ABSTRACT
Efflux of antibiotics is an important survival strategy in bacteria. Mycobacterium tuberculosis has approximately sixty efflux pumps, but little is known about the role of each pump or the substrates they efflux. The putative efflux pump, EfpA, is a member of the major facilitator superfamily and has been shown to be essential by saturation transposon mutagenesis studies. It has been implicated in the efflux of isoniazid (INH), which is a first-line drug used to treat tuberculosis (TB). This is supported by evidence from transcriptional profiling showing that efpA is induced in response to INH exposure. However, its roles in the physiology and adaptation of M. tuberculosis to antibiotics have yet to be determined. In this study, we describe the repression of efpA in M. tuberculosis, using CRISPR interference (CRISPRi) to knockdown the expression of this essential gene and the direct effect of this on the ability of M. tuberculosis to survive exposure to INH over a 45-day time course. We determined that wild-type levels of efpA were required for recovery of M. tuberculosis following INH exposure and that, after 45 days of INH exposure, only a few viable colonies were recoverable from efpA-repressed M. tuberculosis. We conclude that EfpA is required for recovery of M. tuberculosis following INH exposure, which could reduce the efficacy of INH in vivo, and that EfpA may have a role in the development of resistance during drug therapy.
KEYWORDS: EfpA, efflux, CRISPRi, Mycobacterium tuberculosis, TB, isoniazid, regrowth, antibiotic resistance, early bactericidal activity
INTRODUCTION
Infection with Mycobacterium tuberculosis is the leading cause of death from a single infectious agent. In 2022, approximately 10.6 million people became ill due to tuberculosis (TB) and 1.3 million people died from TB worldwide (1). Multidrug resistance in M. tuberculosis, defined as resistance to at least isoniazid (INH) and rifampicin, has also compounded the burden of TB infections globally, with 410,000 cases estimated to have occurred in 2022 alone (1). The lengthy and toxic nature of TB multidrug therapy highlights the need for improved regimens with lower toxicity and shortened treatment times. This requires greater insights into new and existing mycobacterial drug targets for which promising new therapies will emerge to combat the global TB burden.
Although drug resistance in M. tuberculosis is predominantly caused by alterations in drug-activating enzymes or drug targets through DNA mutation, there is increasing evidence of enhanced expression of efflux pumps (including EfpA) in multidrug-resistant clinical isolates (2–4), which may contribute to the development of drug resistance. Studies by Machado et al. in 2012 (5) and Rodrigues et al. in 2012 (6) demonstrated a relationship between the overexpression of genes encoding efflux pumps and increased efflux activity, with an increased frequency in katG mutations. This supports the hypothesis that efflux of an antibiotic provides a window of opportunity for genetically encoded drug resistance to emerge. Therefore, the discovery of molecules that inhibit the action of efflux pumps, potentially extending the life of current and new therapies, would be an advantage in combating both drug-susceptible and drug-resistant M. tuberculosis infections (7, 8).
The efflux pump, EfpA, which belongs to the major facilitator family, is essential in M. tuberculosis, as demonstrated by transposon mutagenesis studies (9–12). This essentiality brings challenges as the function cannot be studied by deleting the gene. The first study to identify and characterize the gene, efpA, was by Doran et al. in 1997 (13), who found that efpA encodes a putative efflux protein that is predicted to be highly related to the QacA transporter family, with orthologs in M. marinum, M. leprae, M. smegmatis, and M. bovis. It is possible that the differential regulation of efpA impacts the survival of M. tuberculosis in the acidic compartment of the macrophage as its expression is reduced under acidic conditions (14). Transcriptomics studies also revealed that in M. tuberculosis, efpA is more highly expressed during INH exposure in vitro (15–19) and in sputa (20).
Populations of M. tuberculosis exposed to INH, in vitro, exhibit a substantial loss in bacterial viability during the first 2 days followed by a period of regrowth (19, 21–23), which is not entirely explained by an increase in the INH-resistant mutant frequency (19). This biphasic response has also been observed in M. tuberculosis-infected guinea pigs treated with INH and was found to be associated with the emergence of antibiotic-tolerant persisting populations that were not INH-resistant mutants (24). Efflux has been suggested as a mechanism that might contribute to M. tuberculosis drug tolerance and development of resistance (25).
There is direct evidence for the role of efpA in the response of M. tuberculosis to the standard drug regimen during the early stages of treatment; efpA was induced in M. tuberculosis derived from patient sputa 3 days after the start of drug therapy (20). Understanding the contributions of efflux to drug tolerance and emergence of genotypic resistance will drive the development of novel efflux-targeting therapeutics. More specifically, this study aims to help define the role of EfpA, in mediating the efficacy of the frontline drug INH. Since efpA is an essential gene, we used CRISPRi knockdown to determine the functional significance of EfpA in M. tuberculosis after exposure to INH.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions
M. tuberculosis H37Rv strains were grown in Difco Middlebrook 7H9 medium (Sigma-Aldrich), supplemented with 0.5% glycerol (VWR), 0.2% Tween-80 (Sigma-Aldrich), and 10% oleic acid dextrose catalase (OADC) growth enrichment supplement (UKHSA, Porton Down Media services). Middlebrook 7H10 medium was supplemented with 0.5% glycerol and 10% OADC. Strains containing plasmids pRH2521 and pRH2502 were selected for in culture using kanamycin (25 µg mL−1) and hygromycin (50 µg mL−1). All liquid cultures were grown at 37°C, with shaking at 200 rpm. Each strain was grown from frozen stocks for 10 days and standardized to an optical density of 0.05 OD540nm for the inoculation of time course experiments.
Antibiotic stock production
INH (Sigma-Aldrich) and anhydrotetracycline (ATc) (Sigma-Aldrich) liquid stocks were prepared in dimethyl sulfoxide (DMSO), whereas kanamycin (Sigma-Aldrich) and hygromycin (Sigma-Aldrich) liquid stocks were prepared in water. All drug stocks were filter-sterilized using a 0.2-µm filter (Sartorius Stedim).
Plasmid and strain construction
The extrachromosomal pRH2521 plasmid, encoding for single-guide RNA (sgRNA) scaffold sequences, and the integrative pRH2502 plasmid that encoded for a deactivated Cas9 (dCas9) from Streptococcus pyogenes (Table 1) (both under the control of Tet-regulatory promoters) were used for efpA repression. The two-plasmid CRISPRi system was developed for M. tuberculosis by Singh et al. in 2016 (26), and the plasmids pRH2521 and pRH2502 were supplied by Addgene (Addgene plasmid # 84380 and Addgene plasmid # 84379). The efpA-targeting sequence was cloned into the sgRNA (26–29). Initially, a 20-nucleotide DNA sequence that is 98 nucleotides downstream of the start of the efpA open-reading frame (ORF) was identified as a target for sgRNA base-pairing. An efpA-targeting sgRNA pRH2521 plasmid was constructed (27), and this plasmid (1 µg) was electroporated into an electrocompetent M. tuberculosis H37RvdCas9 strain (containing pRH2502 plasmid) using a Gene Pulser Xcell (Bio-Rad) and following a published method (30). A pulse of 2.5 kV, 25 µF, and a resistance setting of 1,000 Ω were used. The successful transformants were selected on Middlebrook 7H10 medium containing 25 µg mL−1 of kanamycin and 50 µg mL−1 of hygromycin. The M. tuberculosis H37Rv strain containing pRH2521 + efpA-targeting sgRNA (referred to as “efpAKD”) was compared to a control strain generated using the same approach, except for a sgRNA with a random base-pairing sequence that was not specific to any M. tuberculosis gene target (referred to as “control strain”).
