ABSTRACT
Mycobacterium abscessus pulmonary infections are increasingly problematic, especially for immunocompromised individuals and those with underlying lung conditions. Currently, there is no reliable standardized treatment, underscoring the need for improved preclinical drug testing. We present a simplified immunosuppressed mouse model using only four injections of cyclophosphamide, which allows for sustained M. abscessus lung burden for up to 16 days. This model proved effective for antibiotic efficacy evaluation, as demonstrated with imipenem or amikacin.
KEYWORDS: M. abscessus, mouse model, lung infection
INTRODUCTION
Mycobacterium abscessus, a rapid-growing nontuberculous mycobacterium, causes lung, skin, and soft tissue infections (1). M. abscessus pulmonary infections are now a major health concern, particularly among immunodeficient patients or those with underlying lung conditions such as bronchiectasis or cystic fibrosis (2–4). M. abscessus resistance to many drugs makes eradication challenging (5–8). Due to the low cure rate of current therapies (9), there is a great interest in developing new drugs and regimens to treat M. abscessus lung infections. Unfortunately, many drugs with promising in vitro potency fail to translate to clinical efficacy (10). Hence, preclinical animal models are vital. While mouse models are common for infection research and drug testing, developing a model for M. abscessus is challenging due to its opportunistic nature. Immunocompetent mouse strains eliminate infection quickly (11–14), whereas certain genetically altered strains such as nude, NOD SCID, or GM-CSF knock-out maintain high bacterial counts (11, 14–17), but are costly. Pharmacological treatment with dexamethasone allows M. abscessus to persist in mice but requires daily injections (13). We present here a simplified model with cyclophosphamide-treated BALB/c mice, which requires only four injections and offers a cost-effective method for antibiotic testing.
Since our initial goal was to use a simple and reliable model to test antibiotic efficacy in a mouse model of M. abscessus lung infection, we first evaluated cyclophosphamide treatment as previously described (18). Seven-week-old female BALB/c mice received intraperitoneal injection of 150 mg/kg cyclophosphamide 4 days and 1 day prior to intranasal infection with 1.0 × 107 CFU of the M. abscessus reference strain CIP104536 (rough variant). Following euthanasia, lungs were harvested, homogenized, serially diluted, and plated on Middlebrook 7H11 selective agar at 37°C for CFU enumeration. Our results showed that the bacterial burden in the lungs increased from 6.7 log10 CFU/lung on day 1 to 8.7 log10 CFU/lung by day 7. However, the mice rapidly appeared moribund, necessitating early termination of the experiment. At the time of sacrifice on day 11, the lung bacterial burden had declined to day 1 levels, indicating that the decline in the mice’s health was due to cyclophosphamide-induced toxicity rather than uncontrolled bacterial proliferation. Lowering the cyclophosphamide doses to 100 mg/kg was better tolerated. However, bacterial lung burden dropped sharply by 2.4 orders of magnitude between day 1 and day 14. These results highlighted the problems with the current dosing scheme: higher drug doses could not be tolerated by the mice, while a lower dose failed to maintain a steady bacterial lung burden over time.
