Summary
Background
The increased demand for induction of labour (IOL) at 39 weeks’ gestation in normal-risk nulliparous patients creates significant logistical challenges for busy maternity units. A potential innovation is commencing induction by means of outpatient cervical ripening, using either a vaginal prostaglandin preparation (Propess) or an osmotic cervical dilator (Dilapan-S).
Methods
A Phase III, open label, single centre non-inferiority trial (EudraCT number 2019-004697-25) randomised healthy nulliparous women who chose elective IOL at 39 weeks to one of three methods of initial cervical ripening, specifically 12 h of Dilapan-S(D12), 24 h of Dilapan-S(D24), or 24 h of Propess(P24) between November 2020 and July 2023. After initial administration of the IOL agent in the hospital, participants returned home for 12 or 24 h, before readmission to complete delivery. The primary outcome was vaginal delivery achieved at any time, and this was compared in a non-inferiority analysis of Dilapan-S compared to Propess, within a 10% non-inferiority margin. Secondary outcomes included pairwise comparisons for each induction agent, and a range of logistical factors, such as time to delivery, the need for an additional cervical ripening agent, and length of hospital stay.
Findings
Of the 327 women randomised at 38 weeks, 271 (83%) completed the induction intervention. The D24 and P24 groups showed similarly high rates of vaginal delivery, 75% and 76% respectively. D12 had a lower vaginal delivery rate of 64% and consequently the overall comparison of Dilapan-S to Propess did not demonstrate non-inferiority (difference = −6%, 95% CI = −17%, 5%) because the lower 95% CI exceeded the −10% threshold of non-inferiority. The majority of participants across all groups were delivered by any means within 72 h of starting the induction process, inclusive of time spent at home (89% of the D24 group, 98% of the D12 group, 95% of the P24 group). There were no differences in rates of adverse events between groups.
Interpretation
There were similarly high vaginal delivery rates for D24 and P24, with at least 75% of patients successfully delivering vaginally following outpatient cervical ripening, with no significant adverse maternal or neonatal outcomes.
Funding
The Rotunda Foundation, Medicem Technology s.r.o.
Keywords: Home induction, Outpatient induction, Induction of labour, Elective induction, Cervical ripening
Research in context.
Evidence before this study
A review of studies that examined elective induction of labour (“elective induction of labour” OR “risk-reducing induction of labour”) between database inception and May 2023, was performed using PubMed. Few studies defined a protocol for inducing labour in the outpatient context, therefore bearing this in mind, we searched PubMed for studies addressing outpatient cervical ripening (“outpatient induction of labour” OR “outpatient cervical ripening). The most recent Cochrane review suggested that there was insufficient evidence to confirm the safety of outpatient induction of labour, and called for further, woman-friendly, outpatient studies to be completed.
Added value of this study
This is among the first randomised trials to compare methods of cervical ripening solely in the outpatient setting of 39-week elective induction of labour for normal-risk nulliparous women. Though non-inferiority was not demonstrated, our study provides evidence for the efficacy and safety of three cervical ripening methods for normal-risk nulliparous women in the outpatient setting.
Implications of all the available evidence
Use of either cervical ripening method enables appropriately selected patients to stay home for a large part of the IOL process. These findings should be explored in further randomised trials with expansion to other cohorts (e.g. multiparous women), paving the way for woman-centred protocols for induction of labour.
Introduction
Since 2018, there has been an increase in the demand for induction of labour (IOL).1, 2, 3 Much of this increase has been attributed to the impact of the US-conducted ARRIVE trial which validated the efficacy and safety of routine 39-week IOL in otherwise healthy nulliparous mothers.4 International professional bodies, such as SMFM and ACOG, have now confirmed that it is reasonable to offer such an intervention to normal-risk nulliparous women.5,6 Whilst many studies have examined the potential benefits and risks of elective IOL (eIOL) at 39 weeks’ gestation in otherwise healthy nulliparous women,4,7, 8, 9 few have assessed potential methods of managing the logistics of this increasing demand, given the practical capacity constraints of most maternity units.
Outpatient IOL is a potential option for normal-risk patients,10 and has been found to be effective and favourable amongst women,11, 12, 13 although systematic reviews have raised the need for further studies, including assessment of effective methods of cervical ripening in the outpatient setting.14, 15, 16
Slow-release prostaglandin vaginal inserts (Propess - 10 mg dinoprostone vaginal delivery system) for cervical ripening have created the option for outpatient IOL compared to traditional use of prostaglandin gel, with the latter requiring 6-hourly assessments as an inpatient. In contrast, Propess does not require reassessment for up to 24 h following insertion. Propess has the added benefit of being easily removable with a short half-life,17 meaning it can be removed by staff or even the patient in the setting of hyperstimulation or non-reassuring fetal testing.
Mechanical (non-pharmacological) methods for cervical ripening have also been assessed in the setting of both inpatient and outpatient IOL.18, 19, 20 Most studies of outpatient IOL have examined the role of mechanical methods, although a small number have assessed prostaglandin-based outpatient IOL.19, 20, 21 Non-pharmacological methods may be associated with reduced risk of uterine hyperstimulation compared with prostaglandin-based cervical ripening methods.22 Options for mechanical IOL include placing a synthetic osmotic dilator (Dilapan-S) or a balloon catheter into the cervix, with both performing similarly, although not having to maintain the osmotic dilator under tension (while a balloon catheter tube must be taped under tension against the patient's leg) has been quoted as a benefit and as possibly more acceptable to the patient.18
To our knowledge, there has not been, as of yet, a randomised trial conducted comparing methods of elective IOL at 39 weeks solely in the outpatient setting in normal-risk nulliparous women. Here, we present the results from the HOME INDUCTION randomised clinical trial: Outpatient induction of labour at 39 weeks with Dilapan-S versus Propess. Our aim was to assess methods of cervical ripening agent in the outpatient setting, focusing on efficacy, safety and logistical consideration.
Prior research has suggested that a Dilapan-S insertion time of up to 12 h might be associated with better outcomes when compared to insertion time of up to 24 h.23 The former involves patients returning to our hospital in the evening time, the latter involves patients returning the following day. In the context of equivalent outcomes, the choice of either 12 or 24 h of Dilapan-S could therefore be based on patient or provider preference.
Methods
Study objectives
The primary objective was to determine whether Dilapan-S, either for 12 h of insertion (D12) or 24 h of insertion (D24), is non-inferior to Propess for 24 h of insertion (P24) in the rate of vaginal delivery. The secondary objectives were to assess safety and maternal satisfaction between Dilapan-S, either for 12 h of insertion (D12) or 24 h of insertion (D24), and Propess for 24 h of insertion (P24).
Study design
We conducted a randomised, single centre, open label trial at Ireland's largest tertiary maternity hospital, with approximately 9000 deliveries annually.24 The trial protocol25 was reviewed and approved by the National Research Ethics Committee and previously published (EudraCT number 2019-004697-25). This trial was sponsored by the Royal College of Surgeons in Ireland (RCSI) and regulated by the Irish Health Products Regulatory Authority (HPRA).
Ethics
The trial protocol and patient information leaflet were reviewed and approved by the Irish National Research Ethics Committee (National Maternity Hospital EC08.2020). All patients signed a written informed consent form prior to enrolment.
