Abstract
Background
Down syndrome (DS), or Trisomy 21, is defined by the existence of an additional chromosome 21. Various physiological considerations in DS patients might lead to challenges in adequate pain management and sedation after surgery. The aim of this systematic review and meta-analysis is to evaluate the variations of the requirement needed for pain management and sedation in patients with DS who have undergone surgery compared to patients without DS.
Methods
A systematic review and meta-analysis of studies were conducted, focusing on critically ill patients with DS who were admitted to Intensive care units (ICUs) post-surgery and received opioids and/or benzodiazepines. Searches were conducted in four databases from their inception to November 18, 2023 (Pubmed, Scopus, Cochrane Library, and Web of Science). The primary outcome measured was the dosage of Oral Morphine Equivalent (OME) administered in the days following surgery. Fixed-effect models were used, an approach advisable when only a limited number of studies are available.
Results
Out of the 992 studies initially screened, the systematic review included ten studies, encompassing 730 patients, while the meta-analysis consisted of seven studies, encompassing 533 patients. Of the seven studies included in the analysis, 298 patients were identified to have DS, and 235 patients served as controls. Patients with DS showed a slight increase in OME needs on the first day, but this increase was not statistically significant (mean difference [MD] = 0.09; 95% Confidence Interval [CI]: [-0.02, 0.20]; P = 0.11). There was also no significant difference in the requirement for Midazolam on the first day among DS patients (MD = 0.01; CI [-0.16, 0.19]; P = 0.88). In addition, the duration of mechanical ventilation was not statistically significant in patients with DS compared with the control group (MD = -1.46 hours; 95% CI [-9.74, 6.82]; P = 0.73).
Conclusion
Patients with Down syndrome did not require more sedation or analgesia in the first three days after surgery than patients without Down syndrome. Additionally, the two groups showed no significant difference in the duration of mechanical ventilation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12887-024-04971-0.
Keywords: Down syndrome, Trisomy 21, Analgesia, Sedation, Critical care, Post surgery
Introduction
Down syndrome (DS) is a genetic disorder characterized by an additional chromosomal copy 21, resulting in the DS-associated phenotypic manifestation [1]. According to one study, up to 6,000 children are diagnosed annually with DS in the United States [2]. Children with DS carry a higher risk of having various congenital abnormalities, especially congenital heart disease, in up to 60% of DS patients [3]. Consequently, cardiac repair surgeries are typically required in infancy for these patients, and recovery in the intensive care unit (ICU) is necessary [4]. Additionally, individuals with DS are susceptible to other comorbidities, including blood disorders and frequent infections that necessitate painful and invasive interventions [5].
Achieving optimal sedation and analgesia is required during their ICU stay to reduce stress and promote recovery [6]. However, it can be challenging, with several studies reporting the need for higher doses of sedation and analgesia in this population [7].
Several theories have been proposed to explain the challenges faced in achieving appropriate pain management and sedation in patients with DS. One of these theories suggests that this condition’s unique physiological and anatomical features may play a role. These features include changes in the GABAergic transmission system, which could affect their response to sedation and analgesia management, possibly leading to increased requirements compared to children without DS [7]. According to the results of sensory neurography studies, patients with DS may react to pain stimuli more slowly and have decreased peripheral sensory nerve conduction. However, the findings regarding the degree of pain sensitivity in children with DS are inconclusive [8].
A clinical perspective remains that achieving optimal pain management and sedation in children with DS is challenging. As multiple studies were done to investigate the analgesia dose requirement in patients with DS, the results were conflicting. A retrospective cohort study reported that patients with DS after cardiac surgery required significantly higher doses of morphine and utilized more sedatives and muscle relaxants when compared to a matched control group [9]. Nevertheless, a retrospective study found no significant correlation between patients with DS and high opioid doses in the first 24–96 hours after surgery [10].
Moreover, pharmacokinetic and pharmacodynamic studies revealed that there is no significant difference in the need for morphine between patients with and without DS after surgery [11, 12]. When comparing children with DS to their peers, the results of their analgesic and sedative needs remain inconsistent.
To optimize post-surgery care in patients with DS, it is necessary to understand the differences in sedation and analgesia requirements in those patients. Hence, our systematic review and meta-analysis aimed to evaluate the literature results on the variation in the requirements for pain management and sedation in critically ill post-surgical children with DS.
Methods
We carried out this systematic review and meta-analysis in accordance with the 2020 Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [13]. Additionally, the study was registered in the Prospective International Register of Systematic Reviews (PROSPERO) under reference number (CRD42024484678).
Search strategy and study selection
We conducted searches in four databases (PubMed, Scopus, Cochrane Library, and Web of Science) dating back to their inception through November 18, 2023, using the terms related to ‘Down syndrome,’ ‘intensive care unit,’ ' post-surgery’ and combined with terms related to analgesia and sedation management. A comprehensive search strategy can be found in Appendix 1.
