Abstract
Introduction:
Fusobacterium necrophorum, a human and animal pathogen, is the primary etiologic agent of bovine liver abscesses and a driving factor for prophylactic antibiotic use in the fed cattle industry. Considering calls to reduce agricultural antibiotic use, we isolated phages capable of killing F. necrophorum as an alternative or complementary biocontrol strategy.
Methods:
Six novel phages (φFN37, φRTG5, φKSUM, φHugo, φPaco, and φBB) were isolated from rumen fluid or ruminal F. necrophorum isolates and subjected to host range testing on both F. necrophorum subspecies. Four F. necrophorum subspecies, necrophorum phages, were tested for cross-resistance and host growth inhibition individually and in pairs. Additionally, genomic sequencing, annotation, and analysis were performed.s
Results:
Four of six isolated phages were able to form lysogens, although all six contained lysogeny-related genes. φKSUM and φBB, did not form lysogens and were able to infect both subspecies. Four phages could infect F. necrophorum 8L1 (a liver abscess model challenge strain) in vitro. Genomic analysis showed that these phages belong to class Caudoviricetes with genome sizes ranging from 35 kbp to 111 kbp and GC values ranging from 26% to 36% and have extremely limited similarity to other deposited phage genomes infecting Fusobacterium or other genera.
Conclusions:
Although all phages isolated contained sequences bearing similarities to genes implicated in lysogeny, the four selected for use in cocktails showed potential in inhibiting host growth, with several demonstrating promising attributes for biocontrol and therapeutic applications. Phage cocktails that may offer enhanced antibacterial activity were also identified, indicating the potential of some lysogenic phages to be adapted for biocontrol or therapeutic purposes when lytic phages are difficult to obtain.
Introduction
There is a pressing need for reliable and precise microbial control technologies to target specific animal pathogens while minimizing potential unintended consequences associated with the excessive use of antibiotics in agriculture.1 In this context, bacteriophages (phages) have attracted considerable attention for their potential use as animal feed additives for biocontrol and microbiome modulation applications.2,3 Our study focuses on isolation, characterization, and potential application of phages against Fusobacterium necrophorum, a Gram-negative pathogen implicated in hepatic abscessation in feedlot cattle4,5 as well as other necrotic infections in humans6 and animals.7
F. necrophorum contains two subspecies that are normal inhabitants of the alimentary tract of ruminants: subsp. necrophorum (biotype A) and subsp. funduliforme (biotype B). The two subspecies differ morphologically, biochemically, and biologically, with the subsp. necrophorum observed to be more virulent and isolated more frequently from infections.8 To date, only one phage has been reported to infect F. necrophorum,9 although its genome was not sequenced.
Using either mitomycin induction or ruminal fluid enrichment, we isolated six double-stranded DNA (dsDNA) phages that infect one or both F. necrophorum subspecies, and evaluated their host ranges, inhibitory efficacy, and biocontrol potential using F. necrophorum subsp. necrophorum 8L1, a strain used in hepatic abscess model studies.10 We also conducted detailed genomic analyses to identify possible virulence factors or antibiotic resistance genes, investigated the phages’ lysogenic potential and to predict their potential phylogeny. Interestingly, nucleic acid similarities to deposited phage genomes were sparse. Although no professionally lytic11 phages were found, this study lays the groundwork for the potential application of phages as part of an integrated approach to controlling F. necrophorum within the agricultural sector and beyond.
Materials and Methods
Sampling and storage of rumen fluid. Over 100 rumen fluid samples were collected from a beef processing plant in Amarillo, Texas, the Beef Cattle Research Center (Kansas State University) and the USDA Livestock Issues Research Unit (Lubbock, TX). Samples shipped overnight on ice were processed within 24 h and stored at −80°C in 10% dimethyl sulfoxide.
Bacterial strains, new strain isolation, and culture conditions. Fusobacterium necrophorum subspecies necrophorum strain ATCC 25286 was obtained from the American Type Culture Collection (ATCC, Manassas, VA). Twelve strains (both subspecies) were kindly shared by Dr. TG Nagaraja (Kansas State University) and six additional strains were isolated by our laboratory as previously described (Supplementary Table S1).12 Fusobacterium strains were grown at 37°C anaerobically in Hungate tubes (Chemglass Life Sciences, Cineland, NJ) containing Brain Heart Infusion (BHI) broth (Teknova, Hollister, CA) supplemented with 1 mg/L resazurin, 5 g/L peptone, and 5 g/L yeast extract.
Mitomycin induction. Mitomycin C (≥98.0%, TCI America), a genotoxic antibiotic, was used to induce two prophages13 from F. necrophorum isolates FN37 and RTG5 by adding 0.5 µg/mL to cultures when they reached logarithmic phase (OD600 = 0.2-0.3) and allowing them to continue growing at 37°C. After an overnight incubation, lysates were centrifuged (10,000 g for 10 min) and filter sterilized using a 0.22 μm polyvinylidene fluoride (PVDF) filter, then used for phage isolation or polyethylene glycol (PEG) precipitated (detailed in the phage isolation, purification and production section below) for later use.
Ruminal content enrichment. Peptone Yeast (PY) broth14 was amended with 50 mM maltose and a selective antibiotic mixture:15 josamycin, vancomycin, and norfloxacin (at 3, 4, and 1 μg/mL, respectively, added after autoclaving; hereafter referred to as JVN) was used to enrich for Fusobacterium species and their phages. Briefly, 100 μL aliquots of rumen digesta were added by syringe to presterilized Hungate tubes containing this enrichment medium and tubes were incubated overnight at 37°C. Subsequently, enrichments were sterilized identically to mitomycin inductions (above). Enrichment lysates were then used for phage isolation or PEG precipitated (detailed in the phage isolation, purification and production section below) for later use.
Phage isolation, purification, and production. Phages were isolated by spotting lysates on a double-layer assay (DLA)l.16 BHI agar plates were overlaid with BHI top agar (0.4% agarose) supplemented with 1% cysteine-HCL,17 Oxyrase®, and lysed horse blood (5% of each by volume) and 5% of host bacteria. Assays were incubated overnight at 37°C under anaerobic conditions in Vacu-Quik Jar System (Almore International, Hickory NC). Clear plaques or zones of clearance were harvested and amplified in susceptible host(s). Phages were purified by plaque assay so that a single plaque could be picked using a sterile 1-mL micropipette tip. Agar plugs containing single plaques were resuspended in SM buffer [50 mM Tris-HCl (pH 7.5), 0.1 M NaCl, 8 mM MgSO4, 0.01% w/v gelatin] and amplified in the corresponding host. Host range was assessed through spot tests on a Fusobacterium strain library (Supplementary Table S1). A small collection of Fusobacterium varium strains were also screened, but no activity was detected. Phages were amplified using DLA or using liquid cultures infected with phage. Phage titers were assayed through serial dilution and spotting on DLA. Clarified phage stocks were concentrated by the addition of 1:1 volume of polyethylene glycol (PEG) solution (20% PEG 8000, 2 M NaCl). After incubation overnight at 4°C, the PEG-phage lysate mixture was pelleted at 12,000 g for 30 min and resuspended in SM buffer. Phage stocks were stored at 4°C under anaerobic conditions and shown to retain activity for over six months.