TABLE 1.
Strains and plasmids used
| Strain or plasmid | Description | Source |
|---|---|---|
| Strains | ||
| E. coli DH5α | High transformation efficiency competent cells (T1 phage resistant and endA deficient) | New England Biolabs |
| M. tuberculosis H37Rv | M. tuberculosis laboratory research reference strain | (31) |
| M. tuberculosis H37RvdCas9 | M. tuberculosis H37Rv containing the pRH2502 integrative plasmid encoding Streptococcus pyogenes dCas9 and KanR | (29) |
| M. tuberculosis H37RvdCas9 sgRNA control (“control strain”) | M. tuberculosis H37RvdCas9 containing the pRH2521 extrachromosomal plasmid with a randomized sgRNA sequence, KanR, and HygR | (29) |
| M. tuberculosis H37RvdCas9efpA knockdown (“efpAKD”) | M. tuberculosis H37RvdCas9 containing the pRH2521 extrachromosomal plasmid with an sgRNA sequence targeting efpA at +98 + 120 bp downstream of start of efpA open reading frame, KanR, and HygR | This study |
| Plasmids | ||
| pRH2502 | Integrative plasmid originating from the pTC-0X-1L backbone, expressing S. pyogenes dCas9 under control of the tetracyline-inducible tetRO promoter (uv15tetO) | (26); pRH2502 (Addgene plasmids # 84379) |
| pRH2521 | Extrachromosomal plasmid originating from the pTE-10M-0X backbone, expressing sgRNA (randomized base-pairing sequence) under control of the tetracyline-inducible tetRO promoter (Pmyc1tetO) | (26); pRH2521 (Addgene plasmids # 84380) |
| pRH2521 efpAKD +98 + 120 | pRH2521 with the sgRNA base-pairing region targeting efpA at +98 + 120 bp downstream of start of the efpA open-reading frame, cloned into the plasmid | This study |
ATc induction of efpA repression
The efpAKD and control strains were grown in Middlebrook 7H9 medium over 14 days in a volume of 13 mL, in universal tubes, in triplicate. At the point of inoculation, ATc was added at a final concentration of 200 ng mL−1. Culture samples were collected at intervals, spun at 6,000 rpm for 10 minutes, and the pellet was resuspended in phosphate-buffered saline (PBS) pH 7.4. Total viable counts (CFU mL−1) were determined using the Miles–Misra technique. The plates were incubated for 3 weeks at 37°C in a static incubator prior to colony enumeration.
M. tuberculosis cultures exposed to INH
To assess the impact of INH exposure, the efpAKD and control strains were grown in Middlebrook 7H9 medium over 45 days, in triplicate. At the point of inoculation, ATc (final concentration of 200 ng mL−1) and/or INH (final concentration of 0.5 µg mL−1) were added to the cultures. The time-kill experiments also included a no-drug condition. Culture samples were collected at intervals, spun at 6,000 rpm for 10 minutes, and the pellets were resuspended in phosphate-buffered saline (pH 7.4). Viability was measured using total viable counts (CFU mL−1), as described previously.
RNA extraction and purification
RNA was extracted from 10 mL of culture that had been sampled at intervals of 0, 1, 3, and 7 days post ATc-addition. Forty milliliters of guanidine thiocyanate (GTC) lysis solution (comprising 5 M GTC (Sigma-Aldrich), 0.5% lauryl sarcosine (Sigma-Aldrich), 25 mM trisodium citrate (Sigma-Aldrich), 0.5% Tween-80, and 0.05% 2-mercaptoethanol (Sigma-Aldrich) was added to the culture samples and incubated for 1 hour at room temperature. The cultures were spun at 3,000 rpm for 10 minutes, the supernatant was removed, and the M. tuberculosis pellets were resuspended in 1.2 mL TRIzol solution (Thermo). The extractions were transferred to tubes containing lysing matrix B beads (0.5 mL of 0.1 mm) (MP bio) and disrupted in a bead beater at full power for 50 seconds. The solution was spun at 13,000 rpm for 10 minutes, resuspended in 240 µL 24:1 chloroform:isoamyl alcohol (Sigma-Aldrich), and shaken vigorously for 20 seconds. Following centrifugation at 13,000 rpm for 10 minutes, the aqueous phase was resuspended in 600 µL chloroform:isoamyl alcohol, spun again, and the step was repeated. The aqueous phase was added to 600 µL isopropanol (Sigma-Aldrich) with 60 µL sodium acetate (Sigma-Aldrich) and incubated overnight at −70°C to precipitate the nucleic acids. The sample was washed with ethanol, resuspended in nuclease-free water, and purified using a Qiagen RNeasy kit with additional on-column DNase (Qiagen) digestion. RNA quantity and purity were assessed by using a spectrophotometer (Nanodrop; Thermo).
Quantitative reverse transcription-PCR (qRT-PCR)
The absolute quantification method was used to determine the number of transcripts (copy number) of efpA in each sample, relative to an endogenous housekeeping control gene, sigA. To achieve a copy number standard curve, full gene fragments of efpA and sigA were amplified from M. tuberculosis H37Rv genomic DNA using the PCR primers outlined in Table 2, GoTaq green polymerase (Promega) and DMSO (Sigma-Aldrich), using conditions of 98°C for 2 minutes, followed by 35 cycles of 94°C for 30 seconds, 59°C for 30 seconds, and 72°C for 2 minutes, with a final cycle of 72°C for 10 minutes. The PCR products were purified using a PCR clean-up kit (Qiagen), and the DNA concentration was quantified using a Qubit dsDNA broad range quantification assay (Thermo). From the quantity and gene length, the copy number of efpA and sigA was calculated using the Thermo Fisher copy number calculator. The copy number values were standardized to 107 and used to generate a standard curve of efpA and sigA abundance. The reverse transcription reactions to synthesize cDNA consisted of 500 ng total RNA, 1 µL Superscript III (Thermo), 4 µL First-Strand Synthesis Buffer (Thermo), 1 µL 10 mM dNTP mixture (Thermo), and 1 µL random primers (Thermo). The reactions also featured a no reverse transcriptase control for each sample. For the qRT-PCR reactions, 10 µL Taqman Universal Master-Mix II with UNG (Thermo), 5 µL of cDNA/control DNA fragment, 1 µL of forward and reverse primers, 0.5 µL Taqman probe, and nuclease-free water were added to reach a total reaction volume of 20 µL in a 96-well MicroAmp plate (Thermo). The reaction cycling conditions used were 2 minutes at 95°C followed by 45 cycles of 95°C for 30 seconds and then 60°C for 30 seconds using the QuantStudio 7 Real-Time PCR system (Thermo). Each qRT-PCR experiment featured non-template controls. The data were analyzed using the QuantStudio Real-Time PCR Software (Thermo), and the efpA copy number was normalized as a ratio to the sigA copy number. The values from the ATc-induced efpAKD reactions were compared to that of the ATc-induced control strain and efpAKD without ATc induction.