Following the identification of studies using predetermined search terms in various databases, we utilized the Rayyan software for abstract screening and reference organization after removing duplicate entries [14]. Two investigators (SA and AA) independently screened all the identified studies for inclusion based on predetermined eligibility criteria, using the titles and abstracts of the articles. In cases of discrepancy between the two reviewers, a third investigator (OA) was consulted to reach a consensus. Studies that were deemed eligible after the initial screening phase were then retrieved, and their full texts were independently reviewed by SA and AA. In cases of inter-reviewer disagreement, a fourth investigator, RA, was consulted to ensure a consistent and unbiased selection process.
Randomized control trials, nonrandomized comparative trials, or observational clinical studies of pediatric patients with DS aged 18 years or younger were considered eligible for inclusion. The studies included patients requiring any surgical or non-surgical sedative or analgesia in the intensive care setting. Eligible studies were included irrespective of the type, dose, or route of administration of the analgosedative agents (opioids, benzodiazepines, or non-benzodiazepines). Opioid analgesics such as (Morphine, Hydromorphone, and Fentanyl), sedatives such as Benzodiazepine (Midazolam, Lorazepam, and Diazepam), and non-benzodiazepine such as (Dexmedetomidine, Ketamine, Propofol and Chloral hydrate) were all considered for inclusion. Studies were excluded if they evaluated the sedative or analgesic required for procedures or general anesthesia, or did not compare DS and non-DS patients for the primary or secondary outcome. Also, lower quality studies including case reports or case series studies were excluded from the review.
Study outcomes
Initially, multiple outcomes were selected based on the preliminary search, including sedation and analgesia dose requirements, mechanical ventilation duration, inotropic scores, and vital signs. However, the primary and secondary outcomes were determined based on the most frequently reported outcome after screening the included studies. In this systematic review and meta-analysis, the primary outcome was the Oral Morphine Equivalent (OME) during the first three days of sedative agent administration. To standardize opioids to an equivalent OME, formula [15, 16] was applied, and to standardize various benzodiazepines to an equivalent Midazolam dose, formula [17] was used. Secondary outcomes included the duration of MV. Pooled analysis was performed when two or more studies provided adequate data on the same outcome.
Data extraction and quality assessment
Two investigators (SA and AA) independently utilized a standardized data extraction form to extract relevant data, while a third investigator (RA) assisted in case of discrepancies. Information about study design, patient numbers, inclusion criteria, interventions, and relevant outcomes were obtained from all included studies.
The methodological quality of the studies was evaluated independently by two investigators (MA and HA) using the Newcastle Ottawa Scale (NOS) for cohort studies. We planned to use the Cochrane Risk of Bias tool for randomized trials, however no such studies were included in this review. The NOS’s star allocation system, as outlined in its coding manuals, was used to assess the risk of bias (ROB) in the included studies. The assessment focused on key criteria such as selection bias, comparability of groups, and outcome reporting to evaluate the risk of bias in each study. It involved evaluating eight criteria, with a maximum of nine stars. Each criterion can get a maximum of one star, except for comparability, which can get up to two stars. Based on this system, studies with 8 to 9 stars were classified as having low ROB, those with 6 to 7 stars as medium ROB, and those with 5 or fewer stars were deemed to have high ROB [18].
Furthermore, the GRADE approach was applied to assess the certainty of evidence regarding the risk of bias, inconsistency, indirectness, imprecision, and publication bias. Two authors (HA, MA) independently assessed the certainty of evidence, resolving any discrepancies through discussion.
Data synthesis & analysis
As all study outcomes were continuous variables and reported on the same scale (days), mean differences (MD) were employed to summarize effect estimates, and their uncertainty was expressed through 95% confidence intervals (CIs). For studies reporting only medians and interquartile ranges, these data were converted to means and standard deviations (SD) using the method outlined by Luo et al., 2018 and Wan et al., 2014 [19, 20]. When SD was not reported, we adhered to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions, either by calculating the SD from other available data (e.g., p-values or CIs) or by computing the average SD when there was insufficient information to impute the missing SDs.
A fixed-effects model was used to pool the studies with no heterogeneity, and the DerSimonian Liard random meta-analysis model was used when there was significant heterogeneity. Heterogeneity among studies was assessed using the Chi-square test (Cochrane Q test) and the I-squared (I²) tests. χ² p-value of < 0.1 indicates significant heterogeneity. The I² values can be interpreted as follows: 0–40% might not be important, 30–60%may represent moderate heterogeneity, 50–90%may represent substantial heterogeneity, and 75–100% considerable heterogeneity. All statistical tests were two-tailed, and the significance criterion was < 0.05.