Transmission electron microscopy
Phage particles were imaged by transmission electron microscopy (TEM) using formvar Carbon 400 mesh grids (Ted Pella Inc., 01754-F). Before grid preparation, 7.5 mg/mL uranyl formate (Polysicences Inc, 24762) was prepared in a fume hood by pipetting boiling Milli-Q water onto uranyl formate in a 15 mL conical tube. The tube was sealed with parafilm and vortexed to solubilize the uranyl formate, then allowed to cool to room temperature. The uranyl formate was then passed through a 0.2 um PES filter (Thermo Scientific, 725–2520) using a 10 mL BD syringe. Grids were glow discharged in an EMS100× glow discharge chamber with a coating current of 10 mA for 1 min. Around 3 uL of sample was applied to each grid for 1 min. The sample was then removed from the grid through capillary action by touching the side of the grid to a filter article (Whatman, 1002-150). While the sample was absorbing, one 30 uL drop of water and two 30 uL drops of uranyl formate were pipetted onto parafilm. After the sample was removed from the grid, the sample side of the grid was touched to the water drop, touched to the first uranyl formate drop, then rolled on top of the second uranyl formate drop for 30 s, with liquid being removed between each step using Whatman filter article. Grids were allowed to dry overnight in a Ted Pella Inc. grid box, then imaged using a JEOL JEM-1400Flash transmission electron microscope equipped with a tungsten filament and 15 megapixel AMT NanoSprint15 sCMOS camera.
Phage–host growth curves. Mid-log bacterial cultures were adjusted to an OD600 = 0.2 and added to BHI medium supplemented with 1% peptone, 0.1% freshly prepared cysteine-HCl,17 and 5% Oxyrase®, to a concentration of 5 × 106 CFU/mL. Depending on the experiment, phages were added to individual hosts at a multiplicity of infection (MOI) of 1, 0.1, 0.01. When evaluated in concert, the phages were mixed in a 1:1 ratio. Plates were sealed using qPCR grade plate films to prevent gas exchange, and incubated at 37°C. All growth curve data were collected for at least 24 h in triplicate in a minimum of two independent experiments on a Tecan Infinite M Plex (Tecan Group Ltd., Männedorf, Switzerland).
Statistical analysis. Data based on two independent experiments conducted in triplicate were analyzed using the two-tailed t-test in Microsoft Excel. Results were considered significant when p < 0.05. Phage Scores (PS) and Phage Total Scores (PTS), a mathematical quantification of lytic activity at the population level, were calculated as previously described using GraphPad Prism 9.18 Briefly, a Phage Score compares optical density of a phage-exposed culture over time to a control measurement. A benefit of this method is it compensates for the heterogeneity and stochasticity of phage infection dynamics that are observed at a single-cell level,19 which can cause discrepancies between replicates even under identical conditions and wide fluctuations over time. Additionally, Phage Score values obtained at several MOIs can be collapsed into a single value, PTS, an attractive quality when examining host–phage dynamics under various conditions.20,21 The PTS also has special sensitivity to the efficiency of the lowest dilution of bacteriophage strain by design, giving a higher value to bacteriophages which work efficiently at lower MOI, which is integral for considering phages for use in therapy.22,23 A further explanation of the methodology is included in the supplementary material.
Evaluation of phage-induced cross-resistance. After phage exposure, surviving F. necrophorum 8L1 isolates (putatively bacteriophage-insensitive mutants, BIMs) were assessed for phage cross-resistance. Resistant strains were isolated from liquid culture exposed to phage at an initial MOI > 1.0 or from mesas formed on DLA spotted with phage lysate over 3–5 days, and survivors were streaked on JVN plates and restreaked three times to eliminate residual phages.24 A single colony isolate was used to test phage sensitivity through DLA, with concurrent testing of the original FN8L1 as a positive control. Suspected lysogens were subjected to mitomycin induction and tested through PCR for phage integration. A minimum of three surviving isolates per phage were evaluated.
Genomic DNA extraction and quantification. DNA was extracted through modification of a published method to optimize for long DNA fragments.25 A cleaning and concentration step was added to improve extracted DNA quality using the Zymo Genomic DNA Clean & Concentrator-10 Kit (Irvine, CA, USA) according to the manufacturer’s instructions. Quantification of DNA concentration and quality was performed using a NanoDrop DN-1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, Massachusetts, United States).
Genome sequencing, assembly, and annotation. To obtain long reads, genome sequencing libraries were prepared using high-molecular-weight DNA using the Ligation Sequencing Kit (SQK-LSK109, Oxford Nanopore Technologies) and sequenced on a MinION sequencer (Oxford Nanopore Technologies, Oxford, United Kingdom) using a Flongle adapter and flow cell (R.9.4 chemistry) according to the manufacturer’s instructions. Reads were base-called and demultiplexed using MinKNOW and trimmed with porechop before assembly. Illumina sequencing was conducted by Molecular Research (MR DNA, Shallowater, TX) to obtain short reads for hybrid assembly (2 × 250 bp, 10 million reads).
Both long-read and hybrid assemblies were generated using Unicycler v0.5.026 and were compared to bacterial genome sequences for accuracy when possible (prophage regions). Assemblies were then polished with the long-read dataset using Racon and Medaka, and the short-read dataset using Polypolish. Viral genomes were initially annotated using Pharokka v1.3.227 and then manually refined using BLASTx within ElasticBLAST 1.1.0,28 using a minimum bit score of 50.29 Genomes were submitted to GenBank30 under the following accessions: OR492271, OR492272, OR492273, OR492274, OR492275, and OR492276. Genomes and annotations were visualized using Proksee31 (Supplementary Figs. S1–6).
Bioinformatic analysis and OrthoANI. BLASTn queries for each phage were performed using the NCBI nr database to identify any similarities to deposited phage or bacterial genomes. The taxonomic relationships between the six isolated phages and a collection of reference genomes were inferred using the web-based Virus Classification and Tree Building Online Resource (VICTOR) (https://victor.dsmz.de), a method for the genome-based phylogeny and classification of prokaryotic viruses.32 All pairwise comparisons of the nucleotide sequences as well comparison of the translation to amino acid sequences were conducted using the Genome-BLAST Distance Phylogeny (GBDP) method33 under settings recommended for prokaryotic viruses. The resulting intergenomic distances were used to infer a balanced minimum evolution trees with branch support through FASTME, including SPR postprocessing34 using formula D0, D6 (nucleotide), and D4 (amino acid). Branch support was inferred from 100 pseudo-bootstrap replicates. Trees were rooted at the midpoint35 and visualized with ggtree.36 Taxon boundaries at the species, genus, and family level were estimated with the OPTSIL program,37 the recommended clustering thresholds,32 and an F value (fraction of links required for cluster fusion) of 0.5.38 The reference genomes, totaling 75, were selected based on the results of NCBI BLAST39 searches limited to preferably complete genomes in the Virus nt database, excluding uncultured/environmental samples seeking highly similar (megablast) and more dissimilar (discontiguous megablast) sequences, with a limit set to 100 matches for each new phage.
OrthANI analysis was performed using the CJ Bioscience’s Orthologous Average Nucleotide Identity Tool (OAT) Graphical User Interface. Briefly, OAT uses OrthoANI to measure overall similarity between two genome sequences by fragmenting the genomes into 1020 bp-long pieces and calculating the average nucleotide identities in an orthologous manner.40
Results
Phage–host ranges and morphologies
Phages φFN37 and φHugo were isolated on F. necrophorum subsp. necrophorum strains (FNC and FN38, respectively) and exhibited lytic activity exclusively against F. necrophorum subsp. necrophorum strains in host range testing. Similarly, φPaco and φRTG5 were restricted to their subspecies of isolation, F. necrophorum subsp. funduliforme (strains FF34 and KL3, respectively). φKSUM and φBB were able to infect both subspecies (Table 1). TEM imaging (Fig. 1) revealed three of the phages (φFN37, φPaco, and φKSUM) to exhibit siphovirus-like morphology, with large icosahedral heads and long, thin tails. φKSUM was particularly large, with a capsid measuring approximately 85 nanometers (nm) in diameter with a tail over 300 nm in length. φHugo and φRTG5 both had thicker, myovirus-like tail sheaths attached to their capsids, and during imaging, often exhibited a tendency to aggregate with compromised viral particles with empty capsids or other cellular detritus. Finally, φBB was the most difficult to visualize using TEM, with the majority of particles being broken or empty. However, when full capsids were observed, no tail was seen, although some images suggested a short stubby process coming off the capsid, suggesting φBB is podovirus-like in morphology.