Participants
The study population included normal-risk nulliparous women between the ages of 18 and 39 years, with a BMI ≥18 kg/m2 and <35 kg/m2 in a singleton pregnancy, with no contraindications to induction of labour and who lived within 10miles (or 30 min) of the hospital. All participants had an ultrasound examination to confirm presentation, normal amniotic fluid volume and normal biophysical score. All participants were required to have an unfavourable cervix, with a Bishop score <6, upon commencement of IOL between 39 + 0 and 39 + 4 weeks’ gestation. All participants signed a consent form.
Exclusion criteria included the presence of, or development of any medical complications before IOL, any known fetal anomaly, women who did not have immediate access to transport to the hospital, and any woman whose labour had already started, or whose amniotic membranes had already ruptured. Furthermore, any woman who had an active genital tract infection, or who had a known allergy to any methods used, were excluded.
Randomisation and masking
Trial participants were randomised using simple random allocation to either Dilapan-S for 12 h (D12), Dilapan-S for 24 h (D24), or Propess for 24 h (P24) in a 1:1:1 ratio using a block size of six via a computer-generated randomisation procedure (software SAS Version 6.4). Randomisation was performed by a dedicated clinical trials pharmacy team, who subsequently prepared the assigned cervical ripening device to be available for the morning of induction. This was an open label trial, as it was not possible to conceal the induction agent to staff or patients.
Procedures
Eligible women were approached from 36 weeks' gestation, and offered the option to participate in a trial of elective IOL at 39 weeks' gestation. Those who were interested received the participant information leaflet and a follow-up contact email if they wished to proceed. They were assessed between 38 and 39 weeks' gestation at a dedicated prenatal clinic, where a detailed history, physical assessment, and ultrasound examination were performed to confirm eligibility. After discussing the IOL process, a written consent form was signed, and women were randomised to one of three groups: 12 h of Dilapan-S (an osmotic hygroscopic cervical dilator, with one to five rods placed intracervically), 24 h of Dilapan-S, or 24 h of Propess (Dinoprostone 10 mg intravaginal device). Women were informed of their randomisation result before IOL began. A date was scheduled for IOL between 39 + 0 and 39 + 4 weeks. Women attended the maternity hospital on their assigned day to begin the IOL process. Once a 30-min cardiotocograph tracing was deemed reassuring, either Dilapan-S or Propess were inserted as per randomisation. Dilapan-S was inserted into the cervical canal as per manufacturers' instructions, with between 1 and 5 rods being placed for each participant as deemed appropriate by the clinician performing the induction. Propess (10 mg vaginal delivery system) was inserted as per manufacturers' instructions. A 30–60 min cardiotocograph was performed immediately after insertion for all patients, before being discharged home. Women were instructed to return as planned in 12 h (after D12) or 24 h (after D24 or P24), or sooner if they had concerns over their own or their baby's wellbeing, if regular painful uterine contractions developed, or if vaginal bleeding or leaking of amniotic fluid (spontaneous rupture of membranes) developed. All participants received a safety checklist which they filled out regularly at home to monitor for each of these concerns. Participants received regular reassurance telephone calls from trial staff after commencing IOL to assess for concerns. Upon readmission after the 12 or 24 h period at home (or sooner based on patient concern), a cardiotocograph tracing, and clinical assessment were completed in order to decide further clinical management. Following repeat vaginal examination, patients were either deemed suitable for immediate amniotomy or requiring further cervical ripening. If further cervical ripening was required, patients received 1 mg prostaglandin gel (Prostin) in an inpatient setting, and were reassessed in approximately 6 h. Up to three 1 mg doses of prostaglandin gel were used as required. When deemed suitable, an amniotomy was then performed on the prenatal ward and, subsequently, the patient was transferred to the labour ward for oxytocin infusion.
Outcomes
Efficacy, safety and maternal satisfaction outcomes were compared between the two modalities of induction, Dilapan-S (D12 plus D24) versus Propess (P24), and between the individual study treatments (D24 vs P24, D12 vs P24 and D24 vs D12).
The primary objective was to compare the vaginal delivery rate using a non-inferiority analysis of Dilapan-S to Propess with a 10% non-inferiority margin. Pairwise comparisons of the individual treatments were also assessed for non-inferiority.
The primary outcome measure was vaginal delivery by any means or, equivalently, failure to achieve vaginal delivery. Secondary outcomes included change in Bishop score after cervical ripening, need for second induction modality, adverse maternal and neonatal outcomes including hyper-stimulation, and maternal satisfaction evaluation.
Initially, the study planned to examine time-dependent rates of vaginal delivery as the primary outcome. However, in a protocol amendment, the primary outcome was modified to remove a time-limit on achieving vaginal delivery, following (1) logistical concerns with timing, similar to the SOLVE trial26 (2) statistical concerns given that our interventions represent different hospital readmission times and (3) to ensure consistency with other relevant studies.18,27 Vaginal delivery within various time-limits was therefore considered less meaningful clinically, although these analyses were still completed as secondary outcome measures.
Statistics
A non-inferiority margin of 10% in vaginal delivery rates was considered to be clinically meaningful, consistent with other trials comparing induction methods in the inpatient setting.18,26,28 Power calculations were based on assumed vaginal delivery rates of 70% in the P24 group, 75% in the D24 group, and 80% in the D12 group, derived from results of previous research. Assuming 90% statistical power, a (one-sided) 2.5% Type I error rate, and equal treatment group allocation, the total study sample size required was 285 for a per-protocol analysis. Allowing for a 15% non-adherence rate, the total sample required for recruitment was 327 (109 per group) for an intention-to-treat (ITT) analysis. The study was sufficiently powered for non-inferiority determination of the pairwise treatment comparisons conditional on a minimum assumed difference of 8% (rather than 5%) between individual treatments. Given that this was a non-inferiority trial, the per-protocol analysis was considered the primary population for analysis, although an ITT analysis was also completed. Median [inter-quartile range] or mean (standard deviation), in the absence of skewness, were used to summarize outcome data. SAS Version 9.2 was used to analyse the data. The safety population (all patients with successful insertion of the investigative medicinal product, as received) was used to evaluate potential adverse reactions to the cervical ripening agents used.
95% confidence intervals were used to describe uncertainty in treatment differences for the primary outcome and to determine non-inferiority (lower confidence limit > −10%). A non-inferiority p-value, using a Wald test, was calculated to aid interpretation. Multiple comparison adjustments were not made for the pairwise treatment comparisons, as this would represent penalisation of efficiencies associated with a multi-arm trial. A non-inferiority result of Dilapan-S (all) to Propess in the presence of a potentially inferior Dilapan-S group could only occur if the vaginal delivery rates differed by more than 24% between the two Dilapan-S groups. Exploratory analyses were performed to (1) determine if BMI is an independent predictor of vaginal delivery (visualised with the cumulative incidence function) and (2) do a sensitivity analysis of the non-inferiority assessment by inclusion of BMI as a covariate (logistic regression).
An independent monitor visited the clinical research site intermittently to validate compliance with the protocol according to good clinical practice, maintenance of study related records, and accuracy of electronic case report form (eCRF) entries compared to source data. The study's clinical trial governance was managed by a Trial Steering Committee, Trial Management Group, and Data and Safety Monitoring Board (DSMB). An independent DSMB was assigned to provide impartial and objective evaluation of clinical trial safety data and to promote greater objectivity regarding clinical risk-benefit assessment.