Results
Literature search results
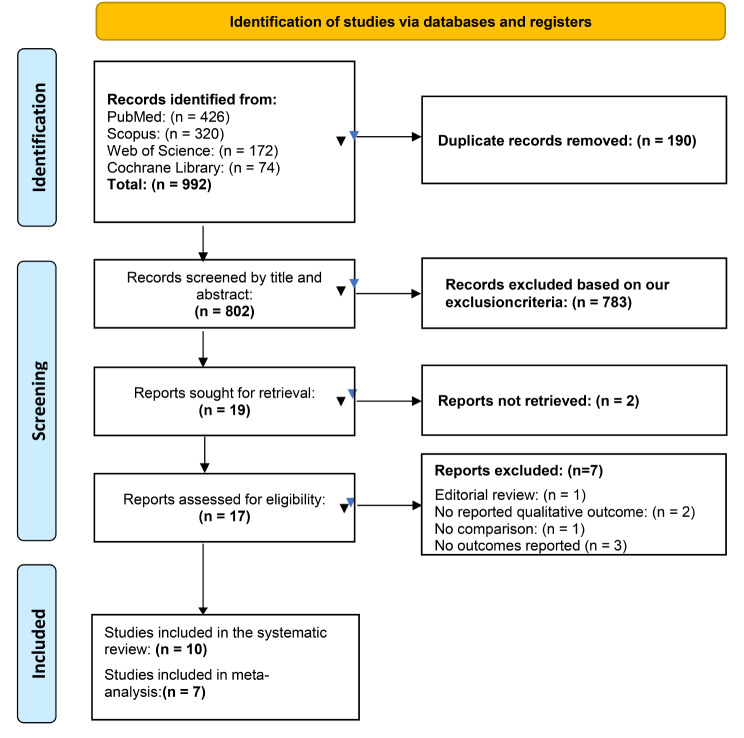
A total of 992 records were retrieved through our literature search process, and after removing 190 duplicate records, we were left with 802 unique records. Additionally, we manually searched the references of the included studies but did not find any additional articles that met our inclusion criteria. Two independent investigators underwent screening of titles and abstracts of these records using Rayyan software. Only 19 studies met the eligibility criteria after this screening phase and were selected for full-text screening. Finally, ten studies were included in the systematic review, seven of which were sufficiently homogeneous in design and outcome to be compared in the meta-analysis. All details regarding the screening and selection process of the included studies are illustrated in the PRISMA flow diagram Fig. 1.
Fig. 1.
PRISMA flow diagram of the study selection process
Characteristics of the included studies
The systematic review included ten studies encompassing 730 patients, while the meta-analysis consisted of seven studies with 533 patients. Of the seven studies included in the analysis, 298 patients were identified to have DS and, 235 patients served as controls. All included studies were observational since no interventional studies were found during the literature search. Additionally, the initial search focused on all pediatric admitted to the ICU. However, all the studies found focused only on postoperative cardiac patients, and no studies were found to assess the desired outcome in non-surgical ICU patients. The detailed baseline characteristics of the included studies are presented in the supplementary materials Table S1.
Quality assessment
The ROB in the analyzed studies varied from high to low risk of bias. It is important to note that the majority of these studies either neglected to assess confounders or omitted key confounders in their analysis, leading to several studies receiving no more than one star for comparability Table S2.
Oral morphine equivalent (OME)
At day one
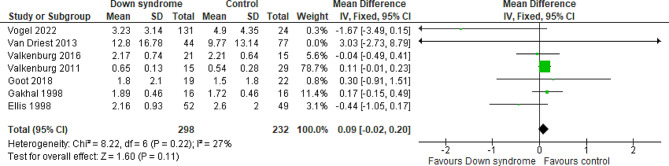
Seven studies compared the patients with DS to the controls regarding the OME on day one with a total of 530 patients. The overall MD between the DS and the control groups showed that the OME at day one was higher in the DS group, but the results did not reach statistical significance (MD = 0.09 mg/kg; 95% CI [-0.02, 0.20]; P = 0.11). The pooled studies were homogeneous (P = 0.22; I2 = 27%) Fig. 2. We downgraded the evidence due to high ROB and imprecision Table 1.
Fig. 2.
Forest plot comparing the DS and the control groups regarding the OME at day 1
Table 1.