Table 1.
Summary of Fusobacterium Phage–Host Ranges
| Subspecies | Strain | Obtained from | φFN37a | φHugob | φPacob | φRTG5a | φKSUMb | φBB |
|---|---|---|---|---|---|---|---|---|
| necrophorum | 25286 | ATCC | + | + | - | - | + | + |
| necrophorum | A | KSU | + | - | - | - | + | + |
| necrophorum | C | KSU | + | + | - | - | + | + |
| necrophorum | FN38 | KSU | - | + | - | - | + | - |
| necrophorum | 8L1 | KSU | + | + | - | - | + | + |
| funduliforme | FF16 | KSU | - | - | - | + | - | - |
| funduliforme | FF34 | KSU | - | - | - | + | - | + |
| funduliforme | KL3 | laboratory | - | - | + | + | + | - |
| funduliforme | RTG5 | laboratory | - | - | - | - | - | - |
Phage obtained through mitomycin induction.
Phage obtained from ruminal enrichment.
FIG. 1.
Transmission electron microscope images of six Fusobacterium necrophorum phages. The bacteriophages, φKSUM, φPaco, and φFN37, as illustrated in the upper row, exhibit the hallmark morphology of siphoviruses, characterized by their elongated, slender tails. φRTG5 and φHugo, depicted in the lower right and center, respectively, display morphological features consistent with myoviruses, notably, a thick tail sheath. Imaging φBB was challenging compared with the other phages; after staining and microscopic examination, it was observed that the majority of the capsids were compromised. Notably, no tailed particles were identified; only capsid structures were observed, accompanied by a scarcely discernible, fuzzy appendage off of the capsid, suggesting it is a podovirus.
Phage isolates repressed native host growth to varying degrees
All phages, except φPaco, significantly repressed the growth of their isolation and production hosts (measured by OD600) at MOIs of 0.1 and 0.01 for at least several hours and up to 24 h (Fig. 2a). φFN37 repressed the growth of FNC for 14 h at a MOI of 0.1 and 21 h at an MOI of 0.01, which is a pattern often observed with temperate phages. The final peak OD600 of phage-treated FNC cultures were about 10% lower than the control (no phage), suggesting a potential fitness cost to phage resistance or carriage, although this was not statistically significant (p > 0.05). φHugo reduced the growth of FN38 for the full 24-h study duration (p < 0.05), with the final OD600 at MOI = 0.1 19% below the control (p < 0.05). From 9–20 h, φHugo inhibited growth by ∼85%, with regrowth observed afterward. A lower MOI (0.01) did not achieve a similar level of growth repression over the course of observation but did exhibit lower terminal OD600 value (56% below control). φRTG5 suppressed the growth of its host FF34 in liquid culture to a significantly lower extent (p < 0.05), with a final OD600 after 24 h that was 51% (MOI = 0.1) and 48% (MOI = 0.01) of that of the controls. φKSUM and φBB also suppressed the growth of their host (ATCC 25286) to a statistically significant extent for 24 h, although in both cases, the optical density began to increase after 20 h. The resulting final growth reductions at the two MOIs (0.1, 0.01) were 20% and 53% for φKSUM and 74% and 63% for φBB. Phage Scores and Phage Total Scores18 were determined for all six phages (Fig. 2b). φBB had the highest Phage Total Score of 0.82, followed by φKSUM (0.72), φHugo (0.47), φRTG5 (0.16), φFN37 (0.11), and finally φPaco (0.03).
FIG. 2.
Growth inhibition of five F. necrophorum strains by six novel Fusobacterium phages. (A) The growth of susceptible F. necrophorum strains (indicated in parentheses) was monitored over 24 h in the presence or absence of phages (multiplicity of infection [MOI] 0.1 or 0.01). Although all six phages presented a plaquing behavior on their isolation hosts in DLA, only five of the six phages showed inhibition of their hosts under liquid culture conditions. (B) Phage Score values and Phage Total Score analysis. The Phage Scores (PS) and Phage Total Scores (PTS, indicated by + symbol) varied greatly among the phages (18). Three of the phages, φRTG5, φPaco, and φFN37 had Pts below 0.2, but the remaining three showed much higher performance, suggesting high suitability of φHugo, φKSUM, and φBB as microbial control agents against these strains.
Growth inhibition of challenge strain 8L1
F. necrophorum subspecies necrophorum 8L1 is a challenge strain of high pathogenicity that is currently used in a liver abscess animal infection model.10 Four of the six phages tested infected 8L1 in DLA spot tests as evidenced by plaque clearances (Table 1) and were evaluated for growth inhibition in liquid culture conditions at MOIs of 5, 1, 0.1, and 0.01. All but φHugo showed inhibition for at least some portion of 24 h of monitored growth (Fig. 3a). φKSUM repressed the growth of F. necrophorum 8L1 at all MOIs to a large extent until regrowth began after 10 h, but OD600 remained below that of the control group until 18 h. The best long-term performance was observed at a MOI of 0.01 with a final average OD600 18% below that of controls. φFN37demonstrated the best killing efficiencies at MOIs below 5, resulting in final OD600 values of 35%, 50%, and 51% for MOIs of 1, 0.1, and 0.01, respectively. Most notably, φFN37 showed better inhibition of strain 8L1 than of its production strain, FNC, achieving a higher Phage Score at MOIs of 0.1 and 0.01 and a higher Phage Total Score. φBB repressed growth of the test strain at all MOIs over the course of the entire 24 h, with the final OD600 reduced by at least 70%. Phage Scores and Phage Total Scores for the four phages are graphically summarized in Figure 3b. φKSUM had the highest PTS (0.62), followed by φBB (0.57), φFN37 (0.49), and Hugo (given a score of zero as no observed or statistically significant inhibition occurred).
FIG. 3.
Growth inhibition of challenge strain F. necrophorum 8L1 by four phages at various MOIs. (A). Four phages were observed to have activity on test strain 8L1 through DLA as evidenced by plaquing clearances, and thus were evaluated for killing efficiency at several MOIs (5, 1, 0.1, 0.01). φHugo had no effect on this strain, while φBB and φKSUM demonstrated similar behavior to that observed in their production strains. Most notably, φFN37 showed better inhibition of the test strain (seen in Fig. 1) than its original host, achieving a higher Phage Scores and a higher Phage Total Score (0.49 vs 0.11) (B). φBB and φKSUM were most effective at suppressing growth of F. necrophorum strain 8L1 over a 24-h period of monitored growth various MOIs, resulting in the highest PS and PTS.