TABLE 2.
Primers and oligonucleotides used in the qRT-PCRa
| Primer or oligonucleotide | Sequence (5’ to 3’) |
|---|---|
| CRISPRi oligonucleotide & target location | |
| efpA +98 + 120 forward | GGGAGTCGTTGAGAGCCGTCATAG |
| efpA +98 + 120 reverse | AAACCTTCAGAGCGCCCCAGCAGG |
| Primers for full gene amplification | |
| efpA full gene forward | ACTGGTGGTTCAACACGGAA |
| efpA full gene reverse | GGATCAGCAGTTTGTGGCTG |
| sigA full gene forward | GACACTTTCGGTTACGCACG |
| sigA full gene reverse | GCTGATTCGAACTCCGATCC |
| qRT-PCR primers and probes | |
| qRT_PCR efpA forward | AGGTTTCATCCCGTTCGTCA |
| qRT_PCR efpA reverse | GCCGAATAGCAGATATCCGC |
| qRT_PCR efpA probe | CCTCGCAGCTGGTGTCCCGGT |
| qRT_PCR sigA forward | CCGGTGATTTCGTCTGGGAT |
| qRT_PCR sigA reverse | ATCTGTTTGAGGTAGGCGCG |
| qRT_PCR sigA probe | ACGAGTCGGAGGCCCTGCGT |
Underlined bases indicate the first four bases of CRISPRi oligonucleotides which are “overhang sequences” used to clone the sgRNA base-pairing sequence into pRH2521.
Gene expression profiling
The quality of the extracted RNA was confirmed using a Bioanalyzer (Agilent Technologies). Cy3-labeled cDNA was produced by mixing 1 µg of total RNA with 1 µL of random primers (Thermo), heated to 70°C for 2 minutes, and then snap-frozen on ice. The RNA was then added to 14 µL of master-mix consisting of 15.3 µL Cy3 dCTP (Sigma-Aldrich), 20.7 µL dNTP mix (Thermo), 22.5 µL DTT (Thermo), 45 µL 5 x First-Strand Buffer (Thermo), and 22.5 µL SuperScript II (Thermo). This reaction mix was incubated in the dark at 25°C for 10 minutes, followed by 42°C for 90 minutes, after which it was purified using a MinElute PCR Purification kit (Qiagen). For hybridization, 18 µL of purified Cy3-labeled cDNA was added to 4.5 µL of 10 x blocking agent (Agilent) and 22.5 µL of 2 x hybridization buffer (Agilent). The reaction mix was incubated at 95°C for 5 minutes, followed by a 1-minute centrifugation at 13,000 rpm. Forty microliters of the sample was pipetted onto a hybridization slide (Agilent) and incubated at 65°C in a hybridization oven set to rotate at 20 rpm. Following overnight incubation, the slides were washed in Oligo aCGH wash buffer 1 (Agilent) at room temperature for 5 minutes with agitation and then in Oligo aCGH wash buffer 2 (Agilent) at 37°C for 1 minute with agitation. Slides were scanned immediately using an Agilent DNA Microarray Scanner (with SureScan 2 µm High-Resolution Technology; G2505C). The microarrays used were custom BµG@S ‘TBv3_0_0’ arrays (accession number A-BUGS-41) that were produced by Agilent Technologies.
Gene expression analyses
Features were extracted from the array images using Agilent Feature Extraction Software (v10.7) with local background correction. The extracted data were analyzed in the RStudio software (version 1.3.1056) using the Limma package (version 3.14) available through Bioconductor (32). These data were log2-transformed and normalized by median absolute deviation (MAD) analysis prior to comparisons in Limma. The data were fitted to a linear model, and pairwise t-statistic comparisons were performed between the expression data for each gene between the conditions at each time point. Using empirical Bayesian statistics and the t-statistics highlighted previously, fold-changes in differential gene expression were determined between paired conditions. The outputs of the Limma analysis were the log2 fold changes in gene expression and the P-values from the t-statistics. These comparisons were further corrected for multiple testing using Benjamini–Hochberg adjustment to provide an adjusted P-value, considering the false discovery rate (FDR). At each timepoint, ATc-induced efpAKD was compared to non-induced efpAKD to define transcriptional changes associated with the repression of efpA. The data sets were filtered to include differentially expressed genes that exhibited an adjusted P-value of <0.05 and a log2 fold-change difference of greater or less than 1 (fold difference of greater or less than 2), detailed in Supplementary Data ”Differential_expression_efpA_knockdown _S1.” The gene functions and names were assigned using DAVID enrichment software (https://david.ncifcrf.gov/) (33) and Mycobrowser (https://mycobrowser.epfl.ch/) (34). The microarray data have been assigned ArrayExpress accession E-MTAB-13755 .
Scanning electron microscopy
efpAKD cultures, with or without ATc induction, were fixed after 7 days of culture, using a final concentration of 4% formaldehyde, spun by centrifugation at 6,000 rpm for 10 minutes, resuspended in water, and imaged by scanning electron microscopy (SEM) using the following method. Cells were immobilized onto glass cover slips, further fixed with osmium tetroxide for 2 hours at room temperature, followed by dehydration by exposure to graded series of ethanol. Samples were then treated with hexamethyldisilane and air-dried. A conductive coating of 20 nm gold was applied using a fine-grain ion-beam sputter coater, prior to imaging using a Zeiss Sigma 300VP Scanning Electron Microscope. To determine changes in the cell dimensions, SEM images were analyzed in ImageJ (35). The proximal widths and lengths of one hundred and eighty-seven randomly selected bacilli were measured using a calibrated drawline function, which was set to the scale bar of each image.
Statistical methods
For the growth curves (Fig. 2), a two-way ANOVA was applied to identify significant differences in the viable count (CFU mL−1) across the time course between strains with or without ATc induction. For the INH time-kill experiments (Fig. 7), a three-way ANOVA was utilized to compare viability (CFU mL−1) between strains, ATc induction, and/or INH exposure. All viable counts were log10-transformed prior to the statistical analyses. A one-way ANOVA with multiple comparisons was applied to compare measurements of the cell dimensions between different conditions (strains; ATc induction). ANOVA was performed using RStudio software (version 1.3.1056).
RESULTS
Targeted repression of efpA results in a loss of viability in M. tuberculosis
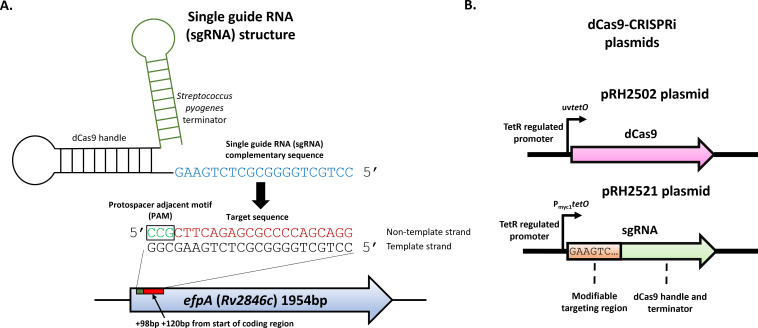
The aim of this study was to determine the role of EfpA in the response of M. tuberculosis to INH. We initially evaluated the effect of efpA repression on the viability of M. tuberculosis in the absence of INH. Since efpA is an essential gene in M. tuberculosis, an efpA knockdown strain (“efpAKD”) was generated by CRISPRi and induced with anhydrotetracycline (ATc), using the two plasmid–dCas9 system (26). The sgRNA targeted efpA in a 20-bp region between +98 bp to +120 bp, relative to the start codon of the efpA coding region, downstream of a 3-bp protospacer adjacent motif (PAM) required for dCas9 binding (Fig. 1). A control strain was generated (“control strain”) that contained the same vectors but with a random sgRNA sequence, rather than an efpA-targeting sgRNA, as a comparator in all experiments (26).