The trial was registered with EudraCT Number 2019-004697-25, on September 14, 2020.
Role of the funding sources
The Rotunda Foundation, a registered charitable organisation (CRA 20079529) associated with The Rotunda Hospital, awarded initial study start-up funding (Grant reference: RF/RCSI/2020/04). An unrestricted educational grant was provided from Medicem Technology s.r.o., the manufacturer of Dilapan-S (Grant reference: Medicemtech). Neither funder had a role in trial design, data collection, data analysis, statistical analysis, report writing, or the decision to submit the manuscript for publication to this Journal.
Results
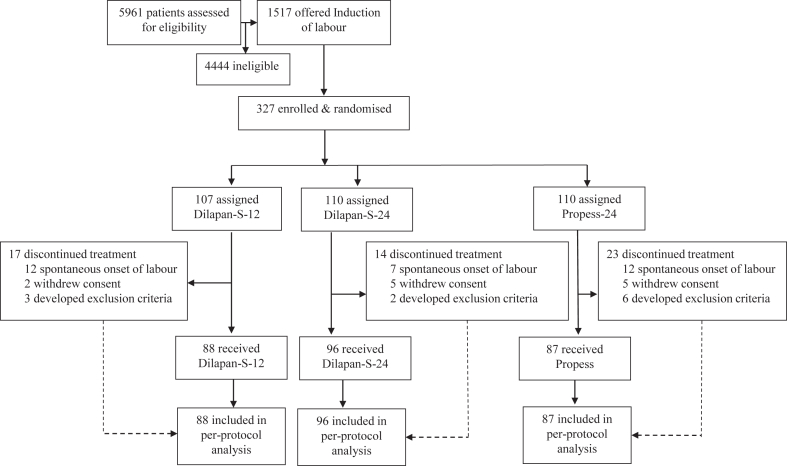
Recruitment took place between November 23, 2020 and August 6, 2023, with the original target of 327 women being successfully recruited and randomised at 38 weeks’ gestation. 110 women were randomised to Dilapan-S for 24 h (D24), 107 women were randomised to Dilapan-S for 12 h (D12), and 110 women were randomised to Propess for 24 h (P24). During the first year of recruitment, the Irish Health Service Executive (HSE) computer system was subject to a cyber-attack which impacted access to electronic health records for a duration of 2 months. A pre-prepared emergency randomisation list was used during this time for 11 trial participants, resulting in slightly different numbers randomised than allowed by a block size of six.
It is inevitable that pregnant patients approaching term might spontaneously progress to labour and delivery prior to a scheduled obstetric intervention. Following randomisation, 9% (31/327) of women were delivered after randomisation but before the time of their scheduled induction date, 4% (12/327) of participants withdrew consent after randomisation, while 3% (11/327) of participants subsequently developed an exclusion criterion which was not present at initial randomisation (Fig. 1). Examples of exclusions recorded prior to induction included development of regular contractions, hypertensive disorders, Bishop score ≥6, oligohydramnios, and COVID-19 infection. All patients received treatments consistent with the randomisation schedule and all remaining patients had successful insertion of the investigational medicinal product, thereby completing the study protocol successfully. The safety population and per-protocol population were therefore identical (N = 271).
Fig. 1.
Patient flow-chart.
Amongst randomised patients (ITT population), the mean (SD) maternal age was 27.3 (5.1) years and body mass index (BMI) was 25.5 (3.9) kg/m2 (Table 1). The most common medical history was a COVID-19 diagnosis (17%), followed by asthma (4%), hypothyroidism (4%), and depression/anxiety (3%). The per-protocol population showed a similar overall distribution in baseline characteristics to the ITT population, and these were comparable between treatment groups, with no statistically significant differences noted.
Table 1.
Baseline characteristics.
| Characteristic | ITT |
Per-Protocol Population |
|||
|---|---|---|---|---|---|
| All (N = 327) | All (N = 271) | D24 Dilapan-S 24 h (N = 96) | D12 Dilapan-S 12 h (N = 88) | P24Propess (N = 87) | |
| Maternal age (years) | |||||
| 18–24 | 116 (35%) | 98 (36%) | 39 (41%) | 32 (36%) | 27 (31%) |
| 25–30 | 94 (29%) | 76 (28%) | 24 (25%) | 22 (25%) | 30 (34%) |
| 30–39 | 117 (36%) | 97 (36%) | 33 (34%) | 34 (39%) | 30 (34%) |
| Mean (SD) | 27.3 (5.1) | 27.2 (5.1) | 27.1 (5.2) | 27.4 (5.3) | 27.3 (4.8) |
| Min-Max | 18–39 | 18–39 | 18–38 | 18–39 | 19–38 |
| Maternal height (m)a | |||||
| Mean (SD) | 166 (6.2) | 166 (6.1) | 166 (5.5) | 166 (6.2) | 167 (6.6) |
| Maternal weight (kg) | |||||
| Mean (SD) | 70.4 (12) | 71.2 (12) | 71.8 (13) | 71.0 (12) | 70.9 (13) |
| BMI (kg/m2)a | |||||
| 18.0–24.9 | 162 (50%) | 130 (48%) | 45 (47%) | 43 (49%) | 42 (48%) |
| 25.0–29.9 | 115 (35%) | 96 (35%) | 35 (36%) | 30 (34%) | 31 (36%) |
| 30.0–34.9 | 49 (15%) | 45 (17%) | 16 (17%) | 15 (17%) | 14 (16%) |
| Mean (SD) | 25.5 (3.9) | 25.7 (4.0) | 25.9 (4.1) | 25.7 (3.8) | 25.5 (4.0) |
| Min-Max | 18.2–34.9 | 18.4–34.9 | 18.4–34.7 | 19.3–34.9 | 18.5–34.7 |
| Medical history | |||||
| Anaemia | 1 (<1%) | 1 (<1%) | 1 (1%) | 0 | 0 |
| Anxiety | 5 (2%) | 4 (1%) | 1 (1%) | 2 (2%) | 1 (1%) |
| Asthma | 12 (4%) | 10 (4%) | 5 (5%) | 3 (3%) | 2 (2%) |
| Depression | 5 (2%) | 5 (2%) | 3 (3%) | 1 (1%) | 1 (1%) |
| Hyperthyroidism | 1 (<1%) | 1 (<1%) | 0 | 0 | 1 (1%) |
| Hypothyroidism | 12 (4%) | 12 (4%) | 4 (4%) | 4 (5%) | 4 (5%) |
| Uterine fibroids | 2 (1%) | 2 (1%) | 1 (1%) | 0 | 1 (1%) |
| Recurrent UTI | 3 (1%) | 3 (1%) | 1 (1%) | 2 (2%) | 0 |
| COVID-19 | 49 (15%) | 46 (17%) | 14 (15%) | 17 (19%) | 15 (17%) |
| Other | 17 (5%) | 15 (6%) | 8 (8%) | 6 (7%) | 1 (1%) |
| Gestational age at screening (weeks) | |||||
| Mean (SD) | 38.5 (0.5) | 38.5 (0.5) | 38.6 (0.5) | 38.5 (0.4) | 38.5 (0.5) |
| Gestational age at induction (weeks) | |||||
| Mean (SD) | 39.2 (0.2) | 39.3 (0.2) | 39.2 (0.2) | 39.2 (0.2) | |
| Bishop Scoreb | |||||
| Median [IQR] | 1 [0,2] | 1 [0,2] | 1 [1,2] | 1 [0,2] | |
ITT, Intention-to-treat population; UTI, urinary tract infection; IQR, inter-quartile range.