Cross-resistance and performance of phage pair cocktails
We evaluated the cross-resistance and synergistic capacity of the four phages (Table 2) able to infect the challenge strain as evidenced by clearances in spot testing. All bacterial clones, which were isolated after phage exposure to φBB and after regrowth was observed as indicated by an increase in culture optical density or mesa formation, were found to be always susceptible to subsequent φBB infection in spot tests and remained sensitive to all other F. necrophorum phages. Most bacterial isolates that survived φKSUM infection remained sensitive to φKSUM in subsequent assays as well as the other phages, indicating that φKSUM resistance does not arise easily. Putative φKSUM bacteriophage-insensitive mutants, when they did arise, were always resistant to φHugo and φFN37. Since φFN37 and φHugo BIMs only occasionally produced cross-resistance to φKSUM and each other, they were used in the second cocktail. Furthermore, φFN37 BIMs were occasionally more sensitive to φKSUM and to φHugo. Thus, we selected two pairs of phages for cocktail combinations: φKSUM + φBB and φHugo + φFN37 (Fig. 4). We also evaluated other possible phage combinations to identify synergistic or antagonistic dynamics, which were not observed. While all cocktail combinations repressed the growth of 8L1 to a statistically significant extent, (p < 0.05), the best combined performance corresponded to the φBB and φKSUM pair, which were able to repress the growth of 8L1 for the full 24 h at all three MOIs of 1.0, 0.1, and 0.0 by 76%, 69%, and 66%, respectively, achieving a PTS of 0.62.
Table 2.
Summary of Phage Cross-Resistance in F. necrophorum Subspecies necrophorum 8L1
| Prior phage exposure | φFN37 | φHugo | φKSUM | φBB |
|---|---|---|---|---|
| φFN37 | A | S | S | N |
| φHugo | S | A | N | N |
| φKSUM | A | A | A | N |
| φBB | N | N | N | N |
A, Always Resistant; S, Sometimes Resistant, N, Never Resistant.
FIG. 4.
Rational cocktail development was predicted by cross-resistance, and no synergistic dynamics were observed. (A) Phage pairs added in a 50:50 ratio were monitored at combined MOIs of 1, 0.1, and 0.01 and monitored over 24 h. Although cross-resistance analysis suggested only two combinations of phages would optimally result in two unique phage cocktails of two phages each, we evaluated two other combinations to validate this assumption. (B) According to the PTS, the best performing combination was, as predicted, φKSUM and φBB, followed by φBB and φHugo. The least successful pairing was φBB and φFN37.
Genome sequencing, annotation, and putative classification
Although gene annotation of phages is difficult due to significant variations in phage protein sequences,41,42 we employed Pharokka and a selection of other tools to generate consensus annotations and characterize the phages directly from their genomes. The six isolates were unenveloped tailed dsDNA phages (class Caudoviricetes), with genome sizes ranging from about 35 kbp up to about 111kbp, and GC content varied from 26–36% (Table 3). All phages were classified as temperate by BacPhlip43 and PhageAI.44 PhageTerm45 was used to predict each phage packaging strategy; only φFN37 was given a definitive classification of headful (pac) packaging and all others were called as “other,” although φHugo was also classified as headful using Li’s method.46 All six genomes contained protein-coding sequence (CDS) regions aligning to hypothetical proteins or various specific domains of unknown function (DUF) found primarily in Fusobacterium necrophorum subsp. funduliforme strain F1260 and F1291 genomes (Accessions CP019306.1 and CP018196.1). All assemblies included hallmark phage genes, e.g., endonucleases,47 terminases, portal proteins, capsid proteins, head–tail connectors and adapters, baseplate/tail proteins, and holins. Encouragingly, the distribution of these genes matched our TEM observations: the two myovirus morphology phages (φRTG5 and φHugo) contained sequences identified as baseplate and tail sheath components, while the three siphovirus morphology phages (φKSUM, φPaco, and φFN37) did not, possessing only tail tube proteins. Interestingly, φBB’s annotation contained a head–tail adaptor, but no tail protein, which may explain the lack of observed tail. Three of the six phages also contained DNA methyltransferases, suggesting a possible defense mechanism against the host-encoded restriction endonucleases.48 YopX was detected in all but one of the phages examined in this study (φRTG5); YopX is believed to be involved in prophage induction.49
Table 3.
Summary of Phage Genome Characteristics
| Phage | Genome size (bp) | GC content (%) | No.CDS | CDS density (%) | No. tRNAs | No. hypothetical protein | Highest scoring BLASTn results (accession)a | |
|---|---|---|---|---|---|---|---|---|
| φFN37 | 85,015 | 29.90 | 163 | 94.52 | 2 | 114 |
Fusobacterium phage FNU_1P, complete genome (MZ503612.1) |
|
| φHugo | 34,681 | 33.35 | 64 | 93.32 | 0 | 27 |
Fusobacterium phage JD-Fnp5, complete genome (ON464762.1) |
|
| φRTG5 | 37,037 | 35.04 | 69 | 95.87 | 0 | 43 |
Fusobacterium phage Funu1, complete genome (KR131710.1) |
|
| φPaco | 43,165 | 36.62 | 62 | 95.03 | 1 | 32 |
Fusobacterium phage FNU_1P, complete genome (MZ503612.1) |
|
| φKSUM | 111,401 | 26.39 | 195 | 91.98 | 1 | 144 |
Fusobacterium phage Fnu1, complete genome (NC_055035.1) |
|
| φBB | 36,404 | 33.62 | 60 | 95.04 | 0 | 38 |
Fusobacterium phage vB_FnuS_FNU4, complete genome (OQ808964.1) |
Obtained using NCBI Blast with the following parameters: limited to the Viruses nt database, complete genomes only, using discontiguous megablast.
The genome of φFN37 (Supplementary Fig. S1) was found to be 85,015bp long, 29.90% GC content, and to contain 163 CDS, 70% of which were identified only as hypothetical proteins. Annotation identified two tRNAs, a queuine tRNA-ribosyltransferase,50 a phosphoadenosine phosphosulfate reductase,51,52 components of type III (AbiQ/ToxN) and type I (SymeE) toxin–antitoxin systems53 and prohibitin family protein,54 in addition to a suite of genes associated with the lysogeny, such as a CI-like repressor and an integrase.
The φHugo annotation (34,681bp, 33.35% GC, 64 CDS) had the most nonhypothetical putative genes identified, with more than 57% of the CDS classified as other than a hypothetical protein (Supplementary Fig. S2). When BLASTed against the NCBI nucleotide database, φHugo had the highest query coverage of the six phages, with 29 matches making up 86% of the genome (93.58% identity) attributed to Fusobacterium necrophorum subsp. funduliforme strain F1260. The largest alignments included over half of the genome, containing a phage terminase (AYV95751.1) and portal protein. Other features detected were a site-specific integrase, two recombinases (YqaJ, recT), and several other genes recognized as repressors and regulators (CopG,55 KilAC,56,57 Xre58) corroborating prediction software indicating that phage was capable of a temperate lifestyle. φHugo lacks a recognizable DNA polymerase, primase, or helicase, but has several genes involved in DNA repair and modification (recA and a IrrE/ImmA family metallo-endopeptidase59).