Fig 1.
A two-plasmid dCas9–sgRNA CRISPRi system adapted to repress efpA expression. Schematic of the single-guide RNA chimeric structure consisting of the terminator region originating from Streptococcus pyogenes, the dCas9 handle region (to enable binding to the dCas9 enzyme), and the sequence complementary to the efpA target sequence at the 5’ end of the gene. The protospacer adjacent motif (PAM) sequence (CCN) is adjacent to the 5’ end of the target sequence (A). Schematic of the CRISPRi genes on two plasmids coding for the CRISPRi system: pRH2502, which encodes the dCas9 endonuclease enzyme under the control of a uvtetO promoter, and pRH2521-efpA, which encodes the efpA sgRNA under the control of a pmyc1tetO promoter (B).
The viability of efpAKD was determined after ATc induction of efpA repression compared to a non-induced state, in triplicate cultures, over a 14-day time course in Middlebrook 7H9 medium. ATc was added on day 0 of the time course at a final concentration of 200 ng mL−1. Samples were removed at days 0, 1, 2, 3, 4, 7, 9, 11, and 14, for total viable counts (Fig. 2). The conditions such as the concentration of ATc and the time of addition were optimized prior to these experiments to maximally reduce efpA expression.
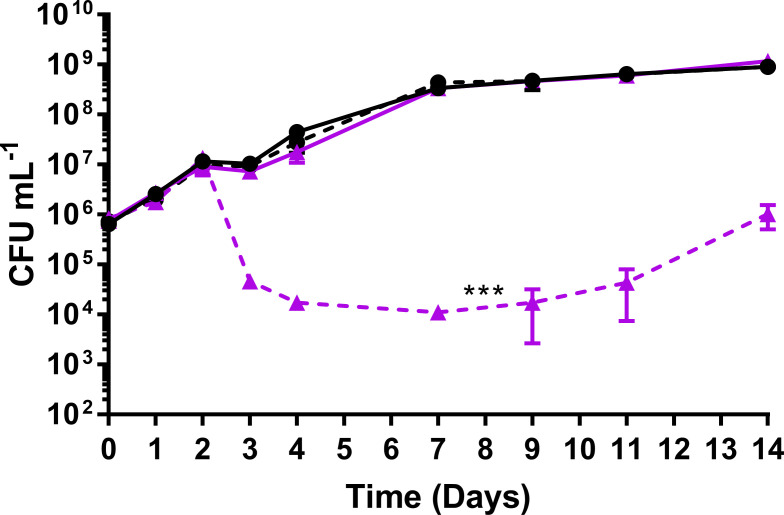
Fig 2.
A comparison of the total viable counts (CFU mL−1) for the control strain (solid line) and efpAKD (dotted line) cultured over a 14-day time course with (purple triangles) or without (black circles) 200 ng/ml ATc to induce efpA knockdown. The ATc was added to respective cultures on day 0. All values represent the mean of three biological replicates. Error bars are ±SD. Statistical significance was determined across the whole time course by two-way ANOVA comparing the responses of each strain with and without ATc addition. *** =P ≤ .001.
The repression of efpA resulted in a steep reduction in the viability of efpAKD between days 2 and 4 of the time course (Fig. 2), and there was a statistically significant difference between the total viable counts compared to those for non-induced efpAKD (P = <0.001). Despite a gradual increase in viability from day 7, by day 14, the efpAKD was unable to recover to the same level of viability as efpAKD without ATc induction. As expected, ATc addition did not affect the viability of the control strain.
Induction of the CRISPRi system targeting efpA leads to a dramatic reduction in efpA expression and changes in cell morphology
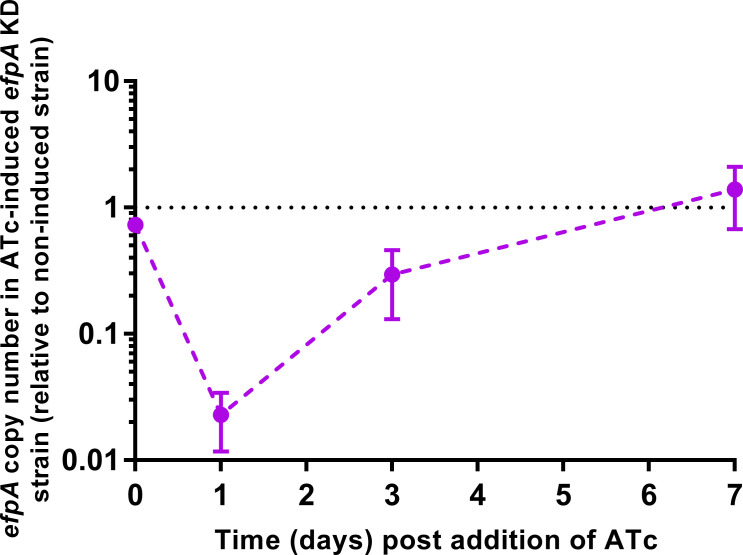
To assess the knockdown of efpA expression, the copy number of efpA mRNA transcripts was determined for efpAKD with or without ATc induction, using qRT-PCR. ATc was added in the mid-exponential phase, and efpA transcript copy numbers were quantified (Fig. 3). At day 1 post-induction, efpA transcript levels were 43-fold lower in the induced efpAKD cultures than in the non-induced cultures, confirming the repression of efpA expression (Fig. 3). Following this, the transcript levels increased over time and were 3-fold lower in the induced efpAKD by day 3 post-induction of knockdown and finally recovered to equivalent expression levels by day 7.
Fig 3.
Repression of efpA was determined by qRT-PCR. ATc was added after 4 days of growth. efpA copy number values were normalized relative to sigA copy number values across the time course. Error bars are ±SD of the mean of three biological replicates.