Maternal height, and subsequently, BMI was not recorded for one patient in the D12 group (ITT population but not in the per-protocol population).
One patient in the D24 group had Bishop score recorded as “<6” at baseline and excluded from the statistical summaries.
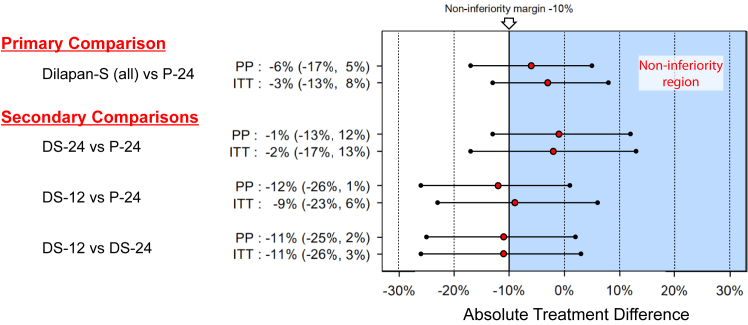
The primary outcome of vaginal delivery by any means occurred in 75% of the D24 group, 64% of the D12 group, 70% of the Dilapan-S (all) groups, and 76% of the P24 group (Table 2). The criteria for non-inferiority of Dilapan-S (all) to Propess were not met (difference = −6%; 95% CI = −17%,5%; non-inferiority p-value = 0.26) in the per-protocol population. The results were the same when re-analysed for the ITT population (difference = −3%; 95% CI = −13%, 8%; non-inferiority p-value = 0.08) (Fig. 2).
Table 2.
Maternal and neonatal outcomes (per protocol population).
| D24 Dilapan-S 24 h (N = 96) | D12 Dilapan-S 12 h (N = 88) | Dilapan-S All (N = 184) | P24 Propess (N = 87) | |
|---|---|---|---|---|
| Primary outcome | ||||
| Vaginal delivery | ||||
| Yes | 72 (75%) | 56 (64%) | 128 (70%) | 66 (76%) |
| No | 24 (25%) | 32 (36%) | 56 (30%) | 21 (24%) |
| Secondary outcomes (maternal) | ||||
| Gestational age at delivery (weeks) | 40 [39,40] | 39 [39,40] | 39 [39,40] | 39 [39,40] |
| Induction commencement to delivery time (hours) | 47 [42,58] | 35 [31,46] | 43 [33,52] | 42 [29,52] |
| Time from removal of induction agent to delivery time (hours)e | 22 [18,32] | 20 [15,29] | 21 [16,31] | 20 [10,29] |
| Delivery within 48 h of starting IOL | 53 (55%) | 72 (82%) | 125 (68%) | 55 (63%) |
| Delivery within 72 h of starting IOL | 85 (89%) | 86 (98%) | 171 (93%) | 83 (95%) |
| Vaginal delivery timing | ||||
| Within 36 h | 10 (10%) | 33 (38%) | 43 (23%) | 27 (31%) |
| Within 48 h | 42 (44%) | 47 (53%) | 89 (48%) | 48 (55%) |
| Within 72 h | 65 (68%) | 55 (63%) | 120 (65%) | 65 (75%) |
| Mode of Delivery | ||||
| SVD | 40 (42%) | 33 (38%) | 73 (40%) | 38 (44%) |
| OVD | 32 (33%) | 23 (26%) | 55 (30%) | 28 (32%) |
| Caesarean delivery | 24 (25%) | 32 (36%) | 56 (30%) | 21 (24%) |
| Indication for emergency intrapartum CSa | ||||
| Failure to progress | 7 (32%) | 12 (38%) | 19 (10%) | 10 (53%) |
| Abnormal CTG | 10 (45%) | 12 (38%) | 22 (12%) | 8 (42%) |
| Failed IOL | 4 (18%) | 7 (22%) | 11 (6%) | 1 (5%) |
| Maternal request | 1 (5%) | 1 (3%) | 2 (1%) | 0 |
| Indication for emergency pre-labour CSa | ||||
| Abnormal CTG | 2 (100%) | 0 | 2 (1%) | 1 (50%) |
| Maternal request | 0 | 0 | 0 | 1 (50%) |
| Shoulder dystocia | 1 (1%) | 1 (1%) | 2 (1%) | 1 (1%) |
| Obstetric anal sphincter injury | 3 (3%) | 1 (1%) | 4 (2%) | 4 (5%) |
| Postpartum haemorrhage >1,000 mls | 4 (4%) | 6 (7%) | 10 (5%) | 7 (8%) |
| Hyperstimulation | 0 | 0 | 0 | 3 (3%) |
| Epidural use | 82 (85%) | 79 (90%) | 161 (88%) | 74 (85%) |
| Hospital stay: Return-visitb to discharge (hours) | 79 [59,104] | 88 [67,111] | 83 [64,105] | 76 [56,98] |
| Secondary outcomes (neonatal) | ||||
| Liveborn | ||||
| Yes | 96 (100%) | 88 (100%) | 184 (100%) | 87 (100%) |
| No | 0 | 0 | 0 | 0 |
| Birthweight (g) | 3507 (353) | 3408 (392) | 3460 (374) | 3473 (307) |
| Apgar <7 at 5 min | 1 (1%) | 2 (2%) | 3 (2%) | 0 |
| NICU admission | 7 (7%) | 4 (5%) | 11 (6%) | 3 (3%) |
| Cord pH (arterial)c | 7.2 (0.1) | 7.2 (0.1) | 7.2 (0.1) | 7.2 (0.1) |
| Cord pH (venous)d | 7.1 (0.8) | 7.3 (0.1) | 7.2 (0.6) | 7.3 (0.1) |
| Antibiotics for suspected infection | 10 (10%) | 6 (7%) | 16 (9%) | 7 (8%) |
| Antibiotics for confirmed infection | 1 (1%) | 0 | 1 (<1%) | 0 |
Results presented are: n (%), mean (SD) and median [IQR].
SVD = spontaneous vaginal delivery; OVD = operative vaginal delivery; CTG = cardiotocograph; NICU = neonatal intensive care unit.
Percentages are based on those with emergency intrapartum or pre-labour CS.
Denotes time in hours from when the patient was re-admitted to hospital after their return with Propess or Dilapan-S in situ, until time of discharge after delivery.
Cord pH (arterial) was recorded in 45, 41 and 44 patients in the D24, D12 and P24 groups, respectively.
Cord pH (venous) was recorded in 48, 42 and 43 patients in the D24, D12 and P24 groups, respectively.
Removal time was missing for 1 patient in the D12 group and 1 patient in the P24 group.
Fig. 2.
Treatment comparisons and non-inferiority assessment for vaginal delivery rate. PP, Per-Protocol Population; ITT, Intention-to-Treat Population.
