dietary supplementation in patients with stage 3 and 4 chronic kidney disease: a 6-month pilot scale trial in Canada. Curr Med Res Opin 2009;25:1919–30. 10.1185/03007990903069249 [DOI] [PubMed] [Google Scholar]
- 156. Kikuchi M, Ueno M, Itoh Y et al. Uremic toxin-producing gut microbiota in rats with chronic kidney disease. Nephron 2017;135:51–60. 10.1159/000450619 [DOI] [PubMed] [Google Scholar]
- 157. Vaziri ND. CKD impairs barrier function and alters microbial flora of the intestine: a major link to inflammation and uremic toxicity. Curr Opin Nephrol Hypertens 2012;21:587–92. 10.1097/MNH.0b013e328358c8d5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Elvers KT, Wilson VJ, Hammond A et al. Antibiotic-induced changes in the human gut microbiota for the most commonly prescribed antibiotics in primary care in the UK: a systematic review. BMJ open 2020;10:e035677. 10.1136/bmjopen-2019-035677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Dangoor I, Stanić D, Reshef L et al. Specific changes in the mammalian gut microbiome as a biomarker for oxytocin-induced behavioral changes. Microorganisms 2021;9: 1–14. 10.3390/microorganisms9091938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Yang T, Richards EM, Pepine CJ et al. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat Rev Nephrol 2018;14:442–56. 10.1038/s41581-018-0018-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Liabeuf S, Pepin M, Franssen CFM et al. Chronic kidney disease and neurological disorders: are uraemic toxins the missing piece of the puzzle? Nephrol Dialysi, Transplant 2021;37:ii33–44. 10.1093/ndt/gfab223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Berger I, Wu S, Masson P et al. Cognition in chronic kidney disease: a systematic review and meta-analysis. BMC Med 2016;14:206. 10.1186/s12916-016-0745-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. El Chamieh C, Larabi IA, Alencar De Pinho N et al. Study of the association between serum levels of kynurenine and cardiovascular outcomes and overall mortality in chronic kidney disease. Clin Kidney J 2023;17: 1–10. 10.1093/ckj/sfad248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Leekha S, Terrell CL, Edson RS. General principles of antimicrobial therapy. Mayo Clin Proc 2011;86:156–67. 10.4065/mcp.2010.0639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Roberts DM, Sevastos J, Carland JE et al. Clinical pharmacokinetics in kidney disease: application to rational design of dosing regimens. Clin J Am Soc Nephrol 2018;13:1254–63. 10.2215/CJN.05150418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Abdul-Aziz MH, Alffenaar J-WC, Bassetti M et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a Position Paper. Intensive Care Med 2020;46:1127–53. 10.1007/s00134-020-06050-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Matusik E, Boidin C, Friggeri A et al. Therapeutic drug monitoring of antibiotic drugs in patients receiving continuous renal replacement therapy or intermittent hemodialysis: a critical review. Ther Drug Monit 2022;44:86–102. 10.1097/FTD.0000000000000941 [DOI] [PubMed] [Google Scholar]
- 168. Song T, Lee M, Jeon HS et al. Linezolid trough concentrations correlate with mitochondrial toxicity-related adverse events in the treatment of chronic extensively drug-resistant tuberculosis. EBioMedicine 2015;2:1627–33. 10.1016/j.ebiom.2015.09.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Summary of product Characteristics: Co-Trimoxazole . Date of revision: July 2021 [Internet] [cited 2023 December 04]. Available from https://www.medicines.org.uk/emc/product/4669 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.