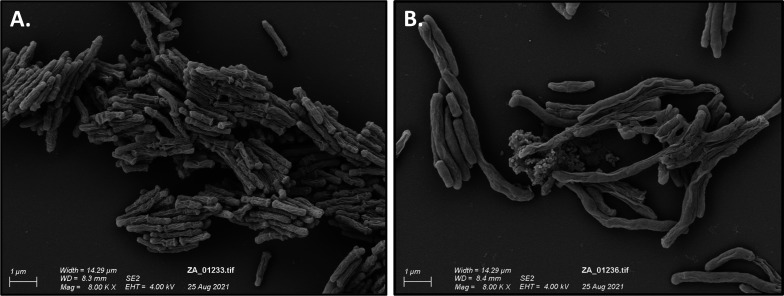
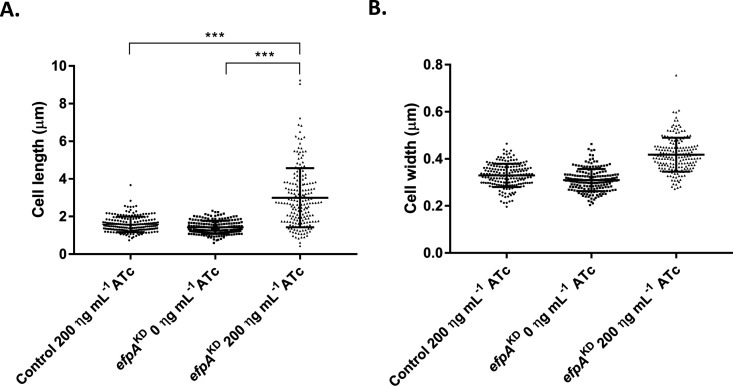
To observe whether the repression of efpA had an impact on cellular morphology, scanning electron microscopy (SEM) was used to study efpAKD with or without ATc addition. Images of the culture samples were taken on day 7; this was the timepoint at which the cell morphology effects were most visible. Repression of efpA led to the formation of elongated bacilli (Fig. 4). We observed a statistically significant increase (P = <0.001) in the average cell length of ATc-induced efpAKD (3 µm ± 1.573 SD) compared to efpAKD without ATc (1.44 µm ± 0.355 SD) (Fig. 5). Visually, the data indicated increased cell widths with efpA repression, although this was not statistically significant. Normal cell morphology was restored by day 14. These results suggest the role of EfpA in cell division.
Fig 4.
Scanning electron micrographs of efpAKD in the absence (A) or presence of 200 ng ml−1 ATc (B). Scanning electron micrographs were imaged at 8,000 x magnification.
Fig 5.
Scatter plots of the cell length (A) and the cell width (B) of either the control strain or efpAKD in the absence or presence of 200 ng mL−1 ATc. The cell length and width were measured from one hundred and eighty-seven bacilli randomly selected from SEM images at 2000 x magnification. Error bars are ±SD. Statistical significance was determined between the different conditions (strains, +/-ATc) by one-way ANOVA. *** =P ≤ .001.
Repression of efpA leads to reduced expression of cell wall functions
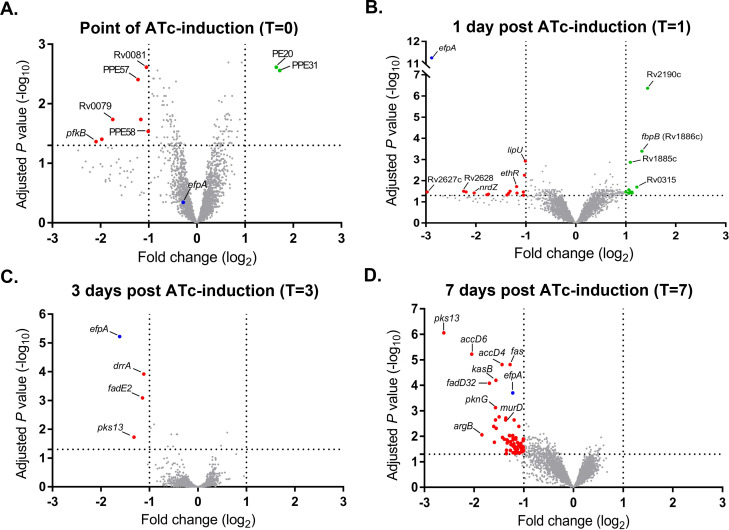
Whole-genome DNA microarray was used to identify pathways differentially expressed after efpA knockdown that could provide insights into the function of EfpA. The impact of efpA knockdown was observed over time, comparing efpAKD with or without ATc addition (Fig. 6). The raw data are available in ArrayExpress (ArrayExpress accession E-MTAB-13755), and the differentially expressed genes for all the timepoint comparisons are captured in the Supplementary Data (Excel file ”Differential_expression_efpA_knockdown _S1”). Reduced expression of efpA was observed in efpAKD at day 1 (−2.88-fold log2 repression), day 3 (−1.62-fold log2 repression), and day 7 (−1.22-fold log2 repression) post-ATc induction. During efpA repression, much of the differential gene expression (reduced expression only) was observed at day 7 (Fig. 6D) for functions (or putative functions) in DNA replication, cell division, redox/energetics, and cell wall lipid biosynthesis; our discussion will focus on these genes.
Fig 6.
Volcano plots describing the M. tuberculosis transcriptional response to efpA repression. Expression data from three biological replicates were analyzed using a pipeline in Limma (30). Plots show the log2 fold-change in gene expression and log10-adjusted P-value for all genes in efpAKD that were induced with 200 ng/mL ATc compared to no induction, at day 0 (A), day 1 (B), day 3 (C), and day 7 (D). Genes with increased expression are highlighted in green, and genes with reduced expression are highlighted in red. Significantly differentially regulated gene names are annotated where known. efpA is indicated in blue.
Repression of efpA prevents regrowth of M. tuberculosis during INH exposure
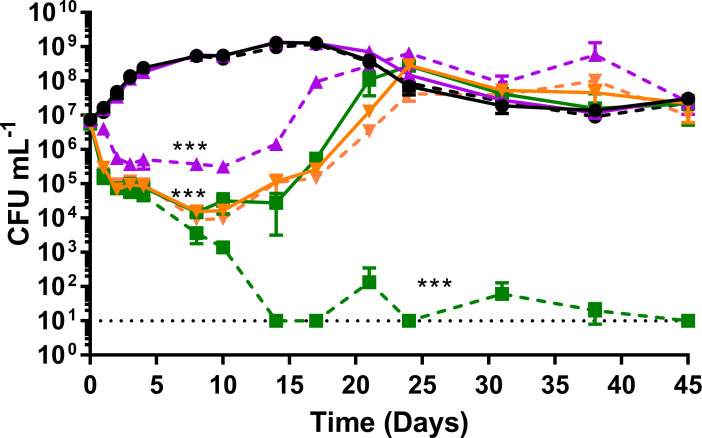
We determined whether efpA repression led to the increased sensitivity of M. tuberculosis to INH. The efpAKD strain and the control strain were cultured for 45 days, in triplicate. At the point of inoculation, the following were added to cultures: either ATc at 200 ng mL−1, INH at 0.5 µg mL−1, a combination of INH/ATc, or no drugs at all. Culture samples were removed at days 0, 1, 2, 3, 4, 8, 10, 14, 17, 21, 24, 31, 38, and 45, for total viable counts (CFU mL−1). As expected, a bactericidal response was observed during INH exposure, which was not observed with ATc exposure. In the absence of INH, the ATc-induced efpAKD cultures exhibited a reduction in viability due to efpA repression (Fig. 7), whereas the control strain grew normally, replicating our earlier findings (Fig. 2). In the presence of INH alone, both the efpAKD and control strains demonstrated an equivalent steep drop in viability (3 logs10 CFU mL−1) over the first 3 days and started to recover by day 5, as expected from previously published in vitro INH exposure experiments (19, 21, 23). By day 24, both strains recovered to the same level of viability (CFU mL−1) as that prior to INH addition. The control strain exposed to a combination of ATc and INH followed the same triphasic pattern of killing, followed by recovery. For efpAKD, a combination of ATc and INH resulted in an initial decline in viability, which was consistent with the response to INH alone. However, between days 7 and 14, efpAKD cultures exhibited a severe reduction in viability and fluctuated at a level of viability around the limit of detection (10 CFU mL−1) over the remainder of the 45-day time course (Fig. 7). In conclusion, the ability of M. tuberculosis to recover from INH exposure was abolished by the repression of efpA.