Pairwise comparisons of the treatment groups showed that, although the vaginal delivery rates for D24 and P24 were very similar, there is some uncertainty in the estimated time difference between the various groups, with wide confidence intervals (95% CI = −13%, 12%; per-protocol analysis). The comparisons of D12 vs D24 (95% CI = −25%, 2%) and D12 vs P24 (95% CI = −26%, 1%) suggest that D12 has a lower vaginal delivery rate than the other groups but the degree to which it may underperform is uncertain (Fig. 2).
The majority of participants across all groups were delivered by any means within 72 h of starting the induction process, inclusive of time spent at home (89% of the D24 group, 98% of the D12 group, 95% of the P24 group) (Table 2).
Adverse maternal and neonatal outcomes were low across all groups (Table 2). Recorded adverse and serious adverse events are summarised in Table S1. The maternal secondary outcomes of shoulder dystocia, obstetric anal sphincter injury (OASI), postpartum haemorrhage (PPH), and neonatal secondary outcomes (Apgar <7 at 5 min, neonatal intensive care unit (NICU) admission, low cord pH or antibiotic use) were similar across the treatment groups. Shoulder dystocia occurred in 3 participants (1%) overall: 1 in the D24 group, 1 in the D12 group and 1 in the P24 group. OASI occurred in 8 participants (3%) overall: 3 in the D24 group, 1 in the D12 group and 4 in the P24 group. PPH occurred in 17 (6%) participants. 235 (87%) participants received an epidural for labour analgesia. Uterine hyperstimulation (defined as more than 7 contractions in a 15 min time period persistently for >30 min and requiring a medical intervention, such as a clinical decision to remove Propess/Dilapan-S or to administer a medication such as terbutaline) was observed in 3 cases, all of which occurred in the P24 group (3%). In each case, the Propess vaginal insert was immediately removed and there were no adverse consequences related to hyperstimulation.
In terms of neonatal outcomes, all infants were liveborn. 14 infants (5%) required NICU admission, with antibiotic administration for suspected infection occurring in 23 infants (8%) overall. Of these cases, one infant in the D24 group had a confirmed infection based on positive blood cultures. A total of 3 infants (1%) had an Apgar of <7 at 5 min.
Tables 2 and 3 describe the logistics associated with the various outpatient IOL protocols, with comparisons described including early return to hospital, requirement for additional prostaglandin for cervical ripening, time to delivery, and overall length of hospital stay. Those in the P24 group were significantly more likely to require early removal of the device (44%) than D24 (13%), or D12 (6%) (p < 0.001). Those in the P24 group were significantly less likely to need an additional administration of prostaglandin gel (34%), than the D12 (58%) or D24 (71%) groups (p < 0.001). There was no significant difference between groups in the requirement for oxytocin (77% in D24, 76% in D12 and 70% in P24).
Table 3.
Return to hospital visit, second induction modality and delivery (Per Protocol Population).
| Outcomes | D24 Dilapan-S 24 hr (N = 96) | D12 Dilapan-S 12 hr (N = 88) | Dilapan-S All (N = 184) | P24 Propess (N = 87) |
|---|---|---|---|---|
| Bishop score | ||||
| Return visitb | 3 [2,4] | 3 [2,5] | 3 [2,5] | 4 [2,5] |
| Change from baselineb,c | 2 [1,3] | 2 [1,3] | 2 [1,3] | 2 [1,4] |
| Earlier induction agent removala | 12 (13%) | 5 (6%) | 17 (9%) | 39 (45%) |
| Additional prostaglandin gel needed | 68 (71%) | 51 (58%) | 119 (65%) | 30 (34%) |
| Oxytocin use | 74 (77%) | 67 (76%) | 141 (77%) | 61 (70%) |
| Additional Prostaglandin subgroupd | N = 68 | N = 51 | N = 119 | N = 30 |
| Vaginal delivery rate | 48 (71%) | 28 (55%) | 76 (64%) | 16 (53%) |
| Induction to delivery time (hrs) | 49 [44,59] | 39 [33,48] | 45 [38,56] | 52 [48,59] |
| Bishop scores | ||||
| Visit 2 (Start of IOL) | 1 [0,2] | 1 [0,2] | 1 [0,2] | 1 [0,2] |
| Visit 3 (Re-assessment visit) | 2 [2,3] | 2 [1,3] | 2 [1,3] | 2 [2,3] |
| Change from Visit 2 to Visit 3 | 1 [1,2] | 1 [0,2] | 1 [0,2] | 1 [0,2] |
| No Additional Prostaglandin subgroup | N = 28 | N = 37 | N = 65 | N = 57 |
| Artificial rupture membranes performed | 25 (89%) | 34 (92%) | 59 (91%) | 33 (58%) |
| Vaginal delivery rate | 24 (86%) | 28 (76%) | 52 (80%) | 50 (88%) |
| Induction to delivery time (hrs) | 43 [37,52] | 30 [26,35] | 34 [28,47] | 34 [23,43] |
| Bishop score | ||||
| Visit 2 (Start of IOL) | 2 [1,2] | 2 [1,3] | 2 [1,3] | 1 [1,3] |
| Visit 3 (Re-assessment visit) | 5 [4,7] | 5 [4,5] | 5 [4,6] | 5 [4,7] |
| Change from Visit 2 to Visit 3 | 4 [2,5] | 3 [2,4] | 3 [2,4] | 3 [2,5] |
Summary statistics are presented as n (%), mean (SD) or median [IQR].
Removal time was missing for 1 patient in the D12 group and 1 patient in the P24 group.
Bishop score was not recorded at Visit 3 in 5, 5 and 11 patients in the D24, D12 and P24 groups, respectively.
One patient in the D24 group had Bishop score recorded as “<6” at baseline and excluded from the statistical summaries.
The subgroup of patients who required administration of an additional dose of prostaglandin gel (Prostin), after removal of Dilapan-S or Propess.
Trial participants who required additional prostaglandin had lower vaginal delivery rates than those who did not require additional prostaglandin (Table 3), reflecting the initial efficacy of the first agent in the group of patients most responsive to induction. For those who needed additional prostaglandin, there was minimal change in Bishop score between visit 2 (start of induction) (median Bishop score 1 [IQR 1,2]) and visit 3 (first reassessment), whereas a greater change in Bishop scores across all groups was observed in those that were suitable for amniotomy from visit 2 (median Bishop score 2 [IQR 1,2]) to visit 3 (median 5 [IQR 4,7]). Of the 122 patients who did not require an additional cervical ripening agent after removal of Dilapan-S or P24, the mean times to delivery for D24, D12 and P24 were 43, 30 and 34 h respectively.
In order to compare findings with an expectant management group, we examined vaginal delivery rates from a previous observational study within the same centre29 of those who were eligible for this trial but declined to participate, which showed similar outcomes (76% vaginal delivery rate, 95% CI: 72%, 80%) to the D24 and P24 groups (Table 4).
Table 4.
HOME INDUCTION TRIAL vaginal delivery rate compared to Rotunda Hospital vaginal delivery rate and the observational cohort (eligible but declined participation) vaginal delivery rate.
| Observational cohort study | |
|---|---|
| Vaginal delivery rate | |
| % (n/N) | 76% (372/490) |
| 95% CIa | 72%–80% |
95% Wald confidence interval for a binomial proportion.