GRADE rating of studies comparing patients with DS to control group with respect to sedation and analgesia requirement
| Outcomes | Anticipated absolute effects* (95% CI) | No of participants (studies) | Certainty of the evidence(GRADE) | Reason for downgrade | |
|---|---|---|---|---|---|
| Risk with control | Risk with down syndrome | ||||
| Oral Morphine Equivalent (OME) at Day One | The mean oral Morphine Equivalent (OME) at Day One was 3.32 Mg/Kg | MD 0.09 Mg/Kg higher (0.02 lower to 0.2 higher) | 530 (7 non-randomised studies) | ⨁⨁◯◯ Low | Downgraded due to high ROB and imprecision |
| Oral Morphine Equivalent (OME) at Day Two | The mean oral Morphine Equivalent (OME) at Day Two was 1.60 Mg/Kg | MD 0.08 Mg/Kg higher (0.02 lower to 0.18 higher) | 323 (5 non-randomised studies) | ⨁⨁◯◯ Low | Downgraded due to high ROB and imprecision |
| Oral Morphine Equivalent (OME) at Day Three | The mean oral Morphine Equivalent (OME) at Day Three was 1.14 Mg/Kg | MD 0.37 Mg/Kg lower (2.07 lower to 1.33 higher) | 187 (2 non-randomised studies) | ⨁◯◯◯ Very low | Downgraded due to high ROB, indirectness, and imprecision |
| Total Cumulative dose of OME for the first two days | The mean total Cumulative dose of OME for the first two days was 3.19 Mg/Kg | MD 0.11 Mg/Kg lower (0.61 lower to 0.39 higher) | 368 (5 non-randomised studies) | ⨁◯◯◯ Very low | Downgraded due to high ROB, indirectness, and imprecision |
| Total Cumulative dose of OME for the first three days | The mean total Cumulative dose of OME for the first three days was 4.56 Mg/Kg | MD 1.87 Mg/Kg lower (7.35 lower to 3.62 higher) | 187 (2 non-randomised studies) | ⨁◯◯◯ Very Low | Downgraded due to high ROB, indirectness, and imprecision |
| Midazolam Requirements at Day One | The mean midazolam Requirement at Day One was 1.00 Mg/Kg |
MD 0.01 Mg/Kg higher (0.16 lower to 0.19 higher) |
270 (3 non-randomised studies) | ⨁⨁◯◯ Low | Downgraded due to high ROB and imprecision |
| Midazolam Requirements at Day Two | The mean midazolam Requirement at Day Two was 0.31 Mg/Kg | MD 0.19 Mg/Kg higher(0.3 lower to 0.68 higher) | 211 (2 non-randomised studies) | ⨁◯◯◯ Very Low | Downgraded due to high ROB, inconsistency, and imprecision |
| Total cumulative dose of Midazolam for the first two days | The mean total cumulative dose of Midazolam for the first two days was 0.95 Mg/Kg | MD 0.09 Mg/Kg higher (0.11 lower to 0.29 higher) | 261 (3 non-randomised studies) | ⨁◯◯◯ Very Low | Downgraded due to high ROB, inconsistency, and imprecision |
| Mechanical Ventilation Duration | The mean mechanical Ventilation Duration was 36.87 Days | MD 1.46 Days fewer(9.74 fewer to 6.82 more) | 400 (5 non-randomised studies) | ⨁⨁◯◯ Low | Downgraded due to high ROB and imprecision |
CI: confidence interval; MD: mean difference
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect
At day two
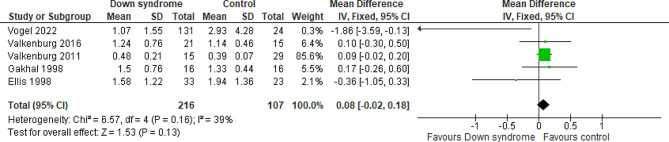
Five studies compared the patients with DS to the controls regarding the OME at day two with a total of 323 patients. The overall MD between the DS and the control groups showed that the OME at day two was higher in the DS group, but the results did not reach statistical significance (MD = 0.08 mg/kg; 95% CI [-0.02, 0.18]; P = 0.13). The pooled studies were homogeneous (P = 0.16; I2 = 39%) Fig. 3. We downgraded the evidence due to high ROB and imprecision Table 1.
Fig. 3.
Forest plot comparing the DS and the control groups regarding the OME at day 2
At day three
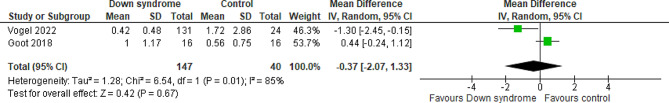
Two studies compared the patients with DS to the controls regarding the OME at day three with a total of 187 patients. The overall MD between the DS and the control groups showed that the OME at day three was almost similar in the two groups with no difference between them (MD= -0.37 mg/kg; 95% CI [-2.07, 1.33]; P = 0.67) and pooled studies showed significant heterogeneity (P = 0.01; I2 = 85%) Fig. 4. We downgraded the evidence due to high ROB, indirectness, and imprecision Table 1.
Fig. 4.