Fig 7.
The impact of efpA repression on INH activity in vitro. The viability of the control strain (solid lines) and efpAKD (dotted lines) over a 45-day time course, exposed to either 0.5 µg mL−1 of INH (orange inverted triangles), 200 µg mL−1 ATc (purple triangles), 0.5 µg mL−1 isoniazid and 200 ng mL−1 ATc in combination (dark green squares), or no drug (black circles). INH/ATc were added at culture inoculation (day 0). The M. tuberculosis viability was measured by viable counts (CFU mL−1). All values represent the mean of three biological replicates. Error bars are ±SD. A three-way ANOVA was used to compare viability (CFU mL−1) between strains with or without ATc induction and/or with or without INH-exposure. *** =P ≤ .001. Minimum CFU mL−1 values were set at the limit of detection (10 CFU mL−1) at days 14, 17, 24, and 45.
DISCUSSION
Targeted repression of efpA using CRISPRi results in a loss of viability in M. tuberculosis
EfpA is a putative efflux pump that may modulate the effectiveness of TB drugs. We used an inducible CRISPRi system to repress efpA expression in M. tuberculosis (26). This resulted in a statistically significant loss in the viability of efpAKD and a triphasic response, that followed a similar trend to the response to INH; cidal, followed by static, and finally regrowth. This loss in viability during efpA repression can be explained by an initial loss of bacteria when efpA is fully repressed, followed by a phase of stalled growth during partial repression of efpA, and finally a period of recovery as the effects of ATc-induced efpA knockdown wane (Fig. 2 and 7 ). This profile is reflected in the levels of the efpA transcript over time. The efpA expression levels immediately decrease post-ATc addition and are at their lowest at day 1, upon which they start to increase. Despite this immediate decrease in efpA, the viability does not start to fall until day 2 post-exposure, which could be explained by the slow growth rate of M. tuberculosis and at least one to two doubling times required to see a population-wide effect. SEM provides supporting evidence for stalled replication (Fig. 4). Bacteria were observed with increased cell length, which appeared not to be dividing normally. Whole-genome transcriptome analyses highlighted reduced expression of genes at day 7 post-ATc induction, which supports a reduction in growth (ATP synthase: atpD–atpF and atpH and ribosomal proteins: rpmD and rpmI), cell division, (peptidoglycan biosynthesis: murD, murF, murX, and ald), and DNA replication and repair (reduction of ribonucleotides: guaB1, nrdB, nrdI, and nrdH). Rv0232, which encodes a TetR-like regulator, which is colocalized (immediately upstream) and coexpressed with nrdB, was also reduced in its expression (36). Efflux pumps have previously been shown to be associated with TetR regulators (37). Another membrane protein, mmpS5, which effluxes bedaquiline, was also downregulated in efpAKD, as was its repressor, Rv0678 (38, 39). It would be interesting to see if either of the regulators described here control the expression of efpA.
Repression of efpA prevented regrowth of M. tuberculosis during INH exposure
The focus of this study was to understand the significance of efpA in the susceptibility of M. tuberculosis to antibiotics, more specifically the recovery in vitro when the organism is exposed to INH. Repression of efpA combined with INH exposure caused a pronounced reduction in viability compared to INH alone and abolished the classic regrowth observed for M. tuberculosis when treated with INH in vitro (Fig. 7).
Regrowth after INH exposure has been observed in a variety of settings including in vitro batch log-phase cultures, continuous culture conditions, murine and guinea pig studies, and in patients with pulmonary tuberculosis (19, 21–24, 40, 41). We have previously sought to understand the reasons for this regrowth, using continuous culture, and addressed the hypothesis that regrowth was due to the persistence of drug-tolerant, slow-growing bacilli or the accumulation of INH drug-resistant mutants. We found through a combination of two studies that regrowth occurred irrespective of growth rate, INH concentration, or INH in combination with other antibiotics. INH resistance mutations only partially explained regrowth (with INH singly or in combination) (19, 23). Transcriptomics studies highlighted a variety of potential tolerance mechanisms, including upregulation of efflux pumps (19). We observed, using chemostat culture, that efpA was more highly expressed during the early bactericidal activity (EBA), at 2 days post-INH addition (19). In the current study, efpA repression led to a longer bactericidal phase as opposed to an increase in the rate of INH-induced killing. Given the upregulation of efpA observed during EBA in chemostats, it seems likely that efpA has a role in enabling the bacilli to survive and recover. Repression of efpA could lead to a reduction in the level of INH expelled from the cell, resulting in increased INH intracellular concentrations, thereby halting regrowth. In 2012, Schmalstieg et al. (25) postulated that efflux pump induction could lead to development of resistance-associated mutations. Alternative explanations could be that cell wall modifications observed after efpA repression may enhance the activity of INH. The transcriptomic analyses at day 7 after ATc addition and in the absence of INH indicated changes in cell wall processes and more specifically alterations in the biosynthesis of mycolic acids or their precursors (kasA, kasB, accD6, accA1, fas, accD4, pks13, and fadD32). INH targets the FAS-II biosynthesis pathway; therefore, a lower expression of these enzymes during efpA repression may be responsible for potentiating the activity of INH.
Is EfpA a good target for therapeutics?
This study provides further evidence that EfpA is essential in M. tuberculosis and may be a legitimate target for drug discovery. Our finding that repression of efpA prevents regrowth of M. tuberculosis after INH exposure is significant, especially for retaining the use of INH in current TB regimens. However, translating these in vitro results to in vivo infection will be challenging. In the first instance, it will be important to see how the inhibition of EfpA impacts INH efficacy in animal models of M. tuberculosis infection and ultimately whether relapse is reduced. The early bactericidal activity of INH reduces the bacterial burden in TB patient sputa substantially over the first few days of therapy; if this killing could be enhanced by an efflux inhibitor, then the use of INH for treatment of susceptible TB could be extended. EfpA was characterized in a recent chemical genetic screen (8, 42), which resulted in the development of two inhibitors that exhibited good activity against EfpA: BRD-8000.3 and BRD-9327. Further studies will determine whether the inhibition of EfpA is a viable strategy to augment multidrug therapy for tuberculosis by repressing efflux activities. These investigations would include experiments to understand the interplay between EfpA repression and the activity of other antibiotics in addition to INH.
ACKNOWLEDGMENTS
This work was funded by the Department of Health and Public Health England (UKHSA) PhD fund, UK. The views expressed in this publication are those of the authors and not necessarily those of the Department of Health or UKHSA.