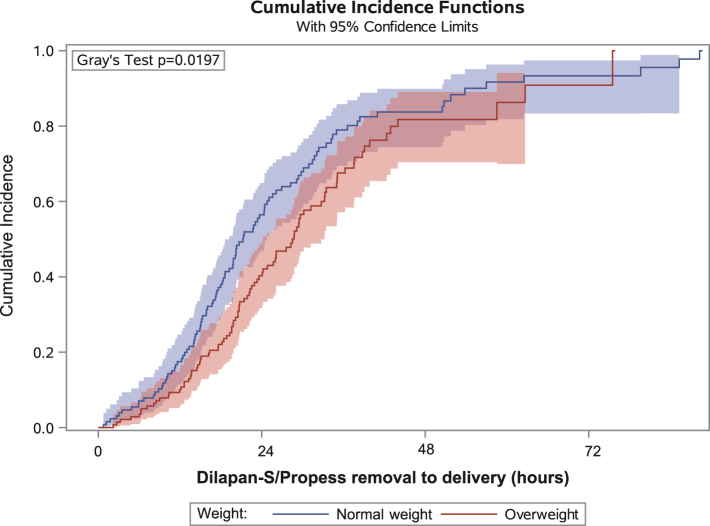
The vaginal delivery rate amongst those with BMI of 18–25 kg/m2 was 80%, BMI of 25–30 kg/m2 was 68%, and BMI of 30–35 kg/m2 was 56%. Adjustment for BMI marginally increased the precision of the treatment group differences for vaginal delivery rates (95% CI: −16%–6%, for Dilapan-S versus Propess. Cumulative incidences curves of vaginal delivery, in the normal BMI (BMI <25 mg/m2) and overweight (BMI >25 kg/m2) participants, are presented in Fig. 3. Time from Dilapan-S/Propess removal to delivery is presented for valid statistical comparisons (i.e. excluding the different set induction times of the trial interventions). Though the median times to vaginal delivery were similar for the groups (19.3 h versus 21.4 h, for normal BMI and overweight groups, respectively), there is evidence of a difference between groups in the risk of achieving vaginal delivery (p = 0.019).
Fig. 3.
Cumulative incidence functions of vaginal delivery according to BMI group. Normal weight is defined as 18 ≤ BMI <25 kg/m2 and overweight as 25 ≤ BMI <35 kg/m2. BMI, Body Mass Index.
Maternal experience was assessed through a postpartum questionnaire (Table 5). The response rate was 42%. There was a significant difference between groups in terms of those that perceived regular painful contractions at home, with 58% of patients in the P24 group and 10% in the Dilapan-S groups experiencing such contractions at home (p < 0.001). This prompted 53% of patients in the P24 group versus 9% of those in the Dilapan-S groups to return to hospital prior to their scheduled time (p < 0.001). Overall, 73% of home induction patients considered induction to be a positive experience, with the vast majority (92%) reporting that they would recommend outpatient IOL to others.
Table 5.
Patient Satisfaction Questionnaire data.
| Question | All (N = 113) | D24 (N = 43) | D12 (N = 27) | Dilapan-S All (N = 70) | P24 (N = 43) |
|---|---|---|---|---|---|
| Did you experience painful regular contractions at home? | |||||
| Yes | 32 (29%) | 5 (12%) | 2 (8%) | 7 (10%) | 25 (58%) |
| No | 79 (71%) | 37 (88%) | 24 (92%) | 61 (90%) | 18 (42%) |
| Did you have to return to hospital before the allocated 12 or 24 h? | |||||
| Yes | 29 (26%) | 5 (12%) | 1 (4%) | 6 (9%) | 23 (53%) |
| No | 83 (74%) | 37 (88%) | 26 (96%) | 63 (91%) | 20 (47%) |
| Would you recommend outpatient IOL to others? | |||||
| Yes | 98 (92%) | 37 (95%) | 24 (92%) | 61 (94%) | 37 (90%) |
| No | 8 (8%) | 2 (5%) | 2 (8%) | 4 (6%) | 4 (10%) |
| Agree/Disagree statements from the HOME INDUCTION RCT Questionnaire | ||||
|---|---|---|---|---|
| Statement | N | Disagree | Unsure | Agree |
| I felt that I was treated as an individual | 107 | 3 (3%) | 7 (6%) | 97 (91%) |
| Staff always took my concerns and questions seriously | 113 | 1 (1%) | 5 (4%) | 107 (95%) |
| Staff reassured me | 113 | 2 (2%) | 5 (4%) | 106 (94%) |
| I felt safe going home for induction | 112 | 2 (2%) | 3 (2%) | 107 (96%) |
| I felt like I got more rest by going home | 113 | 7 (6%) | 8 (7%) | 98 (87%) |
| I felt that I was well looked after | 113 | 7 (6%) | 4 (4%) | 102 (90%) |
| I found the overall induction process to be a positive experience | 113 | 11 (10%) | 20 (17%) | 82 (73%) |
Discussion
This study assessed different methods of cervical ripening for eIOL at 39 weeks’ gestation in the outpatient setting. Whilst the data were inconclusive in terms of non-inferiority of Dilapan-S to Propess, the high vaginal delivery rates highlight the effectiveness of the methods used, particularly in the 24 h cervical ripening groups.
How to manage an otherwise uncomplicated first pregnancy at term has become increasingly controversial in recent years, with two very divergent schools of thought dominating the debate. The traditionalist view is that if a first pregnancy is uncomplicated, no intervention (such as IOL) should be provided, in the hope that spontaneous onset of labour might occur prior to 41–42 weeks' gestation, at which time induction to avoid stillbirth becomes best practice. The advantage of this approach is that it supports a “non-medical” philosophy of maternity care and may be associated with high degrees of maternal satisfaction. It was long assumed that awaiting spontaneous onset of labour in this manner would maximise the probability of successful normal vaginal delivery. However, more recent data has demonstrated that elective induction of labour at 39 weeks' gestation in otherwise uncomplicated nulliparous patients is actually associated with the highest probability of successful vaginal delivery.4 This has resulted in a move to a different philosophy of maternity care amongst otherwise healthy first-time mothers, namely empowering each pregnant woman to make an evidence-based individual choice to either undergo eIOL prior to 40 weeks’ gestation or to await spontaneous onset of labour. The major challenge now facing busy maternity units throughout the world is how to manage the logistical challenges associated with a potentially large increase in patients requesting IOL, which by its nature tends to be more demanding on inpatient resources, such as busy Prenatal Wards or Labour Wards.
While many studies have been performed over the years comparing various forms of pharmacologic methods and mechanical methods of labour induction, to date there have been minimal data available to guide patients, midwives and obstetricians on the role of initiating IOL as an outpatient. Outpatient IOL might enable larger numbers of patients to choose induction if that is their preference, while remaining in the comfort of their own home, provided that this is both safe and effective. The HOME INDUCTION trial was therefore designed to build on the proven benefits of elective IOL for nulliparous patients as shown by the ARRIVE Trial, while enabling patients and their care providers to choose the optimal IOL method that suits their needs. Non-inferiority was not demonstrated and this was due to a partial reversal in the order of magnitude of the vaginal delivery rates, in comparison to those originally assumed. This trial nevertheless provides valuable and practical insights into the efficacy and safety of two potential options for cervical ripening to support elective outpatient IOL at 39 weeks’ gestation. The results lend support to elective IOL as an option for those women who choose it, with no increased risk of caesarean delivery demonstrated in our study, compared to caesarean delivery rates in a same risk observational cohort29 (Table 4).