φKSUM (Supplementary Fig. S3) was both the largest (111,401 bp) and had the lowest GC content (26.39%) among the phages. This phage had the largest percentage of its annotations (195 CDS total) identified only as hypothetical proteins (74%). Annotation identified one tRNA and many proteins putatively involved in metabolism and repair (e.g., Sak460). It also contained a gene identified as IS200/IS605 family element RNA-guided endonuclease TnpB,61 which is suggested to be a predecessor of the CRISPR-Cas9/Cas12 nucleases. Ant and KilAC regulation machinery and transposases are also present. Annotation also indicated similarities to phage genes and morons also found in Fusobacterium nucleatum phage Fnu1: Methyl-CpG-binding domain containing protein 9, a polyscaccharide biosynthesis protein, radical SAM protein, a heat shock protein chaperone homolog DnaJ,62 and NrdD-like anaerobic ribonucleotide reductase.63 Other genes of note included PhoH-like protein typically found in marine phages,64,65 a glutaredoxin domain, a MuGam-like end protection protein sequence,66 and a YddF family protein, which is member of an interesting group of proteins found in three bacterial representatives: Bacillus subtilis, Clostridium beijerincki, and Alkaliphilus metalliredigenes; all other members are found specifically in archaeal viruses.67
φBB (Supplementary Fig. S4) annotation (36,404bp, 33.62% GC) contained 60 CDS with 63% of genes called as hypothetical. Analysis revealed no tRNAs, an integrase, DNA replication proteins, some structural proteins (but no tail tube proteins), and an endolysin. Several CDSs were identified as putative phage proteins with homology to F. nucleatum phage vB_FnuS-FNU4 or unnamed members of Caudoviricetes. Additionally, a single gene typically found in a Cas Type IB Cluster was detected (located 26,858–27,757bp within the genome) using the CRISPRCasFinder tool.68 Blastx identifies this as a type I CRISPR-associated protein Cas8a1/Csx8, the function of which is uncertain but believed to be involved in recognition and binding to DNA sequences and recruitment or activation of Cas3.69
For the two F. necrophorum subspecies funduliforme phages, φPaco (Supplementary Fig. S5) (43,165bp, 36.62% GC) contained a single tRNA(Ser), while none was found in the sequence of φRTG5 (Supplementary Fig. S6) (37,037bp, 35.04% GC). Neither phage annotation revealed morons or auxiliary metabolic genes, virulence factors or resistance genes of interest. φRTG5 contains a baseplate spike similar to the Gp138 family,70 as well as some phage Mu-like transposases and integrases, which putatively led the PhaBOX platform to classify it as a Myovirus, despite its small genome,71 an observation in agreement with morphology observed in TEM imaging.
Bioinformatic analysis indicates these phages are both novel and share little similarity to other phages known to infect the fusobacterium genus
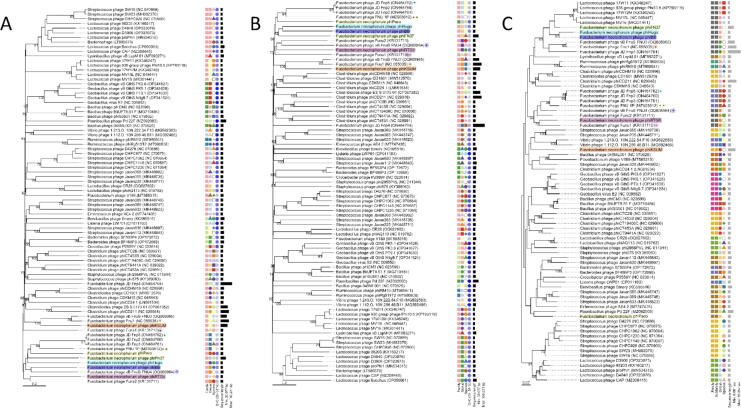
The six phages characterized and sequenced in this study exhibit minimal resemblance to other Fusobacterium-targeting phages listed in the NCBI database. Assessments using the VICTOR phylogenetic tool18 (Fig. 5a, Fig. 5b) categorizes these six phages under a common family cluster. Yet, they are differentiated at the genus and species levels indicating that they are more closely related to one another than to other viral genomes identified through a discontiguous megablast search. Phylogenomic GBDP trees using genomic nucleotide sequences (Fig. 5a, Fig. 5b), constructed with the D0 and D6 formulae, demonstrated an average support of 57% and 60%, respectively. The OPTSIL clustering yielded 79 (D0) and 76 (D6) species clusters, respectively. At the genus level, 37 (D0) and 34 (D6) clusters resulted. The number of clusters determined using both formulae at the family level was five.
FIG. 5.
VICTOR analysis of six novel phages and 75 reference phage genomes. Novel phages are highlighted, and the top complete viral genome hits from an NCBI discontiguous megablast query are marked with a + sign highlighted in a matching color. Different colored and shaped icons denote different clusters as identified by the approach, and do not refer to a particular ICTV classification. (A, B) Nucleotide-based analysis using formulae D0 and D6 identify all six phages as members of the same family cluster but as different genus and species, suggesting they are much more phylogenetically similar to each other than other deposited viral genomes returned with discontiguous megablast. (C) Using the amino acid sequences and the D4 formula, however, the derived tree shows a different clustering pattern, grouping three of the phages together but clustering the remaining three with disparate phage groups.
The analysis was also run using deposited amino acid datasets and formula D4, producing a phylogenomic GDBP tree yielding average support of 53%, which shows a slightly different picture (Fig. 5c). The OPTSIL clustering yielded 72 (D4) species clusters, and at the genus level, 20 clusters resulted. The number of clusters determined at the family and subfamily level was eight, of which the six novel phages presented in this study were members of three. φBB, φHugo, and φFN37 were still shown as being closely related, with φRTG5 identified as sharing their family and subfamily designation. However, φPaco and φKSUM were clustered more closely with phages able to infect Paenibacillus and a group of Streptococcus and Vibrio phages, respectively.
OrthoANI heat maps comparing our six isolates to their closest related deposited sequences (Fig. 6, Supplementary Fig. S7) show high average nucleotide identities (>90) among three phages (φBB, φHugo, and φFN37) but lower or no similarity to the other three phages isolated or other published genomes of Fusobacterium genus-infecting phages (Table 3, column 8). φFN37 had the most nonzero orthANI scores with some similarity (>50%) to 10 of the 11 genomes on which the analysis was performed. The exception was φRTG5, which was the most dissimilar of the six phages isolated, with a OrthoANI of 62.89 to Fusobacterium phage Fnu1 (NCBI Reference Sequence NC_055035.1) (Fig. 6) and 58.99 to Fusobacterium phage vB_FnuS-FNU4 (GenBank OQ808964.1) (Supplementary Fig. S7).
FIG. 6.
OrthoANI heatmaps highlights the variations in similarity among six novel and four reference phages. φBB, φHugo, and φFN37 showed a high orthogonal average nucleotide identity (>93%), corroborating the other metrics suggesting high similarity. However, φFN37 showed a moderate similarity (60–71%) to each of the deposited phages used as reference, while φBB had only similarity to vB_FnuS-FNU4 (65.93%, Supplementary Fig. S1). φKSUM, with the longest genome, had similarities to three of the new phages (excluding φHugo and φRTG5) and all five reference genomes. Overall, φRTG5 was found to be the most dissimilar of the six phages isolated, having a moderate similarity to Fusobacterium phage Fnu1 (above, 62.89%) and vB_FnuS-FNU4 (Supplementary Fig. S1, Fig. S58.99%).
Discussion
We isolated six genetically distinct F. necrophorum phages with differing morphologies and host specificities that, when combined, infected 79% of our library of contemporary bovine ruminal isolates. Phages φFN37 and φRTG5 are temperate phages obtained from mitomycin inductions of F. necrophorum subsp. necrophorum strain FN37 and F. necrophorum subsp. funduliforme strain RTG5, respectively. Phages φKSUM, φHugo, and φPaco were isolated from three different rumen fluid samples after enrichment. The origin of φBB is unclear; this phage was isolated on an FNC lysogen of φFN37 after a series of serial passages to attempt to isolate a lytic mutant 72–74 of phiFN37 using a coculture of FNC and FF34. Phages φKSUM and φBB showed a broader host range (infecting both subspecies) and robust growth inhibition of the model pathogen challenge strain. A common limitation of phage therapy is low phage titers when the target host density is low; this can be overcome using phages with a broader host range allowing the phages to use other hosts to proliferate. Within the ruminal compartment, the abundance of F. necrophorum subsp. necrophorum can be relatively low; thus, the ability to replicate on the putatively less pathogenic subspecies, funduliforme, may enhance the persistence and efficacy of this antimicrobial treatment.