Forest plot comparing the DS and the control groups regarding the OME at day 3
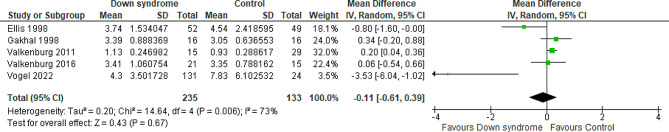
Total cumulative dose of OME for the first two days
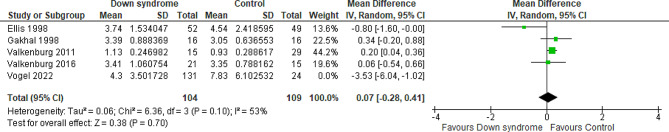
Five studies compared the patients with DS to the controls regarding the cumulative OME for the first two days with a total of 368 patients. The overall MD between the DS and the control groups showed that the cumulative OME for the first two days was almost similar in the two groups with no difference between them (MD= -0.11 mg/kg; 95% CI [-0.61, 0.39]; P = 0.67) and pooled studies showed significant heterogeneity (P = 0.006; I2 = 73%). Figure 5 The heterogeneity was resolved by performing the sensitivity analysis excluding Vogel et al. 2022 study (P = 0.10; I2 = 53%); however, the results still show no difference between the two groups (MD = 0.07 mg/kg; 95% CI [-0.28, 0.41]; P = 0.70) Fig. 6. We downgraded the evidence due to high ROB, indirectness, and imprecision Table 1.
Fig. 5.
Forest plot comparing the DS and the control groups regarding the cumulative OME for the first two days
Fig. 6.
Forest plot comparing the DS and the control groups regarding the cumulative OME for the first two days after resolving the heterogeneity
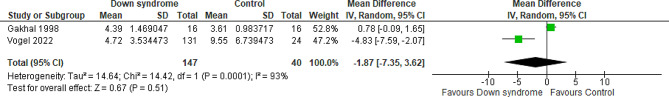
Total cumulative dose of OME for the first three days
Two studies compared the patients with DS to the controls regarding the cumulative OME for the first three days with a total of 187 patients. The overall MD between the DS and the control groups showed that the cumulative one for the first three days was almost similar in the two groups with no difference between them (MD= -1.87 mg/kg; 95% CI [-7.35, 3.62]; P = 0.51) and pooled studies showed significant heterogeneity (P < 0.001; I2 = 93%) Fig. 7. We downgraded the evidence due to high ROB, indirectness, indirectness, and imprecision Table 1.
Fig. 7.
Forest plot comparing the DS and the control groups regarding the cumulative OME for the first three days
Midazolam
At day one
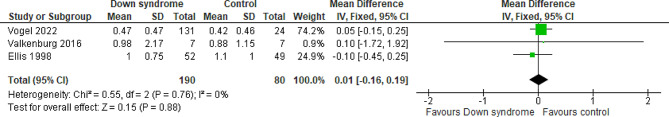
Three studies compared the patients with DS to the controls regarding the midazolam at day one, with a total of 270 patients. The overall MD between the DS and the control groups showed that the midazolam at day one was almost similar in the two groups with no difference between them (MD = 0.01 mg/kg; 95% CI [-0.16, 0.19]; P = 0.88). The pooled studies were homogeneous (P = 0.76; I2 = 0%) Fig. 8. We downgraded the evidence due to high ROB, and imprecision Table 1.
Fig. 8.
Forest plot comparing the DS and the control groups regarding the midazolam at day 1
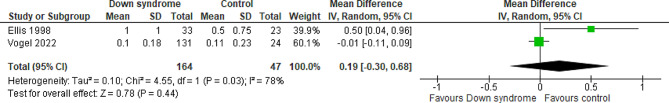
At day two
Two studies compared the patients with DS to the controls regarding midazolam at day two with a total of 211 patients. The overall MD between the DS and the control groups showed that the midazolam at day two was slightly higher, but not statistically significant, in the DS group compared to the control group (MD = 0.19 mg/kg; 95% CI [-0.30, 0.68]; P = 0.44). The pooled studies were not homogeneous (P = 0.03; I2 = 78%) Fig. 9. We downgraded the evidence due to high ROB, indirectness, and imprecision Table 1.
Fig. 9.
Forest plot comparing the DS and the control groups regarding the midazolam at day 2
At day three
Midazolam at day three was reported only by one study with a total of 155 patients. The overall MD between the DS and the control groups showed that the midazolam at day three was lower, but not statistically significant, in the DS group compared to the control group (MD= -0.08 mg/kg; 95% CI [-0.16, 0.00]; P = 0.05). Figure 10.
Fig. 10.