When it comes to the logistical concerns associated with managing an increased demand for eIOL, it is clear that for normal-risk women, outpatient cervical ripening is an effective and well-tolerated option. Availing of this option can minimise the time spent in hospital during the often prolonged process of cervical ripening, allowing for better utilisation of hospital resources.
With respect to efficacy between groups, P24 and D24 performed similarly, meaning either option is reasonable for use in the setting of outpatient IOL. D12 had lower vaginal delivery rates, although it had a similar safety profile. While some hospitals internationally employ prostaglandin vaginal inserts as an outpatient method of cervical ripening, others are hesitant to use such pharmacologic methods in the outpatient setting, given the possibility of uterine hyperstimulation. In this clinical trial, uterine hyperstimulation was uncommon (3%), and was not associated with adverse outcomes in any of the three cases of hyperstimulation. Providing patients with clear information on how to respond to contractions at home, protocols on when to return to the hospital, and having a low threshold for re-admission ensured the relative safety of outpatient P24 in this trial setting.
In hospitals that would prefer to avoid the use of pharmacologic methods as an outpatient, use of a mechanical dilator such as Dilapan-S for 24 h is a potentially attractive option, as it has similarly high vaginal delivery rates, and a similar safety profile, without the risk of hyperstimulation. However, the Dilapan-S mechanical ripening group was less likely to result in labour and more likely to require additional prostaglandin compared with the Propess pharmacologic ripening group. For hospitals that wish to minimise potential medico-legal risks associated with uterine hyperstimulation, these features might be acceptable in terms of Labour Ward resource utilisation, while representing a safe and effective means to achieve cervical ripening.
Examining subgroups of those who required additional prostaglandin to complete the IOL process, compared to those who did not, highlights the importance of the initial response to any cervical ripening agent. Although initially Bishop scores were similar across groups, there was a difference in vaginal delivery rates based on initial response to cervical ripening. Those who had a good response to the initial cervical ripening medium (irrespective of medium) and did not require additional prostaglandin, had higher vaginal delivery rates (84%) compared to those who required further prostaglandin (62%). In general, the P24 group was less likely to require additional prostaglandin, suggesting that in terms of initial efficacy, this agent performs very well. However, for those in the P24 group who did require additional prostaglandin, there was a dramatic fall in subsequent vaginal delivery rates. This suggests that there is a subgroup of women who do not respond as effectively to prostaglandins in the form of either a slow-release vaginal insert or repeated prostaglandin gel. There were no significant differences in the Bishop score between groups at the outset, nor in subsequent need for oxytocin in each group. It remains challenging however to identify in advance which patient might preferentially respond to pharmacologic or mechanical methods of cervical ripening.
Strengths of this trial include its randomised nature, at a single centre with a standardised protocol for labour management, meaning individual midwife or obstetrician differences in practice are unlikely to be confounding factors. In addition, a limited number of obstetricians were responsible for insertion of the induction devices over the course of the trial, reducing interobserver variability, and ensuring experience level or difficulties in placing the device were unlikely to affect outcomes. Our experience during this study has allowed us to develop an excellent model of care for women who wish to have outpatient IOL, and could be adopted as a pathway of care for those who wish to offer this service.
As regards potential trial limitations, it was not possible to achieve blinding in this trial to the specific induction agent used, and therefore the possibility of bias was present, particularly in terms of decisions regarding amniotomy or need for further cervical ripening agents. Non-inferiority was not demonstrated in the study and the primary results should be considered tentative. Additionally, a fundamental principle of this trial was to empower women with choice as to how to manage their own pregnancy. It was not surprising therefore that a small number of women who were eligible opted to take part in the trial but then changed their mind after randomisation and withdrew from the study. This is in keeping with real-world studies of labour management, although it is gratifying to note that for those women who did choose to proceed with eIOL, it was a largely very positive experience. We would accept that a sample size of 327 may be too small to identify potential rare but serious complications attributable to IOL, although we believe this has been addressed by previous large randomised controlled trials of outpatient cervical ripening. Our study nevertheless adds to the cumulative data attesting to the safety of outpatient IOL.
The importance of shared decision making in obstetrics has been acknowledged as a worthy goal. In keeping with this principle, our study supports offering women a choice when it comes to managing late pregnancy. Our data builds on prior RCT evidence supporting informed choice regarding IOL at term, and provides practical, experiential data that can be used by pregnant women, midwives, obstetricians and hospital managers to make rational decisions on how to optimise delivery experience. While it was already clear that it is reasonable to discuss and offer a choice of elective IOL to normal-risk nulliparous women at 39 weeks’ gestation, it is now also clear that commencing this process in the outpatient setting is an effective and well-tolerated option which should optimise resource utilisation and maternal satisfaction. This option has the potential to transform our approach to IOL in routine clinical practice.
Contributors
FM was the principal investigator; he conceptualized the study and led the proposal, methodology development and maintained trial supervision. SN, OS, KF, and ZM contributed to the study design, methodology, protocol development and subsequent amendments, as well as and development of the funding proposals. SN, OS, CO, EW, SEN, IS, BK, RV, GG were sub-investigators and performed clinical duties. CL and EF managed resources. SN and PD reviewed trial data. PD was the lead trial statistician, managed software components, and conducted the formal analysis. ZM was project administrator. SN wrote the original draft of the manuscript. SN, PD, ZM, KF, FM were responsible for visualization, data presentation, review and editing of final manuscript. SN, PD, and FM have verified the underlying data. All authors read and approved the final manuscript.
Data sharing statement
The datasets generated and/or analysed during the current study are not publicly available due to limits set during the ethical review process but are available from the corresponding author on reasonable request.
Declaration of interests
An educational grant was provided to the research department from Medicem Technology s.r.o. and The Rotunda Foundation. The trial has been peer-reviewed by the funding bodies. Neither funder had a role in trial design. The funder had no role in the data collection, data analysis, data interpretation, report writing, or the decision to submit the manuscript for publication.
Acknowledgements
The Home Induction Trial team would like to acknowledge the funders, The Rotunda Foundation and Medicem Technology s.r.o. The team would like to thank the RCSI Office of Research and Innovation, and the RCSI Obstetrics and Gynaecology research team for their input in the running of the trial. We would also like to acknowledge the work of the pharmacy team at the Rotunda Hospital, and the staff at the Rotunda Hospital for their continued care in looking after those who took part. Finally, we would like to thank the patients that took part in this RCT.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102741.