The scarcity of professionally lytic phages against F. necrophorum, despite extensive sampling and enrichment, and the presence of genes associated with lysogeny in all six phages presented in this study, is in line with recent research into the commonality of lysogeny and pseudolysogeny in the gut microbiota.75,76 Despite containing genes associated with a temperate lifestyle, four of our phages repressed growth of our target strain, corroborating that phages with putative lysogenic potential may be an untapped resource in phage therapy.77 Although the genomic sequences of phages isolated in this study showed potential for a lysogenic lifestyle, two did not lysogenize any host tested. After phage exposure, permanent and heritable bacterial resistance did not develop easily against φKSUM or, in the case of φBB, never occurred, despite many rounds of exposure, isolation and testing of surviving bacterial clones. φBB’s improved killing activity and inability to lysogenize supports the use of lysogenic phages as a resource for phage therapy and biocontrol applications.
Although the host range of four of the six phages appeared to be limited to the isolation host subspecies, BLASTn of all six phages produced alignments to genomic sequences of both F. necrophorum subspecies, as well as short alignments to other members of the genus. However, similarities to deposited phage genomes shown to infect other members of the Fusobacterium genera were extremely low, highlighting the novelty of these phages. Annotations were replete with hypothetical proteins, although specific structural proteins were detected and were in agreement with our TEM observations. VICTOR taxonomic analysis and OrthoANI heat maps further confirmed their unique nature. φRTG5 stands out as having one of the narrowest host ranges and is most genetically dissimilar to the other isolated phages and deposited sequences, suggesting that it may represent a distinct lineage. There was a consistent lack of similarity to deposited phage genomes. However, the majority of the phages, which were returned from the discontiguous megablast are reported as infecting Gram-positive hosts (e.g., Streptococcus, Clostridium, Bacillus), which suggests that the mosaic theory of the Fusobacterium genus may have merit.78,79
Recent related research revealed that the ruminal microbiome and liver abscess etiology may be a more complex picture than previously described,5,6 although F. necrophorum is still considered a key player meriting further study and new methods of control. Although lytic phages are preferred for phage-based antimicrobials, there are scenarios, such as in the case of Clostridium difficile, where lytic phages for a particular target are yet to be discovered; therefore, lysogenic phages able to mitigate bacterial proliferation should be considered.80 However, thorough gene analysis is necessary to ensure that the selected phages do not contribute to the propagation of undesired traits in bacterial populations (e.g., pathogenicity or antibiotic resistance) through lysogenic conversion and transfection. Among the phages we isolated, no virulence genes were detected, and the two most effective antimicrobial isolates were never observed to lysogenize their hosts, perhaps due to a mutation within the integration genes or a phage–host incompatibility. Another strategy may be to evolve or engineer these and similar phages to be obligately lytic or perhaps, in the future, to transfer certain genes (e.g., tail fibers for broader host recognition, or specific lysis genes) from phages such as these to a universal chassis.
Conclusions
This study provides valuable insights into the genetic and phenotypic diversity of F. necrophorum phages, contributing to the broader understanding of phage biology and ecology, particularly within the bovine rumen. The genome sequences and associated annotations also offer a valuable resource for future comparative genomic and evolutionary studies. Furthermore, the phages isolated in this study effectively repressed a commonly used challenge strain in vitro, indicating potential utility for future biocontrol applications against F. necrophorum ruminal species. This study’s significance extends beyond the characterization of novel F. necrophorum bacteriophages, as it supports previous work suggesting only temperate phages might be easily isolated for certain bacterial species. Thus, understanding the biology and ecology of such phages helps establish a foundation for harnessing lysogenic phages for antimicrobial and microbiome engineering applications.
Supplementary Material
Authors’ Contributions
J.M., C.S., and J.L.G. designed the experiments. C.S., M.M., M.T., and J.L.G. performed the experiments. C.H. prepared grids and performed T.E.M. C.S., J.L.G., and J.M. analyzed the data. C.S., J.L.G., J.M., and P.A. contributed to the writing of this article. All authors have reviewed and approved the article before submission and agree to be accountable for all aspects of the work.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was funded by Small Business Innovation Research (SBIR) grants from the National Science Foundation (2025980) and the United States Department of Agriculture (2022–04378).
References
- 1. Mann A, Nehra K, Rana JS, et al. Antibiotic resistance in agriculture: Perspectives on upcoming strategies to overcome upsurge in resistance. Curr Res Microb Sci 2021;2:100030; doi: 10.1016/j.crmicr.2021.100030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gambino M, Brøndsted L. Looking into the future of phage-based control of zoonotic pathogens in food and animal production. Curr Opin Biotechnol 2021;68:96–103; doi: 10.1016/j.copbio.2020.10.003 [DOI] [PubMed] [Google Scholar]
- 3. Schwarz C, Mathieu J, Laverde Gomez JA, et al. Renaissance for phage-based bacterial control. Environ Sci Technol 2021;56(8):4691–4701; doi: 10.1021/acs.est.1c06232 [DOI] [PubMed] [Google Scholar]
- 4. Nagaraja TG, Lechtenberg KF. Liver abscesses in feedlot cattle. Vet Clin North Am Food Anim Pract 2007;23(2):351–369, ix; doi: 10.1016/j.cvfa.2007.05.002 [DOI] [PubMed] [Google Scholar]
- 5. Pinnell LJ, Whitlow CW, Huebner KL, et al. Not all liver abscesses are created equal: The impact of tylosin and antibiotic alternatives on bovine liver abscess microbial communities and a first look at bacteroidetes-dominated communities. Front Microbiol 2022;13:882419; doi: 10.3389/fmicb.2022.882419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wright WF, Shiner CN, Ribes JA. Lemierre syndrome. South Med J 2012;105(5):283–288. [DOI] [PubMed] [Google Scholar]
- 7. Brooks JW, Kumar A, Narayanan S, et al. Characterization of fusobacterium isolates from the respiratory tract of white-tailed deer (Odocoileus virginianus). J Vet Diagn Invest 2014;26(2):213–220; doi: 10.1177/1040638714523613 [DOI] [PubMed] [Google Scholar]
- 8. Nagaraja TG, Narayanan SK, Stewart GC, et al. Fusobacterium necrophorum infections in animals: Pathogenesis and pathogenic mechanisms. Anaerobe 2005;11(4):239–246; doi: 10.1016/j.anaerobe.2005.01.007 [DOI] [PubMed] [Google Scholar]
- 9. Tamada H, Harasawa R, Shinjo T. Isolation of a bacteriophage in fusobacterium necrophorum. Nihon Juigaku Zasshi 1985;47(3):483–486; doi: 10.1292/jvms1939.47.483 [DOI] [PubMed] [Google Scholar]
- 10. Narayanan S, Nagaraja TG, Okwumabua O, et al. Ribotyping to compare Fusobacterium necrophorum isolates from bovine liver abscesses, ruminal walls, and ruminal contents. Appl Environ Microbiol 1997;63(12):4671–4678; doi: 10.1128/aem.63.12.4671-4678.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hobbs Z, Abedon ST. Diversity of phage infection types and associated terminology: The problem with ‘Lytic or lysogenic’. FEMS Microbiol Lett 2016;363(7):fnw047. [DOI] [PubMed] [Google Scholar]