Forest plot comparing the DS and the control groups regarding the midazolam at day 3
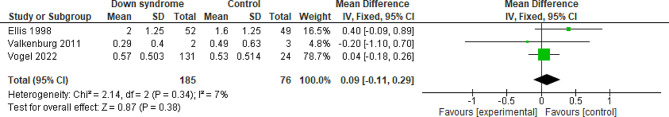
Total cumulative dose of midazolam for the first two days
Three studies compared the patients with DS to the controls regarding the total cumulative dose of Midazolam for the first two days with a total of 261 patients. The overall MD between the DS and the control groups showed that the total cumulative dose of Midazolam for the first two days was slightly higher, but not statistically significant, in the DS group compared to the control group (MD = 0.09 mg/kg; 95% CI [-0.11, 0.29]; P = 0.38). The pooled studies were homogeneous (P = 0.34; I2 = 7%). Figure 1. We downgraded the evidence due to high ROB, indirectness, and imprecision Table 1, (see Fig. 11).
Fig. 11.
Forest plot comparing the DS and the control groups regarding the total cumulative dose of Midazolam for the first two days
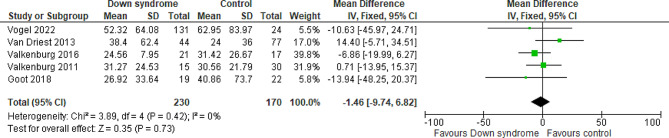
Mechanical ventilation (MV) duration
Five studies reported the MV duration comparing the DS and the control groups with a total of 400 patients. The overall MD showed that there was no significant difference between patients with DS and the controls regarding the MV duration (MD= -1.46 h; 95% CI [-9.74, 6.82]; P = 0.73). The pooled studies were homogeneous (P = 0.42; I2 = 0%) Fig. 12. We downgraded the evidence due to high ROB, and imprecision Table 1.
Fig. 12.
Forest plot comparing the DS and the control groups regarding the mechanical ventilation duration
Discussion
To our knowledge, this is the first meta-analysis to evaluate opioid and benzodiazepine requirements in post-surgical children with DS. The results of this meta-analysis shed light on the inaccurate assumption about increased sedation and analgesia requirements in DS. Such findings are essential as they can provide healthcare professionals with an accurate estimation to optimize patient care. However, future randomized clinical trials with larger cohorts are needed to ensure appropriate dosing in patients with DS.
Our meta-analysis didn’t detect a significant difference in morphine requirement in DS. Findings arise from seven studies, and a total of 533 patients compared OME on day 1 in patients with DS versus the control group. The results indicated that on day 1, OME was higher in the DS group without statistical significance; on day 2, OME was also higher but not statistically significant; and on day 3, OME requirements were similar between the groups with a significant heterogeneity [9–12, 21–23]. Regarding the hypothesis that individuals with DS have a different sensitivity to pain, one study among the studies included in the meta-analysis that had involved 45 infants undergoing duodenal surgery found no significant differences in pain scores or analgesia requirements compared to the control group [22].
Two others among the included studies had reported limitations, including small sample size and variability in underlying cardiac diagnosis between the DS and matched control, leading to patients undergoing different cardiac procedures. This is considered a potential confounder as different cardiac diseases have a significant impact on patient care as well as on clinical outcomes. An additional limitation that was reported in some of the included studies is the use of adjunctive non-opioid medications for pain control, which might lead to a reduced total requirement of opioids among study participants. Therefore, our findings contradict the common belief that DS might require more pain medication than their peers. In alignment with our results, two pharmacokinetics studies have shown that neither morphine volume of distribution nor clearance is different in DS in comparison to patients without DS, meaning that DS shall not be anticipated to require a higher requirement than their peers [12, 22]. While our analysis did report higher OME requirements in the DS on certain days, such a difference did not reach statistical significance. Ultimately, evidence regarding pain sensitivity in children with DS remains inconclusive.
Analysis of sedation requirement on day 1 in trisomy disorder patients across three studies (totaling 270 participants) indicated nearly equivalent use of midazolam between the groups (MD = 0.01 mg/kg; P = 0.88). On day 2, midazolam requirement was slightly higher in the DS group, though not statistically significant in two studies [21, 23]. Conversely, one study found that midazolam requirement on day 3 was lower in the DS group but also didn’t reach statistical significance [21]. Velkenburg et al. developed a population pharmacokinetic model for midazolam, which showed that DS was not considered to be a significant covariate. Their pharmacodynamic analysis concluded that there was no observed difference in midazolam sedative effect between DS and non-DS [22]. Similarly, Vogel et al. demonstrated an insignificant difference in midazolam exposure either preoperatively or intraoperatively between DS and matched control. Additionally, no differences in pediatric intensive care unit or hospital length of stay were observed between the groups. However, limitations include the retrospective nature impacting sample size matching, the challenges in accurately assessing pain in non-verbal children with DS, which might lead to underestimation of their actual needs, and the author noted considerations about the potential impact of other sedative agents, such as ketamine and dexmedetomidine, on the midazolam requirement [21]. The results of our meta-analysis contradict previous studies that reported higher midazolam doses and hypothesized alterations in the GABA transmission system in patients with DS as the cause [7, 22].