Appendix A. Supplementary data
References
- 1.O’ Sullivan C., Wilson E., Beckmann M. Five-year trends in induction of labour in a large Australian metropolitan maternity service. Aust N Z J Obstet Gynaecol. 2022;62(3):407–412. doi: 10.1111/ajo.13486. [DOI] [PubMed] [Google Scholar]
- 2.Swift E.M., Gunnarsdottir J., Zoega H., Bjarnadottir R.I., Steingrimsdottir T., Einarsdottir K. Trends in labor induction indications: a 20-year population-based study. Acta Obstet Gynecol Scand. 2022;101(12):1422–1430. doi: 10.1111/aogs.14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Omerović A., Pajek S., Anzeljc V., Mujezinović F. Labor induction: change of indications and outcomes over time and future trends — a retrospective analysis. Clin Exp Obstet Gynecol. 2022;49(5):102. [Google Scholar]
- 4.Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379(6):513–523. doi: 10.1056/NEJMoa1800566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SMFM statement on elective induction of labor in low-risk nulliparous women at term: the ARRIVE trial. Am J Obstet Gynecol. 2019;221(1):B2–B4. doi: 10.1016/j.ajog.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 6.American College of Obstetricians and Gynecologists . 2018. Practice advisory: clinical guidance for integration of the findings of the ARRIVE trial: labor induction versus expectant management in low-risk nulliparous women.https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2018/08/clinical-guidance-for-integration-of-the-findings-of-the-arrive-trial [Google Scholar]
- 7.El-Sayed Y.Y., Rice M.M., Grobman W.A., et al. Elective labor induction at 39 Weeks of gestation compared with expectant management: factors associated with adverse outcomes in low-risk nulliparous women. Obstet Gynecol. 2020;136(4):692–697. doi: 10.1097/AOG.0000000000004055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailit J.L., Grobman W., Zhao Y., et al. Nonmedically indicated induction vs expectant treatment in term nulliparous women. Am J Obstet Gynecol. 2015;212(1):103.e1–103.e7. doi: 10.1016/j.ajog.2014.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis S., Zhao Z., Schorn M. Elective induction of labor or expectant management: outcomes among nulliparous women with uncomplicated pregnancies. J Midwifery Wom Health. 2022;67(2):170–177. doi: 10.1111/jmwh.13313. [DOI] [PubMed] [Google Scholar]
- 10.Sharp A.N., Stock S.J., Alfirevic Z. Outpatient induction of labour in the UK: a survey of practice. Eur J Obstet Gynecol Reprod Biol. 2016;204:21–23. doi: 10.1016/j.ejogrb.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 11.Coates R., Cupples G., Scamell A., McCourt C., Bhide A. Women's experiences of outpatient induction of labour with double balloon catheter or prostaglandin pessary: a qualitative study. Women Birth. 2021;34(4):e406–e415. doi: 10.1016/j.wombi.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Howard K., Gerard K., Adelson P., Bryce R., Wilkinson C., Turnbull D. Women's preferences for inpatient and outpatient priming for labour induction: a discrete choice experiment. BMC Health Serv Res. 2014;14(1):330. doi: 10.1186/1472-6963-14-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wise M.R., Thompson J.M.D., Battin M., et al. Outpatient balloon catheter vs inpatient prostaglandin for induction of labor: a randomized trial. Am J Obstet Gynecol MFM. 2023;5(6) doi: 10.1016/j.ajogmf.2023.100958. [DOI] [PubMed] [Google Scholar]
- 14.McDonagh M., Skelly A.C., Tilden E., et al. Outpatient cervical ripening: a systematic review and meta-analysis. Obstet Gynecol. 2021;137(6):1091–1101. doi: 10.1097/AOG.0000000000004382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogel J.P., Osoti A.O., Kelly A.J., Livio S., Norman J.E., Alfirevic Z. Pharmacological and mechanical interventions for labour induction in outpatient settings. Cochrane Database Syst Rev. 2017;9(9) doi: 10.1002/14651858.CD007701.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan H., Buaki-Sogo M.A., Barlow P., et al. Efficacy of pharmacological and mechanical cervical priming methods for induction of labour and their applicability for outpatient management: a systematic review of randomised controlled trials. Eur J Obstet Gynecol Reprod Biol. 2023;287:80–92. doi: 10.1016/j.ejogrb.2023.05.037. [DOI] [PubMed] [Google Scholar]
- 17.Ferring Pharmaceuticals LTD . 2021. Propess 10mg vaginal delivery system: SmPC.https://www.medicines.org.uk/emc/product/135/smpc#PRECLINICAL_SAFETY [Google Scholar]
- 18.Saad A.F., Villarreal J., Eid J., et al. A randomized controlled trial of Dilapan-S vs Foley balloon for preinduction cervical ripening (DILAFOL trial) Am J Obstet Gynecol. 2019;220(3):275.e1–275.e9. doi: 10.1016/j.ajog.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Saad A.F., Gavara R., Senguttuvan R.N., et al. Outpatient compared with inpatient preinduction cervical ripening using a synthetic osmotic dilator: a randomized clinical trial. Obstet Gynecol. 2022;140(4):584–590. doi: 10.1097/AOG.0000000000004942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beckmann M., Gibbons K., Flenady V., Kumar S. Induction of labour using prostaglandin E(2) as an inpatient versus balloon catheter as an outpatient: a multicentre randomised controlled trial. BJOG. 2020;127(5):571–579. doi: 10.1111/1471-0528.16030. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson C., Bryce R., Adelson P., Turnbull D. A randomised controlled trial of outpatient compared with inpatient cervical ripening with prostaglandin E 2 (OPRA study) BJOG. 2015;122(1):94–104. doi: 10.1111/1471-0528.12846. [DOI] [PubMed] [Google Scholar]
- 22.de Vaan M.D., Ten Eikelder M.L., Jozwiak M., et al. Mechanical methods for induction of labour. Cochrane Database Syst Rev. 2019;10(10) doi: 10.1002/14651858.CD001233.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta J., Chodankar R., Baev O., et al. Synthetic osmotic dilators in the induction of labour: an international multicentre observational study. Eur J Obstet Gynecol Reprod Biol. 2018;229:70–75. doi: 10.1016/j.ejogrb.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 24.The Rotunda Hospital Dublin . 2023. Annual clinical report 2022.https://rotunda.ie/rotunda-pdfs/Clinical%20Reports/2022Rotunda_AnnualReport.pdf?_t=1693224199 [Google Scholar]
- 25.Nicholson S.M., Smith O., Hatt S., et al. A randomised open-label trial to assess outpatient induction of labour (HOMEIND) and compare efficacy of Propess vs Dilapan-S® for induction of labour at 39 weeks' gestation in normal-risk nulliparous women: study protocol for a randomised controlled trial. Trials. 2023;24(1):135. doi: 10.1186/s13063-023-07174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta J.K., Maher A., Stubbs C., Brocklehurst P., Daniels J.P., Hardy P. A randomized trial of synthetic osmotic cervical dilator for induction of labor vs dinoprostone vaginal insert. Am J Obstet Gynecol MFM. 2022;4(4) doi: 10.1016/j.ajogmf.2022.100628. [DOI] [PubMed] [Google Scholar]
- 27.Saad A.F. 2022. Dilapan-S®: a multicenter us E-registry.https://classic.clinicaltrials.gov/ct2/show/NCT04451109 [Google Scholar]
- 28.Gavara R., Saad A.F., Wapner R.J., et al. Cervical ripening efficacy of synthetic osmotic cervical dilator compared with oral misoprostol at term: a randomized controlled trial. Obstet Gynecol. 2022;139(6):1083–1091. doi: 10.1097/AOG.0000000000004799. [DOI] [PubMed] [Google Scholar]
- 29.Nicholson S.M., Oprescu C., El Nimr S., et al. Labour roulette: probability of achieving spontaneous onset of labour in low risk nulliparous pregnancies. Am J Obstet Gynecol. 2023;228(1):S393–S394. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.