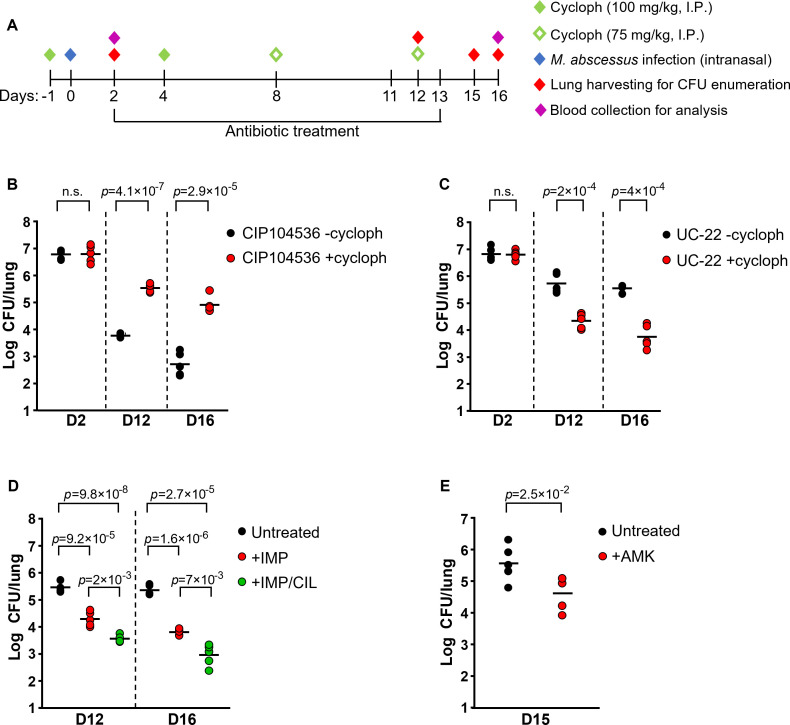
To address these limitations, we devised a new scheme in which 4 doses of pharmaceutical-grade cyclophosphamide (Endoxan®, Baxter) were administered via intraperitoneal injection: two doses of 100 mg/kg 1 day before infection and on day 4 post-infection, followed by two more doses of 75 mg/kg on day 8 and day 12 post-infection, to sustain immunosuppression (Fig. 1A). All mice were infected intranasally with 1 × 107 CFU of M. abscessus CIP104536 (R) and divided into two groups: (i) a control group that did not receive any cyclophosphamide and (ii) a group that received the four doses. Blood was collected on day 2 and day 16 post-infection for hematological analysis. Analysis revealed a decrease in immune cells on day 2 that was sustained until day 16 (Table S1). On day 2 post-infection, the bacterial burden was similar in the untreated and cyclophosphamide-treated groups (Fig. 1B). However, the bacterial burden in immunocompetent animals decreased by three orders of magnitude between day 2 and day 12, with an additional ~1.06 log10 decrease from day 12 to day 16 (Fig. 1B), which was consistent with the literature (13, 16). In the group that received cyclophosphamide, the bacterial burden decreased more modestly by ~1.26 orders of magnitude from day 2 to day 12 and remained high, ~4.9 Log10 CFU/lung, on day 16 (Fig. 1B). These results indicate that the immunosuppression protocol allows for sustained lung colonization while being well-tolerated. Histopathological analyses revealed sustained inflammatory phenotype with neutrophilic and macrophage infiltration, resembling persistent bacterial infection (Fig. S1), indicating some degrees of immunological response despite immunosuppression. Furthermore, we tested whether this model was also suitable for the study of other clinical isolates (19). The same cyclophosphamide treatment scheme (Fig. 1A) also allowed long-term lung colonization with the clinical isolate UC-22 (19) that was otherwise rapidly cleared (Fig. 1C). Together, these results indicate that the protocol is appropriate to allow maintenance of a high level of bacterial lung burden for up to 16 days.
Fig 1.
A cyclophosphamide-treated mouse model of M. abscessus infection suitable for the evaluation of drug efficacy. (A) Schematic of experimental procedure. (B) M. abscessus CIP104536 (R) lung burdens in mice with and without cyclophosphamide treatment. (C) M. abscessus UC-22 lung burdens in mice with and without cyclophosphamide treatment. (D) M. abscessus CIP104536 (R) lung burdens in immunosuppressed mice treated twice daily for 10 days with either 100 mg/kg imipenem, 100 mg/kg imipenem and cilastatin (1:1 dose ratio), or untreated. (E) M. abscessus CIP104536 (R) lung burdens in immunosuppressed mice treated daily for 12 days with 150 mg/kg amikacin (AMK) or untreated. Student t-test was performed to calculate the p-values. Cycloph: cyclophosphamide; IMP: imipenem; CIL: cilastatin; AMK: amikacin; n.s: not significant. The results were repeated at least once.