The authors acknowledge Howard Tolley and Katherine Davies for scanning electron microscopy.
The study was conceptualized by A.H.R., S.J.W., and J.B.; technical support for construction of the CRISPRi system was provided by V.F. and S.L.K.; technical support for the INH exposure experiments was provided by C.W.M. and J.B.; experiments and data analyses were performed by A.H.R.; the manuscript was written by A.H.R. and J.B. and reviewed/edited by all authors.
Contributor Information
Joanna Bacon, Email: Joanna.Bacon@ukhsa.gov.uk.
Sean Wasserman, St. George's, University of London, London, United Kingdom.
DATA AVAILABILITY
UKHSA has an open-access policy; all supplementary data will be available online with the published article. Microarray data are available through Array Express, accession number E-MTAB-13755.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/aac.00261-24.
Tables displaying the log2 fold changes in gene expression and -log10 adjusted P-values for genes in M. tuberculosis efpAKD that has been induced with 200 ng mL -1 ATc compared to M. tuberculosis efpAKD without ATc induction, at days 0, 1, 3, and 7.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. WHO . 2023. Global tuberculosis report.
- 2. Shahi F, Khosravi AD, Tabandeh MR, Salmanzadeh S. 2021. Investigation of the Rv3065, Rv2942, Rv1258C, Rv1410C, and Rv2459 efflux pump genes expression among multidrug-resistant Mycobacterium tuberculosis clinical isolates. Heliyon 7:e07566. doi: 10.1016/j.heliyon.2021.e07566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li G, Zhang J, Guo Q, Jiang Y, Wei J, Zhao L, Zhao X, Lu J, Wan K. 2015. Efflux pump gene expression in multidrug-resistant Mycobacterium tuberculosis clinical isolates. PLoS ONE 10:e0119013. doi: 10.1371/journal.pone.0119013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kardan-Yamchi J, Kazemian H, Haeili M, Harati AA, Amini S, Feizabadi MM. 2019. Expression analysis of 10 efflux pump genes in multidrug-resistant and extensively drug-resistant Mycobacterium tuberculosis clinical isolates. Journal of Global Antimicrobial Resistance 17:201–208. doi: 10.1016/j.jgar.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 5. Machado D, Couto I, Perdigão J, Rodrigues L, Portugal I, Baptista P, Veigas B, Amaral L, Viveiros M. 2012. Contribution of efflux to the emergence of isoniazid and multidrug resistance in Mycobacterium tuberculosis. PLoS ONE 7:e34538. doi: 10.1371/journal.pone.0034538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodrigues L, Machado D, Couto I, Amaral L, Viveiros M. 2012. Contribution of efflux activity to isoniazid resistance in the Mycobacterium tuberculosis complex. Infect Genet Evol 12:695–700. doi: 10.1016/j.meegid.2011.08.009 [DOI] [PubMed] [Google Scholar]
- 7. Laws M, Jin P, Rahman KM. 2022. Efflux pumps in Mycobacterium tuberculosis and their inhibition to tackle antimicrobial resistance. Trends Microbiol 30:57–68. doi: 10.1016/j.tim.2021.05.001 [DOI] [PubMed] [Google Scholar]
- 8. Johnson EO, Office E, Kawate T, Orzechowski M, Hung DT. 2020. Large-scale chemical-genetic strategy enables the design of antimicrobial combination chemotherapy in mycobacteria ACS Infect Dis 6:56–63. doi: 10.1021/acsinfecdis.9b00373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sassetti CM, Boyd DH, Rubin EJ. 2003. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol 48:77–84. doi: 10.1046/j.1365-2958.2003.03425.x [DOI] [PubMed] [Google Scholar]
- 10. Griffin JE, Gawronski JD, Dejesus MA, Ioerger TR, Akerley BJ, Sassetti CM. 2011. High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog 7:e1002251. doi: 10.1371/journal.ppat.1002251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeJesus MA, Gerrick ER, Xu W, Park SW, Long JE, Boutte CC, Rubin EJ, Schnappinger D, Ehrt S, Fortune SM, Sassetti CM, Ioerger TR. 2017. Comprehensive essentiality analysis of the Mycobacterium tuberculosis genome via saturating transposon mutagenesis. mBio 8. doi: 10.1128/mBio.02133-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Minato Y, Gohl DM, Thiede JM, Chacón JM, Harcombe WR, Maruyama F, Baughn AD. 2019. Genomewide assessment of Mycobacterium tuberculosis conditionally essential metabolic pathways. mSystems 4. doi: 10.1128/mSystems.00070-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Doran JL, Pang Y, Mdluli KE, Moran AJ, Victor TC, Stokes RW, Mahenthiralingam E, Kreiswirth BN, Butt JL, Baron GS, Treit JD, Kerr VJ, Van Helden PD, Roberts MC, Nano FE. 1997. Mycobacterium tuberculosis efpA encodes an efflux protein of the QacA transporter family. Clin Diagn Lab Immunol 4:23–32. doi: 10.1128/cdli.4.1.23-32.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fisher MA, Plikaytis BB, Shinnick TM. 2002. Microarray analysis of the Mycobacterium tuberculosis transcriptional response to the acidic conditions found in phagosomes. J Bacteriol 184:4025–4032. doi: 10.1128/JB.184.14.4025-4032.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilson M, DeRisi J, Kristensen HH, Imboden P, Rane S, Brown PO, Schoolnik GK. 1999. Exploring drug-induced alterations in gene expression in Mycobacterium tuberculosis by microarray hybridization. Proc Natl Acad Sci U S A 96:12833–12838. doi: 10.1073/pnas.96.22.12833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boshoff HIM, Myers TG, Copp BR, McNeil MR, Wilson MA, Barry CE. 2004. The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism. Journal of Biological Chemistry 279:40174–40184. doi: 10.1074/jbc.M406796200 [DOI] [PubMed] [Google Scholar]
- 17. Waddell SJ, Stabler RA, Laing K, Kremer L, Reynolds RC, Besra GS. 2004. The use of Microarray analysis to determine the gene expression profiles of Mycobacterium tuberculosis in response to anti-bacterial compounds. Tuberculosis (Edinb) 84:263–274. doi: 10.1016/j.tube.2003.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gupta AK, Katoch VM, Chauhan DS, Sharma R, Singh M, Venkatesan K, Sharma VD. 2010. Microarray analysis of efflux pump genes in multidrug-resistant Mycobacterium tuberculosis during stress induced by common anti-tuberculous drugs. Microb Drug Resist 16:21–28. doi: 10.1089/mdr.2009.0054 [DOI] [PubMed] [Google Scholar]
- 19. Jeeves RE, Marriott AAN, Pullan ST, Hatch KA, Allnutt JC, Freire-Martin I, Hendon-Dunn CL, Watson R, Witney AA, Tyler RH, Arnold C, Marsh PD, McHugh TD, Bacon J. 2015. Mycobacterium tuberculosis is resistant to isoniazid at a slow growth rate by single nucleotide polymorphisms in katg codon ser315. PLoS One 10:e0138253. doi: 10.1371/journal.pone.0138253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Honeyborne I, McHugh TD, Kuittinen I, Cichonska A, Evangelopoulos D, Ronacher K, van Helden PD, Gillespie SH, Fernandez-Reyes D, Walzl G, Rousu J, Butcher PD, Waddell SJ. 2016. Profiling persistent tubercule bacilli from patient sputa during therapy predicts early drug efficacy. BMC Med 14:68. doi: 10.1186/s12916-016-0609-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Steenwinkel JEM, de Knegt GJ, ten Kate MT, van Belkum A, Verbrugh HA, Kremer K, van Soolingen D, Bakker-Woudenberg IAJM. 2010. Time-kill kinetics of anti-tuberculosis drugs, and emergence of resistance, in relation to metabolic activity of Mycobacterium tuberculosis. J Antimicrob Chemother 65:2582–2589. doi: 10.1093/jac/dkq374 [DOI] [PubMed] [Google Scholar]