The duration of MV was assessed in our meta-analysis through the inclusion of five studies [10–12, 21, 22]. The overall findings revealed that there was no difference in MV duration between patients with DS and the controls. However, a study by Goot et al. reported that the length of stay for an individual with DS was longer than that of their peers, which might be indirectly linked to delayed respiratory recovery or feeding intolerance. It should be noted that this study was limited by its sample size [11]. Additionally, prolonged length of stay in patients with DS might be attributed to their underlying disease. A review involving 488 patients with DS found that anesthetic-related consequences such as severe bradycardia occurred in 3.66% of the patients and up to 1.83% occurrence rate of obstruction in the airway. This complication might have an impact on the duration of MV in comparison to patients without DS [24].
We acknowledge that this meta-analysis has considerable limitations. Firstly, it includes only a total of 10 studies in the systemic review and 7 in the meta-analysis, all of which were retrospectively designed. In addition, sedation and analgesia assessment tools were highly diverse across the analyzed studies. Selecting an accurate tool is crucial, particularly considering reports that children with DS had restricted ability in verbal and behavioral expression to painful stimuli [25]. This is of great significance as it could affect the amounts of analgesia actually administered to those patients. The lack of consideration of non-opioid agents or adjunctive sedative agents further adds to the limitation of our findings. Thus, randomized clinical trials or prospective cohort studies accounting for the additional use of non-opioid medication and using standardized assessment tools are needed to address the gap in knowledge about sedation and analgesia requirements in patients with DS.
Conclusion
Our meta-analysis confirms the inaccurate clinical assumption about the use of opioids in children with DS. Our conclusion indicates that patients with DS didn’t necessitate a higher requirement of sedation or analgesia in the first three days postoperatively compared to children without DS. Furthermore, there was no significant difference in the duration of MV between the two groups.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We express our appreciation to all researchers affiliated with the Saudi Critical Care Pharmacy Research (SCAPE) platform, as well as the supporters from the Saudi Society for Multidisciplinary Research Development and Education, for their invaluable assistance in this project.
Author contributions
All authors contributed significantly to the work reported, including in the study conception, design, and execution; the acquisition, analysis, and interpretation of data; and to all aspects of study orchestration. Additionally, all authors participated in drafting, revising, and/or critically reviewing the article; provided final approval of the version to be published; agreed on the journal to which the article has been submitted; and committed to being accountable for all aspects of the work.
Funding
Not applicable.
Data availability
All data generated or analyzed during this study are included in the published article.
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.What is Down Syndrome? - Down Syndrome Resource Foundation. (2023, November 1). Down Syndrome Resource Foundation. https://www.dsrf.org/resources/information/what-is-down-syndrome/
- 2.Mai CT, Isenburg JL, Canfield MA, Meyer RE, Correa A, Alverson CJ, Lupo PJ, Riehle-Colarusso T, Cho SJ, Aggarwal D, Kirby RS. October 3). National population‐based estimates for major birth defects, 2010–2014. Birth Defects Res. 2019;111(18):1420–35. 10.1002/bdr2.1589. Tubman, T. R., Shields, M. D., Craig, B. G. 10.1002/bdr2.1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulholland HC, Nevin NC. (1991, June 15). Congenital heart disease in Down’s syndrome: two year prospective early screening study. BMJ, 302(6790), 1425–1427.10.1136/bmj.302.6790.1425 [DOI] [PMC free article] [PubMed]
- 4.Antonarakis SE, Skotko BG, Rafii MS, Strydom A, Pape SE, Bianchi DW, Sherman SL, Reeves RH. February 6). Down syndrome. Nat Reviews Disease Primers. 2020;6(1). 10.1038/s41572-019-0143-7. [DOI] [PMC free article] [PubMed]
- 5.Bull MJ. Down syndrome. N Engl J Med. 2020;382(24):2344–52. 10.1056/nejmra1706537. 10.1056/nejmra1706537 [DOI] [PubMed] [Google Scholar]