- 12. Schwarz C, Mathieu J, Laverde Gomez J, et al. Unexpected finding of Fusobacterium varium as the dominant Fusobacterium species in cattle rumen: Potential implications for liver abscess etiology and interventions. J Anim Sci 2023;101; doi: 10.1093/jas/skad130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Henrot C, Petit M-A. Signals triggering prophage induction in the gut microbiota. Mol Microbiol 2022;118(5):494–502; doi: 10.1111/mmi.14983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peptone Yeast Extract Broth (PY) AS-821. 2017. Available from: https://anaerobesystems.com/products/broth-media/peptone-yeast/
- 15. Brazier JS, Citron DM, Goldstein EJ. A selective medium for Fusobacterium spp. J Appl Bacteriol 1991;71(4):343–346; doi: 10.1111/j.1365-2672.1991.tb03798.x [DOI] [PubMed] [Google Scholar]
- 16. Adams MH. Enumeration of bacteriophage particles. Bacteriophages 1959:27–30. [Google Scholar]
- 17. Peluso EA, Scheible M, Ton-That H, et al. Genetic manipulation and virulence assessment of fusobacterium nucleatum. Curr Protoc Microbiol 2020;57(1):e104; doi: 10.1002/cpmc.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Konopacki M, Grygorcewicz B, Dołęgowska B, et al. PhageScore: A simple method for comparative evaluation of bacteriophages lytic activity. Biochemical Engineering Journal 2020;161:107652; doi: 10.1016/j.bej.2020.107652 [DOI] [Google Scholar]
- 19. Unterer M, Mirzaei MK, Deng L. Targeted single-phage isolation reveals phage-dependent heterogeneous infection dynamics. Microbiol Spectr 2023;11(3):e05149-22; doi: 10.1128/spectrum.05149-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sørensen PE, Ng DYK, Duchateau L, et al. Classification of in vitro phage-host population growth dynamics. Microorganisms 2021;9(12); doi: 10.3390/microorganisms9122470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grygorcewicz B, Roszak M, Rakoczy R, et al. PhageScore-based analysis of Acinetobacter baumannii infecting phages antibiotic interaction in liquid medium. Arch Microbiol 2022;204(7):421; doi: 10.1007/s00203-022-03020-7 [DOI] [PubMed] [Google Scholar]
- 22. Gelman D, Yerushalmy O, Alkalay-Oren S, et al. Clinical phage microbiology: A suggested framework and recommendations for the in-vitro matching steps of phage therapy. Lancet Microbe 2021;2(10):e555–e563; doi: 10.1016/S2666-5247(21)00127-0 [DOI] [PubMed] [Google Scholar]
- 23. Glonti T, Pirnay JP. In vitro techniques and measurements of phage characteristics that are important for phage therapy success. Viruses 2022;14(7):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Altamirano FLG, Barr JJ. Screening for lysogen activity in therapeutically relevant bacteriophages. Bio Protoc 2021;11(8):e3997; doi: 10.21769/BioProtoc.3997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Summer EJ. Preparation of a phage DNA fragment library for whole genome shotgun sequencing. Methods Mol Biol 2009;502:27–46; doi: 10.1007/978-1-60327-565-1_4 [DOI] [PubMed] [Google Scholar]
- 26. Wick RR, Judd LM, Gorrie CL, et al. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol 2017;13(6):e1005595; doi: 10.1371/journal.pcbi.1005595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bouras G, Nepal R, Houtak G, et al. Pharokka: A fast scalable bacteriophage annotation tool. Bioinformatics 2022;39(1); doi: 10.1093/bioinformatics/btac776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Camacho C, Boratyn GM, Joukov V, et al. ElasticBLAST: Accelerating sequence search via cloud computing. BMC Bioinformatics 2023;24(1):117; doi: 10.1186/s12859-023-05245-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pearson WR. An introduction to sequence similarity (“homology”) searching. Curr Protoc Bioinformatics 2013; Chapter 3:3.1.1–3.1.8; doi: 10.1002/0471250953.bi0301s42Chapter 3; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Benson DA, Cavanaugh M, Clark K, et al. GenBank. Nucleic Acids Res 2013;41(Database issue):D36–42; doi: 10.1093/nar/gks1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grant JR, Enns E, Marinier E, et al. Proksee: In-depth characterization and visualization of bacterial genomes. Nucleic Acids Res 2023;51(W1):W484–W492; doi: 10.1093/nar/gkad326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meier-Kolthoff JP, Göker M. VICTOR: Genome-based phylogeny and classification of prokaryotic viruses. Bioinformatics 2017;33(21):3396–3404; doi: 10.1093/bioinformatics/btx440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meier-Kolthoff JP, Auch AF, Klenk H-P, et al. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics 2013;14(1):60; doi: 10.1186/1471-2105-14-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lefort V, Desper R, Gascuel O. FastME 2.0: A comprehensive, accurate, and fast distance-based phylogeny inference program. Mol Biol Evol 2015;32(10):2798–2800; doi: 10.1093/molbev/msv150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Farris JS. Estimating phylogenetic trees from distance matrices. The American Naturalist 1972;106(951):645–668. [Google Scholar]
- 36. Yu G. Using ggtree to visualize data on tree-like structures. Curr Protoc Bioinformatics 2020;69(1):e96; doi: 10.1002/cpbi.96 [DOI] [PubMed] [Google Scholar]
- 37. Göker M, García-Blázquez G, Voglmayr H, et al. Molecular taxonomy of phytopathogenic fungi: A case study in peronospora. PLoS One 2009;4(7):e6319; doi: 10.1371/journal.pone.0006319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meier-Kolthoff JP, Hahnke RL, Petersen J, et al. Complete genome sequence of DSM 30083(T), the type strain [U5/41(T)] of Escherichia coli, and a proposal for delineating subspecies in microbial taxonomy. Stand Genomic Sci 2014;9:2; doi: 10.1186/1944-3277-9-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Altschul SF, Gish W, Miller W, et al. Basic local alignment search tool. J Mol Biol 1990;215(3):403–410; doi: 10.1016/s0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 40. Lee I, Ouk Kim Y, Park SC, et al. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int J Syst Evol Microbiol 2016;66(2):1100–1103; doi: 10.1099/ijsem.0.000760 [DOI] [PubMed] [Google Scholar]
- 41. McNair K, Aziz RK, Pusch GD, et al. Phage genome annotation using the RAST pipeline. Methods Mol Biol 2018;1681:231–238; doi: 10.1007/978-1-4939-7343-9_17 [DOI] [PubMed] [Google Scholar]
- 42. McNair K, Zhou C, Dinsdale EA, et al. Phanotate: A novel approach to gene identification in phage genomes. Bioinformatics 2019;35(22):4537–4542; doi: 10.1093/bioinformatics/btz265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hockenberry AJ, Wilke CO. BACPHLIP: Predicting bacteriophage lifestyle from conserved protein domains. PeerJ 2021;9:e11396; doi: 10.7717/peerj.11396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tynecki P, Guziński A, Kazimierczak J, et al. PhageAI - bacteriophage life cycle recognition with machine learning and natural language Processing. bioRxiv 2020;2020.07.11.198606, doi: 10.1101/2020.07.11.198606 [DOI]