- 22. Gumbo T, Louie A, Liu W, Ambrose PG, Bhavnani SM, Brown D, Drusano GL. 2007. Isoniazid’s bactericidal activity ceases because of the emergence of resistance, not depletion of Mycobacterium tuberculosis in the log phase of growth. J Infect Dis 195:194–201. doi: 10.1086/510247 [DOI] [PubMed] [Google Scholar]
- 23. Hendon-Dunn CL, Pertinez H, Marriott AAN, Hatch KA, Allnutt JC, Davies G, Bacon J, Bacon J. 2019. Regrowth of Mycobacterium tuberculosis populations exposed to antibiotic combinations is due to the presence of isoniazid and not bacterial growth rate. Antimicrob Agents Chemother 63:1–10. doi: 10.1128/AAC.00570-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ahmad Z, Klinkenberg LG, Pinn ML, Fraig MM, Peloquin CA, Bishai WR, Nuermberger EL, Grosset JH, Karakousis PC. 2009. Biphasic kill curve of isoniazid reveals the presence of drug-tolerant, not drug-resistant, Mycobacterium tuberculosis in the guinea pig. J Infect Dis 200:1136–1143. doi: 10.1086/605605 [DOI] [PubMed] [Google Scholar]
- 25. Schmalstieg AM, Srivastava S, Belkaya S, Deshpande D, Meek C, Leff R, van Oers NSC, Gumbo T. 2012. The antibiotic resistance arrow of time: efflux pump induction is a general first step in the evolution of mycobacterial drug resistance. Antimicrob Agents Chemother 56:4806–4815. doi: 10.1128/AAC.05546-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Singh AK, Carette X, Potluri LP, Sharp JD, Xu R, Prisic S, Husson RN. 2016. Investigating essential gene function in Mycobacterium tuberculosis using an efficient CRISPR interference system. Nucleic Acids Res 44:e143. doi: 10.1093/nar/gkw625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Larson MH, Gilbert LA, Wang X, Lim WA, Weissman JS, Qi LS. 2013. CRISPR interference (Crispri) for sequence-specific control of gene expression. Nat Protoc 8:2180–2196. doi: 10.1038/nprot.2013.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Faulkner V, Cox AA, Goh S, van Bohemen A, Gibson AJ, Liebster O, Wren BW, Willcocks S, Kendall SL. 2020. Re-sensitization of Mycobacterium smegmatis to Rifampicin using CRISPR interference demonstrates its utility for the study of non-essential drug resistance traits. Front Microbiol 11:619427. doi: 10.3389/fmicb.2020.619427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gibson AJ, Passmore IJ, Faulkner V, Xia D, Nobeli I, Stiens J, Willcocks S, Clark TG, Sobkowiak B, Werling D, Villarreal-Ramos B, Wren BW, Kendall SL. 2021. Probing differences in gene essentiality between the human and animal adapted lineages of the Mycobacterium tuberculosis complex using TnSeq. Front Vet Sci 8:760717. doi: 10.3389/fvets.2021.760717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Parish T, Brown AC. 2009. Mycobacteria protocols. In Methods in molecular biology. Vol. 101. Journal of Medical Microbiology. [Google Scholar]
- 31. Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE 3rd, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537–544. doi: 10.1038/31159 [DOI] [PubMed] [Google Scholar]
- 32. Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. 2015. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43:e47. doi: 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang DW, Sherman BT, Lempicki RA. 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57. doi: 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- 34. Kapopoulou A, Lew JM, Cole ST. 2011. The mycobrowser portal: a comprehensive and manually annotated resource for mycobacterial genomes. Tuberculosis (Edinb) 91:8–13. doi: 10.1016/j.tube.2010.09.006 [DOI] [PubMed] [Google Scholar]
- 35. Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH image to Imagej: 25 years of image analysis. Nat Methods 9:671–675. doi: 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stiens J, Tan YY, Joyce R, Arnvig KB, Kendall SL, Nobeli I. 2023. Using a whole genome co-expression network to inform the functional characterisation of predicted genomic elements from Mycobacterium tuberculosis transcriptomic data. Mol Microbiol 119:381–400. doi: 10.1111/mmi.15055 [DOI] [PubMed] [Google Scholar]
- 37. Balhana RJC, Singla A, Sikder MH, Withers M, Kendall SL. 2015. Global analyses of TetR family transcriptional regulators in mycobacteria indicates conservation across species and diversity in regulated functions. BMC Genomics 16:479. doi: 10.1186/s12864-015-1696-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Andries K, Villellas C, Coeck N, Thys K, Gevers T, Vranckx L, Lounis N, de Jong BC, Koul A. 2014. Acquired resistance of Mycobacterium tuberculosis to bedaquiline. PLoS One 9:e102135. doi: 10.1371/journal.pone.0102135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saeed DK, Shakoor S, Razzak SA, Hasan Z, Sabzwari SF, Azizullah Z, Kanji A, Nasir A, Shafiq S, Ghanchi NK, Hasan R. 2022. Variants associated with bedaquiline (BDQ) resistance identified in Rv0678 and efflux pump genes in Mycobacterium tuberculosis isolates from BDQ Naïve TB patients in Pakistan. BMC Microbiol 22:62. doi: 10.1186/s12866-022-02475-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jindani A, Aber VR, Edwards EA, Mitchison DA. 1980. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am Rev Respir Dis 121:939–949. doi: 10.1164/arrd.1980.121.6.939 [DOI] [PubMed] [Google Scholar]
- 41. Klemens SP, Cynamon MH. 1996. Activity of KRM-1648 in combination with isoniazid against Mycobacterium tuberculosis in a murine model. Antimicrob Agents Chemother 40:298–301. doi: 10.1128/AAC.40.2.298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Johnson EO, LaVerriere E, Office E, Stanley M, Meyer E, Kawate T, Gomez JE, Audette RE, Bandyopadhyay N, Betancourt N, et al. 2019. Large-scale chemical–genetics yields new M. tuberculosis inhibitor classes. Nature 571:72–78. doi: 10.1038/s41586-019-1315-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables displaying the log2 fold changes in gene expression and -log10 adjusted P-values for genes in M. tuberculosis efpAKD that has been induced with 200 ng mL -1 ATc compared to M. tuberculosis efpAKD without ATc induction, at days 0, 1, 3, and 7.
Data Availability Statement
UKHSA has an open-access policy; all supplementary data will be available online with the published article. Microarray data are available through Array Express, accession number E-MTAB-13755.