- 6.Barnes S, Yaster M, Kudchadkar SR. (2016, May 1). Pediatric Sedation Management. Pediatrics in Review, 37(5), 203–212. 10.1542/pir.2014-0116 [DOI] [PMC free article] [PubMed]
- 7.Haydar TF, Reeves RH. Trisomy 21 and early brain development. Trends Neurosci. 2012, February;35(2):81–91. 10.1016/j.tins.2011.11.001. [DOI] [PMC free article] [PubMed]
- 8.Shaikh A, Li Y, Lu J. Perspectives on pain in Down Syndrome. Med Res Rev. 2023b;43(5):1411–37. 10.1002/med.21954. 10.1002/med.21954 [DOI] [PubMed] [Google Scholar]
- 9.SCOTT -GAKHALB, C. S., MACNAB AJ. Comparison of morphine requirements for sedation in Down’s syndrome and non-down’s patients following paediatric cardiac surgery. Pediatr Anesth. 1998, May;8(3):229–33. 10.1046/j.1460-9592.1998.00764.x. [DOI] [PubMed]
- 10.Van Driest SL, Shah A, Marshall MD, Xu H, Smith AH, McGregor TL, Kannankeril PJ. November). Opioid use after cardiac surgery in Children with Down Syndrome*. Pediatr Crit Care Med. 2013;14(9):862–8. 10.1097/PCC.0b013e31829f5d9d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goot BH, Kaufman J, Pan Z, Bourne DWA, Hickey F, Twite M, Galinkin J, Christians U, Zuk J, da Cruz EM. Morphine Pharmacokinetics in Children with Down Syndrome following cardiac surgery. Pediatr Crit Care Med. 2018, May;19(5):459–67. 10.1097/pcc.0000000000001537. [DOI] [PubMed]
- 12.Valkenburg AJ, Calvier EAM, van Dijk M, Krekels EHJ, O’Hare BP, Casey WF, Mathôt RAA, Knibbe CAJ, Tibboel D, Breatnach CV. Pharmacodynamics and pharmacokinetics of Morphine after Cardiac surgery in children with and without Down Syndrome. Pediatr Crit Care Med. 2016, October;17(10):930–8. 10.1097/pcc.0000000000000904. [DOI] [PubMed]
- 13.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, Moher D. March 29). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;71. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed]
- 14.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Reviews. 2016, December;5(1). 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed]
- 15.Equianalgesic Doses of Opioid Analgesics, Manual MSD. Retrieved November 29, 2023, from https://www.msdmanuals.com/en-jp/professional/multimedia/table/equianalgesic-doses-of-opioid-analgesics
- 16.Morphine 10 mg/mL injection, 1 mg/mL oral solution. South Australian Neonatal Medication Guidelines. Retrieved November 29. 2023, from https://www.sahealth.sa.gov.au/wps/wcm/connect/public+content/sa+health+internet/clinical+resources/clinical+programs+and+practice+guidelines/womens+and+babies+health/neonatal+medication+guidelines/neonatal+medication+guidelines
- 17.Kane SP. Benzodiazepine Equivalents Conversion Calculator - ClinCalc.com. https://clincalc.com/Benzodiazepine/
- 18.Wells, George & Shea, Beverley & O’Connell, D & Peterson, je & Welch, Vivian & Losos,M & Tugwell, Peter. (2000). The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis
- 19.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2016;27(6):1785–805. 10.1177/0962280216669183. 10.1177/0962280216669183 [DOI] [PubMed] [Google Scholar]
- 20.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014, December;14(1). 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed]
- 21.Vogel ER, Staffa SJ, DiNardo JA, Brown ML. Dosing of opioid medications during and after Pediatric Cardiac surgery for children with Down Syndrome. J Cardiothorac Vasc Anesth. 2022, January;36(1):195–9. 10.1053/j.jvca.2021.08.019. [DOI] [PubMed]
- 22.Valkenburg A, van Dijk M, de Leeuw T, Meeussen C, Knibbe C, Tibboel D. Anaesthesia and postoperative analgesia in surgical neonates with or without Down’s syndrome: is it really different? Br J Anaesth. 2012, February;108(2):295–301. 10.1093/bja/aer421. [DOI] [PubMed]
- 23.Ellis AP, Kanter RK, Requirements for post-operative analgesics and sedatives in children with down syndrome. January. Dosing. Crit Care Med. 1998;26(Supplement):97A. 10.1097/00003246-199801001-00263. 10.1097/00003246-199801001-00263 [DOI] [Google Scholar]
- 24.Borland LM, Colligan J, Brandom BW. (2004, August 26). Frequency of anesthesia-related complications in children with Down syndrome under general anesthesia for noncardiac procedures. Pediatric Anesthesia, 14(9), 733–738. 10.1111/j.1460-9592.2004.01329.x [DOI] [PubMed]
- 25.Hennequin M, Morin C, Feine J. Pain expression and stimulus localisation in individuals with Down’s syndrome. Lancet. 2000, December;356(9245):1882–7. 10.1016/s0140-6736(00)03259-1. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in the published article.