Next, we examined whether the model is suitable for the evaluation of antibiotic efficacy. We chose imipenem and amikacin as reference drugs. Imipenem was given alone at 100 mg/kg as reported before (20) or in combination with cilastatin (1:1) at 100 mg/kg to reduce the rapid metabolization of imipenem in rodents (21). Treatment was initiated 2 days post-infection, twice daily for 10 days. Imipenem alone reduced lung bacterial burden by ~90 and ~99% when given alone or in combination with cilastatin, respectively (Fig. 1D). Amikacin at 150 mg/kg once a day (20) for 12 days also caused an ~90% reduction in bacterial burden (Fig. 1E).
In summary, we describe here a model of M. abscessus pulmonary infection, which is suitable for evaluating drug efficacy. One of the limitations of the study is the use of antibiotic at doses not reflecting human drug blood levels. An in-depth analysis of the immune response also remains to be studied. Furthermore, the model may not reflect the immune response experienced in patients with bronchiectasis or cystic fibrosis. Although no single animal model can comprehensively recapitulate all aspects of M. abscessus pulmonary infection and pathology in humans, the simple model described in this study is a valuable and cost-effective addition to M. abscessus preclinical studies.
ACKNOWLEDGMENTS
This research was supported in part by the Singapore Ministry of Education under its Singapore Ministry of Education Academic Research Fund Tier 3 (Grant MOE2018-T3-1-003), by a MOE Tier 1 (Grant RT01/20), by the National Research Foundation (NRF) Singapore under its Investigatorship Program (grant NRF-NRFI06-2020-0004), and under its Campus for Research Excellence and Technological Enterprise (CREATE) programme through the SMART AMR IRG.
All our animal experimental protocols were approved by the Nanyang Technological University Animal Care and Use Committee under the protocol number 21039.
Y.S.: Data curation, Writing | K.Z.: Data curation, Writing | M.D.S.: Data curation | C.J.E.P.: Data curation | Y.Y.: Data curation | M.K.: Data curation | S.J.Shin.: Data curation, Methodology, Resources | M.B.C-P.: Data curation, Funding acquisition, Methodology, Resources | K.P.: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing – review and editing.
Contributor Information
Kevin Pethe, Email: kevin.pethe@ntu.edu.sg.
Sean Wasserman, St. George's, University of London, London, United Kingdom.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/aac.01520-23.
Tables S1 and S2; Fig. S1.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Falkinham JO. 1996. Epidemiology of infection by nontuberculous mycobacteria. Clin Microbiol Rev 9:177–215. doi: 10.1128/CMR.9.2.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Horsburgh CR, Selik RM. 1989. The epidermiology of disseminated nontuberculous mycobacterial infection in the acquired immunodeficiency syndrome (AIDS). Am Rev Respir Dis 139:4–7. doi: 10.1164/ajrccm/139.1.4 [DOI] [PubMed] [Google Scholar]
- 3. Park IK, Olivier KN. 2015. Nontuberculous mycobacteria in cystic fibrosis and non-cystic fibrosis bronchiectasis. Semin Respir Crit Care Med 36:217–224. doi: 10.1055/s-0035-1546751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schuurbiers MMF, Bruno M, Zweijpfenning SMH, Magis-Escurra C, Boeree M, Netea MG, van Ingen J, van de Veerdonk F, Hoefsloot W. 2020. Immune defects in patients with pulmonary Mycobacterium abscessus disease with cystic fibrosis. ERJ Open Res 6:00590–02020. doi: 10.1183/23120541.00590-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen J, Zhao L, Mao Y, Ye M, Guo Q, Zhang Y, Xu L, Zhang Z, Li B, Chu H. 2019. Clinical efficacy and adverse effects of antibiotics used to treat Mycobacterium abscessus pulmonary disease. Front Microbiol 10:1977. doi: 10.3389/fmicb.2019.01977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Falkinham JO. 2018. Challenges of NTM drug development. Front