- 45. Garneau JR, Depardieu F, Fortier L-C, et al. PhageTerm: A tool for fast and accurate determination of phage termini and packaging mechanism using next-generation sequencing data. Sci Rep 2017;7(1):8292; doi: 10.1038/s41598-017-07910-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li S, Fan H, An X, et al. Scrutinizing virus genome termini by high-throughput sequencing. PLoS One 2014;9(1):e85806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kala S, Cumby N, Sadowski PD, et al. HNH proteins are a widespread component of phage DNA packaging machines. Proc Natl Acad Sci U S A 2014;111(16):6022–6027; doi: 10.1073/pnas.1320952111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sun C, Chen J, Jin M, et al. Long-read sequencing reveals extensive DNA methylations in human gut phagenome contributed by prevalently phage-encoded methyltransferases. Adv Sci (Weinh) 2023;10(25):e2302159; doi: 10.1002/advs.202302159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yasmin A, Kenny JG, Shankar J, et al. Comparative genomics and transduction potential of enterococcus faecalis temperate bacteriophages. J Bacteriol 2010;192(4):1122–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Castelle CJ, Méheust R, Jaffe AL, et al. Protein family content uncovers lineage relationships and bacterial pathway maintenance mechanisms in DPANN archaea. Front Microbiol 2021;12:660052; doi: 10.3389/fmicb.2021.660052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Allali I, Delgado S, Marron PI, et al. Gut microbiome compositional and functional differences between tumor and non-tumor adjacent tissues from cohorts from the US and Spain. Gut Microbes 2015;6(3):161–172; doi: 10.1080/19490976.2015.1039223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kushkevych I, Cejnar J, Treml J, et al. Recent advances in metabolic pathways of sulfate reduction in intestinal bacteria. Cells 2020;9(3):698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Unterholzner SJ, Poppenberger B, Rozhon W. Toxin-antitoxin systems: Biology, identification, and application. Mob Genet Elements 2013;3(5):e26219; doi: 10.4161/mge.26219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mann NH, Clokie MRJ, Millard A, et al. The Genome of S-PM2, a “Photosynthetic” T4-Type Bacteriophage That Infects Marine Synechococcus Strains. J Bacteriol 2005;187(9):3188–3200; doi: 10.1128/jb.187.9.3188-3200.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gomis-Rüth FX, Solá M, Acebo P, et al. The structure of plasmid-encoded transcriptional repressor CopG unliganded and bound to its operator. Embo J 1998;17(24):7404–7415; doi: 10.1093/emboj/17.24.7404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Iyer LM, Koonin EV, Aravind L. Extensive domain shuffling in transcription regulators of DNA viruses and implications for the origin of fungal APSES transcription factors. Genome Biol 2002;3(3):research0012.1; doi: 10.1186/gb-2002-3-3-research0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Azulay G, Pasechnek A, Stadnyuk O, et al. A dual-function phage regulator controls the response of cohabiting phage elements via regulation of the bacterial SOS response. Cell Rep 2022;39(3):110723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wood HE, Devine KM, McConnell DJ. Characterisation of a repressor gene (xre) and a temperature-sensitive allele from the Bacillus subtilis prophage, PBSX. Gene 1990;96(1):83–88; doi: 10.1016/0378-1119(90)90344-Q [DOI] [PubMed] [Google Scholar]
- 59. Zheng H, Liu B, Xu Y, et al. An inducible microbacterium prophage vB_MoxS-R1 represents a novel lineage of siphovirus. Viruses 2022;14(4):731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hutinet G, Besle A, Son O, et al. Sak4 of phage HK620 Is a RecA remote homolog with single-strand annealing activity stimulated by its cognate SSB protein. Front Microbiol 2018;9:743; doi: 10.3389/fmicb.2018.00743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Karvelis T, Druteika G, Bigelyte G, et al. Transposon-associated TnpB is a programmable RNA-guided DNA endonuclease. Nature 2021;599(7886):692–696; doi: 10.1038/s41586-021-04058-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Georgopoulos CP, Lundquist-Heil A, Yochem J, et al. Identification of the E. coli dnaJ gene product. Mol Gen Genet 1980;178(3):583–588; doi: 10.1007/BF00337864 [DOI] [PubMed] [Google Scholar]
- 63. Kabwe M, Brown TL, Dashper S, et al. Genomic, morphological and functional characterisation of novel bacteriophage FNU1 capable of disrupting Fusobacterium nucleatum biofilms. Sci Rep 2019;9(1):9107; doi: 10.1038/s41598-019-45549-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Goldsmith DB, Crosti G, Dwivedi B, et al. Development of phoH as a novel signature gene for assessing marine phage diversity. Appl Environ Microbiol 2011;77(21):7730–7739; doi: 10.1128/aem.05531-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Luna AJ, Wood TL, Chamakura KR, et al. Complete genome of salmonella enterica serovar enteritidis myophage Marshall. Genome Announc 2013;1(6); doi: 10.1128/genomea.00867-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bhattacharyya S, Soniat MM, Walker D, et al. Phage Mu Gam protein promotes NHEJ in concert with Escherichia coli ligase. Proc Natl Acad Sci U S A 2018;115(50):E11614–E11622; doi: 10.1073/pnas.1816606115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Prangishvili D, Garrett RA, Koonin EV. Evolutionary genomics of archaeal viruses: Unique viral genomes in the third domain of life. Virus Res 2006;117(1):52–67. [DOI] [PubMed] [Google Scholar]
- 68. Couvin D, Bernheim A, Toffano-Nioche C, et al. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res 2018;46(W1):W246–w251; doi: 10.1093/nar/gky425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Makarova K, Haft D, Barrangou R, et al. Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol 2011;9(6):467–477; doi: 10.1038/nrmicro2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Browning C, Shneider MM, Bowman VD, et al. Phage pierces the host cell membrane with the iron-loaded spike. Structure 2012;20(2):326–339; doi: 10.1016/j.str.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 71. Comeau AM, Tremblay D, Moineau S, et al. Phage morphology recapitulates phylogeny: The comparative genomics of a new group of myoviruses. PLoS One 2012;7(7):e40102; doi: 10.1371/journal.pone.0040102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jesaitis MA, Hutton JJ. Properties of a bacteriophage derived from Escherichia coli K235. J Exp Med 1963;117(2):285–302; doi: 10.1084/jem.117.2.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Poullain V, Gandon S, Brockhurst MA, et al. The evolution of specificity in evolving and coevolving antagonistic interactions between a bacteria and its phage. Evolution 2008;62(1):1–11. [DOI] [PubMed] [Google Scholar]
- 74. Burrowes BH, Molineux IJ, Fralick JA. Directed in vitro evolution of therapeutic bacteriophages: The appelmans protocol. Viruses 2019;11(3):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Łoś M, Węgrzyn G. Chapter 9—pseudolysogeny. In: Łobocka M, Szybalski WT.; eds. Advances in Virus Research. Academic Press: 2012; pp. 339-349. [DOI] [PubMed] [Google Scholar]
- 76. Berg Miller ME, Yeoman CJ, Chia N, et al. Phage-bacteria relationships and CRISPR elements revealed by a metagenomic survey of the rumen microbiome. Environ Microbiol 2012;14(1):207–227; doi: 10.1111/j.1462-2920.2011.02593.x [DOI] [PubMed] [Google Scholar]
- 77. Monteiro R, Pires DP, Costa AR, et al. Phage therapy: Going temperate? Trends Microbiol 2019;27(4):368–378; doi: 10.1016/j.tim.2018.10.008 [DOI] [PubMed] [Google Scholar]
- 78. Dion MB, Oechslin F, Moineau S. Phage diversity, genomics and phylogeny. Nat Rev Microbiol 2020;18(3):125–138; doi: 10.1038/s41579-019-0311-5 [DOI] [PubMed] [Google Scholar]
- 79. Robinson A, Wilde J, Allen-Vercoe E. Chapter 6—fusobacteria: Physiology, form, and function. In: Colorectal Neoplasia and the Colorectal Microbiome. (Floch MH. ed.) Academic Press: 2020; pp. 95-134. [Google Scholar]
- 80. Hargreaves KR, Clokie MRJ. Clostridium difficile phages: Still difficult? Front Microbiol 2014;5:184; doi: 10.3389/fmicb.2014.00184 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.