Microbiol 9:1613. doi: 10.3389/fmicb.2018.01613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luthra S, Rominski A, Sander P. 2018. The role of antibiotic-target-modifying and antibiotic-modifying enzymes in Mycobaterium abscessus drug resistance. Front Microbiol 9:2179. doi: 10.3389/fmicb.2018.02179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maurer FP, Bruderer VL, Ritter C, Castelberg C, Bloemberg GV, Böttger EC. 2014. Lack of antimicrobial bacterial activity in Mycobacterium abscessus. Antimicrob Agents Chemother 58:3828–3836. doi: 10.1128/AAC.02448-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. 2011. Clinicial and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis 52:565–571. doi: 10.1093/cid/ciq237 [DOI] [PubMed] [Google Scholar]
- 10. Abraham E. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:744b–7745. doi: 10.1164/ajrccm.175.7.744b [DOI] [PubMed] [Google Scholar]
- 11. Obregón-Henao A, Arnett KA, Henao-Tamayo M, Massoudi L, Creissen E, Andries K, Lenaerts AJ, Ordway DJ. 2015. Susceptibility of Mycobacterium abscessus to antimycobacterial drugs in preclinical models. Antimicrob Agents Chemother 59:6904–6912. doi: 10.1128/AAC.00459-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Byrd TF, Lyons CR. 1999. Preliminary characterization of a Mycobacterium abscessus mutant in human and murine models of infection. Infect Immun 67:4700–4707. doi: 10.1128/IAI.67.9.4700-4707.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maggioncalda EC, Story-Roller E, Mylius J, Illei P, Basaraba RJ, Lamichhane G. 2020. A mouse model of pulmonary Mycobacteroides abscessus infection. Sci Rep 10:3690. doi: 10.1038/s41598-020-60452-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ordway DJ, Henao-Tamayo M, Smith E, Shanley C, Harton M, Troudt J, Bai X, Basaraba RJ, Orme IM, Chan ED. 2008. Animal model of Mycobacterium abscessus lung infection. J Leukoc Biol 83:1502–1511. doi: 10.1189/jlb.1007696 [DOI] [PubMed] [Google Scholar]
- 15. De Groote MA, Johnson L, Podell B, Brooks E, Basaraba R, Gonzalez-Juarrero M. 2014. GM-CSF knockout mice for preclinical testing of agents with antimicrobial activity against Mycobacterium abscessus. J Antimicrob Chemother 69:1057–1064. doi: 10.1093/jac/dkt451 [DOI] [PubMed] [Google Scholar]
- 16. Lerat I, Cambau E, Roth dit Bettoni R, Gaillard J-L, Jarlier V, Truffot C, Veziris N. 2014. In vivo evaluation of antibiotic activity against Mycobacterium abscessus. J Infect Dis 209:905–912. doi: 10.1093/infdis/jit614 [DOI] [PubMed] [Google Scholar]
- 17. Nicola F, Cirillo DM, Lorè NI. 2023. Preclinial murine models to study lung infection with Mycobacterium abscessus complex. Tuberculosis 138:102301. doi: 10.1016/j.tube.2022.102301 [DOI] [PubMed] [Google Scholar]
- 18. Zhang S, Zou Y, Guo Q, Chen J, Xu L, Wan X, Zhang Z, Li B, Chu H. 2020. AR-12 exhibits direct and host-targeted antibacterial activity toward Mycobacterium abscessus. Antimicrob Agents Chemother 64:e00236–20. doi: 10.1128/AAC.00236-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sohn H, Kim H-J, Kim JM, Jung Kwon O, Koh W-J, Shin SJ. 2009. High virulent clinical isolates of Mycobacterium abscessus from patients with the upper lobe fibrocavitary form of pulmonary disease. Microbial Pathogenesis 47:321–328. doi: 10.1016/j.micpath.2009.09.010 [DOI] [PubMed] [Google Scholar]
- 20. Le Moigne V, Raynaud C, Moreau F, Dupont C, Nigou J, Neyrolles O, Kremer L, Hermann J. 2020. Efficacy of bedaquline, alone or in combination with imipenem, against Mycobacterium abscessus in C3HeB/FeJ mice. Antimicrob Agents Chemother 64:e00114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Story-Roller E, Maggioncalda EC, Lamichhane G. 2019. Synergistic efficacy of β-Lactam combinations against Mycobacterium abscessus pulmonary infection in mice. Antimicrob Agents Chemother 63:e00614–19. doi: 10.1128/AAC.00614-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 and S2; Fig. S1.